User login
Looking beyond the D-dimer
A 44-year-old woman sought care at the emergency department (ED) because she was having difficulty breathing and felt faint. She had been fine until that morning. Three days earlier the patient, who had a history of high blood pressure and elevated cholesterol levels, had driven from Connecticut to New York and back, spending a total of 4 hours in her car. The patient indicated that she’d been taking oral contraceptives (OCPs) for several years, but she did not smoke. There was no history of hemoptysis, recent surgery, or trauma. Neither blood clots nor cancer were part of her or her family’s history.
In the ED, the patient did not have any signs or symptoms of a deep venous thrombosis (DVT). She was obese, with a body mass index of 40.3 kg/m2; other vitals were: blood pressure (BP), 134/88 mm Hg; heart rate (HR), 64 beats per minute (bpm); respiratory rate (RR), 12; and O2 saturation, 99% with ambulation.
The ED physician strongly suspected a pulmonary embolism (PE), but the patient’s score on a clinical probability algorithm (using the Wells criteria) was a 3, indicating only “moderate probability“ of a PE (TABLE 1). (She scored a 3 because an “alternative diagnosis [was] less likely than PE.”) In addition, her D-dimer level was 160 ng/mL using the Triage D-Dimer Test by Biosite, Inc (normal <400 ng/mL), which ruled out a PE. (Many ED physicians at our institution are more cautious when using this D-dimer assay and use a lower cutoff value.)
Given these results, the ED physician did not order imaging studies because the expense and radiation exposure outweighed the probability of the patient having a PE. A subsequent coronary work-up was also negative. The patient was discharged to home and advised to follow up with her primary care physician a few days later.
Two days later we saw the patient at our office. Not only had her dyspnea gotten worse while the presyncope remained, but she now had left-sided pleuritic chest pain. She also reported mild pain in her right calf. On examination, the patient’s BP was 126/86 mm Hg, HR was 82 bpm, RR was 12, and O2 saturation was 96% with ambulation. Her Wells score was now 6, still a moderate probability for PE. (She received another 3 points for the new DVT symptoms—“clinically suspected DVT.”)
Although the patient did not also have signs of a DVT, her additional symptoms along with the original symptoms’ persistence and the existence of other risk factors (OCP use and obesity) led us to reconsider a PE diagnosis. These suspicions prompted us to send the patient back to the ED, where a Doppler ultrasound of the right lower extremity was negative, but the D-dimer was positive at 565 ng/mL.
A pulmonary computed tomography angiogram (CTA) showed 2 small pulmonary emboli within the distal left upper lobe pulmonary arteries.
The patient was treated with heparin and warfarin and discharged without complications.
TABLE 1
Calculating and interpreting the Wells score4,5,7,9,10
| Clinical parameter | Points |
|---|---|
| Clinically suspected DVT | 3.0 |
| Alternative diagnosis less likely than PE | 3.0 |
| Tachycardia | 1.5 |
| Immobilization/surgery (within 4 weeks) | 1.5 |
| History of DVT or PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy (treatment within 6 months, palliative) | 1.0 |
| TOTAL | |
| Score | Traditional interpretation |
| <2.0 | Low probability of PE |
| 2.0-6.0 | Moderate probability of PE |
| >6.0 | High probability of PE |
| Score | Alternative classification scheme |
| ≤4.0 | PE unlikely |
| >4.0 | PE likely |
| DVT, deep venous thrombosis; PE, pulmonary embolism. | |
Discussion
The incidence of PE in the United States varies significantly: Individuals younger than 40 have a risk of 1 in 10,000 compared with 1 in 100 for those older than 80.1 Mortality associated with undiagnosed PE varies widely, from 9.2% to 51%.2 This percentage is significant given that half of all PEs go undiagnosed.3 In addition, when left untreated, PE will recur in 30% to 50% of patients, with a fatality rate of 10% to 45%.1 Further, up to 4% of patients with acute PE develop chronic PE and subsequent pulmonary hypertension.4,5 Given the consequences of failing to diagnose a PE, clinicians must consider this condition in patients who present with unexplained hypotension, dyspnea, or chest pain.6
Not an easy diagnosis
This case report demonstrates the inherent difficulty in diagnosing a PE. Still, certain clinical symptoms/signs can aid in the decision-making process. Fever, crackles, and wheezes decrease the probability of PE, whereas syncope, hemodynamic shock, leg edema, and hemoptysis increase its likelihood.7 Despite the many commonly reported risk factors for PE, only malignancy, recent surgery, or a history of DVT/PE significantly increase the risk of developing a clot.8
The Wells criteria. This scoring system groups patients according to the probability of having a PE: low (score: <2), moderate (score: 2-6), and high (score: >6).6 An alternative classification scheme divides patients into 2 groups: likely to have a PE (score: >4) or unlikely to have a PE (score: ≤4).8
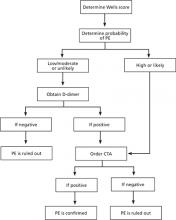
This case report illustrates a key problem with the Wells criteria—the somewhat subjective nature of the scoring. Some physicians find it questionable to award 3 points for “alternative diagnosis less likely than PE,” for example.4 Similarly, with respect to immobilization, some clinicians might have awarded our patient 1.5 points for her recent car trip to New York. We did not think that riding in a car for 2 uninterrupted hours for each leg of the trip was significant enough. However, awarding this patient 1.5 points could have made an important difference in her clinical management if the alternative classification scheme was used. Instead of having a score of 3, the patient would have had a score of 4.5, placing her in the “likely to have a PE” group and prompting us to perform a CTA sooner (FIGURE).
FIGURE
Diagnostic algorithm for pulmonary embolism6,7,10
CTA, computed tomography angiogram; PE, pulmonary embolism.
Inappropriate work-ups are common
Some physicians ignore algorithms when working up a PE and simply order a CTA. In fact, a large multicenter trial showed that 43% of patients suspected of having a PE were inappropriately managed diagnostically.9 Similarly, a meta-analysis of 4 studies including 1660 patients found that only 58% of those with a positive D-dimer had the requisite CTA, as did 7% of patients with a negative D-dimer.2
Physicians should not be concerned about ruling out a PE in the setting of a negative D-dimer, as a meta-analysis found that this diagnostic approach has a negative predictive value (NPV) of 99.7%.2 It is important to note that the NPV is significantly affected by the sensitivity of the D-dimer assay used. If the D-dimer assay is highly sensitive, a negative result in combination with a low, moderate, or unlikely probability Wells score rules out the diagnosis of PE. If the assay is moderately sensitive, however, only a low or unlikely probability Wells score rules out PE.10
The inappropriate work-up of this group of patients is significant and extends beyond the ultimate goal of preventing morbidity and mortality. The unnecessary use of pulmonary CTA is extremely expensive, exposes patients to unnecessary radiation, and results in contrast nephrotoxicity in about 4% of patients.9 Although pulmonary CTA is the standard diagnostic test for PE, other imaging modalities are more appropriate in some cases (TABLE 2).
TABLE 2
Alternative imaging modalities for diagnosing PE1,4,7,11
| Modality | Indication |
|---|---|
| Ventilation-perfusion scanning | Patients with contrast allergies or renal failure; test of choice for diagnosing chronic PE due to limited sensitivity of CT |
| Venous compression ultrasonography | Patients with symptoms of PE and signs/symptoms of DVT |
| Pulmonary angiography | Most invasive test. Should be used only in patients with high probability of PE who may need vascular intervention |
| CT, computed tomography; DVT, deep venous thrombosis; PE, pulmonary embolism. | |
The bottom line
This case report illustrates the importance of using sound clinical judgment when diagnosing a PE. Although our patient initially had a moderate probability Wells score and a negative D-dimer, her symptoms persisted. Her history of OCP use, persistent dyspnea, and new symptoms of a DVT prompted us to reinitiate the diagnostic algorithm and eventually diagnose a PE.
It is always essential to treat the patient and not simply react to laboratory values. To avoid unnecessary testing, however, adhering to the algorithm is equally important.
CORRESPONDENCE
Michael S. Kelleher, MD, University of Connecticut School of Medicine, 263 Farmington Avenue, Farmington, CT 06030; [email protected]
1. Meyer G, Roy PM, Gilberg S, et al. Pulmonary embolism. BMJ. 2010;340:1421.-
2. Pasha SM, Kiok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
3. Taira T, Taira BR, Carmen M, et al. Risk of venous thromboembolism in patients with borderline quantitative D-dimer levels. Am J Emerg Med. 2010;28:450-453.
4. Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. 2010;15:399-406.
5. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
6. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266-274.
7. Gandara E, Wells PS. Diagnosis: use of clinical probability algorithms. Clin Chest Med. 2010;31:629-639.
8. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57:613-621.
9. Gimber LH, Travis RI, Takahashi JM, et al. Computed tomography angiography in patients evaluated for acute pulmonary embolism with low serum D-dimer levels: a prospective study. Perm J. 2009;13:4-10.
10. Agency for Healthcare Research and Quality. Guidelines on the diagnosis and management of acute pulmonary embolism. Available at: http://www.guideline.gov/content.aspx?id=13410#Section420. Accessed February 12, 2011.
11. Kim NH. Chronic thromboembolic pulmonary hypertension: diagnosis. Medscape. Available at: http://www.medscape.org/viewarticle/556058_3. Accessed May 9, 2011.
A 44-year-old woman sought care at the emergency department (ED) because she was having difficulty breathing and felt faint. She had been fine until that morning. Three days earlier the patient, who had a history of high blood pressure and elevated cholesterol levels, had driven from Connecticut to New York and back, spending a total of 4 hours in her car. The patient indicated that she’d been taking oral contraceptives (OCPs) for several years, but she did not smoke. There was no history of hemoptysis, recent surgery, or trauma. Neither blood clots nor cancer were part of her or her family’s history.
In the ED, the patient did not have any signs or symptoms of a deep venous thrombosis (DVT). She was obese, with a body mass index of 40.3 kg/m2; other vitals were: blood pressure (BP), 134/88 mm Hg; heart rate (HR), 64 beats per minute (bpm); respiratory rate (RR), 12; and O2 saturation, 99% with ambulation.
The ED physician strongly suspected a pulmonary embolism (PE), but the patient’s score on a clinical probability algorithm (using the Wells criteria) was a 3, indicating only “moderate probability“ of a PE (TABLE 1). (She scored a 3 because an “alternative diagnosis [was] less likely than PE.”) In addition, her D-dimer level was 160 ng/mL using the Triage D-Dimer Test by Biosite, Inc (normal <400 ng/mL), which ruled out a PE. (Many ED physicians at our institution are more cautious when using this D-dimer assay and use a lower cutoff value.)
Given these results, the ED physician did not order imaging studies because the expense and radiation exposure outweighed the probability of the patient having a PE. A subsequent coronary work-up was also negative. The patient was discharged to home and advised to follow up with her primary care physician a few days later.
Two days later we saw the patient at our office. Not only had her dyspnea gotten worse while the presyncope remained, but she now had left-sided pleuritic chest pain. She also reported mild pain in her right calf. On examination, the patient’s BP was 126/86 mm Hg, HR was 82 bpm, RR was 12, and O2 saturation was 96% with ambulation. Her Wells score was now 6, still a moderate probability for PE. (She received another 3 points for the new DVT symptoms—“clinically suspected DVT.”)
Although the patient did not also have signs of a DVT, her additional symptoms along with the original symptoms’ persistence and the existence of other risk factors (OCP use and obesity) led us to reconsider a PE diagnosis. These suspicions prompted us to send the patient back to the ED, where a Doppler ultrasound of the right lower extremity was negative, but the D-dimer was positive at 565 ng/mL.
A pulmonary computed tomography angiogram (CTA) showed 2 small pulmonary emboli within the distal left upper lobe pulmonary arteries.
The patient was treated with heparin and warfarin and discharged without complications.
TABLE 1
Calculating and interpreting the Wells score4,5,7,9,10
| Clinical parameter | Points |
|---|---|
| Clinically suspected DVT | 3.0 |
| Alternative diagnosis less likely than PE | 3.0 |
| Tachycardia | 1.5 |
| Immobilization/surgery (within 4 weeks) | 1.5 |
| History of DVT or PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy (treatment within 6 months, palliative) | 1.0 |
| TOTAL | |
| Score | Traditional interpretation |
| <2.0 | Low probability of PE |
| 2.0-6.0 | Moderate probability of PE |
| >6.0 | High probability of PE |
| Score | Alternative classification scheme |
| ≤4.0 | PE unlikely |
| >4.0 | PE likely |
| DVT, deep venous thrombosis; PE, pulmonary embolism. | |
Discussion
The incidence of PE in the United States varies significantly: Individuals younger than 40 have a risk of 1 in 10,000 compared with 1 in 100 for those older than 80.1 Mortality associated with undiagnosed PE varies widely, from 9.2% to 51%.2 This percentage is significant given that half of all PEs go undiagnosed.3 In addition, when left untreated, PE will recur in 30% to 50% of patients, with a fatality rate of 10% to 45%.1 Further, up to 4% of patients with acute PE develop chronic PE and subsequent pulmonary hypertension.4,5 Given the consequences of failing to diagnose a PE, clinicians must consider this condition in patients who present with unexplained hypotension, dyspnea, or chest pain.6
Not an easy diagnosis
This case report demonstrates the inherent difficulty in diagnosing a PE. Still, certain clinical symptoms/signs can aid in the decision-making process. Fever, crackles, and wheezes decrease the probability of PE, whereas syncope, hemodynamic shock, leg edema, and hemoptysis increase its likelihood.7 Despite the many commonly reported risk factors for PE, only malignancy, recent surgery, or a history of DVT/PE significantly increase the risk of developing a clot.8
The Wells criteria. This scoring system groups patients according to the probability of having a PE: low (score: <2), moderate (score: 2-6), and high (score: >6).6 An alternative classification scheme divides patients into 2 groups: likely to have a PE (score: >4) or unlikely to have a PE (score: ≤4).8
This case report illustrates a key problem with the Wells criteria—the somewhat subjective nature of the scoring. Some physicians find it questionable to award 3 points for “alternative diagnosis less likely than PE,” for example.4 Similarly, with respect to immobilization, some clinicians might have awarded our patient 1.5 points for her recent car trip to New York. We did not think that riding in a car for 2 uninterrupted hours for each leg of the trip was significant enough. However, awarding this patient 1.5 points could have made an important difference in her clinical management if the alternative classification scheme was used. Instead of having a score of 3, the patient would have had a score of 4.5, placing her in the “likely to have a PE” group and prompting us to perform a CTA sooner (FIGURE).
FIGURE
Diagnostic algorithm for pulmonary embolism6,7,10
CTA, computed tomography angiogram; PE, pulmonary embolism.
Inappropriate work-ups are common
Some physicians ignore algorithms when working up a PE and simply order a CTA. In fact, a large multicenter trial showed that 43% of patients suspected of having a PE were inappropriately managed diagnostically.9 Similarly, a meta-analysis of 4 studies including 1660 patients found that only 58% of those with a positive D-dimer had the requisite CTA, as did 7% of patients with a negative D-dimer.2
Physicians should not be concerned about ruling out a PE in the setting of a negative D-dimer, as a meta-analysis found that this diagnostic approach has a negative predictive value (NPV) of 99.7%.2 It is important to note that the NPV is significantly affected by the sensitivity of the D-dimer assay used. If the D-dimer assay is highly sensitive, a negative result in combination with a low, moderate, or unlikely probability Wells score rules out the diagnosis of PE. If the assay is moderately sensitive, however, only a low or unlikely probability Wells score rules out PE.10
The inappropriate work-up of this group of patients is significant and extends beyond the ultimate goal of preventing morbidity and mortality. The unnecessary use of pulmonary CTA is extremely expensive, exposes patients to unnecessary radiation, and results in contrast nephrotoxicity in about 4% of patients.9 Although pulmonary CTA is the standard diagnostic test for PE, other imaging modalities are more appropriate in some cases (TABLE 2).
TABLE 2
Alternative imaging modalities for diagnosing PE1,4,7,11
| Modality | Indication |
|---|---|
| Ventilation-perfusion scanning | Patients with contrast allergies or renal failure; test of choice for diagnosing chronic PE due to limited sensitivity of CT |
| Venous compression ultrasonography | Patients with symptoms of PE and signs/symptoms of DVT |
| Pulmonary angiography | Most invasive test. Should be used only in patients with high probability of PE who may need vascular intervention |
| CT, computed tomography; DVT, deep venous thrombosis; PE, pulmonary embolism. | |
The bottom line
This case report illustrates the importance of using sound clinical judgment when diagnosing a PE. Although our patient initially had a moderate probability Wells score and a negative D-dimer, her symptoms persisted. Her history of OCP use, persistent dyspnea, and new symptoms of a DVT prompted us to reinitiate the diagnostic algorithm and eventually diagnose a PE.
It is always essential to treat the patient and not simply react to laboratory values. To avoid unnecessary testing, however, adhering to the algorithm is equally important.
CORRESPONDENCE
Michael S. Kelleher, MD, University of Connecticut School of Medicine, 263 Farmington Avenue, Farmington, CT 06030; [email protected]
A 44-year-old woman sought care at the emergency department (ED) because she was having difficulty breathing and felt faint. She had been fine until that morning. Three days earlier the patient, who had a history of high blood pressure and elevated cholesterol levels, had driven from Connecticut to New York and back, spending a total of 4 hours in her car. The patient indicated that she’d been taking oral contraceptives (OCPs) for several years, but she did not smoke. There was no history of hemoptysis, recent surgery, or trauma. Neither blood clots nor cancer were part of her or her family’s history.
In the ED, the patient did not have any signs or symptoms of a deep venous thrombosis (DVT). She was obese, with a body mass index of 40.3 kg/m2; other vitals were: blood pressure (BP), 134/88 mm Hg; heart rate (HR), 64 beats per minute (bpm); respiratory rate (RR), 12; and O2 saturation, 99% with ambulation.
The ED physician strongly suspected a pulmonary embolism (PE), but the patient’s score on a clinical probability algorithm (using the Wells criteria) was a 3, indicating only “moderate probability“ of a PE (TABLE 1). (She scored a 3 because an “alternative diagnosis [was] less likely than PE.”) In addition, her D-dimer level was 160 ng/mL using the Triage D-Dimer Test by Biosite, Inc (normal <400 ng/mL), which ruled out a PE. (Many ED physicians at our institution are more cautious when using this D-dimer assay and use a lower cutoff value.)
Given these results, the ED physician did not order imaging studies because the expense and radiation exposure outweighed the probability of the patient having a PE. A subsequent coronary work-up was also negative. The patient was discharged to home and advised to follow up with her primary care physician a few days later.
Two days later we saw the patient at our office. Not only had her dyspnea gotten worse while the presyncope remained, but she now had left-sided pleuritic chest pain. She also reported mild pain in her right calf. On examination, the patient’s BP was 126/86 mm Hg, HR was 82 bpm, RR was 12, and O2 saturation was 96% with ambulation. Her Wells score was now 6, still a moderate probability for PE. (She received another 3 points for the new DVT symptoms—“clinically suspected DVT.”)
Although the patient did not also have signs of a DVT, her additional symptoms along with the original symptoms’ persistence and the existence of other risk factors (OCP use and obesity) led us to reconsider a PE diagnosis. These suspicions prompted us to send the patient back to the ED, where a Doppler ultrasound of the right lower extremity was negative, but the D-dimer was positive at 565 ng/mL.
A pulmonary computed tomography angiogram (CTA) showed 2 small pulmonary emboli within the distal left upper lobe pulmonary arteries.
The patient was treated with heparin and warfarin and discharged without complications.
TABLE 1
Calculating and interpreting the Wells score4,5,7,9,10
| Clinical parameter | Points |
|---|---|
| Clinically suspected DVT | 3.0 |
| Alternative diagnosis less likely than PE | 3.0 |
| Tachycardia | 1.5 |
| Immobilization/surgery (within 4 weeks) | 1.5 |
| History of DVT or PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy (treatment within 6 months, palliative) | 1.0 |
| TOTAL | |
| Score | Traditional interpretation |
| <2.0 | Low probability of PE |
| 2.0-6.0 | Moderate probability of PE |
| >6.0 | High probability of PE |
| Score | Alternative classification scheme |
| ≤4.0 | PE unlikely |
| >4.0 | PE likely |
| DVT, deep venous thrombosis; PE, pulmonary embolism. | |
Discussion
The incidence of PE in the United States varies significantly: Individuals younger than 40 have a risk of 1 in 10,000 compared with 1 in 100 for those older than 80.1 Mortality associated with undiagnosed PE varies widely, from 9.2% to 51%.2 This percentage is significant given that half of all PEs go undiagnosed.3 In addition, when left untreated, PE will recur in 30% to 50% of patients, with a fatality rate of 10% to 45%.1 Further, up to 4% of patients with acute PE develop chronic PE and subsequent pulmonary hypertension.4,5 Given the consequences of failing to diagnose a PE, clinicians must consider this condition in patients who present with unexplained hypotension, dyspnea, or chest pain.6
Not an easy diagnosis
This case report demonstrates the inherent difficulty in diagnosing a PE. Still, certain clinical symptoms/signs can aid in the decision-making process. Fever, crackles, and wheezes decrease the probability of PE, whereas syncope, hemodynamic shock, leg edema, and hemoptysis increase its likelihood.7 Despite the many commonly reported risk factors for PE, only malignancy, recent surgery, or a history of DVT/PE significantly increase the risk of developing a clot.8
The Wells criteria. This scoring system groups patients according to the probability of having a PE: low (score: <2), moderate (score: 2-6), and high (score: >6).6 An alternative classification scheme divides patients into 2 groups: likely to have a PE (score: >4) or unlikely to have a PE (score: ≤4).8
This case report illustrates a key problem with the Wells criteria—the somewhat subjective nature of the scoring. Some physicians find it questionable to award 3 points for “alternative diagnosis less likely than PE,” for example.4 Similarly, with respect to immobilization, some clinicians might have awarded our patient 1.5 points for her recent car trip to New York. We did not think that riding in a car for 2 uninterrupted hours for each leg of the trip was significant enough. However, awarding this patient 1.5 points could have made an important difference in her clinical management if the alternative classification scheme was used. Instead of having a score of 3, the patient would have had a score of 4.5, placing her in the “likely to have a PE” group and prompting us to perform a CTA sooner (FIGURE).
FIGURE
Diagnostic algorithm for pulmonary embolism6,7,10
CTA, computed tomography angiogram; PE, pulmonary embolism.
Inappropriate work-ups are common
Some physicians ignore algorithms when working up a PE and simply order a CTA. In fact, a large multicenter trial showed that 43% of patients suspected of having a PE were inappropriately managed diagnostically.9 Similarly, a meta-analysis of 4 studies including 1660 patients found that only 58% of those with a positive D-dimer had the requisite CTA, as did 7% of patients with a negative D-dimer.2
Physicians should not be concerned about ruling out a PE in the setting of a negative D-dimer, as a meta-analysis found that this diagnostic approach has a negative predictive value (NPV) of 99.7%.2 It is important to note that the NPV is significantly affected by the sensitivity of the D-dimer assay used. If the D-dimer assay is highly sensitive, a negative result in combination with a low, moderate, or unlikely probability Wells score rules out the diagnosis of PE. If the assay is moderately sensitive, however, only a low or unlikely probability Wells score rules out PE.10
The inappropriate work-up of this group of patients is significant and extends beyond the ultimate goal of preventing morbidity and mortality. The unnecessary use of pulmonary CTA is extremely expensive, exposes patients to unnecessary radiation, and results in contrast nephrotoxicity in about 4% of patients.9 Although pulmonary CTA is the standard diagnostic test for PE, other imaging modalities are more appropriate in some cases (TABLE 2).
TABLE 2
Alternative imaging modalities for diagnosing PE1,4,7,11
| Modality | Indication |
|---|---|
| Ventilation-perfusion scanning | Patients with contrast allergies or renal failure; test of choice for diagnosing chronic PE due to limited sensitivity of CT |
| Venous compression ultrasonography | Patients with symptoms of PE and signs/symptoms of DVT |
| Pulmonary angiography | Most invasive test. Should be used only in patients with high probability of PE who may need vascular intervention |
| CT, computed tomography; DVT, deep venous thrombosis; PE, pulmonary embolism. | |
The bottom line
This case report illustrates the importance of using sound clinical judgment when diagnosing a PE. Although our patient initially had a moderate probability Wells score and a negative D-dimer, her symptoms persisted. Her history of OCP use, persistent dyspnea, and new symptoms of a DVT prompted us to reinitiate the diagnostic algorithm and eventually diagnose a PE.
It is always essential to treat the patient and not simply react to laboratory values. To avoid unnecessary testing, however, adhering to the algorithm is equally important.
CORRESPONDENCE
Michael S. Kelleher, MD, University of Connecticut School of Medicine, 263 Farmington Avenue, Farmington, CT 06030; [email protected]
1. Meyer G, Roy PM, Gilberg S, et al. Pulmonary embolism. BMJ. 2010;340:1421.-
2. Pasha SM, Kiok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
3. Taira T, Taira BR, Carmen M, et al. Risk of venous thromboembolism in patients with borderline quantitative D-dimer levels. Am J Emerg Med. 2010;28:450-453.
4. Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. 2010;15:399-406.
5. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
6. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266-274.
7. Gandara E, Wells PS. Diagnosis: use of clinical probability algorithms. Clin Chest Med. 2010;31:629-639.
8. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57:613-621.
9. Gimber LH, Travis RI, Takahashi JM, et al. Computed tomography angiography in patients evaluated for acute pulmonary embolism with low serum D-dimer levels: a prospective study. Perm J. 2009;13:4-10.
10. Agency for Healthcare Research and Quality. Guidelines on the diagnosis and management of acute pulmonary embolism. Available at: http://www.guideline.gov/content.aspx?id=13410#Section420. Accessed February 12, 2011.
11. Kim NH. Chronic thromboembolic pulmonary hypertension: diagnosis. Medscape. Available at: http://www.medscape.org/viewarticle/556058_3. Accessed May 9, 2011.
1. Meyer G, Roy PM, Gilberg S, et al. Pulmonary embolism. BMJ. 2010;340:1421.-
2. Pasha SM, Kiok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
3. Taira T, Taira BR, Carmen M, et al. Risk of venous thromboembolism in patients with borderline quantitative D-dimer levels. Am J Emerg Med. 2010;28:450-453.
4. Bounameaux H, Perrier A, Righini M. Diagnosis of venous thromboembolism: an update. Vasc Med. 2010;15:399-406.
5. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
6. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med. 2010;363:266-274.
7. Gandara E, Wells PS. Diagnosis: use of clinical probability algorithms. Clin Chest Med. 2010;31:629-639.
8. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57:613-621.
9. Gimber LH, Travis RI, Takahashi JM, et al. Computed tomography angiography in patients evaluated for acute pulmonary embolism with low serum D-dimer levels: a prospective study. Perm J. 2009;13:4-10.
10. Agency for Healthcare Research and Quality. Guidelines on the diagnosis and management of acute pulmonary embolism. Available at: http://www.guideline.gov/content.aspx?id=13410#Section420. Accessed February 12, 2011.
11. Kim NH. Chronic thromboembolic pulmonary hypertension: diagnosis. Medscape. Available at: http://www.medscape.org/viewarticle/556058_3. Accessed May 9, 2011.
Failure to monitor INR leads to severe bleeding, disability ... Rash and hives not taken seriously enough ... More
Failure to monitor INR leads to severe bleeding, disability
A MAN WITH A HISTORY OF DEEP VEIN THROMBOSIS was taking warfarin 10 mg every even day and 7.5 mg every odd day. His physician changed the warfarin dosage while the patient was taking ciprofloxacin, then resumed the original regimen once the patient finished taking the antibiotic.
No new prescriptions were written to confirm the change nor, the patient claimed, was a proper explanation of the new regimen provided. His international normalized ratio (INR) wasn’t checked after the dosage change.
After 2 weeks on the new warfarin dosage, the patient went to the emergency department (ED) complaining of groin pain and a change in urine color. Urinalysis found red blood cells too numerous to count. Although the patient told the ED staff he was taking warfarin, they didn’t check his INR. He was given a diagnosis of urinary tract infection (UTI) and discharged.
Three days later, the patient returned to the ED because of increased bleeding from his Foley catheter. Once again his INR wasn’t checked and he was discharged with a UTI diagnosis and a prescription for antibiotics. Two days afterwards, he was taken back to the hospital bleeding from all orifices. His INR was 75.
The patient spent a month in the hospital, most of it in the intensive care unit, followed by 3 months in a rehabilitation facility before returning home. He remained confined to a hospital bed.
PLAINTIFF’S CLAIM The physician and hospital were negligent for failing to instruct the patient regarding the change in warfarin dosage and neglecting to check his INR.
THE DEFENSE No information about the defense is available.
VERDICT $700,000 Maryland settlement.
COMMENT The management of anticoagulation has numerous pitfalls for the unwary. Careful monitoring can save lives—and lawsuits.
Rash and hives not taken seriously enough
A HISTORY OF 3 SEIZURES in a 7-year-old boy prompted a neurologist to prescribe valproic acid. The neurologist later added lamotrigine because of the child’s behavior problems. After taking both medications for 2 weeks, the child developed a rash, at which point the neurologist discontinued the lamotrigine and started diphenhydramine.
The following day, the child was brought to the ED with an itchy rash and hives on his torso and extremities. An allergic reaction was diagnosed and the child was discharged with instructions to take diphenhydramine along with acetaminophen and ibuprofen as needed. When informed of the ED visit, the neurologist requested a follow-up appointment in 4 weeks.
Two days later, the child was back in the ED because the rash had progressed to include redness and swelling of the face. Once again, he was discharged with a diagnosis of allergic reaction and instructions to take diphenhydramine and acetaminophen.
Two days afterward, the child was taken to a different ED, from which he was airlifted to a tertiary care center and admitted to the intensive care unit for treatment of Stevens-Johnson syndrome. The condition advanced to toxic epidermal necrolysis with sloughing of skin and the lining of the gastrointestinal tract. Several weeks later, the child died.
PLAINTIFF’S CLAIM The neurologist was negligent in prescribing lamotrigine for the behavior problem instead of referring the boy to a child psychologist. The lamotrigine dosage was excessive; the neurologist didn’t respond properly to the report of a rash.
The pharmacist was negligent in failing to contact the neurologist to discuss the excessive dosage. Discharging the child from the ED with a life-threatening drug reaction was unreasonable.
THE DEFENSE The defendants denied that they were negligent or caused the child’s death. They were prepared to present the histories of the parents, whose backgrounds included drug abuse, and state investigations regarding the care of the child.
VERDICT $1.55 million Washington settlement.
COMMENT When prescribing a drug with a potentially serious adverse effect, it’s always prudent to document patient education and follow-up thoroughly. Even though hindsight is 20/20, an “allergic reaction” in a patient on lamotrigine should raise red flags.
Delay in spotting compartment syndrome has permanent consequences
SEVERE NUMBNESS, TINGLING, AND PAIN IN HER LEFT CALF brought a 20-year-old woman to the ED. She couldn’t lift her left foot or bear weight on her left foot or leg. She reported awakening with the symptoms after a New Year’s Eve party the previous evening. After an examination, but no tests, she was discharged with a diagnosis of “floppy foot syndrome” and a prescription for a non-narcotic pain medication.
The young woman went to another ED the next day, complaining of continued pain and swelling in her left calf. She was admitted to the hospital for an orthopedic consultation, which resulted in a diagnosis of compartment syndrome. By that time, the patient had gone into renal failure from rhabdomyolysis caused by tissue breakdown. She underwent a fasciotomy, after which she required hemodialysis (until her kidney function returned) and rehabilitation. Damage to the nerves of her left calf and leg left her with permanent foot drop.
PLAINTIFF’S CLAIM The hospital was negligent in failing to diagnose compartment syndrome when the woman went to the ED. Proper diagnosis and treatment at that time would have prevented the nerve damage and foot drop.
THE DEFENSE No information about the defense is available.
VERDICT $750,000 Maryland settlement.
COMMENT Compartment syndrome can be challenging to recognize. Recently I have come across several allegations of malpractice for untimely diagnosis. Remember this important problem when faced with a patient with leg pain.
Multiple errors end in death from pneumonia
A 24-YEAR-OLD MAN WITH CHEST PAIN AND A COUGH went to his physician, who diagnosed chest wall pain and prescribed a narcotic pain reliever. The young man returned the next day complaining of increased chest pain. He said he’d been spitting up blood-stained sputum. He was perspiring and vomited in the doctor’s waiting room. The doctor diagnosed an upper respiratory infection and prescribed a cough syrup containing more narcotics.
Later that day the patient had a radiograph at a hospital. It revealed pneumonia. Shortly afterward, the hospital confirmed by fax with the doctor’s office that the doctor had received the results. The doctor didn’t read the radiograph results for 2 days.
After the doctor read the radiograph report, his office tried to contact the patient but misdialed his phone number, then made no further attempts at contact. The patient’s former wife found him at home unresponsive. He was admitted to the ED, where he died of pneumonia shortly thereafter.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1.85 million net verdict in Virginia.
COMMENT A cascade of mistakes (sometimes referred to as the Swiss cheese effect) occurs, and a preventable death results. Are you at risk for such an event? What fail-safe measures do you have in place in your practice?
Failure to monitor INR leads to severe bleeding, disability
A MAN WITH A HISTORY OF DEEP VEIN THROMBOSIS was taking warfarin 10 mg every even day and 7.5 mg every odd day. His physician changed the warfarin dosage while the patient was taking ciprofloxacin, then resumed the original regimen once the patient finished taking the antibiotic.
No new prescriptions were written to confirm the change nor, the patient claimed, was a proper explanation of the new regimen provided. His international normalized ratio (INR) wasn’t checked after the dosage change.
After 2 weeks on the new warfarin dosage, the patient went to the emergency department (ED) complaining of groin pain and a change in urine color. Urinalysis found red blood cells too numerous to count. Although the patient told the ED staff he was taking warfarin, they didn’t check his INR. He was given a diagnosis of urinary tract infection (UTI) and discharged.
Three days later, the patient returned to the ED because of increased bleeding from his Foley catheter. Once again his INR wasn’t checked and he was discharged with a UTI diagnosis and a prescription for antibiotics. Two days afterwards, he was taken back to the hospital bleeding from all orifices. His INR was 75.
The patient spent a month in the hospital, most of it in the intensive care unit, followed by 3 months in a rehabilitation facility before returning home. He remained confined to a hospital bed.
PLAINTIFF’S CLAIM The physician and hospital were negligent for failing to instruct the patient regarding the change in warfarin dosage and neglecting to check his INR.
THE DEFENSE No information about the defense is available.
VERDICT $700,000 Maryland settlement.
COMMENT The management of anticoagulation has numerous pitfalls for the unwary. Careful monitoring can save lives—and lawsuits.
Rash and hives not taken seriously enough
A HISTORY OF 3 SEIZURES in a 7-year-old boy prompted a neurologist to prescribe valproic acid. The neurologist later added lamotrigine because of the child’s behavior problems. After taking both medications for 2 weeks, the child developed a rash, at which point the neurologist discontinued the lamotrigine and started diphenhydramine.
The following day, the child was brought to the ED with an itchy rash and hives on his torso and extremities. An allergic reaction was diagnosed and the child was discharged with instructions to take diphenhydramine along with acetaminophen and ibuprofen as needed. When informed of the ED visit, the neurologist requested a follow-up appointment in 4 weeks.
Two days later, the child was back in the ED because the rash had progressed to include redness and swelling of the face. Once again, he was discharged with a diagnosis of allergic reaction and instructions to take diphenhydramine and acetaminophen.
Two days afterward, the child was taken to a different ED, from which he was airlifted to a tertiary care center and admitted to the intensive care unit for treatment of Stevens-Johnson syndrome. The condition advanced to toxic epidermal necrolysis with sloughing of skin and the lining of the gastrointestinal tract. Several weeks later, the child died.
PLAINTIFF’S CLAIM The neurologist was negligent in prescribing lamotrigine for the behavior problem instead of referring the boy to a child psychologist. The lamotrigine dosage was excessive; the neurologist didn’t respond properly to the report of a rash.
The pharmacist was negligent in failing to contact the neurologist to discuss the excessive dosage. Discharging the child from the ED with a life-threatening drug reaction was unreasonable.
THE DEFENSE The defendants denied that they were negligent or caused the child’s death. They were prepared to present the histories of the parents, whose backgrounds included drug abuse, and state investigations regarding the care of the child.
VERDICT $1.55 million Washington settlement.
COMMENT When prescribing a drug with a potentially serious adverse effect, it’s always prudent to document patient education and follow-up thoroughly. Even though hindsight is 20/20, an “allergic reaction” in a patient on lamotrigine should raise red flags.
Delay in spotting compartment syndrome has permanent consequences
SEVERE NUMBNESS, TINGLING, AND PAIN IN HER LEFT CALF brought a 20-year-old woman to the ED. She couldn’t lift her left foot or bear weight on her left foot or leg. She reported awakening with the symptoms after a New Year’s Eve party the previous evening. After an examination, but no tests, she was discharged with a diagnosis of “floppy foot syndrome” and a prescription for a non-narcotic pain medication.
The young woman went to another ED the next day, complaining of continued pain and swelling in her left calf. She was admitted to the hospital for an orthopedic consultation, which resulted in a diagnosis of compartment syndrome. By that time, the patient had gone into renal failure from rhabdomyolysis caused by tissue breakdown. She underwent a fasciotomy, after which she required hemodialysis (until her kidney function returned) and rehabilitation. Damage to the nerves of her left calf and leg left her with permanent foot drop.
PLAINTIFF’S CLAIM The hospital was negligent in failing to diagnose compartment syndrome when the woman went to the ED. Proper diagnosis and treatment at that time would have prevented the nerve damage and foot drop.
THE DEFENSE No information about the defense is available.
VERDICT $750,000 Maryland settlement.
COMMENT Compartment syndrome can be challenging to recognize. Recently I have come across several allegations of malpractice for untimely diagnosis. Remember this important problem when faced with a patient with leg pain.
Multiple errors end in death from pneumonia
A 24-YEAR-OLD MAN WITH CHEST PAIN AND A COUGH went to his physician, who diagnosed chest wall pain and prescribed a narcotic pain reliever. The young man returned the next day complaining of increased chest pain. He said he’d been spitting up blood-stained sputum. He was perspiring and vomited in the doctor’s waiting room. The doctor diagnosed an upper respiratory infection and prescribed a cough syrup containing more narcotics.
Later that day the patient had a radiograph at a hospital. It revealed pneumonia. Shortly afterward, the hospital confirmed by fax with the doctor’s office that the doctor had received the results. The doctor didn’t read the radiograph results for 2 days.
After the doctor read the radiograph report, his office tried to contact the patient but misdialed his phone number, then made no further attempts at contact. The patient’s former wife found him at home unresponsive. He was admitted to the ED, where he died of pneumonia shortly thereafter.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1.85 million net verdict in Virginia.
COMMENT A cascade of mistakes (sometimes referred to as the Swiss cheese effect) occurs, and a preventable death results. Are you at risk for such an event? What fail-safe measures do you have in place in your practice?
Failure to monitor INR leads to severe bleeding, disability
A MAN WITH A HISTORY OF DEEP VEIN THROMBOSIS was taking warfarin 10 mg every even day and 7.5 mg every odd day. His physician changed the warfarin dosage while the patient was taking ciprofloxacin, then resumed the original regimen once the patient finished taking the antibiotic.
No new prescriptions were written to confirm the change nor, the patient claimed, was a proper explanation of the new regimen provided. His international normalized ratio (INR) wasn’t checked after the dosage change.
After 2 weeks on the new warfarin dosage, the patient went to the emergency department (ED) complaining of groin pain and a change in urine color. Urinalysis found red blood cells too numerous to count. Although the patient told the ED staff he was taking warfarin, they didn’t check his INR. He was given a diagnosis of urinary tract infection (UTI) and discharged.
Three days later, the patient returned to the ED because of increased bleeding from his Foley catheter. Once again his INR wasn’t checked and he was discharged with a UTI diagnosis and a prescription for antibiotics. Two days afterwards, he was taken back to the hospital bleeding from all orifices. His INR was 75.
The patient spent a month in the hospital, most of it in the intensive care unit, followed by 3 months in a rehabilitation facility before returning home. He remained confined to a hospital bed.
PLAINTIFF’S CLAIM The physician and hospital were negligent for failing to instruct the patient regarding the change in warfarin dosage and neglecting to check his INR.
THE DEFENSE No information about the defense is available.
VERDICT $700,000 Maryland settlement.
COMMENT The management of anticoagulation has numerous pitfalls for the unwary. Careful monitoring can save lives—and lawsuits.
Rash and hives not taken seriously enough
A HISTORY OF 3 SEIZURES in a 7-year-old boy prompted a neurologist to prescribe valproic acid. The neurologist later added lamotrigine because of the child’s behavior problems. After taking both medications for 2 weeks, the child developed a rash, at which point the neurologist discontinued the lamotrigine and started diphenhydramine.
The following day, the child was brought to the ED with an itchy rash and hives on his torso and extremities. An allergic reaction was diagnosed and the child was discharged with instructions to take diphenhydramine along with acetaminophen and ibuprofen as needed. When informed of the ED visit, the neurologist requested a follow-up appointment in 4 weeks.
Two days later, the child was back in the ED because the rash had progressed to include redness and swelling of the face. Once again, he was discharged with a diagnosis of allergic reaction and instructions to take diphenhydramine and acetaminophen.
Two days afterward, the child was taken to a different ED, from which he was airlifted to a tertiary care center and admitted to the intensive care unit for treatment of Stevens-Johnson syndrome. The condition advanced to toxic epidermal necrolysis with sloughing of skin and the lining of the gastrointestinal tract. Several weeks later, the child died.
PLAINTIFF’S CLAIM The neurologist was negligent in prescribing lamotrigine for the behavior problem instead of referring the boy to a child psychologist. The lamotrigine dosage was excessive; the neurologist didn’t respond properly to the report of a rash.
The pharmacist was negligent in failing to contact the neurologist to discuss the excessive dosage. Discharging the child from the ED with a life-threatening drug reaction was unreasonable.
THE DEFENSE The defendants denied that they were negligent or caused the child’s death. They were prepared to present the histories of the parents, whose backgrounds included drug abuse, and state investigations regarding the care of the child.
VERDICT $1.55 million Washington settlement.
COMMENT When prescribing a drug with a potentially serious adverse effect, it’s always prudent to document patient education and follow-up thoroughly. Even though hindsight is 20/20, an “allergic reaction” in a patient on lamotrigine should raise red flags.
Delay in spotting compartment syndrome has permanent consequences
SEVERE NUMBNESS, TINGLING, AND PAIN IN HER LEFT CALF brought a 20-year-old woman to the ED. She couldn’t lift her left foot or bear weight on her left foot or leg. She reported awakening with the symptoms after a New Year’s Eve party the previous evening. After an examination, but no tests, she was discharged with a diagnosis of “floppy foot syndrome” and a prescription for a non-narcotic pain medication.
The young woman went to another ED the next day, complaining of continued pain and swelling in her left calf. She was admitted to the hospital for an orthopedic consultation, which resulted in a diagnosis of compartment syndrome. By that time, the patient had gone into renal failure from rhabdomyolysis caused by tissue breakdown. She underwent a fasciotomy, after which she required hemodialysis (until her kidney function returned) and rehabilitation. Damage to the nerves of her left calf and leg left her with permanent foot drop.
PLAINTIFF’S CLAIM The hospital was negligent in failing to diagnose compartment syndrome when the woman went to the ED. Proper diagnosis and treatment at that time would have prevented the nerve damage and foot drop.
THE DEFENSE No information about the defense is available.
VERDICT $750,000 Maryland settlement.
COMMENT Compartment syndrome can be challenging to recognize. Recently I have come across several allegations of malpractice for untimely diagnosis. Remember this important problem when faced with a patient with leg pain.
Multiple errors end in death from pneumonia
A 24-YEAR-OLD MAN WITH CHEST PAIN AND A COUGH went to his physician, who diagnosed chest wall pain and prescribed a narcotic pain reliever. The young man returned the next day complaining of increased chest pain. He said he’d been spitting up blood-stained sputum. He was perspiring and vomited in the doctor’s waiting room. The doctor diagnosed an upper respiratory infection and prescribed a cough syrup containing more narcotics.
Later that day the patient had a radiograph at a hospital. It revealed pneumonia. Shortly afterward, the hospital confirmed by fax with the doctor’s office that the doctor had received the results. The doctor didn’t read the radiograph results for 2 days.
After the doctor read the radiograph report, his office tried to contact the patient but misdialed his phone number, then made no further attempts at contact. The patient’s former wife found him at home unresponsive. He was admitted to the ED, where he died of pneumonia shortly thereafter.
PLAINTIFF’S CLAIM No information about the plaintiff’s claim is available.
THE DEFENSE No information about the defense is available.
VERDICT $1.85 million net verdict in Virginia.
COMMENT A cascade of mistakes (sometimes referred to as the Swiss cheese effect) occurs, and a preventable death results. Are you at risk for such an event? What fail-safe measures do you have in place in your practice?
How best to diagnose asthma in infants and toddlers?
NO RELIABLE WAY EXISTS TO DIAGNOSE ASTHMA IN INFANTS AND TODDLERS. Recurrent wheezing, especially apart from colds, combined with physician-diagnosed eczema or atopic dermatitis, eosinophilia, and a parental history of asthma, increase the probability of a subsequent asthma diagnosis in the absence of other causes (strength of recommendation: B, 2 good-quality cohort studies).
Evidence summary
Wheezing in children is common and the differential diagnosis is broad. The many potential causes include upper respiratory infection, asthma, cystic fibrosis, foreign body aspiration, vascular ring, tracheomalacia, primary immunodeficiency, and congenital heart disease.1
Outpatient primary care cohort studies estimate that about half of children wheeze before they reach school age. Only one-third of children who wheeze during the first 3 years of life, however, continue to wheeze into later childhood and young adulthood.2-4
These findings have led some experts to suggest that not all wheezing in children is asthma and that asthma exists in variant forms.5-7 Variant wheezing patterns include transient early wheezing, which seems to be most prevalent in the first 3 years of life; wheezing without atopy, which occurs most often at 3 to 6 years of age; and wheezing with immunoglobulin E-associated atopy, which gradually increases in prevalence from birth and dominates in the over-6 age group. It is children in this last group whom we generally consider to have asthma.
Objective measures of lung function are challenging to perform in young children. Clinical signs and symptoms thus suggest the diagnosis of asthma.
Atopy, rhinitis, and eczema most often accompany persistent wheezing
Primary care cohort studies provide the best available evidence on which findings in infants and toddlers most likely predict persistent airway disease in childhood. A whole-population cohort study followed nearly all children born on the Isle of Wight from January 1989 through February 1990 to evaluate the natural history of childhood wheezing and to study associated risk factors.8 Children were seen at birth and at 1, 2, 4, and 10 years of age.
Findings most associated with current wheezing (within the last year) in 10-year-olds were atopy (odds ratio [OR]=4.38; 95% confidence interval [CI], 3.07-6.25), rhinitis (OR=3.72; 95% CI, 2.21-6.27), and eczema (OR=3.04; 95% CI, 2.05-4.51).8
An index to predict asthma
Since 1980, the Tucson Children’s Respiratory Study has followed 1246 healthy newborns seen by pediatricians affiliated with a large HMO in Tucson, Arizona. Questionnaires about parental asthma history and prenatal smoking history were obtained at enrollment. Childhood wheezing and its frequency, as well as physician-diagnosed allergies or asthma, were assessed at ages 2 and 3. If the child had wheezed in the past year, then the child was considered to be an “early wheezer.” If the frequency was 3 or more on a 5-point scale, then the child was considered to be an “early frequent wheezer.” Questionnaires were re-administered at ages 6, 8, 11, and 13. Three episodes of wheezing within the past year or a physician diagnosis of asthma with symptoms in the past year was considered “active asthma.” Blood specimens for eosinophils were obtained at age 10.
Using these data, the researchers developed stringent and loose criteria (TABLE 1) and odds ratios (TABLES 2 and 3) for childhood factors most predictive of an asthma diagnosis at an older age. The findings of the study may help clinicians care for wheezing infants and toddlers.9
TABLE 1
A clinical index of asthma risk9*
| Major criteria | Minor criteria |
|---|---|
| Parental asthma (history of physician diagnosis of asthma in a parent) | Allergic rhinitis (physician diagnosis of allergic rhinitis as reported in questionnaires at ages 2 or 3 y) |
| Eczema (physician diagnosis of atopic dermatitis as reported in questionnaires at ages 2 or 3 y) | Wheezing apart from colds |
| Eosinophilia (≥4%) | |
| *Stringent index for predicting asthma: Child has early, frequent wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. Loose index for predicting asthma: Child has early wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. | |
TABLE 2
Likelihood of active asthma predicted by stringent index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 9.8 (5.6-17.2) | 27.5 (24.6-30.4) | 96.3 (95.1-97.5) | 47.5 (44.3-50.7) | 91.6 (89.8-93.4) |
| At 8 y | 5.8 (2.9-11.2) | 16.3 (13.7-18.9) | 96.7 (95.4-98.0) | 43.6 (40.1-47.1) | 88.2 (85.9-90.5) |
| At 11 y | 4.3 (2.4-7.8) | 15 (12.6-17.4) | 96.1 (94.8-97.4) | 42.0 (38.7-45.3) | 85.6 (83.3-87.9) |
| At 13 y | 5.7 (2.8-11.6) | 14.8 (12.1-17.5) | 97.0 (95.7-98.3) | 51.5 (47.7-55.3) | 84.2 (81.4-87.0) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
TABLE 3
Likelihood of active asthma predicted by loose index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 5.5 (3.5-8.4) | 56.6 (53.3-59.9) | 80.8 (78.3-83.3) | 26.2 (23.4-29.0) | 93.9 (92.4-95.4) |
| At 8 y | 4.4 (2.8-6.8) | 50.5 (47.0-54.0) | 81.1 (78.3-83.9) | 29.4 (26.2-32.6) | 91.3 (89.3-93.3) |
| At 11 y | 2.6 (1.8-3.8) | 40.1 (36.8-43.4) | 79.6 (76.9-82.3) | 27.1 (24.1-30.1) | 87.5 (85.3-89.7) |
| At 13 y | 3.0 (1.9-4.6) | 39.3 (35.5-43.1) | 82.1 (79.1-85.1) | 31.7 (28.1-35.3) | 86.5 (83.9-89.1) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
Recommendations
A European and United States expert panel guide to the diagnosis and treatment of asthma in childhood, PRACTALL, states that “asthma should be suspected in any infant with recurrent wheezing and cough episodes. Frequently, diagnosis is possible only through long-term follow-up, consideration of the extensive differential diagnoses, and by observing the child’s response to bronchodilator and/or anti-inflammatory treatment.”10
The National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3) notes that diagnostic evaluation for asthma in children 0 to 4 years of age should include history, symptoms, physical examination, and assessment of quality of life.1
1. National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma. NIH publication 07-4051. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 20, 2008.
2. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133-138.
3. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422.
4. Jenkins MA, Hopper JL, Bowes G, et al. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90-93.
5. Rusconi F, Galassi C, Corbo GM, et al. Risk factors for early, persistent, and late-onset wheezing in young children. SIDRIA Collaborative Group. Am J Respir Crit Care Med. 1999;167:1617-1622.
6. Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiologic approach. Paediatr Respir Rev. 2004;5:155-161.
7. Stein RT, Holberg CJ, Morgan WJ, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946-952.
8. Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Early life risk factors for current wheeze, asthma, and bronchial hyper-responsiveness at 10 years of age. Chest. 2005;127:502-508.
9. Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403-1406.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5-34.
NO RELIABLE WAY EXISTS TO DIAGNOSE ASTHMA IN INFANTS AND TODDLERS. Recurrent wheezing, especially apart from colds, combined with physician-diagnosed eczema or atopic dermatitis, eosinophilia, and a parental history of asthma, increase the probability of a subsequent asthma diagnosis in the absence of other causes (strength of recommendation: B, 2 good-quality cohort studies).
Evidence summary
Wheezing in children is common and the differential diagnosis is broad. The many potential causes include upper respiratory infection, asthma, cystic fibrosis, foreign body aspiration, vascular ring, tracheomalacia, primary immunodeficiency, and congenital heart disease.1
Outpatient primary care cohort studies estimate that about half of children wheeze before they reach school age. Only one-third of children who wheeze during the first 3 years of life, however, continue to wheeze into later childhood and young adulthood.2-4
These findings have led some experts to suggest that not all wheezing in children is asthma and that asthma exists in variant forms.5-7 Variant wheezing patterns include transient early wheezing, which seems to be most prevalent in the first 3 years of life; wheezing without atopy, which occurs most often at 3 to 6 years of age; and wheezing with immunoglobulin E-associated atopy, which gradually increases in prevalence from birth and dominates in the over-6 age group. It is children in this last group whom we generally consider to have asthma.
Objective measures of lung function are challenging to perform in young children. Clinical signs and symptoms thus suggest the diagnosis of asthma.
Atopy, rhinitis, and eczema most often accompany persistent wheezing
Primary care cohort studies provide the best available evidence on which findings in infants and toddlers most likely predict persistent airway disease in childhood. A whole-population cohort study followed nearly all children born on the Isle of Wight from January 1989 through February 1990 to evaluate the natural history of childhood wheezing and to study associated risk factors.8 Children were seen at birth and at 1, 2, 4, and 10 years of age.
Findings most associated with current wheezing (within the last year) in 10-year-olds were atopy (odds ratio [OR]=4.38; 95% confidence interval [CI], 3.07-6.25), rhinitis (OR=3.72; 95% CI, 2.21-6.27), and eczema (OR=3.04; 95% CI, 2.05-4.51).8
An index to predict asthma
Since 1980, the Tucson Children’s Respiratory Study has followed 1246 healthy newborns seen by pediatricians affiliated with a large HMO in Tucson, Arizona. Questionnaires about parental asthma history and prenatal smoking history were obtained at enrollment. Childhood wheezing and its frequency, as well as physician-diagnosed allergies or asthma, were assessed at ages 2 and 3. If the child had wheezed in the past year, then the child was considered to be an “early wheezer.” If the frequency was 3 or more on a 5-point scale, then the child was considered to be an “early frequent wheezer.” Questionnaires were re-administered at ages 6, 8, 11, and 13. Three episodes of wheezing within the past year or a physician diagnosis of asthma with symptoms in the past year was considered “active asthma.” Blood specimens for eosinophils were obtained at age 10.
Using these data, the researchers developed stringent and loose criteria (TABLE 1) and odds ratios (TABLES 2 and 3) for childhood factors most predictive of an asthma diagnosis at an older age. The findings of the study may help clinicians care for wheezing infants and toddlers.9
TABLE 1
A clinical index of asthma risk9*
| Major criteria | Minor criteria |
|---|---|
| Parental asthma (history of physician diagnosis of asthma in a parent) | Allergic rhinitis (physician diagnosis of allergic rhinitis as reported in questionnaires at ages 2 or 3 y) |
| Eczema (physician diagnosis of atopic dermatitis as reported in questionnaires at ages 2 or 3 y) | Wheezing apart from colds |
| Eosinophilia (≥4%) | |
| *Stringent index for predicting asthma: Child has early, frequent wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. Loose index for predicting asthma: Child has early wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. | |
TABLE 2
Likelihood of active asthma predicted by stringent index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 9.8 (5.6-17.2) | 27.5 (24.6-30.4) | 96.3 (95.1-97.5) | 47.5 (44.3-50.7) | 91.6 (89.8-93.4) |
| At 8 y | 5.8 (2.9-11.2) | 16.3 (13.7-18.9) | 96.7 (95.4-98.0) | 43.6 (40.1-47.1) | 88.2 (85.9-90.5) |
| At 11 y | 4.3 (2.4-7.8) | 15 (12.6-17.4) | 96.1 (94.8-97.4) | 42.0 (38.7-45.3) | 85.6 (83.3-87.9) |
| At 13 y | 5.7 (2.8-11.6) | 14.8 (12.1-17.5) | 97.0 (95.7-98.3) | 51.5 (47.7-55.3) | 84.2 (81.4-87.0) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
TABLE 3
Likelihood of active asthma predicted by loose index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 5.5 (3.5-8.4) | 56.6 (53.3-59.9) | 80.8 (78.3-83.3) | 26.2 (23.4-29.0) | 93.9 (92.4-95.4) |
| At 8 y | 4.4 (2.8-6.8) | 50.5 (47.0-54.0) | 81.1 (78.3-83.9) | 29.4 (26.2-32.6) | 91.3 (89.3-93.3) |
| At 11 y | 2.6 (1.8-3.8) | 40.1 (36.8-43.4) | 79.6 (76.9-82.3) | 27.1 (24.1-30.1) | 87.5 (85.3-89.7) |
| At 13 y | 3.0 (1.9-4.6) | 39.3 (35.5-43.1) | 82.1 (79.1-85.1) | 31.7 (28.1-35.3) | 86.5 (83.9-89.1) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
Recommendations
A European and United States expert panel guide to the diagnosis and treatment of asthma in childhood, PRACTALL, states that “asthma should be suspected in any infant with recurrent wheezing and cough episodes. Frequently, diagnosis is possible only through long-term follow-up, consideration of the extensive differential diagnoses, and by observing the child’s response to bronchodilator and/or anti-inflammatory treatment.”10
The National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3) notes that diagnostic evaluation for asthma in children 0 to 4 years of age should include history, symptoms, physical examination, and assessment of quality of life.1
NO RELIABLE WAY EXISTS TO DIAGNOSE ASTHMA IN INFANTS AND TODDLERS. Recurrent wheezing, especially apart from colds, combined with physician-diagnosed eczema or atopic dermatitis, eosinophilia, and a parental history of asthma, increase the probability of a subsequent asthma diagnosis in the absence of other causes (strength of recommendation: B, 2 good-quality cohort studies).
Evidence summary
Wheezing in children is common and the differential diagnosis is broad. The many potential causes include upper respiratory infection, asthma, cystic fibrosis, foreign body aspiration, vascular ring, tracheomalacia, primary immunodeficiency, and congenital heart disease.1
Outpatient primary care cohort studies estimate that about half of children wheeze before they reach school age. Only one-third of children who wheeze during the first 3 years of life, however, continue to wheeze into later childhood and young adulthood.2-4
These findings have led some experts to suggest that not all wheezing in children is asthma and that asthma exists in variant forms.5-7 Variant wheezing patterns include transient early wheezing, which seems to be most prevalent in the first 3 years of life; wheezing without atopy, which occurs most often at 3 to 6 years of age; and wheezing with immunoglobulin E-associated atopy, which gradually increases in prevalence from birth and dominates in the over-6 age group. It is children in this last group whom we generally consider to have asthma.
Objective measures of lung function are challenging to perform in young children. Clinical signs and symptoms thus suggest the diagnosis of asthma.
Atopy, rhinitis, and eczema most often accompany persistent wheezing
Primary care cohort studies provide the best available evidence on which findings in infants and toddlers most likely predict persistent airway disease in childhood. A whole-population cohort study followed nearly all children born on the Isle of Wight from January 1989 through February 1990 to evaluate the natural history of childhood wheezing and to study associated risk factors.8 Children were seen at birth and at 1, 2, 4, and 10 years of age.
Findings most associated with current wheezing (within the last year) in 10-year-olds were atopy (odds ratio [OR]=4.38; 95% confidence interval [CI], 3.07-6.25), rhinitis (OR=3.72; 95% CI, 2.21-6.27), and eczema (OR=3.04; 95% CI, 2.05-4.51).8
An index to predict asthma
Since 1980, the Tucson Children’s Respiratory Study has followed 1246 healthy newborns seen by pediatricians affiliated with a large HMO in Tucson, Arizona. Questionnaires about parental asthma history and prenatal smoking history were obtained at enrollment. Childhood wheezing and its frequency, as well as physician-diagnosed allergies or asthma, were assessed at ages 2 and 3. If the child had wheezed in the past year, then the child was considered to be an “early wheezer.” If the frequency was 3 or more on a 5-point scale, then the child was considered to be an “early frequent wheezer.” Questionnaires were re-administered at ages 6, 8, 11, and 13. Three episodes of wheezing within the past year or a physician diagnosis of asthma with symptoms in the past year was considered “active asthma.” Blood specimens for eosinophils were obtained at age 10.
Using these data, the researchers developed stringent and loose criteria (TABLE 1) and odds ratios (TABLES 2 and 3) for childhood factors most predictive of an asthma diagnosis at an older age. The findings of the study may help clinicians care for wheezing infants and toddlers.9
TABLE 1
A clinical index of asthma risk9*
| Major criteria | Minor criteria |
|---|---|
| Parental asthma (history of physician diagnosis of asthma in a parent) | Allergic rhinitis (physician diagnosis of allergic rhinitis as reported in questionnaires at ages 2 or 3 y) |
| Eczema (physician diagnosis of atopic dermatitis as reported in questionnaires at ages 2 or 3 y) | Wheezing apart from colds |
| Eosinophilia (≥4%) | |
| *Stringent index for predicting asthma: Child has early, frequent wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. Loose index for predicting asthma: Child has early wheezing plus at least 1 of the 2 major criteria or 2 of the 3 minor criteria. | |
TABLE 2
Likelihood of active asthma predicted by stringent index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 9.8 (5.6-17.2) | 27.5 (24.6-30.4) | 96.3 (95.1-97.5) | 47.5 (44.3-50.7) | 91.6 (89.8-93.4) |
| At 8 y | 5.8 (2.9-11.2) | 16.3 (13.7-18.9) | 96.7 (95.4-98.0) | 43.6 (40.1-47.1) | 88.2 (85.9-90.5) |
| At 11 y | 4.3 (2.4-7.8) | 15 (12.6-17.4) | 96.1 (94.8-97.4) | 42.0 (38.7-45.3) | 85.6 (83.3-87.9) |
| At 13 y | 5.7 (2.8-11.6) | 14.8 (12.1-17.5) | 97.0 (95.7-98.3) | 51.5 (47.7-55.3) | 84.2 (81.4-87.0) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
TABLE 3
Likelihood of active asthma predicted by loose index9
| Active asthma | OR (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|---|
| At 6 y | 5.5 (3.5-8.4) | 56.6 (53.3-59.9) | 80.8 (78.3-83.3) | 26.2 (23.4-29.0) | 93.9 (92.4-95.4) |
| At 8 y | 4.4 (2.8-6.8) | 50.5 (47.0-54.0) | 81.1 (78.3-83.9) | 29.4 (26.2-32.6) | 91.3 (89.3-93.3) |
| At 11 y | 2.6 (1.8-3.8) | 40.1 (36.8-43.4) | 79.6 (76.9-82.3) | 27.1 (24.1-30.1) | 87.5 (85.3-89.7) |
| At 13 y | 3.0 (1.9-4.6) | 39.3 (35.5-43.1) | 82.1 (79.1-85.1) | 31.7 (28.1-35.3) | 86.5 (83.9-89.1) |
| CI, confidence interval; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value. | |||||
Recommendations
A European and United States expert panel guide to the diagnosis and treatment of asthma in childhood, PRACTALL, states that “asthma should be suspected in any infant with recurrent wheezing and cough episodes. Frequently, diagnosis is possible only through long-term follow-up, consideration of the extensive differential diagnoses, and by observing the child’s response to bronchodilator and/or anti-inflammatory treatment.”10
The National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3) notes that diagnostic evaluation for asthma in children 0 to 4 years of age should include history, symptoms, physical examination, and assessment of quality of life.1
1. National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma. NIH publication 07-4051. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 20, 2008.
2. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133-138.
3. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422.
4. Jenkins MA, Hopper JL, Bowes G, et al. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90-93.
5. Rusconi F, Galassi C, Corbo GM, et al. Risk factors for early, persistent, and late-onset wheezing in young children. SIDRIA Collaborative Group. Am J Respir Crit Care Med. 1999;167:1617-1622.
6. Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiologic approach. Paediatr Respir Rev. 2004;5:155-161.
7. Stein RT, Holberg CJ, Morgan WJ, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946-952.
8. Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Early life risk factors for current wheeze, asthma, and bronchial hyper-responsiveness at 10 years of age. Chest. 2005;127:502-508.
9. Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403-1406.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5-34.
1. National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma. NIH publication 07-4051. Bethesda, Md: National Heart, Lung, and Blood Institute; 2007. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 20, 2008.
2. Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133-138.
3. Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414-1422.
4. Jenkins MA, Hopper JL, Bowes G, et al. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90-93.
5. Rusconi F, Galassi C, Corbo GM, et al. Risk factors for early, persistent, and late-onset wheezing in young children. SIDRIA Collaborative Group. Am J Respir Crit Care Med. 1999;167:1617-1622.
6. Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from an epidemiologic approach. Paediatr Respir Rev. 2004;5:155-161.
7. Stein RT, Holberg CJ, Morgan WJ, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946-952.
8. Arshad SH, Kurukulaaratchy RJ, Fenn M, et al. Early life risk factors for current wheeze, asthma, and bronchial hyper-responsiveness at 10 years of age. Chest. 2005;127:502-508.
9. Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403-1406.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63:5-34.
Evidence-based answers from the Family Physicians Inquiries Network
Responsibility for delayed Dx cuts both ways ... Missed pulmonary embolism proves fatal ... more
Responsibility for delayed Dx cuts both ways
A 44-YEAR-OLD WOMAN went to a university medical clinic complaining of weight gain and fatigue. The clinic was staffed by residents supervised by clinical faculty. The resident who examined the woman found a 1.5-cm mobile mass in one of her breasts. After consultation with the supervising physician, a mammogram with ultrasound was ordered. The supervising physician didn’t see the patient, but signed off on the treatment plan.
The mammogram was performed 2 days later and the mass was evaluated as probably benign. The patient was advised to follow up in 6 months. A month later, a second resident consulted with the patient and told her that she could have a biopsy or follow her condition on her own. The patient decided against a biopsy.
Eight months later, the clinic sent a reminder to the patient to return for follow-up, which she did. At that time, the skin on her breast had the texture of orange rind and the mass had grown. Metastatic breast cancer was diagnosed. Aggressive treatment was recommended, but the patient opted for herbal and other homeopathic remedies.
The initial trial of the case ended in a defense verdict, which was appealed after the patient died. A second trial led to a verdict finding the supervising physician 99% at fault and the patient 1% at fault. The jury award was set aside by the trial court.
PLAINTIFF’S CLAIM Failure to diagnose breast cancer promptly constituted negligence. A needle biopsy was needed.
THE DEFENSE The follow-up plan was reasonable; the patient didn’t return for evaluation when her condition changed.
VERDICT $2.4 million verdict in the second trial, set aside by a Tennessee judge.
COMMENT Failure to appropriately diagnose breast cancer is a leading cause of medical malpractice. A persistent breast mass, no matter the mammographic findings, needs to be followed aggressively and appropriate evaluation and referral pursued.
Missed pulmonary embolism proves fatal
TWO FAINTING EPISODES caused a 41-year-old man to be transported to the emergency department (ED), where he was found to have decreased blood oxygenation, increased respiratory rate, and heart strain. The patient had hypertension and had recently taken 2 4-hour airplane trips.
An ED physician examined the man initially and admitted him to the hospital. About 12 hours after admission, an attending family physician saw the patient, but didn’t order any immediate testing. The patient subsequently died from a pulmonary embolism.
PLAINTIFFS’ CLAIM Prompt testing was needed to rule out pulmonary embolism.
THE DEFENSE Fainting isn’t a common sign of pulmonary embolism. A 4-hour plane ride usually isn’t sufficient to cause deep vein thrombosis.
VERDICT $975,000 New Jersey settlement.
COMMENT Although pulmonary embolism certainly has more classic presentations than this one, the combination of the patient’s history and clinical findings were of sufficient concern to warrant prompt evaluation.
Warfarin + a twisted back = bad outcome
A FALL DOWN A FLIGHT OF STAIRS in her home caused an 85-year-old woman to twist her back when she grabbed for the bannister (she caught herself before landing). She was taken to an emergency department, where the staff noted that she was taking warfarin; she was diagnosed with acute low back pain and strain. The patient continued to receive anticoagulation therapy.
Because the patient also had decreased sensation in her lower legs, a magnetic resonance imaging (MRI) scan of the lumbosacral spine was ordered. The wet read of the MRI reported degenerative joint disease at L4-5 and mild-to-moderate spinal stenosis at L1-2, L2-3, L3-4, and L4-5, with no other abnormalities. The radiologist who issued the formal report described similar findings.
The next morning, the patient complained of numbness in her legs. She couldn’t move either leg and needed help to turn in bed. By noon, she had minimal motor control of her legs and couldn’t stand.
The attending physician was notified, but didn’t assess the woman. When a nurse called the doctor to let her know that the physical therapist had concerns about the patient, the doctor said that she’d address the concerns the following morning.
A neurologist ultimately assessed the patient and reported that she had neurologic deficits in her legs that interfered with her ability to walk. The patient continues to have significantly impaired function in her legs.
PLAINTIFF’S CLAIM The radiologists failed to identify abnormal signal intensity on the MRI, which should have raised concerns about bleeding and prompted an immediate assessment. The patient’s warfarin therapy wasn’t managed properly.
THE DEFENSE Subdural bleeding in the spine is rare. The fall caused the neurologic impairment, which was unlikely to improve regardless of the timing of diagnosis or treatment. The proper orders were given based on the reported MRI results. Discontinuing warfarin posed a risk in light of the patient’s history of mini-strokes.
VERDICT $1.5 million Massachusetts settlement.
COMMENT Although we could debate the cause of this patient’s disability, anyone on warfarin is at risk for occult bleeding and requires careful assessment after a fall or injury.
Colon cancer blamed on failure to screen
AFTER HER PHYSICIAN LEFT HIS PRACTICE, a woman started seeing another doctor in the practice almost exclusively. The second doctor never discussed or recommended colon cancer screening. Seven years later, at 66 years of age, the patient was diagnosed with stage IIB adenocarcinoma of the colon. She underwent surgery to remove part of the large intestine and required 6 months of chemotherapy.
PLAINTIFF’S CLAIM The doctor was negligent for failing to recommend colon cancer screening. The patient wouldn’t have developed cancer if she’d undergone screening.
THE DEFENSE A screening recommendation wasn’t required because the patient visited the doctor’s office only for acute-care issues.
VERDICT $357,130 Illinois verdict.
COMMENT Even patients who are casual users of our practices should receive clearly documented screening recommendations or requests to have a complete physical.
Quinolone leads to tendon damage in patient with known allergy
SINUSITIS PROMPTED A 35-YEAR-OLD WOMAN to visit an otolaryngologist. The physician prescribed moxifloxacin, despite the woman’s well-documented history of allergy to quinolone antibiotics.
After 2 doses of the drug, the patient developed a reaction marked by tendon damage in the hips. She suffered ongoing limited mobility, which affected her work and interfered with her ability to pursue her hobbies.
PLAINTIFF’S CLAIM The doctor was negligent in prescribing moxifloxacin.
THE DEFENSE Although moxifloxacin belongs to the quinolone antibiotic class, it has differences that make prescribing it a matter of judgment.
VERDICT $203,614 Kentucky verdict.
COMMENT Although we don’t know the exact nature of the patient’s “allergy” to quinolone antibiotics—we all know of cases in which allergy is defined as a bit of diarrhea or stomach upset. I have to wonder whether the decision-making process that led to using moxifloxacin (instead of another antibiotic) was documented clearly.
Responsibility for delayed Dx cuts both ways
A 44-YEAR-OLD WOMAN went to a university medical clinic complaining of weight gain and fatigue. The clinic was staffed by residents supervised by clinical faculty. The resident who examined the woman found a 1.5-cm mobile mass in one of her breasts. After consultation with the supervising physician, a mammogram with ultrasound was ordered. The supervising physician didn’t see the patient, but signed off on the treatment plan.
The mammogram was performed 2 days later and the mass was evaluated as probably benign. The patient was advised to follow up in 6 months. A month later, a second resident consulted with the patient and told her that she could have a biopsy or follow her condition on her own. The patient decided against a biopsy.
Eight months later, the clinic sent a reminder to the patient to return for follow-up, which she did. At that time, the skin on her breast had the texture of orange rind and the mass had grown. Metastatic breast cancer was diagnosed. Aggressive treatment was recommended, but the patient opted for herbal and other homeopathic remedies.
The initial trial of the case ended in a defense verdict, which was appealed after the patient died. A second trial led to a verdict finding the supervising physician 99% at fault and the patient 1% at fault. The jury award was set aside by the trial court.
PLAINTIFF’S CLAIM Failure to diagnose breast cancer promptly constituted negligence. A needle biopsy was needed.
THE DEFENSE The follow-up plan was reasonable; the patient didn’t return for evaluation when her condition changed.
VERDICT $2.4 million verdict in the second trial, set aside by a Tennessee judge.
COMMENT Failure to appropriately diagnose breast cancer is a leading cause of medical malpractice. A persistent breast mass, no matter the mammographic findings, needs to be followed aggressively and appropriate evaluation and referral pursued.
Missed pulmonary embolism proves fatal
TWO FAINTING EPISODES caused a 41-year-old man to be transported to the emergency department (ED), where he was found to have decreased blood oxygenation, increased respiratory rate, and heart strain. The patient had hypertension and had recently taken 2 4-hour airplane trips.
An ED physician examined the man initially and admitted him to the hospital. About 12 hours after admission, an attending family physician saw the patient, but didn’t order any immediate testing. The patient subsequently died from a pulmonary embolism.
PLAINTIFFS’ CLAIM Prompt testing was needed to rule out pulmonary embolism.
THE DEFENSE Fainting isn’t a common sign of pulmonary embolism. A 4-hour plane ride usually isn’t sufficient to cause deep vein thrombosis.
VERDICT $975,000 New Jersey settlement.
COMMENT Although pulmonary embolism certainly has more classic presentations than this one, the combination of the patient’s history and clinical findings were of sufficient concern to warrant prompt evaluation.
Warfarin + a twisted back = bad outcome
A FALL DOWN A FLIGHT OF STAIRS in her home caused an 85-year-old woman to twist her back when she grabbed for the bannister (she caught herself before landing). She was taken to an emergency department, where the staff noted that she was taking warfarin; she was diagnosed with acute low back pain and strain. The patient continued to receive anticoagulation therapy.
Because the patient also had decreased sensation in her lower legs, a magnetic resonance imaging (MRI) scan of the lumbosacral spine was ordered. The wet read of the MRI reported degenerative joint disease at L4-5 and mild-to-moderate spinal stenosis at L1-2, L2-3, L3-4, and L4-5, with no other abnormalities. The radiologist who issued the formal report described similar findings.
The next morning, the patient complained of numbness in her legs. She couldn’t move either leg and needed help to turn in bed. By noon, she had minimal motor control of her legs and couldn’t stand.
The attending physician was notified, but didn’t assess the woman. When a nurse called the doctor to let her know that the physical therapist had concerns about the patient, the doctor said that she’d address the concerns the following morning.
A neurologist ultimately assessed the patient and reported that she had neurologic deficits in her legs that interfered with her ability to walk. The patient continues to have significantly impaired function in her legs.
PLAINTIFF’S CLAIM The radiologists failed to identify abnormal signal intensity on the MRI, which should have raised concerns about bleeding and prompted an immediate assessment. The patient’s warfarin therapy wasn’t managed properly.
THE DEFENSE Subdural bleeding in the spine is rare. The fall caused the neurologic impairment, which was unlikely to improve regardless of the timing of diagnosis or treatment. The proper orders were given based on the reported MRI results. Discontinuing warfarin posed a risk in light of the patient’s history of mini-strokes.
VERDICT $1.5 million Massachusetts settlement.
COMMENT Although we could debate the cause of this patient’s disability, anyone on warfarin is at risk for occult bleeding and requires careful assessment after a fall or injury.
Colon cancer blamed on failure to screen
AFTER HER PHYSICIAN LEFT HIS PRACTICE, a woman started seeing another doctor in the practice almost exclusively. The second doctor never discussed or recommended colon cancer screening. Seven years later, at 66 years of age, the patient was diagnosed with stage IIB adenocarcinoma of the colon. She underwent surgery to remove part of the large intestine and required 6 months of chemotherapy.
PLAINTIFF’S CLAIM The doctor was negligent for failing to recommend colon cancer screening. The patient wouldn’t have developed cancer if she’d undergone screening.
THE DEFENSE A screening recommendation wasn’t required because the patient visited the doctor’s office only for acute-care issues.
VERDICT $357,130 Illinois verdict.
COMMENT Even patients who are casual users of our practices should receive clearly documented screening recommendations or requests to have a complete physical.
Quinolone leads to tendon damage in patient with known allergy
SINUSITIS PROMPTED A 35-YEAR-OLD WOMAN to visit an otolaryngologist. The physician prescribed moxifloxacin, despite the woman’s well-documented history of allergy to quinolone antibiotics.
After 2 doses of the drug, the patient developed a reaction marked by tendon damage in the hips. She suffered ongoing limited mobility, which affected her work and interfered with her ability to pursue her hobbies.
PLAINTIFF’S CLAIM The doctor was negligent in prescribing moxifloxacin.
THE DEFENSE Although moxifloxacin belongs to the quinolone antibiotic class, it has differences that make prescribing it a matter of judgment.
VERDICT $203,614 Kentucky verdict.
COMMENT Although we don’t know the exact nature of the patient’s “allergy” to quinolone antibiotics—we all know of cases in which allergy is defined as a bit of diarrhea or stomach upset. I have to wonder whether the decision-making process that led to using moxifloxacin (instead of another antibiotic) was documented clearly.
Responsibility for delayed Dx cuts both ways
A 44-YEAR-OLD WOMAN went to a university medical clinic complaining of weight gain and fatigue. The clinic was staffed by residents supervised by clinical faculty. The resident who examined the woman found a 1.5-cm mobile mass in one of her breasts. After consultation with the supervising physician, a mammogram with ultrasound was ordered. The supervising physician didn’t see the patient, but signed off on the treatment plan.
The mammogram was performed 2 days later and the mass was evaluated as probably benign. The patient was advised to follow up in 6 months. A month later, a second resident consulted with the patient and told her that she could have a biopsy or follow her condition on her own. The patient decided against a biopsy.
Eight months later, the clinic sent a reminder to the patient to return for follow-up, which she did. At that time, the skin on her breast had the texture of orange rind and the mass had grown. Metastatic breast cancer was diagnosed. Aggressive treatment was recommended, but the patient opted for herbal and other homeopathic remedies.
The initial trial of the case ended in a defense verdict, which was appealed after the patient died. A second trial led to a verdict finding the supervising physician 99% at fault and the patient 1% at fault. The jury award was set aside by the trial court.
PLAINTIFF’S CLAIM Failure to diagnose breast cancer promptly constituted negligence. A needle biopsy was needed.
THE DEFENSE The follow-up plan was reasonable; the patient didn’t return for evaluation when her condition changed.
VERDICT $2.4 million verdict in the second trial, set aside by a Tennessee judge.
COMMENT Failure to appropriately diagnose breast cancer is a leading cause of medical malpractice. A persistent breast mass, no matter the mammographic findings, needs to be followed aggressively and appropriate evaluation and referral pursued.
Missed pulmonary embolism proves fatal
TWO FAINTING EPISODES caused a 41-year-old man to be transported to the emergency department (ED), where he was found to have decreased blood oxygenation, increased respiratory rate, and heart strain. The patient had hypertension and had recently taken 2 4-hour airplane trips.
An ED physician examined the man initially and admitted him to the hospital. About 12 hours after admission, an attending family physician saw the patient, but didn’t order any immediate testing. The patient subsequently died from a pulmonary embolism.
PLAINTIFFS’ CLAIM Prompt testing was needed to rule out pulmonary embolism.
THE DEFENSE Fainting isn’t a common sign of pulmonary embolism. A 4-hour plane ride usually isn’t sufficient to cause deep vein thrombosis.
VERDICT $975,000 New Jersey settlement.
COMMENT Although pulmonary embolism certainly has more classic presentations than this one, the combination of the patient’s history and clinical findings were of sufficient concern to warrant prompt evaluation.
Warfarin + a twisted back = bad outcome
A FALL DOWN A FLIGHT OF STAIRS in her home caused an 85-year-old woman to twist her back when she grabbed for the bannister (she caught herself before landing). She was taken to an emergency department, where the staff noted that she was taking warfarin; she was diagnosed with acute low back pain and strain. The patient continued to receive anticoagulation therapy.
Because the patient also had decreased sensation in her lower legs, a magnetic resonance imaging (MRI) scan of the lumbosacral spine was ordered. The wet read of the MRI reported degenerative joint disease at L4-5 and mild-to-moderate spinal stenosis at L1-2, L2-3, L3-4, and L4-5, with no other abnormalities. The radiologist who issued the formal report described similar findings.
The next morning, the patient complained of numbness in her legs. She couldn’t move either leg and needed help to turn in bed. By noon, she had minimal motor control of her legs and couldn’t stand.
The attending physician was notified, but didn’t assess the woman. When a nurse called the doctor to let her know that the physical therapist had concerns about the patient, the doctor said that she’d address the concerns the following morning.
A neurologist ultimately assessed the patient and reported that she had neurologic deficits in her legs that interfered with her ability to walk. The patient continues to have significantly impaired function in her legs.
PLAINTIFF’S CLAIM The radiologists failed to identify abnormal signal intensity on the MRI, which should have raised concerns about bleeding and prompted an immediate assessment. The patient’s warfarin therapy wasn’t managed properly.
THE DEFENSE Subdural bleeding in the spine is rare. The fall caused the neurologic impairment, which was unlikely to improve regardless of the timing of diagnosis or treatment. The proper orders were given based on the reported MRI results. Discontinuing warfarin posed a risk in light of the patient’s history of mini-strokes.
VERDICT $1.5 million Massachusetts settlement.
COMMENT Although we could debate the cause of this patient’s disability, anyone on warfarin is at risk for occult bleeding and requires careful assessment after a fall or injury.
Colon cancer blamed on failure to screen
AFTER HER PHYSICIAN LEFT HIS PRACTICE, a woman started seeing another doctor in the practice almost exclusively. The second doctor never discussed or recommended colon cancer screening. Seven years later, at 66 years of age, the patient was diagnosed with stage IIB adenocarcinoma of the colon. She underwent surgery to remove part of the large intestine and required 6 months of chemotherapy.
PLAINTIFF’S CLAIM The doctor was negligent for failing to recommend colon cancer screening. The patient wouldn’t have developed cancer if she’d undergone screening.
THE DEFENSE A screening recommendation wasn’t required because the patient visited the doctor’s office only for acute-care issues.
VERDICT $357,130 Illinois verdict.
COMMENT Even patients who are casual users of our practices should receive clearly documented screening recommendations or requests to have a complete physical.
Quinolone leads to tendon damage in patient with known allergy
SINUSITIS PROMPTED A 35-YEAR-OLD WOMAN to visit an otolaryngologist. The physician prescribed moxifloxacin, despite the woman’s well-documented history of allergy to quinolone antibiotics.
After 2 doses of the drug, the patient developed a reaction marked by tendon damage in the hips. She suffered ongoing limited mobility, which affected her work and interfered with her ability to pursue her hobbies.
PLAINTIFF’S CLAIM The doctor was negligent in prescribing moxifloxacin.
THE DEFENSE Although moxifloxacin belongs to the quinolone antibiotic class, it has differences that make prescribing it a matter of judgment.
VERDICT $203,614 Kentucky verdict.
COMMENT Although we don’t know the exact nature of the patient’s “allergy” to quinolone antibiotics—we all know of cases in which allergy is defined as a bit of diarrhea or stomach upset. I have to wonder whether the decision-making process that led to using moxifloxacin (instead of another antibiotic) was documented clearly.
Birth control prescription blamed for stroke...Removal of mole without follow-up leads to death...more
Birth control prescription blamed for stroke
A 29-YEAR-OLD WOMAN SUFFERED A BLOOD CLOT in her leg. Her family physician advised her to start taking aspirin, which she did, and counseled her to use birth control that didn’t contain estrogen. She was taking norgestimate/ ethinyl estradiol at the time of the clot.
The woman subsequently went to an obstetrician-gynecologist (ob-gyn), whom she said she told about her family physician’s advice to avoid estrogen-containing birth control medication. The ob-gyn prescribed and inserted an etonogestrel/ethinyl estradiol vaginal ring.
A few months later the patient was hospitalized with severe headaches. She had blood clots in her brain and had suffered a stroke, which affected her speech and executive functions.
PLAINTIFF’S CLAIM The ob-gyn was negligent in prescribing the vaginal ring.
THE DEFENSE The cause of the first clot was an injury; the vaginal ring didn’t cause the second clot and stroke.
VERDICT $523,000 Georgia verdict.
COMMENT A comprehensive history, and clear documentation of communicating the potential risks of therapy, might have prevented this judgment.
Elevated PSA without referral delays diagnosis
ROUTINE BLOOD WORK before orthopedic surgery revealed an elevated prostate-specific antigen (PSA) of 7.4 in a 53-year-old man. A medical assistant who was directed to refer the patient to a urologist didn’t do so. Widespread metastatic prostate cancer was diagnosed 18 months later.
PLAINTIFF’S CLAIM Diagnosing the cancer 18 months earlier would have given the patient a >50% chance of 5-year survival. Because of the delay, he was terminal. The clinic was negligent in having no written procedure or system for tracking adverse lab test results.
THE DEFENSE The patient already had metastatic disease when the PSA level was discovered and would have required the same treatment.
VERDICT $1 million Washington settlement.
COMMENT A clear system for tracking test results is imperative in today’s litigious society.
Removal of mole without follow-up leads to death
A MOLE ON THE UPPER BACK prompted a 26-year-old man to visit a dermatologist, who performed a complete excision. The pathologist who examined the excised tissue suggested that the patient return for follow-up. During the next 6 months, the patient saw the dermatologist twice but didn’t receive proper follow-up.
Two years later, the patient noticed a suspicious area on his back near the scar from the excision. A hospital biopsy resulted in a diagnosis of metastatic melanoma. A review of the slides from the original biopsy found “melanoma, superficial spreading type, invasive to a depth of a minimum of 1.0 mm anatomic level IV, extending to inked deep resection margin.”
The patient underwent a wide local excision and was given a diagnosis of stage III melanoma. The patient underwent neck and back radiation and high-dose treatment with alpha interferon, followed by high-dose interleukin-2 and chemotherapy. Nevertheless, the patient died.
PLAINTIFF’S CLAIM The dermatologist’s office had no system to contact the patient when he didn’t return. The chances for cure would have been between 73% and 94% if the melanoma had been diagnosed at the time of the original excision.
THE DEFENSE No information about the defense is available.
VERDICT $1.7 million Massachusetts settlement.
COMMENT Failure to follow up on abnormal results is a potentially preventable cause of malpractice. Do you have a mechanism to track such testing?
Suggestive symptoms, but no Dx until it was too late
A 42-YEAR-OLD WOMAN went to the hospital in February for chest pain, dizziness, and shortness of breath. The emergency room physician diagnosed sinusitis and bronchitis and discharged the patient in stable condition. In April, the woman visited her primary care physician complaining of fatigue and shortness of breath. She claimed that her physician knew about the February emergency room visit. Later in April, she again went to her physician with shortness of breath; in July, she reported an irregular heart rhythm.
In October, the patient was found unresponsive after suffering cardiorespiratory arrest, hypoxic ischemic brain injury, and static encephalopathy. She has since been in a vegetative state.
PLAINTIFF’S CLAIM The patient had gone to her primary care physician many times during the 2 years before her emergency room visit with complaints suggesting an underlying cardiac condition, including shortness of breath, dizziness, light-headedness, vertigo, chest tightness, fatigue, and an irregular heart rhythm. The defendants were negligent in failing to diagnose the patient’s condition and provide proper treatment, failing to order proper diagnostic testing, and failing to perform a cardiac workup.
THE DEFENSE No negligence occurred.
VERDICT $6.3 million Florida verdict.
COMMENT Comprehensive documentation, including your medical decision making, can help prevent multimillion dollar judgments.
A serendipitous finding—to no avail
A FALL ON THE ICE sent a 74-year-old woman to the hospital with a fractured ankle. A preoperative chest radiograph taken before open reduction and internal fixation to repair the fracture showed a 2-cm nodular opacity in the right upper hemithorax. The radiologist recommended a computed tomography scan to rule out lung cancer, but the treating internists didn’t order a scan or refer the patient for biopsy.
The nodule appeared again on a second radiograph taken 2 days later. The patient wasn’t informed, and the attending internist at the time didn’t order follow-up testing or refer the patient to a specialist. The attending physicians continued to treat the patient without further testing or referral for the nodule.
Two years after the fracture, the patient was admitted to the hospital with complaints of sweating and shortness of breath. A chest radiograph showed pneumonia and the previously noted nodule. The patient was diagnosed with metastatic, inoperable small-cell lung cancer. She died after receiving extensive chemotherapy and radiation.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the lung cancer in a timely manner.
THE DEFENSE No information about the defense is available.
VERDICT $325,000 Michigan settlement.
COMMENT Could this happen to you? How many times have you serendipitously noted an abnormal result that was not followed up adequately?
Birth control prescription blamed for stroke
A 29-YEAR-OLD WOMAN SUFFERED A BLOOD CLOT in her leg. Her family physician advised her to start taking aspirin, which she did, and counseled her to use birth control that didn’t contain estrogen. She was taking norgestimate/ ethinyl estradiol at the time of the clot.
The woman subsequently went to an obstetrician-gynecologist (ob-gyn), whom she said she told about her family physician’s advice to avoid estrogen-containing birth control medication. The ob-gyn prescribed and inserted an etonogestrel/ethinyl estradiol vaginal ring.
A few months later the patient was hospitalized with severe headaches. She had blood clots in her brain and had suffered a stroke, which affected her speech and executive functions.
PLAINTIFF’S CLAIM The ob-gyn was negligent in prescribing the vaginal ring.
THE DEFENSE The cause of the first clot was an injury; the vaginal ring didn’t cause the second clot and stroke.
VERDICT $523,000 Georgia verdict.
COMMENT A comprehensive history, and clear documentation of communicating the potential risks of therapy, might have prevented this judgment.
Elevated PSA without referral delays diagnosis
ROUTINE BLOOD WORK before orthopedic surgery revealed an elevated prostate-specific antigen (PSA) of 7.4 in a 53-year-old man. A medical assistant who was directed to refer the patient to a urologist didn’t do so. Widespread metastatic prostate cancer was diagnosed 18 months later.
PLAINTIFF’S CLAIM Diagnosing the cancer 18 months earlier would have given the patient a >50% chance of 5-year survival. Because of the delay, he was terminal. The clinic was negligent in having no written procedure or system for tracking adverse lab test results.
THE DEFENSE The patient already had metastatic disease when the PSA level was discovered and would have required the same treatment.
VERDICT $1 million Washington settlement.
COMMENT A clear system for tracking test results is imperative in today’s litigious society.
Removal of mole without follow-up leads to death
A MOLE ON THE UPPER BACK prompted a 26-year-old man to visit a dermatologist, who performed a complete excision. The pathologist who examined the excised tissue suggested that the patient return for follow-up. During the next 6 months, the patient saw the dermatologist twice but didn’t receive proper follow-up.
Two years later, the patient noticed a suspicious area on his back near the scar from the excision. A hospital biopsy resulted in a diagnosis of metastatic melanoma. A review of the slides from the original biopsy found “melanoma, superficial spreading type, invasive to a depth of a minimum of 1.0 mm anatomic level IV, extending to inked deep resection margin.”
The patient underwent a wide local excision and was given a diagnosis of stage III melanoma. The patient underwent neck and back radiation and high-dose treatment with alpha interferon, followed by high-dose interleukin-2 and chemotherapy. Nevertheless, the patient died.
PLAINTIFF’S CLAIM The dermatologist’s office had no system to contact the patient when he didn’t return. The chances for cure would have been between 73% and 94% if the melanoma had been diagnosed at the time of the original excision.
THE DEFENSE No information about the defense is available.
VERDICT $1.7 million Massachusetts settlement.
COMMENT Failure to follow up on abnormal results is a potentially preventable cause of malpractice. Do you have a mechanism to track such testing?
Suggestive symptoms, but no Dx until it was too late
A 42-YEAR-OLD WOMAN went to the hospital in February for chest pain, dizziness, and shortness of breath. The emergency room physician diagnosed sinusitis and bronchitis and discharged the patient in stable condition. In April, the woman visited her primary care physician complaining of fatigue and shortness of breath. She claimed that her physician knew about the February emergency room visit. Later in April, she again went to her physician with shortness of breath; in July, she reported an irregular heart rhythm.
In October, the patient was found unresponsive after suffering cardiorespiratory arrest, hypoxic ischemic brain injury, and static encephalopathy. She has since been in a vegetative state.
PLAINTIFF’S CLAIM The patient had gone to her primary care physician many times during the 2 years before her emergency room visit with complaints suggesting an underlying cardiac condition, including shortness of breath, dizziness, light-headedness, vertigo, chest tightness, fatigue, and an irregular heart rhythm. The defendants were negligent in failing to diagnose the patient’s condition and provide proper treatment, failing to order proper diagnostic testing, and failing to perform a cardiac workup.
THE DEFENSE No negligence occurred.
VERDICT $6.3 million Florida verdict.
COMMENT Comprehensive documentation, including your medical decision making, can help prevent multimillion dollar judgments.
A serendipitous finding—to no avail
A FALL ON THE ICE sent a 74-year-old woman to the hospital with a fractured ankle. A preoperative chest radiograph taken before open reduction and internal fixation to repair the fracture showed a 2-cm nodular opacity in the right upper hemithorax. The radiologist recommended a computed tomography scan to rule out lung cancer, but the treating internists didn’t order a scan or refer the patient for biopsy.
The nodule appeared again on a second radiograph taken 2 days later. The patient wasn’t informed, and the attending internist at the time didn’t order follow-up testing or refer the patient to a specialist. The attending physicians continued to treat the patient without further testing or referral for the nodule.
Two years after the fracture, the patient was admitted to the hospital with complaints of sweating and shortness of breath. A chest radiograph showed pneumonia and the previously noted nodule. The patient was diagnosed with metastatic, inoperable small-cell lung cancer. She died after receiving extensive chemotherapy and radiation.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the lung cancer in a timely manner.
THE DEFENSE No information about the defense is available.
VERDICT $325,000 Michigan settlement.
COMMENT Could this happen to you? How many times have you serendipitously noted an abnormal result that was not followed up adequately?
Birth control prescription blamed for stroke
A 29-YEAR-OLD WOMAN SUFFERED A BLOOD CLOT in her leg. Her family physician advised her to start taking aspirin, which she did, and counseled her to use birth control that didn’t contain estrogen. She was taking norgestimate/ ethinyl estradiol at the time of the clot.
The woman subsequently went to an obstetrician-gynecologist (ob-gyn), whom she said she told about her family physician’s advice to avoid estrogen-containing birth control medication. The ob-gyn prescribed and inserted an etonogestrel/ethinyl estradiol vaginal ring.
A few months later the patient was hospitalized with severe headaches. She had blood clots in her brain and had suffered a stroke, which affected her speech and executive functions.
PLAINTIFF’S CLAIM The ob-gyn was negligent in prescribing the vaginal ring.
THE DEFENSE The cause of the first clot was an injury; the vaginal ring didn’t cause the second clot and stroke.
VERDICT $523,000 Georgia verdict.
COMMENT A comprehensive history, and clear documentation of communicating the potential risks of therapy, might have prevented this judgment.
Elevated PSA without referral delays diagnosis
ROUTINE BLOOD WORK before orthopedic surgery revealed an elevated prostate-specific antigen (PSA) of 7.4 in a 53-year-old man. A medical assistant who was directed to refer the patient to a urologist didn’t do so. Widespread metastatic prostate cancer was diagnosed 18 months later.
PLAINTIFF’S CLAIM Diagnosing the cancer 18 months earlier would have given the patient a >50% chance of 5-year survival. Because of the delay, he was terminal. The clinic was negligent in having no written procedure or system for tracking adverse lab test results.
THE DEFENSE The patient already had metastatic disease when the PSA level was discovered and would have required the same treatment.
VERDICT $1 million Washington settlement.
COMMENT A clear system for tracking test results is imperative in today’s litigious society.
Removal of mole without follow-up leads to death
A MOLE ON THE UPPER BACK prompted a 26-year-old man to visit a dermatologist, who performed a complete excision. The pathologist who examined the excised tissue suggested that the patient return for follow-up. During the next 6 months, the patient saw the dermatologist twice but didn’t receive proper follow-up.
Two years later, the patient noticed a suspicious area on his back near the scar from the excision. A hospital biopsy resulted in a diagnosis of metastatic melanoma. A review of the slides from the original biopsy found “melanoma, superficial spreading type, invasive to a depth of a minimum of 1.0 mm anatomic level IV, extending to inked deep resection margin.”
The patient underwent a wide local excision and was given a diagnosis of stage III melanoma. The patient underwent neck and back radiation and high-dose treatment with alpha interferon, followed by high-dose interleukin-2 and chemotherapy. Nevertheless, the patient died.
PLAINTIFF’S CLAIM The dermatologist’s office had no system to contact the patient when he didn’t return. The chances for cure would have been between 73% and 94% if the melanoma had been diagnosed at the time of the original excision.
THE DEFENSE No information about the defense is available.
VERDICT $1.7 million Massachusetts settlement.
COMMENT Failure to follow up on abnormal results is a potentially preventable cause of malpractice. Do you have a mechanism to track such testing?
Suggestive symptoms, but no Dx until it was too late
A 42-YEAR-OLD WOMAN went to the hospital in February for chest pain, dizziness, and shortness of breath. The emergency room physician diagnosed sinusitis and bronchitis and discharged the patient in stable condition. In April, the woman visited her primary care physician complaining of fatigue and shortness of breath. She claimed that her physician knew about the February emergency room visit. Later in April, she again went to her physician with shortness of breath; in July, she reported an irregular heart rhythm.
In October, the patient was found unresponsive after suffering cardiorespiratory arrest, hypoxic ischemic brain injury, and static encephalopathy. She has since been in a vegetative state.
PLAINTIFF’S CLAIM The patient had gone to her primary care physician many times during the 2 years before her emergency room visit with complaints suggesting an underlying cardiac condition, including shortness of breath, dizziness, light-headedness, vertigo, chest tightness, fatigue, and an irregular heart rhythm. The defendants were negligent in failing to diagnose the patient’s condition and provide proper treatment, failing to order proper diagnostic testing, and failing to perform a cardiac workup.
THE DEFENSE No negligence occurred.
VERDICT $6.3 million Florida verdict.
COMMENT Comprehensive documentation, including your medical decision making, can help prevent multimillion dollar judgments.
A serendipitous finding—to no avail
A FALL ON THE ICE sent a 74-year-old woman to the hospital with a fractured ankle. A preoperative chest radiograph taken before open reduction and internal fixation to repair the fracture showed a 2-cm nodular opacity in the right upper hemithorax. The radiologist recommended a computed tomography scan to rule out lung cancer, but the treating internists didn’t order a scan or refer the patient for biopsy.
The nodule appeared again on a second radiograph taken 2 days later. The patient wasn’t informed, and the attending internist at the time didn’t order follow-up testing or refer the patient to a specialist. The attending physicians continued to treat the patient without further testing or referral for the nodule.
Two years after the fracture, the patient was admitted to the hospital with complaints of sweating and shortness of breath. A chest radiograph showed pneumonia and the previously noted nodule. The patient was diagnosed with metastatic, inoperable small-cell lung cancer. She died after receiving extensive chemotherapy and radiation.
PLAINTIFF’S CLAIM The doctors were negligent in failing to diagnose and treat the lung cancer in a timely manner.
THE DEFENSE No information about the defense is available.
VERDICT $325,000 Michigan settlement.
COMMENT Could this happen to you? How many times have you serendipitously noted an abnormal result that was not followed up adequately?
Back pain, then sudden death...Increase in morphine dose has fatal results...more
Back pain, then sudden death
BACK AND CHEST PAIN prompted a 42-year-old man to see a doctor. The family physician took the man’s blood pressure, which was 184/130, but performed no other testing. He prescribed pain medication and sent the patient home. The man died 3 days later of an aortic dissection.
PLAINTIFF’S CLAIM The physician was negligent because he did not try to lower the patient’s blood pressure or order a radiograph before sending him home.
THE DEFENSE There was no reason to suspect aortic dissection; the rupture was sudden and catastrophic.
VERDICT Alabama defense verdict.
COMMENT Although the defense prevailed, this case reminds us to always consider less common causes of low back pain.
Increase in morphine dose has fatal results
BREATHING DIFFICULTIES associated with chronic obstructive pulmonary disease led to the hospitalization of a 79-year-old woman. While there, she suffered respiratory arrest and a code was called. The pulmonologist on duty and the attending physician responded. After the patient was bagged, she started breathing on her own.
The attending physician subsequently discussed the patient’s treatment plan and prognosis with her daughter, who agreed to a do-not-resuscitate order. He ordered 2 mg morphine as needed for comfort.
Minutes later, the pulmonologist overrode the order and ordered 20 mg morphine by IV push. After it was given, the patient lost consciousness while talking to her daughter and granddaughter. She died about 3 hours later without regaining consciousness.
PLAINTIFF’S CLAIM The patient was improving until the night before her arrest, when she failed to get her scheduled breathing treatment. The pulmonologist was negligent in ordering 20 mg morphine, and the hospital nurses were negligent in administering it.
THE DEFENSE No negligence occurred. The patient would have died sooner than 3 hours after the morphine dose if morphine was, indeed, the cause of death.
VERDICT $3 million Georgia verdict.
COMMENT Do not resuscitate does not mean negligible risk of malpractice. Orders for 20 mg (!) of morphine will always be difficult to defend—even in a terminally ill patient.
Breast cancer diagnosis falls through the cracks
AFTER NOTICING A LUMP IN HER LEFT BREAST, a woman in her 40s underwent a screening mammogram rather than a diagnostic mammogram at a local facility. The mammogram showed no abnormalities, but an ultrasound examination the following year was abnormal. The report was faxed to her physician, who reportedly didn’t receive it. No follow-up occurred.
A year later, the patient made a follow-up appointment on her own and underwent diagnostic mammography and surgical biopsy, which revealed advanced breast cancer. A vacuum-assisted core biopsy and clip localization the following month revealed infiltrating ductal carcinoma. Neoadjuvant chemotherapy resulted in complications and hospitalization. The patient subsequently underwent additional chemotherapy and radiation treatments.
PLAINTIFF’S CLAIM The defendant health care facility didn’t properly evaluate the patient for breast cancer.
THE DEFENSE The defendant denied liability and asserted that its personnel acted within the standard of care.
VERDICT $575,000 South Carolina settlement.
COMMENT Follow-up and tracking of results remain key in preventing malpractice.
Back pain, then sudden death
BACK AND CHEST PAIN prompted a 42-year-old man to see a doctor. The family physician took the man’s blood pressure, which was 184/130, but performed no other testing. He prescribed pain medication and sent the patient home. The man died 3 days later of an aortic dissection.
PLAINTIFF’S CLAIM The physician was negligent because he did not try to lower the patient’s blood pressure or order a radiograph before sending him home.
THE DEFENSE There was no reason to suspect aortic dissection; the rupture was sudden and catastrophic.
VERDICT Alabama defense verdict.
COMMENT Although the defense prevailed, this case reminds us to always consider less common causes of low back pain.
Increase in morphine dose has fatal results
BREATHING DIFFICULTIES associated with chronic obstructive pulmonary disease led to the hospitalization of a 79-year-old woman. While there, she suffered respiratory arrest and a code was called. The pulmonologist on duty and the attending physician responded. After the patient was bagged, she started breathing on her own.
The attending physician subsequently discussed the patient’s treatment plan and prognosis with her daughter, who agreed to a do-not-resuscitate order. He ordered 2 mg morphine as needed for comfort.
Minutes later, the pulmonologist overrode the order and ordered 20 mg morphine by IV push. After it was given, the patient lost consciousness while talking to her daughter and granddaughter. She died about 3 hours later without regaining consciousness.
PLAINTIFF’S CLAIM The patient was improving until the night before her arrest, when she failed to get her scheduled breathing treatment. The pulmonologist was negligent in ordering 20 mg morphine, and the hospital nurses were negligent in administering it.
THE DEFENSE No negligence occurred. The patient would have died sooner than 3 hours after the morphine dose if morphine was, indeed, the cause of death.
VERDICT $3 million Georgia verdict.
COMMENT Do not resuscitate does not mean negligible risk of malpractice. Orders for 20 mg (!) of morphine will always be difficult to defend—even in a terminally ill patient.
Breast cancer diagnosis falls through the cracks
AFTER NOTICING A LUMP IN HER LEFT BREAST, a woman in her 40s underwent a screening mammogram rather than a diagnostic mammogram at a local facility. The mammogram showed no abnormalities, but an ultrasound examination the following year was abnormal. The report was faxed to her physician, who reportedly didn’t receive it. No follow-up occurred.
A year later, the patient made a follow-up appointment on her own and underwent diagnostic mammography and surgical biopsy, which revealed advanced breast cancer. A vacuum-assisted core biopsy and clip localization the following month revealed infiltrating ductal carcinoma. Neoadjuvant chemotherapy resulted in complications and hospitalization. The patient subsequently underwent additional chemotherapy and radiation treatments.
PLAINTIFF’S CLAIM The defendant health care facility didn’t properly evaluate the patient for breast cancer.
THE DEFENSE The defendant denied liability and asserted that its personnel acted within the standard of care.
VERDICT $575,000 South Carolina settlement.
COMMENT Follow-up and tracking of results remain key in preventing malpractice.
Back pain, then sudden death
BACK AND CHEST PAIN prompted a 42-year-old man to see a doctor. The family physician took the man’s blood pressure, which was 184/130, but performed no other testing. He prescribed pain medication and sent the patient home. The man died 3 days later of an aortic dissection.
PLAINTIFF’S CLAIM The physician was negligent because he did not try to lower the patient’s blood pressure or order a radiograph before sending him home.
THE DEFENSE There was no reason to suspect aortic dissection; the rupture was sudden and catastrophic.
VERDICT Alabama defense verdict.
COMMENT Although the defense prevailed, this case reminds us to always consider less common causes of low back pain.
Increase in morphine dose has fatal results
BREATHING DIFFICULTIES associated with chronic obstructive pulmonary disease led to the hospitalization of a 79-year-old woman. While there, she suffered respiratory arrest and a code was called. The pulmonologist on duty and the attending physician responded. After the patient was bagged, she started breathing on her own.
The attending physician subsequently discussed the patient’s treatment plan and prognosis with her daughter, who agreed to a do-not-resuscitate order. He ordered 2 mg morphine as needed for comfort.
Minutes later, the pulmonologist overrode the order and ordered 20 mg morphine by IV push. After it was given, the patient lost consciousness while talking to her daughter and granddaughter. She died about 3 hours later without regaining consciousness.
PLAINTIFF’S CLAIM The patient was improving until the night before her arrest, when she failed to get her scheduled breathing treatment. The pulmonologist was negligent in ordering 20 mg morphine, and the hospital nurses were negligent in administering it.
THE DEFENSE No negligence occurred. The patient would have died sooner than 3 hours after the morphine dose if morphine was, indeed, the cause of death.
VERDICT $3 million Georgia verdict.
COMMENT Do not resuscitate does not mean negligible risk of malpractice. Orders for 20 mg (!) of morphine will always be difficult to defend—even in a terminally ill patient.
Breast cancer diagnosis falls through the cracks
AFTER NOTICING A LUMP IN HER LEFT BREAST, a woman in her 40s underwent a screening mammogram rather than a diagnostic mammogram at a local facility. The mammogram showed no abnormalities, but an ultrasound examination the following year was abnormal. The report was faxed to her physician, who reportedly didn’t receive it. No follow-up occurred.
A year later, the patient made a follow-up appointment on her own and underwent diagnostic mammography and surgical biopsy, which revealed advanced breast cancer. A vacuum-assisted core biopsy and clip localization the following month revealed infiltrating ductal carcinoma. Neoadjuvant chemotherapy resulted in complications and hospitalization. The patient subsequently underwent additional chemotherapy and radiation treatments.
PLAINTIFF’S CLAIM The defendant health care facility didn’t properly evaluate the patient for breast cancer.
THE DEFENSE The defendant denied liability and asserted that its personnel acted within the standard of care.
VERDICT $575,000 South Carolina settlement.
COMMENT Follow-up and tracking of results remain key in preventing malpractice.
Does office spirometry improve quit rates in smokers?
IT DEPENDS. Simply performing spirometry and offering cessation advice doesn’t improve quit rates in patients who smoke (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]). However, when the spirometry results are communicated in terms of “lung age,” smokers are more likely to quit (SOR: B, large RCT). Patients with abnormal spirometry results may be more likely to quit than patients with normal results (SOR: B, cohort studies).
Evidence summary
A systematic review of 3 RCTs with a total of 649 participants evaluated office spirometry as a motivational tool to improve quit rates by comparing spirometry plus cessation advice with cessation advice alone. All participants were men and women 19 to 75 years of age recruited from outpatient clinics.1
In 1 trial, the intervention group received repeated counseling at 4 visits and underwent spirometry; the control group had 1 counseling session and was given a brochure. In the other 2 trials, the intervention group had both carbon monoxide measurements and spirometry, and all participants received more extensive counseling, including cessation skills training.
At 9 to 12 months’ follow-up, quit rates ranged from 6% to 24% in the intervention groups vs 5% to 14% in the control groups (not significantly different).1
A subsequent study randomized 221 smokers to receive either spirometry plus brief cessation advice or advice alone. Researchers recruited patients 15 to 80 years of age who were willing to quit smoking from 16 general practice clinics in Belgium. Fifty-one percent of patients in both groups used nicotine replacement therapy (a larger percentage than is typical in studies done in the United States). At 6, 12, and 24 months, 5%, 2%, and 5% more smokers, respectively, from the spirometry group quit smoking compared with the control group, but this difference was not significant.2
Reporting spirometry results in terms of lung age may spur quitting
One RCT found significantly improved quit rates when patients who smoked were given their office spirometry results in terms of “lung age” (the age of an average healthy person with similar spirometry results) rather than as forced expiratory volume in 1 second (FEV1). Investigators performed office spirometry and gave smoking cessation advice to 561 smokers older than 35 years who were recruited from 5 general practices. They randomized patients to receive their spirometry results as either lung age or FEV1 and recorded quit rates at 12 months (smoking cessation was verified by measuring blood levels of carbon monoxide).
Patients whose spirometry results were reported as lung age were significantly more likely to quit than smokers whose results were given as FEV1 (13.6% vs 6.4%; P=.005; number needed to treat [NNT]=14 smokers counseled using lung age to cause 1 more patient to quit). Smokers with normal lung ages were no more likely to quit than smokers with abnormal results.3
Abnormal results also may be a motivator
However, 3 prospective cohort studies demonstrated that patients with abnormal spirometry results were more likely to quit than patients with normal spirometry. In the first study, 4494 patients with at least 10 pack-years of smoking from 10 outpatient chest clinics in Poland underwent spirometry and were counseled to quit smoking; 1177 had abnormal spirometry results.
One year later, 16.3% of smokers with abnormal results had quit smoking, compared with 12% in the group with normal spirometry (P=.0003; NNT=23).4
The second study, also at outpatient chest clinics in Poland, evaluated spirometry plus cessation advice among 558 smokers, 297 of whom had abnormal spirometry results. At 1 year, 10.6% of patients with abnormal results had quit, compared with 8.4% of patients with normal lung function. A subgroup of 109 patients with moderate to severe airflow limitation showed significantly higher quit rates when compared with patients with mildly abnormal spirometry (16.5% vs 6.4%; P<.0001; NNT=10).5
In the third study, 6 primary care sites in Sweden provided spirometry and brief cessation advice to 445 smokers, 119 of whom were found to have abnormal lung function. At 3-year follow-up, 29% of patients with abnormal lung function had quit smoking, compared with 14% of patients with normal lung function (P=.001; NNT=7). Forty-five smokers with mildly abnormal lung function were recruited from this study to participate in another study, which may have biased the results toward higher quit rates among smokers with worse spirometry results.6
Recommendations
The US Preventive Services Task Force recommends against using spirometry to screen for chronic obstructive pulmonary disease, but advocates screening all adults for tobacco use and encouraging cessation.7
The authors of a Cochrane review found insufficient evidence to recommend using biomedical risk assessment (carbon monoxide blood levels, spirometry, genetic testing for alpha-1 antitrypsin deficiency) as a smoking cessation aid.8
1. Wilt TJ, Niewoehner D, Kane RL, et al. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res. 2007;9:21-32.
2. Buffels J, Degryse J, Decramer M, et al. Spirometry and smoking cessation advice in general practice: a randomised clinical trial. Respir Med. 2006;100:2012-2017.
3. Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598-600.
4. Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869-873.
5. Gorecka D, Bednarek M, Nowinski A, et al. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest. 2003;123:1916-1923.
6. Stratelis G, Molstad S, Jakobsson P, et al. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scand J Prim Health Care. 2006;24:133-139.
7. Task force recommends against screening for chronic obstructive pulmonary disease using spirometry [press release] Rockville, Md: Agency for Healthcare Research and Quality; March 3, 2008. Available at: www.ahrq.gov/news/press/pr2008/tfcopdpr.htm. Accessed September 4, 2008.
8. Bize R, Burnand B, Mueller Y, et al. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2009;(2):CD004705.-
IT DEPENDS. Simply performing spirometry and offering cessation advice doesn’t improve quit rates in patients who smoke (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]). However, when the spirometry results are communicated in terms of “lung age,” smokers are more likely to quit (SOR: B, large RCT). Patients with abnormal spirometry results may be more likely to quit than patients with normal results (SOR: B, cohort studies).
Evidence summary
A systematic review of 3 RCTs with a total of 649 participants evaluated office spirometry as a motivational tool to improve quit rates by comparing spirometry plus cessation advice with cessation advice alone. All participants were men and women 19 to 75 years of age recruited from outpatient clinics.1
In 1 trial, the intervention group received repeated counseling at 4 visits and underwent spirometry; the control group had 1 counseling session and was given a brochure. In the other 2 trials, the intervention group had both carbon monoxide measurements and spirometry, and all participants received more extensive counseling, including cessation skills training.
At 9 to 12 months’ follow-up, quit rates ranged from 6% to 24% in the intervention groups vs 5% to 14% in the control groups (not significantly different).1
A subsequent study randomized 221 smokers to receive either spirometry plus brief cessation advice or advice alone. Researchers recruited patients 15 to 80 years of age who were willing to quit smoking from 16 general practice clinics in Belgium. Fifty-one percent of patients in both groups used nicotine replacement therapy (a larger percentage than is typical in studies done in the United States). At 6, 12, and 24 months, 5%, 2%, and 5% more smokers, respectively, from the spirometry group quit smoking compared with the control group, but this difference was not significant.2
Reporting spirometry results in terms of lung age may spur quitting
One RCT found significantly improved quit rates when patients who smoked were given their office spirometry results in terms of “lung age” (the age of an average healthy person with similar spirometry results) rather than as forced expiratory volume in 1 second (FEV1). Investigators performed office spirometry and gave smoking cessation advice to 561 smokers older than 35 years who were recruited from 5 general practices. They randomized patients to receive their spirometry results as either lung age or FEV1 and recorded quit rates at 12 months (smoking cessation was verified by measuring blood levels of carbon monoxide).
Patients whose spirometry results were reported as lung age were significantly more likely to quit than smokers whose results were given as FEV1 (13.6% vs 6.4%; P=.005; number needed to treat [NNT]=14 smokers counseled using lung age to cause 1 more patient to quit). Smokers with normal lung ages were no more likely to quit than smokers with abnormal results.3
Abnormal results also may be a motivator
However, 3 prospective cohort studies demonstrated that patients with abnormal spirometry results were more likely to quit than patients with normal spirometry. In the first study, 4494 patients with at least 10 pack-years of smoking from 10 outpatient chest clinics in Poland underwent spirometry and were counseled to quit smoking; 1177 had abnormal spirometry results.
One year later, 16.3% of smokers with abnormal results had quit smoking, compared with 12% in the group with normal spirometry (P=.0003; NNT=23).4
The second study, also at outpatient chest clinics in Poland, evaluated spirometry plus cessation advice among 558 smokers, 297 of whom had abnormal spirometry results. At 1 year, 10.6% of patients with abnormal results had quit, compared with 8.4% of patients with normal lung function. A subgroup of 109 patients with moderate to severe airflow limitation showed significantly higher quit rates when compared with patients with mildly abnormal spirometry (16.5% vs 6.4%; P<.0001; NNT=10).5
In the third study, 6 primary care sites in Sweden provided spirometry and brief cessation advice to 445 smokers, 119 of whom were found to have abnormal lung function. At 3-year follow-up, 29% of patients with abnormal lung function had quit smoking, compared with 14% of patients with normal lung function (P=.001; NNT=7). Forty-five smokers with mildly abnormal lung function were recruited from this study to participate in another study, which may have biased the results toward higher quit rates among smokers with worse spirometry results.6
Recommendations
The US Preventive Services Task Force recommends against using spirometry to screen for chronic obstructive pulmonary disease, but advocates screening all adults for tobacco use and encouraging cessation.7
The authors of a Cochrane review found insufficient evidence to recommend using biomedical risk assessment (carbon monoxide blood levels, spirometry, genetic testing for alpha-1 antitrypsin deficiency) as a smoking cessation aid.8
IT DEPENDS. Simply performing spirometry and offering cessation advice doesn’t improve quit rates in patients who smoke (strength of recommendation [SOR]: A, systematic review of randomized controlled trials [RCTs]). However, when the spirometry results are communicated in terms of “lung age,” smokers are more likely to quit (SOR: B, large RCT). Patients with abnormal spirometry results may be more likely to quit than patients with normal results (SOR: B, cohort studies).
Evidence summary
A systematic review of 3 RCTs with a total of 649 participants evaluated office spirometry as a motivational tool to improve quit rates by comparing spirometry plus cessation advice with cessation advice alone. All participants were men and women 19 to 75 years of age recruited from outpatient clinics.1
In 1 trial, the intervention group received repeated counseling at 4 visits and underwent spirometry; the control group had 1 counseling session and was given a brochure. In the other 2 trials, the intervention group had both carbon monoxide measurements and spirometry, and all participants received more extensive counseling, including cessation skills training.
At 9 to 12 months’ follow-up, quit rates ranged from 6% to 24% in the intervention groups vs 5% to 14% in the control groups (not significantly different).1
A subsequent study randomized 221 smokers to receive either spirometry plus brief cessation advice or advice alone. Researchers recruited patients 15 to 80 years of age who were willing to quit smoking from 16 general practice clinics in Belgium. Fifty-one percent of patients in both groups used nicotine replacement therapy (a larger percentage than is typical in studies done in the United States). At 6, 12, and 24 months, 5%, 2%, and 5% more smokers, respectively, from the spirometry group quit smoking compared with the control group, but this difference was not significant.2
Reporting spirometry results in terms of lung age may spur quitting
One RCT found significantly improved quit rates when patients who smoked were given their office spirometry results in terms of “lung age” (the age of an average healthy person with similar spirometry results) rather than as forced expiratory volume in 1 second (FEV1). Investigators performed office spirometry and gave smoking cessation advice to 561 smokers older than 35 years who were recruited from 5 general practices. They randomized patients to receive their spirometry results as either lung age or FEV1 and recorded quit rates at 12 months (smoking cessation was verified by measuring blood levels of carbon monoxide).
Patients whose spirometry results were reported as lung age were significantly more likely to quit than smokers whose results were given as FEV1 (13.6% vs 6.4%; P=.005; number needed to treat [NNT]=14 smokers counseled using lung age to cause 1 more patient to quit). Smokers with normal lung ages were no more likely to quit than smokers with abnormal results.3
Abnormal results also may be a motivator
However, 3 prospective cohort studies demonstrated that patients with abnormal spirometry results were more likely to quit than patients with normal spirometry. In the first study, 4494 patients with at least 10 pack-years of smoking from 10 outpatient chest clinics in Poland underwent spirometry and were counseled to quit smoking; 1177 had abnormal spirometry results.
One year later, 16.3% of smokers with abnormal results had quit smoking, compared with 12% in the group with normal spirometry (P=.0003; NNT=23).4
The second study, also at outpatient chest clinics in Poland, evaluated spirometry plus cessation advice among 558 smokers, 297 of whom had abnormal spirometry results. At 1 year, 10.6% of patients with abnormal results had quit, compared with 8.4% of patients with normal lung function. A subgroup of 109 patients with moderate to severe airflow limitation showed significantly higher quit rates when compared with patients with mildly abnormal spirometry (16.5% vs 6.4%; P<.0001; NNT=10).5
In the third study, 6 primary care sites in Sweden provided spirometry and brief cessation advice to 445 smokers, 119 of whom were found to have abnormal lung function. At 3-year follow-up, 29% of patients with abnormal lung function had quit smoking, compared with 14% of patients with normal lung function (P=.001; NNT=7). Forty-five smokers with mildly abnormal lung function were recruited from this study to participate in another study, which may have biased the results toward higher quit rates among smokers with worse spirometry results.6
Recommendations
The US Preventive Services Task Force recommends against using spirometry to screen for chronic obstructive pulmonary disease, but advocates screening all adults for tobacco use and encouraging cessation.7
The authors of a Cochrane review found insufficient evidence to recommend using biomedical risk assessment (carbon monoxide blood levels, spirometry, genetic testing for alpha-1 antitrypsin deficiency) as a smoking cessation aid.8
1. Wilt TJ, Niewoehner D, Kane RL, et al. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res. 2007;9:21-32.
2. Buffels J, Degryse J, Decramer M, et al. Spirometry and smoking cessation advice in general practice: a randomised clinical trial. Respir Med. 2006;100:2012-2017.
3. Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598-600.
4. Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869-873.
5. Gorecka D, Bednarek M, Nowinski A, et al. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest. 2003;123:1916-1923.
6. Stratelis G, Molstad S, Jakobsson P, et al. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scand J Prim Health Care. 2006;24:133-139.
7. Task force recommends against screening for chronic obstructive pulmonary disease using spirometry [press release] Rockville, Md: Agency for Healthcare Research and Quality; March 3, 2008. Available at: www.ahrq.gov/news/press/pr2008/tfcopdpr.htm. Accessed September 4, 2008.
8. Bize R, Burnand B, Mueller Y, et al. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2009;(2):CD004705.-
1. Wilt TJ, Niewoehner D, Kane RL, et al. Spirometry as a motivational tool to improve smoking cessation rates: a systematic review of the literature. Nicotine Tob Res. 2007;9:21-32.
2. Buffels J, Degryse J, Decramer M, et al. Spirometry and smoking cessation advice in general practice: a randomised clinical trial. Respir Med. 2006;100:2012-2017.
3. Parkes G, Greenhalgh T, Griffin M, et al. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598-600.
4. Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006;61:869-873.
5. Gorecka D, Bednarek M, Nowinski A, et al. Diagnosis of airflow limitation combined with smoking cessation advice increases stop-smoking rate. Chest. 2003;123:1916-1923.
6. Stratelis G, Molstad S, Jakobsson P, et al. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scand J Prim Health Care. 2006;24:133-139.
7. Task force recommends against screening for chronic obstructive pulmonary disease using spirometry [press release] Rockville, Md: Agency for Healthcare Research and Quality; March 3, 2008. Available at: www.ahrq.gov/news/press/pr2008/tfcopdpr.htm. Accessed September 4, 2008.
8. Bize R, Burnand B, Mueller Y, et al. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2009;(2):CD004705.-
Evidence-based answers from the Family Physicians Inquiries Network
Excessive opioids blamed for respiratory arrest…A rising PSA, but no evaluation…A hemorrhoid…or something else?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
Excessive opioids blamed for respiratory arrest
A MIDNIGHT VISIT TO THE HOSPITAL prompted by abdominal pain, nausea, and vomiting led to a diagnosis of acute pancreatitis and secondary conditions in a 67-year-old woman. She was admitted to the intensive care unit (ICU) and given pain medication, including Demerol, morphine, and a fentanyl transdermal patch, despite the fact that she was opioid naïve, with no tolerance to strong opioid-based medications. A black box warning for fentanyl specifies that it should not be administered to opioid-naïve patients for acute or short-term pain.
During her stay in the ICU, the patient received increasing amounts of pain medication. On the third day, a physician prescribed almost 10 times the dose given on the previous day. The patient subsequently suffered respiratory arrest, resulting in brain damage that left her with no short-term memory and in need of full-time care.
PLAINTIFF’S CLAIM Excessive administration of opioids caused respiratory arrest and brain damage.
THE DEFENSE Respiratory arrest resulted from the patient’s underlying illnesses, not opioid overdose. The patient did not show typical signs of overdose, such as slowed heart rate and decreased breathing, and was, in fact, agitated up to the time she went into respiratory arrest.
VERDICT Confidential Missouri settlement.
COMMENT I’m seeing many malpractice suits involving the prescription of opioids. Caution and due diligence are essential.
A rising PSA, but no evaluation
A 59-YEAR-OLD MAN received a prostate-specific antigen (PSA) score of 2.0 in 2003. In 2006, his score was 5.26. His primary care physician didn’t evaluate him for prostate cancer.
A year later, the patient complained of frequent, slow urination. A digital rectal examination revealed a hardened, nodular prostate. The patient’s PSA was 209. A biopsy showed stage 4 terminal prostate cancer. Computed tomography and bone scans of the abdomen and pelvis indicated metastasis to lymph nodes and bones. The patient wasn’t a candidate for surgery or radiation.
PLAINTIFF’S CLAIM The patient had been diagnosed with benign prostatic hypertrophy in 2005 and 2006, but had received no further evaluation. A biopsy should have been performed in 2003, at the time of the initial PSA test. If the cancer had been diagnosed and treated with radiation then, the patient’s condition wouldn’t have become terminal.
THE DEFENSE No information about the defense is available.
VERDICT $500,000 California settlement.
COMMENT We may disagree with the assessment that more aggressive evaluation would have been lifesaving. Nonetheless, the lack of follow-up and discussion with the patient makes for a very unfortunate situation.
A hemorrhoid…or something else?
WHILE GIVING BIRTH TO HER SECOND CHILD, a 35-year-old woman sustained a second-degree vaginal tear that required repair. The physician who performed the repair noticed a large hemorrhoid and told a nurse midwife to have it evaluated with a possible gastroenterological consult to rule out a mass. The next day, another doctor and midwife examined the patient. They agreed with the patient to defer a gastroenterology consult and have the patient follow up with her primary care physician in a few weeks.
When the patient saw her primary care physician 3 weeks after delivery, her exam revealed no hemorrhoids; she was instructed to call back if the hemorrhoids recurred. The hemorrhoids didn’t recur, and the patient didn’t follow up with her primary care physician.
During the next 4 years, the patient received care from her gynecologist that didn’t include rectal examinations. Five years after delivery, the patient went to her primary care physician complaining of rectal bleeding with bowel movements. The physician found no external hemorrhoids but noted a rectal mass.
He referred the patient for a gastroenterology consult and biopsy, which revealed intramucosal adenocarcinoma. A computed tomography (CT) scan of the chest showed a nodule in the lower lobe of the right lung, which was suspected to be a metastasis. An abdominal CT scan and a positron-emission tomography scan indicated likely liver metastasis. A liver biopsy confi rmed adenocarcinoma.
The patient underwent chemotherapy and chemoradiation followed several months later by abdominal perineal resection, left lateral segmentectomy of the liver, cholecystectomy, and appendectomy. At the time of the settlement, she was doing well and receiving no cancer treatment.
PLAINTIFF’S CLAIM The primary care physician should have followed up on the rectal finding, which would have led to earlier diagnosis and treatment of the cancer.
THE DEFENSE The finding made at the time of the delivery was a simple hemorrhoid, which went away after delivery. The absence of symptoms for 4½ years indicated that the cancer couldn’t have been present at the time of delivery. The diagnosed cancer was in a different place than the original hemorrhoid.
VERDICT $1 million Massachusetts settlement.
COMMENT The folly of the failed hand off. One of the most common root causes of litigation is poor communication that results in a bad outcome. How many lives could be saved simply by phone calls between physicians?
When to suspect atypical cystic fibrosis
• Don’t dismiss a cystic fibrosis diagnosis just because a patient’s sweat chloride levels are <60 mmol/L. A
• Suspect atypical cystic fibrosis in adults with single organ involvement, including mild lung disease, nasal polyposis, recurrent pancreatitis, biliary cirrhosis, portal hypertension, or obstructive azoospermia. A
• Consider respiratory therapies such as tobramycin, hypertonic saline, and recombinant human DNase in cystic fibrosis patients with relatively mild or atypical disease. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: Lauren W*
Two years ago, Lauren W, a 57-year-old Caucasian woman, sought care at our medical center after learning that her pregnant daughter tested positive during a prenatal cystic fibrosis mutation genetic screen. Lauren had clinical symptoms of malodorous and greasy bowel movements, dyspepsia, early satiety, and a history of recurrent bronchitis since childhood.
According to her history, she did not suffer from failure to thrive as a child. She’d had 5 episodes of adult-onset acute pancreatitis and had 2 surgeries for sinusitis.
On physical exam, we heard no crackles during lung auscultation. Lauren also had mild digital clubbing.
Testing: We ordered a chest x-ray, which revealed left upper lobe atelectasis, but there was no bronchiectasis.
Pulmonary function tests indicated mild obstructive lung disease with forced vital capacity (FVC) 2.39 L or 84% predicted; forced expiratory volume in 1 second (FEV1) 1.59 L or 68% predicted; and an FEV1/FVC of 0.66.
A sweat chloride test was positive on both arms: 77 and 83 mmol/L. Genetic testing revealed compound heterozygosity for cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations Δ F508 and R117H with a 5T allele. To test for pancreatic insufficiency, we performed a 72-hour fecal fat testing while she was on a low-fat diet; it revealed 5.5 g of fat per 24-hour period, suggesting fat malabsorption. Based on positive quantitative sweat test ≥60 mmol/L and the presence of 2 cystic fibrosis–causing mutations, we made the diagnosis of cystic fibrosis.
Treatment: We put Lauren on albuterol and recombinant human DNase respiratory treatments, pancreatic enzymes, and multivitamins with extra lipid-soluble vitamins and calcium supplements. We also continued her low-fat diet of 1500 to 1800 calories per day due to a diagnosis of coronary artery disease.
*Patients’ names have been changed to protect their privacy.
CASE 2: Zack P*
Zack P, a 6-year-old Caucasian boy, was admitted to the hospital with what we suspected was acute gastroenteritis. Serum testing revealed elevated pancreatic enzymes. He had recently sustained an injury to the mid-abdomen during a soccer game and also had a history of chronic sinusitis. One month after his release from the hospital, his symptoms resolved, but his pancreatic enzymes remained elevated (amylase 130 U/L, lipase 177 U/L).
Testing: He underwent 2 sweat chloride tests, which were borderline elevated at 49 mmol/L on the right arm and 40 mmol/L on the left arm; repeat testing was 49 mmol/L on the left arm, 43 mmol/L on the right arm. Genetic testing revealed heterozygosity for CFTR gene mutation Δ F508 with the presence of 7T and 9T allele variants. Over an 8-month period, Zack remained asymptomatic, but his pancreatic enzymes were persistently elevated.
Zack’s physical exam showed that his weight had dropped from the 75th to the 50th percentile. A magnetic resonance cholangiopancreatography indicated homogeneous parenchyma and normal enhancement throughout his pancreas. Similarly, we could not find evidence of acute pancreatitis or biliary or pancreatic duct dilation, and a 72-hour fecal fat study was normal. Given Zack’s borderline sweat chloride, compound heterozygosity of cystic fibrosis mutations, and his phenotype of recurrent pancreatitis, he was given a diagnosis of atypical cystic fibrosis.
Treatment: Based on our diagnostic workup, pancreatic enzyme therapy was not warranted.
*Patients’ names have been changed to protect their privacy.
These 2 cases illustrate how clinically diverse cystic fibrosis can be. The cystic fibrosis phenotype can range from a patient with 2 disease-causing cystic fibrosis mutations with significant sweat gland dysfunction and childhood onset of mild cystic fibrosis symptomatology with normal growth—Lauren—to a patient who is CFTR heterozygous with pancreatitis and a borderline sweat chloride concentration—Zack. Both cases emphasize the need to think “outside the box” and not expect all patients with cystic fibrosis to come in with typical signs and symptoms.
What does the “nonclassic” cystic fibrosis patient look like?
Patients with cystic fibrosis are usually diagnosed during childhood with pulmonary disease, pancreatic insufficiency, malabsorption, malnutrition, elevated sweat chloride, and male infertility. But more recently, patients have been diagnosed in adulthood because either they lacked significant clinical symptoms in childhood or they came in with atypical signs or symptoms (pancreatic sufficiency and sweat chloride <60 mmol/L).
In the past, cystic fibrosis patients rarely lived past their second decade, but those with atypical cystic fibrosis tend to have milder disease, including less severe respiratory signs and symptoms. The good news is that often translates into a long lifespan.1 As recently as 2005, the Cystic Fibrosis Foundation had listed a median survival age of 37.2 Advances in respiratory, gastrointestinal, and nutritional therapies have significantly contributed to the increased survival of these patients. Unfortunately, such milder cases can easily go undetected.
Sweat chloride testing remains a gold standard for the diagnosis of cystic fibrosis. As previously mentioned, classic cystic fibrosis patients are typically diagnosed during childhood and have a sweat chloride concentration ≥60 mmol/L and more severe multiorgan involvement (sinopulmonary and gastrointestinal disease with failure to thrive).
There are 5 classes of CFTR mutations that result in compromised CFTR function: The presence of 2 severe CFTR mutations (classes I-III) completely abolishes CFTR function. It is diagnosed in childhood and usually results in the classic clinical features of pancreatic insufficiency and failure to thrive. However, patients who are compound heterozygous for the milder CFTR mutations (classes IV and V) experience partial CFTR function, which in turn results in atypical features such as pancreatic sufficiency and normal or borderline sweat chloride concentrations. That makes the diagnosis more elusive during early childhood.1,3
The severity of sweat gland and exocrine pancreatic dysfunction produced by a cystic fibrosis mutation depends on the class of CFTR gene mutation and the level of CFTR gene and protein expression.4,5 In certain genetic backgrounds, the 5T allele associated with a high number of TG (thymine/guanine) repeats found in compound heterozygosity with a disease-causing CFTR mutation acts as a “mild CFTR mutation,” resulting in the nonclassic cystic fibrosis phenotype.3 Individuals with atypical cystic fibrosis diagnosed later in life may have single organ involvement, such as mild lung disease, nasal polyposis, recurrent pancreatitis, biliary cirrhosis, portal hypertension, or obstructive azoospermia.1
Lauren had several classic cystic fibrosis features, including recurrent lung disease, pancreatic insufficiency, and sweat gland dysfunction, but it’s likely that her relatively mild pulmonary presentation, normal body mass index, and lack of failure to thrive led to a delay in her diagnosis.
Some patients with atypical cystic fibrosis seek care for idiopathic chronic pancreatitis (ICP), and researchers have found a link between ICP and CFTR gene mutations. For instance, recent studies of ICP patients compared with geographically and ethnically matched controls revealed a higher frequency of abnormal CFTR alleles in the ICP population.6,7 Milder CFTR mutations resulting in partial CFTR function have also been associated with ICP.7,8
Zack was heterozygous for Δ F508, a common CFTR mutation in the Caucasian population. Cohn et al found a higher frequency of this mutation in ICP patients from Europe (mostly English, Italian, and Czech).9 Poly T allele variants such as 5T, 7T, and 9T have not been associated with a higher frequency of ICP.6,7
Early detection may translate into better treatment
Although patients diagnosed with cystic fibrosis as adults are less likely to present with classic clinical features, they may develop bronchiectasis and advanced lung disease.10,11 Those who are identified early on—including those who are asymptomatic and have normal lung function—may benefit from respiratory therapy to prevent or delay lung disease.12 Several studies have shown that patients with mild cystic fibrosis disease and stable spirometry results have evidence of bronchiectasis on their x-rays and advanced lung disease that appears on high-resolution CT.13,14
Judge et al have suggested that mucus plugging occurs early in cystic fibrosis lung disease and at a milder stage of lung function impairment, and that bronchiectasis may be an end result of such abnormalities.14 Nonclassic cystic fibrosis patients often have episodes of “bronchitis,” and once the practitioner becomes concerned, the radiographic image may already show evidence of bronchiectasis. Once again, this emphasizes the importance of early detection and prompt treatment.15
CASE 1: Lauren
Unfortunately, Lauren was unable to benefit from early use of respiratory therapies like tobramycin, hypertonic saline, and recombinant human DNase. She began these treatments after developing advanced lung disease. Studies have shown that tobramycin, long-term inhaled hypertonic saline, and recombinant human DNase can reduce the number of pulmonary exacerbations and increase both FVC and FEV1 values in previously stable cystic fibrosis patients.16-18
Similarly, because Lauren’s pancreatitis was due to pancreatic insufficiency, early recognition of pancreatic insufficiency and enzyme therapy may have greatly reduced the number and severity of her pancreatic episodes.19
Unfortunately, over the last few years, Lauren’s lung function has declined and she has been hospitalized for cystic fibrosis exacerbations and sinusitis; she has had 3 additional episodes of acute pancreatitis. Although her FEV1 is lower than on initial evaluation, she is clinically stable.
CASE 2: Zack
Clinically, Zack is stable and his recent amylase and lipase are elevated at 92 U/L and 71 U/L, respectively. He has had no acute exacerbations.
Patients like Lauren and Zack serve to remind us of the need to recognize and closely monitor patients with nonclassic cystic fibrosis. These patients may come to the office with “asthma-like” symptoms, bronchitis, polyps, pancreatitis, cholelithiasis, constipation, abdominal bloating/flatus, and infertility. Because their symptoms may not be severe enough to be referred to a subspecialist, family physicians play a critical role in recognizing these overlooked cases early on.
CORRESPONDENCE Anupama Chawla, MD, Director, Division of Pediatric Gastroenterology and Nutrition, Stony Brook University Hospital, Stony Brook, NY 11794; [email protected]
1. Kerem E. Atypical CF and CF related diseases. Paediatr Respir Rev. 2006;7(suppl 1):S144-S146.
2. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Annual Patient Registry Data Report 2005. Bethesda, Md; 2006.
3. Castellani C, Cuppens H, Macek M, Jr, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179-196.
4. Wilchanski M, Zielenski J, Markiewicz D. Correlation of sweat chloride concentration with classes of the cystic fibrosis trans-membrane conductance regulator gene mutation, J Pediatr. 1995;127:705-710.
5. Mickle JE, Cutting GR. Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am. 2000;84:597-607.
6. Weiss FU, Simon P, Bogdanova MJ, et al. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic pancreatitis and controls. Gut. 2005;54:1456-1460.
7. Chang MC, Chang YT, Wei S, et al. Spectrum of mutations and variants/haplotypes of CFTR and genotype-phenotype correlation in idiopathic chronic pancreatitis and controls in Chinese by complete analysis. Clin Genet. 2007;71:530-539.
8. Noone PG, Knowles MR. “CFTR-opathies”: disease phenotypes associated with cystic fibrosis transmembrane regulator gene mutations. Respir Res. 2001;2:328-332.
9. Cohn JA, Neoptolemos JP, Feng J, et al. Increased risk of idiopathic pancreatitis in cystic fibrosis carriers. Hum Mutat. 2005;26:303-307.
10. Nick JA, Rodman DM. Manifestations of cystic fibrosis diagnosed in adulthood. Curr Opin Pulm Med 2005;11:513-518.
11. Poller W, Farber JP, Scholz S, et al. Sequence analysis of the cystic fibrosis gene in patients with disseminated bronchiectatic lung disease. Application in the identification of a cystic fibrosis patient with atypical clinical course. Klin Wochenschr. 1991;69:657-663.
12. Quan JM, Tiddens HA, Sy JP, et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813-820.
13. Tiddens HA. Detecting early structural lung damage in cystic fibrosis. Pediatr Pulmonol. 2002;34:228-231.
14. Judge EP, Dodd JD, Masterson JB, et al. Pulmonary abnormalities on high-resolution CT demonstrate more rapid decline than FEV1 in adults with cystic fibrosis. Chest. 2006;130:1424-1432.
15. Robinson TE, Goris ML, Zhu HJ, et al. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease. Chest. 2005;128:2327-2335.
16. Fuchs HJ, Borowitz DS, Christiansen DH, et al. The Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637-642.
17. Ramsey BW, Pepe MS, Quan JM, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23-30.
18. Elkins MR, Robinson M, Rose BR, et al. The National Hyper-tonic Saline in Cystic Fibrosis Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229-240.
19. Witt H. Chronic pancreatitis and cystic fibrosis. Gut. 2003;52(suppl 2):ii31-ii41.
• Don’t dismiss a cystic fibrosis diagnosis just because a patient’s sweat chloride levels are <60 mmol/L. A
• Suspect atypical cystic fibrosis in adults with single organ involvement, including mild lung disease, nasal polyposis, recurrent pancreatitis, biliary cirrhosis, portal hypertension, or obstructive azoospermia. A
• Consider respiratory therapies such as tobramycin, hypertonic saline, and recombinant human DNase in cystic fibrosis patients with relatively mild or atypical disease. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: Lauren W*
Two years ago, Lauren W, a 57-year-old Caucasian woman, sought care at our medical center after learning that her pregnant daughter tested positive during a prenatal cystic fibrosis mutation genetic screen. Lauren had clinical symptoms of malodorous and greasy bowel movements, dyspepsia, early satiety, and a history of recurrent bronchitis since childhood.
According to her history, she did not suffer from failure to thrive as a child. She’d had 5 episodes of adult-onset acute pancreatitis and had 2 surgeries for sinusitis.
On physical exam, we heard no crackles during lung auscultation. Lauren also had mild digital clubbing.
Testing: We ordered a chest x-ray, which revealed left upper lobe atelectasis, but there was no bronchiectasis.
Pulmonary function tests indicated mild obstructive lung disease with forced vital capacity (FVC) 2.39 L or 84% predicted; forced expiratory volume in 1 second (FEV1) 1.59 L or 68% predicted; and an FEV1/FVC of 0.66.
A sweat chloride test was positive on both arms: 77 and 83 mmol/L. Genetic testing revealed compound heterozygosity for cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations Δ F508 and R117H with a 5T allele. To test for pancreatic insufficiency, we performed a 72-hour fecal fat testing while she was on a low-fat diet; it revealed 5.5 g of fat per 24-hour period, suggesting fat malabsorption. Based on positive quantitative sweat test ≥60 mmol/L and the presence of 2 cystic fibrosis–causing mutations, we made the diagnosis of cystic fibrosis.
Treatment: We put Lauren on albuterol and recombinant human DNase respiratory treatments, pancreatic enzymes, and multivitamins with extra lipid-soluble vitamins and calcium supplements. We also continued her low-fat diet of 1500 to 1800 calories per day due to a diagnosis of coronary artery disease.
*Patients’ names have been changed to protect their privacy.
CASE 2: Zack P*
Zack P, a 6-year-old Caucasian boy, was admitted to the hospital with what we suspected was acute gastroenteritis. Serum testing revealed elevated pancreatic enzymes. He had recently sustained an injury to the mid-abdomen during a soccer game and also had a history of chronic sinusitis. One month after his release from the hospital, his symptoms resolved, but his pancreatic enzymes remained elevated (amylase 130 U/L, lipase 177 U/L).
Testing: He underwent 2 sweat chloride tests, which were borderline elevated at 49 mmol/L on the right arm and 40 mmol/L on the left arm; repeat testing was 49 mmol/L on the left arm, 43 mmol/L on the right arm. Genetic testing revealed heterozygosity for CFTR gene mutation Δ F508 with the presence of 7T and 9T allele variants. Over an 8-month period, Zack remained asymptomatic, but his pancreatic enzymes were persistently elevated.
Zack’s physical exam showed that his weight had dropped from the 75th to the 50th percentile. A magnetic resonance cholangiopancreatography indicated homogeneous parenchyma and normal enhancement throughout his pancreas. Similarly, we could not find evidence of acute pancreatitis or biliary or pancreatic duct dilation, and a 72-hour fecal fat study was normal. Given Zack’s borderline sweat chloride, compound heterozygosity of cystic fibrosis mutations, and his phenotype of recurrent pancreatitis, he was given a diagnosis of atypical cystic fibrosis.
Treatment: Based on our diagnostic workup, pancreatic enzyme therapy was not warranted.
*Patients’ names have been changed to protect their privacy.
These 2 cases illustrate how clinically diverse cystic fibrosis can be. The cystic fibrosis phenotype can range from a patient with 2 disease-causing cystic fibrosis mutations with significant sweat gland dysfunction and childhood onset of mild cystic fibrosis symptomatology with normal growth—Lauren—to a patient who is CFTR heterozygous with pancreatitis and a borderline sweat chloride concentration—Zack. Both cases emphasize the need to think “outside the box” and not expect all patients with cystic fibrosis to come in with typical signs and symptoms.
What does the “nonclassic” cystic fibrosis patient look like?
Patients with cystic fibrosis are usually diagnosed during childhood with pulmonary disease, pancreatic insufficiency, malabsorption, malnutrition, elevated sweat chloride, and male infertility. But more recently, patients have been diagnosed in adulthood because either they lacked significant clinical symptoms in childhood or they came in with atypical signs or symptoms (pancreatic sufficiency and sweat chloride <60 mmol/L).
In the past, cystic fibrosis patients rarely lived past their second decade, but those with atypical cystic fibrosis tend to have milder disease, including less severe respiratory signs and symptoms. The good news is that often translates into a long lifespan.1 As recently as 2005, the Cystic Fibrosis Foundation had listed a median survival age of 37.2 Advances in respiratory, gastrointestinal, and nutritional therapies have significantly contributed to the increased survival of these patients. Unfortunately, such milder cases can easily go undetected.
Sweat chloride testing remains a gold standard for the diagnosis of cystic fibrosis. As previously mentioned, classic cystic fibrosis patients are typically diagnosed during childhood and have a sweat chloride concentration ≥60 mmol/L and more severe multiorgan involvement (sinopulmonary and gastrointestinal disease with failure to thrive).
There are 5 classes of CFTR mutations that result in compromised CFTR function: The presence of 2 severe CFTR mutations (classes I-III) completely abolishes CFTR function. It is diagnosed in childhood and usually results in the classic clinical features of pancreatic insufficiency and failure to thrive. However, patients who are compound heterozygous for the milder CFTR mutations (classes IV and V) experience partial CFTR function, which in turn results in atypical features such as pancreatic sufficiency and normal or borderline sweat chloride concentrations. That makes the diagnosis more elusive during early childhood.1,3
The severity of sweat gland and exocrine pancreatic dysfunction produced by a cystic fibrosis mutation depends on the class of CFTR gene mutation and the level of CFTR gene and protein expression.4,5 In certain genetic backgrounds, the 5T allele associated with a high number of TG (thymine/guanine) repeats found in compound heterozygosity with a disease-causing CFTR mutation acts as a “mild CFTR mutation,” resulting in the nonclassic cystic fibrosis phenotype.3 Individuals with atypical cystic fibrosis diagnosed later in life may have single organ involvement, such as mild lung disease, nasal polyposis, recurrent pancreatitis, biliary cirrhosis, portal hypertension, or obstructive azoospermia.1
Lauren had several classic cystic fibrosis features, including recurrent lung disease, pancreatic insufficiency, and sweat gland dysfunction, but it’s likely that her relatively mild pulmonary presentation, normal body mass index, and lack of failure to thrive led to a delay in her diagnosis.
Some patients with atypical cystic fibrosis seek care for idiopathic chronic pancreatitis (ICP), and researchers have found a link between ICP and CFTR gene mutations. For instance, recent studies of ICP patients compared with geographically and ethnically matched controls revealed a higher frequency of abnormal CFTR alleles in the ICP population.6,7 Milder CFTR mutations resulting in partial CFTR function have also been associated with ICP.7,8
Zack was heterozygous for Δ F508, a common CFTR mutation in the Caucasian population. Cohn et al found a higher frequency of this mutation in ICP patients from Europe (mostly English, Italian, and Czech).9 Poly T allele variants such as 5T, 7T, and 9T have not been associated with a higher frequency of ICP.6,7
Early detection may translate into better treatment
Although patients diagnosed with cystic fibrosis as adults are less likely to present with classic clinical features, they may develop bronchiectasis and advanced lung disease.10,11 Those who are identified early on—including those who are asymptomatic and have normal lung function—may benefit from respiratory therapy to prevent or delay lung disease.12 Several studies have shown that patients with mild cystic fibrosis disease and stable spirometry results have evidence of bronchiectasis on their x-rays and advanced lung disease that appears on high-resolution CT.13,14
Judge et al have suggested that mucus plugging occurs early in cystic fibrosis lung disease and at a milder stage of lung function impairment, and that bronchiectasis may be an end result of such abnormalities.14 Nonclassic cystic fibrosis patients often have episodes of “bronchitis,” and once the practitioner becomes concerned, the radiographic image may already show evidence of bronchiectasis. Once again, this emphasizes the importance of early detection and prompt treatment.15
CASE 1: Lauren
Unfortunately, Lauren was unable to benefit from early use of respiratory therapies like tobramycin, hypertonic saline, and recombinant human DNase. She began these treatments after developing advanced lung disease. Studies have shown that tobramycin, long-term inhaled hypertonic saline, and recombinant human DNase can reduce the number of pulmonary exacerbations and increase both FVC and FEV1 values in previously stable cystic fibrosis patients.16-18
Similarly, because Lauren’s pancreatitis was due to pancreatic insufficiency, early recognition of pancreatic insufficiency and enzyme therapy may have greatly reduced the number and severity of her pancreatic episodes.19
Unfortunately, over the last few years, Lauren’s lung function has declined and she has been hospitalized for cystic fibrosis exacerbations and sinusitis; she has had 3 additional episodes of acute pancreatitis. Although her FEV1 is lower than on initial evaluation, she is clinically stable.
CASE 2: Zack
Clinically, Zack is stable and his recent amylase and lipase are elevated at 92 U/L and 71 U/L, respectively. He has had no acute exacerbations.
Patients like Lauren and Zack serve to remind us of the need to recognize and closely monitor patients with nonclassic cystic fibrosis. These patients may come to the office with “asthma-like” symptoms, bronchitis, polyps, pancreatitis, cholelithiasis, constipation, abdominal bloating/flatus, and infertility. Because their symptoms may not be severe enough to be referred to a subspecialist, family physicians play a critical role in recognizing these overlooked cases early on.
CORRESPONDENCE Anupama Chawla, MD, Director, Division of Pediatric Gastroenterology and Nutrition, Stony Brook University Hospital, Stony Brook, NY 11794; [email protected]
• Don’t dismiss a cystic fibrosis diagnosis just because a patient’s sweat chloride levels are <60 mmol/L. A
• Suspect atypical cystic fibrosis in adults with single organ involvement, including mild lung disease, nasal polyposis, recurrent pancreatitis, biliary cirrhosis, portal hypertension, or obstructive azoospermia. A
• Consider respiratory therapies such as tobramycin, hypertonic saline, and recombinant human DNase in cystic fibrosis patients with relatively mild or atypical disease. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1: Lauren W*
Two years ago, Lauren W, a 57-year-old Caucasian woman, sought care at our medical center after learning that her pregnant daughter tested positive during a prenatal cystic fibrosis mutation genetic screen. Lauren had clinical symptoms of malodorous and greasy bowel movements, dyspepsia, early satiety, and a history of recurrent bronchitis since childhood.
According to her history, she did not suffer from failure to thrive as a child. She’d had 5 episodes of adult-onset acute pancreatitis and had 2 surgeries for sinusitis.
On physical exam, we heard no crackles during lung auscultation. Lauren also had mild digital clubbing.
Testing: We ordered a chest x-ray, which revealed left upper lobe atelectasis, but there was no bronchiectasis.
Pulmonary function tests indicated mild obstructive lung disease with forced vital capacity (FVC) 2.39 L or 84% predicted; forced expiratory volume in 1 second (FEV1) 1.59 L or 68% predicted; and an FEV1/FVC of 0.66.
A sweat chloride test was positive on both arms: 77 and 83 mmol/L. Genetic testing revealed compound heterozygosity for cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations Δ F508 and R117H with a 5T allele. To test for pancreatic insufficiency, we performed a 72-hour fecal fat testing while she was on a low-fat diet; it revealed 5.5 g of fat per 24-hour period, suggesting fat malabsorption. Based on positive quantitative sweat test ≥60 mmol/L and the presence of 2 cystic fibrosis–causing mutations, we made the diagnosis of cystic fibrosis.
Treatment: We put Lauren on albuterol and recombinant human DNase respiratory treatments, pancreatic enzymes, and multivitamins with extra lipid-soluble vitamins and calcium supplements. We also continued her low-fat diet of 1500 to 1800 calories per day due to a diagnosis of coronary artery disease.
*Patients’ names have been changed to protect their privacy.
CASE 2: Zack P*
Zack P, a 6-year-old Caucasian boy, was admitted to the hospital with what we suspected was acute gastroenteritis. Serum testing revealed elevated pancreatic enzymes. He had recently sustained an injury to the mid-abdomen during a soccer game and also had a history of chronic sinusitis. One month after his release from the hospital, his symptoms resolved, but his pancreatic enzymes remained elevated (amylase 130 U/L, lipase 177 U/L).
Testing: He underwent 2 sweat chloride tests, which were borderline elevated at 49 mmol/L on the right arm and 40 mmol/L on the left arm; repeat testing was 49 mmol/L on the left arm, 43 mmol/L on the right arm. Genetic testing revealed heterozygosity for CFTR gene mutation Δ F508 with the presence of 7T and 9T allele variants. Over an 8-month period, Zack remained asymptomatic, but his pancreatic enzymes were persistently elevated.
Zack’s physical exam showed that his weight had dropped from the 75th to the 50th percentile. A magnetic resonance cholangiopancreatography indicated homogeneous parenchyma and normal enhancement throughout his pancreas. Similarly, we could not find evidence of acute pancreatitis or biliary or pancreatic duct dilation, and a 72-hour fecal fat study was normal. Given Zack’s borderline sweat chloride, compound heterozygosity of cystic fibrosis mutations, and his phenotype of recurrent pancreatitis, he was given a diagnosis of atypical cystic fibrosis.
Treatment: Based on our diagnostic workup, pancreatic enzyme therapy was not warranted.
*Patients’ names have been changed to protect their privacy.
These 2 cases illustrate how clinically diverse cystic fibrosis can be. The cystic fibrosis phenotype can range from a patient with 2 disease-causing cystic fibrosis mutations with significant sweat gland dysfunction and childhood onset of mild cystic fibrosis symptomatology with normal growth—Lauren—to a patient who is CFTR heterozygous with pancreatitis and a borderline sweat chloride concentration—Zack. Both cases emphasize the need to think “outside the box” and not expect all patients with cystic fibrosis to come in with typical signs and symptoms.
What does the “nonclassic” cystic fibrosis patient look like?
Patients with cystic fibrosis are usually diagnosed during childhood with pulmonary disease, pancreatic insufficiency, malabsorption, malnutrition, elevated sweat chloride, and male infertility. But more recently, patients have been diagnosed in adulthood because either they lacked significant clinical symptoms in childhood or they came in with atypical signs or symptoms (pancreatic sufficiency and sweat chloride <60 mmol/L).
In the past, cystic fibrosis patients rarely lived past their second decade, but those with atypical cystic fibrosis tend to have milder disease, including less severe respiratory signs and symptoms. The good news is that often translates into a long lifespan.1 As recently as 2005, the Cystic Fibrosis Foundation had listed a median survival age of 37.2 Advances in respiratory, gastrointestinal, and nutritional therapies have significantly contributed to the increased survival of these patients. Unfortunately, such milder cases can easily go undetected.
Sweat chloride testing remains a gold standard for the diagnosis of cystic fibrosis. As previously mentioned, classic cystic fibrosis patients are typically diagnosed during childhood and have a sweat chloride concentration ≥60 mmol/L and more severe multiorgan involvement (sinopulmonary and gastrointestinal disease with failure to thrive).
There are 5 classes of CFTR mutations that result in compromised CFTR function: The presence of 2 severe CFTR mutations (classes I-III) completely abolishes CFTR function. It is diagnosed in childhood and usually results in the classic clinical features of pancreatic insufficiency and failure to thrive. However, patients who are compound heterozygous for the milder CFTR mutations (classes IV and V) experience partial CFTR function, which in turn results in atypical features such as pancreatic sufficiency and normal or borderline sweat chloride concentrations. That makes the diagnosis more elusive during early childhood.1,3
The severity of sweat gland and exocrine pancreatic dysfunction produced by a cystic fibrosis mutation depends on the class of CFTR gene mutation and the level of CFTR gene and protein expression.4,5 In certain genetic backgrounds, the 5T allele associated with a high number of TG (thymine/guanine) repeats found in compound heterozygosity with a disease-causing CFTR mutation acts as a “mild CFTR mutation,” resulting in the nonclassic cystic fibrosis phenotype.3 Individuals with atypical cystic fibrosis diagnosed later in life may have single organ involvement, such as mild lung disease, nasal polyposis, recurrent pancreatitis, biliary cirrhosis, portal hypertension, or obstructive azoospermia.1
Lauren had several classic cystic fibrosis features, including recurrent lung disease, pancreatic insufficiency, and sweat gland dysfunction, but it’s likely that her relatively mild pulmonary presentation, normal body mass index, and lack of failure to thrive led to a delay in her diagnosis.
Some patients with atypical cystic fibrosis seek care for idiopathic chronic pancreatitis (ICP), and researchers have found a link between ICP and CFTR gene mutations. For instance, recent studies of ICP patients compared with geographically and ethnically matched controls revealed a higher frequency of abnormal CFTR alleles in the ICP population.6,7 Milder CFTR mutations resulting in partial CFTR function have also been associated with ICP.7,8
Zack was heterozygous for Δ F508, a common CFTR mutation in the Caucasian population. Cohn et al found a higher frequency of this mutation in ICP patients from Europe (mostly English, Italian, and Czech).9 Poly T allele variants such as 5T, 7T, and 9T have not been associated with a higher frequency of ICP.6,7
Early detection may translate into better treatment
Although patients diagnosed with cystic fibrosis as adults are less likely to present with classic clinical features, they may develop bronchiectasis and advanced lung disease.10,11 Those who are identified early on—including those who are asymptomatic and have normal lung function—may benefit from respiratory therapy to prevent or delay lung disease.12 Several studies have shown that patients with mild cystic fibrosis disease and stable spirometry results have evidence of bronchiectasis on their x-rays and advanced lung disease that appears on high-resolution CT.13,14
Judge et al have suggested that mucus plugging occurs early in cystic fibrosis lung disease and at a milder stage of lung function impairment, and that bronchiectasis may be an end result of such abnormalities.14 Nonclassic cystic fibrosis patients often have episodes of “bronchitis,” and once the practitioner becomes concerned, the radiographic image may already show evidence of bronchiectasis. Once again, this emphasizes the importance of early detection and prompt treatment.15
CASE 1: Lauren
Unfortunately, Lauren was unable to benefit from early use of respiratory therapies like tobramycin, hypertonic saline, and recombinant human DNase. She began these treatments after developing advanced lung disease. Studies have shown that tobramycin, long-term inhaled hypertonic saline, and recombinant human DNase can reduce the number of pulmonary exacerbations and increase both FVC and FEV1 values in previously stable cystic fibrosis patients.16-18
Similarly, because Lauren’s pancreatitis was due to pancreatic insufficiency, early recognition of pancreatic insufficiency and enzyme therapy may have greatly reduced the number and severity of her pancreatic episodes.19
Unfortunately, over the last few years, Lauren’s lung function has declined and she has been hospitalized for cystic fibrosis exacerbations and sinusitis; she has had 3 additional episodes of acute pancreatitis. Although her FEV1 is lower than on initial evaluation, she is clinically stable.
CASE 2: Zack
Clinically, Zack is stable and his recent amylase and lipase are elevated at 92 U/L and 71 U/L, respectively. He has had no acute exacerbations.
Patients like Lauren and Zack serve to remind us of the need to recognize and closely monitor patients with nonclassic cystic fibrosis. These patients may come to the office with “asthma-like” symptoms, bronchitis, polyps, pancreatitis, cholelithiasis, constipation, abdominal bloating/flatus, and infertility. Because their symptoms may not be severe enough to be referred to a subspecialist, family physicians play a critical role in recognizing these overlooked cases early on.
CORRESPONDENCE Anupama Chawla, MD, Director, Division of Pediatric Gastroenterology and Nutrition, Stony Brook University Hospital, Stony Brook, NY 11794; [email protected]
1. Kerem E. Atypical CF and CF related diseases. Paediatr Respir Rev. 2006;7(suppl 1):S144-S146.
2. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Annual Patient Registry Data Report 2005. Bethesda, Md; 2006.
3. Castellani C, Cuppens H, Macek M, Jr, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179-196.
4. Wilchanski M, Zielenski J, Markiewicz D. Correlation of sweat chloride concentration with classes of the cystic fibrosis trans-membrane conductance regulator gene mutation, J Pediatr. 1995;127:705-710.
5. Mickle JE, Cutting GR. Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am. 2000;84:597-607.
6. Weiss FU, Simon P, Bogdanova MJ, et al. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic pancreatitis and controls. Gut. 2005;54:1456-1460.
7. Chang MC, Chang YT, Wei S, et al. Spectrum of mutations and variants/haplotypes of CFTR and genotype-phenotype correlation in idiopathic chronic pancreatitis and controls in Chinese by complete analysis. Clin Genet. 2007;71:530-539.
8. Noone PG, Knowles MR. “CFTR-opathies”: disease phenotypes associated with cystic fibrosis transmembrane regulator gene mutations. Respir Res. 2001;2:328-332.
9. Cohn JA, Neoptolemos JP, Feng J, et al. Increased risk of idiopathic pancreatitis in cystic fibrosis carriers. Hum Mutat. 2005;26:303-307.
10. Nick JA, Rodman DM. Manifestations of cystic fibrosis diagnosed in adulthood. Curr Opin Pulm Med 2005;11:513-518.
11. Poller W, Farber JP, Scholz S, et al. Sequence analysis of the cystic fibrosis gene in patients with disseminated bronchiectatic lung disease. Application in the identification of a cystic fibrosis patient with atypical clinical course. Klin Wochenschr. 1991;69:657-663.
12. Quan JM, Tiddens HA, Sy JP, et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813-820.
13. Tiddens HA. Detecting early structural lung damage in cystic fibrosis. Pediatr Pulmonol. 2002;34:228-231.
14. Judge EP, Dodd JD, Masterson JB, et al. Pulmonary abnormalities on high-resolution CT demonstrate more rapid decline than FEV1 in adults with cystic fibrosis. Chest. 2006;130:1424-1432.
15. Robinson TE, Goris ML, Zhu HJ, et al. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease. Chest. 2005;128:2327-2335.
16. Fuchs HJ, Borowitz DS, Christiansen DH, et al. The Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637-642.
17. Ramsey BW, Pepe MS, Quan JM, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23-30.
18. Elkins MR, Robinson M, Rose BR, et al. The National Hyper-tonic Saline in Cystic Fibrosis Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229-240.
19. Witt H. Chronic pancreatitis and cystic fibrosis. Gut. 2003;52(suppl 2):ii31-ii41.
1. Kerem E. Atypical CF and CF related diseases. Paediatr Respir Rev. 2006;7(suppl 1):S144-S146.
2. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Annual Patient Registry Data Report 2005. Bethesda, Md; 2006.
3. Castellani C, Cuppens H, Macek M, Jr, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179-196.
4. Wilchanski M, Zielenski J, Markiewicz D. Correlation of sweat chloride concentration with classes of the cystic fibrosis trans-membrane conductance regulator gene mutation, J Pediatr. 1995;127:705-710.
5. Mickle JE, Cutting GR. Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am. 2000;84:597-607.
6. Weiss FU, Simon P, Bogdanova MJ, et al. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic pancreatitis and controls. Gut. 2005;54:1456-1460.
7. Chang MC, Chang YT, Wei S, et al. Spectrum of mutations and variants/haplotypes of CFTR and genotype-phenotype correlation in idiopathic chronic pancreatitis and controls in Chinese by complete analysis. Clin Genet. 2007;71:530-539.
8. Noone PG, Knowles MR. “CFTR-opathies”: disease phenotypes associated with cystic fibrosis transmembrane regulator gene mutations. Respir Res. 2001;2:328-332.
9. Cohn JA, Neoptolemos JP, Feng J, et al. Increased risk of idiopathic pancreatitis in cystic fibrosis carriers. Hum Mutat. 2005;26:303-307.
10. Nick JA, Rodman DM. Manifestations of cystic fibrosis diagnosed in adulthood. Curr Opin Pulm Med 2005;11:513-518.
11. Poller W, Farber JP, Scholz S, et al. Sequence analysis of the cystic fibrosis gene in patients with disseminated bronchiectatic lung disease. Application in the identification of a cystic fibrosis patient with atypical clinical course. Klin Wochenschr. 1991;69:657-663.
12. Quan JM, Tiddens HA, Sy JP, et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813-820.
13. Tiddens HA. Detecting early structural lung damage in cystic fibrosis. Pediatr Pulmonol. 2002;34:228-231.
14. Judge EP, Dodd JD, Masterson JB, et al. Pulmonary abnormalities on high-resolution CT demonstrate more rapid decline than FEV1 in adults with cystic fibrosis. Chest. 2006;130:1424-1432.
15. Robinson TE, Goris ML, Zhu HJ, et al. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease. Chest. 2005;128:2327-2335.
16. Fuchs HJ, Borowitz DS, Christiansen DH, et al. The Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637-642.
17. Ramsey BW, Pepe MS, Quan JM, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23-30.
18. Elkins MR, Robinson M, Rose BR, et al. The National Hyper-tonic Saline in Cystic Fibrosis Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229-240.
19. Witt H. Chronic pancreatitis and cystic fibrosis. Gut. 2003;52(suppl 2):ii31-ii41.
Recurrent pleural effusions and a normal cardiac CT
CASE An 84-year-old man was admitted to our family medicine inpatient service with increasing weakness, fatigue, nausea, jaundice, and anorexia after undergoing thoracentesis for recurrent large right-sided pleural effusion twice within the past 6 weeks. The patient had no fever, chills, night sweats, chest pain, or chronic cough.
His past medical history included coronary artery disease and hyperlipidemia, and he had undergone coronary artery bypass grafting 6 years prior to this admission.
Initial vital signs included a blood pressure of 116/68 mm hg and a pulse rate of 78 beats per minute. physical examination revealed a thin but otherwise active and functional man, with the presence of scleral icterus and a 2/6 systolic murmur that was loudest at the left upper sternal border, with no pericardial knocks or rubs. There was no jugular venous distension. The patient had decreased breath sounds at the right base, but no appreciable rales, rhonchi, or wheezes, and an enlarged liver with an irregular edge approximately 7 cm below the costal margin. he had bilateral trace pitting edema of the lower extremities and an erythematous chronic venous stasis ulcer on his left lower leg that was being treated with sulfamethoxazole and trimethoprim and rifampin.
Laboratory findings included a sodium level of 130 mEq/L; creatinine, 1.6 mg/dL; total bilirubin, 4.2 mg/dL; and an international normalized ratio (INR) of 1.6, with an otherwise normal liver profile. pleural fluid was transudative by light’s criteria, with negative pleural fluid cultures.
A chest x-ray showed right-sided airspace disease, with an associated small effusion (FIGURE 1), and an electrocardiogram (EKG) revealed a right axis deviation with flipped T waves in V3-V6 (FIGURE 2). A computed tomography (CT) scan of the chest performed 2 months prior to admission revealed a small right pleural effusion, moderate emphysema, and pleural plaques. A transthoracic echocardiogram performed a week earlier was significant for a normal ejection fraction without pericardial thickening, and mild dilation of the left and right atria and the right ventricle.
Our patient had recurrent transudative pleural effusions with no history of congestive heart failure, a normal thyroid-stimulating hormone, no signs of nephrotic syndrome, no risk factors for lung cancer, and a presentation that was not consistent with an acute pulmonary embolus. he had jaundice, but no risk factors for cirrhosis. A comprehensive hepatology consult suggested that the jaundice was associated with recent rifampin use, along with hepatic congestion likely due to cardiac etiology.
The patient also had a documented history of constrictive pericarditis. Although a cardiac CT scan 15 months prior to his hospital admission showed normal pericardial thickness, constrictive pericarditis remained high on our differential.
To further evaluate the patient’s cardiac function, a cardiologist performed a right-sided cardiac catheterization. in constrictive pericarditis, equalization of end-diastolic pressures occurs due to limitation of the total volume of the ventricles by a rigid pericardium. in our patient, these pressures did not equalize. his right and left ventricular pressure tracings did, however, have the classic square root sign often seen in constrictive pericarditis (FIGURE 3). This “dip and plateau” is the result of rapid filling of the ventricles during early diastole, with the inflexible pericardium causing an abrupt halt in ventricular filling.1
We considered restrictive cardiomyopathy in the differential, too, but the signs and symptoms weren’t a perfect fit there, either. equalization of left and right ventricular end-diastolic pressures in cardiac catheterization is usually not seen in restrictive cardiomyopathy, but neither is the square-root sign evident on ventricular pressure monitors.1
FIGURE 1
X-ray shows right-sided airspace disease
FIGURE 2
Right axis deviation with flipped T waves
FIGURE 3
Classic square root sign
WHAT IS THE MOST LIKELY EXPLANATION FOR HIS CONDITION?
Constrictive pericarditis
On the advice of a cardiologist who specializes in advanced cardiac imaging, our patient underwent tissue Doppler velocity echocardiography—a diagnostic test that provides evidence of a diseased myocardium (as in restrictive cardiomyopathy), as well as changes in diastolic blood flow that differentiate constrictive pericarditis from restrictive cardiomyopathy. Our patient’s tissue Doppler velocity echocardiogram revealed a normal myocardium with an abrupt cessation of early left ventricular filling, consistent with constrictive pericarditis. Along with his clinical presentation and history, this test was conclusive enough to diagnose constrictive pericarditis.
Making the diagnosis in more “typical” cases
Symptoms of constrictive pericarditis include fluid overload (eg, recurrent pleural effusions2-4) hepatic dysfunction, and peripheral edema. Studies show that 44% to 54% of patients with constrictive pericarditis present with pleural effusions;5 chest pain and dyspnea are other common symptoms.6 Past medical history is also important, considering the 3 most common risks for constrictive pericarditis: cardiac surgery, pericarditis, and irradiation of the mediastinum (TABLE).7
Typical physical exam findings in patients with constrictive pericarditis include an elevated jugular venous pressure, third spacing of fluids, a pericardial knock, and Kussmaul’s sign. Our patient had third spacing of fluids, including mild peripheral edema and the recurrent effusions as noted, as well as signs of hepatic dysfunction that were initially attributed to his rifampin use. While these symptoms raise the suspicion of constrictive pericarditis, none is specific to that condition alone.
Studies that help in the diagnosis of constrictive pericarditis include chest x-ray, cardiac CT, echocardiography, cardiac magnetic resonance imaging (MRI), and cardiac catheterization. EKG has no specific findings, but cardiac arrhythmias (eg, atrial fibrillation) are common.8 Pericardial thickening on cardiac CT scan is a definitive but not universal finding; in a Mayo Clinic study, such thickening was not evident in 26 of 143 patients with confirmed constrictive pericarditis.8 Cardiac MRI has been shown to have 88% sensitivity and 100% specificity,9 but will miss up to 18% of patients with constrictive pericarditis.8
Differentiating between restriction and constriction. It can be difficult to distinguish between constrictive pericarditis and restrictive cardiomyopathy. Although these conditions can present in a similar manner, they require different modes of treatment. Laboratory testing, cardiac catheterization, and tissue Doppler velocity echocardiography (which we relied on) can help to distinguish between them.
TABLE
Causes of constrictive pericarditis7
| More common |
|---|
| Idiopathic Postcardiac surgery (coronary artery bypass grafting, valve replacement) Postradiation therapy (eg, for breast cancer, lymphoma) Viral (pericarditis) |
| Less common |
| Asbestosis Cancer and myeloproliferative disorders Connective tissue disorders (rheumatoid arthritis, systemic lupus erythematosus) Adverse drug reaction Infection (tuberculosis, fungal) Sarcoidosis Trauma Uremia |
Pericardiectomy is the treatment of choice
Pericardiectomy, the optimal treatment for most patients with constrictive pericarditis, carries a 30-day perioperative mortality of approximately 6%.5,7 Patients with minimal symptoms can be monitored for up to 2 months, but only 17% of cases are self-limited.6 Patients with end-stage disease or those who have radiation-induced constrictive pericarditis experience poor surgical outcomes, and may be better served by medical management.7
In light of our patient’s excellent baseline functional status, clinical presentation, and Doppler test results, a cardiothoracic surgeon performed a pericardiectomy. His symptoms improved postoperatively, and he has had no further pleural effusions. The patient’s fatigue, anorexia, weight loss, and dyspnea fully resolved, as well.
- Include constrictive pericarditis in the differential diagnosis of patients with recurrent pleural effusions, an important presenting symptom in 44% to 54% of patients with this condition.
- Consider multiple testing modalities to arrive at a diagnosis of constrictive pericarditis, including cardiac CT or MRI, tissue Doppler echocardiography, and cardiac catheterization.
- Do not rule out constrictive pericarditis if pericardial thickening is not found on cardiac CT scan; in 1 study, this finding was not present in 18% of patients with a confirmed diagnosis.
CORRESPONDENCE Jeffrey S. Morgeson, MD; 2123 Auburn Avenue, 340 MOB, Cincinnati, OH 45219; [email protected]
1. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
2. Akhter MW, Nuño IN, Rahimtoola SH. Constrictive pericarditis masquerading as chronic idiopathic pleural effusion: importance of physical examination. Am J Med. 2006;119:e1-e4.
3. Ramar K, Daniels CA. Constrictive pericarditis presenting as unexplained dyspnea with recurrent pleural effusion. Respir Care. 2008;53:912-915.
4. Sadikot RT, Fredi JL, Light RW. A 43-year-old man with a large recurrent right-sided pleural effusion. Diagnosis: constrictive pericarditis. Chest. 2000;117:1191-1194.
5. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445-1452.
6. Haley JH, Tajik AJ, Danielson GK, et al. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43:271-275.
7. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
8. Talreja DP, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
9. Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369-373.
CASE An 84-year-old man was admitted to our family medicine inpatient service with increasing weakness, fatigue, nausea, jaundice, and anorexia after undergoing thoracentesis for recurrent large right-sided pleural effusion twice within the past 6 weeks. The patient had no fever, chills, night sweats, chest pain, or chronic cough.
His past medical history included coronary artery disease and hyperlipidemia, and he had undergone coronary artery bypass grafting 6 years prior to this admission.
Initial vital signs included a blood pressure of 116/68 mm hg and a pulse rate of 78 beats per minute. physical examination revealed a thin but otherwise active and functional man, with the presence of scleral icterus and a 2/6 systolic murmur that was loudest at the left upper sternal border, with no pericardial knocks or rubs. There was no jugular venous distension. The patient had decreased breath sounds at the right base, but no appreciable rales, rhonchi, or wheezes, and an enlarged liver with an irregular edge approximately 7 cm below the costal margin. he had bilateral trace pitting edema of the lower extremities and an erythematous chronic venous stasis ulcer on his left lower leg that was being treated with sulfamethoxazole and trimethoprim and rifampin.
Laboratory findings included a sodium level of 130 mEq/L; creatinine, 1.6 mg/dL; total bilirubin, 4.2 mg/dL; and an international normalized ratio (INR) of 1.6, with an otherwise normal liver profile. pleural fluid was transudative by light’s criteria, with negative pleural fluid cultures.
A chest x-ray showed right-sided airspace disease, with an associated small effusion (FIGURE 1), and an electrocardiogram (EKG) revealed a right axis deviation with flipped T waves in V3-V6 (FIGURE 2). A computed tomography (CT) scan of the chest performed 2 months prior to admission revealed a small right pleural effusion, moderate emphysema, and pleural plaques. A transthoracic echocardiogram performed a week earlier was significant for a normal ejection fraction without pericardial thickening, and mild dilation of the left and right atria and the right ventricle.
Our patient had recurrent transudative pleural effusions with no history of congestive heart failure, a normal thyroid-stimulating hormone, no signs of nephrotic syndrome, no risk factors for lung cancer, and a presentation that was not consistent with an acute pulmonary embolus. he had jaundice, but no risk factors for cirrhosis. A comprehensive hepatology consult suggested that the jaundice was associated with recent rifampin use, along with hepatic congestion likely due to cardiac etiology.
The patient also had a documented history of constrictive pericarditis. Although a cardiac CT scan 15 months prior to his hospital admission showed normal pericardial thickness, constrictive pericarditis remained high on our differential.
To further evaluate the patient’s cardiac function, a cardiologist performed a right-sided cardiac catheterization. in constrictive pericarditis, equalization of end-diastolic pressures occurs due to limitation of the total volume of the ventricles by a rigid pericardium. in our patient, these pressures did not equalize. his right and left ventricular pressure tracings did, however, have the classic square root sign often seen in constrictive pericarditis (FIGURE 3). This “dip and plateau” is the result of rapid filling of the ventricles during early diastole, with the inflexible pericardium causing an abrupt halt in ventricular filling.1
We considered restrictive cardiomyopathy in the differential, too, but the signs and symptoms weren’t a perfect fit there, either. equalization of left and right ventricular end-diastolic pressures in cardiac catheterization is usually not seen in restrictive cardiomyopathy, but neither is the square-root sign evident on ventricular pressure monitors.1
FIGURE 1
X-ray shows right-sided airspace disease
FIGURE 2
Right axis deviation with flipped T waves
FIGURE 3
Classic square root sign
WHAT IS THE MOST LIKELY EXPLANATION FOR HIS CONDITION?
Constrictive pericarditis
On the advice of a cardiologist who specializes in advanced cardiac imaging, our patient underwent tissue Doppler velocity echocardiography—a diagnostic test that provides evidence of a diseased myocardium (as in restrictive cardiomyopathy), as well as changes in diastolic blood flow that differentiate constrictive pericarditis from restrictive cardiomyopathy. Our patient’s tissue Doppler velocity echocardiogram revealed a normal myocardium with an abrupt cessation of early left ventricular filling, consistent with constrictive pericarditis. Along with his clinical presentation and history, this test was conclusive enough to diagnose constrictive pericarditis.
Making the diagnosis in more “typical” cases
Symptoms of constrictive pericarditis include fluid overload (eg, recurrent pleural effusions2-4) hepatic dysfunction, and peripheral edema. Studies show that 44% to 54% of patients with constrictive pericarditis present with pleural effusions;5 chest pain and dyspnea are other common symptoms.6 Past medical history is also important, considering the 3 most common risks for constrictive pericarditis: cardiac surgery, pericarditis, and irradiation of the mediastinum (TABLE).7
Typical physical exam findings in patients with constrictive pericarditis include an elevated jugular venous pressure, third spacing of fluids, a pericardial knock, and Kussmaul’s sign. Our patient had third spacing of fluids, including mild peripheral edema and the recurrent effusions as noted, as well as signs of hepatic dysfunction that were initially attributed to his rifampin use. While these symptoms raise the suspicion of constrictive pericarditis, none is specific to that condition alone.
Studies that help in the diagnosis of constrictive pericarditis include chest x-ray, cardiac CT, echocardiography, cardiac magnetic resonance imaging (MRI), and cardiac catheterization. EKG has no specific findings, but cardiac arrhythmias (eg, atrial fibrillation) are common.8 Pericardial thickening on cardiac CT scan is a definitive but not universal finding; in a Mayo Clinic study, such thickening was not evident in 26 of 143 patients with confirmed constrictive pericarditis.8 Cardiac MRI has been shown to have 88% sensitivity and 100% specificity,9 but will miss up to 18% of patients with constrictive pericarditis.8
Differentiating between restriction and constriction. It can be difficult to distinguish between constrictive pericarditis and restrictive cardiomyopathy. Although these conditions can present in a similar manner, they require different modes of treatment. Laboratory testing, cardiac catheterization, and tissue Doppler velocity echocardiography (which we relied on) can help to distinguish between them.
TABLE
Causes of constrictive pericarditis7
| More common |
|---|
| Idiopathic Postcardiac surgery (coronary artery bypass grafting, valve replacement) Postradiation therapy (eg, for breast cancer, lymphoma) Viral (pericarditis) |
| Less common |
| Asbestosis Cancer and myeloproliferative disorders Connective tissue disorders (rheumatoid arthritis, systemic lupus erythematosus) Adverse drug reaction Infection (tuberculosis, fungal) Sarcoidosis Trauma Uremia |
Pericardiectomy is the treatment of choice
Pericardiectomy, the optimal treatment for most patients with constrictive pericarditis, carries a 30-day perioperative mortality of approximately 6%.5,7 Patients with minimal symptoms can be monitored for up to 2 months, but only 17% of cases are self-limited.6 Patients with end-stage disease or those who have radiation-induced constrictive pericarditis experience poor surgical outcomes, and may be better served by medical management.7
In light of our patient’s excellent baseline functional status, clinical presentation, and Doppler test results, a cardiothoracic surgeon performed a pericardiectomy. His symptoms improved postoperatively, and he has had no further pleural effusions. The patient’s fatigue, anorexia, weight loss, and dyspnea fully resolved, as well.
- Include constrictive pericarditis in the differential diagnosis of patients with recurrent pleural effusions, an important presenting symptom in 44% to 54% of patients with this condition.
- Consider multiple testing modalities to arrive at a diagnosis of constrictive pericarditis, including cardiac CT or MRI, tissue Doppler echocardiography, and cardiac catheterization.
- Do not rule out constrictive pericarditis if pericardial thickening is not found on cardiac CT scan; in 1 study, this finding was not present in 18% of patients with a confirmed diagnosis.
CORRESPONDENCE Jeffrey S. Morgeson, MD; 2123 Auburn Avenue, 340 MOB, Cincinnati, OH 45219; [email protected]
CASE An 84-year-old man was admitted to our family medicine inpatient service with increasing weakness, fatigue, nausea, jaundice, and anorexia after undergoing thoracentesis for recurrent large right-sided pleural effusion twice within the past 6 weeks. The patient had no fever, chills, night sweats, chest pain, or chronic cough.
His past medical history included coronary artery disease and hyperlipidemia, and he had undergone coronary artery bypass grafting 6 years prior to this admission.
Initial vital signs included a blood pressure of 116/68 mm hg and a pulse rate of 78 beats per minute. physical examination revealed a thin but otherwise active and functional man, with the presence of scleral icterus and a 2/6 systolic murmur that was loudest at the left upper sternal border, with no pericardial knocks or rubs. There was no jugular venous distension. The patient had decreased breath sounds at the right base, but no appreciable rales, rhonchi, or wheezes, and an enlarged liver with an irregular edge approximately 7 cm below the costal margin. he had bilateral trace pitting edema of the lower extremities and an erythematous chronic venous stasis ulcer on his left lower leg that was being treated with sulfamethoxazole and trimethoprim and rifampin.
Laboratory findings included a sodium level of 130 mEq/L; creatinine, 1.6 mg/dL; total bilirubin, 4.2 mg/dL; and an international normalized ratio (INR) of 1.6, with an otherwise normal liver profile. pleural fluid was transudative by light’s criteria, with negative pleural fluid cultures.
A chest x-ray showed right-sided airspace disease, with an associated small effusion (FIGURE 1), and an electrocardiogram (EKG) revealed a right axis deviation with flipped T waves in V3-V6 (FIGURE 2). A computed tomography (CT) scan of the chest performed 2 months prior to admission revealed a small right pleural effusion, moderate emphysema, and pleural plaques. A transthoracic echocardiogram performed a week earlier was significant for a normal ejection fraction without pericardial thickening, and mild dilation of the left and right atria and the right ventricle.
Our patient had recurrent transudative pleural effusions with no history of congestive heart failure, a normal thyroid-stimulating hormone, no signs of nephrotic syndrome, no risk factors for lung cancer, and a presentation that was not consistent with an acute pulmonary embolus. he had jaundice, but no risk factors for cirrhosis. A comprehensive hepatology consult suggested that the jaundice was associated with recent rifampin use, along with hepatic congestion likely due to cardiac etiology.
The patient also had a documented history of constrictive pericarditis. Although a cardiac CT scan 15 months prior to his hospital admission showed normal pericardial thickness, constrictive pericarditis remained high on our differential.
To further evaluate the patient’s cardiac function, a cardiologist performed a right-sided cardiac catheterization. in constrictive pericarditis, equalization of end-diastolic pressures occurs due to limitation of the total volume of the ventricles by a rigid pericardium. in our patient, these pressures did not equalize. his right and left ventricular pressure tracings did, however, have the classic square root sign often seen in constrictive pericarditis (FIGURE 3). This “dip and plateau” is the result of rapid filling of the ventricles during early diastole, with the inflexible pericardium causing an abrupt halt in ventricular filling.1
We considered restrictive cardiomyopathy in the differential, too, but the signs and symptoms weren’t a perfect fit there, either. equalization of left and right ventricular end-diastolic pressures in cardiac catheterization is usually not seen in restrictive cardiomyopathy, but neither is the square-root sign evident on ventricular pressure monitors.1
FIGURE 1
X-ray shows right-sided airspace disease
FIGURE 2
Right axis deviation with flipped T waves
FIGURE 3
Classic square root sign
WHAT IS THE MOST LIKELY EXPLANATION FOR HIS CONDITION?
Constrictive pericarditis
On the advice of a cardiologist who specializes in advanced cardiac imaging, our patient underwent tissue Doppler velocity echocardiography—a diagnostic test that provides evidence of a diseased myocardium (as in restrictive cardiomyopathy), as well as changes in diastolic blood flow that differentiate constrictive pericarditis from restrictive cardiomyopathy. Our patient’s tissue Doppler velocity echocardiogram revealed a normal myocardium with an abrupt cessation of early left ventricular filling, consistent with constrictive pericarditis. Along with his clinical presentation and history, this test was conclusive enough to diagnose constrictive pericarditis.
Making the diagnosis in more “typical” cases
Symptoms of constrictive pericarditis include fluid overload (eg, recurrent pleural effusions2-4) hepatic dysfunction, and peripheral edema. Studies show that 44% to 54% of patients with constrictive pericarditis present with pleural effusions;5 chest pain and dyspnea are other common symptoms.6 Past medical history is also important, considering the 3 most common risks for constrictive pericarditis: cardiac surgery, pericarditis, and irradiation of the mediastinum (TABLE).7
Typical physical exam findings in patients with constrictive pericarditis include an elevated jugular venous pressure, third spacing of fluids, a pericardial knock, and Kussmaul’s sign. Our patient had third spacing of fluids, including mild peripheral edema and the recurrent effusions as noted, as well as signs of hepatic dysfunction that were initially attributed to his rifampin use. While these symptoms raise the suspicion of constrictive pericarditis, none is specific to that condition alone.
Studies that help in the diagnosis of constrictive pericarditis include chest x-ray, cardiac CT, echocardiography, cardiac magnetic resonance imaging (MRI), and cardiac catheterization. EKG has no specific findings, but cardiac arrhythmias (eg, atrial fibrillation) are common.8 Pericardial thickening on cardiac CT scan is a definitive but not universal finding; in a Mayo Clinic study, such thickening was not evident in 26 of 143 patients with confirmed constrictive pericarditis.8 Cardiac MRI has been shown to have 88% sensitivity and 100% specificity,9 but will miss up to 18% of patients with constrictive pericarditis.8
Differentiating between restriction and constriction. It can be difficult to distinguish between constrictive pericarditis and restrictive cardiomyopathy. Although these conditions can present in a similar manner, they require different modes of treatment. Laboratory testing, cardiac catheterization, and tissue Doppler velocity echocardiography (which we relied on) can help to distinguish between them.
TABLE
Causes of constrictive pericarditis7
| More common |
|---|
| Idiopathic Postcardiac surgery (coronary artery bypass grafting, valve replacement) Postradiation therapy (eg, for breast cancer, lymphoma) Viral (pericarditis) |
| Less common |
| Asbestosis Cancer and myeloproliferative disorders Connective tissue disorders (rheumatoid arthritis, systemic lupus erythematosus) Adverse drug reaction Infection (tuberculosis, fungal) Sarcoidosis Trauma Uremia |
Pericardiectomy is the treatment of choice
Pericardiectomy, the optimal treatment for most patients with constrictive pericarditis, carries a 30-day perioperative mortality of approximately 6%.5,7 Patients with minimal symptoms can be monitored for up to 2 months, but only 17% of cases are self-limited.6 Patients with end-stage disease or those who have radiation-induced constrictive pericarditis experience poor surgical outcomes, and may be better served by medical management.7
In light of our patient’s excellent baseline functional status, clinical presentation, and Doppler test results, a cardiothoracic surgeon performed a pericardiectomy. His symptoms improved postoperatively, and he has had no further pleural effusions. The patient’s fatigue, anorexia, weight loss, and dyspnea fully resolved, as well.
- Include constrictive pericarditis in the differential diagnosis of patients with recurrent pleural effusions, an important presenting symptom in 44% to 54% of patients with this condition.
- Consider multiple testing modalities to arrive at a diagnosis of constrictive pericarditis, including cardiac CT or MRI, tissue Doppler echocardiography, and cardiac catheterization.
- Do not rule out constrictive pericarditis if pericardial thickening is not found on cardiac CT scan; in 1 study, this finding was not present in 18% of patients with a confirmed diagnosis.
CORRESPONDENCE Jeffrey S. Morgeson, MD; 2123 Auburn Avenue, 340 MOB, Cincinnati, OH 45219; [email protected]
1. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
2. Akhter MW, Nuño IN, Rahimtoola SH. Constrictive pericarditis masquerading as chronic idiopathic pleural effusion: importance of physical examination. Am J Med. 2006;119:e1-e4.
3. Ramar K, Daniels CA. Constrictive pericarditis presenting as unexplained dyspnea with recurrent pleural effusion. Respir Care. 2008;53:912-915.
4. Sadikot RT, Fredi JL, Light RW. A 43-year-old man with a large recurrent right-sided pleural effusion. Diagnosis: constrictive pericarditis. Chest. 2000;117:1191-1194.
5. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445-1452.
6. Haley JH, Tajik AJ, Danielson GK, et al. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43:271-275.
7. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
8. Talreja DP, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
9. Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369-373.
1. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25:587-610.
2. Akhter MW, Nuño IN, Rahimtoola SH. Constrictive pericarditis masquerading as chronic idiopathic pleural effusion: importance of physical examination. Am J Med. 2006;119:e1-e4.
3. Ramar K, Daniels CA. Constrictive pericarditis presenting as unexplained dyspnea with recurrent pleural effusion. Respir Care. 2008;53:912-915.
4. Sadikot RT, Fredi JL, Light RW. A 43-year-old man with a large recurrent right-sided pleural effusion. Diagnosis: constrictive pericarditis. Chest. 2000;117:1191-1194.
5. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445-1452.
6. Haley JH, Tajik AJ, Danielson GK, et al. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004;43:271-275.
7. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386.
8. Talreja DP, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857.
9. Masui T, Finck S, Higgins CB. Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology. 1992;182:369-373.