User login
ADOLESCENT DEPRESSION: Help your patient emerge from the darkness
Last month, we introduced you to 15-year-old Jane, a teenager whose once bubbly personality had in the last few months been reduced to a mood of quiet sadness. Her responses to your questions were muted, unenthusiastic. While Jane gets to school every day and can often shake off her down mood when she’s with friends, her responses to the Kutcher Adolescent Depression scale suggest that she’s struggling. You conclude that Jane is experiencing an episode of mild depressive disorder.
How would you manage Jane’s case? And what would you do if her symptoms worsened?
What’s the preference of patient and family?
Begin your initial management of a patient like Jane by considering the treatment preferences of the patient and her family, the severity and urgency of the case, the availability of mental health services, and your own comfort level with managing mental health disorders. A key conclusion of the GLAD-PC (GuideLines for Adolescent Depression in Primary Care) collaborative, described in Part 1 of this series, was that family physicians, alone or in collaboration with mental health professionals, are competent to manage adolescent depression.1 You may or may not choose to manage a patient like Jane yourself, but even if you refer, your initial management provides an essential bridge until the patient and her family are seen by mental health professionals.
Your initial management should include the following:
- education
- a treatment plan
- safety planning.
Step 1: Educate patient and parents
Help your patient to better understand what it means to have depression. Describe the signs and symptoms that led to the diagnosis of depression and review the natural history of the illness, including the chronic nature of the disorder and its tendency to recur. Explain, too, the impact that depression can have on different areas of functioning, such as school performance and peer relationships, and then review the treatment options. You or someone on your staff can provide this patient education initially, but it is also critical to connect the family to specific community resources for additional education, advocacy, and peer support.1
To do this effectively, you need to establish links with mental health resources in the community, including mental health service providers, as well as patients and families who have dealt with adolescent depression and are willing to serve as resources to other teens and their families. The GLAD-PC toolkit, available at www.gladpc.org, provides patient education handouts and links to reputable Web sites, advocacy organizations, and peer support groups. Additional online resources are listed in TABLE 1.
TABLE 1
Online resources
| SOURCE | WEBSITE |
|---|---|
| American Academy of Child and Adolescent Psychiatry | http://www.aacap.org/cs/root/facts_for_families/the_depressed_child |
| Families for Depression Awareness | www.familyaware.org |
| National Alliance on Mental Illness | http://www.nami.org/depression |
| National Institute of Mental Health | http://www.nimh.nih.gov/health/publications/depression |
Step 2: Work out a treatment plan
Developing a treatment plan that the patient and her parents can accept is critical. A plan that includes psychotherapy with a mental health provider, for example, won’t be acceptable to some patients and parents. They may refuse to participate, or their underlying mistrust may affect the outcome of treatment.2,3 Other families may reject any therapeutic approach that includes psychotropic drugs.
Expectations about the benefits of treatment influence outcomes significantly, so that, too, is a topic to explore as the treatment plan is worked out.3,4 Finally, the plan should include agreed-upon goals of treatment. For Jane, planned goals might include getting back into gymnastics or trying out for the school play.
Step 3: Plan for safety
Suicidality, including ideation, behaviors, or attempts, is common among adolescents with depression.5,6 In studies of completed suicide, more than 50% of the victims had a diagnosis of depression.5 To keep your patient safe, develop an emergency communication mechanism for handling increased suicidality or acute crises. If the patient’s risk is high, as shown by a clear plan or intent, immediate hospitalization may be necessary.
If you determine that inpatient treatment is not needed, you need to be sure that adequate adult supervision and support are available; that the teenager does not have access to potentially lethal medications, knives and other sharp objects, or firearms; and that both the patient and parents understand that drugs and alcohol weaken inhibitions. You need to set up a contingency plan with the family that includes checking in with you at reasonable intervals to assure the teen’s safety.5
Establishing a safety plan is especially important during the period of diagnosis and initial treatment, when suicide risk is highest.6 Confidentiality is the norm in adolescent medicine, but a patient like Jane must understand that you will breach confidentiality if that is necessary to keep her safe from harm.
GLAD-PC Recommendation II: Family physicians should develop a treatment plan with patients and families (SOR: C, expert opinion) and set specific treatment goals in key areas of functioning, including home, peer, and school settings (SOR: C, expert opinion).
GLAD-PC Recommendation III: The family physician should establish relevant links/collaboration with mental health resources in the community (SOR: C, expert opinion), which may include patients and families who have dealt with adolescent depression and are willing to serve as resources to other affected adolescents and their family members (SOR: C, expert opinion).
GLAD-PC Recommendation IV: Management must include the establishment of a safety plan, which includes restricting lethal means, engaging a concerned third party, and implementing an emergency communication mechanism should the patient deteriorate, become actively suicidal or dangerous to others, or experience an acute crisis associated with psychosocial stressors, especially during the period of initial treatment when safety concerns are highest (SOR: C, case control study and expert opinion).
GLAD-PC Recommendation V: After initial diagnosis in cases of mild depression, family physicians should consider a period of active support and monitoring before starting other evidence-based treatments (SOR: C, expert opinion).
GLAD-PC Recommendation VI: If a family physician identifies an adolescent with moderate or severe depression or complicating factors/conditions such as co-existing substance abuse or psychosis, consultation with a mental health specialist should be considered (SOR: C, expert opinion). Appropriate roles and responsibilities for ongoing management by the family physician and mental health provider should be communicated and agreed upon (SOR: C, expert opinion).
The patient and family should be consulted and approve of the roles negotiated by the family physician and mental health professionals (SOR: C, expert opinion).
GLAD-PC Recommendation VII: Family physicians should recommend scientifically tested and proven treatments (eg, psychotherapies such as cognitive behavioral therapy or interpersonal therapy, and/or antidepressant treatment such as SSRIs) whenever possible and appropriate to achieve the goals of the treatment plan (SOR: A, RCTs).
GLAD-PC Recommendation VIII: Family physicians should monitor for the emergence of adverse events during antidepressant treatment (SSRIs) (SOR: C, expert opinion).
Treatment options: When active support is best
Selecting the appropriate treatment modality for your patient hinges, of course, on the severity of the teen’s depression. (For more information on how to determine the severity of a depressive episode, see the first installment of this series, “Adolescent depression: Is your young patient suffering in silence?” J Fam Pract. 2009;58:187-192.)
When caring for a patient like Jane who is suffering from mild depression, consider providing active support and monitoring during 6 to 8 weekly or biweekly visits before recommending antidepressant medication or psychotherapy. This approach is also indicated when depressed patients or their parents refuse other treatments.7
Active support and monitoring may include education, frequent follow-up, a prescribed regimen of exercise and leisure activities, referral to a peer support group, and review of self-management goals. Other resources for active monitoring can be found in the GLAD-PC toolkit (available at www.gladpc.org). Evidence from randomized controlled trials (RCTs) shows that a sizable percentage of young people with depression respond to nondirective supportive therapy and regular symptom monitoring.7 Furthermore, emerging data from the research literature, expert opinion, and patient and family preferences indicate that active support and monitoring from family physicians is an important therapeutic strategy.7,8
Is therapy needed—and if so, what kind?
Adolescents with moderate or severe depression or patients with mild depression whose symptoms do not improve with active support and monitoring alone will likely require treatment with one of the evidenced-based treatments, such as psychotherapy or antidepressants. Referral to a mental health provider for further assessment or treatment may also be required, depending on the training of the physician.7,8 If so, you and the mental health provider will need to negotiate your roles and responsibilities for ongoing management, with the input and approval of the patient and family.
Both cognitive behavioral therapy (CBT) and interpersonal therapy (IPT) have been adapted to address major depressive disorder (MDD) in adolescents and have been shown to be effective in community as well as specialized settings.9-11
CBT is time-limited and delivered individually or by 1 or 2 clinicians working with a group. Clinicians follow a manual to guide each session.12 (A manual for therapists and a workbook for adolescents and parents can be downloaded from the Kaiser Permanente Center for Health Research Web site at http://www.kpchr.org/public/acwd/acwd.html.)
The focus of CBT is to change patients’ perception of themselves, their world, and others. CBT treats depression by identifying behavioral and cognitive patterns associated with depressive cycles. Examples of such patterns include the propensity to withdraw from pleasurable activities, or irritability that alienates family and friends just when the teenager needs them most. CBT helps teens identify these self-defeating patterns, encourages them to take part in activities they enjoy, helps develop or reactivate social skills important for maintaining positive social interactions, and helps teens to develop problem-solving strategies for resolving stressful situations.
CBT also aims to correct maladaptive beliefs associated with the patient’s depression. If, for instance, a patient believes she is worthless if she’s not accepted by the “popular” group at school, she is likely to become depressed and stay depressed as long as she is having difficulty connecting with her peers. CBT would help her examine that belief and learn to feel worthwhile even if she is not accepted by the “in” group. In general, CBT sessions are scheduled on a weekly basis for 12 to 16 weeks. In each session, the therapist and patient complete specific tasks and exercises that are provided in a CBT manual. There are also tasks for the patient to complete between sessions and review later with the therapist. CBT has been used in primary care with preliminary positive results.13,14 However, the results of a recent RCT conducted in psychiatric settings demonstrated superior efficacy of combination therapy (fluoxetine and CBT) vs CBT alone.15
IPT for adolescents (IPT-A) is like CBT in that it is time-limited and clinicians are guided by a manual.16 A course of therapy can last anywhere from 12 to 16 sessions with optional maintenance treatment. The theoretical basis for IPT-A is the observed negative impact of depressive symptoms on interpersonal relationships, and the effect poor relationships have in causing and perpetuating depression. In deciding whether a patient may be suitable for IPT-A, you need to find out whether she would be willing to share her experiences of ongoing relationship conflicts with a therapist or therapeutic group. The relationship difficulties IPT-A is designed to help with arise from 1 of 4 sources: grief, fights with peers or family members (interpersonal disputes), transitions from one social surround to another (role transition), and friendlessness (interpersonal deficits).
IPT-A focuses on grief only when someone of significance to the patient has died. Therapy for teens who quarrel frequently with peers or family members is focused on interpersonal disputes, and this is the most common focus in IPT-A. A focus on role transition is called for when the teen’s social world has undergone a drastic change, such as a when a teen has moved to a new school or broken up with a boyfriend. Finally, therapy for a teen with no significant relationships outside the immediate family focuses on interpersonal deficits. In these cases, the goal of therapy is to increase social contact and help the patient build relationships. If your preliminary assessment identifies your patient’s difficulties as rooted in 1 of these 4 areas, IPT-A may be for her.
Because few family physicians are trained in CBT or IPT-A, most psychotherapy will be provided by mental health professionals. What you can provide is familiarity with available community mental health resources. To get to know the therapists in your community, you may want to reach out to a few of them and ask them the questions in TABLE 2. You may also want to share this list with parents who want to find their own therapist.
TABLE 2
6 questions to ask prospective therapists
| 1. What type of therapy can you provide—cognitive behavioral therapy (CBT), interpersonal therapy for adolescents (IPT-A), psychodynamic psychotherapy, supportive therapy, counseling, or eclectic (including elements of IPT-A and CBT)? The evidence suggests that CBT and IPT-A are the treatments of choice for teens with depression. |
| 2. Have you received training in that therapy for adolescents with depression? Where and when? The therapist should have been trained in a clinical program (social work, nursing, psychology) that involved adolescents. |
| 3. Have you received clinical supervision in that therapy? Where? For how long? How many cases? Generally, therapists should be supervised for at least 3 to 4 cases before they are considered pro? cient. |
| 4. Are there specific tasks scheduled for each session? There should be for CBT, but not for IPT-A. |
| 5. Is the therapy time-limited? CBT and IPT-A are both time-limited. |
| 6. What are the goals of the therapy? The goals for both CBT and IPT-A should be the resolution of depressive symptoms. |
| Source: This list has been adapted by Amy Cheung, MD, from her contributions to the forthcoming book tentatively entitled Assessment and Treatment of Pediatric Depression: State of the Science; Best Practices (Editors: Peter S. Jensen, MD, Amy Cheung, MD, Ruth Stein, MD, and Rachel A. Zuckerbrot, MD), to be published by Civic Research Institute, Inc. All rights reserved. |
Choose an antidepressant, monitor with care
Studies have shown that up to 42% of family physicians in the United States had recently prescribed selective serotonin reuptake inhibitors (SSRIs) for more than 1 adolescent under the age of 18.17 When the diagnosis of MDD without comorbid conditions is clear and the patient and family are amenable, you may want to prescribe an SSRI.7,8
If you do, warn the patient and family that antidepressants can sometimes have adverse effects, including a switch from depressive to manic symptoms, signs of behavioral activation including agitation, hostility or restlessness, and suicidal ideation or behavior. If the patient can tolerate the medication without significant adverse effects, you need to prescribe the effective dose for at least 6 to 8 weeks to ensure an adequate trial.7
TABLE 3 provides some guidance for prescribing antidepressants for adolescents with depression.7 Among the antidepressants, only fluoxetine has been approved by the FDA for children and adolescents with depression. Fluoxetine is also the SSRI with the strongest evidence for efficacy in the adolescent population, as demonstrated in 4 RCTs.18 Two studies involving fluoxetine for depression have also shown efficacy in children as young as age 7 (range, 7-12 years).19
Effective dosages for antidepressants are lower for adolescents than for adults. Initiate medications at a low dose and increase in recommended increments every 2 weeks if no significant adverse effects emerge. With the exception of fluoxetine, SSRI medications must be discontinued slowly to minimize the risk of discontinuation effects.
Once treatment begins, you or a member of your staff will need to stay in touch with the patient and family to review their continued adherence to the treatment plan. An FDA black-box warning recommends observing for “clinical worsening, suicidality, and unusual changes in behavior” during initial visits or “at times of dose changes, either increases or decreases.” Develop a regular, frequent monitoring schedule with input from the teen and her (or his) parents to ensure compliance.7,20
Make sure follow-up appointments are not missed, using flags in patient records or in the clinic schedule. The duration of treatment for teens with depression is yet to be determined through clinical trials. Most guidelines suggest drug therapy be continued at the same dosage for 6 to 12 months after symptoms resolve. Guidelines for the treatment of adolescent depression can be found at www.gladpc.org.
Keeping teenagers on an antidepressant regimen can be challenging, given the side effects, the amount of time it takes before they experience an improvement, and the lengthy duration of treatment. Families that know what to expect and are getting continuing support from you and others are most likely to stay with treatment for the duration.
TABLE 3
A guide to prescribing antidepressants for adolescents
| MEDICATION | STARTING DOSE | EFFECTIVE DOSE | MAXIMUM DOSE | NOT TO BE USED WITH | COMMON ADVERSE EFFECTS |
|---|---|---|---|---|---|
| Citalopram | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia |
| Fluoxetine | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia, agitation, anxiety |
| Fluvoxamine | 25-50 mg/d | 150 mg | 300 mg | MAOIs and pimozide | Headache, GI upset, drowsiness |
| Paroxetine | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia |
| Sertraline | 25 mg/d | 100 mg | 200 mg | MAOIs | Headache, GI upset, insomnia |
| Escitalopram | 5 mg/d | 10-20 mg | 20 mg | MAOIs | Headache, GI upset, insomnia |
| MAOI, monoamine oxidase inhibitor. | |||||
| Source: This table has been adapted by Amy Cheung, MD, from her contributions to the forthcoming book tentatively entitled, Assessment and Treatment of Pediatric Depression: State of the Science; Best Practices (Editors: Peter S. Jensen, MD, Amy Cheung, MD, Ruth Stein, MD, and Rachel A. Zuckerbrot, MD), to be published by Civic Research Institute, Inc. All rights reserved. | |||||
What about Jane?
As the family’s physician, your initial management began with you educating Jane and her parents about mild depressive disorder and its likely course. You set up a series of weekly visits to monitor her symptoms and provide active support. You helped Jane find a peer support group and encouraged her to get back into gymnastics. You taught Jane and her family about the importance of keeping her safe while she is depressed, and they were cooperative about safety-proofing their home and setting up a plan to handle emergencies.
The US Preventive Services Task Force now recommends screening all adolescents (12-18 years of age) for major depressive disorder when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive behavioral therapy or interpersonal therapy), and follow-up. Previously, the Task Force concluded that the evidence was insufficient to recommend for or against the practice. For more on the Task Force’s recommendations, go to www.ahrq.gov/clinic/uspstf09/depression/chdeprrs.htm.
Jane’s depressive symptoms gradually ebbed, and she returned to her previous level of energy and social activity. You warned her and her family about the possibility that the disorder might recur, so they would be prepared.
Correspondence
Amy Cheung, MD, 33 Russell Street, 3rd Floor Tower, Toronto, Ontario, Canada MSS 2S1; [email protected]
1. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care–GLAD PC – Part I. Pediatrics. 2007;120:e1299-e1312.
2. Richardson LP, Lewis CW, Casey-Goldstein M, et al. Pediatric primary care providers and adolescent depression. J Adolesc Health. 2007;40:433-439.
3. Myers SS, Phillips RS, Davis RB, et al. Patient expectations as predictors of outcome in patients with acute low back pain. J Gen Intern Med. 2008;23:1525-1497.
4. Aikens JE, Nease DE, Jr, Nau DP, et al. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med. 2005;3:23-30.
5. Brent DA, Perper JA, Moritz G, et al. Psychiatric risk factors for adolescent suicide: a case-control study. J Am Acad Child Adolesc Psychiatry. 1993;32:521-529.
6. American Academy of Child and Adolescent Psychiatry. Summary of the practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40:495-499.
7. Cheung A, Zuckerbrot RA, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care–GLAD PC – Part II. Pediatrics. 2007;120:e1313-e1326.
8. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Expert survey for the management of adolescent depression in primary care. Pediatrics. 2008;121:e101-e107.
9. Compton SN, March JS, Brent D, et al. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930-959.
10. Mufson L, Weissman MM, Moreau D, et al. Efficacy of interpersonal psychotherapy for depressed adolescents. Arch Gen Psychiatry. 1999;56:573-579.
11. Mufson L, Dorta KP, Wickramaratne P, et al. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Arch Genl Psychiatry. 2004;61:577-584.
12. Clarke GN, Rohde P, Lewinsohn PM, et al. Cognitive-behavioral treatment of adolescent depression: efficacy of acute group treatment and booster session. J Am Acad Child Adolesc Psychiatry. 1999;38:272-279.
13. Asarnow JR, Jaycox LH, Duan N, et al. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: a randomized controlled trial. JAMA. 2005;293:311-319.
14. Clarke G, Debar L, Lynch F, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. J Am Acad Child Adolesc Psychiatry. 2005;44:888-898.
15. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for adolescents with depression study (TADS) randomized controlled trial. JAMA. 2004;292:807-820.
16. Mufson L, Moreau D, Weissman M. Interpersonal Psychotherapy for Depressed Adolescents. New York: Guildford Press; 2004.
17. Olson AL, Kelleher KJ, Kemper KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1:91-98.
18. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696.
19. Mayes TL, Tao R, Rintelmann JW, et al. Do children and adolescents have differential response rates in placebo-controlled trials of fluoxetine? CNS Spectr. 2007;12:147-154.
20. Birmaher B, Brent D. And the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503-1526.
Last month, we introduced you to 15-year-old Jane, a teenager whose once bubbly personality had in the last few months been reduced to a mood of quiet sadness. Her responses to your questions were muted, unenthusiastic. While Jane gets to school every day and can often shake off her down mood when she’s with friends, her responses to the Kutcher Adolescent Depression scale suggest that she’s struggling. You conclude that Jane is experiencing an episode of mild depressive disorder.
How would you manage Jane’s case? And what would you do if her symptoms worsened?
What’s the preference of patient and family?
Begin your initial management of a patient like Jane by considering the treatment preferences of the patient and her family, the severity and urgency of the case, the availability of mental health services, and your own comfort level with managing mental health disorders. A key conclusion of the GLAD-PC (GuideLines for Adolescent Depression in Primary Care) collaborative, described in Part 1 of this series, was that family physicians, alone or in collaboration with mental health professionals, are competent to manage adolescent depression.1 You may or may not choose to manage a patient like Jane yourself, but even if you refer, your initial management provides an essential bridge until the patient and her family are seen by mental health professionals.
Your initial management should include the following:
- education
- a treatment plan
- safety planning.
Step 1: Educate patient and parents
Help your patient to better understand what it means to have depression. Describe the signs and symptoms that led to the diagnosis of depression and review the natural history of the illness, including the chronic nature of the disorder and its tendency to recur. Explain, too, the impact that depression can have on different areas of functioning, such as school performance and peer relationships, and then review the treatment options. You or someone on your staff can provide this patient education initially, but it is also critical to connect the family to specific community resources for additional education, advocacy, and peer support.1
To do this effectively, you need to establish links with mental health resources in the community, including mental health service providers, as well as patients and families who have dealt with adolescent depression and are willing to serve as resources to other teens and their families. The GLAD-PC toolkit, available at www.gladpc.org, provides patient education handouts and links to reputable Web sites, advocacy organizations, and peer support groups. Additional online resources are listed in TABLE 1.
TABLE 1
Online resources
| SOURCE | WEBSITE |
|---|---|
| American Academy of Child and Adolescent Psychiatry | http://www.aacap.org/cs/root/facts_for_families/the_depressed_child |
| Families for Depression Awareness | www.familyaware.org |
| National Alliance on Mental Illness | http://www.nami.org/depression |
| National Institute of Mental Health | http://www.nimh.nih.gov/health/publications/depression |
Step 2: Work out a treatment plan
Developing a treatment plan that the patient and her parents can accept is critical. A plan that includes psychotherapy with a mental health provider, for example, won’t be acceptable to some patients and parents. They may refuse to participate, or their underlying mistrust may affect the outcome of treatment.2,3 Other families may reject any therapeutic approach that includes psychotropic drugs.
Expectations about the benefits of treatment influence outcomes significantly, so that, too, is a topic to explore as the treatment plan is worked out.3,4 Finally, the plan should include agreed-upon goals of treatment. For Jane, planned goals might include getting back into gymnastics or trying out for the school play.
Step 3: Plan for safety
Suicidality, including ideation, behaviors, or attempts, is common among adolescents with depression.5,6 In studies of completed suicide, more than 50% of the victims had a diagnosis of depression.5 To keep your patient safe, develop an emergency communication mechanism for handling increased suicidality or acute crises. If the patient’s risk is high, as shown by a clear plan or intent, immediate hospitalization may be necessary.
If you determine that inpatient treatment is not needed, you need to be sure that adequate adult supervision and support are available; that the teenager does not have access to potentially lethal medications, knives and other sharp objects, or firearms; and that both the patient and parents understand that drugs and alcohol weaken inhibitions. You need to set up a contingency plan with the family that includes checking in with you at reasonable intervals to assure the teen’s safety.5
Establishing a safety plan is especially important during the period of diagnosis and initial treatment, when suicide risk is highest.6 Confidentiality is the norm in adolescent medicine, but a patient like Jane must understand that you will breach confidentiality if that is necessary to keep her safe from harm.
GLAD-PC Recommendation II: Family physicians should develop a treatment plan with patients and families (SOR: C, expert opinion) and set specific treatment goals in key areas of functioning, including home, peer, and school settings (SOR: C, expert opinion).
GLAD-PC Recommendation III: The family physician should establish relevant links/collaboration with mental health resources in the community (SOR: C, expert opinion), which may include patients and families who have dealt with adolescent depression and are willing to serve as resources to other affected adolescents and their family members (SOR: C, expert opinion).
GLAD-PC Recommendation IV: Management must include the establishment of a safety plan, which includes restricting lethal means, engaging a concerned third party, and implementing an emergency communication mechanism should the patient deteriorate, become actively suicidal or dangerous to others, or experience an acute crisis associated with psychosocial stressors, especially during the period of initial treatment when safety concerns are highest (SOR: C, case control study and expert opinion).
GLAD-PC Recommendation V: After initial diagnosis in cases of mild depression, family physicians should consider a period of active support and monitoring before starting other evidence-based treatments (SOR: C, expert opinion).
GLAD-PC Recommendation VI: If a family physician identifies an adolescent with moderate or severe depression or complicating factors/conditions such as co-existing substance abuse or psychosis, consultation with a mental health specialist should be considered (SOR: C, expert opinion). Appropriate roles and responsibilities for ongoing management by the family physician and mental health provider should be communicated and agreed upon (SOR: C, expert opinion).
The patient and family should be consulted and approve of the roles negotiated by the family physician and mental health professionals (SOR: C, expert opinion).
GLAD-PC Recommendation VII: Family physicians should recommend scientifically tested and proven treatments (eg, psychotherapies such as cognitive behavioral therapy or interpersonal therapy, and/or antidepressant treatment such as SSRIs) whenever possible and appropriate to achieve the goals of the treatment plan (SOR: A, RCTs).
GLAD-PC Recommendation VIII: Family physicians should monitor for the emergence of adverse events during antidepressant treatment (SSRIs) (SOR: C, expert opinion).
Treatment options: When active support is best
Selecting the appropriate treatment modality for your patient hinges, of course, on the severity of the teen’s depression. (For more information on how to determine the severity of a depressive episode, see the first installment of this series, “Adolescent depression: Is your young patient suffering in silence?” J Fam Pract. 2009;58:187-192.)
When caring for a patient like Jane who is suffering from mild depression, consider providing active support and monitoring during 6 to 8 weekly or biweekly visits before recommending antidepressant medication or psychotherapy. This approach is also indicated when depressed patients or their parents refuse other treatments.7
Active support and monitoring may include education, frequent follow-up, a prescribed regimen of exercise and leisure activities, referral to a peer support group, and review of self-management goals. Other resources for active monitoring can be found in the GLAD-PC toolkit (available at www.gladpc.org). Evidence from randomized controlled trials (RCTs) shows that a sizable percentage of young people with depression respond to nondirective supportive therapy and regular symptom monitoring.7 Furthermore, emerging data from the research literature, expert opinion, and patient and family preferences indicate that active support and monitoring from family physicians is an important therapeutic strategy.7,8
Is therapy needed—and if so, what kind?
Adolescents with moderate or severe depression or patients with mild depression whose symptoms do not improve with active support and monitoring alone will likely require treatment with one of the evidenced-based treatments, such as psychotherapy or antidepressants. Referral to a mental health provider for further assessment or treatment may also be required, depending on the training of the physician.7,8 If so, you and the mental health provider will need to negotiate your roles and responsibilities for ongoing management, with the input and approval of the patient and family.
Both cognitive behavioral therapy (CBT) and interpersonal therapy (IPT) have been adapted to address major depressive disorder (MDD) in adolescents and have been shown to be effective in community as well as specialized settings.9-11
CBT is time-limited and delivered individually or by 1 or 2 clinicians working with a group. Clinicians follow a manual to guide each session.12 (A manual for therapists and a workbook for adolescents and parents can be downloaded from the Kaiser Permanente Center for Health Research Web site at http://www.kpchr.org/public/acwd/acwd.html.)
The focus of CBT is to change patients’ perception of themselves, their world, and others. CBT treats depression by identifying behavioral and cognitive patterns associated with depressive cycles. Examples of such patterns include the propensity to withdraw from pleasurable activities, or irritability that alienates family and friends just when the teenager needs them most. CBT helps teens identify these self-defeating patterns, encourages them to take part in activities they enjoy, helps develop or reactivate social skills important for maintaining positive social interactions, and helps teens to develop problem-solving strategies for resolving stressful situations.
CBT also aims to correct maladaptive beliefs associated with the patient’s depression. If, for instance, a patient believes she is worthless if she’s not accepted by the “popular” group at school, she is likely to become depressed and stay depressed as long as she is having difficulty connecting with her peers. CBT would help her examine that belief and learn to feel worthwhile even if she is not accepted by the “in” group. In general, CBT sessions are scheduled on a weekly basis for 12 to 16 weeks. In each session, the therapist and patient complete specific tasks and exercises that are provided in a CBT manual. There are also tasks for the patient to complete between sessions and review later with the therapist. CBT has been used in primary care with preliminary positive results.13,14 However, the results of a recent RCT conducted in psychiatric settings demonstrated superior efficacy of combination therapy (fluoxetine and CBT) vs CBT alone.15
IPT for adolescents (IPT-A) is like CBT in that it is time-limited and clinicians are guided by a manual.16 A course of therapy can last anywhere from 12 to 16 sessions with optional maintenance treatment. The theoretical basis for IPT-A is the observed negative impact of depressive symptoms on interpersonal relationships, and the effect poor relationships have in causing and perpetuating depression. In deciding whether a patient may be suitable for IPT-A, you need to find out whether she would be willing to share her experiences of ongoing relationship conflicts with a therapist or therapeutic group. The relationship difficulties IPT-A is designed to help with arise from 1 of 4 sources: grief, fights with peers or family members (interpersonal disputes), transitions from one social surround to another (role transition), and friendlessness (interpersonal deficits).
IPT-A focuses on grief only when someone of significance to the patient has died. Therapy for teens who quarrel frequently with peers or family members is focused on interpersonal disputes, and this is the most common focus in IPT-A. A focus on role transition is called for when the teen’s social world has undergone a drastic change, such as a when a teen has moved to a new school or broken up with a boyfriend. Finally, therapy for a teen with no significant relationships outside the immediate family focuses on interpersonal deficits. In these cases, the goal of therapy is to increase social contact and help the patient build relationships. If your preliminary assessment identifies your patient’s difficulties as rooted in 1 of these 4 areas, IPT-A may be for her.
Because few family physicians are trained in CBT or IPT-A, most psychotherapy will be provided by mental health professionals. What you can provide is familiarity with available community mental health resources. To get to know the therapists in your community, you may want to reach out to a few of them and ask them the questions in TABLE 2. You may also want to share this list with parents who want to find their own therapist.
TABLE 2
6 questions to ask prospective therapists
| 1. What type of therapy can you provide—cognitive behavioral therapy (CBT), interpersonal therapy for adolescents (IPT-A), psychodynamic psychotherapy, supportive therapy, counseling, or eclectic (including elements of IPT-A and CBT)? The evidence suggests that CBT and IPT-A are the treatments of choice for teens with depression. |
| 2. Have you received training in that therapy for adolescents with depression? Where and when? The therapist should have been trained in a clinical program (social work, nursing, psychology) that involved adolescents. |
| 3. Have you received clinical supervision in that therapy? Where? For how long? How many cases? Generally, therapists should be supervised for at least 3 to 4 cases before they are considered pro? cient. |
| 4. Are there specific tasks scheduled for each session? There should be for CBT, but not for IPT-A. |
| 5. Is the therapy time-limited? CBT and IPT-A are both time-limited. |
| 6. What are the goals of the therapy? The goals for both CBT and IPT-A should be the resolution of depressive symptoms. |
| Source: This list has been adapted by Amy Cheung, MD, from her contributions to the forthcoming book tentatively entitled Assessment and Treatment of Pediatric Depression: State of the Science; Best Practices (Editors: Peter S. Jensen, MD, Amy Cheung, MD, Ruth Stein, MD, and Rachel A. Zuckerbrot, MD), to be published by Civic Research Institute, Inc. All rights reserved. |
Choose an antidepressant, monitor with care
Studies have shown that up to 42% of family physicians in the United States had recently prescribed selective serotonin reuptake inhibitors (SSRIs) for more than 1 adolescent under the age of 18.17 When the diagnosis of MDD without comorbid conditions is clear and the patient and family are amenable, you may want to prescribe an SSRI.7,8
If you do, warn the patient and family that antidepressants can sometimes have adverse effects, including a switch from depressive to manic symptoms, signs of behavioral activation including agitation, hostility or restlessness, and suicidal ideation or behavior. If the patient can tolerate the medication without significant adverse effects, you need to prescribe the effective dose for at least 6 to 8 weeks to ensure an adequate trial.7
TABLE 3 provides some guidance for prescribing antidepressants for adolescents with depression.7 Among the antidepressants, only fluoxetine has been approved by the FDA for children and adolescents with depression. Fluoxetine is also the SSRI with the strongest evidence for efficacy in the adolescent population, as demonstrated in 4 RCTs.18 Two studies involving fluoxetine for depression have also shown efficacy in children as young as age 7 (range, 7-12 years).19
Effective dosages for antidepressants are lower for adolescents than for adults. Initiate medications at a low dose and increase in recommended increments every 2 weeks if no significant adverse effects emerge. With the exception of fluoxetine, SSRI medications must be discontinued slowly to minimize the risk of discontinuation effects.
Once treatment begins, you or a member of your staff will need to stay in touch with the patient and family to review their continued adherence to the treatment plan. An FDA black-box warning recommends observing for “clinical worsening, suicidality, and unusual changes in behavior” during initial visits or “at times of dose changes, either increases or decreases.” Develop a regular, frequent monitoring schedule with input from the teen and her (or his) parents to ensure compliance.7,20
Make sure follow-up appointments are not missed, using flags in patient records or in the clinic schedule. The duration of treatment for teens with depression is yet to be determined through clinical trials. Most guidelines suggest drug therapy be continued at the same dosage for 6 to 12 months after symptoms resolve. Guidelines for the treatment of adolescent depression can be found at www.gladpc.org.
Keeping teenagers on an antidepressant regimen can be challenging, given the side effects, the amount of time it takes before they experience an improvement, and the lengthy duration of treatment. Families that know what to expect and are getting continuing support from you and others are most likely to stay with treatment for the duration.
TABLE 3
A guide to prescribing antidepressants for adolescents
| MEDICATION | STARTING DOSE | EFFECTIVE DOSE | MAXIMUM DOSE | NOT TO BE USED WITH | COMMON ADVERSE EFFECTS |
|---|---|---|---|---|---|
| Citalopram | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia |
| Fluoxetine | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia, agitation, anxiety |
| Fluvoxamine | 25-50 mg/d | 150 mg | 300 mg | MAOIs and pimozide | Headache, GI upset, drowsiness |
| Paroxetine | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia |
| Sertraline | 25 mg/d | 100 mg | 200 mg | MAOIs | Headache, GI upset, insomnia |
| Escitalopram | 5 mg/d | 10-20 mg | 20 mg | MAOIs | Headache, GI upset, insomnia |
| MAOI, monoamine oxidase inhibitor. | |||||
| Source: This table has been adapted by Amy Cheung, MD, from her contributions to the forthcoming book tentatively entitled, Assessment and Treatment of Pediatric Depression: State of the Science; Best Practices (Editors: Peter S. Jensen, MD, Amy Cheung, MD, Ruth Stein, MD, and Rachel A. Zuckerbrot, MD), to be published by Civic Research Institute, Inc. All rights reserved. | |||||
What about Jane?
As the family’s physician, your initial management began with you educating Jane and her parents about mild depressive disorder and its likely course. You set up a series of weekly visits to monitor her symptoms and provide active support. You helped Jane find a peer support group and encouraged her to get back into gymnastics. You taught Jane and her family about the importance of keeping her safe while she is depressed, and they were cooperative about safety-proofing their home and setting up a plan to handle emergencies.
The US Preventive Services Task Force now recommends screening all adolescents (12-18 years of age) for major depressive disorder when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive behavioral therapy or interpersonal therapy), and follow-up. Previously, the Task Force concluded that the evidence was insufficient to recommend for or against the practice. For more on the Task Force’s recommendations, go to www.ahrq.gov/clinic/uspstf09/depression/chdeprrs.htm.
Jane’s depressive symptoms gradually ebbed, and she returned to her previous level of energy and social activity. You warned her and her family about the possibility that the disorder might recur, so they would be prepared.
Correspondence
Amy Cheung, MD, 33 Russell Street, 3rd Floor Tower, Toronto, Ontario, Canada MSS 2S1; [email protected]
Last month, we introduced you to 15-year-old Jane, a teenager whose once bubbly personality had in the last few months been reduced to a mood of quiet sadness. Her responses to your questions were muted, unenthusiastic. While Jane gets to school every day and can often shake off her down mood when she’s with friends, her responses to the Kutcher Adolescent Depression scale suggest that she’s struggling. You conclude that Jane is experiencing an episode of mild depressive disorder.
How would you manage Jane’s case? And what would you do if her symptoms worsened?
What’s the preference of patient and family?
Begin your initial management of a patient like Jane by considering the treatment preferences of the patient and her family, the severity and urgency of the case, the availability of mental health services, and your own comfort level with managing mental health disorders. A key conclusion of the GLAD-PC (GuideLines for Adolescent Depression in Primary Care) collaborative, described in Part 1 of this series, was that family physicians, alone or in collaboration with mental health professionals, are competent to manage adolescent depression.1 You may or may not choose to manage a patient like Jane yourself, but even if you refer, your initial management provides an essential bridge until the patient and her family are seen by mental health professionals.
Your initial management should include the following:
- education
- a treatment plan
- safety planning.
Step 1: Educate patient and parents
Help your patient to better understand what it means to have depression. Describe the signs and symptoms that led to the diagnosis of depression and review the natural history of the illness, including the chronic nature of the disorder and its tendency to recur. Explain, too, the impact that depression can have on different areas of functioning, such as school performance and peer relationships, and then review the treatment options. You or someone on your staff can provide this patient education initially, but it is also critical to connect the family to specific community resources for additional education, advocacy, and peer support.1
To do this effectively, you need to establish links with mental health resources in the community, including mental health service providers, as well as patients and families who have dealt with adolescent depression and are willing to serve as resources to other teens and their families. The GLAD-PC toolkit, available at www.gladpc.org, provides patient education handouts and links to reputable Web sites, advocacy organizations, and peer support groups. Additional online resources are listed in TABLE 1.
TABLE 1
Online resources
| SOURCE | WEBSITE |
|---|---|
| American Academy of Child and Adolescent Psychiatry | http://www.aacap.org/cs/root/facts_for_families/the_depressed_child |
| Families for Depression Awareness | www.familyaware.org |
| National Alliance on Mental Illness | http://www.nami.org/depression |
| National Institute of Mental Health | http://www.nimh.nih.gov/health/publications/depression |
Step 2: Work out a treatment plan
Developing a treatment plan that the patient and her parents can accept is critical. A plan that includes psychotherapy with a mental health provider, for example, won’t be acceptable to some patients and parents. They may refuse to participate, or their underlying mistrust may affect the outcome of treatment.2,3 Other families may reject any therapeutic approach that includes psychotropic drugs.
Expectations about the benefits of treatment influence outcomes significantly, so that, too, is a topic to explore as the treatment plan is worked out.3,4 Finally, the plan should include agreed-upon goals of treatment. For Jane, planned goals might include getting back into gymnastics or trying out for the school play.
Step 3: Plan for safety
Suicidality, including ideation, behaviors, or attempts, is common among adolescents with depression.5,6 In studies of completed suicide, more than 50% of the victims had a diagnosis of depression.5 To keep your patient safe, develop an emergency communication mechanism for handling increased suicidality or acute crises. If the patient’s risk is high, as shown by a clear plan or intent, immediate hospitalization may be necessary.
If you determine that inpatient treatment is not needed, you need to be sure that adequate adult supervision and support are available; that the teenager does not have access to potentially lethal medications, knives and other sharp objects, or firearms; and that both the patient and parents understand that drugs and alcohol weaken inhibitions. You need to set up a contingency plan with the family that includes checking in with you at reasonable intervals to assure the teen’s safety.5
Establishing a safety plan is especially important during the period of diagnosis and initial treatment, when suicide risk is highest.6 Confidentiality is the norm in adolescent medicine, but a patient like Jane must understand that you will breach confidentiality if that is necessary to keep her safe from harm.
GLAD-PC Recommendation II: Family physicians should develop a treatment plan with patients and families (SOR: C, expert opinion) and set specific treatment goals in key areas of functioning, including home, peer, and school settings (SOR: C, expert opinion).
GLAD-PC Recommendation III: The family physician should establish relevant links/collaboration with mental health resources in the community (SOR: C, expert opinion), which may include patients and families who have dealt with adolescent depression and are willing to serve as resources to other affected adolescents and their family members (SOR: C, expert opinion).
GLAD-PC Recommendation IV: Management must include the establishment of a safety plan, which includes restricting lethal means, engaging a concerned third party, and implementing an emergency communication mechanism should the patient deteriorate, become actively suicidal or dangerous to others, or experience an acute crisis associated with psychosocial stressors, especially during the period of initial treatment when safety concerns are highest (SOR: C, case control study and expert opinion).
GLAD-PC Recommendation V: After initial diagnosis in cases of mild depression, family physicians should consider a period of active support and monitoring before starting other evidence-based treatments (SOR: C, expert opinion).
GLAD-PC Recommendation VI: If a family physician identifies an adolescent with moderate or severe depression or complicating factors/conditions such as co-existing substance abuse or psychosis, consultation with a mental health specialist should be considered (SOR: C, expert opinion). Appropriate roles and responsibilities for ongoing management by the family physician and mental health provider should be communicated and agreed upon (SOR: C, expert opinion).
The patient and family should be consulted and approve of the roles negotiated by the family physician and mental health professionals (SOR: C, expert opinion).
GLAD-PC Recommendation VII: Family physicians should recommend scientifically tested and proven treatments (eg, psychotherapies such as cognitive behavioral therapy or interpersonal therapy, and/or antidepressant treatment such as SSRIs) whenever possible and appropriate to achieve the goals of the treatment plan (SOR: A, RCTs).
GLAD-PC Recommendation VIII: Family physicians should monitor for the emergence of adverse events during antidepressant treatment (SSRIs) (SOR: C, expert opinion).
Treatment options: When active support is best
Selecting the appropriate treatment modality for your patient hinges, of course, on the severity of the teen’s depression. (For more information on how to determine the severity of a depressive episode, see the first installment of this series, “Adolescent depression: Is your young patient suffering in silence?” J Fam Pract. 2009;58:187-192.)
When caring for a patient like Jane who is suffering from mild depression, consider providing active support and monitoring during 6 to 8 weekly or biweekly visits before recommending antidepressant medication or psychotherapy. This approach is also indicated when depressed patients or their parents refuse other treatments.7
Active support and monitoring may include education, frequent follow-up, a prescribed regimen of exercise and leisure activities, referral to a peer support group, and review of self-management goals. Other resources for active monitoring can be found in the GLAD-PC toolkit (available at www.gladpc.org). Evidence from randomized controlled trials (RCTs) shows that a sizable percentage of young people with depression respond to nondirective supportive therapy and regular symptom monitoring.7 Furthermore, emerging data from the research literature, expert opinion, and patient and family preferences indicate that active support and monitoring from family physicians is an important therapeutic strategy.7,8
Is therapy needed—and if so, what kind?
Adolescents with moderate or severe depression or patients with mild depression whose symptoms do not improve with active support and monitoring alone will likely require treatment with one of the evidenced-based treatments, such as psychotherapy or antidepressants. Referral to a mental health provider for further assessment or treatment may also be required, depending on the training of the physician.7,8 If so, you and the mental health provider will need to negotiate your roles and responsibilities for ongoing management, with the input and approval of the patient and family.
Both cognitive behavioral therapy (CBT) and interpersonal therapy (IPT) have been adapted to address major depressive disorder (MDD) in adolescents and have been shown to be effective in community as well as specialized settings.9-11
CBT is time-limited and delivered individually or by 1 or 2 clinicians working with a group. Clinicians follow a manual to guide each session.12 (A manual for therapists and a workbook for adolescents and parents can be downloaded from the Kaiser Permanente Center for Health Research Web site at http://www.kpchr.org/public/acwd/acwd.html.)
The focus of CBT is to change patients’ perception of themselves, their world, and others. CBT treats depression by identifying behavioral and cognitive patterns associated with depressive cycles. Examples of such patterns include the propensity to withdraw from pleasurable activities, or irritability that alienates family and friends just when the teenager needs them most. CBT helps teens identify these self-defeating patterns, encourages them to take part in activities they enjoy, helps develop or reactivate social skills important for maintaining positive social interactions, and helps teens to develop problem-solving strategies for resolving stressful situations.
CBT also aims to correct maladaptive beliefs associated with the patient’s depression. If, for instance, a patient believes she is worthless if she’s not accepted by the “popular” group at school, she is likely to become depressed and stay depressed as long as she is having difficulty connecting with her peers. CBT would help her examine that belief and learn to feel worthwhile even if she is not accepted by the “in” group. In general, CBT sessions are scheduled on a weekly basis for 12 to 16 weeks. In each session, the therapist and patient complete specific tasks and exercises that are provided in a CBT manual. There are also tasks for the patient to complete between sessions and review later with the therapist. CBT has been used in primary care with preliminary positive results.13,14 However, the results of a recent RCT conducted in psychiatric settings demonstrated superior efficacy of combination therapy (fluoxetine and CBT) vs CBT alone.15
IPT for adolescents (IPT-A) is like CBT in that it is time-limited and clinicians are guided by a manual.16 A course of therapy can last anywhere from 12 to 16 sessions with optional maintenance treatment. The theoretical basis for IPT-A is the observed negative impact of depressive symptoms on interpersonal relationships, and the effect poor relationships have in causing and perpetuating depression. In deciding whether a patient may be suitable for IPT-A, you need to find out whether she would be willing to share her experiences of ongoing relationship conflicts with a therapist or therapeutic group. The relationship difficulties IPT-A is designed to help with arise from 1 of 4 sources: grief, fights with peers or family members (interpersonal disputes), transitions from one social surround to another (role transition), and friendlessness (interpersonal deficits).
IPT-A focuses on grief only when someone of significance to the patient has died. Therapy for teens who quarrel frequently with peers or family members is focused on interpersonal disputes, and this is the most common focus in IPT-A. A focus on role transition is called for when the teen’s social world has undergone a drastic change, such as a when a teen has moved to a new school or broken up with a boyfriend. Finally, therapy for a teen with no significant relationships outside the immediate family focuses on interpersonal deficits. In these cases, the goal of therapy is to increase social contact and help the patient build relationships. If your preliminary assessment identifies your patient’s difficulties as rooted in 1 of these 4 areas, IPT-A may be for her.
Because few family physicians are trained in CBT or IPT-A, most psychotherapy will be provided by mental health professionals. What you can provide is familiarity with available community mental health resources. To get to know the therapists in your community, you may want to reach out to a few of them and ask them the questions in TABLE 2. You may also want to share this list with parents who want to find their own therapist.
TABLE 2
6 questions to ask prospective therapists
| 1. What type of therapy can you provide—cognitive behavioral therapy (CBT), interpersonal therapy for adolescents (IPT-A), psychodynamic psychotherapy, supportive therapy, counseling, or eclectic (including elements of IPT-A and CBT)? The evidence suggests that CBT and IPT-A are the treatments of choice for teens with depression. |
| 2. Have you received training in that therapy for adolescents with depression? Where and when? The therapist should have been trained in a clinical program (social work, nursing, psychology) that involved adolescents. |
| 3. Have you received clinical supervision in that therapy? Where? For how long? How many cases? Generally, therapists should be supervised for at least 3 to 4 cases before they are considered pro? cient. |
| 4. Are there specific tasks scheduled for each session? There should be for CBT, but not for IPT-A. |
| 5. Is the therapy time-limited? CBT and IPT-A are both time-limited. |
| 6. What are the goals of the therapy? The goals for both CBT and IPT-A should be the resolution of depressive symptoms. |
| Source: This list has been adapted by Amy Cheung, MD, from her contributions to the forthcoming book tentatively entitled Assessment and Treatment of Pediatric Depression: State of the Science; Best Practices (Editors: Peter S. Jensen, MD, Amy Cheung, MD, Ruth Stein, MD, and Rachel A. Zuckerbrot, MD), to be published by Civic Research Institute, Inc. All rights reserved. |
Choose an antidepressant, monitor with care
Studies have shown that up to 42% of family physicians in the United States had recently prescribed selective serotonin reuptake inhibitors (SSRIs) for more than 1 adolescent under the age of 18.17 When the diagnosis of MDD without comorbid conditions is clear and the patient and family are amenable, you may want to prescribe an SSRI.7,8
If you do, warn the patient and family that antidepressants can sometimes have adverse effects, including a switch from depressive to manic symptoms, signs of behavioral activation including agitation, hostility or restlessness, and suicidal ideation or behavior. If the patient can tolerate the medication without significant adverse effects, you need to prescribe the effective dose for at least 6 to 8 weeks to ensure an adequate trial.7
TABLE 3 provides some guidance for prescribing antidepressants for adolescents with depression.7 Among the antidepressants, only fluoxetine has been approved by the FDA for children and adolescents with depression. Fluoxetine is also the SSRI with the strongest evidence for efficacy in the adolescent population, as demonstrated in 4 RCTs.18 Two studies involving fluoxetine for depression have also shown efficacy in children as young as age 7 (range, 7-12 years).19
Effective dosages for antidepressants are lower for adolescents than for adults. Initiate medications at a low dose and increase in recommended increments every 2 weeks if no significant adverse effects emerge. With the exception of fluoxetine, SSRI medications must be discontinued slowly to minimize the risk of discontinuation effects.
Once treatment begins, you or a member of your staff will need to stay in touch with the patient and family to review their continued adherence to the treatment plan. An FDA black-box warning recommends observing for “clinical worsening, suicidality, and unusual changes in behavior” during initial visits or “at times of dose changes, either increases or decreases.” Develop a regular, frequent monitoring schedule with input from the teen and her (or his) parents to ensure compliance.7,20
Make sure follow-up appointments are not missed, using flags in patient records or in the clinic schedule. The duration of treatment for teens with depression is yet to be determined through clinical trials. Most guidelines suggest drug therapy be continued at the same dosage for 6 to 12 months after symptoms resolve. Guidelines for the treatment of adolescent depression can be found at www.gladpc.org.
Keeping teenagers on an antidepressant regimen can be challenging, given the side effects, the amount of time it takes before they experience an improvement, and the lengthy duration of treatment. Families that know what to expect and are getting continuing support from you and others are most likely to stay with treatment for the duration.
TABLE 3
A guide to prescribing antidepressants for adolescents
| MEDICATION | STARTING DOSE | EFFECTIVE DOSE | MAXIMUM DOSE | NOT TO BE USED WITH | COMMON ADVERSE EFFECTS |
|---|---|---|---|---|---|
| Citalopram | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia |
| Fluoxetine | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia, agitation, anxiety |
| Fluvoxamine | 25-50 mg/d | 150 mg | 300 mg | MAOIs and pimozide | Headache, GI upset, drowsiness |
| Paroxetine | 10 mg/d | 20 mg | 60 mg | MAOIs | Headache, GI upset, insomnia |
| Sertraline | 25 mg/d | 100 mg | 200 mg | MAOIs | Headache, GI upset, insomnia |
| Escitalopram | 5 mg/d | 10-20 mg | 20 mg | MAOIs | Headache, GI upset, insomnia |
| MAOI, monoamine oxidase inhibitor. | |||||
| Source: This table has been adapted by Amy Cheung, MD, from her contributions to the forthcoming book tentatively entitled, Assessment and Treatment of Pediatric Depression: State of the Science; Best Practices (Editors: Peter S. Jensen, MD, Amy Cheung, MD, Ruth Stein, MD, and Rachel A. Zuckerbrot, MD), to be published by Civic Research Institute, Inc. All rights reserved. | |||||
What about Jane?
As the family’s physician, your initial management began with you educating Jane and her parents about mild depressive disorder and its likely course. You set up a series of weekly visits to monitor her symptoms and provide active support. You helped Jane find a peer support group and encouraged her to get back into gymnastics. You taught Jane and her family about the importance of keeping her safe while she is depressed, and they were cooperative about safety-proofing their home and setting up a plan to handle emergencies.
The US Preventive Services Task Force now recommends screening all adolescents (12-18 years of age) for major depressive disorder when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive behavioral therapy or interpersonal therapy), and follow-up. Previously, the Task Force concluded that the evidence was insufficient to recommend for or against the practice. For more on the Task Force’s recommendations, go to www.ahrq.gov/clinic/uspstf09/depression/chdeprrs.htm.
Jane’s depressive symptoms gradually ebbed, and she returned to her previous level of energy and social activity. You warned her and her family about the possibility that the disorder might recur, so they would be prepared.
Correspondence
Amy Cheung, MD, 33 Russell Street, 3rd Floor Tower, Toronto, Ontario, Canada MSS 2S1; [email protected]
1. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care–GLAD PC – Part I. Pediatrics. 2007;120:e1299-e1312.
2. Richardson LP, Lewis CW, Casey-Goldstein M, et al. Pediatric primary care providers and adolescent depression. J Adolesc Health. 2007;40:433-439.
3. Myers SS, Phillips RS, Davis RB, et al. Patient expectations as predictors of outcome in patients with acute low back pain. J Gen Intern Med. 2008;23:1525-1497.
4. Aikens JE, Nease DE, Jr, Nau DP, et al. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med. 2005;3:23-30.
5. Brent DA, Perper JA, Moritz G, et al. Psychiatric risk factors for adolescent suicide: a case-control study. J Am Acad Child Adolesc Psychiatry. 1993;32:521-529.
6. American Academy of Child and Adolescent Psychiatry. Summary of the practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40:495-499.
7. Cheung A, Zuckerbrot RA, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care–GLAD PC – Part II. Pediatrics. 2007;120:e1313-e1326.
8. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Expert survey for the management of adolescent depression in primary care. Pediatrics. 2008;121:e101-e107.
9. Compton SN, March JS, Brent D, et al. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930-959.
10. Mufson L, Weissman MM, Moreau D, et al. Efficacy of interpersonal psychotherapy for depressed adolescents. Arch Gen Psychiatry. 1999;56:573-579.
11. Mufson L, Dorta KP, Wickramaratne P, et al. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Arch Genl Psychiatry. 2004;61:577-584.
12. Clarke GN, Rohde P, Lewinsohn PM, et al. Cognitive-behavioral treatment of adolescent depression: efficacy of acute group treatment and booster session. J Am Acad Child Adolesc Psychiatry. 1999;38:272-279.
13. Asarnow JR, Jaycox LH, Duan N, et al. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: a randomized controlled trial. JAMA. 2005;293:311-319.
14. Clarke G, Debar L, Lynch F, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. J Am Acad Child Adolesc Psychiatry. 2005;44:888-898.
15. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for adolescents with depression study (TADS) randomized controlled trial. JAMA. 2004;292:807-820.
16. Mufson L, Moreau D, Weissman M. Interpersonal Psychotherapy for Depressed Adolescents. New York: Guildford Press; 2004.
17. Olson AL, Kelleher KJ, Kemper KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1:91-98.
18. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696.
19. Mayes TL, Tao R, Rintelmann JW, et al. Do children and adolescents have differential response rates in placebo-controlled trials of fluoxetine? CNS Spectr. 2007;12:147-154.
20. Birmaher B, Brent D. And the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503-1526.
1. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care–GLAD PC – Part I. Pediatrics. 2007;120:e1299-e1312.
2. Richardson LP, Lewis CW, Casey-Goldstein M, et al. Pediatric primary care providers and adolescent depression. J Adolesc Health. 2007;40:433-439.
3. Myers SS, Phillips RS, Davis RB, et al. Patient expectations as predictors of outcome in patients with acute low back pain. J Gen Intern Med. 2008;23:1525-1497.
4. Aikens JE, Nease DE, Jr, Nau DP, et al. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med. 2005;3:23-30.
5. Brent DA, Perper JA, Moritz G, et al. Psychiatric risk factors for adolescent suicide: a case-control study. J Am Acad Child Adolesc Psychiatry. 1993;32:521-529.
6. American Academy of Child and Adolescent Psychiatry. Summary of the practice parameters for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40:495-499.
7. Cheung A, Zuckerbrot RA, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care–GLAD PC – Part II. Pediatrics. 2007;120:e1313-e1326.
8. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Expert survey for the management of adolescent depression in primary care. Pediatrics. 2008;121:e101-e107.
9. Compton SN, March JS, Brent D, et al. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930-959.
10. Mufson L, Weissman MM, Moreau D, et al. Efficacy of interpersonal psychotherapy for depressed adolescents. Arch Gen Psychiatry. 1999;56:573-579.
11. Mufson L, Dorta KP, Wickramaratne P, et al. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Arch Genl Psychiatry. 2004;61:577-584.
12. Clarke GN, Rohde P, Lewinsohn PM, et al. Cognitive-behavioral treatment of adolescent depression: efficacy of acute group treatment and booster session. J Am Acad Child Adolesc Psychiatry. 1999;38:272-279.
13. Asarnow JR, Jaycox LH, Duan N, et al. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: a randomized controlled trial. JAMA. 2005;293:311-319.
14. Clarke G, Debar L, Lynch F, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. J Am Acad Child Adolesc Psychiatry. 2005;44:888-898.
15. March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for adolescents with depression study (TADS) randomized controlled trial. JAMA. 2004;292:807-820.
16. Mufson L, Moreau D, Weissman M. Interpersonal Psychotherapy for Depressed Adolescents. New York: Guildford Press; 2004.
17. Olson AL, Kelleher KJ, Kemper KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1:91-98.
18. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696.
19. Mayes TL, Tao R, Rintelmann JW, et al. Do children and adolescents have differential response rates in placebo-controlled trials of fluoxetine? CNS Spectr. 2007;12:147-154.
20. Birmaher B, Brent D. And the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503-1526.
DMPA’s effect on bone mineral density: A particular concern for adolescents
- Discuss the potential for decreased bone mineral density in using depot-medroxyprogesterone acetate (DMPA) with any woman who is thinking of it as a means of contraception (C).
- Recommend to women that they take 1300 mg of calcium and 400 IU of vitamin D when using DMPA (C).
- Consider prescribing estrogen replacement if DMPA is going to be used for more than 2 years (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
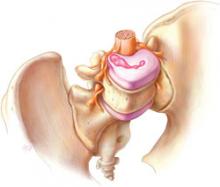
Among adolescent women who use contraception, the injectable progestin-only depot-medroxyprogesterone acetate (DMPA, Depo-Provera) is second in popularity only to oral contraceptive pills.1 A very real drawback with DMPA, however, is a resultant hypoestrogenic state that has been linked to lowered bone mineral density (BMD).
Although several studies have demonstrated a relationship between DMPA use and lower BMD among adults and adolescents (strength of recommendation [SOR]: B), many of them had small sample sizes and methodological flaws. Moreover, most studies have shown that BMD change is reversible after discontinuation of DMPA.
Experts recommend counseling young women about DMPA’s possible effects on bone. But they caution against limiting its use based on the insufficient research to date (SOR: C). Analysts estimate that the availability of DMPA has contributed significantly to decreased adolescent pregnancy rates in the United States over the last 10 years.2 This article reports on a systematic review of the literature concerning DMPA and BMD.
Reason for concern
A 1991 study by Cundy et al3 was the first to examine the relationship between DMPA and BMD and found that DMPA users had significantly lower BMD than nonusers. DMPA delivers high doses of progestin and inhibits ovulation in most women. Consequently, DMPA can decrease serum estradiol levels. Low serum estradiol levels have also been linked to lower BMD levels in women who are in menopause or who have eating disorders.
Adolescence is a time of bone building. The chief reason for interest in the association between DMPA and decreased BMD is the potential risk of future osteoporosis and osteoporotic fractures for women using DMPA during adolescence. A mature woman’s BMD at any given time is related to her peak bone mass and subsequent rate of decline. Ninety percent of peak bone mass (the highest level of BMD achieved during one’s lifetime) is determined by age 18 in women.4 Between the ages of 18 and 30, women gain the last 10% of their maximum bone density. After age 30, bone resorption outpaces bone formation and women start to lose bone slowly.5 This decline continues until menopause, when women experience a more rapid decline in BMD related to sudden withdrawal of estrogen.
Factors that affect peak BMD. Several factors influence the level of peak bone mass a woman will reach—genetics, race, hormonal milieu, and lifestyle factors.4,5 As for lifestyle, it’s been shown that both anorexia and the female athlete triad cause low estrogen levels, and the resultant loss of BMD may not be recovered.6,7
Pregnancy, too, is known to be a state of increased bone turnover and resorption,8,9 and pregnancy during adolescence may also negatively impact BMD. A small 2002 study compared teenagers who had been pregnant with age-matched controls who had not been pregnant, and found that hip bone density in the adolescent mothers was lower by approximately 10%.10
Use of bone-affecting medications by adolescents is worrisome because they are still building bone at a high rate.
What the literature tells us
Studies of adult women. Studies examining the relationship between DMPA use and BMD have yielded varying results ( TABLE 1 ). Most of them show that using DMPA over a course of 2 years decreases BMD by 5% to 10%. New users have the most significant decreases in BMD, suggesting the decline levels off after 2 years of use (SOR: B).11-13 However, most early studies were cross-sectional and small, and thus had limited power to determine causality. In addition, these trials were not randomized, and they may have suffered from bias because treatment groups were volunteers.
Three recent prospective studies12,14,15 found that bone density losses recover after discontinuation of DMPA. Kaunitz15 followed women for up to 2 years after DMPA discontinuation and found that BMD recovered almost completely (-0.2% at hip and -1.19% at lumbosacral [LS] spine at 2 years). However, only a small number of women were studied post-discontinuation for the full 2 years. Clark12 followed women for up to 18 months after discontinuation and found that those who had used DMPA still had significantly lower BMD (-4.7% at the hip and -2.9% at the spine).
These studies established that bone density decreases with the initiation of DMPA, but none of them addressed the key issue of whether BMD remains at lower levels long term (ie, decades) and thereby increases future fracture risk.
Studies of adolescents. Fewer studies have examined the relationship between DMPA use and BMD in adolescents ( TABLE 2 ). Most available studies have small sample sizes and methodological limitations (high dropout rates, different age criteria, and significant differences in the comparison groups). In this population, DMPA seems to cause a mild decrease in BMD. There are not enough data to evaluate BMD recovery after DMPA discontinuation. Therefore, it is hard to extrapolate the information about BMD in an adolescent to future fracture risk.
One study examined serum estradiol levels and BMD in 22 adolescents ages 15 to 19 years who were new users of DMPA.16 Only 6 participants were still using DMPA at 1 year, and 4 used it throughout the 2 years of the study. The trend over 2 years was toward decreasing BMD. Serum estradiol levels were low, but were not correlated with BMD.
Another related study measured bone biochemical markers in 3 groups: 53 adolescents ages 12 to 18 starting DMPA; 165 adolescents starting oral contraceptive pills; and 152 adolescent women not using hormonal contraception.17 There was no relationship between bone biochemical markers and BMD at either the LS spine or the femoral neck.
TABLE 1
DMPA’s effect on BMD in adult women: What the studies reveal
| AUTHOR (TYPE OF STUDY) | # OF PARTICIPANTS/ POPULATION DESCRIPTION | OUTCOME MEASURE | RESULTS | COMMENTS |
|---|---|---|---|---|
| Gbolade, 199824 (cross-sectional) | N=185 Ages 17-52 (mean 33) Using DMPA for 1-16 years | DEXA of LS spine and femoral neck | Z-score lower at LS spine (P<.001) but not at the femoral neck (P=.25) | No significant association between duration of DMPA use and Z-score |
| Ryan, 200225 (cross-sectional) | N=32 Ages 19-53 Using DMPA >2 years Low serum estradiol level or menopausal symptoms | DEXA of LS spine and femoral neck | Z-scores were lower at both femoral neck (-0.84; 95% confidence interval [CI], -1.17 to -0.52) and LS spine (-0.32; 95% CI, -0.62 to -0.02) | 18 women had osteopenia at LS spine 3 women had osteoporosis at LS spine |
| Petitti, 200026 (cross-sectional) | n=350 (DMPA) n=695 (control) Ages 30-34 Using DMPA ≥2 years Control group: women who never used hormonal contraception | SXA of wrist | BMD was lower for DMPA current users vs nonusers 0.465 vs 0.471 g/cm2 in midshaft ulna (P<.001) 0.369 vs 0.382 g/cm2 in distal radius (P<.001) | Large WHO-sponsored, multinational study Past users of DMPA had bone densities not significantly different from nonusers Large variations in BMD among sites |
| Wanichsetakul, 200227 (cross-sectional) | n=34 (DMPA) n=62 (comparison) Ages 30-34 Using DMPA ≥2 years Comparison groups of women on no steroid contraception in prior 6 months | DEXA of LS spine, distal radius, and femoral neck | BMD at femoral neck and distal radius was not different between DMPA users and controls (P=.335 and P=.398) DMPA users had lower BMD at LS spine (P=.007) | Study conducted in Thailand |
| Beksinska, 200528 (cross-sectional) | n=127 (DMPA) n=161 (comparison) Ages 40-49 Using DMPA ≥1 year | DEXA of radius and ulna | No significant difference in BMD at distal radius (P=.26) or ulna (P=.21) | Higher BMD was associated with higher BMI Higher FSH levels were associated with lower BMD |
| Tang, 200029 (cohort) | N=59 Ages 37-49 Using DMPA for a mean of 10 years | DEXA of LS spine and femoral neck Annual measurements for 3 years | Small annual decreases in BMD at LS spine (-0.44%), femoral neck (-0.4%), and Ward’s triangle (-1.05%) | Duration of DMPA use not related to BMD Decreases in BMD less than projected for age Study conducted in China |
| Scholes, 200213 (cohort) | n=183 (DMPA) n=258 (comparison) Ages 18-39 Comparison group not exposed to DMPA | DEXA of LS spine and proximal femur Measurements every 6 months for 4 years | Total hip and LS spine BMD were lower for DMPA users (P=.002 at LS spine; P<.005 for proximal femur) | New users lost bone faster than longer-term users Women who discontinued DMPA showed increasing BMD levels, which reached levels of nonusers after 30 months 33% dropout rate among both groups at 3 years, 44% of DMPA users discontinued use within first 6 months of the study |
| Cundy, 199411 (cohort) | n=36 (DMPA) n=18 (comparison) Ages 25-51 (mean 41-45) 14 women had used DMPA for ≥3 years and discontinued 22 women were long-term DMPA users Individuals in comparison group were never users of DMPA | DEXA of LS spine and femoral neck Measured twice in each woman | Group I (discontinuers) BMD change at LS spine 3.4% per year (1.6% to 5.2%) and at femoral neck 0.8% per year (-1.8% to 3.4%) Group II (long-term users) BMD change at LS spine -0.2% per year (-2.0% to 1.6%) and at femoral neck -1.1% per year (-2.6% to 0.4%) Group III (nonusers) BMD change at LS spine 0.3% per year (-2.2% to 2.8%) and at femoral neck -1.5% per year (-3.2% to 0.2%) | BMD in LS spine in both groups of DMPA users was 9% lower than control group at baseline |
| Berenson, 200130 (cohort) | n=33 (DMPA) n=59 (comparison) Ages 18-33 New users of DMPA Comparison group not using any hormonal contraception | DEXA at LS spine 2 measurements for each participant 12 months apart | Adjusted percent change in BMD for DMPA users was -2.7% (-4.44% to -1.05%) and in nonusers was -0.37% (-1.98% to 1.25%), P=.01 | 39% dropout rate among both groups |
| Merki-Feld, 200031 (cohort) | N=36 Ages 30-45 Using DMPA ≥6 months | Quantitative CT of radius Measured twice over 12 months | Trabecular bone mass increased 1.6% (P=.8) Cortical bone mass decreased 0.6% (P<.04) | Duration of DMPA use was not associated with BMD change |
| Clark, 200414 (cohort) | n=178 (DMPA) n=145 (comparison) Ages 18-35 New users of DMPA Comparison group not using hormonal contraception | DEXA of LS spine and total hip Measured every 3 months for 2 years | At 24 months, change in BMD in DMPA users was -5.8% (SE=0.096) at hip and -5.7% (SE=0.034) at LS spine Significant difference between DMPA group and comparison group (P=.001) | Dropout rate 22% in both groups over 2 years Duration of use predicted decrease in BMD Among DMPA users, increasing BMI was protective against BMD loss at hip |
| Kaunitz, 200615 (cohort) | n=248 (DMPA) n=360 (comparison) Ages 25-35 New users of DMPA Comparison group not using hormonal contraception | DEXA LS spine, total hip, femoral neck, and trochanter Measured at baseline and every 48 weeks for up to 5 years | Mean decrease in BMD in DMPA users was 5.16% (±3.6) at hip and 5.38% (±3.57) at LS spine At 96 weeks after discontinuation, change was -0.20% at hip and -1.19% at LS spine | Decreases in BMD were linearly associated with duration of use up to 5 years 17% of DMPA group and 33% of comparison group completed entire 5 years of study |
| Clark, 200612 (cohort) | n=178 (DMPA) n=145 (comparison) Ages 18-35 New DMPA users Comparison group not using hormonal contraception | DEXA total hip and LS spine Measured every 3 months for up to 4 years | Mean change in BMD in DMPA users was -7.7% (±0.11) at hip and -6.4% (±0.36) at LS spine DMPA users of 24-36 months had BMD of -4.7% (hip) and -2.9% (spine) compared with baseline 18 months after discontinuation | Most loss was noted first 2 years after initiation of DMPA Most users of DMPA up to 2 years returned to baseline BMD by 3 years 36% dropout rate in both groups after second year of study Only 45% of DMPA group completed 4 years of study |
| BMD, bone mineral density; BMI, body mass index; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; DMPA, depot- medroxyprogesterone acetate; FSH, follicle-stimulating hormone; LS, lumbosacral; SE, standard error; SXA, single-energy x-ray absorptiometry; WHO, World Health organization. | ||||
TABLE 2
DMPA’s effect on BMD in adolescent women: What the studies reveal
| AUTHOR (TYPE OF STUDY) | # OF PARTICIPANTS/ POPULATION DESCRIPTION | OUTCOME MEASURE | RESULTS | COMMENTS |
|---|---|---|---|---|
| Scholes, 200432 (cross-sectional) | n=81 (DMPA) n=93 (comparison) Ages 14-18 Current users of DMPA, range of 1-13 injections (mean 3) | DEXA proximal femur and LS spine | Neither total hip (P=.1) nor spine (P=.19) BMD was significantly lower in DMPA users | 17 non-DMPA users were taking OCPs DMPA users were more likely to be African American and to have a previous pregnancy |
| Beksinska, 200733 (cohort) | n=115 (DMPA) n=144 (comparison) Ages 15-19 New users of DMPA Comparison group not using hormonal contraception | DEXA of distal radius and ulna | No significant difference in BMD between groups (P=.88) | 51 DMPA users completed the study vs 91 nonusers of hormonal contraception Majority of cohort was African American |
| Cromer, 200434 (cohort) | n=53 (DMPA) n=152 (comparison) Ages 12-18 New users of DMPA Comparison group not using hormonal contraception | DEXA of femoral neck and LS spine Measured at baseline, 6 months, and 12 months | LS spine BMD decreased in DMPA group 1.4% and increased in control group 3.8% (P<.001); femoral neck BMD decreased in DMPA group 2.2% and increased in control group 2.3% (P<.001) | 45% dropout rate by 12 months in the DMPA group |
| Lara-Torre, 200435 (cohort) | n=58 (DMPA) n=19 (comparison) Ages 12-21 New DMPA users Comparison group ages 15-19 not using any hormonal contraception | DEXA of LS spine Measured at baseline and every 6 months for 2 years | DMPA group had significantly more BMD changes than control group at each check: -3.02% at 6 months (P=.014); -3.38% at 12 months (P=.001); -4.81% at 18 months (P<.001); -6.81% at 24 months (P=.01) | DMPA group was more likely to be African American DMPA group had dropout rates of 54% at 12 months and 64% at 24 months |
| Scholes, 200536 (cohort) | n=80 (DMPA) n=90 (comparison) Ages 14-18 Baseline users of DMPA (duration of use from 1 to 13 injections) | DEXA of hip, spine, and whole body Measured at baseline and every 6 months for 24-36 months | Significant BMD decreases in DMPA users at each check vs comparison group in hip and spine (P=.001), but not in whole-body BMD (P=.78) Most discontinuers had regained BMD back to baseline by 12 months | 18.9% of non-DMPA users were taking OCPs 61 participants discontinued DMPA during the study DMPA group more likely to smoke and to have been pregnant |
| BMD, bone mineral density; DEXA, dual-energy x-ray absorptiometry; DMPA, depot-medroxyprogesterone acetate; LS, lumbosacral; OCPs, oral contraceptive pills. | ||||
Can estrogen therapy counteract DMPA’s effect?
If decreased BMD in women taking DMPA is due to low estradiol levels, it is logical that a trial of estradiol supplementation would mitigate the negative effect. Indeed, a bone-protective effect of supplemental estrogen therapy has been found in studies of young women with amenorrhea secondary to the female athlete triad. Similarly, in postmenopausal women with low serum estradiol levels, supplemental estrogen therapy helps maintain BMD.18
Two randomized trials have evaluated the use of supplemental estrogen on the adverse effects of DMPA on bone.19,20 The trial by Cromer et al19 randomized 123 adolescent women ages 12 to 18 to receive either estrogen supplementation or placebo. They found that the participants in the estrogen group had BMD gains vs BMD losses among those in the placebo group over the 2-year period of the study (2.8% vs -1.8% at the LS spine, and 4.7% vs -5.1% at the femoral neck; P<.001 for both). The limitations to this study include a high dropout rate (53 participants had left by 24 months) and incomplete data collection due to early stoppage of the study.
Cundy et al20 studied 38 adult women who had been on DMPA for at least 2 years and had below-average LS spine BMD. Nineteen women were randomized to receive estrogen supplementation and underwent bone density tests every 6 months; 19 women were also in the comparison placebo group. In the estrogen supplementation group, there was significant attenuation of lowering BMD that increased throughout the trial. However, only 26 subjects completed the 2-year study.
Limit DMPA use to 2 years? Experts disagree
The FDA, in 2004, placed a black box warning on DMPA: “Women who use Depo-Provera Contraceptive Injection may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible. It is unknown if use of Depo-Provera Contraceptive Injection during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk of osteoporotic fracture later in life. Depo-Provera Contraceptive Injection should be used as a long-term birth control method (eg, longer than 2 years) only if other birth-control methods are inadequate.”21 In light of these FDA guidelines, many practitioners have started limiting patients’ use of DMPA to 2 years.
The Society of Adolescent Medicine has produced clinical guidelines for treating adolescents who do well on DMPA for contraception (SOR: C, expert opinion).22 The guidelines recommend, among other things, that physicians:
- continue prescribing DMPA to adolescent girls needing contraception, while providing adequate explanation of benefits and potential risks.
- consider ordering a dual-energy x-ray absorptiometry (DEXA) scan to evaluate a patient’s risk.
- keep in mind that duration of use need not be restricted to 2 years.
- recommend 1300 mg calcium plus 400 IU vitamin D and daily exercise to all adolescents receiving DMPA.
- consider estrogen supplementation in those girls with osteopenia (or those at high risk of osteopenia who have not had a DEXA scan) who are otherwise doing well on DMPA and have no contraindication to estrogen.
The World Health Organization similarly published recommendations stating that no restriction should be placed on the use of DMPA due to bone effects (SOR: C, expert opinion).23
Formulate a reasonable approach
As with any other potentially harmful medication, weigh the risks and benefits of DMPA for the individual patient. It is unclear whether BMD lost during DMPA use completely recovers or even what the time frame for that recovery is. Whether the potential risk for future fracture is increased is unknown, but it certainly is cause for concern. Discuss potential risks with any woman who wants to use DMPA for contraception. Routine calcium and vitamin D supplementation for women using DMPA may be helpful and is unlikely to be harmful.
A search of PubMed, the Cochrane database, and all references from primary reviewed articles was performed in 2007 using the terms depot-medroxyprogesterone acetate, bone mineral density, osteoporosis, osteopenia, injectable contraception, progestin-only contraception, Depo-Provera, and DMPA. Studies qualified for analysis if they contained data about bone density in women who had used some type of progestin-only injectable contraception. All types of studies were included. Excluded were studies that did not use BMD as an outcome measure or that re-analyzed data published elsewhere.
Bone mineral density is traditionally used as a surrogate measure of fracture risk in postmenopausal women. However, most of the women included in the reviewed studies were young and at low risk of fracture. The relationship between bone density in premenopausal women and fracture risk later in life is unclear. There are no available studies relating injectable progestin-only contraception with future osteoporotic fractures.
There is not enough evidence to recommend for or against routine screening of BMD in long-term users of DMPA. Research should evaluate the efficacy of estrogen supplementation in women on prolonged DMPA. Long-term studies could provide more information regarding BMD recovery over several years.
Correspondence
Sarina Schrager, MD, MS, Department of Family Medicine, University of Wisconsin, 777 South Mills Street, Madison, WI 53715; [email protected]
1. Piccinino LJ, Mosher WK. Trends in contraceptive use in the United States: 1982-1995. Fam Plann Perspect. 1998;30:4-10.
2. Donovan P. Falling teen pregnancy, birthrates. What’s behind the declines? The Guttmacher Report on Public Policy. 1998;1(5).-
3. Cundy T, Evans M, Roberts H. Bone density in women receiving depot medroxyprogesterone acetate for contraception. BMJ. 1991;303:13-16.
4. Soyka LA, Fairfield WP, Klibanski A. Hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85:3951-3963.
5. Tudor-locke C, McColl RS. Factors related to variation in premenopausal bone mineral status: a health promotion approach. Osteoporos Int. 2000;11:1-24.
6. Bachrach LK, Katzman DK, Litt IF, et al. Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 1991;72:602-606.
7. Miller KK, Lee EE, Lawson Ea, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931-2937.
8. Silva HG, Tortora RP, Farias ML. Increased bone turnover during the third trimester of pregnancy and decreased bone mineral density after parturition in adolescents as compared to age-matched control patients. Gynecol Endocrinol. 2005;21:174-179.
9. Ulrich U, Miller PB, Eyre DR, et al. Bone remodeling and bone mineral density during pregnancy. Arch Gynecol Obstet. 2003;268:309-316.
10. Lloyd T, Beck TJ, Lin HM, et al. Modifiable determinants of bone status in young women. Bone. 2002;30:416-421.
11. Cundy T, Cornish J, Evans MC, et al. Recovery of bone density in women who stop using medroxyprogesterone acetate. BMJ. 1994;308:247-248.
12. Clark MK, Sowers M, Levy B, et al. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86:1466-1474.
13. Scholes D, LaCroix A Z, Ichikawa LE, et al. Injectable hormone contraception and bone density: results from a prospective study [erratum appears in Epidemiology.2002;13:749]. Epidemiology. 2002;13:581-587.
14. Clark MK, Sowers MR, Nichols S, et al. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2004;82:1580-1586.
15. Kaunitz AM, Miller PD, Rice VM, et al. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception. 2006;74:90-99.
16. Busen NH, Britt RB, Rianon N. Bone mineral density in a cohort of adolescent women using depot medroxyprogesterone acetate for one to two years. Adolesc Health. 2003;32:257-259.
17. Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive [see comment]. J Pediatr Adolesc Gynecol. 2004;17:373-377.
18. Cumming DC. Exercise associated amenorrhea, low bone density, and estrogen replacement therapy. Arch Intern Med. 1996;156:2193-2195.
19. Cromer B, Lazebnik R, Rome E, et al. Double-blinded randomized controlled trial of estrogen supplementation in adolescent girls who receive depot medroxyprogesterone acetate for contraception. Am J Obstet. Gynecol. 2005;192:42-47.
20. Cundy T, Ames R, Horne A, et al. A randomized controlled trial of estrogen replacement therapy in long-term users of depot medroxyprogesterone acetate. J Clin Endocrinol Metab. 2003;88:78-81.
21. U.S. Food and Drug Administration. Black box warning added concerning long-term use of Depo-Provera Contraceptive Injection. 2004. Available at: http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01325.html. Accessed April 7, 2009.
22. Cromer BA, Scholes D, Berenson A, et al. Depot medroxyprogesterone acetate and bone mineral density in adolescents: the black box warning: a position paper of the Society for Adolescent Medicine. Adolesc Health. 2006;39:296-301.
23. d’Arcanques D. WHO statement on hormonal contraception and bone health. Contraception. 2006;73:443-444.
24. Gbolade B, Ellis S, Murby B, et al. Bone density in long term users of depot medroxyprogesterone acetate. Brit J Obstet Gynaecol. 1998;105:790-794.
25. Ryan PJ, Singh SP, Guillebaud J. Depot medroxyprogesterone and bone mineral density. J Fam Plann Reproduct Health Care. 2002;28:12-15.
26. Petitti DB, Piaggio G, Mehta S, et al. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. The WHO Study of Hormonal Contraception and Bone Health. Obstet Gynecol. 2000;95:736-744.
27. Wanichsetakul P, Kamudhamas A, Watanaruangkovit P, et al. Bone mineral density at various anatomic bone sites in women receiving combined oral contraceptives and depot-medroxyprogesterone acetate for contraception. Contraception. 2002;65:407-410.
28. Beksinska ME, Smit JA, Kleinschmidt I, et al. Bone mineral density in women aged 40-49 years using depot-medroxyprogesterone acetate, norethisterone enanthate or combined oral contraceptives for contraception. Contraception. 2005;71:170-175.
29. Tang OS, Tang G, YIP PS, et al. Further evaluation on long-term depot-medroxyprogesterone acetate use and bone mineral density: a longitudinal cohort study. Contraception. 2000;62:161-164.
30. Berenson AB, Radecki CM, Grady JJ, et al. A prospective, controlled study of the effects of hormonal contraception on bone mineral density. Obstet Gynecol. 2001;98:576-582.
31. Merki-Feld GS, Neff M, Keller PJ. A prospective study on the effects of depot medroxyprogesterone acetate on trabecular and cortical bone after attainment of peak bone mass. BJOG. 2000;107:863-869.
32. Scholes D, LaCroix AZ, Ichikawa LE, et al. The association between depot medroxyprogesterone acetate contraception and bone mineral density in adolescent women. Contraception. 2004;69:99-104.
33. Beksinska ME, Kleinschmidt I, Smit JA, et al. Bone mineral density in adolescents using norethisterone enanthate, depot-medroxyprogesterone acetate or combined oral contraceptives for contraception. Contraception. 2007;75:438-443.
34. Cromer BA, Stager M, Bonny A, et al. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls [see comment]. Adolesc Health. 2004;35:434-441.
35. Lara-Torre E, Edwards CP, Perlman S, et al. Bone mineral density in adolescent females using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2004;17:17-21.
36. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
- Discuss the potential for decreased bone mineral density in using depot-medroxyprogesterone acetate (DMPA) with any woman who is thinking of it as a means of contraception (C).
- Recommend to women that they take 1300 mg of calcium and 400 IU of vitamin D when using DMPA (C).
- Consider prescribing estrogen replacement if DMPA is going to be used for more than 2 years (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Among adolescent women who use contraception, the injectable progestin-only depot-medroxyprogesterone acetate (DMPA, Depo-Provera) is second in popularity only to oral contraceptive pills.1 A very real drawback with DMPA, however, is a resultant hypoestrogenic state that has been linked to lowered bone mineral density (BMD).
Although several studies have demonstrated a relationship between DMPA use and lower BMD among adults and adolescents (strength of recommendation [SOR]: B), many of them had small sample sizes and methodological flaws. Moreover, most studies have shown that BMD change is reversible after discontinuation of DMPA.
Experts recommend counseling young women about DMPA’s possible effects on bone. But they caution against limiting its use based on the insufficient research to date (SOR: C). Analysts estimate that the availability of DMPA has contributed significantly to decreased adolescent pregnancy rates in the United States over the last 10 years.2 This article reports on a systematic review of the literature concerning DMPA and BMD.
Reason for concern
A 1991 study by Cundy et al3 was the first to examine the relationship between DMPA and BMD and found that DMPA users had significantly lower BMD than nonusers. DMPA delivers high doses of progestin and inhibits ovulation in most women. Consequently, DMPA can decrease serum estradiol levels. Low serum estradiol levels have also been linked to lower BMD levels in women who are in menopause or who have eating disorders.
Adolescence is a time of bone building. The chief reason for interest in the association between DMPA and decreased BMD is the potential risk of future osteoporosis and osteoporotic fractures for women using DMPA during adolescence. A mature woman’s BMD at any given time is related to her peak bone mass and subsequent rate of decline. Ninety percent of peak bone mass (the highest level of BMD achieved during one’s lifetime) is determined by age 18 in women.4 Between the ages of 18 and 30, women gain the last 10% of their maximum bone density. After age 30, bone resorption outpaces bone formation and women start to lose bone slowly.5 This decline continues until menopause, when women experience a more rapid decline in BMD related to sudden withdrawal of estrogen.
Factors that affect peak BMD. Several factors influence the level of peak bone mass a woman will reach—genetics, race, hormonal milieu, and lifestyle factors.4,5 As for lifestyle, it’s been shown that both anorexia and the female athlete triad cause low estrogen levels, and the resultant loss of BMD may not be recovered.6,7
Pregnancy, too, is known to be a state of increased bone turnover and resorption,8,9 and pregnancy during adolescence may also negatively impact BMD. A small 2002 study compared teenagers who had been pregnant with age-matched controls who had not been pregnant, and found that hip bone density in the adolescent mothers was lower by approximately 10%.10
Use of bone-affecting medications by adolescents is worrisome because they are still building bone at a high rate.
What the literature tells us
Studies of adult women. Studies examining the relationship between DMPA use and BMD have yielded varying results ( TABLE 1 ). Most of them show that using DMPA over a course of 2 years decreases BMD by 5% to 10%. New users have the most significant decreases in BMD, suggesting the decline levels off after 2 years of use (SOR: B).11-13 However, most early studies were cross-sectional and small, and thus had limited power to determine causality. In addition, these trials were not randomized, and they may have suffered from bias because treatment groups were volunteers.
Three recent prospective studies12,14,15 found that bone density losses recover after discontinuation of DMPA. Kaunitz15 followed women for up to 2 years after DMPA discontinuation and found that BMD recovered almost completely (-0.2% at hip and -1.19% at lumbosacral [LS] spine at 2 years). However, only a small number of women were studied post-discontinuation for the full 2 years. Clark12 followed women for up to 18 months after discontinuation and found that those who had used DMPA still had significantly lower BMD (-4.7% at the hip and -2.9% at the spine).
These studies established that bone density decreases with the initiation of DMPA, but none of them addressed the key issue of whether BMD remains at lower levels long term (ie, decades) and thereby increases future fracture risk.
Studies of adolescents. Fewer studies have examined the relationship between DMPA use and BMD in adolescents ( TABLE 2 ). Most available studies have small sample sizes and methodological limitations (high dropout rates, different age criteria, and significant differences in the comparison groups). In this population, DMPA seems to cause a mild decrease in BMD. There are not enough data to evaluate BMD recovery after DMPA discontinuation. Therefore, it is hard to extrapolate the information about BMD in an adolescent to future fracture risk.
One study examined serum estradiol levels and BMD in 22 adolescents ages 15 to 19 years who were new users of DMPA.16 Only 6 participants were still using DMPA at 1 year, and 4 used it throughout the 2 years of the study. The trend over 2 years was toward decreasing BMD. Serum estradiol levels were low, but were not correlated with BMD.
Another related study measured bone biochemical markers in 3 groups: 53 adolescents ages 12 to 18 starting DMPA; 165 adolescents starting oral contraceptive pills; and 152 adolescent women not using hormonal contraception.17 There was no relationship between bone biochemical markers and BMD at either the LS spine or the femoral neck.
TABLE 1
DMPA’s effect on BMD in adult women: What the studies reveal
| AUTHOR (TYPE OF STUDY) | # OF PARTICIPANTS/ POPULATION DESCRIPTION | OUTCOME MEASURE | RESULTS | COMMENTS |
|---|---|---|---|---|
| Gbolade, 199824 (cross-sectional) | N=185 Ages 17-52 (mean 33) Using DMPA for 1-16 years | DEXA of LS spine and femoral neck | Z-score lower at LS spine (P<.001) but not at the femoral neck (P=.25) | No significant association between duration of DMPA use and Z-score |
| Ryan, 200225 (cross-sectional) | N=32 Ages 19-53 Using DMPA >2 years Low serum estradiol level or menopausal symptoms | DEXA of LS spine and femoral neck | Z-scores were lower at both femoral neck (-0.84; 95% confidence interval [CI], -1.17 to -0.52) and LS spine (-0.32; 95% CI, -0.62 to -0.02) | 18 women had osteopenia at LS spine 3 women had osteoporosis at LS spine |
| Petitti, 200026 (cross-sectional) | n=350 (DMPA) n=695 (control) Ages 30-34 Using DMPA ≥2 years Control group: women who never used hormonal contraception | SXA of wrist | BMD was lower for DMPA current users vs nonusers 0.465 vs 0.471 g/cm2 in midshaft ulna (P<.001) 0.369 vs 0.382 g/cm2 in distal radius (P<.001) | Large WHO-sponsored, multinational study Past users of DMPA had bone densities not significantly different from nonusers Large variations in BMD among sites |
| Wanichsetakul, 200227 (cross-sectional) | n=34 (DMPA) n=62 (comparison) Ages 30-34 Using DMPA ≥2 years Comparison groups of women on no steroid contraception in prior 6 months | DEXA of LS spine, distal radius, and femoral neck | BMD at femoral neck and distal radius was not different between DMPA users and controls (P=.335 and P=.398) DMPA users had lower BMD at LS spine (P=.007) | Study conducted in Thailand |
| Beksinska, 200528 (cross-sectional) | n=127 (DMPA) n=161 (comparison) Ages 40-49 Using DMPA ≥1 year | DEXA of radius and ulna | No significant difference in BMD at distal radius (P=.26) or ulna (P=.21) | Higher BMD was associated with higher BMI Higher FSH levels were associated with lower BMD |
| Tang, 200029 (cohort) | N=59 Ages 37-49 Using DMPA for a mean of 10 years | DEXA of LS spine and femoral neck Annual measurements for 3 years | Small annual decreases in BMD at LS spine (-0.44%), femoral neck (-0.4%), and Ward’s triangle (-1.05%) | Duration of DMPA use not related to BMD Decreases in BMD less than projected for age Study conducted in China |
| Scholes, 200213 (cohort) | n=183 (DMPA) n=258 (comparison) Ages 18-39 Comparison group not exposed to DMPA | DEXA of LS spine and proximal femur Measurements every 6 months for 4 years | Total hip and LS spine BMD were lower for DMPA users (P=.002 at LS spine; P<.005 for proximal femur) | New users lost bone faster than longer-term users Women who discontinued DMPA showed increasing BMD levels, which reached levels of nonusers after 30 months 33% dropout rate among both groups at 3 years, 44% of DMPA users discontinued use within first 6 months of the study |
| Cundy, 199411 (cohort) | n=36 (DMPA) n=18 (comparison) Ages 25-51 (mean 41-45) 14 women had used DMPA for ≥3 years and discontinued 22 women were long-term DMPA users Individuals in comparison group were never users of DMPA | DEXA of LS spine and femoral neck Measured twice in each woman | Group I (discontinuers) BMD change at LS spine 3.4% per year (1.6% to 5.2%) and at femoral neck 0.8% per year (-1.8% to 3.4%) Group II (long-term users) BMD change at LS spine -0.2% per year (-2.0% to 1.6%) and at femoral neck -1.1% per year (-2.6% to 0.4%) Group III (nonusers) BMD change at LS spine 0.3% per year (-2.2% to 2.8%) and at femoral neck -1.5% per year (-3.2% to 0.2%) | BMD in LS spine in both groups of DMPA users was 9% lower than control group at baseline |
| Berenson, 200130 (cohort) | n=33 (DMPA) n=59 (comparison) Ages 18-33 New users of DMPA Comparison group not using any hormonal contraception | DEXA at LS spine 2 measurements for each participant 12 months apart | Adjusted percent change in BMD for DMPA users was -2.7% (-4.44% to -1.05%) and in nonusers was -0.37% (-1.98% to 1.25%), P=.01 | 39% dropout rate among both groups |
| Merki-Feld, 200031 (cohort) | N=36 Ages 30-45 Using DMPA ≥6 months | Quantitative CT of radius Measured twice over 12 months | Trabecular bone mass increased 1.6% (P=.8) Cortical bone mass decreased 0.6% (P<.04) | Duration of DMPA use was not associated with BMD change |
| Clark, 200414 (cohort) | n=178 (DMPA) n=145 (comparison) Ages 18-35 New users of DMPA Comparison group not using hormonal contraception | DEXA of LS spine and total hip Measured every 3 months for 2 years | At 24 months, change in BMD in DMPA users was -5.8% (SE=0.096) at hip and -5.7% (SE=0.034) at LS spine Significant difference between DMPA group and comparison group (P=.001) | Dropout rate 22% in both groups over 2 years Duration of use predicted decrease in BMD Among DMPA users, increasing BMI was protective against BMD loss at hip |
| Kaunitz, 200615 (cohort) | n=248 (DMPA) n=360 (comparison) Ages 25-35 New users of DMPA Comparison group not using hormonal contraception | DEXA LS spine, total hip, femoral neck, and trochanter Measured at baseline and every 48 weeks for up to 5 years | Mean decrease in BMD in DMPA users was 5.16% (±3.6) at hip and 5.38% (±3.57) at LS spine At 96 weeks after discontinuation, change was -0.20% at hip and -1.19% at LS spine | Decreases in BMD were linearly associated with duration of use up to 5 years 17% of DMPA group and 33% of comparison group completed entire 5 years of study |
| Clark, 200612 (cohort) | n=178 (DMPA) n=145 (comparison) Ages 18-35 New DMPA users Comparison group not using hormonal contraception | DEXA total hip and LS spine Measured every 3 months for up to 4 years | Mean change in BMD in DMPA users was -7.7% (±0.11) at hip and -6.4% (±0.36) at LS spine DMPA users of 24-36 months had BMD of -4.7% (hip) and -2.9% (spine) compared with baseline 18 months after discontinuation | Most loss was noted first 2 years after initiation of DMPA Most users of DMPA up to 2 years returned to baseline BMD by 3 years 36% dropout rate in both groups after second year of study Only 45% of DMPA group completed 4 years of study |
| BMD, bone mineral density; BMI, body mass index; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; DMPA, depot- medroxyprogesterone acetate; FSH, follicle-stimulating hormone; LS, lumbosacral; SE, standard error; SXA, single-energy x-ray absorptiometry; WHO, World Health organization. | ||||
TABLE 2
DMPA’s effect on BMD in adolescent women: What the studies reveal
| AUTHOR (TYPE OF STUDY) | # OF PARTICIPANTS/ POPULATION DESCRIPTION | OUTCOME MEASURE | RESULTS | COMMENTS |
|---|---|---|---|---|
| Scholes, 200432 (cross-sectional) | n=81 (DMPA) n=93 (comparison) Ages 14-18 Current users of DMPA, range of 1-13 injections (mean 3) | DEXA proximal femur and LS spine | Neither total hip (P=.1) nor spine (P=.19) BMD was significantly lower in DMPA users | 17 non-DMPA users were taking OCPs DMPA users were more likely to be African American and to have a previous pregnancy |
| Beksinska, 200733 (cohort) | n=115 (DMPA) n=144 (comparison) Ages 15-19 New users of DMPA Comparison group not using hormonal contraception | DEXA of distal radius and ulna | No significant difference in BMD between groups (P=.88) | 51 DMPA users completed the study vs 91 nonusers of hormonal contraception Majority of cohort was African American |
| Cromer, 200434 (cohort) | n=53 (DMPA) n=152 (comparison) Ages 12-18 New users of DMPA Comparison group not using hormonal contraception | DEXA of femoral neck and LS spine Measured at baseline, 6 months, and 12 months | LS spine BMD decreased in DMPA group 1.4% and increased in control group 3.8% (P<.001); femoral neck BMD decreased in DMPA group 2.2% and increased in control group 2.3% (P<.001) | 45% dropout rate by 12 months in the DMPA group |
| Lara-Torre, 200435 (cohort) | n=58 (DMPA) n=19 (comparison) Ages 12-21 New DMPA users Comparison group ages 15-19 not using any hormonal contraception | DEXA of LS spine Measured at baseline and every 6 months for 2 years | DMPA group had significantly more BMD changes than control group at each check: -3.02% at 6 months (P=.014); -3.38% at 12 months (P=.001); -4.81% at 18 months (P<.001); -6.81% at 24 months (P=.01) | DMPA group was more likely to be African American DMPA group had dropout rates of 54% at 12 months and 64% at 24 months |
| Scholes, 200536 (cohort) | n=80 (DMPA) n=90 (comparison) Ages 14-18 Baseline users of DMPA (duration of use from 1 to 13 injections) | DEXA of hip, spine, and whole body Measured at baseline and every 6 months for 24-36 months | Significant BMD decreases in DMPA users at each check vs comparison group in hip and spine (P=.001), but not in whole-body BMD (P=.78) Most discontinuers had regained BMD back to baseline by 12 months | 18.9% of non-DMPA users were taking OCPs 61 participants discontinued DMPA during the study DMPA group more likely to smoke and to have been pregnant |
| BMD, bone mineral density; DEXA, dual-energy x-ray absorptiometry; DMPA, depot-medroxyprogesterone acetate; LS, lumbosacral; OCPs, oral contraceptive pills. | ||||
Can estrogen therapy counteract DMPA’s effect?
If decreased BMD in women taking DMPA is due to low estradiol levels, it is logical that a trial of estradiol supplementation would mitigate the negative effect. Indeed, a bone-protective effect of supplemental estrogen therapy has been found in studies of young women with amenorrhea secondary to the female athlete triad. Similarly, in postmenopausal women with low serum estradiol levels, supplemental estrogen therapy helps maintain BMD.18
Two randomized trials have evaluated the use of supplemental estrogen on the adverse effects of DMPA on bone.19,20 The trial by Cromer et al19 randomized 123 adolescent women ages 12 to 18 to receive either estrogen supplementation or placebo. They found that the participants in the estrogen group had BMD gains vs BMD losses among those in the placebo group over the 2-year period of the study (2.8% vs -1.8% at the LS spine, and 4.7% vs -5.1% at the femoral neck; P<.001 for both). The limitations to this study include a high dropout rate (53 participants had left by 24 months) and incomplete data collection due to early stoppage of the study.
Cundy et al20 studied 38 adult women who had been on DMPA for at least 2 years and had below-average LS spine BMD. Nineteen women were randomized to receive estrogen supplementation and underwent bone density tests every 6 months; 19 women were also in the comparison placebo group. In the estrogen supplementation group, there was significant attenuation of lowering BMD that increased throughout the trial. However, only 26 subjects completed the 2-year study.
Limit DMPA use to 2 years? Experts disagree
The FDA, in 2004, placed a black box warning on DMPA: “Women who use Depo-Provera Contraceptive Injection may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible. It is unknown if use of Depo-Provera Contraceptive Injection during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk of osteoporotic fracture later in life. Depo-Provera Contraceptive Injection should be used as a long-term birth control method (eg, longer than 2 years) only if other birth-control methods are inadequate.”21 In light of these FDA guidelines, many practitioners have started limiting patients’ use of DMPA to 2 years.
The Society of Adolescent Medicine has produced clinical guidelines for treating adolescents who do well on DMPA for contraception (SOR: C, expert opinion).22 The guidelines recommend, among other things, that physicians:
- continue prescribing DMPA to adolescent girls needing contraception, while providing adequate explanation of benefits and potential risks.
- consider ordering a dual-energy x-ray absorptiometry (DEXA) scan to evaluate a patient’s risk.
- keep in mind that duration of use need not be restricted to 2 years.
- recommend 1300 mg calcium plus 400 IU vitamin D and daily exercise to all adolescents receiving DMPA.
- consider estrogen supplementation in those girls with osteopenia (or those at high risk of osteopenia who have not had a DEXA scan) who are otherwise doing well on DMPA and have no contraindication to estrogen.
The World Health Organization similarly published recommendations stating that no restriction should be placed on the use of DMPA due to bone effects (SOR: C, expert opinion).23
Formulate a reasonable approach
As with any other potentially harmful medication, weigh the risks and benefits of DMPA for the individual patient. It is unclear whether BMD lost during DMPA use completely recovers or even what the time frame for that recovery is. Whether the potential risk for future fracture is increased is unknown, but it certainly is cause for concern. Discuss potential risks with any woman who wants to use DMPA for contraception. Routine calcium and vitamin D supplementation for women using DMPA may be helpful and is unlikely to be harmful.
A search of PubMed, the Cochrane database, and all references from primary reviewed articles was performed in 2007 using the terms depot-medroxyprogesterone acetate, bone mineral density, osteoporosis, osteopenia, injectable contraception, progestin-only contraception, Depo-Provera, and DMPA. Studies qualified for analysis if they contained data about bone density in women who had used some type of progestin-only injectable contraception. All types of studies were included. Excluded were studies that did not use BMD as an outcome measure or that re-analyzed data published elsewhere.
Bone mineral density is traditionally used as a surrogate measure of fracture risk in postmenopausal women. However, most of the women included in the reviewed studies were young and at low risk of fracture. The relationship between bone density in premenopausal women and fracture risk later in life is unclear. There are no available studies relating injectable progestin-only contraception with future osteoporotic fractures.
There is not enough evidence to recommend for or against routine screening of BMD in long-term users of DMPA. Research should evaluate the efficacy of estrogen supplementation in women on prolonged DMPA. Long-term studies could provide more information regarding BMD recovery over several years.
Correspondence
Sarina Schrager, MD, MS, Department of Family Medicine, University of Wisconsin, 777 South Mills Street, Madison, WI 53715; [email protected]
- Discuss the potential for decreased bone mineral density in using depot-medroxyprogesterone acetate (DMPA) with any woman who is thinking of it as a means of contraception (C).
- Recommend to women that they take 1300 mg of calcium and 400 IU of vitamin D when using DMPA (C).
- Consider prescribing estrogen replacement if DMPA is going to be used for more than 2 years (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Among adolescent women who use contraception, the injectable progestin-only depot-medroxyprogesterone acetate (DMPA, Depo-Provera) is second in popularity only to oral contraceptive pills.1 A very real drawback with DMPA, however, is a resultant hypoestrogenic state that has been linked to lowered bone mineral density (BMD).
Although several studies have demonstrated a relationship between DMPA use and lower BMD among adults and adolescents (strength of recommendation [SOR]: B), many of them had small sample sizes and methodological flaws. Moreover, most studies have shown that BMD change is reversible after discontinuation of DMPA.
Experts recommend counseling young women about DMPA’s possible effects on bone. But they caution against limiting its use based on the insufficient research to date (SOR: C). Analysts estimate that the availability of DMPA has contributed significantly to decreased adolescent pregnancy rates in the United States over the last 10 years.2 This article reports on a systematic review of the literature concerning DMPA and BMD.
Reason for concern
A 1991 study by Cundy et al3 was the first to examine the relationship between DMPA and BMD and found that DMPA users had significantly lower BMD than nonusers. DMPA delivers high doses of progestin and inhibits ovulation in most women. Consequently, DMPA can decrease serum estradiol levels. Low serum estradiol levels have also been linked to lower BMD levels in women who are in menopause or who have eating disorders.
Adolescence is a time of bone building. The chief reason for interest in the association between DMPA and decreased BMD is the potential risk of future osteoporosis and osteoporotic fractures for women using DMPA during adolescence. A mature woman’s BMD at any given time is related to her peak bone mass and subsequent rate of decline. Ninety percent of peak bone mass (the highest level of BMD achieved during one’s lifetime) is determined by age 18 in women.4 Between the ages of 18 and 30, women gain the last 10% of their maximum bone density. After age 30, bone resorption outpaces bone formation and women start to lose bone slowly.5 This decline continues until menopause, when women experience a more rapid decline in BMD related to sudden withdrawal of estrogen.
Factors that affect peak BMD. Several factors influence the level of peak bone mass a woman will reach—genetics, race, hormonal milieu, and lifestyle factors.4,5 As for lifestyle, it’s been shown that both anorexia and the female athlete triad cause low estrogen levels, and the resultant loss of BMD may not be recovered.6,7
Pregnancy, too, is known to be a state of increased bone turnover and resorption,8,9 and pregnancy during adolescence may also negatively impact BMD. A small 2002 study compared teenagers who had been pregnant with age-matched controls who had not been pregnant, and found that hip bone density in the adolescent mothers was lower by approximately 10%.10
Use of bone-affecting medications by adolescents is worrisome because they are still building bone at a high rate.
What the literature tells us
Studies of adult women. Studies examining the relationship between DMPA use and BMD have yielded varying results ( TABLE 1 ). Most of them show that using DMPA over a course of 2 years decreases BMD by 5% to 10%. New users have the most significant decreases in BMD, suggesting the decline levels off after 2 years of use (SOR: B).11-13 However, most early studies were cross-sectional and small, and thus had limited power to determine causality. In addition, these trials were not randomized, and they may have suffered from bias because treatment groups were volunteers.
Three recent prospective studies12,14,15 found that bone density losses recover after discontinuation of DMPA. Kaunitz15 followed women for up to 2 years after DMPA discontinuation and found that BMD recovered almost completely (-0.2% at hip and -1.19% at lumbosacral [LS] spine at 2 years). However, only a small number of women were studied post-discontinuation for the full 2 years. Clark12 followed women for up to 18 months after discontinuation and found that those who had used DMPA still had significantly lower BMD (-4.7% at the hip and -2.9% at the spine).
These studies established that bone density decreases with the initiation of DMPA, but none of them addressed the key issue of whether BMD remains at lower levels long term (ie, decades) and thereby increases future fracture risk.
Studies of adolescents. Fewer studies have examined the relationship between DMPA use and BMD in adolescents ( TABLE 2 ). Most available studies have small sample sizes and methodological limitations (high dropout rates, different age criteria, and significant differences in the comparison groups). In this population, DMPA seems to cause a mild decrease in BMD. There are not enough data to evaluate BMD recovery after DMPA discontinuation. Therefore, it is hard to extrapolate the information about BMD in an adolescent to future fracture risk.
One study examined serum estradiol levels and BMD in 22 adolescents ages 15 to 19 years who were new users of DMPA.16 Only 6 participants were still using DMPA at 1 year, and 4 used it throughout the 2 years of the study. The trend over 2 years was toward decreasing BMD. Serum estradiol levels were low, but were not correlated with BMD.
Another related study measured bone biochemical markers in 3 groups: 53 adolescents ages 12 to 18 starting DMPA; 165 adolescents starting oral contraceptive pills; and 152 adolescent women not using hormonal contraception.17 There was no relationship between bone biochemical markers and BMD at either the LS spine or the femoral neck.
TABLE 1
DMPA’s effect on BMD in adult women: What the studies reveal
| AUTHOR (TYPE OF STUDY) | # OF PARTICIPANTS/ POPULATION DESCRIPTION | OUTCOME MEASURE | RESULTS | COMMENTS |
|---|---|---|---|---|
| Gbolade, 199824 (cross-sectional) | N=185 Ages 17-52 (mean 33) Using DMPA for 1-16 years | DEXA of LS spine and femoral neck | Z-score lower at LS spine (P<.001) but not at the femoral neck (P=.25) | No significant association between duration of DMPA use and Z-score |
| Ryan, 200225 (cross-sectional) | N=32 Ages 19-53 Using DMPA >2 years Low serum estradiol level or menopausal symptoms | DEXA of LS spine and femoral neck | Z-scores were lower at both femoral neck (-0.84; 95% confidence interval [CI], -1.17 to -0.52) and LS spine (-0.32; 95% CI, -0.62 to -0.02) | 18 women had osteopenia at LS spine 3 women had osteoporosis at LS spine |
| Petitti, 200026 (cross-sectional) | n=350 (DMPA) n=695 (control) Ages 30-34 Using DMPA ≥2 years Control group: women who never used hormonal contraception | SXA of wrist | BMD was lower for DMPA current users vs nonusers 0.465 vs 0.471 g/cm2 in midshaft ulna (P<.001) 0.369 vs 0.382 g/cm2 in distal radius (P<.001) | Large WHO-sponsored, multinational study Past users of DMPA had bone densities not significantly different from nonusers Large variations in BMD among sites |
| Wanichsetakul, 200227 (cross-sectional) | n=34 (DMPA) n=62 (comparison) Ages 30-34 Using DMPA ≥2 years Comparison groups of women on no steroid contraception in prior 6 months | DEXA of LS spine, distal radius, and femoral neck | BMD at femoral neck and distal radius was not different between DMPA users and controls (P=.335 and P=.398) DMPA users had lower BMD at LS spine (P=.007) | Study conducted in Thailand |
| Beksinska, 200528 (cross-sectional) | n=127 (DMPA) n=161 (comparison) Ages 40-49 Using DMPA ≥1 year | DEXA of radius and ulna | No significant difference in BMD at distal radius (P=.26) or ulna (P=.21) | Higher BMD was associated with higher BMI Higher FSH levels were associated with lower BMD |
| Tang, 200029 (cohort) | N=59 Ages 37-49 Using DMPA for a mean of 10 years | DEXA of LS spine and femoral neck Annual measurements for 3 years | Small annual decreases in BMD at LS spine (-0.44%), femoral neck (-0.4%), and Ward’s triangle (-1.05%) | Duration of DMPA use not related to BMD Decreases in BMD less than projected for age Study conducted in China |
| Scholes, 200213 (cohort) | n=183 (DMPA) n=258 (comparison) Ages 18-39 Comparison group not exposed to DMPA | DEXA of LS spine and proximal femur Measurements every 6 months for 4 years | Total hip and LS spine BMD were lower for DMPA users (P=.002 at LS spine; P<.005 for proximal femur) | New users lost bone faster than longer-term users Women who discontinued DMPA showed increasing BMD levels, which reached levels of nonusers after 30 months 33% dropout rate among both groups at 3 years, 44% of DMPA users discontinued use within first 6 months of the study |
| Cundy, 199411 (cohort) | n=36 (DMPA) n=18 (comparison) Ages 25-51 (mean 41-45) 14 women had used DMPA for ≥3 years and discontinued 22 women were long-term DMPA users Individuals in comparison group were never users of DMPA | DEXA of LS spine and femoral neck Measured twice in each woman | Group I (discontinuers) BMD change at LS spine 3.4% per year (1.6% to 5.2%) and at femoral neck 0.8% per year (-1.8% to 3.4%) Group II (long-term users) BMD change at LS spine -0.2% per year (-2.0% to 1.6%) and at femoral neck -1.1% per year (-2.6% to 0.4%) Group III (nonusers) BMD change at LS spine 0.3% per year (-2.2% to 2.8%) and at femoral neck -1.5% per year (-3.2% to 0.2%) | BMD in LS spine in both groups of DMPA users was 9% lower than control group at baseline |
| Berenson, 200130 (cohort) | n=33 (DMPA) n=59 (comparison) Ages 18-33 New users of DMPA Comparison group not using any hormonal contraception | DEXA at LS spine 2 measurements for each participant 12 months apart | Adjusted percent change in BMD for DMPA users was -2.7% (-4.44% to -1.05%) and in nonusers was -0.37% (-1.98% to 1.25%), P=.01 | 39% dropout rate among both groups |
| Merki-Feld, 200031 (cohort) | N=36 Ages 30-45 Using DMPA ≥6 months | Quantitative CT of radius Measured twice over 12 months | Trabecular bone mass increased 1.6% (P=.8) Cortical bone mass decreased 0.6% (P<.04) | Duration of DMPA use was not associated with BMD change |
| Clark, 200414 (cohort) | n=178 (DMPA) n=145 (comparison) Ages 18-35 New users of DMPA Comparison group not using hormonal contraception | DEXA of LS spine and total hip Measured every 3 months for 2 years | At 24 months, change in BMD in DMPA users was -5.8% (SE=0.096) at hip and -5.7% (SE=0.034) at LS spine Significant difference between DMPA group and comparison group (P=.001) | Dropout rate 22% in both groups over 2 years Duration of use predicted decrease in BMD Among DMPA users, increasing BMI was protective against BMD loss at hip |
| Kaunitz, 200615 (cohort) | n=248 (DMPA) n=360 (comparison) Ages 25-35 New users of DMPA Comparison group not using hormonal contraception | DEXA LS spine, total hip, femoral neck, and trochanter Measured at baseline and every 48 weeks for up to 5 years | Mean decrease in BMD in DMPA users was 5.16% (±3.6) at hip and 5.38% (±3.57) at LS spine At 96 weeks after discontinuation, change was -0.20% at hip and -1.19% at LS spine | Decreases in BMD were linearly associated with duration of use up to 5 years 17% of DMPA group and 33% of comparison group completed entire 5 years of study |
| Clark, 200612 (cohort) | n=178 (DMPA) n=145 (comparison) Ages 18-35 New DMPA users Comparison group not using hormonal contraception | DEXA total hip and LS spine Measured every 3 months for up to 4 years | Mean change in BMD in DMPA users was -7.7% (±0.11) at hip and -6.4% (±0.36) at LS spine DMPA users of 24-36 months had BMD of -4.7% (hip) and -2.9% (spine) compared with baseline 18 months after discontinuation | Most loss was noted first 2 years after initiation of DMPA Most users of DMPA up to 2 years returned to baseline BMD by 3 years 36% dropout rate in both groups after second year of study Only 45% of DMPA group completed 4 years of study |
| BMD, bone mineral density; BMI, body mass index; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; DMPA, depot- medroxyprogesterone acetate; FSH, follicle-stimulating hormone; LS, lumbosacral; SE, standard error; SXA, single-energy x-ray absorptiometry; WHO, World Health organization. | ||||
TABLE 2
DMPA’s effect on BMD in adolescent women: What the studies reveal
| AUTHOR (TYPE OF STUDY) | # OF PARTICIPANTS/ POPULATION DESCRIPTION | OUTCOME MEASURE | RESULTS | COMMENTS |
|---|---|---|---|---|
| Scholes, 200432 (cross-sectional) | n=81 (DMPA) n=93 (comparison) Ages 14-18 Current users of DMPA, range of 1-13 injections (mean 3) | DEXA proximal femur and LS spine | Neither total hip (P=.1) nor spine (P=.19) BMD was significantly lower in DMPA users | 17 non-DMPA users were taking OCPs DMPA users were more likely to be African American and to have a previous pregnancy |
| Beksinska, 200733 (cohort) | n=115 (DMPA) n=144 (comparison) Ages 15-19 New users of DMPA Comparison group not using hormonal contraception | DEXA of distal radius and ulna | No significant difference in BMD between groups (P=.88) | 51 DMPA users completed the study vs 91 nonusers of hormonal contraception Majority of cohort was African American |
| Cromer, 200434 (cohort) | n=53 (DMPA) n=152 (comparison) Ages 12-18 New users of DMPA Comparison group not using hormonal contraception | DEXA of femoral neck and LS spine Measured at baseline, 6 months, and 12 months | LS spine BMD decreased in DMPA group 1.4% and increased in control group 3.8% (P<.001); femoral neck BMD decreased in DMPA group 2.2% and increased in control group 2.3% (P<.001) | 45% dropout rate by 12 months in the DMPA group |
| Lara-Torre, 200435 (cohort) | n=58 (DMPA) n=19 (comparison) Ages 12-21 New DMPA users Comparison group ages 15-19 not using any hormonal contraception | DEXA of LS spine Measured at baseline and every 6 months for 2 years | DMPA group had significantly more BMD changes than control group at each check: -3.02% at 6 months (P=.014); -3.38% at 12 months (P=.001); -4.81% at 18 months (P<.001); -6.81% at 24 months (P=.01) | DMPA group was more likely to be African American DMPA group had dropout rates of 54% at 12 months and 64% at 24 months |
| Scholes, 200536 (cohort) | n=80 (DMPA) n=90 (comparison) Ages 14-18 Baseline users of DMPA (duration of use from 1 to 13 injections) | DEXA of hip, spine, and whole body Measured at baseline and every 6 months for 24-36 months | Significant BMD decreases in DMPA users at each check vs comparison group in hip and spine (P=.001), but not in whole-body BMD (P=.78) Most discontinuers had regained BMD back to baseline by 12 months | 18.9% of non-DMPA users were taking OCPs 61 participants discontinued DMPA during the study DMPA group more likely to smoke and to have been pregnant |
| BMD, bone mineral density; DEXA, dual-energy x-ray absorptiometry; DMPA, depot-medroxyprogesterone acetate; LS, lumbosacral; OCPs, oral contraceptive pills. | ||||
Can estrogen therapy counteract DMPA’s effect?
If decreased BMD in women taking DMPA is due to low estradiol levels, it is logical that a trial of estradiol supplementation would mitigate the negative effect. Indeed, a bone-protective effect of supplemental estrogen therapy has been found in studies of young women with amenorrhea secondary to the female athlete triad. Similarly, in postmenopausal women with low serum estradiol levels, supplemental estrogen therapy helps maintain BMD.18
Two randomized trials have evaluated the use of supplemental estrogen on the adverse effects of DMPA on bone.19,20 The trial by Cromer et al19 randomized 123 adolescent women ages 12 to 18 to receive either estrogen supplementation or placebo. They found that the participants in the estrogen group had BMD gains vs BMD losses among those in the placebo group over the 2-year period of the study (2.8% vs -1.8% at the LS spine, and 4.7% vs -5.1% at the femoral neck; P<.001 for both). The limitations to this study include a high dropout rate (53 participants had left by 24 months) and incomplete data collection due to early stoppage of the study.
Cundy et al20 studied 38 adult women who had been on DMPA for at least 2 years and had below-average LS spine BMD. Nineteen women were randomized to receive estrogen supplementation and underwent bone density tests every 6 months; 19 women were also in the comparison placebo group. In the estrogen supplementation group, there was significant attenuation of lowering BMD that increased throughout the trial. However, only 26 subjects completed the 2-year study.
Limit DMPA use to 2 years? Experts disagree
The FDA, in 2004, placed a black box warning on DMPA: “Women who use Depo-Provera Contraceptive Injection may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible. It is unknown if use of Depo-Provera Contraceptive Injection during adolescence or early adulthood, a critical period of bone accretion, will reduce peak bone mass and increase the risk of osteoporotic fracture later in life. Depo-Provera Contraceptive Injection should be used as a long-term birth control method (eg, longer than 2 years) only if other birth-control methods are inadequate.”21 In light of these FDA guidelines, many practitioners have started limiting patients’ use of DMPA to 2 years.
The Society of Adolescent Medicine has produced clinical guidelines for treating adolescents who do well on DMPA for contraception (SOR: C, expert opinion).22 The guidelines recommend, among other things, that physicians:
- continue prescribing DMPA to adolescent girls needing contraception, while providing adequate explanation of benefits and potential risks.
- consider ordering a dual-energy x-ray absorptiometry (DEXA) scan to evaluate a patient’s risk.
- keep in mind that duration of use need not be restricted to 2 years.
- recommend 1300 mg calcium plus 400 IU vitamin D and daily exercise to all adolescents receiving DMPA.
- consider estrogen supplementation in those girls with osteopenia (or those at high risk of osteopenia who have not had a DEXA scan) who are otherwise doing well on DMPA and have no contraindication to estrogen.
The World Health Organization similarly published recommendations stating that no restriction should be placed on the use of DMPA due to bone effects (SOR: C, expert opinion).23
Formulate a reasonable approach
As with any other potentially harmful medication, weigh the risks and benefits of DMPA for the individual patient. It is unclear whether BMD lost during DMPA use completely recovers or even what the time frame for that recovery is. Whether the potential risk for future fracture is increased is unknown, but it certainly is cause for concern. Discuss potential risks with any woman who wants to use DMPA for contraception. Routine calcium and vitamin D supplementation for women using DMPA may be helpful and is unlikely to be harmful.
A search of PubMed, the Cochrane database, and all references from primary reviewed articles was performed in 2007 using the terms depot-medroxyprogesterone acetate, bone mineral density, osteoporosis, osteopenia, injectable contraception, progestin-only contraception, Depo-Provera, and DMPA. Studies qualified for analysis if they contained data about bone density in women who had used some type of progestin-only injectable contraception. All types of studies were included. Excluded were studies that did not use BMD as an outcome measure or that re-analyzed data published elsewhere.
Bone mineral density is traditionally used as a surrogate measure of fracture risk in postmenopausal women. However, most of the women included in the reviewed studies were young and at low risk of fracture. The relationship between bone density in premenopausal women and fracture risk later in life is unclear. There are no available studies relating injectable progestin-only contraception with future osteoporotic fractures.
There is not enough evidence to recommend for or against routine screening of BMD in long-term users of DMPA. Research should evaluate the efficacy of estrogen supplementation in women on prolonged DMPA. Long-term studies could provide more information regarding BMD recovery over several years.
Correspondence
Sarina Schrager, MD, MS, Department of Family Medicine, University of Wisconsin, 777 South Mills Street, Madison, WI 53715; [email protected]
1. Piccinino LJ, Mosher WK. Trends in contraceptive use in the United States: 1982-1995. Fam Plann Perspect. 1998;30:4-10.
2. Donovan P. Falling teen pregnancy, birthrates. What’s behind the declines? The Guttmacher Report on Public Policy. 1998;1(5).-
3. Cundy T, Evans M, Roberts H. Bone density in women receiving depot medroxyprogesterone acetate for contraception. BMJ. 1991;303:13-16.
4. Soyka LA, Fairfield WP, Klibanski A. Hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85:3951-3963.
5. Tudor-locke C, McColl RS. Factors related to variation in premenopausal bone mineral status: a health promotion approach. Osteoporos Int. 2000;11:1-24.
6. Bachrach LK, Katzman DK, Litt IF, et al. Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 1991;72:602-606.
7. Miller KK, Lee EE, Lawson Ea, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931-2937.
8. Silva HG, Tortora RP, Farias ML. Increased bone turnover during the third trimester of pregnancy and decreased bone mineral density after parturition in adolescents as compared to age-matched control patients. Gynecol Endocrinol. 2005;21:174-179.
9. Ulrich U, Miller PB, Eyre DR, et al. Bone remodeling and bone mineral density during pregnancy. Arch Gynecol Obstet. 2003;268:309-316.
10. Lloyd T, Beck TJ, Lin HM, et al. Modifiable determinants of bone status in young women. Bone. 2002;30:416-421.
11. Cundy T, Cornish J, Evans MC, et al. Recovery of bone density in women who stop using medroxyprogesterone acetate. BMJ. 1994;308:247-248.
12. Clark MK, Sowers M, Levy B, et al. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86:1466-1474.
13. Scholes D, LaCroix A Z, Ichikawa LE, et al. Injectable hormone contraception and bone density: results from a prospective study [erratum appears in Epidemiology.2002;13:749]. Epidemiology. 2002;13:581-587.
14. Clark MK, Sowers MR, Nichols S, et al. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2004;82:1580-1586.
15. Kaunitz AM, Miller PD, Rice VM, et al. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception. 2006;74:90-99.
16. Busen NH, Britt RB, Rianon N. Bone mineral density in a cohort of adolescent women using depot medroxyprogesterone acetate for one to two years. Adolesc Health. 2003;32:257-259.
17. Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive [see comment]. J Pediatr Adolesc Gynecol. 2004;17:373-377.
18. Cumming DC. Exercise associated amenorrhea, low bone density, and estrogen replacement therapy. Arch Intern Med. 1996;156:2193-2195.
19. Cromer B, Lazebnik R, Rome E, et al. Double-blinded randomized controlled trial of estrogen supplementation in adolescent girls who receive depot medroxyprogesterone acetate for contraception. Am J Obstet. Gynecol. 2005;192:42-47.
20. Cundy T, Ames R, Horne A, et al. A randomized controlled trial of estrogen replacement therapy in long-term users of depot medroxyprogesterone acetate. J Clin Endocrinol Metab. 2003;88:78-81.
21. U.S. Food and Drug Administration. Black box warning added concerning long-term use of Depo-Provera Contraceptive Injection. 2004. Available at: http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01325.html. Accessed April 7, 2009.
22. Cromer BA, Scholes D, Berenson A, et al. Depot medroxyprogesterone acetate and bone mineral density in adolescents: the black box warning: a position paper of the Society for Adolescent Medicine. Adolesc Health. 2006;39:296-301.
23. d’Arcanques D. WHO statement on hormonal contraception and bone health. Contraception. 2006;73:443-444.
24. Gbolade B, Ellis S, Murby B, et al. Bone density in long term users of depot medroxyprogesterone acetate. Brit J Obstet Gynaecol. 1998;105:790-794.
25. Ryan PJ, Singh SP, Guillebaud J. Depot medroxyprogesterone and bone mineral density. J Fam Plann Reproduct Health Care. 2002;28:12-15.
26. Petitti DB, Piaggio G, Mehta S, et al. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. The WHO Study of Hormonal Contraception and Bone Health. Obstet Gynecol. 2000;95:736-744.
27. Wanichsetakul P, Kamudhamas A, Watanaruangkovit P, et al. Bone mineral density at various anatomic bone sites in women receiving combined oral contraceptives and depot-medroxyprogesterone acetate for contraception. Contraception. 2002;65:407-410.
28. Beksinska ME, Smit JA, Kleinschmidt I, et al. Bone mineral density in women aged 40-49 years using depot-medroxyprogesterone acetate, norethisterone enanthate or combined oral contraceptives for contraception. Contraception. 2005;71:170-175.
29. Tang OS, Tang G, YIP PS, et al. Further evaluation on long-term depot-medroxyprogesterone acetate use and bone mineral density: a longitudinal cohort study. Contraception. 2000;62:161-164.
30. Berenson AB, Radecki CM, Grady JJ, et al. A prospective, controlled study of the effects of hormonal contraception on bone mineral density. Obstet Gynecol. 2001;98:576-582.
31. Merki-Feld GS, Neff M, Keller PJ. A prospective study on the effects of depot medroxyprogesterone acetate on trabecular and cortical bone after attainment of peak bone mass. BJOG. 2000;107:863-869.
32. Scholes D, LaCroix AZ, Ichikawa LE, et al. The association between depot medroxyprogesterone acetate contraception and bone mineral density in adolescent women. Contraception. 2004;69:99-104.
33. Beksinska ME, Kleinschmidt I, Smit JA, et al. Bone mineral density in adolescents using norethisterone enanthate, depot-medroxyprogesterone acetate or combined oral contraceptives for contraception. Contraception. 2007;75:438-443.
34. Cromer BA, Stager M, Bonny A, et al. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls [see comment]. Adolesc Health. 2004;35:434-441.
35. Lara-Torre E, Edwards CP, Perlman S, et al. Bone mineral density in adolescent females using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2004;17:17-21.
36. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
1. Piccinino LJ, Mosher WK. Trends in contraceptive use in the United States: 1982-1995. Fam Plann Perspect. 1998;30:4-10.
2. Donovan P. Falling teen pregnancy, birthrates. What’s behind the declines? The Guttmacher Report on Public Policy. 1998;1(5).-
3. Cundy T, Evans M, Roberts H. Bone density in women receiving depot medroxyprogesterone acetate for contraception. BMJ. 1991;303:13-16.
4. Soyka LA, Fairfield WP, Klibanski A. Hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85:3951-3963.
5. Tudor-locke C, McColl RS. Factors related to variation in premenopausal bone mineral status: a health promotion approach. Osteoporos Int. 2000;11:1-24.
6. Bachrach LK, Katzman DK, Litt IF, et al. Recovery from osteopenia in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 1991;72:602-606.
7. Miller KK, Lee EE, Lawson Ea, et al. Determinants of skeletal loss and recovery in anorexia nervosa. J Clin Endocrinol Metab. 2006;91:2931-2937.
8. Silva HG, Tortora RP, Farias ML. Increased bone turnover during the third trimester of pregnancy and decreased bone mineral density after parturition in adolescents as compared to age-matched control patients. Gynecol Endocrinol. 2005;21:174-179.
9. Ulrich U, Miller PB, Eyre DR, et al. Bone remodeling and bone mineral density during pregnancy. Arch Gynecol Obstet. 2003;268:309-316.
10. Lloyd T, Beck TJ, Lin HM, et al. Modifiable determinants of bone status in young women. Bone. 2002;30:416-421.
11. Cundy T, Cornish J, Evans MC, et al. Recovery of bone density in women who stop using medroxyprogesterone acetate. BMJ. 1994;308:247-248.
12. Clark MK, Sowers M, Levy B, et al. Bone mineral density loss and recovery during 48 months in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2006;86:1466-1474.
13. Scholes D, LaCroix A Z, Ichikawa LE, et al. Injectable hormone contraception and bone density: results from a prospective study [erratum appears in Epidemiology.2002;13:749]. Epidemiology. 2002;13:581-587.
14. Clark MK, Sowers MR, Nichols S, et al. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertil Steril. 2004;82:1580-1586.
15. Kaunitz AM, Miller PD, Rice VM, et al. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception. 2006;74:90-99.
16. Busen NH, Britt RB, Rianon N. Bone mineral density in a cohort of adolescent women using depot medroxyprogesterone acetate for one to two years. Adolesc Health. 2003;32:257-259.
17. Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive [see comment]. J Pediatr Adolesc Gynecol. 2004;17:373-377.
18. Cumming DC. Exercise associated amenorrhea, low bone density, and estrogen replacement therapy. Arch Intern Med. 1996;156:2193-2195.
19. Cromer B, Lazebnik R, Rome E, et al. Double-blinded randomized controlled trial of estrogen supplementation in adolescent girls who receive depot medroxyprogesterone acetate for contraception. Am J Obstet. Gynecol. 2005;192:42-47.
20. Cundy T, Ames R, Horne A, et al. A randomized controlled trial of estrogen replacement therapy in long-term users of depot medroxyprogesterone acetate. J Clin Endocrinol Metab. 2003;88:78-81.
21. U.S. Food and Drug Administration. Black box warning added concerning long-term use of Depo-Provera Contraceptive Injection. 2004. Available at: http://www.fda.gov/bbs/topics/ANSWERS/2004/ANS01325.html. Accessed April 7, 2009.
22. Cromer BA, Scholes D, Berenson A, et al. Depot medroxyprogesterone acetate and bone mineral density in adolescents: the black box warning: a position paper of the Society for Adolescent Medicine. Adolesc Health. 2006;39:296-301.
23. d’Arcanques D. WHO statement on hormonal contraception and bone health. Contraception. 2006;73:443-444.
24. Gbolade B, Ellis S, Murby B, et al. Bone density in long term users of depot medroxyprogesterone acetate. Brit J Obstet Gynaecol. 1998;105:790-794.
25. Ryan PJ, Singh SP, Guillebaud J. Depot medroxyprogesterone and bone mineral density. J Fam Plann Reproduct Health Care. 2002;28:12-15.
26. Petitti DB, Piaggio G, Mehta S, et al. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. The WHO Study of Hormonal Contraception and Bone Health. Obstet Gynecol. 2000;95:736-744.
27. Wanichsetakul P, Kamudhamas A, Watanaruangkovit P, et al. Bone mineral density at various anatomic bone sites in women receiving combined oral contraceptives and depot-medroxyprogesterone acetate for contraception. Contraception. 2002;65:407-410.
28. Beksinska ME, Smit JA, Kleinschmidt I, et al. Bone mineral density in women aged 40-49 years using depot-medroxyprogesterone acetate, norethisterone enanthate or combined oral contraceptives for contraception. Contraception. 2005;71:170-175.
29. Tang OS, Tang G, YIP PS, et al. Further evaluation on long-term depot-medroxyprogesterone acetate use and bone mineral density: a longitudinal cohort study. Contraception. 2000;62:161-164.
30. Berenson AB, Radecki CM, Grady JJ, et al. A prospective, controlled study of the effects of hormonal contraception on bone mineral density. Obstet Gynecol. 2001;98:576-582.
31. Merki-Feld GS, Neff M, Keller PJ. A prospective study on the effects of depot medroxyprogesterone acetate on trabecular and cortical bone after attainment of peak bone mass. BJOG. 2000;107:863-869.
32. Scholes D, LaCroix AZ, Ichikawa LE, et al. The association between depot medroxyprogesterone acetate contraception and bone mineral density in adolescent women. Contraception. 2004;69:99-104.
33. Beksinska ME, Kleinschmidt I, Smit JA, et al. Bone mineral density in adolescents using norethisterone enanthate, depot-medroxyprogesterone acetate or combined oral contraceptives for contraception. Contraception. 2007;75:438-443.
34. Cromer BA, Stager M, Bonny A, et al. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls [see comment]. Adolesc Health. 2004;35:434-441.
35. Lara-Torre E, Edwards CP, Perlman S, et al. Bone mineral density in adolescent females using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2004;17:17-21.
36. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
Diabetes: Rethinking risk and the Dx that fits
- Routinely screen adult patients with a sustained blood pressure >135/80 mm Hg for type 2 diabetes (SOR: B).
- Closely monitor pregnant women with 1 or more elevated glucose test results; although a diagnosis of gestational diabetes mellitus (GDM) requires 2 or more abnormal values, even 1 may be associated with a higher risk of adverse outcomes (SOR: C).
- Include latent autoimmune diabetes in adults (LADA), a progressive form of type 1 with a slower onset, in the differential diagnosis for symptomatic patients who don’t fit the classic patterns for type 1 or type 2 diabetes (SOR: B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
The youngest Americans—those born in the year 2000 or thereafter—have more than a 1 in 3 lifetime risk of developing diabetes, according to the Centers for Disease Control and Prevention.1 That estimate, coupled with the fact that more than 2 out of 3 adults and 1 in 6 children between the ages of 2 and 19 years are overweight,2 would seem to indicate a need for widespread diabetes screening. But limited health care resources, a lack of evidence that mass screening improves outcomes, and differences among leading medical associations about whom and when to screen argue against it.
At the same time, widespread obesity is making the presentation of hyperglycemia more complex and the forms of diabetes harder to classify. Many cases don’t follow the classic patterns, in which type 1 (formerly called juvenile diabetes) virtually always emerges in childhood and type 2 (previously known as adult-onset diabetes) is strictly an adult disease. Our evolving understanding of diabetes has led researchers to focus on prediabetes (defined as impaired fasting glucose, impaired glucose tolerance, or both) and latent autoimmune diabetes in adults (LADA), a recently reported type 1 variant that some have labeled type 1.5.3
In the face of growing complexity, the US Preventive Services Task Force (USPSTF) last year upgraded its recommendation for screening for type 2 diabetes, and researchers have developed new risk calculation tools. We’ve taken a look at the changing clinical landscape and sorted through the latest evidence to help you make sense of the latest risk and diagnostic developments in diabetes care.
Screening for type 2: A look at guidelines
Type 2 diabetes accounts for approximately 90% of the cases you’ll see.1,4,5 The American Diabetes Association (ADA) calls for routine screening, starting at 45 years of age and continuing every 3 years thereafter in the absence of risk. But for those who are overweight or obese and have 1 or more additional risk factors, screening is recommended at any age.5 In addition to a body mass index (BMI) ≥25, risks include physical inactivity, a first-degree relative with type 2 diabetes, blood pressure >135/80 mm Hg (or controlled with an antihypertensive), high-density lipoproteins (HDL) <35 mg/dL, triglycerides >250 mg/dL, polycystic ovary syndrome, impaired glucose tolerance or impaired fasting glucose, and acanthosis nigricans, a pigmented thickening of the skin folds of the neck (TABLE).6,7 Patients with the metabolic syndrome—abdominal obesity (defined as a waist circumference of >40” in men and >35” in women) and ≥2 of the following: raised triglyceride levels, elevated blood pressure, elevated fasting plasma glucose, and reduced HDL cholesterol—are at especially high risk of both cardiovascular disease and type 2 diabetes.8
The ADA screening recommendations, however, are not based on prospective outcome studies, nor are they widely followed. Until recently, the USPSTF only recommended screening adults with hypertension and hyperlipidemia.
In 2008, after an assessment of new findings and research updates, the USPSTF revised its recommendation: The task force now calls for screening asymptomatic adults with sustained blood pressure >135/80 mm Hg—regardless of lipid profile.7 For patients with diabetes and hypertension, the USPSTF concluded, evidence shows that early intervention—including lowering blood pressure below conventional targets—can prevent long-term adverse outcomes of diabetes and reduce the risk of cardiovascular events.
Although mass screening remains controversial, regular assessment of risk factors and targeting individuals with established risk is clearly indicated (PATIENT HANDOUT). The importance of early detection was highlighted by the United Kingdom Prospective Diabetes Study, in which approximately half of the patients with newly diagnosed type 2 diabetes already had evidence of complications.9
TABLE
Type 1, type 2, and gestational diabetes: Diagnostic clues
| TYPE 1 DIABETES | TYPE 2 DIABETES | GESTATIONAL DIABETES MELLITUS (GDM) | |
|---|---|---|---|
| Risk factors/characteristics | Patient/family history of autoimmune disease 1st-degree relative with type 1 diabetes Normal weight with symptoms of hyperglycemia | BMI ≥25 Physical inactivity 1st-degree relative with type 2 diabetes High-risk ethnic group (African American, Hispanic, Native American, Asian American, Pacific Islander) History of GDM and/or delivery of an LGA infant BP >135/80 mm Hg or being treated for HTN Polycystic ovary syndrome IGT or IFG Acanthosis nigricans | BMI ≥30 History of GDM and/or delivery of an LGA infant (or poor outcome) 1st-degree relative with type 2 diabetes High-risk ethnic group (African American, Hispanic, Native American, Asian American, Pacific Islander) Glycosuria Age >25 years Polycystic ovary syndrome IGT |
| Laboratory tests/positive results | Specific antibodies to islet cell, insulin, and/or GAD* Tyrosine phosphatase-like auto antigen IA-2 (marker of autoimmune islet cell disease) C-peptide (low or absent); if in normal range, may indicate early disease and partial β-cell activity | FPG >126 mg/dL Random plasma glucose >200 mg/dL (test repeated next day) 2-hr 75-g OGTT >200 mg/dL HDL <35 mg/dL TG >250 mg/dL C-peptide (normal or elevated; may be low initially due to glucose toxicity) | Fasting: ≥95 mg/dL 1-hr OGTT: ≥180 mg/dL 2-hr OGTT: ≥155 mg/dL 3-hr OGTT: ≥140 mg/dL |
| BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; FPG, fasting plasma glucose; GAD, glutamic acid decarboxylase; HDL, high-density lipoproteins; HTN, hypertension; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LGA, large for gestational age; OGTT, oral glucose tolerance test; TG, triglycerides. | |||
| *GAD65 is most specific. | |||
Eating well, maintaining your weight, and engaging in physical activity are essential to good health. If you have risk factors for diabetes, diet and exercise are important steps you can take to help keep the disease at bay.
Making changes to your diet and increasing the amount of exercise you engage in need not be a daunting task. It helps to remember that it’s not necessary to take giant steps. You can improve your health and help prevent diabetes with a series of small changes. For best results, keep each goal small, manageable, and as specific as possible.
Eating. Do you eat fast food frequently, or snack on ice cream or potato chips when you watch TV at night? Pick a “bad habit” that is of particular concern and try to “turn it around.” You might, for instance, promise yourself that:
For the next 4 weeks, I will replace my unhealthy evening snacks with fresh fruit, a small bowl of cereal, or (insert another healthy snack here).
Getting active. Have you stopped working out? Are you concerned that working out will require a big time commitment? Think again. Start small and promise yourself that:
For the next 3 weeks, I will take a 20-minute walk 3 mornings a week.
Each time you set a goal, monitor your progress. When you succeed, give yourself a reward—it can be something as simple as a long bath or a trip to the movies—and vow to continue that lifestyle change and to add another. If you aren’t successful, think about why and revise your goal. If you find you’re too busy getting the kids off to school to walk in the morning, for example, change your schedule and start going out during your lunch break. Or, if it’s too cold or rainy, find a nearby mall where you can walk (or a treadmill at a local gym) instead. It also helps to get a step counter, or pedometer. The American Diabetes Association (ADA) recommends taking 10,000 steps per day.
For additional ideas, visit the ADA Web site (www.diabetes.org) and click on Fitness. Or call our office at __________ and make an appointment to come in and discuss additional lifestyle changes—small and large—that you can make with our help.
Validated risk calculators can boost detection rates
In an attempt to improve detection rates of type 2 diabetes and prediabetes, researchers in both the United States and the United Kingdom recently developed easy-to-use risk calculation tools. The Diabetes Risk Calculator (available at http://www.diabetes.org/food-nutrition-lifestyle/lifestyle-prevention/risk-test.jsp), published in 2008, was validated with findings from the Third National Health and Nutrition Survey.10 The calculator uses answers to questions about age, waist circumference, history of gestational diabetes mellitus (GDM), height, race/ethnicity, hypertension, family history, and exercise to determine whether an individual is at high risk for undetected diabetes. The tool has a low positive predictive value (14%), but a negative predictive value >99%.10
The QDScore Diabetes Risk Calculator (www.qdscore.org), another new tool, is designed to estimate an individual’s 10-year risk of developing type 2 diabetes.11 The program, which calculates risk based on answers to questions about family history of diabetes, patient history of cardiovascular disease, smoking, treatment for hypertension, BMI, ethnicity, and steroid use, was validated with data collected from 2.5 million patients in practices throughout England and Wales. The screening tool showed a high degree of discrimination in reflecting differences in disease prevalence related to ethnic and socioeconomic risk factors.11
Pinning down a type 2 (or prediabetes) diagnosis
The ADA, American Association of Clinical Endocrinologists (AACE), USPSTF, and World Health Organization/International Diabetes Federation agree on the diagnostic criteria for type 2 diabetes: a fasting glucose >126 mg/dL, a random plasma glucose ≥200 mg/dL (that must be confirmed on a subsequent day), or both.5,7,12,13 Patient history, risk factors, and additional laboratory tests can help clinicians distinguish between type 1 and type 2 diabetes.
An oral glucose tolerance test (OGTT) is also an option for diagnosis, but time and scheduling difficulties limit the routine use of this test in primary care. Hemoglobin A1c is not recommended as a diagnostic test because of a lack of standardization.1
Prediabetes and type 2 risk. One in 4 (25.9%) US adults 20 years of age or older and more than 1 in 3 (35.9%) of those 60 years of age or older have prediabetes,14 defined as impaired fasting glucose (100-125 mg/dL), impaired glucose tolerance (2-hour glucose test results of 140-199 mg/dL), or both. Prediabetes increases the risk of developing type 2 diabetes by an estimated 30% over a 4-year period,15 and 70% over 30 years,16 although lifestyle interventions can substantially lower the risk. In a recently released consensus statement, an AACE task force noted that in addition to the increased risk of type 2 diabetes, patients with prediabetes face a greater risk of macrovascular complications.17
Type 2 in kids can be mistaken for type 1
As childhood obesity has surged, type 2 diabetes has been diagnosed at an increasingly early age—even in children younger than 10 years.18 Minority youth, primarily African Americans, Hispanics, and Asians/Pacific Islanders, are at increased risk.14 Symptoms can be insidious in children and adolescents and easily missed or mistaken for type 1 diabetes, in part because type 2 diabetes is still relatively rare in this age group.19
Preteens at risk. In a recent study of BMI and metabolic syndrome risk factors in 8- to 14-year-olds, however, researchers concluded that children who are overweight in early adolescence may be at risk for type 2 diabetes as well as cardiovascular disease before they reach their teens.20 There is evidence of a genetic predisposition for type 2 diabetes and defects of β-cell function,5,21 and family history, in addition to weight, is an important consideration in identifying type 2 diabetes in young patients.
Although young adults with type 1 and type 2 diabetes can present with similar symptoms, there may be certain clues to a type 2 diagnosis. Acanthosis nigricans, which is related to insulin resistance and occurs most frequently in obese adolescents, points to a type 2 diagnosis. Increased insulin and C-peptide levels are indicators of type 2 diabetes. Low levels are not necessarily an indication of type 1, however, because patients with type 2 diabetes may have low levels of insulin and C-peptide because of glucose toxicity and lipotoxicity at the time of diagnosis.22 Treatment with insulin may be necessary until glucose toxicity resolves.
Type 1 diabetes: Beyond childhood
Approximately 5% to 10% of patients with diabetes have type 1, which is defined as idiopathic or cellular immune-mediated autoimmune β-cell destruction.5 The rate of destruction is variable—it generally progresses more rapidly in infants and children than in adults. Some people with type 1 diabetes retain residual β-cell function, but have little or no insulin secretion; this manifests as a low or undetectable level of serum C-peptide.
Most cases of type 1 diabetes are diagnosed in patients younger than 18 years. But type 1 diabetes is increasingly recognized as a disorder that also develops in early adulthood, usually before the age of 40.
Arriving at a type 1 diagnosis
Patients with type 1 diabetes often present with modest hyperglycemia, but may rapidly progress to severe hyperglycemia and diabetic ketoacidosis (DKA) when infection or other physical stressors occur.
While screening for autoantibodies in asymptomatic individuals is not recommended,5 patients with blood glucose levels ≥200 mg/dL and symptoms of polydipsia, polyuria, and polyphagia who do not meet the profile for type 2 diabetes may be candidates for additional laboratory work. Approximately 85% to 90% of patients with type 1 diabetes will have antibodies to islet cells or glutamic acid decarboxylase (GAD).5,23
Even without antibody testing, there are distinguishing characteristics that help support a type 1 diagnosis. As a general rule, individuals who develop type 1 diabetes—especially children—are not obese, although patients usually gain weight over time. In addition, many patients with type 1 diabetes have an auto-immune disease, such as celiac or Graves’ disease, hypothyroidism, adrenal anemia, or pernicious anemia; and a first-degree relative with type 1 diabetes. DKA, with acute symptoms of polydipsia and/or polyuria and recent, unintentional weight loss, is suggestive of—but not definitive for—type 1 diabetes.
A recently validated type 1 risk calculator may be particularly useful for screening patients who have a sibling, parent, or child with type 1 diabetes. Using age, BMI, C-peptide concentration, and OGTT results, the algorithm was highly predictive of type 1 diabetes in family members of patients who tested positive for islet cell antibodies.24
Patient doesn’t “fit” type 1 or 2? Consider LADA
LADA, a gradual, progressive form of type 1 diabetes, can be difficult to identify. Circulating GAD or islet cell antibodies are present, but patients don’t have an absolute need for insulin at the time of diagnosis. Thus, they’re often thought to have type 2 diabetes.25 Individuals with LADA show no signs of insulin resistance, however, and over time, β cells decline and insulin usually becomes necessary.
There are no universal recommendations for testing for LADA. Rather, the diagnosis should be considered in those who don’t fit the classic profile for type 1 or type 2 diabetes,26 but have some of the following features:
- age <50 years
- acute symptoms of polydipsia, polyuria, and/or unintentional weight loss
- BMI <25
- a personal history of autoimmune disease
- a family history of autoimmune disease.27
A prospective analysis found that the majority of LADA patients had at least 2 of these distinguishing characteristics.28 Other recent research found heterogeneity among patients with LADA. Noting that not all patients with LADA become insulin-dependent, researchers concluded that the need for insulin is linked to the degree of autoimmunity and β-cell failure.29
When GDM complicates prenatal care
Any degree of carbohydrate intolerance that is first recognized during pregnancy is classified as GDM, whether or not the condition resolves after delivery. A GDM diagnosis does not preclude the possibility of undiagnosed type 2 diabetes or prediabetes, or (rarely) type 1 diabetes.
Approximately 7% of all pregnancies in the United States are complicated by GDM, totaling more than 200,000 cases annually.5 The rate of GDM is in direct proportion to the prevalence of type 2 diabetes in the population in question, and ranges from 1% to 14%. GDM is the diagnosis in nearly 90% of pregnancies complicated by diabetes.5
The GDM screening controversy
Screening for GDM—whether it should be done universally or selectively on the basis of risk factors—is highly controversial. The USPSTF maintains that there is insufficient evidence to recommend for or against screening women with no history of GDM. The American College of Obstetricians and Gynecologists (ACOG)30 and ADA5 recommend selective screening based on patient history, clinical presentation, and, possibly, prior impaired glucose test results or other abnormal laboratory values. AACE calls for universal screening of pregnant women, starting at 20 weeks for high-risk individuals and between 24 and 28 weeks for those at low risk.12
Identifying patients at risk. Maternal age (>25 years), obesity (BMI ≥30), prior GDM or delivery of a large-forgestational-age infant, belonging to a high-risk ethnic group, glycosuria, history of glucose resistance or glucose tolerance, and a first-degree relative with diabetes (TABLE) are risk factors for GDM. Women at high risk—those who meet all or most of these criteria—should undergo early screening: at the first prenatal visit, according to ACOG;30 upon confirmation of pregnancy (ADA);5 at 20 weeks’ gestation (AACE);12 or between 24 and 28 weeks’ gestation (USPSTF).7 ADA and ACOG recommend a 2-stage approach, starting with a 50-g 1-hour OGTT and following up with a 100-g 3-hour OGTT if the first test results are not definitive.5,30 Testing for patients at average risk—which includes any pregnant woman with even a single risk factor, such as being older than 25 years—should be done between 24 and 28 weeks’ gestation, according to ACOG and ADA; testing is not required for women who are <25 years, have a normal body weight, and no other risk factors.
GDM screening in primary care. Because most women fit the criteria for average or high risk,31 family physicians may find universal screening to be more practical than individual risk assessment. Universal screening is associated with favorable outcomes,32 but screening limited to those at high and average risk also has evidence to support it. In a study of 25,118 deliveries, only 4% of women with GDM were missed by the exclusion of low-risk patients.33
In the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, researchers tracked 25,505 women from 9 countries and found a continuous relationship between the risk of macrosomia and the rise in maternal glucose levels.34 The impact on the developing fetus of varying degrees of glucose was studied after a 75-g 2-hour OGTT. The risk of macrosomia increased with fasting blood glucose >75 mg/dL, 1-hour glucose levels >105 mg/dL, and 2-hour glucose concentration >90 mg/dL.35 The most compelling results for adverse effects were associated with fasting glucose levels, rather than glucose tolerance tests.
2 abnormal results needed for a GDM diagnosis
In the absence of unequivocal hyperglycemia, there are 2 diagnostic standards for GDM: The Carpenter-Coustan Conversion and the National Diabetes Data Group Conversion. The Carpenter-Coustan Conversion uses lower glucose values for fasting (≥95 mg/dL) and subsequent 1-, 2-, and 3-hour levels (≥180, 155, and 140 mg/dL, respectively) and is more widely used. But expert opinion also supports the National Diabetes Data Group Conversion criteria (fasting plasma glucose, ≥105 mg/dL; ≥190, 165, and 145 mg/dL for 1-, 2-, and 3-hour OGTT, respectively), and there are no data from clinical trials to prove the superiority of either standard.30
Both sets of standards require 2 or more thresholds to be met or exceeded for a GDM diagnosis. Women with only 1 abnormal value should be monitored carefully, however, as they, too, may be at increased risk for macrosomia and other morbidities.30
Postpartum follow-up. Obtain a fasting glucose reading or perform an OGTT around the time of the postpartum checkup for any patient who was diagnosed with GDM. ACOG recommends using an OGTT to more accurately diagnose type 2 diabetes or prediabetes in these patients, who are at significantly elevated risk.30
Acknowledgement
The authors wish to thank Carol Hildebrandt, a research assistant with no potential conflict of interest, for her help with this manuscript.
Correspondence
Julienne K. Kirk, PharmD, CDE, Department of Family and Community Medicine, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected]
1. Williamson DF, Vinicor F, Bowman BA. Centers of Disease Control and Prevention Primary Prevention Working Group. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med 2004;140:951-957.
2. Centers for Disease Control and Prevention/National Center for Health Statistics. FastStats. Over-weight prevalence. Available at: http://www.cdc.gov/nchs/fastats/overwt.htm. Accessed March 28, 2009.
3. Palmer JP, Hirsch IB. What’s in a name? Diabetes Care. 2003;26:536-538.
4. Centers for Disease Control and Prevention. Health, United States, 2007. Available at: http://www.cdc.gov/nchs/data/hus/hus07.pdf#executivesummary. Accessed October 4, 2008.
5. American Diabetes Association. Clinical practice recommendations 2009. Diabetes Care. 2009;32(suppl 1):S1-S61.
6. National Diabetes Information Clearinghouse. National Institute of Diabetes and Digestive and Kidney Diseases. Am I at risk for type 2 diabetes? Available at: http://diabetes.niddk.nih.gov/DM/pubs/riskfortype2/. Accessed April 10, 2009.
7. US Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: Recommendations and rationale. Available at: http://www.ahrq.gov/clinic/uspstf08/type2/type2summ.htm. Accessed October 4, 2008.
8. International Diabetes Federation. Backgrounder 1: The IDF consensus worldwide definition of the metabolic syndrome. Brussels, Belgium; 2005.
9. Genuth S, Estman R, Kahn R, et al. American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2003;26(suppl 1):S28-S32.
10. Heikes KE, Eddy DM, Arondekar B, et al. Diabetes risk calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31:1040-1045.
11. Hippisley-Cox J, Coupland C, Robson J, et al. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ. 2009;338:b880.-
12. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):S3-S68.
13. World Health Organization. Screening for type 2 diabetes. Report of a World Health Organization and International Diabetes Federation meeting. 2003. http://www.who.int/diabetes/publications/en/screening_mnc03.pdf. Accessed October 4, 2008.
14. National Diabetes Information Clearinghouse. National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007. Available at: http://diabetes.niddk.nih.gov/DM/PUBS/statistics/. Accessed March 27, 2009.
15. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
16. Eddy DM, Schlessinger L, Khan R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251-264.
17. American College of Endocrinology Task Force on Prediabetes. Diagnosis and management of prediabetes in the continuum of hyperglycemia - When do the risks of diabetes begin? Available at: www.aace.com/meetings/consensus/hyperglycemia/hyperglycemia.pdf. Accessed October 4, 2008.
18. SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510-1518.
19. Centers for Disease Control and Prevention. CDC’s Diabetes Program-Diabetes Projects-Children and Diabetes. Available at: http://www.cdc.gov/diabetes/projects/cda2.htm. Accessed March 27, 2009.
20. Messiah SE, Arheart KL, Luke B, et al. Relationship between body mass index and metabolic syndrome risk factors among US 8- to 14-year-olds, 1999 to 2002. J Pediatr. 2008;153:215-221.
21. Fowler MJ. Classification of diabetes: not all hyperglycemia is the same. Clin Diabetes. 2007;25:74-76.
22. Kitabachi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in diabetes. Diabetes Care. 2004;27(suppl):S94-S102.
23. Borg H, Gottsäter A, Landin-Olsson M, et al. High levels of antigen-specific islet antibodies predict future beta-cell failure in patients with onset of diabetes in adult age. J Clin Endocrinol Metab. 2001;86:3032-3238.
24. Sosenko JM, Krischer JP, Palmer JP, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008;31:528-533.
25. Brophy S, Brunt H, Davies H, et al. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Data Syst Rev. 2007(3);CD006165.-
26. Appel SJ, Wadas TM, Rosenthal RS, et al. Latent autoimmune diabetes of adulthood (LADA): an often misdiagnosed type of diabetes mellitus. J Am Acad Nurse Pract. 2009;21:156-159.
27. Unger J. Diagnosing and managing latent autoimmune diabetes in adults. Pract Diabet. 2008;27:32-37.
28. Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31:1546-1549.
29. Radtke MA, Midthjell K, Nilsen TI, et al. Heterogeneity of patients with latent autoimmune diabetes in adults: linkage to autoimmunity is apparent only in those with perceived need for insulin treatment: results from the Nord-Trondelag Health (HUNT) study. Diabetes Care. 2009;32:245-250.
30. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for ObstetricianGynecologists Number 30, September 2001 (Replaces Technical Bulletin Number 200, December 1994): Gestational diabetes. Obstet Gynecol. 2001;98:525-538.
31. Danilenko-Dixon DR, Van Winter JT, Nelson RL, et al. Universal versus selective gestational diabetes screening: application of 1997 American Diabetes Association recommendations. Am J Obstet Gynecol. 1997;81:798-802.
32. Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab. 2006;32:140-146.
33. Williams CB, Iqbal S, Zawacki CM, et al. Effect of selective screening for gestational diabetes. Diabetes Care. 1999;22:418-421.
34. Holt RI. The Hyperglycemia and Adverse Pregnancy Outcomes trial: answers but still more questions about the management of gestational diabetes. Diabet Med. 2008;25:1013-1014.
35. The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
- Routinely screen adult patients with a sustained blood pressure >135/80 mm Hg for type 2 diabetes (SOR: B).
- Closely monitor pregnant women with 1 or more elevated glucose test results; although a diagnosis of gestational diabetes mellitus (GDM) requires 2 or more abnormal values, even 1 may be associated with a higher risk of adverse outcomes (SOR: C).
- Include latent autoimmune diabetes in adults (LADA), a progressive form of type 1 with a slower onset, in the differential diagnosis for symptomatic patients who don’t fit the classic patterns for type 1 or type 2 diabetes (SOR: B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
The youngest Americans—those born in the year 2000 or thereafter—have more than a 1 in 3 lifetime risk of developing diabetes, according to the Centers for Disease Control and Prevention.1 That estimate, coupled with the fact that more than 2 out of 3 adults and 1 in 6 children between the ages of 2 and 19 years are overweight,2 would seem to indicate a need for widespread diabetes screening. But limited health care resources, a lack of evidence that mass screening improves outcomes, and differences among leading medical associations about whom and when to screen argue against it.
At the same time, widespread obesity is making the presentation of hyperglycemia more complex and the forms of diabetes harder to classify. Many cases don’t follow the classic patterns, in which type 1 (formerly called juvenile diabetes) virtually always emerges in childhood and type 2 (previously known as adult-onset diabetes) is strictly an adult disease. Our evolving understanding of diabetes has led researchers to focus on prediabetes (defined as impaired fasting glucose, impaired glucose tolerance, or both) and latent autoimmune diabetes in adults (LADA), a recently reported type 1 variant that some have labeled type 1.5.3
In the face of growing complexity, the US Preventive Services Task Force (USPSTF) last year upgraded its recommendation for screening for type 2 diabetes, and researchers have developed new risk calculation tools. We’ve taken a look at the changing clinical landscape and sorted through the latest evidence to help you make sense of the latest risk and diagnostic developments in diabetes care.
Screening for type 2: A look at guidelines
Type 2 diabetes accounts for approximately 90% of the cases you’ll see.1,4,5 The American Diabetes Association (ADA) calls for routine screening, starting at 45 years of age and continuing every 3 years thereafter in the absence of risk. But for those who are overweight or obese and have 1 or more additional risk factors, screening is recommended at any age.5 In addition to a body mass index (BMI) ≥25, risks include physical inactivity, a first-degree relative with type 2 diabetes, blood pressure >135/80 mm Hg (or controlled with an antihypertensive), high-density lipoproteins (HDL) <35 mg/dL, triglycerides >250 mg/dL, polycystic ovary syndrome, impaired glucose tolerance or impaired fasting glucose, and acanthosis nigricans, a pigmented thickening of the skin folds of the neck (TABLE).6,7 Patients with the metabolic syndrome—abdominal obesity (defined as a waist circumference of >40” in men and >35” in women) and ≥2 of the following: raised triglyceride levels, elevated blood pressure, elevated fasting plasma glucose, and reduced HDL cholesterol—are at especially high risk of both cardiovascular disease and type 2 diabetes.8
The ADA screening recommendations, however, are not based on prospective outcome studies, nor are they widely followed. Until recently, the USPSTF only recommended screening adults with hypertension and hyperlipidemia.
In 2008, after an assessment of new findings and research updates, the USPSTF revised its recommendation: The task force now calls for screening asymptomatic adults with sustained blood pressure >135/80 mm Hg—regardless of lipid profile.7 For patients with diabetes and hypertension, the USPSTF concluded, evidence shows that early intervention—including lowering blood pressure below conventional targets—can prevent long-term adverse outcomes of diabetes and reduce the risk of cardiovascular events.
Although mass screening remains controversial, regular assessment of risk factors and targeting individuals with established risk is clearly indicated (PATIENT HANDOUT). The importance of early detection was highlighted by the United Kingdom Prospective Diabetes Study, in which approximately half of the patients with newly diagnosed type 2 diabetes already had evidence of complications.9
TABLE
Type 1, type 2, and gestational diabetes: Diagnostic clues
| TYPE 1 DIABETES | TYPE 2 DIABETES | GESTATIONAL DIABETES MELLITUS (GDM) | |
|---|---|---|---|
| Risk factors/characteristics | Patient/family history of autoimmune disease 1st-degree relative with type 1 diabetes Normal weight with symptoms of hyperglycemia | BMI ≥25 Physical inactivity 1st-degree relative with type 2 diabetes High-risk ethnic group (African American, Hispanic, Native American, Asian American, Pacific Islander) History of GDM and/or delivery of an LGA infant BP >135/80 mm Hg or being treated for HTN Polycystic ovary syndrome IGT or IFG Acanthosis nigricans | BMI ≥30 History of GDM and/or delivery of an LGA infant (or poor outcome) 1st-degree relative with type 2 diabetes High-risk ethnic group (African American, Hispanic, Native American, Asian American, Pacific Islander) Glycosuria Age >25 years Polycystic ovary syndrome IGT |
| Laboratory tests/positive results | Specific antibodies to islet cell, insulin, and/or GAD* Tyrosine phosphatase-like auto antigen IA-2 (marker of autoimmune islet cell disease) C-peptide (low or absent); if in normal range, may indicate early disease and partial β-cell activity | FPG >126 mg/dL Random plasma glucose >200 mg/dL (test repeated next day) 2-hr 75-g OGTT >200 mg/dL HDL <35 mg/dL TG >250 mg/dL C-peptide (normal or elevated; may be low initially due to glucose toxicity) | Fasting: ≥95 mg/dL 1-hr OGTT: ≥180 mg/dL 2-hr OGTT: ≥155 mg/dL 3-hr OGTT: ≥140 mg/dL |
| BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; FPG, fasting plasma glucose; GAD, glutamic acid decarboxylase; HDL, high-density lipoproteins; HTN, hypertension; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LGA, large for gestational age; OGTT, oral glucose tolerance test; TG, triglycerides. | |||
| *GAD65 is most specific. | |||
Eating well, maintaining your weight, and engaging in physical activity are essential to good health. If you have risk factors for diabetes, diet and exercise are important steps you can take to help keep the disease at bay.
Making changes to your diet and increasing the amount of exercise you engage in need not be a daunting task. It helps to remember that it’s not necessary to take giant steps. You can improve your health and help prevent diabetes with a series of small changes. For best results, keep each goal small, manageable, and as specific as possible.
Eating. Do you eat fast food frequently, or snack on ice cream or potato chips when you watch TV at night? Pick a “bad habit” that is of particular concern and try to “turn it around.” You might, for instance, promise yourself that:
For the next 4 weeks, I will replace my unhealthy evening snacks with fresh fruit, a small bowl of cereal, or (insert another healthy snack here).
Getting active. Have you stopped working out? Are you concerned that working out will require a big time commitment? Think again. Start small and promise yourself that:
For the next 3 weeks, I will take a 20-minute walk 3 mornings a week.
Each time you set a goal, monitor your progress. When you succeed, give yourself a reward—it can be something as simple as a long bath or a trip to the movies—and vow to continue that lifestyle change and to add another. If you aren’t successful, think about why and revise your goal. If you find you’re too busy getting the kids off to school to walk in the morning, for example, change your schedule and start going out during your lunch break. Or, if it’s too cold or rainy, find a nearby mall where you can walk (or a treadmill at a local gym) instead. It also helps to get a step counter, or pedometer. The American Diabetes Association (ADA) recommends taking 10,000 steps per day.
For additional ideas, visit the ADA Web site (www.diabetes.org) and click on Fitness. Or call our office at __________ and make an appointment to come in and discuss additional lifestyle changes—small and large—that you can make with our help.
Validated risk calculators can boost detection rates
In an attempt to improve detection rates of type 2 diabetes and prediabetes, researchers in both the United States and the United Kingdom recently developed easy-to-use risk calculation tools. The Diabetes Risk Calculator (available at http://www.diabetes.org/food-nutrition-lifestyle/lifestyle-prevention/risk-test.jsp), published in 2008, was validated with findings from the Third National Health and Nutrition Survey.10 The calculator uses answers to questions about age, waist circumference, history of gestational diabetes mellitus (GDM), height, race/ethnicity, hypertension, family history, and exercise to determine whether an individual is at high risk for undetected diabetes. The tool has a low positive predictive value (14%), but a negative predictive value >99%.10
The QDScore Diabetes Risk Calculator (www.qdscore.org), another new tool, is designed to estimate an individual’s 10-year risk of developing type 2 diabetes.11 The program, which calculates risk based on answers to questions about family history of diabetes, patient history of cardiovascular disease, smoking, treatment for hypertension, BMI, ethnicity, and steroid use, was validated with data collected from 2.5 million patients in practices throughout England and Wales. The screening tool showed a high degree of discrimination in reflecting differences in disease prevalence related to ethnic and socioeconomic risk factors.11
Pinning down a type 2 (or prediabetes) diagnosis
The ADA, American Association of Clinical Endocrinologists (AACE), USPSTF, and World Health Organization/International Diabetes Federation agree on the diagnostic criteria for type 2 diabetes: a fasting glucose >126 mg/dL, a random plasma glucose ≥200 mg/dL (that must be confirmed on a subsequent day), or both.5,7,12,13 Patient history, risk factors, and additional laboratory tests can help clinicians distinguish between type 1 and type 2 diabetes.
An oral glucose tolerance test (OGTT) is also an option for diagnosis, but time and scheduling difficulties limit the routine use of this test in primary care. Hemoglobin A1c is not recommended as a diagnostic test because of a lack of standardization.1
Prediabetes and type 2 risk. One in 4 (25.9%) US adults 20 years of age or older and more than 1 in 3 (35.9%) of those 60 years of age or older have prediabetes,14 defined as impaired fasting glucose (100-125 mg/dL), impaired glucose tolerance (2-hour glucose test results of 140-199 mg/dL), or both. Prediabetes increases the risk of developing type 2 diabetes by an estimated 30% over a 4-year period,15 and 70% over 30 years,16 although lifestyle interventions can substantially lower the risk. In a recently released consensus statement, an AACE task force noted that in addition to the increased risk of type 2 diabetes, patients with prediabetes face a greater risk of macrovascular complications.17
Type 2 in kids can be mistaken for type 1
As childhood obesity has surged, type 2 diabetes has been diagnosed at an increasingly early age—even in children younger than 10 years.18 Minority youth, primarily African Americans, Hispanics, and Asians/Pacific Islanders, are at increased risk.14 Symptoms can be insidious in children and adolescents and easily missed or mistaken for type 1 diabetes, in part because type 2 diabetes is still relatively rare in this age group.19
Preteens at risk. In a recent study of BMI and metabolic syndrome risk factors in 8- to 14-year-olds, however, researchers concluded that children who are overweight in early adolescence may be at risk for type 2 diabetes as well as cardiovascular disease before they reach their teens.20 There is evidence of a genetic predisposition for type 2 diabetes and defects of β-cell function,5,21 and family history, in addition to weight, is an important consideration in identifying type 2 diabetes in young patients.
Although young adults with type 1 and type 2 diabetes can present with similar symptoms, there may be certain clues to a type 2 diagnosis. Acanthosis nigricans, which is related to insulin resistance and occurs most frequently in obese adolescents, points to a type 2 diagnosis. Increased insulin and C-peptide levels are indicators of type 2 diabetes. Low levels are not necessarily an indication of type 1, however, because patients with type 2 diabetes may have low levels of insulin and C-peptide because of glucose toxicity and lipotoxicity at the time of diagnosis.22 Treatment with insulin may be necessary until glucose toxicity resolves.
Type 1 diabetes: Beyond childhood
Approximately 5% to 10% of patients with diabetes have type 1, which is defined as idiopathic or cellular immune-mediated autoimmune β-cell destruction.5 The rate of destruction is variable—it generally progresses more rapidly in infants and children than in adults. Some people with type 1 diabetes retain residual β-cell function, but have little or no insulin secretion; this manifests as a low or undetectable level of serum C-peptide.
Most cases of type 1 diabetes are diagnosed in patients younger than 18 years. But type 1 diabetes is increasingly recognized as a disorder that also develops in early adulthood, usually before the age of 40.
Arriving at a type 1 diagnosis
Patients with type 1 diabetes often present with modest hyperglycemia, but may rapidly progress to severe hyperglycemia and diabetic ketoacidosis (DKA) when infection or other physical stressors occur.
While screening for autoantibodies in asymptomatic individuals is not recommended,5 patients with blood glucose levels ≥200 mg/dL and symptoms of polydipsia, polyuria, and polyphagia who do not meet the profile for type 2 diabetes may be candidates for additional laboratory work. Approximately 85% to 90% of patients with type 1 diabetes will have antibodies to islet cells or glutamic acid decarboxylase (GAD).5,23
Even without antibody testing, there are distinguishing characteristics that help support a type 1 diagnosis. As a general rule, individuals who develop type 1 diabetes—especially children—are not obese, although patients usually gain weight over time. In addition, many patients with type 1 diabetes have an auto-immune disease, such as celiac or Graves’ disease, hypothyroidism, adrenal anemia, or pernicious anemia; and a first-degree relative with type 1 diabetes. DKA, with acute symptoms of polydipsia and/or polyuria and recent, unintentional weight loss, is suggestive of—but not definitive for—type 1 diabetes.
A recently validated type 1 risk calculator may be particularly useful for screening patients who have a sibling, parent, or child with type 1 diabetes. Using age, BMI, C-peptide concentration, and OGTT results, the algorithm was highly predictive of type 1 diabetes in family members of patients who tested positive for islet cell antibodies.24
Patient doesn’t “fit” type 1 or 2? Consider LADA
LADA, a gradual, progressive form of type 1 diabetes, can be difficult to identify. Circulating GAD or islet cell antibodies are present, but patients don’t have an absolute need for insulin at the time of diagnosis. Thus, they’re often thought to have type 2 diabetes.25 Individuals with LADA show no signs of insulin resistance, however, and over time, β cells decline and insulin usually becomes necessary.
There are no universal recommendations for testing for LADA. Rather, the diagnosis should be considered in those who don’t fit the classic profile for type 1 or type 2 diabetes,26 but have some of the following features:
- age <50 years
- acute symptoms of polydipsia, polyuria, and/or unintentional weight loss
- BMI <25
- a personal history of autoimmune disease
- a family history of autoimmune disease.27
A prospective analysis found that the majority of LADA patients had at least 2 of these distinguishing characteristics.28 Other recent research found heterogeneity among patients with LADA. Noting that not all patients with LADA become insulin-dependent, researchers concluded that the need for insulin is linked to the degree of autoimmunity and β-cell failure.29
When GDM complicates prenatal care
Any degree of carbohydrate intolerance that is first recognized during pregnancy is classified as GDM, whether or not the condition resolves after delivery. A GDM diagnosis does not preclude the possibility of undiagnosed type 2 diabetes or prediabetes, or (rarely) type 1 diabetes.
Approximately 7% of all pregnancies in the United States are complicated by GDM, totaling more than 200,000 cases annually.5 The rate of GDM is in direct proportion to the prevalence of type 2 diabetes in the population in question, and ranges from 1% to 14%. GDM is the diagnosis in nearly 90% of pregnancies complicated by diabetes.5
The GDM screening controversy
Screening for GDM—whether it should be done universally or selectively on the basis of risk factors—is highly controversial. The USPSTF maintains that there is insufficient evidence to recommend for or against screening women with no history of GDM. The American College of Obstetricians and Gynecologists (ACOG)30 and ADA5 recommend selective screening based on patient history, clinical presentation, and, possibly, prior impaired glucose test results or other abnormal laboratory values. AACE calls for universal screening of pregnant women, starting at 20 weeks for high-risk individuals and between 24 and 28 weeks for those at low risk.12
Identifying patients at risk. Maternal age (>25 years), obesity (BMI ≥30), prior GDM or delivery of a large-forgestational-age infant, belonging to a high-risk ethnic group, glycosuria, history of glucose resistance or glucose tolerance, and a first-degree relative with diabetes (TABLE) are risk factors for GDM. Women at high risk—those who meet all or most of these criteria—should undergo early screening: at the first prenatal visit, according to ACOG;30 upon confirmation of pregnancy (ADA);5 at 20 weeks’ gestation (AACE);12 or between 24 and 28 weeks’ gestation (USPSTF).7 ADA and ACOG recommend a 2-stage approach, starting with a 50-g 1-hour OGTT and following up with a 100-g 3-hour OGTT if the first test results are not definitive.5,30 Testing for patients at average risk—which includes any pregnant woman with even a single risk factor, such as being older than 25 years—should be done between 24 and 28 weeks’ gestation, according to ACOG and ADA; testing is not required for women who are <25 years, have a normal body weight, and no other risk factors.
GDM screening in primary care. Because most women fit the criteria for average or high risk,31 family physicians may find universal screening to be more practical than individual risk assessment. Universal screening is associated with favorable outcomes,32 but screening limited to those at high and average risk also has evidence to support it. In a study of 25,118 deliveries, only 4% of women with GDM were missed by the exclusion of low-risk patients.33
In the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, researchers tracked 25,505 women from 9 countries and found a continuous relationship between the risk of macrosomia and the rise in maternal glucose levels.34 The impact on the developing fetus of varying degrees of glucose was studied after a 75-g 2-hour OGTT. The risk of macrosomia increased with fasting blood glucose >75 mg/dL, 1-hour glucose levels >105 mg/dL, and 2-hour glucose concentration >90 mg/dL.35 The most compelling results for adverse effects were associated with fasting glucose levels, rather than glucose tolerance tests.
2 abnormal results needed for a GDM diagnosis
In the absence of unequivocal hyperglycemia, there are 2 diagnostic standards for GDM: The Carpenter-Coustan Conversion and the National Diabetes Data Group Conversion. The Carpenter-Coustan Conversion uses lower glucose values for fasting (≥95 mg/dL) and subsequent 1-, 2-, and 3-hour levels (≥180, 155, and 140 mg/dL, respectively) and is more widely used. But expert opinion also supports the National Diabetes Data Group Conversion criteria (fasting plasma glucose, ≥105 mg/dL; ≥190, 165, and 145 mg/dL for 1-, 2-, and 3-hour OGTT, respectively), and there are no data from clinical trials to prove the superiority of either standard.30
Both sets of standards require 2 or more thresholds to be met or exceeded for a GDM diagnosis. Women with only 1 abnormal value should be monitored carefully, however, as they, too, may be at increased risk for macrosomia and other morbidities.30
Postpartum follow-up. Obtain a fasting glucose reading or perform an OGTT around the time of the postpartum checkup for any patient who was diagnosed with GDM. ACOG recommends using an OGTT to more accurately diagnose type 2 diabetes or prediabetes in these patients, who are at significantly elevated risk.30
Acknowledgement
The authors wish to thank Carol Hildebrandt, a research assistant with no potential conflict of interest, for her help with this manuscript.
Correspondence
Julienne K. Kirk, PharmD, CDE, Department of Family and Community Medicine, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected]
- Routinely screen adult patients with a sustained blood pressure >135/80 mm Hg for type 2 diabetes (SOR: B).
- Closely monitor pregnant women with 1 or more elevated glucose test results; although a diagnosis of gestational diabetes mellitus (GDM) requires 2 or more abnormal values, even 1 may be associated with a higher risk of adverse outcomes (SOR: C).
- Include latent autoimmune diabetes in adults (LADA), a progressive form of type 1 with a slower onset, in the differential diagnosis for symptomatic patients who don’t fit the classic patterns for type 1 or type 2 diabetes (SOR: B).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
The youngest Americans—those born in the year 2000 or thereafter—have more than a 1 in 3 lifetime risk of developing diabetes, according to the Centers for Disease Control and Prevention.1 That estimate, coupled with the fact that more than 2 out of 3 adults and 1 in 6 children between the ages of 2 and 19 years are overweight,2 would seem to indicate a need for widespread diabetes screening. But limited health care resources, a lack of evidence that mass screening improves outcomes, and differences among leading medical associations about whom and when to screen argue against it.
At the same time, widespread obesity is making the presentation of hyperglycemia more complex and the forms of diabetes harder to classify. Many cases don’t follow the classic patterns, in which type 1 (formerly called juvenile diabetes) virtually always emerges in childhood and type 2 (previously known as adult-onset diabetes) is strictly an adult disease. Our evolving understanding of diabetes has led researchers to focus on prediabetes (defined as impaired fasting glucose, impaired glucose tolerance, or both) and latent autoimmune diabetes in adults (LADA), a recently reported type 1 variant that some have labeled type 1.5.3
In the face of growing complexity, the US Preventive Services Task Force (USPSTF) last year upgraded its recommendation for screening for type 2 diabetes, and researchers have developed new risk calculation tools. We’ve taken a look at the changing clinical landscape and sorted through the latest evidence to help you make sense of the latest risk and diagnostic developments in diabetes care.
Screening for type 2: A look at guidelines
Type 2 diabetes accounts for approximately 90% of the cases you’ll see.1,4,5 The American Diabetes Association (ADA) calls for routine screening, starting at 45 years of age and continuing every 3 years thereafter in the absence of risk. But for those who are overweight or obese and have 1 or more additional risk factors, screening is recommended at any age.5 In addition to a body mass index (BMI) ≥25, risks include physical inactivity, a first-degree relative with type 2 diabetes, blood pressure >135/80 mm Hg (or controlled with an antihypertensive), high-density lipoproteins (HDL) <35 mg/dL, triglycerides >250 mg/dL, polycystic ovary syndrome, impaired glucose tolerance or impaired fasting glucose, and acanthosis nigricans, a pigmented thickening of the skin folds of the neck (TABLE).6,7 Patients with the metabolic syndrome—abdominal obesity (defined as a waist circumference of >40” in men and >35” in women) and ≥2 of the following: raised triglyceride levels, elevated blood pressure, elevated fasting plasma glucose, and reduced HDL cholesterol—are at especially high risk of both cardiovascular disease and type 2 diabetes.8
The ADA screening recommendations, however, are not based on prospective outcome studies, nor are they widely followed. Until recently, the USPSTF only recommended screening adults with hypertension and hyperlipidemia.
In 2008, after an assessment of new findings and research updates, the USPSTF revised its recommendation: The task force now calls for screening asymptomatic adults with sustained blood pressure >135/80 mm Hg—regardless of lipid profile.7 For patients with diabetes and hypertension, the USPSTF concluded, evidence shows that early intervention—including lowering blood pressure below conventional targets—can prevent long-term adverse outcomes of diabetes and reduce the risk of cardiovascular events.
Although mass screening remains controversial, regular assessment of risk factors and targeting individuals with established risk is clearly indicated (PATIENT HANDOUT). The importance of early detection was highlighted by the United Kingdom Prospective Diabetes Study, in which approximately half of the patients with newly diagnosed type 2 diabetes already had evidence of complications.9
TABLE
Type 1, type 2, and gestational diabetes: Diagnostic clues
| TYPE 1 DIABETES | TYPE 2 DIABETES | GESTATIONAL DIABETES MELLITUS (GDM) | |
|---|---|---|---|
| Risk factors/characteristics | Patient/family history of autoimmune disease 1st-degree relative with type 1 diabetes Normal weight with symptoms of hyperglycemia | BMI ≥25 Physical inactivity 1st-degree relative with type 2 diabetes High-risk ethnic group (African American, Hispanic, Native American, Asian American, Pacific Islander) History of GDM and/or delivery of an LGA infant BP >135/80 mm Hg or being treated for HTN Polycystic ovary syndrome IGT or IFG Acanthosis nigricans | BMI ≥30 History of GDM and/or delivery of an LGA infant (or poor outcome) 1st-degree relative with type 2 diabetes High-risk ethnic group (African American, Hispanic, Native American, Asian American, Pacific Islander) Glycosuria Age >25 years Polycystic ovary syndrome IGT |
| Laboratory tests/positive results | Specific antibodies to islet cell, insulin, and/or GAD* Tyrosine phosphatase-like auto antigen IA-2 (marker of autoimmune islet cell disease) C-peptide (low or absent); if in normal range, may indicate early disease and partial β-cell activity | FPG >126 mg/dL Random plasma glucose >200 mg/dL (test repeated next day) 2-hr 75-g OGTT >200 mg/dL HDL <35 mg/dL TG >250 mg/dL C-peptide (normal or elevated; may be low initially due to glucose toxicity) | Fasting: ≥95 mg/dL 1-hr OGTT: ≥180 mg/dL 2-hr OGTT: ≥155 mg/dL 3-hr OGTT: ≥140 mg/dL |
| BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; FPG, fasting plasma glucose; GAD, glutamic acid decarboxylase; HDL, high-density lipoproteins; HTN, hypertension; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LGA, large for gestational age; OGTT, oral glucose tolerance test; TG, triglycerides. | |||
| *GAD65 is most specific. | |||
Eating well, maintaining your weight, and engaging in physical activity are essential to good health. If you have risk factors for diabetes, diet and exercise are important steps you can take to help keep the disease at bay.
Making changes to your diet and increasing the amount of exercise you engage in need not be a daunting task. It helps to remember that it’s not necessary to take giant steps. You can improve your health and help prevent diabetes with a series of small changes. For best results, keep each goal small, manageable, and as specific as possible.
Eating. Do you eat fast food frequently, or snack on ice cream or potato chips when you watch TV at night? Pick a “bad habit” that is of particular concern and try to “turn it around.” You might, for instance, promise yourself that:
For the next 4 weeks, I will replace my unhealthy evening snacks with fresh fruit, a small bowl of cereal, or (insert another healthy snack here).
Getting active. Have you stopped working out? Are you concerned that working out will require a big time commitment? Think again. Start small and promise yourself that:
For the next 3 weeks, I will take a 20-minute walk 3 mornings a week.
Each time you set a goal, monitor your progress. When you succeed, give yourself a reward—it can be something as simple as a long bath or a trip to the movies—and vow to continue that lifestyle change and to add another. If you aren’t successful, think about why and revise your goal. If you find you’re too busy getting the kids off to school to walk in the morning, for example, change your schedule and start going out during your lunch break. Or, if it’s too cold or rainy, find a nearby mall where you can walk (or a treadmill at a local gym) instead. It also helps to get a step counter, or pedometer. The American Diabetes Association (ADA) recommends taking 10,000 steps per day.
For additional ideas, visit the ADA Web site (www.diabetes.org) and click on Fitness. Or call our office at __________ and make an appointment to come in and discuss additional lifestyle changes—small and large—that you can make with our help.
Validated risk calculators can boost detection rates
In an attempt to improve detection rates of type 2 diabetes and prediabetes, researchers in both the United States and the United Kingdom recently developed easy-to-use risk calculation tools. The Diabetes Risk Calculator (available at http://www.diabetes.org/food-nutrition-lifestyle/lifestyle-prevention/risk-test.jsp), published in 2008, was validated with findings from the Third National Health and Nutrition Survey.10 The calculator uses answers to questions about age, waist circumference, history of gestational diabetes mellitus (GDM), height, race/ethnicity, hypertension, family history, and exercise to determine whether an individual is at high risk for undetected diabetes. The tool has a low positive predictive value (14%), but a negative predictive value >99%.10
The QDScore Diabetes Risk Calculator (www.qdscore.org), another new tool, is designed to estimate an individual’s 10-year risk of developing type 2 diabetes.11 The program, which calculates risk based on answers to questions about family history of diabetes, patient history of cardiovascular disease, smoking, treatment for hypertension, BMI, ethnicity, and steroid use, was validated with data collected from 2.5 million patients in practices throughout England and Wales. The screening tool showed a high degree of discrimination in reflecting differences in disease prevalence related to ethnic and socioeconomic risk factors.11
Pinning down a type 2 (or prediabetes) diagnosis
The ADA, American Association of Clinical Endocrinologists (AACE), USPSTF, and World Health Organization/International Diabetes Federation agree on the diagnostic criteria for type 2 diabetes: a fasting glucose >126 mg/dL, a random plasma glucose ≥200 mg/dL (that must be confirmed on a subsequent day), or both.5,7,12,13 Patient history, risk factors, and additional laboratory tests can help clinicians distinguish between type 1 and type 2 diabetes.
An oral glucose tolerance test (OGTT) is also an option for diagnosis, but time and scheduling difficulties limit the routine use of this test in primary care. Hemoglobin A1c is not recommended as a diagnostic test because of a lack of standardization.1
Prediabetes and type 2 risk. One in 4 (25.9%) US adults 20 years of age or older and more than 1 in 3 (35.9%) of those 60 years of age or older have prediabetes,14 defined as impaired fasting glucose (100-125 mg/dL), impaired glucose tolerance (2-hour glucose test results of 140-199 mg/dL), or both. Prediabetes increases the risk of developing type 2 diabetes by an estimated 30% over a 4-year period,15 and 70% over 30 years,16 although lifestyle interventions can substantially lower the risk. In a recently released consensus statement, an AACE task force noted that in addition to the increased risk of type 2 diabetes, patients with prediabetes face a greater risk of macrovascular complications.17
Type 2 in kids can be mistaken for type 1
As childhood obesity has surged, type 2 diabetes has been diagnosed at an increasingly early age—even in children younger than 10 years.18 Minority youth, primarily African Americans, Hispanics, and Asians/Pacific Islanders, are at increased risk.14 Symptoms can be insidious in children and adolescents and easily missed or mistaken for type 1 diabetes, in part because type 2 diabetes is still relatively rare in this age group.19
Preteens at risk. In a recent study of BMI and metabolic syndrome risk factors in 8- to 14-year-olds, however, researchers concluded that children who are overweight in early adolescence may be at risk for type 2 diabetes as well as cardiovascular disease before they reach their teens.20 There is evidence of a genetic predisposition for type 2 diabetes and defects of β-cell function,5,21 and family history, in addition to weight, is an important consideration in identifying type 2 diabetes in young patients.
Although young adults with type 1 and type 2 diabetes can present with similar symptoms, there may be certain clues to a type 2 diagnosis. Acanthosis nigricans, which is related to insulin resistance and occurs most frequently in obese adolescents, points to a type 2 diagnosis. Increased insulin and C-peptide levels are indicators of type 2 diabetes. Low levels are not necessarily an indication of type 1, however, because patients with type 2 diabetes may have low levels of insulin and C-peptide because of glucose toxicity and lipotoxicity at the time of diagnosis.22 Treatment with insulin may be necessary until glucose toxicity resolves.
Type 1 diabetes: Beyond childhood
Approximately 5% to 10% of patients with diabetes have type 1, which is defined as idiopathic or cellular immune-mediated autoimmune β-cell destruction.5 The rate of destruction is variable—it generally progresses more rapidly in infants and children than in adults. Some people with type 1 diabetes retain residual β-cell function, but have little or no insulin secretion; this manifests as a low or undetectable level of serum C-peptide.
Most cases of type 1 diabetes are diagnosed in patients younger than 18 years. But type 1 diabetes is increasingly recognized as a disorder that also develops in early adulthood, usually before the age of 40.
Arriving at a type 1 diagnosis
Patients with type 1 diabetes often present with modest hyperglycemia, but may rapidly progress to severe hyperglycemia and diabetic ketoacidosis (DKA) when infection or other physical stressors occur.
While screening for autoantibodies in asymptomatic individuals is not recommended,5 patients with blood glucose levels ≥200 mg/dL and symptoms of polydipsia, polyuria, and polyphagia who do not meet the profile for type 2 diabetes may be candidates for additional laboratory work. Approximately 85% to 90% of patients with type 1 diabetes will have antibodies to islet cells or glutamic acid decarboxylase (GAD).5,23
Even without antibody testing, there are distinguishing characteristics that help support a type 1 diagnosis. As a general rule, individuals who develop type 1 diabetes—especially children—are not obese, although patients usually gain weight over time. In addition, many patients with type 1 diabetes have an auto-immune disease, such as celiac or Graves’ disease, hypothyroidism, adrenal anemia, or pernicious anemia; and a first-degree relative with type 1 diabetes. DKA, with acute symptoms of polydipsia and/or polyuria and recent, unintentional weight loss, is suggestive of—but not definitive for—type 1 diabetes.
A recently validated type 1 risk calculator may be particularly useful for screening patients who have a sibling, parent, or child with type 1 diabetes. Using age, BMI, C-peptide concentration, and OGTT results, the algorithm was highly predictive of type 1 diabetes in family members of patients who tested positive for islet cell antibodies.24
Patient doesn’t “fit” type 1 or 2? Consider LADA
LADA, a gradual, progressive form of type 1 diabetes, can be difficult to identify. Circulating GAD or islet cell antibodies are present, but patients don’t have an absolute need for insulin at the time of diagnosis. Thus, they’re often thought to have type 2 diabetes.25 Individuals with LADA show no signs of insulin resistance, however, and over time, β cells decline and insulin usually becomes necessary.
There are no universal recommendations for testing for LADA. Rather, the diagnosis should be considered in those who don’t fit the classic profile for type 1 or type 2 diabetes,26 but have some of the following features:
- age <50 years
- acute symptoms of polydipsia, polyuria, and/or unintentional weight loss
- BMI <25
- a personal history of autoimmune disease
- a family history of autoimmune disease.27
A prospective analysis found that the majority of LADA patients had at least 2 of these distinguishing characteristics.28 Other recent research found heterogeneity among patients with LADA. Noting that not all patients with LADA become insulin-dependent, researchers concluded that the need for insulin is linked to the degree of autoimmunity and β-cell failure.29
When GDM complicates prenatal care
Any degree of carbohydrate intolerance that is first recognized during pregnancy is classified as GDM, whether or not the condition resolves after delivery. A GDM diagnosis does not preclude the possibility of undiagnosed type 2 diabetes or prediabetes, or (rarely) type 1 diabetes.
Approximately 7% of all pregnancies in the United States are complicated by GDM, totaling more than 200,000 cases annually.5 The rate of GDM is in direct proportion to the prevalence of type 2 diabetes in the population in question, and ranges from 1% to 14%. GDM is the diagnosis in nearly 90% of pregnancies complicated by diabetes.5
The GDM screening controversy
Screening for GDM—whether it should be done universally or selectively on the basis of risk factors—is highly controversial. The USPSTF maintains that there is insufficient evidence to recommend for or against screening women with no history of GDM. The American College of Obstetricians and Gynecologists (ACOG)30 and ADA5 recommend selective screening based on patient history, clinical presentation, and, possibly, prior impaired glucose test results or other abnormal laboratory values. AACE calls for universal screening of pregnant women, starting at 20 weeks for high-risk individuals and between 24 and 28 weeks for those at low risk.12
Identifying patients at risk. Maternal age (>25 years), obesity (BMI ≥30), prior GDM or delivery of a large-forgestational-age infant, belonging to a high-risk ethnic group, glycosuria, history of glucose resistance or glucose tolerance, and a first-degree relative with diabetes (TABLE) are risk factors for GDM. Women at high risk—those who meet all or most of these criteria—should undergo early screening: at the first prenatal visit, according to ACOG;30 upon confirmation of pregnancy (ADA);5 at 20 weeks’ gestation (AACE);12 or between 24 and 28 weeks’ gestation (USPSTF).7 ADA and ACOG recommend a 2-stage approach, starting with a 50-g 1-hour OGTT and following up with a 100-g 3-hour OGTT if the first test results are not definitive.5,30 Testing for patients at average risk—which includes any pregnant woman with even a single risk factor, such as being older than 25 years—should be done between 24 and 28 weeks’ gestation, according to ACOG and ADA; testing is not required for women who are <25 years, have a normal body weight, and no other risk factors.
GDM screening in primary care. Because most women fit the criteria for average or high risk,31 family physicians may find universal screening to be more practical than individual risk assessment. Universal screening is associated with favorable outcomes,32 but screening limited to those at high and average risk also has evidence to support it. In a study of 25,118 deliveries, only 4% of women with GDM were missed by the exclusion of low-risk patients.33
In the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, researchers tracked 25,505 women from 9 countries and found a continuous relationship between the risk of macrosomia and the rise in maternal glucose levels.34 The impact on the developing fetus of varying degrees of glucose was studied after a 75-g 2-hour OGTT. The risk of macrosomia increased with fasting blood glucose >75 mg/dL, 1-hour glucose levels >105 mg/dL, and 2-hour glucose concentration >90 mg/dL.35 The most compelling results for adverse effects were associated with fasting glucose levels, rather than glucose tolerance tests.
2 abnormal results needed for a GDM diagnosis
In the absence of unequivocal hyperglycemia, there are 2 diagnostic standards for GDM: The Carpenter-Coustan Conversion and the National Diabetes Data Group Conversion. The Carpenter-Coustan Conversion uses lower glucose values for fasting (≥95 mg/dL) and subsequent 1-, 2-, and 3-hour levels (≥180, 155, and 140 mg/dL, respectively) and is more widely used. But expert opinion also supports the National Diabetes Data Group Conversion criteria (fasting plasma glucose, ≥105 mg/dL; ≥190, 165, and 145 mg/dL for 1-, 2-, and 3-hour OGTT, respectively), and there are no data from clinical trials to prove the superiority of either standard.30
Both sets of standards require 2 or more thresholds to be met or exceeded for a GDM diagnosis. Women with only 1 abnormal value should be monitored carefully, however, as they, too, may be at increased risk for macrosomia and other morbidities.30
Postpartum follow-up. Obtain a fasting glucose reading or perform an OGTT around the time of the postpartum checkup for any patient who was diagnosed with GDM. ACOG recommends using an OGTT to more accurately diagnose type 2 diabetes or prediabetes in these patients, who are at significantly elevated risk.30
Acknowledgement
The authors wish to thank Carol Hildebrandt, a research assistant with no potential conflict of interest, for her help with this manuscript.
Correspondence
Julienne K. Kirk, PharmD, CDE, Department of Family and Community Medicine, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1084; [email protected]
1. Williamson DF, Vinicor F, Bowman BA. Centers of Disease Control and Prevention Primary Prevention Working Group. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med 2004;140:951-957.
2. Centers for Disease Control and Prevention/National Center for Health Statistics. FastStats. Over-weight prevalence. Available at: http://www.cdc.gov/nchs/fastats/overwt.htm. Accessed March 28, 2009.
3. Palmer JP, Hirsch IB. What’s in a name? Diabetes Care. 2003;26:536-538.
4. Centers for Disease Control and Prevention. Health, United States, 2007. Available at: http://www.cdc.gov/nchs/data/hus/hus07.pdf#executivesummary. Accessed October 4, 2008.
5. American Diabetes Association. Clinical practice recommendations 2009. Diabetes Care. 2009;32(suppl 1):S1-S61.
6. National Diabetes Information Clearinghouse. National Institute of Diabetes and Digestive and Kidney Diseases. Am I at risk for type 2 diabetes? Available at: http://diabetes.niddk.nih.gov/DM/pubs/riskfortype2/. Accessed April 10, 2009.
7. US Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: Recommendations and rationale. Available at: http://www.ahrq.gov/clinic/uspstf08/type2/type2summ.htm. Accessed October 4, 2008.
8. International Diabetes Federation. Backgrounder 1: The IDF consensus worldwide definition of the metabolic syndrome. Brussels, Belgium; 2005.
9. Genuth S, Estman R, Kahn R, et al. American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2003;26(suppl 1):S28-S32.
10. Heikes KE, Eddy DM, Arondekar B, et al. Diabetes risk calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31:1040-1045.
11. Hippisley-Cox J, Coupland C, Robson J, et al. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ. 2009;338:b880.-
12. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):S3-S68.
13. World Health Organization. Screening for type 2 diabetes. Report of a World Health Organization and International Diabetes Federation meeting. 2003. http://www.who.int/diabetes/publications/en/screening_mnc03.pdf. Accessed October 4, 2008.
14. National Diabetes Information Clearinghouse. National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007. Available at: http://diabetes.niddk.nih.gov/DM/PUBS/statistics/. Accessed March 27, 2009.
15. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
16. Eddy DM, Schlessinger L, Khan R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251-264.
17. American College of Endocrinology Task Force on Prediabetes. Diagnosis and management of prediabetes in the continuum of hyperglycemia - When do the risks of diabetes begin? Available at: www.aace.com/meetings/consensus/hyperglycemia/hyperglycemia.pdf. Accessed October 4, 2008.
18. SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510-1518.
19. Centers for Disease Control and Prevention. CDC’s Diabetes Program-Diabetes Projects-Children and Diabetes. Available at: http://www.cdc.gov/diabetes/projects/cda2.htm. Accessed March 27, 2009.
20. Messiah SE, Arheart KL, Luke B, et al. Relationship between body mass index and metabolic syndrome risk factors among US 8- to 14-year-olds, 1999 to 2002. J Pediatr. 2008;153:215-221.
21. Fowler MJ. Classification of diabetes: not all hyperglycemia is the same. Clin Diabetes. 2007;25:74-76.
22. Kitabachi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in diabetes. Diabetes Care. 2004;27(suppl):S94-S102.
23. Borg H, Gottsäter A, Landin-Olsson M, et al. High levels of antigen-specific islet antibodies predict future beta-cell failure in patients with onset of diabetes in adult age. J Clin Endocrinol Metab. 2001;86:3032-3238.
24. Sosenko JM, Krischer JP, Palmer JP, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008;31:528-533.
25. Brophy S, Brunt H, Davies H, et al. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Data Syst Rev. 2007(3);CD006165.-
26. Appel SJ, Wadas TM, Rosenthal RS, et al. Latent autoimmune diabetes of adulthood (LADA): an often misdiagnosed type of diabetes mellitus. J Am Acad Nurse Pract. 2009;21:156-159.
27. Unger J. Diagnosing and managing latent autoimmune diabetes in adults. Pract Diabet. 2008;27:32-37.
28. Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31:1546-1549.
29. Radtke MA, Midthjell K, Nilsen TI, et al. Heterogeneity of patients with latent autoimmune diabetes in adults: linkage to autoimmunity is apparent only in those with perceived need for insulin treatment: results from the Nord-Trondelag Health (HUNT) study. Diabetes Care. 2009;32:245-250.
30. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for ObstetricianGynecologists Number 30, September 2001 (Replaces Technical Bulletin Number 200, December 1994): Gestational diabetes. Obstet Gynecol. 2001;98:525-538.
31. Danilenko-Dixon DR, Van Winter JT, Nelson RL, et al. Universal versus selective gestational diabetes screening: application of 1997 American Diabetes Association recommendations. Am J Obstet Gynecol. 1997;81:798-802.
32. Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab. 2006;32:140-146.
33. Williams CB, Iqbal S, Zawacki CM, et al. Effect of selective screening for gestational diabetes. Diabetes Care. 1999;22:418-421.
34. Holt RI. The Hyperglycemia and Adverse Pregnancy Outcomes trial: answers but still more questions about the management of gestational diabetes. Diabet Med. 2008;25:1013-1014.
35. The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
1. Williamson DF, Vinicor F, Bowman BA. Centers of Disease Control and Prevention Primary Prevention Working Group. Primary prevention of type 2 diabetes mellitus by lifestyle intervention: implications for health policy. Ann Intern Med 2004;140:951-957.
2. Centers for Disease Control and Prevention/National Center for Health Statistics. FastStats. Over-weight prevalence. Available at: http://www.cdc.gov/nchs/fastats/overwt.htm. Accessed March 28, 2009.
3. Palmer JP, Hirsch IB. What’s in a name? Diabetes Care. 2003;26:536-538.
4. Centers for Disease Control and Prevention. Health, United States, 2007. Available at: http://www.cdc.gov/nchs/data/hus/hus07.pdf#executivesummary. Accessed October 4, 2008.
5. American Diabetes Association. Clinical practice recommendations 2009. Diabetes Care. 2009;32(suppl 1):S1-S61.
6. National Diabetes Information Clearinghouse. National Institute of Diabetes and Digestive and Kidney Diseases. Am I at risk for type 2 diabetes? Available at: http://diabetes.niddk.nih.gov/DM/pubs/riskfortype2/. Accessed April 10, 2009.
7. US Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: Recommendations and rationale. Available at: http://www.ahrq.gov/clinic/uspstf08/type2/type2summ.htm. Accessed October 4, 2008.
8. International Diabetes Federation. Backgrounder 1: The IDF consensus worldwide definition of the metabolic syndrome. Brussels, Belgium; 2005.
9. Genuth S, Estman R, Kahn R, et al. American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2003;26(suppl 1):S28-S32.
10. Heikes KE, Eddy DM, Arondekar B, et al. Diabetes risk calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31:1040-1045.
11. Hippisley-Cox J, Coupland C, Robson J, et al. Predicting risk of type 2 diabetes in England and Wales: prospective derivation and validation of QDScore. BMJ. 2009;338:b880.-
12. AACE Diabetes Mellitus Clinical Practice Guidelines Task Force. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):S3-S68.
13. World Health Organization. Screening for type 2 diabetes. Report of a World Health Organization and International Diabetes Federation meeting. 2003. http://www.who.int/diabetes/publications/en/screening_mnc03.pdf. Accessed October 4, 2008.
14. National Diabetes Information Clearinghouse. National Institute of Diabetes and Digestive and Kidney Diseases. National Diabetes Statistics, 2007. Available at: http://diabetes.niddk.nih.gov/DM/PUBS/statistics/. Accessed March 27, 2009.
15. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
16. Eddy DM, Schlessinger L, Khan R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251-264.
17. American College of Endocrinology Task Force on Prediabetes. Diagnosis and management of prediabetes in the continuum of hyperglycemia - When do the risks of diabetes begin? Available at: www.aace.com/meetings/consensus/hyperglycemia/hyperglycemia.pdf. Accessed October 4, 2008.
18. SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510-1518.
19. Centers for Disease Control and Prevention. CDC’s Diabetes Program-Diabetes Projects-Children and Diabetes. Available at: http://www.cdc.gov/diabetes/projects/cda2.htm. Accessed March 27, 2009.
20. Messiah SE, Arheart KL, Luke B, et al. Relationship between body mass index and metabolic syndrome risk factors among US 8- to 14-year-olds, 1999 to 2002. J Pediatr. 2008;153:215-221.
21. Fowler MJ. Classification of diabetes: not all hyperglycemia is the same. Clin Diabetes. 2007;25:74-76.
22. Kitabachi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in diabetes. Diabetes Care. 2004;27(suppl):S94-S102.
23. Borg H, Gottsäter A, Landin-Olsson M, et al. High levels of antigen-specific islet antibodies predict future beta-cell failure in patients with onset of diabetes in adult age. J Clin Endocrinol Metab. 2001;86:3032-3238.
24. Sosenko JM, Krischer JP, Palmer JP, et al. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008;31:528-533.
25. Brophy S, Brunt H, Davies H, et al. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Data Syst Rev. 2007(3);CD006165.-
26. Appel SJ, Wadas TM, Rosenthal RS, et al. Latent autoimmune diabetes of adulthood (LADA): an often misdiagnosed type of diabetes mellitus. J Am Acad Nurse Pract. 2009;21:156-159.
27. Unger J. Diagnosing and managing latent autoimmune diabetes in adults. Pract Diabet. 2008;27:32-37.
28. Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care. 2008;31:1546-1549.
29. Radtke MA, Midthjell K, Nilsen TI, et al. Heterogeneity of patients with latent autoimmune diabetes in adults: linkage to autoimmunity is apparent only in those with perceived need for insulin treatment: results from the Nord-Trondelag Health (HUNT) study. Diabetes Care. 2009;32:245-250.
30. American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. Clinical Management Guidelines for ObstetricianGynecologists Number 30, September 2001 (Replaces Technical Bulletin Number 200, December 1994): Gestational diabetes. Obstet Gynecol. 2001;98:525-538.
31. Danilenko-Dixon DR, Van Winter JT, Nelson RL, et al. Universal versus selective gestational diabetes screening: application of 1997 American Diabetes Association recommendations. Am J Obstet Gynecol. 1997;81:798-802.
32. Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab. 2006;32:140-146.
33. Williams CB, Iqbal S, Zawacki CM, et al. Effect of selective screening for gestational diabetes. Diabetes Care. 1999;22:418-421.
34. Holt RI. The Hyperglycemia and Adverse Pregnancy Outcomes trial: answers but still more questions about the management of gestational diabetes. Diabet Med. 2008;25:1013-1014.
35. The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002.
What’s best for your patient with BPH?
- Watchful waiting is recommended for patients with benign prostatic hyperplasia (BPH) whose clinical symptoms do not affect their quality of life (B).
- Use a validated patient questionnaire, such as the American Urological Association’s Symptom Index, to establish the severity of BPH symptoms and follow their progression (B).
- α-Adrenergic blockers (either selective or nonselective) or 5-α reductase inhibitors are appropriate first-line therapies for patients bothered by BPH symptoms (A).
- Consider surgery for patients with severe obstructive symptoms who have not benefited from medical therapy or who prefer surgery as first-line treatment (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
By age 60, more than half of men have histopathologic benign prostatic hyperplasia (BPH).1 And, given the publicity BPH is receiving today, it’s quite possible that those who are experiencing symptoms will be less reticent to discuss it than before.
So what does the evidence tell us about how to best manage these patients? Specifically, do you know what the minimal assessment is for those who are experiencing symptoms? When might advanced testing methods be helpful?
Furthermore, among men who are now 50 years old, the expected lifetime incidence for any type of surgical intervention for BPH is approximately 35%.2 What are the first-line treatments available to these patients? Who might be a candidate for combination drug therapy? Are herbal preparations worth considering? When might surgery be a first choice?
These questions underscore the importance of a proper primary care framework for evaluating and treating BPH, which we can develop based on a consensus guideline released by the American Urological Association (AUA)1 and on more recent research.
Assessing symptoms: 2 tools can help
Symptoms of BPH can include urinary frequency, nocturia, urgency, hesitancy, weak or intermittent urine stream, straining to void, and a sensation of incomplete voiding.1 Each patient experiences a unique constellation of these symptoms. Using a urinary symptom scoring system can help define the severity of BPH and be useful in monitoring the success of subsequent therapy. Available instruments for this purpose include the AUA’s 7-question Symptom Index for BPH (http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph) and the International Prostate Symptom Score, which adds an eighth question to the AUA list to gauge the extent to which symptoms bother a patient (http://www.usli.net/uro/Forms/ipss.pdf).1-5 If the patient is unclear about the pattern of his symptoms, consider asking him to keep a voiding diary.
Ruling out other causes of BPH-like symptoms
TABLE 1 lists the differential diagnoses of obstructive urinary symptoms, otherwise known as lower urinary tract symptoms (LUTS).
Look for clues in the history. Ask the patient whether he uses medications known to cause obstructive urinary symptoms—tricyclic antidepressants, first-generation antihistamines, anticholinergic agents, diuretics, narcotics, and decongestants. Does he have any first-degree relatives with prostate cancer? If the answer is yes, how young was he when the cancer was diagnosed?
Focus your examination. Perform a digital rectal examination (DRE) to check prostate size and to detect palpable nodules, induration, or irregularities associated with malignancy or infection. An enlarged prostate is commonly found on rectal examination; however, the degree of hypertrophy does not necessarily correlate with the degree of obstruction or the severity of symptoms. Any irregularity suggestive of cancer requires that you talk to your patient about his preferences for further investigation.6
Conduct a neurologic exam to check mental status, gait, lower extremity strength, and anal sphincter tone to assess for conditions that could cause a neurogenic bladder.
TABLE 1
Lower urinary tract symptoms are also seen with these disorders31
| DISORDER | FINDINGS |
|---|---|
| Bladder calculi | Hematuria, ultrasonography finding |
| Bladder neck dyssynergia | LUTS in younger patients with normal prostate size, diagnosed by cystoscopy or VCUG |
| Overactive bladder | Urgency with possible urge incontinence |
| Prostate cancer | Finding in DRE, elevated serum PSA |
| Prostatitis | Tender prostate gland |
| Stricture of the bladder neck | Prior invasive treatment |
| Urinary bladder cancer | Hematuria, abnormal cytological finding |
| Urethral stricture | Box-shaped flow curve on urinary flow-rate measurement |
| DRE, digital rectal examination; LUTS, lower urinary tract symptoms; PSA, prostate-specific antigen; VCUG, voiding cystourethrogram. | |
Consider these tests. Urinary tract infection (UTI) or bladder cancer may produce symptoms similar to those of BPH. For any patient who has LUTS, perform a urinalysis to screen for infection or hematuria. If a UTI is found, treat it and re-evaluate the patient. If you detect microscopic hematuria, do a further work-up to rule out bladder cancer. If DRE findings are suggestive of prostate cancer, you’ll need an ultrasound-guided biopsy and histological examination to make the diagnosis.
Optional tests in the work-up of LUTS include urinary flow rate measurements, post-void residual urine measurements, and pressure flow studies. These tests may be informative if the diagnosis is unclear based on the history and physical exam or when patients do not respond to initial therapy. Ultrasonography, intravenous pyelography, filling cystometrography, and cystoscopy are not routinely recommended for the evaluation of suspected BPH. However, they may be helpful if a patient has a complex medical history (eg, neurologic disorder or other disease known to affect bladder function, or prior failure of BPH therapy), or if he wants to pursue invasive therapy.1
Talk to your patient about the controversial PSA test. Measuring serum prostate-specific antigen (PSA) levels to screen for prostate cancer is controversial, even for patients with LUTS. Although PSA testing can effectively detect prostate cancer in its early pathologic stages, researchers continue to investigate whether early detection significantly improves outcomes. Quite recently, 2 studies demonstrated that the test saves few lives;7,8 1410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer.
A subset of detected cancers appears to be clinically significant, but many cancers will not progress during a patient’s lifetime.3,5 The United States Preventive Services Task Force (USPSTF) has concluded that the evidence is insufficient to recommend for or against routine prostate cancer screening due to the uncertainty of the balance of potential benefits (reduction of prostate cancer morbidity and mortality) and risks (false-positive results, unnecessary biopsies, and possible complications) of treatment for early disease.9-12 Furthermore, in its 2008 update, the USPSTF specifically recommended against screening for prostate cancer in men 75 years of age and older.
The American Cancer Society says that discouraging or not offering testing is inappropriate: Men who ask their physicians to make the decision on their behalf should be tested.9 A 2006 Cochrane review of this topic found only 2 eligible randomized trials, both of which had high risk of bias. They concluded that insufficient high-quality evidence exists to support or refute the use of any screening for prostate cancer in any patient population, including those with BPH.13
Given this conflicting advice, discuss the benefits and limitations of screening with your patient before deciding whether or not to test.
What is the optimal Approach to treatment?
Patients who are not bothered by their urinary symptoms—even those with moderate to severe symptom scores—can be managed with watchful waiting. Because of the cost and frequent side effects of medications for BPH, these patients generally will not benefit from drug therapy.3,5,14,15 However, follow-up monitoring is important, because the severity of BPH can change even without treatment.
Even if patients with moderate or severe AUA symptom scores are not bothered by their symptoms, inform them of appropriate treatment options.3 When urinary obstruction symptoms from BPH significantly interfere with daily living and sleep activities, treatment is justified.1
Medical management, yes, but which option?
Medical therapies are not as effective as surgical intervention,16 but they often provide adequate symptom relief and cause fewer, less severe, and less permanent adverse effects than surgery. Initiate treatment with medical therapy if (1) the patient is bothered by his symptoms, (2) no significant urinary obstruction exists, and (3) you have followed the patient’s preference for prostate cancer evaluation. TABLE 2 lists prescription medications for the treatment of BPH.
Nonselective α-adrenergic blockers, such as doxazosin and terazosin, reduce prostatic smooth muscle tone, thereby improving urinary flow. A Cochrane systematic review and a subsequent large randomized trial found terazosin to be superior to placebo in improving urinary flow and decreasing symptoms in men with BPH.16,17
Selective α-adrenergic blockers, such as alfuzosin and tamsulosin, are highly selective α-1A-adrenergic antagonists. They are believed to be as effective as the nonselective agents, and patients may experience fewer side effects than with nonselective agents.1,18 However, these drugs are considerably more expensive than their nonselective counterparts.
5-α Reductase inhibitors, such as finasteride and dutasteride, are more effective than placebo for patients with LUTS associated with demonstrable prostate enlargement. In a Cochrane review and subsequent large randomized trial, finasteride proved inferior to terazosin.16,17 However, finasteride reduces the progression to urinary obstruction and the need for invasive therapy; terazosin does not.17 Finasteride achieves this effect by reducing prostatic volume by about 20% over 3 to 6 months of treatment.
Finasteride decreases PSA levels by 40% to 50%. If you conduct PSA screening for prostate cancer in a patient taking finasteride, double the PSA level before comparing it with age-related norms. Handled this way, PSA screening will not lose its sensitivity or specificity for the diagnosis of prostate cancer.19
Though a 5-mg daily regimen of finasteride reduces the overall risk of prostate cancer from 24.4% to 18.4%, it increases the risk of high-grade disease associated with higher mortality from 5.1% to 6.4%. Warn patients of this risk.20
Combining an α-adrenergic blocker and 5-α reductase inhibitor. Combination therapy is appropriate and effective for patients with LUTS associated with demonstrable prostate enlargement for whom monotherapy has failed.9 Taken together, a 5-α reductase inhibitor and a nonselective α-1A-adrenergic blocker alleviate symptoms more effectively than either drug can do alone.21,22 The incidence of most adverse drug reactions with the combination is similar to the baseline risk for each drug. However, ejaculatory abnormalities are reported in 7% of patients in the combination therapy group vs 2% or less in the monotherapy groups. Discontinuation rates for combination therapy are comparable to those in the nonselective α-adrenergic blocker group.22 The adverse effect profile of combining selective α-adrenergic blockers with 5-α reductase inhibitors has not been reported; however, the combination is often used in practice.
For patients to make an informed decision about treatment, discuss with them the common adverse reactions from these agents (TABLE 2) and the need for long-term daily therapy. Also give the patient a reasonable estimate of the risk of his retention symptoms progressing.
TABLE 2
BPH medications: How they compare
| AUA RECOMMENDATION FOR USE WITH LUTS SECONDARY TO BPH1 | DRUG | DOSE | MOST COMMON SIDE EFFECTS (%) | COSTS |
|---|---|---|---|---|
| Nonselective α-adrenergic blockers | ||||
| Useful as first-line therapy due to efficacy and low cost α-Aadrenergic blockers can be used with other therapies as needed | Doxazosin (Cardura, generics) | Start at 1 mg; titrate by doubling dose every 1-2 wk. Goal: 4-8 mg. Maximum dose, 8 mg | Dizziness (16), headache (10), fatigue (8), edema (3), dyspnea (3), orthostatic hypotension (2), abdominal pain (2)* | $4 for 30-day supply for both generic agents† |
| Terazosin (Hytrin, generics) | Start at 1 mg at bedtime, increase PRN over 4-6 wk; most patients require 10 mg. If no response at 10 mg, may increase to 20 mg | Dizziness (9), fatigue (7), headache (5), orthostatic hypotension (4), somnolence (4), nasal congestion (2), ED (2)‡ | ||
| Selective α-adrenergic blockers | ||||
| All believed to be equal in clinical effectiveness | Alfuzosin (Uroxatral or Xatral) | 10 mg/d with the same meal | Dizziness (6), headache (3), upper respiratory infection (3), fatigue (3)§ | $112 for 30-day supply// |
| Tamsulosin (Flomax) | 0.4 mg/d (30 min after same meal); may increase after 2-4 wk to 0.8 mg/d if no response | Headache (19), dizziness (15), rhinitis (13), infection (9), fatigue (8), abnormal ejaculation (8)¶ | $110 for 30-day supply// | |
| 5-α Reductase inhibitors | ||||
| All believed to be appropriate and effective treatments for patients with demonstrable prostate enlargement | Finasteride (Proscar) | 5 mg/d | ED (8), decreased libido (6), decreased volume of ejaculate (4)# | $70 for 30-day generic supply// |
| Dutasteride (Avodart) | 0.5 mg/d | ED (5), decreased libido (3), ejaculation disorder (1), gynecomastia (1)** | $20-$30 for 30-day generic supply†† | |
| AUA, American Urological Association; BPH, benign prostatic hypertrophy; ED, erectile dysfunction; LUTS, lower urinary tract symptoms. | ||||
| * http://www.fda.gov/medwatch/SAFETY/2006/Feb_PI/Cardura_PI.pdf. | ||||
| † Prices listed on Walmart.com as of April 6, 2009. | ||||
| ‡http://www.fda.gov/medwatch/SAFETY/2006/Feb_PI/Hytrin%20Caps_PI.pdf. | ||||
| §http://www.fda.gov/medwatch/safety/2008/Sep_PI/Uroxatral_PI.pdf. | ||||
| // Prices listed on Drugstore.com as of April 6, 2009. | ||||
| ¶http://www.fda.gov/medwatch/SAFETY/2008/Apr_PI/Flomax_PI.pdf. | ||||
| #http://www.fda.gov/medwatch/SAFETY/2004/apr_PI/Proscar_PI.pdf. | ||||
| ** http://www.fda.gov/medwatch/SAFETY/2004/sep_PI/Avodart_PI.pdf. | ||||
| †† Prices listed on Pharmacychecker.com as of April 6, 2009. | ||||
Complementary medicine: Information is still limited
Herbal or complementary medicines are used worldwide to treat BPH. These products are not regulated by the US Food and Drug Administration (FDA), and therefore no standardized formulation or dosing exists. Although a few substances appear to have some positive effects, high-quality clinical trials on clinical outcomes are lacking.
The AUA guideline does not recommend the use of phytotherapy.1 Despite this, many patients—and any number of physicians—turn to phytotherapy to treat LUTS associated with BPH.
Some patients turn to phytotherapy without their physician’s knowledge, so it’s important to ask whether they are using any herbal preparations. Agents currently used include saw palmetto, African plum, South African star grass, and Cernilton.
Saw palmetto (Serenoa repens) has been used by more than 2 million men in the United States. In 2006, Bent et al conducted a rigorously designed double-blinded trial in which 225 men older than 49 years with moderate-to-severe symptoms of BPH were treated for 1 year with saw palmetto extract (160 mg twice a day) or placebo.23 Saw palmetto did not ameliorate the symptoms of BPH. In contrast, a Cochrane systematic review last updated in 2002 asserted that this substance caused mild-to-moderate reductions in urologic symptoms and flow measures when given to men with symptomatic BPH.24 Long-term efficacy and safety of this product are unknown. Given the efficacy of saw palmetto, it is a reasonable option for men who prefer a nonprescription product to treat symptoms of BPH.
African plum (Pygeum africanum) is more effective than placebo in reducing symptoms of BPH and has few side effects, based on poorly designed small studies.25,26 Comparative data with finasteride or the α-adrenergic blockers are lacking.
South African star grass (Hypoxis rooperi and certain species of Pinus and Picea) contain beta-sitosterols and are sources for phytotherapeutic treatments for BPH. A systematic review analyzed the effects of beta-sitosterols in men and found improved urinary symptom scores and flow measures (n=519; 4 randomized, controlled, double-blind trials; duration, 4-26 weeks).27 Their long-term effectiveness and safety are not known.
Cernilton, prepared from the ryegrass pollen Secale cereale, is marketed for the treatment of BPH. A Cochrane systematic review demonstrated that comparative trials lacked a proven active control. Available evidence suggests that short-term use of cernilton is well tolerated and modestly decreases overall urologic symptoms, including nocturia. Additional randomized placebo and active-controlled trials are needed to evaluate the long-term clinical effectiveness and safety of Cernilton.28
Acupuncture was not effective in treating LUTS in men in randomized controlled (single-blinded) trials.29
When patients don’t respond to medical treatment
Surgery is recommended for patients who have not responded to medical treatment, who have refractory retention with a failed attempt at catheter removal, or who experience recurrent UTIs, persistent hematuria, bladder stones, or renal insufficiency.3 In addition, surgery can be the initial treatment choice for patients with high AUA symptom scores who opt for this intervention and are good operative candidates.
The specific surgical approach (open or endoscopic; electrocautery or laser) are technical decisions based on the patient’s prostate size, the individual surgeon’s judgment, and the patient’s comorbidities.1,30 For patients who are not surgical candidates, treatment with intermittent catheterization, an indwelling catheter, or a stent is recommended.
Acknowledgements
The authors thank Jen Creer, MA, of Edit Rx, LLC, for her assistance in the preparation of this manuscript. The University of Nebraska Medical Center, with which both authors were previously affiliated, funded this writing assistance.
Correspondence
Drew M. Keister, MD, Lehigh Valley Health Network, PO Box 7017, Allentown, PA 18105-7017; [email protected]
1. American Urological Association. AUA guideline on the management of benign prostatic hyperplasia: diagnosis and treatment recommendations. 2003/updated 2006. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinicalguidelines.cfm?sub=bph. Accessed April 2, 2009.
2. Oesterling JE. Benign prostatic hyperplasia: medical and minimally invasive treatment options. N Engl J Med. 1995;332:99-109.
3. McConnell JD. Clinical practice guideline no. 8: Benign prostatic hyperplasia: diagnosis and treatment. Benign Prostatic Hyperplasia Guideline Panel. Rockville, Md: US Dept of Health and Human Services, Agency for Health Care Policy and Research; 1994. AHCPR publication 94-0582.
4. Austin O, Ricer RE. (1996). Prostate cancer screening: an appraisal of the PSA test. Fam Pract Recert. 1996;18:81-91.
5. Barry MJ, Fowler FJ, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557.
6. Denis LJ. Diagnosing benign prostatic hyperplasia versus prostate cancer. Br J Urol. 1995;76(suppl 1):S17-S23.
7. Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310-1319.
8. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
9. American Cancer Society Guidelines for the Early Detection of Cancer. Revised March 5, 2008. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp. Accessed April 6, 2009.
10. US Preventive Services Task Force. Screening for prostate cancer: recommendation statement. Available at http://www.ahrq.gov/clinic/uspstf08/prostate/prostaters.htm. Accessed April 3, 2009.
11. American Cancer Society. Cancer Facts & Figures. Atlanta, Ga: 2008. Available at http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. Accessed on April 3, 2009.
12. Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2006;56:11-25.
13. Ilic D, O’Connor D, Green S, et al. Screening for prostate cancer. Cochrane Database Syst Rev. 2006;(3):CD004720.-
14. Barry MJ. Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology. 2001;58(suppl 1):S25-S32.
15. Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery—what we have learned and where we are going. J Urol. 1999;162:293-306.
16. Wilt T, Howe RW, Rutks I, et al. Terazosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(4):CD003851.-
17. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-2398.
18. Wilt T, MacDonald R, Rutks I. Tamsulosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2003;(1):CD002081.-
19. Guess HA, Gormley GJ, Stoner E, et al. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996;155:3-9.
20. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-224.
21. Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469-471.
22. Lepor HL, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533-540.
23. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
24. Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 1999;(1):CD001423.-
25. Ishani A, MacDonald R, Nelson D, et al. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med. 2000;109:654-664.
26. Wilt T, Ishani A, MacDonald R, et al. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(1):CD001044.-
27. Wilt T, Ishani A, MacDonald R, et al. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst Rev. 1999;(4):CD001043.-
28. Wilt T, MacDonald R, Ishani A, et al. Cernilton for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2000;(2):CD001042.-
29. Johnstone PA, Bloom TL, Niemtzow RC, et al. A prospective, randomized pilot trial of acupuncture of the kidney-bladder distinct meridian for lower urinary tract symptoms. J Urol. 2003;169:1037-1039.
30. Keister DM, Neal R. Managing BPH: when to consider surgery. Am Fam Physician. 2008;77:1391-1392.
31. Finnish Medical Society Duodecim. Benign prostatic hyperplasia. In: EBM Guidelines. Evidence-Based Medicine [CD-ROM]. Helsinki, Finland: Duodecim Medical Publications Ltd; 2005.
- Watchful waiting is recommended for patients with benign prostatic hyperplasia (BPH) whose clinical symptoms do not affect their quality of life (B).
- Use a validated patient questionnaire, such as the American Urological Association’s Symptom Index, to establish the severity of BPH symptoms and follow their progression (B).
- α-Adrenergic blockers (either selective or nonselective) or 5-α reductase inhibitors are appropriate first-line therapies for patients bothered by BPH symptoms (A).
- Consider surgery for patients with severe obstructive symptoms who have not benefited from medical therapy or who prefer surgery as first-line treatment (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
By age 60, more than half of men have histopathologic benign prostatic hyperplasia (BPH).1 And, given the publicity BPH is receiving today, it’s quite possible that those who are experiencing symptoms will be less reticent to discuss it than before.
So what does the evidence tell us about how to best manage these patients? Specifically, do you know what the minimal assessment is for those who are experiencing symptoms? When might advanced testing methods be helpful?
Furthermore, among men who are now 50 years old, the expected lifetime incidence for any type of surgical intervention for BPH is approximately 35%.2 What are the first-line treatments available to these patients? Who might be a candidate for combination drug therapy? Are herbal preparations worth considering? When might surgery be a first choice?
These questions underscore the importance of a proper primary care framework for evaluating and treating BPH, which we can develop based on a consensus guideline released by the American Urological Association (AUA)1 and on more recent research.
Assessing symptoms: 2 tools can help
Symptoms of BPH can include urinary frequency, nocturia, urgency, hesitancy, weak or intermittent urine stream, straining to void, and a sensation of incomplete voiding.1 Each patient experiences a unique constellation of these symptoms. Using a urinary symptom scoring system can help define the severity of BPH and be useful in monitoring the success of subsequent therapy. Available instruments for this purpose include the AUA’s 7-question Symptom Index for BPH (http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph) and the International Prostate Symptom Score, which adds an eighth question to the AUA list to gauge the extent to which symptoms bother a patient (http://www.usli.net/uro/Forms/ipss.pdf).1-5 If the patient is unclear about the pattern of his symptoms, consider asking him to keep a voiding diary.
Ruling out other causes of BPH-like symptoms
TABLE 1 lists the differential diagnoses of obstructive urinary symptoms, otherwise known as lower urinary tract symptoms (LUTS).
Look for clues in the history. Ask the patient whether he uses medications known to cause obstructive urinary symptoms—tricyclic antidepressants, first-generation antihistamines, anticholinergic agents, diuretics, narcotics, and decongestants. Does he have any first-degree relatives with prostate cancer? If the answer is yes, how young was he when the cancer was diagnosed?
Focus your examination. Perform a digital rectal examination (DRE) to check prostate size and to detect palpable nodules, induration, or irregularities associated with malignancy or infection. An enlarged prostate is commonly found on rectal examination; however, the degree of hypertrophy does not necessarily correlate with the degree of obstruction or the severity of symptoms. Any irregularity suggestive of cancer requires that you talk to your patient about his preferences for further investigation.6
Conduct a neurologic exam to check mental status, gait, lower extremity strength, and anal sphincter tone to assess for conditions that could cause a neurogenic bladder.
TABLE 1
Lower urinary tract symptoms are also seen with these disorders31
| DISORDER | FINDINGS |
|---|---|
| Bladder calculi | Hematuria, ultrasonography finding |
| Bladder neck dyssynergia | LUTS in younger patients with normal prostate size, diagnosed by cystoscopy or VCUG |
| Overactive bladder | Urgency with possible urge incontinence |
| Prostate cancer | Finding in DRE, elevated serum PSA |
| Prostatitis | Tender prostate gland |
| Stricture of the bladder neck | Prior invasive treatment |
| Urinary bladder cancer | Hematuria, abnormal cytological finding |
| Urethral stricture | Box-shaped flow curve on urinary flow-rate measurement |
| DRE, digital rectal examination; LUTS, lower urinary tract symptoms; PSA, prostate-specific antigen; VCUG, voiding cystourethrogram. | |
Consider these tests. Urinary tract infection (UTI) or bladder cancer may produce symptoms similar to those of BPH. For any patient who has LUTS, perform a urinalysis to screen for infection or hematuria. If a UTI is found, treat it and re-evaluate the patient. If you detect microscopic hematuria, do a further work-up to rule out bladder cancer. If DRE findings are suggestive of prostate cancer, you’ll need an ultrasound-guided biopsy and histological examination to make the diagnosis.
Optional tests in the work-up of LUTS include urinary flow rate measurements, post-void residual urine measurements, and pressure flow studies. These tests may be informative if the diagnosis is unclear based on the history and physical exam or when patients do not respond to initial therapy. Ultrasonography, intravenous pyelography, filling cystometrography, and cystoscopy are not routinely recommended for the evaluation of suspected BPH. However, they may be helpful if a patient has a complex medical history (eg, neurologic disorder or other disease known to affect bladder function, or prior failure of BPH therapy), or if he wants to pursue invasive therapy.1
Talk to your patient about the controversial PSA test. Measuring serum prostate-specific antigen (PSA) levels to screen for prostate cancer is controversial, even for patients with LUTS. Although PSA testing can effectively detect prostate cancer in its early pathologic stages, researchers continue to investigate whether early detection significantly improves outcomes. Quite recently, 2 studies demonstrated that the test saves few lives;7,8 1410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer.
A subset of detected cancers appears to be clinically significant, but many cancers will not progress during a patient’s lifetime.3,5 The United States Preventive Services Task Force (USPSTF) has concluded that the evidence is insufficient to recommend for or against routine prostate cancer screening due to the uncertainty of the balance of potential benefits (reduction of prostate cancer morbidity and mortality) and risks (false-positive results, unnecessary biopsies, and possible complications) of treatment for early disease.9-12 Furthermore, in its 2008 update, the USPSTF specifically recommended against screening for prostate cancer in men 75 years of age and older.
The American Cancer Society says that discouraging or not offering testing is inappropriate: Men who ask their physicians to make the decision on their behalf should be tested.9 A 2006 Cochrane review of this topic found only 2 eligible randomized trials, both of which had high risk of bias. They concluded that insufficient high-quality evidence exists to support or refute the use of any screening for prostate cancer in any patient population, including those with BPH.13
Given this conflicting advice, discuss the benefits and limitations of screening with your patient before deciding whether or not to test.
What is the optimal Approach to treatment?
Patients who are not bothered by their urinary symptoms—even those with moderate to severe symptom scores—can be managed with watchful waiting. Because of the cost and frequent side effects of medications for BPH, these patients generally will not benefit from drug therapy.3,5,14,15 However, follow-up monitoring is important, because the severity of BPH can change even without treatment.
Even if patients with moderate or severe AUA symptom scores are not bothered by their symptoms, inform them of appropriate treatment options.3 When urinary obstruction symptoms from BPH significantly interfere with daily living and sleep activities, treatment is justified.1
Medical management, yes, but which option?
Medical therapies are not as effective as surgical intervention,16 but they often provide adequate symptom relief and cause fewer, less severe, and less permanent adverse effects than surgery. Initiate treatment with medical therapy if (1) the patient is bothered by his symptoms, (2) no significant urinary obstruction exists, and (3) you have followed the patient’s preference for prostate cancer evaluation. TABLE 2 lists prescription medications for the treatment of BPH.
Nonselective α-adrenergic blockers, such as doxazosin and terazosin, reduce prostatic smooth muscle tone, thereby improving urinary flow. A Cochrane systematic review and a subsequent large randomized trial found terazosin to be superior to placebo in improving urinary flow and decreasing symptoms in men with BPH.16,17
Selective α-adrenergic blockers, such as alfuzosin and tamsulosin, are highly selective α-1A-adrenergic antagonists. They are believed to be as effective as the nonselective agents, and patients may experience fewer side effects than with nonselective agents.1,18 However, these drugs are considerably more expensive than their nonselective counterparts.
5-α Reductase inhibitors, such as finasteride and dutasteride, are more effective than placebo for patients with LUTS associated with demonstrable prostate enlargement. In a Cochrane review and subsequent large randomized trial, finasteride proved inferior to terazosin.16,17 However, finasteride reduces the progression to urinary obstruction and the need for invasive therapy; terazosin does not.17 Finasteride achieves this effect by reducing prostatic volume by about 20% over 3 to 6 months of treatment.
Finasteride decreases PSA levels by 40% to 50%. If you conduct PSA screening for prostate cancer in a patient taking finasteride, double the PSA level before comparing it with age-related norms. Handled this way, PSA screening will not lose its sensitivity or specificity for the diagnosis of prostate cancer.19
Though a 5-mg daily regimen of finasteride reduces the overall risk of prostate cancer from 24.4% to 18.4%, it increases the risk of high-grade disease associated with higher mortality from 5.1% to 6.4%. Warn patients of this risk.20
Combining an α-adrenergic blocker and 5-α reductase inhibitor. Combination therapy is appropriate and effective for patients with LUTS associated with demonstrable prostate enlargement for whom monotherapy has failed.9 Taken together, a 5-α reductase inhibitor and a nonselective α-1A-adrenergic blocker alleviate symptoms more effectively than either drug can do alone.21,22 The incidence of most adverse drug reactions with the combination is similar to the baseline risk for each drug. However, ejaculatory abnormalities are reported in 7% of patients in the combination therapy group vs 2% or less in the monotherapy groups. Discontinuation rates for combination therapy are comparable to those in the nonselective α-adrenergic blocker group.22 The adverse effect profile of combining selective α-adrenergic blockers with 5-α reductase inhibitors has not been reported; however, the combination is often used in practice.
For patients to make an informed decision about treatment, discuss with them the common adverse reactions from these agents (TABLE 2) and the need for long-term daily therapy. Also give the patient a reasonable estimate of the risk of his retention symptoms progressing.
TABLE 2
BPH medications: How they compare
| AUA RECOMMENDATION FOR USE WITH LUTS SECONDARY TO BPH1 | DRUG | DOSE | MOST COMMON SIDE EFFECTS (%) | COSTS |
|---|---|---|---|---|
| Nonselective α-adrenergic blockers | ||||
| Useful as first-line therapy due to efficacy and low cost α-Aadrenergic blockers can be used with other therapies as needed | Doxazosin (Cardura, generics) | Start at 1 mg; titrate by doubling dose every 1-2 wk. Goal: 4-8 mg. Maximum dose, 8 mg | Dizziness (16), headache (10), fatigue (8), edema (3), dyspnea (3), orthostatic hypotension (2), abdominal pain (2)* | $4 for 30-day supply for both generic agents† |
| Terazosin (Hytrin, generics) | Start at 1 mg at bedtime, increase PRN over 4-6 wk; most patients require 10 mg. If no response at 10 mg, may increase to 20 mg | Dizziness (9), fatigue (7), headache (5), orthostatic hypotension (4), somnolence (4), nasal congestion (2), ED (2)‡ | ||
| Selective α-adrenergic blockers | ||||
| All believed to be equal in clinical effectiveness | Alfuzosin (Uroxatral or Xatral) | 10 mg/d with the same meal | Dizziness (6), headache (3), upper respiratory infection (3), fatigue (3)§ | $112 for 30-day supply// |
| Tamsulosin (Flomax) | 0.4 mg/d (30 min after same meal); may increase after 2-4 wk to 0.8 mg/d if no response | Headache (19), dizziness (15), rhinitis (13), infection (9), fatigue (8), abnormal ejaculation (8)¶ | $110 for 30-day supply// | |
| 5-α Reductase inhibitors | ||||
| All believed to be appropriate and effective treatments for patients with demonstrable prostate enlargement | Finasteride (Proscar) | 5 mg/d | ED (8), decreased libido (6), decreased volume of ejaculate (4)# | $70 for 30-day generic supply// |
| Dutasteride (Avodart) | 0.5 mg/d | ED (5), decreased libido (3), ejaculation disorder (1), gynecomastia (1)** | $20-$30 for 30-day generic supply†† | |
| AUA, American Urological Association; BPH, benign prostatic hypertrophy; ED, erectile dysfunction; LUTS, lower urinary tract symptoms. | ||||
| * http://www.fda.gov/medwatch/SAFETY/2006/Feb_PI/Cardura_PI.pdf. | ||||
| † Prices listed on Walmart.com as of April 6, 2009. | ||||
| ‡http://www.fda.gov/medwatch/SAFETY/2006/Feb_PI/Hytrin%20Caps_PI.pdf. | ||||
| §http://www.fda.gov/medwatch/safety/2008/Sep_PI/Uroxatral_PI.pdf. | ||||
| // Prices listed on Drugstore.com as of April 6, 2009. | ||||
| ¶http://www.fda.gov/medwatch/SAFETY/2008/Apr_PI/Flomax_PI.pdf. | ||||
| #http://www.fda.gov/medwatch/SAFETY/2004/apr_PI/Proscar_PI.pdf. | ||||
| ** http://www.fda.gov/medwatch/SAFETY/2004/sep_PI/Avodart_PI.pdf. | ||||
| †† Prices listed on Pharmacychecker.com as of April 6, 2009. | ||||
Complementary medicine: Information is still limited
Herbal or complementary medicines are used worldwide to treat BPH. These products are not regulated by the US Food and Drug Administration (FDA), and therefore no standardized formulation or dosing exists. Although a few substances appear to have some positive effects, high-quality clinical trials on clinical outcomes are lacking.
The AUA guideline does not recommend the use of phytotherapy.1 Despite this, many patients—and any number of physicians—turn to phytotherapy to treat LUTS associated with BPH.
Some patients turn to phytotherapy without their physician’s knowledge, so it’s important to ask whether they are using any herbal preparations. Agents currently used include saw palmetto, African plum, South African star grass, and Cernilton.
Saw palmetto (Serenoa repens) has been used by more than 2 million men in the United States. In 2006, Bent et al conducted a rigorously designed double-blinded trial in which 225 men older than 49 years with moderate-to-severe symptoms of BPH were treated for 1 year with saw palmetto extract (160 mg twice a day) or placebo.23 Saw palmetto did not ameliorate the symptoms of BPH. In contrast, a Cochrane systematic review last updated in 2002 asserted that this substance caused mild-to-moderate reductions in urologic symptoms and flow measures when given to men with symptomatic BPH.24 Long-term efficacy and safety of this product are unknown. Given the efficacy of saw palmetto, it is a reasonable option for men who prefer a nonprescription product to treat symptoms of BPH.
African plum (Pygeum africanum) is more effective than placebo in reducing symptoms of BPH and has few side effects, based on poorly designed small studies.25,26 Comparative data with finasteride or the α-adrenergic blockers are lacking.
South African star grass (Hypoxis rooperi and certain species of Pinus and Picea) contain beta-sitosterols and are sources for phytotherapeutic treatments for BPH. A systematic review analyzed the effects of beta-sitosterols in men and found improved urinary symptom scores and flow measures (n=519; 4 randomized, controlled, double-blind trials; duration, 4-26 weeks).27 Their long-term effectiveness and safety are not known.
Cernilton, prepared from the ryegrass pollen Secale cereale, is marketed for the treatment of BPH. A Cochrane systematic review demonstrated that comparative trials lacked a proven active control. Available evidence suggests that short-term use of cernilton is well tolerated and modestly decreases overall urologic symptoms, including nocturia. Additional randomized placebo and active-controlled trials are needed to evaluate the long-term clinical effectiveness and safety of Cernilton.28
Acupuncture was not effective in treating LUTS in men in randomized controlled (single-blinded) trials.29
When patients don’t respond to medical treatment
Surgery is recommended for patients who have not responded to medical treatment, who have refractory retention with a failed attempt at catheter removal, or who experience recurrent UTIs, persistent hematuria, bladder stones, or renal insufficiency.3 In addition, surgery can be the initial treatment choice for patients with high AUA symptom scores who opt for this intervention and are good operative candidates.
The specific surgical approach (open or endoscopic; electrocautery or laser) are technical decisions based on the patient’s prostate size, the individual surgeon’s judgment, and the patient’s comorbidities.1,30 For patients who are not surgical candidates, treatment with intermittent catheterization, an indwelling catheter, or a stent is recommended.
Acknowledgements
The authors thank Jen Creer, MA, of Edit Rx, LLC, for her assistance in the preparation of this manuscript. The University of Nebraska Medical Center, with which both authors were previously affiliated, funded this writing assistance.
Correspondence
Drew M. Keister, MD, Lehigh Valley Health Network, PO Box 7017, Allentown, PA 18105-7017; [email protected]
- Watchful waiting is recommended for patients with benign prostatic hyperplasia (BPH) whose clinical symptoms do not affect their quality of life (B).
- Use a validated patient questionnaire, such as the American Urological Association’s Symptom Index, to establish the severity of BPH symptoms and follow their progression (B).
- α-Adrenergic blockers (either selective or nonselective) or 5-α reductase inhibitors are appropriate first-line therapies for patients bothered by BPH symptoms (A).
- Consider surgery for patients with severe obstructive symptoms who have not benefited from medical therapy or who prefer surgery as first-line treatment (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
By age 60, more than half of men have histopathologic benign prostatic hyperplasia (BPH).1 And, given the publicity BPH is receiving today, it’s quite possible that those who are experiencing symptoms will be less reticent to discuss it than before.
So what does the evidence tell us about how to best manage these patients? Specifically, do you know what the minimal assessment is for those who are experiencing symptoms? When might advanced testing methods be helpful?
Furthermore, among men who are now 50 years old, the expected lifetime incidence for any type of surgical intervention for BPH is approximately 35%.2 What are the first-line treatments available to these patients? Who might be a candidate for combination drug therapy? Are herbal preparations worth considering? When might surgery be a first choice?
These questions underscore the importance of a proper primary care framework for evaluating and treating BPH, which we can develop based on a consensus guideline released by the American Urological Association (AUA)1 and on more recent research.
Assessing symptoms: 2 tools can help
Symptoms of BPH can include urinary frequency, nocturia, urgency, hesitancy, weak or intermittent urine stream, straining to void, and a sensation of incomplete voiding.1 Each patient experiences a unique constellation of these symptoms. Using a urinary symptom scoring system can help define the severity of BPH and be useful in monitoring the success of subsequent therapy. Available instruments for this purpose include the AUA’s 7-question Symptom Index for BPH (http://www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines.cfm?sub=bph) and the International Prostate Symptom Score, which adds an eighth question to the AUA list to gauge the extent to which symptoms bother a patient (http://www.usli.net/uro/Forms/ipss.pdf).1-5 If the patient is unclear about the pattern of his symptoms, consider asking him to keep a voiding diary.
Ruling out other causes of BPH-like symptoms
TABLE 1 lists the differential diagnoses of obstructive urinary symptoms, otherwise known as lower urinary tract symptoms (LUTS).
Look for clues in the history. Ask the patient whether he uses medications known to cause obstructive urinary symptoms—tricyclic antidepressants, first-generation antihistamines, anticholinergic agents, diuretics, narcotics, and decongestants. Does he have any first-degree relatives with prostate cancer? If the answer is yes, how young was he when the cancer was diagnosed?
Focus your examination. Perform a digital rectal examination (DRE) to check prostate size and to detect palpable nodules, induration, or irregularities associated with malignancy or infection. An enlarged prostate is commonly found on rectal examination; however, the degree of hypertrophy does not necessarily correlate with the degree of obstruction or the severity of symptoms. Any irregularity suggestive of cancer requires that you talk to your patient about his preferences for further investigation.6
Conduct a neurologic exam to check mental status, gait, lower extremity strength, and anal sphincter tone to assess for conditions that could cause a neurogenic bladder.
TABLE 1
Lower urinary tract symptoms are also seen with these disorders31
| DISORDER | FINDINGS |
|---|---|
| Bladder calculi | Hematuria, ultrasonography finding |
| Bladder neck dyssynergia | LUTS in younger patients with normal prostate size, diagnosed by cystoscopy or VCUG |
| Overactive bladder | Urgency with possible urge incontinence |
| Prostate cancer | Finding in DRE, elevated serum PSA |
| Prostatitis | Tender prostate gland |
| Stricture of the bladder neck | Prior invasive treatment |
| Urinary bladder cancer | Hematuria, abnormal cytological finding |
| Urethral stricture | Box-shaped flow curve on urinary flow-rate measurement |
| DRE, digital rectal examination; LUTS, lower urinary tract symptoms; PSA, prostate-specific antigen; VCUG, voiding cystourethrogram. | |
Consider these tests. Urinary tract infection (UTI) or bladder cancer may produce symptoms similar to those of BPH. For any patient who has LUTS, perform a urinalysis to screen for infection or hematuria. If a UTI is found, treat it and re-evaluate the patient. If you detect microscopic hematuria, do a further work-up to rule out bladder cancer. If DRE findings are suggestive of prostate cancer, you’ll need an ultrasound-guided biopsy and histological examination to make the diagnosis.
Optional tests in the work-up of LUTS include urinary flow rate measurements, post-void residual urine measurements, and pressure flow studies. These tests may be informative if the diagnosis is unclear based on the history and physical exam or when patients do not respond to initial therapy. Ultrasonography, intravenous pyelography, filling cystometrography, and cystoscopy are not routinely recommended for the evaluation of suspected BPH. However, they may be helpful if a patient has a complex medical history (eg, neurologic disorder or other disease known to affect bladder function, or prior failure of BPH therapy), or if he wants to pursue invasive therapy.1
Talk to your patient about the controversial PSA test. Measuring serum prostate-specific antigen (PSA) levels to screen for prostate cancer is controversial, even for patients with LUTS. Although PSA testing can effectively detect prostate cancer in its early pathologic stages, researchers continue to investigate whether early detection significantly improves outcomes. Quite recently, 2 studies demonstrated that the test saves few lives;7,8 1410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated to prevent 1 death from prostate cancer.
A subset of detected cancers appears to be clinically significant, but many cancers will not progress during a patient’s lifetime.3,5 The United States Preventive Services Task Force (USPSTF) has concluded that the evidence is insufficient to recommend for or against routine prostate cancer screening due to the uncertainty of the balance of potential benefits (reduction of prostate cancer morbidity and mortality) and risks (false-positive results, unnecessary biopsies, and possible complications) of treatment for early disease.9-12 Furthermore, in its 2008 update, the USPSTF specifically recommended against screening for prostate cancer in men 75 years of age and older.
The American Cancer Society says that discouraging or not offering testing is inappropriate: Men who ask their physicians to make the decision on their behalf should be tested.9 A 2006 Cochrane review of this topic found only 2 eligible randomized trials, both of which had high risk of bias. They concluded that insufficient high-quality evidence exists to support or refute the use of any screening for prostate cancer in any patient population, including those with BPH.13
Given this conflicting advice, discuss the benefits and limitations of screening with your patient before deciding whether or not to test.
What is the optimal Approach to treatment?
Patients who are not bothered by their urinary symptoms—even those with moderate to severe symptom scores—can be managed with watchful waiting. Because of the cost and frequent side effects of medications for BPH, these patients generally will not benefit from drug therapy.3,5,14,15 However, follow-up monitoring is important, because the severity of BPH can change even without treatment.
Even if patients with moderate or severe AUA symptom scores are not bothered by their symptoms, inform them of appropriate treatment options.3 When urinary obstruction symptoms from BPH significantly interfere with daily living and sleep activities, treatment is justified.1
Medical management, yes, but which option?
Medical therapies are not as effective as surgical intervention,16 but they often provide adequate symptom relief and cause fewer, less severe, and less permanent adverse effects than surgery. Initiate treatment with medical therapy if (1) the patient is bothered by his symptoms, (2) no significant urinary obstruction exists, and (3) you have followed the patient’s preference for prostate cancer evaluation. TABLE 2 lists prescription medications for the treatment of BPH.
Nonselective α-adrenergic blockers, such as doxazosin and terazosin, reduce prostatic smooth muscle tone, thereby improving urinary flow. A Cochrane systematic review and a subsequent large randomized trial found terazosin to be superior to placebo in improving urinary flow and decreasing symptoms in men with BPH.16,17
Selective α-adrenergic blockers, such as alfuzosin and tamsulosin, are highly selective α-1A-adrenergic antagonists. They are believed to be as effective as the nonselective agents, and patients may experience fewer side effects than with nonselective agents.1,18 However, these drugs are considerably more expensive than their nonselective counterparts.
5-α Reductase inhibitors, such as finasteride and dutasteride, are more effective than placebo for patients with LUTS associated with demonstrable prostate enlargement. In a Cochrane review and subsequent large randomized trial, finasteride proved inferior to terazosin.16,17 However, finasteride reduces the progression to urinary obstruction and the need for invasive therapy; terazosin does not.17 Finasteride achieves this effect by reducing prostatic volume by about 20% over 3 to 6 months of treatment.
Finasteride decreases PSA levels by 40% to 50%. If you conduct PSA screening for prostate cancer in a patient taking finasteride, double the PSA level before comparing it with age-related norms. Handled this way, PSA screening will not lose its sensitivity or specificity for the diagnosis of prostate cancer.19
Though a 5-mg daily regimen of finasteride reduces the overall risk of prostate cancer from 24.4% to 18.4%, it increases the risk of high-grade disease associated with higher mortality from 5.1% to 6.4%. Warn patients of this risk.20
Combining an α-adrenergic blocker and 5-α reductase inhibitor. Combination therapy is appropriate and effective for patients with LUTS associated with demonstrable prostate enlargement for whom monotherapy has failed.9 Taken together, a 5-α reductase inhibitor and a nonselective α-1A-adrenergic blocker alleviate symptoms more effectively than either drug can do alone.21,22 The incidence of most adverse drug reactions with the combination is similar to the baseline risk for each drug. However, ejaculatory abnormalities are reported in 7% of patients in the combination therapy group vs 2% or less in the monotherapy groups. Discontinuation rates for combination therapy are comparable to those in the nonselective α-adrenergic blocker group.22 The adverse effect profile of combining selective α-adrenergic blockers with 5-α reductase inhibitors has not been reported; however, the combination is often used in practice.
For patients to make an informed decision about treatment, discuss with them the common adverse reactions from these agents (TABLE 2) and the need for long-term daily therapy. Also give the patient a reasonable estimate of the risk of his retention symptoms progressing.
TABLE 2
BPH medications: How they compare
| AUA RECOMMENDATION FOR USE WITH LUTS SECONDARY TO BPH1 | DRUG | DOSE | MOST COMMON SIDE EFFECTS (%) | COSTS |
|---|---|---|---|---|
| Nonselective α-adrenergic blockers | ||||
| Useful as first-line therapy due to efficacy and low cost α-Aadrenergic blockers can be used with other therapies as needed | Doxazosin (Cardura, generics) | Start at 1 mg; titrate by doubling dose every 1-2 wk. Goal: 4-8 mg. Maximum dose, 8 mg | Dizziness (16), headache (10), fatigue (8), edema (3), dyspnea (3), orthostatic hypotension (2), abdominal pain (2)* | $4 for 30-day supply for both generic agents† |
| Terazosin (Hytrin, generics) | Start at 1 mg at bedtime, increase PRN over 4-6 wk; most patients require 10 mg. If no response at 10 mg, may increase to 20 mg | Dizziness (9), fatigue (7), headache (5), orthostatic hypotension (4), somnolence (4), nasal congestion (2), ED (2)‡ | ||
| Selective α-adrenergic blockers | ||||
| All believed to be equal in clinical effectiveness | Alfuzosin (Uroxatral or Xatral) | 10 mg/d with the same meal | Dizziness (6), headache (3), upper respiratory infection (3), fatigue (3)§ | $112 for 30-day supply// |
| Tamsulosin (Flomax) | 0.4 mg/d (30 min after same meal); may increase after 2-4 wk to 0.8 mg/d if no response | Headache (19), dizziness (15), rhinitis (13), infection (9), fatigue (8), abnormal ejaculation (8)¶ | $110 for 30-day supply// | |
| 5-α Reductase inhibitors | ||||
| All believed to be appropriate and effective treatments for patients with demonstrable prostate enlargement | Finasteride (Proscar) | 5 mg/d | ED (8), decreased libido (6), decreased volume of ejaculate (4)# | $70 for 30-day generic supply// |
| Dutasteride (Avodart) | 0.5 mg/d | ED (5), decreased libido (3), ejaculation disorder (1), gynecomastia (1)** | $20-$30 for 30-day generic supply†† | |
| AUA, American Urological Association; BPH, benign prostatic hypertrophy; ED, erectile dysfunction; LUTS, lower urinary tract symptoms. | ||||
| * http://www.fda.gov/medwatch/SAFETY/2006/Feb_PI/Cardura_PI.pdf. | ||||
| † Prices listed on Walmart.com as of April 6, 2009. | ||||
| ‡http://www.fda.gov/medwatch/SAFETY/2006/Feb_PI/Hytrin%20Caps_PI.pdf. | ||||
| §http://www.fda.gov/medwatch/safety/2008/Sep_PI/Uroxatral_PI.pdf. | ||||
| // Prices listed on Drugstore.com as of April 6, 2009. | ||||
| ¶http://www.fda.gov/medwatch/SAFETY/2008/Apr_PI/Flomax_PI.pdf. | ||||
| #http://www.fda.gov/medwatch/SAFETY/2004/apr_PI/Proscar_PI.pdf. | ||||
| ** http://www.fda.gov/medwatch/SAFETY/2004/sep_PI/Avodart_PI.pdf. | ||||
| †† Prices listed on Pharmacychecker.com as of April 6, 2009. | ||||
Complementary medicine: Information is still limited
Herbal or complementary medicines are used worldwide to treat BPH. These products are not regulated by the US Food and Drug Administration (FDA), and therefore no standardized formulation or dosing exists. Although a few substances appear to have some positive effects, high-quality clinical trials on clinical outcomes are lacking.
The AUA guideline does not recommend the use of phytotherapy.1 Despite this, many patients—and any number of physicians—turn to phytotherapy to treat LUTS associated with BPH.
Some patients turn to phytotherapy without their physician’s knowledge, so it’s important to ask whether they are using any herbal preparations. Agents currently used include saw palmetto, African plum, South African star grass, and Cernilton.
Saw palmetto (Serenoa repens) has been used by more than 2 million men in the United States. In 2006, Bent et al conducted a rigorously designed double-blinded trial in which 225 men older than 49 years with moderate-to-severe symptoms of BPH were treated for 1 year with saw palmetto extract (160 mg twice a day) or placebo.23 Saw palmetto did not ameliorate the symptoms of BPH. In contrast, a Cochrane systematic review last updated in 2002 asserted that this substance caused mild-to-moderate reductions in urologic symptoms and flow measures when given to men with symptomatic BPH.24 Long-term efficacy and safety of this product are unknown. Given the efficacy of saw palmetto, it is a reasonable option for men who prefer a nonprescription product to treat symptoms of BPH.
African plum (Pygeum africanum) is more effective than placebo in reducing symptoms of BPH and has few side effects, based on poorly designed small studies.25,26 Comparative data with finasteride or the α-adrenergic blockers are lacking.
South African star grass (Hypoxis rooperi and certain species of Pinus and Picea) contain beta-sitosterols and are sources for phytotherapeutic treatments for BPH. A systematic review analyzed the effects of beta-sitosterols in men and found improved urinary symptom scores and flow measures (n=519; 4 randomized, controlled, double-blind trials; duration, 4-26 weeks).27 Their long-term effectiveness and safety are not known.
Cernilton, prepared from the ryegrass pollen Secale cereale, is marketed for the treatment of BPH. A Cochrane systematic review demonstrated that comparative trials lacked a proven active control. Available evidence suggests that short-term use of cernilton is well tolerated and modestly decreases overall urologic symptoms, including nocturia. Additional randomized placebo and active-controlled trials are needed to evaluate the long-term clinical effectiveness and safety of Cernilton.28
Acupuncture was not effective in treating LUTS in men in randomized controlled (single-blinded) trials.29
When patients don’t respond to medical treatment
Surgery is recommended for patients who have not responded to medical treatment, who have refractory retention with a failed attempt at catheter removal, or who experience recurrent UTIs, persistent hematuria, bladder stones, or renal insufficiency.3 In addition, surgery can be the initial treatment choice for patients with high AUA symptom scores who opt for this intervention and are good operative candidates.
The specific surgical approach (open or endoscopic; electrocautery or laser) are technical decisions based on the patient’s prostate size, the individual surgeon’s judgment, and the patient’s comorbidities.1,30 For patients who are not surgical candidates, treatment with intermittent catheterization, an indwelling catheter, or a stent is recommended.
Acknowledgements
The authors thank Jen Creer, MA, of Edit Rx, LLC, for her assistance in the preparation of this manuscript. The University of Nebraska Medical Center, with which both authors were previously affiliated, funded this writing assistance.
Correspondence
Drew M. Keister, MD, Lehigh Valley Health Network, PO Box 7017, Allentown, PA 18105-7017; [email protected]
1. American Urological Association. AUA guideline on the management of benign prostatic hyperplasia: diagnosis and treatment recommendations. 2003/updated 2006. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinicalguidelines.cfm?sub=bph. Accessed April 2, 2009.
2. Oesterling JE. Benign prostatic hyperplasia: medical and minimally invasive treatment options. N Engl J Med. 1995;332:99-109.
3. McConnell JD. Clinical practice guideline no. 8: Benign prostatic hyperplasia: diagnosis and treatment. Benign Prostatic Hyperplasia Guideline Panel. Rockville, Md: US Dept of Health and Human Services, Agency for Health Care Policy and Research; 1994. AHCPR publication 94-0582.
4. Austin O, Ricer RE. (1996). Prostate cancer screening: an appraisal of the PSA test. Fam Pract Recert. 1996;18:81-91.
5. Barry MJ, Fowler FJ, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557.
6. Denis LJ. Diagnosing benign prostatic hyperplasia versus prostate cancer. Br J Urol. 1995;76(suppl 1):S17-S23.
7. Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310-1319.
8. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
9. American Cancer Society Guidelines for the Early Detection of Cancer. Revised March 5, 2008. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp. Accessed April 6, 2009.
10. US Preventive Services Task Force. Screening for prostate cancer: recommendation statement. Available at http://www.ahrq.gov/clinic/uspstf08/prostate/prostaters.htm. Accessed April 3, 2009.
11. American Cancer Society. Cancer Facts & Figures. Atlanta, Ga: 2008. Available at http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. Accessed on April 3, 2009.
12. Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2006;56:11-25.
13. Ilic D, O’Connor D, Green S, et al. Screening for prostate cancer. Cochrane Database Syst Rev. 2006;(3):CD004720.-
14. Barry MJ. Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology. 2001;58(suppl 1):S25-S32.
15. Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery—what we have learned and where we are going. J Urol. 1999;162:293-306.
16. Wilt T, Howe RW, Rutks I, et al. Terazosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(4):CD003851.-
17. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-2398.
18. Wilt T, MacDonald R, Rutks I. Tamsulosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2003;(1):CD002081.-
19. Guess HA, Gormley GJ, Stoner E, et al. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996;155:3-9.
20. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-224.
21. Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469-471.
22. Lepor HL, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533-540.
23. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
24. Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 1999;(1):CD001423.-
25. Ishani A, MacDonald R, Nelson D, et al. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med. 2000;109:654-664.
26. Wilt T, Ishani A, MacDonald R, et al. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(1):CD001044.-
27. Wilt T, Ishani A, MacDonald R, et al. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst Rev. 1999;(4):CD001043.-
28. Wilt T, MacDonald R, Ishani A, et al. Cernilton for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2000;(2):CD001042.-
29. Johnstone PA, Bloom TL, Niemtzow RC, et al. A prospective, randomized pilot trial of acupuncture of the kidney-bladder distinct meridian for lower urinary tract symptoms. J Urol. 2003;169:1037-1039.
30. Keister DM, Neal R. Managing BPH: when to consider surgery. Am Fam Physician. 2008;77:1391-1392.
31. Finnish Medical Society Duodecim. Benign prostatic hyperplasia. In: EBM Guidelines. Evidence-Based Medicine [CD-ROM]. Helsinki, Finland: Duodecim Medical Publications Ltd; 2005.
1. American Urological Association. AUA guideline on the management of benign prostatic hyperplasia: diagnosis and treatment recommendations. 2003/updated 2006. Available at: http://www.auanet.org/content/guidelines-and-quality-care/clinicalguidelines.cfm?sub=bph. Accessed April 2, 2009.
2. Oesterling JE. Benign prostatic hyperplasia: medical and minimally invasive treatment options. N Engl J Med. 1995;332:99-109.
3. McConnell JD. Clinical practice guideline no. 8: Benign prostatic hyperplasia: diagnosis and treatment. Benign Prostatic Hyperplasia Guideline Panel. Rockville, Md: US Dept of Health and Human Services, Agency for Health Care Policy and Research; 1994. AHCPR publication 94-0582.
4. Austin O, Ricer RE. (1996). Prostate cancer screening: an appraisal of the PSA test. Fam Pract Recert. 1996;18:81-91.
5. Barry MJ, Fowler FJ, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557.
6. Denis LJ. Diagnosing benign prostatic hyperplasia versus prostate cancer. Br J Urol. 1995;76(suppl 1):S17-S23.
7. Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310-1319.
8. Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
9. American Cancer Society Guidelines for the Early Detection of Cancer. Revised March 5, 2008. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp. Accessed April 6, 2009.
10. US Preventive Services Task Force. Screening for prostate cancer: recommendation statement. Available at http://www.ahrq.gov/clinic/uspstf08/prostate/prostaters.htm. Accessed April 3, 2009.
11. American Cancer Society. Cancer Facts & Figures. Atlanta, Ga: 2008. Available at http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. Accessed on April 3, 2009.
12. Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2006;56:11-25.
13. Ilic D, O’Connor D, Green S, et al. Screening for prostate cancer. Cochrane Database Syst Rev. 2006;(3):CD004720.-
14. Barry MJ. Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology. 2001;58(suppl 1):S25-S32.
15. Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery—what we have learned and where we are going. J Urol. 1999;162:293-306.
16. Wilt T, Howe RW, Rutks I, et al. Terazosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(4):CD003851.-
17. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-2398.
18. Wilt T, MacDonald R, Rutks I. Tamsulosin for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2003;(1):CD002081.-
19. Guess HA, Gormley GJ, Stoner E, et al. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996;155:3-9.
20. Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215-224.
21. Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469-471.
22. Lepor HL, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Engl J Med. 1996;335:533-540.
23. Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006;354:557-566.
24. Wilt T, Ishani A, MacDonald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 1999;(1):CD001423.-
25. Ishani A, MacDonald R, Nelson D, et al. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med. 2000;109:654-664.
26. Wilt T, Ishani A, MacDonald R, et al. Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2002;(1):CD001044.-
27. Wilt T, Ishani A, MacDonald R, et al. Beta-sitosterols for benign prostatic hyperplasia. Cochrane Database Syst Rev. 1999;(4):CD001043.-
28. Wilt T, MacDonald R, Ishani A, et al. Cernilton for benign prostatic hyperplasia. Cochrane Database Syst Rev. 2000;(2):CD001042.-
29. Johnstone PA, Bloom TL, Niemtzow RC, et al. A prospective, randomized pilot trial of acupuncture of the kidney-bladder distinct meridian for lower urinary tract symptoms. J Urol. 2003;169:1037-1039.
30. Keister DM, Neal R. Managing BPH: when to consider surgery. Am Fam Physician. 2008;77:1391-1392.
31. Finnish Medical Society Duodecim. Benign prostatic hyperplasia. In: EBM Guidelines. Evidence-Based Medicine [CD-ROM]. Helsinki, Finland: Duodecim Medical Publications Ltd; 2005.
What caused this case of asymptomatic hyperthyroidism?
- When taking a medication history, always ask specifically about the use of all nonprescription products—including all over-the-counter remedies, vitamins, “natural” herbal supplements, and dietary aids (C).
- Counsel patients about the need for caution when taking dietary supplements and herbal remedies, which lack regulation and standardization and may contain ingredients not listed on the label (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
When Mary J,* an overweight 47-year-old Caucasian woman, came in for an annual physical examination, she appeared to be in good health. She denied any recent illness, and reported that an oral contraceptive was the only medication she was taking. The patient’s only complaint: She was having difficulty losing weight despite complying with a low-calorie diet and exercise regimen for the last 6 months. Her comments prompted her physician (CC) to order a thyroid-stimulating hormone (TSH) test to rule out hypothyroidism.
The test showed a TSH of 0.2 mIU/L (normal range is 0.35-5.0 mIU/L). Her physician ordered retesting a week later and this time, Mary’s TSH was normal (1.99 mIU/L). The laboratory report also showed elevated free triiodothyronine (T3) of 8.1 pmol/L (normal range 2.6-5.7 pmol/L) and free thyroxine (T4) >70 pmol/L (normal, 10-20 pmol/L); negative antithyroid peroxidase and antithyroglobulin antibodies; normal complete blood count, calcium, and alkaline phosphatase; and low levels of thyroglobulin. The patient had no symptoms and no personal or family history of thyroid disease. She also denied taking thyroid medications.
* The patient’s name has been changed to protect her privacy.
In search of an explanation
On examination, her physician found no significant thyroid enlargement or tenderness. However, the patient’s thyroid was somewhat boggy on palpation. There was no exopthalmos or pretibial myxedema. Mary’s blood pressure was 144/98 mm Hg (with no prior history of hypertension), her heart rate was 84, and she was afebrile. Her physician found no obvious tremors, hyperdynamic apex, or hyperreflexia on physical exam.
To rule out laboratory error from the second set of tests, her physician ordered yet another round of blood work. A diagnosis of hyperthyroidism was confirmed by elevated T4 (>70 pmol/L) and T3 (6.2 pmol/L). As in the previous test, the patient’s TSH was in the normal range (1.54 mIU/L).
Detailed questioning solves the mystery
At follow-up, the patient was again asked about exogenous thyroid intake, which she had initially denied. After further questioning about what she was ingesting, Mary acknowledged that she had been taking Pu Erh—a European dietary supplement marketed as a means of increasing metabolism to help with weight loss—3 times daily for more than 3 months. She hadn’t mentioned it before because it hadn’t occurred to her to question its safety.
Her physician advised her to discontinue the supplement immediately, and to have her blood work retested in a month. Within 5 weeks, all her lab values returned to normal. (For more on lab values, see “Investigating thyroid dysfunction: What to test for” on page 205. )
TABLE
Investigating thyroid dysfunction: What to test for
The single most useful screening test for thyroid dysfunction is serum TSH. Normal TSH levels effectively rule out hyperthyroidism and hypothyroidism, and obtaining serum T3 and T4 levels is usually not indicated.8 Circulating levels of free T3 or T4 are increased in hyperthyroidism and thyrotoxicosis, while TSH levels are low to immeasurable (<0.01 mU/L).9 The term “thyrotoxicosis” is used to denote the excess of thyroid hormone levels without thyroid hyperfunction or increased biosynthesis—ie, excess intake, excess release without synthesis, or syndromes of pituitary resistance to thyroid hormones.10 Low thyroglobulin in association with hyperthyroidism is a hallmark of exogenous thyroid intake, also known as thyrotoxicosis factitia.11 |
Natural does not=safe
With an ever-increasing overweight population, there is growing concern about the misuse of diet aids. It is important for patients to be cautious when using dietary supplements because of the lack of regulation and standardization. Yet such products are often marketed as “natural,” which may be interpreted as an assurance of safety.1 Family physicians can play a crucial role in primary prevention by inquiring about the use of over-the-counter substances including “natural” herbal supplements and dietary aids, advising patients of the risks associated with their use, and being alert to potentially dangerous side effects.
Thyrotoxicosis factitia: An exogenous cause
Thyroid hyperactivity can occur when excessive quantities of thyroid hormone are ingested. The excessive intake may be associated with treatment for hypothyroidism, or, as in Mary’s case, may be linked to overuse (or abuse) of a diet aid in an attempt to lose weight.2 Often, the condition can be traced to an iodine-containing substance such as kelp—a widely used dietary aid that we’ll discuss in greater detail in a bit.
Iodide is an inorganic salt that is absorbed through the gastrointestinal mucosa and transported to the thyroid gland, where it is trapped and concentrated for thyroid hormone synthesis.3 Consuming large quantities of foods or other products that contain iodine—such as iodized salt, shellfish, cough syrups, multivitamins, or medications such as amiodarone and interferon alpha, as well as kelp—can cause hyperthyroidism.
What’s in that weight-loss aid?
Pu Erh, the dietary supplement Mary was taking, was not available for analysis; it was purchased in Poland and the patient had exhausted her supply by the time her physician told her to stop taking it. Each capsule contains 400 mg of red tea extract with 15 mcg chromium, according to the label.
Red tea and chromium. There are no reports associating red tea with thyroid dysfunction, but chromium is a popular weight-loss supplement whose efficacy and long-term safety are uncertain.4 It is unlikely that Mary’s hyperthyroidism was associated with chromium in-take, however, as multivitamins often contain 100 mcg of chromium—nearly 7 times the quantity in each Pu Erh capsule—with no reported thyroid side effects.
Did the supplement contain a thyroid extract? It’s possible, of course, that the supplement contained a thyroid extract, which would explain the resolution of symptoms after Mary stopped taking it. But without analysis of the product, we can’t be sure. Our suspicion, of course, is that it did.
Another possible, but unlikely, explanation. Theoretically, the fluctuating TSH could have been related to silent or subacute thyroiditis, in which T4 can remain elevated for 1 to 3 months. The fact that Mary had no history of cold or flu symptoms in the month preceding the initial TSH was inconsistent with this alternative diagnosis, however. Painless thyroiditis is also unlikely, as it is autoimmune in origin and the patient’s antithyroid antibodies were negative. The low thyroglobulin level supported an exogenous cause of hyperthyroidism.
Other supplements and thyroid dysfunction
Mary’s presentation is a single case indicating a possible link between a weight-loss supplement and asymptomatic hyperthyroidism—a clinically important condition that may be associated with disorders such as paroxysmal atrial fibrillation and osteoporosis. This is only one of a number of case reports of patients taking dietary supplements who have developed thyroid dysfunction.
Kelp. Long used as a dietary supplement, especially in Asia, kelp has been linked to thyroid dysfunction. One case report describes a 72-year-old woman with a history of thyroid disease having typical symptoms of hyperthyroidism while ingesting 4 to 6 kelp tablets daily for 1 year. Her TSH concentration was low, while total T3 and T4 levels were high. After discontinuing the kelp tablets, the hyperthyroidism resolved, and thyroid function tests returned to normal.5 Another report describes an instance of probable transient hyperthyroidism in a patient taking kelp in 2 different diet supplements.6
Tiratricol (Triac), a substance that has weak thyromimetic effects, resulted in a case of documented hyperthyroidism secondary to its use.1
Other reports. In Japan, the weight-reducing herbal medicines, Dream Shape and Ever Youth, became available in 2000. Twelve patients subsequently developed thyrotoxicosis after taking these herbal medicines, both of which were found to contain triiodothyronine and thyroxine.7
As early as 1986, researchers have described several patients who developed thyrotoxicosis from Enzo-Caps, a nonprescription diet aid manufactured in Peru. The product was touted as “a natural food product of papaya, garlic, and kelp” to assist with weight reduction. Researchers obtained the supplement for biochemical analysis and found that it had been adulterated with thyroid hormones, which led to thyrotoxicosis factitia.2
Patients in the report complained of palpitations, weakness, fatigue, headache, diaphoresis, irritability, and nervousness, and had elevated serum T3 and T4 levels. Thyroglobulin levels were not determined, but would have been useful in differentiating thyrotoxicosis factitia from hyperthyroxinemia (Graves’ disease, thyroiditis, nodular goiter).2
These reports, as well as our own experience, leave little doubt as to the importance of asking pointed questions about all prescription and nonprescription products a patient is taking. As we can attest, a little persistence goes a long way when it comes to identifying that agent your patient didn’t think was worth mentioning.
Correspondence
Taryn Taylor, MD, CCFP, Bruyere Family Medicine Center, University of Ottawa, 206 Monterey Drive, Ottawa, Ontario, Canada K2H 7A8; [email protected]
1. Bauer BA, Elkin PL, Erickson D, et al. Symptomatic hyperthyroidism in a patient taking the dietary supplement tiratricol. Mayo Clin Proc. 2002;77:587-590.
2. Braunstein GD, Koblin R, Sugawara M, et al. Unintentional thyrotoxicosis factitia due to a diet pill. Western J Med. 1986;145:388-391.
3. Clark CD, Cliford D, Bertram B, et al. Effects of kelp supplementation on thyroid function in euthyroid subjects. Endocr Pract. 2003;9:363-369.
4. Saper RB. Common dietary supplements for weight loss. Am Fam Physician. 2004;70:1731-1738.
5. Shilo S, Hirsch HJ. Iodine-induced hyperthyroidism in a patient with a normal thyroid gland. Postgrad Med J. 1986;62:661-662.
6. Eliason BC. Transient hyperthyroidism in a patient taking dietary supplements containing kelp. J Am Board Fam Pract. 1998;11:478-480.
7. Ohye H, Fukata S, Kanoh M, et al. Thyrotoxicosis caused by weight-reducing herbal medicines. Arch Intern Med. 2005;165:831-834.
8. Demers LM. Thyroid disease: pathophysiology and diagnosis. Clin Lab Med. 2004;24:19-28.
9. Goichot B, Perrin AE. Thyrotropin as first-line thyroid test. Lancet. 2001;358:509.-
10. Monaco F. Classification of thyroid diseases: suggestions for a revision. J Clin Endocrinol Metab. 2003;88:1428-1432.
11. Parmar MS, Sturge C. Recurrent hamburger thyrotoxicosis. CMAJ. 2003;169:415-417.
- When taking a medication history, always ask specifically about the use of all nonprescription products—including all over-the-counter remedies, vitamins, “natural” herbal supplements, and dietary aids (C).
- Counsel patients about the need for caution when taking dietary supplements and herbal remedies, which lack regulation and standardization and may contain ingredients not listed on the label (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
When Mary J,* an overweight 47-year-old Caucasian woman, came in for an annual physical examination, she appeared to be in good health. She denied any recent illness, and reported that an oral contraceptive was the only medication she was taking. The patient’s only complaint: She was having difficulty losing weight despite complying with a low-calorie diet and exercise regimen for the last 6 months. Her comments prompted her physician (CC) to order a thyroid-stimulating hormone (TSH) test to rule out hypothyroidism.
The test showed a TSH of 0.2 mIU/L (normal range is 0.35-5.0 mIU/L). Her physician ordered retesting a week later and this time, Mary’s TSH was normal (1.99 mIU/L). The laboratory report also showed elevated free triiodothyronine (T3) of 8.1 pmol/L (normal range 2.6-5.7 pmol/L) and free thyroxine (T4) >70 pmol/L (normal, 10-20 pmol/L); negative antithyroid peroxidase and antithyroglobulin antibodies; normal complete blood count, calcium, and alkaline phosphatase; and low levels of thyroglobulin. The patient had no symptoms and no personal or family history of thyroid disease. She also denied taking thyroid medications.
* The patient’s name has been changed to protect her privacy.
In search of an explanation
On examination, her physician found no significant thyroid enlargement or tenderness. However, the patient’s thyroid was somewhat boggy on palpation. There was no exopthalmos or pretibial myxedema. Mary’s blood pressure was 144/98 mm Hg (with no prior history of hypertension), her heart rate was 84, and she was afebrile. Her physician found no obvious tremors, hyperdynamic apex, or hyperreflexia on physical exam.
To rule out laboratory error from the second set of tests, her physician ordered yet another round of blood work. A diagnosis of hyperthyroidism was confirmed by elevated T4 (>70 pmol/L) and T3 (6.2 pmol/L). As in the previous test, the patient’s TSH was in the normal range (1.54 mIU/L).
Detailed questioning solves the mystery
At follow-up, the patient was again asked about exogenous thyroid intake, which she had initially denied. After further questioning about what she was ingesting, Mary acknowledged that she had been taking Pu Erh—a European dietary supplement marketed as a means of increasing metabolism to help with weight loss—3 times daily for more than 3 months. She hadn’t mentioned it before because it hadn’t occurred to her to question its safety.
Her physician advised her to discontinue the supplement immediately, and to have her blood work retested in a month. Within 5 weeks, all her lab values returned to normal. (For more on lab values, see “Investigating thyroid dysfunction: What to test for” on page 205. )
TABLE
Investigating thyroid dysfunction: What to test for
The single most useful screening test for thyroid dysfunction is serum TSH. Normal TSH levels effectively rule out hyperthyroidism and hypothyroidism, and obtaining serum T3 and T4 levels is usually not indicated.8 Circulating levels of free T3 or T4 are increased in hyperthyroidism and thyrotoxicosis, while TSH levels are low to immeasurable (<0.01 mU/L).9 The term “thyrotoxicosis” is used to denote the excess of thyroid hormone levels without thyroid hyperfunction or increased biosynthesis—ie, excess intake, excess release without synthesis, or syndromes of pituitary resistance to thyroid hormones.10 Low thyroglobulin in association with hyperthyroidism is a hallmark of exogenous thyroid intake, also known as thyrotoxicosis factitia.11 |
Natural does not=safe
With an ever-increasing overweight population, there is growing concern about the misuse of diet aids. It is important for patients to be cautious when using dietary supplements because of the lack of regulation and standardization. Yet such products are often marketed as “natural,” which may be interpreted as an assurance of safety.1 Family physicians can play a crucial role in primary prevention by inquiring about the use of over-the-counter substances including “natural” herbal supplements and dietary aids, advising patients of the risks associated with their use, and being alert to potentially dangerous side effects.
Thyrotoxicosis factitia: An exogenous cause
Thyroid hyperactivity can occur when excessive quantities of thyroid hormone are ingested. The excessive intake may be associated with treatment for hypothyroidism, or, as in Mary’s case, may be linked to overuse (or abuse) of a diet aid in an attempt to lose weight.2 Often, the condition can be traced to an iodine-containing substance such as kelp—a widely used dietary aid that we’ll discuss in greater detail in a bit.
Iodide is an inorganic salt that is absorbed through the gastrointestinal mucosa and transported to the thyroid gland, where it is trapped and concentrated for thyroid hormone synthesis.3 Consuming large quantities of foods or other products that contain iodine—such as iodized salt, shellfish, cough syrups, multivitamins, or medications such as amiodarone and interferon alpha, as well as kelp—can cause hyperthyroidism.
What’s in that weight-loss aid?
Pu Erh, the dietary supplement Mary was taking, was not available for analysis; it was purchased in Poland and the patient had exhausted her supply by the time her physician told her to stop taking it. Each capsule contains 400 mg of red tea extract with 15 mcg chromium, according to the label.
Red tea and chromium. There are no reports associating red tea with thyroid dysfunction, but chromium is a popular weight-loss supplement whose efficacy and long-term safety are uncertain.4 It is unlikely that Mary’s hyperthyroidism was associated with chromium in-take, however, as multivitamins often contain 100 mcg of chromium—nearly 7 times the quantity in each Pu Erh capsule—with no reported thyroid side effects.
Did the supplement contain a thyroid extract? It’s possible, of course, that the supplement contained a thyroid extract, which would explain the resolution of symptoms after Mary stopped taking it. But without analysis of the product, we can’t be sure. Our suspicion, of course, is that it did.
Another possible, but unlikely, explanation. Theoretically, the fluctuating TSH could have been related to silent or subacute thyroiditis, in which T4 can remain elevated for 1 to 3 months. The fact that Mary had no history of cold or flu symptoms in the month preceding the initial TSH was inconsistent with this alternative diagnosis, however. Painless thyroiditis is also unlikely, as it is autoimmune in origin and the patient’s antithyroid antibodies were negative. The low thyroglobulin level supported an exogenous cause of hyperthyroidism.
Other supplements and thyroid dysfunction
Mary’s presentation is a single case indicating a possible link between a weight-loss supplement and asymptomatic hyperthyroidism—a clinically important condition that may be associated with disorders such as paroxysmal atrial fibrillation and osteoporosis. This is only one of a number of case reports of patients taking dietary supplements who have developed thyroid dysfunction.
Kelp. Long used as a dietary supplement, especially in Asia, kelp has been linked to thyroid dysfunction. One case report describes a 72-year-old woman with a history of thyroid disease having typical symptoms of hyperthyroidism while ingesting 4 to 6 kelp tablets daily for 1 year. Her TSH concentration was low, while total T3 and T4 levels were high. After discontinuing the kelp tablets, the hyperthyroidism resolved, and thyroid function tests returned to normal.5 Another report describes an instance of probable transient hyperthyroidism in a patient taking kelp in 2 different diet supplements.6
Tiratricol (Triac), a substance that has weak thyromimetic effects, resulted in a case of documented hyperthyroidism secondary to its use.1
Other reports. In Japan, the weight-reducing herbal medicines, Dream Shape and Ever Youth, became available in 2000. Twelve patients subsequently developed thyrotoxicosis after taking these herbal medicines, both of which were found to contain triiodothyronine and thyroxine.7
As early as 1986, researchers have described several patients who developed thyrotoxicosis from Enzo-Caps, a nonprescription diet aid manufactured in Peru. The product was touted as “a natural food product of papaya, garlic, and kelp” to assist with weight reduction. Researchers obtained the supplement for biochemical analysis and found that it had been adulterated with thyroid hormones, which led to thyrotoxicosis factitia.2
Patients in the report complained of palpitations, weakness, fatigue, headache, diaphoresis, irritability, and nervousness, and had elevated serum T3 and T4 levels. Thyroglobulin levels were not determined, but would have been useful in differentiating thyrotoxicosis factitia from hyperthyroxinemia (Graves’ disease, thyroiditis, nodular goiter).2
These reports, as well as our own experience, leave little doubt as to the importance of asking pointed questions about all prescription and nonprescription products a patient is taking. As we can attest, a little persistence goes a long way when it comes to identifying that agent your patient didn’t think was worth mentioning.
Correspondence
Taryn Taylor, MD, CCFP, Bruyere Family Medicine Center, University of Ottawa, 206 Monterey Drive, Ottawa, Ontario, Canada K2H 7A8; [email protected]
- When taking a medication history, always ask specifically about the use of all nonprescription products—including all over-the-counter remedies, vitamins, “natural” herbal supplements, and dietary aids (C).
- Counsel patients about the need for caution when taking dietary supplements and herbal remedies, which lack regulation and standardization and may contain ingredients not listed on the label (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
When Mary J,* an overweight 47-year-old Caucasian woman, came in for an annual physical examination, she appeared to be in good health. She denied any recent illness, and reported that an oral contraceptive was the only medication she was taking. The patient’s only complaint: She was having difficulty losing weight despite complying with a low-calorie diet and exercise regimen for the last 6 months. Her comments prompted her physician (CC) to order a thyroid-stimulating hormone (TSH) test to rule out hypothyroidism.
The test showed a TSH of 0.2 mIU/L (normal range is 0.35-5.0 mIU/L). Her physician ordered retesting a week later and this time, Mary’s TSH was normal (1.99 mIU/L). The laboratory report also showed elevated free triiodothyronine (T3) of 8.1 pmol/L (normal range 2.6-5.7 pmol/L) and free thyroxine (T4) >70 pmol/L (normal, 10-20 pmol/L); negative antithyroid peroxidase and antithyroglobulin antibodies; normal complete blood count, calcium, and alkaline phosphatase; and low levels of thyroglobulin. The patient had no symptoms and no personal or family history of thyroid disease. She also denied taking thyroid medications.
* The patient’s name has been changed to protect her privacy.
In search of an explanation
On examination, her physician found no significant thyroid enlargement or tenderness. However, the patient’s thyroid was somewhat boggy on palpation. There was no exopthalmos or pretibial myxedema. Mary’s blood pressure was 144/98 mm Hg (with no prior history of hypertension), her heart rate was 84, and she was afebrile. Her physician found no obvious tremors, hyperdynamic apex, or hyperreflexia on physical exam.
To rule out laboratory error from the second set of tests, her physician ordered yet another round of blood work. A diagnosis of hyperthyroidism was confirmed by elevated T4 (>70 pmol/L) and T3 (6.2 pmol/L). As in the previous test, the patient’s TSH was in the normal range (1.54 mIU/L).
Detailed questioning solves the mystery
At follow-up, the patient was again asked about exogenous thyroid intake, which she had initially denied. After further questioning about what she was ingesting, Mary acknowledged that she had been taking Pu Erh—a European dietary supplement marketed as a means of increasing metabolism to help with weight loss—3 times daily for more than 3 months. She hadn’t mentioned it before because it hadn’t occurred to her to question its safety.
Her physician advised her to discontinue the supplement immediately, and to have her blood work retested in a month. Within 5 weeks, all her lab values returned to normal. (For more on lab values, see “Investigating thyroid dysfunction: What to test for” on page 205. )
TABLE
Investigating thyroid dysfunction: What to test for
The single most useful screening test for thyroid dysfunction is serum TSH. Normal TSH levels effectively rule out hyperthyroidism and hypothyroidism, and obtaining serum T3 and T4 levels is usually not indicated.8 Circulating levels of free T3 or T4 are increased in hyperthyroidism and thyrotoxicosis, while TSH levels are low to immeasurable (<0.01 mU/L).9 The term “thyrotoxicosis” is used to denote the excess of thyroid hormone levels without thyroid hyperfunction or increased biosynthesis—ie, excess intake, excess release without synthesis, or syndromes of pituitary resistance to thyroid hormones.10 Low thyroglobulin in association with hyperthyroidism is a hallmark of exogenous thyroid intake, also known as thyrotoxicosis factitia.11 |
Natural does not=safe
With an ever-increasing overweight population, there is growing concern about the misuse of diet aids. It is important for patients to be cautious when using dietary supplements because of the lack of regulation and standardization. Yet such products are often marketed as “natural,” which may be interpreted as an assurance of safety.1 Family physicians can play a crucial role in primary prevention by inquiring about the use of over-the-counter substances including “natural” herbal supplements and dietary aids, advising patients of the risks associated with their use, and being alert to potentially dangerous side effects.
Thyrotoxicosis factitia: An exogenous cause
Thyroid hyperactivity can occur when excessive quantities of thyroid hormone are ingested. The excessive intake may be associated with treatment for hypothyroidism, or, as in Mary’s case, may be linked to overuse (or abuse) of a diet aid in an attempt to lose weight.2 Often, the condition can be traced to an iodine-containing substance such as kelp—a widely used dietary aid that we’ll discuss in greater detail in a bit.
Iodide is an inorganic salt that is absorbed through the gastrointestinal mucosa and transported to the thyroid gland, where it is trapped and concentrated for thyroid hormone synthesis.3 Consuming large quantities of foods or other products that contain iodine—such as iodized salt, shellfish, cough syrups, multivitamins, or medications such as amiodarone and interferon alpha, as well as kelp—can cause hyperthyroidism.
What’s in that weight-loss aid?
Pu Erh, the dietary supplement Mary was taking, was not available for analysis; it was purchased in Poland and the patient had exhausted her supply by the time her physician told her to stop taking it. Each capsule contains 400 mg of red tea extract with 15 mcg chromium, according to the label.
Red tea and chromium. There are no reports associating red tea with thyroid dysfunction, but chromium is a popular weight-loss supplement whose efficacy and long-term safety are uncertain.4 It is unlikely that Mary’s hyperthyroidism was associated with chromium in-take, however, as multivitamins often contain 100 mcg of chromium—nearly 7 times the quantity in each Pu Erh capsule—with no reported thyroid side effects.
Did the supplement contain a thyroid extract? It’s possible, of course, that the supplement contained a thyroid extract, which would explain the resolution of symptoms after Mary stopped taking it. But without analysis of the product, we can’t be sure. Our suspicion, of course, is that it did.
Another possible, but unlikely, explanation. Theoretically, the fluctuating TSH could have been related to silent or subacute thyroiditis, in which T4 can remain elevated for 1 to 3 months. The fact that Mary had no history of cold or flu symptoms in the month preceding the initial TSH was inconsistent with this alternative diagnosis, however. Painless thyroiditis is also unlikely, as it is autoimmune in origin and the patient’s antithyroid antibodies were negative. The low thyroglobulin level supported an exogenous cause of hyperthyroidism.
Other supplements and thyroid dysfunction
Mary’s presentation is a single case indicating a possible link between a weight-loss supplement and asymptomatic hyperthyroidism—a clinically important condition that may be associated with disorders such as paroxysmal atrial fibrillation and osteoporosis. This is only one of a number of case reports of patients taking dietary supplements who have developed thyroid dysfunction.
Kelp. Long used as a dietary supplement, especially in Asia, kelp has been linked to thyroid dysfunction. One case report describes a 72-year-old woman with a history of thyroid disease having typical symptoms of hyperthyroidism while ingesting 4 to 6 kelp tablets daily for 1 year. Her TSH concentration was low, while total T3 and T4 levels were high. After discontinuing the kelp tablets, the hyperthyroidism resolved, and thyroid function tests returned to normal.5 Another report describes an instance of probable transient hyperthyroidism in a patient taking kelp in 2 different diet supplements.6
Tiratricol (Triac), a substance that has weak thyromimetic effects, resulted in a case of documented hyperthyroidism secondary to its use.1
Other reports. In Japan, the weight-reducing herbal medicines, Dream Shape and Ever Youth, became available in 2000. Twelve patients subsequently developed thyrotoxicosis after taking these herbal medicines, both of which were found to contain triiodothyronine and thyroxine.7
As early as 1986, researchers have described several patients who developed thyrotoxicosis from Enzo-Caps, a nonprescription diet aid manufactured in Peru. The product was touted as “a natural food product of papaya, garlic, and kelp” to assist with weight reduction. Researchers obtained the supplement for biochemical analysis and found that it had been adulterated with thyroid hormones, which led to thyrotoxicosis factitia.2
Patients in the report complained of palpitations, weakness, fatigue, headache, diaphoresis, irritability, and nervousness, and had elevated serum T3 and T4 levels. Thyroglobulin levels were not determined, but would have been useful in differentiating thyrotoxicosis factitia from hyperthyroxinemia (Graves’ disease, thyroiditis, nodular goiter).2
These reports, as well as our own experience, leave little doubt as to the importance of asking pointed questions about all prescription and nonprescription products a patient is taking. As we can attest, a little persistence goes a long way when it comes to identifying that agent your patient didn’t think was worth mentioning.
Correspondence
Taryn Taylor, MD, CCFP, Bruyere Family Medicine Center, University of Ottawa, 206 Monterey Drive, Ottawa, Ontario, Canada K2H 7A8; [email protected]
1. Bauer BA, Elkin PL, Erickson D, et al. Symptomatic hyperthyroidism in a patient taking the dietary supplement tiratricol. Mayo Clin Proc. 2002;77:587-590.
2. Braunstein GD, Koblin R, Sugawara M, et al. Unintentional thyrotoxicosis factitia due to a diet pill. Western J Med. 1986;145:388-391.
3. Clark CD, Cliford D, Bertram B, et al. Effects of kelp supplementation on thyroid function in euthyroid subjects. Endocr Pract. 2003;9:363-369.
4. Saper RB. Common dietary supplements for weight loss. Am Fam Physician. 2004;70:1731-1738.
5. Shilo S, Hirsch HJ. Iodine-induced hyperthyroidism in a patient with a normal thyroid gland. Postgrad Med J. 1986;62:661-662.
6. Eliason BC. Transient hyperthyroidism in a patient taking dietary supplements containing kelp. J Am Board Fam Pract. 1998;11:478-480.
7. Ohye H, Fukata S, Kanoh M, et al. Thyrotoxicosis caused by weight-reducing herbal medicines. Arch Intern Med. 2005;165:831-834.
8. Demers LM. Thyroid disease: pathophysiology and diagnosis. Clin Lab Med. 2004;24:19-28.
9. Goichot B, Perrin AE. Thyrotropin as first-line thyroid test. Lancet. 2001;358:509.-
10. Monaco F. Classification of thyroid diseases: suggestions for a revision. J Clin Endocrinol Metab. 2003;88:1428-1432.
11. Parmar MS, Sturge C. Recurrent hamburger thyrotoxicosis. CMAJ. 2003;169:415-417.
1. Bauer BA, Elkin PL, Erickson D, et al. Symptomatic hyperthyroidism in a patient taking the dietary supplement tiratricol. Mayo Clin Proc. 2002;77:587-590.
2. Braunstein GD, Koblin R, Sugawara M, et al. Unintentional thyrotoxicosis factitia due to a diet pill. Western J Med. 1986;145:388-391.
3. Clark CD, Cliford D, Bertram B, et al. Effects of kelp supplementation on thyroid function in euthyroid subjects. Endocr Pract. 2003;9:363-369.
4. Saper RB. Common dietary supplements for weight loss. Am Fam Physician. 2004;70:1731-1738.
5. Shilo S, Hirsch HJ. Iodine-induced hyperthyroidism in a patient with a normal thyroid gland. Postgrad Med J. 1986;62:661-662.
6. Eliason BC. Transient hyperthyroidism in a patient taking dietary supplements containing kelp. J Am Board Fam Pract. 1998;11:478-480.
7. Ohye H, Fukata S, Kanoh M, et al. Thyrotoxicosis caused by weight-reducing herbal medicines. Arch Intern Med. 2005;165:831-834.
8. Demers LM. Thyroid disease: pathophysiology and diagnosis. Clin Lab Med. 2004;24:19-28.
9. Goichot B, Perrin AE. Thyrotropin as first-line thyroid test. Lancet. 2001;358:509.-
10. Monaco F. Classification of thyroid diseases: suggestions for a revision. J Clin Endocrinol Metab. 2003;88:1428-1432.
11. Parmar MS, Sturge C. Recurrent hamburger thyrotoxicosis. CMAJ. 2003;169:415-417.
The preoperative consult: A coding quiz
As family physicians, we’re accustomed to seeing patients shortly before they’re scheduled for surgery—in the office, the hospital, or other settings. But not all preoperative (preop) visits are created equal in terms of the level of care, the coding, and the documentation required. Test your knowledge:
- A preop evaluation can be coded as a consultation visit if a request for the evaluation was initiated by:
- a surgeon.
- a patient or patient’s family member.
- physician self-referral.
- all of the above.
- The best reason to code a preop evaluation as a consultation is:
- more accurate Current Procedural Terminology Evaluation and Management (CPT E/M) coding.
- more accurate diagnostic coding per the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) system.
- reimbursement is (usually) better.
- all of the above.
- For outpatient consults for established patients, 2 out of the 3 key components of an encounter must be provided and documented.
- True.
- False.
- The correct way to report the primary diagnosis for a preop consultation is to use:
- the ICD-9-CM code for the patient’s acute or chronic medical condition that will likely be a concern in the perioperative period (eg, diabetes mellitus, coronary artery disease).
- the ICD-9-CM code for the acute or chronic condition for which the patient requires surgery (eg, osteoarthritis for an elective joint replacement, or cholelithiasis for a laparoscopic cholecystectomy).
- V codes V72.81-V72.84 (preop exams).
- none of the above.
- A comprehensive level of examination is required for:
- a level 4 office consultation.
- a level 3 inpatient consultation.
- a level 4 established patient office visit.
- none of the above.
- Preop consultations conducted in the hospital setting should be coded using inpatient consultation codes.
- True.
- False.
- It depends.
QUESTION 1: When can a preop evaluation be coded as a consultation?
Answer: A When a surgeon requests the consult. Here’s why.
A consultation is defined as a type of service provided by a physician whose opinion or advice regarding evaluation and/or management of a specific problem is requested by another physician, or other appropriate source. In order to qualify as a consultation—CPT E/M codes 99241-99245 for outpatients and 99251-99255 for inpatients (TABLE 1)—the evaluation must be requested by any of the following:1
- a physician
- physician assistant
- nurse practitioner
- chiropractor
- physical therapist
- occupational therapist
- speech-language pathologist
- psychologist
- social worker
- lawyer
- insurance company.
If the consultation is mandated by a third-party payer, use modifier -32 to report it.
If the preop encounter does not meet this requirement, use the customary E/M codes instead.
The physician providing the consult must clearly document the request from the surgeon or other source in the medical record.1 Our office satisfies this requirement by using a form that is faxed to the surgeon’s office at the time the preop visit is scheduled. The surgeon completes and signs the form (sometimes with a little prodding from our office staff) and faxes it back. The signed form is affixed to the patient’s chart and available at the time of the consultation visit.
TABLE 1
Consultation codes: The right way to use them
| CPT CODE | HISTORY | EXAM | MEDICAL DECISION-MAKING COMPLEXITY | TIME* (MIN) |
|---|---|---|---|---|
| OUTPATIENT† | ||||
| 99241 | PF | PF | Straightforward | 15 |
| 99242 | EPF | EPF | Straightforward | 30 |
| 99243 | D | D | Low | 40 |
| 99244 | C | C | Moderate | 60 |
| 99245 | C | C | High | 80 |
| INPATIENT† | ||||
| 99251 | PF | PF | Straightforward | 20 |
| 99252 | EPF | EPF | Straightforward | 40 |
| 99253 | D | D | Low | 55 |
| 99254 | C | C | Moderate | 80 |
| 99255 | C | C | High | 110 |
| CPT, Current Procedural Terminology; C, comprehensive; D, detailed; EPF, expanded problem focused; PF, problem focused. | ||||
| * When the physician documents total time and that counseling or care coordination accounted for > 50% of the encounter, time may determine the level of service. | ||||
| †All 3 components of an encounter are required. | ||||
| Source: American Medical Association; 2008.1 | ||||
QUESTION 2: Why should you code a preop evaluation as a consult?
Answer: D There are several reasons to code a preop evaluation performed at the request of a surgeon or other source as a consultation: Doing so offers more accurate E/M coding, more accurate diagnostic coding, and, in most cases, better reimbursement.
The preop evaluation is usually a consultation, sought by a surgeon, regarding the risks to the patient of undergoing the operative procedure and anesthesia, and strategies to provide optimal management of medical problems such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, or asthma in the perioperative period. In general, consultation codes provide significantly better reimbursement than other comparable E/M codes.
For instance, the 2009 Medicare payment for a level 2 outpatient consultation (99242) in the Ohio region is $88.88. In contrast, the fee for a level 2 new patient visit (99202) is $61.71.
Include the 4 Ws: Who, why, what, and where. To bill for a consultation, however, you not only need to provide information about risks and management strategies to the clinician who requests it; you also have to clearly document that you did so. In providing the proper documentation, there are 4 aspects of the consult to consider:
- Who requested the consult. As noted earlier, our practice requires a signed request from the surgeon for the medical record. (While a note documenting a verbal request would probably satisfy this requirement, a written request would provide much stronger evidence if an audit was done.)
- Why the consult is being performed. Remember that a consult is initiated as a request for opinion or advice. If you are simply asked to manage a patient’s medical problems in the postoperative (postop) period, you should charge for concurrent management, not for a consultation.
- What services you provided. Basically, this requirement simply calls for documenting your history, exam, assessment (opinion), and plan (advice). If you provide nonpreop care (such as medication refills or addressing unrelated medical issues) during the consult visit, you can bill separately for these services using modifier -25.
- Where you sent the results of your evaluation. It is also necessary to document that you completed the loop by sending your report to the surgeon who requested the consultation. Often, I complete a handwritten consult on a history and physical (H&P) form at the request of the surgeon. I document in my note that a copy of the H&P form was faxed to the surgeon, another copy was put into the patient’s medical record in my office, and the original was given to the patient to give to the surgeon on the scheduled day of the procedure. (Electronic health records would accomplish the same thing without paper, of course.)
QUESTION 3: True or false: Outpatient consults for established patients require 2 components of an encounter.
Answer: B False. Unlike other outpatient E/M codes, the consultation codes require that all 3 components of an encounter—history, examination, and medical decision making—be provided and documented for the appropriate level of service for both new and established patients (TABLE 1).
All 3 must be included in an inpatient consultation as well.
QUESTION 4: What’s the primary diagnosis code for a preop consult?
Answer: C V codes for preop exams (V72.81-V72.84) should be used as the primary diagnosis. In general, V codes are used “on occasions when circumstances other than a disease or an injury justify an encounter with the health care delivery system or influence the patient’s current condition.”2 The 4 allowable V codes for preoperative visits are:
- V72.81 (preop cardiovascular exam)
- V72.82 (preop respiratory exam)
- V72.83 (other specified preop exam)
- V72.84 (unspecified preop exam)
The acute or chronic medical condition for which the patient requires surgery should be listed as the secondary ICD-9-CM code.3 Additional codes may be used for the patient’s other acute or chronic medical conditions.
QUESTION 5: When is a comprehensive exam required?
Answer: A A level 4 (99244) office consult requires a comprehensive exam level; a level 3 (99253) inpatient consult does not.
The 1997 E/M guidelines4 specify that a level 4 office consult in which a general multisystem examination is conducted requires a comprehensive level—with documentation of 2 exam points from each of 9 systems (for a total of 18 points) and performance of all exam points in those 9 systems. The level 3 inpatient consult and level 4 established patient office visit codes require only a detailed exam, which entails documentation of 12 or more of the allowable exam points. Although the 1995 E/M guidelines can be used as a source to ensure that all the requirements are met, the 1997 guidelines are much more specific about the documentation needed for each exam level.
When to conduct a single-system exam. While family physicians frequently use the requirements of the general multisystem exam to determine their level of coding, the CPT rules allow the option of performing certain single-organ system exams. Because the cardiovascular system is the most common concern with a preop consult, it is often easier, and more appropriate, to document the elements of the cardiovascular system exam (TABLE 2) than the general multisystem exam.
In this instance, the V code (V72.81, preop cardiovascular exam) would be used for diagnosis. For patients with COPD or other respiratory problems, it would be appropriate to document the elements of the respiratory system exam (V72.82) instead (TABLE 3).
TABLE 2
The cardiovascular exam: What’s included*
| SYSTEM/BODY AREA | ELEMENTS |
|---|---|
| Constitutional | • Measurement of any 3 of the following 7 vital signs: 1) sitting or standing BP 2) supine BP 3) pulse rate and regularity 4) respiration 5) temperature 6) height 7) weight • General appearance of patient (eg, development, nutrition, body habitus, deformities, attention to grooming) |
| Head and face | |
| Eyes | • Inspection of conjunctivae and lids |
| Ears, nose, mouth, and throat | • Inspection of teeth, gums, and palate • Inspection of oral mucosa with notation of presence of pallor or cyanosis |
| Neck | • Examination of jugular veins • Examination of thyroid |
| Respiratory | • Assessment of respiratory effort • Auscultation of lungs |
| Cardiovascular | • Palpation of heart (eg, location, size, and forcefulness of the point of maximal impact; thrills; lifts; palpable S3 or S4) • Auscultation of heart, including sounds, abnormal sounds, and murmurs • Measurement of BP in 2 or more extremities when indicated Examination of: • Carotid arteries (eg, waveform, pulse amplitude, bruits, apical-carotid delay) • Abdominal aorta (eg, size, bruits) • Femoral arteries (eg, pulse amplitude, bruits) • Pedal pulses (eg, pulse amplitude) • Extremities for peripheral edema and/or varicosities |
| Chest (breasts) | |
| Gastrointestinal (abdomen) | • Examination of abdomen with notation of presence of masses or tenderness • Examination of liver and spleen • Stool sample for occult blood from patients being considered for thrombolytic or anticoagulant therapy |
| Genitourinary (abdomen) | |
| Lymphatic | |
| Musculoskeletal | • Examination of the back with notation of kyphosis or scoliosis • Examination of gait with notation of ability to undergo exercise testing and/or participation in exercise programs • Assessment of muscle strength and tone, with notation of any atrophy and abnormal movements |
| Extremities | • Inspection and palpation of digits and nails (eg, clubbing, cyanosis, inflammation, petechiae, ischemia, infections, Osler’s nodes) |
| Skin | • Inspection and/or palpation of skin and subcutaneous tissues |
| Neurological/psychiatric | Brief assessment of mental status, including • Orientation to time, place, and person • Mood and affect |
| BP, blood pressure. | |
| * What you are required to do: | |
| Level of exam Perform and document Problem focused: 1-5 elements identified by a bullet Expanded problem focused: ≥6 elements Detailed: ≥12 elements Comprehensive: Perform all elements, document every element in each shaded box and ≥1 element in each unshaded box. | |
| Source: American Medical Association; 2008.1 | |
TABLE 3
The respiratory exam: What’s included*
| SYSTEM/BODY AREA | ELEMENTS |
|---|---|
| Constitutional | • Measurement of any 3 of the following 7 vital signs: 1) sitting or standing BP 2) supine BP 3) pulse rate and regularity 4) respiration 5) temperature 6) height 7) weight • General appearance of patient (eg, development, nutrition, body habitus, deformities, attention to grooming) |
| Head and face | |
| Eyes | |
| Ears, nose, mouth, and throat | • Inspection of nasal mucosa, septum, and turbinates • Inspection of teeth and gums • Inspection of oropharynx (eg, oral mucosa, hard and soft palates, tongue, tonsils, and posterior pharynx) |
| Neck | • Examination of neck • Examination of thyroid • Examination of jugular veins |
| Respiratory | • Inspection of chest with notation of symmetry and expansion • Assessment of respiratory effort (eg, intercostal retractions, use of accessory muscles, diaphragmatic movement) • Percussion of chest (eg, dullness, flatness, hyperresonance) • Palpation of chest (eg, tactile fremitus) • Auscultation of lungs (eg, breath sounds, adventitious sounds, rubs) |
| Cardiovascular | • Auscultation of heart, including sounds, abnormal sounds, and murmurs • Examination of peripheral vascular system by observation and palpation |
| Chest (breasts) | |
| Gastrointestinal (abdomen) | • Examination of abdomen with notation of presence of masses or tenderness • Examination of liver and spleen |
| Genitourinary (abdomen) | |
| Lymphatic | • Palpation of lymph nodes in neck, axillae, groin, and/or other location |
| Musculoskeletal | • Assessment of muscle strength and tone (eg, flaccid, cog wheel, spastic) with notation of any atrophy and abnormal movements • Examination of gait and station |
| Extremities | • Inspection and palpation of digits and nails (eg, clubbing, cyanosis, inflammation, petechiae, ischemia, infections, nodes) |
| Skin | • Inspection and/or palpation of skin and subcutaneous tissue (eg, rashes, lesions, ulcers) |
| Neurological/psychiatric | Brief assessment of mental status, including • Orientation to time, place, and person • Mood and affect |
| BP, blood pressure. | |
| * What you are required to do: | |
| Level of exam: Perform and document Problem focused: 1-5 elements identified by a bullet Expanded problem focused: ≥6 elements Detailed: ≥12 elements Comprehensive: Perform all elements, document every element in each shaded box and ≥1 element in each unshaded box. | |
| Source: American Medical Association; 2008.1 | |
QUESTION 6: Should inpatient codes be used for preop consults in a hospital?
Answer: C It depends. While you’ll typically use inpatient codes, there are exceptions. Patients who are in the hospital but assigned to observation status, in the outpatient surgery area, or in the emergency department and not subsequently admitted, are considered outpatients. Thus, encounters with patients under such circumstances should be billed using outpatient codes.
What’s your score?
Give yourself 1 point for each question you answered correctly. If you scored 5 or better, you’re a coding genius. Please come to my office and help me run my practice!
If you scored 4 or lower, take the opportunity to learn more about coding. Go to http://www.cms.hhs.gov/MLNEdWebGuide, a Centers for Medicare and Medicaid Services site featuring downloadable publications, interactive tutorials, and other coding tools (click on “Documentation Guidelines for E&M Services”). The American Medical Association Web site is also a valuable source of E/M coding. At www.ama-assn.org/ama/pub/category/3113.html, you’ll find CPT/RVU Search, a free search engine you can use to learn more about the relative value unit system and review reimbursement rates for your geographic region.
Correspondence
Edward Onusko, MD, Clinton Memorial Hospital/University of Cincinnati Family Medicine Residency, 825 West Locust, Wilmington, OH 45123; [email protected]
1. Beebe M, Dalton JA, Espronceda M, et al. Current Procedural Terminology 2008 Standard Edition. Chicago: American Medical Association; 2008.
2. Ingenix. Coders’ Desk Reference for Diagnoses 2008. Eden Prairie, Minn: Ingenix; 2008.
3. Centers for Medicare and Medicaid. 1997 Documentation Guidelines for Evaluation and Management Services. Available at: http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed February 23, 2009.
4. Hughes C. A refresher on coding consultations. Fam Pract Manag. 2007;14:45-47.
As family physicians, we’re accustomed to seeing patients shortly before they’re scheduled for surgery—in the office, the hospital, or other settings. But not all preoperative (preop) visits are created equal in terms of the level of care, the coding, and the documentation required. Test your knowledge:
- A preop evaluation can be coded as a consultation visit if a request for the evaluation was initiated by:
- a surgeon.
- a patient or patient’s family member.
- physician self-referral.
- all of the above.
- The best reason to code a preop evaluation as a consultation is:
- more accurate Current Procedural Terminology Evaluation and Management (CPT E/M) coding.
- more accurate diagnostic coding per the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) system.
- reimbursement is (usually) better.
- all of the above.
- For outpatient consults for established patients, 2 out of the 3 key components of an encounter must be provided and documented.
- True.
- False.
- The correct way to report the primary diagnosis for a preop consultation is to use:
- the ICD-9-CM code for the patient’s acute or chronic medical condition that will likely be a concern in the perioperative period (eg, diabetes mellitus, coronary artery disease).
- the ICD-9-CM code for the acute or chronic condition for which the patient requires surgery (eg, osteoarthritis for an elective joint replacement, or cholelithiasis for a laparoscopic cholecystectomy).
- V codes V72.81-V72.84 (preop exams).
- none of the above.
- A comprehensive level of examination is required for:
- a level 4 office consultation.
- a level 3 inpatient consultation.
- a level 4 established patient office visit.
- none of the above.
- Preop consultations conducted in the hospital setting should be coded using inpatient consultation codes.
- True.
- False.
- It depends.
QUESTION 1: When can a preop evaluation be coded as a consultation?
Answer: A When a surgeon requests the consult. Here’s why.
A consultation is defined as a type of service provided by a physician whose opinion or advice regarding evaluation and/or management of a specific problem is requested by another physician, or other appropriate source. In order to qualify as a consultation—CPT E/M codes 99241-99245 for outpatients and 99251-99255 for inpatients (TABLE 1)—the evaluation must be requested by any of the following:1
- a physician
- physician assistant
- nurse practitioner
- chiropractor
- physical therapist
- occupational therapist
- speech-language pathologist
- psychologist
- social worker
- lawyer
- insurance company.
If the consultation is mandated by a third-party payer, use modifier -32 to report it.
If the preop encounter does not meet this requirement, use the customary E/M codes instead.
The physician providing the consult must clearly document the request from the surgeon or other source in the medical record.1 Our office satisfies this requirement by using a form that is faxed to the surgeon’s office at the time the preop visit is scheduled. The surgeon completes and signs the form (sometimes with a little prodding from our office staff) and faxes it back. The signed form is affixed to the patient’s chart and available at the time of the consultation visit.
TABLE 1
Consultation codes: The right way to use them
| CPT CODE | HISTORY | EXAM | MEDICAL DECISION-MAKING COMPLEXITY | TIME* (MIN) |
|---|---|---|---|---|
| OUTPATIENT† | ||||
| 99241 | PF | PF | Straightforward | 15 |
| 99242 | EPF | EPF | Straightforward | 30 |
| 99243 | D | D | Low | 40 |
| 99244 | C | C | Moderate | 60 |
| 99245 | C | C | High | 80 |
| INPATIENT† | ||||
| 99251 | PF | PF | Straightforward | 20 |
| 99252 | EPF | EPF | Straightforward | 40 |
| 99253 | D | D | Low | 55 |
| 99254 | C | C | Moderate | 80 |
| 99255 | C | C | High | 110 |
| CPT, Current Procedural Terminology; C, comprehensive; D, detailed; EPF, expanded problem focused; PF, problem focused. | ||||
| * When the physician documents total time and that counseling or care coordination accounted for > 50% of the encounter, time may determine the level of service. | ||||
| †All 3 components of an encounter are required. | ||||
| Source: American Medical Association; 2008.1 | ||||
QUESTION 2: Why should you code a preop evaluation as a consult?
Answer: D There are several reasons to code a preop evaluation performed at the request of a surgeon or other source as a consultation: Doing so offers more accurate E/M coding, more accurate diagnostic coding, and, in most cases, better reimbursement.
The preop evaluation is usually a consultation, sought by a surgeon, regarding the risks to the patient of undergoing the operative procedure and anesthesia, and strategies to provide optimal management of medical problems such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, or asthma in the perioperative period. In general, consultation codes provide significantly better reimbursement than other comparable E/M codes.
For instance, the 2009 Medicare payment for a level 2 outpatient consultation (99242) in the Ohio region is $88.88. In contrast, the fee for a level 2 new patient visit (99202) is $61.71.
Include the 4 Ws: Who, why, what, and where. To bill for a consultation, however, you not only need to provide information about risks and management strategies to the clinician who requests it; you also have to clearly document that you did so. In providing the proper documentation, there are 4 aspects of the consult to consider:
- Who requested the consult. As noted earlier, our practice requires a signed request from the surgeon for the medical record. (While a note documenting a verbal request would probably satisfy this requirement, a written request would provide much stronger evidence if an audit was done.)
- Why the consult is being performed. Remember that a consult is initiated as a request for opinion or advice. If you are simply asked to manage a patient’s medical problems in the postoperative (postop) period, you should charge for concurrent management, not for a consultation.
- What services you provided. Basically, this requirement simply calls for documenting your history, exam, assessment (opinion), and plan (advice). If you provide nonpreop care (such as medication refills or addressing unrelated medical issues) during the consult visit, you can bill separately for these services using modifier -25.
- Where you sent the results of your evaluation. It is also necessary to document that you completed the loop by sending your report to the surgeon who requested the consultation. Often, I complete a handwritten consult on a history and physical (H&P) form at the request of the surgeon. I document in my note that a copy of the H&P form was faxed to the surgeon, another copy was put into the patient’s medical record in my office, and the original was given to the patient to give to the surgeon on the scheduled day of the procedure. (Electronic health records would accomplish the same thing without paper, of course.)
QUESTION 3: True or false: Outpatient consults for established patients require 2 components of an encounter.
Answer: B False. Unlike other outpatient E/M codes, the consultation codes require that all 3 components of an encounter—history, examination, and medical decision making—be provided and documented for the appropriate level of service for both new and established patients (TABLE 1).
All 3 must be included in an inpatient consultation as well.
QUESTION 4: What’s the primary diagnosis code for a preop consult?
Answer: C V codes for preop exams (V72.81-V72.84) should be used as the primary diagnosis. In general, V codes are used “on occasions when circumstances other than a disease or an injury justify an encounter with the health care delivery system or influence the patient’s current condition.”2 The 4 allowable V codes for preoperative visits are:
- V72.81 (preop cardiovascular exam)
- V72.82 (preop respiratory exam)
- V72.83 (other specified preop exam)
- V72.84 (unspecified preop exam)
The acute or chronic medical condition for which the patient requires surgery should be listed as the secondary ICD-9-CM code.3 Additional codes may be used for the patient’s other acute or chronic medical conditions.
QUESTION 5: When is a comprehensive exam required?
Answer: A A level 4 (99244) office consult requires a comprehensive exam level; a level 3 (99253) inpatient consult does not.
The 1997 E/M guidelines4 specify that a level 4 office consult in which a general multisystem examination is conducted requires a comprehensive level—with documentation of 2 exam points from each of 9 systems (for a total of 18 points) and performance of all exam points in those 9 systems. The level 3 inpatient consult and level 4 established patient office visit codes require only a detailed exam, which entails documentation of 12 or more of the allowable exam points. Although the 1995 E/M guidelines can be used as a source to ensure that all the requirements are met, the 1997 guidelines are much more specific about the documentation needed for each exam level.
When to conduct a single-system exam. While family physicians frequently use the requirements of the general multisystem exam to determine their level of coding, the CPT rules allow the option of performing certain single-organ system exams. Because the cardiovascular system is the most common concern with a preop consult, it is often easier, and more appropriate, to document the elements of the cardiovascular system exam (TABLE 2) than the general multisystem exam.
In this instance, the V code (V72.81, preop cardiovascular exam) would be used for diagnosis. For patients with COPD or other respiratory problems, it would be appropriate to document the elements of the respiratory system exam (V72.82) instead (TABLE 3).
TABLE 2
The cardiovascular exam: What’s included*
| SYSTEM/BODY AREA | ELEMENTS |
|---|---|
| Constitutional | • Measurement of any 3 of the following 7 vital signs: 1) sitting or standing BP 2) supine BP 3) pulse rate and regularity 4) respiration 5) temperature 6) height 7) weight • General appearance of patient (eg, development, nutrition, body habitus, deformities, attention to grooming) |
| Head and face | |
| Eyes | • Inspection of conjunctivae and lids |
| Ears, nose, mouth, and throat | • Inspection of teeth, gums, and palate • Inspection of oral mucosa with notation of presence of pallor or cyanosis |
| Neck | • Examination of jugular veins • Examination of thyroid |
| Respiratory | • Assessment of respiratory effort • Auscultation of lungs |
| Cardiovascular | • Palpation of heart (eg, location, size, and forcefulness of the point of maximal impact; thrills; lifts; palpable S3 or S4) • Auscultation of heart, including sounds, abnormal sounds, and murmurs • Measurement of BP in 2 or more extremities when indicated Examination of: • Carotid arteries (eg, waveform, pulse amplitude, bruits, apical-carotid delay) • Abdominal aorta (eg, size, bruits) • Femoral arteries (eg, pulse amplitude, bruits) • Pedal pulses (eg, pulse amplitude) • Extremities for peripheral edema and/or varicosities |
| Chest (breasts) | |
| Gastrointestinal (abdomen) | • Examination of abdomen with notation of presence of masses or tenderness • Examination of liver and spleen • Stool sample for occult blood from patients being considered for thrombolytic or anticoagulant therapy |
| Genitourinary (abdomen) | |
| Lymphatic | |
| Musculoskeletal | • Examination of the back with notation of kyphosis or scoliosis • Examination of gait with notation of ability to undergo exercise testing and/or participation in exercise programs • Assessment of muscle strength and tone, with notation of any atrophy and abnormal movements |
| Extremities | • Inspection and palpation of digits and nails (eg, clubbing, cyanosis, inflammation, petechiae, ischemia, infections, Osler’s nodes) |
| Skin | • Inspection and/or palpation of skin and subcutaneous tissues |
| Neurological/psychiatric | Brief assessment of mental status, including • Orientation to time, place, and person • Mood and affect |
| BP, blood pressure. | |
| * What you are required to do: | |
| Level of exam Perform and document Problem focused: 1-5 elements identified by a bullet Expanded problem focused: ≥6 elements Detailed: ≥12 elements Comprehensive: Perform all elements, document every element in each shaded box and ≥1 element in each unshaded box. | |
| Source: American Medical Association; 2008.1 | |
TABLE 3
The respiratory exam: What’s included*
| SYSTEM/BODY AREA | ELEMENTS |
|---|---|
| Constitutional | • Measurement of any 3 of the following 7 vital signs: 1) sitting or standing BP 2) supine BP 3) pulse rate and regularity 4) respiration 5) temperature 6) height 7) weight • General appearance of patient (eg, development, nutrition, body habitus, deformities, attention to grooming) |
| Head and face | |
| Eyes | |
| Ears, nose, mouth, and throat | • Inspection of nasal mucosa, septum, and turbinates • Inspection of teeth and gums • Inspection of oropharynx (eg, oral mucosa, hard and soft palates, tongue, tonsils, and posterior pharynx) |
| Neck | • Examination of neck • Examination of thyroid • Examination of jugular veins |
| Respiratory | • Inspection of chest with notation of symmetry and expansion • Assessment of respiratory effort (eg, intercostal retractions, use of accessory muscles, diaphragmatic movement) • Percussion of chest (eg, dullness, flatness, hyperresonance) • Palpation of chest (eg, tactile fremitus) • Auscultation of lungs (eg, breath sounds, adventitious sounds, rubs) |
| Cardiovascular | • Auscultation of heart, including sounds, abnormal sounds, and murmurs • Examination of peripheral vascular system by observation and palpation |
| Chest (breasts) | |
| Gastrointestinal (abdomen) | • Examination of abdomen with notation of presence of masses or tenderness • Examination of liver and spleen |
| Genitourinary (abdomen) | |
| Lymphatic | • Palpation of lymph nodes in neck, axillae, groin, and/or other location |
| Musculoskeletal | • Assessment of muscle strength and tone (eg, flaccid, cog wheel, spastic) with notation of any atrophy and abnormal movements • Examination of gait and station |
| Extremities | • Inspection and palpation of digits and nails (eg, clubbing, cyanosis, inflammation, petechiae, ischemia, infections, nodes) |
| Skin | • Inspection and/or palpation of skin and subcutaneous tissue (eg, rashes, lesions, ulcers) |
| Neurological/psychiatric | Brief assessment of mental status, including • Orientation to time, place, and person • Mood and affect |
| BP, blood pressure. | |
| * What you are required to do: | |
| Level of exam: Perform and document Problem focused: 1-5 elements identified by a bullet Expanded problem focused: ≥6 elements Detailed: ≥12 elements Comprehensive: Perform all elements, document every element in each shaded box and ≥1 element in each unshaded box. | |
| Source: American Medical Association; 2008.1 | |
QUESTION 6: Should inpatient codes be used for preop consults in a hospital?
Answer: C It depends. While you’ll typically use inpatient codes, there are exceptions. Patients who are in the hospital but assigned to observation status, in the outpatient surgery area, or in the emergency department and not subsequently admitted, are considered outpatients. Thus, encounters with patients under such circumstances should be billed using outpatient codes.
What’s your score?
Give yourself 1 point for each question you answered correctly. If you scored 5 or better, you’re a coding genius. Please come to my office and help me run my practice!
If you scored 4 or lower, take the opportunity to learn more about coding. Go to http://www.cms.hhs.gov/MLNEdWebGuide, a Centers for Medicare and Medicaid Services site featuring downloadable publications, interactive tutorials, and other coding tools (click on “Documentation Guidelines for E&M Services”). The American Medical Association Web site is also a valuable source of E/M coding. At www.ama-assn.org/ama/pub/category/3113.html, you’ll find CPT/RVU Search, a free search engine you can use to learn more about the relative value unit system and review reimbursement rates for your geographic region.
Correspondence
Edward Onusko, MD, Clinton Memorial Hospital/University of Cincinnati Family Medicine Residency, 825 West Locust, Wilmington, OH 45123; [email protected]
As family physicians, we’re accustomed to seeing patients shortly before they’re scheduled for surgery—in the office, the hospital, or other settings. But not all preoperative (preop) visits are created equal in terms of the level of care, the coding, and the documentation required. Test your knowledge:
- A preop evaluation can be coded as a consultation visit if a request for the evaluation was initiated by:
- a surgeon.
- a patient or patient’s family member.
- physician self-referral.
- all of the above.
- The best reason to code a preop evaluation as a consultation is:
- more accurate Current Procedural Terminology Evaluation and Management (CPT E/M) coding.
- more accurate diagnostic coding per the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) system.
- reimbursement is (usually) better.
- all of the above.
- For outpatient consults for established patients, 2 out of the 3 key components of an encounter must be provided and documented.
- True.
- False.
- The correct way to report the primary diagnosis for a preop consultation is to use:
- the ICD-9-CM code for the patient’s acute or chronic medical condition that will likely be a concern in the perioperative period (eg, diabetes mellitus, coronary artery disease).
- the ICD-9-CM code for the acute or chronic condition for which the patient requires surgery (eg, osteoarthritis for an elective joint replacement, or cholelithiasis for a laparoscopic cholecystectomy).
- V codes V72.81-V72.84 (preop exams).
- none of the above.
- A comprehensive level of examination is required for:
- a level 4 office consultation.
- a level 3 inpatient consultation.
- a level 4 established patient office visit.
- none of the above.
- Preop consultations conducted in the hospital setting should be coded using inpatient consultation codes.
- True.
- False.
- It depends.
QUESTION 1: When can a preop evaluation be coded as a consultation?
Answer: A When a surgeon requests the consult. Here’s why.
A consultation is defined as a type of service provided by a physician whose opinion or advice regarding evaluation and/or management of a specific problem is requested by another physician, or other appropriate source. In order to qualify as a consultation—CPT E/M codes 99241-99245 for outpatients and 99251-99255 for inpatients (TABLE 1)—the evaluation must be requested by any of the following:1
- a physician
- physician assistant
- nurse practitioner
- chiropractor
- physical therapist
- occupational therapist
- speech-language pathologist
- psychologist
- social worker
- lawyer
- insurance company.
If the consultation is mandated by a third-party payer, use modifier -32 to report it.
If the preop encounter does not meet this requirement, use the customary E/M codes instead.
The physician providing the consult must clearly document the request from the surgeon or other source in the medical record.1 Our office satisfies this requirement by using a form that is faxed to the surgeon’s office at the time the preop visit is scheduled. The surgeon completes and signs the form (sometimes with a little prodding from our office staff) and faxes it back. The signed form is affixed to the patient’s chart and available at the time of the consultation visit.
TABLE 1
Consultation codes: The right way to use them
| CPT CODE | HISTORY | EXAM | MEDICAL DECISION-MAKING COMPLEXITY | TIME* (MIN) |
|---|---|---|---|---|
| OUTPATIENT† | ||||
| 99241 | PF | PF | Straightforward | 15 |
| 99242 | EPF | EPF | Straightforward | 30 |
| 99243 | D | D | Low | 40 |
| 99244 | C | C | Moderate | 60 |
| 99245 | C | C | High | 80 |
| INPATIENT† | ||||
| 99251 | PF | PF | Straightforward | 20 |
| 99252 | EPF | EPF | Straightforward | 40 |
| 99253 | D | D | Low | 55 |
| 99254 | C | C | Moderate | 80 |
| 99255 | C | C | High | 110 |
| CPT, Current Procedural Terminology; C, comprehensive; D, detailed; EPF, expanded problem focused; PF, problem focused. | ||||
| * When the physician documents total time and that counseling or care coordination accounted for > 50% of the encounter, time may determine the level of service. | ||||
| †All 3 components of an encounter are required. | ||||
| Source: American Medical Association; 2008.1 | ||||
QUESTION 2: Why should you code a preop evaluation as a consult?
Answer: D There are several reasons to code a preop evaluation performed at the request of a surgeon or other source as a consultation: Doing so offers more accurate E/M coding, more accurate diagnostic coding, and, in most cases, better reimbursement.
The preop evaluation is usually a consultation, sought by a surgeon, regarding the risks to the patient of undergoing the operative procedure and anesthesia, and strategies to provide optimal management of medical problems such as chronic obstructive pulmonary disease (COPD), diabetes mellitus, or asthma in the perioperative period. In general, consultation codes provide significantly better reimbursement than other comparable E/M codes.
For instance, the 2009 Medicare payment for a level 2 outpatient consultation (99242) in the Ohio region is $88.88. In contrast, the fee for a level 2 new patient visit (99202) is $61.71.
Include the 4 Ws: Who, why, what, and where. To bill for a consultation, however, you not only need to provide information about risks and management strategies to the clinician who requests it; you also have to clearly document that you did so. In providing the proper documentation, there are 4 aspects of the consult to consider:
- Who requested the consult. As noted earlier, our practice requires a signed request from the surgeon for the medical record. (While a note documenting a verbal request would probably satisfy this requirement, a written request would provide much stronger evidence if an audit was done.)
- Why the consult is being performed. Remember that a consult is initiated as a request for opinion or advice. If you are simply asked to manage a patient’s medical problems in the postoperative (postop) period, you should charge for concurrent management, not for a consultation.
- What services you provided. Basically, this requirement simply calls for documenting your history, exam, assessment (opinion), and plan (advice). If you provide nonpreop care (such as medication refills or addressing unrelated medical issues) during the consult visit, you can bill separately for these services using modifier -25.
- Where you sent the results of your evaluation. It is also necessary to document that you completed the loop by sending your report to the surgeon who requested the consultation. Often, I complete a handwritten consult on a history and physical (H&P) form at the request of the surgeon. I document in my note that a copy of the H&P form was faxed to the surgeon, another copy was put into the patient’s medical record in my office, and the original was given to the patient to give to the surgeon on the scheduled day of the procedure. (Electronic health records would accomplish the same thing without paper, of course.)
QUESTION 3: True or false: Outpatient consults for established patients require 2 components of an encounter.
Answer: B False. Unlike other outpatient E/M codes, the consultation codes require that all 3 components of an encounter—history, examination, and medical decision making—be provided and documented for the appropriate level of service for both new and established patients (TABLE 1).
All 3 must be included in an inpatient consultation as well.
QUESTION 4: What’s the primary diagnosis code for a preop consult?
Answer: C V codes for preop exams (V72.81-V72.84) should be used as the primary diagnosis. In general, V codes are used “on occasions when circumstances other than a disease or an injury justify an encounter with the health care delivery system or influence the patient’s current condition.”2 The 4 allowable V codes for preoperative visits are:
- V72.81 (preop cardiovascular exam)
- V72.82 (preop respiratory exam)
- V72.83 (other specified preop exam)
- V72.84 (unspecified preop exam)
The acute or chronic medical condition for which the patient requires surgery should be listed as the secondary ICD-9-CM code.3 Additional codes may be used for the patient’s other acute or chronic medical conditions.
QUESTION 5: When is a comprehensive exam required?
Answer: A A level 4 (99244) office consult requires a comprehensive exam level; a level 3 (99253) inpatient consult does not.
The 1997 E/M guidelines4 specify that a level 4 office consult in which a general multisystem examination is conducted requires a comprehensive level—with documentation of 2 exam points from each of 9 systems (for a total of 18 points) and performance of all exam points in those 9 systems. The level 3 inpatient consult and level 4 established patient office visit codes require only a detailed exam, which entails documentation of 12 or more of the allowable exam points. Although the 1995 E/M guidelines can be used as a source to ensure that all the requirements are met, the 1997 guidelines are much more specific about the documentation needed for each exam level.
When to conduct a single-system exam. While family physicians frequently use the requirements of the general multisystem exam to determine their level of coding, the CPT rules allow the option of performing certain single-organ system exams. Because the cardiovascular system is the most common concern with a preop consult, it is often easier, and more appropriate, to document the elements of the cardiovascular system exam (TABLE 2) than the general multisystem exam.
In this instance, the V code (V72.81, preop cardiovascular exam) would be used for diagnosis. For patients with COPD or other respiratory problems, it would be appropriate to document the elements of the respiratory system exam (V72.82) instead (TABLE 3).
TABLE 2
The cardiovascular exam: What’s included*
| SYSTEM/BODY AREA | ELEMENTS |
|---|---|
| Constitutional | • Measurement of any 3 of the following 7 vital signs: 1) sitting or standing BP 2) supine BP 3) pulse rate and regularity 4) respiration 5) temperature 6) height 7) weight • General appearance of patient (eg, development, nutrition, body habitus, deformities, attention to grooming) |
| Head and face | |
| Eyes | • Inspection of conjunctivae and lids |
| Ears, nose, mouth, and throat | • Inspection of teeth, gums, and palate • Inspection of oral mucosa with notation of presence of pallor or cyanosis |
| Neck | • Examination of jugular veins • Examination of thyroid |
| Respiratory | • Assessment of respiratory effort • Auscultation of lungs |
| Cardiovascular | • Palpation of heart (eg, location, size, and forcefulness of the point of maximal impact; thrills; lifts; palpable S3 or S4) • Auscultation of heart, including sounds, abnormal sounds, and murmurs • Measurement of BP in 2 or more extremities when indicated Examination of: • Carotid arteries (eg, waveform, pulse amplitude, bruits, apical-carotid delay) • Abdominal aorta (eg, size, bruits) • Femoral arteries (eg, pulse amplitude, bruits) • Pedal pulses (eg, pulse amplitude) • Extremities for peripheral edema and/or varicosities |
| Chest (breasts) | |
| Gastrointestinal (abdomen) | • Examination of abdomen with notation of presence of masses or tenderness • Examination of liver and spleen • Stool sample for occult blood from patients being considered for thrombolytic or anticoagulant therapy |
| Genitourinary (abdomen) | |
| Lymphatic | |
| Musculoskeletal | • Examination of the back with notation of kyphosis or scoliosis • Examination of gait with notation of ability to undergo exercise testing and/or participation in exercise programs • Assessment of muscle strength and tone, with notation of any atrophy and abnormal movements |
| Extremities | • Inspection and palpation of digits and nails (eg, clubbing, cyanosis, inflammation, petechiae, ischemia, infections, Osler’s nodes) |
| Skin | • Inspection and/or palpation of skin and subcutaneous tissues |
| Neurological/psychiatric | Brief assessment of mental status, including • Orientation to time, place, and person • Mood and affect |
| BP, blood pressure. | |
| * What you are required to do: | |
| Level of exam Perform and document Problem focused: 1-5 elements identified by a bullet Expanded problem focused: ≥6 elements Detailed: ≥12 elements Comprehensive: Perform all elements, document every element in each shaded box and ≥1 element in each unshaded box. | |
| Source: American Medical Association; 2008.1 | |
TABLE 3
The respiratory exam: What’s included*
| SYSTEM/BODY AREA | ELEMENTS |
|---|---|
| Constitutional | • Measurement of any 3 of the following 7 vital signs: 1) sitting or standing BP 2) supine BP 3) pulse rate and regularity 4) respiration 5) temperature 6) height 7) weight • General appearance of patient (eg, development, nutrition, body habitus, deformities, attention to grooming) |
| Head and face | |
| Eyes | |
| Ears, nose, mouth, and throat | • Inspection of nasal mucosa, septum, and turbinates • Inspection of teeth and gums • Inspection of oropharynx (eg, oral mucosa, hard and soft palates, tongue, tonsils, and posterior pharynx) |
| Neck | • Examination of neck • Examination of thyroid • Examination of jugular veins |
| Respiratory | • Inspection of chest with notation of symmetry and expansion • Assessment of respiratory effort (eg, intercostal retractions, use of accessory muscles, diaphragmatic movement) • Percussion of chest (eg, dullness, flatness, hyperresonance) • Palpation of chest (eg, tactile fremitus) • Auscultation of lungs (eg, breath sounds, adventitious sounds, rubs) |
| Cardiovascular | • Auscultation of heart, including sounds, abnormal sounds, and murmurs • Examination of peripheral vascular system by observation and palpation |
| Chest (breasts) | |
| Gastrointestinal (abdomen) | • Examination of abdomen with notation of presence of masses or tenderness • Examination of liver and spleen |
| Genitourinary (abdomen) | |
| Lymphatic | • Palpation of lymph nodes in neck, axillae, groin, and/or other location |
| Musculoskeletal | • Assessment of muscle strength and tone (eg, flaccid, cog wheel, spastic) with notation of any atrophy and abnormal movements • Examination of gait and station |
| Extremities | • Inspection and palpation of digits and nails (eg, clubbing, cyanosis, inflammation, petechiae, ischemia, infections, nodes) |
| Skin | • Inspection and/or palpation of skin and subcutaneous tissue (eg, rashes, lesions, ulcers) |
| Neurological/psychiatric | Brief assessment of mental status, including • Orientation to time, place, and person • Mood and affect |
| BP, blood pressure. | |
| * What you are required to do: | |
| Level of exam: Perform and document Problem focused: 1-5 elements identified by a bullet Expanded problem focused: ≥6 elements Detailed: ≥12 elements Comprehensive: Perform all elements, document every element in each shaded box and ≥1 element in each unshaded box. | |
| Source: American Medical Association; 2008.1 | |
QUESTION 6: Should inpatient codes be used for preop consults in a hospital?
Answer: C It depends. While you’ll typically use inpatient codes, there are exceptions. Patients who are in the hospital but assigned to observation status, in the outpatient surgery area, or in the emergency department and not subsequently admitted, are considered outpatients. Thus, encounters with patients under such circumstances should be billed using outpatient codes.
What’s your score?
Give yourself 1 point for each question you answered correctly. If you scored 5 or better, you’re a coding genius. Please come to my office and help me run my practice!
If you scored 4 or lower, take the opportunity to learn more about coding. Go to http://www.cms.hhs.gov/MLNEdWebGuide, a Centers for Medicare and Medicaid Services site featuring downloadable publications, interactive tutorials, and other coding tools (click on “Documentation Guidelines for E&M Services”). The American Medical Association Web site is also a valuable source of E/M coding. At www.ama-assn.org/ama/pub/category/3113.html, you’ll find CPT/RVU Search, a free search engine you can use to learn more about the relative value unit system and review reimbursement rates for your geographic region.
Correspondence
Edward Onusko, MD, Clinton Memorial Hospital/University of Cincinnati Family Medicine Residency, 825 West Locust, Wilmington, OH 45123; [email protected]
1. Beebe M, Dalton JA, Espronceda M, et al. Current Procedural Terminology 2008 Standard Edition. Chicago: American Medical Association; 2008.
2. Ingenix. Coders’ Desk Reference for Diagnoses 2008. Eden Prairie, Minn: Ingenix; 2008.
3. Centers for Medicare and Medicaid. 1997 Documentation Guidelines for Evaluation and Management Services. Available at: http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed February 23, 2009.
4. Hughes C. A refresher on coding consultations. Fam Pract Manag. 2007;14:45-47.
1. Beebe M, Dalton JA, Espronceda M, et al. Current Procedural Terminology 2008 Standard Edition. Chicago: American Medical Association; 2008.
2. Ingenix. Coders’ Desk Reference for Diagnoses 2008. Eden Prairie, Minn: Ingenix; 2008.
3. Centers for Medicare and Medicaid. 1997 Documentation Guidelines for Evaluation and Management Services. Available at: http://www.cms.hhs.gov/MLNProducts/Downloads/MASTER1.pdf. Accessed February 23, 2009.
4. Hughes C. A refresher on coding consultations. Fam Pract Manag. 2007;14:45-47.
ADOLESCENT DEPRESSION: Is your young patient suffering in silence?
You’ve known Jane since infancy. Now she’s 15 and in your office for her yearly checkup. As she comes into the exam room, you notice she’s gained a lot of weight since you saw her a year ago. She’s also missing the energy and sparkle that have always been such an engaging part of her personality. When you trot out your usual questions for teens—How’s school? Keeping up your grades? Going out for a team?—her answers are disquieting. School’s dull, her grades have gone downhill, and she’s dropped out of gymnastics. Her mother says Jane is irritable and sleeping a lot, and that worries her.
Could Jane be going through a bout of clinical depression?
Teen depression: Common, and commonly untreated
In North America, about 9% of all teenagers meet the criteria for depression at any given time, and prevalence rates in primary care are very likely higher.1 One study in the 1990s found approximately 28% of teens presenting to a primary care office met criteria for depression.2
Although adolescents with depression frequently seek care in the primary care setting, most are not identified or treated because of any number of barriers.3,4 First, mental illness continues to be highly stigmatized. As a result, many troubled teens (and parents of these teens) do not seek help.4 Second, mental health professionals trained to treat adolescents are in short supply, and most family physicians and other primary care clinicians feel inadequately trained, supported, or reimbursed for the management of this disorder.5 Third, the controversy over the safety and efficacy of antidepressants in the pediatric population has created an additional barrier to care.
In addition, while clinical guidelines for diagnosing and treating adolescent depression have been developed for specialty care settings,6 they are not easily transferred to primary care because of the significant differences between the primary and specialty care settings. Recognizing this gap in clinical guidance, a group of researchers and clinicians (including the authors of this report) from the United States and Canada established a collaborative to formulate primary care guidelines for adolescent depression (GuideLines for Adolescent Depression in Primary Care, or GLAD-PC). Details about the collaborative’s methods and recommendations were published in Pediatrics in 2007.7,8 The accompanying clinician toolkit is available at www.gladpc.org.
This review summarizes the collaborative’s key findings and recommendations and includes evidence from additional research published since the completion of GLAD-PC in 2007. For simplicity’s sake, we use the term “depression” to refer to what is more formally known as major depressive disorder (MDD).
Red flags that you are well positioned to spot
As a family physician, you have the advantage of knowing the families in your practice well and over a long time span. Drawing on that knowledge, you are well placed to spot the red flags that may signal depression in an adolescent patient.
Risk factors for the disorder are well known: a previous episode of depression, a family history of depression, the presence of other psychiatric disorders such as anxiety or attention deficit hyperactivity disorder (ADHD), substance abuse, or life stressors such as bereavement, abuse, or divorce. Teens with depression may complain of emotional problems, or turn up with repeated somatic complaints—headaches, stomach aches, fatigue—that have no apparent physiologic explanation. Their responses to general questions, such as “How is your mood?” or “Have you been sad?” may be worrisome. Or they may screen positive on self-report checklists such as the Beck Depression Inventory (BDI) or the Kutcher Adolescent Depression Scale (KADS), available for download at www.cprf.ca/education/Openmind2006/KADS11.pdf and free for use with permission.9,10
GLAD-PC Recommendation II: Family physicians should consider the diagnosis of depression in high-risk adolescents and those who present with emotional problems as their chief complaints (SOR: B, cohort studies and randomized controlled trials [RCTs]).
Routine screening of all adolescents for depression may be feasible, but the US Preventive Services Task Force concluded in 2002 that the evidence was insufficient to recommend for or against the practice.7,11,12 Expert opinion suggests that among adolescents at elevated risk for depression, depression checklists are useful during well-child and urgent care visits. However, families will likely find general questions more acceptable during acute care visits.10
“SIGECAPS” mnemonic can help as you evaluate the patient
When you suspect depression, take a detailed history. The diagnostic criteria for depression given in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) are shown in TABLE 1 .7,10,13 Bear in mind, however, that adolescents who do not meet the full criteria may still be quite impaired and in need of help. The SIGECAPS mnemonic (sleep, interest, guilt, energy, concentration, appetite changes, psychomotor agitation or retardation, suicidality) can help you recall the neurovegetative symptoms in the depression criteria.
Ask about bereavement, manic symptoms (eg, feeling irritable/giddy/silly, hyperactive, racing thoughts), substance use, and life stressors. Ask, too, whether the teen has been treated for mental health problems in the past, and if there is any history of physical or sexual abuse or a family history of mental illness. Because depression is often comorbid with other conditions, you should also inquire about other psychiatric disorders, such as ADHD and anxiety disorders.
The next step. When risk factors or checklists alert you to the possibility of depression, the next step is a more formal evaluation. Because teens and parents often feel uncomfortable disclosing information in the presence of the other, separate interviews are a good idea. Information crucial to the diagnosis may be available only from the adolescent or only from the parent or caregiver, and then only if they are interviewed separately.7
Parents may—or may not—pick up on their child’s depression. On the one hand, parents will often have important clues to their child’s diagnosis, such as recent withdrawal from social or extracurricular activities. On the other hand, they may attribute their teen’s behavior to normal adolescent moodiness. Or they might not recognize their teenager’s depression because teens don’t need to be “sad” to be depressed. Sometimes irritability is the major symptom in a depressed teen. (See “How teenage depression is different from that of adults” on page 188.)
Further compounding matters: Since depression is an internalizing disorder, teens may not share their innermost thoughts and emotions with their parents.
Teenage depression may not look like adult depression. Teens are more often irritable than sad, and their moods vary with their surroundings (ie, mood reactivity): They may be fine when they’re hanging out with friends, and become depressed again at home or in school. The depressive symptoms they exhibit can range from complaints about stomach aches to fights with family and friends, skipping school, getting poor grades, or substance use.
TABLE 1
Diagnostic criteria for major depressive episode (DSM-IV-TR)
| A. | Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous functioning; at least 1 of the symptoms is either depressed mood or loss of interest.
|
| B. | The symptoms do not meet criteria for mixed episode. |
| C. | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| D. | The symptoms are not due to the direct physiological effects of a substance (eg, a drug of abuse, or a medication) or a general medical condition (eg, hypothyroidism). |
| E. | The symptoms are not better accounted for by bereavement, that is after a loss of a loved one, the symptoms persist for longer than 2 months or are characterized by marked functional impairment, morbid preoccupation with worthlessness, suicidal ideation, psychotic symptoms, or psychomotor retardation. |
Is it MDD, or something else?
Although most of the literature on depression is focused on MDD, you should be aware that there are many subtypes of depression, including dysthymia (in which patients have longstanding depressive symptoms but with less functional impairment than major depression) and adjustment disorder (in which patients develop depressive symptoms in response to an acute stressor). As mentioned above, physicians should also assess for psychiatric disorders that are commonly comorbid with depression, because their presence can affect management. These include anxiety disorders, ADHD, eating disorders, and substance abuse.
Ruling out alternative diagnoses. In assessing potentially depressed teenagers like Jane, ruling out conditions with similar symptoms is essential. Medical conditions to be considered in the differential diagnosis are anemia, malignancies, hypothyroidism, and mononucleosis—as well as other viral conditions. There is, however, no evidence to support routine lab testing (including for hypothyroidism) of adolescent patients. Laboratory and other diagnostic evaluation should, instead, be guided by history and targeted physical exam. TABLE 2 presents common medical causes of symptoms of depression that must be considered in the differential diagnosis.
Consider bipolar disorder. Depressive symptoms may also be part of a cycling mood disorder, such as bipolar disorder. In fact, most teens with bipolar disorder will first present with depressive symptoms. Adolescents with depression as part of a bipolar disorder are more likely to have adverse effects with antidepressants than are teens with depression alone. In order to adequately rule out bipolar depression, ask about:
- rapid onset of depressive symptoms: “She just woke up one day and couldn’t stop crying,” for instance
- psychotic symptoms
- family history of bipolar disorder, especially in first-degree relatives
- previous symptoms of mania while on antidepressant treatment (eg, hyperactive, rapid speech, decreased need for sleep).
If a patient has these symptoms or a history of bipolar disorder, refer her or him for a mental health consultation before starting antidepressant treatment.
TABLE 2
Is a medical cause to blame for those symptoms of depression?
| MEDICAL CAUSES | SYMPTOMS | INVESTIGATIONS |
|---|---|---|
| Hyper- or hypothyroidism | Insomnia, agitation, weight loss or gain | Thyroid function tests |
| Anemia | Fatigue, hypersomnia | Complete blood count |
| Sleep disorder | Fatigue, insomnia | Sleep assessment |
| Mononucleosis/viral infections | Fatigue, hypersomnia | EBV test |
| Medications | ||
| Steroids | • Low mood, weight gain, increased appetite | Complete history of medication use (temporal relationship to onset of symptoms) Medication re-challenge test |
| Albuterol sulfate (Ventolin) | • Irritability, insomnia | |
| Isotretinoin (Accutane) | • Low mood, suicidality |
Help in classifying the severity of depression
The severity of depression can vary considerably from one patient to another, and distinguishing mild, moderate, and severe depression has significant implications for treatment. Guidelines for grading depression severity are given in TABLE 3 . A common way to classify the severity of a depressive episode is to count the number of symptoms the teenager is displaying.7 If all 9 symptoms in the DSM-IV-TR criteria are present, the depression would be classified as severe. But even with fewer symptoms, depression should be considered severe if the teenager is suicidal (has a specific suicide plan, a clear intent, or has made a recent attempt); has psychotic symptoms; or functioning is severely impaired (eg, patient is unable to go to school). The Diagnostic and Statistical Manual of Mental Disorders: Primary Care Version (DSM-PC) is also a useful resource for distinguishing between transient depressive responses and depressive disorders.
TABLE 3
Grading the severity of depressive episodes
| In both the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), severity of depressive episodes is based on the number, type, and severity of symptoms, as well as the degree of functional impairment. The DSM-IV-TR guidelines are summarized in the table below. | |||
|---|---|---|---|
| DSM-IV-TR GUIDELINES FOR GRADING DEPRESSION SEVERITY | |||
| MILD | MODERATE | SEVERE | |
| Number of symptoms | 5-6 | * | Most† |
| Severity of symptoms | Mild | * | Severe |
| Degree of functional impairment | Mild impairment or normal functioning but with “substantial and unusual” effort | * | “Clear-cut, observable disability” |
Ask yourself: Is this teenager impaired?
Symptoms, in themselves, are not enough to clinch the diagnosis. The fundamental question is whether the symptoms prevent your patient from normal functioning. To judge the extent of a patient’s impairment, you need to assess overall functioning and ask about school, home, friends, and leisure activities. Impairment can be determined by asking the patient and parents the simple questions that every family physician is familiar with:
- How is Jane doing in school? Have her grades slipped lately?
- How is life at home? Does Jane’s mood affect family relationships?
- Does Jane have good friends she can talk to?
- Has her mood affected her ability to maintain friendships?
- What does Jane do for fun? Has she been doing those things lately?
First and foremost, keep your patient safe. Even if you can’t do a complete assessment, your evaluation must at least include the determination of acute risk of harm, either from self-inflicted injury or from impaired judgment. At minimum, assess for suicidality, self-injurious behavior, altered sensorium, substance use, and access to firearms.7 Again, this can be aided by the teen’s answers to symptom checklists.
GLAD-PC Recommendation IV: Assessment for depression should include direct interviews with the patients and families/care-givers separately (SOR: B, cohort studies) and should include the assessment of functional impairment in different domains (SOR: C, expert opinion) and other existing psychiatric conditions (SOR: B, cohort studies).
CORRESPONDENCE
Amy Cheung, MD, 33 Russell Street, 3rd Floor Tower, Toronto, Ontario, Canada MSS 2S1; [email protected]
1. Cheung A, Dewa C. Canadian Community Health Survey: major depressive disorder and suicidality in adolescents. Healthcare Policy. 2006;2:76-89.
2. Kramer T, Garralda ME. Psychiatric disorders in adolescents in primary care. Br J Psychiatr. 1998;173:508-513.
3. Cheung A, Dewa C. Service use among youth with major depressive disorder and suicidality. Can J Psychiatr. 2007;52:228-232.
4. Hirschfeld RMA, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement of the undertreatment of depression. JAMA. 1997;277:333-340.
5. Olson AL, Kelleher KJ, Kemper KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1:91-98.
6. Birmaher B, Brent D. and the AACAP Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatr. 2007;46:1503-1526.
7. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care – GLAD PC – Part I. Pediatrics. 2007;120:e1299-e1312.
8. Cheung A, Zuckerbrot RA, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care – GLAD PC – Part II. Pediatrics. 2007;120:e1313-e1326.
9. Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987.
10. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Expert survey for the management of adolescent depression in primary care. Pediatrics. 2008;121(1):e101-e107.
11. Zuckerbrot RA, Jensen PS. Improving recognition of adolescent depression in primary care. Arch Pediatr Adolesc Med. 2006;160:694-704.
12. US Preventive Services Task Force. Screening for depression. Available at: http://www.ahrq.gov/clinic/uspstf/uspsdepr.htm. Accessed June 16, 2008.
13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
You’ve known Jane since infancy. Now she’s 15 and in your office for her yearly checkup. As she comes into the exam room, you notice she’s gained a lot of weight since you saw her a year ago. She’s also missing the energy and sparkle that have always been such an engaging part of her personality. When you trot out your usual questions for teens—How’s school? Keeping up your grades? Going out for a team?—her answers are disquieting. School’s dull, her grades have gone downhill, and she’s dropped out of gymnastics. Her mother says Jane is irritable and sleeping a lot, and that worries her.
Could Jane be going through a bout of clinical depression?
Teen depression: Common, and commonly untreated
In North America, about 9% of all teenagers meet the criteria for depression at any given time, and prevalence rates in primary care are very likely higher.1 One study in the 1990s found approximately 28% of teens presenting to a primary care office met criteria for depression.2
Although adolescents with depression frequently seek care in the primary care setting, most are not identified or treated because of any number of barriers.3,4 First, mental illness continues to be highly stigmatized. As a result, many troubled teens (and parents of these teens) do not seek help.4 Second, mental health professionals trained to treat adolescents are in short supply, and most family physicians and other primary care clinicians feel inadequately trained, supported, or reimbursed for the management of this disorder.5 Third, the controversy over the safety and efficacy of antidepressants in the pediatric population has created an additional barrier to care.
In addition, while clinical guidelines for diagnosing and treating adolescent depression have been developed for specialty care settings,6 they are not easily transferred to primary care because of the significant differences between the primary and specialty care settings. Recognizing this gap in clinical guidance, a group of researchers and clinicians (including the authors of this report) from the United States and Canada established a collaborative to formulate primary care guidelines for adolescent depression (GuideLines for Adolescent Depression in Primary Care, or GLAD-PC). Details about the collaborative’s methods and recommendations were published in Pediatrics in 2007.7,8 The accompanying clinician toolkit is available at www.gladpc.org.
This review summarizes the collaborative’s key findings and recommendations and includes evidence from additional research published since the completion of GLAD-PC in 2007. For simplicity’s sake, we use the term “depression” to refer to what is more formally known as major depressive disorder (MDD).
Red flags that you are well positioned to spot
As a family physician, you have the advantage of knowing the families in your practice well and over a long time span. Drawing on that knowledge, you are well placed to spot the red flags that may signal depression in an adolescent patient.
Risk factors for the disorder are well known: a previous episode of depression, a family history of depression, the presence of other psychiatric disorders such as anxiety or attention deficit hyperactivity disorder (ADHD), substance abuse, or life stressors such as bereavement, abuse, or divorce. Teens with depression may complain of emotional problems, or turn up with repeated somatic complaints—headaches, stomach aches, fatigue—that have no apparent physiologic explanation. Their responses to general questions, such as “How is your mood?” or “Have you been sad?” may be worrisome. Or they may screen positive on self-report checklists such as the Beck Depression Inventory (BDI) or the Kutcher Adolescent Depression Scale (KADS), available for download at www.cprf.ca/education/Openmind2006/KADS11.pdf and free for use with permission.9,10
GLAD-PC Recommendation II: Family physicians should consider the diagnosis of depression in high-risk adolescents and those who present with emotional problems as their chief complaints (SOR: B, cohort studies and randomized controlled trials [RCTs]).
Routine screening of all adolescents for depression may be feasible, but the US Preventive Services Task Force concluded in 2002 that the evidence was insufficient to recommend for or against the practice.7,11,12 Expert opinion suggests that among adolescents at elevated risk for depression, depression checklists are useful during well-child and urgent care visits. However, families will likely find general questions more acceptable during acute care visits.10
“SIGECAPS” mnemonic can help as you evaluate the patient
When you suspect depression, take a detailed history. The diagnostic criteria for depression given in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) are shown in TABLE 1 .7,10,13 Bear in mind, however, that adolescents who do not meet the full criteria may still be quite impaired and in need of help. The SIGECAPS mnemonic (sleep, interest, guilt, energy, concentration, appetite changes, psychomotor agitation or retardation, suicidality) can help you recall the neurovegetative symptoms in the depression criteria.
Ask about bereavement, manic symptoms (eg, feeling irritable/giddy/silly, hyperactive, racing thoughts), substance use, and life stressors. Ask, too, whether the teen has been treated for mental health problems in the past, and if there is any history of physical or sexual abuse or a family history of mental illness. Because depression is often comorbid with other conditions, you should also inquire about other psychiatric disorders, such as ADHD and anxiety disorders.
The next step. When risk factors or checklists alert you to the possibility of depression, the next step is a more formal evaluation. Because teens and parents often feel uncomfortable disclosing information in the presence of the other, separate interviews are a good idea. Information crucial to the diagnosis may be available only from the adolescent or only from the parent or caregiver, and then only if they are interviewed separately.7
Parents may—or may not—pick up on their child’s depression. On the one hand, parents will often have important clues to their child’s diagnosis, such as recent withdrawal from social or extracurricular activities. On the other hand, they may attribute their teen’s behavior to normal adolescent moodiness. Or they might not recognize their teenager’s depression because teens don’t need to be “sad” to be depressed. Sometimes irritability is the major symptom in a depressed teen. (See “How teenage depression is different from that of adults” on page 188.)
Further compounding matters: Since depression is an internalizing disorder, teens may not share their innermost thoughts and emotions with their parents.
Teenage depression may not look like adult depression. Teens are more often irritable than sad, and their moods vary with their surroundings (ie, mood reactivity): They may be fine when they’re hanging out with friends, and become depressed again at home or in school. The depressive symptoms they exhibit can range from complaints about stomach aches to fights with family and friends, skipping school, getting poor grades, or substance use.
TABLE 1
Diagnostic criteria for major depressive episode (DSM-IV-TR)
| A. | Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous functioning; at least 1 of the symptoms is either depressed mood or loss of interest.
|
| B. | The symptoms do not meet criteria for mixed episode. |
| C. | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| D. | The symptoms are not due to the direct physiological effects of a substance (eg, a drug of abuse, or a medication) or a general medical condition (eg, hypothyroidism). |
| E. | The symptoms are not better accounted for by bereavement, that is after a loss of a loved one, the symptoms persist for longer than 2 months or are characterized by marked functional impairment, morbid preoccupation with worthlessness, suicidal ideation, psychotic symptoms, or psychomotor retardation. |
Is it MDD, or something else?
Although most of the literature on depression is focused on MDD, you should be aware that there are many subtypes of depression, including dysthymia (in which patients have longstanding depressive symptoms but with less functional impairment than major depression) and adjustment disorder (in which patients develop depressive symptoms in response to an acute stressor). As mentioned above, physicians should also assess for psychiatric disorders that are commonly comorbid with depression, because their presence can affect management. These include anxiety disorders, ADHD, eating disorders, and substance abuse.
Ruling out alternative diagnoses. In assessing potentially depressed teenagers like Jane, ruling out conditions with similar symptoms is essential. Medical conditions to be considered in the differential diagnosis are anemia, malignancies, hypothyroidism, and mononucleosis—as well as other viral conditions. There is, however, no evidence to support routine lab testing (including for hypothyroidism) of adolescent patients. Laboratory and other diagnostic evaluation should, instead, be guided by history and targeted physical exam. TABLE 2 presents common medical causes of symptoms of depression that must be considered in the differential diagnosis.
Consider bipolar disorder. Depressive symptoms may also be part of a cycling mood disorder, such as bipolar disorder. In fact, most teens with bipolar disorder will first present with depressive symptoms. Adolescents with depression as part of a bipolar disorder are more likely to have adverse effects with antidepressants than are teens with depression alone. In order to adequately rule out bipolar depression, ask about:
- rapid onset of depressive symptoms: “She just woke up one day and couldn’t stop crying,” for instance
- psychotic symptoms
- family history of bipolar disorder, especially in first-degree relatives
- previous symptoms of mania while on antidepressant treatment (eg, hyperactive, rapid speech, decreased need for sleep).
If a patient has these symptoms or a history of bipolar disorder, refer her or him for a mental health consultation before starting antidepressant treatment.
TABLE 2
Is a medical cause to blame for those symptoms of depression?
| MEDICAL CAUSES | SYMPTOMS | INVESTIGATIONS |
|---|---|---|
| Hyper- or hypothyroidism | Insomnia, agitation, weight loss or gain | Thyroid function tests |
| Anemia | Fatigue, hypersomnia | Complete blood count |
| Sleep disorder | Fatigue, insomnia | Sleep assessment |
| Mononucleosis/viral infections | Fatigue, hypersomnia | EBV test |
| Medications | ||
| Steroids | • Low mood, weight gain, increased appetite | Complete history of medication use (temporal relationship to onset of symptoms) Medication re-challenge test |
| Albuterol sulfate (Ventolin) | • Irritability, insomnia | |
| Isotretinoin (Accutane) | • Low mood, suicidality |
Help in classifying the severity of depression
The severity of depression can vary considerably from one patient to another, and distinguishing mild, moderate, and severe depression has significant implications for treatment. Guidelines for grading depression severity are given in TABLE 3 . A common way to classify the severity of a depressive episode is to count the number of symptoms the teenager is displaying.7 If all 9 symptoms in the DSM-IV-TR criteria are present, the depression would be classified as severe. But even with fewer symptoms, depression should be considered severe if the teenager is suicidal (has a specific suicide plan, a clear intent, or has made a recent attempt); has psychotic symptoms; or functioning is severely impaired (eg, patient is unable to go to school). The Diagnostic and Statistical Manual of Mental Disorders: Primary Care Version (DSM-PC) is also a useful resource for distinguishing between transient depressive responses and depressive disorders.
TABLE 3
Grading the severity of depressive episodes
| In both the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), severity of depressive episodes is based on the number, type, and severity of symptoms, as well as the degree of functional impairment. The DSM-IV-TR guidelines are summarized in the table below. | |||
|---|---|---|---|
| DSM-IV-TR GUIDELINES FOR GRADING DEPRESSION SEVERITY | |||
| MILD | MODERATE | SEVERE | |
| Number of symptoms | 5-6 | * | Most† |
| Severity of symptoms | Mild | * | Severe |
| Degree of functional impairment | Mild impairment or normal functioning but with “substantial and unusual” effort | * | “Clear-cut, observable disability” |
Ask yourself: Is this teenager impaired?
Symptoms, in themselves, are not enough to clinch the diagnosis. The fundamental question is whether the symptoms prevent your patient from normal functioning. To judge the extent of a patient’s impairment, you need to assess overall functioning and ask about school, home, friends, and leisure activities. Impairment can be determined by asking the patient and parents the simple questions that every family physician is familiar with:
- How is Jane doing in school? Have her grades slipped lately?
- How is life at home? Does Jane’s mood affect family relationships?
- Does Jane have good friends she can talk to?
- Has her mood affected her ability to maintain friendships?
- What does Jane do for fun? Has she been doing those things lately?
First and foremost, keep your patient safe. Even if you can’t do a complete assessment, your evaluation must at least include the determination of acute risk of harm, either from self-inflicted injury or from impaired judgment. At minimum, assess for suicidality, self-injurious behavior, altered sensorium, substance use, and access to firearms.7 Again, this can be aided by the teen’s answers to symptom checklists.
GLAD-PC Recommendation IV: Assessment for depression should include direct interviews with the patients and families/care-givers separately (SOR: B, cohort studies) and should include the assessment of functional impairment in different domains (SOR: C, expert opinion) and other existing psychiatric conditions (SOR: B, cohort studies).
CORRESPONDENCE
Amy Cheung, MD, 33 Russell Street, 3rd Floor Tower, Toronto, Ontario, Canada MSS 2S1; [email protected]
You’ve known Jane since infancy. Now she’s 15 and in your office for her yearly checkup. As she comes into the exam room, you notice she’s gained a lot of weight since you saw her a year ago. She’s also missing the energy and sparkle that have always been such an engaging part of her personality. When you trot out your usual questions for teens—How’s school? Keeping up your grades? Going out for a team?—her answers are disquieting. School’s dull, her grades have gone downhill, and she’s dropped out of gymnastics. Her mother says Jane is irritable and sleeping a lot, and that worries her.
Could Jane be going through a bout of clinical depression?
Teen depression: Common, and commonly untreated
In North America, about 9% of all teenagers meet the criteria for depression at any given time, and prevalence rates in primary care are very likely higher.1 One study in the 1990s found approximately 28% of teens presenting to a primary care office met criteria for depression.2
Although adolescents with depression frequently seek care in the primary care setting, most are not identified or treated because of any number of barriers.3,4 First, mental illness continues to be highly stigmatized. As a result, many troubled teens (and parents of these teens) do not seek help.4 Second, mental health professionals trained to treat adolescents are in short supply, and most family physicians and other primary care clinicians feel inadequately trained, supported, or reimbursed for the management of this disorder.5 Third, the controversy over the safety and efficacy of antidepressants in the pediatric population has created an additional barrier to care.
In addition, while clinical guidelines for diagnosing and treating adolescent depression have been developed for specialty care settings,6 they are not easily transferred to primary care because of the significant differences between the primary and specialty care settings. Recognizing this gap in clinical guidance, a group of researchers and clinicians (including the authors of this report) from the United States and Canada established a collaborative to formulate primary care guidelines for adolescent depression (GuideLines for Adolescent Depression in Primary Care, or GLAD-PC). Details about the collaborative’s methods and recommendations were published in Pediatrics in 2007.7,8 The accompanying clinician toolkit is available at www.gladpc.org.
This review summarizes the collaborative’s key findings and recommendations and includes evidence from additional research published since the completion of GLAD-PC in 2007. For simplicity’s sake, we use the term “depression” to refer to what is more formally known as major depressive disorder (MDD).
Red flags that you are well positioned to spot
As a family physician, you have the advantage of knowing the families in your practice well and over a long time span. Drawing on that knowledge, you are well placed to spot the red flags that may signal depression in an adolescent patient.
Risk factors for the disorder are well known: a previous episode of depression, a family history of depression, the presence of other psychiatric disorders such as anxiety or attention deficit hyperactivity disorder (ADHD), substance abuse, or life stressors such as bereavement, abuse, or divorce. Teens with depression may complain of emotional problems, or turn up with repeated somatic complaints—headaches, stomach aches, fatigue—that have no apparent physiologic explanation. Their responses to general questions, such as “How is your mood?” or “Have you been sad?” may be worrisome. Or they may screen positive on self-report checklists such as the Beck Depression Inventory (BDI) or the Kutcher Adolescent Depression Scale (KADS), available for download at www.cprf.ca/education/Openmind2006/KADS11.pdf and free for use with permission.9,10
GLAD-PC Recommendation II: Family physicians should consider the diagnosis of depression in high-risk adolescents and those who present with emotional problems as their chief complaints (SOR: B, cohort studies and randomized controlled trials [RCTs]).
Routine screening of all adolescents for depression may be feasible, but the US Preventive Services Task Force concluded in 2002 that the evidence was insufficient to recommend for or against the practice.7,11,12 Expert opinion suggests that among adolescents at elevated risk for depression, depression checklists are useful during well-child and urgent care visits. However, families will likely find general questions more acceptable during acute care visits.10
“SIGECAPS” mnemonic can help as you evaluate the patient
When you suspect depression, take a detailed history. The diagnostic criteria for depression given in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) are shown in TABLE 1 .7,10,13 Bear in mind, however, that adolescents who do not meet the full criteria may still be quite impaired and in need of help. The SIGECAPS mnemonic (sleep, interest, guilt, energy, concentration, appetite changes, psychomotor agitation or retardation, suicidality) can help you recall the neurovegetative symptoms in the depression criteria.
Ask about bereavement, manic symptoms (eg, feeling irritable/giddy/silly, hyperactive, racing thoughts), substance use, and life stressors. Ask, too, whether the teen has been treated for mental health problems in the past, and if there is any history of physical or sexual abuse or a family history of mental illness. Because depression is often comorbid with other conditions, you should also inquire about other psychiatric disorders, such as ADHD and anxiety disorders.
The next step. When risk factors or checklists alert you to the possibility of depression, the next step is a more formal evaluation. Because teens and parents often feel uncomfortable disclosing information in the presence of the other, separate interviews are a good idea. Information crucial to the diagnosis may be available only from the adolescent or only from the parent or caregiver, and then only if they are interviewed separately.7
Parents may—or may not—pick up on their child’s depression. On the one hand, parents will often have important clues to their child’s diagnosis, such as recent withdrawal from social or extracurricular activities. On the other hand, they may attribute their teen’s behavior to normal adolescent moodiness. Or they might not recognize their teenager’s depression because teens don’t need to be “sad” to be depressed. Sometimes irritability is the major symptom in a depressed teen. (See “How teenage depression is different from that of adults” on page 188.)
Further compounding matters: Since depression is an internalizing disorder, teens may not share their innermost thoughts and emotions with their parents.
Teenage depression may not look like adult depression. Teens are more often irritable than sad, and their moods vary with their surroundings (ie, mood reactivity): They may be fine when they’re hanging out with friends, and become depressed again at home or in school. The depressive symptoms they exhibit can range from complaints about stomach aches to fights with family and friends, skipping school, getting poor grades, or substance use.
TABLE 1
Diagnostic criteria for major depressive episode (DSM-IV-TR)
| A. | Five (or more) of the following symptoms have been present during the same 2-week period and represent a change from previous functioning; at least 1 of the symptoms is either depressed mood or loss of interest.
|
| B. | The symptoms do not meet criteria for mixed episode. |
| C. | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| D. | The symptoms are not due to the direct physiological effects of a substance (eg, a drug of abuse, or a medication) or a general medical condition (eg, hypothyroidism). |
| E. | The symptoms are not better accounted for by bereavement, that is after a loss of a loved one, the symptoms persist for longer than 2 months or are characterized by marked functional impairment, morbid preoccupation with worthlessness, suicidal ideation, psychotic symptoms, or psychomotor retardation. |
Is it MDD, or something else?
Although most of the literature on depression is focused on MDD, you should be aware that there are many subtypes of depression, including dysthymia (in which patients have longstanding depressive symptoms but with less functional impairment than major depression) and adjustment disorder (in which patients develop depressive symptoms in response to an acute stressor). As mentioned above, physicians should also assess for psychiatric disorders that are commonly comorbid with depression, because their presence can affect management. These include anxiety disorders, ADHD, eating disorders, and substance abuse.
Ruling out alternative diagnoses. In assessing potentially depressed teenagers like Jane, ruling out conditions with similar symptoms is essential. Medical conditions to be considered in the differential diagnosis are anemia, malignancies, hypothyroidism, and mononucleosis—as well as other viral conditions. There is, however, no evidence to support routine lab testing (including for hypothyroidism) of adolescent patients. Laboratory and other diagnostic evaluation should, instead, be guided by history and targeted physical exam. TABLE 2 presents common medical causes of symptoms of depression that must be considered in the differential diagnosis.
Consider bipolar disorder. Depressive symptoms may also be part of a cycling mood disorder, such as bipolar disorder. In fact, most teens with bipolar disorder will first present with depressive symptoms. Adolescents with depression as part of a bipolar disorder are more likely to have adverse effects with antidepressants than are teens with depression alone. In order to adequately rule out bipolar depression, ask about:
- rapid onset of depressive symptoms: “She just woke up one day and couldn’t stop crying,” for instance
- psychotic symptoms
- family history of bipolar disorder, especially in first-degree relatives
- previous symptoms of mania while on antidepressant treatment (eg, hyperactive, rapid speech, decreased need for sleep).
If a patient has these symptoms or a history of bipolar disorder, refer her or him for a mental health consultation before starting antidepressant treatment.
TABLE 2
Is a medical cause to blame for those symptoms of depression?
| MEDICAL CAUSES | SYMPTOMS | INVESTIGATIONS |
|---|---|---|
| Hyper- or hypothyroidism | Insomnia, agitation, weight loss or gain | Thyroid function tests |
| Anemia | Fatigue, hypersomnia | Complete blood count |
| Sleep disorder | Fatigue, insomnia | Sleep assessment |
| Mononucleosis/viral infections | Fatigue, hypersomnia | EBV test |
| Medications | ||
| Steroids | • Low mood, weight gain, increased appetite | Complete history of medication use (temporal relationship to onset of symptoms) Medication re-challenge test |
| Albuterol sulfate (Ventolin) | • Irritability, insomnia | |
| Isotretinoin (Accutane) | • Low mood, suicidality |
Help in classifying the severity of depression
The severity of depression can vary considerably from one patient to another, and distinguishing mild, moderate, and severe depression has significant implications for treatment. Guidelines for grading depression severity are given in TABLE 3 . A common way to classify the severity of a depressive episode is to count the number of symptoms the teenager is displaying.7 If all 9 symptoms in the DSM-IV-TR criteria are present, the depression would be classified as severe. But even with fewer symptoms, depression should be considered severe if the teenager is suicidal (has a specific suicide plan, a clear intent, or has made a recent attempt); has psychotic symptoms; or functioning is severely impaired (eg, patient is unable to go to school). The Diagnostic and Statistical Manual of Mental Disorders: Primary Care Version (DSM-PC) is also a useful resource for distinguishing between transient depressive responses and depressive disorders.
TABLE 3
Grading the severity of depressive episodes
| In both the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) and the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), severity of depressive episodes is based on the number, type, and severity of symptoms, as well as the degree of functional impairment. The DSM-IV-TR guidelines are summarized in the table below. | |||
|---|---|---|---|
| DSM-IV-TR GUIDELINES FOR GRADING DEPRESSION SEVERITY | |||
| MILD | MODERATE | SEVERE | |
| Number of symptoms | 5-6 | * | Most† |
| Severity of symptoms | Mild | * | Severe |
| Degree of functional impairment | Mild impairment or normal functioning but with “substantial and unusual” effort | * | “Clear-cut, observable disability” |
Ask yourself: Is this teenager impaired?
Symptoms, in themselves, are not enough to clinch the diagnosis. The fundamental question is whether the symptoms prevent your patient from normal functioning. To judge the extent of a patient’s impairment, you need to assess overall functioning and ask about school, home, friends, and leisure activities. Impairment can be determined by asking the patient and parents the simple questions that every family physician is familiar with:
- How is Jane doing in school? Have her grades slipped lately?
- How is life at home? Does Jane’s mood affect family relationships?
- Does Jane have good friends she can talk to?
- Has her mood affected her ability to maintain friendships?
- What does Jane do for fun? Has she been doing those things lately?
First and foremost, keep your patient safe. Even if you can’t do a complete assessment, your evaluation must at least include the determination of acute risk of harm, either from self-inflicted injury or from impaired judgment. At minimum, assess for suicidality, self-injurious behavior, altered sensorium, substance use, and access to firearms.7 Again, this can be aided by the teen’s answers to symptom checklists.
GLAD-PC Recommendation IV: Assessment for depression should include direct interviews with the patients and families/care-givers separately (SOR: B, cohort studies) and should include the assessment of functional impairment in different domains (SOR: C, expert opinion) and other existing psychiatric conditions (SOR: B, cohort studies).
CORRESPONDENCE
Amy Cheung, MD, 33 Russell Street, 3rd Floor Tower, Toronto, Ontario, Canada MSS 2S1; [email protected]
1. Cheung A, Dewa C. Canadian Community Health Survey: major depressive disorder and suicidality in adolescents. Healthcare Policy. 2006;2:76-89.
2. Kramer T, Garralda ME. Psychiatric disorders in adolescents in primary care. Br J Psychiatr. 1998;173:508-513.
3. Cheung A, Dewa C. Service use among youth with major depressive disorder and suicidality. Can J Psychiatr. 2007;52:228-232.
4. Hirschfeld RMA, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement of the undertreatment of depression. JAMA. 1997;277:333-340.
5. Olson AL, Kelleher KJ, Kemper KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1:91-98.
6. Birmaher B, Brent D. and the AACAP Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatr. 2007;46:1503-1526.
7. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care – GLAD PC – Part I. Pediatrics. 2007;120:e1299-e1312.
8. Cheung A, Zuckerbrot RA, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care – GLAD PC – Part II. Pediatrics. 2007;120:e1313-e1326.
9. Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987.
10. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Expert survey for the management of adolescent depression in primary care. Pediatrics. 2008;121(1):e101-e107.
11. Zuckerbrot RA, Jensen PS. Improving recognition of adolescent depression in primary care. Arch Pediatr Adolesc Med. 2006;160:694-704.
12. US Preventive Services Task Force. Screening for depression. Available at: http://www.ahrq.gov/clinic/uspstf/uspsdepr.htm. Accessed June 16, 2008.
13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
1. Cheung A, Dewa C. Canadian Community Health Survey: major depressive disorder and suicidality in adolescents. Healthcare Policy. 2006;2:76-89.
2. Kramer T, Garralda ME. Psychiatric disorders in adolescents in primary care. Br J Psychiatr. 1998;173:508-513.
3. Cheung A, Dewa C. Service use among youth with major depressive disorder and suicidality. Can J Psychiatr. 2007;52:228-232.
4. Hirschfeld RMA, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement of the undertreatment of depression. JAMA. 1997;277:333-340.
5. Olson AL, Kelleher KJ, Kemper KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1:91-98.
6. Birmaher B, Brent D. and the AACAP Work Group on Quality Issues Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatr. 2007;46:1503-1526.
7. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care – GLAD PC – Part I. Pediatrics. 2007;120:e1299-e1312.
8. Cheung A, Zuckerbrot RA, Jensen PS, et al. Guidelines for Adolescent Depression in Primary Care – GLAD PC – Part II. Pediatrics. 2007;120:e1313-e1326.
9. Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1987.
10. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Expert survey for the management of adolescent depression in primary care. Pediatrics. 2008;121(1):e101-e107.
11. Zuckerbrot RA, Jensen PS. Improving recognition of adolescent depression in primary care. Arch Pediatr Adolesc Med. 2006;160:694-704.
12. US Preventive Services Task Force. Screening for depression. Available at: http://www.ahrq.gov/clinic/uspstf/uspsdepr.htm. Accessed June 16, 2008.
13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
Managing lower back pain: You may be doing too much
A 32-year-old construction worker seeks treatment for the lower back pain (LBP) he’s been experiencing since painting his house a few days ago.
A 48-year-old man with a history of LBP comes in because he needs a refill of his hydrocodone prescription.
Both patients are probably pretty typical of the back pain patients you see on a regular basis. But how would you care for each of these patients, and how does your care compare to the latest evidence? This review will help you to find out. In this article we take a look at guidelines from the American College of Physicians (ACP) and the American Pain Society (APS),1 as well as findings from other recent studies, and apply them to these 2 patient cases.
But first, a word about the ACP/APS guidelines.
What’s new?
ACP/APS conducted a systematic review of studies of LBP epidemiology, clinical diagnosis, utility of imaging, and outcomes of pharmacologic2 and nonpharmacologic interventions.3 Whereas previous guidelines dealt with either acute or chronic pain, the ACP/APS guidelines synthesized the literature to apply to both.
Moreover, rather than focusing mostly on pain reduction, the ACP/APS panel was interested in functional outcomes such as back-specific functioning, general health status, disability, and patient satisfaction.
Finally, the panel’s recommendations ( TABLE ) considered the unique environment of primary care (including presentations typically seen in this setting), the ability of primary care physicians to advise and counsel patients, continuity of care, and the role of the physician in coordinating care.
TABLE
Recommendations from the ACP/APS guidelines for low back pain1
| Conduct a focused history and exam to place patients into 1 of 3 broad categories: nonspecific LBP, LBP potentially associated with radiculopathy or spinal stenosis, or LBP associated with another specific cause (strength of recommendation [SOR]: B). |
| Assess for psychosocial factors and emotional distress, as they are stronger predictors of LBP outcomes, including disability, than physical exam findings and severity of pain (SOR: B). |
| Do not routinely obtain imaging for patients with nonspecific LBP. MRI or CT is recommended for patients with LBP associated with a specific cause, for those with severe or progressive neurologic deficits or persistent radiculopathy/spinal stenosis symptoms, and for those who are candidates for surgical interventions (SOR: B). |
| Advise patients with nonspecific LBP to remain active and provide information on LBP’s expected course and effective self-care options (SOR: B). |
| Consider the addition of nonpharmacologic treatments, including selective alternative modalities, when self-care fails. These treatments include spinal manipulation for acute LBP and acupuncture for chronic LBP (SOR: B). |
| Consider acetaminophen or nonsteroidal anti-inflammatory drugs as first-line medication options for most patients. Keep in mind the limited effectiveness and potential harm of others, including opioids (SOR: B). |
| Strength of recommendation (SOR) A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented evidence, case series |
CASE 1
Patient with acute nonspecific LBP
While painting his home, a 32-year-old construction worker felt a twinge in his lower back as he stepped off a ladder. He remained active, relying on over-the-counter ibuprofen and heat packs to relieve soreness. Two days later he visits his physician because the soreness has not abated. He reports no bowel or bladder complaints, worsening of pain, radiation of symptoms, nausea, vomiting, abdominal pain, or fever. He tells his doctor that he strained his back before and that this “feels the same way it did before.” The last time this happened he received physical therapy (PT), which helped. He thinks he may need PT again, but wants to discuss it with his physician.
Physical exam reveals mild tenderness to palpation over the right lumbar paraspinal muscle, but no spasms are apparent. Otherwise, his musculoskeletal exam—including range-of-motion testing—is within normal limits. The neurologic exam also is within normal limits, including normal deep tendon reflexes of the lower extremities and negative straight-leg-raise testing. His gait is normal, with no sign of discomfort.
Specific anatomic diagnoses are elusive. In the primary care setting, fewer than 15% of LBP cases have an identifiable underlying disease or spinal abnormality.4 An exhaustive search for a specific anatomic diagnosis lacks utility in selecting initial therapy or affecting patient outcomes. Instead, when caring for a patient like the one in our case, it’s important to focus on a thorough medical history and examination that assess the location and duration of symptoms, as well as uncover symptoms suggestive of radiculopathy or spinal stenosis.
Of course, you’ll need to rule out potentially serious conditions, such as cancer, vertebral infection, cauda equina syndrome, compression fracture, and ankylosing spondylitis. You’ll also need to check for rapidly progressing or severe neurologic deficits, such as motor deficits at more than one level or a patient’s report of incontinence or bladder dysfunction.
Straight-leg-raise testing and neurologic assessment of the lower extremity—specifically strength and reflex testing of the knee, ankle, foot, and great toe to assess nerve foot level involvement—are key. With this assessment, you should categorize a patient’s LBP as nonspecific, as potentially associated with radiculopathy or spinal stenosis, or as potentially associated with another specific cause (ACP/APS recommendation; strength of recommendation [SOR]: B).1
Pursue imaging—or not? While plain radiography is certainly an option if you suspect a vertebral compression fracture in a high-risk patient, it would not be necessary for a patient like the one in our case. This patient has a classic presentation of acute nonspecific LBP, for which neither routine plain radiography nor advanced imaging (CT or MRI) improves patient outcomes. Given this lack of proven benefit and the unnecessary radiation exposure with certain tests, routine imaging is not recommended for nonspecific LBP (ACP/APS recommendation; SOR: B).1,4-6
Action steps. The evidence supports a number of steps when caring for a patient like the one in our case. Some steps are targeted to patients with nonspecific LBP—and we’ve labeled them as such. Others more broadly apply to patients with LBP.
Explore the possible contribution of psychosocial factors and emotional distress to back pain (ACP/APS recommendation; SOR: B).1 These factors are stronger predictors of low back pain outcomes, including chronic back pain disability, than physical exam findings and duration or severity of pain.7,8 Predictors of poorer outcomes include depression, passive coping strategies, job dissatisfaction, somatization, higher disability levels, and disputed compensation claims. The effectiveness of specific tools for gathering such information has not been demonstrated in the primary care setting. Therefore, fully investigate psychosocial information in the patient interview.
Provide patients with nonspecific LBP with evidence-based information regarding its expected course; advise them to remain active and suggest effective self-care options (ACP/APS recommendation; SOR: B).1 This recommendation is based on findings that the typical course and prognosis of LBP are generally favorable, on studies comparing bed rest versus remaining active, and on outcome studies for self-care interventions.
Self-care includes a variety of interventions patients can implement without a clinical visit—patient education, including self-care books, and patient-structured physical activities. This approach is much less expensive than—and has equivalent or nearly equivalent effectiveness to—costlier interventions such as physical therapy, massage, spinal manipulation, or acupuncture.
Regarding work limitations, there is insufficient evidence for specific guidance. Routinely assess patient age, health, and physical demands and job tasks, and recommend restrictions based on clinical judgment.
Try nonpharmacologic therapies that have proven benefits in the event that self-care fails (ACP/APS recommendation; SOR: B).1 These include spinal manipulation, defined as manual therapy in which loads are applied to the spine by using short- or long-lever methods and high-velocity thrusts are applied to a spinal joint beyond its restricted range of motion. Serious adverse events are extremely rare.3
If medication is needed for acute LBP, first-line drugs include nonopioids with proven benefits, such as acetaminophen, nonsteroidal anti-inflammatory agents, or skeletal muscle relaxants (ACP/APS recommendation; SOR: B).2
CASE 1
The patient’s course
The physician carefully reviews the 32-year-old patient’s psychosocial factors and finds that he is positive about his job, enjoys his work, and is not seeking compensation. He uses exercise and prayer to manage stress and is in a stable relationship. He does not smoke, use recreational drugs, or have a history of psychiatric disorders, including depression. He says he drinks 2 to 3 beers on the weekends.
In discussing treatment, the patient considers PT as the optimal intervention. His doctor does not recommend it, and instead encourages him to remain active, gives him a self-care booklet on stretching and exercises, and advises him to check in again as needed, reassuring him that most cases of nonspecific acute low back pain resolve spontaneously.
Initially the patient does well with self-care and he returns to activity. However, 6 weeks after his office visit, the patient returns with pain that has worsened over the last 2 weeks. He has also begun experiencing tingling sensations down his right leg, trouble standing for short intervals because of pain, and weakness in his back. On physical exam, he still has minimal tenderness to palpation over the right lumbar region. The right straight-leg-raise test yields a positive result, and the right patellar reflex is diminished compared with the left. His rectal tone is normal. His gait is antalgic.
When the patient requests imaging, the physician advises him of the risks associated with imaging and the unlikely prospect that it will change management—despite the change in neurologic symptoms. After considering such evidence-based options as massage therapy, yoga, and spinal manipulation, they agree on a trial of PT. The patient’s current level of function is reviewed, and work limitations are set.
After 8 weeks of PT, the patient experiences an improvement in overall function, pain level, and weakness. His straight-leg-raise test—the physical exam finding with the most sensitivity for disc herniation—returns to normal, as does his patellar reflex. Although frustrated with the length of recovery time, he is appreciative of his physician and therapists.
Follow this physician’s lead: Be prudent with imaging. Many primary care physicians caring for a patient like this one would consider imaging studies to assess the worsening signs and symptoms. The evidence, however, does not clearly support that decision. Given the potential harm of testing and varying benefit in outcomes, the ACP/APS offers different recommendations on imaging and other diagnostic tests, depending on the category of LBP. Prompt evaluation with advanced imaging (MRI or CT) is recommended for severe or progressive neurologic deficits, and with suspicion of a serious underlying condition, such as vertebral infection, cauda equine syndrome, or cancer with spinal cord compression, given that delayed treatment may lead to poor outcomes (ACP/APS recommendation; SOR: B).1
For many patients with herniated lumbar discs, symptoms can improve within 4 weeks.9,10 Thus, there is no compelling evidence that routine imaging changes treatment decisions or outcomes.11 For patients with persistent symptoms of radiculopathy or spinal stenosis who have not responded to conservative therapy, invasive procedures (surgery or epidural injections) become potential treatment options, and thus imaging with MRI (preferred) or CT may be warranted.
CASE 2
Patient with chronic LBP
A 48-year-old man new to the practice comes in complaining of persistent pain in the lower back, which he ranks at 6 on a scale of 1 to 10. Approximately 5 years ago he underwent an L4-L5 laminectomy/fusion for herniated nucleus pulposus. The surgery relieved shooting pains down his left leg, but he has since had progressive problems with lumbar pain and stiffness. Two courses of PT and a series of facet joint injections over the years have provided only temporary relief. The patient had been followed by a pain management clinic, but was discharged after exhausting his insurance benefit. A recent MRI ordered by the pain management clinic showed mild to moderate degenerative changes in L2 to S1 with a healed fusion.
The reason for his visit this day is to request a refill of his hydrocodone, initiated by the pain clinic. He is worried that he will not get better and is afraid of injuring himself and has, as a result, been avoiding activities. He denies depressive symptoms except for decreased self-worth and pain-related sleep disturbance. He says that he was once more vigorous and felt competent, but is now passive and feels helpless about his pain. He is concerned that his physical capabilities will worsen even more and asks if there are any other therapies that might be helpful.
No easy answers. Patients with chronic LBP—pain lasting for longer than 2 months—present unique challenges. They have often seen several clinicians, including pain management specialists, have undergone repeated imaging, and are frustrated by their persistent symptoms. Many have had 1 or more surgeries, and most have tried numerous medications to gain relief.
This patient is seeking a refill of his opioid. Before agreeing to such a request, weigh the risks and benefits of opioids and the potential benefits of alternative therapies (SOR: B).1 Although the chronic use of opioids is an option for a select group of patients with chronic LBP,12 these agents can be expensive, lead to habituation and addiction, be easily redirected for monetary gain, and have untoward side effects. Evidence does not show that long-term opioid use improves functioning in patients with chronic LBP.
Nonpharmacologic therapies that have proven beneficial for such patients include acupuncture, cognitive-behavioral therapy, PT, exercise therapy (defined as any supervised or formal exercise program), and therapeutic massage (SOR: B).1 It would be preferable to start a therapeutic plan incorporating 1 or more of these modalities, based on a patient’s psychosocial history, insurance status, and preferences. Suggesting these therapies with guarded optimism can lead to a decreased need for opioids and increased functioning.
Again, psychosocial evaluation is important. When initially assessing a patient with chronic LBP, it is imperative to evaluate psychosocial factors. As noted earlier, psychosocial factors are better predictors of treatment outcomes than physical findings. Identifying factors related to poor outcomes (eg, anxiety, poor work history, passive attitude toward rehabilitation) can direct therapy and avoid polypharmacy.
Cognitive-behavioral and educational interventions will be more effective when targeting specific psychological and social factors (SOR: C).13,14 Fear-avoidance beliefs, distress, somatization, and pain catastrophizing place patients at the highest risk for poor outcomes. Primary objectives in psychosocial intervention are providing encouragement for overcoming demoralization; helping the patient make the connection between thoughts, feelings, and behaviors; and teaching the patient coping strategies and techniques to adapt to pain and resultant problems.
The ultimate goal for a patient like the one in this second case is to change his perception of chronic pain from overwhelming to manageable and to get him to see himself as resourceful and competent.15 Physician counseling has produced small positive effects in undifferentiated primary care patients with LBP, and it may therefore be more powerful when targeted to patients with specific psychosocial issues such as fear avoidance.16
Provide patients with a realistic outlook. Another key element is to direct patients’ expectations. Most people with chronic LBP will not become pain free, and patients need to know this fact. Aim treatment at improving function as well as reducing pain. You can assess functional status and improvement using patient questionnaires such as the Roland-Morris Disability Questionnaire (http://www.rmdq.org/) or the Oswestry Disability Index 2.0 (ODI, http://www.cpta.ab.ca/resources/Measurement%20Tools/Evaluative_Oswestry%20Disability%20Index.doc).17,18 Although these measures have not demonstrated utility in primary care practice, they have sufficient scale width to reliably detect change in most patients, and serial use can measure change clinically. These measures are used in research examining LBP functional outcomes in primary care; they are easy to use and score (SOR: C).19
What role for medications? Because of complex trade-offs between benefits and harms, evidence is insufficient to say one medication offers a clear net advantage over others in the treatment of patients with LBP. ACP/APS has identified good evidence for tricyclic antidepressants in chronic LBP (ACP/APS recommendation; SOR: B).2 Chronic LBP may exhibit periods of relative quiescence alternating with episodes of exacerbation.20 You can assist your patients in preparing for these occurrences. As with exacerbations in other conditions (eg, chronic obstructive pulmonary disease), you may want to prescribe short-term use of nonpharmacologic or pharmacologic therapies that can be tapered and discontinued after the exacerbation subsides. Patients are likely to differ in how they weigh potential benefits, harms, and cost of various medications. Such a strategy should limit financial burden and potential negative side effects of chronic therapy.
CASE 2
The patient’s course
The physician offers to partner with the patient in working toward a goal of improved functioning. The patient’s spouse accompanies him on 1 visit to discuss steps the family can take to improve fitness. With the ODI, the physician establishes the patient’s baseline function and tracks improvement over the period of care. The patient receives clinical massage therapy once a week, and his hydrocodone is tapered over the course of 6 visits. At the end of the period of care, the patient reports decreased pain and improved hopefulness.
Acknowledgements
The authors thank Honey Elder for organizing our work on this article and for her editorial assistance. Our efforts were supported in part by Grant Number R25 AT00682 from the National Institutes of Health (NIH). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Correspondence
William G. Elder Jr, PhD, University of Kentucky, K309 Kentucky Clinic, Lexington, KY 40536; [email protected]
1. Chou R, Qaseen A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
2. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
3. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
4. Van Tudler MW, Assendelft WJ, Koes BW, et al. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22:427-434.
5. Kerry S, Hilton S, Dundas D, et al. Radiography for low back pain: a randomised controlled trial and observational study in primary care. Br J Gen Pract. 2002;52:469-474.
6. Jarvik JG. Imaging of adults with low back pain in the primary care setting. Neuroimaging Clin N Am. 2003;13:293-305.
7. Gilbert F, Grant A, Gillan M, et al. Scottish Back Trial Group. Low back pain: influence of early MR imaging or CT on treatment and outcome—multicenter randomized trial. Radiology. 2004;231:343-351.
8. Pengel LH, Herbert RD, Maher CG, et al. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327:323.
9. Fayad F, Lefevre-Colau MM, Poiraudeau S, et al. Chronicity, recurrence, and return to work in low back pain: common prognostic factors. Ann Readapt Med Phys. 2004;47:179-189. [In French]
10. Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119-123.
11. Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131-140.
12. Modic MT, Obuchowski NA, Ross JS, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237:597-604.
13. Dillie KS, Fleming MF, Mundt MP, et al. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21:108-117.
14. Dance KA, Neufeld RW. Aptitude treatment interaction research in the clinical setting: a review of attempts to dispel the “patient uniformity” myth. Psychol Bull. 1988;104:192-213.
15. Jellema P, Van der Horst HE, Vlaeyen JW, et al. Predictors of outcome in patients with (sub) acute low back pain differ across treatment groups. Spine. 2006;31:1699-1705.
16. Main CJ, Sullivan MJ, Watson PJ, eds. Pain Management: Practical Applications of the Biopsychosocial Perspective in Clinical and Occupational Settings. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2007.
17. Van der Windt D, Hay E, Jellema P, et al. Psychosocial interventions for low back pain in primary care: Lessons learned from recent trials. Spine. 2008;33:81-89.
18. Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115-3124.
19. Lauridsen HH, Hartvigsen J, Manniche C, et al. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord. 2006;7:82.
20. Resnik L, Dobrykowski E. Outcomes measurement for patients with low back pain. Orthop Nurs. 2005;24:14-24.
A 32-year-old construction worker seeks treatment for the lower back pain (LBP) he’s been experiencing since painting his house a few days ago.
A 48-year-old man with a history of LBP comes in because he needs a refill of his hydrocodone prescription.
Both patients are probably pretty typical of the back pain patients you see on a regular basis. But how would you care for each of these patients, and how does your care compare to the latest evidence? This review will help you to find out. In this article we take a look at guidelines from the American College of Physicians (ACP) and the American Pain Society (APS),1 as well as findings from other recent studies, and apply them to these 2 patient cases.
But first, a word about the ACP/APS guidelines.
What’s new?
ACP/APS conducted a systematic review of studies of LBP epidemiology, clinical diagnosis, utility of imaging, and outcomes of pharmacologic2 and nonpharmacologic interventions.3 Whereas previous guidelines dealt with either acute or chronic pain, the ACP/APS guidelines synthesized the literature to apply to both.
Moreover, rather than focusing mostly on pain reduction, the ACP/APS panel was interested in functional outcomes such as back-specific functioning, general health status, disability, and patient satisfaction.
Finally, the panel’s recommendations ( TABLE ) considered the unique environment of primary care (including presentations typically seen in this setting), the ability of primary care physicians to advise and counsel patients, continuity of care, and the role of the physician in coordinating care.
TABLE
Recommendations from the ACP/APS guidelines for low back pain1
| Conduct a focused history and exam to place patients into 1 of 3 broad categories: nonspecific LBP, LBP potentially associated with radiculopathy or spinal stenosis, or LBP associated with another specific cause (strength of recommendation [SOR]: B). |
| Assess for psychosocial factors and emotional distress, as they are stronger predictors of LBP outcomes, including disability, than physical exam findings and severity of pain (SOR: B). |
| Do not routinely obtain imaging for patients with nonspecific LBP. MRI or CT is recommended for patients with LBP associated with a specific cause, for those with severe or progressive neurologic deficits or persistent radiculopathy/spinal stenosis symptoms, and for those who are candidates for surgical interventions (SOR: B). |
| Advise patients with nonspecific LBP to remain active and provide information on LBP’s expected course and effective self-care options (SOR: B). |
| Consider the addition of nonpharmacologic treatments, including selective alternative modalities, when self-care fails. These treatments include spinal manipulation for acute LBP and acupuncture for chronic LBP (SOR: B). |
| Consider acetaminophen or nonsteroidal anti-inflammatory drugs as first-line medication options for most patients. Keep in mind the limited effectiveness and potential harm of others, including opioids (SOR: B). |
| Strength of recommendation (SOR) A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented evidence, case series |
CASE 1
Patient with acute nonspecific LBP
While painting his home, a 32-year-old construction worker felt a twinge in his lower back as he stepped off a ladder. He remained active, relying on over-the-counter ibuprofen and heat packs to relieve soreness. Two days later he visits his physician because the soreness has not abated. He reports no bowel or bladder complaints, worsening of pain, radiation of symptoms, nausea, vomiting, abdominal pain, or fever. He tells his doctor that he strained his back before and that this “feels the same way it did before.” The last time this happened he received physical therapy (PT), which helped. He thinks he may need PT again, but wants to discuss it with his physician.
Physical exam reveals mild tenderness to palpation over the right lumbar paraspinal muscle, but no spasms are apparent. Otherwise, his musculoskeletal exam—including range-of-motion testing—is within normal limits. The neurologic exam also is within normal limits, including normal deep tendon reflexes of the lower extremities and negative straight-leg-raise testing. His gait is normal, with no sign of discomfort.
Specific anatomic diagnoses are elusive. In the primary care setting, fewer than 15% of LBP cases have an identifiable underlying disease or spinal abnormality.4 An exhaustive search for a specific anatomic diagnosis lacks utility in selecting initial therapy or affecting patient outcomes. Instead, when caring for a patient like the one in our case, it’s important to focus on a thorough medical history and examination that assess the location and duration of symptoms, as well as uncover symptoms suggestive of radiculopathy or spinal stenosis.
Of course, you’ll need to rule out potentially serious conditions, such as cancer, vertebral infection, cauda equina syndrome, compression fracture, and ankylosing spondylitis. You’ll also need to check for rapidly progressing or severe neurologic deficits, such as motor deficits at more than one level or a patient’s report of incontinence or bladder dysfunction.
Straight-leg-raise testing and neurologic assessment of the lower extremity—specifically strength and reflex testing of the knee, ankle, foot, and great toe to assess nerve foot level involvement—are key. With this assessment, you should categorize a patient’s LBP as nonspecific, as potentially associated with radiculopathy or spinal stenosis, or as potentially associated with another specific cause (ACP/APS recommendation; strength of recommendation [SOR]: B).1
Pursue imaging—or not? While plain radiography is certainly an option if you suspect a vertebral compression fracture in a high-risk patient, it would not be necessary for a patient like the one in our case. This patient has a classic presentation of acute nonspecific LBP, for which neither routine plain radiography nor advanced imaging (CT or MRI) improves patient outcomes. Given this lack of proven benefit and the unnecessary radiation exposure with certain tests, routine imaging is not recommended for nonspecific LBP (ACP/APS recommendation; SOR: B).1,4-6
Action steps. The evidence supports a number of steps when caring for a patient like the one in our case. Some steps are targeted to patients with nonspecific LBP—and we’ve labeled them as such. Others more broadly apply to patients with LBP.
Explore the possible contribution of psychosocial factors and emotional distress to back pain (ACP/APS recommendation; SOR: B).1 These factors are stronger predictors of low back pain outcomes, including chronic back pain disability, than physical exam findings and duration or severity of pain.7,8 Predictors of poorer outcomes include depression, passive coping strategies, job dissatisfaction, somatization, higher disability levels, and disputed compensation claims. The effectiveness of specific tools for gathering such information has not been demonstrated in the primary care setting. Therefore, fully investigate psychosocial information in the patient interview.
Provide patients with nonspecific LBP with evidence-based information regarding its expected course; advise them to remain active and suggest effective self-care options (ACP/APS recommendation; SOR: B).1 This recommendation is based on findings that the typical course and prognosis of LBP are generally favorable, on studies comparing bed rest versus remaining active, and on outcome studies for self-care interventions.
Self-care includes a variety of interventions patients can implement without a clinical visit—patient education, including self-care books, and patient-structured physical activities. This approach is much less expensive than—and has equivalent or nearly equivalent effectiveness to—costlier interventions such as physical therapy, massage, spinal manipulation, or acupuncture.
Regarding work limitations, there is insufficient evidence for specific guidance. Routinely assess patient age, health, and physical demands and job tasks, and recommend restrictions based on clinical judgment.
Try nonpharmacologic therapies that have proven benefits in the event that self-care fails (ACP/APS recommendation; SOR: B).1 These include spinal manipulation, defined as manual therapy in which loads are applied to the spine by using short- or long-lever methods and high-velocity thrusts are applied to a spinal joint beyond its restricted range of motion. Serious adverse events are extremely rare.3
If medication is needed for acute LBP, first-line drugs include nonopioids with proven benefits, such as acetaminophen, nonsteroidal anti-inflammatory agents, or skeletal muscle relaxants (ACP/APS recommendation; SOR: B).2
CASE 1
The patient’s course
The physician carefully reviews the 32-year-old patient’s psychosocial factors and finds that he is positive about his job, enjoys his work, and is not seeking compensation. He uses exercise and prayer to manage stress and is in a stable relationship. He does not smoke, use recreational drugs, or have a history of psychiatric disorders, including depression. He says he drinks 2 to 3 beers on the weekends.
In discussing treatment, the patient considers PT as the optimal intervention. His doctor does not recommend it, and instead encourages him to remain active, gives him a self-care booklet on stretching and exercises, and advises him to check in again as needed, reassuring him that most cases of nonspecific acute low back pain resolve spontaneously.
Initially the patient does well with self-care and he returns to activity. However, 6 weeks after his office visit, the patient returns with pain that has worsened over the last 2 weeks. He has also begun experiencing tingling sensations down his right leg, trouble standing for short intervals because of pain, and weakness in his back. On physical exam, he still has minimal tenderness to palpation over the right lumbar region. The right straight-leg-raise test yields a positive result, and the right patellar reflex is diminished compared with the left. His rectal tone is normal. His gait is antalgic.
When the patient requests imaging, the physician advises him of the risks associated with imaging and the unlikely prospect that it will change management—despite the change in neurologic symptoms. After considering such evidence-based options as massage therapy, yoga, and spinal manipulation, they agree on a trial of PT. The patient’s current level of function is reviewed, and work limitations are set.
After 8 weeks of PT, the patient experiences an improvement in overall function, pain level, and weakness. His straight-leg-raise test—the physical exam finding with the most sensitivity for disc herniation—returns to normal, as does his patellar reflex. Although frustrated with the length of recovery time, he is appreciative of his physician and therapists.
Follow this physician’s lead: Be prudent with imaging. Many primary care physicians caring for a patient like this one would consider imaging studies to assess the worsening signs and symptoms. The evidence, however, does not clearly support that decision. Given the potential harm of testing and varying benefit in outcomes, the ACP/APS offers different recommendations on imaging and other diagnostic tests, depending on the category of LBP. Prompt evaluation with advanced imaging (MRI or CT) is recommended for severe or progressive neurologic deficits, and with suspicion of a serious underlying condition, such as vertebral infection, cauda equine syndrome, or cancer with spinal cord compression, given that delayed treatment may lead to poor outcomes (ACP/APS recommendation; SOR: B).1
For many patients with herniated lumbar discs, symptoms can improve within 4 weeks.9,10 Thus, there is no compelling evidence that routine imaging changes treatment decisions or outcomes.11 For patients with persistent symptoms of radiculopathy or spinal stenosis who have not responded to conservative therapy, invasive procedures (surgery or epidural injections) become potential treatment options, and thus imaging with MRI (preferred) or CT may be warranted.
CASE 2
Patient with chronic LBP
A 48-year-old man new to the practice comes in complaining of persistent pain in the lower back, which he ranks at 6 on a scale of 1 to 10. Approximately 5 years ago he underwent an L4-L5 laminectomy/fusion for herniated nucleus pulposus. The surgery relieved shooting pains down his left leg, but he has since had progressive problems with lumbar pain and stiffness. Two courses of PT and a series of facet joint injections over the years have provided only temporary relief. The patient had been followed by a pain management clinic, but was discharged after exhausting his insurance benefit. A recent MRI ordered by the pain management clinic showed mild to moderate degenerative changes in L2 to S1 with a healed fusion.
The reason for his visit this day is to request a refill of his hydrocodone, initiated by the pain clinic. He is worried that he will not get better and is afraid of injuring himself and has, as a result, been avoiding activities. He denies depressive symptoms except for decreased self-worth and pain-related sleep disturbance. He says that he was once more vigorous and felt competent, but is now passive and feels helpless about his pain. He is concerned that his physical capabilities will worsen even more and asks if there are any other therapies that might be helpful.
No easy answers. Patients with chronic LBP—pain lasting for longer than 2 months—present unique challenges. They have often seen several clinicians, including pain management specialists, have undergone repeated imaging, and are frustrated by their persistent symptoms. Many have had 1 or more surgeries, and most have tried numerous medications to gain relief.
This patient is seeking a refill of his opioid. Before agreeing to such a request, weigh the risks and benefits of opioids and the potential benefits of alternative therapies (SOR: B).1 Although the chronic use of opioids is an option for a select group of patients with chronic LBP,12 these agents can be expensive, lead to habituation and addiction, be easily redirected for monetary gain, and have untoward side effects. Evidence does not show that long-term opioid use improves functioning in patients with chronic LBP.
Nonpharmacologic therapies that have proven beneficial for such patients include acupuncture, cognitive-behavioral therapy, PT, exercise therapy (defined as any supervised or formal exercise program), and therapeutic massage (SOR: B).1 It would be preferable to start a therapeutic plan incorporating 1 or more of these modalities, based on a patient’s psychosocial history, insurance status, and preferences. Suggesting these therapies with guarded optimism can lead to a decreased need for opioids and increased functioning.
Again, psychosocial evaluation is important. When initially assessing a patient with chronic LBP, it is imperative to evaluate psychosocial factors. As noted earlier, psychosocial factors are better predictors of treatment outcomes than physical findings. Identifying factors related to poor outcomes (eg, anxiety, poor work history, passive attitude toward rehabilitation) can direct therapy and avoid polypharmacy.
Cognitive-behavioral and educational interventions will be more effective when targeting specific psychological and social factors (SOR: C).13,14 Fear-avoidance beliefs, distress, somatization, and pain catastrophizing place patients at the highest risk for poor outcomes. Primary objectives in psychosocial intervention are providing encouragement for overcoming demoralization; helping the patient make the connection between thoughts, feelings, and behaviors; and teaching the patient coping strategies and techniques to adapt to pain and resultant problems.
The ultimate goal for a patient like the one in this second case is to change his perception of chronic pain from overwhelming to manageable and to get him to see himself as resourceful and competent.15 Physician counseling has produced small positive effects in undifferentiated primary care patients with LBP, and it may therefore be more powerful when targeted to patients with specific psychosocial issues such as fear avoidance.16
Provide patients with a realistic outlook. Another key element is to direct patients’ expectations. Most people with chronic LBP will not become pain free, and patients need to know this fact. Aim treatment at improving function as well as reducing pain. You can assess functional status and improvement using patient questionnaires such as the Roland-Morris Disability Questionnaire (http://www.rmdq.org/) or the Oswestry Disability Index 2.0 (ODI, http://www.cpta.ab.ca/resources/Measurement%20Tools/Evaluative_Oswestry%20Disability%20Index.doc).17,18 Although these measures have not demonstrated utility in primary care practice, they have sufficient scale width to reliably detect change in most patients, and serial use can measure change clinically. These measures are used in research examining LBP functional outcomes in primary care; they are easy to use and score (SOR: C).19
What role for medications? Because of complex trade-offs between benefits and harms, evidence is insufficient to say one medication offers a clear net advantage over others in the treatment of patients with LBP. ACP/APS has identified good evidence for tricyclic antidepressants in chronic LBP (ACP/APS recommendation; SOR: B).2 Chronic LBP may exhibit periods of relative quiescence alternating with episodes of exacerbation.20 You can assist your patients in preparing for these occurrences. As with exacerbations in other conditions (eg, chronic obstructive pulmonary disease), you may want to prescribe short-term use of nonpharmacologic or pharmacologic therapies that can be tapered and discontinued after the exacerbation subsides. Patients are likely to differ in how they weigh potential benefits, harms, and cost of various medications. Such a strategy should limit financial burden and potential negative side effects of chronic therapy.
CASE 2
The patient’s course
The physician offers to partner with the patient in working toward a goal of improved functioning. The patient’s spouse accompanies him on 1 visit to discuss steps the family can take to improve fitness. With the ODI, the physician establishes the patient’s baseline function and tracks improvement over the period of care. The patient receives clinical massage therapy once a week, and his hydrocodone is tapered over the course of 6 visits. At the end of the period of care, the patient reports decreased pain and improved hopefulness.
Acknowledgements
The authors thank Honey Elder for organizing our work on this article and for her editorial assistance. Our efforts were supported in part by Grant Number R25 AT00682 from the National Institutes of Health (NIH). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Correspondence
William G. Elder Jr, PhD, University of Kentucky, K309 Kentucky Clinic, Lexington, KY 40536; [email protected]
A 32-year-old construction worker seeks treatment for the lower back pain (LBP) he’s been experiencing since painting his house a few days ago.
A 48-year-old man with a history of LBP comes in because he needs a refill of his hydrocodone prescription.
Both patients are probably pretty typical of the back pain patients you see on a regular basis. But how would you care for each of these patients, and how does your care compare to the latest evidence? This review will help you to find out. In this article we take a look at guidelines from the American College of Physicians (ACP) and the American Pain Society (APS),1 as well as findings from other recent studies, and apply them to these 2 patient cases.
But first, a word about the ACP/APS guidelines.
What’s new?
ACP/APS conducted a systematic review of studies of LBP epidemiology, clinical diagnosis, utility of imaging, and outcomes of pharmacologic2 and nonpharmacologic interventions.3 Whereas previous guidelines dealt with either acute or chronic pain, the ACP/APS guidelines synthesized the literature to apply to both.
Moreover, rather than focusing mostly on pain reduction, the ACP/APS panel was interested in functional outcomes such as back-specific functioning, general health status, disability, and patient satisfaction.
Finally, the panel’s recommendations ( TABLE ) considered the unique environment of primary care (including presentations typically seen in this setting), the ability of primary care physicians to advise and counsel patients, continuity of care, and the role of the physician in coordinating care.
TABLE
Recommendations from the ACP/APS guidelines for low back pain1
| Conduct a focused history and exam to place patients into 1 of 3 broad categories: nonspecific LBP, LBP potentially associated with radiculopathy or spinal stenosis, or LBP associated with another specific cause (strength of recommendation [SOR]: B). |
| Assess for psychosocial factors and emotional distress, as they are stronger predictors of LBP outcomes, including disability, than physical exam findings and severity of pain (SOR: B). |
| Do not routinely obtain imaging for patients with nonspecific LBP. MRI or CT is recommended for patients with LBP associated with a specific cause, for those with severe or progressive neurologic deficits or persistent radiculopathy/spinal stenosis symptoms, and for those who are candidates for surgical interventions (SOR: B). |
| Advise patients with nonspecific LBP to remain active and provide information on LBP’s expected course and effective self-care options (SOR: B). |
| Consider the addition of nonpharmacologic treatments, including selective alternative modalities, when self-care fails. These treatments include spinal manipulation for acute LBP and acupuncture for chronic LBP (SOR: B). |
| Consider acetaminophen or nonsteroidal anti-inflammatory drugs as first-line medication options for most patients. Keep in mind the limited effectiveness and potential harm of others, including opioids (SOR: B). |
| Strength of recommendation (SOR) A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented evidence, case series |
CASE 1
Patient with acute nonspecific LBP
While painting his home, a 32-year-old construction worker felt a twinge in his lower back as he stepped off a ladder. He remained active, relying on over-the-counter ibuprofen and heat packs to relieve soreness. Two days later he visits his physician because the soreness has not abated. He reports no bowel or bladder complaints, worsening of pain, radiation of symptoms, nausea, vomiting, abdominal pain, or fever. He tells his doctor that he strained his back before and that this “feels the same way it did before.” The last time this happened he received physical therapy (PT), which helped. He thinks he may need PT again, but wants to discuss it with his physician.
Physical exam reveals mild tenderness to palpation over the right lumbar paraspinal muscle, but no spasms are apparent. Otherwise, his musculoskeletal exam—including range-of-motion testing—is within normal limits. The neurologic exam also is within normal limits, including normal deep tendon reflexes of the lower extremities and negative straight-leg-raise testing. His gait is normal, with no sign of discomfort.
Specific anatomic diagnoses are elusive. In the primary care setting, fewer than 15% of LBP cases have an identifiable underlying disease or spinal abnormality.4 An exhaustive search for a specific anatomic diagnosis lacks utility in selecting initial therapy or affecting patient outcomes. Instead, when caring for a patient like the one in our case, it’s important to focus on a thorough medical history and examination that assess the location and duration of symptoms, as well as uncover symptoms suggestive of radiculopathy or spinal stenosis.
Of course, you’ll need to rule out potentially serious conditions, such as cancer, vertebral infection, cauda equina syndrome, compression fracture, and ankylosing spondylitis. You’ll also need to check for rapidly progressing or severe neurologic deficits, such as motor deficits at more than one level or a patient’s report of incontinence or bladder dysfunction.
Straight-leg-raise testing and neurologic assessment of the lower extremity—specifically strength and reflex testing of the knee, ankle, foot, and great toe to assess nerve foot level involvement—are key. With this assessment, you should categorize a patient’s LBP as nonspecific, as potentially associated with radiculopathy or spinal stenosis, or as potentially associated with another specific cause (ACP/APS recommendation; strength of recommendation [SOR]: B).1
Pursue imaging—or not? While plain radiography is certainly an option if you suspect a vertebral compression fracture in a high-risk patient, it would not be necessary for a patient like the one in our case. This patient has a classic presentation of acute nonspecific LBP, for which neither routine plain radiography nor advanced imaging (CT or MRI) improves patient outcomes. Given this lack of proven benefit and the unnecessary radiation exposure with certain tests, routine imaging is not recommended for nonspecific LBP (ACP/APS recommendation; SOR: B).1,4-6
Action steps. The evidence supports a number of steps when caring for a patient like the one in our case. Some steps are targeted to patients with nonspecific LBP—and we’ve labeled them as such. Others more broadly apply to patients with LBP.
Explore the possible contribution of psychosocial factors and emotional distress to back pain (ACP/APS recommendation; SOR: B).1 These factors are stronger predictors of low back pain outcomes, including chronic back pain disability, than physical exam findings and duration or severity of pain.7,8 Predictors of poorer outcomes include depression, passive coping strategies, job dissatisfaction, somatization, higher disability levels, and disputed compensation claims. The effectiveness of specific tools for gathering such information has not been demonstrated in the primary care setting. Therefore, fully investigate psychosocial information in the patient interview.
Provide patients with nonspecific LBP with evidence-based information regarding its expected course; advise them to remain active and suggest effective self-care options (ACP/APS recommendation; SOR: B).1 This recommendation is based on findings that the typical course and prognosis of LBP are generally favorable, on studies comparing bed rest versus remaining active, and on outcome studies for self-care interventions.
Self-care includes a variety of interventions patients can implement without a clinical visit—patient education, including self-care books, and patient-structured physical activities. This approach is much less expensive than—and has equivalent or nearly equivalent effectiveness to—costlier interventions such as physical therapy, massage, spinal manipulation, or acupuncture.
Regarding work limitations, there is insufficient evidence for specific guidance. Routinely assess patient age, health, and physical demands and job tasks, and recommend restrictions based on clinical judgment.
Try nonpharmacologic therapies that have proven benefits in the event that self-care fails (ACP/APS recommendation; SOR: B).1 These include spinal manipulation, defined as manual therapy in which loads are applied to the spine by using short- or long-lever methods and high-velocity thrusts are applied to a spinal joint beyond its restricted range of motion. Serious adverse events are extremely rare.3
If medication is needed for acute LBP, first-line drugs include nonopioids with proven benefits, such as acetaminophen, nonsteroidal anti-inflammatory agents, or skeletal muscle relaxants (ACP/APS recommendation; SOR: B).2
CASE 1
The patient’s course
The physician carefully reviews the 32-year-old patient’s psychosocial factors and finds that he is positive about his job, enjoys his work, and is not seeking compensation. He uses exercise and prayer to manage stress and is in a stable relationship. He does not smoke, use recreational drugs, or have a history of psychiatric disorders, including depression. He says he drinks 2 to 3 beers on the weekends.
In discussing treatment, the patient considers PT as the optimal intervention. His doctor does not recommend it, and instead encourages him to remain active, gives him a self-care booklet on stretching and exercises, and advises him to check in again as needed, reassuring him that most cases of nonspecific acute low back pain resolve spontaneously.
Initially the patient does well with self-care and he returns to activity. However, 6 weeks after his office visit, the patient returns with pain that has worsened over the last 2 weeks. He has also begun experiencing tingling sensations down his right leg, trouble standing for short intervals because of pain, and weakness in his back. On physical exam, he still has minimal tenderness to palpation over the right lumbar region. The right straight-leg-raise test yields a positive result, and the right patellar reflex is diminished compared with the left. His rectal tone is normal. His gait is antalgic.
When the patient requests imaging, the physician advises him of the risks associated with imaging and the unlikely prospect that it will change management—despite the change in neurologic symptoms. After considering such evidence-based options as massage therapy, yoga, and spinal manipulation, they agree on a trial of PT. The patient’s current level of function is reviewed, and work limitations are set.
After 8 weeks of PT, the patient experiences an improvement in overall function, pain level, and weakness. His straight-leg-raise test—the physical exam finding with the most sensitivity for disc herniation—returns to normal, as does his patellar reflex. Although frustrated with the length of recovery time, he is appreciative of his physician and therapists.
Follow this physician’s lead: Be prudent with imaging. Many primary care physicians caring for a patient like this one would consider imaging studies to assess the worsening signs and symptoms. The evidence, however, does not clearly support that decision. Given the potential harm of testing and varying benefit in outcomes, the ACP/APS offers different recommendations on imaging and other diagnostic tests, depending on the category of LBP. Prompt evaluation with advanced imaging (MRI or CT) is recommended for severe or progressive neurologic deficits, and with suspicion of a serious underlying condition, such as vertebral infection, cauda equine syndrome, or cancer with spinal cord compression, given that delayed treatment may lead to poor outcomes (ACP/APS recommendation; SOR: B).1
For many patients with herniated lumbar discs, symptoms can improve within 4 weeks.9,10 Thus, there is no compelling evidence that routine imaging changes treatment decisions or outcomes.11 For patients with persistent symptoms of radiculopathy or spinal stenosis who have not responded to conservative therapy, invasive procedures (surgery or epidural injections) become potential treatment options, and thus imaging with MRI (preferred) or CT may be warranted.
CASE 2
Patient with chronic LBP
A 48-year-old man new to the practice comes in complaining of persistent pain in the lower back, which he ranks at 6 on a scale of 1 to 10. Approximately 5 years ago he underwent an L4-L5 laminectomy/fusion for herniated nucleus pulposus. The surgery relieved shooting pains down his left leg, but he has since had progressive problems with lumbar pain and stiffness. Two courses of PT and a series of facet joint injections over the years have provided only temporary relief. The patient had been followed by a pain management clinic, but was discharged after exhausting his insurance benefit. A recent MRI ordered by the pain management clinic showed mild to moderate degenerative changes in L2 to S1 with a healed fusion.
The reason for his visit this day is to request a refill of his hydrocodone, initiated by the pain clinic. He is worried that he will not get better and is afraid of injuring himself and has, as a result, been avoiding activities. He denies depressive symptoms except for decreased self-worth and pain-related sleep disturbance. He says that he was once more vigorous and felt competent, but is now passive and feels helpless about his pain. He is concerned that his physical capabilities will worsen even more and asks if there are any other therapies that might be helpful.
No easy answers. Patients with chronic LBP—pain lasting for longer than 2 months—present unique challenges. They have often seen several clinicians, including pain management specialists, have undergone repeated imaging, and are frustrated by their persistent symptoms. Many have had 1 or more surgeries, and most have tried numerous medications to gain relief.
This patient is seeking a refill of his opioid. Before agreeing to such a request, weigh the risks and benefits of opioids and the potential benefits of alternative therapies (SOR: B).1 Although the chronic use of opioids is an option for a select group of patients with chronic LBP,12 these agents can be expensive, lead to habituation and addiction, be easily redirected for monetary gain, and have untoward side effects. Evidence does not show that long-term opioid use improves functioning in patients with chronic LBP.
Nonpharmacologic therapies that have proven beneficial for such patients include acupuncture, cognitive-behavioral therapy, PT, exercise therapy (defined as any supervised or formal exercise program), and therapeutic massage (SOR: B).1 It would be preferable to start a therapeutic plan incorporating 1 or more of these modalities, based on a patient’s psychosocial history, insurance status, and preferences. Suggesting these therapies with guarded optimism can lead to a decreased need for opioids and increased functioning.
Again, psychosocial evaluation is important. When initially assessing a patient with chronic LBP, it is imperative to evaluate psychosocial factors. As noted earlier, psychosocial factors are better predictors of treatment outcomes than physical findings. Identifying factors related to poor outcomes (eg, anxiety, poor work history, passive attitude toward rehabilitation) can direct therapy and avoid polypharmacy.
Cognitive-behavioral and educational interventions will be more effective when targeting specific psychological and social factors (SOR: C).13,14 Fear-avoidance beliefs, distress, somatization, and pain catastrophizing place patients at the highest risk for poor outcomes. Primary objectives in psychosocial intervention are providing encouragement for overcoming demoralization; helping the patient make the connection between thoughts, feelings, and behaviors; and teaching the patient coping strategies and techniques to adapt to pain and resultant problems.
The ultimate goal for a patient like the one in this second case is to change his perception of chronic pain from overwhelming to manageable and to get him to see himself as resourceful and competent.15 Physician counseling has produced small positive effects in undifferentiated primary care patients with LBP, and it may therefore be more powerful when targeted to patients with specific psychosocial issues such as fear avoidance.16
Provide patients with a realistic outlook. Another key element is to direct patients’ expectations. Most people with chronic LBP will not become pain free, and patients need to know this fact. Aim treatment at improving function as well as reducing pain. You can assess functional status and improvement using patient questionnaires such as the Roland-Morris Disability Questionnaire (http://www.rmdq.org/) or the Oswestry Disability Index 2.0 (ODI, http://www.cpta.ab.ca/resources/Measurement%20Tools/Evaluative_Oswestry%20Disability%20Index.doc).17,18 Although these measures have not demonstrated utility in primary care practice, they have sufficient scale width to reliably detect change in most patients, and serial use can measure change clinically. These measures are used in research examining LBP functional outcomes in primary care; they are easy to use and score (SOR: C).19
What role for medications? Because of complex trade-offs between benefits and harms, evidence is insufficient to say one medication offers a clear net advantage over others in the treatment of patients with LBP. ACP/APS has identified good evidence for tricyclic antidepressants in chronic LBP (ACP/APS recommendation; SOR: B).2 Chronic LBP may exhibit periods of relative quiescence alternating with episodes of exacerbation.20 You can assist your patients in preparing for these occurrences. As with exacerbations in other conditions (eg, chronic obstructive pulmonary disease), you may want to prescribe short-term use of nonpharmacologic or pharmacologic therapies that can be tapered and discontinued after the exacerbation subsides. Patients are likely to differ in how they weigh potential benefits, harms, and cost of various medications. Such a strategy should limit financial burden and potential negative side effects of chronic therapy.
CASE 2
The patient’s course
The physician offers to partner with the patient in working toward a goal of improved functioning. The patient’s spouse accompanies him on 1 visit to discuss steps the family can take to improve fitness. With the ODI, the physician establishes the patient’s baseline function and tracks improvement over the period of care. The patient receives clinical massage therapy once a week, and his hydrocodone is tapered over the course of 6 visits. At the end of the period of care, the patient reports decreased pain and improved hopefulness.
Acknowledgements
The authors thank Honey Elder for organizing our work on this article and for her editorial assistance. Our efforts were supported in part by Grant Number R25 AT00682 from the National Institutes of Health (NIH). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Correspondence
William G. Elder Jr, PhD, University of Kentucky, K309 Kentucky Clinic, Lexington, KY 40536; [email protected]
1. Chou R, Qaseen A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
2. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
3. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
4. Van Tudler MW, Assendelft WJ, Koes BW, et al. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22:427-434.
5. Kerry S, Hilton S, Dundas D, et al. Radiography for low back pain: a randomised controlled trial and observational study in primary care. Br J Gen Pract. 2002;52:469-474.
6. Jarvik JG. Imaging of adults with low back pain in the primary care setting. Neuroimaging Clin N Am. 2003;13:293-305.
7. Gilbert F, Grant A, Gillan M, et al. Scottish Back Trial Group. Low back pain: influence of early MR imaging or CT on treatment and outcome—multicenter randomized trial. Radiology. 2004;231:343-351.
8. Pengel LH, Herbert RD, Maher CG, et al. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327:323.
9. Fayad F, Lefevre-Colau MM, Poiraudeau S, et al. Chronicity, recurrence, and return to work in low back pain: common prognostic factors. Ann Readapt Med Phys. 2004;47:179-189. [In French]
10. Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119-123.
11. Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131-140.
12. Modic MT, Obuchowski NA, Ross JS, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237:597-604.
13. Dillie KS, Fleming MF, Mundt MP, et al. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21:108-117.
14. Dance KA, Neufeld RW. Aptitude treatment interaction research in the clinical setting: a review of attempts to dispel the “patient uniformity” myth. Psychol Bull. 1988;104:192-213.
15. Jellema P, Van der Horst HE, Vlaeyen JW, et al. Predictors of outcome in patients with (sub) acute low back pain differ across treatment groups. Spine. 2006;31:1699-1705.
16. Main CJ, Sullivan MJ, Watson PJ, eds. Pain Management: Practical Applications of the Biopsychosocial Perspective in Clinical and Occupational Settings. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2007.
17. Van der Windt D, Hay E, Jellema P, et al. Psychosocial interventions for low back pain in primary care: Lessons learned from recent trials. Spine. 2008;33:81-89.
18. Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115-3124.
19. Lauridsen HH, Hartvigsen J, Manniche C, et al. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord. 2006;7:82.
20. Resnik L, Dobrykowski E. Outcomes measurement for patients with low back pain. Orthop Nurs. 2005;24:14-24.
1. Chou R, Qaseen A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
2. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505-514.
3. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492-504.
4. Van Tudler MW, Assendelft WJ, Koes BW, et al. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22:427-434.
5. Kerry S, Hilton S, Dundas D, et al. Radiography for low back pain: a randomised controlled trial and observational study in primary care. Br J Gen Pract. 2002;52:469-474.
6. Jarvik JG. Imaging of adults with low back pain in the primary care setting. Neuroimaging Clin N Am. 2003;13:293-305.
7. Gilbert F, Grant A, Gillan M, et al. Scottish Back Trial Group. Low back pain: influence of early MR imaging or CT on treatment and outcome—multicenter randomized trial. Radiology. 2004;231:343-351.
8. Pengel LH, Herbert RD, Maher CG, et al. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327:323.
9. Fayad F, Lefevre-Colau MM, Poiraudeau S, et al. Chronicity, recurrence, and return to work in low back pain: common prognostic factors. Ann Readapt Med Phys. 2004;47:179-189. [In French]
10. Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119-123.
11. Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131-140.
12. Modic MT, Obuchowski NA, Ross JS, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237:597-604.
13. Dillie KS, Fleming MF, Mundt MP, et al. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med. 2008;21:108-117.
14. Dance KA, Neufeld RW. Aptitude treatment interaction research in the clinical setting: a review of attempts to dispel the “patient uniformity” myth. Psychol Bull. 1988;104:192-213.
15. Jellema P, Van der Horst HE, Vlaeyen JW, et al. Predictors of outcome in patients with (sub) acute low back pain differ across treatment groups. Spine. 2006;31:1699-1705.
16. Main CJ, Sullivan MJ, Watson PJ, eds. Pain Management: Practical Applications of the Biopsychosocial Perspective in Clinical and Occupational Settings. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2007.
17. Van der Windt D, Hay E, Jellema P, et al. Psychosocial interventions for low back pain in primary care: Lessons learned from recent trials. Spine. 2008;33:81-89.
18. Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115-3124.
19. Lauridsen HH, Hartvigsen J, Manniche C, et al. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord. 2006;7:82.
20. Resnik L, Dobrykowski E. Outcomes measurement for patients with low back pain. Orthop Nurs. 2005;24:14-24.
You can do more to slow the progression of heart failure
- Use B-type natriuretic peptide (BNP) levels as an aid not only in the diagnosis of heart failure (HF), but to track its progression as well (A).
- Prescribe exercise training for patients with stable heart failure; exercising at 40% to 70% of maximum capacity for 20 to 45 minutes several times a week offers benefits on par with pharmacotherapy (A).
- Consider using the Simplified Treatment Intervention to Control Hypertension (STITCH) algorithm for hypertensive patients or those who are at risk of developing HF; this step-care strategy is effective in treating hypertension, a leading cause of HF (C).
- Consult a specialist before prescribing both an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) for a patient with advanced HF; studies of combination therapy for this patient population have had mixed results (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Family physicians are all too familiar with heart failure (HF). This debilitating condition accounts for approximately 3.4 million outpatient visits to US physicians annually,1 and fully two-thirds of HF patients are cared for by primary care physicians.2
A host of comorbid conditions—coronary artery disease, valvular heart disease, diabetes, dyslipidemia, metabolic syndrome, obesity, chronic renal insufficiency, and hypertension chief among them—contribute to the development of HF.3 Of these, hypertension is the most important factor. In more than 75% of cases, high blood pressure precedes HF,1 and an individual’s lifetime risk of developing HF is strongly associated with poor blood pressure control.4 Hypertension is the most significant controllable factor in the management of HF as well. Because of the nexus between hypertension and HF, we encourage physicians to think of these 2 conditions as a single entity—and to recognize that a reduction of even a few millimeters of mercury can have huge clinical benefits.
This review, which highlights a recently tested hypertension algorithm along with other recent developments and long-established treatment strategies, will help you do everything possible to slow the progression of this debilitating and deadly disease.
BNP’s increasing role in evaluating heart failure
A diagnosis of HF in patients with known heart disease is based on functionality and symptoms, assessed with the help of 2 classification schemes5,6 ( TABLE 1 ) and a variety of tests. (Patients who present with the signs and symptoms of HF but no evidence of the comorbid conditions typically associated with it should be screened for other, noncardiac causes—human immunodeficiency virus, hepatitis C, hemochromatosis, hypothyroidism, and substance abuse among them.6 )
Diagnostic testing. Baseline serum chemistries include a complete blood count, urinalysis, electrolytes, magnesium, blood urea nitrogen, creatinine, and blood glucose levels, and liver and thyroid function tests.
B-type natriuretic peptide (BNP), a homeostatic marker secreted by the heart in an attempt to maintain stable blood pressure and plasma volume and avoid fluid retention, is increasingly recognized as an important aid, not only in diagnosing HF but in gauging its severity, managing symptoms, and determining the prognosis.7,8 BNP concentrations <80 pg/mL have been found to have a negative predictive value of 98%, and are also highly sensitive (98%) and specific (98%) for the diagnosis of HF.9,10
Testing may also include a 12-lead electrocardiogram as well as a posterior-anterior/lateral chest x-ray. Echocardiography is often used to evaluate left ventricular function and ejection fraction6 —a key to establishing whether the patient has systolic (reduced ejection fraction) or diastolic (preserved ejection fraction) HF.
An ejection fraction ≤40% is characteristic of systolic HF, which affects approximately 60% of patients with heart failure11 and is the focus of the following discussion of treatments.
TABLE 1
Classifying heart failure: 2 systems
| NEW YORK HEART ASSOCIATION |
| Class I: No limitation of physical activity; ordinary activity does not cause undue fatigue or dyspnea. Class II: Slight limitation of activity; comfortable at rest, but ordinary physical activity results in fatigue or dyspnea. Class III: Marked limitation in activity. Class IV: Unable to carry on any physical activity without symptoms; symptoms present even at rest. |
| AMERICAN COLLEGE OF CARDIOLOGY/AMERICAN HEART ASSOCIATION |
| Stage A: Conditions strongly associated with heart failure (HF); at high risk of HF. No identified structural or functional abnormalities of the pericardium, myocardium, or cardiac valves; no signs or symptoms of HF. Stage B: Structural heart disease strongly associated with HF, but no known signs or symptoms. Stage C: Current or prior symptoms of HF associated with underlying structural heart disease. Stage D: Advanced structural heart disease, with marked symptoms of HF at rest despite maximal medical therapy. Specialized interventions required. |
| Sources: Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. 1964;5 Hunt et al. Circulation. 2005.6 |
Early interventions: Get patients moving
For all patients with stable HF—and those at high risk of developingit—behavioral modification is a key component of treatment. Lifestyle intervention should be directed at weight loss and diet, including control of salt intake; increased physical activity; and smoking cessation.
Don’t shy away from exercise. Although many physicians hesitate to prescribe exercise to patients with HF, physical activity should be a routine recommendation for all but the most debilitated patients.6 Regular exercise has been shown to decrease symptoms, increase functional capacity, and improve the quality of life, with benefits comparable to those of pharmacotherapy.6,12,13
Studies of the beneficial effects of exercise were based on sustaining 40% to 70% of maximum capacity for 20 to 45 minutes, 3 to 5 days a week.6 A good walking program—of at least 30 minutes 4 to 5 days each week—should not be difficult for patients to maintain.
BP treatment guidelines: The old and the new
As noted earlier, controlling hypertension is crucial, not only to prevent HF but to attenuate its progress. But blood pressure management is suboptimal in the United States, with many patients failing to achieve recommended levels of pressure reduction. It’s been suggested that the complexity of standard treatment guidelines may be part of the problem.
STITCH step care is a newer option. Researchers designed the Simplified Treatment Intervention to Control Hypertension (STITCH) Trial, a cluster randomized study of patients at multiple family medicine clinics in Canada, to evaluate whether a simplified step-care algorithm would yield better results.
The STITCH algorithm has 4 treatment steps:
Step 1: Initiate therapy by pairing a diuretic with either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Step 2: Increase combination therapy to the highest dose tolerated.
Step 3: Add a calcium channel blocker and increase to the highest tolerated dose.
Step 4: Add a non-first-line antihypertensive agent (alpha-blocker, beta-blocker, or spironolactone).
Researchers found that after 6 months, 64.7% of patients on the STITCH protocol had achieved target blood pressure, compared with 52.7% of those whose treatment was based on the Canadian Hypertension Education Program (CHEP) guidelines (P=.03).14 The CHEP protocol is similar to that of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7);15 both offer numerous options for initial treatment.16
In presenting the STITCH results at the 2007 annual meeting of the American Heart Association, the lead author described the use of a simple step-care approach as “an important way forward in the treatment of hypertension [which] may be a paradigm for managing a range of chronic diseases.”16 Yet the STITCH algorithm has yet to be widely embraced; outside of the research community, most US physicians are relying on the JNC 7 guidelines.
ACC/AHA recommendations indicate that for patients at stage A—that is, those with conditions strongly associated with, and at high risk for, HF—management of hypertension should conform to national standards such as JNC 7. The JNC 7 guidelines recommend the use of a thiazide diuretic as the initial drug of choice for patients with essential hypertension. For those with diabetes, ACE inhibitors and ARBs are the first-line antihypertensive agents of choice.
Glucose control is also essential for stage A patients with diabetes. Treatment of lipid disorders and pharmacotherapy for metabolic syndrome are also recommended for stage A patients, as needed.
Treatment escalates as HF progresses
ACE inhibitors, ARBs, and beta-blockers are the preferred pharmacologic interventions for patients at stage B—those who have structural heart disease strongly associated with HF but are not yet symptomatic. Anyone who has had a myocardial infarction (MI) should be started on a beta-blocker and an ACE inhibitor, ACC/AHA recommends, unless a contraindication exists.6 Similarly, any patient with a reduced ejection fraction should be started on an ACE inhibitor regardless of symptoms.6
The Heart Outcomes Prevention Evaluation (HOPE) study demonstrated a 23% relative risk (RR) reduction with the use of an ACE inhibitor in patients with coronary artery disease, peripheral vascular disease, or diabetes, compared with patients receiving a placebo.17 The importance of a beta-blocker was established in a subanalysis of the Survival and Ventricular Enlargement Trial (SAVE), which found that patients taking beta-blockers in addition to an ACE inhibitor had a 32% RR reduction in progression of HF, compared with patients on an ACE inhibitor alone.18
We recommend an ACE inhibitor or an ARB and a beta-blocker, when appropriate, to slow the progression of HF pathophysiology. It is important to be aware of the potential adverse effects of certain beta-blockers in patients with HF. Only 3 beta-blockers are approved for use in this patient population in the United States—bisoprolol, carvedilol, and metoprolol succinate, which have been found to provide benefits that other beta-blockers do not.6,15
Stages C and D: Tx considerations and controversies
Treatment for patients at stage C should include all components of therapy for patients at stages A and B, but with a more aggressive use of pharmacotherapy ( TABLE 2 ). Patients with stage C HF, by definition, are symptomatic, and the ACC/AHA recommendations reflect concern about their increasingly compromised status. Thus, in addition to the use of ACE inhibitors or ARBs and beta-blockers, modest use of diuretics is recommended, as needed, for fluid volume control.6 Diuretics should be used judiciously, though, with ongoing evaluation to avoid the excessive loss of potassium and magnesium, which can lead to volume depletion and lethal arrhythmias. Limiting sodium consumption is an important dietary restriction for stage C patients.
Aldosterone antagonists may also be considered on a case-by-case basis for patients with stage C HF. Due to their potassium-sparing effects, aldosterone antagonists, used in conjunction with standard therapies, may have a positive effect on electrolyte balance. Potassium levels must be carefully monitored, however, and potassium supplementation reevaluated for patients who are put on an aldosterone antagonist.19
Digitalis may also be helpful in select patients who remain symptomatic despite maximal pharmacotherapy.20 While it does not affect mortality, digitalis has been shown to reduce hospitalizations.21
ACE inhibitor-ARB combination therapy, another possible treatment for advanced HF, remains controversial. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), detailed in “ACE inhibitors and ARBs: One or the other—not both” in the January 2009 issue of The Journal of Family Practice, evaluated use of this dual therapy; the trial was also designed to determine whether telmisartan (an ARB) is inferior to ramipril (an ACE inhibitor) in patients at high risk for vascular events.22 The researchers found that telmisartan is not, in fact, inferior to ramipril, and reported that for patients with HF, an ACE-ARB combination offers a potential benefit.
However, the clinical benefit of an ACE-ARB combination in this patient population was not clarified in this study, and may be potentially harmful. In the Valsartan Heart Failure Trial (ValHeFT), the combination of valsartan, an ARB, and an ACE inhibitor decreased hospitalizations but did not improve mortality.23 Indeed, an increase in mortality was found when an ACEARB combination was used in conjunction with beta-blockers. Because beta-blockers are indicated for routine use in patients with HF, this finding was of particular concern.
In a meta-analysis of randomized trials using both an ACE inhibitor and an ARB in patients with left ventricular dysfunction, researchers found a “marked” increase in adverse effects, including deteriorating renal function (RR=2.17), hyperkalemia (RR=4.87), and symptomatic hypotension (RR=1.05).24 Although an ACE-ARB combination may benefit a subset of patients with HF, it is best to initiate such treatment only with the guidance of an HF specialist.
TABLE 2
Treating heart failure: How the different drugs and devices rate
| STAGE | PHARMACOTHERAPY | LOE | DEVICE/INTERVENTION | LOE |
|---|---|---|---|---|
| A | Treat BP per JNC 7 ACE inhibitor or ARB for patients with vascular disease or diabetes | A | None | N/A |
| B | ACE inhibitor or ARB BB | A | None | N/A |
| C | Routine use: Diuretics ACE inhibitor BB Select use: Aldosterone antagonist ARB Digitalis | A | Consider: Biventricular pacer or ICD or both | B |
| D | Same as C | B | Consider: Heart transplant or LVAD; experimental protocols | C |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta-blocker; BP, blood pressure; ICD, implantable cardioverter defibrillator; JNC 7, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LOE, level of evidence; LVAD, left ventricular assist device. | ||||
| Adapted from: Hunt SA, et al. Circulation. 2005.6 | ||||
Beyond drug therapy: Assistive devices
Refractory end-stage HF requires a clear treatment plan, and should involve the recommendations of an HF specialist. Careful maintenance of fluid status is required, and an evaluation for cardiac transplantation may be considered.
A left ventricular assist device (LVAD) should also be considered for patients with an estimated 1-year mortality of >50%.6 LVADs are mechanical heart pumps that were initially utilized as a “bridge” to transplant, but are increasingly being used as a palliative alternative for severely ill patients.25
Other devices—an implantable cardioverter defibrillator (ICD) or a biventricular pacer—should also be considered for patients at stage D, as well as stage C patients who are at increased risk of sudden death despite maximal drug therapy.6 Patients who have had a previous MI or ventricular arrhythmia are at risk for a repeat episode.6
Use of an ICD can reduce mortality by 23% in selected patients.26 Potential candidates for the device are patients who have an ejection fraction of <30%, mild to moderate symptoms, and a life expectancy of at least 1 year.6
Biventricular pacing, also known as cardiac resynchronization therapy (CRT), has been found to improve the quality of life, functional status, and exercise capacity in some patients with advanced disease. CRT, which reduces symptoms of HF and improves cardiac function by reestablishing the mechanical sequence of ventricular activation and ventricular contraction, has also been associated with reductions in hospitalization and death from progressive HF.27,28
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial demonstrated a 20% reduction in the 12-month risk of death or hospitalization from any cause with CRT, and the Cardiac Resynchronization-Heart Failure (CARE-HF) trial established that patients receiving CRT had a significantly lower risk of death than those receiving medical therapy alone (40% reduction).29,30
However, not all patients with HF have problems with conduction delay that result in a dyssynchronous heart beat. CRT is indicated only for patients who are in sinus rhythm and have:
- NYHA class III or IV HF
- an ejection fraction of <35%
- a prolonged QRS complex (>120 m/sec), and
- continued symptoms despite maximal medical therapy.6
Under these criteria, approximately 10% of patients with HF would qualify for CRT.31 The restrictive criteria are due, in part, to the fact that this modality is relatively new and has been studied only in a small subset of patients.
Options for patients who are running out of them
For acutely decompensated hospitalized patients with volume overload, ultrafiltration (UF) is a useful alternative to diuretics. UF uses high pressure to “force” volume through the kidneys;32,33 the technique maximizes diuresis, and is best suited for patients who have significant renal dysfunction or are not responding to standard diuretic therapy. UF makes it easier to remove the desired amount of fluid, and has a positive impact on pulmonary wedge pressure and cardiac output.34 Its use in diuretic-resistant patients can decrease the length of stay and produce positive clinical benefits that may last up to 3 months.34
There are also a number of experimental strategies, surgical and otherwise. Among them are:
Cardiac wrap surgery, in which the heart is encased in a mesh bag attached with stitches, in an attempt to stop the progression of end-stage HF by preventing further dilation;25
Ventricular restoration surgery, a procedure in which scar tissue caused by MI is removed from the ventricular muscle and the left ventricle is reshaped and its size reduced in an attempt to restore some of the heart’s pumping ability;25 and
Enhanced external counterpulsation, or EECP, a noninvasive technique in which pressure cuffs are placed on the calves, thighs, and buttocks and inflated and deflated in an attempt to increase blood flow back to the heart.25
Correspondence
Randy Wexler, MD, MPH, FAAFP, The Ohio State University, B0902B Cramblett Hall, 456 W. 10th Avenue, Columbus, OH 43210; [email protected]
1. Rosamond W, Flegal K, Friday G, et al. American Heart Association: heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2. Lloyd-Jones DM, Larson MG, Leip EP, et al. Framingham heart study. Lifetime risk for developing congestive heart failure. Circulation. 2002;106:3068-3072.
3. Burt CW, Schappert SM. Ambulatory care visits to physicians offices, hospital outpatient departments, and emergency departments: United states, 1999-2000. Vital Health Stat 13. 2004;157:1-70
4. Levit K, Stranges E, Ryan K, et al. HCUP facts and figures, 2006: Statistics on Hospital-based Care in the United States. Rockville, MD: Agency for Healthcare Research and Quality, 2008. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. Accessed February 9, 2009.
5. Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. Boston: Little, Brown and Company; 1964.
6. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112:e154-e235.
7. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74:1893-1898.
8. Maisel A, Krishnaswamy P, Nowak R, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-167.
9. Maisel AS. B-type natriuretic peptide (BNP) levels: diagnostic and therapeutic potential. Rev Cardiovasc Med. 2001;2(suppl 2):S13-S18.
10. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide (BNP) in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379-385.
11. Philbin EF, Rocco TA, Jr, Lindenmuth NW, et al. Systolic versus diastolic heart failure in a community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605-613.
12. Piepoli MF, Flather M, Coats AJ. Overview of studies of exercise training in chronic heart failure: the need for a prospective randomized multicentre European trial. Eur Heart J. 1998;19:830-841.
13. Coats AJ, Adamopoulos S, Meyer TE, et al. Effects of physical training in chronic heart failure. Lancet. 1990;335:63-66.
14. Feldman RD, Zou G, Feagen BG, et al. The STITCH Investigators. The Simplified Treatment Intervention to Control Hypertension (STITCH) trial: A cluster randomized controlled trial of a step-care algorithm using initial fixed dose combination therapy for the management of hypertension. Presented at Scientific Sessions 2007 of the American Heart Association, November 4-7, 2007; Orlando, Fla.
15. Chobanian A, Bakris G, Black H, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Hypertension. 2003;42:1206-1252.
16. Brookes L. Hypertension highlights: new drug algorithms, new drug approvals, new drugs. Available at: http://canadiancpd.medscape.com/viewarticle/568786_print. Accessed January 27, 2009.
17. Yusuf S, Sleight P, Pogue J, et al. The Heart Outcomes Prevention Evaluation Study Investigators: effects of angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145-153.
18. Vantrimpont P, Rouleau JL, Wun CC, et al. For the SAVE Investigators. Additive beneficial effects of beta-blockers to angiotensin converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) study. J Am Coll Cardiol. 1997;29:229-236.
19. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
20. Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942-2946.
21. Cayley W. Digitalis for the treatment of congestive heart failure in patients in sinus rhythm. Am Fam Physician. 2004;69:71-73.
22. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
23. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
24. Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction. Arch Intern Med. 2007;167:1930-1936.
25. Mayo Clinic. Heart failure: treatments and drugs. January 3, 2008. Available at: http://www.mayoclinic.com/health/heart-failure.DS00061/DSECTION=treatments-and-drugs. Accessed January 30, 2009.
26. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
27. Blanc J-J, Bertault-Valls V, Fatemi M, et al. Mid-term benefits of left univentricular pacing in patients with congestive heart failure. Circulation. 2004;109:1741-1744.
28. Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730-740.
29. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
30. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
31. Farwell D, Patel NR, Hall A, et al. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246-1250.
32. Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the relief for acutely fluid-overloaded patients with decompensated congestive heart failure (RAPD-HF). J Am Coll Cardiol. 2005;46:2043-2046.
33. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with acutely decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047-2051.
34. Agostini PG, Marenzi GC, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency; failure of furosemide to provide same results. Am J Med. 1994;96:191-199.
- Use B-type natriuretic peptide (BNP) levels as an aid not only in the diagnosis of heart failure (HF), but to track its progression as well (A).
- Prescribe exercise training for patients with stable heart failure; exercising at 40% to 70% of maximum capacity for 20 to 45 minutes several times a week offers benefits on par with pharmacotherapy (A).
- Consider using the Simplified Treatment Intervention to Control Hypertension (STITCH) algorithm for hypertensive patients or those who are at risk of developing HF; this step-care strategy is effective in treating hypertension, a leading cause of HF (C).
- Consult a specialist before prescribing both an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) for a patient with advanced HF; studies of combination therapy for this patient population have had mixed results (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Family physicians are all too familiar with heart failure (HF). This debilitating condition accounts for approximately 3.4 million outpatient visits to US physicians annually,1 and fully two-thirds of HF patients are cared for by primary care physicians.2
A host of comorbid conditions—coronary artery disease, valvular heart disease, diabetes, dyslipidemia, metabolic syndrome, obesity, chronic renal insufficiency, and hypertension chief among them—contribute to the development of HF.3 Of these, hypertension is the most important factor. In more than 75% of cases, high blood pressure precedes HF,1 and an individual’s lifetime risk of developing HF is strongly associated with poor blood pressure control.4 Hypertension is the most significant controllable factor in the management of HF as well. Because of the nexus between hypertension and HF, we encourage physicians to think of these 2 conditions as a single entity—and to recognize that a reduction of even a few millimeters of mercury can have huge clinical benefits.
This review, which highlights a recently tested hypertension algorithm along with other recent developments and long-established treatment strategies, will help you do everything possible to slow the progression of this debilitating and deadly disease.
BNP’s increasing role in evaluating heart failure
A diagnosis of HF in patients with known heart disease is based on functionality and symptoms, assessed with the help of 2 classification schemes5,6 ( TABLE 1 ) and a variety of tests. (Patients who present with the signs and symptoms of HF but no evidence of the comorbid conditions typically associated with it should be screened for other, noncardiac causes—human immunodeficiency virus, hepatitis C, hemochromatosis, hypothyroidism, and substance abuse among them.6 )
Diagnostic testing. Baseline serum chemistries include a complete blood count, urinalysis, electrolytes, magnesium, blood urea nitrogen, creatinine, and blood glucose levels, and liver and thyroid function tests.
B-type natriuretic peptide (BNP), a homeostatic marker secreted by the heart in an attempt to maintain stable blood pressure and plasma volume and avoid fluid retention, is increasingly recognized as an important aid, not only in diagnosing HF but in gauging its severity, managing symptoms, and determining the prognosis.7,8 BNP concentrations <80 pg/mL have been found to have a negative predictive value of 98%, and are also highly sensitive (98%) and specific (98%) for the diagnosis of HF.9,10
Testing may also include a 12-lead electrocardiogram as well as a posterior-anterior/lateral chest x-ray. Echocardiography is often used to evaluate left ventricular function and ejection fraction6 —a key to establishing whether the patient has systolic (reduced ejection fraction) or diastolic (preserved ejection fraction) HF.
An ejection fraction ≤40% is characteristic of systolic HF, which affects approximately 60% of patients with heart failure11 and is the focus of the following discussion of treatments.
TABLE 1
Classifying heart failure: 2 systems
| NEW YORK HEART ASSOCIATION |
| Class I: No limitation of physical activity; ordinary activity does not cause undue fatigue or dyspnea. Class II: Slight limitation of activity; comfortable at rest, but ordinary physical activity results in fatigue or dyspnea. Class III: Marked limitation in activity. Class IV: Unable to carry on any physical activity without symptoms; symptoms present even at rest. |
| AMERICAN COLLEGE OF CARDIOLOGY/AMERICAN HEART ASSOCIATION |
| Stage A: Conditions strongly associated with heart failure (HF); at high risk of HF. No identified structural or functional abnormalities of the pericardium, myocardium, or cardiac valves; no signs or symptoms of HF. Stage B: Structural heart disease strongly associated with HF, but no known signs or symptoms. Stage C: Current or prior symptoms of HF associated with underlying structural heart disease. Stage D: Advanced structural heart disease, with marked symptoms of HF at rest despite maximal medical therapy. Specialized interventions required. |
| Sources: Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. 1964;5 Hunt et al. Circulation. 2005.6 |
Early interventions: Get patients moving
For all patients with stable HF—and those at high risk of developingit—behavioral modification is a key component of treatment. Lifestyle intervention should be directed at weight loss and diet, including control of salt intake; increased physical activity; and smoking cessation.
Don’t shy away from exercise. Although many physicians hesitate to prescribe exercise to patients with HF, physical activity should be a routine recommendation for all but the most debilitated patients.6 Regular exercise has been shown to decrease symptoms, increase functional capacity, and improve the quality of life, with benefits comparable to those of pharmacotherapy.6,12,13
Studies of the beneficial effects of exercise were based on sustaining 40% to 70% of maximum capacity for 20 to 45 minutes, 3 to 5 days a week.6 A good walking program—of at least 30 minutes 4 to 5 days each week—should not be difficult for patients to maintain.
BP treatment guidelines: The old and the new
As noted earlier, controlling hypertension is crucial, not only to prevent HF but to attenuate its progress. But blood pressure management is suboptimal in the United States, with many patients failing to achieve recommended levels of pressure reduction. It’s been suggested that the complexity of standard treatment guidelines may be part of the problem.
STITCH step care is a newer option. Researchers designed the Simplified Treatment Intervention to Control Hypertension (STITCH) Trial, a cluster randomized study of patients at multiple family medicine clinics in Canada, to evaluate whether a simplified step-care algorithm would yield better results.
The STITCH algorithm has 4 treatment steps:
Step 1: Initiate therapy by pairing a diuretic with either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Step 2: Increase combination therapy to the highest dose tolerated.
Step 3: Add a calcium channel blocker and increase to the highest tolerated dose.
Step 4: Add a non-first-line antihypertensive agent (alpha-blocker, beta-blocker, or spironolactone).
Researchers found that after 6 months, 64.7% of patients on the STITCH protocol had achieved target blood pressure, compared with 52.7% of those whose treatment was based on the Canadian Hypertension Education Program (CHEP) guidelines (P=.03).14 The CHEP protocol is similar to that of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7);15 both offer numerous options for initial treatment.16
In presenting the STITCH results at the 2007 annual meeting of the American Heart Association, the lead author described the use of a simple step-care approach as “an important way forward in the treatment of hypertension [which] may be a paradigm for managing a range of chronic diseases.”16 Yet the STITCH algorithm has yet to be widely embraced; outside of the research community, most US physicians are relying on the JNC 7 guidelines.
ACC/AHA recommendations indicate that for patients at stage A—that is, those with conditions strongly associated with, and at high risk for, HF—management of hypertension should conform to national standards such as JNC 7. The JNC 7 guidelines recommend the use of a thiazide diuretic as the initial drug of choice for patients with essential hypertension. For those with diabetes, ACE inhibitors and ARBs are the first-line antihypertensive agents of choice.
Glucose control is also essential for stage A patients with diabetes. Treatment of lipid disorders and pharmacotherapy for metabolic syndrome are also recommended for stage A patients, as needed.
Treatment escalates as HF progresses
ACE inhibitors, ARBs, and beta-blockers are the preferred pharmacologic interventions for patients at stage B—those who have structural heart disease strongly associated with HF but are not yet symptomatic. Anyone who has had a myocardial infarction (MI) should be started on a beta-blocker and an ACE inhibitor, ACC/AHA recommends, unless a contraindication exists.6 Similarly, any patient with a reduced ejection fraction should be started on an ACE inhibitor regardless of symptoms.6
The Heart Outcomes Prevention Evaluation (HOPE) study demonstrated a 23% relative risk (RR) reduction with the use of an ACE inhibitor in patients with coronary artery disease, peripheral vascular disease, or diabetes, compared with patients receiving a placebo.17 The importance of a beta-blocker was established in a subanalysis of the Survival and Ventricular Enlargement Trial (SAVE), which found that patients taking beta-blockers in addition to an ACE inhibitor had a 32% RR reduction in progression of HF, compared with patients on an ACE inhibitor alone.18
We recommend an ACE inhibitor or an ARB and a beta-blocker, when appropriate, to slow the progression of HF pathophysiology. It is important to be aware of the potential adverse effects of certain beta-blockers in patients with HF. Only 3 beta-blockers are approved for use in this patient population in the United States—bisoprolol, carvedilol, and metoprolol succinate, which have been found to provide benefits that other beta-blockers do not.6,15
Stages C and D: Tx considerations and controversies
Treatment for patients at stage C should include all components of therapy for patients at stages A and B, but with a more aggressive use of pharmacotherapy ( TABLE 2 ). Patients with stage C HF, by definition, are symptomatic, and the ACC/AHA recommendations reflect concern about their increasingly compromised status. Thus, in addition to the use of ACE inhibitors or ARBs and beta-blockers, modest use of diuretics is recommended, as needed, for fluid volume control.6 Diuretics should be used judiciously, though, with ongoing evaluation to avoid the excessive loss of potassium and magnesium, which can lead to volume depletion and lethal arrhythmias. Limiting sodium consumption is an important dietary restriction for stage C patients.
Aldosterone antagonists may also be considered on a case-by-case basis for patients with stage C HF. Due to their potassium-sparing effects, aldosterone antagonists, used in conjunction with standard therapies, may have a positive effect on electrolyte balance. Potassium levels must be carefully monitored, however, and potassium supplementation reevaluated for patients who are put on an aldosterone antagonist.19
Digitalis may also be helpful in select patients who remain symptomatic despite maximal pharmacotherapy.20 While it does not affect mortality, digitalis has been shown to reduce hospitalizations.21
ACE inhibitor-ARB combination therapy, another possible treatment for advanced HF, remains controversial. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), detailed in “ACE inhibitors and ARBs: One or the other—not both” in the January 2009 issue of The Journal of Family Practice, evaluated use of this dual therapy; the trial was also designed to determine whether telmisartan (an ARB) is inferior to ramipril (an ACE inhibitor) in patients at high risk for vascular events.22 The researchers found that telmisartan is not, in fact, inferior to ramipril, and reported that for patients with HF, an ACE-ARB combination offers a potential benefit.
However, the clinical benefit of an ACE-ARB combination in this patient population was not clarified in this study, and may be potentially harmful. In the Valsartan Heart Failure Trial (ValHeFT), the combination of valsartan, an ARB, and an ACE inhibitor decreased hospitalizations but did not improve mortality.23 Indeed, an increase in mortality was found when an ACEARB combination was used in conjunction with beta-blockers. Because beta-blockers are indicated for routine use in patients with HF, this finding was of particular concern.
In a meta-analysis of randomized trials using both an ACE inhibitor and an ARB in patients with left ventricular dysfunction, researchers found a “marked” increase in adverse effects, including deteriorating renal function (RR=2.17), hyperkalemia (RR=4.87), and symptomatic hypotension (RR=1.05).24 Although an ACE-ARB combination may benefit a subset of patients with HF, it is best to initiate such treatment only with the guidance of an HF specialist.
TABLE 2
Treating heart failure: How the different drugs and devices rate
| STAGE | PHARMACOTHERAPY | LOE | DEVICE/INTERVENTION | LOE |
|---|---|---|---|---|
| A | Treat BP per JNC 7 ACE inhibitor or ARB for patients with vascular disease or diabetes | A | None | N/A |
| B | ACE inhibitor or ARB BB | A | None | N/A |
| C | Routine use: Diuretics ACE inhibitor BB Select use: Aldosterone antagonist ARB Digitalis | A | Consider: Biventricular pacer or ICD or both | B |
| D | Same as C | B | Consider: Heart transplant or LVAD; experimental protocols | C |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta-blocker; BP, blood pressure; ICD, implantable cardioverter defibrillator; JNC 7, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LOE, level of evidence; LVAD, left ventricular assist device. | ||||
| Adapted from: Hunt SA, et al. Circulation. 2005.6 | ||||
Beyond drug therapy: Assistive devices
Refractory end-stage HF requires a clear treatment plan, and should involve the recommendations of an HF specialist. Careful maintenance of fluid status is required, and an evaluation for cardiac transplantation may be considered.
A left ventricular assist device (LVAD) should also be considered for patients with an estimated 1-year mortality of >50%.6 LVADs are mechanical heart pumps that were initially utilized as a “bridge” to transplant, but are increasingly being used as a palliative alternative for severely ill patients.25
Other devices—an implantable cardioverter defibrillator (ICD) or a biventricular pacer—should also be considered for patients at stage D, as well as stage C patients who are at increased risk of sudden death despite maximal drug therapy.6 Patients who have had a previous MI or ventricular arrhythmia are at risk for a repeat episode.6
Use of an ICD can reduce mortality by 23% in selected patients.26 Potential candidates for the device are patients who have an ejection fraction of <30%, mild to moderate symptoms, and a life expectancy of at least 1 year.6
Biventricular pacing, also known as cardiac resynchronization therapy (CRT), has been found to improve the quality of life, functional status, and exercise capacity in some patients with advanced disease. CRT, which reduces symptoms of HF and improves cardiac function by reestablishing the mechanical sequence of ventricular activation and ventricular contraction, has also been associated with reductions in hospitalization and death from progressive HF.27,28
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial demonstrated a 20% reduction in the 12-month risk of death or hospitalization from any cause with CRT, and the Cardiac Resynchronization-Heart Failure (CARE-HF) trial established that patients receiving CRT had a significantly lower risk of death than those receiving medical therapy alone (40% reduction).29,30
However, not all patients with HF have problems with conduction delay that result in a dyssynchronous heart beat. CRT is indicated only for patients who are in sinus rhythm and have:
- NYHA class III or IV HF
- an ejection fraction of <35%
- a prolonged QRS complex (>120 m/sec), and
- continued symptoms despite maximal medical therapy.6
Under these criteria, approximately 10% of patients with HF would qualify for CRT.31 The restrictive criteria are due, in part, to the fact that this modality is relatively new and has been studied only in a small subset of patients.
Options for patients who are running out of them
For acutely decompensated hospitalized patients with volume overload, ultrafiltration (UF) is a useful alternative to diuretics. UF uses high pressure to “force” volume through the kidneys;32,33 the technique maximizes diuresis, and is best suited for patients who have significant renal dysfunction or are not responding to standard diuretic therapy. UF makes it easier to remove the desired amount of fluid, and has a positive impact on pulmonary wedge pressure and cardiac output.34 Its use in diuretic-resistant patients can decrease the length of stay and produce positive clinical benefits that may last up to 3 months.34
There are also a number of experimental strategies, surgical and otherwise. Among them are:
Cardiac wrap surgery, in which the heart is encased in a mesh bag attached with stitches, in an attempt to stop the progression of end-stage HF by preventing further dilation;25
Ventricular restoration surgery, a procedure in which scar tissue caused by MI is removed from the ventricular muscle and the left ventricle is reshaped and its size reduced in an attempt to restore some of the heart’s pumping ability;25 and
Enhanced external counterpulsation, or EECP, a noninvasive technique in which pressure cuffs are placed on the calves, thighs, and buttocks and inflated and deflated in an attempt to increase blood flow back to the heart.25
Correspondence
Randy Wexler, MD, MPH, FAAFP, The Ohio State University, B0902B Cramblett Hall, 456 W. 10th Avenue, Columbus, OH 43210; [email protected]
- Use B-type natriuretic peptide (BNP) levels as an aid not only in the diagnosis of heart failure (HF), but to track its progression as well (A).
- Prescribe exercise training for patients with stable heart failure; exercising at 40% to 70% of maximum capacity for 20 to 45 minutes several times a week offers benefits on par with pharmacotherapy (A).
- Consider using the Simplified Treatment Intervention to Control Hypertension (STITCH) algorithm for hypertensive patients or those who are at risk of developing HF; this step-care strategy is effective in treating hypertension, a leading cause of HF (C).
- Consult a specialist before prescribing both an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) for a patient with advanced HF; studies of combination therapy for this patient population have had mixed results (C).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
Family physicians are all too familiar with heart failure (HF). This debilitating condition accounts for approximately 3.4 million outpatient visits to US physicians annually,1 and fully two-thirds of HF patients are cared for by primary care physicians.2
A host of comorbid conditions—coronary artery disease, valvular heart disease, diabetes, dyslipidemia, metabolic syndrome, obesity, chronic renal insufficiency, and hypertension chief among them—contribute to the development of HF.3 Of these, hypertension is the most important factor. In more than 75% of cases, high blood pressure precedes HF,1 and an individual’s lifetime risk of developing HF is strongly associated with poor blood pressure control.4 Hypertension is the most significant controllable factor in the management of HF as well. Because of the nexus between hypertension and HF, we encourage physicians to think of these 2 conditions as a single entity—and to recognize that a reduction of even a few millimeters of mercury can have huge clinical benefits.
This review, which highlights a recently tested hypertension algorithm along with other recent developments and long-established treatment strategies, will help you do everything possible to slow the progression of this debilitating and deadly disease.
BNP’s increasing role in evaluating heart failure
A diagnosis of HF in patients with known heart disease is based on functionality and symptoms, assessed with the help of 2 classification schemes5,6 ( TABLE 1 ) and a variety of tests. (Patients who present with the signs and symptoms of HF but no evidence of the comorbid conditions typically associated with it should be screened for other, noncardiac causes—human immunodeficiency virus, hepatitis C, hemochromatosis, hypothyroidism, and substance abuse among them.6 )
Diagnostic testing. Baseline serum chemistries include a complete blood count, urinalysis, electrolytes, magnesium, blood urea nitrogen, creatinine, and blood glucose levels, and liver and thyroid function tests.
B-type natriuretic peptide (BNP), a homeostatic marker secreted by the heart in an attempt to maintain stable blood pressure and plasma volume and avoid fluid retention, is increasingly recognized as an important aid, not only in diagnosing HF but in gauging its severity, managing symptoms, and determining the prognosis.7,8 BNP concentrations <80 pg/mL have been found to have a negative predictive value of 98%, and are also highly sensitive (98%) and specific (98%) for the diagnosis of HF.9,10
Testing may also include a 12-lead electrocardiogram as well as a posterior-anterior/lateral chest x-ray. Echocardiography is often used to evaluate left ventricular function and ejection fraction6 —a key to establishing whether the patient has systolic (reduced ejection fraction) or diastolic (preserved ejection fraction) HF.
An ejection fraction ≤40% is characteristic of systolic HF, which affects approximately 60% of patients with heart failure11 and is the focus of the following discussion of treatments.
TABLE 1
Classifying heart failure: 2 systems
| NEW YORK HEART ASSOCIATION |
| Class I: No limitation of physical activity; ordinary activity does not cause undue fatigue or dyspnea. Class II: Slight limitation of activity; comfortable at rest, but ordinary physical activity results in fatigue or dyspnea. Class III: Marked limitation in activity. Class IV: Unable to carry on any physical activity without symptoms; symptoms present even at rest. |
| AMERICAN COLLEGE OF CARDIOLOGY/AMERICAN HEART ASSOCIATION |
| Stage A: Conditions strongly associated with heart failure (HF); at high risk of HF. No identified structural or functional abnormalities of the pericardium, myocardium, or cardiac valves; no signs or symptoms of HF. Stage B: Structural heart disease strongly associated with HF, but no known signs or symptoms. Stage C: Current or prior symptoms of HF associated with underlying structural heart disease. Stage D: Advanced structural heart disease, with marked symptoms of HF at rest despite maximal medical therapy. Specialized interventions required. |
| Sources: Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. 1964;5 Hunt et al. Circulation. 2005.6 |
Early interventions: Get patients moving
For all patients with stable HF—and those at high risk of developingit—behavioral modification is a key component of treatment. Lifestyle intervention should be directed at weight loss and diet, including control of salt intake; increased physical activity; and smoking cessation.
Don’t shy away from exercise. Although many physicians hesitate to prescribe exercise to patients with HF, physical activity should be a routine recommendation for all but the most debilitated patients.6 Regular exercise has been shown to decrease symptoms, increase functional capacity, and improve the quality of life, with benefits comparable to those of pharmacotherapy.6,12,13
Studies of the beneficial effects of exercise were based on sustaining 40% to 70% of maximum capacity for 20 to 45 minutes, 3 to 5 days a week.6 A good walking program—of at least 30 minutes 4 to 5 days each week—should not be difficult for patients to maintain.
BP treatment guidelines: The old and the new
As noted earlier, controlling hypertension is crucial, not only to prevent HF but to attenuate its progress. But blood pressure management is suboptimal in the United States, with many patients failing to achieve recommended levels of pressure reduction. It’s been suggested that the complexity of standard treatment guidelines may be part of the problem.
STITCH step care is a newer option. Researchers designed the Simplified Treatment Intervention to Control Hypertension (STITCH) Trial, a cluster randomized study of patients at multiple family medicine clinics in Canada, to evaluate whether a simplified step-care algorithm would yield better results.
The STITCH algorithm has 4 treatment steps:
Step 1: Initiate therapy by pairing a diuretic with either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB).
Step 2: Increase combination therapy to the highest dose tolerated.
Step 3: Add a calcium channel blocker and increase to the highest tolerated dose.
Step 4: Add a non-first-line antihypertensive agent (alpha-blocker, beta-blocker, or spironolactone).
Researchers found that after 6 months, 64.7% of patients on the STITCH protocol had achieved target blood pressure, compared with 52.7% of those whose treatment was based on the Canadian Hypertension Education Program (CHEP) guidelines (P=.03).14 The CHEP protocol is similar to that of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7);15 both offer numerous options for initial treatment.16
In presenting the STITCH results at the 2007 annual meeting of the American Heart Association, the lead author described the use of a simple step-care approach as “an important way forward in the treatment of hypertension [which] may be a paradigm for managing a range of chronic diseases.”16 Yet the STITCH algorithm has yet to be widely embraced; outside of the research community, most US physicians are relying on the JNC 7 guidelines.
ACC/AHA recommendations indicate that for patients at stage A—that is, those with conditions strongly associated with, and at high risk for, HF—management of hypertension should conform to national standards such as JNC 7. The JNC 7 guidelines recommend the use of a thiazide diuretic as the initial drug of choice for patients with essential hypertension. For those with diabetes, ACE inhibitors and ARBs are the first-line antihypertensive agents of choice.
Glucose control is also essential for stage A patients with diabetes. Treatment of lipid disorders and pharmacotherapy for metabolic syndrome are also recommended for stage A patients, as needed.
Treatment escalates as HF progresses
ACE inhibitors, ARBs, and beta-blockers are the preferred pharmacologic interventions for patients at stage B—those who have structural heart disease strongly associated with HF but are not yet symptomatic. Anyone who has had a myocardial infarction (MI) should be started on a beta-blocker and an ACE inhibitor, ACC/AHA recommends, unless a contraindication exists.6 Similarly, any patient with a reduced ejection fraction should be started on an ACE inhibitor regardless of symptoms.6
The Heart Outcomes Prevention Evaluation (HOPE) study demonstrated a 23% relative risk (RR) reduction with the use of an ACE inhibitor in patients with coronary artery disease, peripheral vascular disease, or diabetes, compared with patients receiving a placebo.17 The importance of a beta-blocker was established in a subanalysis of the Survival and Ventricular Enlargement Trial (SAVE), which found that patients taking beta-blockers in addition to an ACE inhibitor had a 32% RR reduction in progression of HF, compared with patients on an ACE inhibitor alone.18
We recommend an ACE inhibitor or an ARB and a beta-blocker, when appropriate, to slow the progression of HF pathophysiology. It is important to be aware of the potential adverse effects of certain beta-blockers in patients with HF. Only 3 beta-blockers are approved for use in this patient population in the United States—bisoprolol, carvedilol, and metoprolol succinate, which have been found to provide benefits that other beta-blockers do not.6,15
Stages C and D: Tx considerations and controversies
Treatment for patients at stage C should include all components of therapy for patients at stages A and B, but with a more aggressive use of pharmacotherapy ( TABLE 2 ). Patients with stage C HF, by definition, are symptomatic, and the ACC/AHA recommendations reflect concern about their increasingly compromised status. Thus, in addition to the use of ACE inhibitors or ARBs and beta-blockers, modest use of diuretics is recommended, as needed, for fluid volume control.6 Diuretics should be used judiciously, though, with ongoing evaluation to avoid the excessive loss of potassium and magnesium, which can lead to volume depletion and lethal arrhythmias. Limiting sodium consumption is an important dietary restriction for stage C patients.
Aldosterone antagonists may also be considered on a case-by-case basis for patients with stage C HF. Due to their potassium-sparing effects, aldosterone antagonists, used in conjunction with standard therapies, may have a positive effect on electrolyte balance. Potassium levels must be carefully monitored, however, and potassium supplementation reevaluated for patients who are put on an aldosterone antagonist.19
Digitalis may also be helpful in select patients who remain symptomatic despite maximal pharmacotherapy.20 While it does not affect mortality, digitalis has been shown to reduce hospitalizations.21
ACE inhibitor-ARB combination therapy, another possible treatment for advanced HF, remains controversial. The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), detailed in “ACE inhibitors and ARBs: One or the other—not both” in the January 2009 issue of The Journal of Family Practice, evaluated use of this dual therapy; the trial was also designed to determine whether telmisartan (an ARB) is inferior to ramipril (an ACE inhibitor) in patients at high risk for vascular events.22 The researchers found that telmisartan is not, in fact, inferior to ramipril, and reported that for patients with HF, an ACE-ARB combination offers a potential benefit.
However, the clinical benefit of an ACE-ARB combination in this patient population was not clarified in this study, and may be potentially harmful. In the Valsartan Heart Failure Trial (ValHeFT), the combination of valsartan, an ARB, and an ACE inhibitor decreased hospitalizations but did not improve mortality.23 Indeed, an increase in mortality was found when an ACEARB combination was used in conjunction with beta-blockers. Because beta-blockers are indicated for routine use in patients with HF, this finding was of particular concern.
In a meta-analysis of randomized trials using both an ACE inhibitor and an ARB in patients with left ventricular dysfunction, researchers found a “marked” increase in adverse effects, including deteriorating renal function (RR=2.17), hyperkalemia (RR=4.87), and symptomatic hypotension (RR=1.05).24 Although an ACE-ARB combination may benefit a subset of patients with HF, it is best to initiate such treatment only with the guidance of an HF specialist.
TABLE 2
Treating heart failure: How the different drugs and devices rate
| STAGE | PHARMACOTHERAPY | LOE | DEVICE/INTERVENTION | LOE |
|---|---|---|---|---|
| A | Treat BP per JNC 7 ACE inhibitor or ARB for patients with vascular disease or diabetes | A | None | N/A |
| B | ACE inhibitor or ARB BB | A | None | N/A |
| C | Routine use: Diuretics ACE inhibitor BB Select use: Aldosterone antagonist ARB Digitalis | A | Consider: Biventricular pacer or ICD or both | B |
| D | Same as C | B | Consider: Heart transplant or LVAD; experimental protocols | C |
| ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BB, beta-blocker; BP, blood pressure; ICD, implantable cardioverter defibrillator; JNC 7, the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LOE, level of evidence; LVAD, left ventricular assist device. | ||||
| Adapted from: Hunt SA, et al. Circulation. 2005.6 | ||||
Beyond drug therapy: Assistive devices
Refractory end-stage HF requires a clear treatment plan, and should involve the recommendations of an HF specialist. Careful maintenance of fluid status is required, and an evaluation for cardiac transplantation may be considered.
A left ventricular assist device (LVAD) should also be considered for patients with an estimated 1-year mortality of >50%.6 LVADs are mechanical heart pumps that were initially utilized as a “bridge” to transplant, but are increasingly being used as a palliative alternative for severely ill patients.25
Other devices—an implantable cardioverter defibrillator (ICD) or a biventricular pacer—should also be considered for patients at stage D, as well as stage C patients who are at increased risk of sudden death despite maximal drug therapy.6 Patients who have had a previous MI or ventricular arrhythmia are at risk for a repeat episode.6
Use of an ICD can reduce mortality by 23% in selected patients.26 Potential candidates for the device are patients who have an ejection fraction of <30%, mild to moderate symptoms, and a life expectancy of at least 1 year.6
Biventricular pacing, also known as cardiac resynchronization therapy (CRT), has been found to improve the quality of life, functional status, and exercise capacity in some patients with advanced disease. CRT, which reduces symptoms of HF and improves cardiac function by reestablishing the mechanical sequence of ventricular activation and ventricular contraction, has also been associated with reductions in hospitalization and death from progressive HF.27,28
The Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) trial demonstrated a 20% reduction in the 12-month risk of death or hospitalization from any cause with CRT, and the Cardiac Resynchronization-Heart Failure (CARE-HF) trial established that patients receiving CRT had a significantly lower risk of death than those receiving medical therapy alone (40% reduction).29,30
However, not all patients with HF have problems with conduction delay that result in a dyssynchronous heart beat. CRT is indicated only for patients who are in sinus rhythm and have:
- NYHA class III or IV HF
- an ejection fraction of <35%
- a prolonged QRS complex (>120 m/sec), and
- continued symptoms despite maximal medical therapy.6
Under these criteria, approximately 10% of patients with HF would qualify for CRT.31 The restrictive criteria are due, in part, to the fact that this modality is relatively new and has been studied only in a small subset of patients.
Options for patients who are running out of them
For acutely decompensated hospitalized patients with volume overload, ultrafiltration (UF) is a useful alternative to diuretics. UF uses high pressure to “force” volume through the kidneys;32,33 the technique maximizes diuresis, and is best suited for patients who have significant renal dysfunction or are not responding to standard diuretic therapy. UF makes it easier to remove the desired amount of fluid, and has a positive impact on pulmonary wedge pressure and cardiac output.34 Its use in diuretic-resistant patients can decrease the length of stay and produce positive clinical benefits that may last up to 3 months.34
There are also a number of experimental strategies, surgical and otherwise. Among them are:
Cardiac wrap surgery, in which the heart is encased in a mesh bag attached with stitches, in an attempt to stop the progression of end-stage HF by preventing further dilation;25
Ventricular restoration surgery, a procedure in which scar tissue caused by MI is removed from the ventricular muscle and the left ventricle is reshaped and its size reduced in an attempt to restore some of the heart’s pumping ability;25 and
Enhanced external counterpulsation, or EECP, a noninvasive technique in which pressure cuffs are placed on the calves, thighs, and buttocks and inflated and deflated in an attempt to increase blood flow back to the heart.25
Correspondence
Randy Wexler, MD, MPH, FAAFP, The Ohio State University, B0902B Cramblett Hall, 456 W. 10th Avenue, Columbus, OH 43210; [email protected]
1. Rosamond W, Flegal K, Friday G, et al. American Heart Association: heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2. Lloyd-Jones DM, Larson MG, Leip EP, et al. Framingham heart study. Lifetime risk for developing congestive heart failure. Circulation. 2002;106:3068-3072.
3. Burt CW, Schappert SM. Ambulatory care visits to physicians offices, hospital outpatient departments, and emergency departments: United states, 1999-2000. Vital Health Stat 13. 2004;157:1-70
4. Levit K, Stranges E, Ryan K, et al. HCUP facts and figures, 2006: Statistics on Hospital-based Care in the United States. Rockville, MD: Agency for Healthcare Research and Quality, 2008. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. Accessed February 9, 2009.
5. Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. Boston: Little, Brown and Company; 1964.
6. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112:e154-e235.
7. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74:1893-1898.
8. Maisel A, Krishnaswamy P, Nowak R, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-167.
9. Maisel AS. B-type natriuretic peptide (BNP) levels: diagnostic and therapeutic potential. Rev Cardiovasc Med. 2001;2(suppl 2):S13-S18.
10. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide (BNP) in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379-385.
11. Philbin EF, Rocco TA, Jr, Lindenmuth NW, et al. Systolic versus diastolic heart failure in a community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605-613.
12. Piepoli MF, Flather M, Coats AJ. Overview of studies of exercise training in chronic heart failure: the need for a prospective randomized multicentre European trial. Eur Heart J. 1998;19:830-841.
13. Coats AJ, Adamopoulos S, Meyer TE, et al. Effects of physical training in chronic heart failure. Lancet. 1990;335:63-66.
14. Feldman RD, Zou G, Feagen BG, et al. The STITCH Investigators. The Simplified Treatment Intervention to Control Hypertension (STITCH) trial: A cluster randomized controlled trial of a step-care algorithm using initial fixed dose combination therapy for the management of hypertension. Presented at Scientific Sessions 2007 of the American Heart Association, November 4-7, 2007; Orlando, Fla.
15. Chobanian A, Bakris G, Black H, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Hypertension. 2003;42:1206-1252.
16. Brookes L. Hypertension highlights: new drug algorithms, new drug approvals, new drugs. Available at: http://canadiancpd.medscape.com/viewarticle/568786_print. Accessed January 27, 2009.
17. Yusuf S, Sleight P, Pogue J, et al. The Heart Outcomes Prevention Evaluation Study Investigators: effects of angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145-153.
18. Vantrimpont P, Rouleau JL, Wun CC, et al. For the SAVE Investigators. Additive beneficial effects of beta-blockers to angiotensin converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) study. J Am Coll Cardiol. 1997;29:229-236.
19. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
20. Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942-2946.
21. Cayley W. Digitalis for the treatment of congestive heart failure in patients in sinus rhythm. Am Fam Physician. 2004;69:71-73.
22. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
23. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
24. Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction. Arch Intern Med. 2007;167:1930-1936.
25. Mayo Clinic. Heart failure: treatments and drugs. January 3, 2008. Available at: http://www.mayoclinic.com/health/heart-failure.DS00061/DSECTION=treatments-and-drugs. Accessed January 30, 2009.
26. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
27. Blanc J-J, Bertault-Valls V, Fatemi M, et al. Mid-term benefits of left univentricular pacing in patients with congestive heart failure. Circulation. 2004;109:1741-1744.
28. Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730-740.
29. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
30. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
31. Farwell D, Patel NR, Hall A, et al. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246-1250.
32. Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the relief for acutely fluid-overloaded patients with decompensated congestive heart failure (RAPD-HF). J Am Coll Cardiol. 2005;46:2043-2046.
33. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with acutely decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047-2051.
34. Agostini PG, Marenzi GC, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency; failure of furosemide to provide same results. Am J Med. 1994;96:191-199.
1. Rosamond W, Flegal K, Friday G, et al. American Heart Association: heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
2. Lloyd-Jones DM, Larson MG, Leip EP, et al. Framingham heart study. Lifetime risk for developing congestive heart failure. Circulation. 2002;106:3068-3072.
3. Burt CW, Schappert SM. Ambulatory care visits to physicians offices, hospital outpatient departments, and emergency departments: United states, 1999-2000. Vital Health Stat 13. 2004;157:1-70
4. Levit K, Stranges E, Ryan K, et al. HCUP facts and figures, 2006: Statistics on Hospital-based Care in the United States. Rockville, MD: Agency for Healthcare Research and Quality, 2008. Available at: http://www.hcup-us.ahrq.gov/reports.jsp. Accessed February 9, 2009.
5. Criteria Committee of the New York Heart Association. Diseases of the Heart and Blood Vessels–Nomenclature and Criteria for Diagnosis. 6th ed. Boston: Little, Brown and Company; 1964.
6. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation. 2005;112:e154-e235.
7. Doust J, Lehman R, Glasziou P. The role of BNP testing in heart failure. Am Fam Physician. 2006;74:1893-1898.
8. Maisel A, Krishnaswamy P, Nowak R, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161-167.
9. Maisel AS. B-type natriuretic peptide (BNP) levels: diagnostic and therapeutic potential. Rev Cardiovasc Med. 2001;2(suppl 2):S13-S18.
10. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide (BNP) in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379-385.
11. Philbin EF, Rocco TA, Jr, Lindenmuth NW, et al. Systolic versus diastolic heart failure in a community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605-613.
12. Piepoli MF, Flather M, Coats AJ. Overview of studies of exercise training in chronic heart failure: the need for a prospective randomized multicentre European trial. Eur Heart J. 1998;19:830-841.
13. Coats AJ, Adamopoulos S, Meyer TE, et al. Effects of physical training in chronic heart failure. Lancet. 1990;335:63-66.
14. Feldman RD, Zou G, Feagen BG, et al. The STITCH Investigators. The Simplified Treatment Intervention to Control Hypertension (STITCH) trial: A cluster randomized controlled trial of a step-care algorithm using initial fixed dose combination therapy for the management of hypertension. Presented at Scientific Sessions 2007 of the American Heart Association, November 4-7, 2007; Orlando, Fla.
15. Chobanian A, Bakris G, Black H, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Hypertension. 2003;42:1206-1252.
16. Brookes L. Hypertension highlights: new drug algorithms, new drug approvals, new drugs. Available at: http://canadiancpd.medscape.com/viewarticle/568786_print. Accessed January 27, 2009.
17. Yusuf S, Sleight P, Pogue J, et al. The Heart Outcomes Prevention Evaluation Study Investigators: effects of angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145-153.
18. Vantrimpont P, Rouleau JL, Wun CC, et al. For the SAVE Investigators. Additive beneficial effects of beta-blockers to angiotensin converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) study. J Am Coll Cardiol. 1997;29:229-236.
19. Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA. 2003;289:1652-1658.
20. Rahimtoola SH. Digitalis therapy for patients in clinical heart failure. Circulation. 2004;109:2942-2946.
21. Cayley W. Digitalis for the treatment of congestive heart failure in patients in sinus rhythm. Am Fam Physician. 2004;69:71-73.
22. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
23. Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
24. Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction. Arch Intern Med. 2007;167:1930-1936.
25. Mayo Clinic. Heart failure: treatments and drugs. January 3, 2008. Available at: http://www.mayoclinic.com/health/heart-failure.DS00061/DSECTION=treatments-and-drugs. Accessed January 30, 2009.
26. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225-237.
27. Blanc J-J, Bertault-Valls V, Fatemi M, et al. Mid-term benefits of left univentricular pacing in patients with congestive heart failure. Circulation. 2004;109:1741-1744.
28. Bradley DJ, Bradley EA, Baughman KL, et al. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. JAMA. 2003;289:730-740.
29. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140-2150.
30. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
31. Farwell D, Patel NR, Hall A, et al. How many people with heart failure are appropriate for biventricular resynchronization? Eur Heart J. 2000;21:1246-1250.
32. Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the relief for acutely fluid-overloaded patients with decompensated congestive heart failure (RAPD-HF). J Am Coll Cardiol. 2005;46:2043-2046.
33. Costanzo MR, Saltzberg M, O’Sullivan J, et al. Early ultrafiltration in patients with acutely decompensated heart failure and diuretic resistance. J Am Coll Cardiol. 2005;46:2047-2051.
34. Agostini PG, Marenzi GC, Lauri G, et al. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency; failure of furosemide to provide same results. Am J Med. 1994;96:191-199.
Abnormal uterine bleeding: Avoid the rush to hysterectomy
- The levonorgestrel-IUS is the most effective treatment for heavy menstrual bleeding, reducing blood loss by close to 100% (A).
- Endometrial ablation is an effective treatment for women who want to avoid major surgery and preserve their uterus, but have no wish to become pregnant in the future (A).
- Endometrial biopsy should be part of the evaluation of abnormal uterine bleeding in all women over age 35 (B).
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Ms. M, a 39-year-old mother of 3, runs a busy day-care center, cares for her sick mother, and shuttles her children among their myriad activities. During today’s office visit, she seems anxious. She says her periods are regular, but have become increasingly heavy in recent years and may last as long as 9 days. The bleeding is very heavy, with a lot of clots and some cramps during the first few days. In the past year, painful periods have caused her to miss work on several occasions. She says she often feels tired, and worries that she may be anemic. Preserving fertility is not a concern; her husband has had a vasectomy.
On exam she is without orthostasis and appears well. Her uterus is top-normal size, nontender, and there are no adnexal masses or cervical or vaginal abnormalities. You note a normal Pap at your office 5 months ago. Her office hemoglobin is 9.8 mg/dL.
She asks you to refer her to a gynecologist for a hysterectomy because she “just can’t take it anymore.” Her heavy menses are disrupting her life. Since she does not want any more children, she feels that if someone could “just take it out,” her problem would be solved. But she isn’t really enthusiastic about a hysterectomy because her life is too busy to allow time for a lengthy recovery.
You explain that there are a number of options you’d like her to consider first. Then you review the options and some of the research behind them, having recently read an article on evidence-based therapy for abnormal uterine bleeding.
A common complaint in primary care
Abnormal uterine bleeding is a common reason for women to visit their primary care clinician, accounting for about 20% of gynecologic primary care visits.1 Women are understandably concerned about any disruption of their normal bleeding pattern. Many, however, are unaware of common causes of abnormal bleeding and available treatment options.
Most cases of chronic abnormal bleeding can be classified as either heavy and regular (menorrhagia) or heavy and irregular (menometrorrhagia). A rule of thumb to help guide diagnostic testing is that menorrhagia often results from anatomic problems of the uterus or endometrium, such as polyps. Menometrorrhagia is more likely to result from hormonal abnormalities, such as polycystic ovarian syndrome (PCOS). This review will focus on medical and minimally invasive surgical therapy for chronic abnormal bleeding.
First step: Pregnancy test
Many conditions can cause abnormal uterine bleeding (TABLE 1). In women of reproductive age, the first step in the diagnostic process should be a urine or serum pregnancy test. Urine pregnancy tests have a sensitivity of 90% one day after a missed period and approximately 97% after one week.2 In addition to a pregnancy test, consider testing for thyroid dysfunction and obtaining serum prolactin levels for women presenting with anovulatory bleeding.3 If anemia is suspected, a hematocrit or hemogram is indicated. All patients over the age of 35 who present with abnormal uterine bleeding should have an office endometrial biopsy to rule out endometrial hyperplasia or cancer.4
Is she ovulating? Most cases of heavy bleeding with irregular periods are the result of anovulation, which is common soon after menarche and at the approach of menopause. Other causes of anovulatory bleeding include PCOS, hypothyroidism, and elevated prolactin levels. Chronic, irregular bleeding without a known anatomic cause is termed “dysfunctional uterine bleeding,” or DUB.5
Are periods regular? Heavy bleeding with a regular menstrual cycle (ovulatory bleeding) usually has a different etiology. It most often occurs because of anatomic abnormalities such as endometrial polyps, fibroids, and adenomyosis (pockets of endometrium found within the uterine myometrium). Heavy menstrual bleeding that occurs at the onset of or shortly after menarche may be due to a coagulopathy, such as von Willebrand disease. Therefore, consider platelet function analysis for adolescents who present with heavy menses, particularly if they require blood transfusion.
What else do you observe? When evaluating patients with abnormal uterine bleeding, don’t let the obvious focus on the gynecologic organs cause you to overlook other possibly significant findings. Look for acanthosis nigrican and an elevated body mass index (BMI), signs of PCOS, and also check for evidence of hyperthyroidism or galactorrhea. The bimanual exam should determine if the patient has an enlarged uterus, suggesting fibroids or adenomyosis.
TABLE 1
Abnormal uterine bleeding: 4 categories, many causes
| CATEGORY | MOST COMMON TYPE OF BLEEDING | SELECTED CAUSES |
|---|---|---|
| Bleeding associated with uterine pathology | Heavy bleeding, regular cycle (menorrhagia) | Endometrial polyps Adenomyosis Uterine fibroids Endometrial hyperplasia Uterine cancer |
| Dysfunctional uterine bleeding (DUB) without anatomic abnormalities | Heavy bleeding, irregular cycle (menometrorrhagia) | Polycystic ovarian syndrome Hypothalamic dysfunction
|
| Bleeding with a systemic illness | Usually menometrorrhagia | Thyroid dysfunction Elevated prolactin levels Liver or renal disease Coagulopathy Leukemia |
| Iatrogenic bleeding | Usually menometrorrhagia | Oral contraceptives Depot medroxyprogesterone acetate Postmenopausal hormone therapy Anticoagulants Herbal supplements |
Refer for a look inside the uterus
Patients with a pelvic exam that is inconclusive or suggests an enlarged uterus will likely benefit from referral for transvaginal sonography. This procedure is considered by many to be the test of choice for abnormal uterine bleeding.6 Saline infusion vaginal sonography, however, is considered a more sensitive test by some authorities.7 In saline infusion sonography, the clinician infuses a small amount of sterile saline into the uterus via a small catheter, which distends the normally compressed uterine walls and allows visualization of any endometrial cavity abnormalities, such as polyps or fibroids (FIGURE).
Office or outpatient hysteroscopy can also help visualize the endometrial cavity to diagnose cavity defects. Although hysteroscopy is an excellent and usually well-tolerated technique for visualizing the endometrial cavity, it cannot visualize the myometrium or ovaries as saline infusion sonography can. (TABLE 2) details the pros and cons of these diagnostic procedures.
FIGURE
What a difference saline can make
Routine transvaginal sonography shows the endometrium (calipers) without apparent abnormality. A previously undiagnosed endometrial polyp (calipers) in the same patient, revealed after saline infusion.
TABLE 2
Diagnostic studies: The pros, the cons
| STUDY | BENEFITS | POTENTIAL DRAWBACKS |
|---|---|---|
| Transvaginal sonography |
| May miss small or flaccid lesions (polyps) |
| Saline infusion vaginal sonography |
|
|
| Hysteroscopy |
|
|
What’s to be done?
In many cases clinicians can direct a plan of care on the basis of an accurate diagnosis. For example, patients with endometrial polyps or submucous uterine fibroids will benefit from referral to a gynecologist for outpatient surgical intervention. Otherwise, a variety of medical or minimally invasive surgical options are available.
Patients unaware of other options may come in asking about a hysterectomy, the second most common surgical procedure in the United States.8 Although this procedure is the definitive treatment for abnormal uterine bleeding, it carries the risk of surgical bleeding, ureteral or intestinal damage, incision breakdown, venous thromboembolism, and other intra- and postoperative problems.
While it is certainly appropriate to counsel the patient that hysterectomy is an option, there are many other options to consider. We now have a number of randomized trials that provide evidence-based guidance for the management of chronic abnormal uterine bleeding without hysterectomy (TABLE 3). These options can allow the patient to avoid the risks of major surgery and return to work and normal activities more rapidly.
TABLE 3
Beyond hysterectomy: Other treatment options to consider
| TREATMENT | COMMON REGIMENS | SOR | COMMENTS |
|---|---|---|---|
| Combined oral contraceptives9,12 | Cyclic or daily | A, B |
|
| Cyclic progestins9 |
| A | Side effects include spotting, weight gain, nausea, edema, and exacerbation of depression |
| NSAIDs14 | Mefenamic acid (Ponstel) 500 mg orally TID during menses; ibuprofen 800 mg orally TID during menses | A |
|
| Levonorgestrel IUS9,10 | Device provides 20 mcg/24 hours continuously for 5 years | A |
|
| Global endometrial ablation15,16 | Office or outpatient procedure | A |
|
| IUS, intrauterine system; NSAID, nonsteroidal anti-inflammatory drug. | |||
| Strength of recommendation (SOR) A. Good-quality patient-oriented evidence | |||
First choice: LNG-IUS
The levonorgestrel intrauterine system (LNG-IUS) appears to be the most effective medical therapy for treating menorrhagia, with studies showing a 94% reduction in menstrual blood loss.9 The LNG-IUS secretes a small amount of levonorgestrel, which acts locally to keep the endometrium from proliferating. The IUS has not been compared with placebo or no treatment, but it has been found to be more successful than 21-day progestin therapy and somewhat less successful than balloon endometrial ablation.10
An interesting study suggested that women given the choice between an LNG-IUS and hysterectomy will choose the intrauterine system rather than undergo invasive surgery. In this study, researchers randomized 56 women waiting to undergo hysterectomy for heavy menstrual bleeding to either continuation of their existing medical treatment or an LNG-IUS. At 6 months, 64% of the women in the IUS group canceled their hysterectomy, whereas only 14% in the medical therapy group did so.11
IUS candidates should have a uterus free of congenital abnormalities that measures between 6 and 9 centimeters by uterine sound, and be at low risk for sexually transmitted infections. Patients who have never been pregnant may use the LNG-IUS. Side effects of the LNGIUS may include irregular menses, amenorrhea, pelvic inflammatory disease, and uterine perforation at insertion.
Hormonal therapy is another option
Various types of hormonal therapy are also effective options for treating abnormal menstrual bleeding.
Combined oral contraceptives (COCs). Although many clinicians use COCs to treat menorrhagia, data supporting this indication are actually limited.12 COCs are proven to reduce mean menstrual blood loss but have not been well evaluated for patients who complain of heavy menstrual bleeding.9
All COCs contain relatively more progestin than estrogen, which benefits patients with abnormal uterine bleeding by thinning the uterine lining, leading to less menstrual blood loss. Newer types of oral contraceptives, such as lower dose pills or extended cycle formulations where patients take 3 months of pills before having a menstrual cycle, have not been studied for treatment of heavy menstrual bleeding.
Progestin alone. There are several formulations of progestins, including intramuscular injections, oral preparations, vaginal suppositories, creams, and the LNG-IUS. Although there are many potential oral progestin regimens—for example, a 10-day course vs a 21-day course—it appears that the 21-day regimen is most effective. Analysis of several trials shows that bleeding was reduced in 86% of women using a 21-day course of oral progestins.9
Possible side effects include spotting, weight gain, peripheral edema, and exacerbation of depression. Depot-medroxyprogesterone acetate is an injectable progestin that may cause abnormal bleeding or amenorrhea. It has not been studied as a treatment for heavy menstrual bleeding.
Danazol therapy. Danazol is a synthetic steroid that opposes progesterone and estrogen, leading to endometrial atrophy. Although possibly effective for reducing blood loss, danazol has lost favor due to androgenic side effects such as acne, weight gain, and voice deepening.13
NSAIDs reduce bleeding
Although NSAIDs are associated with gastrointestinal bleeding, their effect on uterine bleeding is different. At pharmacologic doses, NSAIDs reduce uterine bleeding. These drugs appear to slow uterine bleeding by helping to constrict the uterine vasculature, reduce prostaglandins, and improve platelet aggregation.14 Several studies show successful use of NSAIDs for abnormal uterine bleeding. A meta-analysis of NSAID therapy concluded that about half of the menorrhagia patients studied benefitted, and these patients had about a 30% reduction in blood loss.9 Mefenamic acid (Ponstel) is the only NSAID currently approved by the US Food and Drug Administration for treating menorrhagia, although all NSAIDs are likely effective.
Consider minimally invasive surgery
The term global endometrial ablation refers to a group of minimally invasive, outpatient procedures designed to destroy the endometrial lining, leading to either reduced bleeding or amenorrhea. These procedures are less invasive than hysterectomy and are best suited for patients with abnormal uterine bleeding who do not have other uterine abnormalities, such as prolapse, dyspareunia, or painful fibroids.
Examples of global ablation procedures include microwave ablation, cryotherapy, thermal balloon ablation, bipolar radiofrequency ablation, and hydrothermal ablation. Some OB/GYNs perform these procedures in the office.
Ablation procedures have some drawbacks. None of the ablation procedures can guarantee complete amenorrhea. Published amenorrhea rates range from 14% to 55%.15 Outpatient ablation procedures are relatively fast (usually under 1 hour) and allow the patient to return to normal activity quickly. There is also a cost advantage: endometrial ablation may be 80% less expensive than hysterectomy.
Not all patients who undergo these procedures remain satisfied with the outcome, however. One study concluded that some patients undergoing hysterectomy were more satisfied after 4 years than those who had an endometrial ablation. Thus, you should counsel patients that they may need to return later for more definitive therapy.16 In addition, because the uterine environment after ablation is not hospitable to fetal development, you’ll want to caution patients that future childbearing is contraindicated and advise them to undergo some type of permanent sterilization procedure.
Uterine artery embolization (UAE). This interventional radiology technique uses embolic particles placed via the femoral artery to occlude the blood supply to the uterine arteries. The procedure is most commonly used to treat heavy bleeding from uterine fibroids, gynecologic malignancies, or postpartum hemorrhage. Although useful for treating heavy menstrual bleeding from uterine fibroids, this technique has not been fully evaluated as a treatment option for heavy menstrual bleeding caused by other disorders.17
Ms. M keeps her uterus
Because Ms. M is older than 35, your first step is a brief, office endometrial pipelle biopsy. The results indicate a benign, proliferative endometrium. You discuss the various treatment options with your patient, and she decides on an LNG-IUS. Her urine pregnancy test is negative, and you place the IUS uneventfully on the last day of her next menses.
Five months later she has amenorrhea, except for rare spotting, and is symptom-free. Her hemoglobin returns to normal levels using oral iron therapy. She is extremely pleased that she was finally able to get some relief without disrupting her busy schedule or undergoing major surgery.
Correspondence
D. Ashley Hill, MD, Associate Director, Dept. of Obstetrics and Gynecology, Florida Hospital Orlando & Loch Haven OB/Gyn Group, 235 Princeton Street, Suite 200, Orlando, FL 32804; d.ashley.hill.md.flhosp.org
1. Nicholson WK, Ellison SA, Grason H, et al. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Obstet Gynecol. 2001;184:523-530.
2. Wilcox A, Baird DD, Dunson D, et al. Natural limits of pregnancy testing in relation to the expected menstrual period. JAMA. 2001;286:1759-1761.
3. Ely JW, Kennedy CM, Clark EC, et al. Abnormal uterine bleeding: a management algorithm. J Am Board Fam Med. 2006;19:590-602.
4. Dijkhuizen F, Mol B, Brölmann H, et al. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia—a meta-analysis. Cancer. 2000;89:1765-1762.
5. Bayer SR, DeCherney AH. Clinical manifestations and treatment of dysfunctional uterine bleeding. JAMA. 1993;269:1823-1828.
6. Oehler MK, Rees MC. Menorrhagia: an update. Acta Obstet Gynecol Scand. 2003;82:405-422.
7. Hill DA. Abnormal uterine bleeding. In: Henningsen C, ed. Clinical Guide to Ultrasonography. St. Louis, Mo: Mosby; 2004:173-196.
8. Keshavarz H, Hillis SD, Kieke BA, et al. Hysterectomy surveillance—United States, 1994–1999. MMWR CDC Surveill Summ. 2002;51(SS-5):1-8.
9. Working Party for Guidelines for the Management of Heavy Menstrual Bleeding. An evidence-based guideline for the management of heavy menstrual bleeding. N Z Med J. 1999;112:174-177.
10. Lethaby AE, Cooke I, Rees M. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
11. Lähteenmäki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316:1122-1126.
12. American College of Obstetricians and Gynecologists (ACOG). Management of anovulatory bleeding. Washington, DC: ACOG; 2000 Mar. 9. ACOG practice bulletin; no. 14.
13. Beaumont H, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2007;(3):CD001017.-
14. Dawood MY. Nonsteroidal antiinflammatory drugs and reproduction. Am J Obstet Gynecol. 1993;169:1255-1265.
15. Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006;108:990-1003.
16. Lethaby A, Shepperd S, Cooke I, et al. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD000329.-
17. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
- The levonorgestrel-IUS is the most effective treatment for heavy menstrual bleeding, reducing blood loss by close to 100% (A).
- Endometrial ablation is an effective treatment for women who want to avoid major surgery and preserve their uterus, but have no wish to become pregnant in the future (A).
- Endometrial biopsy should be part of the evaluation of abnormal uterine bleeding in all women over age 35 (B).
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Ms. M, a 39-year-old mother of 3, runs a busy day-care center, cares for her sick mother, and shuttles her children among their myriad activities. During today’s office visit, she seems anxious. She says her periods are regular, but have become increasingly heavy in recent years and may last as long as 9 days. The bleeding is very heavy, with a lot of clots and some cramps during the first few days. In the past year, painful periods have caused her to miss work on several occasions. She says she often feels tired, and worries that she may be anemic. Preserving fertility is not a concern; her husband has had a vasectomy.
On exam she is without orthostasis and appears well. Her uterus is top-normal size, nontender, and there are no adnexal masses or cervical or vaginal abnormalities. You note a normal Pap at your office 5 months ago. Her office hemoglobin is 9.8 mg/dL.
She asks you to refer her to a gynecologist for a hysterectomy because she “just can’t take it anymore.” Her heavy menses are disrupting her life. Since she does not want any more children, she feels that if someone could “just take it out,” her problem would be solved. But she isn’t really enthusiastic about a hysterectomy because her life is too busy to allow time for a lengthy recovery.
You explain that there are a number of options you’d like her to consider first. Then you review the options and some of the research behind them, having recently read an article on evidence-based therapy for abnormal uterine bleeding.
A common complaint in primary care
Abnormal uterine bleeding is a common reason for women to visit their primary care clinician, accounting for about 20% of gynecologic primary care visits.1 Women are understandably concerned about any disruption of their normal bleeding pattern. Many, however, are unaware of common causes of abnormal bleeding and available treatment options.
Most cases of chronic abnormal bleeding can be classified as either heavy and regular (menorrhagia) or heavy and irregular (menometrorrhagia). A rule of thumb to help guide diagnostic testing is that menorrhagia often results from anatomic problems of the uterus or endometrium, such as polyps. Menometrorrhagia is more likely to result from hormonal abnormalities, such as polycystic ovarian syndrome (PCOS). This review will focus on medical and minimally invasive surgical therapy for chronic abnormal bleeding.
First step: Pregnancy test
Many conditions can cause abnormal uterine bleeding (TABLE 1). In women of reproductive age, the first step in the diagnostic process should be a urine or serum pregnancy test. Urine pregnancy tests have a sensitivity of 90% one day after a missed period and approximately 97% after one week.2 In addition to a pregnancy test, consider testing for thyroid dysfunction and obtaining serum prolactin levels for women presenting with anovulatory bleeding.3 If anemia is suspected, a hematocrit or hemogram is indicated. All patients over the age of 35 who present with abnormal uterine bleeding should have an office endometrial biopsy to rule out endometrial hyperplasia or cancer.4
Is she ovulating? Most cases of heavy bleeding with irregular periods are the result of anovulation, which is common soon after menarche and at the approach of menopause. Other causes of anovulatory bleeding include PCOS, hypothyroidism, and elevated prolactin levels. Chronic, irregular bleeding without a known anatomic cause is termed “dysfunctional uterine bleeding,” or DUB.5
Are periods regular? Heavy bleeding with a regular menstrual cycle (ovulatory bleeding) usually has a different etiology. It most often occurs because of anatomic abnormalities such as endometrial polyps, fibroids, and adenomyosis (pockets of endometrium found within the uterine myometrium). Heavy menstrual bleeding that occurs at the onset of or shortly after menarche may be due to a coagulopathy, such as von Willebrand disease. Therefore, consider platelet function analysis for adolescents who present with heavy menses, particularly if they require blood transfusion.
What else do you observe? When evaluating patients with abnormal uterine bleeding, don’t let the obvious focus on the gynecologic organs cause you to overlook other possibly significant findings. Look for acanthosis nigrican and an elevated body mass index (BMI), signs of PCOS, and also check for evidence of hyperthyroidism or galactorrhea. The bimanual exam should determine if the patient has an enlarged uterus, suggesting fibroids or adenomyosis.
TABLE 1
Abnormal uterine bleeding: 4 categories, many causes
| CATEGORY | MOST COMMON TYPE OF BLEEDING | SELECTED CAUSES |
|---|---|---|
| Bleeding associated with uterine pathology | Heavy bleeding, regular cycle (menorrhagia) | Endometrial polyps Adenomyosis Uterine fibroids Endometrial hyperplasia Uterine cancer |
| Dysfunctional uterine bleeding (DUB) without anatomic abnormalities | Heavy bleeding, irregular cycle (menometrorrhagia) | Polycystic ovarian syndrome Hypothalamic dysfunction
|
| Bleeding with a systemic illness | Usually menometrorrhagia | Thyroid dysfunction Elevated prolactin levels Liver or renal disease Coagulopathy Leukemia |
| Iatrogenic bleeding | Usually menometrorrhagia | Oral contraceptives Depot medroxyprogesterone acetate Postmenopausal hormone therapy Anticoagulants Herbal supplements |
Refer for a look inside the uterus
Patients with a pelvic exam that is inconclusive or suggests an enlarged uterus will likely benefit from referral for transvaginal sonography. This procedure is considered by many to be the test of choice for abnormal uterine bleeding.6 Saline infusion vaginal sonography, however, is considered a more sensitive test by some authorities.7 In saline infusion sonography, the clinician infuses a small amount of sterile saline into the uterus via a small catheter, which distends the normally compressed uterine walls and allows visualization of any endometrial cavity abnormalities, such as polyps or fibroids (FIGURE).
Office or outpatient hysteroscopy can also help visualize the endometrial cavity to diagnose cavity defects. Although hysteroscopy is an excellent and usually well-tolerated technique for visualizing the endometrial cavity, it cannot visualize the myometrium or ovaries as saline infusion sonography can. (TABLE 2) details the pros and cons of these diagnostic procedures.
FIGURE
What a difference saline can make
Routine transvaginal sonography shows the endometrium (calipers) without apparent abnormality. A previously undiagnosed endometrial polyp (calipers) in the same patient, revealed after saline infusion.
TABLE 2
Diagnostic studies: The pros, the cons
| STUDY | BENEFITS | POTENTIAL DRAWBACKS |
|---|---|---|
| Transvaginal sonography |
| May miss small or flaccid lesions (polyps) |
| Saline infusion vaginal sonography |
|
|
| Hysteroscopy |
|
|
What’s to be done?
In many cases clinicians can direct a plan of care on the basis of an accurate diagnosis. For example, patients with endometrial polyps or submucous uterine fibroids will benefit from referral to a gynecologist for outpatient surgical intervention. Otherwise, a variety of medical or minimally invasive surgical options are available.
Patients unaware of other options may come in asking about a hysterectomy, the second most common surgical procedure in the United States.8 Although this procedure is the definitive treatment for abnormal uterine bleeding, it carries the risk of surgical bleeding, ureteral or intestinal damage, incision breakdown, venous thromboembolism, and other intra- and postoperative problems.
While it is certainly appropriate to counsel the patient that hysterectomy is an option, there are many other options to consider. We now have a number of randomized trials that provide evidence-based guidance for the management of chronic abnormal uterine bleeding without hysterectomy (TABLE 3). These options can allow the patient to avoid the risks of major surgery and return to work and normal activities more rapidly.
TABLE 3
Beyond hysterectomy: Other treatment options to consider
| TREATMENT | COMMON REGIMENS | SOR | COMMENTS |
|---|---|---|---|
| Combined oral contraceptives9,12 | Cyclic or daily | A, B |
|
| Cyclic progestins9 |
| A | Side effects include spotting, weight gain, nausea, edema, and exacerbation of depression |
| NSAIDs14 | Mefenamic acid (Ponstel) 500 mg orally TID during menses; ibuprofen 800 mg orally TID during menses | A |
|
| Levonorgestrel IUS9,10 | Device provides 20 mcg/24 hours continuously for 5 years | A |
|
| Global endometrial ablation15,16 | Office or outpatient procedure | A |
|
| IUS, intrauterine system; NSAID, nonsteroidal anti-inflammatory drug. | |||
| Strength of recommendation (SOR) A. Good-quality patient-oriented evidence | |||
First choice: LNG-IUS
The levonorgestrel intrauterine system (LNG-IUS) appears to be the most effective medical therapy for treating menorrhagia, with studies showing a 94% reduction in menstrual blood loss.9 The LNG-IUS secretes a small amount of levonorgestrel, which acts locally to keep the endometrium from proliferating. The IUS has not been compared with placebo or no treatment, but it has been found to be more successful than 21-day progestin therapy and somewhat less successful than balloon endometrial ablation.10
An interesting study suggested that women given the choice between an LNG-IUS and hysterectomy will choose the intrauterine system rather than undergo invasive surgery. In this study, researchers randomized 56 women waiting to undergo hysterectomy for heavy menstrual bleeding to either continuation of their existing medical treatment or an LNG-IUS. At 6 months, 64% of the women in the IUS group canceled their hysterectomy, whereas only 14% in the medical therapy group did so.11
IUS candidates should have a uterus free of congenital abnormalities that measures between 6 and 9 centimeters by uterine sound, and be at low risk for sexually transmitted infections. Patients who have never been pregnant may use the LNG-IUS. Side effects of the LNGIUS may include irregular menses, amenorrhea, pelvic inflammatory disease, and uterine perforation at insertion.
Hormonal therapy is another option
Various types of hormonal therapy are also effective options for treating abnormal menstrual bleeding.
Combined oral contraceptives (COCs). Although many clinicians use COCs to treat menorrhagia, data supporting this indication are actually limited.12 COCs are proven to reduce mean menstrual blood loss but have not been well evaluated for patients who complain of heavy menstrual bleeding.9
All COCs contain relatively more progestin than estrogen, which benefits patients with abnormal uterine bleeding by thinning the uterine lining, leading to less menstrual blood loss. Newer types of oral contraceptives, such as lower dose pills or extended cycle formulations where patients take 3 months of pills before having a menstrual cycle, have not been studied for treatment of heavy menstrual bleeding.
Progestin alone. There are several formulations of progestins, including intramuscular injections, oral preparations, vaginal suppositories, creams, and the LNG-IUS. Although there are many potential oral progestin regimens—for example, a 10-day course vs a 21-day course—it appears that the 21-day regimen is most effective. Analysis of several trials shows that bleeding was reduced in 86% of women using a 21-day course of oral progestins.9
Possible side effects include spotting, weight gain, peripheral edema, and exacerbation of depression. Depot-medroxyprogesterone acetate is an injectable progestin that may cause abnormal bleeding or amenorrhea. It has not been studied as a treatment for heavy menstrual bleeding.
Danazol therapy. Danazol is a synthetic steroid that opposes progesterone and estrogen, leading to endometrial atrophy. Although possibly effective for reducing blood loss, danazol has lost favor due to androgenic side effects such as acne, weight gain, and voice deepening.13
NSAIDs reduce bleeding
Although NSAIDs are associated with gastrointestinal bleeding, their effect on uterine bleeding is different. At pharmacologic doses, NSAIDs reduce uterine bleeding. These drugs appear to slow uterine bleeding by helping to constrict the uterine vasculature, reduce prostaglandins, and improve platelet aggregation.14 Several studies show successful use of NSAIDs for abnormal uterine bleeding. A meta-analysis of NSAID therapy concluded that about half of the menorrhagia patients studied benefitted, and these patients had about a 30% reduction in blood loss.9 Mefenamic acid (Ponstel) is the only NSAID currently approved by the US Food and Drug Administration for treating menorrhagia, although all NSAIDs are likely effective.
Consider minimally invasive surgery
The term global endometrial ablation refers to a group of minimally invasive, outpatient procedures designed to destroy the endometrial lining, leading to either reduced bleeding or amenorrhea. These procedures are less invasive than hysterectomy and are best suited for patients with abnormal uterine bleeding who do not have other uterine abnormalities, such as prolapse, dyspareunia, or painful fibroids.
Examples of global ablation procedures include microwave ablation, cryotherapy, thermal balloon ablation, bipolar radiofrequency ablation, and hydrothermal ablation. Some OB/GYNs perform these procedures in the office.
Ablation procedures have some drawbacks. None of the ablation procedures can guarantee complete amenorrhea. Published amenorrhea rates range from 14% to 55%.15 Outpatient ablation procedures are relatively fast (usually under 1 hour) and allow the patient to return to normal activity quickly. There is also a cost advantage: endometrial ablation may be 80% less expensive than hysterectomy.
Not all patients who undergo these procedures remain satisfied with the outcome, however. One study concluded that some patients undergoing hysterectomy were more satisfied after 4 years than those who had an endometrial ablation. Thus, you should counsel patients that they may need to return later for more definitive therapy.16 In addition, because the uterine environment after ablation is not hospitable to fetal development, you’ll want to caution patients that future childbearing is contraindicated and advise them to undergo some type of permanent sterilization procedure.
Uterine artery embolization (UAE). This interventional radiology technique uses embolic particles placed via the femoral artery to occlude the blood supply to the uterine arteries. The procedure is most commonly used to treat heavy bleeding from uterine fibroids, gynecologic malignancies, or postpartum hemorrhage. Although useful for treating heavy menstrual bleeding from uterine fibroids, this technique has not been fully evaluated as a treatment option for heavy menstrual bleeding caused by other disorders.17
Ms. M keeps her uterus
Because Ms. M is older than 35, your first step is a brief, office endometrial pipelle biopsy. The results indicate a benign, proliferative endometrium. You discuss the various treatment options with your patient, and she decides on an LNG-IUS. Her urine pregnancy test is negative, and you place the IUS uneventfully on the last day of her next menses.
Five months later she has amenorrhea, except for rare spotting, and is symptom-free. Her hemoglobin returns to normal levels using oral iron therapy. She is extremely pleased that she was finally able to get some relief without disrupting her busy schedule or undergoing major surgery.
Correspondence
D. Ashley Hill, MD, Associate Director, Dept. of Obstetrics and Gynecology, Florida Hospital Orlando & Loch Haven OB/Gyn Group, 235 Princeton Street, Suite 200, Orlando, FL 32804; d.ashley.hill.md.flhosp.org
- The levonorgestrel-IUS is the most effective treatment for heavy menstrual bleeding, reducing blood loss by close to 100% (A).
- Endometrial ablation is an effective treatment for women who want to avoid major surgery and preserve their uterus, but have no wish to become pregnant in the future (A).
- Endometrial biopsy should be part of the evaluation of abnormal uterine bleeding in all women over age 35 (B).
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Ms. M, a 39-year-old mother of 3, runs a busy day-care center, cares for her sick mother, and shuttles her children among their myriad activities. During today’s office visit, she seems anxious. She says her periods are regular, but have become increasingly heavy in recent years and may last as long as 9 days. The bleeding is very heavy, with a lot of clots and some cramps during the first few days. In the past year, painful periods have caused her to miss work on several occasions. She says she often feels tired, and worries that she may be anemic. Preserving fertility is not a concern; her husband has had a vasectomy.
On exam she is without orthostasis and appears well. Her uterus is top-normal size, nontender, and there are no adnexal masses or cervical or vaginal abnormalities. You note a normal Pap at your office 5 months ago. Her office hemoglobin is 9.8 mg/dL.
She asks you to refer her to a gynecologist for a hysterectomy because she “just can’t take it anymore.” Her heavy menses are disrupting her life. Since she does not want any more children, she feels that if someone could “just take it out,” her problem would be solved. But she isn’t really enthusiastic about a hysterectomy because her life is too busy to allow time for a lengthy recovery.
You explain that there are a number of options you’d like her to consider first. Then you review the options and some of the research behind them, having recently read an article on evidence-based therapy for abnormal uterine bleeding.
A common complaint in primary care
Abnormal uterine bleeding is a common reason for women to visit their primary care clinician, accounting for about 20% of gynecologic primary care visits.1 Women are understandably concerned about any disruption of their normal bleeding pattern. Many, however, are unaware of common causes of abnormal bleeding and available treatment options.
Most cases of chronic abnormal bleeding can be classified as either heavy and regular (menorrhagia) or heavy and irregular (menometrorrhagia). A rule of thumb to help guide diagnostic testing is that menorrhagia often results from anatomic problems of the uterus or endometrium, such as polyps. Menometrorrhagia is more likely to result from hormonal abnormalities, such as polycystic ovarian syndrome (PCOS). This review will focus on medical and minimally invasive surgical therapy for chronic abnormal bleeding.
First step: Pregnancy test
Many conditions can cause abnormal uterine bleeding (TABLE 1). In women of reproductive age, the first step in the diagnostic process should be a urine or serum pregnancy test. Urine pregnancy tests have a sensitivity of 90% one day after a missed period and approximately 97% after one week.2 In addition to a pregnancy test, consider testing for thyroid dysfunction and obtaining serum prolactin levels for women presenting with anovulatory bleeding.3 If anemia is suspected, a hematocrit or hemogram is indicated. All patients over the age of 35 who present with abnormal uterine bleeding should have an office endometrial biopsy to rule out endometrial hyperplasia or cancer.4
Is she ovulating? Most cases of heavy bleeding with irregular periods are the result of anovulation, which is common soon after menarche and at the approach of menopause. Other causes of anovulatory bleeding include PCOS, hypothyroidism, and elevated prolactin levels. Chronic, irregular bleeding without a known anatomic cause is termed “dysfunctional uterine bleeding,” or DUB.5
Are periods regular? Heavy bleeding with a regular menstrual cycle (ovulatory bleeding) usually has a different etiology. It most often occurs because of anatomic abnormalities such as endometrial polyps, fibroids, and adenomyosis (pockets of endometrium found within the uterine myometrium). Heavy menstrual bleeding that occurs at the onset of or shortly after menarche may be due to a coagulopathy, such as von Willebrand disease. Therefore, consider platelet function analysis for adolescents who present with heavy menses, particularly if they require blood transfusion.
What else do you observe? When evaluating patients with abnormal uterine bleeding, don’t let the obvious focus on the gynecologic organs cause you to overlook other possibly significant findings. Look for acanthosis nigrican and an elevated body mass index (BMI), signs of PCOS, and also check for evidence of hyperthyroidism or galactorrhea. The bimanual exam should determine if the patient has an enlarged uterus, suggesting fibroids or adenomyosis.
TABLE 1
Abnormal uterine bleeding: 4 categories, many causes
| CATEGORY | MOST COMMON TYPE OF BLEEDING | SELECTED CAUSES |
|---|---|---|
| Bleeding associated with uterine pathology | Heavy bleeding, regular cycle (menorrhagia) | Endometrial polyps Adenomyosis Uterine fibroids Endometrial hyperplasia Uterine cancer |
| Dysfunctional uterine bleeding (DUB) without anatomic abnormalities | Heavy bleeding, irregular cycle (menometrorrhagia) | Polycystic ovarian syndrome Hypothalamic dysfunction
|
| Bleeding with a systemic illness | Usually menometrorrhagia | Thyroid dysfunction Elevated prolactin levels Liver or renal disease Coagulopathy Leukemia |
| Iatrogenic bleeding | Usually menometrorrhagia | Oral contraceptives Depot medroxyprogesterone acetate Postmenopausal hormone therapy Anticoagulants Herbal supplements |
Refer for a look inside the uterus
Patients with a pelvic exam that is inconclusive or suggests an enlarged uterus will likely benefit from referral for transvaginal sonography. This procedure is considered by many to be the test of choice for abnormal uterine bleeding.6 Saline infusion vaginal sonography, however, is considered a more sensitive test by some authorities.7 In saline infusion sonography, the clinician infuses a small amount of sterile saline into the uterus via a small catheter, which distends the normally compressed uterine walls and allows visualization of any endometrial cavity abnormalities, such as polyps or fibroids (FIGURE).
Office or outpatient hysteroscopy can also help visualize the endometrial cavity to diagnose cavity defects. Although hysteroscopy is an excellent and usually well-tolerated technique for visualizing the endometrial cavity, it cannot visualize the myometrium or ovaries as saline infusion sonography can. (TABLE 2) details the pros and cons of these diagnostic procedures.
FIGURE
What a difference saline can make
Routine transvaginal sonography shows the endometrium (calipers) without apparent abnormality. A previously undiagnosed endometrial polyp (calipers) in the same patient, revealed after saline infusion.
TABLE 2
Diagnostic studies: The pros, the cons
| STUDY | BENEFITS | POTENTIAL DRAWBACKS |
|---|---|---|
| Transvaginal sonography |
| May miss small or flaccid lesions (polyps) |
| Saline infusion vaginal sonography |
|
|
| Hysteroscopy |
|
|
What’s to be done?
In many cases clinicians can direct a plan of care on the basis of an accurate diagnosis. For example, patients with endometrial polyps or submucous uterine fibroids will benefit from referral to a gynecologist for outpatient surgical intervention. Otherwise, a variety of medical or minimally invasive surgical options are available.
Patients unaware of other options may come in asking about a hysterectomy, the second most common surgical procedure in the United States.8 Although this procedure is the definitive treatment for abnormal uterine bleeding, it carries the risk of surgical bleeding, ureteral or intestinal damage, incision breakdown, venous thromboembolism, and other intra- and postoperative problems.
While it is certainly appropriate to counsel the patient that hysterectomy is an option, there are many other options to consider. We now have a number of randomized trials that provide evidence-based guidance for the management of chronic abnormal uterine bleeding without hysterectomy (TABLE 3). These options can allow the patient to avoid the risks of major surgery and return to work and normal activities more rapidly.
TABLE 3
Beyond hysterectomy: Other treatment options to consider
| TREATMENT | COMMON REGIMENS | SOR | COMMENTS |
|---|---|---|---|
| Combined oral contraceptives9,12 | Cyclic or daily | A, B |
|
| Cyclic progestins9 |
| A | Side effects include spotting, weight gain, nausea, edema, and exacerbation of depression |
| NSAIDs14 | Mefenamic acid (Ponstel) 500 mg orally TID during menses; ibuprofen 800 mg orally TID during menses | A |
|
| Levonorgestrel IUS9,10 | Device provides 20 mcg/24 hours continuously for 5 years | A |
|
| Global endometrial ablation15,16 | Office or outpatient procedure | A |
|
| IUS, intrauterine system; NSAID, nonsteroidal anti-inflammatory drug. | |||
| Strength of recommendation (SOR) A. Good-quality patient-oriented evidence | |||
First choice: LNG-IUS
The levonorgestrel intrauterine system (LNG-IUS) appears to be the most effective medical therapy for treating menorrhagia, with studies showing a 94% reduction in menstrual blood loss.9 The LNG-IUS secretes a small amount of levonorgestrel, which acts locally to keep the endometrium from proliferating. The IUS has not been compared with placebo or no treatment, but it has been found to be more successful than 21-day progestin therapy and somewhat less successful than balloon endometrial ablation.10
An interesting study suggested that women given the choice between an LNG-IUS and hysterectomy will choose the intrauterine system rather than undergo invasive surgery. In this study, researchers randomized 56 women waiting to undergo hysterectomy for heavy menstrual bleeding to either continuation of their existing medical treatment or an LNG-IUS. At 6 months, 64% of the women in the IUS group canceled their hysterectomy, whereas only 14% in the medical therapy group did so.11
IUS candidates should have a uterus free of congenital abnormalities that measures between 6 and 9 centimeters by uterine sound, and be at low risk for sexually transmitted infections. Patients who have never been pregnant may use the LNG-IUS. Side effects of the LNGIUS may include irregular menses, amenorrhea, pelvic inflammatory disease, and uterine perforation at insertion.
Hormonal therapy is another option
Various types of hormonal therapy are also effective options for treating abnormal menstrual bleeding.
Combined oral contraceptives (COCs). Although many clinicians use COCs to treat menorrhagia, data supporting this indication are actually limited.12 COCs are proven to reduce mean menstrual blood loss but have not been well evaluated for patients who complain of heavy menstrual bleeding.9
All COCs contain relatively more progestin than estrogen, which benefits patients with abnormal uterine bleeding by thinning the uterine lining, leading to less menstrual blood loss. Newer types of oral contraceptives, such as lower dose pills or extended cycle formulations where patients take 3 months of pills before having a menstrual cycle, have not been studied for treatment of heavy menstrual bleeding.
Progestin alone. There are several formulations of progestins, including intramuscular injections, oral preparations, vaginal suppositories, creams, and the LNG-IUS. Although there are many potential oral progestin regimens—for example, a 10-day course vs a 21-day course—it appears that the 21-day regimen is most effective. Analysis of several trials shows that bleeding was reduced in 86% of women using a 21-day course of oral progestins.9
Possible side effects include spotting, weight gain, peripheral edema, and exacerbation of depression. Depot-medroxyprogesterone acetate is an injectable progestin that may cause abnormal bleeding or amenorrhea. It has not been studied as a treatment for heavy menstrual bleeding.
Danazol therapy. Danazol is a synthetic steroid that opposes progesterone and estrogen, leading to endometrial atrophy. Although possibly effective for reducing blood loss, danazol has lost favor due to androgenic side effects such as acne, weight gain, and voice deepening.13
NSAIDs reduce bleeding
Although NSAIDs are associated with gastrointestinal bleeding, their effect on uterine bleeding is different. At pharmacologic doses, NSAIDs reduce uterine bleeding. These drugs appear to slow uterine bleeding by helping to constrict the uterine vasculature, reduce prostaglandins, and improve platelet aggregation.14 Several studies show successful use of NSAIDs for abnormal uterine bleeding. A meta-analysis of NSAID therapy concluded that about half of the menorrhagia patients studied benefitted, and these patients had about a 30% reduction in blood loss.9 Mefenamic acid (Ponstel) is the only NSAID currently approved by the US Food and Drug Administration for treating menorrhagia, although all NSAIDs are likely effective.
Consider minimally invasive surgery
The term global endometrial ablation refers to a group of minimally invasive, outpatient procedures designed to destroy the endometrial lining, leading to either reduced bleeding or amenorrhea. These procedures are less invasive than hysterectomy and are best suited for patients with abnormal uterine bleeding who do not have other uterine abnormalities, such as prolapse, dyspareunia, or painful fibroids.
Examples of global ablation procedures include microwave ablation, cryotherapy, thermal balloon ablation, bipolar radiofrequency ablation, and hydrothermal ablation. Some OB/GYNs perform these procedures in the office.
Ablation procedures have some drawbacks. None of the ablation procedures can guarantee complete amenorrhea. Published amenorrhea rates range from 14% to 55%.15 Outpatient ablation procedures are relatively fast (usually under 1 hour) and allow the patient to return to normal activity quickly. There is also a cost advantage: endometrial ablation may be 80% less expensive than hysterectomy.
Not all patients who undergo these procedures remain satisfied with the outcome, however. One study concluded that some patients undergoing hysterectomy were more satisfied after 4 years than those who had an endometrial ablation. Thus, you should counsel patients that they may need to return later for more definitive therapy.16 In addition, because the uterine environment after ablation is not hospitable to fetal development, you’ll want to caution patients that future childbearing is contraindicated and advise them to undergo some type of permanent sterilization procedure.
Uterine artery embolization (UAE). This interventional radiology technique uses embolic particles placed via the femoral artery to occlude the blood supply to the uterine arteries. The procedure is most commonly used to treat heavy bleeding from uterine fibroids, gynecologic malignancies, or postpartum hemorrhage. Although useful for treating heavy menstrual bleeding from uterine fibroids, this technique has not been fully evaluated as a treatment option for heavy menstrual bleeding caused by other disorders.17
Ms. M keeps her uterus
Because Ms. M is older than 35, your first step is a brief, office endometrial pipelle biopsy. The results indicate a benign, proliferative endometrium. You discuss the various treatment options with your patient, and she decides on an LNG-IUS. Her urine pregnancy test is negative, and you place the IUS uneventfully on the last day of her next menses.
Five months later she has amenorrhea, except for rare spotting, and is symptom-free. Her hemoglobin returns to normal levels using oral iron therapy. She is extremely pleased that she was finally able to get some relief without disrupting her busy schedule or undergoing major surgery.
Correspondence
D. Ashley Hill, MD, Associate Director, Dept. of Obstetrics and Gynecology, Florida Hospital Orlando & Loch Haven OB/Gyn Group, 235 Princeton Street, Suite 200, Orlando, FL 32804; d.ashley.hill.md.flhosp.org
1. Nicholson WK, Ellison SA, Grason H, et al. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Obstet Gynecol. 2001;184:523-530.
2. Wilcox A, Baird DD, Dunson D, et al. Natural limits of pregnancy testing in relation to the expected menstrual period. JAMA. 2001;286:1759-1761.
3. Ely JW, Kennedy CM, Clark EC, et al. Abnormal uterine bleeding: a management algorithm. J Am Board Fam Med. 2006;19:590-602.
4. Dijkhuizen F, Mol B, Brölmann H, et al. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia—a meta-analysis. Cancer. 2000;89:1765-1762.
5. Bayer SR, DeCherney AH. Clinical manifestations and treatment of dysfunctional uterine bleeding. JAMA. 1993;269:1823-1828.
6. Oehler MK, Rees MC. Menorrhagia: an update. Acta Obstet Gynecol Scand. 2003;82:405-422.
7. Hill DA. Abnormal uterine bleeding. In: Henningsen C, ed. Clinical Guide to Ultrasonography. St. Louis, Mo: Mosby; 2004:173-196.
8. Keshavarz H, Hillis SD, Kieke BA, et al. Hysterectomy surveillance—United States, 1994–1999. MMWR CDC Surveill Summ. 2002;51(SS-5):1-8.
9. Working Party for Guidelines for the Management of Heavy Menstrual Bleeding. An evidence-based guideline for the management of heavy menstrual bleeding. N Z Med J. 1999;112:174-177.
10. Lethaby AE, Cooke I, Rees M. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
11. Lähteenmäki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316:1122-1126.
12. American College of Obstetricians and Gynecologists (ACOG). Management of anovulatory bleeding. Washington, DC: ACOG; 2000 Mar. 9. ACOG practice bulletin; no. 14.
13. Beaumont H, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2007;(3):CD001017.-
14. Dawood MY. Nonsteroidal antiinflammatory drugs and reproduction. Am J Obstet Gynecol. 1993;169:1255-1265.
15. Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006;108:990-1003.
16. Lethaby A, Shepperd S, Cooke I, et al. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD000329.-
17. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
1. Nicholson WK, Ellison SA, Grason H, et al. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Obstet Gynecol. 2001;184:523-530.
2. Wilcox A, Baird DD, Dunson D, et al. Natural limits of pregnancy testing in relation to the expected menstrual period. JAMA. 2001;286:1759-1761.
3. Ely JW, Kennedy CM, Clark EC, et al. Abnormal uterine bleeding: a management algorithm. J Am Board Fam Med. 2006;19:590-602.
4. Dijkhuizen F, Mol B, Brölmann H, et al. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia—a meta-analysis. Cancer. 2000;89:1765-1762.
5. Bayer SR, DeCherney AH. Clinical manifestations and treatment of dysfunctional uterine bleeding. JAMA. 1993;269:1823-1828.
6. Oehler MK, Rees MC. Menorrhagia: an update. Acta Obstet Gynecol Scand. 2003;82:405-422.
7. Hill DA. Abnormal uterine bleeding. In: Henningsen C, ed. Clinical Guide to Ultrasonography. St. Louis, Mo: Mosby; 2004:173-196.
8. Keshavarz H, Hillis SD, Kieke BA, et al. Hysterectomy surveillance—United States, 1994–1999. MMWR CDC Surveill Summ. 2002;51(SS-5):1-8.
9. Working Party for Guidelines for the Management of Heavy Menstrual Bleeding. An evidence-based guideline for the management of heavy menstrual bleeding. N Z Med J. 1999;112:174-177.
10. Lethaby AE, Cooke I, Rees M. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
11. Lähteenmäki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316:1122-1126.
12. American College of Obstetricians and Gynecologists (ACOG). Management of anovulatory bleeding. Washington, DC: ACOG; 2000 Mar. 9. ACOG practice bulletin; no. 14.
13. Beaumont H, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2007;(3):CD001017.-
14. Dawood MY. Nonsteroidal antiinflammatory drugs and reproduction. Am J Obstet Gynecol. 1993;169:1255-1265.
15. Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006;108:990-1003.
16. Lethaby A, Shepperd S, Cooke I, et al. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD000329.-
17. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.