User login
The Multiple Sclerosis Surveillance Registry: A Novel Interactive Database Within the Veterans Health Administration (FULL)
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
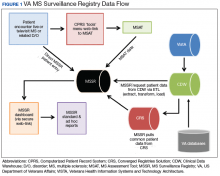
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
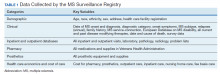
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
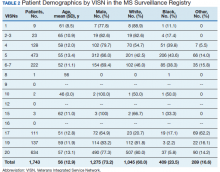
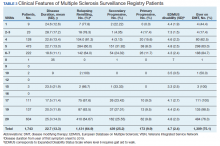
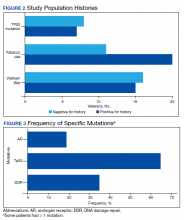
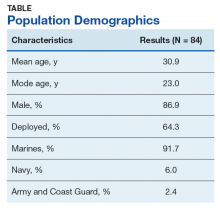
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
The VA MS Surveillance Registry combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
A number of large registries exist for multiple sclerosis (MS) in North America and Europe. The Scandinavian countries have some of the longest running and integrated MS registries to date. The Danish MS Registry was initiated in 1948 and has been consistently maintained to track MS epidemiologic trends.2 Similar databases exist in Swedenand Norway that were created in the later 20th century.3,4 The Rochester Epidemiology Project, launched by Len Kurland at the Mayo Clinic, has tracked the morbidity of MS and many other conditions in Olmsted county Minnesota for > 60 years.5
The Canadian provinces of British Columbia, Ontario, and Manitoba also have long standing MS registries.6-8 Other North American MS registries have gathered state-wide cases, such as the New York State MS Consortium.9 Some registries have gathered a population-based sample throughout the US, such as the Sonya Slifka MS Study.10 The North American Research Consortium on MS (NARCOMS) registry is a patient-driven registry within the US that has enrolled > 30,000 cases.11 The MSBase is the largest online registry to date utilizing data from several countries.12 The MS Bioscreen, based at the University of California San Francisco, is a recent effort to create a longitudinal clinical dataset.13 This electronic registry integrates clinical disease morbidity scales, neuroimaging, genetics and laboratory data for individual patients with the goal of providing predictive tools.
The US military provides a unique population to study MS and has the oldest and largest nation-wide MS cohort in existence starting with World War I service members and continuing through the recent Gulf War Era.14 With the advent of EHRs in the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA) in the mid-1990s and large clinical databases, the possibility of an integrated registry for chronic conditions was created. In this report, we describe the creation of the VA MS Surveillance Registry (MSSR) and the initial roll out to several VA medical centers within the MS Center of Excellence (MSCoE). The MSSR is a unique platform with potential for improving MS patient care and clinical research.
Methods
The MSSR was designed by MSCoE health care providers in conjunction with IT specialists from the VA Northwest Innovation Center. Between 2012 and 2013, the team developed and tested a core template for data entry and refined an efficient data dashboard display to optimize clinical decisions. IT programmers created data entry templates that were tested by 4 to 5 clinicians who provided feedback in biweekly meetings. Technical problems were addressed and enhancements added and the trial process was repeated.
After creation of the prototype MS Assessment Tool (MSAT) data entry template that fed into the prototype MSSR, our team received a grant in 2013 for national development and sustainment. The MSSR was established on the VA Converged Registries Solution (CRS) platform, which is a hardware and software architecture designed to host individual clinical registries and eliminate duplicative development effort while maximizing the ability to create new patient registries. The common platform includes a relational database, Health Level 7 messaging, software classes, security modules, extraction services, and other components. The CR obtains data from the VA Corporate Data Warehouse (CDW), directly from the Veterans Health Information Systems and Technology Architecture (VISTA) and via direct user input using MSAT.
From 2016 to 2019, data from patients with MS followed in several VA MS regional programs were inputted into MSSR. A roll-out process to start patient data entry at VA medical centers began in 2017 that included an orientation, technical support, and quality assurance review. Twelve sites from Veteran Integrated Service Network (VISN) 5 (mid-Atlantic) and VISN 20 (Pacific Northwest) were included in the initial roll-out.
Results
After a live or remote telehealth or telephone visit, a clinician can access MSAT from the Computerized Patient Record System (CPRS) or directly from the MSSR online portal (Figure 1). The tool uses radio buttons and pull-down menus and takes about 5 to 15 minutes to complete with a list of required variables. Data is auto-saved for efficiency, and the key variables that are collected in MSAT are noted in Table 1. The MSAT subsequently creates a text integration utility progress note with health factors that is processed through an integration engine and eventually transmitted to VISTA and becomes part of the EHR and available to all health care providers involved in that patient’s care. Additionally, data from VA outpatient and inpatient utilization files, pharmacy, prosthetics, laboratory, and radiology databases are included in the CDW and are included in MSSR. With data from 1998 to the present, the MSAT and CDW databases can provide longitudinal data analysis.
Between 18,000 and 20,000 patients with MS are evaluated in the VHA annually, and 56,000 unique patients have been assessed since 1998. From 2016 to 2019, 1,743 patients with MS or related disorders were enrolled in MSSR (Table 2 and Figure 2). The mean (SD) age of patients was 56.0 (12.9) years and the male:female ratio was 2.7. Racial minorities make up 40% of the cohort. Among those with definite and possible MS, the mean disease duration was 22.7 years and the mean (SD) European Database for MS disability score was 4.7 (2.4) (Table 3). Three-quarters of the MSSR cohort have used ≥ 1 MS disease modifying therapy and 65% were classified as relapsing-remitting MS. An electronic dashboard was developed for health care providers to easily access demographic and clinical data for individuals and groups of patients (Figure 3). Standard and ad hoc reports can be generated from the MSSR. Larger longitudinal analyses can be performed with MSAT and clinical data from CDW. Data on comorbid conditions, pharmacy, radiology and prosthetics utilization, outpatient clinic and inpatient admission can be accessed for each patient or a group of patients.
In 2015, MSCoE published a larger national survey of the VA MS population.15 This study revealed that the majority of clinical features and demographics of the MSSR were not significantly different from other major US MS registries including the North American Research Committee on MS, the New York State MS Consortium, and the Sonya Slifka Study.16-18
Discussion
The MSSR is novel in that it combines a traditional MS registry with individual clinical and utilization data within the largest integrated health system in the US. This new registry leverages the existing databases related to cost of care, utilization, and pharmacy services to provide surveillance tools for longitudinal follow-up of the MS population within the VHA. Because the structure of the MSAT and MSSR were developed in a partnership between IT developers and clinicians, there has been mutual buy-in for those who use it and maintain it. This registry can be a test bed for standardized patient outcomes including the recently released MS Quality measures from the American Academy of Neurology.19
To achieve greater numbers across populations, there has been efforts in Europe to combine registries into a common European Register for MS. A recent survey found that although many European registries were heterogeneous, it would be possible to have a minimum common data set for limited epidemiologic studies.20 Still many registries do not have environmental or genetic data to evaluate etiologic questions.21 Additionally, most registries are not set up to evaluate cost or quality of care within a health care system.
Recommendations for maximizing the impact of existing MS registries were recently released by a panel of MS clinicians and researchers.22 The first recommendation was to create a broad network of registries that would communicate and collaborate. This group of MS registries would have strategic oversight and direction that would greatly streamline and leverage existing and future efforts. Second, registries should standardize data collection and management thereby enhancing the ability to share data and perform meta-analyses with aggregated data. Third, the collection of physician- and patient-reported outcomes should be encouraged to provide a more complete picture of MS. Finally, registries should prioritize research questions and utilize new technologies for data collection. These recommendations would help to coordinate existing registries and accelerate knowledge discovery.
The MSSR will contribute to the growing registry network of data. The MSSR can address questions about clinical outcomes, cost, quality with a growing data repository and linked biobank. Based on the CR platform, the MSSR allows for integration with other VA clinical registries, including registries for traumatic brain injuries, oncology, HIV, hepatitis C virus, and eye injuries. Identifying case outcomes related to other registries is optimized with the CR common structure.
Conclusion
The MSSR has been a useful tool for clinicians managing individual patients and their regional referral populations with real-time access to clinical and utilization data. It will also be a useful research tool in tracking epidemiological trends for the military population. The MSSR has enhanced clinical management of MS and serves as a national source for clinical outcomes.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
1. Flachenecker P. Multiple sclerosis databases: present and future. Eur Neurol. 2014;72(suppl 1):29-31.
2. Koch-Henriksen N, Magyari M, Laursen B. Registers of multiple sclerosis in Denmark. Acta Neurol Scand. 2015;132(199):4-10.
3. Alping P, Piehl F, Langer-Gould A, Frisell T; COMBAT-MS Study Group. Validation of the Swedish Multiple Sclerosis Register: further improving a resource for pharmacoepidemiologic evaluations. Epidemiology. 2019;30(2):230-233.
4. Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422.
5. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202-1213.
6. Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991-2010). J Neurol. 2015;262(10):2352-2363.
7. Scalfari A, Neuhaus A, Degenhardt A, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. 2010;133(Pt 7):1914-1929.
8. Mahmud SM, Bozat-Emre S, Mostaço-Guidolin LC, Marrie RA. Registry cohort study to determine risk for multiple sclerosis after vaccination for pandemic influenza A(H1N1) with Arepanrix, Manitoba, Canada. Emerg Infect Dis. 2018;24(7):1267-1274.
9. Kister I, Chamot E, Bacon JH, Cutter G, Herbert J; New York State Multiple Sclerosis Consortium. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler. 2011;17(6):725-733.
10. Minden SL, Frankel D, Hadden L, Perloffp J, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: methods and sample characteristics. Mult Scler. 2006;12(1):24-38.
11. Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: survey results from the NARCOMS registry. Mult Scler Relat Disord. 2015;4(3):241-249.
12. Kalincik T, Butzkueven H. The MSBase registry: Informing clinical practice. Mult Scler. 2019;25(14):1828-1834.
13. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol. 2014;76(5):633-642.
14. Wallin MT, Culpepper WJ, Coffman P, et al. The Gulf War era multiple sclerosis cohort: age and incidence rates by race, sex and service. Brain. 2012;135(Pt 6):1778-1785.
15. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272.
16. Salter A, Stahmann A, Ellenberger D, et al. Data harmonization for collaborative research among MS registries: a case study in employment [published online ahead of print, 2020 Mar 12]. Mult Scler. 2020;1352458520910499.
17. Vaughn CB, Kavak KS, Dwyer MG, et al. Fatigue at enrollment predicts EDSS worsening in the New York State Multiple Sclerosis Consortium. Mult Scler. 2020;26(1):99-108.
18. Minden SL, Kinkel RP, Machado HT, et al. Use and cost of disease-modifying therapies by Sonya Slifka Study participants: has anything really changed since 2000 and 2009? Mult Scler J Exp Transl Clin. 2019;5(1):2055217318820888.
19. Rae-Grant A, Bennett A, Sanders AE, Phipps M, Cheng E, Bever C. Quality improvement in neurology: multiple sclerosis quality measures: Executive summary [published correction appears in Neurology. 2016;86(15):1465]. Neurology. 2015;85(21):1904-1908.
20. Flachenecker P, Buckow K, Pugliatti M, et al; EUReMS Consortium. Multiple sclerosis registries in Europe - results of a systematic survey. Mult Scler. 2014;20(11):1523-1532.
21. Traboulsee A, McMullen K. How useful are MS registries?. Mult Scler. 2014;20(11):1423-1424.
22. Bebo BF Jr, Fox RJ, Lee K, Utz U, Thompson AJ. Landscape of MS patient cohorts and registries: Recommendations for maximizing impact. Mult Scler. 2018;24(5):579-586.
The Multiple Sclerosis Centers of Excellence: A Model of Excellence in the VA (FULL)
The Veterans Health Administration (VHA) has established a number of centers of excellence (CoEs), including centers focused on posttraumatic stress disorder, suicide prevention, epilepsy, and, most recently, the Senator Elizabeth Dole CoE for Veteran and Caregiver Research. Some VA CoE serve as centralized locations for specialty care. For example, the VA Epilepsy CoE is a network of 16 facilities that provide comprehensive epilepsy care for veterans with seizure disorders, including expert and presurgical evaluations and inpatient monitoring.
In contrast, other CoEs, including the multiple sclerosis (MS) CoE, achieve their missions by serving as a resource center to a network of regional and supporting various programs to optimize the care of veterans across the nation within their home US Department of Veterans Affairs (VA) medical center (VAMC). The MSCoE are charged, through VHA Directive 1011.06, with establishing at least 1 VA MS Regional Program in each of the 21 Veteran Integrated Service Networks (VISNs) across the country and integrating these and affiliated MS Support Programs into the MS National Network. Currently, there are 29 MS regional programs and 49 MS support programs across the US.1
Established in 2003, the MSCoE is dedicated to furthering the understanding of MS, its impact on veterans, and effective treatments to help manage the disease and its symptoms. In 2002, 2 coordinating centers were selected based on a competitive review process. The MSCoE-East is located at the Baltimore, Maryland and Washington, DC VAMC and serves VISNs 1 to 10. The MSCoE-West serves VISNs 11 to 23 and is jointly-based at VA Puget Sound Health Care System in Seattle, Washington and VA Portland Health Care System in Portland, Oregon. The MSCoEs were made permanent by The Veteran’ Benefits, Healthcare and Information Technology Act of 2006 (38 USC §7330). By partnering with veterans, caregivers, health care professionals, and other affiliates, the MSCoE endeavor to optimize health, activities, participation and quality of life for veterans with MS.
Core Functions
The MSCoE has a 3-part mission. First, the MSCoE seeks to expand care coordination between VAMCs by developing a national network of VA MSCoE Regional and Support Programs. Second, the MSCoE provides resources to VA health care providers (HCPs) through a collaborative approach to clinical care, education, research, and informatics. Third, the MSCoE improves the quality and consistency of health care services delivered to veterans diagnosed with MS nationwide. To meet its objectives, the MSCoE activities are organized around 4 functional cores: clinical care, research, education and training, and informatics and telemedicine.
Clinical Care
The MSCoE delivers high-quality clinical care by identifying veterans with MS who use VA services, understanding their needs, and facilitating appropriate interventions. Veterans with MS are a special cohort for many reasons including that about 70% are male. Men and women veterans not only have different genetics, but also may have different environmental exposures and other risk factors for MS. Since 1998, the VHA has evaluated > 50,000 veterans with MS. Over the past decade, between 18,000 and 20,000 veterans with MS have accessed care within the VHA annually.
The MSCoE advocates for appropriate and safe use of currently available MS disease modifying therapies through collaborations with the VA Pharmacy Benefits Management Service (PBM). The MSCoE partners with PBM to develop and disseminate Criteria For Use, safety, and economic monitoring of the impacts of the MS therapies. The MSCoE also provide national consultation services for complex MS cases, clinical education to VA HCPs, and mentors fellows, residents, and medical students.
The VA provides numerous resources that are not readily available in other health care systems and facilitate the care for patients with chronic diseases, including providing low or no co-pays to patients for MS disease modifying agents and other MS related medications, access to medically necessary adaptive equipment at no charge, the Home Improvement and Structural Alteration (HISA) grant for assistance with safe home ingress and egress, respite care, access to a homemaker/home health aide, and caregiver support programs. Eligible veterans also can access additional resources such as adaptive housing and an automobile grant. The VA also provides substantial hands-on assistance to veterans who are homeless. The clinical team and a veteran with MS can leverage VA resources through the National MS Society (NMSS) Navigator Program as well as other community resources.2
The VHA encourages physical activity and wellness through sports and leisure. Veterans with MS can participate in sports programs and special events, including the National Veterans Wheelchair Games, the National Disabled Veterans Winter Sports Clinic, the National Disabled Veterans TEE (Training, Exposure and Experience) golf tournament, the National Veterans Summer Sports Clinic, the National Veterans Golden Age Games, and the National Veterans Creative Sports Festival. HCPs or veterans who are not sure how to access any of these programs can contact the MSCoE or their local VA social workers.
Research
The primary goal of the MSCoE research core is to conduct clinical, health services, epidemiologic, and basic science research relevant to veterans with MS. The MSCoE serves to enhance collaboration among VAMCs, increase the participation of veterans in research, and provide research mentorship for the next generation of VA MS scientists. MSCoE research is carried out by investigators at the MSCoE and the MS Regional Programs, often in collaboration with investigators at academic institutions. This research is supported by competitive grant awards from a variety of funding agencies including the VA Research and Development Service (R&D) and the NMSS. Results from about 40 research grants in Fiscal Year 2019 were disseminated through 34 peer-reviewed publications, 30 posters, presentations, abstracts, and clinical practice guidelines.
There are many examples of recent high impact MS research performed by MSCoE investigators. For example, MSCoE researchers noted an increase in the estimated prevalence of MS to 1 million individuals in the US, about twice the previously estimated prevalence.3-5 In addition, a multicenter study highlighted the prevalence of MS misdiagnosis and common confounders for MS.6 Other research includes pilot clinical trials evaluating lipoic acid as a potential disease modifying therapy in people with secondary progressive MS and the impact of a multicomponent walking aid selection, fitting, and training program for preventing falls in people with MS.7,8 Clinical trial also are investigating telehealth counseling to improve physical activity in MS and a systematic review of rehabilitation interventions in MS.9,10
Education and Training
A unified program of education is essential to effective management of MS nationally. The primary goal of the education and training core is to provide a national program of MS education for HCPs, veterans, and caregivers to improve knowledge, enhance access to resources, and promote effective management strategies. The MSCoE collaborate with the Paralyzed Veterans of America (PVA), the Consortium of MS Centers (CMSC), the NMSS, and other national service organizations to increase educational opportunities, share knowledge, and expand participation.
The MSCoE education and training core produces a range of products both veterans, HCPs, and others affected by MS. The MSCoE sends a biannual patient newsletter to > 20,000 veterans and a monthly email to > 1,000 VA HCPs. Specific opportunities for HCP education include accredited multidisciplinary MS webinars, sponsored symposia and workshops at the CMSC and PVA Summit annual meetings, and presentations at other university and professional conferences. Enduring educational opportunities for veterans, caregivers, and HCPs can also be found by visiting www.va.gov/ms.
The MSCoE coordinate postdoctoral fellowship training programs to develop expertise in MS health care for the future. It offers VA physician fellowships for neurologists in Baltimore and Portland and for physiatrists in Seattle as well as NMSS fellowships for education and research. In 2019, MSCoE had 6 MD Fellows and 1 PhD Fellow.
Clinical Informatics and Telehealth
The primary goal of the informatics and telemedicine core is to employ state-of-the-art informatics, telemedicine technology, and the MSCoE website, to improve MS health care delivery. The VA has a integrated electronic health record and various data repositories are stored in the VHA Corporate Data Warehouse (CDW). MSCoE utilizes the CDW to maintain a national MS administrative data repository to understand the VHA care provided to veterans with MS. Data from the CDW have also served as an important resource to facilitate a wide range of veteran-focused MS research. This research has addressed clinical conditions like pain and obesity; health behaviors like smoking, alcohol use, and exercise as well as issues related to care delivery such as specialty care access, medication adherence, and appointment attendance.11-19
Monitoring the health of veterans with MS in the VA requires additional data not available in the CDW. To this end, we have developed the MS Surveillance Registry (MSSR), funded and maintained by the VA Office of Information Technology as part of their Veteran Integrated Registry Platform (VIRP). The purpose of the MSSR is to understand the unique characteristics and treatment patterns of veterans with MS in order to optimize their VHA care. HCPs input MS-specific clinical data on their patients into the MSSR, either through the MS Assessment Tool (MSAT) in the Computerized Patient Record System (CPRS) or through a secure online portal. Other data from existing databases from the CDW is also automatically fed into the MSSR. The MSSR continues to be developed and populated to serve as a resource for the future.
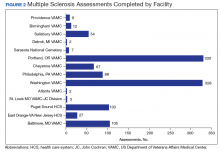
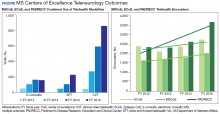
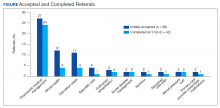
Neurologists, physiatrists, psychologists, and rehabilitation specialists can use telehealth to evaluate and treat veterans who have difficulty accessing outpatient clinics, either because of mobility limitations, or distance. Between 2012 and 2015, the VA MSCoE, together with the Epilepsy CoE and the Parkinson’s Disease Research and Clinical Centers in VISNs 5, 6 (mid-Atlantic) and 20 (Pacific Northwest) initiated an integrated teleneurology project. The goal of this project was to improve patient access to care at 4 tertiary and 12 regional VAMCs. A study team, with administrators and key clinical stakeholders, followed a traditional project management approach to design, plan, implement and evaluate an optimal model for communication and referrals with both live visits and telehealth (Table). Major outcomes of the project included: delivering subspecialty teleneurology to 47 patient sites, increasing interfacility consultation by 133% while reducing wait times by roughly 40%, and increasing telemedicine workload at these centers from 95 annual encounters in 2012 to 1,245 annual encounters in 2015 (Figure).
Today, telehealth for veterans with MS can be delivered to nearby VA facilities closer to their home, within their home, or anywhere else the veteran can use a cellphone or tablet. Telehealth visits can save travel time and expenses and optimize VA productivity and clinic use. The MSCoE and many of the MS regional programs are using telehealth for MS physician follow-up and therapies. The VA Office of Rural Health is also currently working with the MS network to use telehealth to increase access to physical therapy to those who have difficulty coming into clinic.
MSCoE Resources
The MSCoE is funded by VA Central Office through the Office of Specialty Care by Special Purpose funds. The directive specifies that funding for the regional and support programs is through Veterans Equitable Resource Allocation based on VISN and facility workload and complexity. Any research is funded separately through grants, some from VA R&D and others from other sources including the National Institutes of Health, the Patient Centered Outcome Research Institute, affiliated universities, the NMSS, the MS Society of Canada, the Consortium of MS Centers, foundations, and industry.
In 2019, MSCoE investigators received grants totaling > $18 million in funding. In-kind support also is provided by the PVA, the CMSC, the NMSS, and others. The first 3 foundations have been supporters since the inception of the MSCoE and have provided opportunities for the dissemination of education and research for HCPs, fellows, residents and medical students; travel; meeting rooms for MSCoE national meetings; exhibit space for HCP outreach; competitive research and educational grant support; programming and resources for veterans and significant others; organizational expertise; and opportunities for VA HCPs, veterans, and caregivers to learn how to navigate MS with others in the private sector.
Conclusion
The MSCoE had a tremendous impact on improving the consistency and quality of care for veterans with MS through clinical care, research, education and informatics and telehealth. Since opening in 2003, there has been an increase in the number of MS specialty clinics, served veterans with MS, and veterans receiving specialty neurologic and rehabilitation services in VA. Research programs in MS have been initiated to address key questions relevant to veterans with MS, including immunology, epidemiology, clinical care, and rehabilitation. Educational programs and products have evolved with technology and had a greater impact through partnerships with veteran and MS nonprofit organizations.
MSCoE strives to minimize impairment and maximize quality of life for veterans with MS by leveraging integrated electronic health records, data repositories, and telehealth services. These efforts have all improved veteran health, access and safety. We look forward to continuing into the next decade by bringing fresh ideas to the care of veterans with MS, their families and caregivers.
1. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Multiple Sclerosis System of Care-VHA Directive 1101.06 and Multiple Sclerosis Centers of Excellence network facilities. https://www.va.gov/MS/veterans/find_a_clinic/index_clinics.asp. Updated February 26, 2020. Accessed March 6, 2020.
2. National MS Society. MS navigator program. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/MS-Navigator-Program. Accessed March 6, 2020.
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92:e1029-e1040.
4. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285.
5. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480.
6. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
7. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374.
8. Martini DN, Zeeboer E, Hildebrand A, Fling BW, Hugos CL, Cameron MH. ADSTEP: preliminary investigation of a multicomponent walking aid program in people with multiple sclerosis. Arch Phys Med Rehabil. 2018;99(10):2050-2058.
9. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
10. Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
11. Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90(4):646-651.
12. Khurana SR, Bamer AM, Turner AP, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88(2):83-91.
13. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
14. Turner AP, Hawkins EJ, Haselkorn JK, Kivlahan DR. Alcohol misuse and multiple sclerosis. Arch Phys Med Rehabil. 2009;90(5):842-848.
15. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
16. Turner AP, Chapko MK, Yanez D, et al. Access to multiple sclerosis specialty care. PM R. 2013;5(12):1044-1050.
17. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Risk factors for suboptimal medication adherence in persons with multiple sclerosis: development of an electronic health record-based explanatory model for disease-modifying therapy use [published online ahead of print, 2019 Dec 3]. Arch Phys Med Rehabil. 2019;S0003-9993(19)31430-3143.
18. Settle JR, Maloni H, Bedra M, Finkelstein J, Zhan M, Wallin M. Monitoring medication adherence in multiple sclerosis using a novel web-based tool. J Telemed Telecare. 2016;22:225-233.
19. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Who is not coming to clinic? A predictive model of excessive missed appointments in persons with multiple sclerosis. Mult Scler Rel Dis. In Press.
The Veterans Health Administration (VHA) has established a number of centers of excellence (CoEs), including centers focused on posttraumatic stress disorder, suicide prevention, epilepsy, and, most recently, the Senator Elizabeth Dole CoE for Veteran and Caregiver Research. Some VA CoE serve as centralized locations for specialty care. For example, the VA Epilepsy CoE is a network of 16 facilities that provide comprehensive epilepsy care for veterans with seizure disorders, including expert and presurgical evaluations and inpatient monitoring.
In contrast, other CoEs, including the multiple sclerosis (MS) CoE, achieve their missions by serving as a resource center to a network of regional and supporting various programs to optimize the care of veterans across the nation within their home US Department of Veterans Affairs (VA) medical center (VAMC). The MSCoE are charged, through VHA Directive 1011.06, with establishing at least 1 VA MS Regional Program in each of the 21 Veteran Integrated Service Networks (VISNs) across the country and integrating these and affiliated MS Support Programs into the MS National Network. Currently, there are 29 MS regional programs and 49 MS support programs across the US.1
Established in 2003, the MSCoE is dedicated to furthering the understanding of MS, its impact on veterans, and effective treatments to help manage the disease and its symptoms. In 2002, 2 coordinating centers were selected based on a competitive review process. The MSCoE-East is located at the Baltimore, Maryland and Washington, DC VAMC and serves VISNs 1 to 10. The MSCoE-West serves VISNs 11 to 23 and is jointly-based at VA Puget Sound Health Care System in Seattle, Washington and VA Portland Health Care System in Portland, Oregon. The MSCoEs were made permanent by The Veteran’ Benefits, Healthcare and Information Technology Act of 2006 (38 USC §7330). By partnering with veterans, caregivers, health care professionals, and other affiliates, the MSCoE endeavor to optimize health, activities, participation and quality of life for veterans with MS.
Core Functions
The MSCoE has a 3-part mission. First, the MSCoE seeks to expand care coordination between VAMCs by developing a national network of VA MSCoE Regional and Support Programs. Second, the MSCoE provides resources to VA health care providers (HCPs) through a collaborative approach to clinical care, education, research, and informatics. Third, the MSCoE improves the quality and consistency of health care services delivered to veterans diagnosed with MS nationwide. To meet its objectives, the MSCoE activities are organized around 4 functional cores: clinical care, research, education and training, and informatics and telemedicine.
Clinical Care
The MSCoE delivers high-quality clinical care by identifying veterans with MS who use VA services, understanding their needs, and facilitating appropriate interventions. Veterans with MS are a special cohort for many reasons including that about 70% are male. Men and women veterans not only have different genetics, but also may have different environmental exposures and other risk factors for MS. Since 1998, the VHA has evaluated > 50,000 veterans with MS. Over the past decade, between 18,000 and 20,000 veterans with MS have accessed care within the VHA annually.
The MSCoE advocates for appropriate and safe use of currently available MS disease modifying therapies through collaborations with the VA Pharmacy Benefits Management Service (PBM). The MSCoE partners with PBM to develop and disseminate Criteria For Use, safety, and economic monitoring of the impacts of the MS therapies. The MSCoE also provide national consultation services for complex MS cases, clinical education to VA HCPs, and mentors fellows, residents, and medical students.
The VA provides numerous resources that are not readily available in other health care systems and facilitate the care for patients with chronic diseases, including providing low or no co-pays to patients for MS disease modifying agents and other MS related medications, access to medically necessary adaptive equipment at no charge, the Home Improvement and Structural Alteration (HISA) grant for assistance with safe home ingress and egress, respite care, access to a homemaker/home health aide, and caregiver support programs. Eligible veterans also can access additional resources such as adaptive housing and an automobile grant. The VA also provides substantial hands-on assistance to veterans who are homeless. The clinical team and a veteran with MS can leverage VA resources through the National MS Society (NMSS) Navigator Program as well as other community resources.2
The VHA encourages physical activity and wellness through sports and leisure. Veterans with MS can participate in sports programs and special events, including the National Veterans Wheelchair Games, the National Disabled Veterans Winter Sports Clinic, the National Disabled Veterans TEE (Training, Exposure and Experience) golf tournament, the National Veterans Summer Sports Clinic, the National Veterans Golden Age Games, and the National Veterans Creative Sports Festival. HCPs or veterans who are not sure how to access any of these programs can contact the MSCoE or their local VA social workers.
Research
The primary goal of the MSCoE research core is to conduct clinical, health services, epidemiologic, and basic science research relevant to veterans with MS. The MSCoE serves to enhance collaboration among VAMCs, increase the participation of veterans in research, and provide research mentorship for the next generation of VA MS scientists. MSCoE research is carried out by investigators at the MSCoE and the MS Regional Programs, often in collaboration with investigators at academic institutions. This research is supported by competitive grant awards from a variety of funding agencies including the VA Research and Development Service (R&D) and the NMSS. Results from about 40 research grants in Fiscal Year 2019 were disseminated through 34 peer-reviewed publications, 30 posters, presentations, abstracts, and clinical practice guidelines.
There are many examples of recent high impact MS research performed by MSCoE investigators. For example, MSCoE researchers noted an increase in the estimated prevalence of MS to 1 million individuals in the US, about twice the previously estimated prevalence.3-5 In addition, a multicenter study highlighted the prevalence of MS misdiagnosis and common confounders for MS.6 Other research includes pilot clinical trials evaluating lipoic acid as a potential disease modifying therapy in people with secondary progressive MS and the impact of a multicomponent walking aid selection, fitting, and training program for preventing falls in people with MS.7,8 Clinical trial also are investigating telehealth counseling to improve physical activity in MS and a systematic review of rehabilitation interventions in MS.9,10
Education and Training
A unified program of education is essential to effective management of MS nationally. The primary goal of the education and training core is to provide a national program of MS education for HCPs, veterans, and caregivers to improve knowledge, enhance access to resources, and promote effective management strategies. The MSCoE collaborate with the Paralyzed Veterans of America (PVA), the Consortium of MS Centers (CMSC), the NMSS, and other national service organizations to increase educational opportunities, share knowledge, and expand participation.
The MSCoE education and training core produces a range of products both veterans, HCPs, and others affected by MS. The MSCoE sends a biannual patient newsletter to > 20,000 veterans and a monthly email to > 1,000 VA HCPs. Specific opportunities for HCP education include accredited multidisciplinary MS webinars, sponsored symposia and workshops at the CMSC and PVA Summit annual meetings, and presentations at other university and professional conferences. Enduring educational opportunities for veterans, caregivers, and HCPs can also be found by visiting www.va.gov/ms.
The MSCoE coordinate postdoctoral fellowship training programs to develop expertise in MS health care for the future. It offers VA physician fellowships for neurologists in Baltimore and Portland and for physiatrists in Seattle as well as NMSS fellowships for education and research. In 2019, MSCoE had 6 MD Fellows and 1 PhD Fellow.
Clinical Informatics and Telehealth
The primary goal of the informatics and telemedicine core is to employ state-of-the-art informatics, telemedicine technology, and the MSCoE website, to improve MS health care delivery. The VA has a integrated electronic health record and various data repositories are stored in the VHA Corporate Data Warehouse (CDW). MSCoE utilizes the CDW to maintain a national MS administrative data repository to understand the VHA care provided to veterans with MS. Data from the CDW have also served as an important resource to facilitate a wide range of veteran-focused MS research. This research has addressed clinical conditions like pain and obesity; health behaviors like smoking, alcohol use, and exercise as well as issues related to care delivery such as specialty care access, medication adherence, and appointment attendance.11-19
Monitoring the health of veterans with MS in the VA requires additional data not available in the CDW. To this end, we have developed the MS Surveillance Registry (MSSR), funded and maintained by the VA Office of Information Technology as part of their Veteran Integrated Registry Platform (VIRP). The purpose of the MSSR is to understand the unique characteristics and treatment patterns of veterans with MS in order to optimize their VHA care. HCPs input MS-specific clinical data on their patients into the MSSR, either through the MS Assessment Tool (MSAT) in the Computerized Patient Record System (CPRS) or through a secure online portal. Other data from existing databases from the CDW is also automatically fed into the MSSR. The MSSR continues to be developed and populated to serve as a resource for the future.
Neurologists, physiatrists, psychologists, and rehabilitation specialists can use telehealth to evaluate and treat veterans who have difficulty accessing outpatient clinics, either because of mobility limitations, or distance. Between 2012 and 2015, the VA MSCoE, together with the Epilepsy CoE and the Parkinson’s Disease Research and Clinical Centers in VISNs 5, 6 (mid-Atlantic) and 20 (Pacific Northwest) initiated an integrated teleneurology project. The goal of this project was to improve patient access to care at 4 tertiary and 12 regional VAMCs. A study team, with administrators and key clinical stakeholders, followed a traditional project management approach to design, plan, implement and evaluate an optimal model for communication and referrals with both live visits and telehealth (Table). Major outcomes of the project included: delivering subspecialty teleneurology to 47 patient sites, increasing interfacility consultation by 133% while reducing wait times by roughly 40%, and increasing telemedicine workload at these centers from 95 annual encounters in 2012 to 1,245 annual encounters in 2015 (Figure).
Today, telehealth for veterans with MS can be delivered to nearby VA facilities closer to their home, within their home, or anywhere else the veteran can use a cellphone or tablet. Telehealth visits can save travel time and expenses and optimize VA productivity and clinic use. The MSCoE and many of the MS regional programs are using telehealth for MS physician follow-up and therapies. The VA Office of Rural Health is also currently working with the MS network to use telehealth to increase access to physical therapy to those who have difficulty coming into clinic.
MSCoE Resources
The MSCoE is funded by VA Central Office through the Office of Specialty Care by Special Purpose funds. The directive specifies that funding for the regional and support programs is through Veterans Equitable Resource Allocation based on VISN and facility workload and complexity. Any research is funded separately through grants, some from VA R&D and others from other sources including the National Institutes of Health, the Patient Centered Outcome Research Institute, affiliated universities, the NMSS, the MS Society of Canada, the Consortium of MS Centers, foundations, and industry.
In 2019, MSCoE investigators received grants totaling > $18 million in funding. In-kind support also is provided by the PVA, the CMSC, the NMSS, and others. The first 3 foundations have been supporters since the inception of the MSCoE and have provided opportunities for the dissemination of education and research for HCPs, fellows, residents and medical students; travel; meeting rooms for MSCoE national meetings; exhibit space for HCP outreach; competitive research and educational grant support; programming and resources for veterans and significant others; organizational expertise; and opportunities for VA HCPs, veterans, and caregivers to learn how to navigate MS with others in the private sector.
Conclusion
The MSCoE had a tremendous impact on improving the consistency and quality of care for veterans with MS through clinical care, research, education and informatics and telehealth. Since opening in 2003, there has been an increase in the number of MS specialty clinics, served veterans with MS, and veterans receiving specialty neurologic and rehabilitation services in VA. Research programs in MS have been initiated to address key questions relevant to veterans with MS, including immunology, epidemiology, clinical care, and rehabilitation. Educational programs and products have evolved with technology and had a greater impact through partnerships with veteran and MS nonprofit organizations.
MSCoE strives to minimize impairment and maximize quality of life for veterans with MS by leveraging integrated electronic health records, data repositories, and telehealth services. These efforts have all improved veteran health, access and safety. We look forward to continuing into the next decade by bringing fresh ideas to the care of veterans with MS, their families and caregivers.
The Veterans Health Administration (VHA) has established a number of centers of excellence (CoEs), including centers focused on posttraumatic stress disorder, suicide prevention, epilepsy, and, most recently, the Senator Elizabeth Dole CoE for Veteran and Caregiver Research. Some VA CoE serve as centralized locations for specialty care. For example, the VA Epilepsy CoE is a network of 16 facilities that provide comprehensive epilepsy care for veterans with seizure disorders, including expert and presurgical evaluations and inpatient monitoring.
In contrast, other CoEs, including the multiple sclerosis (MS) CoE, achieve their missions by serving as a resource center to a network of regional and supporting various programs to optimize the care of veterans across the nation within their home US Department of Veterans Affairs (VA) medical center (VAMC). The MSCoE are charged, through VHA Directive 1011.06, with establishing at least 1 VA MS Regional Program in each of the 21 Veteran Integrated Service Networks (VISNs) across the country and integrating these and affiliated MS Support Programs into the MS National Network. Currently, there are 29 MS regional programs and 49 MS support programs across the US.1
Established in 2003, the MSCoE is dedicated to furthering the understanding of MS, its impact on veterans, and effective treatments to help manage the disease and its symptoms. In 2002, 2 coordinating centers were selected based on a competitive review process. The MSCoE-East is located at the Baltimore, Maryland and Washington, DC VAMC and serves VISNs 1 to 10. The MSCoE-West serves VISNs 11 to 23 and is jointly-based at VA Puget Sound Health Care System in Seattle, Washington and VA Portland Health Care System in Portland, Oregon. The MSCoEs were made permanent by The Veteran’ Benefits, Healthcare and Information Technology Act of 2006 (38 USC §7330). By partnering with veterans, caregivers, health care professionals, and other affiliates, the MSCoE endeavor to optimize health, activities, participation and quality of life for veterans with MS.
Core Functions
The MSCoE has a 3-part mission. First, the MSCoE seeks to expand care coordination between VAMCs by developing a national network of VA MSCoE Regional and Support Programs. Second, the MSCoE provides resources to VA health care providers (HCPs) through a collaborative approach to clinical care, education, research, and informatics. Third, the MSCoE improves the quality and consistency of health care services delivered to veterans diagnosed with MS nationwide. To meet its objectives, the MSCoE activities are organized around 4 functional cores: clinical care, research, education and training, and informatics and telemedicine.
Clinical Care
The MSCoE delivers high-quality clinical care by identifying veterans with MS who use VA services, understanding their needs, and facilitating appropriate interventions. Veterans with MS are a special cohort for many reasons including that about 70% are male. Men and women veterans not only have different genetics, but also may have different environmental exposures and other risk factors for MS. Since 1998, the VHA has evaluated > 50,000 veterans with MS. Over the past decade, between 18,000 and 20,000 veterans with MS have accessed care within the VHA annually.
The MSCoE advocates for appropriate and safe use of currently available MS disease modifying therapies through collaborations with the VA Pharmacy Benefits Management Service (PBM). The MSCoE partners with PBM to develop and disseminate Criteria For Use, safety, and economic monitoring of the impacts of the MS therapies. The MSCoE also provide national consultation services for complex MS cases, clinical education to VA HCPs, and mentors fellows, residents, and medical students.
The VA provides numerous resources that are not readily available in other health care systems and facilitate the care for patients with chronic diseases, including providing low or no co-pays to patients for MS disease modifying agents and other MS related medications, access to medically necessary adaptive equipment at no charge, the Home Improvement and Structural Alteration (HISA) grant for assistance with safe home ingress and egress, respite care, access to a homemaker/home health aide, and caregiver support programs. Eligible veterans also can access additional resources such as adaptive housing and an automobile grant. The VA also provides substantial hands-on assistance to veterans who are homeless. The clinical team and a veteran with MS can leverage VA resources through the National MS Society (NMSS) Navigator Program as well as other community resources.2
The VHA encourages physical activity and wellness through sports and leisure. Veterans with MS can participate in sports programs and special events, including the National Veterans Wheelchair Games, the National Disabled Veterans Winter Sports Clinic, the National Disabled Veterans TEE (Training, Exposure and Experience) golf tournament, the National Veterans Summer Sports Clinic, the National Veterans Golden Age Games, and the National Veterans Creative Sports Festival. HCPs or veterans who are not sure how to access any of these programs can contact the MSCoE or their local VA social workers.
Research
The primary goal of the MSCoE research core is to conduct clinical, health services, epidemiologic, and basic science research relevant to veterans with MS. The MSCoE serves to enhance collaboration among VAMCs, increase the participation of veterans in research, and provide research mentorship for the next generation of VA MS scientists. MSCoE research is carried out by investigators at the MSCoE and the MS Regional Programs, often in collaboration with investigators at academic institutions. This research is supported by competitive grant awards from a variety of funding agencies including the VA Research and Development Service (R&D) and the NMSS. Results from about 40 research grants in Fiscal Year 2019 were disseminated through 34 peer-reviewed publications, 30 posters, presentations, abstracts, and clinical practice guidelines.
There are many examples of recent high impact MS research performed by MSCoE investigators. For example, MSCoE researchers noted an increase in the estimated prevalence of MS to 1 million individuals in the US, about twice the previously estimated prevalence.3-5 In addition, a multicenter study highlighted the prevalence of MS misdiagnosis and common confounders for MS.6 Other research includes pilot clinical trials evaluating lipoic acid as a potential disease modifying therapy in people with secondary progressive MS and the impact of a multicomponent walking aid selection, fitting, and training program for preventing falls in people with MS.7,8 Clinical trial also are investigating telehealth counseling to improve physical activity in MS and a systematic review of rehabilitation interventions in MS.9,10
Education and Training
A unified program of education is essential to effective management of MS nationally. The primary goal of the education and training core is to provide a national program of MS education for HCPs, veterans, and caregivers to improve knowledge, enhance access to resources, and promote effective management strategies. The MSCoE collaborate with the Paralyzed Veterans of America (PVA), the Consortium of MS Centers (CMSC), the NMSS, and other national service organizations to increase educational opportunities, share knowledge, and expand participation.
The MSCoE education and training core produces a range of products both veterans, HCPs, and others affected by MS. The MSCoE sends a biannual patient newsletter to > 20,000 veterans and a monthly email to > 1,000 VA HCPs. Specific opportunities for HCP education include accredited multidisciplinary MS webinars, sponsored symposia and workshops at the CMSC and PVA Summit annual meetings, and presentations at other university and professional conferences. Enduring educational opportunities for veterans, caregivers, and HCPs can also be found by visiting www.va.gov/ms.
The MSCoE coordinate postdoctoral fellowship training programs to develop expertise in MS health care for the future. It offers VA physician fellowships for neurologists in Baltimore and Portland and for physiatrists in Seattle as well as NMSS fellowships for education and research. In 2019, MSCoE had 6 MD Fellows and 1 PhD Fellow.
Clinical Informatics and Telehealth
The primary goal of the informatics and telemedicine core is to employ state-of-the-art informatics, telemedicine technology, and the MSCoE website, to improve MS health care delivery. The VA has a integrated electronic health record and various data repositories are stored in the VHA Corporate Data Warehouse (CDW). MSCoE utilizes the CDW to maintain a national MS administrative data repository to understand the VHA care provided to veterans with MS. Data from the CDW have also served as an important resource to facilitate a wide range of veteran-focused MS research. This research has addressed clinical conditions like pain and obesity; health behaviors like smoking, alcohol use, and exercise as well as issues related to care delivery such as specialty care access, medication adherence, and appointment attendance.11-19
Monitoring the health of veterans with MS in the VA requires additional data not available in the CDW. To this end, we have developed the MS Surveillance Registry (MSSR), funded and maintained by the VA Office of Information Technology as part of their Veteran Integrated Registry Platform (VIRP). The purpose of the MSSR is to understand the unique characteristics and treatment patterns of veterans with MS in order to optimize their VHA care. HCPs input MS-specific clinical data on their patients into the MSSR, either through the MS Assessment Tool (MSAT) in the Computerized Patient Record System (CPRS) or through a secure online portal. Other data from existing databases from the CDW is also automatically fed into the MSSR. The MSSR continues to be developed and populated to serve as a resource for the future.
Neurologists, physiatrists, psychologists, and rehabilitation specialists can use telehealth to evaluate and treat veterans who have difficulty accessing outpatient clinics, either because of mobility limitations, or distance. Between 2012 and 2015, the VA MSCoE, together with the Epilepsy CoE and the Parkinson’s Disease Research and Clinical Centers in VISNs 5, 6 (mid-Atlantic) and 20 (Pacific Northwest) initiated an integrated teleneurology project. The goal of this project was to improve patient access to care at 4 tertiary and 12 regional VAMCs. A study team, with administrators and key clinical stakeholders, followed a traditional project management approach to design, plan, implement and evaluate an optimal model for communication and referrals with both live visits and telehealth (Table). Major outcomes of the project included: delivering subspecialty teleneurology to 47 patient sites, increasing interfacility consultation by 133% while reducing wait times by roughly 40%, and increasing telemedicine workload at these centers from 95 annual encounters in 2012 to 1,245 annual encounters in 2015 (Figure).
Today, telehealth for veterans with MS can be delivered to nearby VA facilities closer to their home, within their home, or anywhere else the veteran can use a cellphone or tablet. Telehealth visits can save travel time and expenses and optimize VA productivity and clinic use. The MSCoE and many of the MS regional programs are using telehealth for MS physician follow-up and therapies. The VA Office of Rural Health is also currently working with the MS network to use telehealth to increase access to physical therapy to those who have difficulty coming into clinic.
MSCoE Resources
The MSCoE is funded by VA Central Office through the Office of Specialty Care by Special Purpose funds. The directive specifies that funding for the regional and support programs is through Veterans Equitable Resource Allocation based on VISN and facility workload and complexity. Any research is funded separately through grants, some from VA R&D and others from other sources including the National Institutes of Health, the Patient Centered Outcome Research Institute, affiliated universities, the NMSS, the MS Society of Canada, the Consortium of MS Centers, foundations, and industry.
In 2019, MSCoE investigators received grants totaling > $18 million in funding. In-kind support also is provided by the PVA, the CMSC, the NMSS, and others. The first 3 foundations have been supporters since the inception of the MSCoE and have provided opportunities for the dissemination of education and research for HCPs, fellows, residents and medical students; travel; meeting rooms for MSCoE national meetings; exhibit space for HCP outreach; competitive research and educational grant support; programming and resources for veterans and significant others; organizational expertise; and opportunities for VA HCPs, veterans, and caregivers to learn how to navigate MS with others in the private sector.
Conclusion
The MSCoE had a tremendous impact on improving the consistency and quality of care for veterans with MS through clinical care, research, education and informatics and telehealth. Since opening in 2003, there has been an increase in the number of MS specialty clinics, served veterans with MS, and veterans receiving specialty neurologic and rehabilitation services in VA. Research programs in MS have been initiated to address key questions relevant to veterans with MS, including immunology, epidemiology, clinical care, and rehabilitation. Educational programs and products have evolved with technology and had a greater impact through partnerships with veteran and MS nonprofit organizations.
MSCoE strives to minimize impairment and maximize quality of life for veterans with MS by leveraging integrated electronic health records, data repositories, and telehealth services. These efforts have all improved veteran health, access and safety. We look forward to continuing into the next decade by bringing fresh ideas to the care of veterans with MS, their families and caregivers.
1. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Multiple Sclerosis System of Care-VHA Directive 1101.06 and Multiple Sclerosis Centers of Excellence network facilities. https://www.va.gov/MS/veterans/find_a_clinic/index_clinics.asp. Updated February 26, 2020. Accessed March 6, 2020.
2. National MS Society. MS navigator program. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/MS-Navigator-Program. Accessed March 6, 2020.
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92:e1029-e1040.
4. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285.
5. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480.
6. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
7. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374.
8. Martini DN, Zeeboer E, Hildebrand A, Fling BW, Hugos CL, Cameron MH. ADSTEP: preliminary investigation of a multicomponent walking aid program in people with multiple sclerosis. Arch Phys Med Rehabil. 2018;99(10):2050-2058.
9. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
10. Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
11. Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90(4):646-651.
12. Khurana SR, Bamer AM, Turner AP, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88(2):83-91.
13. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
14. Turner AP, Hawkins EJ, Haselkorn JK, Kivlahan DR. Alcohol misuse and multiple sclerosis. Arch Phys Med Rehabil. 2009;90(5):842-848.
15. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
16. Turner AP, Chapko MK, Yanez D, et al. Access to multiple sclerosis specialty care. PM R. 2013;5(12):1044-1050.
17. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Risk factors for suboptimal medication adherence in persons with multiple sclerosis: development of an electronic health record-based explanatory model for disease-modifying therapy use [published online ahead of print, 2019 Dec 3]. Arch Phys Med Rehabil. 2019;S0003-9993(19)31430-3143.
18. Settle JR, Maloni H, Bedra M, Finkelstein J, Zhan M, Wallin M. Monitoring medication adherence in multiple sclerosis using a novel web-based tool. J Telemed Telecare. 2016;22:225-233.
19. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Who is not coming to clinic? A predictive model of excessive missed appointments in persons with multiple sclerosis. Mult Scler Rel Dis. In Press.
1. US Department of Veterans Affairs, Multiple Sclerosis Centers of Excellence. Multiple Sclerosis System of Care-VHA Directive 1101.06 and Multiple Sclerosis Centers of Excellence network facilities. https://www.va.gov/MS/veterans/find_a_clinic/index_clinics.asp. Updated February 26, 2020. Accessed March 6, 2020.
2. National MS Society. MS navigator program. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/MS-Navigator-Program. Accessed March 6, 2020.
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92:e1029-e1040.
4. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269-285.
5. GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480.
6. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
7. Spain R, Powers K, Murchison C, et al. Lipoic acid in secondary progressive MS: a randomized controlled pilot trial. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374.
8. Martini DN, Zeeboer E, Hildebrand A, Fling BW, Hugos CL, Cameron MH. ADSTEP: preliminary investigation of a multicomponent walking aid program in people with multiple sclerosis. Arch Phys Med Rehabil. 2018;99(10):2050-2058.
9. Turner AP, Hartoonian N, Sloan AP, et al. Improving fatigue and depression in individuals with multiple sclerosis using telephone-administered physical activity counseling. J Consult Clin Psychol. 2016;84(4):297-309.
10. Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
11. Hirsh AT, Turner AP, Ehde DM, Haselkorn JK. Prevalence and impact of pain in multiple sclerosis: physical and psychologic contributors. Arch Phys Med Rehabil. 2009;90(4):646-651.
12. Khurana SR, Bamer AM, Turner AP, et al. The prevalence of overweight and obesity in veterans with multiple sclerosis. Am J Phys Med Rehabil. 2009;88(2):83-91.
13. Turner AP, Kivlahan DR, Kazis LE, Haselkorn JK. Smoking among veterans with multiple sclerosis: prevalence correlates, quit attempts, and unmet need for services. Arch Phys Med Rehabil. 2007;88(11):1394-1399.
14. Turner AP, Hawkins EJ, Haselkorn JK, Kivlahan DR. Alcohol misuse and multiple sclerosis. Arch Phys Med Rehabil. 2009;90(5):842-848.
15. Turner AP, Kivlahan DR, Haselkorn JK. Exercise and quality of life among people with multiple sclerosis: looking beyond physical functioning to mental health and participation in life. Arch Phys Med Rehabil. 2009;90(3):420-428.
16. Turner AP, Chapko MK, Yanez D, et al. Access to multiple sclerosis specialty care. PM R. 2013;5(12):1044-1050.
17. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Risk factors for suboptimal medication adherence in persons with multiple sclerosis: development of an electronic health record-based explanatory model for disease-modifying therapy use [published online ahead of print, 2019 Dec 3]. Arch Phys Med Rehabil. 2019;S0003-9993(19)31430-3143.
18. Settle JR, Maloni H, Bedra M, Finkelstein J, Zhan M, Wallin M. Monitoring medication adherence in multiple sclerosis using a novel web-based tool. J Telemed Telecare. 2016;22:225-233.
19. Gromisch ES, Turner AP, Leipertz SL, Beauvais J, Haselkorn JK. Who is not coming to clinic? A predictive model of excessive missed appointments in persons with multiple sclerosis. Mult Scler Rel Dis. In Press.
Role of Speech Pathology in a Multidisciplinary Approach to a Patient With Mild Traumatic Brain Injury
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists can fill a unique need in the treatment of patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
Speech-language pathologists (SLPs) are integral to the comprehensive treatment of mild traumatic brain injury (mTBI), yet the evaluation and treatment options they offer may not be known to all primary care providers (PCPs). As the research on the management and treatment of mTBI continues to evolve, the PCPs role in referring patients with mTBI to the appropriate resources becomes imperative.
mTBI is a common injury in both military and civilian settings, but it can be difficult to diagnose and is not always well understood. Long-term debilitating effects have been associated with mTBI, with literature linking it to an increased risk of developing Alzheimer disease, motor neuron disease, and Parkinson disease.1 In addition, mTBI is a strong predictor for the development of posttraumatic stress disorder (PTSD). Among returning Iraq and Afghanistan service members, the incidence of mTBI associated mental health conditions have been reported to be as high as 22.8%, affecting > 320,000 veterans.2-5
The US Department of Veteran Affairs (VA) health care system offers these returning veterans a comprehensive, multidisciplinary treatment strategy. The care is often coordinated by the veteran’s patient aligned care team (PACT) that consists of a PCP, nurses, and a medical support associate. The US Department of Defense (DoD) and VA also facilitated the development of a clinical practice guideline (CPG) that can be used by the PACT and other health care providers to support evidence based patient-centered care. This CPG is extensive and has recommendations for evaluation and treatment of mTBI and the symptoms associated such as impaired memory and alterations in executive function.6
The following hypothetical case is based on an actual patient. This case illustrates the role of speech pathology in caring for patients with mTBI.
Case Presentation
A 25-year-old male combat veteran presented to his VA PACT team for a new patient visit. As part of the screening of his medical history, mTBI was fully defined for the patient to include “alteration” in consciousness. This reminded the patient of an injury that occurred 1 year prior to presentation during a routine convoy mission. He was riding in the back of a Humvee when it hit a large pothole slamming his head into the side of the vehicle. He reported that he felt “dazed and dizzy” with “ringing” in his ears immediately following the event, without an overt loss of consciousness. He was unable to seek medical attention secondary to the urgency of the convoy mission, so he “shook it off” and kept going. Later that week he noted headache and insomnia. He was seen and evaluated by his health care provider for insomnia, but when questioned he reported no head trauma as he had forgotten the incident. Upon follow-up with his PCP, he reported his headaches were manageable, and his insomnia was somewhat improved with recommended life-style modifications and good sleep hygiene.
He still had frequent headaches, dizziness, and some insomnia. However, his chief concern was that he was struggling with new schoolwork. He noted that he was a straight-A student prior to his military service. A review of his medical history in his medical chart showed that a previous PCP had treated his associated symptoms of insomnia and headache without improvement. In addition, he had recently been diagnosed with PTSD. As his symptoms had lasted > 90 days, not resolved with initial treatment in primary care, and were causing a significant impact on his activities of daily living, his PCP placed a consult to Speech Pathology for cognitive-linguistic assessment and treatment, if indicated, noting that he may have had a mTBI.6 Although not intended to be comprehensive, Table 1 describes several clinical areas where a speech pathology referral may be appropriate.
The Role of the Speech-Language Pathologist
The speech-language pathologist takes an additional history of the patient. This better quantifies specific details of the veteran’s functional concerns pertaining to possible difficulty with attention, memory, executive function, visuospatial awareness, etc. Examples might include difficulty with attention/memory, including not remembering what to get at the store, forgetting to take medications, forgetting appointments, and difficulty in school, among many others. Reports of feeling “stupid” also are common. Assessment varies by clinician, but it is not uncommon for the SLP to administer a battery of evaluations to help identify a range of possible impairments. Choosing testing that is sensitive to even mild impairment is important and should be used in combination with subjective complaints. Mild deficits can sometimes be missed in those with average performance, but whose premorbid intelligence was above average. One combination of test batteries sometimes utilized is the Wechsler Test of Adult Reading (WTAR), the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the Ruff Figural Fluency Test (RFFT), the Controlled Oral Word Association Test (COWAT), and Trails A and B (Table 2).
The initial testing results are discussed with the veteran. If patient concerns and/or testing reveal impairment that is amenable to treatment and the veteran wishes to proceed, subsequent treatment sessions are scheduled. The first treatment session is spent establishing and prioritizing functional goals specific to that individual and their needs (eg, for daily life, work, school). In a case of subacute or older mTBI, as is often seen in veterans coming to the VA, intervention often targets strategies and techniques that can help the individual compensate for current deficits.
Many patients already own a smartphone, so this device often is used functionally as a cognitive prosthetic as early as the first treatment session. In an effort to immediately start addressing important issues like medication management and attending appointments, the veteran is educated to the benefit of entering important information into the calendar and/or reminder apps on their phone and setting associated alarms that would serve as a reminder for what was entered. Patients are often encouraged by the positive impact of these initial strategies and look forward to future treatment sessions to address compensation for their functional deficits.
If a veteran with TBI has numerous needs, it can be beneficial for the care team to discuss the care plan at an interdisciplinary team meeting. It is not uncommon for veterans like the one discussed above to be referred to neurology (persistent headaches and further neurological evaluation); mental health (PTSD treatment and family support/counseling options); occupational therapy (visuospatial needs); and audiology (vestibular concerns). Social work involvement is often extremely beneficial for coordination of care in more complex cases. If patient is having difficulty making healthy eating choices or with meal preparation, a consult to a dietitian may prove invaluable. Concerns related to trouble with medication adherence (beyond memory-related adherence issues that speech pathology would address) or polypharmacy can be directed to a clinical pharmacy specialist, who could prepare a medication chart, review optimal medication timing, and provide education on adverse effects. A veteran's communication with the team can be facilitated through secure messaging (a method of secure emailing) and encouraging use of the My HealtheVet portal. With this modality, patients could review chart notes and results and share them with non-VA health care providers and/or family members as indicated.
A whole health approach also may appeal to some mTBI patients. This approach focuses on the totality of patient needs for healthy living and on patient-centered goal setting. Services provided may differ at various VA medical centers, but the PACT team can connect the veteran to the services of interest.
Conclusions
A team approach to veterans with mTBI provides a comprehensive way to treat the various problems associated with the condition. Further research into the role of multidisciplinary teams in the management of mTBI was recommended in the 2016 CPG.6 The unique role that the speech-language pathologist plays as part of this team has been highlighted, as it is important that PCP’s be aware of the extent of evaluation and treatment services they offer. Beyond mTBI, speech pathologists evaluate and treat patients with several conditions that are seen regularly in primary care.
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
1. McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10(3 suppl):S242-S253. doi:10.1016/j.jalz.2014.04.003
2. Yurgil KA, Barkauskas DA, Vasterling JJ, et al. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty Marines. JAMA Psychiatry. 2014;71(2):149-157. doi:10.1001/jamapsychiatry.2013.3080
3. Chin DL, Zeber JE. Mental Health Outcomes Among Military Service Members After Severe Injury in Combat and TBI. Mil Med. 2020;185(5-6):e711-e718. doi:10.1093/milmed/usz440
4. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA. 2006;295(9):1023-1032. doi:10.1001/jama.295.9.1023
5. Miles SR, Harik JM, Hundt NE, et al. Delivery of mental health treatment to combat veterans with psychiatric diagnoses and TBI histories. PLoS One. 2017;12(9):e0184265. Published 2017 Sep 8. doi:10.1371/journal.pone.0184265
6. US Department of Defense, US Department of Veterans Affairs; Management of Concussion/mTBI Working Group. VA/DoD clinical practice guideline for management of concussion/mild traumatic brain injury. Version 2.0. Published February 2016. Accessed February 8, 2021. https://www.healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf
Physician Responsiveness to Positive Blood Culture Results at the Minneapolis Veterans Affairs Hospital—Is Anyone Paying Attention?
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
A Stepwise Approach for Preventing Suicide by Lethal Poisoning
Suicide is a global phenomenon and a worldwide public health concern.1 The World Health Organization estimates that > 800,000 people die by suicide every year. In the US, suicide is the 10th leading cause of death, and on average, 129 Americans die by suicide each day.2 In 2018, the suicide rate for all veterans was 1.5 times higher than the rate for nonveterans, after adjusting for population differences in age and sex. Among female veterans, the rate of suicide was 2.1 times higher than the rate for female nonveterans.3
In light of this disparity, suicide prevention is one of the highest priorities for the US Department of Veterans Affairs (VA). In 2018, the VA developed and published the National Strategy for Preventing Veteran Suicide.4 One major goal of this strategy is to reduce access to lethal means (ie, firearms, medications, chemicals, or poisons) among veterans at high risk for suicide. Reducing access to lethal means has been found to decrease suicide rates.4,5
Drug overdose is a leading method for suicide attempts, especially for female veterans.3,6 Although the overall case fatality ratio for overdose is < 2%, drug overdose accounted for 59.4% of suicide attempts and 13.5% of deaths by suicide from 2007 to 2014.6,7 Within the veteran population, the majority of suicide deaths in 2018 were due to self-inflicted firearm injury for both male and female veterans, followed by poisoning via substances and pharmaceutical agents for female veterans (Figure 1).3 Notably, when compared with men, women were more likely to choose drug overdose as a method for suicide. One study found that women aged < 45 years used drug and poison ingestion in 9 out of 10 suicide attempts.6 Since some medications are more lethal than others, interventions to limit the availability of lethal medications may prevent deaths and reduce the severity of suicide attempts. This article will provide a stepwise approach to help clinicians identify and limit lethal medications for patients at high risk for suicide.
Step 1: Determine Suicide Risk
Although it is impossible to predict with certainty an individual’s risk of suicide, several patient characteristics and life circumstances have been identified as risk factors. The strongest predictor of suicide is the presence of psychiatric disease.8 More than 90% of those who have had a death by suicide have a psychiatric diagnosis at the time of death, and suicide rates in those with mental illness are at least 10 times as high as in the general population.9,10 Depression is the leading cause of death by suicide worldwide, followed by substance-related disorders (22.4%), personality disorders (11.6%), schizophrenia (10.6%), and anxiety/somatoform disorders (6.1%).8,11-13
Clinicians also can use various risk assessment tools to identify patients at high risk for suicide. The Veterans Health Administration (VHA) Stratification Tool for Opioid Risk Mitigation (STORM) calculates patients’ risk based on data extracted from the electronic health record and is less time intensive, more easily refined, and may be more powerful than standard risk assessment tools because it can be deployed on a large scale.14,15 The VHA also developed the Suicide Prevention Population Risk Identification and Tracking for Exigencies (SPPRITE) tool to assist clinicians in tracking patients with current (or recent) high levels of suicide risk. This tool unifies specific patient information gathered from the patient’s electronic health record and from other predictive model dashboards (such as STORM).
Step 2: Identify Substances Strongly Associated With Fatalities
According to the American Association of Poison Control Centers (AAPCC), the pharmaceutical classes associated with the largest number of fatalities are analgesics, followed by stimulants and street drugs, cardiovascular agents, antidepressants, antipsychotics, and sedatives/hypnotics (Table 1).16 Stimulants and street drugs accounted for 694 fatalities of 39,238 single-substance exposures (mortality rate: 1.8%).16 Drugs of abuse, including cocaine, hallucinogenic amphetamines, heroin, and kratom, have shown an increased trend in use.16
In 2018 there were 834 fatalities from 174,269 single-substance exposure to analgesics, which include opioids and acetaminophen, for a mortality rate of 0.5%.16 The opioid epidemic is one of the main drivers of the increase in drug overdose deaths in the US.16,17 The opioid with the highest drug overdose fatality rate is illicitly manufactured fentanyl, which often is combined with other substances, such as heroin, to increase its potency at a low cost.18 These combinations also increase the risk of overdose fatality.
Acetaminophen is unique among the top substances associated with fatalities because it is obtained easily without a prescription. An acetaminophen overdose can cause hepatic injury, which may progress to fulminant hepatic failure and death.19 The recommended maximum dose of acetaminophen is 4 g/d in an adult and 50 to 75 mg/kg/d in children. A single acute ingestion of > 7.5 g in an adult or 150 mg/kg in children has been considered potentially toxic.19,20 The use of combination analgesics that contain both an opioid and acetaminophen can pose an even greater risk due to the potential for respiratory depression and hepatotoxicity.
Cardiovascular drugs accounted for 232 fatalities from 46,499 single-substance exposures (mortality rate: 0.5%).16 According to the AAPCC, calcium channel blockers (CCB) and β-blockers accounted for 63% of overdose deaths by cardiovascular drugs because they can cause severe hypotension, bradycardia, and hemodynamic collapse.16,21,22
In the past, the nondihydropyridine CCBs verapamil and diltiazem were associated with increased overdose fatalities. However, the most recent data show that dihydropyridine CCBs such as amlodipine also have significant risk for lethality.16 Metoprolol was associated with more overdose deaths in the past year among β-blockers. However, caution also should be used with agents such as propranolol and labetalol, which can antagonize sodium channels in overdose and may be associated with a higher risk of mortality than other β-blockers.22
Antidepressants accounted for 144 fatalities from 56,891 single-substance exposures (mortality rate: 0.3%).16 Nelson and Spyker performed a study to determine the morbidity and mortality index for psychotropic agents based on exposure reports from the National Poison Data system and found that tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) had the highest morbidity and mortality rates among all drugs used to treat depression.23 As a class, TCAs have a mortality index of 40.7 per 10,000 exposures and are associated with higher rates of acidosis, cardiac conduction problems, respiratory depression, and seizures (Table 2).23 Amitriptyline accounted for 39.5% of deaths from antidepressants.23 Among newer antidepressants, citalopram, venlafaxine, and bupropion have been found to be the most hazardous.23 Citalopram and venlafaxine have morbidity indices that are 4- to 5-fold higher than sertraline. Adverse events associated with bupropion, venlafaxine, and citalopram, such as seizures, conduction disturbances, hallucinations, and tachycardia contribute to the morbidity and mortality related to these medications (Table 3).
Of the atypical antipsychotics, olanzapine, quetiapine, and ziprasidone have the highest mortality rates.23 Cardiac conduction problems were more frequent with olanzapine and ziprasidone, and respiratory depression was more frequent with olanzapine and quetiapine. Aripiprazole had the lowest rates of morbidity and mortality.23
Of the mood stabilizers, lithium, valproic acid, and carbamazepine have narrow therapeutic indices and, therefore, moderately high mortality rates.23 Lithium was associated with higher rates of bradycardia, confusion, and renal problems. Valproic acid had relatively high levels of acidosis and coma. Carbamazepine had high rates of coma and the highest rate of nystagmus.
Sedatives and hypnotics accounted for 97 fatalities of 51,495 single-substance exposures (mortality rate 0.2%).16 Within this category, benzodiazepines (BZDs), particularly alprazolam, clonazepam, and diazepam, were associated with the highest number of overdose deaths.16 Although fatalities from single-substance exposure to this category are low, it should be noted that BZDs are primarily metabolized by the CYP2C19 and CYP3A4 enzymes. Interactions with other drugs also metabolized by the same CYP enzymes may lead to prolonged effects of BZDs, such as sedation, and respiratory depression, which significantly increase the risk of overdose death. Furthermore, lipophilic BZDs, such as diazepam, can accumulate in the tissue after multiple doses and have impaired clearance in older patients.
Step 3: Consider Potential Drug-Drug Interactions
Suicide attempts involving multiple substances carry increased risk. Only 12.1% of all fatal overdoses, according to AAPCC, involved single-substance exposure, whereas 56.3% were attributed to multiple substance exposures.16 It is important for clinicians to be aware of and avoid possibly fatal drug-drug interactions, such as the combination of opioids and sedative-hypnotics, like BZDs, which can lead to fatal respiratory depression. Clinicians also should be aware of a patient’s history of illicit opioid and alcohol use before prescribing opioids and BZDs. Clinicians can use various online databases to detect potential drug-drug interactions.
Step 4: Address Risks
If a patient is deemed to be at high risk for suicide, but it is not imminent and the patient will be managed as an outpatient, then it may be preferential to prescribe medications that are less lethal, such as SSRIs, instead of TCAs or MAOIs. If a potentially lethal medication is indicated, such as lithium or clozapine, both of which have been found to reduce suicidal behavior, then dispensing a limited quantity of pills and having more frequent follow-up visits are some ways to lessen risk.24,25 A clinical pearl published in Current Psychiatry provided an equation to determine the lethality of a 30-day supply of medications.26 This equation uses lethal dose 50 (LD50), which is the dose of a medication that results in the death of 50% of the animals used in a controlled experiment, and the maximum daily dose of the medication (D) to find the human equivalent dose (HED) relative lethality. The HED relative lethality calculation may help prescribers determine which medications should have a limited quantity dispensed to patients at risk of medication-related suicide. Any value for the HED relative lethality that is > 100% is considered a lethal dose for humans. Therefore, it would be appropriate to avoid or limit the quantity of medications with a HED relative lethality > 100%. Table 4 lists the psychotropic agents with the highest relative lethality for a 30-day supply. The psychotropic agents with the lowest HED relative lethality are SSRIs: desvenlafaxine, mirtazapine, topiramate, and aripiprazole.26
Limiting drugs with a narrow therapeutic index should be considered when aiming to reduce the risk of medication-related suicide. These drugs present a high risk in the event of an overdose. Clinicians can monitor the levels of lithium, clozapine, or TCAs to ensure that a patient is taking the medication as prescribed rather than stockpiling it at home. If the patient is in a monitored setting, such as a partial hospital program or intensive outpatient program, then the medication can be given while under direct observation.
Clinicians should obtain an accurate and detailed medication and illicit drug use history from patients. It also is important to review the prescription drug monitoring program to limit access to potentially lethal combinations of medications.27 Clinicians can additionally employ risk mitigation strategies (eg, providing naloxone kits) for patients who are prescribed or abuse opioids.
Finally, all patients with a high risk of suicide should receive lethal means counseling, which involves first determining whether patients have access to lethal means, such as firearms or medications with high lethality, then limiting their access to these lethal means. This includes advising patients and family members to safely dispose medications that are no longer in use and in some cases recommending that a family member keep medications locked and dispense them on a daily basis.
Conclusions
Suicide is a major public health concern that affects tens of thousands of Americans annually. Furthermore, veterans are more likely to die by suicide than those in the general population. Firearms continue to be the most lethal means for suicide. However, intentional poisoning with medications or substances also is a common method for suicide, especially in female veterans. Having knowledge of medications with high lethality and limiting access to these agents can be a successful strategy for reducing suicide deaths.
1. World Health Organization. Preventing suicide: a global imperative. Published 2014. Accessed January 16, 2021. https://apps.who.int/iris/bitstream/handle/10665/131056/9789241564779_eng.pdf
2. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Violence Prevention. National Violent Death Reporting System (NVDRS). Updated November 7, 2019. Accessed January 7, 2021. https://www.cdc.gov/violenceprevention/datasources/nvdrs/
3. US Department of Veteran Affairs, Office of Mental Health and Suicide Prevention. 2020 national veteran suicide prevention annual report. Accessed January 16, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
4. US Department of Veteran Affairs. National strategy for preventing veteran suicide 2018-2028. Accessed January 7, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
5. Zalsman G, Hawton K, Wasserman D, et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry. 2016;3(7):646-659. doi:10.1016/S2215-0366(16)30030-X
6. Spicer RS, Miller TR. Suicide acts in 8 states: incidence and case fatality rates by demographics and method. Am J Public Health. 2000;90(12):1885-1891. doi:10.2105/ajph.90.12.1885
7. Conner A, Azrael D, Miller M. Suicide Case-Fatality Rates in the United States, 2007 to 2014: A Nationwide Population-Based Study. Ann Intern Med. 2019;171(12):885-895. doi:10.7326/M19-1324
8. Bertolote JM, Fleischmann A. Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiatry. 2002;1(3):181-185.
9. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics. 1999;40(1):18-27. doi:10.1016/S0033-3182(99)71267-3
10. Bachmann S. Epidemiology of Suicide and the Psychiatric Perspective. Int J Environ Res Public Health. 2018;15(7):1425. Published 2018 Jul 6. doi:10.3390/ijerph15071425
11. Hoertel N, Franco S, Wall MM, et al. Mental disorders and risk of suicide attempt: a national prospective study. Mol Psychiatry. 2015;20(6):718-726. doi:10.1038/mp.2015.19
12. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi:10.1371/journal.pmed.1001547
13. World Health Organization. Mental health atlas. Accessed January 7, 2021. https://apps.who.int/iris/bitstream/handle/10665/178879/9789241565011_eng.pdf
14. Velupillai S, Hadlaczky G, Baca-Garcia E, et al. Risk assessment tools and data-driven approaches for predicting and preventing suicidal behavior. Front Psychiatry. 2019;10:36. Published 2019 Feb 13. doi:10.3389/fpsyt.2019.00036
15. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
16. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report [published correction appears in Clin Toxicol (Phila). 2019 Dec;57(12):e1]. Clin Toxicol (Phila). 2019;57(12):1220-1413. doi:10.1080/15563650.2019.1677022
17. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief. 2020(356).
18. Kuczyn´ska K, Grzonkowski P, Kacprzak Ł, Zawilska JB. Abuse of fentanyl: An emerging problem to face. Forensic Sci Int. 2018;289:207-214. doi:10.1016/j.forsciint.2018.05.042
19. Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499-516. doi:10.1016/j.ccc.2012.07.006
20. Chiew AL, Gluud C, Brok J, Buckley NA. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2018;2(2):CD003328. Published 2018 Feb 23. doi:10.1002/14651858.CD003328.pub3
21. Graudins A, Lee HM, Druda D. Calcium channel antagonist and beta-blocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol. 2016;81(3):453-461. doi:10.1111/bcp.12763
22. DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol Rev. 2004;23(4):223-238. doi:10.2165/00139709-200423040-00003
23. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450. doi:10.1176/appi.ajp.2016.16050523
24. Sarai SK, Mekala HM, Lippmann S. Lithium suicide prevention: a brief review and reminder. Innov Clin Neurosci. 2018;15(11-12):30-32.
25. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [published correction appears in Arch Gen Psychiatry. 2003 Jul;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91. doi:10.1001/archpsyc.60.1.82
26. Giurca D. Decreasing suicide risk with math. Curr Psychiatry. 2018;17(2):57-61.
27. Malte CA, Berger D, Saxon AJ, et al. Electronic medical record alert associated with reduced opioid and benzodiazepine coprescribing in high-risk veteran patients. Med Care. 2018;56(2):171-178. doi:10.1097/MLR.0000000000000861
Suicide is a global phenomenon and a worldwide public health concern.1 The World Health Organization estimates that > 800,000 people die by suicide every year. In the US, suicide is the 10th leading cause of death, and on average, 129 Americans die by suicide each day.2 In 2018, the suicide rate for all veterans was 1.5 times higher than the rate for nonveterans, after adjusting for population differences in age and sex. Among female veterans, the rate of suicide was 2.1 times higher than the rate for female nonveterans.3
In light of this disparity, suicide prevention is one of the highest priorities for the US Department of Veterans Affairs (VA). In 2018, the VA developed and published the National Strategy for Preventing Veteran Suicide.4 One major goal of this strategy is to reduce access to lethal means (ie, firearms, medications, chemicals, or poisons) among veterans at high risk for suicide. Reducing access to lethal means has been found to decrease suicide rates.4,5
Drug overdose is a leading method for suicide attempts, especially for female veterans.3,6 Although the overall case fatality ratio for overdose is < 2%, drug overdose accounted for 59.4% of suicide attempts and 13.5% of deaths by suicide from 2007 to 2014.6,7 Within the veteran population, the majority of suicide deaths in 2018 were due to self-inflicted firearm injury for both male and female veterans, followed by poisoning via substances and pharmaceutical agents for female veterans (Figure 1).3 Notably, when compared with men, women were more likely to choose drug overdose as a method for suicide. One study found that women aged < 45 years used drug and poison ingestion in 9 out of 10 suicide attempts.6 Since some medications are more lethal than others, interventions to limit the availability of lethal medications may prevent deaths and reduce the severity of suicide attempts. This article will provide a stepwise approach to help clinicians identify and limit lethal medications for patients at high risk for suicide.
Step 1: Determine Suicide Risk
Although it is impossible to predict with certainty an individual’s risk of suicide, several patient characteristics and life circumstances have been identified as risk factors. The strongest predictor of suicide is the presence of psychiatric disease.8 More than 90% of those who have had a death by suicide have a psychiatric diagnosis at the time of death, and suicide rates in those with mental illness are at least 10 times as high as in the general population.9,10 Depression is the leading cause of death by suicide worldwide, followed by substance-related disorders (22.4%), personality disorders (11.6%), schizophrenia (10.6%), and anxiety/somatoform disorders (6.1%).8,11-13
Clinicians also can use various risk assessment tools to identify patients at high risk for suicide. The Veterans Health Administration (VHA) Stratification Tool for Opioid Risk Mitigation (STORM) calculates patients’ risk based on data extracted from the electronic health record and is less time intensive, more easily refined, and may be more powerful than standard risk assessment tools because it can be deployed on a large scale.14,15 The VHA also developed the Suicide Prevention Population Risk Identification and Tracking for Exigencies (SPPRITE) tool to assist clinicians in tracking patients with current (or recent) high levels of suicide risk. This tool unifies specific patient information gathered from the patient’s electronic health record and from other predictive model dashboards (such as STORM).
Step 2: Identify Substances Strongly Associated With Fatalities
According to the American Association of Poison Control Centers (AAPCC), the pharmaceutical classes associated with the largest number of fatalities are analgesics, followed by stimulants and street drugs, cardiovascular agents, antidepressants, antipsychotics, and sedatives/hypnotics (Table 1).16 Stimulants and street drugs accounted for 694 fatalities of 39,238 single-substance exposures (mortality rate: 1.8%).16 Drugs of abuse, including cocaine, hallucinogenic amphetamines, heroin, and kratom, have shown an increased trend in use.16
In 2018 there were 834 fatalities from 174,269 single-substance exposure to analgesics, which include opioids and acetaminophen, for a mortality rate of 0.5%.16 The opioid epidemic is one of the main drivers of the increase in drug overdose deaths in the US.16,17 The opioid with the highest drug overdose fatality rate is illicitly manufactured fentanyl, which often is combined with other substances, such as heroin, to increase its potency at a low cost.18 These combinations also increase the risk of overdose fatality.
Acetaminophen is unique among the top substances associated with fatalities because it is obtained easily without a prescription. An acetaminophen overdose can cause hepatic injury, which may progress to fulminant hepatic failure and death.19 The recommended maximum dose of acetaminophen is 4 g/d in an adult and 50 to 75 mg/kg/d in children. A single acute ingestion of > 7.5 g in an adult or 150 mg/kg in children has been considered potentially toxic.19,20 The use of combination analgesics that contain both an opioid and acetaminophen can pose an even greater risk due to the potential for respiratory depression and hepatotoxicity.
Cardiovascular drugs accounted for 232 fatalities from 46,499 single-substance exposures (mortality rate: 0.5%).16 According to the AAPCC, calcium channel blockers (CCB) and β-blockers accounted for 63% of overdose deaths by cardiovascular drugs because they can cause severe hypotension, bradycardia, and hemodynamic collapse.16,21,22
In the past, the nondihydropyridine CCBs verapamil and diltiazem were associated with increased overdose fatalities. However, the most recent data show that dihydropyridine CCBs such as amlodipine also have significant risk for lethality.16 Metoprolol was associated with more overdose deaths in the past year among β-blockers. However, caution also should be used with agents such as propranolol and labetalol, which can antagonize sodium channels in overdose and may be associated with a higher risk of mortality than other β-blockers.22
Antidepressants accounted for 144 fatalities from 56,891 single-substance exposures (mortality rate: 0.3%).16 Nelson and Spyker performed a study to determine the morbidity and mortality index for psychotropic agents based on exposure reports from the National Poison Data system and found that tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) had the highest morbidity and mortality rates among all drugs used to treat depression.23 As a class, TCAs have a mortality index of 40.7 per 10,000 exposures and are associated with higher rates of acidosis, cardiac conduction problems, respiratory depression, and seizures (Table 2).23 Amitriptyline accounted for 39.5% of deaths from antidepressants.23 Among newer antidepressants, citalopram, venlafaxine, and bupropion have been found to be the most hazardous.23 Citalopram and venlafaxine have morbidity indices that are 4- to 5-fold higher than sertraline. Adverse events associated with bupropion, venlafaxine, and citalopram, such as seizures, conduction disturbances, hallucinations, and tachycardia contribute to the morbidity and mortality related to these medications (Table 3).
Of the atypical antipsychotics, olanzapine, quetiapine, and ziprasidone have the highest mortality rates.23 Cardiac conduction problems were more frequent with olanzapine and ziprasidone, and respiratory depression was more frequent with olanzapine and quetiapine. Aripiprazole had the lowest rates of morbidity and mortality.23
Of the mood stabilizers, lithium, valproic acid, and carbamazepine have narrow therapeutic indices and, therefore, moderately high mortality rates.23 Lithium was associated with higher rates of bradycardia, confusion, and renal problems. Valproic acid had relatively high levels of acidosis and coma. Carbamazepine had high rates of coma and the highest rate of nystagmus.
Sedatives and hypnotics accounted for 97 fatalities of 51,495 single-substance exposures (mortality rate 0.2%).16 Within this category, benzodiazepines (BZDs), particularly alprazolam, clonazepam, and diazepam, were associated with the highest number of overdose deaths.16 Although fatalities from single-substance exposure to this category are low, it should be noted that BZDs are primarily metabolized by the CYP2C19 and CYP3A4 enzymes. Interactions with other drugs also metabolized by the same CYP enzymes may lead to prolonged effects of BZDs, such as sedation, and respiratory depression, which significantly increase the risk of overdose death. Furthermore, lipophilic BZDs, such as diazepam, can accumulate in the tissue after multiple doses and have impaired clearance in older patients.
Step 3: Consider Potential Drug-Drug Interactions
Suicide attempts involving multiple substances carry increased risk. Only 12.1% of all fatal overdoses, according to AAPCC, involved single-substance exposure, whereas 56.3% were attributed to multiple substance exposures.16 It is important for clinicians to be aware of and avoid possibly fatal drug-drug interactions, such as the combination of opioids and sedative-hypnotics, like BZDs, which can lead to fatal respiratory depression. Clinicians also should be aware of a patient’s history of illicit opioid and alcohol use before prescribing opioids and BZDs. Clinicians can use various online databases to detect potential drug-drug interactions.
Step 4: Address Risks
If a patient is deemed to be at high risk for suicide, but it is not imminent and the patient will be managed as an outpatient, then it may be preferential to prescribe medications that are less lethal, such as SSRIs, instead of TCAs or MAOIs. If a potentially lethal medication is indicated, such as lithium or clozapine, both of which have been found to reduce suicidal behavior, then dispensing a limited quantity of pills and having more frequent follow-up visits are some ways to lessen risk.24,25 A clinical pearl published in Current Psychiatry provided an equation to determine the lethality of a 30-day supply of medications.26 This equation uses lethal dose 50 (LD50), which is the dose of a medication that results in the death of 50% of the animals used in a controlled experiment, and the maximum daily dose of the medication (D) to find the human equivalent dose (HED) relative lethality. The HED relative lethality calculation may help prescribers determine which medications should have a limited quantity dispensed to patients at risk of medication-related suicide. Any value for the HED relative lethality that is > 100% is considered a lethal dose for humans. Therefore, it would be appropriate to avoid or limit the quantity of medications with a HED relative lethality > 100%. Table 4 lists the psychotropic agents with the highest relative lethality for a 30-day supply. The psychotropic agents with the lowest HED relative lethality are SSRIs: desvenlafaxine, mirtazapine, topiramate, and aripiprazole.26
Limiting drugs with a narrow therapeutic index should be considered when aiming to reduce the risk of medication-related suicide. These drugs present a high risk in the event of an overdose. Clinicians can monitor the levels of lithium, clozapine, or TCAs to ensure that a patient is taking the medication as prescribed rather than stockpiling it at home. If the patient is in a monitored setting, such as a partial hospital program or intensive outpatient program, then the medication can be given while under direct observation.
Clinicians should obtain an accurate and detailed medication and illicit drug use history from patients. It also is important to review the prescription drug monitoring program to limit access to potentially lethal combinations of medications.27 Clinicians can additionally employ risk mitigation strategies (eg, providing naloxone kits) for patients who are prescribed or abuse opioids.
Finally, all patients with a high risk of suicide should receive lethal means counseling, which involves first determining whether patients have access to lethal means, such as firearms or medications with high lethality, then limiting their access to these lethal means. This includes advising patients and family members to safely dispose medications that are no longer in use and in some cases recommending that a family member keep medications locked and dispense them on a daily basis.
Conclusions
Suicide is a major public health concern that affects tens of thousands of Americans annually. Furthermore, veterans are more likely to die by suicide than those in the general population. Firearms continue to be the most lethal means for suicide. However, intentional poisoning with medications or substances also is a common method for suicide, especially in female veterans. Having knowledge of medications with high lethality and limiting access to these agents can be a successful strategy for reducing suicide deaths.
Suicide is a global phenomenon and a worldwide public health concern.1 The World Health Organization estimates that > 800,000 people die by suicide every year. In the US, suicide is the 10th leading cause of death, and on average, 129 Americans die by suicide each day.2 In 2018, the suicide rate for all veterans was 1.5 times higher than the rate for nonveterans, after adjusting for population differences in age and sex. Among female veterans, the rate of suicide was 2.1 times higher than the rate for female nonveterans.3
In light of this disparity, suicide prevention is one of the highest priorities for the US Department of Veterans Affairs (VA). In 2018, the VA developed and published the National Strategy for Preventing Veteran Suicide.4 One major goal of this strategy is to reduce access to lethal means (ie, firearms, medications, chemicals, or poisons) among veterans at high risk for suicide. Reducing access to lethal means has been found to decrease suicide rates.4,5
Drug overdose is a leading method for suicide attempts, especially for female veterans.3,6 Although the overall case fatality ratio for overdose is < 2%, drug overdose accounted for 59.4% of suicide attempts and 13.5% of deaths by suicide from 2007 to 2014.6,7 Within the veteran population, the majority of suicide deaths in 2018 were due to self-inflicted firearm injury for both male and female veterans, followed by poisoning via substances and pharmaceutical agents for female veterans (Figure 1).3 Notably, when compared with men, women were more likely to choose drug overdose as a method for suicide. One study found that women aged < 45 years used drug and poison ingestion in 9 out of 10 suicide attempts.6 Since some medications are more lethal than others, interventions to limit the availability of lethal medications may prevent deaths and reduce the severity of suicide attempts. This article will provide a stepwise approach to help clinicians identify and limit lethal medications for patients at high risk for suicide.
Step 1: Determine Suicide Risk
Although it is impossible to predict with certainty an individual’s risk of suicide, several patient characteristics and life circumstances have been identified as risk factors. The strongest predictor of suicide is the presence of psychiatric disease.8 More than 90% of those who have had a death by suicide have a psychiatric diagnosis at the time of death, and suicide rates in those with mental illness are at least 10 times as high as in the general population.9,10 Depression is the leading cause of death by suicide worldwide, followed by substance-related disorders (22.4%), personality disorders (11.6%), schizophrenia (10.6%), and anxiety/somatoform disorders (6.1%).8,11-13
Clinicians also can use various risk assessment tools to identify patients at high risk for suicide. The Veterans Health Administration (VHA) Stratification Tool for Opioid Risk Mitigation (STORM) calculates patients’ risk based on data extracted from the electronic health record and is less time intensive, more easily refined, and may be more powerful than standard risk assessment tools because it can be deployed on a large scale.14,15 The VHA also developed the Suicide Prevention Population Risk Identification and Tracking for Exigencies (SPPRITE) tool to assist clinicians in tracking patients with current (or recent) high levels of suicide risk. This tool unifies specific patient information gathered from the patient’s electronic health record and from other predictive model dashboards (such as STORM).
Step 2: Identify Substances Strongly Associated With Fatalities
According to the American Association of Poison Control Centers (AAPCC), the pharmaceutical classes associated with the largest number of fatalities are analgesics, followed by stimulants and street drugs, cardiovascular agents, antidepressants, antipsychotics, and sedatives/hypnotics (Table 1).16 Stimulants and street drugs accounted for 694 fatalities of 39,238 single-substance exposures (mortality rate: 1.8%).16 Drugs of abuse, including cocaine, hallucinogenic amphetamines, heroin, and kratom, have shown an increased trend in use.16
In 2018 there were 834 fatalities from 174,269 single-substance exposure to analgesics, which include opioids and acetaminophen, for a mortality rate of 0.5%.16 The opioid epidemic is one of the main drivers of the increase in drug overdose deaths in the US.16,17 The opioid with the highest drug overdose fatality rate is illicitly manufactured fentanyl, which often is combined with other substances, such as heroin, to increase its potency at a low cost.18 These combinations also increase the risk of overdose fatality.
Acetaminophen is unique among the top substances associated with fatalities because it is obtained easily without a prescription. An acetaminophen overdose can cause hepatic injury, which may progress to fulminant hepatic failure and death.19 The recommended maximum dose of acetaminophen is 4 g/d in an adult and 50 to 75 mg/kg/d in children. A single acute ingestion of > 7.5 g in an adult or 150 mg/kg in children has been considered potentially toxic.19,20 The use of combination analgesics that contain both an opioid and acetaminophen can pose an even greater risk due to the potential for respiratory depression and hepatotoxicity.
Cardiovascular drugs accounted for 232 fatalities from 46,499 single-substance exposures (mortality rate: 0.5%).16 According to the AAPCC, calcium channel blockers (CCB) and β-blockers accounted for 63% of overdose deaths by cardiovascular drugs because they can cause severe hypotension, bradycardia, and hemodynamic collapse.16,21,22
In the past, the nondihydropyridine CCBs verapamil and diltiazem were associated with increased overdose fatalities. However, the most recent data show that dihydropyridine CCBs such as amlodipine also have significant risk for lethality.16 Metoprolol was associated with more overdose deaths in the past year among β-blockers. However, caution also should be used with agents such as propranolol and labetalol, which can antagonize sodium channels in overdose and may be associated with a higher risk of mortality than other β-blockers.22
Antidepressants accounted for 144 fatalities from 56,891 single-substance exposures (mortality rate: 0.3%).16 Nelson and Spyker performed a study to determine the morbidity and mortality index for psychotropic agents based on exposure reports from the National Poison Data system and found that tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) had the highest morbidity and mortality rates among all drugs used to treat depression.23 As a class, TCAs have a mortality index of 40.7 per 10,000 exposures and are associated with higher rates of acidosis, cardiac conduction problems, respiratory depression, and seizures (Table 2).23 Amitriptyline accounted for 39.5% of deaths from antidepressants.23 Among newer antidepressants, citalopram, venlafaxine, and bupropion have been found to be the most hazardous.23 Citalopram and venlafaxine have morbidity indices that are 4- to 5-fold higher than sertraline. Adverse events associated with bupropion, venlafaxine, and citalopram, such as seizures, conduction disturbances, hallucinations, and tachycardia contribute to the morbidity and mortality related to these medications (Table 3).
Of the atypical antipsychotics, olanzapine, quetiapine, and ziprasidone have the highest mortality rates.23 Cardiac conduction problems were more frequent with olanzapine and ziprasidone, and respiratory depression was more frequent with olanzapine and quetiapine. Aripiprazole had the lowest rates of morbidity and mortality.23
Of the mood stabilizers, lithium, valproic acid, and carbamazepine have narrow therapeutic indices and, therefore, moderately high mortality rates.23 Lithium was associated with higher rates of bradycardia, confusion, and renal problems. Valproic acid had relatively high levels of acidosis and coma. Carbamazepine had high rates of coma and the highest rate of nystagmus.
Sedatives and hypnotics accounted for 97 fatalities of 51,495 single-substance exposures (mortality rate 0.2%).16 Within this category, benzodiazepines (BZDs), particularly alprazolam, clonazepam, and diazepam, were associated with the highest number of overdose deaths.16 Although fatalities from single-substance exposure to this category are low, it should be noted that BZDs are primarily metabolized by the CYP2C19 and CYP3A4 enzymes. Interactions with other drugs also metabolized by the same CYP enzymes may lead to prolonged effects of BZDs, such as sedation, and respiratory depression, which significantly increase the risk of overdose death. Furthermore, lipophilic BZDs, such as diazepam, can accumulate in the tissue after multiple doses and have impaired clearance in older patients.
Step 3: Consider Potential Drug-Drug Interactions
Suicide attempts involving multiple substances carry increased risk. Only 12.1% of all fatal overdoses, according to AAPCC, involved single-substance exposure, whereas 56.3% were attributed to multiple substance exposures.16 It is important for clinicians to be aware of and avoid possibly fatal drug-drug interactions, such as the combination of opioids and sedative-hypnotics, like BZDs, which can lead to fatal respiratory depression. Clinicians also should be aware of a patient’s history of illicit opioid and alcohol use before prescribing opioids and BZDs. Clinicians can use various online databases to detect potential drug-drug interactions.
Step 4: Address Risks
If a patient is deemed to be at high risk for suicide, but it is not imminent and the patient will be managed as an outpatient, then it may be preferential to prescribe medications that are less lethal, such as SSRIs, instead of TCAs or MAOIs. If a potentially lethal medication is indicated, such as lithium or clozapine, both of which have been found to reduce suicidal behavior, then dispensing a limited quantity of pills and having more frequent follow-up visits are some ways to lessen risk.24,25 A clinical pearl published in Current Psychiatry provided an equation to determine the lethality of a 30-day supply of medications.26 This equation uses lethal dose 50 (LD50), which is the dose of a medication that results in the death of 50% of the animals used in a controlled experiment, and the maximum daily dose of the medication (D) to find the human equivalent dose (HED) relative lethality. The HED relative lethality calculation may help prescribers determine which medications should have a limited quantity dispensed to patients at risk of medication-related suicide. Any value for the HED relative lethality that is > 100% is considered a lethal dose for humans. Therefore, it would be appropriate to avoid or limit the quantity of medications with a HED relative lethality > 100%. Table 4 lists the psychotropic agents with the highest relative lethality for a 30-day supply. The psychotropic agents with the lowest HED relative lethality are SSRIs: desvenlafaxine, mirtazapine, topiramate, and aripiprazole.26
Limiting drugs with a narrow therapeutic index should be considered when aiming to reduce the risk of medication-related suicide. These drugs present a high risk in the event of an overdose. Clinicians can monitor the levels of lithium, clozapine, or TCAs to ensure that a patient is taking the medication as prescribed rather than stockpiling it at home. If the patient is in a monitored setting, such as a partial hospital program or intensive outpatient program, then the medication can be given while under direct observation.
Clinicians should obtain an accurate and detailed medication and illicit drug use history from patients. It also is important to review the prescription drug monitoring program to limit access to potentially lethal combinations of medications.27 Clinicians can additionally employ risk mitigation strategies (eg, providing naloxone kits) for patients who are prescribed or abuse opioids.
Finally, all patients with a high risk of suicide should receive lethal means counseling, which involves first determining whether patients have access to lethal means, such as firearms or medications with high lethality, then limiting their access to these lethal means. This includes advising patients and family members to safely dispose medications that are no longer in use and in some cases recommending that a family member keep medications locked and dispense them on a daily basis.
Conclusions
Suicide is a major public health concern that affects tens of thousands of Americans annually. Furthermore, veterans are more likely to die by suicide than those in the general population. Firearms continue to be the most lethal means for suicide. However, intentional poisoning with medications or substances also is a common method for suicide, especially in female veterans. Having knowledge of medications with high lethality and limiting access to these agents can be a successful strategy for reducing suicide deaths.
1. World Health Organization. Preventing suicide: a global imperative. Published 2014. Accessed January 16, 2021. https://apps.who.int/iris/bitstream/handle/10665/131056/9789241564779_eng.pdf
2. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Violence Prevention. National Violent Death Reporting System (NVDRS). Updated November 7, 2019. Accessed January 7, 2021. https://www.cdc.gov/violenceprevention/datasources/nvdrs/
3. US Department of Veteran Affairs, Office of Mental Health and Suicide Prevention. 2020 national veteran suicide prevention annual report. Accessed January 16, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
4. US Department of Veteran Affairs. National strategy for preventing veteran suicide 2018-2028. Accessed January 7, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
5. Zalsman G, Hawton K, Wasserman D, et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry. 2016;3(7):646-659. doi:10.1016/S2215-0366(16)30030-X
6. Spicer RS, Miller TR. Suicide acts in 8 states: incidence and case fatality rates by demographics and method. Am J Public Health. 2000;90(12):1885-1891. doi:10.2105/ajph.90.12.1885
7. Conner A, Azrael D, Miller M. Suicide Case-Fatality Rates in the United States, 2007 to 2014: A Nationwide Population-Based Study. Ann Intern Med. 2019;171(12):885-895. doi:10.7326/M19-1324
8. Bertolote JM, Fleischmann A. Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiatry. 2002;1(3):181-185.
9. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics. 1999;40(1):18-27. doi:10.1016/S0033-3182(99)71267-3
10. Bachmann S. Epidemiology of Suicide and the Psychiatric Perspective. Int J Environ Res Public Health. 2018;15(7):1425. Published 2018 Jul 6. doi:10.3390/ijerph15071425
11. Hoertel N, Franco S, Wall MM, et al. Mental disorders and risk of suicide attempt: a national prospective study. Mol Psychiatry. 2015;20(6):718-726. doi:10.1038/mp.2015.19
12. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi:10.1371/journal.pmed.1001547
13. World Health Organization. Mental health atlas. Accessed January 7, 2021. https://apps.who.int/iris/bitstream/handle/10665/178879/9789241565011_eng.pdf
14. Velupillai S, Hadlaczky G, Baca-Garcia E, et al. Risk assessment tools and data-driven approaches for predicting and preventing suicidal behavior. Front Psychiatry. 2019;10:36. Published 2019 Feb 13. doi:10.3389/fpsyt.2019.00036
15. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
16. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report [published correction appears in Clin Toxicol (Phila). 2019 Dec;57(12):e1]. Clin Toxicol (Phila). 2019;57(12):1220-1413. doi:10.1080/15563650.2019.1677022
17. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief. 2020(356).
18. Kuczyn´ska K, Grzonkowski P, Kacprzak Ł, Zawilska JB. Abuse of fentanyl: An emerging problem to face. Forensic Sci Int. 2018;289:207-214. doi:10.1016/j.forsciint.2018.05.042
19. Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499-516. doi:10.1016/j.ccc.2012.07.006
20. Chiew AL, Gluud C, Brok J, Buckley NA. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2018;2(2):CD003328. Published 2018 Feb 23. doi:10.1002/14651858.CD003328.pub3
21. Graudins A, Lee HM, Druda D. Calcium channel antagonist and beta-blocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol. 2016;81(3):453-461. doi:10.1111/bcp.12763
22. DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol Rev. 2004;23(4):223-238. doi:10.2165/00139709-200423040-00003
23. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450. doi:10.1176/appi.ajp.2016.16050523
24. Sarai SK, Mekala HM, Lippmann S. Lithium suicide prevention: a brief review and reminder. Innov Clin Neurosci. 2018;15(11-12):30-32.
25. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [published correction appears in Arch Gen Psychiatry. 2003 Jul;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91. doi:10.1001/archpsyc.60.1.82
26. Giurca D. Decreasing suicide risk with math. Curr Psychiatry. 2018;17(2):57-61.
27. Malte CA, Berger D, Saxon AJ, et al. Electronic medical record alert associated with reduced opioid and benzodiazepine coprescribing in high-risk veteran patients. Med Care. 2018;56(2):171-178. doi:10.1097/MLR.0000000000000861
1. World Health Organization. Preventing suicide: a global imperative. Published 2014. Accessed January 16, 2021. https://apps.who.int/iris/bitstream/handle/10665/131056/9789241564779_eng.pdf
2. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, Division of Violence Prevention. National Violent Death Reporting System (NVDRS). Updated November 7, 2019. Accessed January 7, 2021. https://www.cdc.gov/violenceprevention/datasources/nvdrs/
3. US Department of Veteran Affairs, Office of Mental Health and Suicide Prevention. 2020 national veteran suicide prevention annual report. Accessed January 16, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2020/2020-National-Veteran-Suicide-Prevention-Annual-Report-11-2020-508.pdf
4. US Department of Veteran Affairs. National strategy for preventing veteran suicide 2018-2028. Accessed January 7, 2021. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
5. Zalsman G, Hawton K, Wasserman D, et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry. 2016;3(7):646-659. doi:10.1016/S2215-0366(16)30030-X
6. Spicer RS, Miller TR. Suicide acts in 8 states: incidence and case fatality rates by demographics and method. Am J Public Health. 2000;90(12):1885-1891. doi:10.2105/ajph.90.12.1885
7. Conner A, Azrael D, Miller M. Suicide Case-Fatality Rates in the United States, 2007 to 2014: A Nationwide Population-Based Study. Ann Intern Med. 2019;171(12):885-895. doi:10.7326/M19-1324
8. Bertolote JM, Fleischmann A. Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiatry. 2002;1(3):181-185.
9. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics. 1999;40(1):18-27. doi:10.1016/S0033-3182(99)71267-3
10. Bachmann S. Epidemiology of Suicide and the Psychiatric Perspective. Int J Environ Res Public Health. 2018;15(7):1425. Published 2018 Jul 6. doi:10.3390/ijerph15071425
11. Hoertel N, Franco S, Wall MM, et al. Mental disorders and risk of suicide attempt: a national prospective study. Mol Psychiatry. 2015;20(6):718-726. doi:10.1038/mp.2015.19
12. Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi:10.1371/journal.pmed.1001547
13. World Health Organization. Mental health atlas. Accessed January 7, 2021. https://apps.who.int/iris/bitstream/handle/10665/178879/9789241565011_eng.pdf
14. Velupillai S, Hadlaczky G, Baca-Garcia E, et al. Risk assessment tools and data-driven approaches for predicting and preventing suicidal behavior. Front Psychiatry. 2019;10:36. Published 2019 Feb 13. doi:10.3389/fpsyt.2019.00036
15. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
16. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report [published correction appears in Clin Toxicol (Phila). 2019 Dec;57(12):e1]. Clin Toxicol (Phila). 2019;57(12):1220-1413. doi:10.1080/15563650.2019.1677022
17. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief. 2020(356).
18. Kuczyn´ska K, Grzonkowski P, Kacprzak Ł, Zawilska JB. Abuse of fentanyl: An emerging problem to face. Forensic Sci Int. 2018;289:207-214. doi:10.1016/j.forsciint.2018.05.042
19. Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28(4):499-516. doi:10.1016/j.ccc.2012.07.006
20. Chiew AL, Gluud C, Brok J, Buckley NA. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev. 2018;2(2):CD003328. Published 2018 Feb 23. doi:10.1002/14651858.CD003328.pub3
21. Graudins A, Lee HM, Druda D. Calcium channel antagonist and beta-blocker overdose: antidotes and adjunct therapies. Br J Clin Pharmacol. 2016;81(3):453-461. doi:10.1111/bcp.12763
22. DeWitt CR, Waksman JC. Pharmacology, pathophysiology and management of calcium channel blocker and beta-blocker toxicity. Toxicol Rev. 2004;23(4):223-238. doi:10.2165/00139709-200423040-00003
23. Nelson JC, Spyker DA. Morbidity and mortality associated with medications used in the treatment of depression: an analysis of cases reported to U.S. Poison Control Centers, 2000-2014. Am J Psychiatry. 2017;174(5):438-450. doi:10.1176/appi.ajp.2016.16050523
24. Sarai SK, Mekala HM, Lippmann S. Lithium suicide prevention: a brief review and reminder. Innov Clin Neurosci. 2018;15(11-12):30-32.
25. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [published correction appears in Arch Gen Psychiatry. 2003 Jul;60(7):735]. Arch Gen Psychiatry. 2003;60(1):82-91. doi:10.1001/archpsyc.60.1.82
26. Giurca D. Decreasing suicide risk with math. Curr Psychiatry. 2018;17(2):57-61.
27. Malte CA, Berger D, Saxon AJ, et al. Electronic medical record alert associated with reduced opioid and benzodiazepine coprescribing in high-risk veteran patients. Med Care. 2018;56(2):171-178. doi:10.1097/MLR.0000000000000861
Management of Do Not Resuscitate Orders Before Invasive Procedures
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
In January 2017, the US Department of Veterans Affairs (VA), led by the National Center of Ethics in Health Care, created the Life-Sustaining Treatment Decisions Initiative (LSTDI). The VA gradually implemented the LSTDI in its facilities nationwide. In a format similar to the standardized form of portable medical orders, provider orders for life-sustaining treatments (POLST), the initiative promotes discussions with veterans and encourages but does not require health care professionals (HCPs) to complete a template for documentation (life-sustaining treatment [LST] note) of a patient’s preferences.1 The HCP enters a code status into the electronic health record (EHR), creating a portable and durable note and order.
With a new durable code status, the HCPs performing these procedures (eg, colonoscopies, coronary catheterization, or percutaneous biopsies) need to acknowledge and can potentially rescind a do not resuscitate (DNR) order. Although the risk of cardiac arrest or intubation is low, all invasive procedures carry these risks to some degree.2,3 Some HCPs advocate the automatic discontinuation of DNR orders before any procedure, but multiple professional societies recommend that patients be included in these discussions to honor their wishes.4-7 Although no procedures at the VA require the suspension of a DNR status, it is important to establish which life-sustaining measures are acceptable to patients.
As part of the informed consent process, proceduralists (HCPs who perform a procedure) should discuss the option of temporary suspension of DNR in the periprocedural period and document the outcome of this discussion (eg, rescinded DNR, acknowledgment of continued DNR status). These discussions need to be documented clearly to ensure accurate communication with other HCPs, particularly those caring for the patient postprocedure. Without the documentation, the risk that the patient’s wishes will not be honored is high.8 Code status is usually addressed before intubation of general anesthesia; however, nonsurgical procedures have a lower likelihood of DNR acknowledgment.
This study aimed to examine and improve the rate of acknowledgment of DNR status before nonsurgical procedures. We hypothesized that the rate of DNR acknowledgment before nonsurgical invasive procedures is low; and the rate can be raised with an intervention designed to educate proceduralists and improve and simplify this documentation.9
Methods
This was a single center, before/after quasi-experimental study. The study was considered clinical operations and institutional review board approval was unnecessary.
A retrospective chart review was performed of patients who underwent an inpatient or outpatient, nonsurgical invasive procedure at the Minneapolis VA Medical Center in Minnesota. The preintervention period was defined as the first 6 months after implementation of the LSTDI between May 8, 2018 and October 31, 2018. The intervention was presented in December 2018 and January 2019. The postintervention period was from February 1, 2019 to April 30, 2019.
Patients who underwent a nonsurgical invasive procedure were reviewed in 3 procedural areas. These areas were chosen based on high patient volumes and the need for rapid patient turnover, including gastroenterology, cardiology, and interventional radiology. An invasive procedure was defined as any procedure requiring patient consent. Those patients who had a completed LST note and who had a DNR order were recorded.
The intervention was composed of 2 elements: (1) an addendum to the LST note, which temporarily suspended resuscitation orders (Figure). We developed the addendum based on templates and orders in use before LSTDI implementation. Physicians from the procedural areas reviewed the addendum and provided feedback and the facility chief-of-staff provided approval. Part 2 was an educational presentation to proceduralists in each procedural area. The presentation included a brief introduction to the LSTDI, where to find a life-sustaining treatment note, code status, the importance of addressing code status, and a description of the addendum. The proceduralists were advised to use the addendum only after discussion with the patient and obtaining verbal consent for DNR suspension. If the patient elected to remain DNR, proceduralists were encouraged to document the conversation acknowledging the DNR.
Outcomes
The primary outcome of the study was proceduralist acknowledgment of DNR status before nonsurgical invasive procedures. DNR status was considered acknowledged if the proceduralist provided any type of documentation.
Statistical Analysis
Model predicted percentages of DNR acknowledgment are reported from a logistic regression model with both procedural area, time (before vs after) and the interaction between these 2 variables in the model. The simple main effects comparing before vs after within the procedural area based on post hoc contrasts of the interaction term also are shown.
Results
During the first 6 months following LSTDI implementation (the preintervention phase), 5,362 invasive procedures were performed in gastroenterology, interventional radiology, and cardiology. A total of 211 procedures were performed on patients who had a prior LST note indicating DNR. Of those, 68 (32.2%) had documentation acknowledging their DNR status. The educational presentation was given to each of the 3 departments with about 75% faculty attendance in each department. After the intervention, 1,932 invasive procedures were performed, identifying 143 LST notes with a DNR status. Sixty-five (45.5%) had documentation of a discussion regarding their DNR status.
The interaction between procedural areas and time (before, after) was examined. Of the 3 procedural areas, only interventional radiology had significant differences before vs after, 7.5% vs 26.3%, respectively (P = .01). Model-adjusted percentages before vs after for cardiology were 75.6% vs 91.7% (P = .12) and for gastroenterology were 46% vs 53.5% (P = .40) (Table). When all 3 procedural areas were combined, there was a significant improvement in the overall percentage of DNR acknowledgment postintervention from 38.6% to 61.1.% (P = .01).
Discussion
With the LSTDI, DNR orders remain in place and are valid in the inpatient and outpatient setting until reversed by the patient. This creates new challenges for proceduralists. Before our intervention, only about one-third of proceduralists’ recognized DNR status before procedures. This low rate of preprocedural DNR acknowledgments is not unique to the VA. A pilot study assessing rate of documentation of code status discussions in patients undergoing venting gastrostomy tube for malignant bowel obstruction showed documentation in only 22% of cases before the procedure.10 Another simulation-based study of anesthesiologist showed only 57% of subjects addressed resuscitation before starting the procedure.11
Despite the low initial rates of DNR acknowledgment, our intervention successfully improved these rates, although with variation between procedural areas. Prior studies looking at improving adherence to guidelines have shown the benefit of physician education.12,13 Improving code status acknowledgment before an invasive procedure not only involves increasing awareness of a preexisting code status, but also developing a system to incorporate the documentation process efficiently into the procedural workflow and ensuring that providers are aware of the appropriate process. Although the largest improvement was in interventional radiology, many patients postintervention still did not have their DNR orders acknowledged. Confusion is created when the patient is cared for by a different HCP or when the resuscitation team is called during a cardiac arrest. Cardiopulmonary resuscitation may be started or withheld incorrectly if the patient’s most recent wishes for resuscitation are unclear.14
Outside of using education to raise awareness, other improvements could utilize informatics solutions, such as developing an alert on opening a patient chart if a DNR status exists (such as a pop-up screen) or adding code status as an item to a preprocedural checklist. Similar to our study, previous studies also have found that a systematic approach with guidelines and templates improved rates of documentation of code status and DNR decisions.15,16 A large proportion of the LST notes and procedures done on patients with a DNR in our study occurred in the inpatient setting without any involvement of the primary care provider in the discussion. Having an automated way to alert the primary care provider that a new LST note has been completed may be helpful in guiding future care. Future work could identify additional systematic methods to increase acknowledgment of DNR.
Limitations
Our single-center results may not be generalizable. Although the interaction between procedural area and time was tested, it is possible that improvement in DNR acknowledgment was attributable to secular trends and not the intervention. Other limitations included the decreased generalizability of a VA health care initiative and its unique electronic health record, incomplete attendance rates at our educational sessions, and a lack of patient-centered outcomes.
Conclusions
A templated addendum combined with targeted staff education improved the percentage of DNR acknowledgments before nonsurgical invasive procedures, an important step in establishing patient preferences for life-sustaining treatment in procedures with potential complications. Further research is needed to assess whether these improvements also lead to improved patient-centered outcomes.
Acknowledgments
The authors would like to acknowledge the invaluable help of Dr. Kathryn Rice and Dr. Anne Melzer for their guidance in the manuscript revision process
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
1. Physician Orders for Life-Sustaining Treatment Paradigm. Honoring the wishes of those with serious illness and frailty. Accessed January 11, 2021.
2. Arepally A, Oechsle D, Kirkwood S, Savader S. Safety of conscious sedation in interventional radiology. Cardiovasc Intervent Radiol. 2001;24(3):185-190. doi:10.1007/s002700002549
3. Arrowsmith J, Gertsman B, Fleischer D, Benjamin S. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427. doi:10.1016/s0016-5107(91)70773-6
4. Burkle C, Swetz K, Armstrong M, Keegan M. Patient and doctor attitudes and beliefs concerning perioperative do not resuscitate orders: anesthesiologists’ growing compliance with patient autonomy and self-determination guidelines. BMC Anesthesiol. 2013;13:2. doi:10.1186/1471-2253-13-2
5. American College of Surgeons. Statement on advance directives by patients: “do not resuscitate” in the operative room. Published January 3, 2014. Accessed January 11, 2021. https://bulletin.facs.org/2014/01/statement-on-advance-directives-by-patients-do-not-resuscitate-in-the-operating-room
6. Association of periOperative Registered Nurses. AORN position statement on perioperative care of patients with do-not-resuscitate or allow-natural death orders. Reaffirmed February 2020. Accessed June 16, 2020. https://www.aorn.org/guidelines/clinical-resources/position-statements
7. Bastron DR. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment. Published 1996. Accessed January 11, 2021. https://pubs.asahq.org/anesthesiology/article/85/5/1190/35862/Ethical-Concerns-in-Anesthetic-Care-for-Patients
8. Baxter L, Hancox J, King B, Powell A, Tolley T. Stop! Patients receiving CPR despite valid DNACPR documentation. Eur J Pall Car. 2018;23(3):125-127.
9. Agency for Healthcare Research and Quality. Practice facilitation handbook, module 10: academic detailing as a quality improvement tool. Last reviewed May 2013. Accessed January 11, 2021. 2021. https://www.ahrq.gov/ncepcr/tools/pf-handbook/mod10.html
10. Urman R, Lilley E, Changala M, Lindvall C, Hepner D, Bader A. A pilot study to evaluate compliance with guidelines for preprocedural reconsideration of code status limitations. J Palliat Med. 2018;21(8):1152-1156. doi:10.1089/jpm.2017.0601
11. Waisel D, Simon R, Truog R, Baboolal H, Raemer D. Anesthesiologist management of perioperative do-not-resuscitate orders: a simulation-based experiment. Simul Healthc. 2009;4(2):70-76. doi:10.1097/SIH.0b013e31819e137b
12. Lozano P, Finkelstein J, Carey V, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic-asthma care. Arch Pediatr Adolesc Med. 2004;158(9):875-883. doi:10.1001/archpedi.158.9.875
13. Brunström M, Ng N, Dahlström J, et al. Association of physician education and feedback on hypertension management with patient blood pressure and hypertension control. JAMA Netw Open. 2020;3(1):e1918625. doi:10.1001/jamanetworkopen.2019.18625
14. Wong J, Duane P, Ingraham N. A case series of patients who were do not resuscitate but underwent cardiopulmonary resuscitation. Resuscitation. 2020;146:145-146. doi:10.1016/j.resuscitation.2019.11.020
15. Mittelberger J, Lo B, Martin D, Uhlmann R. Impact of a procedure-specific do not resuscitate order form on documentation of do not resuscitate orders. Arch Intern Med. 1993;153(2):228-232.
16. Neubauer M, Taniguchi C, Hoverman J. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-266. doi:10.1200/JOP.2014.001438
Minimizing Opioids After Joint Operation: Protocol to Decrease Postoperative Opioid Use After Primary Total Knee Arthroplasty
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
For decades, opioids have been a mainstay in the management of pain after total joint arthroplasty. In the past 10 years, however, opioid prescribing has come under increased scrutiny due to a rise in rates of opioid abuse, pill diversion, and opioid-related deaths.1,2 Opioids are associated with adverse effects, including nausea, vomiting, constipation, apathy, and respiratory depression, all of which influence arthroplasty outcomes and affect the patient experience. Although primary care groups account for nearly half of prescriptions written, orthopedic surgeons have the third highest per capita rate of opioid prescribing of all medical specialties.3,4 This puts orthopedic surgeons, particularly those who perform routine procedures, in an opportune but challenging position to confront this problem through novel pain management strategies.
Approximately 1 million total knee arthroplasties (TKAs) are performed in the US every year, and the US Department of Veterans Affairs (VA) health system performs about 10,000 hip and knee joint replacements.5,6 There is no standardization of opioid prescribing in the postoperative period following these procedures, and studies have reported a wide variation in prescribing habits even within a single institution for a specific surgery.7 Patients who undergo TKA are at particularly high risk of long-term opioid use if they are on continuous opioids at the time of surgery; this is problematic in a VA patient population in which at least 16% of patients are prescribed opioids in a given year.8 Furthermore, veterans are twice as likely as nonveterans to die of an accidental overdose.9 Despite these risks, opioids remain a cornerstone of postoperative pain management both within and outside of the VA.10
In 2018, to limit unnecessary prescribing of opioid pain medication, the total joint service at the VA Portland Health Care System (VAPHCS) in Oregon implemented the Minimizing Opioids after Joint Operation (MOJO) postoperative pain protocol. The goal of the protocol was to reduce opioid use following TKA. The objectives were to provide safe, appropriate analgesia while allowing early mobilization and discharge without a concomitant increase in readmissions or emergency department (ED) visits. The purpose of this retrospective chart review was to compare the efficacy of the MOJO protocol with our historical experience and report our preliminary results.
Methods
Institutional review board approval was obtained to retrospectively review the medical records of patients who had undergone TKA surgery during 2018 at VAPHCS. The MOJO protocol was composed of several simultaneous changes. The centerpiece of the new protocol was a drastic decrease in routine prescription of postoperative opioids (Table 1). Other changes included instructing patients to reduce the use of preoperative opioid pain medication 6 weeks before surgery with a goal of no opioid consumption, perform daily sets of preoperative exercises, and attend a preoperative consultation/education session with a nurse coordinator to emphasize early recovery and discharge. In patients with chronic use of opioid pain medication (particularly those for whom the medication had been prescribed for other sources of pain, such as lumbar back pain), the goal was daily opioid use of ≤ 30 morphine equivalent doses (MEDs). During the inpatient stay, we stopped prescribing prophylactic pain medication prior to physical therapy (PT).
We encouraged preoperative optimization of muscle strength by giving instructions for 4 to 8 weeks of daily exercises (Appendix). We introduced perioperative adductor canal blocks (at the discretion of the anesthesia team) and transitioned to surgery without a tourniquet. Patients in both groups received intraoperative antibiotics and IV tranexamic acid (TXA); the MOJO group also received topical TXA.
Further patient care optimization included providing patients with a team-based approach, which consisted of nurse coordinators, physician assistants and nurse practitioners, residents, and the attending surgeon. Our team reviews the planned pain management protocol, perioperative expectations, criteria for discharge, and anticipated surgical outcomes with the patient during their preoperative visits. On postoperative day 1, these members round as a team to encourage patients in their immediate postoperative recovery and rehabilitation. During rounds, the team assesses whether the patient meets the criteria for discharge, adjusting the pain management protocol if necessary.
Changes in surgical technique included arthrotomy with electrocautery, minimizing traumatic dissection or resection of the synovial tissue, and intra-articular injection of a cocktail of ropivacaine 5 mg/mL 40 mL, epinephrine 1:1,000 0.5 mL, and methylprednisolone sodium 40 mg diluted with normal saline to a total volume of 120 mL.
The new routine was gradually implemented beginning January 2017 and fully implemented by July 2018. This study compared the first 20 consecutive patients undergoing primary TKA after July 2018 to the last 20 consecutive patients undergoing primary TKA prior to January 2017. Exclusion criteria included bilateral TKA, death before 90 days, and revision as the indication for surgery. The senior attending surgeon performed all surgeries using a standard midline approach. The majority of surgeries were performed using a cemented Vanguard total knee system (Zimmer Biomet); 4 patients in the historical group had a NexGen knee system, cementless monoblock tibial components (Zimmer Biomet); and 1 patient had a Logic knee system (Exactech). Surgical selection criteria for patients did not differ between groups.
Electronic health records were reviewed and data were abstracted. The data included demographic information (age, gender, body mass index [BMI], diagnosis, and procedure), surgical factors (American Society of Anesthesiologists score, Risk Assessment and Predictive Tool score, operative time, tourniquet time, estimated blood loss), hospital factors (length of stay [LOS], discharge location), postoperative pain scores (measured on postoperative day 1 and on day of discharge), and postdischarge events (90-day complications, telephone calls reporting pain, reoperations, returns to the ED, 90-day readmissions).
The primary outcome was the mean postoperative daily MED during the inpatient stay. Secondary outcomes included pain on postoperative day 1, pain at the time of discharge, LOS, hospital readmissions, and ED visits within 90 days of surgery. Because different opioid pain medications were used by patients postoperatively, all opioids were converted to MED prior to the final analysis. Collected patient data were de-identified prior to analysis.
Power analysis was conducted to determine whether the study had sufficient population size to reject the null hypothesis for the primary outcome measure. Because practitioners controlled postoperative opioid use, a Cohen’s d of 1.0 was used so that a very large effect size was needed to reach clinical significance. Statistical significance was set to 0.05, and patient groups were set at 20 patients each. This yielded an appropriate power of 0.87. Population characteristics were compared between groups using t tests and χ2 tests as appropriate. To analyze the primary outcome, comparisons were made between the 2 cohorts using 2-tailed t tests. Secondary outcomes were compared between groups using t tests or χ2 tests. All statistics were performed using R version 3.5.2. Power analysis was conducted using the package pwr.11 Statistical significance was set at
Results
Forty patients met the inclusion criteria, evenly divided between those undergoing TKA before and after instituting the MOJO protocol (Table 2). A single patient in the MOJO group died and was excluded. A patient who underwent bilateral TKA also was excluded. Both groups reflected the male predominance of the VA patient population. MOJO patients tended to have lower BMIs (34 vs 30, P < .01). All patients indicated for surgery with preoperative opioid use were able to titrate down to their preoperative goal as verified by prescriptions filled at VA pharmacies. Twelve of the patients in the MOJO group received adductor canal blocks.
Results of t tests and χ2 tests comparing primary and secondary endpoints are listed in Table 3. Differences between the daily MEDs given in the historical and MOJO groups are shown. There were significant differences between the pre-MOJO and MOJO groups with regard to daily inpatient MEDs (82 mg vs 29 mg, P < .01) and total inpatient MEDs (306 mg vs 32 mg, P < .01). There was less self-reported pain on postoperative day 1 in the MOJO group (5.5 vs 3.9, P < .01), decreased LOS (4.4 days vs 1.2 days, P < .01), a trend toward fewer total ED visits (6 vs 2, P = .24), and fewer discharges to skilled nursing facilities (12 vs 0, P < .01). There were no blood transfusions in either group.
There were no readmissions due to uncontrolled pain. There was 1 readmission for shortness of breath in the MOJO group. The patient was discharged home the following day after ruling out thromboembolic and cardiovascular events. One patient from the control group was readmitted after missing a step on a staircase and falling. The patient sustained a quadriceps tendon rupture and underwent primary suture repair.
Discussion
Our results demonstrate that a multimodal approach to significantly reduce postoperative opioid use in patients with TKA is possible without increasing readmissions or ED visits for pain control. The patients in the MOJO group had a faster recovery, earlier discharge, and less use of postoperative opioid medication. Our approach to postoperative pain management was divided into 2 main categories: patient optimization and surgical optimization.
Patient Selection
Besides the standard evaluation and optimization of patients’ medical conditions, identifying and optimizing at-risk patients before surgery was a critical component of our protocol. Managing postoperative pain in patients with prior opioid use is an intractable challenge in orthopedic surgery. Patients with a history of chronic pain and preoperative use of opioid medications remain at higher risk of postoperative chronic pain and persistent use of opioid medication despite no obvious surgical complications.8 In a sample of > 6,000 veterans who underwent TKA at VA hospitals in 2014, 57% of the patients with daily use of opioids in the 90 days before surgery remained on opioids 1 year after surgery (vs 2 % in patients not on long-term opioids).8 This relationship between pre- and postoperative opioid use also was dose dependent.12
Furthermore, those with high preoperative use may experience worse outcomes relative to the opioid naive population as measured by arthritis-specific pain indices.13 In a well-powered retrospective study of patients who underwent elective orthopedic procedures, preoperative opioid abuse or dependence (determined by the International Classification of Diseases, Ninth Revision diagnosis) increased inpatient mortality, aggregate morbidity, surgical site infection, myocardial infarction, and LOS.14 Preoperative opioid use also has been associated with increased risk of ED visits, readmission, infection, stiffness, and aseptic revision.15 In patients with TKA in the VA specifically, preoperative opioid use (> 3 months in the prior year) was associated with increased revision rates that were even higher than those for patients with diabetes mellitus.16
Patient Education
Based on this evidence, we instruct patients to reduce their preoperative opioid dosing to zero (for patients with joint pain) or < 30 MED (for patients using opioids for other reasons). Although preoperative reduction of opioid use has been shown to improve outcomes after TKA, pain subspecialty recommendations for patients with chronic opioid use recommend considering adjunctive therapies, including transcutaneous electrical nerve stimulation, cognitive behavioral therapy, gabapentin, or ketamine.17,18 Through patient education our team has been successful in decreasing preoperative opioid use without adding other drugs or modalities.
Patient Optimization
Preoperative patient optimization included 4 to 8 weeks of daily sets of physical activity instructions (prehab) to improve the musculoskeletal function. These instructions are given to patients 4 to 8 weeks before surgery and aim to improve the patient’s balance, mobility, and functional ability (Appendix). Meta-analysis has shown that patients who undergo preoperative PT have a small but statistically significant decrease in postoperative pain at 4 weeks, though this does not persist beyond that period.19
We did note a lower BMI in patients in the MOJO group. Though this has the potential to be a confounder, a study of BMI in > 4,000 patients who underwent joint replacement surgery has shown that BMI is not associated with differences in postoperative pain.20
Surgeon and Surgical-Related Variables
Patients in the MOJO group had increased use of adductor canal blocks. A 2017 meta-analysis of 12,530 patients comparing analgesic modalities found that peripheral nerve blocks targeting multiple nerves (eg, femoral/sciatic) decreased pain at rest, decreased opioid consumption, and improved range of motion postoperatively.21 Also, these were found to be superior to single nerve blocks, periarticular infiltration, and epidural blocks.21 However, major nerve and epidural blocks affecting the lower extremity may increase the risk of falls and prolong LOS.22,23 The preferred peripheral block at VAPHCS is a single shot ultrasound-guided adductor canal block before the induction of general or spinal anesthesia. A randomized controlled trial has demonstrated superiority of this block to the femoral nerve block with regard to postoperative quadriceps strength, conferring the theoretical advantage of decreased fall risk and ability to participate in immediate PT.24 Although we are unable to confirm an association between anesthetic modalities and opioid burden, our clinical impression is that blocks were effective at reducing immediate postoperative pain. However, among MOJO patients there were no differences in patients with and without blocks for either pain (4.2 vs 3.8, P = .69) or opioid consumption (28.8 vs 33.0, P = .72) after surgery, though our study was not powered to detect a difference in this restricted subgroup.
Patients who frequently had reported postoperative thigh pain prompted us to make changes in our surgical technique, performing TKA without use of a tourniquet. Tourniquet use has been associated with an increased risk of thigh pain after TKA by multiple authors.25,26 Postoperative thigh pain also is pressure dependent.27 In addition, its use may be associated with a slightly increased risk of thromboembolic events and delayed functional recovery.28,29
Because postoperative hemarthrosis is associated with more pain and reduced joint recovery function, we used topical TXA to reduce postoperative surgical site and joint hematoma. TXA (either oral, IV, or topical) during TKA is used to control postoperative bleeding primarily and decrease the need for transfusion without concomitant increase in thromboembolic events.30,31 Topical TXA may be more effective than IV, particularly in the immediate postoperative period.32 Although pain typically is not an endpoint in studies of TXA, a prospective study of 48 patients showed evidence that its use may be associated with decreased postoperative pain in the first 24 hours after surgery (though not after).33 Finally, the use of intra-articular injection has evolved in our clinical practice, but literature is lacking with regard to its efficacy; more studies are needed to determine its effect relative to no injection. We have not seen any benefits to using
Limitations
This is a nonrandomized retrospective single-institution study. Our study population is composed of mostly males with military experience and is not necessarily a representative sample of the general population eligible for joint arthroplasty. Our primary endpoint (reduction of opioid use postoperatively) also was a cornerstone of our intervention. To account for this, we set a very large effect size in our power analysis and evaluated multiple secondary endpoints to determine whether postoperative pain remained well controlled and complications/readmission minimized with our interventions. Because our intervention was multimodal, our study cannot make conclusions about the effect of a particular component of our treatment strategy. We did not measure or compare functional outcomes between both groups, which offers an opportunity for further research.
These limitations are balanced by several strengths. Our cohort was well controlled with respect to the dose and type of drug used. There is staff dedicated to postoperative telephone follow-up after discharge, and veterans are apt to seek care within the VA health care system, which improves case finding for complications and ED visits. No patients were lost to follow-up. Moreover, our drastic reduction in opioid use is promising enough to warrant reporting, while the broader orthopedic literature explores the relative impact of each variable.
Conclusions
The MOJO protocol has been effective for reducing postoperative opioid use after TKA without compromising effective pain management. The drastic reduction in the postoperative use of opioid pain medications and LOS have contributed to a cultural shift within our department, comprehensive team approach, multimodal pain management, and preoperative patient optimization. Further investigations are required to assess the impact of each intervention on observed outcomes. However, the framework and routines are applicable to other institutions and surgical specialties.
Acknowledgments
The authors recognize Derek Bond, MD, for his help in creating the MOJO acronym.
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
1. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics Data Brief No. 329. Published November 2018. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief No. 294. Published December 2017. Accessed January 12, 2021. https://www.cdc.gov/nchs/data/databriefs/db294.pdf
3. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic–prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413. doi:10.1016/j.amepre.2015.02.020
4. Guy GP, Zhang K. Opioid prescribing by specialty and volume in the U.S. Am J Prev Med. 2018;55(5):e153-155. doi:10.1016/j.amepre.2018.06.008
5. Kremers HM, Larson DR, Crowson CS, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surgery Am. 2015;17:1386-1397. doi:10.2106/JBJS.N.01141
6. Giori NJ, Amanatullah DF, Gupta S, Bowe T, Harris AHS. Risk reduction compared with access to care: quantifying the trade-off of enforcing a body mass index eligibility criterion for joint replacement. J Bone Joint Surg Am. 2018; 4(100):539-545. doi:10.2106/JBJS.17.00120
7. Sabatino MJ, Kunkel ST, Ramkumar DB, Keeney BJ, Jevsevar DS. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100(3):180-188. doi:10.2106/JBJS.17.00672
8. Hadlandsmyth K, Vander Weg MW, McCoy KD, Mosher HJ, Vaughan-Sarrazin MS, Lund BC. Risk for prolonged opioid use following total knee arthroplasty in veterans. J Arthroplasty. 2018;33(1):119-123. doi:10.1016/j.arth.2017.08.022
9. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. doi:10.1001/jama.2011.370
10. Hall MJ, Schwartzman A, Zhang J, Liu X. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017(102):1-15.
11. Champely S. pwr: basic functions for power analysis. R package version 1.2-2; 2018. Accessed January 13, 2021. https://rdrr.io/cran/pwr/
12. Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi:10.1097/j.pain.0000000000000516
13. Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am. 2017;99(10):803-808. doi:10.2106/JBJS.16.01200
14. Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473(7):2402-412. doi:10.1007/s11999-015-4173-5
15. Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113-118. doi:10.1016/j.arth.2017.08.006
16. Ben-Ari A, Chansky H, Rozet I. Preoperative opioid use is associated with early revision after total knee arthroplasty: a study of male patients treated in the Veterans Affairs System. J Bone Joint Surg Am. 2017;99(1):1-9. doi:10.2106/JBJS.16.00167
17. Nguyen L-CL, Sing DC, Bozic KJ. Preoperative reduction of opioid use before total joint arthroplasty. J Arthroplasty. 2016;31(suppl 9):282-287. doi:10.1016/j.arth.2016.01.068
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157. doi:10.1016/j.jpain.2015.12.008
19. Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J. Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2016;6(2):e009857. doi:10.1136/bmjopen-2015-009857
20. Li W, Ayers DC, Lewis CG, Bowen TR, Allison JJ, Franklin PD. Functional gain and pain relief after total joint replacement according to obesity status. J Bone Joint Surg. 2017;99(14):1183-1189. doi:10.2106/JBJS.16.00960
21. Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology. 2017;126(5):923-937. doi:10.1097/ALN.0000000000001607
22. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111(6):1552-1554. doi:10.1213/ANE.0b013e3181fb9507
23. Elkassabany NM, Antosh S, Ahmed M, et al. The risk of falls after total knee arthroplasty with the use of a femoral nerve block versus an adductor canal block. Anest Analg. 2016;122(5):1696-1703. doi:10.1213/ane.0000000000001237
24. Wang D, Yang Y, Li Q, et al. Adductor canal block versus femoral nerve block for total knee arthroplasty: a meta-analysis of randomized controlled trials. Sci Rep. 2017;7:40721. doi:10.1038/srep40721
25. Liu D, Graham D, Gillies K, Gillies RM. Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res. 2014;26(4):207-213. doi:10.5792/ksrr.2014.26.4.207
26. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
27. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Thigh pain following tourniquet application in simultaneous bilateral total knee replacement arthroplasty. J Arthroplasty. 1997;12(8):848-852. doi:10.1016/s0883-5403(97)90153-4
28. Tai T-W, Lin C-J, Jou I-M, Chang C-W, Lai K-A, Yang C-Y. Tourniquet use in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol, Arthrosc. 2011;19(7):1121-1130. doi:10.1007/s00167-010-1342-7
29. Jiang F-Z, Zhong H-M, Hong Y-C, Zhao G-F. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20(21):110-123. doi:10.1007/s00776-014-0664-6
30. Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93(12):1577-1585. doi:10.1302/0301-620X.93B12.26989
31. Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20(5):300-309. doi:10.1016/j.knee.2013.05.014
32. Wang J, Wang Q, Zhang X, Wang Q. Intra-articular application is more effective than intravenous application of tranexamic acid in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2017;32(11):3385-3389. doi:10.1016/j.arth.2017.06.024
33. Guerreiro JPF, Badaro BS, Balbino JRM, Danieli MV, Queiroz AO, Cataneo DC. Application of tranexamic acid in total knee arthroplasty – prospective randomized trial. J Open Orthop J. 2017;11:1049-1057. doi:10.2174/1874325001711011049
Liquid Biopsies in a Veteran Patient Population With Advanced Prostate and Lung Non-Small Cell Carcinomas: A New Paradigm and Unique Challenge in Personalized Medicine
The advent of liquid biopsies targeting genetic mutations in solid tumors is a major milestone in the field of precision oncology.1 Conventional methods of obtaining tissue for molecular studies are limited by sample size and often do not represent the entire bulk of the tumor.2 This newer minimally invasive, revolutionary technique analyzes circulating cell-free DNA carrying tumor-specific alterations (circulating tumor DNA [ctDNA]) in peripheral blood and detects signature genomic alterations.1 Tp53 mutations have been reported in 25 to 40% of prostatic cancers and > 50% of non-small cell lung cancers (NSCLC), being more common in late-stage and hormone refractory prostate cancers.3,4 Tp53 mutation has been found to be associated with poor prognosis and increased germline mutations.5
The veteran patient population has distinct demographic characteristics that make veterans more vulnerable to genetic mutations and malignancies, including risk of exposure to Agent Orange, smoking, substance abuse, and asbestos. This area is understudied and extremely sparse in the literature for frequency of genetic mutations, risk factors in solid malignancies occurring in the veteran patient population, and the clinical impact of these risk factors. We herein present a quality assurance study for the utility of liquid biopsies regarding the frequency of DNA damage repair (DDR) gene, Tp53, and androgen receptor (AR) mutations. The clinical impact in advanced lung and prostate cancers in the veteran patient population and frequency are the quality assurance observations that are the study endpoints.
Methods
We reviewed for quality assurance documentation from the Foundation Medicine (www.foundationmedicine.com) cancer biomarker tests on liquid biopsies performed at the Corporal Michael J. Crescenz Veteran Affairs Medical Center in Philadelphia, Pennsylvania from May 2019 to April 15, 2020. All biopsies were performed on cancers with biochemical, imaging or tissue evidence of advanced tumor progression. The testing was performed on advanced solid malignancies, including NSCLC, prostate adenocarcinoma, and metastatic colon cancer. Statistical data for adequacy; cases with notable mutations; frequency; and type of mutations of AR, DDR, and Tp53 were noted. General and specific risk factors associated with the veteran patient population were studied and matched with the type of mutations (Table 1).
Results
Thirty-one liquid biopsies were performed over this period—23 for prostate cancer, 7 for patients with lung cancer patients, and 1 for a patient with colon cancer. Of 31 cases, sensitivity/adequacy of liquid biopsy for genetic mutation was detected in 29 (93.5%) cases (Figure 1). Two inadequate biopsies (both from patients with prostate cancer) were excluded from the study, leaving 29 liquid biopsies with adequate ctDNA for analysis that were considered for further statistical purpose—21 prostate, 7 lung, and 1 colon cancer.
Multiple (common and different) genetic mutations were identified; however, our study subcategorized the mutations into the those that were related to prostate cancer, lung cancer, and some common mutations that occur in both cancers. Only the significant ones will be discussed in this review and equivocal result for AR is excluded from this study. Of the 21 prostate cancers, 4 (19.0%) had directed the targeted therapy to driver mutation (AR being most common in prostate cancer), while KRAS mutation, which was more common in lung cancer, was detected in 2/7 (28.6%) lung cancers. Mutations common to both cancer types were DDR gene mutations, which is a broad name for numerous genes including CDK12, ATM, and CHEK2.
Of all cases irrespective of the cancer type, 23/29 (79.3%) showed notable mutations. DDR gene mutations were found in 6 of 21 (28.5%) patients with prostate cancer and 8 of 23 (34.7%) patients with advanced prostate and lung cancers, indicating poor outcome and possible resistance to the current therapy. Of 23 patients showing mutations irrespective of the cancer type, 15 (65.2%) harbored Tp53 mutations, which is much more frequent in veteran patient population when compared with the literature. Fifteen of the 31 (48.4%) total patients were Vietnam War-era veterans who were potentially exposed to Agent Orange and 20 (64.5%) patients who were not Vietnam War-era veterans had a history that included smoking (Figure 2).
Discussion
The veteran patient population is a unique cohort due to its distinct demographic characteristics with a high volume of cancer cases diagnosed each year. According to data from VA Central Cancer Registry (VACCR), the most frequently diagnosed cancers are prostate (29%) and lung (18%).6
Liquid biopsy is a novel, promising technology that uses ctDNA and circulating tumor cells in peripheral blood for detecting genetic alterations through next generation sequencing.7-9 The advent of this minimally invasive, revolutionary technology has been a breakthrough in the field of precision oncology for prognosis, to monitor treatment response or resistance to therapy and further personalize cancer therapy.9,10
Comprehensive genomic profiling by liquid biopsy has many advantages over the molecular studies performed on tissue biopsy. Due to the tumor heterogeneity, tissue samples may not represent the full profile of the tumor genomics of cancer, while liquid biopsy has full presentation of the disease.11,12 Many times, tissue biopsy may be limited by a sample size that precludes full genetic profiling in addition to higher total cost, potential technical issues during processing, and possible side effects of the biopsy procedure.7,13 Additionally, as the tumor progresses, new driver mutations other than the ones previously detected on the primary tissue may emerge, which can confer resistance to the existing therapy.7,13
Advanced prostatic and lung carcinomas with biochemical, distant organ, or bony progression harbor unique signature genetic mutations indicating poor prognosis, lack of response or resistance to the existing therapy, and high risk of relapse.14,15 Some of the unique characteristics of the veteran patient population include a more aged patient population multiple comorbidities, higher frequency of > 1 type of cancer, advanced cancer stage at presentation, and specific risks factors such as exposure to Agent Orange in veterans who served during the Vietnam War era.16,17 We studied the utility of liquid biopsy in cancer care, including type and incidence of genomic alterations associated with advanced prostate and lung cancers, in this unique patient population.
The amount of cell-free DNA (cfDNA), also known as ctDNA varies widely in cancer patients. Some of the factors associated with low concentration of cfDNA are disease stage, intervening therapy, proliferation rates, and tumor vascularization.18,19 In the peripheral blood, of the total cfDNA, fractions of cfDNA varies from 0.01 to 90%.18,19 All samples containing ≥ 20 ng cfDNA (20 - 100 ng) were subjected to the hybrid capture-based NGS FoundationACT assay.20 In our study, 2 specimens did not meet the minimum criteria of adequacy (20 ng cfDNA); however, the overall adequacy rate for the detection of mutation, irrespective of the cancer type was 29 of 31 (93.5%) with only 2 inadequate samples. This rate is higher than the rate reported in the literature, which is about 70%.20
Significant differences were encountered in the incidence of DNA damage repair genes including Tp53 mutations when compared with those in the general patient population (Table 2). According to recent National Comprehensive Cancer Network (NCCN) guidelines, all prostate cancers should be screened for DDR gene mutations as these genes are common in aggressive prostate cancers and strongly associated with poor outcomes and shortened survival. Due to relatively high frequency of DDR gene mutations in advanced prostatic cancers, liquid biopsy in patients with these advanced stage prostate cancers may be a useful tool in clinical decision making and exploring targeted therapy.20
Mutations in BRCA2, ATM, CDK12, and CHEK2 (DDR gene family) are common. Incidence of ATM and CDK12 mutations in the literature is 3 to 6% of cases.21 Of 21 liquid biopsies of advanced prostate cancer patients, we found combined DDR gene mutation of ATM, CHEK2, and CDK12 genes in 6 (28.5%) cases, which is substantially higher than the 3 to 6% rate reported in the literature.21-24 Of the 23 patients who had notable mutations in our liquid biopsies, including both advanced prostate and lung cancer cases, 8 (34.7%) also showed mutation of the genes of DDR family. Our study did not show BRCA2 mutation, which is otherwise common in the literature.
We also evaluated the frequency of the most commonly occurring genetic mutations, Tp53 in advanced solid malignancies, especially advanced prostate and NSCLC. Previous studies have reported Tp53 mutation in association with risk factors (carcinogens) of cancer and have been a surrogate marker of poor survival or lack of response of therapy.25 Knowledge of Tp53 mutation is crucial for closer disease monitoring, preparing the patient for rapid progression, and encouraging the physician to prepare future lines of therapy.25-27 Although Tp53 mutation varies with histologic type and tissue of origin, Beltran and colleagues reported it in 30 to 40% of tumors, while Robles and colleagues reported about 40 to 42% incidence.25,27
Our study showed notable mutations in 23 of 29 adequate cases. Further, our study showed a high frequency of mutated Tp53 in 65.2% of combined advanced prostate and NSCLC cases. We then correlated cases of Vietnam War-era veterans with risk potential of Agent Orange exposure and Tp53 mutation. We found 7 of 15 Vietnam War-era veterans were positive for Tp53 mutations irrespective of the cancer type. The high incidence of Tp53 mutations in advanced prostate and lung carcinomas in the veteran patient population makes this tumor marker an aspiration not only as a surrogate of aggressive disease and tumor progression, but also as a key marker for targeted therapy in advanced prostate and lung cancers with loss of Tp53 function (Figure 3).
Mutations and amplifications in the AR gene are fundamental to progression of prostate cancer associated with advanced, hormone-refractory prostate cancer with the potential for targeted therapy with AR inhibitors. In our study, AR amplification was detected in 4 of 21 (19%) advanced prostate cancer cases, which is significantly lower than the 30 to 50% previously reported in the literature.28-32 Neither AR amplification or mutation was noted in advanced NSCLC in our study as previously reported in literature by Brennan and colleagues and Wang and colleagues.33-35 This is significant as it provides a pathway for future studies to focus on additional driver mutations for targeted therapies in advanced prostate carcinoma. To date, AR gene mutation does not play a role for personalized therapy in advanced NSCLC. Perhaps, a large cohort study with longitudinal analysis is needed for absolutely ruling out the possibility of personalized medicine in advanced lung cancer using this biomarker.
Conclusions
Liquid biopsy successfully provides precision-based oncology and information for decision making in this unique population of veterans. Difference in frequency of the genetic mutations in this cohort can provide future insight into disease progression, lack of response, and mechanism of resistance to the implemented therapy. Future studies focused on this veteran patient population are needed for developing targeted therapies and patient tailored oncologic therapy. ctDNA has a high potential for monitoring clinically relevant cancer-related genetic and epigenetic modifications for discovering more detailed information on the tumor characterization. Although larger cohort trial with longitudinal analyses are needed, high prevalence of DDR gene and Tp53 mutation in our study instills promising hope for therapeutic interventions in this unique cohort.
The minimally invasive liquid biopsy shows a great promise as both diagnostic and prognostic tool in the personalized clinical management of advanced prostate, and NSCLC in the veteran patient population with unique demographic characteristics. De novo metastatic prostate cancer is more common in veterans when compared with the general population, and therefore veterans may benefit by liquid biopsy. Differences in the frequency of genetic mutations (DDR, TP53, AR) in this cohort provides valuable information for disease progression, lack of response, mechanism of resistance to the implemented therapy and clinical decision making. Precision oncology can be further tailored for this cohort by focusing on DNA repair genes and Tp53 mutations for future targeted therapy.
1
9
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Fourth Biennial Update). Veterans and Agent Orange: Update 2002. National Academies Press (US); 2003.
17. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
18. Saarenheimo J, Eigeliene N, Andersen H, Tiirola M, Jekunen A. The value of liquid biopsies for guiding therapy decisions in non-small cell lung cancer. Front Oncol. 2019;9:129. Published 2019 Mar 5.doi:10.3389/fonc.2019.00129
19
20
21
22
23
24
25
26
27
28
29
30
31. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149-2156. doi:10.1200/JCO.2016.70.1961
32

33. Jung A, Kirchner T. Liquid biopsy in tumor genetic diagnosis. Dtsch Arztebl Int. 2018;115(10):169-174. doi:10.3238/arztebl.2018.0169
34. Brennan S, Wang AR, Beyer H, et al. Androgen receptor as a potential target in non-small cell lung cancer. Cancer Res. 2017;77(Suppl13): abstract nr 4121. doi:10.1158/1538-7445.AM2017-4121
35. Wang AR, Beyer H, Brennan S, et al. Androgen receptor drives differential gene expression in KRAS-mediated non-small cell lung cancer. Cancer Res. 2018;78(Suppl 13): abstract nr 3946. doi:10.1158/1538-7445.AM2018-3946
The advent of liquid biopsies targeting genetic mutations in solid tumors is a major milestone in the field of precision oncology.1 Conventional methods of obtaining tissue for molecular studies are limited by sample size and often do not represent the entire bulk of the tumor.2 This newer minimally invasive, revolutionary technique analyzes circulating cell-free DNA carrying tumor-specific alterations (circulating tumor DNA [ctDNA]) in peripheral blood and detects signature genomic alterations.1 Tp53 mutations have been reported in 25 to 40% of prostatic cancers and > 50% of non-small cell lung cancers (NSCLC), being more common in late-stage and hormone refractory prostate cancers.3,4 Tp53 mutation has been found to be associated with poor prognosis and increased germline mutations.5
The veteran patient population has distinct demographic characteristics that make veterans more vulnerable to genetic mutations and malignancies, including risk of exposure to Agent Orange, smoking, substance abuse, and asbestos. This area is understudied and extremely sparse in the literature for frequency of genetic mutations, risk factors in solid malignancies occurring in the veteran patient population, and the clinical impact of these risk factors. We herein present a quality assurance study for the utility of liquid biopsies regarding the frequency of DNA damage repair (DDR) gene, Tp53, and androgen receptor (AR) mutations. The clinical impact in advanced lung and prostate cancers in the veteran patient population and frequency are the quality assurance observations that are the study endpoints.
Methods
We reviewed for quality assurance documentation from the Foundation Medicine (www.foundationmedicine.com) cancer biomarker tests on liquid biopsies performed at the Corporal Michael J. Crescenz Veteran Affairs Medical Center in Philadelphia, Pennsylvania from May 2019 to April 15, 2020. All biopsies were performed on cancers with biochemical, imaging or tissue evidence of advanced tumor progression. The testing was performed on advanced solid malignancies, including NSCLC, prostate adenocarcinoma, and metastatic colon cancer. Statistical data for adequacy; cases with notable mutations; frequency; and type of mutations of AR, DDR, and Tp53 were noted. General and specific risk factors associated with the veteran patient population were studied and matched with the type of mutations (Table 1).
Results
Thirty-one liquid biopsies were performed over this period—23 for prostate cancer, 7 for patients with lung cancer patients, and 1 for a patient with colon cancer. Of 31 cases, sensitivity/adequacy of liquid biopsy for genetic mutation was detected in 29 (93.5%) cases (Figure 1). Two inadequate biopsies (both from patients with prostate cancer) were excluded from the study, leaving 29 liquid biopsies with adequate ctDNA for analysis that were considered for further statistical purpose—21 prostate, 7 lung, and 1 colon cancer.
Multiple (common and different) genetic mutations were identified; however, our study subcategorized the mutations into the those that were related to prostate cancer, lung cancer, and some common mutations that occur in both cancers. Only the significant ones will be discussed in this review and equivocal result for AR is excluded from this study. Of the 21 prostate cancers, 4 (19.0%) had directed the targeted therapy to driver mutation (AR being most common in prostate cancer), while KRAS mutation, which was more common in lung cancer, was detected in 2/7 (28.6%) lung cancers. Mutations common to both cancer types were DDR gene mutations, which is a broad name for numerous genes including CDK12, ATM, and CHEK2.
Of all cases irrespective of the cancer type, 23/29 (79.3%) showed notable mutations. DDR gene mutations were found in 6 of 21 (28.5%) patients with prostate cancer and 8 of 23 (34.7%) patients with advanced prostate and lung cancers, indicating poor outcome and possible resistance to the current therapy. Of 23 patients showing mutations irrespective of the cancer type, 15 (65.2%) harbored Tp53 mutations, which is much more frequent in veteran patient population when compared with the literature. Fifteen of the 31 (48.4%) total patients were Vietnam War-era veterans who were potentially exposed to Agent Orange and 20 (64.5%) patients who were not Vietnam War-era veterans had a history that included smoking (Figure 2).
Discussion
The veteran patient population is a unique cohort due to its distinct demographic characteristics with a high volume of cancer cases diagnosed each year. According to data from VA Central Cancer Registry (VACCR), the most frequently diagnosed cancers are prostate (29%) and lung (18%).6
Liquid biopsy is a novel, promising technology that uses ctDNA and circulating tumor cells in peripheral blood for detecting genetic alterations through next generation sequencing.7-9 The advent of this minimally invasive, revolutionary technology has been a breakthrough in the field of precision oncology for prognosis, to monitor treatment response or resistance to therapy and further personalize cancer therapy.9,10
Comprehensive genomic profiling by liquid biopsy has many advantages over the molecular studies performed on tissue biopsy. Due to the tumor heterogeneity, tissue samples may not represent the full profile of the tumor genomics of cancer, while liquid biopsy has full presentation of the disease.11,12 Many times, tissue biopsy may be limited by a sample size that precludes full genetic profiling in addition to higher total cost, potential technical issues during processing, and possible side effects of the biopsy procedure.7,13 Additionally, as the tumor progresses, new driver mutations other than the ones previously detected on the primary tissue may emerge, which can confer resistance to the existing therapy.7,13
Advanced prostatic and lung carcinomas with biochemical, distant organ, or bony progression harbor unique signature genetic mutations indicating poor prognosis, lack of response or resistance to the existing therapy, and high risk of relapse.14,15 Some of the unique characteristics of the veteran patient population include a more aged patient population multiple comorbidities, higher frequency of > 1 type of cancer, advanced cancer stage at presentation, and specific risks factors such as exposure to Agent Orange in veterans who served during the Vietnam War era.16,17 We studied the utility of liquid biopsy in cancer care, including type and incidence of genomic alterations associated with advanced prostate and lung cancers, in this unique patient population.
The amount of cell-free DNA (cfDNA), also known as ctDNA varies widely in cancer patients. Some of the factors associated with low concentration of cfDNA are disease stage, intervening therapy, proliferation rates, and tumor vascularization.18,19 In the peripheral blood, of the total cfDNA, fractions of cfDNA varies from 0.01 to 90%.18,19 All samples containing ≥ 20 ng cfDNA (20 - 100 ng) were subjected to the hybrid capture-based NGS FoundationACT assay.20 In our study, 2 specimens did not meet the minimum criteria of adequacy (20 ng cfDNA); however, the overall adequacy rate for the detection of mutation, irrespective of the cancer type was 29 of 31 (93.5%) with only 2 inadequate samples. This rate is higher than the rate reported in the literature, which is about 70%.20
Significant differences were encountered in the incidence of DNA damage repair genes including Tp53 mutations when compared with those in the general patient population (Table 2). According to recent National Comprehensive Cancer Network (NCCN) guidelines, all prostate cancers should be screened for DDR gene mutations as these genes are common in aggressive prostate cancers and strongly associated with poor outcomes and shortened survival. Due to relatively high frequency of DDR gene mutations in advanced prostatic cancers, liquid biopsy in patients with these advanced stage prostate cancers may be a useful tool in clinical decision making and exploring targeted therapy.20
Mutations in BRCA2, ATM, CDK12, and CHEK2 (DDR gene family) are common. Incidence of ATM and CDK12 mutations in the literature is 3 to 6% of cases.21 Of 21 liquid biopsies of advanced prostate cancer patients, we found combined DDR gene mutation of ATM, CHEK2, and CDK12 genes in 6 (28.5%) cases, which is substantially higher than the 3 to 6% rate reported in the literature.21-24 Of the 23 patients who had notable mutations in our liquid biopsies, including both advanced prostate and lung cancer cases, 8 (34.7%) also showed mutation of the genes of DDR family. Our study did not show BRCA2 mutation, which is otherwise common in the literature.
We also evaluated the frequency of the most commonly occurring genetic mutations, Tp53 in advanced solid malignancies, especially advanced prostate and NSCLC. Previous studies have reported Tp53 mutation in association with risk factors (carcinogens) of cancer and have been a surrogate marker of poor survival or lack of response of therapy.25 Knowledge of Tp53 mutation is crucial for closer disease monitoring, preparing the patient for rapid progression, and encouraging the physician to prepare future lines of therapy.25-27 Although Tp53 mutation varies with histologic type and tissue of origin, Beltran and colleagues reported it in 30 to 40% of tumors, while Robles and colleagues reported about 40 to 42% incidence.25,27
Our study showed notable mutations in 23 of 29 adequate cases. Further, our study showed a high frequency of mutated Tp53 in 65.2% of combined advanced prostate and NSCLC cases. We then correlated cases of Vietnam War-era veterans with risk potential of Agent Orange exposure and Tp53 mutation. We found 7 of 15 Vietnam War-era veterans were positive for Tp53 mutations irrespective of the cancer type. The high incidence of Tp53 mutations in advanced prostate and lung carcinomas in the veteran patient population makes this tumor marker an aspiration not only as a surrogate of aggressive disease and tumor progression, but also as a key marker for targeted therapy in advanced prostate and lung cancers with loss of Tp53 function (Figure 3).
Mutations and amplifications in the AR gene are fundamental to progression of prostate cancer associated with advanced, hormone-refractory prostate cancer with the potential for targeted therapy with AR inhibitors. In our study, AR amplification was detected in 4 of 21 (19%) advanced prostate cancer cases, which is significantly lower than the 30 to 50% previously reported in the literature.28-32 Neither AR amplification or mutation was noted in advanced NSCLC in our study as previously reported in literature by Brennan and colleagues and Wang and colleagues.33-35 This is significant as it provides a pathway for future studies to focus on additional driver mutations for targeted therapies in advanced prostate carcinoma. To date, AR gene mutation does not play a role for personalized therapy in advanced NSCLC. Perhaps, a large cohort study with longitudinal analysis is needed for absolutely ruling out the possibility of personalized medicine in advanced lung cancer using this biomarker.
Conclusions
Liquid biopsy successfully provides precision-based oncology and information for decision making in this unique population of veterans. Difference in frequency of the genetic mutations in this cohort can provide future insight into disease progression, lack of response, and mechanism of resistance to the implemented therapy. Future studies focused on this veteran patient population are needed for developing targeted therapies and patient tailored oncologic therapy. ctDNA has a high potential for monitoring clinically relevant cancer-related genetic and epigenetic modifications for discovering more detailed information on the tumor characterization. Although larger cohort trial with longitudinal analyses are needed, high prevalence of DDR gene and Tp53 mutation in our study instills promising hope for therapeutic interventions in this unique cohort.
The minimally invasive liquid biopsy shows a great promise as both diagnostic and prognostic tool in the personalized clinical management of advanced prostate, and NSCLC in the veteran patient population with unique demographic characteristics. De novo metastatic prostate cancer is more common in veterans when compared with the general population, and therefore veterans may benefit by liquid biopsy. Differences in the frequency of genetic mutations (DDR, TP53, AR) in this cohort provides valuable information for disease progression, lack of response, mechanism of resistance to the implemented therapy and clinical decision making. Precision oncology can be further tailored for this cohort by focusing on DNA repair genes and Tp53 mutations for future targeted therapy.
The advent of liquid biopsies targeting genetic mutations in solid tumors is a major milestone in the field of precision oncology.1 Conventional methods of obtaining tissue for molecular studies are limited by sample size and often do not represent the entire bulk of the tumor.2 This newer minimally invasive, revolutionary technique analyzes circulating cell-free DNA carrying tumor-specific alterations (circulating tumor DNA [ctDNA]) in peripheral blood and detects signature genomic alterations.1 Tp53 mutations have been reported in 25 to 40% of prostatic cancers and > 50% of non-small cell lung cancers (NSCLC), being more common in late-stage and hormone refractory prostate cancers.3,4 Tp53 mutation has been found to be associated with poor prognosis and increased germline mutations.5
The veteran patient population has distinct demographic characteristics that make veterans more vulnerable to genetic mutations and malignancies, including risk of exposure to Agent Orange, smoking, substance abuse, and asbestos. This area is understudied and extremely sparse in the literature for frequency of genetic mutations, risk factors in solid malignancies occurring in the veteran patient population, and the clinical impact of these risk factors. We herein present a quality assurance study for the utility of liquid biopsies regarding the frequency of DNA damage repair (DDR) gene, Tp53, and androgen receptor (AR) mutations. The clinical impact in advanced lung and prostate cancers in the veteran patient population and frequency are the quality assurance observations that are the study endpoints.
Methods
We reviewed for quality assurance documentation from the Foundation Medicine (www.foundationmedicine.com) cancer biomarker tests on liquid biopsies performed at the Corporal Michael J. Crescenz Veteran Affairs Medical Center in Philadelphia, Pennsylvania from May 2019 to April 15, 2020. All biopsies were performed on cancers with biochemical, imaging or tissue evidence of advanced tumor progression. The testing was performed on advanced solid malignancies, including NSCLC, prostate adenocarcinoma, and metastatic colon cancer. Statistical data for adequacy; cases with notable mutations; frequency; and type of mutations of AR, DDR, and Tp53 were noted. General and specific risk factors associated with the veteran patient population were studied and matched with the type of mutations (Table 1).
Results
Thirty-one liquid biopsies were performed over this period—23 for prostate cancer, 7 for patients with lung cancer patients, and 1 for a patient with colon cancer. Of 31 cases, sensitivity/adequacy of liquid biopsy for genetic mutation was detected in 29 (93.5%) cases (Figure 1). Two inadequate biopsies (both from patients with prostate cancer) were excluded from the study, leaving 29 liquid biopsies with adequate ctDNA for analysis that were considered for further statistical purpose—21 prostate, 7 lung, and 1 colon cancer.
Multiple (common and different) genetic mutations were identified; however, our study subcategorized the mutations into the those that were related to prostate cancer, lung cancer, and some common mutations that occur in both cancers. Only the significant ones will be discussed in this review and equivocal result for AR is excluded from this study. Of the 21 prostate cancers, 4 (19.0%) had directed the targeted therapy to driver mutation (AR being most common in prostate cancer), while KRAS mutation, which was more common in lung cancer, was detected in 2/7 (28.6%) lung cancers. Mutations common to both cancer types were DDR gene mutations, which is a broad name for numerous genes including CDK12, ATM, and CHEK2.
Of all cases irrespective of the cancer type, 23/29 (79.3%) showed notable mutations. DDR gene mutations were found in 6 of 21 (28.5%) patients with prostate cancer and 8 of 23 (34.7%) patients with advanced prostate and lung cancers, indicating poor outcome and possible resistance to the current therapy. Of 23 patients showing mutations irrespective of the cancer type, 15 (65.2%) harbored Tp53 mutations, which is much more frequent in veteran patient population when compared with the literature. Fifteen of the 31 (48.4%) total patients were Vietnam War-era veterans who were potentially exposed to Agent Orange and 20 (64.5%) patients who were not Vietnam War-era veterans had a history that included smoking (Figure 2).
Discussion
The veteran patient population is a unique cohort due to its distinct demographic characteristics with a high volume of cancer cases diagnosed each year. According to data from VA Central Cancer Registry (VACCR), the most frequently diagnosed cancers are prostate (29%) and lung (18%).6
Liquid biopsy is a novel, promising technology that uses ctDNA and circulating tumor cells in peripheral blood for detecting genetic alterations through next generation sequencing.7-9 The advent of this minimally invasive, revolutionary technology has been a breakthrough in the field of precision oncology for prognosis, to monitor treatment response or resistance to therapy and further personalize cancer therapy.9,10
Comprehensive genomic profiling by liquid biopsy has many advantages over the molecular studies performed on tissue biopsy. Due to the tumor heterogeneity, tissue samples may not represent the full profile of the tumor genomics of cancer, while liquid biopsy has full presentation of the disease.11,12 Many times, tissue biopsy may be limited by a sample size that precludes full genetic profiling in addition to higher total cost, potential technical issues during processing, and possible side effects of the biopsy procedure.7,13 Additionally, as the tumor progresses, new driver mutations other than the ones previously detected on the primary tissue may emerge, which can confer resistance to the existing therapy.7,13
Advanced prostatic and lung carcinomas with biochemical, distant organ, or bony progression harbor unique signature genetic mutations indicating poor prognosis, lack of response or resistance to the existing therapy, and high risk of relapse.14,15 Some of the unique characteristics of the veteran patient population include a more aged patient population multiple comorbidities, higher frequency of > 1 type of cancer, advanced cancer stage at presentation, and specific risks factors such as exposure to Agent Orange in veterans who served during the Vietnam War era.16,17 We studied the utility of liquid biopsy in cancer care, including type and incidence of genomic alterations associated with advanced prostate and lung cancers, in this unique patient population.
The amount of cell-free DNA (cfDNA), also known as ctDNA varies widely in cancer patients. Some of the factors associated with low concentration of cfDNA are disease stage, intervening therapy, proliferation rates, and tumor vascularization.18,19 In the peripheral blood, of the total cfDNA, fractions of cfDNA varies from 0.01 to 90%.18,19 All samples containing ≥ 20 ng cfDNA (20 - 100 ng) were subjected to the hybrid capture-based NGS FoundationACT assay.20 In our study, 2 specimens did not meet the minimum criteria of adequacy (20 ng cfDNA); however, the overall adequacy rate for the detection of mutation, irrespective of the cancer type was 29 of 31 (93.5%) with only 2 inadequate samples. This rate is higher than the rate reported in the literature, which is about 70%.20
Significant differences were encountered in the incidence of DNA damage repair genes including Tp53 mutations when compared with those in the general patient population (Table 2). According to recent National Comprehensive Cancer Network (NCCN) guidelines, all prostate cancers should be screened for DDR gene mutations as these genes are common in aggressive prostate cancers and strongly associated with poor outcomes and shortened survival. Due to relatively high frequency of DDR gene mutations in advanced prostatic cancers, liquid biopsy in patients with these advanced stage prostate cancers may be a useful tool in clinical decision making and exploring targeted therapy.20
Mutations in BRCA2, ATM, CDK12, and CHEK2 (DDR gene family) are common. Incidence of ATM and CDK12 mutations in the literature is 3 to 6% of cases.21 Of 21 liquid biopsies of advanced prostate cancer patients, we found combined DDR gene mutation of ATM, CHEK2, and CDK12 genes in 6 (28.5%) cases, which is substantially higher than the 3 to 6% rate reported in the literature.21-24 Of the 23 patients who had notable mutations in our liquid biopsies, including both advanced prostate and lung cancer cases, 8 (34.7%) also showed mutation of the genes of DDR family. Our study did not show BRCA2 mutation, which is otherwise common in the literature.
We also evaluated the frequency of the most commonly occurring genetic mutations, Tp53 in advanced solid malignancies, especially advanced prostate and NSCLC. Previous studies have reported Tp53 mutation in association with risk factors (carcinogens) of cancer and have been a surrogate marker of poor survival or lack of response of therapy.25 Knowledge of Tp53 mutation is crucial for closer disease monitoring, preparing the patient for rapid progression, and encouraging the physician to prepare future lines of therapy.25-27 Although Tp53 mutation varies with histologic type and tissue of origin, Beltran and colleagues reported it in 30 to 40% of tumors, while Robles and colleagues reported about 40 to 42% incidence.25,27
Our study showed notable mutations in 23 of 29 adequate cases. Further, our study showed a high frequency of mutated Tp53 in 65.2% of combined advanced prostate and NSCLC cases. We then correlated cases of Vietnam War-era veterans with risk potential of Agent Orange exposure and Tp53 mutation. We found 7 of 15 Vietnam War-era veterans were positive for Tp53 mutations irrespective of the cancer type. The high incidence of Tp53 mutations in advanced prostate and lung carcinomas in the veteran patient population makes this tumor marker an aspiration not only as a surrogate of aggressive disease and tumor progression, but also as a key marker for targeted therapy in advanced prostate and lung cancers with loss of Tp53 function (Figure 3).
Mutations and amplifications in the AR gene are fundamental to progression of prostate cancer associated with advanced, hormone-refractory prostate cancer with the potential for targeted therapy with AR inhibitors. In our study, AR amplification was detected in 4 of 21 (19%) advanced prostate cancer cases, which is significantly lower than the 30 to 50% previously reported in the literature.28-32 Neither AR amplification or mutation was noted in advanced NSCLC in our study as previously reported in literature by Brennan and colleagues and Wang and colleagues.33-35 This is significant as it provides a pathway for future studies to focus on additional driver mutations for targeted therapies in advanced prostate carcinoma. To date, AR gene mutation does not play a role for personalized therapy in advanced NSCLC. Perhaps, a large cohort study with longitudinal analysis is needed for absolutely ruling out the possibility of personalized medicine in advanced lung cancer using this biomarker.
Conclusions
Liquid biopsy successfully provides precision-based oncology and information for decision making in this unique population of veterans. Difference in frequency of the genetic mutations in this cohort can provide future insight into disease progression, lack of response, and mechanism of resistance to the implemented therapy. Future studies focused on this veteran patient population are needed for developing targeted therapies and patient tailored oncologic therapy. ctDNA has a high potential for monitoring clinically relevant cancer-related genetic and epigenetic modifications for discovering more detailed information on the tumor characterization. Although larger cohort trial with longitudinal analyses are needed, high prevalence of DDR gene and Tp53 mutation in our study instills promising hope for therapeutic interventions in this unique cohort.
The minimally invasive liquid biopsy shows a great promise as both diagnostic and prognostic tool in the personalized clinical management of advanced prostate, and NSCLC in the veteran patient population with unique demographic characteristics. De novo metastatic prostate cancer is more common in veterans when compared with the general population, and therefore veterans may benefit by liquid biopsy. Differences in the frequency of genetic mutations (DDR, TP53, AR) in this cohort provides valuable information for disease progression, lack of response, mechanism of resistance to the implemented therapy and clinical decision making. Precision oncology can be further tailored for this cohort by focusing on DNA repair genes and Tp53 mutations for future targeted therapy.
1
9
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Fourth Biennial Update). Veterans and Agent Orange: Update 2002. National Academies Press (US); 2003.
17. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
18. Saarenheimo J, Eigeliene N, Andersen H, Tiirola M, Jekunen A. The value of liquid biopsies for guiding therapy decisions in non-small cell lung cancer. Front Oncol. 2019;9:129. Published 2019 Mar 5.doi:10.3389/fonc.2019.00129
19
20
21
22
23
24
25
26
27
28
29
30
31. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149-2156. doi:10.1200/JCO.2016.70.1961
32

33. Jung A, Kirchner T. Liquid biopsy in tumor genetic diagnosis. Dtsch Arztebl Int. 2018;115(10):169-174. doi:10.3238/arztebl.2018.0169
34. Brennan S, Wang AR, Beyer H, et al. Androgen receptor as a potential target in non-small cell lung cancer. Cancer Res. 2017;77(Suppl13): abstract nr 4121. doi:10.1158/1538-7445.AM2017-4121
35. Wang AR, Beyer H, Brennan S, et al. Androgen receptor drives differential gene expression in KRAS-mediated non-small cell lung cancer. Cancer Res. 2018;78(Suppl 13): abstract nr 3946. doi:10.1158/1538-7445.AM2018-3946
1
9
16. Institute of Medicine (US) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides (Fourth Biennial Update). Veterans and Agent Orange: Update 2002. National Academies Press (US); 2003.
17. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
18. Saarenheimo J, Eigeliene N, Andersen H, Tiirola M, Jekunen A. The value of liquid biopsies for guiding therapy decisions in non-small cell lung cancer. Front Oncol. 2019;9:129. Published 2019 Mar 5.doi:10.3389/fonc.2019.00129
19
20
21
22
23
24
25
26
27
28
29
30
31. Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149-2156. doi:10.1200/JCO.2016.70.1961
32

33. Jung A, Kirchner T. Liquid biopsy in tumor genetic diagnosis. Dtsch Arztebl Int. 2018;115(10):169-174. doi:10.3238/arztebl.2018.0169
34. Brennan S, Wang AR, Beyer H, et al. Androgen receptor as a potential target in non-small cell lung cancer. Cancer Res. 2017;77(Suppl13): abstract nr 4121. doi:10.1158/1538-7445.AM2017-4121
35. Wang AR, Beyer H, Brennan S, et al. Androgen receptor drives differential gene expression in KRAS-mediated non-small cell lung cancer. Cancer Res. 2018;78(Suppl 13): abstract nr 3946. doi:10.1158/1538-7445.AM2018-3946
Standardizing the Use of Mental Health Screening Instruments in Patients With Pain (FULL)
Chronic pain is more prevalent in the US than diabetes mellitus, cancer, and cardiovascular disease combined, impacting about 100 million adults.1 The annual cost of all that pain in the US is between $560 and $635 billion.1
The high prevalence of chronic pain among active duty service members and veterans remains a pressing concern given its negative impact on military readiness, health care utilization, productivity, quality of life, and chronic disability rates.2 Pain was found to be the leading complaint of service members returning from Operations Iraqi Freedom and Enduring Freedomand 44% of veterans returning from deployment suffered with chronic pain.3,4
Chronic pain often occurs in the presence of comorbidities. In one study for example, 45% of primary care patients with chronic pain (N = 250) screened positive for ≥ 1 of the 5 types of common anxiety disorders, and those with anxiety disorder had higher pain scores.5 Another study involving almost 6000 participants found that anxiety disorders were present in 35% of people with chronic pain compared with 18% in the general population.6
In addition, military members are prone to depression with a rate of major depressive disorder that is 5% higher than that of civilians.7 Depression often is underdiagnosed and undertreated. According to a National Center for Health Statistics, only 35% of those with symptoms of severe depression in the US saw a mental health provider in the previous year.8 Comorbid depression, anxiety, and chronic pain are strongly associated with more severe pain, greater disability, and poorer health-related quality of life.9
As a result, there was a call for system-level interventions to increase access to, and continuity of, mental health care services for active duty service members and veterans.1 It has been recommended that depression and anxiety screenings take place in primary and secondary care clinics.10 Standardized referral processes also are needed to enhance mental health diagnosis and referral techniques.11 Although various screening tools are available that have excellent reliability and construct validity (eg, General Anxiety Disorder-7 [GAD-7], Patient Health Questionnaire-9 [PHQ-9]), they are underutilized.12 I have witnessed a noticeable gap between clinical practice guidelines and current practice associated with chronic pain and screening for anxiety and depression within the Pain Management Clinic at Navy Medical Center of Camp Lejeune (NMCCL) in North Carolina.
Methods
The premise of this performance improvement (PI) project was to reduce missed opportunities of screening for anxiety and depression, and to examine the impact of the standardized use of the GAD-7 and PHQ-9 on the rate of mental health care referrals. The Theory of Unpleasant Symptoms was chosen as the underpinning of the project because it suggests that symptoms often cluster, and that the occurrence of multiple symptoms makes each of those, as well as other symptoms, worse.13 The PI model used the find, organize, clarify, understand, select (FOCUS), and plan, do, check, act (PDCA) models.14 The facility institutional review board ruled that this performance improvement project did not qualify as human research.
Inclusion and exclusion criteria
Patients were included if they were active duty service members aged 18 to 56 years at the initial patient encounter. Veterans and dependents were not part of the sample because of the high clinic volume. Patients who received mental health care services within the previous 90 days were excluded.
Registered nurses, licensed practical nurses, US Navy corpsman, medical assistants, and nurse aides were educated on the purpose of the GAD-7 and PHQ-9 and were instructed to have patients complete them upon every new patient encounter. A retrospective chart review was conducted over a 6-week time frame to collect and analyze de-identified demographic data including age, gender, prior deployment (yes or no), and branch of service. The review also examined whether the patient had received mental health care services, whether the screening instruments were completed, and whether a mental health referral was made. The clinic providers were asked to consider mental health care referrals for patients who scored ≥ 10 on either the GAD-7 or PHQ-9. The frequency of the use of the instruments and the number of mental health referrals made was calculated during the 3-week period before and after the standardized use of the instruments. The author conducted audits of the new patient charts at the end of each work day to assess whether the GAD-7 and PHQ-9 were completed.
Results
There were 117 new patient encounters during the 6-week project period. Thirty-three patients were excluded from the sample, leaving a remaining sample of 84. Thirty-two patients were included in the sample prior to the standardized use of the instruments, and 52 were included afterward (Table).
Prior to the standardized use of the screening tools, the GAD-7 was used during 75% of patient visits for pain and the PHQ-9 was used during 25%, reinforcing the premise of unpredictable utilization of the screening tools. Three mental health referrals were made during the 3-week period prior to the standardized use of the anxiety and depression instruments (3/32, 10%). After the standardized implementation of the GAD-7 and PHQ-9 tools, both instruments were used 98% of the time, and mental health referrals were made for 12 of 52 patients (23.1%). Eleven of the referrals were made based upon the trigger score of 10 on either the GAD-7 or PHQ-9. One referral was made for a patient with a score of 9 on the PHQ-9 because the provider determined a need for pain-related psychological services.
It was important to provide a link to mental health care because, as one study found, patients with a specific anxiety diagnosis are much more likely than those diagnosed with a not otherwise specified anxiety disorder to receive mental health care services (60% to 67% vs 37%).11 Similarly, patients diagnosed in specialty mental health care settings are more likely to receive mental health services than are those diagnosed in primary care.11 By the same token, experts estimate that 50% of those with severe depression symptoms are not properly diagnosed or treated in primary care.15
Strengths and Limitations
Utilization of the screening tools has led to further dialogue between patients and providers that anecdotally revealed suicidal ideation in some patients. Future studies could incorporate a qualitative component to include clinician and patient perceptions of mental health care services.
The study was limited by the lack of follow-up data to determine the effect of mental health care services on pain, function, or military readiness. Also, it is unclear whether education alone impacted the referral rate.
The author shared the outcomes of this PI project with fellow professionals at NMCCL. As a team, we explored ways for military to link with mental health care within their commands. The process of using these instruments is easily transferable to other clinics with no extraordinary cost.
Conclusion
The economic burden of major depressive disorder in the US has risen 21.5% from 2005 to 2010.16 Unfortunately, only 35% of those with symptoms of severe depression had contact with a mental health professional in the past year.8 Avoiding missing opportunities to screen for mental health conditions can decrease the disease burden. The GAD-7 and PHQ-9 are relatively cost free and are deemed reliable and valid for screening for, and determining the severity of, symptoms of anxiety and depression.12 The evidence suggests that screening for, and early recognition of, mental illness, are critical parts of evidence-based practice and provide the most cost-effective care.16
This PI project demonstrated that the standardized use of the GAD-7 and PHQ-9 during patient visits for pain did improve adherence to guidelines and resulted in a significant increase in the rate of mental health referrals from 10% to 23.1%. This information is valuable because a score of ≥ 10 on either screening instrument is considered the optimal cutoff for diagnosing and determining severity of anxiety and depression symptoms.12 The US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have jointly developed clinical practice guidelines, which recommend that interventions, such as behavioral therapies or first-line pharmacologic treatment, be offered to patients with mild to moderate symptoms of depression.17 The VA/DoD guidelines for low back pain suggest screening for mental health disorders.2 For these reasons, the standardized use of the screening instruments remains in place within the pain management clinic at NMCCL.
1. Board on Health Sciences Policy. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press: Washington, DC; 2011.
2. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines for diagnosis and treatment of low back pain. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPG092917.pdf. Published October 21, 2016. Accessed September 26, 2019.
3. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7(4):339-343.
4. Arlotta CJ. New recommendations for pain management among active duty service military and veterans. Forbes. February 13, 2015. https://www.forbes.com/sites/cjarlotta/2015/02/13/managing-chronic-pain-in-the-active-military-and-veteran-populations/#7d7dd7d93fc3. Accessed September 26, 2019.
5. Kroenke K, Outcalt S, Krebs E, et al. Association between anxiety, health-related quality of life and functional impairment in primry care patients with chronic pain. Gen Hosp Psychiatry. 2013;35(4):359-365.
6. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127-133.
7. Lazar SG. The mental health needs of active duty service members and veterans. Psychodynamic Psychiatry. 2014;42(3):459-478.
8. Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed September 26, 2019.
9. Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
10. National Institute for Clinical Health and Care Excellence. Common mental health problems: identification and pathways to care. https://www.nice.org.uk/guidance/CG123/chapter/1-Guidance#step-1-identification-and-assessment. Published May 2011. Accessed September 26, 2019.
11. Barrera TL, Mott JM, Hundt NE, et al. Diagnostic specificity and mental health service utilization among veterans with newly diagnosed anxiety disorders. Gen Hosp Psychiatry. 2014;36(2):192-198.
12. Kroenke K, Spitzer RL, Williams JBW, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359.
13. Smith MJ, Liehr PR. The Theory of Unpleasant Symptoms. Middle Range Theory for Nursing. New York, NY: Springer Publishing Company, 2014:165-195.
14. Substance Abuse and Mental Health Services Administration, Health Resources and Services Administration. FOCUS PDCA: plan-do-check-act. https://www.integration.samhsa.gov/pbhci-learning-community/Cross-site_TA_slides_-_FOCUSPDCA_Final.pdf. Published September 19, 2017. Accessed September 26, 2019.
15. Bridges KW, Goldberg DP. Somatic presentation of DSM III psychiatric disorders in primary care. J Psychosom Res. 1985;29(6):563-569.
16. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-162.
17. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines. Management of major depressive disorder (MDD) https://www.healthquality.va.gov/guidelines/MH/mdd/. Updated October 12, 2017. Accessed September 26, 2019.
Chronic pain is more prevalent in the US than diabetes mellitus, cancer, and cardiovascular disease combined, impacting about 100 million adults.1 The annual cost of all that pain in the US is between $560 and $635 billion.1
The high prevalence of chronic pain among active duty service members and veterans remains a pressing concern given its negative impact on military readiness, health care utilization, productivity, quality of life, and chronic disability rates.2 Pain was found to be the leading complaint of service members returning from Operations Iraqi Freedom and Enduring Freedomand 44% of veterans returning from deployment suffered with chronic pain.3,4
Chronic pain often occurs in the presence of comorbidities. In one study for example, 45% of primary care patients with chronic pain (N = 250) screened positive for ≥ 1 of the 5 types of common anxiety disorders, and those with anxiety disorder had higher pain scores.5 Another study involving almost 6000 participants found that anxiety disorders were present in 35% of people with chronic pain compared with 18% in the general population.6
In addition, military members are prone to depression with a rate of major depressive disorder that is 5% higher than that of civilians.7 Depression often is underdiagnosed and undertreated. According to a National Center for Health Statistics, only 35% of those with symptoms of severe depression in the US saw a mental health provider in the previous year.8 Comorbid depression, anxiety, and chronic pain are strongly associated with more severe pain, greater disability, and poorer health-related quality of life.9
As a result, there was a call for system-level interventions to increase access to, and continuity of, mental health care services for active duty service members and veterans.1 It has been recommended that depression and anxiety screenings take place in primary and secondary care clinics.10 Standardized referral processes also are needed to enhance mental health diagnosis and referral techniques.11 Although various screening tools are available that have excellent reliability and construct validity (eg, General Anxiety Disorder-7 [GAD-7], Patient Health Questionnaire-9 [PHQ-9]), they are underutilized.12 I have witnessed a noticeable gap between clinical practice guidelines and current practice associated with chronic pain and screening for anxiety and depression within the Pain Management Clinic at Navy Medical Center of Camp Lejeune (NMCCL) in North Carolina.
Methods
The premise of this performance improvement (PI) project was to reduce missed opportunities of screening for anxiety and depression, and to examine the impact of the standardized use of the GAD-7 and PHQ-9 on the rate of mental health care referrals. The Theory of Unpleasant Symptoms was chosen as the underpinning of the project because it suggests that symptoms often cluster, and that the occurrence of multiple symptoms makes each of those, as well as other symptoms, worse.13 The PI model used the find, organize, clarify, understand, select (FOCUS), and plan, do, check, act (PDCA) models.14 The facility institutional review board ruled that this performance improvement project did not qualify as human research.
Inclusion and exclusion criteria
Patients were included if they were active duty service members aged 18 to 56 years at the initial patient encounter. Veterans and dependents were not part of the sample because of the high clinic volume. Patients who received mental health care services within the previous 90 days were excluded.
Registered nurses, licensed practical nurses, US Navy corpsman, medical assistants, and nurse aides were educated on the purpose of the GAD-7 and PHQ-9 and were instructed to have patients complete them upon every new patient encounter. A retrospective chart review was conducted over a 6-week time frame to collect and analyze de-identified demographic data including age, gender, prior deployment (yes or no), and branch of service. The review also examined whether the patient had received mental health care services, whether the screening instruments were completed, and whether a mental health referral was made. The clinic providers were asked to consider mental health care referrals for patients who scored ≥ 10 on either the GAD-7 or PHQ-9. The frequency of the use of the instruments and the number of mental health referrals made was calculated during the 3-week period before and after the standardized use of the instruments. The author conducted audits of the new patient charts at the end of each work day to assess whether the GAD-7 and PHQ-9 were completed.
Results
There were 117 new patient encounters during the 6-week project period. Thirty-three patients were excluded from the sample, leaving a remaining sample of 84. Thirty-two patients were included in the sample prior to the standardized use of the instruments, and 52 were included afterward (Table).
Prior to the standardized use of the screening tools, the GAD-7 was used during 75% of patient visits for pain and the PHQ-9 was used during 25%, reinforcing the premise of unpredictable utilization of the screening tools. Three mental health referrals were made during the 3-week period prior to the standardized use of the anxiety and depression instruments (3/32, 10%). After the standardized implementation of the GAD-7 and PHQ-9 tools, both instruments were used 98% of the time, and mental health referrals were made for 12 of 52 patients (23.1%). Eleven of the referrals were made based upon the trigger score of 10 on either the GAD-7 or PHQ-9. One referral was made for a patient with a score of 9 on the PHQ-9 because the provider determined a need for pain-related psychological services.
It was important to provide a link to mental health care because, as one study found, patients with a specific anxiety diagnosis are much more likely than those diagnosed with a not otherwise specified anxiety disorder to receive mental health care services (60% to 67% vs 37%).11 Similarly, patients diagnosed in specialty mental health care settings are more likely to receive mental health services than are those diagnosed in primary care.11 By the same token, experts estimate that 50% of those with severe depression symptoms are not properly diagnosed or treated in primary care.15
Strengths and Limitations
Utilization of the screening tools has led to further dialogue between patients and providers that anecdotally revealed suicidal ideation in some patients. Future studies could incorporate a qualitative component to include clinician and patient perceptions of mental health care services.
The study was limited by the lack of follow-up data to determine the effect of mental health care services on pain, function, or military readiness. Also, it is unclear whether education alone impacted the referral rate.
The author shared the outcomes of this PI project with fellow professionals at NMCCL. As a team, we explored ways for military to link with mental health care within their commands. The process of using these instruments is easily transferable to other clinics with no extraordinary cost.
Conclusion
The economic burden of major depressive disorder in the US has risen 21.5% from 2005 to 2010.16 Unfortunately, only 35% of those with symptoms of severe depression had contact with a mental health professional in the past year.8 Avoiding missing opportunities to screen for mental health conditions can decrease the disease burden. The GAD-7 and PHQ-9 are relatively cost free and are deemed reliable and valid for screening for, and determining the severity of, symptoms of anxiety and depression.12 The evidence suggests that screening for, and early recognition of, mental illness, are critical parts of evidence-based practice and provide the most cost-effective care.16
This PI project demonstrated that the standardized use of the GAD-7 and PHQ-9 during patient visits for pain did improve adherence to guidelines and resulted in a significant increase in the rate of mental health referrals from 10% to 23.1%. This information is valuable because a score of ≥ 10 on either screening instrument is considered the optimal cutoff for diagnosing and determining severity of anxiety and depression symptoms.12 The US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have jointly developed clinical practice guidelines, which recommend that interventions, such as behavioral therapies or first-line pharmacologic treatment, be offered to patients with mild to moderate symptoms of depression.17 The VA/DoD guidelines for low back pain suggest screening for mental health disorders.2 For these reasons, the standardized use of the screening instruments remains in place within the pain management clinic at NMCCL.
Chronic pain is more prevalent in the US than diabetes mellitus, cancer, and cardiovascular disease combined, impacting about 100 million adults.1 The annual cost of all that pain in the US is between $560 and $635 billion.1
The high prevalence of chronic pain among active duty service members and veterans remains a pressing concern given its negative impact on military readiness, health care utilization, productivity, quality of life, and chronic disability rates.2 Pain was found to be the leading complaint of service members returning from Operations Iraqi Freedom and Enduring Freedomand 44% of veterans returning from deployment suffered with chronic pain.3,4
Chronic pain often occurs in the presence of comorbidities. In one study for example, 45% of primary care patients with chronic pain (N = 250) screened positive for ≥ 1 of the 5 types of common anxiety disorders, and those with anxiety disorder had higher pain scores.5 Another study involving almost 6000 participants found that anxiety disorders were present in 35% of people with chronic pain compared with 18% in the general population.6
In addition, military members are prone to depression with a rate of major depressive disorder that is 5% higher than that of civilians.7 Depression often is underdiagnosed and undertreated. According to a National Center for Health Statistics, only 35% of those with symptoms of severe depression in the US saw a mental health provider in the previous year.8 Comorbid depression, anxiety, and chronic pain are strongly associated with more severe pain, greater disability, and poorer health-related quality of life.9
As a result, there was a call for system-level interventions to increase access to, and continuity of, mental health care services for active duty service members and veterans.1 It has been recommended that depression and anxiety screenings take place in primary and secondary care clinics.10 Standardized referral processes also are needed to enhance mental health diagnosis and referral techniques.11 Although various screening tools are available that have excellent reliability and construct validity (eg, General Anxiety Disorder-7 [GAD-7], Patient Health Questionnaire-9 [PHQ-9]), they are underutilized.12 I have witnessed a noticeable gap between clinical practice guidelines and current practice associated with chronic pain and screening for anxiety and depression within the Pain Management Clinic at Navy Medical Center of Camp Lejeune (NMCCL) in North Carolina.
Methods
The premise of this performance improvement (PI) project was to reduce missed opportunities of screening for anxiety and depression, and to examine the impact of the standardized use of the GAD-7 and PHQ-9 on the rate of mental health care referrals. The Theory of Unpleasant Symptoms was chosen as the underpinning of the project because it suggests that symptoms often cluster, and that the occurrence of multiple symptoms makes each of those, as well as other symptoms, worse.13 The PI model used the find, organize, clarify, understand, select (FOCUS), and plan, do, check, act (PDCA) models.14 The facility institutional review board ruled that this performance improvement project did not qualify as human research.
Inclusion and exclusion criteria
Patients were included if they were active duty service members aged 18 to 56 years at the initial patient encounter. Veterans and dependents were not part of the sample because of the high clinic volume. Patients who received mental health care services within the previous 90 days were excluded.
Registered nurses, licensed practical nurses, US Navy corpsman, medical assistants, and nurse aides were educated on the purpose of the GAD-7 and PHQ-9 and were instructed to have patients complete them upon every new patient encounter. A retrospective chart review was conducted over a 6-week time frame to collect and analyze de-identified demographic data including age, gender, prior deployment (yes or no), and branch of service. The review also examined whether the patient had received mental health care services, whether the screening instruments were completed, and whether a mental health referral was made. The clinic providers were asked to consider mental health care referrals for patients who scored ≥ 10 on either the GAD-7 or PHQ-9. The frequency of the use of the instruments and the number of mental health referrals made was calculated during the 3-week period before and after the standardized use of the instruments. The author conducted audits of the new patient charts at the end of each work day to assess whether the GAD-7 and PHQ-9 were completed.
Results
There were 117 new patient encounters during the 6-week project period. Thirty-three patients were excluded from the sample, leaving a remaining sample of 84. Thirty-two patients were included in the sample prior to the standardized use of the instruments, and 52 were included afterward (Table).
Prior to the standardized use of the screening tools, the GAD-7 was used during 75% of patient visits for pain and the PHQ-9 was used during 25%, reinforcing the premise of unpredictable utilization of the screening tools. Three mental health referrals were made during the 3-week period prior to the standardized use of the anxiety and depression instruments (3/32, 10%). After the standardized implementation of the GAD-7 and PHQ-9 tools, both instruments were used 98% of the time, and mental health referrals were made for 12 of 52 patients (23.1%). Eleven of the referrals were made based upon the trigger score of 10 on either the GAD-7 or PHQ-9. One referral was made for a patient with a score of 9 on the PHQ-9 because the provider determined a need for pain-related psychological services.
It was important to provide a link to mental health care because, as one study found, patients with a specific anxiety diagnosis are much more likely than those diagnosed with a not otherwise specified anxiety disorder to receive mental health care services (60% to 67% vs 37%).11 Similarly, patients diagnosed in specialty mental health care settings are more likely to receive mental health services than are those diagnosed in primary care.11 By the same token, experts estimate that 50% of those with severe depression symptoms are not properly diagnosed or treated in primary care.15
Strengths and Limitations
Utilization of the screening tools has led to further dialogue between patients and providers that anecdotally revealed suicidal ideation in some patients. Future studies could incorporate a qualitative component to include clinician and patient perceptions of mental health care services.
The study was limited by the lack of follow-up data to determine the effect of mental health care services on pain, function, or military readiness. Also, it is unclear whether education alone impacted the referral rate.
The author shared the outcomes of this PI project with fellow professionals at NMCCL. As a team, we explored ways for military to link with mental health care within their commands. The process of using these instruments is easily transferable to other clinics with no extraordinary cost.
Conclusion
The economic burden of major depressive disorder in the US has risen 21.5% from 2005 to 2010.16 Unfortunately, only 35% of those with symptoms of severe depression had contact with a mental health professional in the past year.8 Avoiding missing opportunities to screen for mental health conditions can decrease the disease burden. The GAD-7 and PHQ-9 are relatively cost free and are deemed reliable and valid for screening for, and determining the severity of, symptoms of anxiety and depression.12 The evidence suggests that screening for, and early recognition of, mental illness, are critical parts of evidence-based practice and provide the most cost-effective care.16
This PI project demonstrated that the standardized use of the GAD-7 and PHQ-9 during patient visits for pain did improve adherence to guidelines and resulted in a significant increase in the rate of mental health referrals from 10% to 23.1%. This information is valuable because a score of ≥ 10 on either screening instrument is considered the optimal cutoff for diagnosing and determining severity of anxiety and depression symptoms.12 The US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) have jointly developed clinical practice guidelines, which recommend that interventions, such as behavioral therapies or first-line pharmacologic treatment, be offered to patients with mild to moderate symptoms of depression.17 The VA/DoD guidelines for low back pain suggest screening for mental health disorders.2 For these reasons, the standardized use of the screening instruments remains in place within the pain management clinic at NMCCL.
1. Board on Health Sciences Policy. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press: Washington, DC; 2011.
2. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines for diagnosis and treatment of low back pain. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPG092917.pdf. Published October 21, 2016. Accessed September 26, 2019.
3. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7(4):339-343.
4. Arlotta CJ. New recommendations for pain management among active duty service military and veterans. Forbes. February 13, 2015. https://www.forbes.com/sites/cjarlotta/2015/02/13/managing-chronic-pain-in-the-active-military-and-veteran-populations/#7d7dd7d93fc3. Accessed September 26, 2019.
5. Kroenke K, Outcalt S, Krebs E, et al. Association between anxiety, health-related quality of life and functional impairment in primry care patients with chronic pain. Gen Hosp Psychiatry. 2013;35(4):359-365.
6. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127-133.
7. Lazar SG. The mental health needs of active duty service members and veterans. Psychodynamic Psychiatry. 2014;42(3):459-478.
8. Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed September 26, 2019.
9. Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
10. National Institute for Clinical Health and Care Excellence. Common mental health problems: identification and pathways to care. https://www.nice.org.uk/guidance/CG123/chapter/1-Guidance#step-1-identification-and-assessment. Published May 2011. Accessed September 26, 2019.
11. Barrera TL, Mott JM, Hundt NE, et al. Diagnostic specificity and mental health service utilization among veterans with newly diagnosed anxiety disorders. Gen Hosp Psychiatry. 2014;36(2):192-198.
12. Kroenke K, Spitzer RL, Williams JBW, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359.
13. Smith MJ, Liehr PR. The Theory of Unpleasant Symptoms. Middle Range Theory for Nursing. New York, NY: Springer Publishing Company, 2014:165-195.
14. Substance Abuse and Mental Health Services Administration, Health Resources and Services Administration. FOCUS PDCA: plan-do-check-act. https://www.integration.samhsa.gov/pbhci-learning-community/Cross-site_TA_slides_-_FOCUSPDCA_Final.pdf. Published September 19, 2017. Accessed September 26, 2019.
15. Bridges KW, Goldberg DP. Somatic presentation of DSM III psychiatric disorders in primary care. J Psychosom Res. 1985;29(6):563-569.
16. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-162.
17. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines. Management of major depressive disorder (MDD) https://www.healthquality.va.gov/guidelines/MH/mdd/. Updated October 12, 2017. Accessed September 26, 2019.
1. Board on Health Sciences Policy. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. The National Academies Press: Washington, DC; 2011.
2. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines for diagnosis and treatment of low back pain. https://www.healthquality.va.gov/guidelines/Pain/lbp/VADoDLBPCPG092917.pdf. Published October 21, 2016. Accessed September 26, 2019.
3. Gironda RJ, Clark ME, Massengale JP, Walker RL. Pain among veterans of Operations Enduring Freedom and Iraqi Freedom. Pain Med. 2006;7(4):339-343.
4. Arlotta CJ. New recommendations for pain management among active duty service military and veterans. Forbes. February 13, 2015. https://www.forbes.com/sites/cjarlotta/2015/02/13/managing-chronic-pain-in-the-active-military-and-veteran-populations/#7d7dd7d93fc3. Accessed September 26, 2019.
5. Kroenke K, Outcalt S, Krebs E, et al. Association between anxiety, health-related quality of life and functional impairment in primry care patients with chronic pain. Gen Hosp Psychiatry. 2013;35(4):359-365.
6. McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127-133.
7. Lazar SG. The mental health needs of active duty service members and veterans. Psychodynamic Psychiatry. 2014;42(3):459-478.
8. Pratt LA, Brody DJ. Depression in the U.S. household population, 2009-2012. NCHS Data Brief No. 172. https://www.cdc.gov/nchs/data/databriefs/db172.pdf. Published December 2014. Accessed September 26, 2019.
9. Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
10. National Institute for Clinical Health and Care Excellence. Common mental health problems: identification and pathways to care. https://www.nice.org.uk/guidance/CG123/chapter/1-Guidance#step-1-identification-and-assessment. Published May 2011. Accessed September 26, 2019.
11. Barrera TL, Mott JM, Hundt NE, et al. Diagnostic specificity and mental health service utilization among veterans with newly diagnosed anxiety disorders. Gen Hosp Psychiatry. 2014;36(2):192-198.
12. Kroenke K, Spitzer RL, Williams JBW, Lowe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359.
13. Smith MJ, Liehr PR. The Theory of Unpleasant Symptoms. Middle Range Theory for Nursing. New York, NY: Springer Publishing Company, 2014:165-195.
14. Substance Abuse and Mental Health Services Administration, Health Resources and Services Administration. FOCUS PDCA: plan-do-check-act. https://www.integration.samhsa.gov/pbhci-learning-community/Cross-site_TA_slides_-_FOCUSPDCA_Final.pdf. Published September 19, 2017. Accessed September 26, 2019.
15. Bridges KW, Goldberg DP. Somatic presentation of DSM III psychiatric disorders in primary care. J Psychosom Res. 1985;29(6):563-569.
16. Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155-162.
17. US Department of Defense, US Department of Veterans Affairs. VA/DoD clinical practice guidelines. Management of major depressive disorder (MDD) https://www.healthquality.va.gov/guidelines/MH/mdd/. Updated October 12, 2017. Accessed September 26, 2019.
Implementation of a Protocol to Manage Patients at Risk for Hospitalization Due to an Ambulatory Care Sensitive Condition
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
Hospitalizations related to ambulatory care sensitive conditions (ACSCs) are potentially avoidable if timely and effective care is provided to the patient. The Agency of Healthcare Research and Quality has identified type 2 diabetes mellitus (T2DM), chronic obstructive pulmonary disease (COPD), hypertension, congestive heart failure (CHF), urinary tract infections (UTIs), asthma, dehydration, bacterial pneumonia, angina without an inhospital procedure, and perforated appendix as ACSCs.1,2 Identifying patients with ACSCs who are at risk for hospitalization is a potential measure to enhance primary care delivery and reduce preventable hospitalizations
The US Department of Veterans Affairs (VA) Clinical Pharmacy Practice Office implemented a guidance statement describing the role and impact of a clinical pharmacy specialist (CPS) in managing ACSCs.1 Within the Veterans Health Administration, the CPS may function under a scope of practice within their area of expertise with the ability to prescribe medications, place consults, and order laboratory tests and additional referrals as appropriate. As hospitalizations related to ACSCs are potentially preventable with effective primary care, the CPS can play an essential primary care role to implement interventions targeted at reducing these hospitalizations.
At the William S. Middleton Memorial Veterans Hospital, in Madison, Wisconsin, multiple transitions of care and postdischarge services have been established to capture those patients who are at a high risk of rehospitalization. Studies have been completed regarding implementation of intensive case management programs for high-risk patients.3 Currently though, no standardized process or protocol exists that can identify and optimize primary care for patients with ACSCs who have been hospitalized but are predicted to be at low risk for rehospitalization. Although these patients may not require intensive case management like that of those at high risk, improvements can be made to optimize clinical resources, education, and patient self-monitoring to mitigate risk for hospitalization or rehospitalization. Therefore, this project aimed to evaluate the implementation of offering further referrals and care for patients who have been hospitalized but are considered low risk for hospitalization from ACSCs.
Methods
This quality improvement project to offer further referrals and care to patients considered low risk for hospitalization was implemented to enhance ambulatory-care provided services. All patients identified as being a low risk for hospitalization via a VA dashboard from July through September 2018 were included. Patients were identified based on age, chronic diseases, gender, and other patient-specific factors predetermined by the VA dashboard algorithm. Patients receiving hospice or palliative care and those no longer receiving primary care through the facility were excluded.
A pharmacy resident conducted a baseline chart review using a standardized template in the computerized patient record system (CPRS) to identify additional referrals or interventions a patient may benefit from based on any identified ACSC. Potential referral options included a CPS or nurse care manager disease management, whole health/wellness, educational classes, home monitoring equipment, specialty clinics, nutrition, cardiac or pulmonary rehabilitation, social work, and mental health. A pharmacy resident or the patient aligned care team (PACT) CPS reviewed the identified referrals with PACT members at interdisciplinary team meetings and determined which referrals to offer the patient. The pharmacy resident or designated PACT member reached out to the patient via telephone or during a clinic visit to offer and enter the referrals. If the patient agreed to any referrals, a chart review was conducted 3 months later to determine the percentage of initially agreed-upon referrals that the patient completed. Additionally, the number of emergency department (ED) visits and hospitalizations related to an ACSC at 3 months was collected.
Feasibility was assessed to evaluate potential service implementation and was measured by the time in minutes to complete the baseline chart review, time in minutes to offer referrals to the patient, and proportion of referrals that were completed at 3 months.4 As this quality improvement project was undertaken for programmatic evaluation, the University of Wisconsin-Madison Health Sciences Institutional Review Board determined that this project did not meet the federal definition of research and therefore review was not required. Data were analyzed using descriptive statistics.
Results
A total of 78 veterans who had ≥ 1 ACSC-related hospitalization in the past year and who were categorized as low risk were identified, and 69 veterans were reviewed. Nine patients were not included based on hospice care and no longer receiving primary care through the facility. Eight patients were found to have optimized care with no further action warranted after review. Based on their assigned PACT, there was a range of 0 to 5 patients identified per team. Fifty-one patients were contacted, and 37 accepted ≥ 1 referral. Most of the patients were white and male (Table). The most common ACSCs were hypertension (68%), COPD (46%), and T2DM (30%); additional ACSCs included angina (18%), pneumonia (15%), UTIs (10%), CHF (6%), and asthma, dehydration, and perforated appendix (1.5% for each). Any ACSC listed as a diagnosis for a patient was included, regardless of whether it was related to a hospitalization. Most referrals were offered by pharmacists (pharmacy resident, 41%; CPS, 29%), followed by the nurse care manager (18%) and the primary care provider (12%). One patient passed away related to heart failure complications prior to being contacted to offer additional referrals. Of the 9 patients that were unable to be contacted, 4 did not respond to 3 phone call attempts and 5 had no documentation of referrals being offered after the initial chart review and recommendation was completed.
Most of the initially accepted referrals (n = 68) were for CPS disease management, whole health/wellness, and educational classes (Figure). Of the 28 initially accepted referrals for CPS disease management, most were for COPD (10) and hypertension (8), followed by neuropathic pain (3), vitamin D deficiency (3), hyperlipidemia (2), and T2DM (2). At 3 months, all referrals were completed except for 1 hypertension, 1 vitamin D deficiency, and 2 hyperlipidemia referrals. There were 6 COPD, 4 T2DM self-management, and 1 chronic pain class referrals made with 3 COPD and 1 T2DM referrals completed at 3 months. Two tobacco treatment and 2 palliative care referrals were specialty referrals accepted by patients with 1 palliative care referral completed at 3 months.
In terms of feasibility, the chart review took an average (SD) of 13 (4) minutes, and contacting the patient to offer referrals took an average of 8 (5) minutes. Most of the accepted referrals were completed by 3 months (42/68, 62%).
Comparing the 3 months prior to and the 3 months after offering referrals, there was a cumulative quantitative decrease in the number of ED visits (5 to 1) and hospitalizations (11 to 5). The 1 ED visit was for a patient who was unable to be contacted to offer additional referrals as were 4 of the hospitalizations. One of the hospitalizations was for a patient who was deemed to have optimized care with no additional referrals necessary.
Discussion
Evaluation of the review and referral process for patients at low risk for hospitalization from an ACSC was a proactive approach toward optimizing primary care for veterans, and the process increased patient access to education and primary care. There was a high initial patient acceptance rate of referrals and a high completion rate when offered by PACT members. Based on the number of identified patients, the time spent completing chart reviews and contacting patients to offer referrals for each PACT CPS and team was feasible to conduct.
As there were 69 eligible patients identified over a 3-month period for a single VA facility, including all community-based outpatient clinics serving an estimated 130,000 veterans, the additional time and workload for an individual PACT to reach out to these patients is minimal. Completing the review and outreach process for an average of 21 minutes per patient for at most 5 patients per primary care provider team is feasible to complete during the recommended 4 hours of weekly CPS population health management responsibilities.
Limitations
Several limitations were identified with the implementation of the project. A variety of PACT members completed initial outreach to veterans regarding additional referrals, which may have resulted in a lack of consistency in the approach and discussion of offering referrals to patients. Although there may be a difference in how the team members made referral offers to patients and therefore varying acceptance rates by patients, the process was thought to be more generalizable to the PACT approach for providing care in the VA. In addition, the time to contact patients to offer referrals was not always documented in the electronic health record, making the documented time an estimate. Given that patients identified were managed by a variety of PACT members, there were differences noted among PACTs in terms of acceptability of offering referrals to patients.
While there was a decrease noted in ED visits and hospitalizations when comparing 3 months before and afterward, additional data are needed to provide further insight into this relationship. As the patients identified were at low risk for hospitalization from an ACSC and had 1 or 2 hospitalizations within the year prior, additional time is warranted to compare 12-month ED visits and hospitalization rates postintervention. Finally, these findings may be limited in generalizability to other health care systems as the project was conducted among a specific, veteran patient population with PACT CPSs practicing independently within an established broad scope of practice.
Future Directions
Future directions include incorporating the review and referral process into the PACT CPS population health management responsibilities as a way to use all PACT members to enhance primary care delivered to veterans. To further elucidate the relationship between the referral process and hospitalization rates, a longer data collection period is needed.
Conclusions
Identifying patients at risk for hospitalization from an ACSC via a review and referral process by using the VA PACT structure and team members was feasible and led to increased patient access to primary care and additional services. The PACT CPS would benefit from using a similar approach for population health management for low risk for hospitalization patients or other identified chronic conditions.
Acknowledgments
Presented at the Wisconsin Pharmacy Residency Conference at the Pharmacy Society of Wisconsin Educational Conference April 10, 2019, in Madison, Wisconsin.
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7
1. US Department of Veterans Affairs, Veterans Health Administration, Pharmacy Benefits Management Service, Clinical Pharmacy Practice Office. Clinical pharmacy specialist (CPS) role in management of ambulatory care sensitive conditions (ACSC). [Nonpublic source.]
2. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. https://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf. Revised April 17, 2002. Accessed July 16, 2020.
3. Yoon J, Chang E, Rubenstein L, et al. Impact of primary care intensive management on high-risk veterans’ costs and utilization. Ann Intern Med. 2018;168(12):846-854. doi:10.7326/M17-3039
4. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65-76. doi:10.1007/s10488-010-0319-7