User login
Not your garden variety neck pain ... Untimely death blamed on undiagnosed PE ... More
Not your garden variety neck pain
PERSISTENT BILATERAL NECK PAIN so severe that he couldn’t sit down brought a man to the emergency department (ED), where he was given ketorolac and diazepam. About an hour later, he said that the pain was better and was discharged with a diagnosis of neck strain and spasm and instructions to see his primary care physician if the pain persisted or worsened.
Four days later, the patient went to his primary care physician complaining of neck pain radiating down both arms, numbness in the right thumb, fever, chills, dysuria, and myalgia in his legs. The doctor observed decreased range of motion of the neck in all directions and diagnosed likely prostatitis. He ordered co-trimoxazole (trimethoprim and sulfamethoxazole), a nonemergent magnetic resonance imaging (MRI) scan, and physical therapy.
Fourteen hours after the doctor visit, the patient went back to the ED in a wheelchair. An emergency MRI showed epidural disease up and down the cervical spine and extending into the thoracic spine. An epidural abscess with spinal cord compression was diagnosed and decompression and evacuation surgery with spinal fusion was performed.
After several weeks in the hospital, the patient was referred to rehabilitation for partial quadriplegia. He has no use of his legs and very limited use of his hands and fingers. He’s confined to a wheelchair and needs help with most activities of daily living.
PLAINTIFF’S CLAIM When the patient visited his primary care physician, he had a classic presentation of a spinal abscess and should have undergone an emergent MRI, which would have revealed the abscess and allowed treatment with antibiotics and surgery before permanent damage occurred.
THE DEFENSE The patient’s symptoms weren’t a typical presentation of spinal abscess. There was no way the physician could have known what would happen the next day.
VERDICT $3 million Massachusetts settlement.
COMMENT Yes, there are zebras among the horses. We have to be vigilant to diagnose the rare serious cause of common problems such as neck pain. The combination of neck pain, patchy neurologic findings, signs of infection, and bladder symptoms should have raised red flags.
Untimely death blamed on undiagnosed PE
A 28-YEAR-OLD MAN went to the emergency department (ED) complaining of low-grade fever, nonproductive cough, and dizziness for 2 days. He also had tachycardia and significant hypoxia. An ED physician who saw the patient an hour after his arrival noted that he complained of weakness, shortness of breath, and light-headedness. The differential diagnosis included pneumonia, congestive heart failure, and pulmonary embolism.
After reviewing an electrocardiogram, chest radiograph, and laboratory studies, the ED doctor diagnosed pneumonia and renal insufficiency. The patient was admitted to the hospital, then transferred to another hospital about 8 hours later. He wasn’t evaluated by a physician when he was admitted to the second hospital.
About 5 hours after admission, the patient got out of bed and collapsed in the presence of his wife. A code was called, but the patient never regained consciousness and died about an hour and a half later. An autopsy established a pulmonary embolism as the cause of death.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the pulmonary embolism. Proper treatment would have allowed the patient to survive.
THE DEFENSE There was no negligence; heparin therapy wouldn’t have prevented the patient’s death.
VERDICT $6.1 million Maryland verdict.
COMMENT It isn’t enough to think of pulmonary embolism; a prompt definitive diagnostic work-up and timely treatment are key to preventing such a catastrophic outcome.
Delayed herpes diagnosis leads to lifelong consequences
A 10-DAY-OLD INFANT was examined by a pediatrician, who noted vesicles dotting the baby’s tongue, a possible manifestation of herpes, and observed herpes labialis on the mother’s lips. The pediatrician concluded that the vesicles didn’t indicate herpes and discharged the baby, instructing the parents to have him reexamined if he developed a fever, irritability, or lethargy. The next day the pediatrician consulted a neonatologist, who advised immediate reexamination. The baby was taken to a hospital, but then was immediately transported to another hospital.
At the second hospital, a physician examined the baby and consulted an oral surgeon. The surgeon believed that the vesicles were caused by burns from a hot baby bottle. The baby was discharged.
Six days later, the mother brought the baby to his regular pediatrician. She reported that the infant had been feverish and lethargic. The pediatrician didn’t find vesicles or other abnormalities. She ordered a complete blood count and blood culture, gave antibiotics, and told the parents to bring the baby back to see her the next day.
Very early the next day, the parents brought the baby to a hospital with a temperature of 101.2°F. The examining physician contacted the child’s pediatrician, who said she wanted to see the baby at 8:00 AM. When the pediatrician examined him, the infant’s temperature was 100.5°F. She gave antibiotics and instructed the parents to bring the baby back the next day, when his test results would be available.
The next day, the parents told the pediatrician’s assistant who examined the baby that his arms and legs had been twitching the previous evening. The infant received antibiotics but began to exhibit jerky movements. The parents were told to take him to a hospital, where he was diagnosed with herpes simplex and residual brain damage.
The child has quadriparesis and can’t talk, walk, or feed himself. He can eat only pureed food.
PLAINTIFF’S CLAIM The herpes simplex infection should have been diagnosed earlier. The pediatrician who examined the infant initially should have cultured the vesicles (and made sure that acyclovir was given) or consulted with, or referred the child to, a specialist. The physician who saw the child at the second hospital should have consulted a specialist, which would have led to the administration of acyclovir.
THE DEFENSE Hospitalization wasn’t necessary and a culture wasn’t appropriate. The appearance of the vesicles when the baby was examined at the second hospital didn’t suggest herpes.
VERDICT Multiple New York settlements totaling $10.2 million.
COMMENT As with many malpractice cases, there were many opportunities to prevent an egregious outcome. I wonder whether anyone involved stopped to entertain a differential diagnosis and note the urgent conditions the presentation clearly suggested.
Not your garden variety neck pain
PERSISTENT BILATERAL NECK PAIN so severe that he couldn’t sit down brought a man to the emergency department (ED), where he was given ketorolac and diazepam. About an hour later, he said that the pain was better and was discharged with a diagnosis of neck strain and spasm and instructions to see his primary care physician if the pain persisted or worsened.
Four days later, the patient went to his primary care physician complaining of neck pain radiating down both arms, numbness in the right thumb, fever, chills, dysuria, and myalgia in his legs. The doctor observed decreased range of motion of the neck in all directions and diagnosed likely prostatitis. He ordered co-trimoxazole (trimethoprim and sulfamethoxazole), a nonemergent magnetic resonance imaging (MRI) scan, and physical therapy.
Fourteen hours after the doctor visit, the patient went back to the ED in a wheelchair. An emergency MRI showed epidural disease up and down the cervical spine and extending into the thoracic spine. An epidural abscess with spinal cord compression was diagnosed and decompression and evacuation surgery with spinal fusion was performed.
After several weeks in the hospital, the patient was referred to rehabilitation for partial quadriplegia. He has no use of his legs and very limited use of his hands and fingers. He’s confined to a wheelchair and needs help with most activities of daily living.
PLAINTIFF’S CLAIM When the patient visited his primary care physician, he had a classic presentation of a spinal abscess and should have undergone an emergent MRI, which would have revealed the abscess and allowed treatment with antibiotics and surgery before permanent damage occurred.
THE DEFENSE The patient’s symptoms weren’t a typical presentation of spinal abscess. There was no way the physician could have known what would happen the next day.
VERDICT $3 million Massachusetts settlement.
COMMENT Yes, there are zebras among the horses. We have to be vigilant to diagnose the rare serious cause of common problems such as neck pain. The combination of neck pain, patchy neurologic findings, signs of infection, and bladder symptoms should have raised red flags.
Untimely death blamed on undiagnosed PE
A 28-YEAR-OLD MAN went to the emergency department (ED) complaining of low-grade fever, nonproductive cough, and dizziness for 2 days. He also had tachycardia and significant hypoxia. An ED physician who saw the patient an hour after his arrival noted that he complained of weakness, shortness of breath, and light-headedness. The differential diagnosis included pneumonia, congestive heart failure, and pulmonary embolism.
After reviewing an electrocardiogram, chest radiograph, and laboratory studies, the ED doctor diagnosed pneumonia and renal insufficiency. The patient was admitted to the hospital, then transferred to another hospital about 8 hours later. He wasn’t evaluated by a physician when he was admitted to the second hospital.
About 5 hours after admission, the patient got out of bed and collapsed in the presence of his wife. A code was called, but the patient never regained consciousness and died about an hour and a half later. An autopsy established a pulmonary embolism as the cause of death.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the pulmonary embolism. Proper treatment would have allowed the patient to survive.
THE DEFENSE There was no negligence; heparin therapy wouldn’t have prevented the patient’s death.
VERDICT $6.1 million Maryland verdict.
COMMENT It isn’t enough to think of pulmonary embolism; a prompt definitive diagnostic work-up and timely treatment are key to preventing such a catastrophic outcome.
Delayed herpes diagnosis leads to lifelong consequences
A 10-DAY-OLD INFANT was examined by a pediatrician, who noted vesicles dotting the baby’s tongue, a possible manifestation of herpes, and observed herpes labialis on the mother’s lips. The pediatrician concluded that the vesicles didn’t indicate herpes and discharged the baby, instructing the parents to have him reexamined if he developed a fever, irritability, or lethargy. The next day the pediatrician consulted a neonatologist, who advised immediate reexamination. The baby was taken to a hospital, but then was immediately transported to another hospital.
At the second hospital, a physician examined the baby and consulted an oral surgeon. The surgeon believed that the vesicles were caused by burns from a hot baby bottle. The baby was discharged.
Six days later, the mother brought the baby to his regular pediatrician. She reported that the infant had been feverish and lethargic. The pediatrician didn’t find vesicles or other abnormalities. She ordered a complete blood count and blood culture, gave antibiotics, and told the parents to bring the baby back to see her the next day.
Very early the next day, the parents brought the baby to a hospital with a temperature of 101.2°F. The examining physician contacted the child’s pediatrician, who said she wanted to see the baby at 8:00 AM. When the pediatrician examined him, the infant’s temperature was 100.5°F. She gave antibiotics and instructed the parents to bring the baby back the next day, when his test results would be available.
The next day, the parents told the pediatrician’s assistant who examined the baby that his arms and legs had been twitching the previous evening. The infant received antibiotics but began to exhibit jerky movements. The parents were told to take him to a hospital, where he was diagnosed with herpes simplex and residual brain damage.
The child has quadriparesis and can’t talk, walk, or feed himself. He can eat only pureed food.
PLAINTIFF’S CLAIM The herpes simplex infection should have been diagnosed earlier. The pediatrician who examined the infant initially should have cultured the vesicles (and made sure that acyclovir was given) or consulted with, or referred the child to, a specialist. The physician who saw the child at the second hospital should have consulted a specialist, which would have led to the administration of acyclovir.
THE DEFENSE Hospitalization wasn’t necessary and a culture wasn’t appropriate. The appearance of the vesicles when the baby was examined at the second hospital didn’t suggest herpes.
VERDICT Multiple New York settlements totaling $10.2 million.
COMMENT As with many malpractice cases, there were many opportunities to prevent an egregious outcome. I wonder whether anyone involved stopped to entertain a differential diagnosis and note the urgent conditions the presentation clearly suggested.
Not your garden variety neck pain
PERSISTENT BILATERAL NECK PAIN so severe that he couldn’t sit down brought a man to the emergency department (ED), where he was given ketorolac and diazepam. About an hour later, he said that the pain was better and was discharged with a diagnosis of neck strain and spasm and instructions to see his primary care physician if the pain persisted or worsened.
Four days later, the patient went to his primary care physician complaining of neck pain radiating down both arms, numbness in the right thumb, fever, chills, dysuria, and myalgia in his legs. The doctor observed decreased range of motion of the neck in all directions and diagnosed likely prostatitis. He ordered co-trimoxazole (trimethoprim and sulfamethoxazole), a nonemergent magnetic resonance imaging (MRI) scan, and physical therapy.
Fourteen hours after the doctor visit, the patient went back to the ED in a wheelchair. An emergency MRI showed epidural disease up and down the cervical spine and extending into the thoracic spine. An epidural abscess with spinal cord compression was diagnosed and decompression and evacuation surgery with spinal fusion was performed.
After several weeks in the hospital, the patient was referred to rehabilitation for partial quadriplegia. He has no use of his legs and very limited use of his hands and fingers. He’s confined to a wheelchair and needs help with most activities of daily living.
PLAINTIFF’S CLAIM When the patient visited his primary care physician, he had a classic presentation of a spinal abscess and should have undergone an emergent MRI, which would have revealed the abscess and allowed treatment with antibiotics and surgery before permanent damage occurred.
THE DEFENSE The patient’s symptoms weren’t a typical presentation of spinal abscess. There was no way the physician could have known what would happen the next day.
VERDICT $3 million Massachusetts settlement.
COMMENT Yes, there are zebras among the horses. We have to be vigilant to diagnose the rare serious cause of common problems such as neck pain. The combination of neck pain, patchy neurologic findings, signs of infection, and bladder symptoms should have raised red flags.
Untimely death blamed on undiagnosed PE
A 28-YEAR-OLD MAN went to the emergency department (ED) complaining of low-grade fever, nonproductive cough, and dizziness for 2 days. He also had tachycardia and significant hypoxia. An ED physician who saw the patient an hour after his arrival noted that he complained of weakness, shortness of breath, and light-headedness. The differential diagnosis included pneumonia, congestive heart failure, and pulmonary embolism.
After reviewing an electrocardiogram, chest radiograph, and laboratory studies, the ED doctor diagnosed pneumonia and renal insufficiency. The patient was admitted to the hospital, then transferred to another hospital about 8 hours later. He wasn’t evaluated by a physician when he was admitted to the second hospital.
About 5 hours after admission, the patient got out of bed and collapsed in the presence of his wife. A code was called, but the patient never regained consciousness and died about an hour and a half later. An autopsy established a pulmonary embolism as the cause of death.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the pulmonary embolism. Proper treatment would have allowed the patient to survive.
THE DEFENSE There was no negligence; heparin therapy wouldn’t have prevented the patient’s death.
VERDICT $6.1 million Maryland verdict.
COMMENT It isn’t enough to think of pulmonary embolism; a prompt definitive diagnostic work-up and timely treatment are key to preventing such a catastrophic outcome.
Delayed herpes diagnosis leads to lifelong consequences
A 10-DAY-OLD INFANT was examined by a pediatrician, who noted vesicles dotting the baby’s tongue, a possible manifestation of herpes, and observed herpes labialis on the mother’s lips. The pediatrician concluded that the vesicles didn’t indicate herpes and discharged the baby, instructing the parents to have him reexamined if he developed a fever, irritability, or lethargy. The next day the pediatrician consulted a neonatologist, who advised immediate reexamination. The baby was taken to a hospital, but then was immediately transported to another hospital.
At the second hospital, a physician examined the baby and consulted an oral surgeon. The surgeon believed that the vesicles were caused by burns from a hot baby bottle. The baby was discharged.
Six days later, the mother brought the baby to his regular pediatrician. She reported that the infant had been feverish and lethargic. The pediatrician didn’t find vesicles or other abnormalities. She ordered a complete blood count and blood culture, gave antibiotics, and told the parents to bring the baby back to see her the next day.
Very early the next day, the parents brought the baby to a hospital with a temperature of 101.2°F. The examining physician contacted the child’s pediatrician, who said she wanted to see the baby at 8:00 AM. When the pediatrician examined him, the infant’s temperature was 100.5°F. She gave antibiotics and instructed the parents to bring the baby back the next day, when his test results would be available.
The next day, the parents told the pediatrician’s assistant who examined the baby that his arms and legs had been twitching the previous evening. The infant received antibiotics but began to exhibit jerky movements. The parents were told to take him to a hospital, where he was diagnosed with herpes simplex and residual brain damage.
The child has quadriparesis and can’t talk, walk, or feed himself. He can eat only pureed food.
PLAINTIFF’S CLAIM The herpes simplex infection should have been diagnosed earlier. The pediatrician who examined the infant initially should have cultured the vesicles (and made sure that acyclovir was given) or consulted with, or referred the child to, a specialist. The physician who saw the child at the second hospital should have consulted a specialist, which would have led to the administration of acyclovir.
THE DEFENSE Hospitalization wasn’t necessary and a culture wasn’t appropriate. The appearance of the vesicles when the baby was examined at the second hospital didn’t suggest herpes.
VERDICT Multiple New York settlements totaling $10.2 million.
COMMENT As with many malpractice cases, there were many opportunities to prevent an egregious outcome. I wonder whether anyone involved stopped to entertain a differential diagnosis and note the urgent conditions the presentation clearly suggested.
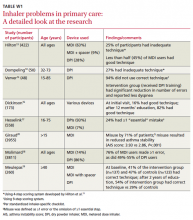
Combatting the cough that won’t quit
• Always include postnasal drip, asthma, and gastroesophageal reflux disease in the differential diagnosis for persistent cough, regardless of clinical signs and symptoms. B
• Do not rely on a patient’s description of the character and timing of the cough or the absence (or presence) of sputum to narrow down the differential diagnosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Margaret M, a 52-year-old nonsmoker, came to our clinic because of a persistent cough that had started about 4 weeks earlier. She had tried multiple over-the-counter cough suppressants, including dextromethorphan and guaifenesin, as well as cough drops, but none had been effective.
Margaret denied having had a cold or respiratory infection in the past few months or being in close contact with anyone with a chronic cough, and she had never had an asthma diagnosis. In response to a question about previous coughing episodes, the patient recalled having had several bouts of chronic cough in the past, including one about a year ago.
While Margaret had no known allergies, she did have occasional heartburn, which an antacid—or, at times, a drink of water—always relieved. Thyroid medication and calcium were the only things she took on a regular basis, separated by several hours to avoid problems with absorption.
Patients like Margaret, who seek help from their primary care physician only after attempting to combat a persistent cough on their own, may be quite frustrated by the time they arrive in your office. They’re counting on you to provide a cure. Fortunately, you’re likely to find it, as the differential diagnosis for subacute cough (a cough of 3-8 weeks’ duration) is limited.
Nonetheless, finding the cause of a subacute or chronic cough (lasting >8 weeks) is sometimes a matter of trial and error. Postnasal drip (also known as upper airway cough syndrome, or UACS), asthma, and gastroesophageal reflux disease (GERD) are the most common causes,1,2 followed by postinfectious cough, nonasthmatic eosinophilic bronchitis (NAEB), and pertussis.3 Although these conditions are all relatively well known, they are not always easy to detect: Some disorders, including UACS, asthma, and GERD, may be “silent,” with persistent cough the only presenting sign or symptom.4 In other cases, more than one condition may be contributing to the cough.

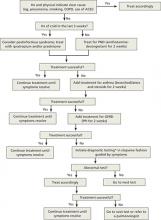
Starting with trials of empiric therapy for the most common causes of persistent cough—with sequential therapy and diagnostic tests, as needed—is far more effective than searching for relatively uncommon or obscure conditions. Following such a protocol, as detailed in the algorithm (FIGURE)4-7 we’ve developed and in the text that follows, can help you combat subacute and chronic cough in a cost-effective, timely way.
FIGURE
Dx and treatment when persistent cough is the only symptom4-7
*May include CXR, PPD, B pertussis IgG or IgA, spirometry with methacholine inhalation challenge, barium swallow, prolonged pH monitoring, sinus CT, and sputum eosinophil count, excluding any tests that have already been performed.
ACEI, angiotensin-converting enzyme inhibitor; CT, computed tomography; COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; GERD, gastroesophageal reflux disease; IgA, immunoglobulin A; IgG, immunoglobulin G; PND, postnasal drip; PPD, purified protein derivative; PPI, proton pump inhibitor.
Treat all patients for upper airway cough syndrome
Postnasal drip—renamed UACS by the guideline committee of the American Association of Chest Physicians because it isn’t clear whether the cough is caused by irritation from direct contact with postnasal drip or by inflammation of cough receptors in the upper airway—is the most common cause of chronic cough.6
The differential diagnosis for UACS, which is implicated in about 34% of cases of persistent cough, includes allergic, postinfectious, and occupational rhinitis; rhinitis due to anatomic abnormalities or physical or chemical irritants, rhinitis medicamentosa, and rhinitis of pregnancy; bacterial sinusitis; and allergic fungal sinusitis.8
The signs and symptoms of UACS are nonspecific, and a definitive diagnosis typically cannot be made from the medical history and physical examination alone. What’s more, the absence of any of the usual clinical findings—eg, rhinorrhea and excess sputum production—should not preclude an empiric trial with a first-generation antihistamine-decongestant combination such as brompheniramine/sustained-release pseudoephedrine. Second-and third-generation combination products, such as fexofenadine/pseudoephedrine, should not be used, as they are not effective in treating UACS.4
CASE Margaret’s physical exam was unremarkable. Her vital signs were stable, she had no cervical lymphadenopathy, and her chest was clear on auscultation. She had a dry cough that occurred twice during the exam, but not on inspiration.
The patient’s work-up included office spirometry, which was normal; a nasopharyngeal culture for Bordetella pertussis was negative. We prescribed a 2-week course of therapy with brompheniramine/sustained-release pseudoephedrine and scheduled a return visit shortly after it was completed.
There is no gold standard diagnostic test to confirm or rule out postnasal drip as the cause of cough. CT scanning of sinuses has a poor positive predictive value and is no longer recommended as part of an initial work-up,9 but may be useful for patients whose symptoms persist longer than 3 weeks.
Consider bronchodilator Tx when asthma is suspected
Cough-variant asthma is the second most common cause of persistent cough, and is responsible for an estimated 28% of cases.6 Asthma is the easiest of the conditions included in the differential diagnosis for persistent cough to establish in an office setting. The challenge is to remember to consider it in patients who present with cough but no sign of the classic expiratory wheezing. When you suspect that a patient has asthma, consider empiric bronchodilator therapy—or conduct spirometry testing.
Spirometric values of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70% and a positive bronchodilator response (≥12%) are consistent with an asthma diagnosis. Management of asthma depends on severity, and patients should be evaluated based on the National Heart, Lung, and Blood Institute’s National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma.10
It is crucial to ask patients with asthma (and, indeed, to ask all patients with a persistent cough) about exposure to secondhand smoke, and to stress the importance of avoiding smoking and secondary exposure. Individuals who are regularly exposed to secondhand smoke report more nasal symptoms and greater use of nasal decongestants compared with people with no exposure to smoke;11 they also have poor control of asthma.12-14
Cough unresolved? Add therapy for GERD
Although GERD is primarily associated with heartburn and gastrointestinal distress, it is not unusual for cough to be its only sign or symptom.15 In fact, GERD is the third most common cause of subacute cough—affecting about 21% of patients who seek help for cough at primary care practices.3
CASE Margaret returned to the clinic shortly after completion of a 2-week course of brompheniramine/sustained-release pseudoephedrine, and reported that she was still coughing frequently—and that the medication had brought little improvement. Because of her history of heartburn, we added a 2-week trial with a proton pump inhibitor (PPI)—omeprazole 20 mg/d.
While there are diagnostic tests for GERD, including a pH probe of the esophagus, a barium esophagogram, and manometry testing, empiric therapy with a PPI—starting with a trial of at least 2 weeks—often eliminates the troublesome cough.16 If the patient responds to treatment, the medication can be continued. Risks associated with long-term PPI therapy include osteoporosis and interference with calcium and magnesium absorption,17 so it is important to monitor patients taking them and to discontinue treatment as soon as the cough symptoms resolve.
Have you ruled out postinfectious cough?
If a patient has a cough that has lingered for 3 to 8 weeks after his or her recovery from an acute upper respiratory infection (URI), postinfectious cough may be the reason.18,19 Such a cough is subacute and self-limiting. (If the cough lasts >8 weeks after an acute illness, other diagnoses, such as chronic infection, are more likely.)
The pathogenesis for postinfectious cough may be related to postviral airway inflammation or bronchial hyperresponsiveness, and antibiotics are not indicated.4 Patients may be treated with a bronchodilator such as ipratropium rather than a beta-agonist or inhaled corticosteroids; oral tapered prednisone can be prescribed, if needed, for severe paroxysms, although there is limited evidence of its efficacy.20 Central antitussive agents such as codeine and dextromethorphan can be used when other measures fail to bring relief.
Nonasthmatic eosinophilic bronchitis does not impede airflow
NAEB is less well known than the conditions discussed thus far, but it is a relatively common cause of persistent cough.21-23 In some studies, up to 13% of patients with subacute cough were diagnosed with NAEB.6
Unlike asthma, NAEB is not associated with abnormalities in airway function; patients have no dyspnea and no wheezing, and no obstruction of airflow.24 Patients will have FEV1 >80% and FEV1/FVC >75% on spirometric examination, a negative response to bronchoprovocation, and, typically, an elevated sputum eosinophil count of >3%. Because induced sputum or bronchoscopic washings are difficult, exhaled nitric oxide testing is another option. If these tests are not available, a trial of inhaled steroids is indicated, even if neither spirometry nor bronchoprovocation testing was abnormal.9
Patients with NAEB respond well to inhaled corticosteroids, and budesonide 400 mcg twice a day or prednisolone 30 mg daily may be prescribed. It is also important to remove airway irritants. Long-term follow-up studies of patients with NAEB have had conflicting results. One study found that most cases resolve completely;23 another showed a need for long-term treatment, and suggested that patients with NAEB may be at increased risk for asthma and chronic obstructive pulmonary disease. 25
Paroxysmal cough, whoops point to pertussis
When a patient has paroxysms of cough, posttussive vomiting, and/or an inspiratory whooping sound, B pertussis infection is the likely culprit.26-28 A definitive diagnosis of pertussis, or whooping cough, may be based on a positive culture from a nasopharyngeal aspirate swab.29 Suspected cases can be confirmed with a polymerase chain reaction test, and a presumptive diagnosis may be made as a result of a 4-fold increase in immunoglobulin G or immunoglobulin A antibodies for B pertussis.4
A macrolide antibiotic, usually azithromycin, is the standard treatment for pertussis.30-32 Patients should be isolated for 5 days from the start of treatment. Antibiotic therapy will reduce the risk of transmission, but will not affect the duration of the cough, which may be 6 to 8 weeks. Long-acting beta-agonists, antihistamines, and corticosteroids should not be used to treat pertussis.4
CASE After a 2-week course of omeprazole 20 mg daily, Margaret was coughing much less. We extended the prescription, and by the end of the next 4 weeks, she was no longer coughing. After 2 months, both the PPI and the antihistamine/decongestant were discontinued. We advised her to institute antireflux measures, such as elevating her head at night and not eating after 6 pm, and she has not had a relapse.
CORRESPONDENCE Rebecca H. Gladu, MD, FAAFP, San Jacinto Methodist Hospital, 4401 Garth Road, Baytown, TX 77521; [email protected]
1. Corrao WM. Chronic persistent cough: diagnosis and treatment update. Pediatr Ann. 1996;25:162-168.
2. Holmes RL, Fadden CT. Evaluation of the patient with chronic cough. Am Fam Physician. 2004;69:2159-2166.
3. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640-647.
4. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough. Executive summary: ACCP evidence-based practice guideline. Chest. 2006;129(1 suppl):1S-23S.
5. Pratter MR, Bartter T, Akers S, et al. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977-983.
6. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):222S-231S.
7. Irwin RS, Madison JM. Anatomical diagnostic protocol in evaluating chronic cough with specific reference to gastroesophageal reflux disease. Am J Med. 2000;108(suppl 4a):126S-130S.
8. Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123 (4 Pt 1):413-417.
9. Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183:708-715.
10. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
11. Reh DD, Lin SY, Clipp SL, et al. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009;23:562-567.
12. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24:313-322.
13. Hersoug LG, Husemoen LL, Sigsgaard T, et al. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010;15:993-1000.
14. Self TH, Wallace JL, Gray LA, et al. Are we failing to document adequate smoking histories? A brief review 1999-2009. Curr Med Res Opin. 2010;26:1691-1696.
15. Sontag SJ. The spectrum of pulmonary symptoms due to gastroesophageal reflux. Thorac Surg Clin. 2005;15:353-368.
16. Irwin RS. Chronic cough due to gastroesophageal reflux. ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):80S-94S.
17. Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93-114.
18. Braman SS. Postinfectious cough: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):138S-146S.
19. Pratter MR. Cough and the common cold: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):72S-74S.
20. Chang AB, McKean M, Morris P. Inhaled anticholinergics for prolonged non-specific cough in children. Cochrane Database Syst Rev. 2004;(1):CD004358.-
21. Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406-410.
22. Gonlugur U, Gonlugur TE. Eosinophilic bronchitis without asthma. Int Arch Allergy Immunol. 2008;147:1-5.
23. Brightling CE. Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung. 2010;188 (suppl 1):S13-S17.
24. Gibson PG, Hargreave FE, Girgis-Gabardo, et al. Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy. 1995;25:127-132.
25. Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598-601.
26. Antico A, Fabozzi F, Scipiotti C. Pertussis in adults. A study in an Italian population with chronic cough. Monaldi Arch Chest Dis. 2002;57:247-252.
27. Birkebaek NH, Kristiansen M, Seefeldt T, et al. Bordetella pertussis and chronic cough in adults. Clin Infect Dis. 1999;29:1239-1242.
28. Kapaskelis AM, Vouloumanou EK, Rafailidis PI, et al. High prevalence of antibody titers against Bordetella pertussis in an adult population with prolonged cough. Respir Med. 2008;102:1586-1591.
29. Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304:890-896.
30. Devasia RA, Jones TF, Collier B, et al. Compliance with azithromycin versus erythromycin in the setting of a pertussis outbreak. Am J Med Sci. 2009;337:176-178.
31. Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest. 1989;95:723-728.
32. Altunaji SM, Kukuruzovic RH, Curtis NC, et al. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007;(3):CD004404.-
• Always include postnasal drip, asthma, and gastroesophageal reflux disease in the differential diagnosis for persistent cough, regardless of clinical signs and symptoms. B
• Do not rely on a patient’s description of the character and timing of the cough or the absence (or presence) of sputum to narrow down the differential diagnosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Margaret M, a 52-year-old nonsmoker, came to our clinic because of a persistent cough that had started about 4 weeks earlier. She had tried multiple over-the-counter cough suppressants, including dextromethorphan and guaifenesin, as well as cough drops, but none had been effective.
Margaret denied having had a cold or respiratory infection in the past few months or being in close contact with anyone with a chronic cough, and she had never had an asthma diagnosis. In response to a question about previous coughing episodes, the patient recalled having had several bouts of chronic cough in the past, including one about a year ago.
While Margaret had no known allergies, she did have occasional heartburn, which an antacid—or, at times, a drink of water—always relieved. Thyroid medication and calcium were the only things she took on a regular basis, separated by several hours to avoid problems with absorption.
Patients like Margaret, who seek help from their primary care physician only after attempting to combat a persistent cough on their own, may be quite frustrated by the time they arrive in your office. They’re counting on you to provide a cure. Fortunately, you’re likely to find it, as the differential diagnosis for subacute cough (a cough of 3-8 weeks’ duration) is limited.
Nonetheless, finding the cause of a subacute or chronic cough (lasting >8 weeks) is sometimes a matter of trial and error. Postnasal drip (also known as upper airway cough syndrome, or UACS), asthma, and gastroesophageal reflux disease (GERD) are the most common causes,1,2 followed by postinfectious cough, nonasthmatic eosinophilic bronchitis (NAEB), and pertussis.3 Although these conditions are all relatively well known, they are not always easy to detect: Some disorders, including UACS, asthma, and GERD, may be “silent,” with persistent cough the only presenting sign or symptom.4 In other cases, more than one condition may be contributing to the cough.

Starting with trials of empiric therapy for the most common causes of persistent cough—with sequential therapy and diagnostic tests, as needed—is far more effective than searching for relatively uncommon or obscure conditions. Following such a protocol, as detailed in the algorithm (FIGURE)4-7 we’ve developed and in the text that follows, can help you combat subacute and chronic cough in a cost-effective, timely way.
FIGURE
Dx and treatment when persistent cough is the only symptom4-7
*May include CXR, PPD, B pertussis IgG or IgA, spirometry with methacholine inhalation challenge, barium swallow, prolonged pH monitoring, sinus CT, and sputum eosinophil count, excluding any tests that have already been performed.
ACEI, angiotensin-converting enzyme inhibitor; CT, computed tomography; COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; GERD, gastroesophageal reflux disease; IgA, immunoglobulin A; IgG, immunoglobulin G; PND, postnasal drip; PPD, purified protein derivative; PPI, proton pump inhibitor.
Treat all patients for upper airway cough syndrome
Postnasal drip—renamed UACS by the guideline committee of the American Association of Chest Physicians because it isn’t clear whether the cough is caused by irritation from direct contact with postnasal drip or by inflammation of cough receptors in the upper airway—is the most common cause of chronic cough.6
The differential diagnosis for UACS, which is implicated in about 34% of cases of persistent cough, includes allergic, postinfectious, and occupational rhinitis; rhinitis due to anatomic abnormalities or physical or chemical irritants, rhinitis medicamentosa, and rhinitis of pregnancy; bacterial sinusitis; and allergic fungal sinusitis.8
The signs and symptoms of UACS are nonspecific, and a definitive diagnosis typically cannot be made from the medical history and physical examination alone. What’s more, the absence of any of the usual clinical findings—eg, rhinorrhea and excess sputum production—should not preclude an empiric trial with a first-generation antihistamine-decongestant combination such as brompheniramine/sustained-release pseudoephedrine. Second-and third-generation combination products, such as fexofenadine/pseudoephedrine, should not be used, as they are not effective in treating UACS.4
CASE Margaret’s physical exam was unremarkable. Her vital signs were stable, she had no cervical lymphadenopathy, and her chest was clear on auscultation. She had a dry cough that occurred twice during the exam, but not on inspiration.
The patient’s work-up included office spirometry, which was normal; a nasopharyngeal culture for Bordetella pertussis was negative. We prescribed a 2-week course of therapy with brompheniramine/sustained-release pseudoephedrine and scheduled a return visit shortly after it was completed.
There is no gold standard diagnostic test to confirm or rule out postnasal drip as the cause of cough. CT scanning of sinuses has a poor positive predictive value and is no longer recommended as part of an initial work-up,9 but may be useful for patients whose symptoms persist longer than 3 weeks.
Consider bronchodilator Tx when asthma is suspected
Cough-variant asthma is the second most common cause of persistent cough, and is responsible for an estimated 28% of cases.6 Asthma is the easiest of the conditions included in the differential diagnosis for persistent cough to establish in an office setting. The challenge is to remember to consider it in patients who present with cough but no sign of the classic expiratory wheezing. When you suspect that a patient has asthma, consider empiric bronchodilator therapy—or conduct spirometry testing.
Spirometric values of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70% and a positive bronchodilator response (≥12%) are consistent with an asthma diagnosis. Management of asthma depends on severity, and patients should be evaluated based on the National Heart, Lung, and Blood Institute’s National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma.10
It is crucial to ask patients with asthma (and, indeed, to ask all patients with a persistent cough) about exposure to secondhand smoke, and to stress the importance of avoiding smoking and secondary exposure. Individuals who are regularly exposed to secondhand smoke report more nasal symptoms and greater use of nasal decongestants compared with people with no exposure to smoke;11 they also have poor control of asthma.12-14
Cough unresolved? Add therapy for GERD
Although GERD is primarily associated with heartburn and gastrointestinal distress, it is not unusual for cough to be its only sign or symptom.15 In fact, GERD is the third most common cause of subacute cough—affecting about 21% of patients who seek help for cough at primary care practices.3
CASE Margaret returned to the clinic shortly after completion of a 2-week course of brompheniramine/sustained-release pseudoephedrine, and reported that she was still coughing frequently—and that the medication had brought little improvement. Because of her history of heartburn, we added a 2-week trial with a proton pump inhibitor (PPI)—omeprazole 20 mg/d.
While there are diagnostic tests for GERD, including a pH probe of the esophagus, a barium esophagogram, and manometry testing, empiric therapy with a PPI—starting with a trial of at least 2 weeks—often eliminates the troublesome cough.16 If the patient responds to treatment, the medication can be continued. Risks associated with long-term PPI therapy include osteoporosis and interference with calcium and magnesium absorption,17 so it is important to monitor patients taking them and to discontinue treatment as soon as the cough symptoms resolve.
Have you ruled out postinfectious cough?
If a patient has a cough that has lingered for 3 to 8 weeks after his or her recovery from an acute upper respiratory infection (URI), postinfectious cough may be the reason.18,19 Such a cough is subacute and self-limiting. (If the cough lasts >8 weeks after an acute illness, other diagnoses, such as chronic infection, are more likely.)
The pathogenesis for postinfectious cough may be related to postviral airway inflammation or bronchial hyperresponsiveness, and antibiotics are not indicated.4 Patients may be treated with a bronchodilator such as ipratropium rather than a beta-agonist or inhaled corticosteroids; oral tapered prednisone can be prescribed, if needed, for severe paroxysms, although there is limited evidence of its efficacy.20 Central antitussive agents such as codeine and dextromethorphan can be used when other measures fail to bring relief.
Nonasthmatic eosinophilic bronchitis does not impede airflow
NAEB is less well known than the conditions discussed thus far, but it is a relatively common cause of persistent cough.21-23 In some studies, up to 13% of patients with subacute cough were diagnosed with NAEB.6
Unlike asthma, NAEB is not associated with abnormalities in airway function; patients have no dyspnea and no wheezing, and no obstruction of airflow.24 Patients will have FEV1 >80% and FEV1/FVC >75% on spirometric examination, a negative response to bronchoprovocation, and, typically, an elevated sputum eosinophil count of >3%. Because induced sputum or bronchoscopic washings are difficult, exhaled nitric oxide testing is another option. If these tests are not available, a trial of inhaled steroids is indicated, even if neither spirometry nor bronchoprovocation testing was abnormal.9
Patients with NAEB respond well to inhaled corticosteroids, and budesonide 400 mcg twice a day or prednisolone 30 mg daily may be prescribed. It is also important to remove airway irritants. Long-term follow-up studies of patients with NAEB have had conflicting results. One study found that most cases resolve completely;23 another showed a need for long-term treatment, and suggested that patients with NAEB may be at increased risk for asthma and chronic obstructive pulmonary disease. 25
Paroxysmal cough, whoops point to pertussis
When a patient has paroxysms of cough, posttussive vomiting, and/or an inspiratory whooping sound, B pertussis infection is the likely culprit.26-28 A definitive diagnosis of pertussis, or whooping cough, may be based on a positive culture from a nasopharyngeal aspirate swab.29 Suspected cases can be confirmed with a polymerase chain reaction test, and a presumptive diagnosis may be made as a result of a 4-fold increase in immunoglobulin G or immunoglobulin A antibodies for B pertussis.4
A macrolide antibiotic, usually azithromycin, is the standard treatment for pertussis.30-32 Patients should be isolated for 5 days from the start of treatment. Antibiotic therapy will reduce the risk of transmission, but will not affect the duration of the cough, which may be 6 to 8 weeks. Long-acting beta-agonists, antihistamines, and corticosteroids should not be used to treat pertussis.4
CASE After a 2-week course of omeprazole 20 mg daily, Margaret was coughing much less. We extended the prescription, and by the end of the next 4 weeks, she was no longer coughing. After 2 months, both the PPI and the antihistamine/decongestant were discontinued. We advised her to institute antireflux measures, such as elevating her head at night and not eating after 6 pm, and she has not had a relapse.
CORRESPONDENCE Rebecca H. Gladu, MD, FAAFP, San Jacinto Methodist Hospital, 4401 Garth Road, Baytown, TX 77521; [email protected]
• Always include postnasal drip, asthma, and gastroesophageal reflux disease in the differential diagnosis for persistent cough, regardless of clinical signs and symptoms. B
• Do not rely on a patient’s description of the character and timing of the cough or the absence (or presence) of sputum to narrow down the differential diagnosis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Margaret M, a 52-year-old nonsmoker, came to our clinic because of a persistent cough that had started about 4 weeks earlier. She had tried multiple over-the-counter cough suppressants, including dextromethorphan and guaifenesin, as well as cough drops, but none had been effective.
Margaret denied having had a cold or respiratory infection in the past few months or being in close contact with anyone with a chronic cough, and she had never had an asthma diagnosis. In response to a question about previous coughing episodes, the patient recalled having had several bouts of chronic cough in the past, including one about a year ago.
While Margaret had no known allergies, she did have occasional heartburn, which an antacid—or, at times, a drink of water—always relieved. Thyroid medication and calcium were the only things she took on a regular basis, separated by several hours to avoid problems with absorption.
Patients like Margaret, who seek help from their primary care physician only after attempting to combat a persistent cough on their own, may be quite frustrated by the time they arrive in your office. They’re counting on you to provide a cure. Fortunately, you’re likely to find it, as the differential diagnosis for subacute cough (a cough of 3-8 weeks’ duration) is limited.
Nonetheless, finding the cause of a subacute or chronic cough (lasting >8 weeks) is sometimes a matter of trial and error. Postnasal drip (also known as upper airway cough syndrome, or UACS), asthma, and gastroesophageal reflux disease (GERD) are the most common causes,1,2 followed by postinfectious cough, nonasthmatic eosinophilic bronchitis (NAEB), and pertussis.3 Although these conditions are all relatively well known, they are not always easy to detect: Some disorders, including UACS, asthma, and GERD, may be “silent,” with persistent cough the only presenting sign or symptom.4 In other cases, more than one condition may be contributing to the cough.

Starting with trials of empiric therapy for the most common causes of persistent cough—with sequential therapy and diagnostic tests, as needed—is far more effective than searching for relatively uncommon or obscure conditions. Following such a protocol, as detailed in the algorithm (FIGURE)4-7 we’ve developed and in the text that follows, can help you combat subacute and chronic cough in a cost-effective, timely way.
FIGURE
Dx and treatment when persistent cough is the only symptom4-7
*May include CXR, PPD, B pertussis IgG or IgA, spirometry with methacholine inhalation challenge, barium swallow, prolonged pH monitoring, sinus CT, and sputum eosinophil count, excluding any tests that have already been performed.
ACEI, angiotensin-converting enzyme inhibitor; CT, computed tomography; COPD, chronic obstructive pulmonary disease; CXR, chest x-ray; GERD, gastroesophageal reflux disease; IgA, immunoglobulin A; IgG, immunoglobulin G; PND, postnasal drip; PPD, purified protein derivative; PPI, proton pump inhibitor.
Treat all patients for upper airway cough syndrome
Postnasal drip—renamed UACS by the guideline committee of the American Association of Chest Physicians because it isn’t clear whether the cough is caused by irritation from direct contact with postnasal drip or by inflammation of cough receptors in the upper airway—is the most common cause of chronic cough.6
The differential diagnosis for UACS, which is implicated in about 34% of cases of persistent cough, includes allergic, postinfectious, and occupational rhinitis; rhinitis due to anatomic abnormalities or physical or chemical irritants, rhinitis medicamentosa, and rhinitis of pregnancy; bacterial sinusitis; and allergic fungal sinusitis.8
The signs and symptoms of UACS are nonspecific, and a definitive diagnosis typically cannot be made from the medical history and physical examination alone. What’s more, the absence of any of the usual clinical findings—eg, rhinorrhea and excess sputum production—should not preclude an empiric trial with a first-generation antihistamine-decongestant combination such as brompheniramine/sustained-release pseudoephedrine. Second-and third-generation combination products, such as fexofenadine/pseudoephedrine, should not be used, as they are not effective in treating UACS.4
CASE Margaret’s physical exam was unremarkable. Her vital signs were stable, she had no cervical lymphadenopathy, and her chest was clear on auscultation. She had a dry cough that occurred twice during the exam, but not on inspiration.
The patient’s work-up included office spirometry, which was normal; a nasopharyngeal culture for Bordetella pertussis was negative. We prescribed a 2-week course of therapy with brompheniramine/sustained-release pseudoephedrine and scheduled a return visit shortly after it was completed.
There is no gold standard diagnostic test to confirm or rule out postnasal drip as the cause of cough. CT scanning of sinuses has a poor positive predictive value and is no longer recommended as part of an initial work-up,9 but may be useful for patients whose symptoms persist longer than 3 weeks.
Consider bronchodilator Tx when asthma is suspected
Cough-variant asthma is the second most common cause of persistent cough, and is responsible for an estimated 28% of cases.6 Asthma is the easiest of the conditions included in the differential diagnosis for persistent cough to establish in an office setting. The challenge is to remember to consider it in patients who present with cough but no sign of the classic expiratory wheezing. When you suspect that a patient has asthma, consider empiric bronchodilator therapy—or conduct spirometry testing.
Spirometric values of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70% and a positive bronchodilator response (≥12%) are consistent with an asthma diagnosis. Management of asthma depends on severity, and patients should be evaluated based on the National Heart, Lung, and Blood Institute’s National Asthma Education and Prevention Program Guidelines for the Diagnosis and Management of Asthma.10
It is crucial to ask patients with asthma (and, indeed, to ask all patients with a persistent cough) about exposure to secondhand smoke, and to stress the importance of avoiding smoking and secondary exposure. Individuals who are regularly exposed to secondhand smoke report more nasal symptoms and greater use of nasal decongestants compared with people with no exposure to smoke;11 they also have poor control of asthma.12-14
Cough unresolved? Add therapy for GERD
Although GERD is primarily associated with heartburn and gastrointestinal distress, it is not unusual for cough to be its only sign or symptom.15 In fact, GERD is the third most common cause of subacute cough—affecting about 21% of patients who seek help for cough at primary care practices.3
CASE Margaret returned to the clinic shortly after completion of a 2-week course of brompheniramine/sustained-release pseudoephedrine, and reported that she was still coughing frequently—and that the medication had brought little improvement. Because of her history of heartburn, we added a 2-week trial with a proton pump inhibitor (PPI)—omeprazole 20 mg/d.
While there are diagnostic tests for GERD, including a pH probe of the esophagus, a barium esophagogram, and manometry testing, empiric therapy with a PPI—starting with a trial of at least 2 weeks—often eliminates the troublesome cough.16 If the patient responds to treatment, the medication can be continued. Risks associated with long-term PPI therapy include osteoporosis and interference with calcium and magnesium absorption,17 so it is important to monitor patients taking them and to discontinue treatment as soon as the cough symptoms resolve.
Have you ruled out postinfectious cough?
If a patient has a cough that has lingered for 3 to 8 weeks after his or her recovery from an acute upper respiratory infection (URI), postinfectious cough may be the reason.18,19 Such a cough is subacute and self-limiting. (If the cough lasts >8 weeks after an acute illness, other diagnoses, such as chronic infection, are more likely.)
The pathogenesis for postinfectious cough may be related to postviral airway inflammation or bronchial hyperresponsiveness, and antibiotics are not indicated.4 Patients may be treated with a bronchodilator such as ipratropium rather than a beta-agonist or inhaled corticosteroids; oral tapered prednisone can be prescribed, if needed, for severe paroxysms, although there is limited evidence of its efficacy.20 Central antitussive agents such as codeine and dextromethorphan can be used when other measures fail to bring relief.
Nonasthmatic eosinophilic bronchitis does not impede airflow
NAEB is less well known than the conditions discussed thus far, but it is a relatively common cause of persistent cough.21-23 In some studies, up to 13% of patients with subacute cough were diagnosed with NAEB.6
Unlike asthma, NAEB is not associated with abnormalities in airway function; patients have no dyspnea and no wheezing, and no obstruction of airflow.24 Patients will have FEV1 >80% and FEV1/FVC >75% on spirometric examination, a negative response to bronchoprovocation, and, typically, an elevated sputum eosinophil count of >3%. Because induced sputum or bronchoscopic washings are difficult, exhaled nitric oxide testing is another option. If these tests are not available, a trial of inhaled steroids is indicated, even if neither spirometry nor bronchoprovocation testing was abnormal.9
Patients with NAEB respond well to inhaled corticosteroids, and budesonide 400 mcg twice a day or prednisolone 30 mg daily may be prescribed. It is also important to remove airway irritants. Long-term follow-up studies of patients with NAEB have had conflicting results. One study found that most cases resolve completely;23 another showed a need for long-term treatment, and suggested that patients with NAEB may be at increased risk for asthma and chronic obstructive pulmonary disease. 25
Paroxysmal cough, whoops point to pertussis
When a patient has paroxysms of cough, posttussive vomiting, and/or an inspiratory whooping sound, B pertussis infection is the likely culprit.26-28 A definitive diagnosis of pertussis, or whooping cough, may be based on a positive culture from a nasopharyngeal aspirate swab.29 Suspected cases can be confirmed with a polymerase chain reaction test, and a presumptive diagnosis may be made as a result of a 4-fold increase in immunoglobulin G or immunoglobulin A antibodies for B pertussis.4
A macrolide antibiotic, usually azithromycin, is the standard treatment for pertussis.30-32 Patients should be isolated for 5 days from the start of treatment. Antibiotic therapy will reduce the risk of transmission, but will not affect the duration of the cough, which may be 6 to 8 weeks. Long-acting beta-agonists, antihistamines, and corticosteroids should not be used to treat pertussis.4
CASE After a 2-week course of omeprazole 20 mg daily, Margaret was coughing much less. We extended the prescription, and by the end of the next 4 weeks, she was no longer coughing. After 2 months, both the PPI and the antihistamine/decongestant were discontinued. We advised her to institute antireflux measures, such as elevating her head at night and not eating after 6 pm, and she has not had a relapse.
CORRESPONDENCE Rebecca H. Gladu, MD, FAAFP, San Jacinto Methodist Hospital, 4401 Garth Road, Baytown, TX 77521; [email protected]
1. Corrao WM. Chronic persistent cough: diagnosis and treatment update. Pediatr Ann. 1996;25:162-168.
2. Holmes RL, Fadden CT. Evaluation of the patient with chronic cough. Am Fam Physician. 2004;69:2159-2166.
3. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640-647.
4. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough. Executive summary: ACCP evidence-based practice guideline. Chest. 2006;129(1 suppl):1S-23S.
5. Pratter MR, Bartter T, Akers S, et al. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977-983.
6. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):222S-231S.
7. Irwin RS, Madison JM. Anatomical diagnostic protocol in evaluating chronic cough with specific reference to gastroesophageal reflux disease. Am J Med. 2000;108(suppl 4a):126S-130S.
8. Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123 (4 Pt 1):413-417.
9. Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183:708-715.
10. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
11. Reh DD, Lin SY, Clipp SL, et al. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009;23:562-567.
12. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24:313-322.
13. Hersoug LG, Husemoen LL, Sigsgaard T, et al. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010;15:993-1000.
14. Self TH, Wallace JL, Gray LA, et al. Are we failing to document adequate smoking histories? A brief review 1999-2009. Curr Med Res Opin. 2010;26:1691-1696.
15. Sontag SJ. The spectrum of pulmonary symptoms due to gastroesophageal reflux. Thorac Surg Clin. 2005;15:353-368.
16. Irwin RS. Chronic cough due to gastroesophageal reflux. ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):80S-94S.
17. Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93-114.
18. Braman SS. Postinfectious cough: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):138S-146S.
19. Pratter MR. Cough and the common cold: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):72S-74S.
20. Chang AB, McKean M, Morris P. Inhaled anticholinergics for prolonged non-specific cough in children. Cochrane Database Syst Rev. 2004;(1):CD004358.-
21. Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406-410.
22. Gonlugur U, Gonlugur TE. Eosinophilic bronchitis without asthma. Int Arch Allergy Immunol. 2008;147:1-5.
23. Brightling CE. Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung. 2010;188 (suppl 1):S13-S17.
24. Gibson PG, Hargreave FE, Girgis-Gabardo, et al. Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy. 1995;25:127-132.
25. Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598-601.
26. Antico A, Fabozzi F, Scipiotti C. Pertussis in adults. A study in an Italian population with chronic cough. Monaldi Arch Chest Dis. 2002;57:247-252.
27. Birkebaek NH, Kristiansen M, Seefeldt T, et al. Bordetella pertussis and chronic cough in adults. Clin Infect Dis. 1999;29:1239-1242.
28. Kapaskelis AM, Vouloumanou EK, Rafailidis PI, et al. High prevalence of antibody titers against Bordetella pertussis in an adult population with prolonged cough. Respir Med. 2008;102:1586-1591.
29. Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304:890-896.
30. Devasia RA, Jones TF, Collier B, et al. Compliance with azithromycin versus erythromycin in the setting of a pertussis outbreak. Am J Med Sci. 2009;337:176-178.
31. Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest. 1989;95:723-728.
32. Altunaji SM, Kukuruzovic RH, Curtis NC, et al. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007;(3):CD004404.-
1. Corrao WM. Chronic persistent cough: diagnosis and treatment update. Pediatr Ann. 1996;25:162-168.
2. Holmes RL, Fadden CT. Evaluation of the patient with chronic cough. Am Fam Physician. 2004;69:2159-2166.
3. Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis. 1990;141:640-647.
4. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough. Executive summary: ACCP evidence-based practice guideline. Chest. 2006;129(1 suppl):1S-23S.
5. Pratter MR, Bartter T, Akers S, et al. An algorithmic approach to chronic cough. Ann Intern Med. 1993;119:977-983.
6. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129 (1 suppl):222S-231S.
7. Irwin RS, Madison JM. Anatomical diagnostic protocol in evaluating chronic cough with specific reference to gastroesophageal reflux disease. Am J Med. 2000;108(suppl 4a):126S-130S.
8. Irwin RS, Corrao WM, Pratter MR. Chronic persistent cough in the adult: the spectrum and frequency of causes and successful outcome of specific therapy. Am Rev Respir Dis. 1981;123 (4 Pt 1):413-417.
9. Birring SS. Controversies in the evaluation and management of chronic cough. Am J Respir Crit Care Med. 2011;183:708-715.
10. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
11. Reh DD, Lin SY, Clipp SL, et al. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009;23:562-567.
12. Stapleton M, Howard-Thompson A, George C, et al. Smoking and asthma. J Am Board Fam Med. 2011;24:313-322.
13. Hersoug LG, Husemoen LL, Sigsgaard T, et al. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology. 2010;15:993-1000.
14. Self TH, Wallace JL, Gray LA, et al. Are we failing to document adequate smoking histories? A brief review 1999-2009. Curr Med Res Opin. 2010;26:1691-1696.
15. Sontag SJ. The spectrum of pulmonary symptoms due to gastroesophageal reflux. Thorac Surg Clin. 2005;15:353-368.
16. Irwin RS. Chronic cough due to gastroesophageal reflux. ACCP evidence-based clinical practice guidelines. Chest. 2006;129(suppl 1):80S-94S.
17. Chen J, Yuan YC, Leontiadis GI, et al. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93-114.
18. Braman SS. Postinfectious cough: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):138S-146S.
19. Pratter MR. Cough and the common cold: ACCP evidence-based practice guidelines. Chest. 2006;129(suppl 1):72S-74S.
20. Chang AB, McKean M, Morris P. Inhaled anticholinergics for prolonged non-specific cough in children. Cochrane Database Syst Rev. 2004;(1):CD004358.-
21. Brightling CE, Ward R, Goh KL, et al. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160:406-410.
22. Gonlugur U, Gonlugur TE. Eosinophilic bronchitis without asthma. Int Arch Allergy Immunol. 2008;147:1-5.
23. Brightling CE. Cough due to asthma and nonasthmatic eosinophilic bronchitis. Lung. 2010;188 (suppl 1):S13-S17.
24. Gibson PG, Hargreave FE, Girgis-Gabardo, et al. Chronic cough with eosinophilic bronchitis: examination for variable airflow obstruction and response to corticosteroid. Clin Exp Allergy. 1995;25:127-132.
25. Berry MA, Hargadon B, McKenna S, et al. Observational study of the natural history of eosinophilic bronchitis. Clin Exp Allergy. 2005;35:598-601.
26. Antico A, Fabozzi F, Scipiotti C. Pertussis in adults. A study in an Italian population with chronic cough. Monaldi Arch Chest Dis. 2002;57:247-252.
27. Birkebaek NH, Kristiansen M, Seefeldt T, et al. Bordetella pertussis and chronic cough in adults. Clin Infect Dis. 1999;29:1239-1242.
28. Kapaskelis AM, Vouloumanou EK, Rafailidis PI, et al. High prevalence of antibody titers against Bordetella pertussis in an adult population with prolonged cough. Respir Med. 2008;102:1586-1591.
29. Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304:890-896.
30. Devasia RA, Jones TF, Collier B, et al. Compliance with azithromycin versus erythromycin in the setting of a pertussis outbreak. Am J Med Sci. 2009;337:176-178.
31. Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough. Experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest. 1989;95:723-728.
32. Altunaji SM, Kukuruzovic RH, Curtis NC, et al. Antibiotics for whooping cough (pertussis). Cochrane Database Syst Rev. 2007;(3):CD004404.-
Inadequate differential proves fatal ... Death by fentanyl patch and methadone ... more
Culture results go undiscussed, man suffers stroke
TWO WEEKS AFTER PROSTATE SURGERY, a 76-year-old man went to the ED because he was having trouble urinating. The ED physician catheterized the patient, ordered a urine culture, and discharged him.
The culture results, showing methicillin-resistant Staphylococcus aureus, were sent to a printer in the ED twice, as was the usual practice, but evidently no one saw them.
The patient returned to the ED 2 weeks after his initial visit with the same complaint of difficult urination and was seen by the same physician. The physician again discharged him with a catheter but without mentioning the culture results. Two days later, the patient suffered a stroke, which paralyzed his left side.
PLAINTIFF’S CLAIM The bacteria had spread from the patient’s urine to his bloodstream, sparking a cascade of events that led to the stroke.
THE DEFENSE No information about the defense is available.
VERDICT $2.25 million New Jersey settlement.
COMMENT The repeated missed opportunities to diagnose and treat this patient’s infection were regrettable—and costly.
Inadequate differential proves fatal
SHORTNESS OF BREATH led a 52-year-old woman to visit her medical group, where she was a long-time patient. The family practitioner who saw her noted tachycardia and ordered an electrocardiogram, which was abnormal. The physician also ordered a chest x-ray and, because the woman had a history of anemia, a complete blood count and a number of other blood tests. He subsequently called the patient at home to tell her that the blood tests were normal and she didn’t have anemia.
Three days later, the patient went to an urgent care center complaining of shortness of breath and tightness in her chest. A pulmonary embolism was diagnosed, and she was transferred to a hospital ED. Later that evening, a code blue was called and the patient was resuscitated. She died the following day.
PLAINTIFF’S CLAIM The doctor assumed that the patient had anemia and failed to develop a differential diagnosis. The patient had risk factors for pulmonary embolism—obesity and the use of an ethinyl estradiol-etonogestrel vaginal contraceptive ring—which should have prompted the doctor to consider that possibility. If he had done so, the pulmonary embolism would have been diagnosed and the patient’s death prevented.
THE DEFENSE The patient’s presentation wasn’t typical for pulmonary embolism, and there wasn’t any way to know whether an earlier diagnosis would have resulted in survival.
VERDICT $1.9 million California verdict.
COMMENT Although pulmonary embolism can be a challenging diagnosis to make, it needs to be considered carefully in all patients with shortness of breath, chest pain, or poorly defined pulmonary or cardiac symptoms.
The correct diagnosis comes too late
FLU-LIKE SYMPTOMS AND AN IRREGULAR HEART RATE prompted a man to go to the ED, where the physician diagnosed a viral infection, prescribed pain medication, and discharged him. The following day, a laboratory report indicating a staph infection was sent to an ED secretary, but the patient wasn’t told the results.
The patient returned to the hospital 2 days later in a confused state. Tests revealed a staph infection and meningitis, for which the patient received antibiotics. A week later, the patient suffered a stroke, resulting in diminished cognitive ability, impaired vision, and right-sided motor deficits.
PLAINTIFF’S CLAIM The white blood cell count and C-reactive protein level measured at the patient’s first visit to the ED would have led to a diagnosis of bacterial infection. The patient should have been admitted to the hospital and given antibiotics at that time.
THE DEFENSE The original diagnosis was reasonable.
VERDICT Confidential settlement with the hospital. $900,000 net verdict against the physician in New Jersey.
COMMENT Lab reports gone awry and the lack of a fail-safe for abnormal tests result in a $900,000 judgment. Do you have adequate systems in place to avoid a communication failure like this one?
Slow response turns a bad situation into a disaster
A 66-YEAR-OLD MAN on warfarin therapy for chronic atrial fibrillation and a transient ischemic attack underwent lithotripsy for kidney stones. Three days after the lithotripsy, he went to the ED complaining of severe flank pain. A computed tomography (CT) scan of the abdomen showed a large retroperitoneal hematoma and prominent perinephric and pararenal hemorrhages.
The patient remained on a gurney in the hallway of the ED in deteriorating condition until he was admitted to the intensive care unit, by which time his condition was critical. He died the next day.
PLAINTIFF’S CLAIM The ED physician and admitting urologists failed to monitor and treat the patient’s active hemorrhage for 9 hours. They didn’t order coagulation studies or respond to signs of escalating hemorrhagic shock. They failed to seek timely consults from surgery and interventional radiology.
THE DEFENSE No information about the defense is available.
VERDICT $825,000 Virginia settlement.
COMMENT Preventing complications of anticoagulation is hard enough; the lack of a timely response in this case made a bad outcome disastrous.
Were steps taken quickly enough?
SEVERE LOWER ABDOMINAL PAIN prompted a 52-year-old woman to go to the ED. She said she hadn’t had a bowel movement in almost a week. The ED physician, in consultation with the attending physician, admitted her to the hospital and ordered intravenous fluids and a soap suds enema, which didn’t relieve the constipation. The patient’s vital signs deteriorated, and she was crying and restless.
When the attending physician saw the patient almost 3 hours after admission, she had a fever of 101.4°F. He ordered additional tests, a computed tomography (CT) scan, and antibiotics, but didn’t order them STAT.
About 1½ hours later, a house physician examined the patient, and, after speaking with the attending physician, transferred her to a step-down telemetry unit. About 1½ hours after the transfer, a nurse called the house physician to report that the patient’s condition was worsening. The house physician ordered pain relievers and a second enema but didn’t come to the hospital.
Because the patient wasn’t in the intensive care unit, no one checked on her again for 3½ hours. When the nurse did check, she found the patient pale, cold, and turning blue. The nurse called the house physician, who came to the hospital. The patient had a fever of 102.4°F and her blood pressure couldn’t be measured.
After speaking with the attending physician, the house physician had the patient admitted to the ICU and also ordered a STAT surgical consultation and CT scan. In the meantime, the patient went into cardiac arrest and couldn’t be revived. Death was caused by peritonitis with sepsis resulting from a large intestinal obstruction.
PLAINTIFF’S CLAIM The patient showed early signs of sepsis. She should have undergone testing sooner and been transferred to the ICU earlier.
THE DEFENSE The doctors claimed that all their actions were appropriate and that the actions suggested by the plaintiff wouldn’t have resulted in the patient’s survival.
VERDICT $3.8 million Pennsylvania verdict.
COMMENT Prompt evaluation and monitoring of this patient might have prevented death and a substantial verdict.
2 analgesic calamities: Death by fentanyl patch …
AFTER A WEEK OF INCREASING BACK PAIN, which had begun to shoot down his right leg, a 37-year-old man went to the ED. He was examined and given prescriptions for pain killers, including acetaminophen and hydrocodone, and muscle relaxants and discharged with instructions to return in 3 days for magnetic resonance imaging (MRI).
While he was at the hospital for the MRI, the patient returned to the ED because he was still in pain and his acetaminophen-hydrocodone prescription was running out. The ED physician prescribed a 0.75-mg fentanyl transdermal patch and instructed the patient to put it on his chest.
Three days later, the patient filled the prescription and applied the patch. The following day, his girlfriend found him dead in bed. Postmortem toxicology results showed a blood fentanyl level of 9.85 ng/mL, markedly higher than the therapeutic level. Respiratory failure caused by fentanyl toxicity was cited as the cause of death.
PLAINTIFF’S CLAIM The ED physician prescribed an excessive dose of fentanyl.
THE DEFENSE A defective patch or misuse of the patch caused the patient’s death.
VERDICT $1.2 million Indiana verdict.
… and methadone
A 36-YEAR-OLD MAN started treatment with a pain specialist for pain arising from a back problem, for which he had taken pain medication previously. The pain specialist prescribed methadone, 360 10-mg tablets. The prescription limited the patient to 2 tablets every 4 hours for a maximum dosage of 12 tablets (120 mg) per day.
Three days after the patient filled the prescription, he was found dead. An autopsy determined the cause of death to be drug toxicity from methadone. At the time the patient died, the bottle of methadone tablets contained 342 tablets, indicating that he had taken only 18 tablets, well within the maximum dosage authorized by the prescription.
PLAINTIFF’S CLAIM The prescribed methadone dosage was excessive for a patient just beginning to use the drug. A proper initial dosage is between 2.5 and 10 mg every 8 to 12 hours for a maximum of 30 mg per day.
THE DEFENSE No information about the defense is available.
VERDICT Confidential Utah settlement.
COMMENT These 2 cases have a common thread. The effects of opioids are often idiosyncratic. A plan for careful monitoring and follow-up should be prepared at initiation of treatment and when escalating the dosage.
Culture results go undiscussed, man suffers stroke
TWO WEEKS AFTER PROSTATE SURGERY, a 76-year-old man went to the ED because he was having trouble urinating. The ED physician catheterized the patient, ordered a urine culture, and discharged him.
The culture results, showing methicillin-resistant Staphylococcus aureus, were sent to a printer in the ED twice, as was the usual practice, but evidently no one saw them.
The patient returned to the ED 2 weeks after his initial visit with the same complaint of difficult urination and was seen by the same physician. The physician again discharged him with a catheter but without mentioning the culture results. Two days later, the patient suffered a stroke, which paralyzed his left side.
PLAINTIFF’S CLAIM The bacteria had spread from the patient’s urine to his bloodstream, sparking a cascade of events that led to the stroke.
THE DEFENSE No information about the defense is available.
VERDICT $2.25 million New Jersey settlement.
COMMENT The repeated missed opportunities to diagnose and treat this patient’s infection were regrettable—and costly.
Inadequate differential proves fatal
SHORTNESS OF BREATH led a 52-year-old woman to visit her medical group, where she was a long-time patient. The family practitioner who saw her noted tachycardia and ordered an electrocardiogram, which was abnormal. The physician also ordered a chest x-ray and, because the woman had a history of anemia, a complete blood count and a number of other blood tests. He subsequently called the patient at home to tell her that the blood tests were normal and she didn’t have anemia.
Three days later, the patient went to an urgent care center complaining of shortness of breath and tightness in her chest. A pulmonary embolism was diagnosed, and she was transferred to a hospital ED. Later that evening, a code blue was called and the patient was resuscitated. She died the following day.
PLAINTIFF’S CLAIM The doctor assumed that the patient had anemia and failed to develop a differential diagnosis. The patient had risk factors for pulmonary embolism—obesity and the use of an ethinyl estradiol-etonogestrel vaginal contraceptive ring—which should have prompted the doctor to consider that possibility. If he had done so, the pulmonary embolism would have been diagnosed and the patient’s death prevented.
THE DEFENSE The patient’s presentation wasn’t typical for pulmonary embolism, and there wasn’t any way to know whether an earlier diagnosis would have resulted in survival.
VERDICT $1.9 million California verdict.
COMMENT Although pulmonary embolism can be a challenging diagnosis to make, it needs to be considered carefully in all patients with shortness of breath, chest pain, or poorly defined pulmonary or cardiac symptoms.
The correct diagnosis comes too late
FLU-LIKE SYMPTOMS AND AN IRREGULAR HEART RATE prompted a man to go to the ED, where the physician diagnosed a viral infection, prescribed pain medication, and discharged him. The following day, a laboratory report indicating a staph infection was sent to an ED secretary, but the patient wasn’t told the results.
The patient returned to the hospital 2 days later in a confused state. Tests revealed a staph infection and meningitis, for which the patient received antibiotics. A week later, the patient suffered a stroke, resulting in diminished cognitive ability, impaired vision, and right-sided motor deficits.
PLAINTIFF’S CLAIM The white blood cell count and C-reactive protein level measured at the patient’s first visit to the ED would have led to a diagnosis of bacterial infection. The patient should have been admitted to the hospital and given antibiotics at that time.
THE DEFENSE The original diagnosis was reasonable.
VERDICT Confidential settlement with the hospital. $900,000 net verdict against the physician in New Jersey.
COMMENT Lab reports gone awry and the lack of a fail-safe for abnormal tests result in a $900,000 judgment. Do you have adequate systems in place to avoid a communication failure like this one?
Slow response turns a bad situation into a disaster
A 66-YEAR-OLD MAN on warfarin therapy for chronic atrial fibrillation and a transient ischemic attack underwent lithotripsy for kidney stones. Three days after the lithotripsy, he went to the ED complaining of severe flank pain. A computed tomography (CT) scan of the abdomen showed a large retroperitoneal hematoma and prominent perinephric and pararenal hemorrhages.
The patient remained on a gurney in the hallway of the ED in deteriorating condition until he was admitted to the intensive care unit, by which time his condition was critical. He died the next day.
PLAINTIFF’S CLAIM The ED physician and admitting urologists failed to monitor and treat the patient’s active hemorrhage for 9 hours. They didn’t order coagulation studies or respond to signs of escalating hemorrhagic shock. They failed to seek timely consults from surgery and interventional radiology.
THE DEFENSE No information about the defense is available.
VERDICT $825,000 Virginia settlement.
COMMENT Preventing complications of anticoagulation is hard enough; the lack of a timely response in this case made a bad outcome disastrous.
Were steps taken quickly enough?
SEVERE LOWER ABDOMINAL PAIN prompted a 52-year-old woman to go to the ED. She said she hadn’t had a bowel movement in almost a week. The ED physician, in consultation with the attending physician, admitted her to the hospital and ordered intravenous fluids and a soap suds enema, which didn’t relieve the constipation. The patient’s vital signs deteriorated, and she was crying and restless.
When the attending physician saw the patient almost 3 hours after admission, she had a fever of 101.4°F. He ordered additional tests, a computed tomography (CT) scan, and antibiotics, but didn’t order them STAT.
About 1½ hours later, a house physician examined the patient, and, after speaking with the attending physician, transferred her to a step-down telemetry unit. About 1½ hours after the transfer, a nurse called the house physician to report that the patient’s condition was worsening. The house physician ordered pain relievers and a second enema but didn’t come to the hospital.
Because the patient wasn’t in the intensive care unit, no one checked on her again for 3½ hours. When the nurse did check, she found the patient pale, cold, and turning blue. The nurse called the house physician, who came to the hospital. The patient had a fever of 102.4°F and her blood pressure couldn’t be measured.
After speaking with the attending physician, the house physician had the patient admitted to the ICU and also ordered a STAT surgical consultation and CT scan. In the meantime, the patient went into cardiac arrest and couldn’t be revived. Death was caused by peritonitis with sepsis resulting from a large intestinal obstruction.
PLAINTIFF’S CLAIM The patient showed early signs of sepsis. She should have undergone testing sooner and been transferred to the ICU earlier.
THE DEFENSE The doctors claimed that all their actions were appropriate and that the actions suggested by the plaintiff wouldn’t have resulted in the patient’s survival.
VERDICT $3.8 million Pennsylvania verdict.
COMMENT Prompt evaluation and monitoring of this patient might have prevented death and a substantial verdict.
2 analgesic calamities: Death by fentanyl patch …
AFTER A WEEK OF INCREASING BACK PAIN, which had begun to shoot down his right leg, a 37-year-old man went to the ED. He was examined and given prescriptions for pain killers, including acetaminophen and hydrocodone, and muscle relaxants and discharged with instructions to return in 3 days for magnetic resonance imaging (MRI).
While he was at the hospital for the MRI, the patient returned to the ED because he was still in pain and his acetaminophen-hydrocodone prescription was running out. The ED physician prescribed a 0.75-mg fentanyl transdermal patch and instructed the patient to put it on his chest.
Three days later, the patient filled the prescription and applied the patch. The following day, his girlfriend found him dead in bed. Postmortem toxicology results showed a blood fentanyl level of 9.85 ng/mL, markedly higher than the therapeutic level. Respiratory failure caused by fentanyl toxicity was cited as the cause of death.
PLAINTIFF’S CLAIM The ED physician prescribed an excessive dose of fentanyl.
THE DEFENSE A defective patch or misuse of the patch caused the patient’s death.
VERDICT $1.2 million Indiana verdict.
… and methadone
A 36-YEAR-OLD MAN started treatment with a pain specialist for pain arising from a back problem, for which he had taken pain medication previously. The pain specialist prescribed methadone, 360 10-mg tablets. The prescription limited the patient to 2 tablets every 4 hours for a maximum dosage of 12 tablets (120 mg) per day.
Three days after the patient filled the prescription, he was found dead. An autopsy determined the cause of death to be drug toxicity from methadone. At the time the patient died, the bottle of methadone tablets contained 342 tablets, indicating that he had taken only 18 tablets, well within the maximum dosage authorized by the prescription.
PLAINTIFF’S CLAIM The prescribed methadone dosage was excessive for a patient just beginning to use the drug. A proper initial dosage is between 2.5 and 10 mg every 8 to 12 hours for a maximum of 30 mg per day.
THE DEFENSE No information about the defense is available.
VERDICT Confidential Utah settlement.
COMMENT These 2 cases have a common thread. The effects of opioids are often idiosyncratic. A plan for careful monitoring and follow-up should be prepared at initiation of treatment and when escalating the dosage.
Culture results go undiscussed, man suffers stroke
TWO WEEKS AFTER PROSTATE SURGERY, a 76-year-old man went to the ED because he was having trouble urinating. The ED physician catheterized the patient, ordered a urine culture, and discharged him.
The culture results, showing methicillin-resistant Staphylococcus aureus, were sent to a printer in the ED twice, as was the usual practice, but evidently no one saw them.
The patient returned to the ED 2 weeks after his initial visit with the same complaint of difficult urination and was seen by the same physician. The physician again discharged him with a catheter but without mentioning the culture results. Two days later, the patient suffered a stroke, which paralyzed his left side.
PLAINTIFF’S CLAIM The bacteria had spread from the patient’s urine to his bloodstream, sparking a cascade of events that led to the stroke.
THE DEFENSE No information about the defense is available.
VERDICT $2.25 million New Jersey settlement.
COMMENT The repeated missed opportunities to diagnose and treat this patient’s infection were regrettable—and costly.
Inadequate differential proves fatal
SHORTNESS OF BREATH led a 52-year-old woman to visit her medical group, where she was a long-time patient. The family practitioner who saw her noted tachycardia and ordered an electrocardiogram, which was abnormal. The physician also ordered a chest x-ray and, because the woman had a history of anemia, a complete blood count and a number of other blood tests. He subsequently called the patient at home to tell her that the blood tests were normal and she didn’t have anemia.
Three days later, the patient went to an urgent care center complaining of shortness of breath and tightness in her chest. A pulmonary embolism was diagnosed, and she was transferred to a hospital ED. Later that evening, a code blue was called and the patient was resuscitated. She died the following day.
PLAINTIFF’S CLAIM The doctor assumed that the patient had anemia and failed to develop a differential diagnosis. The patient had risk factors for pulmonary embolism—obesity and the use of an ethinyl estradiol-etonogestrel vaginal contraceptive ring—which should have prompted the doctor to consider that possibility. If he had done so, the pulmonary embolism would have been diagnosed and the patient’s death prevented.
THE DEFENSE The patient’s presentation wasn’t typical for pulmonary embolism, and there wasn’t any way to know whether an earlier diagnosis would have resulted in survival.
VERDICT $1.9 million California verdict.
COMMENT Although pulmonary embolism can be a challenging diagnosis to make, it needs to be considered carefully in all patients with shortness of breath, chest pain, or poorly defined pulmonary or cardiac symptoms.
The correct diagnosis comes too late
FLU-LIKE SYMPTOMS AND AN IRREGULAR HEART RATE prompted a man to go to the ED, where the physician diagnosed a viral infection, prescribed pain medication, and discharged him. The following day, a laboratory report indicating a staph infection was sent to an ED secretary, but the patient wasn’t told the results.
The patient returned to the hospital 2 days later in a confused state. Tests revealed a staph infection and meningitis, for which the patient received antibiotics. A week later, the patient suffered a stroke, resulting in diminished cognitive ability, impaired vision, and right-sided motor deficits.
PLAINTIFF’S CLAIM The white blood cell count and C-reactive protein level measured at the patient’s first visit to the ED would have led to a diagnosis of bacterial infection. The patient should have been admitted to the hospital and given antibiotics at that time.
THE DEFENSE The original diagnosis was reasonable.
VERDICT Confidential settlement with the hospital. $900,000 net verdict against the physician in New Jersey.
COMMENT Lab reports gone awry and the lack of a fail-safe for abnormal tests result in a $900,000 judgment. Do you have adequate systems in place to avoid a communication failure like this one?
Slow response turns a bad situation into a disaster
A 66-YEAR-OLD MAN on warfarin therapy for chronic atrial fibrillation and a transient ischemic attack underwent lithotripsy for kidney stones. Three days after the lithotripsy, he went to the ED complaining of severe flank pain. A computed tomography (CT) scan of the abdomen showed a large retroperitoneal hematoma and prominent perinephric and pararenal hemorrhages.
The patient remained on a gurney in the hallway of the ED in deteriorating condition until he was admitted to the intensive care unit, by which time his condition was critical. He died the next day.
PLAINTIFF’S CLAIM The ED physician and admitting urologists failed to monitor and treat the patient’s active hemorrhage for 9 hours. They didn’t order coagulation studies or respond to signs of escalating hemorrhagic shock. They failed to seek timely consults from surgery and interventional radiology.
THE DEFENSE No information about the defense is available.
VERDICT $825,000 Virginia settlement.
COMMENT Preventing complications of anticoagulation is hard enough; the lack of a timely response in this case made a bad outcome disastrous.
Were steps taken quickly enough?
SEVERE LOWER ABDOMINAL PAIN prompted a 52-year-old woman to go to the ED. She said she hadn’t had a bowel movement in almost a week. The ED physician, in consultation with the attending physician, admitted her to the hospital and ordered intravenous fluids and a soap suds enema, which didn’t relieve the constipation. The patient’s vital signs deteriorated, and she was crying and restless.
When the attending physician saw the patient almost 3 hours after admission, she had a fever of 101.4°F. He ordered additional tests, a computed tomography (CT) scan, and antibiotics, but didn’t order them STAT.
About 1½ hours later, a house physician examined the patient, and, after speaking with the attending physician, transferred her to a step-down telemetry unit. About 1½ hours after the transfer, a nurse called the house physician to report that the patient’s condition was worsening. The house physician ordered pain relievers and a second enema but didn’t come to the hospital.
Because the patient wasn’t in the intensive care unit, no one checked on her again for 3½ hours. When the nurse did check, she found the patient pale, cold, and turning blue. The nurse called the house physician, who came to the hospital. The patient had a fever of 102.4°F and her blood pressure couldn’t be measured.
After speaking with the attending physician, the house physician had the patient admitted to the ICU and also ordered a STAT surgical consultation and CT scan. In the meantime, the patient went into cardiac arrest and couldn’t be revived. Death was caused by peritonitis with sepsis resulting from a large intestinal obstruction.
PLAINTIFF’S CLAIM The patient showed early signs of sepsis. She should have undergone testing sooner and been transferred to the ICU earlier.
THE DEFENSE The doctors claimed that all their actions were appropriate and that the actions suggested by the plaintiff wouldn’t have resulted in the patient’s survival.
VERDICT $3.8 million Pennsylvania verdict.
COMMENT Prompt evaluation and monitoring of this patient might have prevented death and a substantial verdict.
2 analgesic calamities: Death by fentanyl patch …
AFTER A WEEK OF INCREASING BACK PAIN, which had begun to shoot down his right leg, a 37-year-old man went to the ED. He was examined and given prescriptions for pain killers, including acetaminophen and hydrocodone, and muscle relaxants and discharged with instructions to return in 3 days for magnetic resonance imaging (MRI).
While he was at the hospital for the MRI, the patient returned to the ED because he was still in pain and his acetaminophen-hydrocodone prescription was running out. The ED physician prescribed a 0.75-mg fentanyl transdermal patch and instructed the patient to put it on his chest.
Three days later, the patient filled the prescription and applied the patch. The following day, his girlfriend found him dead in bed. Postmortem toxicology results showed a blood fentanyl level of 9.85 ng/mL, markedly higher than the therapeutic level. Respiratory failure caused by fentanyl toxicity was cited as the cause of death.
PLAINTIFF’S CLAIM The ED physician prescribed an excessive dose of fentanyl.
THE DEFENSE A defective patch or misuse of the patch caused the patient’s death.
VERDICT $1.2 million Indiana verdict.
… and methadone
A 36-YEAR-OLD MAN started treatment with a pain specialist for pain arising from a back problem, for which he had taken pain medication previously. The pain specialist prescribed methadone, 360 10-mg tablets. The prescription limited the patient to 2 tablets every 4 hours for a maximum dosage of 12 tablets (120 mg) per day.
Three days after the patient filled the prescription, he was found dead. An autopsy determined the cause of death to be drug toxicity from methadone. At the time the patient died, the bottle of methadone tablets contained 342 tablets, indicating that he had taken only 18 tablets, well within the maximum dosage authorized by the prescription.
PLAINTIFF’S CLAIM The prescribed methadone dosage was excessive for a patient just beginning to use the drug. A proper initial dosage is between 2.5 and 10 mg every 8 to 12 hours for a maximum of 30 mg per day.
THE DEFENSE No information about the defense is available.
VERDICT Confidential Utah settlement.
COMMENT These 2 cases have a common thread. The effects of opioids are often idiosyncratic. A plan for careful monitoring and follow-up should be prepared at initiation of treatment and when escalating the dosage.
Inhalation therapy: Help patients avoid these mistakes
• Stress the importance of exhaling gently for a few seconds before inhaling (deeply and slowly for a metered dose inhaler, and deeply and rapidly for most dry powder inhalers). C
• Observe the inhaler technique of every patient receiving inhalation therapy on more than one occasion. C
• Don’t rely on self-reports regarding inhaler technique; despite claims of proficiency, most patients make at least one mistake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For patients with asthma or chronic obstructive pulmonary disease (COPD), inhalation therapy is the foundation of treatment. Yet all too often, patients don’t get the full value of their inhaled medications because they use their inhaler incorrectly. When technique is markedly flawed, suboptimal outcomes typically result.
Given the number of Americans with asthma (at least 22 million)1 and COPD (more than 13 million adults),2 faulty inhaler technique is a major public health problem. In fact, the number of people suffering from COPD may be even larger: Close to 24 million US adults are believed to have impaired lung function.3,4 For patients with asthma or COPD—many of whom are treated by family physicians—comprehensive education with a focus on correct use of an inhaler is essential.
In this review, we present evidence of frequent inhaler errors (from clinical studies) and highlight some of the more common mistakes (based on our clinical experience [TABLE]5). Finally, we offer ‘‘time-efficient’’ solutions to inhaler problems—steps that physicians in busy primary care practices can take to ensure that patients with asthma or COPD get the maximum benefit from inhalation therapy.
TABLE
Caution patients about these device-specific mistakes*
| Metered dose inhaler |
|---|
|
| Metered dose inhaler plus spacer/VHC |
|
| Dry powder inhaler |
|
| *These are examples based on the experience of the authors; other errors are possible. †Timing is not as crucial as it is for an MDI without a spacer, but the drug is still lost if inhalation is delayed. ‡Correct use varies by type of product (see product literature for specifics). DPI, dry powder inhaler; MDI, metered dose inhaler; VHC, valved holding chamber. Source: Adapted with permission from Self TH, et al. Consultant. 2003.5 |
Inhaler error is well documented
Since 1965, when it was first reported that many patients used metered dose inhalers (MDIs) incorrectly,6 evidence has accumulated supporting the magnitude of the problem.7-12 (Studies conducted in family practice settings are described in “Researchers look at inhaler problems in primary care” and in TABLE W1.13-20)
A number of studies of various sizes (from 41 to 3955 patients) have assessed inhaler technique in patients being treated by clinicians in primary care. The researchers used a variety of scoring methods, as well. Among them were a simple 4-step (0-4) rating system, a 9-step system, a standardized inhaler-specific checklist, and a system that tracked the number of omissions patients made.13-20 All found significant problems with inhaler technique. (You’ll find a detailed look at the studies in TABLE W1 at jfponline.com.)
In one study of 422 patients,13 including young children, adolescents, and adults, participants received one point for correctly performing each of the following steps:
- Adequate preparation (shaking well for those using a metered dose inhaler [MDI]; loading correctly for patients using a dry powder inhaler [DPI])
- Adequate expiration, correct head position
- Adequate inspiratory technique
- Holding breath afterwards.
The researchers found that 25% of the patients had inadequate technique (≤2 on a 0-4 point scale). In this study, as in others that included patients using various types of devices, use of an MDI was associated with a higher rate of incorrect technique.
Another much-smaller study14 used the same 4-step system to assess the technique of 50 patients, all of whom had the same type of DPI and had received extensive training in the correct use of the device. Despite the training, 27% of the patients received scores of ≤2 (inadequate technique). Sixty-eight percent received a score of 3 (adequate); only 5% received a score of 4 (good).
The 2 largest studies—one including 3955 patients using MDIs20 and the other looking at 3811 patients using various kinds of devices18—found high levels of errors, as well. In the latter study, 76% of patients with MDIs made at least one error vs 49% to 55% of patients using DPIs.18 The results convinced a large majority of the physicians caring for these patients of the need to check inhaler technique more frequently. In the study of MDI users alone, 71% of the patients made at least one mistake.20 inhaler misuse was associated with higher asthma instability scores, this study showed.
More recently, a researcher assessed the effects of an integrated primary care model on the management of asthma and/or COPD in middle-aged and elderly patients, in a study of 260 patients in 44 family practices.19 The study included an evaluation of inhaler technique.
Participants were divided into an intervention group—137 patients who received education regarding inhaler use from a nurse—and a usual care group (123 patients). After 2 years, correct inhaler technique among those in the intervention group went from 41% at baseline to 54%. At the same time, the proportion of those in the usual care group with correct technique fell from 47% to 29%.19
Error rates vary widely from one clinical trial to another, depending on study criteria, type of device, and extent of patient education, among other factors. Nonetheless, several studies (spanning 3 decades) found the error rate to be close to, or greater than, 90%.7,10,21
The most recent of these, published in 2009,21 was based on observation of the inhaler technique used by patients with asthma or COPD directly following appointments in an outpatient clinic. The authors found that, although >98% of the study participants claimed to know how to use their inhalers, 94% committed at least one error. In this study and a number of others, user error was more likely in patients using MDIs.13,18,21,22
Adding a spacer (eg, a valved holding chamber such as the AeroChamber) can be helpful, as the spacer affords the patient more time to inhale the medication. But patients who use an MDI with a spacer often make mistakes, too, and patient education is essential.23-26
Breath-activated dry powder inhalers (DPIs)—such as the Flexhaler, HandiHaler, Aerolizer, and Diskus—also reduce the likelihood of error. DPIs eliminate a step that MDI users often struggle with: the need to simultaneously press down on the canister and begin a slow, deep inhalation.
What’s more, DPIs do not have to be shaken before use. Nonetheless, using a DPI still involves a series of actions. For the HandiHaler and Aerolizer, patients must load the dose, and some patients fail to read the directions and swallow the capsule instead of loading it into the device. Patients must remember to exhale away from the device (ie, not into the dry powder) before inhaling, then hold their breath for approximately 10 seconds. There is potential for error at each step.
Stress the need to exhale before using the inhaler
Forgetting to exhale before inhaling is a common, and significant, mistake regardless of the type of device. It is paramount to stress the need to exhale gently for a few seconds before inhaling (slowly and deeply for patients using an MDI, rapidly and deeply with most DPIs). For MDI users, poor timing, described earlier, is another common and serious mistake. Patients using an MDI with a valved holding chamber sometimes inhale for too long before pressing down on the inhaler, then are unable to continue inhaling although the aerosol is still in the chamber. A common error made by patients using multidose DPIs is simply to forget to load the dose.
Physicians need to brush up on their skills, too
It’s not just patients who lack proficiency in inhaler technique. Numerous studies have demonstrated poor skill among physicians and other health care professionals.27-34 Evidence also shows that targeted education results in substantial improvement.32,35
In one study undertaken to evaluate family medicine residents’ proficiency in using asthma inhalers, participants (an intervention group at one clinic and a control group at another) all were given a pretest. The intervention group then received educational materials and a tutorial, as well as the opportunity for hands-on practice, after which both groups were given a post-test. The residents who received the training had a 170% jump, on average, in proficiency score, vs a 55% increase for the control group (P<.001).35
Inhaled Medication Instructional Videos
Courtesy of: National Jewish Health
Go to http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos
Another study—this one involving first-year interns—looked at level of improvement based on the type of education provided. Initially, only 5% of the interns could use an MDI without error. After a lecture and demonstration, 13% had an error-free technique. But when each intern participated in an intensive one-on-one session, the error-free rate reached 73%. The researchers’ conclusion: Lectures are relatively ineffective in teaching interns inhaler technique compared with a one-on-one approach.32
The Chicago Breathe Project,36 a new program aimed at improving education in the use of asthma inhalers for physicians and minority patients, provides further evidence of the value of clinician education. After a series of workshops for residents at 5 academic institutions, the physicians’ knowledge of proper use of inhalers rose dramatically—from just 5% preprogram to 91% postprogram (P<.001). Six months after the educational activity, the residents (n=161) were more likely (44% vs 11% preprogram) to assess patients’ inhaler technique.36
Teaching patients when time is tight
National and international guidelines stress the need to teach patients correct use of asthma and COPD inhalers.1,37,38 Providing the requisite education includes observation of each patient’s inhaler technique with proper use demonstrated, as needed.
The problem, of course, is how to provide that level of patient education within the time constraints of a busy family practice. We recommend these time-efficient solutions:
Enlist the help of other clinicians. While it is important that someone in your office be well trained and able to instruct patients in the proper use of inhalers, that individual need not be you. The National Institutes of Health recommends that the “principal clinician” introduce key educational messages, which can be reinforced and expanded on by other members of the health care team.1
After you advise patients that it is crucial for them to be trained in and adhere to proper inhaler technique, another health care professional—often a clinic nurse or pharmacist who has had special training—can provide the hands-on education. Studies have shown that when pharmacists who are competent in asthma management, including inhaler technique, work with physicians to optimize the education and overall management of patients with asthma, better outcomes often result, including a reduction in both emergency department visits and hospitalizations.1,39,40
Use videos to demonstrate correct technique. Videos are an effective teaching tool,9 and many of them are device-specific. National Jewish Health, which is world renowned for its asthma care, has a set of instructional videos posted on You-Tube and accessible from its Web site (http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos). In addition to videos that demonstrate the use of an MDI alone and an MDI plus a valved holding chamber, the site has links to 6 DPI videos, each covering a different device.
Use intermittent observation. After the patient views the appropriate video, you or a member of your staff will still need to observe the patient’s inhaler technique to ensure correct use. Ideally, this should occur at every visit.1,37 When that’s not possible, use intermittent observation, starting with the first 2 or 3 visits after the introduction of inhalation therapy and then switching to periodic observation to ensure that the patient is maintaining good technique.
In determining how often observation is necessary, keep in mind that simply asking patients whether they are having inhaler problems is not sufficient.1 Patients tend to say they have little or no trouble when, in fact, most struggle, at times, with the devices. What’s more, good technique tends to decrease over time, and repetitive education is important.
To motivate patients, try this communication technique
Motivational interviewing, a technique that has been used to help patients battle obesity, quit smoking, and control hypertension,41-43 among other health problems, can help you identify inhaler problems that need to be addressed. It involves the use of open-ended questions (eg, “What worries you most about your asthma?”), affirmations (“You’ve done a great job testing your peak flow level every morning”), reflective listening (“You’re tired of taking medicine every day”), and summary statements (“You know you should take your medicine every day but you’re having trouble remembering it. Is that right?”).
A pilot study44 showed that when this technique was incorporated into an asthma education session, patient motivation increased. The ratio of perceived advantages vs disadvantages of taking asthma medication correctly improved, as well. Another study45 found that when motivational interviewing was used during home visits to inner-city African American adolescents for asthma care, the patients’ motivation, readiness to adhere to treatment, and asthma-related quality of life improved, although self-reported adherence to asthma medication did not. Further studies involving patients with asthma are under way (www.clinicaltrials.gov/ct2/results?term=asthma).
It is important to note that the use of motivational interviewing does not require a lengthy visit. One study found that on average, visits in which primary care physicians used this communication technique lasted less than 10 minutes.46
CORRESPONDENCE Timothy H. Self, PharmD, University of Tennessee Health Science Center, 881 Madison Avenue, Room 235, Memphis, TN 38163; [email protected]
1. National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007.
2. Centers for Disease Control and Prevention. National Center for Health Statistics: National health interview survey raw data, 2008. Analysis performed by American Lung Association Research and Program Services.
3. American Lung Association. COPD—Helping the missing millions. February 24, 2010. Available at: http://www.lungusa.org/about-us/our-impact/top-stories/copd-helping-the-missing.html. Accessed November 9, 2011.
4. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1-16.
5. Self TH, Kilgore KE, Shelton V. MDIs, spacers, and dry powder inhalers: what patients are likely to do wrong. Consultant. 2003;49:702-705.
6. Saunders KB. Misuse of inhaled bronchodilator agents. Br Med J. 1965;1:1037-1038.
7. Epstein SW, Manning CPR, Ashley MJ, et al. Survey of the clinical uses of pressurized aerosol inhalers. Can Med Assoc J. 1979;120:813-816.
8. Shim C, Williams MH. The adequacy of inhalation of aerosol from canister nebulizers. Am J Med. 1980;69:891-894.
9. Self TH, Brooks JB, Lieberman P, et al. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Can Med Assoc J. 1983;128:129-131.
10. Hartert TV, Windom HH, Peeples RS, et al. Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am J Med. 1996;100:386-394.
11. Goodman DE, Israel E, Rosenberg M, et al. The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med. 1994;150:1256-1261.
12. Newman SP, Pavia D, Clarke SW. How should a pressurized beta-adrenergic bronchodilator be inhaled? Eur J Respir Dis. 1981;62:3-21.
13. Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br J Gen Pract. 1990;40:505-506.
14. Dompeling E, Van Grunsven PM, Van Schayck GP, et al. Treatment with inhaled steroids in asthma and chronic bronchitis: long-term compliance and inhaler technique. Fam Pract. 1992;9:161-166.
15. Verver S, Poelman M, Bogels A, et al. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam Pract. 1996;13:35-40.
16. Dickinson J, Hutton S, Atkin A, et al. Reducing asthma morbidity in the community: the effect of a targeted nurse-run asthma clinic in an English general practice. Respir Med. 1997;91:634-640.
17. Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care. 2001;19:255-260.
18. Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249-254.
19. Meulepas MA, Jacobs JE, Smeenk FW, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scand J Prim Health Care. 2007;25:186-192.
20. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246-251.
21. Souza ML, Meneghini AC, Ferraz E, et al. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35:824-831.
22. Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323-328.
23. Rachelefsky GS, Rohr AS, Wo J, et al. Use of a tube spacer to improve the efficacy of a metered dose inhaler in asthmatic children. Am J Dis Child. 1986;140:1191-1193.
24. Demirkan K, Tolley E, Mastin T, et al. Salmeterol administration by metered-dose inhaler alone vs metered-dose inhaler plus valved holding chamber. Chest. 2000;117:1314-1318.
25. Pedersen S, Ostergaard PA. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983;38:191-194.
26. Dolovich MD, Ahrens RS, Hess DR, et al. Device selection an outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335-371.
27. Interiano B, Guntupalli KK. Metered-dose inhalers: do health care providers know what to teach? Arch Intern Med. 1993;153:81-85.
28. Hanania NA, Wittman R, Kesten S, et al. Medical personnel’s knowledge of and ability to use inhaling devices. Metered-dose inhalers, spacing chambers, and breath-actuated dry powder inhalers. Chest. 1994;105:111-116.
29. Amirav I, Goren A, Pawlowski NA. What do pediatricians in training know about the correct use of inhalers and spacer devices? J Allergy Clin Immunol. 1994;94:669-675.
30. Chopra N, Oprescu N, Fask A, et al. Does introduction of new “easy to use” inhalational devices improve medical personnel’s knowledge of their proper use? Ann Allergy Asthma Immunol. 2002;88:395-400.
31. Self TH, Arnold LB, Czosnowski LM, et al. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44:593-598.
32. Lee-Wong M, Mayo PH. Results of a programme to improve house staff use of metered dose inhalers and spacers. Postgrad Med J. 2003;79:221-225.
33. Muchao FP, Pern SL, Rodriques JC, et al. Evaluation of the knowledge of health professionals at a pediatric hospital regarding the use of metered dose inhalers. J Bras Pneumol. 2008;34:4-12.
34. Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, Diskus, and Turbuhaler): changes after a single teaching session. J Asthma. 2009;46:944-950.
35. Kelcher S, Brownoff R. Teaching residents to use asthma devices. Assessing family residents’ skill and a brief intervention. Can Fam Physician. 1994;40:2090-2095.
36. Press VG, Pincayage AT, Pappalardo AA, et al. The Chicago Breathe Project: a regional approach to improving education on asthma inhalers for resident physicians and minority patients. J Natl Med Assoc. 2010;102:548-555.
37. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2010. Available at: www.ginasthma.org. Accessed November 9, 2011.
38. Executive Summary: global strategy on the diagnosis and management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report, 2009. Available at: www.goldcopd.com. Accessed November 9, 2011.
39. Self TH, Chrisman CR, Mason DL, et al. Reducing emergency department visits and hospitalizations in African American and Hispanic patients: a 15-year review. J Asthma. 2005;42:807-812.
40. Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy asthma care program (PACP) improves outcomes for patients in the community. Thorax. 2007;62:496-502.
41. DiLillo V, Nicole J, West DS. Incorporating motivational interviewing into behavioral obesity treatment. Cogn Behav Pract. 2003;10:120-130.
42. Borrelli B, Novak S, Hecht J, et al. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking). Prev Med. 2005;41:815-821.
43. Woollard L, Beilin L, Lord T, et al. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466-468.
44. Schmaling K, Blume A, Afari N. A randomized controlled pilot study of motivational interviewing to change attitudes about adherence to medications for asthma. J Clin Psych Med Settings. 2001;8:167-172.
45. Riekert KA, Borrelli B, Bilderback A, et al. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
46. Butler C, Rollnick S, Cohen D, et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49:611-616.
• Stress the importance of exhaling gently for a few seconds before inhaling (deeply and slowly for a metered dose inhaler, and deeply and rapidly for most dry powder inhalers). C
• Observe the inhaler technique of every patient receiving inhalation therapy on more than one occasion. C
• Don’t rely on self-reports regarding inhaler technique; despite claims of proficiency, most patients make at least one mistake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For patients with asthma or chronic obstructive pulmonary disease (COPD), inhalation therapy is the foundation of treatment. Yet all too often, patients don’t get the full value of their inhaled medications because they use their inhaler incorrectly. When technique is markedly flawed, suboptimal outcomes typically result.
Given the number of Americans with asthma (at least 22 million)1 and COPD (more than 13 million adults),2 faulty inhaler technique is a major public health problem. In fact, the number of people suffering from COPD may be even larger: Close to 24 million US adults are believed to have impaired lung function.3,4 For patients with asthma or COPD—many of whom are treated by family physicians—comprehensive education with a focus on correct use of an inhaler is essential.
In this review, we present evidence of frequent inhaler errors (from clinical studies) and highlight some of the more common mistakes (based on our clinical experience [TABLE]5). Finally, we offer ‘‘time-efficient’’ solutions to inhaler problems—steps that physicians in busy primary care practices can take to ensure that patients with asthma or COPD get the maximum benefit from inhalation therapy.
TABLE
Caution patients about these device-specific mistakes*
| Metered dose inhaler |
|---|
|
| Metered dose inhaler plus spacer/VHC |
|
| Dry powder inhaler |
|
| *These are examples based on the experience of the authors; other errors are possible. †Timing is not as crucial as it is for an MDI without a spacer, but the drug is still lost if inhalation is delayed. ‡Correct use varies by type of product (see product literature for specifics). DPI, dry powder inhaler; MDI, metered dose inhaler; VHC, valved holding chamber. Source: Adapted with permission from Self TH, et al. Consultant. 2003.5 |
Inhaler error is well documented
Since 1965, when it was first reported that many patients used metered dose inhalers (MDIs) incorrectly,6 evidence has accumulated supporting the magnitude of the problem.7-12 (Studies conducted in family practice settings are described in “Researchers look at inhaler problems in primary care” and in TABLE W1.13-20)
A number of studies of various sizes (from 41 to 3955 patients) have assessed inhaler technique in patients being treated by clinicians in primary care. The researchers used a variety of scoring methods, as well. Among them were a simple 4-step (0-4) rating system, a 9-step system, a standardized inhaler-specific checklist, and a system that tracked the number of omissions patients made.13-20 All found significant problems with inhaler technique. (You’ll find a detailed look at the studies in TABLE W1 at jfponline.com.)
In one study of 422 patients,13 including young children, adolescents, and adults, participants received one point for correctly performing each of the following steps:
- Adequate preparation (shaking well for those using a metered dose inhaler [MDI]; loading correctly for patients using a dry powder inhaler [DPI])
- Adequate expiration, correct head position
- Adequate inspiratory technique
- Holding breath afterwards.
The researchers found that 25% of the patients had inadequate technique (≤2 on a 0-4 point scale). In this study, as in others that included patients using various types of devices, use of an MDI was associated with a higher rate of incorrect technique.
Another much-smaller study14 used the same 4-step system to assess the technique of 50 patients, all of whom had the same type of DPI and had received extensive training in the correct use of the device. Despite the training, 27% of the patients received scores of ≤2 (inadequate technique). Sixty-eight percent received a score of 3 (adequate); only 5% received a score of 4 (good).
The 2 largest studies—one including 3955 patients using MDIs20 and the other looking at 3811 patients using various kinds of devices18—found high levels of errors, as well. In the latter study, 76% of patients with MDIs made at least one error vs 49% to 55% of patients using DPIs.18 The results convinced a large majority of the physicians caring for these patients of the need to check inhaler technique more frequently. In the study of MDI users alone, 71% of the patients made at least one mistake.20 inhaler misuse was associated with higher asthma instability scores, this study showed.
More recently, a researcher assessed the effects of an integrated primary care model on the management of asthma and/or COPD in middle-aged and elderly patients, in a study of 260 patients in 44 family practices.19 The study included an evaluation of inhaler technique.
Participants were divided into an intervention group—137 patients who received education regarding inhaler use from a nurse—and a usual care group (123 patients). After 2 years, correct inhaler technique among those in the intervention group went from 41% at baseline to 54%. At the same time, the proportion of those in the usual care group with correct technique fell from 47% to 29%.19
Error rates vary widely from one clinical trial to another, depending on study criteria, type of device, and extent of patient education, among other factors. Nonetheless, several studies (spanning 3 decades) found the error rate to be close to, or greater than, 90%.7,10,21
The most recent of these, published in 2009,21 was based on observation of the inhaler technique used by patients with asthma or COPD directly following appointments in an outpatient clinic. The authors found that, although >98% of the study participants claimed to know how to use their inhalers, 94% committed at least one error. In this study and a number of others, user error was more likely in patients using MDIs.13,18,21,22
Adding a spacer (eg, a valved holding chamber such as the AeroChamber) can be helpful, as the spacer affords the patient more time to inhale the medication. But patients who use an MDI with a spacer often make mistakes, too, and patient education is essential.23-26
Breath-activated dry powder inhalers (DPIs)—such as the Flexhaler, HandiHaler, Aerolizer, and Diskus—also reduce the likelihood of error. DPIs eliminate a step that MDI users often struggle with: the need to simultaneously press down on the canister and begin a slow, deep inhalation.
What’s more, DPIs do not have to be shaken before use. Nonetheless, using a DPI still involves a series of actions. For the HandiHaler and Aerolizer, patients must load the dose, and some patients fail to read the directions and swallow the capsule instead of loading it into the device. Patients must remember to exhale away from the device (ie, not into the dry powder) before inhaling, then hold their breath for approximately 10 seconds. There is potential for error at each step.
Stress the need to exhale before using the inhaler
Forgetting to exhale before inhaling is a common, and significant, mistake regardless of the type of device. It is paramount to stress the need to exhale gently for a few seconds before inhaling (slowly and deeply for patients using an MDI, rapidly and deeply with most DPIs). For MDI users, poor timing, described earlier, is another common and serious mistake. Patients using an MDI with a valved holding chamber sometimes inhale for too long before pressing down on the inhaler, then are unable to continue inhaling although the aerosol is still in the chamber. A common error made by patients using multidose DPIs is simply to forget to load the dose.
Physicians need to brush up on their skills, too
It’s not just patients who lack proficiency in inhaler technique. Numerous studies have demonstrated poor skill among physicians and other health care professionals.27-34 Evidence also shows that targeted education results in substantial improvement.32,35
In one study undertaken to evaluate family medicine residents’ proficiency in using asthma inhalers, participants (an intervention group at one clinic and a control group at another) all were given a pretest. The intervention group then received educational materials and a tutorial, as well as the opportunity for hands-on practice, after which both groups were given a post-test. The residents who received the training had a 170% jump, on average, in proficiency score, vs a 55% increase for the control group (P<.001).35
Inhaled Medication Instructional Videos
Courtesy of: National Jewish Health
Go to http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos
Another study—this one involving first-year interns—looked at level of improvement based on the type of education provided. Initially, only 5% of the interns could use an MDI without error. After a lecture and demonstration, 13% had an error-free technique. But when each intern participated in an intensive one-on-one session, the error-free rate reached 73%. The researchers’ conclusion: Lectures are relatively ineffective in teaching interns inhaler technique compared with a one-on-one approach.32
The Chicago Breathe Project,36 a new program aimed at improving education in the use of asthma inhalers for physicians and minority patients, provides further evidence of the value of clinician education. After a series of workshops for residents at 5 academic institutions, the physicians’ knowledge of proper use of inhalers rose dramatically—from just 5% preprogram to 91% postprogram (P<.001). Six months after the educational activity, the residents (n=161) were more likely (44% vs 11% preprogram) to assess patients’ inhaler technique.36
Teaching patients when time is tight
National and international guidelines stress the need to teach patients correct use of asthma and COPD inhalers.1,37,38 Providing the requisite education includes observation of each patient’s inhaler technique with proper use demonstrated, as needed.
The problem, of course, is how to provide that level of patient education within the time constraints of a busy family practice. We recommend these time-efficient solutions:
Enlist the help of other clinicians. While it is important that someone in your office be well trained and able to instruct patients in the proper use of inhalers, that individual need not be you. The National Institutes of Health recommends that the “principal clinician” introduce key educational messages, which can be reinforced and expanded on by other members of the health care team.1
After you advise patients that it is crucial for them to be trained in and adhere to proper inhaler technique, another health care professional—often a clinic nurse or pharmacist who has had special training—can provide the hands-on education. Studies have shown that when pharmacists who are competent in asthma management, including inhaler technique, work with physicians to optimize the education and overall management of patients with asthma, better outcomes often result, including a reduction in both emergency department visits and hospitalizations.1,39,40
Use videos to demonstrate correct technique. Videos are an effective teaching tool,9 and many of them are device-specific. National Jewish Health, which is world renowned for its asthma care, has a set of instructional videos posted on You-Tube and accessible from its Web site (http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos). In addition to videos that demonstrate the use of an MDI alone and an MDI plus a valved holding chamber, the site has links to 6 DPI videos, each covering a different device.
Use intermittent observation. After the patient views the appropriate video, you or a member of your staff will still need to observe the patient’s inhaler technique to ensure correct use. Ideally, this should occur at every visit.1,37 When that’s not possible, use intermittent observation, starting with the first 2 or 3 visits after the introduction of inhalation therapy and then switching to periodic observation to ensure that the patient is maintaining good technique.
In determining how often observation is necessary, keep in mind that simply asking patients whether they are having inhaler problems is not sufficient.1 Patients tend to say they have little or no trouble when, in fact, most struggle, at times, with the devices. What’s more, good technique tends to decrease over time, and repetitive education is important.
To motivate patients, try this communication technique
Motivational interviewing, a technique that has been used to help patients battle obesity, quit smoking, and control hypertension,41-43 among other health problems, can help you identify inhaler problems that need to be addressed. It involves the use of open-ended questions (eg, “What worries you most about your asthma?”), affirmations (“You’ve done a great job testing your peak flow level every morning”), reflective listening (“You’re tired of taking medicine every day”), and summary statements (“You know you should take your medicine every day but you’re having trouble remembering it. Is that right?”).
A pilot study44 showed that when this technique was incorporated into an asthma education session, patient motivation increased. The ratio of perceived advantages vs disadvantages of taking asthma medication correctly improved, as well. Another study45 found that when motivational interviewing was used during home visits to inner-city African American adolescents for asthma care, the patients’ motivation, readiness to adhere to treatment, and asthma-related quality of life improved, although self-reported adherence to asthma medication did not. Further studies involving patients with asthma are under way (www.clinicaltrials.gov/ct2/results?term=asthma).
It is important to note that the use of motivational interviewing does not require a lengthy visit. One study found that on average, visits in which primary care physicians used this communication technique lasted less than 10 minutes.46
CORRESPONDENCE Timothy H. Self, PharmD, University of Tennessee Health Science Center, 881 Madison Avenue, Room 235, Memphis, TN 38163; [email protected]
• Stress the importance of exhaling gently for a few seconds before inhaling (deeply and slowly for a metered dose inhaler, and deeply and rapidly for most dry powder inhalers). C
• Observe the inhaler technique of every patient receiving inhalation therapy on more than one occasion. C
• Don’t rely on self-reports regarding inhaler technique; despite claims of proficiency, most patients make at least one mistake. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For patients with asthma or chronic obstructive pulmonary disease (COPD), inhalation therapy is the foundation of treatment. Yet all too often, patients don’t get the full value of their inhaled medications because they use their inhaler incorrectly. When technique is markedly flawed, suboptimal outcomes typically result.
Given the number of Americans with asthma (at least 22 million)1 and COPD (more than 13 million adults),2 faulty inhaler technique is a major public health problem. In fact, the number of people suffering from COPD may be even larger: Close to 24 million US adults are believed to have impaired lung function.3,4 For patients with asthma or COPD—many of whom are treated by family physicians—comprehensive education with a focus on correct use of an inhaler is essential.
In this review, we present evidence of frequent inhaler errors (from clinical studies) and highlight some of the more common mistakes (based on our clinical experience [TABLE]5). Finally, we offer ‘‘time-efficient’’ solutions to inhaler problems—steps that physicians in busy primary care practices can take to ensure that patients with asthma or COPD get the maximum benefit from inhalation therapy.
TABLE
Caution patients about these device-specific mistakes*
| Metered dose inhaler |
|---|
|
| Metered dose inhaler plus spacer/VHC |
|
| Dry powder inhaler |
|
| *These are examples based on the experience of the authors; other errors are possible. †Timing is not as crucial as it is for an MDI without a spacer, but the drug is still lost if inhalation is delayed. ‡Correct use varies by type of product (see product literature for specifics). DPI, dry powder inhaler; MDI, metered dose inhaler; VHC, valved holding chamber. Source: Adapted with permission from Self TH, et al. Consultant. 2003.5 |
Inhaler error is well documented
Since 1965, when it was first reported that many patients used metered dose inhalers (MDIs) incorrectly,6 evidence has accumulated supporting the magnitude of the problem.7-12 (Studies conducted in family practice settings are described in “Researchers look at inhaler problems in primary care” and in TABLE W1.13-20)
A number of studies of various sizes (from 41 to 3955 patients) have assessed inhaler technique in patients being treated by clinicians in primary care. The researchers used a variety of scoring methods, as well. Among them were a simple 4-step (0-4) rating system, a 9-step system, a standardized inhaler-specific checklist, and a system that tracked the number of omissions patients made.13-20 All found significant problems with inhaler technique. (You’ll find a detailed look at the studies in TABLE W1 at jfponline.com.)
In one study of 422 patients,13 including young children, adolescents, and adults, participants received one point for correctly performing each of the following steps:
- Adequate preparation (shaking well for those using a metered dose inhaler [MDI]; loading correctly for patients using a dry powder inhaler [DPI])
- Adequate expiration, correct head position
- Adequate inspiratory technique
- Holding breath afterwards.
The researchers found that 25% of the patients had inadequate technique (≤2 on a 0-4 point scale). In this study, as in others that included patients using various types of devices, use of an MDI was associated with a higher rate of incorrect technique.
Another much-smaller study14 used the same 4-step system to assess the technique of 50 patients, all of whom had the same type of DPI and had received extensive training in the correct use of the device. Despite the training, 27% of the patients received scores of ≤2 (inadequate technique). Sixty-eight percent received a score of 3 (adequate); only 5% received a score of 4 (good).
The 2 largest studies—one including 3955 patients using MDIs20 and the other looking at 3811 patients using various kinds of devices18—found high levels of errors, as well. In the latter study, 76% of patients with MDIs made at least one error vs 49% to 55% of patients using DPIs.18 The results convinced a large majority of the physicians caring for these patients of the need to check inhaler technique more frequently. In the study of MDI users alone, 71% of the patients made at least one mistake.20 inhaler misuse was associated with higher asthma instability scores, this study showed.
More recently, a researcher assessed the effects of an integrated primary care model on the management of asthma and/or COPD in middle-aged and elderly patients, in a study of 260 patients in 44 family practices.19 The study included an evaluation of inhaler technique.
Participants were divided into an intervention group—137 patients who received education regarding inhaler use from a nurse—and a usual care group (123 patients). After 2 years, correct inhaler technique among those in the intervention group went from 41% at baseline to 54%. At the same time, the proportion of those in the usual care group with correct technique fell from 47% to 29%.19
Error rates vary widely from one clinical trial to another, depending on study criteria, type of device, and extent of patient education, among other factors. Nonetheless, several studies (spanning 3 decades) found the error rate to be close to, or greater than, 90%.7,10,21
The most recent of these, published in 2009,21 was based on observation of the inhaler technique used by patients with asthma or COPD directly following appointments in an outpatient clinic. The authors found that, although >98% of the study participants claimed to know how to use their inhalers, 94% committed at least one error. In this study and a number of others, user error was more likely in patients using MDIs.13,18,21,22
Adding a spacer (eg, a valved holding chamber such as the AeroChamber) can be helpful, as the spacer affords the patient more time to inhale the medication. But patients who use an MDI with a spacer often make mistakes, too, and patient education is essential.23-26
Breath-activated dry powder inhalers (DPIs)—such as the Flexhaler, HandiHaler, Aerolizer, and Diskus—also reduce the likelihood of error. DPIs eliminate a step that MDI users often struggle with: the need to simultaneously press down on the canister and begin a slow, deep inhalation.
What’s more, DPIs do not have to be shaken before use. Nonetheless, using a DPI still involves a series of actions. For the HandiHaler and Aerolizer, patients must load the dose, and some patients fail to read the directions and swallow the capsule instead of loading it into the device. Patients must remember to exhale away from the device (ie, not into the dry powder) before inhaling, then hold their breath for approximately 10 seconds. There is potential for error at each step.
Stress the need to exhale before using the inhaler
Forgetting to exhale before inhaling is a common, and significant, mistake regardless of the type of device. It is paramount to stress the need to exhale gently for a few seconds before inhaling (slowly and deeply for patients using an MDI, rapidly and deeply with most DPIs). For MDI users, poor timing, described earlier, is another common and serious mistake. Patients using an MDI with a valved holding chamber sometimes inhale for too long before pressing down on the inhaler, then are unable to continue inhaling although the aerosol is still in the chamber. A common error made by patients using multidose DPIs is simply to forget to load the dose.
Physicians need to brush up on their skills, too
It’s not just patients who lack proficiency in inhaler technique. Numerous studies have demonstrated poor skill among physicians and other health care professionals.27-34 Evidence also shows that targeted education results in substantial improvement.32,35
In one study undertaken to evaluate family medicine residents’ proficiency in using asthma inhalers, participants (an intervention group at one clinic and a control group at another) all were given a pretest. The intervention group then received educational materials and a tutorial, as well as the opportunity for hands-on practice, after which both groups were given a post-test. The residents who received the training had a 170% jump, on average, in proficiency score, vs a 55% increase for the control group (P<.001).35
Inhaled Medication Instructional Videos
Courtesy of: National Jewish Health
Go to http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos
Another study—this one involving first-year interns—looked at level of improvement based on the type of education provided. Initially, only 5% of the interns could use an MDI without error. After a lecture and demonstration, 13% had an error-free technique. But when each intern participated in an intensive one-on-one session, the error-free rate reached 73%. The researchers’ conclusion: Lectures are relatively ineffective in teaching interns inhaler technique compared with a one-on-one approach.32
The Chicago Breathe Project,36 a new program aimed at improving education in the use of asthma inhalers for physicians and minority patients, provides further evidence of the value of clinician education. After a series of workshops for residents at 5 academic institutions, the physicians’ knowledge of proper use of inhalers rose dramatically—from just 5% preprogram to 91% postprogram (P<.001). Six months after the educational activity, the residents (n=161) were more likely (44% vs 11% preprogram) to assess patients’ inhaler technique.36
Teaching patients when time is tight
National and international guidelines stress the need to teach patients correct use of asthma and COPD inhalers.1,37,38 Providing the requisite education includes observation of each patient’s inhaler technique with proper use demonstrated, as needed.
The problem, of course, is how to provide that level of patient education within the time constraints of a busy family practice. We recommend these time-efficient solutions:
Enlist the help of other clinicians. While it is important that someone in your office be well trained and able to instruct patients in the proper use of inhalers, that individual need not be you. The National Institutes of Health recommends that the “principal clinician” introduce key educational messages, which can be reinforced and expanded on by other members of the health care team.1
After you advise patients that it is crucial for them to be trained in and adhere to proper inhaler technique, another health care professional—often a clinic nurse or pharmacist who has had special training—can provide the hands-on education. Studies have shown that when pharmacists who are competent in asthma management, including inhaler technique, work with physicians to optimize the education and overall management of patients with asthma, better outcomes often result, including a reduction in both emergency department visits and hospitalizations.1,39,40
Use videos to demonstrate correct technique. Videos are an effective teaching tool,9 and many of them are device-specific. National Jewish Health, which is world renowned for its asthma care, has a set of instructional videos posted on You-Tube and accessible from its Web site (http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos). In addition to videos that demonstrate the use of an MDI alone and an MDI plus a valved holding chamber, the site has links to 6 DPI videos, each covering a different device.
Use intermittent observation. After the patient views the appropriate video, you or a member of your staff will still need to observe the patient’s inhaler technique to ensure correct use. Ideally, this should occur at every visit.1,37 When that’s not possible, use intermittent observation, starting with the first 2 or 3 visits after the introduction of inhalation therapy and then switching to periodic observation to ensure that the patient is maintaining good technique.
In determining how often observation is necessary, keep in mind that simply asking patients whether they are having inhaler problems is not sufficient.1 Patients tend to say they have little or no trouble when, in fact, most struggle, at times, with the devices. What’s more, good technique tends to decrease over time, and repetitive education is important.
To motivate patients, try this communication technique
Motivational interviewing, a technique that has been used to help patients battle obesity, quit smoking, and control hypertension,41-43 among other health problems, can help you identify inhaler problems that need to be addressed. It involves the use of open-ended questions (eg, “What worries you most about your asthma?”), affirmations (“You’ve done a great job testing your peak flow level every morning”), reflective listening (“You’re tired of taking medicine every day”), and summary statements (“You know you should take your medicine every day but you’re having trouble remembering it. Is that right?”).
A pilot study44 showed that when this technique was incorporated into an asthma education session, patient motivation increased. The ratio of perceived advantages vs disadvantages of taking asthma medication correctly improved, as well. Another study45 found that when motivational interviewing was used during home visits to inner-city African American adolescents for asthma care, the patients’ motivation, readiness to adhere to treatment, and asthma-related quality of life improved, although self-reported adherence to asthma medication did not. Further studies involving patients with asthma are under way (www.clinicaltrials.gov/ct2/results?term=asthma).
It is important to note that the use of motivational interviewing does not require a lengthy visit. One study found that on average, visits in which primary care physicians used this communication technique lasted less than 10 minutes.46
CORRESPONDENCE Timothy H. Self, PharmD, University of Tennessee Health Science Center, 881 Madison Avenue, Room 235, Memphis, TN 38163; [email protected]
1. National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007.
2. Centers for Disease Control and Prevention. National Center for Health Statistics: National health interview survey raw data, 2008. Analysis performed by American Lung Association Research and Program Services.
3. American Lung Association. COPD—Helping the missing millions. February 24, 2010. Available at: http://www.lungusa.org/about-us/our-impact/top-stories/copd-helping-the-missing.html. Accessed November 9, 2011.
4. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1-16.
5. Self TH, Kilgore KE, Shelton V. MDIs, spacers, and dry powder inhalers: what patients are likely to do wrong. Consultant. 2003;49:702-705.
6. Saunders KB. Misuse of inhaled bronchodilator agents. Br Med J. 1965;1:1037-1038.
7. Epstein SW, Manning CPR, Ashley MJ, et al. Survey of the clinical uses of pressurized aerosol inhalers. Can Med Assoc J. 1979;120:813-816.
8. Shim C, Williams MH. The adequacy of inhalation of aerosol from canister nebulizers. Am J Med. 1980;69:891-894.
9. Self TH, Brooks JB, Lieberman P, et al. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Can Med Assoc J. 1983;128:129-131.
10. Hartert TV, Windom HH, Peeples RS, et al. Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am J Med. 1996;100:386-394.
11. Goodman DE, Israel E, Rosenberg M, et al. The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med. 1994;150:1256-1261.
12. Newman SP, Pavia D, Clarke SW. How should a pressurized beta-adrenergic bronchodilator be inhaled? Eur J Respir Dis. 1981;62:3-21.
13. Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br J Gen Pract. 1990;40:505-506.
14. Dompeling E, Van Grunsven PM, Van Schayck GP, et al. Treatment with inhaled steroids in asthma and chronic bronchitis: long-term compliance and inhaler technique. Fam Pract. 1992;9:161-166.
15. Verver S, Poelman M, Bogels A, et al. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam Pract. 1996;13:35-40.
16. Dickinson J, Hutton S, Atkin A, et al. Reducing asthma morbidity in the community: the effect of a targeted nurse-run asthma clinic in an English general practice. Respir Med. 1997;91:634-640.
17. Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care. 2001;19:255-260.
18. Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249-254.
19. Meulepas MA, Jacobs JE, Smeenk FW, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scand J Prim Health Care. 2007;25:186-192.
20. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246-251.
21. Souza ML, Meneghini AC, Ferraz E, et al. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35:824-831.
22. Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323-328.
23. Rachelefsky GS, Rohr AS, Wo J, et al. Use of a tube spacer to improve the efficacy of a metered dose inhaler in asthmatic children. Am J Dis Child. 1986;140:1191-1193.
24. Demirkan K, Tolley E, Mastin T, et al. Salmeterol administration by metered-dose inhaler alone vs metered-dose inhaler plus valved holding chamber. Chest. 2000;117:1314-1318.
25. Pedersen S, Ostergaard PA. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983;38:191-194.
26. Dolovich MD, Ahrens RS, Hess DR, et al. Device selection an outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335-371.
27. Interiano B, Guntupalli KK. Metered-dose inhalers: do health care providers know what to teach? Arch Intern Med. 1993;153:81-85.
28. Hanania NA, Wittman R, Kesten S, et al. Medical personnel’s knowledge of and ability to use inhaling devices. Metered-dose inhalers, spacing chambers, and breath-actuated dry powder inhalers. Chest. 1994;105:111-116.
29. Amirav I, Goren A, Pawlowski NA. What do pediatricians in training know about the correct use of inhalers and spacer devices? J Allergy Clin Immunol. 1994;94:669-675.
30. Chopra N, Oprescu N, Fask A, et al. Does introduction of new “easy to use” inhalational devices improve medical personnel’s knowledge of their proper use? Ann Allergy Asthma Immunol. 2002;88:395-400.
31. Self TH, Arnold LB, Czosnowski LM, et al. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44:593-598.
32. Lee-Wong M, Mayo PH. Results of a programme to improve house staff use of metered dose inhalers and spacers. Postgrad Med J. 2003;79:221-225.
33. Muchao FP, Pern SL, Rodriques JC, et al. Evaluation of the knowledge of health professionals at a pediatric hospital regarding the use of metered dose inhalers. J Bras Pneumol. 2008;34:4-12.
34. Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, Diskus, and Turbuhaler): changes after a single teaching session. J Asthma. 2009;46:944-950.
35. Kelcher S, Brownoff R. Teaching residents to use asthma devices. Assessing family residents’ skill and a brief intervention. Can Fam Physician. 1994;40:2090-2095.
36. Press VG, Pincayage AT, Pappalardo AA, et al. The Chicago Breathe Project: a regional approach to improving education on asthma inhalers for resident physicians and minority patients. J Natl Med Assoc. 2010;102:548-555.
37. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2010. Available at: www.ginasthma.org. Accessed November 9, 2011.
38. Executive Summary: global strategy on the diagnosis and management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report, 2009. Available at: www.goldcopd.com. Accessed November 9, 2011.
39. Self TH, Chrisman CR, Mason DL, et al. Reducing emergency department visits and hospitalizations in African American and Hispanic patients: a 15-year review. J Asthma. 2005;42:807-812.
40. Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy asthma care program (PACP) improves outcomes for patients in the community. Thorax. 2007;62:496-502.
41. DiLillo V, Nicole J, West DS. Incorporating motivational interviewing into behavioral obesity treatment. Cogn Behav Pract. 2003;10:120-130.
42. Borrelli B, Novak S, Hecht J, et al. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking). Prev Med. 2005;41:815-821.
43. Woollard L, Beilin L, Lord T, et al. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466-468.
44. Schmaling K, Blume A, Afari N. A randomized controlled pilot study of motivational interviewing to change attitudes about adherence to medications for asthma. J Clin Psych Med Settings. 2001;8:167-172.
45. Riekert KA, Borrelli B, Bilderback A, et al. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
46. Butler C, Rollnick S, Cohen D, et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49:611-616.
1. National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; 2007.
2. Centers for Disease Control and Prevention. National Center for Health Statistics: National health interview survey raw data, 2008. Analysis performed by American Lung Association Research and Program Services.
3. American Lung Association. COPD—Helping the missing millions. February 24, 2010. Available at: http://www.lungusa.org/about-us/our-impact/top-stories/copd-helping-the-missing.html. Accessed November 9, 2011.
4. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease surveillance—United States, 1971-2000. MMWR Surveill Summ. 2002;51(6):1-16.
5. Self TH, Kilgore KE, Shelton V. MDIs, spacers, and dry powder inhalers: what patients are likely to do wrong. Consultant. 2003;49:702-705.
6. Saunders KB. Misuse of inhaled bronchodilator agents. Br Med J. 1965;1:1037-1038.
7. Epstein SW, Manning CPR, Ashley MJ, et al. Survey of the clinical uses of pressurized aerosol inhalers. Can Med Assoc J. 1979;120:813-816.
8. Shim C, Williams MH. The adequacy of inhalation of aerosol from canister nebulizers. Am J Med. 1980;69:891-894.
9. Self TH, Brooks JB, Lieberman P, et al. The value of demonstration and role of the pharmacist in teaching the correct use of pressurized bronchodilators. Can Med Assoc J. 1983;128:129-131.
10. Hartert TV, Windom HH, Peeples RS, et al. Inadequate outpatient medical therapy for patients with asthma admitted to two urban hospitals. Am J Med. 1996;100:386-394.
11. Goodman DE, Israel E, Rosenberg M, et al. The influence of age, diagnosis, and gender on proper use of metered-dose inhalers. Am J Respir Crit Care Med. 1994;150:1256-1261.
12. Newman SP, Pavia D, Clarke SW. How should a pressurized beta-adrenergic bronchodilator be inhaled? Eur J Respir Dis. 1981;62:3-21.
13. Hilton S. An audit of inhaler technique among asthma patients of 34 general practitioners. Br J Gen Pract. 1990;40:505-506.
14. Dompeling E, Van Grunsven PM, Van Schayck GP, et al. Treatment with inhaled steroids in asthma and chronic bronchitis: long-term compliance and inhaler technique. Fam Pract. 1992;9:161-166.
15. Verver S, Poelman M, Bogels A, et al. Effects of instruction by practice assistants on inhaler technique and respiratory symptoms of patients. A controlled randomized videotaped intervention study. Fam Pract. 1996;13:35-40.
16. Dickinson J, Hutton S, Atkin A, et al. Reducing asthma morbidity in the community: the effect of a targeted nurse-run asthma clinic in an English general practice. Respir Med. 1997;91:634-640.
17. Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care. 2001;19:255-260.
18. Molimard M, Raherison C, Lignot S, et al. Assessment of handling of inhaler devices in real life: An observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249-254.
19. Meulepas MA, Jacobs JE, Smeenk FW, et al. Effect of an integrated primary care model on the management of middle-aged and old patients with obstructive lung diseases. Scand J Prim Health Care. 2007;25:186-192.
20. Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246-251.
21. Souza ML, Meneghini AC, Ferraz E, et al. Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol. 2009;35:824-831.
22. Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323-328.
23. Rachelefsky GS, Rohr AS, Wo J, et al. Use of a tube spacer to improve the efficacy of a metered dose inhaler in asthmatic children. Am J Dis Child. 1986;140:1191-1193.
24. Demirkan K, Tolley E, Mastin T, et al. Salmeterol administration by metered-dose inhaler alone vs metered-dose inhaler plus valved holding chamber. Chest. 2000;117:1314-1318.
25. Pedersen S, Ostergaard PA. Nasal inhalation as a cause of inefficient pulmonal aerosol inhalation technique in children. Allergy. 1983;38:191-194.
26. Dolovich MD, Ahrens RS, Hess DR, et al. Device selection an outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335-371.
27. Interiano B, Guntupalli KK. Metered-dose inhalers: do health care providers know what to teach? Arch Intern Med. 1993;153:81-85.
28. Hanania NA, Wittman R, Kesten S, et al. Medical personnel’s knowledge of and ability to use inhaling devices. Metered-dose inhalers, spacing chambers, and breath-actuated dry powder inhalers. Chest. 1994;105:111-116.
29. Amirav I, Goren A, Pawlowski NA. What do pediatricians in training know about the correct use of inhalers and spacer devices? J Allergy Clin Immunol. 1994;94:669-675.
30. Chopra N, Oprescu N, Fask A, et al. Does introduction of new “easy to use” inhalational devices improve medical personnel’s knowledge of their proper use? Ann Allergy Asthma Immunol. 2002;88:395-400.
31. Self TH, Arnold LB, Czosnowski LM, et al. Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma. 2007;44:593-598.
32. Lee-Wong M, Mayo PH. Results of a programme to improve house staff use of metered dose inhalers and spacers. Postgrad Med J. 2003;79:221-225.
33. Muchao FP, Pern SL, Rodriques JC, et al. Evaluation of the knowledge of health professionals at a pediatric hospital regarding the use of metered dose inhalers. J Bras Pneumol. 2008;34:4-12.
34. Kim SH, Kwak HJ, Kim TB, et al. Inappropriate techniques used by internal medicine residents with three kinds of inhalers (a metered dose inhaler, Diskus, and Turbuhaler): changes after a single teaching session. J Asthma. 2009;46:944-950.
35. Kelcher S, Brownoff R. Teaching residents to use asthma devices. Assessing family residents’ skill and a brief intervention. Can Fam Physician. 1994;40:2090-2095.
36. Press VG, Pincayage AT, Pappalardo AA, et al. The Chicago Breathe Project: a regional approach to improving education on asthma inhalers for resident physicians and minority patients. J Natl Med Assoc. 2010;102:548-555.
37. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2010. Available at: www.ginasthma.org. Accessed November 9, 2011.
38. Executive Summary: global strategy on the diagnosis and management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Workshop Report, 2009. Available at: www.goldcopd.com. Accessed November 9, 2011.
39. Self TH, Chrisman CR, Mason DL, et al. Reducing emergency department visits and hospitalizations in African American and Hispanic patients: a 15-year review. J Asthma. 2005;42:807-812.
40. Armour C, Bosnic-Anticevich S, Brillant M, et al. Pharmacy asthma care program (PACP) improves outcomes for patients in the community. Thorax. 2007;62:496-502.
41. DiLillo V, Nicole J, West DS. Incorporating motivational interviewing into behavioral obesity treatment. Cogn Behav Pract. 2003;10:120-130.
42. Borrelli B, Novak S, Hecht J, et al. Home health care nurses as a new channel for smoking cessation treatment: outcomes from project CARES (Community-nurse Assisted Research and Education on Smoking). Prev Med. 2005;41:815-821.
43. Woollard L, Beilin L, Lord T, et al. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clin Exp Pharmacol Physiol. 1995;22:466-468.
44. Schmaling K, Blume A, Afari N. A randomized controlled pilot study of motivational interviewing to change attitudes about adherence to medications for asthma. J Clin Psych Med Settings. 2001;8:167-172.
45. Riekert KA, Borrelli B, Bilderback A, et al. The development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Educ Couns. 2011;82:117-122.
46. Butler C, Rollnick S, Cohen D, et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract. 1999;49:611-616.
Patient unaware of abnormal scans until it was too late ... For want of steroids, sight is lost ... more
Patient unaware of abnormal scans until it was too late
A COMPUTED TOMOGRAPHY (CT) SCAN of a patient’s chest ordered by his physician revealed a cancerous nodule on the right lung. The physician’s office received the report but didn’t notify the patient of the finding. Nor was the patient informed of the CT report during a visit to the physician 2 months later, or during several visits the following year.
A second CT scan a year after the first showed a larger cancerous area in the lung. The patient and his wife went to the physician several days after the scan to discuss the results. While reviewing the patient’s chart, the doctor asked how long the man had been his patient and said, “We should have been on this a year ago.” He then left the office, and the building, without speaking further to the patient or his wife or explaining his departure. The patient tried unsuccessfully to get a copy of his medical records from the practice.
Two months later, the patient went to the emergency department (ED) with abdominal pain, shortness of breath, and dizziness. He was diagnosed with stage 4 lung cancer. The patient died about 7 weeks later.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1 million South Carolina settlement.
COMMENT Fail-safes to assure the appropriate communication of abnormal test results are essential. I was pleased when my personal physician called recently concerning an abnormal lab test; too often timely communication doesn’t occur.
A cystic mass, then breast cancer
AFTER 6 MONTHS OF BREAST PAIN that became worse during menses, a 36-year-old woman, who had recently come to the United States from Iraq, consulted her family physician. The physician had been recommended because she was female, as the patient had requested, and, like the patient, was Iraqi.
The physician palpated the right breast and documented cystic fullness with no discrete masses or axillary nodes. She ordered a screening mammogram but was told by a radiologist that a 36-year-old woman could have screening mammography only if a mass was present. The physician changed the order to a diagnostic mammogram for a painful cystic mass. At the time of the mammogram, the patient told the technician that the lump came and went with her menstrual period. The results were reported as normal.
The physician continued to see the patient over the next 3 years for various health issues. At the patient’s final visit, the physician performed a clinical breast exam, which she documented as negative. The patient claimed that the physician hadn’t done any follow-up related to the right breast between her first visit and the final breast exam 3 years later.
Two years afterward, the now 41-year-old patient was diagnosed with cancer in her right breast after a mammogram, ultrasound, and biopsy. According to records at the hospital where she received the diagnosis, she’d discovered the lump 3 months earlier. The patient underwent a right mastectomy with chemotherapy and radiation and was cancer-free at the time of the trial.
PLAINTIFF’S CLAIM An ultrasound and biopsy should have been performed when the patient first consulted the family physician. The family physician didn’t perform any follow-up on the right breast until 3 years after she diagnosed the cystic fullness.
THE DEFENSE The family physician claimed that she tried twice to perform breast examinations during office visits in the 3 years she saw the patient, but the patient refused. The claim wasn’t documented. The patient’s cancer didn’t become palpable until after she left the doctor’s care. She had a fast-growing tumor, and the location of the cancerous mass differed from the area of cystic fullness the doctor originally discovered.
VERDICT $500,000 Illinois verdict.
COMMENT Failure to diagnose breast cancer continues to be a frequent and vexing allegation. Better documentation and follow-up could help obviate many of these claims.
For want of steroids, sight is lost
A 78-YEAR-OLD MAN was diagnosed with polymyalgia rheumatica (painful inflammation of the arteries, usually in the shoulders and hips) by his longtime primary care physician. The doctor treated the condition with low-dose steroids and monitored the patient’s erythrocyte sedimentation rate and C-reactive protein.
Two years after diagnosis, the patient complained to the physician of jaw pain and transient vision loss in the left eye. Three days later, he called the doctor to say that he had developed a headache. The physician lowered the steroid dosage but didn’t order blood tests or a biopsy. The following day the patient woke up and discovered he’d gone blind.
PLAINTIFF’S CLAIM The patient had giant cell arteritis and should have been treated with high-dose steroids. Starting treatment even one day earlier would have prevented blindness.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Washington settlement.
COMMENT Timely diagnosis and appropriate treatment of temporal arteritis remain essential.
Sudden chest pain, sudden death, but not the usual suspects
SUDDEN ONSET OF CHEST PAIN brought a 41-year-old woman to the ED. Results of an electrocardiogram, chest radiograph, and lab tests were all normal. While in the ED, the patient developed diarrhea and was diagnosed with a gastrointestinal bleed.
She was admitted to the hospital, but no bed was available, so she remained in the ED, where she was found dead 7 hours later. Autopsy revealed a type A dissecting aorta to the level of the renal arteries.
PLAINTIFF’S CLAIM The ED physician failed to rule out all potential life-threatening causes of the chest pain and didn’t order a CT scan, which would have showed the aortic dissection.
DOCTOR’S DEFENSE Aortic dissection is a rare condition; the patient didn’t fit the profile of an individual at risk. A chest radiograph almost always reveals such abnormalities; no duty existed to rule out aortic dissection.
VERDICT $1.4 million Ohio verdict.
COMMENT Even though the details of this case are sketchy—and any death is a tragedy—I can’t help but sympathize with the defendant. While as physicians we should not chase zebras, we still have to consider the possibility of rare conditions.
Misdiagnosed cold foot leads to amputation
NUMBNESS IN HER RIGHT FOOT prompted 2 visits to the emergency department by a woman in her early 40s. The foot was cold and discolored. By the second visit, the patient was screaming with pain. A sprain was diagnosed without consulting a vascular surgeon, and the patient was sent home.
Ten days later, the patient had a computed tomography scan at another hospital, which found a blockage of the popliteal artery. Her right leg was amputated below the knee the following day and she was fitted with a prosthesis.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1.25 million New Jersey settlement.
COMMENT I have seen a rash of cases in which peripheral vascular disease was inappropriately diagnosed. One wonders how an alert clinician could miss vascular disease and diagnose a sprain when faced with pain and a cold discolored foot.
Patient unaware of abnormal scans until it was too late
A COMPUTED TOMOGRAPHY (CT) SCAN of a patient’s chest ordered by his physician revealed a cancerous nodule on the right lung. The physician’s office received the report but didn’t notify the patient of the finding. Nor was the patient informed of the CT report during a visit to the physician 2 months later, or during several visits the following year.
A second CT scan a year after the first showed a larger cancerous area in the lung. The patient and his wife went to the physician several days after the scan to discuss the results. While reviewing the patient’s chart, the doctor asked how long the man had been his patient and said, “We should have been on this a year ago.” He then left the office, and the building, without speaking further to the patient or his wife or explaining his departure. The patient tried unsuccessfully to get a copy of his medical records from the practice.
Two months later, the patient went to the emergency department (ED) with abdominal pain, shortness of breath, and dizziness. He was diagnosed with stage 4 lung cancer. The patient died about 7 weeks later.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1 million South Carolina settlement.
COMMENT Fail-safes to assure the appropriate communication of abnormal test results are essential. I was pleased when my personal physician called recently concerning an abnormal lab test; too often timely communication doesn’t occur.
A cystic mass, then breast cancer
AFTER 6 MONTHS OF BREAST PAIN that became worse during menses, a 36-year-old woman, who had recently come to the United States from Iraq, consulted her family physician. The physician had been recommended because she was female, as the patient had requested, and, like the patient, was Iraqi.
The physician palpated the right breast and documented cystic fullness with no discrete masses or axillary nodes. She ordered a screening mammogram but was told by a radiologist that a 36-year-old woman could have screening mammography only if a mass was present. The physician changed the order to a diagnostic mammogram for a painful cystic mass. At the time of the mammogram, the patient told the technician that the lump came and went with her menstrual period. The results were reported as normal.
The physician continued to see the patient over the next 3 years for various health issues. At the patient’s final visit, the physician performed a clinical breast exam, which she documented as negative. The patient claimed that the physician hadn’t done any follow-up related to the right breast between her first visit and the final breast exam 3 years later.
Two years afterward, the now 41-year-old patient was diagnosed with cancer in her right breast after a mammogram, ultrasound, and biopsy. According to records at the hospital where she received the diagnosis, she’d discovered the lump 3 months earlier. The patient underwent a right mastectomy with chemotherapy and radiation and was cancer-free at the time of the trial.
PLAINTIFF’S CLAIM An ultrasound and biopsy should have been performed when the patient first consulted the family physician. The family physician didn’t perform any follow-up on the right breast until 3 years after she diagnosed the cystic fullness.
THE DEFENSE The family physician claimed that she tried twice to perform breast examinations during office visits in the 3 years she saw the patient, but the patient refused. The claim wasn’t documented. The patient’s cancer didn’t become palpable until after she left the doctor’s care. She had a fast-growing tumor, and the location of the cancerous mass differed from the area of cystic fullness the doctor originally discovered.
VERDICT $500,000 Illinois verdict.
COMMENT Failure to diagnose breast cancer continues to be a frequent and vexing allegation. Better documentation and follow-up could help obviate many of these claims.
For want of steroids, sight is lost
A 78-YEAR-OLD MAN was diagnosed with polymyalgia rheumatica (painful inflammation of the arteries, usually in the shoulders and hips) by his longtime primary care physician. The doctor treated the condition with low-dose steroids and monitored the patient’s erythrocyte sedimentation rate and C-reactive protein.
Two years after diagnosis, the patient complained to the physician of jaw pain and transient vision loss in the left eye. Three days later, he called the doctor to say that he had developed a headache. The physician lowered the steroid dosage but didn’t order blood tests or a biopsy. The following day the patient woke up and discovered he’d gone blind.
PLAINTIFF’S CLAIM The patient had giant cell arteritis and should have been treated with high-dose steroids. Starting treatment even one day earlier would have prevented blindness.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Washington settlement.
COMMENT Timely diagnosis and appropriate treatment of temporal arteritis remain essential.
Sudden chest pain, sudden death, but not the usual suspects
SUDDEN ONSET OF CHEST PAIN brought a 41-year-old woman to the ED. Results of an electrocardiogram, chest radiograph, and lab tests were all normal. While in the ED, the patient developed diarrhea and was diagnosed with a gastrointestinal bleed.
She was admitted to the hospital, but no bed was available, so she remained in the ED, where she was found dead 7 hours later. Autopsy revealed a type A dissecting aorta to the level of the renal arteries.
PLAINTIFF’S CLAIM The ED physician failed to rule out all potential life-threatening causes of the chest pain and didn’t order a CT scan, which would have showed the aortic dissection.
DOCTOR’S DEFENSE Aortic dissection is a rare condition; the patient didn’t fit the profile of an individual at risk. A chest radiograph almost always reveals such abnormalities; no duty existed to rule out aortic dissection.
VERDICT $1.4 million Ohio verdict.
COMMENT Even though the details of this case are sketchy—and any death is a tragedy—I can’t help but sympathize with the defendant. While as physicians we should not chase zebras, we still have to consider the possibility of rare conditions.
Misdiagnosed cold foot leads to amputation
NUMBNESS IN HER RIGHT FOOT prompted 2 visits to the emergency department by a woman in her early 40s. The foot was cold and discolored. By the second visit, the patient was screaming with pain. A sprain was diagnosed without consulting a vascular surgeon, and the patient was sent home.
Ten days later, the patient had a computed tomography scan at another hospital, which found a blockage of the popliteal artery. Her right leg was amputated below the knee the following day and she was fitted with a prosthesis.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1.25 million New Jersey settlement.
COMMENT I have seen a rash of cases in which peripheral vascular disease was inappropriately diagnosed. One wonders how an alert clinician could miss vascular disease and diagnose a sprain when faced with pain and a cold discolored foot.
Patient unaware of abnormal scans until it was too late
A COMPUTED TOMOGRAPHY (CT) SCAN of a patient’s chest ordered by his physician revealed a cancerous nodule on the right lung. The physician’s office received the report but didn’t notify the patient of the finding. Nor was the patient informed of the CT report during a visit to the physician 2 months later, or during several visits the following year.
A second CT scan a year after the first showed a larger cancerous area in the lung. The patient and his wife went to the physician several days after the scan to discuss the results. While reviewing the patient’s chart, the doctor asked how long the man had been his patient and said, “We should have been on this a year ago.” He then left the office, and the building, without speaking further to the patient or his wife or explaining his departure. The patient tried unsuccessfully to get a copy of his medical records from the practice.
Two months later, the patient went to the emergency department (ED) with abdominal pain, shortness of breath, and dizziness. He was diagnosed with stage 4 lung cancer. The patient died about 7 weeks later.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1 million South Carolina settlement.
COMMENT Fail-safes to assure the appropriate communication of abnormal test results are essential. I was pleased when my personal physician called recently concerning an abnormal lab test; too often timely communication doesn’t occur.
A cystic mass, then breast cancer
AFTER 6 MONTHS OF BREAST PAIN that became worse during menses, a 36-year-old woman, who had recently come to the United States from Iraq, consulted her family physician. The physician had been recommended because she was female, as the patient had requested, and, like the patient, was Iraqi.
The physician palpated the right breast and documented cystic fullness with no discrete masses or axillary nodes. She ordered a screening mammogram but was told by a radiologist that a 36-year-old woman could have screening mammography only if a mass was present. The physician changed the order to a diagnostic mammogram for a painful cystic mass. At the time of the mammogram, the patient told the technician that the lump came and went with her menstrual period. The results were reported as normal.
The physician continued to see the patient over the next 3 years for various health issues. At the patient’s final visit, the physician performed a clinical breast exam, which she documented as negative. The patient claimed that the physician hadn’t done any follow-up related to the right breast between her first visit and the final breast exam 3 years later.
Two years afterward, the now 41-year-old patient was diagnosed with cancer in her right breast after a mammogram, ultrasound, and biopsy. According to records at the hospital where she received the diagnosis, she’d discovered the lump 3 months earlier. The patient underwent a right mastectomy with chemotherapy and radiation and was cancer-free at the time of the trial.
PLAINTIFF’S CLAIM An ultrasound and biopsy should have been performed when the patient first consulted the family physician. The family physician didn’t perform any follow-up on the right breast until 3 years after she diagnosed the cystic fullness.
THE DEFENSE The family physician claimed that she tried twice to perform breast examinations during office visits in the 3 years she saw the patient, but the patient refused. The claim wasn’t documented. The patient’s cancer didn’t become palpable until after she left the doctor’s care. She had a fast-growing tumor, and the location of the cancerous mass differed from the area of cystic fullness the doctor originally discovered.
VERDICT $500,000 Illinois verdict.
COMMENT Failure to diagnose breast cancer continues to be a frequent and vexing allegation. Better documentation and follow-up could help obviate many of these claims.
For want of steroids, sight is lost
A 78-YEAR-OLD MAN was diagnosed with polymyalgia rheumatica (painful inflammation of the arteries, usually in the shoulders and hips) by his longtime primary care physician. The doctor treated the condition with low-dose steroids and monitored the patient’s erythrocyte sedimentation rate and C-reactive protein.
Two years after diagnosis, the patient complained to the physician of jaw pain and transient vision loss in the left eye. Three days later, he called the doctor to say that he had developed a headache. The physician lowered the steroid dosage but didn’t order blood tests or a biopsy. The following day the patient woke up and discovered he’d gone blind.
PLAINTIFF’S CLAIM The patient had giant cell arteritis and should have been treated with high-dose steroids. Starting treatment even one day earlier would have prevented blindness.
THE DEFENSE No information about the defense is available.
VERDICT $3 million Washington settlement.
COMMENT Timely diagnosis and appropriate treatment of temporal arteritis remain essential.
Sudden chest pain, sudden death, but not the usual suspects
SUDDEN ONSET OF CHEST PAIN brought a 41-year-old woman to the ED. Results of an electrocardiogram, chest radiograph, and lab tests were all normal. While in the ED, the patient developed diarrhea and was diagnosed with a gastrointestinal bleed.
She was admitted to the hospital, but no bed was available, so she remained in the ED, where she was found dead 7 hours later. Autopsy revealed a type A dissecting aorta to the level of the renal arteries.
PLAINTIFF’S CLAIM The ED physician failed to rule out all potential life-threatening causes of the chest pain and didn’t order a CT scan, which would have showed the aortic dissection.
DOCTOR’S DEFENSE Aortic dissection is a rare condition; the patient didn’t fit the profile of an individual at risk. A chest radiograph almost always reveals such abnormalities; no duty existed to rule out aortic dissection.
VERDICT $1.4 million Ohio verdict.
COMMENT Even though the details of this case are sketchy—and any death is a tragedy—I can’t help but sympathize with the defendant. While as physicians we should not chase zebras, we still have to consider the possibility of rare conditions.
Misdiagnosed cold foot leads to amputation
NUMBNESS IN HER RIGHT FOOT prompted 2 visits to the emergency department by a woman in her early 40s. The foot was cold and discolored. By the second visit, the patient was screaming with pain. A sprain was diagnosed without consulting a vascular surgeon, and the patient was sent home.
Ten days later, the patient had a computed tomography scan at another hospital, which found a blockage of the popliteal artery. Her right leg was amputated below the knee the following day and she was fitted with a prosthesis.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1.25 million New Jersey settlement.
COMMENT I have seen a rash of cases in which peripheral vascular disease was inappropriately diagnosed. One wonders how an alert clinician could miss vascular disease and diagnose a sprain when faced with pain and a cold discolored foot.
What’s best for croup?
A SINGLE DOSE OF CORTICOSTEROIDS is the first-line treatment for croup, resulting in fewer return visits and hospital admissions, shorter lengths of stay in the emergency department (ED) or hospital, and less need for supplemental medication (strength of recommendation [SOR]: A, meta-analysis and randomized controlled trials [RCTs]). A 0.15 mg/kg dose of oral dexamethasone is as effective as larger doses (SOR: B, small RCTs).
Nebulized racemic or L-epinephrine reduces severity of symptoms in moderate-to-severe croup (SOR: C, limited-quality disease-oriented evidence).
The role of heliox in moderate to severe croup remains uncertain. Studies to date have been inadequate (SOR: C, limited-quality disease-oriented evidence).
Humidified air provides no demonstrable benefit in the acute setting (SOR: A, meta-analysis).
Evidence summary
Standard management for croup has included glucocorticoids, nebulized racemic epinephrine, humidified air, and, for patients with severe respiratory distress and impending respiratory failure, helium-oxygen mixtures.
Glucocorticoids have significant benefits
A 2011 Cochrane review of glucocorticoids in children with croup identified 38 RCTs with 4299 patients.1 Effective treatments included dexamethasone (oral, subcutaneous, intramuscular, nebulized), budesonide (inhaled), and prednisolone (oral). Meta-analysis revealed a significant decrease in the rate of return visits and (re)admissions for patients treated with glucocorticoids compared with placebo (relative risk=0.5; 95% confidence interval [CI], 0.3-0.7). Glucocorticoid-treated children spent less time in the ED or hospital (weighted mean difference=-12 hours; 95% CI, -5 to -19) and were less likely to need epinephrine (risk difference=10%; 95% CI, 1%-20%).
The standardized improvement in the Westley score (TABLE) for all glucocorticoid treatments compared with placebo was -1.2 (95% CI, -1.6 to -0.8) at 6 hours and -1.9 (95% CI, -2.4 to -1.3) at 12 hours. No statistically significant difference was found at 24 hours (-1.3; 95% CI, -2.7 to 0.2). The combined studies favored glucocorticoids over placebo with a number needed to treat of 5. Meta-regression analysis didn’t demonstrate superiority for any single glucocorticoid.
A single 0.15 mg/kg dose of oral dexamethasone proved as effective as higher doses of 0.3 to 0.6 mg/kg in 3 RCTs (N=100, 120, and 99).2-4
TABLE
Westley Croup Score5
| Score | ||||||
|---|---|---|---|---|---|---|
| Symptom | 0 | 1 | 2 | 3 | 4 | 5 |
| Level of consciousness | Normal, including sleep | ________ | __________ | __________ | __________ | Disoriented |
| Cyanosis | None | ________ | __________ | __________ | With agitation | At rest |
| Stridor | None | With agitation | At rest | __________ | __________ | __________ |
| Air entry | Normal | Decreased | Markedly decreased | __________ | __________ | __________ |
| Retractions | None | Mild | Moderate | Severe | __________ | __________ |
| Scoring: Mild croup=≤2; moderate croup=3-7; severe croup=≥8. | ||||||
Nebulized epinephrine improves moderate to severe croup
Three RCTs (N=54, 20, and 13) found that in moderate to severe croup, treatment with nebulized racemic epinephrine improved croup score within 10 to 30 minutes.5
A small RCT (31 children, 6 months to 6 years of age) demonstrated L-epinephrine [1:1000] to be as effective and well tolerated as racemic epinephrine in moderate to severe croup. Improvement in croup score and respiratory rate peaked at 30 minutes. The effect of epinephrine (racemic or L-form) didn’t last beyond 120 minutes.6
In a retrospective study of 50 children with croup who were given aerosolized racemic epinephrine and observed in the ED for 2 hours after treatment, 58% received steroids during observation and 34% were prescribed prednisolone at discharge. Only 1 child required a return visit within 48 hours.7
Effect of helium-oxygen mixtures isn’t clear
A 2010 Cochrane review identified 2 RCTs of heliox in acute croup. No significant differences in croup score changes were found when heliox was compared with 30% oxygen (n=15, mild to moderate croup) and 100% oxygen with prn nebulized racemic epinephrine (n=29, moderate to severe croup).8 Both studies were underpowered and had significant methodological limitations.
Humidified air shows no benefit
A Cochrane review of 3 RCTs comparing humidified air with room air in emergency settings (n=135) found no evidence of benefit in croup score, oxygen saturation, or pulse rate.9
Recommendations
The 2008 Alberta Medical Association guideline recommends that all children with croup be treated with 0.6 mg/kg oral dexamethasone.10 Children with mild croup can be discharged home without further observation. Children with moderate croup should be observed for at least 4 hours. Hospitalization should be considered for children who fail to show adequate improvement. The guideline advises giving both steroids and nebulized epinephrine to children with severe croup.
The Advanced Pediatric Life Support (APLS) course of the American Academy of Pediatrics and the American College of Emergency Physicians recommends treatment with corticosteroids.11 For severe croup, the APLS advocates racemic or L-epinephrine, followed by observation for 3 or 4 hours and hospital admission in the event of inadequate response or recurrence of severe distress.
1. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.-
2. Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double-blind placebo-controlled clinical trial. BMJ. 1996;313:140-142.
3. Geelhoed GC, Macdonald WBG. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995;20:362-368.
4. Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup. Emerg Med Australas. 2007;19:51-58.
5. Johnson D. Croup. BMJ Clin Evid [monograph online]. London: BMJ Publishing Group; Updated March 2009. Available at: http://clinicalevidence.com. Accessed July 12, 2010.
6. Waisman Y, Klein BL, Boenning DA, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics. 1992;89:302-306.
7. Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. Am J Emerg Med. 1992;10:181-183.
8. Vorwerk C, Coats T. Heliox for croup in children. Cochrane Database Syst Rev. 2010;(2):CD006822.-
9. Moore M, Little P. Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006;(3):CD002870.-
10. Alberta Medical Association. Guideline for the diagnosis and management of croup. 2008 update. Alberta Medical Association. Available at: www.topalbertadoctors.org. Accessed May 11, 2011.
11. American Academy of Pediatrics and American College of Emergency Physicians APLS: The pediatric emergency medicine Resource (American Academy of Pediatrics). 4th ed, rev. Boston, Mass: Jones and Bartlett Publishers; 2007:61–64.
A SINGLE DOSE OF CORTICOSTEROIDS is the first-line treatment for croup, resulting in fewer return visits and hospital admissions, shorter lengths of stay in the emergency department (ED) or hospital, and less need for supplemental medication (strength of recommendation [SOR]: A, meta-analysis and randomized controlled trials [RCTs]). A 0.15 mg/kg dose of oral dexamethasone is as effective as larger doses (SOR: B, small RCTs).
Nebulized racemic or L-epinephrine reduces severity of symptoms in moderate-to-severe croup (SOR: C, limited-quality disease-oriented evidence).
The role of heliox in moderate to severe croup remains uncertain. Studies to date have been inadequate (SOR: C, limited-quality disease-oriented evidence).
Humidified air provides no demonstrable benefit in the acute setting (SOR: A, meta-analysis).
Evidence summary
Standard management for croup has included glucocorticoids, nebulized racemic epinephrine, humidified air, and, for patients with severe respiratory distress and impending respiratory failure, helium-oxygen mixtures.
Glucocorticoids have significant benefits
A 2011 Cochrane review of glucocorticoids in children with croup identified 38 RCTs with 4299 patients.1 Effective treatments included dexamethasone (oral, subcutaneous, intramuscular, nebulized), budesonide (inhaled), and prednisolone (oral). Meta-analysis revealed a significant decrease in the rate of return visits and (re)admissions for patients treated with glucocorticoids compared with placebo (relative risk=0.5; 95% confidence interval [CI], 0.3-0.7). Glucocorticoid-treated children spent less time in the ED or hospital (weighted mean difference=-12 hours; 95% CI, -5 to -19) and were less likely to need epinephrine (risk difference=10%; 95% CI, 1%-20%).
The standardized improvement in the Westley score (TABLE) for all glucocorticoid treatments compared with placebo was -1.2 (95% CI, -1.6 to -0.8) at 6 hours and -1.9 (95% CI, -2.4 to -1.3) at 12 hours. No statistically significant difference was found at 24 hours (-1.3; 95% CI, -2.7 to 0.2). The combined studies favored glucocorticoids over placebo with a number needed to treat of 5. Meta-regression analysis didn’t demonstrate superiority for any single glucocorticoid.
A single 0.15 mg/kg dose of oral dexamethasone proved as effective as higher doses of 0.3 to 0.6 mg/kg in 3 RCTs (N=100, 120, and 99).2-4
TABLE
Westley Croup Score5
| Score | ||||||
|---|---|---|---|---|---|---|
| Symptom | 0 | 1 | 2 | 3 | 4 | 5 |
| Level of consciousness | Normal, including sleep | ________ | __________ | __________ | __________ | Disoriented |
| Cyanosis | None | ________ | __________ | __________ | With agitation | At rest |
| Stridor | None | With agitation | At rest | __________ | __________ | __________ |
| Air entry | Normal | Decreased | Markedly decreased | __________ | __________ | __________ |
| Retractions | None | Mild | Moderate | Severe | __________ | __________ |
| Scoring: Mild croup=≤2; moderate croup=3-7; severe croup=≥8. | ||||||
Nebulized epinephrine improves moderate to severe croup
Three RCTs (N=54, 20, and 13) found that in moderate to severe croup, treatment with nebulized racemic epinephrine improved croup score within 10 to 30 minutes.5
A small RCT (31 children, 6 months to 6 years of age) demonstrated L-epinephrine [1:1000] to be as effective and well tolerated as racemic epinephrine in moderate to severe croup. Improvement in croup score and respiratory rate peaked at 30 minutes. The effect of epinephrine (racemic or L-form) didn’t last beyond 120 minutes.6
In a retrospective study of 50 children with croup who were given aerosolized racemic epinephrine and observed in the ED for 2 hours after treatment, 58% received steroids during observation and 34% were prescribed prednisolone at discharge. Only 1 child required a return visit within 48 hours.7
Effect of helium-oxygen mixtures isn’t clear
A 2010 Cochrane review identified 2 RCTs of heliox in acute croup. No significant differences in croup score changes were found when heliox was compared with 30% oxygen (n=15, mild to moderate croup) and 100% oxygen with prn nebulized racemic epinephrine (n=29, moderate to severe croup).8 Both studies were underpowered and had significant methodological limitations.
Humidified air shows no benefit
A Cochrane review of 3 RCTs comparing humidified air with room air in emergency settings (n=135) found no evidence of benefit in croup score, oxygen saturation, or pulse rate.9
Recommendations
The 2008 Alberta Medical Association guideline recommends that all children with croup be treated with 0.6 mg/kg oral dexamethasone.10 Children with mild croup can be discharged home without further observation. Children with moderate croup should be observed for at least 4 hours. Hospitalization should be considered for children who fail to show adequate improvement. The guideline advises giving both steroids and nebulized epinephrine to children with severe croup.
The Advanced Pediatric Life Support (APLS) course of the American Academy of Pediatrics and the American College of Emergency Physicians recommends treatment with corticosteroids.11 For severe croup, the APLS advocates racemic or L-epinephrine, followed by observation for 3 or 4 hours and hospital admission in the event of inadequate response or recurrence of severe distress.
A SINGLE DOSE OF CORTICOSTEROIDS is the first-line treatment for croup, resulting in fewer return visits and hospital admissions, shorter lengths of stay in the emergency department (ED) or hospital, and less need for supplemental medication (strength of recommendation [SOR]: A, meta-analysis and randomized controlled trials [RCTs]). A 0.15 mg/kg dose of oral dexamethasone is as effective as larger doses (SOR: B, small RCTs).
Nebulized racemic or L-epinephrine reduces severity of symptoms in moderate-to-severe croup (SOR: C, limited-quality disease-oriented evidence).
The role of heliox in moderate to severe croup remains uncertain. Studies to date have been inadequate (SOR: C, limited-quality disease-oriented evidence).
Humidified air provides no demonstrable benefit in the acute setting (SOR: A, meta-analysis).
Evidence summary
Standard management for croup has included glucocorticoids, nebulized racemic epinephrine, humidified air, and, for patients with severe respiratory distress and impending respiratory failure, helium-oxygen mixtures.
Glucocorticoids have significant benefits
A 2011 Cochrane review of glucocorticoids in children with croup identified 38 RCTs with 4299 patients.1 Effective treatments included dexamethasone (oral, subcutaneous, intramuscular, nebulized), budesonide (inhaled), and prednisolone (oral). Meta-analysis revealed a significant decrease in the rate of return visits and (re)admissions for patients treated with glucocorticoids compared with placebo (relative risk=0.5; 95% confidence interval [CI], 0.3-0.7). Glucocorticoid-treated children spent less time in the ED or hospital (weighted mean difference=-12 hours; 95% CI, -5 to -19) and were less likely to need epinephrine (risk difference=10%; 95% CI, 1%-20%).
The standardized improvement in the Westley score (TABLE) for all glucocorticoid treatments compared with placebo was -1.2 (95% CI, -1.6 to -0.8) at 6 hours and -1.9 (95% CI, -2.4 to -1.3) at 12 hours. No statistically significant difference was found at 24 hours (-1.3; 95% CI, -2.7 to 0.2). The combined studies favored glucocorticoids over placebo with a number needed to treat of 5. Meta-regression analysis didn’t demonstrate superiority for any single glucocorticoid.
A single 0.15 mg/kg dose of oral dexamethasone proved as effective as higher doses of 0.3 to 0.6 mg/kg in 3 RCTs (N=100, 120, and 99).2-4
TABLE
Westley Croup Score5
| Score | ||||||
|---|---|---|---|---|---|---|
| Symptom | 0 | 1 | 2 | 3 | 4 | 5 |
| Level of consciousness | Normal, including sleep | ________ | __________ | __________ | __________ | Disoriented |
| Cyanosis | None | ________ | __________ | __________ | With agitation | At rest |
| Stridor | None | With agitation | At rest | __________ | __________ | __________ |
| Air entry | Normal | Decreased | Markedly decreased | __________ | __________ | __________ |
| Retractions | None | Mild | Moderate | Severe | __________ | __________ |
| Scoring: Mild croup=≤2; moderate croup=3-7; severe croup=≥8. | ||||||
Nebulized epinephrine improves moderate to severe croup
Three RCTs (N=54, 20, and 13) found that in moderate to severe croup, treatment with nebulized racemic epinephrine improved croup score within 10 to 30 minutes.5
A small RCT (31 children, 6 months to 6 years of age) demonstrated L-epinephrine [1:1000] to be as effective and well tolerated as racemic epinephrine in moderate to severe croup. Improvement in croup score and respiratory rate peaked at 30 minutes. The effect of epinephrine (racemic or L-form) didn’t last beyond 120 minutes.6
In a retrospective study of 50 children with croup who were given aerosolized racemic epinephrine and observed in the ED for 2 hours after treatment, 58% received steroids during observation and 34% were prescribed prednisolone at discharge. Only 1 child required a return visit within 48 hours.7
Effect of helium-oxygen mixtures isn’t clear
A 2010 Cochrane review identified 2 RCTs of heliox in acute croup. No significant differences in croup score changes were found when heliox was compared with 30% oxygen (n=15, mild to moderate croup) and 100% oxygen with prn nebulized racemic epinephrine (n=29, moderate to severe croup).8 Both studies were underpowered and had significant methodological limitations.
Humidified air shows no benefit
A Cochrane review of 3 RCTs comparing humidified air with room air in emergency settings (n=135) found no evidence of benefit in croup score, oxygen saturation, or pulse rate.9
Recommendations
The 2008 Alberta Medical Association guideline recommends that all children with croup be treated with 0.6 mg/kg oral dexamethasone.10 Children with mild croup can be discharged home without further observation. Children with moderate croup should be observed for at least 4 hours. Hospitalization should be considered for children who fail to show adequate improvement. The guideline advises giving both steroids and nebulized epinephrine to children with severe croup.
The Advanced Pediatric Life Support (APLS) course of the American Academy of Pediatrics and the American College of Emergency Physicians recommends treatment with corticosteroids.11 For severe croup, the APLS advocates racemic or L-epinephrine, followed by observation for 3 or 4 hours and hospital admission in the event of inadequate response or recurrence of severe distress.
1. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.-
2. Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double-blind placebo-controlled clinical trial. BMJ. 1996;313:140-142.
3. Geelhoed GC, Macdonald WBG. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995;20:362-368.
4. Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup. Emerg Med Australas. 2007;19:51-58.
5. Johnson D. Croup. BMJ Clin Evid [monograph online]. London: BMJ Publishing Group; Updated March 2009. Available at: http://clinicalevidence.com. Accessed July 12, 2010.
6. Waisman Y, Klein BL, Boenning DA, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics. 1992;89:302-306.
7. Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. Am J Emerg Med. 1992;10:181-183.
8. Vorwerk C, Coats T. Heliox for croup in children. Cochrane Database Syst Rev. 2010;(2):CD006822.-
9. Moore M, Little P. Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006;(3):CD002870.-
10. Alberta Medical Association. Guideline for the diagnosis and management of croup. 2008 update. Alberta Medical Association. Available at: www.topalbertadoctors.org. Accessed May 11, 2011.
11. American Academy of Pediatrics and American College of Emergency Physicians APLS: The pediatric emergency medicine Resource (American Academy of Pediatrics). 4th ed, rev. Boston, Mass: Jones and Bartlett Publishers; 2007:61–64.
1. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011;(1):CD001955.-
2. Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double-blind placebo-controlled clinical trial. BMJ. 1996;313:140-142.
3. Geelhoed GC, Macdonald WBG. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatr Pulmonol. 1995;20:362-368.
4. Fifoot AA, Ting JY. Comparison between single-dose oral prednisolone and oral dexamethasone in the treatment of croup. Emerg Med Australas. 2007;19:51-58.
5. Johnson D. Croup. BMJ Clin Evid [monograph online]. London: BMJ Publishing Group; Updated March 2009. Available at: http://clinicalevidence.com. Accessed July 12, 2010.
6. Waisman Y, Klein BL, Boenning DA, et al. Prospective randomized double-blind study comparing L-epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics. 1992;89:302-306.
7. Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. Am J Emerg Med. 1992;10:181-183.
8. Vorwerk C, Coats T. Heliox for croup in children. Cochrane Database Syst Rev. 2010;(2):CD006822.-
9. Moore M, Little P. Humidified air inhalation for treating croup. Cochrane Database Syst Rev. 2006;(3):CD002870.-
10. Alberta Medical Association. Guideline for the diagnosis and management of croup. 2008 update. Alberta Medical Association. Available at: www.topalbertadoctors.org. Accessed May 11, 2011.
11. American Academy of Pediatrics and American College of Emergency Physicians APLS: The pediatric emergency medicine Resource (American Academy of Pediatrics). 4th ed, rev. Boston, Mass: Jones and Bartlett Publishers; 2007:61–64.
Evidence-based answers from the Family Physicians Inquiries Network
Does the D-dimer get too much or too little weight?
“Looking Beyond the D-dimer” (J Fam Pract. 2011;60:400-403) left me quite confused. The authors described a patient for whom the Wells criteria and a D-dimer were negative for pulmonary embolism (PE) initially but who did, in fact, have a PE. They point out “a key problem with the Wells criteria” and show that the D-dimer was inaccurate, at least relatively early on. Yet they conclude that physicians should use the Wells criteria to evaluate patients and should not work up a patient with a negative D-dimer—which was less than reliable in the case they described.
If, after all of our training and experience as physicians, we are being taught to rely on algorithms and moderately and/or inconsistently reliable tests, we can all retire and let our computers do our jobs. Although I am concerned that much of modern medicine is dictated by health insurers or driven by fear of malpractice claims, we should not exclude clinical judgment and professional acumen. Nor should we read and live by articles that offer contradictory advice.
Doctors, you can’t have it both ways.
Barry Marged, DO, MA
Alliance, Ohio
The authors respond
My colleague and I read your comments with interest. We believe that our case study provides an important message: Utilize evidence-based algorithms that exist in the literature to the best of your ability, but never lose sight of your clinical instincts. These “resources” are complementary, not mutually exclusive.
Good communication with patients affords us the opportunity to stay involved with the evolution of their clinical status and always be ready to reassess. In our case presentation, the Wells criteria allowed us to incorporate the algorithmic thinking into our clinical judgment, but not to replace it. The patient’s ongoing symptoms required a reevaluation, and the Wells criteria proved their worth the second time around. No harm was done to the patient as the PE turned out to be distal and small.
In the end, no clinical algorithm can deliver a guaranteed outcome. In this era of rigorous scrutiny, evidence-based medicine, and cost-effective care, criteria such as the Wells are particularly important. Avoiding unnecessary CT angiography while maintaining close contact with a patient, or assuring immediate follow-up (in the case of an emergency department evaluation) saves valuable resources that can then be deployed elsewhere. Thoughtful rigor, combined with open-mindedness and trust in our clinical instincts, is the way to deliver value-driven, high-quality care.
H. Andrew Selinger, MD
Bristol, Conn
“Looking Beyond the D-dimer” (J Fam Pract. 2011;60:400-403) left me quite confused. The authors described a patient for whom the Wells criteria and a D-dimer were negative for pulmonary embolism (PE) initially but who did, in fact, have a PE. They point out “a key problem with the Wells criteria” and show that the D-dimer was inaccurate, at least relatively early on. Yet they conclude that physicians should use the Wells criteria to evaluate patients and should not work up a patient with a negative D-dimer—which was less than reliable in the case they described.
If, after all of our training and experience as physicians, we are being taught to rely on algorithms and moderately and/or inconsistently reliable tests, we can all retire and let our computers do our jobs. Although I am concerned that much of modern medicine is dictated by health insurers or driven by fear of malpractice claims, we should not exclude clinical judgment and professional acumen. Nor should we read and live by articles that offer contradictory advice.
Doctors, you can’t have it both ways.
Barry Marged, DO, MA
Alliance, Ohio
The authors respond
My colleague and I read your comments with interest. We believe that our case study provides an important message: Utilize evidence-based algorithms that exist in the literature to the best of your ability, but never lose sight of your clinical instincts. These “resources” are complementary, not mutually exclusive.
Good communication with patients affords us the opportunity to stay involved with the evolution of their clinical status and always be ready to reassess. In our case presentation, the Wells criteria allowed us to incorporate the algorithmic thinking into our clinical judgment, but not to replace it. The patient’s ongoing symptoms required a reevaluation, and the Wells criteria proved their worth the second time around. No harm was done to the patient as the PE turned out to be distal and small.
In the end, no clinical algorithm can deliver a guaranteed outcome. In this era of rigorous scrutiny, evidence-based medicine, and cost-effective care, criteria such as the Wells are particularly important. Avoiding unnecessary CT angiography while maintaining close contact with a patient, or assuring immediate follow-up (in the case of an emergency department evaluation) saves valuable resources that can then be deployed elsewhere. Thoughtful rigor, combined with open-mindedness and trust in our clinical instincts, is the way to deliver value-driven, high-quality care.
H. Andrew Selinger, MD
Bristol, Conn
“Looking Beyond the D-dimer” (J Fam Pract. 2011;60:400-403) left me quite confused. The authors described a patient for whom the Wells criteria and a D-dimer were negative for pulmonary embolism (PE) initially but who did, in fact, have a PE. They point out “a key problem with the Wells criteria” and show that the D-dimer was inaccurate, at least relatively early on. Yet they conclude that physicians should use the Wells criteria to evaluate patients and should not work up a patient with a negative D-dimer—which was less than reliable in the case they described.
If, after all of our training and experience as physicians, we are being taught to rely on algorithms and moderately and/or inconsistently reliable tests, we can all retire and let our computers do our jobs. Although I am concerned that much of modern medicine is dictated by health insurers or driven by fear of malpractice claims, we should not exclude clinical judgment and professional acumen. Nor should we read and live by articles that offer contradictory advice.
Doctors, you can’t have it both ways.
Barry Marged, DO, MA
Alliance, Ohio
The authors respond
My colleague and I read your comments with interest. We believe that our case study provides an important message: Utilize evidence-based algorithms that exist in the literature to the best of your ability, but never lose sight of your clinical instincts. These “resources” are complementary, not mutually exclusive.
Good communication with patients affords us the opportunity to stay involved with the evolution of their clinical status and always be ready to reassess. In our case presentation, the Wells criteria allowed us to incorporate the algorithmic thinking into our clinical judgment, but not to replace it. The patient’s ongoing symptoms required a reevaluation, and the Wells criteria proved their worth the second time around. No harm was done to the patient as the PE turned out to be distal and small.
In the end, no clinical algorithm can deliver a guaranteed outcome. In this era of rigorous scrutiny, evidence-based medicine, and cost-effective care, criteria such as the Wells are particularly important. Avoiding unnecessary CT angiography while maintaining close contact with a patient, or assuring immediate follow-up (in the case of an emergency department evaluation) saves valuable resources that can then be deployed elsewhere. Thoughtful rigor, combined with open-mindedness and trust in our clinical instincts, is the way to deliver value-driven, high-quality care.
H. Andrew Selinger, MD
Bristol, Conn
Compartment syndrome Dx delayed... Failure to suspect endocarditis ends in heart surgery and memory deficit
Delayed diagnosis renders dominant hand and wrist useless
A WOMAN HOSPITALIZED WITH RESPIRATORY SYMPTOMS was treated and released 4 days later. She returned by ambulance the next day and was readmitted for chronic obstructive pulmonary disease and respiratory failure. She had a history of tobacco use. It turned out she had suffered a myocardial infarction. After a cardiac consultation, she was started on 3 anticoagulants, including enoxaparin.
When her condition failed to improve after 4 days, she was transferred to another hospital. Before the transfer, bruising and slight swelling were observed on the patient’s left side and chest, and a physician reportedly ordered that the enoxaparin be discontinued. The plaintiff received another dose of enoxaparin just after she arrived at the second hospital and 3 more doses before the drug was discontinued 2 days later. On the day after admission, the patient’s right forearm, her dominant arm, was noted to be swollen, firm, and painful; her torso was bruised. No immediate evaluation was performed.
An orthopedic consultation the following day led to a diagnosis of compartment syndrome. Emergency surgery resulted in loss of muscle and nerves in the arm and chronic pain. The patient also developed anemia, hypovolemic shock, and retroperitoneal hemorrhage requiring a number of blood transfusions. The patient lost almost all function in her right wrist and hand.
PLAINTIFF’S CLAIM The defendants were negligent in failing to promptly diagnose compartment syndrome and subsequent hemorrhaging.
THE DEFENSE No negligence occurred.
VERDICT $1.525 million Ohio verdict.
COMMENT Subtle and nonspecific findings make compartment syndrome a challenging diagnosis. The combination of extremity pain, swelling, and bruising in the context of anticoagulation should trigger consideration of this condition.
Failure to suspect endocarditis ends in heart surgery and memory deficit
GENERAL ACHES, FATIGUE, AND OCCASIONAL FEVER of 102.5°F led a 43-year-old woman to seek treatment at a local clinic. The nurse practitioner who examined her suspected influenza. Six days later the patient returned, complaining that her symptoms were making it difficult to care for her 4 children. She didn’t have a fever at the time. The nurse practitioner suggested that the woman might want to go to the local hospital for an examination; she also said she could prescribe oral antibiotics to see if they helped. The patient chose the antibiotics.
Her symptoms improved over the next week but then reappeared, prompting her to return to the clinic with complaints of headache, muscle aches, fatigue, chest tightening, an unproductive cough, and night sweats so severe she had to wrap herself in a towel to avoid soaking her bed. Although she was still having regular periods, a physician told her she was probably premenopausal. He also told her that overweight people often sweat at night and attributed her fatigue to her 4 children. He prescribed rizatriptan on the theory that the headaches might be migraines. Because the woman didn’t have a fever at the time of the visit and had just finished a course of antibiotics, the physician said he was sure that she didn’t have an infection.
After 6 days with no improvement, the patient went to a hospital emergency department (ED) for a complete checkup because she was planning to drive to Arizona with her family and wanted to make sure she was all right before leaving. The ED physician ordered scans, a spinal tap, and blood tests; he diagnosed a viral infection.
Three days later, the patient went to the clinic, accompanied by her entire family, to find out the results of the blood tests. She still had symptoms and had developed a swollen, tender sternum. The nurse practitioner noted a positive culture result for Streptococcus veridans on the test report; she allegedly told the patient, in the presence of her 10-year-old son, that it must be a skin contaminant. She advised the patient to go on vacation and have additional blood work if she didn’t feel better.
The nurse practitioner gave the patient another pack of oral antibiotics in case she had a lingering low-grade infection. The patient also received another prescription for rizatriptan and an acetaminophen and oxycodone prescription for pain.
The nurse practitioner claimed that she suggested that the patient could stop by the hospital for a blood test before leaving on vacation, but the patient denied that the nurse made the suggestion, and no notes supported the claim. The oral antibiotics relieved the patient’s symptoms only temporarily. The family cut short their vacation so the patient could return to the clinic, where she received another ineffectual antibiotic. When her condition continued to deteriorate, her husband took her to the ED of a larger hospital in the area.
The ED physician diagnosed subacute endocarditis, which was confirmed by subsequent tests. Testing also identified a bicuspid aortic valve, which increased the patient’s susceptibility to endocarditis. She was started on appropriate intravenous antibiotics and improved initially.
The patient subsequently noticed red patches on her hand and forearm. She also experienced problems with mental processing. She returned to the hospital, where a scan showed increased vegetative growth on her aortic valve. Pieces of the growth were breaking off, causing embolic injury to the patient’s brain, hand, and other areas of her body. The patient underwent open heart surgery to replace the aortic valve and prevent further embolic injury. She continues to suffer from significant short-term memory loss and will require warfarin for the rest of her life to prevent blood clotting.
PLAINTIFF’S CLAIM The patient should have been referred earlier for a complete workup, and the nurse practitioner should have taken seriously the culture showing S veridans. The nurse practitioner was mistaken in thinking that S veridans was found on the skin. Had she looked it up, which she should have done, she would have discovered that the organism is the most common bacterial cause of subacute endocarditis.
The patient had the classic symptoms of subacute endocarditis. The delay in diagnosis allowed bacteria to build up on her aortic valve, forming a biofilm barrier that inhibited the effect of the IV antibiotics and the body’s natural defenses and precipitated the embolic injury.
THE DEFENSE The patient was responsible for the delay in diagnosis, especially in light of the fact that she had a nursing background. Any negligence on the part of the nurse practitioner had no effect on the outcome.
VERDICT $1 million Washington settlement.
COMMENT Subacute bacterial endocarditis remains a challenging diagnosis with potentially devastating consequences. Be on the alert for this subtle masquerader.
Delayed diagnosis renders dominant hand and wrist useless
A WOMAN HOSPITALIZED WITH RESPIRATORY SYMPTOMS was treated and released 4 days later. She returned by ambulance the next day and was readmitted for chronic obstructive pulmonary disease and respiratory failure. She had a history of tobacco use. It turned out she had suffered a myocardial infarction. After a cardiac consultation, she was started on 3 anticoagulants, including enoxaparin.
When her condition failed to improve after 4 days, she was transferred to another hospital. Before the transfer, bruising and slight swelling were observed on the patient’s left side and chest, and a physician reportedly ordered that the enoxaparin be discontinued. The plaintiff received another dose of enoxaparin just after she arrived at the second hospital and 3 more doses before the drug was discontinued 2 days later. On the day after admission, the patient’s right forearm, her dominant arm, was noted to be swollen, firm, and painful; her torso was bruised. No immediate evaluation was performed.
An orthopedic consultation the following day led to a diagnosis of compartment syndrome. Emergency surgery resulted in loss of muscle and nerves in the arm and chronic pain. The patient also developed anemia, hypovolemic shock, and retroperitoneal hemorrhage requiring a number of blood transfusions. The patient lost almost all function in her right wrist and hand.
PLAINTIFF’S CLAIM The defendants were negligent in failing to promptly diagnose compartment syndrome and subsequent hemorrhaging.
THE DEFENSE No negligence occurred.
VERDICT $1.525 million Ohio verdict.
COMMENT Subtle and nonspecific findings make compartment syndrome a challenging diagnosis. The combination of extremity pain, swelling, and bruising in the context of anticoagulation should trigger consideration of this condition.
Failure to suspect endocarditis ends in heart surgery and memory deficit
GENERAL ACHES, FATIGUE, AND OCCASIONAL FEVER of 102.5°F led a 43-year-old woman to seek treatment at a local clinic. The nurse practitioner who examined her suspected influenza. Six days later the patient returned, complaining that her symptoms were making it difficult to care for her 4 children. She didn’t have a fever at the time. The nurse practitioner suggested that the woman might want to go to the local hospital for an examination; she also said she could prescribe oral antibiotics to see if they helped. The patient chose the antibiotics.
Her symptoms improved over the next week but then reappeared, prompting her to return to the clinic with complaints of headache, muscle aches, fatigue, chest tightening, an unproductive cough, and night sweats so severe she had to wrap herself in a towel to avoid soaking her bed. Although she was still having regular periods, a physician told her she was probably premenopausal. He also told her that overweight people often sweat at night and attributed her fatigue to her 4 children. He prescribed rizatriptan on the theory that the headaches might be migraines. Because the woman didn’t have a fever at the time of the visit and had just finished a course of antibiotics, the physician said he was sure that she didn’t have an infection.
After 6 days with no improvement, the patient went to a hospital emergency department (ED) for a complete checkup because she was planning to drive to Arizona with her family and wanted to make sure she was all right before leaving. The ED physician ordered scans, a spinal tap, and blood tests; he diagnosed a viral infection.
Three days later, the patient went to the clinic, accompanied by her entire family, to find out the results of the blood tests. She still had symptoms and had developed a swollen, tender sternum. The nurse practitioner noted a positive culture result for Streptococcus veridans on the test report; she allegedly told the patient, in the presence of her 10-year-old son, that it must be a skin contaminant. She advised the patient to go on vacation and have additional blood work if she didn’t feel better.
The nurse practitioner gave the patient another pack of oral antibiotics in case she had a lingering low-grade infection. The patient also received another prescription for rizatriptan and an acetaminophen and oxycodone prescription for pain.
The nurse practitioner claimed that she suggested that the patient could stop by the hospital for a blood test before leaving on vacation, but the patient denied that the nurse made the suggestion, and no notes supported the claim. The oral antibiotics relieved the patient’s symptoms only temporarily. The family cut short their vacation so the patient could return to the clinic, where she received another ineffectual antibiotic. When her condition continued to deteriorate, her husband took her to the ED of a larger hospital in the area.
The ED physician diagnosed subacute endocarditis, which was confirmed by subsequent tests. Testing also identified a bicuspid aortic valve, which increased the patient’s susceptibility to endocarditis. She was started on appropriate intravenous antibiotics and improved initially.
The patient subsequently noticed red patches on her hand and forearm. She also experienced problems with mental processing. She returned to the hospital, where a scan showed increased vegetative growth on her aortic valve. Pieces of the growth were breaking off, causing embolic injury to the patient’s brain, hand, and other areas of her body. The patient underwent open heart surgery to replace the aortic valve and prevent further embolic injury. She continues to suffer from significant short-term memory loss and will require warfarin for the rest of her life to prevent blood clotting.
PLAINTIFF’S CLAIM The patient should have been referred earlier for a complete workup, and the nurse practitioner should have taken seriously the culture showing S veridans. The nurse practitioner was mistaken in thinking that S veridans was found on the skin. Had she looked it up, which she should have done, she would have discovered that the organism is the most common bacterial cause of subacute endocarditis.
The patient had the classic symptoms of subacute endocarditis. The delay in diagnosis allowed bacteria to build up on her aortic valve, forming a biofilm barrier that inhibited the effect of the IV antibiotics and the body’s natural defenses and precipitated the embolic injury.
THE DEFENSE The patient was responsible for the delay in diagnosis, especially in light of the fact that she had a nursing background. Any negligence on the part of the nurse practitioner had no effect on the outcome.
VERDICT $1 million Washington settlement.
COMMENT Subacute bacterial endocarditis remains a challenging diagnosis with potentially devastating consequences. Be on the alert for this subtle masquerader.
Delayed diagnosis renders dominant hand and wrist useless
A WOMAN HOSPITALIZED WITH RESPIRATORY SYMPTOMS was treated and released 4 days later. She returned by ambulance the next day and was readmitted for chronic obstructive pulmonary disease and respiratory failure. She had a history of tobacco use. It turned out she had suffered a myocardial infarction. After a cardiac consultation, she was started on 3 anticoagulants, including enoxaparin.
When her condition failed to improve after 4 days, she was transferred to another hospital. Before the transfer, bruising and slight swelling were observed on the patient’s left side and chest, and a physician reportedly ordered that the enoxaparin be discontinued. The plaintiff received another dose of enoxaparin just after she arrived at the second hospital and 3 more doses before the drug was discontinued 2 days later. On the day after admission, the patient’s right forearm, her dominant arm, was noted to be swollen, firm, and painful; her torso was bruised. No immediate evaluation was performed.
An orthopedic consultation the following day led to a diagnosis of compartment syndrome. Emergency surgery resulted in loss of muscle and nerves in the arm and chronic pain. The patient also developed anemia, hypovolemic shock, and retroperitoneal hemorrhage requiring a number of blood transfusions. The patient lost almost all function in her right wrist and hand.
PLAINTIFF’S CLAIM The defendants were negligent in failing to promptly diagnose compartment syndrome and subsequent hemorrhaging.
THE DEFENSE No negligence occurred.
VERDICT $1.525 million Ohio verdict.
COMMENT Subtle and nonspecific findings make compartment syndrome a challenging diagnosis. The combination of extremity pain, swelling, and bruising in the context of anticoagulation should trigger consideration of this condition.
Failure to suspect endocarditis ends in heart surgery and memory deficit
GENERAL ACHES, FATIGUE, AND OCCASIONAL FEVER of 102.5°F led a 43-year-old woman to seek treatment at a local clinic. The nurse practitioner who examined her suspected influenza. Six days later the patient returned, complaining that her symptoms were making it difficult to care for her 4 children. She didn’t have a fever at the time. The nurse practitioner suggested that the woman might want to go to the local hospital for an examination; she also said she could prescribe oral antibiotics to see if they helped. The patient chose the antibiotics.
Her symptoms improved over the next week but then reappeared, prompting her to return to the clinic with complaints of headache, muscle aches, fatigue, chest tightening, an unproductive cough, and night sweats so severe she had to wrap herself in a towel to avoid soaking her bed. Although she was still having regular periods, a physician told her she was probably premenopausal. He also told her that overweight people often sweat at night and attributed her fatigue to her 4 children. He prescribed rizatriptan on the theory that the headaches might be migraines. Because the woman didn’t have a fever at the time of the visit and had just finished a course of antibiotics, the physician said he was sure that she didn’t have an infection.
After 6 days with no improvement, the patient went to a hospital emergency department (ED) for a complete checkup because she was planning to drive to Arizona with her family and wanted to make sure she was all right before leaving. The ED physician ordered scans, a spinal tap, and blood tests; he diagnosed a viral infection.
Three days later, the patient went to the clinic, accompanied by her entire family, to find out the results of the blood tests. She still had symptoms and had developed a swollen, tender sternum. The nurse practitioner noted a positive culture result for Streptococcus veridans on the test report; she allegedly told the patient, in the presence of her 10-year-old son, that it must be a skin contaminant. She advised the patient to go on vacation and have additional blood work if she didn’t feel better.
The nurse practitioner gave the patient another pack of oral antibiotics in case she had a lingering low-grade infection. The patient also received another prescription for rizatriptan and an acetaminophen and oxycodone prescription for pain.
The nurse practitioner claimed that she suggested that the patient could stop by the hospital for a blood test before leaving on vacation, but the patient denied that the nurse made the suggestion, and no notes supported the claim. The oral antibiotics relieved the patient’s symptoms only temporarily. The family cut short their vacation so the patient could return to the clinic, where she received another ineffectual antibiotic. When her condition continued to deteriorate, her husband took her to the ED of a larger hospital in the area.
The ED physician diagnosed subacute endocarditis, which was confirmed by subsequent tests. Testing also identified a bicuspid aortic valve, which increased the patient’s susceptibility to endocarditis. She was started on appropriate intravenous antibiotics and improved initially.
The patient subsequently noticed red patches on her hand and forearm. She also experienced problems with mental processing. She returned to the hospital, where a scan showed increased vegetative growth on her aortic valve. Pieces of the growth were breaking off, causing embolic injury to the patient’s brain, hand, and other areas of her body. The patient underwent open heart surgery to replace the aortic valve and prevent further embolic injury. She continues to suffer from significant short-term memory loss and will require warfarin for the rest of her life to prevent blood clotting.
PLAINTIFF’S CLAIM The patient should have been referred earlier for a complete workup, and the nurse practitioner should have taken seriously the culture showing S veridans. The nurse practitioner was mistaken in thinking that S veridans was found on the skin. Had she looked it up, which she should have done, she would have discovered that the organism is the most common bacterial cause of subacute endocarditis.
The patient had the classic symptoms of subacute endocarditis. The delay in diagnosis allowed bacteria to build up on her aortic valve, forming a biofilm barrier that inhibited the effect of the IV antibiotics and the body’s natural defenses and precipitated the embolic injury.
THE DEFENSE The patient was responsible for the delay in diagnosis, especially in light of the fact that she had a nursing background. Any negligence on the part of the nurse practitioner had no effect on the outcome.
VERDICT $1 million Washington settlement.
COMMENT Subacute bacterial endocarditis remains a challenging diagnosis with potentially devastating consequences. Be on the alert for this subtle masquerader.
Hoarseness and chronic cough: Would you suspect reflux?
• Recommend dietary and behavioral modifications as a first step in treating patients with symptoms suggestive of laryngopharyngeal reflux disease (LPRD). C
• When medications are needed, prescribe a high-dose proton-pump inhibitor, a histamine-2 blocker at bedtime, and prophylactic antacids for reflux-inducing activities, such as exercising and eating. B
• Avoid the rebound effect associated with abrupt cessation of medications prescribed for LPRD with a gradual, 16-week taper. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE When Joan C, a 35-year-old patient whom you’ve known for years, comes in for a physical, you notice that she’s coughing frequently. Upon questioning, Joan says she first noticed the cough several months ago; she also reports that she’s frequently hoarse, but has no other symptoms. Joan is a former smoker, and quit 4 years ago.
If Joan were your patient, would you suspect that she had an upper respiratory infection and prescribe an antibiotic such as azithromycin? Would you include laryngopharyngeal reflux disease in the differential diagnosis?
Laryngopharyngeal reflux disease (LPRD) is a common condition that most primary care physicians encounter frequently. It is also frequently misdiagnosed by clinicians who are unfamiliar with the differences between LPRD and gastroesophageal reflux disease (GERD).
The American Academy of Otolaryngology–Head and Neck Surgery defines laryngopharyngeal reflux as the retrograde movement of gastric contents into the laryngopharynx.1 Common symptoms include hoarseness/dysphonia, chronic throat clearing, dysphagia, globus pharyngeus, and chronic cough, as well as postnasal drip, paroxysmal laryngospasm, odynophagia, excessive throat mucus, and a strange taste in the mouth.2
The diversity and vagueness of these symptoms, as well as the lack of a gold standard diagnostic test for LPRD, make it difficult to estimate its prevalence. In addition, signs of gastroesophageal reflux can be found in the laryngopharynx of up to 86% of healthy individuals, further complicating the clinical picture.3 To avoid missing this often overlooked reflux disease, you need to know how it develops, what signs and symptoms to look for, and which distinguishing features to keep in mind.
Pathophysiology and distinguishing features
The precise way in which LPRD develops is not known, but there are 2 proposed means of laryngeal injury—direct and indirect. In the first case, chemical irritants in the gastric refluxate enter the laryngopharynx and cause local mucosal injury. In the second, gastric reflux irritates the esophageal tissue enough to evoke laryngeal reflexes without ever reaching the larynx—a vagally mediated response associated with symptoms such as chronic cough, throat-clearing sensations, and bronchoconstriction.4
Unlike the esophageal lining, laryngeal epithelium is not protected against chemical injury from gastric acid, as it lacks both the stripping motion of esophageal peristalsis and the neutralizing bicarbonate in saliva.4 Thus, while far smaller amounts of gastric reflux make it into the laryngopharynx, the acid remains there longer and may cause greater injury.5 In some cases, this occurs as often as 50 times a day, although as few as 3 episodes per week have been known to cause LPRD.5
Heartburn is not the rule
Heartburn is a primary complaint of patients with GERD. It is reported by little more than a third (35%) of those with LPRD,5,6 however, (which is why it is sometimes called the “silent” reflux disease). This is because heartburn is caused by esophagitis due to esophageal dysmotility and lower esophageal sphincter dysfunction,3 while most patients with LPRD have normal esophageal motor function and upper esophageal sphincter dysfunction. The fact that only a minimal amount of reflux enters the laryngopharynx may be part of the reason heartburn is less likely in patients with LPRD.
Onset of symptoms. When reflux occurs is another thing that distinguishes LPRD and GERD. Symptoms of GERD typically worsen when the individual is supine, while laryngopharyngeal reflux usually occurs when he or she is upright.7 The frequency with which these 2 conditions overlap is debatable, as there are few studies differentiating LPRD and GERD based on standardized signs and symptoms.7
Making sense of signs and symptoms
Most patients with LPRD seek treatment from their primary care physician, typically reporting symptoms that they don’t associate with gastric reflux, such as hoarseness, a chronic cough or sore throat, or the sensation of a lump in the throat (TABLE 1). Less common manifestations include “water brash”—excessive mucus in the mouth caused by a release of salivary bicarbonate to help neutralize acidity8—otitis media, sinus disease, and dental caries.5
Laryngeal endoscopy may reveal many changes from diffuse irritation. Diffuse erythema, edema, and interarytenoid hypertrophy/cobblestoning are the most useful findings for an LPRD diagnosis.9,10 But in most cases, only a few nonspecific signs with a number of possible causes (infection, environmental irritants, allergies, temperature/climate change, among others) are seen on endoscopic examination, with little correlation with symptom severity. In fact, 74% of otolaryngologists responding to a recent survey said they relied more on patient symptoms than on laryngeal signs for an LPRD diagnosis.10
The Reflux Finding Score (RFS), available at http://www.nature.com/gimo/contents/pt1/fig_tab/gimo46_T3.html, is a clinical tool developed to quantify laryngeal inflammation and standardize objective endoscopic findings. The RFS incorporates the following endolaryngeal signs:
- subglottic edema
- ventricular obliteration
- erythema/hyperemia
- vocal cord edema
- diffuse laryngeal edema
- posterior commissure hypertrophy
- granuloma/granulation tissue
- thick endolaryngeal mucus.
A numeric value is assigned to each, based on whether it is present or absent; partial or complete; local or diffuse; or mild or severe. However, the RFS, too, is an imperfect tool. Clinicians who have used the RFS report that a score higher than 7 identifies LPRD with 95% sensitivity.11 But laryngeal findings may be due to other causes, such as infection, autoimmune reaction, or even allergies, and studies have found the RFS to have poor specificity and inter-rater reliability.12-14
Ambulatory dual probe pH monitoring was considered to be the gold standard test for LPRD at one time, but newer studies have raised questions about its validity and usefulness, especially in patients taking proton-pump inhibitors (PPIs).1,5,7 Newer advanced probes featuring less invasive data collection and greater sensitivity are under development. Ambulatory 24-hour multichannel intraluminal impedance with pH monitoring is the most promising new diagnostic tool, as it can monitor both acidic and nonacidic reflux and distinguish between gas and liquid.15
TABLE 1
When to suspect laryngopharyngeal reflux disease1,5,24
| Finding | Frequency among patients with LPRD (%)* |
|---|---|
| Dysphonia/hoarseness (intermittent) | 71 |
| Chronic cough | 51 |
| Globus pharyngeus | 47 |
| Chronic throat clearing | 42 |
| Dysphagia | 35 |
| Heartburn | 35 |
| *The frequency of other symptoms associated with LPRD is not known. | |
Treatment, like diagnosis, is not clear-cut
LPRD is often called a diagnosis of exclusion, because of the nonspecific nature of its signs and symptoms and the importance of considering a range of other etiologies. The differential diagnosis includes excessive voice use, postnasal drip, upper respiratory infection, habitual throat clearing, allergic rhinitis, environmental irritants, temperature/climate change, chronic or episodic use of alcohol and/or tobacco, and psychological problems related to tics, such as habitual throat clearing or coughing.5
Diagnosis is often based on an empiric trial of high-dose PPIs, with confirmation dependent on symptom relief. Because there have been few placebo-controlled trials with PPIs and those that have been completed had conflicting results, diagnosis based on a combination of medical history and endoscopic laryngeal examination may be a better approach.16,17
Acid suppression therapy with either PPIs or histamine-2 (H2) receptor blockers such as ranitidine or famotidine is the mainstay of treatment for LPRD. But medical societies offer conflicting advice. The American Gastroenterological Association cautions clinicians not to prescribe acid-suppression therapy for patients with LPRD unless they also have GERD.6 The American Academy of Otolaryngology–Head and Neck Surgery recommends twice-daily PPI use for ≥6 months.1,13 The general consensus, based on clinical experience alone, is that patients should be treated with high doses of PPIs (eg, 40 mg omeprazole twice a day) for ≥6 months, with the addition of an H2 receptor blocker to help reduce overnight acid production.1,18 Prophylactic antacid use is also recommended in anticipation of reflux, such as before exercising and right after a meal.
Symptoms should start to improve within 6 to 8 weeks, and patients should be reassessed in about 3 months. To avoid a rebound effect from the abrupt cessation of medications, we suggest a gradual taper over 16 weeks. For the first 8 weeks, the H2 blocker should be discontinued and the PPI decreased from twice a day to once. If symptoms are still controlled, the PPI dose can be reduced to once every other day for another 8 weeks, then stopped if symptoms do not recur.18
Lifestyle and dietary changes (TABLE 2), such as smoking cessation, weight loss, and avoidance of alcohol, are an important part of LPRD treatment, and may be used as a first-line therapy before prescribing medication.19 In fact, some studies have found PPI therapy to be inferior to behavioral/lifestyle modifications.17
Fundoplication surgery, a procedure in which the gastric fundus of the stomach is wrapped around the lower end of the esophagus and stitched in place to prevent reflux, may be an option for patients who do not respond to, or cannot tolerate, aggressive medical treatment for LPRD. A 2006 prospective controlled study found that surgical fundoplication did not consistently relieve laryngeal symptoms.20 But other studies have found that a carefully selected population with medically unresponsive laryngopharyngeal symptoms can benefit from this procedure.21,22 One study showed a significant improvement within one month of fundoplication, with continued improvement observed during a 3-year follow-up.21 In another prospective study, researchers showed that while LPRD-related laryngeal symptoms such as coughing and throat-clearing improved with both medical therapy and laparoscopic fundoplication, voice quality and endoscopic laryngeal/pharyngeal findings improved significantly only with the surgical procedure.23
TABLE 2
Recommend these lifestyle modifications19
| Stop smoking |
Avoid:
|
| Eat smaller, more frequent meals |
| Avoid eating within 3 hours of bedtime |
| Lose weight |
CORRESPONDENCE
Shoib Sana, DO, Detroit Medical Center, Otolaryngology-Head and Neck Surgery, 6533 East Jefferson Avenue, Apartment 316, Detroit, MI 48207; [email protected]
1. Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35.
2. Papakonstantinou L, Leslie P, Gray J, et al. Laryngopharyngeal reflux: a prospective analysis of a 34 item symptom questionnaire. Clin Otolaryngol. 2009;34:455-459.
3. Hicks DM, Ours TM, Abelson TI, et al. The prevalence of hypopharynx findings associated with gastroesophageal reflux in normal volunteers. J Voice. 2002;16:564.-
4. Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481-491.
5. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24 hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1-78.
6. Kahrilas PJ, Shaheen NJ, Vaezi M, et al. American Gastroenterological Association Institute (AGAI) medical position statement: management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383.-
7. Postma GN, Tomek MS, Belafsky PC, et al. Esophageal motor function in laryngopharyngeal reflux is superior to that in classic gastroesophageal reflux disease. Ann Otol Rhinol Laryngol. 2001;111:1114-1116.
8. Helen JF, Dodds WJ, Hogan WJ. Salivary response to esophageal acid in normal subjects and patients with reflux esophagitis. Gastroenterology. 1998;94:1394-1398.
9. Belafsky PC. Abnormal endoscopic pharyngeal and laryngeal findings attributable to reflux. Am J Med 2003;116(suppl 3A):91S-97S.
10. Ahmed TF, Khandwala F, Abelson, et al. Chronic laryngitis associated with gastroesophageal reflux: prospective assessment of differences in practice patterns between gastroenterologists and ENT physicians. Am J Gastroenterol. 2006;102:470-478.
11. Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111:1313-1317.
12. Koufman JA, Sataloff RT, Toohill R. Laryngopharyngeal reflux: consensus conference report. J Voice. 1996;10:215-216.
13. Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope 2001;111:979-981.
14. Reichel O, Dressel H, Wiederanders K, et al. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2008;139:414-420.
15. Muderris T, Gokcan MK, Yorulmaz I. The clinical value of pharyngeal pH monitoring using a double-probe, triple-sensor catheter in patients with laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg. 2009;135:163-167.
16. Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131:342-350.
17. Wo JM, Koopman J, Harrell SP, et al. Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol. 2006;101:1972-1978.
18. Park W, Hicks DM, Khandwala F, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005;116:1230-1238.
19. Maceri DR, Zim S. Laryngospasm: an atypical manifestation of severe gastroesophageal reflux disease. Laryngoscope. 2001;111:1976-1979.
20. Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol. 2006;4:433-441.
21. Catania RA, Kavic SM, Roth JS, et al. Laparoscopic Nissen fundoplication effectively relieves symptoms in patients with laryngopharyngeal reflux. J Gastrointest Surg. 2007;11:1579-1587.
22. Ogut F, Ersin S, Engin EZ, et al. The effect of laparoscopic Nissen fundoplication on laryngeal findings and voice quality. Surg Endosc. 2007;21:549-554.
23. Sala E, Salminen P, Simberg S, et al. Laryngopharyngeal reflux disease treated with laparoscopic fundoplication. Dig Dis Sci. 2008;53:2397-2404.
24. Koufman JA, Sataloff RT, Toohill R. Laryngopharyngeal reflux: consensus conference report. J Voice. 1996;10:215-216.
• Recommend dietary and behavioral modifications as a first step in treating patients with symptoms suggestive of laryngopharyngeal reflux disease (LPRD). C
• When medications are needed, prescribe a high-dose proton-pump inhibitor, a histamine-2 blocker at bedtime, and prophylactic antacids for reflux-inducing activities, such as exercising and eating. B
• Avoid the rebound effect associated with abrupt cessation of medications prescribed for LPRD with a gradual, 16-week taper. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE When Joan C, a 35-year-old patient whom you’ve known for years, comes in for a physical, you notice that she’s coughing frequently. Upon questioning, Joan says she first noticed the cough several months ago; she also reports that she’s frequently hoarse, but has no other symptoms. Joan is a former smoker, and quit 4 years ago.
If Joan were your patient, would you suspect that she had an upper respiratory infection and prescribe an antibiotic such as azithromycin? Would you include laryngopharyngeal reflux disease in the differential diagnosis?
Laryngopharyngeal reflux disease (LPRD) is a common condition that most primary care physicians encounter frequently. It is also frequently misdiagnosed by clinicians who are unfamiliar with the differences between LPRD and gastroesophageal reflux disease (GERD).
The American Academy of Otolaryngology–Head and Neck Surgery defines laryngopharyngeal reflux as the retrograde movement of gastric contents into the laryngopharynx.1 Common symptoms include hoarseness/dysphonia, chronic throat clearing, dysphagia, globus pharyngeus, and chronic cough, as well as postnasal drip, paroxysmal laryngospasm, odynophagia, excessive throat mucus, and a strange taste in the mouth.2
The diversity and vagueness of these symptoms, as well as the lack of a gold standard diagnostic test for LPRD, make it difficult to estimate its prevalence. In addition, signs of gastroesophageal reflux can be found in the laryngopharynx of up to 86% of healthy individuals, further complicating the clinical picture.3 To avoid missing this often overlooked reflux disease, you need to know how it develops, what signs and symptoms to look for, and which distinguishing features to keep in mind.
Pathophysiology and distinguishing features
The precise way in which LPRD develops is not known, but there are 2 proposed means of laryngeal injury—direct and indirect. In the first case, chemical irritants in the gastric refluxate enter the laryngopharynx and cause local mucosal injury. In the second, gastric reflux irritates the esophageal tissue enough to evoke laryngeal reflexes without ever reaching the larynx—a vagally mediated response associated with symptoms such as chronic cough, throat-clearing sensations, and bronchoconstriction.4
Unlike the esophageal lining, laryngeal epithelium is not protected against chemical injury from gastric acid, as it lacks both the stripping motion of esophageal peristalsis and the neutralizing bicarbonate in saliva.4 Thus, while far smaller amounts of gastric reflux make it into the laryngopharynx, the acid remains there longer and may cause greater injury.5 In some cases, this occurs as often as 50 times a day, although as few as 3 episodes per week have been known to cause LPRD.5
Heartburn is not the rule
Heartburn is a primary complaint of patients with GERD. It is reported by little more than a third (35%) of those with LPRD,5,6 however, (which is why it is sometimes called the “silent” reflux disease). This is because heartburn is caused by esophagitis due to esophageal dysmotility and lower esophageal sphincter dysfunction,3 while most patients with LPRD have normal esophageal motor function and upper esophageal sphincter dysfunction. The fact that only a minimal amount of reflux enters the laryngopharynx may be part of the reason heartburn is less likely in patients with LPRD.
Onset of symptoms. When reflux occurs is another thing that distinguishes LPRD and GERD. Symptoms of GERD typically worsen when the individual is supine, while laryngopharyngeal reflux usually occurs when he or she is upright.7 The frequency with which these 2 conditions overlap is debatable, as there are few studies differentiating LPRD and GERD based on standardized signs and symptoms.7
Making sense of signs and symptoms
Most patients with LPRD seek treatment from their primary care physician, typically reporting symptoms that they don’t associate with gastric reflux, such as hoarseness, a chronic cough or sore throat, or the sensation of a lump in the throat (TABLE 1). Less common manifestations include “water brash”—excessive mucus in the mouth caused by a release of salivary bicarbonate to help neutralize acidity8—otitis media, sinus disease, and dental caries.5
Laryngeal endoscopy may reveal many changes from diffuse irritation. Diffuse erythema, edema, and interarytenoid hypertrophy/cobblestoning are the most useful findings for an LPRD diagnosis.9,10 But in most cases, only a few nonspecific signs with a number of possible causes (infection, environmental irritants, allergies, temperature/climate change, among others) are seen on endoscopic examination, with little correlation with symptom severity. In fact, 74% of otolaryngologists responding to a recent survey said they relied more on patient symptoms than on laryngeal signs for an LPRD diagnosis.10
The Reflux Finding Score (RFS), available at http://www.nature.com/gimo/contents/pt1/fig_tab/gimo46_T3.html, is a clinical tool developed to quantify laryngeal inflammation and standardize objective endoscopic findings. The RFS incorporates the following endolaryngeal signs:
- subglottic edema
- ventricular obliteration
- erythema/hyperemia
- vocal cord edema
- diffuse laryngeal edema
- posterior commissure hypertrophy
- granuloma/granulation tissue
- thick endolaryngeal mucus.
A numeric value is assigned to each, based on whether it is present or absent; partial or complete; local or diffuse; or mild or severe. However, the RFS, too, is an imperfect tool. Clinicians who have used the RFS report that a score higher than 7 identifies LPRD with 95% sensitivity.11 But laryngeal findings may be due to other causes, such as infection, autoimmune reaction, or even allergies, and studies have found the RFS to have poor specificity and inter-rater reliability.12-14
Ambulatory dual probe pH monitoring was considered to be the gold standard test for LPRD at one time, but newer studies have raised questions about its validity and usefulness, especially in patients taking proton-pump inhibitors (PPIs).1,5,7 Newer advanced probes featuring less invasive data collection and greater sensitivity are under development. Ambulatory 24-hour multichannel intraluminal impedance with pH monitoring is the most promising new diagnostic tool, as it can monitor both acidic and nonacidic reflux and distinguish between gas and liquid.15
TABLE 1
When to suspect laryngopharyngeal reflux disease1,5,24
| Finding | Frequency among patients with LPRD (%)* |
|---|---|
| Dysphonia/hoarseness (intermittent) | 71 |
| Chronic cough | 51 |
| Globus pharyngeus | 47 |
| Chronic throat clearing | 42 |
| Dysphagia | 35 |
| Heartburn | 35 |
| *The frequency of other symptoms associated with LPRD is not known. | |
Treatment, like diagnosis, is not clear-cut
LPRD is often called a diagnosis of exclusion, because of the nonspecific nature of its signs and symptoms and the importance of considering a range of other etiologies. The differential diagnosis includes excessive voice use, postnasal drip, upper respiratory infection, habitual throat clearing, allergic rhinitis, environmental irritants, temperature/climate change, chronic or episodic use of alcohol and/or tobacco, and psychological problems related to tics, such as habitual throat clearing or coughing.5
Diagnosis is often based on an empiric trial of high-dose PPIs, with confirmation dependent on symptom relief. Because there have been few placebo-controlled trials with PPIs and those that have been completed had conflicting results, diagnosis based on a combination of medical history and endoscopic laryngeal examination may be a better approach.16,17
Acid suppression therapy with either PPIs or histamine-2 (H2) receptor blockers such as ranitidine or famotidine is the mainstay of treatment for LPRD. But medical societies offer conflicting advice. The American Gastroenterological Association cautions clinicians not to prescribe acid-suppression therapy for patients with LPRD unless they also have GERD.6 The American Academy of Otolaryngology–Head and Neck Surgery recommends twice-daily PPI use for ≥6 months.1,13 The general consensus, based on clinical experience alone, is that patients should be treated with high doses of PPIs (eg, 40 mg omeprazole twice a day) for ≥6 months, with the addition of an H2 receptor blocker to help reduce overnight acid production.1,18 Prophylactic antacid use is also recommended in anticipation of reflux, such as before exercising and right after a meal.
Symptoms should start to improve within 6 to 8 weeks, and patients should be reassessed in about 3 months. To avoid a rebound effect from the abrupt cessation of medications, we suggest a gradual taper over 16 weeks. For the first 8 weeks, the H2 blocker should be discontinued and the PPI decreased from twice a day to once. If symptoms are still controlled, the PPI dose can be reduced to once every other day for another 8 weeks, then stopped if symptoms do not recur.18
Lifestyle and dietary changes (TABLE 2), such as smoking cessation, weight loss, and avoidance of alcohol, are an important part of LPRD treatment, and may be used as a first-line therapy before prescribing medication.19 In fact, some studies have found PPI therapy to be inferior to behavioral/lifestyle modifications.17
Fundoplication surgery, a procedure in which the gastric fundus of the stomach is wrapped around the lower end of the esophagus and stitched in place to prevent reflux, may be an option for patients who do not respond to, or cannot tolerate, aggressive medical treatment for LPRD. A 2006 prospective controlled study found that surgical fundoplication did not consistently relieve laryngeal symptoms.20 But other studies have found that a carefully selected population with medically unresponsive laryngopharyngeal symptoms can benefit from this procedure.21,22 One study showed a significant improvement within one month of fundoplication, with continued improvement observed during a 3-year follow-up.21 In another prospective study, researchers showed that while LPRD-related laryngeal symptoms such as coughing and throat-clearing improved with both medical therapy and laparoscopic fundoplication, voice quality and endoscopic laryngeal/pharyngeal findings improved significantly only with the surgical procedure.23
TABLE 2
Recommend these lifestyle modifications19
| Stop smoking |
Avoid:
|
| Eat smaller, more frequent meals |
| Avoid eating within 3 hours of bedtime |
| Lose weight |
CORRESPONDENCE
Shoib Sana, DO, Detroit Medical Center, Otolaryngology-Head and Neck Surgery, 6533 East Jefferson Avenue, Apartment 316, Detroit, MI 48207; [email protected]
• Recommend dietary and behavioral modifications as a first step in treating patients with symptoms suggestive of laryngopharyngeal reflux disease (LPRD). C
• When medications are needed, prescribe a high-dose proton-pump inhibitor, a histamine-2 blocker at bedtime, and prophylactic antacids for reflux-inducing activities, such as exercising and eating. B
• Avoid the rebound effect associated with abrupt cessation of medications prescribed for LPRD with a gradual, 16-week taper. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE When Joan C, a 35-year-old patient whom you’ve known for years, comes in for a physical, you notice that she’s coughing frequently. Upon questioning, Joan says she first noticed the cough several months ago; she also reports that she’s frequently hoarse, but has no other symptoms. Joan is a former smoker, and quit 4 years ago.
If Joan were your patient, would you suspect that she had an upper respiratory infection and prescribe an antibiotic such as azithromycin? Would you include laryngopharyngeal reflux disease in the differential diagnosis?
Laryngopharyngeal reflux disease (LPRD) is a common condition that most primary care physicians encounter frequently. It is also frequently misdiagnosed by clinicians who are unfamiliar with the differences between LPRD and gastroesophageal reflux disease (GERD).
The American Academy of Otolaryngology–Head and Neck Surgery defines laryngopharyngeal reflux as the retrograde movement of gastric contents into the laryngopharynx.1 Common symptoms include hoarseness/dysphonia, chronic throat clearing, dysphagia, globus pharyngeus, and chronic cough, as well as postnasal drip, paroxysmal laryngospasm, odynophagia, excessive throat mucus, and a strange taste in the mouth.2
The diversity and vagueness of these symptoms, as well as the lack of a gold standard diagnostic test for LPRD, make it difficult to estimate its prevalence. In addition, signs of gastroesophageal reflux can be found in the laryngopharynx of up to 86% of healthy individuals, further complicating the clinical picture.3 To avoid missing this often overlooked reflux disease, you need to know how it develops, what signs and symptoms to look for, and which distinguishing features to keep in mind.
Pathophysiology and distinguishing features
The precise way in which LPRD develops is not known, but there are 2 proposed means of laryngeal injury—direct and indirect. In the first case, chemical irritants in the gastric refluxate enter the laryngopharynx and cause local mucosal injury. In the second, gastric reflux irritates the esophageal tissue enough to evoke laryngeal reflexes without ever reaching the larynx—a vagally mediated response associated with symptoms such as chronic cough, throat-clearing sensations, and bronchoconstriction.4
Unlike the esophageal lining, laryngeal epithelium is not protected against chemical injury from gastric acid, as it lacks both the stripping motion of esophageal peristalsis and the neutralizing bicarbonate in saliva.4 Thus, while far smaller amounts of gastric reflux make it into the laryngopharynx, the acid remains there longer and may cause greater injury.5 In some cases, this occurs as often as 50 times a day, although as few as 3 episodes per week have been known to cause LPRD.5
Heartburn is not the rule
Heartburn is a primary complaint of patients with GERD. It is reported by little more than a third (35%) of those with LPRD,5,6 however, (which is why it is sometimes called the “silent” reflux disease). This is because heartburn is caused by esophagitis due to esophageal dysmotility and lower esophageal sphincter dysfunction,3 while most patients with LPRD have normal esophageal motor function and upper esophageal sphincter dysfunction. The fact that only a minimal amount of reflux enters the laryngopharynx may be part of the reason heartburn is less likely in patients with LPRD.
Onset of symptoms. When reflux occurs is another thing that distinguishes LPRD and GERD. Symptoms of GERD typically worsen when the individual is supine, while laryngopharyngeal reflux usually occurs when he or she is upright.7 The frequency with which these 2 conditions overlap is debatable, as there are few studies differentiating LPRD and GERD based on standardized signs and symptoms.7
Making sense of signs and symptoms
Most patients with LPRD seek treatment from their primary care physician, typically reporting symptoms that they don’t associate with gastric reflux, such as hoarseness, a chronic cough or sore throat, or the sensation of a lump in the throat (TABLE 1). Less common manifestations include “water brash”—excessive mucus in the mouth caused by a release of salivary bicarbonate to help neutralize acidity8—otitis media, sinus disease, and dental caries.5
Laryngeal endoscopy may reveal many changes from diffuse irritation. Diffuse erythema, edema, and interarytenoid hypertrophy/cobblestoning are the most useful findings for an LPRD diagnosis.9,10 But in most cases, only a few nonspecific signs with a number of possible causes (infection, environmental irritants, allergies, temperature/climate change, among others) are seen on endoscopic examination, with little correlation with symptom severity. In fact, 74% of otolaryngologists responding to a recent survey said they relied more on patient symptoms than on laryngeal signs for an LPRD diagnosis.10
The Reflux Finding Score (RFS), available at http://www.nature.com/gimo/contents/pt1/fig_tab/gimo46_T3.html, is a clinical tool developed to quantify laryngeal inflammation and standardize objective endoscopic findings. The RFS incorporates the following endolaryngeal signs:
- subglottic edema
- ventricular obliteration
- erythema/hyperemia
- vocal cord edema
- diffuse laryngeal edema
- posterior commissure hypertrophy
- granuloma/granulation tissue
- thick endolaryngeal mucus.
A numeric value is assigned to each, based on whether it is present or absent; partial or complete; local or diffuse; or mild or severe. However, the RFS, too, is an imperfect tool. Clinicians who have used the RFS report that a score higher than 7 identifies LPRD with 95% sensitivity.11 But laryngeal findings may be due to other causes, such as infection, autoimmune reaction, or even allergies, and studies have found the RFS to have poor specificity and inter-rater reliability.12-14
Ambulatory dual probe pH monitoring was considered to be the gold standard test for LPRD at one time, but newer studies have raised questions about its validity and usefulness, especially in patients taking proton-pump inhibitors (PPIs).1,5,7 Newer advanced probes featuring less invasive data collection and greater sensitivity are under development. Ambulatory 24-hour multichannel intraluminal impedance with pH monitoring is the most promising new diagnostic tool, as it can monitor both acidic and nonacidic reflux and distinguish between gas and liquid.15
TABLE 1
When to suspect laryngopharyngeal reflux disease1,5,24
| Finding | Frequency among patients with LPRD (%)* |
|---|---|
| Dysphonia/hoarseness (intermittent) | 71 |
| Chronic cough | 51 |
| Globus pharyngeus | 47 |
| Chronic throat clearing | 42 |
| Dysphagia | 35 |
| Heartburn | 35 |
| *The frequency of other symptoms associated with LPRD is not known. | |
Treatment, like diagnosis, is not clear-cut
LPRD is often called a diagnosis of exclusion, because of the nonspecific nature of its signs and symptoms and the importance of considering a range of other etiologies. The differential diagnosis includes excessive voice use, postnasal drip, upper respiratory infection, habitual throat clearing, allergic rhinitis, environmental irritants, temperature/climate change, chronic or episodic use of alcohol and/or tobacco, and psychological problems related to tics, such as habitual throat clearing or coughing.5
Diagnosis is often based on an empiric trial of high-dose PPIs, with confirmation dependent on symptom relief. Because there have been few placebo-controlled trials with PPIs and those that have been completed had conflicting results, diagnosis based on a combination of medical history and endoscopic laryngeal examination may be a better approach.16,17
Acid suppression therapy with either PPIs or histamine-2 (H2) receptor blockers such as ranitidine or famotidine is the mainstay of treatment for LPRD. But medical societies offer conflicting advice. The American Gastroenterological Association cautions clinicians not to prescribe acid-suppression therapy for patients with LPRD unless they also have GERD.6 The American Academy of Otolaryngology–Head and Neck Surgery recommends twice-daily PPI use for ≥6 months.1,13 The general consensus, based on clinical experience alone, is that patients should be treated with high doses of PPIs (eg, 40 mg omeprazole twice a day) for ≥6 months, with the addition of an H2 receptor blocker to help reduce overnight acid production.1,18 Prophylactic antacid use is also recommended in anticipation of reflux, such as before exercising and right after a meal.
Symptoms should start to improve within 6 to 8 weeks, and patients should be reassessed in about 3 months. To avoid a rebound effect from the abrupt cessation of medications, we suggest a gradual taper over 16 weeks. For the first 8 weeks, the H2 blocker should be discontinued and the PPI decreased from twice a day to once. If symptoms are still controlled, the PPI dose can be reduced to once every other day for another 8 weeks, then stopped if symptoms do not recur.18
Lifestyle and dietary changes (TABLE 2), such as smoking cessation, weight loss, and avoidance of alcohol, are an important part of LPRD treatment, and may be used as a first-line therapy before prescribing medication.19 In fact, some studies have found PPI therapy to be inferior to behavioral/lifestyle modifications.17
Fundoplication surgery, a procedure in which the gastric fundus of the stomach is wrapped around the lower end of the esophagus and stitched in place to prevent reflux, may be an option for patients who do not respond to, or cannot tolerate, aggressive medical treatment for LPRD. A 2006 prospective controlled study found that surgical fundoplication did not consistently relieve laryngeal symptoms.20 But other studies have found that a carefully selected population with medically unresponsive laryngopharyngeal symptoms can benefit from this procedure.21,22 One study showed a significant improvement within one month of fundoplication, with continued improvement observed during a 3-year follow-up.21 In another prospective study, researchers showed that while LPRD-related laryngeal symptoms such as coughing and throat-clearing improved with both medical therapy and laparoscopic fundoplication, voice quality and endoscopic laryngeal/pharyngeal findings improved significantly only with the surgical procedure.23
TABLE 2
Recommend these lifestyle modifications19
| Stop smoking |
Avoid:
|
| Eat smaller, more frequent meals |
| Avoid eating within 3 hours of bedtime |
| Lose weight |
CORRESPONDENCE
Shoib Sana, DO, Detroit Medical Center, Otolaryngology-Head and Neck Surgery, 6533 East Jefferson Avenue, Apartment 316, Detroit, MI 48207; [email protected]
1. Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35.
2. Papakonstantinou L, Leslie P, Gray J, et al. Laryngopharyngeal reflux: a prospective analysis of a 34 item symptom questionnaire. Clin Otolaryngol. 2009;34:455-459.
3. Hicks DM, Ours TM, Abelson TI, et al. The prevalence of hypopharynx findings associated with gastroesophageal reflux in normal volunteers. J Voice. 2002;16:564.-
4. Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481-491.
5. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24 hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1-78.
6. Kahrilas PJ, Shaheen NJ, Vaezi M, et al. American Gastroenterological Association Institute (AGAI) medical position statement: management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383.-
7. Postma GN, Tomek MS, Belafsky PC, et al. Esophageal motor function in laryngopharyngeal reflux is superior to that in classic gastroesophageal reflux disease. Ann Otol Rhinol Laryngol. 2001;111:1114-1116.
8. Helen JF, Dodds WJ, Hogan WJ. Salivary response to esophageal acid in normal subjects and patients with reflux esophagitis. Gastroenterology. 1998;94:1394-1398.
9. Belafsky PC. Abnormal endoscopic pharyngeal and laryngeal findings attributable to reflux. Am J Med 2003;116(suppl 3A):91S-97S.
10. Ahmed TF, Khandwala F, Abelson, et al. Chronic laryngitis associated with gastroesophageal reflux: prospective assessment of differences in practice patterns between gastroenterologists and ENT physicians. Am J Gastroenterol. 2006;102:470-478.
11. Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111:1313-1317.
12. Koufman JA, Sataloff RT, Toohill R. Laryngopharyngeal reflux: consensus conference report. J Voice. 1996;10:215-216.
13. Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope 2001;111:979-981.
14. Reichel O, Dressel H, Wiederanders K, et al. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2008;139:414-420.
15. Muderris T, Gokcan MK, Yorulmaz I. The clinical value of pharyngeal pH monitoring using a double-probe, triple-sensor catheter in patients with laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg. 2009;135:163-167.
16. Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131:342-350.
17. Wo JM, Koopman J, Harrell SP, et al. Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol. 2006;101:1972-1978.
18. Park W, Hicks DM, Khandwala F, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005;116:1230-1238.
19. Maceri DR, Zim S. Laryngospasm: an atypical manifestation of severe gastroesophageal reflux disease. Laryngoscope. 2001;111:1976-1979.
20. Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol. 2006;4:433-441.
21. Catania RA, Kavic SM, Roth JS, et al. Laparoscopic Nissen fundoplication effectively relieves symptoms in patients with laryngopharyngeal reflux. J Gastrointest Surg. 2007;11:1579-1587.
22. Ogut F, Ersin S, Engin EZ, et al. The effect of laparoscopic Nissen fundoplication on laryngeal findings and voice quality. Surg Endosc. 2007;21:549-554.
23. Sala E, Salminen P, Simberg S, et al. Laryngopharyngeal reflux disease treated with laparoscopic fundoplication. Dig Dis Sci. 2008;53:2397-2404.
24. Koufman JA, Sataloff RT, Toohill R. Laryngopharyngeal reflux: consensus conference report. J Voice. 1996;10:215-216.
1. Koufman JA, Aviv JE, Casiano RR, et al. Laryngopharyngeal reflux: position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32-35.
2. Papakonstantinou L, Leslie P, Gray J, et al. Laryngopharyngeal reflux: a prospective analysis of a 34 item symptom questionnaire. Clin Otolaryngol. 2009;34:455-459.
3. Hicks DM, Ours TM, Abelson TI, et al. The prevalence of hypopharynx findings associated with gastroesophageal reflux in normal volunteers. J Voice. 2002;16:564.-
4. Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol. 2003;112:481-491.
5. Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24 hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1-78.
6. Kahrilas PJ, Shaheen NJ, Vaezi M, et al. American Gastroenterological Association Institute (AGAI) medical position statement: management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1383.-
7. Postma GN, Tomek MS, Belafsky PC, et al. Esophageal motor function in laryngopharyngeal reflux is superior to that in classic gastroesophageal reflux disease. Ann Otol Rhinol Laryngol. 2001;111:1114-1116.
8. Helen JF, Dodds WJ, Hogan WJ. Salivary response to esophageal acid in normal subjects and patients with reflux esophagitis. Gastroenterology. 1998;94:1394-1398.
9. Belafsky PC. Abnormal endoscopic pharyngeal and laryngeal findings attributable to reflux. Am J Med 2003;116(suppl 3A):91S-97S.
10. Ahmed TF, Khandwala F, Abelson, et al. Chronic laryngitis associated with gastroesophageal reflux: prospective assessment of differences in practice patterns between gastroenterologists and ENT physicians. Am J Gastroenterol. 2006;102:470-478.
11. Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111:1313-1317.
12. Koufman JA, Sataloff RT, Toohill R. Laryngopharyngeal reflux: consensus conference report. J Voice. 1996;10:215-216.
13. Belafsky PC, Postma GN, Koufman JA. Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope 2001;111:979-981.
14. Reichel O, Dressel H, Wiederanders K, et al. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2008;139:414-420.
15. Muderris T, Gokcan MK, Yorulmaz I. The clinical value of pharyngeal pH monitoring using a double-probe, triple-sensor catheter in patients with laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg. 2009;135:163-167.
16. Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131:342-350.
17. Wo JM, Koopman J, Harrell SP, et al. Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol. 2006;101:1972-1978.
18. Park W, Hicks DM, Khandwala F, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005;116:1230-1238.
19. Maceri DR, Zim S. Laryngospasm: an atypical manifestation of severe gastroesophageal reflux disease. Laryngoscope. 2001;111:1976-1979.
20. Swoger J, Ponsky J, Hicks DM, et al. Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol. 2006;4:433-441.
21. Catania RA, Kavic SM, Roth JS, et al. Laparoscopic Nissen fundoplication effectively relieves symptoms in patients with laryngopharyngeal reflux. J Gastrointest Surg. 2007;11:1579-1587.
22. Ogut F, Ersin S, Engin EZ, et al. The effect of laparoscopic Nissen fundoplication on laryngeal findings and voice quality. Surg Endosc. 2007;21:549-554.
23. Sala E, Salminen P, Simberg S, et al. Laryngopharyngeal reflux disease treated with laparoscopic fundoplication. Dig Dis Sci. 2008;53:2397-2404.
24. Koufman JA, Sataloff RT, Toohill R. Laryngopharyngeal reflux: consensus conference report. J Voice. 1996;10:215-216.
Missed aortic aneurysm proves fatal ... Too-late cancer Dx blamed on neglected x-ray findings... More
Missed dissecting aortic aneurysm proves fatal
A 43-YEAR-OLD MAN was admitted to the hospital complaining of severe chest pain, shortness of breath, sweating, and dry mouth. After being seen by several physicians, the patient suffered an aortic dissection, which caused bleeding in the wall of the aorta, an aortic rupture, and bleeding into the pericardium. He died 2 days later.
PLAINTIFF’S CLAIM The defendants failed to order tests to rule out a dissecting aortic aneurysm and did not include aortic dissection in the differential diagnosis. They failed to provide appropriate drug therapy to decrease cardiac impulse and lower the systolic blood pressure. They did not obtain an emergency cardiac consultation or admit the patient to a cardiovascular surgical intensive care unit.
THE DEFENSE The defendants denied negligence and claimed that nothing they did or failed to do contributed to the patient’s death.
VERDICT $250,000 Michigan settlement.
COMMENT Just yesterday, a malpractice lawyer presented me with a case very similar to this one: a patient with unexplained chest pain who died of a dissecting aneurysm. Remember, not all chest pain is caused by coronary artery disease.
Too-late cancer Dx blamed on neglected x-ray findings
A LONG-TERM CIGARETTE SMOKER IN HER 50s saw a physician in 2001 for symptoms of pneumonia. The doctor prescribed antibiotics and referred her to another facility for a chest radiograph.
Five days later, she returned to the physician’s office, where she was seen by another internist in the practice. The internist noted that the chest radiograph showed parenchymal densities in the right lung. Parenchymal densities had also showed up on 2 previous chest radiographs, but were more prevalent on the latest film. The internist advised the patient to finish her antibiotic regimen; he did not prescribe further tests or treatment.
Over the following 40 months, doctors in the patient’s medical group examined her 8 times. Each time she complained of impaired respiration. The internist believed that the symptoms were caused by asthma.
In 2004, the patient was diagnosed with stage IV cancer of the right lung, which had spread to her bones and was untreatable. She died several weeks later.
PLAINTIFF’S CLAIM A proper diagnosis in 2001 would have allowed the cancer to be cured. A computed tomography scan should have been performed and a pulmonologist consulted at that time.
THE DEFENSE Findings from the radiograph from 2001 did not necessitate further action. Because the patient’s cancer had metastasized before that radiograph, treatment then (or later) would not have changed the outcome.
VERDICT $850,000 New York verdict.
COMMENT Careful follow-up and diagnosis of chest radiograph abnormalities is paramount.
Yes, it was a stroke
WEAKNESS, NUMBNESS, AND TINGLING IN HIS RIGHT ARM prompted a 56-year-old man to visit his primary care physician. The physician sent the patient to the emergency department (ED) for testing because he believed the man was experiencing stroke-like symptoms. As the patient and his wife drove to the hospital, the physician faxed the patient’s medical records to the ED.
When the patient’s wife tried to give ED employees the physician’s orders for tests and tell them of the doctor’s concern about a stroke, they told her that all the beds were full and she should sit down and wait.
The patient was eventually evaluated as a low-priority patient with numbness in his right hand. The examining doctor ordered radiographs of the right wrist and discharged the patient with a diagnosis of carpal tunnel syndrome.
Twenty minutes later, a nurse left a message telling the patient to return to the hospital for the stroke-related tests that had been ordered by his primary care physician. An ED physician other than the one who first examined the patient performed the tests—except for a test of blood flow to the brain. The physician diagnosed stroke-like symptoms and requested a consultation with another physician, which never happened. The patient was discharged about 6 hours after his first discharge.
About 16 hours later, the patient suffered a stroke. Subsequent testing revealed an obstruction in the left carotid artery. The stroke resulted in permanent neurologic injury.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE The defendants denied negligence and disputed the extent of the patient’s injuries.
VERDICT $1.123 million Maryland verdict.
COMMENT Coordination of care remains critical, particularly between our outpatient offices and the busy ED.
Missed dissecting aortic aneurysm proves fatal
A 43-YEAR-OLD MAN was admitted to the hospital complaining of severe chest pain, shortness of breath, sweating, and dry mouth. After being seen by several physicians, the patient suffered an aortic dissection, which caused bleeding in the wall of the aorta, an aortic rupture, and bleeding into the pericardium. He died 2 days later.
PLAINTIFF’S CLAIM The defendants failed to order tests to rule out a dissecting aortic aneurysm and did not include aortic dissection in the differential diagnosis. They failed to provide appropriate drug therapy to decrease cardiac impulse and lower the systolic blood pressure. They did not obtain an emergency cardiac consultation or admit the patient to a cardiovascular surgical intensive care unit.
THE DEFENSE The defendants denied negligence and claimed that nothing they did or failed to do contributed to the patient’s death.
VERDICT $250,000 Michigan settlement.
COMMENT Just yesterday, a malpractice lawyer presented me with a case very similar to this one: a patient with unexplained chest pain who died of a dissecting aneurysm. Remember, not all chest pain is caused by coronary artery disease.
Too-late cancer Dx blamed on neglected x-ray findings
A LONG-TERM CIGARETTE SMOKER IN HER 50s saw a physician in 2001 for symptoms of pneumonia. The doctor prescribed antibiotics and referred her to another facility for a chest radiograph.
Five days later, she returned to the physician’s office, where she was seen by another internist in the practice. The internist noted that the chest radiograph showed parenchymal densities in the right lung. Parenchymal densities had also showed up on 2 previous chest radiographs, but were more prevalent on the latest film. The internist advised the patient to finish her antibiotic regimen; he did not prescribe further tests or treatment.
Over the following 40 months, doctors in the patient’s medical group examined her 8 times. Each time she complained of impaired respiration. The internist believed that the symptoms were caused by asthma.
In 2004, the patient was diagnosed with stage IV cancer of the right lung, which had spread to her bones and was untreatable. She died several weeks later.
PLAINTIFF’S CLAIM A proper diagnosis in 2001 would have allowed the cancer to be cured. A computed tomography scan should have been performed and a pulmonologist consulted at that time.
THE DEFENSE Findings from the radiograph from 2001 did not necessitate further action. Because the patient’s cancer had metastasized before that radiograph, treatment then (or later) would not have changed the outcome.
VERDICT $850,000 New York verdict.
COMMENT Careful follow-up and diagnosis of chest radiograph abnormalities is paramount.
Yes, it was a stroke
WEAKNESS, NUMBNESS, AND TINGLING IN HIS RIGHT ARM prompted a 56-year-old man to visit his primary care physician. The physician sent the patient to the emergency department (ED) for testing because he believed the man was experiencing stroke-like symptoms. As the patient and his wife drove to the hospital, the physician faxed the patient’s medical records to the ED.
When the patient’s wife tried to give ED employees the physician’s orders for tests and tell them of the doctor’s concern about a stroke, they told her that all the beds were full and she should sit down and wait.
The patient was eventually evaluated as a low-priority patient with numbness in his right hand. The examining doctor ordered radiographs of the right wrist and discharged the patient with a diagnosis of carpal tunnel syndrome.
Twenty minutes later, a nurse left a message telling the patient to return to the hospital for the stroke-related tests that had been ordered by his primary care physician. An ED physician other than the one who first examined the patient performed the tests—except for a test of blood flow to the brain. The physician diagnosed stroke-like symptoms and requested a consultation with another physician, which never happened. The patient was discharged about 6 hours after his first discharge.
About 16 hours later, the patient suffered a stroke. Subsequent testing revealed an obstruction in the left carotid artery. The stroke resulted in permanent neurologic injury.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE The defendants denied negligence and disputed the extent of the patient’s injuries.
VERDICT $1.123 million Maryland verdict.
COMMENT Coordination of care remains critical, particularly between our outpatient offices and the busy ED.
Missed dissecting aortic aneurysm proves fatal
A 43-YEAR-OLD MAN was admitted to the hospital complaining of severe chest pain, shortness of breath, sweating, and dry mouth. After being seen by several physicians, the patient suffered an aortic dissection, which caused bleeding in the wall of the aorta, an aortic rupture, and bleeding into the pericardium. He died 2 days later.
PLAINTIFF’S CLAIM The defendants failed to order tests to rule out a dissecting aortic aneurysm and did not include aortic dissection in the differential diagnosis. They failed to provide appropriate drug therapy to decrease cardiac impulse and lower the systolic blood pressure. They did not obtain an emergency cardiac consultation or admit the patient to a cardiovascular surgical intensive care unit.
THE DEFENSE The defendants denied negligence and claimed that nothing they did or failed to do contributed to the patient’s death.
VERDICT $250,000 Michigan settlement.
COMMENT Just yesterday, a malpractice lawyer presented me with a case very similar to this one: a patient with unexplained chest pain who died of a dissecting aneurysm. Remember, not all chest pain is caused by coronary artery disease.
Too-late cancer Dx blamed on neglected x-ray findings
A LONG-TERM CIGARETTE SMOKER IN HER 50s saw a physician in 2001 for symptoms of pneumonia. The doctor prescribed antibiotics and referred her to another facility for a chest radiograph.
Five days later, she returned to the physician’s office, where she was seen by another internist in the practice. The internist noted that the chest radiograph showed parenchymal densities in the right lung. Parenchymal densities had also showed up on 2 previous chest radiographs, but were more prevalent on the latest film. The internist advised the patient to finish her antibiotic regimen; he did not prescribe further tests or treatment.
Over the following 40 months, doctors in the patient’s medical group examined her 8 times. Each time she complained of impaired respiration. The internist believed that the symptoms were caused by asthma.
In 2004, the patient was diagnosed with stage IV cancer of the right lung, which had spread to her bones and was untreatable. She died several weeks later.
PLAINTIFF’S CLAIM A proper diagnosis in 2001 would have allowed the cancer to be cured. A computed tomography scan should have been performed and a pulmonologist consulted at that time.
THE DEFENSE Findings from the radiograph from 2001 did not necessitate further action. Because the patient’s cancer had metastasized before that radiograph, treatment then (or later) would not have changed the outcome.
VERDICT $850,000 New York verdict.
COMMENT Careful follow-up and diagnosis of chest radiograph abnormalities is paramount.
Yes, it was a stroke
WEAKNESS, NUMBNESS, AND TINGLING IN HIS RIGHT ARM prompted a 56-year-old man to visit his primary care physician. The physician sent the patient to the emergency department (ED) for testing because he believed the man was experiencing stroke-like symptoms. As the patient and his wife drove to the hospital, the physician faxed the patient’s medical records to the ED.
When the patient’s wife tried to give ED employees the physician’s orders for tests and tell them of the doctor’s concern about a stroke, they told her that all the beds were full and she should sit down and wait.
The patient was eventually evaluated as a low-priority patient with numbness in his right hand. The examining doctor ordered radiographs of the right wrist and discharged the patient with a diagnosis of carpal tunnel syndrome.
Twenty minutes later, a nurse left a message telling the patient to return to the hospital for the stroke-related tests that had been ordered by his primary care physician. An ED physician other than the one who first examined the patient performed the tests—except for a test of blood flow to the brain. The physician diagnosed stroke-like symptoms and requested a consultation with another physician, which never happened. The patient was discharged about 6 hours after his first discharge.
About 16 hours later, the patient suffered a stroke. Subsequent testing revealed an obstruction in the left carotid artery. The stroke resulted in permanent neurologic injury.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE The defendants denied negligence and disputed the extent of the patient’s injuries.
VERDICT $1.123 million Maryland verdict.
COMMENT Coordination of care remains critical, particularly between our outpatient offices and the busy ED.