User login
Help patients control their asthma
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
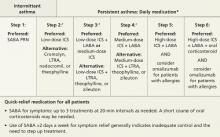
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; [email protected]
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; [email protected]
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; [email protected]
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
Allergic rhinitis: What’s best for your patient?
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
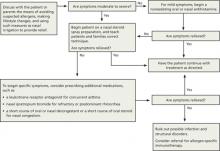
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]
A spoonful of honey helps a coughing child sleep
When a parent brings in a child (ages 1-5 years) with cough, runny nose, and other symptoms of a viral upper respiratory infection (URI), recommend that honey be given at bedtime.1
STRENGTH OF RECOMMENDATION
A: Based on a well-designed, randomized controlled trial (RCT)
Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130:465-471.
ILLUSTRATIVE CASE
A mother brings in her 18-month-old son because he’s had a runny nose and low-grade fever for the past 4 days—and a cough that kept them both up last night. You diagnose a viral URI, and she requests a strong cough medicine so he (and she) can get a good night’s sleep. What can you recommend that is both safe and effective for a child of this age?
For primary care physicians, office visits for coughing kids with URIs are commonplace. In addition to the cost of such visits, Americans spend some $3.5 billion a year on over-the-counter (OTC) cough and cold remedies, and often give them to young children.
It’s not enough to tell parents what not to do
As physicians (and parents), we understand the desire to give a coughing child something to ease the symptoms. We also know that OTC cough and cold medications can lead to serious complications, and even death. Between 1983 and 2007, 118 pediatric deaths were attributed to the misuse of such preparations.2 And, in a 3-year span (2005-2008), the American Association of Poison Control Centers received 64,658 calls for exposures to cough and cold remedies in children younger than 2 years of age, 28 of which resulted in a major adverse reaction or death.3
The US Food and Drug Administration recommends against the use of OTC cough and cold medications in children younger than 2 years,4 and the American Academy of Pediatrics has issued strict warnings about the use of OTC cough and cold preparations in children younger than 6 years.5 But warning parents of the dangers of giving them to young children without offering an alternative doesn’t satisfy anyone’s needs, and many parents continue to use these medications.
What about honey?
A study published in 2007 evaluated buckwheat honey and found it to be superior to no treatment and equal to honey-flavored dextromethorphan in reducing cough severity and improving sleep for children and their parents.6 Honey is known to have both antioxidant and antimicrobial properties—a possible scientific explanation for its effect. Before recommending honey for kids with URIs, however, more evidence of its efficacy was needed.
STUDY SUMMARY: Honey reduces cough frequency and severity
Cohen et al sought to determine whether honey, administered before bedtime, would decrease coughing in children between the ages of one and 5 years—and improve sleep for both the children and their caregivers.1 They enrolled 300 children with a nocturnal cough of <7 days’ duration and a diagnosis of URI in a one-night study.
Children were excluded if they had any signs or symptoms of asthma, pneumonia, sinusitis, allergic rhinitis, or laryngotracheobronchitis, or if they had been given any cough remedy, including honey, the night before. Parents completed a 5-question survey, using a 7-point Likert scale to assess the child’s cough and both the child’s and parents’ sleep the previous night. Only children whose parents rated their child’s cough severity ≥3 in 2 of the 3 questions related to cough were included in the trial.
The study had a double-blind randomized design, with 4 treatment arms. Three groups received 10 g (about 1.5 tsp) of one of 3 types of honey: eucalyptus, citrus, or labiatae (derived from plants including sage, mint, and thyme); the fourth group received a placebo of silan date extract, which is similar to honey in color, texture, and taste.
Children in all 4 groups received the preparation 30 minutes before bedtime. Neither the parents nor the physicians or study coordinators knew which preparation the children received. The following day, research assistants telephoned the parent who had completed the initial survey and asked the same 5 questions. The primary outcome measure was the change in cough frequency from the night before to the night after treatment. Secondary measures included cough severity and the effect on sleep for both the child and the parent.
Of the 300 children initially enrolled, 270 (90%) completed the trial, with an even distribution among the groups. While there were improvements across all outcomes for both the treatment and placebo groups, the changes were statistically significant only in the treatment groups. There were no significant differences in efficacy noted among the 3 types of honey. Adverse effects of stomachache, nausea, or vomiting were noted by 4 parents in the treatment groups and one in the placebo group, a difference that was not statistically significant.
WHAT’S NEW?: We have more evidence of honey’s efficacy
For children older than one year with a viral URI, we can now recommend 1.5 tsp honey to be given prior to bedtime as a cough remedy. This may reduce the use of potentially harmful and often ineffective OTC cough and cold remedies.
CAVEATS: Honey is unsafe for the youngest children
An obvious limitation of this study was its brevity. Although one night of improved cough and sleep is important, a study that showed honey’s sustained benefit as a cough suppressant would be more convincing. What’s more, there are safety concerns that are age-related.
Honey is considered unsafe for children younger than one year because of the risk of botulism. And honey has the potential to increase dental caries if it is given nightly for a prolonged period of time.
We do not know whether all varieties of honey will have the same benefit, and the source of store-bought honey is not always identified. The authors of this study received funding from the Honey Board of Israel.
CHALLENGES TO IMPLEMENTATION: Parents may be reluctant to abandon OTCs
Changing the behavior of parents and other caregivers who are accustomed to treating children with OTC cough and cold remedies is likely to be an uphill battle. Because honey is readily available, however—often as close as the pantry—and perceived to be safe and nutritious, a recommendation from a trusted physician could go a long way toward its implementation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130:465-471.
2. Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med. 2009;53:411-417.
3. Srinivasan A, Budnitz D, Shehab N, et al. Infant deaths associated with cough and cold medications—two states, 2005. JAMA. 2007;297:800-801.
4. US Food and Drug Administration. Public Health Advisory: FDA recommends that over-the-counter (OTC) cough and cold products not be used for Infants and children under 2 years of age. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051137.htm. Accessed February 14, 2013.
5. American Academy of Pediatrics. Withdrawal of cold medicines: addressing parent concerns. Available at: http://www.aap.org/en-us/professional-resources/practice-support/Pages/Withdrawal-of-Cold-Medicines-Addressing-Parent-Concerns.aspx. Accessed February 14, 2013.
6. Paul IM, Beiler J, McMonagle A, et al. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161:1140-1146.
When a parent brings in a child (ages 1-5 years) with cough, runny nose, and other symptoms of a viral upper respiratory infection (URI), recommend that honey be given at bedtime.1
STRENGTH OF RECOMMENDATION
A: Based on a well-designed, randomized controlled trial (RCT)
Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130:465-471.
ILLUSTRATIVE CASE
A mother brings in her 18-month-old son because he’s had a runny nose and low-grade fever for the past 4 days—and a cough that kept them both up last night. You diagnose a viral URI, and she requests a strong cough medicine so he (and she) can get a good night’s sleep. What can you recommend that is both safe and effective for a child of this age?
For primary care physicians, office visits for coughing kids with URIs are commonplace. In addition to the cost of such visits, Americans spend some $3.5 billion a year on over-the-counter (OTC) cough and cold remedies, and often give them to young children.
It’s not enough to tell parents what not to do
As physicians (and parents), we understand the desire to give a coughing child something to ease the symptoms. We also know that OTC cough and cold medications can lead to serious complications, and even death. Between 1983 and 2007, 118 pediatric deaths were attributed to the misuse of such preparations.2 And, in a 3-year span (2005-2008), the American Association of Poison Control Centers received 64,658 calls for exposures to cough and cold remedies in children younger than 2 years of age, 28 of which resulted in a major adverse reaction or death.3
The US Food and Drug Administration recommends against the use of OTC cough and cold medications in children younger than 2 years,4 and the American Academy of Pediatrics has issued strict warnings about the use of OTC cough and cold preparations in children younger than 6 years.5 But warning parents of the dangers of giving them to young children without offering an alternative doesn’t satisfy anyone’s needs, and many parents continue to use these medications.
What about honey?
A study published in 2007 evaluated buckwheat honey and found it to be superior to no treatment and equal to honey-flavored dextromethorphan in reducing cough severity and improving sleep for children and their parents.6 Honey is known to have both antioxidant and antimicrobial properties—a possible scientific explanation for its effect. Before recommending honey for kids with URIs, however, more evidence of its efficacy was needed.
STUDY SUMMARY: Honey reduces cough frequency and severity
Cohen et al sought to determine whether honey, administered before bedtime, would decrease coughing in children between the ages of one and 5 years—and improve sleep for both the children and their caregivers.1 They enrolled 300 children with a nocturnal cough of <7 days’ duration and a diagnosis of URI in a one-night study.
Children were excluded if they had any signs or symptoms of asthma, pneumonia, sinusitis, allergic rhinitis, or laryngotracheobronchitis, or if they had been given any cough remedy, including honey, the night before. Parents completed a 5-question survey, using a 7-point Likert scale to assess the child’s cough and both the child’s and parents’ sleep the previous night. Only children whose parents rated their child’s cough severity ≥3 in 2 of the 3 questions related to cough were included in the trial.
The study had a double-blind randomized design, with 4 treatment arms. Three groups received 10 g (about 1.5 tsp) of one of 3 types of honey: eucalyptus, citrus, or labiatae (derived from plants including sage, mint, and thyme); the fourth group received a placebo of silan date extract, which is similar to honey in color, texture, and taste.
Children in all 4 groups received the preparation 30 minutes before bedtime. Neither the parents nor the physicians or study coordinators knew which preparation the children received. The following day, research assistants telephoned the parent who had completed the initial survey and asked the same 5 questions. The primary outcome measure was the change in cough frequency from the night before to the night after treatment. Secondary measures included cough severity and the effect on sleep for both the child and the parent.
Of the 300 children initially enrolled, 270 (90%) completed the trial, with an even distribution among the groups. While there were improvements across all outcomes for both the treatment and placebo groups, the changes were statistically significant only in the treatment groups. There were no significant differences in efficacy noted among the 3 types of honey. Adverse effects of stomachache, nausea, or vomiting were noted by 4 parents in the treatment groups and one in the placebo group, a difference that was not statistically significant.
WHAT’S NEW?: We have more evidence of honey’s efficacy
For children older than one year with a viral URI, we can now recommend 1.5 tsp honey to be given prior to bedtime as a cough remedy. This may reduce the use of potentially harmful and often ineffective OTC cough and cold remedies.
CAVEATS: Honey is unsafe for the youngest children
An obvious limitation of this study was its brevity. Although one night of improved cough and sleep is important, a study that showed honey’s sustained benefit as a cough suppressant would be more convincing. What’s more, there are safety concerns that are age-related.
Honey is considered unsafe for children younger than one year because of the risk of botulism. And honey has the potential to increase dental caries if it is given nightly for a prolonged period of time.
We do not know whether all varieties of honey will have the same benefit, and the source of store-bought honey is not always identified. The authors of this study received funding from the Honey Board of Israel.
CHALLENGES TO IMPLEMENTATION: Parents may be reluctant to abandon OTCs
Changing the behavior of parents and other caregivers who are accustomed to treating children with OTC cough and cold remedies is likely to be an uphill battle. Because honey is readily available, however—often as close as the pantry—and perceived to be safe and nutritious, a recommendation from a trusted physician could go a long way toward its implementation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
When a parent brings in a child (ages 1-5 years) with cough, runny nose, and other symptoms of a viral upper respiratory infection (URI), recommend that honey be given at bedtime.1
STRENGTH OF RECOMMENDATION
A: Based on a well-designed, randomized controlled trial (RCT)
Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130:465-471.
ILLUSTRATIVE CASE
A mother brings in her 18-month-old son because he’s had a runny nose and low-grade fever for the past 4 days—and a cough that kept them both up last night. You diagnose a viral URI, and she requests a strong cough medicine so he (and she) can get a good night’s sleep. What can you recommend that is both safe and effective for a child of this age?
For primary care physicians, office visits for coughing kids with URIs are commonplace. In addition to the cost of such visits, Americans spend some $3.5 billion a year on over-the-counter (OTC) cough and cold remedies, and often give them to young children.
It’s not enough to tell parents what not to do
As physicians (and parents), we understand the desire to give a coughing child something to ease the symptoms. We also know that OTC cough and cold medications can lead to serious complications, and even death. Between 1983 and 2007, 118 pediatric deaths were attributed to the misuse of such preparations.2 And, in a 3-year span (2005-2008), the American Association of Poison Control Centers received 64,658 calls for exposures to cough and cold remedies in children younger than 2 years of age, 28 of which resulted in a major adverse reaction or death.3
The US Food and Drug Administration recommends against the use of OTC cough and cold medications in children younger than 2 years,4 and the American Academy of Pediatrics has issued strict warnings about the use of OTC cough and cold preparations in children younger than 6 years.5 But warning parents of the dangers of giving them to young children without offering an alternative doesn’t satisfy anyone’s needs, and many parents continue to use these medications.
What about honey?
A study published in 2007 evaluated buckwheat honey and found it to be superior to no treatment and equal to honey-flavored dextromethorphan in reducing cough severity and improving sleep for children and their parents.6 Honey is known to have both antioxidant and antimicrobial properties—a possible scientific explanation for its effect. Before recommending honey for kids with URIs, however, more evidence of its efficacy was needed.
STUDY SUMMARY: Honey reduces cough frequency and severity
Cohen et al sought to determine whether honey, administered before bedtime, would decrease coughing in children between the ages of one and 5 years—and improve sleep for both the children and their caregivers.1 They enrolled 300 children with a nocturnal cough of <7 days’ duration and a diagnosis of URI in a one-night study.
Children were excluded if they had any signs or symptoms of asthma, pneumonia, sinusitis, allergic rhinitis, or laryngotracheobronchitis, or if they had been given any cough remedy, including honey, the night before. Parents completed a 5-question survey, using a 7-point Likert scale to assess the child’s cough and both the child’s and parents’ sleep the previous night. Only children whose parents rated their child’s cough severity ≥3 in 2 of the 3 questions related to cough were included in the trial.
The study had a double-blind randomized design, with 4 treatment arms. Three groups received 10 g (about 1.5 tsp) of one of 3 types of honey: eucalyptus, citrus, or labiatae (derived from plants including sage, mint, and thyme); the fourth group received a placebo of silan date extract, which is similar to honey in color, texture, and taste.
Children in all 4 groups received the preparation 30 minutes before bedtime. Neither the parents nor the physicians or study coordinators knew which preparation the children received. The following day, research assistants telephoned the parent who had completed the initial survey and asked the same 5 questions. The primary outcome measure was the change in cough frequency from the night before to the night after treatment. Secondary measures included cough severity and the effect on sleep for both the child and the parent.
Of the 300 children initially enrolled, 270 (90%) completed the trial, with an even distribution among the groups. While there were improvements across all outcomes for both the treatment and placebo groups, the changes were statistically significant only in the treatment groups. There were no significant differences in efficacy noted among the 3 types of honey. Adverse effects of stomachache, nausea, or vomiting were noted by 4 parents in the treatment groups and one in the placebo group, a difference that was not statistically significant.
WHAT’S NEW?: We have more evidence of honey’s efficacy
For children older than one year with a viral URI, we can now recommend 1.5 tsp honey to be given prior to bedtime as a cough remedy. This may reduce the use of potentially harmful and often ineffective OTC cough and cold remedies.
CAVEATS: Honey is unsafe for the youngest children
An obvious limitation of this study was its brevity. Although one night of improved cough and sleep is important, a study that showed honey’s sustained benefit as a cough suppressant would be more convincing. What’s more, there are safety concerns that are age-related.
Honey is considered unsafe for children younger than one year because of the risk of botulism. And honey has the potential to increase dental caries if it is given nightly for a prolonged period of time.
We do not know whether all varieties of honey will have the same benefit, and the source of store-bought honey is not always identified. The authors of this study received funding from the Honey Board of Israel.
CHALLENGES TO IMPLEMENTATION: Parents may be reluctant to abandon OTCs
Changing the behavior of parents and other caregivers who are accustomed to treating children with OTC cough and cold remedies is likely to be an uphill battle. Because honey is readily available, however—often as close as the pantry—and perceived to be safe and nutritious, a recommendation from a trusted physician could go a long way toward its implementation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130:465-471.
2. Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med. 2009;53:411-417.
3. Srinivasan A, Budnitz D, Shehab N, et al. Infant deaths associated with cough and cold medications—two states, 2005. JAMA. 2007;297:800-801.
4. US Food and Drug Administration. Public Health Advisory: FDA recommends that over-the-counter (OTC) cough and cold products not be used for Infants and children under 2 years of age. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051137.htm. Accessed February 14, 2013.
5. American Academy of Pediatrics. Withdrawal of cold medicines: addressing parent concerns. Available at: http://www.aap.org/en-us/professional-resources/practice-support/Pages/Withdrawal-of-Cold-Medicines-Addressing-Parent-Concerns.aspx. Accessed February 14, 2013.
6. Paul IM, Beiler J, McMonagle A, et al. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161:1140-1146.
1. Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130:465-471.
2. Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med. 2009;53:411-417.
3. Srinivasan A, Budnitz D, Shehab N, et al. Infant deaths associated with cough and cold medications—two states, 2005. JAMA. 2007;297:800-801.
4. US Food and Drug Administration. Public Health Advisory: FDA recommends that over-the-counter (OTC) cough and cold products not be used for Infants and children under 2 years of age. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051137.htm. Accessed February 14, 2013.
5. American Academy of Pediatrics. Withdrawal of cold medicines: addressing parent concerns. Available at: http://www.aap.org/en-us/professional-resources/practice-support/Pages/Withdrawal-of-Cold-Medicines-Addressing-Parent-Concerns.aspx. Accessed February 14, 2013.
6. Paul IM, Beiler J, McMonagle A, et al. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161:1140-1146.
Copyright © 2013 The Family Physicians Inquiries Network. All rights reserved.
Community-acquired pneumonia in children: A look at the IDSA guidelines
• Chest x-rays and lab testing may be optional for children with community-acquired pneumonia (CAP) who are not seriously ill. A
• Start amoxicillin empirically for any child with mild-to-moderate CAP. B
• If an atypical bacterial pneumonia is suspected, azithromycin is the first-line treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What are the recommended antibiotic choices for children with mild-to-moderate bacterial community-acquired pneumonia (CAP) in the outpatient setting? How much diagnostic testing is required? When might hospitalization and combination antibiotic therapy be warranted?
Evidence-based answers to these and other questions relevant to the management of CAP in infants and children older than 3 months are provided in a set of guidelines jointly published by the Infectious Diseases Society of America (IDSA) and the Pediatric Infectious Diseases Society (PIDS) in 2011.1 We summarize them here.
What the guidelines do, and don’t, address
The IDSA/PIDS guidelines, which focus on the care of otherwise healthy children with CAP in both outpatient and inpatient settings, seek to decrease morbidity and mortality rates associated with this respiratory infection. The guidelines do not apply to children younger than 3 months, immunocompromised patients, children receiving home mechanical ventilation, or children with chronic conditions or underlying lung disease, such as cystic fibrosis.
The need for evidence-based guidance. Globally each year, 1.5 million children 5 years of age and younger suffer a pneumonia-related death, particularly in developing countries.2-5 This is more than the number of deaths associated with any other disease in the world, including acquired immune deficiency syndrome (AIDS), tuberculosis (TB), or malaria.2 In 2010, pneumonia was ranked in the United States as the sixth leading cause of death for children one to 4 years of age and the 10th leading cause of death in adolescents.5 It is estimated that out of every 1000 infants and children in North America and Europe, 35 to 40 will be affected by CAP.2
How the guidelines define CAP. Pneumonia can be broadly defined as a lower respiratory tract infection, but definitions vary depending on the organization, institution, or health care setting. For instance, the World Health Organization (WHO) defines pneumonia solely on the basis of clinical findings obtained by visual inspection and timing of the respiratory rate.6 Another definition published by Bone and colleagues states that pneumonia is the “inflammation of the pulmonary parenchyma brought about by the presence of virulent pathogens; usually differentiated from isolated infections of the major airways.”7 The new pediatric guidelines define CAP as “the presence of signs and symptoms of pneumonia in a previously healthy child caused by an infection that has been acquired outside the hospital.”1
CAP pathogens vary with the child’s age
Typically, diagnostic testing of children will reveal several microbes, viral and bacterial, making it difficult to determine which might be the pathogen.1 Viral pathogens are more common causes of CAP in children younger than 2 years, accounting for 80% of cases1; bacterial pathogens are more common in older children.1
The virus detected most often among children younger than 2 years is respiratory syncytial virus (RSV).1,8-12 Less common viruses include adenovirus, influenza types A and B, parainfluenza 1, 2, and 3, and rhinovirus. Streptococcus pneumoniae is the most common bacterial pathogen identified in older children.1,13 The overall incidence of pneumonia decreases with age, but it has been reported that the proportion of cases from atypical bacterial pathogens—Chlamydia pneumoniae and Mycoplasma pneumoniae—may increase among older children.1,13
Signs and symptoms also vary
Signs and symptoms of CAP differ depending on the severity of the infection and the age of the child. In general, respiratory distress (tachypnea, nasal flaring, decreased breath sounds, cough, and rales) with fever are the prominent symptoms associated with pneumonia.1,13,14
Infants and children with mild to moderate infection most commonly exhibit a temperature <38°C and a respiratory rate <50 breaths per minute (bpm).
Children with severe CAP commonly present with a temperature >38°C, flaring of nostrils, grunting with breathing, tachypnea, tachycardia, and cyanosis. Tachypnea is defined as >60 bpm in infants younger than 2 months, >50 bpm in infants 2 to 12 months, and >40 bpm in children ages 1 to 5 years.8 Although respiratory rate is a valuable clinical sign, the work of breathing (as evidenced by nasal flaring, breathlessness, cough, or wheeze) required by the infant or child may be more indicative of pneumonia.15
Utilize diagnostic testing judiciously
Not all patients with suspected CAP require the same amount of diagnostic testing. In fact, IDSA/PIDS recommendations vary for hospitalized patients and for outpatients.1 In all cases, conduct testing quickly to expedite diagnosis and minimize the need for additional testing, to help validate treatment choices, and to reduce time spent in the hospital.1
Blood and sputum cultures not always indicated. The IDSA/PIDS guidelines strongly recommend obtaining blood cultures for hospitalized patients with moderate-to-severe pneumonia, particularly those with complications.1
The guidelines strongly recommend against blood cultures for fully immunized children with CAP who are treated as outpatients. However, blood cultures are strongly recommended for any child who fails to improve after initiation of antibiotic therapy.1 These recommendations are consistent with clinical data, expert opinion, and other treatment guidelines.1,8,13-18
A weak recommendation from the new guidelines states that if a hospitalized child with CAP can produce sputum, gram staining of the specimen may be warranted.1,8,13,15
Use pulse oximetry. The guidelines strongly recommend using pulse oximetry with all children who have pneumonia or suspected hypoxemia.1,18
When chest radiography can help. Routine chest radiography may not be warranted for suspected CAP treated in the outpatient setting. Order chest films for patients with suspected or confirmed hypoxemia or respiratory distress (who tend to have worse outcomes), and for patients who do not respond to initial antibiotic treatment.1,18 Follow-up radiographs are recommended for patients with advancing symptoms 2 to 3 days after starting antibiotics, complicated pneumonia with worsening respiratory distress, or clinical symptoms without improvement.1
Other diagnostic tests mentioned in the guidelines include complete blood cell counts, which are recommended in severe cases of pneumonia.1
Acute-phase reactants such as erythrocyte sedimentation rate (ESR), serum procalcitonin, and C-reactive protein concentrations cannot distinguish between viral and bacterial causes of CAP, and are not routinely recommended for patients treated in the outpatient setting.1,13
For patients requiring endotracheal intubation, gram staining and cultures of aspirates of the trachea and virus testing are recommended.1
Immunocompetent patients hospitalized with severe CAP may be candidates for percutaneous lung aspiration, open lung biopsy, bronchoalveolar lavage (BAL), or bronchoscopic or blind protected brush specimen collection if prior diagnostic tests are negative.1
CAP treatment and prevention
The guidelines provide recommendations for treating bacterial and viral CAP in either inpatient or outpatient settings, and discuss appropriate preventive techniques.
Antiviral therapy. As mentioned earlier, children less than 2 years of age are commonly infected with viral pathogens. Those with mild cases of viral CAP do not require anti-microbial therapy. For children with moderate-to-severe CAP consistent with influenza infection, administer influenza antiviral therapy as soon as possible, especially during a widespread local circulation of influenza viruses. Some influenza A strains will be susceptible to antiviral therapy, even though genetic variability is high each year. The guidelines’ recommended agents for treating influenza in pediatric patients are listed in TABLE 1.1
TABLE 1
Influenza antiviral therapy in pediatric patients*1
| Drug (brand name) | Formulation | Dosing |
|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg capsule; 60 mg/5 mL suspension | 4-8 mo: 6 mg/kg/d in 2 doses 9-23 mo: 7 mg/kg/d in 2 doses ≥24 mo: ~4 mg/kg/d in 2 doses, for 5 days ≤15 kg: 60 mg/d in 2 divided doses >15-23 kg: 90 mg/d in 2 divided doses >23-40 kg: 120 mg/d in 2 divided doses >40 kg: 150 mg/d in 2 divided doses |
| Zanamivir (Relenza) | 5 mg per inhalation, using a Diskhaler | ≥7 y: 2 inhalations (10 mg total per dose), twice daily for 5 days |
| Amantadine (Symmetrel)† | 100 mg tablet; 50 mg/5 mL suspension | 1-9 y: 5-8 mg/kg/d as single daily dose or in 2 doses; not to exceed 150 mg/d 9-12 y: 200 mg/d in 2 doses (not studied as a single dose) |
| Rimantadine (Flumadine)† | 100 mg tablet; 50 mg/5 mL suspension | Not FDA approved for treatment in children, but published data exist on safety and efficacy in children Suspension: 1-9 y: 6.6 mg/kg/d (max 150 mg/kg/d) in 2 doses ≥10 y: 200 mg/d, as single daily dose or in 2 doses |
| *In children for whom prophylaxis is indicated, antiviral drugs should be continued for the duration of known influenza activity in the community (because of the potential for repeated exposures) or until immunity can be achieved as a result of immunization. †Amantadine and rimantadine should be used for treatment and prophylaxis only in the winter, when most isolated influenza A virus strains are susceptible to adamantine; the adamantines should not be used for primary therapy because of the rapid emergence of resistance. However, for patients requiring adamantine therapy, a treatment course of about 7 days is suggested, or one that runs until a day or 2 after the signs and symptoms have disappeared. | ||
Antibacterial therapy. For patients with a suspected bacterial pathogen, start empiric antibiotic therapy as soon as possible. Preferred and alternative agents for specific age groups, immunization status, and specific pathogen(s) appear in TABLE 2.1,19
TABLE 2
Empiric outpatient antibiotic therapy for pediatric CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y, regardless of immunization status | Preferred: amoxicillin 90 mg/kg/d PO in 2 divided doses Alternative: amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses | For all children regardless of age and immunization status: Preferred: azithromycin 10 mg/kg PO on Day 1, followed by 5 mg/kg PO once daily on Days 2-5 Alternative: clarithromycin 15 mg/kg/d PO in 2 divided doses OR In children >7 y: erythromycin 40 mg/kg/d PO in 4 divided doses; or doxycycline 2-4 mg/kg/d PO in 2 divided doses |
| ≥5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a maximum 4 g/d, with or without a macrolide antibiotic Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)†OR linezolid (<12 y) 30 mg/kg/d PO (max 1200 mg/d) in 3 divided doses; or (≥12 y) 20 mg/kg/d (max 1200 mg/d) in 2 divided doses | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a max of 4 g/d; or amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)† | |
| CAP, community-acquired pneumonia. *Preferred treatments of choice change in areas of high S pneumoniae resistance. Refer to the complete guidelines for specific recommendations. †The guidelines do not fully address the controversy concerning the use of quinolones in children. The use of quinolones in infants and children is considered a risk vs benefit decision. | ||
Patients with mild or moderate CAP may be treated first in the outpatient setting with amoxicillin. This antibiotic has been the agent of choice for many years and continues to be the empiric therapy recommended in the guidelines.1 Appropriate dosing depends on the age of the patient.
TABLE 2 also includes treatment alternatives to amoxicillin for patients with drug allergies, treatment failures, or suspected atypical pathogens. Amoxicillin and the alternative treatments provide coverage for S pneumoniae, the most common invasive bacterial pathogen in older children.1,20 When atypical pathogens are suspected, macrolide antibiotics become the antibiotic drug class of choice, with azithromycin being the preferred first-line agent.1,21-23
Bacterial CAP necessitating hospitalization. The guidelines strongly recommend hospitalization for infants and children with respiratory distress or hypoxemia (oxygen saturation <90%); for suspicion of infection caused by community-acquired methicillin-resistant Staphylococcus aureus (MRSA) or any pathogen with high virulence; or for infants 3 to 6 months old.1
Treat with parenteral antibiotics to provide reliable blood and tissue concentrations (TABLE 3).1,19 Ampicillin or penicillin G may be given to fully immunized children; however, take into account the local resistance pattern of S pneumoniae to drugs within the penicillin class. For hospitalized children who are not yet fully immunized, who have life-threatening infections, or who are in a facility with a documented high rate of penicillin resistance, administer a third-generation parenteral cephalosporin such as ceftriaxone or cefotaxime empirically.1,24 In monotherapy treatment of pneumococcal pneumonia, non–beta-lactam agents such as vancomycin have not been shown to be more effective than the third-generation cephalosporins.1
TABLE 3
Empiric antibiotic therapy for hospitalized patients with CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | For all children regardless of age and immunization status: Preferred: azithromycin, 10 mg/kg IV (max of 500 mg) on Days 1 and 2, then transition to oral therapy 10 mg/kg/d for remaining 7-10 days of therapy Alternatives: erythromycin lactobionate 20 mg/kg/d IV divided every 6 h; or levofloxacin 16-20 mg/kg/d IV divided every 12 h to a max of 750 mg/d† |
| <5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternative: levofloxacin (6 mo–<5 y) 16-20 mg/kg/d IV divided every 12 h† | |
| ≥5 y and fully immunized against S pneumoniae and H influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternatives: ampicillin 150-200 mg/kg/d IV divided every 6 h; or levofloxacin 8-10 mg/kg IV once daily (max of 750 mg/d)† | |
| CAP, community-acquired pneumonia. *The addition of clindamycin 40 mg/kg/d IV divided every 6-8 hours or vancomycin 40-60 mg/kg/day IV divided every 6-8 hours is recommended for suspected or confirmed community-acquired methicillin-resistant Staphylococcus aureus. †The guidelines do not fully address the controversy concerning the use of quinolones in children. Use of quinolones in infants and children is considered a risk vs benefit decision. | ||
If S aureus is the suspected microorganism or is confirmed with clinical, laboratory, or imaging characteristics, give vancomycin or clindamycin with a beta-lactam agent.1,25-26 If you suspect an atypical pathogen such as M pneumoniae or C pneumoniae, start empiric therapy with an oral or parenteral macrolide in combination with a beta-lactam.1
Once a pathogen has been identified, adjust antimicrobial therapy as needed to target the specific microbe, to limit empiric antibiotic exposure, and to help limit the potential for antibiotic resistance.
Duration of treatment. The recommended duration of treatment for CAP is 10 days, supported by clinical data and the practice guidelines.1,27-29 Shorter treatment courses may be effective, especially in mild cases or outpatient treatment.1 Specific pathogens, such as MRSA, may need to be treated longer.30
If a patient is receiving intravenous antibiotics, switch to an oral agent as soon as clinically feasible to decrease risks from parenteral administration, and plan for the earliest possible discharge from the hospital to limit exposure to nosocomial pathogens. Hospital discharge may be considered when a child is clinically stable (improved appetite and activity level, afebrile for 24 hours), mental status is back to baseline or stable, and the pulse oximetry level is >90% on room air for at least 24 hours.1
Children receiving adequate therapy regimens should demonstrate both clinical and laboratory signs of improvement within 48 to 72 hours.1 If improvement does not occur, further your investigation with additional cultures, laboratory tests, and imaging evaluation.
For preventive measures, the guidelines recommend properly immunizing children with vaccines for bacterial pathogens such as S pneumoniae, Haemophilus influenzae, and Bordetella pertussis.1 Influenza vaccine should also be offered to prevent CAP in infants and children 6 months of age and older. Offer influenza and pertussis vaccines to adults and those caring for infants and children, to help prevent the spread of disease. Also consider immune prophylaxis with RSV-specific monoclonal antibody for premature infants or those with bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency, to decrease the risk of severe pneumonia and hospitalization. For detailed recommendations on the use of prophylaxis against RSV, refer to the 2003 American Academy of Pediatrics statement.31
CORRESPONDENCE
Stephanie Schauner, PharmD, BCPS, University of Missouri-Kansas City, Health Science Building, Room 2241, 2464 Charlotte Street, Kansas City, MO 64108-2792; [email protected]
1. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.Available at: http://cid.oxfordjournals.org/content/53/7/e25.long. Accessed December 17, 2012.
2. Centers for Disease Control and Prevention Pneumonia Can Be Prevented–Vaccines Can Help. Available at: http://www.cdc.gov/features/pneumonia. Accessed January 17, 2012.
3. Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Organ. 1978;56:481-498.
4. Baqui AH, Black RE, Arifeen SE, et al. Causes of childhood deaths in Bangladesh: results of a nationwide verbal autopsy study. Bull World Health Organ. 1998;76:161-171.
5. Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary data for 2010. National vital statistics reports; vol 60 no 4. Hyattsville, Md: National Center for Health Statistics. 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed May 12, 2012.
6. Clinical management of acute respiratory infections in children: a WHO memorandum. Bull World Health Organ. 1981;59:707-716.
7. Feldman C, Anderson R. Community-acquired pneumonia. In; Bone RC, Dantzker DR, George RB, et al, eds. Pulmonary and Critical Care Medicine. Vol 2. St. Louis, Mo: Mosby-Year Book, Inc; 1997:719–733.
8. Davies HD. Community-acquired pneumonia in children. Paediatr Child Health. 2003;8:616-619.
9. Alexander ER, Foy HM, Kenny GE, et al. Pneumonia due to Mycoplasma pneumoniae. Its incidence in the membership of a co-operative medical group. N Engl J Med. 1966;275:131-136.
10. Foy HM, Cooney MK, Maletzky AJ, et al. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical group over a four-year period. Am J Epidemiol. 1973;97:80-92.
11. Murphy TF, Henderson FW, Clyde WA, Jr, et al. Pneumonia: An eleven-year study in a pediatric practice. Am J Epidemiol. 1981;113:12-21.
12. Denny FW, Clyde WA. Acute lower respiratory tract infections in non-hospitalized children. J Pediatr. 1986;108:635-646.
13. Ostapchuk M, Roberts DM, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Phys. 2004;70:899-908.
14. Margolis P, Gadomski A. The rational clinical examination. Does this infant have pneumonia? JAMA. 1998;279:308-313.
15. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66 (suppl 2):ii1-ii23.
16. Gaston B. Pneumonia. Pediatr Rev. 2002;23:132-140.
17. McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
18. Skolnik N, Tien P. Managing community-acquired pneumonia in infants and children. Fam Pract News. November 10, 2011. Available at: http://www.familypracticenews.com/views/clinical-guidelines-for-family-physicians-by-dr-skolnik/blog/managing-community-acquired-pneumonia-in-infants-and-children/3a77ebb81a.html. Accessed January 17, 2012.
19. O’Mara N. Empiric treatment for pediatric community-acquired pneumonia. Pharmacist’s Letter. November 2011. Available at: http://www.pharmacistletter.com. Accessed February 25, 2012.
20. Klein JO. Bacterial pneumonias. In: Cherry J, Kaplan S, Demmler-Harrison G, eds. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Vol 1. Philadelphia, Pa: Saunders/Elsevier; 2009:302–314.
21. Morita JY, Kahn E, Thompson T, et al. Impact of azithromycin on oropharyngeal carriage of group A Streptococcus and nasopharyngeal carriage of macrolide-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 2000;19:41-46.
22. Block S, Hedrick J, Hammerschlag MR, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
23. Harris JA, Kolokathis A, Campbell M, et al. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17:865-871.
24. Pallares R, Capdevila O, Linares J, et al. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am J Med. 2002;113:120-126.
25. Roson B, Carratala J, Tubau F, et al. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7:85-96.
26. Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am. 2009;23:35-52.
27. Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;(2):CD005976.-
28. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651-1672.
29. Bradley JS, Ching DK, Hart CL. Invasive bacterial disease in childhood: efficacy of oral antibiotic therapy following short course parenteral therapy in non-central nervous system infections. Pediatr Infect Dis J. 1987;6:821-825.
30. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289-294.
31. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and RSV immune globulin intravenous for the prevention of respiratory syncytial virus infection. Pediatrics. 2003;112:1442-1446.
• Chest x-rays and lab testing may be optional for children with community-acquired pneumonia (CAP) who are not seriously ill. A
• Start amoxicillin empirically for any child with mild-to-moderate CAP. B
• If an atypical bacterial pneumonia is suspected, azithromycin is the first-line treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What are the recommended antibiotic choices for children with mild-to-moderate bacterial community-acquired pneumonia (CAP) in the outpatient setting? How much diagnostic testing is required? When might hospitalization and combination antibiotic therapy be warranted?
Evidence-based answers to these and other questions relevant to the management of CAP in infants and children older than 3 months are provided in a set of guidelines jointly published by the Infectious Diseases Society of America (IDSA) and the Pediatric Infectious Diseases Society (PIDS) in 2011.1 We summarize them here.
What the guidelines do, and don’t, address
The IDSA/PIDS guidelines, which focus on the care of otherwise healthy children with CAP in both outpatient and inpatient settings, seek to decrease morbidity and mortality rates associated with this respiratory infection. The guidelines do not apply to children younger than 3 months, immunocompromised patients, children receiving home mechanical ventilation, or children with chronic conditions or underlying lung disease, such as cystic fibrosis.
The need for evidence-based guidance. Globally each year, 1.5 million children 5 years of age and younger suffer a pneumonia-related death, particularly in developing countries.2-5 This is more than the number of deaths associated with any other disease in the world, including acquired immune deficiency syndrome (AIDS), tuberculosis (TB), or malaria.2 In 2010, pneumonia was ranked in the United States as the sixth leading cause of death for children one to 4 years of age and the 10th leading cause of death in adolescents.5 It is estimated that out of every 1000 infants and children in North America and Europe, 35 to 40 will be affected by CAP.2
How the guidelines define CAP. Pneumonia can be broadly defined as a lower respiratory tract infection, but definitions vary depending on the organization, institution, or health care setting. For instance, the World Health Organization (WHO) defines pneumonia solely on the basis of clinical findings obtained by visual inspection and timing of the respiratory rate.6 Another definition published by Bone and colleagues states that pneumonia is the “inflammation of the pulmonary parenchyma brought about by the presence of virulent pathogens; usually differentiated from isolated infections of the major airways.”7 The new pediatric guidelines define CAP as “the presence of signs and symptoms of pneumonia in a previously healthy child caused by an infection that has been acquired outside the hospital.”1
CAP pathogens vary with the child’s age
Typically, diagnostic testing of children will reveal several microbes, viral and bacterial, making it difficult to determine which might be the pathogen.1 Viral pathogens are more common causes of CAP in children younger than 2 years, accounting for 80% of cases1; bacterial pathogens are more common in older children.1
The virus detected most often among children younger than 2 years is respiratory syncytial virus (RSV).1,8-12 Less common viruses include adenovirus, influenza types A and B, parainfluenza 1, 2, and 3, and rhinovirus. Streptococcus pneumoniae is the most common bacterial pathogen identified in older children.1,13 The overall incidence of pneumonia decreases with age, but it has been reported that the proportion of cases from atypical bacterial pathogens—Chlamydia pneumoniae and Mycoplasma pneumoniae—may increase among older children.1,13
Signs and symptoms also vary
Signs and symptoms of CAP differ depending on the severity of the infection and the age of the child. In general, respiratory distress (tachypnea, nasal flaring, decreased breath sounds, cough, and rales) with fever are the prominent symptoms associated with pneumonia.1,13,14
Infants and children with mild to moderate infection most commonly exhibit a temperature <38°C and a respiratory rate <50 breaths per minute (bpm).
Children with severe CAP commonly present with a temperature >38°C, flaring of nostrils, grunting with breathing, tachypnea, tachycardia, and cyanosis. Tachypnea is defined as >60 bpm in infants younger than 2 months, >50 bpm in infants 2 to 12 months, and >40 bpm in children ages 1 to 5 years.8 Although respiratory rate is a valuable clinical sign, the work of breathing (as evidenced by nasal flaring, breathlessness, cough, or wheeze) required by the infant or child may be more indicative of pneumonia.15
Utilize diagnostic testing judiciously
Not all patients with suspected CAP require the same amount of diagnostic testing. In fact, IDSA/PIDS recommendations vary for hospitalized patients and for outpatients.1 In all cases, conduct testing quickly to expedite diagnosis and minimize the need for additional testing, to help validate treatment choices, and to reduce time spent in the hospital.1
Blood and sputum cultures not always indicated. The IDSA/PIDS guidelines strongly recommend obtaining blood cultures for hospitalized patients with moderate-to-severe pneumonia, particularly those with complications.1
The guidelines strongly recommend against blood cultures for fully immunized children with CAP who are treated as outpatients. However, blood cultures are strongly recommended for any child who fails to improve after initiation of antibiotic therapy.1 These recommendations are consistent with clinical data, expert opinion, and other treatment guidelines.1,8,13-18
A weak recommendation from the new guidelines states that if a hospitalized child with CAP can produce sputum, gram staining of the specimen may be warranted.1,8,13,15
Use pulse oximetry. The guidelines strongly recommend using pulse oximetry with all children who have pneumonia or suspected hypoxemia.1,18
When chest radiography can help. Routine chest radiography may not be warranted for suspected CAP treated in the outpatient setting. Order chest films for patients with suspected or confirmed hypoxemia or respiratory distress (who tend to have worse outcomes), and for patients who do not respond to initial antibiotic treatment.1,18 Follow-up radiographs are recommended for patients with advancing symptoms 2 to 3 days after starting antibiotics, complicated pneumonia with worsening respiratory distress, or clinical symptoms without improvement.1
Other diagnostic tests mentioned in the guidelines include complete blood cell counts, which are recommended in severe cases of pneumonia.1
Acute-phase reactants such as erythrocyte sedimentation rate (ESR), serum procalcitonin, and C-reactive protein concentrations cannot distinguish between viral and bacterial causes of CAP, and are not routinely recommended for patients treated in the outpatient setting.1,13
For patients requiring endotracheal intubation, gram staining and cultures of aspirates of the trachea and virus testing are recommended.1
Immunocompetent patients hospitalized with severe CAP may be candidates for percutaneous lung aspiration, open lung biopsy, bronchoalveolar lavage (BAL), or bronchoscopic or blind protected brush specimen collection if prior diagnostic tests are negative.1
CAP treatment and prevention
The guidelines provide recommendations for treating bacterial and viral CAP in either inpatient or outpatient settings, and discuss appropriate preventive techniques.
Antiviral therapy. As mentioned earlier, children less than 2 years of age are commonly infected with viral pathogens. Those with mild cases of viral CAP do not require anti-microbial therapy. For children with moderate-to-severe CAP consistent with influenza infection, administer influenza antiviral therapy as soon as possible, especially during a widespread local circulation of influenza viruses. Some influenza A strains will be susceptible to antiviral therapy, even though genetic variability is high each year. The guidelines’ recommended agents for treating influenza in pediatric patients are listed in TABLE 1.1
TABLE 1
Influenza antiviral therapy in pediatric patients*1
| Drug (brand name) | Formulation | Dosing |
|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg capsule; 60 mg/5 mL suspension | 4-8 mo: 6 mg/kg/d in 2 doses 9-23 mo: 7 mg/kg/d in 2 doses ≥24 mo: ~4 mg/kg/d in 2 doses, for 5 days ≤15 kg: 60 mg/d in 2 divided doses >15-23 kg: 90 mg/d in 2 divided doses >23-40 kg: 120 mg/d in 2 divided doses >40 kg: 150 mg/d in 2 divided doses |
| Zanamivir (Relenza) | 5 mg per inhalation, using a Diskhaler | ≥7 y: 2 inhalations (10 mg total per dose), twice daily for 5 days |
| Amantadine (Symmetrel)† | 100 mg tablet; 50 mg/5 mL suspension | 1-9 y: 5-8 mg/kg/d as single daily dose or in 2 doses; not to exceed 150 mg/d 9-12 y: 200 mg/d in 2 doses (not studied as a single dose) |
| Rimantadine (Flumadine)† | 100 mg tablet; 50 mg/5 mL suspension | Not FDA approved for treatment in children, but published data exist on safety and efficacy in children Suspension: 1-9 y: 6.6 mg/kg/d (max 150 mg/kg/d) in 2 doses ≥10 y: 200 mg/d, as single daily dose or in 2 doses |
| *In children for whom prophylaxis is indicated, antiviral drugs should be continued for the duration of known influenza activity in the community (because of the potential for repeated exposures) or until immunity can be achieved as a result of immunization. †Amantadine and rimantadine should be used for treatment and prophylaxis only in the winter, when most isolated influenza A virus strains are susceptible to adamantine; the adamantines should not be used for primary therapy because of the rapid emergence of resistance. However, for patients requiring adamantine therapy, a treatment course of about 7 days is suggested, or one that runs until a day or 2 after the signs and symptoms have disappeared. | ||
Antibacterial therapy. For patients with a suspected bacterial pathogen, start empiric antibiotic therapy as soon as possible. Preferred and alternative agents for specific age groups, immunization status, and specific pathogen(s) appear in TABLE 2.1,19
TABLE 2
Empiric outpatient antibiotic therapy for pediatric CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y, regardless of immunization status | Preferred: amoxicillin 90 mg/kg/d PO in 2 divided doses Alternative: amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses | For all children regardless of age and immunization status: Preferred: azithromycin 10 mg/kg PO on Day 1, followed by 5 mg/kg PO once daily on Days 2-5 Alternative: clarithromycin 15 mg/kg/d PO in 2 divided doses OR In children >7 y: erythromycin 40 mg/kg/d PO in 4 divided doses; or doxycycline 2-4 mg/kg/d PO in 2 divided doses |
| ≥5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a maximum 4 g/d, with or without a macrolide antibiotic Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)†OR linezolid (<12 y) 30 mg/kg/d PO (max 1200 mg/d) in 3 divided doses; or (≥12 y) 20 mg/kg/d (max 1200 mg/d) in 2 divided doses | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a max of 4 g/d; or amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)† | |
| CAP, community-acquired pneumonia. *Preferred treatments of choice change in areas of high S pneumoniae resistance. Refer to the complete guidelines for specific recommendations. †The guidelines do not fully address the controversy concerning the use of quinolones in children. The use of quinolones in infants and children is considered a risk vs benefit decision. | ||
Patients with mild or moderate CAP may be treated first in the outpatient setting with amoxicillin. This antibiotic has been the agent of choice for many years and continues to be the empiric therapy recommended in the guidelines.1 Appropriate dosing depends on the age of the patient.
TABLE 2 also includes treatment alternatives to amoxicillin for patients with drug allergies, treatment failures, or suspected atypical pathogens. Amoxicillin and the alternative treatments provide coverage for S pneumoniae, the most common invasive bacterial pathogen in older children.1,20 When atypical pathogens are suspected, macrolide antibiotics become the antibiotic drug class of choice, with azithromycin being the preferred first-line agent.1,21-23
Bacterial CAP necessitating hospitalization. The guidelines strongly recommend hospitalization for infants and children with respiratory distress or hypoxemia (oxygen saturation <90%); for suspicion of infection caused by community-acquired methicillin-resistant Staphylococcus aureus (MRSA) or any pathogen with high virulence; or for infants 3 to 6 months old.1
Treat with parenteral antibiotics to provide reliable blood and tissue concentrations (TABLE 3).1,19 Ampicillin or penicillin G may be given to fully immunized children; however, take into account the local resistance pattern of S pneumoniae to drugs within the penicillin class. For hospitalized children who are not yet fully immunized, who have life-threatening infections, or who are in a facility with a documented high rate of penicillin resistance, administer a third-generation parenteral cephalosporin such as ceftriaxone or cefotaxime empirically.1,24 In monotherapy treatment of pneumococcal pneumonia, non–beta-lactam agents such as vancomycin have not been shown to be more effective than the third-generation cephalosporins.1
TABLE 3
Empiric antibiotic therapy for hospitalized patients with CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | For all children regardless of age and immunization status: Preferred: azithromycin, 10 mg/kg IV (max of 500 mg) on Days 1 and 2, then transition to oral therapy 10 mg/kg/d for remaining 7-10 days of therapy Alternatives: erythromycin lactobionate 20 mg/kg/d IV divided every 6 h; or levofloxacin 16-20 mg/kg/d IV divided every 12 h to a max of 750 mg/d† |
| <5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternative: levofloxacin (6 mo–<5 y) 16-20 mg/kg/d IV divided every 12 h† | |
| ≥5 y and fully immunized against S pneumoniae and H influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternatives: ampicillin 150-200 mg/kg/d IV divided every 6 h; or levofloxacin 8-10 mg/kg IV once daily (max of 750 mg/d)† | |
| CAP, community-acquired pneumonia. *The addition of clindamycin 40 mg/kg/d IV divided every 6-8 hours or vancomycin 40-60 mg/kg/day IV divided every 6-8 hours is recommended for suspected or confirmed community-acquired methicillin-resistant Staphylococcus aureus. †The guidelines do not fully address the controversy concerning the use of quinolones in children. Use of quinolones in infants and children is considered a risk vs benefit decision. | ||
If S aureus is the suspected microorganism or is confirmed with clinical, laboratory, or imaging characteristics, give vancomycin or clindamycin with a beta-lactam agent.1,25-26 If you suspect an atypical pathogen such as M pneumoniae or C pneumoniae, start empiric therapy with an oral or parenteral macrolide in combination with a beta-lactam.1
Once a pathogen has been identified, adjust antimicrobial therapy as needed to target the specific microbe, to limit empiric antibiotic exposure, and to help limit the potential for antibiotic resistance.
Duration of treatment. The recommended duration of treatment for CAP is 10 days, supported by clinical data and the practice guidelines.1,27-29 Shorter treatment courses may be effective, especially in mild cases or outpatient treatment.1 Specific pathogens, such as MRSA, may need to be treated longer.30
If a patient is receiving intravenous antibiotics, switch to an oral agent as soon as clinically feasible to decrease risks from parenteral administration, and plan for the earliest possible discharge from the hospital to limit exposure to nosocomial pathogens. Hospital discharge may be considered when a child is clinically stable (improved appetite and activity level, afebrile for 24 hours), mental status is back to baseline or stable, and the pulse oximetry level is >90% on room air for at least 24 hours.1
Children receiving adequate therapy regimens should demonstrate both clinical and laboratory signs of improvement within 48 to 72 hours.1 If improvement does not occur, further your investigation with additional cultures, laboratory tests, and imaging evaluation.
For preventive measures, the guidelines recommend properly immunizing children with vaccines for bacterial pathogens such as S pneumoniae, Haemophilus influenzae, and Bordetella pertussis.1 Influenza vaccine should also be offered to prevent CAP in infants and children 6 months of age and older. Offer influenza and pertussis vaccines to adults and those caring for infants and children, to help prevent the spread of disease. Also consider immune prophylaxis with RSV-specific monoclonal antibody for premature infants or those with bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency, to decrease the risk of severe pneumonia and hospitalization. For detailed recommendations on the use of prophylaxis against RSV, refer to the 2003 American Academy of Pediatrics statement.31
CORRESPONDENCE
Stephanie Schauner, PharmD, BCPS, University of Missouri-Kansas City, Health Science Building, Room 2241, 2464 Charlotte Street, Kansas City, MO 64108-2792; [email protected]
• Chest x-rays and lab testing may be optional for children with community-acquired pneumonia (CAP) who are not seriously ill. A
• Start amoxicillin empirically for any child with mild-to-moderate CAP. B
• If an atypical bacterial pneumonia is suspected, azithromycin is the first-line treatment. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What are the recommended antibiotic choices for children with mild-to-moderate bacterial community-acquired pneumonia (CAP) in the outpatient setting? How much diagnostic testing is required? When might hospitalization and combination antibiotic therapy be warranted?
Evidence-based answers to these and other questions relevant to the management of CAP in infants and children older than 3 months are provided in a set of guidelines jointly published by the Infectious Diseases Society of America (IDSA) and the Pediatric Infectious Diseases Society (PIDS) in 2011.1 We summarize them here.
What the guidelines do, and don’t, address
The IDSA/PIDS guidelines, which focus on the care of otherwise healthy children with CAP in both outpatient and inpatient settings, seek to decrease morbidity and mortality rates associated with this respiratory infection. The guidelines do not apply to children younger than 3 months, immunocompromised patients, children receiving home mechanical ventilation, or children with chronic conditions or underlying lung disease, such as cystic fibrosis.
The need for evidence-based guidance. Globally each year, 1.5 million children 5 years of age and younger suffer a pneumonia-related death, particularly in developing countries.2-5 This is more than the number of deaths associated with any other disease in the world, including acquired immune deficiency syndrome (AIDS), tuberculosis (TB), or malaria.2 In 2010, pneumonia was ranked in the United States as the sixth leading cause of death for children one to 4 years of age and the 10th leading cause of death in adolescents.5 It is estimated that out of every 1000 infants and children in North America and Europe, 35 to 40 will be affected by CAP.2
How the guidelines define CAP. Pneumonia can be broadly defined as a lower respiratory tract infection, but definitions vary depending on the organization, institution, or health care setting. For instance, the World Health Organization (WHO) defines pneumonia solely on the basis of clinical findings obtained by visual inspection and timing of the respiratory rate.6 Another definition published by Bone and colleagues states that pneumonia is the “inflammation of the pulmonary parenchyma brought about by the presence of virulent pathogens; usually differentiated from isolated infections of the major airways.”7 The new pediatric guidelines define CAP as “the presence of signs and symptoms of pneumonia in a previously healthy child caused by an infection that has been acquired outside the hospital.”1
CAP pathogens vary with the child’s age
Typically, diagnostic testing of children will reveal several microbes, viral and bacterial, making it difficult to determine which might be the pathogen.1 Viral pathogens are more common causes of CAP in children younger than 2 years, accounting for 80% of cases1; bacterial pathogens are more common in older children.1
The virus detected most often among children younger than 2 years is respiratory syncytial virus (RSV).1,8-12 Less common viruses include adenovirus, influenza types A and B, parainfluenza 1, 2, and 3, and rhinovirus. Streptococcus pneumoniae is the most common bacterial pathogen identified in older children.1,13 The overall incidence of pneumonia decreases with age, but it has been reported that the proportion of cases from atypical bacterial pathogens—Chlamydia pneumoniae and Mycoplasma pneumoniae—may increase among older children.1,13
Signs and symptoms also vary
Signs and symptoms of CAP differ depending on the severity of the infection and the age of the child. In general, respiratory distress (tachypnea, nasal flaring, decreased breath sounds, cough, and rales) with fever are the prominent symptoms associated with pneumonia.1,13,14
Infants and children with mild to moderate infection most commonly exhibit a temperature <38°C and a respiratory rate <50 breaths per minute (bpm).
Children with severe CAP commonly present with a temperature >38°C, flaring of nostrils, grunting with breathing, tachypnea, tachycardia, and cyanosis. Tachypnea is defined as >60 bpm in infants younger than 2 months, >50 bpm in infants 2 to 12 months, and >40 bpm in children ages 1 to 5 years.8 Although respiratory rate is a valuable clinical sign, the work of breathing (as evidenced by nasal flaring, breathlessness, cough, or wheeze) required by the infant or child may be more indicative of pneumonia.15
Utilize diagnostic testing judiciously
Not all patients with suspected CAP require the same amount of diagnostic testing. In fact, IDSA/PIDS recommendations vary for hospitalized patients and for outpatients.1 In all cases, conduct testing quickly to expedite diagnosis and minimize the need for additional testing, to help validate treatment choices, and to reduce time spent in the hospital.1
Blood and sputum cultures not always indicated. The IDSA/PIDS guidelines strongly recommend obtaining blood cultures for hospitalized patients with moderate-to-severe pneumonia, particularly those with complications.1
The guidelines strongly recommend against blood cultures for fully immunized children with CAP who are treated as outpatients. However, blood cultures are strongly recommended for any child who fails to improve after initiation of antibiotic therapy.1 These recommendations are consistent with clinical data, expert opinion, and other treatment guidelines.1,8,13-18
A weak recommendation from the new guidelines states that if a hospitalized child with CAP can produce sputum, gram staining of the specimen may be warranted.1,8,13,15
Use pulse oximetry. The guidelines strongly recommend using pulse oximetry with all children who have pneumonia or suspected hypoxemia.1,18
When chest radiography can help. Routine chest radiography may not be warranted for suspected CAP treated in the outpatient setting. Order chest films for patients with suspected or confirmed hypoxemia or respiratory distress (who tend to have worse outcomes), and for patients who do not respond to initial antibiotic treatment.1,18 Follow-up radiographs are recommended for patients with advancing symptoms 2 to 3 days after starting antibiotics, complicated pneumonia with worsening respiratory distress, or clinical symptoms without improvement.1
Other diagnostic tests mentioned in the guidelines include complete blood cell counts, which are recommended in severe cases of pneumonia.1
Acute-phase reactants such as erythrocyte sedimentation rate (ESR), serum procalcitonin, and C-reactive protein concentrations cannot distinguish between viral and bacterial causes of CAP, and are not routinely recommended for patients treated in the outpatient setting.1,13
For patients requiring endotracheal intubation, gram staining and cultures of aspirates of the trachea and virus testing are recommended.1
Immunocompetent patients hospitalized with severe CAP may be candidates for percutaneous lung aspiration, open lung biopsy, bronchoalveolar lavage (BAL), or bronchoscopic or blind protected brush specimen collection if prior diagnostic tests are negative.1
CAP treatment and prevention
The guidelines provide recommendations for treating bacterial and viral CAP in either inpatient or outpatient settings, and discuss appropriate preventive techniques.
Antiviral therapy. As mentioned earlier, children less than 2 years of age are commonly infected with viral pathogens. Those with mild cases of viral CAP do not require anti-microbial therapy. For children with moderate-to-severe CAP consistent with influenza infection, administer influenza antiviral therapy as soon as possible, especially during a widespread local circulation of influenza viruses. Some influenza A strains will be susceptible to antiviral therapy, even though genetic variability is high each year. The guidelines’ recommended agents for treating influenza in pediatric patients are listed in TABLE 1.1
TABLE 1
Influenza antiviral therapy in pediatric patients*1
| Drug (brand name) | Formulation | Dosing |
|---|---|---|
| Oseltamivir (Tamiflu) | 75 mg capsule; 60 mg/5 mL suspension | 4-8 mo: 6 mg/kg/d in 2 doses 9-23 mo: 7 mg/kg/d in 2 doses ≥24 mo: ~4 mg/kg/d in 2 doses, for 5 days ≤15 kg: 60 mg/d in 2 divided doses >15-23 kg: 90 mg/d in 2 divided doses >23-40 kg: 120 mg/d in 2 divided doses >40 kg: 150 mg/d in 2 divided doses |
| Zanamivir (Relenza) | 5 mg per inhalation, using a Diskhaler | ≥7 y: 2 inhalations (10 mg total per dose), twice daily for 5 days |
| Amantadine (Symmetrel)† | 100 mg tablet; 50 mg/5 mL suspension | 1-9 y: 5-8 mg/kg/d as single daily dose or in 2 doses; not to exceed 150 mg/d 9-12 y: 200 mg/d in 2 doses (not studied as a single dose) |
| Rimantadine (Flumadine)† | 100 mg tablet; 50 mg/5 mL suspension | Not FDA approved for treatment in children, but published data exist on safety and efficacy in children Suspension: 1-9 y: 6.6 mg/kg/d (max 150 mg/kg/d) in 2 doses ≥10 y: 200 mg/d, as single daily dose or in 2 doses |
| *In children for whom prophylaxis is indicated, antiviral drugs should be continued for the duration of known influenza activity in the community (because of the potential for repeated exposures) or until immunity can be achieved as a result of immunization. †Amantadine and rimantadine should be used for treatment and prophylaxis only in the winter, when most isolated influenza A virus strains are susceptible to adamantine; the adamantines should not be used for primary therapy because of the rapid emergence of resistance. However, for patients requiring adamantine therapy, a treatment course of about 7 days is suggested, or one that runs until a day or 2 after the signs and symptoms have disappeared. | ||
Antibacterial therapy. For patients with a suspected bacterial pathogen, start empiric antibiotic therapy as soon as possible. Preferred and alternative agents for specific age groups, immunization status, and specific pathogen(s) appear in TABLE 2.1,19
TABLE 2
Empiric outpatient antibiotic therapy for pediatric CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y, regardless of immunization status | Preferred: amoxicillin 90 mg/kg/d PO in 2 divided doses Alternative: amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses | For all children regardless of age and immunization status: Preferred: azithromycin 10 mg/kg PO on Day 1, followed by 5 mg/kg PO once daily on Days 2-5 Alternative: clarithromycin 15 mg/kg/d PO in 2 divided doses OR In children >7 y: erythromycin 40 mg/kg/d PO in 4 divided doses; or doxycycline 2-4 mg/kg/d PO in 2 divided doses |
| ≥5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a maximum 4 g/d, with or without a macrolide antibiotic Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)†OR linezolid (<12 y) 30 mg/kg/d PO (max 1200 mg/d) in 3 divided doses; or (≥12 y) 20 mg/kg/d (max 1200 mg/d) in 2 divided doses | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* amoxicillin 90 mg/kg/d PO in 2 divided doses to a max of 4 g/d; or amoxicillin clavulanate 90 mg/kg/d PO in 2 divided doses Alternatives: Second- or third-generation cephalosporins such as oral cefpodoxime, cefuroxime, or cefprozil OR levofloxacin (5-16 y) 8-10 mg/kg PO once daily (max 750 mg/d)† | |
| CAP, community-acquired pneumonia. *Preferred treatments of choice change in areas of high S pneumoniae resistance. Refer to the complete guidelines for specific recommendations. †The guidelines do not fully address the controversy concerning the use of quinolones in children. The use of quinolones in infants and children is considered a risk vs benefit decision. | ||
Patients with mild or moderate CAP may be treated first in the outpatient setting with amoxicillin. This antibiotic has been the agent of choice for many years and continues to be the empiric therapy recommended in the guidelines.1 Appropriate dosing depends on the age of the patient.
TABLE 2 also includes treatment alternatives to amoxicillin for patients with drug allergies, treatment failures, or suspected atypical pathogens. Amoxicillin and the alternative treatments provide coverage for S pneumoniae, the most common invasive bacterial pathogen in older children.1,20 When atypical pathogens are suspected, macrolide antibiotics become the antibiotic drug class of choice, with azithromycin being the preferred first-line agent.1,21-23
Bacterial CAP necessitating hospitalization. The guidelines strongly recommend hospitalization for infants and children with respiratory distress or hypoxemia (oxygen saturation <90%); for suspicion of infection caused by community-acquired methicillin-resistant Staphylococcus aureus (MRSA) or any pathogen with high virulence; or for infants 3 to 6 months old.1
Treat with parenteral antibiotics to provide reliable blood and tissue concentrations (TABLE 3).1,19 Ampicillin or penicillin G may be given to fully immunized children; however, take into account the local resistance pattern of S pneumoniae to drugs within the penicillin class. For hospitalized children who are not yet fully immunized, who have life-threatening infections, or who are in a facility with a documented high rate of penicillin resistance, administer a third-generation parenteral cephalosporin such as ceftriaxone or cefotaxime empirically.1,24 In monotherapy treatment of pneumococcal pneumonia, non–beta-lactam agents such as vancomycin have not been shown to be more effective than the third-generation cephalosporins.1
TABLE 3
Empiric antibiotic therapy for hospitalized patients with CAP1,19
Duration of treatment is 10 days unless otherwise noted
| Patient age | Presumed bacterial pneumonia | Presumed atypical pneumonia |
|---|---|---|
| 3 mo to <5 y and fully immunized against Streptococcus pneumoniae and Haemophilus influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | For all children regardless of age and immunization status: Preferred: azithromycin, 10 mg/kg IV (max of 500 mg) on Days 1 and 2, then transition to oral therapy 10 mg/kg/d for remaining 7-10 days of therapy Alternatives: erythromycin lactobionate 20 mg/kg/d IV divided every 6 h; or levofloxacin 16-20 mg/kg/d IV divided every 12 h to a max of 750 mg/d† |
| <5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternative: levofloxacin (6 mo–<5 y) 16-20 mg/kg/d IV divided every 12 h† | |
| ≥5 y and fully immunized against S pneumoniae and H influenzae | Preferred:* ampicillin 150-200 mg/kg/d IV divided every 6 h; or penicillin G 200,000-250,000 units/kg/d IV divided every 4-6 h Alternatives: ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h | |
| ≥5 y and NOT fully immunized against S pneumoniae and H influenzae | Preferred:* ceftriaxone 50-100 mg/kg/d IV/IM divided every 12-24 h; or cefotaxime 150 mg/kg/d IV divided every 8 h Alternatives: ampicillin 150-200 mg/kg/d IV divided every 6 h; or levofloxacin 8-10 mg/kg IV once daily (max of 750 mg/d)† | |
| CAP, community-acquired pneumonia. *The addition of clindamycin 40 mg/kg/d IV divided every 6-8 hours or vancomycin 40-60 mg/kg/day IV divided every 6-8 hours is recommended for suspected or confirmed community-acquired methicillin-resistant Staphylococcus aureus. †The guidelines do not fully address the controversy concerning the use of quinolones in children. Use of quinolones in infants and children is considered a risk vs benefit decision. | ||
If S aureus is the suspected microorganism or is confirmed with clinical, laboratory, or imaging characteristics, give vancomycin or clindamycin with a beta-lactam agent.1,25-26 If you suspect an atypical pathogen such as M pneumoniae or C pneumoniae, start empiric therapy with an oral or parenteral macrolide in combination with a beta-lactam.1
Once a pathogen has been identified, adjust antimicrobial therapy as needed to target the specific microbe, to limit empiric antibiotic exposure, and to help limit the potential for antibiotic resistance.
Duration of treatment. The recommended duration of treatment for CAP is 10 days, supported by clinical data and the practice guidelines.1,27-29 Shorter treatment courses may be effective, especially in mild cases or outpatient treatment.1 Specific pathogens, such as MRSA, may need to be treated longer.30
If a patient is receiving intravenous antibiotics, switch to an oral agent as soon as clinically feasible to decrease risks from parenteral administration, and plan for the earliest possible discharge from the hospital to limit exposure to nosocomial pathogens. Hospital discharge may be considered when a child is clinically stable (improved appetite and activity level, afebrile for 24 hours), mental status is back to baseline or stable, and the pulse oximetry level is >90% on room air for at least 24 hours.1
Children receiving adequate therapy regimens should demonstrate both clinical and laboratory signs of improvement within 48 to 72 hours.1 If improvement does not occur, further your investigation with additional cultures, laboratory tests, and imaging evaluation.
For preventive measures, the guidelines recommend properly immunizing children with vaccines for bacterial pathogens such as S pneumoniae, Haemophilus influenzae, and Bordetella pertussis.1 Influenza vaccine should also be offered to prevent CAP in infants and children 6 months of age and older. Offer influenza and pertussis vaccines to adults and those caring for infants and children, to help prevent the spread of disease. Also consider immune prophylaxis with RSV-specific monoclonal antibody for premature infants or those with bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency, to decrease the risk of severe pneumonia and hospitalization. For detailed recommendations on the use of prophylaxis against RSV, refer to the 2003 American Academy of Pediatrics statement.31
CORRESPONDENCE
Stephanie Schauner, PharmD, BCPS, University of Missouri-Kansas City, Health Science Building, Room 2241, 2464 Charlotte Street, Kansas City, MO 64108-2792; [email protected]
1. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.Available at: http://cid.oxfordjournals.org/content/53/7/e25.long. Accessed December 17, 2012.
2. Centers for Disease Control and Prevention Pneumonia Can Be Prevented–Vaccines Can Help. Available at: http://www.cdc.gov/features/pneumonia. Accessed January 17, 2012.
3. Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Organ. 1978;56:481-498.
4. Baqui AH, Black RE, Arifeen SE, et al. Causes of childhood deaths in Bangladesh: results of a nationwide verbal autopsy study. Bull World Health Organ. 1998;76:161-171.
5. Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary data for 2010. National vital statistics reports; vol 60 no 4. Hyattsville, Md: National Center for Health Statistics. 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed May 12, 2012.
6. Clinical management of acute respiratory infections in children: a WHO memorandum. Bull World Health Organ. 1981;59:707-716.
7. Feldman C, Anderson R. Community-acquired pneumonia. In; Bone RC, Dantzker DR, George RB, et al, eds. Pulmonary and Critical Care Medicine. Vol 2. St. Louis, Mo: Mosby-Year Book, Inc; 1997:719–733.
8. Davies HD. Community-acquired pneumonia in children. Paediatr Child Health. 2003;8:616-619.
9. Alexander ER, Foy HM, Kenny GE, et al. Pneumonia due to Mycoplasma pneumoniae. Its incidence in the membership of a co-operative medical group. N Engl J Med. 1966;275:131-136.
10. Foy HM, Cooney MK, Maletzky AJ, et al. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical group over a four-year period. Am J Epidemiol. 1973;97:80-92.
11. Murphy TF, Henderson FW, Clyde WA, Jr, et al. Pneumonia: An eleven-year study in a pediatric practice. Am J Epidemiol. 1981;113:12-21.
12. Denny FW, Clyde WA. Acute lower respiratory tract infections in non-hospitalized children. J Pediatr. 1986;108:635-646.
13. Ostapchuk M, Roberts DM, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Phys. 2004;70:899-908.
14. Margolis P, Gadomski A. The rational clinical examination. Does this infant have pneumonia? JAMA. 1998;279:308-313.
15. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66 (suppl 2):ii1-ii23.
16. Gaston B. Pneumonia. Pediatr Rev. 2002;23:132-140.
17. McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
18. Skolnik N, Tien P. Managing community-acquired pneumonia in infants and children. Fam Pract News. November 10, 2011. Available at: http://www.familypracticenews.com/views/clinical-guidelines-for-family-physicians-by-dr-skolnik/blog/managing-community-acquired-pneumonia-in-infants-and-children/3a77ebb81a.html. Accessed January 17, 2012.
19. O’Mara N. Empiric treatment for pediatric community-acquired pneumonia. Pharmacist’s Letter. November 2011. Available at: http://www.pharmacistletter.com. Accessed February 25, 2012.
20. Klein JO. Bacterial pneumonias. In: Cherry J, Kaplan S, Demmler-Harrison G, eds. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Vol 1. Philadelphia, Pa: Saunders/Elsevier; 2009:302–314.
21. Morita JY, Kahn E, Thompson T, et al. Impact of azithromycin on oropharyngeal carriage of group A Streptococcus and nasopharyngeal carriage of macrolide-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 2000;19:41-46.
22. Block S, Hedrick J, Hammerschlag MR, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
23. Harris JA, Kolokathis A, Campbell M, et al. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17:865-871.
24. Pallares R, Capdevila O, Linares J, et al. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am J Med. 2002;113:120-126.
25. Roson B, Carratala J, Tubau F, et al. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7:85-96.
26. Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am. 2009;23:35-52.
27. Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;(2):CD005976.-
28. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651-1672.
29. Bradley JS, Ching DK, Hart CL. Invasive bacterial disease in childhood: efficacy of oral antibiotic therapy following short course parenteral therapy in non-central nervous system infections. Pediatr Infect Dis J. 1987;6:821-825.
30. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289-294.
31. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and RSV immune globulin intravenous for the prevention of respiratory syncytial virus infection. Pediatrics. 2003;112:1442-1446.
1. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25-e76.Available at: http://cid.oxfordjournals.org/content/53/7/e25.long. Accessed December 17, 2012.
2. Centers for Disease Control and Prevention Pneumonia Can Be Prevented–Vaccines Can Help. Available at: http://www.cdc.gov/features/pneumonia. Accessed January 17, 2012.
3. Bulla A, Hitze KL. Acute respiratory infections: a review. Bull World Health Organ. 1978;56:481-498.
4. Baqui AH, Black RE, Arifeen SE, et al. Causes of childhood deaths in Bangladesh: results of a nationwide verbal autopsy study. Bull World Health Organ. 1998;76:161-171.
5. Murphy SL, Xu JQ, Kochanek KD. Deaths: Preliminary data for 2010. National vital statistics reports; vol 60 no 4. Hyattsville, Md: National Center for Health Statistics. 2012. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf. Accessed May 12, 2012.
6. Clinical management of acute respiratory infections in children: a WHO memorandum. Bull World Health Organ. 1981;59:707-716.
7. Feldman C, Anderson R. Community-acquired pneumonia. In; Bone RC, Dantzker DR, George RB, et al, eds. Pulmonary and Critical Care Medicine. Vol 2. St. Louis, Mo: Mosby-Year Book, Inc; 1997:719–733.
8. Davies HD. Community-acquired pneumonia in children. Paediatr Child Health. 2003;8:616-619.
9. Alexander ER, Foy HM, Kenny GE, et al. Pneumonia due to Mycoplasma pneumoniae. Its incidence in the membership of a co-operative medical group. N Engl J Med. 1966;275:131-136.
10. Foy HM, Cooney MK, Maletzky AJ, et al. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical group over a four-year period. Am J Epidemiol. 1973;97:80-92.
11. Murphy TF, Henderson FW, Clyde WA, Jr, et al. Pneumonia: An eleven-year study in a pediatric practice. Am J Epidemiol. 1981;113:12-21.
12. Denny FW, Clyde WA. Acute lower respiratory tract infections in non-hospitalized children. J Pediatr. 1986;108:635-646.
13. Ostapchuk M, Roberts DM, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Phys. 2004;70:899-908.
14. Margolis P, Gadomski A. The rational clinical examination. Does this infant have pneumonia? JAMA. 1998;279:308-313.
15. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66 (suppl 2):ii1-ii23.
16. Gaston B. Pneumonia. Pediatr Rev. 2002;23:132-140.
17. McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-437.
18. Skolnik N, Tien P. Managing community-acquired pneumonia in infants and children. Fam Pract News. November 10, 2011. Available at: http://www.familypracticenews.com/views/clinical-guidelines-for-family-physicians-by-dr-skolnik/blog/managing-community-acquired-pneumonia-in-infants-and-children/3a77ebb81a.html. Accessed January 17, 2012.
19. O’Mara N. Empiric treatment for pediatric community-acquired pneumonia. Pharmacist’s Letter. November 2011. Available at: http://www.pharmacistletter.com. Accessed February 25, 2012.
20. Klein JO. Bacterial pneumonias. In: Cherry J, Kaplan S, Demmler-Harrison G, eds. Feigin & Cherry’s Textbook of Pediatric Infectious Diseases. 6th ed. Vol 1. Philadelphia, Pa: Saunders/Elsevier; 2009:302–314.
21. Morita JY, Kahn E, Thompson T, et al. Impact of azithromycin on oropharyngeal carriage of group A Streptococcus and nasopharyngeal carriage of macrolide-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 2000;19:41-46.
22. Block S, Hedrick J, Hammerschlag MR, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
23. Harris JA, Kolokathis A, Campbell M, et al. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J. 1998;17:865-871.
24. Pallares R, Capdevila O, Linares J, et al. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am J Med. 2002;113:120-126.
25. Roson B, Carratala J, Tubau F, et al. Usefulness of betalactam therapy for community-acquired pneumonia in the era of drug-resistant Streptococcus pneumoniae: a randomized study of amoxicillin-clavulanate and ceftriaxone. Microb Drug Resist. 2001;7:85-96.
26. Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am. 2009;23:35-52.
27. Haider BA, Saeed MA, Bhutta ZA. Short-course versus long-course antibiotic therapy for non-severe community-acquired pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2008;(2):CD005976.-
28. Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651-1672.
29. Bradley JS, Ching DK, Hart CL. Invasive bacterial disease in childhood: efficacy of oral antibiotic therapy following short course parenteral therapy in non-central nervous system infections. Pediatr Infect Dis J. 1987;6:821-825.
30. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30:289-294.
31. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and RSV immune globulin intravenous for the prevention of respiratory syncytial virus infection. Pediatrics. 2003;112:1442-1446.
Rethinking antibiotics for sinusitis—again
Practice Changer
Stop prescribing amoxicillin for acute rhinosinusitis. It’s unlikely to provide a speedier recovery than OTC remedies alone.1
Strength of recommendation
B: Based on a single high-quality randomized controlled trial.
ILLUSTRATIVE CASE
A 28-year-old man comes to your clinic after experiencing fatigue, purulent nasal discharge, and unilateral facial pain for nearly
10 days. Overall, he appears healthy, and you diagnose acute rhinosinusitis. You suggest OTC remedies for supportive care and wonder if a course of amoxicillin would speed his
recovery.
Each year, more than 30 million Americans—about one in seven adults—are diagnosed with sinusitis.2 No more than 2% of these cases are thought to be bacterial.3
CDC guidelines for the diagnosis of acute bacterial rhinosinusitis include symptoms that last seven or more days, with maxillary pain or tenderness in the face or teeth and purulent nasal secretions.4 Patients with symptoms lasting less than seven days are unlikely to have a bacterial infection. But the nonspecific signs and symptoms included in the CDC guidelines limit their usefulness in determining whether the cause of the sinusitis is bacterial or viral on clinical grounds alone.
Most cases of sinusitis spontaneously resolve
In patients with acute bacterial sinusitis, the American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) guidelines advocate watchful waiting and symptom relief with nasal oxymetazoline, pseudoephedrine, and saline nasal irrigation.3 The rate of spontaneous resolution is high: 80% of patients with clinically diagnosed sinusitis improve without treatment within two weeks.1,5
Traditional decongestants and mucolytics have not demonstrated efficacy in resolving sinusitis, although rigorous evaluation is lacking. Other treatments, such as saline irrigation and intranasal corticosteroids, are of unclear benefit and need further study.6-8
Lack of evidence has done little to curtail antibiotic use
A previous PURL that was based on a meta-analysis of antibiotic treatment trials for sinusitis recommended that we stop prescribing antibiotics for adults with acute sinusitis unless their symptoms are severe.9,10 Yet antibiotics remain the mainstay of treatment.
Despite the AAO–HNS guidelines, evidence of spontaneous resolution, and accumulating data on the lack of efficacy of antimicrobials for sinusitis, 81% of patients diagnosed with acute sinusitis were given prescriptions for antibiotics, a study of primary care practices showed.11 Frequent use of antibiotics contributes to high rates of drug resistance, and adverse events related to antibiotic use account for an estimated 142,500 emergency department visits annually.12
STUDY SUMMARY
Little benefit from amoxicillin, even for severe cases
Garbutt and colleagues revisited the issue, randomizing 166 patients from 10 primary care practices to receive amoxicillin plus symptomatic treatment or placebo plus symptomatic treatment for acute rhinosinusitis.1
To be eligible for the study, patients had to be between the ages of 18 and 70, meet CDC diagnostic criteria for acute rhinosinusitis, and have moderate to very severe symptoms that were of seven- to-28-day duration and worsening or not improving, or of < 7-day duration but had worsened after an initial improvement. Exclusion criteria included complications from sinusitis, a history of allergy to penicillin or amoxicillin, antibiotic use in the past four weeks, comorbidities that impair immune function, cystic fibrosis, pregnancy, and mild symptoms.
Both groups had similar baseline characteristics, with participants who were predominantly white (79%) and female (64%). All the participants received a supply of symptomatic treatments: acetaminophen, guaifenesin, dextromethorphan, and sustained-release pseudoephedrine. The treatment group also received amoxicillin 1,500 mg/d, divided into three doses; the placebo group received identical-looking placebo pills.
Patients were assessed with the Sino-nasal Outcome Test-16 (SNOT-16), a validated measure that asks patients to assess both the severity and frequency of 16 sinus symptoms. SNOT-16 uses a 0-to-3 rating scale (0 = no problem; 3 = severe problem), with a clinically important difference of ≥ 0.5 on the mean score. The test was administered at enrollment and at days 3, 7, and 10. The disease-specific quality of life at day 3 was the primary outcome.
There was no statistically significantly difference in SNOT-16 scores between the amoxicillin and placebo groups on days 3 and 10. On day 7, there was a small statistically significant improvement in the amoxicillin group, but it did not reach the level of clinical importance (≥ 0.5) based on SNOT-16’s mean score.
The authors also asked participants to retrospectively assess symptom change since enrollment on a six-point scale. Those who reported that their symptoms were “a lot better” or “absent” were characterized as significantly improved. The results correlated with the data from the SNOT-16, showing no difference between the amoxicillin and control groups at days 3 and 10. On day 7, 74% of patients treated with amoxicillin self-
reported significant improvement in symptoms since the start of the study, versus 56% in the control group. The number needed to treat was 6 (95% confidence interval, 3 to 34; P = .02) for a reduction in symptoms at day 7.
Patients in both groups had similar rates of absenteeism, inability to perform usual activities, relapse and recurrence, and use of additional health care. Satisfaction with treatment was similar, as well.
No serious adverse effects occurred. Both groups reported similar frequencies (< 10%) of nausea, diarrhea, abdominal pain, or vaginitis.
WHAT’S NEW
Even severe sinusitis resolves without antibiotics
Previous studies recommended foregoing antibiotics for acute sinusitis, except when symptoms are severe. This study—in which more than half (52%) of patients in each group had symptoms rated severe or very severe—found no benefit to adding amoxicillin to supportive treatments.1 Antibiotics did not shorten the duration of illness, prevent relapse and recurrence, or improve satisfaction with treatment. The researchers found a statistically significant difference between groups on day 7 of 0.19 points, but no clinically meaningful difference (≥ 0.5) based on the SNOT-16 mean score.
CAVEATS
Guidelines, risk of complications may give reason to pause
The 2012 Infectious Diseases Society of America guidelines recommend amoxicillin with clavulanic acid as empiric therapy for acute bacterial rhinosinusitis.7 The findings of the study by Garbutt et al—conducted at a time when the incidence of beta-lactamase-producing organisms was low and amoxicillin was the treatment of choice—suggest otherwise.
Serious complications of sinusitis, such as brain abscess, periorbital cellulitis, and meningitis, can occur, however. Patients who deteriorate clinically or develop high fever or severe headache require close follow-up, which may include further diagnostic evaluation or consultation with an otolaryngologist. Evidence is lacking as to whether antibiotics prevent such complications.5
CHALLENGES TO IMPLEMENTATION
Managing patient expectations
Many patients with symptoms of acute rhinosinusitis think they need an antibiotic. Managing their expectations and providing instructions about supportive treatments are time consuming and may be
difficult.
Nonetheless, we’re optimistic: We think that most patients today are aware of the problems associated with antibiotic resistance and wary of “superbugs,” and will therefore be receptive to this practice change. Clinicians can help by reminding patients of the adverse effects of antibiotics and the natural course of rhinosinusitis, as well as by offering symptomatic treatments.
REFERENCES
1. Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
2. Centers for Disease Control and Prevention. Summary health statistics for US adults: National Health Interview Survey 2010. January 2012. www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed July 9, 2012.
3. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007:137(3 suppl):S1-S31.
4. Hickner JM, Bartlett JG, Besser RE, et al; American Academy of Family Physians; American College of Physicians; American Society of Internal Medicine; Centers for Disease Control; Infectious Disease Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults; background. Ann Intern Med. 2001;134:498-505.
5. Ahovuo-Saloranta A, Borisenk OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Sys Rev. 2008(2):CD000243.
6. Allen G, Kelsberg G, Jankowski TA. Do nasal decongestants relieve symptoms? J Fam Pract. 2003;52:714-724.
7. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
8. Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2009(4):CD005149.
9. Schumann A, Hickner, J. Patients insist on antibiotics for sinusitis? Here is a good reason to say “no.” J Fam Pract. 2008;57:464-468.
10. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
11. Gill JM, Fleischut P, Haas S. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med. 2006;38:349-354.
12. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008; 47:735-743.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2012 The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2012;61(10):610-612.
Practice Changer
Stop prescribing amoxicillin for acute rhinosinusitis. It’s unlikely to provide a speedier recovery than OTC remedies alone.1
Strength of recommendation
B: Based on a single high-quality randomized controlled trial.
ILLUSTRATIVE CASE
A 28-year-old man comes to your clinic after experiencing fatigue, purulent nasal discharge, and unilateral facial pain for nearly
10 days. Overall, he appears healthy, and you diagnose acute rhinosinusitis. You suggest OTC remedies for supportive care and wonder if a course of amoxicillin would speed his
recovery.
Each year, more than 30 million Americans—about one in seven adults—are diagnosed with sinusitis.2 No more than 2% of these cases are thought to be bacterial.3
CDC guidelines for the diagnosis of acute bacterial rhinosinusitis include symptoms that last seven or more days, with maxillary pain or tenderness in the face or teeth and purulent nasal secretions.4 Patients with symptoms lasting less than seven days are unlikely to have a bacterial infection. But the nonspecific signs and symptoms included in the CDC guidelines limit their usefulness in determining whether the cause of the sinusitis is bacterial or viral on clinical grounds alone.
Most cases of sinusitis spontaneously resolve
In patients with acute bacterial sinusitis, the American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) guidelines advocate watchful waiting and symptom relief with nasal oxymetazoline, pseudoephedrine, and saline nasal irrigation.3 The rate of spontaneous resolution is high: 80% of patients with clinically diagnosed sinusitis improve without treatment within two weeks.1,5
Traditional decongestants and mucolytics have not demonstrated efficacy in resolving sinusitis, although rigorous evaluation is lacking. Other treatments, such as saline irrigation and intranasal corticosteroids, are of unclear benefit and need further study.6-8
Lack of evidence has done little to curtail antibiotic use
A previous PURL that was based on a meta-analysis of antibiotic treatment trials for sinusitis recommended that we stop prescribing antibiotics for adults with acute sinusitis unless their symptoms are severe.9,10 Yet antibiotics remain the mainstay of treatment.
Despite the AAO–HNS guidelines, evidence of spontaneous resolution, and accumulating data on the lack of efficacy of antimicrobials for sinusitis, 81% of patients diagnosed with acute sinusitis were given prescriptions for antibiotics, a study of primary care practices showed.11 Frequent use of antibiotics contributes to high rates of drug resistance, and adverse events related to antibiotic use account for an estimated 142,500 emergency department visits annually.12
STUDY SUMMARY
Little benefit from amoxicillin, even for severe cases
Garbutt and colleagues revisited the issue, randomizing 166 patients from 10 primary care practices to receive amoxicillin plus symptomatic treatment or placebo plus symptomatic treatment for acute rhinosinusitis.1
To be eligible for the study, patients had to be between the ages of 18 and 70, meet CDC diagnostic criteria for acute rhinosinusitis, and have moderate to very severe symptoms that were of seven- to-28-day duration and worsening or not improving, or of < 7-day duration but had worsened after an initial improvement. Exclusion criteria included complications from sinusitis, a history of allergy to penicillin or amoxicillin, antibiotic use in the past four weeks, comorbidities that impair immune function, cystic fibrosis, pregnancy, and mild symptoms.
Both groups had similar baseline characteristics, with participants who were predominantly white (79%) and female (64%). All the participants received a supply of symptomatic treatments: acetaminophen, guaifenesin, dextromethorphan, and sustained-release pseudoephedrine. The treatment group also received amoxicillin 1,500 mg/d, divided into three doses; the placebo group received identical-looking placebo pills.
Patients were assessed with the Sino-nasal Outcome Test-16 (SNOT-16), a validated measure that asks patients to assess both the severity and frequency of 16 sinus symptoms. SNOT-16 uses a 0-to-3 rating scale (0 = no problem; 3 = severe problem), with a clinically important difference of ≥ 0.5 on the mean score. The test was administered at enrollment and at days 3, 7, and 10. The disease-specific quality of life at day 3 was the primary outcome.
There was no statistically significantly difference in SNOT-16 scores between the amoxicillin and placebo groups on days 3 and 10. On day 7, there was a small statistically significant improvement in the amoxicillin group, but it did not reach the level of clinical importance (≥ 0.5) based on SNOT-16’s mean score.
The authors also asked participants to retrospectively assess symptom change since enrollment on a six-point scale. Those who reported that their symptoms were “a lot better” or “absent” were characterized as significantly improved. The results correlated with the data from the SNOT-16, showing no difference between the amoxicillin and control groups at days 3 and 10. On day 7, 74% of patients treated with amoxicillin self-
reported significant improvement in symptoms since the start of the study, versus 56% in the control group. The number needed to treat was 6 (95% confidence interval, 3 to 34; P = .02) for a reduction in symptoms at day 7.
Patients in both groups had similar rates of absenteeism, inability to perform usual activities, relapse and recurrence, and use of additional health care. Satisfaction with treatment was similar, as well.
No serious adverse effects occurred. Both groups reported similar frequencies (< 10%) of nausea, diarrhea, abdominal pain, or vaginitis.
WHAT’S NEW
Even severe sinusitis resolves without antibiotics
Previous studies recommended foregoing antibiotics for acute sinusitis, except when symptoms are severe. This study—in which more than half (52%) of patients in each group had symptoms rated severe or very severe—found no benefit to adding amoxicillin to supportive treatments.1 Antibiotics did not shorten the duration of illness, prevent relapse and recurrence, or improve satisfaction with treatment. The researchers found a statistically significant difference between groups on day 7 of 0.19 points, but no clinically meaningful difference (≥ 0.5) based on the SNOT-16 mean score.
CAVEATS
Guidelines, risk of complications may give reason to pause
The 2012 Infectious Diseases Society of America guidelines recommend amoxicillin with clavulanic acid as empiric therapy for acute bacterial rhinosinusitis.7 The findings of the study by Garbutt et al—conducted at a time when the incidence of beta-lactamase-producing organisms was low and amoxicillin was the treatment of choice—suggest otherwise.
Serious complications of sinusitis, such as brain abscess, periorbital cellulitis, and meningitis, can occur, however. Patients who deteriorate clinically or develop high fever or severe headache require close follow-up, which may include further diagnostic evaluation or consultation with an otolaryngologist. Evidence is lacking as to whether antibiotics prevent such complications.5
CHALLENGES TO IMPLEMENTATION
Managing patient expectations
Many patients with symptoms of acute rhinosinusitis think they need an antibiotic. Managing their expectations and providing instructions about supportive treatments are time consuming and may be
difficult.
Nonetheless, we’re optimistic: We think that most patients today are aware of the problems associated with antibiotic resistance and wary of “superbugs,” and will therefore be receptive to this practice change. Clinicians can help by reminding patients of the adverse effects of antibiotics and the natural course of rhinosinusitis, as well as by offering symptomatic treatments.
REFERENCES
1. Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
2. Centers for Disease Control and Prevention. Summary health statistics for US adults: National Health Interview Survey 2010. January 2012. www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed July 9, 2012.
3. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007:137(3 suppl):S1-S31.
4. Hickner JM, Bartlett JG, Besser RE, et al; American Academy of Family Physians; American College of Physicians; American Society of Internal Medicine; Centers for Disease Control; Infectious Disease Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults; background. Ann Intern Med. 2001;134:498-505.
5. Ahovuo-Saloranta A, Borisenk OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Sys Rev. 2008(2):CD000243.
6. Allen G, Kelsberg G, Jankowski TA. Do nasal decongestants relieve symptoms? J Fam Pract. 2003;52:714-724.
7. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
8. Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2009(4):CD005149.
9. Schumann A, Hickner, J. Patients insist on antibiotics for sinusitis? Here is a good reason to say “no.” J Fam Pract. 2008;57:464-468.
10. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
11. Gill JM, Fleischut P, Haas S. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med. 2006;38:349-354.
12. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008; 47:735-743.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2012 The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2012;61(10):610-612.
Practice Changer
Stop prescribing amoxicillin for acute rhinosinusitis. It’s unlikely to provide a speedier recovery than OTC remedies alone.1
Strength of recommendation
B: Based on a single high-quality randomized controlled trial.
ILLUSTRATIVE CASE
A 28-year-old man comes to your clinic after experiencing fatigue, purulent nasal discharge, and unilateral facial pain for nearly
10 days. Overall, he appears healthy, and you diagnose acute rhinosinusitis. You suggest OTC remedies for supportive care and wonder if a course of amoxicillin would speed his
recovery.
Each year, more than 30 million Americans—about one in seven adults—are diagnosed with sinusitis.2 No more than 2% of these cases are thought to be bacterial.3
CDC guidelines for the diagnosis of acute bacterial rhinosinusitis include symptoms that last seven or more days, with maxillary pain or tenderness in the face or teeth and purulent nasal secretions.4 Patients with symptoms lasting less than seven days are unlikely to have a bacterial infection. But the nonspecific signs and symptoms included in the CDC guidelines limit their usefulness in determining whether the cause of the sinusitis is bacterial or viral on clinical grounds alone.
Most cases of sinusitis spontaneously resolve
In patients with acute bacterial sinusitis, the American Academy of Otolaryngology–Head and Neck Surgery (AAO–HNS) guidelines advocate watchful waiting and symptom relief with nasal oxymetazoline, pseudoephedrine, and saline nasal irrigation.3 The rate of spontaneous resolution is high: 80% of patients with clinically diagnosed sinusitis improve without treatment within two weeks.1,5
Traditional decongestants and mucolytics have not demonstrated efficacy in resolving sinusitis, although rigorous evaluation is lacking. Other treatments, such as saline irrigation and intranasal corticosteroids, are of unclear benefit and need further study.6-8
Lack of evidence has done little to curtail antibiotic use
A previous PURL that was based on a meta-analysis of antibiotic treatment trials for sinusitis recommended that we stop prescribing antibiotics for adults with acute sinusitis unless their symptoms are severe.9,10 Yet antibiotics remain the mainstay of treatment.
Despite the AAO–HNS guidelines, evidence of spontaneous resolution, and accumulating data on the lack of efficacy of antimicrobials for sinusitis, 81% of patients diagnosed with acute sinusitis were given prescriptions for antibiotics, a study of primary care practices showed.11 Frequent use of antibiotics contributes to high rates of drug resistance, and adverse events related to antibiotic use account for an estimated 142,500 emergency department visits annually.12
STUDY SUMMARY
Little benefit from amoxicillin, even for severe cases
Garbutt and colleagues revisited the issue, randomizing 166 patients from 10 primary care practices to receive amoxicillin plus symptomatic treatment or placebo plus symptomatic treatment for acute rhinosinusitis.1
To be eligible for the study, patients had to be between the ages of 18 and 70, meet CDC diagnostic criteria for acute rhinosinusitis, and have moderate to very severe symptoms that were of seven- to-28-day duration and worsening or not improving, or of < 7-day duration but had worsened after an initial improvement. Exclusion criteria included complications from sinusitis, a history of allergy to penicillin or amoxicillin, antibiotic use in the past four weeks, comorbidities that impair immune function, cystic fibrosis, pregnancy, and mild symptoms.
Both groups had similar baseline characteristics, with participants who were predominantly white (79%) and female (64%). All the participants received a supply of symptomatic treatments: acetaminophen, guaifenesin, dextromethorphan, and sustained-release pseudoephedrine. The treatment group also received amoxicillin 1,500 mg/d, divided into three doses; the placebo group received identical-looking placebo pills.
Patients were assessed with the Sino-nasal Outcome Test-16 (SNOT-16), a validated measure that asks patients to assess both the severity and frequency of 16 sinus symptoms. SNOT-16 uses a 0-to-3 rating scale (0 = no problem; 3 = severe problem), with a clinically important difference of ≥ 0.5 on the mean score. The test was administered at enrollment and at days 3, 7, and 10. The disease-specific quality of life at day 3 was the primary outcome.
There was no statistically significantly difference in SNOT-16 scores between the amoxicillin and placebo groups on days 3 and 10. On day 7, there was a small statistically significant improvement in the amoxicillin group, but it did not reach the level of clinical importance (≥ 0.5) based on SNOT-16’s mean score.
The authors also asked participants to retrospectively assess symptom change since enrollment on a six-point scale. Those who reported that their symptoms were “a lot better” or “absent” were characterized as significantly improved. The results correlated with the data from the SNOT-16, showing no difference between the amoxicillin and control groups at days 3 and 10. On day 7, 74% of patients treated with amoxicillin self-
reported significant improvement in symptoms since the start of the study, versus 56% in the control group. The number needed to treat was 6 (95% confidence interval, 3 to 34; P = .02) for a reduction in symptoms at day 7.
Patients in both groups had similar rates of absenteeism, inability to perform usual activities, relapse and recurrence, and use of additional health care. Satisfaction with treatment was similar, as well.
No serious adverse effects occurred. Both groups reported similar frequencies (< 10%) of nausea, diarrhea, abdominal pain, or vaginitis.
WHAT’S NEW
Even severe sinusitis resolves without antibiotics
Previous studies recommended foregoing antibiotics for acute sinusitis, except when symptoms are severe. This study—in which more than half (52%) of patients in each group had symptoms rated severe or very severe—found no benefit to adding amoxicillin to supportive treatments.1 Antibiotics did not shorten the duration of illness, prevent relapse and recurrence, or improve satisfaction with treatment. The researchers found a statistically significant difference between groups on day 7 of 0.19 points, but no clinically meaningful difference (≥ 0.5) based on the SNOT-16 mean score.
CAVEATS
Guidelines, risk of complications may give reason to pause
The 2012 Infectious Diseases Society of America guidelines recommend amoxicillin with clavulanic acid as empiric therapy for acute bacterial rhinosinusitis.7 The findings of the study by Garbutt et al—conducted at a time when the incidence of beta-lactamase-producing organisms was low and amoxicillin was the treatment of choice—suggest otherwise.
Serious complications of sinusitis, such as brain abscess, periorbital cellulitis, and meningitis, can occur, however. Patients who deteriorate clinically or develop high fever or severe headache require close follow-up, which may include further diagnostic evaluation or consultation with an otolaryngologist. Evidence is lacking as to whether antibiotics prevent such complications.5
CHALLENGES TO IMPLEMENTATION
Managing patient expectations
Many patients with symptoms of acute rhinosinusitis think they need an antibiotic. Managing their expectations and providing instructions about supportive treatments are time consuming and may be
difficult.
Nonetheless, we’re optimistic: We think that most patients today are aware of the problems associated with antibiotic resistance and wary of “superbugs,” and will therefore be receptive to this practice change. Clinicians can help by reminding patients of the adverse effects of antibiotics and the natural course of rhinosinusitis, as well as by offering symptomatic treatments.
REFERENCES
1. Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
2. Centers for Disease Control and Prevention. Summary health statistics for US adults: National Health Interview Survey 2010. January 2012. www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed July 9, 2012.
3. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007:137(3 suppl):S1-S31.
4. Hickner JM, Bartlett JG, Besser RE, et al; American Academy of Family Physians; American College of Physicians; American Society of Internal Medicine; Centers for Disease Control; Infectious Disease Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults; background. Ann Intern Med. 2001;134:498-505.
5. Ahovuo-Saloranta A, Borisenk OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Sys Rev. 2008(2):CD000243.
6. Allen G, Kelsberg G, Jankowski TA. Do nasal decongestants relieve symptoms? J Fam Pract. 2003;52:714-724.
7. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
8. Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2009(4):CD005149.
9. Schumann A, Hickner, J. Patients insist on antibiotics for sinusitis? Here is a good reason to say “no.” J Fam Pract. 2008;57:464-468.
10. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
11. Gill JM, Fleischut P, Haas S. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med. 2006;38:349-354.
12. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008; 47:735-743.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2012 The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2012;61(10):610-612.
Don't Quit on a Quitter: Helping Your Patient Stop Smoking
Although smoking prevalence among US adults declined by about 42% between 1965 and 2011, this reduction has slowed in recent years. According to 2010 figures from the CDC, 19.3% of US adults (nearly one in five) remain current smokers,1 despite all the evidence of negative effects that tobacco use and smoke exposure exert on good health.2 Each year, 443,000 premature deaths in the US are attributed to cigarette smoking.3
In the face of these discouraging data, and in ongoing efforts to minimize the deadly effects of cigarette smoking, the US Department of Health and Human Services’ program, Healthy People 2020,4 restated its 10-year goals pertaining to tobacco use as put forth in Healthy People 2010.5 These are to reduce the proportion of Americans who currently smoke to 12%, and to increase the proportion of current smokers who have attempted cessation to 80%.4
Though trained to encourage smoking cessation in their patients, health care providers often lack the knowledge, skills, or resources to support patients through the difficulties of discontinuing tobacco use. Whatever barriers clinicians may face in accessing smoking cessation services, the barriers faced by underserved patients are often greater.6 A heightened awareness of these barriers and improved understanding of common smoking cessation methods will help providers better support their patients who are trying to quit.
ASSESSING READINESS AND MOTIVATING PATIENTS
In 2008, the Public Health Service, US Department of Health and Human Services, updated its clinical practice guidelines for clinicians who manage tobacco-dependent patients.7 According to evidence from randomized controlled trials, even brief interventions on the health care provider’s part (such as raising the issue of smoking cessation at each patient visit to determine whether the patient is ready to quit) can prompt the patient to seriously consider smoking abstinence.3,8 Being asked repeatedly can advance a patient’s readiness, and any attempts patients make to quit must be robustly supported.9
The Public Health Service’s five A’s3,7 outline the recommended conversation with patients:
Ask about tobacco use;
Advise tobacco users to quit in strong, clear terms;
Assess readiness for tobacco cessation;
Assist in developing a plan to stop tobacco use; and
Arrange a follow-up consultation to review the patient’s success or to reassess cessation readiness.
Whenever a patient expresses any interest in smoking cessation, it is essential for the clinician to respond with motivation. To help the patient prepare to stop, the clinician must address the five R’s7:
Relevance—the importance of smoking cessation to the patient;
Risks of continuing to smoke, particularly health concerns;
Rewards of smoking cessation, especially alleviation of health complaints;
Roadblocks that contribute to the threat of relapse—but which can be overcome with sufficient motivation; and
Repetition of support and reinforcement for this healthy lifestyle choice, be it from family, friends, coworkers, or the clinician. To prepare for times when support seems to wane, patients should be encouraged to phone 1-800-QUITNOW, a number that will connect the caller to his or her state’s quit line.
Repetition may also imply repeated cessation attempts if the first (or most recent) was unsuccessful. According to findings from a literature review, the reasons smokers relapse are numerous, including cravings (the most common) and withdrawal symptoms, weight gain, stress, and exposure to other smokers.10,11 Interventions based on patients’ given reasons for relapse have no apparent impact on the rate of return to smoking.12 Nevertheless, clinicians must take responsibility to motivate patients and reinforce their successes at every encounter.
UNAIDED CESSATION
In a culture demanding quick results and in the context of ongoing pharmaceutical advertisements and aisles lined with quit-smoking products, it may be easy to dismiss unaided cessation. Options range from gradual cutting down to the abrupt discontinuation of tobacco—going cold turkey. Although little research has been devoted to these strategies,13 unaided cessation is the method patients most often cite in their attempts to quit—and the method successful quitters report as most effective.14,15 The patients most likely to succeed at quitting are those who do not ask for help.
ASSISTED CESSATION
Patients with a significant dependence on nicotine are likely to request assistance with cessation.15 Identifying those who struggle to quit smoking and offering appropriate support may represent the difference between their success and failure.9
Several available tools to support patients’ “quit smoking” efforts, including pharmacologic options (see table7), are described here.
Nicotine Replacement Therapy
Nicotine replacement therapy (NRT) is used to reduce nicotine withdrawal symptoms by replacing smoking-produced nicotine with an alternate source of delivery. NRT is currently available in several forms: a transdermal patch, gum, lozenge, or the electronic cigarette. The chance of successful quitting is increased 50% to 70% when NRT is used, compared with patients using placebo.16 While the various forms of NRT share the same goal, they are not equally effective.17
Nicotine transdermal patches have been used in the US since 1991. The patches are used in a stepwise fashion; each patch delivers nicotine at a consistent quantity per hour, and over time, patches with increasingly lower doses of nicotine are substituted. There is some evidence that the patch is more efficacious for maintenance after smoking cessation than for the initial effort to quit.18
Nicotine gum and lozenges, orally absorbed forms of NRT, are used as needed, depending on patients’ withdrawal symptoms. Japuntich et al18 found that these products alone are not beneficial. However, combining bupropion with gum or lozenge therapy was found more effective for patients attempting to stop smoking than either agent alone.18 Lozenges have also been described as increasingly beneficial when combined with a longer-acting NRT, such as a transdermal patch, when cravings increase and rapid delivery of nicotine is required.16
The electronic cigarette (e-cigarette) is a battery-operated device that aerosolizes liquid nicotine, which is then orally absorbed. In a 2011 study, Siegel et al19 found that more than two-thirds of smokers reduced the number of cigarettes smoked after using an e-cigarette. Six months after subjects first purchased e-cigarettes, 31% remained tobacco-abstinent.
Since e-cigarettes are flameless, their use has been suggested in areas where smoking was previously prohibited. This short-acting NRT may benefit a patient when craving is provoked by forced denial of nicotine.
Current research is under way to examine two newer potential NRT tools: a nicotine mouth spray and a nicotine vaccine.20,21 In a randomized, double-blind, placebo-controlled study, Tønnesen et al20 found that use of a nicotine mouth spray was associated with significantly higher rates of tobacco abstinence at six, 24, and 52 weeks, compared with patients receiving placebo; however, rates of adverse effects were high in both groups (88% and 71%, respectively).
NRT is inexpensive and easily accessible to patients. Since its forms are all available OTC, consultation with health care providers is unnecessary. For patients who have tried to quit smoking unaided and who need short-term or immediate assistance to prevent a smoking relapse, NRT can be a helpful resource.
Bupropion
For smokers who want to quit without using a nicotine-based intervention, the antidepressant bupropion can be a promising smoking cessation aid. It is not clear what mechanism of action helps smokers who take bupropion to stop, although its chemical structure resembles that of diethylpropion, a drug used as an appetite suppressant.22 Bupropion does hinder norepinephrine and dopamine reuptake in the nervous system—opposing an effect of nicotine withdrawal.
Bupropion’s effects as an antidepressant and as a smoking cessation aid do not appear to be related.22 For this reason, even a patient who has not responded to bupropion for treatment of depression may benefit from using it as a smoking cessation aid.
Bupropion may be used alone or with other agents to stop nicotine use. Many study groups report that a combination of medications is more effective than monotherapy, and this is true for combinations that include bupropion.23-26 When used with nicotine lozenges, bupropion has been found effective in preventing a return to tobacco after previous lapses in smoking abstinence. Aside from a nicotine patch, no other monotherapy or combination was effective at achieving this goal.18 Thus, bupropion may be best utilized as a component in combination therapy.
Varenicline
Approved for use in the US in 2006, varenicline is the newest pharmaceutical therapy for smoking cessation. As a partial nicotinic receptor agonist,7 varenicline prevents nicotine from activating the mesolimbic dopamine system, which is associated with pleasure and reward (among other functions). By stimulating the nervous system’s dopamine (though to a lesser extent than nicotine), this agent reduces cravings for tobacco and symptoms of nicotine withdrawal—which are among the greatest barriers to smoking cessation.10 Because of its mechanism of action, varenicline is not often used in combination with NRT.
Varenicline has been shown to be as effective as the combination therapy of bupropion with nicotine lozenges.7,27 UK investigators Hajek et al27 found that using varenicline for four weeks before attempting to stop smoking had minimal effect on smoking urges and withdrawal symptoms, compared with using varenicline for just one week before attempting to quit. However, those who used varenicline for four weeks before stopping smoking were more likely to be smoke-free at 12 weeks than those who had used it for just one week before quitting.27
Other Pharmaceutical Options
Clonidine, long recognized as an effective antihypertensive medication, was determined by Gourlay et al9 in a 2004 review to have potential for use in supporting smoking cessation. Because significant adverse events (including drowsiness, sedation, and postural hypotension) have been associated with clonidine use7,28 and the FDA has not yet approved it for the indication of smoking cessation, its use may be most appropriate as a second-line treatment option, in combination with bupropion or nortriptyline, or for specialists’ use.28 Clonidine should not be discontinued suddenly.
Like bupropion, the tricyclic antidepressant nortriptyline has been investigated for its potential in tobacco cessation therapy. While a significant amount is known about plasma concentrations of nortriptyline needed to treat depression, levels required for effective tobacco cessation are less clear. Mooney et al29 found that therapeutic plasma concentrations of nortriptyline varied among subjects by race and smoking habits; although a lower concentration was usually required to assist smoking cessation than to treat depression, adverse effects were common even at lower concentrations. Thus, it was recommended that nortriptyline be reserved for second-line treatment.
This summer, researchers for the Cochrane Tobacco Addiction Review Group published a review of the literature (including phase II and phase III trials conducted by pharmaceutical companies—making the risk for bias “high or unclear”) pertaining to two nicotine vaccines in development.21 In two studies, the level of development of nicotine antibodies was associated with commensurate cessation rates; in two others, the outcome measure (12 months’ abstinence from smoking) was met in 11% of subjects, whether they received the vaccine or placebo. Thus, no strong evidence yet exists that nicotine vaccination supports smoking cessation in the long term; further research is needed.
NONPHARMACEUTICAL
INTERVENTIONS
Acupuncture
Variations and modifications of the traditional Chinese therapy of acupuncture, including acupressure and electrostimulation, have been examined in a number of clinical trials. Despite the supporting rhetoric, objective research of good quality in this area is limited. However, one systematic literature review showed acupuncture to be only slightly more effective than sham interventions and less effective than NRT.30 Insufficient evidence was reported on acupressure and laser stimulation, and acupressure was no more effective than psychological treatments. Considering questionable study quality and other limitations in the currently available research, practitioners should not consider acupuncture or related interventions as first-line options—nor should their potential be dismissed altogether.
Hypnotherapy
Conclusive research findings regarding hypnotherapy as an effective treatment for tobacco dependence are also limited. In 2010, Barnes at al31 reviewed 11 studies comparing hypnotherapy with various alternate methods and found little difference in effectiveness among hypnotherapy, psychological counseling, and rapid smoking therapy. Despite the limitations in these data, however, hypnotherapy may be appropriate for some patients.
CONCLUSION
Tobacco dependence is not the same for any two patients. Just as health care providers do not use the same treatment option for every patient with hypertension or diabetes, treatment for tobacco-dependent patients must also be individualized.
Our professional goal is to care for the health of patients. We clinicians must recommend cessation to our patients who smoke at every encounter—and offer support often. When we miss an opportunity to counsel a patient on the importance of quitting, the patient may interpret our silence as condoning the behavior. Empowering patients with an understanding of the options can contribute to their success—a significant move toward better health.
The authors of Healthy People 2020 hope that 80% of current smokers will have tried to stop smoking by that year. Have 80% of your patients been counseled and offered assistance to stop?
REFERENCES
1. CDC. Current cigarette smoking prevalence among working adults—United States, 2004-2010. MMWR Morb Mortal Wkly Rep. 2011;60(38):1305-1309.
2. CDC. Vital signs: current cigarette smoking among adults aged ≥ 18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135-1140.
3. Jamal A, Dube SR, Malarcher AM, et al; CDC. Tobacco use screening and counseling during physician office visits among adults—National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61 suppl: 38-45.
4. US Department of Health and Human Services. Healthy People 2020 summary of objectives: tobacco use. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/TobaccoUse.pdf. Accessed October 18, 2012.
5. US Department of Health and Human Services. Healthy People 2010 archives. www.healthypeople.gov/2010. Accessed October 18, 2012.
6. Blumenthal DS. Barriers to the provision of smoking cessation services reported by clinicians in underserved communities. J Am Board Fam Med. 2007;20(3):272-279.
7. Fiore MC, Jaén CR, Baker TB, et al; Public Health Service, US Department of Health and Human Services. Clinical practice guideline: treating tobacco use and dependence: 2008 update. www.ahrq.gov/clinic/tobacco/treating_tobacco_use08.pdf. Accessed October 18, 2012.
8. Carson KV, Verbiest ME, Crone MR, et al. Training health professionals in smoking cessation. Cochrane Database Syst Rev. 2012 May 16; 5:CD000214.
9. Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane Database Syst Rev. 2004;(3):CD000058.
10. Guirguis AB, Ray SM, Zingone MM, et al. Smoking cessation: barriers to success and readiness to change. Tenn Med. 2010;103(9):45-49.
11. Nørregaard J, Tønnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Prev Med. 1993;22(2):261-271.
12. Lancaster T, Hajek P, Stead LF, et al. Prevention of relapse after quitting smoking: a systematic review of trials. Arch Intern Med. 2006;166(8): 828-835.
13. Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLoS Med. 2010;7(2):e1000216.
14. Hung WT, Dunlop SM, Perez D, Cotter T. Use and perceived helpfulness of smoking cessation methods: results from a population survey of recent quitters. BMC Public Health. 2011;11:592.
15. Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102-111.
16. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD000146.
17. Robles GI, Singh-Franco D, Ghin HL. A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations. Clin Ther. 2008;30(5):800-812.
18. Japuntich SJ, Piper ME, Leventhal AM, et al. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79(1):34-42.
19. Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: results from an online survey. Am J Prev Med. 2011;40 (4):472-475.
20. Tønnesen P, Lauri H, Perfekt R, et al. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J. 2012;40(3):548-554.
21. Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2012 Aug 15;8:CD007072.
22. Roddy E. Bupropion and other non-nicotine pharmacotherapies. BMJ. 2004;328(7438):
509-511.
23. Loh WY, Piper ME, Schlam TR, et al. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine Tob Res. 2012;14(2):131-141.
24. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):44-65.
25. McNeil JJ, Piccenna L, Ioannides-Demos LL. Smoking cessation: recent advances. Cardiovasc Drugs Ther. 2010;24(4):359-367.
26. Ebbert JO, Hays JT, Hurt RD. Combination pharmacotherapy for stopping smoking: what advantages does it offer? Drugs. 2010;70(6):643-650.
27. Hajek P, McRobbie HJ, Myers KE, et al. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171 (8):770-777.
28. Bentz CJ. Review: clonidine is more effective than placebo for long-term smoking cessation, but has side effects. ACP J Club. 2005;142(1):12.
29. Mooney ME, Reus VI, Gorecki J, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clin Pharmacol Ther. 2008;83(3):436-442.
30. White AR, Rampes H, Liu JP, et al. Acupuncture and related interventions for smoking cessation. Cochrane Database Syst Rev. 2011;(1): CD000009.
31. Barnes J, Dong CY, McRobbie H, et al. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev. 2010;(10):CD001008.
Although smoking prevalence among US adults declined by about 42% between 1965 and 2011, this reduction has slowed in recent years. According to 2010 figures from the CDC, 19.3% of US adults (nearly one in five) remain current smokers,1 despite all the evidence of negative effects that tobacco use and smoke exposure exert on good health.2 Each year, 443,000 premature deaths in the US are attributed to cigarette smoking.3
In the face of these discouraging data, and in ongoing efforts to minimize the deadly effects of cigarette smoking, the US Department of Health and Human Services’ program, Healthy People 2020,4 restated its 10-year goals pertaining to tobacco use as put forth in Healthy People 2010.5 These are to reduce the proportion of Americans who currently smoke to 12%, and to increase the proportion of current smokers who have attempted cessation to 80%.4
Though trained to encourage smoking cessation in their patients, health care providers often lack the knowledge, skills, or resources to support patients through the difficulties of discontinuing tobacco use. Whatever barriers clinicians may face in accessing smoking cessation services, the barriers faced by underserved patients are often greater.6 A heightened awareness of these barriers and improved understanding of common smoking cessation methods will help providers better support their patients who are trying to quit.
ASSESSING READINESS AND MOTIVATING PATIENTS
In 2008, the Public Health Service, US Department of Health and Human Services, updated its clinical practice guidelines for clinicians who manage tobacco-dependent patients.7 According to evidence from randomized controlled trials, even brief interventions on the health care provider’s part (such as raising the issue of smoking cessation at each patient visit to determine whether the patient is ready to quit) can prompt the patient to seriously consider smoking abstinence.3,8 Being asked repeatedly can advance a patient’s readiness, and any attempts patients make to quit must be robustly supported.9
The Public Health Service’s five A’s3,7 outline the recommended conversation with patients:
Ask about tobacco use;
Advise tobacco users to quit in strong, clear terms;
Assess readiness for tobacco cessation;
Assist in developing a plan to stop tobacco use; and
Arrange a follow-up consultation to review the patient’s success or to reassess cessation readiness.
Whenever a patient expresses any interest in smoking cessation, it is essential for the clinician to respond with motivation. To help the patient prepare to stop, the clinician must address the five R’s7:
Relevance—the importance of smoking cessation to the patient;
Risks of continuing to smoke, particularly health concerns;
Rewards of smoking cessation, especially alleviation of health complaints;
Roadblocks that contribute to the threat of relapse—but which can be overcome with sufficient motivation; and
Repetition of support and reinforcement for this healthy lifestyle choice, be it from family, friends, coworkers, or the clinician. To prepare for times when support seems to wane, patients should be encouraged to phone 1-800-QUITNOW, a number that will connect the caller to his or her state’s quit line.
Repetition may also imply repeated cessation attempts if the first (or most recent) was unsuccessful. According to findings from a literature review, the reasons smokers relapse are numerous, including cravings (the most common) and withdrawal symptoms, weight gain, stress, and exposure to other smokers.10,11 Interventions based on patients’ given reasons for relapse have no apparent impact on the rate of return to smoking.12 Nevertheless, clinicians must take responsibility to motivate patients and reinforce their successes at every encounter.
UNAIDED CESSATION
In a culture demanding quick results and in the context of ongoing pharmaceutical advertisements and aisles lined with quit-smoking products, it may be easy to dismiss unaided cessation. Options range from gradual cutting down to the abrupt discontinuation of tobacco—going cold turkey. Although little research has been devoted to these strategies,13 unaided cessation is the method patients most often cite in their attempts to quit—and the method successful quitters report as most effective.14,15 The patients most likely to succeed at quitting are those who do not ask for help.
ASSISTED CESSATION
Patients with a significant dependence on nicotine are likely to request assistance with cessation.15 Identifying those who struggle to quit smoking and offering appropriate support may represent the difference between their success and failure.9
Several available tools to support patients’ “quit smoking” efforts, including pharmacologic options (see table7), are described here.
Nicotine Replacement Therapy
Nicotine replacement therapy (NRT) is used to reduce nicotine withdrawal symptoms by replacing smoking-produced nicotine with an alternate source of delivery. NRT is currently available in several forms: a transdermal patch, gum, lozenge, or the electronic cigarette. The chance of successful quitting is increased 50% to 70% when NRT is used, compared with patients using placebo.16 While the various forms of NRT share the same goal, they are not equally effective.17
Nicotine transdermal patches have been used in the US since 1991. The patches are used in a stepwise fashion; each patch delivers nicotine at a consistent quantity per hour, and over time, patches with increasingly lower doses of nicotine are substituted. There is some evidence that the patch is more efficacious for maintenance after smoking cessation than for the initial effort to quit.18
Nicotine gum and lozenges, orally absorbed forms of NRT, are used as needed, depending on patients’ withdrawal symptoms. Japuntich et al18 found that these products alone are not beneficial. However, combining bupropion with gum or lozenge therapy was found more effective for patients attempting to stop smoking than either agent alone.18 Lozenges have also been described as increasingly beneficial when combined with a longer-acting NRT, such as a transdermal patch, when cravings increase and rapid delivery of nicotine is required.16
The electronic cigarette (e-cigarette) is a battery-operated device that aerosolizes liquid nicotine, which is then orally absorbed. In a 2011 study, Siegel et al19 found that more than two-thirds of smokers reduced the number of cigarettes smoked after using an e-cigarette. Six months after subjects first purchased e-cigarettes, 31% remained tobacco-abstinent.
Since e-cigarettes are flameless, their use has been suggested in areas where smoking was previously prohibited. This short-acting NRT may benefit a patient when craving is provoked by forced denial of nicotine.
Current research is under way to examine two newer potential NRT tools: a nicotine mouth spray and a nicotine vaccine.20,21 In a randomized, double-blind, placebo-controlled study, Tønnesen et al20 found that use of a nicotine mouth spray was associated with significantly higher rates of tobacco abstinence at six, 24, and 52 weeks, compared with patients receiving placebo; however, rates of adverse effects were high in both groups (88% and 71%, respectively).
NRT is inexpensive and easily accessible to patients. Since its forms are all available OTC, consultation with health care providers is unnecessary. For patients who have tried to quit smoking unaided and who need short-term or immediate assistance to prevent a smoking relapse, NRT can be a helpful resource.
Bupropion
For smokers who want to quit without using a nicotine-based intervention, the antidepressant bupropion can be a promising smoking cessation aid. It is not clear what mechanism of action helps smokers who take bupropion to stop, although its chemical structure resembles that of diethylpropion, a drug used as an appetite suppressant.22 Bupropion does hinder norepinephrine and dopamine reuptake in the nervous system—opposing an effect of nicotine withdrawal.
Bupropion’s effects as an antidepressant and as a smoking cessation aid do not appear to be related.22 For this reason, even a patient who has not responded to bupropion for treatment of depression may benefit from using it as a smoking cessation aid.
Bupropion may be used alone or with other agents to stop nicotine use. Many study groups report that a combination of medications is more effective than monotherapy, and this is true for combinations that include bupropion.23-26 When used with nicotine lozenges, bupropion has been found effective in preventing a return to tobacco after previous lapses in smoking abstinence. Aside from a nicotine patch, no other monotherapy or combination was effective at achieving this goal.18 Thus, bupropion may be best utilized as a component in combination therapy.
Varenicline
Approved for use in the US in 2006, varenicline is the newest pharmaceutical therapy for smoking cessation. As a partial nicotinic receptor agonist,7 varenicline prevents nicotine from activating the mesolimbic dopamine system, which is associated with pleasure and reward (among other functions). By stimulating the nervous system’s dopamine (though to a lesser extent than nicotine), this agent reduces cravings for tobacco and symptoms of nicotine withdrawal—which are among the greatest barriers to smoking cessation.10 Because of its mechanism of action, varenicline is not often used in combination with NRT.
Varenicline has been shown to be as effective as the combination therapy of bupropion with nicotine lozenges.7,27 UK investigators Hajek et al27 found that using varenicline for four weeks before attempting to stop smoking had minimal effect on smoking urges and withdrawal symptoms, compared with using varenicline for just one week before attempting to quit. However, those who used varenicline for four weeks before stopping smoking were more likely to be smoke-free at 12 weeks than those who had used it for just one week before quitting.27
Other Pharmaceutical Options
Clonidine, long recognized as an effective antihypertensive medication, was determined by Gourlay et al9 in a 2004 review to have potential for use in supporting smoking cessation. Because significant adverse events (including drowsiness, sedation, and postural hypotension) have been associated with clonidine use7,28 and the FDA has not yet approved it for the indication of smoking cessation, its use may be most appropriate as a second-line treatment option, in combination with bupropion or nortriptyline, or for specialists’ use.28 Clonidine should not be discontinued suddenly.
Like bupropion, the tricyclic antidepressant nortriptyline has been investigated for its potential in tobacco cessation therapy. While a significant amount is known about plasma concentrations of nortriptyline needed to treat depression, levels required for effective tobacco cessation are less clear. Mooney et al29 found that therapeutic plasma concentrations of nortriptyline varied among subjects by race and smoking habits; although a lower concentration was usually required to assist smoking cessation than to treat depression, adverse effects were common even at lower concentrations. Thus, it was recommended that nortriptyline be reserved for second-line treatment.
This summer, researchers for the Cochrane Tobacco Addiction Review Group published a review of the literature (including phase II and phase III trials conducted by pharmaceutical companies—making the risk for bias “high or unclear”) pertaining to two nicotine vaccines in development.21 In two studies, the level of development of nicotine antibodies was associated with commensurate cessation rates; in two others, the outcome measure (12 months’ abstinence from smoking) was met in 11% of subjects, whether they received the vaccine or placebo. Thus, no strong evidence yet exists that nicotine vaccination supports smoking cessation in the long term; further research is needed.
NONPHARMACEUTICAL
INTERVENTIONS
Acupuncture
Variations and modifications of the traditional Chinese therapy of acupuncture, including acupressure and electrostimulation, have been examined in a number of clinical trials. Despite the supporting rhetoric, objective research of good quality in this area is limited. However, one systematic literature review showed acupuncture to be only slightly more effective than sham interventions and less effective than NRT.30 Insufficient evidence was reported on acupressure and laser stimulation, and acupressure was no more effective than psychological treatments. Considering questionable study quality and other limitations in the currently available research, practitioners should not consider acupuncture or related interventions as first-line options—nor should their potential be dismissed altogether.
Hypnotherapy
Conclusive research findings regarding hypnotherapy as an effective treatment for tobacco dependence are also limited. In 2010, Barnes at al31 reviewed 11 studies comparing hypnotherapy with various alternate methods and found little difference in effectiveness among hypnotherapy, psychological counseling, and rapid smoking therapy. Despite the limitations in these data, however, hypnotherapy may be appropriate for some patients.
CONCLUSION
Tobacco dependence is not the same for any two patients. Just as health care providers do not use the same treatment option for every patient with hypertension or diabetes, treatment for tobacco-dependent patients must also be individualized.
Our professional goal is to care for the health of patients. We clinicians must recommend cessation to our patients who smoke at every encounter—and offer support often. When we miss an opportunity to counsel a patient on the importance of quitting, the patient may interpret our silence as condoning the behavior. Empowering patients with an understanding of the options can contribute to their success—a significant move toward better health.
The authors of Healthy People 2020 hope that 80% of current smokers will have tried to stop smoking by that year. Have 80% of your patients been counseled and offered assistance to stop?
REFERENCES
1. CDC. Current cigarette smoking prevalence among working adults—United States, 2004-2010. MMWR Morb Mortal Wkly Rep. 2011;60(38):1305-1309.
2. CDC. Vital signs: current cigarette smoking among adults aged ≥ 18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135-1140.
3. Jamal A, Dube SR, Malarcher AM, et al; CDC. Tobacco use screening and counseling during physician office visits among adults—National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61 suppl: 38-45.
4. US Department of Health and Human Services. Healthy People 2020 summary of objectives: tobacco use. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/TobaccoUse.pdf. Accessed October 18, 2012.
5. US Department of Health and Human Services. Healthy People 2010 archives. www.healthypeople.gov/2010. Accessed October 18, 2012.
6. Blumenthal DS. Barriers to the provision of smoking cessation services reported by clinicians in underserved communities. J Am Board Fam Med. 2007;20(3):272-279.
7. Fiore MC, Jaén CR, Baker TB, et al; Public Health Service, US Department of Health and Human Services. Clinical practice guideline: treating tobacco use and dependence: 2008 update. www.ahrq.gov/clinic/tobacco/treating_tobacco_use08.pdf. Accessed October 18, 2012.
8. Carson KV, Verbiest ME, Crone MR, et al. Training health professionals in smoking cessation. Cochrane Database Syst Rev. 2012 May 16; 5:CD000214.
9. Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane Database Syst Rev. 2004;(3):CD000058.
10. Guirguis AB, Ray SM, Zingone MM, et al. Smoking cessation: barriers to success and readiness to change. Tenn Med. 2010;103(9):45-49.
11. Nørregaard J, Tønnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Prev Med. 1993;22(2):261-271.
12. Lancaster T, Hajek P, Stead LF, et al. Prevention of relapse after quitting smoking: a systematic review of trials. Arch Intern Med. 2006;166(8): 828-835.
13. Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLoS Med. 2010;7(2):e1000216.
14. Hung WT, Dunlop SM, Perez D, Cotter T. Use and perceived helpfulness of smoking cessation methods: results from a population survey of recent quitters. BMC Public Health. 2011;11:592.
15. Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102-111.
16. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD000146.
17. Robles GI, Singh-Franco D, Ghin HL. A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations. Clin Ther. 2008;30(5):800-812.
18. Japuntich SJ, Piper ME, Leventhal AM, et al. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79(1):34-42.
19. Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: results from an online survey. Am J Prev Med. 2011;40 (4):472-475.
20. Tønnesen P, Lauri H, Perfekt R, et al. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J. 2012;40(3):548-554.
21. Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2012 Aug 15;8:CD007072.
22. Roddy E. Bupropion and other non-nicotine pharmacotherapies. BMJ. 2004;328(7438):
509-511.
23. Loh WY, Piper ME, Schlam TR, et al. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine Tob Res. 2012;14(2):131-141.
24. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):44-65.
25. McNeil JJ, Piccenna L, Ioannides-Demos LL. Smoking cessation: recent advances. Cardiovasc Drugs Ther. 2010;24(4):359-367.
26. Ebbert JO, Hays JT, Hurt RD. Combination pharmacotherapy for stopping smoking: what advantages does it offer? Drugs. 2010;70(6):643-650.
27. Hajek P, McRobbie HJ, Myers KE, et al. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171 (8):770-777.
28. Bentz CJ. Review: clonidine is more effective than placebo for long-term smoking cessation, but has side effects. ACP J Club. 2005;142(1):12.
29. Mooney ME, Reus VI, Gorecki J, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clin Pharmacol Ther. 2008;83(3):436-442.
30. White AR, Rampes H, Liu JP, et al. Acupuncture and related interventions for smoking cessation. Cochrane Database Syst Rev. 2011;(1): CD000009.
31. Barnes J, Dong CY, McRobbie H, et al. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev. 2010;(10):CD001008.
Although smoking prevalence among US adults declined by about 42% between 1965 and 2011, this reduction has slowed in recent years. According to 2010 figures from the CDC, 19.3% of US adults (nearly one in five) remain current smokers,1 despite all the evidence of negative effects that tobacco use and smoke exposure exert on good health.2 Each year, 443,000 premature deaths in the US are attributed to cigarette smoking.3
In the face of these discouraging data, and in ongoing efforts to minimize the deadly effects of cigarette smoking, the US Department of Health and Human Services’ program, Healthy People 2020,4 restated its 10-year goals pertaining to tobacco use as put forth in Healthy People 2010.5 These are to reduce the proportion of Americans who currently smoke to 12%, and to increase the proportion of current smokers who have attempted cessation to 80%.4
Though trained to encourage smoking cessation in their patients, health care providers often lack the knowledge, skills, or resources to support patients through the difficulties of discontinuing tobacco use. Whatever barriers clinicians may face in accessing smoking cessation services, the barriers faced by underserved patients are often greater.6 A heightened awareness of these barriers and improved understanding of common smoking cessation methods will help providers better support their patients who are trying to quit.
ASSESSING READINESS AND MOTIVATING PATIENTS
In 2008, the Public Health Service, US Department of Health and Human Services, updated its clinical practice guidelines for clinicians who manage tobacco-dependent patients.7 According to evidence from randomized controlled trials, even brief interventions on the health care provider’s part (such as raising the issue of smoking cessation at each patient visit to determine whether the patient is ready to quit) can prompt the patient to seriously consider smoking abstinence.3,8 Being asked repeatedly can advance a patient’s readiness, and any attempts patients make to quit must be robustly supported.9
The Public Health Service’s five A’s3,7 outline the recommended conversation with patients:
Ask about tobacco use;
Advise tobacco users to quit in strong, clear terms;
Assess readiness for tobacco cessation;
Assist in developing a plan to stop tobacco use; and
Arrange a follow-up consultation to review the patient’s success or to reassess cessation readiness.
Whenever a patient expresses any interest in smoking cessation, it is essential for the clinician to respond with motivation. To help the patient prepare to stop, the clinician must address the five R’s7:
Relevance—the importance of smoking cessation to the patient;
Risks of continuing to smoke, particularly health concerns;
Rewards of smoking cessation, especially alleviation of health complaints;
Roadblocks that contribute to the threat of relapse—but which can be overcome with sufficient motivation; and
Repetition of support and reinforcement for this healthy lifestyle choice, be it from family, friends, coworkers, or the clinician. To prepare for times when support seems to wane, patients should be encouraged to phone 1-800-QUITNOW, a number that will connect the caller to his or her state’s quit line.
Repetition may also imply repeated cessation attempts if the first (or most recent) was unsuccessful. According to findings from a literature review, the reasons smokers relapse are numerous, including cravings (the most common) and withdrawal symptoms, weight gain, stress, and exposure to other smokers.10,11 Interventions based on patients’ given reasons for relapse have no apparent impact on the rate of return to smoking.12 Nevertheless, clinicians must take responsibility to motivate patients and reinforce their successes at every encounter.
UNAIDED CESSATION
In a culture demanding quick results and in the context of ongoing pharmaceutical advertisements and aisles lined with quit-smoking products, it may be easy to dismiss unaided cessation. Options range from gradual cutting down to the abrupt discontinuation of tobacco—going cold turkey. Although little research has been devoted to these strategies,13 unaided cessation is the method patients most often cite in their attempts to quit—and the method successful quitters report as most effective.14,15 The patients most likely to succeed at quitting are those who do not ask for help.
ASSISTED CESSATION
Patients with a significant dependence on nicotine are likely to request assistance with cessation.15 Identifying those who struggle to quit smoking and offering appropriate support may represent the difference between their success and failure.9
Several available tools to support patients’ “quit smoking” efforts, including pharmacologic options (see table7), are described here.
Nicotine Replacement Therapy
Nicotine replacement therapy (NRT) is used to reduce nicotine withdrawal symptoms by replacing smoking-produced nicotine with an alternate source of delivery. NRT is currently available in several forms: a transdermal patch, gum, lozenge, or the electronic cigarette. The chance of successful quitting is increased 50% to 70% when NRT is used, compared with patients using placebo.16 While the various forms of NRT share the same goal, they are not equally effective.17
Nicotine transdermal patches have been used in the US since 1991. The patches are used in a stepwise fashion; each patch delivers nicotine at a consistent quantity per hour, and over time, patches with increasingly lower doses of nicotine are substituted. There is some evidence that the patch is more efficacious for maintenance after smoking cessation than for the initial effort to quit.18
Nicotine gum and lozenges, orally absorbed forms of NRT, are used as needed, depending on patients’ withdrawal symptoms. Japuntich et al18 found that these products alone are not beneficial. However, combining bupropion with gum or lozenge therapy was found more effective for patients attempting to stop smoking than either agent alone.18 Lozenges have also been described as increasingly beneficial when combined with a longer-acting NRT, such as a transdermal patch, when cravings increase and rapid delivery of nicotine is required.16
The electronic cigarette (e-cigarette) is a battery-operated device that aerosolizes liquid nicotine, which is then orally absorbed. In a 2011 study, Siegel et al19 found that more than two-thirds of smokers reduced the number of cigarettes smoked after using an e-cigarette. Six months after subjects first purchased e-cigarettes, 31% remained tobacco-abstinent.
Since e-cigarettes are flameless, their use has been suggested in areas where smoking was previously prohibited. This short-acting NRT may benefit a patient when craving is provoked by forced denial of nicotine.
Current research is under way to examine two newer potential NRT tools: a nicotine mouth spray and a nicotine vaccine.20,21 In a randomized, double-blind, placebo-controlled study, Tønnesen et al20 found that use of a nicotine mouth spray was associated with significantly higher rates of tobacco abstinence at six, 24, and 52 weeks, compared with patients receiving placebo; however, rates of adverse effects were high in both groups (88% and 71%, respectively).
NRT is inexpensive and easily accessible to patients. Since its forms are all available OTC, consultation with health care providers is unnecessary. For patients who have tried to quit smoking unaided and who need short-term or immediate assistance to prevent a smoking relapse, NRT can be a helpful resource.
Bupropion
For smokers who want to quit without using a nicotine-based intervention, the antidepressant bupropion can be a promising smoking cessation aid. It is not clear what mechanism of action helps smokers who take bupropion to stop, although its chemical structure resembles that of diethylpropion, a drug used as an appetite suppressant.22 Bupropion does hinder norepinephrine and dopamine reuptake in the nervous system—opposing an effect of nicotine withdrawal.
Bupropion’s effects as an antidepressant and as a smoking cessation aid do not appear to be related.22 For this reason, even a patient who has not responded to bupropion for treatment of depression may benefit from using it as a smoking cessation aid.
Bupropion may be used alone or with other agents to stop nicotine use. Many study groups report that a combination of medications is more effective than monotherapy, and this is true for combinations that include bupropion.23-26 When used with nicotine lozenges, bupropion has been found effective in preventing a return to tobacco after previous lapses in smoking abstinence. Aside from a nicotine patch, no other monotherapy or combination was effective at achieving this goal.18 Thus, bupropion may be best utilized as a component in combination therapy.
Varenicline
Approved for use in the US in 2006, varenicline is the newest pharmaceutical therapy for smoking cessation. As a partial nicotinic receptor agonist,7 varenicline prevents nicotine from activating the mesolimbic dopamine system, which is associated with pleasure and reward (among other functions). By stimulating the nervous system’s dopamine (though to a lesser extent than nicotine), this agent reduces cravings for tobacco and symptoms of nicotine withdrawal—which are among the greatest barriers to smoking cessation.10 Because of its mechanism of action, varenicline is not often used in combination with NRT.
Varenicline has been shown to be as effective as the combination therapy of bupropion with nicotine lozenges.7,27 UK investigators Hajek et al27 found that using varenicline for four weeks before attempting to stop smoking had minimal effect on smoking urges and withdrawal symptoms, compared with using varenicline for just one week before attempting to quit. However, those who used varenicline for four weeks before stopping smoking were more likely to be smoke-free at 12 weeks than those who had used it for just one week before quitting.27
Other Pharmaceutical Options
Clonidine, long recognized as an effective antihypertensive medication, was determined by Gourlay et al9 in a 2004 review to have potential for use in supporting smoking cessation. Because significant adverse events (including drowsiness, sedation, and postural hypotension) have been associated with clonidine use7,28 and the FDA has not yet approved it for the indication of smoking cessation, its use may be most appropriate as a second-line treatment option, in combination with bupropion or nortriptyline, or for specialists’ use.28 Clonidine should not be discontinued suddenly.
Like bupropion, the tricyclic antidepressant nortriptyline has been investigated for its potential in tobacco cessation therapy. While a significant amount is known about plasma concentrations of nortriptyline needed to treat depression, levels required for effective tobacco cessation are less clear. Mooney et al29 found that therapeutic plasma concentrations of nortriptyline varied among subjects by race and smoking habits; although a lower concentration was usually required to assist smoking cessation than to treat depression, adverse effects were common even at lower concentrations. Thus, it was recommended that nortriptyline be reserved for second-line treatment.
This summer, researchers for the Cochrane Tobacco Addiction Review Group published a review of the literature (including phase II and phase III trials conducted by pharmaceutical companies—making the risk for bias “high or unclear”) pertaining to two nicotine vaccines in development.21 In two studies, the level of development of nicotine antibodies was associated with commensurate cessation rates; in two others, the outcome measure (12 months’ abstinence from smoking) was met in 11% of subjects, whether they received the vaccine or placebo. Thus, no strong evidence yet exists that nicotine vaccination supports smoking cessation in the long term; further research is needed.
NONPHARMACEUTICAL
INTERVENTIONS
Acupuncture
Variations and modifications of the traditional Chinese therapy of acupuncture, including acupressure and electrostimulation, have been examined in a number of clinical trials. Despite the supporting rhetoric, objective research of good quality in this area is limited. However, one systematic literature review showed acupuncture to be only slightly more effective than sham interventions and less effective than NRT.30 Insufficient evidence was reported on acupressure and laser stimulation, and acupressure was no more effective than psychological treatments. Considering questionable study quality and other limitations in the currently available research, practitioners should not consider acupuncture or related interventions as first-line options—nor should their potential be dismissed altogether.
Hypnotherapy
Conclusive research findings regarding hypnotherapy as an effective treatment for tobacco dependence are also limited. In 2010, Barnes at al31 reviewed 11 studies comparing hypnotherapy with various alternate methods and found little difference in effectiveness among hypnotherapy, psychological counseling, and rapid smoking therapy. Despite the limitations in these data, however, hypnotherapy may be appropriate for some patients.
CONCLUSION
Tobacco dependence is not the same for any two patients. Just as health care providers do not use the same treatment option for every patient with hypertension or diabetes, treatment for tobacco-dependent patients must also be individualized.
Our professional goal is to care for the health of patients. We clinicians must recommend cessation to our patients who smoke at every encounter—and offer support often. When we miss an opportunity to counsel a patient on the importance of quitting, the patient may interpret our silence as condoning the behavior. Empowering patients with an understanding of the options can contribute to their success—a significant move toward better health.
The authors of Healthy People 2020 hope that 80% of current smokers will have tried to stop smoking by that year. Have 80% of your patients been counseled and offered assistance to stop?
REFERENCES
1. CDC. Current cigarette smoking prevalence among working adults—United States, 2004-2010. MMWR Morb Mortal Wkly Rep. 2011;60(38):1305-1309.
2. CDC. Vital signs: current cigarette smoking among adults aged ≥ 18 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(35):1135-1140.
3. Jamal A, Dube SR, Malarcher AM, et al; CDC. Tobacco use screening and counseling during physician office visits among adults—National Ambulatory Medical Care Survey and National Health Interview Survey, United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61 suppl: 38-45.
4. US Department of Health and Human Services. Healthy People 2020 summary of objectives: tobacco use. http://healthypeople.gov/2020/topicsobjectives2020/pdfs/TobaccoUse.pdf. Accessed October 18, 2012.
5. US Department of Health and Human Services. Healthy People 2010 archives. www.healthypeople.gov/2010. Accessed October 18, 2012.
6. Blumenthal DS. Barriers to the provision of smoking cessation services reported by clinicians in underserved communities. J Am Board Fam Med. 2007;20(3):272-279.
7. Fiore MC, Jaén CR, Baker TB, et al; Public Health Service, US Department of Health and Human Services. Clinical practice guideline: treating tobacco use and dependence: 2008 update. www.ahrq.gov/clinic/tobacco/treating_tobacco_use08.pdf. Accessed October 18, 2012.
8. Carson KV, Verbiest ME, Crone MR, et al. Training health professionals in smoking cessation. Cochrane Database Syst Rev. 2012 May 16; 5:CD000214.
9. Gourlay SG, Stead LF, Benowitz NL. Clonidine for smoking cessation. Cochrane Database Syst Rev. 2004;(3):CD000058.
10. Guirguis AB, Ray SM, Zingone MM, et al. Smoking cessation: barriers to success and readiness to change. Tenn Med. 2010;103(9):45-49.
11. Nørregaard J, Tønnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Prev Med. 1993;22(2):261-271.
12. Lancaster T, Hajek P, Stead LF, et al. Prevention of relapse after quitting smoking: a systematic review of trials. Arch Intern Med. 2006;166(8): 828-835.
13. Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: causes and consequences. PLoS Med. 2010;7(2):e1000216.
14. Hung WT, Dunlop SM, Perez D, Cotter T. Use and perceived helpfulness of smoking cessation methods: results from a population survey of recent quitters. BMC Public Health. 2011;11:592.
15. Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102-111.
16. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD000146.
17. Robles GI, Singh-Franco D, Ghin HL. A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations. Clin Ther. 2008;30(5):800-812.
18. Japuntich SJ, Piper ME, Leventhal AM, et al. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. J Consult Clin Psychol. 2011;79(1):34-42.
19. Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: results from an online survey. Am J Prev Med. 2011;40 (4):472-475.
20. Tønnesen P, Lauri H, Perfekt R, et al. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J. 2012;40(3):548-554.
21. Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2012 Aug 15;8:CD007072.
22. Roddy E. Bupropion and other non-nicotine pharmacotherapies. BMJ. 2004;328(7438):
509-511.
23. Loh WY, Piper ME, Schlam TR, et al. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine Tob Res. 2012;14(2):131-141.
24. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving suppression. J Consult Clin Psychol. 2012;80(1):44-65.
25. McNeil JJ, Piccenna L, Ioannides-Demos LL. Smoking cessation: recent advances. Cardiovasc Drugs Ther. 2010;24(4):359-367.
26. Ebbert JO, Hays JT, Hurt RD. Combination pharmacotherapy for stopping smoking: what advantages does it offer? Drugs. 2010;70(6):643-650.
27. Hajek P, McRobbie HJ, Myers KE, et al. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171 (8):770-777.
28. Bentz CJ. Review: clonidine is more effective than placebo for long-term smoking cessation, but has side effects. ACP J Club. 2005;142(1):12.
29. Mooney ME, Reus VI, Gorecki J, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clin Pharmacol Ther. 2008;83(3):436-442.
30. White AR, Rampes H, Liu JP, et al. Acupuncture and related interventions for smoking cessation. Cochrane Database Syst Rev. 2011;(1): CD000009.
31. Barnes J, Dong CY, McRobbie H, et al. Hypnotherapy for smoking cessation. Cochrane Database Syst Rev. 2010;(10):CD001008.
Antibiotics fail to head off sepsis … Failure to address persistent symptoms proves disastrous… more
Antibiotics fail to head off sepsis
SHORTNESS OF BREATH AND RIGHT-SIDED CHEST PAIN prompted a 45-year-old woman to go to the emergency department (ED) early one morning. She had a history of chronic lung problems with multiple diagnoses of pneumonia, pneumothorax, blebs, and bronchiectasis. The ED doctor diagnosed community-acquired pneumonia and admitted her for intravenous antibiotic treatment.
Late that afternoon the patient’s condition deteriorated rapidly. She was transferred to the intensive care unit, where she died of septic shock caused by Pseudomonas aeruginosa 22 hours after she had arrived at the ED.
PLAINTIFF’S CLAIM The patient should have received broader-spectrum antibiotics.
THE DEFENSE The hospitalist who treated the woman as an inpatient claimed that the treatment she received was appropriate and that she probably would have died even if other antibiotics had been prescribed. The hospitalist also claimed that the nursing staff failed to notify her of the patient’s low blood pressure readings until 10 hours after the initial evaluation. A nurse denied this claim, asserting that the hospitalist had been paged several times during the day. The discharge summary and nursing notes on the patient were missing.
VERDICT $5.28 million arbitration award.
COMMENT It surprises me how often key portions of medical records go missing! Here, the absence of a discharge summary and nursing notes may well have contributed to a $5 million award.
Change, and not for the better
AN ATYPICAL MOLE ON THE LEFT CALF was brought to the attention of a primary care physician by a 36-year-old man during a full physical. The mole was 1 3 1 cm; the patient reported that it had been changing. The mole’s appearance didn’t worry the physician, who described it in his notes as either a hemangioma or dermatofibroma. The doctor advised the patient to return in 6 months if he wanted the mole removed for cosmetic reasons.
Over the next 5 months, the patient noticed further changes in the mole and called the doctor’s office. He was seen by a colleague of his physician, who immediately sent the patient for a biopsy and surgical consultation. The mole was removed and diagnosed as an ulcerating melanoma with downward growth.
Shortly thereafter, the patient underwent wide excision and lymph node dissection, which showed clear margins and no lymph node involvement. Twenty months later, a mass was found in the patient’s liver. Biopsy diagnosed metastatic spread of the melanoma. The patient died 2 months later.
PLAINTIFF’S CLAIM The patient should have had a biopsy and received a surgical referral at the time of the physical examination when he first reported the mole.
THE DEFENSE Waiting for 6 months was appropriate because the mole didn’t look like a melanoma when the patient first called it to the physician’s attention. The melanoma had already metastasized at the time of the physical examination and the diagnostic delay didn’t affect the outcome.
VERDICT $1 million Massachusetts settlement.
COMMENT A changing mole should always raise concern. Biopsy, excision, or a referral could have avoided a million-dollar settlement.
Failure to address persistent symptoms proves disastrous
PAIN IN THE BACK AND CHEST along with respiratory difficulty prompted a 49-year-old man to visit his physician. The physician told him to go to a hospital. The doctor who examined the patient at the hospital diagnosed muscle strain and prescribed muscle relaxants.
The following day, the patient returned to his physician complaining of continuing symptoms. The doctor sent him home. He died the next day of an aortic rupture caused by an undiagnosed dissection.
PLAINTIFF’S CLAIM The 2 physicians should have diagnosed the dissection, which would have permitted treatment and prevented death. The patient had been treated previously at the hospital, and his records should have raised suspicion of an aortic aneurysm. The hospital physician was a new hire and hadn’t received proper training in the hospital’s electronic records system. He should have ordered a computed tomography scan or cardiology consult. The patient’s physician failed to address the ongoing symptoms. He should have hospitalized the patient at the time of the second visit.
THE DEFENSE The hospital physician claimed he had intended to contact the cardiologist who had treated the patient, but the patient couldn’t remember the cardiologist’s name. The patient’s symptoms didn’t suggest an aortic dissection, and the dissection occurred after the patient was discharged from the hospital.
VERDICT $3.4 million New York verdict against the hospital physician only.
COMMENT Although the hoofbeats are usually horses, always remember the zebras (or should it be lions?), particularly when a patient returns repeatedly with ongoing symptoms.
Controlled substances out of control
A WOMAN WITH CHRONIC MIGRAINES, anxiety problems, and nausea also had cardiomyopathy and chronic atrial fibrillation, which could be triggered by pain from her other ailments. She came under the care of a physician who prescribed a number of drugs, including meperidine, hydrocodone, tizanidine, diazepam, promethazine, alprazolam, and oxcarbazepine. The doctor prescribed injectable forms of certain medications after the patient told him her next-door neighbor was a nurse and could help administer the drugs.
Four years after coming under the doctor’s care, the patient signed a Controlled Substance Agreement specifying that the physician would discontinue her as a patient if she got controlled substances from another doctor. (Evidence was later found that the patient was receiving prescriptions from other physicians.)
While under treatment by her doctor, the patient was hospitalized a number of times for medication overdoses. The record from one hospitalization reported that she had made angry, profanity-laced requests for meperidine and promethazine.
About 2 years after signing the Controlled Substance Agreement, the patient received prescriptions from her doctor for 210 doses of meperidine, 100 doses of promethazine, and 60 pills each of diazepam, alprazolam, and acetaminophen and hydrocodone. She filled the prescriptions at 2 pharmacies without objections from the pharmacists. She died of an accidental drug overdose the following month.
Postmortem blood testing showed high levels of meperidine and promethazine. The patient had apparently taken the equivalent of 11 “shots” of meperidine (5 times the maximum prescribed amount), probably by injecting herself through a peripherally inserted central catheter rather than by intramuscular injection, as prescribed.
PLAINTIFF’S CLAIM The patient’s doctor was negligent in prescribing large amounts of controlled substances when he should have known that she was a drug seeker with a drug abuse problem. The pharmacies were negligent for filling the prescriptions without question.
THE DEFENSE The patient was solely responsible for her own death because she gave herself a large overdose.
VERDICT $500,000 Alabama verdict. The case against the pharmacies was dismissed.
COMMENT Increasingly it is expected that physicians (and pharmacists) perform due diligence when prescribing opioids, including taking reasonable precautions against the drug-seeking patient.
Antibiotics fail to head off sepsis
SHORTNESS OF BREATH AND RIGHT-SIDED CHEST PAIN prompted a 45-year-old woman to go to the emergency department (ED) early one morning. She had a history of chronic lung problems with multiple diagnoses of pneumonia, pneumothorax, blebs, and bronchiectasis. The ED doctor diagnosed community-acquired pneumonia and admitted her for intravenous antibiotic treatment.
Late that afternoon the patient’s condition deteriorated rapidly. She was transferred to the intensive care unit, where she died of septic shock caused by Pseudomonas aeruginosa 22 hours after she had arrived at the ED.
PLAINTIFF’S CLAIM The patient should have received broader-spectrum antibiotics.
THE DEFENSE The hospitalist who treated the woman as an inpatient claimed that the treatment she received was appropriate and that she probably would have died even if other antibiotics had been prescribed. The hospitalist also claimed that the nursing staff failed to notify her of the patient’s low blood pressure readings until 10 hours after the initial evaluation. A nurse denied this claim, asserting that the hospitalist had been paged several times during the day. The discharge summary and nursing notes on the patient were missing.
VERDICT $5.28 million arbitration award.
COMMENT It surprises me how often key portions of medical records go missing! Here, the absence of a discharge summary and nursing notes may well have contributed to a $5 million award.
Change, and not for the better
AN ATYPICAL MOLE ON THE LEFT CALF was brought to the attention of a primary care physician by a 36-year-old man during a full physical. The mole was 1 3 1 cm; the patient reported that it had been changing. The mole’s appearance didn’t worry the physician, who described it in his notes as either a hemangioma or dermatofibroma. The doctor advised the patient to return in 6 months if he wanted the mole removed for cosmetic reasons.
Over the next 5 months, the patient noticed further changes in the mole and called the doctor’s office. He was seen by a colleague of his physician, who immediately sent the patient for a biopsy and surgical consultation. The mole was removed and diagnosed as an ulcerating melanoma with downward growth.
Shortly thereafter, the patient underwent wide excision and lymph node dissection, which showed clear margins and no lymph node involvement. Twenty months later, a mass was found in the patient’s liver. Biopsy diagnosed metastatic spread of the melanoma. The patient died 2 months later.
PLAINTIFF’S CLAIM The patient should have had a biopsy and received a surgical referral at the time of the physical examination when he first reported the mole.
THE DEFENSE Waiting for 6 months was appropriate because the mole didn’t look like a melanoma when the patient first called it to the physician’s attention. The melanoma had already metastasized at the time of the physical examination and the diagnostic delay didn’t affect the outcome.
VERDICT $1 million Massachusetts settlement.
COMMENT A changing mole should always raise concern. Biopsy, excision, or a referral could have avoided a million-dollar settlement.
Failure to address persistent symptoms proves disastrous
PAIN IN THE BACK AND CHEST along with respiratory difficulty prompted a 49-year-old man to visit his physician. The physician told him to go to a hospital. The doctor who examined the patient at the hospital diagnosed muscle strain and prescribed muscle relaxants.
The following day, the patient returned to his physician complaining of continuing symptoms. The doctor sent him home. He died the next day of an aortic rupture caused by an undiagnosed dissection.
PLAINTIFF’S CLAIM The 2 physicians should have diagnosed the dissection, which would have permitted treatment and prevented death. The patient had been treated previously at the hospital, and his records should have raised suspicion of an aortic aneurysm. The hospital physician was a new hire and hadn’t received proper training in the hospital’s electronic records system. He should have ordered a computed tomography scan or cardiology consult. The patient’s physician failed to address the ongoing symptoms. He should have hospitalized the patient at the time of the second visit.
THE DEFENSE The hospital physician claimed he had intended to contact the cardiologist who had treated the patient, but the patient couldn’t remember the cardiologist’s name. The patient’s symptoms didn’t suggest an aortic dissection, and the dissection occurred after the patient was discharged from the hospital.
VERDICT $3.4 million New York verdict against the hospital physician only.
COMMENT Although the hoofbeats are usually horses, always remember the zebras (or should it be lions?), particularly when a patient returns repeatedly with ongoing symptoms.
Controlled substances out of control
A WOMAN WITH CHRONIC MIGRAINES, anxiety problems, and nausea also had cardiomyopathy and chronic atrial fibrillation, which could be triggered by pain from her other ailments. She came under the care of a physician who prescribed a number of drugs, including meperidine, hydrocodone, tizanidine, diazepam, promethazine, alprazolam, and oxcarbazepine. The doctor prescribed injectable forms of certain medications after the patient told him her next-door neighbor was a nurse and could help administer the drugs.
Four years after coming under the doctor’s care, the patient signed a Controlled Substance Agreement specifying that the physician would discontinue her as a patient if she got controlled substances from another doctor. (Evidence was later found that the patient was receiving prescriptions from other physicians.)
While under treatment by her doctor, the patient was hospitalized a number of times for medication overdoses. The record from one hospitalization reported that she had made angry, profanity-laced requests for meperidine and promethazine.
About 2 years after signing the Controlled Substance Agreement, the patient received prescriptions from her doctor for 210 doses of meperidine, 100 doses of promethazine, and 60 pills each of diazepam, alprazolam, and acetaminophen and hydrocodone. She filled the prescriptions at 2 pharmacies without objections from the pharmacists. She died of an accidental drug overdose the following month.
Postmortem blood testing showed high levels of meperidine and promethazine. The patient had apparently taken the equivalent of 11 “shots” of meperidine (5 times the maximum prescribed amount), probably by injecting herself through a peripherally inserted central catheter rather than by intramuscular injection, as prescribed.
PLAINTIFF’S CLAIM The patient’s doctor was negligent in prescribing large amounts of controlled substances when he should have known that she was a drug seeker with a drug abuse problem. The pharmacies were negligent for filling the prescriptions without question.
THE DEFENSE The patient was solely responsible for her own death because she gave herself a large overdose.
VERDICT $500,000 Alabama verdict. The case against the pharmacies was dismissed.
COMMENT Increasingly it is expected that physicians (and pharmacists) perform due diligence when prescribing opioids, including taking reasonable precautions against the drug-seeking patient.
Antibiotics fail to head off sepsis
SHORTNESS OF BREATH AND RIGHT-SIDED CHEST PAIN prompted a 45-year-old woman to go to the emergency department (ED) early one morning. She had a history of chronic lung problems with multiple diagnoses of pneumonia, pneumothorax, blebs, and bronchiectasis. The ED doctor diagnosed community-acquired pneumonia and admitted her for intravenous antibiotic treatment.
Late that afternoon the patient’s condition deteriorated rapidly. She was transferred to the intensive care unit, where she died of septic shock caused by Pseudomonas aeruginosa 22 hours after she had arrived at the ED.
PLAINTIFF’S CLAIM The patient should have received broader-spectrum antibiotics.
THE DEFENSE The hospitalist who treated the woman as an inpatient claimed that the treatment she received was appropriate and that she probably would have died even if other antibiotics had been prescribed. The hospitalist also claimed that the nursing staff failed to notify her of the patient’s low blood pressure readings until 10 hours after the initial evaluation. A nurse denied this claim, asserting that the hospitalist had been paged several times during the day. The discharge summary and nursing notes on the patient were missing.
VERDICT $5.28 million arbitration award.
COMMENT It surprises me how often key portions of medical records go missing! Here, the absence of a discharge summary and nursing notes may well have contributed to a $5 million award.
Change, and not for the better
AN ATYPICAL MOLE ON THE LEFT CALF was brought to the attention of a primary care physician by a 36-year-old man during a full physical. The mole was 1 3 1 cm; the patient reported that it had been changing. The mole’s appearance didn’t worry the physician, who described it in his notes as either a hemangioma or dermatofibroma. The doctor advised the patient to return in 6 months if he wanted the mole removed for cosmetic reasons.
Over the next 5 months, the patient noticed further changes in the mole and called the doctor’s office. He was seen by a colleague of his physician, who immediately sent the patient for a biopsy and surgical consultation. The mole was removed and diagnosed as an ulcerating melanoma with downward growth.
Shortly thereafter, the patient underwent wide excision and lymph node dissection, which showed clear margins and no lymph node involvement. Twenty months later, a mass was found in the patient’s liver. Biopsy diagnosed metastatic spread of the melanoma. The patient died 2 months later.
PLAINTIFF’S CLAIM The patient should have had a biopsy and received a surgical referral at the time of the physical examination when he first reported the mole.
THE DEFENSE Waiting for 6 months was appropriate because the mole didn’t look like a melanoma when the patient first called it to the physician’s attention. The melanoma had already metastasized at the time of the physical examination and the diagnostic delay didn’t affect the outcome.
VERDICT $1 million Massachusetts settlement.
COMMENT A changing mole should always raise concern. Biopsy, excision, or a referral could have avoided a million-dollar settlement.
Failure to address persistent symptoms proves disastrous
PAIN IN THE BACK AND CHEST along with respiratory difficulty prompted a 49-year-old man to visit his physician. The physician told him to go to a hospital. The doctor who examined the patient at the hospital diagnosed muscle strain and prescribed muscle relaxants.
The following day, the patient returned to his physician complaining of continuing symptoms. The doctor sent him home. He died the next day of an aortic rupture caused by an undiagnosed dissection.
PLAINTIFF’S CLAIM The 2 physicians should have diagnosed the dissection, which would have permitted treatment and prevented death. The patient had been treated previously at the hospital, and his records should have raised suspicion of an aortic aneurysm. The hospital physician was a new hire and hadn’t received proper training in the hospital’s electronic records system. He should have ordered a computed tomography scan or cardiology consult. The patient’s physician failed to address the ongoing symptoms. He should have hospitalized the patient at the time of the second visit.
THE DEFENSE The hospital physician claimed he had intended to contact the cardiologist who had treated the patient, but the patient couldn’t remember the cardiologist’s name. The patient’s symptoms didn’t suggest an aortic dissection, and the dissection occurred after the patient was discharged from the hospital.
VERDICT $3.4 million New York verdict against the hospital physician only.
COMMENT Although the hoofbeats are usually horses, always remember the zebras (or should it be lions?), particularly when a patient returns repeatedly with ongoing symptoms.
Controlled substances out of control
A WOMAN WITH CHRONIC MIGRAINES, anxiety problems, and nausea also had cardiomyopathy and chronic atrial fibrillation, which could be triggered by pain from her other ailments. She came under the care of a physician who prescribed a number of drugs, including meperidine, hydrocodone, tizanidine, diazepam, promethazine, alprazolam, and oxcarbazepine. The doctor prescribed injectable forms of certain medications after the patient told him her next-door neighbor was a nurse and could help administer the drugs.
Four years after coming under the doctor’s care, the patient signed a Controlled Substance Agreement specifying that the physician would discontinue her as a patient if she got controlled substances from another doctor. (Evidence was later found that the patient was receiving prescriptions from other physicians.)
While under treatment by her doctor, the patient was hospitalized a number of times for medication overdoses. The record from one hospitalization reported that she had made angry, profanity-laced requests for meperidine and promethazine.
About 2 years after signing the Controlled Substance Agreement, the patient received prescriptions from her doctor for 210 doses of meperidine, 100 doses of promethazine, and 60 pills each of diazepam, alprazolam, and acetaminophen and hydrocodone. She filled the prescriptions at 2 pharmacies without objections from the pharmacists. She died of an accidental drug overdose the following month.
Postmortem blood testing showed high levels of meperidine and promethazine. The patient had apparently taken the equivalent of 11 “shots” of meperidine (5 times the maximum prescribed amount), probably by injecting herself through a peripherally inserted central catheter rather than by intramuscular injection, as prescribed.
PLAINTIFF’S CLAIM The patient’s doctor was negligent in prescribing large amounts of controlled substances when he should have known that she was a drug seeker with a drug abuse problem. The pharmacies were negligent for filling the prescriptions without question.
THE DEFENSE The patient was solely responsible for her own death because she gave herself a large overdose.
VERDICT $500,000 Alabama verdict. The case against the pharmacies was dismissed.
COMMENT Increasingly it is expected that physicians (and pharmacists) perform due diligence when prescribing opioids, including taking reasonable precautions against the drug-seeking patient.
Rethinking antibiotics for sinusitis—again
Stop prescribing amoxicillin for acute rhinosinusitis. It’s unlikely to provide a speedier recovery than over-the-counter (OTC) remedies alone.1
STRENGTH OF RECOMMENDATION
B: Based on a single high-quality randomized controlled trial.
Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012 ;307:685-692.
ILLUSTRATIVE CASE
A 28-year-old man comes to your clinic after suffering from fatigue, purulent nasal discharge, and unilateral facial pain for nearly 10 days. Overall, he appears healthy, and you diagnose acute rhinosinusitis. You suggest OTC remedies for supportive care and wonder if a course of amoxicillin would speed his recovery.
Each year, more than 30 million Americans—about one in 7 adults—are diagnosed with sinusitis.2 No more than 2% of these cases are thought to be bacterial.3
Centers for Disease Control and Prevention (CDC) guidelines for the diagnosis of acute bacterial rhinosinusitis include symptoms that last 7 days or more, with maxillary pain or tenderness in the face or teeth and purulent nasal secretions.4 Patients with symptoms lasting less than 7 days are unlikely to have a bacterial infection. But the non-specific signs and symptoms included in the CDC guidelines limit their usefulness in determining whether the cause of the sinusitis is bacterial or viral on clinical grounds alone.
Most cases of sinusitis spontaneously resolve
In patients with acute bacterial sinusitis, the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) guidelines advocate watchful waiting and symptom relief with nasal oxymetazoline, pseudoephedrine, and saline nasal irrigation.3 The rate of spontaneous resolution is high: 80% of patients with clinically diagnosed sinusitis improve without treatment within 2 weeks.1,5
Traditional decongestants and mucolytics have not demonstrated efficacy in resolving sinusitis, although rigorous evaluation is lacking. Other treatments, such as saline irrigation and intranasal corticosteroids, are of unclear benefit and need further study.6-8
Lack of evidence has done little to curtail antibiotic use
An earlier PURL based on a meta-analysis of antibiotic treatment trials for sinusitis recommended that we stop prescribing antibiotics for adults with acute sinusitis unless their symptoms are severe.9,10 Yet antibiotics remain the mainstay of treatment.
Despite the AAO-HNS guidelines, evidence of spontaneous resolution, and accumulating data on the lack of efficacy of antimicrobials for sinusitis, 81% of patients diagnosed with acute sinusitis were given prescriptions for antibiotics, a study of primary care practices showed.11 Frequent use of antibiotics contributes to high rates of drug resistance, and adverse events related to antibiotic use account for an estimated 142,500 emergency department visits annually.12
STUDY SUMMARY: Little benefit from amoxicillin, even for severe cases
Garbutt and colleagues revisited the issue, randomizing 166 patients from 10 primary care practices to amoxicillin plus symptomatic treatment or placebo plus symptomatic treatment for acute rhinosinusitis.1 To be eligible for the study, patients had to be between the ages of 18 and 70 years, meet CDC diagnostic criteria for acute rhinosinusitis, and have moderate to very severe symptoms that were of 7- to-28-day duration and worsening or not improving or of <7-day duration but had worsened after an initial improvement. Exclusion criteria included complications from sinusitis, a history of allergy to penicillin or amoxicillin, antibiotic use in the past 4 weeks, comorbidities that impair immune function, cystic fibrosis, pregnancy, and mild symptoms.
Both groups had similar baseline characteristics, with participants who were predominantly white (79%) and female (64%). All the participants received a supply of symptomatic treatments: acetaminophen, guaifenesin, dextromethorphan, and sustained-release pseudoephedrine. The treatment group also received amoxicillin 1500 mg/d, divided into 3 doses; the placebo group received identical-looking placebo pills.
Patients were assessed with the Sino-nasal Outcome Test-16 (SNOT-16), a validated measure that asks patients to assess both the severity and frequency of 16 sinus symptoms. SNOT-16 uses a 0-to-3 rating scale (0=no problem; 3=severe problem), with a clinically important difference of ≥0.5 on the mean score. The test was administered at enrollment and at Days 3, 7, and 10. The disease-specific quality of life at Day 3 was the primary outcome.
There was no statistically significantly difference in SNOT-16 scores between the amoxicillin and placebo groups on Days 3 and 10. On Day 7, there was a small statistically significant improvement in the amoxicillin group, but it did not reach the level of clinical importance (≥0.5) based on SNOT-16’s mean score.
The authors also asked participants to retrospectively assess symptom change since enrollment on a 6-point scale. Those who reported that their symptoms were “a lot better” or “absent” were characterized as significantly improved. The results correlated with the data from the SNOT-16, showing no difference between the amoxicillin and control group at Days 3 and 10. On Day 7, 74% of patients treated with amoxicillin self- reported significant improvement in symptoms since the start of the study, vs 56% in the control group. The number needed to treat was 6 (95% confidence interval, 3-34; P= .02) for a reduction in symptoms at Day 7.
Patients in both groups had similar rates of absenteeism, inability to perform usual activities, relapse and recurrence, and use of additional health care. Satisfaction with treatment was similar, as well.
No serious adverse effects occurred. Both groups reported similar frequencies (<10%) of nausea, diarrhea, abdominal pain, or vaginitis.
WHAT’S NEW: Even severe sinusitis resolves without antibiotics
Previous studies recommended foregoing antibiotics for acute sinusitis, except when symptoms are severe. This study—in which more than half (52%) of patients in each group had symptoms rated severe or very severe—found no benefit to adding amoxicillin to supportive treatments.1 Antibiotics did not shorten the duration of illness, prevent relapse and recurrence, or improve satisfaction with treatment. The researchers found a statistically significant difference between groups on Day 7 of 0.19 points, but no clinically meaningful difference (≥0.5) based on the SNOT-16 mean score.
CAVEATS: Guidelines, risk of complications may give reason to pause
The 2012 Infectious Diseases Society of America guidelines recommend amoxicillin with clavulanic acid as empiric therapy for acute bacterial rhinosinusitis.7 The findings of the study by Garbutt et al—conducted at a time when the incidence of beta-lactamase-producing organisms was low and amoxicillin was the treatment of choice—suggest otherwise.
Serious complications of sinusitis, such as brain abscess, periorbital cellulitis, and meningitis, can occur, however. Patients who deteriorate clinically or develop high fever or severe headache require close follow-up, which may include further diagnostic evaluation or consultation with an otolaryngologist. Evidence is lacking as to whether antibiotics prevent such complications.5
CHALLENGES TO IMPLEMENTATION: Managing patient expectations
Many patients with symptoms of acute rhinosinusitis think they need an antibiotic. Managing their expectations and providing instructions about supportive treatments are time consuming and may be difficult.
Nonetheless, we’re optimistic: We think that most patients today are aware of the problems associated with antibiotic resistance and wary of “superbugs,” and will therefore be receptive to this practice change. Physicians can help by reminding patients of the adverse effects of antibiotics and the natural course of rhino-sinusitis, as well as by offering symptomatic treatments.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
2. Centers for Disease Control and Prevention. Summary health statistics for US adults: National Health Interview Survey 2010. January 2012. Available at: http://www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed July 9, 2012.
3. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
4. Hickner JM, Bartlett JG, Besser RE, et al. American Academy of Family Physians; American College of Physicians; American Society of Internal Medicine; Centers for Disease Control; Infectious Disease Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults; background. Ann Intern Med. 2001;134:498-505.
5. Ahovuo-Saloranta A, Borisenk OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2008;(2):CD000243.-
6. Allen G, Kelsberg G, Jankowski TA. Do nasal decongestants relieve symptoms? J Fam Pract. 2003;52:714-724.
7. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
8. Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2009;(4):CD005149.-
9. Schumann A, Hickner J. Patients insist on antibiotics for sinusitis? Here is a good reason to say “no”. J Fam Pract. Jul:2008;57:464-468.
10. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
11. Gill JM, Fleischut P, Haas S. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med. 2006;38:349-354.
12. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
Stop prescribing amoxicillin for acute rhinosinusitis. It’s unlikely to provide a speedier recovery than over-the-counter (OTC) remedies alone.1
STRENGTH OF RECOMMENDATION
B: Based on a single high-quality randomized controlled trial.
Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012 ;307:685-692.
ILLUSTRATIVE CASE
A 28-year-old man comes to your clinic after suffering from fatigue, purulent nasal discharge, and unilateral facial pain for nearly 10 days. Overall, he appears healthy, and you diagnose acute rhinosinusitis. You suggest OTC remedies for supportive care and wonder if a course of amoxicillin would speed his recovery.
Each year, more than 30 million Americans—about one in 7 adults—are diagnosed with sinusitis.2 No more than 2% of these cases are thought to be bacterial.3
Centers for Disease Control and Prevention (CDC) guidelines for the diagnosis of acute bacterial rhinosinusitis include symptoms that last 7 days or more, with maxillary pain or tenderness in the face or teeth and purulent nasal secretions.4 Patients with symptoms lasting less than 7 days are unlikely to have a bacterial infection. But the non-specific signs and symptoms included in the CDC guidelines limit their usefulness in determining whether the cause of the sinusitis is bacterial or viral on clinical grounds alone.
Most cases of sinusitis spontaneously resolve
In patients with acute bacterial sinusitis, the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) guidelines advocate watchful waiting and symptom relief with nasal oxymetazoline, pseudoephedrine, and saline nasal irrigation.3 The rate of spontaneous resolution is high: 80% of patients with clinically diagnosed sinusitis improve without treatment within 2 weeks.1,5
Traditional decongestants and mucolytics have not demonstrated efficacy in resolving sinusitis, although rigorous evaluation is lacking. Other treatments, such as saline irrigation and intranasal corticosteroids, are of unclear benefit and need further study.6-8
Lack of evidence has done little to curtail antibiotic use
An earlier PURL based on a meta-analysis of antibiotic treatment trials for sinusitis recommended that we stop prescribing antibiotics for adults with acute sinusitis unless their symptoms are severe.9,10 Yet antibiotics remain the mainstay of treatment.
Despite the AAO-HNS guidelines, evidence of spontaneous resolution, and accumulating data on the lack of efficacy of antimicrobials for sinusitis, 81% of patients diagnosed with acute sinusitis were given prescriptions for antibiotics, a study of primary care practices showed.11 Frequent use of antibiotics contributes to high rates of drug resistance, and adverse events related to antibiotic use account for an estimated 142,500 emergency department visits annually.12
STUDY SUMMARY: Little benefit from amoxicillin, even for severe cases
Garbutt and colleagues revisited the issue, randomizing 166 patients from 10 primary care practices to amoxicillin plus symptomatic treatment or placebo plus symptomatic treatment for acute rhinosinusitis.1 To be eligible for the study, patients had to be between the ages of 18 and 70 years, meet CDC diagnostic criteria for acute rhinosinusitis, and have moderate to very severe symptoms that were of 7- to-28-day duration and worsening or not improving or of <7-day duration but had worsened after an initial improvement. Exclusion criteria included complications from sinusitis, a history of allergy to penicillin or amoxicillin, antibiotic use in the past 4 weeks, comorbidities that impair immune function, cystic fibrosis, pregnancy, and mild symptoms.
Both groups had similar baseline characteristics, with participants who were predominantly white (79%) and female (64%). All the participants received a supply of symptomatic treatments: acetaminophen, guaifenesin, dextromethorphan, and sustained-release pseudoephedrine. The treatment group also received amoxicillin 1500 mg/d, divided into 3 doses; the placebo group received identical-looking placebo pills.
Patients were assessed with the Sino-nasal Outcome Test-16 (SNOT-16), a validated measure that asks patients to assess both the severity and frequency of 16 sinus symptoms. SNOT-16 uses a 0-to-3 rating scale (0=no problem; 3=severe problem), with a clinically important difference of ≥0.5 on the mean score. The test was administered at enrollment and at Days 3, 7, and 10. The disease-specific quality of life at Day 3 was the primary outcome.
There was no statistically significantly difference in SNOT-16 scores between the amoxicillin and placebo groups on Days 3 and 10. On Day 7, there was a small statistically significant improvement in the amoxicillin group, but it did not reach the level of clinical importance (≥0.5) based on SNOT-16’s mean score.
The authors also asked participants to retrospectively assess symptom change since enrollment on a 6-point scale. Those who reported that their symptoms were “a lot better” or “absent” were characterized as significantly improved. The results correlated with the data from the SNOT-16, showing no difference between the amoxicillin and control group at Days 3 and 10. On Day 7, 74% of patients treated with amoxicillin self- reported significant improvement in symptoms since the start of the study, vs 56% in the control group. The number needed to treat was 6 (95% confidence interval, 3-34; P= .02) for a reduction in symptoms at Day 7.
Patients in both groups had similar rates of absenteeism, inability to perform usual activities, relapse and recurrence, and use of additional health care. Satisfaction with treatment was similar, as well.
No serious adverse effects occurred. Both groups reported similar frequencies (<10%) of nausea, diarrhea, abdominal pain, or vaginitis.
WHAT’S NEW: Even severe sinusitis resolves without antibiotics
Previous studies recommended foregoing antibiotics for acute sinusitis, except when symptoms are severe. This study—in which more than half (52%) of patients in each group had symptoms rated severe or very severe—found no benefit to adding amoxicillin to supportive treatments.1 Antibiotics did not shorten the duration of illness, prevent relapse and recurrence, or improve satisfaction with treatment. The researchers found a statistically significant difference between groups on Day 7 of 0.19 points, but no clinically meaningful difference (≥0.5) based on the SNOT-16 mean score.
CAVEATS: Guidelines, risk of complications may give reason to pause
The 2012 Infectious Diseases Society of America guidelines recommend amoxicillin with clavulanic acid as empiric therapy for acute bacterial rhinosinusitis.7 The findings of the study by Garbutt et al—conducted at a time when the incidence of beta-lactamase-producing organisms was low and amoxicillin was the treatment of choice—suggest otherwise.
Serious complications of sinusitis, such as brain abscess, periorbital cellulitis, and meningitis, can occur, however. Patients who deteriorate clinically or develop high fever or severe headache require close follow-up, which may include further diagnostic evaluation or consultation with an otolaryngologist. Evidence is lacking as to whether antibiotics prevent such complications.5
CHALLENGES TO IMPLEMENTATION: Managing patient expectations
Many patients with symptoms of acute rhinosinusitis think they need an antibiotic. Managing their expectations and providing instructions about supportive treatments are time consuming and may be difficult.
Nonetheless, we’re optimistic: We think that most patients today are aware of the problems associated with antibiotic resistance and wary of “superbugs,” and will therefore be receptive to this practice change. Physicians can help by reminding patients of the adverse effects of antibiotics and the natural course of rhino-sinusitis, as well as by offering symptomatic treatments.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Stop prescribing amoxicillin for acute rhinosinusitis. It’s unlikely to provide a speedier recovery than over-the-counter (OTC) remedies alone.1
STRENGTH OF RECOMMENDATION
B: Based on a single high-quality randomized controlled trial.
Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012 ;307:685-692.
ILLUSTRATIVE CASE
A 28-year-old man comes to your clinic after suffering from fatigue, purulent nasal discharge, and unilateral facial pain for nearly 10 days. Overall, he appears healthy, and you diagnose acute rhinosinusitis. You suggest OTC remedies for supportive care and wonder if a course of amoxicillin would speed his recovery.
Each year, more than 30 million Americans—about one in 7 adults—are diagnosed with sinusitis.2 No more than 2% of these cases are thought to be bacterial.3
Centers for Disease Control and Prevention (CDC) guidelines for the diagnosis of acute bacterial rhinosinusitis include symptoms that last 7 days or more, with maxillary pain or tenderness in the face or teeth and purulent nasal secretions.4 Patients with symptoms lasting less than 7 days are unlikely to have a bacterial infection. But the non-specific signs and symptoms included in the CDC guidelines limit their usefulness in determining whether the cause of the sinusitis is bacterial or viral on clinical grounds alone.
Most cases of sinusitis spontaneously resolve
In patients with acute bacterial sinusitis, the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) guidelines advocate watchful waiting and symptom relief with nasal oxymetazoline, pseudoephedrine, and saline nasal irrigation.3 The rate of spontaneous resolution is high: 80% of patients with clinically diagnosed sinusitis improve without treatment within 2 weeks.1,5
Traditional decongestants and mucolytics have not demonstrated efficacy in resolving sinusitis, although rigorous evaluation is lacking. Other treatments, such as saline irrigation and intranasal corticosteroids, are of unclear benefit and need further study.6-8
Lack of evidence has done little to curtail antibiotic use
An earlier PURL based on a meta-analysis of antibiotic treatment trials for sinusitis recommended that we stop prescribing antibiotics for adults with acute sinusitis unless their symptoms are severe.9,10 Yet antibiotics remain the mainstay of treatment.
Despite the AAO-HNS guidelines, evidence of spontaneous resolution, and accumulating data on the lack of efficacy of antimicrobials for sinusitis, 81% of patients diagnosed with acute sinusitis were given prescriptions for antibiotics, a study of primary care practices showed.11 Frequent use of antibiotics contributes to high rates of drug resistance, and adverse events related to antibiotic use account for an estimated 142,500 emergency department visits annually.12
STUDY SUMMARY: Little benefit from amoxicillin, even for severe cases
Garbutt and colleagues revisited the issue, randomizing 166 patients from 10 primary care practices to amoxicillin plus symptomatic treatment or placebo plus symptomatic treatment for acute rhinosinusitis.1 To be eligible for the study, patients had to be between the ages of 18 and 70 years, meet CDC diagnostic criteria for acute rhinosinusitis, and have moderate to very severe symptoms that were of 7- to-28-day duration and worsening or not improving or of <7-day duration but had worsened after an initial improvement. Exclusion criteria included complications from sinusitis, a history of allergy to penicillin or amoxicillin, antibiotic use in the past 4 weeks, comorbidities that impair immune function, cystic fibrosis, pregnancy, and mild symptoms.
Both groups had similar baseline characteristics, with participants who were predominantly white (79%) and female (64%). All the participants received a supply of symptomatic treatments: acetaminophen, guaifenesin, dextromethorphan, and sustained-release pseudoephedrine. The treatment group also received amoxicillin 1500 mg/d, divided into 3 doses; the placebo group received identical-looking placebo pills.
Patients were assessed with the Sino-nasal Outcome Test-16 (SNOT-16), a validated measure that asks patients to assess both the severity and frequency of 16 sinus symptoms. SNOT-16 uses a 0-to-3 rating scale (0=no problem; 3=severe problem), with a clinically important difference of ≥0.5 on the mean score. The test was administered at enrollment and at Days 3, 7, and 10. The disease-specific quality of life at Day 3 was the primary outcome.
There was no statistically significantly difference in SNOT-16 scores between the amoxicillin and placebo groups on Days 3 and 10. On Day 7, there was a small statistically significant improvement in the amoxicillin group, but it did not reach the level of clinical importance (≥0.5) based on SNOT-16’s mean score.
The authors also asked participants to retrospectively assess symptom change since enrollment on a 6-point scale. Those who reported that their symptoms were “a lot better” or “absent” were characterized as significantly improved. The results correlated with the data from the SNOT-16, showing no difference between the amoxicillin and control group at Days 3 and 10. On Day 7, 74% of patients treated with amoxicillin self- reported significant improvement in symptoms since the start of the study, vs 56% in the control group. The number needed to treat was 6 (95% confidence interval, 3-34; P= .02) for a reduction in symptoms at Day 7.
Patients in both groups had similar rates of absenteeism, inability to perform usual activities, relapse and recurrence, and use of additional health care. Satisfaction with treatment was similar, as well.
No serious adverse effects occurred. Both groups reported similar frequencies (<10%) of nausea, diarrhea, abdominal pain, or vaginitis.
WHAT’S NEW: Even severe sinusitis resolves without antibiotics
Previous studies recommended foregoing antibiotics for acute sinusitis, except when symptoms are severe. This study—in which more than half (52%) of patients in each group had symptoms rated severe or very severe—found no benefit to adding amoxicillin to supportive treatments.1 Antibiotics did not shorten the duration of illness, prevent relapse and recurrence, or improve satisfaction with treatment. The researchers found a statistically significant difference between groups on Day 7 of 0.19 points, but no clinically meaningful difference (≥0.5) based on the SNOT-16 mean score.
CAVEATS: Guidelines, risk of complications may give reason to pause
The 2012 Infectious Diseases Society of America guidelines recommend amoxicillin with clavulanic acid as empiric therapy for acute bacterial rhinosinusitis.7 The findings of the study by Garbutt et al—conducted at a time when the incidence of beta-lactamase-producing organisms was low and amoxicillin was the treatment of choice—suggest otherwise.
Serious complications of sinusitis, such as brain abscess, periorbital cellulitis, and meningitis, can occur, however. Patients who deteriorate clinically or develop high fever or severe headache require close follow-up, which may include further diagnostic evaluation or consultation with an otolaryngologist. Evidence is lacking as to whether antibiotics prevent such complications.5
CHALLENGES TO IMPLEMENTATION: Managing patient expectations
Many patients with symptoms of acute rhinosinusitis think they need an antibiotic. Managing their expectations and providing instructions about supportive treatments are time consuming and may be difficult.
Nonetheless, we’re optimistic: We think that most patients today are aware of the problems associated with antibiotic resistance and wary of “superbugs,” and will therefore be receptive to this practice change. Physicians can help by reminding patients of the adverse effects of antibiotics and the natural course of rhino-sinusitis, as well as by offering symptomatic treatments.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
2. Centers for Disease Control and Prevention. Summary health statistics for US adults: National Health Interview Survey 2010. January 2012. Available at: http://www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed July 9, 2012.
3. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
4. Hickner JM, Bartlett JG, Besser RE, et al. American Academy of Family Physians; American College of Physicians; American Society of Internal Medicine; Centers for Disease Control; Infectious Disease Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults; background. Ann Intern Med. 2001;134:498-505.
5. Ahovuo-Saloranta A, Borisenk OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2008;(2):CD000243.-
6. Allen G, Kelsberg G, Jankowski TA. Do nasal decongestants relieve symptoms? J Fam Pract. 2003;52:714-724.
7. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
8. Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2009;(4):CD005149.-
9. Schumann A, Hickner J. Patients insist on antibiotics for sinusitis? Here is a good reason to say “no”. J Fam Pract. Jul:2008;57:464-468.
10. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
11. Gill JM, Fleischut P, Haas S. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med. 2006;38:349-354.
12. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
1. Garbutt J, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
2. Centers for Disease Control and Prevention. Summary health statistics for US adults: National Health Interview Survey 2010. January 2012. Available at: http://www.cdc.gov/nchs/data/series/sr_10/sr10_252.pdf. Accessed July 9, 2012.
3. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1-S31.
4. Hickner JM, Bartlett JG, Besser RE, et al. American Academy of Family Physians; American College of Physicians; American Society of Internal Medicine; Centers for Disease Control; Infectious Disease Society of America. Principles of appropriate antibiotic use for acute rhinosinusitis in adults; background. Ann Intern Med. 2001;134:498-505.
5. Ahovuo-Saloranta A, Borisenk OV, Kovanen N, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database Syst Rev. 2008;(2):CD000243.-
6. Allen G, Kelsberg G, Jankowski TA. Do nasal decongestants relieve symptoms? J Fam Pract. 2003;52:714-724.
7. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
8. Zalmanovici A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2009;(4):CD005149.-
9. Schumann A, Hickner J. Patients insist on antibiotics for sinusitis? Here is a good reason to say “no”. J Fam Pract. Jul:2008;57:464-468.
10. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
11. Gill JM, Fleischut P, Haas S. Use of antibiotics for adult upper respiratory infections in outpatient settings: a national ambulatory network study. Fam Med. 2006;38:349-354.
12. Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47:735-743.
Copyright © 2012 The Family Physicians Inquiries Network. All rights reserved.
Macrolide resistance: Cause for concern?
In the PURL, “Consider adding this drug to fight COPD that’s severe”(J Fam Pract. 2012;61:414-416), Drs. Hobbs and Brown state that “there was an increase in the prevalence of macrolide-resistant respiratory pathogens in patients on daily azithromycin.” This statement is technically correct but terribly misleading. It implies that azithromycin caused increased resistance, which it did not.
Prevalence is a proportion, and in this case refers to the proportion of all isolates that were macrolide resistant. An increased proportion may be due to either an increased numerator (resistance) or a decreased denominator (isolates).
In the study in question1 there were actually fewer macrolide-resistant pathogens isolated during treatment with azithromycin compared with placebo. All else being equal, this would have resulted in a decreased prevalence. However, there were also far fewer total isolates in the azithromycin group. This relatively larger decrease in the denominator prevailed, resulting in “increased” prevalence, due to fewer pathogens, not more resistance. This finding (of fewer pathogens isolated) has a clinical correlate. The 2 largest trials comparing azithromycin with placebo both found decreased acute respiratory illnesses in the azithromycin groups compared with the placebo groups.2,3
The correct way to assess resistance would have been to calculate the incidence of newly detected resistant pathogens over a defined period of time in both the azithromycin and placebo groups. In fact, the incidence of macrolide resistance was 24% lower in the azithromycin group (11.1 per 100 patients per year vs 14.9 per 100 per year in the placebo group).4 Thus, the increased “prevalence” referred to by Hobbs and Brown does not indicate increased resistance, but rather decreased pathogens.
David L. Hahn, MD, MS
Madison, Wis
Drs. Hobbs and Mounsey respond
We thank Dr. Hahn for his comments and agree that further clarification of the impact of azithromycin on macrolide resistance is appropriate. As Dr. Hahn notes, the number of colonized patients in the azithromycin group (66/479) was lower than in the placebo group (172/476), as would be expected because they had been on azithromycin for one year.1 Dr. Hahn calculates the incidence of macrolide resistance using as the denominator all the patients in both the azithromycin and placebo groups and shows that the rate is higher in the azithromycin group.
We chose to determine macrolide resistance by comparing resistance rates only in the colonized patients (66 on azithromycin and 172 on placebo), not the whole group—the majority of whom were not colonized at all. Albert et al used similar methodology, reporting that “the incidence of resistance to macrolides was 81% [in the azithromycin group] and 41% [in the placebo group].”1
So as Dr. Hahn states, patients on azithromycin were less likely to become colonized with bacteria, but when they did, the organisms were more likely to be macrolide resistant.
Whichever way the data are presented, the finding of macrolide-resistant organisms in 81% of the isolates after only a year must raise concern about the long-term use of prophylactic azithromycin. In a recent commentary on the use of prophylactic azithromycin, Wenzel et al called this a “major concern” and stated that the Albert trial was not long enough to elucidate the extent or clinical implications of the problem.2
Keia Hobbs, MD
Anne Mounsey, MD
Chapel Hill, NC
In the PURL, “Consider adding this drug to fight COPD that’s severe”(J Fam Pract. 2012;61:414-416), Drs. Hobbs and Brown state that “there was an increase in the prevalence of macrolide-resistant respiratory pathogens in patients on daily azithromycin.” This statement is technically correct but terribly misleading. It implies that azithromycin caused increased resistance, which it did not.
Prevalence is a proportion, and in this case refers to the proportion of all isolates that were macrolide resistant. An increased proportion may be due to either an increased numerator (resistance) or a decreased denominator (isolates).
In the study in question1 there were actually fewer macrolide-resistant pathogens isolated during treatment with azithromycin compared with placebo. All else being equal, this would have resulted in a decreased prevalence. However, there were also far fewer total isolates in the azithromycin group. This relatively larger decrease in the denominator prevailed, resulting in “increased” prevalence, due to fewer pathogens, not more resistance. This finding (of fewer pathogens isolated) has a clinical correlate. The 2 largest trials comparing azithromycin with placebo both found decreased acute respiratory illnesses in the azithromycin groups compared with the placebo groups.2,3
The correct way to assess resistance would have been to calculate the incidence of newly detected resistant pathogens over a defined period of time in both the azithromycin and placebo groups. In fact, the incidence of macrolide resistance was 24% lower in the azithromycin group (11.1 per 100 patients per year vs 14.9 per 100 per year in the placebo group).4 Thus, the increased “prevalence” referred to by Hobbs and Brown does not indicate increased resistance, but rather decreased pathogens.
David L. Hahn, MD, MS
Madison, Wis
Drs. Hobbs and Mounsey respond
We thank Dr. Hahn for his comments and agree that further clarification of the impact of azithromycin on macrolide resistance is appropriate. As Dr. Hahn notes, the number of colonized patients in the azithromycin group (66/479) was lower than in the placebo group (172/476), as would be expected because they had been on azithromycin for one year.1 Dr. Hahn calculates the incidence of macrolide resistance using as the denominator all the patients in both the azithromycin and placebo groups and shows that the rate is higher in the azithromycin group.
We chose to determine macrolide resistance by comparing resistance rates only in the colonized patients (66 on azithromycin and 172 on placebo), not the whole group—the majority of whom were not colonized at all. Albert et al used similar methodology, reporting that “the incidence of resistance to macrolides was 81% [in the azithromycin group] and 41% [in the placebo group].”1
So as Dr. Hahn states, patients on azithromycin were less likely to become colonized with bacteria, but when they did, the organisms were more likely to be macrolide resistant.
Whichever way the data are presented, the finding of macrolide-resistant organisms in 81% of the isolates after only a year must raise concern about the long-term use of prophylactic azithromycin. In a recent commentary on the use of prophylactic azithromycin, Wenzel et al called this a “major concern” and stated that the Albert trial was not long enough to elucidate the extent or clinical implications of the problem.2
Keia Hobbs, MD
Anne Mounsey, MD
Chapel Hill, NC
In the PURL, “Consider adding this drug to fight COPD that’s severe”(J Fam Pract. 2012;61:414-416), Drs. Hobbs and Brown state that “there was an increase in the prevalence of macrolide-resistant respiratory pathogens in patients on daily azithromycin.” This statement is technically correct but terribly misleading. It implies that azithromycin caused increased resistance, which it did not.
Prevalence is a proportion, and in this case refers to the proportion of all isolates that were macrolide resistant. An increased proportion may be due to either an increased numerator (resistance) or a decreased denominator (isolates).
In the study in question1 there were actually fewer macrolide-resistant pathogens isolated during treatment with azithromycin compared with placebo. All else being equal, this would have resulted in a decreased prevalence. However, there were also far fewer total isolates in the azithromycin group. This relatively larger decrease in the denominator prevailed, resulting in “increased” prevalence, due to fewer pathogens, not more resistance. This finding (of fewer pathogens isolated) has a clinical correlate. The 2 largest trials comparing azithromycin with placebo both found decreased acute respiratory illnesses in the azithromycin groups compared with the placebo groups.2,3
The correct way to assess resistance would have been to calculate the incidence of newly detected resistant pathogens over a defined period of time in both the azithromycin and placebo groups. In fact, the incidence of macrolide resistance was 24% lower in the azithromycin group (11.1 per 100 patients per year vs 14.9 per 100 per year in the placebo group).4 Thus, the increased “prevalence” referred to by Hobbs and Brown does not indicate increased resistance, but rather decreased pathogens.
David L. Hahn, MD, MS
Madison, Wis
Drs. Hobbs and Mounsey respond
We thank Dr. Hahn for his comments and agree that further clarification of the impact of azithromycin on macrolide resistance is appropriate. As Dr. Hahn notes, the number of colonized patients in the azithromycin group (66/479) was lower than in the placebo group (172/476), as would be expected because they had been on azithromycin for one year.1 Dr. Hahn calculates the incidence of macrolide resistance using as the denominator all the patients in both the azithromycin and placebo groups and shows that the rate is higher in the azithromycin group.
We chose to determine macrolide resistance by comparing resistance rates only in the colonized patients (66 on azithromycin and 172 on placebo), not the whole group—the majority of whom were not colonized at all. Albert et al used similar methodology, reporting that “the incidence of resistance to macrolides was 81% [in the azithromycin group] and 41% [in the placebo group].”1
So as Dr. Hahn states, patients on azithromycin were less likely to become colonized with bacteria, but when they did, the organisms were more likely to be macrolide resistant.
Whichever way the data are presented, the finding of macrolide-resistant organisms in 81% of the isolates after only a year must raise concern about the long-term use of prophylactic azithromycin. In a recent commentary on the use of prophylactic azithromycin, Wenzel et al called this a “major concern” and stated that the Albert trial was not long enough to elucidate the extent or clinical implications of the problem.2
Keia Hobbs, MD
Anne Mounsey, MD
Chapel Hill, NC
Do antibiotics improve outcomes for patients hospitalized with COPD exacerbations?
YES. Antibiotic use reduced mortality and treatment failure in patients hospitalized with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Giving antibiotics early to hospitalized patients decreased the need for later ventilation and readmission within 30 days for exacerbation of COPD (SOR: B, a retrospective cohort study).
Evidence summary
A systematic review of 4 RCTs with a total of 356 patients found that antibiotic therapy reduced mortality more than placebo in moderately to severely ill hospitalized patients with COPD. Short-term mortality (7 days after treatment to 18 months after hospital discharge) decreased by 77% with antibiotic use in acute exacerbations of COPD (number needed to treat [NNT]=8; 95% confidence interval [CI], 6-17).
This same Cochrane review and a meta-analysis of 4 hospital-based trials with 321 patients evaluated failure to improve, deterioration, or death during the study period.1,2 The results favored treatment with antibiotics over placebo (NNT=3; 95% CI, 3-5).
Don’t wait to give antibiotics
A large retrospective cohort study of 84,621 hospitalized patients compared outcomes in patients given antibiotics for acute exacerbations of COPD during their first 2 days in the hospital with patients treated later or not at all.3 Outcomes assessed included need for later ventilation and readmission within 30 days for acute exacerbations of COPD.
The study included patients 40 years or older with a principal diagnosis, based on ICD 9 codes, of acute exacerbation of COPD, emphysema, or respiratory failure paired with a secondary diagnosis of COPD with acute exacerbation or emphysema. Patients who had been admitted directly to the intensive care unit were excluded, as were patients with other bacterial infections, such as pneumonia or cellulitis, for which they might receive antibiotics.
Early administration of antibiotics delayed the need for subsequent ventilation when compared with no antibiotics or antibiotics given later (1.07% vs 1.80%; P<.001; NNT=137). Giving antibiotics early also lowered readmission rates for acute exacerbations of COPD (7.91% vs 8.79%; P<.001; NNT=114), improved mortality rates (1.04% vs 1.59%; P<.001; NNT=182), and decreased treatment failure (9.77% vs 11.75%; P<.001; NNT=51).
Recommendations
The recommendations of the Global Initiative for Chronic Obstructive Lung Disease (GOLD), updated in 2008, call for antibiotics to be given to patients with 2 or more of the cardinal symptoms of acute exacerbations of COPD (shortness of breath, increased sputum production, and sputum purulence). Patients with severe exacerbations who require a ventilator should also receive antibiotics.4
The Primary Care Consensus Guidelines from 2004, consistent with the GOLD recommendations, state that a newer macrolide, extended-spectrum cephalosporin, or doxycycline is appropriate for moderately severe exacerbations. High-dose amoxicillin/clavulanate or a respiratory fluoroquinolone should be given for severe exacerbations.5
1. Ram FSF, Rodriguez-Rosin R, Granados-Navarrete A, et al. Antibiotics for exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(2):CD004403.
2. Russo RL, D’Aprile M. Role of antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Ann Pharmacother. 2001;35:576-581.
3. Rothenberg M, Pekow P, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
4. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO workshop report. Bethesda, Md: National Heart, Lung and Blood Institute; April 2001. Updated December 2009. Available at: http://www.goldcopd.com. Accessed July 1, 2011.
5. Brunton S, Carmichael P, Colgan R, et al. Acute exacerbation of chronic bronchitis: a primary care consensus guideline. Am J Manag Care. 2004;10:689-696.
YES. Antibiotic use reduced mortality and treatment failure in patients hospitalized with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Giving antibiotics early to hospitalized patients decreased the need for later ventilation and readmission within 30 days for exacerbation of COPD (SOR: B, a retrospective cohort study).
Evidence summary
A systematic review of 4 RCTs with a total of 356 patients found that antibiotic therapy reduced mortality more than placebo in moderately to severely ill hospitalized patients with COPD. Short-term mortality (7 days after treatment to 18 months after hospital discharge) decreased by 77% with antibiotic use in acute exacerbations of COPD (number needed to treat [NNT]=8; 95% confidence interval [CI], 6-17).
This same Cochrane review and a meta-analysis of 4 hospital-based trials with 321 patients evaluated failure to improve, deterioration, or death during the study period.1,2 The results favored treatment with antibiotics over placebo (NNT=3; 95% CI, 3-5).
Don’t wait to give antibiotics
A large retrospective cohort study of 84,621 hospitalized patients compared outcomes in patients given antibiotics for acute exacerbations of COPD during their first 2 days in the hospital with patients treated later or not at all.3 Outcomes assessed included need for later ventilation and readmission within 30 days for acute exacerbations of COPD.
The study included patients 40 years or older with a principal diagnosis, based on ICD 9 codes, of acute exacerbation of COPD, emphysema, or respiratory failure paired with a secondary diagnosis of COPD with acute exacerbation or emphysema. Patients who had been admitted directly to the intensive care unit were excluded, as were patients with other bacterial infections, such as pneumonia or cellulitis, for which they might receive antibiotics.
Early administration of antibiotics delayed the need for subsequent ventilation when compared with no antibiotics or antibiotics given later (1.07% vs 1.80%; P<.001; NNT=137). Giving antibiotics early also lowered readmission rates for acute exacerbations of COPD (7.91% vs 8.79%; P<.001; NNT=114), improved mortality rates (1.04% vs 1.59%; P<.001; NNT=182), and decreased treatment failure (9.77% vs 11.75%; P<.001; NNT=51).
Recommendations
The recommendations of the Global Initiative for Chronic Obstructive Lung Disease (GOLD), updated in 2008, call for antibiotics to be given to patients with 2 or more of the cardinal symptoms of acute exacerbations of COPD (shortness of breath, increased sputum production, and sputum purulence). Patients with severe exacerbations who require a ventilator should also receive antibiotics.4
The Primary Care Consensus Guidelines from 2004, consistent with the GOLD recommendations, state that a newer macrolide, extended-spectrum cephalosporin, or doxycycline is appropriate for moderately severe exacerbations. High-dose amoxicillin/clavulanate or a respiratory fluoroquinolone should be given for severe exacerbations.5
YES. Antibiotic use reduced mortality and treatment failure in patients hospitalized with acute exacerbations of chronic obstructive pulmonary disease (COPD) (strength of recommendation [SOR]: A, systematic reviews of randomized controlled trials [RCTs]).
Giving antibiotics early to hospitalized patients decreased the need for later ventilation and readmission within 30 days for exacerbation of COPD (SOR: B, a retrospective cohort study).
Evidence summary
A systematic review of 4 RCTs with a total of 356 patients found that antibiotic therapy reduced mortality more than placebo in moderately to severely ill hospitalized patients with COPD. Short-term mortality (7 days after treatment to 18 months after hospital discharge) decreased by 77% with antibiotic use in acute exacerbations of COPD (number needed to treat [NNT]=8; 95% confidence interval [CI], 6-17).
This same Cochrane review and a meta-analysis of 4 hospital-based trials with 321 patients evaluated failure to improve, deterioration, or death during the study period.1,2 The results favored treatment with antibiotics over placebo (NNT=3; 95% CI, 3-5).
Don’t wait to give antibiotics
A large retrospective cohort study of 84,621 hospitalized patients compared outcomes in patients given antibiotics for acute exacerbations of COPD during their first 2 days in the hospital with patients treated later or not at all.3 Outcomes assessed included need for later ventilation and readmission within 30 days for acute exacerbations of COPD.
The study included patients 40 years or older with a principal diagnosis, based on ICD 9 codes, of acute exacerbation of COPD, emphysema, or respiratory failure paired with a secondary diagnosis of COPD with acute exacerbation or emphysema. Patients who had been admitted directly to the intensive care unit were excluded, as were patients with other bacterial infections, such as pneumonia or cellulitis, for which they might receive antibiotics.
Early administration of antibiotics delayed the need for subsequent ventilation when compared with no antibiotics or antibiotics given later (1.07% vs 1.80%; P<.001; NNT=137). Giving antibiotics early also lowered readmission rates for acute exacerbations of COPD (7.91% vs 8.79%; P<.001; NNT=114), improved mortality rates (1.04% vs 1.59%; P<.001; NNT=182), and decreased treatment failure (9.77% vs 11.75%; P<.001; NNT=51).
Recommendations
The recommendations of the Global Initiative for Chronic Obstructive Lung Disease (GOLD), updated in 2008, call for antibiotics to be given to patients with 2 or more of the cardinal symptoms of acute exacerbations of COPD (shortness of breath, increased sputum production, and sputum purulence). Patients with severe exacerbations who require a ventilator should also receive antibiotics.4
The Primary Care Consensus Guidelines from 2004, consistent with the GOLD recommendations, state that a newer macrolide, extended-spectrum cephalosporin, or doxycycline is appropriate for moderately severe exacerbations. High-dose amoxicillin/clavulanate or a respiratory fluoroquinolone should be given for severe exacerbations.5
1. Ram FSF, Rodriguez-Rosin R, Granados-Navarrete A, et al. Antibiotics for exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(2):CD004403.
2. Russo RL, D’Aprile M. Role of antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Ann Pharmacother. 2001;35:576-581.
3. Rothenberg M, Pekow P, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
4. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO workshop report. Bethesda, Md: National Heart, Lung and Blood Institute; April 2001. Updated December 2009. Available at: http://www.goldcopd.com. Accessed July 1, 2011.
5. Brunton S, Carmichael P, Colgan R, et al. Acute exacerbation of chronic bronchitis: a primary care consensus guideline. Am J Manag Care. 2004;10:689-696.
1. Ram FSF, Rodriguez-Rosin R, Granados-Navarrete A, et al. Antibiotics for exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(2):CD004403.
2. Russo RL, D’Aprile M. Role of antimicrobial therapy in acute exacerbations of chronic obstructive pulmonary disease. Ann Pharmacother. 2001;35:576-581.
3. Rothenberg M, Pekow P, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
4. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO workshop report. Bethesda, Md: National Heart, Lung and Blood Institute; April 2001. Updated December 2009. Available at: http://www.goldcopd.com. Accessed July 1, 2011.
5. Brunton S, Carmichael P, Colgan R, et al. Acute exacerbation of chronic bronchitis: a primary care consensus guideline. Am J Manag Care. 2004;10:689-696.
Evidence-based answers from the Family Physicians Inquiries Network