User login
ACIP approves flu vaccine recommendations for 2020-2021
– Fluzone high-dose quadrivalent, which replaces the trivalent Fluzone high-dose and Fluad quadrivalent (Seqirus), according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
At a virtual meeting on June 24, the committee voted unanimously to approve the vaccine recommendations for annual influenza immunization of all individuals aged 6 months and older. They also voted to accept some guidance and language changes to the recommendations.
The past flu season was unique in its overlap with the emergence of the COVID-19 coronavirus, which likely contributed to a third peak in reported cases of influenza-like illness at approximately week 14 of last season, said Lisa Grohskopf, MD, of the CDC’s influenza division, who presented data on last year’s activity and the updates for next season.
The CDC estimates that 39,000,000-56,000,000 flu illnesses occurred in the United States from Oct. 1, 2019, to April 4, 2020, said Dr. Grohskopf. Estimates also suggest as many as 740,000 hospitalizations and 62,000 deaths related to the seasonal flu.
Preliminary results of vaccine effectiveness showed 39% overall for the 2019-2020 season, with more substantial protection against influenza B and lower protection against A/H1N1pmd09.
Vaccine safety data from the Vaccine Adverse Event Reporting System and Vaccine Safety Datalink showed no new safety concerns for any flu vaccine types used last year, Dr. Grohskopf noted.
Based on this information, three components (A/H1N1pdm09, A/H3N2, and B/Victoria) have been updated for the 2020-2021 vaccines, said Dr. Grohskopf. The egg-based influenza vaccines will include hemagglutinin derived from an A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09–like virus, an A/Hong Kong/2671/2019(H3N2)–like virus and a B/Washington/02/2019 (Victoria lineage)–like virus, and (for quadrivalent vaccines) a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Nonegg vaccines will contain hemagglutinin derived from an A/Hawaii/70/2019 (H1N1)pdm09–like virus, an A/Hong Kong/45/2019 (H3N2)–like virus, a B/Washington/02/2019 (Victoria lineage)–like virus, and a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
New guidance for next year’s flu season includes a change to the language in the contraindications and precautions table to simply read “Contraindications,” with more details in the text explaining package insert contraindications and ACIP recommendations, Dr. Grohskopf said. In addition, updated guidance clarifies that live-attenuated influenza vaccine quadravalents (LAIV4) should not be used in patients with cochlear implants, active cerebrospinal fluid leaks, and anatomical or functional asplenia, based on ACIP’s review of the latest evidence and the availability of alternative vaccines.
ACIP also updated guidance on the use of antivirals and LAIV4. Based on half-lives, language was added indicating that clinicians should assume interference if antivirals are given within certain intervals of LAIV4, Dr. Grohskopf explained. “Newer antivirals peramivir and baloxavir have longer half-lives than oseltamivir and zanamivir, and insufficient data are available on the use of LAIV4 in the setting of antiviral use.”
The ACIP members had no financial conflicts to disclose.
– Fluzone high-dose quadrivalent, which replaces the trivalent Fluzone high-dose and Fluad quadrivalent (Seqirus), according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
At a virtual meeting on June 24, the committee voted unanimously to approve the vaccine recommendations for annual influenza immunization of all individuals aged 6 months and older. They also voted to accept some guidance and language changes to the recommendations.
The past flu season was unique in its overlap with the emergence of the COVID-19 coronavirus, which likely contributed to a third peak in reported cases of influenza-like illness at approximately week 14 of last season, said Lisa Grohskopf, MD, of the CDC’s influenza division, who presented data on last year’s activity and the updates for next season.
The CDC estimates that 39,000,000-56,000,000 flu illnesses occurred in the United States from Oct. 1, 2019, to April 4, 2020, said Dr. Grohskopf. Estimates also suggest as many as 740,000 hospitalizations and 62,000 deaths related to the seasonal flu.
Preliminary results of vaccine effectiveness showed 39% overall for the 2019-2020 season, with more substantial protection against influenza B and lower protection against A/H1N1pmd09.
Vaccine safety data from the Vaccine Adverse Event Reporting System and Vaccine Safety Datalink showed no new safety concerns for any flu vaccine types used last year, Dr. Grohskopf noted.
Based on this information, three components (A/H1N1pdm09, A/H3N2, and B/Victoria) have been updated for the 2020-2021 vaccines, said Dr. Grohskopf. The egg-based influenza vaccines will include hemagglutinin derived from an A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09–like virus, an A/Hong Kong/2671/2019(H3N2)–like virus and a B/Washington/02/2019 (Victoria lineage)–like virus, and (for quadrivalent vaccines) a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Nonegg vaccines will contain hemagglutinin derived from an A/Hawaii/70/2019 (H1N1)pdm09–like virus, an A/Hong Kong/45/2019 (H3N2)–like virus, a B/Washington/02/2019 (Victoria lineage)–like virus, and a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
New guidance for next year’s flu season includes a change to the language in the contraindications and precautions table to simply read “Contraindications,” with more details in the text explaining package insert contraindications and ACIP recommendations, Dr. Grohskopf said. In addition, updated guidance clarifies that live-attenuated influenza vaccine quadravalents (LAIV4) should not be used in patients with cochlear implants, active cerebrospinal fluid leaks, and anatomical or functional asplenia, based on ACIP’s review of the latest evidence and the availability of alternative vaccines.
ACIP also updated guidance on the use of antivirals and LAIV4. Based on half-lives, language was added indicating that clinicians should assume interference if antivirals are given within certain intervals of LAIV4, Dr. Grohskopf explained. “Newer antivirals peramivir and baloxavir have longer half-lives than oseltamivir and zanamivir, and insufficient data are available on the use of LAIV4 in the setting of antiviral use.”
The ACIP members had no financial conflicts to disclose.
– Fluzone high-dose quadrivalent, which replaces the trivalent Fluzone high-dose and Fluad quadrivalent (Seqirus), according to the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
At a virtual meeting on June 24, the committee voted unanimously to approve the vaccine recommendations for annual influenza immunization of all individuals aged 6 months and older. They also voted to accept some guidance and language changes to the recommendations.
The past flu season was unique in its overlap with the emergence of the COVID-19 coronavirus, which likely contributed to a third peak in reported cases of influenza-like illness at approximately week 14 of last season, said Lisa Grohskopf, MD, of the CDC’s influenza division, who presented data on last year’s activity and the updates for next season.
The CDC estimates that 39,000,000-56,000,000 flu illnesses occurred in the United States from Oct. 1, 2019, to April 4, 2020, said Dr. Grohskopf. Estimates also suggest as many as 740,000 hospitalizations and 62,000 deaths related to the seasonal flu.
Preliminary results of vaccine effectiveness showed 39% overall for the 2019-2020 season, with more substantial protection against influenza B and lower protection against A/H1N1pmd09.
Vaccine safety data from the Vaccine Adverse Event Reporting System and Vaccine Safety Datalink showed no new safety concerns for any flu vaccine types used last year, Dr. Grohskopf noted.
Based on this information, three components (A/H1N1pdm09, A/H3N2, and B/Victoria) have been updated for the 2020-2021 vaccines, said Dr. Grohskopf. The egg-based influenza vaccines will include hemagglutinin derived from an A/Guangdong-Maonan/SWL1536/2019(H1N1)pdm09–like virus, an A/Hong Kong/2671/2019(H3N2)–like virus and a B/Washington/02/2019 (Victoria lineage)–like virus, and (for quadrivalent vaccines) a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Nonegg vaccines will contain hemagglutinin derived from an A/Hawaii/70/2019 (H1N1)pdm09–like virus, an A/Hong Kong/45/2019 (H3N2)–like virus, a B/Washington/02/2019 (Victoria lineage)–like virus, and a B/Phuket/3073/2013 (Yamagata lineage)–like virus.
New guidance for next year’s flu season includes a change to the language in the contraindications and precautions table to simply read “Contraindications,” with more details in the text explaining package insert contraindications and ACIP recommendations, Dr. Grohskopf said. In addition, updated guidance clarifies that live-attenuated influenza vaccine quadravalents (LAIV4) should not be used in patients with cochlear implants, active cerebrospinal fluid leaks, and anatomical or functional asplenia, based on ACIP’s review of the latest evidence and the availability of alternative vaccines.
ACIP also updated guidance on the use of antivirals and LAIV4. Based on half-lives, language was added indicating that clinicians should assume interference if antivirals are given within certain intervals of LAIV4, Dr. Grohskopf explained. “Newer antivirals peramivir and baloxavir have longer half-lives than oseltamivir and zanamivir, and insufficient data are available on the use of LAIV4 in the setting of antiviral use.”
The ACIP members had no financial conflicts to disclose.
Lung ultrasound works well in children with COVID-19
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
researchers wrote in Pediatrics.
They also noted the benefits that modality provides over other imaging techniques.
Marco Denina, MD, and colleagues from the pediatric infectious diseases unit at Regina Margherita Children’s Hospital in Turin, Italy, performed an observational study of eight children aged 0-17 years who were admitted to the hospital for COVID-19 between March 8 and 26, 2020. In seven of eight patients, the findings were concordant between imaging modalities; in the remaining patient, lung ultrasound (LUS) found an interstitial B-lines pattern that was not seen on radiography. In seven patients with pathologic ultrasound findings at baseline, the improvement or resolution of the subpleural consolidations or interstitial patterns was consistent with concomitant radiologic findings.
The authors cited the benefits of using point-of-care ultrasound instead of other modalities, such as CT. “First, it may reduce the number of radiologic examinations, lowering the radiation exposure of the patients,” they wrote. “Secondly, when performed at the bedside, LUS allows for the reduction of the patient’s movement within the hospital; thus, it lowers the number of health care workers and medical devices exposed to [SARS-CoV-2].”
One limitation of the study is the small sample size; however, the researchers felt the high concordance still suggests LUS is a reasonable method for COVID-19 patients.
There was no external funding for this study and the investigators had no relevant financial disclosures.
SOURCE: Denina M et al. Pediatrics. 2020 Jun. doi: 10.1542/peds.2020-1157.
FROM PEDIATRICS
Preventing arrhythmias and QTc prolongation in COVID-19 patients on psychotropics
Over the last few weeks, several conflicting reports about the efficacy of SARS-CoV-2 treatments have emerged, including high-profile papers that were placed in the limelight and groundbreaking retractions that were issued by the Lancet and New England Journal of Medicine, involving the potential dangers of COVID therapy with findings derived from the Surgisphere database. Hydroxychloroquine has garnered considerable media attention and was touted earlier by President Trump for its therapeutic effects.1 Naturally, there are political connotations associated with the agent, and it is unlikely that hydroxychloroquine will be supplanted in the near future as ongoing clinical trials have demonstrated mixed results amid the controversy.
As clinicians navigating unchartered territory within the hospital setting, we have to come to terms with these new challenges, tailoring treatment protocols accordingly with the best clinical practices in mind. Patients with preexisting mental health conditions and who are being treated for COVID-19 are particularly susceptible to clinical deterioration. Recent studies have indicated that psychiatric patients are more prone to feelings of isolation and/or estrangement as well as exacerbation of symptoms such as paranoia.2 Even more concerning is the medication regimen, namely, the novel combination therapies that arise when agents such as hydroxychloroquine are used in tandem with certain antipsychotics or antidepressants.
What’s at stake for COVID-19–positive mental health care patients?
Although the efficacy of hydroxychloroquine is currently being investigated,3 the antimalarial is usually prescribed in tandem with azithromycin for people with COVID-19. The National Institute of Allergy and Infectious Diseases has advised against that particular combination therapy because of ongoing concerns about toxicities.3,4
In another study, azithromycin was effectively substituted with doxycycline to help minimize systemic effects for patients with cardiac and/or pulmonary issues.5 Azithromycin is notorious in the literature for influencing the electrical activity of the heart with the potential for fatal arrhythmia and sudden cardiac death in individuals at risk for cardiovascular disease.5,6,7 It should be noted that both of these commonly prescribed COVID-19 medications (for example, hydroxychloroquine and azithromycin) could lead to QT interval prolongation especially within the context of combination therapy. This is largely concerning for psychiatrists and various other mental health practitioners for the following reasons: (1) higher rates of metabolic syndrome and cardiovascular diseases among psychiatric patients8 and/or (2) effects of certain antipsychotics (for example, IV haloperidol, thioridazine, and ziprasidone) and antidepressants (for example, citalopram and escitalopram) on the QT interval.9
SARS-CoV-2 and clinical judgment: Evaluating patients at higher risk
Although COVID-19 medication guidelines are still being actively developed, hydroxychloroquine appears to be commonly prescribed by physicians. The medication is known myriad untoward effects, including potential behavioral dysfunction (for example, irritability, agitation, suicidal ideation)10 as well as the aforementioned issues concerning arrhythmia (for example, torsades de pointes). Health care professionals might not have much control over the choice of COVID-19 agents because of a lack of available resources or limited options, but they can exercise clinical judgment with respect to selecting the appropriate psychotropic medications.
Treatment recommendations
1. Establish a baseline EKG
A baseline 12-lead EKG is the standard of care for patients currently being screened for COVID-19. It is necessary to rule out the presence of an underlying cardiovascular disease or a rhythm irregularity. A prolonged QTc interval is generally regarded as being around greater than 450-470 msecs with variations attributable to gender;11 numerous studies have affirmed that the risk of acquiring torsades de pointes is substantial when the QTc interval exceeds 500 msecs.12
2. Medical management and risk assessment
Commonly prescribed antipsychotics such as IV haloperidol and ziprasidone are known for exerting a negative effect on the interval and should readily be substituted with other agents in patients who are being treated for COVID-19; the combination of these antipsychotics alongside some COVID-19 medication regimens (for example, hydroxychloroquine/azithromycin) might prove to be fatal. The same logic applies to COVID-19 patients previously on antidepressant therapeutics such as citalopram and escitalopram.
3. Embrace an individually tailored approach to therapeutics
While American Psychiatric Association guidelines historically supported a cessation or reduction in the offending agent under normal circumstances,12 our team is recommending that the psychotropics associated with QTc interval prolongation are discontinued altogether (or substituted with a low-risk agent) in the event that a patient presents with suspected COVID-19. However, after the patients tests negative with COVID-19, they may resume therapy as indicated under the discretion of the mental health practitioner.
References
1. Offard C. “Lancet, NEJM Retract Surgisphere Studies on COVID-19 Patients.” The Scientist Magazine. 2020 Jun 4.
2. Shigemura J et al. Psychiatry Clin Neurosci. 2020 Apr;74(4):281-2.
3. Keshtkar-Jahromi M and Bavari S. Am J Trop Med Hyg. 2020 May;102(5):932-3.
4. Palca J. “NIH panel recommends against drug combination promoted by Trump for COVID-19.” NPR. 2020 Apr 21.
5. Mongelli L. “Long Island doctor tries new twist on hydroxychloroquine for elderly COVID-19 patients.” New York Post. 2020 Apr 4.
6. Hancox JC et al. Ther Adv Infect Dis. 2013 Oct;(5):155-65.
7. Giudicessi JR and Ackerman MJ. Cleve Clin J Med. 2013 Sep;80(9):539-44.
8. Casey DE. Am J Med. 2005 Apr 1;118(Suppl 2):15S-22S.
9. Beach SR et al. Psychosomatics. 2013 Jan 1;54(1):1-3.
10. Bogaczewicz A and Sobów T. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-4.
11. Chohan PS et al. Pak J Med Sci. 2015 Sep-Oct;31(5):1269-71.
12. Lieberman JA et al. APA guidance on the use of antipsychotic drugs and cardiac sudden death. NYS Office of Mental Health. 2012.
Dr. Faisal A. Islam is medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Faisal Islam disclosed no relevant financial relationships.
Dr. Mohammed Islam is affiliated with the department of psychiatry at the Interfaith Medical Center, New York. He disclosed no relevant financial relationships.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He disclosed no relevant financial relationships.
Over the last few weeks, several conflicting reports about the efficacy of SARS-CoV-2 treatments have emerged, including high-profile papers that were placed in the limelight and groundbreaking retractions that were issued by the Lancet and New England Journal of Medicine, involving the potential dangers of COVID therapy with findings derived from the Surgisphere database. Hydroxychloroquine has garnered considerable media attention and was touted earlier by President Trump for its therapeutic effects.1 Naturally, there are political connotations associated with the agent, and it is unlikely that hydroxychloroquine will be supplanted in the near future as ongoing clinical trials have demonstrated mixed results amid the controversy.
As clinicians navigating unchartered territory within the hospital setting, we have to come to terms with these new challenges, tailoring treatment protocols accordingly with the best clinical practices in mind. Patients with preexisting mental health conditions and who are being treated for COVID-19 are particularly susceptible to clinical deterioration. Recent studies have indicated that psychiatric patients are more prone to feelings of isolation and/or estrangement as well as exacerbation of symptoms such as paranoia.2 Even more concerning is the medication regimen, namely, the novel combination therapies that arise when agents such as hydroxychloroquine are used in tandem with certain antipsychotics or antidepressants.
What’s at stake for COVID-19–positive mental health care patients?
Although the efficacy of hydroxychloroquine is currently being investigated,3 the antimalarial is usually prescribed in tandem with azithromycin for people with COVID-19. The National Institute of Allergy and Infectious Diseases has advised against that particular combination therapy because of ongoing concerns about toxicities.3,4
In another study, azithromycin was effectively substituted with doxycycline to help minimize systemic effects for patients with cardiac and/or pulmonary issues.5 Azithromycin is notorious in the literature for influencing the electrical activity of the heart with the potential for fatal arrhythmia and sudden cardiac death in individuals at risk for cardiovascular disease.5,6,7 It should be noted that both of these commonly prescribed COVID-19 medications (for example, hydroxychloroquine and azithromycin) could lead to QT interval prolongation especially within the context of combination therapy. This is largely concerning for psychiatrists and various other mental health practitioners for the following reasons: (1) higher rates of metabolic syndrome and cardiovascular diseases among psychiatric patients8 and/or (2) effects of certain antipsychotics (for example, IV haloperidol, thioridazine, and ziprasidone) and antidepressants (for example, citalopram and escitalopram) on the QT interval.9
SARS-CoV-2 and clinical judgment: Evaluating patients at higher risk
Although COVID-19 medication guidelines are still being actively developed, hydroxychloroquine appears to be commonly prescribed by physicians. The medication is known myriad untoward effects, including potential behavioral dysfunction (for example, irritability, agitation, suicidal ideation)10 as well as the aforementioned issues concerning arrhythmia (for example, torsades de pointes). Health care professionals might not have much control over the choice of COVID-19 agents because of a lack of available resources or limited options, but they can exercise clinical judgment with respect to selecting the appropriate psychotropic medications.
Treatment recommendations
1. Establish a baseline EKG
A baseline 12-lead EKG is the standard of care for patients currently being screened for COVID-19. It is necessary to rule out the presence of an underlying cardiovascular disease or a rhythm irregularity. A prolonged QTc interval is generally regarded as being around greater than 450-470 msecs with variations attributable to gender;11 numerous studies have affirmed that the risk of acquiring torsades de pointes is substantial when the QTc interval exceeds 500 msecs.12
2. Medical management and risk assessment
Commonly prescribed antipsychotics such as IV haloperidol and ziprasidone are known for exerting a negative effect on the interval and should readily be substituted with other agents in patients who are being treated for COVID-19; the combination of these antipsychotics alongside some COVID-19 medication regimens (for example, hydroxychloroquine/azithromycin) might prove to be fatal. The same logic applies to COVID-19 patients previously on antidepressant therapeutics such as citalopram and escitalopram.
3. Embrace an individually tailored approach to therapeutics
While American Psychiatric Association guidelines historically supported a cessation or reduction in the offending agent under normal circumstances,12 our team is recommending that the psychotropics associated with QTc interval prolongation are discontinued altogether (or substituted with a low-risk agent) in the event that a patient presents with suspected COVID-19. However, after the patients tests negative with COVID-19, they may resume therapy as indicated under the discretion of the mental health practitioner.
References
1. Offard C. “Lancet, NEJM Retract Surgisphere Studies on COVID-19 Patients.” The Scientist Magazine. 2020 Jun 4.
2. Shigemura J et al. Psychiatry Clin Neurosci. 2020 Apr;74(4):281-2.
3. Keshtkar-Jahromi M and Bavari S. Am J Trop Med Hyg. 2020 May;102(5):932-3.
4. Palca J. “NIH panel recommends against drug combination promoted by Trump for COVID-19.” NPR. 2020 Apr 21.
5. Mongelli L. “Long Island doctor tries new twist on hydroxychloroquine for elderly COVID-19 patients.” New York Post. 2020 Apr 4.
6. Hancox JC et al. Ther Adv Infect Dis. 2013 Oct;(5):155-65.
7. Giudicessi JR and Ackerman MJ. Cleve Clin J Med. 2013 Sep;80(9):539-44.
8. Casey DE. Am J Med. 2005 Apr 1;118(Suppl 2):15S-22S.
9. Beach SR et al. Psychosomatics. 2013 Jan 1;54(1):1-3.
10. Bogaczewicz A and Sobów T. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-4.
11. Chohan PS et al. Pak J Med Sci. 2015 Sep-Oct;31(5):1269-71.
12. Lieberman JA et al. APA guidance on the use of antipsychotic drugs and cardiac sudden death. NYS Office of Mental Health. 2012.
Dr. Faisal A. Islam is medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Faisal Islam disclosed no relevant financial relationships.
Dr. Mohammed Islam is affiliated with the department of psychiatry at the Interfaith Medical Center, New York. He disclosed no relevant financial relationships.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He disclosed no relevant financial relationships.
Over the last few weeks, several conflicting reports about the efficacy of SARS-CoV-2 treatments have emerged, including high-profile papers that were placed in the limelight and groundbreaking retractions that were issued by the Lancet and New England Journal of Medicine, involving the potential dangers of COVID therapy with findings derived from the Surgisphere database. Hydroxychloroquine has garnered considerable media attention and was touted earlier by President Trump for its therapeutic effects.1 Naturally, there are political connotations associated with the agent, and it is unlikely that hydroxychloroquine will be supplanted in the near future as ongoing clinical trials have demonstrated mixed results amid the controversy.
As clinicians navigating unchartered territory within the hospital setting, we have to come to terms with these new challenges, tailoring treatment protocols accordingly with the best clinical practices in mind. Patients with preexisting mental health conditions and who are being treated for COVID-19 are particularly susceptible to clinical deterioration. Recent studies have indicated that psychiatric patients are more prone to feelings of isolation and/or estrangement as well as exacerbation of symptoms such as paranoia.2 Even more concerning is the medication regimen, namely, the novel combination therapies that arise when agents such as hydroxychloroquine are used in tandem with certain antipsychotics or antidepressants.
What’s at stake for COVID-19–positive mental health care patients?
Although the efficacy of hydroxychloroquine is currently being investigated,3 the antimalarial is usually prescribed in tandem with azithromycin for people with COVID-19. The National Institute of Allergy and Infectious Diseases has advised against that particular combination therapy because of ongoing concerns about toxicities.3,4
In another study, azithromycin was effectively substituted with doxycycline to help minimize systemic effects for patients with cardiac and/or pulmonary issues.5 Azithromycin is notorious in the literature for influencing the electrical activity of the heart with the potential for fatal arrhythmia and sudden cardiac death in individuals at risk for cardiovascular disease.5,6,7 It should be noted that both of these commonly prescribed COVID-19 medications (for example, hydroxychloroquine and azithromycin) could lead to QT interval prolongation especially within the context of combination therapy. This is largely concerning for psychiatrists and various other mental health practitioners for the following reasons: (1) higher rates of metabolic syndrome and cardiovascular diseases among psychiatric patients8 and/or (2) effects of certain antipsychotics (for example, IV haloperidol, thioridazine, and ziprasidone) and antidepressants (for example, citalopram and escitalopram) on the QT interval.9
SARS-CoV-2 and clinical judgment: Evaluating patients at higher risk
Although COVID-19 medication guidelines are still being actively developed, hydroxychloroquine appears to be commonly prescribed by physicians. The medication is known myriad untoward effects, including potential behavioral dysfunction (for example, irritability, agitation, suicidal ideation)10 as well as the aforementioned issues concerning arrhythmia (for example, torsades de pointes). Health care professionals might not have much control over the choice of COVID-19 agents because of a lack of available resources or limited options, but they can exercise clinical judgment with respect to selecting the appropriate psychotropic medications.
Treatment recommendations
1. Establish a baseline EKG
A baseline 12-lead EKG is the standard of care for patients currently being screened for COVID-19. It is necessary to rule out the presence of an underlying cardiovascular disease or a rhythm irregularity. A prolonged QTc interval is generally regarded as being around greater than 450-470 msecs with variations attributable to gender;11 numerous studies have affirmed that the risk of acquiring torsades de pointes is substantial when the QTc interval exceeds 500 msecs.12
2. Medical management and risk assessment
Commonly prescribed antipsychotics such as IV haloperidol and ziprasidone are known for exerting a negative effect on the interval and should readily be substituted with other agents in patients who are being treated for COVID-19; the combination of these antipsychotics alongside some COVID-19 medication regimens (for example, hydroxychloroquine/azithromycin) might prove to be fatal. The same logic applies to COVID-19 patients previously on antidepressant therapeutics such as citalopram and escitalopram.
3. Embrace an individually tailored approach to therapeutics
While American Psychiatric Association guidelines historically supported a cessation or reduction in the offending agent under normal circumstances,12 our team is recommending that the psychotropics associated with QTc interval prolongation are discontinued altogether (or substituted with a low-risk agent) in the event that a patient presents with suspected COVID-19. However, after the patients tests negative with COVID-19, they may resume therapy as indicated under the discretion of the mental health practitioner.
References
1. Offard C. “Lancet, NEJM Retract Surgisphere Studies on COVID-19 Patients.” The Scientist Magazine. 2020 Jun 4.
2. Shigemura J et al. Psychiatry Clin Neurosci. 2020 Apr;74(4):281-2.
3. Keshtkar-Jahromi M and Bavari S. Am J Trop Med Hyg. 2020 May;102(5):932-3.
4. Palca J. “NIH panel recommends against drug combination promoted by Trump for COVID-19.” NPR. 2020 Apr 21.
5. Mongelli L. “Long Island doctor tries new twist on hydroxychloroquine for elderly COVID-19 patients.” New York Post. 2020 Apr 4.
6. Hancox JC et al. Ther Adv Infect Dis. 2013 Oct;(5):155-65.
7. Giudicessi JR and Ackerman MJ. Cleve Clin J Med. 2013 Sep;80(9):539-44.
8. Casey DE. Am J Med. 2005 Apr 1;118(Suppl 2):15S-22S.
9. Beach SR et al. Psychosomatics. 2013 Jan 1;54(1):1-3.
10. Bogaczewicz A and Sobów T. Psychiatria i Psychologia Kliniczna. 2017;17(2):111-4.
11. Chohan PS et al. Pak J Med Sci. 2015 Sep-Oct;31(5):1269-71.
12. Lieberman JA et al. APA guidance on the use of antipsychotic drugs and cardiac sudden death. NYS Office of Mental Health. 2012.
Dr. Faisal A. Islam is medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Faisal Islam disclosed no relevant financial relationships.
Dr. Mohammed Islam is affiliated with the department of psychiatry at the Interfaith Medical Center, New York. He disclosed no relevant financial relationships.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He disclosed no relevant financial relationships.
Survey: 26% of parents hesitant about influenza vaccine
according to a nationally representative survey.
Influenza vaccination hesitancy may be driven by concerns about vaccine effectiveness, researchers wrote in Pediatrics. These findings “underscore the importance of better communicating to providers and parents the effectiveness of influenza vaccines in reducing severity and morbidity from influenza, even in years when the vaccine has relatively low effectiveness,” noted Allison Kempe, MD, MPH, professor of pediatrics and director of the Adult and Child Consortium for Health Outcomes Research and Delivery Science at the University of Colorado at Denver, Aurora, and colleagues.
The World Health Organization considers vaccine hesitancy a leading threat to global health, but national data about vaccine hesitancy in the United States are limited. To assess hesitancy about routine childhood and influenza vaccinations and related factors, Dr. Kempe and colleagues surveyed more than 2,000 parents in February 2019.
The investigators used an online panel to survey a nationally representative sample of families with children aged between 6 months and 18 years. Parents completed a modified version of the Vaccine Hesitancy Scale, which measures confidence in and concerns about vaccines. Parents with an average score greater than 3 on the scale were considered hesitant.
Factors associated with vaccine hesitancy
Of 4,445 parents sampled, 2,176 completed the survey and 2,052 were eligible respondents. For routine childhood vaccines, the average score on the modified Vaccine Hesitancy Scale was 2 and the percentage of hesitant parents was 6%. For influenza vaccine, the average score was 2 and the percentage of hesitant parents was 26%.
Among hesitant parents, 68% had deferred or refused routine childhood vaccination, compared with 9% of nonhesitant parents (risk ratio, 8.0). For the influenza vaccine, 70% of hesitant parents had deferred or refused influenza vaccination for their child versus 10% of nonhesitant parents (RR, 7.0). Parents were more likely to strongly agree that routine childhood vaccines are effective, compared with the influenza vaccine (70% vs. 26%). “Hesitancy about influenza vaccination is largely driven by concerns about low vaccine effectiveness,” Dr. Kempe and associates wrote.
Although concern about serious side effects was the factor most associated with hesitancy, the percentage of parents who were strongly (12%) or somewhat (27%) concerned about serious side effects was the same for routine childhood vaccines and influenza vaccines. Other factors associated with hesitancy for both routine childhood vaccines and influenza vaccines included lower educational level and household income less than 400% of the federal poverty level.
The survey data may be subject to reporting bias based on social desirability, the authors noted. In addition, the exclusion of infants younger than 6 months may have resulted in an underestimate of hesitancy.
“Although influenza vaccine could be included as a ‘routine’ vaccine, in that it is recommended yearly, we hypothesized that parents view it differently from other childhood vaccines because each year it needs to be given again, its content and effectiveness vary, and it addresses a disease that is often perceived as minor, compared with other childhood diseases,” Dr. Kempe and colleagues wrote. Interventions to counter hesitancy have “a surprising lack of evidence,” and “more work needs to be done to develop methods that are practical and effective for convincing vaccine-hesitant parents to vaccinate.”
Logical next step
“From the pragmatic standpoint of improving immunization rates and disease control, determining the correct evidence-based messaging to counter these perceptions is the next logical step,” Annabelle de St. Maurice, MD, MPH, an assistant professor of pediatrics in the division of infectious diseases at University of California, Los Angeles, and Kathryn Edwards, MD, a professor of pediatrics and director of the vaccine research program at Vanderbilt University, Nashville, wrote in an accompanying editorial.
“Communications should be focused on the burden of influenza in children, rebranding influenza vaccine as a ‘routine’ childhood immunization, reassurance on influenza vaccine safety, and discussion of the efficacy of influenza vaccine in preventing severe disease,” they wrote. “Even in the years when there is a poor match, the vaccine is impactful.”
The research was supported by the National Institutes of Health. Two study authors disclosed financial ties to Sanofi Pasteur, with one also disclosing financial ties to Merck, for work related to vaccinations. The remaining investigators had no relevant financial disclosures. Dr. de St. Maurice indicated that she had no relevant financial disclosures. Dr. Edwards disclosed grants from the Centers for Disease Control and Prevention and the NIH; consulting for Merck, Bionet, and IBM; and serving on data safety and monitoring boards for Sanofi, X4 Pharmaceuticals, Seqirus, Moderna, and Pfizer.
SOURCE: Kempe A et al. Pediatrics. 2020 Jun 15. doi: 10.1542/peds.2019-3852.
according to a nationally representative survey.
Influenza vaccination hesitancy may be driven by concerns about vaccine effectiveness, researchers wrote in Pediatrics. These findings “underscore the importance of better communicating to providers and parents the effectiveness of influenza vaccines in reducing severity and morbidity from influenza, even in years when the vaccine has relatively low effectiveness,” noted Allison Kempe, MD, MPH, professor of pediatrics and director of the Adult and Child Consortium for Health Outcomes Research and Delivery Science at the University of Colorado at Denver, Aurora, and colleagues.
The World Health Organization considers vaccine hesitancy a leading threat to global health, but national data about vaccine hesitancy in the United States are limited. To assess hesitancy about routine childhood and influenza vaccinations and related factors, Dr. Kempe and colleagues surveyed more than 2,000 parents in February 2019.
The investigators used an online panel to survey a nationally representative sample of families with children aged between 6 months and 18 years. Parents completed a modified version of the Vaccine Hesitancy Scale, which measures confidence in and concerns about vaccines. Parents with an average score greater than 3 on the scale were considered hesitant.
Factors associated with vaccine hesitancy
Of 4,445 parents sampled, 2,176 completed the survey and 2,052 were eligible respondents. For routine childhood vaccines, the average score on the modified Vaccine Hesitancy Scale was 2 and the percentage of hesitant parents was 6%. For influenza vaccine, the average score was 2 and the percentage of hesitant parents was 26%.
Among hesitant parents, 68% had deferred or refused routine childhood vaccination, compared with 9% of nonhesitant parents (risk ratio, 8.0). For the influenza vaccine, 70% of hesitant parents had deferred or refused influenza vaccination for their child versus 10% of nonhesitant parents (RR, 7.0). Parents were more likely to strongly agree that routine childhood vaccines are effective, compared with the influenza vaccine (70% vs. 26%). “Hesitancy about influenza vaccination is largely driven by concerns about low vaccine effectiveness,” Dr. Kempe and associates wrote.
Although concern about serious side effects was the factor most associated with hesitancy, the percentage of parents who were strongly (12%) or somewhat (27%) concerned about serious side effects was the same for routine childhood vaccines and influenza vaccines. Other factors associated with hesitancy for both routine childhood vaccines and influenza vaccines included lower educational level and household income less than 400% of the federal poverty level.
The survey data may be subject to reporting bias based on social desirability, the authors noted. In addition, the exclusion of infants younger than 6 months may have resulted in an underestimate of hesitancy.
“Although influenza vaccine could be included as a ‘routine’ vaccine, in that it is recommended yearly, we hypothesized that parents view it differently from other childhood vaccines because each year it needs to be given again, its content and effectiveness vary, and it addresses a disease that is often perceived as minor, compared with other childhood diseases,” Dr. Kempe and colleagues wrote. Interventions to counter hesitancy have “a surprising lack of evidence,” and “more work needs to be done to develop methods that are practical and effective for convincing vaccine-hesitant parents to vaccinate.”
Logical next step
“From the pragmatic standpoint of improving immunization rates and disease control, determining the correct evidence-based messaging to counter these perceptions is the next logical step,” Annabelle de St. Maurice, MD, MPH, an assistant professor of pediatrics in the division of infectious diseases at University of California, Los Angeles, and Kathryn Edwards, MD, a professor of pediatrics and director of the vaccine research program at Vanderbilt University, Nashville, wrote in an accompanying editorial.
“Communications should be focused on the burden of influenza in children, rebranding influenza vaccine as a ‘routine’ childhood immunization, reassurance on influenza vaccine safety, and discussion of the efficacy of influenza vaccine in preventing severe disease,” they wrote. “Even in the years when there is a poor match, the vaccine is impactful.”
The research was supported by the National Institutes of Health. Two study authors disclosed financial ties to Sanofi Pasteur, with one also disclosing financial ties to Merck, for work related to vaccinations. The remaining investigators had no relevant financial disclosures. Dr. de St. Maurice indicated that she had no relevant financial disclosures. Dr. Edwards disclosed grants from the Centers for Disease Control and Prevention and the NIH; consulting for Merck, Bionet, and IBM; and serving on data safety and monitoring boards for Sanofi, X4 Pharmaceuticals, Seqirus, Moderna, and Pfizer.
SOURCE: Kempe A et al. Pediatrics. 2020 Jun 15. doi: 10.1542/peds.2019-3852.
according to a nationally representative survey.
Influenza vaccination hesitancy may be driven by concerns about vaccine effectiveness, researchers wrote in Pediatrics. These findings “underscore the importance of better communicating to providers and parents the effectiveness of influenza vaccines in reducing severity and morbidity from influenza, even in years when the vaccine has relatively low effectiveness,” noted Allison Kempe, MD, MPH, professor of pediatrics and director of the Adult and Child Consortium for Health Outcomes Research and Delivery Science at the University of Colorado at Denver, Aurora, and colleagues.
The World Health Organization considers vaccine hesitancy a leading threat to global health, but national data about vaccine hesitancy in the United States are limited. To assess hesitancy about routine childhood and influenza vaccinations and related factors, Dr. Kempe and colleagues surveyed more than 2,000 parents in February 2019.
The investigators used an online panel to survey a nationally representative sample of families with children aged between 6 months and 18 years. Parents completed a modified version of the Vaccine Hesitancy Scale, which measures confidence in and concerns about vaccines. Parents with an average score greater than 3 on the scale were considered hesitant.
Factors associated with vaccine hesitancy
Of 4,445 parents sampled, 2,176 completed the survey and 2,052 were eligible respondents. For routine childhood vaccines, the average score on the modified Vaccine Hesitancy Scale was 2 and the percentage of hesitant parents was 6%. For influenza vaccine, the average score was 2 and the percentage of hesitant parents was 26%.
Among hesitant parents, 68% had deferred or refused routine childhood vaccination, compared with 9% of nonhesitant parents (risk ratio, 8.0). For the influenza vaccine, 70% of hesitant parents had deferred or refused influenza vaccination for their child versus 10% of nonhesitant parents (RR, 7.0). Parents were more likely to strongly agree that routine childhood vaccines are effective, compared with the influenza vaccine (70% vs. 26%). “Hesitancy about influenza vaccination is largely driven by concerns about low vaccine effectiveness,” Dr. Kempe and associates wrote.
Although concern about serious side effects was the factor most associated with hesitancy, the percentage of parents who were strongly (12%) or somewhat (27%) concerned about serious side effects was the same for routine childhood vaccines and influenza vaccines. Other factors associated with hesitancy for both routine childhood vaccines and influenza vaccines included lower educational level and household income less than 400% of the federal poverty level.
The survey data may be subject to reporting bias based on social desirability, the authors noted. In addition, the exclusion of infants younger than 6 months may have resulted in an underestimate of hesitancy.
“Although influenza vaccine could be included as a ‘routine’ vaccine, in that it is recommended yearly, we hypothesized that parents view it differently from other childhood vaccines because each year it needs to be given again, its content and effectiveness vary, and it addresses a disease that is often perceived as minor, compared with other childhood diseases,” Dr. Kempe and colleagues wrote. Interventions to counter hesitancy have “a surprising lack of evidence,” and “more work needs to be done to develop methods that are practical and effective for convincing vaccine-hesitant parents to vaccinate.”
Logical next step
“From the pragmatic standpoint of improving immunization rates and disease control, determining the correct evidence-based messaging to counter these perceptions is the next logical step,” Annabelle de St. Maurice, MD, MPH, an assistant professor of pediatrics in the division of infectious diseases at University of California, Los Angeles, and Kathryn Edwards, MD, a professor of pediatrics and director of the vaccine research program at Vanderbilt University, Nashville, wrote in an accompanying editorial.
“Communications should be focused on the burden of influenza in children, rebranding influenza vaccine as a ‘routine’ childhood immunization, reassurance on influenza vaccine safety, and discussion of the efficacy of influenza vaccine in preventing severe disease,” they wrote. “Even in the years when there is a poor match, the vaccine is impactful.”
The research was supported by the National Institutes of Health. Two study authors disclosed financial ties to Sanofi Pasteur, with one also disclosing financial ties to Merck, for work related to vaccinations. The remaining investigators had no relevant financial disclosures. Dr. de St. Maurice indicated that she had no relevant financial disclosures. Dr. Edwards disclosed grants from the Centers for Disease Control and Prevention and the NIH; consulting for Merck, Bionet, and IBM; and serving on data safety and monitoring boards for Sanofi, X4 Pharmaceuticals, Seqirus, Moderna, and Pfizer.
SOURCE: Kempe A et al. Pediatrics. 2020 Jun 15. doi: 10.1542/peds.2019-3852.
FROM PEDIATRICS
FDA revokes emergency use of hydroxychloroquine
The U.S. Food and Drug Administration revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
“Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA,” the agency announced in a June 15 statement.
The FDA also warned today that the use of hydroxychloroquine or chloroquine may have a potential drug interaction with the investigational antiviral drug remdesivir that limits its effectiveness against COVID-19.
Remdesivir was granted emergency use authorization by the FDA on May 1.
“Based on a recently completed nonclinical laboratory study, the FDA is revising the fact sheet for healthcare providers that accompanies the drug to state that coadministration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended as it may result in reduced antiviral activity of remdesivir. The agency is not aware of instances of this reduced activity occurring in the clinical setting but is continuing to evaluate all data related to remdesivir,” the FDA said in a news release.
Controversy over hydroxychloroquine
Even with such federal permission, since late March the use of these two agents has been mired in controversy.
President Donald J. Trump promoted the use of hydroxychloroquine and chloroquine to treat Americans with COVID-19, while scientific studies raised questions about their safety and effectiveness. Recent research, for example, pointed to elevated cardiovascular risks, as reported by Medscape Medical News.
The FDA acknowledged this recent evidence. “Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
The full suspension of the EUA follows a warning the agency issued on April 24. The FDA’s Safety Communication cautioned against use of the two agents outside of a hospital setting, citing an increase in outpatient prescriptions and “reports of serious heart rhythm problems.”
“While additional clinical trials continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, we determined the emergency use authorization was no longer appropriate,” based on a rigorous assessment by scientists in our Center for Drug Evaluation and Research,” Patrizia Cavazzoni, MD, acting director of CDER, noted in the FDA statement.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
“Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA,” the agency announced in a June 15 statement.
The FDA also warned today that the use of hydroxychloroquine or chloroquine may have a potential drug interaction with the investigational antiviral drug remdesivir that limits its effectiveness against COVID-19.
Remdesivir was granted emergency use authorization by the FDA on May 1.
“Based on a recently completed nonclinical laboratory study, the FDA is revising the fact sheet for healthcare providers that accompanies the drug to state that coadministration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended as it may result in reduced antiviral activity of remdesivir. The agency is not aware of instances of this reduced activity occurring in the clinical setting but is continuing to evaluate all data related to remdesivir,” the FDA said in a news release.
Controversy over hydroxychloroquine
Even with such federal permission, since late March the use of these two agents has been mired in controversy.
President Donald J. Trump promoted the use of hydroxychloroquine and chloroquine to treat Americans with COVID-19, while scientific studies raised questions about their safety and effectiveness. Recent research, for example, pointed to elevated cardiovascular risks, as reported by Medscape Medical News.
The FDA acknowledged this recent evidence. “Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
The full suspension of the EUA follows a warning the agency issued on April 24. The FDA’s Safety Communication cautioned against use of the two agents outside of a hospital setting, citing an increase in outpatient prescriptions and “reports of serious heart rhythm problems.”
“While additional clinical trials continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, we determined the emergency use authorization was no longer appropriate,” based on a rigorous assessment by scientists in our Center for Drug Evaluation and Research,” Patrizia Cavazzoni, MD, acting director of CDER, noted in the FDA statement.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
“Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA,” the agency announced in a June 15 statement.
The FDA also warned today that the use of hydroxychloroquine or chloroquine may have a potential drug interaction with the investigational antiviral drug remdesivir that limits its effectiveness against COVID-19.
Remdesivir was granted emergency use authorization by the FDA on May 1.
“Based on a recently completed nonclinical laboratory study, the FDA is revising the fact sheet for healthcare providers that accompanies the drug to state that coadministration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended as it may result in reduced antiviral activity of remdesivir. The agency is not aware of instances of this reduced activity occurring in the clinical setting but is continuing to evaluate all data related to remdesivir,” the FDA said in a news release.
Controversy over hydroxychloroquine
Even with such federal permission, since late March the use of these two agents has been mired in controversy.
President Donald J. Trump promoted the use of hydroxychloroquine and chloroquine to treat Americans with COVID-19, while scientific studies raised questions about their safety and effectiveness. Recent research, for example, pointed to elevated cardiovascular risks, as reported by Medscape Medical News.
The FDA acknowledged this recent evidence. “Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
The full suspension of the EUA follows a warning the agency issued on April 24. The FDA’s Safety Communication cautioned against use of the two agents outside of a hospital setting, citing an increase in outpatient prescriptions and “reports of serious heart rhythm problems.”
“While additional clinical trials continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, we determined the emergency use authorization was no longer appropriate,” based on a rigorous assessment by scientists in our Center for Drug Evaluation and Research,” Patrizia Cavazzoni, MD, acting director of CDER, noted in the FDA statement.
This article first appeared on Medscape.com.
Perfect storm of SARS-CoV-2 during flu season
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.
Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.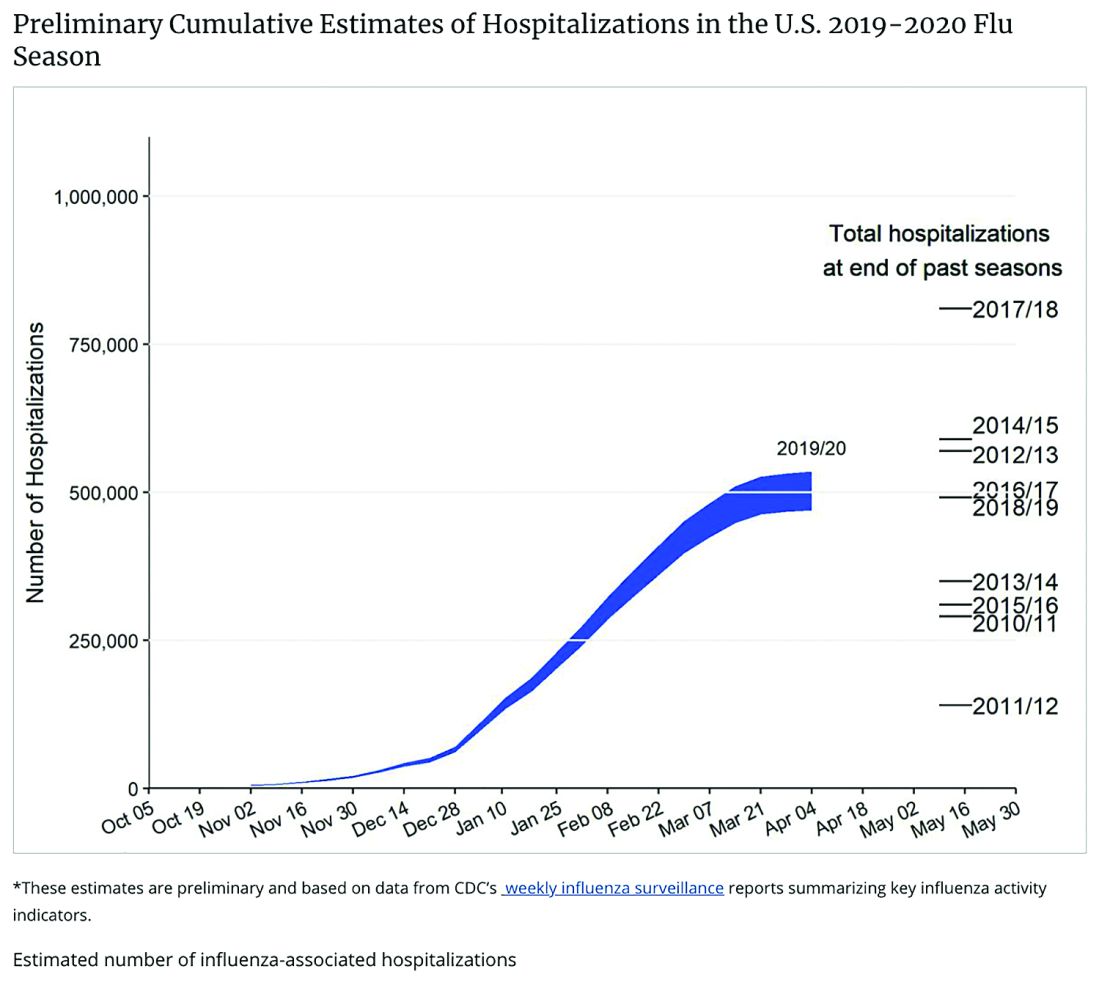
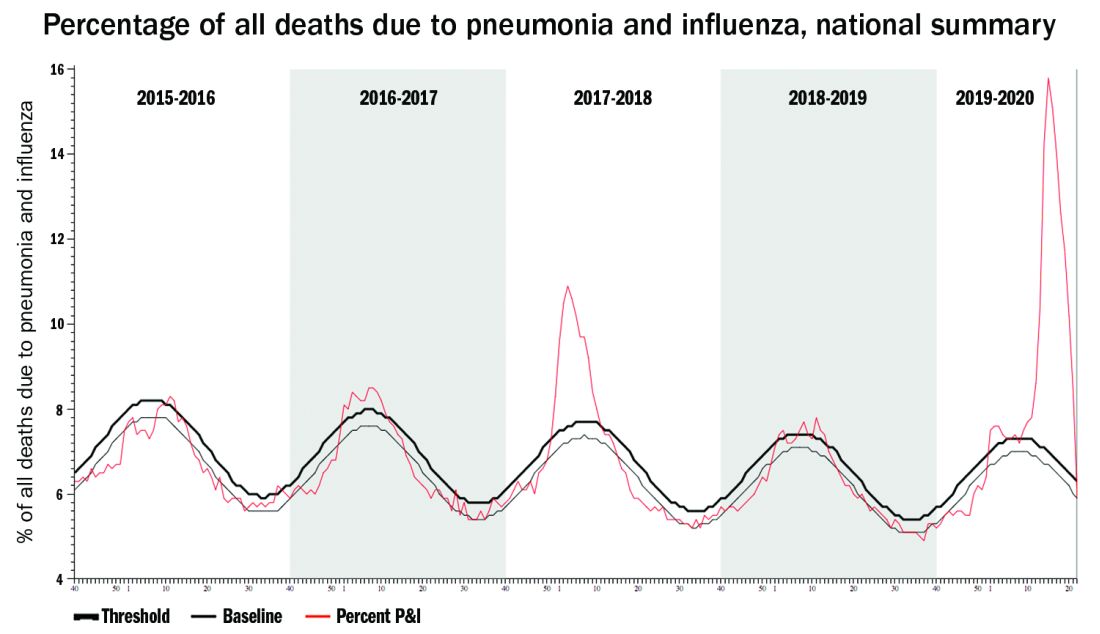
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
If you reopen it, will they come?
On April 16, the White House released federal guidelines for reopening American businesses – followed 3 days later by specific recommendations from the Centers for Medicare and Medicaid Services for .
Depending on where you live, you may have already reopened (or even never closed), or you may be awaiting the relaxation of restrictions in your state. (As I write this on June 10, the stay-at-home order in my state, New Jersey, is being rescinded.)
The big question, of course, is whether patients can be convinced that it is safe to leave their homes and come to your office. The answer may depend on how well you time your reopening and adhere to the appropriate federal, state, and independent guidelines.
The federal guidelines have three sections: criteria, which outline conditions each region or state should satisfy before reopening; preparedness, which lists how states should prepare for reopening; and phase guidelines, which detail responsibilities of individuals and employers during distinct reopening phases.
You should pay the most attention to the “criteria” section. The key question to ask: “Has my state or region satisfied the basic criteria for reopening?”
Those criteria are as follows:
- Symptoms reported within a 14-day period should be on a downward trajectory.
- Cases documented (or positive tests as a percentage of total tests) within a 14-day period should also be on a downward trajectory.
- Hospitals should be treating all patients without crisis care. They should also have a robust testing program in place for at-risk health care workers.
If your area meets these criteria, you can proceed to the CMS recommendations. They cover general advice related to personal protective equipment (PPE), workforce availability, facility considerations, sanitation protocols, supplies, and testing capacity.
The key takeaway: As long as your area has the resources to quickly respond to a surge of COVID-19 cases, you can start offering care to non-COVID patients. Keep seeing patients via telehealth as often as possible, and prioritize surgical/procedural care and high-complexity chronic disease management before moving on to preventive and cosmetic services.
The American Medical Association has issued its own checklist of criteria for reopening your practice to supplement the federal guidelines. Highlights include the following:
- Sit down with a calendar and pick an expected reopening day. Ideally, this should include a “soft reopening.” Make a plan to stock necessary PPE and write down plans for cleaning and staffing if an employee or patient is diagnosed with COVID-19 after visiting your office.
- Take a stepwise approach so you can identify challenges early and address them. It’s important to figure out which visits can continue via telehealth, and begin with just a few in-person visits each day. Plan out a schedule and clearly communicate it to patients, clinicians, and staff.
- Patient safety is your top concern. Encourage patients to visit without companions whenever possible, and of course, all individuals who visit the office should wear a cloth face covering.
- Screen employees for fevers and other symptoms of COVID-19; remember that those records are subject to HIPAA rules and must be kept confidential. Minimize contact between employees as much as possible.
- Do your best to screen patients before in-person visits, to verify they don’t have symptoms of COVID-19. Consider creating a script that office staff can use to contact patients 24 hours before they come in. Use this as a chance to ask about symptoms, and explain any reopening logistics they should know about.
- Contact your malpractice insurance carrier to discuss whether you need to make any changes to your coverage.
This would also be a great time to review your confidentiality, privacy, and data security protocols. COVID-19 presents new challenges for data privacy – for example, if you must inform coworkers or patients that they have come into contact with someone who tested positive. Make a plan that follows HIPAA guidelines during COVID-19. Also, make sure you have a plan for handling issues like paid sick leave or reporting COVID-19 cases to your local health department.
Another useful resource is the Medical Group Management Association’s COVID-19 Medical Practice Reopening Checklist. You can use it to confirm that you are addressing all the important items, and that you haven’t missed anything.
As for me, I am advising patients who are reluctant to seek treatment that many medical problems pose more risk than COVID-19, faster treatment means better outcomes, and because we maintain strict disinfection protocols, they are far less likely to be infected with COVID-19 in my office than, say, at a grocery store.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
On April 16, the White House released federal guidelines for reopening American businesses – followed 3 days later by specific recommendations from the Centers for Medicare and Medicaid Services for .
Depending on where you live, you may have already reopened (or even never closed), or you may be awaiting the relaxation of restrictions in your state. (As I write this on June 10, the stay-at-home order in my state, New Jersey, is being rescinded.)
The big question, of course, is whether patients can be convinced that it is safe to leave their homes and come to your office. The answer may depend on how well you time your reopening and adhere to the appropriate federal, state, and independent guidelines.
The federal guidelines have three sections: criteria, which outline conditions each region or state should satisfy before reopening; preparedness, which lists how states should prepare for reopening; and phase guidelines, which detail responsibilities of individuals and employers during distinct reopening phases.
You should pay the most attention to the “criteria” section. The key question to ask: “Has my state or region satisfied the basic criteria for reopening?”
Those criteria are as follows:
- Symptoms reported within a 14-day period should be on a downward trajectory.
- Cases documented (or positive tests as a percentage of total tests) within a 14-day period should also be on a downward trajectory.
- Hospitals should be treating all patients without crisis care. They should also have a robust testing program in place for at-risk health care workers.
If your area meets these criteria, you can proceed to the CMS recommendations. They cover general advice related to personal protective equipment (PPE), workforce availability, facility considerations, sanitation protocols, supplies, and testing capacity.
The key takeaway: As long as your area has the resources to quickly respond to a surge of COVID-19 cases, you can start offering care to non-COVID patients. Keep seeing patients via telehealth as often as possible, and prioritize surgical/procedural care and high-complexity chronic disease management before moving on to preventive and cosmetic services.
The American Medical Association has issued its own checklist of criteria for reopening your practice to supplement the federal guidelines. Highlights include the following:
- Sit down with a calendar and pick an expected reopening day. Ideally, this should include a “soft reopening.” Make a plan to stock necessary PPE and write down plans for cleaning and staffing if an employee or patient is diagnosed with COVID-19 after visiting your office.
- Take a stepwise approach so you can identify challenges early and address them. It’s important to figure out which visits can continue via telehealth, and begin with just a few in-person visits each day. Plan out a schedule and clearly communicate it to patients, clinicians, and staff.
- Patient safety is your top concern. Encourage patients to visit without companions whenever possible, and of course, all individuals who visit the office should wear a cloth face covering.
- Screen employees for fevers and other symptoms of COVID-19; remember that those records are subject to HIPAA rules and must be kept confidential. Minimize contact between employees as much as possible.
- Do your best to screen patients before in-person visits, to verify they don’t have symptoms of COVID-19. Consider creating a script that office staff can use to contact patients 24 hours before they come in. Use this as a chance to ask about symptoms, and explain any reopening logistics they should know about.
- Contact your malpractice insurance carrier to discuss whether you need to make any changes to your coverage.
This would also be a great time to review your confidentiality, privacy, and data security protocols. COVID-19 presents new challenges for data privacy – for example, if you must inform coworkers or patients that they have come into contact with someone who tested positive. Make a plan that follows HIPAA guidelines during COVID-19. Also, make sure you have a plan for handling issues like paid sick leave or reporting COVID-19 cases to your local health department.
Another useful resource is the Medical Group Management Association’s COVID-19 Medical Practice Reopening Checklist. You can use it to confirm that you are addressing all the important items, and that you haven’t missed anything.
As for me, I am advising patients who are reluctant to seek treatment that many medical problems pose more risk than COVID-19, faster treatment means better outcomes, and because we maintain strict disinfection protocols, they are far less likely to be infected with COVID-19 in my office than, say, at a grocery store.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
On April 16, the White House released federal guidelines for reopening American businesses – followed 3 days later by specific recommendations from the Centers for Medicare and Medicaid Services for .
Depending on where you live, you may have already reopened (or even never closed), or you may be awaiting the relaxation of restrictions in your state. (As I write this on June 10, the stay-at-home order in my state, New Jersey, is being rescinded.)
The big question, of course, is whether patients can be convinced that it is safe to leave their homes and come to your office. The answer may depend on how well you time your reopening and adhere to the appropriate federal, state, and independent guidelines.
The federal guidelines have three sections: criteria, which outline conditions each region or state should satisfy before reopening; preparedness, which lists how states should prepare for reopening; and phase guidelines, which detail responsibilities of individuals and employers during distinct reopening phases.
You should pay the most attention to the “criteria” section. The key question to ask: “Has my state or region satisfied the basic criteria for reopening?”
Those criteria are as follows:
- Symptoms reported within a 14-day period should be on a downward trajectory.
- Cases documented (or positive tests as a percentage of total tests) within a 14-day period should also be on a downward trajectory.
- Hospitals should be treating all patients without crisis care. They should also have a robust testing program in place for at-risk health care workers.
If your area meets these criteria, you can proceed to the CMS recommendations. They cover general advice related to personal protective equipment (PPE), workforce availability, facility considerations, sanitation protocols, supplies, and testing capacity.
The key takeaway: As long as your area has the resources to quickly respond to a surge of COVID-19 cases, you can start offering care to non-COVID patients. Keep seeing patients via telehealth as often as possible, and prioritize surgical/procedural care and high-complexity chronic disease management before moving on to preventive and cosmetic services.
The American Medical Association has issued its own checklist of criteria for reopening your practice to supplement the federal guidelines. Highlights include the following:
- Sit down with a calendar and pick an expected reopening day. Ideally, this should include a “soft reopening.” Make a plan to stock necessary PPE and write down plans for cleaning and staffing if an employee or patient is diagnosed with COVID-19 after visiting your office.
- Take a stepwise approach so you can identify challenges early and address them. It’s important to figure out which visits can continue via telehealth, and begin with just a few in-person visits each day. Plan out a schedule and clearly communicate it to patients, clinicians, and staff.
- Patient safety is your top concern. Encourage patients to visit without companions whenever possible, and of course, all individuals who visit the office should wear a cloth face covering.
- Screen employees for fevers and other symptoms of COVID-19; remember that those records are subject to HIPAA rules and must be kept confidential. Minimize contact between employees as much as possible.
- Do your best to screen patients before in-person visits, to verify they don’t have symptoms of COVID-19. Consider creating a script that office staff can use to contact patients 24 hours before they come in. Use this as a chance to ask about symptoms, and explain any reopening logistics they should know about.
- Contact your malpractice insurance carrier to discuss whether you need to make any changes to your coverage.
This would also be a great time to review your confidentiality, privacy, and data security protocols. COVID-19 presents new challenges for data privacy – for example, if you must inform coworkers or patients that they have come into contact with someone who tested positive. Make a plan that follows HIPAA guidelines during COVID-19. Also, make sure you have a plan for handling issues like paid sick leave or reporting COVID-19 cases to your local health department.
Another useful resource is the Medical Group Management Association’s COVID-19 Medical Practice Reopening Checklist. You can use it to confirm that you are addressing all the important items, and that you haven’t missed anything.
As for me, I am advising patients who are reluctant to seek treatment that many medical problems pose more risk than COVID-19, faster treatment means better outcomes, and because we maintain strict disinfection protocols, they are far less likely to be infected with COVID-19 in my office than, say, at a grocery store.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Baloxavir effective, well tolerated for influenza treatment in children
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Kids with food allergies the newest victims of COVID-19?
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Food insecurity is not knowing how you will get your next meal. This pandemic has led to a lot of it, especially as a result of massive unemployment. Now imagine being in that situation with a food-allergic child. It would be frightening.
There is always a level of anxiety for parents of food-allergic children, but the Food and Drug Administration–mandated labeling of food allergens has helped to allay some of those concerns. Shopping can feel safer, even if it’s not foolproof.
Now, that fear for the safety of food-allergic children is going to be compounded by the FDA’s latest announcement, made at the behest of the food industry.
Disruptions in the food supply chain caused by the COVID-19 pandemic have created some problems for the food industry. The industry sought – and received – relief from the FDA; they are now allowing some ingredient substitutions without mandating a change in labeling. These changes were made without opportunity for public comment, according to the FDA, because of the exigency of the situation. Furthermore, the changes may stay in effect for an indeterminate period of time after the pandemic is deemed under control.
Labeling of gluten and the major eight allergens (peanuts, tree nuts, milk, eggs, soy, wheat, fish, and crustacean shellfish) cannot change under the new guidelines. The FDA also advised “consideration” of major food allergens recognized in other countries (sesame, celery, lupin, buckwheat, molluscan shellfish, and mustard). Of these, lupin is known to cross-react with peanut, and sesame seed allergy is increasingly prevalent. In fact, the FDA has considered adding it to the list of major allergens.
Meanwhile, according to this temporary FDA policy, substitutions should be limited to no more than 2% of the weight of the final product unless it is a variety of the same ingredient. The example provided is substitution of one type of mushroom for another, but even that could be an issue for the rare patient. And what if this is misinterpreted – as will surely happen somewhere – and one seed is substituted for another?
A friend of mine is a pediatrician and mother of a child who is allergic to sesame, peanuts, tree nuts, and garbanzo beans. Naturally, she had grave concerns about these changes. She also wondered what the liability would be for the food manufacturing company in the current situation despite the FDA notice, which seems like a valid point. It is worth noting that, at the very top of this FDA notice, are the words “contains nonbinding recommendations,” so manufacturers may want to think twice about how they approach this. A minority of companies have pledged to relabel foods if necessary. Meanwhile, without any alert in advance, it is now up to patients and their physicians to sort out the attendant risks.
The FDA should have advised or mandated that food manufacturers give notice to online and physical retailers of ingredient changes. A simple sign in front of a display or alert online would be a very reasonable solution and pose no burden to those involved. It should be self-evident that mistakes always happen, especially under duress, and that the loosening of these regulations will have unintended consequences. To the severe problem of food insecurity, we can add one more concern for the parents of allergic children: food-allergen insecurity.
A version of this article originally appeared on Medscape.com.
Elevated inflammation common in children’s severe COVID-19 disease
according to data from 50 patients at a single tertiary care center.
“Risk factors for severe disease in pediatric populations have not been clearly identified and the high prevalence of SARS-CoV-2 in NYC offers an opportunity to describe severe pediatric disease in more detail,” wrote Philip Zachariah, MD, of New York–Presbyterian Hospital, New York, and colleagues.
In a retrospective case series published in JAMA Pediatrics, the researchers reviewed data from 50 patients: 41 classified as severe and 9 classified as nonsevere. Among the patients, 27 were male and 25 were Hispanic. The patient population had a median of 2 days from symptom onset to hospital admission. The most common symptoms were fever (80%) and respiratory symptoms (64%). Seventy-six percent of patients had a median length of stay of 3 days (range 1-30 days).
At hospital admission, children with severe disease had significantly higher levels of several inflammatory markers compared with those without severe disease, notably C-reactive protein (median 8.978 mg/dL vs. 0.64 mg/dL) and procalcitonin (median 0.31 ng/mL vs. 0.17 ng/mL, (P < .001 for both). High mean peak levels of C-reactive protein, procalcitonin, interleukin 6, ferritin, and D-dimer were seen among the nine children (16%) who required mechanical ventilation, Dr. Zachariah and associates said.
None of the 14 infants and 1 of the 8 immunocompromised children in the study had severe disease, the researchers wrote.
Bacterial coinfections detected while patients were hospitalized were bacteremia in 6%, suspected bacterial pneumonia in 18%, urinary tract infections in 10%, skin and soft tissue infections in 6%, and streptococcus pharyngitis in 2%, Dr. Zachariah and associates reported.
Overall, 61% of the children had comorbidities identified in previous COVID-19 studies, of which obesity was the most common (22%); other comorbidities included asthma, sickle cell disease, cardiac disease, and diabetes. Obesity also was significantly associated with the need for mechanical ventilation in children aged 2 years and older (67%). A total of 16 patients required respiratory support, 9 of these were placed on mechanical ventilation; 6 of these 9 children were obese.
Fifteen patients (30%) who met criteria for increased oxygen requirements and respiratory distress received hydroxychloroquine, but the small sample size did not allow for assessment of treatment efficacy, the researchers said.
“Expanding our knowledge of COVID-19 [disease] in children will potentially permit early recognition of SARS-CoV-2 infection, understanding of the natural history of disease, and potential complications, said Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. This review of 50 SARS-CoV-2 infected children (less than 21 years of age) “provides insight into the short period of symptoms prior to hospitalization, challenges the concept that infants less than 1 year are at greatest risk of severe disease (as from the experience in China), and suggests rapid recovery in many children, as median length of stay was 3 days.
“The review revealed two findings that were surprising to me. First, the median length of stay of 3 days. As nearly 20% of the children required mechanical ventilation, it suggests many of the children were discharged quickly after evaluation, suggesting that we need to identify markers of severity to predict those children likely to have progressive disease and require respiratory support,” Dr. Pelton noted.
“The second observation suggests high rates of bacterial infection (bacteremia, pneumonia, UTI, and skin and soft tissue infection). I do not think this has been widely reported in adults, and may represent a difference between child and adult disease. More studies such as this will be required to identify how common coinfection with bacteria is,” he said.
“The take-home message is that although most children with COVID-19 have a mild or even asymptomatic course, some become severely ill requiring ventilator support and potentially ECMO [extracorporeal membrane oxygenation]. Potential predictors of severity include high C-reactive protein, obesity, and older age [adolescence], said Dr. Pelton, who was not involved in the study.
What additional research is needed? Dr. Pelton said that better markers of severe disease are needed, as well as an understanding of why obesity is a risk factor for severe disease in both children and adults. Are these prediabetic patients? he asked.
The study findings were limited by the small sample size and high proportion of Hispanic patients, which may limit generalizability, and some symptoms and comorbidities may have been missed because of the retrospective nature of the study, the researchers noted. However, the results support the need for hospitals to remain vigilant to the variable presentations of COVID-19 infections in children.
“Therapeutic considerations need to [include] the risk of toxicity, control of antiviral replication, and early recognition and management of immune dysregulation,” they concluded.
The study received no outside funding. Dr. Zachariah had no financial conflicts to disclose. Two coauthors reported ties with various pharmaceutical companies and organizations. Dr. Pelton said he had no relevant financial disclosures.
SOURCE: Zachariah P et al. JAMA Pediatr. 2020 June 3. doi:10.1001/jamapediatrics.2020.2430.
according to data from 50 patients at a single tertiary care center.
“Risk factors for severe disease in pediatric populations have not been clearly identified and the high prevalence of SARS-CoV-2 in NYC offers an opportunity to describe severe pediatric disease in more detail,” wrote Philip Zachariah, MD, of New York–Presbyterian Hospital, New York, and colleagues.
In a retrospective case series published in JAMA Pediatrics, the researchers reviewed data from 50 patients: 41 classified as severe and 9 classified as nonsevere. Among the patients, 27 were male and 25 were Hispanic. The patient population had a median of 2 days from symptom onset to hospital admission. The most common symptoms were fever (80%) and respiratory symptoms (64%). Seventy-six percent of patients had a median length of stay of 3 days (range 1-30 days).
At hospital admission, children with severe disease had significantly higher levels of several inflammatory markers compared with those without severe disease, notably C-reactive protein (median 8.978 mg/dL vs. 0.64 mg/dL) and procalcitonin (median 0.31 ng/mL vs. 0.17 ng/mL, (P < .001 for both). High mean peak levels of C-reactive protein, procalcitonin, interleukin 6, ferritin, and D-dimer were seen among the nine children (16%) who required mechanical ventilation, Dr. Zachariah and associates said.
None of the 14 infants and 1 of the 8 immunocompromised children in the study had severe disease, the researchers wrote.
Bacterial coinfections detected while patients were hospitalized were bacteremia in 6%, suspected bacterial pneumonia in 18%, urinary tract infections in 10%, skin and soft tissue infections in 6%, and streptococcus pharyngitis in 2%, Dr. Zachariah and associates reported.
Overall, 61% of the children had comorbidities identified in previous COVID-19 studies, of which obesity was the most common (22%); other comorbidities included asthma, sickle cell disease, cardiac disease, and diabetes. Obesity also was significantly associated with the need for mechanical ventilation in children aged 2 years and older (67%). A total of 16 patients required respiratory support, 9 of these were placed on mechanical ventilation; 6 of these 9 children were obese.
Fifteen patients (30%) who met criteria for increased oxygen requirements and respiratory distress received hydroxychloroquine, but the small sample size did not allow for assessment of treatment efficacy, the researchers said.
“Expanding our knowledge of COVID-19 [disease] in children will potentially permit early recognition of SARS-CoV-2 infection, understanding of the natural history of disease, and potential complications, said Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. This review of 50 SARS-CoV-2 infected children (less than 21 years of age) “provides insight into the short period of symptoms prior to hospitalization, challenges the concept that infants less than 1 year are at greatest risk of severe disease (as from the experience in China), and suggests rapid recovery in many children, as median length of stay was 3 days.
“The review revealed two findings that were surprising to me. First, the median length of stay of 3 days. As nearly 20% of the children required mechanical ventilation, it suggests many of the children were discharged quickly after evaluation, suggesting that we need to identify markers of severity to predict those children likely to have progressive disease and require respiratory support,” Dr. Pelton noted.
“The second observation suggests high rates of bacterial infection (bacteremia, pneumonia, UTI, and skin and soft tissue infection). I do not think this has been widely reported in adults, and may represent a difference between child and adult disease. More studies such as this will be required to identify how common coinfection with bacteria is,” he said.
“The take-home message is that although most children with COVID-19 have a mild or even asymptomatic course, some become severely ill requiring ventilator support and potentially ECMO [extracorporeal membrane oxygenation]. Potential predictors of severity include high C-reactive protein, obesity, and older age [adolescence], said Dr. Pelton, who was not involved in the study.
What additional research is needed? Dr. Pelton said that better markers of severe disease are needed, as well as an understanding of why obesity is a risk factor for severe disease in both children and adults. Are these prediabetic patients? he asked.
The study findings were limited by the small sample size and high proportion of Hispanic patients, which may limit generalizability, and some symptoms and comorbidities may have been missed because of the retrospective nature of the study, the researchers noted. However, the results support the need for hospitals to remain vigilant to the variable presentations of COVID-19 infections in children.
“Therapeutic considerations need to [include] the risk of toxicity, control of antiviral replication, and early recognition and management of immune dysregulation,” they concluded.
The study received no outside funding. Dr. Zachariah had no financial conflicts to disclose. Two coauthors reported ties with various pharmaceutical companies and organizations. Dr. Pelton said he had no relevant financial disclosures.
SOURCE: Zachariah P et al. JAMA Pediatr. 2020 June 3. doi:10.1001/jamapediatrics.2020.2430.
according to data from 50 patients at a single tertiary care center.
“Risk factors for severe disease in pediatric populations have not been clearly identified and the high prevalence of SARS-CoV-2 in NYC offers an opportunity to describe severe pediatric disease in more detail,” wrote Philip Zachariah, MD, of New York–Presbyterian Hospital, New York, and colleagues.
In a retrospective case series published in JAMA Pediatrics, the researchers reviewed data from 50 patients: 41 classified as severe and 9 classified as nonsevere. Among the patients, 27 were male and 25 were Hispanic. The patient population had a median of 2 days from symptom onset to hospital admission. The most common symptoms were fever (80%) and respiratory symptoms (64%). Seventy-six percent of patients had a median length of stay of 3 days (range 1-30 days).
At hospital admission, children with severe disease had significantly higher levels of several inflammatory markers compared with those without severe disease, notably C-reactive protein (median 8.978 mg/dL vs. 0.64 mg/dL) and procalcitonin (median 0.31 ng/mL vs. 0.17 ng/mL, (P < .001 for both). High mean peak levels of C-reactive protein, procalcitonin, interleukin 6, ferritin, and D-dimer were seen among the nine children (16%) who required mechanical ventilation, Dr. Zachariah and associates said.
None of the 14 infants and 1 of the 8 immunocompromised children in the study had severe disease, the researchers wrote.
Bacterial coinfections detected while patients were hospitalized were bacteremia in 6%, suspected bacterial pneumonia in 18%, urinary tract infections in 10%, skin and soft tissue infections in 6%, and streptococcus pharyngitis in 2%, Dr. Zachariah and associates reported.
Overall, 61% of the children had comorbidities identified in previous COVID-19 studies, of which obesity was the most common (22%); other comorbidities included asthma, sickle cell disease, cardiac disease, and diabetes. Obesity also was significantly associated with the need for mechanical ventilation in children aged 2 years and older (67%). A total of 16 patients required respiratory support, 9 of these were placed on mechanical ventilation; 6 of these 9 children were obese.
Fifteen patients (30%) who met criteria for increased oxygen requirements and respiratory distress received hydroxychloroquine, but the small sample size did not allow for assessment of treatment efficacy, the researchers said.
“Expanding our knowledge of COVID-19 [disease] in children will potentially permit early recognition of SARS-CoV-2 infection, understanding of the natural history of disease, and potential complications, said Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. This review of 50 SARS-CoV-2 infected children (less than 21 years of age) “provides insight into the short period of symptoms prior to hospitalization, challenges the concept that infants less than 1 year are at greatest risk of severe disease (as from the experience in China), and suggests rapid recovery in many children, as median length of stay was 3 days.
“The review revealed two findings that were surprising to me. First, the median length of stay of 3 days. As nearly 20% of the children required mechanical ventilation, it suggests many of the children were discharged quickly after evaluation, suggesting that we need to identify markers of severity to predict those children likely to have progressive disease and require respiratory support,” Dr. Pelton noted.
“The second observation suggests high rates of bacterial infection (bacteremia, pneumonia, UTI, and skin and soft tissue infection). I do not think this has been widely reported in adults, and may represent a difference between child and adult disease. More studies such as this will be required to identify how common coinfection with bacteria is,” he said.
“The take-home message is that although most children with COVID-19 have a mild or even asymptomatic course, some become severely ill requiring ventilator support and potentially ECMO [extracorporeal membrane oxygenation]. Potential predictors of severity include high C-reactive protein, obesity, and older age [adolescence], said Dr. Pelton, who was not involved in the study.
What additional research is needed? Dr. Pelton said that better markers of severe disease are needed, as well as an understanding of why obesity is a risk factor for severe disease in both children and adults. Are these prediabetic patients? he asked.
The study findings were limited by the small sample size and high proportion of Hispanic patients, which may limit generalizability, and some symptoms and comorbidities may have been missed because of the retrospective nature of the study, the researchers noted. However, the results support the need for hospitals to remain vigilant to the variable presentations of COVID-19 infections in children.
“Therapeutic considerations need to [include] the risk of toxicity, control of antiviral replication, and early recognition and management of immune dysregulation,” they concluded.
The study received no outside funding. Dr. Zachariah had no financial conflicts to disclose. Two coauthors reported ties with various pharmaceutical companies and organizations. Dr. Pelton said he had no relevant financial disclosures.
SOURCE: Zachariah P et al. JAMA Pediatr. 2020 June 3. doi:10.1001/jamapediatrics.2020.2430.
FROM JAMA PEDIATRICS