User login
Multiple Keratoacanthomas Occurring in Surgical Margins and De Novo Treated With Intralesional Methotrexate
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
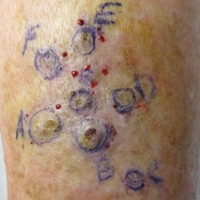
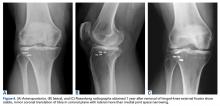
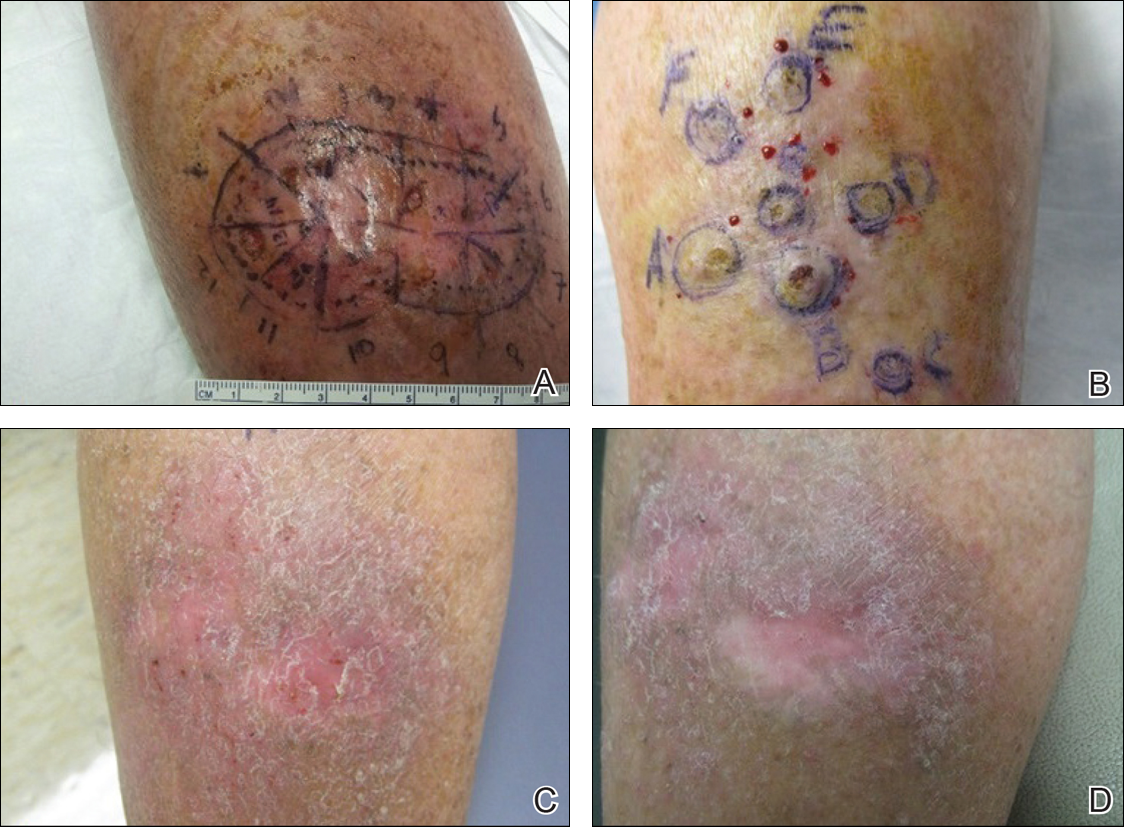
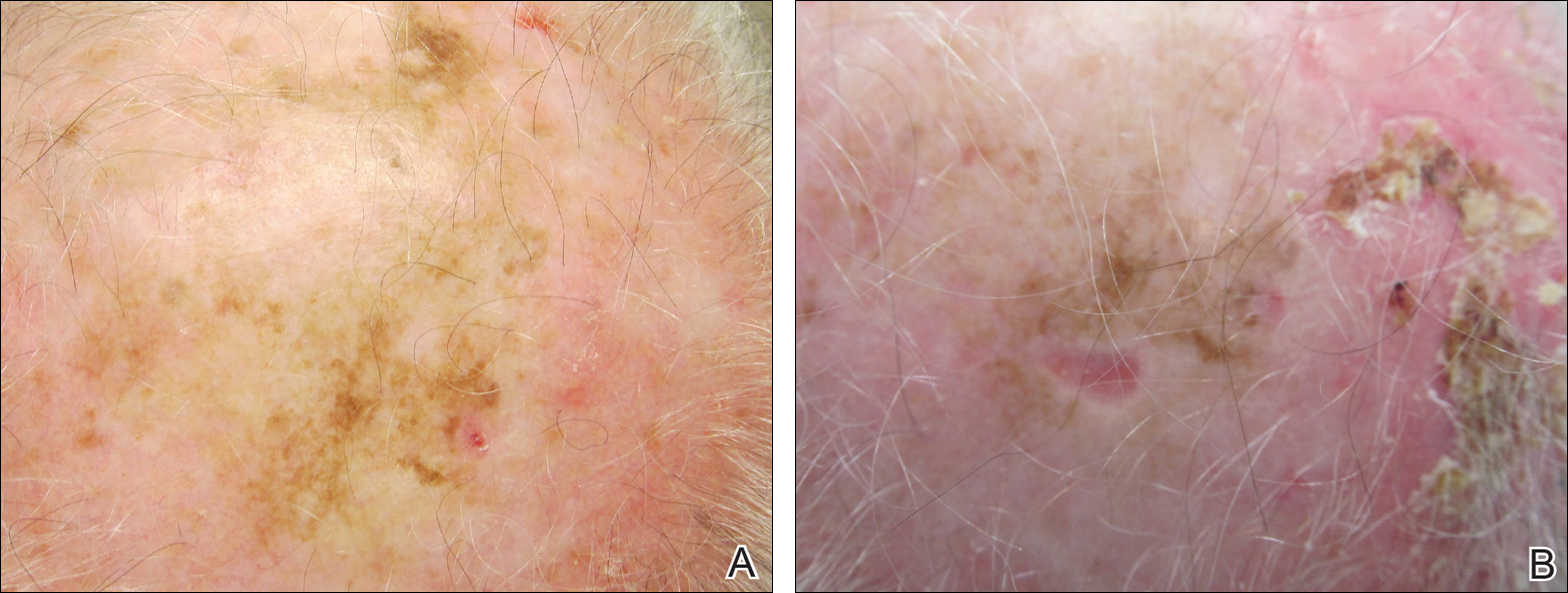
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
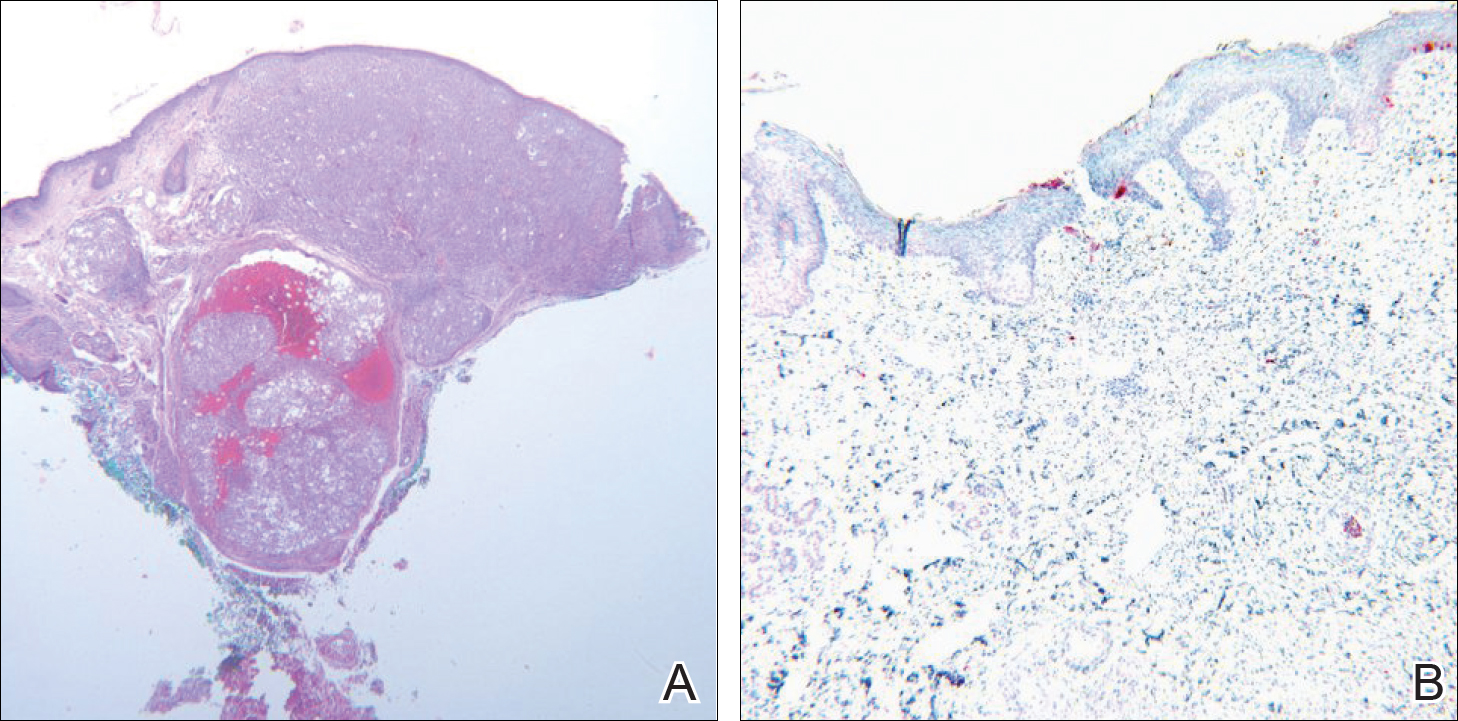
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
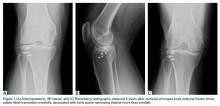
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.
Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
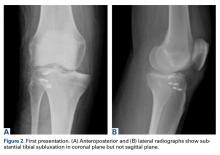
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas of the skin. The normal progression of a KA is to show rapid growth followed by spontaneous resolution.1 Most KAs are solitary; however, there are several variants of multiple KAs including the familial Ferguson-Smith type, Gryzbowski syndrome (generalized eruptive KAs), KA centrifugum marginatum, Muir-Torre syndrome, and xeroderma pigmentosum.2-4 Keratoacanthomas also may develop in areas of trauma, including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.5 Treatment of multiple KAs can be difficult due to a potentially large field size and number of lesions.6 We present a case of multiple KAs developing both in the surgical margins and de novo that responded dramatically to treatment with intralesional methotrexate (MTX).
Case Report
A 55-year-old man with a history of a surgically treated squamous cell carcinoma (SCC) on the anterior aspect of the right leg developed multiple nodules involving the surgical scar. He previously underwent Mohs micrographic surgery (MMS); within a month after the second surgery the patient noticed increased pruritus along with scaly pink changes at the site of the surgical scar.
One month prior to presentation, biopsies from the anterior aspect of the right leg demonstrated well-differentiated SCC and he was subsequently treated with MMS; however, examination 1 month after MMS revealed an 11×7-cm indurated plaque with multiple nodules ranging from 1 to 2 cm near the periphery of the plaque with central atrophy and scarring, reminiscent of KA centrifugum marginatum (Figure, A). In a similar fashion, an 8×5-cm plaque composed of 7 nodular areas was noted on the posterior aspect of the right leg (Figure, B). The patient denied any history of trauma to this area. There was no palpable regional lymphadenopathy and the remainder of the skin examination was normal, except for signs of venous stasis in both legs.
Based on the location and morphology of the lesions, the clinical presentation was consistent with multiple KAs. Histologic examination from punch biopsies taken from the plaque's periphery demonstrated well-differentiated SCC (KA type), as well as a lichenoid inflammatory process, epidermal hyperplasia, and cystic and endophytic squamous proliferation suggestive of hypertrophic lichen planus (HLP).
In consideration of the size and number of the lesions as well as the prolonged wound healing with prior surgery, the patient consented to treatment with intralesional MTX (1 mL of 12.5 mg/mL every 2 weeks) rather than undergoing further surgery. The MTX injection was distributed between the lesions on the anterior and posterior aspects of the lower right leg. At each injection session, the size, thickness, and nodularity of the tumor decreased with markedly less pruritus and symptomatic relief was achieved. After 3 injection sessions, resulting in a total of 3 mL of 12.5 mg/mL of MTX, biopsies were taken from the residual atrophic scar on the anterior aspect of the right leg and the remaining 3 papules on the posterior aspect of the right leg to rule out HLP and invasive SCC. The pathology report commented on the presence of prurigo nodules without any evidence of SCC.
At 3-month follow-up, the patient demonstrated no new lesions or recurrence (Figure, C and D). The right leg continued to heal with scarring and postinflammatory pigmentary changes. The patient was monitored for recurrence and to determine the diagnosis of HLP.

Comment
We report the development of multiple KAs arising both from within surgical margins and de novo, and resolution with intralesional MTX. Keratoacanthomas, especially various KA types, have been observed to develop due to various types of trauma, including sites of surgical scars, lichen planus, tattoos, thermal burns, radiation, and discoid lupus erythematosus, and within skin grafts and donor sites.5-19
Hypertrophic lichen planus is a chronic variant of lichen planus that often is found on the pretibial areas of the lower legs.13 Both SCC and reactive KAs have been observed to develop within lesions of HLP.14 Our pathologist commented on the presence of a lichenoid infiltrate with necrotic keratinocytes and epidermal hyperplasia suspicious for HLP, with a small focus of cystic and endophytic squamous proliferation. The latter lacked notable atypia or an invasive component and could represent an irritated infundibular cyst versus an early evolving KA.
The lichenoid inflammation is suspicious for HLP, which has been associated with eruptive KAs13-16 and may have contributed to the development of persistent KAs in our patient, both in sites of surgical scars (the anterior aspect of the leg) and in uninvolved skin (the posterior aspect of the leg). Trauma from the prior surgery may have stimulated a local inflammatory response and, if coupled with a preexisting underlying chronic inflammatory condition such as HLP, may have triggered the development of new lesions on the posterior leg. Skin pathergy reactions also are caused by an upregulated inflammatory response, which is reduced with immunosuppressive agents such as MTX.12
In our patient, there was both an isotopic and isomorphic response. The term isotopic response refers to the occurrence of a new skin disorder at the site of another unrelated and already healed skin disease. It was first defined by Wolf and Wolf20 in 1985 and hence is also known as Wolf isotopic response. The isotopic response in our patient occurred in the setting of lichen planus. The isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of other skin injuries.
Initially, we thought the patient had recurrence of SCC, but with the rapid development of multiple lesions, the diagnosis of multiple KAs was more likely. Kimyai-Asadi et al8 demonstrated that surgical trauma can precede the development of KAs, as they reported a patient who developed a KA at an excision site. Tamir et al7 reported the simultaneous appearance of KAs in burn scars and skin graft donor sites 4 months after a 40% total body surface area burn. Hamilton et al11 described surgical trauma from a split-skin graft donor site as a trigger for the onset of a KA.
Multiple treatment alternatives exist for KAs, with the standard of care for large or high-risk KAs being excisional surgery21,22; however, other approaches may need to be considered in certain cases, such as with multiple KAs in which lesions may be large and extensive, thereby yielding poor cosmetic outcomes, or with increased surgical risk.23 Furthermore, multiple KAs that develop in the setting of surgical scars require special consideration. Topical 5-fluorouracil, various systemic and intralesional agents (eg, retinoids, interferon, bleomycin, MTX), laser therapy, electrodesiccation and curettage, radiotherapy, and photodynamic therapy all have been reported as methods employed for the treatment of KA.23-27 Goldberg et al5 reported cases of resolution of eruptive KAs arising in both surgical and nonsurgical sites with a combination of deep shave excision, MMS, curettage and desiccation, and oral isotretinoin.
For our patient, we opted for treatment with intralesional MTX, both due to its effectiveness for solitary KAs and reasonably decreased risk of morbidity compared to surgical excision of regions of the pretibial calves. Treatment with MTX would not have been attempted if there was any clinical doubt that the lesions were not the well-differentiated KA type. Also, we had a low threshold for discontinuing therapy and reverting to MMS treatment if any of the lesions displayed a paradoxical growth post-MTX treatment or failed to respond after 3 treatments. Intralesional MTX is less invasive, relatively inexpensive, and a treatment modality with decreased morbidity for KAs, especially for multiple KAs. It should be considered as a potential alternative to surgery in such cases.23-27
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
- Schwartz RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1-19.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46:77-79.
- Ereaux LP, Schopflocher P, Fornier CJ. Keratoacanthoma. Arch Dermatol. 1955;71:73-83.
- Lloyd KM, Madsen DK, Lin PY. Grzybowski's eruptive keratoacanthoma. J Am Acad Dermatol. 1989;21(5, pt 1):1023-1024.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753-758.
- Pillsbury DM, Beerman H. Multiple keratoacanthoma. Am J Med Sci. 1958;236:614-623.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;400(5, pt 2):870-871.
- Kimyai-Asadi A, Shaffer C, Levine VJ, et al. Keratoacanthomas arising from an excisional surgery scar. J Drugs Dermatol. 2004;3:193-194.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(suppl 2):S35-S38.
- Hendricks WM. Sudden appearance of multiple keratoacanthomas three weeks after thermal burns. Cutis. 1991;47:410-412.
- Hamilton SA, Dickson WA, O'Brien CJ. Keratoacanthoma developing in a split skin graft donor site. Br J Plast Surg. 1997;50:560-561.
- Bangash SJ, Green WH, Dolson DJ, et al. Eruptive postoperative squamous cell carcinomas exhibiting a pathergy-like reaction around surgical wound sites. J Am Acad Dermatol. 2009;61:892-897.
- Badell A, Marcoval J, Gallego I, et al. Keratoacanthomas arising in hypertrophic lichen planus. Br J Dermatol. 2000;142:370-393.
- Chave TA, Graham-Brown RAC. Keratoacanthoma developing in hypertrophic lichen planus. Br J Dermatol. 2003;148:592.
- Epstein R. Treatment of keratoacanthoma arising from hypertrophic lichen planus. J Am Acad Dermatol. 2010;62(3, suppl 1):AB28.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Toll A, Salgado R, Espinet B, et al. "Eruptive postoperative squamous cell carcinomas" or "Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia"? J Am Acad Dermatol. 2010;63:910-911.
- Fanti PA, Tosti A, Peluso AM, et al. Multiple keratoacanthoma in discoid lupus erythematosus. J Am Acad Dermatol. 1989;21(4, pt 1):809-810.
- Kossard S, Thompson C, Duncan GM. Hypertrophic lichen planus-like reactions combined with infundibulocystic hyperplasia: pathway to neoplasia. Arch Dermatol. 2004;140:1262-1267.
- Wolf R, Wolf D. Tinea in a site of healed herpes zoster (Isoloci response). Int J Dermatol. 1985;24:539.
- Larson PO. Keratoacanthomas treated with Mohs' micrographic surgery (chemosurgery): a review of forty-three cases. J Am Acad Dermatol. 1987;16:1040-1044.
- Benest L, Kaplan RP, Salit R, et al. Keratoacanthoma centrifugum marginatum of the lower extremity treated with Mohs micrographic surgery. J Am Acad Dermatol. 1994;31:501-502.
- Remling R, Mempel M, Schnopp N, et al. Intralesional methotrexate injection: an effective time and cost saving therapy alternative in keratoacanthomas that are difficult to treat surgically. Hautarzt. 2000;51:612-614.
- Annest NM, VanBeek MJ, Arpey CJ, et al. Intralesional methotrexate treatment for keratoacanthoma tumors: a retrospective study and review of the literature. J Am Acad Dermatol. 2007;56:989-993.
- Melton JL, Nelson BR, Stough DB, et al. Treatment of keratoacanthoma with intralesional methotrexate. J Am Acad Dermatol. 1991;25:1017-1023.
- Cuesta-Romero C, de Grado-Pena J. Intralesional methotrexate in solitary keratoacanthoma. Arch Dermatol. 1998;134:513-514.
- Richard MA, Gachon J, Choux R, et al. Treatment of keratoacanthoma with intralesional methotrexate injections. An Dermatol Venereol. 2000;127:1097.
Practice Points
- Keratoacanthomas (KAs) are rapidly growing tumors most prominently found on sun-exposed areas but also may develop in areas of trauma including burns, laser treatment, radiation, and surgical margins from excisional biopsies or skin grafting.
- Intralesional methotrexate is a potential alternative to surgical treatment of KAs as a less invasive and less costly treatment modality with decreased morbidity for multiple KAs.
- Isotopic response refers to the occurrence of a new skin disorder arising at the site of another unrelated and already healed skin disease. Isomorphic response indicates the appearance of typical skin lesions of an existing dermatosis at sites of injuries.
Topical Imiquimod Clears Invasive Melanoma
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
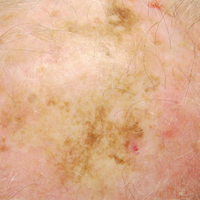
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.
Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7
Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Practice Points
- Topical imiquimod may clear invasive melanoma as well as melanoma in situ.
- Further study is required to confirm the role of topical imiquimod in melanoma treatment.
Primary Cutaneous Mycobacterium avium Complex Infection Following Squamous Cell Carcinoma Excision
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
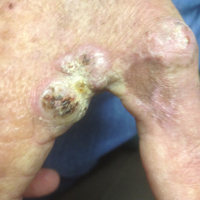
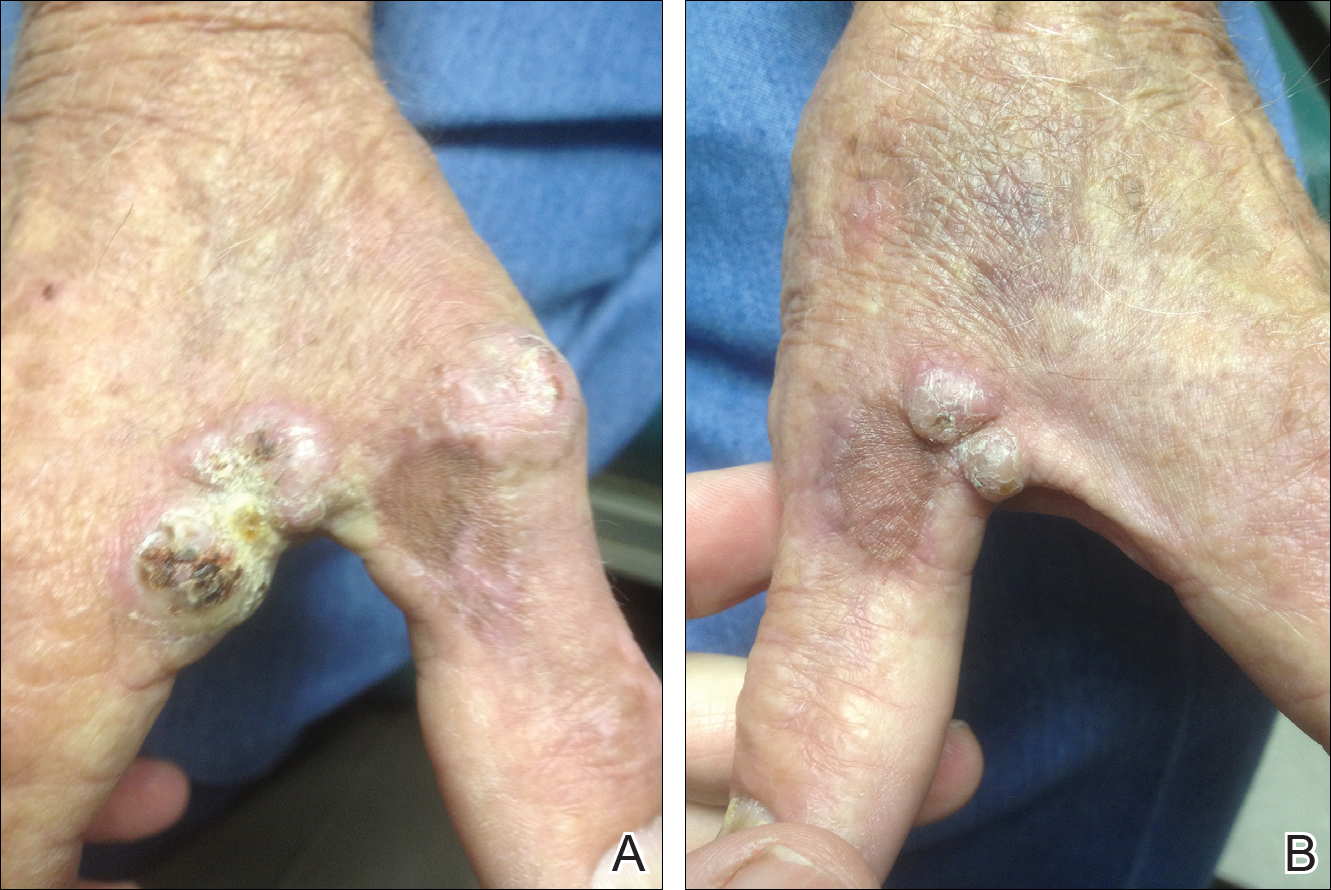
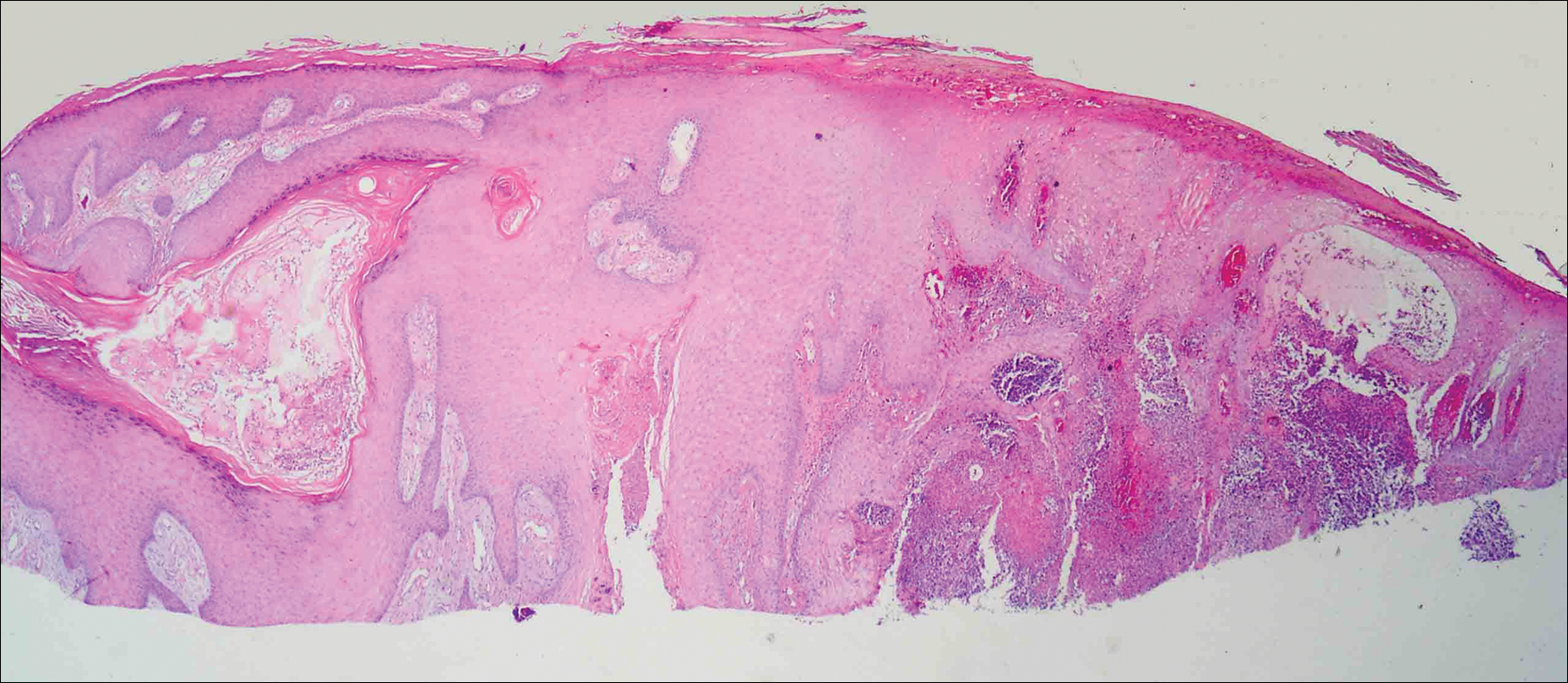
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.
Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
Case Report
A 78-year-old man presented for evaluation of 4 painful keratotic nodules that had appeared on the dorsal aspect of the right thumb, the first web space of the right hand, and the first web space of the left hand. The nodules developed in pericicatricial skin following Mohs micrographic surgery to the affected areas for treatment of invasive squamous cell carcinomas (SCCs) 2 months prior. The patient had worked in lawn maintenance for decades and continued to garden on an avocational basis. He denied exposure to angling or aquariums.
On physical examination the lesions appeared as firm, dusky-violaceous, crusted nodules (Figure 1). Brown patches of hyperpigmentation or characteristic cornlike elevations of the palm were not present to implicate arsenic exposure. Extensive sun damage to the face, neck, forearms, and dorsal aspect of the hands was noted. Epitrochlear lymphadenopathy or lymphangitic streaking were not appreciated. Routine hematologic parameters including leukocyte count were normal, except for chronic thrombocytopenia. Computerized tomography of the abdomen demonstrated no hepatosplenomegaly or enlarged lymph nodes. Hematoxylin and eosin staining of biopsy specimens from the right thumb showed irregular squamous epithelial hyperplasia with an impetiginized scale crust and pustular tissue reaction, including suppurative abscess formation in the dermis (Figure 2). Initial acid-fast staining performed on the biopsy from the right thumb was negative for microorganisms. Given the concerning histologic features indicating infection, a tissue culture was performed. Subsequent growth on Lowenstein-Jensen culture medium confirmed infection with Mycobacterium avium complex (MAC). The patient was started on clarithromycin 500 mg twice daily in accordance with laboratory susceptibilities, and the cutaneous nodules improved. Unfortunately, the patient died 6 months later secondary to cardiac arrest.


Comment
The genus Mycobacterium comprises more than 130 described bacteria, including the precipitants of tuberculosis and leprosy. Mycobacterium avium complex--an umbrella term for M avium, Mycobacterium intracellulare, and other close relatives--is a member of the genus that maintains a low pathogenicity for healthy individuals.1,2 Nonetheless, MAC accounts for more than 70% of cases of nontuberculous mycobacterial disease in the United States.3 Mycobacterium avium complex typically acts as a respiratory pathogen, but infection may manifest with lymphadenitis, osteomyelitis, hepatosplenomegaly, or skin involvement. Disseminated MAC infection can occur in patients with defective immune systems, including those with conditions such as AIDS or hairy cell leukemia and those undergoing immunosuppressive therapy.1,4 Although uncommon, cutaneous infection with MAC occurs via 3 possible mechanisms: (1) primary inoculation, (2) lymphogenous extension, or (3) hematologic dissemination.4 According to a PubMed search of articles indexed for MEDLINE using the terms primary cutaneous Mycobacterium avium complex and MAC skin infection, only 11 known cases of primary cutaneous MAC infection have been reported in the English-language literature,4-14 the most recent being a report by Landriscina et al.11
A Runyon group III bacillus, MAC is a slow-growing nonchromogen that is ubiquitous in nature.15 It has been isolated from soil, water, house dust, vegetables, eggs, and milk. According to Reed et al,3 occupational exposure to soil is an independent risk factor for MAC infection, with individuals reporting more than 6 years of cumulative participation in lawn and landscaping services, farming, or other occupations involving substantial exposure to dirt or dust most likely to be MAC-positive. Cutaneous MAC infection may be associated with water exposure, as Sugita et al2 described one familial outbreak of cutaneous MAC infection linked to use of a circulating, constantly heated bathwater system. With respect to US geography, individuals living in rural areas of the South seem most prone to MAC infection.3
Primary cutaneous infection with MAC occurs after a breach in the skin surface, though this fact may not be elicited by history. Modes of entry include minor abrasions after falling,1 small wounds,2 traumatic inoculation,15 and intramuscular injection.16 Clinically, cutaneous lesions of MAC are protean. In the literature, clinical presentation is described as a polymorphous appearance with scaling plaques, verrucous nodules, crusted ulcers, inflammatory nodules, dermatitis, panniculitis, draining sinuses, ecthymatous lesions, sporotrichoid growth patterns, or rosacealike papulopustules.1,15,17 Lesions may affect the arms and legs, trunk, buttocks, and face.18
The differential diagnosis of MAC infection includes lupus vulgaris, Mycobacterium marinum infection (also known as swimming pool granuloma), sporotrichosis, nocardiosis, sarcoidosis, neutrophilic dermatosis, pyoderma gangrenosum, and cutaneous blastomycosis. Given its rarity and variability, diagnosis of MAC infection requires a high index of suspicion. Cutaneous MAC infection should be considered if a nodule, plaque, or ulcer fails to respond to conventional treatment, especially in patients with a history of environmental exposure and possible injury to the skin.
We report a rare case of primary cutaneous MAC infection arising in SCC excision sites in a patient without known immune deficiency. This presentation may have occurred for several reasons. First, the surgical excision sites coupled with the substantial occupational and recreational exposure to soil experienced by our patient may have served as portals for infection. Although SCCs are common on the hands, Mohs micrographic surgery is not always performed for excision; in our patient's case, this approach allowed for maximum tissue conservation and preserved manual function given the number and location of the lesions. Second, despite an overtly intact immune system, our patient may have harbored an occult immune deficiency, predisposing him to dermatologic infection with a microorganism of low intrinsic virulence and recurrent malignant neoplasms. This presentation may have been the first clinical indication of subtle immune compromise. For example, inadequate proinflammatory cytokines may contribute to both mycobacterial and malignant disease. A potential risk of inhibition of tumor necrosis factor α is the unmasking of tuberculosis or lymphoma.19,20 Likewise, IFN-γ is vital in suppressing mycobacteria and malignancy. Yonekura et al21 found that IFN-γ induces apoptosis in oral SCC lines. It follows that a paucity of IFN-γ could allow neoplastic growth. Normal function of IFN-γ prompts microbicidal activity in macrophages and stimulates granuloma formation, both of which combat mycobacterial infection.19 A final postulation is that a simmering cutaneous MAC infection precipitated neoplastic degeneration into SCC, much the same way that the human papillomavirus has been correlated in the carcinogenesis of cervical cancer. As an intracellular microbe, MAC could cause the genetic machinery of skin cells to go awry. Kullavanijaya et al18 described a patient with cutaneous MAC in association with cervical cancer.
Conclusion
This association of primary cutaneous MAC infection and cutaneous malignancy in a reportedly immunocompetent patient is rare. Cancer patients, as noted by Feld et al,22 are 3 times more likely to develop infections with mycobacteria, with SCC, lymphoma, and leukemia being most commonly indicated. A specific immune deficit in the IFN-γ receptor is known to confer a selective predisposition to mycobacterial infection.23,24 Toyoda et al25 outlined the case of a pediatric patient with IFN-γ receptor 2 deficiency who presented with disseminated MAC infection and later succumbed to multiple SCCs of the hands and face. The authors' assertion was that inherited disorders of IFN-γ-mediated immunity may be associated with SCCs.25 Unfortunately, our patient died before more specific immunological testing could be conducted. This case highlights the remarkable singularity of primary cutaneous MAC infection in association with multiple SCCs with seemingly intact immune status and offers some intriguing hypotheses regarding its occurrence.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
- Hong BK, Kumar C, Marottoli RA. "MAC" attack. Am J Med. 2009;122:1096-1098.
- Sugita Y, Ishii N, Katsuno M, et al. Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol. 2000;142:789-793.
- Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex [published online May 4, 2006]. Am J Epidemiol. 2006;164:32-40.
- Ichiki Y, Hirose M, Akiyama T, et al. Skin infection caused by Mycobacterium avium. Br J Dermatol. 1997;136:260-263.
- Aboutalebi A, Shen A, Katta R, et al. Primary cutaneous infection by Mycobacterium avium: a case report and literature review. Cutis. 2012;89:175-179.
- Nassar D, Ortonne N, Grégoire-Krikorian B, et al. Chronic granulomatous Mycobacterium avium skin pseudotumor. Lancet Infect Dis. 2009;9:136.
- Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23:214-221.
- Lugo-Janer G, Cruz A, Sanchez JL. Disseminated cutaneous infection caused by Mycobacterium avium complex. Arch Dermatol. 1990;126:1108-1110.
- Schmidt JD, Yeager H Jr, Smith EB, et al. Cutaneous infection due to a Runyon group 3 atypical Mycobacterium. Am Rev Respir Dis. 1972;106:469-471.
- Carlos C, Tang YW, Adler DJ, et al. Mycobacterial infection identified with broad-range PCR amplification and suspension array identification. J Clin Pathol. 2012;39:795-797.
- Landriscina A, Musaev T, Amin B, et al. A surprising case of Mycobacterium avium complex skin infection in an immunocompetent patient. J Drugs Dermatol. 2014;13:1491-1493.
- Zhou L, Wang HS, Feng SY, et al. Cutaneous Mycobacterium intracellulare infection in an immunocompetent person. Acta Derm Venereol. 2013;93:711-714.
- Cox S, Strausbaugh L. Chronic cutaneous infection caused by Mycobacterium intracellulare. Arch Dermatol. 1981;117:794-796.
- Sachs M, Fraimow HF, Staros EB, et al. Mycobacterium intracellulare soft tissue infection. J Am Acad Dermatol. 1992;27:1019-1021.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17:491-498.
- Meadows JR, Carter R, Katner HP. Cutaneous Mycobacterium avium complex infection at an intramuscular injection site in a patient with AIDS. Clin Infect Dis. 1997;24:1273-1274.
- Kayal JD, McCall CO. Sporotrichoid cutaneous Mycobacterium avium complex infection. J Am Acad Dermatol. 2002;47(5 suppl):S249-S250.
- Kullavanijaya P, Sirimachan S, Surarak S. Primary cutaneous infection with Mycobacterium avium intracellulare complex resembling lupus vulgaris. Br J Dermatol. 1997;136:264-266.
- Netea MG, Kullberg BJ, Van der Meer JW. Proinflammatory cytokines in the treatment of bacterial and fungal infections. BioDrugs. 2004;18:9-22.
- Dommasch E, Gelfand JM. Is there truly a risk of lymphoma from biologic therapies? Dermatol Ther. 2009;22:418-430.
- Yonekura N, Yokota S, Yonekura K, et al. Interferon-γ downregulates Hsp27 expression and suppresses the negative regulation of cell death in oral squamous cell carcinoma lines. Cell Death Differ. 2003;10:313-322.
- Feld R, Bodey GP, Groschel D. Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976;136:67-70.
- Dorman S, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
- Storgaard M, Varming K, Herlin T, et al. Novel mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infections. Scand J Immunol. 2006;64:137-139.
- Toyoda H, Ido M, Nakanishi K, et al. Multiple cutaneous squamous cell carcinomas in a patient with interferon γ receptor 2 (IFNγR2) deficiency [published online June 18, 2010]. J Med Genet. 2010;47:631-634.
Practice Points
- Mycobacterium avium complex (MAC) is a ubiquitous bacterium that commonly infects the lungs and less commonly infects the skin.
- Clinically, cutaneous MAC infection is polymorphous and may present as a nodule, plaque, or ulcer.
- Standard treatment of primary cutaneous MAC includes systemic antibiotics with or without surgical excision.
Hinged-Knee External Fixator Used to Reduce and Maintain Subacute Tibiofemoral Coronal Subluxation
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Dislocation of the knee is a severe injury that usually results from high-energy blunt trauma.1 Recognition of knee dislocations has increased with expansion of the definition beyond radiographically confirmed loss of tibiofemoral articulation to include injury of multiple knee ligaments with multidirectional joint instability, or the rupture of the anterior and posterior cruciate ligaments (ACL, PCL) when no gross dislocation can be identified2 (though knee dislocations without rupture of either ligament have been reported3,4). Knee dislocations account for 0.02% to 0.2% of orthopedic injuries.5 These multiligamentous injuries are rare, but their clinical outcomes are often complicated by arthrofibrosis, pain, and instability, as surgeons contend with the competing interests of long-term joint stability and range of motion (ROM).6-9
Whereas treatment standards for acute knee dislocations are becoming clearer, treatment of subacute and chronic tibiofemoral dislocations and subluxations is less defined.5 Success with articulated external fixation originally across the ankle and elbow inspired interest in its use for the knee.10-12 Richter and Lobenhoffer13 and Simonian and colleagues14 were the first to report on the postoperative use of a hinged external fixation device to help maintain the reduction of chronic fixed posterior knee dislocations. The literature has even supported nonoperative reduction of small fixed anterior or posterior (sagittal) subluxations with knee bracing alone.15,16 However, there are no reports on treatment of chronic tibial subluxation in the coronal plane.
We report a case of a hinged-knee external fixator (HEF) used alone to reduce a chronic medial tibia subluxation that presented after initial repair of a knee dislocation sustained in a motor vehicle accident. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old healthy woman who was traveling out of state sustained multiple orthopedic injuries in a motor vehicle accident. She had a pelvic fracture, a contralateral femoral shaft fracture, significant multiligamentous damage to the right knee, and a cavitary impaction fracture of the tibial eminence with resultant coronal tibial subluxation. Initial magnetic resonance imaging (MRI) showed the tibia injury likely was the result of varus translation, as the medial femoral condyle impacted the tibial spine, disrupting the ACL (Figures 1A, 1B).
On initial presentation to our clinic 5 weeks after injury, x-rays showed progressive medial subluxation of the tibia in relation to the femur with translation of about a third of the tibial width medially (Figures 2A, 2B).
Given the worsening tibial subluxation and resultant instability, the patient was taken to the operating room for examination under anesthesia, and planned closed reduction and spanning external fixation. Fluoroscopy of the lateral translation and external rotation of the tibia allowed us to reduce the joint, with the lateral tibial plateau and lateral femoral condyle relatively but not completely concentric. A rigid spanning multiplanar external fixator was then placed to maintain the knee joint in a more reduced position.
A week later, the patient was taken back to the operating room for arthroscopic evaluation of the knee joint. At the time of her index operation at the outside institution, she had undergone arthroscopic débridement of intra-articular loose bodies and lateral meniscus repair. Now it was found that the meniscus was not healed but had displaced. A bucket-handle lateral meniscus tear appeared to be blocking lateral translation of the tibia, thus impeding complete reduction.
Given the meniscus deformity that resulted from the chronicity of the injury and the resultant subluxation, a sub-total lateral meniscectomy was performed. As the patient was now noted to have an intact medial collateral ligament and an intact en masse lateral repair, we converted the spanning external fixator to a Compass Universal Hinge (Smith & Nephew) to maintain reduction without further ligamentous reconstruction (Figure 4).
After HEF placement, the patient spent a short time recovering at an inpatient rehabilitation facility before starting aggressive twice-a-week outpatient physical therapy. Initially after HEF placement, she could not actively flex the knee to about 40° or fully extend it concentrically. Given these limitations and concern about interval development of arthrofibrosis, manipulation under anesthesia was performed, 3 weeks after surgery, and 90° of flexion was obtained.
Six weeks after HEF removal, the patient was ambulating well with a cane, pain was minimal, and knee ROM was up to 110° of flexion. Tibiofemoral stability remained constant—no change in medial or lateral joint space opening. Full-extension radiographs showed medial translation of about 5 mm, which decreased to 1 mm on Rosenberg view. This represents marked improvement over the severe subluxation on initial presentation.
Follow-up over the next months revealed continued improvement in the right lower extremity strength, increased tolerance for physical activity, and stable right medial tibial translation.
At 5-year follow-up, the patient was asymptomatic, had continued coronal and sagittal stability, and was tolerating regular aerobic exercise, including hiking, weight training, and cycling. Physical examination revealed grade 1B Lachman, grade 0 pivot shift, and grade 0 posterior drawer. There was 3 mm increased lateral compartment opening in full extension, which increased to about 6 mm at 30° with endpoint.
Discussion
Although knee dislocations with multiligamentous involvement are rare, their outcomes can be poor. Fortunately, the principles of managing these complex injuries in the acute stage are becoming clearer. In a systematic review, Levy and colleagues18 found that operative treatment of a dislocated knee within 3 weeks after injury, compared with nonoperative or delayed treatment, resulted in improved functional outcomes. Ligament repair and reconstruction yielded similar outcomes, though repair of the posterolateral corner had a comparatively higher rate of failure. For associated lateral injuries, Shelbourne and colleagues17 advocated en masse repair in which the healing tissue complex is reattached to the tibia nonanatomically, without dissecting individual structures—a technique used in the original repair of our patient’s injuries.
Originally designed for other joints, hinged external fixators are now occasionally used for rehabilitation after traumatic knee injury. Stannard and colleagues9 recently confirmed the utility of the HEF as a supplement to ligament reconstruction for recovery from acute knee dislocation.9 Compared with postoperative use of a hinged-knee brace, HEF use resulted in fewer failed ligament reconstructions as well as equivalent joint ROM and Lysholm and IKDC scores at final follow-up. This clinical outcome is supported by results of kinematic studies of these hinged devices, which are capable of rigid fixation in all planes except sagittal and can reduce stress on intra-articular and periarticular ligaments when placed on the appropriate flexion-extension axis of the knee.19,20Unfortunately, the situation is more complicated for subacute or chronic tibial subluxation than for acute subluxation. Maak and colleagues16 described 3 operative steps that are crucial in obtaining desired outcomes in this setting: complete release of scar tissue, re-creation of knee axis through ACL and PCL reconstruction, and postoperative application of a HEF or knee brace. These recommendations mimic the management course described by Richter and Lobenhoffer13 and Simonian and colleagues,14 who treated chronic fixed posterior tibial subluxations with arthrolysis, ligament reconstruction, and use of HEFs for 6 weeks, supporting postoperative rehabilitation. All cases maintained reduction at follow-up after fixator removal.
It is also possible for small fixed anterior or posterior tibial subluxations to be managed nonoperatively. Strobel and colleagues15 described a series of 109 patients with fixed posterior subluxations treated at night with posterior tibial support braces. Mean subluxation was reduced from 6.93 mm to 2.58 mm after an average treatment period of 180 days. Although 60% of all subluxations were completely reduced, reductions were significantly more successful for those displaced <10 mm.
Management of subacute or chronic fixed coronal tibial subluxations is yet to be described. In this article, we have reported on acceptable reduction of a subacute medial tibial subluxation with use of a HEF for 6 weeks after arthroscopic débridement of a deformed subacute bucket-handle lateral meniscus tear. Our case report is unique in that it describes use of a HEF alone for the reduction of a subacute tibial subluxation in any plane without the need for more extensive ligament reconstruction.
The injury here was primarily a lateral ligamentous injury. In the nonanatomical repair that was performed, the LCL and the iliotibial band were reattached to the proximal-lateral tibia. Had we started treating this injury from the time of the patient’s accident, then, depending on repair integrity, we might have considered acute augmentation of the anatomical repair of LCL with Larson-type reconstruction of the LCL and the popliteofibular ligament. Alternatively, acute reconstruction of the LCL and popliteus would be considered if the lateral structures were either irreparable or of very poor quality. In addition, had we initially seen the coronal instability/translation, we might have acutely considered either a staged procedure of a multiplanar external fixator or a HEF.
Given the narrowed lateral joint space, the débridement of the lateral meniscus, and the risk of developing posttraumatic arthritis, our patient will probably need total knee arthroplasty (TKA) at some point. We informed her that she had advanced lateral compartment joint space narrowing and arthritic progression and that she would eventually need TKA based on pain or dysfunction. We think the longevity of that TKA will be predictable and good, as she now had improved tibiofemoral alignment and stability of the collateral ligamentous structures. If she had been allowed to maintain the coronally subluxed position, it would have led to medial ligamentous attenuation and would have compromised the success and longevity of the TKA. In essence, a crucial part of the utility of the HEF was improved coronal tibiofemoral alignment and, therefore, decreased abnormal forces on both the repaired lateral ligaments and the native medial ligamentous structures. Although temporary external fixation issues related to infection risk and patient discomfort are recognized,21-23 use of HEF alone can be part of the treatment considerations for fixed tibial subluxations in any plane when they present after treatment for multiligamentous injury.
Am J Orthop. 2016;45(7):E497-E502. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
1. Stannard JP, Sheils TM, McGwin G, Volgas DA, Alonso JE. Use of a hinged external knee fixator after surgery for knee dislocation. Arthroscopy. 2003;19(6):626-631.
2. Yeh WL, Tu YK, Su JY, Hsu RW. Knee dislocation: treatment of high-velocity knee dislocation. J Trauma. 1999;46(4):693-701.
3. Bellabarba C, Bush-Joseph CA, Bach BR Jr. Knee dislocation without anterior cruciate ligament disruption. A report of three cases. Am J Knee Surg. 1996;9(4):167-170.
4. Cooper DE, Speer KP, Wickiewicz TL, Warren RF. Complete knee dislocation without posterior cruciate ligament disruption. A report of four cases and review of the literature. Clin Orthop Relat Res. 1992;(284):228-233.
5. Howells NR, Brunton LR, Robinson J, Porteus AJ, Eldridge JD, Murray JR. Acute knee dislocation: an evidence based approach to the management of the multiligament injured knee. Injury. 2011;42(11):1198-1204.
6. Magit D, Wolff A, Sutton K, Medvecky MJ. Arthrofibrosis of the knee. J Am Acad Orthop Surg. 2007;15(11):682-694.
7. Medvecky MJ, Zazulak BT, Hewett TE. A multidisciplinary approach to the evaluation, reconstruction and rehabilitation of the multi-ligament injured athlete. Sports Med. 2007;37(2):169-187.
8. Noyes FR, Barber-Westin SD. Reconstruction of the anterior and posterior cruciate ligaments after knee dislocation. Use of early protected postoperative motion to decrease arthrofibrosis. Am J Sports Med. 1997;25(6):769-778.
9. Stannard JP, Nuelle CW, McGwin G, Volgas DA. Hinged external fixation in the treatment of knee dislocations: a prospective randomized study. J Bone Joint Surg Am. 2014;96(3):184-191.
10. Bottlang M, Marsh JL, Brown TD. Articulated external fixation of the ankle: minimizing motion resistance by accurate axis alignment. J Biomech. 1999;32(1):63-70.
11. Madey SM, Bottlang M, Steyers CM, Marsh JL, Brown TD. Hinged external fixation of the elbow: optimal axis alignment to minimize motion resistance. J Orthop Trauma. 2000;14(1):41-47.
12. Jupiter JB, Ring D. Treatment of unreduced elbow dislocations with hinged external fixation. J Bone Joint Surg Am. 2002;84(9):1630-1635.
13. Richter M, Lobenhoffer P. Chronic posterior knee dislocation: treatment with arthrolysis, posterior cruciate ligament reconstruction and hinged external fixation device. Injury. 1998;29(7):546-549.
14. Simonian PT, Wickiewicz TL, Hotchkiss RN, Warren RF. Chronic knee dislocation: reduction, reconstruction, and application of a skeletally fixed knee hinge. A report of two cases. Am J Sports Med. 1998;26(4):591-596.
15. Strobel MJ, Weiler A, Schulz MS, Russe K, Eichhorn HJ. Fixed posterior subluxation in posterior cruciate ligament-deficient knees: diagnosis and treatment of a new clinical sign. Am J Sports Med. 2002;30(1):32-38.
16. Maak TG, Marx RG, Wickiewicz TL. Management of chronic tibial subluxation in the multiple-ligament injured knee. Sports Med Arthrosc Rev. 2011;19(2):147-152.
17. Shelbourne KD, Haro MS, Gray T. Knee dislocation with lateral side injury: results of an en masse surgical repair technique of the lateral side. Am J Sports Med. 2007;35(7):1105-1116.
18. Levy BA, Fanelli GC, Whelan DB, et al. Controversies in the treatment of knee dislocations and multiligament reconstruction. J Am Acad Orthop Surg. 2009;17(4):197-206.
19. Fitzpatrick DC, Sommers MB, Kam BC, Marsh JL, Bottlang M. Knee stability after articulated external fixation. Am J Sports Med. 2005;33(11):1735-1741.
20. Sommers MB, Fitzpatrick DC, Kahn KM, Marsh JL, Bottlang M. Hinged external fixation of the knee: intrinsic factors influencing passive joint motion. J Orthop Trauma. 2004;18(3):163-169.
21. Anglen JO, Aleto T. Temporary transarticular external fixation of the knee and ankle. J Orthop Trauma. 1998;12(6):431-434.
22. Behrens F. General theory and principles of external fixation. Clin Orthop Relat Res. 1989;(241):15-23.
23. Haidukewych GJ. Temporary external fixation for the management of complex intra- and periarticular fractures of the lower extremity. J Orthop Trauma. 2002;16(9):678-685.
Fat Embolism Syndrome With Cerebral Fat Embolism Associated With Long-Bone Fracture
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Fat embolism syndrome (FES) occurs in long-bone fractures and classically presents with the triad of hypoxia, petechia, and altered mental status, or the criteria of Gurd and Wilson.1 The Lindeque criteria (femur fracture, pH <7.3, increased work of breathing) are also used.1,2 FES is a potentially fatal complication, with mortality rates ranging from 10% to 36%.1,3 FES typically occurs within 24 to 72 hours after initial insult, with one study finding an average of 48.5 hours after injury and an incidence of 0.15% to 2.4%.4 The overall FES rate is <1% in retrospective reviews and 11% to 29% in prospective studies.5 FES may present without one or all of the Gurd and Wilson criteria,6 and cerebral fat embolism (CFE) can be even more difficult to diagnose. Patients with CFE typically present with a wide array of postoperative neurologic deficits, commonly in the 24- to 72-hour window in which FES typically occurs. Correct diagnosis and management of CFE require a high index of suspicion and knowledge of the diagnostic work-up. In the postoperative setting, it can be difficult to distinguish CFE-related neurologic deficits from the normal sequelae of anesthesia, pain medications, and other factors.
In this article, we report the case of a 42-year-old woman who developed CFE after reamed intramedullary nail fixation of femoral and tibial shaft fractures. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old woman with no past medical history was injured when a horse reared and fell on her. Initial emergent computed tomography (CT) was negative for intracranial hemorrhage, and injury radiographs were obtained (Figures 1A, 1B).
About 9 hours after surgery and 36 hours after injury, the patient was unresponsive. Vital signs, including oxygen saturation, were within normal limits, but she was unable to verbalize. Physical examination revealed symmetric facial musculature but also generalized weakness and diffuse hypertonicity and hyperreflexia. Initial laboratory results, including complete blood cell count, electrolyte panel, and troponin levels, were unremarkable. Naloxone was administered to rule out opioid overdose. An immediate code stroke and neurology consultation was requested. An emergent CT scan of the brain was negative; an urgent magnetic resonance imaging (MRI) scan showed multiple punctate T2/FLAIR (fluid attenuated inversion recovery) hyperintensities with restricted diffusion, predominantly in a parasagittal white matter distribution (Figure 2).
The patient slowly and steadily improved. She was verbal by postoperative day 3 (POD-3), upper motor neuron signs resolved by POD-4, encephalopathy resolved by POD-7, and she was discharged to a rehabilitation center. Unresolved post-stroke symptoms included mild visual field deficits in the right eye (20/25 vision, central scotoma) and amnesia regarding the events immediately surrounding the surgery. There were no other neurologic or cognitive deficits. The patient was non-weight-bearing on the operative extremity and ambulating with assistance, and she started range-of-motion exercises. After 1 week, she was discharged home with crutches.
The patient followed up with neurology and ophthalmology for routine post-stroke care. At 2- and 6-month neurology follow-ups, she was still amnestic regarding her acute stroke event but did not exhibit any confusion, memory problems, speech deficits, facial droop, headaches, or weakness. According to neurology, the encephalopathy was completely resolved, and the patient was completely recovered from the event. Levetiracetam and aspirin were discontinued at 2 months. At the 2-month ophthalmology follow-up, the patient had 20/20 vision in both eyes and nearly complete resolution of the central scotoma. Ophthalmology confirmed symptom relief and recommended return to routine eye care and 1-year follow-up.
The patient began weight-bearing as tolerated on POD-14 and had no hardware or other complications. At 6-month orthopedics follow-up, range of motion of the affected knee was 0° to 120°, and rotation, length, and varus/valgus and anteroposterior knee laxity were all symmetric to the contralateral extremity. The patient walked with a cane for balance and had a mild limp. The affected thigh still had mild atrophy, but strength was 5/5 throughout. The patient denied pain or hardware sensitivity in the affected extremity and was very pleased with the result.
Discussion
Postoperative Acute Mental Status Change
There are many causes of postoperative mental status change after intramedullary nailing. Change may be cardiogenic, infectious, pharmacologic, or neurologic in origin. Age should be considered in the work-up of postoperative mental status change, as common complications differ between older and younger patients, with geriatric patients at particularly high risk for delirium.
Next to be evaluated are vital signs—particularly hypoxia, as isolated tachycardia may simply be a manifestation of pain. The cardiac system is then assessed with EKG and cardiac-specific laboratory tests, including a troponin level test if there is suspicion of myocardial infarction. PE and FES are complications with a higher prevalence in intramedullary nailing, and pulmonary involvement can be ruled out with the CT with PE protocol. Skin examination is important as well, as FES presents with petechial rash in 60% of patients8 (rash was absent in our patient’s case). Narcotic overdose is easily ruled out with administration of naloxone. Infection and sepsis can cause mental changes, though more commonly in the elderly and seldom so soon after surgery. Evaluation for infection and sepsis involves urinalysis and culturing of blood, urine, and other bodily fluids. If there is concern about surgical site infection, the postoperative dressing should be immediately removed and the wound examined. Last, neurologic and embolic phenomena can be initially investigated with CT to rule out hemorrhagic stroke. If CT of the brain is negative, MRI should be performed. MRI is the gold standard for diagnosing ischemic stroke and CFE caused by FES.9
Prevalence of Fat Embolism Syndrome
Development of intramedullary fat release in patients with long-bone injuries is common. A prospective study found circulating fat globules in 95% of 43 patients with femur fractures.10 In another study, transesophageal EKG showed cardiac embolism in 62% of patients who had undergone intramedullary nail fixation.11 Despite this high rate, only 0.9% to 2.2% of patients developed symptomatic FES. Risk factors for FES include younger age, multiple fractures, closed fractures, and nonoperative or delayed management of long-bone fractures.2 As already mentioned, average time to FES presentation after long-bone fracture is about 48 hours. One study found that FES typically occurs within 24 to 72 hours after initial insult (average, 48.5 hours) and that the incidence of FES is 0.15% in tibia fractures, 0.78% in femur fractures, and 2.4% in multiple long-bone fractures.4 The timeline is consistent with the present case—our patient developed symptoms about 36 hours after injury. In addition, other studies have found a higher mortality rate (5%-15%) for patients with bilateral femur fractures than for patients with only one fracture.7,12,13 Patients with a floating knee injury (ipsilateral tibia and femur fractures) are at higher risk for FES and have higher overall morbidity and mortality rates in comparison with patients with an isolated femur or tibia fracture, though the increased risk has not been quantified.
Review of Case Literature: FES With CFE
Few cases of FES with symptomatic CFE in the setting of long-bone fracture or long-bone surgery have been reported in the literature. There is wide variation in the development of FES with respect to preoperative or postoperative status and mechanism of injury. Duran and colleagues14 described a 20-year-old man with ipsilateral tibia and femur fractures caused by gunshots. Twenty-four hours after presentation, he developed tonic-clonic seizures and the classic triad of rash, hypoxia, and altered mental status. MRI confirmed CFE secondary to FES. The patient was optimized neurologically before definitive fixation and was discharged with minimal neurologic deficits on POD-27. Chang and colleagues15 and Yeo and colleagues16 described CFE in patients who underwent bilateral total knee arthroplasty. Symptoms developed 9 hours and 2 days after surgery, respectively. Both patients had fat emboli in the lungs and brain, underwent intensive care treatment, and recovered from the initial insult. After discharge at 44 days and 2 weeks, respectively, they fully recovered.
Other patients with CFE have had less favorable outcomes. Chen and colleagues6 reported the case of a 31-year-old man who sustained closed femur and tibia fractures in an automobile collision and experienced an acute decline in neurologic status 1 hour after arrival in the emergency department. The patient was intubated, CFE was diagnosed on the basis of MRI findings, and the orthopedic injuries were treated with external fixation. After 2 weeks, the patient was discharged with persistent neurologic deficits and the need for long-term tube feeding. Walshe and colleagues17 reported the case of a 19-year-old woman who sustained multiple long-bone injuries and traumatic brain injury and showed fat emboli on MRI. The patient experienced brain herniation while in the intensive care unit and later was declared brain-dead. According to the literature, it is important to maintain high suspicion for FES and possible CFE in the setting of high-energy fracture but also to be aware that FES may develop even with nondisplaced fracture or with reaming of the intramedullary canal in elective total joint arthroplasty.18
Pathophysiology of Fat Embolism Syndrome
The pathophysiology of FES and specifically of CFE is not widely understood. Two main theories on the development of FES have been advanced.
The mechanical theory suggests that exposing intramedullary long-bone contents allows fat to mobilize into the bloodstream.19 This occurs in the setting of long-bone fracture and in canal preparation during joint replacement surgery. More fat extravasates into the venous system after femur fracture than after tibia fracture, which accounts for the higher risk for FES in femoral shaft fractures and the even higher risk in concomitant femur and tibia fractures.4 In addition to there being a risk of fat embolism from the fracture itself, placing the patient in traction or reaming the intramedullary canal may exacerbate this effect by increased extravasation of fat from the medullary canal. With extravasation of fatty bone marrow into the venous system, fat emboli are free to travel back to the lungs, where they can cause infarcts within the lung parenchyma.
In the mechanical theory, presence of PFO may allow fat globules to pass into the systemic circulation and cause end-organ emboli. In the event of cerebral emboli, neurologic symptoms may vary widely and may include diffuse encephalopathy and global deficits.20 Dog studies have found a possible mechanism for CFE in the absence of PFO. One such study, which used femoral pressurization to replicate cemented femoral arthroplasty, found that many fat globules had traversed the lungs after release into bone marrow,21 supporting the theory that fat droplets can traverse the pulmonary system without sequestration in the lung parenchyma. Riding and colleagues22 reported finding pulmonary arteriovenous shunts, which are thought to allow CFE to occur in the absence of PFO. More studies are needed to determine the prevalence of shunts and their overall contribution to CFE development in patients with long-bone fracture.
The biochemical theory holds that bodily trauma induces the release of free fatty acids (FFAs) from the capillaries into the bloodstream.23 This stress response is mediated by catecholamines, which activate the adenyl cyclase pathway, which activates lipase, which hydrolyzes stored triglycerides to FFAs and glycerol. The concentration of circulating FFA was increased in 9 of 10 patients in one study.23 Increased FFAs in the bloodstream can accelerate local and end-organ clotting, leading to thrombocytopenia and endothelial injury. In addition, patients with hypercoagulable diseases are at higher risk for postoperative thromboembolism.24 However, with a negative hypercoagulable work-up and with negative chest helical CT and EKG, which did not demonstrate PFO, the explanation for CFE in our patient may more likely reside with the arteriovenous shunt theory proposed by Riding and colleagues.22
Diagnosis and Treatment
Proper care of orthopedic patients who potentially have FES/CFE involves prompt diagnosis, immediate symptomatic care, and early coordination with neurology and medical services to rule out other causes of symptoms. Obtaining advanced imaging to rule out other potential causes and to confirm the diagnosis is crucial. The patient in this case report did not exhibit any focal neurologic deficits, but emergent CT of the brain was indicated to rule out a hemorrhagic event. If a stroke secondary to FES is clinically suspected, MRI should be obtained as soon as possible. Multiple studies have found that the “starfield” pattern, which is best seen as multiple punctate hyperintensities on T2 imaging, is the typical radiographic manifestation of CFE.9 This applies to patients who are in the 24- to 72-hour window after long-bone fracture or fixation and who fit Gurd and Wilson1 criteria or Lindeque1,2criteria, or who exhibit a change in mental status but have a negative CT scan of the brain, as was the case with our patient. Once the diagnosis is made, treatment involves addressing the symptoms (Figure 4).
Fat Embolism Syndrome in Reamed and Unreamed Nailing
Over the past several decades, the number of long bones fixed with intramedullary nails has increased significantly.26 There is debate regarding whether use of reamed intramedullary nails increases the risk of fat emboli relative to use of unreamed nails, but multiple studies have found no significant difference.26,27 Pulmonary shunting occurs in both reamed and unreamed nailing; neither technique has an advantage in terms of cardiopulmonary complications. In multiple studies, reamed nails have the advantage of improved healing rates.27 A sheep study compared reamed and unreamed femoral nailing.28 After nailing, sheep lungs were examined histologically for the presence of bone marrow fat embolism. The embolism rate was higher with unreamed nailing (10.25%) than with reamed nailing (6.66%). One large study of the adverse effects of reamed and unreamed nailing in 1226 patients with tibial shaft fracture found that those with open fractures had higher rates of a negative event (nonunion, infection, fasciotomy, hardware failure, need for dynamization) after reamed nailing.29 Patients with closed fractures had fewer events after reamed nailing. The authors concluded there is a potential benefit in outcome with reamed intramedullary nailing in patients with closed tibial shaft fractures, but they did not comment on development of FES. In a study of the effect of subject position on intramedullary pressure and fat embolism release, dogs were positioned either supine or lateral for tibial and femoral reaming.30 The authors measured various physiologic parameters, including cardiac output, pulmonary arterial wedge pressure, arterial and venous blood gas, and blood cell counts. There were no statistically significant differences in values between the 2 groups in any variable, indicating that position does not affect FES development in the orthopedic trauma setting.
Conclusion
FES and CFE are potential devastating sequelae of both long-bone fracture and long-bone instrumentation. It is important to recognize these entities in the acute setting and to consider them in the differential diagnosis of a trauma or postoperative patient who experiences sudden onset of altered mental status with or without dyspnea or a petechial rash. If CFE is suspected, early advanced imaging (including urgent MRI) should be obtained with rapid involvement of a multidisciplinary team that can optimize the chance for successful recovery of both neurologic and physical function. The best treatment, early prevention and diagnosis, maximizes care of symptoms. As is evidenced in this case report, rapid diagnosis and treatment often result in recovery from a majority of the symptoms of FES and CFE.
Am J Orthop. 2016;45(7):E515-E521. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
1. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
2. Schonfeld SA, Ploysongsang Y, DiLisio R, et al. Fat embolism prophylaxis with corticosteroids. A prospective study in high-risk patients. Ann Intern Med. 1983;99(4):438-443.
3. Robinson CM. Current concepts of respiratory insufficiency syndromes after fracture. J Bone Joint Surg Br. 2001;83(6):781-791.
4. Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture—clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73(8):407-410.
5. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37(1):5-8.
6. Chen PC, Hsu CW, Liao WI, Chen YL, Ho CH, Tsai SH. Hyperacute cerebral fat embolism in a patient with femoral shaft fracture. Am J Emerg Med. 2013;31(9):1420.e1-e3.
7. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
8. Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38(1):52-55.
9. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
10. Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: a prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14(11):955-962.
11. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
12. Wildsmith JA, Masson AH. Severe fat embolism: a review of 24 cases. Scott Med J. 1978;23(2):141-148.
13. Nork SE, Agel J, Russell GV, Mills WJ, Holt S, Routt ML Jr. Mortality after reamed intramedullary nailing of bilateral femur fractures. Clin Orthop Relat Res. 2003;(415):272-278.
14. Duran L, Kayhan S, Kati C, Akdemir HU, Balci K, Yavuz Y. Cerebral fat embolism syndrome after long bone fracture due to gunshot injury. Indian J Crit Care Med. 2014;18(3):167-169.
15. Chang RN, Kim JH, Lee H, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty. A case report. Korean J Anesthesiol. 2010;59(suppl):S207-S210.
16. Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5(3):239-242.
17. Walshe CM, Cooper JD, Kossmann T, Hayes I, Iles L. Cerebral fat embolism syndrome causing brain death after long-bone fractures and acetazolamide therapy. Crit Care Resusc. 2007;9(2):184-186.
18. Kamano M, Honda Y, Kitaguchi M, Kazuki K. Cerebral fat embolism after a nondisplaced tibial fracture: case report. Clin Orthop Relat Res. 2001;(389):206-209.
19. Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329(13):961-963.
20. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(suppl 4):S68-S73.
21. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994;150(5 pt 1):1416-1422.
22. Riding G, Daly K, Hutchinson S, Rao S, Lovell M, McCollum C. Paradoxical cerebral embolisation. An explanation for fat embolism syndrome. J Bone Joint Surg Br. 2004;86(1):95-98.
23. Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11(12):1026-1030.
24. Rodríguez-Erdmann F. Bleeding due to increased intravascular blood coagulation. Hemorrhagic syndromes caused by consumption of blood-clotting factors (consumption-coagulopathies). N Engl J Med. 1965;273(25):1370-1378.
25. Satoh H, Kurisu K, Ohtani M, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial Doppler sonography, and single photon emission computed tomography: case report. J Trauma. 1997;43(2):345-348.
26. Deleanu B, Prejbeanu R, Poenaru D, Vermesan D, Haragus H. Reamed versus unreamed intramedullary locked nailing in tibial fractures. Eur J Orthop Surg Traumatol. 2014;24(8):1597-1601.
27. Helttula I, Karanko M, Gullichsen E. Similar central hemodynamics but increased postoperative oxygen consumption in unreamed versus reamed intramedullary nailing of femoral fractures. J Trauma. 2006;61(5):1178-1185.
28. Högel F, Gerlach UV, Südkamp NP, Müller CA. Pulmonary fat embolism after reamed and unreamed nailing of femoral fractures. Injury. 2010;41(12):1317-1322.
29. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients With Tibial Fractures Investigators; Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
30. Syed KA, Blankstein M, Bhandari M, Nakane M, Zdero R, Schemitsch EH. The effect of patient position during trauma surgery on fat embolism syndrome: an experimental study. Indian J Orthop. 2014;48(2):203-210.
Mycotic Septic Arthritis of the Ankle Joint
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Septic arthritis is a common orthopedic emergency. The most common causative organism is Staphylococcus aureus. Mycotic infections, such as those involving Candida organisms, are much less common but just as debilitating. Delayed diagnosis of septic arthritis caused by Candida infection may result in increased morbidity, making treatment more challenging. Here we report a case of Candida albicans septic arthritis of the ankle and subtalar joint in a patient with diabetes mellitus (DM) and rheumatoid arthritis (RA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 52-year-old woman with type 2 DM (requiring subcutaneous insulin analogue therapy) and RA presented to a local emergency department with a 3-day history of right ankle pain after having the subtalar joint injected with steroid by a rheumatologist 4 weeks earlier. For about 2 weeks, there was purulent discharge from the peroneal sheath. The patient’s RA was being treated with prednisolone (maintenance therapy). Physical examination revealed low-grade pyrexia (37.8°C) and difficulty bearing full weight on the ankle. Clinically, the joint was not erythematous, but active and passive movements were painful. Blood tests revealed a C-reactive protein level of 98 mg/dL and a white blood cell (WBC) count of 11.3 × 109/L. Erythrocyte sedimentation rate (ESR) was not checked. The ankle underwent magnetic resonance imaging (Figures A-D).
Mycotic screening of the fluid was positive for C albicans. The patient was referred to the orthopedic team, which performed urgent arthroscopic surgical débridement, biopsy, and washout of the subtalar joint. After surgery, a 6-week course of antifungal therapy with anidulafungin was started, per specialist microbiology advice.
The septic ankle was successfully managed with arthroscopic surgical débridement followed by treatment with anidulafungin. The patient continued to make good progress and was weight-bearing when discharged home from the orthopedic unit.
Discussion
Worldwide, about 1 in 6 people has arthritis, which affects daily lifestyle and reduces quality of life. Degenerative, inflammatory, and septic arthritis each has its management challenges.1
Septic arthritis is an acute infection of the joint, usually of bacterial etiology. It can present as a polyarticular arthropathy (~15% of cases),2,3 but a monoarthropathy of the hip, knee, or ankle is more common.4The Kocher criteria are often applied to cases of suspected septic arthritis of joints, even though they were initially used to distinguish septic arthritis from transient synovitis in pediatric hip joints.5 Kocher and colleagues5 reported 4 key clinical criteria: inability to bear weight, WBC count over 12 × 109/L, ESR over 40 mm/h, and temperature over 38.5°C. When all 4 criteria are met, the predictive value is 99.6%. These criteria are now widely applied to adult joints, and not only the hips.
In septic arthritis, the most common causative pathogen is S aureus.3,6Streptococcus, Neisseria, and Pseudomonas also are common.7 Although much rarer, Candida variants and other mycotic pathogens have been implicated as well.8C albicans is a well-known fungus that colonizes mucosal surfaces. Research indicates increased oral C albicans colonization in rheumatoid patients.9 Although most Candida septic arthritis cases are caused by C albicans, there is no large body of data showing the true incidence of fungal pathogens in septic arthritis.
Our literature search yielded 2 case reports on Candida septic arthritis involving the ankle, but the causative organisms were Candida parapsilosis and Candida glabrata.9,10 Cases of Candida septic arthritis involving the knee or shoulder have also been reported.11-15 Case reports demonstrate that Candida fungal arthritis is extremely rare.9 Etiology reportedly includes direct intra-articular inoculation by surgery or secondary to hematogenous seeding, particularly in immunocompromised patients.10 Risk factors include immunosuppression and joint suppression. DM and RA are common comorbidities in patients with septic arthritis.6,16 The pathophysiology of RA is inflammatory pannus formation of the periarticular surface with subsequent articular cartilage destruction and erosion, as well as progressive deformity and functional debilitation.1Patients with DM are at increased risk for developing fungal and other infections. Factors increasing this risk include disruption of skin-barrier integrity; reduced peripheral oxygen and blood supply, which also disrupts antibiotic delivery; and hyperglycemia-induced reduction in antibody function and disruption of phagocytosis and chemotaxis.17Fungi are eukaryotic, and infections caused by these organisms are difficult to treat.18 As fungal infections are more prevalent among immunosuppressed patients, they often result in prolonged treatment without guarantee of eradication, as spores may persist subclinically.
Literature on C albicans septic arthritis is lacking in general but especially in rheumatoid patients. Delayed diagnosis and suboptimal treatment may result in fungal osteomyelitis. There is little evidence on treating this rare fungal complication, and outcomes historically have been poor.19In an animal model, Marijnissen and colleagues20 found that C albicans infection can increase destruction in an arthritic joint by cytokine environment modification. The result was advanced destruction of the joint and debilitation. For disease management, the authors considered these essential: early diagnosis, prompt treatment, and, as indicated, surgical débridement.
Treatment of Candida septic arthritis largely involves use of antifungal medication, either with surgical débridement, as in our patient’s case, or without. Which antifungal medication to use should be based on sensitivities, identified from wound aspirate, and microbiology advice about treatment duration. The antibiotic should be a broad-spectrum antifungal cover, in keeping with local antibiotic prescribing guidelines, which can be refined once definitive organism culture and sensitivity results are known. However, early aggressive treatment is essential. Periprosthetic fungal infection is rarely resolved without implant removal.21
Conclusion
This case reflects the complexities of septic arthritis caused by atypical pathogens and highlights the need for clinical vigilance in the setting of comorbidities, such as DM and RA. Failure to consider the diagnosis early on might result in delayed and inadequate treatment, increased joint destruction, and, potentially, osteomyelitis with subsequent increased morbidity. Early diagnosis (based on joint aspirate findings), surgical débridement, and prolonged aggressive treatment with antifungal medication are the mainstays of treatment.
Am J Orthop. 2016;45(7):E478-E480. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.
1. Auday BC, Buratovich MA, Marrocco, GF, Moglia P, eds. Magill’s Medical Guide. 7th ed. Ipswich, MA: Salem Press; 2014.
2. Dhaliwal S, LeBel ME. Rapidly progressing polyarticular septic arthritis in a patient with rheumatoid arthritis. Am J Orthop. 2012;41(7):E100-E101.
3. Mateo Soria L, Olivé Marqués A, García Casares E, García Melchor E, Holgado Pérez S, Tena Marsà X. Polyarticular septic arthritis: analysis of 19 cases [in Spanish]. Reumatol Clin. 2009;5(1):18-22.
4. Caksen H, Oztürk MK, Uzüm K, Yüksel S, Ustünbaş HB, Per H. Septic arthritis in childhood. Pediatr Int. 2000;42(5):534-540.
5. Kocher MS, Zurakowski D, Kasser JR. Differentiating between septic arthritis and transient synovitis of the hip in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 1999;81(12):1662-1670.
6. Madruga Dias J, Costa MM, Pereira da Silva JA, Viana de Queiroz M. Septic arthritis: patients with or without isolated infectious agents have similar characteristics. Infection. 2014;42(2):385-391.
7. Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol. 2014;20(2):74-78.
8. Zmierczak H, Goemaere S, Mielants H, Verbruggen G, Veys EM. Candida glabrata arthritis: case report and review of the literature of Candida arthritis. Clin Rheumatol. 1999;18(5):406-409.
9. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans–specific Th17 responses. Arthritis Res Ther. 2014;16(1):R50.
10. Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
11. Sung J, Chun K. Candida parapsilosis arthritis involving the ankle in a diabetes patient. J Korean Soc Radiol. 2011;64:587-591.
12. Marmor L, Peter JB. Candida arthritis of the knee joint. Clin Orthop Relat Res. 1976;(118):133-135.
13. Turgut B, Vural O, Demir M, Kaldir M. Candida arthritis in a patient with chronic myelogenous leukemia (CML) in blastic transformation, unresponsive to fluconazole, but treated effectively with liposomal amphotericin B. Ann Hematol. 2002;81(9):529-531.
14. Christensson B, Ryd L, Dahlberg L, Lohmander S. Candida albicans arthritis in a nonimmunocompromised patient. Complication of placebo intraarticular injections. Acta Orthop Scand. 1993;64(6):695-698.
15. Jeong YM, Cho HY, Lee SW, Hwang YM, Kim YK. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465-469.
16. Favero M, Schiavon R, Riato L, Carraro V, Punzi L. Septic arthritis: a 12 years retrospective study in a rheumatological university clinic [in Italian]. Reumatismo. 2008;60(4):260-267.
17. Leslie D, Lansang C, Coppack S, Kennedy L. Diabetes: Clinician’s Desk Reference. Boca Raton, FL: CRC Press; 2012.
18. Silva PM, Gonçalves S, Santos NC. Defensins: antifungal lesions from eukaryotes. Front Microbiol. 2014;5:97.
19. Bariteau JT, Waryasz GR, McDonnell M, Fischer SA, Hayda RA, Born CT. Fungal osteomyelitis and septic arthritis. J Am Acad Orthop Surg. 2014;22(6):390-401.
20. Marijnissen RJ, Koenders MI, van de Veerdonk FL, et al. Exposure to Candida albicans polarizes a T-cell driven arthritis model towards Th17 responses, resulting in a more destructive arthritis. PLoS One. 2012;7(6):e38889.
21. International Consensus on Periprosthetic Joint Infection. Musculoskeletal Infection Society website. http://www.msis-na.org/international-consensus. Published August 1, 2013. Accessed October 16, 2016.
Emergency Imaging: Facial Trauma After a Fall
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).
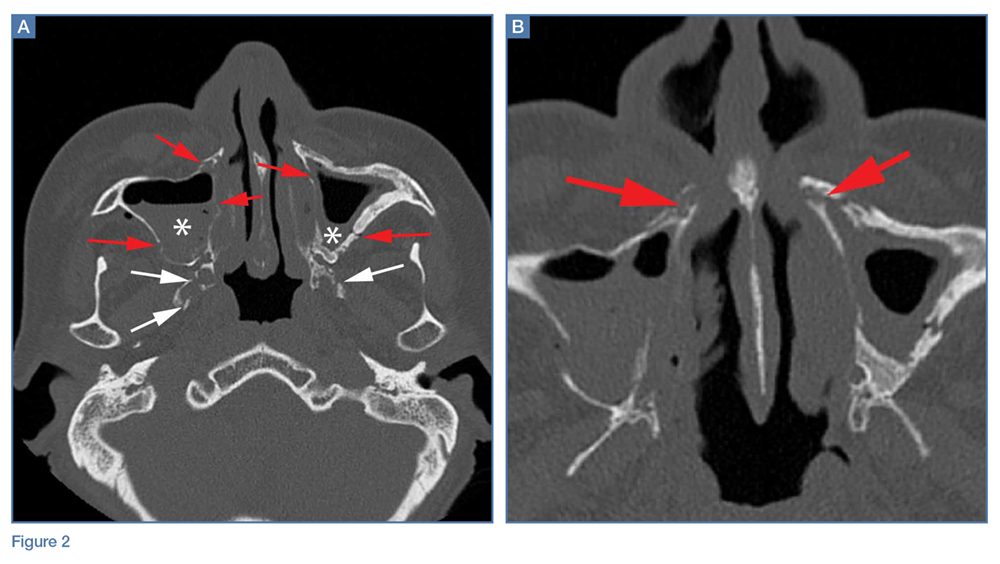
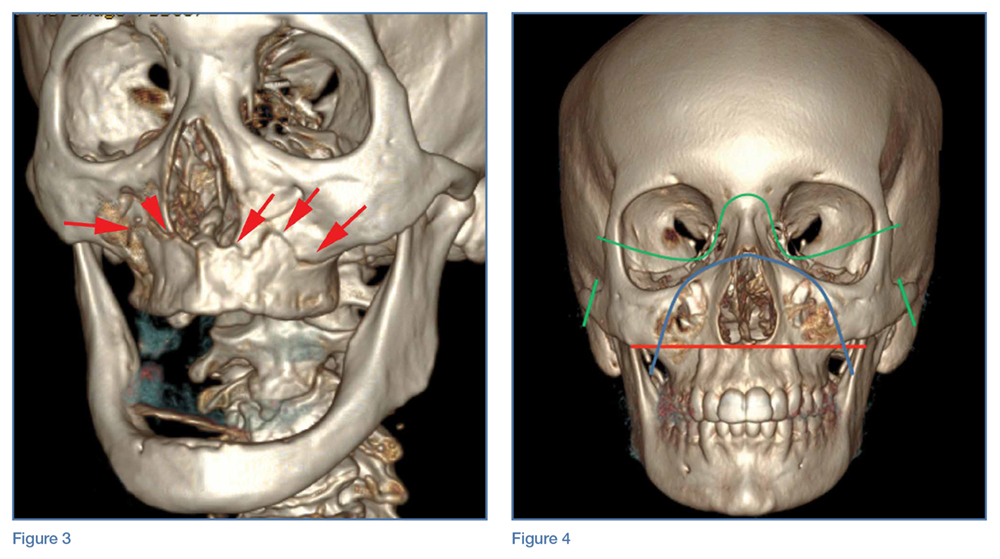
Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
An 89-year-old man presented to the ED with facial trauma due to a mechanical fall after losing his balance on uneven pavement and hitting the right side of his face. Physical examination revealed an ecchymosis inferior to the right eye and tenderness to palpation at the right maxilla and bilateral nasolabial folds. Maxillofacial computed tomography (CT) was ordered for further evaluation; representative images are presented above (Figure 1a and 1b).

What is the diagnosis?
Answer
A noncontrast CT of the maxillofacial bones demonstrated acute fractures through the bilateral pterygoid plates (white arrows, Figure 2a). The fractures extended through the medial and lateral walls of the bilateral maxillary sinuses (red arrows, Figure 2a), and propagated to the frontal processes of the maxilla (red arrows, Figure 2b), extending toward the alveolar process, indicating involvement of the anterolateral margin of the nasal fossa. The full extent of the fracture is best seen on a 3D-reconstructed image (red arrows, Figure 3). Additional images (not presented here) confirmed no fracture involvement of the orbital floors, nasal bones, or zygomatic arches. Expected posttraumatic hemorrhage was appreciated within the maxillary sinuses (white asterisks, Figure 2a).


Le Fort Fractures
The findings described above are characteristic of a Le Fort I fracture pattern. Initially described in 1901 by René Le Fort, a French surgeon, the Le Fort classification system details somewhat predictable midface fracture patterns resulting in various degrees of craniofacial disassociation.1 Using weights that were dropped on cadaveric heads, Le Fort discovered that the pterygoid plates must be disrupted in order for the midface facial bones to separate from the skull base. As such, when diagnosing a Le Fort fracture, fracture of the pterygoid plate must be present, regardless of the fracture type (Le Fort I, II, and III).2
Le Fort I Fracture. This fracture pattern (red line, Figure 4) is referred to as a “floating palate” and involves separation of the hard palate from the skull base via fracture extension from the pterygoid plates into the maxillary sinus walls, as demonstrated in this case. The key distinguisher of the Le Fort I pattern is involvement of the anterolateral margin of the nasal fossa.2
Le Fort II Fracture. This fracture pattern (blue line, Figure 4) describes a “floating maxilla” wherein the pterygoid plate fractures are met with a pyramidal-type fracture pattern of the midface. The maxillary teeth form the base of the pyramid, and the fracture extends superiorly through the infraorbital rims bilaterally and toward the nasofrontal suture.2,3 Le Fort II fractures result in the maxilla floating freely from the rest of the midface and skull base.
Le Fort III Fracture. This fracture pattern (green lines, Figure 4) describes a “floating face” with complete craniofacial disjunction resulting from fracture of the pterygoid plates, nasofrontal suture, maxillofrontal suture, orbital wall, and zygomatic arch/zygomaticofrontal suture.2,3
It is important to note that midface trauma represents a complex spectrum of injuries, and Le Fort fractures only account for a small percentage of facial bone fractures that present through Level 1 trauma centers.2 Le Fort fracture patterns can coexist with other fracture patterns and also can be seen in combination with each other. For example, one side of the face may demonstrate a Le Fort II pattern while the other side concurrently demonstrates a Le Fort III pattern. Though not robust enough for complete description of and surgical planning for facial fractures, this classification system is a succinct and well-accepted means of describing major fracture planes.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
1. Le Fort R. Etude experimentale sur les fractures de la machoire superieure. Rev Chir. 1901;23:208-227, 360-379, 479-507.
2. Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le Fort fractures. AJR Am J Roentgenol. 2005;184(5):1700-1705.
3. Hopper RA, Salemy S, Sze RW. Diagnosis of midface fractures with CT: what the surgeon needs to know. Radiographics. 2006;26(3):783-793.
Too Much of a Good Thing: Weakness, Dysphagia, and Stridor After Botulinum Toxin Injections
Case
A 68-year-old woman presented to the ED 5 days after receiving onabotulinumtoxinA cosmetic injections for wrinkles of the face and neck. She stated that she was unable to raise her head while in a supine position and that her head felt heavy when standing. She also experienced spasms and strain of the posterior cervical neck muscles. In addition, the patient described a constant need to swallow forcefully throughout the day, and felt an intermittent heavy sensation over her larynx that was associated with stridor. She noted these symptoms began 5 days after the onabotulinumtoxinA injections and had peaked 2 days prior to presentation. She also complained of dysphagia without odynophagia, but denied any changes in her voice.
The patient first began onabotulinumtoxinA injections 12 years earlier for aesthetic treatment of glabellar and peri-orbital wrinkles. She initially received the injections at a regular interval of 90 to 100 days. During the course of the first 2 years of treatment, the patient was under the care of a plastic surgeon; thereafter, she sought treatment at a physician-owned medical spa because it offered onabotulinumtoxinA at a lower price. The injections at the medical spa were administered by a physician assistant (PA). The patient stated that although the PA had steadily increased the dose of onabotulinumtoxinA to maintain the desired aesthetic effect, this was the first time she had experienced any side effects from the treatment.
The ED staff contacted the medical spa provider, who reviewed the patient’s medical record over the telephone. The PA stated that he had been the only practitioner at the facility to administer the onabotulinumtoxinA injections to the patient over her past 10 years there as a client. He further informed the emergency physician (EP) that 12 days prior to presentation, he had given the patient a total of 50 IU of onabotulinumtoxinA, in five separate injections, into the mid frontalis muscle; a total of 35 IU, in seven separate injections, into the glabellar region (procerus and corrugator muscles bilaterally); 20 IU into the lateral and inferior-lateral orbicularis oculi bilaterally, in four separate injections per side, (40 IU total); and a total of 100 IU in the anterior platysma, in 20 separate injections, for a total 1-day onabotulinumtoxinA dose of 225 IU.
The PA explained to the EP that he mixed the onabotulinumtoxinA in the patient’s room and had shown her the vials and dilution standard as recommended by the manufacturer because she had been requiring increased dosages and had previously questioned whether the onabotulinumtoxinA was diluted. The PA denied any other patients experiencing similar adverse events as those of the patient’s.
Over the last 10 years, the patient had received onabotulinumtoxinA in the nasolabial folds, upper and lower lip wrinkles, mentalis, depressor angular oris, buccal, nasalis, lateral brow, masseter, and calf muscles. The dosage of onabotulinumtoxinA at this most recent injection cycle was unchanged from her previous visit 3 months prior. According to the PA, the practice did not use abobotulinumtoxinA or incobotulinumtoxinA.
Regarding the patient’s medical history, she had no health issues suggestive of myasthenia gravis, multiple sclerosis, or Guillain-Barré syndrome. Examination of the face revealed decreased muscle excursion of the frontalis muscle from mid-brow to mid-brow, and stair-step wrinkle formation bilaterally. The procerus muscle was very weak, and the corrugator muscles were moderately diminished in strength. The lateral orbicularis oculi were very weak at each canthus. The extra-ocular muscles were intact. She had full mandibular excursion, and powerful movement of the tongue. The oropharynx and floor of the mouth were normal. She was noted to purposefully swallow and extend her neck every 90 to 120 seconds to “clear her throat,” though she did not drool and was able to handle her secretions and swallow fluids without aspiration. Her voice was normal and she was able to recite the letters “KKKKK,” “OOOOO,” and “EEEEE” in rapid fashion without breathiness or stridor. The rest of her facial muscles were normal.
While examining the patient, the EP asked her to refrain from swallowing whenever she extended her neck. Upon complying with this request, her neck extension precipitated swallowing and, by not swallowing, she did not accumulate secretions. Once during the examination, the patient began swallowing and breathing rapidly with stridor. This less than 15-second episode was abated by full-neck extensions, which relieved the patient’s sensation of heaviness over the larynx. Her breathing and voice were normal immediately after this episode.
Examination of the anterior neck revealed four platysmal bands (Figure). One band measured 10 cm in length and extended from the mandible inferiorly; two bands measured 2 cm lateral to the midline bilaterally; and the fourth band extended 4 cm in length from the mandible immediately lateral to the longer platysmal band. The platysma and dermis were flaccid and redundant at rest and with exertion. The sternocleidomastoid muscles were weak with exertion. The larynx moved cephalad with swallowing. The posterior cervical neck and trapezius muscles were of normal tone and strength. No spasms or fasciculations were noted during the examination period.
While supine, the patient strained to lift her head and complained of a suffocating sensation over the larynx. She had no rashes or edema, and the remainder of the physical examination, vital signs, and pulse oximetry were normal. Laboratory evaluation, which included a complete blood count and serum electrolytes, was also normal.
An otolaryngologist consultation for laryngoscopy was obtained. After reviewing the patient’s case, the otolaryngologist concluded that given the patient’s history, intermittent stridor, and an absence of signs or symptoms suggestive of an impending upper airway obstruction (UAO), laryngoscopy was not warranted.
A plastic surgery consultation was then obtained. The patient’s examination was as noted above, and her vital signs and pulse oximetry remained normal throughout her ED stay. Although botulinum and botulinum antibody titers were ordered, the patient refused testing due to cost concerns. She was discharged home by plastic surgery services with a diagnosis of floppy neck and dysphagia secondary to aesthetic botulinum toxin paralysis of the bilateral sternocleidomastoid muscles and platysma. She was given a prescription for metoclopramide hydrochloride to stimulate motility of the upper gastrointestinal tract and to potentially improve swallowing.10
The patient was scheduled for a follow-up evaluation with the plastic surgeon 2 days after discharge. She was instructed to call 911 if she experienced stridor, shortness of breath, drooling, or if any airway issues arose. The patient did not return for her follow-up appointment with the plastic surgeon.
Discussion
Clostridium Botulinum Toxins
Clostridium botulinum is a gram-positive spore-forming anaerobic bacterium that produces extremely potent neuro-exotoxins. C botulinum is found in soil, contaminated foods, and in illicit injectable drugs (eg, heroin). Seven distinct antigenic botulinum toxins (A, B, C1, D, E, F, and G) are produced by several strains of C botulinum. Systemically, each neurotoxin is able to produce severe morbidity and mortality by causing generalized muscle paralysis and death by respiratory failure. The lethal dose of these agents is approximating 10(-9) g/kg body weight. Botulinum toxin type A is the most potent.1,2
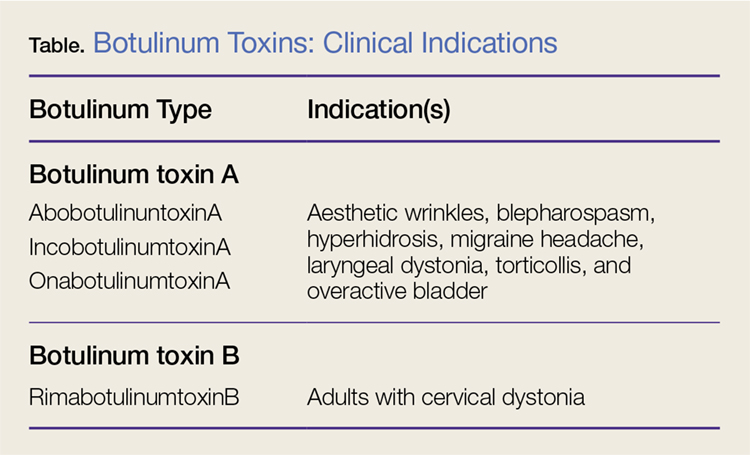
Nonetheless, botulinum toxin has been used clinically since the early 1970s. Currently, there are three FDA-approved botulinum toxin type A agents and one type B formulation (rimabotulinumtoxinB) (Table). Each formulation is unique, proprietary, and differs in molecular weight, toxin-complex size, protein content, and inactive ingredients. The effectiveness and adverse event profile for these four botulinum toxins is individually dependent upon the different dilutions and potency, onset of action, duration of effect, diffusion, and migration potential. Hence, the effective dose of one botulinum toxin does not equate to any other, resulting in a lack of interchangeability between botulinum toxins (eg, 5 IU of incobotulinumtoxinA does not equal 5 IU of onabotulinumtoxinA).
Aesthetic Indications
Historically, the use of botulinum toxin for aesthetic treatment of wrinkles and platysmal bands was first reported by Blitzer3 in 1993.Subsequently, the use of botulinum toxin for the aesthetic treatment of facial wrinkles, hypertrophic platysmal bands and horizontal neck lines gained popularity within the public and medical community.3-5
Anatomically, the platysma is a thin sheet-like muscle that originates in the superior fascia of the pectoralis and deltoid fascia, and extends over the full length of the neck up past the mandible and continuing into the submuscular aponeurotic system. The platysma is innervated by the seventh cranial nerve and functions to pull the jaw downward. The platysma muscle is attached directly to the skin. With normal aging, the anterior neck skin becomes flaccid, the central platysmal bands thicken and contract—forming bands, horizontal wrinkles, and loss of definition of the neck noticed at rest and with contraction of the platysma muscle. These vertical bands are known as platysmal bands. The platysmal bands are benign consequences of aging and as such are targets of correction through surgery or botulinum toxin injection.6,7
Mechanism of Action
Platysmal band and horizontal line injection techniques with botulinum toxin have been reported in the literature with dosages ranging from 15 IU to 200 IU used to block the Soluble N-ethylmaleimide-sensitive factor activating protein receptors. Typical onset of action begins at 3 days, with full paralytic effect at 7 days. Repeat injections every 3 to 4 months are required with prolonged effects seen with each subsequent injection due to chemodenervation-induced muscle atrophy.4,7,8
Adverse Effects
Commercial botulinum toxin type A has been associated with minor and transient side effects. Moderate complications seen in the neck region include transient soft-tissue edema, dermal ecchymoses, intramuscular hematoma, diffuse muscle soreness, neck flexor weakness, and headaches.4,8,9
The use of botulinum toxin for chemodenervation of the platysma can produce significant weakness of other neck muscles, including the sternocleidomastoid, cricothyroid, sternothyroid, and sternohyoid. Floppy neck and dysphagia may be due to diffusion of the toxin into the muscles of deglutition of the larynx; injection directly into the sternocleidomastoid muscle; or a result of the systemic effects of large dosages. Hoarseness, breathiness, and dysphagia may occur 3 to 4 days after injection, especially with doses over 75 IU.10
The recommended concentration of botulinum toxin type A causes a diffusion average of 1 cm in all directions from the injection sites. However, as the dilution increases, so does the zone of diffusion. Typical discharge instructions for platysma treatment include the overuse of the neck muscles for 2 to 4 hours after injection to encourage the botulinum toxin uptake for optimal result. Site manipulation (rubbing or massaging) also increases diffusion. For botulinum toxin type B, the zone of diffusion is greater because its molecular weight is less than the type A toxins, thus making it an undesirable agent for aesthetic facial chemodenervation.4,11
Toxin Resistance
Botulinum toxin resistance is a known complication that occurs normally as a result of the body recognizing the neurotoxin as a foreign substance and producing neutralizing antibodies (NAb). Primary botulinum toxin failure is known in patients who require high doses of the neurotoxin for treatment of neuromuscular disorders.12 Complete secondary therapy failure is known to occur in cosmetic patients after a single dose and those who have been receiving low-dose botulinum toxin regularly. The risk of NAb development increases with long-term treatment and high doses.12-18
Floppy Neck and Dysphagia
As previously noted, floppy neck and dysphagia are adverse clinical findings of botulinum toxin effect on the platysma, sternocleidomastoid, or the paralaryngeal muscles. In this case, the patient was fortunate to have only sustained weakness of the platysma and sternocleidomastoid muscles despite both a large neck and total body dose. Paralaryngeal muscle paralysis is not life-threatening, but the distress may precipitate paradoxical vocal cord motion and stridor.
Stridor
Stridor is typically a symptom of an upper airway obstruction (UAO) process. Typical UAO conditions encountered in the ED are infections (eg, epiglottitis, croup), foreign body, allergy, and laryngeal trauma. The age of the patient, onset of stridor, course of the stridor (ie, intermittent, continuous, worsening), associated symptoms (eg, fever, rash, swelling of oral soft tissues), and bruising must be ascertained.
In differentiating the etiology of stridor, the EP should observe the patient for any associated change in voice, inability to handle secretions, and position of comfort. Patients with stridor require admission and evaluation by an otolaryngologist as expeditiously as possible because impending UAO may quickly progress to complete UAO necessitating emergent intubation.
An atypical presentation of stridor to the ED is sporadic stridor. Sporadic attacks of stridor during activity have been associated with the entity of paradoxical vocal cord motion. Patients usually describe a choking sensation with inability to breathe resulting in an audible inspiratory and/or expiratory sound—ie, stridor. Wheezing may or may not be present. Patients may also describe tightness in the neck and sometimes in the chest. The attacks are usually seconds to minutes in duration. More often, there is a precipitating or an inducing factor such as hyperventilation, cough, panting, phonatory tasks, or the inhalation of irritants or perfume, or an oropharyngeal or laryngeal manipulation prior or postextubation. The feeling of stress alone is commonly reported prior to the attacks. When evaluating patients presenting with floppy neck, dysphagia, and stridor, it is imperative that the clinician conduct a thorough history and physical examination to determine if the symptoms are secondary to a systemic or local effect, and whether the patient will progress to an acute UAO (vocal cord paralysis) necessitating intubation in the ED and subsequent tracheostomy.19,20
Conclusion
The ready availability of botulinum toxins and their low-cost-benefit ratio continue to promote over-utilization for treatment of facial wrinkles, platysmal bands, and horizontal lines; migraine headache; and hyperhidrosis. Complications associated with overuse of botulinum toxins are due to either administration of a large single dose or from regional diffusion. With the increasing number of patients receiving botulinum injections, EPs should be aware of the four available toxin types onset of action, adverse events, and potential life-threatening complications of regional neck injections.
References
1. Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43(2 Pt 1):249-259. doi:10.1067/mjd.2000.105567.
2. Lamanna C. The most poisonous poison. Science. 1959;130(3378):763-772.
3. Blitzer A, Brin MF, Keen MS, Aviv JE. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg. 1993;119(9):1018-1022.
4. Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatol Surg. 1998;24(11):1189-1194.
5. Carruthers J, Carruthers A. Botox use in the mid and lower face and neck. Semin Cutan Med Surg. 2001;20(2):85-92. doi:10.1053/sder.2001.25139
6. Hoefflin SM. Anatomy of the platysma and lip depressor muscles. A simplified mnemonic approach. Dermatol Surg. 1998;24(11):1225-1231.
7. Brandt FS, Bellman B. Cosmetic use of botulinum A exotoxin for the aging neck. Dermatol Surg. 1998;24(11):1232-1234.
8. Klein AW. Complications and adverse reactions with the use of botulinum toxin. Semin Cutan Med Surg. 2001;20(2):109-120. doi:10.1053/sder.2001.25964.
9. Carruthers A, Kiene K, Carruthers J. Botulinum A exotoxin use in clinical dermatology. J Am Acad Dermatol. 1996;34(5 Pt 1):788-797.
10. Howell K, Selber P, Graham HK, Reddihough D. Botulinum neurotoxin A: an unusual systemic effect. J Paediatr Child Health. 2007:43(6):499-501. doi:10.1111/j.1440-1754.2007.01122.x.
11. Carruthers A, Carruthers J. Toxins 99, new information about the botulinum neurotoxins. Dermatol Surg. 2000;26(3):174-176.
12. Dressler D, Adib Saberi F. New formulation of Botox: complete antibody-induced treatment failure in cervical dystonia. J Neurol Neurosurg Psychiatry. 2007;78(1):108-109. doi:10.1136/jnnp.2006.093419.
13. Borodic G. Immunologic resistance after repeated botulinum toxin type a injections for facial rhytides. Ophthal Plast Reconstr Surg. 2006;22:239-240. doi:10.1097/01.iop.0000217703.80859.a3.
14. Goschel H, Wohlfarth K, Frevert J, Dengler R, Bigalke H. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies—therapeutic consequences. Exp Neurol. 1997;147(1):96-102. doi:10.1006/exnr.1997.6580.
15. Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3(1):66-98.
16. Smith LA. Development of recombinant vaccines for botulinum neurotoxin. Toxicon. 1998;36(11):1539-1548.
17. Houser MK, Sheean GL, Lees AJ. Further studies using higher doses of botulinum toxin type F for torticollis resistant to botulinum toxin type A. J Neurol Neurosurg Psychiatry. 1998;64(5):577-580.
18. Dressler D, Wohlfahrt K, Meyer-Rogge E, Wiest L, Bigalke H. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36 Suppl 4:2182-2187. doi:10.1111/j.1524-4725.2010.01710.x.
19. Maschka DA, Bauman NM, McCray PB Jr, Hoffman HT, Karnell MP, Smith RJ. A classification scheme for paradoxical vocal cord motion. Laryngoscope. 1997;107(11 Pt 1):1429-1435.
20. Altman KW, Simpson CB, Amin MR, Abaza M, Balkissoon R, Casiano RR. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511. doi:10.1067/mhn.2002.127589.
Case
A 68-year-old woman presented to the ED 5 days after receiving onabotulinumtoxinA cosmetic injections for wrinkles of the face and neck. She stated that she was unable to raise her head while in a supine position and that her head felt heavy when standing. She also experienced spasms and strain of the posterior cervical neck muscles. In addition, the patient described a constant need to swallow forcefully throughout the day, and felt an intermittent heavy sensation over her larynx that was associated with stridor. She noted these symptoms began 5 days after the onabotulinumtoxinA injections and had peaked 2 days prior to presentation. She also complained of dysphagia without odynophagia, but denied any changes in her voice.
The patient first began onabotulinumtoxinA injections 12 years earlier for aesthetic treatment of glabellar and peri-orbital wrinkles. She initially received the injections at a regular interval of 90 to 100 days. During the course of the first 2 years of treatment, the patient was under the care of a plastic surgeon; thereafter, she sought treatment at a physician-owned medical spa because it offered onabotulinumtoxinA at a lower price. The injections at the medical spa were administered by a physician assistant (PA). The patient stated that although the PA had steadily increased the dose of onabotulinumtoxinA to maintain the desired aesthetic effect, this was the first time she had experienced any side effects from the treatment.
The ED staff contacted the medical spa provider, who reviewed the patient’s medical record over the telephone. The PA stated that he had been the only practitioner at the facility to administer the onabotulinumtoxinA injections to the patient over her past 10 years there as a client. He further informed the emergency physician (EP) that 12 days prior to presentation, he had given the patient a total of 50 IU of onabotulinumtoxinA, in five separate injections, into the mid frontalis muscle; a total of 35 IU, in seven separate injections, into the glabellar region (procerus and corrugator muscles bilaterally); 20 IU into the lateral and inferior-lateral orbicularis oculi bilaterally, in four separate injections per side, (40 IU total); and a total of 100 IU in the anterior platysma, in 20 separate injections, for a total 1-day onabotulinumtoxinA dose of 225 IU.
The PA explained to the EP that he mixed the onabotulinumtoxinA in the patient’s room and had shown her the vials and dilution standard as recommended by the manufacturer because she had been requiring increased dosages and had previously questioned whether the onabotulinumtoxinA was diluted. The PA denied any other patients experiencing similar adverse events as those of the patient’s.
Over the last 10 years, the patient had received onabotulinumtoxinA in the nasolabial folds, upper and lower lip wrinkles, mentalis, depressor angular oris, buccal, nasalis, lateral brow, masseter, and calf muscles. The dosage of onabotulinumtoxinA at this most recent injection cycle was unchanged from her previous visit 3 months prior. According to the PA, the practice did not use abobotulinumtoxinA or incobotulinumtoxinA.
Regarding the patient’s medical history, she had no health issues suggestive of myasthenia gravis, multiple sclerosis, or Guillain-Barré syndrome. Examination of the face revealed decreased muscle excursion of the frontalis muscle from mid-brow to mid-brow, and stair-step wrinkle formation bilaterally. The procerus muscle was very weak, and the corrugator muscles were moderately diminished in strength. The lateral orbicularis oculi were very weak at each canthus. The extra-ocular muscles were intact. She had full mandibular excursion, and powerful movement of the tongue. The oropharynx and floor of the mouth were normal. She was noted to purposefully swallow and extend her neck every 90 to 120 seconds to “clear her throat,” though she did not drool and was able to handle her secretions and swallow fluids without aspiration. Her voice was normal and she was able to recite the letters “KKKKK,” “OOOOO,” and “EEEEE” in rapid fashion without breathiness or stridor. The rest of her facial muscles were normal.
While examining the patient, the EP asked her to refrain from swallowing whenever she extended her neck. Upon complying with this request, her neck extension precipitated swallowing and, by not swallowing, she did not accumulate secretions. Once during the examination, the patient began swallowing and breathing rapidly with stridor. This less than 15-second episode was abated by full-neck extensions, which relieved the patient’s sensation of heaviness over the larynx. Her breathing and voice were normal immediately after this episode.
Examination of the anterior neck revealed four platysmal bands (Figure). One band measured 10 cm in length and extended from the mandible inferiorly; two bands measured 2 cm lateral to the midline bilaterally; and the fourth band extended 4 cm in length from the mandible immediately lateral to the longer platysmal band. The platysma and dermis were flaccid and redundant at rest and with exertion. The sternocleidomastoid muscles were weak with exertion. The larynx moved cephalad with swallowing. The posterior cervical neck and trapezius muscles were of normal tone and strength. No spasms or fasciculations were noted during the examination period.
While supine, the patient strained to lift her head and complained of a suffocating sensation over the larynx. She had no rashes or edema, and the remainder of the physical examination, vital signs, and pulse oximetry were normal. Laboratory evaluation, which included a complete blood count and serum electrolytes, was also normal.
An otolaryngologist consultation for laryngoscopy was obtained. After reviewing the patient’s case, the otolaryngologist concluded that given the patient’s history, intermittent stridor, and an absence of signs or symptoms suggestive of an impending upper airway obstruction (UAO), laryngoscopy was not warranted.
A plastic surgery consultation was then obtained. The patient’s examination was as noted above, and her vital signs and pulse oximetry remained normal throughout her ED stay. Although botulinum and botulinum antibody titers were ordered, the patient refused testing due to cost concerns. She was discharged home by plastic surgery services with a diagnosis of floppy neck and dysphagia secondary to aesthetic botulinum toxin paralysis of the bilateral sternocleidomastoid muscles and platysma. She was given a prescription for metoclopramide hydrochloride to stimulate motility of the upper gastrointestinal tract and to potentially improve swallowing.10
The patient was scheduled for a follow-up evaluation with the plastic surgeon 2 days after discharge. She was instructed to call 911 if she experienced stridor, shortness of breath, drooling, or if any airway issues arose. The patient did not return for her follow-up appointment with the plastic surgeon.
Discussion
Clostridium Botulinum Toxins
Clostridium botulinum is a gram-positive spore-forming anaerobic bacterium that produces extremely potent neuro-exotoxins. C botulinum is found in soil, contaminated foods, and in illicit injectable drugs (eg, heroin). Seven distinct antigenic botulinum toxins (A, B, C1, D, E, F, and G) are produced by several strains of C botulinum. Systemically, each neurotoxin is able to produce severe morbidity and mortality by causing generalized muscle paralysis and death by respiratory failure. The lethal dose of these agents is approximating 10(-9) g/kg body weight. Botulinum toxin type A is the most potent.1,2

Nonetheless, botulinum toxin has been used clinically since the early 1970s. Currently, there are three FDA-approved botulinum toxin type A agents and one type B formulation (rimabotulinumtoxinB) (Table). Each formulation is unique, proprietary, and differs in molecular weight, toxin-complex size, protein content, and inactive ingredients. The effectiveness and adverse event profile for these four botulinum toxins is individually dependent upon the different dilutions and potency, onset of action, duration of effect, diffusion, and migration potential. Hence, the effective dose of one botulinum toxin does not equate to any other, resulting in a lack of interchangeability between botulinum toxins (eg, 5 IU of incobotulinumtoxinA does not equal 5 IU of onabotulinumtoxinA).
Aesthetic Indications
Historically, the use of botulinum toxin for aesthetic treatment of wrinkles and platysmal bands was first reported by Blitzer3 in 1993.Subsequently, the use of botulinum toxin for the aesthetic treatment of facial wrinkles, hypertrophic platysmal bands and horizontal neck lines gained popularity within the public and medical community.3-5
Anatomically, the platysma is a thin sheet-like muscle that originates in the superior fascia of the pectoralis and deltoid fascia, and extends over the full length of the neck up past the mandible and continuing into the submuscular aponeurotic system. The platysma is innervated by the seventh cranial nerve and functions to pull the jaw downward. The platysma muscle is attached directly to the skin. With normal aging, the anterior neck skin becomes flaccid, the central platysmal bands thicken and contract—forming bands, horizontal wrinkles, and loss of definition of the neck noticed at rest and with contraction of the platysma muscle. These vertical bands are known as platysmal bands. The platysmal bands are benign consequences of aging and as such are targets of correction through surgery or botulinum toxin injection.6,7
Mechanism of Action
Platysmal band and horizontal line injection techniques with botulinum toxin have been reported in the literature with dosages ranging from 15 IU to 200 IU used to block the Soluble N-ethylmaleimide-sensitive factor activating protein receptors. Typical onset of action begins at 3 days, with full paralytic effect at 7 days. Repeat injections every 3 to 4 months are required with prolonged effects seen with each subsequent injection due to chemodenervation-induced muscle atrophy.4,7,8
Adverse Effects
Commercial botulinum toxin type A has been associated with minor and transient side effects. Moderate complications seen in the neck region include transient soft-tissue edema, dermal ecchymoses, intramuscular hematoma, diffuse muscle soreness, neck flexor weakness, and headaches.4,8,9
The use of botulinum toxin for chemodenervation of the platysma can produce significant weakness of other neck muscles, including the sternocleidomastoid, cricothyroid, sternothyroid, and sternohyoid. Floppy neck and dysphagia may be due to diffusion of the toxin into the muscles of deglutition of the larynx; injection directly into the sternocleidomastoid muscle; or a result of the systemic effects of large dosages. Hoarseness, breathiness, and dysphagia may occur 3 to 4 days after injection, especially with doses over 75 IU.10
The recommended concentration of botulinum toxin type A causes a diffusion average of 1 cm in all directions from the injection sites. However, as the dilution increases, so does the zone of diffusion. Typical discharge instructions for platysma treatment include the overuse of the neck muscles for 2 to 4 hours after injection to encourage the botulinum toxin uptake for optimal result. Site manipulation (rubbing or massaging) also increases diffusion. For botulinum toxin type B, the zone of diffusion is greater because its molecular weight is less than the type A toxins, thus making it an undesirable agent for aesthetic facial chemodenervation.4,11
Toxin Resistance
Botulinum toxin resistance is a known complication that occurs normally as a result of the body recognizing the neurotoxin as a foreign substance and producing neutralizing antibodies (NAb). Primary botulinum toxin failure is known in patients who require high doses of the neurotoxin for treatment of neuromuscular disorders.12 Complete secondary therapy failure is known to occur in cosmetic patients after a single dose and those who have been receiving low-dose botulinum toxin regularly. The risk of NAb development increases with long-term treatment and high doses.12-18
Floppy Neck and Dysphagia
As previously noted, floppy neck and dysphagia are adverse clinical findings of botulinum toxin effect on the platysma, sternocleidomastoid, or the paralaryngeal muscles. In this case, the patient was fortunate to have only sustained weakness of the platysma and sternocleidomastoid muscles despite both a large neck and total body dose. Paralaryngeal muscle paralysis is not life-threatening, but the distress may precipitate paradoxical vocal cord motion and stridor.
Stridor
Stridor is typically a symptom of an upper airway obstruction (UAO) process. Typical UAO conditions encountered in the ED are infections (eg, epiglottitis, croup), foreign body, allergy, and laryngeal trauma. The age of the patient, onset of stridor, course of the stridor (ie, intermittent, continuous, worsening), associated symptoms (eg, fever, rash, swelling of oral soft tissues), and bruising must be ascertained.
In differentiating the etiology of stridor, the EP should observe the patient for any associated change in voice, inability to handle secretions, and position of comfort. Patients with stridor require admission and evaluation by an otolaryngologist as expeditiously as possible because impending UAO may quickly progress to complete UAO necessitating emergent intubation.
An atypical presentation of stridor to the ED is sporadic stridor. Sporadic attacks of stridor during activity have been associated with the entity of paradoxical vocal cord motion. Patients usually describe a choking sensation with inability to breathe resulting in an audible inspiratory and/or expiratory sound—ie, stridor. Wheezing may or may not be present. Patients may also describe tightness in the neck and sometimes in the chest. The attacks are usually seconds to minutes in duration. More often, there is a precipitating or an inducing factor such as hyperventilation, cough, panting, phonatory tasks, or the inhalation of irritants or perfume, or an oropharyngeal or laryngeal manipulation prior or postextubation. The feeling of stress alone is commonly reported prior to the attacks. When evaluating patients presenting with floppy neck, dysphagia, and stridor, it is imperative that the clinician conduct a thorough history and physical examination to determine if the symptoms are secondary to a systemic or local effect, and whether the patient will progress to an acute UAO (vocal cord paralysis) necessitating intubation in the ED and subsequent tracheostomy.19,20
Conclusion
The ready availability of botulinum toxins and their low-cost-benefit ratio continue to promote over-utilization for treatment of facial wrinkles, platysmal bands, and horizontal lines; migraine headache; and hyperhidrosis. Complications associated with overuse of botulinum toxins are due to either administration of a large single dose or from regional diffusion. With the increasing number of patients receiving botulinum injections, EPs should be aware of the four available toxin types onset of action, adverse events, and potential life-threatening complications of regional neck injections.
Case
A 68-year-old woman presented to the ED 5 days after receiving onabotulinumtoxinA cosmetic injections for wrinkles of the face and neck. She stated that she was unable to raise her head while in a supine position and that her head felt heavy when standing. She also experienced spasms and strain of the posterior cervical neck muscles. In addition, the patient described a constant need to swallow forcefully throughout the day, and felt an intermittent heavy sensation over her larynx that was associated with stridor. She noted these symptoms began 5 days after the onabotulinumtoxinA injections and had peaked 2 days prior to presentation. She also complained of dysphagia without odynophagia, but denied any changes in her voice.
The patient first began onabotulinumtoxinA injections 12 years earlier for aesthetic treatment of glabellar and peri-orbital wrinkles. She initially received the injections at a regular interval of 90 to 100 days. During the course of the first 2 years of treatment, the patient was under the care of a plastic surgeon; thereafter, she sought treatment at a physician-owned medical spa because it offered onabotulinumtoxinA at a lower price. The injections at the medical spa were administered by a physician assistant (PA). The patient stated that although the PA had steadily increased the dose of onabotulinumtoxinA to maintain the desired aesthetic effect, this was the first time she had experienced any side effects from the treatment.
The ED staff contacted the medical spa provider, who reviewed the patient’s medical record over the telephone. The PA stated that he had been the only practitioner at the facility to administer the onabotulinumtoxinA injections to the patient over her past 10 years there as a client. He further informed the emergency physician (EP) that 12 days prior to presentation, he had given the patient a total of 50 IU of onabotulinumtoxinA, in five separate injections, into the mid frontalis muscle; a total of 35 IU, in seven separate injections, into the glabellar region (procerus and corrugator muscles bilaterally); 20 IU into the lateral and inferior-lateral orbicularis oculi bilaterally, in four separate injections per side, (40 IU total); and a total of 100 IU in the anterior platysma, in 20 separate injections, for a total 1-day onabotulinumtoxinA dose of 225 IU.
The PA explained to the EP that he mixed the onabotulinumtoxinA in the patient’s room and had shown her the vials and dilution standard as recommended by the manufacturer because she had been requiring increased dosages and had previously questioned whether the onabotulinumtoxinA was diluted. The PA denied any other patients experiencing similar adverse events as those of the patient’s.
Over the last 10 years, the patient had received onabotulinumtoxinA in the nasolabial folds, upper and lower lip wrinkles, mentalis, depressor angular oris, buccal, nasalis, lateral brow, masseter, and calf muscles. The dosage of onabotulinumtoxinA at this most recent injection cycle was unchanged from her previous visit 3 months prior. According to the PA, the practice did not use abobotulinumtoxinA or incobotulinumtoxinA.
Regarding the patient’s medical history, she had no health issues suggestive of myasthenia gravis, multiple sclerosis, or Guillain-Barré syndrome. Examination of the face revealed decreased muscle excursion of the frontalis muscle from mid-brow to mid-brow, and stair-step wrinkle formation bilaterally. The procerus muscle was very weak, and the corrugator muscles were moderately diminished in strength. The lateral orbicularis oculi were very weak at each canthus. The extra-ocular muscles were intact. She had full mandibular excursion, and powerful movement of the tongue. The oropharynx and floor of the mouth were normal. She was noted to purposefully swallow and extend her neck every 90 to 120 seconds to “clear her throat,” though she did not drool and was able to handle her secretions and swallow fluids without aspiration. Her voice was normal and she was able to recite the letters “KKKKK,” “OOOOO,” and “EEEEE” in rapid fashion without breathiness or stridor. The rest of her facial muscles were normal.
While examining the patient, the EP asked her to refrain from swallowing whenever she extended her neck. Upon complying with this request, her neck extension precipitated swallowing and, by not swallowing, she did not accumulate secretions. Once during the examination, the patient began swallowing and breathing rapidly with stridor. This less than 15-second episode was abated by full-neck extensions, which relieved the patient’s sensation of heaviness over the larynx. Her breathing and voice were normal immediately after this episode.
Examination of the anterior neck revealed four platysmal bands (Figure). One band measured 10 cm in length and extended from the mandible inferiorly; two bands measured 2 cm lateral to the midline bilaterally; and the fourth band extended 4 cm in length from the mandible immediately lateral to the longer platysmal band. The platysma and dermis were flaccid and redundant at rest and with exertion. The sternocleidomastoid muscles were weak with exertion. The larynx moved cephalad with swallowing. The posterior cervical neck and trapezius muscles were of normal tone and strength. No spasms or fasciculations were noted during the examination period.
While supine, the patient strained to lift her head and complained of a suffocating sensation over the larynx. She had no rashes or edema, and the remainder of the physical examination, vital signs, and pulse oximetry were normal. Laboratory evaluation, which included a complete blood count and serum electrolytes, was also normal.
An otolaryngologist consultation for laryngoscopy was obtained. After reviewing the patient’s case, the otolaryngologist concluded that given the patient’s history, intermittent stridor, and an absence of signs or symptoms suggestive of an impending upper airway obstruction (UAO), laryngoscopy was not warranted.
A plastic surgery consultation was then obtained. The patient’s examination was as noted above, and her vital signs and pulse oximetry remained normal throughout her ED stay. Although botulinum and botulinum antibody titers were ordered, the patient refused testing due to cost concerns. She was discharged home by plastic surgery services with a diagnosis of floppy neck and dysphagia secondary to aesthetic botulinum toxin paralysis of the bilateral sternocleidomastoid muscles and platysma. She was given a prescription for metoclopramide hydrochloride to stimulate motility of the upper gastrointestinal tract and to potentially improve swallowing.10
The patient was scheduled for a follow-up evaluation with the plastic surgeon 2 days after discharge. She was instructed to call 911 if she experienced stridor, shortness of breath, drooling, or if any airway issues arose. The patient did not return for her follow-up appointment with the plastic surgeon.
Discussion
Clostridium Botulinum Toxins
Clostridium botulinum is a gram-positive spore-forming anaerobic bacterium that produces extremely potent neuro-exotoxins. C botulinum is found in soil, contaminated foods, and in illicit injectable drugs (eg, heroin). Seven distinct antigenic botulinum toxins (A, B, C1, D, E, F, and G) are produced by several strains of C botulinum. Systemically, each neurotoxin is able to produce severe morbidity and mortality by causing generalized muscle paralysis and death by respiratory failure. The lethal dose of these agents is approximating 10(-9) g/kg body weight. Botulinum toxin type A is the most potent.1,2

Nonetheless, botulinum toxin has been used clinically since the early 1970s. Currently, there are three FDA-approved botulinum toxin type A agents and one type B formulation (rimabotulinumtoxinB) (Table). Each formulation is unique, proprietary, and differs in molecular weight, toxin-complex size, protein content, and inactive ingredients. The effectiveness and adverse event profile for these four botulinum toxins is individually dependent upon the different dilutions and potency, onset of action, duration of effect, diffusion, and migration potential. Hence, the effective dose of one botulinum toxin does not equate to any other, resulting in a lack of interchangeability between botulinum toxins (eg, 5 IU of incobotulinumtoxinA does not equal 5 IU of onabotulinumtoxinA).
Aesthetic Indications
Historically, the use of botulinum toxin for aesthetic treatment of wrinkles and platysmal bands was first reported by Blitzer3 in 1993.Subsequently, the use of botulinum toxin for the aesthetic treatment of facial wrinkles, hypertrophic platysmal bands and horizontal neck lines gained popularity within the public and medical community.3-5
Anatomically, the platysma is a thin sheet-like muscle that originates in the superior fascia of the pectoralis and deltoid fascia, and extends over the full length of the neck up past the mandible and continuing into the submuscular aponeurotic system. The platysma is innervated by the seventh cranial nerve and functions to pull the jaw downward. The platysma muscle is attached directly to the skin. With normal aging, the anterior neck skin becomes flaccid, the central platysmal bands thicken and contract—forming bands, horizontal wrinkles, and loss of definition of the neck noticed at rest and with contraction of the platysma muscle. These vertical bands are known as platysmal bands. The platysmal bands are benign consequences of aging and as such are targets of correction through surgery or botulinum toxin injection.6,7
Mechanism of Action
Platysmal band and horizontal line injection techniques with botulinum toxin have been reported in the literature with dosages ranging from 15 IU to 200 IU used to block the Soluble N-ethylmaleimide-sensitive factor activating protein receptors. Typical onset of action begins at 3 days, with full paralytic effect at 7 days. Repeat injections every 3 to 4 months are required with prolonged effects seen with each subsequent injection due to chemodenervation-induced muscle atrophy.4,7,8
Adverse Effects
Commercial botulinum toxin type A has been associated with minor and transient side effects. Moderate complications seen in the neck region include transient soft-tissue edema, dermal ecchymoses, intramuscular hematoma, diffuse muscle soreness, neck flexor weakness, and headaches.4,8,9
The use of botulinum toxin for chemodenervation of the platysma can produce significant weakness of other neck muscles, including the sternocleidomastoid, cricothyroid, sternothyroid, and sternohyoid. Floppy neck and dysphagia may be due to diffusion of the toxin into the muscles of deglutition of the larynx; injection directly into the sternocleidomastoid muscle; or a result of the systemic effects of large dosages. Hoarseness, breathiness, and dysphagia may occur 3 to 4 days after injection, especially with doses over 75 IU.10
The recommended concentration of botulinum toxin type A causes a diffusion average of 1 cm in all directions from the injection sites. However, as the dilution increases, so does the zone of diffusion. Typical discharge instructions for platysma treatment include the overuse of the neck muscles for 2 to 4 hours after injection to encourage the botulinum toxin uptake for optimal result. Site manipulation (rubbing or massaging) also increases diffusion. For botulinum toxin type B, the zone of diffusion is greater because its molecular weight is less than the type A toxins, thus making it an undesirable agent for aesthetic facial chemodenervation.4,11
Toxin Resistance
Botulinum toxin resistance is a known complication that occurs normally as a result of the body recognizing the neurotoxin as a foreign substance and producing neutralizing antibodies (NAb). Primary botulinum toxin failure is known in patients who require high doses of the neurotoxin for treatment of neuromuscular disorders.12 Complete secondary therapy failure is known to occur in cosmetic patients after a single dose and those who have been receiving low-dose botulinum toxin regularly. The risk of NAb development increases with long-term treatment and high doses.12-18
Floppy Neck and Dysphagia
As previously noted, floppy neck and dysphagia are adverse clinical findings of botulinum toxin effect on the platysma, sternocleidomastoid, or the paralaryngeal muscles. In this case, the patient was fortunate to have only sustained weakness of the platysma and sternocleidomastoid muscles despite both a large neck and total body dose. Paralaryngeal muscle paralysis is not life-threatening, but the distress may precipitate paradoxical vocal cord motion and stridor.
Stridor
Stridor is typically a symptom of an upper airway obstruction (UAO) process. Typical UAO conditions encountered in the ED are infections (eg, epiglottitis, croup), foreign body, allergy, and laryngeal trauma. The age of the patient, onset of stridor, course of the stridor (ie, intermittent, continuous, worsening), associated symptoms (eg, fever, rash, swelling of oral soft tissues), and bruising must be ascertained.
In differentiating the etiology of stridor, the EP should observe the patient for any associated change in voice, inability to handle secretions, and position of comfort. Patients with stridor require admission and evaluation by an otolaryngologist as expeditiously as possible because impending UAO may quickly progress to complete UAO necessitating emergent intubation.
An atypical presentation of stridor to the ED is sporadic stridor. Sporadic attacks of stridor during activity have been associated with the entity of paradoxical vocal cord motion. Patients usually describe a choking sensation with inability to breathe resulting in an audible inspiratory and/or expiratory sound—ie, stridor. Wheezing may or may not be present. Patients may also describe tightness in the neck and sometimes in the chest. The attacks are usually seconds to minutes in duration. More often, there is a precipitating or an inducing factor such as hyperventilation, cough, panting, phonatory tasks, or the inhalation of irritants or perfume, or an oropharyngeal or laryngeal manipulation prior or postextubation. The feeling of stress alone is commonly reported prior to the attacks. When evaluating patients presenting with floppy neck, dysphagia, and stridor, it is imperative that the clinician conduct a thorough history and physical examination to determine if the symptoms are secondary to a systemic or local effect, and whether the patient will progress to an acute UAO (vocal cord paralysis) necessitating intubation in the ED and subsequent tracheostomy.19,20
Conclusion
The ready availability of botulinum toxins and their low-cost-benefit ratio continue to promote over-utilization for treatment of facial wrinkles, platysmal bands, and horizontal lines; migraine headache; and hyperhidrosis. Complications associated with overuse of botulinum toxins are due to either administration of a large single dose or from regional diffusion. With the increasing number of patients receiving botulinum injections, EPs should be aware of the four available toxin types onset of action, adverse events, and potential life-threatening complications of regional neck injections.
References
1. Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43(2 Pt 1):249-259. doi:10.1067/mjd.2000.105567.
2. Lamanna C. The most poisonous poison. Science. 1959;130(3378):763-772.
3. Blitzer A, Brin MF, Keen MS, Aviv JE. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg. 1993;119(9):1018-1022.
4. Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatol Surg. 1998;24(11):1189-1194.
5. Carruthers J, Carruthers A. Botox use in the mid and lower face and neck. Semin Cutan Med Surg. 2001;20(2):85-92. doi:10.1053/sder.2001.25139
6. Hoefflin SM. Anatomy of the platysma and lip depressor muscles. A simplified mnemonic approach. Dermatol Surg. 1998;24(11):1225-1231.
7. Brandt FS, Bellman B. Cosmetic use of botulinum A exotoxin for the aging neck. Dermatol Surg. 1998;24(11):1232-1234.
8. Klein AW. Complications and adverse reactions with the use of botulinum toxin. Semin Cutan Med Surg. 2001;20(2):109-120. doi:10.1053/sder.2001.25964.
9. Carruthers A, Kiene K, Carruthers J. Botulinum A exotoxin use in clinical dermatology. J Am Acad Dermatol. 1996;34(5 Pt 1):788-797.
10. Howell K, Selber P, Graham HK, Reddihough D. Botulinum neurotoxin A: an unusual systemic effect. J Paediatr Child Health. 2007:43(6):499-501. doi:10.1111/j.1440-1754.2007.01122.x.
11. Carruthers A, Carruthers J. Toxins 99, new information about the botulinum neurotoxins. Dermatol Surg. 2000;26(3):174-176.
12. Dressler D, Adib Saberi F. New formulation of Botox: complete antibody-induced treatment failure in cervical dystonia. J Neurol Neurosurg Psychiatry. 2007;78(1):108-109. doi:10.1136/jnnp.2006.093419.
13. Borodic G. Immunologic resistance after repeated botulinum toxin type a injections for facial rhytides. Ophthal Plast Reconstr Surg. 2006;22:239-240. doi:10.1097/01.iop.0000217703.80859.a3.
14. Goschel H, Wohlfarth K, Frevert J, Dengler R, Bigalke H. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies—therapeutic consequences. Exp Neurol. 1997;147(1):96-102. doi:10.1006/exnr.1997.6580.
15. Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3(1):66-98.
16. Smith LA. Development of recombinant vaccines for botulinum neurotoxin. Toxicon. 1998;36(11):1539-1548.
17. Houser MK, Sheean GL, Lees AJ. Further studies using higher doses of botulinum toxin type F for torticollis resistant to botulinum toxin type A. J Neurol Neurosurg Psychiatry. 1998;64(5):577-580.
18. Dressler D, Wohlfahrt K, Meyer-Rogge E, Wiest L, Bigalke H. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36 Suppl 4:2182-2187. doi:10.1111/j.1524-4725.2010.01710.x.
19. Maschka DA, Bauman NM, McCray PB Jr, Hoffman HT, Karnell MP, Smith RJ. A classification scheme for paradoxical vocal cord motion. Laryngoscope. 1997;107(11 Pt 1):1429-1435.
20. Altman KW, Simpson CB, Amin MR, Abaza M, Balkissoon R, Casiano RR. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511. doi:10.1067/mhn.2002.127589.
References
1. Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43(2 Pt 1):249-259. doi:10.1067/mjd.2000.105567.
2. Lamanna C. The most poisonous poison. Science. 1959;130(3378):763-772.
3. Blitzer A, Brin MF, Keen MS, Aviv JE. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg. 1993;119(9):1018-1022.
4. Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatol Surg. 1998;24(11):1189-1194.
5. Carruthers J, Carruthers A. Botox use in the mid and lower face and neck. Semin Cutan Med Surg. 2001;20(2):85-92. doi:10.1053/sder.2001.25139
6. Hoefflin SM. Anatomy of the platysma and lip depressor muscles. A simplified mnemonic approach. Dermatol Surg. 1998;24(11):1225-1231.
7. Brandt FS, Bellman B. Cosmetic use of botulinum A exotoxin for the aging neck. Dermatol Surg. 1998;24(11):1232-1234.
8. Klein AW. Complications and adverse reactions with the use of botulinum toxin. Semin Cutan Med Surg. 2001;20(2):109-120. doi:10.1053/sder.2001.25964.
9. Carruthers A, Kiene K, Carruthers J. Botulinum A exotoxin use in clinical dermatology. J Am Acad Dermatol. 1996;34(5 Pt 1):788-797.
10. Howell K, Selber P, Graham HK, Reddihough D. Botulinum neurotoxin A: an unusual systemic effect. J Paediatr Child Health. 2007:43(6):499-501. doi:10.1111/j.1440-1754.2007.01122.x.
11. Carruthers A, Carruthers J. Toxins 99, new information about the botulinum neurotoxins. Dermatol Surg. 2000;26(3):174-176.
12. Dressler D, Adib Saberi F. New formulation of Botox: complete antibody-induced treatment failure in cervical dystonia. J Neurol Neurosurg Psychiatry. 2007;78(1):108-109. doi:10.1136/jnnp.2006.093419.
13. Borodic G. Immunologic resistance after repeated botulinum toxin type a injections for facial rhytides. Ophthal Plast Reconstr Surg. 2006;22:239-240. doi:10.1097/01.iop.0000217703.80859.a3.
14. Goschel H, Wohlfarth K, Frevert J, Dengler R, Bigalke H. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies—therapeutic consequences. Exp Neurol. 1997;147(1):96-102. doi:10.1006/exnr.1997.6580.
15. Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3(1):66-98.
16. Smith LA. Development of recombinant vaccines for botulinum neurotoxin. Toxicon. 1998;36(11):1539-1548.
17. Houser MK, Sheean GL, Lees AJ. Further studies using higher doses of botulinum toxin type F for torticollis resistant to botulinum toxin type A. J Neurol Neurosurg Psychiatry. 1998;64(5):577-580.
18. Dressler D, Wohlfahrt K, Meyer-Rogge E, Wiest L, Bigalke H. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36 Suppl 4:2182-2187. doi:10.1111/j.1524-4725.2010.01710.x.
19. Maschka DA, Bauman NM, McCray PB Jr, Hoffman HT, Karnell MP, Smith RJ. A classification scheme for paradoxical vocal cord motion. Laryngoscope. 1997;107(11 Pt 1):1429-1435.
20. Altman KW, Simpson CB, Amin MR, Abaza M, Balkissoon R, Casiano RR. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511. doi:10.1067/mhn.2002.127589.
Dung Lung: Reactive Airway Disease Syndrome From Yak-Dung Biomass Fuel Smoke
Case
A 30-year-old man without prior respiratory illness presented with coughing, wheezing, dyspnea on exertion, and decreased exercise tolerance after a 7-hour overnight exposure to yak-dung smoke. This episode took place at 4,240 m elevation in Pheriche village, along the Everest Base Camp trekking route within the Khumbu region of the Nepali Himalayas. Prior to going to bed that evening, the group of five cohabitants had a difficult time igniting the potbelly heating stove filled with yak-dung biomass fuel in the common room. Each time they tried to light it, the fire would smolder and go out within a few minutes, despite the group’s attempts at adjusting the flue and air intake. Eventually, they abandoned further attempts and retired to bed at approximately 9:30
The differential diagnosis included altitude illness, airway mucociliary dysfunction (commonly known as Khumbu cough),1 carbon monoxide (CO) poisoning, acute inhalation injury (AII) resulting in reactive airway disease syndrome (RADS), and high-altitude pulmonary edema (HAPE). Although the group hiked to Kongma La Pass (elevation, 5,545 m), and slept at 5,200 m (960 m higher than their starting point), the patient had not exhibited any symptoms of altitude illness (eg, headache, dizziness, fatigue, sleep disturbances, anorexia, nausea). Auscultation of the patient by the two physicians who accompanied him on the hike noted mild expiratory wheezing without rales or rhonchi, making HAPE unlikely in the differential diagnosis.
Although it is likely the patient had significant CO exposure, he did not display profound symptoms of CO toxicity (eg, light-headedness, headache, vertigo, nausea, or confusion). It is unclear whether the symptoms of decreased exercise tolerance and fatigue were due to CO poisoning as no co-oximeter was available to assess the patient’s CO levels.2,3 Upon return from the trip, pulse oximetry showed the patient to have an oxygen (O2) saturation of 89% on room air, which was within appropriate range for their altitude.
One of the physicians offered the patient an albuterol metered-dose inhaler, which provided profound and immediate relief of his coughing and wheezing. The patient continued to use the albuterol inhaler every 2 to 4 hours over the next 2 days. The dyspnea on exertion and decreased exercise tolerance improved after 24 hours of treatment; the rest of his respiratory symptoms resolved after approximately 5 days at the starting elevation, and he returned to his usual baseline state of health. No follow-up chest X-rays were obtained, and the patient has had no subsequent recurrence of these symptoms despite return to higher altitude in the subsequent year.
Discussion
Nearly one-third to one-half of the world’s population relies on biomass fuels for domestic heating or cooking, with developing countries accounting for 99% of its use.4 These fuels consist of dried dung cakes or patties, agricultural products, coal, and firewood. In the Khumbu region of Nepal above timberline, yak-dung patties are used exclusively for heating and frequently for cooking. Most guesthouses in this region have potbelly-style stoves in the common dining areas, which are fueled by yak dung and ventilated with a chimney.
Pulmonary Pathophysiology of Inhaled Irritants
Biomass fuels are responsible for numerous air pollutants due to incomplete combustion. These fuels suspend particulate matter, CO, nitrogen dioxide, polycyclic aromatic hydrocarbons, and volatile organic compounds, including acetone, methyl ethyl ketone, benzene, formaldehyde, and toluene.5 Compared to other biomass fuel sources, dung-cake combustion results in higher emissions of relatively very small particulate matter with peak concentrations ranging from 0.23 to 0.3 μm in size, which penetrate and affect the distal airway. 6 Their combustion also releases volatile organic compounds and CO.7,8 Aside from indoor air pollution, yak-dung combustion in the Nepali Himalayan valley contributes significantly to the ambient airborne concentrations of lead, copper, aluminum, magnesium, and elemental and organic carbon.9
Emergency physicians (EPs) are often the first-line treating physician for patients exposed not only to biomass fuels, but also home, forest, or occupational fires resulting in smoke inhalation or AII.10 These terms refer to the wide number of substances that may be present in the smoke and collectively affect the patient. Inhaled substances classified as irritants, such as smoke and particulate matter, can harm the epithelium of the respiratory tract, with highly water-soluble or larger particles (>10 μm) mostly affecting the upper airways. These irritants cause symptoms of progressive coughing, and wheezing; or stridor resulting in tracheitis, bronchitis, bronchiolitis, alveolitis, pulmonary edema, and/or airway obstruction. Smaller particles (<2.5 μm) can penetrate further into the lung and affect the distal airway to a greater degree. These particles are able to infiltrate the terminal bronchioles and alveoli, leading to localized inflammatory reaction and bronchospasm.11Smoke may also contain chemical asphyxiants such as CO or hydrogen cyanide, which can be absorbed, leading to systemic toxicity and interfering with O2 delivery or utilization. Importantly, high concentrations of any gas can act as an asphyxiant due to displacement of O2.12 Thermal injuries are also possible from fire and smoke exposure, typically affecting the upper airways. Steam inhalation can even cause irritation and burns below the vocal cords.13
Reactive Airway Disease Syndrome
Reactive airway disease syndrome is a constellation of symptoms presenting similar to asthma with persistent airway reactivity after an AII, and is the most common sequelae of exposure to biomass fuel combustion. This syndrome is not specifically caused by one type of particulate, irritant, or chemical component of the smoke.
Symptoms
Symptoms such as cough, dyspnea, and wheezing may begin minutes after exposure, and can persist for years due to bronchial hyperresponsiveness.14 These chronic symptoms of RADS have been well highlighted by New York Fire Department rescue workers from the World Trade Center collapse, of whom 16% continued to show symptoms of RADS 1 year later.15
Treatment
Bronchodilator therapy is the mainstay of treatment for RADS. Patients who have RADS often respond well to treatment, and show improvement in symptoms and spirometry testing.
Sequelae Associated With Biomass Fuel Exposure
A cross-sectional study showed significant reductions (P < .001) in all pulmonary function testing parameters for cow-dung fuel users compared to those who use modern energy sources: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and mid-flow rate between the first 25% and 75% of forced expiratory flow. Linear regression showed a 12.4% reduction in FVC of cow-dung users, and 36% (compared to 20% in modern energy-source users) were noted to have pulmonary infections.16
Due to these emissions, biomass fuel exposure causes high levels of morbidity and mortality in developing countries, with nearly 2 million attributable deaths annually.1 Chronic exposure to biomass fuel emissions can lead to increased risk of diseases, including respiratory problems (eg, pneumonia, tuberculosis and chronic obstructive pulmonary disease, lung cancer, asthma), low birthweight, cataracts, and cardiovascular events.2,17 Women are at higher risk compared to other family members, as they typically spend approximately 3 to 4 hours longer daily in tents,5 and perform the majoring of the cooking duties. For pregnant women, the developing fetus may also be exposed, which can lead to increased rates of fetal demise.18
Conclusion
Our report represents the first reported case of “dung lung” or RADS from yak-dung biomass fuel combustion exposure. In the medical literature, there has been one previous case report of dung lung by Osbern and Crapo19 in 1981 in which the authors described three patients who died from aspiration of liquid manure in a storage facility.Our case highlights the prevalence of biomass fuel combustion in the third world, the dangerous air pollutants from their emissions, and the morbidity associated with improper ventilation of biomass fuel combustion.
1. Rodway GW, Windsor JS. Airway mucociliary function at high altitude. Wilderness Environ Med. 2006;17(4):271-275.
2. Leigh-Smith S. Carbon monoxide poisoning in tents—a review. Wilderness Environ Med. 2004;15(3):157-163.
3. Lipman GL. Carbon monoxide toxicity at high altitude [Commentary]. Wilderness Environ Med. 2006;17(2):144-145.
4. Prasad R, Singh A, Garg R, Giridhar GB. Biomass fuel exposure and respiratory diseases in India. Biosci Trends. 2012;6(5):219-228.
5. Kim KH, Jahan SA, Kabir E. A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192(2):425-431.
6. Park D, Barabad ML, Lee G, et al. Emission characteristics of particulate matter and volatile organic compounds in cow dung combustion. Environ Sci Technol. 2013;47(22):12952-12957.
7. Venkataraman C, Rao GU. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ Sci Technol. 2001;35(10):2100-2107.
8. Chen PF, Li CL, Kang SC, et al. [Indoor air pollution in the Nam Co and Ando Regions in the Tibetan Plateau]. [Article in Chinese]. Huan Jing Ke Xue. 2011;32(5):1231-1236.
9. avidson CI, Grimm TC, Nasta MA. Airborne lead and other elements derived from local fires in the himalayas. Science. 1981;214(4527):1344-1366.
10. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28-35.
11. Ainslie G. Inhalational injuries produced by smoke and nitrogen dioxide. Respir Med. 1993;87(3):169-174.
12. Glazer CS. Acute inhalational injury. In: Hanley ME, Welsh CH, eds. Current Diagnosis & Treatment in Pulmonary Medicine. International Ed. New York, NY: McGraw Hill; 2003:354-360.
13. Gu TL, Liou SH, Hsu CH, Hsu JC, Wu TN. Acute health hazards of firefighters after fighting a department store fire. Indust Health. 1996;34(1):13-23.
14. Alberts WM, do Picco GA. Reactive airways dysfunction syndrome. Chest. 1996;109(6):1618-1626.
15. Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 Suppl):S102-S106.
16. Sümer H, Turaçlar UT, Onarlioğlu T, Ozdemir L, Zwahlen M. The association of biomass fuel combustion on pulmonary function tests in the adult population of Mid-Anatolia. Soz Praventivmed. 2004;49(4):247-253.
17. Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412.
18. de Koning HW, Smith KR, Last JM. Biomass fuel combustion and health. Bull World Health Organ. 1985;63(1):11-26.
19. Osbern LN, Crapo RO. Dung lung: a report of toxic exposure to liquid manure. Ann Intern Med. 1981;95(3):312-314.
Case
A 30-year-old man without prior respiratory illness presented with coughing, wheezing, dyspnea on exertion, and decreased exercise tolerance after a 7-hour overnight exposure to yak-dung smoke. This episode took place at 4,240 m elevation in Pheriche village, along the Everest Base Camp trekking route within the Khumbu region of the Nepali Himalayas. Prior to going to bed that evening, the group of five cohabitants had a difficult time igniting the potbelly heating stove filled with yak-dung biomass fuel in the common room. Each time they tried to light it, the fire would smolder and go out within a few minutes, despite the group’s attempts at adjusting the flue and air intake. Eventually, they abandoned further attempts and retired to bed at approximately 9:30
The differential diagnosis included altitude illness, airway mucociliary dysfunction (commonly known as Khumbu cough),1 carbon monoxide (CO) poisoning, acute inhalation injury (AII) resulting in reactive airway disease syndrome (RADS), and high-altitude pulmonary edema (HAPE). Although the group hiked to Kongma La Pass (elevation, 5,545 m), and slept at 5,200 m (960 m higher than their starting point), the patient had not exhibited any symptoms of altitude illness (eg, headache, dizziness, fatigue, sleep disturbances, anorexia, nausea). Auscultation of the patient by the two physicians who accompanied him on the hike noted mild expiratory wheezing without rales or rhonchi, making HAPE unlikely in the differential diagnosis.
Although it is likely the patient had significant CO exposure, he did not display profound symptoms of CO toxicity (eg, light-headedness, headache, vertigo, nausea, or confusion). It is unclear whether the symptoms of decreased exercise tolerance and fatigue were due to CO poisoning as no co-oximeter was available to assess the patient’s CO levels.2,3 Upon return from the trip, pulse oximetry showed the patient to have an oxygen (O2) saturation of 89% on room air, which was within appropriate range for their altitude.
One of the physicians offered the patient an albuterol metered-dose inhaler, which provided profound and immediate relief of his coughing and wheezing. The patient continued to use the albuterol inhaler every 2 to 4 hours over the next 2 days. The dyspnea on exertion and decreased exercise tolerance improved after 24 hours of treatment; the rest of his respiratory symptoms resolved after approximately 5 days at the starting elevation, and he returned to his usual baseline state of health. No follow-up chest X-rays were obtained, and the patient has had no subsequent recurrence of these symptoms despite return to higher altitude in the subsequent year.
Discussion
Nearly one-third to one-half of the world’s population relies on biomass fuels for domestic heating or cooking, with developing countries accounting for 99% of its use.4 These fuels consist of dried dung cakes or patties, agricultural products, coal, and firewood. In the Khumbu region of Nepal above timberline, yak-dung patties are used exclusively for heating and frequently for cooking. Most guesthouses in this region have potbelly-style stoves in the common dining areas, which are fueled by yak dung and ventilated with a chimney.
Pulmonary Pathophysiology of Inhaled Irritants
Biomass fuels are responsible for numerous air pollutants due to incomplete combustion. These fuels suspend particulate matter, CO, nitrogen dioxide, polycyclic aromatic hydrocarbons, and volatile organic compounds, including acetone, methyl ethyl ketone, benzene, formaldehyde, and toluene.5 Compared to other biomass fuel sources, dung-cake combustion results in higher emissions of relatively very small particulate matter with peak concentrations ranging from 0.23 to 0.3 μm in size, which penetrate and affect the distal airway. 6 Their combustion also releases volatile organic compounds and CO.7,8 Aside from indoor air pollution, yak-dung combustion in the Nepali Himalayan valley contributes significantly to the ambient airborne concentrations of lead, copper, aluminum, magnesium, and elemental and organic carbon.9
Emergency physicians (EPs) are often the first-line treating physician for patients exposed not only to biomass fuels, but also home, forest, or occupational fires resulting in smoke inhalation or AII.10 These terms refer to the wide number of substances that may be present in the smoke and collectively affect the patient. Inhaled substances classified as irritants, such as smoke and particulate matter, can harm the epithelium of the respiratory tract, with highly water-soluble or larger particles (>10 μm) mostly affecting the upper airways. These irritants cause symptoms of progressive coughing, and wheezing; or stridor resulting in tracheitis, bronchitis, bronchiolitis, alveolitis, pulmonary edema, and/or airway obstruction. Smaller particles (<2.5 μm) can penetrate further into the lung and affect the distal airway to a greater degree. These particles are able to infiltrate the terminal bronchioles and alveoli, leading to localized inflammatory reaction and bronchospasm.11Smoke may also contain chemical asphyxiants such as CO or hydrogen cyanide, which can be absorbed, leading to systemic toxicity and interfering with O2 delivery or utilization. Importantly, high concentrations of any gas can act as an asphyxiant due to displacement of O2.12 Thermal injuries are also possible from fire and smoke exposure, typically affecting the upper airways. Steam inhalation can even cause irritation and burns below the vocal cords.13
Reactive Airway Disease Syndrome
Reactive airway disease syndrome is a constellation of symptoms presenting similar to asthma with persistent airway reactivity after an AII, and is the most common sequelae of exposure to biomass fuel combustion. This syndrome is not specifically caused by one type of particulate, irritant, or chemical component of the smoke.
Symptoms
Symptoms such as cough, dyspnea, and wheezing may begin minutes after exposure, and can persist for years due to bronchial hyperresponsiveness.14 These chronic symptoms of RADS have been well highlighted by New York Fire Department rescue workers from the World Trade Center collapse, of whom 16% continued to show symptoms of RADS 1 year later.15
Treatment
Bronchodilator therapy is the mainstay of treatment for RADS. Patients who have RADS often respond well to treatment, and show improvement in symptoms and spirometry testing.
Sequelae Associated With Biomass Fuel Exposure
A cross-sectional study showed significant reductions (P < .001) in all pulmonary function testing parameters for cow-dung fuel users compared to those who use modern energy sources: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and mid-flow rate between the first 25% and 75% of forced expiratory flow. Linear regression showed a 12.4% reduction in FVC of cow-dung users, and 36% (compared to 20% in modern energy-source users) were noted to have pulmonary infections.16
Due to these emissions, biomass fuel exposure causes high levels of morbidity and mortality in developing countries, with nearly 2 million attributable deaths annually.1 Chronic exposure to biomass fuel emissions can lead to increased risk of diseases, including respiratory problems (eg, pneumonia, tuberculosis and chronic obstructive pulmonary disease, lung cancer, asthma), low birthweight, cataracts, and cardiovascular events.2,17 Women are at higher risk compared to other family members, as they typically spend approximately 3 to 4 hours longer daily in tents,5 and perform the majoring of the cooking duties. For pregnant women, the developing fetus may also be exposed, which can lead to increased rates of fetal demise.18
Conclusion
Our report represents the first reported case of “dung lung” or RADS from yak-dung biomass fuel combustion exposure. In the medical literature, there has been one previous case report of dung lung by Osbern and Crapo19 in 1981 in which the authors described three patients who died from aspiration of liquid manure in a storage facility.Our case highlights the prevalence of biomass fuel combustion in the third world, the dangerous air pollutants from their emissions, and the morbidity associated with improper ventilation of biomass fuel combustion.
Case
A 30-year-old man without prior respiratory illness presented with coughing, wheezing, dyspnea on exertion, and decreased exercise tolerance after a 7-hour overnight exposure to yak-dung smoke. This episode took place at 4,240 m elevation in Pheriche village, along the Everest Base Camp trekking route within the Khumbu region of the Nepali Himalayas. Prior to going to bed that evening, the group of five cohabitants had a difficult time igniting the potbelly heating stove filled with yak-dung biomass fuel in the common room. Each time they tried to light it, the fire would smolder and go out within a few minutes, despite the group’s attempts at adjusting the flue and air intake. Eventually, they abandoned further attempts and retired to bed at approximately 9:30
The differential diagnosis included altitude illness, airway mucociliary dysfunction (commonly known as Khumbu cough),1 carbon monoxide (CO) poisoning, acute inhalation injury (AII) resulting in reactive airway disease syndrome (RADS), and high-altitude pulmonary edema (HAPE). Although the group hiked to Kongma La Pass (elevation, 5,545 m), and slept at 5,200 m (960 m higher than their starting point), the patient had not exhibited any symptoms of altitude illness (eg, headache, dizziness, fatigue, sleep disturbances, anorexia, nausea). Auscultation of the patient by the two physicians who accompanied him on the hike noted mild expiratory wheezing without rales or rhonchi, making HAPE unlikely in the differential diagnosis.
Although it is likely the patient had significant CO exposure, he did not display profound symptoms of CO toxicity (eg, light-headedness, headache, vertigo, nausea, or confusion). It is unclear whether the symptoms of decreased exercise tolerance and fatigue were due to CO poisoning as no co-oximeter was available to assess the patient’s CO levels.2,3 Upon return from the trip, pulse oximetry showed the patient to have an oxygen (O2) saturation of 89% on room air, which was within appropriate range for their altitude.
One of the physicians offered the patient an albuterol metered-dose inhaler, which provided profound and immediate relief of his coughing and wheezing. The patient continued to use the albuterol inhaler every 2 to 4 hours over the next 2 days. The dyspnea on exertion and decreased exercise tolerance improved after 24 hours of treatment; the rest of his respiratory symptoms resolved after approximately 5 days at the starting elevation, and he returned to his usual baseline state of health. No follow-up chest X-rays were obtained, and the patient has had no subsequent recurrence of these symptoms despite return to higher altitude in the subsequent year.
Discussion
Nearly one-third to one-half of the world’s population relies on biomass fuels for domestic heating or cooking, with developing countries accounting for 99% of its use.4 These fuels consist of dried dung cakes or patties, agricultural products, coal, and firewood. In the Khumbu region of Nepal above timberline, yak-dung patties are used exclusively for heating and frequently for cooking. Most guesthouses in this region have potbelly-style stoves in the common dining areas, which are fueled by yak dung and ventilated with a chimney.
Pulmonary Pathophysiology of Inhaled Irritants
Biomass fuels are responsible for numerous air pollutants due to incomplete combustion. These fuels suspend particulate matter, CO, nitrogen dioxide, polycyclic aromatic hydrocarbons, and volatile organic compounds, including acetone, methyl ethyl ketone, benzene, formaldehyde, and toluene.5 Compared to other biomass fuel sources, dung-cake combustion results in higher emissions of relatively very small particulate matter with peak concentrations ranging from 0.23 to 0.3 μm in size, which penetrate and affect the distal airway. 6 Their combustion also releases volatile organic compounds and CO.7,8 Aside from indoor air pollution, yak-dung combustion in the Nepali Himalayan valley contributes significantly to the ambient airborne concentrations of lead, copper, aluminum, magnesium, and elemental and organic carbon.9
Emergency physicians (EPs) are often the first-line treating physician for patients exposed not only to biomass fuels, but also home, forest, or occupational fires resulting in smoke inhalation or AII.10 These terms refer to the wide number of substances that may be present in the smoke and collectively affect the patient. Inhaled substances classified as irritants, such as smoke and particulate matter, can harm the epithelium of the respiratory tract, with highly water-soluble or larger particles (>10 μm) mostly affecting the upper airways. These irritants cause symptoms of progressive coughing, and wheezing; or stridor resulting in tracheitis, bronchitis, bronchiolitis, alveolitis, pulmonary edema, and/or airway obstruction. Smaller particles (<2.5 μm) can penetrate further into the lung and affect the distal airway to a greater degree. These particles are able to infiltrate the terminal bronchioles and alveoli, leading to localized inflammatory reaction and bronchospasm.11Smoke may also contain chemical asphyxiants such as CO or hydrogen cyanide, which can be absorbed, leading to systemic toxicity and interfering with O2 delivery or utilization. Importantly, high concentrations of any gas can act as an asphyxiant due to displacement of O2.12 Thermal injuries are also possible from fire and smoke exposure, typically affecting the upper airways. Steam inhalation can even cause irritation and burns below the vocal cords.13
Reactive Airway Disease Syndrome
Reactive airway disease syndrome is a constellation of symptoms presenting similar to asthma with persistent airway reactivity after an AII, and is the most common sequelae of exposure to biomass fuel combustion. This syndrome is not specifically caused by one type of particulate, irritant, or chemical component of the smoke.
Symptoms
Symptoms such as cough, dyspnea, and wheezing may begin minutes after exposure, and can persist for years due to bronchial hyperresponsiveness.14 These chronic symptoms of RADS have been well highlighted by New York Fire Department rescue workers from the World Trade Center collapse, of whom 16% continued to show symptoms of RADS 1 year later.15
Treatment
Bronchodilator therapy is the mainstay of treatment for RADS. Patients who have RADS often respond well to treatment, and show improvement in symptoms and spirometry testing.
Sequelae Associated With Biomass Fuel Exposure
A cross-sectional study showed significant reductions (P < .001) in all pulmonary function testing parameters for cow-dung fuel users compared to those who use modern energy sources: forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and mid-flow rate between the first 25% and 75% of forced expiratory flow. Linear regression showed a 12.4% reduction in FVC of cow-dung users, and 36% (compared to 20% in modern energy-source users) were noted to have pulmonary infections.16
Due to these emissions, biomass fuel exposure causes high levels of morbidity and mortality in developing countries, with nearly 2 million attributable deaths annually.1 Chronic exposure to biomass fuel emissions can lead to increased risk of diseases, including respiratory problems (eg, pneumonia, tuberculosis and chronic obstructive pulmonary disease, lung cancer, asthma), low birthweight, cataracts, and cardiovascular events.2,17 Women are at higher risk compared to other family members, as they typically spend approximately 3 to 4 hours longer daily in tents,5 and perform the majoring of the cooking duties. For pregnant women, the developing fetus may also be exposed, which can lead to increased rates of fetal demise.18
Conclusion
Our report represents the first reported case of “dung lung” or RADS from yak-dung biomass fuel combustion exposure. In the medical literature, there has been one previous case report of dung lung by Osbern and Crapo19 in 1981 in which the authors described three patients who died from aspiration of liquid manure in a storage facility.Our case highlights the prevalence of biomass fuel combustion in the third world, the dangerous air pollutants from their emissions, and the morbidity associated with improper ventilation of biomass fuel combustion.
1. Rodway GW, Windsor JS. Airway mucociliary function at high altitude. Wilderness Environ Med. 2006;17(4):271-275.
2. Leigh-Smith S. Carbon monoxide poisoning in tents—a review. Wilderness Environ Med. 2004;15(3):157-163.
3. Lipman GL. Carbon monoxide toxicity at high altitude [Commentary]. Wilderness Environ Med. 2006;17(2):144-145.
4. Prasad R, Singh A, Garg R, Giridhar GB. Biomass fuel exposure and respiratory diseases in India. Biosci Trends. 2012;6(5):219-228.
5. Kim KH, Jahan SA, Kabir E. A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192(2):425-431.
6. Park D, Barabad ML, Lee G, et al. Emission characteristics of particulate matter and volatile organic compounds in cow dung combustion. Environ Sci Technol. 2013;47(22):12952-12957.
7. Venkataraman C, Rao GU. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ Sci Technol. 2001;35(10):2100-2107.
8. Chen PF, Li CL, Kang SC, et al. [Indoor air pollution in the Nam Co and Ando Regions in the Tibetan Plateau]. [Article in Chinese]. Huan Jing Ke Xue. 2011;32(5):1231-1236.
9. avidson CI, Grimm TC, Nasta MA. Airborne lead and other elements derived from local fires in the himalayas. Science. 1981;214(4527):1344-1366.
10. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28-35.
11. Ainslie G. Inhalational injuries produced by smoke and nitrogen dioxide. Respir Med. 1993;87(3):169-174.
12. Glazer CS. Acute inhalational injury. In: Hanley ME, Welsh CH, eds. Current Diagnosis & Treatment in Pulmonary Medicine. International Ed. New York, NY: McGraw Hill; 2003:354-360.
13. Gu TL, Liou SH, Hsu CH, Hsu JC, Wu TN. Acute health hazards of firefighters after fighting a department store fire. Indust Health. 1996;34(1):13-23.
14. Alberts WM, do Picco GA. Reactive airways dysfunction syndrome. Chest. 1996;109(6):1618-1626.
15. Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 Suppl):S102-S106.
16. Sümer H, Turaçlar UT, Onarlioğlu T, Ozdemir L, Zwahlen M. The association of biomass fuel combustion on pulmonary function tests in the adult population of Mid-Anatolia. Soz Praventivmed. 2004;49(4):247-253.
17. Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412.
18. de Koning HW, Smith KR, Last JM. Biomass fuel combustion and health. Bull World Health Organ. 1985;63(1):11-26.
19. Osbern LN, Crapo RO. Dung lung: a report of toxic exposure to liquid manure. Ann Intern Med. 1981;95(3):312-314.
1. Rodway GW, Windsor JS. Airway mucociliary function at high altitude. Wilderness Environ Med. 2006;17(4):271-275.
2. Leigh-Smith S. Carbon monoxide poisoning in tents—a review. Wilderness Environ Med. 2004;15(3):157-163.
3. Lipman GL. Carbon monoxide toxicity at high altitude [Commentary]. Wilderness Environ Med. 2006;17(2):144-145.
4. Prasad R, Singh A, Garg R, Giridhar GB. Biomass fuel exposure and respiratory diseases in India. Biosci Trends. 2012;6(5):219-228.
5. Kim KH, Jahan SA, Kabir E. A review of diseases associated with household air pollution due to the use of biomass fuels. J Hazard Mater. 2011;192(2):425-431.
6. Park D, Barabad ML, Lee G, et al. Emission characteristics of particulate matter and volatile organic compounds in cow dung combustion. Environ Sci Technol. 2013;47(22):12952-12957.
7. Venkataraman C, Rao GU. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ Sci Technol. 2001;35(10):2100-2107.
8. Chen PF, Li CL, Kang SC, et al. [Indoor air pollution in the Nam Co and Ando Regions in the Tibetan Plateau]. [Article in Chinese]. Huan Jing Ke Xue. 2011;32(5):1231-1236.
9. avidson CI, Grimm TC, Nasta MA. Airborne lead and other elements derived from local fires in the himalayas. Science. 1981;214(4527):1344-1366.
10. Gorguner M, Akgun M. Acute inhalation injury. Eurasian J Med. 2010;42(1):28-35.
11. Ainslie G. Inhalational injuries produced by smoke and nitrogen dioxide. Respir Med. 1993;87(3):169-174.
12. Glazer CS. Acute inhalational injury. In: Hanley ME, Welsh CH, eds. Current Diagnosis & Treatment in Pulmonary Medicine. International Ed. New York, NY: McGraw Hill; 2003:354-360.
13. Gu TL, Liou SH, Hsu CH, Hsu JC, Wu TN. Acute health hazards of firefighters after fighting a department store fire. Indust Health. 1996;34(1):13-23.
14. Alberts WM, do Picco GA. Reactive airways dysfunction syndrome. Chest. 1996;109(6):1618-1626.
15. Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 Suppl):S102-S106.
16. Sümer H, Turaçlar UT, Onarlioğlu T, Ozdemir L, Zwahlen M. The association of biomass fuel combustion on pulmonary function tests in the adult population of Mid-Anatolia. Soz Praventivmed. 2004;49(4):247-253.
17. Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412.
18. de Koning HW, Smith KR, Last JM. Biomass fuel combustion and health. Bull World Health Organ. 1985;63(1):11-26.
19. Osbern LN, Crapo RO. Dung lung: a report of toxic exposure to liquid manure. Ann Intern Med. 1981;95(3):312-314.
Worsening of longstanding headaches, dizziness, visual symptoms • Dx?
THE CASE
A 59-year-old woman from the Democratic Republic of the Congo presented to our family medicine clinic with acute worsening of longstanding headaches. Using a Swahili interpreter, the patient reported a 15-year history of recurrent, intermittent headaches that had been previously diagnosed as migraines. Over the prior 2 months, the headaches had intensified with new symptoms of dizziness, ocular pain, and blurred vision with red flashes. She had no hemiplegia, dysarthria, respiratory symptoms, night sweats, or weight loss. A neurologic exam was negative.
Before immigrating to the United States 14 years earlier, the patient lived for 6 months in a refugee camp in the Congo. At the time of her immigration, she was negative for human immunodeficiency virus (HIV), and a tuberculosis (TB) skin test was positive. A chest x-ray was normal and she had no respiratory symptoms. Shortly after her immigration, she completed 6 months of isoniazid treatment for latent TB.
THE DIAGNOSIS
A computed tomography (CT) scan of the patient’s head demonstrated a large right frontal mass. The differential diagnosis included neoplasm, sarcoidosis, or, less likely, an infectious etiology. A contrast-enhanced magnetic resonance image (MRI) of the brain showed multiple heterogeneous enhancing lesions, with the largest measuring 4.4 cm x 4.6 cm x 3 cm (FIGURE 1). Significant surrounding edema caused a 1.6-cm midline shift, subfalcine herniation, and impending uncal herniation. A CT of the abdomen and chest showed no pulmonary masses or metastatic disease, but did reveal a single 1-cm lymph node in the mediastinum and a 1.2-cm right axillary node.
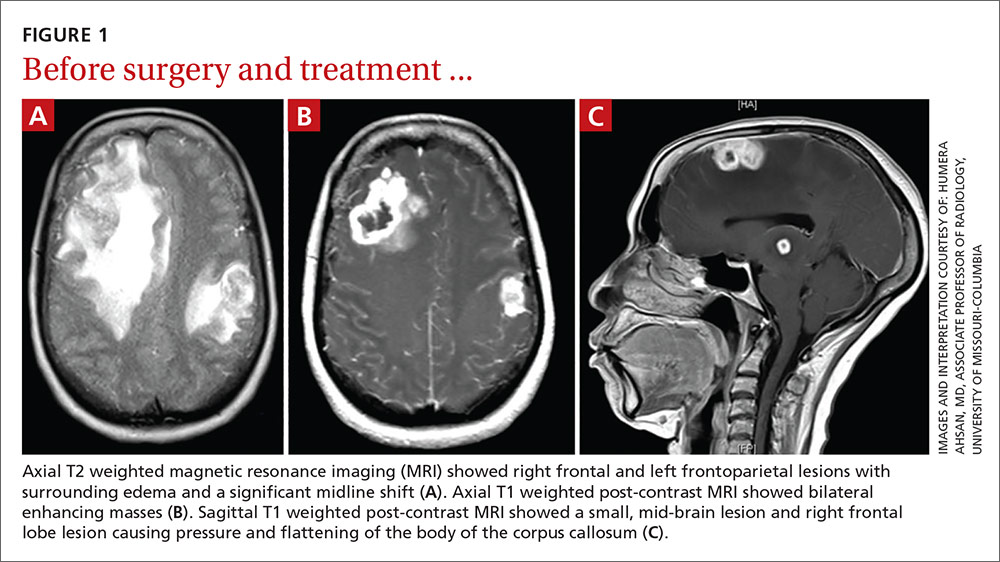
A craniotomy was performed, which confirmed a large mass adhered to the dura. Surgeons removed the mass en bloc; pathology was consistent with a necrotizing granuloma. Acid-fast bacilli (AFB) staining of 3 specimens was negative. Because the tissue was preserved in formalin, mycobacterial cultures could not be obtained. A cerebrospinal fluid analysis showed lymphocytosis and elevated protein, consistent with neurotuberculosis. Blood testing for Mycobacterium tuberculosis with interferon gamma release assay (IGRA) was negative, as was testing for HIV 1 and 2. In addition, induced sputum was AFB-smear negative, as was an M tuberculosis polymerase chain reaction test.
Despite the negative AFB stain and negative IGRA, the patient’s findings were suspicious for TB, so we began to treat her empirically for neurotuberculosis with a 4-drug regimen (isoniazid, rifampin, pyrazinamide, and ethambutol).
In an attempt to confirm the diagnosis of TB and determine sensitivities, we performed a right axillary lymph node biopsy and sent it to the Centers for Disease Control and Prevention (CDC), along with the preserved neural tissue. Using a newly developed technique, the CDC amplified and sequenced mycobacterial DNA from both the central nervous system (CNS) mass and the axillary node, confirming M tuberculosis complex species. Cultures from the axillary node grew pan-sensitive M tuberculosis.
DISCUSSION
About one-third of the world’s population has either active or latent TB.1 In areas where TB is endemic, tuberculomas have accounted for up to 20% of intracranial masses.2 In non-endemic regions, however, they are relatively uncommon. The 3 manifestations of active CNS TB are meningitis, tuberculoma, and abscess.3 The clinical presentation and imaging studies of CNS TB are often indistinguishable from those of patients with malignant neoplasms or metastatic disease. Biopsies may be necessary to distinguish tuberculomas from other intracranial lesions such as pyogenic abscesses or necrotic tumors.4 Mycobacterial cultures were not done on the brain biopsies of our patient because of the high clinical suspicion for neoplasm. Axillary lymph node tissue ultimately confirmed the diagnosis and provided sensitivities.
A diagnosis of CNS tuberculoma without meningitis can be challenging because the clinical presentation is often vague, mild, or even asymptomatic. Constitutional symptoms may include headache, fever, and anorexia.5
In our patient, IGRA testing was also negative. For latent TB, IGRAs are considered to be at least as sensitive as, and considerably more specific than TB skin testing, but their use in CNS TB is less well understood. Studies evaluating IGRA sensitivity for TB meningitis show variable results. In one study, IGRAs were positive in only 50% of culture-confirmed cases of TB meningitis in an HIV-negative population.6
Obtain sputum samples for all patients with extrapulmonary TB
The CDC recommends sputum sampling for all patients with extrapulmonary TB, even in the absence of pulmonary symptoms or radiographic findings, to determine the level of infectivity and potential need for a contact investigation.7
Due to low sensitivity of currently available rapid diagnostic tests and high mortality associated with delayed treatment, initiation of empiric treatment is recommended when the probability of CNS TB is high.5
Treatment duration for CNS tuberculomas is based on one randomized controlled trial,8 a small number of observational studies, a prospective cohort study looking at radiographic resolution,9 and expert opinion. Treatment recommendations often do not distinguish CNS tuberculomas from TB meningitis.10 CNS tuberculomas are commonly treated with a minimum of 12 months of therapy, generally using the same medications and dosages used in the treatment of pulmonary TB, starting with 4 first-line agents: isoniazid, rifampin, pyrazinamide, and ethambutol. Modification of the treatment regimen may be made once sensitivities are available.10
Our patient. After cultures were determined to be pan-sensitive, our patient’s treatment regimen was simplified to rifampin and isoniazid, which she continued for the remainder of her treatment course. Her treatment was discontinued after 18 months when quarterly MRIs showed stabilization of the tuberculomas (FIGURE 2).
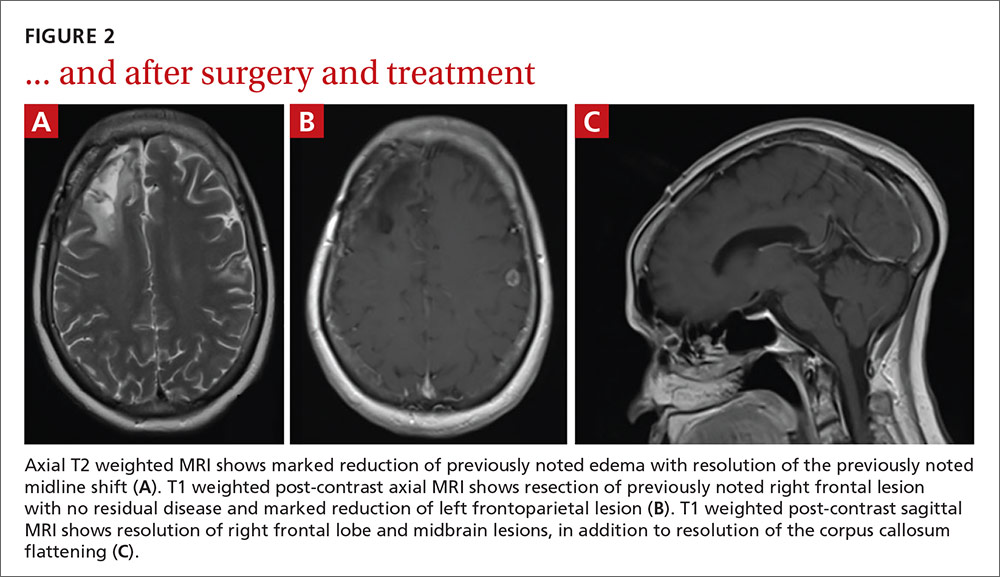
Following her surgery, she was started on levetiractam for seizure prophylaxis. She subsequently had a seizure on 2 occasions when the medication was discontinued or decreased, so we chose to continue it. The patient is asymptomatic from her disease with no residual deficits.
THE TAKEAWAY
A change in headache patterns in a patient over the age of 50 is a red flag that warrants imaging. In patients from countries where TB is endemic,11 consider neurotuberculosis in the differential diagnosis of worsening headaches and progressive neurologic symptoms.
A diagnosis of CNS TB can be difficult and requires a high level of clinical suspicion, but early diagnosis and treatment of neurotuberculosis can minimize the high risk of morbidity and mortality. Treatment for TB shouldn’t be withheld in cases in which there’s a strong clinical suspicion for TB, but for which a definitive diagnosis is still pending.
1. World Health Organization. 10 facts on tuberculosis. Available at: http://www.who.int/features/factfiles/tuberculosis/en/. Accessed September 19, 2014.
2. Dastur DK, Iyer CG. Pathological analysis of 450 intracranial space-occupying lesions. Ind J Cancer. 1966;3:105-115.
3. Chin JH, Mateen FJ. Central nervous system tuberculosis: Challenges and advances in diagnosis and treatment. Curr Infect Dis Rep. 2013;15:631-635.
4. Bayindir C, Mete O, Bilgic B. Retrospective study of 23 pathologically proven cases of central nervous system tuberculomas. Clin Neurol Neurosurg. 2006;108:353-357.
5. Thwaites G, Fisher M, Hemingway C, et al; British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167-187.
6. Simmons CP, Thwaites GE, Quyen NT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176:2007-2014.
7. Centers for Disease Control and Prevention (CDC). Core curriculum on tuberculosis: What the clinician should know. 6th ed. Centers for Disease Control and Prevention, Atlanta, GA; 2013.
8. Rajeswari R, Sivasubramanian S, Balambal R, et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
9. Poonnoose SI, Rajshekhar V. Rate of resolution of histologically verified intracranial tuberculomas. Neurosurgery. 2003;53:873-878.
10. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77. Erratum in: MMWR Recomm Rep. 2005;53:1203.
11. Stop TB Partnership. High burden countries. Available at: http://www.stoptb.org/countries/tbdata.asp. Accessed November 7, 2016.
THE CASE
A 59-year-old woman from the Democratic Republic of the Congo presented to our family medicine clinic with acute worsening of longstanding headaches. Using a Swahili interpreter, the patient reported a 15-year history of recurrent, intermittent headaches that had been previously diagnosed as migraines. Over the prior 2 months, the headaches had intensified with new symptoms of dizziness, ocular pain, and blurred vision with red flashes. She had no hemiplegia, dysarthria, respiratory symptoms, night sweats, or weight loss. A neurologic exam was negative.
Before immigrating to the United States 14 years earlier, the patient lived for 6 months in a refugee camp in the Congo. At the time of her immigration, she was negative for human immunodeficiency virus (HIV), and a tuberculosis (TB) skin test was positive. A chest x-ray was normal and she had no respiratory symptoms. Shortly after her immigration, she completed 6 months of isoniazid treatment for latent TB.
THE DIAGNOSIS
A computed tomography (CT) scan of the patient’s head demonstrated a large right frontal mass. The differential diagnosis included neoplasm, sarcoidosis, or, less likely, an infectious etiology. A contrast-enhanced magnetic resonance image (MRI) of the brain showed multiple heterogeneous enhancing lesions, with the largest measuring 4.4 cm x 4.6 cm x 3 cm (FIGURE 1). Significant surrounding edema caused a 1.6-cm midline shift, subfalcine herniation, and impending uncal herniation. A CT of the abdomen and chest showed no pulmonary masses or metastatic disease, but did reveal a single 1-cm lymph node in the mediastinum and a 1.2-cm right axillary node.

A craniotomy was performed, which confirmed a large mass adhered to the dura. Surgeons removed the mass en bloc; pathology was consistent with a necrotizing granuloma. Acid-fast bacilli (AFB) staining of 3 specimens was negative. Because the tissue was preserved in formalin, mycobacterial cultures could not be obtained. A cerebrospinal fluid analysis showed lymphocytosis and elevated protein, consistent with neurotuberculosis. Blood testing for Mycobacterium tuberculosis with interferon gamma release assay (IGRA) was negative, as was testing for HIV 1 and 2. In addition, induced sputum was AFB-smear negative, as was an M tuberculosis polymerase chain reaction test.
Despite the negative AFB stain and negative IGRA, the patient’s findings were suspicious for TB, so we began to treat her empirically for neurotuberculosis with a 4-drug regimen (isoniazid, rifampin, pyrazinamide, and ethambutol).
In an attempt to confirm the diagnosis of TB and determine sensitivities, we performed a right axillary lymph node biopsy and sent it to the Centers for Disease Control and Prevention (CDC), along with the preserved neural tissue. Using a newly developed technique, the CDC amplified and sequenced mycobacterial DNA from both the central nervous system (CNS) mass and the axillary node, confirming M tuberculosis complex species. Cultures from the axillary node grew pan-sensitive M tuberculosis.
DISCUSSION
About one-third of the world’s population has either active or latent TB.1 In areas where TB is endemic, tuberculomas have accounted for up to 20% of intracranial masses.2 In non-endemic regions, however, they are relatively uncommon. The 3 manifestations of active CNS TB are meningitis, tuberculoma, and abscess.3 The clinical presentation and imaging studies of CNS TB are often indistinguishable from those of patients with malignant neoplasms or metastatic disease. Biopsies may be necessary to distinguish tuberculomas from other intracranial lesions such as pyogenic abscesses or necrotic tumors.4 Mycobacterial cultures were not done on the brain biopsies of our patient because of the high clinical suspicion for neoplasm. Axillary lymph node tissue ultimately confirmed the diagnosis and provided sensitivities.
A diagnosis of CNS tuberculoma without meningitis can be challenging because the clinical presentation is often vague, mild, or even asymptomatic. Constitutional symptoms may include headache, fever, and anorexia.5
In our patient, IGRA testing was also negative. For latent TB, IGRAs are considered to be at least as sensitive as, and considerably more specific than TB skin testing, but their use in CNS TB is less well understood. Studies evaluating IGRA sensitivity for TB meningitis show variable results. In one study, IGRAs were positive in only 50% of culture-confirmed cases of TB meningitis in an HIV-negative population.6
Obtain sputum samples for all patients with extrapulmonary TB
The CDC recommends sputum sampling for all patients with extrapulmonary TB, even in the absence of pulmonary symptoms or radiographic findings, to determine the level of infectivity and potential need for a contact investigation.7
Due to low sensitivity of currently available rapid diagnostic tests and high mortality associated with delayed treatment, initiation of empiric treatment is recommended when the probability of CNS TB is high.5
Treatment duration for CNS tuberculomas is based on one randomized controlled trial,8 a small number of observational studies, a prospective cohort study looking at radiographic resolution,9 and expert opinion. Treatment recommendations often do not distinguish CNS tuberculomas from TB meningitis.10 CNS tuberculomas are commonly treated with a minimum of 12 months of therapy, generally using the same medications and dosages used in the treatment of pulmonary TB, starting with 4 first-line agents: isoniazid, rifampin, pyrazinamide, and ethambutol. Modification of the treatment regimen may be made once sensitivities are available.10
Our patient. After cultures were determined to be pan-sensitive, our patient’s treatment regimen was simplified to rifampin and isoniazid, which she continued for the remainder of her treatment course. Her treatment was discontinued after 18 months when quarterly MRIs showed stabilization of the tuberculomas (FIGURE 2).

Following her surgery, she was started on levetiractam for seizure prophylaxis. She subsequently had a seizure on 2 occasions when the medication was discontinued or decreased, so we chose to continue it. The patient is asymptomatic from her disease with no residual deficits.
THE TAKEAWAY
A change in headache patterns in a patient over the age of 50 is a red flag that warrants imaging. In patients from countries where TB is endemic,11 consider neurotuberculosis in the differential diagnosis of worsening headaches and progressive neurologic symptoms.
A diagnosis of CNS TB can be difficult and requires a high level of clinical suspicion, but early diagnosis and treatment of neurotuberculosis can minimize the high risk of morbidity and mortality. Treatment for TB shouldn’t be withheld in cases in which there’s a strong clinical suspicion for TB, but for which a definitive diagnosis is still pending.
THE CASE
A 59-year-old woman from the Democratic Republic of the Congo presented to our family medicine clinic with acute worsening of longstanding headaches. Using a Swahili interpreter, the patient reported a 15-year history of recurrent, intermittent headaches that had been previously diagnosed as migraines. Over the prior 2 months, the headaches had intensified with new symptoms of dizziness, ocular pain, and blurred vision with red flashes. She had no hemiplegia, dysarthria, respiratory symptoms, night sweats, or weight loss. A neurologic exam was negative.
Before immigrating to the United States 14 years earlier, the patient lived for 6 months in a refugee camp in the Congo. At the time of her immigration, she was negative for human immunodeficiency virus (HIV), and a tuberculosis (TB) skin test was positive. A chest x-ray was normal and she had no respiratory symptoms. Shortly after her immigration, she completed 6 months of isoniazid treatment for latent TB.
THE DIAGNOSIS
A computed tomography (CT) scan of the patient’s head demonstrated a large right frontal mass. The differential diagnosis included neoplasm, sarcoidosis, or, less likely, an infectious etiology. A contrast-enhanced magnetic resonance image (MRI) of the brain showed multiple heterogeneous enhancing lesions, with the largest measuring 4.4 cm x 4.6 cm x 3 cm (FIGURE 1). Significant surrounding edema caused a 1.6-cm midline shift, subfalcine herniation, and impending uncal herniation. A CT of the abdomen and chest showed no pulmonary masses or metastatic disease, but did reveal a single 1-cm lymph node in the mediastinum and a 1.2-cm right axillary node.

A craniotomy was performed, which confirmed a large mass adhered to the dura. Surgeons removed the mass en bloc; pathology was consistent with a necrotizing granuloma. Acid-fast bacilli (AFB) staining of 3 specimens was negative. Because the tissue was preserved in formalin, mycobacterial cultures could not be obtained. A cerebrospinal fluid analysis showed lymphocytosis and elevated protein, consistent with neurotuberculosis. Blood testing for Mycobacterium tuberculosis with interferon gamma release assay (IGRA) was negative, as was testing for HIV 1 and 2. In addition, induced sputum was AFB-smear negative, as was an M tuberculosis polymerase chain reaction test.
Despite the negative AFB stain and negative IGRA, the patient’s findings were suspicious for TB, so we began to treat her empirically for neurotuberculosis with a 4-drug regimen (isoniazid, rifampin, pyrazinamide, and ethambutol).
In an attempt to confirm the diagnosis of TB and determine sensitivities, we performed a right axillary lymph node biopsy and sent it to the Centers for Disease Control and Prevention (CDC), along with the preserved neural tissue. Using a newly developed technique, the CDC amplified and sequenced mycobacterial DNA from both the central nervous system (CNS) mass and the axillary node, confirming M tuberculosis complex species. Cultures from the axillary node grew pan-sensitive M tuberculosis.
DISCUSSION
About one-third of the world’s population has either active or latent TB.1 In areas where TB is endemic, tuberculomas have accounted for up to 20% of intracranial masses.2 In non-endemic regions, however, they are relatively uncommon. The 3 manifestations of active CNS TB are meningitis, tuberculoma, and abscess.3 The clinical presentation and imaging studies of CNS TB are often indistinguishable from those of patients with malignant neoplasms or metastatic disease. Biopsies may be necessary to distinguish tuberculomas from other intracranial lesions such as pyogenic abscesses or necrotic tumors.4 Mycobacterial cultures were not done on the brain biopsies of our patient because of the high clinical suspicion for neoplasm. Axillary lymph node tissue ultimately confirmed the diagnosis and provided sensitivities.
A diagnosis of CNS tuberculoma without meningitis can be challenging because the clinical presentation is often vague, mild, or even asymptomatic. Constitutional symptoms may include headache, fever, and anorexia.5
In our patient, IGRA testing was also negative. For latent TB, IGRAs are considered to be at least as sensitive as, and considerably more specific than TB skin testing, but their use in CNS TB is less well understood. Studies evaluating IGRA sensitivity for TB meningitis show variable results. In one study, IGRAs were positive in only 50% of culture-confirmed cases of TB meningitis in an HIV-negative population.6
Obtain sputum samples for all patients with extrapulmonary TB
The CDC recommends sputum sampling for all patients with extrapulmonary TB, even in the absence of pulmonary symptoms or radiographic findings, to determine the level of infectivity and potential need for a contact investigation.7
Due to low sensitivity of currently available rapid diagnostic tests and high mortality associated with delayed treatment, initiation of empiric treatment is recommended when the probability of CNS TB is high.5
Treatment duration for CNS tuberculomas is based on one randomized controlled trial,8 a small number of observational studies, a prospective cohort study looking at radiographic resolution,9 and expert opinion. Treatment recommendations often do not distinguish CNS tuberculomas from TB meningitis.10 CNS tuberculomas are commonly treated with a minimum of 12 months of therapy, generally using the same medications and dosages used in the treatment of pulmonary TB, starting with 4 first-line agents: isoniazid, rifampin, pyrazinamide, and ethambutol. Modification of the treatment regimen may be made once sensitivities are available.10
Our patient. After cultures were determined to be pan-sensitive, our patient’s treatment regimen was simplified to rifampin and isoniazid, which she continued for the remainder of her treatment course. Her treatment was discontinued after 18 months when quarterly MRIs showed stabilization of the tuberculomas (FIGURE 2).

Following her surgery, she was started on levetiractam for seizure prophylaxis. She subsequently had a seizure on 2 occasions when the medication was discontinued or decreased, so we chose to continue it. The patient is asymptomatic from her disease with no residual deficits.
THE TAKEAWAY
A change in headache patterns in a patient over the age of 50 is a red flag that warrants imaging. In patients from countries where TB is endemic,11 consider neurotuberculosis in the differential diagnosis of worsening headaches and progressive neurologic symptoms.
A diagnosis of CNS TB can be difficult and requires a high level of clinical suspicion, but early diagnosis and treatment of neurotuberculosis can minimize the high risk of morbidity and mortality. Treatment for TB shouldn’t be withheld in cases in which there’s a strong clinical suspicion for TB, but for which a definitive diagnosis is still pending.
1. World Health Organization. 10 facts on tuberculosis. Available at: http://www.who.int/features/factfiles/tuberculosis/en/. Accessed September 19, 2014.
2. Dastur DK, Iyer CG. Pathological analysis of 450 intracranial space-occupying lesions. Ind J Cancer. 1966;3:105-115.
3. Chin JH, Mateen FJ. Central nervous system tuberculosis: Challenges and advances in diagnosis and treatment. Curr Infect Dis Rep. 2013;15:631-635.
4. Bayindir C, Mete O, Bilgic B. Retrospective study of 23 pathologically proven cases of central nervous system tuberculomas. Clin Neurol Neurosurg. 2006;108:353-357.
5. Thwaites G, Fisher M, Hemingway C, et al; British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167-187.
6. Simmons CP, Thwaites GE, Quyen NT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176:2007-2014.
7. Centers for Disease Control and Prevention (CDC). Core curriculum on tuberculosis: What the clinician should know. 6th ed. Centers for Disease Control and Prevention, Atlanta, GA; 2013.
8. Rajeswari R, Sivasubramanian S, Balambal R, et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
9. Poonnoose SI, Rajshekhar V. Rate of resolution of histologically verified intracranial tuberculomas. Neurosurgery. 2003;53:873-878.
10. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77. Erratum in: MMWR Recomm Rep. 2005;53:1203.
11. Stop TB Partnership. High burden countries. Available at: http://www.stoptb.org/countries/tbdata.asp. Accessed November 7, 2016.
1. World Health Organization. 10 facts on tuberculosis. Available at: http://www.who.int/features/factfiles/tuberculosis/en/. Accessed September 19, 2014.
2. Dastur DK, Iyer CG. Pathological analysis of 450 intracranial space-occupying lesions. Ind J Cancer. 1966;3:105-115.
3. Chin JH, Mateen FJ. Central nervous system tuberculosis: Challenges and advances in diagnosis and treatment. Curr Infect Dis Rep. 2013;15:631-635.
4. Bayindir C, Mete O, Bilgic B. Retrospective study of 23 pathologically proven cases of central nervous system tuberculomas. Clin Neurol Neurosurg. 2006;108:353-357.
5. Thwaites G, Fisher M, Hemingway C, et al; British Infection Society. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009;59:167-187.
6. Simmons CP, Thwaites GE, Quyen NT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol. 2006;176:2007-2014.
7. Centers for Disease Control and Prevention (CDC). Core curriculum on tuberculosis: What the clinician should know. 6th ed. Centers for Disease Control and Prevention, Atlanta, GA; 2013.
8. Rajeswari R, Sivasubramanian S, Balambal R, et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
9. Poonnoose SI, Rajshekhar V. Rate of resolution of histologically verified intracranial tuberculomas. Neurosurgery. 2003;53:873-878.
10. American Thoracic Society; CDC; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1-77. Erratum in: MMWR Recomm Rep. 2005;53:1203.
11. Stop TB Partnership. High burden countries. Available at: http://www.stoptb.org/countries/tbdata.asp. Accessed November 7, 2016.