User login
Self-mutilation after recent-onset psychosis
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
CASE Bleeding, bewildered
Mr. K, age 23, a South Asian male, is discovered in the bathroom bleeding profusely. Mr. K’s parents inform emergency medical services (EMS) personnel that Mr. K is “not in his right mind” and speculate that he is depressed. EMS personnel find Mr. K sitting in a pool of blood in the bathtub, holding a cloth over his pubic area and complaining of significant pain. They estimate that Mr. K has lost approximately 1 L of blood. Cursory evaluation reveals that his penis is severed; no other injuries or lacerations are notable. Mr. K states, “I did not want it anymore.” A kitchen knife that he used to self-amputate is found nearby. He is awake, alert, and able to follow simple directives.
In the emergency room, Mr. K is in mild-to-moderate distress. He has no history of medical illness, but his parents report that he previously required psychiatric treatment. Mr. K is not able to elaborate. He reluctantly discloses an intermittent history of Cannabis use. Physical examination reveals tachycardia (heart rate: 115 to 120 beats per minute), and despite blood loss, systolic hypertension (blood pressure: 142/70 to 167/70 mm Hg). His pulse oximetry is 97% to 99%; he is afebrile. Laboratory tests are notable for anemia (hemoglobin, 7.2 g/dL [reference range, 14.0 to 17.5 g/dL]; hematocrit, 21.2% [reference range, 41% to 50%]) and serum toxicology screen is positive for benzodiazepines, which had been administered en route to allay his distress.
Mr. K continues to hold pressure on his pubic area. When pressure is released, active arterial spurting of bright red blood is notable. Genital examination reveals a cleanly amputated phallus. Emergent surgical intervention is required to stop the hemorrhage and reattach the penis. Initially, Mr. K is opposed to reattachment, but after a brief discussion with his parents, he consents to surgery. Urology and plastic surgery consultations are elicited to perform the microvascular portion of the procedure.
[polldaddy:9881368]
The authors’ observations
Self-injurious behaviors occur in approximately 1% to 4% of adults in the United States, with chronic and severe self-injury occurring among approximately 1% of the U.S. population.1,2 Intentional GSM is a relatively rare catastrophic event that is often, but not solely, associated with severe mental illness. Because many cases go unreported, the prevalence of GSM is difficult to estimate.3,4 Although GSM has been described in both men and women, the literature has predominantly focused on GSM among men.5 Genital self-injury has been described in several (ie, ethnic/racial and religious) contexts and has been legally sanctioned.6-8
Psychiatric disorders associated with, and precipitating factors underlying, GSM have long remained elusive.8 GSM has been described in case reports and small case series in both psychiatric and urologic literature. These reports provide incomplete descriptions of the diagnostic conditions and psychosocial factors underlying male GSM.
A recent systematic review of 173 cases of men who engaged in GSM published in the past 115 years (since the first case of GSM was published in the psychiatric literature9) revealed that having some form of psychopathology elevates the probability of GSM10,11; rarely the individual did not have a psychiatric condition.11-17 Nearly one-half of the men had psychosis; most had a schizophrenia spectrum disorder diagnosis. Other psychiatric conditions associated with GSM include personality disorders, substance use disorder, and gender dysphoria. GSM is rarely associated with anxiety or mood disorders.
GSM is a heterogeneous form of self-injury that ranges from superficial genital lacerations, amputation, or castration to combinations of these injuries. Compared with individuals with other psychiatric disorders, a significantly greater proportion of individuals with schizophrenia spectrum disorders engage in self-amputation (auto-penectomy). By contrast, persons with gender dysphoria tend to engage in self-castration at significantly higher rates than those with other psychiatric conditions.11 Despite these trends, clinicians should not infer a specific psychiatric diagnosis based on the severity or type of self-inflicted injury.
HISTORY Command hallucinations
Postoperatively, Mr. K is managed in the trauma intensive care unit. During psychiatric consultation, Mr. K demonstrates a blunted affect. His speech is low in volume but clear and coherent. His thoughts are generally linear for specific lines of inquiry (eg, about perceived level of pain) but otherwise are impoverished. Mr. K often digresses into repetitively mumbled prayers. He appears distracted, as if responding to internal stimuli. Although he acknowledges the GSM, he does not discuss the factors underlying his decision to proceed with auto-penectomy. Over successive evaluations, he reluctantly discloses that he had been experiencing disparaging auditory hallucinations that told him that his penis “was too small” and commanded him to “cut it off.”
Psychiatric history reveals that Mr. K required psychiatric hospitalization 7 months earlier due to new-onset auditory hallucinations, paranoia, and thought disorganization, in the context of daily Cannabis use. At the time, the differential diagnosis included new-onset schizophrenia and substance-induced psychosis. His symptoms improved quickly with risperidone, 2 mg/d, and he was discharged in a stable condition with referrals for outpatient care. Mr. K admits he had stopped taking risperidone several weeks before the GSM because he was convinced that he had been cured. At that time, Mr. K had told his parents he was no longer required to take medication or engage in outpatient psychiatric treatment, and they did not question this. Mr. K struggled to sustain part-time employment (in a family business), having taken a leave of absence from graduate school after his first hospitalization. He continued to use Cannabis regularly but denies being intoxicated at the time of the GSM. Throughout his surgical hospitalization, Mr. K’s thoughts remain disorganized. He denies that the GSM was a suicide attempt or having current suicidal thoughts, intent, or plans. He also denies having religious preoccupations, over-valued religious beliefs, or delusions.
Mr. K identifies as heterosexual, and denies experiencing distress related to sexual orientation or gender identity or guilt related to sexual impulses or actions. He also denies having a history of trauma or victimization and does not report any symptoms of posttraumatic stress disorder or body dysmorphic disorder.
The authors’ observations
Little is known about how many individuals who engage in GSM eventually complete suicide. Although suicidal ideation and intent have been infrequently associated with GSM, suicide has been most notably reported among patients with schizophrenia spectrum disorders and psychotic mood disorders.11,18,23-26 For these individuals, suicidal ideation co-occurred with delusions, hallucinations, and pathological guilt preoccupations. Significant self-inflicted injury can be harbinger of distress that could lead to suicide if not optimally treated. Other psychosocial stressors, such as disruptions in interpersonal functioning arising from changes in or loss of social support or perceived rejection, may contribute to a patient’s level of distress, complicating underlying psychiatric disturbances and increasing vulnerability toward GSM.11,27
Substance use also increases vulnerability toward GSM.11,18,24,28 As is the case with patients who engage in various non-GSM self-injurious behaviors,29,30 substance use or intoxication likely contribute to disinhibition or a dissociative state, which enables individuals to engage in self-injury.30
A lack of access to treatment is a rare precipitant for GSM, except among individuals with gender dysphoria. Studies have found that many patients with gender dysphoria who performed self-castration did so in a premeditated manner with low suicidal intent, and the behavior often was related to a lack of or refusal for gender confirmation surgery.31-34
In the hospital setting, surgical/urological interventions need to be directed at the potentially life-threatening sequelae of self-injury. Although complications vary, depending on the type of injury incurred, urgent measures are needed to manage blood loss because hemorrhage can be fatal.23,35,36 Other consequences that can arise include urinary fistulae, urethral strictures, mummification of the glans penis, and development of sensory abnormalities after repair of the injured tissues or reattachment.8 More superficial injuries may require only hemostasis and simple suturing, whereas extensive injuries, such as complete amputation, can be addressed through microvascular techniques.
The psychiatrist’s role. The psychiatrist should act as an advocate for the GSM patient to create an environment conducive to healing. A patient who is experiencing hallucinations or delusions may feel overwhelmed by medical and familial attention. Pharmacologic treatment for prevailing mental illness, such as psychosis, should be initiated in the inpatient setting. An estimated 20% to 25% of those who self-inflict genital injury may repeatedly mutilate their genitals.19,28 Patients unduly influenced by command hallucinations, delusional thought processes, mood disturbances, or suicidal ideation may attempt to complete the injury, or reinjure themselves after surgical/urological intervention, which may require safety measures, such as 1:1 observation, restraints, or physical barriers, to prevent reinjury.37
Self-injury elicits strong, emotional responses from health care professionals, including fascination, apprehension, and hopelessness. Psychiatrists who care for such patients should monitor members of the patient’s treatment team for psychological reactions. In addition, the patient’s behavior while hospitalized may stir feelings of retaliation, anger, fear, and frustration.11,24,37 Collaborative relationships with medical and surgical specialties can help staff manage emotional reactions and avoid the inadvertent expression of those feelings in their interactions with the patient; these reactions might otherwise undermine treatment.24,34 Family education can help mitigate any guilt family members may harbor for not preventing the injury.37
Although efforts to understand the intended goal(s) and precipitants of the self-injury are likely to be worthwhile, the overwhelming distress associated with GSM and its emergent treatment may preclude intensive exploration.
TREATMENT Restarting medication
While on the surgical unit, Mr. K is restarted on risperidone, 2 mg/d. He appears to tolerate the medication without adverse effects. However, because Mr. K continues to experience auditory hallucinations, and the treatment team remains concerned that he might again experience commands to harm himself, he is transferred to an acute psychiatric inpatient setting.
Urology follow-up reveals necrosis/mummification of the replanted penis and an open scrotal wound. After discussing options with the patient and family, the urologist transfers Mr. K back to the surgical unit for wound closure and removal of the replanted penis. A urethrostomy is performed to allow for bladder emptying.
[polldaddy:9881371]
The authors’ observations
Because most published case reports of GSM among men have focused on acute treatment, there is a dearth of literature available on the long-term course of GSM to inform treatment strategies. Because recovery is a non-static process and a patient’s reactions to his injury will likely evolve over time, a multifaceted approach invoking psychiatric and psychotherapeutic interventions is necessary to help patients after initial injury and surgical management37,40-43 (Table 211,20,27,41).
OUTCOME Return to school, work
Mr. K is discharged with close follow-up at a specialized clinic for new-onset psychosis. Post-discharge treatment consists of education about the course of schizophrenia and the need for medication adherence to prevent relapse. Mr. K also is educated on the relationship between Cannabis use and psychosis, and he abstains from illicit substance use. Family involvement is encouraged to help with medication compliance and monitoring for symptom reemergence.
Therapy focuses on exploring the antecedents of the auto-penectomy, Mr. K’s body image issue concerns, and his feelings related to eventual prosthesis implantation. He insists that he cannot recall any precipitating factors for his self-injury other than the command hallucinations. He does not report sexual guilt, although he had been sexually active with his girlfriend in the months prior to his GSM, which goes against his family’s religious beliefs. He reports significant regret and shame for the self-mutilation, and blames himself for not informing family members about his hallucinations. Therapy involves addressing his attribution of blame using cognitive techniques and focuses on measures that can be taken to prevent further self-harm. Efforts are directed at exploring whether cultural and religious traditions impacted the therapeutic alliance, medication adherence, self-esteem and body image, sexuality, and future goals. Over the course of 1 year, he resumes his graduate studies and part-time work, and explores prosthetic placement for cosmetic purposes.
The authors’ observations
Research suggests that major self-mutilation among patients with psychotic illness is likely to occur during the first episode or early in the course of illness and/or with suboptimal treatment.44,45 Mr. K was enlisted in an intensive outpatient treatment program involving biweekly psychotherapy sessions and psychiatric follow-up. Initial sessions focused on education regarding the importance of medication adherence and exploration of signs and symptoms that might suggest reemergence of a psychotic decompensation. The psychiatrist monitored Mr. K closely to ensure he was able to tolerate his medications to mitigate the possibility that adverse effects would undermine adherence. Mr. K’s reactions to having a psychiatric illness also were explored because of concerns that such self-appraisals might trigger shame, embarrassment, denial, and other responses that might undermine treatment adherence. His family members were apprised of treatment goals and enlisted to foster adherence with medication and follow-up appointments.
Mr. K’s Cannabis use was addressed because ongoing use likely had a negative impact on his schizophrenia (ie, a greater propensity toward relapse and rehospitalization and a poorer therapeutic response to antipsychotic medication).46,47 He was strongly encouraged to avoid Cannabis and other illicit substances.
Psychiatrists can help in examining the meaning behind the injury while helping the patient to adapt to the sequelae and cultivate skills to meet functional demands.41 Once Mr. K’s psychotic symptoms were in remission, treatment began to address the antecedents of the GSM, as well as the resultant physical consequences. It was reasonable to explore how Mr. K now viewed his actions, as well as the consequences that his actions produced in terms of his physical appearance, sexual functioning, capacity for sexual intimacy, and reproductive potential. It was also important to recognize how such highly intimate and deeply personal self-schema are framed and organized against his cultural and religious background.27,33
Body image concerns and expectations for future urologic intervention also should be explored. Although Mr. K was not averse to such exploration, he did not spontaneously address such topics in great depth. The discussion was unforced and effectively left open as an issue that could be explored in future sessions.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
1. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68(4):609-620.
2. Klonsky ED, Oltmanns TF, Turkheimer E. Deliberate self-harm in a nonclinical population: prevalence and psychological correlates. Am J Psychiatry. 2003;160(8):1501-1508.
3. Krasucki C, Kemp R, David A. A case study of female genital self-mutilation in schizophrenia. Br J Med Psychol. 1995;68(pt 2):179-186.
4. Lennon S. Genital self-mutilation in acute mania. Med J Aust. 1963;50(1):79-81.
5. Schweitzer I. Genital self-amputation and the Klingsor syndrome. Aust N Z J Psychiatry. 1990;24(4):566-569.
6. Anumonye A. Self-inflicted amputation of the penis in two Nigerian males. Niger Med J. 1973;3(1):51-52.
7. Bowman KM, Crook GH. Emotional changes following castration. Psychiatr Res Rep Am Psychiatr Assoc. 1960;12:81-96.
8. Eke N. Genital self-mutilation: there is no method in this madness. BJU Int. 2000;85(3):295-298.
9. Stroch D. Self-castration. JAMA. 1901;36(4):270.
10. Veeder TA, Leo RJ. Male genital self-mutilation: a comprehensive review of psychiatric disorders. Poster presented at: Academy of Psychosomatic Medicine Meeting, Austin, Texas, November 10, 2016.
11. Veeder TA, Leo RJ. Male genital self-mutilation: a systematic review of psychiatric disorders and psychosocial factors. Gen Hosp Psychiatry. 2017;44:43-50.
12. Battle AO. The psychological appraisal of a patient who had performed self-castration. British Journal of Projective Psychology & Personality Study. 1973;18(2):5-17.
13. Bhatia MS, Arora S. Penile self-mutilation. Br J Psychiatry. 2001;178(1):86-87.
14. Gleeson MJ, Connolly J, Grainger R. Self-castration as treatment for alopecia. Br J Urol. 1993;71(5):614-615.
15. Hendershot E, Stutson AC, Adair TW. A case of extreme sexual self-mutilation. J Forensic Sci. 2010;55(1):245-247.
16. Hermann M, Thorstenson A. A rare case of male‐to‐eunuch gender dysphoria. Sex Med. 2015;3(4):331-333.
17. Nerli RB, Ravish IR, Amarkhed SS, et al. Genital self-mutilation in nonpsychotic heterosexual males: case report of two cases. Indian J Psychiatry. 2008;50(4):285-287.
18. Blacker KH, Wong N. Four cases of autocastration. Arch Gen Psychiatry. 1963;8:169-176.
19. Catalano G, Catalano MC, Carroll KM. Repetitive male genital self-mutilation: a case report and discussion of possible risk factors. J Sex Marital Ther. 2002;28(1):27-37.
20. Martin T, Gattaz WF. Psychiatric aspects of male genital self-mutilation. Psychopathology. 1991;24(3):170-178.
21. Money J. The Skoptic syndrome: castration and genital self-mutilation as an example of sexual body-image pathology. J Psychol Human Sex. 1988;1(1):113-128.
22. Nakaya M. On background factors of male genital self-mutilation. Psychopathology. 1996;29(4):242-248.
23. Borenstein A, Yaffe B, Seidman DS, et al. Successful microvascular replantation of an amputated penis. Isr J Med Sci. 1991;27(7):395-398.
24. Greilsheimer H, Groves JE. Male genital self-mutilation. Arch Gen Psychiatry. 1979;36(4):441-446.
25. Mendez R, Kiely WF, Morrow JW. Self-emasculation. J Urol. 1972;107(6):981-985.
26. Siddique RA, Deshpande S. A case of genital self-mutilation in a patient with psychosis. German J Psychiatry. 2007;10(1):25-28.
27. Qureshi NA. Male genital self-mutilation with special emphasis on the sociocultural meanings. Neurosciences (Riyadh). 2009;14(2):178-181.
28. Romilly CS, Isaac MT. Male genital self-mutilation. Br J Hosp Med. 1996;55(7):427-431.
29. Gahr M, Plener PL, Kölle MA, et al. Self-mutilation induced by psychotropic substances: a systematic review. Psychiatry Res. 2012;200(2-3):977-983.
30. Evren C, Sar V, Evren B, et al. Self-mutilation among male patients with alcohol dependency: the role of dissociation. Compr Psychiatry. 2008;49(5):489-495.
31. Brown GR. Autocastration and autopenectomy as surgical self-treatment in incarcerated persons with gender identity disorder. Int J Transgend. 2010;12(1):31-39.
32. Master VA, McAninch JW, Santucci RA. Genital self-mutilation and the Internet. J Urol. 2000;164(5):1656.
33. Premand NE, Eytan A. A case of non-psychotic autocastration: the importance of cultural factors. Psychiatry. 2005;68(2):174-178.
34. Simopoulos EF, Trinidad AC. Two cases of male genital self-mutilation: an examination of liaison dynamics. Psychosomatics. 2012;53(2):178-180.
35. Darewicz B, Galek L, Darewicz J, et al. Successful microsurgical replantation of an amputated penis. Int Urol Nephrol. 2001;33(2):385-386.
36. Raheem OA, Mirheydar HS, Patel ND, et al. Surgical management of traumatic penile amputation: a case report and review of the world literature. Sex Med. 2015;3(1):49-53.
37. Young LD, Feinsilver DL. Male genital self-mutilation: combined surgical and psychiatric care. Psychosomatics. 1986;27(7):513-517.
38. Walsh B. Clinical assessment of self-injury: a practical guide. J Clin Psychol. 2007;63(11):1057-1066.
39. Nafisi N, Stanley B. Developing and maintaining the therapeutic alliance with self-injuring patients. J Clin Psychol. 2007;63(11):1069-1079.
40. Fisch RZ. Genital self-mutilation in males: psychodynamic anatomy of a psychosis. Am J Psychother. 1987;41(3):453-458.
41. King PR. Cognitive-behavioral intervention in a case of self-mutilation. Clin Case Stud. 2014;13(2):181-189.
42. Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. J Ment Health Couns. 2006;28(2):166-185.
43. Walsh BW. Treating self-injury: a practical guide. New York, NY: The Guilford Press; 2006.
44. Large M, Babidge N, Andrews D, et al. Major self-mutilation in the first episode of psychosis. Schizophr Bull. 2009;35(5):1012-1021.
45. Large MM, Nielssen OB, Babidge N. Untreated psychosis is the main cause of major self-mutilation. Isr J Psychiatry Relat Sci. 2011;48(1):65.
46. Fergusson DM, Horwood LJ, Swain-Campbell NR. Cannabis dependence and psychotic symptoms in young people. Psychol Med. 2003;33(1):15-21.
47. Bowers MB Jr, Mazure CM, Nelson JC, et al. Psychotogenic drug use and neuroleptic response. Schizophr Bull. 1990;16(1):81-85.
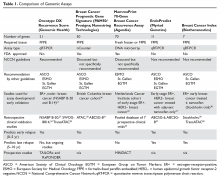
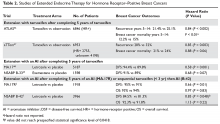
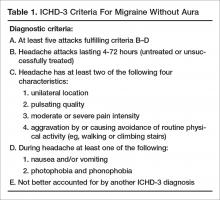
Genomic Testing in Women with Early-Stage Hormone Receptor–Positive, HER2-Negative Breast Cancer
Introduction
Over the past several decades, while the incidence of breast cancer has increased, breast cancer mortality has decreased. This decrease is likely due to both early detection and advances in systemic therapy. However, with more widespread use of screening mammography, there are increasing concerns about potential overdiagnosis of cancer.1 One key challenge is that breast cancer is a heterogeneous disease. Improved tools for determining breast cancer biology can help physicians individualize treatments. Patients with low-risk cancers can be approached with less aggressive treatments, thus preventing unnecessary toxicities, while those with higher-risk cancers remain treated appropriately with more aggressive therapies.
Traditionally, adjuvant chemotherapy was recommended based on tumor features such as stage (tumor size, regional nodal involvement), grade, expression of hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor-2 (HER2), and patient features (age, menopausal status). However, this approach is not accurate enough to guide individualized treatment approaches, which are based on the risk for recurrence and the reduction in this risk that can be achieved with various systemic treatments. In particular, women with low-risk hormone receptor (HR)–positive, HER2-negative breast cancers could be spared the toxicities of cytotoxic chemotherapies without compromising the prognosis.
Beyond chemotherapy, endocrine therapies also have risks, especially when given over extended periods of time. Recently, extended endocrine therapy has been shown to prevent late recurrences of HR-positive breast cancers. In the National Cancer Institute of Canada Clinical Trials Group’s MA.17R study, extended endocrine therapy with letrozole for a total of 10 years (beyond 5 years of an aromatase inhibitor [AI]) decreased the risk for breast cancer recurrence or the occurrence of contralateral breast cancer by 34%.2 However, the overall survival was similar between the 2 groups and the disease-free survival benefits were not confirmed in other studies.3–5 Identifying the subgroup of patients who benefit from this extended AI therapy is important in the era of personalized medicine. Several tumor genomic assays have been developed to provide additional prognostic and predictive information with the goal of individualizing adjuvant therapies for breast cancer. Although assays are also being evaluated in HER2-positive and triple-negative breast cancer, this review will focus on HR-positive, HER2-negative breast cancer.
Tests for Guiding Adjuvant Chemotherapy Decisions
Case Study
Initial Presentation
A 54-year-old postmenopausal woman with no significant past medical history presents with an abnormal screening mammogram, which shows a focal asymmetry in the 10 o’clock position at middle depth of the left breast. Further work-up with a diagnostic mammogram and ultrasound of the left breast shows a suspicious hypoechoic solid mass with irregular margins measuring 17 mm. The patient undergoes an ultrasound-guided core needle biopsy of the suspicious mass, the results of which are consistent with an invasive ductal carcinoma, Nottingham grade 2, ER strongly positive (95%), PR weakly positive (5%), HER2-negative, and Ki-67 of 15%. She undergoes a left partial mastectomy and sentinel lymph node biopsy, with final pathology demonstrating a single focus of invasive ductal carcinoma, measuring 2.2 cm in greatest dimension with no evidence of lymphovascular invasion. Margins are clear and 2 sentinel lymph nodes are negative for metastatic disease (final pathologic stage IIA, pT2 pN0 cM0). She is referred to medical oncology to discuss adjuvant systemic therapy.
- Can additional testing be used to determine prognosis and guide systemic therapy recommendations for early-stage HR-positive/HER2-negative breast cancer?
After a diagnosis of early-stage breast cancer, the key clinical question faced by the patient and medical oncologist is: what is the individual’s risk for a metastatic breast cancer recurrence and thus the risk for death due to breast cancer? Once the risk for recurrence is established, systemic adjuvant chemotherapy, endocrine therapy, and/or HER2-directed therapy are considered based on the receptor status (ER/PR and HER2) to reduce this risk. HR-positive, HER2-negative breast cancer is the most common type of breast cancer. Although adjuvant endocrine therapy has significantly reduced the risk for recurrence and improved survival for patients with HR-positive breast cancer,6 the role of adjuvant chemotherapy for this subset of breast cancer remains unclear. Prior to genomic testing, the recommendation for adjuvant chemotherapy for HR-positive/HER2-negative tumors was primarily based on patient age and tumor stage and grade. However, chemotherapy overtreatment remained a concern given the potential short- and long-term risks of chemotherapy. Further studies into HR-positive/HER2-negative tumors have shown that these tumors can be divided into 2 main subtypes, luminal A and luminal B.7 These subtypes represent unique biology and differ in terms of prognosis and response to endocrine therapy and chemotherapy. Luminal A tumors are strongly endocrine responsive and have a good prognosis, while luminal B tumors are less endocrine responsive and are associated with a poorer prognosis; the addition of adjuvant chemotherapy is often considered for luminal B tumors.8 Several tests, including tumor genomic assays, are now available to help with delineating the tumor subtype and aid in decision-making regarding adjuvant chemotherapy for HR-positive/HER2-negative breast cancers.
Ki-67 Assays, Including IHC4 and PEPI
Proliferation is a hallmark of cancer cells.9 Ki-67, a nuclear nonhistone protein whose expression varies in intensity throughout the cell cycle, has been used as a measurement of tumor cell proliferation.10 Two large meta-analyses have demonstrated that high Ki-67 expression in breast tumors is independently associated with worse disease-free and overall survival rates.11,12 Ki-67 expression has also been used to classify HR-positive tumors as luminal A or B. After classifying tumor subtypes based on intrinsic gene expression profiling, Cheang and colleagues determined that a Ki-67 cut point of 13.25% differentiated luminal A and B tumors.13 However, the ideal cut point for Ki-67 remains unclear, as the sensitivity and specificity in this study was 77% and 78%, respectively. Others have combined Ki-67 with standard ER, PR, and HER2 testing. This immunohistochemical 4 (IHC4) score, which weighs each of these variables, was validated in postmenopausal patients from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial who had ER-positive tumors and did not receive chemotherapy.14 The prognostic information from the IHC4 was similar to that seen with the 21-gene recurrence score (Oncotype DX), which is discussed later in this article. The key challenge with Ki-67 testing currently is the lack of a validated test methodology and intra-observer variability in interpreting the Ki-67 results.15 Recent series have suggested that Ki-67 be considered as a continuous marker rather than a set cut point.16 These issues continue to impact the clinical utility of Ki-67 for decision-making for adjuvant chemotherapy.
Ki-67 and the preoperative endocrine prognostic index (PEPI) score have been explored in the neoadjuvant setting to separate postmenopausal women with endocrine-sensitive versus intrinsically resistant disease and identify patients at risk for recurrent disease.17 The on-treatment levels of Ki-67 in response to endocrine therapy have been shown to be more prognostic than baseline values, and a decrease in Ki-67 as early as 2 weeks after initiation of neoadjuvant endocrine therapy is associated with endocrine-sensitive tumors and improved outcome. The PEPI score was developed through retrospective analysis of the P024 trial18 to evaluate the relationship between post-neoadjuvant endocrine therapy tumor characteristics and risk for early relapse. The score was subsequently validated in an independent data set from the IMPACT (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen) trial.19 Patients with low pathological stage (0 or 1) and a favorable biomarker profile (PEPI score 0) at surgery had the best prognosis in the absence of chemotherapy. On the other hand, higher pathological stage at surgery and a poor biomarker profile with loss of ER positivity or persistently elevated Ki-67 (PEPI score of 3) identified de novo endocrine-resistant tumors that are higher risk for early relapse.20 The ongoing Alliance A011106 ALTERNATE trial (ALTernate approaches for clinical stage II or III Estrogen Receptor positive breast cancer NeoAdjuvant TrEatment in postmenopausal women, NCT01953588) is a phase 3 study to prospectively test this hypothesis.
21-Gene Recurrence Score (Onco type DX Assay)
The 21-gene Oncotype DX assay is conducted on paraffin-embedded tumor tissue and measures the expression of 16 cancer related genes and 5 reference genes using quantitative polymerase chain reaction (PCR). The genes included in this assay are mainly related to proliferation (including Ki-67), invasion, and HER2 or estrogen signaling.21 Originally, the 21-gene recurrence score assay was analyzed as a prognostic biomarker tool in a prospective-retrospective biomarker substudy of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 clinical trial in which patients with node-negative, ER-positive tumors were randomly assigned to receive tamoxifen or placebo without chemotherapy.22 Using the standard reported values of low risk (< 18), intermediate risk (18–30), or high risk (≥ 31) for recurrence, among the tamoxifen-treated patients, cancers with a high-risk recurrence score had a significantly worse rate of distant recurrence and overall survival.21 Inferior breast cancer survival in cancers with a high recurrence score was also confirmed in other series of endocrine-treated patients with node-negative and node-positive disease.23–25
The predictive utility of the 21-gene recurrence score for endocrine therapy has also been evaluated. A comparison of the placebo- and tamoxifen-treated patients from the NSABP B-14 trial demonstrated that the 21-gene recurrence score predicted benefit from tamoxifen in cancers with low- or intermediate-risk recurrence scores.26 However, there was no benefit from the use of tamoxifen over placebo in cancers with high-risk recurrence scores. To date, this intriguing data has not been prospectively confirmed, and thus the 21-gene recurrence score is not used to avoid endocrine therapy.
The 21-gene recurrence score is primarily used by oncologists to aid in decision-making regarding adjuvant chemotherapy in patients with node-negative and node-positive (with up to 3 positive lymph nodes), HR-positive/HER2-negative breast cancers. The predictive utility of the 21-gene recurrence score for adjuvant chemotherapy was initially tested using tumor samples from the NSABP B-20 study. This study initially compared adjuvant tamoxifen alone with tamoxifen plus chemotherapy in patients with node-negative, HR-positive tumors. The prospective-retrospective biomarker analysis showed that the patients with high-risk 21-gene recurrence scores benefited from the addition of chemotherapy, whereas those with low or intermediate risk did not have an improved freedom from distant recurrence with chemotherapy.27 Similarly, an analysis from the prospective phase 3 Southwest Oncology Group (SWOG) 8814 trial comparing tamoxifen to tamoxifen with chemotherapy showed that for node-positive tumors, chemotherapy benefit was only seen in those with high 21-gene recurrence scores.24
Prospective studies are now starting to report results regarding the predictive role of the 21-gene recurrence score. The TAILORx (Trial Assigning Individualized Options for Treatment) trial includes women with node-negative, HR-positive/HER2-negative tumors measuring 0.6 to 5 cm. All patients were treated with standard-of-care endocrine therapy for at least 5 years. Chemotherapy was determined based on the 21-gene recurrence score results on the primary tumor. The 21-gene recurrence score cutoffs were changed to low (0–10), intermediate (11–25), and high (≥ 26). Patients with scores of 26 or higher were treated with chemotherapy, and those with intermediate scores were randomly assigned to chemotherapy or no chemotherapy; results from this cohort are still pending. However, excellent breast cancer outcomes with endocrine therapy alone were reported from the 1626 (15.9% of total cohort) prospectively followed patients with low recurrence score tumors. The 5-year invasive disease-free survival was 93.8%, with overall survival of 98%.28 Given that 5 years is appropriate follow-up to see any chemotherapy benefit, this data supports the recommendation for no chemotherapy in this cohort of patients with very low 21-gene recurrence scores.
The RxPONDER (Rx for Positive Node, Endocrine Responsive Breast Cancer) trial is evaluating women with 1 to 3 node-positive, HR-positive, HER2-negative tumors. In this trial, patients with 21-gene recurrence scores of 0 to 25 were assigned to adjuvant chemotherapy or none. Those with scores of 26 or higher were assigned to chemotherapy. All patients received standard adjuvant endocrine therapy. This study has completed accrual and results are pending. Of note, TAILORx and RxPONDER did not investigate the potential lack of benefit of endocrine therapy in cancers with high recurrence scores. Furthermore, despite data suggesting that chemotherapy may not even benefit women with 4 or more nodes involved but who have a low recurrence score,24 due to the lack of prospective data in this cohort and the quite high risk for distant recurrence, chemotherapy continues to be the standard of care for these patients.
PAM50 (Breast Cancer Prognostic Gene Signature)
Using microarray and quantitative reverse transcriptase PCR (RT-PCR) on formalin-fixed paraffin-embedded (FFPE) tissues, the Breast Cancer Prognostic Gene Signature (PAM50) assay was initially developed to identify intrinsic breast cancer subtypes, including luminal A, luminal B, HER2-enriched, and basal-like.7,29 Based on the prediction analysis of microarray (PAM) method, the assay measures the expression levels of 50 genes, provides a risk category (low, intermediate, and high), and generates a numerical risk of recurrence score (ROR). The intrinsic subtype and ROR have been shown to add significant prognostic value to the clinicopathological characteristics of tumors. Clinical validity of PAM50 was evaluated in postmenopausal women with HR-positive early-stage breast cancer treated in the prospective ATAC and ABCSG-8 (Austrian Breast and Colorectal Cancer Study Group 8) trials.30,31 In 1017 patients with ER-positive breast cancer treated with anastrozole or tamoxifen in the ATAC trial, ROR added significant prognostic information beyond the clinical treatment score (integrated prognostic information from nodal status, tumor size, histopathologic grade, age, and anastrozole or tamoxifen treatment) in all patients. Also, compared with the 21-gene recurrence score, ROR provided more prognostic information in ER-positive, node-negative disease and better differentiation of intermediate- and higher-risk groups. Fewer patients were categorized as intermediate risk by ROR and more as high risk, which could reduce the uncertainty in the estimate of clinical benefit from chemotherapy.30 The clinical utility of PAM50 as a prognostic model was also validated in 1478 postmenopausal women with ER-positive early-stage breast cancer enrolled in the ABCSG-8 trial. In this study, ROR assigned 47% of patients with node-negative disease to the low-risk category. In this low-risk group, the 10-year metastasis risk was less than 3.5%, indicating lack of benefit from additional chemotherapy.31 A key limitation of the PAM50 is the lack of any prospective studies with this assay.
PAM50 has been designed to be carried out in any qualified pathology laboratory. Moreover, the ROR score provides additional prognostic information about risk of late recurrence, which will be discussed in the next section.
70-Gene Breast Cancer Recurrence Assay (MammaPrint)
MammaPrint is a 70-gene assay that was initially developed using an unsupervised, hierarchical clustering algorithm on whole-genome expression arrays with early-stage breast cancer. Among 295 consecutive patients who had MammaPrint testing, those classified with a good-prognosis tumor signature (n = 115) had an excellent 10-year survival rate (94.5%) compared to those with a poor-prognosis signature (54.5%), and the signature remained prognostic upon multivariate analysis.32 Subsequently, a pooled analysis comparing outcomes by MammaPrint score in patients with node-negative or 1 to 3 node-positive breast cancers treated as per discretion of their medical team with either adjuvant chemotherapy plus endocrine therapy or endocrine therapy alone reported that only those patients with a high-risk score benefited from chemotherapy.33 Recently, a prospective phase 3 study (MINDACT [Microarray In Node negative Disease may Avoid ChemoTherapy]) evaluating the utility of MammaPrint for adjuvant chemotherapy decision-making reported results.34 In this study, 6693 women with early-stage breast cancer were assessed by clinical risk and genomic risk using MammaPrint. Those with low clinical and genomic risk did not receive chemotherapy, while those with high clinical and genomic risk all received chemotherapy. The primary goal of the study was to assess whether forgoing chemotherapy would be associated with a low rate of recurrence in those patients with a low-risk prognostic MammaPrint signature but high clinical risk. A total of 1550 patients (23.2%) were in the discordant group, and the majority of these patients had HR-positive disease (98.1%). Without chemotherapy, the rate of survival without distant metastasis at 5 years in this group was 94.7% (95% confidence interval [CI] 92.5% to 96.2%), which met the primary endpoint. Of note, initially, MammaPrint was only available for fresh tissue analysis, but recent advances in RNA processing now allow for this analysis on FFPE tissue.35
Summary
These genomic and biomarker assays can identify different subsets of HR-positive breast cancers, including those patients who have tumors with an excellent prognosis with endocrine therapies alone. Thus, we now have the tools to help avoid the toxicities of chemotherapy in many women with early-stage breast cancer.
Tests for Assessing Risk for Late Recurrence
Case Continued
The patient undergoes 21-gene recurrence score testing, which shows a low recurrence score of 10, estimating the 10-year risk of distant recurrence to be approximately 7% with 5 years of tamoxifen. Chemotherapy is not recommended. The patient completes adjuvant whole breast radiation therapy, and then, based on data supporting AIs over tamoxifen in postmenopausal women, she is started on anastrozole.41 She initially experiences mild side effects from treatment, including fatigue, arthralgia, and vaginal dryness, but her symptoms are able to be managed. As she approaches 5 years of adjuvant endocrine therapy with anastrozole, she is struggling with rotator cuff injury and is anxious about recurrence, but has no evidence of recurrent cancer. Her bone density scan in the beginning of her fourth year of therapy shows a decrease in bone mineral density, with the lowest T score of –1.5 at the left femoral neck, consistent with osteopenia. She has been treated with calcium and vitamin D supplements.
- How long should this patient continue treatment with anastrozole?
The risk for recurrence is highest during the first 5 years after diagnosis for all patients with early breast cancer.42 Although HR-positive breast cancers have a better prognosis than HR-negative disease, the pattern of recurrence is different between the 2 groups, and it is estimated that approximately half of the recurrences among patients with HR-positive early breast cancer occur after the first 5 years from diagnosis. Annualized hazard of recurrence in HR-positive breast cancer has been shown to remain elevated and fairly stable beyond 10 years, even for those with low tumor burden and node-negative disease.43 Prospective trials showed that for women with HR-positive early breast cancer, 5 years of adjuvant tamoxifen could substantially reduce recurrence rates and improve survival, and this became the standard of care.44 AIs are considered the standard of care for adjuvant endocrine therapy in most postmenopausal women, as they result in a significantly lower recurrence rate compared with tamoxifen, either as initial adjuvant therapy or sequentially following 2 to 3 years of tamoxifen.45
Due to the risk for later recurrences with HR-positive breast cancer, more patients and oncologists are considering extended endocrine therapy. This is based on results from the ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) and aTTOM (Adjuvant Tamoxifen–To Offer More?) studies, both of which showed that women with HR-positive breast cancer who continued tamoxifen for 10 years had a lower late recurrence rate and a lower breast cancer mortality rate compared with those who stopped at 5 years.46,47 Furthermore, the NCIC MA.17 trial evaluated extended endocrine therapy in postmenopausal women with 5 years of letrozole following 5 years of tamoxifen. Letrozole was shown to improve both disease-free and distant disease-free survival. The overall survival benefit was limited to patients with node-positive disease.48 A summary of studies of extended endocrine therapy for HR-positive breast cancers is shown in Table 2.2,3,46–49
However, extending AI therapy from 5 years to 10 years is not clearly beneficial. In the MA.17R trial, although longer AI therapy resulted in significantly better disease-free survival (95% versus 91%, hazard ratio 0.66, P = 0.01), this was primarily due to a lower incidence of contralateral breast cancer in those taking the AI compared with placebo. The distant recurrence risks were similar and low (4.4% versus 5.5%), and there was no overall survival difference.2 Also, the NSABP B-42 study, which was presented at the 2016 San Antonio Breast Cancer Symposium, did not meet its predefined endpoint for benefit from extending adjuvant AI therapy with letrozole beyond 5 years.3 Thus, the absolute benefit from extended endocrine therapy has been modest across these studies. Although endocrine therapy is considered relatively safe and well tolerated, side effects can be significant and even associated with morbidity. Ideally, extended endocrine therapy should be offered to the subset of patients who would benefit the most. Several genomic diagnostic assays, including the EndoPredict test, PAM50, and the Breast Cancer Index (BCI) tests, specifically assess the risk for late recurrence in HR-positive cancers.
PAM50
Studies suggest that the ROR score also has value in predicting late recurrences. Analysis of data in patients enrolled in the ABCSG-8 trial showed that ROR could identify patients with endocrine-sensitive disease who are at low risk for late relapse and could be spared from unwanted toxicities of extended endocrine therapies. In 1246 ABCSG-8 patients between years 5 and 15, the PAM50 ROR demonstrated an absolute risk of distant recurrence of 2.4% in the low-risk group, as compared with 17.5% in the high-risk group.50 Also, a combined analysis of patients from both the ATAC and ABCSG-8 trials demonstrated the utility of ROR in identifying this subgroup of patients with low risk for late relapse.51
EndoPredict
EndoPredict is another quantitative RT-PCR–based assay which uses FFPE tissues to calculate a risk score based on 8 cancer-related and 3 reference genes. The score is combined with clinicopathological factors including tumor size and nodal status to make a comprehensive risk score (EPclin). EPclin is used to dichotomize patients into EndoPredict low- and high-risk groups. EndoPredict has been validated in 2 cohorts of patients enrolled in separate randomized studies, ABCSG-6 and ABCSG-8. EP provided prognostic information beyond clinicopathological variables to predict distant recurrence in patients with HR-positive/HER2-negative early breast cancer.37 More important, EndoPredict has been shown to predict early (years 0–5) versus late (> 5 years after diagnosis) recurrences and identify a low-risk subset of patients who would not be expected to benefit from further treatment beyond 5 years of endocrine therapy.52 Recently, EndoPredict and EPclin were compared with the 21-gene (Oncotype DX) recurrence score in a patient population from the TransATAC study. Both EndoPredict and EPclin provided more prognostic information compared to the 21-gene recurrence score and identified early and late relapse events.53 EndoPredict is the first multigene expression assay that could be routinely performed in decentralized molecular pathological laboratories with a short turnaround time.54
Breast Cancer Index
The BCI is a RT-PCR–based gene expression assay that consists of 2 gene expression biomarkers: molecular grade index (MGI) and HOXB13/IL17BR (H/I). The BCI was developed as a prognostic test to assess risk for breast cancer recurrence using a cohort of ER-positive patients (n = 588) treated with adjuvant tamoxifen versus observation from the prospective randomized Stockholm trial.38 In this blinded retrospective study, H/I and MGI were measured and a continuous risk model (BCI) was developed in the tamoxifen-treated group. More than 50% of the patients in this group were classified as having a low risk of recurrence. The rate of distant recurrence or death in this low-risk group at 10 years was less than 3%. The performance of the BCI model was then tested in the untreated arm of the Stockholm trial. In the untreated arm, BCI classified 53%, 27%, and 20% of patients as low, intermediate, and high risk, respectively. The rate of distant metastasis at 10 years in these risk groups was 8.3% (95% CI 4.7% to 14.4%), 22.9% (95% CI 14.5% to 35.2%), and 28.5% (95% CI 17.9% to 43.6%), respectively, and the rate of breast cancer–specific mortality was 5.1% (95% CI 1.3% to 8.7%), 19.8% (95% CI 10.0% to 28.6%), and 28.8% (95% CI 15.3% to 40.2%).38
The prognostic and predictive values of the BCI have been validated in other large, randomized studies and in patients with both node-negative and node-positive disease.39,55 The predictive value of the endocrine-response biomarker, the H/I ratio, has been demonstrated in randomized studies. In the MA.17 trial, a high H/I ratio was associated with increased risk for late recurrence in the absence of letrozole. However, extended endocrine therapy with letrozole in patients with high H/I ratios predicted benefit from therapy and decreased the probability of late disease recurrence.56 BCI was also compared to IHC4 and the 21-gene recurrence score in the TransATAC study and was the only test to show prognostic significance for both early (0–5 years) and late (5–10 year) recurrence.40
The impact of the BCI results on physicians’ recommendations for extended endocrine therapy was assessed by a prospective study. This study showed that the test result had a significant effect on both physician treatment recommendation and patient satisfaction. BCI testing resulted in a change in physician recommendations for extended endocrine therapy, with an overall decrease in recommendations for extended endocrine therapy from 74% to 54%. Knowledge of the test result also led to improved patient satisfaction and decreased anxiety.57
Summary
Due to the risk for late recurrence, extended endocrine therapy is being recommended for many patients with HR-positive breast cancers. Multiple genomic assays are being developed to better understand an individual’s risk for late recurrence and the potential for benefit from extended endocrine therapies. However, none of the assays has been validated in prospective randomized studies. Further validation is needed prior to routine use of these assays.
Case Continued
A BCI test is done and the result shows 4.3% BCI low-risk category in years 5–10, which is consistent with a low likelihood of benefit from extended endocrine therapy. After discussing the results of the BCI test in the context of no survival benefit from extending AIs beyond 5 years, both the patient and her oncologist feel comfortable with discontinuing endocrine therapy at the end of 5 years.
Conclusion
Reduction in breast cancer mortality is mainly the result of improved systemic treatments. With advances in breast cancer screening tools in recent years, the rate of cancer detection has increased. This has raised concerns regarding overdiagnosis. To prevent unwanted toxicities associated with overtreatment, better treatment decision tools are needed. Several genomic assays are currently available and widely used to provide prognostic and predictive information and aid in decisions regarding appropriate use of adjuvant chemotherapy in HR-positive/HER2-negative early-stage breast cancer. Ongoing studies are refining the cutoffs for these assays and expanding the applicability to node-positive breast cancers. Furthermore, with several studies now showing benefit from the use of extended endocrine therapy, some of these assays may be able to identify the subset of patients who are at increased risk for late recurrence and who might benefit from extended endocrine therapy. Advances in molecular testing has enabled clinicians to offer more personalized treatments to their patients, improve patients’ compliance, and decrease anxiety and conflict associated with management decisions. Although small numbers of patients with HER2-positive and triple-negative breast cancers were also included in some of these studies, use of genomic assays in this subset of patients is very limited and currently not recommended.
1. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–47.
2. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19.
3. Mamounas E, Bandos H, Lembersky B. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy with letrozole in postmenopausal women with hormone-receptor-positive breast cancer who have completed previous adjuvant treatment with an aromatase inhibitor. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-05.
4. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, et al: First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-03.
5. Blok EJ, Van de Velde CJH, Meershoek-Klein Kranenbarg EM, et al: Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-04.
6. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2005;365:1687–717.
7. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
8. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46.
9. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
10. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212–20.
11. de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13.
12. Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–91.
13. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50.
14. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and com-parison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–8.
15. Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol 2013;66:512–6.
16. Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 2015; 24 Suppl 2:S67–72.
17. Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol 2016;882:125–54.
18. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32.
19. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anas-trozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16.
20. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–8.
21. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–26.
22. Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004;364:858–68.
23. Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25.
24. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65.
25. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829–34.
26. Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol 2005;23: suppl:510.
27. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34.
28. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14.
29. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
30. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783–90.
31. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 post-menopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339–45.
32. van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009.
33. Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 2010;120:655–61.
34. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29.
35. Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2014;16:190–7.
36. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222–32.
37. Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011;17:6012–20.
38. Jerevall PL, Ma XJ, Li H, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 2011;104:1762–9.
39. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205.
40. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067–76.
41. Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract 2010;6:243–6.
42. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46.
43. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 2016;34:927–35.
44. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84.
45. Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52.
46. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16.
47. Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31 (suppl):5.
48. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262–71.
49. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71.
50. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298–305.
51. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–22.
52. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013;109:2959–64.
53. Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX Recurrence Score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 2016;108:djw149.
54. Muller BM, Keil E, Lehmann A, et al. The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PLoS One 2013;8:e68252.
55. Sgroi DC, Chapman JA, Badovinac-Crnjevic T, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016;18:1.
56. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42.
57. Sanft T, Aktas B, Schroeder B, et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat 2015;154:533–41.
Introduction
Over the past several decades, while the incidence of breast cancer has increased, breast cancer mortality has decreased. This decrease is likely due to both early detection and advances in systemic therapy. However, with more widespread use of screening mammography, there are increasing concerns about potential overdiagnosis of cancer.1 One key challenge is that breast cancer is a heterogeneous disease. Improved tools for determining breast cancer biology can help physicians individualize treatments. Patients with low-risk cancers can be approached with less aggressive treatments, thus preventing unnecessary toxicities, while those with higher-risk cancers remain treated appropriately with more aggressive therapies.
Traditionally, adjuvant chemotherapy was recommended based on tumor features such as stage (tumor size, regional nodal involvement), grade, expression of hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor-2 (HER2), and patient features (age, menopausal status). However, this approach is not accurate enough to guide individualized treatment approaches, which are based on the risk for recurrence and the reduction in this risk that can be achieved with various systemic treatments. In particular, women with low-risk hormone receptor (HR)–positive, HER2-negative breast cancers could be spared the toxicities of cytotoxic chemotherapies without compromising the prognosis.
Beyond chemotherapy, endocrine therapies also have risks, especially when given over extended periods of time. Recently, extended endocrine therapy has been shown to prevent late recurrences of HR-positive breast cancers. In the National Cancer Institute of Canada Clinical Trials Group’s MA.17R study, extended endocrine therapy with letrozole for a total of 10 years (beyond 5 years of an aromatase inhibitor [AI]) decreased the risk for breast cancer recurrence or the occurrence of contralateral breast cancer by 34%.2 However, the overall survival was similar between the 2 groups and the disease-free survival benefits were not confirmed in other studies.3–5 Identifying the subgroup of patients who benefit from this extended AI therapy is important in the era of personalized medicine. Several tumor genomic assays have been developed to provide additional prognostic and predictive information with the goal of individualizing adjuvant therapies for breast cancer. Although assays are also being evaluated in HER2-positive and triple-negative breast cancer, this review will focus on HR-positive, HER2-negative breast cancer.
Tests for Guiding Adjuvant Chemotherapy Decisions
Case Study
Initial Presentation
A 54-year-old postmenopausal woman with no significant past medical history presents with an abnormal screening mammogram, which shows a focal asymmetry in the 10 o’clock position at middle depth of the left breast. Further work-up with a diagnostic mammogram and ultrasound of the left breast shows a suspicious hypoechoic solid mass with irregular margins measuring 17 mm. The patient undergoes an ultrasound-guided core needle biopsy of the suspicious mass, the results of which are consistent with an invasive ductal carcinoma, Nottingham grade 2, ER strongly positive (95%), PR weakly positive (5%), HER2-negative, and Ki-67 of 15%. She undergoes a left partial mastectomy and sentinel lymph node biopsy, with final pathology demonstrating a single focus of invasive ductal carcinoma, measuring 2.2 cm in greatest dimension with no evidence of lymphovascular invasion. Margins are clear and 2 sentinel lymph nodes are negative for metastatic disease (final pathologic stage IIA, pT2 pN0 cM0). She is referred to medical oncology to discuss adjuvant systemic therapy.
- Can additional testing be used to determine prognosis and guide systemic therapy recommendations for early-stage HR-positive/HER2-negative breast cancer?
After a diagnosis of early-stage breast cancer, the key clinical question faced by the patient and medical oncologist is: what is the individual’s risk for a metastatic breast cancer recurrence and thus the risk for death due to breast cancer? Once the risk for recurrence is established, systemic adjuvant chemotherapy, endocrine therapy, and/or HER2-directed therapy are considered based on the receptor status (ER/PR and HER2) to reduce this risk. HR-positive, HER2-negative breast cancer is the most common type of breast cancer. Although adjuvant endocrine therapy has significantly reduced the risk for recurrence and improved survival for patients with HR-positive breast cancer,6 the role of adjuvant chemotherapy for this subset of breast cancer remains unclear. Prior to genomic testing, the recommendation for adjuvant chemotherapy for HR-positive/HER2-negative tumors was primarily based on patient age and tumor stage and grade. However, chemotherapy overtreatment remained a concern given the potential short- and long-term risks of chemotherapy. Further studies into HR-positive/HER2-negative tumors have shown that these tumors can be divided into 2 main subtypes, luminal A and luminal B.7 These subtypes represent unique biology and differ in terms of prognosis and response to endocrine therapy and chemotherapy. Luminal A tumors are strongly endocrine responsive and have a good prognosis, while luminal B tumors are less endocrine responsive and are associated with a poorer prognosis; the addition of adjuvant chemotherapy is often considered for luminal B tumors.8 Several tests, including tumor genomic assays, are now available to help with delineating the tumor subtype and aid in decision-making regarding adjuvant chemotherapy for HR-positive/HER2-negative breast cancers.
Ki-67 Assays, Including IHC4 and PEPI
Proliferation is a hallmark of cancer cells.9 Ki-67, a nuclear nonhistone protein whose expression varies in intensity throughout the cell cycle, has been used as a measurement of tumor cell proliferation.10 Two large meta-analyses have demonstrated that high Ki-67 expression in breast tumors is independently associated with worse disease-free and overall survival rates.11,12 Ki-67 expression has also been used to classify HR-positive tumors as luminal A or B. After classifying tumor subtypes based on intrinsic gene expression profiling, Cheang and colleagues determined that a Ki-67 cut point of 13.25% differentiated luminal A and B tumors.13 However, the ideal cut point for Ki-67 remains unclear, as the sensitivity and specificity in this study was 77% and 78%, respectively. Others have combined Ki-67 with standard ER, PR, and HER2 testing. This immunohistochemical 4 (IHC4) score, which weighs each of these variables, was validated in postmenopausal patients from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial who had ER-positive tumors and did not receive chemotherapy.14 The prognostic information from the IHC4 was similar to that seen with the 21-gene recurrence score (Oncotype DX), which is discussed later in this article. The key challenge with Ki-67 testing currently is the lack of a validated test methodology and intra-observer variability in interpreting the Ki-67 results.15 Recent series have suggested that Ki-67 be considered as a continuous marker rather than a set cut point.16 These issues continue to impact the clinical utility of Ki-67 for decision-making for adjuvant chemotherapy.
Ki-67 and the preoperative endocrine prognostic index (PEPI) score have been explored in the neoadjuvant setting to separate postmenopausal women with endocrine-sensitive versus intrinsically resistant disease and identify patients at risk for recurrent disease.17 The on-treatment levels of Ki-67 in response to endocrine therapy have been shown to be more prognostic than baseline values, and a decrease in Ki-67 as early as 2 weeks after initiation of neoadjuvant endocrine therapy is associated with endocrine-sensitive tumors and improved outcome. The PEPI score was developed through retrospective analysis of the P024 trial18 to evaluate the relationship between post-neoadjuvant endocrine therapy tumor characteristics and risk for early relapse. The score was subsequently validated in an independent data set from the IMPACT (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen) trial.19 Patients with low pathological stage (0 or 1) and a favorable biomarker profile (PEPI score 0) at surgery had the best prognosis in the absence of chemotherapy. On the other hand, higher pathological stage at surgery and a poor biomarker profile with loss of ER positivity or persistently elevated Ki-67 (PEPI score of 3) identified de novo endocrine-resistant tumors that are higher risk for early relapse.20 The ongoing Alliance A011106 ALTERNATE trial (ALTernate approaches for clinical stage II or III Estrogen Receptor positive breast cancer NeoAdjuvant TrEatment in postmenopausal women, NCT01953588) is a phase 3 study to prospectively test this hypothesis.
21-Gene Recurrence Score (Onco type DX Assay)
The 21-gene Oncotype DX assay is conducted on paraffin-embedded tumor tissue and measures the expression of 16 cancer related genes and 5 reference genes using quantitative polymerase chain reaction (PCR). The genes included in this assay are mainly related to proliferation (including Ki-67), invasion, and HER2 or estrogen signaling.21 Originally, the 21-gene recurrence score assay was analyzed as a prognostic biomarker tool in a prospective-retrospective biomarker substudy of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 clinical trial in which patients with node-negative, ER-positive tumors were randomly assigned to receive tamoxifen or placebo without chemotherapy.22 Using the standard reported values of low risk (< 18), intermediate risk (18–30), or high risk (≥ 31) for recurrence, among the tamoxifen-treated patients, cancers with a high-risk recurrence score had a significantly worse rate of distant recurrence and overall survival.21 Inferior breast cancer survival in cancers with a high recurrence score was also confirmed in other series of endocrine-treated patients with node-negative and node-positive disease.23–25
The predictive utility of the 21-gene recurrence score for endocrine therapy has also been evaluated. A comparison of the placebo- and tamoxifen-treated patients from the NSABP B-14 trial demonstrated that the 21-gene recurrence score predicted benefit from tamoxifen in cancers with low- or intermediate-risk recurrence scores.26 However, there was no benefit from the use of tamoxifen over placebo in cancers with high-risk recurrence scores. To date, this intriguing data has not been prospectively confirmed, and thus the 21-gene recurrence score is not used to avoid endocrine therapy.
The 21-gene recurrence score is primarily used by oncologists to aid in decision-making regarding adjuvant chemotherapy in patients with node-negative and node-positive (with up to 3 positive lymph nodes), HR-positive/HER2-negative breast cancers. The predictive utility of the 21-gene recurrence score for adjuvant chemotherapy was initially tested using tumor samples from the NSABP B-20 study. This study initially compared adjuvant tamoxifen alone with tamoxifen plus chemotherapy in patients with node-negative, HR-positive tumors. The prospective-retrospective biomarker analysis showed that the patients with high-risk 21-gene recurrence scores benefited from the addition of chemotherapy, whereas those with low or intermediate risk did not have an improved freedom from distant recurrence with chemotherapy.27 Similarly, an analysis from the prospective phase 3 Southwest Oncology Group (SWOG) 8814 trial comparing tamoxifen to tamoxifen with chemotherapy showed that for node-positive tumors, chemotherapy benefit was only seen in those with high 21-gene recurrence scores.24
Prospective studies are now starting to report results regarding the predictive role of the 21-gene recurrence score. The TAILORx (Trial Assigning Individualized Options for Treatment) trial includes women with node-negative, HR-positive/HER2-negative tumors measuring 0.6 to 5 cm. All patients were treated with standard-of-care endocrine therapy for at least 5 years. Chemotherapy was determined based on the 21-gene recurrence score results on the primary tumor. The 21-gene recurrence score cutoffs were changed to low (0–10), intermediate (11–25), and high (≥ 26). Patients with scores of 26 or higher were treated with chemotherapy, and those with intermediate scores were randomly assigned to chemotherapy or no chemotherapy; results from this cohort are still pending. However, excellent breast cancer outcomes with endocrine therapy alone were reported from the 1626 (15.9% of total cohort) prospectively followed patients with low recurrence score tumors. The 5-year invasive disease-free survival was 93.8%, with overall survival of 98%.28 Given that 5 years is appropriate follow-up to see any chemotherapy benefit, this data supports the recommendation for no chemotherapy in this cohort of patients with very low 21-gene recurrence scores.
The RxPONDER (Rx for Positive Node, Endocrine Responsive Breast Cancer) trial is evaluating women with 1 to 3 node-positive, HR-positive, HER2-negative tumors. In this trial, patients with 21-gene recurrence scores of 0 to 25 were assigned to adjuvant chemotherapy or none. Those with scores of 26 or higher were assigned to chemotherapy. All patients received standard adjuvant endocrine therapy. This study has completed accrual and results are pending. Of note, TAILORx and RxPONDER did not investigate the potential lack of benefit of endocrine therapy in cancers with high recurrence scores. Furthermore, despite data suggesting that chemotherapy may not even benefit women with 4 or more nodes involved but who have a low recurrence score,24 due to the lack of prospective data in this cohort and the quite high risk for distant recurrence, chemotherapy continues to be the standard of care for these patients.
PAM50 (Breast Cancer Prognostic Gene Signature)
Using microarray and quantitative reverse transcriptase PCR (RT-PCR) on formalin-fixed paraffin-embedded (FFPE) tissues, the Breast Cancer Prognostic Gene Signature (PAM50) assay was initially developed to identify intrinsic breast cancer subtypes, including luminal A, luminal B, HER2-enriched, and basal-like.7,29 Based on the prediction analysis of microarray (PAM) method, the assay measures the expression levels of 50 genes, provides a risk category (low, intermediate, and high), and generates a numerical risk of recurrence score (ROR). The intrinsic subtype and ROR have been shown to add significant prognostic value to the clinicopathological characteristics of tumors. Clinical validity of PAM50 was evaluated in postmenopausal women with HR-positive early-stage breast cancer treated in the prospective ATAC and ABCSG-8 (Austrian Breast and Colorectal Cancer Study Group 8) trials.30,31 In 1017 patients with ER-positive breast cancer treated with anastrozole or tamoxifen in the ATAC trial, ROR added significant prognostic information beyond the clinical treatment score (integrated prognostic information from nodal status, tumor size, histopathologic grade, age, and anastrozole or tamoxifen treatment) in all patients. Also, compared with the 21-gene recurrence score, ROR provided more prognostic information in ER-positive, node-negative disease and better differentiation of intermediate- and higher-risk groups. Fewer patients were categorized as intermediate risk by ROR and more as high risk, which could reduce the uncertainty in the estimate of clinical benefit from chemotherapy.30 The clinical utility of PAM50 as a prognostic model was also validated in 1478 postmenopausal women with ER-positive early-stage breast cancer enrolled in the ABCSG-8 trial. In this study, ROR assigned 47% of patients with node-negative disease to the low-risk category. In this low-risk group, the 10-year metastasis risk was less than 3.5%, indicating lack of benefit from additional chemotherapy.31 A key limitation of the PAM50 is the lack of any prospective studies with this assay.
PAM50 has been designed to be carried out in any qualified pathology laboratory. Moreover, the ROR score provides additional prognostic information about risk of late recurrence, which will be discussed in the next section.
70-Gene Breast Cancer Recurrence Assay (MammaPrint)
MammaPrint is a 70-gene assay that was initially developed using an unsupervised, hierarchical clustering algorithm on whole-genome expression arrays with early-stage breast cancer. Among 295 consecutive patients who had MammaPrint testing, those classified with a good-prognosis tumor signature (n = 115) had an excellent 10-year survival rate (94.5%) compared to those with a poor-prognosis signature (54.5%), and the signature remained prognostic upon multivariate analysis.32 Subsequently, a pooled analysis comparing outcomes by MammaPrint score in patients with node-negative or 1 to 3 node-positive breast cancers treated as per discretion of their medical team with either adjuvant chemotherapy plus endocrine therapy or endocrine therapy alone reported that only those patients with a high-risk score benefited from chemotherapy.33 Recently, a prospective phase 3 study (MINDACT [Microarray In Node negative Disease may Avoid ChemoTherapy]) evaluating the utility of MammaPrint for adjuvant chemotherapy decision-making reported results.34 In this study, 6693 women with early-stage breast cancer were assessed by clinical risk and genomic risk using MammaPrint. Those with low clinical and genomic risk did not receive chemotherapy, while those with high clinical and genomic risk all received chemotherapy. The primary goal of the study was to assess whether forgoing chemotherapy would be associated with a low rate of recurrence in those patients with a low-risk prognostic MammaPrint signature but high clinical risk. A total of 1550 patients (23.2%) were in the discordant group, and the majority of these patients had HR-positive disease (98.1%). Without chemotherapy, the rate of survival without distant metastasis at 5 years in this group was 94.7% (95% confidence interval [CI] 92.5% to 96.2%), which met the primary endpoint. Of note, initially, MammaPrint was only available for fresh tissue analysis, but recent advances in RNA processing now allow for this analysis on FFPE tissue.35
Summary
These genomic and biomarker assays can identify different subsets of HR-positive breast cancers, including those patients who have tumors with an excellent prognosis with endocrine therapies alone. Thus, we now have the tools to help avoid the toxicities of chemotherapy in many women with early-stage breast cancer.
Tests for Assessing Risk for Late Recurrence
Case Continued
The patient undergoes 21-gene recurrence score testing, which shows a low recurrence score of 10, estimating the 10-year risk of distant recurrence to be approximately 7% with 5 years of tamoxifen. Chemotherapy is not recommended. The patient completes adjuvant whole breast radiation therapy, and then, based on data supporting AIs over tamoxifen in postmenopausal women, she is started on anastrozole.41 She initially experiences mild side effects from treatment, including fatigue, arthralgia, and vaginal dryness, but her symptoms are able to be managed. As she approaches 5 years of adjuvant endocrine therapy with anastrozole, she is struggling with rotator cuff injury and is anxious about recurrence, but has no evidence of recurrent cancer. Her bone density scan in the beginning of her fourth year of therapy shows a decrease in bone mineral density, with the lowest T score of –1.5 at the left femoral neck, consistent with osteopenia. She has been treated with calcium and vitamin D supplements.
- How long should this patient continue treatment with anastrozole?
The risk for recurrence is highest during the first 5 years after diagnosis for all patients with early breast cancer.42 Although HR-positive breast cancers have a better prognosis than HR-negative disease, the pattern of recurrence is different between the 2 groups, and it is estimated that approximately half of the recurrences among patients with HR-positive early breast cancer occur after the first 5 years from diagnosis. Annualized hazard of recurrence in HR-positive breast cancer has been shown to remain elevated and fairly stable beyond 10 years, even for those with low tumor burden and node-negative disease.43 Prospective trials showed that for women with HR-positive early breast cancer, 5 years of adjuvant tamoxifen could substantially reduce recurrence rates and improve survival, and this became the standard of care.44 AIs are considered the standard of care for adjuvant endocrine therapy in most postmenopausal women, as they result in a significantly lower recurrence rate compared with tamoxifen, either as initial adjuvant therapy or sequentially following 2 to 3 years of tamoxifen.45
Due to the risk for later recurrences with HR-positive breast cancer, more patients and oncologists are considering extended endocrine therapy. This is based on results from the ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) and aTTOM (Adjuvant Tamoxifen–To Offer More?) studies, both of which showed that women with HR-positive breast cancer who continued tamoxifen for 10 years had a lower late recurrence rate and a lower breast cancer mortality rate compared with those who stopped at 5 years.46,47 Furthermore, the NCIC MA.17 trial evaluated extended endocrine therapy in postmenopausal women with 5 years of letrozole following 5 years of tamoxifen. Letrozole was shown to improve both disease-free and distant disease-free survival. The overall survival benefit was limited to patients with node-positive disease.48 A summary of studies of extended endocrine therapy for HR-positive breast cancers is shown in Table 2.2,3,46–49
However, extending AI therapy from 5 years to 10 years is not clearly beneficial. In the MA.17R trial, although longer AI therapy resulted in significantly better disease-free survival (95% versus 91%, hazard ratio 0.66, P = 0.01), this was primarily due to a lower incidence of contralateral breast cancer in those taking the AI compared with placebo. The distant recurrence risks were similar and low (4.4% versus 5.5%), and there was no overall survival difference.2 Also, the NSABP B-42 study, which was presented at the 2016 San Antonio Breast Cancer Symposium, did not meet its predefined endpoint for benefit from extending adjuvant AI therapy with letrozole beyond 5 years.3 Thus, the absolute benefit from extended endocrine therapy has been modest across these studies. Although endocrine therapy is considered relatively safe and well tolerated, side effects can be significant and even associated with morbidity. Ideally, extended endocrine therapy should be offered to the subset of patients who would benefit the most. Several genomic diagnostic assays, including the EndoPredict test, PAM50, and the Breast Cancer Index (BCI) tests, specifically assess the risk for late recurrence in HR-positive cancers.
PAM50
Studies suggest that the ROR score also has value in predicting late recurrences. Analysis of data in patients enrolled in the ABCSG-8 trial showed that ROR could identify patients with endocrine-sensitive disease who are at low risk for late relapse and could be spared from unwanted toxicities of extended endocrine therapies. In 1246 ABCSG-8 patients between years 5 and 15, the PAM50 ROR demonstrated an absolute risk of distant recurrence of 2.4% in the low-risk group, as compared with 17.5% in the high-risk group.50 Also, a combined analysis of patients from both the ATAC and ABCSG-8 trials demonstrated the utility of ROR in identifying this subgroup of patients with low risk for late relapse.51
EndoPredict
EndoPredict is another quantitative RT-PCR–based assay which uses FFPE tissues to calculate a risk score based on 8 cancer-related and 3 reference genes. The score is combined with clinicopathological factors including tumor size and nodal status to make a comprehensive risk score (EPclin). EPclin is used to dichotomize patients into EndoPredict low- and high-risk groups. EndoPredict has been validated in 2 cohorts of patients enrolled in separate randomized studies, ABCSG-6 and ABCSG-8. EP provided prognostic information beyond clinicopathological variables to predict distant recurrence in patients with HR-positive/HER2-negative early breast cancer.37 More important, EndoPredict has been shown to predict early (years 0–5) versus late (> 5 years after diagnosis) recurrences and identify a low-risk subset of patients who would not be expected to benefit from further treatment beyond 5 years of endocrine therapy.52 Recently, EndoPredict and EPclin were compared with the 21-gene (Oncotype DX) recurrence score in a patient population from the TransATAC study. Both EndoPredict and EPclin provided more prognostic information compared to the 21-gene recurrence score and identified early and late relapse events.53 EndoPredict is the first multigene expression assay that could be routinely performed in decentralized molecular pathological laboratories with a short turnaround time.54
Breast Cancer Index
The BCI is a RT-PCR–based gene expression assay that consists of 2 gene expression biomarkers: molecular grade index (MGI) and HOXB13/IL17BR (H/I). The BCI was developed as a prognostic test to assess risk for breast cancer recurrence using a cohort of ER-positive patients (n = 588) treated with adjuvant tamoxifen versus observation from the prospective randomized Stockholm trial.38 In this blinded retrospective study, H/I and MGI were measured and a continuous risk model (BCI) was developed in the tamoxifen-treated group. More than 50% of the patients in this group were classified as having a low risk of recurrence. The rate of distant recurrence or death in this low-risk group at 10 years was less than 3%. The performance of the BCI model was then tested in the untreated arm of the Stockholm trial. In the untreated arm, BCI classified 53%, 27%, and 20% of patients as low, intermediate, and high risk, respectively. The rate of distant metastasis at 10 years in these risk groups was 8.3% (95% CI 4.7% to 14.4%), 22.9% (95% CI 14.5% to 35.2%), and 28.5% (95% CI 17.9% to 43.6%), respectively, and the rate of breast cancer–specific mortality was 5.1% (95% CI 1.3% to 8.7%), 19.8% (95% CI 10.0% to 28.6%), and 28.8% (95% CI 15.3% to 40.2%).38
The prognostic and predictive values of the BCI have been validated in other large, randomized studies and in patients with both node-negative and node-positive disease.39,55 The predictive value of the endocrine-response biomarker, the H/I ratio, has been demonstrated in randomized studies. In the MA.17 trial, a high H/I ratio was associated with increased risk for late recurrence in the absence of letrozole. However, extended endocrine therapy with letrozole in patients with high H/I ratios predicted benefit from therapy and decreased the probability of late disease recurrence.56 BCI was also compared to IHC4 and the 21-gene recurrence score in the TransATAC study and was the only test to show prognostic significance for both early (0–5 years) and late (5–10 year) recurrence.40
The impact of the BCI results on physicians’ recommendations for extended endocrine therapy was assessed by a prospective study. This study showed that the test result had a significant effect on both physician treatment recommendation and patient satisfaction. BCI testing resulted in a change in physician recommendations for extended endocrine therapy, with an overall decrease in recommendations for extended endocrine therapy from 74% to 54%. Knowledge of the test result also led to improved patient satisfaction and decreased anxiety.57
Summary
Due to the risk for late recurrence, extended endocrine therapy is being recommended for many patients with HR-positive breast cancers. Multiple genomic assays are being developed to better understand an individual’s risk for late recurrence and the potential for benefit from extended endocrine therapies. However, none of the assays has been validated in prospective randomized studies. Further validation is needed prior to routine use of these assays.
Case Continued
A BCI test is done and the result shows 4.3% BCI low-risk category in years 5–10, which is consistent with a low likelihood of benefit from extended endocrine therapy. After discussing the results of the BCI test in the context of no survival benefit from extending AIs beyond 5 years, both the patient and her oncologist feel comfortable with discontinuing endocrine therapy at the end of 5 years.
Conclusion
Reduction in breast cancer mortality is mainly the result of improved systemic treatments. With advances in breast cancer screening tools in recent years, the rate of cancer detection has increased. This has raised concerns regarding overdiagnosis. To prevent unwanted toxicities associated with overtreatment, better treatment decision tools are needed. Several genomic assays are currently available and widely used to provide prognostic and predictive information and aid in decisions regarding appropriate use of adjuvant chemotherapy in HR-positive/HER2-negative early-stage breast cancer. Ongoing studies are refining the cutoffs for these assays and expanding the applicability to node-positive breast cancers. Furthermore, with several studies now showing benefit from the use of extended endocrine therapy, some of these assays may be able to identify the subset of patients who are at increased risk for late recurrence and who might benefit from extended endocrine therapy. Advances in molecular testing has enabled clinicians to offer more personalized treatments to their patients, improve patients’ compliance, and decrease anxiety and conflict associated with management decisions. Although small numbers of patients with HER2-positive and triple-negative breast cancers were also included in some of these studies, use of genomic assays in this subset of patients is very limited and currently not recommended.
Introduction
Over the past several decades, while the incidence of breast cancer has increased, breast cancer mortality has decreased. This decrease is likely due to both early detection and advances in systemic therapy. However, with more widespread use of screening mammography, there are increasing concerns about potential overdiagnosis of cancer.1 One key challenge is that breast cancer is a heterogeneous disease. Improved tools for determining breast cancer biology can help physicians individualize treatments. Patients with low-risk cancers can be approached with less aggressive treatments, thus preventing unnecessary toxicities, while those with higher-risk cancers remain treated appropriately with more aggressive therapies.
Traditionally, adjuvant chemotherapy was recommended based on tumor features such as stage (tumor size, regional nodal involvement), grade, expression of hormone receptors (estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor-2 (HER2), and patient features (age, menopausal status). However, this approach is not accurate enough to guide individualized treatment approaches, which are based on the risk for recurrence and the reduction in this risk that can be achieved with various systemic treatments. In particular, women with low-risk hormone receptor (HR)–positive, HER2-negative breast cancers could be spared the toxicities of cytotoxic chemotherapies without compromising the prognosis.
Beyond chemotherapy, endocrine therapies also have risks, especially when given over extended periods of time. Recently, extended endocrine therapy has been shown to prevent late recurrences of HR-positive breast cancers. In the National Cancer Institute of Canada Clinical Trials Group’s MA.17R study, extended endocrine therapy with letrozole for a total of 10 years (beyond 5 years of an aromatase inhibitor [AI]) decreased the risk for breast cancer recurrence or the occurrence of contralateral breast cancer by 34%.2 However, the overall survival was similar between the 2 groups and the disease-free survival benefits were not confirmed in other studies.3–5 Identifying the subgroup of patients who benefit from this extended AI therapy is important in the era of personalized medicine. Several tumor genomic assays have been developed to provide additional prognostic and predictive information with the goal of individualizing adjuvant therapies for breast cancer. Although assays are also being evaluated in HER2-positive and triple-negative breast cancer, this review will focus on HR-positive, HER2-negative breast cancer.
Tests for Guiding Adjuvant Chemotherapy Decisions
Case Study
Initial Presentation
A 54-year-old postmenopausal woman with no significant past medical history presents with an abnormal screening mammogram, which shows a focal asymmetry in the 10 o’clock position at middle depth of the left breast. Further work-up with a diagnostic mammogram and ultrasound of the left breast shows a suspicious hypoechoic solid mass with irregular margins measuring 17 mm. The patient undergoes an ultrasound-guided core needle biopsy of the suspicious mass, the results of which are consistent with an invasive ductal carcinoma, Nottingham grade 2, ER strongly positive (95%), PR weakly positive (5%), HER2-negative, and Ki-67 of 15%. She undergoes a left partial mastectomy and sentinel lymph node biopsy, with final pathology demonstrating a single focus of invasive ductal carcinoma, measuring 2.2 cm in greatest dimension with no evidence of lymphovascular invasion. Margins are clear and 2 sentinel lymph nodes are negative for metastatic disease (final pathologic stage IIA, pT2 pN0 cM0). She is referred to medical oncology to discuss adjuvant systemic therapy.
- Can additional testing be used to determine prognosis and guide systemic therapy recommendations for early-stage HR-positive/HER2-negative breast cancer?
After a diagnosis of early-stage breast cancer, the key clinical question faced by the patient and medical oncologist is: what is the individual’s risk for a metastatic breast cancer recurrence and thus the risk for death due to breast cancer? Once the risk for recurrence is established, systemic adjuvant chemotherapy, endocrine therapy, and/or HER2-directed therapy are considered based on the receptor status (ER/PR and HER2) to reduce this risk. HR-positive, HER2-negative breast cancer is the most common type of breast cancer. Although adjuvant endocrine therapy has significantly reduced the risk for recurrence and improved survival for patients with HR-positive breast cancer,6 the role of adjuvant chemotherapy for this subset of breast cancer remains unclear. Prior to genomic testing, the recommendation for adjuvant chemotherapy for HR-positive/HER2-negative tumors was primarily based on patient age and tumor stage and grade. However, chemotherapy overtreatment remained a concern given the potential short- and long-term risks of chemotherapy. Further studies into HR-positive/HER2-negative tumors have shown that these tumors can be divided into 2 main subtypes, luminal A and luminal B.7 These subtypes represent unique biology and differ in terms of prognosis and response to endocrine therapy and chemotherapy. Luminal A tumors are strongly endocrine responsive and have a good prognosis, while luminal B tumors are less endocrine responsive and are associated with a poorer prognosis; the addition of adjuvant chemotherapy is often considered for luminal B tumors.8 Several tests, including tumor genomic assays, are now available to help with delineating the tumor subtype and aid in decision-making regarding adjuvant chemotherapy for HR-positive/HER2-negative breast cancers.
Ki-67 Assays, Including IHC4 and PEPI
Proliferation is a hallmark of cancer cells.9 Ki-67, a nuclear nonhistone protein whose expression varies in intensity throughout the cell cycle, has been used as a measurement of tumor cell proliferation.10 Two large meta-analyses have demonstrated that high Ki-67 expression in breast tumors is independently associated with worse disease-free and overall survival rates.11,12 Ki-67 expression has also been used to classify HR-positive tumors as luminal A or B. After classifying tumor subtypes based on intrinsic gene expression profiling, Cheang and colleagues determined that a Ki-67 cut point of 13.25% differentiated luminal A and B tumors.13 However, the ideal cut point for Ki-67 remains unclear, as the sensitivity and specificity in this study was 77% and 78%, respectively. Others have combined Ki-67 with standard ER, PR, and HER2 testing. This immunohistochemical 4 (IHC4) score, which weighs each of these variables, was validated in postmenopausal patients from the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial who had ER-positive tumors and did not receive chemotherapy.14 The prognostic information from the IHC4 was similar to that seen with the 21-gene recurrence score (Oncotype DX), which is discussed later in this article. The key challenge with Ki-67 testing currently is the lack of a validated test methodology and intra-observer variability in interpreting the Ki-67 results.15 Recent series have suggested that Ki-67 be considered as a continuous marker rather than a set cut point.16 These issues continue to impact the clinical utility of Ki-67 for decision-making for adjuvant chemotherapy.
Ki-67 and the preoperative endocrine prognostic index (PEPI) score have been explored in the neoadjuvant setting to separate postmenopausal women with endocrine-sensitive versus intrinsically resistant disease and identify patients at risk for recurrent disease.17 The on-treatment levels of Ki-67 in response to endocrine therapy have been shown to be more prognostic than baseline values, and a decrease in Ki-67 as early as 2 weeks after initiation of neoadjuvant endocrine therapy is associated with endocrine-sensitive tumors and improved outcome. The PEPI score was developed through retrospective analysis of the P024 trial18 to evaluate the relationship between post-neoadjuvant endocrine therapy tumor characteristics and risk for early relapse. The score was subsequently validated in an independent data set from the IMPACT (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen) trial.19 Patients with low pathological stage (0 or 1) and a favorable biomarker profile (PEPI score 0) at surgery had the best prognosis in the absence of chemotherapy. On the other hand, higher pathological stage at surgery and a poor biomarker profile with loss of ER positivity or persistently elevated Ki-67 (PEPI score of 3) identified de novo endocrine-resistant tumors that are higher risk for early relapse.20 The ongoing Alliance A011106 ALTERNATE trial (ALTernate approaches for clinical stage II or III Estrogen Receptor positive breast cancer NeoAdjuvant TrEatment in postmenopausal women, NCT01953588) is a phase 3 study to prospectively test this hypothesis.
21-Gene Recurrence Score (Onco type DX Assay)
The 21-gene Oncotype DX assay is conducted on paraffin-embedded tumor tissue and measures the expression of 16 cancer related genes and 5 reference genes using quantitative polymerase chain reaction (PCR). The genes included in this assay are mainly related to proliferation (including Ki-67), invasion, and HER2 or estrogen signaling.21 Originally, the 21-gene recurrence score assay was analyzed as a prognostic biomarker tool in a prospective-retrospective biomarker substudy of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 clinical trial in which patients with node-negative, ER-positive tumors were randomly assigned to receive tamoxifen or placebo without chemotherapy.22 Using the standard reported values of low risk (< 18), intermediate risk (18–30), or high risk (≥ 31) for recurrence, among the tamoxifen-treated patients, cancers with a high-risk recurrence score had a significantly worse rate of distant recurrence and overall survival.21 Inferior breast cancer survival in cancers with a high recurrence score was also confirmed in other series of endocrine-treated patients with node-negative and node-positive disease.23–25
The predictive utility of the 21-gene recurrence score for endocrine therapy has also been evaluated. A comparison of the placebo- and tamoxifen-treated patients from the NSABP B-14 trial demonstrated that the 21-gene recurrence score predicted benefit from tamoxifen in cancers with low- or intermediate-risk recurrence scores.26 However, there was no benefit from the use of tamoxifen over placebo in cancers with high-risk recurrence scores. To date, this intriguing data has not been prospectively confirmed, and thus the 21-gene recurrence score is not used to avoid endocrine therapy.
The 21-gene recurrence score is primarily used by oncologists to aid in decision-making regarding adjuvant chemotherapy in patients with node-negative and node-positive (with up to 3 positive lymph nodes), HR-positive/HER2-negative breast cancers. The predictive utility of the 21-gene recurrence score for adjuvant chemotherapy was initially tested using tumor samples from the NSABP B-20 study. This study initially compared adjuvant tamoxifen alone with tamoxifen plus chemotherapy in patients with node-negative, HR-positive tumors. The prospective-retrospective biomarker analysis showed that the patients with high-risk 21-gene recurrence scores benefited from the addition of chemotherapy, whereas those with low or intermediate risk did not have an improved freedom from distant recurrence with chemotherapy.27 Similarly, an analysis from the prospective phase 3 Southwest Oncology Group (SWOG) 8814 trial comparing tamoxifen to tamoxifen with chemotherapy showed that for node-positive tumors, chemotherapy benefit was only seen in those with high 21-gene recurrence scores.24
Prospective studies are now starting to report results regarding the predictive role of the 21-gene recurrence score. The TAILORx (Trial Assigning Individualized Options for Treatment) trial includes women with node-negative, HR-positive/HER2-negative tumors measuring 0.6 to 5 cm. All patients were treated with standard-of-care endocrine therapy for at least 5 years. Chemotherapy was determined based on the 21-gene recurrence score results on the primary tumor. The 21-gene recurrence score cutoffs were changed to low (0–10), intermediate (11–25), and high (≥ 26). Patients with scores of 26 or higher were treated with chemotherapy, and those with intermediate scores were randomly assigned to chemotherapy or no chemotherapy; results from this cohort are still pending. However, excellent breast cancer outcomes with endocrine therapy alone were reported from the 1626 (15.9% of total cohort) prospectively followed patients with low recurrence score tumors. The 5-year invasive disease-free survival was 93.8%, with overall survival of 98%.28 Given that 5 years is appropriate follow-up to see any chemotherapy benefit, this data supports the recommendation for no chemotherapy in this cohort of patients with very low 21-gene recurrence scores.
The RxPONDER (Rx for Positive Node, Endocrine Responsive Breast Cancer) trial is evaluating women with 1 to 3 node-positive, HR-positive, HER2-negative tumors. In this trial, patients with 21-gene recurrence scores of 0 to 25 were assigned to adjuvant chemotherapy or none. Those with scores of 26 or higher were assigned to chemotherapy. All patients received standard adjuvant endocrine therapy. This study has completed accrual and results are pending. Of note, TAILORx and RxPONDER did not investigate the potential lack of benefit of endocrine therapy in cancers with high recurrence scores. Furthermore, despite data suggesting that chemotherapy may not even benefit women with 4 or more nodes involved but who have a low recurrence score,24 due to the lack of prospective data in this cohort and the quite high risk for distant recurrence, chemotherapy continues to be the standard of care for these patients.
PAM50 (Breast Cancer Prognostic Gene Signature)
Using microarray and quantitative reverse transcriptase PCR (RT-PCR) on formalin-fixed paraffin-embedded (FFPE) tissues, the Breast Cancer Prognostic Gene Signature (PAM50) assay was initially developed to identify intrinsic breast cancer subtypes, including luminal A, luminal B, HER2-enriched, and basal-like.7,29 Based on the prediction analysis of microarray (PAM) method, the assay measures the expression levels of 50 genes, provides a risk category (low, intermediate, and high), and generates a numerical risk of recurrence score (ROR). The intrinsic subtype and ROR have been shown to add significant prognostic value to the clinicopathological characteristics of tumors. Clinical validity of PAM50 was evaluated in postmenopausal women with HR-positive early-stage breast cancer treated in the prospective ATAC and ABCSG-8 (Austrian Breast and Colorectal Cancer Study Group 8) trials.30,31 In 1017 patients with ER-positive breast cancer treated with anastrozole or tamoxifen in the ATAC trial, ROR added significant prognostic information beyond the clinical treatment score (integrated prognostic information from nodal status, tumor size, histopathologic grade, age, and anastrozole or tamoxifen treatment) in all patients. Also, compared with the 21-gene recurrence score, ROR provided more prognostic information in ER-positive, node-negative disease and better differentiation of intermediate- and higher-risk groups. Fewer patients were categorized as intermediate risk by ROR and more as high risk, which could reduce the uncertainty in the estimate of clinical benefit from chemotherapy.30 The clinical utility of PAM50 as a prognostic model was also validated in 1478 postmenopausal women with ER-positive early-stage breast cancer enrolled in the ABCSG-8 trial. In this study, ROR assigned 47% of patients with node-negative disease to the low-risk category. In this low-risk group, the 10-year metastasis risk was less than 3.5%, indicating lack of benefit from additional chemotherapy.31 A key limitation of the PAM50 is the lack of any prospective studies with this assay.
PAM50 has been designed to be carried out in any qualified pathology laboratory. Moreover, the ROR score provides additional prognostic information about risk of late recurrence, which will be discussed in the next section.
70-Gene Breast Cancer Recurrence Assay (MammaPrint)
MammaPrint is a 70-gene assay that was initially developed using an unsupervised, hierarchical clustering algorithm on whole-genome expression arrays with early-stage breast cancer. Among 295 consecutive patients who had MammaPrint testing, those classified with a good-prognosis tumor signature (n = 115) had an excellent 10-year survival rate (94.5%) compared to those with a poor-prognosis signature (54.5%), and the signature remained prognostic upon multivariate analysis.32 Subsequently, a pooled analysis comparing outcomes by MammaPrint score in patients with node-negative or 1 to 3 node-positive breast cancers treated as per discretion of their medical team with either adjuvant chemotherapy plus endocrine therapy or endocrine therapy alone reported that only those patients with a high-risk score benefited from chemotherapy.33 Recently, a prospective phase 3 study (MINDACT [Microarray In Node negative Disease may Avoid ChemoTherapy]) evaluating the utility of MammaPrint for adjuvant chemotherapy decision-making reported results.34 In this study, 6693 women with early-stage breast cancer were assessed by clinical risk and genomic risk using MammaPrint. Those with low clinical and genomic risk did not receive chemotherapy, while those with high clinical and genomic risk all received chemotherapy. The primary goal of the study was to assess whether forgoing chemotherapy would be associated with a low rate of recurrence in those patients with a low-risk prognostic MammaPrint signature but high clinical risk. A total of 1550 patients (23.2%) were in the discordant group, and the majority of these patients had HR-positive disease (98.1%). Without chemotherapy, the rate of survival without distant metastasis at 5 years in this group was 94.7% (95% confidence interval [CI] 92.5% to 96.2%), which met the primary endpoint. Of note, initially, MammaPrint was only available for fresh tissue analysis, but recent advances in RNA processing now allow for this analysis on FFPE tissue.35
Summary
These genomic and biomarker assays can identify different subsets of HR-positive breast cancers, including those patients who have tumors with an excellent prognosis with endocrine therapies alone. Thus, we now have the tools to help avoid the toxicities of chemotherapy in many women with early-stage breast cancer.
Tests for Assessing Risk for Late Recurrence
Case Continued
The patient undergoes 21-gene recurrence score testing, which shows a low recurrence score of 10, estimating the 10-year risk of distant recurrence to be approximately 7% with 5 years of tamoxifen. Chemotherapy is not recommended. The patient completes adjuvant whole breast radiation therapy, and then, based on data supporting AIs over tamoxifen in postmenopausal women, she is started on anastrozole.41 She initially experiences mild side effects from treatment, including fatigue, arthralgia, and vaginal dryness, but her symptoms are able to be managed. As she approaches 5 years of adjuvant endocrine therapy with anastrozole, she is struggling with rotator cuff injury and is anxious about recurrence, but has no evidence of recurrent cancer. Her bone density scan in the beginning of her fourth year of therapy shows a decrease in bone mineral density, with the lowest T score of –1.5 at the left femoral neck, consistent with osteopenia. She has been treated with calcium and vitamin D supplements.
- How long should this patient continue treatment with anastrozole?
The risk for recurrence is highest during the first 5 years after diagnosis for all patients with early breast cancer.42 Although HR-positive breast cancers have a better prognosis than HR-negative disease, the pattern of recurrence is different between the 2 groups, and it is estimated that approximately half of the recurrences among patients with HR-positive early breast cancer occur after the first 5 years from diagnosis. Annualized hazard of recurrence in HR-positive breast cancer has been shown to remain elevated and fairly stable beyond 10 years, even for those with low tumor burden and node-negative disease.43 Prospective trials showed that for women with HR-positive early breast cancer, 5 years of adjuvant tamoxifen could substantially reduce recurrence rates and improve survival, and this became the standard of care.44 AIs are considered the standard of care for adjuvant endocrine therapy in most postmenopausal women, as they result in a significantly lower recurrence rate compared with tamoxifen, either as initial adjuvant therapy or sequentially following 2 to 3 years of tamoxifen.45
Due to the risk for later recurrences with HR-positive breast cancer, more patients and oncologists are considering extended endocrine therapy. This is based on results from the ATLAS (Adjuvant Tamoxifen: Longer Against Shorter) and aTTOM (Adjuvant Tamoxifen–To Offer More?) studies, both of which showed that women with HR-positive breast cancer who continued tamoxifen for 10 years had a lower late recurrence rate and a lower breast cancer mortality rate compared with those who stopped at 5 years.46,47 Furthermore, the NCIC MA.17 trial evaluated extended endocrine therapy in postmenopausal women with 5 years of letrozole following 5 years of tamoxifen. Letrozole was shown to improve both disease-free and distant disease-free survival. The overall survival benefit was limited to patients with node-positive disease.48 A summary of studies of extended endocrine therapy for HR-positive breast cancers is shown in Table 2.2,3,46–49
However, extending AI therapy from 5 years to 10 years is not clearly beneficial. In the MA.17R trial, although longer AI therapy resulted in significantly better disease-free survival (95% versus 91%, hazard ratio 0.66, P = 0.01), this was primarily due to a lower incidence of contralateral breast cancer in those taking the AI compared with placebo. The distant recurrence risks were similar and low (4.4% versus 5.5%), and there was no overall survival difference.2 Also, the NSABP B-42 study, which was presented at the 2016 San Antonio Breast Cancer Symposium, did not meet its predefined endpoint for benefit from extending adjuvant AI therapy with letrozole beyond 5 years.3 Thus, the absolute benefit from extended endocrine therapy has been modest across these studies. Although endocrine therapy is considered relatively safe and well tolerated, side effects can be significant and even associated with morbidity. Ideally, extended endocrine therapy should be offered to the subset of patients who would benefit the most. Several genomic diagnostic assays, including the EndoPredict test, PAM50, and the Breast Cancer Index (BCI) tests, specifically assess the risk for late recurrence in HR-positive cancers.
PAM50
Studies suggest that the ROR score also has value in predicting late recurrences. Analysis of data in patients enrolled in the ABCSG-8 trial showed that ROR could identify patients with endocrine-sensitive disease who are at low risk for late relapse and could be spared from unwanted toxicities of extended endocrine therapies. In 1246 ABCSG-8 patients between years 5 and 15, the PAM50 ROR demonstrated an absolute risk of distant recurrence of 2.4% in the low-risk group, as compared with 17.5% in the high-risk group.50 Also, a combined analysis of patients from both the ATAC and ABCSG-8 trials demonstrated the utility of ROR in identifying this subgroup of patients with low risk for late relapse.51
EndoPredict
EndoPredict is another quantitative RT-PCR–based assay which uses FFPE tissues to calculate a risk score based on 8 cancer-related and 3 reference genes. The score is combined with clinicopathological factors including tumor size and nodal status to make a comprehensive risk score (EPclin). EPclin is used to dichotomize patients into EndoPredict low- and high-risk groups. EndoPredict has been validated in 2 cohorts of patients enrolled in separate randomized studies, ABCSG-6 and ABCSG-8. EP provided prognostic information beyond clinicopathological variables to predict distant recurrence in patients with HR-positive/HER2-negative early breast cancer.37 More important, EndoPredict has been shown to predict early (years 0–5) versus late (> 5 years after diagnosis) recurrences and identify a low-risk subset of patients who would not be expected to benefit from further treatment beyond 5 years of endocrine therapy.52 Recently, EndoPredict and EPclin were compared with the 21-gene (Oncotype DX) recurrence score in a patient population from the TransATAC study. Both EndoPredict and EPclin provided more prognostic information compared to the 21-gene recurrence score and identified early and late relapse events.53 EndoPredict is the first multigene expression assay that could be routinely performed in decentralized molecular pathological laboratories with a short turnaround time.54
Breast Cancer Index
The BCI is a RT-PCR–based gene expression assay that consists of 2 gene expression biomarkers: molecular grade index (MGI) and HOXB13/IL17BR (H/I). The BCI was developed as a prognostic test to assess risk for breast cancer recurrence using a cohort of ER-positive patients (n = 588) treated with adjuvant tamoxifen versus observation from the prospective randomized Stockholm trial.38 In this blinded retrospective study, H/I and MGI were measured and a continuous risk model (BCI) was developed in the tamoxifen-treated group. More than 50% of the patients in this group were classified as having a low risk of recurrence. The rate of distant recurrence or death in this low-risk group at 10 years was less than 3%. The performance of the BCI model was then tested in the untreated arm of the Stockholm trial. In the untreated arm, BCI classified 53%, 27%, and 20% of patients as low, intermediate, and high risk, respectively. The rate of distant metastasis at 10 years in these risk groups was 8.3% (95% CI 4.7% to 14.4%), 22.9% (95% CI 14.5% to 35.2%), and 28.5% (95% CI 17.9% to 43.6%), respectively, and the rate of breast cancer–specific mortality was 5.1% (95% CI 1.3% to 8.7%), 19.8% (95% CI 10.0% to 28.6%), and 28.8% (95% CI 15.3% to 40.2%).38
The prognostic and predictive values of the BCI have been validated in other large, randomized studies and in patients with both node-negative and node-positive disease.39,55 The predictive value of the endocrine-response biomarker, the H/I ratio, has been demonstrated in randomized studies. In the MA.17 trial, a high H/I ratio was associated with increased risk for late recurrence in the absence of letrozole. However, extended endocrine therapy with letrozole in patients with high H/I ratios predicted benefit from therapy and decreased the probability of late disease recurrence.56 BCI was also compared to IHC4 and the 21-gene recurrence score in the TransATAC study and was the only test to show prognostic significance for both early (0–5 years) and late (5–10 year) recurrence.40
The impact of the BCI results on physicians’ recommendations for extended endocrine therapy was assessed by a prospective study. This study showed that the test result had a significant effect on both physician treatment recommendation and patient satisfaction. BCI testing resulted in a change in physician recommendations for extended endocrine therapy, with an overall decrease in recommendations for extended endocrine therapy from 74% to 54%. Knowledge of the test result also led to improved patient satisfaction and decreased anxiety.57
Summary
Due to the risk for late recurrence, extended endocrine therapy is being recommended for many patients with HR-positive breast cancers. Multiple genomic assays are being developed to better understand an individual’s risk for late recurrence and the potential for benefit from extended endocrine therapies. However, none of the assays has been validated in prospective randomized studies. Further validation is needed prior to routine use of these assays.
Case Continued
A BCI test is done and the result shows 4.3% BCI low-risk category in years 5–10, which is consistent with a low likelihood of benefit from extended endocrine therapy. After discussing the results of the BCI test in the context of no survival benefit from extending AIs beyond 5 years, both the patient and her oncologist feel comfortable with discontinuing endocrine therapy at the end of 5 years.
Conclusion
Reduction in breast cancer mortality is mainly the result of improved systemic treatments. With advances in breast cancer screening tools in recent years, the rate of cancer detection has increased. This has raised concerns regarding overdiagnosis. To prevent unwanted toxicities associated with overtreatment, better treatment decision tools are needed. Several genomic assays are currently available and widely used to provide prognostic and predictive information and aid in decisions regarding appropriate use of adjuvant chemotherapy in HR-positive/HER2-negative early-stage breast cancer. Ongoing studies are refining the cutoffs for these assays and expanding the applicability to node-positive breast cancers. Furthermore, with several studies now showing benefit from the use of extended endocrine therapy, some of these assays may be able to identify the subset of patients who are at increased risk for late recurrence and who might benefit from extended endocrine therapy. Advances in molecular testing has enabled clinicians to offer more personalized treatments to their patients, improve patients’ compliance, and decrease anxiety and conflict associated with management decisions. Although small numbers of patients with HER2-positive and triple-negative breast cancers were also included in some of these studies, use of genomic assays in this subset of patients is very limited and currently not recommended.
1. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–47.
2. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19.
3. Mamounas E, Bandos H, Lembersky B. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy with letrozole in postmenopausal women with hormone-receptor-positive breast cancer who have completed previous adjuvant treatment with an aromatase inhibitor. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-05.
4. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, et al: First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-03.
5. Blok EJ, Van de Velde CJH, Meershoek-Klein Kranenbarg EM, et al: Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-04.
6. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2005;365:1687–717.
7. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
8. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46.
9. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
10. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212–20.
11. de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13.
12. Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–91.
13. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50.
14. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and com-parison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–8.
15. Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol 2013;66:512–6.
16. Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 2015; 24 Suppl 2:S67–72.
17. Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol 2016;882:125–54.
18. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32.
19. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anas-trozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16.
20. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–8.
21. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–26.
22. Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004;364:858–68.
23. Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25.
24. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65.
25. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829–34.
26. Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol 2005;23: suppl:510.
27. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34.
28. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14.
29. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
30. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783–90.
31. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 post-menopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339–45.
32. van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009.
33. Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 2010;120:655–61.
34. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29.
35. Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2014;16:190–7.
36. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222–32.
37. Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011;17:6012–20.
38. Jerevall PL, Ma XJ, Li H, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 2011;104:1762–9.
39. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205.
40. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067–76.
41. Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract 2010;6:243–6.
42. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46.
43. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 2016;34:927–35.
44. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84.
45. Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52.
46. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16.
47. Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31 (suppl):5.
48. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262–71.
49. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71.
50. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298–305.
51. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–22.
52. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013;109:2959–64.
53. Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX Recurrence Score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 2016;108:djw149.
54. Muller BM, Keil E, Lehmann A, et al. The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PLoS One 2013;8:e68252.
55. Sgroi DC, Chapman JA, Badovinac-Crnjevic T, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016;18:1.
56. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42.
57. Sanft T, Aktas B, Schroeder B, et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat 2015;154:533–41.
1. Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–47.
2. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19.
3. Mamounas E, Bandos H, Lembersky B. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy with letrozole in postmenopausal women with hormone-receptor-positive breast cancer who have completed previous adjuvant treatment with an aromatase inhibitor. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-05.
4. Tjan-Heijnen VC, Van Hellemond IE, Peer PG, et al: First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2-3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-03.
5. Blok EJ, Van de Velde CJH, Meershoek-Klein Kranenbarg EM, et al: Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy. In: Proceedings from the San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstract S1-04.
6. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 2005;365:1687–717.
7. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
8. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–46.
9. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70.
10. Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 2005;23:7212–20.
11. de Azambuja E, Cardoso F, de Castro G Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13.
12. Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 2015;153:477–91.
13. Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101:736–50.
14. Cuzick J, Dowsett M, Pineda S, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and com-parison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–8.
15. Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol 2013;66:512–6.
16. Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 2015; 24 Suppl 2:S67–72.
17. Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol 2016;882:125–54.
18. Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 2001;12:1527–32.
19. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anas-trozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108–16.
20. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 2008;100:1380–8.
21. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351:2817–26.
22. Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 2004;364:858–68.
23. Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8:R25.
24. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55–65.
25. Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010;28:1829–34.
26. Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the recurrence score assay and tamoxifen clinical benefit in the NSABP study B-14 of node negative, estrogen receptor positive breast cancer. J Clin Oncol 2005;23: suppl:510.
27. Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006;24:3726–34.
28. Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 2015;373:2005–14.
29. Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
30. Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783–90.
31. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 post-menopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339–45.
32. van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009.
33. Knauer M, Mook S, Rutgers EJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat 2010;120:655–61.
34. Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016;375:717–29.
35. Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn 2014;16:190–7.
36. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res 2010;16:5222–32.
37. Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011;17:6012–20.
38. Jerevall PL, Ma XJ, Li H, et al. Prognostic utility of HOXB13:IL17BR and molecular grade index in early-stage breast cancer patients from the Stockholm trial. Br J Cancer 2011;104:1762–9.
39. Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205.
40. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067–76.
41. Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American society of clinical oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract 2010;6:243–6.
42. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996;14:2738–46.
43. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the International Breast Cancer Study Group Trials I to V. J Clin Oncol 2016;34:927–35.
44. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84.
45. Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341–52.
46. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16.
47. Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31 (suppl):5.
48. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262–71.
49. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71.
50. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298–305.
51. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–22.
52. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013;109:2959–64.
53. Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX Recurrence Score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst 2016;108:djw149.
54. Muller BM, Keil E, Lehmann A, et al. The EndoPredict gene-expression assay in clinical practice - performance and impact on clinical decisions. PLoS One 2013;8:e68252.
55. Sgroi DC, Chapman JA, Badovinac-Crnjevic T, et al. Assessment of the prognostic and predictive utility of the Breast Cancer Index (BCI): an NCIC CTG MA.14 study. Breast Cancer Res 2016;18:1.
56. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42.
57. Sanft T, Aktas B, Schroeder B, et al. Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast Cancer Res Treat 2015;154:533–41.
Diagnosis and Treatment of Migraine
From the Department of Neurology, Medstar Georgetown University Hospital, Washington, DC.
Abstract
- Objective: To review the epidemiology, pathophysiology, diagnosis, and treatment of migraine.
- Methods: Review of the literature.
- Results: Migraine is a common disorder associated with significant morbidity. Diagnosis of migraine is performed according to the International Classification of Headache Disorders. Comorbidities are commonly seen with migraine and include mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease. Comorbid conditions can increase migraine disability. Management of migraine with lifestyle modifications, trigger management, and acute and preventive medications can help reduce the frequency, duration, and severity of attacks. Overuse of medications such as opiates, barbiturates, and caffeine-containing medications can increase headache frequency. Educating patients about limiting use of these medications is important.
- Conclusion: Migraine is a common neurologic disease that can be very disabling. Recognizing the condition, making an accurate diagnosis, and starting patients on migraine-specific treatments can help improve patient outcomes.
Migraine is a common neurologic disease that affects 1 in 10 people worldwide [1]. It is 2 to 3 times more prevalent in women than in men [2]. The prevalence of migraine peaks in both sexes during the most productive years of adulthood (age 25 to 55 years) [3]. The Global Burden of Diseases, Injuries, and Risk Factors Study considers it to be the 7th most disabling disease in the world [4]. Over 36 million people in the United States have migraine [5]. However, just 56% of migraineurs have ever been diagnosed [6].
Migraine is associated with a high rate of years lived with disability [7] and the rate has been steadily increasing since 1990. At least 50% of migraine sufferers are severely disabled, many requiring bed rest, during individual migraine attacks lasting hours to days [8]. The total U.S. annual economic costs from headache disorders, including the indirect costs from lost productivity and workplace performance, has been estimated at $31 billion [9,10].
Despite the profound impact of migraine on patients and society, there are numerous barriers to migraine care. Lipton et al [11] identified 3 steps that were minimally necessary to achieve guideline-defined appropriate acute pharmacologic therapy: (1) consulting a prescribing health care professional; (2) receiving a migraine diagnosis; and (3) using migraine-specific or other appropriate acute treatments. In a study they conducted in patients with episodic migraine, 45.5% had consulted health care professional for headache in the preceding year; of these, 86.7% reported receiving a medical diagnosis of migraine, and among the diagnosed consulters, 66.7% currently used acute migraine-specific treatments, resulting in only 26.3% individuals successfully completing all 3 steps. In the recent CaMEO study [12], the proportion patients with chronic migraine that overcame all 3 barriers was less than 5%.
The stigma of migraine often makes it difficult for people to discuss symptoms with their health care providers and family members [13]. When they do discuss their headaches with their provider, often they are not given a diagnosis [14] or do not understand what their diagnosis means [15]. It is important for health care providers to be vigilant about the diagnosis of migraine, discuss treatment goals and strategies, and prescribe appropriate migraine treatment. Migraine is often comorbid with a number of medical, neurological, and psychiatric conditions, and identifying and managing comorbidities is necessary to reduce headache burden and disability. In this article, we provide a review of the diagnosis and treatment of migraine, using a case illustration to highlight key points.
Case Study
Initial Presentation
A 24-year-old woman presents for an evaluation of her headaches.
History and Physical Examination
She initially noted headaches at age 19, which were not memorable and did not cause disability. Her current headaches are a severe throbbing pain over her right forehead. They are associated with light and sound sensitivity and stomach upset. Headaches last 6 to 7 hours without medications and occur 4 to 8 days per month.
She denies vomiting and autonomic symptoms such as runny nose or eye tearing. She also denies preceding aura. She reports headache relief with intake of tablets that contain acetaminophen/aspirin/caffeine and states that she takes between 4 to 15 tablets/month depending on headache frequency. She reports having tried acetaminophen and naproxen with no significant benefit. Aggravating factors include bright lights, strong smells, and soy/ high-sodium foods.
She had no significant past medical problems and denied a history of depression or anxiety. Family history was significant for both her father and sister having a history of headaches. The patient lived alone and denied any major life stressors. She exercises 2 times a week and denies smoking or alcohol use. Review of systems was positive for trouble sleeping, which she described as difficulty falling asleep.
On physical examination, vitals were within normal limits. BMI was 23. Chest, cardiac, abdomen, and general physical examination were all within normal limits. Neurological examination revealed no evidence of papilledema or focal neurological deficits.
What is the pathophysiology of migraine?
Migraine was thought to be a primary vascular disorder of the brain, with the origins of the vascular theory of migraine dating back to 1684 [16]. Trials performed by Wolff concluded that migraine is of vascular origin [17], and this remained the predominant theory over several decades. Current evidence suggests that migraine is unlikely to be a pure vascular disorder and instead may be related to changes in the central or peripheral nervous system [18,19].
Migraine is complex brain network disorder with a strong genetic basis [19]. The trigemino-vascular system, along with neurogenically induced inflammation of the dura mater, mast cell degranulation and release of histamine, are the likely causes of migraine pain. Trigeminal fibers arise from neurons in the trigeminal ganglion that contain substance P and calcitonin gene-related peptide (CGRP) [20]. CGRP is a neuropeptide widely expressed in both peripheral and central neurons. Elevation of CGRP in migraine is linked to diminution of the inhibitory pathways which in turn leads to migraine susceptibility [21]. These findings have led to the development of new drugs that target the CGRP pathway.
In the brainstem, periaqueductal grey matter and the dorsolateral pons have been found to be “migraine generators,” or the driver of changes of cortical activity during migraine [22]. Brainstem nuclei are involved in modulating trigemino-vascular pain transmission and autonomic responses in migraine [23].
The hypothalamus has also been implicated in migraine pathogenesis, particularly its role in nociceptive and autonomic modulation in migraine patients. Schulte and May hypothesized that there is a network change between the hypothalamus and the areas of the brainstem generator leading to the migraine attacks [24].
The thalamus plays a central role for the processing and integration of pain stimuli from the dura mater and cutaneous regions. It maintains complex connections with the somatosensory, motor, visual, auditory, olfactory and limbic regions [25]. The structural and functional alterations in the system play a role in the development of migraine attacks, and also in the sensory hypersensitivity to visual stimuli and mechanical allodynia [26].
Experimental studies in rats show that cortical spreading depression can trigger neurogenic meningeal inflammation and subsequently activate the trigemino-vascular system [27]. It has been observed that between migraine episodes a time-dependent amplitude increase of scalp-evoked potentials to repeated stereotyped stimuli, such as visual, auditory, and somaticstimuli, occurs. This phenomenon is described as “deficient habituation.” In episodic migraine, studies show 2 characteristic changes: a deficient habituation between attacks and sensitization during the attack [28]. Genetic studies have hypothesized an involvement of glutamatergic neurotransmitters and synaptic dysplasticity in causing abnormal cortical excitability in migraine [27].
What are diagnostic criteria for migraine?
Diagnosis of migraine is performed according to the International Classification of Headache Disorders (ICHD) [29]. Based on the number of headache days that the patient reports, migraine is classified into episodic or chronic migraine. Migraines that occur on fewer than 15 days/month are categorized as episodic migraines.
Episodic migraine is divided into 2 categories: migraine with aura (Table 1) and migraine without aura. Migraine without aura is described as recurrent headaches consisting of at least 5 attacks, each lasting 4 to 72 hours if left untreated. At least 2 of the following 4 characteristics must be present: unilateral location, pulsating quality, moderate or severe pain intensity, with aggravation by or causing avoidance of routine physical activity. During headache, at least 1 of nausea and/or vomiting or photophobia and phonophobia should be present.
In migraine with aura (Table 2), headache characteristics are the same, but in addition there are at least 2 lifetime attacks with fully reversible aura symptoms (visual, sensory, speech/language). In addition, these auras have at least 2 of the following 4 characteristics: at least 1 aura symptom spreads gradually over 5 minutes, and/or 2 or more symptoms occur in succession; each individual aura symptom lasts 5 to 60 minutes; aura symptom is unilateral; and aura is accompanied, or followed within 60 minutes, by headache. Migraine with aura is uncommon, occurring in 20% of patients with migraine [30]. Visual aura is the most common type of aura, occurring in up to 90% of patients [31]. There is also aura without migraine, called typical aura without headache. Patients can present with non-migraine headache with aura, categorized as typical aura with headache [29].
Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month, is classified as chronic migraine (Table 3). Evidence indicates that 2.5% of episodic migraine progresses to chronic migraine over 1-year follow-up [32]. There are several risk factors for chronification of migraine. Nonmodifiable factors include female sex, white European heritage, head/neck injury, low education/socioeconomic status, and stressful life events (divorce, moving, work changes, problems with children). Modifiable risk factors are headache frequency, acute medication overuse, caffeine overuse, obesity, comorbid mood disorders, and allodynia. Acute medication use and headache frequency are independent risk factors for development of chronic migraine [33]. The risk of chronic migraine increases exponentially with increased attack frequency, usually when the frequency is ≥ 3 headaches/month. Repetitive episodes of pain may increase central sensitization and result in anatomical changes in the brain and brainstem [34].
What information should be elicited during the history?
Specific questions about the headaches can help with making an accurate diagnosis. These include:
- Length of attacks and their frequency
- Pain characteristics (location, quality, intensity)
- Actions that trigger or aggravate headaches (eg, stress, movement, bright lights, menses, certain foods and smells)
- Associated symptoms that accompany headaches (eg, nausea, vomiting)
- How the headaches impact their life (eg, missed days at work or school, missed life events, avoidance of social activities, emergency room visits due to headache)
To assess headache frequency, it is helpful to ask about the number of headache-free days in a month, eg, “how many days a month do you NOT have a headache.” To assist with headache assessment, patients can be asked to keep a calendar in which they mark days of use of medications, including over the counter medications, menses, and headache days. The calendar can be used to assess for migraine patterns, headache frequency, and response to treatment.
When asking about headache history, it is important for patients to describe their untreated headaches. Patients taking medications may have pain that is less severe or disabling or have reduced associated symptoms. Understanding what the headaches were like when they did not treat is important in making a diagnosis.
Other important questions include when was the first time they recall ever experiencing a headache. Migraine is often present early in life, and understanding the change in headache over time is important. Also ask patients about what they want to do when they have a headache. Often patients want to lie down in a cool dark room. Ask what they would prefer to do if they didn’t have any pending responsibilities.
Comorbidities
Comorbidities are commonly seen with migraine. Common comorbidities are mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease.
Comorbid conditions can increase migraine disability and also can provide information about the pathophysiology of migraine and guide treatment. Management of the underlying comorbidity often leads to improved migraine outcomes. For example, serotonergic dysfunction is a possible pathway involved in both migraine and mood disorders. Treatment with medications that alter the serotonin system may help both migraine and coexisting mood disorders. Bigal et al proposed that activation of the HPA axis with reduced serotonin synthesis is a main pathway involved in affective disorders, migraine, and obesity [35].
In the early 1950s, Wolff conceptualized migraine as a psychophysiologic disorder [36]. The relationship between migraine and psychiatric conditions is complex, and comorbid psychiatric disorders are risk factors for headache progression and chronicity. Psychiatric conditions also play a role in nonadherence to headache medication, which contributes to poor outcome in these patients. Hence, there is a need for assessment and treatment of psychiatric disorders in people with migraine. A study by Guidetti et al found that headache patients with multiple psychiatric conditions have poor outcomes, with 86 % of these headache patients having no improvement and even deterioration in their headache [37]. Another study by Mongini et al concluded that psychiatric disorder appears to influence the result of treatment on a long-term basis [38].
In addition, migraine has been shown to impact mood disorders. Worsening headache was found to be associated with poorer prognosis for depression. Patients with active migraine not on medications with comorbid major depressive disorder (MDD) had more severe anxiety and somatic symptoms as compared with MDD patients without migraine [39].
Case Continued
Our patient has a normal neurologic examination and classic migraine headache history and stable frequency. The physician tells her she meets criteria for episodic migraine without aura. The patient asks if she needs a “brain scan” to see if something more serious may be causing her symptoms.
What workup is recommended for patients with migraine?
If patient symptoms fit the criteria for migraine and there is a normal neurologic examination, the differential is often limited. When there are neurologic abnormalities on examination (eg, papilledema), or if the patient has concerning signs or symptoms (see below), then neuroimaging should be obtained to rule out secondary causes of headache.
In 2014, the American Academy of Neurology (AAN) published practice parameters on the evaluation of adults with recurrent headache based on guidelines published by the US Headache Consortium [40]. As per AAN guidelines, routine laboratory studies, lumbar puncture, and electroencephalogram are not recommended in the evaluation of non-acute migraines. Neuroimaging is not warranted in patients with migraine and a normal neurologic examination (grade B recommendation). Imaging may need to be considered in patients with non-acute headache and an unexplained abnormal finding on the neurologic examination (grade B recommendation).
When patients exhibit particular warning signs, or headache “red flags,” it is recommended that neuroimaging be considered. Red flags include patients with recurrent headaches and systemic symptoms (fever, weight loss), neurologic symptoms or abnormal signs (confusion, impaired alertness or consciousness), sudden onset, abrupt, or split second in nature, patients age > 50 with new onset or progressive headache, previous headache history with new or different headache (change in frequency, severity, or clinical features) and if there are secondary risk factors (HIV, cancer) [41].
Case Continued
Our patient has no red flags and can be reassured that given her normal physical examination and history suggestive of a migraine, a secondary cause of her headache is unlikely. The physician describes the treatments available, including implementing lifestyles changes and preventive and abortive medications. The patient expresses apprehension about being on prescription medications. She is concerned about side effects as well as the need to take daily medication over a long period of time. She reports that these were the main reasons she did not take the rizatriptan and propranolol that was prescribed by her previous doctor.
How is migraine treated?
Migraine is managed with a combination of lifestyle changes and pharmacologic therapy. Pharmacologic management targets treating an attack when it occurs (abortive medication), as well as reducing the frequency and severity of future attacks (preventive medication).
Lifestyle Changes
Patients should be advised that making healthy lifestyle choices, eg, regular sleep, balanced meals, proper hydration, and regular exercise, can mitigate migraine [42–44]. Other lifestyle changes that can be helpful include weight loss in the obese population, as weight loss appears to result in migraine improvement. People who are obese also are at higher risk for the progression to chronic migraine.
Acute Therapy
There are varieties of abortive therapies [45] (Table 4) that are commonly used in clinical practice. Abortive therapy can be taken as needed and is most effective if used within the first 2 hours of headache. For patients with daily or frequent headache, these medications need to be restricted to 8 to 12 days a month of use and their use should be restricted to when headache is worsening. This usually works well in patients with moderate level pain, and especially in patients with no associated nausea. Selective migraine treatments, like triptans and ergots, are used when nonspecific treatments fail, or when headache is more severe. It is preferable that patients avoid opioids, butalbital, and caffeine-containing medications. In the real world, it is difficult to convince patient to stop these medications; it is more realistic to discuss use limitation with patients, who often run out their weekly limit for triptans.
Triptans are effective medications for acute management of migraine but headache recurrence rate is high, occurring in 15% to 40 % of patients taking oral triptans. It is difficult to predict the response to a triptan [46]. The choice of an abortive agent is often directed partially by patient preference (side effect profile, cost, non-sedating vs. prefers to sleep, long vs short half-life), comorbid conditions (avoid triptans and ergots in uncontrolled hypertension, cardiovascular disease, or peripheral vascular disease or stroke/aneurysm; avoid NSAIDS in patients with cardiovascular disease), and migraine-associated symptoms (nausea and/or vomiting). Consider non-oral formulations via subcutaneous or nasal routes in patients who have nausea or vomiting with their migraine attacks. Some patients may require more than one type of abortive medication. The high recurrence rate is similar across different triptans and so switching from one triptan to another has not been found to be useful. Adding NSAIDS to triptans has been found to be more useful than switching between triptans.Overuse of acute medications has been associated with transformation of headache from episodic to chronic (medication overuse headache or rebound headache). The risk of transformation appears to be greatest with medications containing caffeine, opiates, or barbiturates [47]. Use of acute medications should be limited based on the type of medication. Patients should take triptans for no more than 10 days a month. Combined medications and opioids should be used fewer than 8 days a month, and butalbital-containing medications should be avoided or used fewer than 5 days a month [48]. Use of acute therapy should be monitored with headache calendars. It is unclear if and to what degree NSAIDS and acetaminophen cause overuse headaches.
Medication overuse headache can be difficult to treat as patients have to stop using the medication causing rebound. Further, headaches often resemble migraine and it can be difficult to differentiate them from the patients’ routine headache. Vigilance with medication use in patients with frequent headache is an essential part of migraine management, and patients should receive clear instructions regarding how to use acute medications.
Prevention
Patients presenting with more than 4 headaches per month, or headaches that last longer than 12 hours, require preventive therapy. The goals of preventive therapy is to reduce attack frequency, severity, and duration, to improve responsiveness to treatment of acute attacks, to improve function and reduce disability, and to prevent progression or transformation of episodic migraine to chronic migraine. Preventive medications usually need to be taken daily to reduce frequency or severity of the headache. The goal in this approach is 50% reduction of headache frequency and severity. Migraine preventive medications usually belong to 1 of 3 categories of drugs: antihypertensives, antiepileptics, and antidepressants. At present there are many medications for migraine prevention with different levels of evidence [49] (Table 5). Onabotulinuma toxin is the only approved medication for chronic migraine based on promising results of the PREEMPT trial [50].
Other Considerations
A multidisciplinary approach to treatment may be warranted. Psychiatric evaluation and management of underlying depression and mood disorders can help reduce headache frequency and severity. Physical therapy should be prescribed for neck and shoulder pain. Sleep specialists should be consulted if ongoing sleep issues continue despite behavioral management.
How common is nonadherence with migraine medication?
One third of patients who are prescribed triptans discontinue the medication within a year. Lack of efficacy and concerns over medication side effects are 2 of the most common reasons for poor adherence [51]. In addition, age plays a significant role in discontinuing medication, with the elderly population more likely to stop taking triptans [52]. Seng et al reported that among patients with migraine, being male, being single, having frequent headache, and having mild pain are all associated with medication nonadherence [53]. Formulary restrictions and type of insurance coverage also were associated with nonadherence. Among adherent patients, some individuals were found to be hoarding their tablets and waiting until they were sure it was a migraine. Delaying administration of abortive medications increases the chance of incomplete treatment response, leading to patients taking more medication and in turn have more side effects [53].
Educating patients about their medications and how they need to be taken (preventive vs. abortive, when to administer) can help with adherence (Table 6). Monitoring medication use and headache frequency is an essential part of continued care for migraine patients. Maintain follow up with patients to review how they are doing with the medication and avoid providing refills without visits. The patient may not be taking medication consistently or may be using more medication than prescribed.
What is the role of nonpharmacologic therapy?
Most patients respond to pharmacologic treatment, but some patients with mood disorder, anxiety, difficulties or disability associated with headache, and patients with difficulty managing stress or other triggers may benefit from the addition of behavioral treatments (eg, relaxation, biofeedback, cognitive behavioral therapy, stress management) [54].
Cognitive behavioral therapy and mindfulness are techniques that have been found to be effective in decreasing intensity of pain and associated disability. The goal of these techniques is to manage the cognitive, affective, and behavioral precipitants of headache. In this process, patients are helped to identify the thoughts and behavior that play a role in generating headache. These techniques have been found to improve many headache-related outcomes like pain intensity, headache-related disability, measures of quality of life, mood and medication consumption [55]. A multidisciplinary intervention that included group exercise, stress management and relaxation lectures, and massage therapy was found to reduce self-perceived pain intensity, frequency, and duration of the headache, and improve functional status and quality of life in migraineurs [56]. A randomized controlled trial of yoga therapy compared with self care showed that yoga led to significant reduction in migraine headache frequency and improved overall outcome [57].
Overall, results from studies of nonpharmacologic techniques have been mixed [58,59]. A systematic review by Sullivan et al found a large range in the efficacy of psychological interventions for migraine [60]. A 2015 systematic review that examined if cognitive behavioral therapy (CBT) can reduce the physical symptoms of chronic headache and migraines obtained mixed results [58]. Holryod et al’s study [61] found that behavioral management combined with a ß blocker is useful in improving outcomes, but neither the ß blocker alone or behavioral migraine management alone was. Also, a trial by Penzien et al showed that nonpharmacological management helped reduce migraines by 40% to 50% and this was similar to results seen with preventive drugs [62].
Patient education may be helpful in improving outcomes. Smith et al reported a 50% reduction in headache frequency at 12 months in 46% of patients who received migraine education [63]. A randomized controlled trial by Rothrock et al involving 100 migraine patients found that patients who attended a “headache school” consisting of three 90-minute educational sessions focused on topics such as acute treatment and prevention of migraine had a significant reduction in mean migraine disability assessment score (MIDAS) than the group randomized to routine medical management only. The patients also experienced a reduction in functionally incapacitating headache days per month, less need for abortive therapy and were more compliant with prophylactic therapy [64].
Case Conclusion
Our patient is a young woman with a history of headaches suggestive of migraine without aura. Since her headache frequency ranges from 4-8 headaches month, she has episodic migraines. She also has a strong family history of headaches. She denies any other medical or psychiatric comorbidity. She reports an intake of a caffeine-containing medication of 4 to 15 tablets per month.
The physician recommended that she limit her intake of the caffeine-containing medication to 5 days or less per month given the risk of migraine transformation. The physician also recommended maintaining a good sleep schedule, limiting excessive caffeine intake, a stress reduction program, regular cardiovascular exercise, and avoiding skipping or delaying meals. The patient was educated about migraine and its underlying mechanisms and the benefits of taking medications, and her fears regarding medication use and side effects were allayed. Sumatriptan 100 mg oral tablets were prescribed to be taken at headache onset. She was hesitant to be started on an antihypertensive or antiseizure medication, so she was prescribed amitriptyline 30 mg at night for headache prevention. She was also asked to maintain a headache diary. The patient was agreeable with this plan.
Summary
Migraine is often underdiagnosed and undertreated. Primary care providers are often the first point of contact for these patients. Identifying the type and frequency of migraine and comorbidities is necessary to guide appropriate management in terms of medications and lifestyle modifications. Often no testing or imaging is required. Educating patients about this chronic disease, treatment expectations, and limiting intake of medication is essential.
Corresponding author: Pooja Mohan Rao, MBBS, MD, Georgetown University Hospital, 3800 Reservoir Rd. NW, 7 PHC, Washington, DC 20007, [email protected].
Financial disclosures: Dr. Ailani reports receiving honoraria for speaking and consulting for Allergan, Avanir, and Eli Lilly.
1. Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants, J Neurol Sci 2017;372:307–15.
2. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87.
3. Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45 Suppl 1:S3–S13.
4. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602.
5. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention Headache 2015;55 Suppl 2:103–22.
6. Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47:355–63.
7. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study. Lancet 2012;380:2163–96.
8. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–57.
9. Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain in the US workforce. JAMA 2003;290:2443–54.
10. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache 2008;48:553–63.
11. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013;53:81–92.
12. Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: Results from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache 2016;56:821–34.
13. Young WB, Park JE, Tian IX, Kempner J. The stigma of migraine. PLoS One 2013;8(1):e54074.
14. Mia M, Ashna S, Audrey H. A migraine management training program for primary care providers: an overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache 2016;56:725–40.
15. Lipton RB, Amatniek JC, Ferrari MD, Gross M. Migraine: identifying and removing barriers to care. Neurology 1994;44(6 Suppl 4):S63–8.
16. Knapp RD Jr. Reports from the past 2. Headache 1963;3:112–22.
17. Levine M, Wolff HG. Cerebral circulation: afferent impulses from the blood vessels of the pia. Arch Neurol Psychiat 1932;28:140.
18. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross sectional study. Lancet Neurol 2013;12:454–61.
19. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 2017;97:553–622.
20. Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol 2012;15(Suppl 1):S15–S22.
21. Puledda F, Messina R, Goadsby PJ, et al. An update on migraine: current understanding and future J Neurol 2017 Mar 20.
22. Vinogradova LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 2015;35:979–86.
23. Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–7.
24. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93.
25. Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011;31:14204–17.
26. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–45.
27. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol 2017 Mar 20.
28. Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain 2013;14:65.
29. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
30. Yusheng H, Y Li. Typical aura without headache: a case report and review of the literature J Med Case Rep 2015;9:40.
31. Buture A, Khalil M, Ahmed F. Iatrogenic visual aura: a case report and a brief review of the literature Ther Clin Risk Manag 2017;13:643–6.
32. Lipton RB. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009;72:S3–7.
33. Lipton RB. Headache 2011;51;S2:77–83.
34. Scher AI, Stewart WF, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003;106:81–9.
35. Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology 2007;68:1851–61.
36. Wolff HG. Stress and disease. Springfield, IL: Charles C. Thomas; 1953.
37. Guidetti V, Galli F, Fabrizi P, et al. Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998;18:455–62.
38. Mongini F, Keller R, Deregibus A, et al. Personality traits, depression and migraine in women: a longitudinal study. Cephalalgia 2003;23:186–92.
39. Hung CI, Liu CY, Yang CH, Wang SJ. The impacts of migraine among outpatients with major depressive disorder at a two-year follow-up. PLoS One 2015;10:e0128087.
40. Frishberg BM, Rosenberg JH, Matchar DB, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. St Paul: US Headache Consortium; 2000.
41. Headache Measurement Set 2014 Revised. American Academy of Neurology. Accessed at www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/2.Quality_Improvement/1.Quality_Measures/1.All_Measures/2014.
42. Taylor FR. Lifestyle changes, dietary restrictions, and nutraceuticals in migraine prevention. Techn Reg Anesth Pain Manage 2009;13:28–37.
43. Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia 2011;14:1428–38.
44. Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep 2013;17:379.
45. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015;55:3–20.
46. Belvis R, Mas N, Aceituno A. Migraine attack treatment : a tailor-made suit, not one size fits all. Recent Pat CNS Drug Discov 2014;9:26–40.
47. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008;48:1157–68.
48. Bigal ME, Rapoport AM, Sheftell FD, et al. Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes Cephalalgia 2004;24:483–90.
49. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–45.
50. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial Cephalagia year;30:804–14.
51. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache 2014;54:278–89.
52. Holland S, Fanning KM, Serrano D, et al. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci 2013;326:10–7.
53. Seng EK, Rains JA, Nicholson RA, Lipton RB. Improving medication adherence in migraine treatment. Curr Pain Headache Rep 2015;19:24.
54. Nicholson RA, Buse DC, Andrasik F, Lipton RB. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treatment Opt Neurol 2011;13:28–40.
55. Probyn K, Bowers H, Mistry D, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components BMJ Open 2017;7:e016670.
56. Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002;42:845–54.
57. John PJ, Sharma N, Sharma CM, Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache 2007;47:654–61.
58. Harris P, Loveman E, Clegg A, et al. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults Br J Pain 2015;9:213–24.
59. Kropp P, Meyer B, Meyer W, Dresler T. An update on behavioral treatments in migraine - current knowledge and future options. Expert Rev Neurother 2017:1–10.
60. Sullivan A, Cousins S, Ridsdale L. Psychological interventions for migraine: a systematic review. J Neurol 2016;263:2369–77.
61. Holroyd KA, Cottrell CK, O’Donnell FJ, et al. Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ 2010;341:c4871.
62. Penzien DB, Rains JC, Andrasik F. Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback 2002;27:163–81.
63. Smith TR, Nicholson RA, Banks JW. A primary care migraine education program has benefit on headache impact and quality of life: results from the mercy migraine management program. Headache 2010;50:600–12.
64. Rothrock JF, Parada VA, Sims C, et al.The impact of intensive patient education on clinical outcome in a clinic-based migraine population. Headache 2006;46:726–31.
From the Department of Neurology, Medstar Georgetown University Hospital, Washington, DC.
Abstract
- Objective: To review the epidemiology, pathophysiology, diagnosis, and treatment of migraine.
- Methods: Review of the literature.
- Results: Migraine is a common disorder associated with significant morbidity. Diagnosis of migraine is performed according to the International Classification of Headache Disorders. Comorbidities are commonly seen with migraine and include mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease. Comorbid conditions can increase migraine disability. Management of migraine with lifestyle modifications, trigger management, and acute and preventive medications can help reduce the frequency, duration, and severity of attacks. Overuse of medications such as opiates, barbiturates, and caffeine-containing medications can increase headache frequency. Educating patients about limiting use of these medications is important.
- Conclusion: Migraine is a common neurologic disease that can be very disabling. Recognizing the condition, making an accurate diagnosis, and starting patients on migraine-specific treatments can help improve patient outcomes.
Migraine is a common neurologic disease that affects 1 in 10 people worldwide [1]. It is 2 to 3 times more prevalent in women than in men [2]. The prevalence of migraine peaks in both sexes during the most productive years of adulthood (age 25 to 55 years) [3]. The Global Burden of Diseases, Injuries, and Risk Factors Study considers it to be the 7th most disabling disease in the world [4]. Over 36 million people in the United States have migraine [5]. However, just 56% of migraineurs have ever been diagnosed [6].
Migraine is associated with a high rate of years lived with disability [7] and the rate has been steadily increasing since 1990. At least 50% of migraine sufferers are severely disabled, many requiring bed rest, during individual migraine attacks lasting hours to days [8]. The total U.S. annual economic costs from headache disorders, including the indirect costs from lost productivity and workplace performance, has been estimated at $31 billion [9,10].
Despite the profound impact of migraine on patients and society, there are numerous barriers to migraine care. Lipton et al [11] identified 3 steps that were minimally necessary to achieve guideline-defined appropriate acute pharmacologic therapy: (1) consulting a prescribing health care professional; (2) receiving a migraine diagnosis; and (3) using migraine-specific or other appropriate acute treatments. In a study they conducted in patients with episodic migraine, 45.5% had consulted health care professional for headache in the preceding year; of these, 86.7% reported receiving a medical diagnosis of migraine, and among the diagnosed consulters, 66.7% currently used acute migraine-specific treatments, resulting in only 26.3% individuals successfully completing all 3 steps. In the recent CaMEO study [12], the proportion patients with chronic migraine that overcame all 3 barriers was less than 5%.
The stigma of migraine often makes it difficult for people to discuss symptoms with their health care providers and family members [13]. When they do discuss their headaches with their provider, often they are not given a diagnosis [14] or do not understand what their diagnosis means [15]. It is important for health care providers to be vigilant about the diagnosis of migraine, discuss treatment goals and strategies, and prescribe appropriate migraine treatment. Migraine is often comorbid with a number of medical, neurological, and psychiatric conditions, and identifying and managing comorbidities is necessary to reduce headache burden and disability. In this article, we provide a review of the diagnosis and treatment of migraine, using a case illustration to highlight key points.
Case Study
Initial Presentation
A 24-year-old woman presents for an evaluation of her headaches.
History and Physical Examination
She initially noted headaches at age 19, which were not memorable and did not cause disability. Her current headaches are a severe throbbing pain over her right forehead. They are associated with light and sound sensitivity and stomach upset. Headaches last 6 to 7 hours without medications and occur 4 to 8 days per month.
She denies vomiting and autonomic symptoms such as runny nose or eye tearing. She also denies preceding aura. She reports headache relief with intake of tablets that contain acetaminophen/aspirin/caffeine and states that she takes between 4 to 15 tablets/month depending on headache frequency. She reports having tried acetaminophen and naproxen with no significant benefit. Aggravating factors include bright lights, strong smells, and soy/ high-sodium foods.
She had no significant past medical problems and denied a history of depression or anxiety. Family history was significant for both her father and sister having a history of headaches. The patient lived alone and denied any major life stressors. She exercises 2 times a week and denies smoking or alcohol use. Review of systems was positive for trouble sleeping, which she described as difficulty falling asleep.
On physical examination, vitals were within normal limits. BMI was 23. Chest, cardiac, abdomen, and general physical examination were all within normal limits. Neurological examination revealed no evidence of papilledema or focal neurological deficits.
What is the pathophysiology of migraine?
Migraine was thought to be a primary vascular disorder of the brain, with the origins of the vascular theory of migraine dating back to 1684 [16]. Trials performed by Wolff concluded that migraine is of vascular origin [17], and this remained the predominant theory over several decades. Current evidence suggests that migraine is unlikely to be a pure vascular disorder and instead may be related to changes in the central or peripheral nervous system [18,19].
Migraine is complex brain network disorder with a strong genetic basis [19]. The trigemino-vascular system, along with neurogenically induced inflammation of the dura mater, mast cell degranulation and release of histamine, are the likely causes of migraine pain. Trigeminal fibers arise from neurons in the trigeminal ganglion that contain substance P and calcitonin gene-related peptide (CGRP) [20]. CGRP is a neuropeptide widely expressed in both peripheral and central neurons. Elevation of CGRP in migraine is linked to diminution of the inhibitory pathways which in turn leads to migraine susceptibility [21]. These findings have led to the development of new drugs that target the CGRP pathway.
In the brainstem, periaqueductal grey matter and the dorsolateral pons have been found to be “migraine generators,” or the driver of changes of cortical activity during migraine [22]. Brainstem nuclei are involved in modulating trigemino-vascular pain transmission and autonomic responses in migraine [23].
The hypothalamus has also been implicated in migraine pathogenesis, particularly its role in nociceptive and autonomic modulation in migraine patients. Schulte and May hypothesized that there is a network change between the hypothalamus and the areas of the brainstem generator leading to the migraine attacks [24].
The thalamus plays a central role for the processing and integration of pain stimuli from the dura mater and cutaneous regions. It maintains complex connections with the somatosensory, motor, visual, auditory, olfactory and limbic regions [25]. The structural and functional alterations in the system play a role in the development of migraine attacks, and also in the sensory hypersensitivity to visual stimuli and mechanical allodynia [26].
Experimental studies in rats show that cortical spreading depression can trigger neurogenic meningeal inflammation and subsequently activate the trigemino-vascular system [27]. It has been observed that between migraine episodes a time-dependent amplitude increase of scalp-evoked potentials to repeated stereotyped stimuli, such as visual, auditory, and somaticstimuli, occurs. This phenomenon is described as “deficient habituation.” In episodic migraine, studies show 2 characteristic changes: a deficient habituation between attacks and sensitization during the attack [28]. Genetic studies have hypothesized an involvement of glutamatergic neurotransmitters and synaptic dysplasticity in causing abnormal cortical excitability in migraine [27].
What are diagnostic criteria for migraine?
Diagnosis of migraine is performed according to the International Classification of Headache Disorders (ICHD) [29]. Based on the number of headache days that the patient reports, migraine is classified into episodic or chronic migraine. Migraines that occur on fewer than 15 days/month are categorized as episodic migraines.
Episodic migraine is divided into 2 categories: migraine with aura (Table 1) and migraine without aura. Migraine without aura is described as recurrent headaches consisting of at least 5 attacks, each lasting 4 to 72 hours if left untreated. At least 2 of the following 4 characteristics must be present: unilateral location, pulsating quality, moderate or severe pain intensity, with aggravation by or causing avoidance of routine physical activity. During headache, at least 1 of nausea and/or vomiting or photophobia and phonophobia should be present.
In migraine with aura (Table 2), headache characteristics are the same, but in addition there are at least 2 lifetime attacks with fully reversible aura symptoms (visual, sensory, speech/language). In addition, these auras have at least 2 of the following 4 characteristics: at least 1 aura symptom spreads gradually over 5 minutes, and/or 2 or more symptoms occur in succession; each individual aura symptom lasts 5 to 60 minutes; aura symptom is unilateral; and aura is accompanied, or followed within 60 minutes, by headache. Migraine with aura is uncommon, occurring in 20% of patients with migraine [30]. Visual aura is the most common type of aura, occurring in up to 90% of patients [31]. There is also aura without migraine, called typical aura without headache. Patients can present with non-migraine headache with aura, categorized as typical aura with headache [29].
Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month, is classified as chronic migraine (Table 3). Evidence indicates that 2.5% of episodic migraine progresses to chronic migraine over 1-year follow-up [32]. There are several risk factors for chronification of migraine. Nonmodifiable factors include female sex, white European heritage, head/neck injury, low education/socioeconomic status, and stressful life events (divorce, moving, work changes, problems with children). Modifiable risk factors are headache frequency, acute medication overuse, caffeine overuse, obesity, comorbid mood disorders, and allodynia. Acute medication use and headache frequency are independent risk factors for development of chronic migraine [33]. The risk of chronic migraine increases exponentially with increased attack frequency, usually when the frequency is ≥ 3 headaches/month. Repetitive episodes of pain may increase central sensitization and result in anatomical changes in the brain and brainstem [34].
What information should be elicited during the history?
Specific questions about the headaches can help with making an accurate diagnosis. These include:
- Length of attacks and their frequency
- Pain characteristics (location, quality, intensity)
- Actions that trigger or aggravate headaches (eg, stress, movement, bright lights, menses, certain foods and smells)
- Associated symptoms that accompany headaches (eg, nausea, vomiting)
- How the headaches impact their life (eg, missed days at work or school, missed life events, avoidance of social activities, emergency room visits due to headache)
To assess headache frequency, it is helpful to ask about the number of headache-free days in a month, eg, “how many days a month do you NOT have a headache.” To assist with headache assessment, patients can be asked to keep a calendar in which they mark days of use of medications, including over the counter medications, menses, and headache days. The calendar can be used to assess for migraine patterns, headache frequency, and response to treatment.
When asking about headache history, it is important for patients to describe their untreated headaches. Patients taking medications may have pain that is less severe or disabling or have reduced associated symptoms. Understanding what the headaches were like when they did not treat is important in making a diagnosis.
Other important questions include when was the first time they recall ever experiencing a headache. Migraine is often present early in life, and understanding the change in headache over time is important. Also ask patients about what they want to do when they have a headache. Often patients want to lie down in a cool dark room. Ask what they would prefer to do if they didn’t have any pending responsibilities.
Comorbidities
Comorbidities are commonly seen with migraine. Common comorbidities are mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease.
Comorbid conditions can increase migraine disability and also can provide information about the pathophysiology of migraine and guide treatment. Management of the underlying comorbidity often leads to improved migraine outcomes. For example, serotonergic dysfunction is a possible pathway involved in both migraine and mood disorders. Treatment with medications that alter the serotonin system may help both migraine and coexisting mood disorders. Bigal et al proposed that activation of the HPA axis with reduced serotonin synthesis is a main pathway involved in affective disorders, migraine, and obesity [35].
In the early 1950s, Wolff conceptualized migraine as a psychophysiologic disorder [36]. The relationship between migraine and psychiatric conditions is complex, and comorbid psychiatric disorders are risk factors for headache progression and chronicity. Psychiatric conditions also play a role in nonadherence to headache medication, which contributes to poor outcome in these patients. Hence, there is a need for assessment and treatment of psychiatric disorders in people with migraine. A study by Guidetti et al found that headache patients with multiple psychiatric conditions have poor outcomes, with 86 % of these headache patients having no improvement and even deterioration in their headache [37]. Another study by Mongini et al concluded that psychiatric disorder appears to influence the result of treatment on a long-term basis [38].
In addition, migraine has been shown to impact mood disorders. Worsening headache was found to be associated with poorer prognosis for depression. Patients with active migraine not on medications with comorbid major depressive disorder (MDD) had more severe anxiety and somatic symptoms as compared with MDD patients without migraine [39].
Case Continued
Our patient has a normal neurologic examination and classic migraine headache history and stable frequency. The physician tells her she meets criteria for episodic migraine without aura. The patient asks if she needs a “brain scan” to see if something more serious may be causing her symptoms.
What workup is recommended for patients with migraine?
If patient symptoms fit the criteria for migraine and there is a normal neurologic examination, the differential is often limited. When there are neurologic abnormalities on examination (eg, papilledema), or if the patient has concerning signs or symptoms (see below), then neuroimaging should be obtained to rule out secondary causes of headache.
In 2014, the American Academy of Neurology (AAN) published practice parameters on the evaluation of adults with recurrent headache based on guidelines published by the US Headache Consortium [40]. As per AAN guidelines, routine laboratory studies, lumbar puncture, and electroencephalogram are not recommended in the evaluation of non-acute migraines. Neuroimaging is not warranted in patients with migraine and a normal neurologic examination (grade B recommendation). Imaging may need to be considered in patients with non-acute headache and an unexplained abnormal finding on the neurologic examination (grade B recommendation).
When patients exhibit particular warning signs, or headache “red flags,” it is recommended that neuroimaging be considered. Red flags include patients with recurrent headaches and systemic symptoms (fever, weight loss), neurologic symptoms or abnormal signs (confusion, impaired alertness or consciousness), sudden onset, abrupt, or split second in nature, patients age > 50 with new onset or progressive headache, previous headache history with new or different headache (change in frequency, severity, or clinical features) and if there are secondary risk factors (HIV, cancer) [41].
Case Continued
Our patient has no red flags and can be reassured that given her normal physical examination and history suggestive of a migraine, a secondary cause of her headache is unlikely. The physician describes the treatments available, including implementing lifestyles changes and preventive and abortive medications. The patient expresses apprehension about being on prescription medications. She is concerned about side effects as well as the need to take daily medication over a long period of time. She reports that these were the main reasons she did not take the rizatriptan and propranolol that was prescribed by her previous doctor.
How is migraine treated?
Migraine is managed with a combination of lifestyle changes and pharmacologic therapy. Pharmacologic management targets treating an attack when it occurs (abortive medication), as well as reducing the frequency and severity of future attacks (preventive medication).
Lifestyle Changes
Patients should be advised that making healthy lifestyle choices, eg, regular sleep, balanced meals, proper hydration, and regular exercise, can mitigate migraine [42–44]. Other lifestyle changes that can be helpful include weight loss in the obese population, as weight loss appears to result in migraine improvement. People who are obese also are at higher risk for the progression to chronic migraine.
Acute Therapy
There are varieties of abortive therapies [45] (Table 4) that are commonly used in clinical practice. Abortive therapy can be taken as needed and is most effective if used within the first 2 hours of headache. For patients with daily or frequent headache, these medications need to be restricted to 8 to 12 days a month of use and their use should be restricted to when headache is worsening. This usually works well in patients with moderate level pain, and especially in patients with no associated nausea. Selective migraine treatments, like triptans and ergots, are used when nonspecific treatments fail, or when headache is more severe. It is preferable that patients avoid opioids, butalbital, and caffeine-containing medications. In the real world, it is difficult to convince patient to stop these medications; it is more realistic to discuss use limitation with patients, who often run out their weekly limit for triptans.
Triptans are effective medications for acute management of migraine but headache recurrence rate is high, occurring in 15% to 40 % of patients taking oral triptans. It is difficult to predict the response to a triptan [46]. The choice of an abortive agent is often directed partially by patient preference (side effect profile, cost, non-sedating vs. prefers to sleep, long vs short half-life), comorbid conditions (avoid triptans and ergots in uncontrolled hypertension, cardiovascular disease, or peripheral vascular disease or stroke/aneurysm; avoid NSAIDS in patients with cardiovascular disease), and migraine-associated symptoms (nausea and/or vomiting). Consider non-oral formulations via subcutaneous or nasal routes in patients who have nausea or vomiting with their migraine attacks. Some patients may require more than one type of abortive medication. The high recurrence rate is similar across different triptans and so switching from one triptan to another has not been found to be useful. Adding NSAIDS to triptans has been found to be more useful than switching between triptans.Overuse of acute medications has been associated with transformation of headache from episodic to chronic (medication overuse headache or rebound headache). The risk of transformation appears to be greatest with medications containing caffeine, opiates, or barbiturates [47]. Use of acute medications should be limited based on the type of medication. Patients should take triptans for no more than 10 days a month. Combined medications and opioids should be used fewer than 8 days a month, and butalbital-containing medications should be avoided or used fewer than 5 days a month [48]. Use of acute therapy should be monitored with headache calendars. It is unclear if and to what degree NSAIDS and acetaminophen cause overuse headaches.
Medication overuse headache can be difficult to treat as patients have to stop using the medication causing rebound. Further, headaches often resemble migraine and it can be difficult to differentiate them from the patients’ routine headache. Vigilance with medication use in patients with frequent headache is an essential part of migraine management, and patients should receive clear instructions regarding how to use acute medications.
Prevention
Patients presenting with more than 4 headaches per month, or headaches that last longer than 12 hours, require preventive therapy. The goals of preventive therapy is to reduce attack frequency, severity, and duration, to improve responsiveness to treatment of acute attacks, to improve function and reduce disability, and to prevent progression or transformation of episodic migraine to chronic migraine. Preventive medications usually need to be taken daily to reduce frequency or severity of the headache. The goal in this approach is 50% reduction of headache frequency and severity. Migraine preventive medications usually belong to 1 of 3 categories of drugs: antihypertensives, antiepileptics, and antidepressants. At present there are many medications for migraine prevention with different levels of evidence [49] (Table 5). Onabotulinuma toxin is the only approved medication for chronic migraine based on promising results of the PREEMPT trial [50].
Other Considerations
A multidisciplinary approach to treatment may be warranted. Psychiatric evaluation and management of underlying depression and mood disorders can help reduce headache frequency and severity. Physical therapy should be prescribed for neck and shoulder pain. Sleep specialists should be consulted if ongoing sleep issues continue despite behavioral management.
How common is nonadherence with migraine medication?
One third of patients who are prescribed triptans discontinue the medication within a year. Lack of efficacy and concerns over medication side effects are 2 of the most common reasons for poor adherence [51]. In addition, age plays a significant role in discontinuing medication, with the elderly population more likely to stop taking triptans [52]. Seng et al reported that among patients with migraine, being male, being single, having frequent headache, and having mild pain are all associated with medication nonadherence [53]. Formulary restrictions and type of insurance coverage also were associated with nonadherence. Among adherent patients, some individuals were found to be hoarding their tablets and waiting until they were sure it was a migraine. Delaying administration of abortive medications increases the chance of incomplete treatment response, leading to patients taking more medication and in turn have more side effects [53].
Educating patients about their medications and how they need to be taken (preventive vs. abortive, when to administer) can help with adherence (Table 6). Monitoring medication use and headache frequency is an essential part of continued care for migraine patients. Maintain follow up with patients to review how they are doing with the medication and avoid providing refills without visits. The patient may not be taking medication consistently or may be using more medication than prescribed.
What is the role of nonpharmacologic therapy?
Most patients respond to pharmacologic treatment, but some patients with mood disorder, anxiety, difficulties or disability associated with headache, and patients with difficulty managing stress or other triggers may benefit from the addition of behavioral treatments (eg, relaxation, biofeedback, cognitive behavioral therapy, stress management) [54].
Cognitive behavioral therapy and mindfulness are techniques that have been found to be effective in decreasing intensity of pain and associated disability. The goal of these techniques is to manage the cognitive, affective, and behavioral precipitants of headache. In this process, patients are helped to identify the thoughts and behavior that play a role in generating headache. These techniques have been found to improve many headache-related outcomes like pain intensity, headache-related disability, measures of quality of life, mood and medication consumption [55]. A multidisciplinary intervention that included group exercise, stress management and relaxation lectures, and massage therapy was found to reduce self-perceived pain intensity, frequency, and duration of the headache, and improve functional status and quality of life in migraineurs [56]. A randomized controlled trial of yoga therapy compared with self care showed that yoga led to significant reduction in migraine headache frequency and improved overall outcome [57].
Overall, results from studies of nonpharmacologic techniques have been mixed [58,59]. A systematic review by Sullivan et al found a large range in the efficacy of psychological interventions for migraine [60]. A 2015 systematic review that examined if cognitive behavioral therapy (CBT) can reduce the physical symptoms of chronic headache and migraines obtained mixed results [58]. Holryod et al’s study [61] found that behavioral management combined with a ß blocker is useful in improving outcomes, but neither the ß blocker alone or behavioral migraine management alone was. Also, a trial by Penzien et al showed that nonpharmacological management helped reduce migraines by 40% to 50% and this was similar to results seen with preventive drugs [62].
Patient education may be helpful in improving outcomes. Smith et al reported a 50% reduction in headache frequency at 12 months in 46% of patients who received migraine education [63]. A randomized controlled trial by Rothrock et al involving 100 migraine patients found that patients who attended a “headache school” consisting of three 90-minute educational sessions focused on topics such as acute treatment and prevention of migraine had a significant reduction in mean migraine disability assessment score (MIDAS) than the group randomized to routine medical management only. The patients also experienced a reduction in functionally incapacitating headache days per month, less need for abortive therapy and were more compliant with prophylactic therapy [64].
Case Conclusion
Our patient is a young woman with a history of headaches suggestive of migraine without aura. Since her headache frequency ranges from 4-8 headaches month, she has episodic migraines. She also has a strong family history of headaches. She denies any other medical or psychiatric comorbidity. She reports an intake of a caffeine-containing medication of 4 to 15 tablets per month.
The physician recommended that she limit her intake of the caffeine-containing medication to 5 days or less per month given the risk of migraine transformation. The physician also recommended maintaining a good sleep schedule, limiting excessive caffeine intake, a stress reduction program, regular cardiovascular exercise, and avoiding skipping or delaying meals. The patient was educated about migraine and its underlying mechanisms and the benefits of taking medications, and her fears regarding medication use and side effects were allayed. Sumatriptan 100 mg oral tablets were prescribed to be taken at headache onset. She was hesitant to be started on an antihypertensive or antiseizure medication, so she was prescribed amitriptyline 30 mg at night for headache prevention. She was also asked to maintain a headache diary. The patient was agreeable with this plan.
Summary
Migraine is often underdiagnosed and undertreated. Primary care providers are often the first point of contact for these patients. Identifying the type and frequency of migraine and comorbidities is necessary to guide appropriate management in terms of medications and lifestyle modifications. Often no testing or imaging is required. Educating patients about this chronic disease, treatment expectations, and limiting intake of medication is essential.
Corresponding author: Pooja Mohan Rao, MBBS, MD, Georgetown University Hospital, 3800 Reservoir Rd. NW, 7 PHC, Washington, DC 20007, [email protected].
Financial disclosures: Dr. Ailani reports receiving honoraria for speaking and consulting for Allergan, Avanir, and Eli Lilly.
From the Department of Neurology, Medstar Georgetown University Hospital, Washington, DC.
Abstract
- Objective: To review the epidemiology, pathophysiology, diagnosis, and treatment of migraine.
- Methods: Review of the literature.
- Results: Migraine is a common disorder associated with significant morbidity. Diagnosis of migraine is performed according to the International Classification of Headache Disorders. Comorbidities are commonly seen with migraine and include mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease. Comorbid conditions can increase migraine disability. Management of migraine with lifestyle modifications, trigger management, and acute and preventive medications can help reduce the frequency, duration, and severity of attacks. Overuse of medications such as opiates, barbiturates, and caffeine-containing medications can increase headache frequency. Educating patients about limiting use of these medications is important.
- Conclusion: Migraine is a common neurologic disease that can be very disabling. Recognizing the condition, making an accurate diagnosis, and starting patients on migraine-specific treatments can help improve patient outcomes.
Migraine is a common neurologic disease that affects 1 in 10 people worldwide [1]. It is 2 to 3 times more prevalent in women than in men [2]. The prevalence of migraine peaks in both sexes during the most productive years of adulthood (age 25 to 55 years) [3]. The Global Burden of Diseases, Injuries, and Risk Factors Study considers it to be the 7th most disabling disease in the world [4]. Over 36 million people in the United States have migraine [5]. However, just 56% of migraineurs have ever been diagnosed [6].
Migraine is associated with a high rate of years lived with disability [7] and the rate has been steadily increasing since 1990. At least 50% of migraine sufferers are severely disabled, many requiring bed rest, during individual migraine attacks lasting hours to days [8]. The total U.S. annual economic costs from headache disorders, including the indirect costs from lost productivity and workplace performance, has been estimated at $31 billion [9,10].
Despite the profound impact of migraine on patients and society, there are numerous barriers to migraine care. Lipton et al [11] identified 3 steps that were minimally necessary to achieve guideline-defined appropriate acute pharmacologic therapy: (1) consulting a prescribing health care professional; (2) receiving a migraine diagnosis; and (3) using migraine-specific or other appropriate acute treatments. In a study they conducted in patients with episodic migraine, 45.5% had consulted health care professional for headache in the preceding year; of these, 86.7% reported receiving a medical diagnosis of migraine, and among the diagnosed consulters, 66.7% currently used acute migraine-specific treatments, resulting in only 26.3% individuals successfully completing all 3 steps. In the recent CaMEO study [12], the proportion patients with chronic migraine that overcame all 3 barriers was less than 5%.
The stigma of migraine often makes it difficult for people to discuss symptoms with their health care providers and family members [13]. When they do discuss their headaches with their provider, often they are not given a diagnosis [14] or do not understand what their diagnosis means [15]. It is important for health care providers to be vigilant about the diagnosis of migraine, discuss treatment goals and strategies, and prescribe appropriate migraine treatment. Migraine is often comorbid with a number of medical, neurological, and psychiatric conditions, and identifying and managing comorbidities is necessary to reduce headache burden and disability. In this article, we provide a review of the diagnosis and treatment of migraine, using a case illustration to highlight key points.
Case Study
Initial Presentation
A 24-year-old woman presents for an evaluation of her headaches.
History and Physical Examination
She initially noted headaches at age 19, which were not memorable and did not cause disability. Her current headaches are a severe throbbing pain over her right forehead. They are associated with light and sound sensitivity and stomach upset. Headaches last 6 to 7 hours without medications and occur 4 to 8 days per month.
She denies vomiting and autonomic symptoms such as runny nose or eye tearing. She also denies preceding aura. She reports headache relief with intake of tablets that contain acetaminophen/aspirin/caffeine and states that she takes between 4 to 15 tablets/month depending on headache frequency. She reports having tried acetaminophen and naproxen with no significant benefit. Aggravating factors include bright lights, strong smells, and soy/ high-sodium foods.
She had no significant past medical problems and denied a history of depression or anxiety. Family history was significant for both her father and sister having a history of headaches. The patient lived alone and denied any major life stressors. She exercises 2 times a week and denies smoking or alcohol use. Review of systems was positive for trouble sleeping, which she described as difficulty falling asleep.
On physical examination, vitals were within normal limits. BMI was 23. Chest, cardiac, abdomen, and general physical examination were all within normal limits. Neurological examination revealed no evidence of papilledema or focal neurological deficits.
What is the pathophysiology of migraine?
Migraine was thought to be a primary vascular disorder of the brain, with the origins of the vascular theory of migraine dating back to 1684 [16]. Trials performed by Wolff concluded that migraine is of vascular origin [17], and this remained the predominant theory over several decades. Current evidence suggests that migraine is unlikely to be a pure vascular disorder and instead may be related to changes in the central or peripheral nervous system [18,19].
Migraine is complex brain network disorder with a strong genetic basis [19]. The trigemino-vascular system, along with neurogenically induced inflammation of the dura mater, mast cell degranulation and release of histamine, are the likely causes of migraine pain. Trigeminal fibers arise from neurons in the trigeminal ganglion that contain substance P and calcitonin gene-related peptide (CGRP) [20]. CGRP is a neuropeptide widely expressed in both peripheral and central neurons. Elevation of CGRP in migraine is linked to diminution of the inhibitory pathways which in turn leads to migraine susceptibility [21]. These findings have led to the development of new drugs that target the CGRP pathway.
In the brainstem, periaqueductal grey matter and the dorsolateral pons have been found to be “migraine generators,” or the driver of changes of cortical activity during migraine [22]. Brainstem nuclei are involved in modulating trigemino-vascular pain transmission and autonomic responses in migraine [23].
The hypothalamus has also been implicated in migraine pathogenesis, particularly its role in nociceptive and autonomic modulation in migraine patients. Schulte and May hypothesized that there is a network change between the hypothalamus and the areas of the brainstem generator leading to the migraine attacks [24].
The thalamus plays a central role for the processing and integration of pain stimuli from the dura mater and cutaneous regions. It maintains complex connections with the somatosensory, motor, visual, auditory, olfactory and limbic regions [25]. The structural and functional alterations in the system play a role in the development of migraine attacks, and also in the sensory hypersensitivity to visual stimuli and mechanical allodynia [26].
Experimental studies in rats show that cortical spreading depression can trigger neurogenic meningeal inflammation and subsequently activate the trigemino-vascular system [27]. It has been observed that between migraine episodes a time-dependent amplitude increase of scalp-evoked potentials to repeated stereotyped stimuli, such as visual, auditory, and somaticstimuli, occurs. This phenomenon is described as “deficient habituation.” In episodic migraine, studies show 2 characteristic changes: a deficient habituation between attacks and sensitization during the attack [28]. Genetic studies have hypothesized an involvement of glutamatergic neurotransmitters and synaptic dysplasticity in causing abnormal cortical excitability in migraine [27].
What are diagnostic criteria for migraine?
Diagnosis of migraine is performed according to the International Classification of Headache Disorders (ICHD) [29]. Based on the number of headache days that the patient reports, migraine is classified into episodic or chronic migraine. Migraines that occur on fewer than 15 days/month are categorized as episodic migraines.
Episodic migraine is divided into 2 categories: migraine with aura (Table 1) and migraine without aura. Migraine without aura is described as recurrent headaches consisting of at least 5 attacks, each lasting 4 to 72 hours if left untreated. At least 2 of the following 4 characteristics must be present: unilateral location, pulsating quality, moderate or severe pain intensity, with aggravation by or causing avoidance of routine physical activity. During headache, at least 1 of nausea and/or vomiting or photophobia and phonophobia should be present.
In migraine with aura (Table 2), headache characteristics are the same, but in addition there are at least 2 lifetime attacks with fully reversible aura symptoms (visual, sensory, speech/language). In addition, these auras have at least 2 of the following 4 characteristics: at least 1 aura symptom spreads gradually over 5 minutes, and/or 2 or more symptoms occur in succession; each individual aura symptom lasts 5 to 60 minutes; aura symptom is unilateral; and aura is accompanied, or followed within 60 minutes, by headache. Migraine with aura is uncommon, occurring in 20% of patients with migraine [30]. Visual aura is the most common type of aura, occurring in up to 90% of patients [31]. There is also aura without migraine, called typical aura without headache. Patients can present with non-migraine headache with aura, categorized as typical aura with headache [29].
Headache occurring on 15 or more days per month for more than 3 months, which has the features of migraine headache on at least 8 days per month, is classified as chronic migraine (Table 3). Evidence indicates that 2.5% of episodic migraine progresses to chronic migraine over 1-year follow-up [32]. There are several risk factors for chronification of migraine. Nonmodifiable factors include female sex, white European heritage, head/neck injury, low education/socioeconomic status, and stressful life events (divorce, moving, work changes, problems with children). Modifiable risk factors are headache frequency, acute medication overuse, caffeine overuse, obesity, comorbid mood disorders, and allodynia. Acute medication use and headache frequency are independent risk factors for development of chronic migraine [33]. The risk of chronic migraine increases exponentially with increased attack frequency, usually when the frequency is ≥ 3 headaches/month. Repetitive episodes of pain may increase central sensitization and result in anatomical changes in the brain and brainstem [34].
What information should be elicited during the history?
Specific questions about the headaches can help with making an accurate diagnosis. These include:
- Length of attacks and their frequency
- Pain characteristics (location, quality, intensity)
- Actions that trigger or aggravate headaches (eg, stress, movement, bright lights, menses, certain foods and smells)
- Associated symptoms that accompany headaches (eg, nausea, vomiting)
- How the headaches impact their life (eg, missed days at work or school, missed life events, avoidance of social activities, emergency room visits due to headache)
To assess headache frequency, it is helpful to ask about the number of headache-free days in a month, eg, “how many days a month do you NOT have a headache.” To assist with headache assessment, patients can be asked to keep a calendar in which they mark days of use of medications, including over the counter medications, menses, and headache days. The calendar can be used to assess for migraine patterns, headache frequency, and response to treatment.
When asking about headache history, it is important for patients to describe their untreated headaches. Patients taking medications may have pain that is less severe or disabling or have reduced associated symptoms. Understanding what the headaches were like when they did not treat is important in making a diagnosis.
Other important questions include when was the first time they recall ever experiencing a headache. Migraine is often present early in life, and understanding the change in headache over time is important. Also ask patients about what they want to do when they have a headache. Often patients want to lie down in a cool dark room. Ask what they would prefer to do if they didn’t have any pending responsibilities.
Comorbidities
Comorbidities are commonly seen with migraine. Common comorbidities are mood disorders (depression, anxiety, post-traumatic stress disorder), musculoskeletal disorders (neck pain, fibromyalgia, Ehlors-Danlos syndrome), sleep disorders, asthma, allergies, thyroid dysfunction, obesity, irritable bowel syndrome, epilepsy, stroke, and heart disease.
Comorbid conditions can increase migraine disability and also can provide information about the pathophysiology of migraine and guide treatment. Management of the underlying comorbidity often leads to improved migraine outcomes. For example, serotonergic dysfunction is a possible pathway involved in both migraine and mood disorders. Treatment with medications that alter the serotonin system may help both migraine and coexisting mood disorders. Bigal et al proposed that activation of the HPA axis with reduced serotonin synthesis is a main pathway involved in affective disorders, migraine, and obesity [35].
In the early 1950s, Wolff conceptualized migraine as a psychophysiologic disorder [36]. The relationship between migraine and psychiatric conditions is complex, and comorbid psychiatric disorders are risk factors for headache progression and chronicity. Psychiatric conditions also play a role in nonadherence to headache medication, which contributes to poor outcome in these patients. Hence, there is a need for assessment and treatment of psychiatric disorders in people with migraine. A study by Guidetti et al found that headache patients with multiple psychiatric conditions have poor outcomes, with 86 % of these headache patients having no improvement and even deterioration in their headache [37]. Another study by Mongini et al concluded that psychiatric disorder appears to influence the result of treatment on a long-term basis [38].
In addition, migraine has been shown to impact mood disorders. Worsening headache was found to be associated with poorer prognosis for depression. Patients with active migraine not on medications with comorbid major depressive disorder (MDD) had more severe anxiety and somatic symptoms as compared with MDD patients without migraine [39].
Case Continued
Our patient has a normal neurologic examination and classic migraine headache history and stable frequency. The physician tells her she meets criteria for episodic migraine without aura. The patient asks if she needs a “brain scan” to see if something more serious may be causing her symptoms.
What workup is recommended for patients with migraine?
If patient symptoms fit the criteria for migraine and there is a normal neurologic examination, the differential is often limited. When there are neurologic abnormalities on examination (eg, papilledema), or if the patient has concerning signs or symptoms (see below), then neuroimaging should be obtained to rule out secondary causes of headache.
In 2014, the American Academy of Neurology (AAN) published practice parameters on the evaluation of adults with recurrent headache based on guidelines published by the US Headache Consortium [40]. As per AAN guidelines, routine laboratory studies, lumbar puncture, and electroencephalogram are not recommended in the evaluation of non-acute migraines. Neuroimaging is not warranted in patients with migraine and a normal neurologic examination (grade B recommendation). Imaging may need to be considered in patients with non-acute headache and an unexplained abnormal finding on the neurologic examination (grade B recommendation).
When patients exhibit particular warning signs, or headache “red flags,” it is recommended that neuroimaging be considered. Red flags include patients with recurrent headaches and systemic symptoms (fever, weight loss), neurologic symptoms or abnormal signs (confusion, impaired alertness or consciousness), sudden onset, abrupt, or split second in nature, patients age > 50 with new onset or progressive headache, previous headache history with new or different headache (change in frequency, severity, or clinical features) and if there are secondary risk factors (HIV, cancer) [41].
Case Continued
Our patient has no red flags and can be reassured that given her normal physical examination and history suggestive of a migraine, a secondary cause of her headache is unlikely. The physician describes the treatments available, including implementing lifestyles changes and preventive and abortive medications. The patient expresses apprehension about being on prescription medications. She is concerned about side effects as well as the need to take daily medication over a long period of time. She reports that these were the main reasons she did not take the rizatriptan and propranolol that was prescribed by her previous doctor.
How is migraine treated?
Migraine is managed with a combination of lifestyle changes and pharmacologic therapy. Pharmacologic management targets treating an attack when it occurs (abortive medication), as well as reducing the frequency and severity of future attacks (preventive medication).
Lifestyle Changes
Patients should be advised that making healthy lifestyle choices, eg, regular sleep, balanced meals, proper hydration, and regular exercise, can mitigate migraine [42–44]. Other lifestyle changes that can be helpful include weight loss in the obese population, as weight loss appears to result in migraine improvement. People who are obese also are at higher risk for the progression to chronic migraine.
Acute Therapy
There are varieties of abortive therapies [45] (Table 4) that are commonly used in clinical practice. Abortive therapy can be taken as needed and is most effective if used within the first 2 hours of headache. For patients with daily or frequent headache, these medications need to be restricted to 8 to 12 days a month of use and their use should be restricted to when headache is worsening. This usually works well in patients with moderate level pain, and especially in patients with no associated nausea. Selective migraine treatments, like triptans and ergots, are used when nonspecific treatments fail, or when headache is more severe. It is preferable that patients avoid opioids, butalbital, and caffeine-containing medications. In the real world, it is difficult to convince patient to stop these medications; it is more realistic to discuss use limitation with patients, who often run out their weekly limit for triptans.
Triptans are effective medications for acute management of migraine but headache recurrence rate is high, occurring in 15% to 40 % of patients taking oral triptans. It is difficult to predict the response to a triptan [46]. The choice of an abortive agent is often directed partially by patient preference (side effect profile, cost, non-sedating vs. prefers to sleep, long vs short half-life), comorbid conditions (avoid triptans and ergots in uncontrolled hypertension, cardiovascular disease, or peripheral vascular disease or stroke/aneurysm; avoid NSAIDS in patients with cardiovascular disease), and migraine-associated symptoms (nausea and/or vomiting). Consider non-oral formulations via subcutaneous or nasal routes in patients who have nausea or vomiting with their migraine attacks. Some patients may require more than one type of abortive medication. The high recurrence rate is similar across different triptans and so switching from one triptan to another has not been found to be useful. Adding NSAIDS to triptans has been found to be more useful than switching between triptans.Overuse of acute medications has been associated with transformation of headache from episodic to chronic (medication overuse headache or rebound headache). The risk of transformation appears to be greatest with medications containing caffeine, opiates, or barbiturates [47]. Use of acute medications should be limited based on the type of medication. Patients should take triptans for no more than 10 days a month. Combined medications and opioids should be used fewer than 8 days a month, and butalbital-containing medications should be avoided or used fewer than 5 days a month [48]. Use of acute therapy should be monitored with headache calendars. It is unclear if and to what degree NSAIDS and acetaminophen cause overuse headaches.
Medication overuse headache can be difficult to treat as patients have to stop using the medication causing rebound. Further, headaches often resemble migraine and it can be difficult to differentiate them from the patients’ routine headache. Vigilance with medication use in patients with frequent headache is an essential part of migraine management, and patients should receive clear instructions regarding how to use acute medications.
Prevention
Patients presenting with more than 4 headaches per month, or headaches that last longer than 12 hours, require preventive therapy. The goals of preventive therapy is to reduce attack frequency, severity, and duration, to improve responsiveness to treatment of acute attacks, to improve function and reduce disability, and to prevent progression or transformation of episodic migraine to chronic migraine. Preventive medications usually need to be taken daily to reduce frequency or severity of the headache. The goal in this approach is 50% reduction of headache frequency and severity. Migraine preventive medications usually belong to 1 of 3 categories of drugs: antihypertensives, antiepileptics, and antidepressants. At present there are many medications for migraine prevention with different levels of evidence [49] (Table 5). Onabotulinuma toxin is the only approved medication for chronic migraine based on promising results of the PREEMPT trial [50].
Other Considerations
A multidisciplinary approach to treatment may be warranted. Psychiatric evaluation and management of underlying depression and mood disorders can help reduce headache frequency and severity. Physical therapy should be prescribed for neck and shoulder pain. Sleep specialists should be consulted if ongoing sleep issues continue despite behavioral management.
How common is nonadherence with migraine medication?
One third of patients who are prescribed triptans discontinue the medication within a year. Lack of efficacy and concerns over medication side effects are 2 of the most common reasons for poor adherence [51]. In addition, age plays a significant role in discontinuing medication, with the elderly population more likely to stop taking triptans [52]. Seng et al reported that among patients with migraine, being male, being single, having frequent headache, and having mild pain are all associated with medication nonadherence [53]. Formulary restrictions and type of insurance coverage also were associated with nonadherence. Among adherent patients, some individuals were found to be hoarding their tablets and waiting until they were sure it was a migraine. Delaying administration of abortive medications increases the chance of incomplete treatment response, leading to patients taking more medication and in turn have more side effects [53].
Educating patients about their medications and how they need to be taken (preventive vs. abortive, when to administer) can help with adherence (Table 6). Monitoring medication use and headache frequency is an essential part of continued care for migraine patients. Maintain follow up with patients to review how they are doing with the medication and avoid providing refills without visits. The patient may not be taking medication consistently or may be using more medication than prescribed.
What is the role of nonpharmacologic therapy?
Most patients respond to pharmacologic treatment, but some patients with mood disorder, anxiety, difficulties or disability associated with headache, and patients with difficulty managing stress or other triggers may benefit from the addition of behavioral treatments (eg, relaxation, biofeedback, cognitive behavioral therapy, stress management) [54].
Cognitive behavioral therapy and mindfulness are techniques that have been found to be effective in decreasing intensity of pain and associated disability. The goal of these techniques is to manage the cognitive, affective, and behavioral precipitants of headache. In this process, patients are helped to identify the thoughts and behavior that play a role in generating headache. These techniques have been found to improve many headache-related outcomes like pain intensity, headache-related disability, measures of quality of life, mood and medication consumption [55]. A multidisciplinary intervention that included group exercise, stress management and relaxation lectures, and massage therapy was found to reduce self-perceived pain intensity, frequency, and duration of the headache, and improve functional status and quality of life in migraineurs [56]. A randomized controlled trial of yoga therapy compared with self care showed that yoga led to significant reduction in migraine headache frequency and improved overall outcome [57].
Overall, results from studies of nonpharmacologic techniques have been mixed [58,59]. A systematic review by Sullivan et al found a large range in the efficacy of psychological interventions for migraine [60]. A 2015 systematic review that examined if cognitive behavioral therapy (CBT) can reduce the physical symptoms of chronic headache and migraines obtained mixed results [58]. Holryod et al’s study [61] found that behavioral management combined with a ß blocker is useful in improving outcomes, but neither the ß blocker alone or behavioral migraine management alone was. Also, a trial by Penzien et al showed that nonpharmacological management helped reduce migraines by 40% to 50% and this was similar to results seen with preventive drugs [62].
Patient education may be helpful in improving outcomes. Smith et al reported a 50% reduction in headache frequency at 12 months in 46% of patients who received migraine education [63]. A randomized controlled trial by Rothrock et al involving 100 migraine patients found that patients who attended a “headache school” consisting of three 90-minute educational sessions focused on topics such as acute treatment and prevention of migraine had a significant reduction in mean migraine disability assessment score (MIDAS) than the group randomized to routine medical management only. The patients also experienced a reduction in functionally incapacitating headache days per month, less need for abortive therapy and were more compliant with prophylactic therapy [64].
Case Conclusion
Our patient is a young woman with a history of headaches suggestive of migraine without aura. Since her headache frequency ranges from 4-8 headaches month, she has episodic migraines. She also has a strong family history of headaches. She denies any other medical or psychiatric comorbidity. She reports an intake of a caffeine-containing medication of 4 to 15 tablets per month.
The physician recommended that she limit her intake of the caffeine-containing medication to 5 days or less per month given the risk of migraine transformation. The physician also recommended maintaining a good sleep schedule, limiting excessive caffeine intake, a stress reduction program, regular cardiovascular exercise, and avoiding skipping or delaying meals. The patient was educated about migraine and its underlying mechanisms and the benefits of taking medications, and her fears regarding medication use and side effects were allayed. Sumatriptan 100 mg oral tablets were prescribed to be taken at headache onset. She was hesitant to be started on an antihypertensive or antiseizure medication, so she was prescribed amitriptyline 30 mg at night for headache prevention. She was also asked to maintain a headache diary. The patient was agreeable with this plan.
Summary
Migraine is often underdiagnosed and undertreated. Primary care providers are often the first point of contact for these patients. Identifying the type and frequency of migraine and comorbidities is necessary to guide appropriate management in terms of medications and lifestyle modifications. Often no testing or imaging is required. Educating patients about this chronic disease, treatment expectations, and limiting intake of medication is essential.
Corresponding author: Pooja Mohan Rao, MBBS, MD, Georgetown University Hospital, 3800 Reservoir Rd. NW, 7 PHC, Washington, DC 20007, [email protected].
Financial disclosures: Dr. Ailani reports receiving honoraria for speaking and consulting for Allergan, Avanir, and Eli Lilly.
1. Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants, J Neurol Sci 2017;372:307–15.
2. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87.
3. Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45 Suppl 1:S3–S13.
4. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602.
5. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention Headache 2015;55 Suppl 2:103–22.
6. Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47:355–63.
7. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study. Lancet 2012;380:2163–96.
8. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–57.
9. Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain in the US workforce. JAMA 2003;290:2443–54.
10. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache 2008;48:553–63.
11. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013;53:81–92.
12. Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: Results from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache 2016;56:821–34.
13. Young WB, Park JE, Tian IX, Kempner J. The stigma of migraine. PLoS One 2013;8(1):e54074.
14. Mia M, Ashna S, Audrey H. A migraine management training program for primary care providers: an overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache 2016;56:725–40.
15. Lipton RB, Amatniek JC, Ferrari MD, Gross M. Migraine: identifying and removing barriers to care. Neurology 1994;44(6 Suppl 4):S63–8.
16. Knapp RD Jr. Reports from the past 2. Headache 1963;3:112–22.
17. Levine M, Wolff HG. Cerebral circulation: afferent impulses from the blood vessels of the pia. Arch Neurol Psychiat 1932;28:140.
18. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross sectional study. Lancet Neurol 2013;12:454–61.
19. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 2017;97:553–622.
20. Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol 2012;15(Suppl 1):S15–S22.
21. Puledda F, Messina R, Goadsby PJ, et al. An update on migraine: current understanding and future J Neurol 2017 Mar 20.
22. Vinogradova LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 2015;35:979–86.
23. Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–7.
24. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93.
25. Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011;31:14204–17.
26. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–45.
27. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol 2017 Mar 20.
28. Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain 2013;14:65.
29. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
30. Yusheng H, Y Li. Typical aura without headache: a case report and review of the literature J Med Case Rep 2015;9:40.
31. Buture A, Khalil M, Ahmed F. Iatrogenic visual aura: a case report and a brief review of the literature Ther Clin Risk Manag 2017;13:643–6.
32. Lipton RB. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009;72:S3–7.
33. Lipton RB. Headache 2011;51;S2:77–83.
34. Scher AI, Stewart WF, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003;106:81–9.
35. Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology 2007;68:1851–61.
36. Wolff HG. Stress and disease. Springfield, IL: Charles C. Thomas; 1953.
37. Guidetti V, Galli F, Fabrizi P, et al. Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998;18:455–62.
38. Mongini F, Keller R, Deregibus A, et al. Personality traits, depression and migraine in women: a longitudinal study. Cephalalgia 2003;23:186–92.
39. Hung CI, Liu CY, Yang CH, Wang SJ. The impacts of migraine among outpatients with major depressive disorder at a two-year follow-up. PLoS One 2015;10:e0128087.
40. Frishberg BM, Rosenberg JH, Matchar DB, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. St Paul: US Headache Consortium; 2000.
41. Headache Measurement Set 2014 Revised. American Academy of Neurology. Accessed at www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/2.Quality_Improvement/1.Quality_Measures/1.All_Measures/2014.
42. Taylor FR. Lifestyle changes, dietary restrictions, and nutraceuticals in migraine prevention. Techn Reg Anesth Pain Manage 2009;13:28–37.
43. Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia 2011;14:1428–38.
44. Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep 2013;17:379.
45. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015;55:3–20.
46. Belvis R, Mas N, Aceituno A. Migraine attack treatment : a tailor-made suit, not one size fits all. Recent Pat CNS Drug Discov 2014;9:26–40.
47. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008;48:1157–68.
48. Bigal ME, Rapoport AM, Sheftell FD, et al. Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes Cephalalgia 2004;24:483–90.
49. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–45.
50. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial Cephalagia year;30:804–14.
51. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache 2014;54:278–89.
52. Holland S, Fanning KM, Serrano D, et al. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci 2013;326:10–7.
53. Seng EK, Rains JA, Nicholson RA, Lipton RB. Improving medication adherence in migraine treatment. Curr Pain Headache Rep 2015;19:24.
54. Nicholson RA, Buse DC, Andrasik F, Lipton RB. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treatment Opt Neurol 2011;13:28–40.
55. Probyn K, Bowers H, Mistry D, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components BMJ Open 2017;7:e016670.
56. Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002;42:845–54.
57. John PJ, Sharma N, Sharma CM, Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache 2007;47:654–61.
58. Harris P, Loveman E, Clegg A, et al. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults Br J Pain 2015;9:213–24.
59. Kropp P, Meyer B, Meyer W, Dresler T. An update on behavioral treatments in migraine - current knowledge and future options. Expert Rev Neurother 2017:1–10.
60. Sullivan A, Cousins S, Ridsdale L. Psychological interventions for migraine: a systematic review. J Neurol 2016;263:2369–77.
61. Holroyd KA, Cottrell CK, O’Donnell FJ, et al. Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ 2010;341:c4871.
62. Penzien DB, Rains JC, Andrasik F. Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback 2002;27:163–81.
63. Smith TR, Nicholson RA, Banks JW. A primary care migraine education program has benefit on headache impact and quality of life: results from the mercy migraine management program. Headache 2010;50:600–12.
64. Rothrock JF, Parada VA, Sims C, et al.The impact of intensive patient education on clinical outcome in a clinic-based migraine population. Headache 2006;46:726–31.
1. Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants, J Neurol Sci 2017;372:307–15.
2. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol 2017;16:76–87.
3. Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45 Suppl 1:S3–S13.
4. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602.
5. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention Headache 2015;55 Suppl 2:103–22.
6. Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47:355–63.
7. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study. Lancet 2012;380:2163–96.
8. Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41:646–57.
9. Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain in the US workforce. JAMA 2003;290:2443–54.
10. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache 2008;48:553–63.
11. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013;53:81–92.
12. Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: Results from the chronic migraine epidemiology and outcomes (CaMEO) study. Headache 2016;56:821–34.
13. Young WB, Park JE, Tian IX, Kempner J. The stigma of migraine. PLoS One 2013;8(1):e54074.
14. Mia M, Ashna S, Audrey H. A migraine management training program for primary care providers: an overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache 2016;56:725–40.
15. Lipton RB, Amatniek JC, Ferrari MD, Gross M. Migraine: identifying and removing barriers to care. Neurology 1994;44(6 Suppl 4):S63–8.
16. Knapp RD Jr. Reports from the past 2. Headache 1963;3:112–22.
17. Levine M, Wolff HG. Cerebral circulation: afferent impulses from the blood vessels of the pia. Arch Neurol Psychiat 1932;28:140.
18. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross sectional study. Lancet Neurol 2013;12:454–61.
19. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine—a disorder of sensory processing. Physiol Rev 2017;97:553–622.
20. Goadsby PJ. Pathophysiology of migraine. Ann Indian Acad Neurol 2012;15(Suppl 1):S15–S22.
21. Puledda F, Messina R, Goadsby PJ, et al. An update on migraine: current understanding and future J Neurol 2017 Mar 20.
22. Vinogradova LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: implications for the pathophysiology of migraine aura. Cephalalgia 2015;35:979–86.
23. Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet 2001;357:1016–7.
24. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016;139:1987–93.
25. Noseda R, Jakubowski M, Kainz V, et al. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011;31:14204–17.
26. Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci 2010;13:239–45.
27. Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol 2017 Mar 20.
28. Coppola G, Di Lorenzo C, Schoenen J, Pierelli F. Habituation and sensitization in primary headaches. J Headache Pain 2013;14:65.
29. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
30. Yusheng H, Y Li. Typical aura without headache: a case report and review of the literature J Med Case Rep 2015;9:40.
31. Buture A, Khalil M, Ahmed F. Iatrogenic visual aura: a case report and a brief review of the literature Ther Clin Risk Manag 2017;13:643–6.
32. Lipton RB. Tracing transformation: Chronic migraine classification, progression, and epidemiology. Neurology 2009;72:S3–7.
33. Lipton RB. Headache 2011;51;S2:77–83.
34. Scher AI, Stewart WF, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003;106:81–9.
35. Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology 2007;68:1851–61.
36. Wolff HG. Stress and disease. Springfield, IL: Charles C. Thomas; 1953.
37. Guidetti V, Galli F, Fabrizi P, et al. Headache and psychiatric comorbidity: clinical aspects and outcome in an 8-year follow-up study. Cephalalgia 1998;18:455–62.
38. Mongini F, Keller R, Deregibus A, et al. Personality traits, depression and migraine in women: a longitudinal study. Cephalalgia 2003;23:186–92.
39. Hung CI, Liu CY, Yang CH, Wang SJ. The impacts of migraine among outpatients with major depressive disorder at a two-year follow-up. PLoS One 2015;10:e0128087.
40. Frishberg BM, Rosenberg JH, Matchar DB, et al. Evidence-based guidelines in the primary care setting: neuroimaging in patients with nonacute headache. St Paul: US Headache Consortium; 2000.
41. Headache Measurement Set 2014 Revised. American Academy of Neurology. Accessed at www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3.Practice_Management/2.Quality_Improvement/1.Quality_Measures/1.All_Measures/2014.
42. Taylor FR. Lifestyle changes, dietary restrictions, and nutraceuticals in migraine prevention. Techn Reg Anesth Pain Manage 2009;13:28–37.
43. Varkey E, Cider A, Carlsson J, Linde M. Exercise as migraine prophylaxis: A randomized study using relaxation and topiramate as controls. Cephalalgia 2011;14:1428–38.
44. Ahn AH. Why does increased exercise decrease migraine? Curr Pain Headache Rep 2013;17:379.
45. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015;55:3–20.
46. Belvis R, Mas N, Aceituno A. Migraine attack treatment : a tailor-made suit, not one size fits all. Recent Pat CNS Drug Discov 2014;9:26–40.
47. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: A longitudinal population-based study. Headache 2008;48:1157–68.
48. Bigal ME, Rapoport AM, Sheftell FD, et al. Transformed migraine and medication overuse in a tertiary headache centre--clinical characteristics and treatment outcomes Cephalalgia 2004;24:483–90.
49. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–45.
50. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial Cephalagia year;30:804–14.
51. Wells RE, Markowitz SY, Baron EP, et al. Identifying the factors underlying discontinuation of triptans. Headache 2014;54:278–89.
52. Holland S, Fanning KM, Serrano D, et al. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) study. J Neurol Sci 2013;326:10–7.
53. Seng EK, Rains JA, Nicholson RA, Lipton RB. Improving medication adherence in migraine treatment. Curr Pain Headache Rep 2015;19:24.
54. Nicholson RA, Buse DC, Andrasik F, Lipton RB. Nonpharmacologic treatments for migraine and tension-type headache: how to choose and when to use. Curr Treatment Opt Neurol 2011;13:28–40.
55. Probyn K, Bowers H, Mistry D, et al. Non-pharmacological self-management for people living with migraine or tension-type headache: a systematic review including analysis of intervention components BMJ Open 2017;7:e016670.
56. Lemstra M, Stewart B, Olszynski WP. Effectiveness of multidisciplinary intervention in the treatment of migraine: a randomized clinical trial. Headache 2002;42:845–54.
57. John PJ, Sharma N, Sharma CM, Kankane A. Effectiveness of yoga therapy in the treatment of migraine without aura: a randomized controlled trial. Headache 2007;47:654–61.
58. Harris P, Loveman E, Clegg A, et al. Systematic review of cognitive behavioural therapy for the management of headaches and migraines in adults Br J Pain 2015;9:213–24.
59. Kropp P, Meyer B, Meyer W, Dresler T. An update on behavioral treatments in migraine - current knowledge and future options. Expert Rev Neurother 2017:1–10.
60. Sullivan A, Cousins S, Ridsdale L. Psychological interventions for migraine: a systematic review. J Neurol 2016;263:2369–77.
61. Holroyd KA, Cottrell CK, O’Donnell FJ, et al. Effect of preventive (beta blocker) treatment, behavioural migraine management, or their combination on outcomes of optimised acute treatment in frequent migraine: randomised controlled trial. BMJ 2010;341:c4871.
62. Penzien DB, Rains JC, Andrasik F. Behavioral management of recurrent headache: three decades of experience and empiricism. Appl Psychophysiol Biofeedback 2002;27:163–81.
63. Smith TR, Nicholson RA, Banks JW. A primary care migraine education program has benefit on headache impact and quality of life: results from the mercy migraine management program. Headache 2010;50:600–12.
64. Rothrock JF, Parada VA, Sims C, et al.The impact of intensive patient education on clinical outcome in a clinic-based migraine population. Headache 2006;46:726–31.
Lithium-induced bradycardia: A rare but serious adverse effect
Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.
Mr. C, age 30, with schizoaffective disorder, bipolar type, Cannabis abuse, and nicotine dependence, has been enrolled in a Program of Assertive Community Treatment (PACT) for approximately 5 years. He presents to the PACT clinic for follow-up with his psychiatrist. Mr. C reports dizziness, lightheadedness, blurred vision, and nausea worsening over the last few days, and he appears drowsy and hypoactive. He does not report any chest pain, abdominal pain, swelling, cold extremities, shortness of breath, vomiting, diarrhea, or blood loss. Mr. C admits he has eaten only once daily for several weeks because of delusional ideation that he is responsible for others suffering from anorexia nervosa.
His medical history includes gastroesophageal reflux disease. Mr. C’s medication regimen for the past year included total daily oral doses of
Because of Mr. C’s complaints, appearance, and low HR, the psychiatrist calls emergency medical services (EMS). Although the paramedics recommend emergency transport to the hospital, Mr. C refuses. The psychiatrist instructs Mr. C to stop taking lithium because of suspected lithium-induced bradycardia and a concern that he may be more susceptible to lithium toxicity with prolonged anorexia nervosa. When nursing staff evaluate Mr. C the next day, his vitals are HR 60 bpm, respirations 20 breaths per minute, and blood pressure 124/81 mm Hg; his dizziness, blurred vision, lightheadedness, and nausea are resolved.
Bradycardia is defined as a HR <60 bpm; however, symptoms may not occur until the HR is <50 bpm. Symptoms include fatigue, dizziness, lightheadedness, chest pain, shortness of breath, and syncope. The incidence of bradycardia during lithium treatment is unknown; it is considered a rare but serious adverse effect. A literature review reveals several case reports of bradycardia with lithium treatment,2-4 including symptomatic bradycardia after a single dose of lithium.5 Other possible causes of bradycardia include anorexia nervosa, hypothermia, hypothyroidism, hypoxia, infection, stroke, acute myocardial infarction, sedative or opiate use, increased vagal tone with exercise conditioning, and other medications including fluphenazine.6
Mr. C’s symptoms may have been assumed to be secondary to several possible causes, including bradycardia, dehydration from poor oral intake, lithium toxicity, or an undiagnosed medical condition. The combination of nausea, dizziness, anorexia nervosa, blurred vision, and lightheadedness in a patient receiving lithium would certainly trigger a clinician’s concern for lithium toxicity, but he (she) may not be aware of the risk of bradycardia as an adverse effect of lithium. Because Mr. C refused hospital transportation by EMS, discontinuing lithium appears to have been the safest option. Laboratory studies from the day after Mr. C presented to the clinic appeared to lessen the probability that lithium toxicity, hypothyroidism,
Although psychiatrists may be vigilant about monitoring for signs and symptoms of toxicity with lithium use by utilizing regular laboratory studies, they may not be as vigilant with monitoring vital signs at every patient visit (Table). This case demonstrates the importance of regular vital sign measurements to be able to detect this rare but serious adverse effect.
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
1. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-245.
2. White B, Larry J, Kantharia BK. Protracted presyncope and profound bradycardia due to lithium toxicity. Int J Cardiol. 2008;125(3):e48-e50.
3. Palatnik A, Kates R. Bradycardia and medications: identify the dangerous pace. Nurs Manage. 2003;34(6):56A-56F.
4. La Rocca R, Foschi A, Preston NM, et al. QT interval prolongation and bradycardia in lithium-induced nephrogenic diabetes insipidus. Int J Cardiol. 2012;162(1):e1-e2.
5. Sabharwal MS, Annapureddy N, Agarwal SK, et al. Severe bradycardia caused by a single dose of lithium. Intern Med. 2013;52(7):767-769.
6. Homoud MK. Sinus bradycardia. UpToDate. www.uptodate.com/contents/sinus-bradycardia. Updated June 7, 2017. Accessed August 28, 2017.
‘Self-anesthetizing’ to cope with grief
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23
Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23
Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
CASE Grieving, delusional
Mr. M, age 51, is brought to the emergency department (ED) because of new-onset delusions and decreased self-care over the last 2 weeks following the sudden death of his wife. He has become expansive and grandiose, with pressured speech, increased energy, and markedly reduced sleep. Mr. M is preoccupied with the idea that he is “the first to survive a human reboot process” and says that his and his wife’s bodies and brains had been “split apart.” Mr. M has limited his food and fluid intake and lost 15 lb within the past 2 to 3 weeks.
Mr. M has no history of any affective, psychotic, or other major mental disorders or treatment. He reports that he has regularly used Cannabis over the last 10 years, and a few years ago, he started occasionally using nitrous oxide (N2O). He says that in the week following his wife’s death, he used N2O almost daily and in copious amounts. In an attempt to “self-anesthetize” himself after his wife’s funeral, he isolated himself in his bedroom and used escalating amounts of Cannabis and N2O, while continually working on a book about their life together.
At first, Mr. M shows little emotion and describes his situation as “interesting and fascinating.” He mentions that he thinks he might have been “psychotic” the week after his wife’s death, but he shows no sustained insight and immediately relapses into psychotic thinking. Over several hours in the ED, he is tearful and sad about his wife’s death. Mr. M recalls a similar experience of grief after his mother died when he was a teenager, but at that time he did not abuse substances or have psychotic symptoms. He is fully alert, fully oriented, and has no significant deficits of attention or memory.
[polldaddy:9859135]
The authors’ observations
Grief was a precipitating event, but by itself grief cannot explain psychosis. Psychotic depression is a possibility, but Mr. M’s psychotic features are incongruent with his mood. Mania would be a diagnosis of exclusion. Mr. M had no prior history of major affective illness. Mr. M was abusing Cannabis, which might independently contribute to psychosis1; however, he had been using it recreationally for 10 years without psychiatric problems. N2O, however, can cause symptoms consistent with Mr. M’s presentation.
[polldaddy:9859140]
EVALUATION Laboratory tests
Mr. M’s physical examination is notable only for an elevated blood pressure of 196/120 mm Hg. Neurologic examination is normal. Toxicology is positive for cannabinoids and negative for amphetamines, cocaine, opiates, and phencyclidine. Chemistries are normal except for a potassium of 3.4 mEq/L (reference range, 3.7 to 5.2 mEq/L) and a blood urine nitrogen of 25 mg/dL (reference range, 6 to 20 mg/dL), which are consistent with reduced food and fluid intake. Mr. M shows no signs of anemia. Hematocrit is 42% and mean corpuscular volume is 90 fL. Syphilis screen is negative; a head CT scan is unremarkable.
The authors’ observations
N2O, also known as “laughing gas,” is routinely used by dentists and pediatric anesthesiologists, and has other medical uses. Some studies have examined an adjunctive use of N2O for pain control in the ED and during colonoscopies.3,4
In the 2013 U.S. National Survey on Drug Use and Health, 16% of respondents reported lifetime illicit use of N2O.5,6 It is readily available in tanks used in medicine and industry and in small dispensers called “whippits” that can be legally purchased. Acute effects of N2O include euphoric mood, numbness, feeling of warmth, dizziness, and auditory hallucinations.7 The anesthetic effects of N2O are linked to endogenous release of opiates, and recent research links its anxiolytic activity to the facilitation of GABAergic inhibitory and N-methyl-
Beginning with a 1960 report of a series of patients with “megaloblastic madness,”17 there have been calls for increased awareness of the potential for vitamin B12 deficiency–induced psychiatric disorders, even in the absence of other hematologic or neurologic sequelae that would alert clinicians of the deficiency. In a case series of 141 patients with a broad array of neurologic and psychiatric symptoms associated with vitamin B12 deficiency, 40 (28%) patients had no anemia or macrocytosis.2
Vitamin B12-responsive psychosis has been reported as the sole manifestation of illness, without associated neurologic or hematologic symptoms, in only a few case reports. Vitamin B12 levels in these cases ranged from 75 to 236 pg/mL (reference range, 160 to 950 pg/mL).18-20 In all of these cases, the vitamin B12 deficiency was traced to dietary causes. The clinical evaluation of suspected vitamin B12 deficiency is outlined in the Figure.21 Mr. M had used Cannabis recreationally for a long time, and his Cannabis use acutely escalated with use of N2O. Long-term use of Cannabis alone is a risk factor for psychotic illness.22 Combined abuse of Cannabis and N2O has been reported to provoke psychotic illness. In a case report of a 22-year-old male who was treated for paranoid delusions, using Cannabis and 100 cartridges of N2O daily was associated with low vitamin B12 and elevated homocysteine and methylmalonic acid levels.23
Cannabis use may have played a role in Mr. M’s escalating N2O use. In a study comparing 9 active Cannabis users with 9 non-using controls, users rated the subjective effects of N2O as more intense than non-users.24 In our patient’s case, Cannabis may have played a role in both sustaining his escalating N2O abuse and potentiating its psychotomimetic effects.
It also is possible that Mr. M may have been “self-medicating” his grief with N2O. In a recent placebo-controlled crossover trial of 20 patients with treatment-resistant depression, Nagele et al25 found a significant rapid and week-long antidepressant effect of subanesthetic N2O use. A model involving NMDA receptor activation has been proposed.25,26 Zorumski et al26 further reviewed possible antidepressant mechanisms of N2O. They compared N2O with ketamine as an NMDA receptor antagonist, but also noted its distinct effects on glutaminergic and GABAergic neurotransmitter systems as well as other receptors and channels.26 However, illicit use of N2O poses toxicity dangers and has no current indication for psychiatric treatment.
TREATMENT Supplementation
Mr. M is diagnosed with substance-induced psychotic disorder. His symptoms were precipitated by an acute increase in N2O use, which has been shown to cause vitamin B12 deficiency, which we consider was likely a primary contributor to his presentation. Other potential contributing factors are premorbid hyperthymic temperament, a possible propensity to psychotic thinking under stress, the sudden death of his wife, acute grief, the potentiating role of Cannabis, dehydration, and general malnutrition. The death of a loved one is associated with an increased risk of developing substance use disorders.27
During a 15-day psychiatric hospitalization, Mr. M is given olanzapine, increased to 15 mg/d and oral vitamin B12, 1,000 mcg/d for 4 days, then IM cyanocobalamin for 7 days. Mr. M’s symptoms steadily improve, with normalization of sleep and near-total resolution of delusions. On hospital Day 14, his vitamin B12 levels are within normal limits (844 pg/mL). At discharge, Mr. M shows residual mild grandiosity, with limited insight into his illness and what caused it, but frank delusional ideation has clearly receded. He still shows some signs of grief. Mr. M is advised to stop using Cannabis and N2O and about the potential consequences of continued use.
The authors’ observations
For patients with vitamin B12 deficiency, guidelines from the National Health Service in the United Kingdom and the British Society for Haematology recommend treatment with IM hydroxocobalamin, 1,000 IU, 3 times weekly, for 2 weeks.21,28 For patients with neurologic symptoms, the British National Foundation recommends treatment with IM hydroxocobalamin, 1,000 IU, on alternative days until there is no further improvement.21
This case is a reminder for clinicians to screen for inhalant use, specifically N2O, which can precipitate vitamin B12 deficiency with psychiatric symptoms as the only presenting concern. Clinicians should consider measuring vitamin B12 levels in psychiatric patients at risk of deficiency of this nutrient, including older adults, vegetarians, and those with alimentary disorders.29,30 Dietary sources of vitamin B12 include meat, milk, egg, fish, and shellfish.31 The body can store a total of 2 to 5 mg of vitamin B12; thus, it takes 2 to 5 years to develop vitamin B12 deficiency from malabsorption and can take as long as 20 years to develop vitamin B12 deficiency from vegetarianism.32 However, by chemically inactivating vitamin B12, N2O causes a rapid functional deficiency, as was seen in our patient.
OUTCOME Improved insight
At a 1-week follow-up appointment with a psychiatrist, Mr. M has no evident psychotic symptoms. He reports that he has not used Cannabis or N2O, and he discontinues olanzapine following this visit. Two weeks later, Mr. M shows no psychotic or affective symptoms other than grief, which is appropriately expressed. His insight has improved. He commits to not using Cannabis, N2O, or any other illicit substances. Mr. M is referred back to his long-standing primary care provider with the understanding that if any psychiatric symptoms recur he will see a psychiatrist again.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
1. Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19(2):187-194.
2. Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26)1720-1728.
3. Herres J, Chudnofsky CR, Manur R, et al. The use of inhaled nitrous oxide for analgesia in adult ED patients: a pilot study. Am J Emerg Med. 2016;34(2):269-273.
4. Aboumarzouk OM, Agarwal T, Syed Nong Chek SA, et al. Nitrous oxide for colonoscopy. Cochrane Database Syst Rev. 2011;(8):CD008506.
5. National Institute on Drug Abuse. Drug facts: inhalants. http://www.drugabuse.gov/publications/drugfacts/inhalants. Updated February 2017. Accessed September 30, 2017.
6. SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health 2012 and 2013: Table 1.88C. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2013.pdf. Published September 4, 2017. Accessed September 30, 2017.
7. Brouette T, Anton R. Clinical review of inhalants. Am J Addict. 2001;10(1):79-94.
8. Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54(1):9-18.
9. Garakani A, Jaffe RJ, Savla D, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. 2016;25(5):358-369.
10. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301(1-2):1-8.
11. van Tonder SV, Ruck A, van der Westhuyzen J, et al. Dissociation of methionine synthetase (EC 2.1.1.13) activity and impairment of DNA synthesis in fruit bats (Rousettus aegyptiacus) with nitrous oxide-induced vitamin B12 deficiency. Br J Nutr. 1986;55(1):187-192.
12. Schrier SL, Mentzer WC, Tirnauer JS. Diagnosis and treatment of vitamin B12 and folate deficiency. UpToDate. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-vitamin-b12-and-folate-deficiency. Updated September 30, 2011. Accessed September 8, 2015.
13. Sethi NK, Mullin P, Torgovnick J, et al. Nitrous oxide “whippit” abuse presenting with cobalamin responsive psychosis. J Med Toxicol. 2006;2(2):71-74.
14. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115(7):859-862.
15. Brodsky L, Zuniga J. Nitrous oxide: a psychotogenic agent. Compr Psychiatry. 1975;16(2):185-188.
16. Wong SL, Harrison R, Mattman A, et al. Nitrous oxide (N2O)-induced acute psychosis. Can J Neurol Sci. 2014;41(5):672-674.
17. Smith AD. Megaloblastic madness. Br Med J. 1960;2(5216):1840-1845.
18. Masalha R, Chudakov B, Muhamad M, et al. Cobalamin-responsive psychosis as the sole manifestation of vitamin B12 deficiency. Isr Med Associ J. 2001;3(9):701-703.
19. Kuo SC, Yeh SB, Yeh YW, et al. Schizophrenia-like psychotic episode precipitated by cobalamin deficiency. Gen Hosp Psychiatry. 2009;31(6):586-588.
20. Raveendranathan D, Shiva L, Venkatasubramanian G, et al. Vitamin B12 deficiency masquerading as clozapine-resistant psychotic symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25(2):E34-E35.
21. Devalia V, Hamilton MS, Molloy AM; British Committee for Standards in Haematology. Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol. 2014;166(4):496-513.
22. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
23. Garakani A, Welch AK, Jaffe RJ, et al. Psychosis and low cyanocobalamin in a patient abusing nitrous oxide and cannabis. Psychosomatics. 2014;55(6):715-719.
24. Yajnik S, Thapar P, Lichtor JL, et al. Effects of marijuana history on the subjective, psychomotor, and reinforcing effects of nitrous oxide in human. Drug Alcohol Depend. 1994;36(3):227-236.
25. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78(1):10-18.
26. Zorumski CF, Nagele P, Mennerick S, et al. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172.
27. Shear MK. Clinical practice. Complicated grief. N Engl J Med. 2015;372(2):153-160.
28. Knechtli CJC, Crowe JN. Guidelines for the investigation & management of vitamin B12 deficiency. Royal United Hospital Bath, National Health Service. http://www.ruh.nhs.uk/For_Clinicians/departments_ruh/Pathology/documents/haematology/B12_-_advice_on_investigation_management.pdf. Accessed June 14, 2016.
29. Jayaram N, Rao MG, Narashima A, et al. Vitamin B12 levels and psychiatric symptomatology: a case series. J Neuropsychiatry Clin Neurosci. 2013;25(2):150-152.
30. Marks PW, Zukerberg LR. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 30-2004. A 37-year-old woman with paresthesias of the arms and legs. N Engl J Med. 2004;351(13):1333-1341.
31. Watanabe F. Vitamin B12 sources and bioavailablility. Exp Biol Med (Maywood). 2007;232(10):1266-1274.
32. Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45(8):1435-1440.
Management of Patients with HIV and Hepatitis B Coinfection
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
From UT Southwestern Medical Center, Dallas, TX.
Abstract
- Objective: To review the literature on and provide evidence-based recommendations for management of HIV/ hepatitis B (HBV) coinfection.
- Methods: Review of the literature for clinical trials, guidelines, and cohort studies on HIV/HBV disease management.
- Results: HIV patients should be evaluated for viral hepatitis. Those who do not have evidence of immunity should be vaccinated and monitored for response. Those who have HIV/HBV should have additional serologies checked to evaluate for hepatitis B e antigen status and level of viremia. All HIV/HBV coinfected patients should be started on antiretroviral therapy with tenofovir-based regimens. Those with HIV/HBV and cirrhosis should be screened for hepatocellular cancer every 6 months.
- Conclusion: HIV patients should be vaccinated against hepatitis B; those with coinfection should be treated for both viruses. It is important to monitor for treatment response to both HIV and HBV and liver disease complications.
Key words: incentives; reinforcement; substance abuse treatment; dissemination; implementation.
Morbidity and mortality for HIV-infected patients remain high compared to uninfected patients despite effective virologic suppression. Major contributors to illness and death among these patients include cardiovascular disease, non–AIDS-defining malignancies, and chronic liver disease, specifically viral hepatitis [1]. Hepatitis B virus (HBV) infection is one of the main causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC) globally. Because HIV and HBV can both be acquired through injection drug use and sexual transmission, coinfection occurs frequently. The Joint United Nations Program on HIV/AIDS estimates that 10% of the 33 million HIV-positive patients worldwide have simultaneous chronic HBV infection [2].
HIV/HBV coinfection significantly impacts the natural history, progression, and mortality related to both viruses. HIV infection accelerates HBV-related liver impairment, leading to earlier cirrhosis, end-stage liver disease, and HCC. Conversely, chronic HBV does not have a considerable influence in the progression of HIV; however, antiretroviral treatment (ART) toxicities and/or HBV flares due to immune reconstitution inflammatory syndrome (IRIS) or HBV itself can lead to increased liver-related complications [3,4].
Case Patient 1
Initial Presentation and History
An asymptomatic 38-year-old man diagnosed with HIV infection 1 month ago presents for his initial visit to establish HIV care. The patient is a man who has sex with men (MSM) and is currently sexually active with multiple partners. He reports inconsistent use of condoms. One month ago he underwent routine screening and was found to be HIV-positive. At the time of diagnosis, the patient’s baseline CD4 cell count was 328 cells/µL and his viral load was 182,600 copies/mL. The patient wants to discuss the implications of his new diagnosis of HIV and recommendations for further testing and treatment. He is especially interested in HBV screening, since one of his recent partners was known to be positive. The patient has no relevant past medical history. He does not recall the details of his childhood vaccinations. He denies smoking and injection drug use but reports moderate alcohol consumption.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies show normal complete blood count (CBC) and renal and liver function test results, and a baseline HIV genotype does not show resistance. Hepatitis A total antibody (anti-HAV) testing is positive, while tests for hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), antibody to hepatitis B core antigen (anti-HBc), and hepatitis C antibody (anti-HCV) are negative.
• Which screening tests for HBV should be performed in HIV-infected patients?
Routine screening of all HIV-infected patients for hepatitis A virus, HBV, and hepatitis C virus (HCV) is recommended [5]. Routine HBV screening involves obtaining serologies for HBsAg, anti-HBs, and anti-HBc. With these results, patients can be classified into categories of either active infection, immunity, or no evidence of prior exposure (Table 1).
• How effective is HBV vaccination in the HIV population?
Vaccination
Available HBV vaccines in the United States include 2 single-agent vaccines (Recombivax HB [Merck, Whitehouse Station, NJ] and Engerix-B [GlaxoSmithKline, Research Triangle Park, NC]) as well as a combination HAV/HBV vaccine (Twinrix [GlaxoSmithKline]). For adults (age ≥ 20 years) with an immunocompromising condition such as HIV infection, current Centers for Disease Control and Prevention guidelines recommend three 40 µg/mL doses of single-agent vaccine administered at 0, 1, and 6 months (Recombivax HB), or four 40 µg/mL doses of single-agent vaccine (2 doses of 20 µg/mL administered simultaneously) at 0, 1, 2, and 6 months (Engerix-B) [7].
The immunogenicity to HBV vaccination in HIV-positive patients is decreased, reflected by lower antibody titers, waning immunity, and seroconversion rates of 18% to 65% [8–11]. Factors associated with poor response include low CD4 cell counts, detectable HIV RNA, coinfection with HCV, and the general health status of the patient [8]. Ideally, HBV vaccination should occur prior to decline in CD4 cell count below 350 cells/µL. However, guidelines do not recommend deferring vaccination since some patients with advanced HIV disease will seroconvert [6].
Anti-HBs titers should be checked 1 month after completion of the vaccine series to confirm protective antibody titers. For patients with quantitative anti-HBs levels < 10 IU/mL, a second vaccine cycle is recommended. Some specialists may defer revaccination until a sustained increase in CD4 count is achieved on ART.
Two randomized controlled trials have demonstrated that 4 doses of double-dose (40 µg/mL) vaccine generate higher anti-HBs levels than 3 doses of standard-dose (20 µg/mL) vaccine in HIV-infected adults [12,13]. Another study showed that HIV patients with CD4 counts > 350 cells/µL had better responses when immunized with double- dose vaccines on the usual 3-dose series [14]. Currently, the CDC recommends giving a double dose for either a 3-dose schedule or a 4-dose schedule. However, it remains unclear what dosing schedule to use if a patient fails to respond. Likely waiting until the CD4 cell count has increased and HIV viral load is suppressed will be important to seeing a response.
• What approach for HBV prevention should be taken in this patient?
The patient’s serologies confirm no prior exposure to HBV, and he should be offered HBV vaccination. His CD4 cell count is below 350/µL and he has ongoing HIV viremia, which increases his risk for an inadequate response. However, vaccination should not be delayed, particularly given his high risk of sexual transmission. The patient should be counseled regarding all high-risk behaviors. As discussed above, 3 or 4 doses of the higher dose vaccine (40 µg/mL) should be administered depending on what type of recombinant vaccine is available. An anti-HBs level should be checked 1 month after completion. A full repeat vaccine series using the 40 µg/mL dose should be considered for nonresponders who initially received a standard vaccine series. Experts also recommend checking annual anti-HBs levels to monitor for waning immunity, with a booster dose given if the anti-HBs level drops below the protective range.
• What vaccination strategy should be used in patients with isolated positive anti-HBc?
The clinical implications of an isolated positive anti-HBc for vaccination are still uncertain. This serologic pattern may represent a false-positive test, remote HBV infection with loss of anti-HBs, or occult HBV infection with undetectable HBsAg. The latter scenario appears more commonly in HIV-infected patients, particularly with concomitant HCV infection [15].
A recent study suggested that patients with an isolated positive anti-HBc with a negative anamnestic antibody response (anti-HBs titer of < 10 IU/mL 4 weeks after a single 20 µg dose of recombinant HBV vaccine) should be further vaccinated with the double-dose for a 3-dose schedule [6]. Additionally, another study followed HIV/HCV coinfected patients for 9.5 years and found that an isolated positive anti-HBc was not associated with accelerated liver disease progression [16].
Treatment of HIV-1 Infection
Current HIV guidelines recommend initiation of ART for all HIV-infected patients regardless of their CD4 count [17]. ART for a treatment-naïve patient usually consists of 2 nucleotide/nucleoside reverse-transcriptase inhibitors (NRTIs; the “backbone”) combined with a third agent (the “anchor”), which can be a nonnucleoside reverse-transcriptase inhibitor (NNRTI), a protease inhibitor (PI) boosted with a boosting agent, or an integrase strand-transfer inhibitor (INSTI) [18,19].
There are numerous studies indicating that incident HBV risk can be reduced by placing those at risk for HBV acquisition on ART containing a combination of tenofovir disoproxil fumarate (TDF), lamivudine, or emtricitabine [20,21]. Another study in MSM found those on ART with HIV viral load < 400 copies/mL were protected from developing HBV compared to those not on ART [22]. Given this patient’s higher risk for HBV acquisition, placing him on emtricitabine/TDF backbone as part of ART could be protective against incident HBV [23].
Case Patient 2
Initial Presentation and History
A 32-year-old man diagnosed with HIV infection 8 years ago now on ART presents for follow-up. The patient is an MSM with a history of inconsistent condom use. At the time of HIV diagnosis 8 years ago, the patient had a CD4 cell count of 250 cells/µL and an HIV viral load of 648,000 copies/mL. The patient was initiated on lamivudine/zidovudine and lopinavir/ritonavir, and he achieved complete virologic suppression at 20 weeks, with a CD4 cell count of 455 cells/µL at 1 year. The patient has remained on this regimen without major side effects; however, he reports frequent missed doses over the last 2 years due to “pill fatigue.” Previous testing for HBV and HCV at the time of his initial HIV diagnosis was negative, but he failed to complete the HBV vaccine series. He denies alcohol or injection drug use.
Physical Examination and Laboratory Testing
Physical examination is normal. Laboratory studies reveal normal electrolytes and renal function, hemoglobin of 11 g/dL, and platelet count of 235,000/µL. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels are 34 U/L and 44 U/L, respectively, with an INR of 1.1 and albumin level of 3.4 g/dL. CD4 cell count is 320 cells/µL with an HIV viral load of 24,500 copies/mL. Viral hepatitis serologies show anti-HAV positive, anti-HCV negative, HBsAg positive, anti-HBc IgG positive, hepatitis Be antigen (HBeAg) negative, and hepatitis B e antibody (anti-HBe) positive. HBV DNA viral load is 685,000 IU/mL.
• What is the natural history of HIV/HBV coinfection?
Chronic HBV infection affects about 10% of HIV-infected patients globally. Epidemiologic studies indicate that HIV-infected patients have higher rates of reactivation and progression to chronic HBV infection and chronic liver disease than HIV-negative patients [24–26]. Coinfected patients demonstrate higher serum HBV DNA levels, which lead to more rapid progression of hepatic fibrosis and may increase the risk of cirrhosis and HCC [24,27,28]. HIV infection, however, can mediate the necroinflammatory response through blunting of the immune response that drives pathogenesis in HBV infection. Aminotransferase levels may be only slightly elevated or even normal, particularly in the severely immune suppressed. However, elevation in liver enzymes (hepatitis flare) can occur if a patient stops ART, or if HBV resistance develops. Patients being treated for HIV/HBV coinfection should be counseled that stopping HIV treatment puts them at risk of developing a hepatitis flare.
Large cohort studies of HIV/HBV coinfected patients indicate increased risk of liver-related mortality, most pronounced with lower CD4 cell counts [1,28,29]. Introduction of ART appears to increase rather than attenuate this liver-related mortality, possibly by decreasing AIDS-related mortality and allowing more time for liver disease progression. Finally, a recent meta-analysis including 12,382 patients demonstrated a significant effect of HIV/HBV coinfection on all-cause mortality, with a pooled effect estimate of 1.36 [30].
• What diagnostic testing should be done in coinfected patients?
Diagnostic Testing and Evaluation
Persistence of HBsAg for more than 6 months is diagnostic of chronic HBV infection and warrants further serologic evaluation. Patients with chronic HBV infection should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels measured. Elevation of AST and ALT can be seen with untreated HBV. For those on treatment, a hepatitis flare could be caused by abrupt discontinuation of HBV treatment, development of HBV resistance, or superinfection with another viral pathogen.
HBeAg is a marker of active viral replication and is associated with higher levels of HBV DNA and active liver disease. Seroconversion, or loss of HBeAg and development of anti-HBe, heralds a favorable treatment response for those who were initially HBeAg-positive. However, some HBV variants have precore or core promoter mutations that lead to higher levels of HBV DNA in the absence of HBeAg. This highlights the importance of monitoring HBV DNA levels in all patients with chronic HBV infection. Favorable response to therapy in HBeAg-negative patients is marked by normalization of aminotransferases and HBV DNA suppression.Hepatitis D virus (HDV) is a defective virus particle that can only replicate in the presence of HBV. Coinfected patients should be tested for anti-HDV if they are injection drug users or are from a high-prevalence region such as the Mediterranean and Amazon basins. Newly acquired HDV infection should also be considered in the context of hepatitis flares.
Although these routine diagnostic tests are essential for management, studies show low adherence rates to HBV testing guidelines by HIV providers [31,32].
• What is the role of HBV genotype and resistance testing?
HBV can be classified into 10 different genotypes, A through J, based on genomic sequence variations. Each genotype has a distinct ethnic and geographic distribution, with genotypes A and D predominating in North America. HBV genotyping appears to have important prognostic as well as treatment implications [19]. However, data are still preliminary and the guidelines do not recommend routine genotype testing.
Resistance testing in HBV allows for detection of mutations that decrease effectiveness of antivirals. Exposure to lamivudine can lead to mutations in the YMDD region of the HBV DNA polymerase, resulting in drug resistance. Resistance to lamivudine develops at a rate of approximately 25% after 1 year of drug exposure in HIV/HBV coinfection [33]. On the other hand, studies have shown that entecavir is active against HIV and, most importantly, selects for the M184V mutation in HIV. M184V is associated with lamivudine resistance for HIV treatment, thus limiting treatment options [34,35]. After these findings, the FDA advised against monotherapy with entecavir in patients with HIV/HBV coinfection. The case patient was on lamivudine during the time of HBV acquisition, and therefore YMDD mutation must be taken into consideration for therapy purposes. He should be switched to a regimen that suppresses both viruses. The development of drug resistance should be assessed in all patients with persistent or breakthrough HBV viremia on ART, particularly the nucleoside analogues. HIV providers treating HIV/HBV coinfection should regularly monitor both HIV and HBV viral load to assess for therapeutic efficacy.
• What is the role of liver biopsy in this patient?
Liver biopsy should be considered in all coinfected patients as it remains the gold standard for determining the activity and severity of chronic hepatitis B. However, because it carries inherent risks and is not required prior to treatment in all patients, the decision should be individualized. Liver biopsy can be useful to assess baseline liver histology and may be warranted to rule out significant coexisting genetic or metabolic liver disease. Currently, noninvasive methods to assess liver fibrosis either using elastography or various combinations of serum biomarkers are being evaluated [36] and may be considered in lieu of a liver biopsy [37]. One study compared the accuracy of elastography with liver biopsy in HIV/HBV coinfected patients and demonstrated that the former was proficient in discriminating between absence or mild fibrosis and moderate to severe fibrosis [38]. In general, this test has high accuracy in detecting minimal fibrosis from advanced fibrosis or cirrhosis. For the group in the middle, further investigation with additional methods must be considered [37]. Finally, a recent retrospective study involving 70 HIV/HBV coinfected individuals showed fibrosis regression suggesting beneficial effects of long-term ART on liver stiffness, [39] but further studies are needed to confirm these findings.
Case 2 Continued
The patient has now been diagnosed with chronic HBV infection. His diagnostic testing is negative for HBeAg and reveals modest HBV viremia with abnormal aminotransferases less than 2 times the upper limit of normal. Drug resistance testing reveals a mutation in the YMDD region indicative of HBV lamivudine resistance. HIV genotype demonstrates a wild-type virus without resistance. The patient asks what treatment options exist.
• What medications are currently available to treat hepatitis B?
Treatment
All patients with HIV/HBV coinfection should receive treatment to suppress both viruses, and ART needs to include 2 drugs active against HBV, ideally emtricitabine and TDF. This approach prevents drug resistance, slows progression of HBV infection, and reduces the incidence of IRIS [5]. TDF has been associated with decreased renal function and bone mineral density. Recently, tenofovir alafenamide fumarate (TAF) was approved for the treatment of HIV and HBV. A dose of 10 mg daily is given when coadministered with ritonavir, cobicistat, or protease inhibitors, but a dose of 25 mg should be given when administered with NNRTIs or integrase inhibitors. Compared to TDF, TAF shows less accumulation of tenofovir in kidneys and bones and consequently has reduced renal and bone mineral density effects [40]. All patients should be on a TDF- or TAF-based HIV regimen if they have chronic HBV.
Currently, the following antiviral drugs are FDA-approved for the treatment of HBV infection: interferon alfa-2b, pegylated interferon (peginterferon) alfa-2a, lamivudine and emtricitabine, entecavir, adefovir, TDF, TAF, and telbivudine. Telbivudine and adefovir are no longer recommended due to their association with higher incidence of toxicity and higher rates of HBV treatment failure. A summary of available HBV treatment options is outlined in Table 2.
Interferon
Standard interferon alfa-2b blocks HBV replication through interaction with viral proteins and stimulation of host cellular immunity. Peginterferon alfa-2a has proven efficacy in HBV-monoinfected patients, but efficacy data in HIV/HBV coinfected patients is lacking [41,42]. Studies in hepatitis C treatment demonstrate the safety of peginterferon alfa-2a use in HIV-positive patients and indicate that HIV viral suppression occurs with peginterferon without evidence of selection of resistance mutations that affect future ART options [43]. For HIV/HBV coinfected patients not on ART who will receive only therapy for HBV (which is infrequent since all HIV patients should be on ART), pegylated interferon-alfa-2a alone for 48 weeks is the only option that will not cause ART-associated HIV drug resistance [5]. Interferon therapy is contraindicated in patients with decompensated cirrhosis and should be used with caution in patients with active depression, uncontrolled diabetes, and cardiac and pulmonary disease.
Lamivudine and Emtricitabine
Lamivudine is a nucleoside analogue with efficacy against both HIV and HBV. Clinical trials in HIV/HBV-infected patients have shown up to 87% of patients achieve undetectable HBV DNA levels and about 25% achieve HBeAg seroconversion after 1 to 2 years of therapy [44,45]. However, the major issue limiting use of lamivudine is its low genetic barrier to resistance. Mutation of the YMDD motif of the HBV DNA polymerase confers HBV resistance. HIV/HBV coinfected patients develop resistance at rates of up to 94% after 4 years of therapy [33], heralded by rebounds in HBV DNA levels and often hepatitis flares or precipitation of hepatic failure [16]. Because of resistance, lamivudine monotherapy should be avoided; even in patients on ART, abrupt withdrawal of lamivudine or the development of HBV resistance should be closely monitored.
Emtricitabine is another nucleoside analogue with properties and efficacy similar to lamivudine. It is frequently used as a combination pill with TDF (Truvada) in coinfected patients. The same concerns regarding monotherapy and the emergence of resistance that exist for lamivudine apply to emtricitabine.
Tenofovir
TDF, a nucleotide analogue, is one of the preferred first-line agents for HIV treatment and has proven efficacy against both wild-type and lamivudine-resistant HBV. Since it was first used for HIV, TDF has been more extensively studied in coinfected patients compared to most other agents. In a meta-analysis of patients with HIV/HBV coinfection, TDF suppressed HBV viral load to undetectable titers in approximately 90% of patients [46]. Tenofovir is available in 2 preparations: TDF and TAF. TDF has been reported to cause renal impairment as well as bone loss. TAF has shown less renal toxicity and less bone damage [40,47]. In 2016, TAF became available as part as 4 regimens: stand-alone TAF, elvitegravir-cobicistat-emtricitabine-TAF, rilpivirine-emtricitabine-TAF, and TAF-emtricitabine.
TDF and TAF both suppress HIV. Two large randomized trials of HBV monoinfection demonstrate that TAF is noninferior to TDF for the treatment of naïve and treatment-experienced patients [48,49].
Entecavir
Entecavir, a guanosine analogue, is a potent HBV DNA polymerase inhibitor that results in greater virologic suppression compared to lamivudine and retains activity against lamivudine-resistant HBV [50]. Importantly, entecavir shares some cross-resistance with lamivudine, so an entecavir dose of 1 mg daily is recommended in lamivudine-experienced patients compared to 0.5 mg daily in lamivudine-naïve patients. Entecavir requires dose reduction for patients with creatinine clearance less than 50 mL/min, although it is not associated with renal toxicity. A 1-log10 reduction in HIV RNA levels as well as emergence of M184V mutations on entecavir monotherapy has been reported [51,52]. M184V confers HIV resistance to lamivudine and emtricitabine. Therefore, entecavir should not be used as monotherapy in HIV-coinfected patients and/or patients with evidence of lamivudine-resistant HBV.
Combination Therapy
Recent updates in the guidelines recommend that since emtricitabine, lamivudine, TDF, and TAF are active against both viruses, patients with coinfection should start ART with a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual drug combination TDF plus lamivudine [53]. Most experts recommend the use of combination HBV therapy in patients on ART, particularly with lamivudine given the high rates of resistance.
• When should HBV treatment be started in patients with coinfection?
An ART regimen containing TDF (creatinine clearance > 50 mL/min) or TAF (creatinine clearance > 30 mL/min) with lamivudine or emtricitabine should be used in all HIV/HBV patients as soon as the infection is diagnosed. If TDF or TAF cannot be used, the alternate recommended regimen for HBV is entecavir plus a fully suppressive ART. In those with decreased renal function, entecavir should be adjusted to renal function [19].
Although control of viremia is feasible, clearance of infection as marked by loss of HBsAg and development of anti-HBs is unlikely to occur in the majority of patients. Therefore, the goals of treatment focus on prevention of chronic liver disease complications by suppressing viral replication, which can halt disease progression. A suggested algorithm for the management of coinfected patients is provided (Figure).
• What is the duration of therapy for hepatitis B?
Most patients with HIV/HBV coinfection will require lifelong treatment. All patients on HBV therapy as a part of ART should continue HBV therapy, regardless of seroconversion status. Also, patients should be educated and advised against self-discontinuation as it may trigger hepatitis exacerbations and/or hepatic failure.
Case Patient 3
Initial Presentation and History
Two months after starting treatment for HIV and chronic HBV infection, a 46-year-old Hispanic woman presents to clinic with jaundice and right upper quadrant (RUQ) pain. The patient was recently diagnosed with HIV infection and was naïve to treatment with ART. Her CD4 cell count was 50 cells/µL, and her HIV viral load was 743,000 copies/mL, with no baseline mutations on HIV genotype. The patient was also diagnosed with chronic HBV infection with positive HBsAg and HBeAg and negative HBc IgM serologies, as well as an HBV DNA level of 87 million IU/mL. Routine blood work revealed normal renal function and serum transaminases. The decision was made to start the patient on darunavir/ritonavir and TDF/emtricitabine. The patient was also started on sulfamethoxazole/trimethoprim and azithromycin for opportunistic infection prophylaxis.
Physical Examination and Laboratory Testing
Examination is remarkable for mild tenderness in the RUQ and icteric sclera. Laboratory testing demonstrates the following: AST, 1523 U/L; ALT, 795 U/L; albumin, 2.8 mg/dL; and total bilirubin, 3.5 mg/dL. Her CD4 count has increased to 565 cells/µL, and her HIV viral load is 4320 copies/mL. Results of repeat hepatitis serologies are as follows: HBsAg positive, anti-HBc IgM positive, and an HBV DNA level of 4.2 million IU/mL. Testing for hepatitis A, C, and D is negative, and RUQ sonogram reveals no gallstones.
• What monitoring should be done for coinfected patients on HBV therapy?
Monitoring
Providers should routinely monitor patients’ response to HIV/HBV therapy. Initially, all coinfected patients should have liver function tests and HBV DNA levels checked every 12 weeks on therapy. Frequent monitoring allows early detection of HBV drug resistance as well as drug-related hepatotoxicity. In HBeAg-positive coinfected patients who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. In HBeAg-negative patients, only HBV DNA and liver function tests are needed. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy.
Typically, virologic failure results from either the development of drug resistance or abrupt withdrawal of active HBV therapy due to patient nonadherence or changes to the ART regimen. Virologic failure can result in a rise in serum aminotransferases as well as decompensation in patients with significant underlying liver disease. Due to this risk, providers must counsel patients about the importance of adherence to therapy and should continue medications active against HBV when making a change in ART regimens, unless HBV drug resistance dictates a change in HBV therapy.
• What is the likely cause of this patient’s hepatitis “flare”?
Several studies indicate that patients with HIV/HBV coinfection are at increased risk of drug-related hepatotoxicity and grade 4 liver enzyme elevations [54,55].The first 3 months after initiation of ART is a particularly vulnerable time for liver injury. The differential diagnosis for an acute hepatitis “flare” following the initiation of ART is broad and includes the following: development of HBV drug resistance [16]; withdrawal of HBV-active medications due to nonadherence [54]; ART-related hepatotoxicity; superimposed infection with HAV, HCV, or HDV; other opportunistic infections including cytomegalovirus and mycobacterium avium complex; or IRIS, resulting in an exaggerated cytotoxic response by the recovering immune system [56,57]. Complete evaluation is critical to distinguish between the possible causes.
In this case, several clues point toward HBV-related IRIS as the most likely cause for the hepatitis “flare.” A low pretreatment CD4 cell count with a rapid rise after initiation of ART is associated with a higher rate of IRIS [57]. Serologic testing and imaging excluded superinfection with another hepatotropic virus or biliary tract disease. Appropriate declines in HIV viral load and HBV DNA levels imply patient adherence to therapy and argue against the development of HBV drug resistance. Finally, the emergence of anti-HBc IgM positivity signals HBV reactivation, which is commonly seen in patients with HBV-related IRIS [57]. The preferred treatment for HBV-related IRIS involves continuation of therapy, frequently leading to normalization of aminotransferases and subsequent HBeAg seroconversion. Because IRIS usually manifests within the first 6 to 12 weeks after starting ART, liver enzymes should be monitored closely during this period.
• What health maintenance should be done for coinfected patients?
All patients with HIV/HBV coinfection should be monitored for evidence of portal hypertension or cirrhosis and, if these conditions exist, should undergo endoscopic screening for esophageal varices as well as evaluation of ascites and encephalopathy. Patients with HBV are at increased risk for the development of HCC even in the absence of cirrhosis. A recent study showed low rates of HCC screening in HIV/HBV patients by HIV providers [58]. Whether HIV coinfection potentiates HCC risk is uncertain, though coinfected patients present at younger ages and with more symptoms than HIV-negative comparators [59]. Other risk factors for HCC include HCV infection, alcohol abuse, diabetes, obesity, exposure to environmental toxins, and cirrhosis of any etiology (most commonly non-alcoholic fatty liver disease, primary sclerosing cholangitis, primary biliary cirrhosis and hemochromatosis) [60].
The American Association of Liver Diseases (AASLD) guidelines recommend hepatic ultrasound screening every 6 months in all patients with cirrhosis or chronic HBV who are at increased risk (Asian men over the age of 40 years, Asian women over the age of 50 years, African or North American blacks, and patients with family history of HCC) [61]. They should also be referred for an esophagogastroduodenoscopy to evaluate for esophageal varices. In addition, all HIV/HBV coinfected patients with decompensated liver disease should be evaluated for transplantation. HIV infection is not a contraindication for liver transplant with the use of ART. However, since transplantation does not cure HBV infection, post-transplant HBV immune globulin and HBV treatment are required. Contemporary data suggest comparable survival rates after transplant in coinfected patients compared to HBV-monoinfected patients [51].
Summary
Routine screening with HBsAg, anti-HBs, and anti-HBc serologies is recommended for all HIV-positive individuals. Patients without evidence of prior exposure or vaccination and those with isolated anti-HBc should be offered vaccination. HIV-positive adults should receive three or four 40 µg/mL doses of single agent vaccine depending on the recombinant vaccine type available. Anti-HBsAg titers should be checked 1 month after completion of the immunization series. If quantitative anti-HBsAg levels are < 10 IU/mL, patients should receive a second vaccine cycle.
Patients who test positive for HBsAg should be tested for HBeAg, anti-HBe, and HBV DNA levels and have AST and ALT levels checked as well. All patients with HIV/HBV coinfection should start treatment as soon as HIV infection is diagnosed. ART needs to include 2 drugs against HBV, and therefore a fixed-dose combination of TDF/emtricitabine or TAF/emtricitabine or the individual combination of TDF plus lamivudine should be used.
Coinfected patients on treatment should have liver function tests as well as HBV DNA every 12 weeks. In HBeAg-positive coinfected individuals who achieve HBV DNA suppression, HBeAg and anti-HBe testing should be performed every 6 to 12 months to assess for seroconversion. HBV virologic failure is defined as a greater than 1-log10 rise in HBV DNA levels or development of viremia in a patient with a previously suppressed DNA level on therapy. Those with virologic failure should be tested for HBV resistance thorough HBV genotype. Coinfected patients with cirrhosis should receive ultrasound screening every 6 months for evidence of HCC and esophagogastroduodenoscopy to evaluate for esophageal varices.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
1. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–41.
2. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global change. N Engl J Med 2012;366:1749–52.
3. Bellini C, Keise O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med 2009;10:12–8.
4. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001. AIDS 2003;17:2191–18.
5. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Q1–Q17.
6. Piroth L, Launay O, Michel ML, et al. Vaccination against hepatitis B virus (HBV) in HIV-1-infected patients with isolated anti-HBV core antibody: the ANRS HB EP03 CISOVAC prospective study. J Infect Dis 2016;213:1735–42.
7. Centers for Disease Control and Prevention. Recommended adult immunization schedule for adults aged 19 years or older, by vaccine and age group. United States, 2016. Accessed 22 Dec 2016 at www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
8. Rey D, Krantz V, Partisani M, et al. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine 2000;18:1161–5.
9. Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis 2005;41:1045–8.
10. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
11. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
12. Chaiklang K, Wipasa J, Chaiwarith R, et al. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: a randomized, controlled trial. PLoS One 2013;8:e80409.
13. Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA 2011;305:1432–40.
14. Fonseca MO, Pang LW, de Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine 2005;23:2902–8.
15. Gandhi RT, Wurcel A, Lee H, et al. Response to hepatitis B vaccine in HIV-1-positive subjects who test positive for isolated antibody to hepatitis B core antigen: implications for hepatitis B vaccine strategies. J Infect Dis 2005;191:1435–41.
16. French AL, Hotton A, Young M, et al. Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J Acquir Immune Defic Syndr 2016;72:274–80.
17. INSIGHT START Study Group, Lundgren JD, Babiker GA, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807.
18. Sebaaly JC, Kelley D. Single-tablet regimens for the treatment of HIV-1 infection. Ann Pharmacother 2017;51:332–44.
19. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Accessed 6 Dec 2016 at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
20. Heuft MM, Houba SM, van den Berk GE, et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS 2014;28:999–1005.
21. Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on Hepatitis B virus infection. Clin Infect Dis 2013;56:1812–9.
22. Falade-Nwulia O, Seaberg EC, Snider AE, et al. Incident hepatitis B virus infection in HIV-infected and HIV-uninfected men who have sex with men from pre-HAART to HAART periods: a cohort study. Ann Intern Med 2015;163:673–80.
23. Shilaih M, Marzel A, Scherrer AU, et al. Dually active HIV/HBV antiretrovirals as protection against incident hepatitis B infections: potential for prophylaxis. J Infect Dis 2016;214:599–606.
24. Colin JF, Cazals-Hatem D, Loriot MA, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology 1999;29:1306–10.
25. Gilson RJ, Hawkins AE, Beecham MR, et al. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS 1997;11:597–606.
26. Bodsworth N, Donovan B, Nightingale BN. The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: a
study of 150 homosexual men. J Infect Dis 1989;160:577–82.
27. Di Martino V, Thevenot T, Colin JF, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology 2002;123:1812–22.
28. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002;360(9349):1921–6.
29. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005;19:593–601.
30. Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 2009;48:1763–71.
31. Jain MK, Opio CK, Osuagwu CC, et al. Do HIV care providers appropriately manage hepatitis B in coinfected patients treated with antiretroviral therapy? Clin Infect Dis 2007;44:996–1000.
32. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;61:1742–8.
33. Matthews GV, Bartholomeusz A, Locarnini S, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. AIDS 2006;20:863–70.
34. McMahon M, Jilek B, Brennan T, Thio C. The HBV drug entecavir: effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
35. Jain M, Zoellner C. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007; 21:2365–6.
36. Bottero J, Lacombe K, Guechot J, et al. Performance of 11 biomarkers for liver fibrosis assessment in HIV/HBV coinfected patients. J Hepatol 2009;50:1074–83.
37. Moreno S, Garcia-Samaniego J, Moreno A, et al. Noninvasive diagnosis of liver fibrosis in patients with HIV infection and HCV/HBV co-infection. J Viral Hepat 2009;16:249–58.
38. Miailhes P, Pradat P, Chevallier M, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61–9.
39. Audsley J, Robson C, Aitchison S, et al. Liver fibrosis regression measured by transient elastography in human immunodeficiency virus (HIV)-hepatitis B virus (HBV) coinfected individuals on long-term HBV-active combination antiretroviral therapy. Open Forum Infect Dis 2016;3:ofw035.
40. Achhra AC, Nugent M, Mocroft A, et al. Chronic kidney disease and antiretroviral therapy in HIV-positive individuals: recent developments. Curr HIV/AIDS Rep 2016;13:149–57.
41. Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–17.
42. Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95.
43. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004;351:438–50.44. Dore GJ, Cooper DA, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis 1999;180:607–13.
45. Hoff J, Bani-Sadr F, Gassin M, Raffi F. Evaluation of chronic hepatitis B virus (HBV) infection in coinfected patients receiving lamivudine as a component of antihuman immunodeficiency virus regimens. Clin Infect Dis 2001;32:963–9.
46. Price H, Dunn D, PIllary D, et al. Suppresion of HBV by tenofovir in HIV/HBV coinfected patients: a systemic review and meta-analysis. PLoS One 2013;8:e68152.
47. Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with eltegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomized, double-blind, phase 3, non-inferiority trial. Lancet 2015;385:2606–15.
48. Chan HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-positive chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:185–95.
49. Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAG-negative chronic hepatitis B infection. A randomized, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 2016;1:196–206.
50. Chang TT, Lai CL. Hepatitis B virus with primary resistance to adefovir. N Engl J Med 2006;355:322–3.
51. McMahon MA, Jilek BL, Brennan TP, et al. The HBV drug entecavir -- effects on HIV-1 replication and resistance. N Engl J Med 2007;356:2614–21.
52. Jain MK, Zoellner CL. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naive patient treated for chronic HBV. AIDS 2007;21:2365–6.
53. Nuesch R, Ananworanich J, Srasuebkul P, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS 2008;22:152–4.
54. Gaglio PJ, Sterling R, Daniels E, Tedaldi E. Hepatitis B virus and HIV coinfection: results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis 2007;45:618–23.
55. Reisler RB, Han C, Burman WJ, et al. Grade 4 events are as important as AIDS events in the era of HAART. J Acquir Immune Defic Syndr 2003;34:379–86.
56. Drake A, Mijch A, Sasadeusz J. Immune reconstitution hepatitis in HIV and hepatitis B coinfection, despite lamivudine therapy as part of HAART. Clin Infect Dis 2004;39:129–32.
57. Jain MK, Parekh NK, Hester J, Lee WM. Aminotransferase elevation in HIV/hepatitis B virus co-infected patients treated with two active hepatitis B virus drugs. AIDS Patient Care STDS 2006;20:817–22.
58. Hearn B, Chasan R, Bichoupan K, et al. Low adherence of HIV providers to practice guidelines for hepatocellular carcinoma screening in HIV/hepatitis B coinfection. Clin Infect Dis 2015;611742–8.
59. Brau N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.–Canadian multicenter study. J Hepatol 2007;47:527–37.
60. Yang JD, Harmsen WS, Slettedahl SW, et al. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol 2011;9:617–23.
61. Bruix J, Sherman M. AASLD Practice Guideline. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2.
When should you consider combining 2 long-acting injectable antipsychotics?
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.
Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.
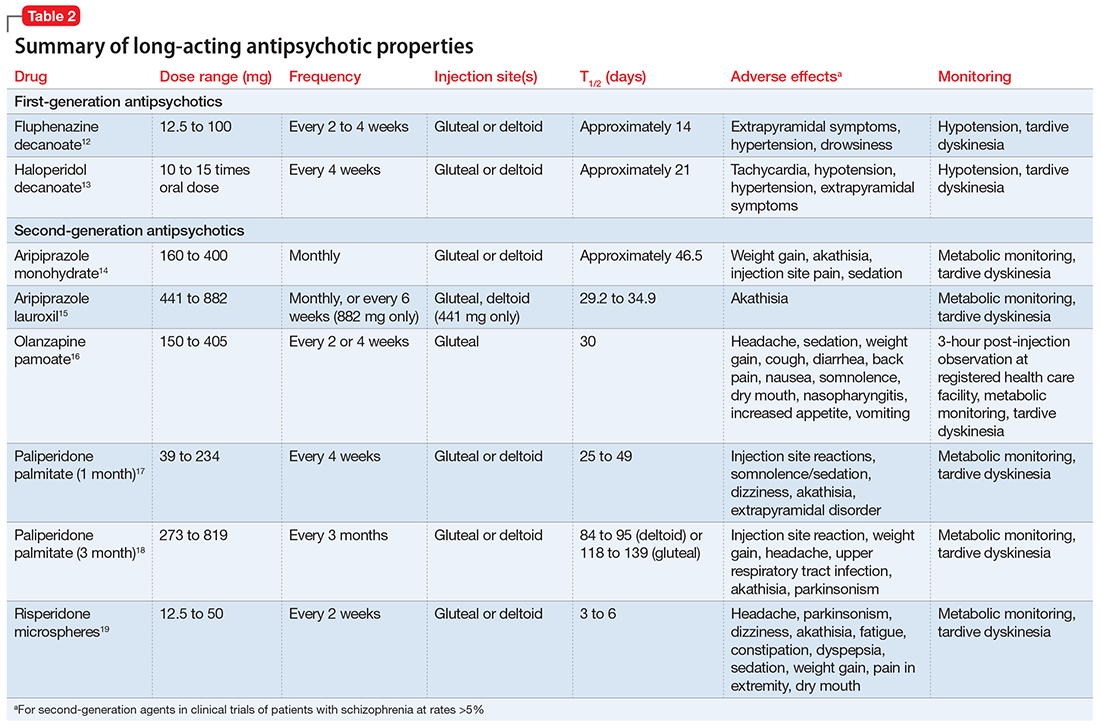
When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.
Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.

When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
Ms. S, age 39, with a 15-year history of schizophrenia and severe paranoid delusions, is admitted after physically assaulting a staff member at a group home. She is receiving paliperidone palmitate, 234 mg every 4 weeks. This has reduced the severity of her symptoms, but she continues to have persistent delusions that affect her ability to accept redirection from staff. Ms. S frequently accuses staff and peers of sexual assault, says that she is pregnant, and does not adhere to treatment recommendations for laboratory monitoring because the “staff uses her blood for experiments.”
Ms. S frequently requires administration of oral and IM haloperidol, as needed, when she becomes aggressive with the staff. She has poor insight into her mental illness and does not believe that she needs medication. Ms. S has a long history of stopping her oral antipsychotic after a few days, reporting that it is “harming her baby.” Monotherapy has been tried with various long-acting injectable antipsychotics (LAIAs), but she still exhibits persistent delusions. The treatment team decides to add a second LAIA, haloperidol decanoate, 200 mg every 4 weeks, to her regimen.
Ladds et al.7 A 49-year-old woman with schizophrenia who was hospitalized for aggressive and bizarre behavior and had been institutionalized for 20 years stopped taking her medication regimen.7 She started taking 8-hour showers with bleach, talking incoherently, and believing that someone was poisoning her. She had poor response to oral risperidone monotherapy; however, 2 months after adding oral fluphenazine and benztropine to her regimen, her symptoms substantially improved (doses not reported). Because she had impaired insight into the need for daily medication, she was started on depot fluphenazine decanoate and risperidone microspheres (doses not reported) before discharge. No substantial adverse effects were noted with this regimen.
Wartelsteiner and Hofer.8 A man who had been diagnosed with paranoid schizophrenia at age 20 presented with thought blocking, incoherence, persecutory delusions, and uncontrolled self-damaging behavior.8 He had been admitted 27 times over 7 years; during this time he received many antipsychotic monotherapies and combination regimens. A total of 8 oral antipsychotics (including clozapine) and 5 LAIAs had been administered during these trials. He significantly improved with the combination of olanzapine and risperidone. Both medications were switched to LAIA formulations to address medication nonadherence. His symptoms remained stable with risperidone microspheres, 100 mg, and olanzapine pamoate, 300 mg, each administered every 2 weeks. He did not experience any adverse effects with this combination therapy.
Scangos et al.9 A 26-year-old Vietnamese man with schizophrenia and an extensive history of unprovoked, psychotically driven assaults was given multiple antipsychotics (including clozapine) during hospitalizations, and his medication regimen consistently included 2 antipsychotics. After contracting viral gastroenteritis, he refused oral medications and required short-acting IM administration of both haloperidol, 5 mg, twice a day, and olanzapine, 10 mg, twice a day. Because of concerns about continuing this regimen, he was switched to haloperidol decanoate (dose not reported) and olanzapine pamoate, 405 mg, administered once per month. The injections were scheduled to alternate so that the patient would receive 1 injection every 2 weeks. The patient’s assaultive behavior was significantly reduced, and no adverse effects were reported.
Ross and Fabian.11 An African American man, age 44, was receiving haloperidol decanoate, 400 mg every 2 weeks, and oral haloperidol, 20 mg/d.11 Because of residual symptoms, a history of nonadherence, and concerns about increasing the haloperidol decanoate dose or frequency, oral haloperidol was discontinued and paliperidone palmitate, 156 mg every 4 weeks, was started. The patient was able to transition into a step-down unit, and no adverse effects were reported.
What to consider before initiating dual LAIA treatment
Evaluate the frequency of administration, flexibility of dosing, administration site, adverse effects, and monitoring requirements of each LAIA (Table 212-19) to ensure the patient’s optimal tolerability of the regimen. Previous tolerability of each medication must be confirmed by evaluating the patient’s medication history or oral or IM administration of each agent prior to initiating the LAIA.

When choosing 2 agents that are each administered once every 4 weeks, consider administering the medications together every 4 weeks or alternating administration so that the patient receives an injection every 2 weeks. Receiving an injection once every 2 weeks might be beneficial for patients who need close follow-up or are more sensitive to injection site reactions, whereas a regimen of once every 4 weeks might be beneficial for patients who are more resistant to receiving the injections, so there is potentially less time spent agitated or anxious leading up to the date of the injection.
Use the lowest effective dose of each LAIA to limit adverse effects and improve tolerability of the regimen. Monitor patients closely for adverse reactions and discontinue the regimen as soon as possible if a severe adverse reaction occurs.
Cost may influence the decision to use 2 LAIAs. The majority of LAIAs in the United States are available only as branded formulations. Insurance companies may require prior authorization for the use of 2 LAIAs.
Although there are no treatment guidelines for combining 2 LAIAs, this practice has been used. A few case reports have described successful use of dual LAIA treatment, but one should consider the risk of the publication’s bias. Overall, the decision to use 2 LAIAs is difficult because there is lack of a large evidence base supporting the practice or direction from treatment guidelines. Because of this, dual LAIA treatment should not be used for most patients. In cases of treatment-resistant schizophrenia where clozapine is not an option and adherence is a concern, it is reasonable to consider this strategy on a case-by-case basis.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Lehman A, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
3. Hasan A, Falkai P, Wobrock T, et al; the WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318-78.
4. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620. 5. Hasan A, Falkai P, Wobrock T, et al; WFSBP Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
6. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al; Schizophrenia Patient Outcomes Research Team (PORT). The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2010;36(1):94-103.
7. Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. The Internet Journal of Psychiatry. 2009;1(1):1-3.
8. Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long-acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35(4):474-475.
9. Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36(3):283-285.
10. Yazdi K, Rosenleitner J, Pischinger B. Combination of two depot antipsychotic drugs [in German]. Nervenarzt. 2014;85(7):870-871.
11. Ross C, Fabian T. High dose haloperidol decanoate augmentation with paliperidone palmitate. Presented at: College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting; April 21-24, 2013; Colorado Springs, CO.
12. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals, LLC; 2010.
13. Haloperidol decanoate [package insert]. Rockford, IL: Mylan; 2014.
14. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2016.
15. Aristada [package insert]. Waltham, MA: Alkermes; 2016.
16. Zyprexa Relprevv [package insert]. Indianapolis, IN: Lilly USA, LLC; 2016.
17. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2009.
18. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2015.
19. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2007.
The girl who couldn’t stop stealing
CASE A lifelong habit
Ms. B, age 14, has diagnoses of attention-deficit/hyperactive disorder (ADHD) and oppositional defiant disorder, and is taking extended-release (ER) methylphenidate, 36 mg/d. Her mother brings her to the hospital with concerns that Ms. B has been stealing small objects, such as money, toys, and pencils from home, school, and her peers, even though she does not need them and her family can afford to buy them for her. Ms. B’s mother routinely searches her daughter when she leaves the house and when she returns and frequently finds things in Ms. B’s possession that do not belong to her.
The mother reports that Ms. B’s stealing has been a lifelong habit that worsened after Ms. B’s father died in a car accident last year.
Ms. B does not volunteer any information about her stealing. She is admitted to a partial hospitalization program for further evaluation and treatment.
[polldaddy:9837962]
EVALUATION Continued stealing
A week later, Ms. B remains reluctant to talk about her stealing habit. However, once a therapeutic alliance is established, she reveals that she experiences increased anxiety before stealing and feels pleasure during the theft. Her methylphenidate ER dosage is increased to 54 mg/d in an attempt to address poor impulse control and subsequent stealing behavior. Her ADHD symptoms are controlled, and she does not exhibit poor impulse control in any situation other than stealing.
However, Ms. B continues to have poor insight and impaired judgment about her behavior. During treatment, Ms. B steals markers from the psychiatrist’s office, which later are found in her bag. When the staff convinces Ms. B to return the markers to the psychiatrist, she denies knowing how they got there. Behavioral interventions, including covert sensitization, systemic desensitization, positive reinforcement, body and bag search, and reminders, occur consistently as part of treatment, but have little effect on her symptoms.
The author’s observations
Risk-taking and novelty-seeking behaviors are common in adolescent patients. Impulsivity, instant reward-seeking behavior, and poor judgment can lead to stealing in this population, but this behavior is not necessarily indicative of kleptomania.
Kleptomania is the recurrent failure to resist impulses to steal objects.2 It differs from other forms of stealing in that the objects stolen by a patient with kleptomania are not needed for personal use or for their monetary value. Kleptomania usually begins in early adolescence, is found in about 0.5% of the general population, and is more common among females.3
There are 2 important theories to explain kleptomania:
- The psychoanalytical theory explains kleptomania as an immature defense against unconscious impulses, conflicts, and desires of destruction. By stealing, the individual protects the self from narcissistic injury and disintegration. The frantic search for objects helps to divert self-destructive aggressiveness and allows for the preservation of the self.4
- The biological model indicates that individuals with kleptomania have a significant deficit of white matter in inferior frontal regions and poor integrity of the tracts connecting the limbic system to the thalamus and to the prefrontal cortex.5 Reward system circuitry (ventral tegmental area–nucleus accumbens–orbital frontal cortex) is likely to be involved in impulse control disorders including kleptomania.6
Comorbidity. Kleptomania often is comorbid with substance use disorder (SUD), obsessive-compulsive disorder (OCD), and compulsive shopping, as well as depression, anxiety disorders, bulimia nervosa, and impulse control and conduct disorders.3,6
Kleptomania shares many characteristics with SUD, including continued engagement in a behavior despite negative consequences and the temporary reduction in urges after the behavior’s completion, followed by a return of the urge to steal. There also is a bidirectional relationship between OCD and kleptomania. Individuals with both disorders frequently engage in excessive and unnecessary rituals even when it is ego-dystonic. First-degree relatives of kleptomania patients have high rates of SUD and OCD.3
Serotonin, dopamine, and opioid pathways play a role in both kleptomania and other behavioral addictions.6 Clinicians should be cautious in treating comorbid disorders with stimulants. These agents may help patients with high impulsivity, but lead to disinhibition and worsen impulse control in patients with low impulsivity.7
TREATMENT Naltrexone
The psychiatrist discusses pharmacologic options to treat kleptomania with Ms. B and her mother. After considering the risks, benefits, adverse effects, and alternative treatments (including the option of no pharmacologic treatment), the mother consents and Ms. B assents to treatment with naltrexone, 25 mg/d. Before starting this medication, both the mother and Ms. B receive detailed psychoeducation describing naltrexone’s interactions with opioids. They are told that if Ms. B has a traumatic injury, they should inform the treatment team that she is taking naltrexone, which can acutely precipitate opiate withdrawal.
Before initiating pharmacotherapy, a comprehensive metabolic profile is obtained, and all values are within the normal range. After 1 week, naltrexone is increased to 50 mg/d. The medication is well tolerated, without any adverse effects.
[polldaddy:9837976]
The author’s observations
Behavioral interventions, such as covert sensitization and systemic desensitization, often are used to treat kleptomania.8 There are no FDA-approved medications for this condition. Opioid antagonists have been considered for the treatment of kleptomania.7
Mu-opioid receptors exist in highest concentrations in presynaptic neurons in the periaqueductal gray region and spinal cord and have high affinity for enkephalins and beta-endorphins. They also are involved in the reward and pleasure pathway. This neurocircuit is implicated in behavioral addiction.9
Naltrexone is an antagonist at μ-opioid receptors. It blocks the binding of endogenous and exogenous opioids at the receptors, particularly at the ventral tegmental area. By blocking the μ-receptor, naltrexone inhibits the processing of the reward and pleasure pathway involved in kleptomania. Naltrexone binds to these receptors, preventing the euphoric effects of behavioral addictions.10 This medication works best in conjunction with behavioral interventions.8
Naltrexone is a Schedule II drug. Use of naltrexone to treat kleptomania or other impulse control disorders is an off-label use of the medication. Naltrexone should not be prescribed to patients who are receiving opiates because it can cause acute opiate withdrawal.
Liver function tests should be monitored in all patients taking naltrexone. If liver function levels begin to rise, naltrexone should be discontinued. Naltrexone should be used with caution in patients with preexisting liver disease.11
OUTCOME Marked improvement
Ms. B’s K-SAS scores are evaluated 2 weeks after starting naltrexone. The results show a marked reduction in the urge to steal and in stealing behavior, and Ms. B’s mother reports no incidents of stealing in the previous week.
Ms. B is maintained on naltrexone, 50 mg/d, for 2 months. On repeated K-SAS scores, her mother rates Ms. B’s symptoms “very much improved” with “occasional” stealing. Ms. B is discharged from the intensive outpatient program.
1. Christianini AR, Conti MA, Hearst N, et al. Treating kleptomania: cross-cultural adaptation of the Kleptomania Symptom Assessment Scale and assessment of an outpatient program. Compr Psychiatry. 2015;56:289-294.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Talih FR. Kleptomania and potential exacerbating factors: a review and case report. Innov Clin Neurosci. 2011;8(10):35-39.
4. Cierpka M. Psychodynamics of neurotically-induced kleptomania [in German]. Psychiatr Prax. 1986;13(3):94-103.
5. Grant JE, Correia S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Res. 2006;147(2-3):233-237.
6. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
7. Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660-671.
8. Grant JE. Understanding and treating kleptomania: new models and new treatments. Isr J Psychiatry Relat Sci. 2006;43(2):81-87.
9. Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101(suppl 1):142-151.
10. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry. 2002;63(4):349-356.
11. Pfohl DN, Allen JI, Atkinson RL, et al. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66-72.
CASE A lifelong habit
Ms. B, age 14, has diagnoses of attention-deficit/hyperactive disorder (ADHD) and oppositional defiant disorder, and is taking extended-release (ER) methylphenidate, 36 mg/d. Her mother brings her to the hospital with concerns that Ms. B has been stealing small objects, such as money, toys, and pencils from home, school, and her peers, even though she does not need them and her family can afford to buy them for her. Ms. B’s mother routinely searches her daughter when she leaves the house and when she returns and frequently finds things in Ms. B’s possession that do not belong to her.
The mother reports that Ms. B’s stealing has been a lifelong habit that worsened after Ms. B’s father died in a car accident last year.
Ms. B does not volunteer any information about her stealing. She is admitted to a partial hospitalization program for further evaluation and treatment.
[polldaddy:9837962]
EVALUATION Continued stealing
A week later, Ms. B remains reluctant to talk about her stealing habit. However, once a therapeutic alliance is established, she reveals that she experiences increased anxiety before stealing and feels pleasure during the theft. Her methylphenidate ER dosage is increased to 54 mg/d in an attempt to address poor impulse control and subsequent stealing behavior. Her ADHD symptoms are controlled, and she does not exhibit poor impulse control in any situation other than stealing.
However, Ms. B continues to have poor insight and impaired judgment about her behavior. During treatment, Ms. B steals markers from the psychiatrist’s office, which later are found in her bag. When the staff convinces Ms. B to return the markers to the psychiatrist, she denies knowing how they got there. Behavioral interventions, including covert sensitization, systemic desensitization, positive reinforcement, body and bag search, and reminders, occur consistently as part of treatment, but have little effect on her symptoms.
The author’s observations
Risk-taking and novelty-seeking behaviors are common in adolescent patients. Impulsivity, instant reward-seeking behavior, and poor judgment can lead to stealing in this population, but this behavior is not necessarily indicative of kleptomania.
Kleptomania is the recurrent failure to resist impulses to steal objects.2 It differs from other forms of stealing in that the objects stolen by a patient with kleptomania are not needed for personal use or for their monetary value. Kleptomania usually begins in early adolescence, is found in about 0.5% of the general population, and is more common among females.3
There are 2 important theories to explain kleptomania:
- The psychoanalytical theory explains kleptomania as an immature defense against unconscious impulses, conflicts, and desires of destruction. By stealing, the individual protects the self from narcissistic injury and disintegration. The frantic search for objects helps to divert self-destructive aggressiveness and allows for the preservation of the self.4
- The biological model indicates that individuals with kleptomania have a significant deficit of white matter in inferior frontal regions and poor integrity of the tracts connecting the limbic system to the thalamus and to the prefrontal cortex.5 Reward system circuitry (ventral tegmental area–nucleus accumbens–orbital frontal cortex) is likely to be involved in impulse control disorders including kleptomania.6
Comorbidity. Kleptomania often is comorbid with substance use disorder (SUD), obsessive-compulsive disorder (OCD), and compulsive shopping, as well as depression, anxiety disorders, bulimia nervosa, and impulse control and conduct disorders.3,6
Kleptomania shares many characteristics with SUD, including continued engagement in a behavior despite negative consequences and the temporary reduction in urges after the behavior’s completion, followed by a return of the urge to steal. There also is a bidirectional relationship between OCD and kleptomania. Individuals with both disorders frequently engage in excessive and unnecessary rituals even when it is ego-dystonic. First-degree relatives of kleptomania patients have high rates of SUD and OCD.3
Serotonin, dopamine, and opioid pathways play a role in both kleptomania and other behavioral addictions.6 Clinicians should be cautious in treating comorbid disorders with stimulants. These agents may help patients with high impulsivity, but lead to disinhibition and worsen impulse control in patients with low impulsivity.7
TREATMENT Naltrexone
The psychiatrist discusses pharmacologic options to treat kleptomania with Ms. B and her mother. After considering the risks, benefits, adverse effects, and alternative treatments (including the option of no pharmacologic treatment), the mother consents and Ms. B assents to treatment with naltrexone, 25 mg/d. Before starting this medication, both the mother and Ms. B receive detailed psychoeducation describing naltrexone’s interactions with opioids. They are told that if Ms. B has a traumatic injury, they should inform the treatment team that she is taking naltrexone, which can acutely precipitate opiate withdrawal.
Before initiating pharmacotherapy, a comprehensive metabolic profile is obtained, and all values are within the normal range. After 1 week, naltrexone is increased to 50 mg/d. The medication is well tolerated, without any adverse effects.
[polldaddy:9837976]
The author’s observations
Behavioral interventions, such as covert sensitization and systemic desensitization, often are used to treat kleptomania.8 There are no FDA-approved medications for this condition. Opioid antagonists have been considered for the treatment of kleptomania.7
Mu-opioid receptors exist in highest concentrations in presynaptic neurons in the periaqueductal gray region and spinal cord and have high affinity for enkephalins and beta-endorphins. They also are involved in the reward and pleasure pathway. This neurocircuit is implicated in behavioral addiction.9
Naltrexone is an antagonist at μ-opioid receptors. It blocks the binding of endogenous and exogenous opioids at the receptors, particularly at the ventral tegmental area. By blocking the μ-receptor, naltrexone inhibits the processing of the reward and pleasure pathway involved in kleptomania. Naltrexone binds to these receptors, preventing the euphoric effects of behavioral addictions.10 This medication works best in conjunction with behavioral interventions.8
Naltrexone is a Schedule II drug. Use of naltrexone to treat kleptomania or other impulse control disorders is an off-label use of the medication. Naltrexone should not be prescribed to patients who are receiving opiates because it can cause acute opiate withdrawal.
Liver function tests should be monitored in all patients taking naltrexone. If liver function levels begin to rise, naltrexone should be discontinued. Naltrexone should be used with caution in patients with preexisting liver disease.11
OUTCOME Marked improvement
Ms. B’s K-SAS scores are evaluated 2 weeks after starting naltrexone. The results show a marked reduction in the urge to steal and in stealing behavior, and Ms. B’s mother reports no incidents of stealing in the previous week.
Ms. B is maintained on naltrexone, 50 mg/d, for 2 months. On repeated K-SAS scores, her mother rates Ms. B’s symptoms “very much improved” with “occasional” stealing. Ms. B is discharged from the intensive outpatient program.
CASE A lifelong habit
Ms. B, age 14, has diagnoses of attention-deficit/hyperactive disorder (ADHD) and oppositional defiant disorder, and is taking extended-release (ER) methylphenidate, 36 mg/d. Her mother brings her to the hospital with concerns that Ms. B has been stealing small objects, such as money, toys, and pencils from home, school, and her peers, even though she does not need them and her family can afford to buy them for her. Ms. B’s mother routinely searches her daughter when she leaves the house and when she returns and frequently finds things in Ms. B’s possession that do not belong to her.
The mother reports that Ms. B’s stealing has been a lifelong habit that worsened after Ms. B’s father died in a car accident last year.
Ms. B does not volunteer any information about her stealing. She is admitted to a partial hospitalization program for further evaluation and treatment.
[polldaddy:9837962]
EVALUATION Continued stealing
A week later, Ms. B remains reluctant to talk about her stealing habit. However, once a therapeutic alliance is established, she reveals that she experiences increased anxiety before stealing and feels pleasure during the theft. Her methylphenidate ER dosage is increased to 54 mg/d in an attempt to address poor impulse control and subsequent stealing behavior. Her ADHD symptoms are controlled, and she does not exhibit poor impulse control in any situation other than stealing.
However, Ms. B continues to have poor insight and impaired judgment about her behavior. During treatment, Ms. B steals markers from the psychiatrist’s office, which later are found in her bag. When the staff convinces Ms. B to return the markers to the psychiatrist, she denies knowing how they got there. Behavioral interventions, including covert sensitization, systemic desensitization, positive reinforcement, body and bag search, and reminders, occur consistently as part of treatment, but have little effect on her symptoms.
The author’s observations
Risk-taking and novelty-seeking behaviors are common in adolescent patients. Impulsivity, instant reward-seeking behavior, and poor judgment can lead to stealing in this population, but this behavior is not necessarily indicative of kleptomania.
Kleptomania is the recurrent failure to resist impulses to steal objects.2 It differs from other forms of stealing in that the objects stolen by a patient with kleptomania are not needed for personal use or for their monetary value. Kleptomania usually begins in early adolescence, is found in about 0.5% of the general population, and is more common among females.3
There are 2 important theories to explain kleptomania:
- The psychoanalytical theory explains kleptomania as an immature defense against unconscious impulses, conflicts, and desires of destruction. By stealing, the individual protects the self from narcissistic injury and disintegration. The frantic search for objects helps to divert self-destructive aggressiveness and allows for the preservation of the self.4
- The biological model indicates that individuals with kleptomania have a significant deficit of white matter in inferior frontal regions and poor integrity of the tracts connecting the limbic system to the thalamus and to the prefrontal cortex.5 Reward system circuitry (ventral tegmental area–nucleus accumbens–orbital frontal cortex) is likely to be involved in impulse control disorders including kleptomania.6
Comorbidity. Kleptomania often is comorbid with substance use disorder (SUD), obsessive-compulsive disorder (OCD), and compulsive shopping, as well as depression, anxiety disorders, bulimia nervosa, and impulse control and conduct disorders.3,6
Kleptomania shares many characteristics with SUD, including continued engagement in a behavior despite negative consequences and the temporary reduction in urges after the behavior’s completion, followed by a return of the urge to steal. There also is a bidirectional relationship between OCD and kleptomania. Individuals with both disorders frequently engage in excessive and unnecessary rituals even when it is ego-dystonic. First-degree relatives of kleptomania patients have high rates of SUD and OCD.3
Serotonin, dopamine, and opioid pathways play a role in both kleptomania and other behavioral addictions.6 Clinicians should be cautious in treating comorbid disorders with stimulants. These agents may help patients with high impulsivity, but lead to disinhibition and worsen impulse control in patients with low impulsivity.7
TREATMENT Naltrexone
The psychiatrist discusses pharmacologic options to treat kleptomania with Ms. B and her mother. After considering the risks, benefits, adverse effects, and alternative treatments (including the option of no pharmacologic treatment), the mother consents and Ms. B assents to treatment with naltrexone, 25 mg/d. Before starting this medication, both the mother and Ms. B receive detailed psychoeducation describing naltrexone’s interactions with opioids. They are told that if Ms. B has a traumatic injury, they should inform the treatment team that she is taking naltrexone, which can acutely precipitate opiate withdrawal.
Before initiating pharmacotherapy, a comprehensive metabolic profile is obtained, and all values are within the normal range. After 1 week, naltrexone is increased to 50 mg/d. The medication is well tolerated, without any adverse effects.
[polldaddy:9837976]
The author’s observations
Behavioral interventions, such as covert sensitization and systemic desensitization, often are used to treat kleptomania.8 There are no FDA-approved medications for this condition. Opioid antagonists have been considered for the treatment of kleptomania.7
Mu-opioid receptors exist in highest concentrations in presynaptic neurons in the periaqueductal gray region and spinal cord and have high affinity for enkephalins and beta-endorphins. They also are involved in the reward and pleasure pathway. This neurocircuit is implicated in behavioral addiction.9
Naltrexone is an antagonist at μ-opioid receptors. It blocks the binding of endogenous and exogenous opioids at the receptors, particularly at the ventral tegmental area. By blocking the μ-receptor, naltrexone inhibits the processing of the reward and pleasure pathway involved in kleptomania. Naltrexone binds to these receptors, preventing the euphoric effects of behavioral addictions.10 This medication works best in conjunction with behavioral interventions.8
Naltrexone is a Schedule II drug. Use of naltrexone to treat kleptomania or other impulse control disorders is an off-label use of the medication. Naltrexone should not be prescribed to patients who are receiving opiates because it can cause acute opiate withdrawal.
Liver function tests should be monitored in all patients taking naltrexone. If liver function levels begin to rise, naltrexone should be discontinued. Naltrexone should be used with caution in patients with preexisting liver disease.11
OUTCOME Marked improvement
Ms. B’s K-SAS scores are evaluated 2 weeks after starting naltrexone. The results show a marked reduction in the urge to steal and in stealing behavior, and Ms. B’s mother reports no incidents of stealing in the previous week.
Ms. B is maintained on naltrexone, 50 mg/d, for 2 months. On repeated K-SAS scores, her mother rates Ms. B’s symptoms “very much improved” with “occasional” stealing. Ms. B is discharged from the intensive outpatient program.
1. Christianini AR, Conti MA, Hearst N, et al. Treating kleptomania: cross-cultural adaptation of the Kleptomania Symptom Assessment Scale and assessment of an outpatient program. Compr Psychiatry. 2015;56:289-294.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Talih FR. Kleptomania and potential exacerbating factors: a review and case report. Innov Clin Neurosci. 2011;8(10):35-39.
4. Cierpka M. Psychodynamics of neurotically-induced kleptomania [in German]. Psychiatr Prax. 1986;13(3):94-103.
5. Grant JE, Correia S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Res. 2006;147(2-3):233-237.
6. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
7. Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660-671.
8. Grant JE. Understanding and treating kleptomania: new models and new treatments. Isr J Psychiatry Relat Sci. 2006;43(2):81-87.
9. Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101(suppl 1):142-151.
10. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry. 2002;63(4):349-356.
11. Pfohl DN, Allen JI, Atkinson RL, et al. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66-72.
1. Christianini AR, Conti MA, Hearst N, et al. Treating kleptomania: cross-cultural adaptation of the Kleptomania Symptom Assessment Scale and assessment of an outpatient program. Compr Psychiatry. 2015;56:289-294.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Talih FR. Kleptomania and potential exacerbating factors: a review and case report. Innov Clin Neurosci. 2011;8(10):35-39.
4. Cierpka M. Psychodynamics of neurotically-induced kleptomania [in German]. Psychiatr Prax. 1986;13(3):94-103.
5. Grant JE, Correia S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Res. 2006;147(2-3):233-237.
6. Grant JE, Odlaug BL, Kim SW. Kleptomania: clinical characteristics and relationship to substance use disorders. Am J Drug Alcohol Abuse. 2010;36(5):291-295.
7. Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660-671.
8. Grant JE. Understanding and treating kleptomania: new models and new treatments. Isr J Psychiatry Relat Sci. 2006;43(2):81-87.
9. Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101(suppl 1):142-151.
10. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry. 2002;63(4):349-356.
11. Pfohl DN, Allen JI, Atkinson RL, et al. Naltrexone hydrochloride (Trexan): a review of serum transaminase elevations at high dosage. NIDA Res Monogr. 1986;67:66-72.
Suspicious, sleepless, and smoking
CASE Sleepless, hallucinating
Mr. F, age 30, is brought to the emergency department (ED) by his brother, with whom he has been living for the last 2 days; his brother says that Mr. F’s wife is afraid of her husband and concerned about her children’s safety. Mr. F has been talking to himself, saying “odd things,” and has an unpredictable temper. He claims that his long-deceased father is alive and telling him “to move to a land that he brought [sic] for him.” In order to follow his father’s instructions, Mr. F says he wants to “see the ambassador so he can get his passport ready.” He also believes his wife and children are intruders in his home. Although he had never smoked before, Mr. F has started smoking ≥2 packs of cigarettes per day, sometimes smoking a pack in 30 minutes. He has not eaten or slept for the last 2 days and lies awake in bed all night staring at the ceiling and smiling to himself.
On examination, Mr. F is short with a slight build and has large, dark eyes, disheveled, short, brown hair, and a scraggly beard. English is not his first language, and he speaks with a thick Eastern European accent. His speech is latent, monotonous, tangential, and illogical. He is alert, oriented only to his person, and says he is 21 or 27 years old and at the hospital for “smoking medication and that’s it.” Despite immigrating to the United States 8 years ago, Mr. F claims he has spent his whole life “here,” although he is unsure of exactly where that is. Cognition and memory are impaired. Regarding his wife and 5 children, he says, “I am a virgin. How then can I have children? That woman is abusing me by forcefully entering my house with 5 kids.” He is fidgety, appears anxious, and does not make eye contact with the examiner during the interview. He is suspicious and irritable. Initial medical workup in the ED is negative.
[polldaddy:9813268]
EVALUATION Labs and observation
Because Mr. F had delusions and hallucinations for the past 2 days and the initial medical workup was negative, brief psychotic disorder is suspected.1 He is admitted to a secure psychiatric floor for further evaluation. He has no documented medical history. A thorough medical workup for a cause of his hallucinations and delusions, including EEG and brain MRI, is negative. Additional collateral interviews with Mr. F’s wife and brother at a family meeting indicate Mr. F had a slow onset of symptoms that began 4 to 5 years ago. Initially, he became isolated, withdrawn, inactive, and had poor sleep. Recently, he also had become suspicious, irritable, delusional, and hallucinatory. Mr. F used to work full-time in construction, then began working intermittently in a warehouse as a day laborer, but has not worked for the last few months. He used to be an involved father and reliable partner, helping with household chores and caring for the children. However, for the last few months, he had become increasingly apathetic and isolated.
During the comprehensive workup for psychosis, Mr. F’s symptoms continue. He is disoriented; although it is 2015, he states it is “2007… I carry a cell phone so I don’t need to know.” On July 31, he is told the date, and for several days after that, he states that it is July 31. When asked his birth date, he looks at his hospital wrist ID. His affect is flat, but he states he feels “fine” and smiles at inappropriate times. He answers open-ended questions briefly, with irrelevant or illogical answers after long pauses, or not at all. His eye contact is poor; he seems preoccupied with internal stimuli, and it is difficult to keep his attention.
Mr. F says he is a “natural-born Bosnian gypsy translator,” and that he needs to finish “building the warehouse” with his father and grandfather (both are deceased). The nurses note that he is withdrawn, inactive, and suspicious; he spends most of the day lying in bed awake, and in the evening he paces in the hallway. Mr. F does not interact with other patients, is guarded when questioned, and does not eat much. He has minimal insight into his condition and says that he is at the hospital for “fevers and a cold,” “ESL treatment,” or because his “right side is thicker” than his left. It is unclear what Mr. F means by “ESL.” It may refer to English as a Second Language, given his apparent perseveration regarding his immigration status and language ability, but this is speculation.
[polldaddy:9813271]
TREATMENT Residual symptoms
With the additional collateral history and a negative medical workup, Mr. F meets DSM-5 criteria for acute, first-episode schizophrenia1 and is started on risperidone, 2 mg/d, titrated up to 2 mg twice daily, and trazodone, 50 mg, as needed, as a sleep aid. He shows significant improvement in his symptoms early in his treatment course. During visiting hours and at family meetings, he recognizes his wife, and during interviews he denies any continuing hallucinations. He initially says that he never failed to recognize his wife and kids, but later explains that he “woke up different…from a dream, and she was a different woman.” When asked specifically about hearing his father’s voice, he is uncertain, saying “No,” “I don’t know,” “I didn’t hear,” or “Not anymore.”
Despite his improvement, Mr. F continues to be disoriented and suspicious, and has minimal insight into his illness. He also continues to exhibit significant negative symptoms and cognitive impairment. Mr. F is withdrawn and has a flat affect, poverty of speech, delayed processing, and poor focus and attention.
On hospital Day 6, Mr. F reports feeling depressed. He misses his children and wants to go home. He has lost several pounds because he had a poor appetite and is now underweight. He is apathetic; interactions with staff and patients are minimal, he declines to attend group therapy sessions, and he still spends most of his time lying in bed awake or pacing the hallway. He also expresses a desire to quit smoking.
[polldaddy:9813273]
The authors’ observations
Despite its lack of specific inclusion in the DSM-5 criteria,1 cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. As demonstrated by Mr. F’s case, the severity of cognitive impairment in schizophrenia has no association with the positive symptoms of schizophrenia; it is a patient’s neurocognitive abilities—not the severity of his (her) psychotic symptoms—that most strongly predict functional outcomes.2
Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia.3,4 Treatment of the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.2 Effective drug therapy regimens are still being developed, and although there are some promising novel targets, no drug is FDA-approved to treat the cognitive symptoms of schizophrenia.2,4 However, it is known that additional treatment modalities, including social skills training and/or vocational rehabilitation, as well as treatment of comorbid conditions, may lead to improved cognitive status and, as a result, improved functional outcomes in schizophrenia.2-4
It is well documented that persons with schizophrenia in households with high expressed emotion (EE) have higher rates of relapse, independent of demographics and pharmacotherapy.5 EE is a measure of the family environment that evaluates how the relatives of a psychiatric patient spontaneously talk about the patient. Relatives are considered to have high EE if they show hostility or marked emotional overinvolvement, or if they make a certain number of critical comments. The tool used to measure EE is the Camberwell Family Interview Schedule.6,7 Rates of first-year relapse in high EE homes when family treatment is employed drop significantly, especially when combined with social skills training.8 The patient’s family members are educated about EE and its potential negative effects on the patient.
Cognitive remediation therapy (CRT) uses therapist-led, computer-based techniques to preserve intact neuroplasticity and has been shown to improve cognition and functional status, especially when paired with vocational rehabilitation or social skills training.2,3 Many trials confirm that CRT produces meaningful, durable improvements in cognition and functioning.3 One systematic review that focused on trials in early schizophrenia found that CRT had a significant effect on functioning and symptoms, and that these effects were larger when CRT was combined with adjunctive psychiatric rehabilitation and small group interventions.3
OUTCOME Gradual improvement
Mr. F is started on nicotine gum, 2 mg/d, for smoking cessation and fluoxetine, 20 mg/d, for depression, and a dietary consult is made for his poor appetite and weight loss. His psychotic symptoms continue to improve, and by hospital Day 10, his depressive symptoms begin to improve as well: his affect brightens, he has increased appetite, and he wants to shave. He also exhibits mildly increased insight into his illness.
Mr. F is discharged with risperidone, 2 mg twice daily, for schizophrenia, fluoxetine, 20 mg/d, for depression, and trazodone, 50 mg, as needed, for sleep, and is referred to a community mental health center for comprehensive follow-up, including vocational rehabilitation and social skills training.
The authors’ observations
A major goal of the National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was to develop a consensus cognitive battery for clinical trials of cognition-enhancing treatments for schizophrenia. The MATRICS Consensus Cognitive Battery (MCCB) is a comprehensive cognitive assessment designed for use in patients with schizophrenia (Table 39). Although the MCCB was developed to be the standard tool for assessing cognitive change in clinical trials of cognition-enhancing drugs for schizophrenia, it also may aid evaluation of cognitive remediation strategies.9
In Mr. F’s case, such testing was not performed, in part because of his improvement. The MoCA was chosen because it is a universally accepted brief cognitive assessment tool used for screening. More robust testing can be administered by the neuropsychiatry team if indicated and if resources are available.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Nasrallah HA, Keefe RS, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(6):S1-S11.
3. Revell ER, Neill JC, Harte M, et al. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168(1-2):213-222.
4. Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
5. Bebbington P, Kuipers L. The predictive utility of expressed emotion in schizophrenia: an aggregate analysis. Psychol Med. 1994;24(3):707-718.
6. Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry. 1998;55(6):547-552.
7. Vaughn C, Leff J. The measurement of expressed emotion in the families of psychiatric patients. Br J Soc Clin Psychol. 1976;15(2):157-165.
8. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. One-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
9. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213.
CASE Sleepless, hallucinating
Mr. F, age 30, is brought to the emergency department (ED) by his brother, with whom he has been living for the last 2 days; his brother says that Mr. F’s wife is afraid of her husband and concerned about her children’s safety. Mr. F has been talking to himself, saying “odd things,” and has an unpredictable temper. He claims that his long-deceased father is alive and telling him “to move to a land that he brought [sic] for him.” In order to follow his father’s instructions, Mr. F says he wants to “see the ambassador so he can get his passport ready.” He also believes his wife and children are intruders in his home. Although he had never smoked before, Mr. F has started smoking ≥2 packs of cigarettes per day, sometimes smoking a pack in 30 minutes. He has not eaten or slept for the last 2 days and lies awake in bed all night staring at the ceiling and smiling to himself.
On examination, Mr. F is short with a slight build and has large, dark eyes, disheveled, short, brown hair, and a scraggly beard. English is not his first language, and he speaks with a thick Eastern European accent. His speech is latent, monotonous, tangential, and illogical. He is alert, oriented only to his person, and says he is 21 or 27 years old and at the hospital for “smoking medication and that’s it.” Despite immigrating to the United States 8 years ago, Mr. F claims he has spent his whole life “here,” although he is unsure of exactly where that is. Cognition and memory are impaired. Regarding his wife and 5 children, he says, “I am a virgin. How then can I have children? That woman is abusing me by forcefully entering my house with 5 kids.” He is fidgety, appears anxious, and does not make eye contact with the examiner during the interview. He is suspicious and irritable. Initial medical workup in the ED is negative.
[polldaddy:9813268]
EVALUATION Labs and observation
Because Mr. F had delusions and hallucinations for the past 2 days and the initial medical workup was negative, brief psychotic disorder is suspected.1 He is admitted to a secure psychiatric floor for further evaluation. He has no documented medical history. A thorough medical workup for a cause of his hallucinations and delusions, including EEG and brain MRI, is negative. Additional collateral interviews with Mr. F’s wife and brother at a family meeting indicate Mr. F had a slow onset of symptoms that began 4 to 5 years ago. Initially, he became isolated, withdrawn, inactive, and had poor sleep. Recently, he also had become suspicious, irritable, delusional, and hallucinatory. Mr. F used to work full-time in construction, then began working intermittently in a warehouse as a day laborer, but has not worked for the last few months. He used to be an involved father and reliable partner, helping with household chores and caring for the children. However, for the last few months, he had become increasingly apathetic and isolated.
During the comprehensive workup for psychosis, Mr. F’s symptoms continue. He is disoriented; although it is 2015, he states it is “2007… I carry a cell phone so I don’t need to know.” On July 31, he is told the date, and for several days after that, he states that it is July 31. When asked his birth date, he looks at his hospital wrist ID. His affect is flat, but he states he feels “fine” and smiles at inappropriate times. He answers open-ended questions briefly, with irrelevant or illogical answers after long pauses, or not at all. His eye contact is poor; he seems preoccupied with internal stimuli, and it is difficult to keep his attention.
Mr. F says he is a “natural-born Bosnian gypsy translator,” and that he needs to finish “building the warehouse” with his father and grandfather (both are deceased). The nurses note that he is withdrawn, inactive, and suspicious; he spends most of the day lying in bed awake, and in the evening he paces in the hallway. Mr. F does not interact with other patients, is guarded when questioned, and does not eat much. He has minimal insight into his condition and says that he is at the hospital for “fevers and a cold,” “ESL treatment,” or because his “right side is thicker” than his left. It is unclear what Mr. F means by “ESL.” It may refer to English as a Second Language, given his apparent perseveration regarding his immigration status and language ability, but this is speculation.
[polldaddy:9813271]
TREATMENT Residual symptoms
With the additional collateral history and a negative medical workup, Mr. F meets DSM-5 criteria for acute, first-episode schizophrenia1 and is started on risperidone, 2 mg/d, titrated up to 2 mg twice daily, and trazodone, 50 mg, as needed, as a sleep aid. He shows significant improvement in his symptoms early in his treatment course. During visiting hours and at family meetings, he recognizes his wife, and during interviews he denies any continuing hallucinations. He initially says that he never failed to recognize his wife and kids, but later explains that he “woke up different…from a dream, and she was a different woman.” When asked specifically about hearing his father’s voice, he is uncertain, saying “No,” “I don’t know,” “I didn’t hear,” or “Not anymore.”
Despite his improvement, Mr. F continues to be disoriented and suspicious, and has minimal insight into his illness. He also continues to exhibit significant negative symptoms and cognitive impairment. Mr. F is withdrawn and has a flat affect, poverty of speech, delayed processing, and poor focus and attention.
On hospital Day 6, Mr. F reports feeling depressed. He misses his children and wants to go home. He has lost several pounds because he had a poor appetite and is now underweight. He is apathetic; interactions with staff and patients are minimal, he declines to attend group therapy sessions, and he still spends most of his time lying in bed awake or pacing the hallway. He also expresses a desire to quit smoking.
[polldaddy:9813273]
The authors’ observations
Despite its lack of specific inclusion in the DSM-5 criteria,1 cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. As demonstrated by Mr. F’s case, the severity of cognitive impairment in schizophrenia has no association with the positive symptoms of schizophrenia; it is a patient’s neurocognitive abilities—not the severity of his (her) psychotic symptoms—that most strongly predict functional outcomes.2
Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia.3,4 Treatment of the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.2 Effective drug therapy regimens are still being developed, and although there are some promising novel targets, no drug is FDA-approved to treat the cognitive symptoms of schizophrenia.2,4 However, it is known that additional treatment modalities, including social skills training and/or vocational rehabilitation, as well as treatment of comorbid conditions, may lead to improved cognitive status and, as a result, improved functional outcomes in schizophrenia.2-4
It is well documented that persons with schizophrenia in households with high expressed emotion (EE) have higher rates of relapse, independent of demographics and pharmacotherapy.5 EE is a measure of the family environment that evaluates how the relatives of a psychiatric patient spontaneously talk about the patient. Relatives are considered to have high EE if they show hostility or marked emotional overinvolvement, or if they make a certain number of critical comments. The tool used to measure EE is the Camberwell Family Interview Schedule.6,7 Rates of first-year relapse in high EE homes when family treatment is employed drop significantly, especially when combined with social skills training.8 The patient’s family members are educated about EE and its potential negative effects on the patient.
Cognitive remediation therapy (CRT) uses therapist-led, computer-based techniques to preserve intact neuroplasticity and has been shown to improve cognition and functional status, especially when paired with vocational rehabilitation or social skills training.2,3 Many trials confirm that CRT produces meaningful, durable improvements in cognition and functioning.3 One systematic review that focused on trials in early schizophrenia found that CRT had a significant effect on functioning and symptoms, and that these effects were larger when CRT was combined with adjunctive psychiatric rehabilitation and small group interventions.3
OUTCOME Gradual improvement
Mr. F is started on nicotine gum, 2 mg/d, for smoking cessation and fluoxetine, 20 mg/d, for depression, and a dietary consult is made for his poor appetite and weight loss. His psychotic symptoms continue to improve, and by hospital Day 10, his depressive symptoms begin to improve as well: his affect brightens, he has increased appetite, and he wants to shave. He also exhibits mildly increased insight into his illness.
Mr. F is discharged with risperidone, 2 mg twice daily, for schizophrenia, fluoxetine, 20 mg/d, for depression, and trazodone, 50 mg, as needed, for sleep, and is referred to a community mental health center for comprehensive follow-up, including vocational rehabilitation and social skills training.
The authors’ observations
A major goal of the National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was to develop a consensus cognitive battery for clinical trials of cognition-enhancing treatments for schizophrenia. The MATRICS Consensus Cognitive Battery (MCCB) is a comprehensive cognitive assessment designed for use in patients with schizophrenia (Table 39). Although the MCCB was developed to be the standard tool for assessing cognitive change in clinical trials of cognition-enhancing drugs for schizophrenia, it also may aid evaluation of cognitive remediation strategies.9
In Mr. F’s case, such testing was not performed, in part because of his improvement. The MoCA was chosen because it is a universally accepted brief cognitive assessment tool used for screening. More robust testing can be administered by the neuropsychiatry team if indicated and if resources are available.
CASE Sleepless, hallucinating
Mr. F, age 30, is brought to the emergency department (ED) by his brother, with whom he has been living for the last 2 days; his brother says that Mr. F’s wife is afraid of her husband and concerned about her children’s safety. Mr. F has been talking to himself, saying “odd things,” and has an unpredictable temper. He claims that his long-deceased father is alive and telling him “to move to a land that he brought [sic] for him.” In order to follow his father’s instructions, Mr. F says he wants to “see the ambassador so he can get his passport ready.” He also believes his wife and children are intruders in his home. Although he had never smoked before, Mr. F has started smoking ≥2 packs of cigarettes per day, sometimes smoking a pack in 30 minutes. He has not eaten or slept for the last 2 days and lies awake in bed all night staring at the ceiling and smiling to himself.
On examination, Mr. F is short with a slight build and has large, dark eyes, disheveled, short, brown hair, and a scraggly beard. English is not his first language, and he speaks with a thick Eastern European accent. His speech is latent, monotonous, tangential, and illogical. He is alert, oriented only to his person, and says he is 21 or 27 years old and at the hospital for “smoking medication and that’s it.” Despite immigrating to the United States 8 years ago, Mr. F claims he has spent his whole life “here,” although he is unsure of exactly where that is. Cognition and memory are impaired. Regarding his wife and 5 children, he says, “I am a virgin. How then can I have children? That woman is abusing me by forcefully entering my house with 5 kids.” He is fidgety, appears anxious, and does not make eye contact with the examiner during the interview. He is suspicious and irritable. Initial medical workup in the ED is negative.
[polldaddy:9813268]
EVALUATION Labs and observation
Because Mr. F had delusions and hallucinations for the past 2 days and the initial medical workup was negative, brief psychotic disorder is suspected.1 He is admitted to a secure psychiatric floor for further evaluation. He has no documented medical history. A thorough medical workup for a cause of his hallucinations and delusions, including EEG and brain MRI, is negative. Additional collateral interviews with Mr. F’s wife and brother at a family meeting indicate Mr. F had a slow onset of symptoms that began 4 to 5 years ago. Initially, he became isolated, withdrawn, inactive, and had poor sleep. Recently, he also had become suspicious, irritable, delusional, and hallucinatory. Mr. F used to work full-time in construction, then began working intermittently in a warehouse as a day laborer, but has not worked for the last few months. He used to be an involved father and reliable partner, helping with household chores and caring for the children. However, for the last few months, he had become increasingly apathetic and isolated.
During the comprehensive workup for psychosis, Mr. F’s symptoms continue. He is disoriented; although it is 2015, he states it is “2007… I carry a cell phone so I don’t need to know.” On July 31, he is told the date, and for several days after that, he states that it is July 31. When asked his birth date, he looks at his hospital wrist ID. His affect is flat, but he states he feels “fine” and smiles at inappropriate times. He answers open-ended questions briefly, with irrelevant or illogical answers after long pauses, or not at all. His eye contact is poor; he seems preoccupied with internal stimuli, and it is difficult to keep his attention.
Mr. F says he is a “natural-born Bosnian gypsy translator,” and that he needs to finish “building the warehouse” with his father and grandfather (both are deceased). The nurses note that he is withdrawn, inactive, and suspicious; he spends most of the day lying in bed awake, and in the evening he paces in the hallway. Mr. F does not interact with other patients, is guarded when questioned, and does not eat much. He has minimal insight into his condition and says that he is at the hospital for “fevers and a cold,” “ESL treatment,” or because his “right side is thicker” than his left. It is unclear what Mr. F means by “ESL.” It may refer to English as a Second Language, given his apparent perseveration regarding his immigration status and language ability, but this is speculation.
[polldaddy:9813271]
TREATMENT Residual symptoms
With the additional collateral history and a negative medical workup, Mr. F meets DSM-5 criteria for acute, first-episode schizophrenia1 and is started on risperidone, 2 mg/d, titrated up to 2 mg twice daily, and trazodone, 50 mg, as needed, as a sleep aid. He shows significant improvement in his symptoms early in his treatment course. During visiting hours and at family meetings, he recognizes his wife, and during interviews he denies any continuing hallucinations. He initially says that he never failed to recognize his wife and kids, but later explains that he “woke up different…from a dream, and she was a different woman.” When asked specifically about hearing his father’s voice, he is uncertain, saying “No,” “I don’t know,” “I didn’t hear,” or “Not anymore.”
Despite his improvement, Mr. F continues to be disoriented and suspicious, and has minimal insight into his illness. He also continues to exhibit significant negative symptoms and cognitive impairment. Mr. F is withdrawn and has a flat affect, poverty of speech, delayed processing, and poor focus and attention.
On hospital Day 6, Mr. F reports feeling depressed. He misses his children and wants to go home. He has lost several pounds because he had a poor appetite and is now underweight. He is apathetic; interactions with staff and patients are minimal, he declines to attend group therapy sessions, and he still spends most of his time lying in bed awake or pacing the hallway. He also expresses a desire to quit smoking.
[polldaddy:9813273]
The authors’ observations
Despite its lack of specific inclusion in the DSM-5 criteria,1 cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. As demonstrated by Mr. F’s case, the severity of cognitive impairment in schizophrenia has no association with the positive symptoms of schizophrenia; it is a patient’s neurocognitive abilities—not the severity of his (her) psychotic symptoms—that most strongly predict functional outcomes.2
Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia.3,4 Treatment of the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.2 Effective drug therapy regimens are still being developed, and although there are some promising novel targets, no drug is FDA-approved to treat the cognitive symptoms of schizophrenia.2,4 However, it is known that additional treatment modalities, including social skills training and/or vocational rehabilitation, as well as treatment of comorbid conditions, may lead to improved cognitive status and, as a result, improved functional outcomes in schizophrenia.2-4
It is well documented that persons with schizophrenia in households with high expressed emotion (EE) have higher rates of relapse, independent of demographics and pharmacotherapy.5 EE is a measure of the family environment that evaluates how the relatives of a psychiatric patient spontaneously talk about the patient. Relatives are considered to have high EE if they show hostility or marked emotional overinvolvement, or if they make a certain number of critical comments. The tool used to measure EE is the Camberwell Family Interview Schedule.6,7 Rates of first-year relapse in high EE homes when family treatment is employed drop significantly, especially when combined with social skills training.8 The patient’s family members are educated about EE and its potential negative effects on the patient.
Cognitive remediation therapy (CRT) uses therapist-led, computer-based techniques to preserve intact neuroplasticity and has been shown to improve cognition and functional status, especially when paired with vocational rehabilitation or social skills training.2,3 Many trials confirm that CRT produces meaningful, durable improvements in cognition and functioning.3 One systematic review that focused on trials in early schizophrenia found that CRT had a significant effect on functioning and symptoms, and that these effects were larger when CRT was combined with adjunctive psychiatric rehabilitation and small group interventions.3
OUTCOME Gradual improvement
Mr. F is started on nicotine gum, 2 mg/d, for smoking cessation and fluoxetine, 20 mg/d, for depression, and a dietary consult is made for his poor appetite and weight loss. His psychotic symptoms continue to improve, and by hospital Day 10, his depressive symptoms begin to improve as well: his affect brightens, he has increased appetite, and he wants to shave. He also exhibits mildly increased insight into his illness.
Mr. F is discharged with risperidone, 2 mg twice daily, for schizophrenia, fluoxetine, 20 mg/d, for depression, and trazodone, 50 mg, as needed, for sleep, and is referred to a community mental health center for comprehensive follow-up, including vocational rehabilitation and social skills training.
The authors’ observations
A major goal of the National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was to develop a consensus cognitive battery for clinical trials of cognition-enhancing treatments for schizophrenia. The MATRICS Consensus Cognitive Battery (MCCB) is a comprehensive cognitive assessment designed for use in patients with schizophrenia (Table 39). Although the MCCB was developed to be the standard tool for assessing cognitive change in clinical trials of cognition-enhancing drugs for schizophrenia, it also may aid evaluation of cognitive remediation strategies.9
In Mr. F’s case, such testing was not performed, in part because of his improvement. The MoCA was chosen because it is a universally accepted brief cognitive assessment tool used for screening. More robust testing can be administered by the neuropsychiatry team if indicated and if resources are available.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Nasrallah HA, Keefe RS, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(6):S1-S11.
3. Revell ER, Neill JC, Harte M, et al. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168(1-2):213-222.
4. Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
5. Bebbington P, Kuipers L. The predictive utility of expressed emotion in schizophrenia: an aggregate analysis. Psychol Med. 1994;24(3):707-718.
6. Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry. 1998;55(6):547-552.
7. Vaughn C, Leff J. The measurement of expressed emotion in the families of psychiatric patients. Br J Soc Clin Psychol. 1976;15(2):157-165.
8. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. One-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
9. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Nasrallah HA, Keefe RS, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(6):S1-S11.
3. Revell ER, Neill JC, Harte M, et al. A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophr Res. 2015;168(1-2):213-222.
4. Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
5. Bebbington P, Kuipers L. The predictive utility of expressed emotion in schizophrenia: an aggregate analysis. Psychol Med. 1994;24(3):707-718.
6. Butzlaff RL, Hooley JM. Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry. 1998;55(6):547-552.
7. Vaughn C, Leff J. The measurement of expressed emotion in the families of psychiatric patients. Br J Soc Clin Psychol. 1976;15(2):157-165.
8. Hogarty GE, Anderson CM, Reiss DJ, et al. Family psychoeducation, social skills training, and maintenance chemotherapy in the aftercare treatment of schizophrenia. I. One-year effects of a controlled study on relapse and expressed emotion. Arch Gen Psychiatry. 1986;43(7):633-642.
9. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213.
Wearable Health Device Dermatitis: A Case of Acrylate-Related Contact Allergy
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).
Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
Mobile health devices enable patients and clinicians to monitor the type, quantity, and quality of everyday activities and hold the promise of improving patient health and health care practices.1 In 2013, 75% of surveyed consumers in the United States owned a fitness technology product, either a dedicated fitness device, application, or portable blood pressure monitor.2 Ownership of dedicated wearable fitness devices among consumers in the United States increased from 3% in 2012 to 9% in 2013. The immense popularity of wearable fitness devices is evident in the trajectory of their reported sales, which increased from $43 million in 2009 to $854 million in 2013.2 Recognizing that “widespread adoption and use of mobile technologies is opening new and innovative ways to improve health,”3 the US Food and Drug Administration (FDA) ruled that “[technologies] that can pose a greater risk to patients will require FDA review.” One popular class of mobile technologies—activity and sleep sensors—falls outside the FDA’s regulatory guidance. To enable continuous monitoring, these sensors often are embedded into wearable devices.
Reports in the media have documented skin rashes arising in conjunction with use of one type of device,4 which may be related to nickel contact allergy, and the manufacturer has reported that the metal housing consists of surgical stainless steel that is known to contain nickel. We report a complication related to continuous use of an unregulated, commercially available, watchlike wearable sensor that was linked not to nickel but to an acrylate-containing component.
Case Report
An otherwise healthy 52-year-old woman with no history of contact allergy presented with an intensely itchy eruption involving the left wrist arising 4 days after continuous use of a new watchlike wearable fitness sensor. By day 11, the eruption evolved into a well-demarcated, erythematous, scaly plaque at the location where the device’s rechargeable battery metal housing came into contact with skin (Figure 1).

Dimethylglyoxime testing of the metal housing and clips was negative, but testing of contacts within the housing was positive for nickel (Figure 2). Epicutaneous patch testing of the patient using a modified North American Contact Dermatitis Group patch test series (Table) demonstrated no reaction to nickel, instead showing a strong positive (2+) reaction at 48 and 72 hours to methyl methacrylate 2% and a positive (1+) reaction at 96 hours to ethyl acrylate 0.1% (Figure 3).


Comment
Acrylates are used as adhesives to bond metal to plastic and as part of lithium ion polymer batteries, presumably similar to the one used in this device.5 Our patient had a history of using acrylic nail polish, which may have been a source of prior sensitization. Exposure to sweat or other moisture could theoretically dissolve such a water-soluble polymer,6 allowing for skin contact. Other acrylate polymers have been reported to break down slowly in contact with water, leading to contact sensitization to the monomer.7 The manufacturer of the device was contacted for additional information but declined to provide specific details regarding the device’s composition (personal communication, January 2014).
Although not considered toxic,8 acrylate was named Allergen of the Year in 2012 by the American Contact Dermatitis Society.9-11 Nickel might be a source of allergy for some other patients who wear mobile health devices, but we concluded that this particular patient developed allergic contact dermatitis from prolonged exposure to low levels of methyl methacrylate or another acrylate due to gradual breakdown of the acrylate polymer used in the rechargeable battery housing for this wearable health device.
Given the FDA’s tailored risk approach to regulation, many wearable sensors that may contain potential contact allergens such as nickel and acrylates do not fall under the FDA regulatory framework. This case should alert physicians to the lack of regulatory oversight for many mobile technologies. They should consider a screening history for contact allergens before recommending wearable sensors and broader testing for contact allergens should exposed patients develop reactions. Future wearable sensor materials and designs should minimize exposure to allergens given prolonged contact with continuous use. In the absence of regulation, manufacturers of these devices should consider due care testing prior to commercialization.
Acknowledgment
We are indebted to Alexander S. Rattner, PhD (State College, Pennsylvania), who provided his engineering expertise and insight during conversations with the authors.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
- Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798.
- Consumer interest in purchasing wearable fitness devices in 2014 quadruples, according to CEA Study [press release]. Arlington, VA: Consumer Electronics Association; December 11, 2013.
- US Food and Drug Administration. Mobile medical applications. http://www.fda.gov/medicaldevices/digitalhealth/mobilemedicalapplications/default.htm. Updated September 22, 2015. Accessed July 26, 2017.
- Northrup L. Fitbit Force is an amazing device, except for my contact dermatitis. Consumerist website. http://consumerist.com/2014/01/13/fitbit-force-is-an-amazing-device-except-for-my-contact-dermatitis/. Published January 13, 2014. Accessed January 12, 2017.
- Stern B. Inside Fitbit Force. Adafruit website. http://learn.adafruit.com/fitbit-force-teardown/inside-fitbit-force. Published December 11, 2013. Updated May 4, 2015. Accessed January 12, 2017.
- Pemberton MA, Lohmann BS. Risk assessment of residual monomer migrating from acrylic polymers and causing allergic contact dermatitis during normal handling and use. Regul Toxicol Pharmacol. 2014;69:467-475.
- Guin JD, Baas K, Nelson-Adesokan P. Contact sensitization to cyanoacrylate adhesive as a cause of severe onychodystrophy. Int J Dermatol. 1998;37:31-36.
- Zondlo Fiume M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int J Toxicol. 2002;21(suppl 3):1-50.
- Sasseville D. Acrylates. Dermatitis. 2012;23:3-5.
- Bowen C, Bidinger J, Hivnor C, et al. Allergic contact dermatitis to 2-octyl cyanoacrylate. Cutis. 2014;94:183-186.
- Spencer A, Gazzani P, Thompson DA. Acrylate and methacrylate contact allergy and allergic contact disease: a 13-year review [published online July 11, 2016]. Contact Dermatitis. 2016;75:157-164.
Practice Points
- Mobile wearable health devices are likely to become an important potential source of contact sensitization as their use increases given their often prolonged contact time with the skin.
- Mobile wearable health devices may pose a risk for allergic contact dermatitis as a result of a variety of components that come into contact with the skin, including but not limited to metals, rubber components, adhesives, and dyes.