User login
Statin therapy: New data suggest effects on plaque volume and stability
| ||||||||||||||||
| ||||||||||||||||
Stop shingles in its tracks
• Initiate antiviral treatment as soon as possible; rapid resolution of acute pain and reduction in the development of postherpetic neuralgia (PHN) are most likely when therapy is started within 72 hours of the outbreak. A
• Discuss herpes zoster (HZ) vaccination with healthy patients 60 years of age and older during their first office visit; the vaccine markedly reduces the incidence of HZ and PHN. A
• Do not prescribe tricyclic antidepressants or corticosteroids in the acute phase of HZ. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
We don’t do enough to protect our patients from the pain of herpes zoster (HZ). Consider:
- Each year in the United States, about 1 million new cases of herpes zoster (HZ) occur.1 The incidence is estimated at 3 to 4 per 1,000 in the general population,2 but climbs to more than 10 per 1000 among people 60 years of age and older.3,4
- Overall, between 13% and 26% of patients with HZ develop postherpetic neuralgia (PHN), defined as pain that continues for more than 1 month after the rash has healed. Among patients who are 70 or older, however, the likelihood that HZ will progress to PHN is approximately 50%.5 (In a study of 7595 patients being treated for HZ or PHN by general practitioners or dermatologists in France, 45% reported pain that was severe or very severe, and 42% reported permanent pain.6 )
- Between 10% and 25% of HZ patients develop ocular complications, which have the potential to result in vision loss, facial scarring, or prolonged or permanent pain.7 Encephalitis, myelitis, and peripheral nerve palsies are potential complications, as well.
Yet HZ and its complications are largely preventable.
A live attenuated vaccine (Zostavax) received US Food and Drug Administration approval in 2006.8 But many patients have not yet heard of it, and many physicians fail to recommend it. (See “Herpes zoster vaccine: Why aren’t more people receiving it?”.)
As a family physician, you can play a key role in reducing the burden of shingles by rapidly identifying and treating HZ, minimizing the risk of prolonged pain, and, notably, by talking to older patients about the benefits of vaccination.
Zostavax, a live attenuated herpes zoster (HZ) vaccine, was licensed by the US Food and Drug Administration in 2006 for use in people 60 years of age and older—the first new vaccine targeting this age group in years. In 2007, researchers at the Centers for Disease Control and Prevention (CDC) conducted a national survey,20 in part to gauge the knowledge of, and interest in, the HZ vaccine among the intended recipients.
Their findings: Of more than 3500 respondents, only 1.9% had received the HZ vaccine. Most (72%) were unaware of the vaccine’s existence, but the majority said they would agree to vaccination if their physician were to recommend it.
Among those who were aware of it, the key reasons for rejecting the vaccine were that it was not needed (cited by 35%), they were not at risk (13%), and a lack of trust in doctors or the US health care system (10%). Both the limited awareness of the vaccine and the lack of physician recommendations are barriers to HZ vaccination, the researchers concluded.20
On its Web site, the CDC broaches another potential barrier to greater use of the HZ vaccine: cost. The vaccine is not covered by Medicare Part B, nor by some private insurers. While it is covered by all Medicare Part D plans, the extent of coverage depends on the particular plan. The CDC recommends that physicians encourage patients to contact their insurers to determine the extent of their coverage.21
Start antiviral therapy without delay
Several meta-analyses and many (though not all) randomized controlled trials (RCTs) of HZ treatment have demonstrated that prompt antiviral therapy—with oral acyclovir (ACV), valacyclovir (VCV), or famciclovir (FCV)—reduces the duration of acute pain and the likelihood that PHN will develop.9,10 Without antiviral therapy, up to 45% of patients over the age of 60 experience pain that persists for 6 months to a year. Even with therapy, studies have found that about 20% of patients older than 50 years continued to have pain for 6 months after their rash appeared.10 Risk factors for PHN include age (>50 years), sex (female), a disseminated rash, a severe pain presentation, and polymerase chain reaction-detectable varicella zoster virus viremia.11
Which agent? What the research reveals
For most people with HZ, any of the 3 antiviral agents can be used, based on physician and patient preference. (See TABLE for treatment guidelines.) Here’s a look at the evidence for each.
Oral ACV has long been the mainstay of treatment for HZ, but its poor bioavailability and the need for 5 daily doses has led to the development of newer antiviral agents.12 When initiated within 48 to 72 hours of the onset of the rash, ACV has demonstrated clinical benefit. (The value of starting ACV therapy beyond the 72-hour mark has not been established, though treatment should be considered if new lesions are still appearing.)
In 1 meta-analysis of 4 placebo-controlled trials, ACV accelerated resolution of acute pain, with the greatest effect in those older than 50 years.13 In a second meta-analysis, treatment with ACV reduced the incidence of PHN at 3 months by 46% (number needed to treat [NNT]=3.2-8).12
VCV is well absorbed in the gastrointestinal tract, providing 3- to 5-fold greater bioavailability compared with ACV.13 VCV’s efficacy was demonstrated in an RCT in which the researchers conducted an intent-to-treat analysis: Compared with ACV therapy for 7 to 14 days, VCV significantly accelerated the resolution of acute pain, reduced the duration of PHN, and decreased the proportion of patients with pain persisting for more than 6 months (19% vs 26%). The incidence of adverse events was similar in both groups.14
FCV has broad activity against varicella-zoster virus.15 In an RCT that evaluated oral FCV in 419 immunocompetent adults (mean age 50 years) with uncomplicated HZ, FCV was well tolerated and accelerated lesion healing compared with placebo. Among those who developed PHN, the pain resolved twice as fast for patients in the FCV group compared with the controls, and the median duration of PHN was reduced by 2 months.15
TABLE
Antiviral therapy dosing guidelines*
| Dosage adjusted for creatinine clearance† (mL/min) | ||||
|---|---|---|---|---|
| Drug | Standard dose | <10 | 10-25 | Duration |
| ACV | 800 mg 5x/d | 1600 mg/d | 2400 mg/d | 7-10 days |
| VCV | 1000 mg tid | 1000 mg/d | 2000 mg/d | 7 days |
| FCV | 750 mg/d or 250 mg tid | 250 mg/d | 500 mg/d | 7 days |
| *All 3 drugs reduce acute pain and development of postherpetic neuralgia, and are most effective when started within 72 hours of onset of rash. | ||||
| †Patients with creatinine clearance >25 mL/min receive the standard dose. | ||||
| ACV, acyclovir; FCV, famciclovir; VCV, valacyclovir. | ||||
Analgesics and other drugs: What to consider
While antiviral therapy helps to relieve the pain of HZ, several trials have shown that none of the available agents completely alleviates it or routinely prevents the development of PHN. As a result, adjunctive therapy, including pain medication, is often required. But prescribing analgesics to frail elderly patients and those who have comorbidities and take multiple medications is not without risk.
The ability of a tricyclic antidepressant to alleviate pain and prevent PHN when therapy is initiated within 48 hours of the eruption of lesions was tested in a double-blind trial in which 72 patients 60 years of age or older were randomized to amitriptyline 25 mg daily for 90 days or placebo.16 Antiviral agents were administered according to the preference of the primary physician. At 6 weeks, the pain prevalence—the primary outcome measure—was reduced by about 50% in the amitriptyline group.16 There is no other evidence to support the use of tricyclics in the acute phase of HZ, however, and concerns about orthostatic hypotension and anticholinergic side effects limit their use, particularly in older patients.
Corticosteroids are sometimes used, too, often in conjunction with antiviral therapy, but there are problems with this approach, as well. One RCT comparing an ACV-prednisone combination with ACV alone in HZ patients over the age of 50 found that patients who received both drugs had faster resolution of acute pain and earlier discontinuation of analgesics.17 But several serious adverse effects of prednisone were reported in patients in the combination therapy group, despite the fact that individuals with contraindications to corticosteroids were excluded from the study. Overall, there is little evidence to suggest that steroids can be safely used to reduce the incidence or severity of PHN. There is no specific recommendation regarding analgesic therapy for PHN, but physicians often adopt a stepwise approach.
Recommend the shingles vaccine
In view of the toll that shingles often takes, vaccination is the best way to prevent HZ and its complications. In a randomized, double-blind, placebo-controlled study involving 38,546 adults who were 60 years of age or older, researchers demonstrated that cell-mediated immunity to the varicella-zoster virus was boosted by the live attenuated HZ vaccine.18 The enhanced immunity was associated with a 51% reduction in the incidence of HZ (NNT=58 to prevent 1 case over 3 years), a 66% reduction in the incidence of PHN (NNT=364 to prevent 1 case of PHN over 3 years), and a 61% reduction in disease burden. The vaccine was well tolerated, and injection site reactions were generally mild.18 Accurate cost-effectiveness analyses of immunization are not available because the duration of vaccine protection is unknown.19
FIGURE
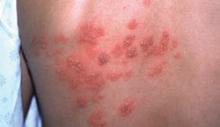
A unilateral vesicular rash
A shingles outbreak, like the rash shown on this patient’s back, usually appears as a patch or band of blisters on 1 side of the body.
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination to prevent both HZ and PHN in healthy adults who are 60 years of age and older (individuals with primary or acquired immunodeficiencies or patients on immunosuppressive therapies should not be vaccinated), and suggests that practitioners offer the HZ vaccine to appropriate patients at their first visit.7 It is not necessary, however, to ask about the patient’s history of varicella or to conduct serologic testing to determine varicella immunity before administering the HZ vaccine.7 A history of shingles is not a contraindication, so advise patients who develop HZ to come in for vaccination soon after the rash and pain resolve.
CORRESPONDENCE
Pierre-Olivier Lang, MD, MPH, PhD, University Hospitals of Geneva, Department of Rehabilitation and Geriatrics, Chemin du Pont-Bochet, 3, CH-1226, Thonex-Geneva, Switzerland; [email protected]
1. Centers for Disease Control and Prevention. Shingles disease – questions and answers (herpes zoster). Available at http://www.cdc.gov/vaccines/vpd-vac/shingles/dis-faqs.htm. Accessed September 1, 2009.
2. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211-1215.
3. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571-575.
4. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33.
5. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine. 2006;24:1308-1314.
6. Chidiac C, Buxelles J, Daures JP, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis. 2001;33:62-69.
7. Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57:1-30.
8. US Food and Drug Administration. FDA licenses new vaccine to reduce older Americans’ risk of shingles. May 26, 2006. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108659.htm. Accessed September 1, 2009.
9. Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748-50.
10. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
11. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545-1551.
12. Jackson JL, Gibbons R, Meyer G, et al. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157:909-912.
13. Wood MJ, Kay R, Dworkin RH, et al. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347.
14. Beutner KR, Friedman DJ, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-1553.
15. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
16. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
17. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
18. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
19. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317-325.
20. Lu P, Euler GL, Jumaan AO, et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2009;27:882-887.
21. Centers for Disease Control and Prevention. Herpes zoster – vaccine Q&As for providers. Available at: http://www.cdc.gov/vaccines/vpd-vac/shingles/vac-faqs-hcp.htm. Accessed September 1, 2009.
• Initiate antiviral treatment as soon as possible; rapid resolution of acute pain and reduction in the development of postherpetic neuralgia (PHN) are most likely when therapy is started within 72 hours of the outbreak. A
• Discuss herpes zoster (HZ) vaccination with healthy patients 60 years of age and older during their first office visit; the vaccine markedly reduces the incidence of HZ and PHN. A
• Do not prescribe tricyclic antidepressants or corticosteroids in the acute phase of HZ. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
We don’t do enough to protect our patients from the pain of herpes zoster (HZ). Consider:
- Each year in the United States, about 1 million new cases of herpes zoster (HZ) occur.1 The incidence is estimated at 3 to 4 per 1,000 in the general population,2 but climbs to more than 10 per 1000 among people 60 years of age and older.3,4
- Overall, between 13% and 26% of patients with HZ develop postherpetic neuralgia (PHN), defined as pain that continues for more than 1 month after the rash has healed. Among patients who are 70 or older, however, the likelihood that HZ will progress to PHN is approximately 50%.5 (In a study of 7595 patients being treated for HZ or PHN by general practitioners or dermatologists in France, 45% reported pain that was severe or very severe, and 42% reported permanent pain.6 )
- Between 10% and 25% of HZ patients develop ocular complications, which have the potential to result in vision loss, facial scarring, or prolonged or permanent pain.7 Encephalitis, myelitis, and peripheral nerve palsies are potential complications, as well.
Yet HZ and its complications are largely preventable.
A live attenuated vaccine (Zostavax) received US Food and Drug Administration approval in 2006.8 But many patients have not yet heard of it, and many physicians fail to recommend it. (See “Herpes zoster vaccine: Why aren’t more people receiving it?”.)
As a family physician, you can play a key role in reducing the burden of shingles by rapidly identifying and treating HZ, minimizing the risk of prolonged pain, and, notably, by talking to older patients about the benefits of vaccination.
Zostavax, a live attenuated herpes zoster (HZ) vaccine, was licensed by the US Food and Drug Administration in 2006 for use in people 60 years of age and older—the first new vaccine targeting this age group in years. In 2007, researchers at the Centers for Disease Control and Prevention (CDC) conducted a national survey,20 in part to gauge the knowledge of, and interest in, the HZ vaccine among the intended recipients.
Their findings: Of more than 3500 respondents, only 1.9% had received the HZ vaccine. Most (72%) were unaware of the vaccine’s existence, but the majority said they would agree to vaccination if their physician were to recommend it.
Among those who were aware of it, the key reasons for rejecting the vaccine were that it was not needed (cited by 35%), they were not at risk (13%), and a lack of trust in doctors or the US health care system (10%). Both the limited awareness of the vaccine and the lack of physician recommendations are barriers to HZ vaccination, the researchers concluded.20
On its Web site, the CDC broaches another potential barrier to greater use of the HZ vaccine: cost. The vaccine is not covered by Medicare Part B, nor by some private insurers. While it is covered by all Medicare Part D plans, the extent of coverage depends on the particular plan. The CDC recommends that physicians encourage patients to contact their insurers to determine the extent of their coverage.21
Start antiviral therapy without delay
Several meta-analyses and many (though not all) randomized controlled trials (RCTs) of HZ treatment have demonstrated that prompt antiviral therapy—with oral acyclovir (ACV), valacyclovir (VCV), or famciclovir (FCV)—reduces the duration of acute pain and the likelihood that PHN will develop.9,10 Without antiviral therapy, up to 45% of patients over the age of 60 experience pain that persists for 6 months to a year. Even with therapy, studies have found that about 20% of patients older than 50 years continued to have pain for 6 months after their rash appeared.10 Risk factors for PHN include age (>50 years), sex (female), a disseminated rash, a severe pain presentation, and polymerase chain reaction-detectable varicella zoster virus viremia.11
Which agent? What the research reveals
For most people with HZ, any of the 3 antiviral agents can be used, based on physician and patient preference. (See TABLE for treatment guidelines.) Here’s a look at the evidence for each.
Oral ACV has long been the mainstay of treatment for HZ, but its poor bioavailability and the need for 5 daily doses has led to the development of newer antiviral agents.12 When initiated within 48 to 72 hours of the onset of the rash, ACV has demonstrated clinical benefit. (The value of starting ACV therapy beyond the 72-hour mark has not been established, though treatment should be considered if new lesions are still appearing.)
In 1 meta-analysis of 4 placebo-controlled trials, ACV accelerated resolution of acute pain, with the greatest effect in those older than 50 years.13 In a second meta-analysis, treatment with ACV reduced the incidence of PHN at 3 months by 46% (number needed to treat [NNT]=3.2-8).12
VCV is well absorbed in the gastrointestinal tract, providing 3- to 5-fold greater bioavailability compared with ACV.13 VCV’s efficacy was demonstrated in an RCT in which the researchers conducted an intent-to-treat analysis: Compared with ACV therapy for 7 to 14 days, VCV significantly accelerated the resolution of acute pain, reduced the duration of PHN, and decreased the proportion of patients with pain persisting for more than 6 months (19% vs 26%). The incidence of adverse events was similar in both groups.14
FCV has broad activity against varicella-zoster virus.15 In an RCT that evaluated oral FCV in 419 immunocompetent adults (mean age 50 years) with uncomplicated HZ, FCV was well tolerated and accelerated lesion healing compared with placebo. Among those who developed PHN, the pain resolved twice as fast for patients in the FCV group compared with the controls, and the median duration of PHN was reduced by 2 months.15
TABLE
Antiviral therapy dosing guidelines*
| Dosage adjusted for creatinine clearance† (mL/min) | ||||
|---|---|---|---|---|
| Drug | Standard dose | <10 | 10-25 | Duration |
| ACV | 800 mg 5x/d | 1600 mg/d | 2400 mg/d | 7-10 days |
| VCV | 1000 mg tid | 1000 mg/d | 2000 mg/d | 7 days |
| FCV | 750 mg/d or 250 mg tid | 250 mg/d | 500 mg/d | 7 days |
| *All 3 drugs reduce acute pain and development of postherpetic neuralgia, and are most effective when started within 72 hours of onset of rash. | ||||
| †Patients with creatinine clearance >25 mL/min receive the standard dose. | ||||
| ACV, acyclovir; FCV, famciclovir; VCV, valacyclovir. | ||||
Analgesics and other drugs: What to consider
While antiviral therapy helps to relieve the pain of HZ, several trials have shown that none of the available agents completely alleviates it or routinely prevents the development of PHN. As a result, adjunctive therapy, including pain medication, is often required. But prescribing analgesics to frail elderly patients and those who have comorbidities and take multiple medications is not without risk.
The ability of a tricyclic antidepressant to alleviate pain and prevent PHN when therapy is initiated within 48 hours of the eruption of lesions was tested in a double-blind trial in which 72 patients 60 years of age or older were randomized to amitriptyline 25 mg daily for 90 days or placebo.16 Antiviral agents were administered according to the preference of the primary physician. At 6 weeks, the pain prevalence—the primary outcome measure—was reduced by about 50% in the amitriptyline group.16 There is no other evidence to support the use of tricyclics in the acute phase of HZ, however, and concerns about orthostatic hypotension and anticholinergic side effects limit their use, particularly in older patients.
Corticosteroids are sometimes used, too, often in conjunction with antiviral therapy, but there are problems with this approach, as well. One RCT comparing an ACV-prednisone combination with ACV alone in HZ patients over the age of 50 found that patients who received both drugs had faster resolution of acute pain and earlier discontinuation of analgesics.17 But several serious adverse effects of prednisone were reported in patients in the combination therapy group, despite the fact that individuals with contraindications to corticosteroids were excluded from the study. Overall, there is little evidence to suggest that steroids can be safely used to reduce the incidence or severity of PHN. There is no specific recommendation regarding analgesic therapy for PHN, but physicians often adopt a stepwise approach.
Recommend the shingles vaccine
In view of the toll that shingles often takes, vaccination is the best way to prevent HZ and its complications. In a randomized, double-blind, placebo-controlled study involving 38,546 adults who were 60 years of age or older, researchers demonstrated that cell-mediated immunity to the varicella-zoster virus was boosted by the live attenuated HZ vaccine.18 The enhanced immunity was associated with a 51% reduction in the incidence of HZ (NNT=58 to prevent 1 case over 3 years), a 66% reduction in the incidence of PHN (NNT=364 to prevent 1 case of PHN over 3 years), and a 61% reduction in disease burden. The vaccine was well tolerated, and injection site reactions were generally mild.18 Accurate cost-effectiveness analyses of immunization are not available because the duration of vaccine protection is unknown.19
FIGURE
A unilateral vesicular rash
A shingles outbreak, like the rash shown on this patient’s back, usually appears as a patch or band of blisters on 1 side of the body.
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination to prevent both HZ and PHN in healthy adults who are 60 years of age and older (individuals with primary or acquired immunodeficiencies or patients on immunosuppressive therapies should not be vaccinated), and suggests that practitioners offer the HZ vaccine to appropriate patients at their first visit.7 It is not necessary, however, to ask about the patient’s history of varicella or to conduct serologic testing to determine varicella immunity before administering the HZ vaccine.7 A history of shingles is not a contraindication, so advise patients who develop HZ to come in for vaccination soon after the rash and pain resolve.
CORRESPONDENCE
Pierre-Olivier Lang, MD, MPH, PhD, University Hospitals of Geneva, Department of Rehabilitation and Geriatrics, Chemin du Pont-Bochet, 3, CH-1226, Thonex-Geneva, Switzerland; [email protected]
• Initiate antiviral treatment as soon as possible; rapid resolution of acute pain and reduction in the development of postherpetic neuralgia (PHN) are most likely when therapy is started within 72 hours of the outbreak. A
• Discuss herpes zoster (HZ) vaccination with healthy patients 60 years of age and older during their first office visit; the vaccine markedly reduces the incidence of HZ and PHN. A
• Do not prescribe tricyclic antidepressants or corticosteroids in the acute phase of HZ. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
We don’t do enough to protect our patients from the pain of herpes zoster (HZ). Consider:
- Each year in the United States, about 1 million new cases of herpes zoster (HZ) occur.1 The incidence is estimated at 3 to 4 per 1,000 in the general population,2 but climbs to more than 10 per 1000 among people 60 years of age and older.3,4
- Overall, between 13% and 26% of patients with HZ develop postherpetic neuralgia (PHN), defined as pain that continues for more than 1 month after the rash has healed. Among patients who are 70 or older, however, the likelihood that HZ will progress to PHN is approximately 50%.5 (In a study of 7595 patients being treated for HZ or PHN by general practitioners or dermatologists in France, 45% reported pain that was severe or very severe, and 42% reported permanent pain.6 )
- Between 10% and 25% of HZ patients develop ocular complications, which have the potential to result in vision loss, facial scarring, or prolonged or permanent pain.7 Encephalitis, myelitis, and peripheral nerve palsies are potential complications, as well.
Yet HZ and its complications are largely preventable.
A live attenuated vaccine (Zostavax) received US Food and Drug Administration approval in 2006.8 But many patients have not yet heard of it, and many physicians fail to recommend it. (See “Herpes zoster vaccine: Why aren’t more people receiving it?”.)
As a family physician, you can play a key role in reducing the burden of shingles by rapidly identifying and treating HZ, minimizing the risk of prolonged pain, and, notably, by talking to older patients about the benefits of vaccination.
Zostavax, a live attenuated herpes zoster (HZ) vaccine, was licensed by the US Food and Drug Administration in 2006 for use in people 60 years of age and older—the first new vaccine targeting this age group in years. In 2007, researchers at the Centers for Disease Control and Prevention (CDC) conducted a national survey,20 in part to gauge the knowledge of, and interest in, the HZ vaccine among the intended recipients.
Their findings: Of more than 3500 respondents, only 1.9% had received the HZ vaccine. Most (72%) were unaware of the vaccine’s existence, but the majority said they would agree to vaccination if their physician were to recommend it.
Among those who were aware of it, the key reasons for rejecting the vaccine were that it was not needed (cited by 35%), they were not at risk (13%), and a lack of trust in doctors or the US health care system (10%). Both the limited awareness of the vaccine and the lack of physician recommendations are barriers to HZ vaccination, the researchers concluded.20
On its Web site, the CDC broaches another potential barrier to greater use of the HZ vaccine: cost. The vaccine is not covered by Medicare Part B, nor by some private insurers. While it is covered by all Medicare Part D plans, the extent of coverage depends on the particular plan. The CDC recommends that physicians encourage patients to contact their insurers to determine the extent of their coverage.21
Start antiviral therapy without delay
Several meta-analyses and many (though not all) randomized controlled trials (RCTs) of HZ treatment have demonstrated that prompt antiviral therapy—with oral acyclovir (ACV), valacyclovir (VCV), or famciclovir (FCV)—reduces the duration of acute pain and the likelihood that PHN will develop.9,10 Without antiviral therapy, up to 45% of patients over the age of 60 experience pain that persists for 6 months to a year. Even with therapy, studies have found that about 20% of patients older than 50 years continued to have pain for 6 months after their rash appeared.10 Risk factors for PHN include age (>50 years), sex (female), a disseminated rash, a severe pain presentation, and polymerase chain reaction-detectable varicella zoster virus viremia.11
Which agent? What the research reveals
For most people with HZ, any of the 3 antiviral agents can be used, based on physician and patient preference. (See TABLE for treatment guidelines.) Here’s a look at the evidence for each.
Oral ACV has long been the mainstay of treatment for HZ, but its poor bioavailability and the need for 5 daily doses has led to the development of newer antiviral agents.12 When initiated within 48 to 72 hours of the onset of the rash, ACV has demonstrated clinical benefit. (The value of starting ACV therapy beyond the 72-hour mark has not been established, though treatment should be considered if new lesions are still appearing.)
In 1 meta-analysis of 4 placebo-controlled trials, ACV accelerated resolution of acute pain, with the greatest effect in those older than 50 years.13 In a second meta-analysis, treatment with ACV reduced the incidence of PHN at 3 months by 46% (number needed to treat [NNT]=3.2-8).12
VCV is well absorbed in the gastrointestinal tract, providing 3- to 5-fold greater bioavailability compared with ACV.13 VCV’s efficacy was demonstrated in an RCT in which the researchers conducted an intent-to-treat analysis: Compared with ACV therapy for 7 to 14 days, VCV significantly accelerated the resolution of acute pain, reduced the duration of PHN, and decreased the proportion of patients with pain persisting for more than 6 months (19% vs 26%). The incidence of adverse events was similar in both groups.14
FCV has broad activity against varicella-zoster virus.15 In an RCT that evaluated oral FCV in 419 immunocompetent adults (mean age 50 years) with uncomplicated HZ, FCV was well tolerated and accelerated lesion healing compared with placebo. Among those who developed PHN, the pain resolved twice as fast for patients in the FCV group compared with the controls, and the median duration of PHN was reduced by 2 months.15
TABLE
Antiviral therapy dosing guidelines*
| Dosage adjusted for creatinine clearance† (mL/min) | ||||
|---|---|---|---|---|
| Drug | Standard dose | <10 | 10-25 | Duration |
| ACV | 800 mg 5x/d | 1600 mg/d | 2400 mg/d | 7-10 days |
| VCV | 1000 mg tid | 1000 mg/d | 2000 mg/d | 7 days |
| FCV | 750 mg/d or 250 mg tid | 250 mg/d | 500 mg/d | 7 days |
| *All 3 drugs reduce acute pain and development of postherpetic neuralgia, and are most effective when started within 72 hours of onset of rash. | ||||
| †Patients with creatinine clearance >25 mL/min receive the standard dose. | ||||
| ACV, acyclovir; FCV, famciclovir; VCV, valacyclovir. | ||||
Analgesics and other drugs: What to consider
While antiviral therapy helps to relieve the pain of HZ, several trials have shown that none of the available agents completely alleviates it or routinely prevents the development of PHN. As a result, adjunctive therapy, including pain medication, is often required. But prescribing analgesics to frail elderly patients and those who have comorbidities and take multiple medications is not without risk.
The ability of a tricyclic antidepressant to alleviate pain and prevent PHN when therapy is initiated within 48 hours of the eruption of lesions was tested in a double-blind trial in which 72 patients 60 years of age or older were randomized to amitriptyline 25 mg daily for 90 days or placebo.16 Antiviral agents were administered according to the preference of the primary physician. At 6 weeks, the pain prevalence—the primary outcome measure—was reduced by about 50% in the amitriptyline group.16 There is no other evidence to support the use of tricyclics in the acute phase of HZ, however, and concerns about orthostatic hypotension and anticholinergic side effects limit their use, particularly in older patients.
Corticosteroids are sometimes used, too, often in conjunction with antiviral therapy, but there are problems with this approach, as well. One RCT comparing an ACV-prednisone combination with ACV alone in HZ patients over the age of 50 found that patients who received both drugs had faster resolution of acute pain and earlier discontinuation of analgesics.17 But several serious adverse effects of prednisone were reported in patients in the combination therapy group, despite the fact that individuals with contraindications to corticosteroids were excluded from the study. Overall, there is little evidence to suggest that steroids can be safely used to reduce the incidence or severity of PHN. There is no specific recommendation regarding analgesic therapy for PHN, but physicians often adopt a stepwise approach.
Recommend the shingles vaccine
In view of the toll that shingles often takes, vaccination is the best way to prevent HZ and its complications. In a randomized, double-blind, placebo-controlled study involving 38,546 adults who were 60 years of age or older, researchers demonstrated that cell-mediated immunity to the varicella-zoster virus was boosted by the live attenuated HZ vaccine.18 The enhanced immunity was associated with a 51% reduction in the incidence of HZ (NNT=58 to prevent 1 case over 3 years), a 66% reduction in the incidence of PHN (NNT=364 to prevent 1 case of PHN over 3 years), and a 61% reduction in disease burden. The vaccine was well tolerated, and injection site reactions were generally mild.18 Accurate cost-effectiveness analyses of immunization are not available because the duration of vaccine protection is unknown.19
FIGURE
A unilateral vesicular rash
A shingles outbreak, like the rash shown on this patient’s back, usually appears as a patch or band of blisters on 1 side of the body.
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination to prevent both HZ and PHN in healthy adults who are 60 years of age and older (individuals with primary or acquired immunodeficiencies or patients on immunosuppressive therapies should not be vaccinated), and suggests that practitioners offer the HZ vaccine to appropriate patients at their first visit.7 It is not necessary, however, to ask about the patient’s history of varicella or to conduct serologic testing to determine varicella immunity before administering the HZ vaccine.7 A history of shingles is not a contraindication, so advise patients who develop HZ to come in for vaccination soon after the rash and pain resolve.
CORRESPONDENCE
Pierre-Olivier Lang, MD, MPH, PhD, University Hospitals of Geneva, Department of Rehabilitation and Geriatrics, Chemin du Pont-Bochet, 3, CH-1226, Thonex-Geneva, Switzerland; [email protected]
1. Centers for Disease Control and Prevention. Shingles disease – questions and answers (herpes zoster). Available at http://www.cdc.gov/vaccines/vpd-vac/shingles/dis-faqs.htm. Accessed September 1, 2009.
2. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211-1215.
3. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571-575.
4. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33.
5. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine. 2006;24:1308-1314.
6. Chidiac C, Buxelles J, Daures JP, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis. 2001;33:62-69.
7. Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57:1-30.
8. US Food and Drug Administration. FDA licenses new vaccine to reduce older Americans’ risk of shingles. May 26, 2006. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108659.htm. Accessed September 1, 2009.
9. Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748-50.
10. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
11. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545-1551.
12. Jackson JL, Gibbons R, Meyer G, et al. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157:909-912.
13. Wood MJ, Kay R, Dworkin RH, et al. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347.
14. Beutner KR, Friedman DJ, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-1553.
15. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
16. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
17. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
18. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
19. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317-325.
20. Lu P, Euler GL, Jumaan AO, et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2009;27:882-887.
21. Centers for Disease Control and Prevention. Herpes zoster – vaccine Q&As for providers. Available at: http://www.cdc.gov/vaccines/vpd-vac/shingles/vac-faqs-hcp.htm. Accessed September 1, 2009.
1. Centers for Disease Control and Prevention. Shingles disease – questions and answers (herpes zoster). Available at http://www.cdc.gov/vaccines/vpd-vac/shingles/dis-faqs.htm. Accessed September 1, 2009.
2. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211-1215.
3. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571-575.
4. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33.
5. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine. 2006;24:1308-1314.
6. Chidiac C, Buxelles J, Daures JP, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis. 2001;33:62-69.
7. Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57:1-30.
8. US Food and Drug Administration. FDA licenses new vaccine to reduce older Americans’ risk of shingles. May 26, 2006. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108659.htm. Accessed September 1, 2009.
9. Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748-50.
10. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
11. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545-1551.
12. Jackson JL, Gibbons R, Meyer G, et al. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157:909-912.
13. Wood MJ, Kay R, Dworkin RH, et al. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347.
14. Beutner KR, Friedman DJ, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-1553.
15. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
16. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
17. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
18. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
19. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317-325.
20. Lu P, Euler GL, Jumaan AO, et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2009;27:882-887.
21. Centers for Disease Control and Prevention. Herpes zoster – vaccine Q&As for providers. Available at: http://www.cdc.gov/vaccines/vpd-vac/shingles/vac-faqs-hcp.htm. Accessed September 1, 2009.
Liver disease: Early signs you may be missing
• Suspect compensated liver cirrhosis in a patient with abnormal liver function tests, a low platelet count, and prolonged prothrombin time. C
• Use ultrasonography as a first-line diagnostic tool for liver cirrhosis. C
• Prescribe beta-blockers as prophylaxis for patients at risk for variceal bleeding. A
• Work collaboratively with hepatic specialists to manage the care of patients with cirrhosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: A patient with mildly elevated ALT and AST
John M., a 63-year-old truck driver with a family history of diabetes and arterial hypertension, is complaining of persistent fatigue—again. He has type 2 diabetes and takes metformin and repaglinide, but his blood pressure is normal. Lab tests reveal a recurrent mild elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of unknown origin; Mr. M. has no history of virus or hepatotoxic drugs, and reports only modest alcohol intake. He is obese, however, with a BMI of 34.5 and a waist circumference of 41 inches.
CASE 2: A patient with abdominal swelling
Anna B., a slim 68-year-old, comes in with 2 acute conditions: About a week ago, she noticed abdominal swelling and low back pain that began suddenly, after she carried a heavy load. She’s taken nonsteroidal anti-inflammatory drugs for 6 days, but the pain has not improved. The patient’s only significant clinical history is a hysterectomy, with oophorectomy, at age 38, and a recurrent elevation of serum transaminase levels that has never been investigated. Examination reveals an important kyphosis, and finger pressure on the vertebral spine or a position change exacerbates the pain. Her abdomen is swollen and tense, with a tympanic sound on the upper abdomen and a dull sound on the lower portion.
If John M. and Anna B. were your patients, would you suspect that they both have advanced liver disease? What diagnostic tests would you order, and how would you manage their care?
Cirrhosis has always been associated with high rates of morbidity and mortality. It is the 12th most common cause of death in the United States;1 in some parts of the world, its ranking as a cause of death is considerably higher.2,3 In recent years, however, cirrhosis has become the focus of greater attention both here and abroad, for 2 reasons: The first is the increasing prevalence of viral hepatitis and steatohepatitis, both of which are prominent causes of cirrhosis. The second is the improvement we’ve made in treatment: Not only can we slow the progression of cirrhosis, but in some cases, we can even restore hepatic function.4
The key to successful management of cirrhosis lies in spotting subtle signs and symptoms well in advance of the serious complications that can arise down the road. Here’s what to look for.
Early warnings you can’t afford to overlook
While the clinical presentation of a patient with liver cirrhosis is often asymptomatic, serum transminases—included in many standard laboratory tests as part of a routine examination—often provide the first sign of a problem.
Mildly elevated ALT in an asymptomatic patient may be transient and benign, or an indication of chronic liver disease.5 In fact, signs suggestive of significant liver disease have been reported in more than 20% of patients with ALT elevation.2 But because abnormal ALT values are common and frequently resolve, many primary care physicians pay little attention to this potentially important finding—and miss a key opportunity for early identification and treatment.6
Look at other lab values, risk factors, as well
Additional lab values that suggest the possibility of cirrhosis include an elevated AST/ALT ratio, a low platelet count (<150,000/L), elevated alkaline phosphatase, elevated bilirubin (>1.1 mg/dL), low serum albumin (<2.5 g/dL), and decreased prothrombin time (<100%). Potential causes include viral hepatitis, heavy alcohol use, hepatotoxic drugs, steatosis, and steatohepatitis.
The next step for a patient with any of these abnormal values is a thorough medication review and medical history. Identify all prescription and nonprescription drugs the patient is taking, as well as any herbal products and supplements, in search of hepatotoxic agents. Amiodarone and valproic acid, among other drugs, may cause steatosis, and some herbal products—particularly kava kava extract, used to treat anxiety and insomnia—have been linked to hepatitis and even liver failure.7 Question the patient about alcohol consumption and a history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders, as well.
At a minimum, schedule follow-up testing of asymptomatic patients with abnormal laboratory findings in no more than 6 months. Persistent ALT elevation in such patients is most commonly caused by major viruses, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), or nonalcoholic steatohepatitis (NASH).8 Nonalcoholic fatty liver is especially likely in patients with clinical and demographic risk factors—those who, like John M., suffer from obesity or diabetes, or both.
Ultrasound yields further information
Further screening should be limited to patients who continue to have abnormal test results for 6 months or more or have multiple risk factors. While biopsy is still considered the gold standard for diagnosing and staging chronic liver disease, it should be considered, according to the American Gastroenterological Association, only if ultrasound and other tests have not been helpful in reaching a diagnosis.9
Often, though, ultrasonography aids in diagnosis. In the case of John M., ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity and signs of severe steatosis and mild splenomegaly, but no increase in portal vein diameter and no ascites. For asymptomatic patients with cirrhosis or an earlier stage of liver disease, ultrasound at 6-month intervals, combined with blood alpha-fetoprotein measurement, can be used to track disease progression and screen for hepatocellular carcinoma.10
Newer, noninvasive methods aid in diagnosis
Noninvasive means of evaluating the presence and extent of liver fibrosis and differentiating cirrhosis from noncirrhosis, developed in recent years, have been found to have positive predictive values greater than 85% to 90%.11 Transient elastography (FibroScan, London, England), which assesses liver stiffness, is 1 such method. Although it is often used successfully, however, morbid obesity, small intercostal spaces, and ascites limit the diagnostic capability of this medical device.12
Fibrosis can also be detected with the use of 1 or more algorithms—each testing blood samples for a different combination of serum surrogate markers for liver disease. Some widely used algorithms include the APRI (AST-to-platelets ratio index), the Fibrotest (aptoglobin, alpha-2 macroglobulin, apolipoprotein A1, gamma-glutamyl transpeptidase, and bilirubin), the Hepascore (bilirubin, gamma-glutamyl transpeptidase, haluronic acid, alpha-2 macroglobulin, age, sex), and the BARD (BMI, AST/ALT ratio, diabetes).
Hepatologists often use the results of ultrasonography, followed by transient elastography in conjunction with findings from 1 or more of these algorithms, to determine which patients are candidates for liver biopsy.11,12
Staging is crucial, with or without biopsy
The decision to perform a liver biopsy should be based on a number of factors, including the patient’s age, lifestyle, liver chemistry abnormalities, desire for prognostic information, and associated comorbidities.9 Despite the value of biopsy, it is a costly procedure with potentially serious side effects and risks—and not always accepted by patients. In a recent survey of 1177 primary care physicians in France, as many as 59% of patients with chronic hepatitis C refused to undergo liver biopsy; what’s more, 22% of the doctors surveyed shared the patients’ hesitancy.13 Whether patients refuse biopsy or it is deemed unnecessary because ultrasound and other noninvasive tests result in a probable diagnosis, staging is necessary, both to guide therapy and to arrive at a prognosis.
Liver enzyme levels reveal little about organ integrity and are not useful for staging. But other parameters (specifically, bilirubin, albumin, and prothrombin time), combined with the presence (or absence) and severity of physical findings such as encephalopathy and ascites, are included in the Child-Pugh classification system ( TABLE 1 ),14 a widely used system that roughly indicates disease severity.15
The Model for End-stage Liver Disease (MELD)—and PELD, the pediatric model—use bilirubin, creatinine, and international normalized ratio values to classify disease severity. MELD and PELD scores are considered more accurate than the Child-Pugh score in determining short-term mortality,16 and are used by the United Network of Organ Sharing (UNOS) for liver allocation. You’ll find a calculator at http://optn.transplant.hrsa.gov/resources/MeldPeldCalculator.asp?index=97.17
Despite the progress in diagnostic techniques, the life expectancy and quality of life for patients with advanced cirrhosis remains poor. Patients routinely experience fatigue, pruritus, ascites, encephalopathy, and bleeding; dyspepsia and malnutrition are common, as well. Cirrhosis also carries the risk of life-threatening complications, partly due to comorbidities—most notably, osteoporosis, malabsorption, and rheumatic diseases. Liver transplantation has the potential to change the life expectancy of these patients, but because of the extensive waiting lists, candidates for transplant often die before a liver becomes available.
But for many patients who are in stable condition—those with compensated cirrhosis, that is—the prognosis is far more hopeful: In addition to providing standard medical care, including immunization, if necessary, and nutritional counseling, targeted therapy is crucial to slow, or stop, disease progression.
TABLE 1
Child-Pugh: Classifying cirrhosis, predicting survival*
| 1 point | 2 points | 3 points | |
|---|---|---|---|
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Prothrombin time (INR) | <4 sec (<1.7) | 4-6 sec (1.7-2.3) | > 6 sec (>2.3) |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| Ascites | Absent | Mild | Severe |
| Encephalopathy | Absent | Mild | Severe |
| INR, international normalized ratio. | |||
| * Total the number of points for all 5 indicators (1 point for every answer in column 1, 2 points for every answer in column 2, and 3 points for every answer in column 3). Patients with ≤6 points (Grade A) have an estimated 1-year survival rate of 100%; patients with 7-9 points (Grade B) have an estimated 1-year survival rate of 80%; and patients with ≥10 points (Grade C) have an estimated 1-year surival rate of 45%. | |||
| Adapted from: Infante-Rivard C, et al. Hepatology. 1987.14 | |||
Treatment for cirrhosis depends on the cause
Although primary care physicians can often provide most, or all, of the care for those in stable condition, a specialist may be helpful in determining further testing to identify the underlying cause of the cirrhosis, which is essential to determining the most appropriate treatment. What’s more, research has shown that patients with cirrhosis whose care is managed by a primary care physician and a hepatologist have better outcomes than those who are treated by a primary care doctor alone.18
What to test for?
Tests to determine the cause of cirrhosis are listed in TABLE 2 . For an individual patient, diagnostic tests would be based on the suspected cause. A patient with a family history of hereditary hemochromatosis would be tested for elevated serum ferritin levels and hepatic iron content on liver biopsy sample; the transferrin saturation index would also be obtained, and the patient might be tested for specific gene mutations. A patient who drinks heavily would be tested for elevated gamma-glutamyl transpeptidase and mean corpuscular volume. For someone with obesity, diabetes, and an enlarged liver, standard lab tests, including high-density lipoprotein (HDL) cholesterol, glucose, and triglycerides, may be sufficient.
Keep in mind, however, that cirrhosis may have more than 1 contributing factor—obesity or chronic alcohol use and a virus, for example; alcohol abuse and metabolic fatty liver; or virus and hemochromatosis. Thus, it may require more than 1 type of treatment.
Alcohol abuse is the cause of 25% of cases of liver cirrhosis, and a contributor to another 25% to 50%.19 The key treatment here—and an ideal role for a family physician—is to refer the patient to a detoxification and treatment program and provide ongoing monitoring and support. Antiviral treatment may be helpful for a recovering alcoholic who also tests positive for hepatitis B or C virus, but because of potential problems with compliance, some physicians delay therapy until the patient has had at least 6 months of continuous abstinence. Although this is not an absolute criterion, the same period of abstinence may be required before a patient becomes eligible for a liver transplant.
NAFLD/NASH is typically diagnosed on the basis of lab values and physical presentation. For a stable patient, the primary treatment includes lifestyle change—a low-calorie, low-carbohydrate diet and an exercise regimen—and a possible switch to insulin for better glycemic control.
For patients who are not candidates for such targeted treatments, either because their disease is too advanced or they’re unable to tolerate the recommended therapy, numerous pharmaceutical preparations claiming antioxidant or anti-inflammatory properties are available. But only 1—an herbal extract known as silymarin, derived from the milk thistle plant and taken with vitamin E—has been found to have some protective effects.20
TABLE 2
Liver cirrhosis: Common causes, diagnostic tests, and treatments4,34-38
| Cause | Test (result) | Therapy |
|---|---|---|
| Alcohol | GGT (↑), MCV (↑) | Abstinence |
| HBV + delta virus infection | HBsAg (+) HBV-DNA(+) HBc-IgM (+) HDV-RNA (+) | Interferon alpha-2b, nucleoside (lamivudine, telbivudine, entecavir) and nucleotide (adefovir, tenofovir) analogs |
| HCV infection | HCV-RNA (+) | Interferon + ribavirin |
| Primary biliary cirrhosis | GGT (↑) Alkaline phosphatase (↑) AMA (+) | Ursodeoxycholate |
| Autoimmune hepatitis | ANA (+) ASMA (+) LKM (+) | Prednisone, azathioprine |
| Hemochromatosis | Ferritin (↑) Transferrin saturation index (>45%) Hepatic iron content (↑) HFE gene mutation (C282Y, H63D) | Phlebotomy, chelating agents |
| Wilson’s disease | Ceruloplasmin (↓) Serum copper (↓) 24h urinary copper excretion (↑) | D-penicillamine, zinc |
| NAFLD/NASH | HDL cholesterol (↓) Glucose (↑) Triglycerides (↑) | Low-calorie diet, physical activity, insulin-sensitizer drugs or insulin |
| AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASMA, anti-smooth-muscle antibody; GGT, gamma–glutamyl transpeptidase; HBc-IgM, immunoglobulin M antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; HCV-RNA, hepatitis C virus RNA; HDL, high-density lipoprotein; HDV-RNA, hepatitis delta virus RNA; LKM, liver kidney microsomes; MCV, mean corpuscular volume, NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. | ||
Address systemic problems along with targeted treatment
Malnutrition is a serious problem for many patients with cirrhosis. Causes range from poor oral intake or malabsorption to ongoing alcohol use, chronic nausea, or early satiety because of compression from ascites. Dental problems that prevent the patient from chewing properly may be a contributing factor, as well.
Regardless of the cause, malnutrition is associated with muscle wasting, hypoalbuminemia, decreased resistance to infections, and variceal bleeding, and addressing it is a key part of treatment. Assess the nutritional status of every patient with cirrhosis, and stress the importance of multivitamin supplementation.21 If dental care is needed, take steps to see that the patient receives it.
Nutritional support, however, should be reserved for severely malnourished patients awaiting transplantation.22
Osteoporosis. Reduced bone formation—the result of vitamin D deficiency, hypoparathyroidism, and hypogonadism—is a well-known complication of end-stage cirrhosis. However, osteopenia may occur in an earlier stage of disease, especially in patients with cholestatic disease and those receiving antiviral therapy. Prescribe bisphosphonates, together with calcium and vitamin D3, to improve bone mineral density.23
Diabetes. The relationship between diabetes and cirrhosis is particularly complex, because diabetes can be both a causal factor and a consequence of cirrhosis. Diabetes is common in patients with NASH, and prevalent among those with hepatitis C and hemochromatosis. Multivariate analyses have found that diabetes has an independent negative effect on the progression of liver disease.24
Diet remains the first-line treatment for hyperglycemia, with metformin as the drug of choice if diet alone is unsuccessful. Sulfonylureas can be used, but require caution to avoid hypoglycemia. Glitazones are a newer alternative, but their value in patients with liver cirrhosis has not been studied. However, the use of any oral antidiabetic agent requires extra caution in patients with cirrhosis, and should be avoided in those with advanced liver disease. Although insulin requires intense self-monitoring of serum glucose levels, it is preferable to oral agents for this patient population.25
Managing complications of cirrhosis
Hospital, home, or long-term care? Whether patients with advanced cirrhosis can be maintained at home or require hospitalization or long-term care is best decided in consultation with patient, family, and other members of the health care team. One helpful tool is the Karnofsky Performance Scale Index (http://www.pennmedicine.org/homecare/hcp/elig_worksheets/Karnofsky-Performance-Status.pdf), which scores patients from 0 to 100 based on their functional impairment.26 (Patients with decompensated liver cirrhosis and limited self-sufficiency typically score <50, indicating that they require home health care, hospice, or institutional care.) Whatever the outcome, the patient may need to be reevaluated as the disease progresses and complications occur.
Ascites, the most common complication of cirrhosis,27 is a primary reason for hospitalization, but may be managed on an outpatient basis, depending on the patient presentation. Determining factors include the presence or absence of portal hypertension, impaired albumin synthesis, decreased plasma oncotic pressure, and sodium retention. Diagnosis is based on physical exam and ultrasonography.
Initial treatment for ascites includes salt restriction28,29 and avoidance of NSAIDs, which promote renal sodium retention, followed by spironolactone (100–400 mg/d). Add furosemide (40-160 mg/d) if the fluid retention does not begin to resolve after 3 to 5 days of treatment. If the condition persists despite maximum tolerable doses of diuretics, large-volume paracentesis to remove transudative fluid (albumin <1 g/dL; serum/ascites albumin gradient >1.1) may be needed. A patient with recurrent or refractory ascites should see a specialist for further evaluation and the possibility of a transjugular intrahepatic portosystemic shunt (TIPS).
Abdominal pain and an ascitic granulocyte count >250/mm3 suggest spontaneous bacterial peritonitis (SBP)—a severe complication of ascites that can result in renal and liver failure. In addition to pain, patients may present with tense ascites and fever, followed by encephalopathy, shock, and increased serum creatinine levels. Hospitalization is required for SBP; therapy includes high-dose albumin and intravenous antibiotics, typically cephalosporin. Long-term prophylaxis with norfloxacin to prevent the recurrence of SBP is indicated.30
If your patient has ascites and is being cared for at home, talk to the patient and his or her family about the importance of a daily weight check. Tell them to contact you if the patient gains more than 4 to 8 lbs within a few days. Frequent electrolyte checks are needed, as well. An albumin infusion is required when serum levels are particularly low, or after large-volume paracentesis.31 Patients with SBP or refractory ascites generally have more advanced disease and a poor prognosis.
Portal hypertension/esophageal varices. The main aim of treating portal hypertension is to prevent esophageal variceal bleeding. The appearance of varices should be checked by endoscopy every 2 to 3 years, or yearly for patients at high risk of bleeding. Patients with varices can be managed with nonselective beta-blockers at doses that are sufficient to elicit a 25% reduction in resting heart rate. Those at high risk for bleeding and patients who have already had esophageal bleeding may require endoscopic band ligation.32 TIPS is an alternative for those whose previous treatments have failed.33
Hepatic encephalopathy. This potentially reversible decrease in neuropsychiatric function mainly affects patients with portal hypertension. Caused by reduced hepatic clearance of gut-deriving neurotoxins, hepatic encephalopathy is associated with a range of signs and symptoms—from subtle personality changes to coma, with flapping tremor as a frequent initial finding. Acid-base and electrolyte disturbances, constipation, infections, gastrointestinal bleeding, and sedatives can precipitate encephalopathy. Hepatic encephalopathy is a diagnosis of exclusion, however, requiring the exclusion of all other etiologies of altered mental status.
Treatment consists of identifying and correcting the precipitating factors, and includes electrolyte correction, colon cleansing, and acidification with lactulose. Dietary protein restriction is no longer advocated, because it may facilitate malnutrition and complications. Oral rifaximine is useful and well tolerated for suppression of intestinal bacterial flora. Venous infusion of branched-chain amino acids or flumazenil may be effective in case of coma.
Fever and sepsis. Infection is a high-risk factor for mortality in patients with cirrhosis, as it can lead to renal and liver failure, variceal bleeding, and hepatic encephalopathy. However, individuals with cirrhosis often do not develop the typical signs and symptoms of infection; leukocytosis may be absent because of severe leukopenia, for instance, and patients may be afebrile.
Thus, the general appearance of systemic illness is an indication for antibiotics, with quinolones and cephalosporins as first-line agents. Infections most commonly involve the urinary tract (25%-55%) or the respiratory tract (20%), or are related to SBP (10%-30%).33 Hospitalization is suggested in case of poor general health status or the appearance of organ dysfunction.
When medical therapy and other interventions fail to control complications, transplantation is the only alternative. Primary care physicians can play a role here, too, in referring potential candidates for liver transplants to specialists for further consideration.
CASE 1: Resolution
As we’ve already seen, John M.’s ultrasound revealed an enlarged liver. The results led to a probable diagnosis of an advanced form of NASH. Other lab tests indicated that he had poorly controlled diabetes, high triglyceride levels, and—for the first time—a low platelet count. His physician stressed the importance of following a low-calorie, low-carbohydrate diet and exercising regularly, prescribed insulin, and referred the patient to a hepatologist for further noninvasive evaluation of fibrosis and to determine whether liver biopsy was needed.
CASE 2: Resolution
Blood tests revealed that Anna B. had a low platelet count (64,000/mm3), elevated liver enzymes (AST 2× upper limit of normal [ULN], ALT 1.5× ULN, GGT 2.5× ULN), and high gamma-globulins (33.6%) with no monoclonal bands. Ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity, portal vein dilatation, and moderate ascites. She also tested positive for HCV and had an HCV-RNA reading of 15×106 IU/mL. No other cause of chronic liver disease emerged. Ms. B.’s physician told her that she had an osteoporotic vertebral fracture—a frequent comorbidity in patients with liver cirrhosis—and decompensated liver cirrhosis from an old HCV infection. He added that her abdomen was distended because of fluid retention. The physician recommended bed rest, prescribed paracetamol (1 g tid) and spironolactone (100 mg/d), and referred the patient to an orthopedist for treatment of the fracture and to a hepatologist to be evaluated for transplantation.
CORRESPONDENCE
Ignazio Grattagliano, MD, Department of Internal Medicine, University Medical School of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
1. Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. National Vital Stat Rep. 2009;57:(14):1-135.Available at: www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. Accessed September 16, 2009.
2. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20:1442-1449.
3. Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762.
4. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851.
5. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110.
6. Sherwood P, Lyburn I, Brown S, et al. How are abnormal results for liver function tests dealt with in primary care? Audit of yield and impact. BMJ. 2001;322:276-278.
7. US Food and Drug Administration. Food. Consumer advisory: Kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm. Accessed September 11, 2009.
8. Grattagliano I, Portincasa P, Palmieri VO, et al. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857-863.
9. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
10. Sherman M, Klein A. AASLD single-topic research conference on hepatocellular carcinoma: conference proceedings. Hepatology. 2004;40:1465-1473.
11. Pinzani M, Vizzutti F, Arena U, et al. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
12. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
13. Bonny C, Rayssiguier R, Ughetto S, et al. Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region [In French]. Gastroenterol Clin Biol. 2003;27:1021-1025.
14. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
15. Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;Aug. 20 [Epub ahead of print].
16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96.
17. United Network of Organ Sharing. Resources. Meld/Peld calculator. Available at: http://www.unos.org/resources/meldPeldCalculator.asp. Accessed September 11, 2009.
18. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
19. Habib A, Bond WM, Heuman DM. Long-term management of cirrhosis. Appropriate supportive care is both critical and difficult. Postgrad Med. 2001;109:101-103.
20. Flora K, Hahn M, Rosen H, et al. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143.
21. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis [In French]. Gastroenterol Clin Biol. 2008;32:265-273.
22. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55.
23. Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(suppl 1):i1-i9.
24. Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75.
25. Garcia-Compean D, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288.
26. Karnofsky Performance Scale Index. Available at: http://www.medal.org/visitor/www%5CActive%5Cch1%5Cch1.01%5Cch1.01.01.aspx. Accessed September 11, 2009.
27. Gentilini P, Bernardi M, Bolondi L, et al. The rational use of albumin in patients with cirrhosis and ascites. A Delphi study for the attainment of a consensus on prescribing standards. Dig Liver Dis. 2004;36:539-546.
28. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
29. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856.
30. Gines P, et al. Pathophysiology, complications, and treatment of ascites. Clin Liver Dis. 1997;1:129-155.
31. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
32. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081-1091.
33. McCormick PA, Greenslade L, Kibbler CC, et al. A prospective randomized trial of ceftazidime versus netilmicin plus mezlocillin in the empirical therapy of presumed sepsis in cirrhotic patients. Hepatology. 1997;25:833-836.
34. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
35. European Association tor the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242.
36. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
37. Portincasa P, Grattagliano I, Palmieri VO, et al. Current pharmacological treatment of nonalcoholic fatty liver. Curr Med Chem. 2006;13:2889-2900.
38. Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328-338.
• Suspect compensated liver cirrhosis in a patient with abnormal liver function tests, a low platelet count, and prolonged prothrombin time. C
• Use ultrasonography as a first-line diagnostic tool for liver cirrhosis. C
• Prescribe beta-blockers as prophylaxis for patients at risk for variceal bleeding. A
• Work collaboratively with hepatic specialists to manage the care of patients with cirrhosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: A patient with mildly elevated ALT and AST
John M., a 63-year-old truck driver with a family history of diabetes and arterial hypertension, is complaining of persistent fatigue—again. He has type 2 diabetes and takes metformin and repaglinide, but his blood pressure is normal. Lab tests reveal a recurrent mild elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of unknown origin; Mr. M. has no history of virus or hepatotoxic drugs, and reports only modest alcohol intake. He is obese, however, with a BMI of 34.5 and a waist circumference of 41 inches.
CASE 2: A patient with abdominal swelling
Anna B., a slim 68-year-old, comes in with 2 acute conditions: About a week ago, she noticed abdominal swelling and low back pain that began suddenly, after she carried a heavy load. She’s taken nonsteroidal anti-inflammatory drugs for 6 days, but the pain has not improved. The patient’s only significant clinical history is a hysterectomy, with oophorectomy, at age 38, and a recurrent elevation of serum transaminase levels that has never been investigated. Examination reveals an important kyphosis, and finger pressure on the vertebral spine or a position change exacerbates the pain. Her abdomen is swollen and tense, with a tympanic sound on the upper abdomen and a dull sound on the lower portion.
If John M. and Anna B. were your patients, would you suspect that they both have advanced liver disease? What diagnostic tests would you order, and how would you manage their care?
Cirrhosis has always been associated with high rates of morbidity and mortality. It is the 12th most common cause of death in the United States;1 in some parts of the world, its ranking as a cause of death is considerably higher.2,3 In recent years, however, cirrhosis has become the focus of greater attention both here and abroad, for 2 reasons: The first is the increasing prevalence of viral hepatitis and steatohepatitis, both of which are prominent causes of cirrhosis. The second is the improvement we’ve made in treatment: Not only can we slow the progression of cirrhosis, but in some cases, we can even restore hepatic function.4
The key to successful management of cirrhosis lies in spotting subtle signs and symptoms well in advance of the serious complications that can arise down the road. Here’s what to look for.
Early warnings you can’t afford to overlook
While the clinical presentation of a patient with liver cirrhosis is often asymptomatic, serum transminases—included in many standard laboratory tests as part of a routine examination—often provide the first sign of a problem.
Mildly elevated ALT in an asymptomatic patient may be transient and benign, or an indication of chronic liver disease.5 In fact, signs suggestive of significant liver disease have been reported in more than 20% of patients with ALT elevation.2 But because abnormal ALT values are common and frequently resolve, many primary care physicians pay little attention to this potentially important finding—and miss a key opportunity for early identification and treatment.6
Look at other lab values, risk factors, as well
Additional lab values that suggest the possibility of cirrhosis include an elevated AST/ALT ratio, a low platelet count (<150,000/L), elevated alkaline phosphatase, elevated bilirubin (>1.1 mg/dL), low serum albumin (<2.5 g/dL), and decreased prothrombin time (<100%). Potential causes include viral hepatitis, heavy alcohol use, hepatotoxic drugs, steatosis, and steatohepatitis.
The next step for a patient with any of these abnormal values is a thorough medication review and medical history. Identify all prescription and nonprescription drugs the patient is taking, as well as any herbal products and supplements, in search of hepatotoxic agents. Amiodarone and valproic acid, among other drugs, may cause steatosis, and some herbal products—particularly kava kava extract, used to treat anxiety and insomnia—have been linked to hepatitis and even liver failure.7 Question the patient about alcohol consumption and a history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders, as well.
At a minimum, schedule follow-up testing of asymptomatic patients with abnormal laboratory findings in no more than 6 months. Persistent ALT elevation in such patients is most commonly caused by major viruses, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), or nonalcoholic steatohepatitis (NASH).8 Nonalcoholic fatty liver is especially likely in patients with clinical and demographic risk factors—those who, like John M., suffer from obesity or diabetes, or both.
Ultrasound yields further information
Further screening should be limited to patients who continue to have abnormal test results for 6 months or more or have multiple risk factors. While biopsy is still considered the gold standard for diagnosing and staging chronic liver disease, it should be considered, according to the American Gastroenterological Association, only if ultrasound and other tests have not been helpful in reaching a diagnosis.9
Often, though, ultrasonography aids in diagnosis. In the case of John M., ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity and signs of severe steatosis and mild splenomegaly, but no increase in portal vein diameter and no ascites. For asymptomatic patients with cirrhosis or an earlier stage of liver disease, ultrasound at 6-month intervals, combined with blood alpha-fetoprotein measurement, can be used to track disease progression and screen for hepatocellular carcinoma.10
Newer, noninvasive methods aid in diagnosis
Noninvasive means of evaluating the presence and extent of liver fibrosis and differentiating cirrhosis from noncirrhosis, developed in recent years, have been found to have positive predictive values greater than 85% to 90%.11 Transient elastography (FibroScan, London, England), which assesses liver stiffness, is 1 such method. Although it is often used successfully, however, morbid obesity, small intercostal spaces, and ascites limit the diagnostic capability of this medical device.12
Fibrosis can also be detected with the use of 1 or more algorithms—each testing blood samples for a different combination of serum surrogate markers for liver disease. Some widely used algorithms include the APRI (AST-to-platelets ratio index), the Fibrotest (aptoglobin, alpha-2 macroglobulin, apolipoprotein A1, gamma-glutamyl transpeptidase, and bilirubin), the Hepascore (bilirubin, gamma-glutamyl transpeptidase, haluronic acid, alpha-2 macroglobulin, age, sex), and the BARD (BMI, AST/ALT ratio, diabetes).
Hepatologists often use the results of ultrasonography, followed by transient elastography in conjunction with findings from 1 or more of these algorithms, to determine which patients are candidates for liver biopsy.11,12
Staging is crucial, with or without biopsy
The decision to perform a liver biopsy should be based on a number of factors, including the patient’s age, lifestyle, liver chemistry abnormalities, desire for prognostic information, and associated comorbidities.9 Despite the value of biopsy, it is a costly procedure with potentially serious side effects and risks—and not always accepted by patients. In a recent survey of 1177 primary care physicians in France, as many as 59% of patients with chronic hepatitis C refused to undergo liver biopsy; what’s more, 22% of the doctors surveyed shared the patients’ hesitancy.13 Whether patients refuse biopsy or it is deemed unnecessary because ultrasound and other noninvasive tests result in a probable diagnosis, staging is necessary, both to guide therapy and to arrive at a prognosis.
Liver enzyme levels reveal little about organ integrity and are not useful for staging. But other parameters (specifically, bilirubin, albumin, and prothrombin time), combined with the presence (or absence) and severity of physical findings such as encephalopathy and ascites, are included in the Child-Pugh classification system ( TABLE 1 ),14 a widely used system that roughly indicates disease severity.15
The Model for End-stage Liver Disease (MELD)—and PELD, the pediatric model—use bilirubin, creatinine, and international normalized ratio values to classify disease severity. MELD and PELD scores are considered more accurate than the Child-Pugh score in determining short-term mortality,16 and are used by the United Network of Organ Sharing (UNOS) for liver allocation. You’ll find a calculator at http://optn.transplant.hrsa.gov/resources/MeldPeldCalculator.asp?index=97.17
Despite the progress in diagnostic techniques, the life expectancy and quality of life for patients with advanced cirrhosis remains poor. Patients routinely experience fatigue, pruritus, ascites, encephalopathy, and bleeding; dyspepsia and malnutrition are common, as well. Cirrhosis also carries the risk of life-threatening complications, partly due to comorbidities—most notably, osteoporosis, malabsorption, and rheumatic diseases. Liver transplantation has the potential to change the life expectancy of these patients, but because of the extensive waiting lists, candidates for transplant often die before a liver becomes available.
But for many patients who are in stable condition—those with compensated cirrhosis, that is—the prognosis is far more hopeful: In addition to providing standard medical care, including immunization, if necessary, and nutritional counseling, targeted therapy is crucial to slow, or stop, disease progression.
TABLE 1
Child-Pugh: Classifying cirrhosis, predicting survival*
| 1 point | 2 points | 3 points | |
|---|---|---|---|
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Prothrombin time (INR) | <4 sec (<1.7) | 4-6 sec (1.7-2.3) | > 6 sec (>2.3) |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| Ascites | Absent | Mild | Severe |
| Encephalopathy | Absent | Mild | Severe |
| INR, international normalized ratio. | |||
| * Total the number of points for all 5 indicators (1 point for every answer in column 1, 2 points for every answer in column 2, and 3 points for every answer in column 3). Patients with ≤6 points (Grade A) have an estimated 1-year survival rate of 100%; patients with 7-9 points (Grade B) have an estimated 1-year survival rate of 80%; and patients with ≥10 points (Grade C) have an estimated 1-year surival rate of 45%. | |||
| Adapted from: Infante-Rivard C, et al. Hepatology. 1987.14 | |||
Treatment for cirrhosis depends on the cause
Although primary care physicians can often provide most, or all, of the care for those in stable condition, a specialist may be helpful in determining further testing to identify the underlying cause of the cirrhosis, which is essential to determining the most appropriate treatment. What’s more, research has shown that patients with cirrhosis whose care is managed by a primary care physician and a hepatologist have better outcomes than those who are treated by a primary care doctor alone.18
What to test for?
Tests to determine the cause of cirrhosis are listed in TABLE 2 . For an individual patient, diagnostic tests would be based on the suspected cause. A patient with a family history of hereditary hemochromatosis would be tested for elevated serum ferritin levels and hepatic iron content on liver biopsy sample; the transferrin saturation index would also be obtained, and the patient might be tested for specific gene mutations. A patient who drinks heavily would be tested for elevated gamma-glutamyl transpeptidase and mean corpuscular volume. For someone with obesity, diabetes, and an enlarged liver, standard lab tests, including high-density lipoprotein (HDL) cholesterol, glucose, and triglycerides, may be sufficient.
Keep in mind, however, that cirrhosis may have more than 1 contributing factor—obesity or chronic alcohol use and a virus, for example; alcohol abuse and metabolic fatty liver; or virus and hemochromatosis. Thus, it may require more than 1 type of treatment.
Alcohol abuse is the cause of 25% of cases of liver cirrhosis, and a contributor to another 25% to 50%.19 The key treatment here—and an ideal role for a family physician—is to refer the patient to a detoxification and treatment program and provide ongoing monitoring and support. Antiviral treatment may be helpful for a recovering alcoholic who also tests positive for hepatitis B or C virus, but because of potential problems with compliance, some physicians delay therapy until the patient has had at least 6 months of continuous abstinence. Although this is not an absolute criterion, the same period of abstinence may be required before a patient becomes eligible for a liver transplant.
NAFLD/NASH is typically diagnosed on the basis of lab values and physical presentation. For a stable patient, the primary treatment includes lifestyle change—a low-calorie, low-carbohydrate diet and an exercise regimen—and a possible switch to insulin for better glycemic control.
For patients who are not candidates for such targeted treatments, either because their disease is too advanced or they’re unable to tolerate the recommended therapy, numerous pharmaceutical preparations claiming antioxidant or anti-inflammatory properties are available. But only 1—an herbal extract known as silymarin, derived from the milk thistle plant and taken with vitamin E—has been found to have some protective effects.20
TABLE 2
Liver cirrhosis: Common causes, diagnostic tests, and treatments4,34-38
| Cause | Test (result) | Therapy |
|---|---|---|
| Alcohol | GGT (↑), MCV (↑) | Abstinence |
| HBV + delta virus infection | HBsAg (+) HBV-DNA(+) HBc-IgM (+) HDV-RNA (+) | Interferon alpha-2b, nucleoside (lamivudine, telbivudine, entecavir) and nucleotide (adefovir, tenofovir) analogs |
| HCV infection | HCV-RNA (+) | Interferon + ribavirin |
| Primary biliary cirrhosis | GGT (↑) Alkaline phosphatase (↑) AMA (+) | Ursodeoxycholate |
| Autoimmune hepatitis | ANA (+) ASMA (+) LKM (+) | Prednisone, azathioprine |
| Hemochromatosis | Ferritin (↑) Transferrin saturation index (>45%) Hepatic iron content (↑) HFE gene mutation (C282Y, H63D) | Phlebotomy, chelating agents |
| Wilson’s disease | Ceruloplasmin (↓) Serum copper (↓) 24h urinary copper excretion (↑) | D-penicillamine, zinc |
| NAFLD/NASH | HDL cholesterol (↓) Glucose (↑) Triglycerides (↑) | Low-calorie diet, physical activity, insulin-sensitizer drugs or insulin |
| AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASMA, anti-smooth-muscle antibody; GGT, gamma–glutamyl transpeptidase; HBc-IgM, immunoglobulin M antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; HCV-RNA, hepatitis C virus RNA; HDL, high-density lipoprotein; HDV-RNA, hepatitis delta virus RNA; LKM, liver kidney microsomes; MCV, mean corpuscular volume, NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. | ||
Address systemic problems along with targeted treatment
Malnutrition is a serious problem for many patients with cirrhosis. Causes range from poor oral intake or malabsorption to ongoing alcohol use, chronic nausea, or early satiety because of compression from ascites. Dental problems that prevent the patient from chewing properly may be a contributing factor, as well.
Regardless of the cause, malnutrition is associated with muscle wasting, hypoalbuminemia, decreased resistance to infections, and variceal bleeding, and addressing it is a key part of treatment. Assess the nutritional status of every patient with cirrhosis, and stress the importance of multivitamin supplementation.21 If dental care is needed, take steps to see that the patient receives it.
Nutritional support, however, should be reserved for severely malnourished patients awaiting transplantation.22
Osteoporosis. Reduced bone formation—the result of vitamin D deficiency, hypoparathyroidism, and hypogonadism—is a well-known complication of end-stage cirrhosis. However, osteopenia may occur in an earlier stage of disease, especially in patients with cholestatic disease and those receiving antiviral therapy. Prescribe bisphosphonates, together with calcium and vitamin D3, to improve bone mineral density.23
Diabetes. The relationship between diabetes and cirrhosis is particularly complex, because diabetes can be both a causal factor and a consequence of cirrhosis. Diabetes is common in patients with NASH, and prevalent among those with hepatitis C and hemochromatosis. Multivariate analyses have found that diabetes has an independent negative effect on the progression of liver disease.24
Diet remains the first-line treatment for hyperglycemia, with metformin as the drug of choice if diet alone is unsuccessful. Sulfonylureas can be used, but require caution to avoid hypoglycemia. Glitazones are a newer alternative, but their value in patients with liver cirrhosis has not been studied. However, the use of any oral antidiabetic agent requires extra caution in patients with cirrhosis, and should be avoided in those with advanced liver disease. Although insulin requires intense self-monitoring of serum glucose levels, it is preferable to oral agents for this patient population.25
Managing complications of cirrhosis
Hospital, home, or long-term care? Whether patients with advanced cirrhosis can be maintained at home or require hospitalization or long-term care is best decided in consultation with patient, family, and other members of the health care team. One helpful tool is the Karnofsky Performance Scale Index (http://www.pennmedicine.org/homecare/hcp/elig_worksheets/Karnofsky-Performance-Status.pdf), which scores patients from 0 to 100 based on their functional impairment.26 (Patients with decompensated liver cirrhosis and limited self-sufficiency typically score <50, indicating that they require home health care, hospice, or institutional care.) Whatever the outcome, the patient may need to be reevaluated as the disease progresses and complications occur.
Ascites, the most common complication of cirrhosis,27 is a primary reason for hospitalization, but may be managed on an outpatient basis, depending on the patient presentation. Determining factors include the presence or absence of portal hypertension, impaired albumin synthesis, decreased plasma oncotic pressure, and sodium retention. Diagnosis is based on physical exam and ultrasonography.
Initial treatment for ascites includes salt restriction28,29 and avoidance of NSAIDs, which promote renal sodium retention, followed by spironolactone (100–400 mg/d). Add furosemide (40-160 mg/d) if the fluid retention does not begin to resolve after 3 to 5 days of treatment. If the condition persists despite maximum tolerable doses of diuretics, large-volume paracentesis to remove transudative fluid (albumin <1 g/dL; serum/ascites albumin gradient >1.1) may be needed. A patient with recurrent or refractory ascites should see a specialist for further evaluation and the possibility of a transjugular intrahepatic portosystemic shunt (TIPS).
Abdominal pain and an ascitic granulocyte count >250/mm3 suggest spontaneous bacterial peritonitis (SBP)—a severe complication of ascites that can result in renal and liver failure. In addition to pain, patients may present with tense ascites and fever, followed by encephalopathy, shock, and increased serum creatinine levels. Hospitalization is required for SBP; therapy includes high-dose albumin and intravenous antibiotics, typically cephalosporin. Long-term prophylaxis with norfloxacin to prevent the recurrence of SBP is indicated.30
If your patient has ascites and is being cared for at home, talk to the patient and his or her family about the importance of a daily weight check. Tell them to contact you if the patient gains more than 4 to 8 lbs within a few days. Frequent electrolyte checks are needed, as well. An albumin infusion is required when serum levels are particularly low, or after large-volume paracentesis.31 Patients with SBP or refractory ascites generally have more advanced disease and a poor prognosis.
Portal hypertension/esophageal varices. The main aim of treating portal hypertension is to prevent esophageal variceal bleeding. The appearance of varices should be checked by endoscopy every 2 to 3 years, or yearly for patients at high risk of bleeding. Patients with varices can be managed with nonselective beta-blockers at doses that are sufficient to elicit a 25% reduction in resting heart rate. Those at high risk for bleeding and patients who have already had esophageal bleeding may require endoscopic band ligation.32 TIPS is an alternative for those whose previous treatments have failed.33
Hepatic encephalopathy. This potentially reversible decrease in neuropsychiatric function mainly affects patients with portal hypertension. Caused by reduced hepatic clearance of gut-deriving neurotoxins, hepatic encephalopathy is associated with a range of signs and symptoms—from subtle personality changes to coma, with flapping tremor as a frequent initial finding. Acid-base and electrolyte disturbances, constipation, infections, gastrointestinal bleeding, and sedatives can precipitate encephalopathy. Hepatic encephalopathy is a diagnosis of exclusion, however, requiring the exclusion of all other etiologies of altered mental status.
Treatment consists of identifying and correcting the precipitating factors, and includes electrolyte correction, colon cleansing, and acidification with lactulose. Dietary protein restriction is no longer advocated, because it may facilitate malnutrition and complications. Oral rifaximine is useful and well tolerated for suppression of intestinal bacterial flora. Venous infusion of branched-chain amino acids or flumazenil may be effective in case of coma.
Fever and sepsis. Infection is a high-risk factor for mortality in patients with cirrhosis, as it can lead to renal and liver failure, variceal bleeding, and hepatic encephalopathy. However, individuals with cirrhosis often do not develop the typical signs and symptoms of infection; leukocytosis may be absent because of severe leukopenia, for instance, and patients may be afebrile.
Thus, the general appearance of systemic illness is an indication for antibiotics, with quinolones and cephalosporins as first-line agents. Infections most commonly involve the urinary tract (25%-55%) or the respiratory tract (20%), or are related to SBP (10%-30%).33 Hospitalization is suggested in case of poor general health status or the appearance of organ dysfunction.
When medical therapy and other interventions fail to control complications, transplantation is the only alternative. Primary care physicians can play a role here, too, in referring potential candidates for liver transplants to specialists for further consideration.
CASE 1: Resolution
As we’ve already seen, John M.’s ultrasound revealed an enlarged liver. The results led to a probable diagnosis of an advanced form of NASH. Other lab tests indicated that he had poorly controlled diabetes, high triglyceride levels, and—for the first time—a low platelet count. His physician stressed the importance of following a low-calorie, low-carbohydrate diet and exercising regularly, prescribed insulin, and referred the patient to a hepatologist for further noninvasive evaluation of fibrosis and to determine whether liver biopsy was needed.
CASE 2: Resolution
Blood tests revealed that Anna B. had a low platelet count (64,000/mm3), elevated liver enzymes (AST 2× upper limit of normal [ULN], ALT 1.5× ULN, GGT 2.5× ULN), and high gamma-globulins (33.6%) with no monoclonal bands. Ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity, portal vein dilatation, and moderate ascites. She also tested positive for HCV and had an HCV-RNA reading of 15×106 IU/mL. No other cause of chronic liver disease emerged. Ms. B.’s physician told her that she had an osteoporotic vertebral fracture—a frequent comorbidity in patients with liver cirrhosis—and decompensated liver cirrhosis from an old HCV infection. He added that her abdomen was distended because of fluid retention. The physician recommended bed rest, prescribed paracetamol (1 g tid) and spironolactone (100 mg/d), and referred the patient to an orthopedist for treatment of the fracture and to a hepatologist to be evaluated for transplantation.
CORRESPONDENCE
Ignazio Grattagliano, MD, Department of Internal Medicine, University Medical School of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
• Suspect compensated liver cirrhosis in a patient with abnormal liver function tests, a low platelet count, and prolonged prothrombin time. C
• Use ultrasonography as a first-line diagnostic tool for liver cirrhosis. C
• Prescribe beta-blockers as prophylaxis for patients at risk for variceal bleeding. A
• Work collaboratively with hepatic specialists to manage the care of patients with cirrhosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: A patient with mildly elevated ALT and AST
John M., a 63-year-old truck driver with a family history of diabetes and arterial hypertension, is complaining of persistent fatigue—again. He has type 2 diabetes and takes metformin and repaglinide, but his blood pressure is normal. Lab tests reveal a recurrent mild elevation of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels of unknown origin; Mr. M. has no history of virus or hepatotoxic drugs, and reports only modest alcohol intake. He is obese, however, with a BMI of 34.5 and a waist circumference of 41 inches.
CASE 2: A patient with abdominal swelling
Anna B., a slim 68-year-old, comes in with 2 acute conditions: About a week ago, she noticed abdominal swelling and low back pain that began suddenly, after she carried a heavy load. She’s taken nonsteroidal anti-inflammatory drugs for 6 days, but the pain has not improved. The patient’s only significant clinical history is a hysterectomy, with oophorectomy, at age 38, and a recurrent elevation of serum transaminase levels that has never been investigated. Examination reveals an important kyphosis, and finger pressure on the vertebral spine or a position change exacerbates the pain. Her abdomen is swollen and tense, with a tympanic sound on the upper abdomen and a dull sound on the lower portion.
If John M. and Anna B. were your patients, would you suspect that they both have advanced liver disease? What diagnostic tests would you order, and how would you manage their care?
Cirrhosis has always been associated with high rates of morbidity and mortality. It is the 12th most common cause of death in the United States;1 in some parts of the world, its ranking as a cause of death is considerably higher.2,3 In recent years, however, cirrhosis has become the focus of greater attention both here and abroad, for 2 reasons: The first is the increasing prevalence of viral hepatitis and steatohepatitis, both of which are prominent causes of cirrhosis. The second is the improvement we’ve made in treatment: Not only can we slow the progression of cirrhosis, but in some cases, we can even restore hepatic function.4
The key to successful management of cirrhosis lies in spotting subtle signs and symptoms well in advance of the serious complications that can arise down the road. Here’s what to look for.
Early warnings you can’t afford to overlook
While the clinical presentation of a patient with liver cirrhosis is often asymptomatic, serum transminases—included in many standard laboratory tests as part of a routine examination—often provide the first sign of a problem.
Mildly elevated ALT in an asymptomatic patient may be transient and benign, or an indication of chronic liver disease.5 In fact, signs suggestive of significant liver disease have been reported in more than 20% of patients with ALT elevation.2 But because abnormal ALT values are common and frequently resolve, many primary care physicians pay little attention to this potentially important finding—and miss a key opportunity for early identification and treatment.6
Look at other lab values, risk factors, as well
Additional lab values that suggest the possibility of cirrhosis include an elevated AST/ALT ratio, a low platelet count (<150,000/L), elevated alkaline phosphatase, elevated bilirubin (>1.1 mg/dL), low serum albumin (<2.5 g/dL), and decreased prothrombin time (<100%). Potential causes include viral hepatitis, heavy alcohol use, hepatotoxic drugs, steatosis, and steatohepatitis.
The next step for a patient with any of these abnormal values is a thorough medication review and medical history. Identify all prescription and nonprescription drugs the patient is taking, as well as any herbal products and supplements, in search of hepatotoxic agents. Amiodarone and valproic acid, among other drugs, may cause steatosis, and some herbal products—particularly kava kava extract, used to treat anxiety and insomnia—have been linked to hepatitis and even liver failure.7 Question the patient about alcohol consumption and a history of conditions associated with liver disease, such as diabetes, hyperlipidemia, and thyroid disorders, as well.
At a minimum, schedule follow-up testing of asymptomatic patients with abnormal laboratory findings in no more than 6 months. Persistent ALT elevation in such patients is most commonly caused by major viruses, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), or nonalcoholic steatohepatitis (NASH).8 Nonalcoholic fatty liver is especially likely in patients with clinical and demographic risk factors—those who, like John M., suffer from obesity or diabetes, or both.
Ultrasound yields further information
Further screening should be limited to patients who continue to have abnormal test results for 6 months or more or have multiple risk factors. While biopsy is still considered the gold standard for diagnosing and staging chronic liver disease, it should be considered, according to the American Gastroenterological Association, only if ultrasound and other tests have not been helpful in reaching a diagnosis.9
Often, though, ultrasonography aids in diagnosis. In the case of John M., ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity and signs of severe steatosis and mild splenomegaly, but no increase in portal vein diameter and no ascites. For asymptomatic patients with cirrhosis or an earlier stage of liver disease, ultrasound at 6-month intervals, combined with blood alpha-fetoprotein measurement, can be used to track disease progression and screen for hepatocellular carcinoma.10
Newer, noninvasive methods aid in diagnosis
Noninvasive means of evaluating the presence and extent of liver fibrosis and differentiating cirrhosis from noncirrhosis, developed in recent years, have been found to have positive predictive values greater than 85% to 90%.11 Transient elastography (FibroScan, London, England), which assesses liver stiffness, is 1 such method. Although it is often used successfully, however, morbid obesity, small intercostal spaces, and ascites limit the diagnostic capability of this medical device.12
Fibrosis can also be detected with the use of 1 or more algorithms—each testing blood samples for a different combination of serum surrogate markers for liver disease. Some widely used algorithms include the APRI (AST-to-platelets ratio index), the Fibrotest (aptoglobin, alpha-2 macroglobulin, apolipoprotein A1, gamma-glutamyl transpeptidase, and bilirubin), the Hepascore (bilirubin, gamma-glutamyl transpeptidase, haluronic acid, alpha-2 macroglobulin, age, sex), and the BARD (BMI, AST/ALT ratio, diabetes).
Hepatologists often use the results of ultrasonography, followed by transient elastography in conjunction with findings from 1 or more of these algorithms, to determine which patients are candidates for liver biopsy.11,12
Staging is crucial, with or without biopsy
The decision to perform a liver biopsy should be based on a number of factors, including the patient’s age, lifestyle, liver chemistry abnormalities, desire for prognostic information, and associated comorbidities.9 Despite the value of biopsy, it is a costly procedure with potentially serious side effects and risks—and not always accepted by patients. In a recent survey of 1177 primary care physicians in France, as many as 59% of patients with chronic hepatitis C refused to undergo liver biopsy; what’s more, 22% of the doctors surveyed shared the patients’ hesitancy.13 Whether patients refuse biopsy or it is deemed unnecessary because ultrasound and other noninvasive tests result in a probable diagnosis, staging is necessary, both to guide therapy and to arrive at a prognosis.
Liver enzyme levels reveal little about organ integrity and are not useful for staging. But other parameters (specifically, bilirubin, albumin, and prothrombin time), combined with the presence (or absence) and severity of physical findings such as encephalopathy and ascites, are included in the Child-Pugh classification system ( TABLE 1 ),14 a widely used system that roughly indicates disease severity.15
The Model for End-stage Liver Disease (MELD)—and PELD, the pediatric model—use bilirubin, creatinine, and international normalized ratio values to classify disease severity. MELD and PELD scores are considered more accurate than the Child-Pugh score in determining short-term mortality,16 and are used by the United Network of Organ Sharing (UNOS) for liver allocation. You’ll find a calculator at http://optn.transplant.hrsa.gov/resources/MeldPeldCalculator.asp?index=97.17
Despite the progress in diagnostic techniques, the life expectancy and quality of life for patients with advanced cirrhosis remains poor. Patients routinely experience fatigue, pruritus, ascites, encephalopathy, and bleeding; dyspepsia and malnutrition are common, as well. Cirrhosis also carries the risk of life-threatening complications, partly due to comorbidities—most notably, osteoporosis, malabsorption, and rheumatic diseases. Liver transplantation has the potential to change the life expectancy of these patients, but because of the extensive waiting lists, candidates for transplant often die before a liver becomes available.
But for many patients who are in stable condition—those with compensated cirrhosis, that is—the prognosis is far more hopeful: In addition to providing standard medical care, including immunization, if necessary, and nutritional counseling, targeted therapy is crucial to slow, or stop, disease progression.
TABLE 1
Child-Pugh: Classifying cirrhosis, predicting survival*
| 1 point | 2 points | 3 points | |
|---|---|---|---|
| Bilirubin (mg/dL) | <2 | 2-3 | >3 |
| Prothrombin time (INR) | <4 sec (<1.7) | 4-6 sec (1.7-2.3) | > 6 sec (>2.3) |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| Ascites | Absent | Mild | Severe |
| Encephalopathy | Absent | Mild | Severe |
| INR, international normalized ratio. | |||
| * Total the number of points for all 5 indicators (1 point for every answer in column 1, 2 points for every answer in column 2, and 3 points for every answer in column 3). Patients with ≤6 points (Grade A) have an estimated 1-year survival rate of 100%; patients with 7-9 points (Grade B) have an estimated 1-year survival rate of 80%; and patients with ≥10 points (Grade C) have an estimated 1-year surival rate of 45%. | |||
| Adapted from: Infante-Rivard C, et al. Hepatology. 1987.14 | |||
Treatment for cirrhosis depends on the cause
Although primary care physicians can often provide most, or all, of the care for those in stable condition, a specialist may be helpful in determining further testing to identify the underlying cause of the cirrhosis, which is essential to determining the most appropriate treatment. What’s more, research has shown that patients with cirrhosis whose care is managed by a primary care physician and a hepatologist have better outcomes than those who are treated by a primary care doctor alone.18
What to test for?
Tests to determine the cause of cirrhosis are listed in TABLE 2 . For an individual patient, diagnostic tests would be based on the suspected cause. A patient with a family history of hereditary hemochromatosis would be tested for elevated serum ferritin levels and hepatic iron content on liver biopsy sample; the transferrin saturation index would also be obtained, and the patient might be tested for specific gene mutations. A patient who drinks heavily would be tested for elevated gamma-glutamyl transpeptidase and mean corpuscular volume. For someone with obesity, diabetes, and an enlarged liver, standard lab tests, including high-density lipoprotein (HDL) cholesterol, glucose, and triglycerides, may be sufficient.
Keep in mind, however, that cirrhosis may have more than 1 contributing factor—obesity or chronic alcohol use and a virus, for example; alcohol abuse and metabolic fatty liver; or virus and hemochromatosis. Thus, it may require more than 1 type of treatment.
Alcohol abuse is the cause of 25% of cases of liver cirrhosis, and a contributor to another 25% to 50%.19 The key treatment here—and an ideal role for a family physician—is to refer the patient to a detoxification and treatment program and provide ongoing monitoring and support. Antiviral treatment may be helpful for a recovering alcoholic who also tests positive for hepatitis B or C virus, but because of potential problems with compliance, some physicians delay therapy until the patient has had at least 6 months of continuous abstinence. Although this is not an absolute criterion, the same period of abstinence may be required before a patient becomes eligible for a liver transplant.
NAFLD/NASH is typically diagnosed on the basis of lab values and physical presentation. For a stable patient, the primary treatment includes lifestyle change—a low-calorie, low-carbohydrate diet and an exercise regimen—and a possible switch to insulin for better glycemic control.
For patients who are not candidates for such targeted treatments, either because their disease is too advanced or they’re unable to tolerate the recommended therapy, numerous pharmaceutical preparations claiming antioxidant or anti-inflammatory properties are available. But only 1—an herbal extract known as silymarin, derived from the milk thistle plant and taken with vitamin E—has been found to have some protective effects.20
TABLE 2
Liver cirrhosis: Common causes, diagnostic tests, and treatments4,34-38
| Cause | Test (result) | Therapy |
|---|---|---|
| Alcohol | GGT (↑), MCV (↑) | Abstinence |
| HBV + delta virus infection | HBsAg (+) HBV-DNA(+) HBc-IgM (+) HDV-RNA (+) | Interferon alpha-2b, nucleoside (lamivudine, telbivudine, entecavir) and nucleotide (adefovir, tenofovir) analogs |
| HCV infection | HCV-RNA (+) | Interferon + ribavirin |
| Primary biliary cirrhosis | GGT (↑) Alkaline phosphatase (↑) AMA (+) | Ursodeoxycholate |
| Autoimmune hepatitis | ANA (+) ASMA (+) LKM (+) | Prednisone, azathioprine |
| Hemochromatosis | Ferritin (↑) Transferrin saturation index (>45%) Hepatic iron content (↑) HFE gene mutation (C282Y, H63D) | Phlebotomy, chelating agents |
| Wilson’s disease | Ceruloplasmin (↓) Serum copper (↓) 24h urinary copper excretion (↑) | D-penicillamine, zinc |
| NAFLD/NASH | HDL cholesterol (↓) Glucose (↑) Triglycerides (↑) | Low-calorie diet, physical activity, insulin-sensitizer drugs or insulin |
| AMA, antimitochondrial antibody; ANA, antinuclear antibody; ASMA, anti-smooth-muscle antibody; GGT, gamma–glutamyl transpeptidase; HBc-IgM, immunoglobulin M antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; HCV-RNA, hepatitis C virus RNA; HDL, high-density lipoprotein; HDV-RNA, hepatitis delta virus RNA; LKM, liver kidney microsomes; MCV, mean corpuscular volume, NAFLD/NASH, nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. | ||
Address systemic problems along with targeted treatment
Malnutrition is a serious problem for many patients with cirrhosis. Causes range from poor oral intake or malabsorption to ongoing alcohol use, chronic nausea, or early satiety because of compression from ascites. Dental problems that prevent the patient from chewing properly may be a contributing factor, as well.
Regardless of the cause, malnutrition is associated with muscle wasting, hypoalbuminemia, decreased resistance to infections, and variceal bleeding, and addressing it is a key part of treatment. Assess the nutritional status of every patient with cirrhosis, and stress the importance of multivitamin supplementation.21 If dental care is needed, take steps to see that the patient receives it.
Nutritional support, however, should be reserved for severely malnourished patients awaiting transplantation.22
Osteoporosis. Reduced bone formation—the result of vitamin D deficiency, hypoparathyroidism, and hypogonadism—is a well-known complication of end-stage cirrhosis. However, osteopenia may occur in an earlier stage of disease, especially in patients with cholestatic disease and those receiving antiviral therapy. Prescribe bisphosphonates, together with calcium and vitamin D3, to improve bone mineral density.23
Diabetes. The relationship between diabetes and cirrhosis is particularly complex, because diabetes can be both a causal factor and a consequence of cirrhosis. Diabetes is common in patients with NASH, and prevalent among those with hepatitis C and hemochromatosis. Multivariate analyses have found that diabetes has an independent negative effect on the progression of liver disease.24
Diet remains the first-line treatment for hyperglycemia, with metformin as the drug of choice if diet alone is unsuccessful. Sulfonylureas can be used, but require caution to avoid hypoglycemia. Glitazones are a newer alternative, but their value in patients with liver cirrhosis has not been studied. However, the use of any oral antidiabetic agent requires extra caution in patients with cirrhosis, and should be avoided in those with advanced liver disease. Although insulin requires intense self-monitoring of serum glucose levels, it is preferable to oral agents for this patient population.25
Managing complications of cirrhosis
Hospital, home, or long-term care? Whether patients with advanced cirrhosis can be maintained at home or require hospitalization or long-term care is best decided in consultation with patient, family, and other members of the health care team. One helpful tool is the Karnofsky Performance Scale Index (http://www.pennmedicine.org/homecare/hcp/elig_worksheets/Karnofsky-Performance-Status.pdf), which scores patients from 0 to 100 based on their functional impairment.26 (Patients with decompensated liver cirrhosis and limited self-sufficiency typically score <50, indicating that they require home health care, hospice, or institutional care.) Whatever the outcome, the patient may need to be reevaluated as the disease progresses and complications occur.
Ascites, the most common complication of cirrhosis,27 is a primary reason for hospitalization, but may be managed on an outpatient basis, depending on the patient presentation. Determining factors include the presence or absence of portal hypertension, impaired albumin synthesis, decreased plasma oncotic pressure, and sodium retention. Diagnosis is based on physical exam and ultrasonography.
Initial treatment for ascites includes salt restriction28,29 and avoidance of NSAIDs, which promote renal sodium retention, followed by spironolactone (100–400 mg/d). Add furosemide (40-160 mg/d) if the fluid retention does not begin to resolve after 3 to 5 days of treatment. If the condition persists despite maximum tolerable doses of diuretics, large-volume paracentesis to remove transudative fluid (albumin <1 g/dL; serum/ascites albumin gradient >1.1) may be needed. A patient with recurrent or refractory ascites should see a specialist for further evaluation and the possibility of a transjugular intrahepatic portosystemic shunt (TIPS).
Abdominal pain and an ascitic granulocyte count >250/mm3 suggest spontaneous bacterial peritonitis (SBP)—a severe complication of ascites that can result in renal and liver failure. In addition to pain, patients may present with tense ascites and fever, followed by encephalopathy, shock, and increased serum creatinine levels. Hospitalization is required for SBP; therapy includes high-dose albumin and intravenous antibiotics, typically cephalosporin. Long-term prophylaxis with norfloxacin to prevent the recurrence of SBP is indicated.30
If your patient has ascites and is being cared for at home, talk to the patient and his or her family about the importance of a daily weight check. Tell them to contact you if the patient gains more than 4 to 8 lbs within a few days. Frequent electrolyte checks are needed, as well. An albumin infusion is required when serum levels are particularly low, or after large-volume paracentesis.31 Patients with SBP or refractory ascites generally have more advanced disease and a poor prognosis.
Portal hypertension/esophageal varices. The main aim of treating portal hypertension is to prevent esophageal variceal bleeding. The appearance of varices should be checked by endoscopy every 2 to 3 years, or yearly for patients at high risk of bleeding. Patients with varices can be managed with nonselective beta-blockers at doses that are sufficient to elicit a 25% reduction in resting heart rate. Those at high risk for bleeding and patients who have already had esophageal bleeding may require endoscopic band ligation.32 TIPS is an alternative for those whose previous treatments have failed.33
Hepatic encephalopathy. This potentially reversible decrease in neuropsychiatric function mainly affects patients with portal hypertension. Caused by reduced hepatic clearance of gut-deriving neurotoxins, hepatic encephalopathy is associated with a range of signs and symptoms—from subtle personality changes to coma, with flapping tremor as a frequent initial finding. Acid-base and electrolyte disturbances, constipation, infections, gastrointestinal bleeding, and sedatives can precipitate encephalopathy. Hepatic encephalopathy is a diagnosis of exclusion, however, requiring the exclusion of all other etiologies of altered mental status.
Treatment consists of identifying and correcting the precipitating factors, and includes electrolyte correction, colon cleansing, and acidification with lactulose. Dietary protein restriction is no longer advocated, because it may facilitate malnutrition and complications. Oral rifaximine is useful and well tolerated for suppression of intestinal bacterial flora. Venous infusion of branched-chain amino acids or flumazenil may be effective in case of coma.
Fever and sepsis. Infection is a high-risk factor for mortality in patients with cirrhosis, as it can lead to renal and liver failure, variceal bleeding, and hepatic encephalopathy. However, individuals with cirrhosis often do not develop the typical signs and symptoms of infection; leukocytosis may be absent because of severe leukopenia, for instance, and patients may be afebrile.
Thus, the general appearance of systemic illness is an indication for antibiotics, with quinolones and cephalosporins as first-line agents. Infections most commonly involve the urinary tract (25%-55%) or the respiratory tract (20%), or are related to SBP (10%-30%).33 Hospitalization is suggested in case of poor general health status or the appearance of organ dysfunction.
When medical therapy and other interventions fail to control complications, transplantation is the only alternative. Primary care physicians can play a role here, too, in referring potential candidates for liver transplants to specialists for further consideration.
CASE 1: Resolution
As we’ve already seen, John M.’s ultrasound revealed an enlarged liver. The results led to a probable diagnosis of an advanced form of NASH. Other lab tests indicated that he had poorly controlled diabetes, high triglyceride levels, and—for the first time—a low platelet count. His physician stressed the importance of following a low-calorie, low-carbohydrate diet and exercising regularly, prescribed insulin, and referred the patient to a hepatologist for further noninvasive evaluation of fibrosis and to determine whether liver biopsy was needed.
CASE 2: Resolution
Blood tests revealed that Anna B. had a low platelet count (64,000/mm3), elevated liver enzymes (AST 2× upper limit of normal [ULN], ALT 1.5× ULN, GGT 2.5× ULN), and high gamma-globulins (33.6%) with no monoclonal bands. Ultrasound revealed an enlarged liver with diffuse echostructural dyshomogeneity, portal vein dilatation, and moderate ascites. She also tested positive for HCV and had an HCV-RNA reading of 15×106 IU/mL. No other cause of chronic liver disease emerged. Ms. B.’s physician told her that she had an osteoporotic vertebral fracture—a frequent comorbidity in patients with liver cirrhosis—and decompensated liver cirrhosis from an old HCV infection. He added that her abdomen was distended because of fluid retention. The physician recommended bed rest, prescribed paracetamol (1 g tid) and spironolactone (100 mg/d), and referred the patient to an orthopedist for treatment of the fracture and to a hepatologist to be evaluated for transplantation.
CORRESPONDENCE
Ignazio Grattagliano, MD, Department of Internal Medicine, University Medical School of Bari, P.zza G. Cesare, 11 – 70124, Bari, Italy; [email protected]
1. Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. National Vital Stat Rep. 2009;57:(14):1-135.Available at: www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. Accessed September 16, 2009.
2. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20:1442-1449.
3. Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762.
4. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851.
5. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110.
6. Sherwood P, Lyburn I, Brown S, et al. How are abnormal results for liver function tests dealt with in primary care? Audit of yield and impact. BMJ. 2001;322:276-278.
7. US Food and Drug Administration. Food. Consumer advisory: Kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm. Accessed September 11, 2009.
8. Grattagliano I, Portincasa P, Palmieri VO, et al. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857-863.
9. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
10. Sherman M, Klein A. AASLD single-topic research conference on hepatocellular carcinoma: conference proceedings. Hepatology. 2004;40:1465-1473.
11. Pinzani M, Vizzutti F, Arena U, et al. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
12. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
13. Bonny C, Rayssiguier R, Ughetto S, et al. Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region [In French]. Gastroenterol Clin Biol. 2003;27:1021-1025.
14. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
15. Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;Aug. 20 [Epub ahead of print].
16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96.
17. United Network of Organ Sharing. Resources. Meld/Peld calculator. Available at: http://www.unos.org/resources/meldPeldCalculator.asp. Accessed September 11, 2009.
18. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
19. Habib A, Bond WM, Heuman DM. Long-term management of cirrhosis. Appropriate supportive care is both critical and difficult. Postgrad Med. 2001;109:101-103.
20. Flora K, Hahn M, Rosen H, et al. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143.
21. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis [In French]. Gastroenterol Clin Biol. 2008;32:265-273.
22. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55.
23. Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(suppl 1):i1-i9.
24. Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75.
25. Garcia-Compean D, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288.
26. Karnofsky Performance Scale Index. Available at: http://www.medal.org/visitor/www%5CActive%5Cch1%5Cch1.01%5Cch1.01.01.aspx. Accessed September 11, 2009.
27. Gentilini P, Bernardi M, Bolondi L, et al. The rational use of albumin in patients with cirrhosis and ascites. A Delphi study for the attainment of a consensus on prescribing standards. Dig Liver Dis. 2004;36:539-546.
28. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
29. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856.
30. Gines P, et al. Pathophysiology, complications, and treatment of ascites. Clin Liver Dis. 1997;1:129-155.
31. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
32. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081-1091.
33. McCormick PA, Greenslade L, Kibbler CC, et al. A prospective randomized trial of ceftazidime versus netilmicin plus mezlocillin in the empirical therapy of presumed sepsis in cirrhotic patients. Hepatology. 1997;25:833-836.
34. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
35. European Association tor the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242.
36. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
37. Portincasa P, Grattagliano I, Palmieri VO, et al. Current pharmacological treatment of nonalcoholic fatty liver. Curr Med Chem. 2006;13:2889-2900.
38. Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328-338.
1. Heron M, Hoyert DL, Murphy SL, et al. Deaths: final data for 2006. National Vital Stat Rep. 2009;57:(14):1-135.Available at: www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf. Accessed September 16, 2009.
2. Bellentani S, Tiribelli C, Saccoccio G, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20:1442-1449.
3. Heidelbaugh JJ, Bruderly M. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 2006;74:756-762.
4. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851.
5. Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105-1110.
6. Sherwood P, Lyburn I, Brown S, et al. How are abnormal results for liver function tests dealt with in primary care? Audit of yield and impact. BMJ. 2001;322:276-278.
7. US Food and Drug Administration. Food. Consumer advisory: Kava-containing dietary supplements may be associated with severe liver injury. March 25, 2002. Available at: http://www.fda.gov/Food/ResourcesForYou/Consumers/ucm085482.htm. Accessed September 11, 2009.
8. Grattagliano I, Portincasa P, Palmieri VO, et al. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857-863.
9. Green RM, Flamm S. AGA technical review on the evaluation of liver chemistry tests. Gastroenterology. 2002;123:1367-1384.
10. Sherman M, Klein A. AASLD single-topic research conference on hepatocellular carcinoma: conference proceedings. Hepatology. 2004;40:1465-1473.
11. Pinzani M, Vizzutti F, Arena U, et al. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95-106.
12. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350.
13. Bonny C, Rayssiguier R, Ughetto S, et al. Medical practices and expectations of general practitioners in relation to hepatitis C virus infection in the Auvergne region [In French]. Gastroenterol Clin Biol. 2003;27:1021-1025.
14. Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting shortterm survival among cirrhotics. Hepatology. 1987;7:660-664.
15. Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;Aug. 20 [Epub ahead of print].
16. Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96.
17. United Network of Organ Sharing. Resources. Meld/Peld calculator. Available at: http://www.unos.org/resources/meldPeldCalculator.asp. Accessed September 11, 2009.
18. Bini EJ, Weinshel EH, Generoso R, et al. Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology. 2001;34:1089-1095.
19. Habib A, Bond WM, Heuman DM. Long-term management of cirrhosis. Appropriate supportive care is both critical and difficult. Postgrad Med. 2001;109:101-103.
20. Flora K, Hahn M, Rosen H, et al. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143.
21. Buyse S, Durand F, Joly F. Nutritional assessment in cirrhosis [In French]. Gastroenterol Clin Biol. 2008;32:265-273.
22. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43-55.
23. Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50(suppl 1):i1-i9.
24. Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006;101:70-75.
25. Garcia-Compean D, et al. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol. 2009;15:280-288.
26. Karnofsky Performance Scale Index. Available at: http://www.medal.org/visitor/www%5CActive%5Cch1%5Cch1.01%5Cch1.01.01.aspx. Accessed September 11, 2009.
27. Gentilini P, Bernardi M, Bolondi L, et al. The rational use of albumin in patients with cirrhosis and ascites. A Delphi study for the attainment of a consensus on prescribing standards. Dig Liver Dis. 2004;36:539-546.
28. Kashani A, Landaverde C, Medici V, et al. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85.
29. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856.
30. Gines P, et al. Pathophysiology, complications, and treatment of ascites. Clin Liver Dis. 1997;1:129-155.
31. Sarin SK, Lamba GS, Kumar M, et al. Comparison of endoscopic ligation and propranolol for the primary prevention of variceal bleeding. N Engl J Med. 1999;340:988-993.
32. Grace ND. Diagnosis and treatment of gastrointestinal bleeding secondary to portal hypertension. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 1997;92:1081-1091.
33. McCormick PA, Greenslade L, Kibbler CC, et al. A prospective randomized trial of ceftazidime versus netilmicin plus mezlocillin in the empirical therapy of presumed sepsis in cirrhotic patients. Hepatology. 1997;25:833-836.
34. Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479-497.
35. European Association tor the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242.
36. Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374.
37. Portincasa P, Grattagliano I, Palmieri VO, et al. Current pharmacological treatment of nonalcoholic fatty liver. Curr Med Chem. 2006;13:2889-2900.
38. Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2008;24:328-338.
DPP-4 Inhibitors: A New Therapeutic Class for the Treatment of Type 2 Diabetes
Type 2 diabetes (T2D) is characterized by altered glucose homeostasis, including decreased insulin sensitivity of target tissues, a gradual decline in β-cell insulin production and secretion, and a progressive inability to suppress pancreatic α-cell glucagon secretion.1 In the past, goals for therapy have focused primarily on insulin secretion, sensitization, and replacement. However, newer T2D medications utilize the incretin gut hormone pathway, a focus of scientific and clinical research for decades.2 The so-called insulin effect, known today as the incretin effect3 —ie, greater insulin secretion in response to nutrient ingestion—was identified in 1964 when Elrick et al4 demonstrated that orally administered glucose produced a significant and sustained increase in plasma insulin, whereas intravenously administered glucose produced a smaller and transient insulin increase. This finding was paramount in bringing incretin-based therapies to clinical practice.
The 2 most well characterized incretin hormones are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) ( Figure 1 ). Currently, therapeutic agents, acting as either an incretin mimetic (via GLP-1 analogs) or to inhibit the breakdown of GLP-1 (via dipeptidyl peptidase-4 [DPP-4] inhibitors) are available for treatment.2,3 Various DPP-4 inhibitors are in development, and 2 are approved by the US Food and Drug Administration (FDA): sitagliptin and the recently approved DPP-4 inhibitor, saxagliptin, are indicated for use in a broad range of patients, including those who are drug naïve or who have inadequate glycemic control on another oral antidiabetic drug (OAD). Both agents are approved as monotherapy and as an add-on to current antihyperglycemic therapy (ie, metformin [MET], sulfonylurea [SU], thiazolidinedione [TZD]), and are also approved as initial combination therapy with MET.5-7 Another DPP-4 inhibitor, alogliptin, failed to gain approval from the FDA, which indicated the need for additional data (Takeda Pharmaceutical Company Limited) ( Table 1 ).
Figure 1
DPP-4 Inhibitors: Mechanism of Glucose Control28
DPP-4, dipeptidyl peptidase-4; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
Post–meal ingestion, GLP-1 and GIP are released from the small intestine and are rapidly degraded by the enzyme DPP-4. Inhibition of DPP-4 prevents the breakdown of GLP-1 and GIP and enhances glucose-stimulated insulin secretion (incretin action). GLP-1 and GIP act on the pancreatic β-cell to increase insulin release. GLP-1 also acts on the α-cell to suppress glucagon release and ultimately suppress hepatic glucose production. Together, the increased cellular glucose uptake and the decreased hepatic glucose output offer physiologic glucose control.
Table 1
DPP-4 Inhibitor Status and Availability
| Drug | Status | Trade name | Pharmaceutical Company |
|---|---|---|---|
| Alogliptin | Failed to gain approval | Not officially disclosed | Takeda Pharmaceutical Company Limited |
| Dutogliptin | Phase 3 | Not officially disclosed | Phenomix/Forest Laboratories, Inc. |
| Linagliptin | Phase 3 | Ondero® | Boehringer Ingelheim |
| Saxagliptin | Approved in the United States and Europe | Onglyza™ | Bristol-Myers Squibb/AstraZeneca |
| Sitagliptin | Approved in the United States and Europe | Januvia™, Janumet® | Merck & Co., Inc. |
| Vildagliptin | Approved in Europe | Galvus® | Novartis AG |
| DPP-4, dipeptidyl peptidase-4. | |||
The current therapeutic options for treating type 2 diabetes (T2D) include drug classes that lower blood glucose levels by different mechanisms of action ( Table ) through various target organs.1,2
Table
Drug classes that lower blood glucose levels
| Agent(s) | Mechanism of Action |
|---|---|
| • Insulin • Sulfonylureas • Glinides | Insulin replacement/secretion |
| • Thiazolidinediones | Insulin sensitization |
| • Biguanides | Decrease of hepatic glucose output |
| • α-Glucosidase inhibitors | Delay of intestinal carbohydrate absorption |
| • DPP-4 inhibitors • GLP-1 analogs | Incretin enhancement/replacement with subsequent effects on insulin and glucagon secretion |
| DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1. | |
Glucagon-like peptide-1 (GLP-1) is known to enhance insulin release from the pancreatic β-cells and inhibit glucagon release through the α-cells in a glucose-dependent manner.3 In the fasted state, circulating levels of GLP are low but rise within minutes of meal ingestion. GLP-1 is released from the L cells of the small intestine within minutes of food consumption; however, incretin hormones are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4).3 By inhibiting DPP-4, the DPP-4 inhibitors enhance the half-life of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), thereby augmenting their levels.1,4 Because the release of GLP-1 is glucose dependent, augmentation of GLP-1 by DPP-4 inhibition minimizes the risk for hypoglycemia, which proves to be clinically important in managing T2D.
The majority of antidiabetic agents act primarily by lowering fasting plasma glucose (FPG) (eg, sulfonylureas), whereas others act primarily by lowering postprandial glucose (PPG).5 DPP-4 inhibitors primarily have a postprandial effect but also show statistically significant reductions in fasting glucose levels.6 FPG and PPG are the essential components of lowering glycosylated hemoglobin (HbA1c), and PPG has a greater effect on lowering HbA1c at values <8.5%.7
References
1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157.
2. Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172:213-226.
3. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165.
4. Stonehouse A, Okerson T, Kendall D, et al. Emerging incretin-based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev. 2008;4:101-109.
5. Leiter LA, Ceriello A, Davidson JA, et al. Postprandial glucose regulation: new data and new implications. Clin Ther. 2005;27(suppl B):S42-S56.
6. Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
7. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881-885.
DPP-4 Inhibitors Offer Comprehensive Glycemic Management
Glycemic control efforts should be directed at the multiple pathophysiologic defects of T2D and should involve routine assessment of HbA1c, monitoring of daily blood glucose values, and treatment with combination regimens that target glucose levels both before and after meals. As monotherapy, DPP-4 inhibitors demonstrate reductions of approximately 0.5% to 0.8% in HbA1c, with concomitant reductions in postprandial glucose (PPG) and fasting plasma glucose (FPG) (eg, PPG reductions of –43 to –45 mg/dL at 120 minutes of an oral glucose tolerance test [OGTT] and FPG reductions of –9 to –15 mg/dL with saxagliptin monotherapy).8,9 Durability of that effect has been demonstrated with up to 2 years of treatment.10,11 DPP-4 inhibitors added to other OADs with complementary mechanisms of action have been proven to be particularly effective in lowering all 3 glycemic parameters in multiple studies ( Table 2 ), regardless of age ( ≤65 and >65 years), gender, race/ethnicity, or body mass index.12-18 DPP-4 inhibitors are also effective when used concurrently (initial combination) with MET in drug-naïve patients, lowering HbA1c by as much as 2.5% with concomitant decreases in FPG and PPG ( Figure 2 ) as demonstrated during a 3-hour OGTT.19
Table 2
DPP-4 Inhibitors in Combination with Other Oral Antidiabetic Drugs*
BL, baseline; DPP-4, dipeptidyl peptidase-4; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; PBO, placebo; PPG, postprandial glucose; Tx, treatment.
*To date, no head-to-head trials with DPP-4 inhibitors have been published.
†Includes placebo group.
‡Values represent placebo-adjusted mean change from baseline. Adjusted mean change from baseline values for vildagliptin treatment groups were not reported.
Figure 2
Changes in Glucose After 24 Weeks of Saxagliptin and Metformin Initial Combination Therapy19
FPG, fasting plasma glucose; MET, metformin; OGTT, oral glucose tolerance test; PPG, postprandial glucose; SAXA, saxagliptin.
Reprinted with permission. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R, for the CV181-039 Investigators. Copyright © 2009 Diabetes, Obesity and Metabolism. Reproduced with permission of Blackwell Publishing Ltd.
TOLERABILITY
DPP-4 inhibitors offer strategic advantages with regard to tolerability, including few side effects or drug interactions with commonly used agents as well as simple oral dosing. These agents have demonstrated low risk for hypoglycemia and are weight neutral.8,20,21 Tolerability profiles have been shown to be similar in patients aged ≤65 years and older patients (aged >65 years) with vildagliptin monotherapy.22 The incidence of discontinuation due to clinical adverse reactions has been shown to be similar to placebo.5 Sitagliptin requires a 2-step dose reduction; one for moderate renal impairment and a second for severe impairment and end-stage renal disease patients.5 Saxagliptin requires only a 1-step dose reduction if creatine clearance is ≤50 mL/min.7 DPP-4 inhibitors in combination with MET, an SU, or a TZD demonstrated favorable tolerability with an overall adverse event profile similar to monotherapy with MET, an SU, or a TZD.13,16-19,23-26 Modest increases in hypoglycemic events have been shown in studies with DPP-4 inhibitors in combination with an SU,17,27 whereas others have shown no increase in hypoglycemic events.14 When used in combination with MET, gastrointestinal side effects are not increased above those seen with MET alone.13
Conclusion
DPP-4 inhibitors are a new class of agents that improve long-term, 24-hour control of HbA1c, FPG (before meal) levels, and PPG (after meal) levels through decreased DPP-4–mediated degradation of incretin hormones. DPP-4 inhibitors provide a complementary mechanism of action to existing OADs and demonstrate significant efficacy when added to MET, an SU, or a TZD, with a well-tolerated profile, including a low risk for hypoglycemia and weight neutrality. DPP-4 inhibitors have been studied in a broad range of patients and have demonstrated similar efficacy, regardless of age, gender, or race/ethnicity. These agents offer an important addition to the treatment of patients with T2D by providing another mechanism to address the multiple pathophysiologic defects present in this disease.
Disclosure
Dr. Cobble has served on advisory boards for Abbott, AstraZeneca, Bristol-Myers Squibb, and Eli Lilly and Company; is the Chief Medical Officer for Atherotech Cardiodiagnostic Lipid Company; and has served as a lecturer for Pri-Med and the American Diabetes Association.
Acknowledgements
Funding for editorial support for this newsletter was provided by Bristol-Myers Squibb and AstraZeneca. Technical writing and editorial assistance for this newsletter was provided by Trina Ricci, PhD, of Innovex Medical Communications. This newsletter has been edited and peer reviewed by The Journal of Family Practice.
1. Ahrén B. β- and α-cell dysfunction in subjects developing impaired glucose tolerance: outcome of a 12-year prospective study in postmenopausal Caucasian women. Diabetes. 2009;58:726-731.
2. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194-206.
3. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157.
4. Elrick H, Stimmler L, Hlad CJ, Jr, et al. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082.
5. Januvia [package insert]. South Granville, NSW: Merck & Co., Inc; 2007.
6. Janumet [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; 2007.
7. Onglyza [package insert]. Princeton, NJ/Wilmington, DE: Bristol-Myers Squibb/AstraZeneca; 2009.
8. Rosenstock J, Aguilar-Salinas C, Klein E, et al. for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
9. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
10. Qi DS, Teng R, Jiang M. Two-year treatment with sitagliptin and initial combination therapy of sitagliptin and metformin provides substantial and durable glycaemic control in patients with type 2 diabetes. Diabetologia. 2008;51(suppl 1):S36.-
11. Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naive patients with type 2 diabetes: comparison with metformin. Horm Metab Res. 2008;40:892-895.
12. Goldstein BJ, Feinglos MN, Lunceford JK, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979-1987.
13. Nauck MA, Ellis GC, Fleck PR, et al. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46-55.
14. Pratley RE, Kipnes MS, Fleck PR, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167-176.
15. Pratley R, Reusch J, Fleck P, et al. Efficacy and safety of alogliptin added to pioglitazone therapy in patients with type 2 diabetes. Poster presented at: 68th Scientific Sessions of the American Diabetes Association; June 6-10, 2008; San Francisco, CA.
16. DeFronzo RA, Hissa MN, Garber AJ, et al. for the Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649-1655.
17. Chacra AR, Tan GH, Apanovitch S, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: A randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
18. Hollander P, Li J, Allen E, et al. for the CV181-013 Investigators. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94(12).
19. Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11:611-622.
20. Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Am J Med. 2009;122(6 suppl):S11-S24.
21. DeFronzo RA, Fleck PR, Wilson CA, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315-2317.
22. Pratley RE, Rosenstock J, Pi-Sunyer FX, et al. Management of type 2 diabetes in treatment-naive elderly patients: benefits and risks of vildagliptin monotherapy. Diabetes Care. 2007;30:3017-3022.
23. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
24. Garber AJ, Schweizer A, Baron MA, et al. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166-174.
25. Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556-1568.
26. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890-895.
27. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
28. Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929-2940.
Type 2 diabetes (T2D) is characterized by altered glucose homeostasis, including decreased insulin sensitivity of target tissues, a gradual decline in β-cell insulin production and secretion, and a progressive inability to suppress pancreatic α-cell glucagon secretion.1 In the past, goals for therapy have focused primarily on insulin secretion, sensitization, and replacement. However, newer T2D medications utilize the incretin gut hormone pathway, a focus of scientific and clinical research for decades.2 The so-called insulin effect, known today as the incretin effect3 —ie, greater insulin secretion in response to nutrient ingestion—was identified in 1964 when Elrick et al4 demonstrated that orally administered glucose produced a significant and sustained increase in plasma insulin, whereas intravenously administered glucose produced a smaller and transient insulin increase. This finding was paramount in bringing incretin-based therapies to clinical practice.
The 2 most well characterized incretin hormones are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) ( Figure 1 ). Currently, therapeutic agents, acting as either an incretin mimetic (via GLP-1 analogs) or to inhibit the breakdown of GLP-1 (via dipeptidyl peptidase-4 [DPP-4] inhibitors) are available for treatment.2,3 Various DPP-4 inhibitors are in development, and 2 are approved by the US Food and Drug Administration (FDA): sitagliptin and the recently approved DPP-4 inhibitor, saxagliptin, are indicated for use in a broad range of patients, including those who are drug naïve or who have inadequate glycemic control on another oral antidiabetic drug (OAD). Both agents are approved as monotherapy and as an add-on to current antihyperglycemic therapy (ie, metformin [MET], sulfonylurea [SU], thiazolidinedione [TZD]), and are also approved as initial combination therapy with MET.5-7 Another DPP-4 inhibitor, alogliptin, failed to gain approval from the FDA, which indicated the need for additional data (Takeda Pharmaceutical Company Limited) ( Table 1 ).
Figure 1
DPP-4 Inhibitors: Mechanism of Glucose Control28
DPP-4, dipeptidyl peptidase-4; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
Post–meal ingestion, GLP-1 and GIP are released from the small intestine and are rapidly degraded by the enzyme DPP-4. Inhibition of DPP-4 prevents the breakdown of GLP-1 and GIP and enhances glucose-stimulated insulin secretion (incretin action). GLP-1 and GIP act on the pancreatic β-cell to increase insulin release. GLP-1 also acts on the α-cell to suppress glucagon release and ultimately suppress hepatic glucose production. Together, the increased cellular glucose uptake and the decreased hepatic glucose output offer physiologic glucose control.
Table 1
DPP-4 Inhibitor Status and Availability
| Drug | Status | Trade name | Pharmaceutical Company |
|---|---|---|---|
| Alogliptin | Failed to gain approval | Not officially disclosed | Takeda Pharmaceutical Company Limited |
| Dutogliptin | Phase 3 | Not officially disclosed | Phenomix/Forest Laboratories, Inc. |
| Linagliptin | Phase 3 | Ondero® | Boehringer Ingelheim |
| Saxagliptin | Approved in the United States and Europe | Onglyza™ | Bristol-Myers Squibb/AstraZeneca |
| Sitagliptin | Approved in the United States and Europe | Januvia™, Janumet® | Merck & Co., Inc. |
| Vildagliptin | Approved in Europe | Galvus® | Novartis AG |
| DPP-4, dipeptidyl peptidase-4. | |||
The current therapeutic options for treating type 2 diabetes (T2D) include drug classes that lower blood glucose levels by different mechanisms of action ( Table ) through various target organs.1,2
Table
Drug classes that lower blood glucose levels
| Agent(s) | Mechanism of Action |
|---|---|
| • Insulin • Sulfonylureas • Glinides | Insulin replacement/secretion |
| • Thiazolidinediones | Insulin sensitization |
| • Biguanides | Decrease of hepatic glucose output |
| • α-Glucosidase inhibitors | Delay of intestinal carbohydrate absorption |
| • DPP-4 inhibitors • GLP-1 analogs | Incretin enhancement/replacement with subsequent effects on insulin and glucagon secretion |
| DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1. | |
Glucagon-like peptide-1 (GLP-1) is known to enhance insulin release from the pancreatic β-cells and inhibit glucagon release through the α-cells in a glucose-dependent manner.3 In the fasted state, circulating levels of GLP are low but rise within minutes of meal ingestion. GLP-1 is released from the L cells of the small intestine within minutes of food consumption; however, incretin hormones are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4).3 By inhibiting DPP-4, the DPP-4 inhibitors enhance the half-life of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), thereby augmenting their levels.1,4 Because the release of GLP-1 is glucose dependent, augmentation of GLP-1 by DPP-4 inhibition minimizes the risk for hypoglycemia, which proves to be clinically important in managing T2D.
The majority of antidiabetic agents act primarily by lowering fasting plasma glucose (FPG) (eg, sulfonylureas), whereas others act primarily by lowering postprandial glucose (PPG).5 DPP-4 inhibitors primarily have a postprandial effect but also show statistically significant reductions in fasting glucose levels.6 FPG and PPG are the essential components of lowering glycosylated hemoglobin (HbA1c), and PPG has a greater effect on lowering HbA1c at values <8.5%.7
References
1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157.
2. Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172:213-226.
3. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165.
4. Stonehouse A, Okerson T, Kendall D, et al. Emerging incretin-based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev. 2008;4:101-109.
5. Leiter LA, Ceriello A, Davidson JA, et al. Postprandial glucose regulation: new data and new implications. Clin Ther. 2005;27(suppl B):S42-S56.
6. Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
7. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881-885.
DPP-4 Inhibitors Offer Comprehensive Glycemic Management
Glycemic control efforts should be directed at the multiple pathophysiologic defects of T2D and should involve routine assessment of HbA1c, monitoring of daily blood glucose values, and treatment with combination regimens that target glucose levels both before and after meals. As monotherapy, DPP-4 inhibitors demonstrate reductions of approximately 0.5% to 0.8% in HbA1c, with concomitant reductions in postprandial glucose (PPG) and fasting plasma glucose (FPG) (eg, PPG reductions of –43 to –45 mg/dL at 120 minutes of an oral glucose tolerance test [OGTT] and FPG reductions of –9 to –15 mg/dL with saxagliptin monotherapy).8,9 Durability of that effect has been demonstrated with up to 2 years of treatment.10,11 DPP-4 inhibitors added to other OADs with complementary mechanisms of action have been proven to be particularly effective in lowering all 3 glycemic parameters in multiple studies ( Table 2 ), regardless of age ( ≤65 and >65 years), gender, race/ethnicity, or body mass index.12-18 DPP-4 inhibitors are also effective when used concurrently (initial combination) with MET in drug-naïve patients, lowering HbA1c by as much as 2.5% with concomitant decreases in FPG and PPG ( Figure 2 ) as demonstrated during a 3-hour OGTT.19
Table 2
DPP-4 Inhibitors in Combination with Other Oral Antidiabetic Drugs*
BL, baseline; DPP-4, dipeptidyl peptidase-4; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; PBO, placebo; PPG, postprandial glucose; Tx, treatment.
*To date, no head-to-head trials with DPP-4 inhibitors have been published.
†Includes placebo group.
‡Values represent placebo-adjusted mean change from baseline. Adjusted mean change from baseline values for vildagliptin treatment groups were not reported.
Figure 2
Changes in Glucose After 24 Weeks of Saxagliptin and Metformin Initial Combination Therapy19
FPG, fasting plasma glucose; MET, metformin; OGTT, oral glucose tolerance test; PPG, postprandial glucose; SAXA, saxagliptin.
Reprinted with permission. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R, for the CV181-039 Investigators. Copyright © 2009 Diabetes, Obesity and Metabolism. Reproduced with permission of Blackwell Publishing Ltd.
TOLERABILITY
DPP-4 inhibitors offer strategic advantages with regard to tolerability, including few side effects or drug interactions with commonly used agents as well as simple oral dosing. These agents have demonstrated low risk for hypoglycemia and are weight neutral.8,20,21 Tolerability profiles have been shown to be similar in patients aged ≤65 years and older patients (aged >65 years) with vildagliptin monotherapy.22 The incidence of discontinuation due to clinical adverse reactions has been shown to be similar to placebo.5 Sitagliptin requires a 2-step dose reduction; one for moderate renal impairment and a second for severe impairment and end-stage renal disease patients.5 Saxagliptin requires only a 1-step dose reduction if creatine clearance is ≤50 mL/min.7 DPP-4 inhibitors in combination with MET, an SU, or a TZD demonstrated favorable tolerability with an overall adverse event profile similar to monotherapy with MET, an SU, or a TZD.13,16-19,23-26 Modest increases in hypoglycemic events have been shown in studies with DPP-4 inhibitors in combination with an SU,17,27 whereas others have shown no increase in hypoglycemic events.14 When used in combination with MET, gastrointestinal side effects are not increased above those seen with MET alone.13
Conclusion
DPP-4 inhibitors are a new class of agents that improve long-term, 24-hour control of HbA1c, FPG (before meal) levels, and PPG (after meal) levels through decreased DPP-4–mediated degradation of incretin hormones. DPP-4 inhibitors provide a complementary mechanism of action to existing OADs and demonstrate significant efficacy when added to MET, an SU, or a TZD, with a well-tolerated profile, including a low risk for hypoglycemia and weight neutrality. DPP-4 inhibitors have been studied in a broad range of patients and have demonstrated similar efficacy, regardless of age, gender, or race/ethnicity. These agents offer an important addition to the treatment of patients with T2D by providing another mechanism to address the multiple pathophysiologic defects present in this disease.
Disclosure
Dr. Cobble has served on advisory boards for Abbott, AstraZeneca, Bristol-Myers Squibb, and Eli Lilly and Company; is the Chief Medical Officer for Atherotech Cardiodiagnostic Lipid Company; and has served as a lecturer for Pri-Med and the American Diabetes Association.
Acknowledgements
Funding for editorial support for this newsletter was provided by Bristol-Myers Squibb and AstraZeneca. Technical writing and editorial assistance for this newsletter was provided by Trina Ricci, PhD, of Innovex Medical Communications. This newsletter has been edited and peer reviewed by The Journal of Family Practice.
Type 2 diabetes (T2D) is characterized by altered glucose homeostasis, including decreased insulin sensitivity of target tissues, a gradual decline in β-cell insulin production and secretion, and a progressive inability to suppress pancreatic α-cell glucagon secretion.1 In the past, goals for therapy have focused primarily on insulin secretion, sensitization, and replacement. However, newer T2D medications utilize the incretin gut hormone pathway, a focus of scientific and clinical research for decades.2 The so-called insulin effect, known today as the incretin effect3 —ie, greater insulin secretion in response to nutrient ingestion—was identified in 1964 when Elrick et al4 demonstrated that orally administered glucose produced a significant and sustained increase in plasma insulin, whereas intravenously administered glucose produced a smaller and transient insulin increase. This finding was paramount in bringing incretin-based therapies to clinical practice.
The 2 most well characterized incretin hormones are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) ( Figure 1 ). Currently, therapeutic agents, acting as either an incretin mimetic (via GLP-1 analogs) or to inhibit the breakdown of GLP-1 (via dipeptidyl peptidase-4 [DPP-4] inhibitors) are available for treatment.2,3 Various DPP-4 inhibitors are in development, and 2 are approved by the US Food and Drug Administration (FDA): sitagliptin and the recently approved DPP-4 inhibitor, saxagliptin, are indicated for use in a broad range of patients, including those who are drug naïve or who have inadequate glycemic control on another oral antidiabetic drug (OAD). Both agents are approved as monotherapy and as an add-on to current antihyperglycemic therapy (ie, metformin [MET], sulfonylurea [SU], thiazolidinedione [TZD]), and are also approved as initial combination therapy with MET.5-7 Another DPP-4 inhibitor, alogliptin, failed to gain approval from the FDA, which indicated the need for additional data (Takeda Pharmaceutical Company Limited) ( Table 1 ).
Figure 1
DPP-4 Inhibitors: Mechanism of Glucose Control28
DPP-4, dipeptidyl peptidase-4; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
Post–meal ingestion, GLP-1 and GIP are released from the small intestine and are rapidly degraded by the enzyme DPP-4. Inhibition of DPP-4 prevents the breakdown of GLP-1 and GIP and enhances glucose-stimulated insulin secretion (incretin action). GLP-1 and GIP act on the pancreatic β-cell to increase insulin release. GLP-1 also acts on the α-cell to suppress glucagon release and ultimately suppress hepatic glucose production. Together, the increased cellular glucose uptake and the decreased hepatic glucose output offer physiologic glucose control.
Table 1
DPP-4 Inhibitor Status and Availability
| Drug | Status | Trade name | Pharmaceutical Company |
|---|---|---|---|
| Alogliptin | Failed to gain approval | Not officially disclosed | Takeda Pharmaceutical Company Limited |
| Dutogliptin | Phase 3 | Not officially disclosed | Phenomix/Forest Laboratories, Inc. |
| Linagliptin | Phase 3 | Ondero® | Boehringer Ingelheim |
| Saxagliptin | Approved in the United States and Europe | Onglyza™ | Bristol-Myers Squibb/AstraZeneca |
| Sitagliptin | Approved in the United States and Europe | Januvia™, Janumet® | Merck & Co., Inc. |
| Vildagliptin | Approved in Europe | Galvus® | Novartis AG |
| DPP-4, dipeptidyl peptidase-4. | |||
The current therapeutic options for treating type 2 diabetes (T2D) include drug classes that lower blood glucose levels by different mechanisms of action ( Table ) through various target organs.1,2
Table
Drug classes that lower blood glucose levels
| Agent(s) | Mechanism of Action |
|---|---|
| • Insulin • Sulfonylureas • Glinides | Insulin replacement/secretion |
| • Thiazolidinediones | Insulin sensitization |
| • Biguanides | Decrease of hepatic glucose output |
| • α-Glucosidase inhibitors | Delay of intestinal carbohydrate absorption |
| • DPP-4 inhibitors • GLP-1 analogs | Incretin enhancement/replacement with subsequent effects on insulin and glucagon secretion |
| DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1. | |
Glucagon-like peptide-1 (GLP-1) is known to enhance insulin release from the pancreatic β-cells and inhibit glucagon release through the α-cells in a glucose-dependent manner.3 In the fasted state, circulating levels of GLP are low but rise within minutes of meal ingestion. GLP-1 is released from the L cells of the small intestine within minutes of food consumption; however, incretin hormones are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4).3 By inhibiting DPP-4, the DPP-4 inhibitors enhance the half-life of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), thereby augmenting their levels.1,4 Because the release of GLP-1 is glucose dependent, augmentation of GLP-1 by DPP-4 inhibition minimizes the risk for hypoglycemia, which proves to be clinically important in managing T2D.
The majority of antidiabetic agents act primarily by lowering fasting plasma glucose (FPG) (eg, sulfonylureas), whereas others act primarily by lowering postprandial glucose (PPG).5 DPP-4 inhibitors primarily have a postprandial effect but also show statistically significant reductions in fasting glucose levels.6 FPG and PPG are the essential components of lowering glycosylated hemoglobin (HbA1c), and PPG has a greater effect on lowering HbA1c at values <8.5%.7
References
1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157.
2. Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172:213-226.
3. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165.
4. Stonehouse A, Okerson T, Kendall D, et al. Emerging incretin-based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev. 2008;4:101-109.
5. Leiter LA, Ceriello A, Davidson JA, et al. Postprandial glucose regulation: new data and new implications. Clin Ther. 2005;27(suppl B):S42-S56.
6. Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
7. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881-885.
DPP-4 Inhibitors Offer Comprehensive Glycemic Management
Glycemic control efforts should be directed at the multiple pathophysiologic defects of T2D and should involve routine assessment of HbA1c, monitoring of daily blood glucose values, and treatment with combination regimens that target glucose levels both before and after meals. As monotherapy, DPP-4 inhibitors demonstrate reductions of approximately 0.5% to 0.8% in HbA1c, with concomitant reductions in postprandial glucose (PPG) and fasting plasma glucose (FPG) (eg, PPG reductions of –43 to –45 mg/dL at 120 minutes of an oral glucose tolerance test [OGTT] and FPG reductions of –9 to –15 mg/dL with saxagliptin monotherapy).8,9 Durability of that effect has been demonstrated with up to 2 years of treatment.10,11 DPP-4 inhibitors added to other OADs with complementary mechanisms of action have been proven to be particularly effective in lowering all 3 glycemic parameters in multiple studies ( Table 2 ), regardless of age ( ≤65 and >65 years), gender, race/ethnicity, or body mass index.12-18 DPP-4 inhibitors are also effective when used concurrently (initial combination) with MET in drug-naïve patients, lowering HbA1c by as much as 2.5% with concomitant decreases in FPG and PPG ( Figure 2 ) as demonstrated during a 3-hour OGTT.19
Table 2
DPP-4 Inhibitors in Combination with Other Oral Antidiabetic Drugs*
BL, baseline; DPP-4, dipeptidyl peptidase-4; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; PBO, placebo; PPG, postprandial glucose; Tx, treatment.
*To date, no head-to-head trials with DPP-4 inhibitors have been published.
†Includes placebo group.
‡Values represent placebo-adjusted mean change from baseline. Adjusted mean change from baseline values for vildagliptin treatment groups were not reported.
Figure 2
Changes in Glucose After 24 Weeks of Saxagliptin and Metformin Initial Combination Therapy19
FPG, fasting plasma glucose; MET, metformin; OGTT, oral glucose tolerance test; PPG, postprandial glucose; SAXA, saxagliptin.
Reprinted with permission. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Jadzinsky M, Pfützner A, Paz-Pacheco E, Xu Z, Allen E, Chen R, for the CV181-039 Investigators. Copyright © 2009 Diabetes, Obesity and Metabolism. Reproduced with permission of Blackwell Publishing Ltd.
TOLERABILITY
DPP-4 inhibitors offer strategic advantages with regard to tolerability, including few side effects or drug interactions with commonly used agents as well as simple oral dosing. These agents have demonstrated low risk for hypoglycemia and are weight neutral.8,20,21 Tolerability profiles have been shown to be similar in patients aged ≤65 years and older patients (aged >65 years) with vildagliptin monotherapy.22 The incidence of discontinuation due to clinical adverse reactions has been shown to be similar to placebo.5 Sitagliptin requires a 2-step dose reduction; one for moderate renal impairment and a second for severe impairment and end-stage renal disease patients.5 Saxagliptin requires only a 1-step dose reduction if creatine clearance is ≤50 mL/min.7 DPP-4 inhibitors in combination with MET, an SU, or a TZD demonstrated favorable tolerability with an overall adverse event profile similar to monotherapy with MET, an SU, or a TZD.13,16-19,23-26 Modest increases in hypoglycemic events have been shown in studies with DPP-4 inhibitors in combination with an SU,17,27 whereas others have shown no increase in hypoglycemic events.14 When used in combination with MET, gastrointestinal side effects are not increased above those seen with MET alone.13
Conclusion
DPP-4 inhibitors are a new class of agents that improve long-term, 24-hour control of HbA1c, FPG (before meal) levels, and PPG (after meal) levels through decreased DPP-4–mediated degradation of incretin hormones. DPP-4 inhibitors provide a complementary mechanism of action to existing OADs and demonstrate significant efficacy when added to MET, an SU, or a TZD, with a well-tolerated profile, including a low risk for hypoglycemia and weight neutrality. DPP-4 inhibitors have been studied in a broad range of patients and have demonstrated similar efficacy, regardless of age, gender, or race/ethnicity. These agents offer an important addition to the treatment of patients with T2D by providing another mechanism to address the multiple pathophysiologic defects present in this disease.
Disclosure
Dr. Cobble has served on advisory boards for Abbott, AstraZeneca, Bristol-Myers Squibb, and Eli Lilly and Company; is the Chief Medical Officer for Atherotech Cardiodiagnostic Lipid Company; and has served as a lecturer for Pri-Med and the American Diabetes Association.
Acknowledgements
Funding for editorial support for this newsletter was provided by Bristol-Myers Squibb and AstraZeneca. Technical writing and editorial assistance for this newsletter was provided by Trina Ricci, PhD, of Innovex Medical Communications. This newsletter has been edited and peer reviewed by The Journal of Family Practice.
1. Ahrén B. β- and α-cell dysfunction in subjects developing impaired glucose tolerance: outcome of a 12-year prospective study in postmenopausal Caucasian women. Diabetes. 2009;58:726-731.
2. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194-206.
3. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157.
4. Elrick H, Stimmler L, Hlad CJ, Jr, et al. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082.
5. Januvia [package insert]. South Granville, NSW: Merck & Co., Inc; 2007.
6. Janumet [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; 2007.
7. Onglyza [package insert]. Princeton, NJ/Wilmington, DE: Bristol-Myers Squibb/AstraZeneca; 2009.
8. Rosenstock J, Aguilar-Salinas C, Klein E, et al. for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
9. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
10. Qi DS, Teng R, Jiang M. Two-year treatment with sitagliptin and initial combination therapy of sitagliptin and metformin provides substantial and durable glycaemic control in patients with type 2 diabetes. Diabetologia. 2008;51(suppl 1):S36.-
11. Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naive patients with type 2 diabetes: comparison with metformin. Horm Metab Res. 2008;40:892-895.
12. Goldstein BJ, Feinglos MN, Lunceford JK, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979-1987.
13. Nauck MA, Ellis GC, Fleck PR, et al. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46-55.
14. Pratley RE, Kipnes MS, Fleck PR, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167-176.
15. Pratley R, Reusch J, Fleck P, et al. Efficacy and safety of alogliptin added to pioglitazone therapy in patients with type 2 diabetes. Poster presented at: 68th Scientific Sessions of the American Diabetes Association; June 6-10, 2008; San Francisco, CA.
16. DeFronzo RA, Hissa MN, Garber AJ, et al. for the Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649-1655.
17. Chacra AR, Tan GH, Apanovitch S, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: A randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
18. Hollander P, Li J, Allen E, et al. for the CV181-013 Investigators. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94(12).
19. Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11:611-622.
20. Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Am J Med. 2009;122(6 suppl):S11-S24.
21. DeFronzo RA, Fleck PR, Wilson CA, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315-2317.
22. Pratley RE, Rosenstock J, Pi-Sunyer FX, et al. Management of type 2 diabetes in treatment-naive elderly patients: benefits and risks of vildagliptin monotherapy. Diabetes Care. 2007;30:3017-3022.
23. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
24. Garber AJ, Schweizer A, Baron MA, et al. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166-174.
25. Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556-1568.
26. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890-895.
27. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
28. Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929-2940.
1. Ahrén B. β- and α-cell dysfunction in subjects developing impaired glucose tolerance: outcome of a 12-year prospective study in postmenopausal Caucasian women. Diabetes. 2009;58:726-731.
2. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194-206.
3. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157.
4. Elrick H, Stimmler L, Hlad CJ, Jr, et al. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076-1082.
5. Januvia [package insert]. South Granville, NSW: Merck & Co., Inc; 2007.
6. Janumet [package insert]. Whitehouse Station, NJ: Merck & Co., Inc; 2007.
7. Onglyza [package insert]. Princeton, NJ/Wilmington, DE: Bristol-Myers Squibb/AstraZeneca; 2009.
8. Rosenstock J, Aguilar-Salinas C, Klein E, et al. for the CV181-011 Study Investigators. Effect of saxagliptin monotherapy in treatment-naïve patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
9. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
10. Qi DS, Teng R, Jiang M. Two-year treatment with sitagliptin and initial combination therapy of sitagliptin and metformin provides substantial and durable glycaemic control in patients with type 2 diabetes. Diabetologia. 2008;51(suppl 1):S36.-
11. Göke B, Hershon K, Kerr D, et al. Efficacy and safety of vildagliptin monotherapy during 2-year treatment of drug-naive patients with type 2 diabetes: comparison with metformin. Horm Metab Res. 2008;40:892-895.
12. Goldstein BJ, Feinglos MN, Lunceford JK, et al. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979-1987.
13. Nauck MA, Ellis GC, Fleck PR, et al. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46-55.
14. Pratley RE, Kipnes MS, Fleck PR, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167-176.
15. Pratley R, Reusch J, Fleck P, et al. Efficacy and safety of alogliptin added to pioglitazone therapy in patients with type 2 diabetes. Poster presented at: 68th Scientific Sessions of the American Diabetes Association; June 6-10, 2008; San Francisco, CA.
16. DeFronzo RA, Hissa MN, Garber AJ, et al. for the Saxagliptin 014 Study Group. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649-1655.
17. Chacra AR, Tan GH, Apanovitch S, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: A randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
18. Hollander P, Li J, Allen E, et al. for the CV181-013 Investigators. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94(12).
19. Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. for the CV181-039 Investigators. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11:611-622.
20. Gilbert MP, Pratley RE. Efficacy and safety of incretin-based therapies in patients with type 2 diabetes mellitus. Am J Med. 2009;122(6 suppl):S11-S24.
21. DeFronzo RA, Fleck PR, Wilson CA, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315-2317.
22. Pratley RE, Rosenstock J, Pi-Sunyer FX, et al. Management of type 2 diabetes in treatment-naive elderly patients: benefits and risks of vildagliptin monotherapy. Diabetes Care. 2007;30:3017-3022.
23. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
24. Garber AJ, Schweizer A, Baron MA, et al. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166-174.
25. Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556-1568.
26. Bosi E, Camisasca RP, Collober C, et al. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890-895.
27. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
28. Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929-2940.
Screening adults for depression in primary care: A position statement of the American College of Preventive Medicine
Position statement
The American College of Preventive Medicine (ACPM) maintains that primary care providers should screen all adults for depression and that all primary care providers should have systems in place, either within the primary care setting itself or through collaborations with mental health professionals, to ensure the accurate diagnosis and treatment of this condition. The earliest and best opportunities to identify depression are in the clinics of primary care providers. Thus, the ACPM supports the recommendations of the US Preventive Services Task Force (USPSTF), and further suggests that all primary care practices should have such systems of care in place.
Why a position statement? The rationale
Primary care physicians have already been urged by the USPSTF and other authorities to consider screening adults for depression an essential aspect of care.1-5 So why is the ACPM issuing a new position statement on the subject? Because, the College believes, controversy over how to apply this mandate in the primary care setting is ongoing. Primary care providers—whether they practice family medicine, internal medicine, obstetrics/gynecology, or are in general practice—need to know what role they should play in screening adults for depression and ensuring adequate diagnosis and treatment.
The USPSTF recommendation
In May 2002, the USPSTF made a category B recommendation (high certainty of moderate net benefit) that adults should be screened for depression in “clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up” of depression1 (emphasis added). The less-than-clear aspect of this recommendation is italicized: Just what constitutes the “system” that primary care providers, the first and often the only point of contact adults have with the health care system, are told to have “in place”? And how can they go about providing such systems? That’s what the College has set out to elucidate.
The toll depression takes
Depression is a potentially life-threatening disorder that affects up to 6.7% of the population 18 years of age and older, or approximately 14.8 million Americans, in a given year.6 Many people younger than age 18 are also affected. The extensive STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study of outpatients with depression found that nearly 40% of respondents had their first depressive episode before the age of 18.7,8
The ripple effects
Depression is the leading cause of disability in the United States for individuals between the ages of 15 and 44 years.9 But the burden of this illness is not borne only by those diagnosed with the disorder; depression has a serious impact on the patient’s family, caregivers, colleagues, and society at large.
Medical costs. Depression contributes to a higher morbidity and mortality of other medical conditions. For example, people who have a myocardial infarction (MI) with comorbid depression have worse outcomes than those having an MI without depression. However, if the depressive episode is treated successfully, medical and surgical outcomes improve.10 Furthermore, studies documenting increased cardiovascular morbidity and mortality in patients with depressive symptoms or major depression suggest that depression is an independent risk factor in the pathophysiologic progression of cardiovascular disease, not just a secondary emotional response to the illness.11
Economic costs. Depression is a leading contributor to lost productivity, not only from worker absenteeism, but also from what is known as “presenteeism”—being physically at work but not fully engaged. Thus, depression may be a leading cause of poor organizational performance. Further, employees diagnosed with depression have a higher incidence and longer duration of both work-related and nonwork-related disabilities.
Screening: Easier than you think
Screening instruments with acceptable sensitivity and specificity are available. These brief, paper-and-pencil instruments can be quickly completed by patients in your waiting room. Some of the most commonly used self-administered measures are the Beck Depression Inventory (BDI), the Center for Epidemiologic Studies Depression Scale, Revised (CES-DR), the Zung Self-Rating Depression Scale, and the Patient Health Questionnaire (PHQ-9).12-16 These tools take approximately 5 to 10 minutes for patients to complete and do not interfere with clinical practice. You can choose the tests that are appropriate for screening your patient population, and you can also use the same instruments for ongoing monitoring of patients receiving treatment for depression.
Two questions. For an even briefer screen, ask your patients these 2 questions:
- Over the past month, have you felt down, depressed, or hopeless?
- Over the past month, have you felt little interest or pleasure in doing things?
Patients who answer Yes may need more in-depth screening and clinical assessment.
When these 2 questions were tested in a primary care setting with patients not receiving psychotropic drugs, they had a sensitivity of 97% and a specificity of 67%.17 Other research also shows that simple questions about depression perform as well as longer questionnaires, further proof that screening for depression need not add undue length to the clinical assessment in primary care.18
Which instrument is best? Selection of a screening measure, whether it be the 2 simple questions noted above or a longer, more comprehensive tool, is the first step in the process of detecting depression in primary care settings. In making your choice, consider characteristics of the population being screened, psychometric properties of the instrument, time required to complete the measure, time required to score the measure, ease of use, and cost. A review of available screening instruments suitable for use by primary care physicians has been published in American Family Physician.19 The review includes screening measures developed specifically for adolescents, such as the Reynolds Adolescent Depression Scale, and those developed for older adults, such as the Geriatric Depression Scale.
What comes next? Making the diagnosis
Screening tools provide only a preliminary assessment. Elevated scores must be confirmed with diagnostic interviewing. Without proper follow-up, false-positive scores can lead to harmful labeling, unnecessary additional testing, and inappropriate treatment.
The diagnostic interview. Primary care physicians may feel competent to perform the diagnostic interview themselves, or they may refer patients identified by screening to a mental health professional. The interview should determine whether a patient meets the diagnostic criteria for a depressive disorder—including major depressive disorder or dysthymic disorder—found in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR).20 Bear in mind that criteria important for diagnosis, such as duration of symptoms, degree of impairment, and comorbid psychiatric or substance use disorders, are not revealed in the screening instruments.
Differential diagnosis. Depressive symptoms often overlap with medical conditions, such as hypothyroidism, and with other psychiatric illnesses, such as generalized anxiety disorder. That’s why the differential diagnosis is crucial.
Screen for bipolar disorder. Patients meeting criteria for a depressive disorder should be screened for bipolar disorder, because the 2 conditions are managed differently. Screening instruments for bipolar disorder have been less extensively studied than the depression screening instruments described earlier. The Mood Disorder Questionnaire is a brief, easy-to-use, self-report screening instrument for bipolar-spectrum disorders.21 This single-page measure screens for a lifetime history (“has there ever been a period of time”) of manic or hypomanic symptoms using 13 yes/no items, as well as 2 items assessing whether several symptoms were experienced during the same time period, and the level of functional impairment associated with such symptoms. As noted earlier, elevated scores on this and other screening instruments must be confirmed with diagnostic interviewing.
Use DSM criteria. Because of the varying clinical manifestations of depression, clinical judgment sometimes must supersede strict adherence to DSM-IV-TR criteria. Nevertheless, reliance on these well-established criteria is generally recommended as the best way to avoid over- or underdiagnosis, billing problems, and legal problems arising from an inaccurate diagnosis or inappropriate use of medications.
Treating depression
Depression is a highly treatable condition with generally good outcomes.22 A variety of antidepressant medications and psychotherapeutic modalities are available. Consensus-based guidelines have been developed to guide clinicians in the evaluation and treatment of depression.23 Remission—not simply treatment response or an improvement in symptoms—should be the targeted endpoint. STAR*D data revealed that “better but not remitted” patients consistently have a worse prognosis and higher relapse rates than those achieving full remission.8,24
Why me?
The answer: Primary care providers are the principal contacts for more than 50% of patients with mental illnesses. Approximately 35% of patients seen in primary care meet criteria for some form of depression and 10% suffer from major depression.25,26 Because individuals with depression use health care more frequently, the prevalence of major depression is 2 to 3 times higher in primary care settings than in the general population.27 Yet, a substantial proportion of primary care patients with major depression go undiagnosed, leading to a dangerous situation in which symptoms may worsen and suicidal ideation can develop.28 That’s why you, as a primary care practitioner, have such an important role to play in assessing, diagnosing, and treating depression.
Making a difference. Interventions initiated in the primary care setting have been shown to be effective for the treatment of depression.29 Findings of the STAR*D study confirm that primary care providers, when given the time, staffing, and reimbursement support, can provide high-quality, appropriate care for patients with depression, especially in uncomplicated cases.8
What you need are “systems in place”
The USPSTF recommended screening for depression in “clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up.” Given the prevalence and gravity of the problem, the ACPM recommends that all primary care practices have such systems in place. These systems may be either:
- within your own practice, using clinical guidelines for the diagnosis and treatment of depression, or
- within an established system of referral to mental health professionals.
If you treat patients with depression within your own practice, keep in mind that using standardized treatments with established efficacy in psychiatric patients has been shown to be more effective than “usual care.”30 If you choose to partner with mental health professionals, you can employ various levels of collaboration. Depending on the size of your practice, you may want to include a mental health professional as an integral part of your staff, or you may prefer to hire a part-time consultant. Another alternative is to establish an ongoing, collaborative relationship with a mental health provider in private practice. In some instances, working with a patient’s employee assistance program may be the best way to ensure that he or she receives treatment and follow-up. Primary care practices serving disadvantaged or impoverished communities may need to develop partnerships with public-sector community mental health centers.
Whatever setup works best for you, the goal is to make sure that your patients with depression have access to ongoing screening, diagnostic, and treatment services. That goal is worthy of your best efforts.
CORRESPONDENCE
Michele Surricchio, MPH, American College of Preventive Medicine, 1307 New York Ave., NW, Suite 200, Washington, DC 20005; [email protected]
1. U.S. Preventive Services Task Force. Recommendations and rationale: screening for depression. Available at http://www.ahrq.gov/clinic/3rduspstf/depression/depressrr.htm. Accessed March 18, 2009.
2. Harris RP, Helfand M, Woolf SH, et al. Current methods of the U.S. Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(suppl 3):S21-S35.
3. American Medical Association. Guidelines for Adolescent Preventive Services (GAPS): Recommendations Monograph. Chicago: American Medical Association, 1997.
4. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care. Washington, DC: American College of Obstetricians and Gynecologists, 2002:126–133, 235–236.
5. American Academy of Family Physicians. Clinical Preventive Services. Revision 6.8, April 2009. Available at http://www.aafp.org/online/en/home/clinical/exam.html. Accessed August 20, 2009.
6. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Arch Gen Psychiatry. 2005;62:617-627.
7. STAR*D: Sequenced Treatment Alternatives to Relieve Depression. Available at http://www.edc.pitt.edu/stard/public/index.html. Accessed March 18, 2009.
8. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164:201-204.
9. World Health Organization. The World Health Report 2004: Changing History, Annex Table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in WHO regions, estimates for 2002. Geneva: WHO; 2004. Available at www.who.int/whr/2004/annex_3_en.pdf. Accessed August 25, 2009.
10. Parashar S, Rumsfeld JS, Spertus JA, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035-2043.
11. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580-592.
12. Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35:785-791.
13. Radloff LS. The CES scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401.
14, Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;2:63-70.
15. Nease DE, Jr, Maloin JM. Depression screening: a practical strategy. J Fam Pract. 2003;52:118-124.
16. Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29:388-395.
17. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
18. Williams JW, Jr, Mulrow CD, Kroenke K, et al. Case-finding for depression in primary care: a randomized trial. Am J Med. 1999;106:36-43.
19. Sharp LK, Lipsky MS. Screening for depression across the lifespan: a review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001-1008.
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
21. Hirschfeld RMA, Williams JBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
22. Gelenberg AJ, Hopkins HS. Assessing and treating depression in primary care medicine. Am J Med. 2007;120:105-108.
23. American Psychiatric Association. Practice guideline for major depressive disorder in adults. Am J Psychiatry. 1993;150(suppl):1-26.
24. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917.
25. Callahan EJ, Bertakis KD, Azari R, et al. The influence of depression on physician-patient interaction in primary care. Fam Med. 1996;28:346-351.
26. AHCPR Depression Guideline Panel. Clinical Practice Guideline Number 5. Depression in Primary Care. Volume 2: Treatment of Major Depression. Rockville, Md: Agency for Health Care Policy and Research, Public Health Services, US Department of Health and Human Services; 1993. AHCPR publication 93-0550.
27. Coyne JC, Fechner-Bates S, Schwenk TL. Prevalence, nature, and comorbidity of depressive disorders in primary care. Gen Hosp Psychiatry. 1994;16:267-276.
28. Rost K, Zhang M, Fortney J, et al. Persistently poor outcomes of undetected major depression in primary care. Gen Hosp Psychiatry. 1998;20:12-20.
29. Dietrich AJ, Oxman TE, Williams JW, et al. Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. BMJ. 2004;329:602-605.
30. Schulberg HC, Block MR, Madonia MJ, et al. Treating major depression in primary care practice: eight-month clinical outcomes. Arch Gen Psychiatry. 1996;53:913-919.
Position statement
The American College of Preventive Medicine (ACPM) maintains that primary care providers should screen all adults for depression and that all primary care providers should have systems in place, either within the primary care setting itself or through collaborations with mental health professionals, to ensure the accurate diagnosis and treatment of this condition. The earliest and best opportunities to identify depression are in the clinics of primary care providers. Thus, the ACPM supports the recommendations of the US Preventive Services Task Force (USPSTF), and further suggests that all primary care practices should have such systems of care in place.
Why a position statement? The rationale
Primary care physicians have already been urged by the USPSTF and other authorities to consider screening adults for depression an essential aspect of care.1-5 So why is the ACPM issuing a new position statement on the subject? Because, the College believes, controversy over how to apply this mandate in the primary care setting is ongoing. Primary care providers—whether they practice family medicine, internal medicine, obstetrics/gynecology, or are in general practice—need to know what role they should play in screening adults for depression and ensuring adequate diagnosis and treatment.
The USPSTF recommendation
In May 2002, the USPSTF made a category B recommendation (high certainty of moderate net benefit) that adults should be screened for depression in “clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up” of depression1 (emphasis added). The less-than-clear aspect of this recommendation is italicized: Just what constitutes the “system” that primary care providers, the first and often the only point of contact adults have with the health care system, are told to have “in place”? And how can they go about providing such systems? That’s what the College has set out to elucidate.
The toll depression takes
Depression is a potentially life-threatening disorder that affects up to 6.7% of the population 18 years of age and older, or approximately 14.8 million Americans, in a given year.6 Many people younger than age 18 are also affected. The extensive STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study of outpatients with depression found that nearly 40% of respondents had their first depressive episode before the age of 18.7,8
The ripple effects
Depression is the leading cause of disability in the United States for individuals between the ages of 15 and 44 years.9 But the burden of this illness is not borne only by those diagnosed with the disorder; depression has a serious impact on the patient’s family, caregivers, colleagues, and society at large.
Medical costs. Depression contributes to a higher morbidity and mortality of other medical conditions. For example, people who have a myocardial infarction (MI) with comorbid depression have worse outcomes than those having an MI without depression. However, if the depressive episode is treated successfully, medical and surgical outcomes improve.10 Furthermore, studies documenting increased cardiovascular morbidity and mortality in patients with depressive symptoms or major depression suggest that depression is an independent risk factor in the pathophysiologic progression of cardiovascular disease, not just a secondary emotional response to the illness.11
Economic costs. Depression is a leading contributor to lost productivity, not only from worker absenteeism, but also from what is known as “presenteeism”—being physically at work but not fully engaged. Thus, depression may be a leading cause of poor organizational performance. Further, employees diagnosed with depression have a higher incidence and longer duration of both work-related and nonwork-related disabilities.
Screening: Easier than you think
Screening instruments with acceptable sensitivity and specificity are available. These brief, paper-and-pencil instruments can be quickly completed by patients in your waiting room. Some of the most commonly used self-administered measures are the Beck Depression Inventory (BDI), the Center for Epidemiologic Studies Depression Scale, Revised (CES-DR), the Zung Self-Rating Depression Scale, and the Patient Health Questionnaire (PHQ-9).12-16 These tools take approximately 5 to 10 minutes for patients to complete and do not interfere with clinical practice. You can choose the tests that are appropriate for screening your patient population, and you can also use the same instruments for ongoing monitoring of patients receiving treatment for depression.
Two questions. For an even briefer screen, ask your patients these 2 questions:
- Over the past month, have you felt down, depressed, or hopeless?
- Over the past month, have you felt little interest or pleasure in doing things?
Patients who answer Yes may need more in-depth screening and clinical assessment.
When these 2 questions were tested in a primary care setting with patients not receiving psychotropic drugs, they had a sensitivity of 97% and a specificity of 67%.17 Other research also shows that simple questions about depression perform as well as longer questionnaires, further proof that screening for depression need not add undue length to the clinical assessment in primary care.18
Which instrument is best? Selection of a screening measure, whether it be the 2 simple questions noted above or a longer, more comprehensive tool, is the first step in the process of detecting depression in primary care settings. In making your choice, consider characteristics of the population being screened, psychometric properties of the instrument, time required to complete the measure, time required to score the measure, ease of use, and cost. A review of available screening instruments suitable for use by primary care physicians has been published in American Family Physician.19 The review includes screening measures developed specifically for adolescents, such as the Reynolds Adolescent Depression Scale, and those developed for older adults, such as the Geriatric Depression Scale.
What comes next? Making the diagnosis
Screening tools provide only a preliminary assessment. Elevated scores must be confirmed with diagnostic interviewing. Without proper follow-up, false-positive scores can lead to harmful labeling, unnecessary additional testing, and inappropriate treatment.
The diagnostic interview. Primary care physicians may feel competent to perform the diagnostic interview themselves, or they may refer patients identified by screening to a mental health professional. The interview should determine whether a patient meets the diagnostic criteria for a depressive disorder—including major depressive disorder or dysthymic disorder—found in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR).20 Bear in mind that criteria important for diagnosis, such as duration of symptoms, degree of impairment, and comorbid psychiatric or substance use disorders, are not revealed in the screening instruments.
Differential diagnosis. Depressive symptoms often overlap with medical conditions, such as hypothyroidism, and with other psychiatric illnesses, such as generalized anxiety disorder. That’s why the differential diagnosis is crucial.
Screen for bipolar disorder. Patients meeting criteria for a depressive disorder should be screened for bipolar disorder, because the 2 conditions are managed differently. Screening instruments for bipolar disorder have been less extensively studied than the depression screening instruments described earlier. The Mood Disorder Questionnaire is a brief, easy-to-use, self-report screening instrument for bipolar-spectrum disorders.21 This single-page measure screens for a lifetime history (“has there ever been a period of time”) of manic or hypomanic symptoms using 13 yes/no items, as well as 2 items assessing whether several symptoms were experienced during the same time period, and the level of functional impairment associated with such symptoms. As noted earlier, elevated scores on this and other screening instruments must be confirmed with diagnostic interviewing.
Use DSM criteria. Because of the varying clinical manifestations of depression, clinical judgment sometimes must supersede strict adherence to DSM-IV-TR criteria. Nevertheless, reliance on these well-established criteria is generally recommended as the best way to avoid over- or underdiagnosis, billing problems, and legal problems arising from an inaccurate diagnosis or inappropriate use of medications.
Treating depression
Depression is a highly treatable condition with generally good outcomes.22 A variety of antidepressant medications and psychotherapeutic modalities are available. Consensus-based guidelines have been developed to guide clinicians in the evaluation and treatment of depression.23 Remission—not simply treatment response or an improvement in symptoms—should be the targeted endpoint. STAR*D data revealed that “better but not remitted” patients consistently have a worse prognosis and higher relapse rates than those achieving full remission.8,24
Why me?
The answer: Primary care providers are the principal contacts for more than 50% of patients with mental illnesses. Approximately 35% of patients seen in primary care meet criteria for some form of depression and 10% suffer from major depression.25,26 Because individuals with depression use health care more frequently, the prevalence of major depression is 2 to 3 times higher in primary care settings than in the general population.27 Yet, a substantial proportion of primary care patients with major depression go undiagnosed, leading to a dangerous situation in which symptoms may worsen and suicidal ideation can develop.28 That’s why you, as a primary care practitioner, have such an important role to play in assessing, diagnosing, and treating depression.
Making a difference. Interventions initiated in the primary care setting have been shown to be effective for the treatment of depression.29 Findings of the STAR*D study confirm that primary care providers, when given the time, staffing, and reimbursement support, can provide high-quality, appropriate care for patients with depression, especially in uncomplicated cases.8
What you need are “systems in place”
The USPSTF recommended screening for depression in “clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up.” Given the prevalence and gravity of the problem, the ACPM recommends that all primary care practices have such systems in place. These systems may be either:
- within your own practice, using clinical guidelines for the diagnosis and treatment of depression, or
- within an established system of referral to mental health professionals.
If you treat patients with depression within your own practice, keep in mind that using standardized treatments with established efficacy in psychiatric patients has been shown to be more effective than “usual care.”30 If you choose to partner with mental health professionals, you can employ various levels of collaboration. Depending on the size of your practice, you may want to include a mental health professional as an integral part of your staff, or you may prefer to hire a part-time consultant. Another alternative is to establish an ongoing, collaborative relationship with a mental health provider in private practice. In some instances, working with a patient’s employee assistance program may be the best way to ensure that he or she receives treatment and follow-up. Primary care practices serving disadvantaged or impoverished communities may need to develop partnerships with public-sector community mental health centers.
Whatever setup works best for you, the goal is to make sure that your patients with depression have access to ongoing screening, diagnostic, and treatment services. That goal is worthy of your best efforts.
CORRESPONDENCE
Michele Surricchio, MPH, American College of Preventive Medicine, 1307 New York Ave., NW, Suite 200, Washington, DC 20005; [email protected]
Position statement
The American College of Preventive Medicine (ACPM) maintains that primary care providers should screen all adults for depression and that all primary care providers should have systems in place, either within the primary care setting itself or through collaborations with mental health professionals, to ensure the accurate diagnosis and treatment of this condition. The earliest and best opportunities to identify depression are in the clinics of primary care providers. Thus, the ACPM supports the recommendations of the US Preventive Services Task Force (USPSTF), and further suggests that all primary care practices should have such systems of care in place.
Why a position statement? The rationale
Primary care physicians have already been urged by the USPSTF and other authorities to consider screening adults for depression an essential aspect of care.1-5 So why is the ACPM issuing a new position statement on the subject? Because, the College believes, controversy over how to apply this mandate in the primary care setting is ongoing. Primary care providers—whether they practice family medicine, internal medicine, obstetrics/gynecology, or are in general practice—need to know what role they should play in screening adults for depression and ensuring adequate diagnosis and treatment.
The USPSTF recommendation
In May 2002, the USPSTF made a category B recommendation (high certainty of moderate net benefit) that adults should be screened for depression in “clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up” of depression1 (emphasis added). The less-than-clear aspect of this recommendation is italicized: Just what constitutes the “system” that primary care providers, the first and often the only point of contact adults have with the health care system, are told to have “in place”? And how can they go about providing such systems? That’s what the College has set out to elucidate.
The toll depression takes
Depression is a potentially life-threatening disorder that affects up to 6.7% of the population 18 years of age and older, or approximately 14.8 million Americans, in a given year.6 Many people younger than age 18 are also affected. The extensive STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study of outpatients with depression found that nearly 40% of respondents had their first depressive episode before the age of 18.7,8
The ripple effects
Depression is the leading cause of disability in the United States for individuals between the ages of 15 and 44 years.9 But the burden of this illness is not borne only by those diagnosed with the disorder; depression has a serious impact on the patient’s family, caregivers, colleagues, and society at large.
Medical costs. Depression contributes to a higher morbidity and mortality of other medical conditions. For example, people who have a myocardial infarction (MI) with comorbid depression have worse outcomes than those having an MI without depression. However, if the depressive episode is treated successfully, medical and surgical outcomes improve.10 Furthermore, studies documenting increased cardiovascular morbidity and mortality in patients with depressive symptoms or major depression suggest that depression is an independent risk factor in the pathophysiologic progression of cardiovascular disease, not just a secondary emotional response to the illness.11
Economic costs. Depression is a leading contributor to lost productivity, not only from worker absenteeism, but also from what is known as “presenteeism”—being physically at work but not fully engaged. Thus, depression may be a leading cause of poor organizational performance. Further, employees diagnosed with depression have a higher incidence and longer duration of both work-related and nonwork-related disabilities.
Screening: Easier than you think
Screening instruments with acceptable sensitivity and specificity are available. These brief, paper-and-pencil instruments can be quickly completed by patients in your waiting room. Some of the most commonly used self-administered measures are the Beck Depression Inventory (BDI), the Center for Epidemiologic Studies Depression Scale, Revised (CES-DR), the Zung Self-Rating Depression Scale, and the Patient Health Questionnaire (PHQ-9).12-16 These tools take approximately 5 to 10 minutes for patients to complete and do not interfere with clinical practice. You can choose the tests that are appropriate for screening your patient population, and you can also use the same instruments for ongoing monitoring of patients receiving treatment for depression.
Two questions. For an even briefer screen, ask your patients these 2 questions:
- Over the past month, have you felt down, depressed, or hopeless?
- Over the past month, have you felt little interest or pleasure in doing things?
Patients who answer Yes may need more in-depth screening and clinical assessment.
When these 2 questions were tested in a primary care setting with patients not receiving psychotropic drugs, they had a sensitivity of 97% and a specificity of 67%.17 Other research also shows that simple questions about depression perform as well as longer questionnaires, further proof that screening for depression need not add undue length to the clinical assessment in primary care.18
Which instrument is best? Selection of a screening measure, whether it be the 2 simple questions noted above or a longer, more comprehensive tool, is the first step in the process of detecting depression in primary care settings. In making your choice, consider characteristics of the population being screened, psychometric properties of the instrument, time required to complete the measure, time required to score the measure, ease of use, and cost. A review of available screening instruments suitable for use by primary care physicians has been published in American Family Physician.19 The review includes screening measures developed specifically for adolescents, such as the Reynolds Adolescent Depression Scale, and those developed for older adults, such as the Geriatric Depression Scale.
What comes next? Making the diagnosis
Screening tools provide only a preliminary assessment. Elevated scores must be confirmed with diagnostic interviewing. Without proper follow-up, false-positive scores can lead to harmful labeling, unnecessary additional testing, and inappropriate treatment.
The diagnostic interview. Primary care physicians may feel competent to perform the diagnostic interview themselves, or they may refer patients identified by screening to a mental health professional. The interview should determine whether a patient meets the diagnostic criteria for a depressive disorder—including major depressive disorder or dysthymic disorder—found in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR).20 Bear in mind that criteria important for diagnosis, such as duration of symptoms, degree of impairment, and comorbid psychiatric or substance use disorders, are not revealed in the screening instruments.
Differential diagnosis. Depressive symptoms often overlap with medical conditions, such as hypothyroidism, and with other psychiatric illnesses, such as generalized anxiety disorder. That’s why the differential diagnosis is crucial.
Screen for bipolar disorder. Patients meeting criteria for a depressive disorder should be screened for bipolar disorder, because the 2 conditions are managed differently. Screening instruments for bipolar disorder have been less extensively studied than the depression screening instruments described earlier. The Mood Disorder Questionnaire is a brief, easy-to-use, self-report screening instrument for bipolar-spectrum disorders.21 This single-page measure screens for a lifetime history (“has there ever been a period of time”) of manic or hypomanic symptoms using 13 yes/no items, as well as 2 items assessing whether several symptoms were experienced during the same time period, and the level of functional impairment associated with such symptoms. As noted earlier, elevated scores on this and other screening instruments must be confirmed with diagnostic interviewing.
Use DSM criteria. Because of the varying clinical manifestations of depression, clinical judgment sometimes must supersede strict adherence to DSM-IV-TR criteria. Nevertheless, reliance on these well-established criteria is generally recommended as the best way to avoid over- or underdiagnosis, billing problems, and legal problems arising from an inaccurate diagnosis or inappropriate use of medications.
Treating depression
Depression is a highly treatable condition with generally good outcomes.22 A variety of antidepressant medications and psychotherapeutic modalities are available. Consensus-based guidelines have been developed to guide clinicians in the evaluation and treatment of depression.23 Remission—not simply treatment response or an improvement in symptoms—should be the targeted endpoint. STAR*D data revealed that “better but not remitted” patients consistently have a worse prognosis and higher relapse rates than those achieving full remission.8,24
Why me?
The answer: Primary care providers are the principal contacts for more than 50% of patients with mental illnesses. Approximately 35% of patients seen in primary care meet criteria for some form of depression and 10% suffer from major depression.25,26 Because individuals with depression use health care more frequently, the prevalence of major depression is 2 to 3 times higher in primary care settings than in the general population.27 Yet, a substantial proportion of primary care patients with major depression go undiagnosed, leading to a dangerous situation in which symptoms may worsen and suicidal ideation can develop.28 That’s why you, as a primary care practitioner, have such an important role to play in assessing, diagnosing, and treating depression.
Making a difference. Interventions initiated in the primary care setting have been shown to be effective for the treatment of depression.29 Findings of the STAR*D study confirm that primary care providers, when given the time, staffing, and reimbursement support, can provide high-quality, appropriate care for patients with depression, especially in uncomplicated cases.8
What you need are “systems in place”
The USPSTF recommended screening for depression in “clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up.” Given the prevalence and gravity of the problem, the ACPM recommends that all primary care practices have such systems in place. These systems may be either:
- within your own practice, using clinical guidelines for the diagnosis and treatment of depression, or
- within an established system of referral to mental health professionals.
If you treat patients with depression within your own practice, keep in mind that using standardized treatments with established efficacy in psychiatric patients has been shown to be more effective than “usual care.”30 If you choose to partner with mental health professionals, you can employ various levels of collaboration. Depending on the size of your practice, you may want to include a mental health professional as an integral part of your staff, or you may prefer to hire a part-time consultant. Another alternative is to establish an ongoing, collaborative relationship with a mental health provider in private practice. In some instances, working with a patient’s employee assistance program may be the best way to ensure that he or she receives treatment and follow-up. Primary care practices serving disadvantaged or impoverished communities may need to develop partnerships with public-sector community mental health centers.
Whatever setup works best for you, the goal is to make sure that your patients with depression have access to ongoing screening, diagnostic, and treatment services. That goal is worthy of your best efforts.
CORRESPONDENCE
Michele Surricchio, MPH, American College of Preventive Medicine, 1307 New York Ave., NW, Suite 200, Washington, DC 20005; [email protected]
1. U.S. Preventive Services Task Force. Recommendations and rationale: screening for depression. Available at http://www.ahrq.gov/clinic/3rduspstf/depression/depressrr.htm. Accessed March 18, 2009.
2. Harris RP, Helfand M, Woolf SH, et al. Current methods of the U.S. Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(suppl 3):S21-S35.
3. American Medical Association. Guidelines for Adolescent Preventive Services (GAPS): Recommendations Monograph. Chicago: American Medical Association, 1997.
4. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care. Washington, DC: American College of Obstetricians and Gynecologists, 2002:126–133, 235–236.
5. American Academy of Family Physicians. Clinical Preventive Services. Revision 6.8, April 2009. Available at http://www.aafp.org/online/en/home/clinical/exam.html. Accessed August 20, 2009.
6. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Arch Gen Psychiatry. 2005;62:617-627.
7. STAR*D: Sequenced Treatment Alternatives to Relieve Depression. Available at http://www.edc.pitt.edu/stard/public/index.html. Accessed March 18, 2009.
8. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164:201-204.
9. World Health Organization. The World Health Report 2004: Changing History, Annex Table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in WHO regions, estimates for 2002. Geneva: WHO; 2004. Available at www.who.int/whr/2004/annex_3_en.pdf. Accessed August 25, 2009.
10. Parashar S, Rumsfeld JS, Spertus JA, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035-2043.
11. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580-592.
12. Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35:785-791.
13. Radloff LS. The CES scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401.
14, Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;2:63-70.
15. Nease DE, Jr, Maloin JM. Depression screening: a practical strategy. J Fam Pract. 2003;52:118-124.
16. Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29:388-395.
17. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
18. Williams JW, Jr, Mulrow CD, Kroenke K, et al. Case-finding for depression in primary care: a randomized trial. Am J Med. 1999;106:36-43.
19. Sharp LK, Lipsky MS. Screening for depression across the lifespan: a review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001-1008.
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
21. Hirschfeld RMA, Williams JBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
22. Gelenberg AJ, Hopkins HS. Assessing and treating depression in primary care medicine. Am J Med. 2007;120:105-108.
23. American Psychiatric Association. Practice guideline for major depressive disorder in adults. Am J Psychiatry. 1993;150(suppl):1-26.
24. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917.
25. Callahan EJ, Bertakis KD, Azari R, et al. The influence of depression on physician-patient interaction in primary care. Fam Med. 1996;28:346-351.
26. AHCPR Depression Guideline Panel. Clinical Practice Guideline Number 5. Depression in Primary Care. Volume 2: Treatment of Major Depression. Rockville, Md: Agency for Health Care Policy and Research, Public Health Services, US Department of Health and Human Services; 1993. AHCPR publication 93-0550.
27. Coyne JC, Fechner-Bates S, Schwenk TL. Prevalence, nature, and comorbidity of depressive disorders in primary care. Gen Hosp Psychiatry. 1994;16:267-276.
28. Rost K, Zhang M, Fortney J, et al. Persistently poor outcomes of undetected major depression in primary care. Gen Hosp Psychiatry. 1998;20:12-20.
29. Dietrich AJ, Oxman TE, Williams JW, et al. Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. BMJ. 2004;329:602-605.
30. Schulberg HC, Block MR, Madonia MJ, et al. Treating major depression in primary care practice: eight-month clinical outcomes. Arch Gen Psychiatry. 1996;53:913-919.
1. U.S. Preventive Services Task Force. Recommendations and rationale: screening for depression. Available at http://www.ahrq.gov/clinic/3rduspstf/depression/depressrr.htm. Accessed March 18, 2009.
2. Harris RP, Helfand M, Woolf SH, et al. Current methods of the U.S. Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(suppl 3):S21-S35.
3. American Medical Association. Guidelines for Adolescent Preventive Services (GAPS): Recommendations Monograph. Chicago: American Medical Association, 1997.
4. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care. Washington, DC: American College of Obstetricians and Gynecologists, 2002:126–133, 235–236.
5. American Academy of Family Physicians. Clinical Preventive Services. Revision 6.8, April 2009. Available at http://www.aafp.org/online/en/home/clinical/exam.html. Accessed August 20, 2009.
6. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R). Arch Gen Psychiatry. 2005;62:617-627.
7. STAR*D: Sequenced Treatment Alternatives to Relieve Depression. Available at http://www.edc.pitt.edu/stard/public/index.html. Accessed March 18, 2009.
8. Rush AJ. STAR*D: what have we learned? Am J Psychiatry. 2007;164:201-204.
9. World Health Organization. The World Health Report 2004: Changing History, Annex Table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in WHO regions, estimates for 2002. Geneva: WHO; 2004. Available at www.who.int/whr/2004/annex_3_en.pdf. Accessed August 25, 2009.
10. Parashar S, Rumsfeld JS, Spertus JA, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med. 2006;166:2035-2043.
11. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580-592.
12. Beck AT, Guth D, Steer RA, et al. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for Primary Care. Behav Res Ther. 1997;35:785-791.
13. Radloff LS. The CES scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401.
14, Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;2:63-70.
15. Nease DE, Jr, Maloin JM. Depression screening: a practical strategy. J Fam Pract. 2003;52:118-124.
16. Wittkampf KA, Naeije L, Schene AH, et al. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29:388-395.
17. Arroll B, Khin N, Kerse N. Screening for depression in primary care with two verbally asked questions: cross sectional study. BMJ. 2003;327:1144-1146.
18. Williams JW, Jr, Mulrow CD, Kroenke K, et al. Case-finding for depression in primary care: a randomized trial. Am J Med. 1999;106:36-43.
19. Sharp LK, Lipsky MS. Screening for depression across the lifespan: a review of measures for use in primary care settings. Am Fam Physician. 2002;66:1001-1008.
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
21. Hirschfeld RMA, Williams JBW, Spitzer RL, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873-1875.
22. Gelenberg AJ, Hopkins HS. Assessing and treating depression in primary care medicine. Am J Med. 2007;120:105-108.
23. American Psychiatric Association. Practice guideline for major depressive disorder in adults. Am J Psychiatry. 1993;150(suppl):1-26.
24. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905-1917.
25. Callahan EJ, Bertakis KD, Azari R, et al. The influence of depression on physician-patient interaction in primary care. Fam Med. 1996;28:346-351.
26. AHCPR Depression Guideline Panel. Clinical Practice Guideline Number 5. Depression in Primary Care. Volume 2: Treatment of Major Depression. Rockville, Md: Agency for Health Care Policy and Research, Public Health Services, US Department of Health and Human Services; 1993. AHCPR publication 93-0550.
27. Coyne JC, Fechner-Bates S, Schwenk TL. Prevalence, nature, and comorbidity of depressive disorders in primary care. Gen Hosp Psychiatry. 1994;16:267-276.
28. Rost K, Zhang M, Fortney J, et al. Persistently poor outcomes of undetected major depression in primary care. Gen Hosp Psychiatry. 1998;20:12-20.
29. Dietrich AJ, Oxman TE, Williams JW, et al. Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. BMJ. 2004;329:602-605.
30. Schulberg HC, Block MR, Madonia MJ, et al. Treating major depression in primary care practice: eight-month clinical outcomes. Arch Gen Psychiatry. 1996;53:913-919.
3 alternatives to standard varicose vein treatment
• Discuss minimally invasive procedures with patients considering surgery for treatment of chronic venous insufficiency. Thermal ablation, in particular, has higher success rates than vein stripping. A
• Consider endovenous chemical ablation for treatment of tortuous saphenous tributary varicosities that cannot be treated with thermal ablation. Foamed sclerosant, injected under ultrasound guidance, allows for direct visualization and has equivalent efficacy rates when compared to venous stripping. A
• Pregnancy, active deep vein thrombosis, poor health with limited mobility, and severe peripheral vascular disease are contraindications for both thermal and chemical ablation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE
Kevin M., a 35-year-old researcher who often works 12- to 14-hour days, is in your office again, following his second trip to the emergency room for bleeding from a protruding varicose vein proximal to his left ankle. He has complained of leg aching and ankle swelling in the past, usually after he’s been on his feet for hours.
A previous ultrasound showed no evidence of deep vein thrombosis (DVT), but did reveal reflux along the left great saphenous vein from the saphenofemoral junction to the ankle. On examination, Kevin’s lower extremity pulses are intact bilaterally, but there are multiple tortuous varicosities branching from the medial thigh to the left medial malleolus, with evidence of hemosiderin hyperpigmentation and dermatitis on the left lower leg. Previous lab studies have been normal, and Kevin has no other chronic conditions. Kevin’s main concern is to minimize “down time” from work, and he asks you about the newer, minimally invasive vein treatments he’s read about on the Internet.
What should you tell him?
Chronic venous insufficiency is a common and costly condition, affecting nearly one-third of the US population.1 While many people mistakenly think of varicose veins primarily as a cosmetic issue, venous insufficiency often results in painful, even debilitating, signs and symptoms—from swelling and aching legs to skin changes that range from stasis dermatitis to open ulceration.
Venous stripping and ambulatory phlebectomy were long considered the primary means of addressing saphenous venous insufficiency,2 and compression therapy with gradient stockings, short stretch bandages, and Unna’s dressings remains a therapeutic cornerstone for every stage of chronic venous disease. Compression has also been shown to dramatically decrease the risk of post-thrombotic syndrome following DVT3 and, when combined with anticoagulation, to prevent DVT after surgery.4
Outcomes of surgical treatment of large varicose veins arising from saphenous truncal vein reflux, however, have historically been poor.5 This is primarily because of neovascularization and residual refluxing veins. Average failure rates of 25% for saphenous vein stripping and 43% for saphenofemoral junction ligation have been reported.6 A subsequent study of 140 patients who underwent saphenous vein stripping found that 20% experienced neovascularization.7
In the last decade, a number of minimally invasive procedures have been developed and tested, giving physicians and their patients more choices for medical management of venous insufficiency. A closer look at 3 procedures, which include 2 types of thermal ablation, will help you direct patients like Kevin to the best possible treatment.
2 thermal ablation procedures use only local anesthesia
Collectively termed endovenous thermal ablation (EVTA), radiofrequency ablation (RFA) and endovenous laser treatment (ELT) are similar. Performed under duplex ultrasound visualization, both offer a number of benefits.
Outpatient treatment. EVTA procedures are typically done on an outpatient basis, with only local anesthesia—0.05% to 0.1% lidocaine, injected as a perivenous tumescent solution under ultrasound guidance.8,9 The physician places and advances an endovenous catheter (for RFA) or a laser fiber (for ELT) into the vein (FIGURE 1). When the catheter or laser is in proper position, the RF generator or laser is activated. Heat, delivered through the tip of the catheter or fiber, ablates the lumen of the vein as the physician withdraws the catheter.
Mild side effects. Temporary discomfort is common with EVTA. Mild bruising, swelling, and nodularity of the treated veins are the most frequent side effects. More serious adverse effects, including skin burns, paresthesias, DVT, and pulmonary embolism, are rare.10-12
High success rates. Successful treatment of both the great and small saphenous veins and long-term maintenance of vein closure are the norm, with rates ranging from 88% to 100% for both RFA13,14 and ELT, regardless of vein size.15-19 Thermal ablation has been shown to be superior to surgery in other ways as well, eliminating the need for general anesthesia and inpatient treatment and resulting in less postoperative pain, a shorter recovery period, an improved quality of life, and lower costs (TABLE).20-23
TABLE
Endovenous thermal ablation vs standard surgery
| Outcomes | |||||
|---|---|---|---|---|---|
| Study | Type | Modalities studied | Follow-up | Return to work | Pain |
| Rautio et al20 | RCT | RFA vs stripping | 8 weeks | Earlier with RFA | Less with RFA |
| Lurie et al21 | RCT | RFA vs stripping | 2 years | N/A | Less with RFA |
| Hinchcliffe et al22 | RCT | RFA vs stripping | 1 year | N/A | Less with RFA |
| Darwood et al23 | RCT | ELT vs stripping | 3 months | Earlier with ELT | Less with ELT |
| ELT, endovenous laser treatment; NA, not available; RCT, randomized controlled trial; RFA, radiofrequency ablation. | |||||
FIGURE 1
Endovenous laser treatment
In preparation for thermal ablation, the physician (A) places a laser catheter tip into the vein and (B) injects perivenous tumescent anesthesia, guided by duplex ultrasound. The laser tip shown here (C) is in the region of the saphenofemoral junction.
Not for every patient
Despite the usefulness of these minimally invasive procedures, RFA and ELT are not always possible. Veins that are tortuous, too small, or too close to the surface of the skin are not well suited for thermal ablation; in some cases, chemical ablation, discussed below, is the only option. However, some conditions—including pregnancy, active DVT, poor health status with limited mobility, and severe peripheral vascular disease—are contraindications for both thermal and chemical ablation.24
Thermal won’t work? Consider chemical ablation
Endovenous chemoablation (ECA), like thermal ablation, is performed on an outpatient basis without sedation. Hypertonic saline, an osmotic agent, has been used for decades in treating small-caliber varicose veins, or “spider veins.” Detergenttype sclerosants, which we’ll discuss here, are now widely used to treat larger veins, including the great and small saphenous vein trunks and their affected tributaries.
Better suited for tortuous veins. Patients who have had prior stripping often have branches that are too tortuous to access with thermal ablation. In such cases, ECA is the only option. Those who have never undergone vein stripping often need a combination of thermal and chemical ablation, as well, with ECA reserved for saphenous veins that are inaccessible by RFA or laser.
Foamed sclerosants allow visualization. When mixed through a 3-way stopcock with an inert gas such as room air or carbon dioxide, the detergent-type sclerosants produce a micro-foamed preparation that is echogenic, and allows direct visualization with ultrasound (FIGURE 2). The foam displaces blood from within the vein and promotes contact with the venous endothelium, and intense vasospasm results. The sclerosant foam mixture, which makes it possible to watch the effect (and extent) of the foam as it disperses throughout the target vessel, has enhanced the treatment of larger viscosities.25-26 Pretreatment venous ultrasound mapping—a process in which the patient stands upright while compression is applied to the saphenous veins and their tributaries to test the valves for normal flow—combined with realtime duplex imaging, minimizes the chance of arterial injection, extravasation, and tissue necrosis.25
Serious adverse effects are rare. Hemosiderin hyperpigmentation and pain at the injection site are among the most common side effects of ultrasound-guided sclerotherapy. Serious adverse events, including anaphylaxis, DVT/pulmonary embolus, cutaneous necrosis, transient ischemic attack, and arterial injection, are rare.27
To date, there is only 1 published prospective, randomized study that directly compares foam sclerotherapy with stripping/ligation surgery. The researchers found the results of both treatments to be comparable, but reported that surgery was more expensive than foam sclerotherapy and produced less improvement in quality of life.28 A meta-analysis of treatment for 12,000 limbs concluded that foam sclerotherapy and EVTA were as effective as surgical stripping.29 There are no prospective, randomized studies comparing foam sclerotherapy with thermal ablation.
FIGURE 2
Endovenous chemoablation
ECA is also conducted under ultrasound guidance: The image at left (A) shows the needle tip inside a refluxing saphenous tributary vein; in the image at right (B), the arrow points to the vein injected with foamed sclerosant.
Advising Kevin M.: What’s his best bet?
You’ve talked to your patient in the past about vein stripping, but he’s been reluctant to undergo surgery. When you describe the minimally invasive approaches to chronic venous insufficiency with him, however, he asks to see a specialist—and to undergo ultrasound mapping.
You give him a prescription for graduated compression stockings for immediate use and a referral to a phlebologist. Soon after, you receive a report from the specialist, indicating that your patient had a combination of ELT of the refluxing left great saphenous vein and subsequent ultrasound-guided ECA of the saphenous tributaries.
The next time you see Kevin, he reports that his leg no longer aches—and you can see that the bulging, tortuous varicosities and swelling have improved significantly.
Acknowledgments
The authors thank John Hickner, MD, MSc, for his input during the manuscript process.
CORRESPONDENCE
Jeffery P. Schoonover, MD, FAAFP, Vein Clinics of America, 9000 Keystone Crossing, Suite 960, Indianapolis, IN 46240; [email protected]
1. McLafferty R, Lohr J, Caprini J, et al. Results of the national pilot screening program for venous disease by the American Venous Forum. J Vasc Surg. 2007;45:142-148.
2. Hagen MD, Johnson ED, Adelman A. What treatments are effective for varicose veins? J Fam Pract. 2003;52:329-331.
3. Partsch H, Flour M, Smith PC. Indications for compression therapy in venous and disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. Int Angiol. 2008;27:193-219.
4. Partsch H. ed. Evidence-based compression therapy. VASA. 2003;32(suppl 63):S3-S39.
5. Fischer R, Chandler JG, de Maeseneer MG, et al. The unresolved problem of recurrent saphenofemoral reflux. J Am Coll Surg. 2002;195:80-94.
6. Perrin MR. Recurrent varices after surgery (REVAS), a consensus document. Cardiovasc Surg. 2000;8:233-245.
7. Perrin MR, Labropoulos M, Leon L. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;43:327-334.
8. Navarro L, Min R, Boné C. Endovenous laser: a new minimally invasive method of treatment for varicose veins—preliminary observations using an 810 nm diode laser. Dermatol Surg. 2001;27:117-122.
9. Min R, Zimmet S, Isaacs M, et al. Endovenous laser treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol. 2001;12:1167-1171.
10. Mundy L, Merlin TL, Fitridge RA, et al. Systematic review of endovenous laser treatment for varicose veins. Br J Surg. 2005;92:1189-1194.
11. van den Bos RR, Kockaert MA, Neumann HAM, et al. Technical review of endovenous laser therapy for varicose veins. Eur J Vasc Endovasc Surg. 2008;35:88-95.
12. Proebstle TM, Moehler T, Gul D, et al. Endovenous treatment of the great saphenous vein using a 1320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31:1380-1385.
13. Pichot O, Kabnick LS, Creton D, et al. Duplex ultrasound scan findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg. 2004;39:189-195.
14. Merchant RF, Pichot O, Myers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31:129-134.
15. Kabnick LS. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006;43:88-93.
16. Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991-996.
17. Proebstle TM, Gul D, Kargl A, et al. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg. 2003;29:357-361.
18. Theivacumar NS, Beale RJ, Mavor AID, et al. Initial experience in endovenous laser ablation (EVLA) of varicose veins due to small saphenous vein reflux. Eur J Vasc Endovasc Surg. 2007;33:614-618.
19. Gibson KD, Ferris BL, Polissar N, et al. Endovenous laser treatment of the short saphenous vein: efficacy and complications. J Vasc Surg. 2007;45:795-803.
20. Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg. 2002;35:958-965.
21. Lurie F, Creton D, Eklof B, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg. 2005;29:67-73.
22. Hinchcliffe RJ, Ubhi J, Beech A, et al. A prospective randomized controlled trial of VNUS closure versus surgery for the treatment of recurrent long saphenous varicose veins. Eur J Vasc Endovasc Surg. 2006;31:212-218.
23. Darwood RJ, Theivacumar N, Dellagrammaticas D, et al. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg. 2008;95:294-301.
24. Sadick NS. Advances in the treatment of varicose veins: ambulatory phlebectomy, foam sclerotherapy, endovascular laser, and radiofrequency closure. Dermatol Clin. 2005;23:443-455.
25. Kakkos SK, Bountouroglou DG, Azzam M, et al. Effectiveness and safety of ultrasound-guided foam sclerotherapy for recurrent varicose veins: immediate results. J Endovasc Ther. 2006;13:357-364.
26. Cabrera J, Cabrera A, Jr, Garcia-Olmedo A. Treatment of varicose long saphenous veins with sclerosant in microfoam form: long-term outcomes. Phlebology. 2000;15:19-23.
27. Jia X, Mowatt G, Burr JM, et al. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;10:307-1308.
28. Belcaro G, Cesarone MR, Di Renzo A, et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO trial). Angiology. 2003;54:307-315.
29. van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
• Discuss minimally invasive procedures with patients considering surgery for treatment of chronic venous insufficiency. Thermal ablation, in particular, has higher success rates than vein stripping. A
• Consider endovenous chemical ablation for treatment of tortuous saphenous tributary varicosities that cannot be treated with thermal ablation. Foamed sclerosant, injected under ultrasound guidance, allows for direct visualization and has equivalent efficacy rates when compared to venous stripping. A
• Pregnancy, active deep vein thrombosis, poor health with limited mobility, and severe peripheral vascular disease are contraindications for both thermal and chemical ablation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE
Kevin M., a 35-year-old researcher who often works 12- to 14-hour days, is in your office again, following his second trip to the emergency room for bleeding from a protruding varicose vein proximal to his left ankle. He has complained of leg aching and ankle swelling in the past, usually after he’s been on his feet for hours.
A previous ultrasound showed no evidence of deep vein thrombosis (DVT), but did reveal reflux along the left great saphenous vein from the saphenofemoral junction to the ankle. On examination, Kevin’s lower extremity pulses are intact bilaterally, but there are multiple tortuous varicosities branching from the medial thigh to the left medial malleolus, with evidence of hemosiderin hyperpigmentation and dermatitis on the left lower leg. Previous lab studies have been normal, and Kevin has no other chronic conditions. Kevin’s main concern is to minimize “down time” from work, and he asks you about the newer, minimally invasive vein treatments he’s read about on the Internet.
What should you tell him?
Chronic venous insufficiency is a common and costly condition, affecting nearly one-third of the US population.1 While many people mistakenly think of varicose veins primarily as a cosmetic issue, venous insufficiency often results in painful, even debilitating, signs and symptoms—from swelling and aching legs to skin changes that range from stasis dermatitis to open ulceration.
Venous stripping and ambulatory phlebectomy were long considered the primary means of addressing saphenous venous insufficiency,2 and compression therapy with gradient stockings, short stretch bandages, and Unna’s dressings remains a therapeutic cornerstone for every stage of chronic venous disease. Compression has also been shown to dramatically decrease the risk of post-thrombotic syndrome following DVT3 and, when combined with anticoagulation, to prevent DVT after surgery.4
Outcomes of surgical treatment of large varicose veins arising from saphenous truncal vein reflux, however, have historically been poor.5 This is primarily because of neovascularization and residual refluxing veins. Average failure rates of 25% for saphenous vein stripping and 43% for saphenofemoral junction ligation have been reported.6 A subsequent study of 140 patients who underwent saphenous vein stripping found that 20% experienced neovascularization.7
In the last decade, a number of minimally invasive procedures have been developed and tested, giving physicians and their patients more choices for medical management of venous insufficiency. A closer look at 3 procedures, which include 2 types of thermal ablation, will help you direct patients like Kevin to the best possible treatment.
2 thermal ablation procedures use only local anesthesia
Collectively termed endovenous thermal ablation (EVTA), radiofrequency ablation (RFA) and endovenous laser treatment (ELT) are similar. Performed under duplex ultrasound visualization, both offer a number of benefits.
Outpatient treatment. EVTA procedures are typically done on an outpatient basis, with only local anesthesia—0.05% to 0.1% lidocaine, injected as a perivenous tumescent solution under ultrasound guidance.8,9 The physician places and advances an endovenous catheter (for RFA) or a laser fiber (for ELT) into the vein (FIGURE 1). When the catheter or laser is in proper position, the RF generator or laser is activated. Heat, delivered through the tip of the catheter or fiber, ablates the lumen of the vein as the physician withdraws the catheter.
Mild side effects. Temporary discomfort is common with EVTA. Mild bruising, swelling, and nodularity of the treated veins are the most frequent side effects. More serious adverse effects, including skin burns, paresthesias, DVT, and pulmonary embolism, are rare.10-12
High success rates. Successful treatment of both the great and small saphenous veins and long-term maintenance of vein closure are the norm, with rates ranging from 88% to 100% for both RFA13,14 and ELT, regardless of vein size.15-19 Thermal ablation has been shown to be superior to surgery in other ways as well, eliminating the need for general anesthesia and inpatient treatment and resulting in less postoperative pain, a shorter recovery period, an improved quality of life, and lower costs (TABLE).20-23
TABLE
Endovenous thermal ablation vs standard surgery
| Outcomes | |||||
|---|---|---|---|---|---|
| Study | Type | Modalities studied | Follow-up | Return to work | Pain |
| Rautio et al20 | RCT | RFA vs stripping | 8 weeks | Earlier with RFA | Less with RFA |
| Lurie et al21 | RCT | RFA vs stripping | 2 years | N/A | Less with RFA |
| Hinchcliffe et al22 | RCT | RFA vs stripping | 1 year | N/A | Less with RFA |
| Darwood et al23 | RCT | ELT vs stripping | 3 months | Earlier with ELT | Less with ELT |
| ELT, endovenous laser treatment; NA, not available; RCT, randomized controlled trial; RFA, radiofrequency ablation. | |||||
FIGURE 1
Endovenous laser treatment
In preparation for thermal ablation, the physician (A) places a laser catheter tip into the vein and (B) injects perivenous tumescent anesthesia, guided by duplex ultrasound. The laser tip shown here (C) is in the region of the saphenofemoral junction.
Not for every patient
Despite the usefulness of these minimally invasive procedures, RFA and ELT are not always possible. Veins that are tortuous, too small, or too close to the surface of the skin are not well suited for thermal ablation; in some cases, chemical ablation, discussed below, is the only option. However, some conditions—including pregnancy, active DVT, poor health status with limited mobility, and severe peripheral vascular disease—are contraindications for both thermal and chemical ablation.24
Thermal won’t work? Consider chemical ablation
Endovenous chemoablation (ECA), like thermal ablation, is performed on an outpatient basis without sedation. Hypertonic saline, an osmotic agent, has been used for decades in treating small-caliber varicose veins, or “spider veins.” Detergenttype sclerosants, which we’ll discuss here, are now widely used to treat larger veins, including the great and small saphenous vein trunks and their affected tributaries.
Better suited for tortuous veins. Patients who have had prior stripping often have branches that are too tortuous to access with thermal ablation. In such cases, ECA is the only option. Those who have never undergone vein stripping often need a combination of thermal and chemical ablation, as well, with ECA reserved for saphenous veins that are inaccessible by RFA or laser.
Foamed sclerosants allow visualization. When mixed through a 3-way stopcock with an inert gas such as room air or carbon dioxide, the detergent-type sclerosants produce a micro-foamed preparation that is echogenic, and allows direct visualization with ultrasound (FIGURE 2). The foam displaces blood from within the vein and promotes contact with the venous endothelium, and intense vasospasm results. The sclerosant foam mixture, which makes it possible to watch the effect (and extent) of the foam as it disperses throughout the target vessel, has enhanced the treatment of larger viscosities.25-26 Pretreatment venous ultrasound mapping—a process in which the patient stands upright while compression is applied to the saphenous veins and their tributaries to test the valves for normal flow—combined with realtime duplex imaging, minimizes the chance of arterial injection, extravasation, and tissue necrosis.25
Serious adverse effects are rare. Hemosiderin hyperpigmentation and pain at the injection site are among the most common side effects of ultrasound-guided sclerotherapy. Serious adverse events, including anaphylaxis, DVT/pulmonary embolus, cutaneous necrosis, transient ischemic attack, and arterial injection, are rare.27
To date, there is only 1 published prospective, randomized study that directly compares foam sclerotherapy with stripping/ligation surgery. The researchers found the results of both treatments to be comparable, but reported that surgery was more expensive than foam sclerotherapy and produced less improvement in quality of life.28 A meta-analysis of treatment for 12,000 limbs concluded that foam sclerotherapy and EVTA were as effective as surgical stripping.29 There are no prospective, randomized studies comparing foam sclerotherapy with thermal ablation.
FIGURE 2
Endovenous chemoablation
ECA is also conducted under ultrasound guidance: The image at left (A) shows the needle tip inside a refluxing saphenous tributary vein; in the image at right (B), the arrow points to the vein injected with foamed sclerosant.
Advising Kevin M.: What’s his best bet?
You’ve talked to your patient in the past about vein stripping, but he’s been reluctant to undergo surgery. When you describe the minimally invasive approaches to chronic venous insufficiency with him, however, he asks to see a specialist—and to undergo ultrasound mapping.
You give him a prescription for graduated compression stockings for immediate use and a referral to a phlebologist. Soon after, you receive a report from the specialist, indicating that your patient had a combination of ELT of the refluxing left great saphenous vein and subsequent ultrasound-guided ECA of the saphenous tributaries.
The next time you see Kevin, he reports that his leg no longer aches—and you can see that the bulging, tortuous varicosities and swelling have improved significantly.
Acknowledgments
The authors thank John Hickner, MD, MSc, for his input during the manuscript process.
CORRESPONDENCE
Jeffery P. Schoonover, MD, FAAFP, Vein Clinics of America, 9000 Keystone Crossing, Suite 960, Indianapolis, IN 46240; [email protected]
• Discuss minimally invasive procedures with patients considering surgery for treatment of chronic venous insufficiency. Thermal ablation, in particular, has higher success rates than vein stripping. A
• Consider endovenous chemical ablation for treatment of tortuous saphenous tributary varicosities that cannot be treated with thermal ablation. Foamed sclerosant, injected under ultrasound guidance, allows for direct visualization and has equivalent efficacy rates when compared to venous stripping. A
• Pregnancy, active deep vein thrombosis, poor health with limited mobility, and severe peripheral vascular disease are contraindications for both thermal and chemical ablation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE
Kevin M., a 35-year-old researcher who often works 12- to 14-hour days, is in your office again, following his second trip to the emergency room for bleeding from a protruding varicose vein proximal to his left ankle. He has complained of leg aching and ankle swelling in the past, usually after he’s been on his feet for hours.
A previous ultrasound showed no evidence of deep vein thrombosis (DVT), but did reveal reflux along the left great saphenous vein from the saphenofemoral junction to the ankle. On examination, Kevin’s lower extremity pulses are intact bilaterally, but there are multiple tortuous varicosities branching from the medial thigh to the left medial malleolus, with evidence of hemosiderin hyperpigmentation and dermatitis on the left lower leg. Previous lab studies have been normal, and Kevin has no other chronic conditions. Kevin’s main concern is to minimize “down time” from work, and he asks you about the newer, minimally invasive vein treatments he’s read about on the Internet.
What should you tell him?
Chronic venous insufficiency is a common and costly condition, affecting nearly one-third of the US population.1 While many people mistakenly think of varicose veins primarily as a cosmetic issue, venous insufficiency often results in painful, even debilitating, signs and symptoms—from swelling and aching legs to skin changes that range from stasis dermatitis to open ulceration.
Venous stripping and ambulatory phlebectomy were long considered the primary means of addressing saphenous venous insufficiency,2 and compression therapy with gradient stockings, short stretch bandages, and Unna’s dressings remains a therapeutic cornerstone for every stage of chronic venous disease. Compression has also been shown to dramatically decrease the risk of post-thrombotic syndrome following DVT3 and, when combined with anticoagulation, to prevent DVT after surgery.4
Outcomes of surgical treatment of large varicose veins arising from saphenous truncal vein reflux, however, have historically been poor.5 This is primarily because of neovascularization and residual refluxing veins. Average failure rates of 25% for saphenous vein stripping and 43% for saphenofemoral junction ligation have been reported.6 A subsequent study of 140 patients who underwent saphenous vein stripping found that 20% experienced neovascularization.7
In the last decade, a number of minimally invasive procedures have been developed and tested, giving physicians and their patients more choices for medical management of venous insufficiency. A closer look at 3 procedures, which include 2 types of thermal ablation, will help you direct patients like Kevin to the best possible treatment.
2 thermal ablation procedures use only local anesthesia
Collectively termed endovenous thermal ablation (EVTA), radiofrequency ablation (RFA) and endovenous laser treatment (ELT) are similar. Performed under duplex ultrasound visualization, both offer a number of benefits.
Outpatient treatment. EVTA procedures are typically done on an outpatient basis, with only local anesthesia—0.05% to 0.1% lidocaine, injected as a perivenous tumescent solution under ultrasound guidance.8,9 The physician places and advances an endovenous catheter (for RFA) or a laser fiber (for ELT) into the vein (FIGURE 1). When the catheter or laser is in proper position, the RF generator or laser is activated. Heat, delivered through the tip of the catheter or fiber, ablates the lumen of the vein as the physician withdraws the catheter.
Mild side effects. Temporary discomfort is common with EVTA. Mild bruising, swelling, and nodularity of the treated veins are the most frequent side effects. More serious adverse effects, including skin burns, paresthesias, DVT, and pulmonary embolism, are rare.10-12
High success rates. Successful treatment of both the great and small saphenous veins and long-term maintenance of vein closure are the norm, with rates ranging from 88% to 100% for both RFA13,14 and ELT, regardless of vein size.15-19 Thermal ablation has been shown to be superior to surgery in other ways as well, eliminating the need for general anesthesia and inpatient treatment and resulting in less postoperative pain, a shorter recovery period, an improved quality of life, and lower costs (TABLE).20-23
TABLE
Endovenous thermal ablation vs standard surgery
| Outcomes | |||||
|---|---|---|---|---|---|
| Study | Type | Modalities studied | Follow-up | Return to work | Pain |
| Rautio et al20 | RCT | RFA vs stripping | 8 weeks | Earlier with RFA | Less with RFA |
| Lurie et al21 | RCT | RFA vs stripping | 2 years | N/A | Less with RFA |
| Hinchcliffe et al22 | RCT | RFA vs stripping | 1 year | N/A | Less with RFA |
| Darwood et al23 | RCT | ELT vs stripping | 3 months | Earlier with ELT | Less with ELT |
| ELT, endovenous laser treatment; NA, not available; RCT, randomized controlled trial; RFA, radiofrequency ablation. | |||||
FIGURE 1
Endovenous laser treatment
In preparation for thermal ablation, the physician (A) places a laser catheter tip into the vein and (B) injects perivenous tumescent anesthesia, guided by duplex ultrasound. The laser tip shown here (C) is in the region of the saphenofemoral junction.
Not for every patient
Despite the usefulness of these minimally invasive procedures, RFA and ELT are not always possible. Veins that are tortuous, too small, or too close to the surface of the skin are not well suited for thermal ablation; in some cases, chemical ablation, discussed below, is the only option. However, some conditions—including pregnancy, active DVT, poor health status with limited mobility, and severe peripheral vascular disease—are contraindications for both thermal and chemical ablation.24
Thermal won’t work? Consider chemical ablation
Endovenous chemoablation (ECA), like thermal ablation, is performed on an outpatient basis without sedation. Hypertonic saline, an osmotic agent, has been used for decades in treating small-caliber varicose veins, or “spider veins.” Detergenttype sclerosants, which we’ll discuss here, are now widely used to treat larger veins, including the great and small saphenous vein trunks and their affected tributaries.
Better suited for tortuous veins. Patients who have had prior stripping often have branches that are too tortuous to access with thermal ablation. In such cases, ECA is the only option. Those who have never undergone vein stripping often need a combination of thermal and chemical ablation, as well, with ECA reserved for saphenous veins that are inaccessible by RFA or laser.
Foamed sclerosants allow visualization. When mixed through a 3-way stopcock with an inert gas such as room air or carbon dioxide, the detergent-type sclerosants produce a micro-foamed preparation that is echogenic, and allows direct visualization with ultrasound (FIGURE 2). The foam displaces blood from within the vein and promotes contact with the venous endothelium, and intense vasospasm results. The sclerosant foam mixture, which makes it possible to watch the effect (and extent) of the foam as it disperses throughout the target vessel, has enhanced the treatment of larger viscosities.25-26 Pretreatment venous ultrasound mapping—a process in which the patient stands upright while compression is applied to the saphenous veins and their tributaries to test the valves for normal flow—combined with realtime duplex imaging, minimizes the chance of arterial injection, extravasation, and tissue necrosis.25
Serious adverse effects are rare. Hemosiderin hyperpigmentation and pain at the injection site are among the most common side effects of ultrasound-guided sclerotherapy. Serious adverse events, including anaphylaxis, DVT/pulmonary embolus, cutaneous necrosis, transient ischemic attack, and arterial injection, are rare.27
To date, there is only 1 published prospective, randomized study that directly compares foam sclerotherapy with stripping/ligation surgery. The researchers found the results of both treatments to be comparable, but reported that surgery was more expensive than foam sclerotherapy and produced less improvement in quality of life.28 A meta-analysis of treatment for 12,000 limbs concluded that foam sclerotherapy and EVTA were as effective as surgical stripping.29 There are no prospective, randomized studies comparing foam sclerotherapy with thermal ablation.
FIGURE 2
Endovenous chemoablation
ECA is also conducted under ultrasound guidance: The image at left (A) shows the needle tip inside a refluxing saphenous tributary vein; in the image at right (B), the arrow points to the vein injected with foamed sclerosant.
Advising Kevin M.: What’s his best bet?
You’ve talked to your patient in the past about vein stripping, but he’s been reluctant to undergo surgery. When you describe the minimally invasive approaches to chronic venous insufficiency with him, however, he asks to see a specialist—and to undergo ultrasound mapping.
You give him a prescription for graduated compression stockings for immediate use and a referral to a phlebologist. Soon after, you receive a report from the specialist, indicating that your patient had a combination of ELT of the refluxing left great saphenous vein and subsequent ultrasound-guided ECA of the saphenous tributaries.
The next time you see Kevin, he reports that his leg no longer aches—and you can see that the bulging, tortuous varicosities and swelling have improved significantly.
Acknowledgments
The authors thank John Hickner, MD, MSc, for his input during the manuscript process.
CORRESPONDENCE
Jeffery P. Schoonover, MD, FAAFP, Vein Clinics of America, 9000 Keystone Crossing, Suite 960, Indianapolis, IN 46240; [email protected]
1. McLafferty R, Lohr J, Caprini J, et al. Results of the national pilot screening program for venous disease by the American Venous Forum. J Vasc Surg. 2007;45:142-148.
2. Hagen MD, Johnson ED, Adelman A. What treatments are effective for varicose veins? J Fam Pract. 2003;52:329-331.
3. Partsch H, Flour M, Smith PC. Indications for compression therapy in venous and disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. Int Angiol. 2008;27:193-219.
4. Partsch H. ed. Evidence-based compression therapy. VASA. 2003;32(suppl 63):S3-S39.
5. Fischer R, Chandler JG, de Maeseneer MG, et al. The unresolved problem of recurrent saphenofemoral reflux. J Am Coll Surg. 2002;195:80-94.
6. Perrin MR. Recurrent varices after surgery (REVAS), a consensus document. Cardiovasc Surg. 2000;8:233-245.
7. Perrin MR, Labropoulos M, Leon L. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;43:327-334.
8. Navarro L, Min R, Boné C. Endovenous laser: a new minimally invasive method of treatment for varicose veins—preliminary observations using an 810 nm diode laser. Dermatol Surg. 2001;27:117-122.
9. Min R, Zimmet S, Isaacs M, et al. Endovenous laser treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol. 2001;12:1167-1171.
10. Mundy L, Merlin TL, Fitridge RA, et al. Systematic review of endovenous laser treatment for varicose veins. Br J Surg. 2005;92:1189-1194.
11. van den Bos RR, Kockaert MA, Neumann HAM, et al. Technical review of endovenous laser therapy for varicose veins. Eur J Vasc Endovasc Surg. 2008;35:88-95.
12. Proebstle TM, Moehler T, Gul D, et al. Endovenous treatment of the great saphenous vein using a 1320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31:1380-1385.
13. Pichot O, Kabnick LS, Creton D, et al. Duplex ultrasound scan findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg. 2004;39:189-195.
14. Merchant RF, Pichot O, Myers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31:129-134.
15. Kabnick LS. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006;43:88-93.
16. Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991-996.
17. Proebstle TM, Gul D, Kargl A, et al. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg. 2003;29:357-361.
18. Theivacumar NS, Beale RJ, Mavor AID, et al. Initial experience in endovenous laser ablation (EVLA) of varicose veins due to small saphenous vein reflux. Eur J Vasc Endovasc Surg. 2007;33:614-618.
19. Gibson KD, Ferris BL, Polissar N, et al. Endovenous laser treatment of the short saphenous vein: efficacy and complications. J Vasc Surg. 2007;45:795-803.
20. Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg. 2002;35:958-965.
21. Lurie F, Creton D, Eklof B, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg. 2005;29:67-73.
22. Hinchcliffe RJ, Ubhi J, Beech A, et al. A prospective randomized controlled trial of VNUS closure versus surgery for the treatment of recurrent long saphenous varicose veins. Eur J Vasc Endovasc Surg. 2006;31:212-218.
23. Darwood RJ, Theivacumar N, Dellagrammaticas D, et al. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg. 2008;95:294-301.
24. Sadick NS. Advances in the treatment of varicose veins: ambulatory phlebectomy, foam sclerotherapy, endovascular laser, and radiofrequency closure. Dermatol Clin. 2005;23:443-455.
25. Kakkos SK, Bountouroglou DG, Azzam M, et al. Effectiveness and safety of ultrasound-guided foam sclerotherapy for recurrent varicose veins: immediate results. J Endovasc Ther. 2006;13:357-364.
26. Cabrera J, Cabrera A, Jr, Garcia-Olmedo A. Treatment of varicose long saphenous veins with sclerosant in microfoam form: long-term outcomes. Phlebology. 2000;15:19-23.
27. Jia X, Mowatt G, Burr JM, et al. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;10:307-1308.
28. Belcaro G, Cesarone MR, Di Renzo A, et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO trial). Angiology. 2003;54:307-315.
29. van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
1. McLafferty R, Lohr J, Caprini J, et al. Results of the national pilot screening program for venous disease by the American Venous Forum. J Vasc Surg. 2007;45:142-148.
2. Hagen MD, Johnson ED, Adelman A. What treatments are effective for varicose veins? J Fam Pract. 2003;52:329-331.
3. Partsch H, Flour M, Smith PC. Indications for compression therapy in venous and disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. Int Angiol. 2008;27:193-219.
4. Partsch H. ed. Evidence-based compression therapy. VASA. 2003;32(suppl 63):S3-S39.
5. Fischer R, Chandler JG, de Maeseneer MG, et al. The unresolved problem of recurrent saphenofemoral reflux. J Am Coll Surg. 2002;195:80-94.
6. Perrin MR. Recurrent varices after surgery (REVAS), a consensus document. Cardiovasc Surg. 2000;8:233-245.
7. Perrin MR, Labropoulos M, Leon L. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;43:327-334.
8. Navarro L, Min R, Boné C. Endovenous laser: a new minimally invasive method of treatment for varicose veins—preliminary observations using an 810 nm diode laser. Dermatol Surg. 2001;27:117-122.
9. Min R, Zimmet S, Isaacs M, et al. Endovenous laser treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol. 2001;12:1167-1171.
10. Mundy L, Merlin TL, Fitridge RA, et al. Systematic review of endovenous laser treatment for varicose veins. Br J Surg. 2005;92:1189-1194.
11. van den Bos RR, Kockaert MA, Neumann HAM, et al. Technical review of endovenous laser therapy for varicose veins. Eur J Vasc Endovasc Surg. 2008;35:88-95.
12. Proebstle TM, Moehler T, Gul D, et al. Endovenous treatment of the great saphenous vein using a 1320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31:1380-1385.
13. Pichot O, Kabnick LS, Creton D, et al. Duplex ultrasound scan findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg. 2004;39:189-195.
14. Merchant RF, Pichot O, Myers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31:129-134.
15. Kabnick LS. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006;43:88-93.
16. Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991-996.
17. Proebstle TM, Gul D, Kargl A, et al. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg. 2003;29:357-361.
18. Theivacumar NS, Beale RJ, Mavor AID, et al. Initial experience in endovenous laser ablation (EVLA) of varicose veins due to small saphenous vein reflux. Eur J Vasc Endovasc Surg. 2007;33:614-618.
19. Gibson KD, Ferris BL, Polissar N, et al. Endovenous laser treatment of the short saphenous vein: efficacy and complications. J Vasc Surg. 2007;45:795-803.
20. Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg. 2002;35:958-965.
21. Lurie F, Creton D, Eklof B, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg. 2005;29:67-73.
22. Hinchcliffe RJ, Ubhi J, Beech A, et al. A prospective randomized controlled trial of VNUS closure versus surgery for the treatment of recurrent long saphenous varicose veins. Eur J Vasc Endovasc Surg. 2006;31:212-218.
23. Darwood RJ, Theivacumar N, Dellagrammaticas D, et al. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg. 2008;95:294-301.
24. Sadick NS. Advances in the treatment of varicose veins: ambulatory phlebectomy, foam sclerotherapy, endovascular laser, and radiofrequency closure. Dermatol Clin. 2005;23:443-455.
25. Kakkos SK, Bountouroglou DG, Azzam M, et al. Effectiveness and safety of ultrasound-guided foam sclerotherapy for recurrent varicose veins: immediate results. J Endovasc Ther. 2006;13:357-364.
26. Cabrera J, Cabrera A, Jr, Garcia-Olmedo A. Treatment of varicose long saphenous veins with sclerosant in microfoam form: long-term outcomes. Phlebology. 2000;15:19-23.
27. Jia X, Mowatt G, Burr JM, et al. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;10:307-1308.
28. Belcaro G, Cesarone MR, Di Renzo A, et al. Foam-sclerotherapy, surgery, sclerotherapy, and combined treatment for varicose veins: a 10-year, prospective, randomized, controlled, trial (VEDICO trial). Angiology. 2003;54:307-315.
29. van den Bos R, Arends L, Kockaert M, et al. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
EYE ON THE ELDERLY—Hypertension care: Striking the proper balance
- Treat systolic hypertension in the elderly to reduce their risk of cardiovascular events and mortality (B).
- Don’t shy away from treating the very old. Hypertension treatment is beneficial even in patients who are 80 years of age or older (B).
- Don’t prescribe an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker for elderly patients without heart failure; the combination increases the risk of adverse effects without reducing cardiovascular events (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Esther V. is an 81-year-old Caucasian woman who has been in your practice for a number of years. Her history is significant for a hip fracture 5 years ago and a subsequent diagnosis of osteoporosis. But she’s still able to live on her own, and takes only 1 medication—alendronate 70 mg once a week. Today Mrs. V.’s blood pressure is 150/80 mm Hg, and a chart review indicates that it has been in that range for the past 2 years. In view of her history and her age, would you proceed with treatment, and if so, how would you address her elevated systolic pressure?
Treating high blood pressure in elderly patients like Esther V. is a complicated proposition: While it’s important to mitigate hypertension’s negative effects, physicians need to be mindful that for this patient population, antihypertensive therapy itself involves elevated risks and unique concerns. These include:
- drug-drug interactions, exacerbated by the multiple medications that many older patients take;
- the side effect profile of antihypertensive agents, including orthostatic hypotension and the possibility of exacerbating an already heightened risk of falls; and
- uncertainty about when to initiate therapy, which drugs to choose if a second antihypertensive agent is needed, and when (or whether) a patient is too old to benefit from treatment.
Noncompliance is another potential complication in the elderly, who may have difficulty following a drug regimen or avoid prescription medications because they’re worried about the adverse effects—or the cost—of the drugs.
Thus, it’s not surprising that hypertension so often remains under- or untreated in older patients, a problem highlighted by a 2003 review in which only 27% of elderly patients were found to have reached their blood pressure goal.1
Despite the difficulty of adequately addressing hypertension in this patient population, the benefits do outweigh the risks. The following practical guide will help you overcome common barriers to treatment, avoid dangerous drug combinations, and customize your patient’s care to maximum benefit.
Treat hypertension, regardless of age
The Seventh Report of the Joint National Committee on Prevention, Evaluation, and Treatment of Hypertension (JNC 7) recommends a goal of <140/90 mm Hg for most individuals, and <130/80 mm Hg for those with diabetes mellitus or chronic kidney disease.2 But as patients age, presentations like that of Mrs. V.—elevated systolic pressure and normal diastolic pressure—are increasingly common. Isolated systolic hypertension is thought to be a consequence of the aging process, which results in a reduction in elasticity and compliance of the large arteries, degradation of arterial elastin, and atherosclerosis-associated accumulation of arterial calcium and collagen.3
Certainly, we know that hypertension of any kind is a major risk factor for a number of conditions with significant morbidity and mortality, including coronary artery disease,4 stroke,5 and heart failure.6 In the elderly, treatment of systolic hypertension, even in the absence of diastolic hypertension, has been proven to reduce cardiovascular and renal disease and death.7-9
Extending lives: What the evidence shows
Treating the very old. Hypertension in the Very Elderly (HYVET), the most recent trial, randomized more than 3800 hypertensive patients over the age of 80 in Europe, China, Australia, and Tunisia to receive either a diuretic or placebo. After 2 years, the treatment group had significant reductions in fatal and nonfatal stroke (number needed to treat [NNT]=19), all-cause mortality (NNT=8), cardiovascular death (NNT=15), and heart failure (NNT=67).10
A subset of this trial (HYVET-COG) reviewed the effects of antihypertensive therapy on the development of dementia in the very old. The researchers did not find a statistically significant reduction in the incidence of dementia in the treatment group. But when the HYVET-COG data were combined in a meta-analysis with data from 3 other antihypertensive trials in the elderly, treatment for hypertension was associated with a 13% relative risk reduction for dementia.11 Despite a major limitation of the HYVET trial—participants were typically healthier than the general population within their age group—the findings highlight the benefits of treating hypertension even in the very old.
Treating systolic hypertension. A Cochrane review conducted more than 10 years ago to assess the effectiveness of treating systolic hypertension in otherwise healthy older patients found that treatment reduced cardiovascular morbidity and mortality by 53 events per 1000 patient-years.12 Several prospective, double-blind, randomized, placebo-controlled studies found that treatment of systolic hypertension in healthy elderly patients reduced the incidence of total mortality (NNT=59), fatal and nonfatal cardiovascular events (NNT=26), and fatal and nonfatal stroke (NNT=79).13
Getting past common barriers to treatment
Despite these findings, many elderly patients with hypertension do not receive adequate treatment, often because of clinicians’ concerns about uncertain parameters and treatment risks. We’ve identified some common barriers and suggested ways to get beyond them.
BARRIER: Unclear parameters, concerns about aggressive Tx
There is little doubt of the value of treating stage 2 systolic hypertension (≥160 mm Hg) in the elderly. Not so for stage 1 systolic hypertension (140-159 mm Hg): A 2004 systematic review of studies evaluating the treatment of elevated systolic pressure in this patient population concluded that evidence for treating stage 1 systolic hypertension was not as strong.14
Observational studies suggesting a link between aggressive blood pressure-lowering and increased mortality in elderly patients have also been a cause for concern among some physicians.15,16 The INDANA meta-analysis, conducted in 1999, reviewed data from patients 80 years of age and older enrolled in antihypertensive trials and found a nonsignificant increase in death rates among patients who received treatment.17
How to respond? Carefully weigh the potential benefits of antihypertensive therapy in view of the overall health, functional status, and risks of side effects for a particular patient. The findings of the INDANA meta-analysis, while not statistically significant, serve as a reminder of the need to individualize treatment.
In the absence of a definitive treatment approach, it is also important to discuss the options with the patient. The authors of the systematic review that evaluated the benefits of treatment for stage 1 systolic hypertension recommended that antihypertensive therapy be based, in part, on patient preference and tolerance of therapy.14
BARRIER: Risks associated with multiple medications
The concurrent use of multiple medications, which is very common in the elderly, can interfere with treatment of hypertension in 2 ways: Some drugs have the potential to cause (or exacerbate) elevated blood pressure. Others may counter the effects of antihypertensive therapy.
Among the drugs that contribute to hypertension are the sympathomimetic agents found in many over-the-counter (OTC) cough and cold preparations in which the mechanism of action is direct vasoconstriction.18 Tricyclic antidepressants, which are commonly used to treat pain syndromes in the elderly, have also been shown to raise both systolic and diastolic pressure.19
On the other hand, nonsteroidal anti-inflammatory drugs (NSAIDs), often used by elderly patients to treat osteoarthritis, may counter the effects of many antihypertensive agents. Both OTC and prescription NSAIDs inhibit cyclo-oxygenase-2 (COX-2) in the kidneys, resulting in a reduction in sodium excretion and an increase in plasma volume.20
How to respond? Take a thorough medication history, including OTC and prescription drugs and supplements, before initiating treatment. Caution patients to avoid NSAIDs, OTC cough and cold preparations, and excessive caffeine, all of which may elevate their blood pressure. Remind patients that any new medication prescribed by another physician may affect their blood pressure, and advise them to maintain a current list of all medications to be reviewed at each visit.
BARRIER: Concerns about side effects
The elderly are more susceptible to orthostatic hypotension than younger patients because of age-related decreases in the baroreflex.21 Estimates suggest that anywhere from 5% to 50% of individuals older than 65 years are affected.21 Orthostatic hypotension, a side effect of many antihypertensive agents, is associated with higher rates of dizziness, syncope, and falls—a particular concern in elderly patients with a history of osteoporosis, pathological fracture, or gait dysfunction (See “Screening for hearing loss, risk of falls”).
How to respond? Before initiating antihypertensive therapy, take your patient’s blood pressure in both a sitting and standing position. Educate the patient and his or her family about the presentation of orthostatic hypotension, and advise the patient to stop taking the new medication and contact you if he or she develops the associated signs and symptoms. Schedule frequent follow-up appointments to closely monitor the effects of antihypertensive therapy, as well.
Stress the importance of lifestyle interventions
JNC 7 recommends several nonpharmacological treatments for all hypertensive patients, regardless of age. These include:
- healthy diet (such as Dietary Approaches to Stop Hypertension [DASH])
- sodium restriction (daily intake of <100 mmol/d)
- limited alcohol intake
- weight reduction (a decrease of 5-20 mm Hg for every 10 kg weight loss has been reported)
- regular physical activity (reported decrease of 4-9 mm Hg).2
No long-term trials have assessed the effects of lifestyle modification on morbidity and mortality. A subset of patients in the original DASH trial with stage 1 systolic hypertension did benefit from the diet, but the subgroup was small and the median age was 54.7 years.22 No large-scale studies have addressed the effectiveness of the diet in older patients with systolic hypertension.
Customizing therapy: What to consider
Evidence supports the use of various classes of antihypertensive medications in the treatment of high blood pressure, including thiazide diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs).10,23-27 JNC 7 recommends starting with 1 medication for patients with stage 1 hypertension (140-159/90-99 mm Hg) and 2 medications for patients with stage 2 hypertension (≥160/100 mm Hg).2 As previously noted, however, clear-cut evidence in support of treating elderly patients with stage 1 hypertension is lacking.
When you do initiate treatment for an elderly patient, begin with a low dose and titrate slowly, carefully monitoring for side effects. If 2 antihypertensive agents are needed, start 1 agent at a time in patients who are at risk for significant side effects. The type of antihypertensive agent you prescribe should be based on your patient’s health status, comorbidities, and treatment for other conditions, as well as on the known effects of the particular class of drugs (TABLE).
Thiazide diuretics. JNC 7 recommends thiazide diuretics as first-line treatment based on cost and effectiveness. Because thiazide diuretics decrease the urinary excretion of calcium, JNC 7 recommends their use in patients with osteoporosis.2
But potential side effects, such as dehydration, orthostatic hypotension, and hypokalemia, are more common in elderly patients, and may limit the use of these drugs in clinical practice. NSAIDs may reduce the antihypertensive effects of thiazide diuretics through a decrease in sodium excretion and an increase in plasma volume, and should be used with caution—if at all—in this patient population.20
Hydrochlorothiazide, a commonly used thiazide diuretic, should not be used in doses >50 mg/d because higher doses increase the risk of side effects without increasing efficacy.28 In the Systolic Hypertension in the Elderly Program (SHEP) study, the positive effects of another thiazide diuretic—chlorthalidone—were lost in elderly patients with serum potassium <3.5 mmol/L, so serum electrolytes should be routinely assessed during therapy.2,7 Thiazide diuretics lose their effectiveness in patients with a glomerular filtration rate of <30 mL/min/1.73 m2 and should not be used in this group.29
Beta-blockers. Beta-blockers are indicated in patients with a previous history of myocardial infarction, as they have been shown to decrease the rate of new coronary events.30 Other comorbidities that may be mitigated by beta-blocker administration include angina pectoris, atrial fibrillation with a rapid ventricular rate, compensated heart failure, preoperative hypertension, and essential tremor.2
Side effects of beta-blockers include sedation, depression, sexual dysfunction, bradycardia, conduction abnormalities, and exacerbation of severe reactive airway disease.2 Central nervous system effects of beta-blockers tend to be lowest in hydrophilic agents, such as atenolol, and highest in lipophilic agents, such as propranolol, which readily cross the blood-brain barrier.31
A 2007 Cochrane review looked at the effectiveness of beta-blockers as first-line agents in uncomplicated hypertension and concluded that the evidence does not support their use. The reviewers noted, however, that the majority of the trials used atenolol and cautioned that the results might not apply to other beta-blockers—or to subgroups, such as the elderly.32
ACE inhibitors and ARBs. Both ACE inhibitors and ARBs inhibit the renin-angiotensin-aldosterone pathway, and both classes are indicated in patients with diabetes, chronic kidney disease, or heart failure.2 Cough is a side effect of ACE inhibitors that affects 5% to 35% of patients,33 but angioedema—estimated to occur in 0.1% to 0.7% of patients taking ACE inhibitors—is the most serious side effect.34
An elevation in serum potassium and serum creatinine may occur after starting an ACE inhibitor in patients with heart failure, dehydration, or significant renal insufficiency, so assess serum creatinine and potassium 1 week after initiating therapy. An increase in serum creatinine >30% may require discontinuation or dose reduction.2,35
Elderly patients who take NSAIDs while on ACE inhibitors are at particular risk for acute renal failure; reviews of iatrogenic acute renal failure in the elderly have shown that most cases are related to the concurrent use of these medications.36,37 This is yet another reason to avoid giving NSAIDs to elderly patients with hypertension, particularly if they’re taking diuretics or ACE inhibitors.
Beware of ACE inhibitor-ARB combinations. The American College of Cardiology and the American Heart Association recommend substituting an ARB if side effects from an ACE inhibitor occur in patients with heart failure, but extreme caution is required if the patient experienced ACE inhibitor-associated angioedema.38 TRANSCEND, a randomized controlled trial of almost 6000 patients, specifically studied the use of ARBs in patients unable to tolerate ACE inhibitors and concluded that they can be safely given to patients who had side effects from ACE inhibitors.39
Since ACE inhibitors and ARBs affect the renin-angiotensin-aldosterone pathway at different points, there has been interest in the effectiveness of combining these agents. The CHARM-Added trial found that the combination of these agents reduced both cardiovascular events and mortality in patients with heart failure.40
Combining ACE inhibitors and ARBs in patients without heart failure was evaluated in the ONTARGET trial. While the combination treatment group had a greater reduction in blood pressure, there was no significant cardiovascular benefit over patients who were on ACE inhibitors alone. But there was an increase in hypotension, syncope, and renal dysfunction among those in the combination treatment group, all of which are significant concerns in the elderly. The authors concluded that the ACE inhibitor-ARB combination should not be used in patients who do not have heart failure.41
Calcium channel blockers. CCBs block the entrance of calcium into vascular cells, producing dilation in the coronary arteries and peripheral vasculature, and are effective in the treatment of hypertension in elderly patients.42 Nondihydropyridine CCBs (diltiazem and verapamil) are useful in patients with atrial fibrillation and supraventricular tachycardia because of their negative chronotropic effects.2 And African American patients respond better to CCBs—with a greater reduction in blood pressure and cardiovascular complications—than to ACE inhibitors.2,25
Dihydropyridine CCBs (amlodipine and long-acting felodipine and nifedipine) are safe in patients with heart failure or chronic stable angina,43 but short-acting nifedipine has been found to increase the risk of mortality.44
Dihydropyridine CCBs have been reported to cause peripheral edema in 7% to 8% of patients taking long-acting nifedipine and up to 16% of elderly patient taking amlodipine.45 Verapamil has the highest incidence of constipation, and should be avoided in elderly patients.2
TABLE
Which drugs, for which patients?2
| DRUG CLASS* | INDICATIONS | CONSIDERATIONS |
|---|---|---|
| ACE inhibitors | Heart failure, post-MI, diabetes, high risk for CAD, chronic kidney disease, recurrent stroke prevention | Avoid concurrent use of ARBs in patients without heart failure. Avoid concurrent use of NSAIDs. If a second drug is needed, add a thiazide diuretic or CCB. |
| ARBs | Heart failure, diabetes, chronic kidney disease | Avoid concurrent use of ACE inhibitors in patients who don’t have heart failure. |
| Beta-blockers | Heart failure, post-MI, high risk for CAD, diabetes | Not recommended as first-line therapy for uncomplicated hypertension. |
| CCBs | High risk for CAD, diabetes | Avoid prescribing verapamil for elderly patients. |
| Thiazide diuretics | Heart failure, high risk for CAD, diabetes, recurrent stroke prevention | Avoid concurrent use of NSAIDs. Will not work with reduced GFR or hypokalemia. Use with caution in patients prone to orthostatic hypotension. |
| *Peripheral alpha-blockers, centrally acting alpha-agonists, and vasodilators should not be routinely used to treat hypertension in elderly patients. | ||
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CCB, calcium channel blocker; GFR, glomerular filtration rate; MI, myocardial infarction; NSAIDs, nonsteroidal anti-inflammatory drugs. | ||
Steer clear of these drugs
Significant side effects, including hypotension, sedation, depression, and dry mouth, limit the use of other antihypertensives, such as peripheral alpha blockers, centrally acting agents, and vasodilators, in elderly patients.2 In the ALLHAT trial, the alpha-blocker arm was stopped prematurely because of an increased rate of cardiovascular events and heart failure compared with the thiazide diuretic arm.25 That finding led to the recommendation that alpha-blockers not be used as first-line agents for treating hypertension.
Centrally acting alpha-agonists (clonidine, methyldopa, and reserpine) have a high incidence of sedation, dry mouth, and depression, and elderly patients are more likely to experience orthostatic hypotension and rebound hypertension if these agents are discontinued abruptly or doses are missed.2 Vasodilators, including minoxidil and hydralazine, cause sodium and fluid retention and reflex tachycardia.2 Because of their unfavorable side effects and lack of outcomes data, alpha-blockers, centrally acting alpha-agonists, and vasodilators should not be used routinely for the treatment of hypertension in elderly patients.
Considerations in selecting a second agent
Patients who fail to achieve adequate blood pressure control despite lifestyle modifications and a single agent, and those who are initially diagnosed with stage 2 hypertension, will require 2 or more medications. An elderly patient who has not responded to a single agent should be asked about any other medications (or substances) that might be interfering with the antihypertensive agent, especially NSAIDs, tricyclic antidepressants, sympathomimetics, and caffeine. Find out, too, whether the patient is following the prescribed regimen.
When you prescribe 2 antihypertensives, choose agents with complementary mechanisms of action—a diuretic and an ACE inhibitor, for example, or an ACE inhibitor and a CCB. In addition to avoiding an ACE/ARB combination in patients who do not have heart failure, avoid concurrent use of beta-blockers and ACE inhibitors46 and a dihydropyridine CCB/thiazide diuretic combination47 in all elderly patients.14
How does the evidence apply to your patient?
As we saw earlier, Esther V. has isolated systolic hypertension, which increases her risk of myocardial infarction, stroke, and heart failure. Even though she is 81 years old, studies such as HYVET support the treatment of her hypertension. Certainly, you’ll encourage her to limit her salt intake, follow a healthy diet, and exercise daily, as tolerated.
But because Mrs. V. has stage 1 hypertension, her preference and her ability to tolerate therapy will play a key role in the decision to initiate treatment. If her blood pressure does not respond to lifestyle modifications and she is agreeable to medical therapy, you would recommend 1 antihypertensive agent.
Which drug class is best? Given her history of osteoporosis, a low-dose thiazide diuretic might be a reasonable choice. But because she has a history of hip fracture from a fall, it would be prudent to avoid agents associated with volume depletion —and to opt for either an ACE inhibitor or a CCB as first-line therapy instead. It is extremely important to obtain a list of any OTC medications Mrs. V. is currently taking and to advise her to avoid the use of NSAIDs.
You would also want to obtain the results of Mrs. V.’s most recent bone mineral density test, and talk to her about the importance of taking calcium with vitamin D. If there are concerns about her gait, you would consider a formal gait evaluation, as well. Finally, you would review potential side effects, including orthostatic hypotension, and tell Mrs. V. to call if they occur, and schedule an appointment to monitor her serum potassium and renal function in a few weeks.
Correspondence Robert C. Langan, MD, St. Luke’s Family Medicine Residency Program, 2830 Easton Avenue, Bethlehem, PA 18017; [email protected]
1. Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003-1010.
2. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Yambe M, Tomiyama H, Yamada J, et al. Arterial stiffness and progression to hypertension in Japanese male subjects with high normal blood pressure. J Hypertens. 2007;25:87-93.
4. Lewington S, Clarke R, Quizilbash N, et al. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
5. He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138(Pt 2):211-219.
6. Levy D, Larson MG, Varsan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557-1562.
7. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP): SHEP Cooperative Research Group. JAMA. 1991;265:3255-3264.
8. Staessen JA, Fargard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension: the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757-764.
9. Izzo JL, Levy D, Black HR. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35:1021-1024.
10. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
11. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683-689.
12. Mulrow C, Lau J, Cornell J, et al. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev. 1998;(2):CD000028.-
13. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000;355:865-872.
14. Chaundry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA. 2004;292:1074-1080.
15. Somes GW, Pahor M, Shorr RI, et al. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004-2009.
16. Rastas S, Pirttila T, Viramo P, et al. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc. 2006;54:912-918.
17. Gueyffier F, Bulpitt C, Boissel JP. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. Lancet. 1999;353:793-796.
18. Pimenta E, Gaddam KK, Oprail S. Mechanisms and treatment of resistant hypertension. J Clin Hypertens. 2008;10:239-244.
19. Licht CM, de Gues EJ, Seldenrjik A. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631-638.
20. White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension. 2007;49:408.-
21. Weiss A, Grossman E, Belooseky Y, et al. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med. 2002;162:2369-2374.
22. Moore TJ, Conlin PR, Ard J, et al. DASH (Dietary Approached to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension. 2001;38:155-158.
23. Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751-1756.
24. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction study (LIFE) : a randomised trial against atenolol. Lancet. 2002;359:995-1003.
25. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
26. Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs. a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. JAMA. 2003;290:2805-2816.
27. Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583-592.
28. Black HR. The evolution of low-dose diuretic therapy: the lessons from clinical trials. Am J Med. 1996;101(suppl 3A):S47-S52.
29. National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Hypertensive Agents in Chronic Kidney Disease. Guideline 12: Use of Diuretics in CKD. Available at http://www.kidney.org/Professionals/Kdoqi/Guidelines_Bp/guide_12.htm. Accessed May 11, 2009.
30. Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina). J Am Coll Cardiol. 2003;41:159-168.
31. McAinsh J, Cruickshank JM. Beta-blockers and central nervous system side effects. Pharmacol Ther. 1990;46:163-197.
32. Wiysonge CSU, Bradley HA, Mayosi BM, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2007(1):CD002003.-
33. Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP Evidence-Based Clinical Practice Guidelines. Chest. 2006;129(suppl 1):S169-S173.
34. Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med. 2002;347:621-622.
35. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
36. Thomas MC. Diuretics, ACE inhibitors and NSAIDS-the triple whammy. Med J Aust. 2000;172:184-185.
37. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797-805.
38. Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977-2016.
39. The TRANSCEND investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174-1183.
40. McMurray JJ, Ostergren J, Swedberg K, et al. For the CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
41. Yusuf S, Teo KK, Pogue J, et al. For the ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
42. Malacco E, Mancia G, Rappelli A, et al. Treatment of isolated systolic hypertension: the SHELL study results. Blood Press. 2003;12:160-167.
43. Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med. 1996;335:1107-1114.
44. Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620-625.
45. Sirker A, Missouris CG, MacGregor GA. Dihydropyridine calcium channel blockers and peripheral side effects. J Human Hypertens. 2001;15:745-746.
46. MacGregor GA, Markandu ND, Banks RA, et al. Captopril in essential hypertension: contrasting effects of adding hydrochlorothiazide or propranolol. BMJ. 1982;284:693-696.
47. MacGregor GA, Cappuccio FP. Lack of effect of a diuretic added to diltiazem. J Hum Hypertens. 1997;11:249-250.
- Treat systolic hypertension in the elderly to reduce their risk of cardiovascular events and mortality (B).
- Don’t shy away from treating the very old. Hypertension treatment is beneficial even in patients who are 80 years of age or older (B).
- Don’t prescribe an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker for elderly patients without heart failure; the combination increases the risk of adverse effects without reducing cardiovascular events (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Esther V. is an 81-year-old Caucasian woman who has been in your practice for a number of years. Her history is significant for a hip fracture 5 years ago and a subsequent diagnosis of osteoporosis. But she’s still able to live on her own, and takes only 1 medication—alendronate 70 mg once a week. Today Mrs. V.’s blood pressure is 150/80 mm Hg, and a chart review indicates that it has been in that range for the past 2 years. In view of her history and her age, would you proceed with treatment, and if so, how would you address her elevated systolic pressure?
Treating high blood pressure in elderly patients like Esther V. is a complicated proposition: While it’s important to mitigate hypertension’s negative effects, physicians need to be mindful that for this patient population, antihypertensive therapy itself involves elevated risks and unique concerns. These include:
- drug-drug interactions, exacerbated by the multiple medications that many older patients take;
- the side effect profile of antihypertensive agents, including orthostatic hypotension and the possibility of exacerbating an already heightened risk of falls; and
- uncertainty about when to initiate therapy, which drugs to choose if a second antihypertensive agent is needed, and when (or whether) a patient is too old to benefit from treatment.
Noncompliance is another potential complication in the elderly, who may have difficulty following a drug regimen or avoid prescription medications because they’re worried about the adverse effects—or the cost—of the drugs.
Thus, it’s not surprising that hypertension so often remains under- or untreated in older patients, a problem highlighted by a 2003 review in which only 27% of elderly patients were found to have reached their blood pressure goal.1
Despite the difficulty of adequately addressing hypertension in this patient population, the benefits do outweigh the risks. The following practical guide will help you overcome common barriers to treatment, avoid dangerous drug combinations, and customize your patient’s care to maximum benefit.
Treat hypertension, regardless of age
The Seventh Report of the Joint National Committee on Prevention, Evaluation, and Treatment of Hypertension (JNC 7) recommends a goal of <140/90 mm Hg for most individuals, and <130/80 mm Hg for those with diabetes mellitus or chronic kidney disease.2 But as patients age, presentations like that of Mrs. V.—elevated systolic pressure and normal diastolic pressure—are increasingly common. Isolated systolic hypertension is thought to be a consequence of the aging process, which results in a reduction in elasticity and compliance of the large arteries, degradation of arterial elastin, and atherosclerosis-associated accumulation of arterial calcium and collagen.3
Certainly, we know that hypertension of any kind is a major risk factor for a number of conditions with significant morbidity and mortality, including coronary artery disease,4 stroke,5 and heart failure.6 In the elderly, treatment of systolic hypertension, even in the absence of diastolic hypertension, has been proven to reduce cardiovascular and renal disease and death.7-9
Extending lives: What the evidence shows
Treating the very old. Hypertension in the Very Elderly (HYVET), the most recent trial, randomized more than 3800 hypertensive patients over the age of 80 in Europe, China, Australia, and Tunisia to receive either a diuretic or placebo. After 2 years, the treatment group had significant reductions in fatal and nonfatal stroke (number needed to treat [NNT]=19), all-cause mortality (NNT=8), cardiovascular death (NNT=15), and heart failure (NNT=67).10
A subset of this trial (HYVET-COG) reviewed the effects of antihypertensive therapy on the development of dementia in the very old. The researchers did not find a statistically significant reduction in the incidence of dementia in the treatment group. But when the HYVET-COG data were combined in a meta-analysis with data from 3 other antihypertensive trials in the elderly, treatment for hypertension was associated with a 13% relative risk reduction for dementia.11 Despite a major limitation of the HYVET trial—participants were typically healthier than the general population within their age group—the findings highlight the benefits of treating hypertension even in the very old.
Treating systolic hypertension. A Cochrane review conducted more than 10 years ago to assess the effectiveness of treating systolic hypertension in otherwise healthy older patients found that treatment reduced cardiovascular morbidity and mortality by 53 events per 1000 patient-years.12 Several prospective, double-blind, randomized, placebo-controlled studies found that treatment of systolic hypertension in healthy elderly patients reduced the incidence of total mortality (NNT=59), fatal and nonfatal cardiovascular events (NNT=26), and fatal and nonfatal stroke (NNT=79).13
Getting past common barriers to treatment
Despite these findings, many elderly patients with hypertension do not receive adequate treatment, often because of clinicians’ concerns about uncertain parameters and treatment risks. We’ve identified some common barriers and suggested ways to get beyond them.
BARRIER: Unclear parameters, concerns about aggressive Tx
There is little doubt of the value of treating stage 2 systolic hypertension (≥160 mm Hg) in the elderly. Not so for stage 1 systolic hypertension (140-159 mm Hg): A 2004 systematic review of studies evaluating the treatment of elevated systolic pressure in this patient population concluded that evidence for treating stage 1 systolic hypertension was not as strong.14
Observational studies suggesting a link between aggressive blood pressure-lowering and increased mortality in elderly patients have also been a cause for concern among some physicians.15,16 The INDANA meta-analysis, conducted in 1999, reviewed data from patients 80 years of age and older enrolled in antihypertensive trials and found a nonsignificant increase in death rates among patients who received treatment.17
How to respond? Carefully weigh the potential benefits of antihypertensive therapy in view of the overall health, functional status, and risks of side effects for a particular patient. The findings of the INDANA meta-analysis, while not statistically significant, serve as a reminder of the need to individualize treatment.
In the absence of a definitive treatment approach, it is also important to discuss the options with the patient. The authors of the systematic review that evaluated the benefits of treatment for stage 1 systolic hypertension recommended that antihypertensive therapy be based, in part, on patient preference and tolerance of therapy.14
BARRIER: Risks associated with multiple medications
The concurrent use of multiple medications, which is very common in the elderly, can interfere with treatment of hypertension in 2 ways: Some drugs have the potential to cause (or exacerbate) elevated blood pressure. Others may counter the effects of antihypertensive therapy.
Among the drugs that contribute to hypertension are the sympathomimetic agents found in many over-the-counter (OTC) cough and cold preparations in which the mechanism of action is direct vasoconstriction.18 Tricyclic antidepressants, which are commonly used to treat pain syndromes in the elderly, have also been shown to raise both systolic and diastolic pressure.19
On the other hand, nonsteroidal anti-inflammatory drugs (NSAIDs), often used by elderly patients to treat osteoarthritis, may counter the effects of many antihypertensive agents. Both OTC and prescription NSAIDs inhibit cyclo-oxygenase-2 (COX-2) in the kidneys, resulting in a reduction in sodium excretion and an increase in plasma volume.20
How to respond? Take a thorough medication history, including OTC and prescription drugs and supplements, before initiating treatment. Caution patients to avoid NSAIDs, OTC cough and cold preparations, and excessive caffeine, all of which may elevate their blood pressure. Remind patients that any new medication prescribed by another physician may affect their blood pressure, and advise them to maintain a current list of all medications to be reviewed at each visit.
BARRIER: Concerns about side effects
The elderly are more susceptible to orthostatic hypotension than younger patients because of age-related decreases in the baroreflex.21 Estimates suggest that anywhere from 5% to 50% of individuals older than 65 years are affected.21 Orthostatic hypotension, a side effect of many antihypertensive agents, is associated with higher rates of dizziness, syncope, and falls—a particular concern in elderly patients with a history of osteoporosis, pathological fracture, or gait dysfunction (See “Screening for hearing loss, risk of falls”).
How to respond? Before initiating antihypertensive therapy, take your patient’s blood pressure in both a sitting and standing position. Educate the patient and his or her family about the presentation of orthostatic hypotension, and advise the patient to stop taking the new medication and contact you if he or she develops the associated signs and symptoms. Schedule frequent follow-up appointments to closely monitor the effects of antihypertensive therapy, as well.
Stress the importance of lifestyle interventions
JNC 7 recommends several nonpharmacological treatments for all hypertensive patients, regardless of age. These include:
- healthy diet (such as Dietary Approaches to Stop Hypertension [DASH])
- sodium restriction (daily intake of <100 mmol/d)
- limited alcohol intake
- weight reduction (a decrease of 5-20 mm Hg for every 10 kg weight loss has been reported)
- regular physical activity (reported decrease of 4-9 mm Hg).2
No long-term trials have assessed the effects of lifestyle modification on morbidity and mortality. A subset of patients in the original DASH trial with stage 1 systolic hypertension did benefit from the diet, but the subgroup was small and the median age was 54.7 years.22 No large-scale studies have addressed the effectiveness of the diet in older patients with systolic hypertension.
Customizing therapy: What to consider
Evidence supports the use of various classes of antihypertensive medications in the treatment of high blood pressure, including thiazide diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs).10,23-27 JNC 7 recommends starting with 1 medication for patients with stage 1 hypertension (140-159/90-99 mm Hg) and 2 medications for patients with stage 2 hypertension (≥160/100 mm Hg).2 As previously noted, however, clear-cut evidence in support of treating elderly patients with stage 1 hypertension is lacking.
When you do initiate treatment for an elderly patient, begin with a low dose and titrate slowly, carefully monitoring for side effects. If 2 antihypertensive agents are needed, start 1 agent at a time in patients who are at risk for significant side effects. The type of antihypertensive agent you prescribe should be based on your patient’s health status, comorbidities, and treatment for other conditions, as well as on the known effects of the particular class of drugs (TABLE).
Thiazide diuretics. JNC 7 recommends thiazide diuretics as first-line treatment based on cost and effectiveness. Because thiazide diuretics decrease the urinary excretion of calcium, JNC 7 recommends their use in patients with osteoporosis.2
But potential side effects, such as dehydration, orthostatic hypotension, and hypokalemia, are more common in elderly patients, and may limit the use of these drugs in clinical practice. NSAIDs may reduce the antihypertensive effects of thiazide diuretics through a decrease in sodium excretion and an increase in plasma volume, and should be used with caution—if at all—in this patient population.20
Hydrochlorothiazide, a commonly used thiazide diuretic, should not be used in doses >50 mg/d because higher doses increase the risk of side effects without increasing efficacy.28 In the Systolic Hypertension in the Elderly Program (SHEP) study, the positive effects of another thiazide diuretic—chlorthalidone—were lost in elderly patients with serum potassium <3.5 mmol/L, so serum electrolytes should be routinely assessed during therapy.2,7 Thiazide diuretics lose their effectiveness in patients with a glomerular filtration rate of <30 mL/min/1.73 m2 and should not be used in this group.29
Beta-blockers. Beta-blockers are indicated in patients with a previous history of myocardial infarction, as they have been shown to decrease the rate of new coronary events.30 Other comorbidities that may be mitigated by beta-blocker administration include angina pectoris, atrial fibrillation with a rapid ventricular rate, compensated heart failure, preoperative hypertension, and essential tremor.2
Side effects of beta-blockers include sedation, depression, sexual dysfunction, bradycardia, conduction abnormalities, and exacerbation of severe reactive airway disease.2 Central nervous system effects of beta-blockers tend to be lowest in hydrophilic agents, such as atenolol, and highest in lipophilic agents, such as propranolol, which readily cross the blood-brain barrier.31
A 2007 Cochrane review looked at the effectiveness of beta-blockers as first-line agents in uncomplicated hypertension and concluded that the evidence does not support their use. The reviewers noted, however, that the majority of the trials used atenolol and cautioned that the results might not apply to other beta-blockers—or to subgroups, such as the elderly.32
ACE inhibitors and ARBs. Both ACE inhibitors and ARBs inhibit the renin-angiotensin-aldosterone pathway, and both classes are indicated in patients with diabetes, chronic kidney disease, or heart failure.2 Cough is a side effect of ACE inhibitors that affects 5% to 35% of patients,33 but angioedema—estimated to occur in 0.1% to 0.7% of patients taking ACE inhibitors—is the most serious side effect.34
An elevation in serum potassium and serum creatinine may occur after starting an ACE inhibitor in patients with heart failure, dehydration, or significant renal insufficiency, so assess serum creatinine and potassium 1 week after initiating therapy. An increase in serum creatinine >30% may require discontinuation or dose reduction.2,35
Elderly patients who take NSAIDs while on ACE inhibitors are at particular risk for acute renal failure; reviews of iatrogenic acute renal failure in the elderly have shown that most cases are related to the concurrent use of these medications.36,37 This is yet another reason to avoid giving NSAIDs to elderly patients with hypertension, particularly if they’re taking diuretics or ACE inhibitors.
Beware of ACE inhibitor-ARB combinations. The American College of Cardiology and the American Heart Association recommend substituting an ARB if side effects from an ACE inhibitor occur in patients with heart failure, but extreme caution is required if the patient experienced ACE inhibitor-associated angioedema.38 TRANSCEND, a randomized controlled trial of almost 6000 patients, specifically studied the use of ARBs in patients unable to tolerate ACE inhibitors and concluded that they can be safely given to patients who had side effects from ACE inhibitors.39
Since ACE inhibitors and ARBs affect the renin-angiotensin-aldosterone pathway at different points, there has been interest in the effectiveness of combining these agents. The CHARM-Added trial found that the combination of these agents reduced both cardiovascular events and mortality in patients with heart failure.40
Combining ACE inhibitors and ARBs in patients without heart failure was evaluated in the ONTARGET trial. While the combination treatment group had a greater reduction in blood pressure, there was no significant cardiovascular benefit over patients who were on ACE inhibitors alone. But there was an increase in hypotension, syncope, and renal dysfunction among those in the combination treatment group, all of which are significant concerns in the elderly. The authors concluded that the ACE inhibitor-ARB combination should not be used in patients who do not have heart failure.41
Calcium channel blockers. CCBs block the entrance of calcium into vascular cells, producing dilation in the coronary arteries and peripheral vasculature, and are effective in the treatment of hypertension in elderly patients.42 Nondihydropyridine CCBs (diltiazem and verapamil) are useful in patients with atrial fibrillation and supraventricular tachycardia because of their negative chronotropic effects.2 And African American patients respond better to CCBs—with a greater reduction in blood pressure and cardiovascular complications—than to ACE inhibitors.2,25
Dihydropyridine CCBs (amlodipine and long-acting felodipine and nifedipine) are safe in patients with heart failure or chronic stable angina,43 but short-acting nifedipine has been found to increase the risk of mortality.44
Dihydropyridine CCBs have been reported to cause peripheral edema in 7% to 8% of patients taking long-acting nifedipine and up to 16% of elderly patient taking amlodipine.45 Verapamil has the highest incidence of constipation, and should be avoided in elderly patients.2
TABLE
Which drugs, for which patients?2
| DRUG CLASS* | INDICATIONS | CONSIDERATIONS |
|---|---|---|
| ACE inhibitors | Heart failure, post-MI, diabetes, high risk for CAD, chronic kidney disease, recurrent stroke prevention | Avoid concurrent use of ARBs in patients without heart failure. Avoid concurrent use of NSAIDs. If a second drug is needed, add a thiazide diuretic or CCB. |
| ARBs | Heart failure, diabetes, chronic kidney disease | Avoid concurrent use of ACE inhibitors in patients who don’t have heart failure. |
| Beta-blockers | Heart failure, post-MI, high risk for CAD, diabetes | Not recommended as first-line therapy for uncomplicated hypertension. |
| CCBs | High risk for CAD, diabetes | Avoid prescribing verapamil for elderly patients. |
| Thiazide diuretics | Heart failure, high risk for CAD, diabetes, recurrent stroke prevention | Avoid concurrent use of NSAIDs. Will not work with reduced GFR or hypokalemia. Use with caution in patients prone to orthostatic hypotension. |
| *Peripheral alpha-blockers, centrally acting alpha-agonists, and vasodilators should not be routinely used to treat hypertension in elderly patients. | ||
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CCB, calcium channel blocker; GFR, glomerular filtration rate; MI, myocardial infarction; NSAIDs, nonsteroidal anti-inflammatory drugs. | ||
Steer clear of these drugs
Significant side effects, including hypotension, sedation, depression, and dry mouth, limit the use of other antihypertensives, such as peripheral alpha blockers, centrally acting agents, and vasodilators, in elderly patients.2 In the ALLHAT trial, the alpha-blocker arm was stopped prematurely because of an increased rate of cardiovascular events and heart failure compared with the thiazide diuretic arm.25 That finding led to the recommendation that alpha-blockers not be used as first-line agents for treating hypertension.
Centrally acting alpha-agonists (clonidine, methyldopa, and reserpine) have a high incidence of sedation, dry mouth, and depression, and elderly patients are more likely to experience orthostatic hypotension and rebound hypertension if these agents are discontinued abruptly or doses are missed.2 Vasodilators, including minoxidil and hydralazine, cause sodium and fluid retention and reflex tachycardia.2 Because of their unfavorable side effects and lack of outcomes data, alpha-blockers, centrally acting alpha-agonists, and vasodilators should not be used routinely for the treatment of hypertension in elderly patients.
Considerations in selecting a second agent
Patients who fail to achieve adequate blood pressure control despite lifestyle modifications and a single agent, and those who are initially diagnosed with stage 2 hypertension, will require 2 or more medications. An elderly patient who has not responded to a single agent should be asked about any other medications (or substances) that might be interfering with the antihypertensive agent, especially NSAIDs, tricyclic antidepressants, sympathomimetics, and caffeine. Find out, too, whether the patient is following the prescribed regimen.
When you prescribe 2 antihypertensives, choose agents with complementary mechanisms of action—a diuretic and an ACE inhibitor, for example, or an ACE inhibitor and a CCB. In addition to avoiding an ACE/ARB combination in patients who do not have heart failure, avoid concurrent use of beta-blockers and ACE inhibitors46 and a dihydropyridine CCB/thiazide diuretic combination47 in all elderly patients.14
How does the evidence apply to your patient?
As we saw earlier, Esther V. has isolated systolic hypertension, which increases her risk of myocardial infarction, stroke, and heart failure. Even though she is 81 years old, studies such as HYVET support the treatment of her hypertension. Certainly, you’ll encourage her to limit her salt intake, follow a healthy diet, and exercise daily, as tolerated.
But because Mrs. V. has stage 1 hypertension, her preference and her ability to tolerate therapy will play a key role in the decision to initiate treatment. If her blood pressure does not respond to lifestyle modifications and she is agreeable to medical therapy, you would recommend 1 antihypertensive agent.
Which drug class is best? Given her history of osteoporosis, a low-dose thiazide diuretic might be a reasonable choice. But because she has a history of hip fracture from a fall, it would be prudent to avoid agents associated with volume depletion —and to opt for either an ACE inhibitor or a CCB as first-line therapy instead. It is extremely important to obtain a list of any OTC medications Mrs. V. is currently taking and to advise her to avoid the use of NSAIDs.
You would also want to obtain the results of Mrs. V.’s most recent bone mineral density test, and talk to her about the importance of taking calcium with vitamin D. If there are concerns about her gait, you would consider a formal gait evaluation, as well. Finally, you would review potential side effects, including orthostatic hypotension, and tell Mrs. V. to call if they occur, and schedule an appointment to monitor her serum potassium and renal function in a few weeks.
Correspondence Robert C. Langan, MD, St. Luke’s Family Medicine Residency Program, 2830 Easton Avenue, Bethlehem, PA 18017; [email protected]
- Treat systolic hypertension in the elderly to reduce their risk of cardiovascular events and mortality (B).
- Don’t shy away from treating the very old. Hypertension treatment is beneficial even in patients who are 80 years of age or older (B).
- Don’t prescribe an angiotensin-converting enzyme inhibitor and an angiotensin receptor blocker for elderly patients without heart failure; the combination increases the risk of adverse effects without reducing cardiovascular events (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Esther V. is an 81-year-old Caucasian woman who has been in your practice for a number of years. Her history is significant for a hip fracture 5 years ago and a subsequent diagnosis of osteoporosis. But she’s still able to live on her own, and takes only 1 medication—alendronate 70 mg once a week. Today Mrs. V.’s blood pressure is 150/80 mm Hg, and a chart review indicates that it has been in that range for the past 2 years. In view of her history and her age, would you proceed with treatment, and if so, how would you address her elevated systolic pressure?
Treating high blood pressure in elderly patients like Esther V. is a complicated proposition: While it’s important to mitigate hypertension’s negative effects, physicians need to be mindful that for this patient population, antihypertensive therapy itself involves elevated risks and unique concerns. These include:
- drug-drug interactions, exacerbated by the multiple medications that many older patients take;
- the side effect profile of antihypertensive agents, including orthostatic hypotension and the possibility of exacerbating an already heightened risk of falls; and
- uncertainty about when to initiate therapy, which drugs to choose if a second antihypertensive agent is needed, and when (or whether) a patient is too old to benefit from treatment.
Noncompliance is another potential complication in the elderly, who may have difficulty following a drug regimen or avoid prescription medications because they’re worried about the adverse effects—or the cost—of the drugs.
Thus, it’s not surprising that hypertension so often remains under- or untreated in older patients, a problem highlighted by a 2003 review in which only 27% of elderly patients were found to have reached their blood pressure goal.1
Despite the difficulty of adequately addressing hypertension in this patient population, the benefits do outweigh the risks. The following practical guide will help you overcome common barriers to treatment, avoid dangerous drug combinations, and customize your patient’s care to maximum benefit.
Treat hypertension, regardless of age
The Seventh Report of the Joint National Committee on Prevention, Evaluation, and Treatment of Hypertension (JNC 7) recommends a goal of <140/90 mm Hg for most individuals, and <130/80 mm Hg for those with diabetes mellitus or chronic kidney disease.2 But as patients age, presentations like that of Mrs. V.—elevated systolic pressure and normal diastolic pressure—are increasingly common. Isolated systolic hypertension is thought to be a consequence of the aging process, which results in a reduction in elasticity and compliance of the large arteries, degradation of arterial elastin, and atherosclerosis-associated accumulation of arterial calcium and collagen.3
Certainly, we know that hypertension of any kind is a major risk factor for a number of conditions with significant morbidity and mortality, including coronary artery disease,4 stroke,5 and heart failure.6 In the elderly, treatment of systolic hypertension, even in the absence of diastolic hypertension, has been proven to reduce cardiovascular and renal disease and death.7-9
Extending lives: What the evidence shows
Treating the very old. Hypertension in the Very Elderly (HYVET), the most recent trial, randomized more than 3800 hypertensive patients over the age of 80 in Europe, China, Australia, and Tunisia to receive either a diuretic or placebo. After 2 years, the treatment group had significant reductions in fatal and nonfatal stroke (number needed to treat [NNT]=19), all-cause mortality (NNT=8), cardiovascular death (NNT=15), and heart failure (NNT=67).10
A subset of this trial (HYVET-COG) reviewed the effects of antihypertensive therapy on the development of dementia in the very old. The researchers did not find a statistically significant reduction in the incidence of dementia in the treatment group. But when the HYVET-COG data were combined in a meta-analysis with data from 3 other antihypertensive trials in the elderly, treatment for hypertension was associated with a 13% relative risk reduction for dementia.11 Despite a major limitation of the HYVET trial—participants were typically healthier than the general population within their age group—the findings highlight the benefits of treating hypertension even in the very old.
Treating systolic hypertension. A Cochrane review conducted more than 10 years ago to assess the effectiveness of treating systolic hypertension in otherwise healthy older patients found that treatment reduced cardiovascular morbidity and mortality by 53 events per 1000 patient-years.12 Several prospective, double-blind, randomized, placebo-controlled studies found that treatment of systolic hypertension in healthy elderly patients reduced the incidence of total mortality (NNT=59), fatal and nonfatal cardiovascular events (NNT=26), and fatal and nonfatal stroke (NNT=79).13
Getting past common barriers to treatment
Despite these findings, many elderly patients with hypertension do not receive adequate treatment, often because of clinicians’ concerns about uncertain parameters and treatment risks. We’ve identified some common barriers and suggested ways to get beyond them.
BARRIER: Unclear parameters, concerns about aggressive Tx
There is little doubt of the value of treating stage 2 systolic hypertension (≥160 mm Hg) in the elderly. Not so for stage 1 systolic hypertension (140-159 mm Hg): A 2004 systematic review of studies evaluating the treatment of elevated systolic pressure in this patient population concluded that evidence for treating stage 1 systolic hypertension was not as strong.14
Observational studies suggesting a link between aggressive blood pressure-lowering and increased mortality in elderly patients have also been a cause for concern among some physicians.15,16 The INDANA meta-analysis, conducted in 1999, reviewed data from patients 80 years of age and older enrolled in antihypertensive trials and found a nonsignificant increase in death rates among patients who received treatment.17
How to respond? Carefully weigh the potential benefits of antihypertensive therapy in view of the overall health, functional status, and risks of side effects for a particular patient. The findings of the INDANA meta-analysis, while not statistically significant, serve as a reminder of the need to individualize treatment.
In the absence of a definitive treatment approach, it is also important to discuss the options with the patient. The authors of the systematic review that evaluated the benefits of treatment for stage 1 systolic hypertension recommended that antihypertensive therapy be based, in part, on patient preference and tolerance of therapy.14
BARRIER: Risks associated with multiple medications
The concurrent use of multiple medications, which is very common in the elderly, can interfere with treatment of hypertension in 2 ways: Some drugs have the potential to cause (or exacerbate) elevated blood pressure. Others may counter the effects of antihypertensive therapy.
Among the drugs that contribute to hypertension are the sympathomimetic agents found in many over-the-counter (OTC) cough and cold preparations in which the mechanism of action is direct vasoconstriction.18 Tricyclic antidepressants, which are commonly used to treat pain syndromes in the elderly, have also been shown to raise both systolic and diastolic pressure.19
On the other hand, nonsteroidal anti-inflammatory drugs (NSAIDs), often used by elderly patients to treat osteoarthritis, may counter the effects of many antihypertensive agents. Both OTC and prescription NSAIDs inhibit cyclo-oxygenase-2 (COX-2) in the kidneys, resulting in a reduction in sodium excretion and an increase in plasma volume.20
How to respond? Take a thorough medication history, including OTC and prescription drugs and supplements, before initiating treatment. Caution patients to avoid NSAIDs, OTC cough and cold preparations, and excessive caffeine, all of which may elevate their blood pressure. Remind patients that any new medication prescribed by another physician may affect their blood pressure, and advise them to maintain a current list of all medications to be reviewed at each visit.
BARRIER: Concerns about side effects
The elderly are more susceptible to orthostatic hypotension than younger patients because of age-related decreases in the baroreflex.21 Estimates suggest that anywhere from 5% to 50% of individuals older than 65 years are affected.21 Orthostatic hypotension, a side effect of many antihypertensive agents, is associated with higher rates of dizziness, syncope, and falls—a particular concern in elderly patients with a history of osteoporosis, pathological fracture, or gait dysfunction (See “Screening for hearing loss, risk of falls”).
How to respond? Before initiating antihypertensive therapy, take your patient’s blood pressure in both a sitting and standing position. Educate the patient and his or her family about the presentation of orthostatic hypotension, and advise the patient to stop taking the new medication and contact you if he or she develops the associated signs and symptoms. Schedule frequent follow-up appointments to closely monitor the effects of antihypertensive therapy, as well.
Stress the importance of lifestyle interventions
JNC 7 recommends several nonpharmacological treatments for all hypertensive patients, regardless of age. These include:
- healthy diet (such as Dietary Approaches to Stop Hypertension [DASH])
- sodium restriction (daily intake of <100 mmol/d)
- limited alcohol intake
- weight reduction (a decrease of 5-20 mm Hg for every 10 kg weight loss has been reported)
- regular physical activity (reported decrease of 4-9 mm Hg).2
No long-term trials have assessed the effects of lifestyle modification on morbidity and mortality. A subset of patients in the original DASH trial with stage 1 systolic hypertension did benefit from the diet, but the subgroup was small and the median age was 54.7 years.22 No large-scale studies have addressed the effectiveness of the diet in older patients with systolic hypertension.
Customizing therapy: What to consider
Evidence supports the use of various classes of antihypertensive medications in the treatment of high blood pressure, including thiazide diuretics, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers (CCBs).10,23-27 JNC 7 recommends starting with 1 medication for patients with stage 1 hypertension (140-159/90-99 mm Hg) and 2 medications for patients with stage 2 hypertension (≥160/100 mm Hg).2 As previously noted, however, clear-cut evidence in support of treating elderly patients with stage 1 hypertension is lacking.
When you do initiate treatment for an elderly patient, begin with a low dose and titrate slowly, carefully monitoring for side effects. If 2 antihypertensive agents are needed, start 1 agent at a time in patients who are at risk for significant side effects. The type of antihypertensive agent you prescribe should be based on your patient’s health status, comorbidities, and treatment for other conditions, as well as on the known effects of the particular class of drugs (TABLE).
Thiazide diuretics. JNC 7 recommends thiazide diuretics as first-line treatment based on cost and effectiveness. Because thiazide diuretics decrease the urinary excretion of calcium, JNC 7 recommends their use in patients with osteoporosis.2
But potential side effects, such as dehydration, orthostatic hypotension, and hypokalemia, are more common in elderly patients, and may limit the use of these drugs in clinical practice. NSAIDs may reduce the antihypertensive effects of thiazide diuretics through a decrease in sodium excretion and an increase in plasma volume, and should be used with caution—if at all—in this patient population.20
Hydrochlorothiazide, a commonly used thiazide diuretic, should not be used in doses >50 mg/d because higher doses increase the risk of side effects without increasing efficacy.28 In the Systolic Hypertension in the Elderly Program (SHEP) study, the positive effects of another thiazide diuretic—chlorthalidone—were lost in elderly patients with serum potassium <3.5 mmol/L, so serum electrolytes should be routinely assessed during therapy.2,7 Thiazide diuretics lose their effectiveness in patients with a glomerular filtration rate of <30 mL/min/1.73 m2 and should not be used in this group.29
Beta-blockers. Beta-blockers are indicated in patients with a previous history of myocardial infarction, as they have been shown to decrease the rate of new coronary events.30 Other comorbidities that may be mitigated by beta-blocker administration include angina pectoris, atrial fibrillation with a rapid ventricular rate, compensated heart failure, preoperative hypertension, and essential tremor.2
Side effects of beta-blockers include sedation, depression, sexual dysfunction, bradycardia, conduction abnormalities, and exacerbation of severe reactive airway disease.2 Central nervous system effects of beta-blockers tend to be lowest in hydrophilic agents, such as atenolol, and highest in lipophilic agents, such as propranolol, which readily cross the blood-brain barrier.31
A 2007 Cochrane review looked at the effectiveness of beta-blockers as first-line agents in uncomplicated hypertension and concluded that the evidence does not support their use. The reviewers noted, however, that the majority of the trials used atenolol and cautioned that the results might not apply to other beta-blockers—or to subgroups, such as the elderly.32
ACE inhibitors and ARBs. Both ACE inhibitors and ARBs inhibit the renin-angiotensin-aldosterone pathway, and both classes are indicated in patients with diabetes, chronic kidney disease, or heart failure.2 Cough is a side effect of ACE inhibitors that affects 5% to 35% of patients,33 but angioedema—estimated to occur in 0.1% to 0.7% of patients taking ACE inhibitors—is the most serious side effect.34
An elevation in serum potassium and serum creatinine may occur after starting an ACE inhibitor in patients with heart failure, dehydration, or significant renal insufficiency, so assess serum creatinine and potassium 1 week after initiating therapy. An increase in serum creatinine >30% may require discontinuation or dose reduction.2,35
Elderly patients who take NSAIDs while on ACE inhibitors are at particular risk for acute renal failure; reviews of iatrogenic acute renal failure in the elderly have shown that most cases are related to the concurrent use of these medications.36,37 This is yet another reason to avoid giving NSAIDs to elderly patients with hypertension, particularly if they’re taking diuretics or ACE inhibitors.
Beware of ACE inhibitor-ARB combinations. The American College of Cardiology and the American Heart Association recommend substituting an ARB if side effects from an ACE inhibitor occur in patients with heart failure, but extreme caution is required if the patient experienced ACE inhibitor-associated angioedema.38 TRANSCEND, a randomized controlled trial of almost 6000 patients, specifically studied the use of ARBs in patients unable to tolerate ACE inhibitors and concluded that they can be safely given to patients who had side effects from ACE inhibitors.39
Since ACE inhibitors and ARBs affect the renin-angiotensin-aldosterone pathway at different points, there has been interest in the effectiveness of combining these agents. The CHARM-Added trial found that the combination of these agents reduced both cardiovascular events and mortality in patients with heart failure.40
Combining ACE inhibitors and ARBs in patients without heart failure was evaluated in the ONTARGET trial. While the combination treatment group had a greater reduction in blood pressure, there was no significant cardiovascular benefit over patients who were on ACE inhibitors alone. But there was an increase in hypotension, syncope, and renal dysfunction among those in the combination treatment group, all of which are significant concerns in the elderly. The authors concluded that the ACE inhibitor-ARB combination should not be used in patients who do not have heart failure.41
Calcium channel blockers. CCBs block the entrance of calcium into vascular cells, producing dilation in the coronary arteries and peripheral vasculature, and are effective in the treatment of hypertension in elderly patients.42 Nondihydropyridine CCBs (diltiazem and verapamil) are useful in patients with atrial fibrillation and supraventricular tachycardia because of their negative chronotropic effects.2 And African American patients respond better to CCBs—with a greater reduction in blood pressure and cardiovascular complications—than to ACE inhibitors.2,25
Dihydropyridine CCBs (amlodipine and long-acting felodipine and nifedipine) are safe in patients with heart failure or chronic stable angina,43 but short-acting nifedipine has been found to increase the risk of mortality.44
Dihydropyridine CCBs have been reported to cause peripheral edema in 7% to 8% of patients taking long-acting nifedipine and up to 16% of elderly patient taking amlodipine.45 Verapamil has the highest incidence of constipation, and should be avoided in elderly patients.2
TABLE
Which drugs, for which patients?2
| DRUG CLASS* | INDICATIONS | CONSIDERATIONS |
|---|---|---|
| ACE inhibitors | Heart failure, post-MI, diabetes, high risk for CAD, chronic kidney disease, recurrent stroke prevention | Avoid concurrent use of ARBs in patients without heart failure. Avoid concurrent use of NSAIDs. If a second drug is needed, add a thiazide diuretic or CCB. |
| ARBs | Heart failure, diabetes, chronic kidney disease | Avoid concurrent use of ACE inhibitors in patients who don’t have heart failure. |
| Beta-blockers | Heart failure, post-MI, high risk for CAD, diabetes | Not recommended as first-line therapy for uncomplicated hypertension. |
| CCBs | High risk for CAD, diabetes | Avoid prescribing verapamil for elderly patients. |
| Thiazide diuretics | Heart failure, high risk for CAD, diabetes, recurrent stroke prevention | Avoid concurrent use of NSAIDs. Will not work with reduced GFR or hypokalemia. Use with caution in patients prone to orthostatic hypotension. |
| *Peripheral alpha-blockers, centrally acting alpha-agonists, and vasodilators should not be routinely used to treat hypertension in elderly patients. | ||
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CAD, coronary artery disease; CCB, calcium channel blocker; GFR, glomerular filtration rate; MI, myocardial infarction; NSAIDs, nonsteroidal anti-inflammatory drugs. | ||
Steer clear of these drugs
Significant side effects, including hypotension, sedation, depression, and dry mouth, limit the use of other antihypertensives, such as peripheral alpha blockers, centrally acting agents, and vasodilators, in elderly patients.2 In the ALLHAT trial, the alpha-blocker arm was stopped prematurely because of an increased rate of cardiovascular events and heart failure compared with the thiazide diuretic arm.25 That finding led to the recommendation that alpha-blockers not be used as first-line agents for treating hypertension.
Centrally acting alpha-agonists (clonidine, methyldopa, and reserpine) have a high incidence of sedation, dry mouth, and depression, and elderly patients are more likely to experience orthostatic hypotension and rebound hypertension if these agents are discontinued abruptly or doses are missed.2 Vasodilators, including minoxidil and hydralazine, cause sodium and fluid retention and reflex tachycardia.2 Because of their unfavorable side effects and lack of outcomes data, alpha-blockers, centrally acting alpha-agonists, and vasodilators should not be used routinely for the treatment of hypertension in elderly patients.
Considerations in selecting a second agent
Patients who fail to achieve adequate blood pressure control despite lifestyle modifications and a single agent, and those who are initially diagnosed with stage 2 hypertension, will require 2 or more medications. An elderly patient who has not responded to a single agent should be asked about any other medications (or substances) that might be interfering with the antihypertensive agent, especially NSAIDs, tricyclic antidepressants, sympathomimetics, and caffeine. Find out, too, whether the patient is following the prescribed regimen.
When you prescribe 2 antihypertensives, choose agents with complementary mechanisms of action—a diuretic and an ACE inhibitor, for example, or an ACE inhibitor and a CCB. In addition to avoiding an ACE/ARB combination in patients who do not have heart failure, avoid concurrent use of beta-blockers and ACE inhibitors46 and a dihydropyridine CCB/thiazide diuretic combination47 in all elderly patients.14
How does the evidence apply to your patient?
As we saw earlier, Esther V. has isolated systolic hypertension, which increases her risk of myocardial infarction, stroke, and heart failure. Even though she is 81 years old, studies such as HYVET support the treatment of her hypertension. Certainly, you’ll encourage her to limit her salt intake, follow a healthy diet, and exercise daily, as tolerated.
But because Mrs. V. has stage 1 hypertension, her preference and her ability to tolerate therapy will play a key role in the decision to initiate treatment. If her blood pressure does not respond to lifestyle modifications and she is agreeable to medical therapy, you would recommend 1 antihypertensive agent.
Which drug class is best? Given her history of osteoporosis, a low-dose thiazide diuretic might be a reasonable choice. But because she has a history of hip fracture from a fall, it would be prudent to avoid agents associated with volume depletion —and to opt for either an ACE inhibitor or a CCB as first-line therapy instead. It is extremely important to obtain a list of any OTC medications Mrs. V. is currently taking and to advise her to avoid the use of NSAIDs.
You would also want to obtain the results of Mrs. V.’s most recent bone mineral density test, and talk to her about the importance of taking calcium with vitamin D. If there are concerns about her gait, you would consider a formal gait evaluation, as well. Finally, you would review potential side effects, including orthostatic hypotension, and tell Mrs. V. to call if they occur, and schedule an appointment to monitor her serum potassium and renal function in a few weeks.
Correspondence Robert C. Langan, MD, St. Luke’s Family Medicine Residency Program, 2830 Easton Avenue, Bethlehem, PA 18017; [email protected]
1. Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003-1010.
2. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Yambe M, Tomiyama H, Yamada J, et al. Arterial stiffness and progression to hypertension in Japanese male subjects with high normal blood pressure. J Hypertens. 2007;25:87-93.
4. Lewington S, Clarke R, Quizilbash N, et al. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
5. He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138(Pt 2):211-219.
6. Levy D, Larson MG, Varsan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557-1562.
7. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP): SHEP Cooperative Research Group. JAMA. 1991;265:3255-3264.
8. Staessen JA, Fargard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension: the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757-764.
9. Izzo JL, Levy D, Black HR. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35:1021-1024.
10. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
11. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683-689.
12. Mulrow C, Lau J, Cornell J, et al. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev. 1998;(2):CD000028.-
13. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000;355:865-872.
14. Chaundry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA. 2004;292:1074-1080.
15. Somes GW, Pahor M, Shorr RI, et al. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004-2009.
16. Rastas S, Pirttila T, Viramo P, et al. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc. 2006;54:912-918.
17. Gueyffier F, Bulpitt C, Boissel JP. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. Lancet. 1999;353:793-796.
18. Pimenta E, Gaddam KK, Oprail S. Mechanisms and treatment of resistant hypertension. J Clin Hypertens. 2008;10:239-244.
19. Licht CM, de Gues EJ, Seldenrjik A. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631-638.
20. White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension. 2007;49:408.-
21. Weiss A, Grossman E, Belooseky Y, et al. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med. 2002;162:2369-2374.
22. Moore TJ, Conlin PR, Ard J, et al. DASH (Dietary Approached to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension. 2001;38:155-158.
23. Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751-1756.
24. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction study (LIFE) : a randomised trial against atenolol. Lancet. 2002;359:995-1003.
25. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
26. Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs. a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. JAMA. 2003;290:2805-2816.
27. Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583-592.
28. Black HR. The evolution of low-dose diuretic therapy: the lessons from clinical trials. Am J Med. 1996;101(suppl 3A):S47-S52.
29. National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Hypertensive Agents in Chronic Kidney Disease. Guideline 12: Use of Diuretics in CKD. Available at http://www.kidney.org/Professionals/Kdoqi/Guidelines_Bp/guide_12.htm. Accessed May 11, 2009.
30. Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina). J Am Coll Cardiol. 2003;41:159-168.
31. McAinsh J, Cruickshank JM. Beta-blockers and central nervous system side effects. Pharmacol Ther. 1990;46:163-197.
32. Wiysonge CSU, Bradley HA, Mayosi BM, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2007(1):CD002003.-
33. Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP Evidence-Based Clinical Practice Guidelines. Chest. 2006;129(suppl 1):S169-S173.
34. Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med. 2002;347:621-622.
35. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
36. Thomas MC. Diuretics, ACE inhibitors and NSAIDS-the triple whammy. Med J Aust. 2000;172:184-185.
37. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797-805.
38. Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977-2016.
39. The TRANSCEND investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174-1183.
40. McMurray JJ, Ostergren J, Swedberg K, et al. For the CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
41. Yusuf S, Teo KK, Pogue J, et al. For the ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
42. Malacco E, Mancia G, Rappelli A, et al. Treatment of isolated systolic hypertension: the SHELL study results. Blood Press. 2003;12:160-167.
43. Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med. 1996;335:1107-1114.
44. Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620-625.
45. Sirker A, Missouris CG, MacGregor GA. Dihydropyridine calcium channel blockers and peripheral side effects. J Human Hypertens. 2001;15:745-746.
46. MacGregor GA, Markandu ND, Banks RA, et al. Captopril in essential hypertension: contrasting effects of adding hydrochlorothiazide or propranolol. BMJ. 1982;284:693-696.
47. MacGregor GA, Cappuccio FP. Lack of effect of a diuretic added to diltiazem. J Hum Hypertens. 1997;11:249-250.
1. Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003-1010.
2. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
3. Yambe M, Tomiyama H, Yamada J, et al. Arterial stiffness and progression to hypertension in Japanese male subjects with high normal blood pressure. J Hypertens. 2007;25:87-93.
4. Lewington S, Clarke R, Quizilbash N, et al. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913.
5. He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138(Pt 2):211-219.
6. Levy D, Larson MG, Varsan RS, et al. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557-1562.
7. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP): SHEP Cooperative Research Group. JAMA. 1991;265:3255-3264.
8. Staessen JA, Fargard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension: the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757-764.
9. Izzo JL, Levy D, Black HR. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35:1021-1024.
10. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
11. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683-689.
12. Mulrow C, Lau J, Cornell J, et al. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev. 1998;(2):CD000028.-
13. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet. 2000;355:865-872.
14. Chaundry SI, Krumholz HM, Foody JM. Systolic hypertension in older persons. JAMA. 2004;292:1074-1080.
15. Somes GW, Pahor M, Shorr RI, et al. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med. 1999;159:2004-2009.
16. Rastas S, Pirttila T, Viramo P, et al. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc. 2006;54:912-918.
17. Gueyffier F, Bulpitt C, Boissel JP. Antihypertensive drugs in very old people: a subgroup meta-analysis of randomised controlled trials. Lancet. 1999;353:793-796.
18. Pimenta E, Gaddam KK, Oprail S. Mechanisms and treatment of resistant hypertension. J Clin Hypertens. 2008;10:239-244.
19. Licht CM, de Gues EJ, Seldenrjik A. Depression is associated with decreased blood pressure, but antidepressant use increases the risk for hypertension. Hypertension. 2009;53:631-638.
20. White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension. 2007;49:408.-
21. Weiss A, Grossman E, Belooseky Y, et al. Orthostatic hypotension in acute geriatric ward: is it a consistent finding? Arch Intern Med. 2002;162:2369-2374.
22. Moore TJ, Conlin PR, Ard J, et al. DASH (Dietary Approached to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension. 2001;38:155-158.
23. Hansson L, Lindholm LH, Ekbom T, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751-1756.
24. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction study (LIFE) : a randomised trial against atenolol. Lancet. 2002;359:995-1003.
25. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997.
26. Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs. a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. JAMA. 2003;290:2805-2816.
27. Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583-592.
28. Black HR. The evolution of low-dose diuretic therapy: the lessons from clinical trials. Am J Med. 1996;101(suppl 3A):S47-S52.
29. National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Hypertensive Agents in Chronic Kidney Disease. Guideline 12: Use of Diuretics in CKD. Available at http://www.kidney.org/Professionals/Kdoqi/Guidelines_Bp/guide_12.htm. Accessed May 11, 2009.
30. Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina—summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina). J Am Coll Cardiol. 2003;41:159-168.
31. McAinsh J, Cruickshank JM. Beta-blockers and central nervous system side effects. Pharmacol Ther. 1990;46:163-197.
32. Wiysonge CSU, Bradley HA, Mayosi BM, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2007(1):CD002003.-
33. Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP Evidence-Based Clinical Practice Guidelines. Chest. 2006;129(suppl 1):S169-S173.
34. Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med. 2002;347:621-622.
35. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
36. Thomas MC. Diuretics, ACE inhibitors and NSAIDS-the triple whammy. Med J Aust. 2000;172:184-185.
37. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797-805.
38. Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977-2016.
39. The TRANSCEND investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174-1183.
40. McMurray JJ, Ostergren J, Swedberg K, et al. For the CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
41. Yusuf S, Teo KK, Pogue J, et al. For the ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
42. Malacco E, Mancia G, Rappelli A, et al. Treatment of isolated systolic hypertension: the SHELL study results. Blood Press. 2003;12:160-167.
43. Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med. 1996;335:1107-1114.
44. Psaty BM, Heckbert SR, Koepsell TD, et al. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620-625.
45. Sirker A, Missouris CG, MacGregor GA. Dihydropyridine calcium channel blockers and peripheral side effects. J Human Hypertens. 2001;15:745-746.
46. MacGregor GA, Markandu ND, Banks RA, et al. Captopril in essential hypertension: contrasting effects of adding hydrochlorothiazide or propranolol. BMJ. 1982;284:693-696.
47. MacGregor GA, Cappuccio FP. Lack of effect of a diuretic added to diltiazem. J Hum Hypertens. 1997;11:249-250.
Minor derm ailments: How good is the evidence for common treatments?
- Oral flucloxacillin is less effective than local antibiotics for impetigo in limited disease (level of evidence [LOE] 1a).
- Topical metronidazole and azelaic acid are effective for rosacea (LOE 1a).
- Betadine is effective for minor infections following partial thickness burns (LOE 1b).
- Terbinafine is effective against fungal infections of the nail (LOE 1a).
- Miconazole is effective against oral thrush (LOE 1a).
Level of evidence (LOE)
1a: Systematic reviews (with homogeneity) of randomized controlled trials (RCTs).
1a-: Systematic review of randomized trials displaying worrisome heterogeneity.
1b: Individual RCT (with a narrow confidence interval).
1b-: Individual RCT (with a wide confidence interval).
1c: All or none RCTs.
2a: Systematic reviews (with homogeneity) of cohort studies.
2a-: Systematic reviews of cohort studies displaying worrisome heterogeneity.
2b: Individual cohort study or low-quality RCTs (<80% follow-up).
2b-: Individual cohort study or low-quality RCTs (<80% follow-up/wide confidence interval).
2c: “outcomes” research; ecological studies.
3a: Systematic review (with homogeneity) of case-control studies.
3a-: Systematic review of case-control studies with worrisome heterogeneity.
3b: Individual case-control study.
4: Case series (and poor-quality cohort and case-control studies).
5: Expert opinion without explicit critical appraisal, or based on physiology, bench research, or “first principles.”
Source: Essential Evidence Plus. Levels of evidence.1
Do you use silver sulfadiazine for partial-thickness burns? If you do, you may be surprised to learn that the evidence for its use in this situation is conflicting. This was just one of the findings of our systematic review of the methodologic quality and statistical and clinical relevance of current therapies for minor dermatologic ailments.
Given that minor ailments, frequently dermatologic, account for 40% to 70% of all consultations in family medicine,2,3 guidelines based on better research are needed. This need is underscored by the increasing delegation of minor treatments to staff nurses, nurse practitioners, and physician assistants, who should undergo comprehensive training, preferably based on valid guidelines.4,5 Moreover, consultations for prevalent minor ailments often lead to prescriptions for medications, thereby generating considerable costs.6,7
Methods
The starting point for this review was the textbook, Minor Ailments in Primary Care: An Evidence-Based Approach,6 which describes 119 minor ailments, selected mainly on the basis of disease prevalence. We selected all dermatologic ailments (International Classification of Primary Care-code ‘S’) (N=42) (TABLE).5
We searched the online databases PubMed, Cochrane Controlled Trials Register, and Clinical Evidence for articles relating to the treatment of these conditions. For each ailment, we used various search terms for indication and treatment.8 (See note at end of Methods section.) We excluded alternative (nonallopathic) and most preventive therapies because they are unusual in the daily practice of family medicine.
We searched only for trials in which treatments were compared with placebo or a reasonable, accepted usual therapy. The search followed a hierarchy of evidence:8 systematic reviews (SRs), then randomized controlled trials (RCTs), then other research articles (nonrandomized clinical trials, case series). When we found a relevant SR published in 2004 or later, we did not search for a lower level of evidence (LOE). Instead, we restricted our subsequent search to RCTs published after the publication date of the SR.8 Two of the authors (SPG and JAHE) selected articles independently, based on article title and abstract. Disagreements in selection were discussed and consensus was reached. If an article contained relevant first-line therapy, we also used the “related articles” option in PubMed to check for more sources. (See note at end of Methods section.)
To evaluate the methodologic quality of SRs and trials, we ranked articles according to the method of infoPOEMs.8 (See key.) Two experienced researchers (JAHE and AKN) scored all articles independently. Consensus was reached in cases of disagreement.9 We deemed evidence convincing if the study showed the intervention was effective and if the LOE of the study was high (levels 1a, 1b, or 2a).
Evaluating breadth of treatment application. To explore whether a treatment for a certain minor ailment could be applied to other ailments with similar symptoms and thus increase the strength of the treatment’s rationale, we clustered ailments, where possible, into bacterial infection, fungal infection, itch, and pain.
We classified the efficacy of therapies as yes, likely (if the result was not convincingly effective or based on small studies, or if the study objective was unclear), or no. Treatments with no trials to support them are so identified. As to whether the evidence was convincing, we indicated yes, no, or conflicting.
Post hoc analysis. For trials with a wide confidence interval and for therapies described as not clearly effective, we performed a post hoc power analysis to explore if the trial was underpowered.10 We compared the number of subjects in the study (n1) with the number we calculated as necessary for the study to have sufficient power (n2). For all studies, we used standardized values (α=0.05 and β=0.20). If n1≥n2 we considered the study design accurate, and if n1< n2 we concluded that the power was insufficient for the study to be able to answer its objectives.
- terms used in searching online databases
- post hoc power analysis
- a summary of treatment rationales, therapies and their effectiveness, country where the research was undertaken, number of authors, and year of article publication for each dermatologic ailment.
Results
We collected 71 articles published in the medical literature between January 1981 and July 2007.11-81 On average, we found 2 articles per minor dermatologic ailment, with a range of 0 to 7. For 7 common ailments, we found no studies on therapies; for 13 ailments we found just 1 trial each.
For 20 of the 42 ailments, we found a SR of treatments (10 Cochrane reviews, 5 Clinical Evidence, and 5 from other sources). Most articles describing RCTs presented results with wide confidence intervals (LOEs 1b- and 2b-), mainly due to small sample sizes. Eleven RCTs (14%) had high dropout rates (LOE 2b or 2b-).
Seventy-four percent of all the trials were conducted in Europe and North America. The United States (24%) and United Kingdom (25%) were the largest contributors. Studies of Asian and South American populations (eg, Indian, Nepalese, Iraqi, Brazilian) tended to focus on problems more prevalent in these countries, such as lice and scabies.
For 26 of the 42 ailments, evidence was unclear (no studies or studies with inconclusive evidence). Very few of the therapies commonly used for minor dermatologic ailments are supported by high-level research evidence. Even some SRs included only methodologically poor RCTs, which indicates that more research is needed.
A look at outcomes. The TABLE summarizes the effectiveness of therapies usually applied to minor dermatologic ailments in daily practice. The columns present, in turn:
- the minor ailment,
- the treatments usually applied in daily practice,
- the number of studies found for these treatments,
- the condition at which treatment was aimed,
- whether the targeted condition belongs to 1 of the 4 categories of main symptoms,
- whether the study/studies reported a positive effect for the treatment,
- whether the evidence for the effectiveness of a particular treatment was (according to the authors) convincing,
- whether the overall rating of evidence was convincing,
- and whether further studies are needed.
Results varied. With partial thickness burns, evidence was conflicting on the effectiveness and the harms of silver sulfadiazine and several types of gauzes. For boils, we could find no trial about therapy. For both warts and mollusca contagiosa, Cochrane reviews were inconclusive on therapies commonly used in general practice. Evidence was also inconclusive for treatments for paronychia, polymorphic light eruption, and dog and cat bites.
TABLE
The treatment of minor dermatologic ailments: What the research tells us
Treatments for which sufficient positive evidence exists are formatted in bold; those for which negative evidence exists are formatted in bold italic.
| Dermatological minor ailment (N*) | Treatment | Target for treatment | Category of treatment target, according to main symptoms | Was treatment effective? | Was the research convincing?† | Overall rating of research evidence | Are further studies required? | |
|---|---|---|---|---|---|---|---|---|
| 1. | Partial thickness burns (4) | Oral antibiotics (flucloxacillin)11 | Infection (bacterial) | Bacterial infection | No trials | No | Moderate | Yes |
| Gauze dressings11 | Skin lesion | Likely | Conflicting | |||||
| Silver sulfadiazine11 | Skin lesion | No | Conflicting | |||||
| Betadine12 | Infection (bacterial) | Bacterial infection | Yes | Yes | ||||
| Cooling13 | Pain | Pain | No | No | ||||
| Honey14 | Pain | Pain | No | No | ||||
| 2. | Polymorphic light eruption (4) | Neutral lotion | Itch | Itch | No trials | No | Poor | Yes |
| Corticosteroids15,18 | Itch | Itch | Yes | Yes | ||||
| Oral antihistamines | Itch | Itch | No trials | No | ||||
| Sunscreens16,17 | Preventive | Yes | No | |||||
| 3. | Acute urticaria (2) | Local ointments | Itch/rash | Itch | No trials | No | Moderate | Yes |
| Oral antihistamines19 | Itch/rash | Itch | Yes | Yes | ||||
| Corticosteroids20 | Itch/rash | Itch | Yes | Yes | ||||
| 4. | Insect bites and stings (0) | Doxycycline | Infection (bacterial) | Bacterial infection | No trials | No | None | Yes |
| Amoxicillin | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| Oral antihistamines | Pain/itch | Itch | No trials | No | ||||
| 5. | Pediculosis (3) | Malathion21 | Infection | Yes | Yes | Good | No | |
| Permethrin22 | Infection | Yes | Yes | |||||
| Lindane | Infection | No trials | No | |||||
| Combing23 | Infection | Yes | Yes | |||||
| 6. | Scabies (3) | Permethrin25,26 | Infection | Yes | Yes | Good | No | |
| Lindane24 | Infection | Yes | Yes | |||||
| Benzyl benzoate | Infection | No trials | No | |||||
| Malathion | Infection | No trials | No | |||||
| 7. | Dog and cat bites (1) | Oral antibiotics (amoxicillin)27 | Infection (bacterial) | Bacterial infection | Yes, for bites to the hands. No, for other bites | No | Moderate | Yes |
| 8. | Abrasions (1) | Paraffin gauze | Skin lesion | No trials | No | Poor | Yes | |
| Non-adherent absorbent compress | Skin lesion | No trials | No | |||||
| Betadine | Skin lesion/infection (bacterial) | Bacterial infection | No trials | No | ||||
| Honey2 | Skin lesion | Likely | No | |||||
| 9. | Warts (4) | Salicylic acid29 | Lump on the skin | Yes | Yes | Moderate | Yes | |
| Cryotherapy29 | Lump on the skin | No | No | |||||
| Duct tape occlusion30-32 | Lump on the skin | No | Yes, treatment was not effective | |||||
| Surgical procedures | Lump on the skin | No trials | No | |||||
| 10. | Molluscum contagiosum (1) | Curettage33 | Lump on the skin | No trials | No | Poor | Yes | |
| Liquid nitrogen33 | Lump on the skin | No trials | No | |||||
| Fusidic acid cream33 | Lump on the skin | No trials | No | |||||
| Betadine33 | Lump on the skin | No | No | |||||
| 11. | Furuncles (0) | Hot compress | Pain | Bacterial infection | No trials | No | None | Yes |
| Antibiotics | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| 12. | Impetigo (1) | Local fusidic acid or mupirocin34 | Infection (bacterial) | Bacterial infection | Yes | Yes | Good | No |
| Oral antibiotics34 | Infection (bacterial) | Bacterial infection | Yes | Yes, but less effective than local treatment in limited disease | ||||
| 13. | Pityriasis versicolor (2) | Selenium sulphide | Infection | Fungal infection | No trials | No | Moderate | Yes |
| Imidazole | Infection | Fungal infection | No trials | No | ||||
| Fluconazole35 | Infection | Fungal infection | Yes | No | ||||
| Itraconazole36 | Infection | Fungal infection | Yes | No | ||||
| 14. | Intertrigo (2) | Miconazole37,38 | Infection | Fungal infection | Yes | Yes | Moderate | Yes |
| Hydrocortisone37 | Infection | Fungal infection | No | No | ||||
| 15. | Erythrasma (1) | Imidazole | Infection (bacterial) | Bacterial infection | No trials | No | Good | No |
| Benzoic acid | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| Erythromycin39 | Infection (bacterial) | Bacterial infection | Yes | Yes | ||||
| 16. | Shingles (6) | Acyclovir40 | Infection (viral) | Yes | Yes | Moderate/Good | Yes | |
| Famcyclovir41 | Infection (viral) | Yes | No | |||||
| Acyclovir + prednisolone42 | Infection (viral) | Yes | No | |||||
| Corticosteroids43,44 | Inflammation | No | Yes, treatment was not effective | |||||
| Amitriptyline45 | Pain | Likely | No | |||||
| 17. | Pruritus in the elderly (1) | Local emollients | Itch | Itch | No trials | No | Moderate | Yes |
| Corticosteroids | Itch | Itch | No trials | No | ||||
| Local antihistamines | Itch | Itch | No trials | No | ||||
| Oral antihistamines46 | Itch | Itch | Yes | Yes | ||||
| 18. | Xeroderma (0) | Emollients | Dry skin | No trials | No | None | Yes | |
| 19. | Androgenic alopecia (5) | Wig | Hair loss | No trials | No | Moderate | Yes | |
| Finasteride49-51 | Hair loss | Yes | Yes | |||||
| Minoxidil47,48 | Hair loss | Likely | Conflicting | |||||
| 20. | Alopecia areata (5) | Minoxidil52,53 | Hair loss | No | No | Moderate | Yes | |
| Oral prednisolone54 | Hair loss | Likely | No | |||||
| Desoxymethasone55 | Hair loss | No | No | |||||
| Betamethasone56 | Hair loss | Likely | No | |||||
| 21. | Dandruff (4) | Zinc pyrithione57 | Infection (yeast) | Fungal infection | Yes | No | Moderate | Yes |
| Ciclopirox58-60 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Ketoconazole61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Selenium sulphide61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Corticosteroids61 | Itch | Itch | Yes | Yes | ||||
| 22. | Seborrhoeic eczema (2) | Zinc pyrithione57 | Infection (yeast) | Fungal infection | Yes | No | Moderate | Yes |
| Ketoconazole61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Coal tar61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Selenium sulphide61 | Infection (yeast) | Fungal infection | Yes | No | ||||
| Corticosteroids61 | Itch | Itch | Yes | Yes | ||||
| 23. | Herpes labialis (1) | Sunscreens62 | Prevention | Yes | Yes | Good | No | |
| Oral antivirals62 | Infection (viral) | Yes | Yes | |||||
| Zinc oxide cream62 | Skin lesion | Likely | No | |||||
| Topical antivirals | Infection (viral) | No trials | No | |||||
| Topical antivirals | Pain | No trials | No | |||||
| 24. | Perioral dermatitis (1) | Clean with water | Prevention | No trials | No | Poor | Yes | |
| Topical metronidazole63 | Infection | Bacterial infection | Likely | No | ||||
| Tetracycline | Infection | Bacterial infection | No | No | ||||
| 25. | Oral thrush (1) | Nystatin64 | Infection | Fungal infection | Less effective than miconazole | No | Good | No |
| Miconazole64 | Infection | Fungal infection | Yes | Yes | ||||
| 26. | Salmon patch (0) | No treatment is needed | None | No | ||||
| 27. | Chloasma (3) | Hydroquinone, tretinoin, hydrocortisone combination65,66 | Skin irritation | Yes | No | Moderate | Yes | |
| Hydroquinone 67 | Skin irritation | Yes | No | |||||
| 28. | Rosacea (2) | Topical metronidazole68 | Infection | Bacterial infection | Yes | Yes | Moderate | Yes |
| Azelaic acid68 | Infection | Bacterial infection | Yes | Yes | ||||
| Zinc-sulphate69 | Infection | Bacterial infection | Yes | No | ||||
| Tetracycline68 | Infection | Bacterial infection | Yes | No | ||||
| 29. | Umbilical problems in infants (0) | Disinfectant liquid | Infection | Bacterial infection | No trials | - | None | Yes |
| Antiseptic dressing | Infection | Bacterial infection | No trials | - | ||||
| Silver nitrate | To stop granulations | No trials | - | |||||
| Electrocauterization | To stop granulations | No trials | - | |||||
| 30. | Nappy rash (2) | Zinc oxide cream70 | Skin lesion | Yes | Yes | Moderate | Yes | |
| Miconazole71 | Infection | Fungal infection | Yes | No | ||||
| Hydrocortisone | Itch | Itch | No trials | No | ||||
| 31. | Fish hook in finger (0) | Local extirpation | Skin lesion | No trials | - | None | No | |
| 32. | Splinter under nail (0) | Splinter removal | Skin lesion | No trials | - | None | No | |
| 33. | Subungual hematoma (1) | Making a hole in the nail72 | Discharging hematoma | Likely | No | Moderate | Yes | |
| 34. | Brittle nails (0) | Terbinafine (oral) | Infection | No trials | No | None | Yes | |
| Itraconazole (oral) | Infection | No trials | No | |||||
| 35. | Paronychia (0) | Antibiotics | IInfection (bacterial) | Bacterial infection | No trials | No | Poor | Yes |
| Drainage | Discharging pus | No trials | No | |||||
| Antifungal cream | Infection | Fungal infection | No trials | No | ||||
| 36. | Fungal infection of the nail (3) | Local treatment (imidazole)73 | Infection | Fungal infection | Yes | Yes | Good | No |
| Oral terbinafine74,75 | Infection | Fungal infection | Yes | Yes | ||||
| 37. | Calluses on the feet (1) | Removing the excess callus76 | Removing callosity | Yes | No | None | Yes | |
| Disinfectant ointment | Infection | No trials | No | |||||
| 38. | Ingrown nail (2) | Wedge excision77,78 | Removing infected tissue | Yes | Yes | Good | Yes | |
| Chemical ablation77,78 | Destruction nail matrix | Yes | Yes | |||||
| 39. | Corns (1) | Salicylic acid | Resolution callosity | No trials | No | None | Yes | |
| Excision76 | Removing callosity | Yes | No | |||||
| 40. | Athlete’s foot (1) | Imidazole79 | Infection | Fungal infection | Yes | Yes | Good | No |
| Imidazole + hydrocortisone79 | Infection/itch | Fungal infection /itch | Yes | Yes | ||||
| Itraconazole79 | Infection | Fungal infection | Yes | Yes | ||||
| 41. | Foot blisters (2) | Betadine | Infection | Bacterial infection | No trials | No | Moderate | Yes |
| Antiperspirant 80,81 | Reducing incidence of blisters | Yes | Conflicting | |||||
| 42. | Plantar warts (4) | Salicylic acid29 | Lump on the skin | Yes | Yes | Moderate/good | Yes | |
| Cryotherapy 29 | Lump on the skin | No | No | |||||
| Duct tape occlusion30-32 | Lump on the skin | No | Yes, treatment was not effective | |||||
| Surgical procedures | Lump on the skin | No trials | No | |||||
| *N=Number of trials. | ||||||||
| † Convincing evidence taken as level of evidence 1a or 1b. | ||||||||
Click here view to PDF version
Clustering by treatment rationale
Bacterial infections. We found trials on antibiotic therapy for 5 of the 12 minor dermatologic ailments caused by or followed by bacterial infection. For the other 7, no trials were available. We found evidence for the effectiveness of treatment in 3 of the 11 indications (impetigo, erythrasma, and rosacea). For the treatment of impetigo (in cases of limited disease), oral flucloxacillin is less effective than local antibiotic treatment (LOE 1a). Betadine for minor infections after partial thickness burns is effective (LOE 1b)or all other dermatological minor ailments in the bacterial infections category, the effectiveness of antibiotic therapy was unclear.
Fungal infections. For 8 of the 9 ailments in which a fungal infection (yeast, fungals, dermatophytes) was one of the main reasons for therapy, we found trials on antimycotic treatment. There were 2 SRs of oral therapy for fungal nail infections, both concluding that terbinafine is an effective antifungal therapy for the condition. Miconazole is effective for infections with Candida albicans or dermatophytes (LOE 1a).
Itch. Itch was a main reason for treating 8 ailments. We found some trials for neutral lotion or oral antihistamines. We also found evidence supporting use of local antihistamines for 2 of the 8 minor ailments. For 4 ailments, we found studies with positive results for local application of steroids; we found no studies for oral steroids. We can therefore conclude that local steroids are effective for ailments in which itch is one of the main symptoms.
Pain. For 5 ailments, relief from pain was the main target of treatment. Trials, however, did not focus on generic pain medications but on treatments aimed at specific causal pathways of the ailment (eg, antiviral treatment for the post-herpetic pain of shingles). Therefore, we cannot draw generalizable conclusions on the treatment of pain in minor dermatologic ailments.
Post hoc power analysis
Most of the 10 trials with LOE 1b- (and effectiveness of treatment described as no or likely) needed many more patients to reach a higher LOE. In only 2 trials,55,66 the number of patients was sufficient. Four of the 10 trials were missing information that would have enabled us to judge whether they were underpowered. In 4 other trials, we considered the number of patients needed to prove treatment effectiveness (n2) unrealistic, and, consequently, the therapy as very likely ineffective.
Conclusions
Study design was poor for more than half of the trials identified. And other studies were so small as to lack statistical power. We found convincing evidence (SRs or good RCTs) for the effectiveness of usual therapy for fewer than half of the ailments selected. Had we extended our search to more databases, such as EMBASE and CINAHL, we may have identified more trials. However, it is unlikely we would have arrived at a different conclusion, given that the number of relevant studies was so low in the databases we did search (PubMed, Cochrane library, Clinical Evidence).
We clustered ailments to determine if a treatment aimed at a particular symptom or complication could be applied to all ailments exhibiting that condition. On the basis of the treatment effect found for 4 ailments, we determined that local steroids would most likely effectively relieve itch associated with all minor dermatologic ailments. For other conditions, grouping by rationale for treatment did not yield any extendable applications.
Generally accepted treatments for minor dermatologic ailments are insufficiently supported by research evidence. This limitation contrasts dramatically with the body of evidence supporting therapies in other aspects of family practice, reportedly having sufficient LOEs in the range of 50% to 80% of treatments.82,83 Given that minor ailments are a substantial portion of a family physician’s workload, and that other primary care providers are increasingly treating these ailments, definitive guidelines based on high-quality research are needed. This aspect of medical care deserves more attention from researchers and funding agencies.
Correspondence J.A.H. Eekhof, MD, PhD, Department of Public Health and Primary Care, Leiden University Medical Center (LUMC), PO Box 9600, 2300 RC Leiden, The Netherlands; [email protected]
1. Essential Evidence Plus. Levels of evidence. Available at: http://www.essentialevidenceplus.com/product/ebm_loe.cfm?show=oxford. Accessed August 18, 2009.
2. Rambihar BV. [e-letter BMJ] 18 May 2001. Available at: bmj.com/cgi/eletters/322/7296/1193. Accessed July 30, 2009.
3. Morris CJ, Cantrill JA, Weiss MC. GPs’ attitudes to minor ailments. Fam Pract. 2001;18:581-585.
4. Pritchard A, Kendrick D. Practice nurse and health visitor management of acute minor illness in a general practice. J Adv Nurs. 2001;36:556-562.
5. Okkes IM, Becker HW, Bernstein RM, et al. The March 2002 update of the electronic version of ICPC-2. A step forward to the use of ICD-10 as a nomenclature and a terminology for ICPC-2. Fam Pract. 2002;19:543-546.
6. Eekhof JAH, Knuistingh NA, Verheij TJM. eds. Minor Ailments in Primary Care: An Evidence-Based Approach. Edinburgh/London: Elsevier Butterworth Heinemann; 2005.
7. Salmon P. The potentially somatizing effect of clinical consultation. CNS Spectr. 2006;11:190-200.
8. Assendelft WJJ, van Tulder MW, Scholten RJ, et al. De praktijk van systematische reviews. II. Zoeken en selecteren van literatuur. [The practice of systematic reviews. II. Searching and selection of studies.] Ned Tijdschr Geneeskd. 1999;143:656-661.
9. Assendelft WJJ, Scholten RJ, van Eijk JT, et al. De Praktijk van systematisch reviews. III. Methodologische beoordeling van onderzoeken. [The practice of systematic reviews. III. Evaluation of methodological quality of research studies.] Ned Tijdschr Geneeskd. 1999;143:714-719.
10. Pocock SJ. The size of a clinical trial. In: Clinical Trials: A Practical Approach. Chichester, England: John Wiley & Sons; 1983:123–141.
11. Wasiak J, Cleland H. Burns (minor thermal). Clin Evid. 2005;14:2388-2396.
12. Han KH, Maitra AK. Management of partial skin thickness burn wounds with Inadine dressings. Burns. 1989;15:399-402.
13. Werner MU, Lassen B, Pedersen JL, et al. Local cooling does not prevent hyperalgesia following burn injury in humans. Pain. 2002;98:297-303.
14. Moore OA, Smith LA, Campbell F, et al. Systematic review of the use of honey as a wound dressing. BMC Complement Altern Med. 2001;1:2.-Available at: http://www.biomedcentral.com/1472-6882/1. Accessed May 8, 2008.
15. Man I, Dawe RS, Ibbotson SH, et al. Is topical steroid effective in polymorphic light eruption? Br J Dermatol. 2000;143(suppl 157):S113.-
16. Proby CM, Baker CS, Morton O, et al. New broad-spectrum sunscreen for polymorphic light eruption. Lancet. 1993;341:1347-1348.
17. Allas S, Lui H, Moyal D, et al. Comparison of the ability of 2 sunscreens to protect against polymorphous light eruption induced by a UV-A/UV-B metal halide lamp. Arch Dermatol. 1999;135:1421-1422.
18. Patel DC, Bellaney GJ, Seed PT, et al. Efficacy of short-course oral prednisolone in polymorphic light eruption: a randomized controlled trial. Br J Dermatol. 2000;143:828-831.
19. Lin RY, Curry A, Pesola GR, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000;36:462-468.
20. Pollack CV, Jr, Romano TJ. Outpatient management of acute urticaria: the role of prednisone. Ann Emerg Med. 1995;26:547-551.
21. Dodd CS. Interventions for treating head lice. Cochrane Database Syst Rev. 2001;(3):CD001165.-
22. Vander Stichele RH, Dezeure EM, Bogaert MG. Systematic review of clinical efficacy of topical treatments for head lice. BMJ. 1995;311:604-608.
23. Hill N, Moor G, Cameron MM, et al. Single blind, randomised, comparative study of the Bug Buster kit and over the counter pediculicide treatments against head lice in the United Kingdom. BMJ. 2005;331:384-387.Epub 2005 Aug 5. Available at: http://www.bmj.com/content/vol331/issue7513/#PRIMARY_CARE. Accessed May 5, 2008.
24. Walker GJ, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev. 2000;(3):CD000320.-
25. Usha V, Gopalakrishnan Nair TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol. 2000;42:236-240.
26. Zargari O, Golchai J, Sobhani A, et al. Comparison of the efficacy of topical 1% lindane vs 5% permethrin in scabies: a randomized, double-blind study. Indian J Dermatol Venereol Leprol. 2006;72:33-36.
27. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.-
28. Ingle R, Levin J, Polinder K. Wound healing with honey—a randomised controlled trial. S Afr Med J. 2006;96:831-835.
29. Gibbs S, Harvey I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2006;(3):CD001781.-
30. Focht DR, 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
31. Wenner R, Askari SK, Cham PM, et al. Duct tape for the treatment of common warts in adults: a double-blind randomized controlled trial. Arch Dermatol. 2007;143:309-313.
32. de Haen M, Spigt MG, van Uden CJ, et al. Efficacy of duct tape vs placebo in the treatment of verruca vulgaris (warts) in primary school children. Arch Pediatr Adolesc Med. 2006;160:1121-1125.
33. van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006;(2):CD004767.-
34. Koning S, Verhagen AP, van Suijlekom-Smit LW, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2004;(2):CD003261.-
35. Partap R, Kaur I, Chakrabarti A, et al. Single-dose fluconazole versus itraconazole in pityriasis versicolor. Dermatology. 2004;208:55-59.
36. Hickman JG. A double-blind, randomized, placebo-controlled evaluation of short-term treatment with oral itraconazole in patients with tinea versicolor. J Am Acad Dermatol. 1996;34:785-787.
37. Hedley K, Tooley P, Williams H. Problems with clinical trials in general practice—a double-blind comparison of cream containing miconazole and hydrocortisone with hydrocortisone alone in the treatment of intertrigo. Br J Clin Pract. 1990;44:131-135.
38. Mistiaen P, Poot E, Hickox S, et al. Preventing and treating intertrigo in the large skin folds of adults: a literature overview. Dermatol Nurs. 2004;16:43-46,49–57.
39. Hamann K, Thorn P. Systemic or local treatment of erythrasma? A comparison between erythromycin tablets and Fucidin cream in general practice. Scand J Prim Health Care. 1991;9:35-39.
40. Lancaster T, Silagy C, Gray S. Primary care management of acute herpes zoster: systematic review of evidence from randomized controlled trials. Br J Gen Pract. 1995;45:39-45.
41. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
42. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
43. Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896-900.
44. van Wijck AJ, Opstelten W, Moons KG, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet. 2006;367:219-224.
45. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
46. Dupont C, de Maubeuge J, Kotlar W, et al. Oxatomide in the treatment of pruritus senilis. A double-blind placebo-controlled trial. Dermatologica. 1984;169:348-353.
47. Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol. 1999;41:717-721.
48. Olsen EA. Topical minoxidil in the treatment of androgenetic alopecia in women. Cutis. 1991;48:243-248.
49. Kawashima M, Hayashi N, Igarashi A. Finasteride in the treatment of Japanese men with male pattern hair loss. Eur J Dermatol. 2004;14:247-254.
50. Price VH, Menefee E, Sanchez M, et al. Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. J Am Acad Dermatol. 2006;55:71-74.
51. Whiting DA, Olsen EA, Savin R, et al. Male Pattern Hair Loss Study Group. Efficacy and tolerability of finasteride 1 mg in men aged 41 to 60 years with male pattern hair loss. Eur J Dermatol. 2003;13:150-160.
52. Fransway AF, Muller SA. 3 percent topical minoxidil compared with placebo for the treatment of chronic severe alopecia areata. Cutis. 1988;41:431-435.
53. Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
54. Kar BR, Handa S, Dogra S, et al. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005;52:287-290.
55. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
56. Mancuso G, Balducci A, Casadio C, et al. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: a multicenter, prospective, randomized, controlled, investigator-blinded trial. Int J Dermatol. 2003;42:572-575.
57. Warner RR, Schwartz JR, Boissy Y, et al. Dandruff has an altered stratum corneum ultrastructure that is improved with zinc pyrithione shampoo. J Am Acad Dermatol. 2001;45:897-903.
58. Altmeyer P, Hoffmann K. Loprox Shampoo Dosing Concentration Study Group. Efficacy of different concentrations of ciclopirox shampoo for the treatment of seborrheic dermatitis of the scalp: results of a randomized, double-blind, vehicle-controlled trial. Int J Dermatol. 2004;43(suppl 1):9-12.
59. Lee JH, Lee HS, Eun HC, et al. Successful treatment of dandruff with 1.5% ciclopirox olamine shampoo in Korea. J Dermatolog Treat. 2003;14:212-215.
60. Squire RA, Goode K. A randomised, single-blind, single-centre clinical trial to evaluate comparative clinical efficacy of shampoos containing ciclopirox olamine (1.5%) and salicylic acid (3%), or ketoconazole (2%, Nizoral) for the treatment of dandruff/seborrhoic dermatitis. J Dermatolog Treat. 2002;13:51-60.
61. Manriquez JJ, Uribe P. Seborrhoeic dermatitis. BMJ Clin Evid. 2007;7:171-173.
62. Worrall G. Herpes labialis. BMJ Clin Evid. 2005;14:2050-2057.
63. Veien NK, Munkvad JM, Nielsen AO, et al. Topical metronidazole in the treatment of perioral dermatitis. J Am Acad Dermatol. 1991;24:258-260.
64. Pankhurst CL. Candidiasis (oropharyngeal). BMJ Clin Evid. 2006;15:1849-1863.
65. Ferreira Cestari T, Hassun K, Sittart A, et al. A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6:36-39.
66. Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
67. Espinal-Perez LE, Moncada B, Castanedo-Cazares JP. A double-blind randomized trial of 5% ascorbic acid vs. 4% hydroquinone in melasma. Int J Dermatol. 2004;43:604-607.
68. van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.-
69. Sharquie KE, Najim RA, Al-Salman HN. Oral zinc sulfate in the treatment of rosacea: a double-blind, placebo-controlled study. Int J Dermatol. 2006;45:857-861.
70. Baldwin S, Odio MR, Haines SL, et al. Skin benefits from continuous topical administration of a zinc oxide/petrolatum formulation by a novel disposable diaper. J Eur Acad Dermatol Venereol. 2001;15(suppl 1):S5-S11.
71. Concannon P, Gisoldi E, Phillips S, et al. Diaper dermatitis: a therapeutic dilemma. Results of a double-blind placebo controlled trial of miconazole nitrate 0.25%. Pediatr Dermatol. 2001;18:149-155.
72. Ciocon D, Gowrishankar TR, Herndon T, et al. How low should you go: novel device for nail trephination. Dermatol Surg. 2006;32:828-833.
73. Crawford F, Hart R, Bell-Syer S, et al. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev. 1999;(3):CD001434.-
74. Bell-Syer SEM, Hart R, Crawford F, et al. Oral treatments for fungal infections of the skin of the foot. Cochrane Database Syst Rev. 2002;(2):CD003584.-
75. Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol. 2004;150:537-544.
76. Davys HJ, Turner DE, Helliwell PS, et al. Debridement of plantar callosities in rheumatoid arthritis: a randomized controlled trial. Rheumatology. 2005;44:207-210.
77. van der Ham AC, Hackeng CA, Yo TI. The treatment of ingrowing toenails. A randomised comparison of wedge excision and phenol cauterisation. J Bone Joint Surg Br. 1990;72:507-509.
78. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.-
79. Crawford F. Athlete’s foot. BMJ Clin Evid. 2005;14:2000-2005.
80. Knapik JJ, Reynolds K, Barson J. Influence of an antiperspirant on foot blister incidence during cross-country hiking. J Am Acad Dermatol. 1998;39:202-206.
81. Reynolds K, Darrigrand A, Roberts D, et al. Effects of an antiperspirant with emollients on foot-sweat accumulation and blister formation while walking in the heat. J Am Acad Dermatol. 1995;33:626-630.
82. Matzen P. How evidence-based is medicine? A systematic literature review. Ugeskr Laeger. 2003;165:1431-1435.
83. Gill P, Dowell AC, Neal RD, et al. Evidence based general practice: a retrospective study of interventions in one training practice. BMJ. 1996;312:819-821.
- Oral flucloxacillin is less effective than local antibiotics for impetigo in limited disease (level of evidence [LOE] 1a).
- Topical metronidazole and azelaic acid are effective for rosacea (LOE 1a).
- Betadine is effective for minor infections following partial thickness burns (LOE 1b).
- Terbinafine is effective against fungal infections of the nail (LOE 1a).
- Miconazole is effective against oral thrush (LOE 1a).
Level of evidence (LOE)
1a: Systematic reviews (with homogeneity) of randomized controlled trials (RCTs).
1a-: Systematic review of randomized trials displaying worrisome heterogeneity.
1b: Individual RCT (with a narrow confidence interval).
1b-: Individual RCT (with a wide confidence interval).
1c: All or none RCTs.
2a: Systematic reviews (with homogeneity) of cohort studies.
2a-: Systematic reviews of cohort studies displaying worrisome heterogeneity.
2b: Individual cohort study or low-quality RCTs (<80% follow-up).
2b-: Individual cohort study or low-quality RCTs (<80% follow-up/wide confidence interval).
2c: “outcomes” research; ecological studies.
3a: Systematic review (with homogeneity) of case-control studies.
3a-: Systematic review of case-control studies with worrisome heterogeneity.
3b: Individual case-control study.
4: Case series (and poor-quality cohort and case-control studies).
5: Expert opinion without explicit critical appraisal, or based on physiology, bench research, or “first principles.”
Source: Essential Evidence Plus. Levels of evidence.1
Do you use silver sulfadiazine for partial-thickness burns? If you do, you may be surprised to learn that the evidence for its use in this situation is conflicting. This was just one of the findings of our systematic review of the methodologic quality and statistical and clinical relevance of current therapies for minor dermatologic ailments.
Given that minor ailments, frequently dermatologic, account for 40% to 70% of all consultations in family medicine,2,3 guidelines based on better research are needed. This need is underscored by the increasing delegation of minor treatments to staff nurses, nurse practitioners, and physician assistants, who should undergo comprehensive training, preferably based on valid guidelines.4,5 Moreover, consultations for prevalent minor ailments often lead to prescriptions for medications, thereby generating considerable costs.6,7
Methods
The starting point for this review was the textbook, Minor Ailments in Primary Care: An Evidence-Based Approach,6 which describes 119 minor ailments, selected mainly on the basis of disease prevalence. We selected all dermatologic ailments (International Classification of Primary Care-code ‘S’) (N=42) (TABLE).5
We searched the online databases PubMed, Cochrane Controlled Trials Register, and Clinical Evidence for articles relating to the treatment of these conditions. For each ailment, we used various search terms for indication and treatment.8 (See note at end of Methods section.) We excluded alternative (nonallopathic) and most preventive therapies because they are unusual in the daily practice of family medicine.
We searched only for trials in which treatments were compared with placebo or a reasonable, accepted usual therapy. The search followed a hierarchy of evidence:8 systematic reviews (SRs), then randomized controlled trials (RCTs), then other research articles (nonrandomized clinical trials, case series). When we found a relevant SR published in 2004 or later, we did not search for a lower level of evidence (LOE). Instead, we restricted our subsequent search to RCTs published after the publication date of the SR.8 Two of the authors (SPG and JAHE) selected articles independently, based on article title and abstract. Disagreements in selection were discussed and consensus was reached. If an article contained relevant first-line therapy, we also used the “related articles” option in PubMed to check for more sources. (See note at end of Methods section.)
To evaluate the methodologic quality of SRs and trials, we ranked articles according to the method of infoPOEMs.8 (See key.) Two experienced researchers (JAHE and AKN) scored all articles independently. Consensus was reached in cases of disagreement.9 We deemed evidence convincing if the study showed the intervention was effective and if the LOE of the study was high (levels 1a, 1b, or 2a).
Evaluating breadth of treatment application. To explore whether a treatment for a certain minor ailment could be applied to other ailments with similar symptoms and thus increase the strength of the treatment’s rationale, we clustered ailments, where possible, into bacterial infection, fungal infection, itch, and pain.
We classified the efficacy of therapies as yes, likely (if the result was not convincingly effective or based on small studies, or if the study objective was unclear), or no. Treatments with no trials to support them are so identified. As to whether the evidence was convincing, we indicated yes, no, or conflicting.
Post hoc analysis. For trials with a wide confidence interval and for therapies described as not clearly effective, we performed a post hoc power analysis to explore if the trial was underpowered.10 We compared the number of subjects in the study (n1) with the number we calculated as necessary for the study to have sufficient power (n2). For all studies, we used standardized values (α=0.05 and β=0.20). If n1≥n2 we considered the study design accurate, and if n1< n2 we concluded that the power was insufficient for the study to be able to answer its objectives.
- terms used in searching online databases
- post hoc power analysis
- a summary of treatment rationales, therapies and their effectiveness, country where the research was undertaken, number of authors, and year of article publication for each dermatologic ailment.
Results
We collected 71 articles published in the medical literature between January 1981 and July 2007.11-81 On average, we found 2 articles per minor dermatologic ailment, with a range of 0 to 7. For 7 common ailments, we found no studies on therapies; for 13 ailments we found just 1 trial each.
For 20 of the 42 ailments, we found a SR of treatments (10 Cochrane reviews, 5 Clinical Evidence, and 5 from other sources). Most articles describing RCTs presented results with wide confidence intervals (LOEs 1b- and 2b-), mainly due to small sample sizes. Eleven RCTs (14%) had high dropout rates (LOE 2b or 2b-).
Seventy-four percent of all the trials were conducted in Europe and North America. The United States (24%) and United Kingdom (25%) were the largest contributors. Studies of Asian and South American populations (eg, Indian, Nepalese, Iraqi, Brazilian) tended to focus on problems more prevalent in these countries, such as lice and scabies.
For 26 of the 42 ailments, evidence was unclear (no studies or studies with inconclusive evidence). Very few of the therapies commonly used for minor dermatologic ailments are supported by high-level research evidence. Even some SRs included only methodologically poor RCTs, which indicates that more research is needed.
A look at outcomes. The TABLE summarizes the effectiveness of therapies usually applied to minor dermatologic ailments in daily practice. The columns present, in turn:
- the minor ailment,
- the treatments usually applied in daily practice,
- the number of studies found for these treatments,
- the condition at which treatment was aimed,
- whether the targeted condition belongs to 1 of the 4 categories of main symptoms,
- whether the study/studies reported a positive effect for the treatment,
- whether the evidence for the effectiveness of a particular treatment was (according to the authors) convincing,
- whether the overall rating of evidence was convincing,
- and whether further studies are needed.
Results varied. With partial thickness burns, evidence was conflicting on the effectiveness and the harms of silver sulfadiazine and several types of gauzes. For boils, we could find no trial about therapy. For both warts and mollusca contagiosa, Cochrane reviews were inconclusive on therapies commonly used in general practice. Evidence was also inconclusive for treatments for paronychia, polymorphic light eruption, and dog and cat bites.
TABLE
The treatment of minor dermatologic ailments: What the research tells us
Treatments for which sufficient positive evidence exists are formatted in bold; those for which negative evidence exists are formatted in bold italic.
| Dermatological minor ailment (N*) | Treatment | Target for treatment | Category of treatment target, according to main symptoms | Was treatment effective? | Was the research convincing?† | Overall rating of research evidence | Are further studies required? | |
|---|---|---|---|---|---|---|---|---|
| 1. | Partial thickness burns (4) | Oral antibiotics (flucloxacillin)11 | Infection (bacterial) | Bacterial infection | No trials | No | Moderate | Yes |
| Gauze dressings11 | Skin lesion | Likely | Conflicting | |||||
| Silver sulfadiazine11 | Skin lesion | No | Conflicting | |||||
| Betadine12 | Infection (bacterial) | Bacterial infection | Yes | Yes | ||||
| Cooling13 | Pain | Pain | No | No | ||||
| Honey14 | Pain | Pain | No | No | ||||
| 2. | Polymorphic light eruption (4) | Neutral lotion | Itch | Itch | No trials | No | Poor | Yes |
| Corticosteroids15,18 | Itch | Itch | Yes | Yes | ||||
| Oral antihistamines | Itch | Itch | No trials | No | ||||
| Sunscreens16,17 | Preventive | Yes | No | |||||
| 3. | Acute urticaria (2) | Local ointments | Itch/rash | Itch | No trials | No | Moderate | Yes |
| Oral antihistamines19 | Itch/rash | Itch | Yes | Yes | ||||
| Corticosteroids20 | Itch/rash | Itch | Yes | Yes | ||||
| 4. | Insect bites and stings (0) | Doxycycline | Infection (bacterial) | Bacterial infection | No trials | No | None | Yes |
| Amoxicillin | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| Oral antihistamines | Pain/itch | Itch | No trials | No | ||||
| 5. | Pediculosis (3) | Malathion21 | Infection | Yes | Yes | Good | No | |
| Permethrin22 | Infection | Yes | Yes | |||||
| Lindane | Infection | No trials | No | |||||
| Combing23 | Infection | Yes | Yes | |||||
| 6. | Scabies (3) | Permethrin25,26 | Infection | Yes | Yes | Good | No | |
| Lindane24 | Infection | Yes | Yes | |||||
| Benzyl benzoate | Infection | No trials | No | |||||
| Malathion | Infection | No trials | No | |||||
| 7. | Dog and cat bites (1) | Oral antibiotics (amoxicillin)27 | Infection (bacterial) | Bacterial infection | Yes, for bites to the hands. No, for other bites | No | Moderate | Yes |
| 8. | Abrasions (1) | Paraffin gauze | Skin lesion | No trials | No | Poor | Yes | |
| Non-adherent absorbent compress | Skin lesion | No trials | No | |||||
| Betadine | Skin lesion/infection (bacterial) | Bacterial infection | No trials | No | ||||
| Honey2 | Skin lesion | Likely | No | |||||
| 9. | Warts (4) | Salicylic acid29 | Lump on the skin | Yes | Yes | Moderate | Yes | |
| Cryotherapy29 | Lump on the skin | No | No | |||||
| Duct tape occlusion30-32 | Lump on the skin | No | Yes, treatment was not effective | |||||
| Surgical procedures | Lump on the skin | No trials | No | |||||
| 10. | Molluscum contagiosum (1) | Curettage33 | Lump on the skin | No trials | No | Poor | Yes | |
| Liquid nitrogen33 | Lump on the skin | No trials | No | |||||
| Fusidic acid cream33 | Lump on the skin | No trials | No | |||||
| Betadine33 | Lump on the skin | No | No | |||||
| 11. | Furuncles (0) | Hot compress | Pain | Bacterial infection | No trials | No | None | Yes |
| Antibiotics | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| 12. | Impetigo (1) | Local fusidic acid or mupirocin34 | Infection (bacterial) | Bacterial infection | Yes | Yes | Good | No |
| Oral antibiotics34 | Infection (bacterial) | Bacterial infection | Yes | Yes, but less effective than local treatment in limited disease | ||||
| 13. | Pityriasis versicolor (2) | Selenium sulphide | Infection | Fungal infection | No trials | No | Moderate | Yes |
| Imidazole | Infection | Fungal infection | No trials | No | ||||
| Fluconazole35 | Infection | Fungal infection | Yes | No | ||||
| Itraconazole36 | Infection | Fungal infection | Yes | No | ||||
| 14. | Intertrigo (2) | Miconazole37,38 | Infection | Fungal infection | Yes | Yes | Moderate | Yes |
| Hydrocortisone37 | Infection | Fungal infection | No | No | ||||
| 15. | Erythrasma (1) | Imidazole | Infection (bacterial) | Bacterial infection | No trials | No | Good | No |
| Benzoic acid | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| Erythromycin39 | Infection (bacterial) | Bacterial infection | Yes | Yes | ||||
| 16. | Shingles (6) | Acyclovir40 | Infection (viral) | Yes | Yes | Moderate/Good | Yes | |
| Famcyclovir41 | Infection (viral) | Yes | No | |||||
| Acyclovir + prednisolone42 | Infection (viral) | Yes | No | |||||
| Corticosteroids43,44 | Inflammation | No | Yes, treatment was not effective | |||||
| Amitriptyline45 | Pain | Likely | No | |||||
| 17. | Pruritus in the elderly (1) | Local emollients | Itch | Itch | No trials | No | Moderate | Yes |
| Corticosteroids | Itch | Itch | No trials | No | ||||
| Local antihistamines | Itch | Itch | No trials | No | ||||
| Oral antihistamines46 | Itch | Itch | Yes | Yes | ||||
| 18. | Xeroderma (0) | Emollients | Dry skin | No trials | No | None | Yes | |
| 19. | Androgenic alopecia (5) | Wig | Hair loss | No trials | No | Moderate | Yes | |
| Finasteride49-51 | Hair loss | Yes | Yes | |||||
| Minoxidil47,48 | Hair loss | Likely | Conflicting | |||||
| 20. | Alopecia areata (5) | Minoxidil52,53 | Hair loss | No | No | Moderate | Yes | |
| Oral prednisolone54 | Hair loss | Likely | No | |||||
| Desoxymethasone55 | Hair loss | No | No | |||||
| Betamethasone56 | Hair loss | Likely | No | |||||
| 21. | Dandruff (4) | Zinc pyrithione57 | Infection (yeast) | Fungal infection | Yes | No | Moderate | Yes |
| Ciclopirox58-60 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Ketoconazole61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Selenium sulphide61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Corticosteroids61 | Itch | Itch | Yes | Yes | ||||
| 22. | Seborrhoeic eczema (2) | Zinc pyrithione57 | Infection (yeast) | Fungal infection | Yes | No | Moderate | Yes |
| Ketoconazole61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Coal tar61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Selenium sulphide61 | Infection (yeast) | Fungal infection | Yes | No | ||||
| Corticosteroids61 | Itch | Itch | Yes | Yes | ||||
| 23. | Herpes labialis (1) | Sunscreens62 | Prevention | Yes | Yes | Good | No | |
| Oral antivirals62 | Infection (viral) | Yes | Yes | |||||
| Zinc oxide cream62 | Skin lesion | Likely | No | |||||
| Topical antivirals | Infection (viral) | No trials | No | |||||
| Topical antivirals | Pain | No trials | No | |||||
| 24. | Perioral dermatitis (1) | Clean with water | Prevention | No trials | No | Poor | Yes | |
| Topical metronidazole63 | Infection | Bacterial infection | Likely | No | ||||
| Tetracycline | Infection | Bacterial infection | No | No | ||||
| 25. | Oral thrush (1) | Nystatin64 | Infection | Fungal infection | Less effective than miconazole | No | Good | No |
| Miconazole64 | Infection | Fungal infection | Yes | Yes | ||||
| 26. | Salmon patch (0) | No treatment is needed | None | No | ||||
| 27. | Chloasma (3) | Hydroquinone, tretinoin, hydrocortisone combination65,66 | Skin irritation | Yes | No | Moderate | Yes | |
| Hydroquinone 67 | Skin irritation | Yes | No | |||||
| 28. | Rosacea (2) | Topical metronidazole68 | Infection | Bacterial infection | Yes | Yes | Moderate | Yes |
| Azelaic acid68 | Infection | Bacterial infection | Yes | Yes | ||||
| Zinc-sulphate69 | Infection | Bacterial infection | Yes | No | ||||
| Tetracycline68 | Infection | Bacterial infection | Yes | No | ||||
| 29. | Umbilical problems in infants (0) | Disinfectant liquid | Infection | Bacterial infection | No trials | - | None | Yes |
| Antiseptic dressing | Infection | Bacterial infection | No trials | - | ||||
| Silver nitrate | To stop granulations | No trials | - | |||||
| Electrocauterization | To stop granulations | No trials | - | |||||
| 30. | Nappy rash (2) | Zinc oxide cream70 | Skin lesion | Yes | Yes | Moderate | Yes | |
| Miconazole71 | Infection | Fungal infection | Yes | No | ||||
| Hydrocortisone | Itch | Itch | No trials | No | ||||
| 31. | Fish hook in finger (0) | Local extirpation | Skin lesion | No trials | - | None | No | |
| 32. | Splinter under nail (0) | Splinter removal | Skin lesion | No trials | - | None | No | |
| 33. | Subungual hematoma (1) | Making a hole in the nail72 | Discharging hematoma | Likely | No | Moderate | Yes | |
| 34. | Brittle nails (0) | Terbinafine (oral) | Infection | No trials | No | None | Yes | |
| Itraconazole (oral) | Infection | No trials | No | |||||
| 35. | Paronychia (0) | Antibiotics | IInfection (bacterial) | Bacterial infection | No trials | No | Poor | Yes |
| Drainage | Discharging pus | No trials | No | |||||
| Antifungal cream | Infection | Fungal infection | No trials | No | ||||
| 36. | Fungal infection of the nail (3) | Local treatment (imidazole)73 | Infection | Fungal infection | Yes | Yes | Good | No |
| Oral terbinafine74,75 | Infection | Fungal infection | Yes | Yes | ||||
| 37. | Calluses on the feet (1) | Removing the excess callus76 | Removing callosity | Yes | No | None | Yes | |
| Disinfectant ointment | Infection | No trials | No | |||||
| 38. | Ingrown nail (2) | Wedge excision77,78 | Removing infected tissue | Yes | Yes | Good | Yes | |
| Chemical ablation77,78 | Destruction nail matrix | Yes | Yes | |||||
| 39. | Corns (1) | Salicylic acid | Resolution callosity | No trials | No | None | Yes | |
| Excision76 | Removing callosity | Yes | No | |||||
| 40. | Athlete’s foot (1) | Imidazole79 | Infection | Fungal infection | Yes | Yes | Good | No |
| Imidazole + hydrocortisone79 | Infection/itch | Fungal infection /itch | Yes | Yes | ||||
| Itraconazole79 | Infection | Fungal infection | Yes | Yes | ||||
| 41. | Foot blisters (2) | Betadine | Infection | Bacterial infection | No trials | No | Moderate | Yes |
| Antiperspirant 80,81 | Reducing incidence of blisters | Yes | Conflicting | |||||
| 42. | Plantar warts (4) | Salicylic acid29 | Lump on the skin | Yes | Yes | Moderate/good | Yes | |
| Cryotherapy 29 | Lump on the skin | No | No | |||||
| Duct tape occlusion30-32 | Lump on the skin | No | Yes, treatment was not effective | |||||
| Surgical procedures | Lump on the skin | No trials | No | |||||
| *N=Number of trials. | ||||||||
| † Convincing evidence taken as level of evidence 1a or 1b. | ||||||||
Click here view to PDF version
Clustering by treatment rationale
Bacterial infections. We found trials on antibiotic therapy for 5 of the 12 minor dermatologic ailments caused by or followed by bacterial infection. For the other 7, no trials were available. We found evidence for the effectiveness of treatment in 3 of the 11 indications (impetigo, erythrasma, and rosacea). For the treatment of impetigo (in cases of limited disease), oral flucloxacillin is less effective than local antibiotic treatment (LOE 1a). Betadine for minor infections after partial thickness burns is effective (LOE 1b)or all other dermatological minor ailments in the bacterial infections category, the effectiveness of antibiotic therapy was unclear.
Fungal infections. For 8 of the 9 ailments in which a fungal infection (yeast, fungals, dermatophytes) was one of the main reasons for therapy, we found trials on antimycotic treatment. There were 2 SRs of oral therapy for fungal nail infections, both concluding that terbinafine is an effective antifungal therapy for the condition. Miconazole is effective for infections with Candida albicans or dermatophytes (LOE 1a).
Itch. Itch was a main reason for treating 8 ailments. We found some trials for neutral lotion or oral antihistamines. We also found evidence supporting use of local antihistamines for 2 of the 8 minor ailments. For 4 ailments, we found studies with positive results for local application of steroids; we found no studies for oral steroids. We can therefore conclude that local steroids are effective for ailments in which itch is one of the main symptoms.
Pain. For 5 ailments, relief from pain was the main target of treatment. Trials, however, did not focus on generic pain medications but on treatments aimed at specific causal pathways of the ailment (eg, antiviral treatment for the post-herpetic pain of shingles). Therefore, we cannot draw generalizable conclusions on the treatment of pain in minor dermatologic ailments.
Post hoc power analysis
Most of the 10 trials with LOE 1b- (and effectiveness of treatment described as no or likely) needed many more patients to reach a higher LOE. In only 2 trials,55,66 the number of patients was sufficient. Four of the 10 trials were missing information that would have enabled us to judge whether they were underpowered. In 4 other trials, we considered the number of patients needed to prove treatment effectiveness (n2) unrealistic, and, consequently, the therapy as very likely ineffective.
Conclusions
Study design was poor for more than half of the trials identified. And other studies were so small as to lack statistical power. We found convincing evidence (SRs or good RCTs) for the effectiveness of usual therapy for fewer than half of the ailments selected. Had we extended our search to more databases, such as EMBASE and CINAHL, we may have identified more trials. However, it is unlikely we would have arrived at a different conclusion, given that the number of relevant studies was so low in the databases we did search (PubMed, Cochrane library, Clinical Evidence).
We clustered ailments to determine if a treatment aimed at a particular symptom or complication could be applied to all ailments exhibiting that condition. On the basis of the treatment effect found for 4 ailments, we determined that local steroids would most likely effectively relieve itch associated with all minor dermatologic ailments. For other conditions, grouping by rationale for treatment did not yield any extendable applications.
Generally accepted treatments for minor dermatologic ailments are insufficiently supported by research evidence. This limitation contrasts dramatically with the body of evidence supporting therapies in other aspects of family practice, reportedly having sufficient LOEs in the range of 50% to 80% of treatments.82,83 Given that minor ailments are a substantial portion of a family physician’s workload, and that other primary care providers are increasingly treating these ailments, definitive guidelines based on high-quality research are needed. This aspect of medical care deserves more attention from researchers and funding agencies.
Correspondence J.A.H. Eekhof, MD, PhD, Department of Public Health and Primary Care, Leiden University Medical Center (LUMC), PO Box 9600, 2300 RC Leiden, The Netherlands; [email protected]
- Oral flucloxacillin is less effective than local antibiotics for impetigo in limited disease (level of evidence [LOE] 1a).
- Topical metronidazole and azelaic acid are effective for rosacea (LOE 1a).
- Betadine is effective for minor infections following partial thickness burns (LOE 1b).
- Terbinafine is effective against fungal infections of the nail (LOE 1a).
- Miconazole is effective against oral thrush (LOE 1a).
Level of evidence (LOE)
1a: Systematic reviews (with homogeneity) of randomized controlled trials (RCTs).
1a-: Systematic review of randomized trials displaying worrisome heterogeneity.
1b: Individual RCT (with a narrow confidence interval).
1b-: Individual RCT (with a wide confidence interval).
1c: All or none RCTs.
2a: Systematic reviews (with homogeneity) of cohort studies.
2a-: Systematic reviews of cohort studies displaying worrisome heterogeneity.
2b: Individual cohort study or low-quality RCTs (<80% follow-up).
2b-: Individual cohort study or low-quality RCTs (<80% follow-up/wide confidence interval).
2c: “outcomes” research; ecological studies.
3a: Systematic review (with homogeneity) of case-control studies.
3a-: Systematic review of case-control studies with worrisome heterogeneity.
3b: Individual case-control study.
4: Case series (and poor-quality cohort and case-control studies).
5: Expert opinion without explicit critical appraisal, or based on physiology, bench research, or “first principles.”
Source: Essential Evidence Plus. Levels of evidence.1
Do you use silver sulfadiazine for partial-thickness burns? If you do, you may be surprised to learn that the evidence for its use in this situation is conflicting. This was just one of the findings of our systematic review of the methodologic quality and statistical and clinical relevance of current therapies for minor dermatologic ailments.
Given that minor ailments, frequently dermatologic, account for 40% to 70% of all consultations in family medicine,2,3 guidelines based on better research are needed. This need is underscored by the increasing delegation of minor treatments to staff nurses, nurse practitioners, and physician assistants, who should undergo comprehensive training, preferably based on valid guidelines.4,5 Moreover, consultations for prevalent minor ailments often lead to prescriptions for medications, thereby generating considerable costs.6,7
Methods
The starting point for this review was the textbook, Minor Ailments in Primary Care: An Evidence-Based Approach,6 which describes 119 minor ailments, selected mainly on the basis of disease prevalence. We selected all dermatologic ailments (International Classification of Primary Care-code ‘S’) (N=42) (TABLE).5
We searched the online databases PubMed, Cochrane Controlled Trials Register, and Clinical Evidence for articles relating to the treatment of these conditions. For each ailment, we used various search terms for indication and treatment.8 (See note at end of Methods section.) We excluded alternative (nonallopathic) and most preventive therapies because they are unusual in the daily practice of family medicine.
We searched only for trials in which treatments were compared with placebo or a reasonable, accepted usual therapy. The search followed a hierarchy of evidence:8 systematic reviews (SRs), then randomized controlled trials (RCTs), then other research articles (nonrandomized clinical trials, case series). When we found a relevant SR published in 2004 or later, we did not search for a lower level of evidence (LOE). Instead, we restricted our subsequent search to RCTs published after the publication date of the SR.8 Two of the authors (SPG and JAHE) selected articles independently, based on article title and abstract. Disagreements in selection were discussed and consensus was reached. If an article contained relevant first-line therapy, we also used the “related articles” option in PubMed to check for more sources. (See note at end of Methods section.)
To evaluate the methodologic quality of SRs and trials, we ranked articles according to the method of infoPOEMs.8 (See key.) Two experienced researchers (JAHE and AKN) scored all articles independently. Consensus was reached in cases of disagreement.9 We deemed evidence convincing if the study showed the intervention was effective and if the LOE of the study was high (levels 1a, 1b, or 2a).
Evaluating breadth of treatment application. To explore whether a treatment for a certain minor ailment could be applied to other ailments with similar symptoms and thus increase the strength of the treatment’s rationale, we clustered ailments, where possible, into bacterial infection, fungal infection, itch, and pain.
We classified the efficacy of therapies as yes, likely (if the result was not convincingly effective or based on small studies, or if the study objective was unclear), or no. Treatments with no trials to support them are so identified. As to whether the evidence was convincing, we indicated yes, no, or conflicting.
Post hoc analysis. For trials with a wide confidence interval and for therapies described as not clearly effective, we performed a post hoc power analysis to explore if the trial was underpowered.10 We compared the number of subjects in the study (n1) with the number we calculated as necessary for the study to have sufficient power (n2). For all studies, we used standardized values (α=0.05 and β=0.20). If n1≥n2 we considered the study design accurate, and if n1< n2 we concluded that the power was insufficient for the study to be able to answer its objectives.
- terms used in searching online databases
- post hoc power analysis
- a summary of treatment rationales, therapies and their effectiveness, country where the research was undertaken, number of authors, and year of article publication for each dermatologic ailment.
Results
We collected 71 articles published in the medical literature between January 1981 and July 2007.11-81 On average, we found 2 articles per minor dermatologic ailment, with a range of 0 to 7. For 7 common ailments, we found no studies on therapies; for 13 ailments we found just 1 trial each.
For 20 of the 42 ailments, we found a SR of treatments (10 Cochrane reviews, 5 Clinical Evidence, and 5 from other sources). Most articles describing RCTs presented results with wide confidence intervals (LOEs 1b- and 2b-), mainly due to small sample sizes. Eleven RCTs (14%) had high dropout rates (LOE 2b or 2b-).
Seventy-four percent of all the trials were conducted in Europe and North America. The United States (24%) and United Kingdom (25%) were the largest contributors. Studies of Asian and South American populations (eg, Indian, Nepalese, Iraqi, Brazilian) tended to focus on problems more prevalent in these countries, such as lice and scabies.
For 26 of the 42 ailments, evidence was unclear (no studies or studies with inconclusive evidence). Very few of the therapies commonly used for minor dermatologic ailments are supported by high-level research evidence. Even some SRs included only methodologically poor RCTs, which indicates that more research is needed.
A look at outcomes. The TABLE summarizes the effectiveness of therapies usually applied to minor dermatologic ailments in daily practice. The columns present, in turn:
- the minor ailment,
- the treatments usually applied in daily practice,
- the number of studies found for these treatments,
- the condition at which treatment was aimed,
- whether the targeted condition belongs to 1 of the 4 categories of main symptoms,
- whether the study/studies reported a positive effect for the treatment,
- whether the evidence for the effectiveness of a particular treatment was (according to the authors) convincing,
- whether the overall rating of evidence was convincing,
- and whether further studies are needed.
Results varied. With partial thickness burns, evidence was conflicting on the effectiveness and the harms of silver sulfadiazine and several types of gauzes. For boils, we could find no trial about therapy. For both warts and mollusca contagiosa, Cochrane reviews were inconclusive on therapies commonly used in general practice. Evidence was also inconclusive for treatments for paronychia, polymorphic light eruption, and dog and cat bites.
TABLE
The treatment of minor dermatologic ailments: What the research tells us
Treatments for which sufficient positive evidence exists are formatted in bold; those for which negative evidence exists are formatted in bold italic.
| Dermatological minor ailment (N*) | Treatment | Target for treatment | Category of treatment target, according to main symptoms | Was treatment effective? | Was the research convincing?† | Overall rating of research evidence | Are further studies required? | |
|---|---|---|---|---|---|---|---|---|
| 1. | Partial thickness burns (4) | Oral antibiotics (flucloxacillin)11 | Infection (bacterial) | Bacterial infection | No trials | No | Moderate | Yes |
| Gauze dressings11 | Skin lesion | Likely | Conflicting | |||||
| Silver sulfadiazine11 | Skin lesion | No | Conflicting | |||||
| Betadine12 | Infection (bacterial) | Bacterial infection | Yes | Yes | ||||
| Cooling13 | Pain | Pain | No | No | ||||
| Honey14 | Pain | Pain | No | No | ||||
| 2. | Polymorphic light eruption (4) | Neutral lotion | Itch | Itch | No trials | No | Poor | Yes |
| Corticosteroids15,18 | Itch | Itch | Yes | Yes | ||||
| Oral antihistamines | Itch | Itch | No trials | No | ||||
| Sunscreens16,17 | Preventive | Yes | No | |||||
| 3. | Acute urticaria (2) | Local ointments | Itch/rash | Itch | No trials | No | Moderate | Yes |
| Oral antihistamines19 | Itch/rash | Itch | Yes | Yes | ||||
| Corticosteroids20 | Itch/rash | Itch | Yes | Yes | ||||
| 4. | Insect bites and stings (0) | Doxycycline | Infection (bacterial) | Bacterial infection | No trials | No | None | Yes |
| Amoxicillin | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| Oral antihistamines | Pain/itch | Itch | No trials | No | ||||
| 5. | Pediculosis (3) | Malathion21 | Infection | Yes | Yes | Good | No | |
| Permethrin22 | Infection | Yes | Yes | |||||
| Lindane | Infection | No trials | No | |||||
| Combing23 | Infection | Yes | Yes | |||||
| 6. | Scabies (3) | Permethrin25,26 | Infection | Yes | Yes | Good | No | |
| Lindane24 | Infection | Yes | Yes | |||||
| Benzyl benzoate | Infection | No trials | No | |||||
| Malathion | Infection | No trials | No | |||||
| 7. | Dog and cat bites (1) | Oral antibiotics (amoxicillin)27 | Infection (bacterial) | Bacterial infection | Yes, for bites to the hands. No, for other bites | No | Moderate | Yes |
| 8. | Abrasions (1) | Paraffin gauze | Skin lesion | No trials | No | Poor | Yes | |
| Non-adherent absorbent compress | Skin lesion | No trials | No | |||||
| Betadine | Skin lesion/infection (bacterial) | Bacterial infection | No trials | No | ||||
| Honey2 | Skin lesion | Likely | No | |||||
| 9. | Warts (4) | Salicylic acid29 | Lump on the skin | Yes | Yes | Moderate | Yes | |
| Cryotherapy29 | Lump on the skin | No | No | |||||
| Duct tape occlusion30-32 | Lump on the skin | No | Yes, treatment was not effective | |||||
| Surgical procedures | Lump on the skin | No trials | No | |||||
| 10. | Molluscum contagiosum (1) | Curettage33 | Lump on the skin | No trials | No | Poor | Yes | |
| Liquid nitrogen33 | Lump on the skin | No trials | No | |||||
| Fusidic acid cream33 | Lump on the skin | No trials | No | |||||
| Betadine33 | Lump on the skin | No | No | |||||
| 11. | Furuncles (0) | Hot compress | Pain | Bacterial infection | No trials | No | None | Yes |
| Antibiotics | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| 12. | Impetigo (1) | Local fusidic acid or mupirocin34 | Infection (bacterial) | Bacterial infection | Yes | Yes | Good | No |
| Oral antibiotics34 | Infection (bacterial) | Bacterial infection | Yes | Yes, but less effective than local treatment in limited disease | ||||
| 13. | Pityriasis versicolor (2) | Selenium sulphide | Infection | Fungal infection | No trials | No | Moderate | Yes |
| Imidazole | Infection | Fungal infection | No trials | No | ||||
| Fluconazole35 | Infection | Fungal infection | Yes | No | ||||
| Itraconazole36 | Infection | Fungal infection | Yes | No | ||||
| 14. | Intertrigo (2) | Miconazole37,38 | Infection | Fungal infection | Yes | Yes | Moderate | Yes |
| Hydrocortisone37 | Infection | Fungal infection | No | No | ||||
| 15. | Erythrasma (1) | Imidazole | Infection (bacterial) | Bacterial infection | No trials | No | Good | No |
| Benzoic acid | Infection (bacterial) | Bacterial infection | No trials | No | ||||
| Erythromycin39 | Infection (bacterial) | Bacterial infection | Yes | Yes | ||||
| 16. | Shingles (6) | Acyclovir40 | Infection (viral) | Yes | Yes | Moderate/Good | Yes | |
| Famcyclovir41 | Infection (viral) | Yes | No | |||||
| Acyclovir + prednisolone42 | Infection (viral) | Yes | No | |||||
| Corticosteroids43,44 | Inflammation | No | Yes, treatment was not effective | |||||
| Amitriptyline45 | Pain | Likely | No | |||||
| 17. | Pruritus in the elderly (1) | Local emollients | Itch | Itch | No trials | No | Moderate | Yes |
| Corticosteroids | Itch | Itch | No trials | No | ||||
| Local antihistamines | Itch | Itch | No trials | No | ||||
| Oral antihistamines46 | Itch | Itch | Yes | Yes | ||||
| 18. | Xeroderma (0) | Emollients | Dry skin | No trials | No | None | Yes | |
| 19. | Androgenic alopecia (5) | Wig | Hair loss | No trials | No | Moderate | Yes | |
| Finasteride49-51 | Hair loss | Yes | Yes | |||||
| Minoxidil47,48 | Hair loss | Likely | Conflicting | |||||
| 20. | Alopecia areata (5) | Minoxidil52,53 | Hair loss | No | No | Moderate | Yes | |
| Oral prednisolone54 | Hair loss | Likely | No | |||||
| Desoxymethasone55 | Hair loss | No | No | |||||
| Betamethasone56 | Hair loss | Likely | No | |||||
| 21. | Dandruff (4) | Zinc pyrithione57 | Infection (yeast) | Fungal infection | Yes | No | Moderate | Yes |
| Ciclopirox58-60 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Ketoconazole61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Selenium sulphide61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Corticosteroids61 | Itch | Itch | Yes | Yes | ||||
| 22. | Seborrhoeic eczema (2) | Zinc pyrithione57 | Infection (yeast) | Fungal infection | Yes | No | Moderate | Yes |
| Ketoconazole61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Coal tar61 | Infection (yeast) | Fungal infection | Yes | Yes | ||||
| Selenium sulphide61 | Infection (yeast) | Fungal infection | Yes | No | ||||
| Corticosteroids61 | Itch | Itch | Yes | Yes | ||||
| 23. | Herpes labialis (1) | Sunscreens62 | Prevention | Yes | Yes | Good | No | |
| Oral antivirals62 | Infection (viral) | Yes | Yes | |||||
| Zinc oxide cream62 | Skin lesion | Likely | No | |||||
| Topical antivirals | Infection (viral) | No trials | No | |||||
| Topical antivirals | Pain | No trials | No | |||||
| 24. | Perioral dermatitis (1) | Clean with water | Prevention | No trials | No | Poor | Yes | |
| Topical metronidazole63 | Infection | Bacterial infection | Likely | No | ||||
| Tetracycline | Infection | Bacterial infection | No | No | ||||
| 25. | Oral thrush (1) | Nystatin64 | Infection | Fungal infection | Less effective than miconazole | No | Good | No |
| Miconazole64 | Infection | Fungal infection | Yes | Yes | ||||
| 26. | Salmon patch (0) | No treatment is needed | None | No | ||||
| 27. | Chloasma (3) | Hydroquinone, tretinoin, hydrocortisone combination65,66 | Skin irritation | Yes | No | Moderate | Yes | |
| Hydroquinone 67 | Skin irritation | Yes | No | |||||
| 28. | Rosacea (2) | Topical metronidazole68 | Infection | Bacterial infection | Yes | Yes | Moderate | Yes |
| Azelaic acid68 | Infection | Bacterial infection | Yes | Yes | ||||
| Zinc-sulphate69 | Infection | Bacterial infection | Yes | No | ||||
| Tetracycline68 | Infection | Bacterial infection | Yes | No | ||||
| 29. | Umbilical problems in infants (0) | Disinfectant liquid | Infection | Bacterial infection | No trials | - | None | Yes |
| Antiseptic dressing | Infection | Bacterial infection | No trials | - | ||||
| Silver nitrate | To stop granulations | No trials | - | |||||
| Electrocauterization | To stop granulations | No trials | - | |||||
| 30. | Nappy rash (2) | Zinc oxide cream70 | Skin lesion | Yes | Yes | Moderate | Yes | |
| Miconazole71 | Infection | Fungal infection | Yes | No | ||||
| Hydrocortisone | Itch | Itch | No trials | No | ||||
| 31. | Fish hook in finger (0) | Local extirpation | Skin lesion | No trials | - | None | No | |
| 32. | Splinter under nail (0) | Splinter removal | Skin lesion | No trials | - | None | No | |
| 33. | Subungual hematoma (1) | Making a hole in the nail72 | Discharging hematoma | Likely | No | Moderate | Yes | |
| 34. | Brittle nails (0) | Terbinafine (oral) | Infection | No trials | No | None | Yes | |
| Itraconazole (oral) | Infection | No trials | No | |||||
| 35. | Paronychia (0) | Antibiotics | IInfection (bacterial) | Bacterial infection | No trials | No | Poor | Yes |
| Drainage | Discharging pus | No trials | No | |||||
| Antifungal cream | Infection | Fungal infection | No trials | No | ||||
| 36. | Fungal infection of the nail (3) | Local treatment (imidazole)73 | Infection | Fungal infection | Yes | Yes | Good | No |
| Oral terbinafine74,75 | Infection | Fungal infection | Yes | Yes | ||||
| 37. | Calluses on the feet (1) | Removing the excess callus76 | Removing callosity | Yes | No | None | Yes | |
| Disinfectant ointment | Infection | No trials | No | |||||
| 38. | Ingrown nail (2) | Wedge excision77,78 | Removing infected tissue | Yes | Yes | Good | Yes | |
| Chemical ablation77,78 | Destruction nail matrix | Yes | Yes | |||||
| 39. | Corns (1) | Salicylic acid | Resolution callosity | No trials | No | None | Yes | |
| Excision76 | Removing callosity | Yes | No | |||||
| 40. | Athlete’s foot (1) | Imidazole79 | Infection | Fungal infection | Yes | Yes | Good | No |
| Imidazole + hydrocortisone79 | Infection/itch | Fungal infection /itch | Yes | Yes | ||||
| Itraconazole79 | Infection | Fungal infection | Yes | Yes | ||||
| 41. | Foot blisters (2) | Betadine | Infection | Bacterial infection | No trials | No | Moderate | Yes |
| Antiperspirant 80,81 | Reducing incidence of blisters | Yes | Conflicting | |||||
| 42. | Plantar warts (4) | Salicylic acid29 | Lump on the skin | Yes | Yes | Moderate/good | Yes | |
| Cryotherapy 29 | Lump on the skin | No | No | |||||
| Duct tape occlusion30-32 | Lump on the skin | No | Yes, treatment was not effective | |||||
| Surgical procedures | Lump on the skin | No trials | No | |||||
| *N=Number of trials. | ||||||||
| † Convincing evidence taken as level of evidence 1a or 1b. | ||||||||
Click here view to PDF version
Clustering by treatment rationale
Bacterial infections. We found trials on antibiotic therapy for 5 of the 12 minor dermatologic ailments caused by or followed by bacterial infection. For the other 7, no trials were available. We found evidence for the effectiveness of treatment in 3 of the 11 indications (impetigo, erythrasma, and rosacea). For the treatment of impetigo (in cases of limited disease), oral flucloxacillin is less effective than local antibiotic treatment (LOE 1a). Betadine for minor infections after partial thickness burns is effective (LOE 1b)or all other dermatological minor ailments in the bacterial infections category, the effectiveness of antibiotic therapy was unclear.
Fungal infections. For 8 of the 9 ailments in which a fungal infection (yeast, fungals, dermatophytes) was one of the main reasons for therapy, we found trials on antimycotic treatment. There were 2 SRs of oral therapy for fungal nail infections, both concluding that terbinafine is an effective antifungal therapy for the condition. Miconazole is effective for infections with Candida albicans or dermatophytes (LOE 1a).
Itch. Itch was a main reason for treating 8 ailments. We found some trials for neutral lotion or oral antihistamines. We also found evidence supporting use of local antihistamines for 2 of the 8 minor ailments. For 4 ailments, we found studies with positive results for local application of steroids; we found no studies for oral steroids. We can therefore conclude that local steroids are effective for ailments in which itch is one of the main symptoms.
Pain. For 5 ailments, relief from pain was the main target of treatment. Trials, however, did not focus on generic pain medications but on treatments aimed at specific causal pathways of the ailment (eg, antiviral treatment for the post-herpetic pain of shingles). Therefore, we cannot draw generalizable conclusions on the treatment of pain in minor dermatologic ailments.
Post hoc power analysis
Most of the 10 trials with LOE 1b- (and effectiveness of treatment described as no or likely) needed many more patients to reach a higher LOE. In only 2 trials,55,66 the number of patients was sufficient. Four of the 10 trials were missing information that would have enabled us to judge whether they were underpowered. In 4 other trials, we considered the number of patients needed to prove treatment effectiveness (n2) unrealistic, and, consequently, the therapy as very likely ineffective.
Conclusions
Study design was poor for more than half of the trials identified. And other studies were so small as to lack statistical power. We found convincing evidence (SRs or good RCTs) for the effectiveness of usual therapy for fewer than half of the ailments selected. Had we extended our search to more databases, such as EMBASE and CINAHL, we may have identified more trials. However, it is unlikely we would have arrived at a different conclusion, given that the number of relevant studies was so low in the databases we did search (PubMed, Cochrane library, Clinical Evidence).
We clustered ailments to determine if a treatment aimed at a particular symptom or complication could be applied to all ailments exhibiting that condition. On the basis of the treatment effect found for 4 ailments, we determined that local steroids would most likely effectively relieve itch associated with all minor dermatologic ailments. For other conditions, grouping by rationale for treatment did not yield any extendable applications.
Generally accepted treatments for minor dermatologic ailments are insufficiently supported by research evidence. This limitation contrasts dramatically with the body of evidence supporting therapies in other aspects of family practice, reportedly having sufficient LOEs in the range of 50% to 80% of treatments.82,83 Given that minor ailments are a substantial portion of a family physician’s workload, and that other primary care providers are increasingly treating these ailments, definitive guidelines based on high-quality research are needed. This aspect of medical care deserves more attention from researchers and funding agencies.
Correspondence J.A.H. Eekhof, MD, PhD, Department of Public Health and Primary Care, Leiden University Medical Center (LUMC), PO Box 9600, 2300 RC Leiden, The Netherlands; [email protected]
1. Essential Evidence Plus. Levels of evidence. Available at: http://www.essentialevidenceplus.com/product/ebm_loe.cfm?show=oxford. Accessed August 18, 2009.
2. Rambihar BV. [e-letter BMJ] 18 May 2001. Available at: bmj.com/cgi/eletters/322/7296/1193. Accessed July 30, 2009.
3. Morris CJ, Cantrill JA, Weiss MC. GPs’ attitudes to minor ailments. Fam Pract. 2001;18:581-585.
4. Pritchard A, Kendrick D. Practice nurse and health visitor management of acute minor illness in a general practice. J Adv Nurs. 2001;36:556-562.
5. Okkes IM, Becker HW, Bernstein RM, et al. The March 2002 update of the electronic version of ICPC-2. A step forward to the use of ICD-10 as a nomenclature and a terminology for ICPC-2. Fam Pract. 2002;19:543-546.
6. Eekhof JAH, Knuistingh NA, Verheij TJM. eds. Minor Ailments in Primary Care: An Evidence-Based Approach. Edinburgh/London: Elsevier Butterworth Heinemann; 2005.
7. Salmon P. The potentially somatizing effect of clinical consultation. CNS Spectr. 2006;11:190-200.
8. Assendelft WJJ, van Tulder MW, Scholten RJ, et al. De praktijk van systematische reviews. II. Zoeken en selecteren van literatuur. [The practice of systematic reviews. II. Searching and selection of studies.] Ned Tijdschr Geneeskd. 1999;143:656-661.
9. Assendelft WJJ, Scholten RJ, van Eijk JT, et al. De Praktijk van systematisch reviews. III. Methodologische beoordeling van onderzoeken. [The practice of systematic reviews. III. Evaluation of methodological quality of research studies.] Ned Tijdschr Geneeskd. 1999;143:714-719.
10. Pocock SJ. The size of a clinical trial. In: Clinical Trials: A Practical Approach. Chichester, England: John Wiley & Sons; 1983:123–141.
11. Wasiak J, Cleland H. Burns (minor thermal). Clin Evid. 2005;14:2388-2396.
12. Han KH, Maitra AK. Management of partial skin thickness burn wounds with Inadine dressings. Burns. 1989;15:399-402.
13. Werner MU, Lassen B, Pedersen JL, et al. Local cooling does not prevent hyperalgesia following burn injury in humans. Pain. 2002;98:297-303.
14. Moore OA, Smith LA, Campbell F, et al. Systematic review of the use of honey as a wound dressing. BMC Complement Altern Med. 2001;1:2.-Available at: http://www.biomedcentral.com/1472-6882/1. Accessed May 8, 2008.
15. Man I, Dawe RS, Ibbotson SH, et al. Is topical steroid effective in polymorphic light eruption? Br J Dermatol. 2000;143(suppl 157):S113.-
16. Proby CM, Baker CS, Morton O, et al. New broad-spectrum sunscreen for polymorphic light eruption. Lancet. 1993;341:1347-1348.
17. Allas S, Lui H, Moyal D, et al. Comparison of the ability of 2 sunscreens to protect against polymorphous light eruption induced by a UV-A/UV-B metal halide lamp. Arch Dermatol. 1999;135:1421-1422.
18. Patel DC, Bellaney GJ, Seed PT, et al. Efficacy of short-course oral prednisolone in polymorphic light eruption: a randomized controlled trial. Br J Dermatol. 2000;143:828-831.
19. Lin RY, Curry A, Pesola GR, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000;36:462-468.
20. Pollack CV, Jr, Romano TJ. Outpatient management of acute urticaria: the role of prednisone. Ann Emerg Med. 1995;26:547-551.
21. Dodd CS. Interventions for treating head lice. Cochrane Database Syst Rev. 2001;(3):CD001165.-
22. Vander Stichele RH, Dezeure EM, Bogaert MG. Systematic review of clinical efficacy of topical treatments for head lice. BMJ. 1995;311:604-608.
23. Hill N, Moor G, Cameron MM, et al. Single blind, randomised, comparative study of the Bug Buster kit and over the counter pediculicide treatments against head lice in the United Kingdom. BMJ. 2005;331:384-387.Epub 2005 Aug 5. Available at: http://www.bmj.com/content/vol331/issue7513/#PRIMARY_CARE. Accessed May 5, 2008.
24. Walker GJ, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev. 2000;(3):CD000320.-
25. Usha V, Gopalakrishnan Nair TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol. 2000;42:236-240.
26. Zargari O, Golchai J, Sobhani A, et al. Comparison of the efficacy of topical 1% lindane vs 5% permethrin in scabies: a randomized, double-blind study. Indian J Dermatol Venereol Leprol. 2006;72:33-36.
27. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.-
28. Ingle R, Levin J, Polinder K. Wound healing with honey—a randomised controlled trial. S Afr Med J. 2006;96:831-835.
29. Gibbs S, Harvey I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2006;(3):CD001781.-
30. Focht DR, 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
31. Wenner R, Askari SK, Cham PM, et al. Duct tape for the treatment of common warts in adults: a double-blind randomized controlled trial. Arch Dermatol. 2007;143:309-313.
32. de Haen M, Spigt MG, van Uden CJ, et al. Efficacy of duct tape vs placebo in the treatment of verruca vulgaris (warts) in primary school children. Arch Pediatr Adolesc Med. 2006;160:1121-1125.
33. van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006;(2):CD004767.-
34. Koning S, Verhagen AP, van Suijlekom-Smit LW, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2004;(2):CD003261.-
35. Partap R, Kaur I, Chakrabarti A, et al. Single-dose fluconazole versus itraconazole in pityriasis versicolor. Dermatology. 2004;208:55-59.
36. Hickman JG. A double-blind, randomized, placebo-controlled evaluation of short-term treatment with oral itraconazole in patients with tinea versicolor. J Am Acad Dermatol. 1996;34:785-787.
37. Hedley K, Tooley P, Williams H. Problems with clinical trials in general practice—a double-blind comparison of cream containing miconazole and hydrocortisone with hydrocortisone alone in the treatment of intertrigo. Br J Clin Pract. 1990;44:131-135.
38. Mistiaen P, Poot E, Hickox S, et al. Preventing and treating intertrigo in the large skin folds of adults: a literature overview. Dermatol Nurs. 2004;16:43-46,49–57.
39. Hamann K, Thorn P. Systemic or local treatment of erythrasma? A comparison between erythromycin tablets and Fucidin cream in general practice. Scand J Prim Health Care. 1991;9:35-39.
40. Lancaster T, Silagy C, Gray S. Primary care management of acute herpes zoster: systematic review of evidence from randomized controlled trials. Br J Gen Pract. 1995;45:39-45.
41. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
42. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
43. Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896-900.
44. van Wijck AJ, Opstelten W, Moons KG, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet. 2006;367:219-224.
45. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
46. Dupont C, de Maubeuge J, Kotlar W, et al. Oxatomide in the treatment of pruritus senilis. A double-blind placebo-controlled trial. Dermatologica. 1984;169:348-353.
47. Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol. 1999;41:717-721.
48. Olsen EA. Topical minoxidil in the treatment of androgenetic alopecia in women. Cutis. 1991;48:243-248.
49. Kawashima M, Hayashi N, Igarashi A. Finasteride in the treatment of Japanese men with male pattern hair loss. Eur J Dermatol. 2004;14:247-254.
50. Price VH, Menefee E, Sanchez M, et al. Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. J Am Acad Dermatol. 2006;55:71-74.
51. Whiting DA, Olsen EA, Savin R, et al. Male Pattern Hair Loss Study Group. Efficacy and tolerability of finasteride 1 mg in men aged 41 to 60 years with male pattern hair loss. Eur J Dermatol. 2003;13:150-160.
52. Fransway AF, Muller SA. 3 percent topical minoxidil compared with placebo for the treatment of chronic severe alopecia areata. Cutis. 1988;41:431-435.
53. Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
54. Kar BR, Handa S, Dogra S, et al. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005;52:287-290.
55. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
56. Mancuso G, Balducci A, Casadio C, et al. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: a multicenter, prospective, randomized, controlled, investigator-blinded trial. Int J Dermatol. 2003;42:572-575.
57. Warner RR, Schwartz JR, Boissy Y, et al. Dandruff has an altered stratum corneum ultrastructure that is improved with zinc pyrithione shampoo. J Am Acad Dermatol. 2001;45:897-903.
58. Altmeyer P, Hoffmann K. Loprox Shampoo Dosing Concentration Study Group. Efficacy of different concentrations of ciclopirox shampoo for the treatment of seborrheic dermatitis of the scalp: results of a randomized, double-blind, vehicle-controlled trial. Int J Dermatol. 2004;43(suppl 1):9-12.
59. Lee JH, Lee HS, Eun HC, et al. Successful treatment of dandruff with 1.5% ciclopirox olamine shampoo in Korea. J Dermatolog Treat. 2003;14:212-215.
60. Squire RA, Goode K. A randomised, single-blind, single-centre clinical trial to evaluate comparative clinical efficacy of shampoos containing ciclopirox olamine (1.5%) and salicylic acid (3%), or ketoconazole (2%, Nizoral) for the treatment of dandruff/seborrhoic dermatitis. J Dermatolog Treat. 2002;13:51-60.
61. Manriquez JJ, Uribe P. Seborrhoeic dermatitis. BMJ Clin Evid. 2007;7:171-173.
62. Worrall G. Herpes labialis. BMJ Clin Evid. 2005;14:2050-2057.
63. Veien NK, Munkvad JM, Nielsen AO, et al. Topical metronidazole in the treatment of perioral dermatitis. J Am Acad Dermatol. 1991;24:258-260.
64. Pankhurst CL. Candidiasis (oropharyngeal). BMJ Clin Evid. 2006;15:1849-1863.
65. Ferreira Cestari T, Hassun K, Sittart A, et al. A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6:36-39.
66. Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
67. Espinal-Perez LE, Moncada B, Castanedo-Cazares JP. A double-blind randomized trial of 5% ascorbic acid vs. 4% hydroquinone in melasma. Int J Dermatol. 2004;43:604-607.
68. van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.-
69. Sharquie KE, Najim RA, Al-Salman HN. Oral zinc sulfate in the treatment of rosacea: a double-blind, placebo-controlled study. Int J Dermatol. 2006;45:857-861.
70. Baldwin S, Odio MR, Haines SL, et al. Skin benefits from continuous topical administration of a zinc oxide/petrolatum formulation by a novel disposable diaper. J Eur Acad Dermatol Venereol. 2001;15(suppl 1):S5-S11.
71. Concannon P, Gisoldi E, Phillips S, et al. Diaper dermatitis: a therapeutic dilemma. Results of a double-blind placebo controlled trial of miconazole nitrate 0.25%. Pediatr Dermatol. 2001;18:149-155.
72. Ciocon D, Gowrishankar TR, Herndon T, et al. How low should you go: novel device for nail trephination. Dermatol Surg. 2006;32:828-833.
73. Crawford F, Hart R, Bell-Syer S, et al. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev. 1999;(3):CD001434.-
74. Bell-Syer SEM, Hart R, Crawford F, et al. Oral treatments for fungal infections of the skin of the foot. Cochrane Database Syst Rev. 2002;(2):CD003584.-
75. Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol. 2004;150:537-544.
76. Davys HJ, Turner DE, Helliwell PS, et al. Debridement of plantar callosities in rheumatoid arthritis: a randomized controlled trial. Rheumatology. 2005;44:207-210.
77. van der Ham AC, Hackeng CA, Yo TI. The treatment of ingrowing toenails. A randomised comparison of wedge excision and phenol cauterisation. J Bone Joint Surg Br. 1990;72:507-509.
78. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.-
79. Crawford F. Athlete’s foot. BMJ Clin Evid. 2005;14:2000-2005.
80. Knapik JJ, Reynolds K, Barson J. Influence of an antiperspirant on foot blister incidence during cross-country hiking. J Am Acad Dermatol. 1998;39:202-206.
81. Reynolds K, Darrigrand A, Roberts D, et al. Effects of an antiperspirant with emollients on foot-sweat accumulation and blister formation while walking in the heat. J Am Acad Dermatol. 1995;33:626-630.
82. Matzen P. How evidence-based is medicine? A systematic literature review. Ugeskr Laeger. 2003;165:1431-1435.
83. Gill P, Dowell AC, Neal RD, et al. Evidence based general practice: a retrospective study of interventions in one training practice. BMJ. 1996;312:819-821.
1. Essential Evidence Plus. Levels of evidence. Available at: http://www.essentialevidenceplus.com/product/ebm_loe.cfm?show=oxford. Accessed August 18, 2009.
2. Rambihar BV. [e-letter BMJ] 18 May 2001. Available at: bmj.com/cgi/eletters/322/7296/1193. Accessed July 30, 2009.
3. Morris CJ, Cantrill JA, Weiss MC. GPs’ attitudes to minor ailments. Fam Pract. 2001;18:581-585.
4. Pritchard A, Kendrick D. Practice nurse and health visitor management of acute minor illness in a general practice. J Adv Nurs. 2001;36:556-562.
5. Okkes IM, Becker HW, Bernstein RM, et al. The March 2002 update of the electronic version of ICPC-2. A step forward to the use of ICD-10 as a nomenclature and a terminology for ICPC-2. Fam Pract. 2002;19:543-546.
6. Eekhof JAH, Knuistingh NA, Verheij TJM. eds. Minor Ailments in Primary Care: An Evidence-Based Approach. Edinburgh/London: Elsevier Butterworth Heinemann; 2005.
7. Salmon P. The potentially somatizing effect of clinical consultation. CNS Spectr. 2006;11:190-200.
8. Assendelft WJJ, van Tulder MW, Scholten RJ, et al. De praktijk van systematische reviews. II. Zoeken en selecteren van literatuur. [The practice of systematic reviews. II. Searching and selection of studies.] Ned Tijdschr Geneeskd. 1999;143:656-661.
9. Assendelft WJJ, Scholten RJ, van Eijk JT, et al. De Praktijk van systematisch reviews. III. Methodologische beoordeling van onderzoeken. [The practice of systematic reviews. III. Evaluation of methodological quality of research studies.] Ned Tijdschr Geneeskd. 1999;143:714-719.
10. Pocock SJ. The size of a clinical trial. In: Clinical Trials: A Practical Approach. Chichester, England: John Wiley & Sons; 1983:123–141.
11. Wasiak J, Cleland H. Burns (minor thermal). Clin Evid. 2005;14:2388-2396.
12. Han KH, Maitra AK. Management of partial skin thickness burn wounds with Inadine dressings. Burns. 1989;15:399-402.
13. Werner MU, Lassen B, Pedersen JL, et al. Local cooling does not prevent hyperalgesia following burn injury in humans. Pain. 2002;98:297-303.
14. Moore OA, Smith LA, Campbell F, et al. Systematic review of the use of honey as a wound dressing. BMC Complement Altern Med. 2001;1:2.-Available at: http://www.biomedcentral.com/1472-6882/1. Accessed May 8, 2008.
15. Man I, Dawe RS, Ibbotson SH, et al. Is topical steroid effective in polymorphic light eruption? Br J Dermatol. 2000;143(suppl 157):S113.-
16. Proby CM, Baker CS, Morton O, et al. New broad-spectrum sunscreen for polymorphic light eruption. Lancet. 1993;341:1347-1348.
17. Allas S, Lui H, Moyal D, et al. Comparison of the ability of 2 sunscreens to protect against polymorphous light eruption induced by a UV-A/UV-B metal halide lamp. Arch Dermatol. 1999;135:1421-1422.
18. Patel DC, Bellaney GJ, Seed PT, et al. Efficacy of short-course oral prednisolone in polymorphic light eruption: a randomized controlled trial. Br J Dermatol. 2000;143:828-831.
19. Lin RY, Curry A, Pesola GR, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000;36:462-468.
20. Pollack CV, Jr, Romano TJ. Outpatient management of acute urticaria: the role of prednisone. Ann Emerg Med. 1995;26:547-551.
21. Dodd CS. Interventions for treating head lice. Cochrane Database Syst Rev. 2001;(3):CD001165.-
22. Vander Stichele RH, Dezeure EM, Bogaert MG. Systematic review of clinical efficacy of topical treatments for head lice. BMJ. 1995;311:604-608.
23. Hill N, Moor G, Cameron MM, et al. Single blind, randomised, comparative study of the Bug Buster kit and over the counter pediculicide treatments against head lice in the United Kingdom. BMJ. 2005;331:384-387.Epub 2005 Aug 5. Available at: http://www.bmj.com/content/vol331/issue7513/#PRIMARY_CARE. Accessed May 5, 2008.
24. Walker GJ, Johnstone PW. Interventions for treating scabies. Cochrane Database Syst Rev. 2000;(3):CD000320.-
25. Usha V, Gopalakrishnan Nair TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. J Am Acad Dermatol. 2000;42:236-240.
26. Zargari O, Golchai J, Sobhani A, et al. Comparison of the efficacy of topical 1% lindane vs 5% permethrin in scabies: a randomized, double-blind study. Indian J Dermatol Venereol Leprol. 2006;72:33-36.
27. Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;(2):CD001738.-
28. Ingle R, Levin J, Polinder K. Wound healing with honey—a randomised controlled trial. S Afr Med J. 2006;96:831-835.
29. Gibbs S, Harvey I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2006;(3):CD001781.-
30. Focht DR, 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
31. Wenner R, Askari SK, Cham PM, et al. Duct tape for the treatment of common warts in adults: a double-blind randomized controlled trial. Arch Dermatol. 2007;143:309-313.
32. de Haen M, Spigt MG, van Uden CJ, et al. Efficacy of duct tape vs placebo in the treatment of verruca vulgaris (warts) in primary school children. Arch Pediatr Adolesc Med. 2006;160:1121-1125.
33. van der Wouden JC, Menke J, Gajadin S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006;(2):CD004767.-
34. Koning S, Verhagen AP, van Suijlekom-Smit LW, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2004;(2):CD003261.-
35. Partap R, Kaur I, Chakrabarti A, et al. Single-dose fluconazole versus itraconazole in pityriasis versicolor. Dermatology. 2004;208:55-59.
36. Hickman JG. A double-blind, randomized, placebo-controlled evaluation of short-term treatment with oral itraconazole in patients with tinea versicolor. J Am Acad Dermatol. 1996;34:785-787.
37. Hedley K, Tooley P, Williams H. Problems with clinical trials in general practice—a double-blind comparison of cream containing miconazole and hydrocortisone with hydrocortisone alone in the treatment of intertrigo. Br J Clin Pract. 1990;44:131-135.
38. Mistiaen P, Poot E, Hickox S, et al. Preventing and treating intertrigo in the large skin folds of adults: a literature overview. Dermatol Nurs. 2004;16:43-46,49–57.
39. Hamann K, Thorn P. Systemic or local treatment of erythrasma? A comparison between erythromycin tablets and Fucidin cream in general practice. Scand J Prim Health Care. 1991;9:35-39.
40. Lancaster T, Silagy C, Gray S. Primary care management of acute herpes zoster: systematic review of evidence from randomized controlled trials. Br J Gen Pract. 1995;45:39-45.
41. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
42. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
43. Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896-900.
44. van Wijck AJ, Opstelten W, Moons KG, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet. 2006;367:219-224.
45. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
46. Dupont C, de Maubeuge J, Kotlar W, et al. Oxatomide in the treatment of pruritus senilis. A double-blind placebo-controlled trial. Dermatologica. 1984;169:348-353.
47. Price VH, Menefee E, Strauss PC. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J Am Acad Dermatol. 1999;41:717-721.
48. Olsen EA. Topical minoxidil in the treatment of androgenetic alopecia in women. Cutis. 1991;48:243-248.
49. Kawashima M, Hayashi N, Igarashi A. Finasteride in the treatment of Japanese men with male pattern hair loss. Eur J Dermatol. 2004;14:247-254.
50. Price VH, Menefee E, Sanchez M, et al. Changes in hair weight in men with androgenetic alopecia after treatment with finasteride (1 mg daily): three- and 4-year results. J Am Acad Dermatol. 2006;55:71-74.
51. Whiting DA, Olsen EA, Savin R, et al. Male Pattern Hair Loss Study Group. Efficacy and tolerability of finasteride 1 mg in men aged 41 to 60 years with male pattern hair loss. Eur J Dermatol. 2003;13:150-160.
52. Fransway AF, Muller SA. 3 percent topical minoxidil compared with placebo for the treatment of chronic severe alopecia areata. Cutis. 1988;41:431-435.
53. Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
54. Kar BR, Handa S, Dogra S, et al. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005;52:287-290.
55. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
56. Mancuso G, Balducci A, Casadio C, et al. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: a multicenter, prospective, randomized, controlled, investigator-blinded trial. Int J Dermatol. 2003;42:572-575.
57. Warner RR, Schwartz JR, Boissy Y, et al. Dandruff has an altered stratum corneum ultrastructure that is improved with zinc pyrithione shampoo. J Am Acad Dermatol. 2001;45:897-903.
58. Altmeyer P, Hoffmann K. Loprox Shampoo Dosing Concentration Study Group. Efficacy of different concentrations of ciclopirox shampoo for the treatment of seborrheic dermatitis of the scalp: results of a randomized, double-blind, vehicle-controlled trial. Int J Dermatol. 2004;43(suppl 1):9-12.
59. Lee JH, Lee HS, Eun HC, et al. Successful treatment of dandruff with 1.5% ciclopirox olamine shampoo in Korea. J Dermatolog Treat. 2003;14:212-215.
60. Squire RA, Goode K. A randomised, single-blind, single-centre clinical trial to evaluate comparative clinical efficacy of shampoos containing ciclopirox olamine (1.5%) and salicylic acid (3%), or ketoconazole (2%, Nizoral) for the treatment of dandruff/seborrhoic dermatitis. J Dermatolog Treat. 2002;13:51-60.
61. Manriquez JJ, Uribe P. Seborrhoeic dermatitis. BMJ Clin Evid. 2007;7:171-173.
62. Worrall G. Herpes labialis. BMJ Clin Evid. 2005;14:2050-2057.
63. Veien NK, Munkvad JM, Nielsen AO, et al. Topical metronidazole in the treatment of perioral dermatitis. J Am Acad Dermatol. 1991;24:258-260.
64. Pankhurst CL. Candidiasis (oropharyngeal). BMJ Clin Evid. 2006;15:1849-1863.
65. Ferreira Cestari T, Hassun K, Sittart A, et al. A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6:36-39.
66. Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
67. Espinal-Perez LE, Moncada B, Castanedo-Cazares JP. A double-blind randomized trial of 5% ascorbic acid vs. 4% hydroquinone in melasma. Int J Dermatol. 2004;43:604-607.
68. van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.-
69. Sharquie KE, Najim RA, Al-Salman HN. Oral zinc sulfate in the treatment of rosacea: a double-blind, placebo-controlled study. Int J Dermatol. 2006;45:857-861.
70. Baldwin S, Odio MR, Haines SL, et al. Skin benefits from continuous topical administration of a zinc oxide/petrolatum formulation by a novel disposable diaper. J Eur Acad Dermatol Venereol. 2001;15(suppl 1):S5-S11.
71. Concannon P, Gisoldi E, Phillips S, et al. Diaper dermatitis: a therapeutic dilemma. Results of a double-blind placebo controlled trial of miconazole nitrate 0.25%. Pediatr Dermatol. 2001;18:149-155.
72. Ciocon D, Gowrishankar TR, Herndon T, et al. How low should you go: novel device for nail trephination. Dermatol Surg. 2006;32:828-833.
73. Crawford F, Hart R, Bell-Syer S, et al. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev. 1999;(3):CD001434.-
74. Bell-Syer SEM, Hart R, Crawford F, et al. Oral treatments for fungal infections of the skin of the foot. Cochrane Database Syst Rev. 2002;(2):CD003584.-
75. Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol. 2004;150:537-544.
76. Davys HJ, Turner DE, Helliwell PS, et al. Debridement of plantar callosities in rheumatoid arthritis: a randomized controlled trial. Rheumatology. 2005;44:207-210.
77. van der Ham AC, Hackeng CA, Yo TI. The treatment of ingrowing toenails. A randomised comparison of wedge excision and phenol cauterisation. J Bone Joint Surg Br. 1990;72:507-509.
78. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.-
79. Crawford F. Athlete’s foot. BMJ Clin Evid. 2005;14:2000-2005.
80. Knapik JJ, Reynolds K, Barson J. Influence of an antiperspirant on foot blister incidence during cross-country hiking. J Am Acad Dermatol. 1998;39:202-206.
81. Reynolds K, Darrigrand A, Roberts D, et al. Effects of an antiperspirant with emollients on foot-sweat accumulation and blister formation while walking in the heat. J Am Acad Dermatol. 1995;33:626-630.
82. Matzen P. How evidence-based is medicine? A systematic literature review. Ugeskr Laeger. 2003;165:1431-1435.
83. Gill P, Dowell AC, Neal RD, et al. Evidence based general practice: a retrospective study of interventions in one training practice. BMJ. 1996;312:819-821.
EYE ON THE ELDERLY—Screening for hearing loss, risk of falls: A hassle-free approach
- Simply asking elderly patients whether they have trouble hearing is an effective start to screening for hearing loss (SOR: B).
- Refer elderly patients with suspected hearing impairment for audiologic diagnosis and nonmedical rehabilitation treatment, including hearing aids (SOR: B).
- To assess a patient’s risk of falling, review gait, balance disorders, weakness, environmental hazards, and medications (SOR: A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“Do you have a hearing problem now? Have you fallen recently?” These 2 simple questions are the first step in assessing a patient’s hearing status and risk of falls—a screening opportunity too often overlooked. Although family physicians are well qualified to address hearing loss and the risk of falls, screening elderly patients for these problems often seems like a lower priority than evaluating for serious, or potentially life-threatening, conditions. In a recent national survey of primary care physicians, most said they had little time to screen for hearing loss or vestibular or balance disorders and did so only if patients broached the subject or showed clear evidence of risk.1
Screening for hearing impairment in patients 65 years of age and older, with referral to appropriate specialists, ranked 15th among services deemed effective by the US Preventive Services Task Force and the Advisory Committee on Immunization Practices2,3—outranking screening for osteoporosis, cholesterol, and diabetes.
There is no evidence favoring any particular screening procedure. As a result, physicians have considerable leeway in assessing elderly patients’ functional ability and safety, including the risk of falls that may be precipitated by an impaired vestibular system and balance disorders.4
Screening for these problems need not be onerous. It can be accomplished as part of your continuity of care with longstanding patients or during the preventive care examination that Medicare offers newly enrolled patients. This review, and the screening tools and strategies that accompany it, will help you get started.
Hearing loss screening: Make it easier to do
Bilateral hearing impairment affects 1 in 3 adults over 65 years of age5 and is the third most common chronic condition among the elderly,6 trailing only arthritis and hypertension. Documented problems associated with hearing loss include social isolation, depression and anxiety, loneliness, diminished self-efficacy, and stressful relationships with family, friends, and coworkers who may experience frustration, impatience, anger, pity, or guilt in trying to communicate with a person who has a hearing loss.7-9
The mental, emotional, and social consequences of untreated hearing loss negatively affect patients’ health-related quality of life (HRQoL).8,10-15 Hearing loss also compromises patients’ ability to interact with you and to understand—and follow—your recommendations.
Simplify screening. A recent study assessed 2 hearing screening methods used with older adults who also underwent audiologic evaluation as part of the biennial examination for the Framingham Heart Study.16 It found that simply asking, “Do you have a hearing problem now?” effectively identified potential deficits. If you use the American Academy of Family Physicians’ (AAFP) Medicare Initial Preventive Physical Examination Encounter Form,17 consider replacing its entry for hearing loss with this simple question (See “Medicare preventive exam: Where the AAFP encounter form falls short”).
We recommend that you pose the question to elderly patients or their family members during regular office visits. If the answer is Yes, immediately assess the patient’s ability to understand conversational speech. If necessary, use an inexpensive amplification device to make it easier for you and your patient to communicate. Referral to an audiologist for a comprehensive evaluation may be indicated, as well.
The American Academy of Family Physicians’ (AAFP) Medicare Initial Preventive Physical Examination Encounter Form17 does not fully address hearing screening. Unlike the Depression Screen and Functional Ability/Safety Screen sections, which require Yes or No responses to questions, the section covering hearing merely presents the term “Hearing Evaluation,” followed by a space for recording information.
Although the form clearly states that a Yes response to any question about depression or function/safety should trigger further evaluation, there is no such recommendation for further evaluation of hearing. Thus, some practitioners short on time may overlook hearing screening entirely, and some elderly patients with sensorineural hearing loss may not receive appropriate education, counseling, or referral.
Furthermore, the second page of the AAFP form that is given to patients and makes recommendations for scheduled follow-up does not even mention hearing or the risk of falling. That’s why it’s important to remember to cover these areas with your elderly patients—and why you may want to ask the questions, “Do you have a hearing problem now?” and “Have you fallen recently?”
A time-saving suggestion. You can save precious consultation time by having elderly patients complete a standardized self-assessment questionnaire such as the Hearing Handicap Inventory for the Elderly-Screening version (HHIE-S) (FIGURE 1) while they’re in the waiting room.18,19 When hearing loss is identified, counsel the patient as appropriate and strongly recommend further audiologic testing and management.20 The HHIE-S can help patients realize the social and emotional consequences of hearing loss, which may encourage them to seek assistance. But many won’t do so without your recommendation.
Let patients know something can be done. Evidence shows that nonmedical management of sensorineural hearing loss, including the fitting of hearing aids, is critical in helping older adults improve communication and reduce psychosocial problems.10,21
Recently, a task force of the American Academy of Audiology conducted a systematic review of the HRQoL benefits of amplification in adults.21 A meta-analysis revealed that hearing aids had at least a small effect on HRQoL, as measured by generic health instruments (eg, the Medical Outcomes Study Short Form-36), and medium-to-large effects when disease-specific hearing outcome measures (eg, the HHIE) were used. Hearing aids, combined with auditory rehabilitation, changed patients’ perception of their handicap to a greater extent than amplification alone, particularly during the initial stage of adjusting to a device.22,23
Preprinted forms can help educate patients about hearing loss and the benefits of amplification. The PATIENT HANDOUT (ARE YOU HAVING HEARING PROBLEMS?) explains the causes and symptoms of sensorineural hearing loss, as well as diagnosis, frequency of audiologic evaluations, and treatments.
Sensorineural hearing loss occurs when sensory receptors (hair cells) in the inner ear (cochlea) or hearing nerve pathways to the brain are damaged. It is usually permanent and cannot be treated medically or surgically, but hearing aids almost always help.
Hearing loss most commonly results from age-related changes in the inner ear. Other possible causes are excessive noise exposure, drugs that are toxic to the auditory system, certain viruses or diseases, head trauma, and genetic or familial disorders.
If you think you may have a hearing loss or are having difficulty communicating, tell your doctor immediately.
What are the signs that I may be losing my hearing?
You may:
- complain that people are mumbling
- continually ask people to repeat themselves
- avoid noisy rooms, social occasions, or family gatherings
- prefer the TV or radio louder than other people do
- have difficulty understanding people when you can’t see their faces
- have trouble hearing at the movies or theater, your house of worship, or other public places
- have difficulty following conversations in a group
- become impatient, irritable, frustrated, or withdrawn.
Why can I hear people talk but not understand what they’re saying?
Hearing loss not only reduces your ability to hear normally audible sound, it interferes with your ability to detect particular sounds. High-pitched consonants (such as d, f, sh, s, t, and th) become harder to hear than low-pitched vowels. The high-pitched consonant sounds carry the meaning of words and help us understand speech, but in normal conversation, they’re softer than the less important low-pitched vowel sounds. So conversation may sound loud enough, but the words may not be clear.
How is hearing loss diagnosed?
Audiologists—health care professionals who specialize in hearing—conduct a comprehensive evaluation to determine the degree and type of hearing loss and its effect on your ability to communicate in everyday life. The exam is conducted with special instruments in a sound-dampened room. Medical diagnosis of ear disease is performed by ear, nose, and throat doctors (also known as ENTs or otolaryngologists).
How often should I have a hearing test?
After age 50, you should have your hearing tested every 2 years. But it’s important to request a hearing test immediately if you notice a sudden change in hearing, increased ringing in the ears (tinnitus), or dizziness.
How is hearing loss treated?
Hearing aids, which work better than ever because of digital technology, help many people. Your audiologist will talk to you about the different kinds of hearing aids available and what you can realistically expect when you use them. Together you’ll decide which one is best for you.
People who use hearing aids report better personal relationships, an easier time communicating, improved mental health, and a greater sense of control over their lives.
Where can I get more information?
American Academy of Audiology
800-AAA-2336
http://www.audiology.org
American Academy of Otolaryngology, Head, and Neck Surgery
703-836-4444
http://www.aaohns.org
American Speech-Language-Hearing Association
800-638-8255
http://www.asha.org
FIGURE 1
Hearing Handicap Inventory for the Elderly—Screening version (HHIE-S)
Source: Ventry IM, et al. ASHA. 1983.18 Reprinted with permission.
Copyright 1983 by American Speech-Language-Hearing Association. All rights reserved.
*If your score is 10 or greater, you may benefit from additional hearing evaluation.
Falls risk: Make use of assessment tools
Each year, approximately 30% to 40% of elderly adults living independently fall, and the incidence increases with age.4 Falls frequently cause injuries, such as hip fractures, and reduce HRQoL.24 Strong evidence supports the efficacy of a multifaceted approach for identifying and reducing risk of falls in this patient population.25,26 A review of more than 3000 falls indicated that about one-third were the result of accidents or environmental hazards, and nearly another third were caused by gait problems, balance disorders, or weakness.27
Means of determining risk of falls. As with hearing assessment, you can gauge older patients’ risk of falls during routine visits or annual exams. For patients’ first Medicare visit, the AAFP Medicare preventive exam encounter form17 includes 2 items in the “Functional Ability/Safety Screen” section that cover the leading causes of falls. Item 1 asks, “Was the patient’s Timed Up & Go test unsteady or longer than 30 seconds?” This simple test requires patients to stand from a sitting position, using their arms for support; walk a few paces, turn around, and return to their chair to sit. The test is sensitive to, and specific for, identifying elderly people living independently who are at risk of falls.28
The Dizziness Handicap Inventory–Screening version (DHI-S) (FIGURE 2),29 is a 10-item patient self-assessment questionnaire that takes fewer than 5 minutes to complete. You can also use the DHI-S to supplement the Timed Up & Go test in judging a patient’s postural control.
Possible reasons for poor performance on the Timed Up & Go test are vestibular dysfunction or other balance disorders that require a careful evaluation. Cross-checking the “Medications, supplements, and vitamins” list in the “Medical/Social History” section of the Medicare encounter form may reveal use of a drug or multiple drugs (particularly likely if the patient sees different physicians) that commonly contribute to falls.30
Patients’ descriptions of “Past personal illnesses and injuries,” also covered in the encounter form, or their accounts of situations that provoked dizziness, may help you identify the presence of a disorder such as benign paroxysmal positional vertigo, which increases the risk of falls.31
In weighing whether to treat such a disorder yourself or to refer, keep in mind that the evidence strongly supports the efficacy of referring patients to otolaryngologists or audiologists for further diagnosis and treatment.32 Vestibular rehabilitation is effective for elderly patients with stable but symptomatic and uncompensated central deficits.33
FIGURE 2
Dizziness Handicap Inventory-Screening version (DHI-S)
Source: Jacobson GP, et al. Am J Otol. 1998.29 Reprinted with permission.
*The higher the score, the greater the need for further evaluation.
Item 3 on the AAFP Medicare encounter form17 probes for hazards in the home, asking: “Does your home have rugs in the hallway, lack grab bars in the bathroom, lack handrails on the stairs or have poor lighting?” Strong evidence supports the efficacy of a home hazard assessment and modification in preventing falls. However, during the brief time allotted for an office visit, asking patients to recall and describe every potential hazard in their homes is impractical. The PATIENT HANDOUT (HOW TO REDUCE YOUR RISK OF FALLS) details the causes of falls in older people and preventive measures that patients or their families may be able to implement at home.
Injuries from a fall can range from bruises and cuts to more serious problems, like a broken hip. Fortunately, most falls can be prevented. If you’ve fallen recently or think you might have a balance problem, tell your doctor immediately.
Am I in danger of falling?
People fall more as they get older because of changes that come with age, such as poor vision, balance problems, weak muscles, and arthritis. Cluttered, poorly lit living quarters also increase the risk. You are more likely to fall if you:
- have fallen before
- don’t get much exercise and have weakness in your legs
- are unsteady when you walk
- are taking medicines that can contribute to falls, such as sedatives or antidepressants, or take 4 or more different medications
- have medical problems such as Parkinson’s disease, osteoporosis, heart disease, or low blood pressure—or if you have had a stroke.
How can I protect myself at home?
You can minimize the risk of falling at home by taking these steps:
- Keep the floors free of clutter. Remove things you can easily trip over, such as throw rugs, electrical cords, piles of paper, and clothing.
- Make sure you have good lighting throughout the house, and put night lights in your bedroom, bathroom, hallways, and stairs.
- Have railings installed in the bathtub and shower and around the toilet, and use nonskid mats in the tub and shower area.
- Keep items within easy reach in the kitchen.
- Put handrails on the stairs. (Using bright paint or strips of tape on the railing will make it easier to see.)
- Wear shoes with firm, nonskid soles. Don’t wear house shoes, such as flip-flops or loose slippers, or shoes with heels higher than 1 inch.
What else can I do to protect myself?
- Get your eyes checked regularly. Wear your glasses as prescribed, and clean them often to improve visibility.
- Stay active and exercise often to keep your muscles and bones strong. Ask your doctor about weight-bearing exercise, and what you can do to improve your balance.
- Eat a healthy diet, with plenty of calcium and vitamin D. Limit consumption of alcoholic beverages (have no more than 2 drinks a day).
- Take care of your feet. If they hurt, tell your doctor.
- Ask the doctor whether you need a cane or other walking aid.
- Have your doctor go over all your medicines to see if you’re taking anything that can make you dizzy or sleepy.
- Get up very slowly. When you’re getting out of bed, sit on the side for a few minutes before you stand up. Getting up too quickly can make you feel dizzy or lose your balance.
- If you live alone, get an emergency alert system that you can wear around your wrist or neck and press to call for help if you fall and can’t reach the phone.
Where can I get more information?
American Geriatrics Society
212-308-1414
http://www.americangeriatrics.org
National Center for Injury Prevention and Control
770-488-1506
http://www.cdc.gov/ncipc/falls/#PDF
National Institute on Aging
http://www.niapublications.org/engagepages/falls.asp
U.S. Consumer Product Safety Commission
http://www.cpsc.gov/CPSCPUB/PUBS/701.html
Sources: Rao SS. Am Fam Physician. 20054; Tremblay KR, et al. Colorado State University. 2005.34
CORRESPONDENCE
Carole E. Johnson, PhD, AuD, Department of Communication Disorders, 1199 Haley Center, Auburn University, Auburn, AL 36849; [email protected].
1. Johnson CE, Danhauer JL, Koch LL, et al. Hearing and balance screening and referrals for Medicare patients: a national survey of primary care physicians. J Am Acad Audiol. 2008;19:171-190.
2. U.S. Preventative Services Task Force Screening for Hearing Impairment. In: Guide to clinical preventative services: a report of the U.S. Preventative Services Task Force. 2nd ed. Baltimore, Md: Williams & Wilkins, 1996:393-405.
3. Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventative services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52-61.
4. Rao SS. Prevention of falls in older patients. Am Fam Physician. 2005;72:81-88.
5. Ries PW. Prevalence and characteristics of persons with hearing trouble: United States, 1990-91. Vital Health Stat 10. 1994;No. 188.-
6. Healthy People 2010. Hearing health progress review. http://www.nidcd.nih.gov/health/healthyhearing/what_hh/progress_review_04.asp. October 20, 2004. Accessed March 19, 2005.
7. Campbell VA, Crews JE, Moriarty DG, et al. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults–United States, 1993-1997. MMWR CDC Surveill Summ. 1999;48:131-156.
8. Keller BK, Morton JL, Thomas VS, et al. The effect of visual and hearing impairments on functional status. J Am Geriatr Soc. 1999;47:1319-1325.
9. Kramer SE, Kapteyn TS, Kuik DJ, et al. The association of hearing impairment and chronic diseases with psychosocial health status in older age. J Aging Health. 2002;14:122-137.
10. National Council on Aging. The consequences of untreated hearing loss in older persons. ORL Head Neck Nurs. 2000;18:12-16.
11. Chia EM, Wang JJ, Rochtchina E, et al. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hear. 2007;28:187-195.
12. Dalton DS, Cruickshanks KJ, Klein BE, et al. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661-668.
13. Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment: a randomized trial. Ann Intern Med. 1990;113:188-194.
14. Pugh KC. Health status attributes of older African-American adults with hearing loss. J Natl Med Assoc. 2004;96:772-779.
15. Strawbridge WJ, Wallhagen MI, Shema SJ, et al. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40:320-326.
16. Gates GA, Murphy M, Rees TS, et al. Screening for handicapping hearing loss in the elderly. J Fam Pract. 2003;52:56-62.
17. Card RO. How to conduct a “Welcome to Medicare” visit. Fam Pract Manag. 2005;12:27-29, 31, 32.
18. Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25:37-42.
19. Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259:2875-2878.
20. Valente M, Abrams H, Benson D, et al. Guidelines for the audiologic management of adult hearing impairment. Audiol Today. 2006;18:32-36.
21. Chisolm TH, Johnson CE, Danhauer JL, et al. A systematic review of health-related quality of life and hearing aids: final report of the American Academy of Audiology Task Force on the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol. 2007;18:151-183.
22. Beynon GJ, Thornton FL, Poole C. A randomized, controlled trial of the efficacy of a communication course for first time hearing aid users. Br J Audiol. 1997;31:345-351.
23. Chisolm TH, Abrams HB, McArdle R. Short- and long-term outcomes of adult audiological rehabilitation. Ear Hear. 2004;25:464-477.
24. Cranney AB, Coyle D, Hopman WM, et al. Prospective evaluation of p and quality of life in women with hip fractures. J Rheumatol. 2005;32:2393-2399.
25. Gillespie LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing falls in elderly. Cochrane Database Syst Rev. 2009(4);CD000340.-
26. Chang JT, Morton SC, Rubenstein LZ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomized clinical trials. BMJ. 2004;328:680.-
27. Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141-158.
28. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896-903.
29. Jacobson GP, Calder JH. A screening version of the Dizziness Handicap Inventory (DHI-S). Am J Otol. 1998;19:804-808.
30. Rocchiccioli JT, Sanford J, Caplinger B. Polymedicine and aging. Enhancing older adult care through advanced practitioners. GNPs and elder care pharmacists can help provide optimal pharmaceutical care. J Gerontol Nurs. 2007;33:19-24.
31. von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78:710-715.
32. Hilton M, Pinder D. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2004;(2):CD003162.-
33. Macias JD, Massingale S, Gerkin RD. Efficacy of vestibular rehabilitation therapy in reducing falls. Otolaryngol Head Neck Surg. 2005;133:323-325.
34. Tremblay KR, Barbe CE. Preventing falls in the elderly. 2005. Colorado State University. http://www.ext.colostate.edu/PUBS/consumer/10242.html. Accessed August 13, 2009.
- Simply asking elderly patients whether they have trouble hearing is an effective start to screening for hearing loss (SOR: B).
- Refer elderly patients with suspected hearing impairment for audiologic diagnosis and nonmedical rehabilitation treatment, including hearing aids (SOR: B).
- To assess a patient’s risk of falling, review gait, balance disorders, weakness, environmental hazards, and medications (SOR: A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“Do you have a hearing problem now? Have you fallen recently?” These 2 simple questions are the first step in assessing a patient’s hearing status and risk of falls—a screening opportunity too often overlooked. Although family physicians are well qualified to address hearing loss and the risk of falls, screening elderly patients for these problems often seems like a lower priority than evaluating for serious, or potentially life-threatening, conditions. In a recent national survey of primary care physicians, most said they had little time to screen for hearing loss or vestibular or balance disorders and did so only if patients broached the subject or showed clear evidence of risk.1
Screening for hearing impairment in patients 65 years of age and older, with referral to appropriate specialists, ranked 15th among services deemed effective by the US Preventive Services Task Force and the Advisory Committee on Immunization Practices2,3—outranking screening for osteoporosis, cholesterol, and diabetes.
There is no evidence favoring any particular screening procedure. As a result, physicians have considerable leeway in assessing elderly patients’ functional ability and safety, including the risk of falls that may be precipitated by an impaired vestibular system and balance disorders.4
Screening for these problems need not be onerous. It can be accomplished as part of your continuity of care with longstanding patients or during the preventive care examination that Medicare offers newly enrolled patients. This review, and the screening tools and strategies that accompany it, will help you get started.
Hearing loss screening: Make it easier to do
Bilateral hearing impairment affects 1 in 3 adults over 65 years of age5 and is the third most common chronic condition among the elderly,6 trailing only arthritis and hypertension. Documented problems associated with hearing loss include social isolation, depression and anxiety, loneliness, diminished self-efficacy, and stressful relationships with family, friends, and coworkers who may experience frustration, impatience, anger, pity, or guilt in trying to communicate with a person who has a hearing loss.7-9
The mental, emotional, and social consequences of untreated hearing loss negatively affect patients’ health-related quality of life (HRQoL).8,10-15 Hearing loss also compromises patients’ ability to interact with you and to understand—and follow—your recommendations.
Simplify screening. A recent study assessed 2 hearing screening methods used with older adults who also underwent audiologic evaluation as part of the biennial examination for the Framingham Heart Study.16 It found that simply asking, “Do you have a hearing problem now?” effectively identified potential deficits. If you use the American Academy of Family Physicians’ (AAFP) Medicare Initial Preventive Physical Examination Encounter Form,17 consider replacing its entry for hearing loss with this simple question (See “Medicare preventive exam: Where the AAFP encounter form falls short”).
We recommend that you pose the question to elderly patients or their family members during regular office visits. If the answer is Yes, immediately assess the patient’s ability to understand conversational speech. If necessary, use an inexpensive amplification device to make it easier for you and your patient to communicate. Referral to an audiologist for a comprehensive evaluation may be indicated, as well.
The American Academy of Family Physicians’ (AAFP) Medicare Initial Preventive Physical Examination Encounter Form17 does not fully address hearing screening. Unlike the Depression Screen and Functional Ability/Safety Screen sections, which require Yes or No responses to questions, the section covering hearing merely presents the term “Hearing Evaluation,” followed by a space for recording information.
Although the form clearly states that a Yes response to any question about depression or function/safety should trigger further evaluation, there is no such recommendation for further evaluation of hearing. Thus, some practitioners short on time may overlook hearing screening entirely, and some elderly patients with sensorineural hearing loss may not receive appropriate education, counseling, or referral.
Furthermore, the second page of the AAFP form that is given to patients and makes recommendations for scheduled follow-up does not even mention hearing or the risk of falling. That’s why it’s important to remember to cover these areas with your elderly patients—and why you may want to ask the questions, “Do you have a hearing problem now?” and “Have you fallen recently?”
A time-saving suggestion. You can save precious consultation time by having elderly patients complete a standardized self-assessment questionnaire such as the Hearing Handicap Inventory for the Elderly-Screening version (HHIE-S) (FIGURE 1) while they’re in the waiting room.18,19 When hearing loss is identified, counsel the patient as appropriate and strongly recommend further audiologic testing and management.20 The HHIE-S can help patients realize the social and emotional consequences of hearing loss, which may encourage them to seek assistance. But many won’t do so without your recommendation.
Let patients know something can be done. Evidence shows that nonmedical management of sensorineural hearing loss, including the fitting of hearing aids, is critical in helping older adults improve communication and reduce psychosocial problems.10,21
Recently, a task force of the American Academy of Audiology conducted a systematic review of the HRQoL benefits of amplification in adults.21 A meta-analysis revealed that hearing aids had at least a small effect on HRQoL, as measured by generic health instruments (eg, the Medical Outcomes Study Short Form-36), and medium-to-large effects when disease-specific hearing outcome measures (eg, the HHIE) were used. Hearing aids, combined with auditory rehabilitation, changed patients’ perception of their handicap to a greater extent than amplification alone, particularly during the initial stage of adjusting to a device.22,23
Preprinted forms can help educate patients about hearing loss and the benefits of amplification. The PATIENT HANDOUT (ARE YOU HAVING HEARING PROBLEMS?) explains the causes and symptoms of sensorineural hearing loss, as well as diagnosis, frequency of audiologic evaluations, and treatments.
Sensorineural hearing loss occurs when sensory receptors (hair cells) in the inner ear (cochlea) or hearing nerve pathways to the brain are damaged. It is usually permanent and cannot be treated medically or surgically, but hearing aids almost always help.
Hearing loss most commonly results from age-related changes in the inner ear. Other possible causes are excessive noise exposure, drugs that are toxic to the auditory system, certain viruses or diseases, head trauma, and genetic or familial disorders.
If you think you may have a hearing loss or are having difficulty communicating, tell your doctor immediately.
What are the signs that I may be losing my hearing?
You may:
- complain that people are mumbling
- continually ask people to repeat themselves
- avoid noisy rooms, social occasions, or family gatherings
- prefer the TV or radio louder than other people do
- have difficulty understanding people when you can’t see their faces
- have trouble hearing at the movies or theater, your house of worship, or other public places
- have difficulty following conversations in a group
- become impatient, irritable, frustrated, or withdrawn.
Why can I hear people talk but not understand what they’re saying?
Hearing loss not only reduces your ability to hear normally audible sound, it interferes with your ability to detect particular sounds. High-pitched consonants (such as d, f, sh, s, t, and th) become harder to hear than low-pitched vowels. The high-pitched consonant sounds carry the meaning of words and help us understand speech, but in normal conversation, they’re softer than the less important low-pitched vowel sounds. So conversation may sound loud enough, but the words may not be clear.
How is hearing loss diagnosed?
Audiologists—health care professionals who specialize in hearing—conduct a comprehensive evaluation to determine the degree and type of hearing loss and its effect on your ability to communicate in everyday life. The exam is conducted with special instruments in a sound-dampened room. Medical diagnosis of ear disease is performed by ear, nose, and throat doctors (also known as ENTs or otolaryngologists).
How often should I have a hearing test?
After age 50, you should have your hearing tested every 2 years. But it’s important to request a hearing test immediately if you notice a sudden change in hearing, increased ringing in the ears (tinnitus), or dizziness.
How is hearing loss treated?
Hearing aids, which work better than ever because of digital technology, help many people. Your audiologist will talk to you about the different kinds of hearing aids available and what you can realistically expect when you use them. Together you’ll decide which one is best for you.
People who use hearing aids report better personal relationships, an easier time communicating, improved mental health, and a greater sense of control over their lives.
Where can I get more information?
American Academy of Audiology
800-AAA-2336
http://www.audiology.org
American Academy of Otolaryngology, Head, and Neck Surgery
703-836-4444
http://www.aaohns.org
American Speech-Language-Hearing Association
800-638-8255
http://www.asha.org
FIGURE 1
Hearing Handicap Inventory for the Elderly—Screening version (HHIE-S)
Source: Ventry IM, et al. ASHA. 1983.18 Reprinted with permission.
Copyright 1983 by American Speech-Language-Hearing Association. All rights reserved.
*If your score is 10 or greater, you may benefit from additional hearing evaluation.
Falls risk: Make use of assessment tools
Each year, approximately 30% to 40% of elderly adults living independently fall, and the incidence increases with age.4 Falls frequently cause injuries, such as hip fractures, and reduce HRQoL.24 Strong evidence supports the efficacy of a multifaceted approach for identifying and reducing risk of falls in this patient population.25,26 A review of more than 3000 falls indicated that about one-third were the result of accidents or environmental hazards, and nearly another third were caused by gait problems, balance disorders, or weakness.27
Means of determining risk of falls. As with hearing assessment, you can gauge older patients’ risk of falls during routine visits or annual exams. For patients’ first Medicare visit, the AAFP Medicare preventive exam encounter form17 includes 2 items in the “Functional Ability/Safety Screen” section that cover the leading causes of falls. Item 1 asks, “Was the patient’s Timed Up & Go test unsteady or longer than 30 seconds?” This simple test requires patients to stand from a sitting position, using their arms for support; walk a few paces, turn around, and return to their chair to sit. The test is sensitive to, and specific for, identifying elderly people living independently who are at risk of falls.28
The Dizziness Handicap Inventory–Screening version (DHI-S) (FIGURE 2),29 is a 10-item patient self-assessment questionnaire that takes fewer than 5 minutes to complete. You can also use the DHI-S to supplement the Timed Up & Go test in judging a patient’s postural control.
Possible reasons for poor performance on the Timed Up & Go test are vestibular dysfunction or other balance disorders that require a careful evaluation. Cross-checking the “Medications, supplements, and vitamins” list in the “Medical/Social History” section of the Medicare encounter form may reveal use of a drug or multiple drugs (particularly likely if the patient sees different physicians) that commonly contribute to falls.30
Patients’ descriptions of “Past personal illnesses and injuries,” also covered in the encounter form, or their accounts of situations that provoked dizziness, may help you identify the presence of a disorder such as benign paroxysmal positional vertigo, which increases the risk of falls.31
In weighing whether to treat such a disorder yourself or to refer, keep in mind that the evidence strongly supports the efficacy of referring patients to otolaryngologists or audiologists for further diagnosis and treatment.32 Vestibular rehabilitation is effective for elderly patients with stable but symptomatic and uncompensated central deficits.33
FIGURE 2
Dizziness Handicap Inventory-Screening version (DHI-S)
Source: Jacobson GP, et al. Am J Otol. 1998.29 Reprinted with permission.
*The higher the score, the greater the need for further evaluation.
Item 3 on the AAFP Medicare encounter form17 probes for hazards in the home, asking: “Does your home have rugs in the hallway, lack grab bars in the bathroom, lack handrails on the stairs or have poor lighting?” Strong evidence supports the efficacy of a home hazard assessment and modification in preventing falls. However, during the brief time allotted for an office visit, asking patients to recall and describe every potential hazard in their homes is impractical. The PATIENT HANDOUT (HOW TO REDUCE YOUR RISK OF FALLS) details the causes of falls in older people and preventive measures that patients or their families may be able to implement at home.
Injuries from a fall can range from bruises and cuts to more serious problems, like a broken hip. Fortunately, most falls can be prevented. If you’ve fallen recently or think you might have a balance problem, tell your doctor immediately.
Am I in danger of falling?
People fall more as they get older because of changes that come with age, such as poor vision, balance problems, weak muscles, and arthritis. Cluttered, poorly lit living quarters also increase the risk. You are more likely to fall if you:
- have fallen before
- don’t get much exercise and have weakness in your legs
- are unsteady when you walk
- are taking medicines that can contribute to falls, such as sedatives or antidepressants, or take 4 or more different medications
- have medical problems such as Parkinson’s disease, osteoporosis, heart disease, or low blood pressure—or if you have had a stroke.
How can I protect myself at home?
You can minimize the risk of falling at home by taking these steps:
- Keep the floors free of clutter. Remove things you can easily trip over, such as throw rugs, electrical cords, piles of paper, and clothing.
- Make sure you have good lighting throughout the house, and put night lights in your bedroom, bathroom, hallways, and stairs.
- Have railings installed in the bathtub and shower and around the toilet, and use nonskid mats in the tub and shower area.
- Keep items within easy reach in the kitchen.
- Put handrails on the stairs. (Using bright paint or strips of tape on the railing will make it easier to see.)
- Wear shoes with firm, nonskid soles. Don’t wear house shoes, such as flip-flops or loose slippers, or shoes with heels higher than 1 inch.
What else can I do to protect myself?
- Get your eyes checked regularly. Wear your glasses as prescribed, and clean them often to improve visibility.
- Stay active and exercise often to keep your muscles and bones strong. Ask your doctor about weight-bearing exercise, and what you can do to improve your balance.
- Eat a healthy diet, with plenty of calcium and vitamin D. Limit consumption of alcoholic beverages (have no more than 2 drinks a day).
- Take care of your feet. If they hurt, tell your doctor.
- Ask the doctor whether you need a cane or other walking aid.
- Have your doctor go over all your medicines to see if you’re taking anything that can make you dizzy or sleepy.
- Get up very slowly. When you’re getting out of bed, sit on the side for a few minutes before you stand up. Getting up too quickly can make you feel dizzy or lose your balance.
- If you live alone, get an emergency alert system that you can wear around your wrist or neck and press to call for help if you fall and can’t reach the phone.
Where can I get more information?
American Geriatrics Society
212-308-1414
http://www.americangeriatrics.org
National Center for Injury Prevention and Control
770-488-1506
http://www.cdc.gov/ncipc/falls/#PDF
National Institute on Aging
http://www.niapublications.org/engagepages/falls.asp
U.S. Consumer Product Safety Commission
http://www.cpsc.gov/CPSCPUB/PUBS/701.html
Sources: Rao SS. Am Fam Physician. 20054; Tremblay KR, et al. Colorado State University. 2005.34
CORRESPONDENCE
Carole E. Johnson, PhD, AuD, Department of Communication Disorders, 1199 Haley Center, Auburn University, Auburn, AL 36849; [email protected].
- Simply asking elderly patients whether they have trouble hearing is an effective start to screening for hearing loss (SOR: B).
- Refer elderly patients with suspected hearing impairment for audiologic diagnosis and nonmedical rehabilitation treatment, including hearing aids (SOR: B).
- To assess a patient’s risk of falling, review gait, balance disorders, weakness, environmental hazards, and medications (SOR: A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“Do you have a hearing problem now? Have you fallen recently?” These 2 simple questions are the first step in assessing a patient’s hearing status and risk of falls—a screening opportunity too often overlooked. Although family physicians are well qualified to address hearing loss and the risk of falls, screening elderly patients for these problems often seems like a lower priority than evaluating for serious, or potentially life-threatening, conditions. In a recent national survey of primary care physicians, most said they had little time to screen for hearing loss or vestibular or balance disorders and did so only if patients broached the subject or showed clear evidence of risk.1
Screening for hearing impairment in patients 65 years of age and older, with referral to appropriate specialists, ranked 15th among services deemed effective by the US Preventive Services Task Force and the Advisory Committee on Immunization Practices2,3—outranking screening for osteoporosis, cholesterol, and diabetes.
There is no evidence favoring any particular screening procedure. As a result, physicians have considerable leeway in assessing elderly patients’ functional ability and safety, including the risk of falls that may be precipitated by an impaired vestibular system and balance disorders.4
Screening for these problems need not be onerous. It can be accomplished as part of your continuity of care with longstanding patients or during the preventive care examination that Medicare offers newly enrolled patients. This review, and the screening tools and strategies that accompany it, will help you get started.
Hearing loss screening: Make it easier to do
Bilateral hearing impairment affects 1 in 3 adults over 65 years of age5 and is the third most common chronic condition among the elderly,6 trailing only arthritis and hypertension. Documented problems associated with hearing loss include social isolation, depression and anxiety, loneliness, diminished self-efficacy, and stressful relationships with family, friends, and coworkers who may experience frustration, impatience, anger, pity, or guilt in trying to communicate with a person who has a hearing loss.7-9
The mental, emotional, and social consequences of untreated hearing loss negatively affect patients’ health-related quality of life (HRQoL).8,10-15 Hearing loss also compromises patients’ ability to interact with you and to understand—and follow—your recommendations.
Simplify screening. A recent study assessed 2 hearing screening methods used with older adults who also underwent audiologic evaluation as part of the biennial examination for the Framingham Heart Study.16 It found that simply asking, “Do you have a hearing problem now?” effectively identified potential deficits. If you use the American Academy of Family Physicians’ (AAFP) Medicare Initial Preventive Physical Examination Encounter Form,17 consider replacing its entry for hearing loss with this simple question (See “Medicare preventive exam: Where the AAFP encounter form falls short”).
We recommend that you pose the question to elderly patients or their family members during regular office visits. If the answer is Yes, immediately assess the patient’s ability to understand conversational speech. If necessary, use an inexpensive amplification device to make it easier for you and your patient to communicate. Referral to an audiologist for a comprehensive evaluation may be indicated, as well.
The American Academy of Family Physicians’ (AAFP) Medicare Initial Preventive Physical Examination Encounter Form17 does not fully address hearing screening. Unlike the Depression Screen and Functional Ability/Safety Screen sections, which require Yes or No responses to questions, the section covering hearing merely presents the term “Hearing Evaluation,” followed by a space for recording information.
Although the form clearly states that a Yes response to any question about depression or function/safety should trigger further evaluation, there is no such recommendation for further evaluation of hearing. Thus, some practitioners short on time may overlook hearing screening entirely, and some elderly patients with sensorineural hearing loss may not receive appropriate education, counseling, or referral.
Furthermore, the second page of the AAFP form that is given to patients and makes recommendations for scheduled follow-up does not even mention hearing or the risk of falling. That’s why it’s important to remember to cover these areas with your elderly patients—and why you may want to ask the questions, “Do you have a hearing problem now?” and “Have you fallen recently?”
A time-saving suggestion. You can save precious consultation time by having elderly patients complete a standardized self-assessment questionnaire such as the Hearing Handicap Inventory for the Elderly-Screening version (HHIE-S) (FIGURE 1) while they’re in the waiting room.18,19 When hearing loss is identified, counsel the patient as appropriate and strongly recommend further audiologic testing and management.20 The HHIE-S can help patients realize the social and emotional consequences of hearing loss, which may encourage them to seek assistance. But many won’t do so without your recommendation.
Let patients know something can be done. Evidence shows that nonmedical management of sensorineural hearing loss, including the fitting of hearing aids, is critical in helping older adults improve communication and reduce psychosocial problems.10,21
Recently, a task force of the American Academy of Audiology conducted a systematic review of the HRQoL benefits of amplification in adults.21 A meta-analysis revealed that hearing aids had at least a small effect on HRQoL, as measured by generic health instruments (eg, the Medical Outcomes Study Short Form-36), and medium-to-large effects when disease-specific hearing outcome measures (eg, the HHIE) were used. Hearing aids, combined with auditory rehabilitation, changed patients’ perception of their handicap to a greater extent than amplification alone, particularly during the initial stage of adjusting to a device.22,23
Preprinted forms can help educate patients about hearing loss and the benefits of amplification. The PATIENT HANDOUT (ARE YOU HAVING HEARING PROBLEMS?) explains the causes and symptoms of sensorineural hearing loss, as well as diagnosis, frequency of audiologic evaluations, and treatments.
Sensorineural hearing loss occurs when sensory receptors (hair cells) in the inner ear (cochlea) or hearing nerve pathways to the brain are damaged. It is usually permanent and cannot be treated medically or surgically, but hearing aids almost always help.
Hearing loss most commonly results from age-related changes in the inner ear. Other possible causes are excessive noise exposure, drugs that are toxic to the auditory system, certain viruses or diseases, head trauma, and genetic or familial disorders.
If you think you may have a hearing loss or are having difficulty communicating, tell your doctor immediately.
What are the signs that I may be losing my hearing?
You may:
- complain that people are mumbling
- continually ask people to repeat themselves
- avoid noisy rooms, social occasions, or family gatherings
- prefer the TV or radio louder than other people do
- have difficulty understanding people when you can’t see their faces
- have trouble hearing at the movies or theater, your house of worship, or other public places
- have difficulty following conversations in a group
- become impatient, irritable, frustrated, or withdrawn.
Why can I hear people talk but not understand what they’re saying?
Hearing loss not only reduces your ability to hear normally audible sound, it interferes with your ability to detect particular sounds. High-pitched consonants (such as d, f, sh, s, t, and th) become harder to hear than low-pitched vowels. The high-pitched consonant sounds carry the meaning of words and help us understand speech, but in normal conversation, they’re softer than the less important low-pitched vowel sounds. So conversation may sound loud enough, but the words may not be clear.
How is hearing loss diagnosed?
Audiologists—health care professionals who specialize in hearing—conduct a comprehensive evaluation to determine the degree and type of hearing loss and its effect on your ability to communicate in everyday life. The exam is conducted with special instruments in a sound-dampened room. Medical diagnosis of ear disease is performed by ear, nose, and throat doctors (also known as ENTs or otolaryngologists).
How often should I have a hearing test?
After age 50, you should have your hearing tested every 2 years. But it’s important to request a hearing test immediately if you notice a sudden change in hearing, increased ringing in the ears (tinnitus), or dizziness.
How is hearing loss treated?
Hearing aids, which work better than ever because of digital technology, help many people. Your audiologist will talk to you about the different kinds of hearing aids available and what you can realistically expect when you use them. Together you’ll decide which one is best for you.
People who use hearing aids report better personal relationships, an easier time communicating, improved mental health, and a greater sense of control over their lives.
Where can I get more information?
American Academy of Audiology
800-AAA-2336
http://www.audiology.org
American Academy of Otolaryngology, Head, and Neck Surgery
703-836-4444
http://www.aaohns.org
American Speech-Language-Hearing Association
800-638-8255
http://www.asha.org
FIGURE 1
Hearing Handicap Inventory for the Elderly—Screening version (HHIE-S)
Source: Ventry IM, et al. ASHA. 1983.18 Reprinted with permission.
Copyright 1983 by American Speech-Language-Hearing Association. All rights reserved.
*If your score is 10 or greater, you may benefit from additional hearing evaluation.
Falls risk: Make use of assessment tools
Each year, approximately 30% to 40% of elderly adults living independently fall, and the incidence increases with age.4 Falls frequently cause injuries, such as hip fractures, and reduce HRQoL.24 Strong evidence supports the efficacy of a multifaceted approach for identifying and reducing risk of falls in this patient population.25,26 A review of more than 3000 falls indicated that about one-third were the result of accidents or environmental hazards, and nearly another third were caused by gait problems, balance disorders, or weakness.27
Means of determining risk of falls. As with hearing assessment, you can gauge older patients’ risk of falls during routine visits or annual exams. For patients’ first Medicare visit, the AAFP Medicare preventive exam encounter form17 includes 2 items in the “Functional Ability/Safety Screen” section that cover the leading causes of falls. Item 1 asks, “Was the patient’s Timed Up & Go test unsteady or longer than 30 seconds?” This simple test requires patients to stand from a sitting position, using their arms for support; walk a few paces, turn around, and return to their chair to sit. The test is sensitive to, and specific for, identifying elderly people living independently who are at risk of falls.28
The Dizziness Handicap Inventory–Screening version (DHI-S) (FIGURE 2),29 is a 10-item patient self-assessment questionnaire that takes fewer than 5 minutes to complete. You can also use the DHI-S to supplement the Timed Up & Go test in judging a patient’s postural control.
Possible reasons for poor performance on the Timed Up & Go test are vestibular dysfunction or other balance disorders that require a careful evaluation. Cross-checking the “Medications, supplements, and vitamins” list in the “Medical/Social History” section of the Medicare encounter form may reveal use of a drug or multiple drugs (particularly likely if the patient sees different physicians) that commonly contribute to falls.30
Patients’ descriptions of “Past personal illnesses and injuries,” also covered in the encounter form, or their accounts of situations that provoked dizziness, may help you identify the presence of a disorder such as benign paroxysmal positional vertigo, which increases the risk of falls.31
In weighing whether to treat such a disorder yourself or to refer, keep in mind that the evidence strongly supports the efficacy of referring patients to otolaryngologists or audiologists for further diagnosis and treatment.32 Vestibular rehabilitation is effective for elderly patients with stable but symptomatic and uncompensated central deficits.33
FIGURE 2
Dizziness Handicap Inventory-Screening version (DHI-S)
Source: Jacobson GP, et al. Am J Otol. 1998.29 Reprinted with permission.
*The higher the score, the greater the need for further evaluation.
Item 3 on the AAFP Medicare encounter form17 probes for hazards in the home, asking: “Does your home have rugs in the hallway, lack grab bars in the bathroom, lack handrails on the stairs or have poor lighting?” Strong evidence supports the efficacy of a home hazard assessment and modification in preventing falls. However, during the brief time allotted for an office visit, asking patients to recall and describe every potential hazard in their homes is impractical. The PATIENT HANDOUT (HOW TO REDUCE YOUR RISK OF FALLS) details the causes of falls in older people and preventive measures that patients or their families may be able to implement at home.
Injuries from a fall can range from bruises and cuts to more serious problems, like a broken hip. Fortunately, most falls can be prevented. If you’ve fallen recently or think you might have a balance problem, tell your doctor immediately.
Am I in danger of falling?
People fall more as they get older because of changes that come with age, such as poor vision, balance problems, weak muscles, and arthritis. Cluttered, poorly lit living quarters also increase the risk. You are more likely to fall if you:
- have fallen before
- don’t get much exercise and have weakness in your legs
- are unsteady when you walk
- are taking medicines that can contribute to falls, such as sedatives or antidepressants, or take 4 or more different medications
- have medical problems such as Parkinson’s disease, osteoporosis, heart disease, or low blood pressure—or if you have had a stroke.
How can I protect myself at home?
You can minimize the risk of falling at home by taking these steps:
- Keep the floors free of clutter. Remove things you can easily trip over, such as throw rugs, electrical cords, piles of paper, and clothing.
- Make sure you have good lighting throughout the house, and put night lights in your bedroom, bathroom, hallways, and stairs.
- Have railings installed in the bathtub and shower and around the toilet, and use nonskid mats in the tub and shower area.
- Keep items within easy reach in the kitchen.
- Put handrails on the stairs. (Using bright paint or strips of tape on the railing will make it easier to see.)
- Wear shoes with firm, nonskid soles. Don’t wear house shoes, such as flip-flops or loose slippers, or shoes with heels higher than 1 inch.
What else can I do to protect myself?
- Get your eyes checked regularly. Wear your glasses as prescribed, and clean them often to improve visibility.
- Stay active and exercise often to keep your muscles and bones strong. Ask your doctor about weight-bearing exercise, and what you can do to improve your balance.
- Eat a healthy diet, with plenty of calcium and vitamin D. Limit consumption of alcoholic beverages (have no more than 2 drinks a day).
- Take care of your feet. If they hurt, tell your doctor.
- Ask the doctor whether you need a cane or other walking aid.
- Have your doctor go over all your medicines to see if you’re taking anything that can make you dizzy or sleepy.
- Get up very slowly. When you’re getting out of bed, sit on the side for a few minutes before you stand up. Getting up too quickly can make you feel dizzy or lose your balance.
- If you live alone, get an emergency alert system that you can wear around your wrist or neck and press to call for help if you fall and can’t reach the phone.
Where can I get more information?
American Geriatrics Society
212-308-1414
http://www.americangeriatrics.org
National Center for Injury Prevention and Control
770-488-1506
http://www.cdc.gov/ncipc/falls/#PDF
National Institute on Aging
http://www.niapublications.org/engagepages/falls.asp
U.S. Consumer Product Safety Commission
http://www.cpsc.gov/CPSCPUB/PUBS/701.html
Sources: Rao SS. Am Fam Physician. 20054; Tremblay KR, et al. Colorado State University. 2005.34
CORRESPONDENCE
Carole E. Johnson, PhD, AuD, Department of Communication Disorders, 1199 Haley Center, Auburn University, Auburn, AL 36849; [email protected].
1. Johnson CE, Danhauer JL, Koch LL, et al. Hearing and balance screening and referrals for Medicare patients: a national survey of primary care physicians. J Am Acad Audiol. 2008;19:171-190.
2. U.S. Preventative Services Task Force Screening for Hearing Impairment. In: Guide to clinical preventative services: a report of the U.S. Preventative Services Task Force. 2nd ed. Baltimore, Md: Williams & Wilkins, 1996:393-405.
3. Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventative services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52-61.
4. Rao SS. Prevention of falls in older patients. Am Fam Physician. 2005;72:81-88.
5. Ries PW. Prevalence and characteristics of persons with hearing trouble: United States, 1990-91. Vital Health Stat 10. 1994;No. 188.-
6. Healthy People 2010. Hearing health progress review. http://www.nidcd.nih.gov/health/healthyhearing/what_hh/progress_review_04.asp. October 20, 2004. Accessed March 19, 2005.
7. Campbell VA, Crews JE, Moriarty DG, et al. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults–United States, 1993-1997. MMWR CDC Surveill Summ. 1999;48:131-156.
8. Keller BK, Morton JL, Thomas VS, et al. The effect of visual and hearing impairments on functional status. J Am Geriatr Soc. 1999;47:1319-1325.
9. Kramer SE, Kapteyn TS, Kuik DJ, et al. The association of hearing impairment and chronic diseases with psychosocial health status in older age. J Aging Health. 2002;14:122-137.
10. National Council on Aging. The consequences of untreated hearing loss in older persons. ORL Head Neck Nurs. 2000;18:12-16.
11. Chia EM, Wang JJ, Rochtchina E, et al. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hear. 2007;28:187-195.
12. Dalton DS, Cruickshanks KJ, Klein BE, et al. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661-668.
13. Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment: a randomized trial. Ann Intern Med. 1990;113:188-194.
14. Pugh KC. Health status attributes of older African-American adults with hearing loss. J Natl Med Assoc. 2004;96:772-779.
15. Strawbridge WJ, Wallhagen MI, Shema SJ, et al. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40:320-326.
16. Gates GA, Murphy M, Rees TS, et al. Screening for handicapping hearing loss in the elderly. J Fam Pract. 2003;52:56-62.
17. Card RO. How to conduct a “Welcome to Medicare” visit. Fam Pract Manag. 2005;12:27-29, 31, 32.
18. Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25:37-42.
19. Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259:2875-2878.
20. Valente M, Abrams H, Benson D, et al. Guidelines for the audiologic management of adult hearing impairment. Audiol Today. 2006;18:32-36.
21. Chisolm TH, Johnson CE, Danhauer JL, et al. A systematic review of health-related quality of life and hearing aids: final report of the American Academy of Audiology Task Force on the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol. 2007;18:151-183.
22. Beynon GJ, Thornton FL, Poole C. A randomized, controlled trial of the efficacy of a communication course for first time hearing aid users. Br J Audiol. 1997;31:345-351.
23. Chisolm TH, Abrams HB, McArdle R. Short- and long-term outcomes of adult audiological rehabilitation. Ear Hear. 2004;25:464-477.
24. Cranney AB, Coyle D, Hopman WM, et al. Prospective evaluation of p and quality of life in women with hip fractures. J Rheumatol. 2005;32:2393-2399.
25. Gillespie LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing falls in elderly. Cochrane Database Syst Rev. 2009(4);CD000340.-
26. Chang JT, Morton SC, Rubenstein LZ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomized clinical trials. BMJ. 2004;328:680.-
27. Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141-158.
28. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896-903.
29. Jacobson GP, Calder JH. A screening version of the Dizziness Handicap Inventory (DHI-S). Am J Otol. 1998;19:804-808.
30. Rocchiccioli JT, Sanford J, Caplinger B. Polymedicine and aging. Enhancing older adult care through advanced practitioners. GNPs and elder care pharmacists can help provide optimal pharmaceutical care. J Gerontol Nurs. 2007;33:19-24.
31. von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78:710-715.
32. Hilton M, Pinder D. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2004;(2):CD003162.-
33. Macias JD, Massingale S, Gerkin RD. Efficacy of vestibular rehabilitation therapy in reducing falls. Otolaryngol Head Neck Surg. 2005;133:323-325.
34. Tremblay KR, Barbe CE. Preventing falls in the elderly. 2005. Colorado State University. http://www.ext.colostate.edu/PUBS/consumer/10242.html. Accessed August 13, 2009.
1. Johnson CE, Danhauer JL, Koch LL, et al. Hearing and balance screening and referrals for Medicare patients: a national survey of primary care physicians. J Am Acad Audiol. 2008;19:171-190.
2. U.S. Preventative Services Task Force Screening for Hearing Impairment. In: Guide to clinical preventative services: a report of the U.S. Preventative Services Task Force. 2nd ed. Baltimore, Md: Williams & Wilkins, 1996:393-405.
3. Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventative services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52-61.
4. Rao SS. Prevention of falls in older patients. Am Fam Physician. 2005;72:81-88.
5. Ries PW. Prevalence and characteristics of persons with hearing trouble: United States, 1990-91. Vital Health Stat 10. 1994;No. 188.-
6. Healthy People 2010. Hearing health progress review. http://www.nidcd.nih.gov/health/healthyhearing/what_hh/progress_review_04.asp. October 20, 2004. Accessed March 19, 2005.
7. Campbell VA, Crews JE, Moriarty DG, et al. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults–United States, 1993-1997. MMWR CDC Surveill Summ. 1999;48:131-156.
8. Keller BK, Morton JL, Thomas VS, et al. The effect of visual and hearing impairments on functional status. J Am Geriatr Soc. 1999;47:1319-1325.
9. Kramer SE, Kapteyn TS, Kuik DJ, et al. The association of hearing impairment and chronic diseases with psychosocial health status in older age. J Aging Health. 2002;14:122-137.
10. National Council on Aging. The consequences of untreated hearing loss in older persons. ORL Head Neck Nurs. 2000;18:12-16.
11. Chia EM, Wang JJ, Rochtchina E, et al. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hear. 2007;28:187-195.
12. Dalton DS, Cruickshanks KJ, Klein BE, et al. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661-668.
13. Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment: a randomized trial. Ann Intern Med. 1990;113:188-194.
14. Pugh KC. Health status attributes of older African-American adults with hearing loss. J Natl Med Assoc. 2004;96:772-779.
15. Strawbridge WJ, Wallhagen MI, Shema SJ, et al. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40:320-326.
16. Gates GA, Murphy M, Rees TS, et al. Screening for handicapping hearing loss in the elderly. J Fam Pract. 2003;52:56-62.
17. Card RO. How to conduct a “Welcome to Medicare” visit. Fam Pract Manag. 2005;12:27-29, 31, 32.
18. Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25:37-42.
19. Lichtenstein MJ, Bess FH, Logan SA. Validation of screening tools for identifying hearing-impaired elderly in primary care. JAMA. 1988;259:2875-2878.
20. Valente M, Abrams H, Benson D, et al. Guidelines for the audiologic management of adult hearing impairment. Audiol Today. 2006;18:32-36.
21. Chisolm TH, Johnson CE, Danhauer JL, et al. A systematic review of health-related quality of life and hearing aids: final report of the American Academy of Audiology Task Force on the Health-Related Quality of Life Benefits of Amplification in Adults. J Am Acad Audiol. 2007;18:151-183.
22. Beynon GJ, Thornton FL, Poole C. A randomized, controlled trial of the efficacy of a communication course for first time hearing aid users. Br J Audiol. 1997;31:345-351.
23. Chisolm TH, Abrams HB, McArdle R. Short- and long-term outcomes of adult audiological rehabilitation. Ear Hear. 2004;25:464-477.
24. Cranney AB, Coyle D, Hopman WM, et al. Prospective evaluation of p and quality of life in women with hip fractures. J Rheumatol. 2005;32:2393-2399.
25. Gillespie LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing falls in elderly. Cochrane Database Syst Rev. 2009(4);CD000340.-
26. Chang JT, Morton SC, Rubenstein LZ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomized clinical trials. BMJ. 2004;328:680.-
27. Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141-158.
28. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896-903.
29. Jacobson GP, Calder JH. A screening version of the Dizziness Handicap Inventory (DHI-S). Am J Otol. 1998;19:804-808.
30. Rocchiccioli JT, Sanford J, Caplinger B. Polymedicine and aging. Enhancing older adult care through advanced practitioners. GNPs and elder care pharmacists can help provide optimal pharmaceutical care. J Gerontol Nurs. 2007;33:19-24.
31. von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78:710-715.
32. Hilton M, Pinder D. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2004;(2):CD003162.-
33. Macias JD, Massingale S, Gerkin RD. Efficacy of vestibular rehabilitation therapy in reducing falls. Otolaryngol Head Neck Surg. 2005;133:323-325.
34. Tremblay KR, Barbe CE. Preventing falls in the elderly. 2005. Colorado State University. http://www.ext.colostate.edu/PUBS/consumer/10242.html. Accessed August 13, 2009.
When an athlete can’t catch his breath
- Don’t rely on self-reported symptoms to diagnose exercise-induced bronchoconstriction (EIB) (A).
- Indirect testing is the best way to diagnose EIB in patients who do not have underlying asthma (A).
- Short-acting β2-agonists should be first-line management in EIB (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Luke, a 16-year-old basketball player, complains that he can’t finish a game without running out of breath. He says things are at their worst when the game is close and when it’s nearing the end. He doesn’t have the problem during practice, or when he is playing other sports. The team physician suggested using an albuterol inhaler half an hour before game time and when he has symptoms, but he gets only minimal relief. Now he has come to you.
His vital signs, lung exam, and cardiac exam are normal. Results of pulmonary function tests with pre- and post-albuterol challenge done a year ago were also normal. Does Luke have exercise-induced bronchoconstriction (EIB)? How can you be sure? And what can you do to help?
Symptoms like Luke’s are common among athletes of all abilities. They may add up to EIB, a condition with an estimated prevalence of 6% to 12% in the general population—or they may not.1 One study showed that only a third of athletes with symptoms or prior diagnosis of EIB had positive objective testing for the condition, and current studies show that reported symptoms are not an accurate guide in athletes like Luke who do not have underlying asthma.2,3 To treat him correctly, you will need to nail down the diagnosis with additional tests.3,4
Shortness of breath that’s worse than expected
EIB can have many different presentations. The most common symptom is cough associated with exercise.3 Other common signs and symptoms include wheezing, chest tightness, and more severe than expected or worsening shortness of breath. More unusual symptoms include a decrease in performance or fatigue out of proportion to workload. Often patients with EIB have other associated medical conditions, such as allergic rhinitis.
Bronchoconstriction usually occurs with maximal or near maximal exertion. Generally, it takes 5 to 8 minutes of exercising at 80% of maximal heart rate to trigger EIB. Classically, the symptoms peak 5 to 10 minutes after exercise begins.5
Rule out cardiac problems. If EIB is the correct diagnosis, the physical exam is usually normal. The importance of the physical exam is to evaluate for other diagnoses with similar presentations. Conditions to rule out include cardiac problems, exercise-induced hyperventilation, upper and lower respiratory infections or abnormalities, exercise-induced laryngeal dysfunction, exercise-induced anaphylaxis, and gastroesophageal reflux disease (GERD). The differential diagnosis for EIB is summarized in TABLE 1.
Test for asthma. Once you have gone through the differential diagnosis and are comfortable that the symptoms are respiratory, the next step should be pulmonary function tests (PFT), pre- and post-albuterol challenge. Findings of obstruction, such as reduced forced expiratory volume in 1 second (FEV1) or increased lung volume, are consistent with a diagnosis of asthma. In that case, no further workup is needed—unless the patient is unresponsive to asthma treatment. In athletes like Luke who do not have asthma and have a normal nonprovocative spirometry, you can move on to either provocative spirometry or empiric treatment.
TABLE 1
Is it EIB, or something else?
| ETIOLOGY | POSSIBLE DIAGNOSES |
|---|---|
| Pulmonary | Exercise-induced hyperventilation (pseudo-asthma syndrome) Restrictive lung disease Cystic fibrosis Upper and lower respiratory infections Foreign body aspiration |
| Cardiac | Coronary artery disease Congenital and acquired heart defects Cardiomyopathy Congestive heart failure |
| Laryngeal | Exercise-induced laryngeal dysfunction Vocal cord dysfunction Laryngeal prolapse Laryngomalacia |
| Gastroesophageal | Gastroesophageal reflux disease |
| Allergic | Exercise-induced anaphylaxis |
| Other | Athlete is out of shape |
| EIB, exercise-induced bronchoconstriction. | |
| Source: Weiler JM, et al. J Allergy Clin Immunol. 2007.4 | |
Perform provocative spirometry
Direct spirometry is commonly done with a methacholine challenge. This test is less sensitive than indirect testing for EIB patients who do not have underlying asthma.
The gold standard for indirect testing is eucapnic voluntary hyperventilation (EVH). Because EVH requires special equipment, however, it may not be an option in your office. The more reasonable choice is exercise challenge testing, which can be done either in your office or in the milieu—the basketball court, for example—where the athlete’s symptoms usually occur. In an exercise challenge, you get a baseline spirometry measurement, have the athlete exercise to 80% to 90% of maximal heart rate, and then repeat spirometry at short intervals after exercise ends. If you do an exercise challenge in the office, you can reduce false-negative results by maintaining an ambient temperature between 68° and 77°F (20°-25°C) with a relative humidity of less than 50%.6,7
Or try empiric treatment
Empiric treatment is a reasonable strategy for athletes with EIB symptoms, worth trying both for athletes who have underlying asthma and for those who do not. If the athlete with asthma responds to treatment, the problem is solved. For the athlete who does not have asthma, however, there are some exceptions to this approach—specifically, the elite athlete.
In the elite athlete, you will need to confirm the diagnosis because many of the substances used to treat EIB are restricted by governing bodies such as the International Olympic Committee (IOC) and require provocative testing to obtain a therapeutic use exemption.8 There is some debate as to whether nonelite athletes also need bronchoprovocative testing. Some recommendations advise testing all elite and competitive athletes and restricting empiric treatment to recreational athletes.1 For more information on banned or restricted medications, see “Is that drug banned from competition?”.
If you take the empiric approach and the athlete does not respond to treatment, consider further testing to rule out other, more serious problems. In Luke’s case, where empiric treatment with albuterol has failed, indirect testing would be the next step.
Certain medications used in the treatment of asthma and exercise-induced bronchoconstriction (EIB) are considered performance-enhancing drugs and either banned or restricted in athletic competition. The regulatory bodies that make these designations in the United States are the National Collegiate Athletic Association (NCAA) and the International Olympic Committee World Anti-Doping Agency (IOC-WADA). These organizations update their list of banned substances yearly and make the current list available on the Web. You can find the NCAA list at www.pace.edu/emplibrary/NCAA%20LIST%20OF%20BANNED%20SUBSTANCESb.doc and the IOC-WADA list at www.wada-ama.org/rtecontent/document/2009_Prohibited_List_ENG_Final_20_Sept_08.pdf.
The IOC-WADA allows competing athletes to use inhaled corticosteroids and β2 agonists, but requires athletes with asthma to provide documentation that the medication is for therapeutic use. Glucocorticosteroids and oral β2 agonists remain prohibited by the IOC-WADA, but only oral β2 agonists are banned by the NCAA. The NCAA warns that student athletes are responsible for knowing which substances are on the banned list and advises them to consult www.drugfreesport.com for more information. To avoid disqualifying a patient from sports participation, check medications you prescribe with the official lists and be sure your EIB patient has the documentation he or she needs to qualify for a therapeutic use exemption.
Medicate before exercise: SABAs and LABAs
Prophylaxis for EIB usually starts with an inhaled short-acting β2 agonist (SABA) such as albuterol or pirbuterol, taken 15 minutes before starting to exercise.9,10 The effectiveness of both short- and long-acting β2 agonists decreases with frequent use, which may be Luke’s problem. For that reason, patients with mild EIB may choose to use pretreatment medication only for more demanding exercise sessions.11 Advise EIB patients who need daily pretreatment to try adjunctive maintenance therapy (discussed at greater length, below.)
Longer-acting β2 agonists (LABAs) such as salmeterol or formoterol may be effective for prolonged or all-day exercise, but may lose their prophylactic effect with prolonged use.12 Furthermore, the US Food and Drug Administration (FDA) has advised against using LABAs alone because of the possibility of severe asthma episodes or death. LABAs should be used only in conjunction with daily maintenance therapy with inhaled corticosteroids. The properties of these and other EIB medications are summarized in TABLE 2.
TABLE 2
EIB medications
| MEDICATION | INDICATION | DOSE | CAUTIONS | COMMENT |
|---|---|---|---|---|
| Short-acting β2 agonists (SABAs) | ||||
| Albuterol, pirbuterol | Pre-exercise prophylaxis, acute treatment | 2 puffs pre-exercise or 2 puffs every 4-6 h as needed | May cause tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | First-line treatment |
| Mast cell stabilizers | ||||
| Cromolyn | Pre-exercise treatment | 2 puffs 30-45 min before exercise | None | Best combined with SABA. Tell patients not to use for rescue. |
| Inhaled corticosteroids | ||||
| Flunisolide, fluticasone, budesonide, triamcinolone, beclomethasone, mometasone | Daily maintenance | Variable | Can cause oral candidiasis, hoarseness. | Tell patients this is not a rescue inhaler. |
| Leukotriene inhibitors | ||||
| Zafirlukast | Daily maintenance | 20 mg PO, bid | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Montelukast | Daily maintenance, pre-exercise prophylaxis | 10 mg PO daily or up to 2 h pre-exercise | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Zileuton | Daily maintenance | 1200 mg PO, bid | Risk of elevated liver function tests. | Variable response. Low side-effect profile. |
| Combinations | ||||
| Inhaled fluticasone and salmeterol | Daily maintenance | Variable doses (100/50, 250/50, 500/50 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| Inhaled budesonide and formoterol | Daily maintenance | Variable doses (80/4.5, 160/4.5 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| EIB, exercise-induced bronchoconstriction. | ||||
| Adapted from the National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma.9 | ||||
Cromolyn, antileukotrienes are options, too
Mast cell stabilizers (cromolyn) can be used with β2 agonists as prophylactic therapy. When these agents are used together, they have an additive effect.13 The athlete may take them 10 minutes to an hour before exercise. Make sure your patient knows that mast cell stabilizers cannot be used as a rescue inhaler or bronchodilator.
Inhaled corticosteroids (flunisolide, fluticasone, others) may be needed for athletes with poorly controlled chronic asthma; they can also be used as adjunct preventive treatment for athletes who have EIB with no underlying chronic asthma.14-16 Often, inhaled corticosteroids are used as combination therapy with a LABA or an antileukotriene agent (montelukast, zafirlukast; see below). Recent research shows that montelukast in combination with inhaled corticosteroids is more efficacious than LABA with inhaled corticosteroids.14,17
Antileukotriene agents can be especially helpful for EIB in patients with mild, stable asthma.18 Patients who do respond to antileukotriene agents usually respond very favorably. Antileukotrienes offer a reasonable alternative to inhaled corticosteroids and LABAs. They have a low side-effect profile and should be considered as daily prophylaxis.19,20 The effects of montelukast are evident as early as 2 hours after administration, and bronchoprotective effects can last as long as 24 hours.21,22 For that reason, montelukast is especially useful in children whose exercise patterns are not always predictable.
Be prepared for acute exacerbations. Prophylactic medication does not always prevent acute exacerbations. When that happens, your EIB patient will need to use a β2 agonist as rescue therapy. Make sure your patient knows that none of the other medications are effective bronchodilators in acute exacerbations.
Remember, too, that EIB cannot be effectively treated if the athlete has poorly controlled chronic asthma. Underlying causes of asthma exacerbations like allergies or respiratory infections must be addressed and stabilized first, following guidelines of the National Asthma Education and Prevention Program (NAEPP).9 You can access the guidelines at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
These tips can help the athlete
Encourage athletes with EIB to keep up their exercise routines, because cardiovascular fitness has a beneficial effect on this condition. Fit individuals breathe more slowly, which reduces the likelihood of exacerbations. Of note, though: Certain sports are easier on patients with EIB. Patients may want to keep this in mind when deciding which team they want to go out for. Specifically, indoor sports, where air temperature, humidity, and exposure to allergens are controlled, and sports like baseball, sprinting, or football, which require less prolonged aerobic endurance, are good options.
Tell athletes whose sports require cold, dry conditions—ice skating, or skiing, for instance—to try breathing through a scarf or mask to keep inspired air warm and less irritating.
And tell all athletes with EIB to warm up properly before they start to compete.23 That means a 15-minute warm-up at moderate exertion, followed by a 15- to 30-minute rest period. The rest period is the time to take their medication.
When therapy fails
When an EIB patient fails to respond despite multiple drug therapy, it’s time to reconsider other diagnoses, such as vocal cord dysfunction and severe GERD, which may mimic symptoms of EIB.
On the horizon. Other therapies for possible treatment of EIB are being studied. These include omega-3 fatty acid dietary supplementation and inhaled enoxaparin.24,25 Data are currently insufficient to recommend use of these agents in clinical practice.
As for Luke, indirect testing via exercise challenge was positive for EIB. Adjunctive therapy with montelukast was added to his albuterol inhaler, and the combination has worked well for him. He’s still playing basketball, and enjoying it.
Acknowledgments
The authors thank Ken Rundell, PhD, for reviewing this article. Dr. Rundell is director of the Human Physiology Laboratory at the Keith J. O’Neill Center of Marywood University, Scranton, Pa.
CORRESPONDENCE
Michael A. Krafczyk, MD, FAAFP, St. Luke’s Sports Medicine, 153 Brodhead Rd, Bethlehem, PA 18017; [email protected]
1. Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med. 2004;14:134-138.
2. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343-348.
3. Rundell KW, Mayers LB, Wilber RL, et al. Self-reported symptoms of exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33:208-213.
4. Weiler JM, Bonini S, Coifman R, et al. Ad Hoc Committee of Sports Medicine Committee, American Academy of Allergy, Asthma, and Immunology Work Group Report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119:1349-1358.
5. Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128:3966-3974.
6. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
7. Rundell KW, Wilber RL, Szmedra L, et al. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenges. Med Sci Sports Exerc. 2000;32:309-316.
8. Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s Consensus Conference, Lausanne Switzerland. January 22-24, 2008. J Allergy Clin Immunol. 2008;122:254-260.
9. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. NIH publication no. 08-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed September 1, 2007.
10. Anderson S, Seale JP, Ferris L, et al. An evaluation of pharmacotherapy for exercise-induced asthma. J Allergy Clin Immunol. 1979;64:612-624.
11. Hancox RJ, Subbarao P, Kamada D, et al. β2-Agonist tolerance and exercise-induced bronchospasm. Am Respir Crit Care Med. 2002;165:1068-1070.
12. Inman M, O’Byrne PM. The effect of regular inhaled albuterol on exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 1996;153:65-69.
13. Latimer KM, O’Byrne PM, Morris MM, et al. Bronchoconstriction stimulated by airway cooling: better protection with combined inhalation of terbutaline sulphate and cromolyn sodium than with either alone. Am Rev Respir Dis. 1983;128:440-443.
14. Stelmach I, Grzelewski T, Majak P, et al. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J Allergy Clin Immunol. 2008;121:383-389.
15. Koh MS, Tee A, Lasserson TJ, et al. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;(3):CD002739.-
16. Jonasson G, Carlsen KH, Hultquist C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr Allergy Immunol. 2000;11:120-125.
17. Storms W, Chervinsky P, Ghannam AF, et al. Challenge-Rescue Study Group. Respir Med. 2004;98:1051-1062.
18. Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147-152.
19. Steinshamn S, Sandsund M, Sue-Chu M, et al. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest. 2004;126:1154-1160.
20. Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis, and exercise-induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173-2187.
21. Pearlman DS, van Adelsberg J, Philip G, et al. Onset and duration of protection against exercise-induced bronchoconstriction by a single oral dose of montelukast. Ann Allergy Asthma Immunol. 2006;97:98-104.
22. Philip G, Villaran C, Pearlman DS, et al. Protection against exercise-induced bronchoconstriction two hours after a single oral dose of montelukast. J Asthma. 2007;44:213-217.
23. Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35:1464-1470.
24. Mickleborough TD, Lindley MR, Ionescu AA, et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39-49.
25. Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160:576-581.
- Don’t rely on self-reported symptoms to diagnose exercise-induced bronchoconstriction (EIB) (A).
- Indirect testing is the best way to diagnose EIB in patients who do not have underlying asthma (A).
- Short-acting β2-agonists should be first-line management in EIB (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Luke, a 16-year-old basketball player, complains that he can’t finish a game without running out of breath. He says things are at their worst when the game is close and when it’s nearing the end. He doesn’t have the problem during practice, or when he is playing other sports. The team physician suggested using an albuterol inhaler half an hour before game time and when he has symptoms, but he gets only minimal relief. Now he has come to you.
His vital signs, lung exam, and cardiac exam are normal. Results of pulmonary function tests with pre- and post-albuterol challenge done a year ago were also normal. Does Luke have exercise-induced bronchoconstriction (EIB)? How can you be sure? And what can you do to help?
Symptoms like Luke’s are common among athletes of all abilities. They may add up to EIB, a condition with an estimated prevalence of 6% to 12% in the general population—or they may not.1 One study showed that only a third of athletes with symptoms or prior diagnosis of EIB had positive objective testing for the condition, and current studies show that reported symptoms are not an accurate guide in athletes like Luke who do not have underlying asthma.2,3 To treat him correctly, you will need to nail down the diagnosis with additional tests.3,4
Shortness of breath that’s worse than expected
EIB can have many different presentations. The most common symptom is cough associated with exercise.3 Other common signs and symptoms include wheezing, chest tightness, and more severe than expected or worsening shortness of breath. More unusual symptoms include a decrease in performance or fatigue out of proportion to workload. Often patients with EIB have other associated medical conditions, such as allergic rhinitis.
Bronchoconstriction usually occurs with maximal or near maximal exertion. Generally, it takes 5 to 8 minutes of exercising at 80% of maximal heart rate to trigger EIB. Classically, the symptoms peak 5 to 10 minutes after exercise begins.5
Rule out cardiac problems. If EIB is the correct diagnosis, the physical exam is usually normal. The importance of the physical exam is to evaluate for other diagnoses with similar presentations. Conditions to rule out include cardiac problems, exercise-induced hyperventilation, upper and lower respiratory infections or abnormalities, exercise-induced laryngeal dysfunction, exercise-induced anaphylaxis, and gastroesophageal reflux disease (GERD). The differential diagnosis for EIB is summarized in TABLE 1.
Test for asthma. Once you have gone through the differential diagnosis and are comfortable that the symptoms are respiratory, the next step should be pulmonary function tests (PFT), pre- and post-albuterol challenge. Findings of obstruction, such as reduced forced expiratory volume in 1 second (FEV1) or increased lung volume, are consistent with a diagnosis of asthma. In that case, no further workup is needed—unless the patient is unresponsive to asthma treatment. In athletes like Luke who do not have asthma and have a normal nonprovocative spirometry, you can move on to either provocative spirometry or empiric treatment.
TABLE 1
Is it EIB, or something else?
| ETIOLOGY | POSSIBLE DIAGNOSES |
|---|---|
| Pulmonary | Exercise-induced hyperventilation (pseudo-asthma syndrome) Restrictive lung disease Cystic fibrosis Upper and lower respiratory infections Foreign body aspiration |
| Cardiac | Coronary artery disease Congenital and acquired heart defects Cardiomyopathy Congestive heart failure |
| Laryngeal | Exercise-induced laryngeal dysfunction Vocal cord dysfunction Laryngeal prolapse Laryngomalacia |
| Gastroesophageal | Gastroesophageal reflux disease |
| Allergic | Exercise-induced anaphylaxis |
| Other | Athlete is out of shape |
| EIB, exercise-induced bronchoconstriction. | |
| Source: Weiler JM, et al. J Allergy Clin Immunol. 2007.4 | |
Perform provocative spirometry
Direct spirometry is commonly done with a methacholine challenge. This test is less sensitive than indirect testing for EIB patients who do not have underlying asthma.
The gold standard for indirect testing is eucapnic voluntary hyperventilation (EVH). Because EVH requires special equipment, however, it may not be an option in your office. The more reasonable choice is exercise challenge testing, which can be done either in your office or in the milieu—the basketball court, for example—where the athlete’s symptoms usually occur. In an exercise challenge, you get a baseline spirometry measurement, have the athlete exercise to 80% to 90% of maximal heart rate, and then repeat spirometry at short intervals after exercise ends. If you do an exercise challenge in the office, you can reduce false-negative results by maintaining an ambient temperature between 68° and 77°F (20°-25°C) with a relative humidity of less than 50%.6,7
Or try empiric treatment
Empiric treatment is a reasonable strategy for athletes with EIB symptoms, worth trying both for athletes who have underlying asthma and for those who do not. If the athlete with asthma responds to treatment, the problem is solved. For the athlete who does not have asthma, however, there are some exceptions to this approach—specifically, the elite athlete.
In the elite athlete, you will need to confirm the diagnosis because many of the substances used to treat EIB are restricted by governing bodies such as the International Olympic Committee (IOC) and require provocative testing to obtain a therapeutic use exemption.8 There is some debate as to whether nonelite athletes also need bronchoprovocative testing. Some recommendations advise testing all elite and competitive athletes and restricting empiric treatment to recreational athletes.1 For more information on banned or restricted medications, see “Is that drug banned from competition?”.
If you take the empiric approach and the athlete does not respond to treatment, consider further testing to rule out other, more serious problems. In Luke’s case, where empiric treatment with albuterol has failed, indirect testing would be the next step.
Certain medications used in the treatment of asthma and exercise-induced bronchoconstriction (EIB) are considered performance-enhancing drugs and either banned or restricted in athletic competition. The regulatory bodies that make these designations in the United States are the National Collegiate Athletic Association (NCAA) and the International Olympic Committee World Anti-Doping Agency (IOC-WADA). These organizations update their list of banned substances yearly and make the current list available on the Web. You can find the NCAA list at www.pace.edu/emplibrary/NCAA%20LIST%20OF%20BANNED%20SUBSTANCESb.doc and the IOC-WADA list at www.wada-ama.org/rtecontent/document/2009_Prohibited_List_ENG_Final_20_Sept_08.pdf.
The IOC-WADA allows competing athletes to use inhaled corticosteroids and β2 agonists, but requires athletes with asthma to provide documentation that the medication is for therapeutic use. Glucocorticosteroids and oral β2 agonists remain prohibited by the IOC-WADA, but only oral β2 agonists are banned by the NCAA. The NCAA warns that student athletes are responsible for knowing which substances are on the banned list and advises them to consult www.drugfreesport.com for more information. To avoid disqualifying a patient from sports participation, check medications you prescribe with the official lists and be sure your EIB patient has the documentation he or she needs to qualify for a therapeutic use exemption.
Medicate before exercise: SABAs and LABAs
Prophylaxis for EIB usually starts with an inhaled short-acting β2 agonist (SABA) such as albuterol or pirbuterol, taken 15 minutes before starting to exercise.9,10 The effectiveness of both short- and long-acting β2 agonists decreases with frequent use, which may be Luke’s problem. For that reason, patients with mild EIB may choose to use pretreatment medication only for more demanding exercise sessions.11 Advise EIB patients who need daily pretreatment to try adjunctive maintenance therapy (discussed at greater length, below.)
Longer-acting β2 agonists (LABAs) such as salmeterol or formoterol may be effective for prolonged or all-day exercise, but may lose their prophylactic effect with prolonged use.12 Furthermore, the US Food and Drug Administration (FDA) has advised against using LABAs alone because of the possibility of severe asthma episodes or death. LABAs should be used only in conjunction with daily maintenance therapy with inhaled corticosteroids. The properties of these and other EIB medications are summarized in TABLE 2.
TABLE 2
EIB medications
| MEDICATION | INDICATION | DOSE | CAUTIONS | COMMENT |
|---|---|---|---|---|
| Short-acting β2 agonists (SABAs) | ||||
| Albuterol, pirbuterol | Pre-exercise prophylaxis, acute treatment | 2 puffs pre-exercise or 2 puffs every 4-6 h as needed | May cause tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | First-line treatment |
| Mast cell stabilizers | ||||
| Cromolyn | Pre-exercise treatment | 2 puffs 30-45 min before exercise | None | Best combined with SABA. Tell patients not to use for rescue. |
| Inhaled corticosteroids | ||||
| Flunisolide, fluticasone, budesonide, triamcinolone, beclomethasone, mometasone | Daily maintenance | Variable | Can cause oral candidiasis, hoarseness. | Tell patients this is not a rescue inhaler. |
| Leukotriene inhibitors | ||||
| Zafirlukast | Daily maintenance | 20 mg PO, bid | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Montelukast | Daily maintenance, pre-exercise prophylaxis | 10 mg PO daily or up to 2 h pre-exercise | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Zileuton | Daily maintenance | 1200 mg PO, bid | Risk of elevated liver function tests. | Variable response. Low side-effect profile. |
| Combinations | ||||
| Inhaled fluticasone and salmeterol | Daily maintenance | Variable doses (100/50, 250/50, 500/50 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| Inhaled budesonide and formoterol | Daily maintenance | Variable doses (80/4.5, 160/4.5 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| EIB, exercise-induced bronchoconstriction. | ||||
| Adapted from the National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma.9 | ||||
Cromolyn, antileukotrienes are options, too
Mast cell stabilizers (cromolyn) can be used with β2 agonists as prophylactic therapy. When these agents are used together, they have an additive effect.13 The athlete may take them 10 minutes to an hour before exercise. Make sure your patient knows that mast cell stabilizers cannot be used as a rescue inhaler or bronchodilator.
Inhaled corticosteroids (flunisolide, fluticasone, others) may be needed for athletes with poorly controlled chronic asthma; they can also be used as adjunct preventive treatment for athletes who have EIB with no underlying chronic asthma.14-16 Often, inhaled corticosteroids are used as combination therapy with a LABA or an antileukotriene agent (montelukast, zafirlukast; see below). Recent research shows that montelukast in combination with inhaled corticosteroids is more efficacious than LABA with inhaled corticosteroids.14,17
Antileukotriene agents can be especially helpful for EIB in patients with mild, stable asthma.18 Patients who do respond to antileukotriene agents usually respond very favorably. Antileukotrienes offer a reasonable alternative to inhaled corticosteroids and LABAs. They have a low side-effect profile and should be considered as daily prophylaxis.19,20 The effects of montelukast are evident as early as 2 hours after administration, and bronchoprotective effects can last as long as 24 hours.21,22 For that reason, montelukast is especially useful in children whose exercise patterns are not always predictable.
Be prepared for acute exacerbations. Prophylactic medication does not always prevent acute exacerbations. When that happens, your EIB patient will need to use a β2 agonist as rescue therapy. Make sure your patient knows that none of the other medications are effective bronchodilators in acute exacerbations.
Remember, too, that EIB cannot be effectively treated if the athlete has poorly controlled chronic asthma. Underlying causes of asthma exacerbations like allergies or respiratory infections must be addressed and stabilized first, following guidelines of the National Asthma Education and Prevention Program (NAEPP).9 You can access the guidelines at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
These tips can help the athlete
Encourage athletes with EIB to keep up their exercise routines, because cardiovascular fitness has a beneficial effect on this condition. Fit individuals breathe more slowly, which reduces the likelihood of exacerbations. Of note, though: Certain sports are easier on patients with EIB. Patients may want to keep this in mind when deciding which team they want to go out for. Specifically, indoor sports, where air temperature, humidity, and exposure to allergens are controlled, and sports like baseball, sprinting, or football, which require less prolonged aerobic endurance, are good options.
Tell athletes whose sports require cold, dry conditions—ice skating, or skiing, for instance—to try breathing through a scarf or mask to keep inspired air warm and less irritating.
And tell all athletes with EIB to warm up properly before they start to compete.23 That means a 15-minute warm-up at moderate exertion, followed by a 15- to 30-minute rest period. The rest period is the time to take their medication.
When therapy fails
When an EIB patient fails to respond despite multiple drug therapy, it’s time to reconsider other diagnoses, such as vocal cord dysfunction and severe GERD, which may mimic symptoms of EIB.
On the horizon. Other therapies for possible treatment of EIB are being studied. These include omega-3 fatty acid dietary supplementation and inhaled enoxaparin.24,25 Data are currently insufficient to recommend use of these agents in clinical practice.
As for Luke, indirect testing via exercise challenge was positive for EIB. Adjunctive therapy with montelukast was added to his albuterol inhaler, and the combination has worked well for him. He’s still playing basketball, and enjoying it.
Acknowledgments
The authors thank Ken Rundell, PhD, for reviewing this article. Dr. Rundell is director of the Human Physiology Laboratory at the Keith J. O’Neill Center of Marywood University, Scranton, Pa.
CORRESPONDENCE
Michael A. Krafczyk, MD, FAAFP, St. Luke’s Sports Medicine, 153 Brodhead Rd, Bethlehem, PA 18017; [email protected]
- Don’t rely on self-reported symptoms to diagnose exercise-induced bronchoconstriction (EIB) (A).
- Indirect testing is the best way to diagnose EIB in patients who do not have underlying asthma (A).
- Short-acting β2-agonists should be first-line management in EIB (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Luke, a 16-year-old basketball player, complains that he can’t finish a game without running out of breath. He says things are at their worst when the game is close and when it’s nearing the end. He doesn’t have the problem during practice, or when he is playing other sports. The team physician suggested using an albuterol inhaler half an hour before game time and when he has symptoms, but he gets only minimal relief. Now he has come to you.
His vital signs, lung exam, and cardiac exam are normal. Results of pulmonary function tests with pre- and post-albuterol challenge done a year ago were also normal. Does Luke have exercise-induced bronchoconstriction (EIB)? How can you be sure? And what can you do to help?
Symptoms like Luke’s are common among athletes of all abilities. They may add up to EIB, a condition with an estimated prevalence of 6% to 12% in the general population—or they may not.1 One study showed that only a third of athletes with symptoms or prior diagnosis of EIB had positive objective testing for the condition, and current studies show that reported symptoms are not an accurate guide in athletes like Luke who do not have underlying asthma.2,3 To treat him correctly, you will need to nail down the diagnosis with additional tests.3,4
Shortness of breath that’s worse than expected
EIB can have many different presentations. The most common symptom is cough associated with exercise.3 Other common signs and symptoms include wheezing, chest tightness, and more severe than expected or worsening shortness of breath. More unusual symptoms include a decrease in performance or fatigue out of proportion to workload. Often patients with EIB have other associated medical conditions, such as allergic rhinitis.
Bronchoconstriction usually occurs with maximal or near maximal exertion. Generally, it takes 5 to 8 minutes of exercising at 80% of maximal heart rate to trigger EIB. Classically, the symptoms peak 5 to 10 minutes after exercise begins.5
Rule out cardiac problems. If EIB is the correct diagnosis, the physical exam is usually normal. The importance of the physical exam is to evaluate for other diagnoses with similar presentations. Conditions to rule out include cardiac problems, exercise-induced hyperventilation, upper and lower respiratory infections or abnormalities, exercise-induced laryngeal dysfunction, exercise-induced anaphylaxis, and gastroesophageal reflux disease (GERD). The differential diagnosis for EIB is summarized in TABLE 1.
Test for asthma. Once you have gone through the differential diagnosis and are comfortable that the symptoms are respiratory, the next step should be pulmonary function tests (PFT), pre- and post-albuterol challenge. Findings of obstruction, such as reduced forced expiratory volume in 1 second (FEV1) or increased lung volume, are consistent with a diagnosis of asthma. In that case, no further workup is needed—unless the patient is unresponsive to asthma treatment. In athletes like Luke who do not have asthma and have a normal nonprovocative spirometry, you can move on to either provocative spirometry or empiric treatment.
TABLE 1
Is it EIB, or something else?
| ETIOLOGY | POSSIBLE DIAGNOSES |
|---|---|
| Pulmonary | Exercise-induced hyperventilation (pseudo-asthma syndrome) Restrictive lung disease Cystic fibrosis Upper and lower respiratory infections Foreign body aspiration |
| Cardiac | Coronary artery disease Congenital and acquired heart defects Cardiomyopathy Congestive heart failure |
| Laryngeal | Exercise-induced laryngeal dysfunction Vocal cord dysfunction Laryngeal prolapse Laryngomalacia |
| Gastroesophageal | Gastroesophageal reflux disease |
| Allergic | Exercise-induced anaphylaxis |
| Other | Athlete is out of shape |
| EIB, exercise-induced bronchoconstriction. | |
| Source: Weiler JM, et al. J Allergy Clin Immunol. 2007.4 | |
Perform provocative spirometry
Direct spirometry is commonly done with a methacholine challenge. This test is less sensitive than indirect testing for EIB patients who do not have underlying asthma.
The gold standard for indirect testing is eucapnic voluntary hyperventilation (EVH). Because EVH requires special equipment, however, it may not be an option in your office. The more reasonable choice is exercise challenge testing, which can be done either in your office or in the milieu—the basketball court, for example—where the athlete’s symptoms usually occur. In an exercise challenge, you get a baseline spirometry measurement, have the athlete exercise to 80% to 90% of maximal heart rate, and then repeat spirometry at short intervals after exercise ends. If you do an exercise challenge in the office, you can reduce false-negative results by maintaining an ambient temperature between 68° and 77°F (20°-25°C) with a relative humidity of less than 50%.6,7
Or try empiric treatment
Empiric treatment is a reasonable strategy for athletes with EIB symptoms, worth trying both for athletes who have underlying asthma and for those who do not. If the athlete with asthma responds to treatment, the problem is solved. For the athlete who does not have asthma, however, there are some exceptions to this approach—specifically, the elite athlete.
In the elite athlete, you will need to confirm the diagnosis because many of the substances used to treat EIB are restricted by governing bodies such as the International Olympic Committee (IOC) and require provocative testing to obtain a therapeutic use exemption.8 There is some debate as to whether nonelite athletes also need bronchoprovocative testing. Some recommendations advise testing all elite and competitive athletes and restricting empiric treatment to recreational athletes.1 For more information on banned or restricted medications, see “Is that drug banned from competition?”.
If you take the empiric approach and the athlete does not respond to treatment, consider further testing to rule out other, more serious problems. In Luke’s case, where empiric treatment with albuterol has failed, indirect testing would be the next step.
Certain medications used in the treatment of asthma and exercise-induced bronchoconstriction (EIB) are considered performance-enhancing drugs and either banned or restricted in athletic competition. The regulatory bodies that make these designations in the United States are the National Collegiate Athletic Association (NCAA) and the International Olympic Committee World Anti-Doping Agency (IOC-WADA). These organizations update their list of banned substances yearly and make the current list available on the Web. You can find the NCAA list at www.pace.edu/emplibrary/NCAA%20LIST%20OF%20BANNED%20SUBSTANCESb.doc and the IOC-WADA list at www.wada-ama.org/rtecontent/document/2009_Prohibited_List_ENG_Final_20_Sept_08.pdf.
The IOC-WADA allows competing athletes to use inhaled corticosteroids and β2 agonists, but requires athletes with asthma to provide documentation that the medication is for therapeutic use. Glucocorticosteroids and oral β2 agonists remain prohibited by the IOC-WADA, but only oral β2 agonists are banned by the NCAA. The NCAA warns that student athletes are responsible for knowing which substances are on the banned list and advises them to consult www.drugfreesport.com for more information. To avoid disqualifying a patient from sports participation, check medications you prescribe with the official lists and be sure your EIB patient has the documentation he or she needs to qualify for a therapeutic use exemption.
Medicate before exercise: SABAs and LABAs
Prophylaxis for EIB usually starts with an inhaled short-acting β2 agonist (SABA) such as albuterol or pirbuterol, taken 15 minutes before starting to exercise.9,10 The effectiveness of both short- and long-acting β2 agonists decreases with frequent use, which may be Luke’s problem. For that reason, patients with mild EIB may choose to use pretreatment medication only for more demanding exercise sessions.11 Advise EIB patients who need daily pretreatment to try adjunctive maintenance therapy (discussed at greater length, below.)
Longer-acting β2 agonists (LABAs) such as salmeterol or formoterol may be effective for prolonged or all-day exercise, but may lose their prophylactic effect with prolonged use.12 Furthermore, the US Food and Drug Administration (FDA) has advised against using LABAs alone because of the possibility of severe asthma episodes or death. LABAs should be used only in conjunction with daily maintenance therapy with inhaled corticosteroids. The properties of these and other EIB medications are summarized in TABLE 2.
TABLE 2
EIB medications
| MEDICATION | INDICATION | DOSE | CAUTIONS | COMMENT |
|---|---|---|---|---|
| Short-acting β2 agonists (SABAs) | ||||
| Albuterol, pirbuterol | Pre-exercise prophylaxis, acute treatment | 2 puffs pre-exercise or 2 puffs every 4-6 h as needed | May cause tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | First-line treatment |
| Mast cell stabilizers | ||||
| Cromolyn | Pre-exercise treatment | 2 puffs 30-45 min before exercise | None | Best combined with SABA. Tell patients not to use for rescue. |
| Inhaled corticosteroids | ||||
| Flunisolide, fluticasone, budesonide, triamcinolone, beclomethasone, mometasone | Daily maintenance | Variable | Can cause oral candidiasis, hoarseness. | Tell patients this is not a rescue inhaler. |
| Leukotriene inhibitors | ||||
| Zafirlukast | Daily maintenance | 20 mg PO, bid | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Montelukast | Daily maintenance, pre-exercise prophylaxis | 10 mg PO daily or up to 2 h pre-exercise | None | Variable response. Works well with inhaled corticosteroids. Low side-effect profile. |
| Zileuton | Daily maintenance | 1200 mg PO, bid | Risk of elevated liver function tests. | Variable response. Low side-effect profile. |
| Combinations | ||||
| Inhaled fluticasone and salmeterol | Daily maintenance | Variable doses (100/50, 250/50, 500/50 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| Inhaled budesonide and formoterol | Daily maintenance | Variable doses (80/4.5, 160/4.5 mcg/spray); 1 puff bid | Can cause oral candidiasis, hoarseness, tachycardia, hypokalemia. Tachyphylaxis can develop with frequent use. | Tell patients this is not a rescue inhaler. |
| EIB, exercise-induced bronchoconstriction. | ||||
| Adapted from the National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma.9 | ||||
Cromolyn, antileukotrienes are options, too
Mast cell stabilizers (cromolyn) can be used with β2 agonists as prophylactic therapy. When these agents are used together, they have an additive effect.13 The athlete may take them 10 minutes to an hour before exercise. Make sure your patient knows that mast cell stabilizers cannot be used as a rescue inhaler or bronchodilator.
Inhaled corticosteroids (flunisolide, fluticasone, others) may be needed for athletes with poorly controlled chronic asthma; they can also be used as adjunct preventive treatment for athletes who have EIB with no underlying chronic asthma.14-16 Often, inhaled corticosteroids are used as combination therapy with a LABA or an antileukotriene agent (montelukast, zafirlukast; see below). Recent research shows that montelukast in combination with inhaled corticosteroids is more efficacious than LABA with inhaled corticosteroids.14,17
Antileukotriene agents can be especially helpful for EIB in patients with mild, stable asthma.18 Patients who do respond to antileukotriene agents usually respond very favorably. Antileukotrienes offer a reasonable alternative to inhaled corticosteroids and LABAs. They have a low side-effect profile and should be considered as daily prophylaxis.19,20 The effects of montelukast are evident as early as 2 hours after administration, and bronchoprotective effects can last as long as 24 hours.21,22 For that reason, montelukast is especially useful in children whose exercise patterns are not always predictable.
Be prepared for acute exacerbations. Prophylactic medication does not always prevent acute exacerbations. When that happens, your EIB patient will need to use a β2 agonist as rescue therapy. Make sure your patient knows that none of the other medications are effective bronchodilators in acute exacerbations.
Remember, too, that EIB cannot be effectively treated if the athlete has poorly controlled chronic asthma. Underlying causes of asthma exacerbations like allergies or respiratory infections must be addressed and stabilized first, following guidelines of the National Asthma Education and Prevention Program (NAEPP).9 You can access the guidelines at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
These tips can help the athlete
Encourage athletes with EIB to keep up their exercise routines, because cardiovascular fitness has a beneficial effect on this condition. Fit individuals breathe more slowly, which reduces the likelihood of exacerbations. Of note, though: Certain sports are easier on patients with EIB. Patients may want to keep this in mind when deciding which team they want to go out for. Specifically, indoor sports, where air temperature, humidity, and exposure to allergens are controlled, and sports like baseball, sprinting, or football, which require less prolonged aerobic endurance, are good options.
Tell athletes whose sports require cold, dry conditions—ice skating, or skiing, for instance—to try breathing through a scarf or mask to keep inspired air warm and less irritating.
And tell all athletes with EIB to warm up properly before they start to compete.23 That means a 15-minute warm-up at moderate exertion, followed by a 15- to 30-minute rest period. The rest period is the time to take their medication.
When therapy fails
When an EIB patient fails to respond despite multiple drug therapy, it’s time to reconsider other diagnoses, such as vocal cord dysfunction and severe GERD, which may mimic symptoms of EIB.
On the horizon. Other therapies for possible treatment of EIB are being studied. These include omega-3 fatty acid dietary supplementation and inhaled enoxaparin.24,25 Data are currently insufficient to recommend use of these agents in clinical practice.
As for Luke, indirect testing via exercise challenge was positive for EIB. Adjunctive therapy with montelukast was added to his albuterol inhaler, and the combination has worked well for him. He’s still playing basketball, and enjoying it.
Acknowledgments
The authors thank Ken Rundell, PhD, for reviewing this article. Dr. Rundell is director of the Human Physiology Laboratory at the Keith J. O’Neill Center of Marywood University, Scranton, Pa.
CORRESPONDENCE
Michael A. Krafczyk, MD, FAAFP, St. Luke’s Sports Medicine, 153 Brodhead Rd, Bethlehem, PA 18017; [email protected]
1. Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med. 2004;14:134-138.
2. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343-348.
3. Rundell KW, Mayers LB, Wilber RL, et al. Self-reported symptoms of exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33:208-213.
4. Weiler JM, Bonini S, Coifman R, et al. Ad Hoc Committee of Sports Medicine Committee, American Academy of Allergy, Asthma, and Immunology Work Group Report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119:1349-1358.
5. Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128:3966-3974.
6. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
7. Rundell KW, Wilber RL, Szmedra L, et al. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenges. Med Sci Sports Exerc. 2000;32:309-316.
8. Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s Consensus Conference, Lausanne Switzerland. January 22-24, 2008. J Allergy Clin Immunol. 2008;122:254-260.
9. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. NIH publication no. 08-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed September 1, 2007.
10. Anderson S, Seale JP, Ferris L, et al. An evaluation of pharmacotherapy for exercise-induced asthma. J Allergy Clin Immunol. 1979;64:612-624.
11. Hancox RJ, Subbarao P, Kamada D, et al. β2-Agonist tolerance and exercise-induced bronchospasm. Am Respir Crit Care Med. 2002;165:1068-1070.
12. Inman M, O’Byrne PM. The effect of regular inhaled albuterol on exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 1996;153:65-69.
13. Latimer KM, O’Byrne PM, Morris MM, et al. Bronchoconstriction stimulated by airway cooling: better protection with combined inhalation of terbutaline sulphate and cromolyn sodium than with either alone. Am Rev Respir Dis. 1983;128:440-443.
14. Stelmach I, Grzelewski T, Majak P, et al. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J Allergy Clin Immunol. 2008;121:383-389.
15. Koh MS, Tee A, Lasserson TJ, et al. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;(3):CD002739.-
16. Jonasson G, Carlsen KH, Hultquist C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr Allergy Immunol. 2000;11:120-125.
17. Storms W, Chervinsky P, Ghannam AF, et al. Challenge-Rescue Study Group. Respir Med. 2004;98:1051-1062.
18. Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147-152.
19. Steinshamn S, Sandsund M, Sue-Chu M, et al. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest. 2004;126:1154-1160.
20. Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis, and exercise-induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173-2187.
21. Pearlman DS, van Adelsberg J, Philip G, et al. Onset and duration of protection against exercise-induced bronchoconstriction by a single oral dose of montelukast. Ann Allergy Asthma Immunol. 2006;97:98-104.
22. Philip G, Villaran C, Pearlman DS, et al. Protection against exercise-induced bronchoconstriction two hours after a single oral dose of montelukast. J Asthma. 2007;44:213-217.
23. Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35:1464-1470.
24. Mickleborough TD, Lindley MR, Ionescu AA, et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39-49.
25. Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160:576-581.
1. Holzer K, Brukner P. Screening of athletes for exercise-induced bronchoconstriction. Clin J Sport Med. 2004;14:134-138.
2. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343-348.
3. Rundell KW, Mayers LB, Wilber RL, et al. Self-reported symptoms of exercise-induced asthma in the elite athlete. Med Sci Sports Exerc. 2001;33:208-213.
4. Weiler JM, Bonini S, Coifman R, et al. Ad Hoc Committee of Sports Medicine Committee, American Academy of Allergy, Asthma, and Immunology Work Group Report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119:1349-1358.
5. Parsons JP, Mastronarde JG. Exercise-induced bronchoconstriction in athletes. Chest. 2005;128:3966-3974.
6. Rundell KW, Slee JB. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J Allergy Clin Immunol. 2008;122:238-246.
7. Rundell KW, Wilber RL, Szmedra L, et al. Exercise-induced asthma screening of elite athletes: field versus laboratory exercise challenges. Med Sci Sports Exerc. 2000;32:309-316.
8. Fitch KD, Sue-Chu M, Anderson SD, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s Consensus Conference, Lausanne Switzerland. January 22-24, 2008. J Allergy Clin Immunol. 2008;122:254-260.
9. National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. NIH publication no. 08-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed September 1, 2007.
10. Anderson S, Seale JP, Ferris L, et al. An evaluation of pharmacotherapy for exercise-induced asthma. J Allergy Clin Immunol. 1979;64:612-624.
11. Hancox RJ, Subbarao P, Kamada D, et al. β2-Agonist tolerance and exercise-induced bronchospasm. Am Respir Crit Care Med. 2002;165:1068-1070.
12. Inman M, O’Byrne PM. The effect of regular inhaled albuterol on exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 1996;153:65-69.
13. Latimer KM, O’Byrne PM, Morris MM, et al. Bronchoconstriction stimulated by airway cooling: better protection with combined inhalation of terbutaline sulphate and cromolyn sodium than with either alone. Am Rev Respir Dis. 1983;128:440-443.
14. Stelmach I, Grzelewski T, Majak P, et al. Effect of different antiasthmatic treatments on exercise-induced bronchoconstriction in children with asthma. J Allergy Clin Immunol. 2008;121:383-389.
15. Koh MS, Tee A, Lasserson TJ, et al. Inhaled corticosteroids compared to placebo for prevention of exercise induced bronchoconstriction. Cochrane Database Syst Rev. 2007;(3):CD002739.-
16. Jonasson G, Carlsen KH, Hultquist C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr Allergy Immunol. 2000;11:120-125.
17. Storms W, Chervinsky P, Ghannam AF, et al. Challenge-Rescue Study Group. Respir Med. 2004;98:1051-1062.
18. Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-receptor antagonist for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147-152.
19. Steinshamn S, Sandsund M, Sue-Chu M, et al. Effects of montelukast and salmeterol on physical performance and exercise economy in adult asthmatics with exercise-induced bronchoconstriction. Chest. 2004;126:1154-1160.
20. Storms W. Update on montelukast and its role in the treatment of asthma, allergic rhinitis, and exercise-induced bronchoconstriction. Expert Opin Pharmacother. 2007;8:2173-2187.
21. Pearlman DS, van Adelsberg J, Philip G, et al. Onset and duration of protection against exercise-induced bronchoconstriction by a single oral dose of montelukast. Ann Allergy Asthma Immunol. 2006;97:98-104.
22. Philip G, Villaran C, Pearlman DS, et al. Protection against exercise-induced bronchoconstriction two hours after a single oral dose of montelukast. J Asthma. 2007;44:213-217.
23. Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc. 2003;35:1464-1470.
24. Mickleborough TD, Lindley MR, Ionescu AA, et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39-49.
25. Ahmed T, Gonzalez BJ, Danta I. Prevention of exercise-induced bronchoconstriction by inhaled low-molecular-weight heparin. Am J Respir Crit Care Med. 1999;160:576-581.