User login
Obstructive sleep apnea: A diagnostic and treatment guide
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
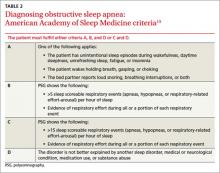
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
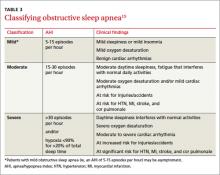
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
CASE 1 Mr. M, age 59, has diabetes, hypertension, hyperlipidemia, and obesity, with a body mass index (BMI) of 37 kg/m2. His hyperlipidemia is well controlled with atorvastatin. He also takes hydrochlorothiazide, lisinopril, metformin, and glyburide, but neither his hypertension nor his glucose levels are well controlled. Mr. M does not exercise, and acknowledges eating a high-calorie diet.
Mr. M reports that his wife has begun complaining about his loud snoring, and that he’s been falling asleep at work. He says he has 2 to 3 alcoholic drinks per week, and doesn’t smoke or take any other drugs.
CASE 2 Ms. C, age 35, is seeking treatment for early morning headaches that began several months ago. She describes a constant dull pain over the frontal area that occurs almost every day and typically resolves in 2 to 3 hours. The pain is not affected by postural changes, she says.
She also reports fatigue, daytime sleepiness, and anxiety. Ms. C has a normal BMI (24 kg/m2), takes no medication, and has no prior history of migraine or tension headaches. She denies any alcohol or recreational drug use.
If Mr. M and Ms. C were your patients, what would your next step be?
Obstructive sleep apnea (OSA) is characterized by repeated cycles of partial airway obstruction, hypoxemia, sympathetic discharge, and arousal to restore ventilation. In addition to fragmented sleep and daytime sleepiness, this common sleep disorder is associated with a decreased functional capacity1-3 and an increased risk for accidents, arrhythmias, myocardial infarction (MI), stroke, and death.4-6 In the Wisconsin Sleep Cohort, an 18-year follow-up study, the estimated hazard ratio for patients with severe OSA was 3.0 for all-cause mortality (95% CI; P-trend <.008) and 5.2 for cardiovascular death (95% CI; P=.003).7
The prevalence of OSA in the United States is 3% to 7% for males and 2% to 5% for females, with higher rates among those older than 65 years. But these figures apply only to those with an OSA diagnosis. An additional 5% of the general population is believed to have undiagnosed OSA.4,8
Arriving at an OSA diagnosis may not be difficult with a patient like Mr. M, who is obese and has classic symptoms. But it is important to consider OSA in patients who, like Ms. C, are not overweight and whose chief complaint appears to be unrelated to sleep.
This review—of risk factors, common (and uncommon) symptoms, diagnostic criteria, and treatment for OSA—highlights key indicators and optimal therapies.
The OSA profile: Risk factors and presenting symptoms
Obesity is perhaps the best-known risk factor for OSA: About 70% of patients with OSA are obese, and 40% of obese individuals have OSA. Approximately 60% of those with OSA have metabolic syndrome.9-12 But it is important to remember that individuals with normal or even low BMI may develop OSA, as well.
Other possible contributing factors include alcohol use, smoking, nasal congestion, menopause, polycystic ovary disease, and a genetic predisposition. And OSA may cause or worsen a wide range of conditions, including hypertension, congestive heart failure, atrial fibrillation, stroke, and nocturnal arrhythmias.8,13
Symptoms may be classic—or not
A bed partner’s complaint about a patient’s snoring, typically followed by arousal and gasping for air, is often the first indication of OSA. Loud snoring and daytime sleepiness are the most common symptoms. But not all patients present with these classic symptoms. Some may complain of irritability, difficulty concentrating, daily headaches, and nocturia.
Because the symptoms of OSA are not highly specific, a wide differential diagnosis must be considered, including numerous causes of excessive daytime sleepiness and conditions, such as panic attacks, pulmonary disease, and gastroesophageal reflux disease, which can interfere with sleep (TABLE 1).8,13
What to include in the medical history and work-up
When you suspect OSA or another sleep disorder, the medical history should include a complete review of systems. The social history needs to include information regarding alcohol intake, use of sedatives, and recreational drugs. If possible, interview the patient’s bed partner, as well.
Examine upper airway anatomy. In performing a physical exam, pay special attention to blood pressure, BMI, and upper airway anatomy. Assess for conditions that can cause obstruction, such as nasal congestion, septal deviation, retrognathia (malocclusion of the mouth due to an abnormal posterior position of the maxilla or mandible), macroglossia (an unusually large tongue), dental malocclusion, enlarged lymphoid tissue and tonsils, large uvula, low hanging soft palate, and a large neck circumference (>40 cm for females and >43 cm for males).13
Order blood tests. Routine blood tests do not support an OSA diagnosis, but they help rule out or identify other conditions associated or mimicking OSA, such as anemia, hypothyroidism, diabetes, liver disease, and kidney disease. Suggested labs include a comprehensive metabolic profile, complete blood count, and thyroid-stimulating hormone test.
Sleep studies are needed for a definitive diagnosis
Conducted overnight in a sleep lab, polysomnography (PSG) uses respiratory effort, respiratory air flow, and peripheral oximetry to identify and quantify episodes of apnea and hypopnea (reported as the apnea-hypopnea index, or AHI). PSG also records brain electrical activity (electroencephalogram), heart rhythm (electrocardiogram), eye movement (electro-oculogram), and muscle activation (electromyogram)—studies used to identify other sleep disorders, such as restless leg syndrome, narcolepsy, parasomnias, and disturbances in rapid-eye movement
(REM) sleep.
Home monitoring. For patients who are unable or unwilling to undergo an overnight sleep study, home portable monitoring is a less expensive alternative. The monitor—a small wireless device—provides data for calculating the AHI and the presence and degree of oxygen desaturation.14 Unlike PSG, which can identify the amount of pressure needed for continuous positive airway pressure (CPAP) therapy, findings from a portable monitor are not sufficient to rule out other sleep disorders to determine whether CPAP is required.15
Evaluating daytime sleepiness. Several tools have been used to evaluate daytime sleepiness. The Epworth Sleepiness Scale (ESS), a quick 8-item screening questionnaire, determines the average person’s level of sleepiness during the day. It ranges from 0 to 24 points, with 10 being normal. Although the ESS has been used extensively in OSA research, recent studies found that it has a low sensitivity (54%) and specificity (57%) for scores >10 and does not correlate well with hypopnea and apnea measurements.16,17
Two additional tools, the Berlin and STOP questionnaires, can also be used to screen for OSA. Both questionnaires have about a 50% positive predictive value and a 70% negative predictive value.18,19
Diagnosing and classifying OSA
Diagnostic criteria developed by the American Academy of Sleep Medicine (AASM) are based on reported and observed symptoms and PSG recordings of hypopnea and apneic episodes. Of the 4 criteria (A through D), patients must meet either A, B, and D or C and D (TABLE 2).15
The AASM further classifies OSA as mild, moderate, or severe (TABLE 3)15 based on the AHI as well as on clinical findings, including oxygen desaturation and arrhythmias. Patients with severe OSA have excessive daytime sleepiness (EDS) that interferes with their normal activities, 15 as well as severe oxygen desaturation, moderate to severe cardiac arrhythmias, and significant risk for hypertension, MI, stroke, and cor pulmonale.
CASE 1 An examination of Mr. M’s upper airway anatomy reveals a neck circumference of 44 cm and normal oropharynx. The results of his lab tests were only significant for elevated blood sugar (234 mg/dL) and glycosylated hemoglobin (9.2%). Because he presents with classic symptoms of OSA, he receives a referral for PSG. He is found to have an AHI of 49, consistent with severe sleep apnea.
CASE 2 A system review of Ms. C finds no fever, nausea, vomiting, weakness, vision changes, or neurological symptoms. A Patient Health Questionnaire-9 (depression screen) is normal, as are her lab tests and a brain MRI with and without contrast. After an extensive work-up for headaches finds nothing, OSA is considered, in light of her daytime sleepiness—and she, too, is referred for PSG. This patient has moderate OSA, with an AHI of 27.
Initiating treatment: What’s best?
Ideally, treatment of OSA would reverse EDS and fatigue, restore full cognitive function, reduce the risk of accidents associated with OSA, and minimize its harmful cardiovascular and pulmonary effects. In fact, while OSA can be managed and its effects ameliorated, all available treatments have limitations and a cure remains elusive.
Let patients know that our understanding of OSA is limited, that treatment may not reverse or eliminate all the risks associated with this condition, and that compliance can be challenging. You can also tell them that, while more and better studies are needed, several modalities have been found to successfully treat OSA.
What to expect from lifestyle modification
Recommend lifestyle changes, such as weight loss, regular exercise early in the day, greater emphasis on sleep hygiene (eg, using the bed only for sleeping and sexual activity), and avoidance of sedating drugs and alcohol for patients with OSA.20,21
The beneficial effect of weight loss on OSA has been demonstrated in studies of both bariatric surgery and conventional weight loss therapies.21-23 While early studies of bariatric surgery were often limited by small size, ambiguous classification of OSA, and selection and follow-up biases, more recent trials show that while OSA symptoms frequently improve postoperatively, the disorder typically persists despite significant reductions in both BMI and AHI.24 Weight reduction should be strongly encouraged for obese patients, however, not only to improve OSA symptoms, but also to reduce the risk for other diseases.
Avoidance of alcohol. Alcohol has adverse effects on sleep: It shortens sleep latency, increases slow-wave sleep, suppresses REM and parasympathetic nerve activity,25 and can exacerbate OSA. Driving simulation studies have found that, compared with healthy individuals, those with untreated OSA are more susceptible to the effects of alcohol and at higher risk for accidents after just one drink.26
CPAP improves sleep, but some problems persist
CPAP supplies a flow of positive air pressure, adjusted to the level needed to keep the airway open, delivered through a facial device best suited to the patient’s anatomy, physiology, and comfort.
Multiple studies have demonstrated the effectiveness of CPAP in reducing symptoms of moderate to severe OSA, compared with placebo and other treatment modalities such as oral devices, surgical procedures, and medications. CPAP reduces AHI, blood pressure, and cardiac arrhythmias. It improves sleep efficiency, oxygen saturation, and self-reported sleep and well-being.27-30 While it ameliorates many of the harmful effects of OSA, it does not improve or reverse all of them. (See “Peripheral neuropathy linked to obstructive sleep apnea?”)
A Cochrane review of 36 randomized controlled trials with a combined total of more than 1700 patients demonstrated the superiority of CPAP vs control in several measures, such as subjective daytime sleepiness, quality of life, cognitive function, and blood pressure.28 On specific parameters of OSA, such as snoring and EDS, studies yielded mixed results.
CPAP has been found to decrease work-related injuries and morbidity and mortality associated with motor vehicle accidents linked to EDS.30-32 However, no study of CPAP has demonstrated a long-term reduction in morbidity and mortality. And no standards define the minimum number of sleep hours and/or frequency of CPAP use that is required to obtain specific benefits.
Patient compliance is poor. Part of the problem is that CPAP is difficult to use, which affects compliance. Poor patient compliance is a major barrier to evaluating its long-term benefits. (The video below, "CPAP Patients Tips from the FDA", can help ensure that patients use CPAP safely and effectively.) Studies estimate that 65% to 89% of patients with CPAP devices use them for at least 4 hours a night for 70% of nights, but that about half of those for whom CPAP is prescribed stop using it after 2 to 3 years.33-36 Several risk factors and comorbid conditions, including advanced age, diabetes, obesity, smoking, and especially, depression, are associated with decreased compliance.13
Improving CPAP compliance continues to be a challenge, highlighting the importance of treating not only OSA but all comorbidities, particularly depression. Short-term studies have found behavioral modification to be a promising means of improving CPAP compliance.33-36
Although not a first-line therapy, bilevel positive airway pressure (BiPAP), which delivers both inspiratory and expiratory pressure via a face device, can be tried in patients unable to tolerate CPAP.37 Studies are limited and it has been used in patients with complex OSA.
Oral appliances are a CPAP alternative
Mandibular repositioning devices (MRDs) and tongue-retaining devices are alternatives to CPAP.38 Although both types of oral appliance are beneficial, they are less effective than CPAP.28
MRDs, which are more commonly used than tongue-retaining devices, are available in several models. An MRD can be custom-made to hold the lower jaw in a forward position during sleep, enlarge the space behind the tongue, and put tension on the walls of the pharynx and the palate to reduce collapse.38
Tongue-retaining devices—splints that hold the tongue in place to keep the airway open—can be used for mild to moderate OSA, and for patients unable to tolerate CPAP.38,39
Studies comparing MRDs and tongue-retaining devices found no statistically significant difference in their ability to reduce AHI, but patients tolerated MRDs better.38,39 Nonetheless, both devices can cause dental discomfort, temporomandibular joint pain, dry mouth or excessive salivation, gum irritation, bruxism, and long-term occlusal changes.38
Is surgery an option?
Numerous surgical techniques are available for treating OSA, all aimed at relieving the obstruction by removing or bypassing it or increasing airway size. These include uvulopalatopharyngoplasty, which resects the uvula, retrolingual, and palatine tonsillar tissue; septoplasty; rhinoplasty; nasal turbinate reduction; nasal polypectomy palatal advancement pharyngoplasty; tonsillectomy; adenoidectomy; palatal implants; tongue reduction; genioglossus advancement; and maxillomandibular advancement.
The choice of modality depends on the patient’s anatomy and physiology, and is selected only after a full evaluation by a head and neck surgeon who specializes in surgical treatment of OSA.40,41 There is a paucity of reliable studies on the results of such procedures, but a Cochrane review of the existing literature concluded that surgery is only indicated for severe cases of sleep apnea in patients who have an anatomic obstruction.40
Too little evidence of medications’ efficacy
Numerous drugs have been tested for the treatment of OSA. Two Cochrane reviews looked at multiple trials of more than 20 drugs, in meta-analyses encompassing more than 500 patients.42,43 Ten drugs—eszopiclone, paroxetine, acetazolamide, ondansetron-fluoxetine combination, naltrexone, and fluticasone nasal spray among them—showed a statistically significant reduction in AHI, and a few showed a subjective benefit in daytime sleepiness. However, the studies were very small and of short duration and the reviewers concluded that evidence is insufficient to recommend drug therapy for OSA.
CASE 1 CPAP was prescribed for Mr. M. He tolerated it well and after 2 weeks, he reported feeling refreshed upon awakening, having less daytime somnolence and being better able to concentrate at work. His hypertension and glucose control improved, but he continues to struggle with his weight.
CASE 2 Ms. C started CPAP and within a week, her morning headaches and irritability resolved.
CORRESPONDENCE
Cecilia Gutierrez, MD, 200 West Arbor Drive, Mail Code 8809, San Diego, CA 92103; [email protected]
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
1. Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47-112.
2. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6:199-204.
3. Satela MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
4. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
5. Yaggi HK, Concato J, Kernan W, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
6. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
7. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;3:1071-1078.
8. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
9. Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735-741.
10. Basta M, Vgontzas AN. Metabolic abnormalities in obesity and sleep apnea are in a continuum. Sleep Med. 2007;8:5-7.
11. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-3021.
12. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467-472.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013-2016.
14. Skomro RP, Gjevre J, Reid J, et al. Outcomes of home-base diagnosis and treatment of obstructive sleep apnea. Chest. 2010;138:257-263.
15. Iber C, Ancoli-Israel S, Chesson AL Jr, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
16. Johns MW. Sensitivity and specificity of the multiple sleep maintenance tests, the maintenance of wakefulness tests and the Epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5-11.
17. Hesselbacher S, Subramanian S, Allen J, et al. Body mass index, gender, and ethnic variations alter the clinical implication of the Epworth sleepiness scale in patients with suspected of obstructive sleep apnea. Open Respir Med J. 2012;6:2020-2027.
18. Ahmadi N, Chung S, Gibbs A, et al. The Berlin questionnaire for sleep apnea in a sleep clinic population: relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath. 2008;12:38-45.
19. Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
20. Shneerson J, Wright J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst Rev. 2001;(1):CD002875.
21. Barvaux VA, Aubert G, Rodenstein DO. Weight loss as treatment for obstructive sleep apnea. Sleep Med Rev. 2000;4:435-452.
22. Buchwald H, Avidor H, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737.
23. Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs. conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142-1149.
24. Greenburg A, Lettieri C, Arn E. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542.
25. Sagawa Y, Kondo H, Matsubuchi N. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35:2093-2100.
26. Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447-455.
27. Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565-571.
28. Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(3):CD001106.
29. Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25:1070-1076.
30. Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430-434.
31. Findley L, Smith C, Hooper J, et al. Treatment with nasal CPAP decreases automobile accidents in patient with sleep apnea. Am J Respir Crit Care Med. 2000;161:857-859.
32. Barbe F, Sunyer J, de la Pena A, et al. Effects of continuous positive airway pressure on the risk of road accidents in sleep apnea patients. Respiration. 2007;74:44-49.
33. Sin DD, Mayers I, Man GC, et al. Long Term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population based study. Chest. 2002;121:430-435.
34. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart. 2005;91:1265-1270.
35. Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample—what are the benefits and the treatment compliance? Sleep Med. 2006;7:553-560.
36. Engleman HM, Wild MR. Improving CPAP use by patients with the sleep apnea-hypopnea syndrome (SAHS). Sleep Med. 2003;7:81-99.
37. Blau A, Minx M, Peter JG, et al. Auto bi-level pressure relief-PAP is as effective as CPAP in OSA patients—a pilot study. Sleep Breath. 2012;16:773-739.
38. Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132:693-699.
39. Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240-243.
40. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;(4):CD001004.
41. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407.
42. Smith I, Lasserson TJ, Wright JJ. Drug therapy for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006;():CD003002. Review.
43. Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2013;(5): CD003002.
Don't mind the noise-- they're just trying to sleep
Two years ago, a house guest apologized to my wife and me as he prepared to turn in for the evening. He was sorry to subject us to his noisy CPAP machine, he said. Last year at a family reunion, my sleepy brother-in-law blamed his lethargy on sleep apnea, and another in-law chimed in to say that CPAP worked great for her.
If my family and friends are any indication, we seem to be surrounded by people with obstructive sleep apnea (OSA), due almost entirely to the steep rise in obesity over the past 30 years.
It is alarming to know that OSA is associated with traffic accidents, worsening of diabetes and hypertension, atrial fibrillation, and a higher risk of death. What we don’t yet know is whether these major health problems are caused by sleep apnea or whether they’re fellow travelers. (In “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565, Gutierrez and Brady present an evidence-based summary of symptoms, diagnostic criteria, and treatment modalities. And in “Peripheral neuropathy linked to obstructive sleep apnea?” on page 577, Schmidt et al highlight a lesser known association with OSA.)
There is excellent evidence that CPAP and, in some cases, dental devices, can alleviate daytime sleepiness in patients with OSA. But more randomized trials are needed to determine whether treatment of OSA can improve hypertension, diabetes control, and atrial fibrillation, as well as prevent excess mortality.
Recent trials offer a glimmer of hope. One found that CPAP therapy led to small but significant blood pressure reductions in patients with treatment-resistant hypertension.1 Another demonstrated that CPAP led to improvement in lipid profiles and hemoglobin A1c in obese patients with diabetes.2 But many more studies are needed to know how effective sleep apnea treatments are in reducing the significant morbidity and mortality associated with OSA.
Sleep medicine is a young specialty, so it is not surprising that high-quality randomized trials of sleep apnea are in their infancy. (The American Academy of Sleep Medicine was founded in 1975 and the American Board of Sleep Medicine was established in 1991.) Because the science is evolving, I encourage you to stay abreast of new developments in the field. In the meantime, nothing works better for improving sleep apnea, hypertension, and diabetes than good old-fashioned weight loss!
1. Lozano L, Tovar JL, Sampo G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161-2168.
2. Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617B-625B.
Two years ago, a house guest apologized to my wife and me as he prepared to turn in for the evening. He was sorry to subject us to his noisy CPAP machine, he said. Last year at a family reunion, my sleepy brother-in-law blamed his lethargy on sleep apnea, and another in-law chimed in to say that CPAP worked great for her.
If my family and friends are any indication, we seem to be surrounded by people with obstructive sleep apnea (OSA), due almost entirely to the steep rise in obesity over the past 30 years.
It is alarming to know that OSA is associated with traffic accidents, worsening of diabetes and hypertension, atrial fibrillation, and a higher risk of death. What we don’t yet know is whether these major health problems are caused by sleep apnea or whether they’re fellow travelers. (In “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565, Gutierrez and Brady present an evidence-based summary of symptoms, diagnostic criteria, and treatment modalities. And in “Peripheral neuropathy linked to obstructive sleep apnea?” on page 577, Schmidt et al highlight a lesser known association with OSA.)
There is excellent evidence that CPAP and, in some cases, dental devices, can alleviate daytime sleepiness in patients with OSA. But more randomized trials are needed to determine whether treatment of OSA can improve hypertension, diabetes control, and atrial fibrillation, as well as prevent excess mortality.
Recent trials offer a glimmer of hope. One found that CPAP therapy led to small but significant blood pressure reductions in patients with treatment-resistant hypertension.1 Another demonstrated that CPAP led to improvement in lipid profiles and hemoglobin A1c in obese patients with diabetes.2 But many more studies are needed to know how effective sleep apnea treatments are in reducing the significant morbidity and mortality associated with OSA.
Sleep medicine is a young specialty, so it is not surprising that high-quality randomized trials of sleep apnea are in their infancy. (The American Academy of Sleep Medicine was founded in 1975 and the American Board of Sleep Medicine was established in 1991.) Because the science is evolving, I encourage you to stay abreast of new developments in the field. In the meantime, nothing works better for improving sleep apnea, hypertension, and diabetes than good old-fashioned weight loss!
Two years ago, a house guest apologized to my wife and me as he prepared to turn in for the evening. He was sorry to subject us to his noisy CPAP machine, he said. Last year at a family reunion, my sleepy brother-in-law blamed his lethargy on sleep apnea, and another in-law chimed in to say that CPAP worked great for her.
If my family and friends are any indication, we seem to be surrounded by people with obstructive sleep apnea (OSA), due almost entirely to the steep rise in obesity over the past 30 years.
It is alarming to know that OSA is associated with traffic accidents, worsening of diabetes and hypertension, atrial fibrillation, and a higher risk of death. What we don’t yet know is whether these major health problems are caused by sleep apnea or whether they’re fellow travelers. (In “Obstructive sleep apnea: A diagnostic and treatment guide” on page 565, Gutierrez and Brady present an evidence-based summary of symptoms, diagnostic criteria, and treatment modalities. And in “Peripheral neuropathy linked to obstructive sleep apnea?” on page 577, Schmidt et al highlight a lesser known association with OSA.)
There is excellent evidence that CPAP and, in some cases, dental devices, can alleviate daytime sleepiness in patients with OSA. But more randomized trials are needed to determine whether treatment of OSA can improve hypertension, diabetes control, and atrial fibrillation, as well as prevent excess mortality.
Recent trials offer a glimmer of hope. One found that CPAP therapy led to small but significant blood pressure reductions in patients with treatment-resistant hypertension.1 Another demonstrated that CPAP led to improvement in lipid profiles and hemoglobin A1c in obese patients with diabetes.2 But many more studies are needed to know how effective sleep apnea treatments are in reducing the significant morbidity and mortality associated with OSA.
Sleep medicine is a young specialty, so it is not surprising that high-quality randomized trials of sleep apnea are in their infancy. (The American Academy of Sleep Medicine was founded in 1975 and the American Board of Sleep Medicine was established in 1991.) Because the science is evolving, I encourage you to stay abreast of new developments in the field. In the meantime, nothing works better for improving sleep apnea, hypertension, and diabetes than good old-fashioned weight loss!
1. Lozano L, Tovar JL, Sampo G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161-2168.
2. Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617B-625B.
1. Lozano L, Tovar JL, Sampo G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161-2168.
2. Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617B-625B.
Dexamethasone improves outcomes for infants with bronchiolitis, atopy history
A 5-day course of dexamethasone significantly shortened hospital stays for infants with bronchiolitis who had eczema or close relatives with asthma.
The randomized, placebo-controlled study suggests that a family history of atopy could identify a subset of babies who would benefit from the addition of a corticosteroid to the usual salbutamol therapy for acute bronchiolitis, according to Dr. Khalid Alansari and colleagues. The report was published in the Sept. 16 issue of Pediatrics.
The researchers examined 7-day outcomes in 200 infants with acute bronchiolitis who were at a high risk of asthma, as determined by having at least one first-degree relative with either asthma or eczema. All of the children (mean age 3.5 months) were admitted to a pediatric hospital for treatment, wrote Dr. Alansari of Weill Cornell Medical College, Doha, Qatar, and coauthors. Infants who received dexamethasone were discharged 8 hours earlier than were those receiving standard treatment. The mean duration of symptoms was 4.5 days (Pediatrics 2013 Sept. 13 [doi: 10.1542/peds.2012-3746]).
The study’s primary outcome was time until discharge. Secondary outcomes included the number of patients who needed epinephrine treatment, readmission for a shorter stay in an infirmary site, and revisiting the emergency department or another clinic for the same illness. A study nurse made daily calls to assess the patients after discharge.
Infants in the dexamethasone group were discharged at a mean of 18.6 hours – significantly sooner than those in the control group (27 hours). Epinephrine was necessary for 19 infants in the dexamethasone group and 31 in the placebo group – again a significant difference.
Similar numbers in each group needed readmission and additional outpatient visits in the week after discharge. During the follow-up week, 22% of the dexamethasone group needed infirmary care and the mean stay was 17 hours, compared with 21% of the placebo group with a mean stay of 18 hours.
Nineteen in the dexamethasone group and 11 in the placebo group made a clinic visit (18.6% vs. 11%); this difference was not significant.
The chest radiograph was normal in about 37% of infants studied. About half showed lesser infiltrates; 15% had a lobar collapse or consolidation.
More than 70% had a full sibling with asthma. About 20% had a parent with the disease; in 5%, both parents had it. About 20% of patients had both eczema and first-degree relative with asthma.
All of the infants received 2.5 mg salbutamol nebulization at baseline and at 30, 60, and 120 minutes, and then every 2 hours until discharge. Nebulized epinephrine (0.5 mL/kg with a maximum dose of 5 mL) was available if needed. In addition, they were randomized to either placebo or to a 5-day course of dexamethasone 1 mg/mL, at a rate of 1 mL/kg on day 1, reduced to 0.6 mL/kg for days 2-5.
The study was sponsored by Hamad Medical Corporation. The authors reported no financial conflicts.
A 5-day course of dexamethasone significantly shortened hospital stays for infants with bronchiolitis who had eczema or close relatives with asthma.
The randomized, placebo-controlled study suggests that a family history of atopy could identify a subset of babies who would benefit from the addition of a corticosteroid to the usual salbutamol therapy for acute bronchiolitis, according to Dr. Khalid Alansari and colleagues. The report was published in the Sept. 16 issue of Pediatrics.
The researchers examined 7-day outcomes in 200 infants with acute bronchiolitis who were at a high risk of asthma, as determined by having at least one first-degree relative with either asthma or eczema. All of the children (mean age 3.5 months) were admitted to a pediatric hospital for treatment, wrote Dr. Alansari of Weill Cornell Medical College, Doha, Qatar, and coauthors. Infants who received dexamethasone were discharged 8 hours earlier than were those receiving standard treatment. The mean duration of symptoms was 4.5 days (Pediatrics 2013 Sept. 13 [doi: 10.1542/peds.2012-3746]).
The study’s primary outcome was time until discharge. Secondary outcomes included the number of patients who needed epinephrine treatment, readmission for a shorter stay in an infirmary site, and revisiting the emergency department or another clinic for the same illness. A study nurse made daily calls to assess the patients after discharge.
Infants in the dexamethasone group were discharged at a mean of 18.6 hours – significantly sooner than those in the control group (27 hours). Epinephrine was necessary for 19 infants in the dexamethasone group and 31 in the placebo group – again a significant difference.
Similar numbers in each group needed readmission and additional outpatient visits in the week after discharge. During the follow-up week, 22% of the dexamethasone group needed infirmary care and the mean stay was 17 hours, compared with 21% of the placebo group with a mean stay of 18 hours.
Nineteen in the dexamethasone group and 11 in the placebo group made a clinic visit (18.6% vs. 11%); this difference was not significant.
The chest radiograph was normal in about 37% of infants studied. About half showed lesser infiltrates; 15% had a lobar collapse or consolidation.
More than 70% had a full sibling with asthma. About 20% had a parent with the disease; in 5%, both parents had it. About 20% of patients had both eczema and first-degree relative with asthma.
All of the infants received 2.5 mg salbutamol nebulization at baseline and at 30, 60, and 120 minutes, and then every 2 hours until discharge. Nebulized epinephrine (0.5 mL/kg with a maximum dose of 5 mL) was available if needed. In addition, they were randomized to either placebo or to a 5-day course of dexamethasone 1 mg/mL, at a rate of 1 mL/kg on day 1, reduced to 0.6 mL/kg for days 2-5.
The study was sponsored by Hamad Medical Corporation. The authors reported no financial conflicts.
A 5-day course of dexamethasone significantly shortened hospital stays for infants with bronchiolitis who had eczema or close relatives with asthma.
The randomized, placebo-controlled study suggests that a family history of atopy could identify a subset of babies who would benefit from the addition of a corticosteroid to the usual salbutamol therapy for acute bronchiolitis, according to Dr. Khalid Alansari and colleagues. The report was published in the Sept. 16 issue of Pediatrics.
The researchers examined 7-day outcomes in 200 infants with acute bronchiolitis who were at a high risk of asthma, as determined by having at least one first-degree relative with either asthma or eczema. All of the children (mean age 3.5 months) were admitted to a pediatric hospital for treatment, wrote Dr. Alansari of Weill Cornell Medical College, Doha, Qatar, and coauthors. Infants who received dexamethasone were discharged 8 hours earlier than were those receiving standard treatment. The mean duration of symptoms was 4.5 days (Pediatrics 2013 Sept. 13 [doi: 10.1542/peds.2012-3746]).
The study’s primary outcome was time until discharge. Secondary outcomes included the number of patients who needed epinephrine treatment, readmission for a shorter stay in an infirmary site, and revisiting the emergency department or another clinic for the same illness. A study nurse made daily calls to assess the patients after discharge.
Infants in the dexamethasone group were discharged at a mean of 18.6 hours – significantly sooner than those in the control group (27 hours). Epinephrine was necessary for 19 infants in the dexamethasone group and 31 in the placebo group – again a significant difference.
Similar numbers in each group needed readmission and additional outpatient visits in the week after discharge. During the follow-up week, 22% of the dexamethasone group needed infirmary care and the mean stay was 17 hours, compared with 21% of the placebo group with a mean stay of 18 hours.
Nineteen in the dexamethasone group and 11 in the placebo group made a clinic visit (18.6% vs. 11%); this difference was not significant.
The chest radiograph was normal in about 37% of infants studied. About half showed lesser infiltrates; 15% had a lobar collapse or consolidation.
More than 70% had a full sibling with asthma. About 20% had a parent with the disease; in 5%, both parents had it. About 20% of patients had both eczema and first-degree relative with asthma.
All of the infants received 2.5 mg salbutamol nebulization at baseline and at 30, 60, and 120 minutes, and then every 2 hours until discharge. Nebulized epinephrine (0.5 mL/kg with a maximum dose of 5 mL) was available if needed. In addition, they were randomized to either placebo or to a 5-day course of dexamethasone 1 mg/mL, at a rate of 1 mL/kg on day 1, reduced to 0.6 mL/kg for days 2-5.
The study was sponsored by Hamad Medical Corporation. The authors reported no financial conflicts.
Lung cancer screening: The USPSTF's latest proposal
Estrogen-related VTE shows low recurrence rate
AMSTERDAM – Women with an unprovoked index venous thromboembolism while they are on estrogen treatment have a low recurrence risk as long as they stop estrogen, and therefore don’t need prolonged anticoagulant treatment, based on a review of 630 cases.
Women whose index venous thromboembolism (VTE) occurred while on estrogen had 4 recurrences for every 10 among the women who had unprovoked VTE and were not on estrogen during an average follow-up of more than 6 years, Dr. Lisbeth Eischer said at the 24th Congress of the International Society on Thrombosis and Haemostasis.
"We propose that these women [whose index VTE occurred while on estrogen] should receive anticoagulant treatment for no longer than 3 months," said Dr. Eischer of the division of hematology and hemostasis at the Medical University of Vienna.
"I think the recommendation [for duration of anticoagulation] should be comparable to women who have undergone surgery, not more than 3 months," she said. In the review she presented, which included 630 women treated for a VTE since 1992, the average duration of anticoagulant treatment was 7 months among women whose VTE occurred when on estrogen as well as those not on estrogen at the time of their VTE.
Dr. Eischer and her associates studied women enrolled in the prospective Austrian Study on Recurrent Venous Thromboembolism (AUREC). The group of 630 included women aged 18 years or older who had a first VTE and received at least 3 months of anticoagulant treatment. The study excluded women with another identifiable cause of VTE, such as surgery, trauma, pregnancy, cancer, or significant thrombophilia. Women were not excluded if they were heterozygous for a factor V Leiden mutation. They averaged 46 years old; 361 (57%) had deep vein thrombosis as their index case, and 269 (43%) had pulmonary embolism as their index case. The average length of follow-up was 76 months (6.3 years).
Three hundred thirty-three (53%) women were on estrogen treatment at the time of their VTE. These women were significantly younger, averaging 38 years compared with an average of 55 among the women not receiving estrogen at the time of their VTE. The prevalence of women with a single factor V Leiden mutation was 48 (16%) among those not on estrogen and 98 (28%) among those on estrogen, a statistically significant difference.
During follow-up, the incidence of recurrent VTE was 22 (7%) among the 333 women whose index VTE occurred while on estrogen, and 49 (16%) among the 297 with an unprovoked index VTE. In a multivariate analysis that adjusted for age, location of the VTE (pulmonary or deep vein), and presence of a factor V Leiden mutation, women whose index VTE occurred while they were taking estrogen had a statistically significant 60% reduced rate of having a recurrent VTE during follow-up compared with the women whose index VTE occurred when they were not on estrogen.
Dr. Eischer said she had no relevant financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Women with an unprovoked index venous thromboembolism while they are on estrogen treatment have a low recurrence risk as long as they stop estrogen, and therefore don’t need prolonged anticoagulant treatment, based on a review of 630 cases.
Women whose index venous thromboembolism (VTE) occurred while on estrogen had 4 recurrences for every 10 among the women who had unprovoked VTE and were not on estrogen during an average follow-up of more than 6 years, Dr. Lisbeth Eischer said at the 24th Congress of the International Society on Thrombosis and Haemostasis.
"We propose that these women [whose index VTE occurred while on estrogen] should receive anticoagulant treatment for no longer than 3 months," said Dr. Eischer of the division of hematology and hemostasis at the Medical University of Vienna.
"I think the recommendation [for duration of anticoagulation] should be comparable to women who have undergone surgery, not more than 3 months," she said. In the review she presented, which included 630 women treated for a VTE since 1992, the average duration of anticoagulant treatment was 7 months among women whose VTE occurred when on estrogen as well as those not on estrogen at the time of their VTE.
Dr. Eischer and her associates studied women enrolled in the prospective Austrian Study on Recurrent Venous Thromboembolism (AUREC). The group of 630 included women aged 18 years or older who had a first VTE and received at least 3 months of anticoagulant treatment. The study excluded women with another identifiable cause of VTE, such as surgery, trauma, pregnancy, cancer, or significant thrombophilia. Women were not excluded if they were heterozygous for a factor V Leiden mutation. They averaged 46 years old; 361 (57%) had deep vein thrombosis as their index case, and 269 (43%) had pulmonary embolism as their index case. The average length of follow-up was 76 months (6.3 years).
Three hundred thirty-three (53%) women were on estrogen treatment at the time of their VTE. These women were significantly younger, averaging 38 years compared with an average of 55 among the women not receiving estrogen at the time of their VTE. The prevalence of women with a single factor V Leiden mutation was 48 (16%) among those not on estrogen and 98 (28%) among those on estrogen, a statistically significant difference.
During follow-up, the incidence of recurrent VTE was 22 (7%) among the 333 women whose index VTE occurred while on estrogen, and 49 (16%) among the 297 with an unprovoked index VTE. In a multivariate analysis that adjusted for age, location of the VTE (pulmonary or deep vein), and presence of a factor V Leiden mutation, women whose index VTE occurred while they were taking estrogen had a statistically significant 60% reduced rate of having a recurrent VTE during follow-up compared with the women whose index VTE occurred when they were not on estrogen.
Dr. Eischer said she had no relevant financial disclosures.
On Twitter @mitchelzoler
AMSTERDAM – Women with an unprovoked index venous thromboembolism while they are on estrogen treatment have a low recurrence risk as long as they stop estrogen, and therefore don’t need prolonged anticoagulant treatment, based on a review of 630 cases.
Women whose index venous thromboembolism (VTE) occurred while on estrogen had 4 recurrences for every 10 among the women who had unprovoked VTE and were not on estrogen during an average follow-up of more than 6 years, Dr. Lisbeth Eischer said at the 24th Congress of the International Society on Thrombosis and Haemostasis.
"We propose that these women [whose index VTE occurred while on estrogen] should receive anticoagulant treatment for no longer than 3 months," said Dr. Eischer of the division of hematology and hemostasis at the Medical University of Vienna.
"I think the recommendation [for duration of anticoagulation] should be comparable to women who have undergone surgery, not more than 3 months," she said. In the review she presented, which included 630 women treated for a VTE since 1992, the average duration of anticoagulant treatment was 7 months among women whose VTE occurred when on estrogen as well as those not on estrogen at the time of their VTE.
Dr. Eischer and her associates studied women enrolled in the prospective Austrian Study on Recurrent Venous Thromboembolism (AUREC). The group of 630 included women aged 18 years or older who had a first VTE and received at least 3 months of anticoagulant treatment. The study excluded women with another identifiable cause of VTE, such as surgery, trauma, pregnancy, cancer, or significant thrombophilia. Women were not excluded if they were heterozygous for a factor V Leiden mutation. They averaged 46 years old; 361 (57%) had deep vein thrombosis as their index case, and 269 (43%) had pulmonary embolism as their index case. The average length of follow-up was 76 months (6.3 years).
Three hundred thirty-three (53%) women were on estrogen treatment at the time of their VTE. These women were significantly younger, averaging 38 years compared with an average of 55 among the women not receiving estrogen at the time of their VTE. The prevalence of women with a single factor V Leiden mutation was 48 (16%) among those not on estrogen and 98 (28%) among those on estrogen, a statistically significant difference.
During follow-up, the incidence of recurrent VTE was 22 (7%) among the 333 women whose index VTE occurred while on estrogen, and 49 (16%) among the 297 with an unprovoked index VTE. In a multivariate analysis that adjusted for age, location of the VTE (pulmonary or deep vein), and presence of a factor V Leiden mutation, women whose index VTE occurred while they were taking estrogen had a statistically significant 60% reduced rate of having a recurrent VTE during follow-up compared with the women whose index VTE occurred when they were not on estrogen.
Dr. Eischer said she had no relevant financial disclosures.
On Twitter @mitchelzoler
AT ISTH 2013
This asthma treatment has a lasting side effect in children
Before prescribing inhaled corticosteroids (ICS) for a child with asthma, tell the patient—and parents—that their use could lead to a small but permanent effect on adult height.1
STRENGTH OF RECOMMENDATIONS
B: Based on one prospective study.
Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
Illustrative case
A 10-year-old boy is brought in by his father for asthma follow-up. The child uses an albuterol inhaler, but has had increased coughing and wheezing recently. You are ready to step up his asthma therapy to include ICS. But the patient’s father questions this, noting that he recently read that steroids may reduce a child’s growth. How should you respond?
Inhaled corticosteroids (ICS) are a mainstay in the treatment of asthma ranging from mild persistent to severe. Standards of care for asthma treatment involve a stepwise approach, with ICS added if symptoms are not controlled with short-acting beta antagonists alone.2 In addition, monotherapy with ICS is more effective for controlling symptoms than leukotriene inhibitors or other controller medications, while also decreasing hospitalizations and nocturnal awakenings and improving quality of life—with few side effects.3
What we know about ICS and children’s growth
One adverse effect of ICS, however, is that of “decreased linear growth velocity”4—ie, slowing the rate at which children grow. Until recently, children were thought to “catch up” later in life, either by growing for a longer period of time than they would had they not taken ICS or by growing at an increased velocity after ICS medications are discontinued.4-6
Study summary: The effect on growth is small, but long-lasting
Kelly et al conducted a prospective observational cohort study that followed 943 (90.7%) participants in the Childhood Asthma Management Program (CAMP) in the years after the randomized controlled trial (RCT) ended.
A double-blind, placebo-controlled RCT, CAMP studied the linear growth of 1041 children with mild-to-moderate persistent asthma who were divided into 3 treatment groups: One group received 200 mcg inhaled budesonide twice daily; a second group received 8 mg inhaled nedocromil twice daily; and a third group received placebo. Albuterol was used symptomatically by all 3 groups.7 The children ranged in age from 5 to 13 years at the start of the study; 98 patients—split evenly among the 3 treatment arms—were lost to follow-up.
During the 4 to 6 years of the CAMP trial, the budesonide group received a mean total of 636 mg ICS, whereas the nedocromil and placebo groups received an average of 88.5 and 109.4 mg ICS, respectively. After the RCT ended, all participants had standardized asthma treatment, receiving mean adjusted total doses of ICS of 381 mg for the budesonide group, 347.9 mg for the nedocromil group, and 355 mg for the placebo group.
Patients’ height was measured every 6 months for the next 4.5 years, and once or twice a year thereafter until they reached adult height (at a mean age of 24.9±2.7 years).
ICS users were a half inch shorter
Long-term ICS use was linked to a lower adult height. The adjusted mean height was 171.1 cm for the budesonide group vs 172.3 cm for those on placebo, a difference of 1.2 cm, or 0.47 inch (95% confidence interval [CI], −1.9 to −0.5; P=.001); the mean adult height in the nedocromil group (172.1 cm) was similar to that of the placebo group (−0.2 cm; 95% CI, −0.9 to 0.5; P=.61).
The lower adult height in the ICS group did not vary significantly based on sex, age at trial entry, race, or duration of asthma prior to trial entry; however, dose was a key factor. A larger daily dose of budesonide—particularly in the first 2 years of the RCT—was associated with a lower adult height (about −0.1 cm for each mcg/kg in that 2-year time frame). This was consistent with results from studies that looked at other types of ICS (beclomethasone, fluticasone, and mometasone).8-11
The study also showed that growth velocity was reduced in the first 2 years of assigned treatment with budesonide, and this was primarily among prepubertal participants. After the initial 2-year slowing in growth rate, the children resumed growing at normal speeds.
What’s new: Now we know: Children don’t “catch up"
Retrospective studies have reported that children on ICS for mild persistent to moderate asthma would have an initial slowing in growth velocity but then “catch up” by growing for a longer period of time.3-5 This is the first prospective study with good follow-up to show that ICS use affects long-term growth and adult height. While the effect is not large, some children and their families might be concerned about it.
Caveats: ICS use was atypical
The randomized controlled portion of the study used a prescribed dose of budesonide without regard to symptoms. This is not the typical pattern of ICS use. In addition, compliance with ICS varies significantly.12 Because the effect on adult height appears to be dose dependent, however, we think the results of this study are valid.
In addition, there was a placebo control group (and big differences in exposure to ICS) only for the duration of the RCT. During the subsequent study, all patients received equivalent doses of ICS. This means that the variation in mean adult height achieved can be primarily ascribed to participants’ use of ICS during the 4- to 6-year CAMP trial. Of note, the effect of ICS was greatest in prepubertal participants, so there may be a diminished effect as teens approach their final height.
The study did not look at the effect of ICS use in patients with severe asthma—the group most likely to use ICS. However, the benefits of ICS for those with severe asthma likely outweigh any negative effects on adult height.
Challenges to implementation: What to tell patients
The message we convey to patients (and parents) about ICS use is a nuanced one. We can stress that ICS remain very important in the treatment of asthma and, while it appears that their use causes a slight decrease in adult height, most children with persistent asthma benefit from ICS.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
2. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health National Heart, Lung and Blood Institute: National Asthma Education and Prevention Program, 2007. Available at: http://www.nhlbi. nih.gov/guidelines/asthma/asthgdln.pdf. Accessed August 15, 2013.
3. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/ or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
4. Agertoft L, Pedersen S. Effect of long- term treatment with budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-1069.
5. Van Bever HP, Desager KN, Lijssens N, et al. Does treatment of asthmatic children with inhaled corticosteroids affect their adult height? Pediatr Pulmonol. 1999;27:369-375.
6. Silverstein MD, Yunginger JW, Reed CE, et al. Attained adult height after childhood asthma: effect of glucocorticoid therapy. J Allergy Clin Immunol. 1997;99:466-474.
7. The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-1063.
8. Tinkelman DG, Reed CE, Nelson HS, et al. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64-77.
9. Verberne AA, Frost C, Roorda RJ, et al. One year treatment with salmeterol compared with beclomethasone in children with asthma. Am J Respir Crit Care Med. 1997;156:688-695.
10. Allen DB, Bronsky EA, LaForce CF, et al. Growth in asthmatic children treated with fluticasone propionate. J Pediatr 1998;132: 472-477.
11. Skoner DP, Meltzer EO, Milgrom H, et al. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo-controlled trial in children 4-9 years old with mild persistent asthma. J Asthma. 2011;48:848-859.
12. Cochrane MG, Bala MV, Downs KE, et al. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542-550.
Before prescribing inhaled corticosteroids (ICS) for a child with asthma, tell the patient—and parents—that their use could lead to a small but permanent effect on adult height.1
STRENGTH OF RECOMMENDATIONS
B: Based on one prospective study.
Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
Illustrative case
A 10-year-old boy is brought in by his father for asthma follow-up. The child uses an albuterol inhaler, but has had increased coughing and wheezing recently. You are ready to step up his asthma therapy to include ICS. But the patient’s father questions this, noting that he recently read that steroids may reduce a child’s growth. How should you respond?
Inhaled corticosteroids (ICS) are a mainstay in the treatment of asthma ranging from mild persistent to severe. Standards of care for asthma treatment involve a stepwise approach, with ICS added if symptoms are not controlled with short-acting beta antagonists alone.2 In addition, monotherapy with ICS is more effective for controlling symptoms than leukotriene inhibitors or other controller medications, while also decreasing hospitalizations and nocturnal awakenings and improving quality of life—with few side effects.3
What we know about ICS and children’s growth
One adverse effect of ICS, however, is that of “decreased linear growth velocity”4—ie, slowing the rate at which children grow. Until recently, children were thought to “catch up” later in life, either by growing for a longer period of time than they would had they not taken ICS or by growing at an increased velocity after ICS medications are discontinued.4-6
Study summary: The effect on growth is small, but long-lasting
Kelly et al conducted a prospective observational cohort study that followed 943 (90.7%) participants in the Childhood Asthma Management Program (CAMP) in the years after the randomized controlled trial (RCT) ended.
A double-blind, placebo-controlled RCT, CAMP studied the linear growth of 1041 children with mild-to-moderate persistent asthma who were divided into 3 treatment groups: One group received 200 mcg inhaled budesonide twice daily; a second group received 8 mg inhaled nedocromil twice daily; and a third group received placebo. Albuterol was used symptomatically by all 3 groups.7 The children ranged in age from 5 to 13 years at the start of the study; 98 patients—split evenly among the 3 treatment arms—were lost to follow-up.
During the 4 to 6 years of the CAMP trial, the budesonide group received a mean total of 636 mg ICS, whereas the nedocromil and placebo groups received an average of 88.5 and 109.4 mg ICS, respectively. After the RCT ended, all participants had standardized asthma treatment, receiving mean adjusted total doses of ICS of 381 mg for the budesonide group, 347.9 mg for the nedocromil group, and 355 mg for the placebo group.
Patients’ height was measured every 6 months for the next 4.5 years, and once or twice a year thereafter until they reached adult height (at a mean age of 24.9±2.7 years).
ICS users were a half inch shorter
Long-term ICS use was linked to a lower adult height. The adjusted mean height was 171.1 cm for the budesonide group vs 172.3 cm for those on placebo, a difference of 1.2 cm, or 0.47 inch (95% confidence interval [CI], −1.9 to −0.5; P=.001); the mean adult height in the nedocromil group (172.1 cm) was similar to that of the placebo group (−0.2 cm; 95% CI, −0.9 to 0.5; P=.61).
The lower adult height in the ICS group did not vary significantly based on sex, age at trial entry, race, or duration of asthma prior to trial entry; however, dose was a key factor. A larger daily dose of budesonide—particularly in the first 2 years of the RCT—was associated with a lower adult height (about −0.1 cm for each mcg/kg in that 2-year time frame). This was consistent with results from studies that looked at other types of ICS (beclomethasone, fluticasone, and mometasone).8-11
The study also showed that growth velocity was reduced in the first 2 years of assigned treatment with budesonide, and this was primarily among prepubertal participants. After the initial 2-year slowing in growth rate, the children resumed growing at normal speeds.
What’s new: Now we know: Children don’t “catch up"
Retrospective studies have reported that children on ICS for mild persistent to moderate asthma would have an initial slowing in growth velocity but then “catch up” by growing for a longer period of time.3-5 This is the first prospective study with good follow-up to show that ICS use affects long-term growth and adult height. While the effect is not large, some children and their families might be concerned about it.
Caveats: ICS use was atypical
The randomized controlled portion of the study used a prescribed dose of budesonide without regard to symptoms. This is not the typical pattern of ICS use. In addition, compliance with ICS varies significantly.12 Because the effect on adult height appears to be dose dependent, however, we think the results of this study are valid.
In addition, there was a placebo control group (and big differences in exposure to ICS) only for the duration of the RCT. During the subsequent study, all patients received equivalent doses of ICS. This means that the variation in mean adult height achieved can be primarily ascribed to participants’ use of ICS during the 4- to 6-year CAMP trial. Of note, the effect of ICS was greatest in prepubertal participants, so there may be a diminished effect as teens approach their final height.
The study did not look at the effect of ICS use in patients with severe asthma—the group most likely to use ICS. However, the benefits of ICS for those with severe asthma likely outweigh any negative effects on adult height.
Challenges to implementation: What to tell patients
The message we convey to patients (and parents) about ICS use is a nuanced one. We can stress that ICS remain very important in the treatment of asthma and, while it appears that their use causes a slight decrease in adult height, most children with persistent asthma benefit from ICS.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Before prescribing inhaled corticosteroids (ICS) for a child with asthma, tell the patient—and parents—that their use could lead to a small but permanent effect on adult height.1
STRENGTH OF RECOMMENDATIONS
B: Based on one prospective study.
Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
Illustrative case
A 10-year-old boy is brought in by his father for asthma follow-up. The child uses an albuterol inhaler, but has had increased coughing and wheezing recently. You are ready to step up his asthma therapy to include ICS. But the patient’s father questions this, noting that he recently read that steroids may reduce a child’s growth. How should you respond?
Inhaled corticosteroids (ICS) are a mainstay in the treatment of asthma ranging from mild persistent to severe. Standards of care for asthma treatment involve a stepwise approach, with ICS added if symptoms are not controlled with short-acting beta antagonists alone.2 In addition, monotherapy with ICS is more effective for controlling symptoms than leukotriene inhibitors or other controller medications, while also decreasing hospitalizations and nocturnal awakenings and improving quality of life—with few side effects.3
What we know about ICS and children’s growth
One adverse effect of ICS, however, is that of “decreased linear growth velocity”4—ie, slowing the rate at which children grow. Until recently, children were thought to “catch up” later in life, either by growing for a longer period of time than they would had they not taken ICS or by growing at an increased velocity after ICS medications are discontinued.4-6
Study summary: The effect on growth is small, but long-lasting
Kelly et al conducted a prospective observational cohort study that followed 943 (90.7%) participants in the Childhood Asthma Management Program (CAMP) in the years after the randomized controlled trial (RCT) ended.
A double-blind, placebo-controlled RCT, CAMP studied the linear growth of 1041 children with mild-to-moderate persistent asthma who were divided into 3 treatment groups: One group received 200 mcg inhaled budesonide twice daily; a second group received 8 mg inhaled nedocromil twice daily; and a third group received placebo. Albuterol was used symptomatically by all 3 groups.7 The children ranged in age from 5 to 13 years at the start of the study; 98 patients—split evenly among the 3 treatment arms—were lost to follow-up.
During the 4 to 6 years of the CAMP trial, the budesonide group received a mean total of 636 mg ICS, whereas the nedocromil and placebo groups received an average of 88.5 and 109.4 mg ICS, respectively. After the RCT ended, all participants had standardized asthma treatment, receiving mean adjusted total doses of ICS of 381 mg for the budesonide group, 347.9 mg for the nedocromil group, and 355 mg for the placebo group.
Patients’ height was measured every 6 months for the next 4.5 years, and once or twice a year thereafter until they reached adult height (at a mean age of 24.9±2.7 years).
ICS users were a half inch shorter
Long-term ICS use was linked to a lower adult height. The adjusted mean height was 171.1 cm for the budesonide group vs 172.3 cm for those on placebo, a difference of 1.2 cm, or 0.47 inch (95% confidence interval [CI], −1.9 to −0.5; P=.001); the mean adult height in the nedocromil group (172.1 cm) was similar to that of the placebo group (−0.2 cm; 95% CI, −0.9 to 0.5; P=.61).
The lower adult height in the ICS group did not vary significantly based on sex, age at trial entry, race, or duration of asthma prior to trial entry; however, dose was a key factor. A larger daily dose of budesonide—particularly in the first 2 years of the RCT—was associated with a lower adult height (about −0.1 cm for each mcg/kg in that 2-year time frame). This was consistent with results from studies that looked at other types of ICS (beclomethasone, fluticasone, and mometasone).8-11
The study also showed that growth velocity was reduced in the first 2 years of assigned treatment with budesonide, and this was primarily among prepubertal participants. After the initial 2-year slowing in growth rate, the children resumed growing at normal speeds.
What’s new: Now we know: Children don’t “catch up"
Retrospective studies have reported that children on ICS for mild persistent to moderate asthma would have an initial slowing in growth velocity but then “catch up” by growing for a longer period of time.3-5 This is the first prospective study with good follow-up to show that ICS use affects long-term growth and adult height. While the effect is not large, some children and their families might be concerned about it.
Caveats: ICS use was atypical
The randomized controlled portion of the study used a prescribed dose of budesonide without regard to symptoms. This is not the typical pattern of ICS use. In addition, compliance with ICS varies significantly.12 Because the effect on adult height appears to be dose dependent, however, we think the results of this study are valid.
In addition, there was a placebo control group (and big differences in exposure to ICS) only for the duration of the RCT. During the subsequent study, all patients received equivalent doses of ICS. This means that the variation in mean adult height achieved can be primarily ascribed to participants’ use of ICS during the 4- to 6-year CAMP trial. Of note, the effect of ICS was greatest in prepubertal participants, so there may be a diminished effect as teens approach their final height.
The study did not look at the effect of ICS use in patients with severe asthma—the group most likely to use ICS. However, the benefits of ICS for those with severe asthma likely outweigh any negative effects on adult height.
Challenges to implementation: What to tell patients
The message we convey to patients (and parents) about ICS use is a nuanced one. We can stress that ICS remain very important in the treatment of asthma and, while it appears that their use causes a slight decrease in adult height, most children with persistent asthma benefit from ICS.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
2. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health National Heart, Lung and Blood Institute: National Asthma Education and Prevention Program, 2007. Available at: http://www.nhlbi. nih.gov/guidelines/asthma/asthgdln.pdf. Accessed August 15, 2013.
3. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/ or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
4. Agertoft L, Pedersen S. Effect of long- term treatment with budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-1069.
5. Van Bever HP, Desager KN, Lijssens N, et al. Does treatment of asthmatic children with inhaled corticosteroids affect their adult height? Pediatr Pulmonol. 1999;27:369-375.
6. Silverstein MD, Yunginger JW, Reed CE, et al. Attained adult height after childhood asthma: effect of glucocorticoid therapy. J Allergy Clin Immunol. 1997;99:466-474.
7. The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-1063.
8. Tinkelman DG, Reed CE, Nelson HS, et al. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64-77.
9. Verberne AA, Frost C, Roorda RJ, et al. One year treatment with salmeterol compared with beclomethasone in children with asthma. Am J Respir Crit Care Med. 1997;156:688-695.
10. Allen DB, Bronsky EA, LaForce CF, et al. Growth in asthmatic children treated with fluticasone propionate. J Pediatr 1998;132: 472-477.
11. Skoner DP, Meltzer EO, Milgrom H, et al. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo-controlled trial in children 4-9 years old with mild persistent asthma. J Asthma. 2011;48:848-859.
12. Cochrane MG, Bala MV, Downs KE, et al. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542-550.
1. Kelly HW, Sternberg AL, Lescher R, et al; CAMP Research Group. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904-912.
2. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health National Heart, Lung and Blood Institute: National Asthma Education and Prevention Program, 2007. Available at: http://www.nhlbi. nih.gov/guidelines/asthma/asthgdln.pdf. Accessed August 15, 2013.
3. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/ or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.
4. Agertoft L, Pedersen S. Effect of long- term treatment with budesonide on adult height in children with asthma. N Engl J Med. 2000;343:1064-1069.
5. Van Bever HP, Desager KN, Lijssens N, et al. Does treatment of asthmatic children with inhaled corticosteroids affect their adult height? Pediatr Pulmonol. 1999;27:369-375.
6. Silverstein MD, Yunginger JW, Reed CE, et al. Attained adult height after childhood asthma: effect of glucocorticoid therapy. J Allergy Clin Immunol. 1997;99:466-474.
7. The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054-1063.
8. Tinkelman DG, Reed CE, Nelson HS, et al. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64-77.
9. Verberne AA, Frost C, Roorda RJ, et al. One year treatment with salmeterol compared with beclomethasone in children with asthma. Am J Respir Crit Care Med. 1997;156:688-695.
10. Allen DB, Bronsky EA, LaForce CF, et al. Growth in asthmatic children treated with fluticasone propionate. J Pediatr 1998;132: 472-477.
11. Skoner DP, Meltzer EO, Milgrom H, et al. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo-controlled trial in children 4-9 years old with mild persistent asthma. J Asthma. 2011;48:848-859.
12. Cochrane MG, Bala MV, Downs KE, et al. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117:542-550.
Copyright © 2013 Family Physicians Inquiries Network. All rights reserved.
Should you clear a child with a URI for surgery?
• Consult the anesthesiologist if a pediatric patient is about to undergo an elective surgical procedure and is febrile or coughing—especially if the child has significant comorbidities. These conditions may warrant postponing the procedure. A
• Avoid surgery in a child with cardiac disease who has inflammatory respiratory disease—especially if he or she has had palliative procedures for cyanotic lesions or has a hypoplastic right or left heart. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE You are seeing a 2-year-old boy with a runny nose in your busy clinic. He was sent to you by a local surgeon who plans to repair a hernia 3 days from now. Other than the upper respiratory tract infection, the child is healthy. The surgeon wants you to clear the boy for surgery to avoid the possibility of the anesthesiologist canceling on the day of the procedure.
What are your next steps?
In our experience, children are regularly brought to the ambulatory surgery suite after having seen their family physician or pediatrician. To better equip you for such visits, we’ve put together the following summary of the risks for a child with an upper respiratory infection (URI) who is about to undergo surgery. We’ve also detailed some of the reasoning and evidence behind the decisions that anesthesiologists make in cases like this.
Making decisions in the absence of consensus
While the American Society of Anesthesiologists has a consensus statement on preoperative fasting to reduce the risk of pulmonary aspiration,1 there is no consensus on how to manage a child scheduled for elective surgery who develops a URI.
Historically, any child with a current or recent URI would not be considered a candidate for elective surgery due to the potential for respiratory complications caused by airway irritability.2 These complications can include bronchospasm, laryngospasm, hypoxemia, croup, pulmonary shunting, atelectasis, postoperative apnea, negative pressure pulmonary edema, and airway or endotracheal tube obstruction from increased secretions.3
This concern has been based on the clinical observation that children with URI-related airway irritability are at a greater risk of having such events during the perioperative period.4 In fact, pulmonary function studies reveal an increase in airway irritability for as long as 6 weeks after a significant URI.
Many children with a URI will have airway edema and increased secretions in the upper nasopharynx and the posterior oropharynx down to the level of the vocal cords. In addition, patients with some viral infections—including respiratory syncytial virus—may experience increased edema in the larynx, trachea, and small and large bronchi. The presence of airway inflammation increases mucus production, which is normally coughed out in an awake patient.
The period between the awake state and surgical anesthesia—referred to as Stage 2— is the time of highest risk for the development of laryngospasm. Stage 2 occurs both during the induction of and the emergence from general anesthesia. Children who develop laryngospasm may be difficult to ventilate by mask, and tracheal intubation can be difficult through the closed glottis. In these clinically emergent situations, patients become hypoxemic rapidly. Ventilation may be possible only if the vocal cords are relaxed with agents such as succinylcholine.5
If the anesthesia team cannot quickly treat such laryngospasm, it can lead to postobstructive pulmonary edema. Negative pressure developed in the thorax during spontaneous ventilation against a closed glottis causes a pressure gradient across the alveolar-capillary membrane, leading to movement of fluid into the alveoli, characterized by a typically pink, frothy transudate. Hypoxia may ensue, and the chest x-ray will reveal pulmonary edema. Mild forms may respond to an increase in ambient oxygen alone, but severe cases may require intubation, ventilation, and diuretics to restore the child to a normal state.6
Certain anesthetic agents may be problematic
Unfortunately, airway irritability is only one of many problems to contend with. Inhalational anesthetic agents have an adverse effect on the mucociliary elevator, as well.7,8 Cilia on the surface of epithelial cells lining the trachea and bronchi act to move mucus from the distal to the proximal airway so that it can be coughed out. Failure of this mechanism in a child with an inflammatory condition in the airway increases the risk of atelectasis from thickened secretions and occasionally from pneumonia.
Most of the potent general anesthetic agents have significant bronchodilatory properties. But desflurane, a commonly used agent, causes bronchoconstriction when used in a patient with an irritated, infected airway.9This agent will produce predictable wheezing from bronchospasm, especially in patients who have confounding pulmonary disease such as asthma.
Talk to the anesthesiologist. With these concerns in mind, clinicians must consider the type of anesthetic and the nature of the surgical procedure and discuss these issues with the anesthesiologist in the preoperative period. Some anesthetic agents and techniques are less irritating to airways.2,3 Avoidance of both desflurane and endotracheal intubation, for instance, will minimize airway irritation.
Brief procedures that do not involve major body cavities (eg, abdominal, thoracic, and intracranial) may be done without instrumenting the trachea. Face masks and laryngeal mask airways have been shown to decrease the incidence of adverse reactions because these forms of airway management are less invasive and physiologically insulting than direct laryngoscopy and endotracheal intubation.
Clinical observations suggest that endotracheal intubation increases pulmonary risks for the child with a URI.10,11 Long procedures, a patient position that limits access to the airway, the anticipated need to use muscle relaxants, airway surgery, and surgery in major cavities all require intubation for airway management. In these circumstances, it’s best to plan the perioperative care of a child suffering from a URI with an anesthesiologist who is comfortable caring for pediatric patients.12
Proceeding with surgery despite the risks
During emergency procedures on infants and children, the anesthesiologist has to do the best possible job under less than ideal conditions. Bowel obstruction, an incarcerated inguinal hernia, or a foreign body in the airway can all be life-threatening. In these cases, the anesthesiologist will counsel the surgeon and parent on the risks of the anesthetic. They likely will proceed with the knowledge that the usual methods of anesthetizing a child may have to be altered to provide the safest possible conditions.
But even certain nonemergent procedures may require taking some risks. Anesthesiologists are likely to anesthetize a child for placement of pressure-equalizing (PE) tubes, for instance, even with a mild infection in the upper oropharynx. This is because the possibility is high that the patient will be infected throughout the winter season, and waiting for a URI-free period might mean that the child would not get the PE tubes at all. Furthermore, PE tube placement is performed very quickly, with no instrumentation of the airway necessary. The anesthesiologist performs a mask anesthetic, always has control and access to the airway, and the procedure can be aborted at any time, with no incision to close.
How long should you wait it a URI is serious?
As mentioned earlier, there is no consensus on how long to wait, but clinical studies have suggested delaying surgery for as long as 6 weeks after the acute episode.4 The thinking was that this long period allowed time for the inflammatory response to dissipate completely. Unfortunately, in the middle of the winter, it’s likely that the child will be exposed to another viral strain and develop yet another URI. Clinical judgment plays a pivotal role here; it is always best to establish a relationship with an anesthesiologist in your community and call him or her with questions about individual patients.
Before you sign off on surgery
There are several other circumstances to consider when approving a child with a URI for surgery.
Children with cardiac disease, especially those who have had palliative procedures for cyanotic lesions or who have a hypoplastic right or left heart, are characteristically unstable in the face of inflammatory respiratory disease. Unless the surgical procedure is an emergency, such patients should not be considered for general anesthesia if they have a URI.13 As an example, bronchiolitis plus cyanotic heart disease can be rapidly fatal, requiring prolonged ventilation or extracorporeal membrane oxygenation in order to save the patient.
Intensive care nursery “graduates” may present to your office for preoperative assessment. Many of these infants and children will have marginally compensated lung disease, some with substantial pulmonary hypertension. Their respiratory function will continue to improve, some until the age of 7 to 10 years. In the meantime, they, too, are at high risk for complications from general anesthesia if they have a URI, and the decision to take them to the operating room should be discussed with other care providers and the parents.
Children with fever, mucopurulent discharge, wheezing, lethargy, and cough are at high risk for complications during the perioperative period, regardless of any comorbidities. Many anesthesiologists would cancel surgery in these circumstances, even if the patient has been seen recently by his or her primary care physician and is taking antibiotics for coverage of a potential bacterial infection.
Other indicators of increased risk of pulmonary complications include a history of reactive airway disease, exposure to tobacco smoke, snoring, nasal congestion, the need for endotracheal intubation, and surgery on the airway.14
CASE You evaluate the 2-year-old and note that he has a history of mucopurulent nasal discharge and a productive cough. The child’s temperature in the clinic is 99.8°F and his chest x-ray is consistent with bronchitis. After talking with a local anesthesiologist and the surgeon, you all agree that the boy’s surgery should be postponed for a month.
1. American Society of Anesthesiologists Task Force on Preoperative Fasting. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896-905.
2. Parnis SJ, Barker DS, Van Der Walt JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
3. Cote CJ. The upper respiratory tract infection URI dilemma: fear of a complication or litigation? Anesthesiology. 2001;95:283-285.
4. Nandwani N, Raphael JH, Langton JA. Effect of an upper respiratory tract infection on airway reactivity. Br J Anaesth. 1997;
78:352-355.
5. Hampson-Evans D, Morgan P, Farrar M. Pediatric laryngospasm. Pediatr Anesth. 2008;18:303-307.
6. Krodel DJ, Bittner BA, Abdulnour R, et al. Case scenario: acute negative pressure pulmonary edema. Anesthesiology. 2010;113: 200-207.
7. Forbes AR. Halothane depresses mucociliary flow in the trachea. Anesthesiology. 1976;45:59-63.
8. Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane, and desflurane anaesthesia.
Anaesthesia. 2003;58:745-748.
9. Forbes AR, Horrigan RW. Mucociliary flow in the trachea during anesthesia with enflurane, ether, nitrous oxide and morphine. Anesthesiology. 1977;46:319-321.
10. Tait AR, Pandit UA, Voepel-Lewis T, et al. Use of the laryngeal mask airway in children with upper respiratory infections: a comparison with endotracheal intubation. Anesth Analg. 1998;
86:701-711.
11. Tait AR, Malviya S, Voepel-Lewis T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
12. Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in pediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773-783.
13. Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628-632.
14. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory infection: still a dilemma? Anesth Analg. 2005;100:59-65.
• Consult the anesthesiologist if a pediatric patient is about to undergo an elective surgical procedure and is febrile or coughing—especially if the child has significant comorbidities. These conditions may warrant postponing the procedure. A
• Avoid surgery in a child with cardiac disease who has inflammatory respiratory disease—especially if he or she has had palliative procedures for cyanotic lesions or has a hypoplastic right or left heart. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE You are seeing a 2-year-old boy with a runny nose in your busy clinic. He was sent to you by a local surgeon who plans to repair a hernia 3 days from now. Other than the upper respiratory tract infection, the child is healthy. The surgeon wants you to clear the boy for surgery to avoid the possibility of the anesthesiologist canceling on the day of the procedure.
What are your next steps?
In our experience, children are regularly brought to the ambulatory surgery suite after having seen their family physician or pediatrician. To better equip you for such visits, we’ve put together the following summary of the risks for a child with an upper respiratory infection (URI) who is about to undergo surgery. We’ve also detailed some of the reasoning and evidence behind the decisions that anesthesiologists make in cases like this.
Making decisions in the absence of consensus
While the American Society of Anesthesiologists has a consensus statement on preoperative fasting to reduce the risk of pulmonary aspiration,1 there is no consensus on how to manage a child scheduled for elective surgery who develops a URI.
Historically, any child with a current or recent URI would not be considered a candidate for elective surgery due to the potential for respiratory complications caused by airway irritability.2 These complications can include bronchospasm, laryngospasm, hypoxemia, croup, pulmonary shunting, atelectasis, postoperative apnea, negative pressure pulmonary edema, and airway or endotracheal tube obstruction from increased secretions.3
This concern has been based on the clinical observation that children with URI-related airway irritability are at a greater risk of having such events during the perioperative period.4 In fact, pulmonary function studies reveal an increase in airway irritability for as long as 6 weeks after a significant URI.
Many children with a URI will have airway edema and increased secretions in the upper nasopharynx and the posterior oropharynx down to the level of the vocal cords. In addition, patients with some viral infections—including respiratory syncytial virus—may experience increased edema in the larynx, trachea, and small and large bronchi. The presence of airway inflammation increases mucus production, which is normally coughed out in an awake patient.
The period between the awake state and surgical anesthesia—referred to as Stage 2— is the time of highest risk for the development of laryngospasm. Stage 2 occurs both during the induction of and the emergence from general anesthesia. Children who develop laryngospasm may be difficult to ventilate by mask, and tracheal intubation can be difficult through the closed glottis. In these clinically emergent situations, patients become hypoxemic rapidly. Ventilation may be possible only if the vocal cords are relaxed with agents such as succinylcholine.5
If the anesthesia team cannot quickly treat such laryngospasm, it can lead to postobstructive pulmonary edema. Negative pressure developed in the thorax during spontaneous ventilation against a closed glottis causes a pressure gradient across the alveolar-capillary membrane, leading to movement of fluid into the alveoli, characterized by a typically pink, frothy transudate. Hypoxia may ensue, and the chest x-ray will reveal pulmonary edema. Mild forms may respond to an increase in ambient oxygen alone, but severe cases may require intubation, ventilation, and diuretics to restore the child to a normal state.6
Certain anesthetic agents may be problematic
Unfortunately, airway irritability is only one of many problems to contend with. Inhalational anesthetic agents have an adverse effect on the mucociliary elevator, as well.7,8 Cilia on the surface of epithelial cells lining the trachea and bronchi act to move mucus from the distal to the proximal airway so that it can be coughed out. Failure of this mechanism in a child with an inflammatory condition in the airway increases the risk of atelectasis from thickened secretions and occasionally from pneumonia.
Most of the potent general anesthetic agents have significant bronchodilatory properties. But desflurane, a commonly used agent, causes bronchoconstriction when used in a patient with an irritated, infected airway.9This agent will produce predictable wheezing from bronchospasm, especially in patients who have confounding pulmonary disease such as asthma.
Talk to the anesthesiologist. With these concerns in mind, clinicians must consider the type of anesthetic and the nature of the surgical procedure and discuss these issues with the anesthesiologist in the preoperative period. Some anesthetic agents and techniques are less irritating to airways.2,3 Avoidance of both desflurane and endotracheal intubation, for instance, will minimize airway irritation.
Brief procedures that do not involve major body cavities (eg, abdominal, thoracic, and intracranial) may be done without instrumenting the trachea. Face masks and laryngeal mask airways have been shown to decrease the incidence of adverse reactions because these forms of airway management are less invasive and physiologically insulting than direct laryngoscopy and endotracheal intubation.
Clinical observations suggest that endotracheal intubation increases pulmonary risks for the child with a URI.10,11 Long procedures, a patient position that limits access to the airway, the anticipated need to use muscle relaxants, airway surgery, and surgery in major cavities all require intubation for airway management. In these circumstances, it’s best to plan the perioperative care of a child suffering from a URI with an anesthesiologist who is comfortable caring for pediatric patients.12
Proceeding with surgery despite the risks
During emergency procedures on infants and children, the anesthesiologist has to do the best possible job under less than ideal conditions. Bowel obstruction, an incarcerated inguinal hernia, or a foreign body in the airway can all be life-threatening. In these cases, the anesthesiologist will counsel the surgeon and parent on the risks of the anesthetic. They likely will proceed with the knowledge that the usual methods of anesthetizing a child may have to be altered to provide the safest possible conditions.
But even certain nonemergent procedures may require taking some risks. Anesthesiologists are likely to anesthetize a child for placement of pressure-equalizing (PE) tubes, for instance, even with a mild infection in the upper oropharynx. This is because the possibility is high that the patient will be infected throughout the winter season, and waiting for a URI-free period might mean that the child would not get the PE tubes at all. Furthermore, PE tube placement is performed very quickly, with no instrumentation of the airway necessary. The anesthesiologist performs a mask anesthetic, always has control and access to the airway, and the procedure can be aborted at any time, with no incision to close.
How long should you wait it a URI is serious?
As mentioned earlier, there is no consensus on how long to wait, but clinical studies have suggested delaying surgery for as long as 6 weeks after the acute episode.4 The thinking was that this long period allowed time for the inflammatory response to dissipate completely. Unfortunately, in the middle of the winter, it’s likely that the child will be exposed to another viral strain and develop yet another URI. Clinical judgment plays a pivotal role here; it is always best to establish a relationship with an anesthesiologist in your community and call him or her with questions about individual patients.
Before you sign off on surgery
There are several other circumstances to consider when approving a child with a URI for surgery.
Children with cardiac disease, especially those who have had palliative procedures for cyanotic lesions or who have a hypoplastic right or left heart, are characteristically unstable in the face of inflammatory respiratory disease. Unless the surgical procedure is an emergency, such patients should not be considered for general anesthesia if they have a URI.13 As an example, bronchiolitis plus cyanotic heart disease can be rapidly fatal, requiring prolonged ventilation or extracorporeal membrane oxygenation in order to save the patient.
Intensive care nursery “graduates” may present to your office for preoperative assessment. Many of these infants and children will have marginally compensated lung disease, some with substantial pulmonary hypertension. Their respiratory function will continue to improve, some until the age of 7 to 10 years. In the meantime, they, too, are at high risk for complications from general anesthesia if they have a URI, and the decision to take them to the operating room should be discussed with other care providers and the parents.
Children with fever, mucopurulent discharge, wheezing, lethargy, and cough are at high risk for complications during the perioperative period, regardless of any comorbidities. Many anesthesiologists would cancel surgery in these circumstances, even if the patient has been seen recently by his or her primary care physician and is taking antibiotics for coverage of a potential bacterial infection.
Other indicators of increased risk of pulmonary complications include a history of reactive airway disease, exposure to tobacco smoke, snoring, nasal congestion, the need for endotracheal intubation, and surgery on the airway.14
CASE You evaluate the 2-year-old and note that he has a history of mucopurulent nasal discharge and a productive cough. The child’s temperature in the clinic is 99.8°F and his chest x-ray is consistent with bronchitis. After talking with a local anesthesiologist and the surgeon, you all agree that the boy’s surgery should be postponed for a month.
• Consult the anesthesiologist if a pediatric patient is about to undergo an elective surgical procedure and is febrile or coughing—especially if the child has significant comorbidities. These conditions may warrant postponing the procedure. A
• Avoid surgery in a child with cardiac disease who has inflammatory respiratory disease—especially if he or she has had palliative procedures for cyanotic lesions or has a hypoplastic right or left heart. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE You are seeing a 2-year-old boy with a runny nose in your busy clinic. He was sent to you by a local surgeon who plans to repair a hernia 3 days from now. Other than the upper respiratory tract infection, the child is healthy. The surgeon wants you to clear the boy for surgery to avoid the possibility of the anesthesiologist canceling on the day of the procedure.
What are your next steps?
In our experience, children are regularly brought to the ambulatory surgery suite after having seen their family physician or pediatrician. To better equip you for such visits, we’ve put together the following summary of the risks for a child with an upper respiratory infection (URI) who is about to undergo surgery. We’ve also detailed some of the reasoning and evidence behind the decisions that anesthesiologists make in cases like this.
Making decisions in the absence of consensus
While the American Society of Anesthesiologists has a consensus statement on preoperative fasting to reduce the risk of pulmonary aspiration,1 there is no consensus on how to manage a child scheduled for elective surgery who develops a URI.
Historically, any child with a current or recent URI would not be considered a candidate for elective surgery due to the potential for respiratory complications caused by airway irritability.2 These complications can include bronchospasm, laryngospasm, hypoxemia, croup, pulmonary shunting, atelectasis, postoperative apnea, negative pressure pulmonary edema, and airway or endotracheal tube obstruction from increased secretions.3
This concern has been based on the clinical observation that children with URI-related airway irritability are at a greater risk of having such events during the perioperative period.4 In fact, pulmonary function studies reveal an increase in airway irritability for as long as 6 weeks after a significant URI.
Many children with a URI will have airway edema and increased secretions in the upper nasopharynx and the posterior oropharynx down to the level of the vocal cords. In addition, patients with some viral infections—including respiratory syncytial virus—may experience increased edema in the larynx, trachea, and small and large bronchi. The presence of airway inflammation increases mucus production, which is normally coughed out in an awake patient.
The period between the awake state and surgical anesthesia—referred to as Stage 2— is the time of highest risk for the development of laryngospasm. Stage 2 occurs both during the induction of and the emergence from general anesthesia. Children who develop laryngospasm may be difficult to ventilate by mask, and tracheal intubation can be difficult through the closed glottis. In these clinically emergent situations, patients become hypoxemic rapidly. Ventilation may be possible only if the vocal cords are relaxed with agents such as succinylcholine.5
If the anesthesia team cannot quickly treat such laryngospasm, it can lead to postobstructive pulmonary edema. Negative pressure developed in the thorax during spontaneous ventilation against a closed glottis causes a pressure gradient across the alveolar-capillary membrane, leading to movement of fluid into the alveoli, characterized by a typically pink, frothy transudate. Hypoxia may ensue, and the chest x-ray will reveal pulmonary edema. Mild forms may respond to an increase in ambient oxygen alone, but severe cases may require intubation, ventilation, and diuretics to restore the child to a normal state.6
Certain anesthetic agents may be problematic
Unfortunately, airway irritability is only one of many problems to contend with. Inhalational anesthetic agents have an adverse effect on the mucociliary elevator, as well.7,8 Cilia on the surface of epithelial cells lining the trachea and bronchi act to move mucus from the distal to the proximal airway so that it can be coughed out. Failure of this mechanism in a child with an inflammatory condition in the airway increases the risk of atelectasis from thickened secretions and occasionally from pneumonia.
Most of the potent general anesthetic agents have significant bronchodilatory properties. But desflurane, a commonly used agent, causes bronchoconstriction when used in a patient with an irritated, infected airway.9This agent will produce predictable wheezing from bronchospasm, especially in patients who have confounding pulmonary disease such as asthma.
Talk to the anesthesiologist. With these concerns in mind, clinicians must consider the type of anesthetic and the nature of the surgical procedure and discuss these issues with the anesthesiologist in the preoperative period. Some anesthetic agents and techniques are less irritating to airways.2,3 Avoidance of both desflurane and endotracheal intubation, for instance, will minimize airway irritation.
Brief procedures that do not involve major body cavities (eg, abdominal, thoracic, and intracranial) may be done without instrumenting the trachea. Face masks and laryngeal mask airways have been shown to decrease the incidence of adverse reactions because these forms of airway management are less invasive and physiologically insulting than direct laryngoscopy and endotracheal intubation.
Clinical observations suggest that endotracheal intubation increases pulmonary risks for the child with a URI.10,11 Long procedures, a patient position that limits access to the airway, the anticipated need to use muscle relaxants, airway surgery, and surgery in major cavities all require intubation for airway management. In these circumstances, it’s best to plan the perioperative care of a child suffering from a URI with an anesthesiologist who is comfortable caring for pediatric patients.12
Proceeding with surgery despite the risks
During emergency procedures on infants and children, the anesthesiologist has to do the best possible job under less than ideal conditions. Bowel obstruction, an incarcerated inguinal hernia, or a foreign body in the airway can all be life-threatening. In these cases, the anesthesiologist will counsel the surgeon and parent on the risks of the anesthetic. They likely will proceed with the knowledge that the usual methods of anesthetizing a child may have to be altered to provide the safest possible conditions.
But even certain nonemergent procedures may require taking some risks. Anesthesiologists are likely to anesthetize a child for placement of pressure-equalizing (PE) tubes, for instance, even with a mild infection in the upper oropharynx. This is because the possibility is high that the patient will be infected throughout the winter season, and waiting for a URI-free period might mean that the child would not get the PE tubes at all. Furthermore, PE tube placement is performed very quickly, with no instrumentation of the airway necessary. The anesthesiologist performs a mask anesthetic, always has control and access to the airway, and the procedure can be aborted at any time, with no incision to close.
How long should you wait it a URI is serious?
As mentioned earlier, there is no consensus on how long to wait, but clinical studies have suggested delaying surgery for as long as 6 weeks after the acute episode.4 The thinking was that this long period allowed time for the inflammatory response to dissipate completely. Unfortunately, in the middle of the winter, it’s likely that the child will be exposed to another viral strain and develop yet another URI. Clinical judgment plays a pivotal role here; it is always best to establish a relationship with an anesthesiologist in your community and call him or her with questions about individual patients.
Before you sign off on surgery
There are several other circumstances to consider when approving a child with a URI for surgery.
Children with cardiac disease, especially those who have had palliative procedures for cyanotic lesions or who have a hypoplastic right or left heart, are characteristically unstable in the face of inflammatory respiratory disease. Unless the surgical procedure is an emergency, such patients should not be considered for general anesthesia if they have a URI.13 As an example, bronchiolitis plus cyanotic heart disease can be rapidly fatal, requiring prolonged ventilation or extracorporeal membrane oxygenation in order to save the patient.
Intensive care nursery “graduates” may present to your office for preoperative assessment. Many of these infants and children will have marginally compensated lung disease, some with substantial pulmonary hypertension. Their respiratory function will continue to improve, some until the age of 7 to 10 years. In the meantime, they, too, are at high risk for complications from general anesthesia if they have a URI, and the decision to take them to the operating room should be discussed with other care providers and the parents.
Children with fever, mucopurulent discharge, wheezing, lethargy, and cough are at high risk for complications during the perioperative period, regardless of any comorbidities. Many anesthesiologists would cancel surgery in these circumstances, even if the patient has been seen recently by his or her primary care physician and is taking antibiotics for coverage of a potential bacterial infection.
Other indicators of increased risk of pulmonary complications include a history of reactive airway disease, exposure to tobacco smoke, snoring, nasal congestion, the need for endotracheal intubation, and surgery on the airway.14
CASE You evaluate the 2-year-old and note that he has a history of mucopurulent nasal discharge and a productive cough. The child’s temperature in the clinic is 99.8°F and his chest x-ray is consistent with bronchitis. After talking with a local anesthesiologist and the surgeon, you all agree that the boy’s surgery should be postponed for a month.
1. American Society of Anesthesiologists Task Force on Preoperative Fasting. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896-905.
2. Parnis SJ, Barker DS, Van Der Walt JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
3. Cote CJ. The upper respiratory tract infection URI dilemma: fear of a complication or litigation? Anesthesiology. 2001;95:283-285.
4. Nandwani N, Raphael JH, Langton JA. Effect of an upper respiratory tract infection on airway reactivity. Br J Anaesth. 1997;
78:352-355.
5. Hampson-Evans D, Morgan P, Farrar M. Pediatric laryngospasm. Pediatr Anesth. 2008;18:303-307.
6. Krodel DJ, Bittner BA, Abdulnour R, et al. Case scenario: acute negative pressure pulmonary edema. Anesthesiology. 2010;113: 200-207.
7. Forbes AR. Halothane depresses mucociliary flow in the trachea. Anesthesiology. 1976;45:59-63.
8. Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane, and desflurane anaesthesia.
Anaesthesia. 2003;58:745-748.
9. Forbes AR, Horrigan RW. Mucociliary flow in the trachea during anesthesia with enflurane, ether, nitrous oxide and morphine. Anesthesiology. 1977;46:319-321.
10. Tait AR, Pandit UA, Voepel-Lewis T, et al. Use of the laryngeal mask airway in children with upper respiratory infections: a comparison with endotracheal intubation. Anesth Analg. 1998;
86:701-711.
11. Tait AR, Malviya S, Voepel-Lewis T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
12. Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in pediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773-783.
13. Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628-632.
14. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory infection: still a dilemma? Anesth Analg. 2005;100:59-65.
1. American Society of Anesthesiologists Task Force on Preoperative Fasting. Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures. Anesthesiology. 1999;90:896-905.
2. Parnis SJ, Barker DS, Van Der Walt JH. Clinical predictors of anaesthetic complications in children with respiratory tract infections. Paediatr Anaesth. 2001;11:29-40.
3. Cote CJ. The upper respiratory tract infection URI dilemma: fear of a complication or litigation? Anesthesiology. 2001;95:283-285.
4. Nandwani N, Raphael JH, Langton JA. Effect of an upper respiratory tract infection on airway reactivity. Br J Anaesth. 1997;
78:352-355.
5. Hampson-Evans D, Morgan P, Farrar M. Pediatric laryngospasm. Pediatr Anesth. 2008;18:303-307.
6. Krodel DJ, Bittner BA, Abdulnour R, et al. Case scenario: acute negative pressure pulmonary edema. Anesthesiology. 2010;113: 200-207.
7. Forbes AR. Halothane depresses mucociliary flow in the trachea. Anesthesiology. 1976;45:59-63.
8. Dikmen Y, Eminoglu E, Salihoglu Z, et al. Pulmonary mechanics during isoflurane, sevoflurane, and desflurane anaesthesia.
Anaesthesia. 2003;58:745-748.
9. Forbes AR, Horrigan RW. Mucociliary flow in the trachea during anesthesia with enflurane, ether, nitrous oxide and morphine. Anesthesiology. 1977;46:319-321.
10. Tait AR, Pandit UA, Voepel-Lewis T, et al. Use of the laryngeal mask airway in children with upper respiratory infections: a comparison with endotracheal intubation. Anesth Analg. 1998;
86:701-711.
11. Tait AR, Malviya S, Voepel-Lewis T, et al. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299-306.
12. Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in pediatric anaesthesia: a prospective cohort study. Lancet. 2010;376:773-783.
13. Malviya S, Voepel-Lewis T, Siewert M, et al. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628-632.
14. Tait AR, Malviya S. Anesthesia for the child with an upper respiratory infection: still a dilemma? Anesth Analg. 2005;100:59-65.
Asthma medications
One of the most common conditions that complicate pregnancy is maternal asthma. Evidence continues to mount that adequate control of asthma, including appropriate use of medications, is the best approach to optimizing outcomes. Yet questions persist about the effect of asthma itself, as well as specific medications and the risk for major congenital malformations. As with any exposure during pregnancy, answering these questions is challenging because of the rarity of specific birth defects and the various and increasing number of medications that might be used to treat or prevent asthma symptoms.
Previously in this column ("Beta2-agonists for asthma, December 2011), we reviewed two studies that suggested short-acting beta-agonists used for the treatment of asthma were associated with an increased risk of oral clefts and that long-acting beta-agonists might be associated with an increased risk of cardiac anomalies (Hum. Reprod. 2011;26:3147-54; Birth Defects Res. A. Clin. Mol. Teratol. 2011;91:937-47).
What have we learned since then? Two studies published in 2013 add to the body of knowledge. The first, a database analysis using the United Kingdom’s General Practice Research Database, assessed pregnancy outcomes between 1991 and 2002 in 7,911 women exposed to asthma medications in the first trimester of pregnancy and 15,840 women who were not exposed (Pharmacotherapy 2013;33:363-8). Major anomalies were identified up to 1 year of age. Minor anomalies, chromosomal anomalies, and those associated with prematurity were excluded.
The overall risk for any exposure, compared with no exposure, for any congenital anomaly was 1.1 (95% confidence interval [CI], 1.0-1.3). No significant differences were found by class of asthma medication. Specific categories of defects also were evaluated, including musculoskeletal anomalies, oral clefts, cardiovascular defects, and multiple anomalies. Some estimates were elevated for specific medication classes.
For example, the relative risk (RR) of cleft lip or palate associated with exposure to long-acting beta-agonists was 2.4, but the confidence interval included 1 (0.3-21.8) based on 424 exposed pregnancies. The authors concluded that they found no significant increased risk of congenital anomalies associated with exposure to asthma, asthma medications, or any specific asthma medication classes in the first trimester. Limitations of the study included the inability to verify that exposure took place and insufficient data to adjust for confounding by vitamin supplementation, alcohol use, socioeconomic status, or markers of disease severity (other than number of medications prescribed).
The second study addressed the issue of maternal asthma itself and the risk for congenital anomalies (BJOG 2013;120:812-22). Using a meta-analysis approach, 21 cohort studies published between 1975 and 2012 met the criteria for inclusion. Combining major and minor congenital anomalies, the authors found a slight but statistically significant increased risk for any defect (RR, 1.11; 95% CI, 1.01-1.68), but when the analysis was restricted to major defects alone, the summary estimate was elevated but no longer significant (RR, 1.31; 95% CI, 0.57-3.02). When the specific defect grouping of oral clefts was examined, however, there was a significantly elevated overall relative risk of 1.30 (95% CI, 1.01-1.68). Limitations of this study include differing quality of studies and the appropriateness of combining data to derive a summary estimate.
Although both studies provide reassurance about the overall risk of major defects in the offspring of women with asthma, both suggest that more work needs to be done to follow up on the risk for oral clefts and whether this is linked to underlying disease severity and/or use of specific medications. Furthermore, safety of specific long-acting beta-agonist medications in pregnancy should be further examined.
The take-home message, however, continues to be that the risk for major defects in the offspring of pregnant women with asthma appears to be low, which supports the recommendation to follow guidelines for appropriate treatment of women with asthma both during and outside of pregnancy to control symptoms.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said that she had no relevant financial disclosures.
One of the most common conditions that complicate pregnancy is maternal asthma. Evidence continues to mount that adequate control of asthma, including appropriate use of medications, is the best approach to optimizing outcomes. Yet questions persist about the effect of asthma itself, as well as specific medications and the risk for major congenital malformations. As with any exposure during pregnancy, answering these questions is challenging because of the rarity of specific birth defects and the various and increasing number of medications that might be used to treat or prevent asthma symptoms.
Previously in this column ("Beta2-agonists for asthma, December 2011), we reviewed two studies that suggested short-acting beta-agonists used for the treatment of asthma were associated with an increased risk of oral clefts and that long-acting beta-agonists might be associated with an increased risk of cardiac anomalies (Hum. Reprod. 2011;26:3147-54; Birth Defects Res. A. Clin. Mol. Teratol. 2011;91:937-47).
What have we learned since then? Two studies published in 2013 add to the body of knowledge. The first, a database analysis using the United Kingdom’s General Practice Research Database, assessed pregnancy outcomes between 1991 and 2002 in 7,911 women exposed to asthma medications in the first trimester of pregnancy and 15,840 women who were not exposed (Pharmacotherapy 2013;33:363-8). Major anomalies were identified up to 1 year of age. Minor anomalies, chromosomal anomalies, and those associated with prematurity were excluded.
The overall risk for any exposure, compared with no exposure, for any congenital anomaly was 1.1 (95% confidence interval [CI], 1.0-1.3). No significant differences were found by class of asthma medication. Specific categories of defects also were evaluated, including musculoskeletal anomalies, oral clefts, cardiovascular defects, and multiple anomalies. Some estimates were elevated for specific medication classes.
For example, the relative risk (RR) of cleft lip or palate associated with exposure to long-acting beta-agonists was 2.4, but the confidence interval included 1 (0.3-21.8) based on 424 exposed pregnancies. The authors concluded that they found no significant increased risk of congenital anomalies associated with exposure to asthma, asthma medications, or any specific asthma medication classes in the first trimester. Limitations of the study included the inability to verify that exposure took place and insufficient data to adjust for confounding by vitamin supplementation, alcohol use, socioeconomic status, or markers of disease severity (other than number of medications prescribed).
The second study addressed the issue of maternal asthma itself and the risk for congenital anomalies (BJOG 2013;120:812-22). Using a meta-analysis approach, 21 cohort studies published between 1975 and 2012 met the criteria for inclusion. Combining major and minor congenital anomalies, the authors found a slight but statistically significant increased risk for any defect (RR, 1.11; 95% CI, 1.01-1.68), but when the analysis was restricted to major defects alone, the summary estimate was elevated but no longer significant (RR, 1.31; 95% CI, 0.57-3.02). When the specific defect grouping of oral clefts was examined, however, there was a significantly elevated overall relative risk of 1.30 (95% CI, 1.01-1.68). Limitations of this study include differing quality of studies and the appropriateness of combining data to derive a summary estimate.
Although both studies provide reassurance about the overall risk of major defects in the offspring of women with asthma, both suggest that more work needs to be done to follow up on the risk for oral clefts and whether this is linked to underlying disease severity and/or use of specific medications. Furthermore, safety of specific long-acting beta-agonist medications in pregnancy should be further examined.
The take-home message, however, continues to be that the risk for major defects in the offspring of pregnant women with asthma appears to be low, which supports the recommendation to follow guidelines for appropriate treatment of women with asthma both during and outside of pregnancy to control symptoms.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said that she had no relevant financial disclosures.
One of the most common conditions that complicate pregnancy is maternal asthma. Evidence continues to mount that adequate control of asthma, including appropriate use of medications, is the best approach to optimizing outcomes. Yet questions persist about the effect of asthma itself, as well as specific medications and the risk for major congenital malformations. As with any exposure during pregnancy, answering these questions is challenging because of the rarity of specific birth defects and the various and increasing number of medications that might be used to treat or prevent asthma symptoms.
Previously in this column ("Beta2-agonists for asthma, December 2011), we reviewed two studies that suggested short-acting beta-agonists used for the treatment of asthma were associated with an increased risk of oral clefts and that long-acting beta-agonists might be associated with an increased risk of cardiac anomalies (Hum. Reprod. 2011;26:3147-54; Birth Defects Res. A. Clin. Mol. Teratol. 2011;91:937-47).
What have we learned since then? Two studies published in 2013 add to the body of knowledge. The first, a database analysis using the United Kingdom’s General Practice Research Database, assessed pregnancy outcomes between 1991 and 2002 in 7,911 women exposed to asthma medications in the first trimester of pregnancy and 15,840 women who were not exposed (Pharmacotherapy 2013;33:363-8). Major anomalies were identified up to 1 year of age. Minor anomalies, chromosomal anomalies, and those associated with prematurity were excluded.
The overall risk for any exposure, compared with no exposure, for any congenital anomaly was 1.1 (95% confidence interval [CI], 1.0-1.3). No significant differences were found by class of asthma medication. Specific categories of defects also were evaluated, including musculoskeletal anomalies, oral clefts, cardiovascular defects, and multiple anomalies. Some estimates were elevated for specific medication classes.
For example, the relative risk (RR) of cleft lip or palate associated with exposure to long-acting beta-agonists was 2.4, but the confidence interval included 1 (0.3-21.8) based on 424 exposed pregnancies. The authors concluded that they found no significant increased risk of congenital anomalies associated with exposure to asthma, asthma medications, or any specific asthma medication classes in the first trimester. Limitations of the study included the inability to verify that exposure took place and insufficient data to adjust for confounding by vitamin supplementation, alcohol use, socioeconomic status, or markers of disease severity (other than number of medications prescribed).
The second study addressed the issue of maternal asthma itself and the risk for congenital anomalies (BJOG 2013;120:812-22). Using a meta-analysis approach, 21 cohort studies published between 1975 and 2012 met the criteria for inclusion. Combining major and minor congenital anomalies, the authors found a slight but statistically significant increased risk for any defect (RR, 1.11; 95% CI, 1.01-1.68), but when the analysis was restricted to major defects alone, the summary estimate was elevated but no longer significant (RR, 1.31; 95% CI, 0.57-3.02). When the specific defect grouping of oral clefts was examined, however, there was a significantly elevated overall relative risk of 1.30 (95% CI, 1.01-1.68). Limitations of this study include differing quality of studies and the appropriateness of combining data to derive a summary estimate.
Although both studies provide reassurance about the overall risk of major defects in the offspring of women with asthma, both suggest that more work needs to be done to follow up on the risk for oral clefts and whether this is linked to underlying disease severity and/or use of specific medications. Furthermore, safety of specific long-acting beta-agonist medications in pregnancy should be further examined.
The take-home message, however, continues to be that the risk for major defects in the offspring of pregnant women with asthma appears to be low, which supports the recommendation to follow guidelines for appropriate treatment of women with asthma both during and outside of pregnancy to control symptoms.
Dr. Chambers is professor of pediatrics and family and preventive medicine at the University of California, San Diego. She is director of the California Teratogen Information Service and Clinical Research Program. Dr. Chambers is a past president of the Organization of Teratology Information Specialists and past president of the Teratology Society. She said that she had no relevant financial disclosures.
Cough and Back Pain in a Man With COPD
ANSWER
The radiograph shows some evidence of hyperinflated lungs, consistent with COPD. There is a small right effusion evident.
Of note is a superior mediastinal mass, which is causing right-sided and anterior displacement of the intrathoracic trachea. The differential includes possible adenopathy related to a carcinoma or a substernal goiter. Further diagnostic studies and surgical evaluation are warranted.
In this particular case, review of the patient’s imaging history showed he had a chest radiograph two years ago, at which time these findings were present. This favors substernal goiter as the diagnosis. Multinodular goiter was later confirmed with a thyroid ultrasound, and referral to general surgery was made.
ANSWER
The radiograph shows some evidence of hyperinflated lungs, consistent with COPD. There is a small right effusion evident.
Of note is a superior mediastinal mass, which is causing right-sided and anterior displacement of the intrathoracic trachea. The differential includes possible adenopathy related to a carcinoma or a substernal goiter. Further diagnostic studies and surgical evaluation are warranted.
In this particular case, review of the patient’s imaging history showed he had a chest radiograph two years ago, at which time these findings were present. This favors substernal goiter as the diagnosis. Multinodular goiter was later confirmed with a thyroid ultrasound, and referral to general surgery was made.
ANSWER
The radiograph shows some evidence of hyperinflated lungs, consistent with COPD. There is a small right effusion evident.
Of note is a superior mediastinal mass, which is causing right-sided and anterior displacement of the intrathoracic trachea. The differential includes possible adenopathy related to a carcinoma or a substernal goiter. Further diagnostic studies and surgical evaluation are warranted.
In this particular case, review of the patient’s imaging history showed he had a chest radiograph two years ago, at which time these findings were present. This favors substernal goiter as the diagnosis. Multinodular goiter was later confirmed with a thyroid ultrasound, and referral to general surgery was made.
A 60-year-old man presents for evaluation of fever, cough, and back pain. His symptoms have been intermittent but have worsened over the past month or so. He has had no treatment prior to today’s visit. His medical history is significant for hypertension, COPD, and chronic renal insufficiency. He denies any history of tobacco use. On physical exam, you see an older man in no obvious distress. His vital signs are stable. He is afe-brile, with a blood pressure of 150/90 mm Hg, a heart rate of 66 beats/min, and a respiratory rate of 18 breaths/min. His O2 saturation is 98% on room air. His neck is supple, with no evidence of ade-nopathy. Lung sounds are slightly decreased bilaterally, with a few crackles heard. The rest of his physical exam, overall, is normal. You order preliminary lab work as well as a chest radiograph (shown). What is your impression?