User login
Long-term Remission of Pyoderma Gangrenosum, Acne, and Hidradenitis Suppurativa Syndrome
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.
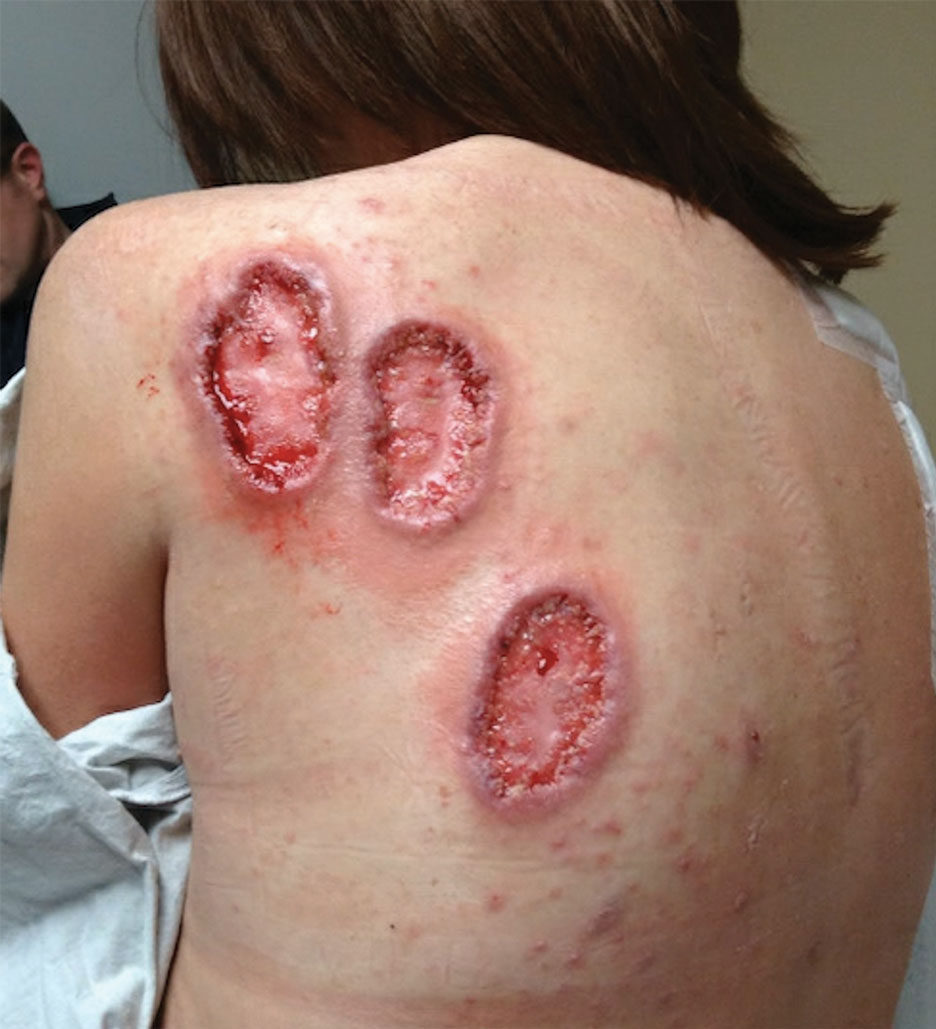
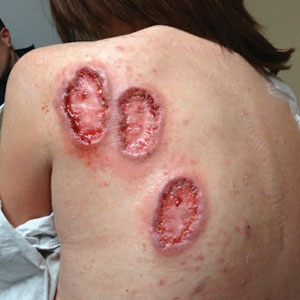
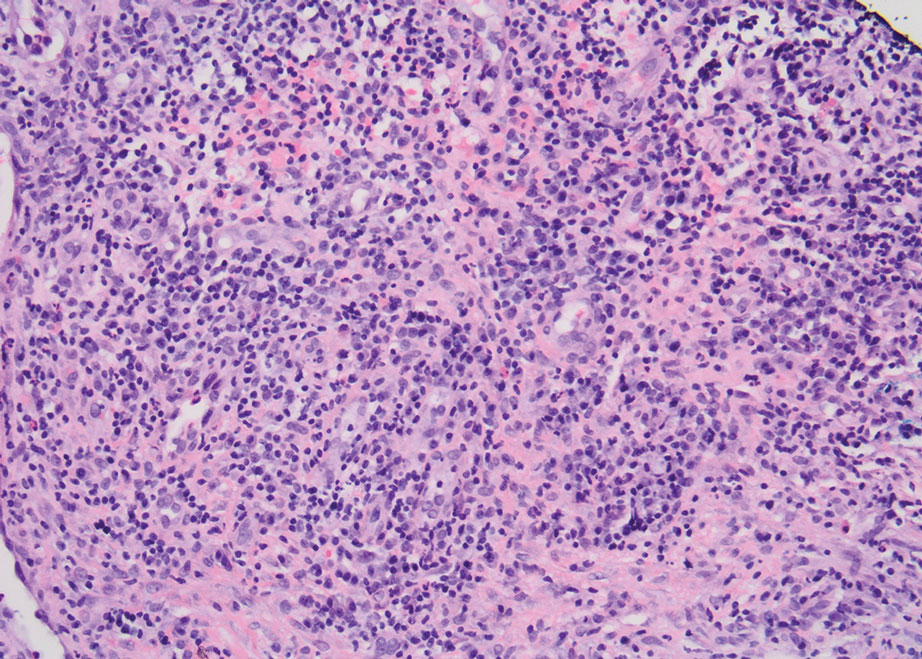
Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.
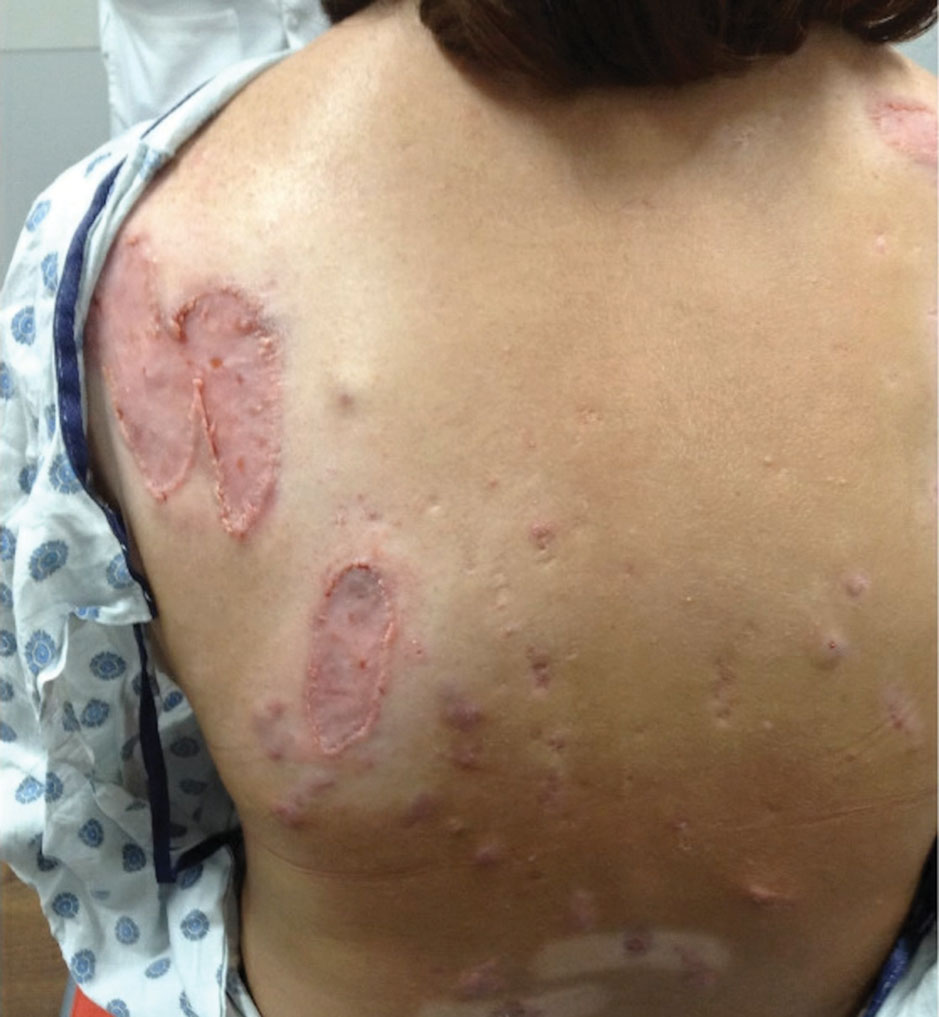
After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
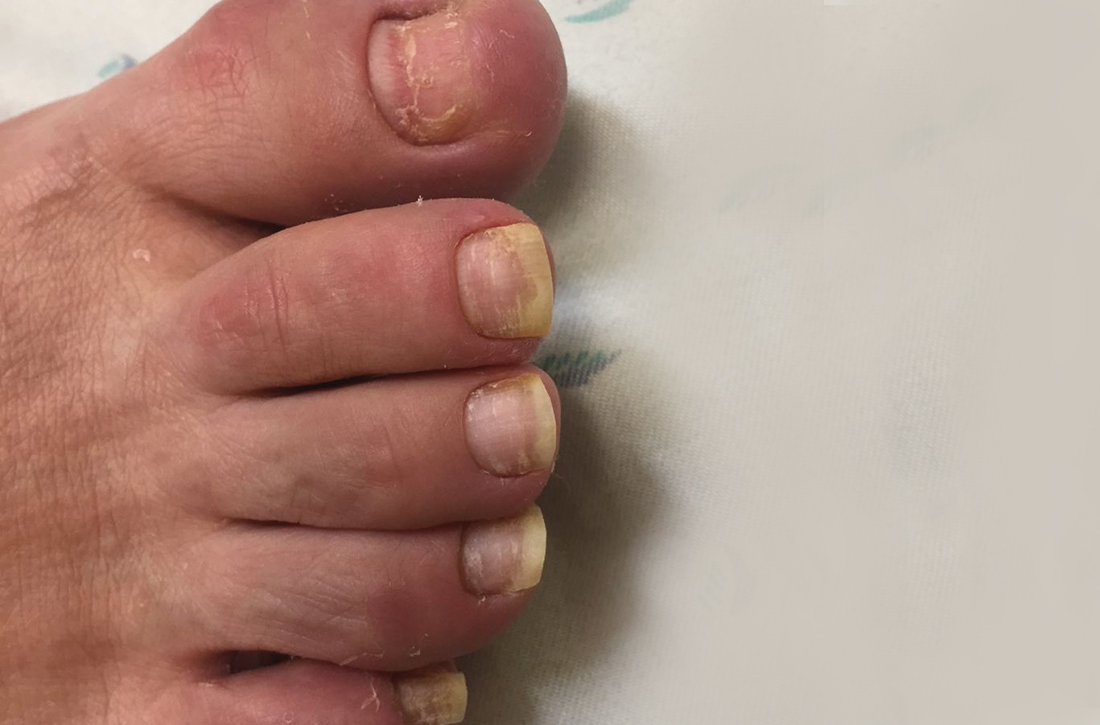
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.
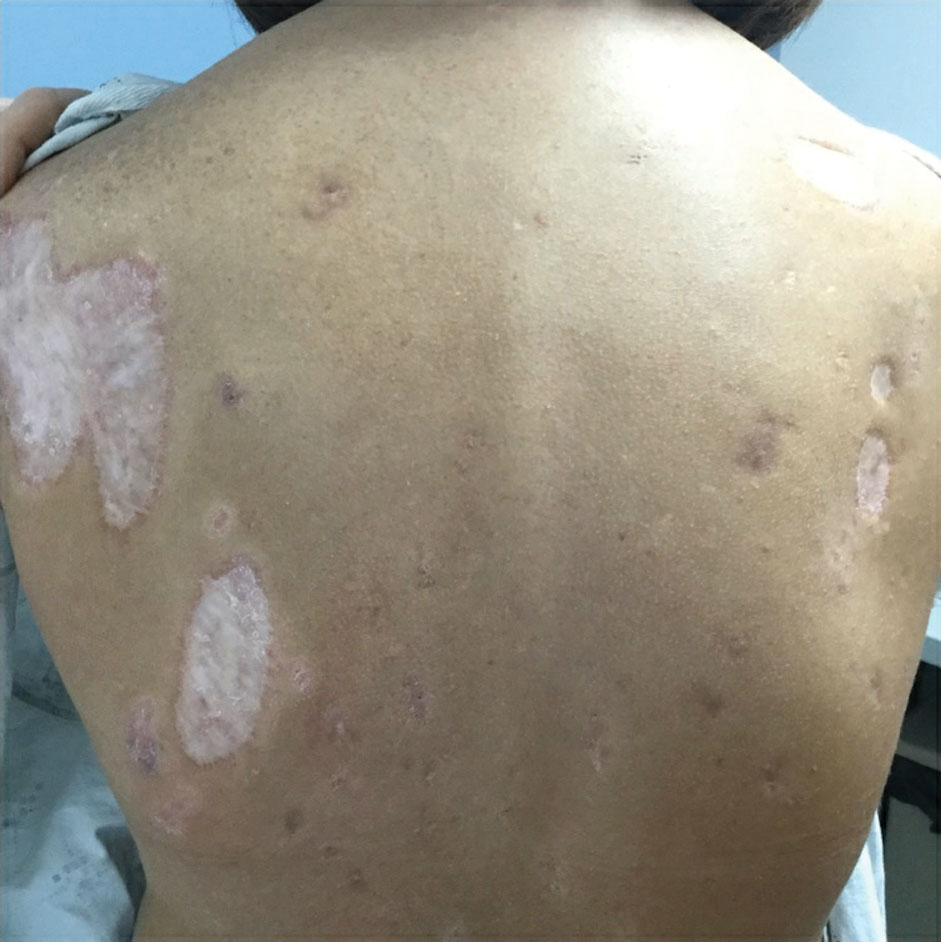
The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.
Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.
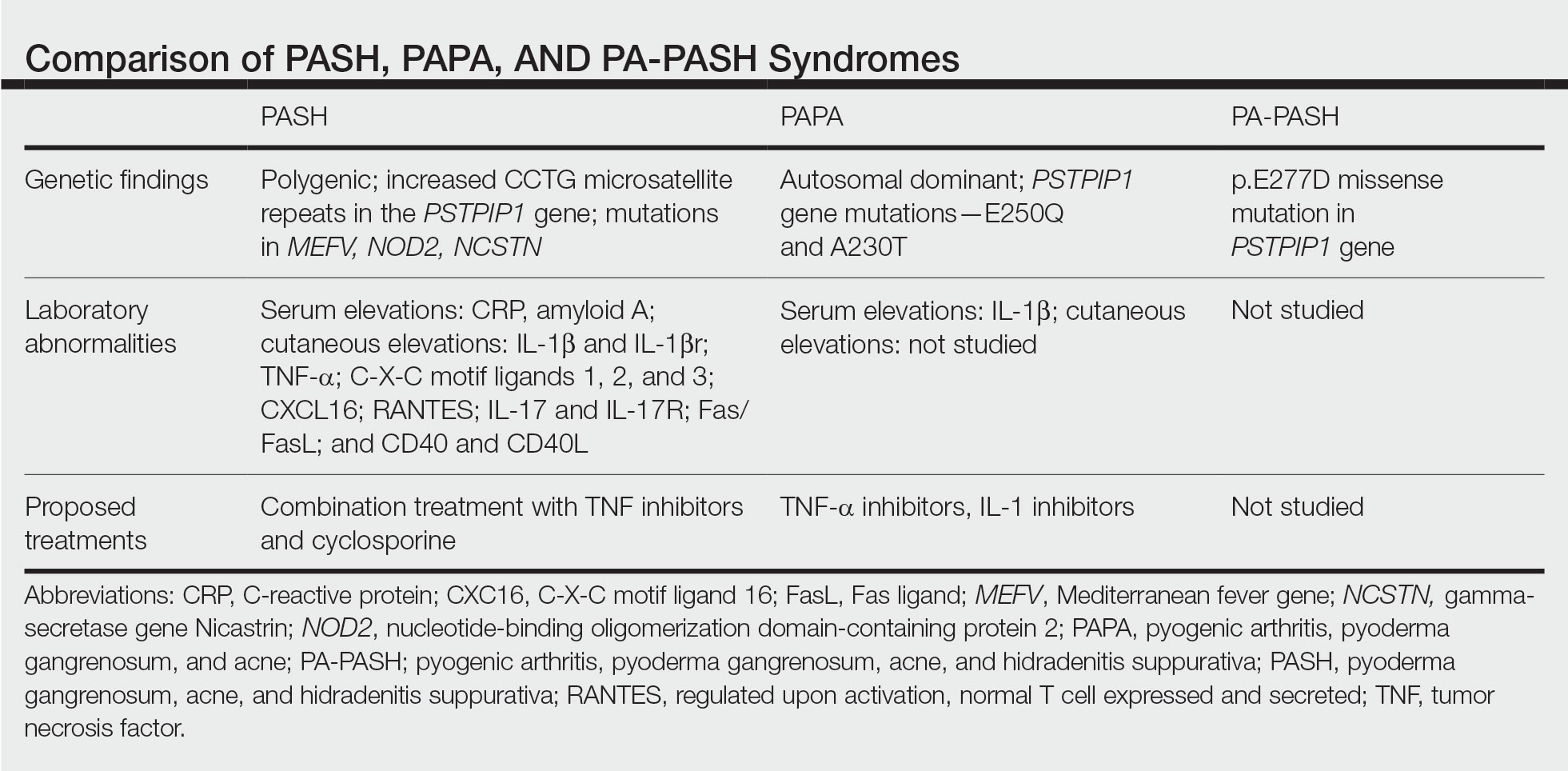
PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
Pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (HS)(PASH) syndrome is a recently identified disease process within the spectrum of autoinflammatory diseases (AIDs), which are distinct from autoimmune, infectious, and allergic syndromes and are gaining increasing interest given their complex pathophysiology and therapeutic resistance.1 Autoinflammatory diseases are defined by a dysregulation of the innate immune system in the absence of typical autoimmune features, including autoantibodies and antigen-specific T lymphocytes.2 Mutations affecting proteins of the inflammasome or proteins involved in regulating inflammasome function have been associated with these AIDs.2
Many AIDs have cutaneous involvement, as seen in PASH syndrome. Pyoderma gangrenosum is a neutrophilic dermatosis presenting as skin ulcers with undermined, erythematous, violaceous borders. It can be isolated, syndromic, or associated with inflammatory conditions (eg, inflammatory bowel disease, rheumatologic disorders, hematologic disorders).1 Acne vulgaris develops because of chronic obstruction of hair follicles as a result of disordered keratinization and abnormal sebaceous stem cell differentiation.2 Propionibacterium acnes can reside and replicate within the biofilm community of the hair follicle and activate the inflammasome.2,3 Hidradenitis suppurativa, a chronic relapsing neutrophilic dermatosis, is a debilitating inflammatory disease of the hair follicles involving apocrine gland–bearing skin (ie, the axillary, inguinal, and anogenital regions).2 Onset often occurs between the ages of 20 and 40 years, with a 3-fold higher incidence in women compared to men.3 Patients experience painful, deep-seated nodules that drain into sinus tracts and abscesses. The condition can be isolated or associated with inflammatory conditions, such as inflammatory bowel disease.4
PASH syndrome has been described as a polygenic autoinflammatory condition that most commonly presents in young adults, with onset of acne beginning years prior to other manifestations. A study analyzing 5 patients with PASH syndrome reported an average age of 32.2 years at diagnosis with a disease duration of 3 to 7 years.5 Pathophysiology of this condition is not well understood, with many hypotheses calling upon dysregulation of the innate immune system, a commonality this syndrome may share with other AIDs. Given its poorly understood pathophysiology, treating PASH syndrome can be especially difficult. We report a novel case of disease remission lasting more than 4 years using adalimumab and cyclosporine. We also discuss prior treatment successes and hypotheses regarding etiologic factors in PASH syndrome.
Case Report
A 36-year-old woman presented for evaluation of open draining ulcerations on the back of 18 months’ duration. She had a 16-year history of scarring cystic acne of the face and HS of the groin. The patient’s family history was remarkable for severe cystic acne in her brother and son as well as HS in her mother and another brother. Her treatment history included isotretinoin, doxycycline, and topical steroids.

Physical examination revealed 2 ulcerations with violaceous borders involving the left upper back (greatest diameter, 5×7 cm)(Figure 1). Evidence of papular and cystic acne with residual scarring was noted on the cheeks. Scarring from HS was noted in the axillae and right groin. A biopsy from the edge of an ulceration on the back demonstrated epidermal spongiosis with acute and chronic inflammation and fibrosis (Figure 2). The clinicopathologic findings were most consistent with PG, and the patient was diagnosed with PASH syndrome, given the constellation of cutaneous lesions.

After treatment with topical and systemic antibiotics for acne and HS for more than 1 year failed, the patient was started on adalimumab. The initial dose was 160 mg subcutaneously, then 80 mg 2 weeks later, then 40 mg weekly thereafter. Doxycycline was continued for treatment of the acne and HS. After 6 weeks of adalimumab, the PG worsened and prednisone was added. She developed tender furuncles on the back, and cultures grew Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus that responded to ciprofloxacin and cephalexin.
Due to progression of PG on adalimumab, switching to an infliximab infusion or anakinra was considered, but these options were not covered by the patient’s health insurance. Three months after the initial presentation, the patient was started on cyclosporine 100 mg 3 times daily (5 mg/kg/d) while adalimumab was continued; the ulcers started to improve within 2.5 weeks. After 3 months (Figure 3), the cyclosporine was reduced to 100 mg twice daily, and adalimumab was continued. She had a slight flare of PG after 8 months of treatment when adalimumab was unavailable to her for 2 months. After 8 months on cyclosporine, the dosage was tapered to 100 mg/d and then completely discontinued after 12 months.

The patient has continued on adalimumab 40 mg weekly with excellent control of the PG (Figure 4), although she did have one HS flare in the left axilla 11 months after the initial treatment. The patient’s cystic acne has intermittently flared and has been managed with spironolactone 100 mg/d for 3 years. After 4 years of management, the patient’s PG and HS remain well controlled on adalimumab.

Comment
Our case represents a major step in refining long-term treatment approaches for PASH syndrome due to the 4-year remission. Prior cases have reported use of anakinra, anakinra-cyclosporine combination, prednisone, azathioprine, topical tacrolimus, etanercept, and dapsone without sustainable success.1-6 The case studies discussed below have achieved remission via alternative drug combinations.
Staub et al4 found greatest success with a combination of infliximab, dapsone, and cyclosporine, and their patient had been in remission for 20 months at time of publication. Their hypothesis proposed that multiple inflammatory signaling pathways are involved in PASH syndrome, and this is why combination therapy is required for remission.4 In 2018, Lamiaux et al7 demonstrated successful treatment with rifampicin and clindamycin. Their patient had been in remission for 22 months at the time of publication—this time frame included 12 months of combination therapy and 10 months without medication. The authors hypothesized that, because of the autoinflammatory nature of these antibiotics, this pharmacologic combination could eradicate pathogenic bacteria from host microbiota while also inhibiting neutrophil function and synthesis of chemokines and cytokines.7
More recently, reports have been published regarding the success of tildrakizumab, an IL-23 antagonist, and ixekizumab, an IL-17 antagonist, in the treatment of PASH syndrome.6,8 Ixekizumab was used in combination with doxycycline, and remission was achieved in 12 months.8 However, tildrakizumab was used alone and achieved greater than 75% improvement in disease manifestations within 2 months.
Marzano et al5 conducted protein arrays and enzyme-linked immunosorbent assay to analyze the expression of cytokine, chemokine, and effector molecule profiles in PASH syndrome. It was determined that serum analysis displayed a normal cytokine/chemokine profile, with the only abnormalities being anemia and elevated C-reactive protein. There were no statistically significant differences in serum levels of IL-1β, tumor necrosis factor (TNF) α, or IL-17 between PASH syndrome and healthy controls. However, cutaneous analysis revealed extensive cytokine and chemokine hyperactivity for IL-1β and IL-1β receptor; TNF-α; C-X-C motif ligands 1, 2, and 3; C-X-C motif ligand 16;
Ead et al3 presented a unique perspective focusing on cutaneous biofilm involvement in PASH syndrome. Microbes within these biofilms induce the migration and proliferation of inflammatory cells that consume factors normally utilized for tissue catabolism. These organisms deplete necessary biochemical cofactors used during healing. This lack of nutrients needed for healing not only slows the process but also promotes favorable conditions for the growth of anerobic species. In conjunction, biofilm formation restricts bacterial access to oxygen and nutrients, thus decreasing the bacterial metabolic rate and preventing the effects of antibiotic therapy. These features of biofilm communities contribute to inflammation and possibly the troubling resistance to many therapeutic options for PASH syndrome.
Each component of PASH syndrome has been associated with biofilm formation. As previously described, PG manifests in the skin as painful ulcerations, often with slough. This slough is hypothesized to be a consequence of increased vascular permeability and exudative byproducts that accompany the inflammatory nature of biofilms.3 Acne vulgaris has well-described associations with P acnes. Ead et al3 described P acnes as a component of the biofilm community within the microcomedone of hair follicles. This biofilm allows for antibiotic resistance occasionally seen in the treatment of acne and is potentially the pathogenic factor that both impedes healing and enhances the inflammatory state. Hidradenitis suppurativa has been associated with biofilm formation.3
In further pursuit of PASH syndrome pathophysiology, many experts have sought to uncover the relationship between PASH syndrome and the previously described pyogenic arthritis, PG, and acne (PAPA) syndrome, another entity within the AIDs spectrum (Table). This condition was first recognized in 1997 in a 3-generation family with 10 affected members.1 It is characterized by PG and acne, similar to PASH; however, PAPA syndrome includes PG arthritis and lacks HS. Pyogenic arthritis manifests as recurrent aseptic inflammation of the joints, mainly the elbows, knees, and ankles. Pyogenic arthritis commonly is the presenting symptom of PAPA syndrome, with onset in childhood.2 As patients age, the arthritic symptoms decrease, and skin manifestations become more prominent.

PAPA syndrome has autosomal-dominant inheritance with mutations on chromosome 15 in the proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) gene.1 This mutation induces hyperphosphorylation of PSTPIP1, allowing for increased binding affinity to pyrin. Both PSTPIP1 and pyrin are co-expressed as parts of the NLRP3 inflammasome in granulocytes and monocytes.1 As a result, pyrin is more highly bound and loses its inhibitory effect on the NLRP3 inflammasome pathway. This lack of inhibition allows for uninhibited cleavage of pro–IL-1β to active IL-1β by the inflammasome.1
Elevated concentrations of IL-1β in patients with PAPA syndrome result in a dysregulation of the innate immune system. IL-1β induces the release of proinflammatory cytokines, namely TNF-α; interferon γ; IL-8; and regulated on activation, normal T cell expressed and secreted (RANTES), all of which activate neutrophils and induce neutrophilic inflammation.2 IL-1β not only initiates this entire cascade but also acts as an antiapoptotic signal for neutrophils.2 When IL-1β reaches a critical threshold, it induces enough inflammation to cause severe tissue damage, thus causing joint and cutaneous disease in PAPA syndrome. IL-1 inhibitors (anakinra) or TNF-α inhibitors (etanercept, adalimumab, infliximab) have been used many times to successfully treat PAPA syndrome, with TNF-α inhibitors providing the most consistent results.
Another AIDs entity with similarities to both PAPA syndrome and PASH syndrome is pyogenic arthritis, PG, acne, and HS (PA-PASH) syndrome. First identified in 2012 by Bruzzese,9 genetic analyses revealed a p.E277D missense mutation in PSTPIP1 in PA-PASH syndrome. Research has suggested that the key molecular feature is neutrophil activation by TH17 cells and the TNF-α axis.9 This syndrome has not been further characterized, and little is known regarding adequate treatment for PA-PASH syndrome.
Although it is similar in phenotype to aspects of PAPA and PA-PASH syndromes, PASH syndrome has distinct genotypic and immunologic abnormalities. Genetic analysis of this condition has shown an increased number of CCTG repeats in proximity to the PSTPIP1 promoter. It is hypothesized that these additional repeats predispose patients to neutrophilic inflammation in a similar manner to a condition described in France, termed aseptic abscess syndrome.1,5 Other mutations have been identified, including those in IL-1N, PSMB8, MEFV, NOD2, NCSTN, and more.2,7 However, it has been determined that the majority of these variants have already been filed in the Single Nucleotide Polymorphism Database or in the Registry of Hereditary Auto-inflammatory Disorders Mutations.2 The question remains regarding the origin of inflammation seen in PASH syndrome; the potential role of biofilms; and the relationship between PASH, PAPA, and PA-PASH syndromes. Much work remains to be done in refining therapeutic options for PASH syndrome. Continued biochemical research is necessary, as well as collaboration among dermatologists worldwide who find success in treating this condition.
Conclusion
There are genotypic and phenotypic similarities between PASH, PAPA, and PA-PASH syndromes, with various mutations within or near the PSTPIP1 gene; however, their genetic discrepancies seem to play a major role in the pathophysiology of each syndrome. Much work remains to be done in PA-PASH syndrome, which has not yet been well described. Meanwhile, PAPA syndrome has been well characterized with mutations affecting proteins of the NLRP3 inflammasome, resulting in elevated IL-1β and excess neutrophilic inflammation. In PASH syndrome, the importance of increased repeats near the PSTPIP1 promoter is yet to be elucidated. It has been shown that these abnormalities predispose individuals to neutrophilic inflammation, but the mechanism by which they do so is unknown. In addition, consideration of biofilms and their predisposition to inflammation within the pathophysiology of PASH syndrome is a possibility that must be considered when discussing therapeutic options. Based on our case study and previous successes in treating PASH syndrome, it is clear that a multidrug approach is necessary for remission. It is likely that the etiology of PASH syndrome is multifaceted and involves hyperactivity in multiple arms of the innate immune system.
Patients with PASH syndrome have severely impaired quality of life and often experience social withdrawal due to the disfiguring sequelae and limited treatment options available. To improve patient outcomes, it is essential for physicians and scientists to report on successful treatment strategies and advances in immunologic understanding. Improved understanding of PASH syndrome calls for further genetic exploration into the role of additional genomic repeats and how these affect the PSTPIP1 gene and inflammasome activity. As medical advances improve understanding of the pathophysiology of this disease entity, it will likely become clear which mechanisms are most important in disease progression and how clinicians can best optimize treatment.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Ead JK, Snyder RJ, Wise J, et al. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30:216-223.
- Staub J, Pfannschmidt N, Strohal R, et al. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol. 2015;29:2243-2247.
- Marzano AV, Ceccherini I, Gattorno M, et al. Association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles with other autoinflammatory diseases. Medicine (Baltimore). 2014;93:E187.
- Kok Y, Nicolopoulos J, Varigos G, et al. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61:E373-E374.
- Lamiaux M, Dabouz F, Wantz M, et al. Successful combined antibiotic therapy with oral clindamycin and oral rifampicin for pyoderma gangrenosum in patient with PASH syndrome. JAAD Case Rep. 2018;4:17-21.
- Gul MI, Singam V, Hanson C, et al. Remission of refractory PASH syndrome using ixekizumab and doxycycline. J Drugs Dermatol. 2020;19:1123.
- Bruzzese V. Pyoderma gangrenosum, acne conglobata, suppurative hidradenitis, and axial spondyloarthritis: efficacy of anti-tumor necrosis factor α therapy. J Clin Rheumatol. 2012;18:413-415.
Practice Points
- Despite phenotypic similarities among pyoderma gangrenosum (PG), acne, and hidradenitis suppurativa (PASH) syndrome; pyogenic arthritis, PG, and acne syndrome; and pyogenic arthritis–PASH syndrome, there are genotypic differences that contribute to unique inflammatory cytokine patterns and the need for distinct pharmacologic considerations within each entity.
- When formulating therapeutic regimens for patients with PASH syndrome, it is essential for dermatologists to consider the likelihood of hyperactivity in multiple pathways of the innate immune system and utilize a combination of multimodal antiinflammatory therapies.
55-year-old woman • unilateral nasal drainage • salty taste • nasal redness • recent COVID-19 nasal swabs • Dx?
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
THE CASE
A 55-year-old woman was evaluated in a family medicine clinic for clear, right-side nasal drainage. She stated that the drainage began 5 months earlier after 2 hospitalizations for severe anxiety leading to emesis and hypokalemia. She reported 3 different COVID-19 nasal swab tests performed on the right nare. Chart review showed 2 negative COVID-19 tests, 6 days apart. Since the hospitalizations, the patient had been given antihistamines for rhinorrhea at an urgent care visit. Despite this treatment, the patient reported a constant drip from the right nare with a salty taste. She also reported experiencing occasional headaches but denied nausea/vomiting.
The patient’s history included uncontrolled hypertension, treatment-resistant anxiety and depression, obstructive sleep apnea, chronic sinus disease (observed on computed tomography [CT] scans), and type 2 diabetes. She was on amlodipine 10 mg/d for hypertension and was not taking any medication for diabetes.
On examination, the patient’s vital signs were within normal limits except for an elevated blood pressure of 158/88 mm Hg. The patient had persistent clear rhinorrhea fluid draining from the right nostril that was exacerbated when she looked down. Right nasal erythema was present.
THE DIAGNOSIS
The patient’s negative COVID-19 tests, lack of improvement on antihistamines, and description of the nasal fluid as salty tasting prompted us to suspect a cerebrospinal fluid (CSF) leak. The clinical work-up included a halo (“double-ring”) sign test, a β-2 transferrin test, and a sinus x-ray.
The halo sign test was negative for CSF fluid. Sinus/skull x-ray did not show a cribriform or other fracture. However, a sample of the nasal fluid collected in a sterile container was positive for β-2 transferrin, the gold-standard laboratory test to confirm a CSF leak.
The patient was sent for a maxillofacial CT scan without contrast. Results showed a 3-mm defect over the right ethmoid roof associated with a 10 × 16–mm low-attenuation structure in the right ethmoid labyrinth, suspicious for encephalocele. This defect, in the setting of the patient’s history of chronic sinus disease, furthered our suspicion of a CSF leak secondary to COVID-19 testing. Radiology confirmed the diagnosis.
DISCUSSION
CSF rhinorrhea is CSF leakage through the nasal cavity due to abnormal communication between the arachnoid membrane and nasal mucosa.1 The most commonly reported risk factors for this include female sex, middle age (fourth to fifth decade), obesity (body mass index > 40), intracranial hypertension, and obstructive sleep apnea.1,2
Continue to: Clear, unilateral rhinorrhea...
Clear, unilateral rhinorrhea drainage that increases at times of relatively increased intracranial pressure and has a metallic or salty taste is suspicious for CSF rhinorrhea.3 It can occur following skull‐base trauma (eg, cribriform plate, temporal bone), endoscopic sinus surgery, or neurosurgical procedures, or have a spontaneous etiology.3,4
Modalities to confirm CSF rhinorrhea include radionuclide cisternography and testing of fluid for the halo sign, glucose, and the CSF-specific proteins β‐2 transferrin and β-trace protein.3,4 High‐resolution CT is the imaging method most commonly used for localizing a CSF leak.4
Treatment is provided in the hospital
Patients with CSF rhinorrhea typically require inpatient management with bed rest, head-of-bed elevation, and frequent neurologic evaluation, as persistent CSF rhinorrhea increases the risk for meningitis, thus necessitating surgical intervention.3,5 Some cases resolve with bed rest alone. Endonasal endoscopic repair of CSF leaks has become the standard of care because of its high success rate and lower morbidity profile.4
The preferred treatment method for encephalocele is surgical removal after diagnosis is confirmed with CT or magnetic resonance imaging.6
Our patient underwent surgery to remove the encephalocele. The surgeons reported no evidence of fracture.
The final cause of her CSF leak is still uncertain. The surgeons felt confident it was due to ethmoidal encephalocele, a form of neural tube defect in which brain tissue herniates through structural weaknesses of the skull.6-8 While more common in infants, encephalocele can manifest in adulthood due to traumatic or iatrogenic causes.7,8
There is a previous report of encephalocele with CSF leak after COVID-19 testing.9 This case report suggests the possibility of a nasal swab causing trauma to a patient’s pre‐existing encephalocele—a probability in our patient’s case. It is unlikely, however, that the nasal swab itself violated the bony skull base.
THE TAKEAWAY
This case exemplifies how unexplained local symptoms, a high index of suspicion, and adequate work-up can lead to a rare diagnosis. Diagnostic strategies employed for cases of CSF rhinorrhea vary widely due to limited evidence-based guidance.4 Unilateral rhinorrhea with clear fluid that increases at times of increased intracranial pressure, such as bending over, should prompt suspicion for CSF rhinorrhea. With millions of people getting nasal swabs daily during the COVID-19 pandemic, it is even more important to keep CSF leak in our differential diagnosis.
CORRESPONDENCE
Eliana Lizeth Garcia, MD, BS, BA, University of New Mexico Health Sciences Center, 1209 University Boulevard NE, Albuquerque, NM 87131-5001; [email protected]
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
1. Keshri A, Jain R, Manogaran RS, et al. Management of spontaneous CSF rhinorrhea: an institutional experience. J Neurol Surg B Skull Base. 2019;80:493-499. doi: 10.1055/s-0038-1676334
2. Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:215-224. doi: 10.1002/lio2.75
3. Van Zele T, Dewaele F. Traumatic CSF leaks of the anterior skull base. B-ENT. 2016;suppl 26:19-27.
4. Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8-16. doi: 10.1002/alr.21637
5. Friedman JA, Ebersold MJ, Quast LM. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066. doi: 10.1007/s00268-001-0059-7
6. Tirumandas M, Sharma A, Gbenimacho I, et al. Nasal encephaloceles: a review of etiology, pathophysiology, clinical presentations, diagnosis, treatment, and complications. Childs Nerv Syst. 2013;29:739-744. doi: 10.1007/s00381-012-1998-z
7. Junaid M, Sobani ZU, Shamim AA, et al. Nasal encephaloceles presenting at later ages: experience of otorhinolaryngology department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012;62:74-76.
8. Dhirawani RB, Gupta R, Pathak S, et al. Frontoethmoidal encephalocele: case report and review on management. Ann Maxillofac Surg. 2014;4:195-197. doi: 10.4103/2231-0746.147140
9. Paquin R, Ryan L, Vale FL, et al. CSF leak after COVID-19 nasopharyngeal swab: a case report. Laryngoscope. 2021;131:1927-1929. doi: 10.1002/lary.29462
► Unilateral nasal drainage
► Salty taste
► Nasal redness
► Recent COVID-19 nasal swabs
64-year-old woman • hot flashes, facial flushing, excessive sweating, and palpitations • daily headaches • history of hypertension • Dx?
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.
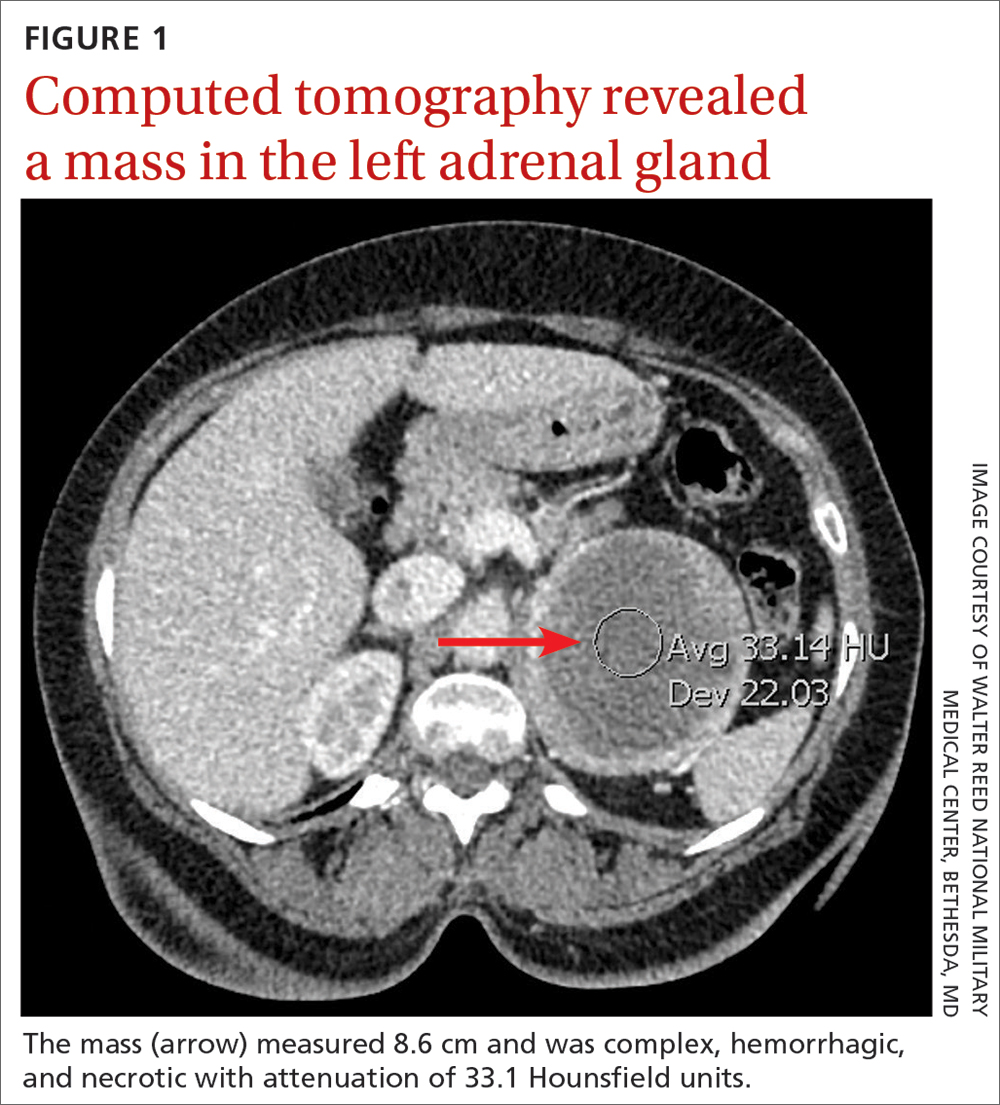
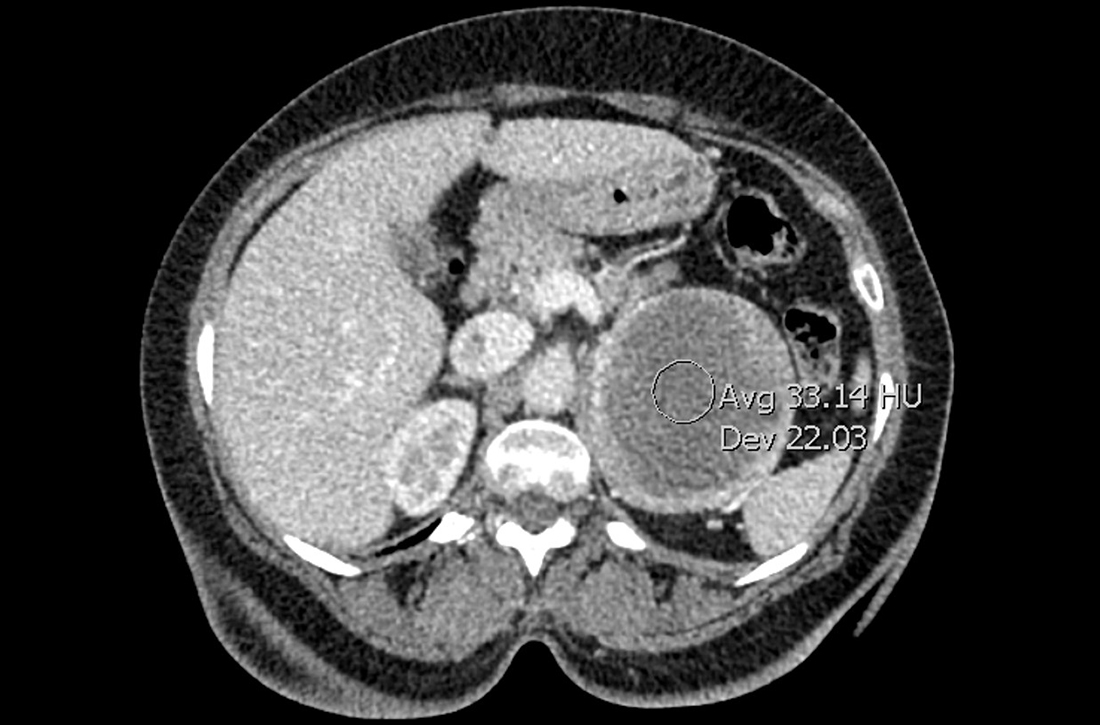
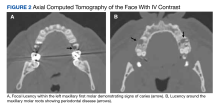
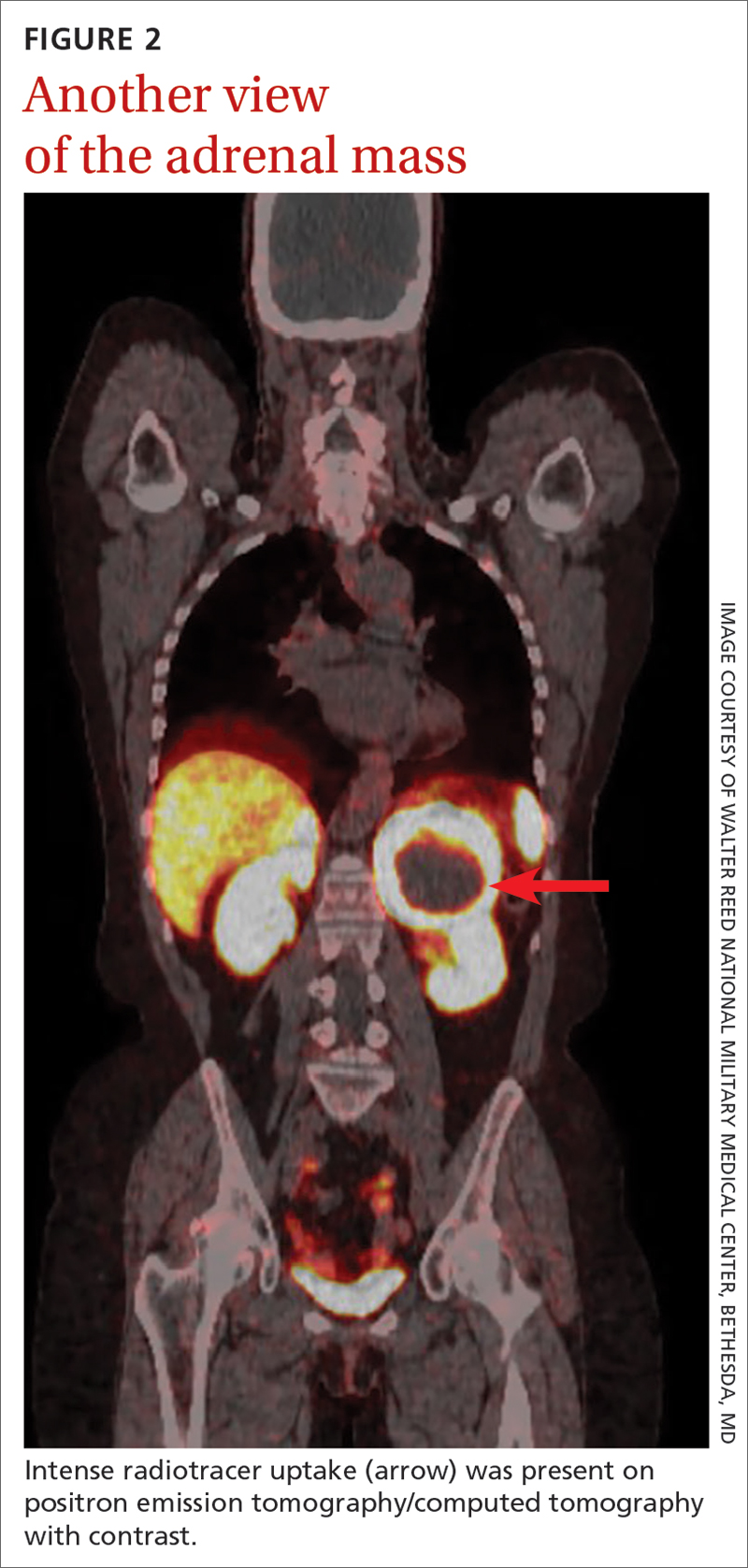
Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).
THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
► Hot flashes, facial flushing, excessive sweating, and palpitations
► Daily headaches
► History of hypertension
Pyogenic Hepatic Abscess in an Immunocompetent Patient With Poor Oral Health and COVID-19 Infection
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
Pyogenic hepatic abscess (PHA) is a collection of pus in the liver caused by bacterial infection of the liver parenchyma. This potentially life-threatening condition has a mortality rate reported to be as high as 47%.1 The incidence of PHA is reported to be 2.3 per 100,000 individuals and is more common in immunosuppressed individuals and those with diabetes mellitus, cancer, and liver transplant.2,3 PHA infections are usually polymicrobial and most commonly include enteric organisms like Escherichia coli and Klebsiella pneumoniae.4
We present a rare cause of PHA with Fusobacterium nucleatum (F nucleatum) in an immunocompetent patient with poor oral health, history of diverticulitis, and recent COVID-19 infection whose only symptoms were chest pain and a 4-week history of fever and malaise.
Case Presentation
A 52-year-old man initially presented to the C.W. Bill Young Veterans Affairs Medical Center (CWBYVAMC) emergency department in Bay Pines, Florida, for fever, malaise, and right-sided chest pain on inspiration. The fever and malaise began while he was on vacation 4 weeks prior. He originally presented to an outside hospital where he tested positive for COVID-19 and was recommended ibuprofen and rest. His symptoms did not improve, and he returned a second time to the outside hospital 2 weeks later and was diagnosed with pneumonia and placed on outpatient antibiotics. The patient subsequently returned to CWBYVAMC 2 weeks after starting antibiotics when he began to develop right-sided inspiratory chest pain. He reported no other recent travel and no abdominal pain. The patient’s history was significant for diverticulitis 2 years before. A colonoscopy was performed during that time and showed no masses.
On presentation, the patient was febrile with a temperature of 100.8 °F; otherwise, his vital signs were stable. Physical examinations, including abdominal, respiratory, and cardiovascular, were unremarkable. The initial laboratory workup revealed a white blood cell (WBC) count of 18.7 K/μL (reference range, 5-10 K/μL) and microcytic anemia with a hemoglobin level of 8.8 g/dL. The comprehensive metabolic panel revealed normal aspartate transaminase, alanine transaminase, and total bilirubin levels and elevated alkaline phosphatase of 215 U/L (reference range, 44-147 U/L), revealing possible mild intrahepatic cholestasis. Urinalysis showed trace proteinuria and urobilinogen. Coagulation studies showed elevated D-dimer and procalcitonin levels at 1.9 ng/mL (reference range, < 0.1 ng/mL) and 1.21 ng/mL (reference range, < 0.5 ng/mL), respectively, with normal prothrombin and partial thromboplastin times. The patient had a normal troponin, fecal, and blood culture; entamoeba serology was negative.
A computed tomograph (CT) angiography of the chest was performed to rule out pulmonary embolism, revealing liver lesions suspicious for abscess or metastatic disease. Minimal pleural effusion was detected bilaterally. A subsequent CT
Following the procedure, the patient developed shaking chills, hypertension, fever, and acute hypoxic respiratory failure. He improved with oxygen and was transferred to the intensive care unit (ICU) where he had an increase in temperature and became septic without shock. A repeat blood culture was negative. An echocardiogram revealed no vegetation. Vancomycin was added for empiric coverage of potentially resistant organisms. The patient clinically improved and was able to leave the ICU 2 days later on hospital day 4.
The patient’s renal function worsened on day 5, and piperacillin-tazobactam and vancomycin were discontinued due to possible acute interstitial nephritis and renal toxicity. He started cefepime and continued metronidazole, and his renal function returned to normal 2 days later. Vancomycin was then re-administered. The results of the culture taken from the abscess came back positive for monomicrobial growth of F nucleatum on hospital day 9.
Due to the patient’s persisting fever and WBC count, a repeat CT of the abdomen on hospital day 10 revealed a partial decrease in the abscess with a persistent collection superior to the location of the initial pigtail catheter placement. A second pigtail catheter was then placed near the dome of the liver 1 day later on hospital day 11. Following the procedure, the patient improved significantly. The repeat CT after 1 week showed marked overall resolution of the abscess, and the repeat culture of the abscess did not reveal any organism growth. Vancomycin was discontinued on day 19, and the drains were removed on hospital day 20. He was discharged home in stable condition on metronidazole and cefdinir for 21 days with follow-up appointments for CT of the abdomen and with primary care, infectious disease, and a dental specialist.
Discussion
F nucleatum is a gram-negative, nonmotile, spindle-shaped rod found in dental plaques.5 The incidence of F nucleatum bacteremia is 0.34 per 100,000 people and increases with age, with the median age being 53.5 years.6 Although our patient did not present with F nucleatum bacteremia, it is possible that bacteremia was present before hospitalization but resolved by the time the sample was drawn for culture. F nucleatum bacteremia can lead to a variety of presentations. The most common primary diagnoses are intra-abdominal infections (eg, PHA, respiratory tract infections, and hematological disorders).1,6
PHA Presentation
The most common presenting symptoms of PHA are fever (88%), abdominal pain (79%), and vomiting (50%).4 The patient’s presentation of inspiratory right-sided chest pain is likely due to irritation of the diaphragmatic pleura of the right lung secondary to the abscess formation. The patient did not experience abdominal pain throughout the course of this disease or on palpation of his right upper quadrant. To our knowledge, this is the only case of PHA in the literature of a patient with inspiratory chest pain without respiratory infection, abdominal pain, and cardiac abnormalities. There was no radiologic evidence or signs of hypoxia on admission to CWBYVAMC, which makes respiratory infection an unlikely cause of the chest pain. Moreover, the patient presented with new-onset chest pain 2 weeks after the diagnosis of pneumonia.
Common laboratory findings of PHA include transaminitis, leukocytosis, and bilirubinemia.4 Of note, increased procalcitonin has also been associated with PHA and extreme elevation (> 200 μg/L) may be a useful biomarker to identify F nucleatum infections before the presence of leukocytosis.3 CT of PHA usually reveals right lobe involvement, and F nucleatum infection usually demonstrates multiple abscesses.4,7
Contributing Factors in F nucleatum PHA
F nucleatum is associated with several oral diseases, such as periodontitis and gingivitis.8 It is important to do an oral inspection on patients with F nucleatum infections because it can spread from oral cavities to different body parts.
F nucleatum is also found in the gut.9 Any disease that can cause a break in the gastrointestinal mucosa may result in F nucleatum bacteremia and PHA. This may be why F nucleatum has been associated with a variety of different diseases, such as diverticulitis, inflammatory bowel disease, appendicitis, and colorectal cancer.10,11 Our patient had a history of diverticulosis with diverticulitis. Bawa and colleagues described a patient with recurrent diverticulitis who developed F nucleatum bacteremia and PHA.11 Our patient did not have any signs of diverticulitis.
Our patient’s COVID-19 infection also had a role in delaying the appropriate treatment of PHA. Without any symptoms of PHA, a diagnosis is difficult in a patient with a positive COVID-19 test, and treatment was delayed 1 month. Moreover, COVID-19 has been reported to delay the diagnosis of PHA even in the absence of a positive COVID-19 test. Collins and Diamond presented a patient during the COVID-19 pandemic who developed a periodontal abscess, which resulted in F nucleatum bacteremia and PHA due to delayed hospital presentation after the patient’s practitioners recommended self-isolation, despite a negative COVID-19 test.12 This highlights the impact that COVID-19 may have on the timely diagnosis and treatment of patients with PHA.
Malignancy has been associated with F nucleatum bacteremia.1,13 Possibly the association is due to gastrointestinal mucosa malignancy’s ability to cause micro-abrasions, resulting in F nucleatum bacteremia.10 Additionally, F nucleatum may promote the development of colorectal neoplasms.8 Due to this association, screening for colorectal cancer in patients with F nucleatum infection is important. In our patient, a colonoscopy was performed during the patient’s hospitalization for diverticulitis 2 years prior. No signs of colorectal neoplasm were noted
Conclusions
PHA due to F nucleatum is a rare but potentially life-threatening condition that must be diagnosed and treated promptly. It usually presents with fever, abdominal pain, and vomiting but can present with chest pain in the absence of a respiratory infection, cardiac abnormalities, and abdominal pain, as in our patient. A wide spectrum of infections can occur with F nucleatum, including PHA.
Suspicion for infection with this organism should be kept high in middle-aged and older individuals who present with an indolent disease course and have risk factors, such as poor oral health and comorbidities. Suspicion should be kept high even in the event of COVID-19 infection, especially in individuals with prolonged fever without other signs indicating respiratory infection. We believe that the most likely causes of this patient’s infection were his dental caries and periodontal disease. The timing of his symptoms is not consistent with his previous episode of diverticulitis. Due to the mortality of PHA, diagnosis and treatment must be prompt. Initial treatment with drainage and empiric anaerobic coverage is recommended, followed by a tailored antibiotic regiment if indicated by culture, and further drainage if suggested by imaging.
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
1. Yang CC, Ye JJ, Hsu PC, et al. Characteristics and outcomes of Fusobacterium nucleatum bacteremia—a 6-year experience at a tertiary care hospital in northern Taiwan. Diagn Microbiol Infect Dis. 2011;70(2):167-174. doi:10.1016/j.diagmicrobio.2010.12.017
2. Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2(11):1032-1038. doi:10.1016/s1542-3565(04)00459-8
3. Cao SA, Hinchey S. Identification and management of fusobacterium nucleatum liver abscess and bacteremia in a young healthy man. Cureus. 2020;12(12):e12303. doi:10.7759/cureus.12303
4. Abbas MT, Khan FY, Muhsin SA, Al-Dehwe B, Abukamar M, Elzouki AN. Epidemiology, clinical features and outcome of liver abscess: a single reference center experience in Qatar. Oman Med J. 2014;29(4):260-263. doi:10.5001/omj.2014.69
5. Bolstad AI, Jensen HB, Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996;9(1):55-71. doi:10.1128/CMR.9.1.55
6. Afra K, Laupland K, Leal J, Lloyd T, Gregson D. Incidence, risk factors, and outcomes of Fusobacterium species bacteremia. BMC Infect Dis. 2013;13:264. doi:10.1186/1471-2334-13-264
7. Crippin JS, Wang KK. An unrecognized etiology for pyogenic hepatic abscesses in normal hosts: dental disease. Am J Gastroenterol. 1992;87(12):1740-1743.
8. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol. 2018;10(3):71-81. doi:10.4251/wjgo.v10.i3.71
9. Allen-Vercoe E, Strauss J, Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes. 2011;2(5):294-298. doi:10.4161/gmic.2.5.18603
10. Han YW. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 2015;23:141-147. doi:10.1016/j.mib.2014.11.013
11. Bawa A, Kainat A, Raza H, George TB, Omer H, Pillai AC. Fusobacterium bacteremia causing hepatic abscess in a patient with diverticulitis. Cureus. 2022;14(7):e26938. doi:10.7759/cureus.26938
12. Collins L, Diamond T. Fusobacterium nucleatum causing a pyogenic liver abscess: a rare complication of periodontal disease that occurred during the COVID-19 pandemic. BMJ Case Rep. 2021;14(1):e240080. doi:10.1136/bcr-2020-240080
13. Nohrstrom E, Mattila T, Pettila V, et al. Clinical spectrum of bacteraemic Fusobacterium infections: from septic shock to nosocomial bacteraemia. Scand J Infect Dis. 2011;43(6-7):463-470. doi:10.3109/00365548.2011.565071
23-year-old woman • fever, fatigue, and sore throat • scleral icterus and hepatosplenomegaly • Dx?
THE CASE
A 23-year-old woman sought care from her primary care physician (PCP) after being sick for 7 days. The illness started with a headache and fatigue, and by Day 6, she also had fever, chills, sore throat, nausea, a poor appetite, and intractable vomiting. The patient had no significant medical history and was socially isolating due to the COVID-19 pandemic. She had no known sick contacts or recent sexual activity and did not use any illicit drugs.
On examination, her vital signs were normal although she appeared ill and diaphoretic. A shallow tonsil ulcer and tonsillar adenopathy were present. Laboratory tests included a complete blood count (CBC), comprehensive metabolic panel, Monospot test, and Epstein-Barr virus (EBV) antibody test. Results were notable for leukocytosis with atypical lymphocytes on her CBC. Her Monospot test and EBV immunoglobulin (Ig) M antibody were positive, and her EBV IgG antibody was negative. She was given a diagnosis of infectious mononucleosis (IM) and told to get adequate rest, drink a lot of fluids, and take ibuprofen or acetaminophen for pain control.
Two days later, she returned to her PCP with scleral icterus (FIGURE 1A), increasingly tender cervical lymphadenopathy, and left-side abdominal pain. Her liver function tests (LFTs) had worsened (TABLE). An abdominal ultrasound revealed mild diffuse decreased hepatic echogenicity and prominent periportal echogenicity, likely related to diffuse hepatic parenchymal disease, as well as splenomegaly and a mildly thickened gallbladder with no gallstones. She also had severe throat discomfort, with bilateral tonsillar exudates and pharyngeal erythema (FIGURE 1B).
THE DIAGNOSIS
Based on her symptoms and the results of her physical examination, LFTs, EBV serologic assays, and abdominal ultrasound, this patient was given a diagnosis of acute EBV hepatitis.
DISCUSSION
EBV infection, which is the most common cause of IM, causes asymptomatic liver enzyme abnormalities in 80% to 90% of patients.1-3 Although not common, patients can develop acute EBV hepatitis and require hospitalization.4
Be aware of potential complications. Prompt assessment of elevated liver enzymes and accurate diagnosis are key.5 Although acute EBV hepatitis is usually self-limiting, there can be serious gastrointestinal complications such as splenic rupture, liver failure due to acute and/or chronic EBV infection, autoimmune hepatitis, and hepatocellular carcinoma.2 It’s rare for EBV hepatitis to lead to acute liver failure, but when that occurs, it can be fatal.6-9 One case series revealed that while primary EBV infection accounts for less than 1% of adult acute liver failure cases, it has a high case fatality rate of 50%.9
Treatment for patients with EBV hepatitis is usually supportive and includes rest, analgesia, and avoidance of vigorous activity for 1 month to reduce the risk for splenic rupture.1 In patients with nausea and vomiting, intravenous fluids may be necessary and can be administered at an outpatient infusion center. For individuals with severe tonsillar hypertrophy, prednisone (40-60 mg/d for 2-3 days, with subsequent tapering over 1-2 weeks) is indicated to prevent airway obstruction.1 Acyclovir may be used to reduce EBV viral shedding; however, it has no significant clinical impact.1
Continue to: Patients who are hemodynamially stable...
Patients who are hemodynamically stable and have appropriate access to follow-up care can be managed at home.2 If follow-up cannot occur remotely within 1 week or the patient’s clinical status begins to worsen (ie, the patient’s liver enzymes or bilirubin levels dramatically increase), hospitalization is necessary.10
Through shared decision-making, our patient was treated as an outpatient based on her hemodynamic stability and her ability to closely follow up in the clinic and by phone and to access an outpatient infusion center. She was reexamined within 2 days and given ondansetron 8 mg IV with 2 L of normal saline at our outpatient infusion center. We also prescribed ibuprofen (400 mg every 6 hours as needed) for analgesia and issued the standard recommendations that she avoid contact sports (for at least 6 weeks) and excessive alcohol consumption.
On Day 11, the patient followed up with her PCP by telephone. The patient was started on oral prednisone (40 mg/d for 3 days with taper over the next week as symptoms improved) for her severe throat discomfort, exudates, difficulty swallowing, and muffled voice. By Day 14, her aminotransferase levels began to decrease (TABLE), and her symptoms steadily improved thereafter.
THE TAKEAWAY
When a patient presents with unexplained elevated liver enzymes or cholestasis, it is important to assess for signs and symptoms of EBV hepatitis. Although EBV hepatitis is typically self-limiting, it can have serious complications or be fatal. Prompt initiation of outpatient management may avoid these complications and hospitalization.
CORRESPONDENCE
Lydia J. Schneider, MD, 225 East Chicago Avenue, Chicago, IL 60611; [email protected]
1. Cohen JI. Chapter 189: Epstein-Barr virus infections, including infectious mononucleosis. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill; 2020. Accessed March 21, 2023. accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192024765
2. Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. doi: 10.1097/01.smj.0000216469.04854.2a
3. Bunchorntavakul C, Reddy KR. Epstein-Barr virus and cytomegalovirus infections of the liver. Gastroenterol Clin North Am. 2020;49:331-346. doi: 10.1016/j.gtc.2020.01.008
4. Leonardsson H, Hreinsson JP, Löve A, et al. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. doi: 10.1080/ 00365521.2017.1319972
5. Banker L, Bowman PE. Epstein-Barr virus: forgotten etiology of hepatic injury. Clinical Advisor. September 23, 2021. Accessed April 18, 2023. www.clinicaladvisor.com/home/topics/infectious-diseases-information-center/epstein-barr-virus-etiology-hepatic-injury/
6. Fugl A, Lykkegaard Andersen C. Epstein-Barr virus and its association with disease: a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3
7. Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210-1217. doi: 10.1016/0016-5085(87)90246-0
8. Zhang W, Chen B, Chen Y, et al. Epstein-Barr virus-associated acute liver failure present in a 67-year-old immunocompetent female. Gastroenterology Res. 2016;9:74-78.
9. Mellinğer J, Rossaro L, Naugler W, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. doi: 10.1007/s10620-014-3029-2
10. Uluğ M, Kemal Celen M, Ayaz C, et al. Acute hepatitis: a rare complication of Epstein-Barr virus (EBV) infection. J Infect Dev Ctries. 2010;4:668-673. doi: 10.3855/jidc.871
THE CASE
A 23-year-old woman sought care from her primary care physician (PCP) after being sick for 7 days. The illness started with a headache and fatigue, and by Day 6, she also had fever, chills, sore throat, nausea, a poor appetite, and intractable vomiting. The patient had no significant medical history and was socially isolating due to the COVID-19 pandemic. She had no known sick contacts or recent sexual activity and did not use any illicit drugs.
On examination, her vital signs were normal although she appeared ill and diaphoretic. A shallow tonsil ulcer and tonsillar adenopathy were present. Laboratory tests included a complete blood count (CBC), comprehensive metabolic panel, Monospot test, and Epstein-Barr virus (EBV) antibody test. Results were notable for leukocytosis with atypical lymphocytes on her CBC. Her Monospot test and EBV immunoglobulin (Ig) M antibody were positive, and her EBV IgG antibody was negative. She was given a diagnosis of infectious mononucleosis (IM) and told to get adequate rest, drink a lot of fluids, and take ibuprofen or acetaminophen for pain control.

Two days later, she returned to her PCP with scleral icterus (FIGURE 1A), increasingly tender cervical lymphadenopathy, and left-side abdominal pain. Her liver function tests (LFTs) had worsened (TABLE). An abdominal ultrasound revealed mild diffuse decreased hepatic echogenicity and prominent periportal echogenicity, likely related to diffuse hepatic parenchymal disease, as well as splenomegaly and a mildly thickened gallbladder with no gallstones. She also had severe throat discomfort, with bilateral tonsillar exudates and pharyngeal erythema (FIGURE 1B).

THE DIAGNOSIS
Based on her symptoms and the results of her physical examination, LFTs, EBV serologic assays, and abdominal ultrasound, this patient was given a diagnosis of acute EBV hepatitis.
DISCUSSION
EBV infection, which is the most common cause of IM, causes asymptomatic liver enzyme abnormalities in 80% to 90% of patients.1-3 Although not common, patients can develop acute EBV hepatitis and require hospitalization.4
Be aware of potential complications. Prompt assessment of elevated liver enzymes and accurate diagnosis are key.5 Although acute EBV hepatitis is usually self-limiting, there can be serious gastrointestinal complications such as splenic rupture, liver failure due to acute and/or chronic EBV infection, autoimmune hepatitis, and hepatocellular carcinoma.2 It’s rare for EBV hepatitis to lead to acute liver failure, but when that occurs, it can be fatal.6-9 One case series revealed that while primary EBV infection accounts for less than 1% of adult acute liver failure cases, it has a high case fatality rate of 50%.9
Treatment for patients with EBV hepatitis is usually supportive and includes rest, analgesia, and avoidance of vigorous activity for 1 month to reduce the risk for splenic rupture.1 In patients with nausea and vomiting, intravenous fluids may be necessary and can be administered at an outpatient infusion center. For individuals with severe tonsillar hypertrophy, prednisone (40-60 mg/d for 2-3 days, with subsequent tapering over 1-2 weeks) is indicated to prevent airway obstruction.1 Acyclovir may be used to reduce EBV viral shedding; however, it has no significant clinical impact.1
Continue to: Patients who are hemodynamially stable...
Patients who are hemodynamically stable and have appropriate access to follow-up care can be managed at home.2 If follow-up cannot occur remotely within 1 week or the patient’s clinical status begins to worsen (ie, the patient’s liver enzymes or bilirubin levels dramatically increase), hospitalization is necessary.10
Through shared decision-making, our patient was treated as an outpatient based on her hemodynamic stability and her ability to closely follow up in the clinic and by phone and to access an outpatient infusion center. She was reexamined within 2 days and given ondansetron 8 mg IV with 2 L of normal saline at our outpatient infusion center. We also prescribed ibuprofen (400 mg every 6 hours as needed) for analgesia and issued the standard recommendations that she avoid contact sports (for at least 6 weeks) and excessive alcohol consumption.
On Day 11, the patient followed up with her PCP by telephone. The patient was started on oral prednisone (40 mg/d for 3 days with taper over the next week as symptoms improved) for her severe throat discomfort, exudates, difficulty swallowing, and muffled voice. By Day 14, her aminotransferase levels began to decrease (TABLE), and her symptoms steadily improved thereafter.
THE TAKEAWAY
When a patient presents with unexplained elevated liver enzymes or cholestasis, it is important to assess for signs and symptoms of EBV hepatitis. Although EBV hepatitis is typically self-limiting, it can have serious complications or be fatal. Prompt initiation of outpatient management may avoid these complications and hospitalization.
CORRESPONDENCE
Lydia J. Schneider, MD, 225 East Chicago Avenue, Chicago, IL 60611; [email protected]
THE CASE
A 23-year-old woman sought care from her primary care physician (PCP) after being sick for 7 days. The illness started with a headache and fatigue, and by Day 6, she also had fever, chills, sore throat, nausea, a poor appetite, and intractable vomiting. The patient had no significant medical history and was socially isolating due to the COVID-19 pandemic. She had no known sick contacts or recent sexual activity and did not use any illicit drugs.
On examination, her vital signs were normal although she appeared ill and diaphoretic. A shallow tonsil ulcer and tonsillar adenopathy were present. Laboratory tests included a complete blood count (CBC), comprehensive metabolic panel, Monospot test, and Epstein-Barr virus (EBV) antibody test. Results were notable for leukocytosis with atypical lymphocytes on her CBC. Her Monospot test and EBV immunoglobulin (Ig) M antibody were positive, and her EBV IgG antibody was negative. She was given a diagnosis of infectious mononucleosis (IM) and told to get adequate rest, drink a lot of fluids, and take ibuprofen or acetaminophen for pain control.

Two days later, she returned to her PCP with scleral icterus (FIGURE 1A), increasingly tender cervical lymphadenopathy, and left-side abdominal pain. Her liver function tests (LFTs) had worsened (TABLE). An abdominal ultrasound revealed mild diffuse decreased hepatic echogenicity and prominent periportal echogenicity, likely related to diffuse hepatic parenchymal disease, as well as splenomegaly and a mildly thickened gallbladder with no gallstones. She also had severe throat discomfort, with bilateral tonsillar exudates and pharyngeal erythema (FIGURE 1B).

THE DIAGNOSIS
Based on her symptoms and the results of her physical examination, LFTs, EBV serologic assays, and abdominal ultrasound, this patient was given a diagnosis of acute EBV hepatitis.
DISCUSSION
EBV infection, which is the most common cause of IM, causes asymptomatic liver enzyme abnormalities in 80% to 90% of patients.1-3 Although not common, patients can develop acute EBV hepatitis and require hospitalization.4
Be aware of potential complications. Prompt assessment of elevated liver enzymes and accurate diagnosis are key.5 Although acute EBV hepatitis is usually self-limiting, there can be serious gastrointestinal complications such as splenic rupture, liver failure due to acute and/or chronic EBV infection, autoimmune hepatitis, and hepatocellular carcinoma.2 It’s rare for EBV hepatitis to lead to acute liver failure, but when that occurs, it can be fatal.6-9 One case series revealed that while primary EBV infection accounts for less than 1% of adult acute liver failure cases, it has a high case fatality rate of 50%.9
Treatment for patients with EBV hepatitis is usually supportive and includes rest, analgesia, and avoidance of vigorous activity for 1 month to reduce the risk for splenic rupture.1 In patients with nausea and vomiting, intravenous fluids may be necessary and can be administered at an outpatient infusion center. For individuals with severe tonsillar hypertrophy, prednisone (40-60 mg/d for 2-3 days, with subsequent tapering over 1-2 weeks) is indicated to prevent airway obstruction.1 Acyclovir may be used to reduce EBV viral shedding; however, it has no significant clinical impact.1
Continue to: Patients who are hemodynamially stable...
Patients who are hemodynamically stable and have appropriate access to follow-up care can be managed at home.2 If follow-up cannot occur remotely within 1 week or the patient’s clinical status begins to worsen (ie, the patient’s liver enzymes or bilirubin levels dramatically increase), hospitalization is necessary.10
Through shared decision-making, our patient was treated as an outpatient based on her hemodynamic stability and her ability to closely follow up in the clinic and by phone and to access an outpatient infusion center. She was reexamined within 2 days and given ondansetron 8 mg IV with 2 L of normal saline at our outpatient infusion center. We also prescribed ibuprofen (400 mg every 6 hours as needed) for analgesia and issued the standard recommendations that she avoid contact sports (for at least 6 weeks) and excessive alcohol consumption.
On Day 11, the patient followed up with her PCP by telephone. The patient was started on oral prednisone (40 mg/d for 3 days with taper over the next week as symptoms improved) for her severe throat discomfort, exudates, difficulty swallowing, and muffled voice. By Day 14, her aminotransferase levels began to decrease (TABLE), and her symptoms steadily improved thereafter.
THE TAKEAWAY
When a patient presents with unexplained elevated liver enzymes or cholestasis, it is important to assess for signs and symptoms of EBV hepatitis. Although EBV hepatitis is typically self-limiting, it can have serious complications or be fatal. Prompt initiation of outpatient management may avoid these complications and hospitalization.
CORRESPONDENCE
Lydia J. Schneider, MD, 225 East Chicago Avenue, Chicago, IL 60611; [email protected]
1. Cohen JI. Chapter 189: Epstein-Barr virus infections, including infectious mononucleosis. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill; 2020. Accessed March 21, 2023. accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192024765
2. Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. doi: 10.1097/01.smj.0000216469.04854.2a
3. Bunchorntavakul C, Reddy KR. Epstein-Barr virus and cytomegalovirus infections of the liver. Gastroenterol Clin North Am. 2020;49:331-346. doi: 10.1016/j.gtc.2020.01.008
4. Leonardsson H, Hreinsson JP, Löve A, et al. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. doi: 10.1080/ 00365521.2017.1319972
5. Banker L, Bowman PE. Epstein-Barr virus: forgotten etiology of hepatic injury. Clinical Advisor. September 23, 2021. Accessed April 18, 2023. www.clinicaladvisor.com/home/topics/infectious-diseases-information-center/epstein-barr-virus-etiology-hepatic-injury/
6. Fugl A, Lykkegaard Andersen C. Epstein-Barr virus and its association with disease: a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3
7. Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210-1217. doi: 10.1016/0016-5085(87)90246-0
8. Zhang W, Chen B, Chen Y, et al. Epstein-Barr virus-associated acute liver failure present in a 67-year-old immunocompetent female. Gastroenterology Res. 2016;9:74-78.
9. Mellinğer J, Rossaro L, Naugler W, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. doi: 10.1007/s10620-014-3029-2
10. Uluğ M, Kemal Celen M, Ayaz C, et al. Acute hepatitis: a rare complication of Epstein-Barr virus (EBV) infection. J Infect Dev Ctries. 2010;4:668-673. doi: 10.3855/jidc.871
1. Cohen JI. Chapter 189: Epstein-Barr virus infections, including infectious mononucleosis. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill; 2020. Accessed March 21, 2023. accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192024765
2. Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. doi: 10.1097/01.smj.0000216469.04854.2a
3. Bunchorntavakul C, Reddy KR. Epstein-Barr virus and cytomegalovirus infections of the liver. Gastroenterol Clin North Am. 2020;49:331-346. doi: 10.1016/j.gtc.2020.01.008
4. Leonardsson H, Hreinsson JP, Löve A, et al. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. doi: 10.1080/ 00365521.2017.1319972
5. Banker L, Bowman PE. Epstein-Barr virus: forgotten etiology of hepatic injury. Clinical Advisor. September 23, 2021. Accessed April 18, 2023. www.clinicaladvisor.com/home/topics/infectious-diseases-information-center/epstein-barr-virus-etiology-hepatic-injury/
6. Fugl A, Lykkegaard Andersen C. Epstein-Barr virus and its association with disease: a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3
7. Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210-1217. doi: 10.1016/0016-5085(87)90246-0
8. Zhang W, Chen B, Chen Y, et al. Epstein-Barr virus-associated acute liver failure present in a 67-year-old immunocompetent female. Gastroenterology Res. 2016;9:74-78.
9. Mellinğer J, Rossaro L, Naugler W, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. doi: 10.1007/s10620-014-3029-2
10. Uluğ M, Kemal Celen M, Ayaz C, et al. Acute hepatitis: a rare complication of Epstein-Barr virus (EBV) infection. J Infect Dev Ctries. 2010;4:668-673. doi: 10.3855/jidc.871
► Fever, fatigue, and sore throat
► Scleral icterus and hepatosplenomegaly
Primary Hepatic Lymphoma: A Rare Form of Diffuse Large B-Cell Lymphoma of the Liver
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Deoxycholic Acid for Dercum Disease: Repurposing a Cosmetic Agent to Treat a Rare Disease
Dercum disease (or adiposis dolorosa) is a rare condition of unknown etiology characterized by multiple painful lipomas localized throughout the body.1,2 It typically presents in adults aged 35 to 50 years and is at least 5 times more common in women.3 It often is associated with comorbidities such as obesity, fatigue and weakness.1 There currently are no approved treatments for Dercum disease, only therapies tried with little to no efficacy for symptom management, including analgesics, excision, liposuction,1 lymphatic drainage,4 hypobaric pressure,5 and frequency rhythmic electrical modulation systems.6 For patients who continually develop widespread lesions, surgical excision is not feasible, which poses a therapeutic challenge. Deoxycholic acid (DCA), a bile acid that is approved to treat submental fat, disrupts the integrity of cell membranes, induces adipocyte lysis, and solubilizes fat when injected subcutaneously.7 We used DCA to mitigate pain and reduce lipoma size in patients with Dercum disease, which demonstrated lipoma reduction via ultrasonography in 3 patients.
Case Reports
Three patients presented to clinic with multiple painful subcutaneous nodules throughout several areas of the body and were screened using radiography. Ultrasonography demonstrated numerous lipomas consistent with Dercum disease. The lipomas were measured by ultrasonography to obtain 3-dimensional measurements of each lesion. The most painful lipomas identified by the patients were either treated with 2 mL of DCA (10 mg/mL) or served as a control with no treatment. Patients returned for symptom monitoring and repeat measurements of both treated and untreated lipomas. Two physicians with expertise in ultrasonography measured lesions in a blinded fashion. Photographs were obtained with patient consent.
Patient 1—A 45-year-old woman with a family history of lipomas was diagnosed with Dercum disease that was confirmed via ultrasonography. A painful 1.63×1.64×0.55-cm lipoma was measured on the volar aspect of the left forearm, and a 1.17×1.26×0.39-cm lipoma was measured on the volar aspect of the right wrist. At a follow-up visit 11 months later, 2 mL of DCA was administered to the lipoma on the volar aspect of the left forearm, while the lipoma on the volar aspect of the right wrist was monitored as an untreated control. Following the procedure, the patient reported 1 week of swelling and tenderness of the treated area. Repeat imaging 4 months after administration of DCA revealed reduction of the treated lesion to 0.80×1.48×0.60 cm and growth of the untreated lesion to 1.32×2.17×0.52 cm. The treated lipoma reduced in volume by 34.55%, while the lipoma in the untreated control increased in volume from its original measurement by 111.11% (Table). The patient also reported decreased pain in the treated area at all follow-up visits in the 1 year following the procedure.
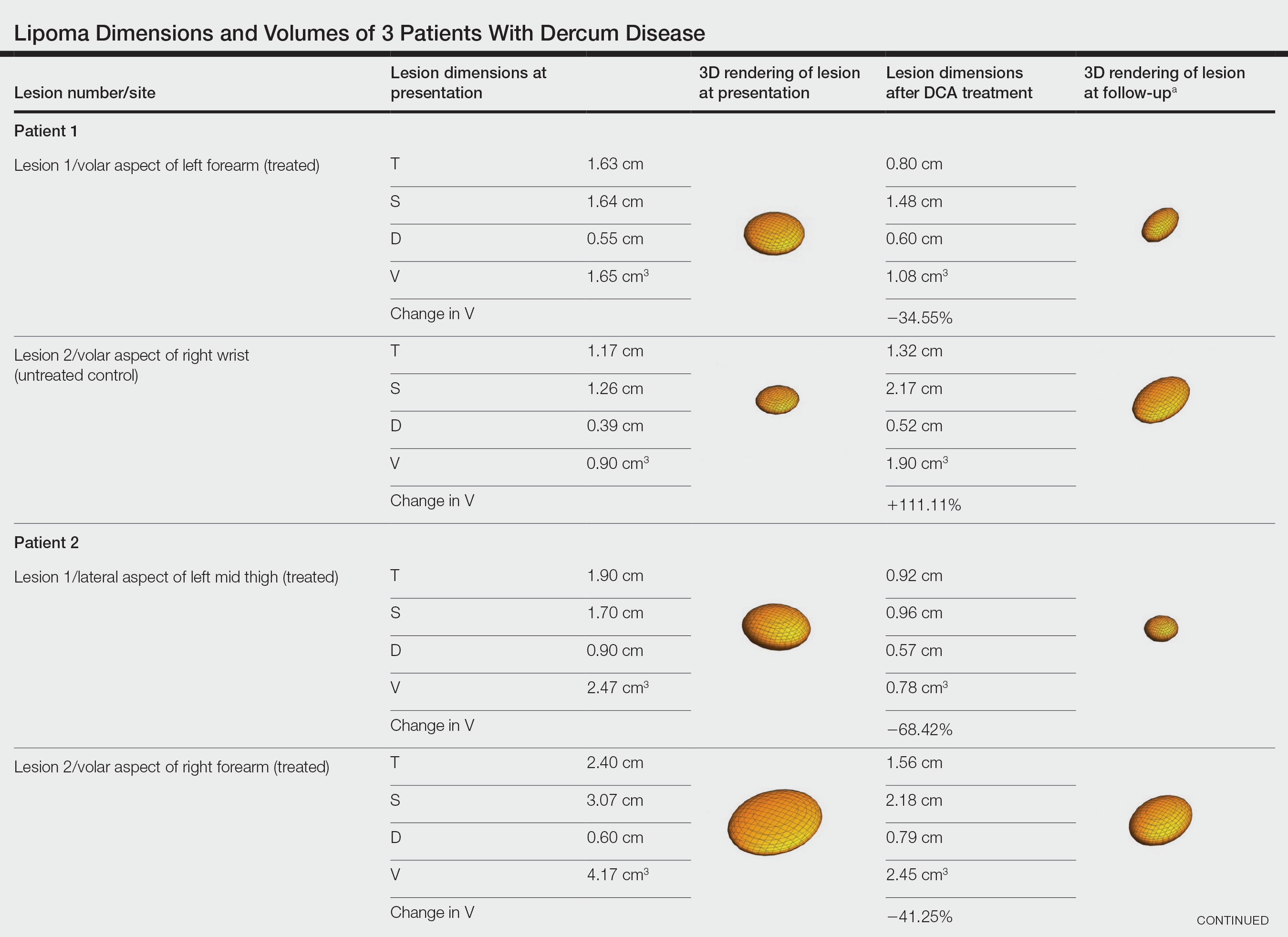
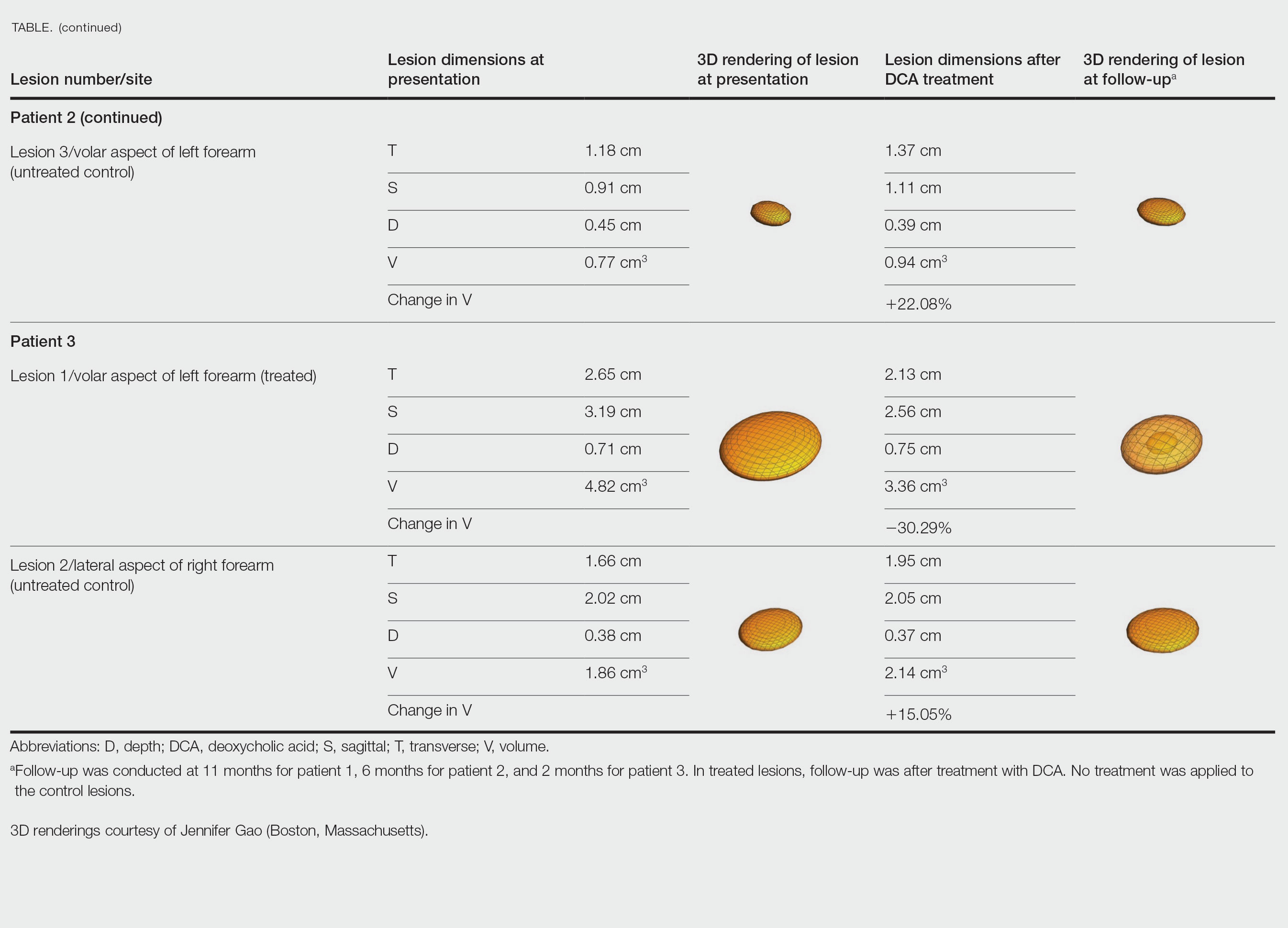
Patient 2—A 42-year-old woman with Dercum disease received administration of 2 mL of DCA to a 1.90×1.70×0.90-cm lipoma of the lateral aspect of the left mid thigh and 2 mL of DCA to a 2.40×3.07×0.60-cm lipoma on the volar aspect of the right forearm 2 weeks later. A 1.18×0.91×0.45-cm lipoma of the volar aspect of the left forearm was monitored as an untreated control. The patient reported bruising and discoloration a few weeks following the procedure. At subsequent 1-month and 3-month follow-ups, the patient reported induration in the volar aspect of the right forearm and noticeable reduction in size of the lesion in the lateral aspect of the left mid thigh. At the 6-month follow-up, the patient reported reduction in size of both lesions and improvement of the previously noted side effects. Repeat ultrasonography approximately 6 months after administration of DCA demonstrated reduction of the treated lesion on the lateral aspect of the left mid thigh to 0.92×0.96×0.57 cm and the volar aspect of the right forearm to 1.56×2.18×0.79 cm, with growth of the untreated lesion on the volar aspect of the left forearm to 1.37×1.11×0.39 cm. The treated lipomas reduced in volume by 68.42% and 41.25%, respectively, and the untreated control increased in volume by 22.08% (Table).
Patient 3—A 75-year-old woman with a family history of lipomas was diagnosed with Dercum disease verified by ultrasonography. The patient was administered 2 mL of DCA to a 2.65×3.19×0.71-cm lipoma of the volar aspect of the left forearm. A 1.66×2.02×0.38-cm lipoma of the lateral aspect of the right forearm was monitored as an untreated control. Following the procedure, the patient reported initial swelling that persisted for a few weeks followed by notable pain relief and a decrease in lipoma size. At 2-month follow-up, the patient reported no pain or other adverse effects, while repeat imaging demonstrated reduction of the treated lesion on the volar aspect of the left forearm to 2.13×2.56×0.75 cm and growth of the untreated lesion on the lateral aspect of the right forearm to 1.95×2.05×0.37 cm. The treated lipoma reduced in volume by 30.29%, and the untreated control increased in volume by 15.05% (Table).
Comment
Deoxycholic acid is a bile acid naturally found in the body that helps to emulsify and solubilize fats in the intestines. When injected subcutaneously, DCA becomes an adipolytic agent that induces inflammation and targets adipose degradation by macrophages, and it has been manufactured to reduce submental fat.7 Off-label use of DCA has been explored for nonsurgical body contouring and lipomas with promising results in some cases; however, these prior studies have been limited by the lack of quantitative objective measurements to effectively demonstrate the impact of treatment.8,9
We present 3 patients who requested treatment for numerous painful lipomas. Given the extent of their disease, surgical options were not feasible, and the patients opted to try a nonsurgical alternative. In each case, the painful lipomas that were chosen for treatment were injected with 2 mL of DCA. Injection-associated symptoms included swelling, tenderness, discoloration, and induration, which resolved over a period of months. Patient 1 had a treated lipoma that reduced in volume by approximately 35%, while the control continued to grow and doubled in volume. In patient 2, the treated lesion on the lateral aspect of the mid thigh reduced in volume by almost 70%, and the treated lesion on the volar aspect of the right forearm reduced in volume by more than 40%, while the control grew by more than 20%. In patient 3, the volume of the treated lipoma decreased by 30%, and the control increased by 15%. The follow-up interval was shortest in patient 3—2 months as opposed to 11 months and 6 months for patients 1 and 2, respectively; therefore, more progress may be seen in patient 3 with more time. Interestingly, a change in shape of the lipoma was noted in patient 3 (Figure)—an increase in its depth while the center became anechoic, which is a sign of hollowing in the center due to the saponification of fat and a possible cause for the change from an elliptical to a more spherical or doughnutlike shape. Intralesional administration of DCA may offer patients with extensive lipomas, such as those seen in patients with Dercum disease, an alternative, less-invasive option to assist with pain and tumor burden when excision is not feasible. Although treatments with DCA can be associated with side effects, including pain, swelling, bruising, erythema, induration, and numbness, all 3 of our patients had ultimate mitigation of pain and reduction in lipoma size within months of the injection. Additional studies should be explored to determine the optimal dose and frequency of administration of DCA that could benefit patients with Dercum disease.
- National Organization for Rare Disorders. Dercum’s disease. Updated March 26, 2020. Accessed March 27, 2023. https://rarediseases.org/rare-diseases/dercums-disease/.
- Kucharz EJ, Kopec´-Me˛drek M, Kramza J, et al. Dercum’s disease (adiposis dolorosa): a review of clinical presentation and management. Reumatologia. 2019;57:281-287. doi:10.5114/reum.2019.89521
- Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis. 2012;7:23. doi:10.1186/1750-1172-7-23
- Lange U, Oelzner P, Uhlemann C. Dercum’s disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29:17-22. doi:10.1007/s00296-008-0635-3
- Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153. doi:10.2147/JPR.S12351
- Martinenghi S, Caretto A, Losio C, et al. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94:e950. doi:10.1097/MD.0000000000000950
- National Center for Biotechnology Information. PubChem compound summary for CID 222528, deoxycholic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Deoxycholic-acid. Accessed November 11, 2021.
- Liu C, Li MK, Alster TS. Alternative cosmetic and medical applications of injectable deoxycholic acid: a systematic review. Dermatol Surg. 2021;47:1466-1472. doi:10.1097/DSS.0000000000003159
- Santiago-Vázquez M, Michelen-Gómez EA, Carrasquillo-Bonilla D, et al. Intralesional deoxycholic acid: a potential therapeutic alternative for the treatment of lipomas arising in the face. JAAD Case Rep. 2021;13:112-114. doi:10.1016/j.jdcr.2021.04.037
Dercum disease (or adiposis dolorosa) is a rare condition of unknown etiology characterized by multiple painful lipomas localized throughout the body.1,2 It typically presents in adults aged 35 to 50 years and is at least 5 times more common in women.3 It often is associated with comorbidities such as obesity, fatigue and weakness.1 There currently are no approved treatments for Dercum disease, only therapies tried with little to no efficacy for symptom management, including analgesics, excision, liposuction,1 lymphatic drainage,4 hypobaric pressure,5 and frequency rhythmic electrical modulation systems.6 For patients who continually develop widespread lesions, surgical excision is not feasible, which poses a therapeutic challenge. Deoxycholic acid (DCA), a bile acid that is approved to treat submental fat, disrupts the integrity of cell membranes, induces adipocyte lysis, and solubilizes fat when injected subcutaneously.7 We used DCA to mitigate pain and reduce lipoma size in patients with Dercum disease, which demonstrated lipoma reduction via ultrasonography in 3 patients.
Case Reports
Three patients presented to clinic with multiple painful subcutaneous nodules throughout several areas of the body and were screened using radiography. Ultrasonography demonstrated numerous lipomas consistent with Dercum disease. The lipomas were measured by ultrasonography to obtain 3-dimensional measurements of each lesion. The most painful lipomas identified by the patients were either treated with 2 mL of DCA (10 mg/mL) or served as a control with no treatment. Patients returned for symptom monitoring and repeat measurements of both treated and untreated lipomas. Two physicians with expertise in ultrasonography measured lesions in a blinded fashion. Photographs were obtained with patient consent.
Patient 1—A 45-year-old woman with a family history of lipomas was diagnosed with Dercum disease that was confirmed via ultrasonography. A painful 1.63×1.64×0.55-cm lipoma was measured on the volar aspect of the left forearm, and a 1.17×1.26×0.39-cm lipoma was measured on the volar aspect of the right wrist. At a follow-up visit 11 months later, 2 mL of DCA was administered to the lipoma on the volar aspect of the left forearm, while the lipoma on the volar aspect of the right wrist was monitored as an untreated control. Following the procedure, the patient reported 1 week of swelling and tenderness of the treated area. Repeat imaging 4 months after administration of DCA revealed reduction of the treated lesion to 0.80×1.48×0.60 cm and growth of the untreated lesion to 1.32×2.17×0.52 cm. The treated lipoma reduced in volume by 34.55%, while the lipoma in the untreated control increased in volume from its original measurement by 111.11% (Table). The patient also reported decreased pain in the treated area at all follow-up visits in the 1 year following the procedure.


Patient 2—A 42-year-old woman with Dercum disease received administration of 2 mL of DCA to a 1.90×1.70×0.90-cm lipoma of the lateral aspect of the left mid thigh and 2 mL of DCA to a 2.40×3.07×0.60-cm lipoma on the volar aspect of the right forearm 2 weeks later. A 1.18×0.91×0.45-cm lipoma of the volar aspect of the left forearm was monitored as an untreated control. The patient reported bruising and discoloration a few weeks following the procedure. At subsequent 1-month and 3-month follow-ups, the patient reported induration in the volar aspect of the right forearm and noticeable reduction in size of the lesion in the lateral aspect of the left mid thigh. At the 6-month follow-up, the patient reported reduction in size of both lesions and improvement of the previously noted side effects. Repeat ultrasonography approximately 6 months after administration of DCA demonstrated reduction of the treated lesion on the lateral aspect of the left mid thigh to 0.92×0.96×0.57 cm and the volar aspect of the right forearm to 1.56×2.18×0.79 cm, with growth of the untreated lesion on the volar aspect of the left forearm to 1.37×1.11×0.39 cm. The treated lipomas reduced in volume by 68.42% and 41.25%, respectively, and the untreated control increased in volume by 22.08% (Table).
Patient 3—A 75-year-old woman with a family history of lipomas was diagnosed with Dercum disease verified by ultrasonography. The patient was administered 2 mL of DCA to a 2.65×3.19×0.71-cm lipoma of the volar aspect of the left forearm. A 1.66×2.02×0.38-cm lipoma of the lateral aspect of the right forearm was monitored as an untreated control. Following the procedure, the patient reported initial swelling that persisted for a few weeks followed by notable pain relief and a decrease in lipoma size. At 2-month follow-up, the patient reported no pain or other adverse effects, while repeat imaging demonstrated reduction of the treated lesion on the volar aspect of the left forearm to 2.13×2.56×0.75 cm and growth of the untreated lesion on the lateral aspect of the right forearm to 1.95×2.05×0.37 cm. The treated lipoma reduced in volume by 30.29%, and the untreated control increased in volume by 15.05% (Table).
Comment
Deoxycholic acid is a bile acid naturally found in the body that helps to emulsify and solubilize fats in the intestines. When injected subcutaneously, DCA becomes an adipolytic agent that induces inflammation and targets adipose degradation by macrophages, and it has been manufactured to reduce submental fat.7 Off-label use of DCA has been explored for nonsurgical body contouring and lipomas with promising results in some cases; however, these prior studies have been limited by the lack of quantitative objective measurements to effectively demonstrate the impact of treatment.8,9
We present 3 patients who requested treatment for numerous painful lipomas. Given the extent of their disease, surgical options were not feasible, and the patients opted to try a nonsurgical alternative. In each case, the painful lipomas that were chosen for treatment were injected with 2 mL of DCA. Injection-associated symptoms included swelling, tenderness, discoloration, and induration, which resolved over a period of months. Patient 1 had a treated lipoma that reduced in volume by approximately 35%, while the control continued to grow and doubled in volume. In patient 2, the treated lesion on the lateral aspect of the mid thigh reduced in volume by almost 70%, and the treated lesion on the volar aspect of the right forearm reduced in volume by more than 40%, while the control grew by more than 20%. In patient 3, the volume of the treated lipoma decreased by 30%, and the control increased by 15%. The follow-up interval was shortest in patient 3—2 months as opposed to 11 months and 6 months for patients 1 and 2, respectively; therefore, more progress may be seen in patient 3 with more time. Interestingly, a change in shape of the lipoma was noted in patient 3 (Figure)—an increase in its depth while the center became anechoic, which is a sign of hollowing in the center due to the saponification of fat and a possible cause for the change from an elliptical to a more spherical or doughnutlike shape. Intralesional administration of DCA may offer patients with extensive lipomas, such as those seen in patients with Dercum disease, an alternative, less-invasive option to assist with pain and tumor burden when excision is not feasible. Although treatments with DCA can be associated with side effects, including pain, swelling, bruising, erythema, induration, and numbness, all 3 of our patients had ultimate mitigation of pain and reduction in lipoma size within months of the injection. Additional studies should be explored to determine the optimal dose and frequency of administration of DCA that could benefit patients with Dercum disease.

Dercum disease (or adiposis dolorosa) is a rare condition of unknown etiology characterized by multiple painful lipomas localized throughout the body.1,2 It typically presents in adults aged 35 to 50 years and is at least 5 times more common in women.3 It often is associated with comorbidities such as obesity, fatigue and weakness.1 There currently are no approved treatments for Dercum disease, only therapies tried with little to no efficacy for symptom management, including analgesics, excision, liposuction,1 lymphatic drainage,4 hypobaric pressure,5 and frequency rhythmic electrical modulation systems.6 For patients who continually develop widespread lesions, surgical excision is not feasible, which poses a therapeutic challenge. Deoxycholic acid (DCA), a bile acid that is approved to treat submental fat, disrupts the integrity of cell membranes, induces adipocyte lysis, and solubilizes fat when injected subcutaneously.7 We used DCA to mitigate pain and reduce lipoma size in patients with Dercum disease, which demonstrated lipoma reduction via ultrasonography in 3 patients.
Case Reports
Three patients presented to clinic with multiple painful subcutaneous nodules throughout several areas of the body and were screened using radiography. Ultrasonography demonstrated numerous lipomas consistent with Dercum disease. The lipomas were measured by ultrasonography to obtain 3-dimensional measurements of each lesion. The most painful lipomas identified by the patients were either treated with 2 mL of DCA (10 mg/mL) or served as a control with no treatment. Patients returned for symptom monitoring and repeat measurements of both treated and untreated lipomas. Two physicians with expertise in ultrasonography measured lesions in a blinded fashion. Photographs were obtained with patient consent.
Patient 1—A 45-year-old woman with a family history of lipomas was diagnosed with Dercum disease that was confirmed via ultrasonography. A painful 1.63×1.64×0.55-cm lipoma was measured on the volar aspect of the left forearm, and a 1.17×1.26×0.39-cm lipoma was measured on the volar aspect of the right wrist. At a follow-up visit 11 months later, 2 mL of DCA was administered to the lipoma on the volar aspect of the left forearm, while the lipoma on the volar aspect of the right wrist was monitored as an untreated control. Following the procedure, the patient reported 1 week of swelling and tenderness of the treated area. Repeat imaging 4 months after administration of DCA revealed reduction of the treated lesion to 0.80×1.48×0.60 cm and growth of the untreated lesion to 1.32×2.17×0.52 cm. The treated lipoma reduced in volume by 34.55%, while the lipoma in the untreated control increased in volume from its original measurement by 111.11% (Table). The patient also reported decreased pain in the treated area at all follow-up visits in the 1 year following the procedure.


Patient 2—A 42-year-old woman with Dercum disease received administration of 2 mL of DCA to a 1.90×1.70×0.90-cm lipoma of the lateral aspect of the left mid thigh and 2 mL of DCA to a 2.40×3.07×0.60-cm lipoma on the volar aspect of the right forearm 2 weeks later. A 1.18×0.91×0.45-cm lipoma of the volar aspect of the left forearm was monitored as an untreated control. The patient reported bruising and discoloration a few weeks following the procedure. At subsequent 1-month and 3-month follow-ups, the patient reported induration in the volar aspect of the right forearm and noticeable reduction in size of the lesion in the lateral aspect of the left mid thigh. At the 6-month follow-up, the patient reported reduction in size of both lesions and improvement of the previously noted side effects. Repeat ultrasonography approximately 6 months after administration of DCA demonstrated reduction of the treated lesion on the lateral aspect of the left mid thigh to 0.92×0.96×0.57 cm and the volar aspect of the right forearm to 1.56×2.18×0.79 cm, with growth of the untreated lesion on the volar aspect of the left forearm to 1.37×1.11×0.39 cm. The treated lipomas reduced in volume by 68.42% and 41.25%, respectively, and the untreated control increased in volume by 22.08% (Table).
Patient 3—A 75-year-old woman with a family history of lipomas was diagnosed with Dercum disease verified by ultrasonography. The patient was administered 2 mL of DCA to a 2.65×3.19×0.71-cm lipoma of the volar aspect of the left forearm. A 1.66×2.02×0.38-cm lipoma of the lateral aspect of the right forearm was monitored as an untreated control. Following the procedure, the patient reported initial swelling that persisted for a few weeks followed by notable pain relief and a decrease in lipoma size. At 2-month follow-up, the patient reported no pain or other adverse effects, while repeat imaging demonstrated reduction of the treated lesion on the volar aspect of the left forearm to 2.13×2.56×0.75 cm and growth of the untreated lesion on the lateral aspect of the right forearm to 1.95×2.05×0.37 cm. The treated lipoma reduced in volume by 30.29%, and the untreated control increased in volume by 15.05% (Table).
Comment
Deoxycholic acid is a bile acid naturally found in the body that helps to emulsify and solubilize fats in the intestines. When injected subcutaneously, DCA becomes an adipolytic agent that induces inflammation and targets adipose degradation by macrophages, and it has been manufactured to reduce submental fat.7 Off-label use of DCA has been explored for nonsurgical body contouring and lipomas with promising results in some cases; however, these prior studies have been limited by the lack of quantitative objective measurements to effectively demonstrate the impact of treatment.8,9
We present 3 patients who requested treatment for numerous painful lipomas. Given the extent of their disease, surgical options were not feasible, and the patients opted to try a nonsurgical alternative. In each case, the painful lipomas that were chosen for treatment were injected with 2 mL of DCA. Injection-associated symptoms included swelling, tenderness, discoloration, and induration, which resolved over a period of months. Patient 1 had a treated lipoma that reduced in volume by approximately 35%, while the control continued to grow and doubled in volume. In patient 2, the treated lesion on the lateral aspect of the mid thigh reduced in volume by almost 70%, and the treated lesion on the volar aspect of the right forearm reduced in volume by more than 40%, while the control grew by more than 20%. In patient 3, the volume of the treated lipoma decreased by 30%, and the control increased by 15%. The follow-up interval was shortest in patient 3—2 months as opposed to 11 months and 6 months for patients 1 and 2, respectively; therefore, more progress may be seen in patient 3 with more time. Interestingly, a change in shape of the lipoma was noted in patient 3 (Figure)—an increase in its depth while the center became anechoic, which is a sign of hollowing in the center due to the saponification of fat and a possible cause for the change from an elliptical to a more spherical or doughnutlike shape. Intralesional administration of DCA may offer patients with extensive lipomas, such as those seen in patients with Dercum disease, an alternative, less-invasive option to assist with pain and tumor burden when excision is not feasible. Although treatments with DCA can be associated with side effects, including pain, swelling, bruising, erythema, induration, and numbness, all 3 of our patients had ultimate mitigation of pain and reduction in lipoma size within months of the injection. Additional studies should be explored to determine the optimal dose and frequency of administration of DCA that could benefit patients with Dercum disease.

- National Organization for Rare Disorders. Dercum’s disease. Updated March 26, 2020. Accessed March 27, 2023. https://rarediseases.org/rare-diseases/dercums-disease/.
- Kucharz EJ, Kopec´-Me˛drek M, Kramza J, et al. Dercum’s disease (adiposis dolorosa): a review of clinical presentation and management. Reumatologia. 2019;57:281-287. doi:10.5114/reum.2019.89521
- Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis. 2012;7:23. doi:10.1186/1750-1172-7-23
- Lange U, Oelzner P, Uhlemann C. Dercum’s disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29:17-22. doi:10.1007/s00296-008-0635-3
- Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153. doi:10.2147/JPR.S12351
- Martinenghi S, Caretto A, Losio C, et al. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94:e950. doi:10.1097/MD.0000000000000950
- National Center for Biotechnology Information. PubChem compound summary for CID 222528, deoxycholic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Deoxycholic-acid. Accessed November 11, 2021.
- Liu C, Li MK, Alster TS. Alternative cosmetic and medical applications of injectable deoxycholic acid: a systematic review. Dermatol Surg. 2021;47:1466-1472. doi:10.1097/DSS.0000000000003159
- Santiago-Vázquez M, Michelen-Gómez EA, Carrasquillo-Bonilla D, et al. Intralesional deoxycholic acid: a potential therapeutic alternative for the treatment of lipomas arising in the face. JAAD Case Rep. 2021;13:112-114. doi:10.1016/j.jdcr.2021.04.037
- National Organization for Rare Disorders. Dercum’s disease. Updated March 26, 2020. Accessed March 27, 2023. https://rarediseases.org/rare-diseases/dercums-disease/.
- Kucharz EJ, Kopec´-Me˛drek M, Kramza J, et al. Dercum’s disease (adiposis dolorosa): a review of clinical presentation and management. Reumatologia. 2019;57:281-287. doi:10.5114/reum.2019.89521
- Hansson E, Svensson H, Brorson H. Review of Dercum’s disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J Rare Dis. 2012;7:23. doi:10.1186/1750-1172-7-23
- Lange U, Oelzner P, Uhlemann C. Dercum’s disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol Int. 2008;29:17-22. doi:10.1007/s00296-008-0635-3
- Herbst KL, Rutledge T. Pilot study: rapidly cycling hypobaric pressure improves pain after 5 days in adiposis dolorosa. J Pain Res. 2010;3:147-153. doi:10.2147/JPR.S12351
- Martinenghi S, Caretto A, Losio C, et al. Successful treatment of Dercum’s disease by transcutaneous electrical stimulation: a case report. Medicine (Baltimore). 2015;94:e950. doi:10.1097/MD.0000000000000950
- National Center for Biotechnology Information. PubChem compound summary for CID 222528, deoxycholic acid. https://pubchem.ncbi.nlm.nih.gov/compound/Deoxycholic-acid. Accessed November 11, 2021.
- Liu C, Li MK, Alster TS. Alternative cosmetic and medical applications of injectable deoxycholic acid: a systematic review. Dermatol Surg. 2021;47:1466-1472. doi:10.1097/DSS.0000000000003159
- Santiago-Vázquez M, Michelen-Gómez EA, Carrasquillo-Bonilla D, et al. Intralesional deoxycholic acid: a potential therapeutic alternative for the treatment of lipomas arising in the face. JAAD Case Rep. 2021;13:112-114. doi:10.1016/j.jdcr.2021.04.037
Practice Points
- Dermatologists should consider Dercum disease when encountering a patient with numerous painful lipomas.
- Subcutaneous administration of deoxycholic acid resulted in a notable reduction in pain and size of lipomas by 30% to 68% per radiographic review.
- Deoxycholic acid may provide an alternative therapeutic option for patients who have Dercum disease with substantial tumor burden.
75-year-old man • recent history of hand-foot-mouth disease • discolored fingernails and toenails lifting from the proximal end • Dx?
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.
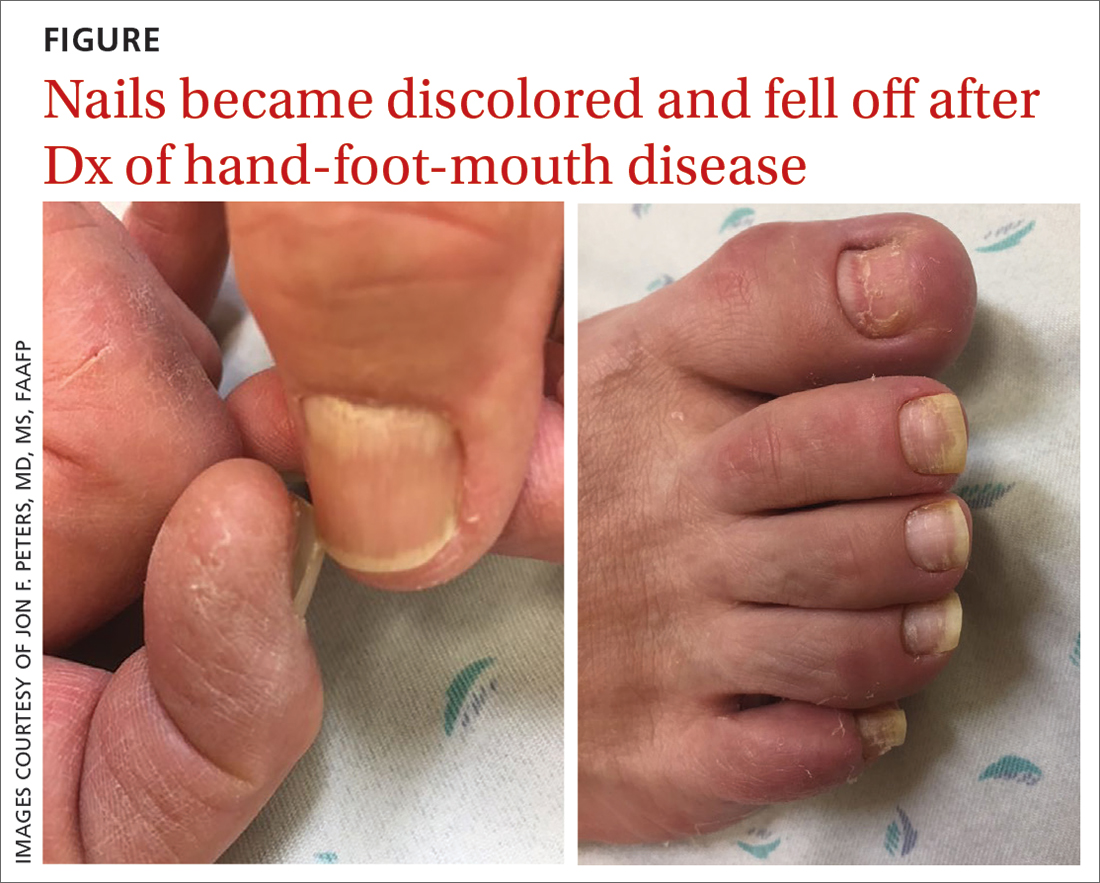
The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.

The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.

The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
► Recent history of hand-foot-mouth disease
► Discolored fingernails and toenails lifting from the proximal end