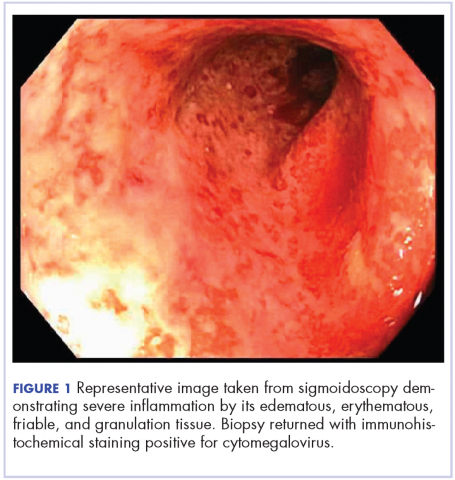
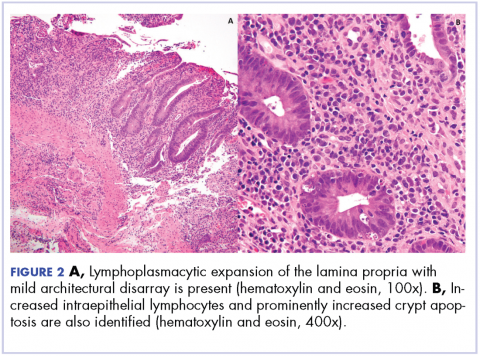
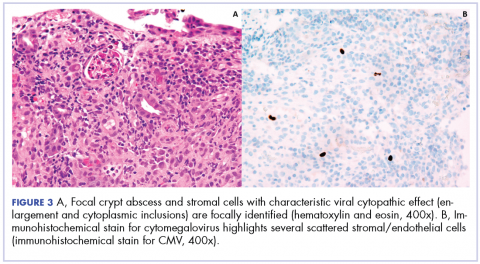
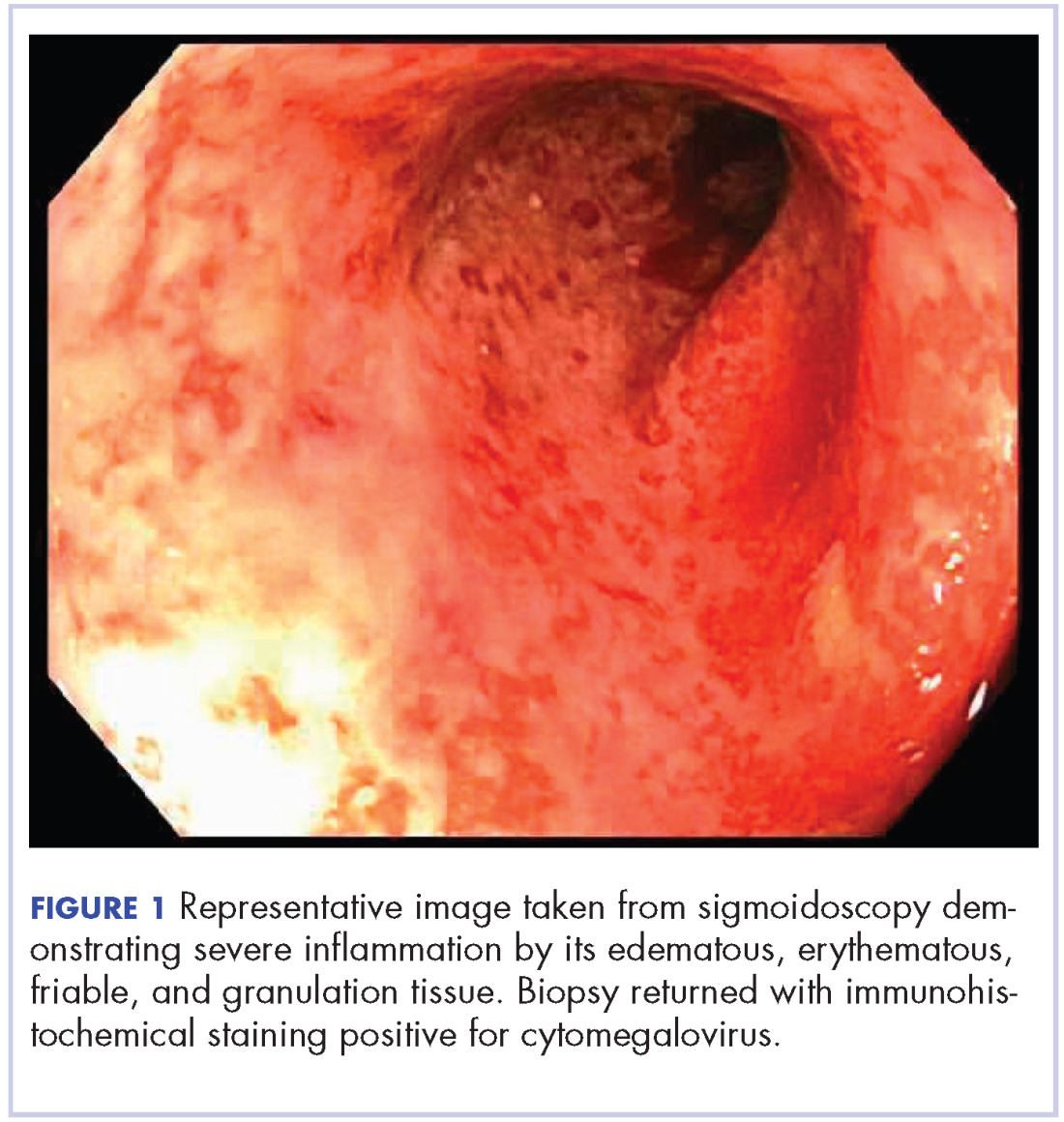
User login
Postherpetic Isotopic Responses With 3 Simultaneously Occurring Reactions Following Herpes Zoster
Postherpetic isotopic response (PHIR) refers to the occurrence of a second disease manifesting at the site of prior herpes infection. Many forms of PHIR have been described (Table), with postzoster granulomatous dermatitis (eg, granuloma annulare, sarcoidosis, granulomatous vasculitis) being the most common.1 Both primary and metastatic malignancies also can occur at the site of a prior herpes infection. Rarely, multiple types of PHIRs occur simultaneously. We report a case of 3 simultaneously occurring postzoster isotopic responses--granulomatous dermatitis, vasculitis, and chronic lymphocytic leukemia (CLL)--and review the various types of PHIRs.
Case Report
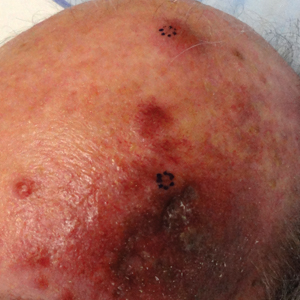
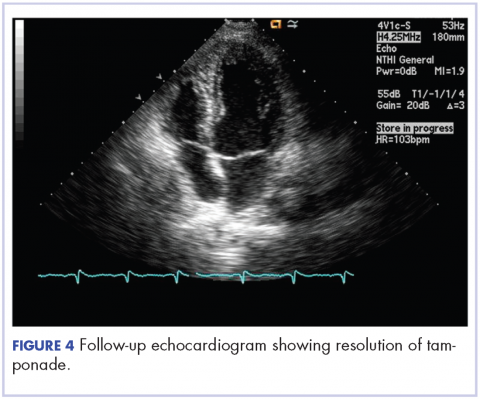
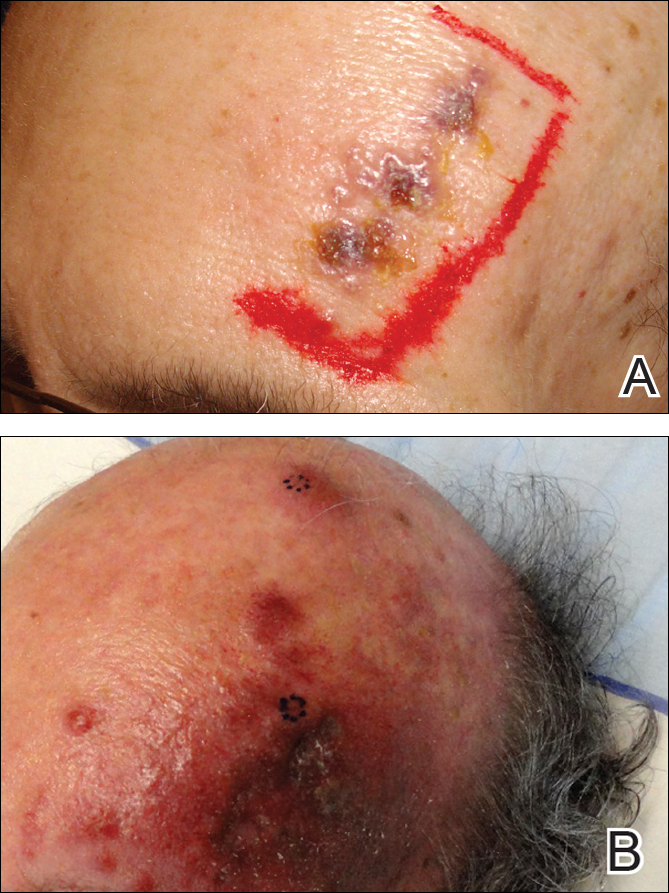
A 55-year-old man with a 4-year history of CLL was admitted to the hospital due to a painful rash on the left side of the face of 2 months' duration. Erythematous to violaceous plaques with surrounding papules and nodules were present on the left side of the forehead and frontal scalp with focal ulceration. Two months prior, the patient had unilateral vesicular lesions in the same distribution (Figure 1A). He initially received a 3-week course of acyclovir for a presumed herpes zoster infection and showed prompt improvement in the vesicular lesions. After resolution of the vesicles, papules and nodules began developing in the prior vesicular areas and he was treated with another course of acyclovir with the addition of clindamycin. When the lesions continued to progress and spread down the left side of the forehead and upper eyelid (Figure 1B), he was admitted to the hospital and assessed by the consultative dermatology team. No fevers, chills, or other systemic symptoms were reported.
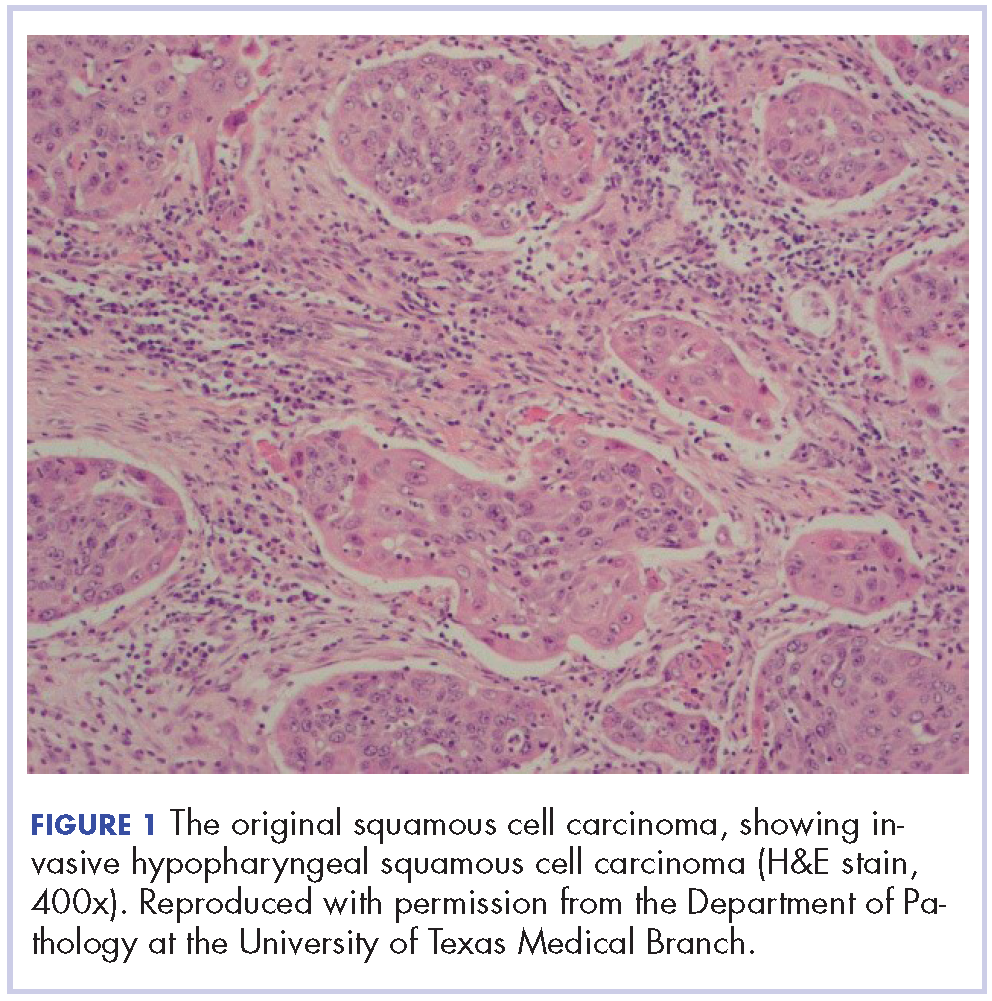
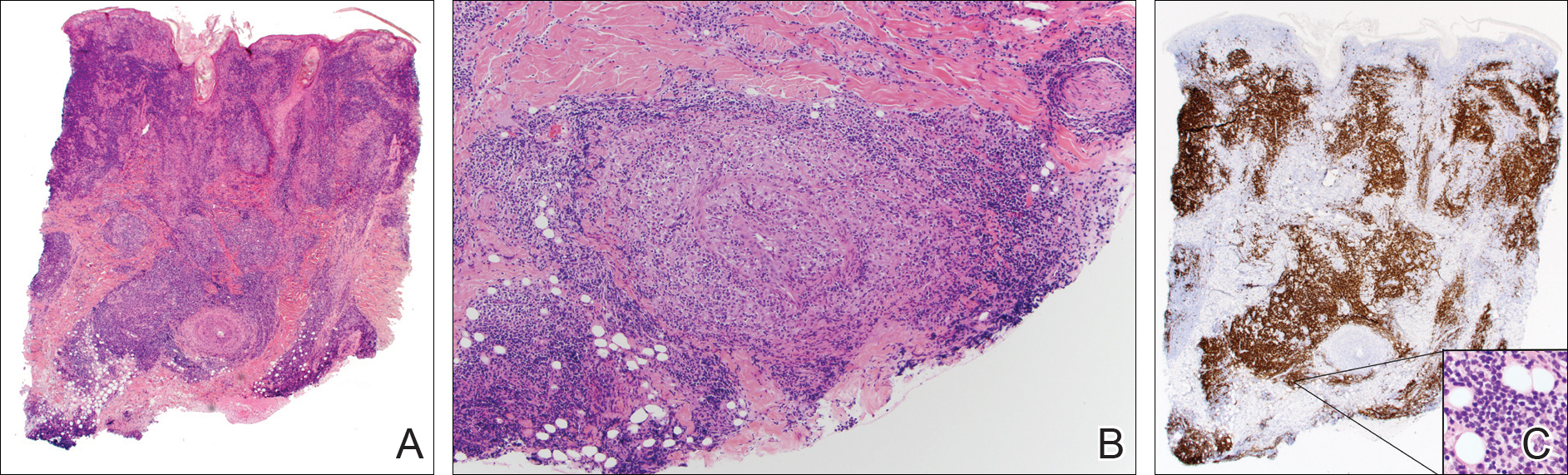
A punch biopsy showed a diffuse lymphocytic infiltrate filling the dermis and extending into the subcutis with nodular collections of histiocytes and some plasma cells scattered throughout (Figure 2A). A medium-vessel vasculitis was present with numerous histiocytes and lymphocytes infiltrating the muscular wall of a blood vessel in the subcutis (Figure 2B). CD3 and CD20 immunostaining showed an overwhelming majority of B cells, some with enlarged atypical nuclei and a smaller number of reactive T lymphocytes (Figure 2C). CD5 and CD43 were diffusely positive in the B cells, confirming the diagnosis of cutaneous CLL. CD23 staining was focally positive. Immunostaining for κ and λ light chains showed a marginal κ predominance. An additional biopsy for tissue culture was negative. A diagnosis of postzoster granulomatous dermatitis with vasculitis and cutaneous CLL was rendered.
Comment
Postherpetic Cutaneous Reactions
Various cutaneous reactions can occur at the site of prior herpes infection. The most frequently reported reactions are granulomatous dermatitides such as granuloma annulare, granulomatous vasculitis, granulomatous folliculitis, sarcoidosis, and nonspecific granulomatous dermatitis.1 Primary cutaneous malignancies and cutaneous metastases, including hematologic malignancies, have also been reported after herpetic infections. In a review of 127 patients with postherpetic cutaneous reactions, 47 had a granulomatous dermatitis, 32 had nonhematologic malignancies, 18 had leukemic or lymphomatous/pseudolymphomatous infiltrates, 10 had acneform lesions, 9 had nongranulomatous dermatitides such as lichen planus and allergic contact dermatitis, and 8 had nonherpetic skin infections; single cases of reactive perforating collagenosis, nodular solar degeneration, and a keloid also were reported.1
Pathogenesis of Cutaneous Reactions
Although postherpetic cutaneous reactions can develop in healthy individuals, they occur more often in immunocompromised patients. Postherpetic isotopic response has been used to describe the development of a nonherpetic disease at the site of prior herpes infection.2 Several different theories have been proposed to explain the pathogenesis of the PHIR, including an unusual delayed-type hypersensitivity reaction to residual viral antigen or host-tissue antigen altered by the virus. This delayed-type hypersensitivity explanation is supported by the presence of helper T cells, activated T lymphocytes, macrophages, varicella major viral envelope glycoproteins, and viral DNA in postherpetic granulomatous lesions3; however, cases that lack detectable virus and viral DNA in these types of lesions also have been reported.4
A second hypothesis proposes that inflammatory or viral-induced alteration of the local microvasculature results in increased site-specific susceptibility to subsequent inflammatory responses and drives these isotopic reactions.2,3 Damage or alteration of local peripheral nerves leading to abnormal release of specific neuromediators involved in regulating cutaneous inflammatory responses also may play a role.5 Varicella-zoster virus utilizes the peripheral nervous system to establish latent infection and can cause destruction of alpha delta and C nerve fibers in the dermis.1 Destruction of nerve fibers may indirectly influence the local immune system by altering the release of neuromediators such as substance P (known to increase blood vessel permeability, increase fibrinolytic activity, and induce mast cell secretion), vasoactive intestinal peptide (enhances monocyte migration, increases histamine release from mast cells, and inhibits natural killer cell activity), calcitonin gene-related peptide (increases vascular permeability, endothelial cell proliferation, and the accumulation of neutrophils), and melanocyte-stimulating hormone (induces anti-inflammatory cytokines). Disruption of the nervous system resulting in an altered local immune response also has been observed in other settings (eg, amputees who develop inflammatory diseases, bacterial and fungal infections, and cutaneous neoplasms confined to stump skin).1
Malignancies in PHIR
The granulomatous inflammation in PHIRs is a nonneoplastic inflammatory reaction with a variable lymphocytic component. Granuloma formation can be seen in both reactive inflammatory infiltrates and in cutaneous involvement of leukemias and lymphomas. Leukemia cutis has been reported in 4% to 20% of patients with CLL/small lymphocytic leukemia.6 In one series of 42 patients with CLL, the malignant cells were confined to the site of postherpetic scars in 14% (6/42) of patients.5 Sixteen percent (7/42) of patients had no prior diagnosis of CLL at the time they developed leukemia cutis, including one patient with leukemia cutis in a postzoster scar. The mechanism involved in the accumulation of neoplastic lymphocytes within postzoster scars has not been fully characterized. The idea that postzoster sites represent a site of least resistance for cutaneous infiltration of CLL due to the changes from prior inflammatory responses has been proposed.7
Combined CLL and granulomatous dermatitis at prior sites of herpes zoster was first reported in 1990.8 In 1995, Cerroni et al9 reported a series of 5 patients with cutaneous CLL following herpes zoster or herpes simplex virus infection. Three of those patients also demonstrated granuloma formation.9 Establishing a new diagnosis of CLL from a biopsy of postzoster granulomatous dermatitis with an associated lymphoid infiltrate also has been reported.10 Cerroni et al9 postulated that cutaneous CLL in post-herpes zoster scars may occur more frequently than reported due to misdiagnoses of CLL as pseudolymphoma. Two additional cases of postherpetic cutaneous CLL and granulomatous dermatitis have been reported since 1995.7,10
Diagnosis of Multiple PHIRs
The presence of 3 concurrent PHIRs is rare. The patient in this report had postzoster cutaneous CLL with an associated granulomatous dermatitis and medium-vessel vasculitis. One other case with these 3 findings was reported by Elgoweini et al.7 Overlooking important diagnoses when multiple findings are present in a biopsy can lead to diagnostic delay and incorrect treatment; we highlighted the importance of careful examination of biopsies in PHIRs to ensure diagnostic accuracy. In cases of postzoster granulomatous dermatitis, assessment of the lymphocytic component should not be overlooked. The presence of a dense lymphocytic infiltrate should raise the possibility of a lymphoproliferative disorder such as CLL, even in patients with no prior history of lymphoma. If initial immunostaining discloses a predominantly B-cell infiltrate, additional immuno-stains (eg, CD5, CD23, CD43) and/or genetic testing for monoclonality should be pursued.
Conclusion
Clinicians and dermatopathologists should be aware of the multiplicity of postherpetic isotopic responses and consider immunohistochemical stains to differentiate between a genuine lymphoma such as CLL and pseudolymphoma in PHIRs with a lymphoid infiltrate.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpes virus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Wolf D, Ruocco E, et al. Wolf's isotopic response. Clin Dermatol. 2011;29:237-240.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Snow J, el-Azhary R, Gibson L, et al. Granulomatous vasculitis associated with herpes virus: a persistent, painful, postherpetic papular eruption. Mayo Clin Proc. 1997;72:851-853.
- Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Elgoweini M, Blessing K, Jackson R, et al. Coexistent granulomatous vasculitis and leukaemia cutis in a patient with resolving herpes zoster. Clin Exp Dermatol. 2011;36:749-751.
- Pujol RM, Matias-Guiu X, Planaguma M, et al. Chronic lymphocytic leukemia and cutaneous granulomas at sites of herpes zoster scars. Int J Dermatol. 1990;29:652-654.
- Cerroni L, Zenahlik P, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia arising at the site of herpes zoster and herpes simplex scars. Cancer. 1995;76:26-31.
- Trojjet S, Hammami H, Zaraa I, et al. Chronic lymphocytic leukemia revealed by a granulomatous zosteriform eruption. Skinmed. 2012;10:50-52.
Postherpetic isotopic response (PHIR) refers to the occurrence of a second disease manifesting at the site of prior herpes infection. Many forms of PHIR have been described (Table), with postzoster granulomatous dermatitis (eg, granuloma annulare, sarcoidosis, granulomatous vasculitis) being the most common.1 Both primary and metastatic malignancies also can occur at the site of a prior herpes infection. Rarely, multiple types of PHIRs occur simultaneously. We report a case of 3 simultaneously occurring postzoster isotopic responses--granulomatous dermatitis, vasculitis, and chronic lymphocytic leukemia (CLL)--and review the various types of PHIRs.

Case Report
A 55-year-old man with a 4-year history of CLL was admitted to the hospital due to a painful rash on the left side of the face of 2 months' duration. Erythematous to violaceous plaques with surrounding papules and nodules were present on the left side of the forehead and frontal scalp with focal ulceration. Two months prior, the patient had unilateral vesicular lesions in the same distribution (Figure 1A). He initially received a 3-week course of acyclovir for a presumed herpes zoster infection and showed prompt improvement in the vesicular lesions. After resolution of the vesicles, papules and nodules began developing in the prior vesicular areas and he was treated with another course of acyclovir with the addition of clindamycin. When the lesions continued to progress and spread down the left side of the forehead and upper eyelid (Figure 1B), he was admitted to the hospital and assessed by the consultative dermatology team. No fevers, chills, or other systemic symptoms were reported.

A punch biopsy showed a diffuse lymphocytic infiltrate filling the dermis and extending into the subcutis with nodular collections of histiocytes and some plasma cells scattered throughout (Figure 2A). A medium-vessel vasculitis was present with numerous histiocytes and lymphocytes infiltrating the muscular wall of a blood vessel in the subcutis (Figure 2B). CD3 and CD20 immunostaining showed an overwhelming majority of B cells, some with enlarged atypical nuclei and a smaller number of reactive T lymphocytes (Figure 2C). CD5 and CD43 were diffusely positive in the B cells, confirming the diagnosis of cutaneous CLL. CD23 staining was focally positive. Immunostaining for κ and λ light chains showed a marginal κ predominance. An additional biopsy for tissue culture was negative. A diagnosis of postzoster granulomatous dermatitis with vasculitis and cutaneous CLL was rendered.

Comment
Postherpetic Cutaneous Reactions
Various cutaneous reactions can occur at the site of prior herpes infection. The most frequently reported reactions are granulomatous dermatitides such as granuloma annulare, granulomatous vasculitis, granulomatous folliculitis, sarcoidosis, and nonspecific granulomatous dermatitis.1 Primary cutaneous malignancies and cutaneous metastases, including hematologic malignancies, have also been reported after herpetic infections. In a review of 127 patients with postherpetic cutaneous reactions, 47 had a granulomatous dermatitis, 32 had nonhematologic malignancies, 18 had leukemic or lymphomatous/pseudolymphomatous infiltrates, 10 had acneform lesions, 9 had nongranulomatous dermatitides such as lichen planus and allergic contact dermatitis, and 8 had nonherpetic skin infections; single cases of reactive perforating collagenosis, nodular solar degeneration, and a keloid also were reported.1
Pathogenesis of Cutaneous Reactions
Although postherpetic cutaneous reactions can develop in healthy individuals, they occur more often in immunocompromised patients. Postherpetic isotopic response has been used to describe the development of a nonherpetic disease at the site of prior herpes infection.2 Several different theories have been proposed to explain the pathogenesis of the PHIR, including an unusual delayed-type hypersensitivity reaction to residual viral antigen or host-tissue antigen altered by the virus. This delayed-type hypersensitivity explanation is supported by the presence of helper T cells, activated T lymphocytes, macrophages, varicella major viral envelope glycoproteins, and viral DNA in postherpetic granulomatous lesions3; however, cases that lack detectable virus and viral DNA in these types of lesions also have been reported.4
A second hypothesis proposes that inflammatory or viral-induced alteration of the local microvasculature results in increased site-specific susceptibility to subsequent inflammatory responses and drives these isotopic reactions.2,3 Damage or alteration of local peripheral nerves leading to abnormal release of specific neuromediators involved in regulating cutaneous inflammatory responses also may play a role.5 Varicella-zoster virus utilizes the peripheral nervous system to establish latent infection and can cause destruction of alpha delta and C nerve fibers in the dermis.1 Destruction of nerve fibers may indirectly influence the local immune system by altering the release of neuromediators such as substance P (known to increase blood vessel permeability, increase fibrinolytic activity, and induce mast cell secretion), vasoactive intestinal peptide (enhances monocyte migration, increases histamine release from mast cells, and inhibits natural killer cell activity), calcitonin gene-related peptide (increases vascular permeability, endothelial cell proliferation, and the accumulation of neutrophils), and melanocyte-stimulating hormone (induces anti-inflammatory cytokines). Disruption of the nervous system resulting in an altered local immune response also has been observed in other settings (eg, amputees who develop inflammatory diseases, bacterial and fungal infections, and cutaneous neoplasms confined to stump skin).1
Malignancies in PHIR
The granulomatous inflammation in PHIRs is a nonneoplastic inflammatory reaction with a variable lymphocytic component. Granuloma formation can be seen in both reactive inflammatory infiltrates and in cutaneous involvement of leukemias and lymphomas. Leukemia cutis has been reported in 4% to 20% of patients with CLL/small lymphocytic leukemia.6 In one series of 42 patients with CLL, the malignant cells were confined to the site of postherpetic scars in 14% (6/42) of patients.5 Sixteen percent (7/42) of patients had no prior diagnosis of CLL at the time they developed leukemia cutis, including one patient with leukemia cutis in a postzoster scar. The mechanism involved in the accumulation of neoplastic lymphocytes within postzoster scars has not been fully characterized. The idea that postzoster sites represent a site of least resistance for cutaneous infiltration of CLL due to the changes from prior inflammatory responses has been proposed.7
Combined CLL and granulomatous dermatitis at prior sites of herpes zoster was first reported in 1990.8 In 1995, Cerroni et al9 reported a series of 5 patients with cutaneous CLL following herpes zoster or herpes simplex virus infection. Three of those patients also demonstrated granuloma formation.9 Establishing a new diagnosis of CLL from a biopsy of postzoster granulomatous dermatitis with an associated lymphoid infiltrate also has been reported.10 Cerroni et al9 postulated that cutaneous CLL in post-herpes zoster scars may occur more frequently than reported due to misdiagnoses of CLL as pseudolymphoma. Two additional cases of postherpetic cutaneous CLL and granulomatous dermatitis have been reported since 1995.7,10
Diagnosis of Multiple PHIRs
The presence of 3 concurrent PHIRs is rare. The patient in this report had postzoster cutaneous CLL with an associated granulomatous dermatitis and medium-vessel vasculitis. One other case with these 3 findings was reported by Elgoweini et al.7 Overlooking important diagnoses when multiple findings are present in a biopsy can lead to diagnostic delay and incorrect treatment; we highlighted the importance of careful examination of biopsies in PHIRs to ensure diagnostic accuracy. In cases of postzoster granulomatous dermatitis, assessment of the lymphocytic component should not be overlooked. The presence of a dense lymphocytic infiltrate should raise the possibility of a lymphoproliferative disorder such as CLL, even in patients with no prior history of lymphoma. If initial immunostaining discloses a predominantly B-cell infiltrate, additional immuno-stains (eg, CD5, CD23, CD43) and/or genetic testing for monoclonality should be pursued.
Conclusion
Clinicians and dermatopathologists should be aware of the multiplicity of postherpetic isotopic responses and consider immunohistochemical stains to differentiate between a genuine lymphoma such as CLL and pseudolymphoma in PHIRs with a lymphoid infiltrate.
Postherpetic isotopic response (PHIR) refers to the occurrence of a second disease manifesting at the site of prior herpes infection. Many forms of PHIR have been described (Table), with postzoster granulomatous dermatitis (eg, granuloma annulare, sarcoidosis, granulomatous vasculitis) being the most common.1 Both primary and metastatic malignancies also can occur at the site of a prior herpes infection. Rarely, multiple types of PHIRs occur simultaneously. We report a case of 3 simultaneously occurring postzoster isotopic responses--granulomatous dermatitis, vasculitis, and chronic lymphocytic leukemia (CLL)--and review the various types of PHIRs.

Case Report
A 55-year-old man with a 4-year history of CLL was admitted to the hospital due to a painful rash on the left side of the face of 2 months' duration. Erythematous to violaceous plaques with surrounding papules and nodules were present on the left side of the forehead and frontal scalp with focal ulceration. Two months prior, the patient had unilateral vesicular lesions in the same distribution (Figure 1A). He initially received a 3-week course of acyclovir for a presumed herpes zoster infection and showed prompt improvement in the vesicular lesions. After resolution of the vesicles, papules and nodules began developing in the prior vesicular areas and he was treated with another course of acyclovir with the addition of clindamycin. When the lesions continued to progress and spread down the left side of the forehead and upper eyelid (Figure 1B), he was admitted to the hospital and assessed by the consultative dermatology team. No fevers, chills, or other systemic symptoms were reported.

A punch biopsy showed a diffuse lymphocytic infiltrate filling the dermis and extending into the subcutis with nodular collections of histiocytes and some plasma cells scattered throughout (Figure 2A). A medium-vessel vasculitis was present with numerous histiocytes and lymphocytes infiltrating the muscular wall of a blood vessel in the subcutis (Figure 2B). CD3 and CD20 immunostaining showed an overwhelming majority of B cells, some with enlarged atypical nuclei and a smaller number of reactive T lymphocytes (Figure 2C). CD5 and CD43 were diffusely positive in the B cells, confirming the diagnosis of cutaneous CLL. CD23 staining was focally positive. Immunostaining for κ and λ light chains showed a marginal κ predominance. An additional biopsy for tissue culture was negative. A diagnosis of postzoster granulomatous dermatitis with vasculitis and cutaneous CLL was rendered.

Comment
Postherpetic Cutaneous Reactions
Various cutaneous reactions can occur at the site of prior herpes infection. The most frequently reported reactions are granulomatous dermatitides such as granuloma annulare, granulomatous vasculitis, granulomatous folliculitis, sarcoidosis, and nonspecific granulomatous dermatitis.1 Primary cutaneous malignancies and cutaneous metastases, including hematologic malignancies, have also been reported after herpetic infections. In a review of 127 patients with postherpetic cutaneous reactions, 47 had a granulomatous dermatitis, 32 had nonhematologic malignancies, 18 had leukemic or lymphomatous/pseudolymphomatous infiltrates, 10 had acneform lesions, 9 had nongranulomatous dermatitides such as lichen planus and allergic contact dermatitis, and 8 had nonherpetic skin infections; single cases of reactive perforating collagenosis, nodular solar degeneration, and a keloid also were reported.1
Pathogenesis of Cutaneous Reactions
Although postherpetic cutaneous reactions can develop in healthy individuals, they occur more often in immunocompromised patients. Postherpetic isotopic response has been used to describe the development of a nonherpetic disease at the site of prior herpes infection.2 Several different theories have been proposed to explain the pathogenesis of the PHIR, including an unusual delayed-type hypersensitivity reaction to residual viral antigen or host-tissue antigen altered by the virus. This delayed-type hypersensitivity explanation is supported by the presence of helper T cells, activated T lymphocytes, macrophages, varicella major viral envelope glycoproteins, and viral DNA in postherpetic granulomatous lesions3; however, cases that lack detectable virus and viral DNA in these types of lesions also have been reported.4
A second hypothesis proposes that inflammatory or viral-induced alteration of the local microvasculature results in increased site-specific susceptibility to subsequent inflammatory responses and drives these isotopic reactions.2,3 Damage or alteration of local peripheral nerves leading to abnormal release of specific neuromediators involved in regulating cutaneous inflammatory responses also may play a role.5 Varicella-zoster virus utilizes the peripheral nervous system to establish latent infection and can cause destruction of alpha delta and C nerve fibers in the dermis.1 Destruction of nerve fibers may indirectly influence the local immune system by altering the release of neuromediators such as substance P (known to increase blood vessel permeability, increase fibrinolytic activity, and induce mast cell secretion), vasoactive intestinal peptide (enhances monocyte migration, increases histamine release from mast cells, and inhibits natural killer cell activity), calcitonin gene-related peptide (increases vascular permeability, endothelial cell proliferation, and the accumulation of neutrophils), and melanocyte-stimulating hormone (induces anti-inflammatory cytokines). Disruption of the nervous system resulting in an altered local immune response also has been observed in other settings (eg, amputees who develop inflammatory diseases, bacterial and fungal infections, and cutaneous neoplasms confined to stump skin).1
Malignancies in PHIR
The granulomatous inflammation in PHIRs is a nonneoplastic inflammatory reaction with a variable lymphocytic component. Granuloma formation can be seen in both reactive inflammatory infiltrates and in cutaneous involvement of leukemias and lymphomas. Leukemia cutis has been reported in 4% to 20% of patients with CLL/small lymphocytic leukemia.6 In one series of 42 patients with CLL, the malignant cells were confined to the site of postherpetic scars in 14% (6/42) of patients.5 Sixteen percent (7/42) of patients had no prior diagnosis of CLL at the time they developed leukemia cutis, including one patient with leukemia cutis in a postzoster scar. The mechanism involved in the accumulation of neoplastic lymphocytes within postzoster scars has not been fully characterized. The idea that postzoster sites represent a site of least resistance for cutaneous infiltration of CLL due to the changes from prior inflammatory responses has been proposed.7
Combined CLL and granulomatous dermatitis at prior sites of herpes zoster was first reported in 1990.8 In 1995, Cerroni et al9 reported a series of 5 patients with cutaneous CLL following herpes zoster or herpes simplex virus infection. Three of those patients also demonstrated granuloma formation.9 Establishing a new diagnosis of CLL from a biopsy of postzoster granulomatous dermatitis with an associated lymphoid infiltrate also has been reported.10 Cerroni et al9 postulated that cutaneous CLL in post-herpes zoster scars may occur more frequently than reported due to misdiagnoses of CLL as pseudolymphoma. Two additional cases of postherpetic cutaneous CLL and granulomatous dermatitis have been reported since 1995.7,10
Diagnosis of Multiple PHIRs
The presence of 3 concurrent PHIRs is rare. The patient in this report had postzoster cutaneous CLL with an associated granulomatous dermatitis and medium-vessel vasculitis. One other case with these 3 findings was reported by Elgoweini et al.7 Overlooking important diagnoses when multiple findings are present in a biopsy can lead to diagnostic delay and incorrect treatment; we highlighted the importance of careful examination of biopsies in PHIRs to ensure diagnostic accuracy. In cases of postzoster granulomatous dermatitis, assessment of the lymphocytic component should not be overlooked. The presence of a dense lymphocytic infiltrate should raise the possibility of a lymphoproliferative disorder such as CLL, even in patients with no prior history of lymphoma. If initial immunostaining discloses a predominantly B-cell infiltrate, additional immuno-stains (eg, CD5, CD23, CD43) and/or genetic testing for monoclonality should be pursued.
Conclusion
Clinicians and dermatopathologists should be aware of the multiplicity of postherpetic isotopic responses and consider immunohistochemical stains to differentiate between a genuine lymphoma such as CLL and pseudolymphoma in PHIRs with a lymphoid infiltrate.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpes virus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Wolf D, Ruocco E, et al. Wolf's isotopic response. Clin Dermatol. 2011;29:237-240.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Snow J, el-Azhary R, Gibson L, et al. Granulomatous vasculitis associated with herpes virus: a persistent, painful, postherpetic papular eruption. Mayo Clin Proc. 1997;72:851-853.
- Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Elgoweini M, Blessing K, Jackson R, et al. Coexistent granulomatous vasculitis and leukaemia cutis in a patient with resolving herpes zoster. Clin Exp Dermatol. 2011;36:749-751.
- Pujol RM, Matias-Guiu X, Planaguma M, et al. Chronic lymphocytic leukemia and cutaneous granulomas at sites of herpes zoster scars. Int J Dermatol. 1990;29:652-654.
- Cerroni L, Zenahlik P, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia arising at the site of herpes zoster and herpes simplex scars. Cancer. 1995;76:26-31.
- Trojjet S, Hammami H, Zaraa I, et al. Chronic lymphocytic leukemia revealed by a granulomatous zosteriform eruption. Skinmed. 2012;10:50-52.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpes virus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Wolf D, Ruocco E, et al. Wolf's isotopic response. Clin Dermatol. 2011;29:237-240.
- Nikkels AF, Debrus S, Delvenne P, et al. Viral glycoproteins in herpesviridae granulomas. Am J Dermatopathol. 1994;16:588-592.
- Snow J, el-Azhary R, Gibson L, et al. Granulomatous vasculitis associated with herpes virus: a persistent, painful, postherpetic papular eruption. Mayo Clin Proc. 1997;72:851-853.
- Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopathologic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000-1010.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Elgoweini M, Blessing K, Jackson R, et al. Coexistent granulomatous vasculitis and leukaemia cutis in a patient with resolving herpes zoster. Clin Exp Dermatol. 2011;36:749-751.
- Pujol RM, Matias-Guiu X, Planaguma M, et al. Chronic lymphocytic leukemia and cutaneous granulomas at sites of herpes zoster scars. Int J Dermatol. 1990;29:652-654.
- Cerroni L, Zenahlik P, Kerl H. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia arising at the site of herpes zoster and herpes simplex scars. Cancer. 1995;76:26-31.
- Trojjet S, Hammami H, Zaraa I, et al. Chronic lymphocytic leukemia revealed by a granulomatous zosteriform eruption. Skinmed. 2012;10:50-52.
Practice Points
- Multiple diseases may present in prior sites of herpes infection (postherpetic isotopic response).
- Granulomatous dermatitis is the most common postherpetic isotopic response, but other inflammatory, neoplastic, or infectious conditions also occur.
- Multiple conditions may present simultaneously at sites of herpes infection.
- Cutaneous involvement by chronic lymphocytic leukemia (CLL) can be easily overlooked in this setting.
Anti-PD-1 therapy with nivolumab in the treatment of metastatic malignant PEComa
Perivascular epithelioid cell neoplasms (PEComas) are an uncommon class of tumors consisting on histology of perivascular epithelioid cells occurring in both localized and metastatic forms at various body sites. The approach to treatment of these tumors generally involves a combination of surgical resection, chemotherapy, and/or radiation therapy.1
Case presentation and summary
A 46-year-old man presented to our institution with a non-tender, slowly enlarging, 8.3 cm mass in his right popliteal fossa. Upon biopsy, the pathologic findings were consistent with an epithelioid malignancy with melanocytic differentiation most consistent with a PEComa. Discussion of the pathologic diagnosis of our patient has been reported by the pathology group at our institution in a separate case report.2
Our patient was initially offered and refused amputation. He was started on therapy with the mechanistic Target of Rapamycin (mTOR) inhibitor everolimus, but was unable to tolerate the side effects after the first week of treatment. He then elected to monitor his symptoms clinically.
Approximately one year after his initial diagnosis, he presented to our facility with sepsis and bleeding from a now fungating tumor on his right knee. At this time, emergent above-knee amputation was performed. Re-staging images now showed the presence of multiple pulmonary nodules in his right lung as well as a lytic rib lesion, a concerning finding for metastatic disease. Video-Assisted Thorascopic Surgery (VATS) and right lower lobe wedge resection were performed and findings confirmed metastatic PEComa.
Given the patient’s intolerance to everolimus, he was started on the growth factor inhibitor, pazopanib. His disease did not progress on pazopanib, and improvement was noted in the dominant pulmonary nodule. Subsequently, however, he developed significant skin irritation and discontinued pazopanib. Repeat imaging approximately 2 months after stopping pazopanib showed significant disease progression.
We elected to start the patient on a non-standard approach to therapy with nivolumab infusions once every 2 weeks and concurrent radiation therapy to the rib lesion. At 2 and 5 months after initiating this treatment approach, CT imaging showed improvement in disease. At 12 months, significant disease response was noted (Figure 1).
The patient is now at 12 months of nivolumab therapy with progression free survival and no new identifiable metastatic lesions. He has been tolerating the medication with minimal side effects and has had an overall improvement in his pain and functional status. He continues to work full time.
Discussion
Our patient’s response presents a unique opportunity to talk about the role of immunotherapy as a treatment modality in patients with PEComa. The efficacy of check-point blockade in soft tissue sarcoma is still unclear predominantly because it is difficult to assess the degree of expression of immunogenic cell surface markers such as programmed cell death protein 1 (PD-1).1,3 Nivolumab has been tried in small cohorts for treatment of soft tissue sarcomas that express PD-1 and results showed some clinical benefit in about half of patients.4 Further, the expression of PD-1 has been assessed in soft tissue sarcomas and has been reported to suggest a negative prognostic role.5
To our knowledge, there has not yet been another reported case of PEComa that has been treated with immunotherapy and achieved a sustained response. Further clinical studies need to be done to assess response to agents such as nivolumab in the treatment of PEComa to bolster our observation that nivolumab is a viable treatment option that may lead to lasting remission. Our patient’s case also brings to light the need for further inquiry into assessing the immune tumor microenvironments, particularly looking at the expression of cell surface proteins such as PD-1, as it ultimately affects treatment options. TSJ
Correspondence
REFERENCES
1. Burgess, Melissa, et al. “Immunotherapy in Sarcoma: Future Horizons.” Current Oncology Reports, vol. 17, no. 11, 2015, doi:10.1007/s11912-015-0476-7.
2. Alnajar, Hussein, et al. “Metastatic Malignant PEComa of the Leg with Identification of ATRX Mutation by next-Generation Sequencing.” Virchows Archiv (2017). https://doi:10.1007/s004280172208-x.
3. Ghosn, Marwan, et al. “Immunotherapies in Sarcoma: Updates and Future Perspectives.” World Journal of Clinical Oncology, vol. 8, no. 2, 2017, p. 145., doi:10.5306/wjco.v8.i2.145.
4. Paoluzzi, L., et al. “Response to Anti-PD1 Therapy with Nivolumab in Metastatic Sarcomas.” Clinical Sarcoma Research, vol. 6, no. 1, 2016, doi:10.1186/s13569-016 0064-0.
5. Kim, Chan, et al. “Prognostic Implications of PD-L1 Expression in Patients with Soft Tissue Sarcoma.” BMC Cancer, BioMed Central 8 July 2016.
Perivascular epithelioid cell neoplasms (PEComas) are an uncommon class of tumors consisting on histology of perivascular epithelioid cells occurring in both localized and metastatic forms at various body sites. The approach to treatment of these tumors generally involves a combination of surgical resection, chemotherapy, and/or radiation therapy.1
Case presentation and summary
A 46-year-old man presented to our institution with a non-tender, slowly enlarging, 8.3 cm mass in his right popliteal fossa. Upon biopsy, the pathologic findings were consistent with an epithelioid malignancy with melanocytic differentiation most consistent with a PEComa. Discussion of the pathologic diagnosis of our patient has been reported by the pathology group at our institution in a separate case report.2
Our patient was initially offered and refused amputation. He was started on therapy with the mechanistic Target of Rapamycin (mTOR) inhibitor everolimus, but was unable to tolerate the side effects after the first week of treatment. He then elected to monitor his symptoms clinically.
Approximately one year after his initial diagnosis, he presented to our facility with sepsis and bleeding from a now fungating tumor on his right knee. At this time, emergent above-knee amputation was performed. Re-staging images now showed the presence of multiple pulmonary nodules in his right lung as well as a lytic rib lesion, a concerning finding for metastatic disease. Video-Assisted Thorascopic Surgery (VATS) and right lower lobe wedge resection were performed and findings confirmed metastatic PEComa.
Given the patient’s intolerance to everolimus, he was started on the growth factor inhibitor, pazopanib. His disease did not progress on pazopanib, and improvement was noted in the dominant pulmonary nodule. Subsequently, however, he developed significant skin irritation and discontinued pazopanib. Repeat imaging approximately 2 months after stopping pazopanib showed significant disease progression.
We elected to start the patient on a non-standard approach to therapy with nivolumab infusions once every 2 weeks and concurrent radiation therapy to the rib lesion. At 2 and 5 months after initiating this treatment approach, CT imaging showed improvement in disease. At 12 months, significant disease response was noted (Figure 1).
The patient is now at 12 months of nivolumab therapy with progression free survival and no new identifiable metastatic lesions. He has been tolerating the medication with minimal side effects and has had an overall improvement in his pain and functional status. He continues to work full time.
Discussion
Our patient’s response presents a unique opportunity to talk about the role of immunotherapy as a treatment modality in patients with PEComa. The efficacy of check-point blockade in soft tissue sarcoma is still unclear predominantly because it is difficult to assess the degree of expression of immunogenic cell surface markers such as programmed cell death protein 1 (PD-1).1,3 Nivolumab has been tried in small cohorts for treatment of soft tissue sarcomas that express PD-1 and results showed some clinical benefit in about half of patients.4 Further, the expression of PD-1 has been assessed in soft tissue sarcomas and has been reported to suggest a negative prognostic role.5
To our knowledge, there has not yet been another reported case of PEComa that has been treated with immunotherapy and achieved a sustained response. Further clinical studies need to be done to assess response to agents such as nivolumab in the treatment of PEComa to bolster our observation that nivolumab is a viable treatment option that may lead to lasting remission. Our patient’s case also brings to light the need for further inquiry into assessing the immune tumor microenvironments, particularly looking at the expression of cell surface proteins such as PD-1, as it ultimately affects treatment options. TSJ
Correspondence
REFERENCES
1. Burgess, Melissa, et al. “Immunotherapy in Sarcoma: Future Horizons.” Current Oncology Reports, vol. 17, no. 11, 2015, doi:10.1007/s11912-015-0476-7.
2. Alnajar, Hussein, et al. “Metastatic Malignant PEComa of the Leg with Identification of ATRX Mutation by next-Generation Sequencing.” Virchows Archiv (2017). https://doi:10.1007/s004280172208-x.
3. Ghosn, Marwan, et al. “Immunotherapies in Sarcoma: Updates and Future Perspectives.” World Journal of Clinical Oncology, vol. 8, no. 2, 2017, p. 145., doi:10.5306/wjco.v8.i2.145.
4. Paoluzzi, L., et al. “Response to Anti-PD1 Therapy with Nivolumab in Metastatic Sarcomas.” Clinical Sarcoma Research, vol. 6, no. 1, 2016, doi:10.1186/s13569-016 0064-0.
5. Kim, Chan, et al. “Prognostic Implications of PD-L1 Expression in Patients with Soft Tissue Sarcoma.” BMC Cancer, BioMed Central 8 July 2016.
Perivascular epithelioid cell neoplasms (PEComas) are an uncommon class of tumors consisting on histology of perivascular epithelioid cells occurring in both localized and metastatic forms at various body sites. The approach to treatment of these tumors generally involves a combination of surgical resection, chemotherapy, and/or radiation therapy.1
Case presentation and summary
A 46-year-old man presented to our institution with a non-tender, slowly enlarging, 8.3 cm mass in his right popliteal fossa. Upon biopsy, the pathologic findings were consistent with an epithelioid malignancy with melanocytic differentiation most consistent with a PEComa. Discussion of the pathologic diagnosis of our patient has been reported by the pathology group at our institution in a separate case report.2
Our patient was initially offered and refused amputation. He was started on therapy with the mechanistic Target of Rapamycin (mTOR) inhibitor everolimus, but was unable to tolerate the side effects after the first week of treatment. He then elected to monitor his symptoms clinically.
Approximately one year after his initial diagnosis, he presented to our facility with sepsis and bleeding from a now fungating tumor on his right knee. At this time, emergent above-knee amputation was performed. Re-staging images now showed the presence of multiple pulmonary nodules in his right lung as well as a lytic rib lesion, a concerning finding for metastatic disease. Video-Assisted Thorascopic Surgery (VATS) and right lower lobe wedge resection were performed and findings confirmed metastatic PEComa.
Given the patient’s intolerance to everolimus, he was started on the growth factor inhibitor, pazopanib. His disease did not progress on pazopanib, and improvement was noted in the dominant pulmonary nodule. Subsequently, however, he developed significant skin irritation and discontinued pazopanib. Repeat imaging approximately 2 months after stopping pazopanib showed significant disease progression.
We elected to start the patient on a non-standard approach to therapy with nivolumab infusions once every 2 weeks and concurrent radiation therapy to the rib lesion. At 2 and 5 months after initiating this treatment approach, CT imaging showed improvement in disease. At 12 months, significant disease response was noted (Figure 1).
The patient is now at 12 months of nivolumab therapy with progression free survival and no new identifiable metastatic lesions. He has been tolerating the medication with minimal side effects and has had an overall improvement in his pain and functional status. He continues to work full time.
Discussion
Our patient’s response presents a unique opportunity to talk about the role of immunotherapy as a treatment modality in patients with PEComa. The efficacy of check-point blockade in soft tissue sarcoma is still unclear predominantly because it is difficult to assess the degree of expression of immunogenic cell surface markers such as programmed cell death protein 1 (PD-1).1,3 Nivolumab has been tried in small cohorts for treatment of soft tissue sarcomas that express PD-1 and results showed some clinical benefit in about half of patients.4 Further, the expression of PD-1 has been assessed in soft tissue sarcomas and has been reported to suggest a negative prognostic role.5
To our knowledge, there has not yet been another reported case of PEComa that has been treated with immunotherapy and achieved a sustained response. Further clinical studies need to be done to assess response to agents such as nivolumab in the treatment of PEComa to bolster our observation that nivolumab is a viable treatment option that may lead to lasting remission. Our patient’s case also brings to light the need for further inquiry into assessing the immune tumor microenvironments, particularly looking at the expression of cell surface proteins such as PD-1, as it ultimately affects treatment options. TSJ
Correspondence
REFERENCES
1. Burgess, Melissa, et al. “Immunotherapy in Sarcoma: Future Horizons.” Current Oncology Reports, vol. 17, no. 11, 2015, doi:10.1007/s11912-015-0476-7.
2. Alnajar, Hussein, et al. “Metastatic Malignant PEComa of the Leg with Identification of ATRX Mutation by next-Generation Sequencing.” Virchows Archiv (2017). https://doi:10.1007/s004280172208-x.
3. Ghosn, Marwan, et al. “Immunotherapies in Sarcoma: Updates and Future Perspectives.” World Journal of Clinical Oncology, vol. 8, no. 2, 2017, p. 145., doi:10.5306/wjco.v8.i2.145.
4. Paoluzzi, L., et al. “Response to Anti-PD1 Therapy with Nivolumab in Metastatic Sarcomas.” Clinical Sarcoma Research, vol. 6, no. 1, 2016, doi:10.1186/s13569-016 0064-0.
5. Kim, Chan, et al. “Prognostic Implications of PD-L1 Expression in Patients with Soft Tissue Sarcoma.” BMC Cancer, BioMed Central 8 July 2016.
Tumor lysis syndrome in an adolescent with recurrence of abdominal rhabdomyosarcoma: A case report and literature review
Introduction
Tumor lysis syndrome (TLS) is a life-threatening oncologic emergency that results when massive cell breakdown occurs either spontaneously or in response to cytotoxic chemotherapy. TLS is characterized by metabolic derangements, including hyperkalemia and hyperphosphatemia, secondary to the release of intracellular components into the systemic circulatory system. In addition, purine degradation can lead to hyperuricemia, and precipitation of calcium phosphate can result in hypocalcemia. Lactate dehydrogenase (LDH) levels are often elevated, especially in higher risk patients; however, this finding is not a specific marker for TLS.
TLS more commonly occurs in patients with rapidly proliferating hematological malignancies, such as acute leukemias with a high white blood cell count and Burkitt’s lymphoma, and is a relatively rare event in patients with solid malignancies.1-3 It is even more rare in patients with tumor recurrence.
There are few reported cases of TLS in children with solid malignancies. To our knowledge, only one case of TLS has previously been reported in a pediatric patient with abdominal rhabdomyosarcoma. We report the second such case, and what we believe to be the only reported case of TLS occurring in a pediatric patient with recurrence of a solid tumor.
Case Description
A 15-year-old male from Saudi Arabia presented to our hospital with confirmed stage IV abdominal rhabdomyosarcoma and lung metastases diagnosed in 2012. His initial treatment consisted of complete surgical resection, lung irradiation, and chemotherapy with intercalating cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, as per the COG-ARST0431 high-risk sarcoma protocol (NCT00354744). He completed treatment without any reported TLS in Saudi Arabia in June 2014. He had no residual tumor at the end of therapy, but six months later he was found to have an abdominal recurrence and started treatment with single-agent topotecan chemotherapy. He experienced worsening abdominal distention, pain, and difficulty voiding, prompting his family to seek further treatment options abroad.
The patient was admitted to our hospital in March 2015. Despite being severely malnourished, he was in stable condition. He was noted to have a markedly enlarged, firm, distended abdomen with dilated veins, abdominal and lower back pain, lower extremity pitting edema, and difficulty urinating.
Initial laboratory findings were unremarkable except for elevated levels of BUN (29 mg/dL), creatinine (1.69 mg/dL), and phosphorus (5.6 mg/dL). MRI revealed a large pelvic mass measuring 15.3 x 15.2 x 21.3 centimeters in transverse, anterior-posterior, and craniocaudal dimensions, respectively; with concomitant severe bilateral hydroureternephrosis (FIGURE 1).
FIGURE 1. Sagittal (A) and Axial (B) T2-weighted MR images of the pelvis (prior to initiating therapy) demonstrating a large heterogeneous mass occupying the entire pelvis. There is evidence of edema involving the soft tissues of the perineum (long arrow) and a large associated hydrocele (short arrow).
Three days following admission, the patient’s urine output decreased and his creatinine level rose rapidly. His worsening abdominal distention was attributed to growing tumor bulk and obstructive nephropathy. He required emergency placement of bilateral nephrostomy tubes. Urine output subsequently improved; although, serum creatinine remained persistently elevated.
Given his worsening condition, chemotherapy was begun three days after nephrostomy tube placement with vinorelbine, cyclophosphamide, and temsirolimus, as per COG-ARST0921 (NCT01222715), at renal-adjusted doses. Laboratory studies approximately 24 hours after chemotherapy initiation demonstrated the presence of TLS (TABLE 1). Potassium level was at the upper end of normal at 4.9 mmol/L, calcium level was decreased to 7.1 mg/dL, phosphorus level elevated to 12 mg/dL, uric acid level was markedly elevated to 19.5 mg/dL, and LDH elevated to 662 unit/L. A dose of 0.15 mg/kg of rasburicase was immediately given with a second dose repeated 14 hours later, after which the uric acid level decreased to less than 0.5 mg/dL. Sevelamer, sodium polystyrene, calcium carbonate, and magnesium gluconate were also administered to treat other electrolyte imbalances. The patient remained at clinical baseline throughout, and the TLS laboratory derangements normalized by three days after the TLS diagnosis; LDH level normalized after one week. The patient continued with chemotherapy, per protocol, with no further TLS-related complications. Over subsequent weeks, his tumor continued to shrink dramatically. Pain related to intra-abdominal compression, lower extremity edema, and difficulty voiding resolved.
Discussion
A literature search was performed using Pubmed/Medline and Scopus from 1950 to July 2016 using key words “TLS,” “tumor lysis syndrome,” “pediatric tumor lysis syndrome,” “tumor lysis syndrome in solid malignancies,” “recurrence,” “solid tumor,” “sarcoma,” “rhabdomyosarcoma,” and their combinations. The references of relevant articles were reviewed. Baeksgaard and Sorensen,3 and Vodopivec, et al4 provide an organized review of reported cases of TLS in solid tumors until 2002 and 2011 respectively; their articles are supported by the 2014 literature review by Mirrakhimov, et al.1 Excluding our case, 13 cases of TLS have been described in pediatric patients with solid tumors, with only one occurring in patient with abdominal rhabdomyosarcoma5. Patients’ ages ranged from 2 days to 23 years; the cases are summarized in the following table (TABLE 2). To our knowledge, ours is the first case of TLS reported in association with a pediatric solid tumor recurrence.
It is important to note that the three reported cases of disseminated rhabdomyosarcoma6,7 were initially believed to be hematologic malignancies because of their presentation with lymphadenopathy, metastases to the bone marrow, and spontaneous onset of TLS. Rhabdomyosarcoma with bone marrow involvement without an obvious primary tumor is easily confused with acute leukemia, particularly of the lymphoblastic type.12 However, this disseminated-hematologic presentation of rhabdomyosarcoma differs from the solid abdominal-pelvic tumor, which we describe.
Cairo and Bishop13 categorize patients as either laboratory TLS, depicted by metabolic abnormalities alone, or clinical TLS, occurring when laboratory imbalances lead to significant, life-threatening clinical manifestations. Hyperkalemia may lead to cardiac arrhythmias such as torsades de pointes and cardiac arrest. Obstructive nephropathy can occur from the precipitation of calcium phosphate or uric acid crystals in the renal tubules. Hypocalcemia may cause neuromuscular irritability including tetany, convulsions, and altered mental status.13, 14The 2015 “Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology”4 state there are well-recognized risk factors for the development of TLS including, but not limited to, high tumor burden, tumors with rapid cell turnover, and pre-existing renal impairment. Cairo and Bishop, on behalf of the TLS expert panel consensus of 20102, classify patients as having low-risk disease (LRD), intermediate-risk disease (IRD), or high-risk disease (HRD) based on the risk factors and type of malignancy. All patients with solid tumors are classified into LRD, unless the tumors are bulky or sensitive to chemotherapy, mentioning specifically that neuroblastomas, germ-cell tumors and small cell lung cancers are classified as IRD. Cairo and Bishop take into account the risk factor of renal dysfunction/ involvement, which if present, increases the risk by one level. For example, if the patient has IRD and has renal dysfunction, risk increases to HRD2. However, these guidelines do not mention or address the significance of recurrence in any kind of malignancy with regards to assessing risk for TLS.
The British Committee’s 2015 Guidelines for management of TLS in hematologic malignancies14 provide recommendations for treatment based on the patient’s risk classification (TABLE 3). Children with HRD are recommended to be treated prophylactically with a single dose of 0.2 mg/kg of rasburicase. Patients with IRD are recommended to be offered up to 7 days of allopurinol prophylaxis with increased hydration post initiation of treatment or until risk of TLS has resolved. Patients with LRD are recommended to be managed essentially with close observation. Patients with established TLS should receive rasburicase 0.2 mg/kg/day - duration to depend on clinical response. If the patient is receiving rasburicase, the addition of allopurinol is not recommended, as it has the potential to reduce the effectiveness of rasburicase. Further, rasburicase is to be avoided in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency14.
Our patient likely developed TLS because of a fast growing tumor that caused significant tumor burden and renal involvement, indicated by an elevated phosphorus level. Despite these risk factors, TLS was not anticipated in the case presented; therefore, a uric acid level was not collected at the time of admission. Review of the literature indicates that the incidence of TLS in a solid tumor recurrence is either unheard of, or is likely under-reported and truly unknown. Further, the TLS expert panel consensus of 20102, which provides guidelines on risk assessment for TLS, does not address the risk of TLS in a malignancy recurrence. The British Committee’s 2015 guidelines14 also do not address hyperuricemia prophylaxis in a solid tumor recurrence.
Our case presents a question regarding the degree of risk for the development of TLS in a solid tumor recurrence. If the guidelines had existed at the time of the case presentation and had been applied, our patient would likely be classified as having IRD because of his renal involvement. This classification would have lead to a different course of management when initiating chemotherapy, likely prevented laboratory TLS, and provided more cost effective treatment, as rasburicase is known to be expensive.
On the other hand, it can also be argued that our patient classifies as LRD, considering the rarity of TLS in a solid tumor recurrence, that the patient had no TLS complication with his initial course of therapy, and also had a normal LDH on admission. LDH is sometimes used to assess risk in hematological malignancies, although it is not used to make the diagnosis of TLS2. However, with such an argument, it is assumed that the risk of TLS in a solid tumor malignancy recurrence, with no previous TLS complication, is less than the risk associated with a new-onset solid tumor malignancy when, truly, the actual risk is not known. Again, the question is raised of the degree of risk for the development of TLS in a case of a malignancy recurrence, and also in a pediatric patient with risk factors.
In our patient’s case, close observation allowed for prompt diagnosis, appropriate treatment of laboratory TLS, and prevented clinical symptoms from developing. However, a screening or baseline uric acid level may have lead to a more conservative approach towards hyperuricemia prophylaxis, similar to treating the patient as IRD. Therefore, we recommend that a screening or baseline uric acid level and LDH level be obtained when initiating chemotherapy, even in patients with LRD.
Our patient was never hyperkalemic, likely because of concomitant administration of furosemide in an attempt to improve his decreased urine output. Hyperuricemia dropped from 19.5 mg/dL to less than 0.5 mg/dL within 24 hours, following two doses of 0.15 mg/kg of rasburicase, confirming the efficacy of this therapy in cases of established TLS, as is recommended by the British Committee’s 2015 guidelines.14
Conclusion
TLS is a relatively rare event in patients with solid malignancies and even more rare in a tumor recurrence. While there is only one previously reported case of TLS occurring in a pediatric patient with abdominal rhabdomyosarcoma, there are not any reported cases to date of TLS occurring in pediatric solid tumor recurrence. This may be because the incidence is truly rare or because cases may be under-reported. Thus, a question is raised regarding the risk for TLS in a solid tumor recurrence, and moreover in a pediatric patient with pre-existing risk factors, such as renal involvement.
TLS remains a life-threatening emergency that can be prevented and reversed if a high index of suspicion is maintained. We recommend all patients with malignancies receiving chemotherapy, especially those with risk factors, have a baseline or screening uric acid and LDH level drawn, as part of the assessment and risk-stratification for TLS which should always be performed. TSJ
Correspondence
References
1. Mirrakhimov AE, Ali AM, Khan M, et al. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6:68-74.
2. Cairo MS, Bertrand C, Reiter A, et al. Recommendation for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
3. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors – a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187-192.
4. Vodopivec D, Rubio J, Fornoni A, et al. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012;2012:1-12.
5. Khan J, Broadbent VA. Tumor lysis syndrome complicating treatment of widespread metastatic abdominal rhabdomyosarcoma. Pediatr Hematol Oncol. 1993;10:151-155.
6. Bien E, Maciejka-Kapuscinka L, Niedzwiecki M, et al. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399-407.
7. Patiroglu T, Isik B, Unal E, et al. Cranial metastatic alveolar rhabdomyosarcoma mimicking hematological malignancy in an adolescent boy. Childs Nerv Syst. 2014;30:1737-1741.
8. Hain RD, Rayner L, Weitzman S, et al. Acute tumour lysis syndrome complicating treatment of stage IVS neuroblastoma in infants under six months old. Med Pediatr Oncol. 1994;23:136-139.
9. Kushner BH, LaQuaglia MP, Modak S, et al. Tumor lysis syndrome, neuroblastoma, and correlation between serum lactate dehydrogenase levels and MYCN-amplification. Med Pediatr Oncol. 2003;41:80-82.
10. Bercovitz RS, Greffe BS, Hunger SP. Acute tumor lysis syndrome in a 7-month-old with hepatoblastoma. Curr Opin Pediatr. 2010;22:113-116.
11. Lobe TE, Karkera MS, Custer MD, et al. Fatal refractory hyperkalemia due to tumor lysis during primary resection for hepatoblastoma. J Pediatr Surg. 1990;25:249-250.
12. Sandberg A, Stone J, Czarnecki L, et al. Hematologic Masquerade of Rhabdomyosarcoma. Am J Hematol. 2001;68:51-57
13. Cairo M, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
14. Jones G, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661-671.
References
1. Mirrakhimov AE, Ali AM, Khan M, et al. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6:68-74.
2. Cairo MS, Bertrand C, Reiter A, et al. Recommendation for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
3. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors – a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187-192.
4. Vodopivec D, Rubio J, Fornoni A, et al. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012;2012:1-12.
5. Khan J, Broadbent VA. Tumor lysis syndrome complicating treatment of widespread metastatic abdominal rhabdomyosarcoma. Pediatr Hematol Oncol. 1993;10:151-155.
6. Bien E, Maciejka-Kapuscinka L, Niedzwiecki M, et al. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399-407.
7. Patiroglu T, Isik B, Unal E, et al. Cranial metastatic alveolar rhabdomyosarcoma mimicking hematological malignancy in an adolescent boy. Childs Nerv Syst. 2014;30:1737-1741.
8. Hain RD, Rayner L, Weitzman S, et al. Acute tumour lysis syndrome complicating treatment of stage IVS neuroblastoma in infants under six months old. Med Pediatr Oncol. 1994;23:136-139.
9. Kushner BH, LaQuaglia MP, Modak S, et al. Tumor lysis syndrome, neuroblastoma, and correlation between serum lactate dehydrogenase levels and MYCN-amplification. Med Pediatr Oncol. 2003;41:80-82.
10. Bercovitz RS, Greffe BS, Hunger SP. Acute tumor lysis syndrome in a 7-month-old with hepatoblastoma. Curr Opin Pediatr. 2010;22:113-116.
11. Lobe TE, Karkera MS, Custer MD, et al. Fatal refractory hyperkalemia due to tumor lysis during primary resection for hepatoblastoma. J Pediatr Surg. 1990;25:249-250.
12. Sandberg A, Stone J, Czarnecki L, et al. Hematologic Masquerade of Rhabdomyosarcoma. Am J Hematol. 2001;68:51-57
13. Cairo M, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
14. Jones G, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661-671.
Introduction
Tumor lysis syndrome (TLS) is a life-threatening oncologic emergency that results when massive cell breakdown occurs either spontaneously or in response to cytotoxic chemotherapy. TLS is characterized by metabolic derangements, including hyperkalemia and hyperphosphatemia, secondary to the release of intracellular components into the systemic circulatory system. In addition, purine degradation can lead to hyperuricemia, and precipitation of calcium phosphate can result in hypocalcemia. Lactate dehydrogenase (LDH) levels are often elevated, especially in higher risk patients; however, this finding is not a specific marker for TLS.
TLS more commonly occurs in patients with rapidly proliferating hematological malignancies, such as acute leukemias with a high white blood cell count and Burkitt’s lymphoma, and is a relatively rare event in patients with solid malignancies.1-3 It is even more rare in patients with tumor recurrence.
There are few reported cases of TLS in children with solid malignancies. To our knowledge, only one case of TLS has previously been reported in a pediatric patient with abdominal rhabdomyosarcoma. We report the second such case, and what we believe to be the only reported case of TLS occurring in a pediatric patient with recurrence of a solid tumor.
Case Description
A 15-year-old male from Saudi Arabia presented to our hospital with confirmed stage IV abdominal rhabdomyosarcoma and lung metastases diagnosed in 2012. His initial treatment consisted of complete surgical resection, lung irradiation, and chemotherapy with intercalating cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, as per the COG-ARST0431 high-risk sarcoma protocol (NCT00354744). He completed treatment without any reported TLS in Saudi Arabia in June 2014. He had no residual tumor at the end of therapy, but six months later he was found to have an abdominal recurrence and started treatment with single-agent topotecan chemotherapy. He experienced worsening abdominal distention, pain, and difficulty voiding, prompting his family to seek further treatment options abroad.
The patient was admitted to our hospital in March 2015. Despite being severely malnourished, he was in stable condition. He was noted to have a markedly enlarged, firm, distended abdomen with dilated veins, abdominal and lower back pain, lower extremity pitting edema, and difficulty urinating.
Initial laboratory findings were unremarkable except for elevated levels of BUN (29 mg/dL), creatinine (1.69 mg/dL), and phosphorus (5.6 mg/dL). MRI revealed a large pelvic mass measuring 15.3 x 15.2 x 21.3 centimeters in transverse, anterior-posterior, and craniocaudal dimensions, respectively; with concomitant severe bilateral hydroureternephrosis (FIGURE 1).
FIGURE 1. Sagittal (A) and Axial (B) T2-weighted MR images of the pelvis (prior to initiating therapy) demonstrating a large heterogeneous mass occupying the entire pelvis. There is evidence of edema involving the soft tissues of the perineum (long arrow) and a large associated hydrocele (short arrow).
Three days following admission, the patient’s urine output decreased and his creatinine level rose rapidly. His worsening abdominal distention was attributed to growing tumor bulk and obstructive nephropathy. He required emergency placement of bilateral nephrostomy tubes. Urine output subsequently improved; although, serum creatinine remained persistently elevated.
Given his worsening condition, chemotherapy was begun three days after nephrostomy tube placement with vinorelbine, cyclophosphamide, and temsirolimus, as per COG-ARST0921 (NCT01222715), at renal-adjusted doses. Laboratory studies approximately 24 hours after chemotherapy initiation demonstrated the presence of TLS (TABLE 1). Potassium level was at the upper end of normal at 4.9 mmol/L, calcium level was decreased to 7.1 mg/dL, phosphorus level elevated to 12 mg/dL, uric acid level was markedly elevated to 19.5 mg/dL, and LDH elevated to 662 unit/L. A dose of 0.15 mg/kg of rasburicase was immediately given with a second dose repeated 14 hours later, after which the uric acid level decreased to less than 0.5 mg/dL. Sevelamer, sodium polystyrene, calcium carbonate, and magnesium gluconate were also administered to treat other electrolyte imbalances. The patient remained at clinical baseline throughout, and the TLS laboratory derangements normalized by three days after the TLS diagnosis; LDH level normalized after one week. The patient continued with chemotherapy, per protocol, with no further TLS-related complications. Over subsequent weeks, his tumor continued to shrink dramatically. Pain related to intra-abdominal compression, lower extremity edema, and difficulty voiding resolved.
Discussion
A literature search was performed using Pubmed/Medline and Scopus from 1950 to July 2016 using key words “TLS,” “tumor lysis syndrome,” “pediatric tumor lysis syndrome,” “tumor lysis syndrome in solid malignancies,” “recurrence,” “solid tumor,” “sarcoma,” “rhabdomyosarcoma,” and their combinations. The references of relevant articles were reviewed. Baeksgaard and Sorensen,3 and Vodopivec, et al4 provide an organized review of reported cases of TLS in solid tumors until 2002 and 2011 respectively; their articles are supported by the 2014 literature review by Mirrakhimov, et al.1 Excluding our case, 13 cases of TLS have been described in pediatric patients with solid tumors, with only one occurring in patient with abdominal rhabdomyosarcoma5. Patients’ ages ranged from 2 days to 23 years; the cases are summarized in the following table (TABLE 2). To our knowledge, ours is the first case of TLS reported in association with a pediatric solid tumor recurrence.
It is important to note that the three reported cases of disseminated rhabdomyosarcoma6,7 were initially believed to be hematologic malignancies because of their presentation with lymphadenopathy, metastases to the bone marrow, and spontaneous onset of TLS. Rhabdomyosarcoma with bone marrow involvement without an obvious primary tumor is easily confused with acute leukemia, particularly of the lymphoblastic type.12 However, this disseminated-hematologic presentation of rhabdomyosarcoma differs from the solid abdominal-pelvic tumor, which we describe.
Cairo and Bishop13 categorize patients as either laboratory TLS, depicted by metabolic abnormalities alone, or clinical TLS, occurring when laboratory imbalances lead to significant, life-threatening clinical manifestations. Hyperkalemia may lead to cardiac arrhythmias such as torsades de pointes and cardiac arrest. Obstructive nephropathy can occur from the precipitation of calcium phosphate or uric acid crystals in the renal tubules. Hypocalcemia may cause neuromuscular irritability including tetany, convulsions, and altered mental status.13, 14The 2015 “Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology”4 state there are well-recognized risk factors for the development of TLS including, but not limited to, high tumor burden, tumors with rapid cell turnover, and pre-existing renal impairment. Cairo and Bishop, on behalf of the TLS expert panel consensus of 20102, classify patients as having low-risk disease (LRD), intermediate-risk disease (IRD), or high-risk disease (HRD) based on the risk factors and type of malignancy. All patients with solid tumors are classified into LRD, unless the tumors are bulky or sensitive to chemotherapy, mentioning specifically that neuroblastomas, germ-cell tumors and small cell lung cancers are classified as IRD. Cairo and Bishop take into account the risk factor of renal dysfunction/ involvement, which if present, increases the risk by one level. For example, if the patient has IRD and has renal dysfunction, risk increases to HRD2. However, these guidelines do not mention or address the significance of recurrence in any kind of malignancy with regards to assessing risk for TLS.
The British Committee’s 2015 Guidelines for management of TLS in hematologic malignancies14 provide recommendations for treatment based on the patient’s risk classification (TABLE 3). Children with HRD are recommended to be treated prophylactically with a single dose of 0.2 mg/kg of rasburicase. Patients with IRD are recommended to be offered up to 7 days of allopurinol prophylaxis with increased hydration post initiation of treatment or until risk of TLS has resolved. Patients with LRD are recommended to be managed essentially with close observation. Patients with established TLS should receive rasburicase 0.2 mg/kg/day - duration to depend on clinical response. If the patient is receiving rasburicase, the addition of allopurinol is not recommended, as it has the potential to reduce the effectiveness of rasburicase. Further, rasburicase is to be avoided in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency14.
Our patient likely developed TLS because of a fast growing tumor that caused significant tumor burden and renal involvement, indicated by an elevated phosphorus level. Despite these risk factors, TLS was not anticipated in the case presented; therefore, a uric acid level was not collected at the time of admission. Review of the literature indicates that the incidence of TLS in a solid tumor recurrence is either unheard of, or is likely under-reported and truly unknown. Further, the TLS expert panel consensus of 20102, which provides guidelines on risk assessment for TLS, does not address the risk of TLS in a malignancy recurrence. The British Committee’s 2015 guidelines14 also do not address hyperuricemia prophylaxis in a solid tumor recurrence.
Our case presents a question regarding the degree of risk for the development of TLS in a solid tumor recurrence. If the guidelines had existed at the time of the case presentation and had been applied, our patient would likely be classified as having IRD because of his renal involvement. This classification would have lead to a different course of management when initiating chemotherapy, likely prevented laboratory TLS, and provided more cost effective treatment, as rasburicase is known to be expensive.
On the other hand, it can also be argued that our patient classifies as LRD, considering the rarity of TLS in a solid tumor recurrence, that the patient had no TLS complication with his initial course of therapy, and also had a normal LDH on admission. LDH is sometimes used to assess risk in hematological malignancies, although it is not used to make the diagnosis of TLS2. However, with such an argument, it is assumed that the risk of TLS in a solid tumor malignancy recurrence, with no previous TLS complication, is less than the risk associated with a new-onset solid tumor malignancy when, truly, the actual risk is not known. Again, the question is raised of the degree of risk for the development of TLS in a case of a malignancy recurrence, and also in a pediatric patient with risk factors.
In our patient’s case, close observation allowed for prompt diagnosis, appropriate treatment of laboratory TLS, and prevented clinical symptoms from developing. However, a screening or baseline uric acid level may have lead to a more conservative approach towards hyperuricemia prophylaxis, similar to treating the patient as IRD. Therefore, we recommend that a screening or baseline uric acid level and LDH level be obtained when initiating chemotherapy, even in patients with LRD.
Our patient was never hyperkalemic, likely because of concomitant administration of furosemide in an attempt to improve his decreased urine output. Hyperuricemia dropped from 19.5 mg/dL to less than 0.5 mg/dL within 24 hours, following two doses of 0.15 mg/kg of rasburicase, confirming the efficacy of this therapy in cases of established TLS, as is recommended by the British Committee’s 2015 guidelines.14
Conclusion
TLS is a relatively rare event in patients with solid malignancies and even more rare in a tumor recurrence. While there is only one previously reported case of TLS occurring in a pediatric patient with abdominal rhabdomyosarcoma, there are not any reported cases to date of TLS occurring in pediatric solid tumor recurrence. This may be because the incidence is truly rare or because cases may be under-reported. Thus, a question is raised regarding the risk for TLS in a solid tumor recurrence, and moreover in a pediatric patient with pre-existing risk factors, such as renal involvement.
TLS remains a life-threatening emergency that can be prevented and reversed if a high index of suspicion is maintained. We recommend all patients with malignancies receiving chemotherapy, especially those with risk factors, have a baseline or screening uric acid and LDH level drawn, as part of the assessment and risk-stratification for TLS which should always be performed. TSJ
Correspondence
References
1. Mirrakhimov AE, Ali AM, Khan M, et al. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6:68-74.
2. Cairo MS, Bertrand C, Reiter A, et al. Recommendation for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
3. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors – a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187-192.
4. Vodopivec D, Rubio J, Fornoni A, et al. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012;2012:1-12.
5. Khan J, Broadbent VA. Tumor lysis syndrome complicating treatment of widespread metastatic abdominal rhabdomyosarcoma. Pediatr Hematol Oncol. 1993;10:151-155.
6. Bien E, Maciejka-Kapuscinka L, Niedzwiecki M, et al. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399-407.
7. Patiroglu T, Isik B, Unal E, et al. Cranial metastatic alveolar rhabdomyosarcoma mimicking hematological malignancy in an adolescent boy. Childs Nerv Syst. 2014;30:1737-1741.
8. Hain RD, Rayner L, Weitzman S, et al. Acute tumour lysis syndrome complicating treatment of stage IVS neuroblastoma in infants under six months old. Med Pediatr Oncol. 1994;23:136-139.
9. Kushner BH, LaQuaglia MP, Modak S, et al. Tumor lysis syndrome, neuroblastoma, and correlation between serum lactate dehydrogenase levels and MYCN-amplification. Med Pediatr Oncol. 2003;41:80-82.
10. Bercovitz RS, Greffe BS, Hunger SP. Acute tumor lysis syndrome in a 7-month-old with hepatoblastoma. Curr Opin Pediatr. 2010;22:113-116.
11. Lobe TE, Karkera MS, Custer MD, et al. Fatal refractory hyperkalemia due to tumor lysis during primary resection for hepatoblastoma. J Pediatr Surg. 1990;25:249-250.
12. Sandberg A, Stone J, Czarnecki L, et al. Hematologic Masquerade of Rhabdomyosarcoma. Am J Hematol. 2001;68:51-57
13. Cairo M, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
14. Jones G, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661-671.
Introduction
Tumor lysis syndrome (TLS) is a life-threatening oncologic emergency that results when massive cell breakdown occurs either spontaneously or in response to cytotoxic chemotherapy. TLS is characterized by metabolic derangements, including hyperkalemia and hyperphosphatemia, secondary to the release of intracellular components into the systemic circulatory system. In addition, purine degradation can lead to hyperuricemia, and precipitation of calcium phosphate can result in hypocalcemia. Lactate dehydrogenase (LDH) levels are often elevated, especially in higher risk patients; however, this finding is not a specific marker for TLS.
TLS more commonly occurs in patients with rapidly proliferating hematological malignancies, such as acute leukemias with a high white blood cell count and Burkitt’s lymphoma, and is a relatively rare event in patients with solid malignancies.1-3 It is even more rare in patients with tumor recurrence.
There are few reported cases of TLS in children with solid malignancies. To our knowledge, only one case of TLS has previously been reported in a pediatric patient with abdominal rhabdomyosarcoma. We report the second such case, and what we believe to be the only reported case of TLS occurring in a pediatric patient with recurrence of a solid tumor.
Case Description
A 15-year-old male from Saudi Arabia presented to our hospital with confirmed stage IV abdominal rhabdomyosarcoma and lung metastases diagnosed in 2012. His initial treatment consisted of complete surgical resection, lung irradiation, and chemotherapy with intercalating cycles of ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide, as per the COG-ARST0431 high-risk sarcoma protocol (NCT00354744). He completed treatment without any reported TLS in Saudi Arabia in June 2014. He had no residual tumor at the end of therapy, but six months later he was found to have an abdominal recurrence and started treatment with single-agent topotecan chemotherapy. He experienced worsening abdominal distention, pain, and difficulty voiding, prompting his family to seek further treatment options abroad.
The patient was admitted to our hospital in March 2015. Despite being severely malnourished, he was in stable condition. He was noted to have a markedly enlarged, firm, distended abdomen with dilated veins, abdominal and lower back pain, lower extremity pitting edema, and difficulty urinating.
Initial laboratory findings were unremarkable except for elevated levels of BUN (29 mg/dL), creatinine (1.69 mg/dL), and phosphorus (5.6 mg/dL). MRI revealed a large pelvic mass measuring 15.3 x 15.2 x 21.3 centimeters in transverse, anterior-posterior, and craniocaudal dimensions, respectively; with concomitant severe bilateral hydroureternephrosis (FIGURE 1).
FIGURE 1. Sagittal (A) and Axial (B) T2-weighted MR images of the pelvis (prior to initiating therapy) demonstrating a large heterogeneous mass occupying the entire pelvis. There is evidence of edema involving the soft tissues of the perineum (long arrow) and a large associated hydrocele (short arrow).
Three days following admission, the patient’s urine output decreased and his creatinine level rose rapidly. His worsening abdominal distention was attributed to growing tumor bulk and obstructive nephropathy. He required emergency placement of bilateral nephrostomy tubes. Urine output subsequently improved; although, serum creatinine remained persistently elevated.
Given his worsening condition, chemotherapy was begun three days after nephrostomy tube placement with vinorelbine, cyclophosphamide, and temsirolimus, as per COG-ARST0921 (NCT01222715), at renal-adjusted doses. Laboratory studies approximately 24 hours after chemotherapy initiation demonstrated the presence of TLS (TABLE 1). Potassium level was at the upper end of normal at 4.9 mmol/L, calcium level was decreased to 7.1 mg/dL, phosphorus level elevated to 12 mg/dL, uric acid level was markedly elevated to 19.5 mg/dL, and LDH elevated to 662 unit/L. A dose of 0.15 mg/kg of rasburicase was immediately given with a second dose repeated 14 hours later, after which the uric acid level decreased to less than 0.5 mg/dL. Sevelamer, sodium polystyrene, calcium carbonate, and magnesium gluconate were also administered to treat other electrolyte imbalances. The patient remained at clinical baseline throughout, and the TLS laboratory derangements normalized by three days after the TLS diagnosis; LDH level normalized after one week. The patient continued with chemotherapy, per protocol, with no further TLS-related complications. Over subsequent weeks, his tumor continued to shrink dramatically. Pain related to intra-abdominal compression, lower extremity edema, and difficulty voiding resolved.
Discussion
A literature search was performed using Pubmed/Medline and Scopus from 1950 to July 2016 using key words “TLS,” “tumor lysis syndrome,” “pediatric tumor lysis syndrome,” “tumor lysis syndrome in solid malignancies,” “recurrence,” “solid tumor,” “sarcoma,” “rhabdomyosarcoma,” and their combinations. The references of relevant articles were reviewed. Baeksgaard and Sorensen,3 and Vodopivec, et al4 provide an organized review of reported cases of TLS in solid tumors until 2002 and 2011 respectively; their articles are supported by the 2014 literature review by Mirrakhimov, et al.1 Excluding our case, 13 cases of TLS have been described in pediatric patients with solid tumors, with only one occurring in patient with abdominal rhabdomyosarcoma5. Patients’ ages ranged from 2 days to 23 years; the cases are summarized in the following table (TABLE 2). To our knowledge, ours is the first case of TLS reported in association with a pediatric solid tumor recurrence.
It is important to note that the three reported cases of disseminated rhabdomyosarcoma6,7 were initially believed to be hematologic malignancies because of their presentation with lymphadenopathy, metastases to the bone marrow, and spontaneous onset of TLS. Rhabdomyosarcoma with bone marrow involvement without an obvious primary tumor is easily confused with acute leukemia, particularly of the lymphoblastic type.12 However, this disseminated-hematologic presentation of rhabdomyosarcoma differs from the solid abdominal-pelvic tumor, which we describe.
Cairo and Bishop13 categorize patients as either laboratory TLS, depicted by metabolic abnormalities alone, or clinical TLS, occurring when laboratory imbalances lead to significant, life-threatening clinical manifestations. Hyperkalemia may lead to cardiac arrhythmias such as torsades de pointes and cardiac arrest. Obstructive nephropathy can occur from the precipitation of calcium phosphate or uric acid crystals in the renal tubules. Hypocalcemia may cause neuromuscular irritability including tetany, convulsions, and altered mental status.13, 14The 2015 “Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology”4 state there are well-recognized risk factors for the development of TLS including, but not limited to, high tumor burden, tumors with rapid cell turnover, and pre-existing renal impairment. Cairo and Bishop, on behalf of the TLS expert panel consensus of 20102, classify patients as having low-risk disease (LRD), intermediate-risk disease (IRD), or high-risk disease (HRD) based on the risk factors and type of malignancy. All patients with solid tumors are classified into LRD, unless the tumors are bulky or sensitive to chemotherapy, mentioning specifically that neuroblastomas, germ-cell tumors and small cell lung cancers are classified as IRD. Cairo and Bishop take into account the risk factor of renal dysfunction/ involvement, which if present, increases the risk by one level. For example, if the patient has IRD and has renal dysfunction, risk increases to HRD2. However, these guidelines do not mention or address the significance of recurrence in any kind of malignancy with regards to assessing risk for TLS.
The British Committee’s 2015 Guidelines for management of TLS in hematologic malignancies14 provide recommendations for treatment based on the patient’s risk classification (TABLE 3). Children with HRD are recommended to be treated prophylactically with a single dose of 0.2 mg/kg of rasburicase. Patients with IRD are recommended to be offered up to 7 days of allopurinol prophylaxis with increased hydration post initiation of treatment or until risk of TLS has resolved. Patients with LRD are recommended to be managed essentially with close observation. Patients with established TLS should receive rasburicase 0.2 mg/kg/day - duration to depend on clinical response. If the patient is receiving rasburicase, the addition of allopurinol is not recommended, as it has the potential to reduce the effectiveness of rasburicase. Further, rasburicase is to be avoided in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency14.
Our patient likely developed TLS because of a fast growing tumor that caused significant tumor burden and renal involvement, indicated by an elevated phosphorus level. Despite these risk factors, TLS was not anticipated in the case presented; therefore, a uric acid level was not collected at the time of admission. Review of the literature indicates that the incidence of TLS in a solid tumor recurrence is either unheard of, or is likely under-reported and truly unknown. Further, the TLS expert panel consensus of 20102, which provides guidelines on risk assessment for TLS, does not address the risk of TLS in a malignancy recurrence. The British Committee’s 2015 guidelines14 also do not address hyperuricemia prophylaxis in a solid tumor recurrence.
Our case presents a question regarding the degree of risk for the development of TLS in a solid tumor recurrence. If the guidelines had existed at the time of the case presentation and had been applied, our patient would likely be classified as having IRD because of his renal involvement. This classification would have lead to a different course of management when initiating chemotherapy, likely prevented laboratory TLS, and provided more cost effective treatment, as rasburicase is known to be expensive.
On the other hand, it can also be argued that our patient classifies as LRD, considering the rarity of TLS in a solid tumor recurrence, that the patient had no TLS complication with his initial course of therapy, and also had a normal LDH on admission. LDH is sometimes used to assess risk in hematological malignancies, although it is not used to make the diagnosis of TLS2. However, with such an argument, it is assumed that the risk of TLS in a solid tumor malignancy recurrence, with no previous TLS complication, is less than the risk associated with a new-onset solid tumor malignancy when, truly, the actual risk is not known. Again, the question is raised of the degree of risk for the development of TLS in a case of a malignancy recurrence, and also in a pediatric patient with risk factors.
In our patient’s case, close observation allowed for prompt diagnosis, appropriate treatment of laboratory TLS, and prevented clinical symptoms from developing. However, a screening or baseline uric acid level may have lead to a more conservative approach towards hyperuricemia prophylaxis, similar to treating the patient as IRD. Therefore, we recommend that a screening or baseline uric acid level and LDH level be obtained when initiating chemotherapy, even in patients with LRD.
Our patient was never hyperkalemic, likely because of concomitant administration of furosemide in an attempt to improve his decreased urine output. Hyperuricemia dropped from 19.5 mg/dL to less than 0.5 mg/dL within 24 hours, following two doses of 0.15 mg/kg of rasburicase, confirming the efficacy of this therapy in cases of established TLS, as is recommended by the British Committee’s 2015 guidelines.14
Conclusion
TLS is a relatively rare event in patients with solid malignancies and even more rare in a tumor recurrence. While there is only one previously reported case of TLS occurring in a pediatric patient with abdominal rhabdomyosarcoma, there are not any reported cases to date of TLS occurring in pediatric solid tumor recurrence. This may be because the incidence is truly rare or because cases may be under-reported. Thus, a question is raised regarding the risk for TLS in a solid tumor recurrence, and moreover in a pediatric patient with pre-existing risk factors, such as renal involvement.
TLS remains a life-threatening emergency that can be prevented and reversed if a high index of suspicion is maintained. We recommend all patients with malignancies receiving chemotherapy, especially those with risk factors, have a baseline or screening uric acid and LDH level drawn, as part of the assessment and risk-stratification for TLS which should always be performed. TSJ
Correspondence
References
1. Mirrakhimov AE, Ali AM, Khan M, et al. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6:68-74.
2. Cairo MS, Bertrand C, Reiter A, et al. Recommendation for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
3. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors – a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187-192.
4. Vodopivec D, Rubio J, Fornoni A, et al. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012;2012:1-12.
5. Khan J, Broadbent VA. Tumor lysis syndrome complicating treatment of widespread metastatic abdominal rhabdomyosarcoma. Pediatr Hematol Oncol. 1993;10:151-155.
6. Bien E, Maciejka-Kapuscinka L, Niedzwiecki M, et al. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399-407.
7. Patiroglu T, Isik B, Unal E, et al. Cranial metastatic alveolar rhabdomyosarcoma mimicking hematological malignancy in an adolescent boy. Childs Nerv Syst. 2014;30:1737-1741.
8. Hain RD, Rayner L, Weitzman S, et al. Acute tumour lysis syndrome complicating treatment of stage IVS neuroblastoma in infants under six months old. Med Pediatr Oncol. 1994;23:136-139.
9. Kushner BH, LaQuaglia MP, Modak S, et al. Tumor lysis syndrome, neuroblastoma, and correlation between serum lactate dehydrogenase levels and MYCN-amplification. Med Pediatr Oncol. 2003;41:80-82.
10. Bercovitz RS, Greffe BS, Hunger SP. Acute tumor lysis syndrome in a 7-month-old with hepatoblastoma. Curr Opin Pediatr. 2010;22:113-116.
11. Lobe TE, Karkera MS, Custer MD, et al. Fatal refractory hyperkalemia due to tumor lysis during primary resection for hepatoblastoma. J Pediatr Surg. 1990;25:249-250.
12. Sandberg A, Stone J, Czarnecki L, et al. Hematologic Masquerade of Rhabdomyosarcoma. Am J Hematol. 2001;68:51-57
13. Cairo M, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
14. Jones G, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661-671.
References
1. Mirrakhimov AE, Ali AM, Khan M, et al. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6:68-74.
2. Cairo MS, Bertrand C, Reiter A, et al. Recommendation for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
3. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors – a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187-192.
4. Vodopivec D, Rubio J, Fornoni A, et al. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012;2012:1-12.
5. Khan J, Broadbent VA. Tumor lysis syndrome complicating treatment of widespread metastatic abdominal rhabdomyosarcoma. Pediatr Hematol Oncol. 1993;10:151-155.
6. Bien E, Maciejka-Kapuscinka L, Niedzwiecki M, et al. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399-407.
7. Patiroglu T, Isik B, Unal E, et al. Cranial metastatic alveolar rhabdomyosarcoma mimicking hematological malignancy in an adolescent boy. Childs Nerv Syst. 2014;30:1737-1741.
8. Hain RD, Rayner L, Weitzman S, et al. Acute tumour lysis syndrome complicating treatment of stage IVS neuroblastoma in infants under six months old. Med Pediatr Oncol. 1994;23:136-139.
9. Kushner BH, LaQuaglia MP, Modak S, et al. Tumor lysis syndrome, neuroblastoma, and correlation between serum lactate dehydrogenase levels and MYCN-amplification. Med Pediatr Oncol. 2003;41:80-82.
10. Bercovitz RS, Greffe BS, Hunger SP. Acute tumor lysis syndrome in a 7-month-old with hepatoblastoma. Curr Opin Pediatr. 2010;22:113-116.
11. Lobe TE, Karkera MS, Custer MD, et al. Fatal refractory hyperkalemia due to tumor lysis during primary resection for hepatoblastoma. J Pediatr Surg. 1990;25:249-250.
12. Sandberg A, Stone J, Czarnecki L, et al. Hematologic Masquerade of Rhabdomyosarcoma. Am J Hematol. 2001;68:51-57
13. Cairo M, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
14. Jones G, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661-671.
References
1. Mirrakhimov AE, Ali AM, Khan M, et al. Tumor lysis syndrome in solid tumors: an up to date review of the literature. Rare Tumors. 2014;6:68-74.
2. Cairo MS, Bertrand C, Reiter A, et al. Recommendation for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
3. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors – a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187-192.
4. Vodopivec D, Rubio J, Fornoni A, et al. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med. 2012;2012:1-12.
5. Khan J, Broadbent VA. Tumor lysis syndrome complicating treatment of widespread metastatic abdominal rhabdomyosarcoma. Pediatr Hematol Oncol. 1993;10:151-155.
6. Bien E, Maciejka-Kapuscinka L, Niedzwiecki M, et al. Childhood rhabdomyosarcoma metastatic to bone marrow presenting with disseminated intravascular coagulation and acute tumour lysis syndrome: review of the literature apropos of two cases. Clin Exp Metastasis. 2010;27:399-407.
7. Patiroglu T, Isik B, Unal E, et al. Cranial metastatic alveolar rhabdomyosarcoma mimicking hematological malignancy in an adolescent boy. Childs Nerv Syst. 2014;30:1737-1741.
8. Hain RD, Rayner L, Weitzman S, et al. Acute tumour lysis syndrome complicating treatment of stage IVS neuroblastoma in infants under six months old. Med Pediatr Oncol. 1994;23:136-139.
9. Kushner BH, LaQuaglia MP, Modak S, et al. Tumor lysis syndrome, neuroblastoma, and correlation between serum lactate dehydrogenase levels and MYCN-amplification. Med Pediatr Oncol. 2003;41:80-82.
10. Bercovitz RS, Greffe BS, Hunger SP. Acute tumor lysis syndrome in a 7-month-old with hepatoblastoma. Curr Opin Pediatr. 2010;22:113-116.
11. Lobe TE, Karkera MS, Custer MD, et al. Fatal refractory hyperkalemia due to tumor lysis during primary resection for hepatoblastoma. J Pediatr Surg. 1990;25:249-250.
12. Sandberg A, Stone J, Czarnecki L, et al. Hematologic Masquerade of Rhabdomyosarcoma. Am J Hematol. 2001;68:51-57
13. Cairo M, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11.
14. Jones G, Will A, Jackson GH, et al. Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661-671.
Cardiac pleomorphic sarcoma after placement of a Dacron graft
Primary cardiac tumors, either benign or malignant, are very rare. The combined incidence is 0.002% on pooled autopsy series.1 The benign tumors account for 63% of primary cardiac tumors and include myxoma, the most common, and followed by papillary fibroelastoma, fibroma, and hemangioma. The remaining 37% are malignant tumors, essentially predominated by sarcomas.1
Although myxoma is the most common tumor arising in the left atrium, we present a case that shows that sarcoma can also arise from the same chamber. In fact, sarcomas could mimic cardiac myxoma.2 The cardiac sarcomas can have similar clinical presentation and more importantly can share similar histopathological features. Sarcomas may have myxoid features.2 Cases diagnosed as cardiac myxomas should be diligently worked up to rule out the presence of sarcomas with myxoid features. In addition, foreign bodies have been found to induce sarcomas in experimental animals.3,4 In particular, 2 case reports have described sarcomas arising in association with Dacron vascular prostheses in humans.5,6 We present here the case of a patient who was diagnosed with cardiac pleomorphic sarcoma 8 years after the placement of a Dacron graft.
Case presentation and summary
A 56-year-old woman with history of left atrial myxoma status after resection in 2005 and placement of a Dacron graft, morbid obesity, hypertension, and asthma presented to the emergency department with progressively worsening shortness of breath and blurry vision over period of 2 months. Acute coronary syndrome was ruled out by electrocardiogram and serial biomarkers. A computed-tomography angiogram was pursued because of her history of left atrial myxoma, and the results suggested the presence of a left atrial tumor. She underwent a transesophageal echocardiogram, which confirmed the presence of a large left atrial mass that likely was attached to the interatrial septum prolapsing across the mitral valve and was suggestive for recurrent left atrial myxoma (Figure 1). The results of a cardiac catheterization showed normal coronaries.
The patient subsequently underwent an excision of the left atrial tumor with profound internal and external myocardial cooling using antegrade blood cardioplegia under mildly hypothermic cardiopulmonary bypass. Frozen sections showed high-grade malignancy in favor of sarcoma. The hematoxylin and eosin stained permanent sections showed sheets of malignant pleomorphic spindle cells focally arranged in a storiform pattern. There were areas of necrosis and abundant mitotic activity. By immunohistochemical (IHC) stains, the tumor cells were diffusely positive for vimentin, and negative for pan-cytokeratin antibody (AE1/AE3), S-100 protein, Melan-A antibody, HMB45, CD34, CD31, myogenin, and MYOD1. IHC stains for CK-OSCAR, desmin, and smooth muscle actin were focally positive, and a ki-67 stain showed a proliferation index of about 80%. The histologic and IHC findings were consistent with a final diagnosis of high-grade undifferentiated pleomorphic sarcoma (Figure 2).
A positron emission tomography scan performed November 2013 did not show any other activity. The patient was scheduled for chemotherapy with adriamycin and ifosfamide with a plan for total of 6 cycles. Before her admission for the chemotherapy, the patient was admitted to the hospital for atrial fibrillation with rapid ventricular response and had multiple complications requiring prolonged hospitalization and rehabilitation. Repeat imaging 2 months later showed diffuse metastatic disease. However, her performance status had declined and she was not eligible for chemotherapy. She was placed under hospice care.
Discussion
This case demonstrates development of a cardiac pleomorphic sarcoma, a rare tumor, after placement of a Dacron graft. Given that foreign bodies have been found to induce sarcomas in experimental animals,3,4 and a few case reports have described sarcomas arising in association with Dacron vascular prostheses, 5-10 it seems that an exuberant host response around the foreign body might represent an important intermediate step in the development of the sarcoma.
There is no clearly defined pathogenesis that explains the link between a Dacron graft and sarcomas. In 1950s, Oppenheimer and colleagues described the formation of malignant tumors by various types of plastics, including Dacron, that were embedded in rats. 3,4 Most of the tumors were some form of sarcomas. It was inferred that physical properties of the plastics may have some role in tumor development. Plastics in sheet form or film that remained in situ for more than 6 months induced significant number of tumors compared with other forms such as sponges, films with holes, or powders.3,4 The 3-dimensional polymeric structure of the Dacron graft seems to play a role in induction of sarcoma as well. A pore diameter of less than 0.4 mm may increase tumorigenicity.11 The removal of the material before the 6-month mark does not lead to malignant tumors, which further supports the link between Dacron graft and formation of tumor. A pocket is formed around the foreign material after a certain period, as has been shown in histologic studies as the site of tumor origin.9,10
At the molecular level, the MDM-2/p53 pathway has been cited as possible mechanism for pathogenesis of intimal sarcoma.12,13 It has been suggested that endothelial dysplasia occurs as a precursor lesion in these sarcomas.14 The Dacron graft may cause a dysplastic effect on the endothelium leading to this precursor lesion and in certain cases transforming into sarcoma. Further definitive studies are required.
The primary treatment for cardiac sarcoma is surgical removal, although it is not always feasible. Findings in a Mayo clinic study showed that the median survival was 17 months for patients who underwent complete surgical excision, compared with 6 months for those who complete resection was not possible.15 In addition, a 10% survival rate at 1 year has been reported in primary cardiac sarcomas that are treated without any type of surgery.16
There is no clear-cut evidence supporting or refuting adjuvant chemotherapy for cardiac sarcoma. Some have inferred a potential benefit of adjuvant chemotherapy although definitive conclusions cannot be drawn. The median survival was 16.5 months in a case series of patients who received adjuvant chemotherapy, compared with 9 months and 11 months in 2 other case series.17,18,19 Multiple chemotherapy regimens have been used in the past for treatment. A retrospective s
Radiation showed some benefit in progression-free survival in a French retrospective study.21 Radiation therapies have been tried in other cases, as well in addition to chemotherapy. However, there is not enough data to support or refute it at this time.15,17,20 Several sporadic cases reported show benefit of cardiac transplantation.21,22
Conclusion
In consideration of the placement of the Dacron graft 8 years before the tumor occurrence, the anatomic proximity of the tumor to the Dacron graft, and the association between sarcoma with Dacron in medical literature, it seems logical to infer that this unusual malignancy in our patient is associated with the Dacron prosthesis. TSJ
Correspondence
1. Patil HR, Singh D, Hajdu M. Cardiac sarcoma presenting as heart failure and diagnosed as recurrent myxoma by echocardiogram. Eur J Echocardiogr. 2010;11(4):E12.
2. Awamleh P, Alberca MT, Gamallo C, Enrech S, Sarraj A. Left atrium myxosarcoma: an exceptional cardiac malignant primary tumor. Clin Cardiol. 2007;30(6):306-308.
3. Oppenheimer BS, Oppenheimer ET, Stout AP, Danishefsky I. Malignant tumors resulting from embedding plastics in rodents. Science. 1953;118:305-306.
4. Oppenheimer BS, Oppenheimer ET, Stout AP, Willhite M, Danishefski, I. The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer. 1958;11(1):204-213.
5. Almeida NJ, Hoang P, Biddle P, Arouni A, Esterbrooks D. Primary cardiac angiosarcoma: in a patient with a Dacron aortic prosthesis. Tex Heart Inst J. 2011;38(1):61-65; discussion 65.
6. Stewart B, Manglik N, Zhao B, et al. Aortic intimal sarcoma: report of two cases with immunohistochemical analysis for pathogenesis. Cardiovasc Pathol. 2013;22(5):351-356.
7. Umscheid TW, Rouhani G, Morlang T, et al. Hemangiosarcoma after endovascular aortic aneurysm repair. J Endovasc Ther. 2007;14(1):101-105.
8. Ben-Izhak O, Vlodavsky E, Ofer A, Engel A, Nitecky S, Hoffman A. Epithelioid angiosarcoma associated with a Dacron vascular graft. Am J Surg Pathol. 1999;23(11):1418-1422.
9. Fyfe BS, Quintana CS, Kaneko M, Griepp RB. Aortic sarcoma four years after Dacron graft insertion. Ann Thorac Surg. 1994;58(6):1752-1754.
10. O’Connell TX, Fee HJ, Golding A. Sarcoma associated with Dacron prosthetic material: case report and review of the literature. J Thorac Cardiovasc Surg. 1976;72(1):94-96.
11. Karp RD, Johnson KH, Buoen LC, et al. Tumorogenesis by millipore filters in mice: histology and ultastructure of tissue reactions, as related to pore size. J Natl Cancer Inst. 1973;51:1275-1285.
12. Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57-65.
13. Zeitz C, Rossle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Surg Pathol. 1998;153:1425-1433.
14. Haber LM, Truong L. Immunohistochemical demonstration of the endothelialnature of aortic intimal sarcoma. Am J Surg Pathol. 1988 Oct;12(10):798-802. PubMed PMID: 3138923.
15. Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112(11):2440-2446.
16. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261-262.
17. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34(4):295-304.
18. Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DC. Primary cardiac sarcomas. Ann Thorac Surg. 1990; 51; 906-910.
19. Murphy WR, Sweeney MS, Putnam JB et al. Surgical treatment of cardiac tumors: a 25-year experience. Ann Thorac Surg. 1990;49;612-618.
20. Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78(12):1624-1628.
21. Isambert N, Ray-Coquard I, Italiano A, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(1):128-136.
22. Agaimy A, Rösch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(9):928-938.
Primary cardiac tumors, either benign or malignant, are very rare. The combined incidence is 0.002% on pooled autopsy series.1 The benign tumors account for 63% of primary cardiac tumors and include myxoma, the most common, and followed by papillary fibroelastoma, fibroma, and hemangioma. The remaining 37% are malignant tumors, essentially predominated by sarcomas.1
Although myxoma is the most common tumor arising in the left atrium, we present a case that shows that sarcoma can also arise from the same chamber. In fact, sarcomas could mimic cardiac myxoma.2 The cardiac sarcomas can have similar clinical presentation and more importantly can share similar histopathological features. Sarcomas may have myxoid features.2 Cases diagnosed as cardiac myxomas should be diligently worked up to rule out the presence of sarcomas with myxoid features. In addition, foreign bodies have been found to induce sarcomas in experimental animals.3,4 In particular, 2 case reports have described sarcomas arising in association with Dacron vascular prostheses in humans.5,6 We present here the case of a patient who was diagnosed with cardiac pleomorphic sarcoma 8 years after the placement of a Dacron graft.
Case presentation and summary
A 56-year-old woman with history of left atrial myxoma status after resection in 2005 and placement of a Dacron graft, morbid obesity, hypertension, and asthma presented to the emergency department with progressively worsening shortness of breath and blurry vision over period of 2 months. Acute coronary syndrome was ruled out by electrocardiogram and serial biomarkers. A computed-tomography angiogram was pursued because of her history of left atrial myxoma, and the results suggested the presence of a left atrial tumor. She underwent a transesophageal echocardiogram, which confirmed the presence of a large left atrial mass that likely was attached to the interatrial septum prolapsing across the mitral valve and was suggestive for recurrent left atrial myxoma (Figure 1). The results of a cardiac catheterization showed normal coronaries.
The patient subsequently underwent an excision of the left atrial tumor with profound internal and external myocardial cooling using antegrade blood cardioplegia under mildly hypothermic cardiopulmonary bypass. Frozen sections showed high-grade malignancy in favor of sarcoma. The hematoxylin and eosin stained permanent sections showed sheets of malignant pleomorphic spindle cells focally arranged in a storiform pattern. There were areas of necrosis and abundant mitotic activity. By immunohistochemical (IHC) stains, the tumor cells were diffusely positive for vimentin, and negative for pan-cytokeratin antibody (AE1/AE3), S-100 protein, Melan-A antibody, HMB45, CD34, CD31, myogenin, and MYOD1. IHC stains for CK-OSCAR, desmin, and smooth muscle actin were focally positive, and a ki-67 stain showed a proliferation index of about 80%. The histologic and IHC findings were consistent with a final diagnosis of high-grade undifferentiated pleomorphic sarcoma (Figure 2).
A positron emission tomography scan performed November 2013 did not show any other activity. The patient was scheduled for chemotherapy with adriamycin and ifosfamide with a plan for total of 6 cycles. Before her admission for the chemotherapy, the patient was admitted to the hospital for atrial fibrillation with rapid ventricular response and had multiple complications requiring prolonged hospitalization and rehabilitation. Repeat imaging 2 months later showed diffuse metastatic disease. However, her performance status had declined and she was not eligible for chemotherapy. She was placed under hospice care.
Discussion
This case demonstrates development of a cardiac pleomorphic sarcoma, a rare tumor, after placement of a Dacron graft. Given that foreign bodies have been found to induce sarcomas in experimental animals,3,4 and a few case reports have described sarcomas arising in association with Dacron vascular prostheses, 5-10 it seems that an exuberant host response around the foreign body might represent an important intermediate step in the development of the sarcoma.
There is no clearly defined pathogenesis that explains the link between a Dacron graft and sarcomas. In 1950s, Oppenheimer and colleagues described the formation of malignant tumors by various types of plastics, including Dacron, that were embedded in rats. 3,4 Most of the tumors were some form of sarcomas. It was inferred that physical properties of the plastics may have some role in tumor development. Plastics in sheet form or film that remained in situ for more than 6 months induced significant number of tumors compared with other forms such as sponges, films with holes, or powders.3,4 The 3-dimensional polymeric structure of the Dacron graft seems to play a role in induction of sarcoma as well. A pore diameter of less than 0.4 mm may increase tumorigenicity.11 The removal of the material before the 6-month mark does not lead to malignant tumors, which further supports the link between Dacron graft and formation of tumor. A pocket is formed around the foreign material after a certain period, as has been shown in histologic studies as the site of tumor origin.9,10
At the molecular level, the MDM-2/p53 pathway has been cited as possible mechanism for pathogenesis of intimal sarcoma.12,13 It has been suggested that endothelial dysplasia occurs as a precursor lesion in these sarcomas.14 The Dacron graft may cause a dysplastic effect on the endothelium leading to this precursor lesion and in certain cases transforming into sarcoma. Further definitive studies are required.
The primary treatment for cardiac sarcoma is surgical removal, although it is not always feasible. Findings in a Mayo clinic study showed that the median survival was 17 months for patients who underwent complete surgical excision, compared with 6 months for those who complete resection was not possible.15 In addition, a 10% survival rate at 1 year has been reported in primary cardiac sarcomas that are treated without any type of surgery.16
There is no clear-cut evidence supporting or refuting adjuvant chemotherapy for cardiac sarcoma. Some have inferred a potential benefit of adjuvant chemotherapy although definitive conclusions cannot be drawn. The median survival was 16.5 months in a case series of patients who received adjuvant chemotherapy, compared with 9 months and 11 months in 2 other case series.17,18,19 Multiple chemotherapy regimens have been used in the past for treatment. A retrospective s
Radiation showed some benefit in progression-free survival in a French retrospective study.21 Radiation therapies have been tried in other cases, as well in addition to chemotherapy. However, there is not enough data to support or refute it at this time.15,17,20 Several sporadic cases reported show benefit of cardiac transplantation.21,22
Conclusion
In consideration of the placement of the Dacron graft 8 years before the tumor occurrence, the anatomic proximity of the tumor to the Dacron graft, and the association between sarcoma with Dacron in medical literature, it seems logical to infer that this unusual malignancy in our patient is associated with the Dacron prosthesis. TSJ
Correspondence
1. Patil HR, Singh D, Hajdu M. Cardiac sarcoma presenting as heart failure and diagnosed as recurrent myxoma by echocardiogram. Eur J Echocardiogr. 2010;11(4):E12.
2. Awamleh P, Alberca MT, Gamallo C, Enrech S, Sarraj A. Left atrium myxosarcoma: an exceptional cardiac malignant primary tumor. Clin Cardiol. 2007;30(6):306-308.
3. Oppenheimer BS, Oppenheimer ET, Stout AP, Danishefsky I. Malignant tumors resulting from embedding plastics in rodents. Science. 1953;118:305-306.
4. Oppenheimer BS, Oppenheimer ET, Stout AP, Willhite M, Danishefski, I. The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer. 1958;11(1):204-213.
5. Almeida NJ, Hoang P, Biddle P, Arouni A, Esterbrooks D. Primary cardiac angiosarcoma: in a patient with a Dacron aortic prosthesis. Tex Heart Inst J. 2011;38(1):61-65; discussion 65.
6. Stewart B, Manglik N, Zhao B, et al. Aortic intimal sarcoma: report of two cases with immunohistochemical analysis for pathogenesis. Cardiovasc Pathol. 2013;22(5):351-356.
7. Umscheid TW, Rouhani G, Morlang T, et al. Hemangiosarcoma after endovascular aortic aneurysm repair. J Endovasc Ther. 2007;14(1):101-105.
8. Ben-Izhak O, Vlodavsky E, Ofer A, Engel A, Nitecky S, Hoffman A. Epithelioid angiosarcoma associated with a Dacron vascular graft. Am J Surg Pathol. 1999;23(11):1418-1422.
9. Fyfe BS, Quintana CS, Kaneko M, Griepp RB. Aortic sarcoma four years after Dacron graft insertion. Ann Thorac Surg. 1994;58(6):1752-1754.
10. O’Connell TX, Fee HJ, Golding A. Sarcoma associated with Dacron prosthetic material: case report and review of the literature. J Thorac Cardiovasc Surg. 1976;72(1):94-96.
11. Karp RD, Johnson KH, Buoen LC, et al. Tumorogenesis by millipore filters in mice: histology and ultastructure of tissue reactions, as related to pore size. J Natl Cancer Inst. 1973;51:1275-1285.
12. Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57-65.
13. Zeitz C, Rossle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Surg Pathol. 1998;153:1425-1433.
14. Haber LM, Truong L. Immunohistochemical demonstration of the endothelialnature of aortic intimal sarcoma. Am J Surg Pathol. 1988 Oct;12(10):798-802. PubMed PMID: 3138923.
15. Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112(11):2440-2446.
16. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261-262.
17. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34(4):295-304.
18. Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DC. Primary cardiac sarcomas. Ann Thorac Surg. 1990; 51; 906-910.
19. Murphy WR, Sweeney MS, Putnam JB et al. Surgical treatment of cardiac tumors: a 25-year experience. Ann Thorac Surg. 1990;49;612-618.
20. Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78(12):1624-1628.
21. Isambert N, Ray-Coquard I, Italiano A, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(1):128-136.
22. Agaimy A, Rösch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(9):928-938.
Primary cardiac tumors, either benign or malignant, are very rare. The combined incidence is 0.002% on pooled autopsy series.1 The benign tumors account for 63% of primary cardiac tumors and include myxoma, the most common, and followed by papillary fibroelastoma, fibroma, and hemangioma. The remaining 37% are malignant tumors, essentially predominated by sarcomas.1
Although myxoma is the most common tumor arising in the left atrium, we present a case that shows that sarcoma can also arise from the same chamber. In fact, sarcomas could mimic cardiac myxoma.2 The cardiac sarcomas can have similar clinical presentation and more importantly can share similar histopathological features. Sarcomas may have myxoid features.2 Cases diagnosed as cardiac myxomas should be diligently worked up to rule out the presence of sarcomas with myxoid features. In addition, foreign bodies have been found to induce sarcomas in experimental animals.3,4 In particular, 2 case reports have described sarcomas arising in association with Dacron vascular prostheses in humans.5,6 We present here the case of a patient who was diagnosed with cardiac pleomorphic sarcoma 8 years after the placement of a Dacron graft.
Case presentation and summary
A 56-year-old woman with history of left atrial myxoma status after resection in 2005 and placement of a Dacron graft, morbid obesity, hypertension, and asthma presented to the emergency department with progressively worsening shortness of breath and blurry vision over period of 2 months. Acute coronary syndrome was ruled out by electrocardiogram and serial biomarkers. A computed-tomography angiogram was pursued because of her history of left atrial myxoma, and the results suggested the presence of a left atrial tumor. She underwent a transesophageal echocardiogram, which confirmed the presence of a large left atrial mass that likely was attached to the interatrial septum prolapsing across the mitral valve and was suggestive for recurrent left atrial myxoma (Figure 1). The results of a cardiac catheterization showed normal coronaries.
The patient subsequently underwent an excision of the left atrial tumor with profound internal and external myocardial cooling using antegrade blood cardioplegia under mildly hypothermic cardiopulmonary bypass. Frozen sections showed high-grade malignancy in favor of sarcoma. The hematoxylin and eosin stained permanent sections showed sheets of malignant pleomorphic spindle cells focally arranged in a storiform pattern. There were areas of necrosis and abundant mitotic activity. By immunohistochemical (IHC) stains, the tumor cells were diffusely positive for vimentin, and negative for pan-cytokeratin antibody (AE1/AE3), S-100 protein, Melan-A antibody, HMB45, CD34, CD31, myogenin, and MYOD1. IHC stains for CK-OSCAR, desmin, and smooth muscle actin were focally positive, and a ki-67 stain showed a proliferation index of about 80%. The histologic and IHC findings were consistent with a final diagnosis of high-grade undifferentiated pleomorphic sarcoma (Figure 2).
A positron emission tomography scan performed November 2013 did not show any other activity. The patient was scheduled for chemotherapy with adriamycin and ifosfamide with a plan for total of 6 cycles. Before her admission for the chemotherapy, the patient was admitted to the hospital for atrial fibrillation with rapid ventricular response and had multiple complications requiring prolonged hospitalization and rehabilitation. Repeat imaging 2 months later showed diffuse metastatic disease. However, her performance status had declined and she was not eligible for chemotherapy. She was placed under hospice care.
Discussion
This case demonstrates development of a cardiac pleomorphic sarcoma, a rare tumor, after placement of a Dacron graft. Given that foreign bodies have been found to induce sarcomas in experimental animals,3,4 and a few case reports have described sarcomas arising in association with Dacron vascular prostheses, 5-10 it seems that an exuberant host response around the foreign body might represent an important intermediate step in the development of the sarcoma.
There is no clearly defined pathogenesis that explains the link between a Dacron graft and sarcomas. In 1950s, Oppenheimer and colleagues described the formation of malignant tumors by various types of plastics, including Dacron, that were embedded in rats. 3,4 Most of the tumors were some form of sarcomas. It was inferred that physical properties of the plastics may have some role in tumor development. Plastics in sheet form or film that remained in situ for more than 6 months induced significant number of tumors compared with other forms such as sponges, films with holes, or powders.3,4 The 3-dimensional polymeric structure of the Dacron graft seems to play a role in induction of sarcoma as well. A pore diameter of less than 0.4 mm may increase tumorigenicity.11 The removal of the material before the 6-month mark does not lead to malignant tumors, which further supports the link between Dacron graft and formation of tumor. A pocket is formed around the foreign material after a certain period, as has been shown in histologic studies as the site of tumor origin.9,10
At the molecular level, the MDM-2/p53 pathway has been cited as possible mechanism for pathogenesis of intimal sarcoma.12,13 It has been suggested that endothelial dysplasia occurs as a precursor lesion in these sarcomas.14 The Dacron graft may cause a dysplastic effect on the endothelium leading to this precursor lesion and in certain cases transforming into sarcoma. Further definitive studies are required.
The primary treatment for cardiac sarcoma is surgical removal, although it is not always feasible. Findings in a Mayo clinic study showed that the median survival was 17 months for patients who underwent complete surgical excision, compared with 6 months for those who complete resection was not possible.15 In addition, a 10% survival rate at 1 year has been reported in primary cardiac sarcomas that are treated without any type of surgery.16
There is no clear-cut evidence supporting or refuting adjuvant chemotherapy for cardiac sarcoma. Some have inferred a potential benefit of adjuvant chemotherapy although definitive conclusions cannot be drawn. The median survival was 16.5 months in a case series of patients who received adjuvant chemotherapy, compared with 9 months and 11 months in 2 other case series.17,18,19 Multiple chemotherapy regimens have been used in the past for treatment. A retrospective s
Radiation showed some benefit in progression-free survival in a French retrospective study.21 Radiation therapies have been tried in other cases, as well in addition to chemotherapy. However, there is not enough data to support or refute it at this time.15,17,20 Several sporadic cases reported show benefit of cardiac transplantation.21,22
Conclusion
In consideration of the placement of the Dacron graft 8 years before the tumor occurrence, the anatomic proximity of the tumor to the Dacron graft, and the association between sarcoma with Dacron in medical literature, it seems logical to infer that this unusual malignancy in our patient is associated with the Dacron prosthesis. TSJ
Correspondence
1. Patil HR, Singh D, Hajdu M. Cardiac sarcoma presenting as heart failure and diagnosed as recurrent myxoma by echocardiogram. Eur J Echocardiogr. 2010;11(4):E12.
2. Awamleh P, Alberca MT, Gamallo C, Enrech S, Sarraj A. Left atrium myxosarcoma: an exceptional cardiac malignant primary tumor. Clin Cardiol. 2007;30(6):306-308.
3. Oppenheimer BS, Oppenheimer ET, Stout AP, Danishefsky I. Malignant tumors resulting from embedding plastics in rodents. Science. 1953;118:305-306.
4. Oppenheimer BS, Oppenheimer ET, Stout AP, Willhite M, Danishefski, I. The latent period in carcinogenesis by plastics in rats and its relation to the presarcomatous stage. Cancer. 1958;11(1):204-213.
5. Almeida NJ, Hoang P, Biddle P, Arouni A, Esterbrooks D. Primary cardiac angiosarcoma: in a patient with a Dacron aortic prosthesis. Tex Heart Inst J. 2011;38(1):61-65; discussion 65.
6. Stewart B, Manglik N, Zhao B, et al. Aortic intimal sarcoma: report of two cases with immunohistochemical analysis for pathogenesis. Cardiovasc Pathol. 2013;22(5):351-356.
7. Umscheid TW, Rouhani G, Morlang T, et al. Hemangiosarcoma after endovascular aortic aneurysm repair. J Endovasc Ther. 2007;14(1):101-105.
8. Ben-Izhak O, Vlodavsky E, Ofer A, Engel A, Nitecky S, Hoffman A. Epithelioid angiosarcoma associated with a Dacron vascular graft. Am J Surg Pathol. 1999;23(11):1418-1422.
9. Fyfe BS, Quintana CS, Kaneko M, Griepp RB. Aortic sarcoma four years after Dacron graft insertion. Ann Thorac Surg. 1994;58(6):1752-1754.
10. O’Connell TX, Fee HJ, Golding A. Sarcoma associated with Dacron prosthetic material: case report and review of the literature. J Thorac Cardiovasc Surg. 1976;72(1):94-96.
11. Karp RD, Johnson KH, Buoen LC, et al. Tumorogenesis by millipore filters in mice: histology and ultastructure of tissue reactions, as related to pore size. J Natl Cancer Inst. 1973;51:1275-1285.
12. Bode-Lesniewska B, Zhao J, Speel EJ, et al. Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57-65.
13. Zeitz C, Rossle M, Haas C, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Surg Pathol. 1998;153:1425-1433.
14. Haber LM, Truong L. Immunohistochemical demonstration of the endothelialnature of aortic intimal sarcoma. Am J Surg Pathol. 1988 Oct;12(10):798-802. PubMed PMID: 3138923.
15. Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112(11):2440-2446.
16. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38(3):261-262.
17. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34(4):295-304.
18. Putnam JB, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DC. Primary cardiac sarcomas. Ann Thorac Surg. 1990; 51; 906-910.
19. Murphy WR, Sweeney MS, Putnam JB et al. Surgical treatment of cardiac tumors: a 25-year experience. Ann Thorac Surg. 1990;49;612-618.
20. Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78(12):1624-1628.
21. Isambert N, Ray-Coquard I, Italiano A, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50(1):128-136.
22. Agaimy A, Rösch J, Weyand M, Strecker T. Primary and metastatic cardiac sarcomas: a 12-year experience at a German heart center. Int J Clin Exp Pathol. 2012;5(9):928-938.
Bilateral wrist pain • limited range of motion • tenderness to palpation • Dx?
THE CASE
A 12-year-old girl presented to my office (JH) with bilateral wrist pain. She had fallen on both wrists palmar-flexed and then, while trying to get up, landed on both wrists dorsi-flexed. The patient did not hear any “pops,” but felt immediate pain when her wrists hyperextended. Hand, wrist, and forearm x-rays were negative bilaterally for fractures. She was placed in bilateral thumb spica splints.
At follow-up one week later, the patient reported 6/10 pain in her left wrist and 7/10 pain in her right wrist. The pain increased to 10/10 bilaterally with movement and was not relieved by icing or nonsteroidal anti-inflammatory drugs. On physical exam, there was bilateral swelling of the wrists without ecchymosis or erythema. The patient had limited passive and active range of motion, especially during wrist extension. She also had tenderness to palpation over the anatomical snuff box, extending proximally to the distal radius bilaterally. She had no tenderness over the ulna or metacarpals, no loss of sensation in any area nerves, and she was neurovascularly intact bilaterally.
Based on the mechanism of injury, undetected fracture or full thickness ligament tear were both possible. Because of this, and because magnetic resonance imaging (MRI) entails no radiation exposure, MRI was chosen for additional imaging of both wrists.
THE DIAGNOSIS
The MRI revealed bilateral, nondisplaced, extra-articular fractures extending through the scaphoid waist, with surrounding bone marrow edema. In the right wrist, the patient also had a low-grade partial tear of the membranous portion of the scapholunate interosseous ligament (SLIL) at the scaphoid attachment (FIGURE 1). In the left wrist, she also had a low-grade sprain of the SLIL without tear (FIGURE 2).
DISCUSSION
Carpal fractures account for 6% of all fractures.1 Scaphoid fractures are the most common carpal bone fracture among all age groups, but account for only 0.4% of all pediatric fractures.1-3 They’re commonly missed on x-rays because they are usually nondisplaced and hidden by other structures superimposed on the image.1,2,4 Undetected, scaphoid fractures can cause prolonged interruption to the bone’s architecture, leading to avascular necrosis of the proximal portion of the scaphoid bone.5,6
Bilateral scaphoid fractures are extremely rare and account for less than 1% of all scaphoid fractures.7 Very few of these cases have been published in the literature, and those that have been published have talked about the fractures being secondary to chronic stress fractures and as being treated with internal fixation (regardless of whether the fractures were nondisplaced or if the ligaments were intact).6-9
Our patient was placed in bilateral fiberglass short-arm thumb spica casts. We tried conservative treatment measures first because she had help with her activities of daily living (ADLs). At a follow-up visit 2 weeks later, we switched the casts to long-arm thumb spica casts because of the patient’s ability to pronate and supinate her wrists in the short-arm versions. After one month of wearing the long-arm casts, we placed her back in bilateral short-arm casts for 2 weeks. Eight weeks after the fall, we removed the short-arm casts for reevaluation.
We obtained x-rays to assess for any new changes to the wrist and specifically the scaphoid bones. The x-rays showed almost completely healed scaphoid bones with good alignment, but the patient still had 5/10 pain in the left wrist and 8/10 pain in the right wrist with movement. We placed her in adjustable thermoformable polymer braces, which were removed when she bathed.
Due to the uniqueness of her injuries, our patient had weekly visits with her primary care provider (PCP) for the first 2 months of treatment, followed by bimonthly visits for the remainder. At 10 weeks after the fall, her pain with movement was almost gone and she began physical therapy. She also began removing the braces during sedentary activity in order to practice range-of-motion exercises to prevent excessive stiffness in her wrists. Our patient regained full strength and range of motion one month later.
One other published case report describes the successful union of bilateral scaphoid fractures using bilateral long-arm casts followed by short-arm casts.7 Similar to our patient’s case, full union of the scaphoid bones was achieved within 12 weeks.7 Together, these cases suggest that conservative treatment methods are a viable alternative to surgery.
TAKEAWAY
For patients presenting with wrist pain after trauma to the wrists, assess anatomical snuffbox tenderness and obtain x-rays. Do not be falsely reassured by negative x-rays in the presence of a positive physical exam, however, as scaphoid fractures are often hidden on x-rays. If tenderness at the anatomical snuffbox is present and doesn’t subside within a few days, apply a short-arm thumb splint and obtain subsequent imaging.
If bilateral, nondisplaced, stable scaphoid fractures are diagnosed, conservative treatment with long-arm and short-arm casts is a viable alternative to surgery. This treatment decision should be made on an individual basis, however, as it requires the patient to have frequent PCP visits, assistance with ADLs, and complete adherence to the treatment plan.
1. Pillai A, Jain M. Management of clinical fractures of the scaphoid: results of an audit and literature review. Eur J Emerg Med. 2005;12:47-51.
2. Evenski AJ, Adamczyk MJ, Steiner RP, et al. Clinically suspected scaphoid fractures in children. J Pediatr Orthop. 2009;29:352-355.
3. Wulff R, Schmidt T. Carpal fractures in children. J Pediatr Orthop. 1998;18:462-465.
4. Nellans KW, Chung KC. Pediatric hand fractures. Hand Clin. 2013;29:569-578.
5. Jernigan EW, Smetana BS, Patterson JM. Pediatric scaphoid proximal pole nonunion with avascular necrosis. J Hand Surgery. 2017;42:299.e1-299.e4.
6. Pidemunt G, Torres-Claramunt R, Ginés A, et al. Bilateral stress fracture of the carpal scaphoid: report in a child and review of the literature. Clin J Sport Med. 2012;22:511-513.
7. Saglam F, Gulabi D, Baysal Ö, et al. Chronic wrist pain in a goalkeeper; bilateral scaphoid stress fracture: a case report. Int J Surg Case Rep. 2015;7:20-22.
8. Muzaffar N, Wani I, Ehsan M, et al. Simultaneous bilateral scaphoid fractures in a soldier managed conservatively by scaphoid casts. Arch Clin Exp Surg. 2016;5:63-64.
9. Mohamed Haflah NH, Mat Nor NF, Abdullah S, et al. Bilateral scaphoid stress fracture in a platform diver presenting with unilateral symptoms. Singapore Med J. 2014;55:e159-e161.
THE CASE
A 12-year-old girl presented to my office (JH) with bilateral wrist pain. She had fallen on both wrists palmar-flexed and then, while trying to get up, landed on both wrists dorsi-flexed. The patient did not hear any “pops,” but felt immediate pain when her wrists hyperextended. Hand, wrist, and forearm x-rays were negative bilaterally for fractures. She was placed in bilateral thumb spica splints.
At follow-up one week later, the patient reported 6/10 pain in her left wrist and 7/10 pain in her right wrist. The pain increased to 10/10 bilaterally with movement and was not relieved by icing or nonsteroidal anti-inflammatory drugs. On physical exam, there was bilateral swelling of the wrists without ecchymosis or erythema. The patient had limited passive and active range of motion, especially during wrist extension. She also had tenderness to palpation over the anatomical snuff box, extending proximally to the distal radius bilaterally. She had no tenderness over the ulna or metacarpals, no loss of sensation in any area nerves, and she was neurovascularly intact bilaterally.
Based on the mechanism of injury, undetected fracture or full thickness ligament tear were both possible. Because of this, and because magnetic resonance imaging (MRI) entails no radiation exposure, MRI was chosen for additional imaging of both wrists.
THE DIAGNOSIS
The MRI revealed bilateral, nondisplaced, extra-articular fractures extending through the scaphoid waist, with surrounding bone marrow edema. In the right wrist, the patient also had a low-grade partial tear of the membranous portion of the scapholunate interosseous ligament (SLIL) at the scaphoid attachment (FIGURE 1). In the left wrist, she also had a low-grade sprain of the SLIL without tear (FIGURE 2).
DISCUSSION
Carpal fractures account for 6% of all fractures.1 Scaphoid fractures are the most common carpal bone fracture among all age groups, but account for only 0.4% of all pediatric fractures.1-3 They’re commonly missed on x-rays because they are usually nondisplaced and hidden by other structures superimposed on the image.1,2,4 Undetected, scaphoid fractures can cause prolonged interruption to the bone’s architecture, leading to avascular necrosis of the proximal portion of the scaphoid bone.5,6
Bilateral scaphoid fractures are extremely rare and account for less than 1% of all scaphoid fractures.7 Very few of these cases have been published in the literature, and those that have been published have talked about the fractures being secondary to chronic stress fractures and as being treated with internal fixation (regardless of whether the fractures were nondisplaced or if the ligaments were intact).6-9
Our patient was placed in bilateral fiberglass short-arm thumb spica casts. We tried conservative treatment measures first because she had help with her activities of daily living (ADLs). At a follow-up visit 2 weeks later, we switched the casts to long-arm thumb spica casts because of the patient’s ability to pronate and supinate her wrists in the short-arm versions. After one month of wearing the long-arm casts, we placed her back in bilateral short-arm casts for 2 weeks. Eight weeks after the fall, we removed the short-arm casts for reevaluation.
We obtained x-rays to assess for any new changes to the wrist and specifically the scaphoid bones. The x-rays showed almost completely healed scaphoid bones with good alignment, but the patient still had 5/10 pain in the left wrist and 8/10 pain in the right wrist with movement. We placed her in adjustable thermoformable polymer braces, which were removed when she bathed.
Due to the uniqueness of her injuries, our patient had weekly visits with her primary care provider (PCP) for the first 2 months of treatment, followed by bimonthly visits for the remainder. At 10 weeks after the fall, her pain with movement was almost gone and she began physical therapy. She also began removing the braces during sedentary activity in order to practice range-of-motion exercises to prevent excessive stiffness in her wrists. Our patient regained full strength and range of motion one month later.
One other published case report describes the successful union of bilateral scaphoid fractures using bilateral long-arm casts followed by short-arm casts.7 Similar to our patient’s case, full union of the scaphoid bones was achieved within 12 weeks.7 Together, these cases suggest that conservative treatment methods are a viable alternative to surgery.
TAKEAWAY
For patients presenting with wrist pain after trauma to the wrists, assess anatomical snuffbox tenderness and obtain x-rays. Do not be falsely reassured by negative x-rays in the presence of a positive physical exam, however, as scaphoid fractures are often hidden on x-rays. If tenderness at the anatomical snuffbox is present and doesn’t subside within a few days, apply a short-arm thumb splint and obtain subsequent imaging.
If bilateral, nondisplaced, stable scaphoid fractures are diagnosed, conservative treatment with long-arm and short-arm casts is a viable alternative to surgery. This treatment decision should be made on an individual basis, however, as it requires the patient to have frequent PCP visits, assistance with ADLs, and complete adherence to the treatment plan.
THE CASE
A 12-year-old girl presented to my office (JH) with bilateral wrist pain. She had fallen on both wrists palmar-flexed and then, while trying to get up, landed on both wrists dorsi-flexed. The patient did not hear any “pops,” but felt immediate pain when her wrists hyperextended. Hand, wrist, and forearm x-rays were negative bilaterally for fractures. She was placed in bilateral thumb spica splints.
At follow-up one week later, the patient reported 6/10 pain in her left wrist and 7/10 pain in her right wrist. The pain increased to 10/10 bilaterally with movement and was not relieved by icing or nonsteroidal anti-inflammatory drugs. On physical exam, there was bilateral swelling of the wrists without ecchymosis or erythema. The patient had limited passive and active range of motion, especially during wrist extension. She also had tenderness to palpation over the anatomical snuff box, extending proximally to the distal radius bilaterally. She had no tenderness over the ulna or metacarpals, no loss of sensation in any area nerves, and she was neurovascularly intact bilaterally.
Based on the mechanism of injury, undetected fracture or full thickness ligament tear were both possible. Because of this, and because magnetic resonance imaging (MRI) entails no radiation exposure, MRI was chosen for additional imaging of both wrists.
THE DIAGNOSIS
The MRI revealed bilateral, nondisplaced, extra-articular fractures extending through the scaphoid waist, with surrounding bone marrow edema. In the right wrist, the patient also had a low-grade partial tear of the membranous portion of the scapholunate interosseous ligament (SLIL) at the scaphoid attachment (FIGURE 1). In the left wrist, she also had a low-grade sprain of the SLIL without tear (FIGURE 2).
DISCUSSION
Carpal fractures account for 6% of all fractures.1 Scaphoid fractures are the most common carpal bone fracture among all age groups, but account for only 0.4% of all pediatric fractures.1-3 They’re commonly missed on x-rays because they are usually nondisplaced and hidden by other structures superimposed on the image.1,2,4 Undetected, scaphoid fractures can cause prolonged interruption to the bone’s architecture, leading to avascular necrosis of the proximal portion of the scaphoid bone.5,6
Bilateral scaphoid fractures are extremely rare and account for less than 1% of all scaphoid fractures.7 Very few of these cases have been published in the literature, and those that have been published have talked about the fractures being secondary to chronic stress fractures and as being treated with internal fixation (regardless of whether the fractures were nondisplaced or if the ligaments were intact).6-9
Our patient was placed in bilateral fiberglass short-arm thumb spica casts. We tried conservative treatment measures first because she had help with her activities of daily living (ADLs). At a follow-up visit 2 weeks later, we switched the casts to long-arm thumb spica casts because of the patient’s ability to pronate and supinate her wrists in the short-arm versions. After one month of wearing the long-arm casts, we placed her back in bilateral short-arm casts for 2 weeks. Eight weeks after the fall, we removed the short-arm casts for reevaluation.
We obtained x-rays to assess for any new changes to the wrist and specifically the scaphoid bones. The x-rays showed almost completely healed scaphoid bones with good alignment, but the patient still had 5/10 pain in the left wrist and 8/10 pain in the right wrist with movement. We placed her in adjustable thermoformable polymer braces, which were removed when she bathed.
Due to the uniqueness of her injuries, our patient had weekly visits with her primary care provider (PCP) for the first 2 months of treatment, followed by bimonthly visits for the remainder. At 10 weeks after the fall, her pain with movement was almost gone and she began physical therapy. She also began removing the braces during sedentary activity in order to practice range-of-motion exercises to prevent excessive stiffness in her wrists. Our patient regained full strength and range of motion one month later.
One other published case report describes the successful union of bilateral scaphoid fractures using bilateral long-arm casts followed by short-arm casts.7 Similar to our patient’s case, full union of the scaphoid bones was achieved within 12 weeks.7 Together, these cases suggest that conservative treatment methods are a viable alternative to surgery.
TAKEAWAY
For patients presenting with wrist pain after trauma to the wrists, assess anatomical snuffbox tenderness and obtain x-rays. Do not be falsely reassured by negative x-rays in the presence of a positive physical exam, however, as scaphoid fractures are often hidden on x-rays. If tenderness at the anatomical snuffbox is present and doesn’t subside within a few days, apply a short-arm thumb splint and obtain subsequent imaging.
If bilateral, nondisplaced, stable scaphoid fractures are diagnosed, conservative treatment with long-arm and short-arm casts is a viable alternative to surgery. This treatment decision should be made on an individual basis, however, as it requires the patient to have frequent PCP visits, assistance with ADLs, and complete adherence to the treatment plan.
1. Pillai A, Jain M. Management of clinical fractures of the scaphoid: results of an audit and literature review. Eur J Emerg Med. 2005;12:47-51.
2. Evenski AJ, Adamczyk MJ, Steiner RP, et al. Clinically suspected scaphoid fractures in children. J Pediatr Orthop. 2009;29:352-355.
3. Wulff R, Schmidt T. Carpal fractures in children. J Pediatr Orthop. 1998;18:462-465.
4. Nellans KW, Chung KC. Pediatric hand fractures. Hand Clin. 2013;29:569-578.
5. Jernigan EW, Smetana BS, Patterson JM. Pediatric scaphoid proximal pole nonunion with avascular necrosis. J Hand Surgery. 2017;42:299.e1-299.e4.
6. Pidemunt G, Torres-Claramunt R, Ginés A, et al. Bilateral stress fracture of the carpal scaphoid: report in a child and review of the literature. Clin J Sport Med. 2012;22:511-513.
7. Saglam F, Gulabi D, Baysal Ö, et al. Chronic wrist pain in a goalkeeper; bilateral scaphoid stress fracture: a case report. Int J Surg Case Rep. 2015;7:20-22.
8. Muzaffar N, Wani I, Ehsan M, et al. Simultaneous bilateral scaphoid fractures in a soldier managed conservatively by scaphoid casts. Arch Clin Exp Surg. 2016;5:63-64.
9. Mohamed Haflah NH, Mat Nor NF, Abdullah S, et al. Bilateral scaphoid stress fracture in a platform diver presenting with unilateral symptoms. Singapore Med J. 2014;55:e159-e161.
1. Pillai A, Jain M. Management of clinical fractures of the scaphoid: results of an audit and literature review. Eur J Emerg Med. 2005;12:47-51.
2. Evenski AJ, Adamczyk MJ, Steiner RP, et al. Clinically suspected scaphoid fractures in children. J Pediatr Orthop. 2009;29:352-355.
3. Wulff R, Schmidt T. Carpal fractures in children. J Pediatr Orthop. 1998;18:462-465.
4. Nellans KW, Chung KC. Pediatric hand fractures. Hand Clin. 2013;29:569-578.
5. Jernigan EW, Smetana BS, Patterson JM. Pediatric scaphoid proximal pole nonunion with avascular necrosis. J Hand Surgery. 2017;42:299.e1-299.e4.
6. Pidemunt G, Torres-Claramunt R, Ginés A, et al. Bilateral stress fracture of the carpal scaphoid: report in a child and review of the literature. Clin J Sport Med. 2012;22:511-513.
7. Saglam F, Gulabi D, Baysal Ö, et al. Chronic wrist pain in a goalkeeper; bilateral scaphoid stress fracture: a case report. Int J Surg Case Rep. 2015;7:20-22.
8. Muzaffar N, Wani I, Ehsan M, et al. Simultaneous bilateral scaphoid fractures in a soldier managed conservatively by scaphoid casts. Arch Clin Exp Surg. 2016;5:63-64.
9. Mohamed Haflah NH, Mat Nor NF, Abdullah S, et al. Bilateral scaphoid stress fracture in a platform diver presenting with unilateral symptoms. Singapore Med J. 2014;55:e159-e161.
Concurrent ipilimumab and CMV colitis refractory to oral steroids
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
Recurrent head and neck cancer presenting as a large retroperitoneal mass
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
Massive liver metastasis from colon adenocarcinoma causing cardiac tamponade
Colorectal cancer is the third most commonly diagnosed cancer in the United States.1 About 5% of Americans will be diagnosed with colorectal cancer in their lifetime, of which 20% will present with distant metastasis.2 The most common sites of metastasis are regional lymph nodes, liver, lung and peritoneum, and patients may present with signs or symptoms related to disease burden at any of these organs.
Case presentation and summary
A 55-year-old man had presented to an outside hospital in August of 2014 with 6 months of hematochezia and a 40-lb weight loss. He was found to be severely anemic on admission (hemoglobin, 4.9 g/dL [normal, 13-17 g/dL], hematocrit, 16% [normal, 35%-45%]). A computed-tomography (CT) scan of the abdomen and pelvis with contrast revealed a mass of 6.9 x 4.7 x 6.3 cm in the rectosigmoid colon and a mass of 10.0 x 12.0 x 10.7 cm in the right hepatic lobe consistent with metastatic disease. The patient was taken to the operating room where the rectosigmoid mass was resected completely. The liver mass was deemed unresectable because of its large size, and surgically directed therapy could not be performed. Pathology was consistent with a T3N1 moderately differentiated adenocarcinoma 11 cm from the anal verge. Further molecular tumor studies revealed wild type KRAS and NRAS, as well as a BRAF mutation.
About 4 weeks after the surgery, the patient was seen at our institution for an initial consultation and was noted to have significant anasarca, including 4+ pitting lower extremity edema and scrotal edema. He complained of dyspnea on exertion, which he attributed to deconditioning. His resting heart rate was found to be 123 beats per minute (normal, 60-100 bpm). Jugular venous distention was present. The patient was sent for an urgent echocardiogram, which showed external compression of the right atrium and ventricle by his liver metastasis resulting in tamponade physiology without the presence of any pericardial effusion (Figure 1).
A CT of the abdomen and pelvis at that time showed that the liver mass had increased to 17.6 x 12.1 x 16.1 cm, exerting pressure on the heart and causing atelectasis of the right middle and lower lung lobes (Figure 2).
Treatment plan
The patient was evaluated by surgical oncology for resection, but his cardiovascular status placed him at high risk for perioperative complications, so such surgery was not pursued. Radioembolization was considered but not pursued because the process needed to evaluate, plan, and treat was not considered sufficiently timely. We consulted with our radiation oncology colleagues about external beam radiotherapy (EBRT) for rapid palliation. They evaluated the patient and recommended the EBRT, and the patient signed consent for treatment. We performed a CT-based simulation and generated an external beam, linear-accelerator–based treatment plan. The plan consisted of three 15-megavoltage photon fields delivering 3,000 cGy in 10 fractions to the whole liver, with appropriate multileaf collimation blocking to minimize dose to adjacent heart, right lung, and bilateral kidneys (Figure 3).
Before initiation of the EBRT, the patient received systemic chemotherapy with a dose-adjusted FOLFOX regimen (5-FU bolus 200 mg/m2, leucovorin 200 mg/m2, oxaliplatin 85 mg/m2, with infusional 5-FU 2,400 mg/m2 over 46 hours). After completing 1 dose of modified FOLFOX, he completed 10 fractions of whole liver radiotherapy with the aforementioned plan. He tolerated the initial treatment well and his subjective symptoms improved. The patient then proceeded to further systemic therapy. After recent data demonstrated improved median progression-free survival and response rates with FOLFOXIRI plus bevacizumab (infusional 5-FU 3200 mg/m2, leucovorin 200 mg/m2, irinotecan 165 mg/m2, and oxaliplatin 85 mg/m2, bevacizumab 5 mg/kg) versus FOLFIRI plus bevacizumab,3 we decided to modify his systemic therapy to FOLFOXIRI with bevacizumab to induce a better response.
Treatment response
After 2 doses of chemotherapy and completion of radiotherapy, the edema and shortness of breath improved. A follow-up echocardiogram performed a month after completion of EBRT, 1 dose of FOLFOX, and 1 dose of FOLFOXIRI showed resolution of the cardiac compression (Figure 4).
A CT scan of the abdomen and pelvis obtained after 3 cycles of FOLFOXIRI showed marked decrease in the size of the right lobe hepatic mass from 17.6 x 12.1 cm to 12.0 x 8.0 cm. Given the survival benefit of VEGF inhibition in colon cancer, bevacizumab (5 mg/kg) was added to the FOLFOXIRI regimen with cycle 4. Unfortunately, after the 5th cycle, a CT scan of the abdomen showed an increase in size of the hepatic lesions. At this time, FOLFOXIRI and bevacizumab were stopped, and given the tumor’s KRAS/NRAS wild type status, systemic therapy was changed to panitumumab (6 mg/kg). The patient initially tolerated treatment well, but after 9 cycles, the total bilirubin started to increase. CT abdomen at this point was consistent with progression of disease. The patient was not eligible for a clinical trial targeting BRAF mutation given the elevated bilirubin. Regorafanib (80 mg daily for 3 weeks on and 1 week off) was started. After the first cycle, the total bilirubin increased further and the regorafanib was dose reduced to 40 mg daily. Unfortunately, a repeat CT scan of the abdomen demonstrated progression of disease, and given that he developed a progressive transaminitis and hyperbilirubinemia, hospice care was recommended. The patient died shortly thereafter, about 15 months after his initial diagnosis.
Discussion
Massive liver metastasis in the setting of disseminated cancer is not an uncommon manifestation of advanced cancer that can have life-threatening consequences. In te present case, a bulky liver metastasis caused extrinsic compression of the right atrium, resulting in obvious clinical and echocardiogram-proven cardiac tamponade physiology. To our knowledge, this is the first reported case of the treatment of a bulky hepatic metastasis causing cardiac tamponade. In this patient’s case, both radiotherapy and chemotherapy were given safely in rapid sequence resulting in quick resolution of the patient’s symptoms and echocardiogram findings. The presence of a BRAF mutation conferred a poor prognosis and poor response to systemic chemotherapy. Nevertheless, the patient showed good response to a FOLFOXIRI regimen, chosen in this emergent situation given its significantly higher response rates compared with the standard FOLFIRI regimen, which was tolerated well with minimal adverse effects.
Findings from randomized controlled trials examining the role of palliative radiotherapy for metastatic liver disease have suggested that dose escalation above 30 Gy to the whole liver may lead to unacceptably high rates of radiation-induced liver disease, which typically leads to mortality.4-8 Two prospective trials comparing twice daily with daily fractionation have shown no benefit to hyperfractionation, with possibly increased rates of acute toxicity in the setting of hepatocellular carcinoma.9,10 There is emerging evidence that partial liver irradiation, in the appropriate setting in the form of boost after whole-liver RT or stereotactic body radiotherapy, may allow for further dose escalation while avoiding clinical hepatitis.11 Although there is no clear consensus about optimal RT dose and fractionation, the aforementioned studies show that dose and fractionation schemes ranging between 21 Gy and 30 Gy in 1.5 Gy to 3 Gy daily fractions likely provide the best therapeutic ratio for whole-liver irradiation.
In conclusion, this case demonstrates the resolution of cardiac tamponade from a massive liver colorectal metastasis after chemoradiation and illustrates the potential utility of adding radiotherapy to chemotherapy in an urgent scenario where the former might not typically be considered.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. Published 2015. Accessed October 10, 2017.
2. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
3. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618.
4. Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27(1):117-123.
5. Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA. 1975;232(6):625-628.
6. Sherman DM, Weichselbaum R, Order SE, Cloud L, Trey C, Piro AJ. Palliation of hepatic metastasis. Cancer. 1978;41(5):2013-2017.
7. Prasad B, Lee MS, Hendrickson FR. Irradiation of hepatic metastases. Int J Radiat Oncol Biol Phys. 1977;2:129-132.
8. Borgelt BB, Gelber R, Brady LW, Griffin T, Hendrickson FR. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7(5):587-591.
9. Raju PI, Maruyama Y, DeSimone P, MacDonald J. Treatment of liver metastases with a combination of chemotherapy and hyperfractionated external radiation therapy. Am J Clin Oncol. 1987;10(1):41-43.
10. Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989;17(6):1223-1229.
11. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996;14(3):722-728.
Colorectal cancer is the third most commonly diagnosed cancer in the United States.1 About 5% of Americans will be diagnosed with colorectal cancer in their lifetime, of which 20% will present with distant metastasis.2 The most common sites of metastasis are regional lymph nodes, liver, lung and peritoneum, and patients may present with signs or symptoms related to disease burden at any of these organs.
Case presentation and summary
A 55-year-old man had presented to an outside hospital in August of 2014 with 6 months of hematochezia and a 40-lb weight loss. He was found to be severely anemic on admission (hemoglobin, 4.9 g/dL [normal, 13-17 g/dL], hematocrit, 16% [normal, 35%-45%]). A computed-tomography (CT) scan of the abdomen and pelvis with contrast revealed a mass of 6.9 x 4.7 x 6.3 cm in the rectosigmoid colon and a mass of 10.0 x 12.0 x 10.7 cm in the right hepatic lobe consistent with metastatic disease. The patient was taken to the operating room where the rectosigmoid mass was resected completely. The liver mass was deemed unresectable because of its large size, and surgically directed therapy could not be performed. Pathology was consistent with a T3N1 moderately differentiated adenocarcinoma 11 cm from the anal verge. Further molecular tumor studies revealed wild type KRAS and NRAS, as well as a BRAF mutation.
About 4 weeks after the surgery, the patient was seen at our institution for an initial consultation and was noted to have significant anasarca, including 4+ pitting lower extremity edema and scrotal edema. He complained of dyspnea on exertion, which he attributed to deconditioning. His resting heart rate was found to be 123 beats per minute (normal, 60-100 bpm). Jugular venous distention was present. The patient was sent for an urgent echocardiogram, which showed external compression of the right atrium and ventricle by his liver metastasis resulting in tamponade physiology without the presence of any pericardial effusion (Figure 1).
A CT of the abdomen and pelvis at that time showed that the liver mass had increased to 17.6 x 12.1 x 16.1 cm, exerting pressure on the heart and causing atelectasis of the right middle and lower lung lobes (Figure 2).
Treatment plan
The patient was evaluated by surgical oncology for resection, but his cardiovascular status placed him at high risk for perioperative complications, so such surgery was not pursued. Radioembolization was considered but not pursued because the process needed to evaluate, plan, and treat was not considered sufficiently timely. We consulted with our radiation oncology colleagues about external beam radiotherapy (EBRT) for rapid palliation. They evaluated the patient and recommended the EBRT, and the patient signed consent for treatment. We performed a CT-based simulation and generated an external beam, linear-accelerator–based treatment plan. The plan consisted of three 15-megavoltage photon fields delivering 3,000 cGy in 10 fractions to the whole liver, with appropriate multileaf collimation blocking to minimize dose to adjacent heart, right lung, and bilateral kidneys (Figure 3).
Before initiation of the EBRT, the patient received systemic chemotherapy with a dose-adjusted FOLFOX regimen (5-FU bolus 200 mg/m2, leucovorin 200 mg/m2, oxaliplatin 85 mg/m2, with infusional 5-FU 2,400 mg/m2 over 46 hours). After completing 1 dose of modified FOLFOX, he completed 10 fractions of whole liver radiotherapy with the aforementioned plan. He tolerated the initial treatment well and his subjective symptoms improved. The patient then proceeded to further systemic therapy. After recent data demonstrated improved median progression-free survival and response rates with FOLFOXIRI plus bevacizumab (infusional 5-FU 3200 mg/m2, leucovorin 200 mg/m2, irinotecan 165 mg/m2, and oxaliplatin 85 mg/m2, bevacizumab 5 mg/kg) versus FOLFIRI plus bevacizumab,3 we decided to modify his systemic therapy to FOLFOXIRI with bevacizumab to induce a better response.
Treatment response
After 2 doses of chemotherapy and completion of radiotherapy, the edema and shortness of breath improved. A follow-up echocardiogram performed a month after completion of EBRT, 1 dose of FOLFOX, and 1 dose of FOLFOXIRI showed resolution of the cardiac compression (Figure 4).
A CT scan of the abdomen and pelvis obtained after 3 cycles of FOLFOXIRI showed marked decrease in the size of the right lobe hepatic mass from 17.6 x 12.1 cm to 12.0 x 8.0 cm. Given the survival benefit of VEGF inhibition in colon cancer, bevacizumab (5 mg/kg) was added to the FOLFOXIRI regimen with cycle 4. Unfortunately, after the 5th cycle, a CT scan of the abdomen showed an increase in size of the hepatic lesions. At this time, FOLFOXIRI and bevacizumab were stopped, and given the tumor’s KRAS/NRAS wild type status, systemic therapy was changed to panitumumab (6 mg/kg). The patient initially tolerated treatment well, but after 9 cycles, the total bilirubin started to increase. CT abdomen at this point was consistent with progression of disease. The patient was not eligible for a clinical trial targeting BRAF mutation given the elevated bilirubin. Regorafanib (80 mg daily for 3 weeks on and 1 week off) was started. After the first cycle, the total bilirubin increased further and the regorafanib was dose reduced to 40 mg daily. Unfortunately, a repeat CT scan of the abdomen demonstrated progression of disease, and given that he developed a progressive transaminitis and hyperbilirubinemia, hospice care was recommended. The patient died shortly thereafter, about 15 months after his initial diagnosis.
Discussion
Massive liver metastasis in the setting of disseminated cancer is not an uncommon manifestation of advanced cancer that can have life-threatening consequences. In te present case, a bulky liver metastasis caused extrinsic compression of the right atrium, resulting in obvious clinical and echocardiogram-proven cardiac tamponade physiology. To our knowledge, this is the first reported case of the treatment of a bulky hepatic metastasis causing cardiac tamponade. In this patient’s case, both radiotherapy and chemotherapy were given safely in rapid sequence resulting in quick resolution of the patient’s symptoms and echocardiogram findings. The presence of a BRAF mutation conferred a poor prognosis and poor response to systemic chemotherapy. Nevertheless, the patient showed good response to a FOLFOXIRI regimen, chosen in this emergent situation given its significantly higher response rates compared with the standard FOLFIRI regimen, which was tolerated well with minimal adverse effects.
Findings from randomized controlled trials examining the role of palliative radiotherapy for metastatic liver disease have suggested that dose escalation above 30 Gy to the whole liver may lead to unacceptably high rates of radiation-induced liver disease, which typically leads to mortality.4-8 Two prospective trials comparing twice daily with daily fractionation have shown no benefit to hyperfractionation, with possibly increased rates of acute toxicity in the setting of hepatocellular carcinoma.9,10 There is emerging evidence that partial liver irradiation, in the appropriate setting in the form of boost after whole-liver RT or stereotactic body radiotherapy, may allow for further dose escalation while avoiding clinical hepatitis.11 Although there is no clear consensus about optimal RT dose and fractionation, the aforementioned studies show that dose and fractionation schemes ranging between 21 Gy and 30 Gy in 1.5 Gy to 3 Gy daily fractions likely provide the best therapeutic ratio for whole-liver irradiation.
In conclusion, this case demonstrates the resolution of cardiac tamponade from a massive liver colorectal metastasis after chemoradiation and illustrates the potential utility of adding radiotherapy to chemotherapy in an urgent scenario where the former might not typically be considered.
Colorectal cancer is the third most commonly diagnosed cancer in the United States.1 About 5% of Americans will be diagnosed with colorectal cancer in their lifetime, of which 20% will present with distant metastasis.2 The most common sites of metastasis are regional lymph nodes, liver, lung and peritoneum, and patients may present with signs or symptoms related to disease burden at any of these organs.
Case presentation and summary
A 55-year-old man had presented to an outside hospital in August of 2014 with 6 months of hematochezia and a 40-lb weight loss. He was found to be severely anemic on admission (hemoglobin, 4.9 g/dL [normal, 13-17 g/dL], hematocrit, 16% [normal, 35%-45%]). A computed-tomography (CT) scan of the abdomen and pelvis with contrast revealed a mass of 6.9 x 4.7 x 6.3 cm in the rectosigmoid colon and a mass of 10.0 x 12.0 x 10.7 cm in the right hepatic lobe consistent with metastatic disease. The patient was taken to the operating room where the rectosigmoid mass was resected completely. The liver mass was deemed unresectable because of its large size, and surgically directed therapy could not be performed. Pathology was consistent with a T3N1 moderately differentiated adenocarcinoma 11 cm from the anal verge. Further molecular tumor studies revealed wild type KRAS and NRAS, as well as a BRAF mutation.
About 4 weeks after the surgery, the patient was seen at our institution for an initial consultation and was noted to have significant anasarca, including 4+ pitting lower extremity edema and scrotal edema. He complained of dyspnea on exertion, which he attributed to deconditioning. His resting heart rate was found to be 123 beats per minute (normal, 60-100 bpm). Jugular venous distention was present. The patient was sent for an urgent echocardiogram, which showed external compression of the right atrium and ventricle by his liver metastasis resulting in tamponade physiology without the presence of any pericardial effusion (Figure 1).
A CT of the abdomen and pelvis at that time showed that the liver mass had increased to 17.6 x 12.1 x 16.1 cm, exerting pressure on the heart and causing atelectasis of the right middle and lower lung lobes (Figure 2).
Treatment plan
The patient was evaluated by surgical oncology for resection, but his cardiovascular status placed him at high risk for perioperative complications, so such surgery was not pursued. Radioembolization was considered but not pursued because the process needed to evaluate, plan, and treat was not considered sufficiently timely. We consulted with our radiation oncology colleagues about external beam radiotherapy (EBRT) for rapid palliation. They evaluated the patient and recommended the EBRT, and the patient signed consent for treatment. We performed a CT-based simulation and generated an external beam, linear-accelerator–based treatment plan. The plan consisted of three 15-megavoltage photon fields delivering 3,000 cGy in 10 fractions to the whole liver, with appropriate multileaf collimation blocking to minimize dose to adjacent heart, right lung, and bilateral kidneys (Figure 3).
Before initiation of the EBRT, the patient received systemic chemotherapy with a dose-adjusted FOLFOX regimen (5-FU bolus 200 mg/m2, leucovorin 200 mg/m2, oxaliplatin 85 mg/m2, with infusional 5-FU 2,400 mg/m2 over 46 hours). After completing 1 dose of modified FOLFOX, he completed 10 fractions of whole liver radiotherapy with the aforementioned plan. He tolerated the initial treatment well and his subjective symptoms improved. The patient then proceeded to further systemic therapy. After recent data demonstrated improved median progression-free survival and response rates with FOLFOXIRI plus bevacizumab (infusional 5-FU 3200 mg/m2, leucovorin 200 mg/m2, irinotecan 165 mg/m2, and oxaliplatin 85 mg/m2, bevacizumab 5 mg/kg) versus FOLFIRI plus bevacizumab,3 we decided to modify his systemic therapy to FOLFOXIRI with bevacizumab to induce a better response.
Treatment response
After 2 doses of chemotherapy and completion of radiotherapy, the edema and shortness of breath improved. A follow-up echocardiogram performed a month after completion of EBRT, 1 dose of FOLFOX, and 1 dose of FOLFOXIRI showed resolution of the cardiac compression (Figure 4).
A CT scan of the abdomen and pelvis obtained after 3 cycles of FOLFOXIRI showed marked decrease in the size of the right lobe hepatic mass from 17.6 x 12.1 cm to 12.0 x 8.0 cm. Given the survival benefit of VEGF inhibition in colon cancer, bevacizumab (5 mg/kg) was added to the FOLFOXIRI regimen with cycle 4. Unfortunately, after the 5th cycle, a CT scan of the abdomen showed an increase in size of the hepatic lesions. At this time, FOLFOXIRI and bevacizumab were stopped, and given the tumor’s KRAS/NRAS wild type status, systemic therapy was changed to panitumumab (6 mg/kg). The patient initially tolerated treatment well, but after 9 cycles, the total bilirubin started to increase. CT abdomen at this point was consistent with progression of disease. The patient was not eligible for a clinical trial targeting BRAF mutation given the elevated bilirubin. Regorafanib (80 mg daily for 3 weeks on and 1 week off) was started. After the first cycle, the total bilirubin increased further and the regorafanib was dose reduced to 40 mg daily. Unfortunately, a repeat CT scan of the abdomen demonstrated progression of disease, and given that he developed a progressive transaminitis and hyperbilirubinemia, hospice care was recommended. The patient died shortly thereafter, about 15 months after his initial diagnosis.
Discussion
Massive liver metastasis in the setting of disseminated cancer is not an uncommon manifestation of advanced cancer that can have life-threatening consequences. In te present case, a bulky liver metastasis caused extrinsic compression of the right atrium, resulting in obvious clinical and echocardiogram-proven cardiac tamponade physiology. To our knowledge, this is the first reported case of the treatment of a bulky hepatic metastasis causing cardiac tamponade. In this patient’s case, both radiotherapy and chemotherapy were given safely in rapid sequence resulting in quick resolution of the patient’s symptoms and echocardiogram findings. The presence of a BRAF mutation conferred a poor prognosis and poor response to systemic chemotherapy. Nevertheless, the patient showed good response to a FOLFOXIRI regimen, chosen in this emergent situation given its significantly higher response rates compared with the standard FOLFIRI regimen, which was tolerated well with minimal adverse effects.
Findings from randomized controlled trials examining the role of palliative radiotherapy for metastatic liver disease have suggested that dose escalation above 30 Gy to the whole liver may lead to unacceptably high rates of radiation-induced liver disease, which typically leads to mortality.4-8 Two prospective trials comparing twice daily with daily fractionation have shown no benefit to hyperfractionation, with possibly increased rates of acute toxicity in the setting of hepatocellular carcinoma.9,10 There is emerging evidence that partial liver irradiation, in the appropriate setting in the form of boost after whole-liver RT or stereotactic body radiotherapy, may allow for further dose escalation while avoiding clinical hepatitis.11 Although there is no clear consensus about optimal RT dose and fractionation, the aforementioned studies show that dose and fractionation schemes ranging between 21 Gy and 30 Gy in 1.5 Gy to 3 Gy daily fractions likely provide the best therapeutic ratio for whole-liver irradiation.
In conclusion, this case demonstrates the resolution of cardiac tamponade from a massive liver colorectal metastasis after chemoradiation and illustrates the potential utility of adding radiotherapy to chemotherapy in an urgent scenario where the former might not typically be considered.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. Published 2015. Accessed October 10, 2017.
2. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
3. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618.
4. Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27(1):117-123.
5. Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA. 1975;232(6):625-628.
6. Sherman DM, Weichselbaum R, Order SE, Cloud L, Trey C, Piro AJ. Palliation of hepatic metastasis. Cancer. 1978;41(5):2013-2017.
7. Prasad B, Lee MS, Hendrickson FR. Irradiation of hepatic metastases. Int J Radiat Oncol Biol Phys. 1977;2:129-132.
8. Borgelt BB, Gelber R, Brady LW, Griffin T, Hendrickson FR. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7(5):587-591.
9. Raju PI, Maruyama Y, DeSimone P, MacDonald J. Treatment of liver metastases with a combination of chemotherapy and hyperfractionated external radiation therapy. Am J Clin Oncol. 1987;10(1):41-43.
10. Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989;17(6):1223-1229.
11. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996;14(3):722-728.
1. American Cancer Society. Cancer Facts & Figures 2015. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. Published 2015. Accessed October 10, 2017.
2. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104-117.
3. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618.
4. Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27(1):117-123.
5. Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA. 1975;232(6):625-628.
6. Sherman DM, Weichselbaum R, Order SE, Cloud L, Trey C, Piro AJ. Palliation of hepatic metastasis. Cancer. 1978;41(5):2013-2017.
7. Prasad B, Lee MS, Hendrickson FR. Irradiation of hepatic metastases. Int J Radiat Oncol Biol Phys. 1977;2:129-132.
8. Borgelt BB, Gelber R, Brady LW, Griffin T, Hendrickson FR. The palliation of hepatic metastases: results of the Radiation Therapy Oncology Group pilot study. Int J Radiat Oncol Biol Phys. 1981;7(5):587-591.
9. Raju PI, Maruyama Y, DeSimone P, MacDonald J. Treatment of liver metastases with a combination of chemotherapy and hyperfractionated external radiation therapy. Am J Clin Oncol. 1987;10(1):41-43.
10. Stillwagon GB, Order SE, Guse C, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989;17(6):1223-1229.
11. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996;14(3):722-728.