User login
Natural History of HPV Infections
Transmission of HPV
Most papillomavirus infections are transmitted through close skin-to-skin or mucosa-to-mucosa contact. Epidemiologic studies clearly indicate that sexual intercourse is the primary route for anogenital HPV infection.1 Infection is relatively uncommon in women who have not had intercourse, and there is a strong and consistent relationship between the number of both lifetime and recent sexual partners and the prevalence of HPV in women. There is also a strong association between having had a recent new sexual partner(s) and incident anogenital HPV infection. Consistent condom use reduces—but does not eliminate—HPV transmission.2 In a prospective study on college students who initiated sexual intercourse either after or immediately prior to enrollment, the overall rate of anogenital HPV infection was 89 per 100 patient-years of follow-up in those whose partners rarely used condoms during sexual intercourse, compared with 38 per 100 patient-years of follow-up among those whose partners always used condoms.
Penetrative sexual intercourse is not a requirement for HPV transmission. Both oral and digital HPV infections occur, and there is evidence that digital-genital and oral-genital contact can result in the transmission of HPV, albeit at relatively low rates. In a study of college students from Seattle, the 2-year cumulative incidence of HPV infections was 38.8% in those who were sexually active at enrollment.3 Among college students who remained virginal, the 2-year cumulative incidence of HPV was 9.7% in those who reported nonpenetrative sexual contact, but only 1.3% in those who reported no sexual contact whatsoever. HPV also can be transmitted perinatally.1
Although the clinical significance of HPV perinatal transmission is unknown, this route of transmission is well documented. A recent study of oral and genital HPV infections in infants born to both HPV-positive and HPV-negative women detected HPV DNA in 6% of the infants at birth, 13% at 6 weeks after birth, and 9% between 3 to 24 months of age.4 Approximately half of the HPV infections in infants were oral and half were genital. Interestingly, persistence of HPV infection was uncommon in the newborns—only 1.4% had the same HPV type detected on 2 or more occasions. Therefore, most of these infections appear to be very transient, and it is unlikely that the majority have adverse clinical consequences.
Initial HPV infections and prevalence of HPV in the population
Most sexually active adolescents and women become infected with HPV within several years of initiating sexual activity. A prospective follow-up study of sexually naïve college students found that within 12 months of initiating sexual intercourse, 30% became HPV positive; within 48 months, 54% were HPV positive.3 Other follow-up studies of adolescents and young women have found that with repeated testing and long-term follow-up, HPV is detected in more than two-thirds over a several-year period.5-7
Women with transient HPV infections often develop cytological abnormalities while they are actively shedding HPV DNA. This occurs because productive HPV infections result in cytological abnormalities in the infected epithelial cells. Cells with these cytological features are found in about one-third of HPV-infected women and result in a diagnosis of either low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of undetermined significance (ASC-US).8 If followed, cytological abnormalities continue to be detected for approximately 1 to 2 years, but by 4 years, the risk of having an abnormal cervical cytology is similar to that of women in the general population.9
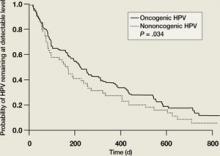
The majority of HPV infections are self-limited and spontaneously clear within a several-year period as a result of cell-mediated immunity. In one study, two-thirds of adolescents infected with low-risk HPV types spontaneously cleared their infections by 12 months, as did over half of those infected with high-risk HPV types ( FIGURE 1 ).5 By 23 months, more than 80% had cleared their HPV infections. In another follow-up study of adolescents and young women with LSIL, 91% of HPV-infected individuals cleared their infections after 36 months of follow-up.10 However, many women who spontaneously clear one specific type of HPV become infected with another HPV type. This is part of the reason that infection with multiple types of HPV is quite common in sexually active adolescents and young women.
The natural history of HPV infections explains the prevalence of HPV infection in women in the general population. Since infection is sexually transmitted and is usually transient, the prevalence of HPV infections is highest among sexually active women in their 20s. With increasing age, women tend to have fewer new sexual partners, and prevalence decreases. After age 45, the prevalence of high-risk HPV infections tends to stabilize, and less than 5% of women in the general population are DNA positive for high-risk types of HPV. The prevalence of HPV DNA positivity drops to less than 3% of women with a normal cervical cytology result.11
It is unclear how many HPV-infected women who become HPV DNA negative actually have complete viral clearance and how many continue to harbor the viral genome in the basal cells of the squamous epithelium, but at such a low copy number that they cannot be detected using standard molecular tests. Such undetectable, low-level infections are usually referred to as “latent infections” and are similar to the latent infections that are seen with herpes simplex virus and varicella zoster. The finding that almost all HIV-infected individuals become HPV DNA positive as they become more profoundly immunosuppressed suggests that HPV viral latency clearly occurs.12
Reactivation of a latent infection secondary to senescence of HPV-directed cellular immunity could easily explain many of the HPV infections that are detected in older women with a previously normal screening history and no new sexual partners.8 Currently, it is impossible to distinguish between reactivation of a latent HPV infection and a newly acquired infection. It should also be noted that the risk for subsequently developing either cervical intraepithelial neoplasia (CIN) 2,3 or cervical cancer after reactivation of a latent infection appears to be relatively low in women who have a history of 3 or more normal cervical cytology results.13 This conclusion is based on the fact that although 4% to 5% of women 45 years and older are at high risk for becoming HPV DNA positive at any single point in time, the risk that these women will have CIN 2,3 or cervical cancer detected during routine screening is minimal (≤0.05%).13
FIGURE 1
Clearance of HPV infections
HPV, human papillomavirus.
Kaplan-Meier estimates of clearance time of high-risk (HR) and low risk (LR) HPV infection. The median clearance time for high-risk HPV was 226 days.
Reprinted with permission from Brown DR, et al. J Infect Dis. 2005:191:182-192. Copyright 2004 by the Infectious Diseases Society of America, University of Chicago Press. All rights reserved.
Persistent HPV infections and the development of CIN 2,3
Only about 10% of HPV infections persist for more than 3 years. The longer a specific HPV infection persists, the lower the probability that the lesion will clear spontaneously and the higher the probability that a CIN 2,3 lesion or cervical cancer will develop.8 Prevalent HPV infections detected at the time of cervical cancer screening tend to persist longer in older women compared to younger women. This may be due to the fact that the infections identified in older women are more likely to represent infections that have already been persistent for several years, whereas infections in younger women are more likely to represent recently acquired infections. There is no established definition of what constitutes clinically important persistence, but most management recommendations consider persistence for 12 months to be clinically significant and therefore warrant colposcopy.
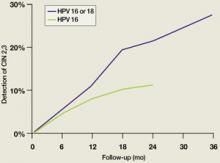
Since high-risk HPV DNA is detected in almost all CIN 2,3 lesions and invasive cervical cancers, it is clear that persistence of infection with a high-risk HPV is a requirement for the development of these lesions. New data demonstrate that the time required for an initial HPV infection to progress to a CIN 2,3 lesion can be quite short. In college-aged women, incident infection associated with any HPV type results in an 11% cumulative incidence of biopsy-confirmed CIN 2,3 by 36 months.14 For incident HPV 16 or HPV 18 infections, the cumulative incidence of CIN 2,3 at 36 months is 27% (FIGURE 2). Similarly, Mao et al followed young women in the placebo arm of an HPV 16 vaccine trial and found that all but one case of CIN 2,3 occurring after an incident HPV 16 infection developed within 12 months (FIGURE 2).15 It should be emphasized, however, that it takes almost a decade for a CIN 2,3 lesion to progress to invasive cervical cancer; therefore, it is safe to extend the screening interval to 3 years or more in women who are found to be both high-risk HPV DNA and cytology negative during routine screening.
We also have a much better understanding of the risk of being diagnosed with CIN 2,3 or cervical cancer in older, high-risk HPV DNA-positive women. In a records linkage study of Danish women who were initially cytologically negative after 3 years, CIN 2,3 or cervical cancer had been diagnosed in 6.3% of high-risk HPV-positive women.16 The cumulative detection of CIN 2,3 was 11.3% and 22.9% after 5 and 10 years of follow-up, respectively. In comparison, CIN 2,3 was diagnosed after 10 years of follow-up in only 1.9% of the HPV-negative women. A Swedish study that included all women, irrespective of cytology results, detected CIN 2,3 in 37% of women who were HPV 16 positive and 26% of those who were HPV 18 positive after 4 years of follow-up ( TABLE ).17 Importantly, in this Swedish study, CIN 2,3 lesions were detected in a substantial number of women infected with other high-risk types of HPV, including HPV 31, 33, 52, and 58. This finding contrasts with the results from a study by the National Cancer Institutes (NCI), at Kaiser, Portland, Oregon.18 In a Kaiser follow-up study of 20,810 women, the cumulative detection of CIN 3 after 10 years of follow-up was 20.7% in HPV 16-positive women >30 years of age with negative cytology; 17.7% for those with HPV 18; 1.5% for those with other high-risk types of HPV; and 0.5% for HPV DNA-negative women.
FIGURE 2
Cumulative detection of CIN 2,3 after incident HPV infections in two studies
HPV, human papillomavirus.
After incident HPV 16 infection (green line) and after incident HPV 16 or 18 infection (blue line).
Modified from Winer RL, et al. J Infect Dis. 2005;191:731-738 (blue line); Mao C, et al. Obstet Gynecol. 2006;107:18-27 (green line).
TABLE
Detection of CIN 2,3 or cancer
| HPV status | Percent with CIN 2+* |
|---|---|
| HPV negative | 0.4% |
| HPV 16 | 37% |
| HPV 18 | 26% |
| HPV 31 | 37% |
| HPV 33 | 48% |
| HPV 52 | 26% |
| HPV 58 | 30% |
| CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. | |
| *Percentage of women diagnosed with CIN 2,3 or cancer during a 4-year follow-up period. | |
| Modified from Naucler P, et al. Br J Cancer. 2007;97:129-132. | |
- HPV infections are common, and approximately half of young women become infected within 4 years of initiating sexual activity.
- The predominant mode of transmission of HPV is by sexual intercourse; consistent use of condoms reduces, but does not prevent, transmission.
- More than 80% of HPV infections spontaneously clear over a 3-year period.
- Less than 5% of women in the general population are high-risk HPV positive by the age of 45 years.
- HPV 16 and HPV 18 are quite oncogenic, and about 1 out of 4 infected individuals will develop CIN 2,3 over a 3-year period.
1. Burchell AN, Winer RL, de Sanjose S, et al. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 (suppl 3):S52-S61.
2. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645-2654.
3. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Castellsague X, Drudis T, Canadas MP, et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74.
5. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182-192.
6. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485-490.
7. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-428.
8. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907.
9. Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer. 2002;95:2145-2151.
10. Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678-1683.
11. Castle PE, Fetterman B, Poitras N, et al. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595-600.
12. Wright TC, Kuhn L. Immunosuppression and the cervix; human immunodeficiency virus (HIV). In: Jordan JA, Singer A, eds. The Cervix. Malden, MA: Blackwell; 2006:450–517.
13. Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349:1501-1509.
14. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
15. Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
16. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
17. Naucler P, Ryd W, Tornberg S, et al. HPV type-specific risks of high-grade CIN during 4 years of follow-up: a population-based prospective study. Br J Cancer. 2007;97:129-132.
18. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
Transmission of HPV
Most papillomavirus infections are transmitted through close skin-to-skin or mucosa-to-mucosa contact. Epidemiologic studies clearly indicate that sexual intercourse is the primary route for anogenital HPV infection.1 Infection is relatively uncommon in women who have not had intercourse, and there is a strong and consistent relationship between the number of both lifetime and recent sexual partners and the prevalence of HPV in women. There is also a strong association between having had a recent new sexual partner(s) and incident anogenital HPV infection. Consistent condom use reduces—but does not eliminate—HPV transmission.2 In a prospective study on college students who initiated sexual intercourse either after or immediately prior to enrollment, the overall rate of anogenital HPV infection was 89 per 100 patient-years of follow-up in those whose partners rarely used condoms during sexual intercourse, compared with 38 per 100 patient-years of follow-up among those whose partners always used condoms.
Penetrative sexual intercourse is not a requirement for HPV transmission. Both oral and digital HPV infections occur, and there is evidence that digital-genital and oral-genital contact can result in the transmission of HPV, albeit at relatively low rates. In a study of college students from Seattle, the 2-year cumulative incidence of HPV infections was 38.8% in those who were sexually active at enrollment.3 Among college students who remained virginal, the 2-year cumulative incidence of HPV was 9.7% in those who reported nonpenetrative sexual contact, but only 1.3% in those who reported no sexual contact whatsoever. HPV also can be transmitted perinatally.1
Although the clinical significance of HPV perinatal transmission is unknown, this route of transmission is well documented. A recent study of oral and genital HPV infections in infants born to both HPV-positive and HPV-negative women detected HPV DNA in 6% of the infants at birth, 13% at 6 weeks after birth, and 9% between 3 to 24 months of age.4 Approximately half of the HPV infections in infants were oral and half were genital. Interestingly, persistence of HPV infection was uncommon in the newborns—only 1.4% had the same HPV type detected on 2 or more occasions. Therefore, most of these infections appear to be very transient, and it is unlikely that the majority have adverse clinical consequences.
Initial HPV infections and prevalence of HPV in the population
Most sexually active adolescents and women become infected with HPV within several years of initiating sexual activity. A prospective follow-up study of sexually naïve college students found that within 12 months of initiating sexual intercourse, 30% became HPV positive; within 48 months, 54% were HPV positive.3 Other follow-up studies of adolescents and young women have found that with repeated testing and long-term follow-up, HPV is detected in more than two-thirds over a several-year period.5-7
Women with transient HPV infections often develop cytological abnormalities while they are actively shedding HPV DNA. This occurs because productive HPV infections result in cytological abnormalities in the infected epithelial cells. Cells with these cytological features are found in about one-third of HPV-infected women and result in a diagnosis of either low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of undetermined significance (ASC-US).8 If followed, cytological abnormalities continue to be detected for approximately 1 to 2 years, but by 4 years, the risk of having an abnormal cervical cytology is similar to that of women in the general population.9
The majority of HPV infections are self-limited and spontaneously clear within a several-year period as a result of cell-mediated immunity. In one study, two-thirds of adolescents infected with low-risk HPV types spontaneously cleared their infections by 12 months, as did over half of those infected with high-risk HPV types ( FIGURE 1 ).5 By 23 months, more than 80% had cleared their HPV infections. In another follow-up study of adolescents and young women with LSIL, 91% of HPV-infected individuals cleared their infections after 36 months of follow-up.10 However, many women who spontaneously clear one specific type of HPV become infected with another HPV type. This is part of the reason that infection with multiple types of HPV is quite common in sexually active adolescents and young women.
The natural history of HPV infections explains the prevalence of HPV infection in women in the general population. Since infection is sexually transmitted and is usually transient, the prevalence of HPV infections is highest among sexually active women in their 20s. With increasing age, women tend to have fewer new sexual partners, and prevalence decreases. After age 45, the prevalence of high-risk HPV infections tends to stabilize, and less than 5% of women in the general population are DNA positive for high-risk types of HPV. The prevalence of HPV DNA positivity drops to less than 3% of women with a normal cervical cytology result.11
It is unclear how many HPV-infected women who become HPV DNA negative actually have complete viral clearance and how many continue to harbor the viral genome in the basal cells of the squamous epithelium, but at such a low copy number that they cannot be detected using standard molecular tests. Such undetectable, low-level infections are usually referred to as “latent infections” and are similar to the latent infections that are seen with herpes simplex virus and varicella zoster. The finding that almost all HIV-infected individuals become HPV DNA positive as they become more profoundly immunosuppressed suggests that HPV viral latency clearly occurs.12
Reactivation of a latent infection secondary to senescence of HPV-directed cellular immunity could easily explain many of the HPV infections that are detected in older women with a previously normal screening history and no new sexual partners.8 Currently, it is impossible to distinguish between reactivation of a latent HPV infection and a newly acquired infection. It should also be noted that the risk for subsequently developing either cervical intraepithelial neoplasia (CIN) 2,3 or cervical cancer after reactivation of a latent infection appears to be relatively low in women who have a history of 3 or more normal cervical cytology results.13 This conclusion is based on the fact that although 4% to 5% of women 45 years and older are at high risk for becoming HPV DNA positive at any single point in time, the risk that these women will have CIN 2,3 or cervical cancer detected during routine screening is minimal (≤0.05%).13
FIGURE 1
Clearance of HPV infections
HPV, human papillomavirus.
Kaplan-Meier estimates of clearance time of high-risk (HR) and low risk (LR) HPV infection. The median clearance time for high-risk HPV was 226 days.
Reprinted with permission from Brown DR, et al. J Infect Dis. 2005:191:182-192. Copyright 2004 by the Infectious Diseases Society of America, University of Chicago Press. All rights reserved.
Persistent HPV infections and the development of CIN 2,3
Only about 10% of HPV infections persist for more than 3 years. The longer a specific HPV infection persists, the lower the probability that the lesion will clear spontaneously and the higher the probability that a CIN 2,3 lesion or cervical cancer will develop.8 Prevalent HPV infections detected at the time of cervical cancer screening tend to persist longer in older women compared to younger women. This may be due to the fact that the infections identified in older women are more likely to represent infections that have already been persistent for several years, whereas infections in younger women are more likely to represent recently acquired infections. There is no established definition of what constitutes clinically important persistence, but most management recommendations consider persistence for 12 months to be clinically significant and therefore warrant colposcopy.
Since high-risk HPV DNA is detected in almost all CIN 2,3 lesions and invasive cervical cancers, it is clear that persistence of infection with a high-risk HPV is a requirement for the development of these lesions. New data demonstrate that the time required for an initial HPV infection to progress to a CIN 2,3 lesion can be quite short. In college-aged women, incident infection associated with any HPV type results in an 11% cumulative incidence of biopsy-confirmed CIN 2,3 by 36 months.14 For incident HPV 16 or HPV 18 infections, the cumulative incidence of CIN 2,3 at 36 months is 27% (FIGURE 2). Similarly, Mao et al followed young women in the placebo arm of an HPV 16 vaccine trial and found that all but one case of CIN 2,3 occurring after an incident HPV 16 infection developed within 12 months (FIGURE 2).15 It should be emphasized, however, that it takes almost a decade for a CIN 2,3 lesion to progress to invasive cervical cancer; therefore, it is safe to extend the screening interval to 3 years or more in women who are found to be both high-risk HPV DNA and cytology negative during routine screening.
We also have a much better understanding of the risk of being diagnosed with CIN 2,3 or cervical cancer in older, high-risk HPV DNA-positive women. In a records linkage study of Danish women who were initially cytologically negative after 3 years, CIN 2,3 or cervical cancer had been diagnosed in 6.3% of high-risk HPV-positive women.16 The cumulative detection of CIN 2,3 was 11.3% and 22.9% after 5 and 10 years of follow-up, respectively. In comparison, CIN 2,3 was diagnosed after 10 years of follow-up in only 1.9% of the HPV-negative women. A Swedish study that included all women, irrespective of cytology results, detected CIN 2,3 in 37% of women who were HPV 16 positive and 26% of those who were HPV 18 positive after 4 years of follow-up ( TABLE ).17 Importantly, in this Swedish study, CIN 2,3 lesions were detected in a substantial number of women infected with other high-risk types of HPV, including HPV 31, 33, 52, and 58. This finding contrasts with the results from a study by the National Cancer Institutes (NCI), at Kaiser, Portland, Oregon.18 In a Kaiser follow-up study of 20,810 women, the cumulative detection of CIN 3 after 10 years of follow-up was 20.7% in HPV 16-positive women >30 years of age with negative cytology; 17.7% for those with HPV 18; 1.5% for those with other high-risk types of HPV; and 0.5% for HPV DNA-negative women.
FIGURE 2
Cumulative detection of CIN 2,3 after incident HPV infections in two studies
HPV, human papillomavirus.
After incident HPV 16 infection (green line) and after incident HPV 16 or 18 infection (blue line).
Modified from Winer RL, et al. J Infect Dis. 2005;191:731-738 (blue line); Mao C, et al. Obstet Gynecol. 2006;107:18-27 (green line).
TABLE
Detection of CIN 2,3 or cancer
| HPV status | Percent with CIN 2+* |
|---|---|
| HPV negative | 0.4% |
| HPV 16 | 37% |
| HPV 18 | 26% |
| HPV 31 | 37% |
| HPV 33 | 48% |
| HPV 52 | 26% |
| HPV 58 | 30% |
| CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. | |
| *Percentage of women diagnosed with CIN 2,3 or cancer during a 4-year follow-up period. | |
| Modified from Naucler P, et al. Br J Cancer. 2007;97:129-132. | |
- HPV infections are common, and approximately half of young women become infected within 4 years of initiating sexual activity.
- The predominant mode of transmission of HPV is by sexual intercourse; consistent use of condoms reduces, but does not prevent, transmission.
- More than 80% of HPV infections spontaneously clear over a 3-year period.
- Less than 5% of women in the general population are high-risk HPV positive by the age of 45 years.
- HPV 16 and HPV 18 are quite oncogenic, and about 1 out of 4 infected individuals will develop CIN 2,3 over a 3-year period.
Transmission of HPV
Most papillomavirus infections are transmitted through close skin-to-skin or mucosa-to-mucosa contact. Epidemiologic studies clearly indicate that sexual intercourse is the primary route for anogenital HPV infection.1 Infection is relatively uncommon in women who have not had intercourse, and there is a strong and consistent relationship between the number of both lifetime and recent sexual partners and the prevalence of HPV in women. There is also a strong association between having had a recent new sexual partner(s) and incident anogenital HPV infection. Consistent condom use reduces—but does not eliminate—HPV transmission.2 In a prospective study on college students who initiated sexual intercourse either after or immediately prior to enrollment, the overall rate of anogenital HPV infection was 89 per 100 patient-years of follow-up in those whose partners rarely used condoms during sexual intercourse, compared with 38 per 100 patient-years of follow-up among those whose partners always used condoms.
Penetrative sexual intercourse is not a requirement for HPV transmission. Both oral and digital HPV infections occur, and there is evidence that digital-genital and oral-genital contact can result in the transmission of HPV, albeit at relatively low rates. In a study of college students from Seattle, the 2-year cumulative incidence of HPV infections was 38.8% in those who were sexually active at enrollment.3 Among college students who remained virginal, the 2-year cumulative incidence of HPV was 9.7% in those who reported nonpenetrative sexual contact, but only 1.3% in those who reported no sexual contact whatsoever. HPV also can be transmitted perinatally.1
Although the clinical significance of HPV perinatal transmission is unknown, this route of transmission is well documented. A recent study of oral and genital HPV infections in infants born to both HPV-positive and HPV-negative women detected HPV DNA in 6% of the infants at birth, 13% at 6 weeks after birth, and 9% between 3 to 24 months of age.4 Approximately half of the HPV infections in infants were oral and half were genital. Interestingly, persistence of HPV infection was uncommon in the newborns—only 1.4% had the same HPV type detected on 2 or more occasions. Therefore, most of these infections appear to be very transient, and it is unlikely that the majority have adverse clinical consequences.
Initial HPV infections and prevalence of HPV in the population
Most sexually active adolescents and women become infected with HPV within several years of initiating sexual activity. A prospective follow-up study of sexually naïve college students found that within 12 months of initiating sexual intercourse, 30% became HPV positive; within 48 months, 54% were HPV positive.3 Other follow-up studies of adolescents and young women have found that with repeated testing and long-term follow-up, HPV is detected in more than two-thirds over a several-year period.5-7
Women with transient HPV infections often develop cytological abnormalities while they are actively shedding HPV DNA. This occurs because productive HPV infections result in cytological abnormalities in the infected epithelial cells. Cells with these cytological features are found in about one-third of HPV-infected women and result in a diagnosis of either low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of undetermined significance (ASC-US).8 If followed, cytological abnormalities continue to be detected for approximately 1 to 2 years, but by 4 years, the risk of having an abnormal cervical cytology is similar to that of women in the general population.9
The majority of HPV infections are self-limited and spontaneously clear within a several-year period as a result of cell-mediated immunity. In one study, two-thirds of adolescents infected with low-risk HPV types spontaneously cleared their infections by 12 months, as did over half of those infected with high-risk HPV types ( FIGURE 1 ).5 By 23 months, more than 80% had cleared their HPV infections. In another follow-up study of adolescents and young women with LSIL, 91% of HPV-infected individuals cleared their infections after 36 months of follow-up.10 However, many women who spontaneously clear one specific type of HPV become infected with another HPV type. This is part of the reason that infection with multiple types of HPV is quite common in sexually active adolescents and young women.
The natural history of HPV infections explains the prevalence of HPV infection in women in the general population. Since infection is sexually transmitted and is usually transient, the prevalence of HPV infections is highest among sexually active women in their 20s. With increasing age, women tend to have fewer new sexual partners, and prevalence decreases. After age 45, the prevalence of high-risk HPV infections tends to stabilize, and less than 5% of women in the general population are DNA positive for high-risk types of HPV. The prevalence of HPV DNA positivity drops to less than 3% of women with a normal cervical cytology result.11
It is unclear how many HPV-infected women who become HPV DNA negative actually have complete viral clearance and how many continue to harbor the viral genome in the basal cells of the squamous epithelium, but at such a low copy number that they cannot be detected using standard molecular tests. Such undetectable, low-level infections are usually referred to as “latent infections” and are similar to the latent infections that are seen with herpes simplex virus and varicella zoster. The finding that almost all HIV-infected individuals become HPV DNA positive as they become more profoundly immunosuppressed suggests that HPV viral latency clearly occurs.12
Reactivation of a latent infection secondary to senescence of HPV-directed cellular immunity could easily explain many of the HPV infections that are detected in older women with a previously normal screening history and no new sexual partners.8 Currently, it is impossible to distinguish between reactivation of a latent HPV infection and a newly acquired infection. It should also be noted that the risk for subsequently developing either cervical intraepithelial neoplasia (CIN) 2,3 or cervical cancer after reactivation of a latent infection appears to be relatively low in women who have a history of 3 or more normal cervical cytology results.13 This conclusion is based on the fact that although 4% to 5% of women 45 years and older are at high risk for becoming HPV DNA positive at any single point in time, the risk that these women will have CIN 2,3 or cervical cancer detected during routine screening is minimal (≤0.05%).13
FIGURE 1
Clearance of HPV infections
HPV, human papillomavirus.
Kaplan-Meier estimates of clearance time of high-risk (HR) and low risk (LR) HPV infection. The median clearance time for high-risk HPV was 226 days.
Reprinted with permission from Brown DR, et al. J Infect Dis. 2005:191:182-192. Copyright 2004 by the Infectious Diseases Society of America, University of Chicago Press. All rights reserved.
Persistent HPV infections and the development of CIN 2,3
Only about 10% of HPV infections persist for more than 3 years. The longer a specific HPV infection persists, the lower the probability that the lesion will clear spontaneously and the higher the probability that a CIN 2,3 lesion or cervical cancer will develop.8 Prevalent HPV infections detected at the time of cervical cancer screening tend to persist longer in older women compared to younger women. This may be due to the fact that the infections identified in older women are more likely to represent infections that have already been persistent for several years, whereas infections in younger women are more likely to represent recently acquired infections. There is no established definition of what constitutes clinically important persistence, but most management recommendations consider persistence for 12 months to be clinically significant and therefore warrant colposcopy.
Since high-risk HPV DNA is detected in almost all CIN 2,3 lesions and invasive cervical cancers, it is clear that persistence of infection with a high-risk HPV is a requirement for the development of these lesions. New data demonstrate that the time required for an initial HPV infection to progress to a CIN 2,3 lesion can be quite short. In college-aged women, incident infection associated with any HPV type results in an 11% cumulative incidence of biopsy-confirmed CIN 2,3 by 36 months.14 For incident HPV 16 or HPV 18 infections, the cumulative incidence of CIN 2,3 at 36 months is 27% (FIGURE 2). Similarly, Mao et al followed young women in the placebo arm of an HPV 16 vaccine trial and found that all but one case of CIN 2,3 occurring after an incident HPV 16 infection developed within 12 months (FIGURE 2).15 It should be emphasized, however, that it takes almost a decade for a CIN 2,3 lesion to progress to invasive cervical cancer; therefore, it is safe to extend the screening interval to 3 years or more in women who are found to be both high-risk HPV DNA and cytology negative during routine screening.
We also have a much better understanding of the risk of being diagnosed with CIN 2,3 or cervical cancer in older, high-risk HPV DNA-positive women. In a records linkage study of Danish women who were initially cytologically negative after 3 years, CIN 2,3 or cervical cancer had been diagnosed in 6.3% of high-risk HPV-positive women.16 The cumulative detection of CIN 2,3 was 11.3% and 22.9% after 5 and 10 years of follow-up, respectively. In comparison, CIN 2,3 was diagnosed after 10 years of follow-up in only 1.9% of the HPV-negative women. A Swedish study that included all women, irrespective of cytology results, detected CIN 2,3 in 37% of women who were HPV 16 positive and 26% of those who were HPV 18 positive after 4 years of follow-up ( TABLE ).17 Importantly, in this Swedish study, CIN 2,3 lesions were detected in a substantial number of women infected with other high-risk types of HPV, including HPV 31, 33, 52, and 58. This finding contrasts with the results from a study by the National Cancer Institutes (NCI), at Kaiser, Portland, Oregon.18 In a Kaiser follow-up study of 20,810 women, the cumulative detection of CIN 3 after 10 years of follow-up was 20.7% in HPV 16-positive women >30 years of age with negative cytology; 17.7% for those with HPV 18; 1.5% for those with other high-risk types of HPV; and 0.5% for HPV DNA-negative women.
FIGURE 2
Cumulative detection of CIN 2,3 after incident HPV infections in two studies
HPV, human papillomavirus.
After incident HPV 16 infection (green line) and after incident HPV 16 or 18 infection (blue line).
Modified from Winer RL, et al. J Infect Dis. 2005;191:731-738 (blue line); Mao C, et al. Obstet Gynecol. 2006;107:18-27 (green line).
TABLE
Detection of CIN 2,3 or cancer
| HPV status | Percent with CIN 2+* |
|---|---|
| HPV negative | 0.4% |
| HPV 16 | 37% |
| HPV 18 | 26% |
| HPV 31 | 37% |
| HPV 33 | 48% |
| HPV 52 | 26% |
| HPV 58 | 30% |
| CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus. | |
| *Percentage of women diagnosed with CIN 2,3 or cancer during a 4-year follow-up period. | |
| Modified from Naucler P, et al. Br J Cancer. 2007;97:129-132. | |
- HPV infections are common, and approximately half of young women become infected within 4 years of initiating sexual activity.
- The predominant mode of transmission of HPV is by sexual intercourse; consistent use of condoms reduces, but does not prevent, transmission.
- More than 80% of HPV infections spontaneously clear over a 3-year period.
- Less than 5% of women in the general population are high-risk HPV positive by the age of 45 years.
- HPV 16 and HPV 18 are quite oncogenic, and about 1 out of 4 infected individuals will develop CIN 2,3 over a 3-year period.
1. Burchell AN, Winer RL, de Sanjose S, et al. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 (suppl 3):S52-S61.
2. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645-2654.
3. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Castellsague X, Drudis T, Canadas MP, et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74.
5. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182-192.
6. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485-490.
7. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-428.
8. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907.
9. Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer. 2002;95:2145-2151.
10. Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678-1683.
11. Castle PE, Fetterman B, Poitras N, et al. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595-600.
12. Wright TC, Kuhn L. Immunosuppression and the cervix; human immunodeficiency virus (HIV). In: Jordan JA, Singer A, eds. The Cervix. Malden, MA: Blackwell; 2006:450–517.
13. Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349:1501-1509.
14. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
15. Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
16. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
17. Naucler P, Ryd W, Tornberg S, et al. HPV type-specific risks of high-grade CIN during 4 years of follow-up: a population-based prospective study. Br J Cancer. 2007;97:129-132.
18. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
1. Burchell AN, Winer RL, de Sanjose S, et al. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 (suppl 3):S52-S61.
2. Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645-2654.
3. Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218-226.
4. Castellsague X, Drudis T, Canadas MP, et al. Human papillomavirus (HPV) infection in pregnant women and mother-to-child transmission of genital HPV genotypes: a prospective study in Spain. BMC Infect Dis. 2009;9:74.
5. Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182-192.
6. Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485-490.
7. Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423-428.
8. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370:890-907.
9. Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer. 2002;95:2145-2151.
10. Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678-1683.
11. Castle PE, Fetterman B, Poitras N, et al. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595-600.
12. Wright TC, Kuhn L. Immunosuppression and the cervix; human immunodeficiency virus (HIV). In: Jordan JA, Singer A, eds. The Cervix. Malden, MA: Blackwell; 2006:450–517.
13. Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med. 2003;349:1501-1509.
14. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
15. Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18-27.
16. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630-10636.
17. Naucler P, Ryd W, Tornberg S, et al. HPV type-specific risks of high-grade CIN during 4 years of follow-up: a population-based prospective study. Br J Cancer. 2007;97:129-132.
18. Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072-1079.
Restless legs syndrome: Diagnostic time-savers, Tx tips
- To diagnose restless legs syndrome (RLS), start with the 4 “essential criteria”—(1) a powerful urge to move the legs that is (2) rest-induced, (3) improves with activity, and (4) worsens in the evening (C).
- Carefully screen for secondary causes of RLS, including renal failure, pregnancy, iron deficiency, and medications that can cause or exacerbate symptoms (A).
- Carbidopa/levodopa is the first-line treatment for patients with intermittent symptoms of RLS; dopamine agonists are recommended for those with daily or refractory symptoms (C).
Restless legs syndrome (RLS) has become increasingly familiar to Americans in recent years, as the medical literature, consumer ads, and lay press have focused on new findings and treatments. Yet much about this movement disorder remains a mystery.
Both the number of people with RLS and the proportion of RLS patients whose symptoms are frequent or severe are among the unknowns. Estimates of prevalence range from approximately 2% of the general population to 15% of adults.1-6
Diagnosing RLS remains complicated. Although 4 key features, or “essential criteria,” have been identified, there is no definitive clinical finding or laboratory test for this syndrome. And, because the symptoms of a number of other movement disorders resemble those of RLS, you need to be alert to other clinical features and distinguishing characteristics to confirm an RLS diagnosis.
The pathophysiology of RLS is certainly not clear-cut, either. Possible mechanisms involve overexcitation of the spinal cord by the brain stem, decreased dopamine signaling, and low iron levels.5-8 Low serum iron levels, and especially low central nervous system ferritin levels, have been closely correlated with the severity of RLS symptoms.5,9,10 Genetic links to RLS are also being studied, but have not yet been clearly established.2,7,10,11
What we do know is that the prevalence of RLS increases with age. In a National Sleep Foundation poll, nearly 25% of people older than 65 reported symptoms of RLS.12 In a more recent study of the elderly conducted under the auspices of the World Health Organization, 9.8% of participants met the criteria for RLS.13
An aging population means you’re likely to see an increasing number of patients with symptoms of RLS, which can range in severity from occasional discomfort to daily leg pain. This update will help you hone your diagnostic skills and provide the best possible care to patients who are affected.
Start with the URGE mnemonic
Initial diagnostic criteria for RLS were developed by the National Institutes of Health (NIH) in 2002 and revised by the International Restless Legs Syndrome Study Group (IRLSSG) in 2005.9 They begin with 4 essential criteria ( TABLE 1 ), easily remembered with this simple mnemonic:
- Urge to move the legs
Rest induced
Gets better with activity
Evening and night accentuation.
Here’s what to keep in mind about each.
Urge. Patients with RLS experience a powerful urge to move their legs, and often their arms or other body parts, as well. Some patients also experience discomfort in their legs,14,15 which arises from deep within the legs rather than from the surface—a characteristic that helps differentiate RLS from other movement disorders.3,6,16
Rest induced. Numerous studies have shown that RLS symptoms worsen during periods of physical and mental inactivity, and when patients are in a seated or lying position.3,5,15,16 The longer the rest period, the more severe the symptoms become. Mentally stimulating activities, such as playing video games or reading, are often enough to prevent the onset of symptoms, at least in the early stage of the disorder.3,5,15,16
Gets better with activity. While inactivity exacerbates RLS symptoms, activity typically brings complete or partial relief. Symptom relief can be the result of physical movement, a mentally stimulating activity, or even a change in temperature. Touching and rubbing the legs often helps, too, although this effect diminishes as RLS progresses.3,6,15,16
Evening accentuation. The severity of RLS symptoms tends to follow the same circadian pattern as body temperature—increasing in the evening and peaking between the hours of 11 PM and 3 AM, and making it difficult, if not impossible, for patients to experience hours of uninterrupted sleep.
TABLE 1
Diagnosing restless legs syndrome2,17,26,33
Essential diagnostic criteria (URGE mnemonic)
|
Supportive features
|
Associated features
|
| RLS, restless legs syndrome. |
Other clues to an RLS diagnosis
In addition to the essential criteria, the NIH and the IRLSSG developed a number of supportive and associated clinical features ( TABLE 1 ) that provide further help in differentiating RLS from conditions with similar symptoms.
Supportive clinical features
Although not every patient with RLS will have all (or possibly any) of the findings that are identified as supportive, their presence will lend support to an RLS diagnosis. These include:
- family history (1st-degree relative with RLS)
- improvement with dopaminergic therapy
- periodic leg movements during sleep (PLMS) in patients <50 years of age
- periodic leg movements while awake in patients of any age.17
In interpreting the second feature—dopaminergic therapy—it is important to note that while patient response often wanes over time, an initial response (often obtained by patient history, if the patient has ever been treated with a dopaminergic agent) has a sensitivity of 80% and a specificity of 100% for diagnosis of RLS.16 Keep in mind, too, that periodic leg movements—typically defined as jerking, repetitive motions—are present in many other disorders, and also tend to increase in elderly patients who do not have RLS.
Associated clinical features
Similarly, a diagnosis of RLS is not dependent on the presence of these findings. They’re noteworthy, however, because they’re experienced by many patients with RLS.16
A natural progression of RLS that follows an identifiable pattern is the first associated feature. The course of RLS varies, however, depending in part on the age of onset. Patients who develop RLS in young adulthood tend to have a slower progression, with long periods of remission, while RLS tends to progress more rapidly in those who develop the condition as older adults.15
Sleep disturbances. Leg movements typically result in frequent awakenings and increased sleep latency. Because of these disruptions, RLS patients often experience daytime somnolence and an inability to pay attention; they also have trouble performing daytime duties.
No abnormal findings. There are no physical exam or lab abnormalities associated with primary (idiopathic) RLS. The presence of abnormal findings should raise questions about the diagnosis, and cause clinicians to explore the possibility of a secondary cause.
CASE STUDY: Would you suspect RLS?
Grace (not her real name), a 54-year-old woman who underwent gastric bypass surgery several years ago, has come in today seeking help for chronic insomnia. She reports that she experiences uncomfortable sensations deep in her legs when she lies down at night. She says that she is able to get some relief from these sensations when she gets up and walks. She also notes that when she tries to lie still, she feels a need to move her legs.
Grace says that when she does fall asleep, she moves her legs so frequently that her husband has begun sleeping in a separate bed—symptoms that immediately arouse suspicion of RLS. If she were your patient, how would you support (or refute) the diagnosis, and how would you treat it?
Rule out conditions that mimic RLS
When evaluating patients like Grace with suspected RLS, it is crucial to be aware of conditions with similar symptoms—some of which may coexist. The differential diagnosis and spectrum of movement disorders that should be considered in patients with RLS symptoms are listed in TABLE 2. While some of the presenting symptoms overlap, keeping the essential criteria of RLS in mind may help in identifying distinguishing characteristics.
Neuropathic pain syndrome may occur at rest or during intense activity, for example, and peripheral vascular disease is provoked by activity, while RLS is brought on by rest.
Symptoms of neuroleptic-induced akathisia may occur day or night; in contrast, RLS typically follows a circadian rhythm.
Similarly, the urge to move the legs that patients with RLS experience is powerful, but the movement itself is voluntary. This feature distinguishes RLS from sleep starts (hypnagogic jerks), for example, which are involuntary movements.
TABLE 2
RLS: Distinguishing features and differential diagnosis6,26,33
| DIFFERENTIAL DIAGNOSIS | CHARACTERISTICS | DISTINGUISHING FEATURES OF RLS |
|---|---|---|
| Positional discomfort | Alleviated by change in body position without need for repetitive body movements. |
|
| Neuropathic pain syndrome | Pain may occur during periods of activity or rest. | |
| Peripheral vascular disease/claudications | Pain evoked by activity. | |
| Painful legs and moving toes syndrome | Continuous to semi-continuous involuntary movement of toes with associated pain in affected extremity. |
|
| Sleep starts (hypnagogic jerks) | Sudden, brief, involuntary jerks of arms or legs. | |
| Sleep-related cramps | Involve specific muscle groups and are relieved (or partially relieved) by stretching. |
|
| Neuroleptic-induced akathisia | Day- or nighttime motor restlessness that is generalized, immediately relieved with movement, and recurs immediately after the patient stops moving. | |
| Rheumatoid arthritis | Pain is chronic, not immediately relieved by moving the affected extremity, and characteristically associated with joint deformities. |
|
| RLS, restless legs syndrome. | ||
Review the need for iron replacement
The role that low levels of iron play in RLS is not entirely clear. One study found the median ferritin level in patients with RLS symptoms to be 33 mcg/L, compared with 59 mcg/L in those without symptoms.18 Another study showed patients with ferritin levels less than 50 mcg/L to have more severe RLS symptoms than those with levels greater than 50 mcg/L.19 Both iron and dopamine have been shown to have circadian rhythms similar to RLS, with their nadir correlating with the time of maximum severity of RLS symptoms. (Low levels of iron may be associated with either idiopathic or secondary RLS.)
Consensus guidelines recommend against initiating iron replacement therapy without checking levels, as this could lead to iron overload.6 Any patient with a plasma ferritin concentration <50 mcg/L and RLS symptoms should be started on iron replacement therapy. The recommended dose of iron is 325 mg ferrous sulfate, taken with 300 mg vitamin C, 3 times a day. Vitamin C allows for better absorption of the iron.8
Monitor ferritin levels at 6-week intervals until they exceed 50 mcg/L, then check iron concentrations every few months. Iron replacement therapy can be decreased or discontinued, provided the ferritin remains at this level.17
Try these strategies for symptom relief
The goal in treating RLS is to decrease the severity and frequency of symptoms, leading to an improvement in sleep quality, a decrease in daytime somnolence, and an overall improvement in quality of life. Treatment guidelines, supported by the IRLSSG and the Medical Advisory Board of the Restless Legs Syndrome Foundation, are based on symptom frequency and severity—whether they are intermittent, daily, or refractory.3,9,17
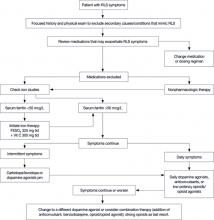
The algorithm ( FIGURE ) provides recommendations for starting and escalating pharmacologic therapy. Nonpharmacologic treatments have not been studied in systematic trials; however, recommendations for their use are based on expert opinion, case series, or anecdotal reports. In patients who do not have severe symptoms, it makes sense to try simpler strategies first.
Review medications and diet. Many medications have been associated with RLS, either as a secondary cause or suspected of exacerbating symptoms. These include dopamine antagonists (neuroleptics, antiemetics); antidepressants, primarily tricyclics and selective serotonin reuptake inhibitors, lithium, and antipsychotics; antihistamines, including diphenhydramine and other over-the-counter cold and allergy remedies; calcium channel blockers; and diuretics.6,8,17
If a patient troubled by symptoms of RLS is taking any of these agents, consider changing the medication. If no suitable substitute is available, it may help to change the dosing schedule to earlier in the day—ideally no later than 3 PM.8,17 Advise patients, too, to avoid caffeine, tobacco, and alcohol, as well as any other food or beverage known to contain stimulants.
Encourage activity. Discuss the importance of exercise in the management of RLS symptoms. Encourage patients to routinely engage in physical activity and to pursue mentally stimulating activities such as reading, puzzles, or games.
Stress good sleep hygiene. Urge patients to follow a consistent sleep schedule, going to sleep and awakening at the same time each day; to use the bed only for rest or intimacy and avoid reading or watching TV in bed; and to establish a relaxing bedtime routine. Some patients have found that hot or cold baths before bed help to relieve symptoms; others report that massaging their legs or stretching leads to an improvement in symptoms.7,9,17
FIGURE
RLS: A treatment algorithm2,7,9,20
FESO4, ferrous sulfate; RLS, restless legs syndrome.
Pharmacologic options: Selecting the right one
In addition to iron replacement, there are 5 major types of RLS treatment:
- dopaminergic agents,
- dopamine agonists
- anticonvulsants,
- opioids,
- benzodiazepines.
Carbidopa/levodopa for intermittent symptoms
Most patients with RLS have a positive response to treatment with dopaminergic agents, at least initially, and for many years carbidopa/levodopa was the usual therapy for RLS. It remains a first-line treatment for patients with intermittent symptoms.
Early studies consistently showed that 70% to 80% of RLS patients treated with carbidopa/levodopa had a significant improvement in symptoms.20-22 But the studies were small, did not always include a placebo arm, and most were crossover trials, making it impossible to do a statistical comparison.5
Typical doses of carbidopa/levodopa as a treatment for RLS are 25/100 mg to 100/400 mg in divided doses, given before bedtime and again, if needed, in the middle of the sleep period.9 These doses are much lower than those used to treat Parkinsonism.
Common side effects of carbidopa/levodopa include nausea, headache, dry mouth, and daytime somnolence.2,21 An increased risk of melanoma has been seen in some studies of carbidopa/levodopa, but the evidence is inconclusive.23,24 Rebound (a worsening of symptom severity when the medication wears off) and augmentation (the development of more severe symptoms early in the day) have also been reported.
Tolerance to carbidopa/levodopa is infrequent among patients with RLS. One early study found that only 3 of 43 patients (7%) required an increase in dosage over time.21 The on/off phenomenon that occurs with this medication in the treatment of Parkinsonism is not mentioned in the literature in reference to RLS.
Because carbidopa/levodopa only provides relief for 4 to 6 hours, a second dose is often needed. If that second dose repeatedly disrupts the patient’s sleep, the recommended approach is to give 2 doses before bedtime—1 dose of regular carbidopa/levodopa and 1 dose of the controlled release form.6,9
Augmentation, the most serious problem associated with carbidopa/levodopa, occurs in 65% to 80% of RLS patients treated with this medication. It is more common in those with refractory symptoms and those taking higher doses, but can affect any RLS patient.
If augmentation develops, discontinue the carbidopa/levodopa and switch the patient to another agent. Augmentation reverses within a few weeks of stopping the medication and treatment can then be resumed, but be aware that the augmentation may reoccur.
Carbidopa/levodopa is a good choice for patients with intermittent RLS symptoms, despite the risks associated with this medication. Not only does it provide quick relief, but it can be used only on the days when symptoms occur.2,5,8
Dopamine agonists
The use of pramipexole or ropinirole as first-line treatment for people with daily or refractory symptoms of RLS is well supported by controlled studies.7,17,25 Dopamine agonists can also be used to treat patients with RLS with varying levels of severity,6 and are sometimes prescribed as the initial treatment for intermittent symptoms.
These newer agents have a longer half-life than carbidopa/levodopa, which eliminates the need for a second dose in the midst of the sleep cycle. They also have much lower rates of augmentation. Studies have been inconsistent with regard to the risk of augmentation associated with these drugs, however, with results ranging from a high of 33%7,26 to a low of 4%.27
Nausea, headache, fatigue, dizziness, orthostatic hypotension, and vomiting—the most common side effects of pramipexole and ropinirole—usually decrease in severity after 7 to 10 days of therapy. In a recent meta-analysis comparing dopamine agonists with placebo in RLS patients, the number needed to harm (NNH) was 77 and the number needed to treat (NNT) was 6.27
Pramipexole. Dosing is started at 0.125 mg at bedtime and slowly titrated up to minimize side effects. Most patients experience relief at an average dose of 0.375 mg, taken daily or intermittently for symptom relief. 6,17,27
Ropinirole. Dosing is started at 0.25 mg at bedtime (or at dinner and bedtime), and then slowly titrated up every few days to every week until a good response is obtained. Most patients respond to a dose between 1 and 2.5 mg/d.6,17,28
Tx alternatives: Anticonvulsants, opioids, and benzodiazepines
When patients are unable to tolerate—or do not respond adequately to—dopaminergic agents or dopamine agonists, anticonvulsants, opioids, or benzodiazepines may be effective alternatives, or adjunctive treatments. They may also be used in patients who have another disorder, such as chronic pain, for which these alternatives will be beneficial.
Anticonvulsants. Of the anticonvulsants studied in RLS patients, gabapentin has been shown to most effectively decrease symptoms.25 Use of the drug should be reserved for patients with daily symptoms or refractory RLS. Gabapentin also appears to be especially effective in patients who perceive their symptoms as painful, and in hemodialysis patients.2,17,28 The average effective daily dose of gabapentin for treatment of RLS is 1855 mg.6
A 2002 double-blind crossover trial found that after 6 weeks of therapy, 16 of 24 (66%) patients taking gabapentin had only mild RLS symptoms, compared with 8 of 24 (33%) of those taking placebo (NNT=3).14 The most common side effects were malaise, somnolence, dry mouth, and nausea (NNH=4). Of note, there was no significant difference in the incidence of side effects among those in the therapy group compared with the controls.
Gabapentin also has fewer side effects and drug interactions than other anticonvulsants. (Both carbamazepine and valproate have also been studied for the treatment of RLS symptoms, but at best, provided only modest improvements.)
Opioids have long been recognized as an effective treatment for RLS, but their use is limited by the potential for abuse.6 In a double-blind crossover trial, 10 of 11 patients preferred opioids over placebo, and significantly more rated their leg sensations as mild after 2 weeks of treatment with opioids (NNT=2). The most common side effects were constipation and sedation (NNH=3).17,29
Many patients obtain symptom relief from low-potency opioids such as codeine, taken at bedtime, and there appears to be a lower abuse potential when bedtime-only dosing is used. However, higher-potency opioids may be necessary for patients with refractory RLS.17
One study did show an increase in symptoms of sleep apnea in RLS patients treated with opioids. If you suspect sleep apnea in an RLS patient taking opioids, provide a referral for a polysomnography evaluation.30
Benzodiazepines. There is limited evidence to support the use of clonazepam in the treatment of RLS. Although a prospective controlled study found clonazepam to be no more effective than placebo in RLS treatment,31 clonazepam has been shown to be an effective treatment in patients with PLMS.32 Because of the association between these 2 movement disorders, clonazepam is considered an option to use alone or as adjunctive therapy in patients with RLS.2,7
CASE STUDY: Grace’s diagnosis and treatment
In addition to having the 4 essential criteria for RLS, Grace reported sleep disturbances and periodic leg movements—2 additional features that are common to RLS. She also had low serum iron levels; however, her iron deficiency was related to her gastric bypass, and she was unable to tolerate iron therapy. We started her on a low dose of pramipexole, and she had a significant—and rapid—improvement in symptoms. When we last saw her, she reported that she usually slept through the night and that her leg movements had diminished so much that her husband no longer found it necessary to sleep in a separate bed.
Correspondence
Darlene E. Moyer, MD, Scottsdale Healthcare Family Medicine Residency Program, University of Arizona School of Medicine, 7301 East 2nd Street, Suite 210, Scottsdale, AZ 85251; [email protected]
1. Karroum E, Konofal E, Arnulf I. Restless-legs syndrome. Rev Neurol. 2008;164:701-721.
2. Vergne-Salle P, Coyral D, Dufauret K, et al. Is restless legs syndrome underrecognized? Current management. Joint Bone Spine. 2006;73:369-373.
3. Fulda S, Wetter TC. Dopamine agonists for the treatment of restless legs syndrome. Expert Opin Pharmacother. 2005;6:2655-2666.
4. Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286-1292.
5. Conti C, de Oliveira MM, Andriolo RB, et al. Levodopa for idiopathic restless legs syndrome: evidence-based review. Mov Disord. 2007;22:1943-1951.
6. Restless Legs Syndrome Foundation. RLS Medical Bulletin. 2005. Available at: http://www.irlssg.org/RLSMB2005pf.pdf. Accessed July 9, 2009.
7. Ryan M, Slevin J. Restless legs syndrome. Am J Health Syst Pharm. 2006;63:1599-1612.
8. Oertel W, Trenkwalder C, Zucconi M, et al. State of the art in restless legs syndrome therapy: practice recommendations for treating restless legs syndrome. Mov Disord. 2007;22(suppl 18):S466-S475.
9. Thorpy M. New paradigms in the treatment of restless legs syndrome. Neurology. 2005;64(12 suppl 3):S28-S33.
10. Winkelman J. A better future for patients with restless legs syndrome. Am J Med. 2007;120(1 suppl 1):S28-S29.
11. Chahine L, Chemali Z. Restless legs syndrome: a review. CNS Spectr. 2006;11:511-520.
12. Johnson E. Omnibus Sleep in America poll. National Sleep Foundation. 1998.-
13. Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Neurology. 2000;54:1064-1068.
14. Garcia-Borreguero D. Time to REST: epidemiology and burden. Eur J Neurol. 2006;13(suppl 3):S15-S20.
15. Schapira A. RLS patients: who are they? Eur J Neurol. 2006;13(suppl 3):S2-S7.
16. Benes H, Walters AS, Allen RP, et al. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. 2007;22(suppl 18):S401-S408.
17. Hening W, Allen R, Tenzer P, et al. Restless legs syndrome: demographics, presentation and differential diagnosis. Geriatrics. 2007;62:26-29.
18. O’Keefe ST, Gavin K, Lavan JN. Iron status and restless legs in the elderly. Age Ageing. 1994;23:200-203.
19. Sun ER, Chen CA, Ho G, et al. Iron and the restless legs syndrome. Sleep. 1998;21:371-377.
20. Akpinar S. Restless legs syndrome treatment with dopaminergic drugs. Clin Neuropharmacol. 1987;10:69-79.
21. Becker P, Jamieson A, Brown D. Dopaminergic agents in restless legs syndrome and periodic limb movements of sleep: response and complications of extended treatment in 49 cases. Sleep. 1993;16:713-716.
22. Von Scheele C, Kempi V. Long-term effect of dopaminergic drugs in restless legs. A 2 year follow-up. Arch Neurol. 1990;47:1223-1224.
23. Bertoni JM, Arlette JP, Fernandez HH, et al. Epidemiologic association of Parkinson’s disease and melanoma. Mov Disord. 2006;21(suppl 15):S610.-
24. Fiala K, Whetteckey J, Manyam B. Malignant melanoma and levodopa in Parkinson’s disease: causality or coincidence? Parkinsonism Relat Disord. 2003;9:321-327.
25. Montagna P. The treatment of restless legs syndrome. Neurol Sci. 2007;28(suppl 1):S61-S66.
26. Kushida C. Clinical presentation, diagnosis and quality of life issues in restless legs syndrome. Am J Med. 2007;120(1 suppl 1):S4-S12.
27. Baker W, White C, Coleman C. Effect of nonergot dopamine agonists on symptoms of restless legs syndrome. Ann Fam Med. 2008;6:253-262.
28. Fulda S, Wetter T. Emerging drugs for restless legs syndrome. Expert Opin Emerg Drugs. 2005;10:527-552.
29. Walters A, Wagner M, Hening W, et al. Successful treatment of idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16:327-332.
30. Walters A, Winkelman J, Trenkwalder C, et al. Long term follow-up on restless legs syndrome patients treated with opioids. Mov Disord. 2001;16:1105-1109.
31. Boghen D, Lamothe L, Elie R. The treatment of restless legs syndrome with clonazepam: a prospective controlled trial. Can J Neurol Sci. 1986;13:245-247.
32. Peled R, Lavie P. Double-blind evaluation of clonazepam on periodic leg movements in sleep. J Neurol Neurosurg Psychiatry. 1987;50:1679-1681.
33. Ferni-Strambi L. RLS-like symptoms: differential diagnosis by history and clinical assessment. Sleep Med. 2007;8(suppl 2):S3-S6.
- To diagnose restless legs syndrome (RLS), start with the 4 “essential criteria”—(1) a powerful urge to move the legs that is (2) rest-induced, (3) improves with activity, and (4) worsens in the evening (C).
- Carefully screen for secondary causes of RLS, including renal failure, pregnancy, iron deficiency, and medications that can cause or exacerbate symptoms (A).
- Carbidopa/levodopa is the first-line treatment for patients with intermittent symptoms of RLS; dopamine agonists are recommended for those with daily or refractory symptoms (C).
Restless legs syndrome (RLS) has become increasingly familiar to Americans in recent years, as the medical literature, consumer ads, and lay press have focused on new findings and treatments. Yet much about this movement disorder remains a mystery.
Both the number of people with RLS and the proportion of RLS patients whose symptoms are frequent or severe are among the unknowns. Estimates of prevalence range from approximately 2% of the general population to 15% of adults.1-6
Diagnosing RLS remains complicated. Although 4 key features, or “essential criteria,” have been identified, there is no definitive clinical finding or laboratory test for this syndrome. And, because the symptoms of a number of other movement disorders resemble those of RLS, you need to be alert to other clinical features and distinguishing characteristics to confirm an RLS diagnosis.
The pathophysiology of RLS is certainly not clear-cut, either. Possible mechanisms involve overexcitation of the spinal cord by the brain stem, decreased dopamine signaling, and low iron levels.5-8 Low serum iron levels, and especially low central nervous system ferritin levels, have been closely correlated with the severity of RLS symptoms.5,9,10 Genetic links to RLS are also being studied, but have not yet been clearly established.2,7,10,11
What we do know is that the prevalence of RLS increases with age. In a National Sleep Foundation poll, nearly 25% of people older than 65 reported symptoms of RLS.12 In a more recent study of the elderly conducted under the auspices of the World Health Organization, 9.8% of participants met the criteria for RLS.13
An aging population means you’re likely to see an increasing number of patients with symptoms of RLS, which can range in severity from occasional discomfort to daily leg pain. This update will help you hone your diagnostic skills and provide the best possible care to patients who are affected.
Start with the URGE mnemonic
Initial diagnostic criteria for RLS were developed by the National Institutes of Health (NIH) in 2002 and revised by the International Restless Legs Syndrome Study Group (IRLSSG) in 2005.9 They begin with 4 essential criteria ( TABLE 1 ), easily remembered with this simple mnemonic:
- Urge to move the legs
Rest induced
Gets better with activity
Evening and night accentuation.
Here’s what to keep in mind about each.
Urge. Patients with RLS experience a powerful urge to move their legs, and often their arms or other body parts, as well. Some patients also experience discomfort in their legs,14,15 which arises from deep within the legs rather than from the surface—a characteristic that helps differentiate RLS from other movement disorders.3,6,16
Rest induced. Numerous studies have shown that RLS symptoms worsen during periods of physical and mental inactivity, and when patients are in a seated or lying position.3,5,15,16 The longer the rest period, the more severe the symptoms become. Mentally stimulating activities, such as playing video games or reading, are often enough to prevent the onset of symptoms, at least in the early stage of the disorder.3,5,15,16
Gets better with activity. While inactivity exacerbates RLS symptoms, activity typically brings complete or partial relief. Symptom relief can be the result of physical movement, a mentally stimulating activity, or even a change in temperature. Touching and rubbing the legs often helps, too, although this effect diminishes as RLS progresses.3,6,15,16
Evening accentuation. The severity of RLS symptoms tends to follow the same circadian pattern as body temperature—increasing in the evening and peaking between the hours of 11 PM and 3 AM, and making it difficult, if not impossible, for patients to experience hours of uninterrupted sleep.
TABLE 1
Diagnosing restless legs syndrome2,17,26,33
Essential diagnostic criteria (URGE mnemonic)
|
Supportive features
|
Associated features
|
| RLS, restless legs syndrome. |
Other clues to an RLS diagnosis
In addition to the essential criteria, the NIH and the IRLSSG developed a number of supportive and associated clinical features ( TABLE 1 ) that provide further help in differentiating RLS from conditions with similar symptoms.
Supportive clinical features
Although not every patient with RLS will have all (or possibly any) of the findings that are identified as supportive, their presence will lend support to an RLS diagnosis. These include:
- family history (1st-degree relative with RLS)
- improvement with dopaminergic therapy
- periodic leg movements during sleep (PLMS) in patients <50 years of age
- periodic leg movements while awake in patients of any age.17
In interpreting the second feature—dopaminergic therapy—it is important to note that while patient response often wanes over time, an initial response (often obtained by patient history, if the patient has ever been treated with a dopaminergic agent) has a sensitivity of 80% and a specificity of 100% for diagnosis of RLS.16 Keep in mind, too, that periodic leg movements—typically defined as jerking, repetitive motions—are present in many other disorders, and also tend to increase in elderly patients who do not have RLS.
Associated clinical features
Similarly, a diagnosis of RLS is not dependent on the presence of these findings. They’re noteworthy, however, because they’re experienced by many patients with RLS.16
A natural progression of RLS that follows an identifiable pattern is the first associated feature. The course of RLS varies, however, depending in part on the age of onset. Patients who develop RLS in young adulthood tend to have a slower progression, with long periods of remission, while RLS tends to progress more rapidly in those who develop the condition as older adults.15
Sleep disturbances. Leg movements typically result in frequent awakenings and increased sleep latency. Because of these disruptions, RLS patients often experience daytime somnolence and an inability to pay attention; they also have trouble performing daytime duties.
No abnormal findings. There are no physical exam or lab abnormalities associated with primary (idiopathic) RLS. The presence of abnormal findings should raise questions about the diagnosis, and cause clinicians to explore the possibility of a secondary cause.
CASE STUDY: Would you suspect RLS?
Grace (not her real name), a 54-year-old woman who underwent gastric bypass surgery several years ago, has come in today seeking help for chronic insomnia. She reports that she experiences uncomfortable sensations deep in her legs when she lies down at night. She says that she is able to get some relief from these sensations when she gets up and walks. She also notes that when she tries to lie still, she feels a need to move her legs.
Grace says that when she does fall asleep, she moves her legs so frequently that her husband has begun sleeping in a separate bed—symptoms that immediately arouse suspicion of RLS. If she were your patient, how would you support (or refute) the diagnosis, and how would you treat it?
Rule out conditions that mimic RLS
When evaluating patients like Grace with suspected RLS, it is crucial to be aware of conditions with similar symptoms—some of which may coexist. The differential diagnosis and spectrum of movement disorders that should be considered in patients with RLS symptoms are listed in TABLE 2. While some of the presenting symptoms overlap, keeping the essential criteria of RLS in mind may help in identifying distinguishing characteristics.
Neuropathic pain syndrome may occur at rest or during intense activity, for example, and peripheral vascular disease is provoked by activity, while RLS is brought on by rest.
Symptoms of neuroleptic-induced akathisia may occur day or night; in contrast, RLS typically follows a circadian rhythm.
Similarly, the urge to move the legs that patients with RLS experience is powerful, but the movement itself is voluntary. This feature distinguishes RLS from sleep starts (hypnagogic jerks), for example, which are involuntary movements.
TABLE 2
RLS: Distinguishing features and differential diagnosis6,26,33
| DIFFERENTIAL DIAGNOSIS | CHARACTERISTICS | DISTINGUISHING FEATURES OF RLS |
|---|---|---|
| Positional discomfort | Alleviated by change in body position without need for repetitive body movements. |
|
| Neuropathic pain syndrome | Pain may occur during periods of activity or rest. | |
| Peripheral vascular disease/claudications | Pain evoked by activity. | |
| Painful legs and moving toes syndrome | Continuous to semi-continuous involuntary movement of toes with associated pain in affected extremity. |
|
| Sleep starts (hypnagogic jerks) | Sudden, brief, involuntary jerks of arms or legs. | |
| Sleep-related cramps | Involve specific muscle groups and are relieved (or partially relieved) by stretching. |
|
| Neuroleptic-induced akathisia | Day- or nighttime motor restlessness that is generalized, immediately relieved with movement, and recurs immediately after the patient stops moving. | |
| Rheumatoid arthritis | Pain is chronic, not immediately relieved by moving the affected extremity, and characteristically associated with joint deformities. |
|
| RLS, restless legs syndrome. | ||
Review the need for iron replacement
The role that low levels of iron play in RLS is not entirely clear. One study found the median ferritin level in patients with RLS symptoms to be 33 mcg/L, compared with 59 mcg/L in those without symptoms.18 Another study showed patients with ferritin levels less than 50 mcg/L to have more severe RLS symptoms than those with levels greater than 50 mcg/L.19 Both iron and dopamine have been shown to have circadian rhythms similar to RLS, with their nadir correlating with the time of maximum severity of RLS symptoms. (Low levels of iron may be associated with either idiopathic or secondary RLS.)
Consensus guidelines recommend against initiating iron replacement therapy without checking levels, as this could lead to iron overload.6 Any patient with a plasma ferritin concentration <50 mcg/L and RLS symptoms should be started on iron replacement therapy. The recommended dose of iron is 325 mg ferrous sulfate, taken with 300 mg vitamin C, 3 times a day. Vitamin C allows for better absorption of the iron.8
Monitor ferritin levels at 6-week intervals until they exceed 50 mcg/L, then check iron concentrations every few months. Iron replacement therapy can be decreased or discontinued, provided the ferritin remains at this level.17
Try these strategies for symptom relief
The goal in treating RLS is to decrease the severity and frequency of symptoms, leading to an improvement in sleep quality, a decrease in daytime somnolence, and an overall improvement in quality of life. Treatment guidelines, supported by the IRLSSG and the Medical Advisory Board of the Restless Legs Syndrome Foundation, are based on symptom frequency and severity—whether they are intermittent, daily, or refractory.3,9,17
The algorithm ( FIGURE ) provides recommendations for starting and escalating pharmacologic therapy. Nonpharmacologic treatments have not been studied in systematic trials; however, recommendations for their use are based on expert opinion, case series, or anecdotal reports. In patients who do not have severe symptoms, it makes sense to try simpler strategies first.
Review medications and diet. Many medications have been associated with RLS, either as a secondary cause or suspected of exacerbating symptoms. These include dopamine antagonists (neuroleptics, antiemetics); antidepressants, primarily tricyclics and selective serotonin reuptake inhibitors, lithium, and antipsychotics; antihistamines, including diphenhydramine and other over-the-counter cold and allergy remedies; calcium channel blockers; and diuretics.6,8,17
If a patient troubled by symptoms of RLS is taking any of these agents, consider changing the medication. If no suitable substitute is available, it may help to change the dosing schedule to earlier in the day—ideally no later than 3 PM.8,17 Advise patients, too, to avoid caffeine, tobacco, and alcohol, as well as any other food or beverage known to contain stimulants.
Encourage activity. Discuss the importance of exercise in the management of RLS symptoms. Encourage patients to routinely engage in physical activity and to pursue mentally stimulating activities such as reading, puzzles, or games.
Stress good sleep hygiene. Urge patients to follow a consistent sleep schedule, going to sleep and awakening at the same time each day; to use the bed only for rest or intimacy and avoid reading or watching TV in bed; and to establish a relaxing bedtime routine. Some patients have found that hot or cold baths before bed help to relieve symptoms; others report that massaging their legs or stretching leads to an improvement in symptoms.7,9,17
FIGURE
RLS: A treatment algorithm2,7,9,20
FESO4, ferrous sulfate; RLS, restless legs syndrome.
Pharmacologic options: Selecting the right one
In addition to iron replacement, there are 5 major types of RLS treatment:
- dopaminergic agents,
- dopamine agonists
- anticonvulsants,
- opioids,
- benzodiazepines.
Carbidopa/levodopa for intermittent symptoms
Most patients with RLS have a positive response to treatment with dopaminergic agents, at least initially, and for many years carbidopa/levodopa was the usual therapy for RLS. It remains a first-line treatment for patients with intermittent symptoms.
Early studies consistently showed that 70% to 80% of RLS patients treated with carbidopa/levodopa had a significant improvement in symptoms.20-22 But the studies were small, did not always include a placebo arm, and most were crossover trials, making it impossible to do a statistical comparison.5
Typical doses of carbidopa/levodopa as a treatment for RLS are 25/100 mg to 100/400 mg in divided doses, given before bedtime and again, if needed, in the middle of the sleep period.9 These doses are much lower than those used to treat Parkinsonism.
Common side effects of carbidopa/levodopa include nausea, headache, dry mouth, and daytime somnolence.2,21 An increased risk of melanoma has been seen in some studies of carbidopa/levodopa, but the evidence is inconclusive.23,24 Rebound (a worsening of symptom severity when the medication wears off) and augmentation (the development of more severe symptoms early in the day) have also been reported.
Tolerance to carbidopa/levodopa is infrequent among patients with RLS. One early study found that only 3 of 43 patients (7%) required an increase in dosage over time.21 The on/off phenomenon that occurs with this medication in the treatment of Parkinsonism is not mentioned in the literature in reference to RLS.
Because carbidopa/levodopa only provides relief for 4 to 6 hours, a second dose is often needed. If that second dose repeatedly disrupts the patient’s sleep, the recommended approach is to give 2 doses before bedtime—1 dose of regular carbidopa/levodopa and 1 dose of the controlled release form.6,9
Augmentation, the most serious problem associated with carbidopa/levodopa, occurs in 65% to 80% of RLS patients treated with this medication. It is more common in those with refractory symptoms and those taking higher doses, but can affect any RLS patient.
If augmentation develops, discontinue the carbidopa/levodopa and switch the patient to another agent. Augmentation reverses within a few weeks of stopping the medication and treatment can then be resumed, but be aware that the augmentation may reoccur.
Carbidopa/levodopa is a good choice for patients with intermittent RLS symptoms, despite the risks associated with this medication. Not only does it provide quick relief, but it can be used only on the days when symptoms occur.2,5,8
Dopamine agonists
The use of pramipexole or ropinirole as first-line treatment for people with daily or refractory symptoms of RLS is well supported by controlled studies.7,17,25 Dopamine agonists can also be used to treat patients with RLS with varying levels of severity,6 and are sometimes prescribed as the initial treatment for intermittent symptoms.
These newer agents have a longer half-life than carbidopa/levodopa, which eliminates the need for a second dose in the midst of the sleep cycle. They also have much lower rates of augmentation. Studies have been inconsistent with regard to the risk of augmentation associated with these drugs, however, with results ranging from a high of 33%7,26 to a low of 4%.27
Nausea, headache, fatigue, dizziness, orthostatic hypotension, and vomiting—the most common side effects of pramipexole and ropinirole—usually decrease in severity after 7 to 10 days of therapy. In a recent meta-analysis comparing dopamine agonists with placebo in RLS patients, the number needed to harm (NNH) was 77 and the number needed to treat (NNT) was 6.27
Pramipexole. Dosing is started at 0.125 mg at bedtime and slowly titrated up to minimize side effects. Most patients experience relief at an average dose of 0.375 mg, taken daily or intermittently for symptom relief. 6,17,27
Ropinirole. Dosing is started at 0.25 mg at bedtime (or at dinner and bedtime), and then slowly titrated up every few days to every week until a good response is obtained. Most patients respond to a dose between 1 and 2.5 mg/d.6,17,28
Tx alternatives: Anticonvulsants, opioids, and benzodiazepines
When patients are unable to tolerate—or do not respond adequately to—dopaminergic agents or dopamine agonists, anticonvulsants, opioids, or benzodiazepines may be effective alternatives, or adjunctive treatments. They may also be used in patients who have another disorder, such as chronic pain, for which these alternatives will be beneficial.
Anticonvulsants. Of the anticonvulsants studied in RLS patients, gabapentin has been shown to most effectively decrease symptoms.25 Use of the drug should be reserved for patients with daily symptoms or refractory RLS. Gabapentin also appears to be especially effective in patients who perceive their symptoms as painful, and in hemodialysis patients.2,17,28 The average effective daily dose of gabapentin for treatment of RLS is 1855 mg.6
A 2002 double-blind crossover trial found that after 6 weeks of therapy, 16 of 24 (66%) patients taking gabapentin had only mild RLS symptoms, compared with 8 of 24 (33%) of those taking placebo (NNT=3).14 The most common side effects were malaise, somnolence, dry mouth, and nausea (NNH=4). Of note, there was no significant difference in the incidence of side effects among those in the therapy group compared with the controls.
Gabapentin also has fewer side effects and drug interactions than other anticonvulsants. (Both carbamazepine and valproate have also been studied for the treatment of RLS symptoms, but at best, provided only modest improvements.)
Opioids have long been recognized as an effective treatment for RLS, but their use is limited by the potential for abuse.6 In a double-blind crossover trial, 10 of 11 patients preferred opioids over placebo, and significantly more rated their leg sensations as mild after 2 weeks of treatment with opioids (NNT=2). The most common side effects were constipation and sedation (NNH=3).17,29
Many patients obtain symptom relief from low-potency opioids such as codeine, taken at bedtime, and there appears to be a lower abuse potential when bedtime-only dosing is used. However, higher-potency opioids may be necessary for patients with refractory RLS.17
One study did show an increase in symptoms of sleep apnea in RLS patients treated with opioids. If you suspect sleep apnea in an RLS patient taking opioids, provide a referral for a polysomnography evaluation.30
Benzodiazepines. There is limited evidence to support the use of clonazepam in the treatment of RLS. Although a prospective controlled study found clonazepam to be no more effective than placebo in RLS treatment,31 clonazepam has been shown to be an effective treatment in patients with PLMS.32 Because of the association between these 2 movement disorders, clonazepam is considered an option to use alone or as adjunctive therapy in patients with RLS.2,7
CASE STUDY: Grace’s diagnosis and treatment
In addition to having the 4 essential criteria for RLS, Grace reported sleep disturbances and periodic leg movements—2 additional features that are common to RLS. She also had low serum iron levels; however, her iron deficiency was related to her gastric bypass, and she was unable to tolerate iron therapy. We started her on a low dose of pramipexole, and she had a significant—and rapid—improvement in symptoms. When we last saw her, she reported that she usually slept through the night and that her leg movements had diminished so much that her husband no longer found it necessary to sleep in a separate bed.
Correspondence
Darlene E. Moyer, MD, Scottsdale Healthcare Family Medicine Residency Program, University of Arizona School of Medicine, 7301 East 2nd Street, Suite 210, Scottsdale, AZ 85251; [email protected]
- To diagnose restless legs syndrome (RLS), start with the 4 “essential criteria”—(1) a powerful urge to move the legs that is (2) rest-induced, (3) improves with activity, and (4) worsens in the evening (C).
- Carefully screen for secondary causes of RLS, including renal failure, pregnancy, iron deficiency, and medications that can cause or exacerbate symptoms (A).
- Carbidopa/levodopa is the first-line treatment for patients with intermittent symptoms of RLS; dopamine agonists are recommended for those with daily or refractory symptoms (C).
Restless legs syndrome (RLS) has become increasingly familiar to Americans in recent years, as the medical literature, consumer ads, and lay press have focused on new findings and treatments. Yet much about this movement disorder remains a mystery.
Both the number of people with RLS and the proportion of RLS patients whose symptoms are frequent or severe are among the unknowns. Estimates of prevalence range from approximately 2% of the general population to 15% of adults.1-6
Diagnosing RLS remains complicated. Although 4 key features, or “essential criteria,” have been identified, there is no definitive clinical finding or laboratory test for this syndrome. And, because the symptoms of a number of other movement disorders resemble those of RLS, you need to be alert to other clinical features and distinguishing characteristics to confirm an RLS diagnosis.
The pathophysiology of RLS is certainly not clear-cut, either. Possible mechanisms involve overexcitation of the spinal cord by the brain stem, decreased dopamine signaling, and low iron levels.5-8 Low serum iron levels, and especially low central nervous system ferritin levels, have been closely correlated with the severity of RLS symptoms.5,9,10 Genetic links to RLS are also being studied, but have not yet been clearly established.2,7,10,11
What we do know is that the prevalence of RLS increases with age. In a National Sleep Foundation poll, nearly 25% of people older than 65 reported symptoms of RLS.12 In a more recent study of the elderly conducted under the auspices of the World Health Organization, 9.8% of participants met the criteria for RLS.13
An aging population means you’re likely to see an increasing number of patients with symptoms of RLS, which can range in severity from occasional discomfort to daily leg pain. This update will help you hone your diagnostic skills and provide the best possible care to patients who are affected.
Start with the URGE mnemonic
Initial diagnostic criteria for RLS were developed by the National Institutes of Health (NIH) in 2002 and revised by the International Restless Legs Syndrome Study Group (IRLSSG) in 2005.9 They begin with 4 essential criteria ( TABLE 1 ), easily remembered with this simple mnemonic:
- Urge to move the legs
Rest induced
Gets better with activity
Evening and night accentuation.
Here’s what to keep in mind about each.
Urge. Patients with RLS experience a powerful urge to move their legs, and often their arms or other body parts, as well. Some patients also experience discomfort in their legs,14,15 which arises from deep within the legs rather than from the surface—a characteristic that helps differentiate RLS from other movement disorders.3,6,16
Rest induced. Numerous studies have shown that RLS symptoms worsen during periods of physical and mental inactivity, and when patients are in a seated or lying position.3,5,15,16 The longer the rest period, the more severe the symptoms become. Mentally stimulating activities, such as playing video games or reading, are often enough to prevent the onset of symptoms, at least in the early stage of the disorder.3,5,15,16
Gets better with activity. While inactivity exacerbates RLS symptoms, activity typically brings complete or partial relief. Symptom relief can be the result of physical movement, a mentally stimulating activity, or even a change in temperature. Touching and rubbing the legs often helps, too, although this effect diminishes as RLS progresses.3,6,15,16
Evening accentuation. The severity of RLS symptoms tends to follow the same circadian pattern as body temperature—increasing in the evening and peaking between the hours of 11 PM and 3 AM, and making it difficult, if not impossible, for patients to experience hours of uninterrupted sleep.
TABLE 1
Diagnosing restless legs syndrome2,17,26,33
Essential diagnostic criteria (URGE mnemonic)
|
Supportive features
|
Associated features
|
| RLS, restless legs syndrome. |
Other clues to an RLS diagnosis
In addition to the essential criteria, the NIH and the IRLSSG developed a number of supportive and associated clinical features ( TABLE 1 ) that provide further help in differentiating RLS from conditions with similar symptoms.
Supportive clinical features
Although not every patient with RLS will have all (or possibly any) of the findings that are identified as supportive, their presence will lend support to an RLS diagnosis. These include:
- family history (1st-degree relative with RLS)
- improvement with dopaminergic therapy
- periodic leg movements during sleep (PLMS) in patients <50 years of age
- periodic leg movements while awake in patients of any age.17
In interpreting the second feature—dopaminergic therapy—it is important to note that while patient response often wanes over time, an initial response (often obtained by patient history, if the patient has ever been treated with a dopaminergic agent) has a sensitivity of 80% and a specificity of 100% for diagnosis of RLS.16 Keep in mind, too, that periodic leg movements—typically defined as jerking, repetitive motions—are present in many other disorders, and also tend to increase in elderly patients who do not have RLS.
Associated clinical features
Similarly, a diagnosis of RLS is not dependent on the presence of these findings. They’re noteworthy, however, because they’re experienced by many patients with RLS.16
A natural progression of RLS that follows an identifiable pattern is the first associated feature. The course of RLS varies, however, depending in part on the age of onset. Patients who develop RLS in young adulthood tend to have a slower progression, with long periods of remission, while RLS tends to progress more rapidly in those who develop the condition as older adults.15
Sleep disturbances. Leg movements typically result in frequent awakenings and increased sleep latency. Because of these disruptions, RLS patients often experience daytime somnolence and an inability to pay attention; they also have trouble performing daytime duties.
No abnormal findings. There are no physical exam or lab abnormalities associated with primary (idiopathic) RLS. The presence of abnormal findings should raise questions about the diagnosis, and cause clinicians to explore the possibility of a secondary cause.
CASE STUDY: Would you suspect RLS?
Grace (not her real name), a 54-year-old woman who underwent gastric bypass surgery several years ago, has come in today seeking help for chronic insomnia. She reports that she experiences uncomfortable sensations deep in her legs when she lies down at night. She says that she is able to get some relief from these sensations when she gets up and walks. She also notes that when she tries to lie still, she feels a need to move her legs.
Grace says that when she does fall asleep, she moves her legs so frequently that her husband has begun sleeping in a separate bed—symptoms that immediately arouse suspicion of RLS. If she were your patient, how would you support (or refute) the diagnosis, and how would you treat it?
Rule out conditions that mimic RLS
When evaluating patients like Grace with suspected RLS, it is crucial to be aware of conditions with similar symptoms—some of which may coexist. The differential diagnosis and spectrum of movement disorders that should be considered in patients with RLS symptoms are listed in TABLE 2. While some of the presenting symptoms overlap, keeping the essential criteria of RLS in mind may help in identifying distinguishing characteristics.
Neuropathic pain syndrome may occur at rest or during intense activity, for example, and peripheral vascular disease is provoked by activity, while RLS is brought on by rest.
Symptoms of neuroleptic-induced akathisia may occur day or night; in contrast, RLS typically follows a circadian rhythm.
Similarly, the urge to move the legs that patients with RLS experience is powerful, but the movement itself is voluntary. This feature distinguishes RLS from sleep starts (hypnagogic jerks), for example, which are involuntary movements.
TABLE 2
RLS: Distinguishing features and differential diagnosis6,26,33
| DIFFERENTIAL DIAGNOSIS | CHARACTERISTICS | DISTINGUISHING FEATURES OF RLS |
|---|---|---|
| Positional discomfort | Alleviated by change in body position without need for repetitive body movements. |
|
| Neuropathic pain syndrome | Pain may occur during periods of activity or rest. | |
| Peripheral vascular disease/claudications | Pain evoked by activity. | |
| Painful legs and moving toes syndrome | Continuous to semi-continuous involuntary movement of toes with associated pain in affected extremity. |
|
| Sleep starts (hypnagogic jerks) | Sudden, brief, involuntary jerks of arms or legs. | |
| Sleep-related cramps | Involve specific muscle groups and are relieved (or partially relieved) by stretching. |
|
| Neuroleptic-induced akathisia | Day- or nighttime motor restlessness that is generalized, immediately relieved with movement, and recurs immediately after the patient stops moving. | |
| Rheumatoid arthritis | Pain is chronic, not immediately relieved by moving the affected extremity, and characteristically associated with joint deformities. |
|
| RLS, restless legs syndrome. | ||
Review the need for iron replacement
The role that low levels of iron play in RLS is not entirely clear. One study found the median ferritin level in patients with RLS symptoms to be 33 mcg/L, compared with 59 mcg/L in those without symptoms.18 Another study showed patients with ferritin levels less than 50 mcg/L to have more severe RLS symptoms than those with levels greater than 50 mcg/L.19 Both iron and dopamine have been shown to have circadian rhythms similar to RLS, with their nadir correlating with the time of maximum severity of RLS symptoms. (Low levels of iron may be associated with either idiopathic or secondary RLS.)
Consensus guidelines recommend against initiating iron replacement therapy without checking levels, as this could lead to iron overload.6 Any patient with a plasma ferritin concentration <50 mcg/L and RLS symptoms should be started on iron replacement therapy. The recommended dose of iron is 325 mg ferrous sulfate, taken with 300 mg vitamin C, 3 times a day. Vitamin C allows for better absorption of the iron.8
Monitor ferritin levels at 6-week intervals until they exceed 50 mcg/L, then check iron concentrations every few months. Iron replacement therapy can be decreased or discontinued, provided the ferritin remains at this level.17
Try these strategies for symptom relief
The goal in treating RLS is to decrease the severity and frequency of symptoms, leading to an improvement in sleep quality, a decrease in daytime somnolence, and an overall improvement in quality of life. Treatment guidelines, supported by the IRLSSG and the Medical Advisory Board of the Restless Legs Syndrome Foundation, are based on symptom frequency and severity—whether they are intermittent, daily, or refractory.3,9,17
The algorithm ( FIGURE ) provides recommendations for starting and escalating pharmacologic therapy. Nonpharmacologic treatments have not been studied in systematic trials; however, recommendations for their use are based on expert opinion, case series, or anecdotal reports. In patients who do not have severe symptoms, it makes sense to try simpler strategies first.
Review medications and diet. Many medications have been associated with RLS, either as a secondary cause or suspected of exacerbating symptoms. These include dopamine antagonists (neuroleptics, antiemetics); antidepressants, primarily tricyclics and selective serotonin reuptake inhibitors, lithium, and antipsychotics; antihistamines, including diphenhydramine and other over-the-counter cold and allergy remedies; calcium channel blockers; and diuretics.6,8,17
If a patient troubled by symptoms of RLS is taking any of these agents, consider changing the medication. If no suitable substitute is available, it may help to change the dosing schedule to earlier in the day—ideally no later than 3 PM.8,17 Advise patients, too, to avoid caffeine, tobacco, and alcohol, as well as any other food or beverage known to contain stimulants.
Encourage activity. Discuss the importance of exercise in the management of RLS symptoms. Encourage patients to routinely engage in physical activity and to pursue mentally stimulating activities such as reading, puzzles, or games.
Stress good sleep hygiene. Urge patients to follow a consistent sleep schedule, going to sleep and awakening at the same time each day; to use the bed only for rest or intimacy and avoid reading or watching TV in bed; and to establish a relaxing bedtime routine. Some patients have found that hot or cold baths before bed help to relieve symptoms; others report that massaging their legs or stretching leads to an improvement in symptoms.7,9,17
FIGURE
RLS: A treatment algorithm2,7,9,20
FESO4, ferrous sulfate; RLS, restless legs syndrome.
Pharmacologic options: Selecting the right one
In addition to iron replacement, there are 5 major types of RLS treatment:
- dopaminergic agents,
- dopamine agonists
- anticonvulsants,
- opioids,
- benzodiazepines.
Carbidopa/levodopa for intermittent symptoms
Most patients with RLS have a positive response to treatment with dopaminergic agents, at least initially, and for many years carbidopa/levodopa was the usual therapy for RLS. It remains a first-line treatment for patients with intermittent symptoms.
Early studies consistently showed that 70% to 80% of RLS patients treated with carbidopa/levodopa had a significant improvement in symptoms.20-22 But the studies were small, did not always include a placebo arm, and most were crossover trials, making it impossible to do a statistical comparison.5
Typical doses of carbidopa/levodopa as a treatment for RLS are 25/100 mg to 100/400 mg in divided doses, given before bedtime and again, if needed, in the middle of the sleep period.9 These doses are much lower than those used to treat Parkinsonism.
Common side effects of carbidopa/levodopa include nausea, headache, dry mouth, and daytime somnolence.2,21 An increased risk of melanoma has been seen in some studies of carbidopa/levodopa, but the evidence is inconclusive.23,24 Rebound (a worsening of symptom severity when the medication wears off) and augmentation (the development of more severe symptoms early in the day) have also been reported.
Tolerance to carbidopa/levodopa is infrequent among patients with RLS. One early study found that only 3 of 43 patients (7%) required an increase in dosage over time.21 The on/off phenomenon that occurs with this medication in the treatment of Parkinsonism is not mentioned in the literature in reference to RLS.
Because carbidopa/levodopa only provides relief for 4 to 6 hours, a second dose is often needed. If that second dose repeatedly disrupts the patient’s sleep, the recommended approach is to give 2 doses before bedtime—1 dose of regular carbidopa/levodopa and 1 dose of the controlled release form.6,9
Augmentation, the most serious problem associated with carbidopa/levodopa, occurs in 65% to 80% of RLS patients treated with this medication. It is more common in those with refractory symptoms and those taking higher doses, but can affect any RLS patient.
If augmentation develops, discontinue the carbidopa/levodopa and switch the patient to another agent. Augmentation reverses within a few weeks of stopping the medication and treatment can then be resumed, but be aware that the augmentation may reoccur.
Carbidopa/levodopa is a good choice for patients with intermittent RLS symptoms, despite the risks associated with this medication. Not only does it provide quick relief, but it can be used only on the days when symptoms occur.2,5,8
Dopamine agonists
The use of pramipexole or ropinirole as first-line treatment for people with daily or refractory symptoms of RLS is well supported by controlled studies.7,17,25 Dopamine agonists can also be used to treat patients with RLS with varying levels of severity,6 and are sometimes prescribed as the initial treatment for intermittent symptoms.
These newer agents have a longer half-life than carbidopa/levodopa, which eliminates the need for a second dose in the midst of the sleep cycle. They also have much lower rates of augmentation. Studies have been inconsistent with regard to the risk of augmentation associated with these drugs, however, with results ranging from a high of 33%7,26 to a low of 4%.27
Nausea, headache, fatigue, dizziness, orthostatic hypotension, and vomiting—the most common side effects of pramipexole and ropinirole—usually decrease in severity after 7 to 10 days of therapy. In a recent meta-analysis comparing dopamine agonists with placebo in RLS patients, the number needed to harm (NNH) was 77 and the number needed to treat (NNT) was 6.27
Pramipexole. Dosing is started at 0.125 mg at bedtime and slowly titrated up to minimize side effects. Most patients experience relief at an average dose of 0.375 mg, taken daily or intermittently for symptom relief. 6,17,27
Ropinirole. Dosing is started at 0.25 mg at bedtime (or at dinner and bedtime), and then slowly titrated up every few days to every week until a good response is obtained. Most patients respond to a dose between 1 and 2.5 mg/d.6,17,28
Tx alternatives: Anticonvulsants, opioids, and benzodiazepines
When patients are unable to tolerate—or do not respond adequately to—dopaminergic agents or dopamine agonists, anticonvulsants, opioids, or benzodiazepines may be effective alternatives, or adjunctive treatments. They may also be used in patients who have another disorder, such as chronic pain, for which these alternatives will be beneficial.
Anticonvulsants. Of the anticonvulsants studied in RLS patients, gabapentin has been shown to most effectively decrease symptoms.25 Use of the drug should be reserved for patients with daily symptoms or refractory RLS. Gabapentin also appears to be especially effective in patients who perceive their symptoms as painful, and in hemodialysis patients.2,17,28 The average effective daily dose of gabapentin for treatment of RLS is 1855 mg.6
A 2002 double-blind crossover trial found that after 6 weeks of therapy, 16 of 24 (66%) patients taking gabapentin had only mild RLS symptoms, compared with 8 of 24 (33%) of those taking placebo (NNT=3).14 The most common side effects were malaise, somnolence, dry mouth, and nausea (NNH=4). Of note, there was no significant difference in the incidence of side effects among those in the therapy group compared with the controls.
Gabapentin also has fewer side effects and drug interactions than other anticonvulsants. (Both carbamazepine and valproate have also been studied for the treatment of RLS symptoms, but at best, provided only modest improvements.)
Opioids have long been recognized as an effective treatment for RLS, but their use is limited by the potential for abuse.6 In a double-blind crossover trial, 10 of 11 patients preferred opioids over placebo, and significantly more rated their leg sensations as mild after 2 weeks of treatment with opioids (NNT=2). The most common side effects were constipation and sedation (NNH=3).17,29
Many patients obtain symptom relief from low-potency opioids such as codeine, taken at bedtime, and there appears to be a lower abuse potential when bedtime-only dosing is used. However, higher-potency opioids may be necessary for patients with refractory RLS.17
One study did show an increase in symptoms of sleep apnea in RLS patients treated with opioids. If you suspect sleep apnea in an RLS patient taking opioids, provide a referral for a polysomnography evaluation.30
Benzodiazepines. There is limited evidence to support the use of clonazepam in the treatment of RLS. Although a prospective controlled study found clonazepam to be no more effective than placebo in RLS treatment,31 clonazepam has been shown to be an effective treatment in patients with PLMS.32 Because of the association between these 2 movement disorders, clonazepam is considered an option to use alone or as adjunctive therapy in patients with RLS.2,7
CASE STUDY: Grace’s diagnosis and treatment
In addition to having the 4 essential criteria for RLS, Grace reported sleep disturbances and periodic leg movements—2 additional features that are common to RLS. She also had low serum iron levels; however, her iron deficiency was related to her gastric bypass, and she was unable to tolerate iron therapy. We started her on a low dose of pramipexole, and she had a significant—and rapid—improvement in symptoms. When we last saw her, she reported that she usually slept through the night and that her leg movements had diminished so much that her husband no longer found it necessary to sleep in a separate bed.
Correspondence
Darlene E. Moyer, MD, Scottsdale Healthcare Family Medicine Residency Program, University of Arizona School of Medicine, 7301 East 2nd Street, Suite 210, Scottsdale, AZ 85251; [email protected]
1. Karroum E, Konofal E, Arnulf I. Restless-legs syndrome. Rev Neurol. 2008;164:701-721.
2. Vergne-Salle P, Coyral D, Dufauret K, et al. Is restless legs syndrome underrecognized? Current management. Joint Bone Spine. 2006;73:369-373.
3. Fulda S, Wetter TC. Dopamine agonists for the treatment of restless legs syndrome. Expert Opin Pharmacother. 2005;6:2655-2666.
4. Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286-1292.
5. Conti C, de Oliveira MM, Andriolo RB, et al. Levodopa for idiopathic restless legs syndrome: evidence-based review. Mov Disord. 2007;22:1943-1951.
6. Restless Legs Syndrome Foundation. RLS Medical Bulletin. 2005. Available at: http://www.irlssg.org/RLSMB2005pf.pdf. Accessed July 9, 2009.
7. Ryan M, Slevin J. Restless legs syndrome. Am J Health Syst Pharm. 2006;63:1599-1612.
8. Oertel W, Trenkwalder C, Zucconi M, et al. State of the art in restless legs syndrome therapy: practice recommendations for treating restless legs syndrome. Mov Disord. 2007;22(suppl 18):S466-S475.
9. Thorpy M. New paradigms in the treatment of restless legs syndrome. Neurology. 2005;64(12 suppl 3):S28-S33.
10. Winkelman J. A better future for patients with restless legs syndrome. Am J Med. 2007;120(1 suppl 1):S28-S29.
11. Chahine L, Chemali Z. Restless legs syndrome: a review. CNS Spectr. 2006;11:511-520.
12. Johnson E. Omnibus Sleep in America poll. National Sleep Foundation. 1998.-
13. Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Neurology. 2000;54:1064-1068.
14. Garcia-Borreguero D. Time to REST: epidemiology and burden. Eur J Neurol. 2006;13(suppl 3):S15-S20.
15. Schapira A. RLS patients: who are they? Eur J Neurol. 2006;13(suppl 3):S2-S7.
16. Benes H, Walters AS, Allen RP, et al. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. 2007;22(suppl 18):S401-S408.
17. Hening W, Allen R, Tenzer P, et al. Restless legs syndrome: demographics, presentation and differential diagnosis. Geriatrics. 2007;62:26-29.
18. O’Keefe ST, Gavin K, Lavan JN. Iron status and restless legs in the elderly. Age Ageing. 1994;23:200-203.
19. Sun ER, Chen CA, Ho G, et al. Iron and the restless legs syndrome. Sleep. 1998;21:371-377.
20. Akpinar S. Restless legs syndrome treatment with dopaminergic drugs. Clin Neuropharmacol. 1987;10:69-79.
21. Becker P, Jamieson A, Brown D. Dopaminergic agents in restless legs syndrome and periodic limb movements of sleep: response and complications of extended treatment in 49 cases. Sleep. 1993;16:713-716.
22. Von Scheele C, Kempi V. Long-term effect of dopaminergic drugs in restless legs. A 2 year follow-up. Arch Neurol. 1990;47:1223-1224.
23. Bertoni JM, Arlette JP, Fernandez HH, et al. Epidemiologic association of Parkinson’s disease and melanoma. Mov Disord. 2006;21(suppl 15):S610.-
24. Fiala K, Whetteckey J, Manyam B. Malignant melanoma and levodopa in Parkinson’s disease: causality or coincidence? Parkinsonism Relat Disord. 2003;9:321-327.
25. Montagna P. The treatment of restless legs syndrome. Neurol Sci. 2007;28(suppl 1):S61-S66.
26. Kushida C. Clinical presentation, diagnosis and quality of life issues in restless legs syndrome. Am J Med. 2007;120(1 suppl 1):S4-S12.
27. Baker W, White C, Coleman C. Effect of nonergot dopamine agonists on symptoms of restless legs syndrome. Ann Fam Med. 2008;6:253-262.
28. Fulda S, Wetter T. Emerging drugs for restless legs syndrome. Expert Opin Emerg Drugs. 2005;10:527-552.
29. Walters A, Wagner M, Hening W, et al. Successful treatment of idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16:327-332.
30. Walters A, Winkelman J, Trenkwalder C, et al. Long term follow-up on restless legs syndrome patients treated with opioids. Mov Disord. 2001;16:1105-1109.
31. Boghen D, Lamothe L, Elie R. The treatment of restless legs syndrome with clonazepam: a prospective controlled trial. Can J Neurol Sci. 1986;13:245-247.
32. Peled R, Lavie P. Double-blind evaluation of clonazepam on periodic leg movements in sleep. J Neurol Neurosurg Psychiatry. 1987;50:1679-1681.
33. Ferni-Strambi L. RLS-like symptoms: differential diagnosis by history and clinical assessment. Sleep Med. 2007;8(suppl 2):S3-S6.
1. Karroum E, Konofal E, Arnulf I. Restless-legs syndrome. Rev Neurol. 2008;164:701-721.
2. Vergne-Salle P, Coyral D, Dufauret K, et al. Is restless legs syndrome underrecognized? Current management. Joint Bone Spine. 2006;73:369-373.
3. Fulda S, Wetter TC. Dopamine agonists for the treatment of restless legs syndrome. Expert Opin Pharmacother. 2005;6:2655-2666.
4. Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286-1292.
5. Conti C, de Oliveira MM, Andriolo RB, et al. Levodopa for idiopathic restless legs syndrome: evidence-based review. Mov Disord. 2007;22:1943-1951.
6. Restless Legs Syndrome Foundation. RLS Medical Bulletin. 2005. Available at: http://www.irlssg.org/RLSMB2005pf.pdf. Accessed July 9, 2009.
7. Ryan M, Slevin J. Restless legs syndrome. Am J Health Syst Pharm. 2006;63:1599-1612.
8. Oertel W, Trenkwalder C, Zucconi M, et al. State of the art in restless legs syndrome therapy: practice recommendations for treating restless legs syndrome. Mov Disord. 2007;22(suppl 18):S466-S475.
9. Thorpy M. New paradigms in the treatment of restless legs syndrome. Neurology. 2005;64(12 suppl 3):S28-S33.
10. Winkelman J. A better future for patients with restless legs syndrome. Am J Med. 2007;120(1 suppl 1):S28-S29.
11. Chahine L, Chemali Z. Restless legs syndrome: a review. CNS Spectr. 2006;11:511-520.
12. Johnson E. Omnibus Sleep in America poll. National Sleep Foundation. 1998.-
13. Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Neurology. 2000;54:1064-1068.
14. Garcia-Borreguero D. Time to REST: epidemiology and burden. Eur J Neurol. 2006;13(suppl 3):S15-S20.
15. Schapira A. RLS patients: who are they? Eur J Neurol. 2006;13(suppl 3):S2-S7.
16. Benes H, Walters AS, Allen RP, et al. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. 2007;22(suppl 18):S401-S408.
17. Hening W, Allen R, Tenzer P, et al. Restless legs syndrome: demographics, presentation and differential diagnosis. Geriatrics. 2007;62:26-29.
18. O’Keefe ST, Gavin K, Lavan JN. Iron status and restless legs in the elderly. Age Ageing. 1994;23:200-203.
19. Sun ER, Chen CA, Ho G, et al. Iron and the restless legs syndrome. Sleep. 1998;21:371-377.
20. Akpinar S. Restless legs syndrome treatment with dopaminergic drugs. Clin Neuropharmacol. 1987;10:69-79.
21. Becker P, Jamieson A, Brown D. Dopaminergic agents in restless legs syndrome and periodic limb movements of sleep: response and complications of extended treatment in 49 cases. Sleep. 1993;16:713-716.
22. Von Scheele C, Kempi V. Long-term effect of dopaminergic drugs in restless legs. A 2 year follow-up. Arch Neurol. 1990;47:1223-1224.
23. Bertoni JM, Arlette JP, Fernandez HH, et al. Epidemiologic association of Parkinson’s disease and melanoma. Mov Disord. 2006;21(suppl 15):S610.-
24. Fiala K, Whetteckey J, Manyam B. Malignant melanoma and levodopa in Parkinson’s disease: causality or coincidence? Parkinsonism Relat Disord. 2003;9:321-327.
25. Montagna P. The treatment of restless legs syndrome. Neurol Sci. 2007;28(suppl 1):S61-S66.
26. Kushida C. Clinical presentation, diagnosis and quality of life issues in restless legs syndrome. Am J Med. 2007;120(1 suppl 1):S4-S12.
27. Baker W, White C, Coleman C. Effect of nonergot dopamine agonists on symptoms of restless legs syndrome. Ann Fam Med. 2008;6:253-262.
28. Fulda S, Wetter T. Emerging drugs for restless legs syndrome. Expert Opin Emerg Drugs. 2005;10:527-552.
29. Walters A, Wagner M, Hening W, et al. Successful treatment of idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16:327-332.
30. Walters A, Winkelman J, Trenkwalder C, et al. Long term follow-up on restless legs syndrome patients treated with opioids. Mov Disord. 2001;16:1105-1109.
31. Boghen D, Lamothe L, Elie R. The treatment of restless legs syndrome with clonazepam: a prospective controlled trial. Can J Neurol Sci. 1986;13:245-247.
32. Peled R, Lavie P. Double-blind evaluation of clonazepam on periodic leg movements in sleep. J Neurol Neurosurg Psychiatry. 1987;50:1679-1681.
33. Ferni-Strambi L. RLS-like symptoms: differential diagnosis by history and clinical assessment. Sleep Med. 2007;8(suppl 2):S3-S6.
Concussion care: Simple strategies, big payoffs
- Consider any alteration of mental status that follows a trauma to be a concussion, whether or not there is also a loss of consciousness (A).
- Don’t order neuroimaging routinely; it is not necessary for diagnosing concussion. Neuroimaging is important, however, for patients who exhibit prolonged unconsciousness, focal neurologic deficits, or worsening symptoms (A).
- Treat post-concussive headache, a common complaint, with acetaminophen or ibuprofen (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s 7 AM and you’re just finishing breakfast when you get a call from an emergency room (ER) resident telling you that Max, a 14-year-old patient of yours, has just been brought in by ambulance after a car accident. When the EMTs arrived at the scene, the car was totaled. The driver, Max’s older brother, had no injuries other than a minor abrasion on his nose, but Max was unconscious.
By the time you get to the hospital, Max is sitting up and talking. He says his head aches and he’s feeling dizzy. He’s having difficulty comprehending what he’s doing in the ER, doesn’t know how he got there, and has no memory of the accident. He has a small contusion on his forehead, but no other apparent injuries.
You question Max’s brother, and he tells you he hydroplaned while driving in the rain, and skidded into a telephone pole. He was OK, but Max was slumped down in his seat and unresponsive. The driver of another car had seen the accident and called 911.
How would you evaluate Max’s condition? What tests would you perform? And what would you tell his worried parents?
A diagnosis with variable—and often subtle—symptoms
Max’s situation fits the American Academy of Neurology’s (AAN) definition of concussion, ie, an alteration in mental status following a trauma that may or may not result in a loss of consciousness.1 According to the AAN, this change in mental status usually lasts less than 24 hours and may be coupled with symptoms of vertigo, headache, nausea, vomiting, tinnitus, photophobia, blurred vision, and anterograde or retrograde amnesia.2-5
The presenting signs and symptoms of concussion are so variable that the condition can be difficult to diagnose. Many patients display nothing more than a so-called vacant stare. Others experience only minor symptoms, such as headache or nausea. Max’s loss of consciousness is relatively rare, occurring in only about 10% of concussion cases.6
At least 1.4 million concussions occur in the United States each year, according to the Centers for Disease Control and Prevention (CDC).7,8 The incidence, though, is probably greater than the CDC reports, because so many cases are unrecognized or unreported. Falls, motor vehicle accidents, and sports injuries are the leading causes, with some 250,000 football-related concussions reported each year.4,7,9,10
Common, yes, but challenging, too
As common as concussion is, it can—at times—be challenging to recognize. These tips can help:
Do a mental status exam. Ask the patient what his name is, what today’s date is, and where he is now. See if he can repeat 3 words immediately after you say them and then 5 minutes later. Can he spell the word “world” backwards?
Get the story. If the patient can’t remember the incident or isn’t able to tell you about it, try to get the details from a witness. If the patient is able to talk, ask if she or he remembers what was happening just before the accident and afterwards. Does the patient seem to have difficulty concentrating on your questions? Seem alert, or confused? Can the patient (or a witness) tell you how the injury occurred, how severe the impact was, and whether there was loss of consciousness—all important factors in the diagnosis.1 Has the patient ever had a concussion before? Were there any sequelae?
Perform a neurologic evaluation. Assess cranial nerves and cerebral, cerebellar, and peripheral nerve function. Check pupils for reactivity and symmetry. See whether the patient has full and appropriate eye movement in all directions. Assess grip strength and muscle strength in arm and leg flexion and extension. Test for abnormalities in reactions to pinprick, temperature, and vibration in all extremities. Ask the patient to close his eyes, extend the arms with palms up, and hold the position for 20 to 30 seconds. If the patient fails this assessment for pronator drift, consider a diagnosis of muscle weakness or cerebellar disease. Check gait for ataxia and speech for fluency and coherence.
When a CT is required
While most cases of suspected concussion do not require imaging, it is appropriate for patients like this 52-year-old man, who had a history of assault and presented with dizziness and vomiting. His CT scan revealed mild hyperattenuation adjacent to the cranial shadow—a finding suggestive of a subarachnoid hemorrhage in the region.
When trauma’s an old story
Patients with head injury do not always seek emergency care. Whenever an office patient tells you about a head injury, however minor it seems, you should always assess for concussion. The diagnosis may be useful in guiding treatment and prevention strategies in the future. It may also help you recognize post-concussive symptoms, which can occur weeks or months after the trauma and cause significant morbidity.
Does your patient really need imaging?
Imaging is usually not necessary for diagnosis when the mental status and neurologic examinations are negative. Abnormal imaging scans are rare in cases of suspected concussion, showing up in fewer than 10% of computed tomography (CT) scans and 30% of magnetic resonance images (MRIs).11 You wouldn’t order imaging for Max, as he passed his neurologic exam with flying colors. However, if he had remained unconscious for a longer period, his mental status changes had continued, or he had neurologic symptoms that persisted for more than a week, you would order neuroimaging to rule out additional pathology.10,12 Neuroimaging may also be indicated in cases of particularly forceful injury—a fall from a height greater than 3 feet, for example, or a pedestrian hit by a car—or for a patient with an open, depressed, or suspected basal skull fracture.
In addition, imaging studies should be done for patients with a score of less than 15 on the Glasgow coma scale, retrograde amnesia for more than 30 minutes before the accident, or more than 2 episodes of vomiting. Imaging options include a CT scan without contrast to evaluate for intracranial bleeding or an MRI without contrast to test for smaller intracranial bleeds or axonal injury.13,14
CT scans are quick, generally available, and reasonably inexpensive, but may not detect all relevant abnormalities. MRIs are more sensitive and better able to detect areas of contusion, petechial hemorrhage, and axonal injury, but are less accessible in emergencies and cost a great deal more.13,14
New research suggests that patients with prolonged neurologic sequelae may benefit from single proton emission computed tomography (SPECT) or positron emission tomography (PET) in addition to conventional CT and MRI studies.13,15 SPECT studies use radioactive tracers that can cross the blood-brain barrier to estimate cerebral blood flow; decreased cerebral blood flow indicates areas of brain damage.11 PET scans are more expensive than SPECT, but have the advantage of being able to demonstrate oxygen and glucose metabolism, which are more sensitive indicators of brain damage. While SPECT and PET images are seldom used in diagnosing concussion, they can benefit patients who continue to have neurologic deficits that require further definition of the areas of brain injury.
How to fine-tune concussion care
First, grade the concussion. Concussion scales are a useful guide for making treatment decisions.4,5,10 The AAN scale presented in TABLE 1 is the most widely used. Keep in mind, though, that this scale is scheduled for revision.1
You conclude from your examination of Max that he does have a concussion, and that his loss of consciousness indicates a grade 3, even though his symptoms of dizziness and confusion lasted less than 15 minutes.
Initiate monitoring. This essential aspect of concussion management can take place at home for most patients, with hospitalization necessary for only a few. Max can go home. You know his parents well, and they’re competent to follow a monitoring protocol. For the first 24 hours, you tell them to wake Max up every 2 hours, so that they can pick up any change in his symptoms without delay. If they have difficulty waking him or he develops signs and symptoms such as vomiting or severe headache, you tell them to call you and bring Max back to the hospital.
Patients who should be monitored in the hospital are those with seizures, evidence of intracranial bleeding or cerebral edema on CT scan, or a history of taking oral anticoagulants. So should any patients whose living situation is not reliable for adequate home monitoring—homeless patients or those with a chaotic home life, for instance.
Return to usual activities: When to say Yes, when to say No. You advise Max’s parents to keep him home from school and have him take a break from homework. Concentrating on schoolwork can aggravate concussive symptoms.12 Strenuous physical activity is out, too, and he shouldn’t be alone for more than short periods until all his symptoms subside.
Sports concussions have their own imperatives, based on the grade of the concussion. Patients with a grade 1 concussion can return to the playing field within 15 minutes, as long as their neurologic exam is normal and they have no symptoms. With a grade 2 concussion, the player should be asymptomatic for 1 week before going back to normal activities. A grade 3 concussion requires that the player stay off the field until he or she has been asymptomatic for 2 weeks.4 This recommendation would apply to Max, who had a grade 3 concussion.
TABLE 1
AAN concussion grading scale1
| GRADE 1 | GRADE 2 | GRADE 3 | |
|---|---|---|---|
| Loss of consciousness | No | No | Yes |
| Symptoms | Lasting <15 minutes | Lasting >15 minutes | Lasting >15 minutes |
When symptoms linger
Most concussion patients will recover fairly rapidly. Unfortunately, however, some 38% of patients who have experienced a concussion with loss of consciousness continue to be plagued with what is called post-concussive syndrome (PCS).16 The International Classification of Diseases (ICD-10, 2nd ed.) defines PCS as a combination of signs and symptoms that occur within 4 weeks of head trauma with loss of consciousness. These include headache, fatigue, depression, emotional lability, difficulty concentrating, insomnia, and a preoccupation with symptoms with a fear of brain damage. The incidence of PCS using criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) is similar to that documented with the ICD-10, which suggests that either definition could be used to evaluate for PCS.17
By far the most common of these post-concussive symptoms is headache, reported by nearly 80% of patients with PCS and occurring most often in those who are headache-prone.18 That’s the case with Max, who has always had a problem with headaches and returns to your office a month after the accident complaining of persistent head pain.
Most post-concussive headaches resolve with rest and over-the-counter medications such as acetaminophen or ibuprofen. You may also consider prescribing antidepressants, particularly the selective serotonin reuptake inhibitor sertraline, which has been shown to decrease the vertigo, blurred vision, visual changes, and headache often associated with PCS.16,19,20 Start sertraline at a dosage of 25 mg/d, then titrate after a week to the recommended 50 mg/d.19 If symptoms persist, slowly titrate to the maximum dose of 200 mg/d while carefully monitoring for potential side effects.19
Patients with post-concussive status migrainosus, a headache lasting longer than 3 days that is unresponsive to conventional treatment, may benefit from a short course of corticosteroids.4 Additional treatment options include triptans, anticonvulsants, and β-blockers, although none of these options has been backed up by a large-scale, randomized controlled trial.16,20
Vigilance needed in cases of repeated trauma
Patients who experience repeated head trauma require particular attention. They are more likely to have detectable signs and symptoms and PCS.21 Also, they are more likely to incur second impact syndrome (SIS), a cascade of symptoms that can occur within 2 to 5 minutes of sustaining a second blow. SIS patients experience a rapid and diffuse cerebral edema, which can lead to brain herniation and death.3 SIS is a primary risk in sports like football, when players are allowed to return to the field too early. TABLE 2 sets out the special diagnostic criteria the AAN has formulated for managing patients who experience repeated concussions.
TABLE 2
Multiple concussions: When can your patient return to play?1
| SYMPTOM SEVERITY | SECOND CONCUSSION | THIRD CONCUSSION (OR MORE) |
|---|---|---|
| Concussive symptoms lasting <15 minutes | Return to play when asymptomatic for 1 week | Return to play when asymptomatic for 1 week |
| Post-traumatic amnesia <30 minutes, without loss of consciousness | Return to play when asymptomatic for 2 weeks | Return to play when asymptomatic for 1 month |
| Post-traumatic amnesia >30 minutes or loss of consciousness | Return to play when asymptomatic for 1 month | Discourage return to play indefinitely |
Help prevent concussion
In your role as educator, you can inform patients, school officials, and community leaders about the importance of protective equipment such as sports helmets, seat belts, and air bags in reducing the incidence of concussion.9,15 As a family physician, you have a special opportunity to teach parents, teachers, coaches, and players to recognize the signs and symptoms of concussion, understand the risks, and stop players from returning to sports activities prematurely.4
CORRESPONDENCE
Amanda McConnell, DO, MPH, Grandview Hospital, Office of Medical Education, 405 Grand Avenue, Dayton, OH 45405; [email protected]
1. Practice parameter: the management of concussion in sports (summary statement) Report of the Quality Standards Subcommittee. Neurology. 1997;48:581-585.
2. Anderson T, Heitger M, Macleod AD. Concussion and mild head injury. Pract Neurol. 2006;6:342-357.
3. Delaney JS, Abuzeyad F, Correa JA, et al. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29:189-197.
4. Harmon KG. Assessment and management of concussion in sports. Am Fam Physician. 1999;60:887-894.
5. Kushner DS. Concussion in sports: minimizing the risk of complications. Am Fam Physician. 2001;64:1007-1014.
6. Cantu RC. Head injuries in sport. Br J Sports Med. 1996;30:289-296.
7. Division of Injury and Disability Outcomes and Programs National Center for Injury Prevention and Control Centers for Disease Control and Prevention Department of Health and Human Services. “Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths.” January 2006. Available at: http://www.cdc.gov/ncipc/pub-res/TBI_in_US_04/TBI_ ED.htm. Accessed November 10, 2007.
8. Centers for Disease Control and Prevention Non-fatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. Morb Mortal Wkly Rep. 2007;56:733-737.
9. Hillary FG, Schatz P, Moelter ST, et al. Motor vehicle collision factors influence severity and type of TBI. Brain Injury. 2002;16:729-741.
10. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association position statement: management of sport-related concussion. J Athl Train. 2004;39:280-297.
11. Ropper AH, Gorson KC. Concussion. N Engl J Med. 2007;356:166-172.
12. McCrory P, Meeuwisse W, Johnston K, et al. Summary and agreement statement of the 3rd international conference on concussion in sport, November 2008. Br J Sports Med. 2009;43:76-84.
13. Bazarian JJ, Blyth B, Cimpello L. Bench to bedside: evidence for brain injury after concussion—looking beyond the computed tomography scan. Acad Emerg Med. 2006;13:199-214.
14. Hughes DG, Jackson A, Mason DL, et al. Abnormalities on magnetic resonance imaging seen acutely following a mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550-558.
15. Ryan L, Warden D. Post concussion syndrome. Int Rev Psychiatry. 2003;15:310-316.
16. Mittenberg W, Canyock E, Condit D, et al. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23:829-836.
17. McCauley SR, Boake C, Pedroza C, et al. Post-concussional disorder: are the DSM-IV criteria an improvement over the ICD-10? J Nerv Ment Dis. 2005;193:540-550.
18. Mickeviciene D, Schrader H, Obelieniene D, et al. A controlled prospective inception cohort study on the post-concussion syndrome outside the medicolegal context. Eur J Neurol. 2004;11:411-419.
19. Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12:226-236.
20. McAllister TW, Arciniegas D. Evaluation and treatment of postconcussive symptoms. NeuroRehabilitation. 2002;17:265-283.
21. Whiteside J. Management of head and neck injury by the sideline physician. Am Fam Physician. 2006;74:1357-1362.
- Consider any alteration of mental status that follows a trauma to be a concussion, whether or not there is also a loss of consciousness (A).
- Don’t order neuroimaging routinely; it is not necessary for diagnosing concussion. Neuroimaging is important, however, for patients who exhibit prolonged unconsciousness, focal neurologic deficits, or worsening symptoms (A).
- Treat post-concussive headache, a common complaint, with acetaminophen or ibuprofen (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s 7 AM and you’re just finishing breakfast when you get a call from an emergency room (ER) resident telling you that Max, a 14-year-old patient of yours, has just been brought in by ambulance after a car accident. When the EMTs arrived at the scene, the car was totaled. The driver, Max’s older brother, had no injuries other than a minor abrasion on his nose, but Max was unconscious.
By the time you get to the hospital, Max is sitting up and talking. He says his head aches and he’s feeling dizzy. He’s having difficulty comprehending what he’s doing in the ER, doesn’t know how he got there, and has no memory of the accident. He has a small contusion on his forehead, but no other apparent injuries.
You question Max’s brother, and he tells you he hydroplaned while driving in the rain, and skidded into a telephone pole. He was OK, but Max was slumped down in his seat and unresponsive. The driver of another car had seen the accident and called 911.
How would you evaluate Max’s condition? What tests would you perform? And what would you tell his worried parents?
A diagnosis with variable—and often subtle—symptoms
Max’s situation fits the American Academy of Neurology’s (AAN) definition of concussion, ie, an alteration in mental status following a trauma that may or may not result in a loss of consciousness.1 According to the AAN, this change in mental status usually lasts less than 24 hours and may be coupled with symptoms of vertigo, headache, nausea, vomiting, tinnitus, photophobia, blurred vision, and anterograde or retrograde amnesia.2-5
The presenting signs and symptoms of concussion are so variable that the condition can be difficult to diagnose. Many patients display nothing more than a so-called vacant stare. Others experience only minor symptoms, such as headache or nausea. Max’s loss of consciousness is relatively rare, occurring in only about 10% of concussion cases.6
At least 1.4 million concussions occur in the United States each year, according to the Centers for Disease Control and Prevention (CDC).7,8 The incidence, though, is probably greater than the CDC reports, because so many cases are unrecognized or unreported. Falls, motor vehicle accidents, and sports injuries are the leading causes, with some 250,000 football-related concussions reported each year.4,7,9,10
Common, yes, but challenging, too
As common as concussion is, it can—at times—be challenging to recognize. These tips can help:
Do a mental status exam. Ask the patient what his name is, what today’s date is, and where he is now. See if he can repeat 3 words immediately after you say them and then 5 minutes later. Can he spell the word “world” backwards?
Get the story. If the patient can’t remember the incident or isn’t able to tell you about it, try to get the details from a witness. If the patient is able to talk, ask if she or he remembers what was happening just before the accident and afterwards. Does the patient seem to have difficulty concentrating on your questions? Seem alert, or confused? Can the patient (or a witness) tell you how the injury occurred, how severe the impact was, and whether there was loss of consciousness—all important factors in the diagnosis.1 Has the patient ever had a concussion before? Were there any sequelae?
Perform a neurologic evaluation. Assess cranial nerves and cerebral, cerebellar, and peripheral nerve function. Check pupils for reactivity and symmetry. See whether the patient has full and appropriate eye movement in all directions. Assess grip strength and muscle strength in arm and leg flexion and extension. Test for abnormalities in reactions to pinprick, temperature, and vibration in all extremities. Ask the patient to close his eyes, extend the arms with palms up, and hold the position for 20 to 30 seconds. If the patient fails this assessment for pronator drift, consider a diagnosis of muscle weakness or cerebellar disease. Check gait for ataxia and speech for fluency and coherence.
When a CT is required
While most cases of suspected concussion do not require imaging, it is appropriate for patients like this 52-year-old man, who had a history of assault and presented with dizziness and vomiting. His CT scan revealed mild hyperattenuation adjacent to the cranial shadow—a finding suggestive of a subarachnoid hemorrhage in the region.
When trauma’s an old story
Patients with head injury do not always seek emergency care. Whenever an office patient tells you about a head injury, however minor it seems, you should always assess for concussion. The diagnosis may be useful in guiding treatment and prevention strategies in the future. It may also help you recognize post-concussive symptoms, which can occur weeks or months after the trauma and cause significant morbidity.
Does your patient really need imaging?
Imaging is usually not necessary for diagnosis when the mental status and neurologic examinations are negative. Abnormal imaging scans are rare in cases of suspected concussion, showing up in fewer than 10% of computed tomography (CT) scans and 30% of magnetic resonance images (MRIs).11 You wouldn’t order imaging for Max, as he passed his neurologic exam with flying colors. However, if he had remained unconscious for a longer period, his mental status changes had continued, or he had neurologic symptoms that persisted for more than a week, you would order neuroimaging to rule out additional pathology.10,12 Neuroimaging may also be indicated in cases of particularly forceful injury—a fall from a height greater than 3 feet, for example, or a pedestrian hit by a car—or for a patient with an open, depressed, or suspected basal skull fracture.
In addition, imaging studies should be done for patients with a score of less than 15 on the Glasgow coma scale, retrograde amnesia for more than 30 minutes before the accident, or more than 2 episodes of vomiting. Imaging options include a CT scan without contrast to evaluate for intracranial bleeding or an MRI without contrast to test for smaller intracranial bleeds or axonal injury.13,14
CT scans are quick, generally available, and reasonably inexpensive, but may not detect all relevant abnormalities. MRIs are more sensitive and better able to detect areas of contusion, petechial hemorrhage, and axonal injury, but are less accessible in emergencies and cost a great deal more.13,14
New research suggests that patients with prolonged neurologic sequelae may benefit from single proton emission computed tomography (SPECT) or positron emission tomography (PET) in addition to conventional CT and MRI studies.13,15 SPECT studies use radioactive tracers that can cross the blood-brain barrier to estimate cerebral blood flow; decreased cerebral blood flow indicates areas of brain damage.11 PET scans are more expensive than SPECT, but have the advantage of being able to demonstrate oxygen and glucose metabolism, which are more sensitive indicators of brain damage. While SPECT and PET images are seldom used in diagnosing concussion, they can benefit patients who continue to have neurologic deficits that require further definition of the areas of brain injury.
How to fine-tune concussion care
First, grade the concussion. Concussion scales are a useful guide for making treatment decisions.4,5,10 The AAN scale presented in TABLE 1 is the most widely used. Keep in mind, though, that this scale is scheduled for revision.1
You conclude from your examination of Max that he does have a concussion, and that his loss of consciousness indicates a grade 3, even though his symptoms of dizziness and confusion lasted less than 15 minutes.
Initiate monitoring. This essential aspect of concussion management can take place at home for most patients, with hospitalization necessary for only a few. Max can go home. You know his parents well, and they’re competent to follow a monitoring protocol. For the first 24 hours, you tell them to wake Max up every 2 hours, so that they can pick up any change in his symptoms without delay. If they have difficulty waking him or he develops signs and symptoms such as vomiting or severe headache, you tell them to call you and bring Max back to the hospital.
Patients who should be monitored in the hospital are those with seizures, evidence of intracranial bleeding or cerebral edema on CT scan, or a history of taking oral anticoagulants. So should any patients whose living situation is not reliable for adequate home monitoring—homeless patients or those with a chaotic home life, for instance.
Return to usual activities: When to say Yes, when to say No. You advise Max’s parents to keep him home from school and have him take a break from homework. Concentrating on schoolwork can aggravate concussive symptoms.12 Strenuous physical activity is out, too, and he shouldn’t be alone for more than short periods until all his symptoms subside.
Sports concussions have their own imperatives, based on the grade of the concussion. Patients with a grade 1 concussion can return to the playing field within 15 minutes, as long as their neurologic exam is normal and they have no symptoms. With a grade 2 concussion, the player should be asymptomatic for 1 week before going back to normal activities. A grade 3 concussion requires that the player stay off the field until he or she has been asymptomatic for 2 weeks.4 This recommendation would apply to Max, who had a grade 3 concussion.
TABLE 1
AAN concussion grading scale1
| GRADE 1 | GRADE 2 | GRADE 3 | |
|---|---|---|---|
| Loss of consciousness | No | No | Yes |
| Symptoms | Lasting <15 minutes | Lasting >15 minutes | Lasting >15 minutes |
When symptoms linger
Most concussion patients will recover fairly rapidly. Unfortunately, however, some 38% of patients who have experienced a concussion with loss of consciousness continue to be plagued with what is called post-concussive syndrome (PCS).16 The International Classification of Diseases (ICD-10, 2nd ed.) defines PCS as a combination of signs and symptoms that occur within 4 weeks of head trauma with loss of consciousness. These include headache, fatigue, depression, emotional lability, difficulty concentrating, insomnia, and a preoccupation with symptoms with a fear of brain damage. The incidence of PCS using criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) is similar to that documented with the ICD-10, which suggests that either definition could be used to evaluate for PCS.17
By far the most common of these post-concussive symptoms is headache, reported by nearly 80% of patients with PCS and occurring most often in those who are headache-prone.18 That’s the case with Max, who has always had a problem with headaches and returns to your office a month after the accident complaining of persistent head pain.
Most post-concussive headaches resolve with rest and over-the-counter medications such as acetaminophen or ibuprofen. You may also consider prescribing antidepressants, particularly the selective serotonin reuptake inhibitor sertraline, which has been shown to decrease the vertigo, blurred vision, visual changes, and headache often associated with PCS.16,19,20 Start sertraline at a dosage of 25 mg/d, then titrate after a week to the recommended 50 mg/d.19 If symptoms persist, slowly titrate to the maximum dose of 200 mg/d while carefully monitoring for potential side effects.19
Patients with post-concussive status migrainosus, a headache lasting longer than 3 days that is unresponsive to conventional treatment, may benefit from a short course of corticosteroids.4 Additional treatment options include triptans, anticonvulsants, and β-blockers, although none of these options has been backed up by a large-scale, randomized controlled trial.16,20
Vigilance needed in cases of repeated trauma
Patients who experience repeated head trauma require particular attention. They are more likely to have detectable signs and symptoms and PCS.21 Also, they are more likely to incur second impact syndrome (SIS), a cascade of symptoms that can occur within 2 to 5 minutes of sustaining a second blow. SIS patients experience a rapid and diffuse cerebral edema, which can lead to brain herniation and death.3 SIS is a primary risk in sports like football, when players are allowed to return to the field too early. TABLE 2 sets out the special diagnostic criteria the AAN has formulated for managing patients who experience repeated concussions.
TABLE 2
Multiple concussions: When can your patient return to play?1
| SYMPTOM SEVERITY | SECOND CONCUSSION | THIRD CONCUSSION (OR MORE) |
|---|---|---|
| Concussive symptoms lasting <15 minutes | Return to play when asymptomatic for 1 week | Return to play when asymptomatic for 1 week |
| Post-traumatic amnesia <30 minutes, without loss of consciousness | Return to play when asymptomatic for 2 weeks | Return to play when asymptomatic for 1 month |
| Post-traumatic amnesia >30 minutes or loss of consciousness | Return to play when asymptomatic for 1 month | Discourage return to play indefinitely |
Help prevent concussion
In your role as educator, you can inform patients, school officials, and community leaders about the importance of protective equipment such as sports helmets, seat belts, and air bags in reducing the incidence of concussion.9,15 As a family physician, you have a special opportunity to teach parents, teachers, coaches, and players to recognize the signs and symptoms of concussion, understand the risks, and stop players from returning to sports activities prematurely.4
CORRESPONDENCE
Amanda McConnell, DO, MPH, Grandview Hospital, Office of Medical Education, 405 Grand Avenue, Dayton, OH 45405; [email protected]
- Consider any alteration of mental status that follows a trauma to be a concussion, whether or not there is also a loss of consciousness (A).
- Don’t order neuroimaging routinely; it is not necessary for diagnosing concussion. Neuroimaging is important, however, for patients who exhibit prolonged unconsciousness, focal neurologic deficits, or worsening symptoms (A).
- Treat post-concussive headache, a common complaint, with acetaminophen or ibuprofen (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
It’s 7 AM and you’re just finishing breakfast when you get a call from an emergency room (ER) resident telling you that Max, a 14-year-old patient of yours, has just been brought in by ambulance after a car accident. When the EMTs arrived at the scene, the car was totaled. The driver, Max’s older brother, had no injuries other than a minor abrasion on his nose, but Max was unconscious.
By the time you get to the hospital, Max is sitting up and talking. He says his head aches and he’s feeling dizzy. He’s having difficulty comprehending what he’s doing in the ER, doesn’t know how he got there, and has no memory of the accident. He has a small contusion on his forehead, but no other apparent injuries.
You question Max’s brother, and he tells you he hydroplaned while driving in the rain, and skidded into a telephone pole. He was OK, but Max was slumped down in his seat and unresponsive. The driver of another car had seen the accident and called 911.
How would you evaluate Max’s condition? What tests would you perform? And what would you tell his worried parents?
A diagnosis with variable—and often subtle—symptoms
Max’s situation fits the American Academy of Neurology’s (AAN) definition of concussion, ie, an alteration in mental status following a trauma that may or may not result in a loss of consciousness.1 According to the AAN, this change in mental status usually lasts less than 24 hours and may be coupled with symptoms of vertigo, headache, nausea, vomiting, tinnitus, photophobia, blurred vision, and anterograde or retrograde amnesia.2-5
The presenting signs and symptoms of concussion are so variable that the condition can be difficult to diagnose. Many patients display nothing more than a so-called vacant stare. Others experience only minor symptoms, such as headache or nausea. Max’s loss of consciousness is relatively rare, occurring in only about 10% of concussion cases.6
At least 1.4 million concussions occur in the United States each year, according to the Centers for Disease Control and Prevention (CDC).7,8 The incidence, though, is probably greater than the CDC reports, because so many cases are unrecognized or unreported. Falls, motor vehicle accidents, and sports injuries are the leading causes, with some 250,000 football-related concussions reported each year.4,7,9,10
Common, yes, but challenging, too
As common as concussion is, it can—at times—be challenging to recognize. These tips can help:
Do a mental status exam. Ask the patient what his name is, what today’s date is, and where he is now. See if he can repeat 3 words immediately after you say them and then 5 minutes later. Can he spell the word “world” backwards?
Get the story. If the patient can’t remember the incident or isn’t able to tell you about it, try to get the details from a witness. If the patient is able to talk, ask if she or he remembers what was happening just before the accident and afterwards. Does the patient seem to have difficulty concentrating on your questions? Seem alert, or confused? Can the patient (or a witness) tell you how the injury occurred, how severe the impact was, and whether there was loss of consciousness—all important factors in the diagnosis.1 Has the patient ever had a concussion before? Were there any sequelae?
Perform a neurologic evaluation. Assess cranial nerves and cerebral, cerebellar, and peripheral nerve function. Check pupils for reactivity and symmetry. See whether the patient has full and appropriate eye movement in all directions. Assess grip strength and muscle strength in arm and leg flexion and extension. Test for abnormalities in reactions to pinprick, temperature, and vibration in all extremities. Ask the patient to close his eyes, extend the arms with palms up, and hold the position for 20 to 30 seconds. If the patient fails this assessment for pronator drift, consider a diagnosis of muscle weakness or cerebellar disease. Check gait for ataxia and speech for fluency and coherence.
When a CT is required
While most cases of suspected concussion do not require imaging, it is appropriate for patients like this 52-year-old man, who had a history of assault and presented with dizziness and vomiting. His CT scan revealed mild hyperattenuation adjacent to the cranial shadow—a finding suggestive of a subarachnoid hemorrhage in the region.
When trauma’s an old story
Patients with head injury do not always seek emergency care. Whenever an office patient tells you about a head injury, however minor it seems, you should always assess for concussion. The diagnosis may be useful in guiding treatment and prevention strategies in the future. It may also help you recognize post-concussive symptoms, which can occur weeks or months after the trauma and cause significant morbidity.
Does your patient really need imaging?
Imaging is usually not necessary for diagnosis when the mental status and neurologic examinations are negative. Abnormal imaging scans are rare in cases of suspected concussion, showing up in fewer than 10% of computed tomography (CT) scans and 30% of magnetic resonance images (MRIs).11 You wouldn’t order imaging for Max, as he passed his neurologic exam with flying colors. However, if he had remained unconscious for a longer period, his mental status changes had continued, or he had neurologic symptoms that persisted for more than a week, you would order neuroimaging to rule out additional pathology.10,12 Neuroimaging may also be indicated in cases of particularly forceful injury—a fall from a height greater than 3 feet, for example, or a pedestrian hit by a car—or for a patient with an open, depressed, or suspected basal skull fracture.
In addition, imaging studies should be done for patients with a score of less than 15 on the Glasgow coma scale, retrograde amnesia for more than 30 minutes before the accident, or more than 2 episodes of vomiting. Imaging options include a CT scan without contrast to evaluate for intracranial bleeding or an MRI without contrast to test for smaller intracranial bleeds or axonal injury.13,14
CT scans are quick, generally available, and reasonably inexpensive, but may not detect all relevant abnormalities. MRIs are more sensitive and better able to detect areas of contusion, petechial hemorrhage, and axonal injury, but are less accessible in emergencies and cost a great deal more.13,14
New research suggests that patients with prolonged neurologic sequelae may benefit from single proton emission computed tomography (SPECT) or positron emission tomography (PET) in addition to conventional CT and MRI studies.13,15 SPECT studies use radioactive tracers that can cross the blood-brain barrier to estimate cerebral blood flow; decreased cerebral blood flow indicates areas of brain damage.11 PET scans are more expensive than SPECT, but have the advantage of being able to demonstrate oxygen and glucose metabolism, which are more sensitive indicators of brain damage. While SPECT and PET images are seldom used in diagnosing concussion, they can benefit patients who continue to have neurologic deficits that require further definition of the areas of brain injury.
How to fine-tune concussion care
First, grade the concussion. Concussion scales are a useful guide for making treatment decisions.4,5,10 The AAN scale presented in TABLE 1 is the most widely used. Keep in mind, though, that this scale is scheduled for revision.1
You conclude from your examination of Max that he does have a concussion, and that his loss of consciousness indicates a grade 3, even though his symptoms of dizziness and confusion lasted less than 15 minutes.
Initiate monitoring. This essential aspect of concussion management can take place at home for most patients, with hospitalization necessary for only a few. Max can go home. You know his parents well, and they’re competent to follow a monitoring protocol. For the first 24 hours, you tell them to wake Max up every 2 hours, so that they can pick up any change in his symptoms without delay. If they have difficulty waking him or he develops signs and symptoms such as vomiting or severe headache, you tell them to call you and bring Max back to the hospital.
Patients who should be monitored in the hospital are those with seizures, evidence of intracranial bleeding or cerebral edema on CT scan, or a history of taking oral anticoagulants. So should any patients whose living situation is not reliable for adequate home monitoring—homeless patients or those with a chaotic home life, for instance.
Return to usual activities: When to say Yes, when to say No. You advise Max’s parents to keep him home from school and have him take a break from homework. Concentrating on schoolwork can aggravate concussive symptoms.12 Strenuous physical activity is out, too, and he shouldn’t be alone for more than short periods until all his symptoms subside.
Sports concussions have their own imperatives, based on the grade of the concussion. Patients with a grade 1 concussion can return to the playing field within 15 minutes, as long as their neurologic exam is normal and they have no symptoms. With a grade 2 concussion, the player should be asymptomatic for 1 week before going back to normal activities. A grade 3 concussion requires that the player stay off the field until he or she has been asymptomatic for 2 weeks.4 This recommendation would apply to Max, who had a grade 3 concussion.
TABLE 1
AAN concussion grading scale1
| GRADE 1 | GRADE 2 | GRADE 3 | |
|---|---|---|---|
| Loss of consciousness | No | No | Yes |
| Symptoms | Lasting <15 minutes | Lasting >15 minutes | Lasting >15 minutes |
When symptoms linger
Most concussion patients will recover fairly rapidly. Unfortunately, however, some 38% of patients who have experienced a concussion with loss of consciousness continue to be plagued with what is called post-concussive syndrome (PCS).16 The International Classification of Diseases (ICD-10, 2nd ed.) defines PCS as a combination of signs and symptoms that occur within 4 weeks of head trauma with loss of consciousness. These include headache, fatigue, depression, emotional lability, difficulty concentrating, insomnia, and a preoccupation with symptoms with a fear of brain damage. The incidence of PCS using criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) is similar to that documented with the ICD-10, which suggests that either definition could be used to evaluate for PCS.17
By far the most common of these post-concussive symptoms is headache, reported by nearly 80% of patients with PCS and occurring most often in those who are headache-prone.18 That’s the case with Max, who has always had a problem with headaches and returns to your office a month after the accident complaining of persistent head pain.
Most post-concussive headaches resolve with rest and over-the-counter medications such as acetaminophen or ibuprofen. You may also consider prescribing antidepressants, particularly the selective serotonin reuptake inhibitor sertraline, which has been shown to decrease the vertigo, blurred vision, visual changes, and headache often associated with PCS.16,19,20 Start sertraline at a dosage of 25 mg/d, then titrate after a week to the recommended 50 mg/d.19 If symptoms persist, slowly titrate to the maximum dose of 200 mg/d while carefully monitoring for potential side effects.19
Patients with post-concussive status migrainosus, a headache lasting longer than 3 days that is unresponsive to conventional treatment, may benefit from a short course of corticosteroids.4 Additional treatment options include triptans, anticonvulsants, and β-blockers, although none of these options has been backed up by a large-scale, randomized controlled trial.16,20
Vigilance needed in cases of repeated trauma
Patients who experience repeated head trauma require particular attention. They are more likely to have detectable signs and symptoms and PCS.21 Also, they are more likely to incur second impact syndrome (SIS), a cascade of symptoms that can occur within 2 to 5 minutes of sustaining a second blow. SIS patients experience a rapid and diffuse cerebral edema, which can lead to brain herniation and death.3 SIS is a primary risk in sports like football, when players are allowed to return to the field too early. TABLE 2 sets out the special diagnostic criteria the AAN has formulated for managing patients who experience repeated concussions.
TABLE 2
Multiple concussions: When can your patient return to play?1
| SYMPTOM SEVERITY | SECOND CONCUSSION | THIRD CONCUSSION (OR MORE) |
|---|---|---|
| Concussive symptoms lasting <15 minutes | Return to play when asymptomatic for 1 week | Return to play when asymptomatic for 1 week |
| Post-traumatic amnesia <30 minutes, without loss of consciousness | Return to play when asymptomatic for 2 weeks | Return to play when asymptomatic for 1 month |
| Post-traumatic amnesia >30 minutes or loss of consciousness | Return to play when asymptomatic for 1 month | Discourage return to play indefinitely |
Help prevent concussion
In your role as educator, you can inform patients, school officials, and community leaders about the importance of protective equipment such as sports helmets, seat belts, and air bags in reducing the incidence of concussion.9,15 As a family physician, you have a special opportunity to teach parents, teachers, coaches, and players to recognize the signs and symptoms of concussion, understand the risks, and stop players from returning to sports activities prematurely.4
CORRESPONDENCE
Amanda McConnell, DO, MPH, Grandview Hospital, Office of Medical Education, 405 Grand Avenue, Dayton, OH 45405; [email protected]
1. Practice parameter: the management of concussion in sports (summary statement) Report of the Quality Standards Subcommittee. Neurology. 1997;48:581-585.
2. Anderson T, Heitger M, Macleod AD. Concussion and mild head injury. Pract Neurol. 2006;6:342-357.
3. Delaney JS, Abuzeyad F, Correa JA, et al. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29:189-197.
4. Harmon KG. Assessment and management of concussion in sports. Am Fam Physician. 1999;60:887-894.
5. Kushner DS. Concussion in sports: minimizing the risk of complications. Am Fam Physician. 2001;64:1007-1014.
6. Cantu RC. Head injuries in sport. Br J Sports Med. 1996;30:289-296.
7. Division of Injury and Disability Outcomes and Programs National Center for Injury Prevention and Control Centers for Disease Control and Prevention Department of Health and Human Services. “Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths.” January 2006. Available at: http://www.cdc.gov/ncipc/pub-res/TBI_in_US_04/TBI_ ED.htm. Accessed November 10, 2007.
8. Centers for Disease Control and Prevention Non-fatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. Morb Mortal Wkly Rep. 2007;56:733-737.
9. Hillary FG, Schatz P, Moelter ST, et al. Motor vehicle collision factors influence severity and type of TBI. Brain Injury. 2002;16:729-741.
10. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association position statement: management of sport-related concussion. J Athl Train. 2004;39:280-297.
11. Ropper AH, Gorson KC. Concussion. N Engl J Med. 2007;356:166-172.
12. McCrory P, Meeuwisse W, Johnston K, et al. Summary and agreement statement of the 3rd international conference on concussion in sport, November 2008. Br J Sports Med. 2009;43:76-84.
13. Bazarian JJ, Blyth B, Cimpello L. Bench to bedside: evidence for brain injury after concussion—looking beyond the computed tomography scan. Acad Emerg Med. 2006;13:199-214.
14. Hughes DG, Jackson A, Mason DL, et al. Abnormalities on magnetic resonance imaging seen acutely following a mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550-558.
15. Ryan L, Warden D. Post concussion syndrome. Int Rev Psychiatry. 2003;15:310-316.
16. Mittenberg W, Canyock E, Condit D, et al. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23:829-836.
17. McCauley SR, Boake C, Pedroza C, et al. Post-concussional disorder: are the DSM-IV criteria an improvement over the ICD-10? J Nerv Ment Dis. 2005;193:540-550.
18. Mickeviciene D, Schrader H, Obelieniene D, et al. A controlled prospective inception cohort study on the post-concussion syndrome outside the medicolegal context. Eur J Neurol. 2004;11:411-419.
19. Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12:226-236.
20. McAllister TW, Arciniegas D. Evaluation and treatment of postconcussive symptoms. NeuroRehabilitation. 2002;17:265-283.
21. Whiteside J. Management of head and neck injury by the sideline physician. Am Fam Physician. 2006;74:1357-1362.
1. Practice parameter: the management of concussion in sports (summary statement) Report of the Quality Standards Subcommittee. Neurology. 1997;48:581-585.
2. Anderson T, Heitger M, Macleod AD. Concussion and mild head injury. Pract Neurol. 2006;6:342-357.
3. Delaney JS, Abuzeyad F, Correa JA, et al. Recognition and characteristics of concussions in the emergency department population. J Emerg Med. 2005;29:189-197.
4. Harmon KG. Assessment and management of concussion in sports. Am Fam Physician. 1999;60:887-894.
5. Kushner DS. Concussion in sports: minimizing the risk of complications. Am Fam Physician. 2001;64:1007-1014.
6. Cantu RC. Head injuries in sport. Br J Sports Med. 1996;30:289-296.
7. Division of Injury and Disability Outcomes and Programs National Center for Injury Prevention and Control Centers for Disease Control and Prevention Department of Health and Human Services. “Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths.” January 2006. Available at: http://www.cdc.gov/ncipc/pub-res/TBI_in_US_04/TBI_ ED.htm. Accessed November 10, 2007.
8. Centers for Disease Control and Prevention Non-fatal traumatic brain injuries from sports and recreation activities—United States, 2001-2005. Morb Mortal Wkly Rep. 2007;56:733-737.
9. Hillary FG, Schatz P, Moelter ST, et al. Motor vehicle collision factors influence severity and type of TBI. Brain Injury. 2002;16:729-741.
10. Guskiewicz KM, Bruce SL, Cantu RC, et al. National Athletic Trainers’ Association position statement: management of sport-related concussion. J Athl Train. 2004;39:280-297.
11. Ropper AH, Gorson KC. Concussion. N Engl J Med. 2007;356:166-172.
12. McCrory P, Meeuwisse W, Johnston K, et al. Summary and agreement statement of the 3rd international conference on concussion in sport, November 2008. Br J Sports Med. 2009;43:76-84.
13. Bazarian JJ, Blyth B, Cimpello L. Bench to bedside: evidence for brain injury after concussion—looking beyond the computed tomography scan. Acad Emerg Med. 2006;13:199-214.
14. Hughes DG, Jackson A, Mason DL, et al. Abnormalities on magnetic resonance imaging seen acutely following a mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550-558.
15. Ryan L, Warden D. Post concussion syndrome. Int Rev Psychiatry. 2003;15:310-316.
16. Mittenberg W, Canyock E, Condit D, et al. Treatment of post-concussion syndrome following mild head injury. J Clin Exp Neuropsychol. 2001;23:829-836.
17. McCauley SR, Boake C, Pedroza C, et al. Post-concussional disorder: are the DSM-IV criteria an improvement over the ICD-10? J Nerv Ment Dis. 2005;193:540-550.
18. Mickeviciene D, Schrader H, Obelieniene D, et al. A controlled prospective inception cohort study on the post-concussion syndrome outside the medicolegal context. Eur J Neurol. 2004;11:411-419.
19. Fann JR, Uomoto JM, Katon WJ. Sertraline in the treatment of major depression following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2000;12:226-236.
20. McAllister TW, Arciniegas D. Evaluation and treatment of postconcussive symptoms. NeuroRehabilitation. 2002;17:265-283.
21. Whiteside J. Management of head and neck injury by the sideline physician. Am Fam Physician. 2006;74:1357-1362.
A survivor’s guide for primary care physicians
- Building strong relationships among physicians and staff improves the practice’s ability to deal with the uncertainties of a rapidly changing environment (B).
- Interacting proactively with the economic, social, political, and cultural environment—the practice landscape—provides opportunities for adaptation and ongoing learning (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“Everyday Primary Care,” a popular, urban 3-physician family medicine office, has served mostly middle- and working-class people for more than 25 years. Most of the patients have grown older with Drs. Newman and Cope and now have a substantial chronic disease burden. Dr. Varimore, Dr. Cope’s son, has recently joined the practice. He replaced a long-time partner who left in frustration to do emergency medicine.
On a typical day, Dr. Cope enters the crowded waiting room, sighs, and walks quickly toward the nurses’ station where her third scheduled patient has just arrived; her first 2 patients are already waiting in examining rooms. In her tiny office, stacks of charts, phone messages, and forms await her attention. Phones ring constantly. Rushing to see her first patient, Dr. Cope squeezes past her nursing assistant in the narrow hallway. She catches a glimpse of her partner, Dr. Newman, at the end of the corridor. They grunt a word of greeting, but say nothing more. In fact, the physicians and their staff have barely spoken to each other in days.
The 2 older physicians were hopeful that Dr. Varimore would infuse fresh energy into the practice, but the only thing that has changed with his arrival is an increase in the number of patients they see and the expenses of running the office. When the door finally closes at the end of a long day, everyone leaves feeling exhausted and alone.
A toxic atmosphere
The situation at Everyday Primary Care is not unusual.1 These are unhealthy times for most primary care practices. Despite the critical role that primary care is expected to play in health care reform, there is tremendous uncertainty about the future viability of primary care practice.1-6 An alarming number of primary care physicians are leaving practice or taking early retirement as frustration and exhaustion move deeply into our community.1,7,8 Staff turnover is high and disruptive. Primary care physicians feel buffeted by conflicting patient demands, insurance coverage restrictions, inadequate Medicare reimbursement, multiple and often inconsistent practice guidelines, and onerous government regulations. Primary care practices suffer from a culture of despair that impedes decision-making. These practices—and the physicians who struggle to keep them viable—need to develop resilience to survive in this hostile climate and improve the quality of care they provide.
Research-based strategies. This article suggests strategies for primary care practices to move forward—whatever proposed reforms emerge from the current debate. The strategies we propose derive from specific, concrete observations gathered during a 15-year program of research that included nearly 500 primary care offices.9-16 (In fact, Everyday Primary Care is an actual practice that participated in 1 of our studies, though we’ve changed its name and the names of the physicians.) Our research was funded by the National Institutes of Health (NIH) and included both descriptive and intervention projects. Our studies provided in-depth descriptions of a wide variety of primary care practices, as well as new models for describing change.10,14,15 The practices varied in how they delivered preventive services, in their cancer-related prevention and screening activities, and in the way they managed chronic disease.11,14,17-19 Yet across all these variations, we found a pattern in which educated, well-trained professionals and staff wanted to provide good care, but found themselves thwarted in their efforts to succeed.
What’s going on here? We sought to understand what was really happening in these primary care practices and to formulate strategies to help them become better for patients, staff, and clinicians.
We came up with 2 fundamental insights:
- Practices that focus on building strong internal relationships are better able to deal with surprise and uncertainty.
- Practices that are proactive in interacting with the changing environment will find multiple ways to achieve effective health care delivery.
Work on building those relationships
In our research, we repeatedly observed that careful attention to the relationships among all the people (clinical and nonclinical staff) working within each practice is critical to improving practice processes and outcomes.20 We wanted to learn why relationships mattered so much and how they could be improved. What we found can best be explained by taking another look at Everyday Primary Care.
The physician-owners of everyday Primary Care, feeling stressed out and recognizing that “things are not good here,” signed up to participate in 1 of our studies. Participation required allowing an outside facilitator to observe practice operations and conduct open-ended interviews with physicians and staff over a 2-week period, followed by a series of 12 weekly meetings. In addition, physicians and staff agreed to fill out multiple surveys during the study process and allow researchers to audit the charts of randomly selected patient samples.
One year after Everyday Primary Care signed up, the office space was still cramped, the financial situation was no better, and environmental pressures were continuing to mount. And yet, the practice felt like a different place, one filled with energy and hope. What had happened?
RAP, huddles, effective teams. Most importantly, the quality and types of relationships within the practice had changed. At our suggestion, the practice formed a RAP (reflective adaptive process) team under the guidance of a facilitator—a nurse we trained in basic facilitation skills, including effective meeting strategies, brainstorming, and conflict resolution. The team consisted of physician leaders (both Drs. Cope and Varimore attended all meetings), the practice manager, representatives from each part of the practice (billing, front desk, nursing staff, insurance clerk), and a patient.15 The RAP intervention was designed to provide members with time and space to reflect and opportunities to learn the value of communication, respectful interaction, and listening to diverse opinions and perspectives.20 The team met with the facilitator for 1 hour every week, reviewed the practice’s vision, and developed and implemented strategies for solving prioritized practice issues and problems.
Brainstorming helped identify recurrent problems. As the RAP meetings progressed, it became clear that despite the close quarters, each part of the practice was isolated from the others and all team members were frustrated by their inability to influence the lead physician, Dr. Cope. Over time, the RAP meetings changed the relationship patterns and the quality of communication, thus helping the practice move forward and get unstuck. Dr. Cope repeatedly commented, “I didn’t know that,” as staff shared their concerns and challenges. For example, Dr. Cope was amazed when the front desk described the amount of time and degree of disruption caused by drug reps constantly coming into the office. Together, the team was able to come up with a solution—setting aside a special time for drug reps, rather than allowing them to arrive whenever they chose—that worked for physicians and staff alike.
Our current project notes from Everyday Primary Care reflect a very different and vibrant practice, in which the atmosphere is charged with hope and everyone reports being more relaxed—though just as busy. Office processes have improved and space is less cluttered. Chart audit scores reveal improved quality of chronic care and preventive services. Because practice members have learned to communicate across the barriers of job classification and hierarchy, they are able to solve problems as they arise without allowing things to fester. These improved relationships led to an enhanced understanding of complex issues like patient triage and scheduling and more numerous and accurate memories of how the practice has operated over the years.21-23
Our research has taught us that practices that pay attention to building strong relationships are better able to deal with the surprise and uncertainty that characterize modern health care delivery.24-26 The primary care management literature has highlighted a number of practical strategies for enhancing relationships and communication, including the use of RAP teams, huddles, effective team meetings, and high-performing clinical teams.15,27-29 In addition, we refer the reader to The Team Handbook, 3rd ed., by Peter R. Scholtes, Brian L. Joiner, and Barbara J. Streibel. The handbook contains a wide range of practical teambuilding strategies in an easily accessible style.30 FIGURE 1 summarizes 5 tips for building critical relationships in your own practice.
FIGURE 1
5 tips for building critical relationships
Interact with the “local fitness landscape”
Our second insight is that practices must learn to interact with what we call the “local fitness landscape.”31-33 To understand what that term implies, imagine your hometown with multiple primary care offices of different sizes, a variety of specialty practices, 2 or 3 competing hospital systems, multiple insurance options, businesses, housing clusters representing different social classes, schools, banks, scattered farms, industries, waterways, animals and plants, transportation systems, and political and religious institutions. The totality of all these elements is the local fitness landscape.
The landscape is a dynamic, fluid system within which the component parts respond to and influence each other. Everyday Primary Care is embedded in such a landscape, acting on and being acted upon by other parts of the system. Unfortunately, like most practices we observed, Everyday tended to ignore or resist the local fitness landscape rather than trying to understand and adapt to it. The physicians felt trapped by environmental constraints and frustrated by the turbulence they observed.
What constraints does Everyday Primary Care face? When we first visited this practice, we could see that the facility was too small for the growing volume of patients. The physician-owners knew the space wasn’t conducive to optimum patient care, but told us they could not afford to pay higher rent for larger quarters. Similarly, they understood the potential of electronic medical records (EMRs), but hadn’t been able to find time or money to support the transition. Rising overhead expenses were outpacing practice productivity, as measured in the number of patients seen per day. What was worse, the need to see so many patients was making it more difficult to address the needs of their aging and medically complex patient population.
Looking outward can help
Despite these constraints, internal conversations generated through RAP sessions led practice staff to reach out to other physicians and physician organizations for information. They compared notes with other practices on questions like how their computerized billing system functions, or how to word a letter to patients announcing a new policy on prescription refills. These external conversations expanded the practice’s notions of what was possible and gave them opportunities to share information and learn of new approaches other practices were developing. The result was a newfound level of energy and hope within the practice and exposure to new ideas from the outside.
Learning from the landscape. Numerous conversations with physician organizations, neighboring practices, and a local hospital system yielded new solutions for recalcitrant problems: How to make better use of existing office space, for example, and where to find support for long-range strategic planning. These contacts exposed Everyday to the experiences of other practices with EMRs, and the practice’s physicians have now selected and implemented their own system. The practice was finally able to address the inevitable retirement of 1 of the physicians and now has a succession plan in place. In sum, Everyday learned how to interact and adjust to the changing environment and no longer worried about survival.
Practices co-evolve with all the other systems in a constantly changing fitness landscape. As practice members navigate the local fitness landscapes, they make decisions among competing demands and priorities to maintain their own financial viability and internal stability. What seems to characterize innovative primary care practices is that they don’t wait to react to the next environmental change. Rather, by paying attention to local relationships, they improve the chances that co-evolution will move the practice in desired ways.
Making much-needed connections
There are a number of ways that practices can engage their fitness landscapes, but perhaps the most powerful is creating the time and space to meet with colleagues—either locally or regionally. The most effective approaches are likely to be those that allow sharing experiences and ideas over time, rather than one-time, opportunistic conversations that occur, say, at national and state academy meetings. Practices can participate in activities of regional Practice-Based Research Networks, local residency programs, or even form their own local support group.34,35 To learn how you can connect with a regional Practice-Based Research Network, go to the AHRQ website (http://pbrn.ahrq.gov/portal/server.pt). FIGURE 2 summarizes 4 strategies for reaching out to your local landscape.
FIGURE 2
4 strategies for reaching out to your local landscape
One size doesn’t fit all: Strategic alternatives
When practices build critical relationships and pay attention to their local fitness landscape, they co-evolve improvements that make sense in the context of their unique characteristics and circumstances. Our research shows that practices use a range of alternative strategies to meet the needs of patients, their communities, and themselves. For example, while we have observed primary care offices using EMRs that have achieved high levels of adherence to diabetes guidelines, we have also found high adherence rates in practices that use paper charts.19 We have seen different, successful approaches to the delivery of preventive health services.11 Some practices involve staff in assuring protocol adherence and others don’t. Some use reminder systems and others don’t. Several practices with higher rates of preventive service delivery use none of these. A recent evaluation of 15 case studies of family practices using teams to implement the chronic care model showed the value of different types of teams in different practices.36
Variability and standardization. The emergence of processes and outcome measures designed to meet the needs of a particular local setting (fitness landscape) appeals to our sense of equity and common sense. Yet variations like these fly in the face of prevailing models and guidelines that emphasize standardized processes. Many health plans and provider organizations insist on evidence-based “best practices” and “optimized models” for delivering primary care.37-39 They assume that if we know the goals, there is a best way to get everyone to achieve them.
A better strategy is to determine when variability and tailoring are more appropriate and then use standardization to help create more time for those processes that require variation. Thus, the practice can use a standardized protocol to turn over immunizations to staff in order to free clinicians to spend more time interacting directly with patients.
Multiple pathways to excellence. Medical practice is full of surprises and complexities. We used to believe that the right tools in the hands of accountable individuals using good management systems would produce best practice outcomes. But we have learned that no single right tool or individual management strategy works consistently in primary care.
We now believe that the relationship system within the practice is a critical element in creating an optimal healing environment. Practices with improved relationship systems exhibit more resilience in weathering a hostile environment, while discovering their own unique model of successful primary care. Such practices can thrive, provide improved quality of patient-centered care, and find professional satisfaction and joy in daily work. We hope that the health care reform plans now being debated in Congress will be informed by these insights and provide space for multiple models of care delivery to emerge.
CORRESPONDENCE
Benjamin F. Crabtree, PhD, Department of Family Medicine, Robert Wood Johnson Medical school, 1 World’s Fair Drive, First Floor, Somerset, NJ 08873; [email protected]
1. Showstack JA, Rothman AA, Hassmiller S. The Future of Primary Care. 1st ed. San Francisco: Jossey-Bass; 2004.
2. Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Institute of Medicine. Division of Health Care services. Committee on the Future of Primary Care. Donaldson MS. Primary Care: America’s Health in a New Era. Washington, DC: National Academy Press; 1996.
4. Martin JC, Avant RF, Bowman MA, et al. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(suppl 1):S3-S32.
5. Society of General Internal Medicine. Future of general internal medicine. Available at: www.sgim.org/index.cfm?section=site&pageId=366. Accessed July 7, 2009.
6. Grumbach K, Bodenheimer T. A primary care home for Americans: putting the house in order. JAMA. 2002;288:889-893.
7. Bodenheimer T, Grumbach K. Improving Primary Care: Strategies and Tools for a Better Practice. New York: Lange Medical Books/McGraw-Hill; 2007.
8. Moore G, Showstack J. Primary care medicine in crisis: toward reconstruction and renewal. Ann Intern Med. 2003;138:244-247.
9. Direct Observation of Primary Care (DOPC) Writing Group. Conducting the direct observation of primary care study. J Fam Pract. 2001;50:345-352.
10. Stange KC, Zyzanski SJ, Jaen CR, et al. Illuminating the ‘black box’. A description of 4454 patient visits to 138 family physicians. J Fam Pract. 1998;46:377-389.
11. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
12. Goodwin MA, Zyzanski SJ, Zronek S, et al. A clinical trial of tailored office systems for preventive service delivery. The Study to Enhance Prevention by Understanding Practice (STEP-UP). Am J Prev Med. 2001;21:20-28.
13. Stange KC, Goodwin MA, Zyzanski SJ, et al. Sustainability of a practice-individualized preventive service delivery intervention. Am J Prev Med. 2003;25:296-300.
14. Cohen D, McDaniel RR, Jr, Crabtree BF, et al. A practice change model for quality improvement in primary care practice. J Healthc Manag. 2004;49:155-168;discussion 169–170.
15. Stroebel CK, McDaniel RR, Jr., Crabtree BF, et al. How complexity science can inform a reflective process for improvement in primary care practices. Jt Comm J Qual Patient Saf. 2005;31:438-446.
16. Stange KC, Jaen CR, Flocke SA, et al. The value of a family physician. J Fam Pract. 1998;46:363-368.
17. Crabtree BF, Miller WL, Aita VA, et al. Primary care practice organization and preventive services delivery: a qualitative analysis. J Fam Pract. 1998;46:403-409.
18. Ohman-Strickland PA, Orzano AJ, Hudson SV, et al. Quality of diabetes care in family medicine practices: influence of nurse-practitioners and physician’s assistants. Ann Fam Med. 2008;6:14-22.
19. Crosson JC, Ohman-Strickland PA, Hahn KA, et al. Electronic medical records and diabetes quality of care: results from a sample of family medicine practices. Ann Fam Med. 2007;5:209-215.
20. Tallia AF, Lanham HJ, McDaniel RR, Jr., et al. 7 characteristics of successful work relationships. Fam Pract Manag. 2006;13:47-50.
21. Weick KE. Sensemaking in Organizations. Thousand Oaks, Calif: Sage Publications; 1995.
22. Weick KE, Sutcliffe KM. Managing the Unexpected: Assuring High Performance in an Age of Complexity. 1st ed. San Francisco: Jossey-Bass; 2001.
23. Weick KE. Making Sense of the Organization. Malden, Mass: Blackwell Publishers; 2001.
24. McDaniel RR, Jr, Jordan ME, Fleeman BF. Surprise, Surprise, Surprise! A complexity science view of the unexpected. Health Care Manage Rev. 2003;28:266-278.
25. Miller WL, Crabtree BF, McDaniel R, et al. Understanding change in primary care practice using complexity theory. J Fam Pract. 1998;46:369-376.
26. Miller WL, McDaniel RR, Jr., Crabtree BF, et al. Practice jazz: understanding variation in family practices using complexity science. J Fam Pract. 2001;50:872-878.
27. Stewart EE, Johnson BC. Improve office efficiency in mere minutes. Fam Pract Manag. 2007;14:27-29.
28. Shenkel R. How to make your meetings more productive. Fam Pract Manag. 2003;10:59-60.
29. Moore LG. Creating a high-performing clinical team. Fam Pract Manag. 2006;13:38-40.
30. Scholtes P, Joiner B, Streibel B. The Team Handbook. 3rd ed. Madison, Wisc: Oriel Incorporated; 2003.
31. Capra F. The Web of Life: A New Scientific Understanding of Living Systems. 1st Anchor Books ed. New York: Anchor Books; 1996.
32. Holland JH. Emergence: from Chaos to Order. Reading, Mass: Addison-Wesley; 1998.
33. Olson EE, Eoyang GH. Facilitating Organization Change: Lessons from Complexity Science. San Francisco: Jossey-Bass/Pfeiffer; 2001.
34. Lanier D. Primary care practice-based research comes of age in the United States. Ann Fam Med. 2005;3(suppl 1):S2-S4.
35. Mold JW, Peterson KA. Primary care practice-based research networks: working at the interface between research and quality improvement. Ann Fam Med. 2005;3(suppl 1):S12-S20.
36. Bodenheimer T. Building teams in primary care: Lessons from 15 case studies. Oakland, Calif: California HealthCare Foundation; July 2007. Available at: www.chcf.org/topics/chronicdisease/index.cfm?itemID=133375. Accessed March 18, 2008.
37. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff. 2002;21:80-90.
38. Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000-1005.
39. McGlynn EA. An evidence-based national quality measurement and reporting system. Med Care. 2003;41(suppl 1):S8-S15.
- Building strong relationships among physicians and staff improves the practice’s ability to deal with the uncertainties of a rapidly changing environment (B).
- Interacting proactively with the economic, social, political, and cultural environment—the practice landscape—provides opportunities for adaptation and ongoing learning (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“Everyday Primary Care,” a popular, urban 3-physician family medicine office, has served mostly middle- and working-class people for more than 25 years. Most of the patients have grown older with Drs. Newman and Cope and now have a substantial chronic disease burden. Dr. Varimore, Dr. Cope’s son, has recently joined the practice. He replaced a long-time partner who left in frustration to do emergency medicine.
On a typical day, Dr. Cope enters the crowded waiting room, sighs, and walks quickly toward the nurses’ station where her third scheduled patient has just arrived; her first 2 patients are already waiting in examining rooms. In her tiny office, stacks of charts, phone messages, and forms await her attention. Phones ring constantly. Rushing to see her first patient, Dr. Cope squeezes past her nursing assistant in the narrow hallway. She catches a glimpse of her partner, Dr. Newman, at the end of the corridor. They grunt a word of greeting, but say nothing more. In fact, the physicians and their staff have barely spoken to each other in days.
The 2 older physicians were hopeful that Dr. Varimore would infuse fresh energy into the practice, but the only thing that has changed with his arrival is an increase in the number of patients they see and the expenses of running the office. When the door finally closes at the end of a long day, everyone leaves feeling exhausted and alone.
A toxic atmosphere
The situation at Everyday Primary Care is not unusual.1 These are unhealthy times for most primary care practices. Despite the critical role that primary care is expected to play in health care reform, there is tremendous uncertainty about the future viability of primary care practice.1-6 An alarming number of primary care physicians are leaving practice or taking early retirement as frustration and exhaustion move deeply into our community.1,7,8 Staff turnover is high and disruptive. Primary care physicians feel buffeted by conflicting patient demands, insurance coverage restrictions, inadequate Medicare reimbursement, multiple and often inconsistent practice guidelines, and onerous government regulations. Primary care practices suffer from a culture of despair that impedes decision-making. These practices—and the physicians who struggle to keep them viable—need to develop resilience to survive in this hostile climate and improve the quality of care they provide.
Research-based strategies. This article suggests strategies for primary care practices to move forward—whatever proposed reforms emerge from the current debate. The strategies we propose derive from specific, concrete observations gathered during a 15-year program of research that included nearly 500 primary care offices.9-16 (In fact, Everyday Primary Care is an actual practice that participated in 1 of our studies, though we’ve changed its name and the names of the physicians.) Our research was funded by the National Institutes of Health (NIH) and included both descriptive and intervention projects. Our studies provided in-depth descriptions of a wide variety of primary care practices, as well as new models for describing change.10,14,15 The practices varied in how they delivered preventive services, in their cancer-related prevention and screening activities, and in the way they managed chronic disease.11,14,17-19 Yet across all these variations, we found a pattern in which educated, well-trained professionals and staff wanted to provide good care, but found themselves thwarted in their efforts to succeed.
What’s going on here? We sought to understand what was really happening in these primary care practices and to formulate strategies to help them become better for patients, staff, and clinicians.
We came up with 2 fundamental insights:
- Practices that focus on building strong internal relationships are better able to deal with surprise and uncertainty.
- Practices that are proactive in interacting with the changing environment will find multiple ways to achieve effective health care delivery.
Work on building those relationships
In our research, we repeatedly observed that careful attention to the relationships among all the people (clinical and nonclinical staff) working within each practice is critical to improving practice processes and outcomes.20 We wanted to learn why relationships mattered so much and how they could be improved. What we found can best be explained by taking another look at Everyday Primary Care.
The physician-owners of everyday Primary Care, feeling stressed out and recognizing that “things are not good here,” signed up to participate in 1 of our studies. Participation required allowing an outside facilitator to observe practice operations and conduct open-ended interviews with physicians and staff over a 2-week period, followed by a series of 12 weekly meetings. In addition, physicians and staff agreed to fill out multiple surveys during the study process and allow researchers to audit the charts of randomly selected patient samples.
One year after Everyday Primary Care signed up, the office space was still cramped, the financial situation was no better, and environmental pressures were continuing to mount. And yet, the practice felt like a different place, one filled with energy and hope. What had happened?
RAP, huddles, effective teams. Most importantly, the quality and types of relationships within the practice had changed. At our suggestion, the practice formed a RAP (reflective adaptive process) team under the guidance of a facilitator—a nurse we trained in basic facilitation skills, including effective meeting strategies, brainstorming, and conflict resolution. The team consisted of physician leaders (both Drs. Cope and Varimore attended all meetings), the practice manager, representatives from each part of the practice (billing, front desk, nursing staff, insurance clerk), and a patient.15 The RAP intervention was designed to provide members with time and space to reflect and opportunities to learn the value of communication, respectful interaction, and listening to diverse opinions and perspectives.20 The team met with the facilitator for 1 hour every week, reviewed the practice’s vision, and developed and implemented strategies for solving prioritized practice issues and problems.
Brainstorming helped identify recurrent problems. As the RAP meetings progressed, it became clear that despite the close quarters, each part of the practice was isolated from the others and all team members were frustrated by their inability to influence the lead physician, Dr. Cope. Over time, the RAP meetings changed the relationship patterns and the quality of communication, thus helping the practice move forward and get unstuck. Dr. Cope repeatedly commented, “I didn’t know that,” as staff shared their concerns and challenges. For example, Dr. Cope was amazed when the front desk described the amount of time and degree of disruption caused by drug reps constantly coming into the office. Together, the team was able to come up with a solution—setting aside a special time for drug reps, rather than allowing them to arrive whenever they chose—that worked for physicians and staff alike.
Our current project notes from Everyday Primary Care reflect a very different and vibrant practice, in which the atmosphere is charged with hope and everyone reports being more relaxed—though just as busy. Office processes have improved and space is less cluttered. Chart audit scores reveal improved quality of chronic care and preventive services. Because practice members have learned to communicate across the barriers of job classification and hierarchy, they are able to solve problems as they arise without allowing things to fester. These improved relationships led to an enhanced understanding of complex issues like patient triage and scheduling and more numerous and accurate memories of how the practice has operated over the years.21-23
Our research has taught us that practices that pay attention to building strong relationships are better able to deal with the surprise and uncertainty that characterize modern health care delivery.24-26 The primary care management literature has highlighted a number of practical strategies for enhancing relationships and communication, including the use of RAP teams, huddles, effective team meetings, and high-performing clinical teams.15,27-29 In addition, we refer the reader to The Team Handbook, 3rd ed., by Peter R. Scholtes, Brian L. Joiner, and Barbara J. Streibel. The handbook contains a wide range of practical teambuilding strategies in an easily accessible style.30 FIGURE 1 summarizes 5 tips for building critical relationships in your own practice.
FIGURE 1
5 tips for building critical relationships
Interact with the “local fitness landscape”
Our second insight is that practices must learn to interact with what we call the “local fitness landscape.”31-33 To understand what that term implies, imagine your hometown with multiple primary care offices of different sizes, a variety of specialty practices, 2 or 3 competing hospital systems, multiple insurance options, businesses, housing clusters representing different social classes, schools, banks, scattered farms, industries, waterways, animals and plants, transportation systems, and political and religious institutions. The totality of all these elements is the local fitness landscape.
The landscape is a dynamic, fluid system within which the component parts respond to and influence each other. Everyday Primary Care is embedded in such a landscape, acting on and being acted upon by other parts of the system. Unfortunately, like most practices we observed, Everyday tended to ignore or resist the local fitness landscape rather than trying to understand and adapt to it. The physicians felt trapped by environmental constraints and frustrated by the turbulence they observed.
What constraints does Everyday Primary Care face? When we first visited this practice, we could see that the facility was too small for the growing volume of patients. The physician-owners knew the space wasn’t conducive to optimum patient care, but told us they could not afford to pay higher rent for larger quarters. Similarly, they understood the potential of electronic medical records (EMRs), but hadn’t been able to find time or money to support the transition. Rising overhead expenses were outpacing practice productivity, as measured in the number of patients seen per day. What was worse, the need to see so many patients was making it more difficult to address the needs of their aging and medically complex patient population.
Looking outward can help
Despite these constraints, internal conversations generated through RAP sessions led practice staff to reach out to other physicians and physician organizations for information. They compared notes with other practices on questions like how their computerized billing system functions, or how to word a letter to patients announcing a new policy on prescription refills. These external conversations expanded the practice’s notions of what was possible and gave them opportunities to share information and learn of new approaches other practices were developing. The result was a newfound level of energy and hope within the practice and exposure to new ideas from the outside.
Learning from the landscape. Numerous conversations with physician organizations, neighboring practices, and a local hospital system yielded new solutions for recalcitrant problems: How to make better use of existing office space, for example, and where to find support for long-range strategic planning. These contacts exposed Everyday to the experiences of other practices with EMRs, and the practice’s physicians have now selected and implemented their own system. The practice was finally able to address the inevitable retirement of 1 of the physicians and now has a succession plan in place. In sum, Everyday learned how to interact and adjust to the changing environment and no longer worried about survival.
Practices co-evolve with all the other systems in a constantly changing fitness landscape. As practice members navigate the local fitness landscapes, they make decisions among competing demands and priorities to maintain their own financial viability and internal stability. What seems to characterize innovative primary care practices is that they don’t wait to react to the next environmental change. Rather, by paying attention to local relationships, they improve the chances that co-evolution will move the practice in desired ways.
Making much-needed connections
There are a number of ways that practices can engage their fitness landscapes, but perhaps the most powerful is creating the time and space to meet with colleagues—either locally or regionally. The most effective approaches are likely to be those that allow sharing experiences and ideas over time, rather than one-time, opportunistic conversations that occur, say, at national and state academy meetings. Practices can participate in activities of regional Practice-Based Research Networks, local residency programs, or even form their own local support group.34,35 To learn how you can connect with a regional Practice-Based Research Network, go to the AHRQ website (http://pbrn.ahrq.gov/portal/server.pt). FIGURE 2 summarizes 4 strategies for reaching out to your local landscape.
FIGURE 2
4 strategies for reaching out to your local landscape
One size doesn’t fit all: Strategic alternatives
When practices build critical relationships and pay attention to their local fitness landscape, they co-evolve improvements that make sense in the context of their unique characteristics and circumstances. Our research shows that practices use a range of alternative strategies to meet the needs of patients, their communities, and themselves. For example, while we have observed primary care offices using EMRs that have achieved high levels of adherence to diabetes guidelines, we have also found high adherence rates in practices that use paper charts.19 We have seen different, successful approaches to the delivery of preventive health services.11 Some practices involve staff in assuring protocol adherence and others don’t. Some use reminder systems and others don’t. Several practices with higher rates of preventive service delivery use none of these. A recent evaluation of 15 case studies of family practices using teams to implement the chronic care model showed the value of different types of teams in different practices.36
Variability and standardization. The emergence of processes and outcome measures designed to meet the needs of a particular local setting (fitness landscape) appeals to our sense of equity and common sense. Yet variations like these fly in the face of prevailing models and guidelines that emphasize standardized processes. Many health plans and provider organizations insist on evidence-based “best practices” and “optimized models” for delivering primary care.37-39 They assume that if we know the goals, there is a best way to get everyone to achieve them.
A better strategy is to determine when variability and tailoring are more appropriate and then use standardization to help create more time for those processes that require variation. Thus, the practice can use a standardized protocol to turn over immunizations to staff in order to free clinicians to spend more time interacting directly with patients.
Multiple pathways to excellence. Medical practice is full of surprises and complexities. We used to believe that the right tools in the hands of accountable individuals using good management systems would produce best practice outcomes. But we have learned that no single right tool or individual management strategy works consistently in primary care.
We now believe that the relationship system within the practice is a critical element in creating an optimal healing environment. Practices with improved relationship systems exhibit more resilience in weathering a hostile environment, while discovering their own unique model of successful primary care. Such practices can thrive, provide improved quality of patient-centered care, and find professional satisfaction and joy in daily work. We hope that the health care reform plans now being debated in Congress will be informed by these insights and provide space for multiple models of care delivery to emerge.
CORRESPONDENCE
Benjamin F. Crabtree, PhD, Department of Family Medicine, Robert Wood Johnson Medical school, 1 World’s Fair Drive, First Floor, Somerset, NJ 08873; [email protected]
- Building strong relationships among physicians and staff improves the practice’s ability to deal with the uncertainties of a rapidly changing environment (B).
- Interacting proactively with the economic, social, political, and cultural environment—the practice landscape—provides opportunities for adaptation and ongoing learning (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
“Everyday Primary Care,” a popular, urban 3-physician family medicine office, has served mostly middle- and working-class people for more than 25 years. Most of the patients have grown older with Drs. Newman and Cope and now have a substantial chronic disease burden. Dr. Varimore, Dr. Cope’s son, has recently joined the practice. He replaced a long-time partner who left in frustration to do emergency medicine.
On a typical day, Dr. Cope enters the crowded waiting room, sighs, and walks quickly toward the nurses’ station where her third scheduled patient has just arrived; her first 2 patients are already waiting in examining rooms. In her tiny office, stacks of charts, phone messages, and forms await her attention. Phones ring constantly. Rushing to see her first patient, Dr. Cope squeezes past her nursing assistant in the narrow hallway. She catches a glimpse of her partner, Dr. Newman, at the end of the corridor. They grunt a word of greeting, but say nothing more. In fact, the physicians and their staff have barely spoken to each other in days.
The 2 older physicians were hopeful that Dr. Varimore would infuse fresh energy into the practice, but the only thing that has changed with his arrival is an increase in the number of patients they see and the expenses of running the office. When the door finally closes at the end of a long day, everyone leaves feeling exhausted and alone.
A toxic atmosphere
The situation at Everyday Primary Care is not unusual.1 These are unhealthy times for most primary care practices. Despite the critical role that primary care is expected to play in health care reform, there is tremendous uncertainty about the future viability of primary care practice.1-6 An alarming number of primary care physicians are leaving practice or taking early retirement as frustration and exhaustion move deeply into our community.1,7,8 Staff turnover is high and disruptive. Primary care physicians feel buffeted by conflicting patient demands, insurance coverage restrictions, inadequate Medicare reimbursement, multiple and often inconsistent practice guidelines, and onerous government regulations. Primary care practices suffer from a culture of despair that impedes decision-making. These practices—and the physicians who struggle to keep them viable—need to develop resilience to survive in this hostile climate and improve the quality of care they provide.
Research-based strategies. This article suggests strategies for primary care practices to move forward—whatever proposed reforms emerge from the current debate. The strategies we propose derive from specific, concrete observations gathered during a 15-year program of research that included nearly 500 primary care offices.9-16 (In fact, Everyday Primary Care is an actual practice that participated in 1 of our studies, though we’ve changed its name and the names of the physicians.) Our research was funded by the National Institutes of Health (NIH) and included both descriptive and intervention projects. Our studies provided in-depth descriptions of a wide variety of primary care practices, as well as new models for describing change.10,14,15 The practices varied in how they delivered preventive services, in their cancer-related prevention and screening activities, and in the way they managed chronic disease.11,14,17-19 Yet across all these variations, we found a pattern in which educated, well-trained professionals and staff wanted to provide good care, but found themselves thwarted in their efforts to succeed.
What’s going on here? We sought to understand what was really happening in these primary care practices and to formulate strategies to help them become better for patients, staff, and clinicians.
We came up with 2 fundamental insights:
- Practices that focus on building strong internal relationships are better able to deal with surprise and uncertainty.
- Practices that are proactive in interacting with the changing environment will find multiple ways to achieve effective health care delivery.
Work on building those relationships
In our research, we repeatedly observed that careful attention to the relationships among all the people (clinical and nonclinical staff) working within each practice is critical to improving practice processes and outcomes.20 We wanted to learn why relationships mattered so much and how they could be improved. What we found can best be explained by taking another look at Everyday Primary Care.
The physician-owners of everyday Primary Care, feeling stressed out and recognizing that “things are not good here,” signed up to participate in 1 of our studies. Participation required allowing an outside facilitator to observe practice operations and conduct open-ended interviews with physicians and staff over a 2-week period, followed by a series of 12 weekly meetings. In addition, physicians and staff agreed to fill out multiple surveys during the study process and allow researchers to audit the charts of randomly selected patient samples.
One year after Everyday Primary Care signed up, the office space was still cramped, the financial situation was no better, and environmental pressures were continuing to mount. And yet, the practice felt like a different place, one filled with energy and hope. What had happened?
RAP, huddles, effective teams. Most importantly, the quality and types of relationships within the practice had changed. At our suggestion, the practice formed a RAP (reflective adaptive process) team under the guidance of a facilitator—a nurse we trained in basic facilitation skills, including effective meeting strategies, brainstorming, and conflict resolution. The team consisted of physician leaders (both Drs. Cope and Varimore attended all meetings), the practice manager, representatives from each part of the practice (billing, front desk, nursing staff, insurance clerk), and a patient.15 The RAP intervention was designed to provide members with time and space to reflect and opportunities to learn the value of communication, respectful interaction, and listening to diverse opinions and perspectives.20 The team met with the facilitator for 1 hour every week, reviewed the practice’s vision, and developed and implemented strategies for solving prioritized practice issues and problems.
Brainstorming helped identify recurrent problems. As the RAP meetings progressed, it became clear that despite the close quarters, each part of the practice was isolated from the others and all team members were frustrated by their inability to influence the lead physician, Dr. Cope. Over time, the RAP meetings changed the relationship patterns and the quality of communication, thus helping the practice move forward and get unstuck. Dr. Cope repeatedly commented, “I didn’t know that,” as staff shared their concerns and challenges. For example, Dr. Cope was amazed when the front desk described the amount of time and degree of disruption caused by drug reps constantly coming into the office. Together, the team was able to come up with a solution—setting aside a special time for drug reps, rather than allowing them to arrive whenever they chose—that worked for physicians and staff alike.
Our current project notes from Everyday Primary Care reflect a very different and vibrant practice, in which the atmosphere is charged with hope and everyone reports being more relaxed—though just as busy. Office processes have improved and space is less cluttered. Chart audit scores reveal improved quality of chronic care and preventive services. Because practice members have learned to communicate across the barriers of job classification and hierarchy, they are able to solve problems as they arise without allowing things to fester. These improved relationships led to an enhanced understanding of complex issues like patient triage and scheduling and more numerous and accurate memories of how the practice has operated over the years.21-23
Our research has taught us that practices that pay attention to building strong relationships are better able to deal with the surprise and uncertainty that characterize modern health care delivery.24-26 The primary care management literature has highlighted a number of practical strategies for enhancing relationships and communication, including the use of RAP teams, huddles, effective team meetings, and high-performing clinical teams.15,27-29 In addition, we refer the reader to The Team Handbook, 3rd ed., by Peter R. Scholtes, Brian L. Joiner, and Barbara J. Streibel. The handbook contains a wide range of practical teambuilding strategies in an easily accessible style.30 FIGURE 1 summarizes 5 tips for building critical relationships in your own practice.
FIGURE 1
5 tips for building critical relationships
Interact with the “local fitness landscape”
Our second insight is that practices must learn to interact with what we call the “local fitness landscape.”31-33 To understand what that term implies, imagine your hometown with multiple primary care offices of different sizes, a variety of specialty practices, 2 or 3 competing hospital systems, multiple insurance options, businesses, housing clusters representing different social classes, schools, banks, scattered farms, industries, waterways, animals and plants, transportation systems, and political and religious institutions. The totality of all these elements is the local fitness landscape.
The landscape is a dynamic, fluid system within which the component parts respond to and influence each other. Everyday Primary Care is embedded in such a landscape, acting on and being acted upon by other parts of the system. Unfortunately, like most practices we observed, Everyday tended to ignore or resist the local fitness landscape rather than trying to understand and adapt to it. The physicians felt trapped by environmental constraints and frustrated by the turbulence they observed.
What constraints does Everyday Primary Care face? When we first visited this practice, we could see that the facility was too small for the growing volume of patients. The physician-owners knew the space wasn’t conducive to optimum patient care, but told us they could not afford to pay higher rent for larger quarters. Similarly, they understood the potential of electronic medical records (EMRs), but hadn’t been able to find time or money to support the transition. Rising overhead expenses were outpacing practice productivity, as measured in the number of patients seen per day. What was worse, the need to see so many patients was making it more difficult to address the needs of their aging and medically complex patient population.
Looking outward can help
Despite these constraints, internal conversations generated through RAP sessions led practice staff to reach out to other physicians and physician organizations for information. They compared notes with other practices on questions like how their computerized billing system functions, or how to word a letter to patients announcing a new policy on prescription refills. These external conversations expanded the practice’s notions of what was possible and gave them opportunities to share information and learn of new approaches other practices were developing. The result was a newfound level of energy and hope within the practice and exposure to new ideas from the outside.
Learning from the landscape. Numerous conversations with physician organizations, neighboring practices, and a local hospital system yielded new solutions for recalcitrant problems: How to make better use of existing office space, for example, and where to find support for long-range strategic planning. These contacts exposed Everyday to the experiences of other practices with EMRs, and the practice’s physicians have now selected and implemented their own system. The practice was finally able to address the inevitable retirement of 1 of the physicians and now has a succession plan in place. In sum, Everyday learned how to interact and adjust to the changing environment and no longer worried about survival.
Practices co-evolve with all the other systems in a constantly changing fitness landscape. As practice members navigate the local fitness landscapes, they make decisions among competing demands and priorities to maintain their own financial viability and internal stability. What seems to characterize innovative primary care practices is that they don’t wait to react to the next environmental change. Rather, by paying attention to local relationships, they improve the chances that co-evolution will move the practice in desired ways.
Making much-needed connections
There are a number of ways that practices can engage their fitness landscapes, but perhaps the most powerful is creating the time and space to meet with colleagues—either locally or regionally. The most effective approaches are likely to be those that allow sharing experiences and ideas over time, rather than one-time, opportunistic conversations that occur, say, at national and state academy meetings. Practices can participate in activities of regional Practice-Based Research Networks, local residency programs, or even form their own local support group.34,35 To learn how you can connect with a regional Practice-Based Research Network, go to the AHRQ website (http://pbrn.ahrq.gov/portal/server.pt). FIGURE 2 summarizes 4 strategies for reaching out to your local landscape.
FIGURE 2
4 strategies for reaching out to your local landscape
One size doesn’t fit all: Strategic alternatives
When practices build critical relationships and pay attention to their local fitness landscape, they co-evolve improvements that make sense in the context of their unique characteristics and circumstances. Our research shows that practices use a range of alternative strategies to meet the needs of patients, their communities, and themselves. For example, while we have observed primary care offices using EMRs that have achieved high levels of adherence to diabetes guidelines, we have also found high adherence rates in practices that use paper charts.19 We have seen different, successful approaches to the delivery of preventive health services.11 Some practices involve staff in assuring protocol adherence and others don’t. Some use reminder systems and others don’t. Several practices with higher rates of preventive service delivery use none of these. A recent evaluation of 15 case studies of family practices using teams to implement the chronic care model showed the value of different types of teams in different practices.36
Variability and standardization. The emergence of processes and outcome measures designed to meet the needs of a particular local setting (fitness landscape) appeals to our sense of equity and common sense. Yet variations like these fly in the face of prevailing models and guidelines that emphasize standardized processes. Many health plans and provider organizations insist on evidence-based “best practices” and “optimized models” for delivering primary care.37-39 They assume that if we know the goals, there is a best way to get everyone to achieve them.
A better strategy is to determine when variability and tailoring are more appropriate and then use standardization to help create more time for those processes that require variation. Thus, the practice can use a standardized protocol to turn over immunizations to staff in order to free clinicians to spend more time interacting directly with patients.
Multiple pathways to excellence. Medical practice is full of surprises and complexities. We used to believe that the right tools in the hands of accountable individuals using good management systems would produce best practice outcomes. But we have learned that no single right tool or individual management strategy works consistently in primary care.
We now believe that the relationship system within the practice is a critical element in creating an optimal healing environment. Practices with improved relationship systems exhibit more resilience in weathering a hostile environment, while discovering their own unique model of successful primary care. Such practices can thrive, provide improved quality of patient-centered care, and find professional satisfaction and joy in daily work. We hope that the health care reform plans now being debated in Congress will be informed by these insights and provide space for multiple models of care delivery to emerge.
CORRESPONDENCE
Benjamin F. Crabtree, PhD, Department of Family Medicine, Robert Wood Johnson Medical school, 1 World’s Fair Drive, First Floor, Somerset, NJ 08873; [email protected]
1. Showstack JA, Rothman AA, Hassmiller S. The Future of Primary Care. 1st ed. San Francisco: Jossey-Bass; 2004.
2. Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Institute of Medicine. Division of Health Care services. Committee on the Future of Primary Care. Donaldson MS. Primary Care: America’s Health in a New Era. Washington, DC: National Academy Press; 1996.
4. Martin JC, Avant RF, Bowman MA, et al. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(suppl 1):S3-S32.
5. Society of General Internal Medicine. Future of general internal medicine. Available at: www.sgim.org/index.cfm?section=site&pageId=366. Accessed July 7, 2009.
6. Grumbach K, Bodenheimer T. A primary care home for Americans: putting the house in order. JAMA. 2002;288:889-893.
7. Bodenheimer T, Grumbach K. Improving Primary Care: Strategies and Tools for a Better Practice. New York: Lange Medical Books/McGraw-Hill; 2007.
8. Moore G, Showstack J. Primary care medicine in crisis: toward reconstruction and renewal. Ann Intern Med. 2003;138:244-247.
9. Direct Observation of Primary Care (DOPC) Writing Group. Conducting the direct observation of primary care study. J Fam Pract. 2001;50:345-352.
10. Stange KC, Zyzanski SJ, Jaen CR, et al. Illuminating the ‘black box’. A description of 4454 patient visits to 138 family physicians. J Fam Pract. 1998;46:377-389.
11. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
12. Goodwin MA, Zyzanski SJ, Zronek S, et al. A clinical trial of tailored office systems for preventive service delivery. The Study to Enhance Prevention by Understanding Practice (STEP-UP). Am J Prev Med. 2001;21:20-28.
13. Stange KC, Goodwin MA, Zyzanski SJ, et al. Sustainability of a practice-individualized preventive service delivery intervention. Am J Prev Med. 2003;25:296-300.
14. Cohen D, McDaniel RR, Jr, Crabtree BF, et al. A practice change model for quality improvement in primary care practice. J Healthc Manag. 2004;49:155-168;discussion 169–170.
15. Stroebel CK, McDaniel RR, Jr., Crabtree BF, et al. How complexity science can inform a reflective process for improvement in primary care practices. Jt Comm J Qual Patient Saf. 2005;31:438-446.
16. Stange KC, Jaen CR, Flocke SA, et al. The value of a family physician. J Fam Pract. 1998;46:363-368.
17. Crabtree BF, Miller WL, Aita VA, et al. Primary care practice organization and preventive services delivery: a qualitative analysis. J Fam Pract. 1998;46:403-409.
18. Ohman-Strickland PA, Orzano AJ, Hudson SV, et al. Quality of diabetes care in family medicine practices: influence of nurse-practitioners and physician’s assistants. Ann Fam Med. 2008;6:14-22.
19. Crosson JC, Ohman-Strickland PA, Hahn KA, et al. Electronic medical records and diabetes quality of care: results from a sample of family medicine practices. Ann Fam Med. 2007;5:209-215.
20. Tallia AF, Lanham HJ, McDaniel RR, Jr., et al. 7 characteristics of successful work relationships. Fam Pract Manag. 2006;13:47-50.
21. Weick KE. Sensemaking in Organizations. Thousand Oaks, Calif: Sage Publications; 1995.
22. Weick KE, Sutcliffe KM. Managing the Unexpected: Assuring High Performance in an Age of Complexity. 1st ed. San Francisco: Jossey-Bass; 2001.
23. Weick KE. Making Sense of the Organization. Malden, Mass: Blackwell Publishers; 2001.
24. McDaniel RR, Jr, Jordan ME, Fleeman BF. Surprise, Surprise, Surprise! A complexity science view of the unexpected. Health Care Manage Rev. 2003;28:266-278.
25. Miller WL, Crabtree BF, McDaniel R, et al. Understanding change in primary care practice using complexity theory. J Fam Pract. 1998;46:369-376.
26. Miller WL, McDaniel RR, Jr., Crabtree BF, et al. Practice jazz: understanding variation in family practices using complexity science. J Fam Pract. 2001;50:872-878.
27. Stewart EE, Johnson BC. Improve office efficiency in mere minutes. Fam Pract Manag. 2007;14:27-29.
28. Shenkel R. How to make your meetings more productive. Fam Pract Manag. 2003;10:59-60.
29. Moore LG. Creating a high-performing clinical team. Fam Pract Manag. 2006;13:38-40.
30. Scholtes P, Joiner B, Streibel B. The Team Handbook. 3rd ed. Madison, Wisc: Oriel Incorporated; 2003.
31. Capra F. The Web of Life: A New Scientific Understanding of Living Systems. 1st Anchor Books ed. New York: Anchor Books; 1996.
32. Holland JH. Emergence: from Chaos to Order. Reading, Mass: Addison-Wesley; 1998.
33. Olson EE, Eoyang GH. Facilitating Organization Change: Lessons from Complexity Science. San Francisco: Jossey-Bass/Pfeiffer; 2001.
34. Lanier D. Primary care practice-based research comes of age in the United States. Ann Fam Med. 2005;3(suppl 1):S2-S4.
35. Mold JW, Peterson KA. Primary care practice-based research networks: working at the interface between research and quality improvement. Ann Fam Med. 2005;3(suppl 1):S12-S20.
36. Bodenheimer T. Building teams in primary care: Lessons from 15 case studies. Oakland, Calif: California HealthCare Foundation; July 2007. Available at: www.chcf.org/topics/chronicdisease/index.cfm?itemID=133375. Accessed March 18, 2008.
37. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff. 2002;21:80-90.
38. Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000-1005.
39. McGlynn EA. An evidence-based national quality measurement and reporting system. Med Care. 2003;41(suppl 1):S8-S15.
1. Showstack JA, Rothman AA, Hassmiller S. The Future of Primary Care. 1st ed. San Francisco: Jossey-Bass; 2004.
2. Institute of Medicine. Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
3. Institute of Medicine. Division of Health Care services. Committee on the Future of Primary Care. Donaldson MS. Primary Care: America’s Health in a New Era. Washington, DC: National Academy Press; 1996.
4. Martin JC, Avant RF, Bowman MA, et al. The future of family medicine: a collaborative project of the family medicine community. Ann Fam Med. 2004;2(suppl 1):S3-S32.
5. Society of General Internal Medicine. Future of general internal medicine. Available at: www.sgim.org/index.cfm?section=site&pageId=366. Accessed July 7, 2009.
6. Grumbach K, Bodenheimer T. A primary care home for Americans: putting the house in order. JAMA. 2002;288:889-893.
7. Bodenheimer T, Grumbach K. Improving Primary Care: Strategies and Tools for a Better Practice. New York: Lange Medical Books/McGraw-Hill; 2007.
8. Moore G, Showstack J. Primary care medicine in crisis: toward reconstruction and renewal. Ann Intern Med. 2003;138:244-247.
9. Direct Observation of Primary Care (DOPC) Writing Group. Conducting the direct observation of primary care study. J Fam Pract. 2001;50:345-352.
10. Stange KC, Zyzanski SJ, Jaen CR, et al. Illuminating the ‘black box’. A description of 4454 patient visits to 138 family physicians. J Fam Pract. 1998;46:377-389.
11. Crabtree BF, Miller WL, Tallia AF, et al. Delivery of clinical preventive services in family medicine offices. Ann Fam Med. 2005;3:430-435.
12. Goodwin MA, Zyzanski SJ, Zronek S, et al. A clinical trial of tailored office systems for preventive service delivery. The Study to Enhance Prevention by Understanding Practice (STEP-UP). Am J Prev Med. 2001;21:20-28.
13. Stange KC, Goodwin MA, Zyzanski SJ, et al. Sustainability of a practice-individualized preventive service delivery intervention. Am J Prev Med. 2003;25:296-300.
14. Cohen D, McDaniel RR, Jr, Crabtree BF, et al. A practice change model for quality improvement in primary care practice. J Healthc Manag. 2004;49:155-168;discussion 169–170.
15. Stroebel CK, McDaniel RR, Jr., Crabtree BF, et al. How complexity science can inform a reflective process for improvement in primary care practices. Jt Comm J Qual Patient Saf. 2005;31:438-446.
16. Stange KC, Jaen CR, Flocke SA, et al. The value of a family physician. J Fam Pract. 1998;46:363-368.
17. Crabtree BF, Miller WL, Aita VA, et al. Primary care practice organization and preventive services delivery: a qualitative analysis. J Fam Pract. 1998;46:403-409.
18. Ohman-Strickland PA, Orzano AJ, Hudson SV, et al. Quality of diabetes care in family medicine practices: influence of nurse-practitioners and physician’s assistants. Ann Fam Med. 2008;6:14-22.
19. Crosson JC, Ohman-Strickland PA, Hahn KA, et al. Electronic medical records and diabetes quality of care: results from a sample of family medicine practices. Ann Fam Med. 2007;5:209-215.
20. Tallia AF, Lanham HJ, McDaniel RR, Jr., et al. 7 characteristics of successful work relationships. Fam Pract Manag. 2006;13:47-50.
21. Weick KE. Sensemaking in Organizations. Thousand Oaks, Calif: Sage Publications; 1995.
22. Weick KE, Sutcliffe KM. Managing the Unexpected: Assuring High Performance in an Age of Complexity. 1st ed. San Francisco: Jossey-Bass; 2001.
23. Weick KE. Making Sense of the Organization. Malden, Mass: Blackwell Publishers; 2001.
24. McDaniel RR, Jr, Jordan ME, Fleeman BF. Surprise, Surprise, Surprise! A complexity science view of the unexpected. Health Care Manage Rev. 2003;28:266-278.
25. Miller WL, Crabtree BF, McDaniel R, et al. Understanding change in primary care practice using complexity theory. J Fam Pract. 1998;46:369-376.
26. Miller WL, McDaniel RR, Jr., Crabtree BF, et al. Practice jazz: understanding variation in family practices using complexity science. J Fam Pract. 2001;50:872-878.
27. Stewart EE, Johnson BC. Improve office efficiency in mere minutes. Fam Pract Manag. 2007;14:27-29.
28. Shenkel R. How to make your meetings more productive. Fam Pract Manag. 2003;10:59-60.
29. Moore LG. Creating a high-performing clinical team. Fam Pract Manag. 2006;13:38-40.
30. Scholtes P, Joiner B, Streibel B. The Team Handbook. 3rd ed. Madison, Wisc: Oriel Incorporated; 2003.
31. Capra F. The Web of Life: A New Scientific Understanding of Living Systems. 1st Anchor Books ed. New York: Anchor Books; 1996.
32. Holland JH. Emergence: from Chaos to Order. Reading, Mass: Addison-Wesley; 1998.
33. Olson EE, Eoyang GH. Facilitating Organization Change: Lessons from Complexity Science. San Francisco: Jossey-Bass/Pfeiffer; 2001.
34. Lanier D. Primary care practice-based research comes of age in the United States. Ann Fam Med. 2005;3(suppl 1):S2-S4.
35. Mold JW, Peterson KA. Primary care practice-based research networks: working at the interface between research and quality improvement. Ann Fam Med. 2005;3(suppl 1):S12-S20.
36. Bodenheimer T. Building teams in primary care: Lessons from 15 case studies. Oakland, Calif: California HealthCare Foundation; July 2007. Available at: www.chcf.org/topics/chronicdisease/index.cfm?itemID=133375. Accessed March 18, 2008.
37. Berwick DM. A user’s manual for the IOM’s ‘Quality Chasm’ report. Health Aff. 2002;21:80-90.
38. Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000-1005.
39. McGlynn EA. An evidence-based national quality measurement and reporting system. Med Care. 2003;41(suppl 1):S8-S15.
Cochrane Musculoskeletal Group review: Acute gout
Gout afflicts about 2% of men over age 30 and women over age 50 and its prevalence appears to be increasing.1 In the United States in 2005, an estimated 3 million adults had suffered an episode of gout in the preceding year.2 Health care utilization costs associated with the disorder are substantial.3
Options for treating acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and intra-articular and systemic corticosteroids.4 Choosing among them can be challenging, however, because the evidence that one or another of these options yields real benefit is of varying strength. Using NSAIDs can be problematic with increasing age, as comorbidities like gastrointestinal (GI) bleeding, renal failure, heart failure, and cardiovascular risk increase and anticoagulant therapy is more likely to be in use.5 That’s where the kind of systematic reviews Cochrane Musculoskeletal Group (CMSG) performs can be of real help.
This article is the first in a series intended to bring the findings of the Cochrane Musculoskeletal Group (CMSG) to the attention of family physicians. CMSG—one of the largest review groups that comprises the Cochrane Collaboration—includes more than 200 active researchers, health care professionals, and consumer representatives from 26 countries. The group synthesizes the results of high-quality clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. Each article in this series will summarize a CMSG review on a single topic, using common clinical scenarios to demonstrate how the information the review supplies can be applied to clinical practice.
Colchicine and steroids: What the reviews tell us
The CMSG has done 2 systematic reviews of acute gout therapy. In 1, Schlesinger and colleagues went over all the available clinical trials to assess the efficacy and adverse effects of colchicine compared to placebo or to other acute gout treatments.6 In the other, Janssens and colleagues performed a similar review to assess the efficacy and safety of systemic corticosteroids in the treatment of acute gout in comparison with placebo, other acute gout treatments, or no therapy.7
Colchicine. Only 1 randomized controlled trial (RCT) of colchicine was identified.6 The trial included 43 participants (mostly men) who were treated with either colchicine or placebo, and the effects of treatment in both groups were compared. Colchicine was given as an initial dose of 1 mg orally followed by 0.5 mg every 2 hours until the acute episode subsided or toxic side effects occurred.
All 22 participants who took colchicine developed diarrhea or vomiting within 24 hours of initiating therapy, after taking a mean dose of 6.7 mg. In other words, the number needed to harm from colchicine therapy given in this way was 1. Three patients had to be treated in order to achieve at least a 50% decrease in pain.
No RCTs comparing colchicine with other treatments of acute gout were found. Case reports suggest that lower doses (0.5 mg, 3 times a day or less) of colchicine may be associated with fewer GI side effects, but there was no RCT evidence to support this approach.8
Systemic corticosteroids. No placebo-controlled trials of either intra-articular or systemic corticosteroids were found. Three trials involving 148 patients compared different systemic corticosteroids with different control drugs.7
Intramuscular (IM) triamcinolone acetonide 60 mg was compared with oral indomethacin 50 mg 3 times daily in 1 trial, and with IM adrenocorticotropic hormone (ACTH) 40 IU in a second trial. In a third trial, oral prednisolone (30 mg daily for 5 days) was compared with an initial single IM injection of 75 mg diclofenac followed by oral indomethacin 50 mg 3 times a day for 3 days, and then a reduced dose of 25 mg indomethacin 3 times a day for another 3 days.
No clinically relevant differences between the corticosteroids and the comparator drugs were found in any of the 3 trials. No important safety problems were attributed to the corticosteroid medications. Most adverse events were related to the comparator drugs—in particular to the NSAIDs. In view of the low quality and variability in the design of the trials, the review authors could not draw firm conclusions about the comparative effectiveness of systemic corticosteroids and the other drugs used.
Another study comes on heels of Cochrane review
A further RCT in progress at the time of the review compared oral prednisolone 35 mg daily with naproxen 500 mg twice daily for 5 days in 120 patients. This trial has since been published.9 It was a double-blind, double-dummy RCT with adequate allocation concealment, low loss to follow up, and thus low risk of bias.10 The results showed that the 2 interventions were clinically equivalent, with a 73% and 78% decrease in pain score over 90 hours in the prednisolone and naproxen groups, respectively. Most of the improvement occurred during the first 42 hours. Adverse events did not differ between groups.
Design the therapy to fit the patient
The study cited above demonstrates that there is no real difference, either in efficacy or safety, between corticosteroids and NSAIDs in the treatment of gout. In theory, that would seem to mean that you could feel equally comfortable prescribing either therapy. But in clinical practice, characteristics of individual patients and the profile of each class of drugs will influence your choice. The following cases illustrate this point. These cases discuss the initial treatment of acute gout, but of course in clinical practice you would go on to consider ongoing prophylactic treatment.
Mr. Peters is a 53-year-old man with a body mass index of 29. He is usually well and not on any medication. He limps into your office one day, complaining of excruciating pain in his left great toe that started suddenly the night before. He’d had a bad night, with pain so severe he couldn’t even tolerate the pressure of the bed sheet on his foot. A dose of acetaminophen failed to control the pain. He has never experienced anything like it.
You look at his foot, and find a swollen, erythematous first metatarsophalangeal joint. On the basis of this classic presentation, you make a provisional diagnosis of gout.
Q. What immediate treatment do you choose?
You consider colchicine, but decide against it because of its side effect profile. Mr. Peters has no contraindication for NSAIDs, so you start him on diclofenac 50 mg orally, 3 times a day. You are aware that NSAIDs can have adverse effects in some patients. If Mr. Peters develops these or fails to improve quickly on diclofenac, you will consider switching him to intra-articular or systemic corticosteroids. Therefore, you ask him to check back with you in 48 hours regarding his progress. At this review, you find his symptoms are settling well, with no adverse effects.
CASE 2
Mrs. Jones, age 81, arrives in your waiting room with an acutely painful, red, and swollen right index finger. Her condition makes it very difficult for her to bathe and prepare meals, a serious problem because she lives alone. She has hypertension, chronic atrial fibrillation, and chronic mild heart failure that has been stable for more than a year.
Mrs. Jones takes warfarin 4 mg daily because of her atrial fibrillation. Her international normalized ratio (INR) has been stable on this dose for the last 6 months. Mrs. Jones also takes perindopril 5 mg and hydrochlorothiazide 6 mg daily for her heart failure and hypertension. Her renal function is normal. You notice that in addition to the inflamed proximal interphalangeal joint of the index finger of the right hand, Mrs. Jones has swelling and what appears to be a tophus over the distal interphalangeal joint of the third finger, which suggests that she has gout. You realize that the thiazide diuretics may have precipitated this problem and that possibility will need to be addressed, but in the short term you are concerned about managing her pain and restoring her hand function.
Q. What treatment do you consider?
There are many reasons why you are extremely reluctant to use NSAIDs. Mrs. Jones’s age and the warfarin she takes create an unacceptably high risk of GI bleeding. Other side effects of NSAIDs, including hypertension and fluid retention, could aggravate her cardiac failure. You are also reluctant to use colchicine at the recommended high dosage for acute gout, because the GI effects associated with this drug may further incapacitate Mrs. Jones, and because the risk of dehydration with or without renal failure is particularly serious in an elderly woman.
You could consider a lower dose of colchicine, but the evidence for effectiveness and rapid onset of action at lower doses is weak and information on the frequency of GI effects at lower doses is not available. While a short course of oral corticosteroids is a possibility, these drugs also carry a risk of GI bleeding when used in combination with warfarin and might worsen her cardiac failure.
A steroid injection is worth considering. No RCTs have examined the effectiveness and safety of intra-articular corticosteroids for gout, but an uncontrolled trial of intra-articular triamcinolone acetonide (10 mg to the knee and 8 mg into small joints) demonstrated pain relief within 48 hours in all 19 patients receiving this treatment.4,11 Further, there is evidence that intra-articular corticosteroids are effective in other inflammatory joint conditions. Intra-articular injection of a corticosteroid carries a small risk of joint hemorrhage in a patient taking warfarin and might be painful when administered into the finger, but if the injection is done carefully with a small needle, this seems to be the safest option. You decide to explain the risks and benefits of the different strategies for treating gout, and recommend a local corticosteroid injection.
Q. If you’re not experienced in this technique and a rheumatologist or other specialist is not immediately available to perform it for you, what would you do then?
Because Mrs. Jones’s heart failure is stable and mild, you can consider a 5-day course of prednisolone together with a proton pump inhibitor to reduce the risk of GI toxicity while monitoring her heart failure and INR carefully. While the dose of prednisolone used in the trials was 30 to 35 mg, you are reluctant to use a dose this high with this patient, and so opt to use a lower dose of 15 mg daily and review her progress in 24 hours. The next day her symptoms are improved and Mrs. Jones continues the prednisolone for the next 4 days.
So where do we go from here?
Although anti-inflammatories, colchicine, and intra-articular and systemic corticosteroids have been mainstays of treatment for acute gout for years, evidence to guide your therapeutic choices is limited. NSAIDs are a reasonable first option, provided there are no contraindications. However, as Case 2 illustrates, when NSAIDs are contraindicated the available evidence provides only limited guidance for treatment choices.
While colchicine has demonstrated efficacy at the standard dosage of 1 mg orally followed by 0.5 mg every 2 hours, the unacceptably high level of GI side effects, together with concerns about more serious toxicity, limits its usefulness.12 No trials have examined the effectiveness and safety of lower doses. Intra-articular corticosteroids may be effective, but this has not been tested in an RCT.
One trial found that oral prednisolone 35 mg daily provided equivalent relief to NSAIDs, and this is another treatment option.9 However, it is unclear whether lower doses of oral corticosteroids might be similarly effective with reduced risks. The bottom line is that more high-quality clinical trials are needed to determine the optimum therapy for acute gout.
Correspondence
Tania Winzenberg, MBBS, Menzies Research Institute, Private Bag 23, Hobart, Tasmania, Australia 7001; [email protected]
1. Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17:341-345.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Wu EQ, Patel PA, Yu AP, et al. Disease-related and all-cause health care costs of elderly patients with gout. J Manag Care Pharm. 2008;14:164-175.
4. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
5. Australian Medicines Handbook. 9th ed. Adelaide, Australia: Australia Pty ltd; 2007.
6. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
7. Janssens HJ, Lucassen PL, Van de Laar FA, et al. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
8. Morris I, Varughese G, Mattingly P. Colchicine in acute gout. BMJ. 2003;327:1275-1276.
9. Janssens HJ, Janssen M, van de Lisdonk EH, et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
10. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42-46.
11. Fernandez C, Noguera R, Gonzalez JA, et al. Treatment of acute attacks of gout with a small dose of intraarticular triamcinolone acetonide. J Rheumatol. 1999;26:2285-2286.
12. Australian Adverse Drug Reactions Committee and the Adverse Drug Reactions unit of the Therapeutic Goods Administration. Fatal interactions and reactions with colchicine: beware CYP3A4 inhibitors. Australian Adverse Drug Reactions Bulletin October 2008. Available at: http://www.tga.gov.au/adr/aadrb/aadr0810.htm#a1. Accessed June 1, 2009.
Gout afflicts about 2% of men over age 30 and women over age 50 and its prevalence appears to be increasing.1 In the United States in 2005, an estimated 3 million adults had suffered an episode of gout in the preceding year.2 Health care utilization costs associated with the disorder are substantial.3
Options for treating acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and intra-articular and systemic corticosteroids.4 Choosing among them can be challenging, however, because the evidence that one or another of these options yields real benefit is of varying strength. Using NSAIDs can be problematic with increasing age, as comorbidities like gastrointestinal (GI) bleeding, renal failure, heart failure, and cardiovascular risk increase and anticoagulant therapy is more likely to be in use.5 That’s where the kind of systematic reviews Cochrane Musculoskeletal Group (CMSG) performs can be of real help.
This article is the first in a series intended to bring the findings of the Cochrane Musculoskeletal Group (CMSG) to the attention of family physicians. CMSG—one of the largest review groups that comprises the Cochrane Collaboration—includes more than 200 active researchers, health care professionals, and consumer representatives from 26 countries. The group synthesizes the results of high-quality clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. Each article in this series will summarize a CMSG review on a single topic, using common clinical scenarios to demonstrate how the information the review supplies can be applied to clinical practice.
Colchicine and steroids: What the reviews tell us
The CMSG has done 2 systematic reviews of acute gout therapy. In 1, Schlesinger and colleagues went over all the available clinical trials to assess the efficacy and adverse effects of colchicine compared to placebo or to other acute gout treatments.6 In the other, Janssens and colleagues performed a similar review to assess the efficacy and safety of systemic corticosteroids in the treatment of acute gout in comparison with placebo, other acute gout treatments, or no therapy.7
Colchicine. Only 1 randomized controlled trial (RCT) of colchicine was identified.6 The trial included 43 participants (mostly men) who were treated with either colchicine or placebo, and the effects of treatment in both groups were compared. Colchicine was given as an initial dose of 1 mg orally followed by 0.5 mg every 2 hours until the acute episode subsided or toxic side effects occurred.
All 22 participants who took colchicine developed diarrhea or vomiting within 24 hours of initiating therapy, after taking a mean dose of 6.7 mg. In other words, the number needed to harm from colchicine therapy given in this way was 1. Three patients had to be treated in order to achieve at least a 50% decrease in pain.
No RCTs comparing colchicine with other treatments of acute gout were found. Case reports suggest that lower doses (0.5 mg, 3 times a day or less) of colchicine may be associated with fewer GI side effects, but there was no RCT evidence to support this approach.8
Systemic corticosteroids. No placebo-controlled trials of either intra-articular or systemic corticosteroids were found. Three trials involving 148 patients compared different systemic corticosteroids with different control drugs.7
Intramuscular (IM) triamcinolone acetonide 60 mg was compared with oral indomethacin 50 mg 3 times daily in 1 trial, and with IM adrenocorticotropic hormone (ACTH) 40 IU in a second trial. In a third trial, oral prednisolone (30 mg daily for 5 days) was compared with an initial single IM injection of 75 mg diclofenac followed by oral indomethacin 50 mg 3 times a day for 3 days, and then a reduced dose of 25 mg indomethacin 3 times a day for another 3 days.
No clinically relevant differences between the corticosteroids and the comparator drugs were found in any of the 3 trials. No important safety problems were attributed to the corticosteroid medications. Most adverse events were related to the comparator drugs—in particular to the NSAIDs. In view of the low quality and variability in the design of the trials, the review authors could not draw firm conclusions about the comparative effectiveness of systemic corticosteroids and the other drugs used.
Another study comes on heels of Cochrane review
A further RCT in progress at the time of the review compared oral prednisolone 35 mg daily with naproxen 500 mg twice daily for 5 days in 120 patients. This trial has since been published.9 It was a double-blind, double-dummy RCT with adequate allocation concealment, low loss to follow up, and thus low risk of bias.10 The results showed that the 2 interventions were clinically equivalent, with a 73% and 78% decrease in pain score over 90 hours in the prednisolone and naproxen groups, respectively. Most of the improvement occurred during the first 42 hours. Adverse events did not differ between groups.
Design the therapy to fit the patient
The study cited above demonstrates that there is no real difference, either in efficacy or safety, between corticosteroids and NSAIDs in the treatment of gout. In theory, that would seem to mean that you could feel equally comfortable prescribing either therapy. But in clinical practice, characteristics of individual patients and the profile of each class of drugs will influence your choice. The following cases illustrate this point. These cases discuss the initial treatment of acute gout, but of course in clinical practice you would go on to consider ongoing prophylactic treatment.
Mr. Peters is a 53-year-old man with a body mass index of 29. He is usually well and not on any medication. He limps into your office one day, complaining of excruciating pain in his left great toe that started suddenly the night before. He’d had a bad night, with pain so severe he couldn’t even tolerate the pressure of the bed sheet on his foot. A dose of acetaminophen failed to control the pain. He has never experienced anything like it.
You look at his foot, and find a swollen, erythematous first metatarsophalangeal joint. On the basis of this classic presentation, you make a provisional diagnosis of gout.
Q. What immediate treatment do you choose?
You consider colchicine, but decide against it because of its side effect profile. Mr. Peters has no contraindication for NSAIDs, so you start him on diclofenac 50 mg orally, 3 times a day. You are aware that NSAIDs can have adverse effects in some patients. If Mr. Peters develops these or fails to improve quickly on diclofenac, you will consider switching him to intra-articular or systemic corticosteroids. Therefore, you ask him to check back with you in 48 hours regarding his progress. At this review, you find his symptoms are settling well, with no adverse effects.
CASE 2
Mrs. Jones, age 81, arrives in your waiting room with an acutely painful, red, and swollen right index finger. Her condition makes it very difficult for her to bathe and prepare meals, a serious problem because she lives alone. She has hypertension, chronic atrial fibrillation, and chronic mild heart failure that has been stable for more than a year.
Mrs. Jones takes warfarin 4 mg daily because of her atrial fibrillation. Her international normalized ratio (INR) has been stable on this dose for the last 6 months. Mrs. Jones also takes perindopril 5 mg and hydrochlorothiazide 6 mg daily for her heart failure and hypertension. Her renal function is normal. You notice that in addition to the inflamed proximal interphalangeal joint of the index finger of the right hand, Mrs. Jones has swelling and what appears to be a tophus over the distal interphalangeal joint of the third finger, which suggests that she has gout. You realize that the thiazide diuretics may have precipitated this problem and that possibility will need to be addressed, but in the short term you are concerned about managing her pain and restoring her hand function.
Q. What treatment do you consider?
There are many reasons why you are extremely reluctant to use NSAIDs. Mrs. Jones’s age and the warfarin she takes create an unacceptably high risk of GI bleeding. Other side effects of NSAIDs, including hypertension and fluid retention, could aggravate her cardiac failure. You are also reluctant to use colchicine at the recommended high dosage for acute gout, because the GI effects associated with this drug may further incapacitate Mrs. Jones, and because the risk of dehydration with or without renal failure is particularly serious in an elderly woman.
You could consider a lower dose of colchicine, but the evidence for effectiveness and rapid onset of action at lower doses is weak and information on the frequency of GI effects at lower doses is not available. While a short course of oral corticosteroids is a possibility, these drugs also carry a risk of GI bleeding when used in combination with warfarin and might worsen her cardiac failure.
A steroid injection is worth considering. No RCTs have examined the effectiveness and safety of intra-articular corticosteroids for gout, but an uncontrolled trial of intra-articular triamcinolone acetonide (10 mg to the knee and 8 mg into small joints) demonstrated pain relief within 48 hours in all 19 patients receiving this treatment.4,11 Further, there is evidence that intra-articular corticosteroids are effective in other inflammatory joint conditions. Intra-articular injection of a corticosteroid carries a small risk of joint hemorrhage in a patient taking warfarin and might be painful when administered into the finger, but if the injection is done carefully with a small needle, this seems to be the safest option. You decide to explain the risks and benefits of the different strategies for treating gout, and recommend a local corticosteroid injection.
Q. If you’re not experienced in this technique and a rheumatologist or other specialist is not immediately available to perform it for you, what would you do then?
Because Mrs. Jones’s heart failure is stable and mild, you can consider a 5-day course of prednisolone together with a proton pump inhibitor to reduce the risk of GI toxicity while monitoring her heart failure and INR carefully. While the dose of prednisolone used in the trials was 30 to 35 mg, you are reluctant to use a dose this high with this patient, and so opt to use a lower dose of 15 mg daily and review her progress in 24 hours. The next day her symptoms are improved and Mrs. Jones continues the prednisolone for the next 4 days.
So where do we go from here?
Although anti-inflammatories, colchicine, and intra-articular and systemic corticosteroids have been mainstays of treatment for acute gout for years, evidence to guide your therapeutic choices is limited. NSAIDs are a reasonable first option, provided there are no contraindications. However, as Case 2 illustrates, when NSAIDs are contraindicated the available evidence provides only limited guidance for treatment choices.
While colchicine has demonstrated efficacy at the standard dosage of 1 mg orally followed by 0.5 mg every 2 hours, the unacceptably high level of GI side effects, together with concerns about more serious toxicity, limits its usefulness.12 No trials have examined the effectiveness and safety of lower doses. Intra-articular corticosteroids may be effective, but this has not been tested in an RCT.
One trial found that oral prednisolone 35 mg daily provided equivalent relief to NSAIDs, and this is another treatment option.9 However, it is unclear whether lower doses of oral corticosteroids might be similarly effective with reduced risks. The bottom line is that more high-quality clinical trials are needed to determine the optimum therapy for acute gout.
Correspondence
Tania Winzenberg, MBBS, Menzies Research Institute, Private Bag 23, Hobart, Tasmania, Australia 7001; [email protected]
Gout afflicts about 2% of men over age 30 and women over age 50 and its prevalence appears to be increasing.1 In the United States in 2005, an estimated 3 million adults had suffered an episode of gout in the preceding year.2 Health care utilization costs associated with the disorder are substantial.3
Options for treating acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and intra-articular and systemic corticosteroids.4 Choosing among them can be challenging, however, because the evidence that one or another of these options yields real benefit is of varying strength. Using NSAIDs can be problematic with increasing age, as comorbidities like gastrointestinal (GI) bleeding, renal failure, heart failure, and cardiovascular risk increase and anticoagulant therapy is more likely to be in use.5 That’s where the kind of systematic reviews Cochrane Musculoskeletal Group (CMSG) performs can be of real help.
This article is the first in a series intended to bring the findings of the Cochrane Musculoskeletal Group (CMSG) to the attention of family physicians. CMSG—one of the largest review groups that comprises the Cochrane Collaboration—includes more than 200 active researchers, health care professionals, and consumer representatives from 26 countries. The group synthesizes the results of high-quality clinical trials to determine whether interventions for the prevention, treatment, and rehabilitation of musculoskeletal disorders are safe and effective. Each article in this series will summarize a CMSG review on a single topic, using common clinical scenarios to demonstrate how the information the review supplies can be applied to clinical practice.
Colchicine and steroids: What the reviews tell us
The CMSG has done 2 systematic reviews of acute gout therapy. In 1, Schlesinger and colleagues went over all the available clinical trials to assess the efficacy and adverse effects of colchicine compared to placebo or to other acute gout treatments.6 In the other, Janssens and colleagues performed a similar review to assess the efficacy and safety of systemic corticosteroids in the treatment of acute gout in comparison with placebo, other acute gout treatments, or no therapy.7
Colchicine. Only 1 randomized controlled trial (RCT) of colchicine was identified.6 The trial included 43 participants (mostly men) who were treated with either colchicine or placebo, and the effects of treatment in both groups were compared. Colchicine was given as an initial dose of 1 mg orally followed by 0.5 mg every 2 hours until the acute episode subsided or toxic side effects occurred.
All 22 participants who took colchicine developed diarrhea or vomiting within 24 hours of initiating therapy, after taking a mean dose of 6.7 mg. In other words, the number needed to harm from colchicine therapy given in this way was 1. Three patients had to be treated in order to achieve at least a 50% decrease in pain.
No RCTs comparing colchicine with other treatments of acute gout were found. Case reports suggest that lower doses (0.5 mg, 3 times a day or less) of colchicine may be associated with fewer GI side effects, but there was no RCT evidence to support this approach.8
Systemic corticosteroids. No placebo-controlled trials of either intra-articular or systemic corticosteroids were found. Three trials involving 148 patients compared different systemic corticosteroids with different control drugs.7
Intramuscular (IM) triamcinolone acetonide 60 mg was compared with oral indomethacin 50 mg 3 times daily in 1 trial, and with IM adrenocorticotropic hormone (ACTH) 40 IU in a second trial. In a third trial, oral prednisolone (30 mg daily for 5 days) was compared with an initial single IM injection of 75 mg diclofenac followed by oral indomethacin 50 mg 3 times a day for 3 days, and then a reduced dose of 25 mg indomethacin 3 times a day for another 3 days.
No clinically relevant differences between the corticosteroids and the comparator drugs were found in any of the 3 trials. No important safety problems were attributed to the corticosteroid medications. Most adverse events were related to the comparator drugs—in particular to the NSAIDs. In view of the low quality and variability in the design of the trials, the review authors could not draw firm conclusions about the comparative effectiveness of systemic corticosteroids and the other drugs used.
Another study comes on heels of Cochrane review
A further RCT in progress at the time of the review compared oral prednisolone 35 mg daily with naproxen 500 mg twice daily for 5 days in 120 patients. This trial has since been published.9 It was a double-blind, double-dummy RCT with adequate allocation concealment, low loss to follow up, and thus low risk of bias.10 The results showed that the 2 interventions were clinically equivalent, with a 73% and 78% decrease in pain score over 90 hours in the prednisolone and naproxen groups, respectively. Most of the improvement occurred during the first 42 hours. Adverse events did not differ between groups.
Design the therapy to fit the patient
The study cited above demonstrates that there is no real difference, either in efficacy or safety, between corticosteroids and NSAIDs in the treatment of gout. In theory, that would seem to mean that you could feel equally comfortable prescribing either therapy. But in clinical practice, characteristics of individual patients and the profile of each class of drugs will influence your choice. The following cases illustrate this point. These cases discuss the initial treatment of acute gout, but of course in clinical practice you would go on to consider ongoing prophylactic treatment.
Mr. Peters is a 53-year-old man with a body mass index of 29. He is usually well and not on any medication. He limps into your office one day, complaining of excruciating pain in his left great toe that started suddenly the night before. He’d had a bad night, with pain so severe he couldn’t even tolerate the pressure of the bed sheet on his foot. A dose of acetaminophen failed to control the pain. He has never experienced anything like it.
You look at his foot, and find a swollen, erythematous first metatarsophalangeal joint. On the basis of this classic presentation, you make a provisional diagnosis of gout.
Q. What immediate treatment do you choose?
You consider colchicine, but decide against it because of its side effect profile. Mr. Peters has no contraindication for NSAIDs, so you start him on diclofenac 50 mg orally, 3 times a day. You are aware that NSAIDs can have adverse effects in some patients. If Mr. Peters develops these or fails to improve quickly on diclofenac, you will consider switching him to intra-articular or systemic corticosteroids. Therefore, you ask him to check back with you in 48 hours regarding his progress. At this review, you find his symptoms are settling well, with no adverse effects.
CASE 2
Mrs. Jones, age 81, arrives in your waiting room with an acutely painful, red, and swollen right index finger. Her condition makes it very difficult for her to bathe and prepare meals, a serious problem because she lives alone. She has hypertension, chronic atrial fibrillation, and chronic mild heart failure that has been stable for more than a year.
Mrs. Jones takes warfarin 4 mg daily because of her atrial fibrillation. Her international normalized ratio (INR) has been stable on this dose for the last 6 months. Mrs. Jones also takes perindopril 5 mg and hydrochlorothiazide 6 mg daily for her heart failure and hypertension. Her renal function is normal. You notice that in addition to the inflamed proximal interphalangeal joint of the index finger of the right hand, Mrs. Jones has swelling and what appears to be a tophus over the distal interphalangeal joint of the third finger, which suggests that she has gout. You realize that the thiazide diuretics may have precipitated this problem and that possibility will need to be addressed, but in the short term you are concerned about managing her pain and restoring her hand function.
Q. What treatment do you consider?
There are many reasons why you are extremely reluctant to use NSAIDs. Mrs. Jones’s age and the warfarin she takes create an unacceptably high risk of GI bleeding. Other side effects of NSAIDs, including hypertension and fluid retention, could aggravate her cardiac failure. You are also reluctant to use colchicine at the recommended high dosage for acute gout, because the GI effects associated with this drug may further incapacitate Mrs. Jones, and because the risk of dehydration with or without renal failure is particularly serious in an elderly woman.
You could consider a lower dose of colchicine, but the evidence for effectiveness and rapid onset of action at lower doses is weak and information on the frequency of GI effects at lower doses is not available. While a short course of oral corticosteroids is a possibility, these drugs also carry a risk of GI bleeding when used in combination with warfarin and might worsen her cardiac failure.
A steroid injection is worth considering. No RCTs have examined the effectiveness and safety of intra-articular corticosteroids for gout, but an uncontrolled trial of intra-articular triamcinolone acetonide (10 mg to the knee and 8 mg into small joints) demonstrated pain relief within 48 hours in all 19 patients receiving this treatment.4,11 Further, there is evidence that intra-articular corticosteroids are effective in other inflammatory joint conditions. Intra-articular injection of a corticosteroid carries a small risk of joint hemorrhage in a patient taking warfarin and might be painful when administered into the finger, but if the injection is done carefully with a small needle, this seems to be the safest option. You decide to explain the risks and benefits of the different strategies for treating gout, and recommend a local corticosteroid injection.
Q. If you’re not experienced in this technique and a rheumatologist or other specialist is not immediately available to perform it for you, what would you do then?
Because Mrs. Jones’s heart failure is stable and mild, you can consider a 5-day course of prednisolone together with a proton pump inhibitor to reduce the risk of GI toxicity while monitoring her heart failure and INR carefully. While the dose of prednisolone used in the trials was 30 to 35 mg, you are reluctant to use a dose this high with this patient, and so opt to use a lower dose of 15 mg daily and review her progress in 24 hours. The next day her symptoms are improved and Mrs. Jones continues the prednisolone for the next 4 days.
So where do we go from here?
Although anti-inflammatories, colchicine, and intra-articular and systemic corticosteroids have been mainstays of treatment for acute gout for years, evidence to guide your therapeutic choices is limited. NSAIDs are a reasonable first option, provided there are no contraindications. However, as Case 2 illustrates, when NSAIDs are contraindicated the available evidence provides only limited guidance for treatment choices.
While colchicine has demonstrated efficacy at the standard dosage of 1 mg orally followed by 0.5 mg every 2 hours, the unacceptably high level of GI side effects, together with concerns about more serious toxicity, limits its usefulness.12 No trials have examined the effectiveness and safety of lower doses. Intra-articular corticosteroids may be effective, but this has not been tested in an RCT.
One trial found that oral prednisolone 35 mg daily provided equivalent relief to NSAIDs, and this is another treatment option.9 However, it is unclear whether lower doses of oral corticosteroids might be similarly effective with reduced risks. The bottom line is that more high-quality clinical trials are needed to determine the optimum therapy for acute gout.
Correspondence
Tania Winzenberg, MBBS, Menzies Research Institute, Private Bag 23, Hobart, Tasmania, Australia 7001; [email protected]
1. Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17:341-345.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Wu EQ, Patel PA, Yu AP, et al. Disease-related and all-cause health care costs of elderly patients with gout. J Manag Care Pharm. 2008;14:164-175.
4. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
5. Australian Medicines Handbook. 9th ed. Adelaide, Australia: Australia Pty ltd; 2007.
6. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
7. Janssens HJ, Lucassen PL, Van de Laar FA, et al. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
8. Morris I, Varughese G, Mattingly P. Colchicine in acute gout. BMJ. 2003;327:1275-1276.
9. Janssens HJ, Janssen M, van de Lisdonk EH, et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
10. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42-46.
11. Fernandez C, Noguera R, Gonzalez JA, et al. Treatment of acute attacks of gout with a small dose of intraarticular triamcinolone acetonide. J Rheumatol. 1999;26:2285-2286.
12. Australian Adverse Drug Reactions Committee and the Adverse Drug Reactions unit of the Therapeutic Goods Administration. Fatal interactions and reactions with colchicine: beware CYP3A4 inhibitors. Australian Adverse Drug Reactions Bulletin October 2008. Available at: http://www.tga.gov.au/adr/aadrb/aadr0810.htm#a1. Accessed June 1, 2009.
1. Choi HK, Curhan G. Gout: epidemiology and lifestyle choices. Curr Opin Rheumatol. 2005;17:341-345.
2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
3. Wu EQ, Patel PA, Yu AP, et al. Disease-related and all-cause health care costs of elderly patients with gout. J Manag Care Pharm. 2008;14:164-175.
4. Zhang W, Doherty M, Bardin T, et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
5. Australian Medicines Handbook. 9th ed. Adelaide, Australia: Australia Pty ltd; 2007.
6. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;(4):CD006190.-
7. Janssens HJ, Lucassen PL, Van de Laar FA, et al. Systemic corticosteroids for acute gout. Cochrane Database Syst Rev. 2008;(2):CD005521.-
8. Morris I, Varughese G, Mattingly P. Colchicine in acute gout. BMJ. 2003;327:1275-1276.
9. Janssens HJ, Janssen M, van de Lisdonk EH, et al. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet. 2008;371:1854-1860.
10. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323:42-46.
11. Fernandez C, Noguera R, Gonzalez JA, et al. Treatment of acute attacks of gout with a small dose of intraarticular triamcinolone acetonide. J Rheumatol. 1999;26:2285-2286.
12. Australian Adverse Drug Reactions Committee and the Adverse Drug Reactions unit of the Therapeutic Goods Administration. Fatal interactions and reactions with colchicine: beware CYP3A4 inhibitors. Australian Adverse Drug Reactions Bulletin October 2008. Available at: http://www.tga.gov.au/adr/aadrb/aadr0810.htm#a1. Accessed June 1, 2009.
Osteoarthritis: Managing without surgery
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
STEPS key: Tolerability/Simplicity: low=less tolerable/more complex; high=more tolerable/less complex.
Osteoarthritis (OA) is a common, almost expected, part of getting old. Some patients require no treatment or have symptoms that are easily controlled with over-the-counter analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs) and lifestyle modifications. Others are so debilitated that surgery is the only way to go.
Where does that leave elderly patients in between—those who don’t make good surgical candidates or find little relief from basic interventions and want to “try everything” before surgery?
The best way to answer this question is to utilize the STEPS (Safety, Tolerability, Efficacy, Price, and Simplicity) format, a helpful mnemonic for an objective way to evaluate drugs or medical therapies.1
The recommendations, strength of recommendation (SOR) ratings, and summaries that follow are presented in this format, and draw upon recent reviews, focused studies, and scholarly analysis provided by the Osteoarthritis Research Society International (OARSI). The interventions are divided into 3 categories:
- over-the-counter remedies
- nonpharmacologic interventions
- injections/prescription drugs.
Over-the-counter remedies
Capsaicin ointment
Recommended (SOR: A).
Safety: High.
Tolerability: Medium. About 50% of patients experience a local (and possibly intense) burning sensation initially, but it generally wanes after several weeks. The number needed to harm (NNH) is about 10 for withdrawal due to adverse effects.2
Efficacy: Medium. Evidence regarding topical capsaicin comes mostly from studies of patients with musculoskeletal conditions in general, not OA specifically. About 40% of patients treated with capsaicin report a 50% decrease in musculoskeletal pain, as do 25% of those using a placebo (number needed to treat [NNT]=8).2
Price: Low. About $15 per month per joint.
Simplicity: Medium. Patients need to apply the ointment 3 or 4 times a day.
Tell patients, too, to use topical capsaicin for a few weeks before evaluating its effectiveness or deciding whether to abandon it. Emphasize that it should be used for base pain rather than for acute pain relief. (For those who can’t tolerate capsaicin, topical salicylate may offer similar benefits.)
Glucosamine
Recommended with or without chondroitin (SOR: B).
Safety: High.
Tolerability: High. Side effects are rare.
Efficacy: Low. There have been numerous trials of glucosamine alone (chondroitin alone is not recommended), mostly limited to knee pain. While many have found that glucosamine offers little or no clinical benefit, some have shown a benefit comparable to or slightly better than acetaminophen. Overall, evidence suggests that up to 40% of those who take glucosamine achieve a clinically significant response,3-5 although the placebo response may be substantial.
One study found that glucosamine was effective only when it was combined with chondroitin, and only when pain was more than mild.6 Another showed no clinical benefit for hip OA.7 There is limited evidence for or against the use of glucosamine and chondroitin to slow disease progression; the 2 agents are not recommended for this purpose.
Price: Low to medium ($5-$35 per month).
Simplicity: Medium to high; patients should take glucosamine (or glucosamine and chondroitin) 3 times a day. Compliance is likely to be variable, depending on results.
Evaluate after 4 months, and advise patients to discontinue if there is no significant improvement. Tell them, too, that while preparations vary by manufacturer and price, the difference between the various products is too small to justify the cost of the higher-priced brands.
Nonpharmacologic interventions
Acupuncture
Not recommended (SOR: A).
Safety: High.
Tolerability: Medium to high. Discomfort from the needles varies from slight to none.
Efficacy: Zero to low. Several studies have indicated some beneficial effect, but the degree of improvement has been small, with a sizable placebo effect.8 Studies typically include weekly sessions over the course of several months, and have demonstrated up to a 20% improvement in pain and function; however, much of that improvement has been attributed to the placebo effect, as demonstrated by studies that compared acupuncture with a sham procedure.8 Most data on acupuncture involve the knee; there is insufficient information about the procedure’s efficacy for other sites and for shorter treatment periods.
Price: Medium to high, approaching $1000 for a series of weekly sessions over 3 to 4 months.
Simplicity: Medium. Frequent office visits are standard. Long-term compliance is likely to be low, given the small benefit and the inconvenience.
Knee braces
Recommended (SOR: C).
Safety: High.
Tolerability: Medium to high. Tolerance depends on the type of brace and its complexity.
Efficacy: Low to medium. Few studies of knee bracing have been done; however, 1 trial showed that 73% of those who used sophisticated taping for 3 weeks reported improvement, vs 10% of no-tape controls.9 Pain decreased by 2 points on a 0- to 10-point scale at 3 weeks, and the benefit persisted for an additional 3 weeks after the taping was discontinued. The taping used in the study is impractical for usual and long-term use, but 45% of those who used a sham taping/wrap reported improvement and about a 1-point decrease in pain at 3 and 6 weeks. This suggests that a less sophisticated wrap, such as an elastic patellar-stabilization sleeve, is likely to provide some benefit.
Price: Low (generally less than $30).
Simplicity: High (for a basic sleeve or wrap).
TENS
Recommended (SOR: B).
Safety/Tolerability: High. Withdrawals due to adverse effects of transcutaneous electrical nerve stimulation (TENS) range from 0% to 14%.10
Efficacy: Medium. The mean improvement with TENS is about 20 points more (on a 100-point scale) than the effect associated with placebo; the data are based on knee OA pain that is moderate to severe. The effect may last 2 to 4 weeks; however, there have been few long-term studies of the benefits of TENS.
Price: Low to medium, depending on availability and insurance coverage.
Simplicity: Medium; TENS units can be used at home, but some patients may be uncomfortable using the apparatus.
Injections/prescription drugs
Corticosteroid injections
Recommended (SOR: A).
Safety: High for short-term use, but data on the degree and frequency of acute post-injection pain is limited. The possibility of harm from repeated injections is similarly uncertain, although 1 study of patients receiving 8 injections over a 2-year period found no progression in ill effects compared with patients receiving placebo.11
Tolerability: Medium to high. Discomfort from the needle is usually mild.
Efficacy: Low to medium. Critical analysis supports a modest benefit, with an NNT of 2 to 3 for short-term improvement (lasting 3-4 weeks).12,13 In 1 study, steroid injections produced an average of a 16-point reduction in pain on a 100-point scale. After 1 month, the benefit from the injection is usually not clinically significant.
Price: Low ($100-$200 per injection).
Simplicity: High. The injection itself is a relatively simple procedure, but patient compliance with a scheduled course of repeat injections is uncertain.
Hyaluronic acid (HA) injections
Recommended with caveats (SOR: A).
Safety: High.
Tolerability: Medium. A small number of patients experience a transitory local flare-up. In addition, some patients have an aversion to injections.
Efficacy: Low. Several recent meta-analyses and systematic reviews of HA injections for knee OA have found a small, often negligible, clinical effect, with placebo usually accounting for up to half of the reported benefit.14-18 In 1 study with no control group, however, patients experienced about a 60% decrease in composite pain score—and 75% were satisfied or very satisfied with the treatment.19 Studies of HA injection for hip OA are very limited, but a recent single-injection trial showed no benefit over placebo.20
Any benefit from an HA injection generally lasts 3 to 4 months.
Price: Medium-high. A course of 3 injections costs $700 to $1000 per joint. There are claims of substantial savings due to delayed joint replacement, but these are speculative.
Simplicity: Medium. Compliance (completing all 3 injections) was high in clinical trials, but is uncertain otherwise. However, injections may frequently be useless because the joint space isn’t entered. One study found that 29% of knee injections were extra-articular; placement was uncertain in another 5%. There was no correlation between successful placement and presumed expertise. This error is not surprising, considering that the articular space is usually narrowed and patients being treated for joint pain are often obese. Being able to aspirate fluid increases the chance of proper placement. (While placement errors may also occur with corticosteroid injections, that possibility is offset by a lower cost and somewhat greater likelihood of beneficial effect.)
- Consider this option only for patients with high-function goals and substantial pain or disability.
- Proceed cautiously with morbidly obese patients and patients without effusion.
- Opt for bacterial HA (Euflexxa), which has been found to be the most cost effective and have the highest efficacy and the fewest adverse effects; evidence suggests that hylan (Synvisc) should be avoided.15,16
HA injections can be repeated every 6 to 12 months if they prove to be highly beneficial. Advise patients, however, that data on repeated courses of the injections are very limited.
Nonsteroidal anti-inflammatory gel or patch
Not recommended (SOR: B).
Safety: High. Absorption is minimal, but there may be some systemic effects.
Tolerability: Medium to high, although some patients develop skin reactions to the patch and the gel.
Efficacy: Low. Pain reduction of up to 50% has been reported, but most of it is attributed to the placebo effect. The actual effect of the gel or patch averages only about 10%.21-24
Price: Medium. The gel sells for $150 to $180 per month for a quantity large enough to be used on 2 hands or 1 knee; the patch is similarly priced.
Simplicity: Low. Manufacturers recommend that the gel be applied 4 times a day, but this can prove difficult over the long term. The patch is applied only twice a day, but the painful area is usually too large or irregular for the patch to cover.
Narcotics for refractory pain
Recommended (SOR: B).
Safety: Medium. The elderly are particularly vulnerable to narcotics’ adverse effects, but the incidence of addiction and abuse in geriatric patients is very low. Recently released guidelines on the management of persistent pain from the American Geriatrics Society recommend that all older patients with moderate to severe pain or diminished quality of life be considered for narcotic therapy.25
Tolerability: Medium. Noncompliance is associated with side effects, which are usually dose-dependent. These include constipation, somnolence, and mental status changes. Reported noncompliance rates vary, in some cases reaching as high as 30%.26
Efficacy: Low to medium for weak narcotics (eg, codeine, tramadol), with about a 10% decrease in pain.26,27 However, studies generally suffer from small numbers of patients, short duration, inconsistency among clinical trials, and limited evaluation of disability and quality of life. One good study of tramadol users showed about a 15% decrease in pain among “young elders”—the total decrease in pain was 30%, including the placebo effect. Sleep quality improved and tolerability was high, with no increase in withdrawal due to adverse effects.28 For stronger narcotics (eg, morphine), direct evidence of efficacy for OA pain is lacking, but it seems reasonable to expect a medium benefit. Low-to-moderate daily doses of opioids for chronic noncancer pain—including arthritis—have been shown to improve the quality of life, compared with no narcotics or high doses.29
Price: Low (less than $20/month) for hydrocodone with acetaminophen; medium to high (more than $150/month) for long-acting morphine and transdermal fentanyl.
Simplicity: Medium to high. Twice-a-day dosing is available for long-acting strong opioids, but some narcotics must be taken 4 times a day.
What else can you do? Encourage activity—again
A clear conclusion from many studies is that nonaquatic exercise involving the affected joints, either specifically or as part of a general exercise routine, is clinically beneficial in decreasing pain and increasing function.30 When it comes to aquatic exercises for OA, the data are very limited, but suggest a small short-term benefit.31 Overall, the optimal type and duration of exercise for OA patients, as well as the best location (home or at a gym), is not known.
If your patient has stopped or markedly cut down on the time spent exercising, explore the feasibility of another attempt, and discuss ways to improve compliance. Stress the “bonus” benefits of exercise, such as mood elevation and an overall sense of well-being. If the patient is not undergoing physical therapy, consider a referral.
Use a chronic pain approach
Patients whose OA symptoms continue to be troublesome may benefit from a chronic pain syndrome approach. Nurse case management, phone contact, and extra attention from you may be helpful, along with educational material that the patient can consult between visits (PATIENT HANDOUT).32
Treat insomnia. Studies are insufficient for hypnotics for OA symptoms, but good sleep is a goal of standard pain management. Cognitive-behavioral therapy has been found to be helpful in alleviating sleep problems in patients with OA.33 At a minimum, take a good sleep history and provide basic sleep hygiene education.
Treat vitamin deficiency and depression. Many elderly patients are deficient in vitamin D, and evidence indicates that supplementation supports muscle strengthening and may aid in pain reduction. The effects are mostly preventive, however, and therefore not easily appreciated, so stress the importance of long-term health.
Depression, too, is common among the elderly, particularly for chronic pain patients. If you prescribe antidepressants or vitamin supplementation, emphasize that they are “for your arthritis.”
Inspect footwear. Despite several studies regarding the effects of shoes with inserts on knee OA, it is unclear whether they provide clinical benefit. OARSI supports insoles for selected patients, but acknowledges that evidence is weak. Advise patients to select shoes that are flexible yet supportive and that moderately priced shoes appear to be as good as more expensive footwear for most OA patients. A podiatry consult may be beneficial for patients who have deformities of the feet or complain of foot pain.
Consider assistive devices. Patients with OA who are unstable may benefit from the use of a cane, walker, or other assistive device. Use common sense and individualize treatment, in consultation with an occupational therapist, if necessary.
Living with arthritis: Do’s and Don’ts
Osteoarthritis is an inflammation of the joint that affects many people as they age, mainly in the knees, hips, and hands. After many years of use, the cartilage that lines the inside of the joint thins and eventually wears off, leaving bone rubbing on bone. At the edge of the joint, the bone may grow into small “spurs” and fluid may increase inside, causing inflammation and pain.
There is no cure for arthritis. But there are many things that you and your doctor can do to make arthritis easier to live with, and to slow its progression. These Do’s and Don’ts may help:
Do get moving. Exercising your arthritic joints, except during acute flare-ups, will strengthen the muscles and help you stay active. Eventually you’ll find that you’re in less pain and can move about more easily.
Do consult a physical therapist to find out what type of exercise is best for you. Ask your doctor for a referral.
Don’t do those exercises during painful flare-ups. This is the time to give your joints a rest.
Do take acetaminophen (Tylenol), as needed, especially during flare-ups. You can take up to 4000 mg a day, but be sure to tell your doctor if you’re taking acetaminophen regularly.
Don’t take acetaminophen without first consulting your doctor if you have liver or kidney disease—or you’re taking a prescription pain medication.
Do ask your doctor about nonsteroidal anti-inflammatory drugs, sometimes called NSAIDs (pronounced N-SEDs). Some people benefit from ibuprofen (Advil, Motrin) or naproxen (Aleve), which are sold over-the-counter. Others take prescription NSAIDs such as Celebrex.
Don’t take NSAIDs without consulting your doctor if you’re over the age of 65. Both prescription and nonprescription NSAIDs can have serious side effects, and should be used with caution, if at all, by older people.
Do consider a trial of glucosamine (1500 mg daily), with or without chondroitin (1200 mg daily). Take it every day for 3 or 4 months without making other changes in treatment before you decide whether it’s working.
Don’t buy an expensive brand of glucosamine. The extra cost probably isn’t worth it.
Don’t take chondroitin without glucosamine, as it is unlikely to help.
Do apply ointments and rubs for pain relief. Start with capsaicin, which is available without a prescription. Buy the lowest strength you can find, and start by applying a very small amount on a small area because it may cause a burning sensation in the beginning. If you can’t tolerate capsaicin ointment, try a salicylate rub instead.
Do consider corticosteroid injections if you continue to have a lot of pain in your knee, especially if the doctor finds that you have fluid build-up. (If you don’t want to be injected with a steroid, ask your doctor if you’re a candidate for injections of hyaluronic acid, an artificial joint fluid that may help some patients.)
Do try TENS (transcutaneous electrical nerve stimulation) if you continue to have severe hip or knee pain. TENS therapy may be administered by a physical therapist, or with a device that you can use at home. A pad that emits a small tingle of electricity is placed over the painful joint to relieve the pain.
Do talk to your doctor about narcotic pain medication if your pain continues to be severe.
ADDITIONAL SELF-CARE TIPS:
Do try a wrap-around knee brace, which you can purchase at your local pharmacy. Ask your doctor about getting a cane or a walker if you need additional support.
Do alert your physician to certain conditions that can make your arthritis pain worse—insomnia, depression, or foot problems, for instance. And, if you have ill-fitting shoes or shoes that don’t provide much support, it’s time to replace them.
Joint replacement: When or whether?
Patients who are considering joint replacement surgery or are interested in learning more about it need a realistic picture of what to expect. Explain that results are usually—but not always—very favorable. However, surgery often brings greater improvement in pain than in function, and recovery can be very strenuous and lengthy. Infection rates average 1%,34,35 and mortality rates are low. In a large study of veterans, the 30-day mortality rates after knee and hip arthroplasty were 0.6% and 0.7%, respectively,36 but thromboembolic complications may occur despite prophylaxis. Overall, 5% of those undergoing joint replacement surgery develop significant complications.34,35
Knowing what surgery entails and the difficult recovery required may help patients be more tolerant of their OA symptoms. Whatever treatment your patients opt for, empathy on your part will help, as well.
CORRESPONDENCE
James Crosby, MD, United Health Services, Wilson Family Medicine Residency, 40 Arch Street, Johnson City, NY 13790; [email protected]
1. Ebell M, Siwek J. AFP: Doing more to help you get the best evidence. Am Fam Physician. 2004;69:483-484.
2. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;228:991.-
3. Fox BA, Schmitz E, Wallace R. Glucosamine and chondroitin for osteoarthritis, Family Practice Inquires Network Clinical Inquires. Am Fam Physician. 2006;73:1245-1247.
4. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.-
5. Herrero-Beaumont G, Ivorra JA, Del Carmen TM, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms. Arthritis Rheum. 2007;56:555-567.
6. Clegg DO, Reda D, Harris CL, et al. Glucosamine, chrondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
7. Rozendaal RM, Koes BW, von Osch GJ, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148:268-277.
8. Manheimer E, Linde K, Lao L, et al. Meta-analysis: acupuncture for OA of the knee. Ann Intern Med. 2007;146:868-877.
9. Hinman RS, Crossley KM, McConnell J, et al. Efficacy of knee tape in the management of osteoarthritis of the knee: blinded randomised controlled trial. BMJ. 2003;327:135.-
10. Bjordal JM, Johnson MI, Lopes-Martins RA, et al. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8:51.-
11. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370-377.
12. Arroll B, Goodinyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869-873.
13. Palacios L, Jones W, Mayo H. Do steroid injections help with osteoarthritis of the knee? J Fam Pract. 2004;53:921-922.
14. Bellamy N, Campell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
15. Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172:1039-1043.
16. Medina JM, Thomas A, Denegar CR. Knee osteoarthritis: should your patient opt for hyaluronic acid injection? J Fam Pract. 2006;55:669-675.
17. Modawal A, Fley J, Shukla R, et al. Hyaluronic acid injections relieve knee pain. J Fam Pract. 2005;54:758-767.
18. Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging. 2007;24:629-642.
19. Kichner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-162.
20. Richette P, Ravaud P, Conrozier T, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: a multicenter, randomized, placebo-controlled trial. Arthritis Rheum. 2009;60:824-830.
21. Underwood M. Advice to use topical or oral ibuprofen for chronic knee pain in older people: randomized controlled trial and patient preference study. BMJ. 2008;336:138-142.
22. Diclofenac gel for osteoarthritis. Medical Letter. 2008;50:31-32.
23. Lin J, Zhang W, Jones A, et al. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomized controlled trials. BMJ. 2004;329:324-329.
24. A diclofenac patch for pain. Medical Letter. 2008;50:1-2.
25. American Geriatrics Society. American Geriatrics Society announces new guidelines to improve pain management, quality of life, and quality of care for older patients. May 1, 2009. Available at: http://www.americangeriatrics.org/news/pain043009.shtml. Accessed June 1, 2009.
26. Peloso PM. Opioid therapy for osteoarthritis of the hip and knee: use it or lose it? J Rheum. 2001;28:6-10.
27. Bannwarth B. Letter: Opioids for osteoarthritis of the hip and knee: which opioids for which patients? J Rheum. 2001;28:1930-1931.
28. Vorsanger G, Xiang J, Jordan D, et al. Post hoc analysis of a randomized, double blind, placebo-controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clin Ther. 2007;29:2520-2535.
29. Dillie KS, Fleming MF, Mundt MP, et al. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21:108-117.
30. Fransen M, McConnell S, Bell M. Exercise for osteoarthritis of the hip or knee. Cochrane Database Syst Rev. 2003;(3):CD004376.-
31. Bartels EM, Lund H, Hagen KB, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2007;(4):CD005523.-
32. Rosemann T, Joos S, Laux G, et al. Case management of arthritis patients in primary care: a cluster-randomized controlled trial. Arthritis Rheum. 2007;57:1390-1397.
33. Vitiello M. CBT for insomnia may reduce osteoarthritis pain. Poster presented at: 60th Annual Meeting of the Gerontological Society of America; November 16-20, 2007; San Francisco, Calif.
34. Erens G, Thronhill T. Complications of total hip arthroplasty. In: Basow DS, ed. UpToDate. Waltham, Mass: UpToDate; 2009. Available at: http://www.uptodate.com. Accessed May 20, 2009.
35. Martin G, Thornhill T. Complications of total knee arthroplasty. In: Basow DS, ed. UpToDate. Waltham, Mass: UpToDate; 2009. Available at: http://www.uptodate.com. Accessed May 20, 2009.
36. Ibrahim SA, Stone RA, Han X, et al. Racial/ethnic differences in surgical outcomes in veterans following knee and hip arthroplasty. Arthritis Rheum. 2005;52:3143-3151.
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
STEPS key: Tolerability/Simplicity: low=less tolerable/more complex; high=more tolerable/less complex.
Osteoarthritis (OA) is a common, almost expected, part of getting old. Some patients require no treatment or have symptoms that are easily controlled with over-the-counter analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs) and lifestyle modifications. Others are so debilitated that surgery is the only way to go.
Where does that leave elderly patients in between—those who don’t make good surgical candidates or find little relief from basic interventions and want to “try everything” before surgery?
The best way to answer this question is to utilize the STEPS (Safety, Tolerability, Efficacy, Price, and Simplicity) format, a helpful mnemonic for an objective way to evaluate drugs or medical therapies.1
The recommendations, strength of recommendation (SOR) ratings, and summaries that follow are presented in this format, and draw upon recent reviews, focused studies, and scholarly analysis provided by the Osteoarthritis Research Society International (OARSI). The interventions are divided into 3 categories:
- over-the-counter remedies
- nonpharmacologic interventions
- injections/prescription drugs.
Over-the-counter remedies
Capsaicin ointment
Recommended (SOR: A).
Safety: High.
Tolerability: Medium. About 50% of patients experience a local (and possibly intense) burning sensation initially, but it generally wanes after several weeks. The number needed to harm (NNH) is about 10 for withdrawal due to adverse effects.2
Efficacy: Medium. Evidence regarding topical capsaicin comes mostly from studies of patients with musculoskeletal conditions in general, not OA specifically. About 40% of patients treated with capsaicin report a 50% decrease in musculoskeletal pain, as do 25% of those using a placebo (number needed to treat [NNT]=8).2
Price: Low. About $15 per month per joint.
Simplicity: Medium. Patients need to apply the ointment 3 or 4 times a day.
Tell patients, too, to use topical capsaicin for a few weeks before evaluating its effectiveness or deciding whether to abandon it. Emphasize that it should be used for base pain rather than for acute pain relief. (For those who can’t tolerate capsaicin, topical salicylate may offer similar benefits.)
Glucosamine
Recommended with or without chondroitin (SOR: B).
Safety: High.
Tolerability: High. Side effects are rare.
Efficacy: Low. There have been numerous trials of glucosamine alone (chondroitin alone is not recommended), mostly limited to knee pain. While many have found that glucosamine offers little or no clinical benefit, some have shown a benefit comparable to or slightly better than acetaminophen. Overall, evidence suggests that up to 40% of those who take glucosamine achieve a clinically significant response,3-5 although the placebo response may be substantial.
One study found that glucosamine was effective only when it was combined with chondroitin, and only when pain was more than mild.6 Another showed no clinical benefit for hip OA.7 There is limited evidence for or against the use of glucosamine and chondroitin to slow disease progression; the 2 agents are not recommended for this purpose.
Price: Low to medium ($5-$35 per month).
Simplicity: Medium to high; patients should take glucosamine (or glucosamine and chondroitin) 3 times a day. Compliance is likely to be variable, depending on results.
Evaluate after 4 months, and advise patients to discontinue if there is no significant improvement. Tell them, too, that while preparations vary by manufacturer and price, the difference between the various products is too small to justify the cost of the higher-priced brands.
Nonpharmacologic interventions
Acupuncture
Not recommended (SOR: A).
Safety: High.
Tolerability: Medium to high. Discomfort from the needles varies from slight to none.
Efficacy: Zero to low. Several studies have indicated some beneficial effect, but the degree of improvement has been small, with a sizable placebo effect.8 Studies typically include weekly sessions over the course of several months, and have demonstrated up to a 20% improvement in pain and function; however, much of that improvement has been attributed to the placebo effect, as demonstrated by studies that compared acupuncture with a sham procedure.8 Most data on acupuncture involve the knee; there is insufficient information about the procedure’s efficacy for other sites and for shorter treatment periods.
Price: Medium to high, approaching $1000 for a series of weekly sessions over 3 to 4 months.
Simplicity: Medium. Frequent office visits are standard. Long-term compliance is likely to be low, given the small benefit and the inconvenience.
Knee braces
Recommended (SOR: C).
Safety: High.
Tolerability: Medium to high. Tolerance depends on the type of brace and its complexity.
Efficacy: Low to medium. Few studies of knee bracing have been done; however, 1 trial showed that 73% of those who used sophisticated taping for 3 weeks reported improvement, vs 10% of no-tape controls.9 Pain decreased by 2 points on a 0- to 10-point scale at 3 weeks, and the benefit persisted for an additional 3 weeks after the taping was discontinued. The taping used in the study is impractical for usual and long-term use, but 45% of those who used a sham taping/wrap reported improvement and about a 1-point decrease in pain at 3 and 6 weeks. This suggests that a less sophisticated wrap, such as an elastic patellar-stabilization sleeve, is likely to provide some benefit.
Price: Low (generally less than $30).
Simplicity: High (for a basic sleeve or wrap).
TENS
Recommended (SOR: B).
Safety/Tolerability: High. Withdrawals due to adverse effects of transcutaneous electrical nerve stimulation (TENS) range from 0% to 14%.10
Efficacy: Medium. The mean improvement with TENS is about 20 points more (on a 100-point scale) than the effect associated with placebo; the data are based on knee OA pain that is moderate to severe. The effect may last 2 to 4 weeks; however, there have been few long-term studies of the benefits of TENS.
Price: Low to medium, depending on availability and insurance coverage.
Simplicity: Medium; TENS units can be used at home, but some patients may be uncomfortable using the apparatus.
Injections/prescription drugs
Corticosteroid injections
Recommended (SOR: A).
Safety: High for short-term use, but data on the degree and frequency of acute post-injection pain is limited. The possibility of harm from repeated injections is similarly uncertain, although 1 study of patients receiving 8 injections over a 2-year period found no progression in ill effects compared with patients receiving placebo.11
Tolerability: Medium to high. Discomfort from the needle is usually mild.
Efficacy: Low to medium. Critical analysis supports a modest benefit, with an NNT of 2 to 3 for short-term improvement (lasting 3-4 weeks).12,13 In 1 study, steroid injections produced an average of a 16-point reduction in pain on a 100-point scale. After 1 month, the benefit from the injection is usually not clinically significant.
Price: Low ($100-$200 per injection).
Simplicity: High. The injection itself is a relatively simple procedure, but patient compliance with a scheduled course of repeat injections is uncertain.
Hyaluronic acid (HA) injections
Recommended with caveats (SOR: A).
Safety: High.
Tolerability: Medium. A small number of patients experience a transitory local flare-up. In addition, some patients have an aversion to injections.
Efficacy: Low. Several recent meta-analyses and systematic reviews of HA injections for knee OA have found a small, often negligible, clinical effect, with placebo usually accounting for up to half of the reported benefit.14-18 In 1 study with no control group, however, patients experienced about a 60% decrease in composite pain score—and 75% were satisfied or very satisfied with the treatment.19 Studies of HA injection for hip OA are very limited, but a recent single-injection trial showed no benefit over placebo.20
Any benefit from an HA injection generally lasts 3 to 4 months.
Price: Medium-high. A course of 3 injections costs $700 to $1000 per joint. There are claims of substantial savings due to delayed joint replacement, but these are speculative.
Simplicity: Medium. Compliance (completing all 3 injections) was high in clinical trials, but is uncertain otherwise. However, injections may frequently be useless because the joint space isn’t entered. One study found that 29% of knee injections were extra-articular; placement was uncertain in another 5%. There was no correlation between successful placement and presumed expertise. This error is not surprising, considering that the articular space is usually narrowed and patients being treated for joint pain are often obese. Being able to aspirate fluid increases the chance of proper placement. (While placement errors may also occur with corticosteroid injections, that possibility is offset by a lower cost and somewhat greater likelihood of beneficial effect.)
- Consider this option only for patients with high-function goals and substantial pain or disability.
- Proceed cautiously with morbidly obese patients and patients without effusion.
- Opt for bacterial HA (Euflexxa), which has been found to be the most cost effective and have the highest efficacy and the fewest adverse effects; evidence suggests that hylan (Synvisc) should be avoided.15,16
HA injections can be repeated every 6 to 12 months if they prove to be highly beneficial. Advise patients, however, that data on repeated courses of the injections are very limited.
Nonsteroidal anti-inflammatory gel or patch
Not recommended (SOR: B).
Safety: High. Absorption is minimal, but there may be some systemic effects.
Tolerability: Medium to high, although some patients develop skin reactions to the patch and the gel.
Efficacy: Low. Pain reduction of up to 50% has been reported, but most of it is attributed to the placebo effect. The actual effect of the gel or patch averages only about 10%.21-24
Price: Medium. The gel sells for $150 to $180 per month for a quantity large enough to be used on 2 hands or 1 knee; the patch is similarly priced.
Simplicity: Low. Manufacturers recommend that the gel be applied 4 times a day, but this can prove difficult over the long term. The patch is applied only twice a day, but the painful area is usually too large or irregular for the patch to cover.
Narcotics for refractory pain
Recommended (SOR: B).
Safety: Medium. The elderly are particularly vulnerable to narcotics’ adverse effects, but the incidence of addiction and abuse in geriatric patients is very low. Recently released guidelines on the management of persistent pain from the American Geriatrics Society recommend that all older patients with moderate to severe pain or diminished quality of life be considered for narcotic therapy.25
Tolerability: Medium. Noncompliance is associated with side effects, which are usually dose-dependent. These include constipation, somnolence, and mental status changes. Reported noncompliance rates vary, in some cases reaching as high as 30%.26
Efficacy: Low to medium for weak narcotics (eg, codeine, tramadol), with about a 10% decrease in pain.26,27 However, studies generally suffer from small numbers of patients, short duration, inconsistency among clinical trials, and limited evaluation of disability and quality of life. One good study of tramadol users showed about a 15% decrease in pain among “young elders”—the total decrease in pain was 30%, including the placebo effect. Sleep quality improved and tolerability was high, with no increase in withdrawal due to adverse effects.28 For stronger narcotics (eg, morphine), direct evidence of efficacy for OA pain is lacking, but it seems reasonable to expect a medium benefit. Low-to-moderate daily doses of opioids for chronic noncancer pain—including arthritis—have been shown to improve the quality of life, compared with no narcotics or high doses.29
Price: Low (less than $20/month) for hydrocodone with acetaminophen; medium to high (more than $150/month) for long-acting morphine and transdermal fentanyl.
Simplicity: Medium to high. Twice-a-day dosing is available for long-acting strong opioids, but some narcotics must be taken 4 times a day.
What else can you do? Encourage activity—again
A clear conclusion from many studies is that nonaquatic exercise involving the affected joints, either specifically or as part of a general exercise routine, is clinically beneficial in decreasing pain and increasing function.30 When it comes to aquatic exercises for OA, the data are very limited, but suggest a small short-term benefit.31 Overall, the optimal type and duration of exercise for OA patients, as well as the best location (home or at a gym), is not known.
If your patient has stopped or markedly cut down on the time spent exercising, explore the feasibility of another attempt, and discuss ways to improve compliance. Stress the “bonus” benefits of exercise, such as mood elevation and an overall sense of well-being. If the patient is not undergoing physical therapy, consider a referral.
Use a chronic pain approach
Patients whose OA symptoms continue to be troublesome may benefit from a chronic pain syndrome approach. Nurse case management, phone contact, and extra attention from you may be helpful, along with educational material that the patient can consult between visits (PATIENT HANDOUT).32
Treat insomnia. Studies are insufficient for hypnotics for OA symptoms, but good sleep is a goal of standard pain management. Cognitive-behavioral therapy has been found to be helpful in alleviating sleep problems in patients with OA.33 At a minimum, take a good sleep history and provide basic sleep hygiene education.
Treat vitamin deficiency and depression. Many elderly patients are deficient in vitamin D, and evidence indicates that supplementation supports muscle strengthening and may aid in pain reduction. The effects are mostly preventive, however, and therefore not easily appreciated, so stress the importance of long-term health.
Depression, too, is common among the elderly, particularly for chronic pain patients. If you prescribe antidepressants or vitamin supplementation, emphasize that they are “for your arthritis.”
Inspect footwear. Despite several studies regarding the effects of shoes with inserts on knee OA, it is unclear whether they provide clinical benefit. OARSI supports insoles for selected patients, but acknowledges that evidence is weak. Advise patients to select shoes that are flexible yet supportive and that moderately priced shoes appear to be as good as more expensive footwear for most OA patients. A podiatry consult may be beneficial for patients who have deformities of the feet or complain of foot pain.
Consider assistive devices. Patients with OA who are unstable may benefit from the use of a cane, walker, or other assistive device. Use common sense and individualize treatment, in consultation with an occupational therapist, if necessary.
Living with arthritis: Do’s and Don’ts
Osteoarthritis is an inflammation of the joint that affects many people as they age, mainly in the knees, hips, and hands. After many years of use, the cartilage that lines the inside of the joint thins and eventually wears off, leaving bone rubbing on bone. At the edge of the joint, the bone may grow into small “spurs” and fluid may increase inside, causing inflammation and pain.
There is no cure for arthritis. But there are many things that you and your doctor can do to make arthritis easier to live with, and to slow its progression. These Do’s and Don’ts may help:
Do get moving. Exercising your arthritic joints, except during acute flare-ups, will strengthen the muscles and help you stay active. Eventually you’ll find that you’re in less pain and can move about more easily.
Do consult a physical therapist to find out what type of exercise is best for you. Ask your doctor for a referral.
Don’t do those exercises during painful flare-ups. This is the time to give your joints a rest.
Do take acetaminophen (Tylenol), as needed, especially during flare-ups. You can take up to 4000 mg a day, but be sure to tell your doctor if you’re taking acetaminophen regularly.
Don’t take acetaminophen without first consulting your doctor if you have liver or kidney disease—or you’re taking a prescription pain medication.
Do ask your doctor about nonsteroidal anti-inflammatory drugs, sometimes called NSAIDs (pronounced N-SEDs). Some people benefit from ibuprofen (Advil, Motrin) or naproxen (Aleve), which are sold over-the-counter. Others take prescription NSAIDs such as Celebrex.
Don’t take NSAIDs without consulting your doctor if you’re over the age of 65. Both prescription and nonprescription NSAIDs can have serious side effects, and should be used with caution, if at all, by older people.
Do consider a trial of glucosamine (1500 mg daily), with or without chondroitin (1200 mg daily). Take it every day for 3 or 4 months without making other changes in treatment before you decide whether it’s working.
Don’t buy an expensive brand of glucosamine. The extra cost probably isn’t worth it.
Don’t take chondroitin without glucosamine, as it is unlikely to help.
Do apply ointments and rubs for pain relief. Start with capsaicin, which is available without a prescription. Buy the lowest strength you can find, and start by applying a very small amount on a small area because it may cause a burning sensation in the beginning. If you can’t tolerate capsaicin ointment, try a salicylate rub instead.
Do consider corticosteroid injections if you continue to have a lot of pain in your knee, especially if the doctor finds that you have fluid build-up. (If you don’t want to be injected with a steroid, ask your doctor if you’re a candidate for injections of hyaluronic acid, an artificial joint fluid that may help some patients.)
Do try TENS (transcutaneous electrical nerve stimulation) if you continue to have severe hip or knee pain. TENS therapy may be administered by a physical therapist, or with a device that you can use at home. A pad that emits a small tingle of electricity is placed over the painful joint to relieve the pain.
Do talk to your doctor about narcotic pain medication if your pain continues to be severe.
ADDITIONAL SELF-CARE TIPS:
Do try a wrap-around knee brace, which you can purchase at your local pharmacy. Ask your doctor about getting a cane or a walker if you need additional support.
Do alert your physician to certain conditions that can make your arthritis pain worse—insomnia, depression, or foot problems, for instance. And, if you have ill-fitting shoes or shoes that don’t provide much support, it’s time to replace them.
Joint replacement: When or whether?
Patients who are considering joint replacement surgery or are interested in learning more about it need a realistic picture of what to expect. Explain that results are usually—but not always—very favorable. However, surgery often brings greater improvement in pain than in function, and recovery can be very strenuous and lengthy. Infection rates average 1%,34,35 and mortality rates are low. In a large study of veterans, the 30-day mortality rates after knee and hip arthroplasty were 0.6% and 0.7%, respectively,36 but thromboembolic complications may occur despite prophylaxis. Overall, 5% of those undergoing joint replacement surgery develop significant complications.34,35
Knowing what surgery entails and the difficult recovery required may help patients be more tolerant of their OA symptoms. Whatever treatment your patients opt for, empathy on your part will help, as well.
CORRESPONDENCE
James Crosby, MD, United Health Services, Wilson Family Medicine Residency, 40 Arch Street, Johnson City, NY 13790; [email protected]
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
STEPS key: Tolerability/Simplicity: low=less tolerable/more complex; high=more tolerable/less complex.
Osteoarthritis (OA) is a common, almost expected, part of getting old. Some patients require no treatment or have symptoms that are easily controlled with over-the-counter analgesics or nonsteroidal anti-inflammatory drugs (NSAIDs) and lifestyle modifications. Others are so debilitated that surgery is the only way to go.
Where does that leave elderly patients in between—those who don’t make good surgical candidates or find little relief from basic interventions and want to “try everything” before surgery?
The best way to answer this question is to utilize the STEPS (Safety, Tolerability, Efficacy, Price, and Simplicity) format, a helpful mnemonic for an objective way to evaluate drugs or medical therapies.1
The recommendations, strength of recommendation (SOR) ratings, and summaries that follow are presented in this format, and draw upon recent reviews, focused studies, and scholarly analysis provided by the Osteoarthritis Research Society International (OARSI). The interventions are divided into 3 categories:
- over-the-counter remedies
- nonpharmacologic interventions
- injections/prescription drugs.
Over-the-counter remedies
Capsaicin ointment
Recommended (SOR: A).
Safety: High.
Tolerability: Medium. About 50% of patients experience a local (and possibly intense) burning sensation initially, but it generally wanes after several weeks. The number needed to harm (NNH) is about 10 for withdrawal due to adverse effects.2
Efficacy: Medium. Evidence regarding topical capsaicin comes mostly from studies of patients with musculoskeletal conditions in general, not OA specifically. About 40% of patients treated with capsaicin report a 50% decrease in musculoskeletal pain, as do 25% of those using a placebo (number needed to treat [NNT]=8).2
Price: Low. About $15 per month per joint.
Simplicity: Medium. Patients need to apply the ointment 3 or 4 times a day.
Tell patients, too, to use topical capsaicin for a few weeks before evaluating its effectiveness or deciding whether to abandon it. Emphasize that it should be used for base pain rather than for acute pain relief. (For those who can’t tolerate capsaicin, topical salicylate may offer similar benefits.)
Glucosamine
Recommended with or without chondroitin (SOR: B).
Safety: High.
Tolerability: High. Side effects are rare.
Efficacy: Low. There have been numerous trials of glucosamine alone (chondroitin alone is not recommended), mostly limited to knee pain. While many have found that glucosamine offers little or no clinical benefit, some have shown a benefit comparable to or slightly better than acetaminophen. Overall, evidence suggests that up to 40% of those who take glucosamine achieve a clinically significant response,3-5 although the placebo response may be substantial.
One study found that glucosamine was effective only when it was combined with chondroitin, and only when pain was more than mild.6 Another showed no clinical benefit for hip OA.7 There is limited evidence for or against the use of glucosamine and chondroitin to slow disease progression; the 2 agents are not recommended for this purpose.
Price: Low to medium ($5-$35 per month).
Simplicity: Medium to high; patients should take glucosamine (or glucosamine and chondroitin) 3 times a day. Compliance is likely to be variable, depending on results.
Evaluate after 4 months, and advise patients to discontinue if there is no significant improvement. Tell them, too, that while preparations vary by manufacturer and price, the difference between the various products is too small to justify the cost of the higher-priced brands.
Nonpharmacologic interventions
Acupuncture
Not recommended (SOR: A).
Safety: High.
Tolerability: Medium to high. Discomfort from the needles varies from slight to none.
Efficacy: Zero to low. Several studies have indicated some beneficial effect, but the degree of improvement has been small, with a sizable placebo effect.8 Studies typically include weekly sessions over the course of several months, and have demonstrated up to a 20% improvement in pain and function; however, much of that improvement has been attributed to the placebo effect, as demonstrated by studies that compared acupuncture with a sham procedure.8 Most data on acupuncture involve the knee; there is insufficient information about the procedure’s efficacy for other sites and for shorter treatment periods.
Price: Medium to high, approaching $1000 for a series of weekly sessions over 3 to 4 months.
Simplicity: Medium. Frequent office visits are standard. Long-term compliance is likely to be low, given the small benefit and the inconvenience.
Knee braces
Recommended (SOR: C).
Safety: High.
Tolerability: Medium to high. Tolerance depends on the type of brace and its complexity.
Efficacy: Low to medium. Few studies of knee bracing have been done; however, 1 trial showed that 73% of those who used sophisticated taping for 3 weeks reported improvement, vs 10% of no-tape controls.9 Pain decreased by 2 points on a 0- to 10-point scale at 3 weeks, and the benefit persisted for an additional 3 weeks after the taping was discontinued. The taping used in the study is impractical for usual and long-term use, but 45% of those who used a sham taping/wrap reported improvement and about a 1-point decrease in pain at 3 and 6 weeks. This suggests that a less sophisticated wrap, such as an elastic patellar-stabilization sleeve, is likely to provide some benefit.
Price: Low (generally less than $30).
Simplicity: High (for a basic sleeve or wrap).
TENS
Recommended (SOR: B).
Safety/Tolerability: High. Withdrawals due to adverse effects of transcutaneous electrical nerve stimulation (TENS) range from 0% to 14%.10
Efficacy: Medium. The mean improvement with TENS is about 20 points more (on a 100-point scale) than the effect associated with placebo; the data are based on knee OA pain that is moderate to severe. The effect may last 2 to 4 weeks; however, there have been few long-term studies of the benefits of TENS.
Price: Low to medium, depending on availability and insurance coverage.
Simplicity: Medium; TENS units can be used at home, but some patients may be uncomfortable using the apparatus.
Injections/prescription drugs
Corticosteroid injections
Recommended (SOR: A).
Safety: High for short-term use, but data on the degree and frequency of acute post-injection pain is limited. The possibility of harm from repeated injections is similarly uncertain, although 1 study of patients receiving 8 injections over a 2-year period found no progression in ill effects compared with patients receiving placebo.11
Tolerability: Medium to high. Discomfort from the needle is usually mild.
Efficacy: Low to medium. Critical analysis supports a modest benefit, with an NNT of 2 to 3 for short-term improvement (lasting 3-4 weeks).12,13 In 1 study, steroid injections produced an average of a 16-point reduction in pain on a 100-point scale. After 1 month, the benefit from the injection is usually not clinically significant.
Price: Low ($100-$200 per injection).
Simplicity: High. The injection itself is a relatively simple procedure, but patient compliance with a scheduled course of repeat injections is uncertain.
Hyaluronic acid (HA) injections
Recommended with caveats (SOR: A).
Safety: High.
Tolerability: Medium. A small number of patients experience a transitory local flare-up. In addition, some patients have an aversion to injections.
Efficacy: Low. Several recent meta-analyses and systematic reviews of HA injections for knee OA have found a small, often negligible, clinical effect, with placebo usually accounting for up to half of the reported benefit.14-18 In 1 study with no control group, however, patients experienced about a 60% decrease in composite pain score—and 75% were satisfied or very satisfied with the treatment.19 Studies of HA injection for hip OA are very limited, but a recent single-injection trial showed no benefit over placebo.20
Any benefit from an HA injection generally lasts 3 to 4 months.
Price: Medium-high. A course of 3 injections costs $700 to $1000 per joint. There are claims of substantial savings due to delayed joint replacement, but these are speculative.
Simplicity: Medium. Compliance (completing all 3 injections) was high in clinical trials, but is uncertain otherwise. However, injections may frequently be useless because the joint space isn’t entered. One study found that 29% of knee injections were extra-articular; placement was uncertain in another 5%. There was no correlation between successful placement and presumed expertise. This error is not surprising, considering that the articular space is usually narrowed and patients being treated for joint pain are often obese. Being able to aspirate fluid increases the chance of proper placement. (While placement errors may also occur with corticosteroid injections, that possibility is offset by a lower cost and somewhat greater likelihood of beneficial effect.)
- Consider this option only for patients with high-function goals and substantial pain or disability.
- Proceed cautiously with morbidly obese patients and patients without effusion.
- Opt for bacterial HA (Euflexxa), which has been found to be the most cost effective and have the highest efficacy and the fewest adverse effects; evidence suggests that hylan (Synvisc) should be avoided.15,16
HA injections can be repeated every 6 to 12 months if they prove to be highly beneficial. Advise patients, however, that data on repeated courses of the injections are very limited.
Nonsteroidal anti-inflammatory gel or patch
Not recommended (SOR: B).
Safety: High. Absorption is minimal, but there may be some systemic effects.
Tolerability: Medium to high, although some patients develop skin reactions to the patch and the gel.
Efficacy: Low. Pain reduction of up to 50% has been reported, but most of it is attributed to the placebo effect. The actual effect of the gel or patch averages only about 10%.21-24
Price: Medium. The gel sells for $150 to $180 per month for a quantity large enough to be used on 2 hands or 1 knee; the patch is similarly priced.
Simplicity: Low. Manufacturers recommend that the gel be applied 4 times a day, but this can prove difficult over the long term. The patch is applied only twice a day, but the painful area is usually too large or irregular for the patch to cover.
Narcotics for refractory pain
Recommended (SOR: B).
Safety: Medium. The elderly are particularly vulnerable to narcotics’ adverse effects, but the incidence of addiction and abuse in geriatric patients is very low. Recently released guidelines on the management of persistent pain from the American Geriatrics Society recommend that all older patients with moderate to severe pain or diminished quality of life be considered for narcotic therapy.25
Tolerability: Medium. Noncompliance is associated with side effects, which are usually dose-dependent. These include constipation, somnolence, and mental status changes. Reported noncompliance rates vary, in some cases reaching as high as 30%.26
Efficacy: Low to medium for weak narcotics (eg, codeine, tramadol), with about a 10% decrease in pain.26,27 However, studies generally suffer from small numbers of patients, short duration, inconsistency among clinical trials, and limited evaluation of disability and quality of life. One good study of tramadol users showed about a 15% decrease in pain among “young elders”—the total decrease in pain was 30%, including the placebo effect. Sleep quality improved and tolerability was high, with no increase in withdrawal due to adverse effects.28 For stronger narcotics (eg, morphine), direct evidence of efficacy for OA pain is lacking, but it seems reasonable to expect a medium benefit. Low-to-moderate daily doses of opioids for chronic noncancer pain—including arthritis—have been shown to improve the quality of life, compared with no narcotics or high doses.29
Price: Low (less than $20/month) for hydrocodone with acetaminophen; medium to high (more than $150/month) for long-acting morphine and transdermal fentanyl.
Simplicity: Medium to high. Twice-a-day dosing is available for long-acting strong opioids, but some narcotics must be taken 4 times a day.
What else can you do? Encourage activity—again
A clear conclusion from many studies is that nonaquatic exercise involving the affected joints, either specifically or as part of a general exercise routine, is clinically beneficial in decreasing pain and increasing function.30 When it comes to aquatic exercises for OA, the data are very limited, but suggest a small short-term benefit.31 Overall, the optimal type and duration of exercise for OA patients, as well as the best location (home or at a gym), is not known.
If your patient has stopped or markedly cut down on the time spent exercising, explore the feasibility of another attempt, and discuss ways to improve compliance. Stress the “bonus” benefits of exercise, such as mood elevation and an overall sense of well-being. If the patient is not undergoing physical therapy, consider a referral.
Use a chronic pain approach
Patients whose OA symptoms continue to be troublesome may benefit from a chronic pain syndrome approach. Nurse case management, phone contact, and extra attention from you may be helpful, along with educational material that the patient can consult between visits (PATIENT HANDOUT).32
Treat insomnia. Studies are insufficient for hypnotics for OA symptoms, but good sleep is a goal of standard pain management. Cognitive-behavioral therapy has been found to be helpful in alleviating sleep problems in patients with OA.33 At a minimum, take a good sleep history and provide basic sleep hygiene education.
Treat vitamin deficiency and depression. Many elderly patients are deficient in vitamin D, and evidence indicates that supplementation supports muscle strengthening and may aid in pain reduction. The effects are mostly preventive, however, and therefore not easily appreciated, so stress the importance of long-term health.
Depression, too, is common among the elderly, particularly for chronic pain patients. If you prescribe antidepressants or vitamin supplementation, emphasize that they are “for your arthritis.”
Inspect footwear. Despite several studies regarding the effects of shoes with inserts on knee OA, it is unclear whether they provide clinical benefit. OARSI supports insoles for selected patients, but acknowledges that evidence is weak. Advise patients to select shoes that are flexible yet supportive and that moderately priced shoes appear to be as good as more expensive footwear for most OA patients. A podiatry consult may be beneficial for patients who have deformities of the feet or complain of foot pain.
Consider assistive devices. Patients with OA who are unstable may benefit from the use of a cane, walker, or other assistive device. Use common sense and individualize treatment, in consultation with an occupational therapist, if necessary.
Living with arthritis: Do’s and Don’ts
Osteoarthritis is an inflammation of the joint that affects many people as they age, mainly in the knees, hips, and hands. After many years of use, the cartilage that lines the inside of the joint thins and eventually wears off, leaving bone rubbing on bone. At the edge of the joint, the bone may grow into small “spurs” and fluid may increase inside, causing inflammation and pain.
There is no cure for arthritis. But there are many things that you and your doctor can do to make arthritis easier to live with, and to slow its progression. These Do’s and Don’ts may help:
Do get moving. Exercising your arthritic joints, except during acute flare-ups, will strengthen the muscles and help you stay active. Eventually you’ll find that you’re in less pain and can move about more easily.
Do consult a physical therapist to find out what type of exercise is best for you. Ask your doctor for a referral.
Don’t do those exercises during painful flare-ups. This is the time to give your joints a rest.
Do take acetaminophen (Tylenol), as needed, especially during flare-ups. You can take up to 4000 mg a day, but be sure to tell your doctor if you’re taking acetaminophen regularly.
Don’t take acetaminophen without first consulting your doctor if you have liver or kidney disease—or you’re taking a prescription pain medication.
Do ask your doctor about nonsteroidal anti-inflammatory drugs, sometimes called NSAIDs (pronounced N-SEDs). Some people benefit from ibuprofen (Advil, Motrin) or naproxen (Aleve), which are sold over-the-counter. Others take prescription NSAIDs such as Celebrex.
Don’t take NSAIDs without consulting your doctor if you’re over the age of 65. Both prescription and nonprescription NSAIDs can have serious side effects, and should be used with caution, if at all, by older people.
Do consider a trial of glucosamine (1500 mg daily), with or without chondroitin (1200 mg daily). Take it every day for 3 or 4 months without making other changes in treatment before you decide whether it’s working.
Don’t buy an expensive brand of glucosamine. The extra cost probably isn’t worth it.
Don’t take chondroitin without glucosamine, as it is unlikely to help.
Do apply ointments and rubs for pain relief. Start with capsaicin, which is available without a prescription. Buy the lowest strength you can find, and start by applying a very small amount on a small area because it may cause a burning sensation in the beginning. If you can’t tolerate capsaicin ointment, try a salicylate rub instead.
Do consider corticosteroid injections if you continue to have a lot of pain in your knee, especially if the doctor finds that you have fluid build-up. (If you don’t want to be injected with a steroid, ask your doctor if you’re a candidate for injections of hyaluronic acid, an artificial joint fluid that may help some patients.)
Do try TENS (transcutaneous electrical nerve stimulation) if you continue to have severe hip or knee pain. TENS therapy may be administered by a physical therapist, or with a device that you can use at home. A pad that emits a small tingle of electricity is placed over the painful joint to relieve the pain.
Do talk to your doctor about narcotic pain medication if your pain continues to be severe.
ADDITIONAL SELF-CARE TIPS:
Do try a wrap-around knee brace, which you can purchase at your local pharmacy. Ask your doctor about getting a cane or a walker if you need additional support.
Do alert your physician to certain conditions that can make your arthritis pain worse—insomnia, depression, or foot problems, for instance. And, if you have ill-fitting shoes or shoes that don’t provide much support, it’s time to replace them.
Joint replacement: When or whether?
Patients who are considering joint replacement surgery or are interested in learning more about it need a realistic picture of what to expect. Explain that results are usually—but not always—very favorable. However, surgery often brings greater improvement in pain than in function, and recovery can be very strenuous and lengthy. Infection rates average 1%,34,35 and mortality rates are low. In a large study of veterans, the 30-day mortality rates after knee and hip arthroplasty were 0.6% and 0.7%, respectively,36 but thromboembolic complications may occur despite prophylaxis. Overall, 5% of those undergoing joint replacement surgery develop significant complications.34,35
Knowing what surgery entails and the difficult recovery required may help patients be more tolerant of their OA symptoms. Whatever treatment your patients opt for, empathy on your part will help, as well.
CORRESPONDENCE
James Crosby, MD, United Health Services, Wilson Family Medicine Residency, 40 Arch Street, Johnson City, NY 13790; [email protected]
1. Ebell M, Siwek J. AFP: Doing more to help you get the best evidence. Am Fam Physician. 2004;69:483-484.
2. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;228:991.-
3. Fox BA, Schmitz E, Wallace R. Glucosamine and chondroitin for osteoarthritis, Family Practice Inquires Network Clinical Inquires. Am Fam Physician. 2006;73:1245-1247.
4. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.-
5. Herrero-Beaumont G, Ivorra JA, Del Carmen TM, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms. Arthritis Rheum. 2007;56:555-567.
6. Clegg DO, Reda D, Harris CL, et al. Glucosamine, chrondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
7. Rozendaal RM, Koes BW, von Osch GJ, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148:268-277.
8. Manheimer E, Linde K, Lao L, et al. Meta-analysis: acupuncture for OA of the knee. Ann Intern Med. 2007;146:868-877.
9. Hinman RS, Crossley KM, McConnell J, et al. Efficacy of knee tape in the management of osteoarthritis of the knee: blinded randomised controlled trial. BMJ. 2003;327:135.-
10. Bjordal JM, Johnson MI, Lopes-Martins RA, et al. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8:51.-
11. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370-377.
12. Arroll B, Goodinyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869-873.
13. Palacios L, Jones W, Mayo H. Do steroid injections help with osteoarthritis of the knee? J Fam Pract. 2004;53:921-922.
14. Bellamy N, Campell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
15. Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172:1039-1043.
16. Medina JM, Thomas A, Denegar CR. Knee osteoarthritis: should your patient opt for hyaluronic acid injection? J Fam Pract. 2006;55:669-675.
17. Modawal A, Fley J, Shukla R, et al. Hyaluronic acid injections relieve knee pain. J Fam Pract. 2005;54:758-767.
18. Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging. 2007;24:629-642.
19. Kichner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-162.
20. Richette P, Ravaud P, Conrozier T, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: a multicenter, randomized, placebo-controlled trial. Arthritis Rheum. 2009;60:824-830.
21. Underwood M. Advice to use topical or oral ibuprofen for chronic knee pain in older people: randomized controlled trial and patient preference study. BMJ. 2008;336:138-142.
22. Diclofenac gel for osteoarthritis. Medical Letter. 2008;50:31-32.
23. Lin J, Zhang W, Jones A, et al. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomized controlled trials. BMJ. 2004;329:324-329.
24. A diclofenac patch for pain. Medical Letter. 2008;50:1-2.
25. American Geriatrics Society. American Geriatrics Society announces new guidelines to improve pain management, quality of life, and quality of care for older patients. May 1, 2009. Available at: http://www.americangeriatrics.org/news/pain043009.shtml. Accessed June 1, 2009.
26. Peloso PM. Opioid therapy for osteoarthritis of the hip and knee: use it or lose it? J Rheum. 2001;28:6-10.
27. Bannwarth B. Letter: Opioids for osteoarthritis of the hip and knee: which opioids for which patients? J Rheum. 2001;28:1930-1931.
28. Vorsanger G, Xiang J, Jordan D, et al. Post hoc analysis of a randomized, double blind, placebo-controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clin Ther. 2007;29:2520-2535.
29. Dillie KS, Fleming MF, Mundt MP, et al. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21:108-117.
30. Fransen M, McConnell S, Bell M. Exercise for osteoarthritis of the hip or knee. Cochrane Database Syst Rev. 2003;(3):CD004376.-
31. Bartels EM, Lund H, Hagen KB, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2007;(4):CD005523.-
32. Rosemann T, Joos S, Laux G, et al. Case management of arthritis patients in primary care: a cluster-randomized controlled trial. Arthritis Rheum. 2007;57:1390-1397.
33. Vitiello M. CBT for insomnia may reduce osteoarthritis pain. Poster presented at: 60th Annual Meeting of the Gerontological Society of America; November 16-20, 2007; San Francisco, Calif.
34. Erens G, Thronhill T. Complications of total hip arthroplasty. In: Basow DS, ed. UpToDate. Waltham, Mass: UpToDate; 2009. Available at: http://www.uptodate.com. Accessed May 20, 2009.
35. Martin G, Thornhill T. Complications of total knee arthroplasty. In: Basow DS, ed. UpToDate. Waltham, Mass: UpToDate; 2009. Available at: http://www.uptodate.com. Accessed May 20, 2009.
36. Ibrahim SA, Stone RA, Han X, et al. Racial/ethnic differences in surgical outcomes in veterans following knee and hip arthroplasty. Arthritis Rheum. 2005;52:3143-3151.
1. Ebell M, Siwek J. AFP: Doing more to help you get the best evidence. Am Fam Physician. 2004;69:483-484.
2. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;228:991.-
3. Fox BA, Schmitz E, Wallace R. Glucosamine and chondroitin for osteoarthritis, Family Practice Inquires Network Clinical Inquires. Am Fam Physician. 2006;73:1245-1247.
4. Towheed TE, Maxwell L, Anastassiades TP, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2005;(2):CD002946.-
5. Herrero-Beaumont G, Ivorra JA, Del Carmen TM, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms. Arthritis Rheum. 2007;56:555-567.
6. Clegg DO, Reda D, Harris CL, et al. Glucosamine, chrondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795-808.
7. Rozendaal RM, Koes BW, von Osch GJ, et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med. 2008;148:268-277.
8. Manheimer E, Linde K, Lao L, et al. Meta-analysis: acupuncture for OA of the knee. Ann Intern Med. 2007;146:868-877.
9. Hinman RS, Crossley KM, McConnell J, et al. Efficacy of knee tape in the management of osteoarthritis of the knee: blinded randomised controlled trial. BMJ. 2003;327:135.-
10. Bjordal JM, Johnson MI, Lopes-Martins RA, et al. Short-term efficacy of physical interventions in osteoarthritic knee pain. A systematic review and meta-analysis of randomised placebo-controlled trials. BMC Musculoskelet Disord. 2007;8:51.-
11. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48:370-377.
12. Arroll B, Goodinyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869-873.
13. Palacios L, Jones W, Mayo H. Do steroid injections help with osteoarthritis of the knee? J Fam Pract. 2004;53:921-922.
14. Bellamy N, Campell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321.-
15. Arrich J, Piribauer F, Mad P, et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172:1039-1043.
16. Medina JM, Thomas A, Denegar CR. Knee osteoarthritis: should your patient opt for hyaluronic acid injection? J Fam Pract. 2006;55:669-675.
17. Modawal A, Fley J, Shukla R, et al. Hyaluronic acid injections relieve knee pain. J Fam Pract. 2005;54:758-767.
18. Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging. 2007;24:629-642.
19. Kichner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-162.
20. Richette P, Ravaud P, Conrozier T, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: a multicenter, randomized, placebo-controlled trial. Arthritis Rheum. 2009;60:824-830.
21. Underwood M. Advice to use topical or oral ibuprofen for chronic knee pain in older people: randomized controlled trial and patient preference study. BMJ. 2008;336:138-142.
22. Diclofenac gel for osteoarthritis. Medical Letter. 2008;50:31-32.
23. Lin J, Zhang W, Jones A, et al. Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomized controlled trials. BMJ. 2004;329:324-329.
24. A diclofenac patch for pain. Medical Letter. 2008;50:1-2.
25. American Geriatrics Society. American Geriatrics Society announces new guidelines to improve pain management, quality of life, and quality of care for older patients. May 1, 2009. Available at: http://www.americangeriatrics.org/news/pain043009.shtml. Accessed June 1, 2009.
26. Peloso PM. Opioid therapy for osteoarthritis of the hip and knee: use it or lose it? J Rheum. 2001;28:6-10.
27. Bannwarth B. Letter: Opioids for osteoarthritis of the hip and knee: which opioids for which patients? J Rheum. 2001;28:1930-1931.
28. Vorsanger G, Xiang J, Jordan D, et al. Post hoc analysis of a randomized, double blind, placebo-controlled efficacy and tolerability study of tramadol extended release for the treatment of osteoarthritis pain in geriatric patients. Clin Ther. 2007;29:2520-2535.
29. Dillie KS, Fleming MF, Mundt MP, et al. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21:108-117.
30. Fransen M, McConnell S, Bell M. Exercise for osteoarthritis of the hip or knee. Cochrane Database Syst Rev. 2003;(3):CD004376.-
31. Bartels EM, Lund H, Hagen KB, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2007;(4):CD005523.-
32. Rosemann T, Joos S, Laux G, et al. Case management of arthritis patients in primary care: a cluster-randomized controlled trial. Arthritis Rheum. 2007;57:1390-1397.
33. Vitiello M. CBT for insomnia may reduce osteoarthritis pain. Poster presented at: 60th Annual Meeting of the Gerontological Society of America; November 16-20, 2007; San Francisco, Calif.
34. Erens G, Thronhill T. Complications of total hip arthroplasty. In: Basow DS, ed. UpToDate. Waltham, Mass: UpToDate; 2009. Available at: http://www.uptodate.com. Accessed May 20, 2009.
35. Martin G, Thornhill T. Complications of total knee arthroplasty. In: Basow DS, ed. UpToDate. Waltham, Mass: UpToDate; 2009. Available at: http://www.uptodate.com. Accessed May 20, 2009.
36. Ibrahim SA, Stone RA, Han X, et al. Racial/ethnic differences in surgical outcomes in veterans following knee and hip arthroplasty. Arthritis Rheum. 2005;52:3143-3151.
What to do when warfarin therapy goes too far
- For patients with an elevated international normalized ratio (INR) with mild or no bleeding, withhold the warfarin and recheck INR in 1 to 2 days; if INR >5, add oral vitamin K supplementation (C).
- For major bleeding and elevated INR, hospital admission, vitamin K, fresh frozen plasma, and frequent monitoring are needed (B).
- Emergent situations call for hospitalization, clotting factor replacement, and vitamin K administered by slow intravenous infusion (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
I feel weak,” reports Mary Jo, a 67-year-old patient who scheduled today’s appointment when she began noticing black, tarry stools 2 days ago. Her chart reveals that she’s on warfarin therapy for chronic atrial fibrillation, and today’s labs show a hematocrit of 18 and an international normalized ratio (INR) of 6.
If Mary Jo were your patient, what would you do?
With some 30.6 million outpatient prescriptions dispensed in the United States in a single year,1 warfarin is among the nation’s most commonly prescribed medications. It is also a dangerous drug. Warfarin’s anticoagulant and antithrombotic effects occur through its ability to inhibit the enzymes responsible for the reduction of vitamin K—an essential cofactor in the normal production of vitamin K-dependent clotting factors II, VII, XI, and X and anticoagulant factors protein C and S. In the presence of warfarin, these clotting factors are produced in a partially carboxylated state with reduced or absent biological activity. The result is a hypocoagulability that can be life-threatening.
Given the sheer number of patients receiving warfarin therapy and the potential for hemorrhage and other adverse effects, primary care physicians need to be familiar with evidence-based recommendations for managing warfarin-induced hypocoagulation. This review will help ensure that when you see patients like Mary Jo, you’ll be prepared to take the best approach to reversing their hypocoagulable state.
Which patients face the highest risk?
The reported incidence of bleeding in patients taking warfarin varies significantly, but is generally in the range of 1% annually.2 Among those who develop warfarin-related major bleeds, however, the fatality rate may be as high as 13.4%.3
The risk of bleeding is highest in the first 30 days of warfarin therapy,3 and increases exponentially once the INR exceeds 5.4 Other risk factors include:
- age (the risk increases to about 5% per year for patients >75 years)5
- hypertension
- cerebrovascular disease
- ischemic stroke
- a history of bleeds.6-8
Multiple medications and herbal substances can interfere with warfarin therapy. Some agents work by augmenting warfarin’s effect; others, such as antiplatelet agents, directly increase the risk of bleeding through unrelated mechanisms; still others may counteract warfarin therapy by enhancing coagulation. Ask patients on warfarin therapy to tell you everything they’re taking, including all over-the-counter medications, supplements, and prescription drugs. TABLE 1 lists herbal substances with the potential to increase or decrease INR. A comprehensive list of drugs that can interact with warfarin is available at http://www.drugs.com/drug-interactions/coumadin_d00022.html.
TABLE 1
Herbal substances that may affect INR38-40
| INCREASE INR | DECREASE INR | |
|---|---|---|
| Angelica root Anise Arnica flower Asafoetida Bogbean Boldo-fenugreek Borage seed oil Bromelain Capsicum Celery Chamomile Clove Dashen Devil’s claw Dong quai Feverfew Fish oil Garlic Ginger Ginkgo | Goldenseal Horse chestnut Licorice root Lovage root Lycium barbarum (wolfberry) Meadowsweet Onion Papain Parsley Passionflower Poplar Quassia Quilinggao Red clover Rue Sweet clover Turmeric Vitamin E Willow bark | Coenzyme Q10 Ginseng Green tea St. John’s wort |
When reversal is needed, how best to achieve it?
The options for reversing warfarin-induced anticoagulation include withholding 1 or more doses of warfarin and providing vitamin K supplementation and clotting factor replacement, as needed. The decision of which combination to use is based on both the urgency ( TABLE 2 ) and completeness of reversal required (target INR range) and the risk of thrombosis when the anticoagulation is reversed.9
Vitamin K is actually a group of lipid-soluble chemicals that are necessary for the production of functional carboxylated clotting factors II, VII, IX, and X. Vitamin K1 (phytonadione), which is available in food and as a supplement, is the particular chemical that competes with warfarin. When it is used as a reversal agent, phytonadione is generally referred to simply as vitamin K.
The oral route of vitamin K is preferred, but its effect is delayed because of the time required for absorption and production of factors. Thus, a slow (15-30 min) infusion of intravenous (IV) vitamin K should be used if reversal is needed within 6 hours—or oral therapy is unavailable. Avoid subcutaneous administration; it is not reliable and may take up to 72 hours to reverse the INR.10-12 Intramuscular (IM) administration of vitamin K should also be avoided in patients taking warfarin because of concerns about hematoma formation, although a 2003 study of patients in teaching hospitals found that the IM route is used about 10% of the time.13
The optimal dose of vitamin K varies, based on patient-specific factors such as comorbidities, metabolic and genetic variation, weight, age, and liver function. Doses as low as 0.5 mg IV or 1 mg oral vitamin K have been effective in reversing an elevated INR to a therapeutic range in nonlife-threatening situations.11,14,15 The American College of Chest Physicians (ACCP), which issued new guidelines in 2008, recommends doses of <5 mg for an INR >5 but <9 if there is a high risk of bleeding; 5 to 10 mg is the recommended dose for all patients with an INR ≥9. In cases of significant bleeding, a dose of 10 mg IV is recommended.16 Excessive vitamin K supplementation may lead to warfarin resistance, making it necessary to use much higher doses of warfarin down the road to achieve therapeutic INR levels.
Fresh frozen plasma (FFP) replaces functional vitamin K-dependent clotting factors that are decreased in patients taking warfarin. The suggested dose is 15 mL/kg,17,18 but patients must be monitored with coagulation laboratory values to assess the amount needed. One unit of FFP is roughly 250 mL, which corresponds to roughly 250 units of clotting factors.
FFP works to offset coagulopathy quickly. But because the plasma is frozen, it has to be thawed and blood type-matched, which is time-consuming. FFP transfusion also may be associated with infections, although the risk is generally believed to be minimal.17 Other limitations in using FFP include the large volume of fluid that must be administered—with the attendant risk of fluid overload—and the possibility of significant infusion reactions that may require slowing the infusion rate.19
Prothrombin complex concentrate (PCC) is pooled from donor plasma and lyophilized to a powder. It is then reconstituted for clotting factor replacement, and is available through the pharmacy rather than the blood bank.20 PCC is dosed in international units of factor IX, although it includes proportional amounts of factors II, VII, and X and proteins C and S. The typical recommended dose is 30 to 50 U/kg.20,21
Although PCC contains human coagulation factors, it does not involve the same risks of fluid overload or infectious transmission as FFP. It can be given IV over 5 to 10 minutes. The risk of thrombogenicity has been reported in patients with hemophilia who receive PCC,22 but studies of PCC use in warfarin reversal have not shown this adverse effect.23,24 Data from the use of PCC for the treatment of hemophilia suggest that the risk of thromboembolic events begins with daily doses >200 U/kg. There is limited information about the safety of giving PCC to patients with mechanical valve replacement, pregnant women, and those in other high-risk situations.
Recombinant activated factor VII (rFVIIa) is also effective in reversing elevated INR.25,26 It replaces 1 of the clotting factors that is decreased in anticoagulated patients (factor VII), but the significance of not replacing factors II, IX, and X is unknown.27 The recommended quantity of rFVIIa ranges from a single dose of 1200 mcg to weight-based dosing (10-160 mcg/kg).25,28-30 (IV vitamin K and FFP are also given in emergent situations in which rFVIIa is administered.) Thrombogenicity is a possible complication with the use of rFVIIa, but data are scarce regarding the incidence of adverse effects.
Neither PCC nor rFVIIa has US Food and Drug Administration approval for use in reversing warfarin-induced anticoagulation. Their use for this purpose may be warranted only in situations that threaten life or limb, and must be guided by clinical judgment.
TABLE 2
How fast? Reversal agents and time of action32
| SPEED/TYPE OF REVERSAL REQUIRED | WHAT TO USE |
|---|---|
| Rapid (complete; within 10-15 minutes) | PCC or rFVIIa + vitamin K IV |
| Fast (partial) | FFP + vitamin K IV |
| Prompt (4-6 hours) | Vitamin K IV |
| Slow (within 24 hours) | Oral vitamin K |
| Ultra-slow (over a period of days) | Omit warfarin dose (no vitamin K) |
| FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII. | |
Severity of bleeding as a treatment guide
Studies of methods used to reverse warfarin’s anticoagulation effect are difficult to compare because of a lack of a standardized approach to the classification of bleeds.18,31,32 We’ve used the following classification system, modified from that of Fihn et al,31 to avoid confusion and inform treatment decisions:
- Minor bleed: Reported, not requiring additional testing
- Major bleed: Requiring medical evaluation and inpatient treatment and/or blood transfusion
- Life-threatening bleed: Leading to cardiac arrest, surgical/angiographic intervention, or irreversible sequelae (loss of limb/sight).
Here’s how to put this classification system—and the ACCP’s 2008 guidelines for managing patients with elevated INR16 ( FIGURE )—into action:
In the case of minor bleeding and elevated INR, withhold the next 1 to 2 doses of warfarin.16 If the patient is considered high risk, give oral vitamin K in small amounts (1-2.5 mg). Keep in mind that excessive amounts of vitamin K will promote warfarin resistance.
FIGURE
Reversal of warfarin-induced anticoagulation
FFP, fresh frozen plasma; INR, international normalized ratio; IV, intravenous; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII.
Adapted from: Ansell J et al. Chest. 2008.16 Ask the patient to return to your office in 1 or 2 days for a recheck of INR. If it remains elevated, give another dose of vitamin K. Warfarin may need to be restarted at a lower dose depending on the clinical situation.17,32
In cases of major bleeding and elevated INR, stop the warfarin, give vitamin K (the administration route will be based on clinical presentation and the urgency of reversal), and arrange for factor replacement and hospital admission.16 Use FFP, if possible, because more is known about its safety than the safety of PCC or rFVIIa. That said, the choice of factor replacement should be based on the urgency of reversal and on clinical condition.16,33,34
INR should be rechecked immediately after factor replacement. Because coagulation factors have varied half-lives, INR should be checked daily for the next 4 days to confirm that it remains at a therapeutic level.16
In cases of life-threatening bleeding, stop the warfarin, give 10 mg vitamin K IV, and replace clotting factors.16 PCC should be considered because it will reverse the anticoagulation in the shortest amount of time, without limitations associated with fluid status, blood type-matching, or infusion reaction.35,36 While the risk of thrombosis needs to be evaluated in each high-risk circumstance, the use of either PCC or rFVIIa, depending on availability, is appropriate for a life-threatening bleed.29,37
Classifying—and treating—our patient
Based on our classification system, we determined that Mary Jo had major bleeding: She needed inpatient monitoring, with the possibility of a blood transfusion, but her condition was not life-threatening. She was hospitalized immediately. Her warfarin was withheld and she was given vitamin K IV and FFP to reverse her hypocoagulable state.
Once Mary Jo’s hypocoagulable state was reversed, which took about 4 hours, she was evaluated and found to have a small bleeding ulcer. The ulcer was cauterized, and her condition remained stable. A detailed investigation of possible reasons for the patient’s elevated INR did not reveal any causes. Three days after the cauterization, Mary Jo was started back on a lower dosing schedule of warfarin. She was discharged after a 5-day stay, with instructions to return to the clinic in 5 days for continued monitoring.
CORRESPONDENCE
Shailendra Prasad, MBBS, MPH, 1020 W. Broadway, Minneapolis, MN 55411; [email protected]
1. Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414-1419.
2. Beyth RJ. Hemorrhagic complications of oral anticoagulant therapy. Clin Geriatr Med. 2001;17:49-56.
3. Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893-900.
4. Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):S287-S310.
5. Pengo V, Legnani C, Noventa F, et al. ISCOAT Study Group. (Italian Study on Complications of Oral Anticoagulant Therapy). Oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and risk of bleeding. A multicenter inception cohort study. Thromb Haemost. 2001;85:418-422.
6. White RH, McKittrick T, Takakuwa J, et al. Management and prognosis of life-threatening bleeding during warfarin therapy. National Consortium of Anticoagulation Clinics. Arch Intern Med. 1996;156:1197-1201.
7. Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91-99.
8. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
9. Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):S204-S233.
10. Crowther MA, Douketis JD, Schnurr T, et al. Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial. Ann Intern Med. 2002;137:251-254.
11. Whitling AM, Bussey HI, Lyons RM. Comparing different routes and doses of phytonadione for reversing excessive anticoagulation. Arch Intern Med. 1998;158:2136-2140.
12. Nee R, Doppenschmidt D, Donovan DJ, et al. Intravenous versus subcutaneous vitamin K1 in reversing excessive oral anticoagulation. Am J Cardiol. 1999;83:286-288.
13. Fan J, Armitstead JA, Adams AG, et al. A retrospective evaluation of vitamin K1 therapy to reverse the anticoagulant effect of warfarin. Pharmacotherapy. 2003;23:1245-1250.
14. Lubetsky A, Yonath H, Olchovsky D, et al. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163:2469-2473.
15. Hung A, Singh S, Tait RC. A prospective randomized study to determine the optimal dose of intravenous vitamin K in reversal of over-warfarinization. Br J Haematol. 2000;109:537-539.
16. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists. Chest. 2008;133(suppl):160S-198S.
17. Dentali F, Ageno W, Crowther M. Treatment of coumarin-associated coagulopathy: a systematic review and proposed algorithms. J Thromb Haemost. 2006;4:1853-1863.
18. Makris M, Watson HG. The management of coumarin-induced over-anticoagulation Annotation [see comment]. Br J Haematol. 2001;114:271-280.
19. O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11-28.
20. Makris M. Optimisation of the prothrombin complex concentrate dose for warfarin reversal. Thromb Res. 2005;115:451-453.
21. Vigue B, Ract C, Tremey B, et al. Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial hemorrhage. Intensive Care Med. 2007;33:721-725.
22. Kohler M. Thrombogenicity of prothrombin complex concentrates. Thromb Res. 1999;95(suppl 1):S13-S17.
23. Lorenz R, Kienast J, Otto U, et al. Successful emergency reversal of phenprocoumon anticoagulation with prothrombin complex concentrate: a prospective clinical study. Blood Coagul Fibrinolysis. 2007;18:565-570.
24. Pabinger I, Brenner B, Kalina U, et al. Beriplex P/N Anticoagulation Reversal Study Group. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622-631.
25. Sorensen B, Johansen P, Nielsen GL, et al. Reversal of the International Normalized Ratio with recombinant activated factor VII in central nervous system bleeding during warfarin thromboprophylaxis: clinical and biochemical aspects. Blood Coagul Fibrinolysis. 2003;14:469-477.
26. Brody DL, Aiyagari V, Shackleford AM, et al. Use of recombinant factor VIIa in patients with warfarin-associated intracranial hemorrhage. Neurocrit Care. 2005;2:263-267.
27. Tanaka KA, Szlam F, Dickneite G, et al. Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb Res. 2008;122:117-123.
28. Lin J, Hanigan WC, Tarantino M, et al. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98:737-740.
29. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777-785.
30. Dager WE, King JH, Regalia RC, et al. Reversal of elevated international normalized ratio and bleeding with low-dose recombinant factor VII in patients receiving warfarin. Pharmacotherapy. 2006;26:1091-1098.
31. Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993;118:511-520.
32. Hanley JP. Warfarin reversal. J Clin Pathol. 2004;57:1132-1139.
33. Yasaka M, Sakata T, Minematsu K, et al. Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complication. Thromb Res. 2002;108:25-30.
34. Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced anticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;115:998-1001.
35. Lubetsky A, Hoffman R, Zimlichman R, et al. Efficacy and safety of complex concentrate (Octaplex) for rapid reversal of oral anticoagulation. Thromb Res. 2004;113:371-378.
36. Cartmill M, Dolan G, Byrne JL, et al. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg. 2000;14:458-461.
37. Deveras RA, Kessler CM. Reversal of warfarin-induced excessive anticoagulation with recombinant human factor VIIa concentrate. Ann Intern Med. 2002;137:884-888.
38. Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106.
39. Haines ST, Zeolla M, Witt DM. Venous Thromboembolism. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiological Approach. 6th ed. New York: McGraw-Hill Companies, Inc.; 2005:373-413.
40. Micromedex Healthcare Series. Drug-REAX System. New York: Thomson Reuters; 2008.
- For patients with an elevated international normalized ratio (INR) with mild or no bleeding, withhold the warfarin and recheck INR in 1 to 2 days; if INR >5, add oral vitamin K supplementation (C).
- For major bleeding and elevated INR, hospital admission, vitamin K, fresh frozen plasma, and frequent monitoring are needed (B).
- Emergent situations call for hospitalization, clotting factor replacement, and vitamin K administered by slow intravenous infusion (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
I feel weak,” reports Mary Jo, a 67-year-old patient who scheduled today’s appointment when she began noticing black, tarry stools 2 days ago. Her chart reveals that she’s on warfarin therapy for chronic atrial fibrillation, and today’s labs show a hematocrit of 18 and an international normalized ratio (INR) of 6.
If Mary Jo were your patient, what would you do?
With some 30.6 million outpatient prescriptions dispensed in the United States in a single year,1 warfarin is among the nation’s most commonly prescribed medications. It is also a dangerous drug. Warfarin’s anticoagulant and antithrombotic effects occur through its ability to inhibit the enzymes responsible for the reduction of vitamin K—an essential cofactor in the normal production of vitamin K-dependent clotting factors II, VII, XI, and X and anticoagulant factors protein C and S. In the presence of warfarin, these clotting factors are produced in a partially carboxylated state with reduced or absent biological activity. The result is a hypocoagulability that can be life-threatening.
Given the sheer number of patients receiving warfarin therapy and the potential for hemorrhage and other adverse effects, primary care physicians need to be familiar with evidence-based recommendations for managing warfarin-induced hypocoagulation. This review will help ensure that when you see patients like Mary Jo, you’ll be prepared to take the best approach to reversing their hypocoagulable state.
Which patients face the highest risk?
The reported incidence of bleeding in patients taking warfarin varies significantly, but is generally in the range of 1% annually.2 Among those who develop warfarin-related major bleeds, however, the fatality rate may be as high as 13.4%.3
The risk of bleeding is highest in the first 30 days of warfarin therapy,3 and increases exponentially once the INR exceeds 5.4 Other risk factors include:
- age (the risk increases to about 5% per year for patients >75 years)5
- hypertension
- cerebrovascular disease
- ischemic stroke
- a history of bleeds.6-8
Multiple medications and herbal substances can interfere with warfarin therapy. Some agents work by augmenting warfarin’s effect; others, such as antiplatelet agents, directly increase the risk of bleeding through unrelated mechanisms; still others may counteract warfarin therapy by enhancing coagulation. Ask patients on warfarin therapy to tell you everything they’re taking, including all over-the-counter medications, supplements, and prescription drugs. TABLE 1 lists herbal substances with the potential to increase or decrease INR. A comprehensive list of drugs that can interact with warfarin is available at http://www.drugs.com/drug-interactions/coumadin_d00022.html.
TABLE 1
Herbal substances that may affect INR38-40
| INCREASE INR | DECREASE INR | |
|---|---|---|
| Angelica root Anise Arnica flower Asafoetida Bogbean Boldo-fenugreek Borage seed oil Bromelain Capsicum Celery Chamomile Clove Dashen Devil’s claw Dong quai Feverfew Fish oil Garlic Ginger Ginkgo | Goldenseal Horse chestnut Licorice root Lovage root Lycium barbarum (wolfberry) Meadowsweet Onion Papain Parsley Passionflower Poplar Quassia Quilinggao Red clover Rue Sweet clover Turmeric Vitamin E Willow bark | Coenzyme Q10 Ginseng Green tea St. John’s wort |
When reversal is needed, how best to achieve it?
The options for reversing warfarin-induced anticoagulation include withholding 1 or more doses of warfarin and providing vitamin K supplementation and clotting factor replacement, as needed. The decision of which combination to use is based on both the urgency ( TABLE 2 ) and completeness of reversal required (target INR range) and the risk of thrombosis when the anticoagulation is reversed.9
Vitamin K is actually a group of lipid-soluble chemicals that are necessary for the production of functional carboxylated clotting factors II, VII, IX, and X. Vitamin K1 (phytonadione), which is available in food and as a supplement, is the particular chemical that competes with warfarin. When it is used as a reversal agent, phytonadione is generally referred to simply as vitamin K.
The oral route of vitamin K is preferred, but its effect is delayed because of the time required for absorption and production of factors. Thus, a slow (15-30 min) infusion of intravenous (IV) vitamin K should be used if reversal is needed within 6 hours—or oral therapy is unavailable. Avoid subcutaneous administration; it is not reliable and may take up to 72 hours to reverse the INR.10-12 Intramuscular (IM) administration of vitamin K should also be avoided in patients taking warfarin because of concerns about hematoma formation, although a 2003 study of patients in teaching hospitals found that the IM route is used about 10% of the time.13
The optimal dose of vitamin K varies, based on patient-specific factors such as comorbidities, metabolic and genetic variation, weight, age, and liver function. Doses as low as 0.5 mg IV or 1 mg oral vitamin K have been effective in reversing an elevated INR to a therapeutic range in nonlife-threatening situations.11,14,15 The American College of Chest Physicians (ACCP), which issued new guidelines in 2008, recommends doses of <5 mg for an INR >5 but <9 if there is a high risk of bleeding; 5 to 10 mg is the recommended dose for all patients with an INR ≥9. In cases of significant bleeding, a dose of 10 mg IV is recommended.16 Excessive vitamin K supplementation may lead to warfarin resistance, making it necessary to use much higher doses of warfarin down the road to achieve therapeutic INR levels.
Fresh frozen plasma (FFP) replaces functional vitamin K-dependent clotting factors that are decreased in patients taking warfarin. The suggested dose is 15 mL/kg,17,18 but patients must be monitored with coagulation laboratory values to assess the amount needed. One unit of FFP is roughly 250 mL, which corresponds to roughly 250 units of clotting factors.
FFP works to offset coagulopathy quickly. But because the plasma is frozen, it has to be thawed and blood type-matched, which is time-consuming. FFP transfusion also may be associated with infections, although the risk is generally believed to be minimal.17 Other limitations in using FFP include the large volume of fluid that must be administered—with the attendant risk of fluid overload—and the possibility of significant infusion reactions that may require slowing the infusion rate.19
Prothrombin complex concentrate (PCC) is pooled from donor plasma and lyophilized to a powder. It is then reconstituted for clotting factor replacement, and is available through the pharmacy rather than the blood bank.20 PCC is dosed in international units of factor IX, although it includes proportional amounts of factors II, VII, and X and proteins C and S. The typical recommended dose is 30 to 50 U/kg.20,21
Although PCC contains human coagulation factors, it does not involve the same risks of fluid overload or infectious transmission as FFP. It can be given IV over 5 to 10 minutes. The risk of thrombogenicity has been reported in patients with hemophilia who receive PCC,22 but studies of PCC use in warfarin reversal have not shown this adverse effect.23,24 Data from the use of PCC for the treatment of hemophilia suggest that the risk of thromboembolic events begins with daily doses >200 U/kg. There is limited information about the safety of giving PCC to patients with mechanical valve replacement, pregnant women, and those in other high-risk situations.
Recombinant activated factor VII (rFVIIa) is also effective in reversing elevated INR.25,26 It replaces 1 of the clotting factors that is decreased in anticoagulated patients (factor VII), but the significance of not replacing factors II, IX, and X is unknown.27 The recommended quantity of rFVIIa ranges from a single dose of 1200 mcg to weight-based dosing (10-160 mcg/kg).25,28-30 (IV vitamin K and FFP are also given in emergent situations in which rFVIIa is administered.) Thrombogenicity is a possible complication with the use of rFVIIa, but data are scarce regarding the incidence of adverse effects.
Neither PCC nor rFVIIa has US Food and Drug Administration approval for use in reversing warfarin-induced anticoagulation. Their use for this purpose may be warranted only in situations that threaten life or limb, and must be guided by clinical judgment.
TABLE 2
How fast? Reversal agents and time of action32
| SPEED/TYPE OF REVERSAL REQUIRED | WHAT TO USE |
|---|---|
| Rapid (complete; within 10-15 minutes) | PCC or rFVIIa + vitamin K IV |
| Fast (partial) | FFP + vitamin K IV |
| Prompt (4-6 hours) | Vitamin K IV |
| Slow (within 24 hours) | Oral vitamin K |
| Ultra-slow (over a period of days) | Omit warfarin dose (no vitamin K) |
| FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII. | |
Severity of bleeding as a treatment guide
Studies of methods used to reverse warfarin’s anticoagulation effect are difficult to compare because of a lack of a standardized approach to the classification of bleeds.18,31,32 We’ve used the following classification system, modified from that of Fihn et al,31 to avoid confusion and inform treatment decisions:
- Minor bleed: Reported, not requiring additional testing
- Major bleed: Requiring medical evaluation and inpatient treatment and/or blood transfusion
- Life-threatening bleed: Leading to cardiac arrest, surgical/angiographic intervention, or irreversible sequelae (loss of limb/sight).
Here’s how to put this classification system—and the ACCP’s 2008 guidelines for managing patients with elevated INR16 ( FIGURE )—into action:
In the case of minor bleeding and elevated INR, withhold the next 1 to 2 doses of warfarin.16 If the patient is considered high risk, give oral vitamin K in small amounts (1-2.5 mg). Keep in mind that excessive amounts of vitamin K will promote warfarin resistance.
FIGURE
Reversal of warfarin-induced anticoagulation
FFP, fresh frozen plasma; INR, international normalized ratio; IV, intravenous; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII.
Adapted from: Ansell J et al. Chest. 2008.16 Ask the patient to return to your office in 1 or 2 days for a recheck of INR. If it remains elevated, give another dose of vitamin K. Warfarin may need to be restarted at a lower dose depending on the clinical situation.17,32
In cases of major bleeding and elevated INR, stop the warfarin, give vitamin K (the administration route will be based on clinical presentation and the urgency of reversal), and arrange for factor replacement and hospital admission.16 Use FFP, if possible, because more is known about its safety than the safety of PCC or rFVIIa. That said, the choice of factor replacement should be based on the urgency of reversal and on clinical condition.16,33,34
INR should be rechecked immediately after factor replacement. Because coagulation factors have varied half-lives, INR should be checked daily for the next 4 days to confirm that it remains at a therapeutic level.16
In cases of life-threatening bleeding, stop the warfarin, give 10 mg vitamin K IV, and replace clotting factors.16 PCC should be considered because it will reverse the anticoagulation in the shortest amount of time, without limitations associated with fluid status, blood type-matching, or infusion reaction.35,36 While the risk of thrombosis needs to be evaluated in each high-risk circumstance, the use of either PCC or rFVIIa, depending on availability, is appropriate for a life-threatening bleed.29,37
Classifying—and treating—our patient
Based on our classification system, we determined that Mary Jo had major bleeding: She needed inpatient monitoring, with the possibility of a blood transfusion, but her condition was not life-threatening. She was hospitalized immediately. Her warfarin was withheld and she was given vitamin K IV and FFP to reverse her hypocoagulable state.
Once Mary Jo’s hypocoagulable state was reversed, which took about 4 hours, she was evaluated and found to have a small bleeding ulcer. The ulcer was cauterized, and her condition remained stable. A detailed investigation of possible reasons for the patient’s elevated INR did not reveal any causes. Three days after the cauterization, Mary Jo was started back on a lower dosing schedule of warfarin. She was discharged after a 5-day stay, with instructions to return to the clinic in 5 days for continued monitoring.
CORRESPONDENCE
Shailendra Prasad, MBBS, MPH, 1020 W. Broadway, Minneapolis, MN 55411; [email protected]
- For patients with an elevated international normalized ratio (INR) with mild or no bleeding, withhold the warfarin and recheck INR in 1 to 2 days; if INR >5, add oral vitamin K supplementation (C).
- For major bleeding and elevated INR, hospital admission, vitamin K, fresh frozen plasma, and frequent monitoring are needed (B).
- Emergent situations call for hospitalization, clotting factor replacement, and vitamin K administered by slow intravenous infusion (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
I feel weak,” reports Mary Jo, a 67-year-old patient who scheduled today’s appointment when she began noticing black, tarry stools 2 days ago. Her chart reveals that she’s on warfarin therapy for chronic atrial fibrillation, and today’s labs show a hematocrit of 18 and an international normalized ratio (INR) of 6.
If Mary Jo were your patient, what would you do?
With some 30.6 million outpatient prescriptions dispensed in the United States in a single year,1 warfarin is among the nation’s most commonly prescribed medications. It is also a dangerous drug. Warfarin’s anticoagulant and antithrombotic effects occur through its ability to inhibit the enzymes responsible for the reduction of vitamin K—an essential cofactor in the normal production of vitamin K-dependent clotting factors II, VII, XI, and X and anticoagulant factors protein C and S. In the presence of warfarin, these clotting factors are produced in a partially carboxylated state with reduced or absent biological activity. The result is a hypocoagulability that can be life-threatening.
Given the sheer number of patients receiving warfarin therapy and the potential for hemorrhage and other adverse effects, primary care physicians need to be familiar with evidence-based recommendations for managing warfarin-induced hypocoagulation. This review will help ensure that when you see patients like Mary Jo, you’ll be prepared to take the best approach to reversing their hypocoagulable state.
Which patients face the highest risk?
The reported incidence of bleeding in patients taking warfarin varies significantly, but is generally in the range of 1% annually.2 Among those who develop warfarin-related major bleeds, however, the fatality rate may be as high as 13.4%.3
The risk of bleeding is highest in the first 30 days of warfarin therapy,3 and increases exponentially once the INR exceeds 5.4 Other risk factors include:
- age (the risk increases to about 5% per year for patients >75 years)5
- hypertension
- cerebrovascular disease
- ischemic stroke
- a history of bleeds.6-8
Multiple medications and herbal substances can interfere with warfarin therapy. Some agents work by augmenting warfarin’s effect; others, such as antiplatelet agents, directly increase the risk of bleeding through unrelated mechanisms; still others may counteract warfarin therapy by enhancing coagulation. Ask patients on warfarin therapy to tell you everything they’re taking, including all over-the-counter medications, supplements, and prescription drugs. TABLE 1 lists herbal substances with the potential to increase or decrease INR. A comprehensive list of drugs that can interact with warfarin is available at http://www.drugs.com/drug-interactions/coumadin_d00022.html.
TABLE 1
Herbal substances that may affect INR38-40
| INCREASE INR | DECREASE INR | |
|---|---|---|
| Angelica root Anise Arnica flower Asafoetida Bogbean Boldo-fenugreek Borage seed oil Bromelain Capsicum Celery Chamomile Clove Dashen Devil’s claw Dong quai Feverfew Fish oil Garlic Ginger Ginkgo | Goldenseal Horse chestnut Licorice root Lovage root Lycium barbarum (wolfberry) Meadowsweet Onion Papain Parsley Passionflower Poplar Quassia Quilinggao Red clover Rue Sweet clover Turmeric Vitamin E Willow bark | Coenzyme Q10 Ginseng Green tea St. John’s wort |
When reversal is needed, how best to achieve it?
The options for reversing warfarin-induced anticoagulation include withholding 1 or more doses of warfarin and providing vitamin K supplementation and clotting factor replacement, as needed. The decision of which combination to use is based on both the urgency ( TABLE 2 ) and completeness of reversal required (target INR range) and the risk of thrombosis when the anticoagulation is reversed.9
Vitamin K is actually a group of lipid-soluble chemicals that are necessary for the production of functional carboxylated clotting factors II, VII, IX, and X. Vitamin K1 (phytonadione), which is available in food and as a supplement, is the particular chemical that competes with warfarin. When it is used as a reversal agent, phytonadione is generally referred to simply as vitamin K.
The oral route of vitamin K is preferred, but its effect is delayed because of the time required for absorption and production of factors. Thus, a slow (15-30 min) infusion of intravenous (IV) vitamin K should be used if reversal is needed within 6 hours—or oral therapy is unavailable. Avoid subcutaneous administration; it is not reliable and may take up to 72 hours to reverse the INR.10-12 Intramuscular (IM) administration of vitamin K should also be avoided in patients taking warfarin because of concerns about hematoma formation, although a 2003 study of patients in teaching hospitals found that the IM route is used about 10% of the time.13
The optimal dose of vitamin K varies, based on patient-specific factors such as comorbidities, metabolic and genetic variation, weight, age, and liver function. Doses as low as 0.5 mg IV or 1 mg oral vitamin K have been effective in reversing an elevated INR to a therapeutic range in nonlife-threatening situations.11,14,15 The American College of Chest Physicians (ACCP), which issued new guidelines in 2008, recommends doses of <5 mg for an INR >5 but <9 if there is a high risk of bleeding; 5 to 10 mg is the recommended dose for all patients with an INR ≥9. In cases of significant bleeding, a dose of 10 mg IV is recommended.16 Excessive vitamin K supplementation may lead to warfarin resistance, making it necessary to use much higher doses of warfarin down the road to achieve therapeutic INR levels.
Fresh frozen plasma (FFP) replaces functional vitamin K-dependent clotting factors that are decreased in patients taking warfarin. The suggested dose is 15 mL/kg,17,18 but patients must be monitored with coagulation laboratory values to assess the amount needed. One unit of FFP is roughly 250 mL, which corresponds to roughly 250 units of clotting factors.
FFP works to offset coagulopathy quickly. But because the plasma is frozen, it has to be thawed and blood type-matched, which is time-consuming. FFP transfusion also may be associated with infections, although the risk is generally believed to be minimal.17 Other limitations in using FFP include the large volume of fluid that must be administered—with the attendant risk of fluid overload—and the possibility of significant infusion reactions that may require slowing the infusion rate.19
Prothrombin complex concentrate (PCC) is pooled from donor plasma and lyophilized to a powder. It is then reconstituted for clotting factor replacement, and is available through the pharmacy rather than the blood bank.20 PCC is dosed in international units of factor IX, although it includes proportional amounts of factors II, VII, and X and proteins C and S. The typical recommended dose is 30 to 50 U/kg.20,21
Although PCC contains human coagulation factors, it does not involve the same risks of fluid overload or infectious transmission as FFP. It can be given IV over 5 to 10 minutes. The risk of thrombogenicity has been reported in patients with hemophilia who receive PCC,22 but studies of PCC use in warfarin reversal have not shown this adverse effect.23,24 Data from the use of PCC for the treatment of hemophilia suggest that the risk of thromboembolic events begins with daily doses >200 U/kg. There is limited information about the safety of giving PCC to patients with mechanical valve replacement, pregnant women, and those in other high-risk situations.
Recombinant activated factor VII (rFVIIa) is also effective in reversing elevated INR.25,26 It replaces 1 of the clotting factors that is decreased in anticoagulated patients (factor VII), but the significance of not replacing factors II, IX, and X is unknown.27 The recommended quantity of rFVIIa ranges from a single dose of 1200 mcg to weight-based dosing (10-160 mcg/kg).25,28-30 (IV vitamin K and FFP are also given in emergent situations in which rFVIIa is administered.) Thrombogenicity is a possible complication with the use of rFVIIa, but data are scarce regarding the incidence of adverse effects.
Neither PCC nor rFVIIa has US Food and Drug Administration approval for use in reversing warfarin-induced anticoagulation. Their use for this purpose may be warranted only in situations that threaten life or limb, and must be guided by clinical judgment.
TABLE 2
How fast? Reversal agents and time of action32
| SPEED/TYPE OF REVERSAL REQUIRED | WHAT TO USE |
|---|---|
| Rapid (complete; within 10-15 minutes) | PCC or rFVIIa + vitamin K IV |
| Fast (partial) | FFP + vitamin K IV |
| Prompt (4-6 hours) | Vitamin K IV |
| Slow (within 24 hours) | Oral vitamin K |
| Ultra-slow (over a period of days) | Omit warfarin dose (no vitamin K) |
| FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII. | |
Severity of bleeding as a treatment guide
Studies of methods used to reverse warfarin’s anticoagulation effect are difficult to compare because of a lack of a standardized approach to the classification of bleeds.18,31,32 We’ve used the following classification system, modified from that of Fihn et al,31 to avoid confusion and inform treatment decisions:
- Minor bleed: Reported, not requiring additional testing
- Major bleed: Requiring medical evaluation and inpatient treatment and/or blood transfusion
- Life-threatening bleed: Leading to cardiac arrest, surgical/angiographic intervention, or irreversible sequelae (loss of limb/sight).
Here’s how to put this classification system—and the ACCP’s 2008 guidelines for managing patients with elevated INR16 ( FIGURE )—into action:
In the case of minor bleeding and elevated INR, withhold the next 1 to 2 doses of warfarin.16 If the patient is considered high risk, give oral vitamin K in small amounts (1-2.5 mg). Keep in mind that excessive amounts of vitamin K will promote warfarin resistance.
FIGURE
Reversal of warfarin-induced anticoagulation
FFP, fresh frozen plasma; INR, international normalized ratio; IV, intravenous; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII.
Adapted from: Ansell J et al. Chest. 2008.16 Ask the patient to return to your office in 1 or 2 days for a recheck of INR. If it remains elevated, give another dose of vitamin K. Warfarin may need to be restarted at a lower dose depending on the clinical situation.17,32
In cases of major bleeding and elevated INR, stop the warfarin, give vitamin K (the administration route will be based on clinical presentation and the urgency of reversal), and arrange for factor replacement and hospital admission.16 Use FFP, if possible, because more is known about its safety than the safety of PCC or rFVIIa. That said, the choice of factor replacement should be based on the urgency of reversal and on clinical condition.16,33,34
INR should be rechecked immediately after factor replacement. Because coagulation factors have varied half-lives, INR should be checked daily for the next 4 days to confirm that it remains at a therapeutic level.16
In cases of life-threatening bleeding, stop the warfarin, give 10 mg vitamin K IV, and replace clotting factors.16 PCC should be considered because it will reverse the anticoagulation in the shortest amount of time, without limitations associated with fluid status, blood type-matching, or infusion reaction.35,36 While the risk of thrombosis needs to be evaluated in each high-risk circumstance, the use of either PCC or rFVIIa, depending on availability, is appropriate for a life-threatening bleed.29,37
Classifying—and treating—our patient
Based on our classification system, we determined that Mary Jo had major bleeding: She needed inpatient monitoring, with the possibility of a blood transfusion, but her condition was not life-threatening. She was hospitalized immediately. Her warfarin was withheld and she was given vitamin K IV and FFP to reverse her hypocoagulable state.
Once Mary Jo’s hypocoagulable state was reversed, which took about 4 hours, she was evaluated and found to have a small bleeding ulcer. The ulcer was cauterized, and her condition remained stable. A detailed investigation of possible reasons for the patient’s elevated INR did not reveal any causes. Three days after the cauterization, Mary Jo was started back on a lower dosing schedule of warfarin. She was discharged after a 5-day stay, with instructions to return to the clinic in 5 days for continued monitoring.
CORRESPONDENCE
Shailendra Prasad, MBBS, MPH, 1020 W. Broadway, Minneapolis, MN 55411; [email protected]
1. Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414-1419.
2. Beyth RJ. Hemorrhagic complications of oral anticoagulant therapy. Clin Geriatr Med. 2001;17:49-56.
3. Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893-900.
4. Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):S287-S310.
5. Pengo V, Legnani C, Noventa F, et al. ISCOAT Study Group. (Italian Study on Complications of Oral Anticoagulant Therapy). Oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and risk of bleeding. A multicenter inception cohort study. Thromb Haemost. 2001;85:418-422.
6. White RH, McKittrick T, Takakuwa J, et al. Management and prognosis of life-threatening bleeding during warfarin therapy. National Consortium of Anticoagulation Clinics. Arch Intern Med. 1996;156:1197-1201.
7. Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91-99.
8. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
9. Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):S204-S233.
10. Crowther MA, Douketis JD, Schnurr T, et al. Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial. Ann Intern Med. 2002;137:251-254.
11. Whitling AM, Bussey HI, Lyons RM. Comparing different routes and doses of phytonadione for reversing excessive anticoagulation. Arch Intern Med. 1998;158:2136-2140.
12. Nee R, Doppenschmidt D, Donovan DJ, et al. Intravenous versus subcutaneous vitamin K1 in reversing excessive oral anticoagulation. Am J Cardiol. 1999;83:286-288.
13. Fan J, Armitstead JA, Adams AG, et al. A retrospective evaluation of vitamin K1 therapy to reverse the anticoagulant effect of warfarin. Pharmacotherapy. 2003;23:1245-1250.
14. Lubetsky A, Yonath H, Olchovsky D, et al. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163:2469-2473.
15. Hung A, Singh S, Tait RC. A prospective randomized study to determine the optimal dose of intravenous vitamin K in reversal of over-warfarinization. Br J Haematol. 2000;109:537-539.
16. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists. Chest. 2008;133(suppl):160S-198S.
17. Dentali F, Ageno W, Crowther M. Treatment of coumarin-associated coagulopathy: a systematic review and proposed algorithms. J Thromb Haemost. 2006;4:1853-1863.
18. Makris M, Watson HG. The management of coumarin-induced over-anticoagulation Annotation [see comment]. Br J Haematol. 2001;114:271-280.
19. O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11-28.
20. Makris M. Optimisation of the prothrombin complex concentrate dose for warfarin reversal. Thromb Res. 2005;115:451-453.
21. Vigue B, Ract C, Tremey B, et al. Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial hemorrhage. Intensive Care Med. 2007;33:721-725.
22. Kohler M. Thrombogenicity of prothrombin complex concentrates. Thromb Res. 1999;95(suppl 1):S13-S17.
23. Lorenz R, Kienast J, Otto U, et al. Successful emergency reversal of phenprocoumon anticoagulation with prothrombin complex concentrate: a prospective clinical study. Blood Coagul Fibrinolysis. 2007;18:565-570.
24. Pabinger I, Brenner B, Kalina U, et al. Beriplex P/N Anticoagulation Reversal Study Group. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622-631.
25. Sorensen B, Johansen P, Nielsen GL, et al. Reversal of the International Normalized Ratio with recombinant activated factor VII in central nervous system bleeding during warfarin thromboprophylaxis: clinical and biochemical aspects. Blood Coagul Fibrinolysis. 2003;14:469-477.
26. Brody DL, Aiyagari V, Shackleford AM, et al. Use of recombinant factor VIIa in patients with warfarin-associated intracranial hemorrhage. Neurocrit Care. 2005;2:263-267.
27. Tanaka KA, Szlam F, Dickneite G, et al. Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb Res. 2008;122:117-123.
28. Lin J, Hanigan WC, Tarantino M, et al. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98:737-740.
29. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777-785.
30. Dager WE, King JH, Regalia RC, et al. Reversal of elevated international normalized ratio and bleeding with low-dose recombinant factor VII in patients receiving warfarin. Pharmacotherapy. 2006;26:1091-1098.
31. Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993;118:511-520.
32. Hanley JP. Warfarin reversal. J Clin Pathol. 2004;57:1132-1139.
33. Yasaka M, Sakata T, Minematsu K, et al. Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complication. Thromb Res. 2002;108:25-30.
34. Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced anticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;115:998-1001.
35. Lubetsky A, Hoffman R, Zimlichman R, et al. Efficacy and safety of complex concentrate (Octaplex) for rapid reversal of oral anticoagulation. Thromb Res. 2004;113:371-378.
36. Cartmill M, Dolan G, Byrne JL, et al. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg. 2000;14:458-461.
37. Deveras RA, Kessler CM. Reversal of warfarin-induced excessive anticoagulation with recombinant human factor VIIa concentrate. Ann Intern Med. 2002;137:884-888.
38. Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106.
39. Haines ST, Zeolla M, Witt DM. Venous Thromboembolism. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiological Approach. 6th ed. New York: McGraw-Hill Companies, Inc.; 2005:373-413.
40. Micromedex Healthcare Series. Drug-REAX System. New York: Thomson Reuters; 2008.
1. Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414-1419.
2. Beyth RJ. Hemorrhagic complications of oral anticoagulant therapy. Clin Geriatr Med. 2001;17:49-56.
3. Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893-900.
4. Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):S287-S310.
5. Pengo V, Legnani C, Noventa F, et al. ISCOAT Study Group. (Italian Study on Complications of Oral Anticoagulant Therapy). Oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and risk of bleeding. A multicenter inception cohort study. Thromb Haemost. 2001;85:418-422.
6. White RH, McKittrick T, Takakuwa J, et al. Management and prognosis of life-threatening bleeding during warfarin therapy. National Consortium of Anticoagulation Clinics. Arch Intern Med. 1996;156:1197-1201.
7. Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91-99.
8. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet. 1993;342:1255-1262.
9. Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl 3):S204-S233.
10. Crowther MA, Douketis JD, Schnurr T, et al. Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial. Ann Intern Med. 2002;137:251-254.
11. Whitling AM, Bussey HI, Lyons RM. Comparing different routes and doses of phytonadione for reversing excessive anticoagulation. Arch Intern Med. 1998;158:2136-2140.
12. Nee R, Doppenschmidt D, Donovan DJ, et al. Intravenous versus subcutaneous vitamin K1 in reversing excessive oral anticoagulation. Am J Cardiol. 1999;83:286-288.
13. Fan J, Armitstead JA, Adams AG, et al. A retrospective evaluation of vitamin K1 therapy to reverse the anticoagulant effect of warfarin. Pharmacotherapy. 2003;23:1245-1250.
14. Lubetsky A, Yonath H, Olchovsky D, et al. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163:2469-2473.
15. Hung A, Singh S, Tait RC. A prospective randomized study to determine the optimal dose of intravenous vitamin K in reversal of over-warfarinization. Br J Haematol. 2000;109:537-539.
16. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists. Chest. 2008;133(suppl):160S-198S.
17. Dentali F, Ageno W, Crowther M. Treatment of coumarin-associated coagulopathy: a systematic review and proposed algorithms. J Thromb Haemost. 2006;4:1853-1863.
18. Makris M, Watson HG. The management of coumarin-induced over-anticoagulation Annotation [see comment]. Br J Haematol. 2001;114:271-280.
19. O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11-28.
20. Makris M. Optimisation of the prothrombin complex concentrate dose for warfarin reversal. Thromb Res. 2005;115:451-453.
21. Vigue B, Ract C, Tremey B, et al. Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial hemorrhage. Intensive Care Med. 2007;33:721-725.
22. Kohler M. Thrombogenicity of prothrombin complex concentrates. Thromb Res. 1999;95(suppl 1):S13-S17.
23. Lorenz R, Kienast J, Otto U, et al. Successful emergency reversal of phenprocoumon anticoagulation with prothrombin complex concentrate: a prospective clinical study. Blood Coagul Fibrinolysis. 2007;18:565-570.
24. Pabinger I, Brenner B, Kalina U, et al. Beriplex P/N Anticoagulation Reversal Study Group. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622-631.
25. Sorensen B, Johansen P, Nielsen GL, et al. Reversal of the International Normalized Ratio with recombinant activated factor VII in central nervous system bleeding during warfarin thromboprophylaxis: clinical and biochemical aspects. Blood Coagul Fibrinolysis. 2003;14:469-477.
26. Brody DL, Aiyagari V, Shackleford AM, et al. Use of recombinant factor VIIa in patients with warfarin-associated intracranial hemorrhage. Neurocrit Care. 2005;2:263-267.
27. Tanaka KA, Szlam F, Dickneite G, et al. Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb Res. 2008;122:117-123.
28. Lin J, Hanigan WC, Tarantino M, et al. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98:737-740.
29. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777-785.
30. Dager WE, King JH, Regalia RC, et al. Reversal of elevated international normalized ratio and bleeding with low-dose recombinant factor VII in patients receiving warfarin. Pharmacotherapy. 2006;26:1091-1098.
31. Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann Intern Med. 1993;118:511-520.
32. Hanley JP. Warfarin reversal. J Clin Pathol. 2004;57:1132-1139.
33. Yasaka M, Sakata T, Minematsu K, et al. Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complication. Thromb Res. 2002;108:25-30.
34. Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced anticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;115:998-1001.
35. Lubetsky A, Hoffman R, Zimlichman R, et al. Efficacy and safety of complex concentrate (Octaplex) for rapid reversal of oral anticoagulation. Thromb Res. 2004;113:371-378.
36. Cartmill M, Dolan G, Byrne JL, et al. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg. 2000;14:458-461.
37. Deveras RA, Kessler CM. Reversal of warfarin-induced excessive anticoagulation with recombinant human factor VIIa concentrate. Ann Intern Med. 2002;137:884-888.
38. Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106.
39. Haines ST, Zeolla M, Witt DM. Venous Thromboembolism. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiological Approach. 6th ed. New York: McGraw-Hill Companies, Inc.; 2005:373-413.
40. Micromedex Healthcare Series. Drug-REAX System. New York: Thomson Reuters; 2008.
Derm diagnoses you can’t afford to miss
- Management of hereditary angioedema should include fresh frozen plasma containing C1 inhibitor (C1-INH), whenever possible; if C1-INH-containing plasma is unavailable, fresh frozen plasma can be used instead (SOR: A).
- Do not give neomycin to patients with suspected cellulitis; the drug may promote antibiotic resistance in Staphylococcus aureus, a pathogen often associated with this condition (SOR: A).
- Whenever a patient presents with erythematous skin lesions and a recent history of receiving penicillin or a cephalosporin antibiotic, a sulfa derivative, or an anticonvulsant, the suspected medication should be stopped until Stevens-Johnson syndrome is ruled out (SOR: A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Skin eruptions are a common reason for visits to primary care physicians. While most are innocuous, some are associated with—or are early warning signs of—severe allergic reactions or other emergent conditions. A 9-year-old patient I’ll call Julie is a case in point.
The first time Julie’s parents brought her to our clinic, she’d been complaining of a sore throat and had a fever that hovered between 102° and 103°F for several days. The physician who examined Julie found mild maxillary tenderness. A rapid streptococcal throat swab was negative; her doctor prescribed a 10-day course of trimethoprim/sulfamethoxazole (TMP/SMX) for presumed acute sinusitis.
Thirteen days later (3 days after the patient completed the course of antibiotics), Julie’s parents brought her back to the clinic. Her throat still hurt, and she had erythematous oval lesions on her trunk and upper extremities. Her physician diagnosed scarlet fever and wrote a prescription for penicillin.
The following day, Julie was taken to the emergency department (ED) with bilateral conjunctival hyperemia and diffuse, confluent erythematous macules throughout her body. The ED physician who examined Julie found a 2.5 × 2.0 cm targetoid lesion with a necrotic, purpuric center on her lower back—a diagnostic clue to the cause of her signs and symptoms.
If you had been Julie’s physician, would you have been alert to that clue?
For family physicians accustomed to seeing relatively mild skin disorders, recognizing and responding to dermatologic conditions with potentially dire outcomes can be challenging. This review, and the images that accompany it, will help you sharpen your dermatologic diagnostic and treatment skills, both for benign disorders and those that are less common and more severe. We’ll also tell you more about Julie and her diagnosis.
Urticaria: A simple case of hives?
This common allergic reaction affects close to 10% of the population at some point in their lives. The affected areas are itchy and have raised, circumscribed red welts with surrounding erythema.1 Urticaria can occur throughout the body, with new lesions often erupting as the old ones disappear.2,3
Despite the persistent itchiness that patients typically complain of, however, urticaria is usually self-limiting, and rarely life-threatening. Acute urticaria normally resolves within 2 to 6 weeks.2,4
In most cases, urticaria arises secondary to exposure to an allergenic substance, chemical, or emotional stress.4,5 In rare instances, systemic diseases, such as hematologic malignancies, can also cause urticarial lesions to erupt throughout the body.4
Body piercing, cosmetics, latex exposure, Helicobacter pylori, insects, and angiotensin-converting enzyme (ACE) inhibitors have been identified as common triggers of urticaria, as have nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotics, animal dander, and foods such as shellfish, nuts, and dairy products.4,6
Treatment of all forms of urticaria should be based on identification and strict avoidance of the causative agent, if it’s known.7 Following withdrawal of the specific agent, symptomatic treatment with medications such as histamine antagonists and corticosteroids remains the mainstay of therapy.4,8 A daily dose of 40 to 60 mg prednisone for 5 days is a reasonable therapeutic regimen for adults; a 5-day course of 1 mg/kg per day is suitable for pediatric patients.4,8,9
In the event that topical or oral therapy is ineffective in mild cases of urticaria, intravenous (IV) diphenhydramine (50 mg) can be administered every 6 to 8 hours.4,8 IV diphenhydramine typically takes 30 minutes to work, while corticosteroids take at least 2 hours to reach full effect.4,8
In an emergency setting, subcutaneous epinephrine (0.3-0.5 mg) can be useful in treating severe urticaria.4,8 And recent clinical trials have demonstrated complete clearance of urticaria with leukotriene inhibitors, such as montelukast (10 mg).10
Chronic urticaria, trigger unknown
Although acute urticaria is responsive to treatment, chronic urticaria—lesions that do not resolve after 6 weeks—poses a greater challenge. In up to 80% of cases of chronic urticaria, no identifiable trigger is found.3,8 Long-term treatment of patients with this chronic condition, including lifestyle changes (to avoid environmental or dietary triggers) and a medication regimen for 6 months or more, leads to complete resolution of symptoms in most cases.7,8
Angioedema: Less common, more dangerous
Angioedema is part of the same disease spectrum as urticaria, but it affects the deeper tissues—involving mucosal and submucosal swelling. Angioedema affects just 0.1% to 0.2% of the general population, but up to 15% of patients with urticaria.8
The risk increases with the use of ACE inhibitors; for every 1000 patients taking ACE inhibitors, 0.4 to 3.5 develop angioedema.11 A recent double-blind study involving 25,642 patients being treated with ACE inhibitors revealed that those taking a combination of ACE inhibitors were more likely to develop angioedema than those on monotherapy.12
Angioedema is characterized by the sudden appearance of painful, localized erythematous wheals with central blanching.13 It results from increased vascular permeability in capillaries of the dermis, which leads to fluid leakage.13,14 Increased accumulation of fluids from the vessels of the skin results in rapidly developing nonpitting edema that most often affects the hands, face (and lips), neck, and oropharynx (FIGURE 1).14 Although the head and neck are the most commonly involved areas, angioedema can also affect the digestive tract, leading to nausea, vomiting, diarrhea, and abdominal pain secondary to bowel edema.13
FIGURE 1
Angioedema: A look at the most commonly affected areas
Airway management is imperative
In severe cases of angioedema, involvement of the oral mucosa results in stridor, followed by upper airway obstruction. To avoid hypoxemia and death,13,14 rapid preparation for emergency intervention to maintain the airway is critical; mortality can be as high as 30% in patients with airway compromise.13,15 In severe cases in which edema engulfs the oropharynx, oral intubation is often impossible, and nasotracheal intubation may be warranted.15,16 A failure to maintain adequate airway function via nasotracheal intubation may signal the need for an emergency tracheotomy.15,16
Is the angioedema hereditary or acquired?
Hereditary and acquired angioedema are treated differently. After ensuring that the patient has a patent airway, distinguishing between them is critical. Hereditary angioedema is the result of an inherited deficiency of plasma protein C1 inhibitor (C1-INH). Acquired angioedema is typically caused by enhanced consumption of endogenous C1-INH,13,17 leading to a net deficit of circulating C1-INH, and can be triggered by food, pharmacologic agents, and, occasionally, by systemic disorders.13 African Americans and patients with renal impairment appear to be at increased risk for acquired angioedema.13 In both hereditary and acquired angioiedema, decreased levels of C1-INH lead to disruption of the complement pathway.
Although the clinical presentation of acquired and hereditary angioedema is similar, a focused patient history can be used to distinguish between them. Age, health status, and medication history are the key considerations. Hereditary angioedema most commonly occurs—in recurrent attacks after minor trauma—in children with no underlying disease, with worsening symptoms during puberty. Acquired angioedema is generally seen in adult and elderly patients with malignancies or other underlying disorders.
Mild to moderate cases of acquired angioedema respond well to oral corticosteroids and antihistamines. Severe cases often require administration of subcutaneous epinephrine, followed by IV steroids. Management of hereditary angioedema should include C1-INH-containing fresh frozen plasma,13,16,18 although fresh frozen plasma can be used if plasma with C1-INH is not available.13
While oral corticosteroids and anti-histamines may be effective adjunctive therapy for hereditary angioedema, they are not likely to reverse acute attacks in this patient population. IV diphenhydramine (50-100 mg) or IV cimetidine (300 mg) every 6 to 8 hours is a reasonable therapeutic regimen for acute attacks of hereditary angioedema.
A recent randomized double-blind trial involving 40 patients with hereditary angioedema studied the administration of ecallantide, a kallikrein inhibitor for which US Food and Drug Administration approval is pending.19 Nearly 3 out of 4 of those who received ecallantide (72.5%) for acute attacks of angioedema showed significant improvement within 4 hours.20 The use of kallikrein inhibitors, which target inflammatory blood components, is not widespread, but may hold promise for the treatment of hereditary angioedema.20
Cellulitis: On the lookout for infiltration
Cellulitis is a bacterial infection of the skin that affects approximately 24.6 in 1000 people and is rarely associated with death.21 It occurs when bacteria enter through disrupted areas in the skin, particularly when skin integrity is compromised by recent surgery, piercing, wounds, athlete’s foot, or even dermatitis.22,23Streptococcus and Staphylococcus are the 2 most common infectious agents, and methicillin-resistant Staphylococcus aureus (MRSA) is increasingly common.22,23
Although cellulitis is primarily superficial in nature, it may progress to a serious condition by infiltrating underlying tissues and spreading to nearby lymphatic tissue and the bloodstream to cause lymphadenitis or bacteremia.21,23 In instances of cellulitis-induced bacteremia, mortality rates increase if prompt, targeted treatment is not provided.23
Raised erythematous plaques are the cardinal features of cellulitis, with the affected areas warm to the touch, red, and tender.21,23 As the condition progresses, the affected area tends to enlarge and expand (FIGURE 2),24 and the patient often becomes febrile.22,24
The risk of developing cellulitis increases with age, compromised immune status, diabetes, obesity, IV drug use, lymphedema, and chronic corticosteroid use.22,24
Cellulitis is often diagnosed solely on the basis of clinical presentation, although aspiration of purulent discharge from the wound and a gram stain of the culture can confirm the diagnosis.25,26 (See “Is it cellulitis or stasis dermatitis?”.)
Direct immunofluorescence can be used when cultures are difficult to obtain, but this technique is seldom necessary.22 If infiltration of underlying soft tissues is suspected based on clinical findings, magnetic resonance imaging can be a useful tool in evaluating the extent of the infection and in directing appropriate debridement and drainage of affected areas.22,27
Patients with venous insufficiency may present with stasis dermatitis, which often results in breakdown of the skin and ulceration that bears a striking resemblance to cellulitis. Thus, these conditions can be easily confused, and may lead to unnecessary antibiotic use and, possibly, hospitalization in patients with venous insufficiency.26,28
Despite the similarities of these conditions, a focused patient history and physical exam can prevent such confusion. Stasis dermatitis arises as a result of venous insufficiency, so it is likely to be accompanied by pitting edema that responds to leg raising and to the use of elastic compression stockings—interventions that are seldom effective for cellulitis.26 In addition, cellulitis tends to be unilateral, while stasis dermatitis often has bilateral involvement.
FIGURE 2
Diagnosing cellulitis based on clinical presentation
The raised erythematous lesions that are a hallmark of cellulitis, shown here on the arm and face, are warm to the touch, red, and tender.
Treatment: Targeted antibiotics and preventive measures
Because of the likelihood of recurrence with cellulitis, treating the condition involves both preventive and curative measures. Mild cases can be treated in an outpatient setting with a 7- to 10-day course of oral cephalosporins or antibiotics with similar coverage.22,25,26 A recent randomized study involving 391 patients found that cure rates for cellulitis treated with cephalexin were between 83% and 92%, depending on the pathogen involved.26
For severe cases of cellulitis, patients who are immunocompromised, and cases that are refractory to oral medications, hospital admission is recommended, and use of IV antibiotics is routinely required.22,25,26 For patients with MRSA, a drug such as vancomycin IV may be warranted; a reasonable dose would be 15 mg/kg every 12 hours.22,26,27,29
A recent randomized, multicenter study demonstrated that vancomycin effectively treated approximately 67% of cases of MRSA-induced cellulitis.26 Neomycin should be strictly avoided whenever cellulitis is suspected, because of its propensity to promote antibiotic resistance to S. aureus.29
Patient education emphasizing preventive measures is critical for minimizing recurrence of cellulitis.22,25,26 Encourage patients to wash with antibacterial soap and water daily, apply topical antibiotic ointment, and keep the wound completely covered at all times. Advise them to change bandages and wash their hands frequently.27,29 Patients with diabetes and others with decreased circulation in the extremities need to take further precautions, such as moisturizing the skin regularly in order to prevent cuts in their skin.22,29
SJS: Triggered by drugs, and infections, too
Stevens-Johnson syndrome (SJS), also known as erythema multiforme major, is an often-debilitating and possibly fatal adverse reaction, typically (but not exclusively) to a drug. It manifests as full-thickness epidermal necrosis of the mucous membranes.30,31 SJS occurs at a rate of about 1 to 7 cases per million people per year, and has a mortality rate of approximately 5%.30-32 The types of medication that most commonly precipitate SJS are anticonvulsants, sulfa drugs, penicillin-related and cephalosporin antibiotics, anti-inflammatory agents, and certain neoplastic drugs.30,32 SJS can develop in response to infections and neoplasms (TABLE) as well, and in many cases a cause is never found.
Patients with widespread involvement often complain of a burning sensation, particularly around the mouth.32-36 In some cases, this is the presenting sign, because the oral mucosa tends to be among the first mucous membranes involved.
TABLE
Stevens-Johnson syndrome: Pinpointing the cause32
| MORE FREQUENT ETIOLOGY |
| Drugs: Allopurinol, anticonvulsants, antiparasitics, barbiturates, NSAIDs, penicillin-related and cephalosporin antibiotics, sulfas, tetracyclines |
| LESS FREQUENT ETIOLOGY |
| Bacterial: Diphtheria, group A Streptococcus, Mycoplasma pneumoniae, tularemia, typhoid |
| Fungal: Coccidiomycosis, dermatophytosis, histoplasmosis, |
| Protozoan: Plasmodium, trichomoniasis |
| Viral: AIDS, Coxsackie, Epstein-Barr, HSV, influenza |
| AIDS, acquired immune deficiency syndrome; HSV, herpes-simplex virus; NSAIDs, nonsteroidal anti-inflammatory drugs. |
Targetoid lesions, Nikolsky sign are diagnostic clues
The characteristic skin lesions seen with SJS consist of initially erythematous macules that rapidly develop central necrosis to form vesiculation, as well as other variable areas of denudation (FIGURE 3). These vesicles tend to demonstrate confluence and often show a positive Nikolsky sign—epidermal detachment of superficial layers of the skin when slight pressure is applied. The lesions typically take on a targetoid appearance. If left untreated, the blisters often result in ulceration and hemorrhagic crusting of affected areas.
Like most skin disorders, SJS is initially diagnosed on the basis of clinical presentation. However, it is a rare disorder, and commonly misdiagnosed. Among the disorders SJS has been mistaken for are staphylococcal scalded skin syndrome, toxic shock syndrome, exfoliative dermatitis, scarlet fever, erythema multiforme, and iatrogenic chemical burns.32,37 (Erythema multiforme, SJS, and toxic epidermal necrolysis [TEN] are considered part of the same disease spectrum; erythema multiforme typically presents with few random lesions and no mucosal involvement, SJS with mucosal involvement on up to 30% of the body surface, and TEN with >30%.)32,37 Skin biopsies and immunofluorescence studies are recommended to confirm the diagnosis.
During the course of SJS, the mucous membranes of the oropharynx, ocular cavity, gastrointestinal system, nasal cavity, genitourinary system, and lower respiratory tracts are typically affected.32-35 As the condition progresses, increased epidermal erosion can lead to the sloughing off of up to 100% of the epidermis, resulting in considerable fluid loss.32,34
Corneal ulceration, anterior uveitis, panophthalmitis, polyarthritis, hematuria, and acute tubular necrosis leading to renal failure may also occur.32,35,37 Scarring within vital ocular structures can result in corneal opacity and lead to significant visual impairment. In severe cases, blood loss and fluid loss increase the risk of bacterial superinfection and sepsis.35,37
FIGURE 3
Targetoid lesions are characteristic of SJS
Characteristic diffuse erythematous macules with necrotic centers and overlying blistering on the back of a patient with Stevens-Johnson syndrome.
Supportive therapy, wound care are key components of treatment
Rapid cessation of the offending agent with targeted dermatologic management can reduce morbidity by promoting rapid re-epithelialization of affected skin. (See “SJS is diagnosed, but not quickly,” [Verdicts] on page 332, for a discussion of the dangers of delayed diagnosis and failure to promptly stop the drug causing the acute reaction.)
Closely monitoring the patient for fluid and electrolyte abnormalities is also crucial. Corticosteroids and IV immunoglobulins have been suggested for early severe cases of SJS, but their efficacy in treating this condition has yet to be established by prospective double-blind studies.32-37
The skin lesions associated with SJS should be treated in the same way you would treat thermal burns, with local wound care, warm compresses, and topical anesthetics for pain reduction.36,37 Oral lesions are managed with diphenhydramine or sodium bicarbonate mouthwashes and glycerin swabs.
An ophthalmologic consultation is mandatory because of the risk of vision loss associated with corneal scarring.
And now, a return to our 9-year-old patient
When we left off our discussion of Julie, the ED physician who examined her had detected a targetoid lesion with a necrotic, purpuric center—a finding that we described as a diagnostic clue. The second diagnostic clue? The presence of the Nikolsky sign, which the doctor detected by applying slight pressure to the lesion. Julie was admitted to the hospital with a presumed diagnosis of SJS, which skin biopsies and immunofluorescence studies later confirmed.
It wasn’t clear whether Julie had had a reaction to the sulfa (which she’d completed) or to the penicillin (which she’d just begun taking), or whether she had a synergistic reaction to both. Although the exact cause remained uncertain, as it often does, the penicillin was stopped immediately. She received dermatologic treatment without delay and was monitored closely for fluid and electrolyte status. Since Julie had signs of ocular involvement, daily erythromycin and corticosteroid eyedrops were administered to minimize the risk of infection and reduce local inflammation. Given the risk of long-term ocular complications in patients with SJS, we recommended continued ophthalmologic care.
Nine days after she was admitted, Julie’s symptoms resolved, with the exception of persistent complaints of dry eye. At discharge, Julie was given artificial tears to minimize ocular irritation. We suspected that she had dry eyes because of SJS-induced corneal scarring, but we were unable to confirm our suspicion because our patient failed to return for scheduled ophthalmologic appointments. She was subsequently lost to follow up.
Acknowledgement
The authors wish to thank Azita Hamedani, MD, MPH, FACEP, for her critical editing of the text.
Correspondence
Ribhi Hazin, MD, 29 Garden Street, Suite 214, Cambridge, MA 02138; [email protected]
1. Noe R, Cohen AL, Lederman E, et al. Skin disorders among construction workers following Hurricane Katrina and Hurricane Rita: an outbreak investigation in New Orleans, Louisiana. Arch Dermatol. 2007;143:1393-1398.
2. Kulthanan K, Jiamton S, Thumpimukvatana N, et al. Chronic idiopathic urticaria: prevalence and clinical course. J Dermatol. 2007;34:294-301.
3. Stanway AD, Cohen SN, Chen C, et al. H1-antihistamines for chronic urticaria. Cochrane Database Syst Rev. 2008(2):CD006137.-
4. Grattan CE, Humphreys F. British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007;157:1116-1123.
5. Kozel MM, Mekkes JR, Bossuyt PM, et al. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-391.
6. Nettis E, Colanardi MC, Soccio AL, et al. Double-blind, placebo-controlled study of sublingual immunotherapy in patients with latex-induced urticaria: a 12-month study. Br J Dermatol. 2007;156:674-681.
7. Lee EE, Maibach HI. Treatment of urticaria. An evidence-based evaluation of antihistamines. Am J Clin Dermatol. 2001;2:27-32.
8. Humphreys F, Hunter JA. The characteristics of urticaria in 390 patients. Br J Dermatol. 1998;138:635-638.
9. Serhat Inaloz H, Ozturk S, Akcali C, et al. Low-dose and short-term cyclosporine treatment in patients with chronic idiopathic urticaria: A clinical and immunological evaluation. J Dermatol. 2008;35:276-282.
10. Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria. A single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110:484-488.
11. Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637-1642.
12. Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-1559.
13. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;115(3 Suppl 2):s483-s523.
14. Bas M, Kirchhartz N, Hochfeld J, et al. Potential role of vasomotor effects of fibrinogen in bradykinin-induced angioedema. J Allergy Clin Immunol. 2008;121:969e2-975e2.
15. Sica DA, Black HR. Angioedema in heart failure: occurrence with ACE inhibitors and safety of angiotensin receptor blocker therapy. Congest Heart Fail. 2002;8:334-341.
16. Banerji A, Clark S, Blanda M, et al. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. 2008;100:327-332.
17. Cicardi M, Zingale LC, Zanichelli A, et al. The use of plasma-derived C1 inhibitor in the treatment of hereditary angioedema. Expert Opin Pharmacother. 2007;8:3173-3181.
18. Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100:153-161.
19. US Food and Drug Administration. Advisory Committee Briefing Document. Kalbitor (ecallantide) for acute attacks of hereditary angioedema. Available at: http://www.fda.gov/ohrms/dockets/AC/09/briefing/2009-4413b1-03-Dyax.pdf. Accessed May 5, 2009.
20. Schneider L, Lumry W, Vegh A, et al. Critical role of kallikrein in hereditary angioedema pathogenesis: a clinical trial of ecallantide, a novel kallikrein inhibitor. J Allergy Clin Immunol. 2007;120:416-422.
21. Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293-299.
22. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
23. Morris A. What are the benefits of treatments? Cellulitis and erysipelas. BMJ Clin Evid. 2005;13:2066-2069.
24. Murray H, Stiell I, Wells G. Treatment failure in emergency department patients with cellulitis. CJEM. 2005;7:228-234.
25. Meier DE, Nkor SK, Aasa D, et al. Prospective randomized comparison of two preoperative skin preparation techniques in a developing world country. World J Surg. 2001;25:441-443.
26. Giordano PA, Elston D, Akinlade BK, et al. Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. Curr Med Res Opin. 2006;22:2419-2428.
27. Halpern J, Holder R, Langford NJ. Ethnicity and other risk factors for acute lower limb cellulitis: a U.K.-based prospective case-control study. Br J Dermatol. 2008;158:1288-1292.
28. Lin YT, Lu PW. Retrospective study of pediatric facial cellulitis of odontogenic origin. Pediatr Infect Dis J. 2006;25:339-342.
29. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemo. 2007;51:4044-4048.
30. Schneck J, Fagot JP, Sekula P, et al. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58:33-40.
31. Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43-47.
32. Hazin R, Ibrahimi OA, Hazin MI, et al. Stevens-Johnson syndrome: pathogenesis, diagnosis, and management. Ann Med, 2008;40:129-138.
33. Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486.-
34. Jette N, Hemming K, Hutton JL, et al. Topiramate add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2008;(3):DC001417.-
35. Gürcan HM, Ahmed AR. Efficacy of various intravenous immunoglobulin therapy protocols in auto-immune and chronic inflammatory disorders. Ann Pharmacother. 2007;41:812-523.
36. Strom BL, Carson JL, Halpern AC, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991;127:831-838.
37. Gravante G, Delogu D, Marianetti M, et al. Toxic epidermal necrolysis and Steven-Johnson syndrome: 11-years experience and outcome. Eur Rev Med Pharmacol Sci. 2007;11:119-127.
- Management of hereditary angioedema should include fresh frozen plasma containing C1 inhibitor (C1-INH), whenever possible; if C1-INH-containing plasma is unavailable, fresh frozen plasma can be used instead (SOR: A).
- Do not give neomycin to patients with suspected cellulitis; the drug may promote antibiotic resistance in Staphylococcus aureus, a pathogen often associated with this condition (SOR: A).
- Whenever a patient presents with erythematous skin lesions and a recent history of receiving penicillin or a cephalosporin antibiotic, a sulfa derivative, or an anticonvulsant, the suspected medication should be stopped until Stevens-Johnson syndrome is ruled out (SOR: A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Skin eruptions are a common reason for visits to primary care physicians. While most are innocuous, some are associated with—or are early warning signs of—severe allergic reactions or other emergent conditions. A 9-year-old patient I’ll call Julie is a case in point.
The first time Julie’s parents brought her to our clinic, she’d been complaining of a sore throat and had a fever that hovered between 102° and 103°F for several days. The physician who examined Julie found mild maxillary tenderness. A rapid streptococcal throat swab was negative; her doctor prescribed a 10-day course of trimethoprim/sulfamethoxazole (TMP/SMX) for presumed acute sinusitis.
Thirteen days later (3 days after the patient completed the course of antibiotics), Julie’s parents brought her back to the clinic. Her throat still hurt, and she had erythematous oval lesions on her trunk and upper extremities. Her physician diagnosed scarlet fever and wrote a prescription for penicillin.
The following day, Julie was taken to the emergency department (ED) with bilateral conjunctival hyperemia and diffuse, confluent erythematous macules throughout her body. The ED physician who examined Julie found a 2.5 × 2.0 cm targetoid lesion with a necrotic, purpuric center on her lower back—a diagnostic clue to the cause of her signs and symptoms.
If you had been Julie’s physician, would you have been alert to that clue?
For family physicians accustomed to seeing relatively mild skin disorders, recognizing and responding to dermatologic conditions with potentially dire outcomes can be challenging. This review, and the images that accompany it, will help you sharpen your dermatologic diagnostic and treatment skills, both for benign disorders and those that are less common and more severe. We’ll also tell you more about Julie and her diagnosis.
Urticaria: A simple case of hives?
This common allergic reaction affects close to 10% of the population at some point in their lives. The affected areas are itchy and have raised, circumscribed red welts with surrounding erythema.1 Urticaria can occur throughout the body, with new lesions often erupting as the old ones disappear.2,3
Despite the persistent itchiness that patients typically complain of, however, urticaria is usually self-limiting, and rarely life-threatening. Acute urticaria normally resolves within 2 to 6 weeks.2,4
In most cases, urticaria arises secondary to exposure to an allergenic substance, chemical, or emotional stress.4,5 In rare instances, systemic diseases, such as hematologic malignancies, can also cause urticarial lesions to erupt throughout the body.4
Body piercing, cosmetics, latex exposure, Helicobacter pylori, insects, and angiotensin-converting enzyme (ACE) inhibitors have been identified as common triggers of urticaria, as have nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotics, animal dander, and foods such as shellfish, nuts, and dairy products.4,6
Treatment of all forms of urticaria should be based on identification and strict avoidance of the causative agent, if it’s known.7 Following withdrawal of the specific agent, symptomatic treatment with medications such as histamine antagonists and corticosteroids remains the mainstay of therapy.4,8 A daily dose of 40 to 60 mg prednisone for 5 days is a reasonable therapeutic regimen for adults; a 5-day course of 1 mg/kg per day is suitable for pediatric patients.4,8,9
In the event that topical or oral therapy is ineffective in mild cases of urticaria, intravenous (IV) diphenhydramine (50 mg) can be administered every 6 to 8 hours.4,8 IV diphenhydramine typically takes 30 minutes to work, while corticosteroids take at least 2 hours to reach full effect.4,8
In an emergency setting, subcutaneous epinephrine (0.3-0.5 mg) can be useful in treating severe urticaria.4,8 And recent clinical trials have demonstrated complete clearance of urticaria with leukotriene inhibitors, such as montelukast (10 mg).10
Chronic urticaria, trigger unknown
Although acute urticaria is responsive to treatment, chronic urticaria—lesions that do not resolve after 6 weeks—poses a greater challenge. In up to 80% of cases of chronic urticaria, no identifiable trigger is found.3,8 Long-term treatment of patients with this chronic condition, including lifestyle changes (to avoid environmental or dietary triggers) and a medication regimen for 6 months or more, leads to complete resolution of symptoms in most cases.7,8
Angioedema: Less common, more dangerous
Angioedema is part of the same disease spectrum as urticaria, but it affects the deeper tissues—involving mucosal and submucosal swelling. Angioedema affects just 0.1% to 0.2% of the general population, but up to 15% of patients with urticaria.8
The risk increases with the use of ACE inhibitors; for every 1000 patients taking ACE inhibitors, 0.4 to 3.5 develop angioedema.11 A recent double-blind study involving 25,642 patients being treated with ACE inhibitors revealed that those taking a combination of ACE inhibitors were more likely to develop angioedema than those on monotherapy.12
Angioedema is characterized by the sudden appearance of painful, localized erythematous wheals with central blanching.13 It results from increased vascular permeability in capillaries of the dermis, which leads to fluid leakage.13,14 Increased accumulation of fluids from the vessels of the skin results in rapidly developing nonpitting edema that most often affects the hands, face (and lips), neck, and oropharynx (FIGURE 1).14 Although the head and neck are the most commonly involved areas, angioedema can also affect the digestive tract, leading to nausea, vomiting, diarrhea, and abdominal pain secondary to bowel edema.13
FIGURE 1
Angioedema: A look at the most commonly affected areas
Airway management is imperative
In severe cases of angioedema, involvement of the oral mucosa results in stridor, followed by upper airway obstruction. To avoid hypoxemia and death,13,14 rapid preparation for emergency intervention to maintain the airway is critical; mortality can be as high as 30% in patients with airway compromise.13,15 In severe cases in which edema engulfs the oropharynx, oral intubation is often impossible, and nasotracheal intubation may be warranted.15,16 A failure to maintain adequate airway function via nasotracheal intubation may signal the need for an emergency tracheotomy.15,16
Is the angioedema hereditary or acquired?
Hereditary and acquired angioedema are treated differently. After ensuring that the patient has a patent airway, distinguishing between them is critical. Hereditary angioedema is the result of an inherited deficiency of plasma protein C1 inhibitor (C1-INH). Acquired angioedema is typically caused by enhanced consumption of endogenous C1-INH,13,17 leading to a net deficit of circulating C1-INH, and can be triggered by food, pharmacologic agents, and, occasionally, by systemic disorders.13 African Americans and patients with renal impairment appear to be at increased risk for acquired angioedema.13 In both hereditary and acquired angioiedema, decreased levels of C1-INH lead to disruption of the complement pathway.
Although the clinical presentation of acquired and hereditary angioedema is similar, a focused patient history can be used to distinguish between them. Age, health status, and medication history are the key considerations. Hereditary angioedema most commonly occurs—in recurrent attacks after minor trauma—in children with no underlying disease, with worsening symptoms during puberty. Acquired angioedema is generally seen in adult and elderly patients with malignancies or other underlying disorders.
Mild to moderate cases of acquired angioedema respond well to oral corticosteroids and antihistamines. Severe cases often require administration of subcutaneous epinephrine, followed by IV steroids. Management of hereditary angioedema should include C1-INH-containing fresh frozen plasma,13,16,18 although fresh frozen plasma can be used if plasma with C1-INH is not available.13
While oral corticosteroids and anti-histamines may be effective adjunctive therapy for hereditary angioedema, they are not likely to reverse acute attacks in this patient population. IV diphenhydramine (50-100 mg) or IV cimetidine (300 mg) every 6 to 8 hours is a reasonable therapeutic regimen for acute attacks of hereditary angioedema.
A recent randomized double-blind trial involving 40 patients with hereditary angioedema studied the administration of ecallantide, a kallikrein inhibitor for which US Food and Drug Administration approval is pending.19 Nearly 3 out of 4 of those who received ecallantide (72.5%) for acute attacks of angioedema showed significant improvement within 4 hours.20 The use of kallikrein inhibitors, which target inflammatory blood components, is not widespread, but may hold promise for the treatment of hereditary angioedema.20
Cellulitis: On the lookout for infiltration
Cellulitis is a bacterial infection of the skin that affects approximately 24.6 in 1000 people and is rarely associated with death.21 It occurs when bacteria enter through disrupted areas in the skin, particularly when skin integrity is compromised by recent surgery, piercing, wounds, athlete’s foot, or even dermatitis.22,23Streptococcus and Staphylococcus are the 2 most common infectious agents, and methicillin-resistant Staphylococcus aureus (MRSA) is increasingly common.22,23
Although cellulitis is primarily superficial in nature, it may progress to a serious condition by infiltrating underlying tissues and spreading to nearby lymphatic tissue and the bloodstream to cause lymphadenitis or bacteremia.21,23 In instances of cellulitis-induced bacteremia, mortality rates increase if prompt, targeted treatment is not provided.23
Raised erythematous plaques are the cardinal features of cellulitis, with the affected areas warm to the touch, red, and tender.21,23 As the condition progresses, the affected area tends to enlarge and expand (FIGURE 2),24 and the patient often becomes febrile.22,24
The risk of developing cellulitis increases with age, compromised immune status, diabetes, obesity, IV drug use, lymphedema, and chronic corticosteroid use.22,24
Cellulitis is often diagnosed solely on the basis of clinical presentation, although aspiration of purulent discharge from the wound and a gram stain of the culture can confirm the diagnosis.25,26 (See “Is it cellulitis or stasis dermatitis?”.)
Direct immunofluorescence can be used when cultures are difficult to obtain, but this technique is seldom necessary.22 If infiltration of underlying soft tissues is suspected based on clinical findings, magnetic resonance imaging can be a useful tool in evaluating the extent of the infection and in directing appropriate debridement and drainage of affected areas.22,27
Patients with venous insufficiency may present with stasis dermatitis, which often results in breakdown of the skin and ulceration that bears a striking resemblance to cellulitis. Thus, these conditions can be easily confused, and may lead to unnecessary antibiotic use and, possibly, hospitalization in patients with venous insufficiency.26,28
Despite the similarities of these conditions, a focused patient history and physical exam can prevent such confusion. Stasis dermatitis arises as a result of venous insufficiency, so it is likely to be accompanied by pitting edema that responds to leg raising and to the use of elastic compression stockings—interventions that are seldom effective for cellulitis.26 In addition, cellulitis tends to be unilateral, while stasis dermatitis often has bilateral involvement.
FIGURE 2
Diagnosing cellulitis based on clinical presentation
The raised erythematous lesions that are a hallmark of cellulitis, shown here on the arm and face, are warm to the touch, red, and tender.
Treatment: Targeted antibiotics and preventive measures
Because of the likelihood of recurrence with cellulitis, treating the condition involves both preventive and curative measures. Mild cases can be treated in an outpatient setting with a 7- to 10-day course of oral cephalosporins or antibiotics with similar coverage.22,25,26 A recent randomized study involving 391 patients found that cure rates for cellulitis treated with cephalexin were between 83% and 92%, depending on the pathogen involved.26
For severe cases of cellulitis, patients who are immunocompromised, and cases that are refractory to oral medications, hospital admission is recommended, and use of IV antibiotics is routinely required.22,25,26 For patients with MRSA, a drug such as vancomycin IV may be warranted; a reasonable dose would be 15 mg/kg every 12 hours.22,26,27,29
A recent randomized, multicenter study demonstrated that vancomycin effectively treated approximately 67% of cases of MRSA-induced cellulitis.26 Neomycin should be strictly avoided whenever cellulitis is suspected, because of its propensity to promote antibiotic resistance to S. aureus.29
Patient education emphasizing preventive measures is critical for minimizing recurrence of cellulitis.22,25,26 Encourage patients to wash with antibacterial soap and water daily, apply topical antibiotic ointment, and keep the wound completely covered at all times. Advise them to change bandages and wash their hands frequently.27,29 Patients with diabetes and others with decreased circulation in the extremities need to take further precautions, such as moisturizing the skin regularly in order to prevent cuts in their skin.22,29
SJS: Triggered by drugs, and infections, too
Stevens-Johnson syndrome (SJS), also known as erythema multiforme major, is an often-debilitating and possibly fatal adverse reaction, typically (but not exclusively) to a drug. It manifests as full-thickness epidermal necrosis of the mucous membranes.30,31 SJS occurs at a rate of about 1 to 7 cases per million people per year, and has a mortality rate of approximately 5%.30-32 The types of medication that most commonly precipitate SJS are anticonvulsants, sulfa drugs, penicillin-related and cephalosporin antibiotics, anti-inflammatory agents, and certain neoplastic drugs.30,32 SJS can develop in response to infections and neoplasms (TABLE) as well, and in many cases a cause is never found.
Patients with widespread involvement often complain of a burning sensation, particularly around the mouth.32-36 In some cases, this is the presenting sign, because the oral mucosa tends to be among the first mucous membranes involved.
TABLE
Stevens-Johnson syndrome: Pinpointing the cause32
| MORE FREQUENT ETIOLOGY |
| Drugs: Allopurinol, anticonvulsants, antiparasitics, barbiturates, NSAIDs, penicillin-related and cephalosporin antibiotics, sulfas, tetracyclines |
| LESS FREQUENT ETIOLOGY |
| Bacterial: Diphtheria, group A Streptococcus, Mycoplasma pneumoniae, tularemia, typhoid |
| Fungal: Coccidiomycosis, dermatophytosis, histoplasmosis, |
| Protozoan: Plasmodium, trichomoniasis |
| Viral: AIDS, Coxsackie, Epstein-Barr, HSV, influenza |
| AIDS, acquired immune deficiency syndrome; HSV, herpes-simplex virus; NSAIDs, nonsteroidal anti-inflammatory drugs. |
Targetoid lesions, Nikolsky sign are diagnostic clues
The characteristic skin lesions seen with SJS consist of initially erythematous macules that rapidly develop central necrosis to form vesiculation, as well as other variable areas of denudation (FIGURE 3). These vesicles tend to demonstrate confluence and often show a positive Nikolsky sign—epidermal detachment of superficial layers of the skin when slight pressure is applied. The lesions typically take on a targetoid appearance. If left untreated, the blisters often result in ulceration and hemorrhagic crusting of affected areas.
Like most skin disorders, SJS is initially diagnosed on the basis of clinical presentation. However, it is a rare disorder, and commonly misdiagnosed. Among the disorders SJS has been mistaken for are staphylococcal scalded skin syndrome, toxic shock syndrome, exfoliative dermatitis, scarlet fever, erythema multiforme, and iatrogenic chemical burns.32,37 (Erythema multiforme, SJS, and toxic epidermal necrolysis [TEN] are considered part of the same disease spectrum; erythema multiforme typically presents with few random lesions and no mucosal involvement, SJS with mucosal involvement on up to 30% of the body surface, and TEN with >30%.)32,37 Skin biopsies and immunofluorescence studies are recommended to confirm the diagnosis.
During the course of SJS, the mucous membranes of the oropharynx, ocular cavity, gastrointestinal system, nasal cavity, genitourinary system, and lower respiratory tracts are typically affected.32-35 As the condition progresses, increased epidermal erosion can lead to the sloughing off of up to 100% of the epidermis, resulting in considerable fluid loss.32,34
Corneal ulceration, anterior uveitis, panophthalmitis, polyarthritis, hematuria, and acute tubular necrosis leading to renal failure may also occur.32,35,37 Scarring within vital ocular structures can result in corneal opacity and lead to significant visual impairment. In severe cases, blood loss and fluid loss increase the risk of bacterial superinfection and sepsis.35,37
FIGURE 3
Targetoid lesions are characteristic of SJS
Characteristic diffuse erythematous macules with necrotic centers and overlying blistering on the back of a patient with Stevens-Johnson syndrome.
Supportive therapy, wound care are key components of treatment
Rapid cessation of the offending agent with targeted dermatologic management can reduce morbidity by promoting rapid re-epithelialization of affected skin. (See “SJS is diagnosed, but not quickly,” [Verdicts] on page 332, for a discussion of the dangers of delayed diagnosis and failure to promptly stop the drug causing the acute reaction.)
Closely monitoring the patient for fluid and electrolyte abnormalities is also crucial. Corticosteroids and IV immunoglobulins have been suggested for early severe cases of SJS, but their efficacy in treating this condition has yet to be established by prospective double-blind studies.32-37
The skin lesions associated with SJS should be treated in the same way you would treat thermal burns, with local wound care, warm compresses, and topical anesthetics for pain reduction.36,37 Oral lesions are managed with diphenhydramine or sodium bicarbonate mouthwashes and glycerin swabs.
An ophthalmologic consultation is mandatory because of the risk of vision loss associated with corneal scarring.
And now, a return to our 9-year-old patient
When we left off our discussion of Julie, the ED physician who examined her had detected a targetoid lesion with a necrotic, purpuric center—a finding that we described as a diagnostic clue. The second diagnostic clue? The presence of the Nikolsky sign, which the doctor detected by applying slight pressure to the lesion. Julie was admitted to the hospital with a presumed diagnosis of SJS, which skin biopsies and immunofluorescence studies later confirmed.
It wasn’t clear whether Julie had had a reaction to the sulfa (which she’d completed) or to the penicillin (which she’d just begun taking), or whether she had a synergistic reaction to both. Although the exact cause remained uncertain, as it often does, the penicillin was stopped immediately. She received dermatologic treatment without delay and was monitored closely for fluid and electrolyte status. Since Julie had signs of ocular involvement, daily erythromycin and corticosteroid eyedrops were administered to minimize the risk of infection and reduce local inflammation. Given the risk of long-term ocular complications in patients with SJS, we recommended continued ophthalmologic care.
Nine days after she was admitted, Julie’s symptoms resolved, with the exception of persistent complaints of dry eye. At discharge, Julie was given artificial tears to minimize ocular irritation. We suspected that she had dry eyes because of SJS-induced corneal scarring, but we were unable to confirm our suspicion because our patient failed to return for scheduled ophthalmologic appointments. She was subsequently lost to follow up.
Acknowledgement
The authors wish to thank Azita Hamedani, MD, MPH, FACEP, for her critical editing of the text.
Correspondence
Ribhi Hazin, MD, 29 Garden Street, Suite 214, Cambridge, MA 02138; [email protected]
- Management of hereditary angioedema should include fresh frozen plasma containing C1 inhibitor (C1-INH), whenever possible; if C1-INH-containing plasma is unavailable, fresh frozen plasma can be used instead (SOR: A).
- Do not give neomycin to patients with suspected cellulitis; the drug may promote antibiotic resistance in Staphylococcus aureus, a pathogen often associated with this condition (SOR: A).
- Whenever a patient presents with erythematous skin lesions and a recent history of receiving penicillin or a cephalosporin antibiotic, a sulfa derivative, or an anticonvulsant, the suspected medication should be stopped until Stevens-Johnson syndrome is ruled out (SOR: A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Skin eruptions are a common reason for visits to primary care physicians. While most are innocuous, some are associated with—or are early warning signs of—severe allergic reactions or other emergent conditions. A 9-year-old patient I’ll call Julie is a case in point.
The first time Julie’s parents brought her to our clinic, she’d been complaining of a sore throat and had a fever that hovered between 102° and 103°F for several days. The physician who examined Julie found mild maxillary tenderness. A rapid streptococcal throat swab was negative; her doctor prescribed a 10-day course of trimethoprim/sulfamethoxazole (TMP/SMX) for presumed acute sinusitis.
Thirteen days later (3 days after the patient completed the course of antibiotics), Julie’s parents brought her back to the clinic. Her throat still hurt, and she had erythematous oval lesions on her trunk and upper extremities. Her physician diagnosed scarlet fever and wrote a prescription for penicillin.
The following day, Julie was taken to the emergency department (ED) with bilateral conjunctival hyperemia and diffuse, confluent erythematous macules throughout her body. The ED physician who examined Julie found a 2.5 × 2.0 cm targetoid lesion with a necrotic, purpuric center on her lower back—a diagnostic clue to the cause of her signs and symptoms.
If you had been Julie’s physician, would you have been alert to that clue?
For family physicians accustomed to seeing relatively mild skin disorders, recognizing and responding to dermatologic conditions with potentially dire outcomes can be challenging. This review, and the images that accompany it, will help you sharpen your dermatologic diagnostic and treatment skills, both for benign disorders and those that are less common and more severe. We’ll also tell you more about Julie and her diagnosis.
Urticaria: A simple case of hives?
This common allergic reaction affects close to 10% of the population at some point in their lives. The affected areas are itchy and have raised, circumscribed red welts with surrounding erythema.1 Urticaria can occur throughout the body, with new lesions often erupting as the old ones disappear.2,3
Despite the persistent itchiness that patients typically complain of, however, urticaria is usually self-limiting, and rarely life-threatening. Acute urticaria normally resolves within 2 to 6 weeks.2,4
In most cases, urticaria arises secondary to exposure to an allergenic substance, chemical, or emotional stress.4,5 In rare instances, systemic diseases, such as hematologic malignancies, can also cause urticarial lesions to erupt throughout the body.4
Body piercing, cosmetics, latex exposure, Helicobacter pylori, insects, and angiotensin-converting enzyme (ACE) inhibitors have been identified as common triggers of urticaria, as have nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotics, animal dander, and foods such as shellfish, nuts, and dairy products.4,6
Treatment of all forms of urticaria should be based on identification and strict avoidance of the causative agent, if it’s known.7 Following withdrawal of the specific agent, symptomatic treatment with medications such as histamine antagonists and corticosteroids remains the mainstay of therapy.4,8 A daily dose of 40 to 60 mg prednisone for 5 days is a reasonable therapeutic regimen for adults; a 5-day course of 1 mg/kg per day is suitable for pediatric patients.4,8,9
In the event that topical or oral therapy is ineffective in mild cases of urticaria, intravenous (IV) diphenhydramine (50 mg) can be administered every 6 to 8 hours.4,8 IV diphenhydramine typically takes 30 minutes to work, while corticosteroids take at least 2 hours to reach full effect.4,8
In an emergency setting, subcutaneous epinephrine (0.3-0.5 mg) can be useful in treating severe urticaria.4,8 And recent clinical trials have demonstrated complete clearance of urticaria with leukotriene inhibitors, such as montelukast (10 mg).10
Chronic urticaria, trigger unknown
Although acute urticaria is responsive to treatment, chronic urticaria—lesions that do not resolve after 6 weeks—poses a greater challenge. In up to 80% of cases of chronic urticaria, no identifiable trigger is found.3,8 Long-term treatment of patients with this chronic condition, including lifestyle changes (to avoid environmental or dietary triggers) and a medication regimen for 6 months or more, leads to complete resolution of symptoms in most cases.7,8
Angioedema: Less common, more dangerous
Angioedema is part of the same disease spectrum as urticaria, but it affects the deeper tissues—involving mucosal and submucosal swelling. Angioedema affects just 0.1% to 0.2% of the general population, but up to 15% of patients with urticaria.8
The risk increases with the use of ACE inhibitors; for every 1000 patients taking ACE inhibitors, 0.4 to 3.5 develop angioedema.11 A recent double-blind study involving 25,642 patients being treated with ACE inhibitors revealed that those taking a combination of ACE inhibitors were more likely to develop angioedema than those on monotherapy.12
Angioedema is characterized by the sudden appearance of painful, localized erythematous wheals with central blanching.13 It results from increased vascular permeability in capillaries of the dermis, which leads to fluid leakage.13,14 Increased accumulation of fluids from the vessels of the skin results in rapidly developing nonpitting edema that most often affects the hands, face (and lips), neck, and oropharynx (FIGURE 1).14 Although the head and neck are the most commonly involved areas, angioedema can also affect the digestive tract, leading to nausea, vomiting, diarrhea, and abdominal pain secondary to bowel edema.13
FIGURE 1
Angioedema: A look at the most commonly affected areas
Airway management is imperative
In severe cases of angioedema, involvement of the oral mucosa results in stridor, followed by upper airway obstruction. To avoid hypoxemia and death,13,14 rapid preparation for emergency intervention to maintain the airway is critical; mortality can be as high as 30% in patients with airway compromise.13,15 In severe cases in which edema engulfs the oropharynx, oral intubation is often impossible, and nasotracheal intubation may be warranted.15,16 A failure to maintain adequate airway function via nasotracheal intubation may signal the need for an emergency tracheotomy.15,16
Is the angioedema hereditary or acquired?
Hereditary and acquired angioedema are treated differently. After ensuring that the patient has a patent airway, distinguishing between them is critical. Hereditary angioedema is the result of an inherited deficiency of plasma protein C1 inhibitor (C1-INH). Acquired angioedema is typically caused by enhanced consumption of endogenous C1-INH,13,17 leading to a net deficit of circulating C1-INH, and can be triggered by food, pharmacologic agents, and, occasionally, by systemic disorders.13 African Americans and patients with renal impairment appear to be at increased risk for acquired angioedema.13 In both hereditary and acquired angioiedema, decreased levels of C1-INH lead to disruption of the complement pathway.
Although the clinical presentation of acquired and hereditary angioedema is similar, a focused patient history can be used to distinguish between them. Age, health status, and medication history are the key considerations. Hereditary angioedema most commonly occurs—in recurrent attacks after minor trauma—in children with no underlying disease, with worsening symptoms during puberty. Acquired angioedema is generally seen in adult and elderly patients with malignancies or other underlying disorders.
Mild to moderate cases of acquired angioedema respond well to oral corticosteroids and antihistamines. Severe cases often require administration of subcutaneous epinephrine, followed by IV steroids. Management of hereditary angioedema should include C1-INH-containing fresh frozen plasma,13,16,18 although fresh frozen plasma can be used if plasma with C1-INH is not available.13
While oral corticosteroids and anti-histamines may be effective adjunctive therapy for hereditary angioedema, they are not likely to reverse acute attacks in this patient population. IV diphenhydramine (50-100 mg) or IV cimetidine (300 mg) every 6 to 8 hours is a reasonable therapeutic regimen for acute attacks of hereditary angioedema.
A recent randomized double-blind trial involving 40 patients with hereditary angioedema studied the administration of ecallantide, a kallikrein inhibitor for which US Food and Drug Administration approval is pending.19 Nearly 3 out of 4 of those who received ecallantide (72.5%) for acute attacks of angioedema showed significant improvement within 4 hours.20 The use of kallikrein inhibitors, which target inflammatory blood components, is not widespread, but may hold promise for the treatment of hereditary angioedema.20
Cellulitis: On the lookout for infiltration
Cellulitis is a bacterial infection of the skin that affects approximately 24.6 in 1000 people and is rarely associated with death.21 It occurs when bacteria enter through disrupted areas in the skin, particularly when skin integrity is compromised by recent surgery, piercing, wounds, athlete’s foot, or even dermatitis.22,23Streptococcus and Staphylococcus are the 2 most common infectious agents, and methicillin-resistant Staphylococcus aureus (MRSA) is increasingly common.22,23
Although cellulitis is primarily superficial in nature, it may progress to a serious condition by infiltrating underlying tissues and spreading to nearby lymphatic tissue and the bloodstream to cause lymphadenitis or bacteremia.21,23 In instances of cellulitis-induced bacteremia, mortality rates increase if prompt, targeted treatment is not provided.23
Raised erythematous plaques are the cardinal features of cellulitis, with the affected areas warm to the touch, red, and tender.21,23 As the condition progresses, the affected area tends to enlarge and expand (FIGURE 2),24 and the patient often becomes febrile.22,24
The risk of developing cellulitis increases with age, compromised immune status, diabetes, obesity, IV drug use, lymphedema, and chronic corticosteroid use.22,24
Cellulitis is often diagnosed solely on the basis of clinical presentation, although aspiration of purulent discharge from the wound and a gram stain of the culture can confirm the diagnosis.25,26 (See “Is it cellulitis or stasis dermatitis?”.)
Direct immunofluorescence can be used when cultures are difficult to obtain, but this technique is seldom necessary.22 If infiltration of underlying soft tissues is suspected based on clinical findings, magnetic resonance imaging can be a useful tool in evaluating the extent of the infection and in directing appropriate debridement and drainage of affected areas.22,27
Patients with venous insufficiency may present with stasis dermatitis, which often results in breakdown of the skin and ulceration that bears a striking resemblance to cellulitis. Thus, these conditions can be easily confused, and may lead to unnecessary antibiotic use and, possibly, hospitalization in patients with venous insufficiency.26,28
Despite the similarities of these conditions, a focused patient history and physical exam can prevent such confusion. Stasis dermatitis arises as a result of venous insufficiency, so it is likely to be accompanied by pitting edema that responds to leg raising and to the use of elastic compression stockings—interventions that are seldom effective for cellulitis.26 In addition, cellulitis tends to be unilateral, while stasis dermatitis often has bilateral involvement.
FIGURE 2
Diagnosing cellulitis based on clinical presentation
The raised erythematous lesions that are a hallmark of cellulitis, shown here on the arm and face, are warm to the touch, red, and tender.
Treatment: Targeted antibiotics and preventive measures
Because of the likelihood of recurrence with cellulitis, treating the condition involves both preventive and curative measures. Mild cases can be treated in an outpatient setting with a 7- to 10-day course of oral cephalosporins or antibiotics with similar coverage.22,25,26 A recent randomized study involving 391 patients found that cure rates for cellulitis treated with cephalexin were between 83% and 92%, depending on the pathogen involved.26
For severe cases of cellulitis, patients who are immunocompromised, and cases that are refractory to oral medications, hospital admission is recommended, and use of IV antibiotics is routinely required.22,25,26 For patients with MRSA, a drug such as vancomycin IV may be warranted; a reasonable dose would be 15 mg/kg every 12 hours.22,26,27,29
A recent randomized, multicenter study demonstrated that vancomycin effectively treated approximately 67% of cases of MRSA-induced cellulitis.26 Neomycin should be strictly avoided whenever cellulitis is suspected, because of its propensity to promote antibiotic resistance to S. aureus.29
Patient education emphasizing preventive measures is critical for minimizing recurrence of cellulitis.22,25,26 Encourage patients to wash with antibacterial soap and water daily, apply topical antibiotic ointment, and keep the wound completely covered at all times. Advise them to change bandages and wash their hands frequently.27,29 Patients with diabetes and others with decreased circulation in the extremities need to take further precautions, such as moisturizing the skin regularly in order to prevent cuts in their skin.22,29
SJS: Triggered by drugs, and infections, too
Stevens-Johnson syndrome (SJS), also known as erythema multiforme major, is an often-debilitating and possibly fatal adverse reaction, typically (but not exclusively) to a drug. It manifests as full-thickness epidermal necrosis of the mucous membranes.30,31 SJS occurs at a rate of about 1 to 7 cases per million people per year, and has a mortality rate of approximately 5%.30-32 The types of medication that most commonly precipitate SJS are anticonvulsants, sulfa drugs, penicillin-related and cephalosporin antibiotics, anti-inflammatory agents, and certain neoplastic drugs.30,32 SJS can develop in response to infections and neoplasms (TABLE) as well, and in many cases a cause is never found.
Patients with widespread involvement often complain of a burning sensation, particularly around the mouth.32-36 In some cases, this is the presenting sign, because the oral mucosa tends to be among the first mucous membranes involved.
TABLE
Stevens-Johnson syndrome: Pinpointing the cause32
| MORE FREQUENT ETIOLOGY |
| Drugs: Allopurinol, anticonvulsants, antiparasitics, barbiturates, NSAIDs, penicillin-related and cephalosporin antibiotics, sulfas, tetracyclines |
| LESS FREQUENT ETIOLOGY |
| Bacterial: Diphtheria, group A Streptococcus, Mycoplasma pneumoniae, tularemia, typhoid |
| Fungal: Coccidiomycosis, dermatophytosis, histoplasmosis, |
| Protozoan: Plasmodium, trichomoniasis |
| Viral: AIDS, Coxsackie, Epstein-Barr, HSV, influenza |
| AIDS, acquired immune deficiency syndrome; HSV, herpes-simplex virus; NSAIDs, nonsteroidal anti-inflammatory drugs. |
Targetoid lesions, Nikolsky sign are diagnostic clues
The characteristic skin lesions seen with SJS consist of initially erythematous macules that rapidly develop central necrosis to form vesiculation, as well as other variable areas of denudation (FIGURE 3). These vesicles tend to demonstrate confluence and often show a positive Nikolsky sign—epidermal detachment of superficial layers of the skin when slight pressure is applied. The lesions typically take on a targetoid appearance. If left untreated, the blisters often result in ulceration and hemorrhagic crusting of affected areas.
Like most skin disorders, SJS is initially diagnosed on the basis of clinical presentation. However, it is a rare disorder, and commonly misdiagnosed. Among the disorders SJS has been mistaken for are staphylococcal scalded skin syndrome, toxic shock syndrome, exfoliative dermatitis, scarlet fever, erythema multiforme, and iatrogenic chemical burns.32,37 (Erythema multiforme, SJS, and toxic epidermal necrolysis [TEN] are considered part of the same disease spectrum; erythema multiforme typically presents with few random lesions and no mucosal involvement, SJS with mucosal involvement on up to 30% of the body surface, and TEN with >30%.)32,37 Skin biopsies and immunofluorescence studies are recommended to confirm the diagnosis.
During the course of SJS, the mucous membranes of the oropharynx, ocular cavity, gastrointestinal system, nasal cavity, genitourinary system, and lower respiratory tracts are typically affected.32-35 As the condition progresses, increased epidermal erosion can lead to the sloughing off of up to 100% of the epidermis, resulting in considerable fluid loss.32,34
Corneal ulceration, anterior uveitis, panophthalmitis, polyarthritis, hematuria, and acute tubular necrosis leading to renal failure may also occur.32,35,37 Scarring within vital ocular structures can result in corneal opacity and lead to significant visual impairment. In severe cases, blood loss and fluid loss increase the risk of bacterial superinfection and sepsis.35,37
FIGURE 3
Targetoid lesions are characteristic of SJS
Characteristic diffuse erythematous macules with necrotic centers and overlying blistering on the back of a patient with Stevens-Johnson syndrome.
Supportive therapy, wound care are key components of treatment
Rapid cessation of the offending agent with targeted dermatologic management can reduce morbidity by promoting rapid re-epithelialization of affected skin. (See “SJS is diagnosed, but not quickly,” [Verdicts] on page 332, for a discussion of the dangers of delayed diagnosis and failure to promptly stop the drug causing the acute reaction.)
Closely monitoring the patient for fluid and electrolyte abnormalities is also crucial. Corticosteroids and IV immunoglobulins have been suggested for early severe cases of SJS, but their efficacy in treating this condition has yet to be established by prospective double-blind studies.32-37
The skin lesions associated with SJS should be treated in the same way you would treat thermal burns, with local wound care, warm compresses, and topical anesthetics for pain reduction.36,37 Oral lesions are managed with diphenhydramine or sodium bicarbonate mouthwashes and glycerin swabs.
An ophthalmologic consultation is mandatory because of the risk of vision loss associated with corneal scarring.
And now, a return to our 9-year-old patient
When we left off our discussion of Julie, the ED physician who examined her had detected a targetoid lesion with a necrotic, purpuric center—a finding that we described as a diagnostic clue. The second diagnostic clue? The presence of the Nikolsky sign, which the doctor detected by applying slight pressure to the lesion. Julie was admitted to the hospital with a presumed diagnosis of SJS, which skin biopsies and immunofluorescence studies later confirmed.
It wasn’t clear whether Julie had had a reaction to the sulfa (which she’d completed) or to the penicillin (which she’d just begun taking), or whether she had a synergistic reaction to both. Although the exact cause remained uncertain, as it often does, the penicillin was stopped immediately. She received dermatologic treatment without delay and was monitored closely for fluid and electrolyte status. Since Julie had signs of ocular involvement, daily erythromycin and corticosteroid eyedrops were administered to minimize the risk of infection and reduce local inflammation. Given the risk of long-term ocular complications in patients with SJS, we recommended continued ophthalmologic care.
Nine days after she was admitted, Julie’s symptoms resolved, with the exception of persistent complaints of dry eye. At discharge, Julie was given artificial tears to minimize ocular irritation. We suspected that she had dry eyes because of SJS-induced corneal scarring, but we were unable to confirm our suspicion because our patient failed to return for scheduled ophthalmologic appointments. She was subsequently lost to follow up.
Acknowledgement
The authors wish to thank Azita Hamedani, MD, MPH, FACEP, for her critical editing of the text.
Correspondence
Ribhi Hazin, MD, 29 Garden Street, Suite 214, Cambridge, MA 02138; [email protected]
1. Noe R, Cohen AL, Lederman E, et al. Skin disorders among construction workers following Hurricane Katrina and Hurricane Rita: an outbreak investigation in New Orleans, Louisiana. Arch Dermatol. 2007;143:1393-1398.
2. Kulthanan K, Jiamton S, Thumpimukvatana N, et al. Chronic idiopathic urticaria: prevalence and clinical course. J Dermatol. 2007;34:294-301.
3. Stanway AD, Cohen SN, Chen C, et al. H1-antihistamines for chronic urticaria. Cochrane Database Syst Rev. 2008(2):CD006137.-
4. Grattan CE, Humphreys F. British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007;157:1116-1123.
5. Kozel MM, Mekkes JR, Bossuyt PM, et al. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-391.
6. Nettis E, Colanardi MC, Soccio AL, et al. Double-blind, placebo-controlled study of sublingual immunotherapy in patients with latex-induced urticaria: a 12-month study. Br J Dermatol. 2007;156:674-681.
7. Lee EE, Maibach HI. Treatment of urticaria. An evidence-based evaluation of antihistamines. Am J Clin Dermatol. 2001;2:27-32.
8. Humphreys F, Hunter JA. The characteristics of urticaria in 390 patients. Br J Dermatol. 1998;138:635-638.
9. Serhat Inaloz H, Ozturk S, Akcali C, et al. Low-dose and short-term cyclosporine treatment in patients with chronic idiopathic urticaria: A clinical and immunological evaluation. J Dermatol. 2008;35:276-282.
10. Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria. A single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110:484-488.
11. Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637-1642.
12. Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-1559.
13. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;115(3 Suppl 2):s483-s523.
14. Bas M, Kirchhartz N, Hochfeld J, et al. Potential role of vasomotor effects of fibrinogen in bradykinin-induced angioedema. J Allergy Clin Immunol. 2008;121:969e2-975e2.
15. Sica DA, Black HR. Angioedema in heart failure: occurrence with ACE inhibitors and safety of angiotensin receptor blocker therapy. Congest Heart Fail. 2002;8:334-341.
16. Banerji A, Clark S, Blanda M, et al. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. 2008;100:327-332.
17. Cicardi M, Zingale LC, Zanichelli A, et al. The use of plasma-derived C1 inhibitor in the treatment of hereditary angioedema. Expert Opin Pharmacother. 2007;8:3173-3181.
18. Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100:153-161.
19. US Food and Drug Administration. Advisory Committee Briefing Document. Kalbitor (ecallantide) for acute attacks of hereditary angioedema. Available at: http://www.fda.gov/ohrms/dockets/AC/09/briefing/2009-4413b1-03-Dyax.pdf. Accessed May 5, 2009.
20. Schneider L, Lumry W, Vegh A, et al. Critical role of kallikrein in hereditary angioedema pathogenesis: a clinical trial of ecallantide, a novel kallikrein inhibitor. J Allergy Clin Immunol. 2007;120:416-422.
21. Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293-299.
22. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
23. Morris A. What are the benefits of treatments? Cellulitis and erysipelas. BMJ Clin Evid. 2005;13:2066-2069.
24. Murray H, Stiell I, Wells G. Treatment failure in emergency department patients with cellulitis. CJEM. 2005;7:228-234.
25. Meier DE, Nkor SK, Aasa D, et al. Prospective randomized comparison of two preoperative skin preparation techniques in a developing world country. World J Surg. 2001;25:441-443.
26. Giordano PA, Elston D, Akinlade BK, et al. Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. Curr Med Res Opin. 2006;22:2419-2428.
27. Halpern J, Holder R, Langford NJ. Ethnicity and other risk factors for acute lower limb cellulitis: a U.K.-based prospective case-control study. Br J Dermatol. 2008;158:1288-1292.
28. Lin YT, Lu PW. Retrospective study of pediatric facial cellulitis of odontogenic origin. Pediatr Infect Dis J. 2006;25:339-342.
29. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemo. 2007;51:4044-4048.
30. Schneck J, Fagot JP, Sekula P, et al. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58:33-40.
31. Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43-47.
32. Hazin R, Ibrahimi OA, Hazin MI, et al. Stevens-Johnson syndrome: pathogenesis, diagnosis, and management. Ann Med, 2008;40:129-138.
33. Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486.-
34. Jette N, Hemming K, Hutton JL, et al. Topiramate add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2008;(3):DC001417.-
35. Gürcan HM, Ahmed AR. Efficacy of various intravenous immunoglobulin therapy protocols in auto-immune and chronic inflammatory disorders. Ann Pharmacother. 2007;41:812-523.
36. Strom BL, Carson JL, Halpern AC, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991;127:831-838.
37. Gravante G, Delogu D, Marianetti M, et al. Toxic epidermal necrolysis and Steven-Johnson syndrome: 11-years experience and outcome. Eur Rev Med Pharmacol Sci. 2007;11:119-127.
1. Noe R, Cohen AL, Lederman E, et al. Skin disorders among construction workers following Hurricane Katrina and Hurricane Rita: an outbreak investigation in New Orleans, Louisiana. Arch Dermatol. 2007;143:1393-1398.
2. Kulthanan K, Jiamton S, Thumpimukvatana N, et al. Chronic idiopathic urticaria: prevalence and clinical course. J Dermatol. 2007;34:294-301.
3. Stanway AD, Cohen SN, Chen C, et al. H1-antihistamines for chronic urticaria. Cochrane Database Syst Rev. 2008(2):CD006137.-
4. Grattan CE, Humphreys F. British Association of Dermatologists Therapy Guidelines and Audit Subcommittee. Guidelines for evaluation and management of urticaria in adults and children. Br J Dermatol. 2007;157:1116-1123.
5. Kozel MM, Mekkes JR, Bossuyt PM, et al. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-391.
6. Nettis E, Colanardi MC, Soccio AL, et al. Double-blind, placebo-controlled study of sublingual immunotherapy in patients with latex-induced urticaria: a 12-month study. Br J Dermatol. 2007;156:674-681.
7. Lee EE, Maibach HI. Treatment of urticaria. An evidence-based evaluation of antihistamines. Am J Clin Dermatol. 2001;2:27-32.
8. Humphreys F, Hunter JA. The characteristics of urticaria in 390 patients. Br J Dermatol. 1998;138:635-638.
9. Serhat Inaloz H, Ozturk S, Akcali C, et al. Low-dose and short-term cyclosporine treatment in patients with chronic idiopathic urticaria: A clinical and immunological evaluation. J Dermatol. 2008;35:276-282.
10. Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria. A single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110:484-488.
11. Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637-1642.
12. Yusuf S, Teo KK, Pogue J, et al. ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-1559.
13. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;115(3 Suppl 2):s483-s523.
14. Bas M, Kirchhartz N, Hochfeld J, et al. Potential role of vasomotor effects of fibrinogen in bradykinin-induced angioedema. J Allergy Clin Immunol. 2008;121:969e2-975e2.
15. Sica DA, Black HR. Angioedema in heart failure: occurrence with ACE inhibitors and safety of angiotensin receptor blocker therapy. Congest Heart Fail. 2002;8:334-341.
16. Banerji A, Clark S, Blanda M, et al. Multicenter study of patients with angiotensin-converting enzyme inhibitor-induced angioedema who present to the emergency department. Ann Allergy Asthma Immunol. 2008;100:327-332.
17. Cicardi M, Zingale LC, Zanichelli A, et al. The use of plasma-derived C1 inhibitor in the treatment of hereditary angioedema. Expert Opin Pharmacother. 2007;8:3173-3181.
18. Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100:153-161.
19. US Food and Drug Administration. Advisory Committee Briefing Document. Kalbitor (ecallantide) for acute attacks of hereditary angioedema. Available at: http://www.fda.gov/ohrms/dockets/AC/09/briefing/2009-4413b1-03-Dyax.pdf. Accessed May 5, 2009.
20. Schneider L, Lumry W, Vegh A, et al. Critical role of kallikrein in hereditary angioedema pathogenesis: a clinical trial of ecallantide, a novel kallikrein inhibitor. J Allergy Clin Immunol. 2007;120:416-422.
21. Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293-299.
22. Stevens DL, Bisno AL, Chambers HF, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
23. Morris A. What are the benefits of treatments? Cellulitis and erysipelas. BMJ Clin Evid. 2005;13:2066-2069.
24. Murray H, Stiell I, Wells G. Treatment failure in emergency department patients with cellulitis. CJEM. 2005;7:228-234.
25. Meier DE, Nkor SK, Aasa D, et al. Prospective randomized comparison of two preoperative skin preparation techniques in a developing world country. World J Surg. 2001;25:441-443.
26. Giordano PA, Elston D, Akinlade BK, et al. Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. Curr Med Res Opin. 2006;22:2419-2428.
27. Halpern J, Holder R, Langford NJ. Ethnicity and other risk factors for acute lower limb cellulitis: a U.K.-based prospective case-control study. Br J Dermatol. 2008;158:1288-1292.
28. Lin YT, Lu PW. Retrospective study of pediatric facial cellulitis of odontogenic origin. Pediatr Infect Dis J. 2006;25:339-342.
29. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemo. 2007;51:4044-4048.
30. Schneck J, Fagot JP, Sekula P, et al. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58:33-40.
31. Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43-47.
32. Hazin R, Ibrahimi OA, Hazin MI, et al. Stevens-Johnson syndrome: pathogenesis, diagnosis, and management. Ann Med, 2008;40:129-138.
33. Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486.-
34. Jette N, Hemming K, Hutton JL, et al. Topiramate add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2008;(3):DC001417.-
35. Gürcan HM, Ahmed AR. Efficacy of various intravenous immunoglobulin therapy protocols in auto-immune and chronic inflammatory disorders. Ann Pharmacother. 2007;41:812-523.
36. Strom BL, Carson JL, Halpern AC, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991;127:831-838.
37. Gravante G, Delogu D, Marianetti M, et al. Toxic epidermal necrolysis and Steven-Johnson syndrome: 11-years experience and outcome. Eur Rev Med Pharmacol Sci. 2007;11:119-127.
How to remove those things children put up their nose
When a parent brings in a young child who has inexplicably used his or her nostril as the repository for an object like a marble or a button, are you prepared to get it out—or do you send them to the ED? If you send patients to the local hospital (or otolaryngologist), you’re not alone. Lack of appropriate equipment prevents many family physicians from handling this all-too-common dilemma. Yet you can usually retrieve a nasal foreign body yourself, if you have several simple instruments (FIGURE 1) and a topical decongestant and anesthetic on hand.
FIGURE 1
Pediatric nasal retrieval tools you’ll need
The equipment you’ll need to remove an object from a child’s nostril includes (left to right): Hartmann forceps, Frazier suction tube, blunt-tipped right-angle ear hook, and pediatric nasal speculum.
What did he put up there? Parental history often yields the specific time of insertion and the item involved. When such information is not available, purulent unilateral nasal discharge strongly suggests the presence of a foreign body. A plain radiograph can help identify a metallic or radiopaque object such as a button-like hearing aid battery. Batteries leak caustic alkali and require rapid removal. (Of course, all objects should be extracted as promptly as possible.)
Start with a topical decongestant
How to proceed? Apply a topical decongestant with 0.05% oxymetazoline, either in the form of a spray or nasal drops, to reduce edema and bleeding and facilitate removal. (I recommend applying the nasal decongestant to both nostrils; the child may have inserted more than 1 foreign object, so you should always examine both sides.)
The decongestant may be supplemented with a 4% lidocaine topical anesthetic, to reduce the discomfort associated with object retrieval—and, possibly, make the procedure less stressful for patient, family, and physician. If you’re using an oxymetazoline spray bottle, remove the top, add an equal amount of the lidocaine, and replace the top before applying it. You can use the same 50-50 formula to mix lidocaine and nasal drops.
Use a “papoose.” For further ease and safety, restrain the patient by wrapping him or her in a bed sheet made into a “papoose.” While there is a trend away from restraining children, my experience suggests that doing so reduces the risk of intranasal injury caused by uncontrolled patient motion while instruments are in the nose. Good lighting is also essential (a headlight is best), along with suction and safety eyewear for the staff.
Have the right equipment on hand
The pediatric nasal speculum, with its shorter blades, is best for small nostrils (FIGURE 1). The blunt-tipped right-angle ear hook is probably the most useful instrument for retrieving nasal foreign bodies because it can reach behind objects such as beads, buttons, or batteries, and hook textile or other fabric-like foreign bodies. The Hartmann forceps are more effective than the smaller jaw “alligator” forceps (FIGURE 2); their longer jaws with their less obtuse angle make it easier to avoid pushing the object posteriorly. Availability of these few instruments will allow you to remove most foreign bodies, reducing the need to refer to another physician.
FIGURE 2
Which forceps are best?
Avoid using the alligator forceps (left) for retrieving a nasal foreign body; the longer jaws of the Hartmann forceps (right) are less likely to push the object farther into the nostril.
No luck? If your efforts fail, referral will of course be necessary. It’s usually better to send the patient to an otolaryngologist than to the ED, for 2 reasons: (1) The otolaryngologist is more likely to have rigid nasal endoscopes on hand, and (2) a visit to the otolaryngologist will typically cost less than a trip to the ED. In addition, an otolaryngic evaluation will help determine whether a particular case warrants an extraction in the operating room under general anesthesia.
When a parent brings in a young child who has inexplicably used his or her nostril as the repository for an object like a marble or a button, are you prepared to get it out—or do you send them to the ED? If you send patients to the local hospital (or otolaryngologist), you’re not alone. Lack of appropriate equipment prevents many family physicians from handling this all-too-common dilemma. Yet you can usually retrieve a nasal foreign body yourself, if you have several simple instruments (FIGURE 1) and a topical decongestant and anesthetic on hand.
FIGURE 1
Pediatric nasal retrieval tools you’ll need
The equipment you’ll need to remove an object from a child’s nostril includes (left to right): Hartmann forceps, Frazier suction tube, blunt-tipped right-angle ear hook, and pediatric nasal speculum.
What did he put up there? Parental history often yields the specific time of insertion and the item involved. When such information is not available, purulent unilateral nasal discharge strongly suggests the presence of a foreign body. A plain radiograph can help identify a metallic or radiopaque object such as a button-like hearing aid battery. Batteries leak caustic alkali and require rapid removal. (Of course, all objects should be extracted as promptly as possible.)
Start with a topical decongestant
How to proceed? Apply a topical decongestant with 0.05% oxymetazoline, either in the form of a spray or nasal drops, to reduce edema and bleeding and facilitate removal. (I recommend applying the nasal decongestant to both nostrils; the child may have inserted more than 1 foreign object, so you should always examine both sides.)
The decongestant may be supplemented with a 4% lidocaine topical anesthetic, to reduce the discomfort associated with object retrieval—and, possibly, make the procedure less stressful for patient, family, and physician. If you’re using an oxymetazoline spray bottle, remove the top, add an equal amount of the lidocaine, and replace the top before applying it. You can use the same 50-50 formula to mix lidocaine and nasal drops.
Use a “papoose.” For further ease and safety, restrain the patient by wrapping him or her in a bed sheet made into a “papoose.” While there is a trend away from restraining children, my experience suggests that doing so reduces the risk of intranasal injury caused by uncontrolled patient motion while instruments are in the nose. Good lighting is also essential (a headlight is best), along with suction and safety eyewear for the staff.
Have the right equipment on hand
The pediatric nasal speculum, with its shorter blades, is best for small nostrils (FIGURE 1). The blunt-tipped right-angle ear hook is probably the most useful instrument for retrieving nasal foreign bodies because it can reach behind objects such as beads, buttons, or batteries, and hook textile or other fabric-like foreign bodies. The Hartmann forceps are more effective than the smaller jaw “alligator” forceps (FIGURE 2); their longer jaws with their less obtuse angle make it easier to avoid pushing the object posteriorly. Availability of these few instruments will allow you to remove most foreign bodies, reducing the need to refer to another physician.
FIGURE 2
Which forceps are best?
Avoid using the alligator forceps (left) for retrieving a nasal foreign body; the longer jaws of the Hartmann forceps (right) are less likely to push the object farther into the nostril.
No luck? If your efforts fail, referral will of course be necessary. It’s usually better to send the patient to an otolaryngologist than to the ED, for 2 reasons: (1) The otolaryngologist is more likely to have rigid nasal endoscopes on hand, and (2) a visit to the otolaryngologist will typically cost less than a trip to the ED. In addition, an otolaryngic evaluation will help determine whether a particular case warrants an extraction in the operating room under general anesthesia.
When a parent brings in a young child who has inexplicably used his or her nostril as the repository for an object like a marble or a button, are you prepared to get it out—or do you send them to the ED? If you send patients to the local hospital (or otolaryngologist), you’re not alone. Lack of appropriate equipment prevents many family physicians from handling this all-too-common dilemma. Yet you can usually retrieve a nasal foreign body yourself, if you have several simple instruments (FIGURE 1) and a topical decongestant and anesthetic on hand.
FIGURE 1
Pediatric nasal retrieval tools you’ll need
The equipment you’ll need to remove an object from a child’s nostril includes (left to right): Hartmann forceps, Frazier suction tube, blunt-tipped right-angle ear hook, and pediatric nasal speculum.
What did he put up there? Parental history often yields the specific time of insertion and the item involved. When such information is not available, purulent unilateral nasal discharge strongly suggests the presence of a foreign body. A plain radiograph can help identify a metallic or radiopaque object such as a button-like hearing aid battery. Batteries leak caustic alkali and require rapid removal. (Of course, all objects should be extracted as promptly as possible.)
Start with a topical decongestant
How to proceed? Apply a topical decongestant with 0.05% oxymetazoline, either in the form of a spray or nasal drops, to reduce edema and bleeding and facilitate removal. (I recommend applying the nasal decongestant to both nostrils; the child may have inserted more than 1 foreign object, so you should always examine both sides.)
The decongestant may be supplemented with a 4% lidocaine topical anesthetic, to reduce the discomfort associated with object retrieval—and, possibly, make the procedure less stressful for patient, family, and physician. If you’re using an oxymetazoline spray bottle, remove the top, add an equal amount of the lidocaine, and replace the top before applying it. You can use the same 50-50 formula to mix lidocaine and nasal drops.
Use a “papoose.” For further ease and safety, restrain the patient by wrapping him or her in a bed sheet made into a “papoose.” While there is a trend away from restraining children, my experience suggests that doing so reduces the risk of intranasal injury caused by uncontrolled patient motion while instruments are in the nose. Good lighting is also essential (a headlight is best), along with suction and safety eyewear for the staff.
Have the right equipment on hand
The pediatric nasal speculum, with its shorter blades, is best for small nostrils (FIGURE 1). The blunt-tipped right-angle ear hook is probably the most useful instrument for retrieving nasal foreign bodies because it can reach behind objects such as beads, buttons, or batteries, and hook textile or other fabric-like foreign bodies. The Hartmann forceps are more effective than the smaller jaw “alligator” forceps (FIGURE 2); their longer jaws with their less obtuse angle make it easier to avoid pushing the object posteriorly. Availability of these few instruments will allow you to remove most foreign bodies, reducing the need to refer to another physician.
FIGURE 2
Which forceps are best?
Avoid using the alligator forceps (left) for retrieving a nasal foreign body; the longer jaws of the Hartmann forceps (right) are less likely to push the object farther into the nostril.
No luck? If your efforts fail, referral will of course be necessary. It’s usually better to send the patient to an otolaryngologist than to the ED, for 2 reasons: (1) The otolaryngologist is more likely to have rigid nasal endoscopes on hand, and (2) a visit to the otolaryngologist will typically cost less than a trip to the ED. In addition, an otolaryngic evaluation will help determine whether a particular case warrants an extraction in the operating room under general anesthesia.
Preconception counseling: Make it part of the annual exam
- Supplementing women’s diets with 400 mg folic acid every day reduces the incidence of neural tube defects in their offspring by up to 72% (A).
- Optimizing diabetic glucose control prior to conception is linked to a reduction in birth defects and pregnancy loss (B).
- Limiting caffeine consumption to no more than 200 mg per day may reduce the risk of miscarriage (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
In the United States most women do not seek out prenatal care until the 8th to 12th week of pregnancy, when the crucial period of organogenesis (4 to 10 weeks after fertilization) has already passed. In addition, women whose pregnancies are unintended—up to half of all pregnancies—may delay seeking care even longer.1 Given these realities, family physicians should consider all visits during the reproductive years—especially yearly exams—as opportunities for preconception counseling.
Preconception health care is essential to the health of our nation. Data from the Centers for Disease Control and Prevention (CDC) are cause for concern:2,3
- 12% of infants are born prematurely.
- 31% of pregnancies are complicated by maternal health issues.
- 11% of women smoke during pregnancy.
- 10% drink alcohol during pregnancy.
- 69% of women do not take folate supplements.
- 31% of women are obese.
- 3% of women take medications and supplements that are known teratogens.
The Healthy People 2000 initiative set a goal of 60% of primary caregivers providing preconception care at routine medical visits, but thus far, only about 25% do so.2,3
As a primary care provider, you can have a huge impact on fetal and maternal health by counseling women to choose healthier lifestyles, helping them to manage their chronic conditions, updating their immunizations, and screening for genetic disorders before they become pregnant.
Start with the hard part: Lifestyle modification
Changing the way patients go about their lives—how they eat, how much they exercise, whether they use alcohol or tobacco—has 2 salient characteristics: It’s the most difficult thing to get patients to do, and it has the biggest payoff in improving maternal and fetal health. Lifestyle issues of greatest significance for the preconception patient include:
Folic acid supplementation. If your patients are like most women, they may not be aware of the importance of folic acid supplementation. Yet by neglecting to supplement their diet with folates, women are passing up an opportunity to reduce the incidence of neural tube defects (NTDs) such as anencephaly and spina bifida by up to 70%.4
4D ultrasound of an open neural tube defect in a developing fetus
Women considering conception or those who do not use contraception should take 400 mg folic acid, a dosage found in most prenatal vitamins, every day. All women of reproductive age should consider folate supplementation because of the high rate of unplanned pregnancies. Women who have previously had a child with an NTD and women who take anti-epileptic drugs should take 4 mg folate per day.5
Use routine office visits as an opportunity to counsel patients about folic acid supplementation, whether via prenatal vitamins or, if a higher dosage is necessary, by prescription. One group has reported that preconception counseling increases folate use in women planning pregnancy.6
Safer sex counseling. Counseling patients about safer sexual practices may reduce the incidence of human immunodeficiency virus (HIV), herpes, gonorrhea, chlamydia, and syphilis—conditions that may increase the incidence of preterm delivery, fetal malformations, neonatal infection, or developmental abnormalities.3
Identification of patients with HIV is essential, as early treatment with zidovudine (azidothymidine [AZT], Retrovir), reduces the risk of vertical transmission from mother to neonate by up to 70%.7
Infection prevention. Infections with the potential to harm the fetus include parvovirus B-19, cytomegalovirus, toxoplasmosis, and hepatitis B. Vaccinations are available for hepatitis B but not for the others, so it is particularly important to warn patients about avoiding exposure.
Women who work in child care, for example, should avoid direct contact with children who have parvovirus infection (“Fifth disease”) or other viral exanthems. Health care workers should use universal precautions at all times, and all pregnant women should avoid direct exposure to cat feces and consumption of uncooked meats.
Weight control. Obesity is an increasingly serious health problem in the United States. Obesity poses significant risks for pregnant women and their fetuses, including NTDs, diabetes, venous thromboembolism (VTE), premature labor or cesarean delivery, preeclampsia, and macrosomia.8,9 The time to start a program of exercise and weight loss is before conception, because some of the adverse effects of obesity can occur during the first few months of pregnancy.
Diet modification. Many patients considering conception ask about foods that may be unsafe during pregnancy. Like most clinicians, you may want to advise patients to avoid soft cheeses because of the risk of Listeria infection, and not to eat raw or undercooked meats because of the risk of toxoplasmosis.
Women considering conception should not eat fish high in methylmercury, which can affect the neurologic development of a fetus. These include swordfish, tilefish, king mackerel, and shark. Fish with lower levels of methylmercury are shrimp, canned light tuna, pollock, catfish, and salmon.
Women may eat 12 ounces (or 2 average meals) of these safer fish each week. Methylmercury exposure is cumulative; in women with initially high levels, it can take as long as a year after reducing consumption of fish high in methylmercury for levels to return to normal.10 An Environmental Protection Agency fact sheet for clinicians and patients is available at http://www.epa.gov/waterscience/fish/advice/factsheet.html.
Hot tubs and spas. Maternal hyperthermia (core temperature greater than 100.4°F) during the first trimester is associated with an increased risk of NTDs, so tell women who are pregnant or trying to conceive to stay out of hot tubs and heated spas.11
Environmental toxins. Pregnant women should avoid solvents, paint thinners, heavy metals, pesticides, ionizing radiation (unless indicated for necessary health care), alcohol, illicit drugs, and cigarette smoke.
Smoking cessation. Smoking even less than 1 pack a day can be very harmful to the developing fetus.12,13 Smoking increases the risks of miscarriage, stillbirth, and other pregnancy complications, and is also associated with increased neonatal mortality and sudden infant death syndrome. About 11% of pregnant women smoke.14
As a primary care physician, you should use every opportunity to help smokers considering pregnancy to quit. Proven methods of smoking cessation include counseling, medications such as bupropion, and over-the-counter smoking cessation aids, such as nicotine replacement gum and lozenges.15
One intervention shown to be particularly useful for women who smoke fewer than 20 cigarettes a day is the “5 As” method (Ask, Advise, Assess, Assist, and Arrange).16 A review of studies on the effects of smoking during pregnancy that includes cessation interventions such as the 5 As method is available in the American College of Obstetricians and Gynecologists Committee Opinion No. 316.15
Substance abuse. Fetal alcohol spectrum disorders (FASDs) are among the most preventable congenital defects and developmental disabilities. Ask patients trying to conceive about their patterns of alcohol use, and tell them there is no known safe amount of alcohol intake during pregnancy.
The US Preventive Services Task Force recommends screening pregnant patients with either the TWEAK or T-ACE instruments, because these tests can detect relatively low levels of alcohol consumption that may still harm a developing fetus.17 All women contemplating pregnancy need to know that exposure to alcohol can cause FASDs, congenital malformations, intrauterine growth restriction, and miscarriages. Problem drinkers should be referred for treatment.
Illegal drugs also pose significant risks to fetal development. Damage to the placenta caused by cocaine, for example, can lead to abruption, miscarriage, growth restriction, and prematurity. Consider screening all patients for illegal drug use and referring for counseling or methadone management, as indicated.
Caffeine. Recent studies have linked excessive caffeine intake (>200 mg/d) with miscarriages during the first trimester (adjusted hazards ratio=2.23). To reduce the risk of miscarriage, counsel pregnant women to eliminate caffeine or to cut back to less than 200 mg/d.18 Amounts of caffeine in various beverages are listed in TABLE 1 .
TABLE 1
How much caffeine is your patient drinking?30,31
| BEVERAGE | SERVING SIZE (OZ) | CAFFEINE CONTENT (MG) |
|---|---|---|
| Decaffeinated coffee | 8 | 2 |
| Caffeinated coffees | ||
| Starbucks Grande Coffee | 16 | 330 |
| Starbucks Caffe Latte | 16 | 150 |
| Plain, brewed coffee | 8 | 95 |
| Espresso | 1 | 64 |
| Teas | ||
| Decaffeinated tea | 8 | 2 |
| Black tea, brewed | 8 | 47 |
| Snapple iced tea | 16 | 18 |
| Caffeinated soft drinks | ||
| Diet Mountain Dew | 12 | 55 |
| Diet Coke | 12 | 46 |
| Diet Pepsi | 12 | 37 |
| Sam’s Diet Cola | 12 | 13 |
| Energy drinks | ||
| SoBe Adrenaline Rush | 16 | 152 |
| Red Bull | 8.3 | 76 |
Avert trouble: Manage chronic conditions now
A number of maternal health conditions have a potential for adverse consequences to the fetus, but optimizing the mother’s condition before and during pregnancy can often avert problems. Maternal disorders to monitor include:
Diabetes mellitus. Improving glycemic control prior to conception is linked to a 3-fold decrease in the prevalence of birth defects.3 Patients entering pregnancy with hemoglobin A1C levels less than 8.5% have a fetal anomaly rate of 3.4%, whereas women with a hemoglobin A1C of more than 8.5% have an anomaly rate of 22.4%.19
According to the American Association of Clinical Endocrinologists, goals for glucose control during pregnancy include a hemoglobin A1C of less than 6% and blood glucose concentrations of between 60 mg/dL fasting and 120 mg/dL 1 hour after a meal. Achieving these levels may require tighter control than patients are accustomed to. Blood pressure for these patients should not exceed 130/80 mm Hg.
The use of oral hypoglycemic medications during pregnancy is somewhat controversial. For that reason, you may want to refer these patients to an endocrinologist or other expert in diabetes management during pregnancy for consideration of scheduled insulin injections or an insulin pump.20
Patients with diabetes should be screened for retinal disease, renal disease, hypertension, and hyperlipidemia prior to conception, including a 24-hour urine collection for protein and creatinine clearance. Because women with type 1 diabetes have up to a 40% incidence of thyroid dysfunction, consider screening for thyroid disorders as well.21
While counseling patients with diabetes, keep in mind that a blood urea nitrogen concentration of more than 30 mg/dL, current coronary artery disease, and creatinine clearance of less than 30 mL/min are considered contraindications for pregnancy.20 All women who have had diabetes for more than 10 years should have an electrocardiogram (EKG).
Hypothyroidism. Poorly controlled hypothyroidism may cause developmental, growth, and neurologic abnormalities. Patients with thyroid abnormalities should have their medication dosage optimized before they conceive.
Epilepsy. Seizure disorders generally do not worsen during pregnancy, but several antiseizure medications have a potential for harming the fetus ( TABLE 2 ). Counsel patients about the increased risk of congenital anomalies (4%-8%) in neonates born to women with seizure disorders, either because of the disorder itself or as a consequence of antiseizure medication.22
Patients taking anti-epileptic drugs should take 4 mg/d of folate supplementation. According to the American Academy of Neurology, best practice is to use a single agent best suited to the type of seizures the patient experiences, at the lowest effective dose. Avoid multiple anti-epileptic drugs, if possible. Do not change an effective medication regimen if the patient becomes pregnant, but do check drug levels.23 Also counsel patients about the possibility of decreased effectiveness of hormonal contraception while taking enzymeinducing (cytochrome P450) antiepileptic drugs.24
Psychiatric disorders. Approximately 1 of every 7 pregnant women meets the diagnostic criteria for depression.25 Depression, anxiety, and other psychiatric conditions can adversely impact the patient and her developing fetus if the condition is undertreated. Unfortunately, some women stop psychiatric medications when they discover they are pregnant. In 1 study of 201 pregnant women with depression, 43% had a relapse during the course of pregnancy. Patients with relapse included 68% of those who stopped medication during the pregnancy, vs 26% of those who continued taking medication.26
Most antidepressants are relatively safe during pregnancy, with the exception of paroxetine (Paxil), which is associated with fetal cardiac defects.27 When a patient taking psychiatric medications comes in for a preconception visit, consider higher doses of 1 psychotropic medication rather than lower doses of multiple medications. This will decrease fetal medication exposure. If a patient on a stable regimen becomes pregnant, do not switch medications. Always collaborate with the patient’s mental health care team.
Venous thromboembolic disease. Some inherited thrombophilias can lead to a VTE during pregnancy or the postpartum period. Consider testing patients who have had a VTE or have a family history of VTE for anticardiolipin antibodies, protein S deficiency, and other thrombophilias before conception, because some of the lab values that indicate these conditions can change during pregnancy.
Patients with a history of a VTE related to hormone use or pregnancy will require prophylactic anticoagulation. When in doubt, refer to a hematologist or perinatologist. Both heparin and low-molecular-weight heparin are safe during pregnancy. Because warfarin is a suspected teratogen, patients taking warfarin should be converted to either heparin or low-molecular-weight heparin.
Hypertension. Hypertensive disorders may lead to pregnancy-induced hypertension, growth restriction, and renal disease. If patients are taking thiazide diuretics, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors ( TABLE 2 ), switch to medications such as methyldopa (Aldomet), nifedipine, or labetalol, which are safer during pregnancy.28 Screen patients with long-standing hypertension for cardiac disease (via EKG) and nephropathy before conception.
TABLE 2
Medications that can harm the unborn child32,33
| MEDICATION | POTENTIAL HARM TO FETUS | FDA CATEGORY* |
|---|---|---|
| Cardiovascular | ||
| ACE inhibitors: captopril, enalapril, lisinopril | Cardiovascular malformations, hypotension, anuria, oligohydramnios | C (1st trimester), D (2nd, 3rd trimesters) |
| Statins: atorvastatin, simvastatin | Polydactyly, cleft lip, club foot | X |
| Antidepressants | ||
| SSRIs: citalopram, fluoxetine, paroxetine, sertraline | Pulmonary hypertension, withdrawal syndrome; paroxetine: cardiac malformations, NTDs | C (D, paroxetine) |
| Anxiolytics | ||
| Benzodiazepines: alprazolam, clonazepam, diazepam, lorazepam | Withdrawal syndrome, congenital anomalies (various), floppy infant syndrome | D |
| Anti-epileptic drugs | ||
| Valproic acid and derivatives | NTDs, facial/cardiac defects | D |
| Carbamazepine | See valproic acid | D |
| Phenobarbital | Cardiac defects, hemorrhagic disease of the newborn | D |
| Phenytoin | Fetal hydantoin syndrome | D |
| Other | ||
| Isotretinoin | Hydrocephalus, microcephaly, limb anomalies, preterm labor, increased spontaneous abortions | X |
| Warfarin | Skeletal defects, intrauterine growth restriction, neurologic defects | X |
| Methotrexate | Fetal malformations, spontaneous abortion | X |
| ACE, angiotensin-converting enzyme; NTDs, neural tube defects; SSRIs, selective serotonin reuptake inhibitors. | ||
| *FDA categories describe the relative risk of medication use during pregnancy. The FDA is currently proposing major revisions to the system to upgrade the usefulness of this information. Please reconfirm categories prior to prescribing. | ||
| A: Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities in any trimester of pregnancy. | ||
| B: Animal studies have revealed no evidence of harm to the fetus, but no adequate and well-controlled studies in pregnant women are available OR animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester. | ||
| C: Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women OR no animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. | ||
| D: Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. | ||
| X: Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant. | ||
| FDA categories source: Meadows M. Pregnancy and the drug dilemma. FDA Consumer Magazine. May-June 2001. Available at www.fda.gov/fdac/features/2001/301_preg.html. Accessed May 6, 2009. | ||
Think ahead: Address immunization status
When a pregnant woman contracts an infectious disease, her developing fetus can be affected. Making sure the immunization status of all your reproductive-age patients is up to date will go a long way toward protecting their offspring from harm.29
Rubella. Also known as German measles, rubella can cause fetal anomalies and spontaneous abortion if contracted during the first half of pregnancy. Because the measles, mumps, and rubella (MMR) vaccine contains live attenuated viruses, susceptible patients should be immunized at least 1 month before they conceive.3
Hepatitis B. Preventing hepatitis B is an important public health issue. Screen patients at risk for hepatitis B infection—health care workers, sex workers, intravenous drug abusers, and nonmonogamous women who do not use barrier protection—with a hepatitis B surface antigen level. Vaccination is safe up to 1 month before conception.3
Varicella. Maternal varicella (chicken pox) can cause fetal harm, particularly if symptoms appear just before or during delivery. Women of reproductive age who have not already had the disease or been vaccinated should be immunized. This is a live virus vaccine and must, therefore, be administered at least 1 month before conception.3
Influenza. Administering influenza vaccine is not contraindicated during pregnancy, although most experts advise waiting until the second trimester. Certainly it is appropriate to administer the vaccine during influenza season or when risk factors such as chronic lung disease are present. Avoid live attenuated vaccine (FluMist) in pregnant women.
Tdap vaccination. The CDC’s Advisory Committee on Immunization Practices recommended in 2008 that susceptible pregnant women receive Tdap during the postpartum period to protect vulnerable infants against pertussis. Tdap is thought to be safe during pregnancy, but it would make sense to administer this vaccine when indicated prior to conception as part of a vaccination screening program.
Screen for genetic conditions prepregnancy
Part of a comprehensive preconception visit includes screening for communicable diseases and genetic conditions.
Communicable diseases. Consider screening all women prior to pregnancy for HIV infection, gonorrhea, chlamydia, hepatitis B, hepatitis C (for health care workers), and syphilis.
Diabetes. Screening guidelines for diabetes are available from the American Association of Clinical Endocrinologists.20 Consider preconception screening for patients who, during a previous pregnancy, had gestational diabetes or who delivered a baby weighing more than 9 pounds.
Genetic screening. Patients from certain ethnic groups are more susceptible to specific genetic mutations. Genetic disorders associated with particular ethnic origins are listed in TABLE 3 . Consider a preconception referral to a genetic counselor or perinatologist when the patient’s family history suggests inherited disorders.
TABLE 3
Genetic disorders: Who to screen, tests to use34
| ETHNIC ORIGIN* | DISORDER | RECOMMENDED TEST |
|---|---|---|
| Ashkenazi Jews | Tay-Sachs disease; Canavan disease | DNA panel, hexosaminidase A |
| African American | Sickle cell trait; beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| French Canadian, Cajun | Tay-Sachs disease | Hexosaminidase A |
| Mediterranean | Alpha-,† beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Indian, Middle Eastern | Sickle cell trait; alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Caucasian | Cystic fibrosis | DNA panel |
| Southeast Asian (Thai, Laotian, Cambodian) | Alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| MCV, mean corpuscular volume. | ||
| *Offer screening to any interested patient. | ||
| †Do not pursue alpha-thalassemia work-up unless patient has a history of pregnancy loss or fetal hydrops. | ||
Correspondence
D. Ashley Hill, MD, Associate Director, Department of Obstetrics and Gynecology, Loch Haven OB/Gyn Group, Florida Hospital Orlando, 235 Princeton Street, Suite 200, Orlando, FL 32804; [email protected]
1. Mayer JP. Unintended childbearing, maternal beliefs, and delay of prenatal care. Birth. 1997;24:247-252.
2. Proceedings of the Preconception Health and Health Care Clinical, Public Health, and Consumer Workgroup Meetings. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities; 2006. Available at: http://www.cdc.gov/ncbddd/preconception/documents/Workgroup%20Proceedings%20June06.pdf. Accessed May 14, 2009.
3. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-06):1-23.Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed May 14, 2009.
4. Lumley J, Watson L, Watson M, et al. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.-
5. Iqbal MM. Prevention of neural tube defects by periconceptional use of folic acid. Pediatr Rev. 2000;21:58-66.
6. de Weerd S, Thomas CM, Cikot RJ, et al. Preconception counseling improves folate status of women planning pregnancy. Obstet Gynecol. 2002;99:45-50.
7. Mofenson LM. Centers for Disease Control and Prevention, US Public Health Service Task Force. US Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 2002;51(RR-18):1-38.
8. Robinson HE, O’Connell CM, Joseph KS, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357-1364.
9. Kiel DW, Dodson EA, Artal R, et al. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstet Gynecol. 2007;110:752-758.
10. US Food and Drug Administration. Food safety for moms-to-be. August 24, 2005. Available at: http://www.cfsan.fda.gov/~pregnant/pregnant.html. Accessed September 18, 2008.
11. Cheschier N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. (Replaces committee opinion number 252, March 2001). Int J Gynaecol Obstet. 2003;83:123-133.
12. Castles A, Adams EK, Melvin CL, et al. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16:208-215.
13. US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. May 27, 2004. Available at: http://www.surgeongeneral.gov/library/smokingconsequences. Accessed July 6, 2008.
14. National Center for Health Statistics. Health, United States, 2004: With Chartbook on Trends in the Health of Americans. Hyattsville, MD: NCHS; 2004. Available at: http://www.cdc.gov/nchs/data/hus/hus04.pdf. Accessed September 18, 2008.
15. ACOG Committee on Health Care for Underdeserved Women; ACOG Committee on Obstetric Practice. ACOG committee opinion. Number 316, October 2005. Smoking cessation during pregnancy. Obstet Gynecol. 2005;106:883-888.
16. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.ahrq.gov/path/tobacco.htm. Accessed May 14, 2009.
17. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
18. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:279.e1-279.e8.
19. Lucas MJ, Leveno KJ, Williams ML, et al. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am J Obstet Gynecol. 1989;161:426-431.
20. American Association of Clinical Endocrinologists Diabetes Mellitus Clinical Practice Guidelines Task Force. AACE diabetes mellitus guidelines. Chapter 9: diabetes and pregnancy. Endocr Pract. 2007;13(suppl 1):55-59.
21. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675-685.
22. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Int J Gynaecol Obstet. 1997;56:279-286.
23. American Academy of Neurology. Guideline summary for clinicians—management issues for women with epilepsy. Available at: www.aan.com/professionals/practice/pdfs/women_epilepsy.pdf. Accessed May 14, 2009.
24. Carl JS, Weaver SP, Tweed E, et al. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
25. Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799-801.
26. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment [published correction appears in JAMA. 2006;296:170]. JAMA. 2006;295:499-507.
27. FDA public health advisory: paroxetine. December 8, 2005. Available at: http://www.fda.gov/CDER/Drug/advisory/paroxetine200512.htm. Accessed May 6, 2009.
28. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Chronic hypertension in pregnancy. Obstet Gynecol. 2001;98(suppl):177-185.
29. Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2009. MMWR. 2009;57(53):Q-1-Q-4.Available at: http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed May 14, 2009.
30. MayoClinic.Com. How much caffeine is in your daily habit? Available at: http://www.mayoclinic.com/health/caffeine/AN01211. Accessed September 18, 2008.
31. Chou KH, Bell LH. Caffeine content of prepackaged national-brand and private-label carbonated beverages. J Food Sci. 2007;72:C337-C342.
32. Physician’s Desk Reference. Montvale, NJ: Thomson Reuters; 2009.
33. Drugs.com. Available at: http://www.drugs.com. Accessed April 26, 2009.
34. National Society of Genetic Counselors, Prenatal Special Interest Group. Ancestry Based Carrier Screening. Chicago, IL: NSGC; 2005. Available at: http://www.nsgc.org. Accessed September 18, 2008.
- Supplementing women’s diets with 400 mg folic acid every day reduces the incidence of neural tube defects in their offspring by up to 72% (A).
- Optimizing diabetic glucose control prior to conception is linked to a reduction in birth defects and pregnancy loss (B).
- Limiting caffeine consumption to no more than 200 mg per day may reduce the risk of miscarriage (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
In the United States most women do not seek out prenatal care until the 8th to 12th week of pregnancy, when the crucial period of organogenesis (4 to 10 weeks after fertilization) has already passed. In addition, women whose pregnancies are unintended—up to half of all pregnancies—may delay seeking care even longer.1 Given these realities, family physicians should consider all visits during the reproductive years—especially yearly exams—as opportunities for preconception counseling.
Preconception health care is essential to the health of our nation. Data from the Centers for Disease Control and Prevention (CDC) are cause for concern:2,3
- 12% of infants are born prematurely.
- 31% of pregnancies are complicated by maternal health issues.
- 11% of women smoke during pregnancy.
- 10% drink alcohol during pregnancy.
- 69% of women do not take folate supplements.
- 31% of women are obese.
- 3% of women take medications and supplements that are known teratogens.
The Healthy People 2000 initiative set a goal of 60% of primary caregivers providing preconception care at routine medical visits, but thus far, only about 25% do so.2,3
As a primary care provider, you can have a huge impact on fetal and maternal health by counseling women to choose healthier lifestyles, helping them to manage their chronic conditions, updating their immunizations, and screening for genetic disorders before they become pregnant.
Start with the hard part: Lifestyle modification
Changing the way patients go about their lives—how they eat, how much they exercise, whether they use alcohol or tobacco—has 2 salient characteristics: It’s the most difficult thing to get patients to do, and it has the biggest payoff in improving maternal and fetal health. Lifestyle issues of greatest significance for the preconception patient include:
Folic acid supplementation. If your patients are like most women, they may not be aware of the importance of folic acid supplementation. Yet by neglecting to supplement their diet with folates, women are passing up an opportunity to reduce the incidence of neural tube defects (NTDs) such as anencephaly and spina bifida by up to 70%.4
4D ultrasound of an open neural tube defect in a developing fetus
Women considering conception or those who do not use contraception should take 400 mg folic acid, a dosage found in most prenatal vitamins, every day. All women of reproductive age should consider folate supplementation because of the high rate of unplanned pregnancies. Women who have previously had a child with an NTD and women who take anti-epileptic drugs should take 4 mg folate per day.5
Use routine office visits as an opportunity to counsel patients about folic acid supplementation, whether via prenatal vitamins or, if a higher dosage is necessary, by prescription. One group has reported that preconception counseling increases folate use in women planning pregnancy.6
Safer sex counseling. Counseling patients about safer sexual practices may reduce the incidence of human immunodeficiency virus (HIV), herpes, gonorrhea, chlamydia, and syphilis—conditions that may increase the incidence of preterm delivery, fetal malformations, neonatal infection, or developmental abnormalities.3
Identification of patients with HIV is essential, as early treatment with zidovudine (azidothymidine [AZT], Retrovir), reduces the risk of vertical transmission from mother to neonate by up to 70%.7
Infection prevention. Infections with the potential to harm the fetus include parvovirus B-19, cytomegalovirus, toxoplasmosis, and hepatitis B. Vaccinations are available for hepatitis B but not for the others, so it is particularly important to warn patients about avoiding exposure.
Women who work in child care, for example, should avoid direct contact with children who have parvovirus infection (“Fifth disease”) or other viral exanthems. Health care workers should use universal precautions at all times, and all pregnant women should avoid direct exposure to cat feces and consumption of uncooked meats.
Weight control. Obesity is an increasingly serious health problem in the United States. Obesity poses significant risks for pregnant women and their fetuses, including NTDs, diabetes, venous thromboembolism (VTE), premature labor or cesarean delivery, preeclampsia, and macrosomia.8,9 The time to start a program of exercise and weight loss is before conception, because some of the adverse effects of obesity can occur during the first few months of pregnancy.
Diet modification. Many patients considering conception ask about foods that may be unsafe during pregnancy. Like most clinicians, you may want to advise patients to avoid soft cheeses because of the risk of Listeria infection, and not to eat raw or undercooked meats because of the risk of toxoplasmosis.
Women considering conception should not eat fish high in methylmercury, which can affect the neurologic development of a fetus. These include swordfish, tilefish, king mackerel, and shark. Fish with lower levels of methylmercury are shrimp, canned light tuna, pollock, catfish, and salmon.
Women may eat 12 ounces (or 2 average meals) of these safer fish each week. Methylmercury exposure is cumulative; in women with initially high levels, it can take as long as a year after reducing consumption of fish high in methylmercury for levels to return to normal.10 An Environmental Protection Agency fact sheet for clinicians and patients is available at http://www.epa.gov/waterscience/fish/advice/factsheet.html.
Hot tubs and spas. Maternal hyperthermia (core temperature greater than 100.4°F) during the first trimester is associated with an increased risk of NTDs, so tell women who are pregnant or trying to conceive to stay out of hot tubs and heated spas.11
Environmental toxins. Pregnant women should avoid solvents, paint thinners, heavy metals, pesticides, ionizing radiation (unless indicated for necessary health care), alcohol, illicit drugs, and cigarette smoke.
Smoking cessation. Smoking even less than 1 pack a day can be very harmful to the developing fetus.12,13 Smoking increases the risks of miscarriage, stillbirth, and other pregnancy complications, and is also associated with increased neonatal mortality and sudden infant death syndrome. About 11% of pregnant women smoke.14
As a primary care physician, you should use every opportunity to help smokers considering pregnancy to quit. Proven methods of smoking cessation include counseling, medications such as bupropion, and over-the-counter smoking cessation aids, such as nicotine replacement gum and lozenges.15
One intervention shown to be particularly useful for women who smoke fewer than 20 cigarettes a day is the “5 As” method (Ask, Advise, Assess, Assist, and Arrange).16 A review of studies on the effects of smoking during pregnancy that includes cessation interventions such as the 5 As method is available in the American College of Obstetricians and Gynecologists Committee Opinion No. 316.15
Substance abuse. Fetal alcohol spectrum disorders (FASDs) are among the most preventable congenital defects and developmental disabilities. Ask patients trying to conceive about their patterns of alcohol use, and tell them there is no known safe amount of alcohol intake during pregnancy.
The US Preventive Services Task Force recommends screening pregnant patients with either the TWEAK or T-ACE instruments, because these tests can detect relatively low levels of alcohol consumption that may still harm a developing fetus.17 All women contemplating pregnancy need to know that exposure to alcohol can cause FASDs, congenital malformations, intrauterine growth restriction, and miscarriages. Problem drinkers should be referred for treatment.
Illegal drugs also pose significant risks to fetal development. Damage to the placenta caused by cocaine, for example, can lead to abruption, miscarriage, growth restriction, and prematurity. Consider screening all patients for illegal drug use and referring for counseling or methadone management, as indicated.
Caffeine. Recent studies have linked excessive caffeine intake (>200 mg/d) with miscarriages during the first trimester (adjusted hazards ratio=2.23). To reduce the risk of miscarriage, counsel pregnant women to eliminate caffeine or to cut back to less than 200 mg/d.18 Amounts of caffeine in various beverages are listed in TABLE 1 .
TABLE 1
How much caffeine is your patient drinking?30,31
| BEVERAGE | SERVING SIZE (OZ) | CAFFEINE CONTENT (MG) |
|---|---|---|
| Decaffeinated coffee | 8 | 2 |
| Caffeinated coffees | ||
| Starbucks Grande Coffee | 16 | 330 |
| Starbucks Caffe Latte | 16 | 150 |
| Plain, brewed coffee | 8 | 95 |
| Espresso | 1 | 64 |
| Teas | ||
| Decaffeinated tea | 8 | 2 |
| Black tea, brewed | 8 | 47 |
| Snapple iced tea | 16 | 18 |
| Caffeinated soft drinks | ||
| Diet Mountain Dew | 12 | 55 |
| Diet Coke | 12 | 46 |
| Diet Pepsi | 12 | 37 |
| Sam’s Diet Cola | 12 | 13 |
| Energy drinks | ||
| SoBe Adrenaline Rush | 16 | 152 |
| Red Bull | 8.3 | 76 |
Avert trouble: Manage chronic conditions now
A number of maternal health conditions have a potential for adverse consequences to the fetus, but optimizing the mother’s condition before and during pregnancy can often avert problems. Maternal disorders to monitor include:
Diabetes mellitus. Improving glycemic control prior to conception is linked to a 3-fold decrease in the prevalence of birth defects.3 Patients entering pregnancy with hemoglobin A1C levels less than 8.5% have a fetal anomaly rate of 3.4%, whereas women with a hemoglobin A1C of more than 8.5% have an anomaly rate of 22.4%.19
According to the American Association of Clinical Endocrinologists, goals for glucose control during pregnancy include a hemoglobin A1C of less than 6% and blood glucose concentrations of between 60 mg/dL fasting and 120 mg/dL 1 hour after a meal. Achieving these levels may require tighter control than patients are accustomed to. Blood pressure for these patients should not exceed 130/80 mm Hg.
The use of oral hypoglycemic medications during pregnancy is somewhat controversial. For that reason, you may want to refer these patients to an endocrinologist or other expert in diabetes management during pregnancy for consideration of scheduled insulin injections or an insulin pump.20
Patients with diabetes should be screened for retinal disease, renal disease, hypertension, and hyperlipidemia prior to conception, including a 24-hour urine collection for protein and creatinine clearance. Because women with type 1 diabetes have up to a 40% incidence of thyroid dysfunction, consider screening for thyroid disorders as well.21
While counseling patients with diabetes, keep in mind that a blood urea nitrogen concentration of more than 30 mg/dL, current coronary artery disease, and creatinine clearance of less than 30 mL/min are considered contraindications for pregnancy.20 All women who have had diabetes for more than 10 years should have an electrocardiogram (EKG).
Hypothyroidism. Poorly controlled hypothyroidism may cause developmental, growth, and neurologic abnormalities. Patients with thyroid abnormalities should have their medication dosage optimized before they conceive.
Epilepsy. Seizure disorders generally do not worsen during pregnancy, but several antiseizure medications have a potential for harming the fetus ( TABLE 2 ). Counsel patients about the increased risk of congenital anomalies (4%-8%) in neonates born to women with seizure disorders, either because of the disorder itself or as a consequence of antiseizure medication.22
Patients taking anti-epileptic drugs should take 4 mg/d of folate supplementation. According to the American Academy of Neurology, best practice is to use a single agent best suited to the type of seizures the patient experiences, at the lowest effective dose. Avoid multiple anti-epileptic drugs, if possible. Do not change an effective medication regimen if the patient becomes pregnant, but do check drug levels.23 Also counsel patients about the possibility of decreased effectiveness of hormonal contraception while taking enzymeinducing (cytochrome P450) antiepileptic drugs.24
Psychiatric disorders. Approximately 1 of every 7 pregnant women meets the diagnostic criteria for depression.25 Depression, anxiety, and other psychiatric conditions can adversely impact the patient and her developing fetus if the condition is undertreated. Unfortunately, some women stop psychiatric medications when they discover they are pregnant. In 1 study of 201 pregnant women with depression, 43% had a relapse during the course of pregnancy. Patients with relapse included 68% of those who stopped medication during the pregnancy, vs 26% of those who continued taking medication.26
Most antidepressants are relatively safe during pregnancy, with the exception of paroxetine (Paxil), which is associated with fetal cardiac defects.27 When a patient taking psychiatric medications comes in for a preconception visit, consider higher doses of 1 psychotropic medication rather than lower doses of multiple medications. This will decrease fetal medication exposure. If a patient on a stable regimen becomes pregnant, do not switch medications. Always collaborate with the patient’s mental health care team.
Venous thromboembolic disease. Some inherited thrombophilias can lead to a VTE during pregnancy or the postpartum period. Consider testing patients who have had a VTE or have a family history of VTE for anticardiolipin antibodies, protein S deficiency, and other thrombophilias before conception, because some of the lab values that indicate these conditions can change during pregnancy.
Patients with a history of a VTE related to hormone use or pregnancy will require prophylactic anticoagulation. When in doubt, refer to a hematologist or perinatologist. Both heparin and low-molecular-weight heparin are safe during pregnancy. Because warfarin is a suspected teratogen, patients taking warfarin should be converted to either heparin or low-molecular-weight heparin.
Hypertension. Hypertensive disorders may lead to pregnancy-induced hypertension, growth restriction, and renal disease. If patients are taking thiazide diuretics, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors ( TABLE 2 ), switch to medications such as methyldopa (Aldomet), nifedipine, or labetalol, which are safer during pregnancy.28 Screen patients with long-standing hypertension for cardiac disease (via EKG) and nephropathy before conception.
TABLE 2
Medications that can harm the unborn child32,33
| MEDICATION | POTENTIAL HARM TO FETUS | FDA CATEGORY* |
|---|---|---|
| Cardiovascular | ||
| ACE inhibitors: captopril, enalapril, lisinopril | Cardiovascular malformations, hypotension, anuria, oligohydramnios | C (1st trimester), D (2nd, 3rd trimesters) |
| Statins: atorvastatin, simvastatin | Polydactyly, cleft lip, club foot | X |
| Antidepressants | ||
| SSRIs: citalopram, fluoxetine, paroxetine, sertraline | Pulmonary hypertension, withdrawal syndrome; paroxetine: cardiac malformations, NTDs | C (D, paroxetine) |
| Anxiolytics | ||
| Benzodiazepines: alprazolam, clonazepam, diazepam, lorazepam | Withdrawal syndrome, congenital anomalies (various), floppy infant syndrome | D |
| Anti-epileptic drugs | ||
| Valproic acid and derivatives | NTDs, facial/cardiac defects | D |
| Carbamazepine | See valproic acid | D |
| Phenobarbital | Cardiac defects, hemorrhagic disease of the newborn | D |
| Phenytoin | Fetal hydantoin syndrome | D |
| Other | ||
| Isotretinoin | Hydrocephalus, microcephaly, limb anomalies, preterm labor, increased spontaneous abortions | X |
| Warfarin | Skeletal defects, intrauterine growth restriction, neurologic defects | X |
| Methotrexate | Fetal malformations, spontaneous abortion | X |
| ACE, angiotensin-converting enzyme; NTDs, neural tube defects; SSRIs, selective serotonin reuptake inhibitors. | ||
| *FDA categories describe the relative risk of medication use during pregnancy. The FDA is currently proposing major revisions to the system to upgrade the usefulness of this information. Please reconfirm categories prior to prescribing. | ||
| A: Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities in any trimester of pregnancy. | ||
| B: Animal studies have revealed no evidence of harm to the fetus, but no adequate and well-controlled studies in pregnant women are available OR animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester. | ||
| C: Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women OR no animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. | ||
| D: Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. | ||
| X: Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant. | ||
| FDA categories source: Meadows M. Pregnancy and the drug dilemma. FDA Consumer Magazine. May-June 2001. Available at www.fda.gov/fdac/features/2001/301_preg.html. Accessed May 6, 2009. | ||
Think ahead: Address immunization status
When a pregnant woman contracts an infectious disease, her developing fetus can be affected. Making sure the immunization status of all your reproductive-age patients is up to date will go a long way toward protecting their offspring from harm.29
Rubella. Also known as German measles, rubella can cause fetal anomalies and spontaneous abortion if contracted during the first half of pregnancy. Because the measles, mumps, and rubella (MMR) vaccine contains live attenuated viruses, susceptible patients should be immunized at least 1 month before they conceive.3
Hepatitis B. Preventing hepatitis B is an important public health issue. Screen patients at risk for hepatitis B infection—health care workers, sex workers, intravenous drug abusers, and nonmonogamous women who do not use barrier protection—with a hepatitis B surface antigen level. Vaccination is safe up to 1 month before conception.3
Varicella. Maternal varicella (chicken pox) can cause fetal harm, particularly if symptoms appear just before or during delivery. Women of reproductive age who have not already had the disease or been vaccinated should be immunized. This is a live virus vaccine and must, therefore, be administered at least 1 month before conception.3
Influenza. Administering influenza vaccine is not contraindicated during pregnancy, although most experts advise waiting until the second trimester. Certainly it is appropriate to administer the vaccine during influenza season or when risk factors such as chronic lung disease are present. Avoid live attenuated vaccine (FluMist) in pregnant women.
Tdap vaccination. The CDC’s Advisory Committee on Immunization Practices recommended in 2008 that susceptible pregnant women receive Tdap during the postpartum period to protect vulnerable infants against pertussis. Tdap is thought to be safe during pregnancy, but it would make sense to administer this vaccine when indicated prior to conception as part of a vaccination screening program.
Screen for genetic conditions prepregnancy
Part of a comprehensive preconception visit includes screening for communicable diseases and genetic conditions.
Communicable diseases. Consider screening all women prior to pregnancy for HIV infection, gonorrhea, chlamydia, hepatitis B, hepatitis C (for health care workers), and syphilis.
Diabetes. Screening guidelines for diabetes are available from the American Association of Clinical Endocrinologists.20 Consider preconception screening for patients who, during a previous pregnancy, had gestational diabetes or who delivered a baby weighing more than 9 pounds.
Genetic screening. Patients from certain ethnic groups are more susceptible to specific genetic mutations. Genetic disorders associated with particular ethnic origins are listed in TABLE 3 . Consider a preconception referral to a genetic counselor or perinatologist when the patient’s family history suggests inherited disorders.
TABLE 3
Genetic disorders: Who to screen, tests to use34
| ETHNIC ORIGIN* | DISORDER | RECOMMENDED TEST |
|---|---|---|
| Ashkenazi Jews | Tay-Sachs disease; Canavan disease | DNA panel, hexosaminidase A |
| African American | Sickle cell trait; beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| French Canadian, Cajun | Tay-Sachs disease | Hexosaminidase A |
| Mediterranean | Alpha-,† beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Indian, Middle Eastern | Sickle cell trait; alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Caucasian | Cystic fibrosis | DNA panel |
| Southeast Asian (Thai, Laotian, Cambodian) | Alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| MCV, mean corpuscular volume. | ||
| *Offer screening to any interested patient. | ||
| †Do not pursue alpha-thalassemia work-up unless patient has a history of pregnancy loss or fetal hydrops. | ||
Correspondence
D. Ashley Hill, MD, Associate Director, Department of Obstetrics and Gynecology, Loch Haven OB/Gyn Group, Florida Hospital Orlando, 235 Princeton Street, Suite 200, Orlando, FL 32804; [email protected]
- Supplementing women’s diets with 400 mg folic acid every day reduces the incidence of neural tube defects in their offspring by up to 72% (A).
- Optimizing diabetic glucose control prior to conception is linked to a reduction in birth defects and pregnancy loss (B).
- Limiting caffeine consumption to no more than 200 mg per day may reduce the risk of miscarriage (B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
In the United States most women do not seek out prenatal care until the 8th to 12th week of pregnancy, when the crucial period of organogenesis (4 to 10 weeks after fertilization) has already passed. In addition, women whose pregnancies are unintended—up to half of all pregnancies—may delay seeking care even longer.1 Given these realities, family physicians should consider all visits during the reproductive years—especially yearly exams—as opportunities for preconception counseling.
Preconception health care is essential to the health of our nation. Data from the Centers for Disease Control and Prevention (CDC) are cause for concern:2,3
- 12% of infants are born prematurely.
- 31% of pregnancies are complicated by maternal health issues.
- 11% of women smoke during pregnancy.
- 10% drink alcohol during pregnancy.
- 69% of women do not take folate supplements.
- 31% of women are obese.
- 3% of women take medications and supplements that are known teratogens.
The Healthy People 2000 initiative set a goal of 60% of primary caregivers providing preconception care at routine medical visits, but thus far, only about 25% do so.2,3
As a primary care provider, you can have a huge impact on fetal and maternal health by counseling women to choose healthier lifestyles, helping them to manage their chronic conditions, updating their immunizations, and screening for genetic disorders before they become pregnant.
Start with the hard part: Lifestyle modification
Changing the way patients go about their lives—how they eat, how much they exercise, whether they use alcohol or tobacco—has 2 salient characteristics: It’s the most difficult thing to get patients to do, and it has the biggest payoff in improving maternal and fetal health. Lifestyle issues of greatest significance for the preconception patient include:
Folic acid supplementation. If your patients are like most women, they may not be aware of the importance of folic acid supplementation. Yet by neglecting to supplement their diet with folates, women are passing up an opportunity to reduce the incidence of neural tube defects (NTDs) such as anencephaly and spina bifida by up to 70%.4
4D ultrasound of an open neural tube defect in a developing fetus
Women considering conception or those who do not use contraception should take 400 mg folic acid, a dosage found in most prenatal vitamins, every day. All women of reproductive age should consider folate supplementation because of the high rate of unplanned pregnancies. Women who have previously had a child with an NTD and women who take anti-epileptic drugs should take 4 mg folate per day.5
Use routine office visits as an opportunity to counsel patients about folic acid supplementation, whether via prenatal vitamins or, if a higher dosage is necessary, by prescription. One group has reported that preconception counseling increases folate use in women planning pregnancy.6
Safer sex counseling. Counseling patients about safer sexual practices may reduce the incidence of human immunodeficiency virus (HIV), herpes, gonorrhea, chlamydia, and syphilis—conditions that may increase the incidence of preterm delivery, fetal malformations, neonatal infection, or developmental abnormalities.3
Identification of patients with HIV is essential, as early treatment with zidovudine (azidothymidine [AZT], Retrovir), reduces the risk of vertical transmission from mother to neonate by up to 70%.7
Infection prevention. Infections with the potential to harm the fetus include parvovirus B-19, cytomegalovirus, toxoplasmosis, and hepatitis B. Vaccinations are available for hepatitis B but not for the others, so it is particularly important to warn patients about avoiding exposure.
Women who work in child care, for example, should avoid direct contact with children who have parvovirus infection (“Fifth disease”) or other viral exanthems. Health care workers should use universal precautions at all times, and all pregnant women should avoid direct exposure to cat feces and consumption of uncooked meats.
Weight control. Obesity is an increasingly serious health problem in the United States. Obesity poses significant risks for pregnant women and their fetuses, including NTDs, diabetes, venous thromboembolism (VTE), premature labor or cesarean delivery, preeclampsia, and macrosomia.8,9 The time to start a program of exercise and weight loss is before conception, because some of the adverse effects of obesity can occur during the first few months of pregnancy.
Diet modification. Many patients considering conception ask about foods that may be unsafe during pregnancy. Like most clinicians, you may want to advise patients to avoid soft cheeses because of the risk of Listeria infection, and not to eat raw or undercooked meats because of the risk of toxoplasmosis.
Women considering conception should not eat fish high in methylmercury, which can affect the neurologic development of a fetus. These include swordfish, tilefish, king mackerel, and shark. Fish with lower levels of methylmercury are shrimp, canned light tuna, pollock, catfish, and salmon.
Women may eat 12 ounces (or 2 average meals) of these safer fish each week. Methylmercury exposure is cumulative; in women with initially high levels, it can take as long as a year after reducing consumption of fish high in methylmercury for levels to return to normal.10 An Environmental Protection Agency fact sheet for clinicians and patients is available at http://www.epa.gov/waterscience/fish/advice/factsheet.html.
Hot tubs and spas. Maternal hyperthermia (core temperature greater than 100.4°F) during the first trimester is associated with an increased risk of NTDs, so tell women who are pregnant or trying to conceive to stay out of hot tubs and heated spas.11
Environmental toxins. Pregnant women should avoid solvents, paint thinners, heavy metals, pesticides, ionizing radiation (unless indicated for necessary health care), alcohol, illicit drugs, and cigarette smoke.
Smoking cessation. Smoking even less than 1 pack a day can be very harmful to the developing fetus.12,13 Smoking increases the risks of miscarriage, stillbirth, and other pregnancy complications, and is also associated with increased neonatal mortality and sudden infant death syndrome. About 11% of pregnant women smoke.14
As a primary care physician, you should use every opportunity to help smokers considering pregnancy to quit. Proven methods of smoking cessation include counseling, medications such as bupropion, and over-the-counter smoking cessation aids, such as nicotine replacement gum and lozenges.15
One intervention shown to be particularly useful for women who smoke fewer than 20 cigarettes a day is the “5 As” method (Ask, Advise, Assess, Assist, and Arrange).16 A review of studies on the effects of smoking during pregnancy that includes cessation interventions such as the 5 As method is available in the American College of Obstetricians and Gynecologists Committee Opinion No. 316.15
Substance abuse. Fetal alcohol spectrum disorders (FASDs) are among the most preventable congenital defects and developmental disabilities. Ask patients trying to conceive about their patterns of alcohol use, and tell them there is no known safe amount of alcohol intake during pregnancy.
The US Preventive Services Task Force recommends screening pregnant patients with either the TWEAK or T-ACE instruments, because these tests can detect relatively low levels of alcohol consumption that may still harm a developing fetus.17 All women contemplating pregnancy need to know that exposure to alcohol can cause FASDs, congenital malformations, intrauterine growth restriction, and miscarriages. Problem drinkers should be referred for treatment.
Illegal drugs also pose significant risks to fetal development. Damage to the placenta caused by cocaine, for example, can lead to abruption, miscarriage, growth restriction, and prematurity. Consider screening all patients for illegal drug use and referring for counseling or methadone management, as indicated.
Caffeine. Recent studies have linked excessive caffeine intake (>200 mg/d) with miscarriages during the first trimester (adjusted hazards ratio=2.23). To reduce the risk of miscarriage, counsel pregnant women to eliminate caffeine or to cut back to less than 200 mg/d.18 Amounts of caffeine in various beverages are listed in TABLE 1 .
TABLE 1
How much caffeine is your patient drinking?30,31
| BEVERAGE | SERVING SIZE (OZ) | CAFFEINE CONTENT (MG) |
|---|---|---|
| Decaffeinated coffee | 8 | 2 |
| Caffeinated coffees | ||
| Starbucks Grande Coffee | 16 | 330 |
| Starbucks Caffe Latte | 16 | 150 |
| Plain, brewed coffee | 8 | 95 |
| Espresso | 1 | 64 |
| Teas | ||
| Decaffeinated tea | 8 | 2 |
| Black tea, brewed | 8 | 47 |
| Snapple iced tea | 16 | 18 |
| Caffeinated soft drinks | ||
| Diet Mountain Dew | 12 | 55 |
| Diet Coke | 12 | 46 |
| Diet Pepsi | 12 | 37 |
| Sam’s Diet Cola | 12 | 13 |
| Energy drinks | ||
| SoBe Adrenaline Rush | 16 | 152 |
| Red Bull | 8.3 | 76 |
Avert trouble: Manage chronic conditions now
A number of maternal health conditions have a potential for adverse consequences to the fetus, but optimizing the mother’s condition before and during pregnancy can often avert problems. Maternal disorders to monitor include:
Diabetes mellitus. Improving glycemic control prior to conception is linked to a 3-fold decrease in the prevalence of birth defects.3 Patients entering pregnancy with hemoglobin A1C levels less than 8.5% have a fetal anomaly rate of 3.4%, whereas women with a hemoglobin A1C of more than 8.5% have an anomaly rate of 22.4%.19
According to the American Association of Clinical Endocrinologists, goals for glucose control during pregnancy include a hemoglobin A1C of less than 6% and blood glucose concentrations of between 60 mg/dL fasting and 120 mg/dL 1 hour after a meal. Achieving these levels may require tighter control than patients are accustomed to. Blood pressure for these patients should not exceed 130/80 mm Hg.
The use of oral hypoglycemic medications during pregnancy is somewhat controversial. For that reason, you may want to refer these patients to an endocrinologist or other expert in diabetes management during pregnancy for consideration of scheduled insulin injections or an insulin pump.20
Patients with diabetes should be screened for retinal disease, renal disease, hypertension, and hyperlipidemia prior to conception, including a 24-hour urine collection for protein and creatinine clearance. Because women with type 1 diabetes have up to a 40% incidence of thyroid dysfunction, consider screening for thyroid disorders as well.21
While counseling patients with diabetes, keep in mind that a blood urea nitrogen concentration of more than 30 mg/dL, current coronary artery disease, and creatinine clearance of less than 30 mL/min are considered contraindications for pregnancy.20 All women who have had diabetes for more than 10 years should have an electrocardiogram (EKG).
Hypothyroidism. Poorly controlled hypothyroidism may cause developmental, growth, and neurologic abnormalities. Patients with thyroid abnormalities should have their medication dosage optimized before they conceive.
Epilepsy. Seizure disorders generally do not worsen during pregnancy, but several antiseizure medications have a potential for harming the fetus ( TABLE 2 ). Counsel patients about the increased risk of congenital anomalies (4%-8%) in neonates born to women with seizure disorders, either because of the disorder itself or as a consequence of antiseizure medication.22
Patients taking anti-epileptic drugs should take 4 mg/d of folate supplementation. According to the American Academy of Neurology, best practice is to use a single agent best suited to the type of seizures the patient experiences, at the lowest effective dose. Avoid multiple anti-epileptic drugs, if possible. Do not change an effective medication regimen if the patient becomes pregnant, but do check drug levels.23 Also counsel patients about the possibility of decreased effectiveness of hormonal contraception while taking enzymeinducing (cytochrome P450) antiepileptic drugs.24
Psychiatric disorders. Approximately 1 of every 7 pregnant women meets the diagnostic criteria for depression.25 Depression, anxiety, and other psychiatric conditions can adversely impact the patient and her developing fetus if the condition is undertreated. Unfortunately, some women stop psychiatric medications when they discover they are pregnant. In 1 study of 201 pregnant women with depression, 43% had a relapse during the course of pregnancy. Patients with relapse included 68% of those who stopped medication during the pregnancy, vs 26% of those who continued taking medication.26
Most antidepressants are relatively safe during pregnancy, with the exception of paroxetine (Paxil), which is associated with fetal cardiac defects.27 When a patient taking psychiatric medications comes in for a preconception visit, consider higher doses of 1 psychotropic medication rather than lower doses of multiple medications. This will decrease fetal medication exposure. If a patient on a stable regimen becomes pregnant, do not switch medications. Always collaborate with the patient’s mental health care team.
Venous thromboembolic disease. Some inherited thrombophilias can lead to a VTE during pregnancy or the postpartum period. Consider testing patients who have had a VTE or have a family history of VTE for anticardiolipin antibodies, protein S deficiency, and other thrombophilias before conception, because some of the lab values that indicate these conditions can change during pregnancy.
Patients with a history of a VTE related to hormone use or pregnancy will require prophylactic anticoagulation. When in doubt, refer to a hematologist or perinatologist. Both heparin and low-molecular-weight heparin are safe during pregnancy. Because warfarin is a suspected teratogen, patients taking warfarin should be converted to either heparin or low-molecular-weight heparin.
Hypertension. Hypertensive disorders may lead to pregnancy-induced hypertension, growth restriction, and renal disease. If patients are taking thiazide diuretics, angiotensin receptor blockers, or angiotensin-converting enzyme inhibitors ( TABLE 2 ), switch to medications such as methyldopa (Aldomet), nifedipine, or labetalol, which are safer during pregnancy.28 Screen patients with long-standing hypertension for cardiac disease (via EKG) and nephropathy before conception.
TABLE 2
Medications that can harm the unborn child32,33
| MEDICATION | POTENTIAL HARM TO FETUS | FDA CATEGORY* |
|---|---|---|
| Cardiovascular | ||
| ACE inhibitors: captopril, enalapril, lisinopril | Cardiovascular malformations, hypotension, anuria, oligohydramnios | C (1st trimester), D (2nd, 3rd trimesters) |
| Statins: atorvastatin, simvastatin | Polydactyly, cleft lip, club foot | X |
| Antidepressants | ||
| SSRIs: citalopram, fluoxetine, paroxetine, sertraline | Pulmonary hypertension, withdrawal syndrome; paroxetine: cardiac malformations, NTDs | C (D, paroxetine) |
| Anxiolytics | ||
| Benzodiazepines: alprazolam, clonazepam, diazepam, lorazepam | Withdrawal syndrome, congenital anomalies (various), floppy infant syndrome | D |
| Anti-epileptic drugs | ||
| Valproic acid and derivatives | NTDs, facial/cardiac defects | D |
| Carbamazepine | See valproic acid | D |
| Phenobarbital | Cardiac defects, hemorrhagic disease of the newborn | D |
| Phenytoin | Fetal hydantoin syndrome | D |
| Other | ||
| Isotretinoin | Hydrocephalus, microcephaly, limb anomalies, preterm labor, increased spontaneous abortions | X |
| Warfarin | Skeletal defects, intrauterine growth restriction, neurologic defects | X |
| Methotrexate | Fetal malformations, spontaneous abortion | X |
| ACE, angiotensin-converting enzyme; NTDs, neural tube defects; SSRIs, selective serotonin reuptake inhibitors. | ||
| *FDA categories describe the relative risk of medication use during pregnancy. The FDA is currently proposing major revisions to the system to upgrade the usefulness of this information. Please reconfirm categories prior to prescribing. | ||
| A: Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities in any trimester of pregnancy. | ||
| B: Animal studies have revealed no evidence of harm to the fetus, but no adequate and well-controlled studies in pregnant women are available OR animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester. | ||
| C: Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women OR no animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. | ||
| D: Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. | ||
| X: Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant. | ||
| FDA categories source: Meadows M. Pregnancy and the drug dilemma. FDA Consumer Magazine. May-June 2001. Available at www.fda.gov/fdac/features/2001/301_preg.html. Accessed May 6, 2009. | ||
Think ahead: Address immunization status
When a pregnant woman contracts an infectious disease, her developing fetus can be affected. Making sure the immunization status of all your reproductive-age patients is up to date will go a long way toward protecting their offspring from harm.29
Rubella. Also known as German measles, rubella can cause fetal anomalies and spontaneous abortion if contracted during the first half of pregnancy. Because the measles, mumps, and rubella (MMR) vaccine contains live attenuated viruses, susceptible patients should be immunized at least 1 month before they conceive.3
Hepatitis B. Preventing hepatitis B is an important public health issue. Screen patients at risk for hepatitis B infection—health care workers, sex workers, intravenous drug abusers, and nonmonogamous women who do not use barrier protection—with a hepatitis B surface antigen level. Vaccination is safe up to 1 month before conception.3
Varicella. Maternal varicella (chicken pox) can cause fetal harm, particularly if symptoms appear just before or during delivery. Women of reproductive age who have not already had the disease or been vaccinated should be immunized. This is a live virus vaccine and must, therefore, be administered at least 1 month before conception.3
Influenza. Administering influenza vaccine is not contraindicated during pregnancy, although most experts advise waiting until the second trimester. Certainly it is appropriate to administer the vaccine during influenza season or when risk factors such as chronic lung disease are present. Avoid live attenuated vaccine (FluMist) in pregnant women.
Tdap vaccination. The CDC’s Advisory Committee on Immunization Practices recommended in 2008 that susceptible pregnant women receive Tdap during the postpartum period to protect vulnerable infants against pertussis. Tdap is thought to be safe during pregnancy, but it would make sense to administer this vaccine when indicated prior to conception as part of a vaccination screening program.
Screen for genetic conditions prepregnancy
Part of a comprehensive preconception visit includes screening for communicable diseases and genetic conditions.
Communicable diseases. Consider screening all women prior to pregnancy for HIV infection, gonorrhea, chlamydia, hepatitis B, hepatitis C (for health care workers), and syphilis.
Diabetes. Screening guidelines for diabetes are available from the American Association of Clinical Endocrinologists.20 Consider preconception screening for patients who, during a previous pregnancy, had gestational diabetes or who delivered a baby weighing more than 9 pounds.
Genetic screening. Patients from certain ethnic groups are more susceptible to specific genetic mutations. Genetic disorders associated with particular ethnic origins are listed in TABLE 3 . Consider a preconception referral to a genetic counselor or perinatologist when the patient’s family history suggests inherited disorders.
TABLE 3
Genetic disorders: Who to screen, tests to use34
| ETHNIC ORIGIN* | DISORDER | RECOMMENDED TEST |
|---|---|---|
| Ashkenazi Jews | Tay-Sachs disease; Canavan disease | DNA panel, hexosaminidase A |
| African American | Sickle cell trait; beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| French Canadian, Cajun | Tay-Sachs disease | Hexosaminidase A |
| Mediterranean | Alpha-,† beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Indian, Middle Eastern | Sickle cell trait; alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| Caucasian | Cystic fibrosis | DNA panel |
| Southeast Asian (Thai, Laotian, Cambodian) | Alpha-, beta-thalassemia | Hemoglobin electrophoresis, MCV <70 fL |
| MCV, mean corpuscular volume. | ||
| *Offer screening to any interested patient. | ||
| †Do not pursue alpha-thalassemia work-up unless patient has a history of pregnancy loss or fetal hydrops. | ||
Correspondence
D. Ashley Hill, MD, Associate Director, Department of Obstetrics and Gynecology, Loch Haven OB/Gyn Group, Florida Hospital Orlando, 235 Princeton Street, Suite 200, Orlando, FL 32804; [email protected]
1. Mayer JP. Unintended childbearing, maternal beliefs, and delay of prenatal care. Birth. 1997;24:247-252.
2. Proceedings of the Preconception Health and Health Care Clinical, Public Health, and Consumer Workgroup Meetings. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities; 2006. Available at: http://www.cdc.gov/ncbddd/preconception/documents/Workgroup%20Proceedings%20June06.pdf. Accessed May 14, 2009.
3. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-06):1-23.Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed May 14, 2009.
4. Lumley J, Watson L, Watson M, et al. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.-
5. Iqbal MM. Prevention of neural tube defects by periconceptional use of folic acid. Pediatr Rev. 2000;21:58-66.
6. de Weerd S, Thomas CM, Cikot RJ, et al. Preconception counseling improves folate status of women planning pregnancy. Obstet Gynecol. 2002;99:45-50.
7. Mofenson LM. Centers for Disease Control and Prevention, US Public Health Service Task Force. US Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 2002;51(RR-18):1-38.
8. Robinson HE, O’Connell CM, Joseph KS, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357-1364.
9. Kiel DW, Dodson EA, Artal R, et al. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstet Gynecol. 2007;110:752-758.
10. US Food and Drug Administration. Food safety for moms-to-be. August 24, 2005. Available at: http://www.cfsan.fda.gov/~pregnant/pregnant.html. Accessed September 18, 2008.
11. Cheschier N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. (Replaces committee opinion number 252, March 2001). Int J Gynaecol Obstet. 2003;83:123-133.
12. Castles A, Adams EK, Melvin CL, et al. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16:208-215.
13. US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. May 27, 2004. Available at: http://www.surgeongeneral.gov/library/smokingconsequences. Accessed July 6, 2008.
14. National Center for Health Statistics. Health, United States, 2004: With Chartbook on Trends in the Health of Americans. Hyattsville, MD: NCHS; 2004. Available at: http://www.cdc.gov/nchs/data/hus/hus04.pdf. Accessed September 18, 2008.
15. ACOG Committee on Health Care for Underdeserved Women; ACOG Committee on Obstetric Practice. ACOG committee opinion. Number 316, October 2005. Smoking cessation during pregnancy. Obstet Gynecol. 2005;106:883-888.
16. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.ahrq.gov/path/tobacco.htm. Accessed May 14, 2009.
17. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
18. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:279.e1-279.e8.
19. Lucas MJ, Leveno KJ, Williams ML, et al. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am J Obstet Gynecol. 1989;161:426-431.
20. American Association of Clinical Endocrinologists Diabetes Mellitus Clinical Practice Guidelines Task Force. AACE diabetes mellitus guidelines. Chapter 9: diabetes and pregnancy. Endocr Pract. 2007;13(suppl 1):55-59.
21. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675-685.
22. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Int J Gynaecol Obstet. 1997;56:279-286.
23. American Academy of Neurology. Guideline summary for clinicians—management issues for women with epilepsy. Available at: www.aan.com/professionals/practice/pdfs/women_epilepsy.pdf. Accessed May 14, 2009.
24. Carl JS, Weaver SP, Tweed E, et al. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
25. Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799-801.
26. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment [published correction appears in JAMA. 2006;296:170]. JAMA. 2006;295:499-507.
27. FDA public health advisory: paroxetine. December 8, 2005. Available at: http://www.fda.gov/CDER/Drug/advisory/paroxetine200512.htm. Accessed May 6, 2009.
28. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Chronic hypertension in pregnancy. Obstet Gynecol. 2001;98(suppl):177-185.
29. Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2009. MMWR. 2009;57(53):Q-1-Q-4.Available at: http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed May 14, 2009.
30. MayoClinic.Com. How much caffeine is in your daily habit? Available at: http://www.mayoclinic.com/health/caffeine/AN01211. Accessed September 18, 2008.
31. Chou KH, Bell LH. Caffeine content of prepackaged national-brand and private-label carbonated beverages. J Food Sci. 2007;72:C337-C342.
32. Physician’s Desk Reference. Montvale, NJ: Thomson Reuters; 2009.
33. Drugs.com. Available at: http://www.drugs.com. Accessed April 26, 2009.
34. National Society of Genetic Counselors, Prenatal Special Interest Group. Ancestry Based Carrier Screening. Chicago, IL: NSGC; 2005. Available at: http://www.nsgc.org. Accessed September 18, 2008.
1. Mayer JP. Unintended childbearing, maternal beliefs, and delay of prenatal care. Birth. 1997;24:247-252.
2. Proceedings of the Preconception Health and Health Care Clinical, Public Health, and Consumer Workgroup Meetings. Atlanta, GA: Centers for Disease Control and Prevention, National Center on Birth Defects and Developmental Disabilities; 2006. Available at: http://www.cdc.gov/ncbddd/preconception/documents/Workgroup%20Proceedings%20June06.pdf. Accessed May 14, 2009.
3. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-06):1-23.Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed May 14, 2009.
4. Lumley J, Watson L, Watson M, et al. Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev. 2001;(3):CD001056.-
5. Iqbal MM. Prevention of neural tube defects by periconceptional use of folic acid. Pediatr Rev. 2000;21:58-66.
6. de Weerd S, Thomas CM, Cikot RJ, et al. Preconception counseling improves folate status of women planning pregnancy. Obstet Gynecol. 2002;99:45-50.
7. Mofenson LM. Centers for Disease Control and Prevention, US Public Health Service Task Force. US Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Recomm Rep. 2002;51(RR-18):1-38.
8. Robinson HE, O’Connell CM, Joseph KS, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106:1357-1364.
9. Kiel DW, Dodson EA, Artal R, et al. Gestational weight gain and pregnancy outcomes in obese women: How much is enough? Obstet Gynecol. 2007;110:752-758.
10. US Food and Drug Administration. Food safety for moms-to-be. August 24, 2005. Available at: http://www.cfsan.fda.gov/~pregnant/pregnant.html. Accessed September 18, 2008.
11. Cheschier N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin. Neural tube defects. Number 44, July 2003. (Replaces committee opinion number 252, March 2001). Int J Gynaecol Obstet. 2003;83:123-133.
12. Castles A, Adams EK, Melvin CL, et al. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16:208-215.
13. US Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. May 27, 2004. Available at: http://www.surgeongeneral.gov/library/smokingconsequences. Accessed July 6, 2008.
14. National Center for Health Statistics. Health, United States, 2004: With Chartbook on Trends in the Health of Americans. Hyattsville, MD: NCHS; 2004. Available at: http://www.cdc.gov/nchs/data/hus/hus04.pdf. Accessed September 18, 2008.
15. ACOG Committee on Health Care for Underdeserved Women; ACOG Committee on Obstetric Practice. ACOG committee opinion. Number 316, October 2005. Smoking cessation during pregnancy. Obstet Gynecol. 2005;106:883-888.
16. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.ahrq.gov/path/tobacco.htm. Accessed May 14, 2009.
17. US Preventive Services Task Force. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med. 2004;140:554-556.
18. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:279.e1-279.e8.
19. Lucas MJ, Leveno KJ, Williams ML, et al. Early pregnancy glycosylated hemoglobin, severity of diabetes, and fetal malformations. Am J Obstet Gynecol. 1989;161:426-431.
20. American Association of Clinical Endocrinologists Diabetes Mellitus Clinical Practice Guidelines Task Force. AACE diabetes mellitus guidelines. Chapter 9: diabetes and pregnancy. Endocr Pract. 2007;13(suppl 1):55-59.
21. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol. 2005;105:675-685.
22. Committee on Educational Bulletins of the American College of Obstetricians and Gynecologists. ACOG educational bulletin. Seizure disorders in pregnancy. Number 231, December 1996. Int J Gynaecol Obstet. 1997;56:279-286.
23. American Academy of Neurology. Guideline summary for clinicians—management issues for women with epilepsy. Available at: www.aan.com/professionals/practice/pdfs/women_epilepsy.pdf. Accessed May 14, 2009.
24. Carl JS, Weaver SP, Tweed E, et al. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
25. Weissman MM, Olfson M. Depression in women: implications for health care research. Science. 1995;269:799-801.
26. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment [published correction appears in JAMA. 2006;296:170]. JAMA. 2006;295:499-507.
27. FDA public health advisory: paroxetine. December 8, 2005. Available at: http://www.fda.gov/CDER/Drug/advisory/paroxetine200512.htm. Accessed May 6, 2009.
28. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin. Chronic hypertension in pregnancy. Obstet Gynecol. 2001;98(suppl):177-185.
29. Centers for Disease Control and Prevention. Recommended adult immunization schedule—United States, 2009. MMWR. 2009;57(53):Q-1-Q-4.Available at: http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed May 14, 2009.
30. MayoClinic.Com. How much caffeine is in your daily habit? Available at: http://www.mayoclinic.com/health/caffeine/AN01211. Accessed September 18, 2008.
31. Chou KH, Bell LH. Caffeine content of prepackaged national-brand and private-label carbonated beverages. J Food Sci. 2007;72:C337-C342.
32. Physician’s Desk Reference. Montvale, NJ: Thomson Reuters; 2009.
33. Drugs.com. Available at: http://www.drugs.com. Accessed April 26, 2009.
34. National Society of Genetic Counselors, Prenatal Special Interest Group. Ancestry Based Carrier Screening. Chicago, IL: NSGC; 2005. Available at: http://www.nsgc.org. Accessed September 18, 2008.