User login
Acute Painful Horner Syndrome as the First Presenting Sign of Carotid Artery Dissection
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
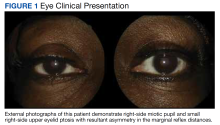
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
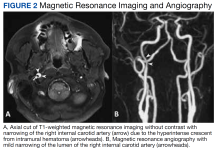
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
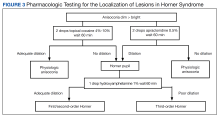
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
Horner syndrome is a rare condition that has no sex or race predilection and is characterized by the clinical triad of a miosis, anhidrosis, and small, unilateral ptosis. The prompt diagnosis and determination of the etiology of Horner syndrome are of utmost importance, as the condition can result from many life-threatening systemic complications. Horner syndrome is often asymptomatic but can have distinct, easily identified characteristics seen with an ophthalmic examination. This report describes a patient who presented with Horner syndrome resulting from an internal carotid artery dissection.
Case Presentation
A 61-year-old woman presented with periorbital pain with onset 3 days prior. The patient described the pain as 7 of 10 that had been worsening and was localized around and behind the right eye. She reported new-onset headaches on the right side over the past week with associated intermittent vision blurriness in the right eye. She had a history of mobility issues and had fallen backward about 1 week before, hitting the back of her head on the floor without direct trauma to the eye. She was symptomatic for light sensitivity, syncope, and dizziness, with reports of a recent history of transient ischemic attacks (TIAs) of unknown etiology, which had occurred in the months preceding her examination. She reported no jaw claudication, scalp tenderness, and neck or shoulder pain. She was unaware of any changes in her perspiration pattern on the right side of her face but mentioned that she had noticed her right upper eyelid drooping while looking in the mirror.
This patient had a routine eye examination 2 months before, which was remarkable for stable, nonfoveal involving adult-onset vitelliform dystrophy in the left eye and nuclear sclerotic cataracts and mild refractive error in both eyes. No iris heterochromia was noted, and her pupils were equal, round, and reactive to light. Her history was remarkable for chest pain, obesity, bipolar disorder, vertigo, transient cerebral ischemia, hypertension, hypercholesterolemia, alcohol use disorder, cocaine use disorder, and asthma. A carotid ultrasound had been performed 1 month before the onset of symptoms due to her history of TIAs, which showed no hemodynamically significant stenosis (> 50% stenosis) of either carotid artery. Her medications included oxybutynin chloride, amlodipine, acetaminophen, sertraline hydrochloride, lidocaine, albuterol, risperidone, hydroxyzine hydrochloride, lisinopril, omeprazole, once-daily baby aspirin, atorvastatin, and calcium.
At the time of presentation, an ophthalmic examination revealed no decrease in visual acuity with a best-corrected visual acuity of 20/20 in the right and left eyes. The patient’s pupil sizes were unequal, with a smaller, more miotic right pupil with a greater difference between the pupil sizes in dim illumination (Figure 1).
As the patient had pathologic miosis, conditions causing pathologic mydriasis, such as Adie tonic pupil and cranial nerve III palsy, were ruled out. The presence of an acute, slight ptosis with pathologic miosis and pain in the ipsilateral eye with no reports of exposure to miotic pharmaceutical agents and no history of trauma to the globe or orbit eliminated other differentials, leading to a diagnosis of right-sided Horner syndrome. Due to concerns of acute onset periorbital and retrobulbar pain, she was referred to the emergency department with recommendations for computed tomography angiography (CTA), magnetic resonance imaging (MRI), and magnetic resonance angiogram (MRA) of the head and neck to rule out a carotid artery dissection.
CTA revealed a focal linear filling defect in the right midinternal carotid artery, likely related to an internal carotid artery vascular flap. There was no evidence of proximal intracranial occlusive disease. MRI revealed a linear area of high-intensity signal projecting over the mid and distal right internal carotid artery lumen (Figure 2A).
Imaging suggested an internal carotid artery dissection, and the patient was admitted to the hospital for observation for 4 days. During this time, the patient was instructed to continue taking 81mg aspirin daily and to begin taking 75 mg clopidogrel bisulfate daily to prevent a cerebrovascular accident. Once stability was established, the patient was discharged with instructions to follow up with neurology and neuro-ophthalmology.
Discussion
Anisocoria is defined as a difference in pupil sizes between the eyes.1 This difference can be physiologic with no underlying pathology as an etiology of the condition. If underlying pathology causes anisocoria, it can result in dysfunction with mydriasis, leading to a more miotic pupil, or it can result from issues with miosis, leading to a more mydriatic pupil.1
To determine whether anisocoria is physiologic or pathologic, one must assess the patient’s pupil sizes in dim and bright illumination. If the difference in the pupil size is the same in both room illuminations (ie, the anisocoria is 2 mm in both bright and dim illumination, pupillary constriction and dilation are functioning normally), then the patient has physiologic anisocoria.1 If anisocoria is different in bright and dim illumination (ie, the anisocoria is 1 mm in bright and 3 mm in dim settings or 3 mm in bright and 1 mm in dim settings), the condition is related to pathology. To determine the underlying pathology of anisocoria in cases that are not physiologic, it is important to first determine whether the anisocoria is related to miotic or mydriatic dysfunction.1
If the anisocoria is greater in dim illumination, this suggests mydriatic dysfunction and could be a result of damage to the sympathetic pupillary pathway.1 The smaller or more miotic pupil in this instance is the pathologic pupil. If the anisocoria is greater in bright illumination, this suggests miotic dysfunction and could be a result of damage to the parasympathetic pathway.1 The larger or more mydriatic pupil in this instance is the pathologic pupil. Congenital abnormalities, such as iris colobomas, aniridia, and ectopic pupils, can result in a wide range of pupil sizes and shapes, including miotic or mydriatic pupils.1
Pathologic Mydriasis
Pathologic mydriatic pupils can result from dysfunction in the parasympathetic nervous system, which results in a pupil that is not sufficiently able to dilate with the removal of a light stimulus. Mydriatic pupils can be related to Adie tonic pupil, Argyll-Robertson pupil, third nerve palsy, trauma, surgeries, or pharmacologic mydriasis.2 The conditions that cause mydriasis can be readily differentiated from one another based on clinical examination.
Adie tonic pupil results from damage to the ciliary ganglion.2 While pupillary constriction in response to light will be absent or sluggish in an Adie pupil, the patient will have an intact but sluggish accommodative pupillary response; therefore, the pupil will still constrict with accommodation and convergence to focus on near objects, although slowly. This is known as light-near dissociation.2
Argyll-Robertson pupils are caused by damage to the Edinger-Westphal nucleus in the rostral midbrain.3 Lesions to this area of the brain are typically associated with neurosyphilis but also can be a result of Lyme disease, multiple sclerosis, encephalitis, neurosarcoidosis, herpes zoster, diabetes mellitus, and chronic alcohol misuse.3 Argyll Robertson pupils can appear very similar to a tonic pupil in that this condition will also have a dilated pupil and light-near dissociation.3 These pupils will differ in that they also tend to have an irregular shape (dyscoria), and the pupils will constrict briskly when focusing on near objects and dilate briskly when focusing on distant objects, not sluggishly, as in Adie tonic pupil.3
Mydriasis due to a third nerve palsy will present with ptosis and extraocular muscle dysfunction (including deficits to the superior rectus, medial rectus, inferior oblique, and inferior rectus), with the classic presentation of a completed palsy with the eye positioned “down and out” or the patient’s inability to look medially and superiorly with the affected eye.2
As in cases of pathologic mydriasis, a thorough and in-depth history can help determine traumatic, surgical and pharmacologic etiologies of a mydriatic pupil. It should be determined whether the patient has had any previous trauma or surgeries to the eye or has been in contact with any of the following: acetylcholine receptor antagonists (atropine, scopolamine, homatropine, cyclopentolate, and tropicamide), motion sickness patches (scopolamine), nasal vasoconstrictors, glycopyrrolate deodorants, and/or various plants (Jimson weed or plants belonging to the digitalis family, such as foxglove).2
Pathologic Miosis
Pathologic miotic pupils can result from dysfunction in the sympathetic nervous system and can be related to blunt or penetrating trauma to the orbit, Horner syndrome, and pharmacologic miosis.2 Horner syndrome will be accompanied by a slight ptosis and sometimes anhidrosis on the ipsilateral side of the face. To differentiate between traumatic and pharmacologic miosis, a detailed history should be obtained, paying close attention to injuries to the eyes or head and/or possible exposure to chemical or pharmaceutical agents, including prostaglandins, pilocarpine, organophosphates, and opiates.2
Horner Syndrome
Horner syndrome is a neurologic condition that results from damage to the oculosympathetic pathway.4 The oculosympathetic pathway is a 3-neuron pathway that begins in the hypothalamus and follows a circuitous route to ultimately innervate the facial sweat glands, the smooth muscles of the blood vessels in the orbit and face, the iris dilator muscle, and the Müller muscles of the superior and inferior eyelids.1,5 Therefore, this pathway’s functions include vasoconstriction of facial blood vessels, facial diaphoresis (sweating), pupillary dilation, and maintaining an open position of the eyelids.1
Oculosympathetic pathway anatomy. To understand the findings associated with Horner syndrome, it is necessary to understand the anatomy of this 3-neuron pathway.5 First-order neurons, or central neurons, arise in the posterolateral aspect of the hypothalamus, where they then descend through the midbrain, pons, medulla, and cervical spinal cord via the intermediolateral gray column.6 The fibers then synapse in the ciliospinal center of Budge at the level of cervical vertebra C8 to thoracic vertebra T2, which give rise to the preganglionic, or second-order neurons.6
Second-order neurons begin at the ciliospinal center of Budge and exit the spinal cord via the central roots, most at the level of thoracic vertebra T1, with the remainder leaving at the levels of cervical vertebra C8 and thoracic vertebra T2.7 After exiting the spinal cord, the second-order neurons loop around the subclavian artery, where they then ascend close to the apex of the lung to synapse with the cell bodies of the third-order neurons at the superior cervical ganglion near cervical vertebrae C2 and C3.7
After arising at the superior cervical ganglion, third-order neurons diverge to follow 2 different courses.7 A portion of the neurons travels along the external carotid artery to ultimately innervate the facial sweat glands, while the other portion of the neurons combines with the carotid plexus and travels within the walls of the internal carotid artery and through the cavernous sinus.7 The fibers then briefly join the abducens nerve before anastomosing with the ophthalmic division of the trigeminal nerve.7 After coursing through the superior orbital fissure, the fibers innervate the iris dilator and Müller muscles via the long ciliary nerves.7
Symptoms and signs. Patients with Horner syndrome can present with a variety of symptoms and signs. Patients may be largely asymptomatic or they may complain of a droopy eyelid and blurry vision. The full Horner syndrome triad consists of ipsilateral miosis, anhidrosis of the face, and mild ptosis of the upper eyelid with reverse ptosis of the lower eyelid.8 The difference in pupil size is greatest 4 to 5 seconds after switching from bright to dim room illumination due to dilation lag in the miotic pupil from poor innervation.1
Although the classical triad of ptosis, miosis, and anhidrosis is emphasized in the literature, the full triad may not always be present.4 This variation is due to the anatomy of the oculosympathetic pathway with branches of the nerve system separating at the superior cervical ganglion and following different pathways along the internal and external carotid arteries, resulting in anhidrosis only in Horner syndrome caused by lesions to the first- or second-order neurons.4,5 Because of this deviation of the nerve fibers in the pathway, the presence of miosis and a slight ptosis in the absence of anhidrosis should still strongly suggest Horner syndrome.
In addition to the classic triad, Horner syndrome can present with other ophthalmic findings, including conjunctival injection, changes in accommodation, and a small decrease in intraocular pressure usually by no more than 1 to 2 mm Hg.4 Congenital Horner syndrome is unique in that it can result in iris heterochromia, with the lighter eye being the affected eye.4
Due to the long and circuitous nature of the oculosympathetic pathway, damage can occur due to a wide variety of conditions (Table) and can present with many neurologic findings.7
Localization of lesions. In Horner syndrome, 13% of lesions were present at first-order neurons, 44% at second-order neurons, and 43% at third-order neurons.7 While all these lesions have similar clinical presentations that can be difficult to differentiate, localization of the lesion within the oculosympathetic pathway is important to determine the underlying cause. This determination can be readily achieved in office with pharmacologic pupil testing (Figure 3).
Management. All acute Horner syndrome presentations should be referred for same-day evaluation to rule out potentially life-threatening conditions, such as a cerebrovascular accident, carotid artery dissection or aneurysm, and giant cell arteritis.10 The urgent evaluation should include CTA and MRI/MRA of the head and neck.5 If giant cell arteritis is suspected, it is also recommended to obtain urgent bloodwork, which should include complete blood count with differential, erythrocyte sedimentation rate, and C-reactive protein.5 Carotid angiography and CT of the chest also are indicated if the aforementioned tests are noncontributory, but these are less urgent and can be deferred for evaluation within 1 to 2 days after the initial diagnosis.10
In this patient’s case, an immediate neurologic evaluation was appropriate due to the acute and painful nature of her presentation. Ultimately, her Horner syndrome was determined to result from an internal carotid artery dissection. As indicated by Schievink, all acute Horner syndrome cases should be considered a result of a carotid artery dissection until proven otherwise, despite the presence or absence of any other signs or symptoms.11 This consideration is not only because of the potentially life-threatening sequelae associated with carotid dissections, but also because dissections have been shown to be the most common cause of ischemic strokes in young and middle-aged patients, accounting for 10% to 25% of all ischemic strokes.4,11
Carotid Artery Dissection
An artery dissection is typically the result of a tear of the
There are many causes of carotid artery dissections, such as structural defects of the arterial wall, fibromuscular dysplasia, cystic medial necrosis, and connective tissue disorders, including Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, and osteogenesis imperfecta type I.13 Many environmental factors also can induce a carotid artery dissection, such as a history of anesthesia use, resuscitation with classic cardiopulmonary resuscitation techniques, head or neck trauma, chiropractic manipulation of the neck, and hyperextension or rotation of the neck, which can occur in activities such as yoga, painting a ceiling, coughing, vomiting, or sneezing.11
Patients with an internal carotid artery dissection typically present with pain on one side of the neck, face, or head, which can be accompanied by a partial Horner syndrome that results from damage to the oculosympathetic neurons traveling with the carotid plexus in the internal carotid artery wall.9,10 Unilateral facial or orbital pain has been noted to be present in half of patients and is typically accompanied by an ipsilateral headache.9 These symptoms are typically followed by cerebral or retinal ischemia within hours or days of onset and other ophthalmic conditions that can cause blindness, such as ischemic optic neuropathy or retinal artery occlusions, although these are rare.9
Due to the potential complications that can arise, carotid artery dissections require prompt treatment with antithrombotic therapy for 3 to 6 months to prevent carotid artery occlusion, which can result in a hemispheric cerebrovascular accident or TIAs.15 The options for antithrombotic therapy include anticoagulants, such as warfarin, and antiplatelets, such as aspirin. Studies have found similar rates of recurrent ischemic strokes in treatment with anticoagulants compared with antiplatelets, so both are reasonable therapeutic options.15,16 Following a carotid artery dissection diagnosis, patients should be evaluated by neurology to minimize other cardiovascular risk factors and prevent other complications.
Conclusions
Due to the potential life-threatening complications that can arise from conditions resulting in Horner syndrome, it is imperative that clinicians have a thorough understanding of the condition and its appropriate treatment and management modalities. Understanding the need for immediate testing to determine the underlying etiology of Horner syndrome can help prevent a decrease in a patient’s vision or quality of life, and in some cases, prevent death.
Acknowledgments
The author recognizes and thanks Kyle Stuard for his invaluable assistance in the editing of this manuscript
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
1. Yanoff M, Duker J. Ophthalmology. 5th ed. Elsevier; 2019.
2. Payne WN, Blair K, Barrett MJ. Anisocoria. StatPearls Publishing; 2022. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK470384
3. Lee A, Bindiganavile SH, Fan J, Al-Zubidi N, Bhatti MT. Argyll Robertson pupils. Accessed February 1, 2023. https://eyewiki.aao.org/Argyll_Robertson_Pupils
4. Kedar S, Prakalapakorn G, Yen M, et al. Horner syndrome. American Academy of Optometry. 2021. Accessed February 1, 2023. https://eyewiki.aao.org/Horner_Syndrome
5. Daroff R, Bradley W, Jankovic J. Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. Elsevier; 2022.
6. Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46. doi:10.2147/EB.S63633
7. Lykstad J, Reddy V, Hanna A. Neuroanatomy, Pupillary Dilation Pathway. StatPearls Publishing; 2022. Updated August 11, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK535421
8. Friedman N, Kaiser P, Pineda R. The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 5th ed. Elsevier; 2020.
9. Silbert PL, Mokri B, Schievink WI. Headache and neck pain in spontaneous internal carotid and vertebral artery dissections. Neurology. 1995;45(8):1517-1522. doi:10.1212/wnl.45.8.1517
10. Gervasio K, Peck T. The Will’s Eye Manual. 8th ed. Walters Kluwer; 2022.
11. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906. doi:10.1056/NEJM200103223441206
12. Hart RG, Easton JD. Dissections of cervical and cerebral arteries. Neurol Clin. 1983;1(1):155-182.
13. Goodfriend SD, Tadi P, Koury R. Carotid Artery Dissection. StatPearls Publishing; 2022. Updated December 24, 2021. Accessed February 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430835
14. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. 2015;2(4):e26670. doi:10.5812/archneurosci.26670
15. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227-276. doi:10.1161/STR.0b013e3181f7d043
16. Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345(20):1444-1451. doi:10.1056/NEJMoa011258
17. Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298. doi:10.1038/eye.2012.281
High-Grade Staphylococcus lugdunensis Bacteremia in a Patient on Home Hemodialysis
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
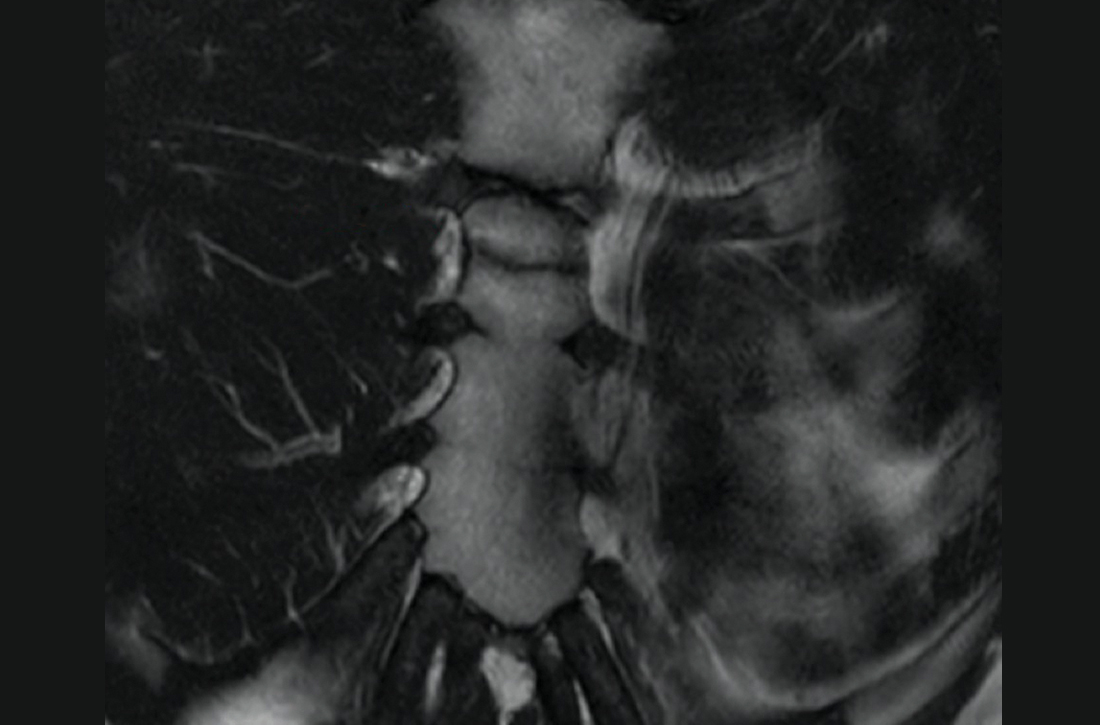
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
85-year-old woman • insomnia • abdominal discomfort • urge to move at night • Dx?
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; [email protected]
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; [email protected]
THE CASE
An 85-year-old woman with hypertension presented to our hospital with a 10-month history of insomnia along with abdominal discomfort. Several months prior, the patient had undergone an esophagogastroduodenoscopy, the results of which were normal, and had received diagnoses of psychogenic insomnia and abdominal pain from her previous physician. At that time, she was prescribed eszopiclone, but her insomnia did not improve. She did not complain of any other gastrointestinal symptoms.
On examination at our hospital, the patient’s abdomen was soft and nontender. Laboratory results were unremarkable. Abdominal computed tomography was performed to exclude obvious malignancy and showed no remarkable findings.
Additional history taking and physical examination were performed. The patient reported that she could sleep for only about 2 hours per night due to persistent severe discomfort around the umbilicus, which she described as “itching.” The discomfort occurred along with an urge to move while she laid in a state of relaxed wakefulness. This discomfort occurred no matter what position she laid in and improved if she walked or tapped around the umbilicus for a while. She denied any unusual or uncomfortable sensations in her lower extremities.
Her symptoms were absent during the daytime and not related to diet. Furthermore, she did not have any symptoms of anxiety and/or depression; a detailed neurologic examination, including cognitive assessment and extrapyramidal system, yielded unremarkable findings. Additional laboratory tests showed a mild iron deficiency (ferritin, 52.6 µ g/L; iron, 10.7 µ mol/L) without anemia.
THE DIAGNOSIS
Given the patient’s presentation and clinical history, the differential diagnosis included restless abdomen (which is a spectrum or a phenotypic variant of restless legs syndrome [RLS]) and its mimics, which include fibromyalgia and gastrointestinal tract diseases. We considered the characteristic symptoms of this case (ie, irresistible symptoms, lengthy duration of symptoms, and sleep problems) to better support the diagnosis of restless abdomen than its mimics.1 In particular, abdominal discomfort that led to insomnia was characteristic of restless abdomen, helping to pinpoint the diagnosis.
DISCUSSION
RLS is a common sensorimotor disorder that is characterized by an unpleasant urge to move the legs.2 RLS may manifest as an idiopathic condition, or it can be secondary to medical conditions such as iron deficiency and Parkinson disease.3,4 Because the unpleasant symptom is exacerbated in the evenings, patients with RLS frequently complain of sleep disturbance.
Cases of RLS-like sensory disorders, with symptoms involving sites other than the lower extremities (eg, arms, mouth, trunk, and genitals) recently have been reported.5-7 Among them is restless abdomen, a rare disorder that manifests with a restless abdominal sensation and worsens the quality of sleep and life.6
Continue to: Restless abdomen meets all...
Restless abdomen meets all other diagnostic criteria for RLS except for the affected anatomy.6,8 In most cases of restless abdomen, the uncomfortable sensation involves the abdomen, as well as other parts of the body (eg, legs and arms). Cases in which the symptoms are confined to the abdomen are rare, with only 7 reported to date. 6,8-10 All of these cases have involved patients older than 40 years. 6,8-10
Treatment is straightforward, but consider iron supplementation, as well
Because RLS or its variants degrade the quality of life and sleep in patients,3,4 appropriate therapy must be initiated early. Although the optimal treatment strategy for restless abdomen is yet to be established, an oral dopamine agonist—specifically, pramipexole—has been used successfully in almost all cases.6,8-10
Previous clinical research has shown that patients with RLS have low levels of iron in the brain and may benefit from iron supplementation, even if they are not anemic.3,4 Iron replacement is suggested for patients with RLS whose fasting serum ferritin level is ≤ 75 µg/L.4 It is not known to what extent iron deficiency is involved in the pathophysiology of restless abdomen, and further research is required to determine the optimal therapy for it.
Our patient was started on oral supplementation with sodium ferrous citrate (50 mg/d) based on an initial suspicion that iron deficiency was the cause of her restless abdomen. We also suggested that the patient undergo a fecal occult blood test or colonoscopy, but she declined because of her advanced age.
After 2 months of iron supplementation, the patient’s serum ferritin levels improved (100 µg/L) and her insomnia and abdominal discomfort improved a bit. However, 3 months after starting on the iron supplementation, her symptoms flared again.
Continue to: We then prescribed...
We then prescribed pramipexole 0.25 mg/d. The patient’s symptoms subsequently resolved, and she no longer experienced insomnia. This favorable response to dopamine agonist therapy supported the diagnosis of restless abdomen. The patient continues to take the pramipexole to prevent a relapse.
THE TAKEAWAY
Insomnia is a common presenting complaint in primary care and sleeping pills may be prescribed without adequate investigation of the cause. However, some patients may have serious underlying diseases.11
Although restless abdomen is a disorder that causes severe sleep disturbance and impairs the patient’s quality of sleep and life, it is not widely recognized by clinicians and may be misdiagnosed. When recognized, insomnia due to restless abdomen can be relieved by a simple therapy: oral dopamine agonists. Therefore, primary care physicians should consider restless abdomen as a potential cause of insomnia with abdominal symptoms.
CORRESPONDENCE
Hirohisa Fujikawa, MD, Department of Medical Education Studies, International Research Center for Medical Education, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan; [email protected]
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
1. Hening WA, Allen RP, Washburn M, et al. The four diagnostic criteria for restless legs syndrome are unable to exclude confounding conditions (“mimics”). Sleep Med. 2009;10:976-981. doi: 10.1016/j.sleep.2008.09.015
2. Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623-634. doi: 10.1016/j.sleep.2010.12.018
3. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125:99-111. doi: 10.3810/pgm.2013.05.2636
4. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96:1921-1937. doi: 10.1016/j.mayocp.2020.12.026
5. Aquino CC, Mestre T, Lang AE. Restless genital syndrome in Parkinson disease. JAMA Neurol. 2014;71:1559-1561. doi: 10.1001/jamaneurol.2014.1326
6. Pérez-Díaz H, Iranzo A, Rye DB, et al. Restless abdomen: a phenotypic variant of restless legs syndrome. Neurology. 2011;77:1283-1286. doi: 10.1212/WNL.0b013e318230207a
7. Sforza E, Hupin D, Roche F. Restless genital syndrome: differential diagnosis and treatment with pramipexole. J Clin Sleep Med. 2017;13:1109-1110. doi: 10.5664/jcsm.6736
8. Wang XX, Zhu XY, Wang Z, et al. Restless abdomen: a spectrum or a phenotype variant of restless legs syndrome? BMC Neurol. 2020;20:298. doi: 10.1186/s12883-020-01875-1
9. Esaki Y, Kitajima T, Tsuchiya A, et al. Periodic abdominal movements. Psychiatry Clin Neurosci. 2014;68:167. doi: 10.1111/pcn.12095
10. Baiardi S, La Morgia C, Mondini S, et al. A restless abdomen and propriospinal myoclonus like at sleep onset: an unusual overlap syndrome. BMJ Case Rep. 2015;2015:bcr2014206679. doi: 10.1136/bcr-2014-206679
11. Pavlova MK, Latreille V. Sleep disorders. Am J Med. 2019;132:292-299. doi: 10.1016/j.amjmed.2018.09.021
Acetaminophen as Renoprotective Treatment in a Patient With Severe Malaria
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Severe Esophageal Lichen Planus Treated With Tofacitinib
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.
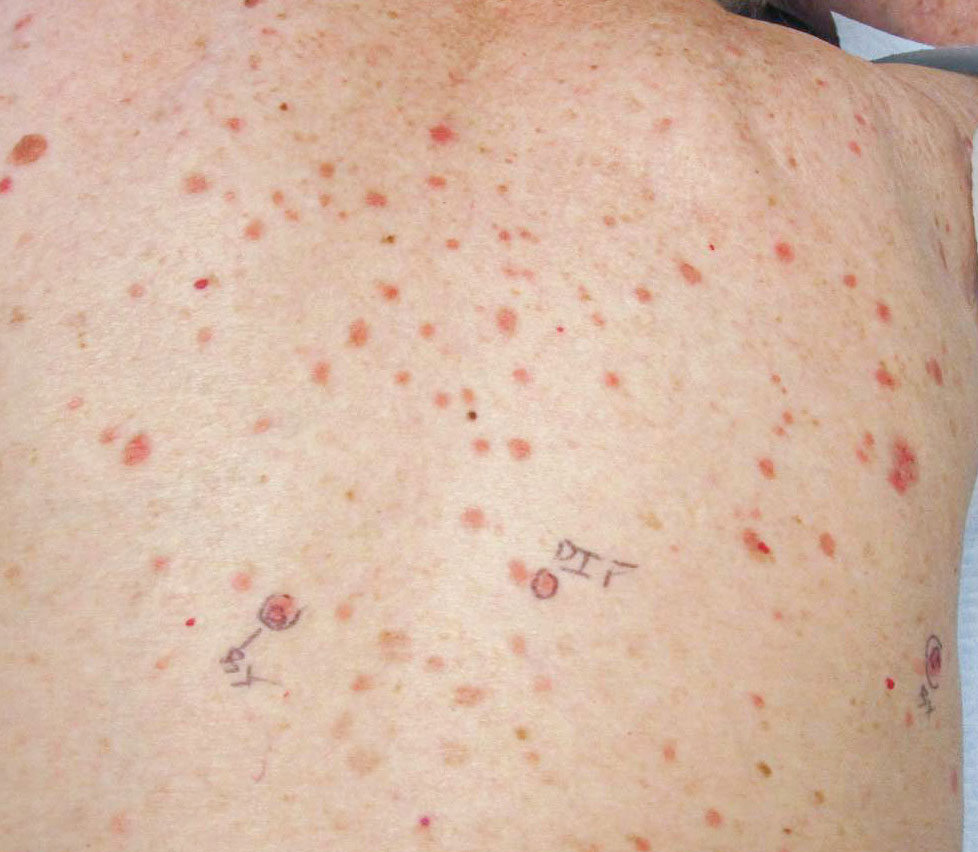
Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.
Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.
The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.
The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.
Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.
Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.

Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.

Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.

The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.

The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.

Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.

Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
To reach early diagnoses and improve outcomes in cases of mucosal and esophageal lichen planus (ELP), patient education along with a multidisciplinary approach centered on collaboration among dermatologists, gastroenterologists, gynecologists, and dental practitioners should be a priority. Tofacitinib therapy should be considered in the treatment of patients presenting with cutaneous lichen planus (CLP), mucosal lichen planus, and ELP.
Lichen planus is a papulosquamous disease of the skin and mucous membranes that is most common on the skin and oral mucosa. Typical lesions of CLP present as purple, pruritic, polygonal papules and plaques on the flexural surfaces of the wrists and ankles as well as areas of friction or trauma due to scratching such as the shins and lower back. Various subtypes of lichen planus can present simultaneously, resulting in extensive involvement that worsens through koebnerization and affects the oral cavity, esophagus, larynx, sclera, genitalia, scalp, and nails.1,2
Esophageal lichen planus can develop with or without the presence of CLP, oral lichen planus (OLP), or genital lichen planus.3 It typically affects women older than 50 years and is linked to OLP and vulvar lichen planus, with 1 study reporting that 87% (63/72) of ELP patients were women with a median age of 61.9 years at the time of diagnosis (range, 22–85 years). Almost all ELP patients in the study had lichen planus symptoms in other locations; 89% (64/72) had OLP, and 42% (30/72) had vulvar lichen planus.4 Consequently, a diagnosis of ELP should be followed by a thorough full-body examination to check for lichen planus at other sites. Studies that examined lichen planus patients for ELP found that 25% to 50% of patients diagnosed with orocutaneous lichen planus also had ELP, with ELP frequently presenting without symptoms.3,5 These findings indicate that ELP likely is underdiagnosed and often misdiagnosed, resulting in an underestimation of its prevalence.

Our case highlights a frequently misdiagnosed condition and underscores the importance of close examination of patients presenting with CLP and OLP for signs and symptoms of ELP. Furthermore, we discuss the importance of patient education and collaboration among different specialties in attaining an early diagnosis to improve patient outcomes. Finally, we review the clinical presentation, diagnosis, and treatment of CLP, OLP, and ELP, as well as the utility of tofacitinib for ELP.

Case Report
An emaciated 89-year-old woman with an 11-year history of CLP, OLP, and genital lichen planus that had been successfully treated with topicals presented with an OLP recurrence alongside difficulties eating and swallowing. Her symptoms lasted 1 year and would recur when treatment was paused. Her medical history included rheumatoid arthritis, hypothyroidism, and hypertension, and she was taking levothyroxine, olmesartan, and vitamin D supplements. Dentures and olmesartan previously were ruled out as potential triggers following a 2-month elimination. None of her remaining natural teeth had fillings. She also reported that neither she nor her partner had ever smoked or chewed tobacco.

The patient’s lichen planus involvement first manifested as red, itchy, polygonal, lichenoid papules on the superior and inferior mid back 11 years prior to the current presentation (Figure 1). Further examination noted erosions on the genitalia, and a subsequent biopsy of the vulva confirmed a diagnosis of lichen planus (Figure 2). Treatment with halobetasol propionate ointment and tacrolimus ointment 0.1% twice daily (BID) resulted in remission of the CLP and vulvar lichen planus. She presented a year later with oral involvement revealing Wickham striae on the buccal mucosa and erosions on the upper palate that resolved after 2 months of treatment with cyclosporine oral solution mixed with a 5-times-daily nystatin swish-and-spit (Figure 3). The CLP did not recur but OLP was punctuated by remissions and recurrences on a yearly basis, often related to the cessation of mouthwash and topical creams. The OLP and vulvar lichen planus were successfully treated with as-needed use of a cyclosporine mouthwash swish-and-spit 3 times daily as well as halobetasol ointment 0.05% 3 times daily, respectively. Six years later, the patient was hospitalized for unrelated causes and was lost to follow-up for 2 years.

The patient experienced worsening dysphagia and odynophagia over a period of 2 years (mild dysphagia was first recorded 7 years prior to the initial presentation) and reported an unintentional weight loss of 20 pounds. An endoscopy was performed 3 years after the initial report of dysphagia and noted esophageal erosions (Figure 4A) and a stricture (Figure 4B), but all abnormal involvement was attributed to active gastroesophageal reflux disease. She underwent 8 esophageal dilations to treat the stricture but noted that the duration of symptomatic relief decreased with every subsequent dilation. An esophageal stent was placed 4 years after the initial concern of dysphagia, but it was not well tolerated and had to be removed soon thereafter. A year later, the patient underwent an esophageal bypass with a substernal gastric conduit that provided relief for 2 months but failed to permanently resolve the condition. In fact, her condition worsened over the next 1.5 years when she presented with extreme emaciation attributed to a low appetite and pain while eating. A review of the slides from a prior hospital esophageal biopsy revealed lichen planus (Figure 5). She was prescribed tofacitinib 5 mg BID as a dual-purpose treatment for the rheumatoid arthritis and OLP/ELP. At 1-month follow-up she noted that she had only taken one 5-mg pill daily without notable improvement, and after the visit she started the initial recommendation of 5 mg BID. Over the next several months, her condition continued to consistently improve; the odynophagia resolved, and she regained the majority of her lost weight. Tofacitinib was well tolerated across the course of treatment, and no adverse side effects were noted. Furthermore, the patient regained a full range of motion in the previously immobile arthritic right shoulder. She has experienced no recurrence of the genital lichen planus, OLP, or CLP since starting tofacitinib. To date, the patient is still taking only tofacitinib 5 mg BID with no recurrence of the cutaneous, mucosal, or esophageal lichen planus and has experienced no adverse events from the medication.

Comment
Clinical Presentation—Lichen planus—CLP and OLP—most frequently presents between the ages of 40 and 60 years, with a slight female predilection.1,2 The lesions typically present with the 5 P’s—purple, pruritic, polygonal papules and plaques—with some lesions revealing white lacy lines overlying them called Wickham striae.6 The lesions may be red at first before turning purple. They often present on the flexural surfaces of the wrists and ankles as well as the shins and back but rarely affect the face, perhaps because of increased chronic sun exposure.2,6 Less common locations include the scalp, nails, and mucosal areas (eg, oral, vulvar, conjunctival, laryngeal, esophageal, anal).1
If CLP is diagnosed, the patient likely will also have oral lesions, which occur in 50% of patients.2 Once any form of lichen planus is found, it is important to examine all of the most frequently involved locations—mucocutaneous and cutaneous as well as the nails and scalp. Special care should be taken when examining OLP and genital lichen planus, as long-standing lesions have a 2% to 5% chance of transforming into squamous cell carcinoma.2
Although cases of traditional OLP and CLP are ubiquitous in the literature, ELP rarely is documented because of frequent misdiagnoses. Esophageal lichen planus has a closer histopathologic resemblance to OLP compared to CLP, and its highly variable presentation often results in an inconclusive diagnosis.3 A review of 27 patients with lichen planus highlighted the difficult nature of diagnosing ELP; ELP manifested up to 20 years after initial lichen planus diagnosis, and patients underwent an average of 2.5 dilations prior to the successful diagnosis of ELP. Interestingly, 2 patients in the study presented with ELP in isolation, which emphasizes the importance of secondary examination for lichen planus in the presence of esophageal strictures.7 The eTable provides common patient demographics and symptoms to more effectively identify ELP.Differential Diagnosis—Because lichen planus can present anywhere on the body, it may be difficult to differentiate it from other skin conditions. Clinical appearance alone often is insufficient for diagnosing lichen planus, and a punch biopsy often is needed.2,20 Cutaneous lichen planus may resemble eczema, lichen simplex chronicus, pityriasis rosea, prurigo nodularis, and psoriasis, while OLP may resemble bite trauma, leukoplakia, pemphigus, and thrush.20 Dermoscopy of the tissue makes Wickham striae easier to visualize and assists in the diagnosis of lichen planus. Furthermore, thickening of the stratum granulosum, a prevalence of lymphocytes in the dermoepidermal junction, and vacuolar alteration of the stratum basale help to distinguish between lichen planus and other inflammatory dermatoses.20 A diagnosis of lichen planus merits a full-body skin examination—hair, nails, eyes, oral mucosa, and genitalia—to rule out additional involvement.
Esophageal lichen planus most frequently presents as dysphagia, odynophagia, and weight loss, but other symptoms including heartburn, hoarseness, choking, and epigastric pain may suggest esophageal involvement.4 Typically, ELP presents in the proximal and/or central esophagus, assisting in the differentiation between ELP and other esophageal conditions.3 Special consideration should be taken when both ELP and gastroesophageal reflux disease are considered in a differential diagnosis, and it is recommended to pair an upper endoscopy with pH monitoring to avoid misdiagnosis.8 Screening endoscopies also are helpful, as they assist in identifying the characteristic white webs, skin peeling, skin surface erosion, and strictures of ELP.4 Taken together, dermatologists should encourage patients with cutaneous or mucocutaneous lichen planus to undergo an esophagogastroduodenoscopy, especially in the presence of any of ELP’s common symptoms (eTable).
Etiology—Although the exact etiology of lichen planus is not well established, there are several known correlative factors, including hepatitis C; increased stress; dental materials; oral medications, most frequently antihypertensives and nonsteroidal anti-inflammatory drugs; systemic diseases; and tobacco usage.6,21
Dental materials used in oral treatments such as silver amalgam, gold, cobalt, palladium, chromium, epoxy resins, and dentures can trigger or exacerbate OLP, and patch testing of a patient’s dental materials can help determine if the reaction was caused by the materials.6,22 The removal of material contributing to lesions often will cause OLP to resolve.22
It also has been suggested that the presence of thyroid disorders, autoimmune disease, various cancers, hypertension, type 2 diabetes mellitus, hyperlipidemia, oral sedative usage, and/or vitamin D deficiency may be associated with OLP.21,23 Although OLP patients who were initially deficient in vitamin D demonstrated marked improvement with supplementation, it is unlikely that vitamin D supplements impacted our patient’s presentation of OLP, as she had been consistently taking them for more than 5 years with no change in OLP presentation.24
Pathogenesis—Lichen planus is thought to be a cytotoxic CD8+ T cell–mediated autoimmune disease to a virally modified epidermal self-antigen on keratinocytes. The cytotoxic T cells target the modified self-antigens on basal keratinocytes and induce apoptosis.25 The cytokine-mediated lymphocyte homing mechanism is human leukocyte antigen dependent and involves tumor necrosis factor α as well as IFN-γ and IL-1. The latter cytokines lead to upregulation of vascular adhesion molecules on endothelial vessels of subepithelial vascular plexus as well as a cascade of nonspecific mechanisms such as mast cell degranulation and matrix metalloproteinase activation, resulting in increased basement membrane disruption.6
Shao et al19 underscored the role of IFN-γ in CD8+ T cell–mediated cytotoxic cellular responses, noting that the Janus kinase (JAK)–signal transducer and activator of transcription pathway may play a key role in the pathogenesis of lichen planus. They proposed using JAK inhibitors for the treatment of lichen planus, specifically tofacitinib, a JAK1/JAK3 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor, as top therapeutic agents for lichen planus (eTable).19 Tofacitinib has been reported to successfully treat conditions such as psoriasis, psoriatic arthritis, alopecia areata, vitiligo, atopic dermatitis, sarcoidosis, pyoderma gangrenosum, and lichen planopilaris.26 Additionally, the efficacy of tofacitinib has been established in patients with erosive lichen planus; tofacitinib resulted in marked improvement while prednisone, acitretin, methotrexate, mycophenolate mofetil, and cyclosporine treatment failed.27 Although more studies on tofacitinib’s long-term efficacy, cost, and safety are necessary, tofacitinib may soon play an integral role in the battle against inflammatory dermatoses.

Conclusion
Esophageal lichen planus is an underreported form of lichen planus that often is misdiagnosed. It frequently causes dysphagia and odynophagia, resulting in a major decrease in a patient’s quality of life. We present the case of an 89-year-old woman who underwent procedures to dilate her esophagus that worsened her condition. We emphasize the importance of considering ELP in the differential diagnosis of patients presenting with lichen planus in another region. In our patient, tofacitinib 5 mg BID resolved her condition without any adverse effects.
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
- Le Cleach L, Chosidow O. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/nejmcp1103641
- Heath L, Matin R. Lichen planus. InnovAiT. 2017;10:133-138. doi:10.1177/1755738016686804
- Oliveira JP, Uribe NC, Abulafia LA, et al. Esophageal lichenplanus. An Bras Dermatol. 2015;90:394-396. doi:10.1590/abd1806-4841.20153255
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-183. doi:10.1016/j.jaad.2010.03.029
- Quispel R, van Boxel O, Schipper M, et al. High prevalence of esophageal involvement in lichen planus: a study using magnification chromoendoscopy. Endoscopy. 2009;41:187-193. doi:10.1055/s-0028-1119590
- Gupta S, Jawanda MK. Oral lichen planus: an update on etiology, pathogenesis, clinical presentation, diagnosis and management. Indian J Dermatol. 2015;60:222-229. doi:10.4103/0019-5154.156315
- Katzka DA, Smyrk TC, Bruce AJ, et al. Variations in presentations of esophageal involvement in lichen planus. Clin Gastroenterol Hepatol. 2010;8:777-782. doi:10.1016/j.cgh.2010.04.024
- Abraham SC, Ravich WJ, Anhalt GJ, et al. Esophageal lichen planus. Am J Surg Pathol. 2000;24:1678-1682. doi:10.1097/00000478-200012000-00014
- Murro D, Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827-830. doi:10.5858/arpa.2014-0111-RS
- Wilcox CM. Infectious esophagitis. Gastroenterol Hepatol (N Y). 2006;2:567-568.
- Cancio A, Cruz C. A case of Kaposi’s sarcoma of the esophagus presenting with odynophagia. Am J Gastroenterol. 2018;113:S995-S996.
- Kokturk A. Clinical and pathological manifestations with differential diagnosis in Behçet’s disease. Patholog Res Int. 2012;2012:690390. doi:10.1155/2012/690390
- Madhusudhan KS, Sharma R. Esophageal lichen planus: a case report and review of literature. Indian J Dermatol. 2008;53:26-27. doi:10.4103/0019-5154.39738
- Bottomley WW, Dakkak M, Walton S, et al. Esophageal involvement in Behçet’s disease. is endoscopy necessary? Dig Dis Sci. 1992;37:594-597. doi:10.1007/BF01307585
- McDonald GB, Sullivan KM, Schuffler MD, et al. Esophageal abnormalities in chronic graft-versus-host disease in humans. Gastroenterology. 1981;80:914-921.
- Trabulo D, Ferreira S, Lage P, et al. Esophageal stenosis with sloughing esophagitis: a curious manifestation of graft-vs-host disease. World J Gastroenterol. 2015;21:9217-9222. doi:10.3748/wjg.v21.i30.9217
- Abbas H, Ghazanfar H, Ul Hussain AN, et al. Atypical presentation of esophageal squamous cell carcinoma masquerading as diffuse severe esophagitis. Case Rep Gastroenterol. 2021;15:533-538. doi:10.1159/000517129
- Ellis A, Risk JM, Maruthappu T, et al. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi:10.1186/s13023-015-0346-2
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med. 2019;11:eaav7561. doi:10.1126/scitranslmed.aav7561
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Dave A, Shariff J, Philipone E. Association between oral lichen planus and systemic conditions and medications: case-control study. Oral Dis. 2020;27:515-524. doi:10.1111/odi.13572
- Krupaa RJ, Sankari SL, Masthan KM, et al. Oral lichen planus: an overview. J Pharm Bioallied Sci. 2015;7(suppl 1):S158-S161. doi:10.4103/0975-7406.155873
- Tak MM, Chalkoo AH. Vitamin D deficiency—a possible contributing factor in the aetiopathogenesis of oral lichen planus. J Evolution Med Dent Sci. 2017;6:4769-4772. doi:10.14260/jemds/2017/1033
- Gupta J, Aggarwal A, Asadullah M, et al. Vitamin D in thetreatment of oral lichen planus: a pilot clinical study. J Indian Acad Oral Med Radiol. 2019;31:222-227. doi:10.4103/jiaomr.jiaomr_97_19
- Shiohara T, Moriya N, Mochizuki T, et al. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II. LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Invest Dermatol. 1987;89:8-14.
- Sonthalia S, Aggarwal P. Oral tofacitinib: contemporary appraisal of its role in dermatology. Indian Dermatol Online J. 2019;10:503-518. doi:10.4103/idoj.idoj_474_18
- Damsky W, Wang A, Olamiju B, et al. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. 2020;145:1708-1710.e2. doi:10.1016/j.jaci.2020.01.031
Practice Points
- Patients diagnosed with lichen planus should be informed about the signs of esophageal lichen planus (ELP).
- Twenty-five percent to 50% of patients with oral lichen planus (OLP) have been shown to have concomitant ELP.
- Esophageal lichen planus may be asymptomatic and often is misdiagnosed.
- Tofacitinib should be considered for the treatment of ELP, OLP, and cutaneous lichen planus.
44-year-old man • elevated total cholesterol • chest pains • ketogenic diet • Dx?
THE CASE
A 44-year-old man with a history of morbid obesity reestablished care in our clinic. He had been treated in our health care system about 5 years previously, and prior lab testing showed a total cholesterol of 203 mg/dL; triglycerides, 191 mg/dL; high-density lipoprotein (HDL), 56 mg/dL; and low-density lipoprotein (LDL), 109 mg/dL. At that time, he weighed 299 lbs (BMI, 39.4). He then started a strict ketogenic diet and a regular exercise program (running ~ 16 miles per week and lifting weights), which he maintained for several years. He had experienced remarkable weight loss; upon reestablishing care, he weighed 199 lbs (BMI, 26.33).
However, lipid testing revealed a severely elevated total cholesterol of 334 mg/dL; LDL, 248 mg/dL; HDL, 67 mg/dL; and triglycerides, 95 mg/dL. He was advised to start statin therapy and to stop his ketogenic diet, but he was hesitant to take either step. He elected to have his lab work reevaluated in 6 months.
About 4 months later, he presented with new and increasing burning pain in his mid chest and upper abdomen. He rated the pain 6/10 in severity and said it occurred during exertion or at night when lying down. Resting would relieve the pain. Reduced intake of spicy foods and caffeine had also helped. He denied dyspnea, diaphoresis, palpitations, or nausea.
The patient was a nonsmoker but did have a strong family history of cardiovascular disease. His vital signs and physical examination were unremarkable, apart from mild epigastric and periumbilical tenderness on palpation.
THE DIAGNOSIS
The patient’s chest pain had features of both gastroesophageal reflux disease (GERD) and coronary artery disease (CAD) with exertional angina. His high-fat diet, nightly symptoms, and the partial relief he achieved by cutting back on spicy foods and caffeine suggested GERD, but the exertional nature of the chest pain and gradual relief with rest was highly suggestive of angina, so an outpatient electrocardiogram treadmill stress test was ordered.
The stress test was markedly abnormal, showing worsening ST depressions and T-wave inversions with exertion, and he experienced chest pain during testing. An urgent left heart catheterization was performed, showing severe multivessel CAD. He subsequently underwent 3-vessel coronary artery bypass grafting. A familial hypercholesterolemia panel failed to reveal any significant variants.
As a result of these findings, the patient received a diagnosis of severe ketogenic diet–associated hypercholesterolemia and early-onset CAD.
Continue to: DISCUSSION
DISCUSSION
Low-carbohydrate (low-carb) and ketogenic diets have grown in popularity throughout the United States over the past decade, particularly for weight loss, and the diet has entered the popular consciousness with several celebrities publicly supporting it.1 Simultaneously, there also has been a growing interest in these diets for the treatment of chronic diseases, such as type 2 diabetes.2 However, the long-term cardiovascular effects of low-carb diets are not well studied, and there is significant heterogeneity among these diets.
Low-carb vs low-fat. Multiple meta-analyses comparing low-carb diets to low-fat diets have found that those following low-carb diets have significantly higher total cholesterol and LDL levels.3,4,5 The National Lipid Association’s review of evidence determined that LDL and total cholesterol responses vary in individuals following a low-carb diet, but that increasing LDL levels in particular were concerning enough to warrant lipid monitoring of patients on low-carb diets.6 Another meta-analysis evaluated the difference in estimated atherosclerotic cardiovascular disease (ASCVD) risk between low-carb and low-fat diets, finding those following a low-carb diet to have a lower estimated ASCVD risk but higher LDL levels.7
Weighing the benefits and harms. Since our patient’s dramatic weight loss and greatly increased exercise level would be expected to lower his LDL levels, the severe worsening of his LDL levels was likely related to his ketogenic diet and was a factor in the early onset of CAD. The benefits of low-carb diets for weight loss, contrasted with the consistent worsening of LDL levels, has prompted a debate about which parameters should be considered in estimating the long-term risk of these diets for patients. Diamond et al8 posit that these diets have beneficial effects on “the most reliable [cardiovascular disease] risk factors,” but long-term, patient-oriented outcome data are lacking, and these diets may not be appropriate for certain patients, as our case demonstrates.
A reasonable strategy for patients contemplating a low-carb diet specifically for weight loss would be to use such a diet for 3 to 6 months to achieve initial and rapid results, then continue with a heart-healthy diet and increased exercise levels to maintain weight loss and reduce long-term cardiovascular risk.
Our patient was started on a postoperative medication regimen of aspirin 81 mg/d, evolocumab 140 mg every 14 days, metoprolol tartrate 25 mg bid, and rosuvastatin 10 mg/d. A year later, he was able to resume a high level of physical activity (6-mile runs) without chest pain. His follow-up lipid panel showed a total cholesterol of 153 mg/dL; LDL, 53 mg/dL; HDL, 89 mg/dL; and triglycerides, 55 mg/dL. He had also switched to a regular diet and had been able to maintain his weight loss.
THE TAKEAWAY
Growing evidence suggests that low-carb diets may have a significant and detrimental effect on LDL levels. The long-term safety of these diets hasn’t been well studied, particularly regarding cardiovascular outcomes. At a minimum, patients who initiate low-carb diets should be counseled on general dietary recommendations regarding saturated fat and cholesterol intake, and they should have a follow-up lipid screening to evaluate for any significant worsening in total cholesterol and LDL levels.
CORRESPONDENCE
Samuel Dickmann, MD, 13611 NW 1st Lane, Suite 200, Newberry, FL 32669; [email protected]
1. Gorin A. What is the keto diet – and is it right for you? NBC News BETTER. February 22, 2018. Accessed February 3, 2023. www.nbcnews.com/better/health/what-keto-diet-it-right-you-ncna847256
2. Tinguely D, Gross J, Kosinski, C. Efficacy of ketogenic diets on type 2 diabetes: a systematic review. Current Diabetes Reports. 2021;21:32. doi: 10.1007/s11892-021-01399-z
3. Mansoor N, Vinknes KJ, Veierod MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466-479. doi: 10.1017/S0007114515004699
4. Bueno NB, de Melo ISV, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. doi: 10.1017/S0007114513000548
5. Chawla S, Tessarolo Silva F, Amaral Medeiros S, et al. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12:3774. doi: 10.3390/nu12123774
6. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13:689-711.e1. doi: 10.1016/j.jacl.2019.08.003
7. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817
8. Diamond DM, O’Neill BJ, Volek JS. Low carbohydrate diet: are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr Opin Endocrinol Diabetes Obes. 2020;27:291-300. doi: 10.1097/MED.0000000000000568
THE CASE
A 44-year-old man with a history of morbid obesity reestablished care in our clinic. He had been treated in our health care system about 5 years previously, and prior lab testing showed a total cholesterol of 203 mg/dL; triglycerides, 191 mg/dL; high-density lipoprotein (HDL), 56 mg/dL; and low-density lipoprotein (LDL), 109 mg/dL. At that time, he weighed 299 lbs (BMI, 39.4). He then started a strict ketogenic diet and a regular exercise program (running ~ 16 miles per week and lifting weights), which he maintained for several years. He had experienced remarkable weight loss; upon reestablishing care, he weighed 199 lbs (BMI, 26.33).
However, lipid testing revealed a severely elevated total cholesterol of 334 mg/dL; LDL, 248 mg/dL; HDL, 67 mg/dL; and triglycerides, 95 mg/dL. He was advised to start statin therapy and to stop his ketogenic diet, but he was hesitant to take either step. He elected to have his lab work reevaluated in 6 months.
About 4 months later, he presented with new and increasing burning pain in his mid chest and upper abdomen. He rated the pain 6/10 in severity and said it occurred during exertion or at night when lying down. Resting would relieve the pain. Reduced intake of spicy foods and caffeine had also helped. He denied dyspnea, diaphoresis, palpitations, or nausea.
The patient was a nonsmoker but did have a strong family history of cardiovascular disease. His vital signs and physical examination were unremarkable, apart from mild epigastric and periumbilical tenderness on palpation.
THE DIAGNOSIS
The patient’s chest pain had features of both gastroesophageal reflux disease (GERD) and coronary artery disease (CAD) with exertional angina. His high-fat diet, nightly symptoms, and the partial relief he achieved by cutting back on spicy foods and caffeine suggested GERD, but the exertional nature of the chest pain and gradual relief with rest was highly suggestive of angina, so an outpatient electrocardiogram treadmill stress test was ordered.
The stress test was markedly abnormal, showing worsening ST depressions and T-wave inversions with exertion, and he experienced chest pain during testing. An urgent left heart catheterization was performed, showing severe multivessel CAD. He subsequently underwent 3-vessel coronary artery bypass grafting. A familial hypercholesterolemia panel failed to reveal any significant variants.
As a result of these findings, the patient received a diagnosis of severe ketogenic diet–associated hypercholesterolemia and early-onset CAD.
Continue to: DISCUSSION
DISCUSSION
Low-carbohydrate (low-carb) and ketogenic diets have grown in popularity throughout the United States over the past decade, particularly for weight loss, and the diet has entered the popular consciousness with several celebrities publicly supporting it.1 Simultaneously, there also has been a growing interest in these diets for the treatment of chronic diseases, such as type 2 diabetes.2 However, the long-term cardiovascular effects of low-carb diets are not well studied, and there is significant heterogeneity among these diets.
Low-carb vs low-fat. Multiple meta-analyses comparing low-carb diets to low-fat diets have found that those following low-carb diets have significantly higher total cholesterol and LDL levels.3,4,5 The National Lipid Association’s review of evidence determined that LDL and total cholesterol responses vary in individuals following a low-carb diet, but that increasing LDL levels in particular were concerning enough to warrant lipid monitoring of patients on low-carb diets.6 Another meta-analysis evaluated the difference in estimated atherosclerotic cardiovascular disease (ASCVD) risk between low-carb and low-fat diets, finding those following a low-carb diet to have a lower estimated ASCVD risk but higher LDL levels.7
Weighing the benefits and harms. Since our patient’s dramatic weight loss and greatly increased exercise level would be expected to lower his LDL levels, the severe worsening of his LDL levels was likely related to his ketogenic diet and was a factor in the early onset of CAD. The benefits of low-carb diets for weight loss, contrasted with the consistent worsening of LDL levels, has prompted a debate about which parameters should be considered in estimating the long-term risk of these diets for patients. Diamond et al8 posit that these diets have beneficial effects on “the most reliable [cardiovascular disease] risk factors,” but long-term, patient-oriented outcome data are lacking, and these diets may not be appropriate for certain patients, as our case demonstrates.
A reasonable strategy for patients contemplating a low-carb diet specifically for weight loss would be to use such a diet for 3 to 6 months to achieve initial and rapid results, then continue with a heart-healthy diet and increased exercise levels to maintain weight loss and reduce long-term cardiovascular risk.
Our patient was started on a postoperative medication regimen of aspirin 81 mg/d, evolocumab 140 mg every 14 days, metoprolol tartrate 25 mg bid, and rosuvastatin 10 mg/d. A year later, he was able to resume a high level of physical activity (6-mile runs) without chest pain. His follow-up lipid panel showed a total cholesterol of 153 mg/dL; LDL, 53 mg/dL; HDL, 89 mg/dL; and triglycerides, 55 mg/dL. He had also switched to a regular diet and had been able to maintain his weight loss.
THE TAKEAWAY
Growing evidence suggests that low-carb diets may have a significant and detrimental effect on LDL levels. The long-term safety of these diets hasn’t been well studied, particularly regarding cardiovascular outcomes. At a minimum, patients who initiate low-carb diets should be counseled on general dietary recommendations regarding saturated fat and cholesterol intake, and they should have a follow-up lipid screening to evaluate for any significant worsening in total cholesterol and LDL levels.
CORRESPONDENCE
Samuel Dickmann, MD, 13611 NW 1st Lane, Suite 200, Newberry, FL 32669; [email protected]
THE CASE
A 44-year-old man with a history of morbid obesity reestablished care in our clinic. He had been treated in our health care system about 5 years previously, and prior lab testing showed a total cholesterol of 203 mg/dL; triglycerides, 191 mg/dL; high-density lipoprotein (HDL), 56 mg/dL; and low-density lipoprotein (LDL), 109 mg/dL. At that time, he weighed 299 lbs (BMI, 39.4). He then started a strict ketogenic diet and a regular exercise program (running ~ 16 miles per week and lifting weights), which he maintained for several years. He had experienced remarkable weight loss; upon reestablishing care, he weighed 199 lbs (BMI, 26.33).
However, lipid testing revealed a severely elevated total cholesterol of 334 mg/dL; LDL, 248 mg/dL; HDL, 67 mg/dL; and triglycerides, 95 mg/dL. He was advised to start statin therapy and to stop his ketogenic diet, but he was hesitant to take either step. He elected to have his lab work reevaluated in 6 months.
About 4 months later, he presented with new and increasing burning pain in his mid chest and upper abdomen. He rated the pain 6/10 in severity and said it occurred during exertion or at night when lying down. Resting would relieve the pain. Reduced intake of spicy foods and caffeine had also helped. He denied dyspnea, diaphoresis, palpitations, or nausea.
The patient was a nonsmoker but did have a strong family history of cardiovascular disease. His vital signs and physical examination were unremarkable, apart from mild epigastric and periumbilical tenderness on palpation.
THE DIAGNOSIS
The patient’s chest pain had features of both gastroesophageal reflux disease (GERD) and coronary artery disease (CAD) with exertional angina. His high-fat diet, nightly symptoms, and the partial relief he achieved by cutting back on spicy foods and caffeine suggested GERD, but the exertional nature of the chest pain and gradual relief with rest was highly suggestive of angina, so an outpatient electrocardiogram treadmill stress test was ordered.
The stress test was markedly abnormal, showing worsening ST depressions and T-wave inversions with exertion, and he experienced chest pain during testing. An urgent left heart catheterization was performed, showing severe multivessel CAD. He subsequently underwent 3-vessel coronary artery bypass grafting. A familial hypercholesterolemia panel failed to reveal any significant variants.
As a result of these findings, the patient received a diagnosis of severe ketogenic diet–associated hypercholesterolemia and early-onset CAD.
Continue to: DISCUSSION
DISCUSSION
Low-carbohydrate (low-carb) and ketogenic diets have grown in popularity throughout the United States over the past decade, particularly for weight loss, and the diet has entered the popular consciousness with several celebrities publicly supporting it.1 Simultaneously, there also has been a growing interest in these diets for the treatment of chronic diseases, such as type 2 diabetes.2 However, the long-term cardiovascular effects of low-carb diets are not well studied, and there is significant heterogeneity among these diets.
Low-carb vs low-fat. Multiple meta-analyses comparing low-carb diets to low-fat diets have found that those following low-carb diets have significantly higher total cholesterol and LDL levels.3,4,5 The National Lipid Association’s review of evidence determined that LDL and total cholesterol responses vary in individuals following a low-carb diet, but that increasing LDL levels in particular were concerning enough to warrant lipid monitoring of patients on low-carb diets.6 Another meta-analysis evaluated the difference in estimated atherosclerotic cardiovascular disease (ASCVD) risk between low-carb and low-fat diets, finding those following a low-carb diet to have a lower estimated ASCVD risk but higher LDL levels.7
Weighing the benefits and harms. Since our patient’s dramatic weight loss and greatly increased exercise level would be expected to lower his LDL levels, the severe worsening of his LDL levels was likely related to his ketogenic diet and was a factor in the early onset of CAD. The benefits of low-carb diets for weight loss, contrasted with the consistent worsening of LDL levels, has prompted a debate about which parameters should be considered in estimating the long-term risk of these diets for patients. Diamond et al8 posit that these diets have beneficial effects on “the most reliable [cardiovascular disease] risk factors,” but long-term, patient-oriented outcome data are lacking, and these diets may not be appropriate for certain patients, as our case demonstrates.
A reasonable strategy for patients contemplating a low-carb diet specifically for weight loss would be to use such a diet for 3 to 6 months to achieve initial and rapid results, then continue with a heart-healthy diet and increased exercise levels to maintain weight loss and reduce long-term cardiovascular risk.
Our patient was started on a postoperative medication regimen of aspirin 81 mg/d, evolocumab 140 mg every 14 days, metoprolol tartrate 25 mg bid, and rosuvastatin 10 mg/d. A year later, he was able to resume a high level of physical activity (6-mile runs) without chest pain. His follow-up lipid panel showed a total cholesterol of 153 mg/dL; LDL, 53 mg/dL; HDL, 89 mg/dL; and triglycerides, 55 mg/dL. He had also switched to a regular diet and had been able to maintain his weight loss.
THE TAKEAWAY
Growing evidence suggests that low-carb diets may have a significant and detrimental effect on LDL levels. The long-term safety of these diets hasn’t been well studied, particularly regarding cardiovascular outcomes. At a minimum, patients who initiate low-carb diets should be counseled on general dietary recommendations regarding saturated fat and cholesterol intake, and they should have a follow-up lipid screening to evaluate for any significant worsening in total cholesterol and LDL levels.
CORRESPONDENCE
Samuel Dickmann, MD, 13611 NW 1st Lane, Suite 200, Newberry, FL 32669; [email protected]
1. Gorin A. What is the keto diet – and is it right for you? NBC News BETTER. February 22, 2018. Accessed February 3, 2023. www.nbcnews.com/better/health/what-keto-diet-it-right-you-ncna847256
2. Tinguely D, Gross J, Kosinski, C. Efficacy of ketogenic diets on type 2 diabetes: a systematic review. Current Diabetes Reports. 2021;21:32. doi: 10.1007/s11892-021-01399-z
3. Mansoor N, Vinknes KJ, Veierod MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466-479. doi: 10.1017/S0007114515004699
4. Bueno NB, de Melo ISV, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. doi: 10.1017/S0007114513000548
5. Chawla S, Tessarolo Silva F, Amaral Medeiros S, et al. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12:3774. doi: 10.3390/nu12123774
6. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13:689-711.e1. doi: 10.1016/j.jacl.2019.08.003
7. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817
8. Diamond DM, O’Neill BJ, Volek JS. Low carbohydrate diet: are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr Opin Endocrinol Diabetes Obes. 2020;27:291-300. doi: 10.1097/MED.0000000000000568
1. Gorin A. What is the keto diet – and is it right for you? NBC News BETTER. February 22, 2018. Accessed February 3, 2023. www.nbcnews.com/better/health/what-keto-diet-it-right-you-ncna847256
2. Tinguely D, Gross J, Kosinski, C. Efficacy of ketogenic diets on type 2 diabetes: a systematic review. Current Diabetes Reports. 2021;21:32. doi: 10.1007/s11892-021-01399-z
3. Mansoor N, Vinknes KJ, Veierod MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466-479. doi: 10.1017/S0007114515004699
4. Bueno NB, de Melo ISV, de Oliveira SL, et al. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. doi: 10.1017/S0007114513000548
5. Chawla S, Tessarolo Silva F, Amaral Medeiros S, et al. The effect of low-fat and low-carbohydrate diets on weight loss and lipid levels: a systematic review and meta-analysis. Nutrients. 2020;12:3774. doi: 10.3390/nu12123774
6. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13:689-711.e1. doi: 10.1016/j.jacl.2019.08.003
7. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10:e0139817. doi: 10.1371/journal.pone.0139817
8. Diamond DM, O’Neill BJ, Volek JS. Low carbohydrate diet: are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr Opin Endocrinol Diabetes Obes. 2020;27:291-300. doi: 10.1097/MED.0000000000000568
Infiltrating Wound Vacuum-Assisted Closure With Topical Amphotericin for Mucormycosis Infection of the Achilles Tendon
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
Vacuum-assisted closure (VAC) of wounds has become a foundational tool in the armamentarium of wound care specialists. Using a system consisting of a sponge, semi-occlusive barrier, and fluid collection device, VAC systems apply constant negative pressure resulting in macro and micro deformation to a wound, stabilization of the wound environment, and removal of inflammatory factors in wound fluid.1 These conditions allow for the removal of drainage and fluid from a wound bed, reduced edema and inflammation, reduced bacterial load, recruitment of healing factors, approximation of wound edges, and increased blood flow to the wound.2
In complex, infected wounds, a variation of negative pressure wound therapy (NPWT) via the instillation of topical antibiotics (instillation VAC) has been used.3 This variation has been advantageous even in soft tissue fungal infections. Early and aggressive treatment of such infections is critical to prevent dissemination, particularly in aggressive infections, such as mucormycosis.4 We present a case of a patient with a mucormycosis infection of his left Achilles tendon and overlying skin who was successfully treated with surgical debridement and wound care with instillation NPWT with topical amphotericin B.
Case Presentation
A 53-year-old man underwent left Achilles tendon reconstruction with allograft after a complete tear during exercise. He had no relevant medical history and was otherwise healthy, which he attributed to working out daily. About a week after the operation, he began having incisional breakdown, prompting presentation to an emergency department. There, he received IV antibiotics along with multiple debridements. After the wound failed to improve and intra-operative cultures grew mucormycosis, he was transferred to our facility for a higher level of care. On admission, he was immediately given IV amphotericin B and scheduled for repeat debridement.
After 1 prior debridement and 10 total days of IV amphotericin, a repeat debridement was performed. After the debridement, the installation VAC was applied to the patient’s left lower extremity wound with an instilling fluid of amphotericin B and the settings as follows: smart phase instill volume, 110 mL; soak time, 3.5 hours; target pressure, 125 mm Hg; intensity, low; and VAC therapy mode, continuous. After 5 days, the wound bed appeared clean without overt signs of infection. However, due to some toxicity to healthy surrounding soft tissue, the instillation VAC was discontinued and standard NPWT was started. The patient underwent 2 additional rounds of debridement with partial delayed closure. Four weeks after discontinuation of the instillation VAC, the wound appeared healthy and granulated so the patient underwent split-thickness skin grafting to the left posterior ankle. He subsequently completed a course of oral antifungal medication as an outpatient.
The patient was seen in the outpatient clinic for 14 months from the initial mucormycosis infection (Figure).
Discussion
Mucormycosis is an infection caused by fungi in the class Zygomycetes and of the order Mucorales that typically occurs in immunocompromised patients, especially those with diabetic ketoacidosis and neutropenia. Given that this patient had no relevant medical history and was otherwise healthy, he was at extremely low risk of this type of infection. In this patient’s case, the spores of this nonseptate hyphae wide-branching species were most likely introduced at the time of left Achilles tendon repair. Mucormycosis is progressive and can be fatal unless treated, with a mortality rate approaching 70%.5 The rarity and heterogeneity of mucormycosis make treatment variable.6 No prospective or randomized clinical trials exist in plastic surgery literature.
The use of wound VAC in combination with the instillation of amphotericin B to treat cutaneous mucormycosis is not well documented. Mucormycosis infections are traditionally addressed with surgical debridement and antifungal therapy, specifically IV amphotericin B.7,8 As previously noted, NPWT has become the gold standard in treating complex wounds.3 Additionally, wound VAC therapy with instillation has been noted in the literature as a reliable method to treat bacteria-infected wounds, providing a shorter treatment period and earlier wound closure.9 Instillation VAC therapy has proven particularly useful in complex, infected wounds, such as aggressive fungal infections.
Mucormycosis treatment is challenging particularly in the extremities as management must balance both mortality and limb salvage. In this case, the use of NPWT with wound VAC and intervals of instilling amphotericin B facilitated infection control in this lower extremity mucormycosis infection. The significant adverse effect profile of amphotericin B, particularly the nephrotoxicity, should be seriously considered when deciding the treatment regimen for patients affected by mucormycosis. Locally, topical amphotericin B has been reported to cause blistering, itchiness, redness, peeling, and dryness. However, topical preparations of amphotericin B are nontoxic unlike their IV counterpart, able to cross the physiological barriers of the skin while simultaneously targeting macrophages in the dermis and epidermis.10
Conclusions
Although the mainstay of treatment for systemic mucormycosis is radical debridement and IV amphotericin B, a more localized infection may benefit from an adjunct like an instillation wound VAC with topical amphotericin B, as presented in this case study. Swift treatment with wound VAC was beneficial in the overall recovery and tissue healing of this patient and may be beneficial in similar cases.
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
1. Normandin S, Safran T, Winocour S, et al. negative pressure wound therapy: mechanism of action and clinical applications. Semin Plast Surg. 2021;35(3):164-170. doi:10.1055/s-0041-1731792
2. Agarwal P, Kukrele R, Sharma D. Vacuum assisted closure (VAC)/negative pressure wound therapy (NPWT) for difficult wounds: a review. J Clin Orthop Trauma. 2019;10(5):845-848. doi:10.1016/j.jcot.2019.06.015
3. Gabriel A, Shores J, Bernstein B, et al. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;6(suppl 2):1-25. doi:10.1111/j.1742-481X.2009.00628.x
4. Guégan S, Lanternier F, Rouzaud C, Dupin N, Lortholary O. Fungal skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):124-130. doi:10.1097/QCO.0000000000000252
5. Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S16-S22. doi:10.1093/cid/cir865
6. Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of mucormycosis. J Fungi (Basel). 2018;4(3):90. Published 2018 Jul 31. doi:10.3390/jof4030090
7. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569. doi:10.1128/CMR.18.3.556-569.2005
8. Losee JE, Selber J, Vega S, Hall C, Scott G, Serletti JM. Primary cutaneous mucormycosis: guide to surgical management. Ann Plast Surg. 2002;49(4):385-390. doi:10.1097/00000637-200210000-00009
9. Webb LX. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10(5):303-311. doi:10.5435/00124635-200209000-00002
10. Varikuti S, Oghumu S, Saljoughian N, et al. Topical treatment with nanoliposomal Amphotericin B reduces early lesion growth but fails to induce cure in an experimental model of cutaneous leishmaniasis caused by Leishmania mexicana. Acta Trop. 2017;173:102-108. doi:10.1016/j.actatropica.2017.06.004
14-year-old boy • aching midsternal pain following a basketball injury • worsening pain with direct pressure and when the patient sneezed • Dx?
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).
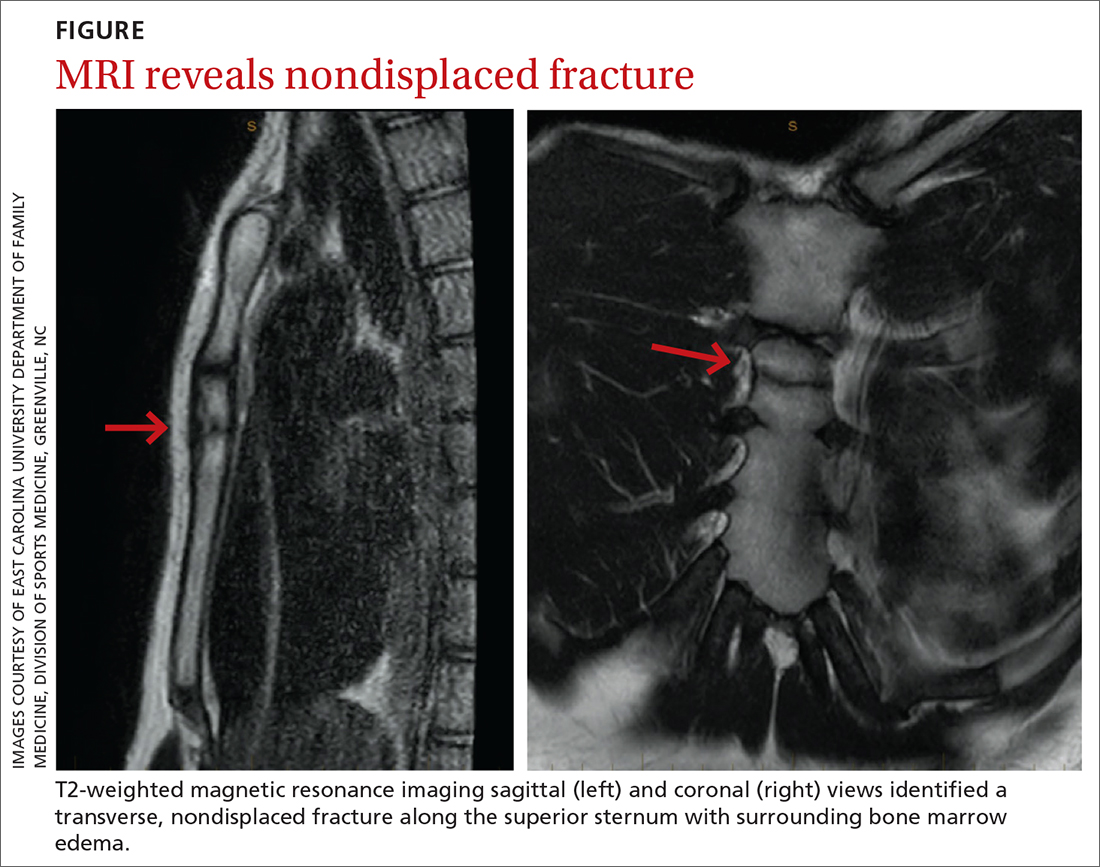
DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).

DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).

DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17