User login
Two men with dyspnea, enlarged lymph nodes • Dx?
CASE 1
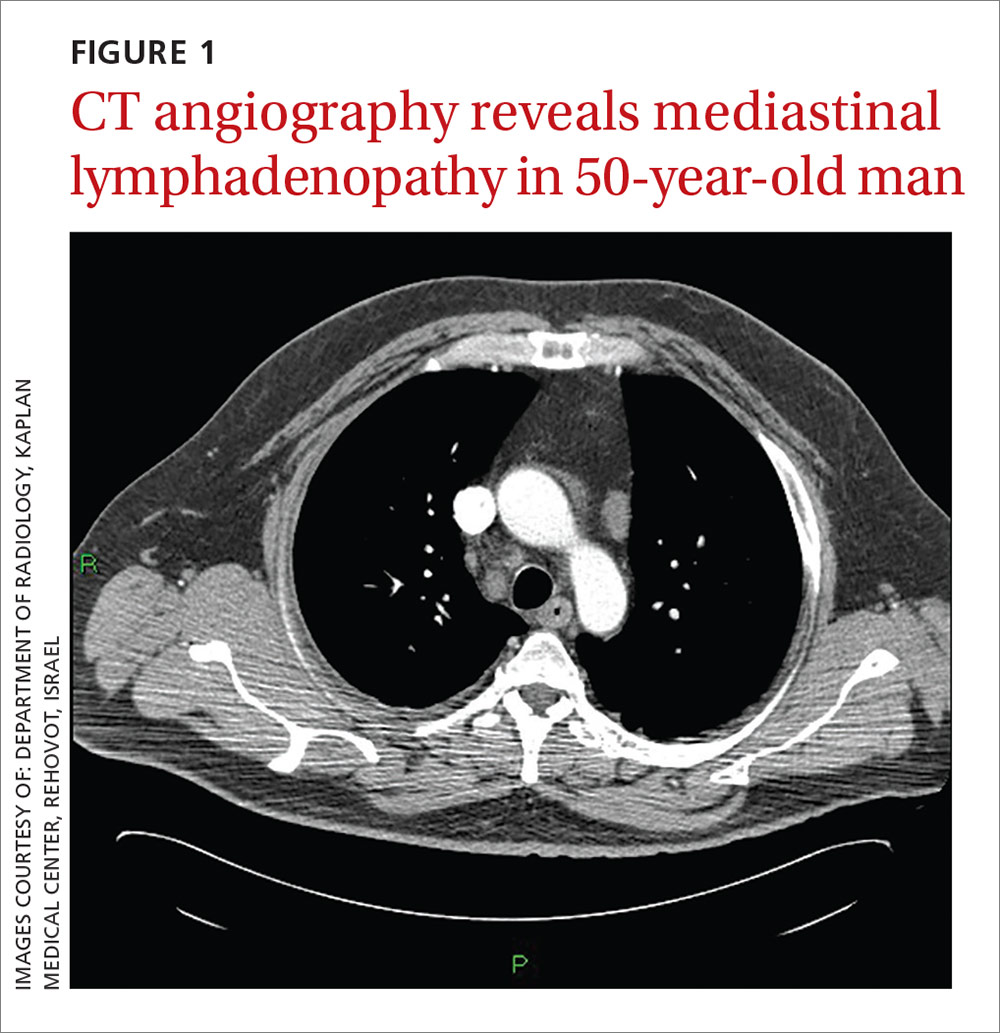
A 50-year-old man sought care for progressive dyspnea on exertion, abdominal bloating, and bilateral leg edema. He had hypertension that was being treated with atenolol, nifedipine, and enalapril. On examination, his blood pressure was 157/80 mm Hg and his heart rate was 50 beats/min. Jugular venous pressure was grossly elevated with occasional cannon A waves. The patient also had decreased breath sounds in both lower lung zones and moderate pitting edema up to the knees. A chest x-ray showed a small bilateral pleural effusion and no cardiomegaly. An electrocardiogram revealed complete atrioventricular (AV) block with a ventricular response of 50 beats/min. Computed tomography (CT) angiography revealed no evidence of a pulmonary embolus, but did show several enlarged (up to 3.5 cm in diameter) lymph nodes in the upper and middle mediastinum (FIGURE 1). We performed an echocardiogram.
CASE 2
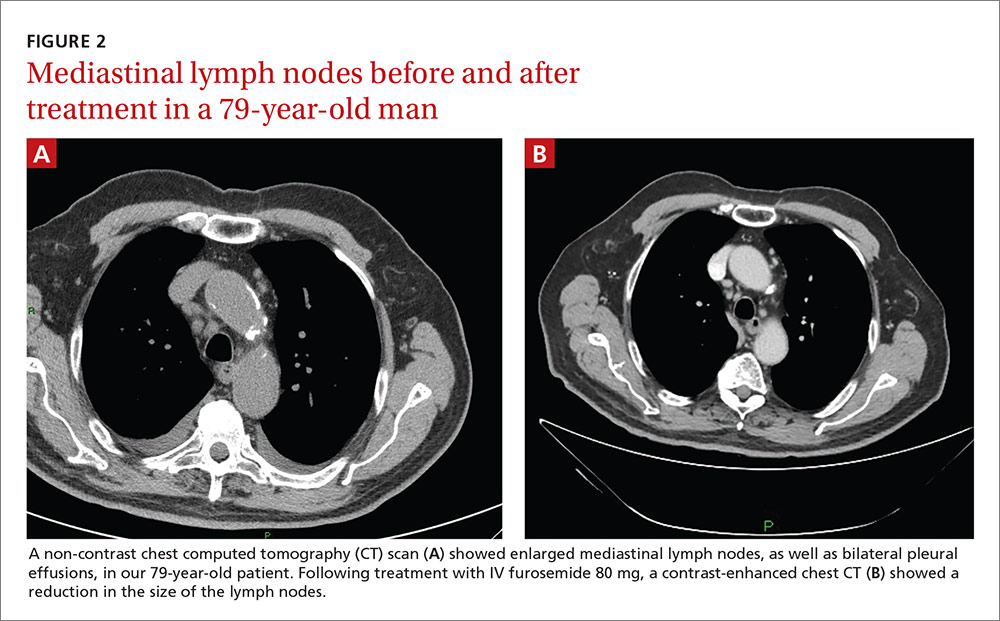
A 79-year-old man with hypertension and diabetes presented to our medical center with acute dyspnea. During the physical examination, we noted bilateral diminished breath sounds with expiratory wheezes and an irregular pulse. Chest x-ray showed mild pulmonary congestion. A chest CT demonstrated bilateral small pleural effusions and multiple enlarged mediastinal lymph nodes with a maximal diameter of 2.4 cm (FIGURE 2A). One week later, the patient’s shortness of breath increased and he was hospitalized. A chest x-ray at that time showed moderate pulmonary congestion, so we performed an echocardiogram.
THE DIAGNOSIS
The echocardiogram for the 50-year-old patient in Case 1 revealed a mildly dilated left ventricle with normal systolic function, diastolic left ventricular (LV) dysfunction, moderate tricuspid regurgitation, and mild pulmonary hypertension. Extensive testing for malignancy and tuberculosis was negative.
For the 79-year-old patient in Case 2, echocardiography demonstrated concentric LV hypertrophy, mild dilatation of the left ventricle, normal LV systolic function, LV diastolic dysfunction with elevated LV diastolic filling pressure, and mild-to-moderate pulmonary hypertension.
Based on these results, we diagnosed both patients with diastolic heart failure. The patient in the second case had features of cardiac asthma, as well. Both patients had also developed reversible mediastinal lymphadenopathy (MLN), of which the diastolic heart failure was the only apparent cause. In both cases, radiologists did not note any suspicious findings for malignancy beyond the MLN.
DISCUSSION
Systolic heart failure has been previously recognized as a cause of MLN.1,2 Other causes of MLN include sarcoidosis, various malignancies, pulmonary infections, and occupational lung diseases. There are, however, no reports of MLN in patients with diastolic heart failure.
Heart failure and MLN. Slanetz et al reported one series of 46 patients who had undergone CT of the chest during periods of congestive heart failure (CHF).1 There was mediastinal lymph node enlargement in 55% of these patients. In a subset of 17 patients who had elevated capillary wedge pressure, 82% had some degree of lymphadenopathy.
Erly et al2 retrospectively studied 44 patients who had a thoracic CT performed before cardiac transplantation. Twenty-nine (66%) had at least one enlarged mediastinal lymph node (>1 cm). Eighty-one percent of patients with an ejection fraction <35% had lymphadenopathy, while none of the patients with an ejection fraction >35% had lymphadenopathy. Most enlarged lymph nodes were pretracheal, with a mean short axis diameter of 1.3 cm.
However, Storto et al reported that an association between CHF and MLN was not found in 7 patients undergoing high-resolution CT imaging.3 There are also cases of MLN in patients with pulmonary hypertension without systolic dysfunction.4
Chabbert et al studied 31 consecutive patients with subacute left heart failure (mean ejection fraction, 39%).5 Enlarged mediastinal lymph nodes were present in 13 patients (42%). Other radiographic features included blurred contour of the lymph nodes in 5 patients (16%) and hazy mediastinal fat in one patient (3%). Follow-up CT showed a significant decrease in the size of the lymph nodes in 8 of 13 patients (62%) following initiation of treatment.
Heart failure and malignancy. A PubMed search with the keywords “diastolic dysfunction” and “lymphoma” found 7 references in the English language. There is a report of 125 survivors of childhood lymphomas treated with mediastinal radiotherapy and anthracyclines,6 another of 44 children treated for acute lymphoblastic leukemia and Hodgkin’s lymphoma7, a report of 294 patients who had received mediastinal irradiation for the treatment of Hodgkin’s disease,8 and another of 106 survivors of non-Hodgkin’s and Hodgkin’s lymphomas.9 None of these reports, however, made any mention of mediastinal lymphadenopathy.
What caused the lymphadenopathy in our patients?
Our 2 patients had volume overload due to diastolic dysfunction with elevated LV end diastolic pressure. Our first patient also had a loss of AV synchronization—which was reversible upon pacemaker insertion—that probably exacerbated the heart failure.
The mechanism for the lymphadenopathy is not clear, but may be due to cardiogenic pulmonary edema causing distension of the pulmonary lymphatic vessels and pulmonary hypertension. In a study of patients with severe systolic dysfunction undergoing evaluation for cardiac transplant, there was a relationship (albeit weak), between MLN and mitral regurgitation, tricuspid regurgitation, elevated mean pulmonary artery pressure, elevated pulmonary capillary wedge pressure, and elevated right atrial pressure.10
How to accurately detect and treat MLN
MLN may be detected by chest x-ray, CT, magnetic resonance imaging, or endoscopic ultrasound examinations. The clinical situation will dictate the imaging modality used. Keep in mind that it is difficult to make a comparison between a finding of lymphadenopathy on one modality and another, especially if one is looking for a change in size.
If clinically appropriate, a trial of diuretics, such as intravenous (IV) furosemide 80 mg,
Our patients. The 50-year-old man in Case 1 responded well to 80 mg of IV furosemide after one hour and improved further upon receipt of a pacemaker the next day. A repeat thoracic CT one month later showed complete resolution of the MLN.
The 79-year-old man in Case 2 also received 80 mg of IV furosemide and improved within 3 hours. A month later, a repeat thoracic CT showed a significant reduction in the size of all the enlarged lymph nodes (FIGURE 2B).
THE TAKEAWAY
The importance of these 2 cases is that they show that heart failure—even diastolic alone—can produce enlarged mediastinal lymph nodes. In patients with heart failure in whom unexpected MLN is detected, consideration should be given to performing a repeat imaging examination after the administration of diuretics.
1. Slanetz PJ, Truong M, Shepard JA, et al. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol. 1998;171:1307-1309.
2. Erly WK, Borders RJ, Outwater EK, et al. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assist Tomogr. 2003;27:485-489.
3. Storto ML, Kee ST, Golden JA, et al. Hydrostatic pulmonary edema: high-resolution CT findings. AJR Am J Roentgenol. 1995;165:817-820.
4. Moua T, Levin DL, Carmona EM, et al. Frequency of mediastinal lymphadenopathy in patients with idiopathic pulmonary arterial hypertension. Chest. 2013;143:344-348.
5. Chabbert V, Canevet G, Baixas C, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol. 2004;14:881-889.
6. Christiansen JR, Hamre H, Massey R, et al. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483-490.
7. Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, et al. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin’s lymphoma. J Paediatr Child Health. 2011;47:455-459.
8. Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977-982.
9. Elbl L, Vasova I, Tomaskova I, et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47:843-851.
10. Pastis NJ Jr, Van Bakel AB, Brand TM, et al. Mediastinal lymphadenopathy in patients undergoing cardiac transplant evaluation. Chest. 2011;139:1451-1457.
CASE 1
A 50-year-old man sought care for progressive dyspnea on exertion, abdominal bloating, and bilateral leg edema. He had hypertension that was being treated with atenolol, nifedipine, and enalapril. On examination, his blood pressure was 157/80 mm Hg and his heart rate was 50 beats/min. Jugular venous pressure was grossly elevated with occasional cannon A waves. The patient also had decreased breath sounds in both lower lung zones and moderate pitting edema up to the knees. A chest x-ray showed a small bilateral pleural effusion and no cardiomegaly. An electrocardiogram revealed complete atrioventricular (AV) block with a ventricular response of 50 beats/min. Computed tomography (CT) angiography revealed no evidence of a pulmonary embolus, but did show several enlarged (up to 3.5 cm in diameter) lymph nodes in the upper and middle mediastinum (FIGURE 1). We performed an echocardiogram.

CASE 2
A 79-year-old man with hypertension and diabetes presented to our medical center with acute dyspnea. During the physical examination, we noted bilateral diminished breath sounds with expiratory wheezes and an irregular pulse. Chest x-ray showed mild pulmonary congestion. A chest CT demonstrated bilateral small pleural effusions and multiple enlarged mediastinal lymph nodes with a maximal diameter of 2.4 cm (FIGURE 2A). One week later, the patient’s shortness of breath increased and he was hospitalized. A chest x-ray at that time showed moderate pulmonary congestion, so we performed an echocardiogram.

THE DIAGNOSIS
The echocardiogram for the 50-year-old patient in Case 1 revealed a mildly dilated left ventricle with normal systolic function, diastolic left ventricular (LV) dysfunction, moderate tricuspid regurgitation, and mild pulmonary hypertension. Extensive testing for malignancy and tuberculosis was negative.
For the 79-year-old patient in Case 2, echocardiography demonstrated concentric LV hypertrophy, mild dilatation of the left ventricle, normal LV systolic function, LV diastolic dysfunction with elevated LV diastolic filling pressure, and mild-to-moderate pulmonary hypertension.
Based on these results, we diagnosed both patients with diastolic heart failure. The patient in the second case had features of cardiac asthma, as well. Both patients had also developed reversible mediastinal lymphadenopathy (MLN), of which the diastolic heart failure was the only apparent cause. In both cases, radiologists did not note any suspicious findings for malignancy beyond the MLN.
DISCUSSION
Systolic heart failure has been previously recognized as a cause of MLN.1,2 Other causes of MLN include sarcoidosis, various malignancies, pulmonary infections, and occupational lung diseases. There are, however, no reports of MLN in patients with diastolic heart failure.
Heart failure and MLN. Slanetz et al reported one series of 46 patients who had undergone CT of the chest during periods of congestive heart failure (CHF).1 There was mediastinal lymph node enlargement in 55% of these patients. In a subset of 17 patients who had elevated capillary wedge pressure, 82% had some degree of lymphadenopathy.
Erly et al2 retrospectively studied 44 patients who had a thoracic CT performed before cardiac transplantation. Twenty-nine (66%) had at least one enlarged mediastinal lymph node (>1 cm). Eighty-one percent of patients with an ejection fraction <35% had lymphadenopathy, while none of the patients with an ejection fraction >35% had lymphadenopathy. Most enlarged lymph nodes were pretracheal, with a mean short axis diameter of 1.3 cm.
However, Storto et al reported that an association between CHF and MLN was not found in 7 patients undergoing high-resolution CT imaging.3 There are also cases of MLN in patients with pulmonary hypertension without systolic dysfunction.4
Chabbert et al studied 31 consecutive patients with subacute left heart failure (mean ejection fraction, 39%).5 Enlarged mediastinal lymph nodes were present in 13 patients (42%). Other radiographic features included blurred contour of the lymph nodes in 5 patients (16%) and hazy mediastinal fat in one patient (3%). Follow-up CT showed a significant decrease in the size of the lymph nodes in 8 of 13 patients (62%) following initiation of treatment.
Heart failure and malignancy. A PubMed search with the keywords “diastolic dysfunction” and “lymphoma” found 7 references in the English language. There is a report of 125 survivors of childhood lymphomas treated with mediastinal radiotherapy and anthracyclines,6 another of 44 children treated for acute lymphoblastic leukemia and Hodgkin’s lymphoma7, a report of 294 patients who had received mediastinal irradiation for the treatment of Hodgkin’s disease,8 and another of 106 survivors of non-Hodgkin’s and Hodgkin’s lymphomas.9 None of these reports, however, made any mention of mediastinal lymphadenopathy.
What caused the lymphadenopathy in our patients?
Our 2 patients had volume overload due to diastolic dysfunction with elevated LV end diastolic pressure. Our first patient also had a loss of AV synchronization—which was reversible upon pacemaker insertion—that probably exacerbated the heart failure.
The mechanism for the lymphadenopathy is not clear, but may be due to cardiogenic pulmonary edema causing distension of the pulmonary lymphatic vessels and pulmonary hypertension. In a study of patients with severe systolic dysfunction undergoing evaluation for cardiac transplant, there was a relationship (albeit weak), between MLN and mitral regurgitation, tricuspid regurgitation, elevated mean pulmonary artery pressure, elevated pulmonary capillary wedge pressure, and elevated right atrial pressure.10
How to accurately detect and treat MLN
MLN may be detected by chest x-ray, CT, magnetic resonance imaging, or endoscopic ultrasound examinations. The clinical situation will dictate the imaging modality used. Keep in mind that it is difficult to make a comparison between a finding of lymphadenopathy on one modality and another, especially if one is looking for a change in size.
If clinically appropriate, a trial of diuretics, such as intravenous (IV) furosemide 80 mg,
Our patients. The 50-year-old man in Case 1 responded well to 80 mg of IV furosemide after one hour and improved further upon receipt of a pacemaker the next day. A repeat thoracic CT one month later showed complete resolution of the MLN.
The 79-year-old man in Case 2 also received 80 mg of IV furosemide and improved within 3 hours. A month later, a repeat thoracic CT showed a significant reduction in the size of all the enlarged lymph nodes (FIGURE 2B).
THE TAKEAWAY
The importance of these 2 cases is that they show that heart failure—even diastolic alone—can produce enlarged mediastinal lymph nodes. In patients with heart failure in whom unexpected MLN is detected, consideration should be given to performing a repeat imaging examination after the administration of diuretics.
CASE 1
A 50-year-old man sought care for progressive dyspnea on exertion, abdominal bloating, and bilateral leg edema. He had hypertension that was being treated with atenolol, nifedipine, and enalapril. On examination, his blood pressure was 157/80 mm Hg and his heart rate was 50 beats/min. Jugular venous pressure was grossly elevated with occasional cannon A waves. The patient also had decreased breath sounds in both lower lung zones and moderate pitting edema up to the knees. A chest x-ray showed a small bilateral pleural effusion and no cardiomegaly. An electrocardiogram revealed complete atrioventricular (AV) block with a ventricular response of 50 beats/min. Computed tomography (CT) angiography revealed no evidence of a pulmonary embolus, but did show several enlarged (up to 3.5 cm in diameter) lymph nodes in the upper and middle mediastinum (FIGURE 1). We performed an echocardiogram.

CASE 2
A 79-year-old man with hypertension and diabetes presented to our medical center with acute dyspnea. During the physical examination, we noted bilateral diminished breath sounds with expiratory wheezes and an irregular pulse. Chest x-ray showed mild pulmonary congestion. A chest CT demonstrated bilateral small pleural effusions and multiple enlarged mediastinal lymph nodes with a maximal diameter of 2.4 cm (FIGURE 2A). One week later, the patient’s shortness of breath increased and he was hospitalized. A chest x-ray at that time showed moderate pulmonary congestion, so we performed an echocardiogram.

THE DIAGNOSIS
The echocardiogram for the 50-year-old patient in Case 1 revealed a mildly dilated left ventricle with normal systolic function, diastolic left ventricular (LV) dysfunction, moderate tricuspid regurgitation, and mild pulmonary hypertension. Extensive testing for malignancy and tuberculosis was negative.
For the 79-year-old patient in Case 2, echocardiography demonstrated concentric LV hypertrophy, mild dilatation of the left ventricle, normal LV systolic function, LV diastolic dysfunction with elevated LV diastolic filling pressure, and mild-to-moderate pulmonary hypertension.
Based on these results, we diagnosed both patients with diastolic heart failure. The patient in the second case had features of cardiac asthma, as well. Both patients had also developed reversible mediastinal lymphadenopathy (MLN), of which the diastolic heart failure was the only apparent cause. In both cases, radiologists did not note any suspicious findings for malignancy beyond the MLN.
DISCUSSION
Systolic heart failure has been previously recognized as a cause of MLN.1,2 Other causes of MLN include sarcoidosis, various malignancies, pulmonary infections, and occupational lung diseases. There are, however, no reports of MLN in patients with diastolic heart failure.
Heart failure and MLN. Slanetz et al reported one series of 46 patients who had undergone CT of the chest during periods of congestive heart failure (CHF).1 There was mediastinal lymph node enlargement in 55% of these patients. In a subset of 17 patients who had elevated capillary wedge pressure, 82% had some degree of lymphadenopathy.
Erly et al2 retrospectively studied 44 patients who had a thoracic CT performed before cardiac transplantation. Twenty-nine (66%) had at least one enlarged mediastinal lymph node (>1 cm). Eighty-one percent of patients with an ejection fraction <35% had lymphadenopathy, while none of the patients with an ejection fraction >35% had lymphadenopathy. Most enlarged lymph nodes were pretracheal, with a mean short axis diameter of 1.3 cm.
However, Storto et al reported that an association between CHF and MLN was not found in 7 patients undergoing high-resolution CT imaging.3 There are also cases of MLN in patients with pulmonary hypertension without systolic dysfunction.4
Chabbert et al studied 31 consecutive patients with subacute left heart failure (mean ejection fraction, 39%).5 Enlarged mediastinal lymph nodes were present in 13 patients (42%). Other radiographic features included blurred contour of the lymph nodes in 5 patients (16%) and hazy mediastinal fat in one patient (3%). Follow-up CT showed a significant decrease in the size of the lymph nodes in 8 of 13 patients (62%) following initiation of treatment.
Heart failure and malignancy. A PubMed search with the keywords “diastolic dysfunction” and “lymphoma” found 7 references in the English language. There is a report of 125 survivors of childhood lymphomas treated with mediastinal radiotherapy and anthracyclines,6 another of 44 children treated for acute lymphoblastic leukemia and Hodgkin’s lymphoma7, a report of 294 patients who had received mediastinal irradiation for the treatment of Hodgkin’s disease,8 and another of 106 survivors of non-Hodgkin’s and Hodgkin’s lymphomas.9 None of these reports, however, made any mention of mediastinal lymphadenopathy.
What caused the lymphadenopathy in our patients?
Our 2 patients had volume overload due to diastolic dysfunction with elevated LV end diastolic pressure. Our first patient also had a loss of AV synchronization—which was reversible upon pacemaker insertion—that probably exacerbated the heart failure.
The mechanism for the lymphadenopathy is not clear, but may be due to cardiogenic pulmonary edema causing distension of the pulmonary lymphatic vessels and pulmonary hypertension. In a study of patients with severe systolic dysfunction undergoing evaluation for cardiac transplant, there was a relationship (albeit weak), between MLN and mitral regurgitation, tricuspid regurgitation, elevated mean pulmonary artery pressure, elevated pulmonary capillary wedge pressure, and elevated right atrial pressure.10
How to accurately detect and treat MLN
MLN may be detected by chest x-ray, CT, magnetic resonance imaging, or endoscopic ultrasound examinations. The clinical situation will dictate the imaging modality used. Keep in mind that it is difficult to make a comparison between a finding of lymphadenopathy on one modality and another, especially if one is looking for a change in size.
If clinically appropriate, a trial of diuretics, such as intravenous (IV) furosemide 80 mg,
Our patients. The 50-year-old man in Case 1 responded well to 80 mg of IV furosemide after one hour and improved further upon receipt of a pacemaker the next day. A repeat thoracic CT one month later showed complete resolution of the MLN.
The 79-year-old man in Case 2 also received 80 mg of IV furosemide and improved within 3 hours. A month later, a repeat thoracic CT showed a significant reduction in the size of all the enlarged lymph nodes (FIGURE 2B).
THE TAKEAWAY
The importance of these 2 cases is that they show that heart failure—even diastolic alone—can produce enlarged mediastinal lymph nodes. In patients with heart failure in whom unexpected MLN is detected, consideration should be given to performing a repeat imaging examination after the administration of diuretics.
1. Slanetz PJ, Truong M, Shepard JA, et al. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol. 1998;171:1307-1309.
2. Erly WK, Borders RJ, Outwater EK, et al. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assist Tomogr. 2003;27:485-489.
3. Storto ML, Kee ST, Golden JA, et al. Hydrostatic pulmonary edema: high-resolution CT findings. AJR Am J Roentgenol. 1995;165:817-820.
4. Moua T, Levin DL, Carmona EM, et al. Frequency of mediastinal lymphadenopathy in patients with idiopathic pulmonary arterial hypertension. Chest. 2013;143:344-348.
5. Chabbert V, Canevet G, Baixas C, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol. 2004;14:881-889.
6. Christiansen JR, Hamre H, Massey R, et al. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483-490.
7. Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, et al. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin’s lymphoma. J Paediatr Child Health. 2011;47:455-459.
8. Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977-982.
9. Elbl L, Vasova I, Tomaskova I, et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47:843-851.
10. Pastis NJ Jr, Van Bakel AB, Brand TM, et al. Mediastinal lymphadenopathy in patients undergoing cardiac transplant evaluation. Chest. 2011;139:1451-1457.
1. Slanetz PJ, Truong M, Shepard JA, et al. Mediastinal lymphadenopathy and hazy mediastinal fat: new CT findings of congestive heart failure. AJR Am J Roentgenol. 1998;171:1307-1309.
2. Erly WK, Borders RJ, Outwater EK, et al. Location, size, and distribution of mediastinal lymph node enlargement in chronic congestive heart failure. J Comput Assist Tomogr. 2003;27:485-489.
3. Storto ML, Kee ST, Golden JA, et al. Hydrostatic pulmonary edema: high-resolution CT findings. AJR Am J Roentgenol. 1995;165:817-820.
4. Moua T, Levin DL, Carmona EM, et al. Frequency of mediastinal lymphadenopathy in patients with idiopathic pulmonary arterial hypertension. Chest. 2013;143:344-348.
5. Chabbert V, Canevet G, Baixas C, et al. Mediastinal lymphadenopathy in congestive heart failure: a sequential CT evaluation with clinical and echocardiographic correlations. Eur Radiol. 2004;14:881-889.
6. Christiansen JR, Hamre H, Massey R, et al. Left ventricular function in long-term survivors of childhood lymphoma. Am J Cardiol. 2014;114:483-490.
7. Krawczuk-Rybak M, Dakowicz L, Hryniewicz A, et al. Cardiac function in survivors of acute lymphoblastic leukaemia and Hodgkin’s lymphoma. J Paediatr Child Health. 2011;47:455-459.
8. Heidenreich PA, Hancock SL, Vagelos RH, et al. Diastolic dysfunction after mediastinal irradiation. Am Heart J. 2005;150:977-982.
9. Elbl L, Vasova I, Tomaskova I, et al. Cardiopulmonary exercise testing in the evaluation of functional capacity after treatment of lymphomas in adults. Leuk Lymphoma. 2006;47:843-851.
10. Pastis NJ Jr, Van Bakel AB, Brand TM, et al. Mediastinal lymphadenopathy in patients undergoing cardiac transplant evaluation. Chest. 2011;139:1451-1457.
Sarcoidosis and Squamous Cell Carcinoma: A Connection Documented in a Case Series of 3 Patients
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
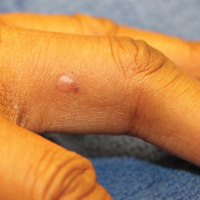
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that most commonly affects the lungs, eyes, and skin. Cutaneous involvement is reported in 25% to 35% of patients with sarcoidosis and may occur in a variety of forms including macules, papules, plaques, and lupus pernio.1,2 Dermatologists commonly are confronted with the diagnosis and management of sarcoidosis because of its high incidence of cutaneous involvement. Due to the protean nature of the disease, skin biopsy plays a key role in confirming the diagnosis. Histological evidence of noncaseating granulomas in combination with an appropriate clinical and radiographic picture is necessary for the diagnosis of sarcoidosis.1,2 Brincker and Wilbek
We describe 3 patients with sarcoidosis who developed squamous cell carcinoma (SCC) of the skin, including 2 black patients, which highlights the potential for SCC development.
Case Reports
Patient 1
A black woman in her 60s with a history of sarcoidosis affecting the lungs and skin that was well controlled with biweekly adalimumab 40 mg subcutaneous injections presented with a new dark painful lesion on the right third finger. She reported the lesion had been present for 1 to 2 years prior to the current presentation and was increasing in size. She had no history of prior skin cancers.
Physical examination revealed a waxy, brown-pigmented papule with overlying scale on the ulnar aspect of the right third digit near the web space (Figure 1A). A shave biopsy revealed atypical keratinocytes involving all layers of the epidermis along with associated parakeratotic scale consistent with a diagnosis of SCC in situ (Figure 1B). Human papillomavirus staining was negative. Due to the location of the lesion, the patient underwent Mohs micrographic surgery and the lesion was completely excised.

Patient 2
A black woman in her 60s with a history of cutaneous sarcoidosis that was maintained on minocycline 100 mg twice daily, chloroquine 250 mg daily, tacrolimus ointment 0.1%, tretinoin cream 0.025%, and intermittent intralesional triamcinolone acetonide injections to the nose, as well as quiescent pulmonary sarcoidosis, developed a new, growing, asymptomatic, hyperpigmented lesion on the left side of the submandibular neck over a period of a few months. A biopsy was performed and the lesion was found to be an SCC, which subsequently was completely excised.
Patient 3
A white man in his 60s with a history of prior quiescent pulmonary sarcoidosis, remote melanoma, and multiple nonmelanoma skin cancers developed scaly papules on the scalp for months, one that was interpreted by an outside pathologist as an invasive SCC (Figure 2A). He was referred to our institution for Mohs micrographic surgery. On presentation when his scalp was shaved for surgery, he was noted to have several violaceous, annular, thin plaques on the scalp (Figure 2B). A biopsy of an annular plaque demonstrated several areas of granulomatous dermatitis consistent with a diagnosis of cutaneous sarcoidosis (Figure 2C). The patient had clinical lymphadenopathy of the neck and supraclavicular region. Given the patient’s history, the differential diagnosis for these lesions included metastatic SCC, lymphoma, and sarcoidosis. The patient underwent a positron emission tomography scan, which demonstrated fluorodeoxyglucose-positive regions in both lungs and the right side of the neck. After evaluation by the pulmonary and otorhinolaryngology departments, including a lymph node biopsy, the positron emission tomography–enhancing lesions were ultimately determined to be consistent with sarcoidosis.
The patient underwent Mohs micrographic surgery for treatment of the scalp SCC and was started on triamcinolone cream 0.1% for the body, clobetasol propionate foam 0.05% for the scalp, and hydroxychloroquine sulfate 400 mg daily for the cutaneous sarcoidosis. His annular scalp lesions resolved, but over the following 12 months the patient had numerous clinically suspicious skin lesions that were biopsied and were consistent with multiple basal cell carcinomas, actinic keratoses, and SCC in situ. They were treated with surgery, cryosurgical destruction with liquid nitrogen, and 5-fluorouracil cream.

Over the 3 years subsequent to initial presentation, the patient developed ocular inflammation attributed to his sarcoidosis and atrial fibrillation, which was determined to be unrelated. He also developed 5 scaly hyperkeratotic plaques on the vertex aspect of the scalp. Biopsy of 2 lesions revealed mild keratinocyte atypia and epidermal hyperplasia, favored to represent SCC over pseudoepitheliomatous hyperplasia overlying associated granulomatous inflammation. These lesions ultimately were believed to represent new SCCs, while biopsies of 2 other lesions revealed isolated granulomatous inflammation that was believed to represent hyperkeratotic cutaneous sarcoidosis clinically resembling his SCCs. The patient was again referred for Mohs micrographic surgery and the malignancies were completely removed, while the cutaneous sarcoidosis was again treated with topical corticosteroids with complete resolution.
Comment
The potential increased risk for malignancy in patients with sarcoidosis has been well documented.3-6 Brincker and Wilbek3 first reported this association after studying 2544 patients with pulmonary sarcoidosis from 1962 to 1971. In particular, they noted a difference between the expected and observed number of cases of malignancy, particularly lung cancer and lymphoma, in the sarcoidosis population.3 In a study of 10,037 hospitalized sarcoidosis patients from 1964 to 2004, Ji et al5 noted a 40% overall increase in the incidence of cancer and found that the risk for malignancy was highest in the year following hospitalization. Interestingly, they found that the risk for developing cutaneous SCC was elevated in sarcoidosis patients even after the first year following hospitalization.5 In a retrospective cohort study examining more than 9000 patients, Askling et al4 also confirmed the increased incidence of malignancy in sarcoidosis patients. Specifically, the authors found a higher than expected occurrence of skin cancer, both melanoma (standardized incidence ratio, 1.6; 95% confidence interval, 1.1-2.3) and nonmelanoma skin cancer (standardized incidence ratio, 2.8; 95% confidence interval, 2.0-3.8) in patients with sarcoidosis.4 Reich et al7 cross-matched 30,000 cases from the Kaiser Permanente Northwest Region Tumor Registry against a sarcoidosis registry of 243 cases to evaluate for evidence of linkage between sarcoidosis and malignancy. They concluded that there may be an etiologic relationship between sarcoidosis and malignancy in at least one-quarter of cases in which both are present and hypothesized that granulomas may be the result of a cell-mediated reaction to tumor antigens.7
Few published studies specifically address the incidence of malignancy in patients with primarily cutaneous sarcoidosis. Cutaneous sarcoidosis includes nonspecific lesions, such as erythema nodosum, as well as specific lesions, such as papules, plaques, nodules, and lupus pernio.8 Alexandrescu et al6 evaluated 110 patients with a diagnosis of both sarcoidosis (cutaneous and noncutaneous) and malignancy. Through their analysis, they found that cutaneous sarcoidosis is seen more commonly in patients presenting with sarcoidosis and malignancy (56.4%) than in the total sarcoidosis population (20%–25%). From these findings, the authors concluded that cutaneous sarcoidosis appears to be a subtype of sarcoidosis associated with cancer.6
We report 3 cases that specifically illustrate a link between cutaneous sarcoidosis and an increased risk for cutaneous SCC. Because sarcoidosis commonly affects the skin, patients often present to dermatologists for care. Once the initial diagnosis of cutaneous sarcoidosis is made via biopsy, it is natural to be tempted to attribute any new skin lesions to worsening or active disease; however, as cutaneous sarcoidosis may take on a variety of nonspecific forms, it is important to biopsy any unusual lesions. In our case series, patient 3 presented at several different points with scaly scalp lesions. Upon biopsy, several of these lesions were found to be SCCs, while others demonstrated regions of granulomatous inflammation consistent with a diagnosis of cutaneous sarcoidosis. On further review of pathology during the preparation of this manuscript after the initial diagnoses were made, it was further noted that it is challenging to distinguish granulomatous inflammation with reactive pseudoepitheliomatous hyperplasia from SCC. The fact that these lesions were clinically indistinguishable illustrates the critical importance of appropriate-depth biopsy in this situation, and the histopathologic challenges highlighted herein are important for pathologists to remember.
Patients 1 and 2 were both black women, and the fact that these patients both presented with cutaneous SCCs—one of whom was immunosuppressed due to treatment with adalimumab, the other without systemic immunosuppression—exemplifies the need for comprehensive skin examinations in sarcoidosis patients as well as for biopsies of new or unusual lesions.
The mechanism for the development of malignancy in patients with sarcoidosis is unknown and likely is multifactorial. Multiple theories have been proposed.1,2,5,6,8 Sarcoidosis is marked by the development of granulomas secondary to the interaction between CD4+ T cells and antigen-presenting cells, which is mediated by various cytokines and chemokines, including IL-2 and IFN-γ. Patients with sarcoidosis have been found to have oligoclonal T-cell lineages with a limited receptor repertoire, suggestive of selective immune system activation, as well as a deficiency of certain types of regulatory cells, namely natural killer cells.1,2 This immune dysregulation has been postulated to play an etiologic role in the development of malignancy in sarcoidosis patients.1,2,5 Furthermore, the chronic inflammation found in the organs commonly affected by both sarcoidosis and malignancy is another possible mechanism.6,8 Finally, immunosuppression and mutagenesis secondary to the treatment modalities used in sarcoidosis may be another contributing factor.6
Conclusion
An association between sarcoidosis and malignancy has been suggested for several decades. We specifically report 3 cases of patients with cutaneous sarcoidosis who presented with concurrent cutaneous SCCs. Given the varied and often nonspecific nature of cutaneous sarcoidosis, these cases highlight the importance of biopsy when sarcoidosis patients present with new and unusual skin lesions. Additionally, they illustrate the importance of thorough skin examinations in sarcoidosis patients as well as some of the challenges these patients pose for dermatologists.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
- Iannuzzi MC, Rybicki BA, Teirsten AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis and therapeutics. JAMA. 2011;305:391-399.
- Brincker H, Wilbek E. The incidence of malignant tumours in patients with respiratory sarcoidosis. Br J Cancer. 1974;29:247-251.
- Askling J, Grunewald J, Eklund A, et al. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160(5, pt 1):1668-1672.
- Ji J, Shu X, Li X, et al. Cancer risk in hospitalized sarcoidosis patients: a follow-up study in Sweden. Ann Oncol. 2009;20:1121-1126.
- Alexandrescu DT, Kauffman CL, Ichim TE, et al. Cutaneous sarcoidosis and malignancy: an association between sarcoidosis with skin manifestations and systemic neoplasia. Dermatol Online J. 2011;17:2.
- Reich JM, Mullooly JP, Johnson RE. Linkage analysis of malignancy-associated sarcoidosis. Chest. 1995;107:605-613.
- Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333.
Practice Points
- There may be an increased risk of skin cancer in patients with sarcoidosis.
- Sarcoidosis may present with multiple morphologies, including verrucous or hyperkeratotic lesions; superficial biopsy of this type of lesion may be mistaken for a squamous cell carcinoma.
- A biopsy diagnosis of squamous cell carcinoma in a black patient with sarcoidosis should be carefully reviewed for evidence of deeper granulomatous inflammation.
Tinea Capitis Caused by Trichophyton rubrum Mimicking Favus
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
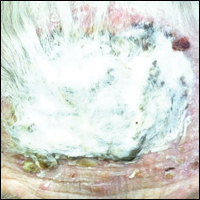
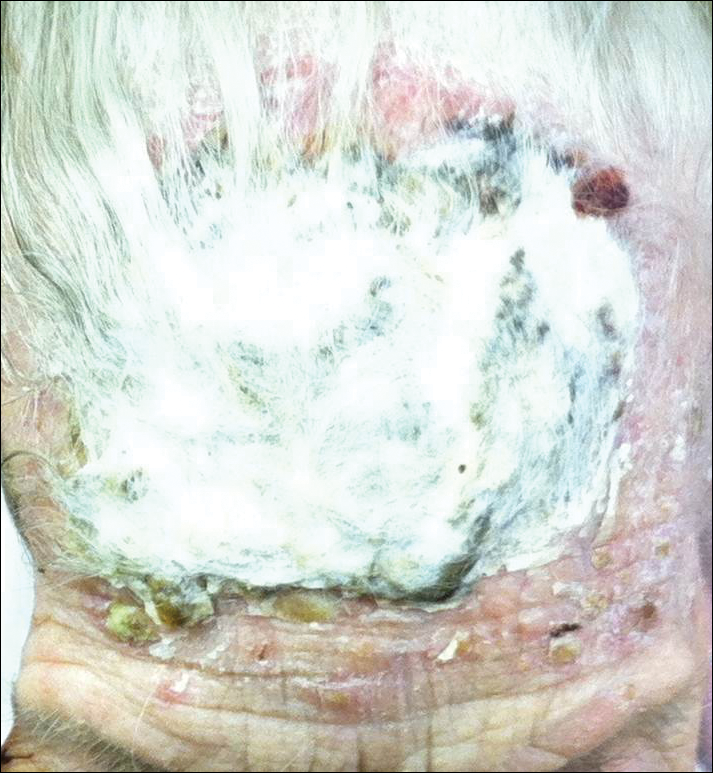
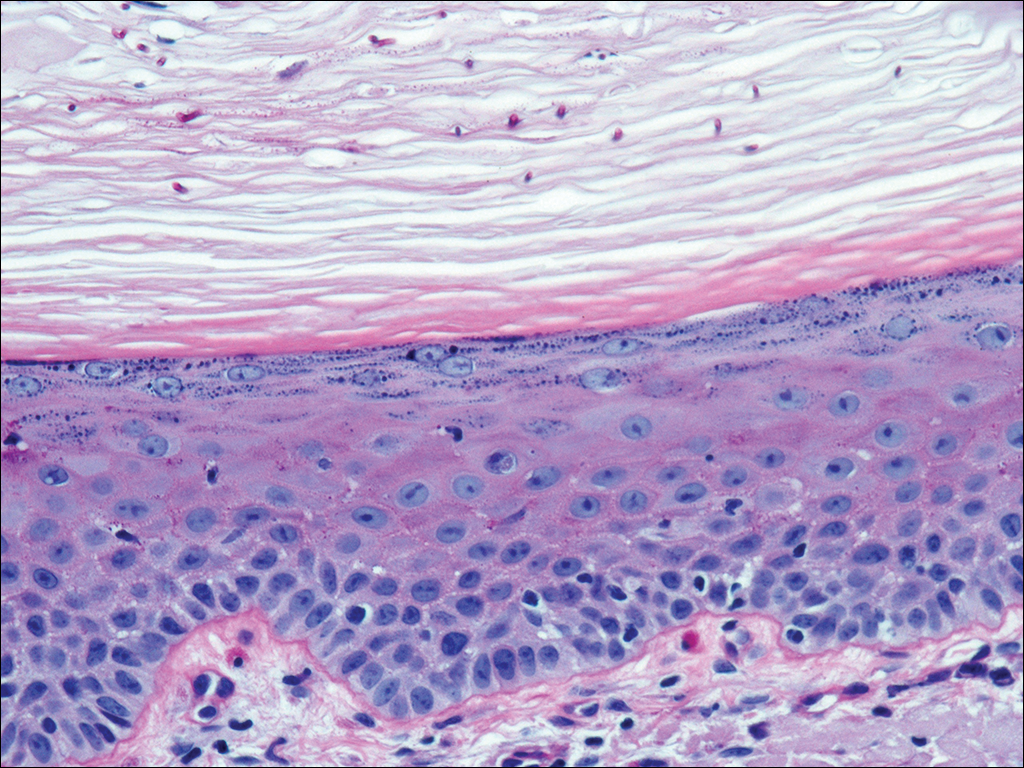
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.
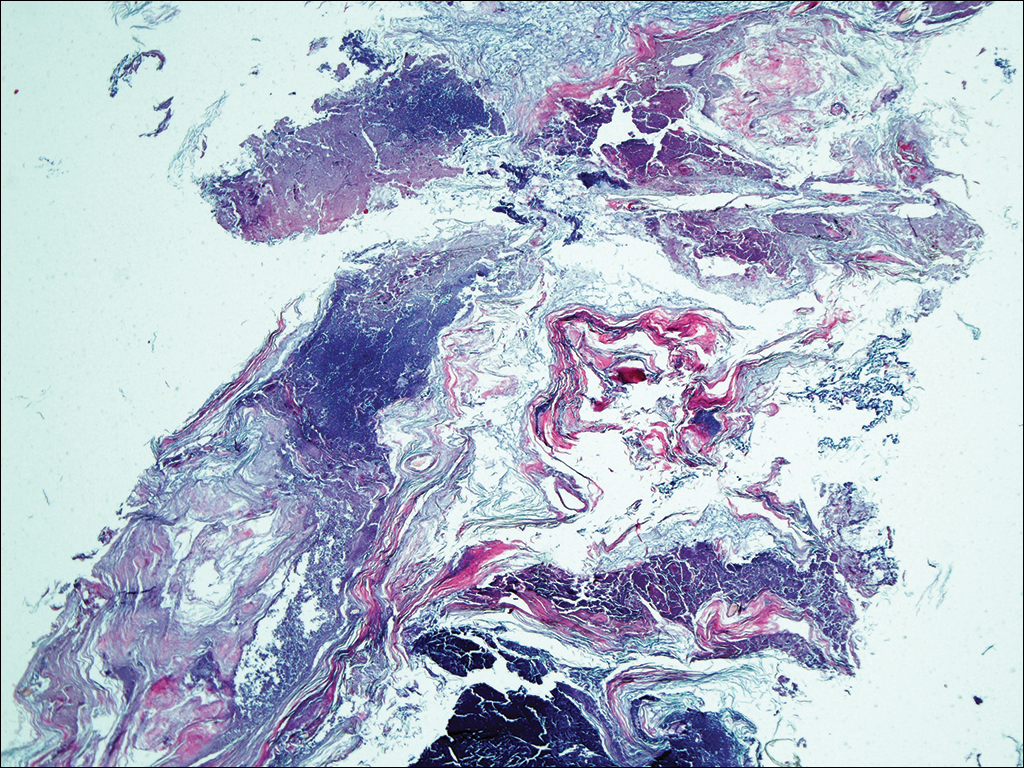
At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).

Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
Practice Points
- Although favus is uncommonly seen in developed countries, it still exists and can mimick other conditions, notably cutaneous malignancies.
- Favus may affect the skin and nails in addition to the hair.
- The lesions of favus may persist for many years.
Kratom: A New Product in an Expanding Substance Abuse Market
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opiates, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most health care providers (HCPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of the “dark Internet” websites and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite these websites’ purported raison d’être, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer and colleagues of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patient’s digital habits, the researchers demonstrated that the majority of subjects used these websites and as a result either increased their drug use or learned about (and tried) new substances!4
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opiate withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and is increasingly popular in the U.S. and in the United Kingdom.2,5,6 In the U.S., this poses a problem for HCPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the U.S. Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined websites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about U.S. $13).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least 9 deaths.7
This article presents a case of kratom abuse and will outline a brief history, the pharmacologic characteristics, clinical presentation of kratom abuse, and conclude with an overview of the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the U.S., a basic working knowledge of the drug is quickly becoming a must for federal HCPs.
Case Presentation
At his employer’s request, a 33-year-old married man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disc resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he thought that he was abusing his opiate medication, and it was discontinued. The patient was transferred to the local hospital and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline. Initial point-of-care admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s additional sequelae had passed, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia (eFigure).2,3 The leaves contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-l
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8 Presently, kratom’s legal status in the U.S. continues to be nebulous: It has not been officially scheduled by the DEA, and it is easily obtained.
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the 2 major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including μ, δ, and κ opioid receptors, leading to its opioid-like ef
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for HCPs to be aware of the clinical presentation of kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from prodrug websites like Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1 g-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5 g-15 g of raw crushed leaves), it is believed that the μ opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed symptomatically with judicious use of benzodiazepines and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of an acute kratom overdose (typically requiring ingestion of > 15 g of crushed leaves) begins with addressing airway blockage, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including point-of-care glucose, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, etc).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong μ agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid detoxification.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, α-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to HCPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurologic and mental status exams.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and permanent psychosis.3-7 Additionally, a 2008 study by Kittirattanapaiboon and colleagues correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥ 14,000 individuals included in the study sample) with statistically significant higher suicide risk.12
Detection
Because kratom is a relatively new compound in the U.S., medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and 2 metabolites of mitragynine in urine.7 Le and colleagues were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours.13 Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the study patient’s urine because a urine test was not done until hospital day 5.
Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden in this highly intelligent individual—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Kratom is an emerging drug of abuse in the Western World. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the U.S. and multiple other Western countries, kratom is easily accessible and is difficult to detect. Health care providers need to be aware of kratom, and during their evaluations, question patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opiates, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most health care providers (HCPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of the “dark Internet” websites and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite these websites’ purported raison d’être, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer and colleagues of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patient’s digital habits, the researchers demonstrated that the majority of subjects used these websites and as a result either increased their drug use or learned about (and tried) new substances!4
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opiate withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and is increasingly popular in the U.S. and in the United Kingdom.2,5,6 In the U.S., this poses a problem for HCPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the U.S. Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined websites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about U.S. $13).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least 9 deaths.7
This article presents a case of kratom abuse and will outline a brief history, the pharmacologic characteristics, clinical presentation of kratom abuse, and conclude with an overview of the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the U.S., a basic working knowledge of the drug is quickly becoming a must for federal HCPs.
Case Presentation
At his employer’s request, a 33-year-old married man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disc resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he thought that he was abusing his opiate medication, and it was discontinued. The patient was transferred to the local hospital and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline. Initial point-of-care admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s additional sequelae had passed, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia (eFigure).2,3 The leaves contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-l
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8 Presently, kratom’s legal status in the U.S. continues to be nebulous: It has not been officially scheduled by the DEA, and it is easily obtained.
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the 2 major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including μ, δ, and κ opioid receptors, leading to its opioid-like ef
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for HCPs to be aware of the clinical presentation of kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from prodrug websites like Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1 g-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5 g-15 g of raw crushed leaves), it is believed that the μ opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed symptomatically with judicious use of benzodiazepines and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of an acute kratom overdose (typically requiring ingestion of > 15 g of crushed leaves) begins with addressing airway blockage, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including point-of-care glucose, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, etc).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong μ agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid detoxification.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, α-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to HCPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurologic and mental status exams.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and permanent psychosis.3-7 Additionally, a 2008 study by Kittirattanapaiboon and colleagues correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥ 14,000 individuals included in the study sample) with statistically significant higher suicide risk.12
Detection
Because kratom is a relatively new compound in the U.S., medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and 2 metabolites of mitragynine in urine.7 Le and colleagues were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours.13 Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the study patient’s urine because a urine test was not done until hospital day 5.
Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden in this highly intelligent individual—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Kratom is an emerging drug of abuse in the Western World. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the U.S. and multiple other Western countries, kratom is easily accessible and is difficult to detect. Health care providers need to be aware of kratom, and during their evaluations, question patients about kratom and other legal highs.
According to the United Nations Office on Drugs and Crime, the last decade saw an alarming rise in the use of recreational substances.1 There was an escalation not only in the use of the more well-known street drugs (cannabis, stimulants, opiates, and hallucinogens), but also an exponential increase in the abuse of novel psychoactive substances. Although most health care providers (HCPs) are at least relatively familiar with some of these designer drugs—often synthesized analogues of common street drugs—region-specific herbal products with psychoactive properties are now entering the market worldwide. Certainly, the cause of this increased use is multifactorial: Ease of access to these drugs and ambiguous legality are believed to be among the largest contributors. Infrastructure established through globalization promotes easy drug transportation and distribution across borders, and widespread Internet use makes knowledge of and accessibility to such substances exceedingly simple.2,3
In particular, widespread online access has permanently altered the acquisition of knowledge in all realms—including drug use. Although Erowid Center remains one of the oldest and best-known of the “dark Internet” websites and bills itself as providing “harm reduction,” others have cropped up online and disseminate information about many forms of potentially psychoactive substances. Despite these websites’ purported raison d’être, recent studies have demonstrated these sites’ efficacy in promoting drug use under the guise of safety, particularly among adolescents and young adults. Among these is a qualitative study by Boyer and colleagues of 12 drug users admitted to a pediatric psychiatry unit. Through extensive questioning about the patient’s digital habits, the researchers demonstrated that the majority of subjects used these websites and as a result either increased their drug use or learned about (and tried) new substances!4
One drug that has benefited from globalization and the Internet is kratom (Mitragyna speciosa korth). This formerly regionally confined herbal psychoactive substance is native to Southeast Asia, where it has been used (and abused) for centuries as a mild stimulant, to prevent opiate withdrawal, and for recreational purposes. In recent years, kratom has been marketed as a psychotropic drug and is increasingly popular in the U.S. and in the United Kingdom.2,5,6 In the U.S., this poses a problem for HCPs who often are unaware of this plant’s existence, much less its abuse potential or health effects.2 Also known as ketum, kakuam, thang, thom, or biak, kratom is marketed in stores and online as a cheap, safe alternative to opioids.
Although considered a “substance of concern” without any approved medical use by the U.S. Drug Enforcement Agency (DEA
To that end, users consider kratom a legal high, and it is easily purchased online. A 2010 study in the United Kingdom examined websites where kratom and many other quasilegal substances (including Salvia divinorum and legal precursors to LSD) could be purchased for an average of £10 (about U.S. $13).5 This study’s authors also noted a significant lack of product information on these marketplaces. As these products are not overseen by any regulatory body, the risk of overdose or adulteration is extremely high.2,3,6-8 In fact, Krypton, a product sold online, was found to be adulterated with O-desmethyltramadol—the active metabolite of the synthetic opiate tramadol—and implicated in at least 9 deaths.7
This article presents a case of kratom abuse and will outline a brief history, the pharmacologic characteristics, clinical presentation of kratom abuse, and conclude with an overview of the treatment of kratom-related illness and evaluation of potential toxic sequelae. In light of the rapid proliferation of kratom in the U.S., a basic working knowledge of the drug is quickly becoming a must for federal HCPs.
Case Presentation
At his employer’s request, a 33-year-old married man presented to his family physician for a worsening of his uncontrolled back pain from a herniated lumbar disc resulting from a motor vehicle collision 3 months before. At his physician’s office he stated, “I don’t care if I live or die, I’m tired of the pain,” and “I’m going to go off on somebody if I can’t get this pain under control.” He also endorsed having auditory hallucinations for several years and a history of violence and homicide. The problem arose precipitously after he thought that he was abusing his opiate medication, and it was discontinued. The patient was transferred to the local hospital and admitted to the psychiatric service for his suicidal ideations and risk of harming self and others.
On admission to the psychiatric service, the patient complained of body aches, chills, rhinorrhea, and significantly worsened irritability from his baseline. Initial point-of-care admission drug testing had been negative as had expanded urine tests looking for synthetic opioids, cannabinoids, and cathinones. The patient reported no opioid use but was unable to explain his current symptom patterns, which were worsening his chronic pain and hampering any attempt to build rapport. On hospital day 3, the patient’s additional sequelae had passed, and psychiatric treatment was able to progress fully. On hospital day 4, the inpatient treatment team received a message from the patient’s primary care manager stating that a friend of the patient had found a bottle of herbal pills in the patient’s car. This was later revealed to be a kratom formulation that he had purchased online.
Background
Kratom is the colloquial name of a tree that is native to Thailand, Malaysia, and other countries in Southeast Asia. These trees, which can grow to 50 feet high and 15 feet wide, have long been the source of herbal remedies in Southeast Asia (eFigure).2,3 The leaves contain psychoactive substances that have a variety of effects when consumed. At low doses, kratom causes a stimulant effect (akin to the leaves of the coca plant in South America); laborers and farmers often use it to help boost their energy. At higher doses, kratom causes an opioid-l
In the United Kingdom, kratom is currently the second most common drug that is considered a legal high, only behind salvia (Salvia divinorum), a hallucinogenic herb that is better known as a result of its use by young celebrities over the past decade.5,8 Presently, kratom’s legal status in the U.S. continues to be nebulous: It has not been officially scheduled by the DEA, and it is easily obtained.
Kratom can be taken in a variety of ways: Crushed leaves often are placed in gel caps and swallowed; it can be drunk as a tea, juice, or boiled syrup; and it can be smoked or insufflated.2,3,5,6
Pharmacology and Clinical Presentation
More than 20 psychoactive compounds have been isolated from kratom. Although a discussion of all these compounds is beyond the scope of this review, the 2 major compounds are mitragynine and 7-hydroxymitragynine.
Mitragynine
Mitragynine, the most abundant psychoactive compound found in kratom, is an indole alkaloid (Figure 1). Extraction and analysis of this compound has demonstrated numerous effects on multiple receptors, including μ, δ, and κ opioid receptors, leading to its opioid-like ef
7-Hydroxymitragynine
7-hydroxymitragynine, despite being far less concentrated in kratom preparations, is about 13 times more potent than morphine and 46 times more potent than mitragynine. It is thought that its hydroxyl side chain added to C7 (Figure 2) adds to its lipophilicity and ability to cross the blood-brain barrier at a far more rapid rate than that of mitragynine.2
Mitragynine and 7-hydroxymitragynine remain the best-studied psychoactive components of kratom at this time. Other compounds that have been isolated, such as speciociliatine, paynantheine, and speciogynine, may play a role in kratom’s analgesic and psychoactive effects. Animal studies have demonstrated antimuscarinic properties in these compounds, but the properties do not seem to have any demonstrable effect at the opioid receptors.2
Intoxication and Withdrawal
Due to its increasing worldwide popularity, it is now imperative for HCPs to be aware of the clinical presentation of kratom abuse as well as the management of withdrawal in light of its dependence potential. However, large-scale studies have not been performed, and much of the evidence comes not from the medical literature but from prodrug websites like Erowid or SageWisdom.2,5-9 To that end, such information will be discussed along with the limited research and expert consensuses available in peer-reviewed medical literature.
Kratom seems to have dose-dependent effects. At low doses (1 g-5 g of raw crushed leaves), kratom abusers often report a mild energizing effect, thought to be secondary to the stimulant properties of kratom’s multiple alkaloids. Users have reported mild euphoria and highs similar to those of the abuse of methylphenidate or modafinil.2,9,10 Also similar to abuse of those substances, users have reported anxiety, irritability, and aggressiveness as a result of the stimulant-like effects.
At moderate-to-high doses (5 g-15 g of raw crushed leaves), it is believed that the μ opiate receptor agonism overtakes the stimulant effects, leading to the euphoria, relaxation, and analgesia seen with conventional opioid use and abuse.2,10 In light of the drug’s substantial binding and agonism of all opioid receptors, constipation and itching also are seen.2 As such, if an individual is intoxicated, he or she should be managed symptomatically with judicious use of benzodiazepines and continuous monitoring of heart rate, blood pressure, respiratory rate, and oxygen saturation.2,10 Kratom intoxication can precipitate psychotic episodes similar to those caused by opiate intoxication, so monitoring for agitation or psychotic behaviors is also indicated.9,10
The medical management of an acute kratom overdose (typically requiring ingestion of > 15 g of crushed leaves) begins with addressing airway blockage, breathing, and circulation along with continuous vital sign monitoring and laboratory testing, including point-of-care glucose, complete blood count, electrolytes, lactate, venous blood gas, and measurable drug levels (ethanol, acetaminophen, tricyclic antidepressants, etc).11 If it is determined that kratom was the intoxicant, the greatest concern of death is similar to that of opioid overdose: respiratory depression. Although there are no large-scale human studies demonstrating efficacy, multiple authors suggest the use of naloxone in kratom-related hypoventilation.9,10
The development of dependence on kratom and its subsequent withdrawal phenomena are thought to be similar to that of opioids, in light of its strong μ agonism.2,5,9,10 Indeed, kratom has a long history of being used by opioid-dependent patients as an attempt to quit drug abuse or stave off debilitating withdrawal symptoms when they are unable to acquire their substance of choice.2,5-10 As such, withdrawal and the treatment thereof will also mimic that of opioid detoxification.
The kratom-dependent individual will often present with rhinorrhea, lacrimation, dry mouth, hostility, aggression, and emotional lability similar to the case study described earlier.2,9,10 Kratom withdrawal, much like intoxication, also may precipitate or worsen psychotic symptoms, and monitoring is necessary throughout the detoxification process.2,5,10 Withdrawal management should proceed along ambulatory clinic or hospital opioid withdrawal protocols that include step-down administration of opioids or with nonopioid medications for symptomatic relief, including muscle relaxants, α-2 agonists, and antidiarrheal agents.5,9,10
Kratom Toxicity
A review of the available medical literature has demonstrated a number of toxic effects with kratom abuse, either as the sole agent or in concert with prescribed medications, recreational coingestants, or as a result of manufacturer’s adulteration with other chemicals or drugs. Of particular interest to HCPs are manic or psychotic episode precipitation, seizure, hypothyroidism, intrahepatic cholestatic injury, and even sudden cardiac death.2,3,5-10 In addition to the basic history, physical, and laboratory examination, the workup of patients identified as kratom users should include the following:
- Fastidious medication reconciliation with drug-interaction check;
- Exhaustive substance abuse history;
- Identification of the brand name and source of kratom purchased, to determine whether there are advertised coingestants or reports of adulteration;
- Electrocardiogram;
- Thyroid function testing;
- Hepatic function testing; and
- Comprehensive neurologic and mental status exams.
In chronic users of kratom, a number of effects have been seen whose etiologies have not yet been determined. These effects include depression, anxiety, tremulousness, weight loss, and permanent psychosis.3-7 Additionally, a 2008 study by Kittirattanapaiboon and colleagues correlated drug use by those with concurrent mental health disorders (in particular, kratom, which was used in 59% of the ≥ 14,000 individuals included in the study sample) with statistically significant higher suicide risk.12
Detection
Because kratom is a relatively new compound in the U.S., medical and forensic laboratories are only now implementing kratom detection protocols. Many laboratories now use high-performance liquid chromatography to analyze for mitragynine, 7-hydroxymitragynine, and 2 metabolites of mitragynine in urine.7 Le and colleagues were able to detect mitragynine in the urine in levels as low as 1 ng/mL, which is clinically useful as mitragynine has a half-life determined in animal studies to be 3.85 hours.13 Similar detection limits for mitragynine and 7-hydroxymitragynine are used only at Naval Medical Center Portsmouth in Virginia; however, kratom was not detected in the study patient’s urine because a urine test was not done until hospital day 5.
Conclusion
When gently confronted about the kratom found in his car, the case study patient admitted that he had purchased kratom online after he was “cut off” from prescription opioids for his pain. He admitted that although it was beneficial for his pain, he did notice worsening in his aggression toward his spouse and coworkers. This progressed to an exacerbation of his psychotic symptoms of hallucinations and persecutory delusions. These symptoms remained well hidden in this highly intelligent individual—but were present for years prior to his presentation at the hospital. The patient was discharged from the inpatient psychiatric unit on hospital day 16 with a diagnosis of schizoaffective disorder, depressive type in addition to opioid use disorder. The patient agreed to seek a pain management specialist and discontinue kratom use.
Kratom is an emerging drug of abuse in the Western World. Although significant research is being conducted on its possible medical uses, little is known about kratom beyond the “trip reports” of kratom users posted online. Because of its technically legal status in the U.S. and multiple other Western countries, kratom is easily accessible and is difficult to detect. Health care providers need to be aware of kratom, and during their evaluations, question patients about kratom and other legal highs.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
1. United Nations Office of Drug and Crime. World Drug Report 2014. https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf. Published June 2014. Accessed September 26, 2016.
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792-799.
3. U.S. Drug Enforcement Administration, Office of Diversion Control. Kratom (Mitragyna speciosa korth). http://www.deadiversion.usdoj.gov/drug _chem_info/kratom.pdf. Published January 2013. Accessed September 26, 2016.
4. Boyer EW, Shannon M, Hibberd PL. The Internet and psychoactive substance use among innovative drug users. Pediatrics. 2005;115(2):302-305.
5. Yusoff NH, Suhaimi FW, Vadivelu RK, et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict Biol. 2016;21(1):98-110.
6. Schmidt MM, Sharma A, Schifano F, Feinmann C. “Legal highs” on the net-evaluation of UK-based websites, products and product information. Forensic Sci Int. 2011;206(1-3):92-97.
7. Kronstrand R, Roman M, Thelander G, Eriksson A. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend Krypton. J Anal Toxicol. 2011;35(4):242-247.
8. Holler JM, Vorce SP, McDonough-Bender PC, Magluilo J Jr, Solomon CJ, Levine B. A drug toxicity death involving propylhexedrine and mitragynine. J Anal Toxicol. 2011;35(1):54-59.
9. Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15-32.
10. Rech MA, Donahey E, Cappiello Dziedzic JM, Oh L, Greenhalgh E. New drugs of abuse. Pharmacotherapy. 2015;35(2):189-197.
11. Silvilotti MLA. Initial management of the critically ill adult with an unknown overdose. http://www.uptodate.com/contents/initial-management-of-the -critically-ill-adult-with-an-unknown-overdose. Updated August 27, 2015. Accessed September 26, 2016.
12. Kittirattanapaiboon P, Suttajit S, Junsirimongkol B, Likhitsathian S, Srisurapanont M. Suicide risk among Thai illicit drug users with and without mental/alcohol use disorders. Neuropsychiatr Dis Treat. 2014;10:453-458.
13. Le D, Goggin MM, Janis GC. Analysis of mitragynine and metabolites in human urine for detecting the use of the psychoactive plant kratom. J Anal Toxicol. 2012;36(9):616-625.
Palliative concurrent chemoradiation for gastrostomy site metastasis
Patients with head and neck squamous cell carcinoma typically present with dysphagia, odynophagia, and weight loss. Treatment of the disease with surgery or concurrent chemoradiation often results in local inflammation and limits further oral intake. Percutaneous endoscopic gastrostomy has been a common and effective means of nutritional support in these patients.
Click on the PDF icon at the top of this introduction to read the full article.
Patients with head and neck squamous cell carcinoma typically present with dysphagia, odynophagia, and weight loss. Treatment of the disease with surgery or concurrent chemoradiation often results in local inflammation and limits further oral intake. Percutaneous endoscopic gastrostomy has been a common and effective means of nutritional support in these patients.
Click on the PDF icon at the top of this introduction to read the full article.
Patients with head and neck squamous cell carcinoma typically present with dysphagia, odynophagia, and weight loss. Treatment of the disease with surgery or concurrent chemoradiation often results in local inflammation and limits further oral intake. Percutaneous endoscopic gastrostomy has been a common and effective means of nutritional support in these patients.
Click on the PDF icon at the top of this introduction to read the full article.
Acute-onset hypokalemic paralysis with arsenic trioxide therapy in patient with acute promyelocytic leukemia
Acute myeloid leukemia (AML) is characterized by clonal proliferation of myeloid precursors with a reduced capacity to differentiate into mature cellular components.1 Acute promyeloctic leukemia (APL; previously called AML-M3), a subtype of AML, is further characterized by a balanced translocation t(15;17) (q24.1;q21.1). It is an interesting model in cancer research because it responds to the differentiation and apoptosis induction therapy using arsenic trioxide (ATO) and all-trans retinoic acid (ATRA).2
Click on the PDF icon at the top of this introduction to read the full article.
Acute myeloid leukemia (AML) is characterized by clonal proliferation of myeloid precursors with a reduced capacity to differentiate into mature cellular components.1 Acute promyeloctic leukemia (APL; previously called AML-M3), a subtype of AML, is further characterized by a balanced translocation t(15;17) (q24.1;q21.1). It is an interesting model in cancer research because it responds to the differentiation and apoptosis induction therapy using arsenic trioxide (ATO) and all-trans retinoic acid (ATRA).2
Click on the PDF icon at the top of this introduction to read the full article.
Acute myeloid leukemia (AML) is characterized by clonal proliferation of myeloid precursors with a reduced capacity to differentiate into mature cellular components.1 Acute promyeloctic leukemia (APL; previously called AML-M3), a subtype of AML, is further characterized by a balanced translocation t(15;17) (q24.1;q21.1). It is an interesting model in cancer research because it responds to the differentiation and apoptosis induction therapy using arsenic trioxide (ATO) and all-trans retinoic acid (ATRA).2
Click on the PDF icon at the top of this introduction to read the full article.
Tibial Tubercle Fracture After Bone–Patellar Tendon–Bone Autograft
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
A fracture occurring after anterior cruciate ligament (ACL) reconstruction is rare, and rarer still when it involves the harvest site of a bone—patellar tendon—bone (BPTB) autograft. The vast majority of fractures described in the literature are patellar, with the weak point along the patellar bone cut. A number of fractures generally also occur through the bone tunnels in both hamstring and BPTB grafts. However, only 2 cases of tibial tubercle fracture after BPTB graft have been published, and we expound on them in this case report.1,2 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
Eight years after undergoing successful left ACL reconstruction with ipsilateral BPTB graft, a 45-year-old man developed a graft rupture and demonstrated recurrent instability. He requested revision reconstruction, again with a BPTB construct. In the operating room, he was prepared and draped in the usual sterile fashion, and left ACL reconstruction was performed with right-knee central-third BPTB graft.
During surgery, the left knee was arthroscopically examined, and residual ACL graft from the initial reconstruction was removed. Notchplasty was performed, and the residual femoral interference screw was removed from the 12:30 position. A transtibial approach was used, with a 10-mm reamer brought through the proximal tibia, the posterior tibial ACL footprint, and the 2:00 distal femoral position, with 30 mm of femoral condyle drilled, leaving 1 mm of posterior femoral cortex.
After the right leg was exsanguinated, a central-third patellar tendon graft was harvested through a longitudinal incision with a 22-mm × 10-mm patellar plug, a 10-mm patellar graft, and a 22-mm × 11-mm tibial plug. The graft was prepared, the left tibia was overreamed, and the graft was passed. The graft was fixed with a 7-mm × 23-mm biointerference screw in the femur, trialed, and fixed with an 8-mm × 23-mm interference screw in the tibia. Excess bone graft was packed in the patellar defect in the right knee. The rent in the patellar tendon was closed. The rest of the incision was closed, and the patient was placed in an immobilizer and a cold therapy device (Polar Care; Breg, Inc).
At 2-week follow-up, the patient reported having slipped on ice and flexed the right knee, causing a pop, pain, and limitation in range of motion (ROM; 0°-70°).
The patient returned to the operating room 5 days later and underwent open reduction and internal fixation (ORIF) of the tibial tubercle avulsion. After sterile preparation and draping, the previous incision was used. The bony fragment was isolated and the hematoma débrided. Repair was performed with two No. 2 running locked FiberWire sutures (Arthrex) placed through bony drill holes in the fragment (1 medial, 1 lateral). The fragment was reduced and the sutures tied, with further fixation provided with a DePuy Synthes small-fragment 3.5-mm cortical screw with washer. A No. 5 Ethibond suture (Ethicon) was then placed as a secondary cerclage figure-of-8 stitch to protect the repair.
The patient was seen in follow-up 6 weeks after right ACL reconstruction and 4 weeks after left tibial tubercle ORIF. He continued with right knee restrictions, with the weight-bearing brace locked in extension. Left knee ROM was more than 0° to 90° even before any formal physical therapy. At this point, the patient began physical therapy on both knees with ROM limited to 0° to 30° and weight-bearing as tolerated on the right knee (no restrictions on the left knee).
Discussion
Cases of tibial tubercle fracture after BPTB autograft harvest are extremely rare in the published literature. PubMed and Cochrane Review searches revealed only 2—1 in the ipsilateral knee as ACL fixation1 and 1 in the contralateral knee.2 The middle third of the patellar tendon has been used for ACL reconstruction for more than 50 years, which supports the extreme rarity of this complication.3 Tibial tubercle fractures are so rare that they are not even mentioned in reviews of ACL complications.4 These fractures are universally treated with ORIF.1,2
Far more common but still rare, fracture-type complications involve the extensor mechanism and the tibial plateau. Patellar fractures have been documented as occurring in 0.2% to 2.3% of cases.5-7 One paper reported a fracture in 1.3% of cases at a mean of 57 days, with roughly half caused by trauma and the other half having atraumatic causes.8 Lee and colleagues9 found a 0.2% complication rate for all BPTB grafts in 1725 consecutive patients. Although some patients were treated nonoperatively, others underwent operative fixation. Time to clinical and radiographic healing was 7 and 10 weeks, respectively.
Tibial plateau fracture after BPTB harvest is a rare complication, with 11 cases reported in the literature.10 In 4 of those cases, the proposed mechanism of fracture was a stress riser resulting from the synergistic weakness of the tibial harvest site combined with the tibial tunnel reducing proximal tibial bone strength.11-14 The mechanism of injury varied from traumatic to insufficiency fracture, with fixation varying with fracture displacement.
Tibial tubercle fracture after BPTB harvest is extremely rare, with the present case being only the third published in the literature. Like most reported post-ACL reconstruction extensor mechanism disruptions, our case resulted from a traumatic event at an interval after surgery. All other tibial tubercle fracture post-ACL reconstruction disruptions occurred within 2 weeks after surgery.1,2 Sudden tension on the extensor mechanism secondary to hyperflexion caused a fracture through a weakened tibial tubercle with avulsion of the remaining tendon in 2 of the 3 cases, with the third being a lower stress popping noise that occurred during a pivot to stand.1
The residual defect after tibial bone block harvest could represent a weakening of the tubercle by loss of structural bone and by development of stress risers. The previous reports of tibial tubercle fracture after BPTB harvest documented a similar methodology: Use a bone saw and osteotomes to harvest a trapezoidal tibial bone plug 10 mm to 11 mm wide and 22 cm to 35 cm long. As previously documented, we suggest taking care with saw cuts and osteotomes so as not to weaken the proximal tibia or distal patella more than is necessary.1,2 Before surgery, patients should be warned about the possibility of extensor mechanism injuries with use of BPTB grafts.
Conclusion
Tibial tubercle fracture after BPTB harvest for ACL reconstruction is an extremely rare complication. Treatment is ORIF of the tubercle fragment, with a delay in ACL rehabilitation in cases involving the ipsilateral knee.
Am J Orthop. 2016;45(7):E469-E471. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
1. Acton KJ, Dowd GS. Fracture of the tibial tubercle following anterior cruciate ligament reconstruction. Knee. 2002;9(2):157-159.
2. Busfield BT, Safran MR, Cannon WD. Extensor mechanism disruption after contralateral middle third patellar tendon harvest for anterior cruciate ligament revision reconstruction. Arthroscopy. 2005;21(10):1268.e1-e1268.e6.
3. Jones KG. Reconstruction of the anterior cruciate ligament. A technique using the central one-third of the patellar ligament. J Bone Joint Surg Am. 1963;45(5):925-932.
4. Tjoumakaris FP, Herz-Brown AL, Bowers AL, Sennett BJ, Bernstein J. Complications in brief: anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2012;470(2):630-636.
5. Morgan-Jones RL, Cross TM, Caldwell B, Cross MJ. “Silent” transverse patellar fracture following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(9):997-999.
6. Viola R, Vianello R. Three cases of patella fracture in 1,320 anterior cruciate ligament reconstructions with bone–patellar tendon–bone autograft. Arthroscopy. 1999;15(1):93-97.
7. Berg EE. Management of patella fractures associated with central third bone–patella tendon–bone autograft ACL reconstructions. Arthroscopy. 1996;12(6):756-759.
8. Stein DA, Hunt SA, Rosen JE, Sherman OH. The incidence and outcome of patella fractures after anterior cruciate ligament reconstruction. Arthroscopy. 2002;18(6):578-583.
9. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
10. Wong JJ, Muir B. Insufficiency fracture of the tibial plateau after anterior cruciate ligament reconstructive surgery: a case report and review of the literature. J Can Chiropr Assoc. 2013;57(2):123-131.
11. Morgan E, Steensen RN. Traumatic proximal tibial fracture following anterior cruciate ligament reconstruction. Am J Knee Surg. 1998;11(3):193-194.
12. Delcogliano A, Chiossi S, Caporaso A, Franzese S, Menghi A. Tibial plateau fracture after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(4):E16.
13. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):325-328.
14. Moen KY, Boynton MD, Raasch WG. Fracture of the proximal tibia after anterior cruciate ligament reconstruction: a case report. Am J Orthop. 1998;27(9):629-630.
Primary chest-wall leiomyosarcoma: a rare mimic of a malignant rib lesion
Primary chest-wall leiomyosarcoma (LMS) is an uncommon, malignant, soft-tissue tumor that most often affects the extremities. Malignant LMS originates from mesenchymal cells with smooth muscle differentiation. It is rare in adults, forming only 7% of all soft-tissue sarcomas (STS), but it is the most common STS. In adults, this type of tumor is usually found in the retroperitoneum and extremities.1 Chest-wall LMS is rare and most often occurs in men aged 50-70 years.2 When LMS is associated with rib destruction, it may mimic a primary bone tumor or metastasis. We present here the case of histologically proven chest-wall sarcoma with associated rib destruction that was initially mistaken on imaging for either a metastasis or primary bone tumor.
Case presentation and summary
A 69-year-old man presented to the emergency department complaining of pain over the right side of the chest. The pain, which was pleuritic in nature, had worsened over the previous 6 months and was severe at presentation. The patient had no fever, shortness of breath, or loss of weight. He had no history of chest trauma or chest wall radiation, and nothing noteworthy was discovered in his medical history. Subsequent test results for hemoglobin, white blood cell count, lymphocyte count, and cardiac enzymes were normal.
A frontal chest radiograph showed an osteolytic destructive lesion involving the posterior right 6th rib (Figure 1). A contrast-enhanced computedtomography (CE-CT) scan of the chest showed a heterogeneously enhancing, ovoid, soft-tissue mass of 5.6 x 3.6 cm (2.2 x 1.2 in) centered on the postero- lateral right 6th rib, with associated rib erosion. There was another 2.0-cm (0.8-in) subpleural nodule in the left upper lobe (Figure 2).
Click on the PDF icon below to read the full article.
Primary chest-wall leiomyosarcoma (LMS) is an uncommon, malignant, soft-tissue tumor that most often affects the extremities. Malignant LMS originates from mesenchymal cells with smooth muscle differentiation. It is rare in adults, forming only 7% of all soft-tissue sarcomas (STS), but it is the most common STS. In adults, this type of tumor is usually found in the retroperitoneum and extremities.1 Chest-wall LMS is rare and most often occurs in men aged 50-70 years.2 When LMS is associated with rib destruction, it may mimic a primary bone tumor or metastasis. We present here the case of histologically proven chest-wall sarcoma with associated rib destruction that was initially mistaken on imaging for either a metastasis or primary bone tumor.
Case presentation and summary
A 69-year-old man presented to the emergency department complaining of pain over the right side of the chest. The pain, which was pleuritic in nature, had worsened over the previous 6 months and was severe at presentation. The patient had no fever, shortness of breath, or loss of weight. He had no history of chest trauma or chest wall radiation, and nothing noteworthy was discovered in his medical history. Subsequent test results for hemoglobin, white blood cell count, lymphocyte count, and cardiac enzymes were normal.
A frontal chest radiograph showed an osteolytic destructive lesion involving the posterior right 6th rib (Figure 1). A contrast-enhanced computedtomography (CE-CT) scan of the chest showed a heterogeneously enhancing, ovoid, soft-tissue mass of 5.6 x 3.6 cm (2.2 x 1.2 in) centered on the postero- lateral right 6th rib, with associated rib erosion. There was another 2.0-cm (0.8-in) subpleural nodule in the left upper lobe (Figure 2).
Click on the PDF icon below to read the full article.
Primary chest-wall leiomyosarcoma (LMS) is an uncommon, malignant, soft-tissue tumor that most often affects the extremities. Malignant LMS originates from mesenchymal cells with smooth muscle differentiation. It is rare in adults, forming only 7% of all soft-tissue sarcomas (STS), but it is the most common STS. In adults, this type of tumor is usually found in the retroperitoneum and extremities.1 Chest-wall LMS is rare and most often occurs in men aged 50-70 years.2 When LMS is associated with rib destruction, it may mimic a primary bone tumor or metastasis. We present here the case of histologically proven chest-wall sarcoma with associated rib destruction that was initially mistaken on imaging for either a metastasis or primary bone tumor.
Case presentation and summary
A 69-year-old man presented to the emergency department complaining of pain over the right side of the chest. The pain, which was pleuritic in nature, had worsened over the previous 6 months and was severe at presentation. The patient had no fever, shortness of breath, or loss of weight. He had no history of chest trauma or chest wall radiation, and nothing noteworthy was discovered in his medical history. Subsequent test results for hemoglobin, white blood cell count, lymphocyte count, and cardiac enzymes were normal.
A frontal chest radiograph showed an osteolytic destructive lesion involving the posterior right 6th rib (Figure 1). A contrast-enhanced computedtomography (CE-CT) scan of the chest showed a heterogeneously enhancing, ovoid, soft-tissue mass of 5.6 x 3.6 cm (2.2 x 1.2 in) centered on the postero- lateral right 6th rib, with associated rib erosion. There was another 2.0-cm (0.8-in) subpleural nodule in the left upper lobe (Figure 2).
Click on the PDF icon below to read the full article.
Central nervous system manifestations of multiple myeloma: risk and prognostic considerations
Multiple myeloma accounts for about 1% of all cancers and for 10% of hematologic malignancies in the United States. This report describes the cases of 2 patients with multiple myeloma who developed CNS involvement after autologous stem cell transplant in the context of extramedullary disease.
Click on the PDF icon at the top of this introduction to read the full article.
Multiple myeloma accounts for about 1% of all cancers and for 10% of hematologic malignancies in the United States. This report describes the cases of 2 patients with multiple myeloma who developed CNS involvement after autologous stem cell transplant in the context of extramedullary disease.
Click on the PDF icon at the top of this introduction to read the full article.
Multiple myeloma accounts for about 1% of all cancers and for 10% of hematologic malignancies in the United States. This report describes the cases of 2 patients with multiple myeloma who developed CNS involvement after autologous stem cell transplant in the context of extramedullary disease.
Click on the PDF icon at the top of this introduction to read the full article.