User login
Bilateral thigh and knee pain • leg weakness • no history of trauma • Dx?
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
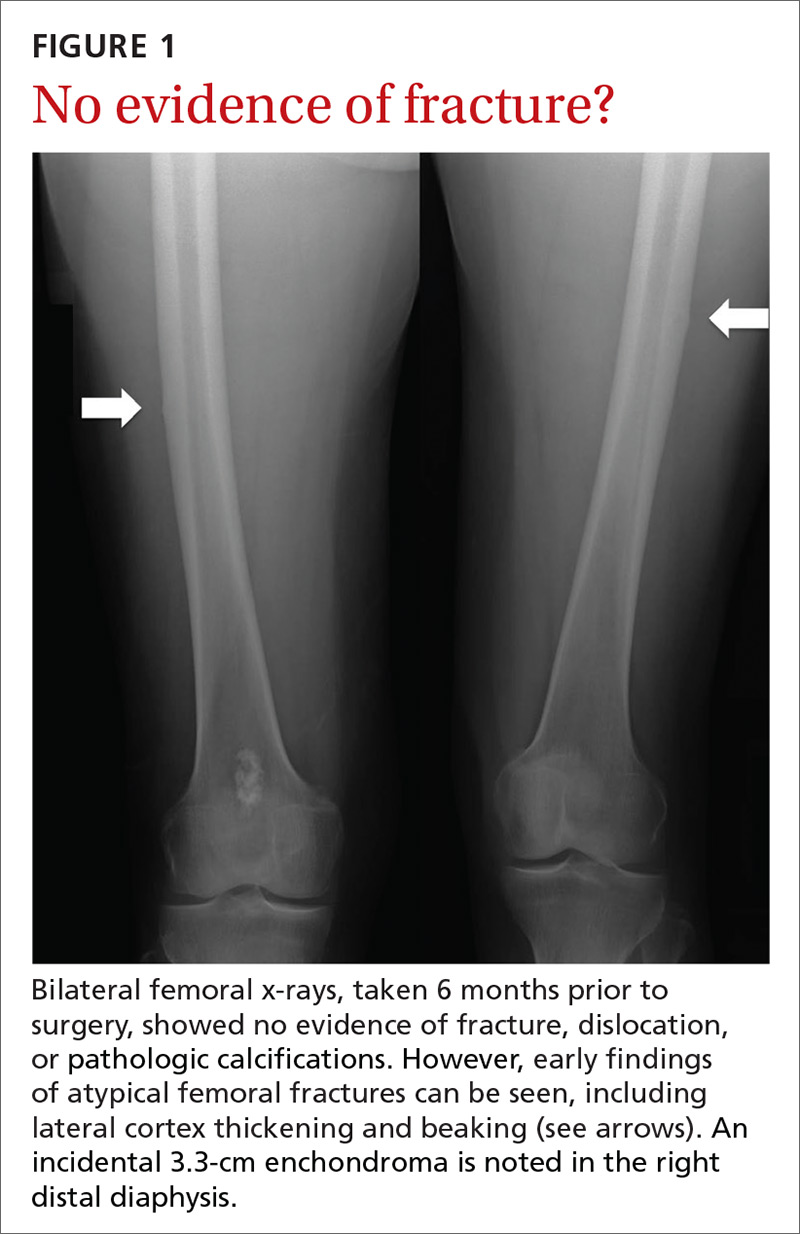
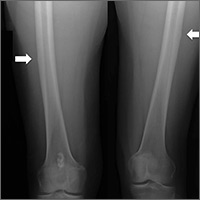
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.
Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
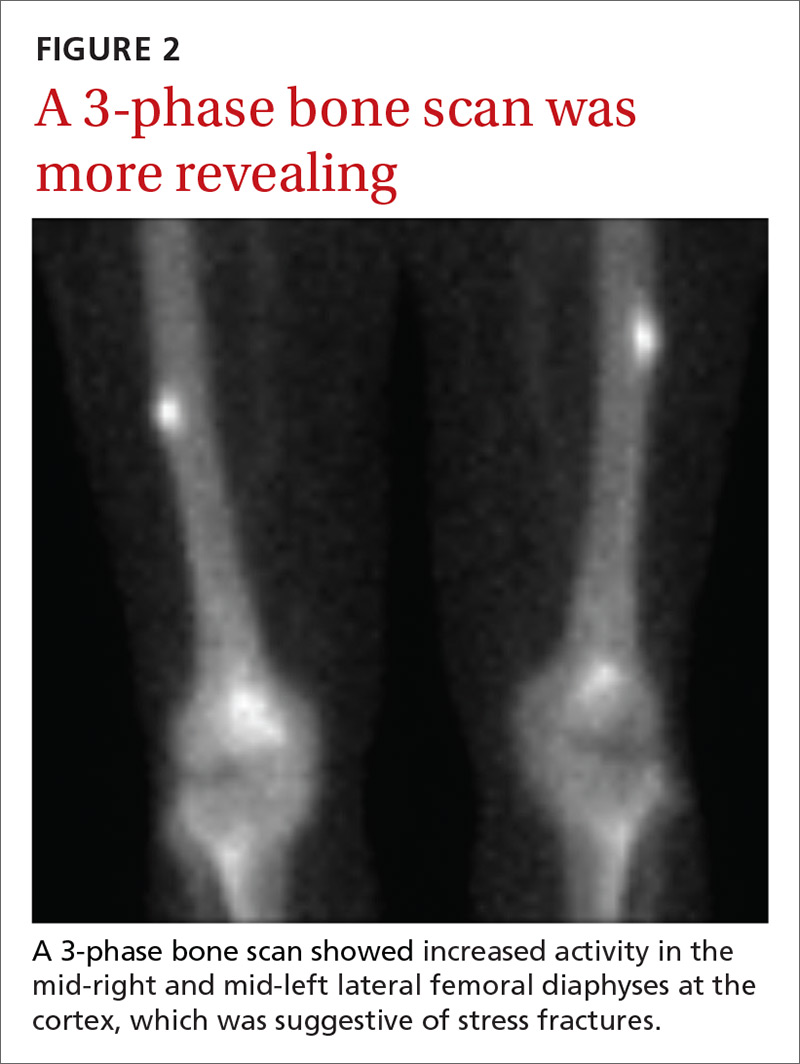
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).
DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.
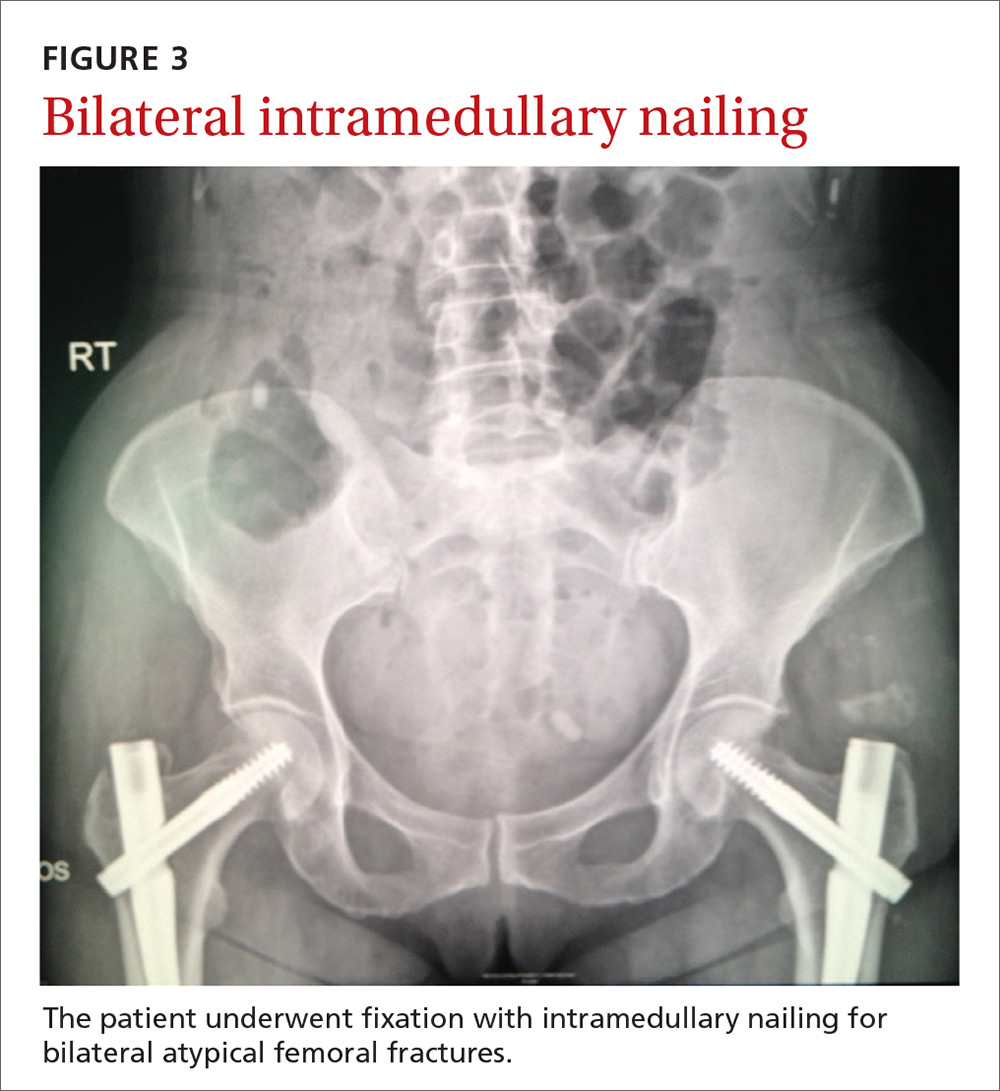
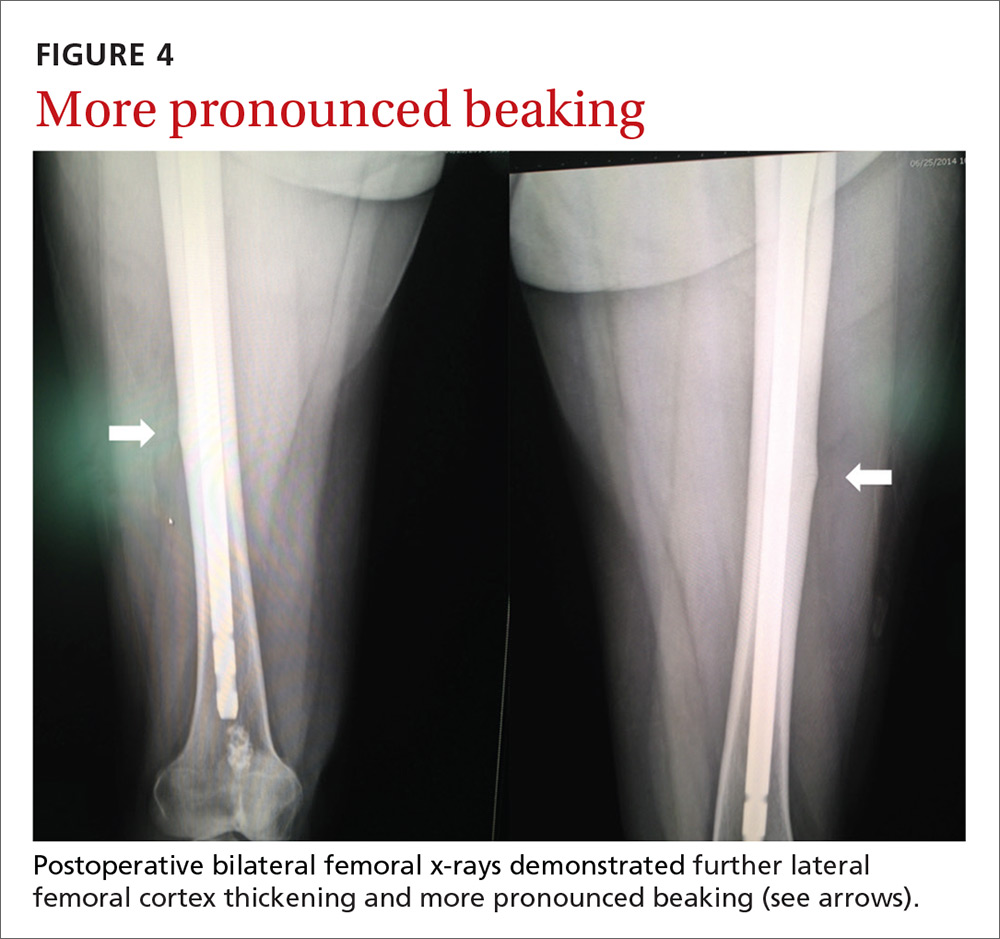
Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.
THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.
Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).
DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.
Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.
THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
THE CASE
A 67-year-old woman presented to our orthopaedic clinic with a 2-year history of bilateral thigh and knee pain and weakness of her legs. She had no history of trauma, and the pain, which was localized to the distal anterior thighs and patellofemoral area, was 7/10 at rest and worse with standing and walking.
Her medical history was significant for osteoporosis (diagnosed in 2004), hypertension, hypothyroidism, gastroesophageal reflux disease, and menopause (age 54). Her original dual-energy x-ray absorptiometry (DEXA) scan did not reveal the presence of any previous fractures. She was started on calcium and vitamin D supplementation and oral alendronate (70 mg once a week). She took alendronate for 4 years until 2008, when it was stopped due to nausea. She was then started on zoledronic acid (5 mg IV annually). She received 5 infusions of zoledronic acid between 2008 and 2013; she did not have an infusion in 2012. Her medication list also included lisinopril, omeprazole, naproxen, cyclobenzaprine, and a multivitamin. She had normal renal function (estimated glomerular filtration rate >60 mL/min/1.73 m2) and she did not drink alcohol or use tobacco.
In the 2 years prior to her visit to our clinic, she had been evaluated by her primary care provider, an orthopedic sports medicine specialist, 2 spinal surgeons, and a physiatrist. She had also undergone 30 physical therapy sessions. Bilateral femur radiographs (FIGURE 1) ordered by her orthopedist 6 months earlier demonstrated no evidence of fracture, but did show an incidental enchondroma in the right distal diaphysis and bilateral thickening of the lateral femoral cortices.
Finally, with no relief in sight, her obstetrician suggested that she might be experiencing myalgias attributable to her zoledronic acid infusions. She was subsequently referred to us.
The physical exam revealed a thin female with a body mass index of 21. She had mild tenderness on palpation of the bilateral anterior thighs and knees. There was no pain with hip or knee range of motion and minimal pain in the bilateral lower extremities with axial loading. The patient had normal sensation, did not have an antalgic gait, and exhibited 5/5 strength bilaterally in all distributions of the lower extremities.
THE DIAGNOSIS
Due to continued pain despite negative x-rays, we obtained a 3-phase bone scan of the pelvis and bilateral femurs. Delayed images showed moderately increased activity in the mid-right and mid-left lateral femoral diaphyses at the cortex and confirmed stress fractures (FIGURE 2).
DISCUSSION
Bisphosphonates are considered first-line therapy for osteoporosis, according to current evidence-based guidelines.1 These medications inhibit osteoclast activity and can bind to the bone for more than 10 years.2,3 (In women with bone mineral density scores ≤ –2.5, the number needed to treat is 21.1,4)
Patients taking bisphosphonates, however, are susceptible to atypical femoral fractures (AFFs), which are stress or insufficiency fractures associated with minimal or no trauma.5 The pathophysiology remains unknown at this time, but AFFs may result from changes in bone remodeling that occur when a bone experiences repetitive microtrauma, leading to lateral cortical thickening of the femur.6,7 Incidence of AFFs in patients taking bisphosphonates is estimated to be between 3.2 and 50 cases per 100,000 person-years; however, this risk increases to approximately 100 per 100,000 person-years with long-term use.5 Other risk factors include low body weight, advancing age, rheumatoid arthritis, long-term glucocorticoid therapy, and excessive alcohol and cigarette use.8
What you’ll see
Symptoms typically include unilateral or bilateral prodromal pain with a sharp or achy character that is localized to the mid-thigh, upper thigh, or groin.9 If an AFF is suspected, we recommend performing a bilateral exam and obtaining radiographs.
If characteristic features are found (eg, signs of focal cortical thickening or beaking) and pain arises in the opposite limb, obtain a radiograph of the contralateral femur. If radiographs are negative but suspicion remains, order magnetic resonance imaging or a bone scan, to identify a cortical fracture line, bone and marrow edema, or hyperemia.5
Begin treatment by discontinuing bisphosphonates
Upon identification of an AFF, discontinue bisphosphonates and initiate calcium and vitamin D supplementation.5 Prophylactic surgical fixation may also be necessary to accelerate healing and prevent fracture propagation and further pain.
Our patient. Due to the longevity of the symptoms and the bilateral stress fractures noted on the bone scan, our patient chose to proceed with intramedullary nailing of the bilateral femurs (FIGURES 3 and 4). On postop Day 1, she was able to ambulate using a walker and to participate in bilateral weight-bearing (as tolerated). She was discharged to a skilled nursing facility, where she progressed to full weight-bearing without aid. On follow-up (one year postop), the patient reported no residual leg pain and was able to work out 5 days per week. Radiographs of her femurs demonstrated healed fractures and stable position of the intramedullary nails.
THE TAKEAWAY
An increased suspicion for AFFs due to bisphosphonate use can lead to earlier diagnosis and decreased morbidity for patients. Use of femoral imaging can promote detection and reduce financial burden.
To help prevent AFFs from occurring, we recommend reevaluating the need for continued bisphosphonate therapy after 2 to 5 years of treatment. Continued surveillance is also advisable throughout the duration of their use.
ACKNOWLEDGMENT
The authors wish to acknowledge Dr. Maurice Manring for his help in preparing this manuscript.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
1. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16 Suppl 3:1-37.
2. Cakmak S, Mahiroğullari M, Keklikci K, et al. Bilateral low-energy sequential femoral shaft fractures in patients on long-term bisphosphonate therapy. Acta Orthop Traumatol Turc. 2013;47:162-172.
3. Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032-1045.
4. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366:2051-2053.
5. Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1-23.
6. Allen MR. Recent advances in understanding bisphosphonate effects on bone mechanical properties. Curr Osteoporos Rep. 2018 Mar 1. [Epub ahead of print]
7. Hagino H, Endo N, Yamamoto T, et al. Treatment status and radiographic features of patients with atypical femoral fractures. J Orthop Sci. 2018;23:316-320.
8. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581-589.
9. Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone. 2010;47:169-180.
Severe right upper chest pain • tender right sternoclavicular joint • Dx?
THE CASE
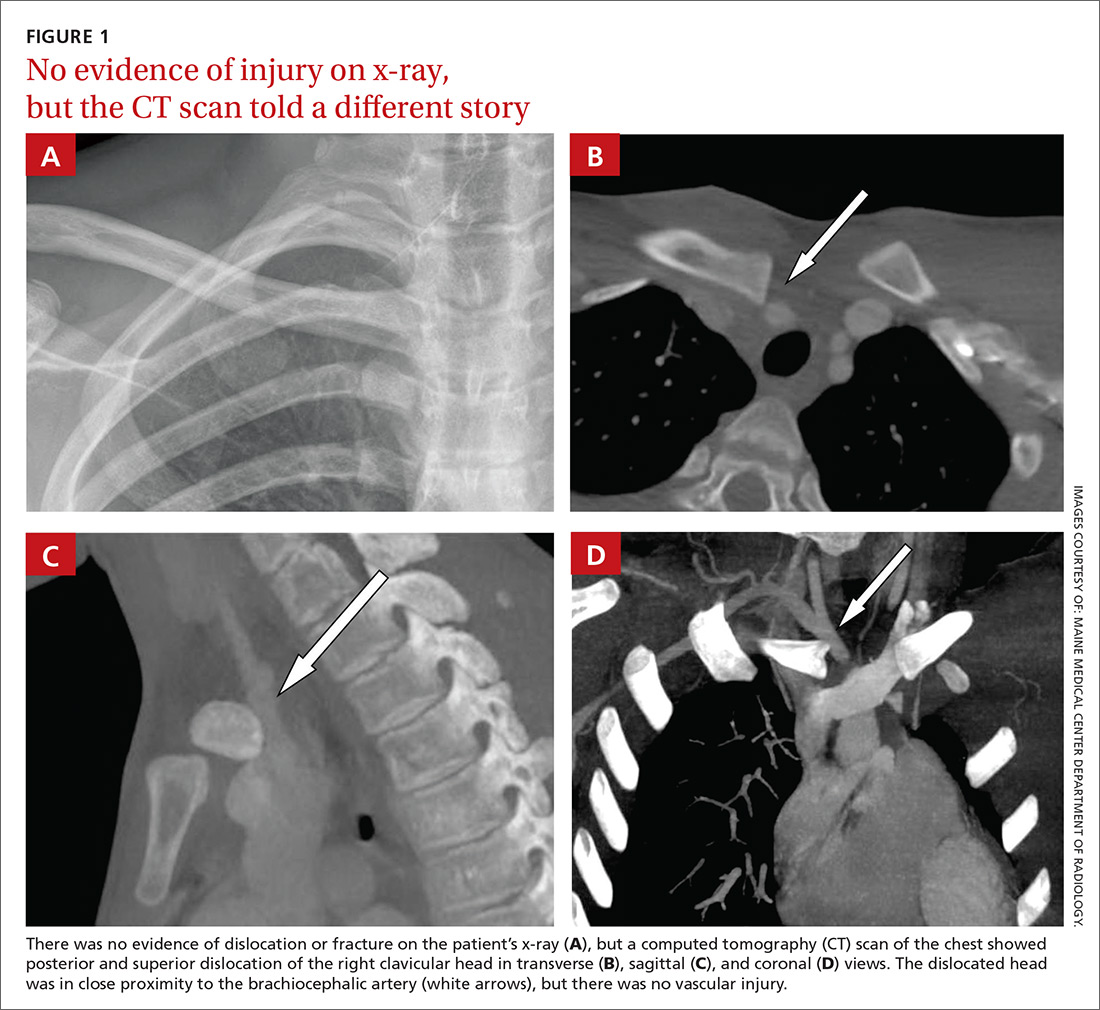
A 16-year-old hockey player presented to our emergency department with sharp pain in his right upper chest after “checking” another player during a game. The pain did not resolve with rest and was worse with movement and breathing. The patient did not have dysphagia, dyspnea, paresthesias, or hoarseness. The physical examination revealed tenderness over the right sternoclavicular joint (SCJ) without swelling or deformity. A distal neurovascular exam was intact, and a chest x-ray showed no evidence of dislocation or fracture (FIGURE 1A). The patient’s pain was refractory to multiple intravenous (IV) pain medications.
THE DIAGNOSIS
A computed tomography (CT) scan with IV contrast of the chest demonstrated posterior and superior dislocation of the right clavicular head. Despite the close proximity of the dislocated head to the brachiocephalic artery (FIGURE 1B-1D), there was no vascular injury.
DISCUSSION
Posterior sternoclavicular dislocations (PSCDs) can be difficult to diagnose. Edema can mask the characteristic skin depression that one would expect with a posterior dislocation.1 Chest radiographs are often normal (as was true in this case). Patients may present with an abnormal pulse, paresthesias, hoarseness, dysphagia, and/or dyspnea. However, for more than half of these patients, their only signs and symptoms will be pain, swelling, and limited range of motion.1 As a result, a PSCD may be misdiagnosed as a ligamentous or soft tissue injury.1
An uncommon injury that can result in serious complications
PSCDs represent 3% to 5% of all SCJ dislocations, which comprise <5% of all shoulder girdle injuries.1 Nevertheless, prompt and accurate diagnosis is critical, as these dislocations involve a high risk for injury to the posterior structures, particularly the brachiocephalic vein, right common carotid artery, and aortic arch.
One study found that nearly 75% of patients had a significant structure <1 cm posterior to the SCJ.2This proximity can result in neurovascular complications—some of which are devastating—in up to 30% of patients with PSCDs.3 A case report from 2011, for example, describes a 19-year-old man who had an undiagnosed PSCD that caused a pseudoaneurysm in his subclavian artery and a subsequent thrombotic cerebrovascular accident.4
Which injuries should raise your suspicions? Injuries in which lateral compression on the shoulder has caused it to roll forward and those in which a posteriorly directed force has been applied to the medial clavicle (as might occur in tackle sports or motor vehicle rollovers) should increase suspicion of a PSCD.1
Proper diagnosis requires CT angiography of the chest to assess the injury and evaluate the underlying structures. If CT is not available, additional chest film views, such as a serendipity view (anteroposterior view with 40° cephalic tilt) or Heinig view (oblique projection perpendicular to SCJ), may be obtained; an ultrasound is also an option.5
PSCD = surgical emergency
Following diagnosis, immediate orthopedic consultation is required. A PSCD is a surgical emergency. Reduction (open or closed) must be performed under general anesthesia with vascular and/or cardiothoracic surgery specialists available, as the reduction itself could injure one of the great vessels. Fortunately, most patients do quite well following surgery, with the majority achieving good-to-excellent results.6
Our patient was admitted to the hospital and underwent orthopedic surgery the following morning. Vascular and cardiothoracic surgeons were consulted and available in the event of a complication. A Salter-Harris type 2 fracture of the medial clavicle was identified intraoperatively, and an open reduction with internal fixation was performed. The patient had an uneventful recovery and resumed his usual activities, including playing hockey.
THE TAKEAWAY
PSCDs, although uncommon, can be life-threatening. Since the physical exam is unreliable and standard radiographs are often normal, accurate diagnosis relies largely on increased clinical suspicion. When there is a history of shoulder trauma, medial clavicle pain, and SCJ edema or tenderness, a PSCD should be suspected.7
Confirm the diagnosis with CT angiogram, and remember that a PSCD is a surgical emergency that requires coordination with orthopedic and vascular/cardiothoracic surgeons.
1. Chaudhry S. Pediatric posterior sternoclavicular joint injuries. J Am Acad Orthop Surg. 2015;23:468-475.
2. Ponce BA, Kundukulam JA, Pflugner R, et al. Sternoclavicular joint surgery: how far does danger lurk below? J Shoulder Elbow Surg. 2013;22:993-999.
3. Daya MR, Bengtzen RR. Shoulder. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:618-642.
4. Marcus MS, Tan V. Cerebrovascular accident in a 19-year-old patient: a case report of posterior sternoclavicular dislocation. J Shoulder Elbow Surg. 2011;20:e1-e4.
5. Morell DJ, Thyagarajan DS. Sternoclavicular joint dislocation and its management: a review of the literature. World J Orthop. 2016;7:244-250.
6. Boesmueller S, Wech M, Tiefenboeck TM, et al. Incidence, characteristics, and long-term follow-up of sternoclavicular injuries: an epidemiologic analysis of 92 cases. J Trauma Acute Care Surg. 2016;80:289-295.
7. Roepke C, Kleiner M, Jhun P, et al. Chest pain bounce-back: posterior sternoclavicular dislocation. Ann Emerg Med. 2015;66:559-561.
THE CASE
A 16-year-old hockey player presented to our emergency department with sharp pain in his right upper chest after “checking” another player during a game. The pain did not resolve with rest and was worse with movement and breathing. The patient did not have dysphagia, dyspnea, paresthesias, or hoarseness. The physical examination revealed tenderness over the right sternoclavicular joint (SCJ) without swelling or deformity. A distal neurovascular exam was intact, and a chest x-ray showed no evidence of dislocation or fracture (FIGURE 1A). The patient’s pain was refractory to multiple intravenous (IV) pain medications.
THE DIAGNOSIS
A computed tomography (CT) scan with IV contrast of the chest demonstrated posterior and superior dislocation of the right clavicular head. Despite the close proximity of the dislocated head to the brachiocephalic artery (FIGURE 1B-1D), there was no vascular injury.
DISCUSSION
Posterior sternoclavicular dislocations (PSCDs) can be difficult to diagnose. Edema can mask the characteristic skin depression that one would expect with a posterior dislocation.1 Chest radiographs are often normal (as was true in this case). Patients may present with an abnormal pulse, paresthesias, hoarseness, dysphagia, and/or dyspnea. However, for more than half of these patients, their only signs and symptoms will be pain, swelling, and limited range of motion.1 As a result, a PSCD may be misdiagnosed as a ligamentous or soft tissue injury.1
An uncommon injury that can result in serious complications
PSCDs represent 3% to 5% of all SCJ dislocations, which comprise <5% of all shoulder girdle injuries.1 Nevertheless, prompt and accurate diagnosis is critical, as these dislocations involve a high risk for injury to the posterior structures, particularly the brachiocephalic vein, right common carotid artery, and aortic arch.
One study found that nearly 75% of patients had a significant structure <1 cm posterior to the SCJ.2This proximity can result in neurovascular complications—some of which are devastating—in up to 30% of patients with PSCDs.3 A case report from 2011, for example, describes a 19-year-old man who had an undiagnosed PSCD that caused a pseudoaneurysm in his subclavian artery and a subsequent thrombotic cerebrovascular accident.4
Which injuries should raise your suspicions? Injuries in which lateral compression on the shoulder has caused it to roll forward and those in which a posteriorly directed force has been applied to the medial clavicle (as might occur in tackle sports or motor vehicle rollovers) should increase suspicion of a PSCD.1
Proper diagnosis requires CT angiography of the chest to assess the injury and evaluate the underlying structures. If CT is not available, additional chest film views, such as a serendipity view (anteroposterior view with 40° cephalic tilt) or Heinig view (oblique projection perpendicular to SCJ), may be obtained; an ultrasound is also an option.5
PSCD = surgical emergency
Following diagnosis, immediate orthopedic consultation is required. A PSCD is a surgical emergency. Reduction (open or closed) must be performed under general anesthesia with vascular and/or cardiothoracic surgery specialists available, as the reduction itself could injure one of the great vessels. Fortunately, most patients do quite well following surgery, with the majority achieving good-to-excellent results.6
Our patient was admitted to the hospital and underwent orthopedic surgery the following morning. Vascular and cardiothoracic surgeons were consulted and available in the event of a complication. A Salter-Harris type 2 fracture of the medial clavicle was identified intraoperatively, and an open reduction with internal fixation was performed. The patient had an uneventful recovery and resumed his usual activities, including playing hockey.
THE TAKEAWAY
PSCDs, although uncommon, can be life-threatening. Since the physical exam is unreliable and standard radiographs are often normal, accurate diagnosis relies largely on increased clinical suspicion. When there is a history of shoulder trauma, medial clavicle pain, and SCJ edema or tenderness, a PSCD should be suspected.7
Confirm the diagnosis with CT angiogram, and remember that a PSCD is a surgical emergency that requires coordination with orthopedic and vascular/cardiothoracic surgeons.
THE CASE
A 16-year-old hockey player presented to our emergency department with sharp pain in his right upper chest after “checking” another player during a game. The pain did not resolve with rest and was worse with movement and breathing. The patient did not have dysphagia, dyspnea, paresthesias, or hoarseness. The physical examination revealed tenderness over the right sternoclavicular joint (SCJ) without swelling or deformity. A distal neurovascular exam was intact, and a chest x-ray showed no evidence of dislocation or fracture (FIGURE 1A). The patient’s pain was refractory to multiple intravenous (IV) pain medications.
THE DIAGNOSIS
A computed tomography (CT) scan with IV contrast of the chest demonstrated posterior and superior dislocation of the right clavicular head. Despite the close proximity of the dislocated head to the brachiocephalic artery (FIGURE 1B-1D), there was no vascular injury.
DISCUSSION
Posterior sternoclavicular dislocations (PSCDs) can be difficult to diagnose. Edema can mask the characteristic skin depression that one would expect with a posterior dislocation.1 Chest radiographs are often normal (as was true in this case). Patients may present with an abnormal pulse, paresthesias, hoarseness, dysphagia, and/or dyspnea. However, for more than half of these patients, their only signs and symptoms will be pain, swelling, and limited range of motion.1 As a result, a PSCD may be misdiagnosed as a ligamentous or soft tissue injury.1
An uncommon injury that can result in serious complications
PSCDs represent 3% to 5% of all SCJ dislocations, which comprise <5% of all shoulder girdle injuries.1 Nevertheless, prompt and accurate diagnosis is critical, as these dislocations involve a high risk for injury to the posterior structures, particularly the brachiocephalic vein, right common carotid artery, and aortic arch.
One study found that nearly 75% of patients had a significant structure <1 cm posterior to the SCJ.2This proximity can result in neurovascular complications—some of which are devastating—in up to 30% of patients with PSCDs.3 A case report from 2011, for example, describes a 19-year-old man who had an undiagnosed PSCD that caused a pseudoaneurysm in his subclavian artery and a subsequent thrombotic cerebrovascular accident.4
Which injuries should raise your suspicions? Injuries in which lateral compression on the shoulder has caused it to roll forward and those in which a posteriorly directed force has been applied to the medial clavicle (as might occur in tackle sports or motor vehicle rollovers) should increase suspicion of a PSCD.1
Proper diagnosis requires CT angiography of the chest to assess the injury and evaluate the underlying structures. If CT is not available, additional chest film views, such as a serendipity view (anteroposterior view with 40° cephalic tilt) or Heinig view (oblique projection perpendicular to SCJ), may be obtained; an ultrasound is also an option.5
PSCD = surgical emergency
Following diagnosis, immediate orthopedic consultation is required. A PSCD is a surgical emergency. Reduction (open or closed) must be performed under general anesthesia with vascular and/or cardiothoracic surgery specialists available, as the reduction itself could injure one of the great vessels. Fortunately, most patients do quite well following surgery, with the majority achieving good-to-excellent results.6
Our patient was admitted to the hospital and underwent orthopedic surgery the following morning. Vascular and cardiothoracic surgeons were consulted and available in the event of a complication. A Salter-Harris type 2 fracture of the medial clavicle was identified intraoperatively, and an open reduction with internal fixation was performed. The patient had an uneventful recovery and resumed his usual activities, including playing hockey.
THE TAKEAWAY
PSCDs, although uncommon, can be life-threatening. Since the physical exam is unreliable and standard radiographs are often normal, accurate diagnosis relies largely on increased clinical suspicion. When there is a history of shoulder trauma, medial clavicle pain, and SCJ edema or tenderness, a PSCD should be suspected.7
Confirm the diagnosis with CT angiogram, and remember that a PSCD is a surgical emergency that requires coordination with orthopedic and vascular/cardiothoracic surgeons.
1. Chaudhry S. Pediatric posterior sternoclavicular joint injuries. J Am Acad Orthop Surg. 2015;23:468-475.
2. Ponce BA, Kundukulam JA, Pflugner R, et al. Sternoclavicular joint surgery: how far does danger lurk below? J Shoulder Elbow Surg. 2013;22:993-999.
3. Daya MR, Bengtzen RR. Shoulder. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:618-642.
4. Marcus MS, Tan V. Cerebrovascular accident in a 19-year-old patient: a case report of posterior sternoclavicular dislocation. J Shoulder Elbow Surg. 2011;20:e1-e4.
5. Morell DJ, Thyagarajan DS. Sternoclavicular joint dislocation and its management: a review of the literature. World J Orthop. 2016;7:244-250.
6. Boesmueller S, Wech M, Tiefenboeck TM, et al. Incidence, characteristics, and long-term follow-up of sternoclavicular injuries: an epidemiologic analysis of 92 cases. J Trauma Acute Care Surg. 2016;80:289-295.
7. Roepke C, Kleiner M, Jhun P, et al. Chest pain bounce-back: posterior sternoclavicular dislocation. Ann Emerg Med. 2015;66:559-561.
1. Chaudhry S. Pediatric posterior sternoclavicular joint injuries. J Am Acad Orthop Surg. 2015;23:468-475.
2. Ponce BA, Kundukulam JA, Pflugner R, et al. Sternoclavicular joint surgery: how far does danger lurk below? J Shoulder Elbow Surg. 2013;22:993-999.
3. Daya MR, Bengtzen RR. Shoulder. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:618-642.
4. Marcus MS, Tan V. Cerebrovascular accident in a 19-year-old patient: a case report of posterior sternoclavicular dislocation. J Shoulder Elbow Surg. 2011;20:e1-e4.
5. Morell DJ, Thyagarajan DS. Sternoclavicular joint dislocation and its management: a review of the literature. World J Orthop. 2016;7:244-250.
6. Boesmueller S, Wech M, Tiefenboeck TM, et al. Incidence, characteristics, and long-term follow-up of sternoclavicular injuries: an epidemiologic analysis of 92 cases. J Trauma Acute Care Surg. 2016;80:289-295.
7. Roepke C, Kleiner M, Jhun P, et al. Chest pain bounce-back: posterior sternoclavicular dislocation. Ann Emerg Med. 2015;66:559-561.
Bedside Ultrasound for Pulsatile Hand Mass
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
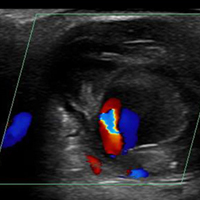
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
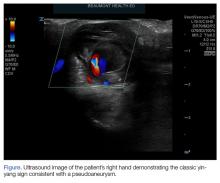
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
Case
A 23-year-old man presented to an outside hospital’s ED for evaluation of a wound on his right hand, which he sustained after he accidentally stabbed himself with a steak knife. At presentation, the patient’s vital signs were: heart rate, 90 beats/min; respiratory rate, 16 breaths/min; blood pressure, 150/92 mm Hg; and temperature, 98.1°F. Oxygen saturation was 98% on room air. Examination revealed a laceration on the patient’s right hand measuring 2 cm in length. The emergency physician (EP) closed the wound using four nylon sutures and administered a Boostrix shot. The patient was discharged home with a prescription for cephalexin capsule 500 mg to be taken four times daily for 5 days. He was instructed to return in 10 days for suture removal, but failed to follow-up.
The patient presented to our ED two months after the initial injury for evaluation of a 1.5-cm round pulsatile mass on his right palm, at the base of the middle finger, from which exuded a small amount of sanguineous fluid. The patient complained of numbness and difficulty extending his right index and middle fingers.
Discussion
Palmar Pseudoaneurysms
A pseudoaneurysm, also referred to as a traumatic aneurysm, develops when a tear of the vessel wall and hemorrhage is contained by a thin-walled capsule, typically following traumatic perforation of the arterial wall. Unlike a true aneurysm, a pseudoaneurysm does not contain all three layers of intima, media, and adventitia. Thin walls lead to inevitable expansion over time; in some cases, a patient will present with a soft-tissue mass years after the initial injury. Compression of nearby structures can cause neuropathy, peripheral edema, venous thrombosis, arterial occlusion or emboli, and even bone erosion.1,2
Hand pseudoaneurysms are more likely to occur on the palmar surface, involving the superficial palmar arch,3 and are due to a penetrating injury or repetitive microtrauma. Hypothenar hammer syndrome occurs when repetitive microtrauma is applied to the ulnar artery as it passes under the hook of the hamate bone into the hand. This condition is also referred to as “hammer hand syndrome” because it frequently occurs in laborers such as mechanics, carpenters, and machinists as a result of repetitive palm trauma. Cases have also been reported in baseball players and cooks who also expose their hands to repetitive trauma.3 Likewise, elderly patients who use walking canes can also present with bilateral hammer hand syndrome,3 and patients who need crutches for a prolonged period of time may also develop axillary artery aneurysms.1,2
Although rare, there have also been cases of spontaneous hand pseudoaneurysms in patients on anticoagulation therapy;4,5 however, pseudoaneurysms are not an absolute contraindication to initiating or continuing use of anticoagulants.
Evaluation
Physical Examination. The patient’s mass in this case was clearly pulsatile on examination, but physical examination alone is not a reliable indicator of pseudoaneurysm, as patients may present only with soft-tissue swelling, pain, erythema, or neurological symptoms.3,6,7
Ultrasound Imaging. In the emergency setting, POC ultrasound should be performed to evaluate any soft-tissue hand mass, especially in the context of trauma or any neurovascular findings, since palmar pseudoaneurysms can easily be confused with an abscess, foreign body, cyst, or even a tendon tear.6 Ultrasound studies using the linear vascular probe should always be done before any attempt to incise and drain the mass.
Three ultrasound characteristics of pseudoaneurysms include expansile pulsatility, turbulent flow with a classic yin-yang sign on Doppler, and a hematoma with variable echogenicity. Variable echogenicity may represent separate episodes of bleeding and rebleeding.8 A “to-and-fro” spectral waveform is pathognomonic for palmar pseudoaneurysms.8
Computed Tomography and Magnetic Resonance Angiography. Definitive imaging for operative management includes computed tomography or magnetic resonance angiography to assess for the exact location and presence of collateral circulation.
Treatment
Treatment of pseudoaneurysms includes conservative compression therapy, surgical excision, or anastomosis, and more recently, ultrasound-guided thrombin injection (UGTI).
Compression Therapy. Compression therapy is often used for femoral artery pseudoaneurysms that develop after iatrogenic injury. However, this technique is time consuming, is uncomfortable for patients, is not effective in treating large pseudoaneurysms, and is contraindicated in patients on anticoagulation therapy. Compression therapy also has a high-failure rate of resolving pseudoaneurysms. Traditionally, surgical excision or anastomosis has been the definitive treatment for palmar pseudoaneurysms.
Ultrasound-Guided Thrombin Injection. A more recent treatment option is UGTI, which is usually performed by an interventional radiologist. Although there is no consensus on exact dose of thrombin for this procedure, the literature describes UGTI to treat both the radial and ulnar arteries.9,10 One study of 83 pseudoaneurysms demonstrated a relationship between the size of the palmar pseudoaneurysm and the number of thrombin injections required to resolve it. Depending on the size of the palmar pseudoaneurysm, the effective thrombin doses ranged from 200 to 2,500 U. Regarding adverse effects and events from treatment, this study reported one case of transient distal ischemia.11
Intravascular balloon occlusion of the pseudoaneurysm neck has also been recommended for UGTI in the femoral artery if the neck is greater than 1 mm, but there is currently nothing in the literature describing its use in palmar pseudoaneurysms.12
Complications
There are more descriptions of palmar, radial, and ulnar pseudoaneurysms in critical care patients due to the frequent, but necessary, use of invasive lines. Emergency physicians frequently place radial or femoral arterial lines for hemodynamic monitoring in critically ill patients. However, the incidence of pseudoaneurysms and its sequelae from these lines are not usually observed in the ED setting.
Radial arterial lines may cause thrombosis in 19% to 57% of cases, and local infection in 1% to 18% of cases.10 In a study of 12,500 patients with radial artery catheters, the rate of radial pseudoaneurysm was only 0.05%.11 Although this is a small complication rate, pseudoaneurysms can lead to significant loss of function. To decrease the number of attempts and penetrating injuries to the arteries, ultrasound guidance for these procedures in the ED is strongly recommended. In addition to decreasing the risk of developing a pseudoaneurysm, ultrasound-guidance decreases the discomfort level of the patient and reduces the risk of bleeding, hematoma formation, and infection. Arterial line placement in the ED using ultrasound guidance decreases the risk of developing pseudoaneurysms and their sequelae, such as distal embolization.
Case Conclusion
The patient in this case underwent an arterial duplex study, which found a partially thrombosed right superficial palmar arch pseudoaneurysm measuring 1.91 cm x 2.08 cm, with an active flow area measuring 0.58 cm x 0.68 cm. The flow to the index finger medial artery and middle finger lateral artery was also diminished. The patient was discharged home with a bulky soft dressing and underwent excision and repair by hand surgery 3 days later. At the 1-month postoperative follow-up visit, the patient had full sensation but mildly decreased range of motion in his fingers.
Summary
Hand pseudoaneurysms are often associated with penetrating injuries—as demonstrated in our case—or repetitive microtrauma. Hand pseudoaneurysms can present with minimal findings such as isolated soft-tissue swelling, pain, or neuropathy. The EP should consider vascular pathology in the differential for patients who present with posttraumatic neuropathy. Regarding imaging studies, ultrasound is the best imaging modality to assess for pseudoaneurysms, and EPs should have a low threshold for its use at bedside—especially prior to attempting any invasive procedure. Patients with a confirmed pseudoaneurysm should be referred to a hand or vascular surgeon for surgical repair, or to an interventional radiologist for UGTI.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
1. Newton EJ, Arora S. Peripheral vascular injury. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. Philadelphia, PA: Elsevier Saunders; 2014:502.
2. Aufderheide TP. Peripheral arteriovascular disease. In: Marx JA, Hockberger RS, Walls RM, et al, eds. Rosen’s Emergency Medicine Concepts and Clinical Practice. Vol 1. 8th ed. 2014:1147-1149.
3. Anderson SE, De Monaco D, Buechler U, et al. Imaging features of pseudoaneurysms of the hand in children and adults. AJR Am J Roentgenol. 2003;180(3):659-664. doi:10.2214/ajr.180.3.1800659.
4. Shah S, Powell-Brett S, Garnham A. Pseudoaneurysm: an unusual cause of post-traumatic hand swelling. BMJ Case Rep. 2015;2015. pii: bcr2014208750. doi:10.1136/bcr-2014-208750.
5. Kitamura A, Mukohara N. Spontaneous pseudoaneurysm of the hand. Ann Vasc Surg. 2014;28(3):739.e1-e3. doi:10.1016/j.avsg.2013.04.033.
6. Huang SW, Wei TS, Liu SY, Wang WT. Spontaneous totally thrombosed pseudoaneurysm mimicking a tendon tear of the wrist. Orthopedics. 2010;33(10):776. doi:10.3928/01477447-20100826-23.
7. Belyayev L, Rich NM, McKay P, Nesti L, Wind G. Traumatic ulnar artery pseudoaneurysm following a grenade blast: report of a case. Mil Med. 2015;180(6):e725-e727. doi:10.7205/MILMED-D-14-00400.
8. Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10(2):89-91.
9. Bosman A, Veger HTC, Doornink F, Hedeman Joosten PPA. A pseudoaneurysm of the deep palmar arch after penetrating trauma to the hand: successful exclusion by ultrasound guided percutaneous thrombin injection. EJVES Short Rep. 2016;31:9-11. doi:10.1016/j.ejvssr.2016.03.002.
10. Komorowska-Timek E, Teruya TH, Abou-Zamzam AM Jr, Papa D, Ballard JL. Treatment of radial and ulnar artery pseudoaneurysms using percutaneous thrombin injection. J Hand Surg. 2004;29A(5):936-942. doi:10.1016/j.jhsa.2004.05.009.
11. Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101(2):490-495.
12. Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31(2):289-298.
A Recalcitrant Case of Toxic Epidermal Necrolysis
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
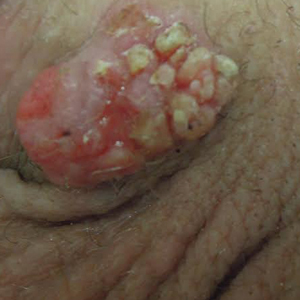
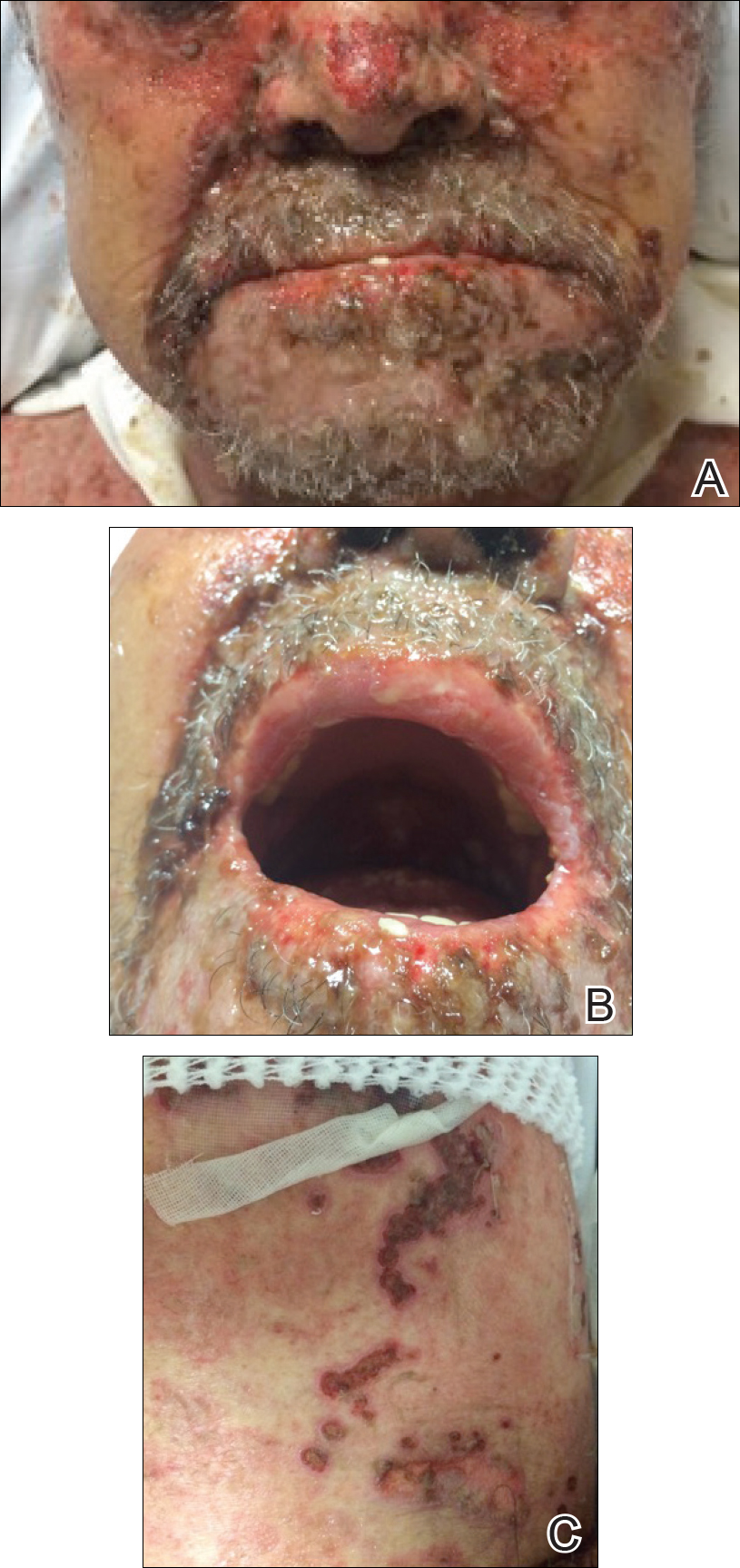
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
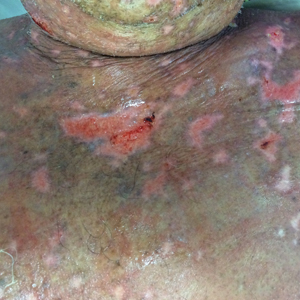
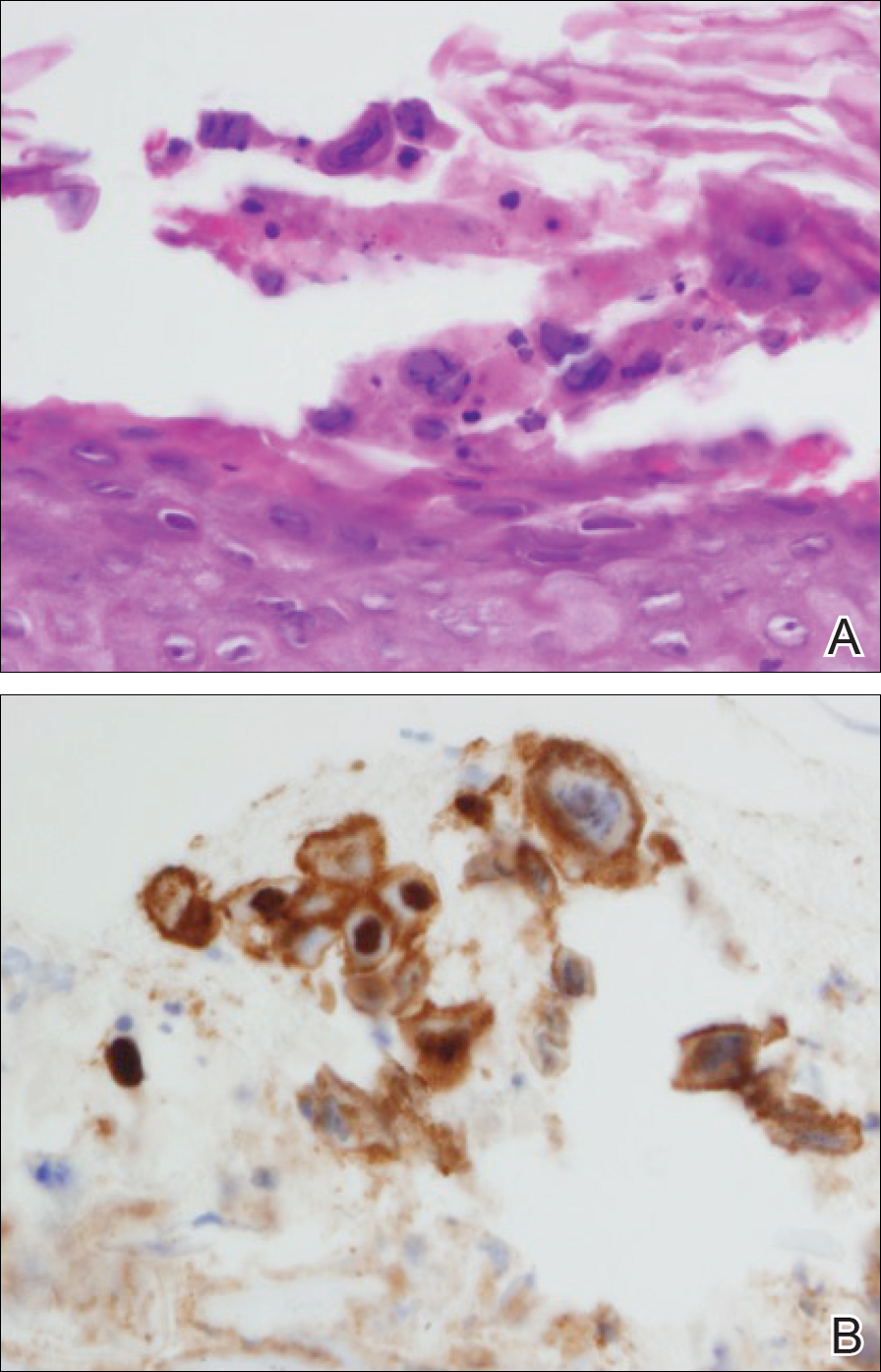
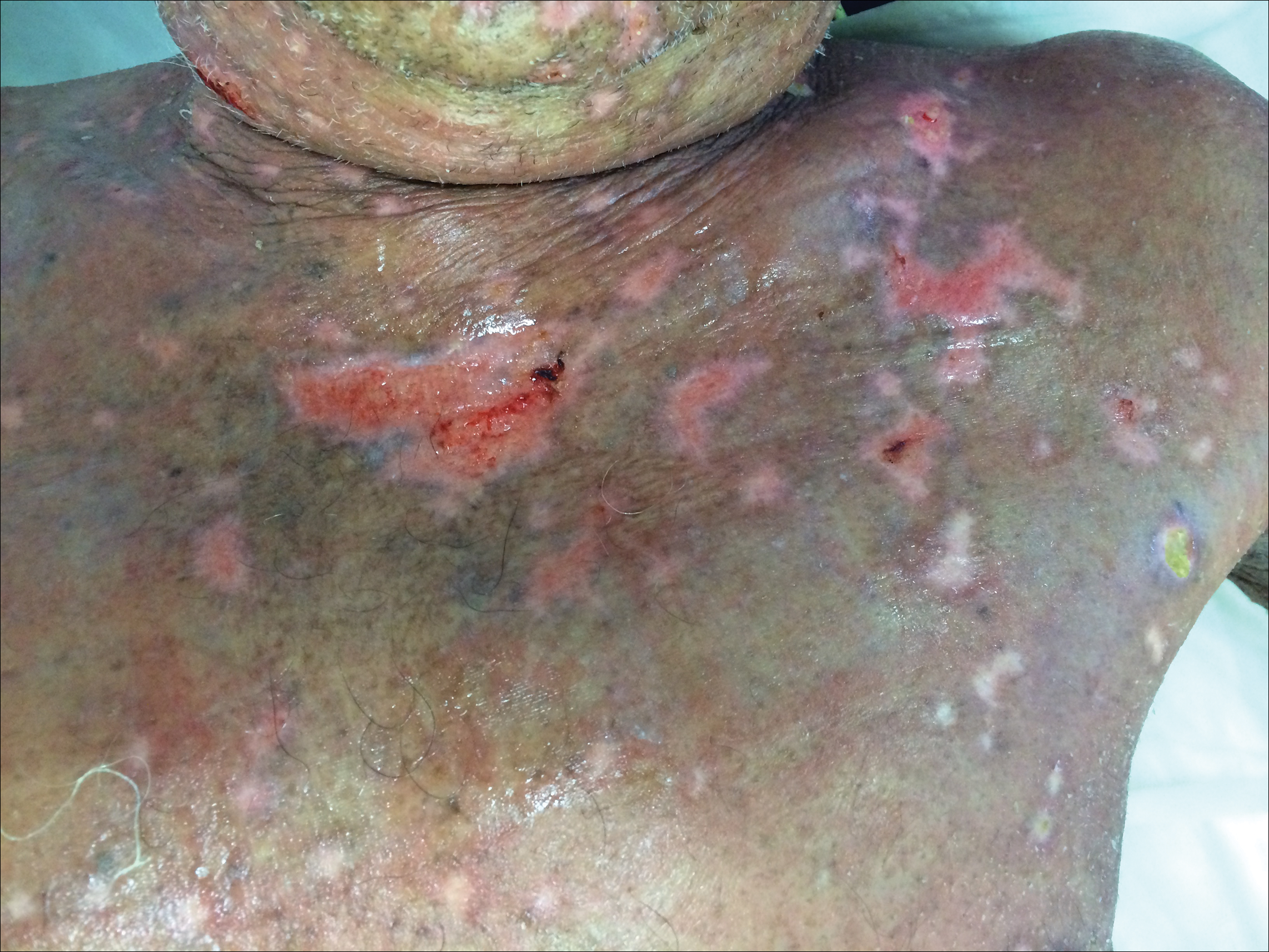
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.
Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
One of the most severe complications of systemic medications is the development of a life-threatening rash, especially toxic epidermal necrolysis (TEN). Most patients can expect a full recovery if the complicating medication is discontinued early on in its course.1 When suspected TEN does not improve despite discontinuation of the detrimental medication, other diseases must be considered, particularly immunobullous and infectious etiologies. Treatment of these diseases differs substantially; therefore, a quick diagnosis is crucial. We present a case of a patient with an acute blistering eruption that was initially diagnosed and managed as TEN but physical examination and histopathologic confirmed another diagnosis. We review key examination findings that can help differentiate the causes of an acute blistering eruption with mucosal involvement, allowing for earlier diagnosis and treatment of these patients.
Case Report
An 85-year-old immunocompetent man was admitted to an outside hospital with a pruritic blistering eruption associated with myalgia, weakness, and fatigue of 3 weeks’ duration. The eruption initiated on the scalp and face and then spread down to the trunk and proximal arms and legs, with oral erosions also reported. An outside dermatologist was consulted on admission and performed a skin biopsy; the initial pathology was read as TEN. The patient was admitted to our institution on the same day, and all potentially complicating medications were stopped. He was treated with intravenous (IV)
At that time, physical examination revealed numerous confluent erosions with honey-colored crust involving the entire face (Figure 1A) and sharp demarcation at the cutaneous lip (Figure 1B). There was a large erosion on the dorsal aspect of the tongue, but the rest of the oral mucosa was spared. The trunk and proximal extremities showed numerous grouped, punched-out erosions with scalloped borders (Figure 1C). A repeat skin biopsy showed an ulcer with viral cytopathic changes. Immunoperoxidase studies demonstrated positive staining for herpes simplex virus (HSV) type 1 (Figure 2). The original slides were a frozen section from an outside facility and could not be obtained. A tissue culture and direct fluorescent antibody also confirmed HSV-1, and the patient was diagnosed with disseminated herpes. He was rapidly tapered off of the steroids and started on IV acyclovir 10 mg/kg every 8 hours for 21 days. All prior erosions reepithelialized within 7 days of treatment (Figure 3). The patient had an otherwise uncomplicated hospital course and was discharged on hospital day 21.



Comment
A patient with an acute generalized blistering eruption requires urgent workup and treatment given the potentially devastating sequelae. Toxic epidermal necrolysis and immunobullous diseases often are the first diagnoses to be ruled out. Certainly infections such as HSV can cause a vesicular and erosive eruption, especially in the setting of a poorly controlled dermatitis, but they typically are not in the same differential as the other diagnoses.
Clinical Presentation
This case highlights 2 key physical examination findings that can alert the clinician to a possible underlying herpetic infection. First, the distribution of this patient’s oral lesions was telling. In most cases of TEN or pemphigus vulgaris, there is notable involvement of the oral mucosa, particularly the buccal and labial mucosa. Although herpes can involve any mucocutaneous surface, it does have a predilection for keratinized tissue, with the tongue and cutaneous lip commonly involved.2,3 Our patient had a solitary linear erosion on the dorsal aspect of the tongue, but the rest of the oral cavity was strikingly spared. In addition, the erosions around the mouth stopped right at the cutaneous lip, sparing the labial mucosa (Figure 1B).
Second, the configuration of the erosions on the trunk, arms, and legs was diagnostic. Herpes classically presents as a cluster of vesicles overlying an erythematous base. When these vesicles rupture, punched-out erosions are left behind. Because these vesicles often are grouped, they can develop a scalloped border, which is a helpful indicator of HSV (Figure 1C). When these erosions become more confluent and irregular, the distinction from other conditions may not be as clear. A careful skin examination often can show areas that have preserved this herpetiform configuration.
Immune Compromise
Additionally, this case is illustrative of how immunosuppression and immunocompromise can affect the clinical presentation of HSV infection. Herpetic infections in the immunocompromised host tend to have a more protracted course, with chronic enlarging ulcers involving multiple sites.
Conclusion
This case is a good reminder that not everything that blisters and involves the mucosa is due to a hypersensitivity state such as TEN and Stevens-Johnson syndrome or an immunobullous disorder such as pemphigus vulgaris and pemphigus vegetans. The fact that this patient was worsening despite drug cessation, high-dose steroids, and IV immunoglobulin should have indicated a misdiagnosis. This case also shows that the early histopathologic findings of disseminated HSV and TEN can be nonspecific, and viral cytopathic changes may not always be obvious early in the disease.
Disseminated HSV should be considered in the differential diagnosis of a patient with an acute blistering eruption with mucosal involvement, and careful history and physical examination should be taken to rule out a viral etiology.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
- Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. New York, NY: Mosby; 2008.
- Woo SB, Lee SF. Oral recrudescent herpes simplex virus infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:239-243.
Practice Points
- Toxic epidermal necrolysis can be difficult to diagnose and treat.
- Patients who are refractory to treatment should prompt further management considerations.
Atraumatic splenic rupture as an initial presentation of chronic myelogenous leukemia
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
Single-Dose Niacin-Induced Hepatitis
Niacin, also known as vitamin B3, is an important cofactor in many metabolic processes necessary to life. Over the past 15 to 20 years, niacin has been prescribed to patients with hyperlipidemia to increase high-density lipoprotein and lower low-density lipoprotein.1 As a naturally occurring vitamin, niacin is also available over-the-counter (OTC) as a dietary supplement, and is also a common ingredient in energy drinks and multivitamins.2
In addition to treating hyperlipidemia and as a nutritional supplement, some anecdotal reports amongst lay-persons suggests that niacin offers other health benefits, such as promoting weight loss and expediting the elimination of alcohol and illicit drugs from one’s system (eg, marijuana).3,4 The increased use of niacin supplementation in the general population for all of the aforementioned reasons has resulted in an increased incidence of niacin toxicity.
Formulations
Niacin is available in three formulations: extended-release (ER, also referred to as intermediate-release), immediate-release (IR), and sustained-release (SR).
The ER formulations of niacin are typically prescribed to treat hyperlipidemia. Patients are usually started on ER niacin at an initial dose of 250 mg once daily. The dose is gradually increased, as tolerated or necessary, to 2 g per day, taken in three doses. It is not uncommon for patients with hyperlipidemia to take more than 1 g of niacin per day after titration by their primary physicians.
Side Effects
Since niacin increases the release of arachidonic acid from cell membranes that metabolizes into prostaglandins, specifically prostaglandins E2 and D2, many patients taking niacin experience uncomfortable flushing and itching.5 Nonsteroidal anti-inflammatory drugs (NSAIDs) prevent this side effect by inhibiting the metabolism of arachidonic acid into those vasodilatory prostaglandins. The newer ER and SR formulations of niacin, which are approved for OTC use as a dietary supplement, are less likely to cause flushing.5
Extended-release niacin, however, is associated with a higher incidence of hepatotoxicity than the other prescription formulations of niacin.6 Toxicity has been well recognized in patients taking niacin chronically for hyperlipidemia, with reports of such cases dating back to the 1980s.7,8 We report a unique case of niacin toxicity following a single-dose ingestion in a young man.
Case
A 22-year-old man presented to the ED for evaluation of a 2-week history of intermittent periumbilical abdominal pain. This visit represented the patient’s second visit to the ED over the past week for the same complaint.
Upon presentation the patient’s vital signs were: blood pressure (BP), 113/64 mm Hg; heart rate, 82 beats/min; respiratory rate, 16 breaths/min; and temperature 36.6°C. Oxygen saturation was 100% on room air. The patient was otherwise healthy and had no significant recent or remote medical history. He denied taking any medications prior to his initial presentation, and reported only occasional alcohol use.
At the patient’s initial presentation 1 week earlier, he was diagnosed with acute gastroenteritis and treated with famotidine and ondansetron in the ED. The patient appeared well clinically at this visit, and laboratory values were within normal limits, including normal blood glucose and urinalysis.
The patient was discharged home from this first visit with prescriptions of famotidine and ondansetron, and was advised to follow up with his primary care physician in 1 week. Throughout the week after discharge from the ED, the patient experienced worsening abdominal pain, and he developed frequent nonbloody emesis, prompting his second presentation to the ED. At this second visit, the patient stated that he had taken one dose of ondansetron at home, without effect. He also noted subjective fevers, but had no diarrhea or melena.
Vital signs remained within normal limits with BPs ranging from 115 to 130 mm Hg systolic and 50 to 89 mm Hg diastolic. The patient was never tachycardic, tachypneic, febrile, or hypoxic. Physical examination was remarkable for periumbilical tenderness. The patient had no jaundice. A more thorough laboratory evaluation revealed elevated anion gap and blood urea nitrogen/creatinine values, and leukocytosis. The patient’s hepatic enzymes were also elevated, with aspartate aminotransferase (AST) over 2,000 U/L and alanine aminotransferase (ALT) of 1,698 U/L. Lipase, bilirubin, and alkaline phosphatase were all within normal limits. The patient’s prothrombin time (PT) was elevated at 14 seconds, and the international normalized ratio (INR) was elevated at 1.28. Laboratory analysis for acetaminophen and alcohol was negative.
A computed tomography (CT) scan of the abdomen/pelvis with intravenous (IV) contrast was unremarkable, demonstrating a liver devoid of any masses, portal or biliary dilation, or cirrhotic changes.
The patient received IV famotidine and ondansetron, and morphine for pain control, and was admitted to the general medical floor for hepatitis of uncertain etiology. A viral hepatitis panel was negative.
On the recommendation of the toxicology service, the patient was given N-acetylcysteine (NAC), and his hepatic enzymes trended down to an AST of 642 U/L and an ALT of 456 U/L by hospital day 2. (The patient essentially completed a positive dechallenge test).9
A gastroenterology consult was ordered, during which additional history-taking and chart review noted that the patient admitted to taking one or two tablets of OTC niacin as a dietary supplement the day before his initial presentation. Although the patient could not recall the exact dosage, he stated that he had been taking supplemental niacin approximately once a month over the past several years without any issues. Since OTC niacin is most commonly available in 500-mg tablets, this suggested the patient’s recent one-time ingested dose was approximately 500 to 1,000 mg.
Based on the patient’s admission to niacin use, additional studies were ordered, including an abdominal ultrasound and a urine drug screen. Ultrasound findings were unremarkable for portal venous thrombosis. The urine drug screen, however, was positive for marijuana and opiates. While the patient denied any history of opioid use, the positive opiate assay could have been attributed to the morphine given in the ED.
Throughout the patient’s hospital course, he remained normotensive and had no change in mental status. His liver enzymes, PT, and INR continued to normalize, and he was discharged home after 3 days, with instructions to follow up with the gastrointestinal clinic within 11 days. An appointment was made for him, which he did not attend.
Given the patient’s negative autoimmune and viral workup, and rapid resolution of symptoms after discontinuing niacin use, it is believed that he had an acute drug-induced hepatitis due to niacin ingestion. Regarding any coingestants that could have contributed to the hepatitis, the patient denied taking other common coingestants such as alcohol and acetaminophen; this assertion was supported by laboratory results.
Since we were unable to attain a qualitative measurement of the patient’s niacin concentration, our diagnosis was primarily based on the patient’s reported history.10 It is possible the patient had been taking more niacin than that to which he admitted, or that he was taking another hepatotoxic substance not detected on our toxicology workup. As previously noted, there are many medications and/or dietary supplements that could cause or contribute to a synergistic effect of drug-induced hepatitis for which the patient was not tested at his initial presentation. The patient could have co-ingested this large dose of niacin with acetaminophen and/or other supplements, energy drinks, or alcohol. A combination such as this could have contributed to his hepatitis, and the metabolites of these other substances would have been eliminated by the time of his second ED presentation.
Discussion
There are over 900 different drugs, toxins, and supplements known to cause hepatic injury.11,12 Clinical manifestations of toxicity range from asymptomatic incidental elevations in transaminases to fulminant liver failure causing mortality. Ingestion of commonly used medications such as statins (although not in overdose quantities) can cause transient asymptomatic transaminitis.13 These elevations are usually mild—ie, less than twice the upper limit of normal. Patients who experience such elevations can usually continue to take the medications with frequent and vigilant monitoring of hepatic function.
Signs and Symptoms
Acute Liver Injury. Acute liver injury is diagnosed when AST and ALT levels are greater than twice the upper limit of normal. Patients also typically have mild-to-moderate abdominal findings, such as pain, nausea, and vomiting—as was experienced by our patient. Along with niacin, angiotensin-converting enzyme inhibitors, NSAIDs, and antifungal medications are examples of other medications that can cause this degree of drug-induced hepatitis.
Severe Liver Injury. Severe liver injury features elevations in not only AST and ALT, but also alkaline phosphate and bilirubin. Patients with severe hepatic injury appear clinically ill and may exhibit altered mental status and jaundice. This type of subfulminant hepatic failure commonly results from acetaminophen toxicity, anesthetic gases, iron toxicity, phosphorus toxicity, and cocaine toxicity. Examples of drugs that result in massive liver necrosis and fulminant hepatitis are acetaminophen, isoniazid, phenelzine, phenytoin, propylthiouracil, and sertraline. Patients with massive hepatic necrosis and hepatitis may require liver transplantation.
Etiology
Identifying the etiology of liver injury is made largely through the patient’s history because there are simply too many possible hepatotoxic agents to test for them all. Diagnostic suspicion of hepatic toxicity should be increased with signs of more serious disease; however, drug-induced liver injury should be included in the differential diagnosis for all cases of abdominal pain.
With respect to the patient in our case, obtaining a more complete history involving supplement and vitamin use would have allowed us to make the diagnosis in the ED. Unfortunately, these subtle aspects of a patient’s history are often overlooked in the emergent care setting.
Treatment
The treatment of niacin-induced liver injury is similar to the guidelines for treating most other drug-induced pathology.14 Removal of the offending agent and providing supportive care is the primary treatment modality.15 In addition, it is important that the clinician exclude and rule-out other causes of hepatitis such as those of viral, autoimmune, or ischemic etiology.
N-acetylcysteine. A medication classically used in patients with acetaminophen overdose, NAC is a safe and effective treatment for non-acetaminophen-induced liver injury, and was given to treat our patient.16L-carnitine. L-carnitine has been shown to be effective in cases of chronic steatosis from hepatitis C and in valproic acid induced hepatitis.17Since L-carnitine is not included on our hospital’s formulary, it was not a treatment option for our patient.Glucocorticoid Therapy. Although glucocorticoids are occasionally given to patients with systemic symptoms of drug reactions, its effectiveness has not been adequately studied.18
Prognosis
The prognosis of patients with acute drug-induced hepatitis is generally good, and most patients fully recover once the offending agent is removed. Poor prognostic factors include the presence of jaundice, requirement for dialysis, underlying chronic liver conditions, or elevated serum creatinine. While most patients will experience a complete recovery, approximately 5% to 10% will develop chronic hepatitis and/or cirrhosis.
Conclusion
Niacin is now available as prescription and OTC formulations and is a potentially hepatotoxic medication and dietary supplement. Niacin can cause an acute hepatitis, especially when taken in conjunction with other hepatotoxic substances. Drug-induced liver injury from niacin ingestion will improve quickly following removal, and the prognosis in otherwise healthy individuals is good.
Patients, especially young, healthy patients who present with symptoms concerning for hepatitis, should be asked specifically about any nutritional, herbal, or other supplement usage. During the history intake, many patients do not consider vitamins or other nutritional or herbal supplements as “medication” or as being significant, and only report prescription and OTC medications.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213.
2. Vivekanandarajah A, Ni S, Waked A. Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep. 2011;5:227. doi:10.1186/1752-1947-5-227.
3. Niacin. U.S. National Library of Medicine. https://medlineplus.gov/druginfo/meds/a682518.html. Updated July 15, 2017. Accessed February 21, 2018.
4. Addiction Resource. Niacin flush for drug detox. https://addictionresource.com/drug-testing/niacin-drug-test/. Accessed February 20, 2018.
5. Kamanna VS, Ganji SH, Kashyap ML. The mechanism and mitigation of niacin-induced flushing. Int J Clin Pract. 2009;63(9):1369-1377. doi:10.1111/j.1742-1241.2009.02099.x.
6. Etchason JA, Miller TD, Squires RW, et al. Niacin-induced hepatitis: a potential side effect with low-dose time-release niacin. Mayo Clin Proc. 1991;66(1):23-28.
7. Patterson DJ, Dew EW, Gyorkey F, Graham DY. Niacin hepatitis. South Med J. 1983;76(2):239-241.
8. Ferenchick G, Rovner D. Hepatitis and hematemesis complicating nicotinic acid use. Am J Med Sci. 1989;298(3):191-193.
9. Henkin Y, Johnson KC, Segrest JP. Rechallenge with crystalline niacin after drug-induced hepatitis from sustained-release niacin. JAMA. 1990;264(2):241-243.
10. Barritt AS 4th, Lee J, Hayashi PH. Detective work in drug-induced liver injury: sometimes it is all about interviewing the right witness. Clin Gastroenterol Hepatol. 2010;8(7):635-637. doi:10.1016/j.cgh.2010.03.020.
11. Chitturi S, Teoh NC, Farrell GC. Hepatic drug metabolism and liver disease caused by drugs. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 10th ed. Philadelphia, PA: Elsevier Saunders; 2016:1442-1477.
12. National Institutes of Health. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Niacin. https://livertox.nih.gov/Niacin.htm. Updated January 18, 2018. Accessed February 21, 2018.
13. Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4):690-695.
14. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966. doi:10.1038/ajg.2014.131.
15. Larson AM. Hepatotoxicity due to herbal medication and dietary supplements. UpToDate Web site. https://www.uptodate.com/contents/hepatotoxicity-due-to-herbal-medications-and-dietary-supplements?source=search_result&search=niacin%20induced%20hepatitis&selectedTitle=3~150. Updated December 21, 2017. Accessed February 21, 2018.
16. Mumtaz K, Azam Z, Hamid S, et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int. 2009;3(4):563-570. doi:10.1007/s12072-009-9151-0.
17. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293. doi:10.1345/aph.1P135.
18. O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439-445.
Niacin, also known as vitamin B3, is an important cofactor in many metabolic processes necessary to life. Over the past 15 to 20 years, niacin has been prescribed to patients with hyperlipidemia to increase high-density lipoprotein and lower low-density lipoprotein.1 As a naturally occurring vitamin, niacin is also available over-the-counter (OTC) as a dietary supplement, and is also a common ingredient in energy drinks and multivitamins.2
In addition to treating hyperlipidemia and as a nutritional supplement, some anecdotal reports amongst lay-persons suggests that niacin offers other health benefits, such as promoting weight loss and expediting the elimination of alcohol and illicit drugs from one’s system (eg, marijuana).3,4 The increased use of niacin supplementation in the general population for all of the aforementioned reasons has resulted in an increased incidence of niacin toxicity.
Formulations
Niacin is available in three formulations: extended-release (ER, also referred to as intermediate-release), immediate-release (IR), and sustained-release (SR).
The ER formulations of niacin are typically prescribed to treat hyperlipidemia. Patients are usually started on ER niacin at an initial dose of 250 mg once daily. The dose is gradually increased, as tolerated or necessary, to 2 g per day, taken in three doses. It is not uncommon for patients with hyperlipidemia to take more than 1 g of niacin per day after titration by their primary physicians.
Side Effects
Since niacin increases the release of arachidonic acid from cell membranes that metabolizes into prostaglandins, specifically prostaglandins E2 and D2, many patients taking niacin experience uncomfortable flushing and itching.5 Nonsteroidal anti-inflammatory drugs (NSAIDs) prevent this side effect by inhibiting the metabolism of arachidonic acid into those vasodilatory prostaglandins. The newer ER and SR formulations of niacin, which are approved for OTC use as a dietary supplement, are less likely to cause flushing.5
Extended-release niacin, however, is associated with a higher incidence of hepatotoxicity than the other prescription formulations of niacin.6 Toxicity has been well recognized in patients taking niacin chronically for hyperlipidemia, with reports of such cases dating back to the 1980s.7,8 We report a unique case of niacin toxicity following a single-dose ingestion in a young man.
Case
A 22-year-old man presented to the ED for evaluation of a 2-week history of intermittent periumbilical abdominal pain. This visit represented the patient’s second visit to the ED over the past week for the same complaint.
Upon presentation the patient’s vital signs were: blood pressure (BP), 113/64 mm Hg; heart rate, 82 beats/min; respiratory rate, 16 breaths/min; and temperature 36.6°C. Oxygen saturation was 100% on room air. The patient was otherwise healthy and had no significant recent or remote medical history. He denied taking any medications prior to his initial presentation, and reported only occasional alcohol use.
At the patient’s initial presentation 1 week earlier, he was diagnosed with acute gastroenteritis and treated with famotidine and ondansetron in the ED. The patient appeared well clinically at this visit, and laboratory values were within normal limits, including normal blood glucose and urinalysis.
The patient was discharged home from this first visit with prescriptions of famotidine and ondansetron, and was advised to follow up with his primary care physician in 1 week. Throughout the week after discharge from the ED, the patient experienced worsening abdominal pain, and he developed frequent nonbloody emesis, prompting his second presentation to the ED. At this second visit, the patient stated that he had taken one dose of ondansetron at home, without effect. He also noted subjective fevers, but had no diarrhea or melena.
Vital signs remained within normal limits with BPs ranging from 115 to 130 mm Hg systolic and 50 to 89 mm Hg diastolic. The patient was never tachycardic, tachypneic, febrile, or hypoxic. Physical examination was remarkable for periumbilical tenderness. The patient had no jaundice. A more thorough laboratory evaluation revealed elevated anion gap and blood urea nitrogen/creatinine values, and leukocytosis. The patient’s hepatic enzymes were also elevated, with aspartate aminotransferase (AST) over 2,000 U/L and alanine aminotransferase (ALT) of 1,698 U/L. Lipase, bilirubin, and alkaline phosphatase were all within normal limits. The patient’s prothrombin time (PT) was elevated at 14 seconds, and the international normalized ratio (INR) was elevated at 1.28. Laboratory analysis for acetaminophen and alcohol was negative.
A computed tomography (CT) scan of the abdomen/pelvis with intravenous (IV) contrast was unremarkable, demonstrating a liver devoid of any masses, portal or biliary dilation, or cirrhotic changes.
The patient received IV famotidine and ondansetron, and morphine for pain control, and was admitted to the general medical floor for hepatitis of uncertain etiology. A viral hepatitis panel was negative.
On the recommendation of the toxicology service, the patient was given N-acetylcysteine (NAC), and his hepatic enzymes trended down to an AST of 642 U/L and an ALT of 456 U/L by hospital day 2. (The patient essentially completed a positive dechallenge test).9
A gastroenterology consult was ordered, during which additional history-taking and chart review noted that the patient admitted to taking one or two tablets of OTC niacin as a dietary supplement the day before his initial presentation. Although the patient could not recall the exact dosage, he stated that he had been taking supplemental niacin approximately once a month over the past several years without any issues. Since OTC niacin is most commonly available in 500-mg tablets, this suggested the patient’s recent one-time ingested dose was approximately 500 to 1,000 mg.
Based on the patient’s admission to niacin use, additional studies were ordered, including an abdominal ultrasound and a urine drug screen. Ultrasound findings were unremarkable for portal venous thrombosis. The urine drug screen, however, was positive for marijuana and opiates. While the patient denied any history of opioid use, the positive opiate assay could have been attributed to the morphine given in the ED.
Throughout the patient’s hospital course, he remained normotensive and had no change in mental status. His liver enzymes, PT, and INR continued to normalize, and he was discharged home after 3 days, with instructions to follow up with the gastrointestinal clinic within 11 days. An appointment was made for him, which he did not attend.
Given the patient’s negative autoimmune and viral workup, and rapid resolution of symptoms after discontinuing niacin use, it is believed that he had an acute drug-induced hepatitis due to niacin ingestion. Regarding any coingestants that could have contributed to the hepatitis, the patient denied taking other common coingestants such as alcohol and acetaminophen; this assertion was supported by laboratory results.
Since we were unable to attain a qualitative measurement of the patient’s niacin concentration, our diagnosis was primarily based on the patient’s reported history.10 It is possible the patient had been taking more niacin than that to which he admitted, or that he was taking another hepatotoxic substance not detected on our toxicology workup. As previously noted, there are many medications and/or dietary supplements that could cause or contribute to a synergistic effect of drug-induced hepatitis for which the patient was not tested at his initial presentation. The patient could have co-ingested this large dose of niacin with acetaminophen and/or other supplements, energy drinks, or alcohol. A combination such as this could have contributed to his hepatitis, and the metabolites of these other substances would have been eliminated by the time of his second ED presentation.
Discussion
There are over 900 different drugs, toxins, and supplements known to cause hepatic injury.11,12 Clinical manifestations of toxicity range from asymptomatic incidental elevations in transaminases to fulminant liver failure causing mortality. Ingestion of commonly used medications such as statins (although not in overdose quantities) can cause transient asymptomatic transaminitis.13 These elevations are usually mild—ie, less than twice the upper limit of normal. Patients who experience such elevations can usually continue to take the medications with frequent and vigilant monitoring of hepatic function.
Signs and Symptoms
Acute Liver Injury. Acute liver injury is diagnosed when AST and ALT levels are greater than twice the upper limit of normal. Patients also typically have mild-to-moderate abdominal findings, such as pain, nausea, and vomiting—as was experienced by our patient. Along with niacin, angiotensin-converting enzyme inhibitors, NSAIDs, and antifungal medications are examples of other medications that can cause this degree of drug-induced hepatitis.
Severe Liver Injury. Severe liver injury features elevations in not only AST and ALT, but also alkaline phosphate and bilirubin. Patients with severe hepatic injury appear clinically ill and may exhibit altered mental status and jaundice. This type of subfulminant hepatic failure commonly results from acetaminophen toxicity, anesthetic gases, iron toxicity, phosphorus toxicity, and cocaine toxicity. Examples of drugs that result in massive liver necrosis and fulminant hepatitis are acetaminophen, isoniazid, phenelzine, phenytoin, propylthiouracil, and sertraline. Patients with massive hepatic necrosis and hepatitis may require liver transplantation.
Etiology
Identifying the etiology of liver injury is made largely through the patient’s history because there are simply too many possible hepatotoxic agents to test for them all. Diagnostic suspicion of hepatic toxicity should be increased with signs of more serious disease; however, drug-induced liver injury should be included in the differential diagnosis for all cases of abdominal pain.
With respect to the patient in our case, obtaining a more complete history involving supplement and vitamin use would have allowed us to make the diagnosis in the ED. Unfortunately, these subtle aspects of a patient’s history are often overlooked in the emergent care setting.
Treatment
The treatment of niacin-induced liver injury is similar to the guidelines for treating most other drug-induced pathology.14 Removal of the offending agent and providing supportive care is the primary treatment modality.15 In addition, it is important that the clinician exclude and rule-out other causes of hepatitis such as those of viral, autoimmune, or ischemic etiology.
N-acetylcysteine. A medication classically used in patients with acetaminophen overdose, NAC is a safe and effective treatment for non-acetaminophen-induced liver injury, and was given to treat our patient.16L-carnitine. L-carnitine has been shown to be effective in cases of chronic steatosis from hepatitis C and in valproic acid induced hepatitis.17Since L-carnitine is not included on our hospital’s formulary, it was not a treatment option for our patient.Glucocorticoid Therapy. Although glucocorticoids are occasionally given to patients with systemic symptoms of drug reactions, its effectiveness has not been adequately studied.18
Prognosis
The prognosis of patients with acute drug-induced hepatitis is generally good, and most patients fully recover once the offending agent is removed. Poor prognostic factors include the presence of jaundice, requirement for dialysis, underlying chronic liver conditions, or elevated serum creatinine. While most patients will experience a complete recovery, approximately 5% to 10% will develop chronic hepatitis and/or cirrhosis.
Conclusion
Niacin is now available as prescription and OTC formulations and is a potentially hepatotoxic medication and dietary supplement. Niacin can cause an acute hepatitis, especially when taken in conjunction with other hepatotoxic substances. Drug-induced liver injury from niacin ingestion will improve quickly following removal, and the prognosis in otherwise healthy individuals is good.
Patients, especially young, healthy patients who present with symptoms concerning for hepatitis, should be asked specifically about any nutritional, herbal, or other supplement usage. During the history intake, many patients do not consider vitamins or other nutritional or herbal supplements as “medication” or as being significant, and only report prescription and OTC medications.
Niacin, also known as vitamin B3, is an important cofactor in many metabolic processes necessary to life. Over the past 15 to 20 years, niacin has been prescribed to patients with hyperlipidemia to increase high-density lipoprotein and lower low-density lipoprotein.1 As a naturally occurring vitamin, niacin is also available over-the-counter (OTC) as a dietary supplement, and is also a common ingredient in energy drinks and multivitamins.2
In addition to treating hyperlipidemia and as a nutritional supplement, some anecdotal reports amongst lay-persons suggests that niacin offers other health benefits, such as promoting weight loss and expediting the elimination of alcohol and illicit drugs from one’s system (eg, marijuana).3,4 The increased use of niacin supplementation in the general population for all of the aforementioned reasons has resulted in an increased incidence of niacin toxicity.
Formulations
Niacin is available in three formulations: extended-release (ER, also referred to as intermediate-release), immediate-release (IR), and sustained-release (SR).
The ER formulations of niacin are typically prescribed to treat hyperlipidemia. Patients are usually started on ER niacin at an initial dose of 250 mg once daily. The dose is gradually increased, as tolerated or necessary, to 2 g per day, taken in three doses. It is not uncommon for patients with hyperlipidemia to take more than 1 g of niacin per day after titration by their primary physicians.
Side Effects
Since niacin increases the release of arachidonic acid from cell membranes that metabolizes into prostaglandins, specifically prostaglandins E2 and D2, many patients taking niacin experience uncomfortable flushing and itching.5 Nonsteroidal anti-inflammatory drugs (NSAIDs) prevent this side effect by inhibiting the metabolism of arachidonic acid into those vasodilatory prostaglandins. The newer ER and SR formulations of niacin, which are approved for OTC use as a dietary supplement, are less likely to cause flushing.5
Extended-release niacin, however, is associated with a higher incidence of hepatotoxicity than the other prescription formulations of niacin.6 Toxicity has been well recognized in patients taking niacin chronically for hyperlipidemia, with reports of such cases dating back to the 1980s.7,8 We report a unique case of niacin toxicity following a single-dose ingestion in a young man.
Case
A 22-year-old man presented to the ED for evaluation of a 2-week history of intermittent periumbilical abdominal pain. This visit represented the patient’s second visit to the ED over the past week for the same complaint.
Upon presentation the patient’s vital signs were: blood pressure (BP), 113/64 mm Hg; heart rate, 82 beats/min; respiratory rate, 16 breaths/min; and temperature 36.6°C. Oxygen saturation was 100% on room air. The patient was otherwise healthy and had no significant recent or remote medical history. He denied taking any medications prior to his initial presentation, and reported only occasional alcohol use.
At the patient’s initial presentation 1 week earlier, he was diagnosed with acute gastroenteritis and treated with famotidine and ondansetron in the ED. The patient appeared well clinically at this visit, and laboratory values were within normal limits, including normal blood glucose and urinalysis.
The patient was discharged home from this first visit with prescriptions of famotidine and ondansetron, and was advised to follow up with his primary care physician in 1 week. Throughout the week after discharge from the ED, the patient experienced worsening abdominal pain, and he developed frequent nonbloody emesis, prompting his second presentation to the ED. At this second visit, the patient stated that he had taken one dose of ondansetron at home, without effect. He also noted subjective fevers, but had no diarrhea or melena.
Vital signs remained within normal limits with BPs ranging from 115 to 130 mm Hg systolic and 50 to 89 mm Hg diastolic. The patient was never tachycardic, tachypneic, febrile, or hypoxic. Physical examination was remarkable for periumbilical tenderness. The patient had no jaundice. A more thorough laboratory evaluation revealed elevated anion gap and blood urea nitrogen/creatinine values, and leukocytosis. The patient’s hepatic enzymes were also elevated, with aspartate aminotransferase (AST) over 2,000 U/L and alanine aminotransferase (ALT) of 1,698 U/L. Lipase, bilirubin, and alkaline phosphatase were all within normal limits. The patient’s prothrombin time (PT) was elevated at 14 seconds, and the international normalized ratio (INR) was elevated at 1.28. Laboratory analysis for acetaminophen and alcohol was negative.
A computed tomography (CT) scan of the abdomen/pelvis with intravenous (IV) contrast was unremarkable, demonstrating a liver devoid of any masses, portal or biliary dilation, or cirrhotic changes.
The patient received IV famotidine and ondansetron, and morphine for pain control, and was admitted to the general medical floor for hepatitis of uncertain etiology. A viral hepatitis panel was negative.
On the recommendation of the toxicology service, the patient was given N-acetylcysteine (NAC), and his hepatic enzymes trended down to an AST of 642 U/L and an ALT of 456 U/L by hospital day 2. (The patient essentially completed a positive dechallenge test).9
A gastroenterology consult was ordered, during which additional history-taking and chart review noted that the patient admitted to taking one or two tablets of OTC niacin as a dietary supplement the day before his initial presentation. Although the patient could not recall the exact dosage, he stated that he had been taking supplemental niacin approximately once a month over the past several years without any issues. Since OTC niacin is most commonly available in 500-mg tablets, this suggested the patient’s recent one-time ingested dose was approximately 500 to 1,000 mg.
Based on the patient’s admission to niacin use, additional studies were ordered, including an abdominal ultrasound and a urine drug screen. Ultrasound findings were unremarkable for portal venous thrombosis. The urine drug screen, however, was positive for marijuana and opiates. While the patient denied any history of opioid use, the positive opiate assay could have been attributed to the morphine given in the ED.
Throughout the patient’s hospital course, he remained normotensive and had no change in mental status. His liver enzymes, PT, and INR continued to normalize, and he was discharged home after 3 days, with instructions to follow up with the gastrointestinal clinic within 11 days. An appointment was made for him, which he did not attend.
Given the patient’s negative autoimmune and viral workup, and rapid resolution of symptoms after discontinuing niacin use, it is believed that he had an acute drug-induced hepatitis due to niacin ingestion. Regarding any coingestants that could have contributed to the hepatitis, the patient denied taking other common coingestants such as alcohol and acetaminophen; this assertion was supported by laboratory results.
Since we were unable to attain a qualitative measurement of the patient’s niacin concentration, our diagnosis was primarily based on the patient’s reported history.10 It is possible the patient had been taking more niacin than that to which he admitted, or that he was taking another hepatotoxic substance not detected on our toxicology workup. As previously noted, there are many medications and/or dietary supplements that could cause or contribute to a synergistic effect of drug-induced hepatitis for which the patient was not tested at his initial presentation. The patient could have co-ingested this large dose of niacin with acetaminophen and/or other supplements, energy drinks, or alcohol. A combination such as this could have contributed to his hepatitis, and the metabolites of these other substances would have been eliminated by the time of his second ED presentation.
Discussion
There are over 900 different drugs, toxins, and supplements known to cause hepatic injury.11,12 Clinical manifestations of toxicity range from asymptomatic incidental elevations in transaminases to fulminant liver failure causing mortality. Ingestion of commonly used medications such as statins (although not in overdose quantities) can cause transient asymptomatic transaminitis.13 These elevations are usually mild—ie, less than twice the upper limit of normal. Patients who experience such elevations can usually continue to take the medications with frequent and vigilant monitoring of hepatic function.
Signs and Symptoms
Acute Liver Injury. Acute liver injury is diagnosed when AST and ALT levels are greater than twice the upper limit of normal. Patients also typically have mild-to-moderate abdominal findings, such as pain, nausea, and vomiting—as was experienced by our patient. Along with niacin, angiotensin-converting enzyme inhibitors, NSAIDs, and antifungal medications are examples of other medications that can cause this degree of drug-induced hepatitis.
Severe Liver Injury. Severe liver injury features elevations in not only AST and ALT, but also alkaline phosphate and bilirubin. Patients with severe hepatic injury appear clinically ill and may exhibit altered mental status and jaundice. This type of subfulminant hepatic failure commonly results from acetaminophen toxicity, anesthetic gases, iron toxicity, phosphorus toxicity, and cocaine toxicity. Examples of drugs that result in massive liver necrosis and fulminant hepatitis are acetaminophen, isoniazid, phenelzine, phenytoin, propylthiouracil, and sertraline. Patients with massive hepatic necrosis and hepatitis may require liver transplantation.
Etiology
Identifying the etiology of liver injury is made largely through the patient’s history because there are simply too many possible hepatotoxic agents to test for them all. Diagnostic suspicion of hepatic toxicity should be increased with signs of more serious disease; however, drug-induced liver injury should be included in the differential diagnosis for all cases of abdominal pain.
With respect to the patient in our case, obtaining a more complete history involving supplement and vitamin use would have allowed us to make the diagnosis in the ED. Unfortunately, these subtle aspects of a patient’s history are often overlooked in the emergent care setting.
Treatment
The treatment of niacin-induced liver injury is similar to the guidelines for treating most other drug-induced pathology.14 Removal of the offending agent and providing supportive care is the primary treatment modality.15 In addition, it is important that the clinician exclude and rule-out other causes of hepatitis such as those of viral, autoimmune, or ischemic etiology.
N-acetylcysteine. A medication classically used in patients with acetaminophen overdose, NAC is a safe and effective treatment for non-acetaminophen-induced liver injury, and was given to treat our patient.16L-carnitine. L-carnitine has been shown to be effective in cases of chronic steatosis from hepatitis C and in valproic acid induced hepatitis.17Since L-carnitine is not included on our hospital’s formulary, it was not a treatment option for our patient.Glucocorticoid Therapy. Although glucocorticoids are occasionally given to patients with systemic symptoms of drug reactions, its effectiveness has not been adequately studied.18
Prognosis
The prognosis of patients with acute drug-induced hepatitis is generally good, and most patients fully recover once the offending agent is removed. Poor prognostic factors include the presence of jaundice, requirement for dialysis, underlying chronic liver conditions, or elevated serum creatinine. While most patients will experience a complete recovery, approximately 5% to 10% will develop chronic hepatitis and/or cirrhosis.
Conclusion
Niacin is now available as prescription and OTC formulations and is a potentially hepatotoxic medication and dietary supplement. Niacin can cause an acute hepatitis, especially when taken in conjunction with other hepatotoxic substances. Drug-induced liver injury from niacin ingestion will improve quickly following removal, and the prognosis in otherwise healthy individuals is good.
Patients, especially young, healthy patients who present with symptoms concerning for hepatitis, should be asked specifically about any nutritional, herbal, or other supplement usage. During the history intake, many patients do not consider vitamins or other nutritional or herbal supplements as “medication” or as being significant, and only report prescription and OTC medications.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213.
2. Vivekanandarajah A, Ni S, Waked A. Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep. 2011;5:227. doi:10.1186/1752-1947-5-227.
3. Niacin. U.S. National Library of Medicine. https://medlineplus.gov/druginfo/meds/a682518.html. Updated July 15, 2017. Accessed February 21, 2018.
4. Addiction Resource. Niacin flush for drug detox. https://addictionresource.com/drug-testing/niacin-drug-test/. Accessed February 20, 2018.
5. Kamanna VS, Ganji SH, Kashyap ML. The mechanism and mitigation of niacin-induced flushing. Int J Clin Pract. 2009;63(9):1369-1377. doi:10.1111/j.1742-1241.2009.02099.x.
6. Etchason JA, Miller TD, Squires RW, et al. Niacin-induced hepatitis: a potential side effect with low-dose time-release niacin. Mayo Clin Proc. 1991;66(1):23-28.
7. Patterson DJ, Dew EW, Gyorkey F, Graham DY. Niacin hepatitis. South Med J. 1983;76(2):239-241.
8. Ferenchick G, Rovner D. Hepatitis and hematemesis complicating nicotinic acid use. Am J Med Sci. 1989;298(3):191-193.
9. Henkin Y, Johnson KC, Segrest JP. Rechallenge with crystalline niacin after drug-induced hepatitis from sustained-release niacin. JAMA. 1990;264(2):241-243.
10. Barritt AS 4th, Lee J, Hayashi PH. Detective work in drug-induced liver injury: sometimes it is all about interviewing the right witness. Clin Gastroenterol Hepatol. 2010;8(7):635-637. doi:10.1016/j.cgh.2010.03.020.
11. Chitturi S, Teoh NC, Farrell GC. Hepatic drug metabolism and liver disease caused by drugs. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 10th ed. Philadelphia, PA: Elsevier Saunders; 2016:1442-1477.
12. National Institutes of Health. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Niacin. https://livertox.nih.gov/Niacin.htm. Updated January 18, 2018. Accessed February 21, 2018.
13. Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4):690-695.
14. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966. doi:10.1038/ajg.2014.131.
15. Larson AM. Hepatotoxicity due to herbal medication and dietary supplements. UpToDate Web site. https://www.uptodate.com/contents/hepatotoxicity-due-to-herbal-medications-and-dietary-supplements?source=search_result&search=niacin%20induced%20hepatitis&selectedTitle=3~150. Updated December 21, 2017. Accessed February 21, 2018.
16. Mumtaz K, Azam Z, Hamid S, et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int. 2009;3(4):563-570. doi:10.1007/s12072-009-9151-0.
17. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293. doi:10.1345/aph.1P135.
18. O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439-445.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213.
2. Vivekanandarajah A, Ni S, Waked A. Acute hepatitis in a woman following excessive ingestion of an energy drink: a case report. J Med Case Rep. 2011;5:227. doi:10.1186/1752-1947-5-227.
3. Niacin. U.S. National Library of Medicine. https://medlineplus.gov/druginfo/meds/a682518.html. Updated July 15, 2017. Accessed February 21, 2018.
4. Addiction Resource. Niacin flush for drug detox. https://addictionresource.com/drug-testing/niacin-drug-test/. Accessed February 20, 2018.
5. Kamanna VS, Ganji SH, Kashyap ML. The mechanism and mitigation of niacin-induced flushing. Int J Clin Pract. 2009;63(9):1369-1377. doi:10.1111/j.1742-1241.2009.02099.x.
6. Etchason JA, Miller TD, Squires RW, et al. Niacin-induced hepatitis: a potential side effect with low-dose time-release niacin. Mayo Clin Proc. 1991;66(1):23-28.
7. Patterson DJ, Dew EW, Gyorkey F, Graham DY. Niacin hepatitis. South Med J. 1983;76(2):239-241.
8. Ferenchick G, Rovner D. Hepatitis and hematemesis complicating nicotinic acid use. Am J Med Sci. 1989;298(3):191-193.
9. Henkin Y, Johnson KC, Segrest JP. Rechallenge with crystalline niacin after drug-induced hepatitis from sustained-release niacin. JAMA. 1990;264(2):241-243.
10. Barritt AS 4th, Lee J, Hayashi PH. Detective work in drug-induced liver injury: sometimes it is all about interviewing the right witness. Clin Gastroenterol Hepatol. 2010;8(7):635-637. doi:10.1016/j.cgh.2010.03.020.
11. Chitturi S, Teoh NC, Farrell GC. Hepatic drug metabolism and liver disease caused by drugs. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 10th ed. Philadelphia, PA: Elsevier Saunders; 2016:1442-1477.
12. National Institutes of Health. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Niacin. https://livertox.nih.gov/Niacin.htm. Updated January 18, 2018. Accessed February 21, 2018.
13. Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4):690-695.
14. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966. doi:10.1038/ajg.2014.131.
15. Larson AM. Hepatotoxicity due to herbal medication and dietary supplements. UpToDate Web site. https://www.uptodate.com/contents/hepatotoxicity-due-to-herbal-medications-and-dietary-supplements?source=search_result&search=niacin%20induced%20hepatitis&selectedTitle=3~150. Updated December 21, 2017. Accessed February 21, 2018.
16. Mumtaz K, Azam Z, Hamid S, et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int. 2009;3(4):563-570. doi:10.1007/s12072-009-9151-0.
17. Perrott J, Murphy NG, Zed PJ. L-carnitine for acute valproic acid overdose: a systematic review of published cases. Ann Pharmacother. 2010;44(7-8):1287-1293. doi:10.1345/aph.1P135.
18. O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97(2):439-445.
Invasive Penile Squamous Cell Carcinoma
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.
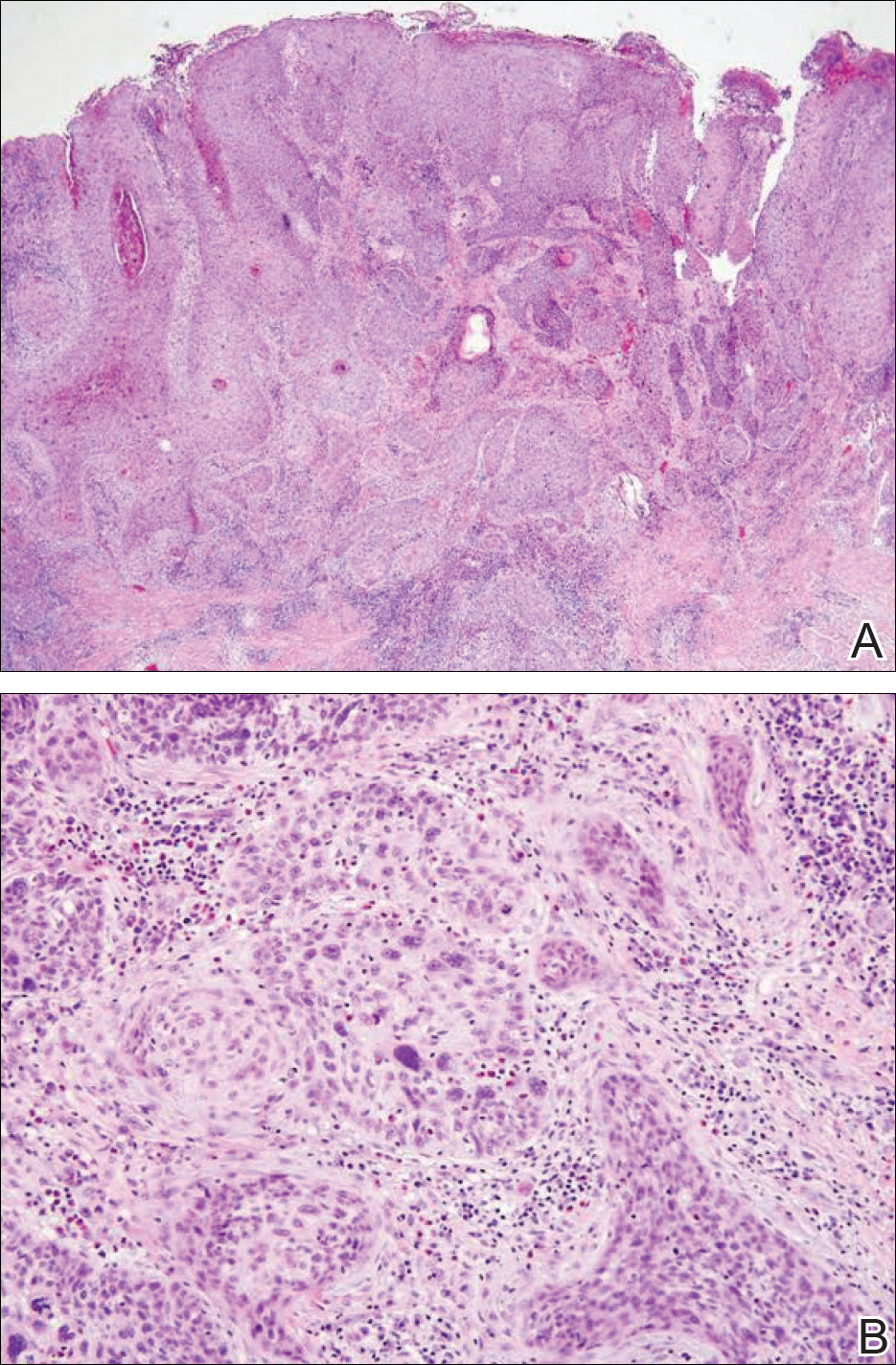
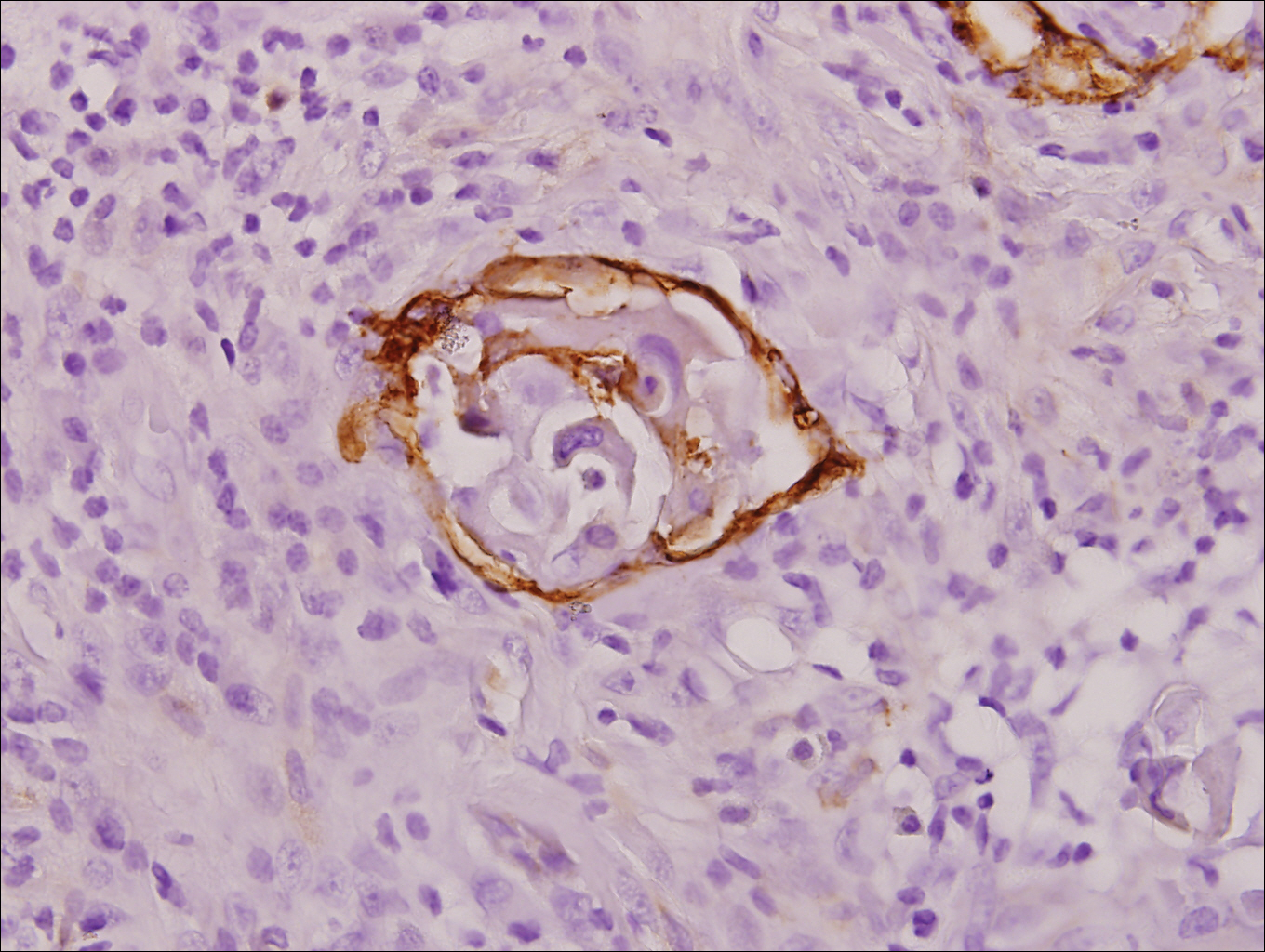
Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
Invasive penile cancer is a rare malignancy with considerable morbidity and mortality. The American Cancer Society estimates that there will be 2320 new cases of invasive penile cancer in the United States in 2018, of which primary penile squamous cell carcinoma (PSCC) represents the majority.1 In one study, the mean age at diagnosis was 60 years, with PSCC occurring only rarely in men younger than 35 years of age (estimated incidence, 0.01 cases per 100,000 individuals).2 Presentation to a physician generally occurs more than 1 year after initial onset of symptoms or clinical lesion(s). This delay in diagnosis and treatment often results in disease progression,3 which can have a devastating outcome.4 Therefore, physicians should maintain a high index of clinical suspicion for PSCC, particularly in young or middle-aged patients in whom presentation of PSCC is uncommon. The most commonly associated risk factors for PSCC include lack of circumcision (specifically during the neonatal period), high-risk human papillomavirus (HPV) infection, and tobacco use.5 Chronic alcoholism also has been linked to PSCC.6 It also is common in patients without health insurance.7 We report the case of a 27-year-old circumcised man who presented with invasive PSCC following a diagnosis of condyloma 8 years prior by an outside physician.
Case Report
A 27-year-old man presented for evaluation of persistent genital warts that had been diagnosed 8 years prior. His medical history was remarkable for intravenous drug use, active hepatitis C infection, tobacco smoking, chronic alcohol use, and mild asthma. Eight years prior to the current presentation, 7 lesions had developed on the penis and were diagnosed by an outside physician as condyloma, which was treated with cryotherapy and topical imiquimod. All of the lesions except for 1 responded to treatment. The residual lesion continued to grow until the size prompted him to contact his primary care physician, who referred him for dermatologic evaluation. The patient cited lack of health insurance as the primary reason he did not seek follow-up treatment after the initial evaluation and treatment 8 years prior.
Physical examination at the current presentation revealed a circumcised man with an asymptomatic, 2.6-cm, pink, friable, verrucous mass on the left lateral penile shaft (Figure 1) and otherwise unremarkable penile architecture. A clinically enlarged, nontender right inguinal lymph node was noted as well as subtle enlargement of a left inguinal lymph node. An excisional biopsy was performed with pathologic evaluation confirming a diagnosis of high-grade invasive squamous cell carcinoma (SCC) arising in the setting of squamous cell carcinoma in situ (Figure 2). Lymphovascular invasion was highlighted on cluster of differentiation 31 and podoplanin immunostaining (Figure 3). The patient was subsequently referred to urology and hematology-oncology specialists for further evaluation. Computed tomography (CT) of the abdomen and pelvis confirmed the contralaterally enlarged right inguinal lymph node discovered during physical examination and mildly enlarged ipsilateral inguinal, obturator, and external iliac nodes. Computed tomography–guided fine-needle aspiration of the right inguinal node confirmed the diagnosis of contralateral locoregional metastasis. Further evaluation with positron emission tomography/CT imaging revealed only a single metabolically active region confined to the right inguinal node. The patient’s history of active hepatitis C complicated proposed neoadjuvant chemotherapy regimens. Ultimately, after discussion with multiple surgical and oncologist specialties within our institution and others, a treatment plan was formulated. The patient underwent robotic laparoscopic bilateral pelvic and inguinal lymph node dissection and re-excision of the primary PSCC, with one of 15 right superficial inguinal nodes testing positive for tumor cells; the left superficial and bilateral deep inguinal lymph nodes were negative for SCC.



Repeat positron emission tomography/CT imaging at 6 months’ follow-up showed no evidence of active disease. On 1-year follow-up, a CT scan did not show any new or residual disease, but the patient continued to have edema of the bilateral legs, which began after lymph node dissection and was managed with physical therapy and compression stockings.
Comment
Prevalence
Penile cancer is rare in industrialized countries. Early detection is a critical factor for both overall survival and organ function. If successful interventions are to be made, physicians should be familiar with known risk factors as well as unusual presentations, such as lesions presenting in young circumcised men, as reported above. Similarly, tumors located on the shaft of the penis represent an uncommon location for tumor presentation, occurring in less than 5% of PSCC cases.8 Penile SCC most commonly develops as a solitary painless lesion on the glans, balanopreputial sulcus and/or prepuce.9 In our case, histopathology confirmed high-grade invasive SCC arising from squamous cell carcinoma in situ, an entity generally associated with older men with a 10% to 20% rate of progression into invasive SCC.9 Our patient denied any clinical change in the appearance of the tumor in the years prior to the current presentation, making it possible that the condyloma treated 8 years prior was squamous cell carcinoma in situ or PSCC. As many as 25% of premalignant lesions are mistaken for benign lesions, which can thus delay treatment and allow progression to malignancy.10
Diagnosis
Penile SCC often is etiologically subcategorized into 2 pathways based on HPV dependence or independence. Recent research suggests that this distinction often is difficult to make, and accurate laboratory and pathologic confirmation of HPV DNA, intact virions, and viral-related cutaneous changes is not always possible, leading to much speculation regarding the exact role of HPV in tumorigenesis.11 Cancers developing in the absence of HPV DNA often occur secondary to chronic inflammatory conditions such as lichen planus or lichen sclerosus. Human papillomavirus DNA has shown to be present in 70% to 100% of all SCC in situ of the penis11; therefore, the transformation of in situ disease to an invasive tumor in our patient most likely occurred via an HPV-dependent pathway. Viral carcinogenesis in the HPV-dependent pathway involves inactivation of host cell cycle regulatory proteins, specifically the retinoblastoma and p53 regulatory proteins by the viral oncoproteins E7 and E6, respectively.12,13 Human papillomavirus–dependent pathways are related to a patient’s age at first sexual intercourse, number of sexual partners, and history of condyloma and other sexually transmitted diseases.14,15 High-risk HPV types 16 and 18 are the most common viral types found in HPV related premalignant lesions, making it possible to decrease the incidence of PSCC with recently developed vaccines.16 Human papillomavirus vaccines have been shown to reduce the incidence of anal intraepithelial neoplasias and genital warts in men.17 While the effects of the HPV vaccine on reducing PSCC could not be assessed in the study due to low incidence of disease (both in the study population and in general), it is thought that HPV vaccination could potentially decrease the incidence of all PSCCs by one third, making it an important resource in the primary prevention of the disease.18
Management
Contemporary surgical management of PSCC has evolved from organ resection in toto for all PSCCs to a more conservative approach based upon tumor stage and grade. The standard margin for surgical resection of PSCC is 2 cm, a procedure often referred to as a partial penectomy. This remains the most common procedure for surgical resection of PSCC and has achieved good local control, with reported recurrence rates of 4% to 8%.19,20 Complication rates of the procedure are moderate one-third of patients experiencing compromise of sexual activity after surgery.21 With evidence that smaller resection margins may result in good local control and a lower incidence of postoperative functional impairment, resection margins of 5, 10, and 15 mm have been advocated for PSCCs of varying histologic grades and tumor stages.22-24 Treatment options for T1 and in situ tumors have expanded to include glansectomy, margin-controlled Mohs micrographic surgery, and ablative laser therapy for local disease control.5,20 More advanced tumors are still treated with partial or complete penectomy given the high risks for locoregional recurrence and distant spread.
Prognosis
The most important factor predicting survival in patients with PSCC is metastasis to inguinal lymph nodes. The 5-year survival rate for patients without nodal involvement is 85% to 100%, while those with pathologically positive lymph nodes have a 5-year survival rate of 15% to 45%.25 Once distant metastasis occurs, the mean time of survival is 7 to 10 months.26 Our patient presented with high-grade PSCC with histologic lymphovascular spread and palpable inguinal lymph nodes. When stratified with other similar cases at presentation, our patient was at a considerable risk for locoregional as well as distant metastasis. Management with regional nodal dissection with a plan for close observation (and deferment of chemotherapeutics) was based upon evaluations from multiple different medical specialties.
Conclusion
Invasive PSCC is rare in young circumcised adults, and a delay in diagnosis can lead to considerable morbidity and mortality. We present a case of invasive PSCC arising in the setting of squamous cell carcinoma in situ in an area previously treated with cryotherapy and imiquimod. Our patient’s young age, concurrent hepatitis C infection, and contralateral locoregional nodal metastasis made this a complex case, involving evaluation and treatment by multiple medical disciplines. This case highlights the importance of biopsy in any lesion recalcitrant to conventional modalities regardless of the patient’s age. Early detection and treatment of PSCC can prevent organ dysfunction, loss of organ, and even death.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
- About penile cancer. American Cancer Society website. https://www.cancer.org/content/dam/CRC/PDF/Public/8783.00.pdf. Revised February 9, 2016. Accessed February 27, 2018.
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361-367.
- Koifman L, Vides AJ, Koifman N, et al. Epidemiological aspects of penile cancer in Rio de Janeiro: evaluation of 230 cases. Int Braz J Urol. 2011;37:231-240.
- Kamat AM, Carpenter SM, Czerniak BA, et al. Metastatic penile cancer in a young Caucasian male: impact of delayed diagnosis. Urol Oncol. 2005;23:130-131.
- Deem S, Keane T, Bhavsar R, et al. Contemporary diagnosis and management of squamous cell carcinoma (SCC) of the penis. BJU Int. 2011;108:1378-1392.
- McIntyre M, Weiss A, Wahlquist A, et al. Penile cancer: an analysis of socioeconomic factors at a southeastern tertiary referral center. Can J Urol. 2011;18:5524-5528.
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19-24.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(suppl 10):2883-2891.
- Ferrandiz-Pulido C, de Torres I, Garcia-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:478-487.
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559-565.
- Mannweiler S, Sygulla S, Winter E, et al. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16, HPV-negative cancers associated with dermatoses express p53, but lack p16 overexpression. J Am Acad Dermatol. 2013;69:73-81.
- Scheffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76-79.
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606-616.
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol. 2009;27:141-150.
- Shabbir M, Barod R, Hegarty PK, et al. Primary prevention and vaccination for penile cancer. Ther Adv Urol. 2013;5:161-169.
- Palefsky J, Giuliano A, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576-1585.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449-457.
- Korets R, Koppie TM, Snyder ME, et al. Partial penectomy for patients with squamous cell carcinoma of the penis: the Memorial Sloan-Kettering experience. Ann Surg Oncol. 2007;14:3614-3619.
- Zukiwskyj M, Daly P, Chung E. Penile cancer and phallus preservation strategies: a review of current literature. BJU Int. 2013;112(suppl 2):21-26.
- Romero FR, Romero KR, Mattos MA, et al. Sexual function after partial penectomy for penile cancer. Urology. 2005;66:1292-1295.
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040-1043.
- Feldman AS, McDougal WS. Long-term outcome of excisional organ sparing surgery for carcinoma of the penis. J Urol. 2011;186:1303-1307.
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol. 2012;188:803-808.
- Brady KL, Mercurio MG, Brown MD. Malignant tumors of the penis. Dermatol Surg. 2013;39:527-547.
- Ornellas AA, Nobrega BL, Wei Kin Chin E, et al. Prognostic factors in invasive squamous cell carcinoma of the penis: analysis of 196 patients treated at the Brazilian National Cancer Institute. J Urol. 2008;180:1354-1359.
Practice Points
- Invasive penile squamous cell carcinoma (PSCC) is a rare malignancy with considerable morbidity and mortality that typically does not present in young men.
- Delayed or incorrect diagnosis of PSCC can have a devastating outcome; therefore, physicians should maintain a high index of clinical suspicion for PSCC in patients presenting with penile lesions, particularly in young or middle-aged patients.
Diffuse Cutaneous Breast Cancer Metastases Resembling Subcutaneous Nodules With No Surface Changes
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.
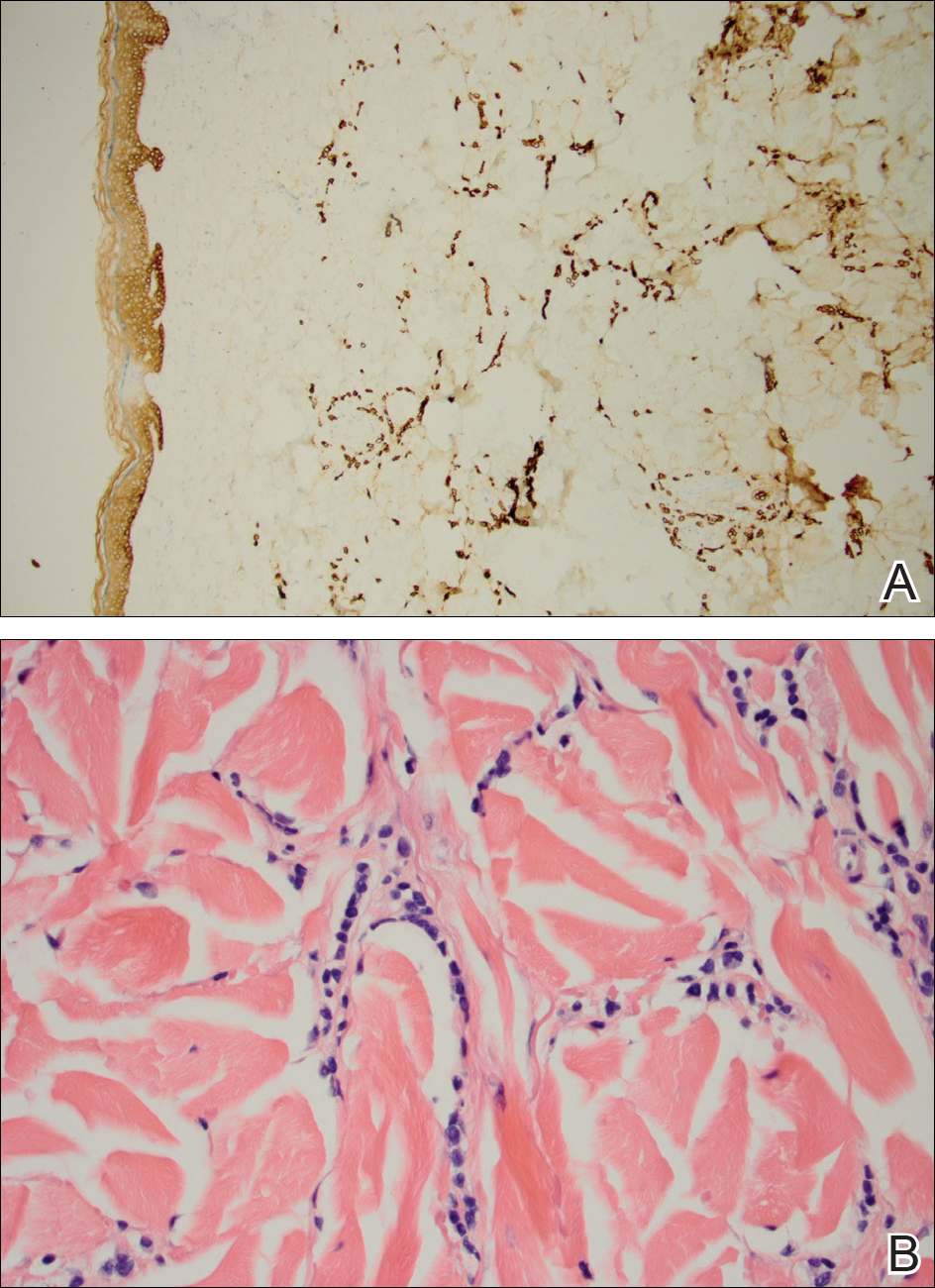
After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.
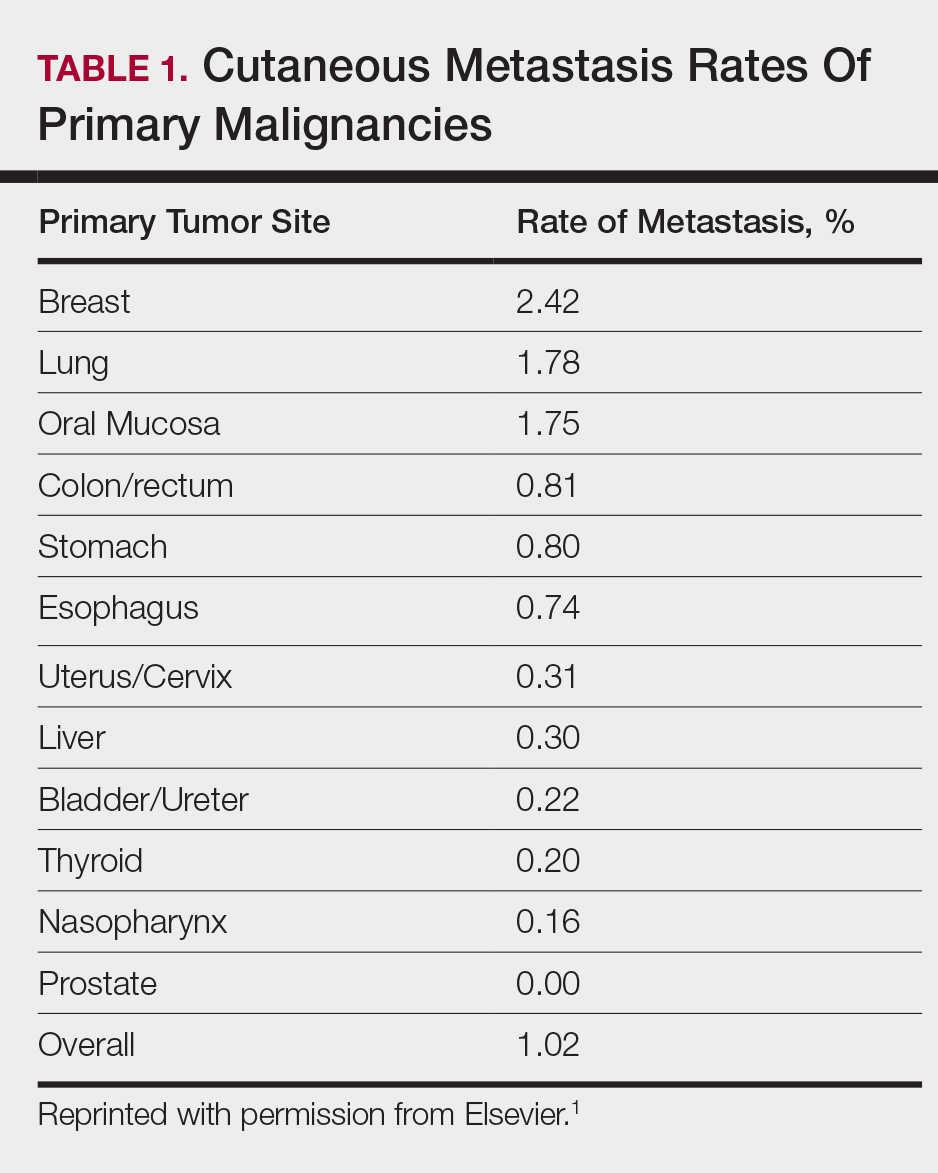
The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.
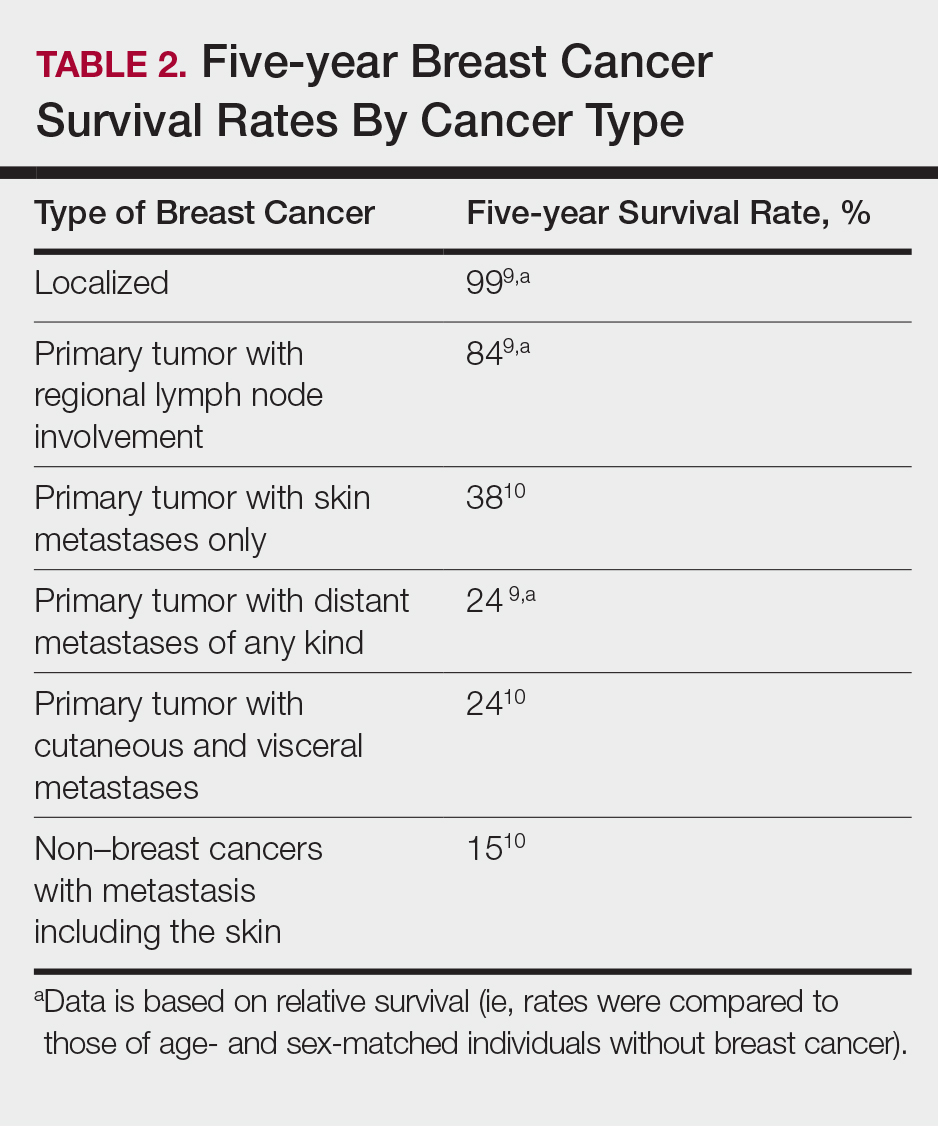
Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
Cutaneous metastases from solid tumors in general occur at a rate of about 1% per primary tumor.1 In breast cancer, cutaneous metastases occur at a rate of about 2.5% per primary tumor. Because of the high incidence of breast cancers relative to other internal malignancies, breast cancer accounts for almost 33% of all cutaneous metastases.2 Infiltrating ductal carcinoma accounts for almost 70% of cutaneous metastases from breast cancers, whereas lobular carcinoma accounts for about 15%.
Cutaneous metastases may be the first presenting sign of primary malignancy. In one retrospective study, 6% of breast carcinomas (N=992) initially presented with only skin manifestations.3 Clinical appearance can vary, but cutaneous metastases from breast adenocarcinomas often present as isolated dermal nodules with superficial discoloration or changes in texture. The most common location of cutaneous metastases is on the chest ipsilateral to the primary breast malignancy.4 We pre-sent a case of metastatic adenocarcinoma of the breast presenting with diffuse cutaneous nodules with no surface changes.
Case Report
A 64-year-old woman who was otherwise in good health presented to her primary care physician for evaluation of recent-onset fatigue. Laboratory testing revealed that she was mildly anemic with mild thrombocytopenia and lymphocytosis. She was referred to a hematologist, who ordered flow cytometry and cytogenetic testing. Blood abnormalities were not considered severe enough to warrant a bone marrow biopsy, and she was monitored clinically for the next 2 years.
Two years after the initial presentation, the primary care physician performed a breast examination that was unremarkable, but enlarged axillary lymph nodes up to 15 mm were discovered in the right breast during routine breast ultrasonography. Additionally, she noted that she had experienced unintentional weight loss of 10 lb over the past year. The hematologist suspected a low-grade lymphoma and performed a bone marrow biopsy. The immunohistochemistry of the bone marrow specimen was consistent with an estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma, which was then confirmed in the right breast on magnetic resonance imaging. The patient denied any history of prior radiation treatment, but she disclosed a family history of breast cancer in her cousin.
Several weeks after the bone marrow biopsy, an oncologist found that the patient also had an abdominal mass and bone metastases of the primary breast cancer. Colonoscopy confirmed metastases to the colon that subsequently led to obstruction and ultimately required a right hemicolectomy. The patient’s oncologist started her on anastrozole, an aromatase inhibitor (AI), for treatment of the metastatic breast cancer and zoledronic acid, a bisphosphonate, along with calcium and vitamin D for the bone involvement.
Shortly after, during a routine annual skin examination, the patient’s dermatologist (H.T.N.) discovered 3 soft, fixed, subcutaneous-appearing nodules—one on the right chest that was 15 mm in diameter, one on the left mid back that was 7 mm, and one on the left upper anterior thigh that was 10 mm. They were discrete with well-defined borders but had only minimal elevation, making them difficult to detect clinically, especially without palpation. The nodules were not visibly apparent because they were flesh-colored with no surface discoloration or texture changes. The patient remembered that the lesions had appeared gradually several months prior, predating the breast cancer diagnosis, and were not associated with pain, itching, or burning, so she was not alarmed by their appearance and never sought medical attention. The dermatologist (H.T.N.) recommended a biopsy at the time of the skin examination, but the patient declined.
One year after the appearance of the first skin lesions, 14 more nodules (Figure 1) progressively erupted on the ipsilateral and contralateral chest (Figure 2A), axillae, arms, shoulders, back (Figure 2B), and thighs (Figure 2C). At this point, the dermatologists performed a punch biopsy on a lesion on the back to confirm the suspicion of cutaneous metastasis of the primary breast cancer. The biopsy showed interstitial dermal proliferation of atypical cells between collagen bundles and stained strongly positive for cytokeratin 7, an epithelial protein common in breast adenocarcinoma (Figure 3). Further immunohistochemical staining returned metastatic estrogen receptor–positive, progesterone receptor–negative, human epidermal growth factor receptor 2–negative invasive lobular breast carcinoma. Therefore, the markers for the cutaneous metastases were consistent with the markers for the original breast cancer.



After 1 year of treatment with anastrozole, the patient’s internal metastases had not changed considerably, but the cutaneous metastases continued to grow—the lesion on the left thigh doubled from 10 to 20 mm in diameter, and new nodules developed on the chest, back, arms, and legs. One year and a half after the initial lesions were documented, several nodules had disappeared and several new ones appeared. The remaining nodules remained relatively constant in size.
After stopping anastrozole, the patient was enrolled in a research trial using bortezomib, a chemotherapeutic agent typically used for multiple myeloma, as well as fulvestrant, an estrogen receptor antagonist; however, because of continued progression of the metastatic cancer, the patient was removed from the trial and switched to the established regimen of everolimus, a chemotherapeutic agent, and exemestane, another AI. Everolimus eventually was stopped, but the patient continued on exemestane as monotherapy. In addition to development of pleural disease, the cutaneous metastases continued to progress. The patient did not receive any local treatment for her cutaneous metastases.
Comment
Typically, cutaneous metastases of breast cancer manifests as a 1- to 3-cm, asymptomatic, firm, pink to red-brown nodule on the chest ipsilateral to the primary tumor. There may be more than 1 nodule, and ulceration may be present.5,6 In addition to nodular metastases, which make up 47% of cases (N=305), other common presentations include alopecia neoplastica (12%), telangiectatic carcinoma (8%), melanomalike lesions (6%), carcinoma erysipeloides (6%), subungual lesions (5%), carcinoma en cuirasse (4%), and zosteriform metastases (4%).6
Although nodular metastases are the most common type of cutaneous breast cancer metastases, our case is unique in that the patient had soft nodules dispersed to both arms and legs, and the nodules had no surface changes. Although cutaneous metastases can present as flesh-colored nodules,7 they typically have an erythematous base, a slight change in coloration, or induration. Additionally, cutaneous metastases most often are few in number and appear in close proximity to the primary breast adenocarcinoma.8 Without the detection of a slight soft elevation on palpation, our patient’s nodules were practically indistinguishable from the normal skin.
Among common internal cancers, breast cancer is the most likely to metastasize to the skin at a rate of 2.42% per primary tumor (Table 1).1 Cutaneous metastases from lobular carcinomas are much rarer than those from ductal carcinomas.4 The metastases also are most often located locally on the chest ipsilateral to the primary malignancy. Distant metastases are relatively rare. In a review of 212 cases of breast cancer patients with skin metastases, only 9 had involvement of the legs and only 4 had involvement of the contralateral chest.4 Our patient had involvement of the ipsilateral chest, both arms and legs, and the contralateral chest.

The 5-year relative survival rate for breast cancer patients varies based on the stage at diagnosis (99% in patients with localized cancer, 84% with regional lymph node involvement, 24% with distant metastases of any kind).9 In a study of 141 patients with cutaneous metastases in a Taiwanese medical center, Hu et al10 found that patients with breast cancer with only cutaneous metastases had a 5-year absolute survival rate of 38%. In the same study, patients with non–breast cancer metastasis including cutaneous metastasis had a 5-year survival rate of 15%.10 This data is summarized in Table 2.

Breast cancer metastasis to soft tissue (eg, the skin) typically indicates a better prognosis than breast cancer metastasis to a visceral organ or bone. In a study of 439 patients with metastatic relapse after surgical resection of a primary breast cancer, those who had soft tissue metastases had a median survival period of 39 months, whereas those who had visceral or bone metastases had a median survival period of 13 and 28 months, respectively.11 Furthermore, cutaneous metastases from breast cancers do not necessarily indicate as poor a prognosis as skin metastases from other internal malignancies. Cutaneous metastases from other internal malignancies carry a relative risk of mortality of 4.3 compared to cutaneous metastases from breast cancer.10
Treatment of cutaneous metastases may be medically or cosmetically indicated. Standard treatments for cutaneous metastases from the breast include surgical excision, external beam radiotherapy, and systemic chemotherapy.6 While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response to hormone therapy or chemotherapy,12 the response may be poorer due to the skin’s relatively weaker blood supply.13
Our patient was first prescribed anastrozole, an AI. For metastatic hormone receptor–positive breast cancer, AIs are a first-line therapy in postmenopausal women. In one meta-analysis, AIs showed greater improvement of survival rates relative to other endocrine therapies such as tamoxifen, an estrogen receptor antagonist (hazard ratio of 0.87).14 After stopping anastrozole, the patient was prescribed fulvestrant, another estrogen receptor antagonist, along with a trial drug. In a randomized, double-blind, placebo-controlled trial, fulvestrant was found to be an effective second-line treatment after anastrozole for hormone receptor–positive breast cancer in postmenopausal women.15 Our patient was then started on everolimus, a chemotherapeutic agent, and exemestane, another AI. After first-line treatment with anastrozole, this regimen also has been found to be an effective second-line treatment with improved progression-free survival.16 For the bone metastases, our patient was treated with zoledronic acid, a bisphosphonate. In a meta-analysis, bisphosphonates were found to reduce skeletal-related complications by a median of 28% in breast cancer patients with bone metastases.17
Some promising new local treatments for cutaneous breast metastases include topical imiquimod and electrochemotherapy. In a small study of 10 patients whose malignancies were refractory to radiotherapy, imiquimod achieved a partial response in 20% (2/10) of patients.18 In another study, 12 patients received electrochemotherapy involving electroporation (applying an electrical field to increase cell membrane permeability and thus increase drug uptake) followed by local administration of bleomycin, an antineoplastic agent. Seventy-five percent (9/12) of the patients received a complete response with disappearance of the metastases.19
This case report provides a rare presentation of diffuse nodular cutaneous metastases of breast adenocarcinoma with no surface changes. The subtle clinical findings in our patient demonstrate the spectrum of clinical manifestations for cutaneous metastases. Our case also serves to highlight the need for close inspection of the skin, including palpation in patients with a history of internal malignancy.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
- Hu SC, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Wong CY, Helm MA, Helm TN, et al. Patterns of skin metastases: a review of 25 years’ experience at a single cancer center. Int J Dermatol. 2014;53:56-60.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma: a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2, part 1):228-236.
- Gan DEH, Teh YC, Ng CH, et al. Cutaneous metastases of breast cancer: a case report. Breast Case. 2012;1:23-36.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Vano-Galvan S, Moreno-Martin P, Salguero I, et al. Cutaneous metastases of breast carcinoma: a case report. Cases J. 2009;2:71.
- Dacso M, Soldano AC, Talbott LB, et al. A solitary neck nodule as late evidence of recurrent lobular breast carcinoma. Case Rep Oncol. 2009;2:24-29.
- Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2010. Table 1.5 Age-Adjusted SEER Incidence and U.S. Death Rates and 5-Year Relative Survival (Percent) By Primary Cancer Site, Sex and Time Period. Bethesda, MD: National Cancer Institute; 2013. https://seer.cancer.gov/archive/csr/1975_2010/results_merged/topic_survival.pdf. Updated June 14, 2014. Accessed February 27, 2018.
- Hu SC, Chen GS, Lu YW, et al. Cutaneous metastases from different internal malignancies: a clinical and prognostic appraisal. J Eur Acad Dermatol Venereol. 2008;22:735-740.
- Insa A, Lluch A, Prosper F, et al. Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat. 1999;56:67-78.
- Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247.
- Kamble R, Kumar L, Kochupillai V, et al. Cutaneous metastases of lung cancer. Postgrad Med J. 1995;71:741-743.
- Mauri D, Pavlidis N, Polyzos N, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285-1291.
- Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664-1670.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2012;366:520-529.
- Wong MH, Stockler M, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474.
- Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748-6757.
- Benevento R, Santoriello A, Perna G, et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12(suppl 1):S6.
Practice Points
- Although breast cancer has the highest rate of cutaneous metastasis among internal malignancies, cutaneous metastases occur in only a small minority of breast cancer patients.
- Cutaneous metastases from breast cancer typically do not carry as poor a prognosis as those in other internal malignancies.
- The clinical presentation of cutaneous metastases from breast cancer can be varied. In our patient, the metastases were subtle and resembled subcutaneous nodules lacking surface changes, thus making them best detectable by palpation.
- While oncologists can use the response of cutaneous metastases to treatment as an indicator of systemic response, the cutaneous response may be poorer due to the skin’s relatively weaker blood supply.
Linear Terra Firma–Forme Dermatosis of the Midline Back
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.
Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.


Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
Terra firma–forme dermatosis (TFFD) was first described by Duncan et al,1 in 1987 and is characterized by brown to black pigmented plaques on the skin that cannot be removed with soap and water but are easily wiped away with isopropyl alcohol. Since that publication, relatively few case reports and case series have been published. We present a case of linear TFFD on the midline back of a 46-year-old woman.
Case Report
A 46-year-old woman presented to our clinic for evaluation of a lesion on the back that had been present for 3 years. An initial diagnosis of acanthosis nigricans or lichen simplex chronicus was made and treatment with topical triamcinolone cream 0.1% was initiated. However, after 8 months of treatment, no improvement was observed and the patient returned to our clinic. Her medical history was notable for obesity, type 2 diabetes mellitus, and hypertension. The patient stated that she maintained good hygiene, including daily to twice-daily showers with soap. Physical examination revealed a linear, hyperkeratotic, dark-brown plaque on the midline back extending from the top of the sacrum to the upper back (Figure 1). No other areas of skin involvement were noted. The hyperpigmented scales were easily removed with an isopropyl alcohol swab, which confirmed a diagnosis of TFFD (Figure 2). The patient was given ammonium lactate lotion 12% to apply to the lesion once daily using an applicator stick if the lesion recurred. She reported some improvement during this treatment. She occasionally had recurrent lesions, which were removed with isopropyl alcohol on subsequent dermatology visits.


Comment
Terra firma–forme dermatosis is an idiopathic condition that, although benign, can cause notable distress to patients. It presents clinically as asymptomatic, brown or black, hyperpigmented, hyperkeratotic, verrucous, or papillomatous plaques or light scaling in some cases.1-4 It can be readily cleared by rubbing with isopropyl alcohol but is resistant to ordinary soap and water.1
Recent reports have shown that TFFD may be more common than once thought.4-6 Although commonly observed in children, TFFD has been reported over a wide range of ages (4–86 years).2-5 The face, ankles, neck, and trunk are the most commonly affected areas.4,7,8 Areas that are less commonly affected often include surgical incision sites as well as the scalp, axillae, back, umbilical area, pubic area, arms, and legs.2-4,8,9 The lesions may be generalized or localized and are sometimes found to be symmetrical.4,10,11
The exact etiology of TFFD is unknown but is believed to be due to melanin retention and alteration or a delay of keratinization that leads to the buildup and compaction of scales.1,2,12 Poor hygiene generally is considered to exclude the diagnosis of TFFD in favor of dermatitis neglecta.6,12,13 Histopathology typically shows epidermal acanthosis, lamellar hyperkeratosis, and orthokeratotic whorls.3,7 However, biopsies seldom are performed due to the ease of diagnosis by removal by cleaning the lesion with isopropyl alcohol.
The diagnosis is confirmed by resolution of the rash after cleaning with isopropyl alcohol.1 Further confirmation of this diagnosis can be achieved through dermoscopy, as large, polygonal, platelike, brown scales can be found arranged together giving a mosaic pattern.6 In addition to cleaning with isopropyl alcohol,5,8 other treatments have shown efficacy for more resistant cases of TFFD, including topical keratolytic agents (eg, lactic acid, urea lotion).4,14
Conclusion
Terra firma–forme dermatosis is a condition that if recognized early, may provide treatment satisfaction through immediate removal of the lesions. Physicians should keep TFFD in their differential during evaluation of patients with asymptomatic, hyperpigmented, hyperkeratotic plaques. Awareness of TFFD is important, as early diagnosis can prevent unnecessary treatment and diagnostic workup.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
- Duncan CW, Tschen JA, Knox JM. Terra firma-forme dermatosis. Arch Dermatol. 1987;123:567-569.
- Browning J, Rosen T. Terra firmaforme dermatosis revisited. Dermatol Online J. 2005;11:11-13.
- Ashique KT, Kaliyadan F, Goyal T. Terra firma-forme dermatosis: report of a series of 11 cases and a brief review of the literature. Int J Dermatol. 2016;55:769-774.
- Berk DR. Terra firma-forme dermatosis: a retrospective review of 31 patients. Pediatr Dermatol. 2012;29:297-300.
- Greywal T, Cohen PR. Terra firma-forme dermatosis: a report of ten individuals with Duncan’s dirty dermatosis and literature review. Dermatol Pract Concept. 2015;5:29-33.
- Abdel-Razek MM, Fathy H. Terra firm-forme dermatosis: case series and dermoscopic features. Dermatol Online J. 2015;21:4-7.
- Akkash L, Badran D, Al-Omari AQ. Terra firma forme dermatosis. case series and review of the literature. J Dtsch Dermatol Ges. 2009;7:102-107.
- O’Brien TJ, Hall AP. Terra firma-forme dermatosis. Aust J Dermatol. 1997;38:163-164.
- Guarneri C, Guarneri F, Cannavò SP. Terra firma-forme dermatosis. Int J Dermatol. 2008;47:482-484.
- Santarpia M, Guarneri C. Terra firma-forme dermatosis. Eur J Intern Med. 2016;34:1-2.
- Panchal K, Bhalla N, Salunke P, et al. Extensive terra firma forme dermatosis (TFFD): a rare presentation. Indian Dermatol Online J. 2015;6:458-459.
- Erkek E, Sahin S, Cetin ED, et al. Terra firmaforme dermatosis revisited. Indian J Dermatol Venereol Leprol. 2012;78:358-360.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Unal E, Guarneri C, Chokoeva AA, et al. Terra firma-forme dermatosis [published online October 21, 2016]. Wien Med Wochenschr. 2017;167:66-69.
Practice Points
- Terra firma-forme dermatosis (TFFD) is an idiopathic condition characterized by asymptomatic hyperpigmented and hyperkeratotic plaques that are resistant to removal with soap and water.
- Diagnosis and cure of TFFD can be achieved through removal by rubbing with isopropyl alcohol.
- Increased awareness of the clinical presentation and treatment of TFFD may help patients avoid unnecessary treatment and workup and leads to immediate resolution of the condition.