User login
Vocal Cord Dysfunction: Unmasking the Asthma Pretender
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
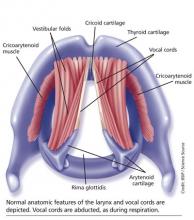
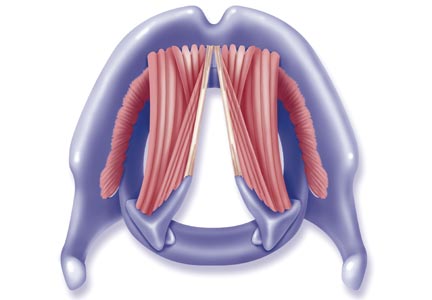
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
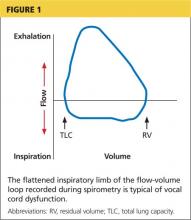
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
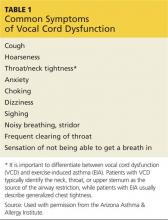
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
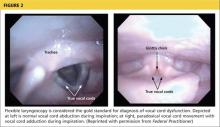
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
Time to Change How We Test for TB?
Public health officials in El Paso, Texas, reported in September 2014 that more than 850 infants and 43 health care workers (HCW) at Providence Memorial Hospital may have been exposed to tuberculosis (TB) by a nurse with active infection. In collaboration with the CDC, the hospital administrators and local and state health officials advised potentially exposed individuals and their families to be screened for TB. According to press reports, five infants tested positive for TB and were to be treated.1,2 This news report highlights the importance of maintaining infection control protocols in health care facilities throughout the United States to reduce the risk for TB transmission.
SCREENING HEALTH CARE WORKERS
In the US, reported cases of TB, a treatable and curable disease, have declined since 1993. A total of 9,582 cases were reported in 2013, with 536 deaths due to TB reported in 2011 (the most recent year for which this data is available).3
Even though TB is on the decline worldwide,4 HCWs remain at increased risk for infection, confirming that TB is an occupational disease.5 It is imperative that health care facilities have effective infection control plans in place, primarily to reduce the risk for transmission of TB to HCWs.
This article reviews the available TB screening tests, CDC recommendations, parameters for evaluating test performance, and recent studies that lend support to the superiority of interferon-γ (IFN-γ) release assays (IGRAs) over tuberculin skin tests (TSTs) as part of HCW screening and infection control for TB. Primary care clinicians need to know about the status of these tests not only because we are HCWs ourselves, but because we are often responsible for the safety of other HCWs in our workplaces.
TB SCREENING TESTS
Diagnosing latent tuberculosis infection (LTBI) is key to overall control of the disease, since treatment decreases risk for conversion to active disease. Until recently, the diagnosis of LTBI relied on the TST, despite its limitations (see discussion under “Accuracy”). Now, immune-based blood tests hold promise for improving LTBI diagnosis.
Tuberculin skin test
In 1934, Florence Seibert developed what is known today as the purified protein derivative (PPD) test, which was adopted as the standard in the US in 1941.6 For 60 years, the PPD TST was the only screening method for Mycobacterium tuberculosis infection. It involves the intradermal injection of PPD; a hypersensitivity response leads to a cutaneous induration at the injection site after 48 to 72 hours.7Hence, the TST is a two-step test, requiring a follow-up visit for the result to be read.
Although strongly predictive of TB, the TST may result in false-positive test results in those previously immunized with the Bacille Calmette-Guerin (BCG) vaccine (a WHO-recommended childhood vaccination against TB, widely used outside the US),8 and in those exposed to certain nontuberculous mycobacteria such as M bovis and M africana.9 Test results may also be false negative in immunocompromised patients.9
Interferon-γ release assays
With the goal of developing a more specific and sensitive test for TB infection, IGRAs were developed in the mid-1990s as a new tool to detect active and latent TB infection.10 The first IGRA test for TB received FDA approval in 2001.11 There are currently two FDA-approved IGRAs—QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB—in use.
QuantiFERON-TB Gold In-Tube test. The QFT-GIT measures cell-mediated immune responses to antigens that simulate mycobacterial proteins. Requiring only one patient visit, whole blood is collected in three different tubes (negative control, TB antigen, and mitogen [positive] control), each containing a single antigen.12 After 16 to 24 hours in a temperature-controlled environment, the tubes are centrifuged to separate the plasma. IFN-γ levels are measured in each tube to calculate the test result.
T-SPOT.TB test. Using one blood sample, the T-SPOT.TB test captures IFN-γ produced by activated T-cells in response to stimulation by two M tuberculosis antigens. Addition of a substrate produces dark blue spots; the number of spots indicates the quantity of M tuberculosis-sensitive effector T-cells in the peripheral blood. A positive result is eight or more spots; a negative result is fewer than four spots, and a borderline result is five to seven spots.13 Unlike the QFT-GIT, the T-SPOT.TB has a “borderline” interpretation category.
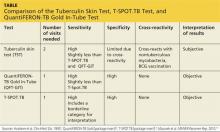
See the Table for a comparison of the TST, QFT-GIT, and T-SPOT.TB tests for TB screening.
Continue for CDC guidelines >>
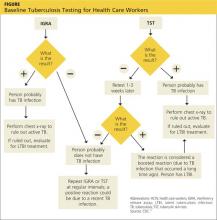
CDC GUIDELINES
Subject to state and federal regulations and Occupational Safety and Health Administration (OSHA) directives, the CDC states that the goals of a TB infection control plan are
• Prompt detection of suspected or confirmed TB infection
• Airborne precautions implemented to reduce risk for TB transmission in areas in which exposure can occur
• Treatment of persons with suspected or confirmed TB.14
TB screening for HCWs has historically been a challenge in that, for new hires, the CDC recommends a baseline two-step process (meaning up to four visits) when TST is used.15 This is because TST-tested individuals may test false negative, even if they have LTBI, if many years have passed since their infection was acquired. As a result, guidelines for baseline testing are that, if the initial TST is negative, TST should be repeated one to three weeks later. If the person is in fact infected with TB, the first TST may stimulate the immune system’s ability to react to the TB antigens and elicit a positive or “boosted” response to the second test.
The above figure has been corrected from the print version as of December 16, 2014.
The 2005 guidelines recommended screening new-hire HCWs with either a baseline two-step TST or with one blood assay for M tuberculosis. After the introduction of the QFT-GIT and T-SPOT.TB tests, the CDC included them in its updated 2010 guidelines and indicated that either IGRAs or TSTs may be used in HCW surveillance programs for occupational exposure to M tuberculosis (see Figure).15,16 The algorithm clearly illustrates how the use of IGRAs in place of TSTs streamlines the process of HCW TB screening.17
EVALUATION OF TESTS
Given the stringent nature of the CDC’s TB infection control goals, it is essential that health care facilities use the most effective and efficient means for timely and thorough screening of HCWs for TB. When evaluating the available tests, the following factors must be considered: accuracy and reproducibility of test results; impact on results when testing is repeated frequently; interpretation of discordant test results; and specificity, sensitivity, and identification of appropriate test cutoff values so that a positive result signifies a new TB infection (ie, conversion, a change from a documented negative to positive test result within a two-year period) rather than a false positive; and costs.18,19
Accuracy
Unlike TSTs, IGRAs do not produce false-positive results in individuals vaccinated with BCG or in those infected with most nontuberculous mycobacteria.9,16 Neither TSTs nor IGRAs, however, can distinguish between active and latent TB infection.19 If either test is positive, a chest radiograph is indicated. If the x-ray reveals abnormalities in the lungs, a sputum smear to detect acid-fast-bacilli (AFB), of which M tuberculosis is one, is indicative of TB. Nuclear acid amplification testing of a respiratory specimen provides rapid laboratory confirmation, but a positive culture for M tuberculosis confirms the diagnosis.22
Specificity and sensitivity
Pai, Zwerling, and Menzies conducted a meta-analysis of 38 studies of TB testing. Most of the studies were small and had limitations, such as the lack of a gold standard test for the diagnosis of LTBI and variable TST methods and cutoff values. Nevertheless, the researchers were able to conclude that IGRAs are significantly more specific than TST and are unaffected by BCG vaccination. Although the sensitivity of IGRAs and TST is inconsistent across test populations, T-SPOT.TB appears to have greater sensitivity than QFT-GIT or TST.23
Further, TST is subject to variability in administration and interpretation, and cut points for TST positivity vary internationally.9 In contrast, the T-SPOT.TB test specifies a borderline result zone. According to the CDC, this increases test accuracy by classifying results near the cut point, making a subsequent test conversion from negative to positive more likely to represent newly acquired infection.16
On the other hand, some studies of IGRAs have found unexpectedly high rates of initial positive results and conversions among HCWs in low-risk settings that are later determined to be false positives.24 However, as noted previously, TSTs are also subject to false-positive results. In addition, the definition of an IGRA conversion is less stringent than the TST conversion definition, which may result in more IGRA conversions.16
Discordant results
Zwerling et al conducted a systematic review of all studies in which IGRAs were used for HCW screening to summarize their performance in cross-sectional and serial testing settings. The prevalence of positive IGRAs was found to be lower than that of positive TSTs. This difference was significant in low- and moderate-TB incidence settings but not in high-incidence settings. A positive association was reported between positive IGRA test results and occupational risk factors, including work in high-risk wards, TB clinics, and geriatric care, as well as length of employment.18
According to Mancuso et al, discordance of results between the TST and IGRAs in populations with low LTBI prevalence suggests that most positive test results are in fact false positives in these populations.25 Although IGRAs were designed to increase specificity, the authors found that IGRA specificity was no better than the specificity of TST. Without a gold standard for detecting M tuberculosis infection, assessing the true significance of discordance between TST and IGRAs is difficult. Further research is needed to determine the significance of test discordance, to obtain data on progression to active TB, and to better define appropriate cut points for interpreting IGRA results.25
Costs
The primary impediment to the widespread use of IGRAs has been cost, which is approximately three times that of a TST.24
Eralp et al studied the cost-effectiveness of IGRAs versus TST for screening for active LTBI in HCWs by using healthy life-years gained—defined as the number of TB cases avoided, yielding an increase in life expectancy—as the benefit metric rather than quality-adjusted life-years. Because testing is completed with a single visit, use of IGRAs increases compliance while minimizing resources needed for a second visit and eliminating loss to follow-up. Also notable is that IGRA testing takes place in a laboratory, where costs can be held in check with focused expertise and optimized staffing structures. The authors concluded that incremental IGRA costs per healthy life-year gained were justified.19
Until publication of the SWITCH (Screening health care Workers with IGRA vs. TST: impact on Costs and adHerence to testing) study in 2012, cost-effectiveness studies had shown inconsistent results. This study, conducted by The Johns Hopkins University (JHU) employee health department, was the first of its kind in the US to systematically analyze test performance and labor costs for TB screening of HCWs. The results showed that the time required to administer a TST is one of the costliest elements. For a sizeable institution such as JHU, TST screening cost more than $1.3 million annually, equivalent to approximately $73 per person; in contrast, IGRA screening amounted to less than $55 per person.26
Further research
The association between IGRA test conversion in HCWs and the risk for active TB disease has not been demonstrated.16 However, this is also true of TSTs27 and further research is needed in this area. Research is also needed to determine the significance of TSA-IGRA results discordance and to better define cut points for IGRA interpretation.25 In addition, more study of factors related to serial (periodic or ongoing) TB testing—which are very important within the context of HCW screening—is needed. As an example, serial TB testing may reveal trends in test conversions and can identify areas of concern within a health care facility. Unfortunately, current CDC recommendations do not provide specific guidelines for serial IGRA testing, such as guidance for accurate interpretation of IGRA results within a serial testing context.16 These areas should be addressed in future CDC updates.
Continue for conclusion >>
CONCLUSION
Occupational health professionals are continuously evaluating how to improve surveillance programs and reduce HCWs’ time away from work. Within this context, four advantages IGRAs offer include
• Elimination of a two-step TST for new hires (which requires up to four separate visits)
• No false-positive results caused by previous BCG vaccination or exposure to most nontuberculous mycobacteria
• Significantly improved HCW compliance because screening requires only one visit
• Cost effectiveness.
From an occupational health perspective, these are significant advantages that support the use of IGRAs to screen HCW for TB.
REFERENCES
1. Wilson J. Five babies test positive for TB in Texas. CNN. September 30, 2014. www.cnn.com/2014/09/29/health/babies-test-positive-tb/index.html. Accessed November 12, 2014.
2. Bailey J. 700 babies exposed to TB at Texas Hospital. Atlanta Journal Constitution. September 22, 2014. www.ajc.com/news/lifestyles/health/700-babies-exposed-TB-texas-hospital/nhRmC/. Accessed November 12, 2014.
3. CDC. Reported tuberculosis in the United States, 2013. www.cdc.gov/tb/statistics/reports/2013/pdf/report2013.pdf. Accessed October 23, 2014.
4. World Health Organization. Global tuberculosis report 2014. www.who.int/tb/publications/global_report/en/. Accessed November 12, 2014.
5. Baussano I, Nunn P, Williams B, et al. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17(3):488-494.
6. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin: past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273-280.
7. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968-975.
8. World Health Organization. Recommendations for vaccine administration. www.who.int/immunization/policy/Immunization_routine_table1.pdf. Accessed November 12, 2014.
9. Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better? Ann Thorac Med. 2009;4(1):5-9.
10. Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5(4): 531-536.
11. FDA. QuantiFERON-TB - P010033. www.fda.gov/MedicalDevices/Products andMedicalProcedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm084025.htm. Accessed November 12, 2014.
12. QuantiFERON-TB Gold In-Tube Test [package insert]. www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf. Accessed November 12, 2014.
13. T-SPOT.TB [package insert]. Marlborough, MA. Oxford Immunotec, Inc. www.tspot.com/wp-content/uploads/2012/01/PI-TB-US-v4.pdf. Accessed November 12, 2014.
14. CDC. Fact sheet: infection control in health-care settings. www.cdc.gov/tb/publications/factsheets/prevention/ichcs.htm. Accessed November 12, 2014.
15. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium TB in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
16. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee, CDC. Updated guidelines for using interferon γ release assays to detect Mycobacterium TB infection: United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
17. CDC. Latent tuberculosis infection: a guide for primary health care providers. www.cdc.gov/tb/publications/ltbi/diagnosis.htm. Accessed November 3, 2014.
18. Zwerling A, van den Hof S, Scholten J, et al. Interferon-γ release assays for TB screening of healthcare workers: a systematic review. Thorax. 2012;67(1): 62-70.
19. Eralp MN, Scholtes S, Martell G, et al. Screening of healthcare workers for TB: development and validation of a new health economic model to inform practice. BMJ Open. 2012;2:e000630.
20. FDA. FDA permits marketing of first US test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin [press release]. July 25, 2013.
21. CDC. Availability of an assay for detecting Mycobacterium TB, including rifampin-resistant strains, and considerations for its use: United States, 2013 [published correction appears in MMWR Morb Mortal Wkly Rep. 2013;62(45):906]. MMWR Morb Mortal Wkly Rep. 2013;62(41):821-827.
22. CDC. Fact sheet: diagnosis of tuberculosis disease. www.cdc.gov/tb/publica tions/factsheets/testing/diagnosis.htm. Accessed November 12, 2014.
23. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent TB infection: an update. Ann Intern Med. 2008;149(3): 177-184.
24. LoBue PA, Castro KG. Is it time to replace the tuberculin skin test with a blood test? JAMA. 2012;308(3):241-242.
25. Mancuso JD, Mazurek GH, Tribble D, et al. Discordance among commercially available diagnostics for latent TB infection. Am J Respir Crit Care Med. 2012;185(4):427-434.
26. Wrighton-Smith P, Sneed L, Humphrey F, et al. Screening health care workers with interferon-γ release assay versus tuberculin skin test: impact on costs and adherence to testing (the SWITCH study). J Occup Environ Med. 2012;54(7):806-815.
27. World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries. www.who.int/tb/
features_archive/policy_statement_igra_oct2011.pdf. Accessed November 12, 2014.
Public health officials in El Paso, Texas, reported in September 2014 that more than 850 infants and 43 health care workers (HCW) at Providence Memorial Hospital may have been exposed to tuberculosis (TB) by a nurse with active infection. In collaboration with the CDC, the hospital administrators and local and state health officials advised potentially exposed individuals and their families to be screened for TB. According to press reports, five infants tested positive for TB and were to be treated.1,2 This news report highlights the importance of maintaining infection control protocols in health care facilities throughout the United States to reduce the risk for TB transmission.
SCREENING HEALTH CARE WORKERS
In the US, reported cases of TB, a treatable and curable disease, have declined since 1993. A total of 9,582 cases were reported in 2013, with 536 deaths due to TB reported in 2011 (the most recent year for which this data is available).3
Even though TB is on the decline worldwide,4 HCWs remain at increased risk for infection, confirming that TB is an occupational disease.5 It is imperative that health care facilities have effective infection control plans in place, primarily to reduce the risk for transmission of TB to HCWs.
This article reviews the available TB screening tests, CDC recommendations, parameters for evaluating test performance, and recent studies that lend support to the superiority of interferon-γ (IFN-γ) release assays (IGRAs) over tuberculin skin tests (TSTs) as part of HCW screening and infection control for TB. Primary care clinicians need to know about the status of these tests not only because we are HCWs ourselves, but because we are often responsible for the safety of other HCWs in our workplaces.
TB SCREENING TESTS
Diagnosing latent tuberculosis infection (LTBI) is key to overall control of the disease, since treatment decreases risk for conversion to active disease. Until recently, the diagnosis of LTBI relied on the TST, despite its limitations (see discussion under “Accuracy”). Now, immune-based blood tests hold promise for improving LTBI diagnosis.
Tuberculin skin test
In 1934, Florence Seibert developed what is known today as the purified protein derivative (PPD) test, which was adopted as the standard in the US in 1941.6 For 60 years, the PPD TST was the only screening method for Mycobacterium tuberculosis infection. It involves the intradermal injection of PPD; a hypersensitivity response leads to a cutaneous induration at the injection site after 48 to 72 hours.7Hence, the TST is a two-step test, requiring a follow-up visit for the result to be read.
Although strongly predictive of TB, the TST may result in false-positive test results in those previously immunized with the Bacille Calmette-Guerin (BCG) vaccine (a WHO-recommended childhood vaccination against TB, widely used outside the US),8 and in those exposed to certain nontuberculous mycobacteria such as M bovis and M africana.9 Test results may also be false negative in immunocompromised patients.9
Interferon-γ release assays
With the goal of developing a more specific and sensitive test for TB infection, IGRAs were developed in the mid-1990s as a new tool to detect active and latent TB infection.10 The first IGRA test for TB received FDA approval in 2001.11 There are currently two FDA-approved IGRAs—QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB—in use.
QuantiFERON-TB Gold In-Tube test. The QFT-GIT measures cell-mediated immune responses to antigens that simulate mycobacterial proteins. Requiring only one patient visit, whole blood is collected in three different tubes (negative control, TB antigen, and mitogen [positive] control), each containing a single antigen.12 After 16 to 24 hours in a temperature-controlled environment, the tubes are centrifuged to separate the plasma. IFN-γ levels are measured in each tube to calculate the test result.
T-SPOT.TB test. Using one blood sample, the T-SPOT.TB test captures IFN-γ produced by activated T-cells in response to stimulation by two M tuberculosis antigens. Addition of a substrate produces dark blue spots; the number of spots indicates the quantity of M tuberculosis-sensitive effector T-cells in the peripheral blood. A positive result is eight or more spots; a negative result is fewer than four spots, and a borderline result is five to seven spots.13 Unlike the QFT-GIT, the T-SPOT.TB has a “borderline” interpretation category.
See the Table for a comparison of the TST, QFT-GIT, and T-SPOT.TB tests for TB screening.
Continue for CDC guidelines >>
CDC GUIDELINES
Subject to state and federal regulations and Occupational Safety and Health Administration (OSHA) directives, the CDC states that the goals of a TB infection control plan are
• Prompt detection of suspected or confirmed TB infection
• Airborne precautions implemented to reduce risk for TB transmission in areas in which exposure can occur
• Treatment of persons with suspected or confirmed TB.14
TB screening for HCWs has historically been a challenge in that, for new hires, the CDC recommends a baseline two-step process (meaning up to four visits) when TST is used.15 This is because TST-tested individuals may test false negative, even if they have LTBI, if many years have passed since their infection was acquired. As a result, guidelines for baseline testing are that, if the initial TST is negative, TST should be repeated one to three weeks later. If the person is in fact infected with TB, the first TST may stimulate the immune system’s ability to react to the TB antigens and elicit a positive or “boosted” response to the second test.
The above figure has been corrected from the print version as of December 16, 2014.
The 2005 guidelines recommended screening new-hire HCWs with either a baseline two-step TST or with one blood assay for M tuberculosis. After the introduction of the QFT-GIT and T-SPOT.TB tests, the CDC included them in its updated 2010 guidelines and indicated that either IGRAs or TSTs may be used in HCW surveillance programs for occupational exposure to M tuberculosis (see Figure).15,16 The algorithm clearly illustrates how the use of IGRAs in place of TSTs streamlines the process of HCW TB screening.17
EVALUATION OF TESTS
Given the stringent nature of the CDC’s TB infection control goals, it is essential that health care facilities use the most effective and efficient means for timely and thorough screening of HCWs for TB. When evaluating the available tests, the following factors must be considered: accuracy and reproducibility of test results; impact on results when testing is repeated frequently; interpretation of discordant test results; and specificity, sensitivity, and identification of appropriate test cutoff values so that a positive result signifies a new TB infection (ie, conversion, a change from a documented negative to positive test result within a two-year period) rather than a false positive; and costs.18,19
Accuracy
Unlike TSTs, IGRAs do not produce false-positive results in individuals vaccinated with BCG or in those infected with most nontuberculous mycobacteria.9,16 Neither TSTs nor IGRAs, however, can distinguish between active and latent TB infection.19 If either test is positive, a chest radiograph is indicated. If the x-ray reveals abnormalities in the lungs, a sputum smear to detect acid-fast-bacilli (AFB), of which M tuberculosis is one, is indicative of TB. Nuclear acid amplification testing of a respiratory specimen provides rapid laboratory confirmation, but a positive culture for M tuberculosis confirms the diagnosis.22
Specificity and sensitivity
Pai, Zwerling, and Menzies conducted a meta-analysis of 38 studies of TB testing. Most of the studies were small and had limitations, such as the lack of a gold standard test for the diagnosis of LTBI and variable TST methods and cutoff values. Nevertheless, the researchers were able to conclude that IGRAs are significantly more specific than TST and are unaffected by BCG vaccination. Although the sensitivity of IGRAs and TST is inconsistent across test populations, T-SPOT.TB appears to have greater sensitivity than QFT-GIT or TST.23
Further, TST is subject to variability in administration and interpretation, and cut points for TST positivity vary internationally.9 In contrast, the T-SPOT.TB test specifies a borderline result zone. According to the CDC, this increases test accuracy by classifying results near the cut point, making a subsequent test conversion from negative to positive more likely to represent newly acquired infection.16
On the other hand, some studies of IGRAs have found unexpectedly high rates of initial positive results and conversions among HCWs in low-risk settings that are later determined to be false positives.24 However, as noted previously, TSTs are also subject to false-positive results. In addition, the definition of an IGRA conversion is less stringent than the TST conversion definition, which may result in more IGRA conversions.16
Discordant results
Zwerling et al conducted a systematic review of all studies in which IGRAs were used for HCW screening to summarize their performance in cross-sectional and serial testing settings. The prevalence of positive IGRAs was found to be lower than that of positive TSTs. This difference was significant in low- and moderate-TB incidence settings but not in high-incidence settings. A positive association was reported between positive IGRA test results and occupational risk factors, including work in high-risk wards, TB clinics, and geriatric care, as well as length of employment.18
According to Mancuso et al, discordance of results between the TST and IGRAs in populations with low LTBI prevalence suggests that most positive test results are in fact false positives in these populations.25 Although IGRAs were designed to increase specificity, the authors found that IGRA specificity was no better than the specificity of TST. Without a gold standard for detecting M tuberculosis infection, assessing the true significance of discordance between TST and IGRAs is difficult. Further research is needed to determine the significance of test discordance, to obtain data on progression to active TB, and to better define appropriate cut points for interpreting IGRA results.25
Costs
The primary impediment to the widespread use of IGRAs has been cost, which is approximately three times that of a TST.24
Eralp et al studied the cost-effectiveness of IGRAs versus TST for screening for active LTBI in HCWs by using healthy life-years gained—defined as the number of TB cases avoided, yielding an increase in life expectancy—as the benefit metric rather than quality-adjusted life-years. Because testing is completed with a single visit, use of IGRAs increases compliance while minimizing resources needed for a second visit and eliminating loss to follow-up. Also notable is that IGRA testing takes place in a laboratory, where costs can be held in check with focused expertise and optimized staffing structures. The authors concluded that incremental IGRA costs per healthy life-year gained were justified.19
Until publication of the SWITCH (Screening health care Workers with IGRA vs. TST: impact on Costs and adHerence to testing) study in 2012, cost-effectiveness studies had shown inconsistent results. This study, conducted by The Johns Hopkins University (JHU) employee health department, was the first of its kind in the US to systematically analyze test performance and labor costs for TB screening of HCWs. The results showed that the time required to administer a TST is one of the costliest elements. For a sizeable institution such as JHU, TST screening cost more than $1.3 million annually, equivalent to approximately $73 per person; in contrast, IGRA screening amounted to less than $55 per person.26
Further research
The association between IGRA test conversion in HCWs and the risk for active TB disease has not been demonstrated.16 However, this is also true of TSTs27 and further research is needed in this area. Research is also needed to determine the significance of TSA-IGRA results discordance and to better define cut points for IGRA interpretation.25 In addition, more study of factors related to serial (periodic or ongoing) TB testing—which are very important within the context of HCW screening—is needed. As an example, serial TB testing may reveal trends in test conversions and can identify areas of concern within a health care facility. Unfortunately, current CDC recommendations do not provide specific guidelines for serial IGRA testing, such as guidance for accurate interpretation of IGRA results within a serial testing context.16 These areas should be addressed in future CDC updates.
Continue for conclusion >>
CONCLUSION
Occupational health professionals are continuously evaluating how to improve surveillance programs and reduce HCWs’ time away from work. Within this context, four advantages IGRAs offer include
• Elimination of a two-step TST for new hires (which requires up to four separate visits)
• No false-positive results caused by previous BCG vaccination or exposure to most nontuberculous mycobacteria
• Significantly improved HCW compliance because screening requires only one visit
• Cost effectiveness.
From an occupational health perspective, these are significant advantages that support the use of IGRAs to screen HCW for TB.
REFERENCES
1. Wilson J. Five babies test positive for TB in Texas. CNN. September 30, 2014. www.cnn.com/2014/09/29/health/babies-test-positive-tb/index.html. Accessed November 12, 2014.
2. Bailey J. 700 babies exposed to TB at Texas Hospital. Atlanta Journal Constitution. September 22, 2014. www.ajc.com/news/lifestyles/health/700-babies-exposed-TB-texas-hospital/nhRmC/. Accessed November 12, 2014.
3. CDC. Reported tuberculosis in the United States, 2013. www.cdc.gov/tb/statistics/reports/2013/pdf/report2013.pdf. Accessed October 23, 2014.
4. World Health Organization. Global tuberculosis report 2014. www.who.int/tb/publications/global_report/en/. Accessed November 12, 2014.
5. Baussano I, Nunn P, Williams B, et al. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17(3):488-494.
6. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin: past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273-280.
7. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968-975.
8. World Health Organization. Recommendations for vaccine administration. www.who.int/immunization/policy/Immunization_routine_table1.pdf. Accessed November 12, 2014.
9. Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better? Ann Thorac Med. 2009;4(1):5-9.
10. Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5(4): 531-536.
11. FDA. QuantiFERON-TB - P010033. www.fda.gov/MedicalDevices/Products andMedicalProcedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm084025.htm. Accessed November 12, 2014.
12. QuantiFERON-TB Gold In-Tube Test [package insert]. www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf. Accessed November 12, 2014.
13. T-SPOT.TB [package insert]. Marlborough, MA. Oxford Immunotec, Inc. www.tspot.com/wp-content/uploads/2012/01/PI-TB-US-v4.pdf. Accessed November 12, 2014.
14. CDC. Fact sheet: infection control in health-care settings. www.cdc.gov/tb/publications/factsheets/prevention/ichcs.htm. Accessed November 12, 2014.
15. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium TB in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
16. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee, CDC. Updated guidelines for using interferon γ release assays to detect Mycobacterium TB infection: United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
17. CDC. Latent tuberculosis infection: a guide for primary health care providers. www.cdc.gov/tb/publications/ltbi/diagnosis.htm. Accessed November 3, 2014.
18. Zwerling A, van den Hof S, Scholten J, et al. Interferon-γ release assays for TB screening of healthcare workers: a systematic review. Thorax. 2012;67(1): 62-70.
19. Eralp MN, Scholtes S, Martell G, et al. Screening of healthcare workers for TB: development and validation of a new health economic model to inform practice. BMJ Open. 2012;2:e000630.
20. FDA. FDA permits marketing of first US test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin [press release]. July 25, 2013.
21. CDC. Availability of an assay for detecting Mycobacterium TB, including rifampin-resistant strains, and considerations for its use: United States, 2013 [published correction appears in MMWR Morb Mortal Wkly Rep. 2013;62(45):906]. MMWR Morb Mortal Wkly Rep. 2013;62(41):821-827.
22. CDC. Fact sheet: diagnosis of tuberculosis disease. www.cdc.gov/tb/publica tions/factsheets/testing/diagnosis.htm. Accessed November 12, 2014.
23. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent TB infection: an update. Ann Intern Med. 2008;149(3): 177-184.
24. LoBue PA, Castro KG. Is it time to replace the tuberculin skin test with a blood test? JAMA. 2012;308(3):241-242.
25. Mancuso JD, Mazurek GH, Tribble D, et al. Discordance among commercially available diagnostics for latent TB infection. Am J Respir Crit Care Med. 2012;185(4):427-434.
26. Wrighton-Smith P, Sneed L, Humphrey F, et al. Screening health care workers with interferon-γ release assay versus tuberculin skin test: impact on costs and adherence to testing (the SWITCH study). J Occup Environ Med. 2012;54(7):806-815.
27. World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries. www.who.int/tb/
features_archive/policy_statement_igra_oct2011.pdf. Accessed November 12, 2014.
Public health officials in El Paso, Texas, reported in September 2014 that more than 850 infants and 43 health care workers (HCW) at Providence Memorial Hospital may have been exposed to tuberculosis (TB) by a nurse with active infection. In collaboration with the CDC, the hospital administrators and local and state health officials advised potentially exposed individuals and their families to be screened for TB. According to press reports, five infants tested positive for TB and were to be treated.1,2 This news report highlights the importance of maintaining infection control protocols in health care facilities throughout the United States to reduce the risk for TB transmission.
SCREENING HEALTH CARE WORKERS
In the US, reported cases of TB, a treatable and curable disease, have declined since 1993. A total of 9,582 cases were reported in 2013, with 536 deaths due to TB reported in 2011 (the most recent year for which this data is available).3
Even though TB is on the decline worldwide,4 HCWs remain at increased risk for infection, confirming that TB is an occupational disease.5 It is imperative that health care facilities have effective infection control plans in place, primarily to reduce the risk for transmission of TB to HCWs.
This article reviews the available TB screening tests, CDC recommendations, parameters for evaluating test performance, and recent studies that lend support to the superiority of interferon-γ (IFN-γ) release assays (IGRAs) over tuberculin skin tests (TSTs) as part of HCW screening and infection control for TB. Primary care clinicians need to know about the status of these tests not only because we are HCWs ourselves, but because we are often responsible for the safety of other HCWs in our workplaces.
TB SCREENING TESTS
Diagnosing latent tuberculosis infection (LTBI) is key to overall control of the disease, since treatment decreases risk for conversion to active disease. Until recently, the diagnosis of LTBI relied on the TST, despite its limitations (see discussion under “Accuracy”). Now, immune-based blood tests hold promise for improving LTBI diagnosis.
Tuberculin skin test
In 1934, Florence Seibert developed what is known today as the purified protein derivative (PPD) test, which was adopted as the standard in the US in 1941.6 For 60 years, the PPD TST was the only screening method for Mycobacterium tuberculosis infection. It involves the intradermal injection of PPD; a hypersensitivity response leads to a cutaneous induration at the injection site after 48 to 72 hours.7Hence, the TST is a two-step test, requiring a follow-up visit for the result to be read.
Although strongly predictive of TB, the TST may result in false-positive test results in those previously immunized with the Bacille Calmette-Guerin (BCG) vaccine (a WHO-recommended childhood vaccination against TB, widely used outside the US),8 and in those exposed to certain nontuberculous mycobacteria such as M bovis and M africana.9 Test results may also be false negative in immunocompromised patients.9
Interferon-γ release assays
With the goal of developing a more specific and sensitive test for TB infection, IGRAs were developed in the mid-1990s as a new tool to detect active and latent TB infection.10 The first IGRA test for TB received FDA approval in 2001.11 There are currently two FDA-approved IGRAs—QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB—in use.
QuantiFERON-TB Gold In-Tube test. The QFT-GIT measures cell-mediated immune responses to antigens that simulate mycobacterial proteins. Requiring only one patient visit, whole blood is collected in three different tubes (negative control, TB antigen, and mitogen [positive] control), each containing a single antigen.12 After 16 to 24 hours in a temperature-controlled environment, the tubes are centrifuged to separate the plasma. IFN-γ levels are measured in each tube to calculate the test result.
T-SPOT.TB test. Using one blood sample, the T-SPOT.TB test captures IFN-γ produced by activated T-cells in response to stimulation by two M tuberculosis antigens. Addition of a substrate produces dark blue spots; the number of spots indicates the quantity of M tuberculosis-sensitive effector T-cells in the peripheral blood. A positive result is eight or more spots; a negative result is fewer than four spots, and a borderline result is five to seven spots.13 Unlike the QFT-GIT, the T-SPOT.TB has a “borderline” interpretation category.
See the Table for a comparison of the TST, QFT-GIT, and T-SPOT.TB tests for TB screening.
Continue for CDC guidelines >>
CDC GUIDELINES
Subject to state and federal regulations and Occupational Safety and Health Administration (OSHA) directives, the CDC states that the goals of a TB infection control plan are
• Prompt detection of suspected or confirmed TB infection
• Airborne precautions implemented to reduce risk for TB transmission in areas in which exposure can occur
• Treatment of persons with suspected or confirmed TB.14
TB screening for HCWs has historically been a challenge in that, for new hires, the CDC recommends a baseline two-step process (meaning up to four visits) when TST is used.15 This is because TST-tested individuals may test false negative, even if they have LTBI, if many years have passed since their infection was acquired. As a result, guidelines for baseline testing are that, if the initial TST is negative, TST should be repeated one to three weeks later. If the person is in fact infected with TB, the first TST may stimulate the immune system’s ability to react to the TB antigens and elicit a positive or “boosted” response to the second test.
The above figure has been corrected from the print version as of December 16, 2014.
The 2005 guidelines recommended screening new-hire HCWs with either a baseline two-step TST or with one blood assay for M tuberculosis. After the introduction of the QFT-GIT and T-SPOT.TB tests, the CDC included them in its updated 2010 guidelines and indicated that either IGRAs or TSTs may be used in HCW surveillance programs for occupational exposure to M tuberculosis (see Figure).15,16 The algorithm clearly illustrates how the use of IGRAs in place of TSTs streamlines the process of HCW TB screening.17
EVALUATION OF TESTS
Given the stringent nature of the CDC’s TB infection control goals, it is essential that health care facilities use the most effective and efficient means for timely and thorough screening of HCWs for TB. When evaluating the available tests, the following factors must be considered: accuracy and reproducibility of test results; impact on results when testing is repeated frequently; interpretation of discordant test results; and specificity, sensitivity, and identification of appropriate test cutoff values so that a positive result signifies a new TB infection (ie, conversion, a change from a documented negative to positive test result within a two-year period) rather than a false positive; and costs.18,19
Accuracy
Unlike TSTs, IGRAs do not produce false-positive results in individuals vaccinated with BCG or in those infected with most nontuberculous mycobacteria.9,16 Neither TSTs nor IGRAs, however, can distinguish between active and latent TB infection.19 If either test is positive, a chest radiograph is indicated. If the x-ray reveals abnormalities in the lungs, a sputum smear to detect acid-fast-bacilli (AFB), of which M tuberculosis is one, is indicative of TB. Nuclear acid amplification testing of a respiratory specimen provides rapid laboratory confirmation, but a positive culture for M tuberculosis confirms the diagnosis.22
Specificity and sensitivity
Pai, Zwerling, and Menzies conducted a meta-analysis of 38 studies of TB testing. Most of the studies were small and had limitations, such as the lack of a gold standard test for the diagnosis of LTBI and variable TST methods and cutoff values. Nevertheless, the researchers were able to conclude that IGRAs are significantly more specific than TST and are unaffected by BCG vaccination. Although the sensitivity of IGRAs and TST is inconsistent across test populations, T-SPOT.TB appears to have greater sensitivity than QFT-GIT or TST.23
Further, TST is subject to variability in administration and interpretation, and cut points for TST positivity vary internationally.9 In contrast, the T-SPOT.TB test specifies a borderline result zone. According to the CDC, this increases test accuracy by classifying results near the cut point, making a subsequent test conversion from negative to positive more likely to represent newly acquired infection.16
On the other hand, some studies of IGRAs have found unexpectedly high rates of initial positive results and conversions among HCWs in low-risk settings that are later determined to be false positives.24 However, as noted previously, TSTs are also subject to false-positive results. In addition, the definition of an IGRA conversion is less stringent than the TST conversion definition, which may result in more IGRA conversions.16
Discordant results
Zwerling et al conducted a systematic review of all studies in which IGRAs were used for HCW screening to summarize their performance in cross-sectional and serial testing settings. The prevalence of positive IGRAs was found to be lower than that of positive TSTs. This difference was significant in low- and moderate-TB incidence settings but not in high-incidence settings. A positive association was reported between positive IGRA test results and occupational risk factors, including work in high-risk wards, TB clinics, and geriatric care, as well as length of employment.18
According to Mancuso et al, discordance of results between the TST and IGRAs in populations with low LTBI prevalence suggests that most positive test results are in fact false positives in these populations.25 Although IGRAs were designed to increase specificity, the authors found that IGRA specificity was no better than the specificity of TST. Without a gold standard for detecting M tuberculosis infection, assessing the true significance of discordance between TST and IGRAs is difficult. Further research is needed to determine the significance of test discordance, to obtain data on progression to active TB, and to better define appropriate cut points for interpreting IGRA results.25
Costs
The primary impediment to the widespread use of IGRAs has been cost, which is approximately three times that of a TST.24
Eralp et al studied the cost-effectiveness of IGRAs versus TST for screening for active LTBI in HCWs by using healthy life-years gained—defined as the number of TB cases avoided, yielding an increase in life expectancy—as the benefit metric rather than quality-adjusted life-years. Because testing is completed with a single visit, use of IGRAs increases compliance while minimizing resources needed for a second visit and eliminating loss to follow-up. Also notable is that IGRA testing takes place in a laboratory, where costs can be held in check with focused expertise and optimized staffing structures. The authors concluded that incremental IGRA costs per healthy life-year gained were justified.19
Until publication of the SWITCH (Screening health care Workers with IGRA vs. TST: impact on Costs and adHerence to testing) study in 2012, cost-effectiveness studies had shown inconsistent results. This study, conducted by The Johns Hopkins University (JHU) employee health department, was the first of its kind in the US to systematically analyze test performance and labor costs for TB screening of HCWs. The results showed that the time required to administer a TST is one of the costliest elements. For a sizeable institution such as JHU, TST screening cost more than $1.3 million annually, equivalent to approximately $73 per person; in contrast, IGRA screening amounted to less than $55 per person.26
Further research
The association between IGRA test conversion in HCWs and the risk for active TB disease has not been demonstrated.16 However, this is also true of TSTs27 and further research is needed in this area. Research is also needed to determine the significance of TSA-IGRA results discordance and to better define cut points for IGRA interpretation.25 In addition, more study of factors related to serial (periodic or ongoing) TB testing—which are very important within the context of HCW screening—is needed. As an example, serial TB testing may reveal trends in test conversions and can identify areas of concern within a health care facility. Unfortunately, current CDC recommendations do not provide specific guidelines for serial IGRA testing, such as guidance for accurate interpretation of IGRA results within a serial testing context.16 These areas should be addressed in future CDC updates.
Continue for conclusion >>
CONCLUSION
Occupational health professionals are continuously evaluating how to improve surveillance programs and reduce HCWs’ time away from work. Within this context, four advantages IGRAs offer include
• Elimination of a two-step TST for new hires (which requires up to four separate visits)
• No false-positive results caused by previous BCG vaccination or exposure to most nontuberculous mycobacteria
• Significantly improved HCW compliance because screening requires only one visit
• Cost effectiveness.
From an occupational health perspective, these are significant advantages that support the use of IGRAs to screen HCW for TB.
REFERENCES
1. Wilson J. Five babies test positive for TB in Texas. CNN. September 30, 2014. www.cnn.com/2014/09/29/health/babies-test-positive-tb/index.html. Accessed November 12, 2014.
2. Bailey J. 700 babies exposed to TB at Texas Hospital. Atlanta Journal Constitution. September 22, 2014. www.ajc.com/news/lifestyles/health/700-babies-exposed-TB-texas-hospital/nhRmC/. Accessed November 12, 2014.
3. CDC. Reported tuberculosis in the United States, 2013. www.cdc.gov/tb/statistics/reports/2013/pdf/report2013.pdf. Accessed October 23, 2014.
4. World Health Organization. Global tuberculosis report 2014. www.who.int/tb/publications/global_report/en/. Accessed November 12, 2014.
5. Baussano I, Nunn P, Williams B, et al. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17(3):488-494.
6. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin: past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273-280.
7. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968-975.
8. World Health Organization. Recommendations for vaccine administration. www.who.int/immunization/policy/Immunization_routine_table1.pdf. Accessed November 12, 2014.
9. Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better? Ann Thorac Med. 2009;4(1):5-9.
10. Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5(4): 531-536.
11. FDA. QuantiFERON-TB - P010033. www.fda.gov/MedicalDevices/Products andMedicalProcedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm084025.htm. Accessed November 12, 2014.
12. QuantiFERON-TB Gold In-Tube Test [package insert]. www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf. Accessed November 12, 2014.
13. T-SPOT.TB [package insert]. Marlborough, MA. Oxford Immunotec, Inc. www.tspot.com/wp-content/uploads/2012/01/PI-TB-US-v4.pdf. Accessed November 12, 2014.
14. CDC. Fact sheet: infection control in health-care settings. www.cdc.gov/tb/publications/factsheets/prevention/ichcs.htm. Accessed November 12, 2014.
15. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium TB in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
16. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee, CDC. Updated guidelines for using interferon γ release assays to detect Mycobacterium TB infection: United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
17. CDC. Latent tuberculosis infection: a guide for primary health care providers. www.cdc.gov/tb/publications/ltbi/diagnosis.htm. Accessed November 3, 2014.
18. Zwerling A, van den Hof S, Scholten J, et al. Interferon-γ release assays for TB screening of healthcare workers: a systematic review. Thorax. 2012;67(1): 62-70.
19. Eralp MN, Scholtes S, Martell G, et al. Screening of healthcare workers for TB: development and validation of a new health economic model to inform practice. BMJ Open. 2012;2:e000630.
20. FDA. FDA permits marketing of first US test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin [press release]. July 25, 2013.
21. CDC. Availability of an assay for detecting Mycobacterium TB, including rifampin-resistant strains, and considerations for its use: United States, 2013 [published correction appears in MMWR Morb Mortal Wkly Rep. 2013;62(45):906]. MMWR Morb Mortal Wkly Rep. 2013;62(41):821-827.
22. CDC. Fact sheet: diagnosis of tuberculosis disease. www.cdc.gov/tb/publica tions/factsheets/testing/diagnosis.htm. Accessed November 12, 2014.
23. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent TB infection: an update. Ann Intern Med. 2008;149(3): 177-184.
24. LoBue PA, Castro KG. Is it time to replace the tuberculin skin test with a blood test? JAMA. 2012;308(3):241-242.
25. Mancuso JD, Mazurek GH, Tribble D, et al. Discordance among commercially available diagnostics for latent TB infection. Am J Respir Crit Care Med. 2012;185(4):427-434.
26. Wrighton-Smith P, Sneed L, Humphrey F, et al. Screening health care workers with interferon-γ release assay versus tuberculin skin test: impact on costs and adherence to testing (the SWITCH study). J Occup Environ Med. 2012;54(7):806-815.
27. World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries. www.who.int/tb/
features_archive/policy_statement_igra_oct2011.pdf. Accessed November 12, 2014.
ACIP Recommends PCV13 for All Adults 65 and Up
All adults who are 65 years or older should receive 13-valent pneumococcal conjugate vaccine (PCV13) routinely in series with 23-valent pneumococcal polysaccharide vaccine (PPSV23), according to a new recommendation from the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). The recommendation appears in the Sept. 19 issue of Morbidity and Mortality Weekly Report.
The ACIP recommendation calls for pneumococcal vaccine-naive adults aged 65 and older to receive one dose of PCV13 vaccine, followed by a dose of PPSV23 6-12 months later (MMWR 2014:63;822-5). Older adults who have previously received only PPSV23 should receive a dose of PCV13 at least 12 months later, wrote Sara Tomczyk of the CDC and her associates.
ACIP has recommended PPSV23 for older adults since 2010. In 2012, the committee made its first recommendation for PCV13, targeting patients 19 years and older who have immunocompromising conditions, functional or anatomic asplenia, cerebrospinal fluid leak, or cochlear implants. The new PCV13 recommendation for all older adults is based on a randomized, placebo-controlled trial of the vaccine in about 85,000 adults aged 65 years and older in the Netherlands who had no prior pneumococcal vaccine exposure. The vaccine showed a moderate level of evidence for efficacy against community-acquired pneumonia in this cohort, ACIP determined. Efficacy against nonbacteremic vaccine-type pneumococcal pneumonia was about 45%, while efficacy against vaccine-type invasive pneumococcal disease was about 75%, the reviewers wrote.
ACIP will reevaluate the recommendations in 2018.
Ms. Tomczyk and her colleagues disclosed no funding sources or conflicts of interest.
All adults who are 65 years or older should receive 13-valent pneumococcal conjugate vaccine (PCV13) routinely in series with 23-valent pneumococcal polysaccharide vaccine (PPSV23), according to a new recommendation from the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). The recommendation appears in the Sept. 19 issue of Morbidity and Mortality Weekly Report.
The ACIP recommendation calls for pneumococcal vaccine-naive adults aged 65 and older to receive one dose of PCV13 vaccine, followed by a dose of PPSV23 6-12 months later (MMWR 2014:63;822-5). Older adults who have previously received only PPSV23 should receive a dose of PCV13 at least 12 months later, wrote Sara Tomczyk of the CDC and her associates.
ACIP has recommended PPSV23 for older adults since 2010. In 2012, the committee made its first recommendation for PCV13, targeting patients 19 years and older who have immunocompromising conditions, functional or anatomic asplenia, cerebrospinal fluid leak, or cochlear implants. The new PCV13 recommendation for all older adults is based on a randomized, placebo-controlled trial of the vaccine in about 85,000 adults aged 65 years and older in the Netherlands who had no prior pneumococcal vaccine exposure. The vaccine showed a moderate level of evidence for efficacy against community-acquired pneumonia in this cohort, ACIP determined. Efficacy against nonbacteremic vaccine-type pneumococcal pneumonia was about 45%, while efficacy against vaccine-type invasive pneumococcal disease was about 75%, the reviewers wrote.
ACIP will reevaluate the recommendations in 2018.
Ms. Tomczyk and her colleagues disclosed no funding sources or conflicts of interest.
All adults who are 65 years or older should receive 13-valent pneumococcal conjugate vaccine (PCV13) routinely in series with 23-valent pneumococcal polysaccharide vaccine (PPSV23), according to a new recommendation from the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). The recommendation appears in the Sept. 19 issue of Morbidity and Mortality Weekly Report.
The ACIP recommendation calls for pneumococcal vaccine-naive adults aged 65 and older to receive one dose of PCV13 vaccine, followed by a dose of PPSV23 6-12 months later (MMWR 2014:63;822-5). Older adults who have previously received only PPSV23 should receive a dose of PCV13 at least 12 months later, wrote Sara Tomczyk of the CDC and her associates.
ACIP has recommended PPSV23 for older adults since 2010. In 2012, the committee made its first recommendation for PCV13, targeting patients 19 years and older who have immunocompromising conditions, functional or anatomic asplenia, cerebrospinal fluid leak, or cochlear implants. The new PCV13 recommendation for all older adults is based on a randomized, placebo-controlled trial of the vaccine in about 85,000 adults aged 65 years and older in the Netherlands who had no prior pneumococcal vaccine exposure. The vaccine showed a moderate level of evidence for efficacy against community-acquired pneumonia in this cohort, ACIP determined. Efficacy against nonbacteremic vaccine-type pneumococcal pneumonia was about 45%, while efficacy against vaccine-type invasive pneumococcal disease was about 75%, the reviewers wrote.
ACIP will reevaluate the recommendations in 2018.
Ms. Tomczyk and her colleagues disclosed no funding sources or conflicts of interest.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Sleeping on Animal Skins Might Protect Against Childhood Asthma, Hay Fever
Babies who slept on animal skins during their first 3 months of life were almost 40% less likely to have asthma by the time they were 10 years old, according to a population-based cohort study.
Sleeping on animal skins during infancy also was linked to lower odds of wheezing and hay fever, but did not seem to affect eczema or sensitivity to airborne antigens, Dr. Christina Tischer reported at the annual meeting of the European Respiratory Society.
"Early exposure to animal fur could be a simple, cheap, and effective way to resemble an environment with higher microbial exposure," said Dr. Tischer, a researcher at the German Institute for Environmental Health in Neuherberg, Germany. "It might follow similar protective mechanisms in relation to asthma and allergy as it has been observed in farm and rural environments."
The investigators studied 2,441 children in Germany who were up to 10 years old; parents answered a series of questionnaires about asthma and respiratory risk factors and health outcomes. In all, 55% of the children slept on animal skins or animal furs during their first 3 months of life, Dr. Tischer and her associates reported.
By age 10 years, children who slept on animal skins or animal fur as infants had a 25% lower odds of ever having wheezed (adjusted odds ratio, 0.75), a 38% lower odds of having been diagnosed with asthma (aOR, 0.62), and a 35% lower odds of having been diagnosed with hay fever (aOR, 0.65) compared with children who did not sleep on animals skins or furs as infants, the investigators reported.
Funding information for the study was not available. Dr. Tischer reported no conflicts of interest.
Babies who slept on animal skins during their first 3 months of life were almost 40% less likely to have asthma by the time they were 10 years old, according to a population-based cohort study.
Sleeping on animal skins during infancy also was linked to lower odds of wheezing and hay fever, but did not seem to affect eczema or sensitivity to airborne antigens, Dr. Christina Tischer reported at the annual meeting of the European Respiratory Society.
"Early exposure to animal fur could be a simple, cheap, and effective way to resemble an environment with higher microbial exposure," said Dr. Tischer, a researcher at the German Institute for Environmental Health in Neuherberg, Germany. "It might follow similar protective mechanisms in relation to asthma and allergy as it has been observed in farm and rural environments."
The investigators studied 2,441 children in Germany who were up to 10 years old; parents answered a series of questionnaires about asthma and respiratory risk factors and health outcomes. In all, 55% of the children slept on animal skins or animal furs during their first 3 months of life, Dr. Tischer and her associates reported.
By age 10 years, children who slept on animal skins or animal fur as infants had a 25% lower odds of ever having wheezed (adjusted odds ratio, 0.75), a 38% lower odds of having been diagnosed with asthma (aOR, 0.62), and a 35% lower odds of having been diagnosed with hay fever (aOR, 0.65) compared with children who did not sleep on animals skins or furs as infants, the investigators reported.
Funding information for the study was not available. Dr. Tischer reported no conflicts of interest.
Babies who slept on animal skins during their first 3 months of life were almost 40% less likely to have asthma by the time they were 10 years old, according to a population-based cohort study.
Sleeping on animal skins during infancy also was linked to lower odds of wheezing and hay fever, but did not seem to affect eczema or sensitivity to airborne antigens, Dr. Christina Tischer reported at the annual meeting of the European Respiratory Society.
"Early exposure to animal fur could be a simple, cheap, and effective way to resemble an environment with higher microbial exposure," said Dr. Tischer, a researcher at the German Institute for Environmental Health in Neuherberg, Germany. "It might follow similar protective mechanisms in relation to asthma and allergy as it has been observed in farm and rural environments."
The investigators studied 2,441 children in Germany who were up to 10 years old; parents answered a series of questionnaires about asthma and respiratory risk factors and health outcomes. In all, 55% of the children slept on animal skins or animal furs during their first 3 months of life, Dr. Tischer and her associates reported.
By age 10 years, children who slept on animal skins or animal fur as infants had a 25% lower odds of ever having wheezed (adjusted odds ratio, 0.75), a 38% lower odds of having been diagnosed with asthma (aOR, 0.62), and a 35% lower odds of having been diagnosed with hay fever (aOR, 0.65) compared with children who did not sleep on animals skins or furs as infants, the investigators reported.
Funding information for the study was not available. Dr. Tischer reported no conflicts of interest.
FROM THE EUROPEAN RESPIRATORY SOCIETY INTERNATIONAL CONGRESS
NHLBI Expert Panel Issues Guideline on Sickle Cell Disease
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
FROM JAMA
When Patients Ask for Antibiotics, Arm Them With Handouts
Drug store and supermarket shelves display aisle after aisle of OTC medications that alleviate the common symptoms of upper respiratory infections. Despite the easy availability of symptom relief, a significant number of people consult clinicians in primary care offices, emergency departments (EDs), and walk-in or convenient care clinics for help when they feel that they’re “coming down with something.” During these visits, many of these patients expect, and sometimes demand, antibiotics.
Antibiotics may be viewed by the patient as a quick fix, with the demand undoubtedly fueled by busy lifestyles, long work hours, and little time for patients to stay home while ill. Patients so inclined may “doctor shop” if their demands are not met; clinicians know better but may feel pressure to “satisfy the customer” and may rationalize that an antibiotic might prove helpful in a particular case.
Lee et al studied outpatient antibiotic prescribing in the United States for acute respiratory tract infections (ARTI), including acute nasopharyngitis, upper respiratory tract infection, bronchitis, influenza, pharyngitis, and sinusitis. In 2000, antibiotics were prescribed during outpatient visits for ARTI to 64% of patients; by 2010, that percentage had increased to 73%.1 Although antibiotics are neither effective nor appropriate for the treatment of ARTI, most of which are viral infections, they are commonly prescribed. Further, while 17% of ARTI prescriptions in 2000 were for broad-spectrum antibiotics, that percentage jumped to 46% in 2010.1,2
Patient insistence on antibiotics may stem either from little knowledge of or little regard for the health problems caused by unnecessary antibiotic use. For example, one study found that 19.3% of drug-related ED visits were related to systemic antibiotics; nearly 80% of those were for allergic reactions.3,4 With an estimated 50% of antibiotic prescriptions considered inappropriate, the overuse of antibiotics creates unnecessary personal health risks and health care expenditures. Further, a more serious consequence of this overuse is the growing public health problem of antibiotic resistance (see Figure 1).1,2
Clinicians are ideally positioned to address these issues by incorporating effective, proactive strategies into selected patient encounters to specifically explain appropriate versus inappropriate antibiotic use.
Continue for patient handouts >>
One approach is to merge accurate, powerful messages about antibiotics with helpful information about effective OTC products to both enlighten patients and offer them the symptomatic relief they seek. The CDC has taken the lead in this area with its “Get Smart: Know When Antibiotics Work” initiative, which includes a variety of materials for both health care providers and patients.5
Inspired by the unmet need for written communications that explain viral and bacterial illnesses in simple terms and the reasons why taking antibiotics for the former is a bad idea, we developed two handouts for adult patients who present to the clinician’s office with viral respiratory illnesses.
The first is entitled “Prescription for Recovery From Your Viral Respiratory Illness” [download PDF]. Intended to be duplicated and designed with primary care use in mind, it can be customized for specialty use as well.
This “prescription” handout addresses common complaints of fever, pain (eg, sore throat, body aches, headache), cough, congestion (chest, nose, sinuses), and sneezing/runny nose. Clinicians can check off the appropriate treatments for an individual patient, who can use it as a handy reference to purchase the recommended OTC products and/or for selecting products or preparing helpful remedies at home. Blank lines at the bottom provide space for you to write in your OTC preferences and allow you to customize patient instructions.
The second patient handout is entitled “Antibiotics: When You Need Them, When You Don’t, and What to Take When You Don’t” [download PDF]. This focused patient teaching tool offers an overview of
• Viral and bacterial respiratory illnesses and what the patient should do if he or she has one or the other (including when to see a clinician)
• Some helpful OTC and home treatments for symptoms of viral respiratory illnesses
• When antibiotics are indicated and when they’re not—and why
• The serious problems caused by unnecessary use of antibiotics.
This brief guide can be used as a general informational handout that can, for example, be given, mailed, or e-mailed to all adult patients at the start of cold and flu season and retained by them for reference. It can also be provided along with the “prescription” handout to patients with ARTIs who visit your office. In the latter situation, the patient leaves your office with concrete information and guidance for appropriate care of his or her viral respiratory illness—but without a prescription for antibiotics. As with the “prescription” handout, this brief guide may be duplicated or customized as needed.
To download the handouts: Click here.
REFERENCES
1. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000-2010. BMC Med. 2014;12:96.
2. CDC. Antibiotics: will they work when you really need them? www.cdc.gov/getsmart/healthcare/factsheets/antibiotics.html#MustAct. Accessed August 14, 2014.
3. Shehab N, Patel P, Srinivasan A, Budnitz D. Emergency department visits for antibiotic associated adverse events. Clin Infect Dis. 2008;47:735-743.
4. CDC. Adverse drug events from select medication classes. www.cdc.gov/MedicationSafety/program_focus_activities.html. Accessed August 4, 2014.
5. CDC. Get smart: know when antibiotics work. www.cdc.gov/getsmart/. Accessed August 14, 2014.
Drug store and supermarket shelves display aisle after aisle of OTC medications that alleviate the common symptoms of upper respiratory infections. Despite the easy availability of symptom relief, a significant number of people consult clinicians in primary care offices, emergency departments (EDs), and walk-in or convenient care clinics for help when they feel that they’re “coming down with something.” During these visits, many of these patients expect, and sometimes demand, antibiotics.
Antibiotics may be viewed by the patient as a quick fix, with the demand undoubtedly fueled by busy lifestyles, long work hours, and little time for patients to stay home while ill. Patients so inclined may “doctor shop” if their demands are not met; clinicians know better but may feel pressure to “satisfy the customer” and may rationalize that an antibiotic might prove helpful in a particular case.
Lee et al studied outpatient antibiotic prescribing in the United States for acute respiratory tract infections (ARTI), including acute nasopharyngitis, upper respiratory tract infection, bronchitis, influenza, pharyngitis, and sinusitis. In 2000, antibiotics were prescribed during outpatient visits for ARTI to 64% of patients; by 2010, that percentage had increased to 73%.1 Although antibiotics are neither effective nor appropriate for the treatment of ARTI, most of which are viral infections, they are commonly prescribed. Further, while 17% of ARTI prescriptions in 2000 were for broad-spectrum antibiotics, that percentage jumped to 46% in 2010.1,2
Patient insistence on antibiotics may stem either from little knowledge of or little regard for the health problems caused by unnecessary antibiotic use. For example, one study found that 19.3% of drug-related ED visits were related to systemic antibiotics; nearly 80% of those were for allergic reactions.3,4 With an estimated 50% of antibiotic prescriptions considered inappropriate, the overuse of antibiotics creates unnecessary personal health risks and health care expenditures. Further, a more serious consequence of this overuse is the growing public health problem of antibiotic resistance (see Figure 1).1,2
Clinicians are ideally positioned to address these issues by incorporating effective, proactive strategies into selected patient encounters to specifically explain appropriate versus inappropriate antibiotic use.
Continue for patient handouts >>
One approach is to merge accurate, powerful messages about antibiotics with helpful information about effective OTC products to both enlighten patients and offer them the symptomatic relief they seek. The CDC has taken the lead in this area with its “Get Smart: Know When Antibiotics Work” initiative, which includes a variety of materials for both health care providers and patients.5
Inspired by the unmet need for written communications that explain viral and bacterial illnesses in simple terms and the reasons why taking antibiotics for the former is a bad idea, we developed two handouts for adult patients who present to the clinician’s office with viral respiratory illnesses.
The first is entitled “Prescription for Recovery From Your Viral Respiratory Illness” [download PDF]. Intended to be duplicated and designed with primary care use in mind, it can be customized for specialty use as well.
This “prescription” handout addresses common complaints of fever, pain (eg, sore throat, body aches, headache), cough, congestion (chest, nose, sinuses), and sneezing/runny nose. Clinicians can check off the appropriate treatments for an individual patient, who can use it as a handy reference to purchase the recommended OTC products and/or for selecting products or preparing helpful remedies at home. Blank lines at the bottom provide space for you to write in your OTC preferences and allow you to customize patient instructions.
The second patient handout is entitled “Antibiotics: When You Need Them, When You Don’t, and What to Take When You Don’t” [download PDF]. This focused patient teaching tool offers an overview of
• Viral and bacterial respiratory illnesses and what the patient should do if he or she has one or the other (including when to see a clinician)
• Some helpful OTC and home treatments for symptoms of viral respiratory illnesses
• When antibiotics are indicated and when they’re not—and why
• The serious problems caused by unnecessary use of antibiotics.
This brief guide can be used as a general informational handout that can, for example, be given, mailed, or e-mailed to all adult patients at the start of cold and flu season and retained by them for reference. It can also be provided along with the “prescription” handout to patients with ARTIs who visit your office. In the latter situation, the patient leaves your office with concrete information and guidance for appropriate care of his or her viral respiratory illness—but without a prescription for antibiotics. As with the “prescription” handout, this brief guide may be duplicated or customized as needed.
To download the handouts: Click here.
REFERENCES
1. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000-2010. BMC Med. 2014;12:96.
2. CDC. Antibiotics: will they work when you really need them? www.cdc.gov/getsmart/healthcare/factsheets/antibiotics.html#MustAct. Accessed August 14, 2014.
3. Shehab N, Patel P, Srinivasan A, Budnitz D. Emergency department visits for antibiotic associated adverse events. Clin Infect Dis. 2008;47:735-743.
4. CDC. Adverse drug events from select medication classes. www.cdc.gov/MedicationSafety/program_focus_activities.html. Accessed August 4, 2014.
5. CDC. Get smart: know when antibiotics work. www.cdc.gov/getsmart/. Accessed August 14, 2014.
Drug store and supermarket shelves display aisle after aisle of OTC medications that alleviate the common symptoms of upper respiratory infections. Despite the easy availability of symptom relief, a significant number of people consult clinicians in primary care offices, emergency departments (EDs), and walk-in or convenient care clinics for help when they feel that they’re “coming down with something.” During these visits, many of these patients expect, and sometimes demand, antibiotics.
Antibiotics may be viewed by the patient as a quick fix, with the demand undoubtedly fueled by busy lifestyles, long work hours, and little time for patients to stay home while ill. Patients so inclined may “doctor shop” if their demands are not met; clinicians know better but may feel pressure to “satisfy the customer” and may rationalize that an antibiotic might prove helpful in a particular case.
Lee et al studied outpatient antibiotic prescribing in the United States for acute respiratory tract infections (ARTI), including acute nasopharyngitis, upper respiratory tract infection, bronchitis, influenza, pharyngitis, and sinusitis. In 2000, antibiotics were prescribed during outpatient visits for ARTI to 64% of patients; by 2010, that percentage had increased to 73%.1 Although antibiotics are neither effective nor appropriate for the treatment of ARTI, most of which are viral infections, they are commonly prescribed. Further, while 17% of ARTI prescriptions in 2000 were for broad-spectrum antibiotics, that percentage jumped to 46% in 2010.1,2
Patient insistence on antibiotics may stem either from little knowledge of or little regard for the health problems caused by unnecessary antibiotic use. For example, one study found that 19.3% of drug-related ED visits were related to systemic antibiotics; nearly 80% of those were for allergic reactions.3,4 With an estimated 50% of antibiotic prescriptions considered inappropriate, the overuse of antibiotics creates unnecessary personal health risks and health care expenditures. Further, a more serious consequence of this overuse is the growing public health problem of antibiotic resistance (see Figure 1).1,2
Clinicians are ideally positioned to address these issues by incorporating effective, proactive strategies into selected patient encounters to specifically explain appropriate versus inappropriate antibiotic use.
Continue for patient handouts >>
One approach is to merge accurate, powerful messages about antibiotics with helpful information about effective OTC products to both enlighten patients and offer them the symptomatic relief they seek. The CDC has taken the lead in this area with its “Get Smart: Know When Antibiotics Work” initiative, which includes a variety of materials for both health care providers and patients.5
Inspired by the unmet need for written communications that explain viral and bacterial illnesses in simple terms and the reasons why taking antibiotics for the former is a bad idea, we developed two handouts for adult patients who present to the clinician’s office with viral respiratory illnesses.
The first is entitled “Prescription for Recovery From Your Viral Respiratory Illness” [download PDF]. Intended to be duplicated and designed with primary care use in mind, it can be customized for specialty use as well.
This “prescription” handout addresses common complaints of fever, pain (eg, sore throat, body aches, headache), cough, congestion (chest, nose, sinuses), and sneezing/runny nose. Clinicians can check off the appropriate treatments for an individual patient, who can use it as a handy reference to purchase the recommended OTC products and/or for selecting products or preparing helpful remedies at home. Blank lines at the bottom provide space for you to write in your OTC preferences and allow you to customize patient instructions.
The second patient handout is entitled “Antibiotics: When You Need Them, When You Don’t, and What to Take When You Don’t” [download PDF]. This focused patient teaching tool offers an overview of
• Viral and bacterial respiratory illnesses and what the patient should do if he or she has one or the other (including when to see a clinician)
• Some helpful OTC and home treatments for symptoms of viral respiratory illnesses
• When antibiotics are indicated and when they’re not—and why
• The serious problems caused by unnecessary use of antibiotics.
This brief guide can be used as a general informational handout that can, for example, be given, mailed, or e-mailed to all adult patients at the start of cold and flu season and retained by them for reference. It can also be provided along with the “prescription” handout to patients with ARTIs who visit your office. In the latter situation, the patient leaves your office with concrete information and guidance for appropriate care of his or her viral respiratory illness—but without a prescription for antibiotics. As with the “prescription” handout, this brief guide may be duplicated or customized as needed.
To download the handouts: Click here.
REFERENCES
1. Lee GC, Reveles KR, Attridge RT, et al. Outpatient antibiotic prescribing in the United States: 2000-2010. BMC Med. 2014;12:96.
2. CDC. Antibiotics: will they work when you really need them? www.cdc.gov/getsmart/healthcare/factsheets/antibiotics.html#MustAct. Accessed August 14, 2014.
3. Shehab N, Patel P, Srinivasan A, Budnitz D. Emergency department visits for antibiotic associated adverse events. Clin Infect Dis. 2008;47:735-743.
4. CDC. Adverse drug events from select medication classes. www.cdc.gov/MedicationSafety/program_focus_activities.html. Accessed August 4, 2014.
5. CDC. Get smart: know when antibiotics work. www.cdc.gov/getsmart/. Accessed August 14, 2014.
Failure to spot CHF leads to heart transplant
Failure to spot CHF leads to heart transplant
A 49-YEAR-OLD MAN SOUGHT TREATMENT AT AN URGENT CARE FACILITY after having shortness of breath every morning for 2 weeks. His heart rate was 119 beats/min, his blood pressure was 170/101 mm Hg, and he did not have chest pain. An electrocardiogram (EKG) was abnormal and chest x-ray showed fluid in the lung. The patient was diagnosed with pneumonia, prescribed antibiotics, and told to follow up with his physician. A follow-up chest x-ray 2 weeks later showed an enlarged heart and more fluid in the lung. A computed tomography scan indicated congestive heart failure and an EKG showed signs of a heart attack. The patient underwent a heart transplant and requires immunosuppressants.
PLAINTIFF'S CLAIM If the physician at the urgent care facility had noticed the patient’s enlarged heart, there would have been less heart damage, and the patient might have required a bypass, rather than a transplant.
THE DEFENSE No information about the defense is available.
VERDICT $1 million New Jersey verdict.
COMMENT When evaluating shortness of breath, always think lungs and heart until you have a definite diagnosis. Remember that neurological disease can present with shortness of breath, too. Consider amyotrophic lateral sclerosis, Guillain-Barré syndrome, and myasthenia gravis.
Infant suffers brain injury after delayed lab results
PARENTS BROUGHT THEIR 2-WEEK-OLD DAUGHTER TO THE EMERGENCY DEPARTMENT (ED) after she had missed several feedings and was short of breath. The ED physician ordered blood tests, but discharged the patient before receiving the results and told the parents to follow up with the infant’s pediatrician. Blood work subsequently revealed that the child had a Group B streptococcus infection, but by the time these results were communicated to the parents and treatment had begun, the infant had developed meningitis. She suffered brain injury, and was diagnosed with cerebral palsy.
PLAINTIFF'S CLAIM There was a delay in the diagnosis and treatment of the infant. Blood test results showing a bacterial infection were available the morning after discharge, but instead of notifying the parents, an additional blood culture was ordered to determine the type of bacteria present. The parents were then contacted 6 hours after the bacteria was identified as Group B streptococcus.
THE DEFENSE The defendants denied any negligence, although a nurse who cared for the infant claimed she had expressed concerns about the decision to discharge the patient.
VERDICT $7.15 million Maryland verdict.
COMMENT In newborns, the differential diagnosis for shortness of breath widens to include infection. In this case, I suspect the problem was a lack of tight follow-up, which can lead to bad outcomes—especially in newborns.
Failure to spot CHF leads to heart transplant
A 49-YEAR-OLD MAN SOUGHT TREATMENT AT AN URGENT CARE FACILITY after having shortness of breath every morning for 2 weeks. His heart rate was 119 beats/min, his blood pressure was 170/101 mm Hg, and he did not have chest pain. An electrocardiogram (EKG) was abnormal and chest x-ray showed fluid in the lung. The patient was diagnosed with pneumonia, prescribed antibiotics, and told to follow up with his physician. A follow-up chest x-ray 2 weeks later showed an enlarged heart and more fluid in the lung. A computed tomography scan indicated congestive heart failure and an EKG showed signs of a heart attack. The patient underwent a heart transplant and requires immunosuppressants.
PLAINTIFF'S CLAIM If the physician at the urgent care facility had noticed the patient’s enlarged heart, there would have been less heart damage, and the patient might have required a bypass, rather than a transplant.
THE DEFENSE No information about the defense is available.
VERDICT $1 million New Jersey verdict.
COMMENT When evaluating shortness of breath, always think lungs and heart until you have a definite diagnosis. Remember that neurological disease can present with shortness of breath, too. Consider amyotrophic lateral sclerosis, Guillain-Barré syndrome, and myasthenia gravis.
Infant suffers brain injury after delayed lab results
PARENTS BROUGHT THEIR 2-WEEK-OLD DAUGHTER TO THE EMERGENCY DEPARTMENT (ED) after she had missed several feedings and was short of breath. The ED physician ordered blood tests, but discharged the patient before receiving the results and told the parents to follow up with the infant’s pediatrician. Blood work subsequently revealed that the child had a Group B streptococcus infection, but by the time these results were communicated to the parents and treatment had begun, the infant had developed meningitis. She suffered brain injury, and was diagnosed with cerebral palsy.
PLAINTIFF'S CLAIM There was a delay in the diagnosis and treatment of the infant. Blood test results showing a bacterial infection were available the morning after discharge, but instead of notifying the parents, an additional blood culture was ordered to determine the type of bacteria present. The parents were then contacted 6 hours after the bacteria was identified as Group B streptococcus.
THE DEFENSE The defendants denied any negligence, although a nurse who cared for the infant claimed she had expressed concerns about the decision to discharge the patient.
VERDICT $7.15 million Maryland verdict.
COMMENT In newborns, the differential diagnosis for shortness of breath widens to include infection. In this case, I suspect the problem was a lack of tight follow-up, which can lead to bad outcomes—especially in newborns.
Failure to spot CHF leads to heart transplant
A 49-YEAR-OLD MAN SOUGHT TREATMENT AT AN URGENT CARE FACILITY after having shortness of breath every morning for 2 weeks. His heart rate was 119 beats/min, his blood pressure was 170/101 mm Hg, and he did not have chest pain. An electrocardiogram (EKG) was abnormal and chest x-ray showed fluid in the lung. The patient was diagnosed with pneumonia, prescribed antibiotics, and told to follow up with his physician. A follow-up chest x-ray 2 weeks later showed an enlarged heart and more fluid in the lung. A computed tomography scan indicated congestive heart failure and an EKG showed signs of a heart attack. The patient underwent a heart transplant and requires immunosuppressants.
PLAINTIFF'S CLAIM If the physician at the urgent care facility had noticed the patient’s enlarged heart, there would have been less heart damage, and the patient might have required a bypass, rather than a transplant.
THE DEFENSE No information about the defense is available.
VERDICT $1 million New Jersey verdict.
COMMENT When evaluating shortness of breath, always think lungs and heart until you have a definite diagnosis. Remember that neurological disease can present with shortness of breath, too. Consider amyotrophic lateral sclerosis, Guillain-Barré syndrome, and myasthenia gravis.
Infant suffers brain injury after delayed lab results
PARENTS BROUGHT THEIR 2-WEEK-OLD DAUGHTER TO THE EMERGENCY DEPARTMENT (ED) after she had missed several feedings and was short of breath. The ED physician ordered blood tests, but discharged the patient before receiving the results and told the parents to follow up with the infant’s pediatrician. Blood work subsequently revealed that the child had a Group B streptococcus infection, but by the time these results were communicated to the parents and treatment had begun, the infant had developed meningitis. She suffered brain injury, and was diagnosed with cerebral palsy.
PLAINTIFF'S CLAIM There was a delay in the diagnosis and treatment of the infant. Blood test results showing a bacterial infection were available the morning after discharge, but instead of notifying the parents, an additional blood culture was ordered to determine the type of bacteria present. The parents were then contacted 6 hours after the bacteria was identified as Group B streptococcus.
THE DEFENSE The defendants denied any negligence, although a nurse who cared for the infant claimed she had expressed concerns about the decision to discharge the patient.
VERDICT $7.15 million Maryland verdict.
COMMENT In newborns, the differential diagnosis for shortness of breath widens to include infection. In this case, I suspect the problem was a lack of tight follow-up, which can lead to bad outcomes—especially in newborns.
Suctioning neonates at birth: Time to change our approach
Stop suctioning neonates at birth. There is no benefit to this practice, and it can cause bradycardia and apnea. Instead, wipe the baby’s mouth and nose with a towel to clear excess secretions and stimulate respiration.1
Strength of recommendation
B: Based on a single randomized equivalency trial.
Kelleher J, Bhat, R, Salas AA, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet. 2013;382:326-330.
Illustrative case
A healthy neonate is born through clear amniotic fluid with no meconium. She is vigorous and has no major congenital anomalies. Does she need oronasopharyngeal suctioning?
No, she does not need suctioning. Although it is still standard practice to perform oronasopharyngeal suctioning with a bulb syringe immediately after delivery, multiple studies have found no benefit to routine suctioning.2-7 Guidelines from the Neonatal Resuscitation Program (NRP) and other organizations recommend against the practice, even for neonates born through meconium-stained amniotic fluid.8,9 Suctioning is done because some clinicians believe it reduces the risk of aspiration, especially if there is meconium, and to stimulate breathing, but the evidence suggests that suctioning can stimulate the vagus nerve, which can lead to bradycardia.2 Studies that compared babies who did and didn’t receive suctioning found that those who received it had lower Apgar scores and oxygen saturation levels.2-4
Wiping the neonate’s mouth and nose with a towel is an alternative to suctioning, but until now no trials have compared the outcomes of these 2 methods. Kelleher et al1 conducted an equivalency trial to determine if wiping the mouth and nose is as effective as oronasopharyngeal suctioning.
STUDY SUMMARY: No difference in breathing after wiping or suctioning
Of 506 neonates randomized, 15 were excluded because they were not vigorous and had meconium-stained fluid, and 3 were excluded when their parents withdrew consent. Baseline characteristics for the 2 groups—including maternal age, presence of chronic medical conditions, and body mass index; vaginal vs cesarean delivery; umbilical artery pH; and neonatal sex, ethnic origin, and birth weight—were similar.
In the first 24 hours after birth, the average respiratory rate in the wiping group was 51 breaths/min (standard deviation [SD] ± 8) vs 50 breaths/min (SD ± 6) in the suctioning group. There was no difference in respiratory rates between the 2 groups at 1, 8, or 16 hours after birth. There was also no difference between the 2 groups in Apgar scores or need for advanced resuscitation. More neonates in the wiping group than in the suctioning group were admitted to the neonatal intensive care unit (45 of 246 [18%] vs 30 of 242 [12%]; P=.07), but the study was not powered to assess this outcome.
WHAT'S NEW: Wiping is as effective as suctioning, but there are no adverse effects
This study gives us evidence that wiping the face, mouth, and nose is equivalent to suctioning newborns at delivery, and it supports the NRP recommendation against routine suctioning in vigorous neonates born at term. Wiping avoids the potential adverse effects on the respiratory mucosa, bradycardia, and lower Apgar scores associated with suctioning via bulb syringes.
CAVEATS: Wiping is not best if a neonate’s airway is obstructed
This study looked only at neonates born after 35 weeks’ gestation who did not have meconium-stained amniotic fluid or congenital abnormalities. Also, NRP guidelines do recommend clearing the airways with a bulb syringe or suction catheter if airway obstruction is evident or positive-pressure ventilation is required.8
Another caveat ... In this study,1 there were 98 treatment crossovers: 64 of the 246 neonates in the wiping group received suctioning, and 34 of the 242 neonates in the suctioning group received wiping. However, this was not likely to change the study’s overall conclusion because a per-treatment analysis also found that wiping and suctioning were equivalent.
CHALLENGES TO IMPLEMENTATION: “We’ve always done it this way”
Practice patterns in a delivery room can be difficult to change. As we work on improving our delivery room environment and changing ingrained habits, the evidence from this study should help support the use of wiping in place of suctioning. The transition from suctioning to wiping also would be facilitated by having easily accessible towels designated for wiping.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kelleher J, Bhat R, Salas AA, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet. 2013;382:326-330.
2. Gungor S, Kurt E, Teksoz E, et al. Oronasopharyngeal suction versus no suction in normal and term infants delivered by elective cesarean section: a prospective randomized controlled trial. Gynecol Obstet Invest. 2006;61:9-14.
3. Gungor S, Teksoz E, Ceyhan T, et al. Oronasopharyngeal suction versus no suction in normal, term and vaginally born infants: a prospective randomized controlled trial. Aust N Z J Obstet Gynaecol. 2005;45:453-456.
4. Carrasco M, Martell M, Estol PC. Oronasopharyngeal suction at birth: effects on arterial oxygen saturation. J Pediatr. 1997;130:832-834.
5. Estol PC, Piriz H, Basalo S, et al. Oro-naso-pharyngeal suction at birth: effects on respiratory adaptation of normal term vaginally born infants. J Perinat Med. 1992;20:297-305.
6. Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics. 2000;105(1 pt 1):1-7.
7. Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomized controlled trial. Lancet. 2004;364:597-602.
8. Kattwinkel J, Perlman JM, Aziz K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 suppl 3):S909-S919.
9. Perlman JM, Wyllie J, Kattwinkel J, et al; Neonatal Resuscitation Chapter Collaborators. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126:e1319-1344.
Stop suctioning neonates at birth. There is no benefit to this practice, and it can cause bradycardia and apnea. Instead, wipe the baby’s mouth and nose with a towel to clear excess secretions and stimulate respiration.1
Strength of recommendation
B: Based on a single randomized equivalency trial.
Kelleher J, Bhat, R, Salas AA, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet. 2013;382:326-330.
Illustrative case
A healthy neonate is born through clear amniotic fluid with no meconium. She is vigorous and has no major congenital anomalies. Does she need oronasopharyngeal suctioning?
No, she does not need suctioning. Although it is still standard practice to perform oronasopharyngeal suctioning with a bulb syringe immediately after delivery, multiple studies have found no benefit to routine suctioning.2-7 Guidelines from the Neonatal Resuscitation Program (NRP) and other organizations recommend against the practice, even for neonates born through meconium-stained amniotic fluid.8,9 Suctioning is done because some clinicians believe it reduces the risk of aspiration, especially if there is meconium, and to stimulate breathing, but the evidence suggests that suctioning can stimulate the vagus nerve, which can lead to bradycardia.2 Studies that compared babies who did and didn’t receive suctioning found that those who received it had lower Apgar scores and oxygen saturation levels.2-4
Wiping the neonate’s mouth and nose with a towel is an alternative to suctioning, but until now no trials have compared the outcomes of these 2 methods. Kelleher et al1 conducted an equivalency trial to determine if wiping the mouth and nose is as effective as oronasopharyngeal suctioning.
STUDY SUMMARY: No difference in breathing after wiping or suctioning
Of 506 neonates randomized, 15 were excluded because they were not vigorous and had meconium-stained fluid, and 3 were excluded when their parents withdrew consent. Baseline characteristics for the 2 groups—including maternal age, presence of chronic medical conditions, and body mass index; vaginal vs cesarean delivery; umbilical artery pH; and neonatal sex, ethnic origin, and birth weight—were similar.
In the first 24 hours after birth, the average respiratory rate in the wiping group was 51 breaths/min (standard deviation [SD] ± 8) vs 50 breaths/min (SD ± 6) in the suctioning group. There was no difference in respiratory rates between the 2 groups at 1, 8, or 16 hours after birth. There was also no difference between the 2 groups in Apgar scores or need for advanced resuscitation. More neonates in the wiping group than in the suctioning group were admitted to the neonatal intensive care unit (45 of 246 [18%] vs 30 of 242 [12%]; P=.07), but the study was not powered to assess this outcome.
WHAT'S NEW: Wiping is as effective as suctioning, but there are no adverse effects
This study gives us evidence that wiping the face, mouth, and nose is equivalent to suctioning newborns at delivery, and it supports the NRP recommendation against routine suctioning in vigorous neonates born at term. Wiping avoids the potential adverse effects on the respiratory mucosa, bradycardia, and lower Apgar scores associated with suctioning via bulb syringes.
CAVEATS: Wiping is not best if a neonate’s airway is obstructed
This study looked only at neonates born after 35 weeks’ gestation who did not have meconium-stained amniotic fluid or congenital abnormalities. Also, NRP guidelines do recommend clearing the airways with a bulb syringe or suction catheter if airway obstruction is evident or positive-pressure ventilation is required.8
Another caveat ... In this study,1 there were 98 treatment crossovers: 64 of the 246 neonates in the wiping group received suctioning, and 34 of the 242 neonates in the suctioning group received wiping. However, this was not likely to change the study’s overall conclusion because a per-treatment analysis also found that wiping and suctioning were equivalent.
CHALLENGES TO IMPLEMENTATION: “We’ve always done it this way”
Practice patterns in a delivery room can be difficult to change. As we work on improving our delivery room environment and changing ingrained habits, the evidence from this study should help support the use of wiping in place of suctioning. The transition from suctioning to wiping also would be facilitated by having easily accessible towels designated for wiping.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Stop suctioning neonates at birth. There is no benefit to this practice, and it can cause bradycardia and apnea. Instead, wipe the baby’s mouth and nose with a towel to clear excess secretions and stimulate respiration.1
Strength of recommendation
B: Based on a single randomized equivalency trial.
Kelleher J, Bhat, R, Salas AA, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet. 2013;382:326-330.
Illustrative case
A healthy neonate is born through clear amniotic fluid with no meconium. She is vigorous and has no major congenital anomalies. Does she need oronasopharyngeal suctioning?
No, she does not need suctioning. Although it is still standard practice to perform oronasopharyngeal suctioning with a bulb syringe immediately after delivery, multiple studies have found no benefit to routine suctioning.2-7 Guidelines from the Neonatal Resuscitation Program (NRP) and other organizations recommend against the practice, even for neonates born through meconium-stained amniotic fluid.8,9 Suctioning is done because some clinicians believe it reduces the risk of aspiration, especially if there is meconium, and to stimulate breathing, but the evidence suggests that suctioning can stimulate the vagus nerve, which can lead to bradycardia.2 Studies that compared babies who did and didn’t receive suctioning found that those who received it had lower Apgar scores and oxygen saturation levels.2-4
Wiping the neonate’s mouth and nose with a towel is an alternative to suctioning, but until now no trials have compared the outcomes of these 2 methods. Kelleher et al1 conducted an equivalency trial to determine if wiping the mouth and nose is as effective as oronasopharyngeal suctioning.
STUDY SUMMARY: No difference in breathing after wiping or suctioning
Of 506 neonates randomized, 15 were excluded because they were not vigorous and had meconium-stained fluid, and 3 were excluded when their parents withdrew consent. Baseline characteristics for the 2 groups—including maternal age, presence of chronic medical conditions, and body mass index; vaginal vs cesarean delivery; umbilical artery pH; and neonatal sex, ethnic origin, and birth weight—were similar.
In the first 24 hours after birth, the average respiratory rate in the wiping group was 51 breaths/min (standard deviation [SD] ± 8) vs 50 breaths/min (SD ± 6) in the suctioning group. There was no difference in respiratory rates between the 2 groups at 1, 8, or 16 hours after birth. There was also no difference between the 2 groups in Apgar scores or need for advanced resuscitation. More neonates in the wiping group than in the suctioning group were admitted to the neonatal intensive care unit (45 of 246 [18%] vs 30 of 242 [12%]; P=.07), but the study was not powered to assess this outcome.
WHAT'S NEW: Wiping is as effective as suctioning, but there are no adverse effects
This study gives us evidence that wiping the face, mouth, and nose is equivalent to suctioning newborns at delivery, and it supports the NRP recommendation against routine suctioning in vigorous neonates born at term. Wiping avoids the potential adverse effects on the respiratory mucosa, bradycardia, and lower Apgar scores associated with suctioning via bulb syringes.
CAVEATS: Wiping is not best if a neonate’s airway is obstructed
This study looked only at neonates born after 35 weeks’ gestation who did not have meconium-stained amniotic fluid or congenital abnormalities. Also, NRP guidelines do recommend clearing the airways with a bulb syringe or suction catheter if airway obstruction is evident or positive-pressure ventilation is required.8
Another caveat ... In this study,1 there were 98 treatment crossovers: 64 of the 246 neonates in the wiping group received suctioning, and 34 of the 242 neonates in the suctioning group received wiping. However, this was not likely to change the study’s overall conclusion because a per-treatment analysis also found that wiping and suctioning were equivalent.
CHALLENGES TO IMPLEMENTATION: “We’ve always done it this way”
Practice patterns in a delivery room can be difficult to change. As we work on improving our delivery room environment and changing ingrained habits, the evidence from this study should help support the use of wiping in place of suctioning. The transition from suctioning to wiping also would be facilitated by having easily accessible towels designated for wiping.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kelleher J, Bhat R, Salas AA, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet. 2013;382:326-330.
2. Gungor S, Kurt E, Teksoz E, et al. Oronasopharyngeal suction versus no suction in normal and term infants delivered by elective cesarean section: a prospective randomized controlled trial. Gynecol Obstet Invest. 2006;61:9-14.
3. Gungor S, Teksoz E, Ceyhan T, et al. Oronasopharyngeal suction versus no suction in normal, term and vaginally born infants: a prospective randomized controlled trial. Aust N Z J Obstet Gynaecol. 2005;45:453-456.
4. Carrasco M, Martell M, Estol PC. Oronasopharyngeal suction at birth: effects on arterial oxygen saturation. J Pediatr. 1997;130:832-834.
5. Estol PC, Piriz H, Basalo S, et al. Oro-naso-pharyngeal suction at birth: effects on respiratory adaptation of normal term vaginally born infants. J Perinat Med. 1992;20:297-305.
6. Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics. 2000;105(1 pt 1):1-7.
7. Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomized controlled trial. Lancet. 2004;364:597-602.
8. Kattwinkel J, Perlman JM, Aziz K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 suppl 3):S909-S919.
9. Perlman JM, Wyllie J, Kattwinkel J, et al; Neonatal Resuscitation Chapter Collaborators. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126:e1319-1344.
1. Kelleher J, Bhat R, Salas AA, et al. Oronasopharyngeal suction versus wiping of the mouth and nose at birth: a randomised equivalency trial. Lancet. 2013;382:326-330.
2. Gungor S, Kurt E, Teksoz E, et al. Oronasopharyngeal suction versus no suction in normal and term infants delivered by elective cesarean section: a prospective randomized controlled trial. Gynecol Obstet Invest. 2006;61:9-14.
3. Gungor S, Teksoz E, Ceyhan T, et al. Oronasopharyngeal suction versus no suction in normal, term and vaginally born infants: a prospective randomized controlled trial. Aust N Z J Obstet Gynaecol. 2005;45:453-456.
4. Carrasco M, Martell M, Estol PC. Oronasopharyngeal suction at birth: effects on arterial oxygen saturation. J Pediatr. 1997;130:832-834.
5. Estol PC, Piriz H, Basalo S, et al. Oro-naso-pharyngeal suction at birth: effects on respiratory adaptation of normal term vaginally born infants. J Perinat Med. 1992;20:297-305.
6. Wiswell TE, Gannon CM, Jacob J, et al. Delivery room management of the apparently vigorous meconium-stained neonate: results of the multicenter, international collaborative trial. Pediatrics. 2000;105(1 pt 1):1-7.
7. Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomized controlled trial. Lancet. 2004;364:597-602.
8. Kattwinkel J, Perlman JM, Aziz K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 suppl 3):S909-S919.
9. Perlman JM, Wyllie J, Kattwinkel J, et al; Neonatal Resuscitation Chapter Collaborators. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126:e1319-1344.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Think Twice About Nebulizers for Asthma Attacks
PRACTICE CHANGER
Stop ordering nebulizers to deliver β-agonists to patients older than 2 who have mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
STRENGTH OF RECOMMENDATION
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs). 1
ILLUSTRATIVE CASE
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35 breaths/min, and an O2 saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled β-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a β-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable or preferable to nebulizers for β-agonist administration in children and adults.6,7 However, based on our experience, clinicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Clinicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added four new studies to those included in their earlier Cochrane meta-analysis and evaluated what, if any, effect these studies had on our understanding of nebulizers versus MDIs with spacers.
STUDY SUMMARY
Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering β-agonists during acute, non–life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1,897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The four new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with β-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving β-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR] = 0.94) and children (RR = 0.71). Duration of hospital stay did not differ between the two delivery methods in adults (mean difference [MD] = –0.60 d) and children (MD = 0.33 d).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD = –33.48 min). There was no difference observed in adults (MD = 1.75 min). The rate of tremor was lower in children using spacers (RR = 0.64) and was similar in adults (RR = 1.12). The rise in pulse rate was lower in children using spacers
(MD = –5.41% change from baseline) and was similar in adults (MD = –1.23%).
On the next page: What's new and challenges to implementation >>
WHAT’S NEW
Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included four new studies, should finally dispel the myth that nebulizers deliver β-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of adverse effects, including tremor and elevated pulse rate.
CAVEATS
Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION
Old habits are hard to break
Clinicians may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
REFERENCES
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9: CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. www.hcup-us.ahrq.gov/reports/stat briefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Accessed June 16, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Accessed June 16, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2): CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction: a meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. National Heart, Lung, and Blood Institute Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma/asth gdln.htm. Accessed June 16, 2014.
7. British Thoracic Society. British guideline of the management of asthma: a national clinical guideline. www.brit-thoracic.org.uk/document-library/clinical-information/asth ma/btssign-guideline-on-the-management-of-asthma/. Accessed June 16, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed June 16, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):321-322, 346.
PRACTICE CHANGER
Stop ordering nebulizers to deliver β-agonists to patients older than 2 who have mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
STRENGTH OF RECOMMENDATION
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs). 1
ILLUSTRATIVE CASE
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35 breaths/min, and an O2 saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled β-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a β-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable or preferable to nebulizers for β-agonist administration in children and adults.6,7 However, based on our experience, clinicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Clinicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added four new studies to those included in their earlier Cochrane meta-analysis and evaluated what, if any, effect these studies had on our understanding of nebulizers versus MDIs with spacers.
STUDY SUMMARY
Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering β-agonists during acute, non–life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1,897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The four new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with β-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving β-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR] = 0.94) and children (RR = 0.71). Duration of hospital stay did not differ between the two delivery methods in adults (mean difference [MD] = –0.60 d) and children (MD = 0.33 d).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD = –33.48 min). There was no difference observed in adults (MD = 1.75 min). The rate of tremor was lower in children using spacers (RR = 0.64) and was similar in adults (RR = 1.12). The rise in pulse rate was lower in children using spacers
(MD = –5.41% change from baseline) and was similar in adults (MD = –1.23%).
On the next page: What's new and challenges to implementation >>
WHAT’S NEW
Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included four new studies, should finally dispel the myth that nebulizers deliver β-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of adverse effects, including tremor and elevated pulse rate.
CAVEATS
Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION
Old habits are hard to break
Clinicians may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
REFERENCES
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9: CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. www.hcup-us.ahrq.gov/reports/stat briefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Accessed June 16, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Accessed June 16, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2): CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction: a meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. National Heart, Lung, and Blood Institute Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma/asth gdln.htm. Accessed June 16, 2014.
7. British Thoracic Society. British guideline of the management of asthma: a national clinical guideline. www.brit-thoracic.org.uk/document-library/clinical-information/asth ma/btssign-guideline-on-the-management-of-asthma/. Accessed June 16, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed June 16, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):321-322, 346.
PRACTICE CHANGER
Stop ordering nebulizers to deliver β-agonists to patients older than 2 who have mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
STRENGTH OF RECOMMENDATION
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs). 1
ILLUSTRATIVE CASE
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35 breaths/min, and an O2 saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled β-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a β-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable or preferable to nebulizers for β-agonist administration in children and adults.6,7 However, based on our experience, clinicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Clinicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added four new studies to those included in their earlier Cochrane meta-analysis and evaluated what, if any, effect these studies had on our understanding of nebulizers versus MDIs with spacers.
STUDY SUMMARY
Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering β-agonists during acute, non–life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1,897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The four new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with β-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving β-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR] = 0.94) and children (RR = 0.71). Duration of hospital stay did not differ between the two delivery methods in adults (mean difference [MD] = –0.60 d) and children (MD = 0.33 d).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD = –33.48 min). There was no difference observed in adults (MD = 1.75 min). The rate of tremor was lower in children using spacers (RR = 0.64) and was similar in adults (RR = 1.12). The rise in pulse rate was lower in children using spacers
(MD = –5.41% change from baseline) and was similar in adults (MD = –1.23%).
On the next page: What's new and challenges to implementation >>
WHAT’S NEW
Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included four new studies, should finally dispel the myth that nebulizers deliver β-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of adverse effects, including tremor and elevated pulse rate.
CAVEATS
Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION
Old habits are hard to break
Clinicians may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
REFERENCES
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9: CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. www.hcup-us.ahrq.gov/reports/stat briefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Accessed June 16, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Accessed June 16, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2): CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction: a meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. National Heart, Lung, and Blood Institute Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma/asth gdln.htm. Accessed June 16, 2014.
7. British Thoracic Society. British guideline of the management of asthma: a national clinical guideline. www.brit-thoracic.org.uk/document-library/clinical-information/asth ma/btssign-guideline-on-the-management-of-asthma/. Accessed June 16, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed June 16, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(6):321-322, 346.
Think twice about nebulizers for asthma attacks
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.