User login
Is it safe to add long-acting β-2 agonists to inhaled corticosteroids in patients with persistent asthma?
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
Possibly. Long-acting β-2 agonists (LABAs) used in combination with inhaled corticosteroids (ICS) don’t appear to increase all-cause mortality or serious adverse events in patients with persistent asthma compared with ICS alone. Studies showing an increase in catastrophic events had serious methodologic issues. A large surveillance study is ongoing (strength of recommendation: A, meta-analysis of randomized controlled trials [RCTs]).
No significant difference in combination therapy vs ICS alone
In 2013, a Cochrane review analyzed the risk of mortality and nonfatal serious adverse events in patients treated with the LABA salmeterol in combination with ICS, compared with patients receiving the same dose of ICS alone.1 The review included 35 RCTs of moderate quality with 13,447 adolescents and adults and 5 RCTs with 1862 children. Patients had all stages of asthma; mean study duration was 34 weeks in adult trials and 15 weeks in trials of children.
Seven deaths from all causes occurred in both the salmeterol-plus-ICS group and the ICS-alone group (35 trials, N=13,447; Peto odds ratio [OR]=0.90; 95% confidence interval [CI], 0.31-2.6). No deaths in children and no asthma-related deaths occurred in any study participants (40 trials, N=15,309).
Adults treated with ICS alone showed no significant difference from adults receiving combination therapy in the frequency of serious adverse events (defined as life threatening, requiring hospitalization or prolongation of existing hospitalization, or resulting in persistent or significant disability or incapacity). Adults on ICS had 21 events per 1000 compared with 24 per 1000 in adults on combination treatment (35 trials, N=13,447; Peto OR=1.2; 95% CI, 0.91-1.4).
Asthma-related serious adverse events were reported in 29 of 6986 adults in the combination group and 23 of 6461 in the ICS-alone group, a nonsignificant difference (35 trials, N=13,447; Peto OR=1.1; 95% CI, 0.65-1.9).
Only one serious asthma-related adverse event occurred in each group of children (ICS- and combination-treated); (5 trials, N=1862; Peto OR=0.99; 95% CI, 0.6-16). Because the number of events was so small and the results were so imprecise, a relative increase in all-cause mortality or nonfatal adverse events can’t be completely ruled out.
Inconsistent dosages mar trials that show more catastrophic events
A systematic review of 7 RCTs with 7253 asthmatic patients compared LABA plus ICS or ICS alone at various doses. All of the trials included at least one catastrophic event, defined as an asthma-related intubation or death.2 The mean ages of the patients varied from 11 to 48 years, and the length of the studies from 12 to 52 weeks. The risk of catastrophic events was greater in the LABA plus ICS groups than ICS alone (OR=3.7; 95% CI, 1.4-9.6).
Only one of the 7 trials was included in the 2013 Cochrane review. The others were excluded because the control groups used different doses of ICS than the LABA-plus-ICS groups. In one trial, for example, the ICS group used 4 times the dose of budesonide used in the LABA-plus-ICS group. The difference in outcomes may therefore reflect the variation in ICS dose rather than the presence or absence of LABA.
Because of these conflicting results, the US Food and Drug Administration has mandated continued evaluation of LABAs by manufacturers.3 Five clinical trials that are multinational, randomized, double-blind, and lasting at least 6 months will evaluate the safety of LABAs plus fixed-dose ICS compared with fixed-dose ICS alone. A total of 6200 children and 46,800 adults will be enrolled in the studies, whose results should be available in 2017.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
1. Cates CJ, Jaeschke R, Schmidt S, et al. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events. Cochrane Database of Syst Rev. 2013;(3):CD006922.
2. Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322-328.
3. Chowdhury BA, Seymour SM, Levenson MS. Assessing the safety of adding LABAs to inhaled corticosteroids for treating asthma. N Engl J Med. 2011;364:2473-2475.
Evidence-based answers from the Family Physicians Inquiries Network
Exercise-induced Anaphylaxis
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Anaphylaxis is a relatively common occurrence for many adolescents. As primary care doctors, we normally see the patient after the acute phase, and then are required to do the detective work to figure out the causes of the episode. The cause may be obvious, but many times we have to hope for another occurrence with similar circumstances to identify it. Surprisingly, the cause may not be what you think. Factors that contribute to an anaphylaxis response may be related to activity, timing of food ingestion, an environmental factor, or medication.
Let’s look at just one type, exercise-induced anaphylaxis. It’s divided into two categories: food dependent and nonfood dependent. Both are described as an induction of itching, urticaria, and fatigue, with progression to angioedema and hypotension, associated with exercise (J. Allergy Clin. Immunol. 1980;66:106-11).
Food-dependent exercise-induced anaphylaxis occurs when exercise is started 30 minutes after ingesting food. This may be difficult to identify because patients react to the food only if they exercise, so food is usually eliminated as a cause. Wheat and wheat flour are common culprits for this type of reaction because of the omega-5 gliadin, which is the protein in gluten (J. Allergy Clin. Immunol. 1991;87:34-40). In one study, larger amounts of the suspected agent were given; hives and angioedema did start to occur in 20% of patients challenged, which suggested that there was likely a baseline allergy to the food, and exercise itself might be a cofactor in augmentation of the allergic reaction.
In nonfood-dependent exercise-induced anaphylaxis, symptoms of itching, urticaria, and fatigue can occur 5-30 minutes after the start of exercise. Although bronchospasm is rare, it can occur along with angioedema, nausea, vomiting, and hypotension, and can even be fatal if exercise continues. If exercise is stopped, it usually resolves. However, many people try to push through it, which only worsens the symptoms.
Cofactors associated with nonfood-dependent exercise-induced anaphylaxis are ingestion of alcohol and an NSAID several hours beforehand. These agents also might be overlooked if well tolerated independently (Br. J. Dermatol. 2001;145:336-9).
Timing of the episode also plays a role. Premenstrual syndrome can be a factor in augmentation of anaphylaxis, so it also should be considered. Knowing the date of the last menstrual cycle and identifying if the anaphylaxis is episodic will identify premenstrual syndrome as a cause.
The work-up should include standard allergy testing and determination of tryptase levels. Skin testing is essential to identify offending agents, and is rarely negative. If a food is suspected and skin testing is negative, repeat the skin testing in 6 months. In one study, wheat extract was found to be positive in only 29% of persons suspected of having a wheat allergy, but when the paste of wheat flour was tested, 80% were identified. The ImmunoCAP Test also was found to have a sensitivity of 80%, so it is a valuable test to try along with the skin prick.
Tryptase levels should be evaluated because in nonfood-dependent exercise-induced anaphylaxis, these levels are slightly elevated at the time of the anaphylaxis, but return to normal. A patient with mastocytosis, a group of disorders characterized by pathologic mast cells infiltrating the skin, will consistently have elevated tryptase levels. Seasonal allergies associated with pollen, and asthma bronchospasm also should be considered as causes.
Although these exercise-induced anaphylaxis episodes can occur at any age, they are most frequent in the adolescent age group, probably because that’s the time most of this population are involved in organized sports. Upon presentation, a careful detailed history will help to identify the cause of anaphylaxis and result in quicker resolution.
Treatment includes avoidance of the offending agent if identified and an antihistamine, and if symptoms do occur, ceasing exercise immediately to avoid a full-blown anaphylactic reaction.
Dr. Pearce is a pediatrician in Frankfort, Ill.
Pleuritic chest pain and globus pharyngeus
A 22-year-old woman with a history of attention-deficit/hyperactivity disorder and childhood asthma came to the emergency department (ED) for treatment of a cramping, substernal, pleuritic chest pain she’d had for a week and the feeling of a “lump in her throat” that made it difficult and painful for her to swallow. The patient’s vital signs were normal and her substernal chest pain was reproducible with palpation. An anteroposterior (AP) chest x-ray (CXR) was unremarkable.
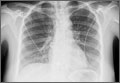
A “GI cocktail” (lidocaine, Mylanta and Donnatal), ketorolac, morphine, and lorazepam were administered in the ED, but did not provide the patient with any relief. She was admitted to the hospital to rule out acute coronary syndrome and was kept NPO overnight. A repeat CXR with posteroanterior (PA) and lateral views was also obtained (FIGURE 1A AND 1B).
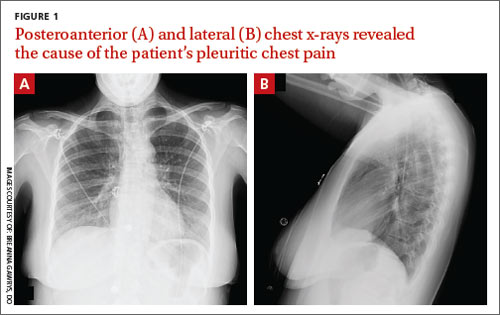
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pneumomediastinum
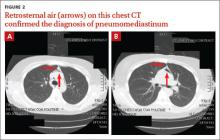
The PA and lateral view CXRs revealed the presence of retrosternal air, suggesting the patient had pneumomediastinum. A computed tomography (CT) scan of the chest also showed retrosternal air (FIGURE 2A AND 2B, arrows) and confirmed this diagnosis. To rule out esophageal perforation, the team ordered Gastrografin and barium swallow studies. The patient was kept NPO until both studies were confirmed to be negative.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by Hamman’s sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Differential diagnosis includes inflammatory conditions
The differential diagnosis for pneumomediastinum includes pericarditis, mediastinitis, Boerhaave syndrome, and acute coronary syndrome.
Pericarditis. In a patient with inflammation of the pericardium, you would hear reduced heart sounds and observe electrocardiogram (EKG) changes (eg, diffuse ST elevation in acute pericarditis). These signs typically would not be present in a patient with pneumomediastinum.1
Mediastinitis. Patients with mediastinitis—inflammation of the mediastinum—are more likely to have hypotension and shock.1
Boerhaave syndrome, or spontaneous esophageal perforation, has a similar presentation to pneumomediastinum but is more likely to be accompanied by hypotension and shock. Additionally, there would be extravasation of the contrast agent during swallow studies.2
Acute coronary syndrome is also part of the differential. However, in ACS, you would see ST changes on the patient’s EKG and elevated cardiac enzymes.1
Lateral x-rays are especially useful in making the diagnosis
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
For most patients, rest and pain control are key
Because pneumomediastinum is generally a self-limiting condition, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home.
If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation. If the result of this test is abnormal, a follow-up study with barium is recommended.3 Gastrografin swallow studies are the preferred initial study.3 A barium swallow study is more sensitive, but has a higher risk of causing pneumomediastinitis if an esophageal perforation is present.2
If the swallow study reveals a perforation, surgical decompression and antibiotics may be necessary.1,4,5
Our patient received subsequent serial CXRs that showed improvement in pneumomediastinum. Once our patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged home after a 3-day hospitalization with close follow-up. One week later, she had no further complaints and her pain had almost entirely resolved.
CORRESPONDENCE
Breanna Gawrys, DO, Fort Belvoir Community Hospital Family Medicine Residency, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:2039–2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057.
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334.
4. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31:1110-1114.
5. Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol. 2001;31:67-75.
A 22-year-old woman with a history of attention-deficit/hyperactivity disorder and childhood asthma came to the emergency department (ED) for treatment of a cramping, substernal, pleuritic chest pain she’d had for a week and the feeling of a “lump in her throat” that made it difficult and painful for her to swallow. The patient’s vital signs were normal and her substernal chest pain was reproducible with palpation. An anteroposterior (AP) chest x-ray (CXR) was unremarkable.
A “GI cocktail” (lidocaine, Mylanta and Donnatal), ketorolac, morphine, and lorazepam were administered in the ED, but did not provide the patient with any relief. She was admitted to the hospital to rule out acute coronary syndrome and was kept NPO overnight. A repeat CXR with posteroanterior (PA) and lateral views was also obtained (FIGURE 1A AND 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pneumomediastinum
The PA and lateral view CXRs revealed the presence of retrosternal air, suggesting the patient had pneumomediastinum. A computed tomography (CT) scan of the chest also showed retrosternal air (FIGURE 2A AND 2B, arrows) and confirmed this diagnosis. To rule out esophageal perforation, the team ordered Gastrografin and barium swallow studies. The patient was kept NPO until both studies were confirmed to be negative.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by Hamman’s sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Differential diagnosis includes inflammatory conditions
The differential diagnosis for pneumomediastinum includes pericarditis, mediastinitis, Boerhaave syndrome, and acute coronary syndrome.
Pericarditis. In a patient with inflammation of the pericardium, you would hear reduced heart sounds and observe electrocardiogram (EKG) changes (eg, diffuse ST elevation in acute pericarditis). These signs typically would not be present in a patient with pneumomediastinum.1
Mediastinitis. Patients with mediastinitis—inflammation of the mediastinum—are more likely to have hypotension and shock.1
Boerhaave syndrome, or spontaneous esophageal perforation, has a similar presentation to pneumomediastinum but is more likely to be accompanied by hypotension and shock. Additionally, there would be extravasation of the contrast agent during swallow studies.2
Acute coronary syndrome is also part of the differential. However, in ACS, you would see ST changes on the patient’s EKG and elevated cardiac enzymes.1
Lateral x-rays are especially useful in making the diagnosis
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
For most patients, rest and pain control are key
Because pneumomediastinum is generally a self-limiting condition, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home.
If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation. If the result of this test is abnormal, a follow-up study with barium is recommended.3 Gastrografin swallow studies are the preferred initial study.3 A barium swallow study is more sensitive, but has a higher risk of causing pneumomediastinitis if an esophageal perforation is present.2
If the swallow study reveals a perforation, surgical decompression and antibiotics may be necessary.1,4,5
Our patient received subsequent serial CXRs that showed improvement in pneumomediastinum. Once our patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged home after a 3-day hospitalization with close follow-up. One week later, she had no further complaints and her pain had almost entirely resolved.
CORRESPONDENCE
Breanna Gawrys, DO, Fort Belvoir Community Hospital Family Medicine Residency, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
A 22-year-old woman with a history of attention-deficit/hyperactivity disorder and childhood asthma came to the emergency department (ED) for treatment of a cramping, substernal, pleuritic chest pain she’d had for a week and the feeling of a “lump in her throat” that made it difficult and painful for her to swallow. The patient’s vital signs were normal and her substernal chest pain was reproducible with palpation. An anteroposterior (AP) chest x-ray (CXR) was unremarkable.
A “GI cocktail” (lidocaine, Mylanta and Donnatal), ketorolac, morphine, and lorazepam were administered in the ED, but did not provide the patient with any relief. She was admitted to the hospital to rule out acute coronary syndrome and was kept NPO overnight. A repeat CXR with posteroanterior (PA) and lateral views was also obtained (FIGURE 1A AND 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pneumomediastinum
The PA and lateral view CXRs revealed the presence of retrosternal air, suggesting the patient had pneumomediastinum. A computed tomography (CT) scan of the chest also showed retrosternal air (FIGURE 2A AND 2B, arrows) and confirmed this diagnosis. To rule out esophageal perforation, the team ordered Gastrografin and barium swallow studies. The patient was kept NPO until both studies were confirmed to be negative.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by Hamman’s sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Differential diagnosis includes inflammatory conditions
The differential diagnosis for pneumomediastinum includes pericarditis, mediastinitis, Boerhaave syndrome, and acute coronary syndrome.
Pericarditis. In a patient with inflammation of the pericardium, you would hear reduced heart sounds and observe electrocardiogram (EKG) changes (eg, diffuse ST elevation in acute pericarditis). These signs typically would not be present in a patient with pneumomediastinum.1
Mediastinitis. Patients with mediastinitis—inflammation of the mediastinum—are more likely to have hypotension and shock.1
Boerhaave syndrome, or spontaneous esophageal perforation, has a similar presentation to pneumomediastinum but is more likely to be accompanied by hypotension and shock. Additionally, there would be extravasation of the contrast agent during swallow studies.2
Acute coronary syndrome is also part of the differential. However, in ACS, you would see ST changes on the patient’s EKG and elevated cardiac enzymes.1
Lateral x-rays are especially useful in making the diagnosis
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
For most patients, rest and pain control are key
Because pneumomediastinum is generally a self-limiting condition, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home.
If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation. If the result of this test is abnormal, a follow-up study with barium is recommended.3 Gastrografin swallow studies are the preferred initial study.3 A barium swallow study is more sensitive, but has a higher risk of causing pneumomediastinitis if an esophageal perforation is present.2
If the swallow study reveals a perforation, surgical decompression and antibiotics may be necessary.1,4,5
Our patient received subsequent serial CXRs that showed improvement in pneumomediastinum. Once our patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged home after a 3-day hospitalization with close follow-up. One week later, she had no further complaints and her pain had almost entirely resolved.
CORRESPONDENCE
Breanna Gawrys, DO, Fort Belvoir Community Hospital Family Medicine Residency, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:2039–2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057.
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334.
4. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31:1110-1114.
5. Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol. 2001;31:67-75.
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:2039–2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057.
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334.
4. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31:1110-1114.
5. Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol. 2001;31:67-75.
Respiratory Disorders Most Common Cause of Childhood Hospitalization
The three most common causes of nonneonatal and nonmaternal hospitalization in 2012 for children under 18 years were all respiratory disorders, according to a report from the Agency for Healthcare Research and Quality.
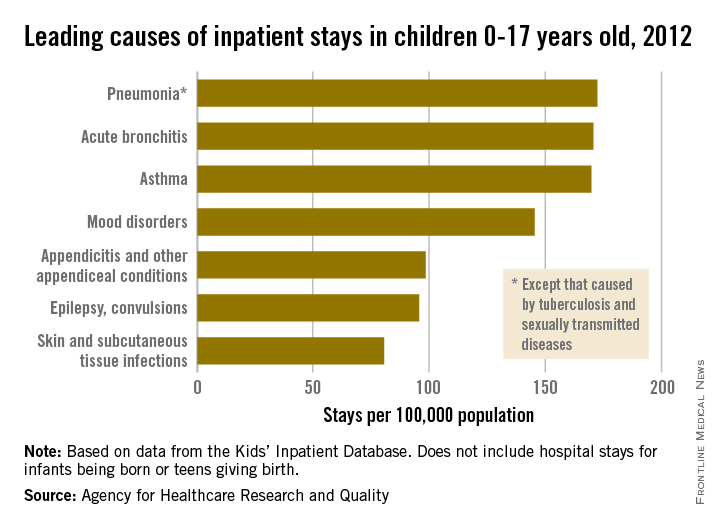
Pneumonia was the most common cause of hospitalization in children, with stays occurring at a rate of 169/100,000 population. There was very little difference in the rate between pneumonia and the next two most common diagnoses, acute bronchitis and asthma, which occurred at a rate of 168/100,000 and 167/100,000, respectively.
Mood disorders were the most common nonrespiratory and nonmaternal hospitalization diagnosis, with a hospital admission incidence of 144/100,000 population. This was followed by appendicitis (97/100,000), epilepsy and convulsions (95/100,000), and skin and subcutaneous tissue infections (80/100,000). The overall rate of hospitalization in children under age 18 years for nonneonatal and nonmaternal diagnoses was just over 2,500/100,000.
The incidence of six of the seven most common causes of hospitalization, and all but 1 of the 24 reported diagnostic categories, either decreased or remained steady from 2000 to 2012. Only skin and subcutaneous tissue conditions saw a significant increase in that time period. “Understanding the reasons why children are hospitalized and examining trends over time is critical to inform clinical practice and health policy,” the researchers commented.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.
The three most common causes of nonneonatal and nonmaternal hospitalization in 2012 for children under 18 years were all respiratory disorders, according to a report from the Agency for Healthcare Research and Quality.
Pneumonia was the most common cause of hospitalization in children, with stays occurring at a rate of 169/100,000 population. There was very little difference in the rate between pneumonia and the next two most common diagnoses, acute bronchitis and asthma, which occurred at a rate of 168/100,000 and 167/100,000, respectively.
Mood disorders were the most common nonrespiratory and nonmaternal hospitalization diagnosis, with a hospital admission incidence of 144/100,000 population. This was followed by appendicitis (97/100,000), epilepsy and convulsions (95/100,000), and skin and subcutaneous tissue infections (80/100,000). The overall rate of hospitalization in children under age 18 years for nonneonatal and nonmaternal diagnoses was just over 2,500/100,000.
The incidence of six of the seven most common causes of hospitalization, and all but 1 of the 24 reported diagnostic categories, either decreased or remained steady from 2000 to 2012. Only skin and subcutaneous tissue conditions saw a significant increase in that time period. “Understanding the reasons why children are hospitalized and examining trends over time is critical to inform clinical practice and health policy,” the researchers commented.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.
The three most common causes of nonneonatal and nonmaternal hospitalization in 2012 for children under 18 years were all respiratory disorders, according to a report from the Agency for Healthcare Research and Quality.
Pneumonia was the most common cause of hospitalization in children, with stays occurring at a rate of 169/100,000 population. There was very little difference in the rate between pneumonia and the next two most common diagnoses, acute bronchitis and asthma, which occurred at a rate of 168/100,000 and 167/100,000, respectively.
Mood disorders were the most common nonrespiratory and nonmaternal hospitalization diagnosis, with a hospital admission incidence of 144/100,000 population. This was followed by appendicitis (97/100,000), epilepsy and convulsions (95/100,000), and skin and subcutaneous tissue infections (80/100,000). The overall rate of hospitalization in children under age 18 years for nonneonatal and nonmaternal diagnoses was just over 2,500/100,000.
The incidence of six of the seven most common causes of hospitalization, and all but 1 of the 24 reported diagnostic categories, either decreased or remained steady from 2000 to 2012. Only skin and subcutaneous tissue conditions saw a significant increase in that time period. “Understanding the reasons why children are hospitalized and examining trends over time is critical to inform clinical practice and health policy,” the researchers commented.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.
Anxiety tied to fear of falling • fatigue • difficulty concentrating • Dx?
THE CASE
A 21-year-old college student was referred to us by the counseling center at our university for a psychiatric evaluation after 11 psychotherapy sessions over 3 months had failed to reduce her feelings of anxiety and panic.
During our evaluation, the patient described feeling “not quite right” for many months. She had been experiencing mental fogginess, fatigue, and worsening concentration/ memory. Her anxiety, which had been gradually increasing, was the result of being unsure about her gait. She first noticed this while walking down some bleachers; she felt dizzy, was afraid of falling, and couldn’t walk down without assistance. All episodes of “panic” occurred in situations where she experienced disequilibrium, unsteady gait, and fear of falling. She grew fearful of driving or going anywhere without assistance.
The patient had celiac disease that was well controlled with a gluten-free diet. She had no personal or family psychiatric history and no history of substance abuse.
THE DIAGNOSIS
Physical exam and lab studies, including a complete blood count, comprehensive metabolic panel, and thyrotropin and folate levels, were normal. Her homocysteine level was 11.8 μmol/L (reference range, 5.4-11.9 μmol/L) and vitamin B12 level was 292 pg/mL (reference range, 200-1100 pg/mL). Her lab report included a note that read, “Although the reference range for vitamin B12 is 200 to 1100 pg/mL, it has been reported that between 5% and 10% of patients with values between 200 and 400 pg/mL may experience neuropsychiatric and hematologic abnormalities due to occult B12 deficiency; <1% of patients with values >400 pg/mL will have symptoms.”
Based on this vitamin B12 level, the patient’s symptoms, and her borderline high homocysteine level, we diagnosed vitamin B12 deficiency.
DISCUSSION
There are no recommendations by the US Preventive Services Task Force or any other major US medical society for routine vitamin B12 screening.1 In Canada, the Medical Services Commission of the British Columbia Ministry of Health recommends B12 screening for patients who present with macrocytic anemia or unexplained neurologic symptoms (eg, paresthesia, numbness, poor motor coordination, memory lapses, or cognitive or personality changes).2
Vitamin B12 deficiency can be caused by numerous conditions, including those that cause malabsorption (such as gastric bypass). It can also be caused by diseases such as human immunodeficiency virus infection or Crohn’s disease, long-term adherence to a vegetarian or vegan diet, or by any other lack of dietary intake.1 The condition can cause hematologic-related signs and symptoms such as megaloblastic anemia, fatigue, and syncope. It also can have neurologic manifestations, including paresthesia, weakness, motor disturbances (including gait abnormalities), vision loss, and a wide range of cognitive and behavioral changes.1 Anemia is uncommon because since 1998, the US Food and Drug Administration has required fortification of all enriched grain and cereal products with folic acid; thus, vitamin B12 deficiency may proceed without anemia revealing its presence.1
A controversial topic. Vitamin B12 deficiency is a complicated and controversial subject. Specifically, there is uncertainty about the clinical importance of lower serum levels of vitamin B12 (200-400 pg/mL), their impact on well-being, and the need for treatment. In addition to measuring a patient’s serum B12 level, testing a second biomarker (such as homocysteine or methylmalonic acid) can be helpful in establishing a diagnosis of B12 deficiency.1 Levels of each of these are elevated in patients with B12 deficiency.1
Although vitamin B12 deficiency has been well studied in older patients,3 little has been published about the condition in young adults. National Health and Nutrition Examination Survey (NHANES) data from 2000 to 2004 shows that almost 40% of people ages 19 to 30 years have a B12 level <400 pg/mL.1 How many of these individuals are at risk of complications of B12 deficiency is unknown.
B12 supplementation might improve depression, anxiety
B12 supplementation is inexpensive and has no significant adverse effects.1 It can be administered orally, parenterally (intramuscularly or subcutaneously), or intranasally.1 A common oral regimen is 1 mg/d; parental regimens vary widely, but might include a 1-mg injection once a week for 8 weeks, then once a month for life.1
Some evidence suggests B12 supplementation may improve symptoms of depression and anxiety. A Pakistani study randomized 73 patients with depression and “low normal” B12 levels (190-300 pg/mL) to an antidepressant only (equivalent to imipramine 100-250 mg/d or fluoxetine 20-40 mg/d) or an antidepressant plus parenteral B12 (1000 mcg once a week).4 At 3 months follow-up, 100% of the treatment group showed at least a 20% reduction in their Hamilton Depression Rating Scale (HAM-D) score, compared to 69% in the control arm (P<.001).4
A Swedish study analyzed the effects of several B vitamins, including 0.5 mg/d of B12 vs placebo on mood in 65 celiac patients on a gluten-free diet who had borderline/low normal B12 levels (>191 pg/mL).5 Patients who scored low on a measure of psychological well-being at the beginning of the study and who received B12 experienced significant improvements in anxiety and depressed mood compared to those who received placebo.
Our patient
Because neurologic and psychiatric symptoms require assured compliance and urgent treatment, our patient received vitamin B12 parenterally as cyanocobalamin 1 mg/mL. She was given this dosage intramuscularly once a day for 5 days, then once a week for 4 weeks. She will continue to receive it once a month indefinitely.
The patient was advised that if she wished to switch to oral therapy, she could do so after several months of parenteral treatment, as long as she had close follow-up with frequent B12 measurements to assure that she was absorbing oral therapy. Her anxiety and mood symptoms resolved within one month, and her disequilibrium was almost entirely resolved within 3 months of treatment.
THE TAKEAWAY
Although more common in older patients, vitamin B12 deficiency can also affect younger patients. “Low normal” B12 levels (200-400 pg/mL) may affect psychological well-being.
Consider testing serum B12 and a second biomarker—such as homocysteine or methylmalonic acid, if indicated—in patients who present with depressed mood, anxiety, cognitive symptoms, and/or fatigue. Vitamin B12 supplementation can be administered orally and has no major adverse effects.
1. Centers for Disease Control and Prevention. Why vitamin B12 deficiency should be on your radar screen. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/b12/. Accessed February 26, 2015.
2. Guidelines and Protocols Advisory Committee, Medical Services Commission, British Columbia Ministry of Health. Cobalamin (vitamin B12) Deficiency - Investigation & Management. British Columbia Ministry of Health Web site. Available at: http://www.bcguidelines.ca/guideline_cobalamin.html. Accessed March 13, 2015.
3. Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197-1204.
4. Syed EU, Wasay M, Awan S. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7:44-48
5. Hallert C, Svensson M, Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Ailment Pharmacol Ther. 2009;29:811-816.
THE CASE
A 21-year-old college student was referred to us by the counseling center at our university for a psychiatric evaluation after 11 psychotherapy sessions over 3 months had failed to reduce her feelings of anxiety and panic.
During our evaluation, the patient described feeling “not quite right” for many months. She had been experiencing mental fogginess, fatigue, and worsening concentration/ memory. Her anxiety, which had been gradually increasing, was the result of being unsure about her gait. She first noticed this while walking down some bleachers; she felt dizzy, was afraid of falling, and couldn’t walk down without assistance. All episodes of “panic” occurred in situations where she experienced disequilibrium, unsteady gait, and fear of falling. She grew fearful of driving or going anywhere without assistance.
The patient had celiac disease that was well controlled with a gluten-free diet. She had no personal or family psychiatric history and no history of substance abuse.
THE DIAGNOSIS
Physical exam and lab studies, including a complete blood count, comprehensive metabolic panel, and thyrotropin and folate levels, were normal. Her homocysteine level was 11.8 μmol/L (reference range, 5.4-11.9 μmol/L) and vitamin B12 level was 292 pg/mL (reference range, 200-1100 pg/mL). Her lab report included a note that read, “Although the reference range for vitamin B12 is 200 to 1100 pg/mL, it has been reported that between 5% and 10% of patients with values between 200 and 400 pg/mL may experience neuropsychiatric and hematologic abnormalities due to occult B12 deficiency; <1% of patients with values >400 pg/mL will have symptoms.”
Based on this vitamin B12 level, the patient’s symptoms, and her borderline high homocysteine level, we diagnosed vitamin B12 deficiency.
DISCUSSION
There are no recommendations by the US Preventive Services Task Force or any other major US medical society for routine vitamin B12 screening.1 In Canada, the Medical Services Commission of the British Columbia Ministry of Health recommends B12 screening for patients who present with macrocytic anemia or unexplained neurologic symptoms (eg, paresthesia, numbness, poor motor coordination, memory lapses, or cognitive or personality changes).2
Vitamin B12 deficiency can be caused by numerous conditions, including those that cause malabsorption (such as gastric bypass). It can also be caused by diseases such as human immunodeficiency virus infection or Crohn’s disease, long-term adherence to a vegetarian or vegan diet, or by any other lack of dietary intake.1 The condition can cause hematologic-related signs and symptoms such as megaloblastic anemia, fatigue, and syncope. It also can have neurologic manifestations, including paresthesia, weakness, motor disturbances (including gait abnormalities), vision loss, and a wide range of cognitive and behavioral changes.1 Anemia is uncommon because since 1998, the US Food and Drug Administration has required fortification of all enriched grain and cereal products with folic acid; thus, vitamin B12 deficiency may proceed without anemia revealing its presence.1
A controversial topic. Vitamin B12 deficiency is a complicated and controversial subject. Specifically, there is uncertainty about the clinical importance of lower serum levels of vitamin B12 (200-400 pg/mL), their impact on well-being, and the need for treatment. In addition to measuring a patient’s serum B12 level, testing a second biomarker (such as homocysteine or methylmalonic acid) can be helpful in establishing a diagnosis of B12 deficiency.1 Levels of each of these are elevated in patients with B12 deficiency.1
Although vitamin B12 deficiency has been well studied in older patients,3 little has been published about the condition in young adults. National Health and Nutrition Examination Survey (NHANES) data from 2000 to 2004 shows that almost 40% of people ages 19 to 30 years have a B12 level <400 pg/mL.1 How many of these individuals are at risk of complications of B12 deficiency is unknown.
B12 supplementation might improve depression, anxiety
B12 supplementation is inexpensive and has no significant adverse effects.1 It can be administered orally, parenterally (intramuscularly or subcutaneously), or intranasally.1 A common oral regimen is 1 mg/d; parental regimens vary widely, but might include a 1-mg injection once a week for 8 weeks, then once a month for life.1
Some evidence suggests B12 supplementation may improve symptoms of depression and anxiety. A Pakistani study randomized 73 patients with depression and “low normal” B12 levels (190-300 pg/mL) to an antidepressant only (equivalent to imipramine 100-250 mg/d or fluoxetine 20-40 mg/d) or an antidepressant plus parenteral B12 (1000 mcg once a week).4 At 3 months follow-up, 100% of the treatment group showed at least a 20% reduction in their Hamilton Depression Rating Scale (HAM-D) score, compared to 69% in the control arm (P<.001).4
A Swedish study analyzed the effects of several B vitamins, including 0.5 mg/d of B12 vs placebo on mood in 65 celiac patients on a gluten-free diet who had borderline/low normal B12 levels (>191 pg/mL).5 Patients who scored low on a measure of psychological well-being at the beginning of the study and who received B12 experienced significant improvements in anxiety and depressed mood compared to those who received placebo.
Our patient
Because neurologic and psychiatric symptoms require assured compliance and urgent treatment, our patient received vitamin B12 parenterally as cyanocobalamin 1 mg/mL. She was given this dosage intramuscularly once a day for 5 days, then once a week for 4 weeks. She will continue to receive it once a month indefinitely.
The patient was advised that if she wished to switch to oral therapy, she could do so after several months of parenteral treatment, as long as she had close follow-up with frequent B12 measurements to assure that she was absorbing oral therapy. Her anxiety and mood symptoms resolved within one month, and her disequilibrium was almost entirely resolved within 3 months of treatment.
THE TAKEAWAY
Although more common in older patients, vitamin B12 deficiency can also affect younger patients. “Low normal” B12 levels (200-400 pg/mL) may affect psychological well-being.
Consider testing serum B12 and a second biomarker—such as homocysteine or methylmalonic acid, if indicated—in patients who present with depressed mood, anxiety, cognitive symptoms, and/or fatigue. Vitamin B12 supplementation can be administered orally and has no major adverse effects.
THE CASE
A 21-year-old college student was referred to us by the counseling center at our university for a psychiatric evaluation after 11 psychotherapy sessions over 3 months had failed to reduce her feelings of anxiety and panic.
During our evaluation, the patient described feeling “not quite right” for many months. She had been experiencing mental fogginess, fatigue, and worsening concentration/ memory. Her anxiety, which had been gradually increasing, was the result of being unsure about her gait. She first noticed this while walking down some bleachers; she felt dizzy, was afraid of falling, and couldn’t walk down without assistance. All episodes of “panic” occurred in situations where she experienced disequilibrium, unsteady gait, and fear of falling. She grew fearful of driving or going anywhere without assistance.
The patient had celiac disease that was well controlled with a gluten-free diet. She had no personal or family psychiatric history and no history of substance abuse.
THE DIAGNOSIS
Physical exam and lab studies, including a complete blood count, comprehensive metabolic panel, and thyrotropin and folate levels, were normal. Her homocysteine level was 11.8 μmol/L (reference range, 5.4-11.9 μmol/L) and vitamin B12 level was 292 pg/mL (reference range, 200-1100 pg/mL). Her lab report included a note that read, “Although the reference range for vitamin B12 is 200 to 1100 pg/mL, it has been reported that between 5% and 10% of patients with values between 200 and 400 pg/mL may experience neuropsychiatric and hematologic abnormalities due to occult B12 deficiency; <1% of patients with values >400 pg/mL will have symptoms.”
Based on this vitamin B12 level, the patient’s symptoms, and her borderline high homocysteine level, we diagnosed vitamin B12 deficiency.
DISCUSSION
There are no recommendations by the US Preventive Services Task Force or any other major US medical society for routine vitamin B12 screening.1 In Canada, the Medical Services Commission of the British Columbia Ministry of Health recommends B12 screening for patients who present with macrocytic anemia or unexplained neurologic symptoms (eg, paresthesia, numbness, poor motor coordination, memory lapses, or cognitive or personality changes).2
Vitamin B12 deficiency can be caused by numerous conditions, including those that cause malabsorption (such as gastric bypass). It can also be caused by diseases such as human immunodeficiency virus infection or Crohn’s disease, long-term adherence to a vegetarian or vegan diet, or by any other lack of dietary intake.1 The condition can cause hematologic-related signs and symptoms such as megaloblastic anemia, fatigue, and syncope. It also can have neurologic manifestations, including paresthesia, weakness, motor disturbances (including gait abnormalities), vision loss, and a wide range of cognitive and behavioral changes.1 Anemia is uncommon because since 1998, the US Food and Drug Administration has required fortification of all enriched grain and cereal products with folic acid; thus, vitamin B12 deficiency may proceed without anemia revealing its presence.1
A controversial topic. Vitamin B12 deficiency is a complicated and controversial subject. Specifically, there is uncertainty about the clinical importance of lower serum levels of vitamin B12 (200-400 pg/mL), their impact on well-being, and the need for treatment. In addition to measuring a patient’s serum B12 level, testing a second biomarker (such as homocysteine or methylmalonic acid) can be helpful in establishing a diagnosis of B12 deficiency.1 Levels of each of these are elevated in patients with B12 deficiency.1
Although vitamin B12 deficiency has been well studied in older patients,3 little has been published about the condition in young adults. National Health and Nutrition Examination Survey (NHANES) data from 2000 to 2004 shows that almost 40% of people ages 19 to 30 years have a B12 level <400 pg/mL.1 How many of these individuals are at risk of complications of B12 deficiency is unknown.
B12 supplementation might improve depression, anxiety
B12 supplementation is inexpensive and has no significant adverse effects.1 It can be administered orally, parenterally (intramuscularly or subcutaneously), or intranasally.1 A common oral regimen is 1 mg/d; parental regimens vary widely, but might include a 1-mg injection once a week for 8 weeks, then once a month for life.1
Some evidence suggests B12 supplementation may improve symptoms of depression and anxiety. A Pakistani study randomized 73 patients with depression and “low normal” B12 levels (190-300 pg/mL) to an antidepressant only (equivalent to imipramine 100-250 mg/d or fluoxetine 20-40 mg/d) or an antidepressant plus parenteral B12 (1000 mcg once a week).4 At 3 months follow-up, 100% of the treatment group showed at least a 20% reduction in their Hamilton Depression Rating Scale (HAM-D) score, compared to 69% in the control arm (P<.001).4
A Swedish study analyzed the effects of several B vitamins, including 0.5 mg/d of B12 vs placebo on mood in 65 celiac patients on a gluten-free diet who had borderline/low normal B12 levels (>191 pg/mL).5 Patients who scored low on a measure of psychological well-being at the beginning of the study and who received B12 experienced significant improvements in anxiety and depressed mood compared to those who received placebo.
Our patient
Because neurologic and psychiatric symptoms require assured compliance and urgent treatment, our patient received vitamin B12 parenterally as cyanocobalamin 1 mg/mL. She was given this dosage intramuscularly once a day for 5 days, then once a week for 4 weeks. She will continue to receive it once a month indefinitely.
The patient was advised that if she wished to switch to oral therapy, she could do so after several months of parenteral treatment, as long as she had close follow-up with frequent B12 measurements to assure that she was absorbing oral therapy. Her anxiety and mood symptoms resolved within one month, and her disequilibrium was almost entirely resolved within 3 months of treatment.
THE TAKEAWAY
Although more common in older patients, vitamin B12 deficiency can also affect younger patients. “Low normal” B12 levels (200-400 pg/mL) may affect psychological well-being.
Consider testing serum B12 and a second biomarker—such as homocysteine or methylmalonic acid, if indicated—in patients who present with depressed mood, anxiety, cognitive symptoms, and/or fatigue. Vitamin B12 supplementation can be administered orally and has no major adverse effects.
1. Centers for Disease Control and Prevention. Why vitamin B12 deficiency should be on your radar screen. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/b12/. Accessed February 26, 2015.
2. Guidelines and Protocols Advisory Committee, Medical Services Commission, British Columbia Ministry of Health. Cobalamin (vitamin B12) Deficiency - Investigation & Management. British Columbia Ministry of Health Web site. Available at: http://www.bcguidelines.ca/guideline_cobalamin.html. Accessed March 13, 2015.
3. Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197-1204.
4. Syed EU, Wasay M, Awan S. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7:44-48
5. Hallert C, Svensson M, Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Ailment Pharmacol Ther. 2009;29:811-816.
1. Centers for Disease Control and Prevention. Why vitamin B12 deficiency should be on your radar screen. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/b12/. Accessed February 26, 2015.
2. Guidelines and Protocols Advisory Committee, Medical Services Commission, British Columbia Ministry of Health. Cobalamin (vitamin B12) Deficiency - Investigation & Management. British Columbia Ministry of Health Web site. Available at: http://www.bcguidelines.ca/guideline_cobalamin.html. Accessed March 13, 2015.
3. Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197-1204.
4. Syed EU, Wasay M, Awan S. Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol J. 2013;7:44-48
5. Hallert C, Svensson M, Tholstrup J, et al. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Ailment Pharmacol Ther. 2009;29:811-816.
Headache • fatigue • blurred vision • Dx?
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
Are inhaled steroids effective for a postviral cough?
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
Evidence-based answers from the Family Physicians Inquiries Network
Asphyxiation by Cake: An Unusual Case of Dyspnea
A 58-year-old man presented to the emergency department (ED) via emergency medical services (EMS) with shortness of breath, lightheadedness, and nausea. Upon arrival to the ED, most of his symptoms had resolved. The patient reported that he had taken a two-hour flight into town the previous day and had spent an uneventful evening at a local hotel. He said that he began experiencing shortness of breath and lightheadedness soon after entering his rental vehicle an hour prior to presentation, explaining that he felt as if he “could not get any air.”
He denied chest pain, leg pain or swelling, abdominal pain, or recent illness. Medical history was significant for hypertension, for which he was taking losartan and amlodipine. He had no drug allergies, surgical history, or smoking history. Of note, when the hotel clerk got in the same rental vehicle to move it, he developed symptoms similar to those of the patient. As with the patient, the clerk’s symptoms quickly resolved after he got out of the vehicle.
The patient’s vital signs at examination included an oral temperature of 97.5°F; pulse, 62 beats/min; respiratory rate (RR), 18 breaths/min; blood pressure, 133/83 mm Hg; and O2 saturation, 100% on room air. He was alert and oriented, in no distress, easily conversational, and without diaphoresis. The lungs were clear to auscultation bilaterally, and there was no calf swelling, tenderness, or palpable cords. The remainder of the physical exam was normal.
Ancillary studies included a normal chest X-ray. An ECG demonstrated sinus bradycardia with a rate of 56 beats/min but no evidence of ischemia or right heart strain. Complete blood count, troponin I, D-dimer, and creatine phosphokinase (CPK) with MB fraction levels were all within normal limits. A serum chemistry panel was also within normal limits, except for a serum glucose level of 181 mg/dL. Venous co-oximetry showed a carboxyhemoglobin level of 0.0, and methemoglobin level of 0.5 gm% (normal range, 0.4-1.5).
Since both the patient’s and hotel clerk’s symptoms started when each was in the rental car, the patient was questioned about the vehicle and its contents. The car was a late-model rental in good condition per report. The patient informed the treating emergency physician that he worked as a decorative cake salesman and had brought cake samples with him to display at a trade show. He further stated that he had left these samples in the car overnight, packed in dry ice.
What's the solution to this unusual case?
Upon learning this information, EMS was contacted and instructed to return to the hotel and rental vehicle. The hotel room was noted to have normal levels of O2 and carbon monoxide (CO) on measurement. Investigation of the car revealed normal levels of CO but O2 levels too low to read on the sensor. The emergency team concluded that the dry ice (the solid form of carbon dioxide [CO2]) sublimed to CO2 gas overnight. This displaced the O2 in the vehicle, resulting in severe hypoxia and the symptoms of both the patient and hotel clerk.
The patient was initially placed on 15 L of O2 via a nonrebreather mask, then switched to 2 L of O2 via nasal cannula. He was observed for a total of four hours after arrival; as he remained symptom-free, he was discharged home. Postdischarge follow-up information was not obtainable.
DISCUSSION
Carbon dioxide is prevalent in everyday life, from an agent in fire extinguishers and carbonation in beverages to byproducts of cellular metabolism. Similar to CO, it is a colorless and odorless gas.
Carbon dioxide is commonly used in the food industry as dry ice to keep items cold. In its solid state, CO2 can cause severe frostbite with direct contact, similar to a burn. However, when dry ice is warmed and sublimated to a gaseous state, large amounts of CO2 are generated, and this heavy gas can accumulate and displace air (ie, atmospheric O2), especially in confined spaces. In low concentrations, gaseous CO2 appears to have minimal toxicologic effects, but at higher concentrations it can cause tachycardia, tachypnea, dyspnea, visual disturbances, arrhythmias, impaired levels of consciousness, and death.
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Compensation for this acidosis is accomplished by an increased RR (ie, respiratory alkalosis), which further worsens the intake of CO2.1,2
The normal concentration of CO2 in the atmosphere is approximately 0.04% (396 ppm). The Occupational Safety and Health Administration (OSHA) has set a maximum safe exposure level of CO2 at 0.5% (5,000 ppm) over an eight-hour day.3 Concentrations as low as 1% (10,000 ppm) may cause drowsiness. Exposure to concentrations of 7% to 10% for several minutes to an hour results in headache, tachycardia, dyspnea, and hyperventilation. At levels of 10% to 15%, dizziness, severe muscle twitching, and loss of consciousness can occur after only a few minutes. Death occurs within minutes at concentrations greater than 30%.2
Carbon dioxide also acts as a potent cerebral vasodilator, which may explain symptoms such as headache and dizziness.2 The severity of symptoms is dependent on the concentration of CO2, the length of the exposure, and the underlying health of the patient. Elevated concentrations of CO2 can occur in areas where there is limited or poor ventilation, such as in a mine (where it is known as blackdamp, stythe, or choke damp),4 submarine, grain silo, or a sealed building without mechanical ventilation.
Continue for other case presentations >>
Other case presentations
Similar cases have been described in the literature. In one case, following Hurricane Ivan, a 34-year-old man placed four 25-pound blocks of dry ice wrapped in paper in the front seat of his truck with the windows closed.5 After driving less than one-quarter of a mile, he developed dyspnea and telephoned for help before losing consciousness. Fortunately, he was found in time and recovered soon after the doors to his truck were opened.5
In another case, a 59-year-old man entered a walk-in freezer that contained dry ice wrapped loosely in plastic. He was found inside the freezer 20 minutes later in cardiac arrest; resuscitation efforts were unsuccessful. Investigation of the freezer found an initial O2 concentration of 13% (normal level, 20.93%) and an estimated CO2 level of 40%.5
Similarly, a 35-year-old woman was inadvertently locked in a bank vault while storing receipts. In a bid for help, she pulled the fire alarm, which triggered a CO2-based fire-extinguishing system. The fire department responded and found the woman dead in the vault 30 minutes later. The cause of death was labeled as CO2 intoxication.6
Natural phenomena
There have also been documented cases of CO2 toxicity associated with volcanic eruption and other natural phenomena; for example, the Lake Nyos, Cameroon, West Africa incident in 1986. In this event, a magma pocket underlying the lake saturated the water with CO2 stored as carbonic acid in the water. When a landslide hit the lake, it caused the carbonic acid stored in the depths of the lake to be upheaved to the surface, where it turned back into CO2 and was released into the atmosphere. Since CO2 is heavier than O2, it displaced the O2 near the ground, resulting in the suffocation and death of 1,700 people in the surrounding villages.2
Next page: Differential diagnosis >>
Differential diagnosis
When CO2 toxicity is suspected, other conditions should be considered, as there may be more than one process involved. For example, other causes of coma or dyspnea should be investigated, including trauma, hypoglycemia, CO, methemoglobinemia, or other metabolic processes. In addition, a patient may have a pre-existing condition, such as a trauma or an altered mental status due to drugs or alcohol, all of which can increase his or her susceptibility to the effects of CO2.
Evaluation and treatment
Useful laboratory testing includes arterial blood gas, venous co-oximetry for carboxyhemoglobin, chemistry panels, ethanol testing, and radiographs or CT, as indicated.
Initial management of suspected CO2 toxicity entails first removing the patient from the source of the gas. Rescuers must exercise caution so as to prevent a mass-casualty incident. Once out of the dangerous environment, as long as the patient is conscious and spontaneously breathing, supportive measures are generally all that are necessary. Oxygen should be applied, after which the spontaneously breathing patient without underlying lung disease should rapidly return to normal.
If there is marked decrease in mental status or poor respiratory drive despite O2 administration, intubation with mechanical ventilation may be required. A higher-than-normal RR will help remove excessive CO2 in this instance.
If a respiratory acidosis is present, IV sodium bicarbonate should be avoided, as this may increase the level of serum CO2. IV fluids and other supportive measures, including treatment for any concurrent conditions, may be indicated.
REFERENCES
1. Nelson LS, Odujebe OA. Simple asphyxiants and pulmonary irritants. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s Toxicologic Emergencies, 9th ed. New York, NY: McGraw-Hill; 2011:1644-1645.
2. Langford NJ. Carbon dioxide poisoning. Toxicol Rev. 2005;24(4):229-235.
3. Occupational Health and Safety Standards. Table Z-1, Limits for air contaminants. Occupational Safety and Health Administration Web site. www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9992. Accessed January 19, 2015.
4. Hedlund FH. The extreme carbon dioxide outburst at the Menzengraben potash mine 7 July 1953. Safety Sci. 2012;50(3):537-553.
5. Dunford JV, Lucas J, Vent N, et al. Asphyxiation due to dry ice in a walk-in freezer. J Emerg Med. 2009;36(4):353-356.
6. Gill JR, Ely SF, Hua Z. Environmental gas displacement: three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002;23(1):26-30.
A 58-year-old man presented to the emergency department (ED) via emergency medical services (EMS) with shortness of breath, lightheadedness, and nausea. Upon arrival to the ED, most of his symptoms had resolved. The patient reported that he had taken a two-hour flight into town the previous day and had spent an uneventful evening at a local hotel. He said that he began experiencing shortness of breath and lightheadedness soon after entering his rental vehicle an hour prior to presentation, explaining that he felt as if he “could not get any air.”
He denied chest pain, leg pain or swelling, abdominal pain, or recent illness. Medical history was significant for hypertension, for which he was taking losartan and amlodipine. He had no drug allergies, surgical history, or smoking history. Of note, when the hotel clerk got in the same rental vehicle to move it, he developed symptoms similar to those of the patient. As with the patient, the clerk’s symptoms quickly resolved after he got out of the vehicle.
The patient’s vital signs at examination included an oral temperature of 97.5°F; pulse, 62 beats/min; respiratory rate (RR), 18 breaths/min; blood pressure, 133/83 mm Hg; and O2 saturation, 100% on room air. He was alert and oriented, in no distress, easily conversational, and without diaphoresis. The lungs were clear to auscultation bilaterally, and there was no calf swelling, tenderness, or palpable cords. The remainder of the physical exam was normal.
Ancillary studies included a normal chest X-ray. An ECG demonstrated sinus bradycardia with a rate of 56 beats/min but no evidence of ischemia or right heart strain. Complete blood count, troponin I, D-dimer, and creatine phosphokinase (CPK) with MB fraction levels were all within normal limits. A serum chemistry panel was also within normal limits, except for a serum glucose level of 181 mg/dL. Venous co-oximetry showed a carboxyhemoglobin level of 0.0, and methemoglobin level of 0.5 gm% (normal range, 0.4-1.5).
Since both the patient’s and hotel clerk’s symptoms started when each was in the rental car, the patient was questioned about the vehicle and its contents. The car was a late-model rental in good condition per report. The patient informed the treating emergency physician that he worked as a decorative cake salesman and had brought cake samples with him to display at a trade show. He further stated that he had left these samples in the car overnight, packed in dry ice.
What's the solution to this unusual case?
Upon learning this information, EMS was contacted and instructed to return to the hotel and rental vehicle. The hotel room was noted to have normal levels of O2 and carbon monoxide (CO) on measurement. Investigation of the car revealed normal levels of CO but O2 levels too low to read on the sensor. The emergency team concluded that the dry ice (the solid form of carbon dioxide [CO2]) sublimed to CO2 gas overnight. This displaced the O2 in the vehicle, resulting in severe hypoxia and the symptoms of both the patient and hotel clerk.
The patient was initially placed on 15 L of O2 via a nonrebreather mask, then switched to 2 L of O2 via nasal cannula. He was observed for a total of four hours after arrival; as he remained symptom-free, he was discharged home. Postdischarge follow-up information was not obtainable.
DISCUSSION
Carbon dioxide is prevalent in everyday life, from an agent in fire extinguishers and carbonation in beverages to byproducts of cellular metabolism. Similar to CO, it is a colorless and odorless gas.
Carbon dioxide is commonly used in the food industry as dry ice to keep items cold. In its solid state, CO2 can cause severe frostbite with direct contact, similar to a burn. However, when dry ice is warmed and sublimated to a gaseous state, large amounts of CO2 are generated, and this heavy gas can accumulate and displace air (ie, atmospheric O2), especially in confined spaces. In low concentrations, gaseous CO2 appears to have minimal toxicologic effects, but at higher concentrations it can cause tachycardia, tachypnea, dyspnea, visual disturbances, arrhythmias, impaired levels of consciousness, and death.
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Compensation for this acidosis is accomplished by an increased RR (ie, respiratory alkalosis), which further worsens the intake of CO2.1,2
The normal concentration of CO2 in the atmosphere is approximately 0.04% (396 ppm). The Occupational Safety and Health Administration (OSHA) has set a maximum safe exposure level of CO2 at 0.5% (5,000 ppm) over an eight-hour day.3 Concentrations as low as 1% (10,000 ppm) may cause drowsiness. Exposure to concentrations of 7% to 10% for several minutes to an hour results in headache, tachycardia, dyspnea, and hyperventilation. At levels of 10% to 15%, dizziness, severe muscle twitching, and loss of consciousness can occur after only a few minutes. Death occurs within minutes at concentrations greater than 30%.2
Carbon dioxide also acts as a potent cerebral vasodilator, which may explain symptoms such as headache and dizziness.2 The severity of symptoms is dependent on the concentration of CO2, the length of the exposure, and the underlying health of the patient. Elevated concentrations of CO2 can occur in areas where there is limited or poor ventilation, such as in a mine (where it is known as blackdamp, stythe, or choke damp),4 submarine, grain silo, or a sealed building without mechanical ventilation.
Continue for other case presentations >>
Other case presentations
Similar cases have been described in the literature. In one case, following Hurricane Ivan, a 34-year-old man placed four 25-pound blocks of dry ice wrapped in paper in the front seat of his truck with the windows closed.5 After driving less than one-quarter of a mile, he developed dyspnea and telephoned for help before losing consciousness. Fortunately, he was found in time and recovered soon after the doors to his truck were opened.5
In another case, a 59-year-old man entered a walk-in freezer that contained dry ice wrapped loosely in plastic. He was found inside the freezer 20 minutes later in cardiac arrest; resuscitation efforts were unsuccessful. Investigation of the freezer found an initial O2 concentration of 13% (normal level, 20.93%) and an estimated CO2 level of 40%.5
Similarly, a 35-year-old woman was inadvertently locked in a bank vault while storing receipts. In a bid for help, she pulled the fire alarm, which triggered a CO2-based fire-extinguishing system. The fire department responded and found the woman dead in the vault 30 minutes later. The cause of death was labeled as CO2 intoxication.6
Natural phenomena
There have also been documented cases of CO2 toxicity associated with volcanic eruption and other natural phenomena; for example, the Lake Nyos, Cameroon, West Africa incident in 1986. In this event, a magma pocket underlying the lake saturated the water with CO2 stored as carbonic acid in the water. When a landslide hit the lake, it caused the carbonic acid stored in the depths of the lake to be upheaved to the surface, where it turned back into CO2 and was released into the atmosphere. Since CO2 is heavier than O2, it displaced the O2 near the ground, resulting in the suffocation and death of 1,700 people in the surrounding villages.2
Next page: Differential diagnosis >>
Differential diagnosis
When CO2 toxicity is suspected, other conditions should be considered, as there may be more than one process involved. For example, other causes of coma or dyspnea should be investigated, including trauma, hypoglycemia, CO, methemoglobinemia, or other metabolic processes. In addition, a patient may have a pre-existing condition, such as a trauma or an altered mental status due to drugs or alcohol, all of which can increase his or her susceptibility to the effects of CO2.
Evaluation and treatment
Useful laboratory testing includes arterial blood gas, venous co-oximetry for carboxyhemoglobin, chemistry panels, ethanol testing, and radiographs or CT, as indicated.
Initial management of suspected CO2 toxicity entails first removing the patient from the source of the gas. Rescuers must exercise caution so as to prevent a mass-casualty incident. Once out of the dangerous environment, as long as the patient is conscious and spontaneously breathing, supportive measures are generally all that are necessary. Oxygen should be applied, after which the spontaneously breathing patient without underlying lung disease should rapidly return to normal.
If there is marked decrease in mental status or poor respiratory drive despite O2 administration, intubation with mechanical ventilation may be required. A higher-than-normal RR will help remove excessive CO2 in this instance.
If a respiratory acidosis is present, IV sodium bicarbonate should be avoided, as this may increase the level of serum CO2. IV fluids and other supportive measures, including treatment for any concurrent conditions, may be indicated.
REFERENCES
1. Nelson LS, Odujebe OA. Simple asphyxiants and pulmonary irritants. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s Toxicologic Emergencies, 9th ed. New York, NY: McGraw-Hill; 2011:1644-1645.
2. Langford NJ. Carbon dioxide poisoning. Toxicol Rev. 2005;24(4):229-235.
3. Occupational Health and Safety Standards. Table Z-1, Limits for air contaminants. Occupational Safety and Health Administration Web site. www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9992. Accessed January 19, 2015.
4. Hedlund FH. The extreme carbon dioxide outburst at the Menzengraben potash mine 7 July 1953. Safety Sci. 2012;50(3):537-553.
5. Dunford JV, Lucas J, Vent N, et al. Asphyxiation due to dry ice in a walk-in freezer. J Emerg Med. 2009;36(4):353-356.
6. Gill JR, Ely SF, Hua Z. Environmental gas displacement: three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002;23(1):26-30.
A 58-year-old man presented to the emergency department (ED) via emergency medical services (EMS) with shortness of breath, lightheadedness, and nausea. Upon arrival to the ED, most of his symptoms had resolved. The patient reported that he had taken a two-hour flight into town the previous day and had spent an uneventful evening at a local hotel. He said that he began experiencing shortness of breath and lightheadedness soon after entering his rental vehicle an hour prior to presentation, explaining that he felt as if he “could not get any air.”
He denied chest pain, leg pain or swelling, abdominal pain, or recent illness. Medical history was significant for hypertension, for which he was taking losartan and amlodipine. He had no drug allergies, surgical history, or smoking history. Of note, when the hotel clerk got in the same rental vehicle to move it, he developed symptoms similar to those of the patient. As with the patient, the clerk’s symptoms quickly resolved after he got out of the vehicle.
The patient’s vital signs at examination included an oral temperature of 97.5°F; pulse, 62 beats/min; respiratory rate (RR), 18 breaths/min; blood pressure, 133/83 mm Hg; and O2 saturation, 100% on room air. He was alert and oriented, in no distress, easily conversational, and without diaphoresis. The lungs were clear to auscultation bilaterally, and there was no calf swelling, tenderness, or palpable cords. The remainder of the physical exam was normal.
Ancillary studies included a normal chest X-ray. An ECG demonstrated sinus bradycardia with a rate of 56 beats/min but no evidence of ischemia or right heart strain. Complete blood count, troponin I, D-dimer, and creatine phosphokinase (CPK) with MB fraction levels were all within normal limits. A serum chemistry panel was also within normal limits, except for a serum glucose level of 181 mg/dL. Venous co-oximetry showed a carboxyhemoglobin level of 0.0, and methemoglobin level of 0.5 gm% (normal range, 0.4-1.5).
Since both the patient’s and hotel clerk’s symptoms started when each was in the rental car, the patient was questioned about the vehicle and its contents. The car was a late-model rental in good condition per report. The patient informed the treating emergency physician that he worked as a decorative cake salesman and had brought cake samples with him to display at a trade show. He further stated that he had left these samples in the car overnight, packed in dry ice.
What's the solution to this unusual case?
Upon learning this information, EMS was contacted and instructed to return to the hotel and rental vehicle. The hotel room was noted to have normal levels of O2 and carbon monoxide (CO) on measurement. Investigation of the car revealed normal levels of CO but O2 levels too low to read on the sensor. The emergency team concluded that the dry ice (the solid form of carbon dioxide [CO2]) sublimed to CO2 gas overnight. This displaced the O2 in the vehicle, resulting in severe hypoxia and the symptoms of both the patient and hotel clerk.
The patient was initially placed on 15 L of O2 via a nonrebreather mask, then switched to 2 L of O2 via nasal cannula. He was observed for a total of four hours after arrival; as he remained symptom-free, he was discharged home. Postdischarge follow-up information was not obtainable.
DISCUSSION
Carbon dioxide is prevalent in everyday life, from an agent in fire extinguishers and carbonation in beverages to byproducts of cellular metabolism. Similar to CO, it is a colorless and odorless gas.
Carbon dioxide is commonly used in the food industry as dry ice to keep items cold. In its solid state, CO2 can cause severe frostbite with direct contact, similar to a burn. However, when dry ice is warmed and sublimated to a gaseous state, large amounts of CO2 are generated, and this heavy gas can accumulate and displace air (ie, atmospheric O2), especially in confined spaces. In low concentrations, gaseous CO2 appears to have minimal toxicologic effects, but at higher concentrations it can cause tachycardia, tachypnea, dyspnea, visual disturbances, arrhythmias, impaired levels of consciousness, and death.
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Compensation for this acidosis is accomplished by an increased RR (ie, respiratory alkalosis), which further worsens the intake of CO2.1,2
The normal concentration of CO2 in the atmosphere is approximately 0.04% (396 ppm). The Occupational Safety and Health Administration (OSHA) has set a maximum safe exposure level of CO2 at 0.5% (5,000 ppm) over an eight-hour day.3 Concentrations as low as 1% (10,000 ppm) may cause drowsiness. Exposure to concentrations of 7% to 10% for several minutes to an hour results in headache, tachycardia, dyspnea, and hyperventilation. At levels of 10% to 15%, dizziness, severe muscle twitching, and loss of consciousness can occur after only a few minutes. Death occurs within minutes at concentrations greater than 30%.2
Carbon dioxide also acts as a potent cerebral vasodilator, which may explain symptoms such as headache and dizziness.2 The severity of symptoms is dependent on the concentration of CO2, the length of the exposure, and the underlying health of the patient. Elevated concentrations of CO2 can occur in areas where there is limited or poor ventilation, such as in a mine (where it is known as blackdamp, stythe, or choke damp),4 submarine, grain silo, or a sealed building without mechanical ventilation.
Continue for other case presentations >>
Other case presentations
Similar cases have been described in the literature. In one case, following Hurricane Ivan, a 34-year-old man placed four 25-pound blocks of dry ice wrapped in paper in the front seat of his truck with the windows closed.5 After driving less than one-quarter of a mile, he developed dyspnea and telephoned for help before losing consciousness. Fortunately, he was found in time and recovered soon after the doors to his truck were opened.5
In another case, a 59-year-old man entered a walk-in freezer that contained dry ice wrapped loosely in plastic. He was found inside the freezer 20 minutes later in cardiac arrest; resuscitation efforts were unsuccessful. Investigation of the freezer found an initial O2 concentration of 13% (normal level, 20.93%) and an estimated CO2 level of 40%.5
Similarly, a 35-year-old woman was inadvertently locked in a bank vault while storing receipts. In a bid for help, she pulled the fire alarm, which triggered a CO2-based fire-extinguishing system. The fire department responded and found the woman dead in the vault 30 minutes later. The cause of death was labeled as CO2 intoxication.6
Natural phenomena
There have also been documented cases of CO2 toxicity associated with volcanic eruption and other natural phenomena; for example, the Lake Nyos, Cameroon, West Africa incident in 1986. In this event, a magma pocket underlying the lake saturated the water with CO2 stored as carbonic acid in the water. When a landslide hit the lake, it caused the carbonic acid stored in the depths of the lake to be upheaved to the surface, where it turned back into CO2 and was released into the atmosphere. Since CO2 is heavier than O2, it displaced the O2 near the ground, resulting in the suffocation and death of 1,700 people in the surrounding villages.2
Next page: Differential diagnosis >>
Differential diagnosis
When CO2 toxicity is suspected, other conditions should be considered, as there may be more than one process involved. For example, other causes of coma or dyspnea should be investigated, including trauma, hypoglycemia, CO, methemoglobinemia, or other metabolic processes. In addition, a patient may have a pre-existing condition, such as a trauma or an altered mental status due to drugs or alcohol, all of which can increase his or her susceptibility to the effects of CO2.
Evaluation and treatment
Useful laboratory testing includes arterial blood gas, venous co-oximetry for carboxyhemoglobin, chemistry panels, ethanol testing, and radiographs or CT, as indicated.
Initial management of suspected CO2 toxicity entails first removing the patient from the source of the gas. Rescuers must exercise caution so as to prevent a mass-casualty incident. Once out of the dangerous environment, as long as the patient is conscious and spontaneously breathing, supportive measures are generally all that are necessary. Oxygen should be applied, after which the spontaneously breathing patient without underlying lung disease should rapidly return to normal.
If there is marked decrease in mental status or poor respiratory drive despite O2 administration, intubation with mechanical ventilation may be required. A higher-than-normal RR will help remove excessive CO2 in this instance.
If a respiratory acidosis is present, IV sodium bicarbonate should be avoided, as this may increase the level of serum CO2. IV fluids and other supportive measures, including treatment for any concurrent conditions, may be indicated.
REFERENCES
1. Nelson LS, Odujebe OA. Simple asphyxiants and pulmonary irritants. In: Nelson LS, Lewin NA, Howland MA, et al, eds. Goldfrank’s Toxicologic Emergencies, 9th ed. New York, NY: McGraw-Hill; 2011:1644-1645.
2. Langford NJ. Carbon dioxide poisoning. Toxicol Rev. 2005;24(4):229-235.
3. Occupational Health and Safety Standards. Table Z-1, Limits for air contaminants. Occupational Safety and Health Administration Web site. www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9992. Accessed January 19, 2015.
4. Hedlund FH. The extreme carbon dioxide outburst at the Menzengraben potash mine 7 July 1953. Safety Sci. 2012;50(3):537-553.
5. Dunford JV, Lucas J, Vent N, et al. Asphyxiation due to dry ice in a walk-in freezer. J Emerg Med. 2009;36(4):353-356.
6. Gill JR, Ely SF, Hua Z. Environmental gas displacement: three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002;23(1):26-30.
CDC: Flu Remains Widespread; Antivirals Underutilized
The approximate midpoint of the 2014-2015 flu season has been reached, disease remains widespread, and antiviral flu medications remain underutilized, according to the Centers for Disease Control and Prevention.
As predicted, this season is proving particularly severe due to the predominance of antigenically drifted H3N2 virus strains, and the CDC is urging clinicians to maintain a high index of suspicion for flu and to prescribe antivirals earlier and more aggressively for patients presenting with flulike illness – especially young children, those over age 65 years, and those with underlying conditions, even if their symptoms are mild, CDC Director Thomas R. Frieden said during a press briefing.
A health advisory on the topic was issued Jan. 9, he noted.
“The CDC has recommended the use of antiviral drugs as an adjunct to vaccination. They are the only medicines that can specifically treat influenza illness, and in the context of an H3N2 predominant season with a less effective vaccine, treatment with antiflu drugs is even more important than usual,” he said, referring to the two neuraminidase inhibitors currently approved for treating influenza: oseltamivir and zanamivir.
About two-thirds of the H3N2 viruses analyzed this season are different from the H3N2 virus included in this year’s vaccine, which is why the vaccine is expected to have reduced effectiveness, he explained.
This makes early antiviral treatment all the more important, he added, noting that treatment within 2 days of symptom onset is optimal, but later treatment can also offer benefit.
“CDC scientists have looked very carefully at the use of influenza drugs in the clinical setting, and the conclusion is clear: They work, but they aren’t being used nearly enough. They can reduce symptoms, shorten duration of illness, and prevent serious complications,” he said.
Continue for early treatments >>
Early treatment may help patients avoid hospitalization and could be life saving, he added.
Many patients are unaware that effective prescription treatments exist, and many doctors are not using the treatments as recommended. In one study, fewer than one in five eligible high-risk patients received treatment.
“So we’re expanding our efforts to reach clinicians about reminders about the importance of these drugs,” he said, noting that if high-risk patients with underlying conditions such as asthma, sickle cell disease, renal disease, or diabetes were treated, “tens of thousands of hospitalizations, and thousands of deaths could potentially be prevented.”
Dr. Frieden suggested that clinicians consider phone triage lines for high-risk patients to discuss symptoms over the phone and to “facilitate early initiation of treatment.”
An antiviral prescription can be provided without testing and before an office visit, he added.
The typical flu season lasts about 13 weeks on average, and the season began about 7 weeks ago. The continued high rate of H3N2 disease has had the greatest impact on older adults; that is typical of such seasons, which often involve a higher rate of hospitalizations and deaths.
In fact, hospitalization rates in the over 65 age group are rising sharply, Dr. Frieden said.
Last week the rate was 52 per 100,000 population, and this week the rate was 92 per 100,000 population. The cumulative rate 2 years ago when H3N2 viruses last predominated was 183 per 100,000, he noted.
“We wouldn’t be surprised to see something very similar this year,” he said.
Young children are also severely affected; thus far in the season there have been 26 reported pediatric flu-related deaths.
However, there are early signs that infection rates are declining in areas where the season started earlier, such as the Southeast, and the number of patients presenting to a doctor with flulike symptoms has also declined slightly, but it is too soon to tell whether disease activity has peaked, he said, stressing that “we still have several weeks of flu activity ahead.”
It’s not too late to get vaccinated, and despite the reduced efficacy of this year’s vaccine against the circulating H3N2 strains, vaccination may still offer some benefit.
“There are other strains out there as well,” he said, noting that a proportion of cases from other strains, such as influenza B, tend to occur late in the season, and this year’s vaccine offers a good match for influenza B viruses.
Pneumococcal vaccination is also important, particularly for older adults and those at high risk , he said.
Last September the CDC announced a new recommendation that all adults over age 65 years should get two different pneumococcal vaccines. Such vaccination may help prevent flu-related pneumonia.
Patients with the flu or flulike illness should be advised to cover their cough and to stay home from work or school to protect those who are most vulnerable to the flu, he said.
The approximate midpoint of the 2014-2015 flu season has been reached, disease remains widespread, and antiviral flu medications remain underutilized, according to the Centers for Disease Control and Prevention.
As predicted, this season is proving particularly severe due to the predominance of antigenically drifted H3N2 virus strains, and the CDC is urging clinicians to maintain a high index of suspicion for flu and to prescribe antivirals earlier and more aggressively for patients presenting with flulike illness – especially young children, those over age 65 years, and those with underlying conditions, even if their symptoms are mild, CDC Director Thomas R. Frieden said during a press briefing.
A health advisory on the topic was issued Jan. 9, he noted.
“The CDC has recommended the use of antiviral drugs as an adjunct to vaccination. They are the only medicines that can specifically treat influenza illness, and in the context of an H3N2 predominant season with a less effective vaccine, treatment with antiflu drugs is even more important than usual,” he said, referring to the two neuraminidase inhibitors currently approved for treating influenza: oseltamivir and zanamivir.
About two-thirds of the H3N2 viruses analyzed this season are different from the H3N2 virus included in this year’s vaccine, which is why the vaccine is expected to have reduced effectiveness, he explained.
This makes early antiviral treatment all the more important, he added, noting that treatment within 2 days of symptom onset is optimal, but later treatment can also offer benefit.
“CDC scientists have looked very carefully at the use of influenza drugs in the clinical setting, and the conclusion is clear: They work, but they aren’t being used nearly enough. They can reduce symptoms, shorten duration of illness, and prevent serious complications,” he said.
Continue for early treatments >>
Early treatment may help patients avoid hospitalization and could be life saving, he added.
Many patients are unaware that effective prescription treatments exist, and many doctors are not using the treatments as recommended. In one study, fewer than one in five eligible high-risk patients received treatment.
“So we’re expanding our efforts to reach clinicians about reminders about the importance of these drugs,” he said, noting that if high-risk patients with underlying conditions such as asthma, sickle cell disease, renal disease, or diabetes were treated, “tens of thousands of hospitalizations, and thousands of deaths could potentially be prevented.”
Dr. Frieden suggested that clinicians consider phone triage lines for high-risk patients to discuss symptoms over the phone and to “facilitate early initiation of treatment.”
An antiviral prescription can be provided without testing and before an office visit, he added.
The typical flu season lasts about 13 weeks on average, and the season began about 7 weeks ago. The continued high rate of H3N2 disease has had the greatest impact on older adults; that is typical of such seasons, which often involve a higher rate of hospitalizations and deaths.
In fact, hospitalization rates in the over 65 age group are rising sharply, Dr. Frieden said.
Last week the rate was 52 per 100,000 population, and this week the rate was 92 per 100,000 population. The cumulative rate 2 years ago when H3N2 viruses last predominated was 183 per 100,000, he noted.
“We wouldn’t be surprised to see something very similar this year,” he said.
Young children are also severely affected; thus far in the season there have been 26 reported pediatric flu-related deaths.
However, there are early signs that infection rates are declining in areas where the season started earlier, such as the Southeast, and the number of patients presenting to a doctor with flulike symptoms has also declined slightly, but it is too soon to tell whether disease activity has peaked, he said, stressing that “we still have several weeks of flu activity ahead.”
It’s not too late to get vaccinated, and despite the reduced efficacy of this year’s vaccine against the circulating H3N2 strains, vaccination may still offer some benefit.
“There are other strains out there as well,” he said, noting that a proportion of cases from other strains, such as influenza B, tend to occur late in the season, and this year’s vaccine offers a good match for influenza B viruses.
Pneumococcal vaccination is also important, particularly for older adults and those at high risk , he said.
Last September the CDC announced a new recommendation that all adults over age 65 years should get two different pneumococcal vaccines. Such vaccination may help prevent flu-related pneumonia.
Patients with the flu or flulike illness should be advised to cover their cough and to stay home from work or school to protect those who are most vulnerable to the flu, he said.
The approximate midpoint of the 2014-2015 flu season has been reached, disease remains widespread, and antiviral flu medications remain underutilized, according to the Centers for Disease Control and Prevention.
As predicted, this season is proving particularly severe due to the predominance of antigenically drifted H3N2 virus strains, and the CDC is urging clinicians to maintain a high index of suspicion for flu and to prescribe antivirals earlier and more aggressively for patients presenting with flulike illness – especially young children, those over age 65 years, and those with underlying conditions, even if their symptoms are mild, CDC Director Thomas R. Frieden said during a press briefing.
A health advisory on the topic was issued Jan. 9, he noted.
“The CDC has recommended the use of antiviral drugs as an adjunct to vaccination. They are the only medicines that can specifically treat influenza illness, and in the context of an H3N2 predominant season with a less effective vaccine, treatment with antiflu drugs is even more important than usual,” he said, referring to the two neuraminidase inhibitors currently approved for treating influenza: oseltamivir and zanamivir.
About two-thirds of the H3N2 viruses analyzed this season are different from the H3N2 virus included in this year’s vaccine, which is why the vaccine is expected to have reduced effectiveness, he explained.
This makes early antiviral treatment all the more important, he added, noting that treatment within 2 days of symptom onset is optimal, but later treatment can also offer benefit.
“CDC scientists have looked very carefully at the use of influenza drugs in the clinical setting, and the conclusion is clear: They work, but they aren’t being used nearly enough. They can reduce symptoms, shorten duration of illness, and prevent serious complications,” he said.
Continue for early treatments >>
Early treatment may help patients avoid hospitalization and could be life saving, he added.
Many patients are unaware that effective prescription treatments exist, and many doctors are not using the treatments as recommended. In one study, fewer than one in five eligible high-risk patients received treatment.
“So we’re expanding our efforts to reach clinicians about reminders about the importance of these drugs,” he said, noting that if high-risk patients with underlying conditions such as asthma, sickle cell disease, renal disease, or diabetes were treated, “tens of thousands of hospitalizations, and thousands of deaths could potentially be prevented.”
Dr. Frieden suggested that clinicians consider phone triage lines for high-risk patients to discuss symptoms over the phone and to “facilitate early initiation of treatment.”
An antiviral prescription can be provided without testing and before an office visit, he added.
The typical flu season lasts about 13 weeks on average, and the season began about 7 weeks ago. The continued high rate of H3N2 disease has had the greatest impact on older adults; that is typical of such seasons, which often involve a higher rate of hospitalizations and deaths.
In fact, hospitalization rates in the over 65 age group are rising sharply, Dr. Frieden said.
Last week the rate was 52 per 100,000 population, and this week the rate was 92 per 100,000 population. The cumulative rate 2 years ago when H3N2 viruses last predominated was 183 per 100,000, he noted.
“We wouldn’t be surprised to see something very similar this year,” he said.
Young children are also severely affected; thus far in the season there have been 26 reported pediatric flu-related deaths.
However, there are early signs that infection rates are declining in areas where the season started earlier, such as the Southeast, and the number of patients presenting to a doctor with flulike symptoms has also declined slightly, but it is too soon to tell whether disease activity has peaked, he said, stressing that “we still have several weeks of flu activity ahead.”
It’s not too late to get vaccinated, and despite the reduced efficacy of this year’s vaccine against the circulating H3N2 strains, vaccination may still offer some benefit.
“There are other strains out there as well,” he said, noting that a proportion of cases from other strains, such as influenza B, tend to occur late in the season, and this year’s vaccine offers a good match for influenza B viruses.
Pneumococcal vaccination is also important, particularly for older adults and those at high risk , he said.
Last September the CDC announced a new recommendation that all adults over age 65 years should get two different pneumococcal vaccines. Such vaccination may help prevent flu-related pneumonia.
Patients with the flu or flulike illness should be advised to cover their cough and to stay home from work or school to protect those who are most vulnerable to the flu, he said.
Normal and Abnormal Vocal Cord Movement
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[Courtesy of Arizona Asthma & Allergy Institute]
Click Here for Free CE/CME on Vocal Cord Dysfunction: Unmasking the Asthma Pretender
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[Courtesy of Arizona Asthma & Allergy Institute]
Click Here for Free CE/CME on Vocal Cord Dysfunction: Unmasking the Asthma Pretender
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[Courtesy of Arizona Asthma & Allergy Institute]
Click Here for Free CE/CME on Vocal Cord Dysfunction: Unmasking the Asthma Pretender