User login
Case Report: Acute Intermittent Porphyria
Case
A 34-year-old woman presented to the ED with severe, persistent abdominal pain that had begun 18 days earlier. She was 7 weeks pregnant and had been seen in the same ED the day before. During that visit, ultrasound had shown a single pregnancy of doubtful viability. Abdominal magnetic resonance imaging was normal. She was given multiple doses of hydromorphone. The discharge diagnosis was “missed abortion.”
Since the onset of her pain, she had been hospitalized twice elsewhere, with no clear diagnosis to explain her pain. Treatment consisted of repeat doses of hydromorphone. During the second hospitalization, a sodium level of 109 mEq/L had been corrected with hypertonic saline, and a urinary tract infection (UTI) had been treated with cephalexin.
Our patient had never experienced similar abdominal pain. Her medical history included depression and asthma. Her family history was notable for an aunt who had died of lung cancer.
On this ED visit, the patient’s vital signs were normal. On examination, she was moaning in pain and clutching her abdomen. The abdomen was tender in both lower quadrants, with guarding but no rebound. Her sodium level was 125 mEq/L; the day before it had been 132 mEq/L.
Urine dipstick testing showed 2+ glucose and 2+ bilirubin; both had been within normal range (negative) the day before. An abdominal/pelvic computed tomography scan with intravenous (IV) and oral contrast did not reveal any potential cause of the patient’s pain. Multiple doses of IV hydromorphone were given, but her pain persisted.
We revisited our patient’s family history. With prompting, she remembered that as a teenager, her mother had had an illness that caused “problems with her nerves and blood vessels and turned her urine red.” When reached by phone, the patient’s mother, who lives outside the United States, said she was familiar with the term “porphyria,” but curiously, she did not state she carried the diagnosis, and had not advised her children they could be at risk.
The patient was admitted to the intensive care unit (ICU) for treatment of hyponatremia. Her mother’s history led us to suspect porphyria, so we sent a urine sample from the ED for porphobilinogen (PBG) testing. Her urine was not red at that time (on further questioning, she remembered she had had an episode of “red urine” recently). Two days later, after the PBG result came back positive, treatment was initiated for porphyria. With further stool and serum testing, the diagnosis of acute intermittent porphyria (AIP) was made.
The patient was treated with glucose loading and hemin therapy. In the ICU, 2 ampules of 50% dextrose in water solution (D50W) was administered, and she was transferred to the hematology-oncology service for hemin therapy. Soon after, she underwent a dilation and curettage. Three weeks later, she was discharged in good condition.
Discussion
The unifying diagnostic concept is that toxins damage all components of the nervous system: intestinal, central, peripheral, and autonomic. Hence, any combination of abdominal pain, vomiting, psychiatric symptoms, vital sign instability, weakness, or sensory loss may occur.1 To further confuse the diagnostician, the constellation of symptoms may vary with each episode.
Two critical laboratory clues are red urine—which often is mistaken for a UTI or hematuria—and hyponatremia.1 Another porphyria hallmark is triggers. These include drugs, carbohydrate deprivation, smoking, and stress. Common chemical inciters include alcohol, ketamine, etomidate, macrodantin, nifedipine, progesterone, and phenytoin.1 The Atkins diet (zero carbohydrates) reportedly caused an uptick in new porphyria cases.1
Attacks usually start after puberty. Women tend to experience flares during the luteal (progesterone) phase of the menstrual cycle.1 Acute intermittent porphyria can mimic Guillain-Barré syndrome and psychosis.2 Delayed diagnosis may lead to irreversible neurological damage or death.2
Despite AIP’s complexity, initial diagnostic testing is simple: a urinary PBG level obtained during an attack is virtually 100% sensitive and specific for AIP and two other acute porphyrias: hereditary coproporphyria (HCP), and variegate porphyria (VP). A positive urine PBG mandates immediate treatment—even while awaiting porphyrin and GK delta-aminolevulinic acid (ALA) levels in stool and serum to identify which porphyria (AIP, HCP, or VP) is present. The fourth (and least common) acute porphyria, ALA dehydratase porphyria (ADP), may produce no PBG elevation and requires separate porphyrin and ALA testing to make the diagnosis.
Treatment
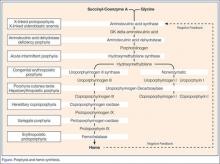
Treatment targets runaway heme precursor synthesis at its start and finish (Figure). Glucose-loading suppresses the initial enzyme, ALA synthase. Since the absence of normal end-product (heme) drives the enzymatic cascade, addition of IV hemin provides the substrate—and negative feedback—to stop it.
Conclusion
This case represents an example of AIP in which a patient presented with the characteristic abdominal pain and hyponatremia, complicated by the fact that she was pregnant and her urine was not red.
Intractable abdominal pain with negative imaging must prompt a search for red urine, neurological symptoms, porphyria medication triggers, and a family history of porphyria. Any constellation of findings should prompt immediate urine PBG testing.
In the appropriate clinical setting, it may be prudent to glucose-load a patient while waiting for confirmatory testing (which can take 1-2 days). Hemin therapy is best instituted by the hematology service after high urine PBG levels are confirmed.
- Sood GK, Anderson KE. Pathogenesis, clinical manifestations, and diagnosis of acute intermittent porphyria.” Available at: http://www.uptodate.com/contents/pathogenesis-clinical-manifestations-and-diagnosis-of-acute-intermittent-porphyria. Accessed February 22, 2016
- Bonkovsky HL, Siao P, Roig Z, Hedley-Whyte ET, Flotte TJ. Case records of the Massachusetts General Hospital. Case 20-2008. A 57-year-old woman with abdominal pain and weakness after gastric bypass surgery. N Engl J Med. 2008;358(26):2813-2825.
Case
A 34-year-old woman presented to the ED with severe, persistent abdominal pain that had begun 18 days earlier. She was 7 weeks pregnant and had been seen in the same ED the day before. During that visit, ultrasound had shown a single pregnancy of doubtful viability. Abdominal magnetic resonance imaging was normal. She was given multiple doses of hydromorphone. The discharge diagnosis was “missed abortion.”
Since the onset of her pain, she had been hospitalized twice elsewhere, with no clear diagnosis to explain her pain. Treatment consisted of repeat doses of hydromorphone. During the second hospitalization, a sodium level of 109 mEq/L had been corrected with hypertonic saline, and a urinary tract infection (UTI) had been treated with cephalexin.
Our patient had never experienced similar abdominal pain. Her medical history included depression and asthma. Her family history was notable for an aunt who had died of lung cancer.
On this ED visit, the patient’s vital signs were normal. On examination, she was moaning in pain and clutching her abdomen. The abdomen was tender in both lower quadrants, with guarding but no rebound. Her sodium level was 125 mEq/L; the day before it had been 132 mEq/L.
Urine dipstick testing showed 2+ glucose and 2+ bilirubin; both had been within normal range (negative) the day before. An abdominal/pelvic computed tomography scan with intravenous (IV) and oral contrast did not reveal any potential cause of the patient’s pain. Multiple doses of IV hydromorphone were given, but her pain persisted.
We revisited our patient’s family history. With prompting, she remembered that as a teenager, her mother had had an illness that caused “problems with her nerves and blood vessels and turned her urine red.” When reached by phone, the patient’s mother, who lives outside the United States, said she was familiar with the term “porphyria,” but curiously, she did not state she carried the diagnosis, and had not advised her children they could be at risk.
The patient was admitted to the intensive care unit (ICU) for treatment of hyponatremia. Her mother’s history led us to suspect porphyria, so we sent a urine sample from the ED for porphobilinogen (PBG) testing. Her urine was not red at that time (on further questioning, she remembered she had had an episode of “red urine” recently). Two days later, after the PBG result came back positive, treatment was initiated for porphyria. With further stool and serum testing, the diagnosis of acute intermittent porphyria (AIP) was made.
The patient was treated with glucose loading and hemin therapy. In the ICU, 2 ampules of 50% dextrose in water solution (D50W) was administered, and she was transferred to the hematology-oncology service for hemin therapy. Soon after, she underwent a dilation and curettage. Three weeks later, she was discharged in good condition.
Discussion
The unifying diagnostic concept is that toxins damage all components of the nervous system: intestinal, central, peripheral, and autonomic. Hence, any combination of abdominal pain, vomiting, psychiatric symptoms, vital sign instability, weakness, or sensory loss may occur.1 To further confuse the diagnostician, the constellation of symptoms may vary with each episode.
Two critical laboratory clues are red urine—which often is mistaken for a UTI or hematuria—and hyponatremia.1 Another porphyria hallmark is triggers. These include drugs, carbohydrate deprivation, smoking, and stress. Common chemical inciters include alcohol, ketamine, etomidate, macrodantin, nifedipine, progesterone, and phenytoin.1 The Atkins diet (zero carbohydrates) reportedly caused an uptick in new porphyria cases.1
Attacks usually start after puberty. Women tend to experience flares during the luteal (progesterone) phase of the menstrual cycle.1 Acute intermittent porphyria can mimic Guillain-Barré syndrome and psychosis.2 Delayed diagnosis may lead to irreversible neurological damage or death.2
Despite AIP’s complexity, initial diagnostic testing is simple: a urinary PBG level obtained during an attack is virtually 100% sensitive and specific for AIP and two other acute porphyrias: hereditary coproporphyria (HCP), and variegate porphyria (VP). A positive urine PBG mandates immediate treatment—even while awaiting porphyrin and GK delta-aminolevulinic acid (ALA) levels in stool and serum to identify which porphyria (AIP, HCP, or VP) is present. The fourth (and least common) acute porphyria, ALA dehydratase porphyria (ADP), may produce no PBG elevation and requires separate porphyrin and ALA testing to make the diagnosis.
Treatment
Treatment targets runaway heme precursor synthesis at its start and finish (Figure). Glucose-loading suppresses the initial enzyme, ALA synthase. Since the absence of normal end-product (heme) drives the enzymatic cascade, addition of IV hemin provides the substrate—and negative feedback—to stop it.
Conclusion
This case represents an example of AIP in which a patient presented with the characteristic abdominal pain and hyponatremia, complicated by the fact that she was pregnant and her urine was not red.
Intractable abdominal pain with negative imaging must prompt a search for red urine, neurological symptoms, porphyria medication triggers, and a family history of porphyria. Any constellation of findings should prompt immediate urine PBG testing.
In the appropriate clinical setting, it may be prudent to glucose-load a patient while waiting for confirmatory testing (which can take 1-2 days). Hemin therapy is best instituted by the hematology service after high urine PBG levels are confirmed.
Case
A 34-year-old woman presented to the ED with severe, persistent abdominal pain that had begun 18 days earlier. She was 7 weeks pregnant and had been seen in the same ED the day before. During that visit, ultrasound had shown a single pregnancy of doubtful viability. Abdominal magnetic resonance imaging was normal. She was given multiple doses of hydromorphone. The discharge diagnosis was “missed abortion.”
Since the onset of her pain, she had been hospitalized twice elsewhere, with no clear diagnosis to explain her pain. Treatment consisted of repeat doses of hydromorphone. During the second hospitalization, a sodium level of 109 mEq/L had been corrected with hypertonic saline, and a urinary tract infection (UTI) had been treated with cephalexin.
Our patient had never experienced similar abdominal pain. Her medical history included depression and asthma. Her family history was notable for an aunt who had died of lung cancer.
On this ED visit, the patient’s vital signs were normal. On examination, she was moaning in pain and clutching her abdomen. The abdomen was tender in both lower quadrants, with guarding but no rebound. Her sodium level was 125 mEq/L; the day before it had been 132 mEq/L.
Urine dipstick testing showed 2+ glucose and 2+ bilirubin; both had been within normal range (negative) the day before. An abdominal/pelvic computed tomography scan with intravenous (IV) and oral contrast did not reveal any potential cause of the patient’s pain. Multiple doses of IV hydromorphone were given, but her pain persisted.
We revisited our patient’s family history. With prompting, she remembered that as a teenager, her mother had had an illness that caused “problems with her nerves and blood vessels and turned her urine red.” When reached by phone, the patient’s mother, who lives outside the United States, said she was familiar with the term “porphyria,” but curiously, she did not state she carried the diagnosis, and had not advised her children they could be at risk.
The patient was admitted to the intensive care unit (ICU) for treatment of hyponatremia. Her mother’s history led us to suspect porphyria, so we sent a urine sample from the ED for porphobilinogen (PBG) testing. Her urine was not red at that time (on further questioning, she remembered she had had an episode of “red urine” recently). Two days later, after the PBG result came back positive, treatment was initiated for porphyria. With further stool and serum testing, the diagnosis of acute intermittent porphyria (AIP) was made.
The patient was treated with glucose loading and hemin therapy. In the ICU, 2 ampules of 50% dextrose in water solution (D50W) was administered, and she was transferred to the hematology-oncology service for hemin therapy. Soon after, she underwent a dilation and curettage. Three weeks later, she was discharged in good condition.
Discussion
The unifying diagnostic concept is that toxins damage all components of the nervous system: intestinal, central, peripheral, and autonomic. Hence, any combination of abdominal pain, vomiting, psychiatric symptoms, vital sign instability, weakness, or sensory loss may occur.1 To further confuse the diagnostician, the constellation of symptoms may vary with each episode.
Two critical laboratory clues are red urine—which often is mistaken for a UTI or hematuria—and hyponatremia.1 Another porphyria hallmark is triggers. These include drugs, carbohydrate deprivation, smoking, and stress. Common chemical inciters include alcohol, ketamine, etomidate, macrodantin, nifedipine, progesterone, and phenytoin.1 The Atkins diet (zero carbohydrates) reportedly caused an uptick in new porphyria cases.1
Attacks usually start after puberty. Women tend to experience flares during the luteal (progesterone) phase of the menstrual cycle.1 Acute intermittent porphyria can mimic Guillain-Barré syndrome and psychosis.2 Delayed diagnosis may lead to irreversible neurological damage or death.2
Despite AIP’s complexity, initial diagnostic testing is simple: a urinary PBG level obtained during an attack is virtually 100% sensitive and specific for AIP and two other acute porphyrias: hereditary coproporphyria (HCP), and variegate porphyria (VP). A positive urine PBG mandates immediate treatment—even while awaiting porphyrin and GK delta-aminolevulinic acid (ALA) levels in stool and serum to identify which porphyria (AIP, HCP, or VP) is present. The fourth (and least common) acute porphyria, ALA dehydratase porphyria (ADP), may produce no PBG elevation and requires separate porphyrin and ALA testing to make the diagnosis.
Treatment
Treatment targets runaway heme precursor synthesis at its start and finish (Figure). Glucose-loading suppresses the initial enzyme, ALA synthase. Since the absence of normal end-product (heme) drives the enzymatic cascade, addition of IV hemin provides the substrate—and negative feedback—to stop it.
Conclusion
This case represents an example of AIP in which a patient presented with the characteristic abdominal pain and hyponatremia, complicated by the fact that she was pregnant and her urine was not red.
Intractable abdominal pain with negative imaging must prompt a search for red urine, neurological symptoms, porphyria medication triggers, and a family history of porphyria. Any constellation of findings should prompt immediate urine PBG testing.
In the appropriate clinical setting, it may be prudent to glucose-load a patient while waiting for confirmatory testing (which can take 1-2 days). Hemin therapy is best instituted by the hematology service after high urine PBG levels are confirmed.
- Sood GK, Anderson KE. Pathogenesis, clinical manifestations, and diagnosis of acute intermittent porphyria.” Available at: http://www.uptodate.com/contents/pathogenesis-clinical-manifestations-and-diagnosis-of-acute-intermittent-porphyria. Accessed February 22, 2016
- Bonkovsky HL, Siao P, Roig Z, Hedley-Whyte ET, Flotte TJ. Case records of the Massachusetts General Hospital. Case 20-2008. A 57-year-old woman with abdominal pain and weakness after gastric bypass surgery. N Engl J Med. 2008;358(26):2813-2825.
- Sood GK, Anderson KE. Pathogenesis, clinical manifestations, and diagnosis of acute intermittent porphyria.” Available at: http://www.uptodate.com/contents/pathogenesis-clinical-manifestations-and-diagnosis-of-acute-intermittent-porphyria. Accessed February 22, 2016
- Bonkovsky HL, Siao P, Roig Z, Hedley-Whyte ET, Flotte TJ. Case records of the Massachusetts General Hospital. Case 20-2008. A 57-year-old woman with abdominal pain and weakness after gastric bypass surgery. N Engl J Med. 2008;358(26):2813-2825.
Swollen lymph nodes • patient is otherwise "healthy" • Dx?
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
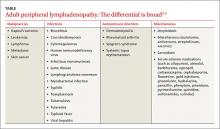
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
Extreme Postinjection Flare in Response to Intra-Articular Triamcinolone Acetonide (Kenalog)
Intra-articular corticosteroid injections (CSIs) have been a common treatment for osteoarthritis since the 1950s and continue to be an option for patients who prefer nonoperative management.1 Although CSIs may improve pain secondary to osteoarthritis temporarily, they do not slow articular cartilage degradation, and many patients request multiple CSIs before total joint arthroplasty ultimately is required.1,2 Therefore, acute and chronic side effects of CSI must be considered when repeatedly administering corticosteroids.
A postinjection flare, the most common acute side effect of intra-articular CSI, is characterized by a localized inflammatory response that can last 2 to 3 days. The flare occurs in 2% to 25% of CSI cases.3-5 Symptoms can range from mild joint effusion to disabling pain.6 In the present case, a severe postinjection flare occurred after intra-articular administration of triamcinolone acetonide (Kenalog). This case is novel in that its acuity of onset, severity of symptoms, and synovial fluid analysis mimicked septic arthritis, which was ultimately ruled out with negative cultures and confirmation of triamcinolone acetonide crystals in the synovial aspirate, viewed by polarized light microscopy. To date, only one other case of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone has been reported.7 As CSIs are often used in the nonoperative treatment of osteoarthritis, it is imperative for the treating physician to be aware of this potential side effect in order to appropriately inform the patient of this risk and guide treatment should the scenario arise. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman with a history of hypertension, hypothyroidism, and moderate bilateral knee osteoarthritis presented with left knee pain. She had been receiving annual hylan injections for 5 years and had no adverse reactions, but the pain gradually worsened over the past 3 months. She was given an intra-articular injection of 2 mL of 1% lidocaine and 2 mL (40 mg) of triamcinolone acetonide in the left knee.
Two hours later, she experienced swelling and intense pain in the knee and was unable to ambulate. Physical examination revealed she was afebrile but was having severe pain in the knee through all range of motion. The knee had no appreciable erythema or warmth. Laboratory data were significant: White blood cell (WBC) count was 14,600, and erythrocyte sedimentation rate was 1 mm/h. The knee was aspirated with a return of 25 mL of “butterscotch”-colored fluid (Figure 1). The patient was admitted to rule out iatrogenic septic arthritis, or chronic, indolent septic arthritis acutely worsened by CSI, until synovial fluid analysis and cultures could be performed (Table 1).
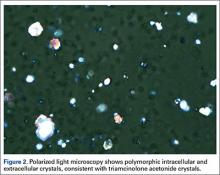
She was treated overnight with a compressive wrap, elevation, ice, and nonsteroidal anti-inflammatory drugs, which provided significant pain relief. Polarized light microscopy revealed polymorphic intracellular and extracellular crystals with crystal morphology consistent with the injection of triamcinolone ester (Figure 2). Gram stain showed many WBCs but no organisms. These findings were thought to represent an exogenous crystal-induced acute inflammatory response. Given the patient’s improving clinical course, she was discharged the next morning.
Twelve days later, at clinic follow-up, she was still experiencing pain above her baseline level. Given the continued effusion, 8 mL of synovial fluid was aspirated, which appeared clear and only slightly blood-tinged. Synovial analysis showed resolution of leukocytosis, confirming a severe postinjection flare in response to triamcinolone acetonide.
Discussion
Although rare, side effects from repeated intra-articular CSIs include hypothalamic-pituitary-adrenal axis dysfunction and steroid-induced myopathy.8,9 Acute side effects are more common and include postinjection flare, iatrogenic septic arthritis, local tissue atrophy, cartilage damage, tendon rupture, nerve atrophy, increased blood glucose, and osteonecrosis.10,11 The present case report describes an extreme example of a postinjection flare in response to triamcinolone acetonide and summarizes the characteristics of injections that cause flares.
The physical properties of corticosteroids have a significant impact on their efficacy and on their potential for adverse events. Corticosteroid preparations can be water-soluble or water-insoluble. Most commonly, water-insoluble preparations that contain insoluble corticosteroid esters (eg, triamcinolone, methylprednisolone) are used in intra-articular injections. These form microcrystalline aggregates in solution, which require the patient’s own hydrolytic enzymes (esterases) to release the active moiety and thus have a longer duration of action. However, they are more commonly associated with postinjection flares compared with their more soluble and faster- acting counterparts (eg. dexamethasone, betamethasone).10 Microcrystalline aggregates, which are larger in size, induce a stronger inflammatory response, and in a dose-dependent manner.6A sterile inflammatory reaction to hydrocortisone, cortisone, dexamethasone, triamcinolone, and prednisolone crystals in normal joints has been previously described,6,12,13 and crystals of the various preparations have been demonstrated within leukocytes by both polarized light and electron microscopy.12,13 Table 2 summarizes previous synovial fluid analyses after intra-articular injections of various corticosteroid preparations in normal healthy joints and in patients experiencing a postinjection flare. To date, there have been no reports of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone acetonide, though there was a report of a postinjection flare in response to triamcinolone hexacetonide (Aristospan),7 and here the synovial fluid WBC count (30,000) was much lower.
Although many cases of corticosteroid hypersensitivity have been reported, in rare cases intra-articular administration of triamcinolone has caused anaphylactic reactions and shock.14,15 Multiple case studies have determined that the specific excipient carboxymethylcellulose (found in many triamcinolone preparations), and not the corticosteroid itself, can cause an immunoglobulin E–mediated anaphylactic reaction.16-18 Therefore, performing skin-prick tests for potential corticosteroids and their excipients in patients with known postinjection flares might help prevent serious adverse reactions.18,19
The present case involved an extreme postinjection flare in response to intra-articular administration of triamcinolone acetonide. Postinjection flares are rare but significant events, and physicians using CSIs in the treatment of arthritis need to be aware of this potential reaction in order to appropriately inform patients of this risk and guide treatment should the scenario arise.
1. Hollander JL, Brown EM Jr, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629-1635.
2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;19(2):CD005328.
3. Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7(6):850-856.
4. Brown EM Jr, Frain JB, Udell L, Hollander JL. Locally administered hydrocortisone in the rheumatic diseases; a summary of its use in 547 patients. Am J Med. 1953;15(5):656-665.
5. Hollander JL, Jessar RA, Brown EM Jr. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:239-240.
6. McCarty DJ Jr, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum. 1964;7(4):359-367.
7. Berger RG, Yount WJ. Immediate “steroid flare” from intraarticular triamcinolone hexacetonide injection: case report and review of the literature. Arthritis Rheum. 1990;33(8):1284-1286.
8. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924-928.
9. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
10. MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647-661.
11. Sparling M, Malleson P, Wood B, Petty R. Radiographic followup of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33(6):821-826.
12. Eymontt MJ, Gordon GV, Schumacher HR, Hansell JR. The effects on synovial permeability and synovial fluid leukocyte counts in symptomatic osteoarthritis after intraarticular corticosteroid administration. J Rheumatol. 1982;9(2):198-203.
13. Gordon GV, Schumacher HR. Electron microscopic study of depot corticosteroid crystals with clinical studies after intra-articular injection. J Rheumatol. 1979;6(1):7-14.
14. Karsh J, Yang WH. An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003;90(2):254-258.
15. Larsson LG. Anaphylactic shock after i.a. administration of triamcinolone acetonide in a 35-year-old female. Scand J Rheumatol. 1989;18(6):441-442.
16. García-Ortega P, Corominas M, Badia M. Carboxymethylcellulose allergy as a cause of suspected corticosteroid anaphylaxis. Ann Allergy Asthma Immunol. 2003;91(4):421.
17. Patterson DL, Yunginger JW, Dunn WF, Jones RT, Hunt LW. Anaphylaxis induced by the carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog). Ann Allergy Asthma Immunol. 1995;74(2):163-166.
18. Steiner UC, Gentinetta T, Hausmann O, Pichler WJ. IgE-mediated anaphylaxis to intraarticular glucocorticoid preparations. AJR Am J Roentgenol. 2009;193(2):W156-W157.
19. Ijsselmuiden OE, Knegt-Junk KJ, van Wijk RG, van Joost T. Cutaneous adverse reactions after intra-articular injection of triamcinolone acetonide. Acta Derm Venereol. 1995;75(1):57-58.
20. Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol. 2002;29(12):2611-2614.
Intra-articular corticosteroid injections (CSIs) have been a common treatment for osteoarthritis since the 1950s and continue to be an option for patients who prefer nonoperative management.1 Although CSIs may improve pain secondary to osteoarthritis temporarily, they do not slow articular cartilage degradation, and many patients request multiple CSIs before total joint arthroplasty ultimately is required.1,2 Therefore, acute and chronic side effects of CSI must be considered when repeatedly administering corticosteroids.
A postinjection flare, the most common acute side effect of intra-articular CSI, is characterized by a localized inflammatory response that can last 2 to 3 days. The flare occurs in 2% to 25% of CSI cases.3-5 Symptoms can range from mild joint effusion to disabling pain.6 In the present case, a severe postinjection flare occurred after intra-articular administration of triamcinolone acetonide (Kenalog). This case is novel in that its acuity of onset, severity of symptoms, and synovial fluid analysis mimicked septic arthritis, which was ultimately ruled out with negative cultures and confirmation of triamcinolone acetonide crystals in the synovial aspirate, viewed by polarized light microscopy. To date, only one other case of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone has been reported.7 As CSIs are often used in the nonoperative treatment of osteoarthritis, it is imperative for the treating physician to be aware of this potential side effect in order to appropriately inform the patient of this risk and guide treatment should the scenario arise. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman with a history of hypertension, hypothyroidism, and moderate bilateral knee osteoarthritis presented with left knee pain. She had been receiving annual hylan injections for 5 years and had no adverse reactions, but the pain gradually worsened over the past 3 months. She was given an intra-articular injection of 2 mL of 1% lidocaine and 2 mL (40 mg) of triamcinolone acetonide in the left knee.
Two hours later, she experienced swelling and intense pain in the knee and was unable to ambulate. Physical examination revealed she was afebrile but was having severe pain in the knee through all range of motion. The knee had no appreciable erythema or warmth. Laboratory data were significant: White blood cell (WBC) count was 14,600, and erythrocyte sedimentation rate was 1 mm/h. The knee was aspirated with a return of 25 mL of “butterscotch”-colored fluid (Figure 1). The patient was admitted to rule out iatrogenic septic arthritis, or chronic, indolent septic arthritis acutely worsened by CSI, until synovial fluid analysis and cultures could be performed (Table 1).
She was treated overnight with a compressive wrap, elevation, ice, and nonsteroidal anti-inflammatory drugs, which provided significant pain relief. Polarized light microscopy revealed polymorphic intracellular and extracellular crystals with crystal morphology consistent with the injection of triamcinolone ester (Figure 2). Gram stain showed many WBCs but no organisms. These findings were thought to represent an exogenous crystal-induced acute inflammatory response. Given the patient’s improving clinical course, she was discharged the next morning.
Twelve days later, at clinic follow-up, she was still experiencing pain above her baseline level. Given the continued effusion, 8 mL of synovial fluid was aspirated, which appeared clear and only slightly blood-tinged. Synovial analysis showed resolution of leukocytosis, confirming a severe postinjection flare in response to triamcinolone acetonide.
Discussion
Although rare, side effects from repeated intra-articular CSIs include hypothalamic-pituitary-adrenal axis dysfunction and steroid-induced myopathy.8,9 Acute side effects are more common and include postinjection flare, iatrogenic septic arthritis, local tissue atrophy, cartilage damage, tendon rupture, nerve atrophy, increased blood glucose, and osteonecrosis.10,11 The present case report describes an extreme example of a postinjection flare in response to triamcinolone acetonide and summarizes the characteristics of injections that cause flares.
The physical properties of corticosteroids have a significant impact on their efficacy and on their potential for adverse events. Corticosteroid preparations can be water-soluble or water-insoluble. Most commonly, water-insoluble preparations that contain insoluble corticosteroid esters (eg, triamcinolone, methylprednisolone) are used in intra-articular injections. These form microcrystalline aggregates in solution, which require the patient’s own hydrolytic enzymes (esterases) to release the active moiety and thus have a longer duration of action. However, they are more commonly associated with postinjection flares compared with their more soluble and faster- acting counterparts (eg. dexamethasone, betamethasone).10 Microcrystalline aggregates, which are larger in size, induce a stronger inflammatory response, and in a dose-dependent manner.6A sterile inflammatory reaction to hydrocortisone, cortisone, dexamethasone, triamcinolone, and prednisolone crystals in normal joints has been previously described,6,12,13 and crystals of the various preparations have been demonstrated within leukocytes by both polarized light and electron microscopy.12,13 Table 2 summarizes previous synovial fluid analyses after intra-articular injections of various corticosteroid preparations in normal healthy joints and in patients experiencing a postinjection flare. To date, there have been no reports of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone acetonide, though there was a report of a postinjection flare in response to triamcinolone hexacetonide (Aristospan),7 and here the synovial fluid WBC count (30,000) was much lower.
Although many cases of corticosteroid hypersensitivity have been reported, in rare cases intra-articular administration of triamcinolone has caused anaphylactic reactions and shock.14,15 Multiple case studies have determined that the specific excipient carboxymethylcellulose (found in many triamcinolone preparations), and not the corticosteroid itself, can cause an immunoglobulin E–mediated anaphylactic reaction.16-18 Therefore, performing skin-prick tests for potential corticosteroids and their excipients in patients with known postinjection flares might help prevent serious adverse reactions.18,19
The present case involved an extreme postinjection flare in response to intra-articular administration of triamcinolone acetonide. Postinjection flares are rare but significant events, and physicians using CSIs in the treatment of arthritis need to be aware of this potential reaction in order to appropriately inform patients of this risk and guide treatment should the scenario arise.
Intra-articular corticosteroid injections (CSIs) have been a common treatment for osteoarthritis since the 1950s and continue to be an option for patients who prefer nonoperative management.1 Although CSIs may improve pain secondary to osteoarthritis temporarily, they do not slow articular cartilage degradation, and many patients request multiple CSIs before total joint arthroplasty ultimately is required.1,2 Therefore, acute and chronic side effects of CSI must be considered when repeatedly administering corticosteroids.
A postinjection flare, the most common acute side effect of intra-articular CSI, is characterized by a localized inflammatory response that can last 2 to 3 days. The flare occurs in 2% to 25% of CSI cases.3-5 Symptoms can range from mild joint effusion to disabling pain.6 In the present case, a severe postinjection flare occurred after intra-articular administration of triamcinolone acetonide (Kenalog). This case is novel in that its acuity of onset, severity of symptoms, and synovial fluid analysis mimicked septic arthritis, which was ultimately ruled out with negative cultures and confirmation of triamcinolone acetonide crystals in the synovial aspirate, viewed by polarized light microscopy. To date, only one other case of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone has been reported.7 As CSIs are often used in the nonoperative treatment of osteoarthritis, it is imperative for the treating physician to be aware of this potential side effect in order to appropriately inform the patient of this risk and guide treatment should the scenario arise. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman with a history of hypertension, hypothyroidism, and moderate bilateral knee osteoarthritis presented with left knee pain. She had been receiving annual hylan injections for 5 years and had no adverse reactions, but the pain gradually worsened over the past 3 months. She was given an intra-articular injection of 2 mL of 1% lidocaine and 2 mL (40 mg) of triamcinolone acetonide in the left knee.
Two hours later, she experienced swelling and intense pain in the knee and was unable to ambulate. Physical examination revealed she was afebrile but was having severe pain in the knee through all range of motion. The knee had no appreciable erythema or warmth. Laboratory data were significant: White blood cell (WBC) count was 14,600, and erythrocyte sedimentation rate was 1 mm/h. The knee was aspirated with a return of 25 mL of “butterscotch”-colored fluid (Figure 1). The patient was admitted to rule out iatrogenic septic arthritis, or chronic, indolent septic arthritis acutely worsened by CSI, until synovial fluid analysis and cultures could be performed (Table 1).
She was treated overnight with a compressive wrap, elevation, ice, and nonsteroidal anti-inflammatory drugs, which provided significant pain relief. Polarized light microscopy revealed polymorphic intracellular and extracellular crystals with crystal morphology consistent with the injection of triamcinolone ester (Figure 2). Gram stain showed many WBCs but no organisms. These findings were thought to represent an exogenous crystal-induced acute inflammatory response. Given the patient’s improving clinical course, she was discharged the next morning.
Twelve days later, at clinic follow-up, she was still experiencing pain above her baseline level. Given the continued effusion, 8 mL of synovial fluid was aspirated, which appeared clear and only slightly blood-tinged. Synovial analysis showed resolution of leukocytosis, confirming a severe postinjection flare in response to triamcinolone acetonide.
Discussion
Although rare, side effects from repeated intra-articular CSIs include hypothalamic-pituitary-adrenal axis dysfunction and steroid-induced myopathy.8,9 Acute side effects are more common and include postinjection flare, iatrogenic septic arthritis, local tissue atrophy, cartilage damage, tendon rupture, nerve atrophy, increased blood glucose, and osteonecrosis.10,11 The present case report describes an extreme example of a postinjection flare in response to triamcinolone acetonide and summarizes the characteristics of injections that cause flares.
The physical properties of corticosteroids have a significant impact on their efficacy and on their potential for adverse events. Corticosteroid preparations can be water-soluble or water-insoluble. Most commonly, water-insoluble preparations that contain insoluble corticosteroid esters (eg, triamcinolone, methylprednisolone) are used in intra-articular injections. These form microcrystalline aggregates in solution, which require the patient’s own hydrolytic enzymes (esterases) to release the active moiety and thus have a longer duration of action. However, they are more commonly associated with postinjection flares compared with their more soluble and faster- acting counterparts (eg. dexamethasone, betamethasone).10 Microcrystalline aggregates, which are larger in size, induce a stronger inflammatory response, and in a dose-dependent manner.6A sterile inflammatory reaction to hydrocortisone, cortisone, dexamethasone, triamcinolone, and prednisolone crystals in normal joints has been previously described,6,12,13 and crystals of the various preparations have been demonstrated within leukocytes by both polarized light and electron microscopy.12,13 Table 2 summarizes previous synovial fluid analyses after intra-articular injections of various corticosteroid preparations in normal healthy joints and in patients experiencing a postinjection flare. To date, there have been no reports of an immediate (<2 hours) and severe postinjection flare in response to triamcinolone acetonide, though there was a report of a postinjection flare in response to triamcinolone hexacetonide (Aristospan),7 and here the synovial fluid WBC count (30,000) was much lower.
Although many cases of corticosteroid hypersensitivity have been reported, in rare cases intra-articular administration of triamcinolone has caused anaphylactic reactions and shock.14,15 Multiple case studies have determined that the specific excipient carboxymethylcellulose (found in many triamcinolone preparations), and not the corticosteroid itself, can cause an immunoglobulin E–mediated anaphylactic reaction.16-18 Therefore, performing skin-prick tests for potential corticosteroids and their excipients in patients with known postinjection flares might help prevent serious adverse reactions.18,19
The present case involved an extreme postinjection flare in response to intra-articular administration of triamcinolone acetonide. Postinjection flares are rare but significant events, and physicians using CSIs in the treatment of arthritis need to be aware of this potential reaction in order to appropriately inform patients of this risk and guide treatment should the scenario arise.
1. Hollander JL, Brown EM Jr, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629-1635.
2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;19(2):CD005328.
3. Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7(6):850-856.
4. Brown EM Jr, Frain JB, Udell L, Hollander JL. Locally administered hydrocortisone in the rheumatic diseases; a summary of its use in 547 patients. Am J Med. 1953;15(5):656-665.
5. Hollander JL, Jessar RA, Brown EM Jr. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:239-240.
6. McCarty DJ Jr, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum. 1964;7(4):359-367.
7. Berger RG, Yount WJ. Immediate “steroid flare” from intraarticular triamcinolone hexacetonide injection: case report and review of the literature. Arthritis Rheum. 1990;33(8):1284-1286.
8. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924-928.
9. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
10. MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647-661.
11. Sparling M, Malleson P, Wood B, Petty R. Radiographic followup of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33(6):821-826.
12. Eymontt MJ, Gordon GV, Schumacher HR, Hansell JR. The effects on synovial permeability and synovial fluid leukocyte counts in symptomatic osteoarthritis after intraarticular corticosteroid administration. J Rheumatol. 1982;9(2):198-203.
13. Gordon GV, Schumacher HR. Electron microscopic study of depot corticosteroid crystals with clinical studies after intra-articular injection. J Rheumatol. 1979;6(1):7-14.
14. Karsh J, Yang WH. An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003;90(2):254-258.
15. Larsson LG. Anaphylactic shock after i.a. administration of triamcinolone acetonide in a 35-year-old female. Scand J Rheumatol. 1989;18(6):441-442.
16. García-Ortega P, Corominas M, Badia M. Carboxymethylcellulose allergy as a cause of suspected corticosteroid anaphylaxis. Ann Allergy Asthma Immunol. 2003;91(4):421.
17. Patterson DL, Yunginger JW, Dunn WF, Jones RT, Hunt LW. Anaphylaxis induced by the carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog). Ann Allergy Asthma Immunol. 1995;74(2):163-166.
18. Steiner UC, Gentinetta T, Hausmann O, Pichler WJ. IgE-mediated anaphylaxis to intraarticular glucocorticoid preparations. AJR Am J Roentgenol. 2009;193(2):W156-W157.
19. Ijsselmuiden OE, Knegt-Junk KJ, van Wijk RG, van Joost T. Cutaneous adverse reactions after intra-articular injection of triamcinolone acetonide. Acta Derm Venereol. 1995;75(1):57-58.
20. Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol. 2002;29(12):2611-2614.
1. Hollander JL, Brown EM Jr, Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints; comparative effects of and use of hydrocortisone as a local antiarthritic agent. J Am Med Assoc. 1951;147(17):1629-1635.
2. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;19(2):CD005328.
3. Friedman DM, Moore ME. The efficacy of intraarticular steroids in osteoarthritis: a double-blind study. J Rheumatol. 1980;7(6):850-856.
4. Brown EM Jr, Frain JB, Udell L, Hollander JL. Locally administered hydrocortisone in the rheumatic diseases; a summary of its use in 547 patients. Am J Med. 1953;15(5):656-665.
5. Hollander JL, Jessar RA, Brown EM Jr. Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis. 1961;11:239-240.
6. McCarty DJ Jr, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum. 1964;7(4):359-367.
7. Berger RG, Yount WJ. Immediate “steroid flare” from intraarticular triamcinolone hexacetonide injection: case report and review of the literature. Arthritis Rheum. 1990;33(8):1284-1286.
8. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924-928.
9. Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370-377.
10. MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 2009;252(3):647-661.
11. Sparling M, Malleson P, Wood B, Petty R. Radiographic followup of joints injected with triamcinolone hexacetonide for the management of childhood arthritis. Arthritis Rheum. 1990;33(6):821-826.
12. Eymontt MJ, Gordon GV, Schumacher HR, Hansell JR. The effects on synovial permeability and synovial fluid leukocyte counts in symptomatic osteoarthritis after intraarticular corticosteroid administration. J Rheumatol. 1982;9(2):198-203.
13. Gordon GV, Schumacher HR. Electron microscopic study of depot corticosteroid crystals with clinical studies after intra-articular injection. J Rheumatol. 1979;6(1):7-14.
14. Karsh J, Yang WH. An anaphylactic reaction to intra-articular triamcinolone: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003;90(2):254-258.
15. Larsson LG. Anaphylactic shock after i.a. administration of triamcinolone acetonide in a 35-year-old female. Scand J Rheumatol. 1989;18(6):441-442.
16. García-Ortega P, Corominas M, Badia M. Carboxymethylcellulose allergy as a cause of suspected corticosteroid anaphylaxis. Ann Allergy Asthma Immunol. 2003;91(4):421.
17. Patterson DL, Yunginger JW, Dunn WF, Jones RT, Hunt LW. Anaphylaxis induced by the carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog). Ann Allergy Asthma Immunol. 1995;74(2):163-166.
18. Steiner UC, Gentinetta T, Hausmann O, Pichler WJ. IgE-mediated anaphylaxis to intraarticular glucocorticoid preparations. AJR Am J Roentgenol. 2009;193(2):W156-W157.
19. Ijsselmuiden OE, Knegt-Junk KJ, van Wijk RG, van Joost T. Cutaneous adverse reactions after intra-articular injection of triamcinolone acetonide. Acta Derm Venereol. 1995;75(1):57-58.
20. Pullman-Mooar S, Mooar P, Sieck M, Clayburne G, Schumacher HR. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections? J Rheumatol. 2002;29(12):2611-2614.
Tibialis Posterior Tendon Entrapment Within Posterior Malleolar Fracture Fragment
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
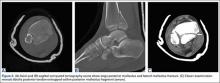
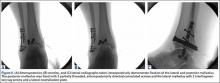
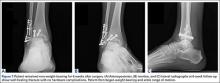
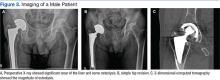
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
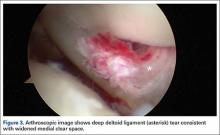
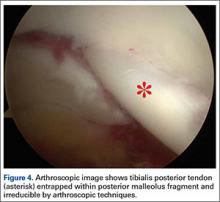
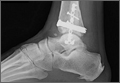
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
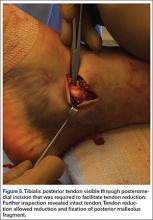
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
Irreducible ankle fracture-dislocation secondary to tibialis posterior tendon interposition is a rare but documented complication most commonly associated with Lauge-Hansen classification pronation–external rotation ankle fractures.1-4 Entrapment of the tibialis posterior tendon has been documented in the syndesmosis (tibiotalar joint)1,2,4 and within a medial malleolus fracture.5 To our knowledge, however, there are no case reports of entrapment of the tibialis posterior tendon in a posterior malleolus fracture.
Ankle arthroscopy performed at time of fracture fixation is gaining in popularity because of its enhanced ability to document and treat intra-articular pathology associated with the initial injury.6,7 In addition, percutaneous fixation of a posterior malleolar fragment with arthroscopic assessment of the articular surface reduction may be valuable, as evaluation of tibial plafond fracture reduction by plain radiographs and fluoroscopy has proved to have limitations.8,9
In this article, we present the case of a patient who underwent attempted arthroscopy-assisted reduction of the posterior malleolus with entrapment of the tibialis posterior tendon within the posterior malleolar fracture fragment. The tendon was irreducible with arthroscopic techniques, necessitating posteromedial incision and subsequent open reduction of the incarcerated structure. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old man slipped and fell on ice while jogging and subsequently presented to the emergency department with a closed bimalleolar ankle fracture-dislocation. Plain radiography (Figure 1) and computed tomography (CT) showed an oblique lateral malleolar fracture and a large posterior malleolar fracture. Further examination of the CT scan revealed entrapment of the tibialis posterior tendon within the posterior malleolar fracture (Figure 2).
Two days after injury, the patient was taken to the operating room for ankle arthroscopy with planned extrication of the entrapped tibialis posterior tendon and possible arthroscopy-assisted percutaneous fixation of the posterior malleolar fracture and open fixation of the distal fibula fracture. Diagnostic arthroscopy revealed a deltoid ligament injury (Figure 3) and a loose piece of articular cartilage (~1 cm in diameter), which was excised. No donor site for this cartilage fragment was identified with further arthroscopic evaluation. During arthroscopic examination, the tibialis posterior tendon was visualized within the joint, incarcerated within the posterior malleolar fracture (Figure 4). Attempts to release the tibialis posterior tendon from the fracture site using arthroscopic instruments and closed reduction techniques were unsuccessful, both with and without noninvasive skeletal traction applied to the ankle.
After multiple unsuccessful attempts to extract the tibialis posterior tendon arthroscopically, traction was removed, and a separate incision was made over the posteromedial aspect of the ankle. The tibialis posterior tendon was identified within the fracture site and was removed using an angled clamp (Figure 5). The fracture was reduced and held provisionally with a large tenaculum clamp. Two anterior-to-posterior, partially threaded cannulated screws were placed for fixation after adequate fracture reduction was confirmed on fluoroscopy. As a medial incision was made to extract the tibialis posterior tendon, the joint could not retain arthroscopic fluid, and visualization of the posterior fracture fragment after tendon removal was difficult. Therefore, arthroscopy-assisted reduction could not be completed.
Next, the lateral malleolus was open-reduced, and fixation was achieved using a standard interfragmentary lag screw and a lateral neutralization plate technique (Figure 6). After surgery, the patient was immobilized in a posterior splint with side gussets. Two weeks later, the incisions were healing well, and the tibialis posterior tendon was functioning normally. The sutures were removed, the patient was transitioned to a controlled ankle movement (CAM) boot, and ankle and subtalar range-of-motion exercises were initiated. The patient remained non-weight-bearing for 6 weeks. Radiographs 6 weeks after surgery showed healing fractures with stable hardware (Figure 7). The patient demonstrated 5/5 strength of the tibialis posterior tendon without subluxation or dislocation. There was no tenderness to palpation over the fracture sites or tibialis posterior tendon. The patient began progressive weight-bearing in a CAM boot and physical therapy for range of motion and strengthening.
Discussion
Tibialis posterior tendon injuries—including rupture, dislocation, and entrapment—are well-described complications of ankle injuries.1,2,5,10 Most commonly, the tibialis posterior tendon has been reported to cause a mechanical block to reduction in lateral subtalar dislocations.11-13 In addition, there are case reports of isolated traumatic dislocations of the tibialis posterior tendon without rupture, requiring operative stabilization and retinaculum repair with or without deepening of the posterior groove.14,15
Posterior malleolar ankle fractures remain controversial, with respect to both need for fixation and fixation methods. Although multiple investigators have advocated operative treatment for such fractures that exceed 25% to 33% of the anteroposterior dimension of the tibial plafond, there are no conclusive studies or evidence-based guidelines for treating these fractures.16,17 Anatomical reduction and plating are important to restore articular congruity and increase syndesmotic stability; recent studies have demonstrated that fixation of posterior malleolar fractures provides more syndesmotic stability than trans-syndesmotic screws do.18,19 Indirect reduction of the posterior malleolar fragment after fibula fixation is often accepted as adequate. Whether indirect or direct reduction is attempted, close attention should be given to plain radiographs after attempted reduction, and consideration should be given to possible soft-tissue or bony interposition if malreduction is identified.16,17 Plain radiographs are unreliable in assessing posterior malleolar fragment size as well as amount of comminution and impaction.8,9 Therefore, an arthroscopy-assisted approach coupled with percutaneous fixation may provide more reliable fracture reduction over indirect fracture reduction with fibular fixation, with less dissection than a formal posterolateral approach with posterior plating.
Not all ankle fractures require CT. However, for posterior malleolus fractures thought to require fixation, preoperative CT may help in determining if percutaneous fixation with or without arthroscopic guidance is a feasible treatment option. Ideally, percutaneous reduction can obviate the need for a larger posterolateral incision and buttress plate and, with arthroscopic assistance, may be superior to indirect reduction with fluoroscopy.
In our patient’s case, arthroscopic assistance facilitated diagnosis of an entrapped structure that would have been difficult to identify, particularly without preoperative CT. It may be difficult to identify imperfect reduction of the posterior malleolus on plain radiographs alone, and arthroscopy-assisted fixation enhances the surgeon’s ability to consider reduction, view incarcerated structures within the joint, and treat articular injuries. We do not routinely use ankle arthroscopy as an adjunct to ankle fracture fixation, but judicious use in select cases can facilitate treatment of intra-articular injuries and facilitate visualization and reduction of posterior malleolar fracture fragments before percutaneous anterior-to-posterior cannulated screw fixation. If an open incision is required, as in the present case, visualization becomes difficult secondary to fluid extravasation. However, we think avoiding the morbidity associated with an open incision is worthwhile for fixation of posterior malleolus fractures.
Conclusion
Close inspection of both preoperative and intraoperative radiographs is required to ensure adequate reduction of a posterior malleolar fragment without soft-tissue or bony interposition in the reduction of ankle fractures. Although not previously reported, posterior tendon entrapment within the posterior malleolus fracture may occur and may require arthroscopic or open techniques to ensure adequate extrication of the tendon to allow for posterior malleolar fracture reduction and fixation. This case report highlights one indication for arthroscopy in the treatment of ankle fractures despite the fact that the tibialis posterior tendon was openly removed. Arthroscopic assistance in acute ankle injuries allows the surgeon to evaluate articular cartilage injuries and ensure there are no interposed structures while checking reduction of the posterior malleolar fracture fragment when present.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
1. Ermis MN, Yagmurlu MF, Kilinc AS, Karakas ES. Irreducible fracture dislocation of the ankle caused by tibialis posterior tendon interposition. J Foot Ankle Surg. 2010;49(2):166-171.
2. Curry EE, O’Brien TS, Johnson JE. Fibular nonunion and equinovarus deformity secondary to posterior tibial tendon incarceration in the syndesmosis: a case report after a bimalleolar fracture-dislocation. Foot Ankle Int. 1999;20(8):527-531.
3. Coonrad RW, Bugg EI Jr. Trapping of the posterior tibial tendon and interposition of soft tissue in severe fractures about the ankle joint. J Bone Joint Surg Am. 1954;36(4):744-750.
4. Pankovich AM. Fracture-dislocation of the ankle. Trapping of the postero-medial ankle tendons and neurovascular bundle in the tibiofibular interosseous space: a case report. J Trauma. 1976;16(11):927-929.
5. Khamaisy S, Leibner ED, Elishoov O. Tibialis posterior entrapment: case report. Foot Ankle Int. 2012;33(5):441-443.
6. Hsu AR, Gross CE, Lee S, Carreira DS. Extended indications for foot and ankle arthroscopy. J Am Acad Orthop Surg. 2014;22(1):10-19.
7. Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92(2):279-286.
8. Büchler L, Tannast M, Bonel HM, Weber M. Reliability of radiologic assessment of the fracture anatomy at the posterior tibial plafond in malleolar fractures. J Orthop Trauma. 2009;23(3):208-212.
9. Ferries JS, DeCoster TA, Firoozbakhsh KK, Garcia JF, Miller RA. Plain radiographic interpretation in trimalleolar ankle fractures poorly assesses posterior fragment size. J Orthop Trauma. 1994;8(4):328-331.
10. Jarvis HC, Cannada LK. Acute tibialis posterior tendon rupture associated with a distal tibial fracture. Orthopedics. 2012;35(4):e595-e597.
11. Woodruff MJ, Brown JN, Mountney J. A mechanism for entrapment of the tibialis posterior tendon in lateral subtalar dislocation. Injury. 1996;27(3):193-194.
12. Leitner B. Obstacles to reduction in subtalar dislocations. J Bone Joint Surg Am. 1954;36(2):299-306.
13. Waldrop J, Ebraheim NA, Shapiro P, Jackson WT. Anatomical considerations of posterior tibialis tendon entrapment in irreducible lateral subtalar dislocation. Foot Ankle. 1992;13(8):458-461.
14. Goucher NR, Coughlin MJ, Kristensen RM. Dislocation of the posterior tibial tendon: a literature review and presentation of two cases. Iowa Orthop J. 2006;26:122-126.
15. Olivé Vilás R, Redón Montojo N, Pino Sorroche S. Traumatic dislocation of tibialis posterior tendon: a case report in a tae-kwon-do athlete. Clin J Sport Med. 2009;19(1):68-69.
16. Gardner MJ, Streubel PN, McCormick JJ, Klein SE, Johnson JE, Ricci WM. Surgeon practices regarding operative treatment of posterior malleolus fractures. Foot Ankle Int. 2011;32(4):385-393.
17. Irwin TA, Lien J, Kadakia AR. Posterior malleolus fracture. J Am Acad Orthop Surg. 2013;21(1):32-40.
18. Gardner MJ, Brodsky A, Briggs SM, Nielson JH, Lorich DG. Fixation of posterior malleolar fractures provides greater syndesmotic stability. Clin Orthop Relat Res. 2006;(447):165-171.
19. Miller AN, Carroll EA, Parker RJ, Helfet DL, Lorich DG. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin Orthop Relat Res. 2010;468(4):1129-1135.
An extremely indolent T-cell leukemia: an 18-year follow-up
T-cell prolymphocytic leukemia (T-PLL) is a rare malignancy that comprises about 2% of all mature lymphoid neoplasms. Patients usually present with prominent peripheral blood lymphocytosis, splenomegaly, hepatomegaly, lymphadenopathy, B symptoms, and occasionally with skin lesions.1 The disease follows an aggressive clinical course with rapid progression and typically has a median survival of less than 1 year. In some cases, the disease is indolent for a period of time before becoming aggressive.2 In 2002, 7 years after initial diagnosis in 1995, the case discussed herein was reported as a rare, indolent form of T-PLL.3 We now present 11 additional years of follow-up of this case, during which time the patient remained asymptomatic with respect to his lymphoid neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
T-cell prolymphocytic leukemia (T-PLL) is a rare malignancy that comprises about 2% of all mature lymphoid neoplasms. Patients usually present with prominent peripheral blood lymphocytosis, splenomegaly, hepatomegaly, lymphadenopathy, B symptoms, and occasionally with skin lesions.1 The disease follows an aggressive clinical course with rapid progression and typically has a median survival of less than 1 year. In some cases, the disease is indolent for a period of time before becoming aggressive.2 In 2002, 7 years after initial diagnosis in 1995, the case discussed herein was reported as a rare, indolent form of T-PLL.3 We now present 11 additional years of follow-up of this case, during which time the patient remained asymptomatic with respect to his lymphoid neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
T-cell prolymphocytic leukemia (T-PLL) is a rare malignancy that comprises about 2% of all mature lymphoid neoplasms. Patients usually present with prominent peripheral blood lymphocytosis, splenomegaly, hepatomegaly, lymphadenopathy, B symptoms, and occasionally with skin lesions.1 The disease follows an aggressive clinical course with rapid progression and typically has a median survival of less than 1 year. In some cases, the disease is indolent for a period of time before becoming aggressive.2 In 2002, 7 years after initial diagnosis in 1995, the case discussed herein was reported as a rare, indolent form of T-PLL.3 We now present 11 additional years of follow-up of this case, during which time the patient remained asymptomatic with respect to his lymphoid neoplasm.
Click on the PDF icon at the top of this introduction to read the full article.
A novel treatment approach prolonging survival in an uncommon metastatic primary bladder adenocarcinoma
Primary bladder adenocarcinoma is an epithelial malignancy with pure glandular differentiation, without evidence of typical urothelial (transitional cell) carcinoma. PBA is rare, accounting for 0.5%-2% of all malignant bladder neoplasms, and it is seen more frequently in men than in women and is commonly diagnosed in the sixth decade of life.1-3 Clinical presentation includes hematuria and symptoms of bladder irritation.2
Click on the PDF icon at the top of this introduction to read the full article.
Primary bladder adenocarcinoma is an epithelial malignancy with pure glandular differentiation, without evidence of typical urothelial (transitional cell) carcinoma. PBA is rare, accounting for 0.5%-2% of all malignant bladder neoplasms, and it is seen more frequently in men than in women and is commonly diagnosed in the sixth decade of life.1-3 Clinical presentation includes hematuria and symptoms of bladder irritation.2
Click on the PDF icon at the top of this introduction to read the full article.
Primary bladder adenocarcinoma is an epithelial malignancy with pure glandular differentiation, without evidence of typical urothelial (transitional cell) carcinoma. PBA is rare, accounting for 0.5%-2% of all malignant bladder neoplasms, and it is seen more frequently in men than in women and is commonly diagnosed in the sixth decade of life.1-3 Clinical presentation includes hematuria and symptoms of bladder irritation.2
Click on the PDF icon at the top of this introduction to read the full article.
Asymptomatic but Time for a Hip Revision
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
Total hip arthroplasty (THA) is considered to be one of the most successful orthopedic interventions of its generation.1 In 2010, 332,000 THAs were performed in the U.S.2 Although used to correct advanced joint diseases in the elderly, the THA procedure has become increasingly common in a younger population for posttraumatic fractures and conditions that lead to early onset secondary arthritis such as avascular necrosis, juvenile rheumatoid arthritis, hip dysplasia, Perthes disease, and femoro-acetabular impingement.
Current hip replacements are expected to function at least 10 to 20 years in 90% of patients.3 As increasing numbers of young patients have these procedures and as seniors continue to live longer, patients will outlast their implants. Younger and more active patients have a higher rate of revision, because the longevity of the prosthesis is usually a function of usage.3 The number of revision THAs is projected to increase 137% by 2030.4
Hip resurfacing has been developed as a bone preserving surgical alternative to THA. The first system for use in the U.S. received FDA approval in 2006, but concerns about the metal on metal bearing surfaces, high failure and revision rates, and early catastrophic modes of failure compared with THAs has resulted in the recall of many of these devices. Hip resurfacing may offer some advantages compared with those of a THA in a carefully selected population, but its use will not be further discussed in this case study.5 Periprosthetic osteolysis and aseptic loosening are 2 of the long-term consequences of THA.6 Bone loss is felt to be secondary to a biologic reaction to particulate debris from implants.6 Some patients, especially those with loosening, complete wear, or fracture, will be symptomatic with pain. However, wear and osteolysis is a silent disease unless there is mechanical failure. Other patients may not experience discomfort. Radiographic studies may reveal significant changes, which warrant the recommendation for a hip revision.
Hip revision surgery has 3 major purposes: relieving pain in the affected joint, restoring the patient’s mobility, and removing a loose or damaged prosthesis before irreversible harm is done to the joint. It’s anticipated that most primary care providers (PCPs) will encounter patients who seek advice on the need for a revision hip arthroplasty.
This case will present an asymptomatic patient who underwent a THA in 1997 at age 37, to address developmental dysplasia of the hip (DDH) and was advised to undergo a revision hip arthroplasty due to abnormal radiographic findings at age 55 years. A discussion will follow that includes a brief review of the history of THA, the materials and bearings commonly used, the presenting symptoms or radiographic changes that signal the need for a revision, and the current options available for a patient such as this.
Case Report
A man aged 55 years presented to a new orthopedic surgeon for his first orthopedic appointment in 10 years. The patient had a left metal-on-polyethylene (M-on-PE) THA 18 years prior due to early onset secondary degenerative joint disease from DDH. The patient’s M-on-PE THA was a titanium acetabular socket and femoral stem with a cobalt-chromium alloy femoral head and a polyethylene liner. The patient remained physically active with an exercise routine consisting of walking, swimming, and weight training.
The patient’s orthopedic history was notable for a right knee arthroscopy for intervention due to a torn medial and lateral meniscus, and birth history was noteworthy for a breech presentation. The physical exam was unremarkable except for a slight leg length discrepancy, but the patient did not exhibit a Trendelenburg gait.
Plain X-rays and a computed tomography (CT) scan showed eccentric PE wear and superior migration of the femoral head, which was indicative of significant PE liner wear. No significant osteolysis or periprosthetic loosening was observed on the X-rays or CT scan. He was advised that a hip revision procedure would need to be done, optimally, within the next 6 months to a year.
Discussion
Hip dysplasia represents a broad group of disorders and generally means abnormal development of the hip joint. The term is most commonly used to refer to DDH with inadequate coverage of the femoral head. In one study, 25% of hip replacements performed in patients aged ≤ 40 years were due to underlying hip dysplasia.7
Developmental dysplasia of the hip occurs more often in children who present in the breech position.8 One theory argues that packaging issues in utero may account for the increased incidence of DDH.9 The earliest recorded attempts at hip replacement occurred in Germany, in 1891, when ivory was used to replace the femoral heads of patients whose hip joints had been destroyed by tuberculosis.1
The orthopedic surgeon Sir John Charnley, who worked at the Manchester Royal Infirmary, is considered the father of the modern THA.1 His low friction arthroplasty, designed in the early 1960s is identical, in principle, to the M-on-PE prosthesis used today.1 The PE liner used was ultrahigh molecular weight polyethylene (UHMWPE).1
Due to the early success of the Charnley prosthesis, the M-on-PE prosthesis became the most widely used. Although PE is the most studied and understood of all acetabular liner materials, it will eventually wear and shed debris. Acetabular cup wear is the most frequent reason for mid-to-long-term revisions, especially in young and active patients.10 More active patients shed more debris.3 The PE debris instigates the release of inflammatory mediators, which results in chronic inflammation and tissue damage that erodes the supporting bone and can lead to implant loosening or fracture.6 Ongoing studies seek to optimize and improve properties of the UHMWPE and to develop alternative bearings. After FDA approval in 1999, highly cross-linked polyethylene liners (HXLPE) rapidly became the standard of care for THAs, at least in the U.S.11 Highly cross-linked polyethylene liners are created from UHMWPE through a process of cross-linking by exposure to gamma radiation, and subsequent heat treatment to neutralize free radicals and limit oxidative degradation.12
In one study, the 5-year annual linear wear rate for a HXLPE liner was only 45% of that seen with the UHMWPE liner, although the qualitative wear pattern was the same.13 In a study that followed patients for 7 years postoperatively, the mean steady-state wear rate of the HXLPE was 0.005 mm/y compared with 0.037 mm/y for UHMWPE.14 In a long-term study (a minimum follow-up of 10 years) of 50 patients who were aged < 50 years and underwent THA using HXLPE liners, there was no radiographic evidence of osteolysis or component loosening, and liner wear was 0.020 ± 0.0047 mm/y.12 In 2005, second-generation HXLPE liners were introduced clinically and have been shown to further reduce wear in vitro compared with both UHMWPE and first-generation HXLPE liners. Callary and colleagues calculated that the wear rates between 1 year and 5 years were all < 0.001 mm/y.15
The use of ceramic for THAs began in 1970, and ceramic heads on polyethylene (C-on-PE) liners and ceramic-on-ceramic (C-on-C) bearings have been in continual use for > 30 years in Europe. Premarket FDA approval based on European data was granted in 1983; however, the manufacturer voluntarily removed it from the market because of a high incidence of stem loosening (> 30% within 3 years in some series).16 FDA approvals came much later for C-on-PE (1989) and C-on-C (2003) bearings.
Ceramic is the hardest implant material used, and it can be concluded from many clinical and laboratory reports that C-on-PE and C-on-C combinations confer a potentially significant reduction in wear on THA bearings.16 Ceramic hips initially had 2 concerns: catastrophic shattering and squeaking. Current ceramic hips have been substantially improved, and some experts feel shattering has been essentially eliminated.16 Other experts note that ceramic brittleness remains a major concern.17 Squeaking remains a problem for some, but it usually abates over time. No study has correlated squeaking with impending failure or increased pain or disability.
While C-on-C bearings are now felt to be a good implant for young active patients, these bearings have generally not resulted in significantly lower wear rates and fewer revisions.18 High rates of wear and osteolysis have been sporadically documented over the 35-year history of ceramic implants.16 The FDA approved the first ceramic-on-metal total hip replacement system on June 13, 2011.
Metal-on-metal (M-on-M) implants have been used by some for decades, although they were not approved by the FDA until the late 1990s. However, some device recalls have brought negative attention to M-on-M implants.19 It was felt that they would generate less wear debris than PE, but reports of pseudotumors (from inflammatory mediators) and metallosis have significantly tempered enthusiasm for these products.20,21 The wear rates are very low, estimated to be only 0.01 mm/y, but concerns about the carcinogenetic potential of systemically increased metal ions remains a possible and much debated concern.19,22,23 In January 2013, FDA issued a safety communication on M-on-M implants.
Many experts feel that modern ceramic or metal on second-generation HXLPE represents the gold standard and the most predictable bearing choice for young, active patients.18 Others feel that the optimal choice of bearing surfaces in THA, particularly in the younger and more active patient, remains controversial.24
Follow-Up
Intermittent orthopedic monitoring is recommended for all patients who have undergone a THA. The frequency of hip X-rays on follow-up appointments is left to the orthopedic surgeon. After the initial recovery, serial images every 2 to 5 years can identify progressive failure, and annual X-rays may be used for closer follow-up in high-risk patients.
Patients who experience dislocations, fractures, infections, or pain usually maintain close orthopedic follow-up. Significant wear of the prosthesis damages the socket; osteolysis can cause irreversible bone loss, fracture, and loosening. Massive acetabular bone loss is very difficult to reverse and creates major reconstruction challenges.
Figure 1A is a 2009 X-ray of a woman aged 44 years who underwent a THA after a motor vehicle accident in 1997 and who was advised to have a revision THA when seen in 2009.
Figure 3A is an X-ray of a man aged 71 years who had undergone THA 21 years earlier and had complied with routine follow-up. When his X-rays showed significant wear of the liner and some osteolysis, he was able to undergo a simple revision (Figure 3B).
Three-dimensional CT is useful for quantifying the presence and severity of osteolytic lesions, because plain radiographs may underestimate the amount of bone loss that is present.25 The CT in Figure 3C shows the magnitude of osteolysis that was underestimated by the preoperative plain X-rays (Figure 3A). Computed tomography scans are crucial for surgical planning in the setting of severe acetabular bone loss.
There is a wide spectrum of signs and symptoms that can occur in the setting of acetabular component failure. Pain is a common presenting symptom. Groin pain can represent acetabular failure; thigh pain may be correlated to femoral component failure.25 The clinical patient presentation ultimately depends on the underlying cause: an infection, polyethylene wear, instability, or aseptic loosening.25 Leg-length discrepancy, joint deformity, location of prior incisions, functional status, and baseline neurologic status should be evaluated and documented during the preoperative evaluation as well.25
Case Study Revision Options
The X-rays and CT scans for this case study patient showed that he was a possible candidate for the simplest revision surgery; an isolated liner exchange and replacement of the femoral head. When the original surgery was performed (1997), the only FDA approved PE liner was UHMWPE. To justify isolated liner exchange, the modular acetabular metallic shell also should be well-fixed and appropriately oriented.26 This is evaluated both preoperatively and intraoperatively.
If found to be well fixed with an appropriate orientation and locking mechanism, the UHMWPE liner could be replaced with a HXLPE liner and a larger metal femoral head for improved wear and stability. Acetabular revision is indicted for an asymptomatic patient who has progressive osteolysis, severe wear, or bone loss that would compromise future reconstruction.
Conclusions
Over the past several decades, THA has become recognized as an effective treatment option for the reduction of pain and disability associated with hip joint disease and is associated with successful clinical outcomes. The most frequently noted recommendations for trying to increase the life expectancy of an artificial hip replacement include maintaining a normal weight, keeping leg muscles strong, and avoiding repetitive squatting and kneeling.
As the number of primary THAs has increased and the average age of those undergoing a primary THA has decreased, the need for revisions has risen. Reviews have demonstrated that the most common causes for early total hip revision, regardless of component, included infection, instability/dislocation, and fracture, whereas wear is the most common reason for mid to late revisions.
The wear of all materials used has been shown to be greatest in the most active patients.
Studies continue to identify ways to potentially prevent or reverse osteolysis from wear debris. Alendronate therapy has been shown to prevent and treat PE debris-induced periprosthetic bone loss in rats.27 It also was successfully used in a case report of an asymptomatic woman aged 39 years who had rapid PE wear and aggressive periprosthetic osteolysis within just 2 years of a bilateral THA.28 Other areas of research on decreasing osteolysis in THA recipients include trials with mesenchymal stem cells, bone morphogenic proteins, and gene therapy.6
In the U.S., 46,000 revisions were performed in 2004 and this number is expected to more than double by 2030.4 Primary care providers are sure to encounter patients who will be in need of a hip revision procedure. It’s important for them to make sure that their patients who have undergone a THA are periodically seen for orthopedic follow-up. Despite the long history of primary THAs, there is still not a single technique and material to suit all patient characteristics.1 Unfortunately, the same currently applies to hip revision procedures.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
1. Knight SR, Aujla R, Biswas SP. Total hip arthroplasty--over 100 years of operative history. Orthop Rev (Pavia). 2011;3(2):e16.
2. Centers for Disease Control and Prevention. FastStats: inpatient surgery. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed January 18, 2016.
3. Joint Revision Surgery-When do I need it? American Academy of Orthopedic Surgeons Website. http://www.tlhoc.com/uploads/documents/when_do_I_need_it.pdf. Accessed January 18, 2016.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Nunley RM, Della Valle CJ, Barrack RL. Is patient selection important for hip resurfacing? Clin Orthop Relat Res. 2009;467(1):56-65.
6. Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312-316.
7. Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82(2):149-154.
8. Salter RB. Etiology, pathogenesis and possible prevention of congenital dislocation of the hip. Can Med Assoc J. 1968;98(20):933-945.
9. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
10. Pace TB, Keith KC, Alvarez E, Snider RG, Tanner, SL, Desjardins JD. Comparison of conventional polyethylene wear and signs of cup failure in two similar total hip designs. Adv Orthop. 2013;2013:710621.
11. Kurtz SM. The UHMWPE Handbook: Ultra-High Molecular Weight Polyethylene in Total Joint Replacement. Academic Press: London; 2014.
12. Babovic N, Trousdale RT. Total hip athroplasty using highly cross-linked polyethylene in patients younger than 50 years with minimum 10-year follow-up. J Arthroplasty. 2013;29(5):815-817.
13. Dorr LD, Wan Z, Shahrdar C, Sirianni L, Boutary M, Yun A. Clinical performance of a Durasal highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816-1821.
14. Thomas G, Simpson D, Mehmmod S, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis. J Bone Joint Surg Am. 2011;93(8):716-722.
15. Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study. Clin Orthop Relat Res. 2013;471(11):3596-3600.
16. Tateiwa T, Clarke IC, Williams PA, et al. Ceramic total hip arthroplasty in the United States: safety and risk issues revisited. Am J Orthop (Belle Mead NJ). 2008;37(2):E26-E31.
17. Traina F, De Fine M, Di Martino A, Faldini C. Fracture of ceramic bearing surfaces following total hip replacement: a systematic review. BioMed Res Int. 2013;2013:157247.
18. Haidukewych GJ, Petrie J. Bearing surface considerations for total hip arthroplasty in young patients. Orthop Clin N Am. 2012;43(3):395-402.
19. Cohen D. How safe are metal-on-metal hip implants? BMJ. 2012;344:e1410.
20. Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468(9):2321-2327.
21. Pritchett JW. Adverse reaction to metal debris: metallosis of the resurfaced hip. Curr Orthop Pract. 2012;23(1):50-58.
22. Smith AJ, Dieppe P, Porter M, Blom AW; National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383.
23. Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33(6):1531-1536.
24. Cash, D, Khanduja V. The case for ceramics-on-polyethylene as the preferred bearing for a young adult hip replacement. Hip Int. 2014;24(5):421-427.
25. Taylor ED, Browne JA. Reconstruction options for acetabular revision. World J Orthop. 2012;3(7):95-100.
26. Lombardi AV, Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg. 2008;16(5):243-248.
27. Millet PJ, Allen MJ, Bostrom MP. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am. 2002;84-A(2):236-249.
28. O'Hara LJ, Nivbrant B, Rohrl S.Cross-linked polyethylene and bisphosphonate therapy for osteolysis in total hip athroplasty: a case report. J Orthop Surg (Hong Kong). 2004;12(1):114-121.
A Case of Bloom Syndrome With Uncommon Clinical Manifestations Confirmed on Genetic Testing
Bloom syndrome, also called congenital telangiectatic erythema and stunted growth, was first described by David Bloom in 1954.1 It is a rare autosomal-recessive disorder (Online Mendelian Inheritance in Man 210900) characterized by specific clinical manifestations including photosensitivity, telangiectatic facial erythema, proportionate growth deficiency, hypogonadism, immunodeficiency, and a tendency to develop various malignancies.2 Linkage analysis revealed that the Bloom syndrome gene locus resides on chromosome arm 15q26.1,3 and the BLM gene in this region has been identified as being responsible for the development of Bloom syndrome.4,5 We report the case of a 12-year-old Chinese girl with Bloom syndrome and detected BLM gene. The evaluation was approved by the Institutional Ethical Review Boards of Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China).
Case Report
We evaluated a Bloom syndrome family, which consisted of the patient and her parents. The patient was a 12-year-old Chinese girl who was apparently healthy until 3 months of age when her parents noticed an erythematous eruption with blisters on the face. Exacerbation after exposure to sunlight is usual, which results in the eruption becoming prominent in summer and fainter in winter.2 Gradually, the patient’s skin lesions became more progressive, extending to the forehead, nose, and ears, with oozing, crusting, atrophy, and telangiectases developing on the face despite treatment. In the last 3 years, no blisters were present on the patient’s face because of her efforts to avoid sun exposure. She had no history of recurrent infections.
On physical examination, the patient was generally healthy with normal intelligence and short stature. She weighed 26 kg and was approximately 122-cm tall. Telangiectatic erythema and slight scaling were noted on the face, which simulated lupus erythematosus (Figures 1A and 1B). She had additional abnormalities including alopecia areata (Figure 1C), eyebrow hair loss, flat nose, reticular pigmentation on the forehead and trunk, and finger swelling. The distal phalanges on all 10 fingers became short and sharpened and the fingernails became wider than they were long (Figure 1D). Laboratory investigations, including a complete blood cell count, liver and kidney function tests, stool examination, serum complement, and albumin and globulin levels, were within reference range.

After informed consent was obtained, a mutation analysis of the BLM gene was performed in the patient and her parents. We used a genomic DNA purification kit to extract genomic DNA from peripheral blood according to the manufacturer’s protocol. Genomic DNA was used to amplify the exons of the BLM gene with intron flanking sequences by polymerase chain reaction with the primer described elsewhere.6 After the amplification, the polymerase chain reaction products were purified and the BLM gene was sequenced. Sequence comparisons and analysis were performed using Phred/Phrap/Consed version 12.0.
The patient was found to carry changes in 2 heterozygous nucleotide sites, including c.2603C>T in exon 13 and c.3961G>A in exon 21 of the BLM gene. The patient’s father was found to carry c.2603C>T and her mother carried c.3961G>A (Figure 2).
Comment
Patients with Bloom syndrome have a characteristic clinical appearance that typically includes photosensitivity, telangiectatic facial erythema, and growth deficiency. Telangiectatic erythema of the face develops during infancy or early childhood as red macules or plaques and may simulate lupus erythematosus. The lesions are described as a butterfly rash affecting the bridge of the nose and cheeks but also may involve the margins of the eyelids, forehead, ears, and sometimes the dorsa of the hands and forearms. Moderate and proportionate growth deficiencies develop both in utero and postnatally. Patients with Bloom syndrome characteristically have narrow, slender, distinct facial features with micrognathism and a relatively prominent nose. They usually may have mild microcephaly, meaning the head is longer and narrower than normal.2,7-10
German and Takebe11 reported 14 Japanese patients with Bloom syndrome. The phenotype differs somewhat from most cases recognized elsewhere in that dolichocephaly was a less constant feature, the facial skin was less prominent, and life-threatening infections were less common. Our patient had typical telangiectatic facial erythema without microcephaly, dolichocephaly, or any infections. She also had some uncommon manifestations such as alopecia areata, eyebrow hair loss, flat nose, reticular pigmentation, and short sharpened distal phalanges with fingernails that were wider than they were long. Although she had no recurrent infections and laboratory tests were within reference range, the alopecia areata and eyebrow hair loss may be associated with an abnormal immune response. The reasons for the short sharpened distal phalanges and the fingernail findings are unclear. The presence of reticular pigmentation also is unclear but may be associated with photosensitivity. Since the BLM gene was discovered to be the disease-causing gene of Bloom syndrome in 1995,4,5 approximately 70 mutations were reported. The BLM gene encodes for the Bloom syndrome protein, a DNA helicase of the highly conserved RecQ subfamily of helicases, a group of nuclear proteins important in the maintenance of genomic stability.12
Mutation analysis of the BLM gene in our patient showed changes in 2 heterozygous nucleotide sites, including c.2603C>T in exon 13 and c.3961G>A in exon 21 of the BLM gene, which altered proline residue with leucine residue at 868 and valine residue with isoleucine residue at 1321, respectively. According to GenBank,13,14 c.2603C>T and c.3961G>A are single nucleotide polymorphisms of the BLM gene. The genotypic distribution of International HapMap Project15 showed that C=602/602 and T=0/602 on c.2603 in 301 unrelated Chinese patients and G=585/602 and A=17/602 on c.3961 in 301 unrelated Chinese patients. Because of the low prevalence of genotypes c.2603T and c.3961A in China, the relationship between clinical features and c.2603C>T and c.3961G>A of the BLM gene in our patient requires further study.
In conclusion, we report a patient with Bloom syndrome with uncommon clinical manifestations. Our findings indicate that c.2603C>T and c.3961G>A of the BLM gene may be the pathogenic nature for Bloom syndrome in China.
Acknowledgments
The authors would like to thank the patient and her family for their participation in the study. The authors also thank Li Qi, BA, Beijing, China, for his contribution to the review of the data in the literature.
- Bloom D. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs; probably a syndrome entity. AMA Am J Dis Child. 1954;88:754-758.
- German J. Bloom’s syndrome, I: genetical and clinical observations in the first twenty-seven patients. Am J Hum Genet. 1969;21:196-227.
- German J, Roe AM, Leppert MF, et al. Bloom syndrome: an analysis of consanguineous families assigns the locus mutated to chromosome band 15q26.1. Proc Natl Acad Sci U S A. 1994;91:6669-6673.
- Passarge E. A DNA helicase in full Bloom. Nat Genet. 1995;11:356-357.
- Ellis NA, Groden J, Ye TZ, et al. The Bloom’s syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655-666.
- German J, Sanz MM, Ciocci S, et al. Syndrome-causing mutations of the BLM gene in persons in the Bloom’s Syndrome Registry. Hum Mutat. 2007;28:743-753.
- Landau JW, Sasaki MS, Newcomer VD, et al. Bloom’s syndrome: the syndrome of telangiectatic erythema and growth retardation. Arch Dermatol. 1966;94:687-694.
- Gretzula JC, Hevia O, Weber PJ. Bloom’s syndrome. J Am Acad Dermatol. 1987;17:479-488.
- Passarge E. Bloom’s syndrome: the German experience. Ann Genet. 1991;34:179-197.
- German J. Bloom’s syndrome. Dermatol Clin. 1995;13:7-18.
- German J, Takebe H. Bloom’s syndrome, XIV: the disorder in Japan. Clin Genet. 1989;35:93-110.
- Bennett RJ, Keck JL. Structure and function of RecQ DNA helicases. Crit Rev Biochem Mol Biol. 2004;39:79-97.
- Reference SNP (refSNP) Cluster Report: rs2227935. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=2227935. Accessed February 3, 2016.
- Reference SNP (refSNP) Cluster Report: rs7167216. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=7167216. Accessed February 3, 2016.
- Homo sapiens:GRCh37.p13 (GCF_000001405.25)Chr 1 (NC_000001.10):1 - 249.3M. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/variationtools/1000genomes/?=%EF%BC%86=. Accessed February 3, 2016.
Bloom syndrome, also called congenital telangiectatic erythema and stunted growth, was first described by David Bloom in 1954.1 It is a rare autosomal-recessive disorder (Online Mendelian Inheritance in Man 210900) characterized by specific clinical manifestations including photosensitivity, telangiectatic facial erythema, proportionate growth deficiency, hypogonadism, immunodeficiency, and a tendency to develop various malignancies.2 Linkage analysis revealed that the Bloom syndrome gene locus resides on chromosome arm 15q26.1,3 and the BLM gene in this region has been identified as being responsible for the development of Bloom syndrome.4,5 We report the case of a 12-year-old Chinese girl with Bloom syndrome and detected BLM gene. The evaluation was approved by the Institutional Ethical Review Boards of Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China).
Case Report
We evaluated a Bloom syndrome family, which consisted of the patient and her parents. The patient was a 12-year-old Chinese girl who was apparently healthy until 3 months of age when her parents noticed an erythematous eruption with blisters on the face. Exacerbation after exposure to sunlight is usual, which results in the eruption becoming prominent in summer and fainter in winter.2 Gradually, the patient’s skin lesions became more progressive, extending to the forehead, nose, and ears, with oozing, crusting, atrophy, and telangiectases developing on the face despite treatment. In the last 3 years, no blisters were present on the patient’s face because of her efforts to avoid sun exposure. She had no history of recurrent infections.
On physical examination, the patient was generally healthy with normal intelligence and short stature. She weighed 26 kg and was approximately 122-cm tall. Telangiectatic erythema and slight scaling were noted on the face, which simulated lupus erythematosus (Figures 1A and 1B). She had additional abnormalities including alopecia areata (Figure 1C), eyebrow hair loss, flat nose, reticular pigmentation on the forehead and trunk, and finger swelling. The distal phalanges on all 10 fingers became short and sharpened and the fingernails became wider than they were long (Figure 1D). Laboratory investigations, including a complete blood cell count, liver and kidney function tests, stool examination, serum complement, and albumin and globulin levels, were within reference range.

After informed consent was obtained, a mutation analysis of the BLM gene was performed in the patient and her parents. We used a genomic DNA purification kit to extract genomic DNA from peripheral blood according to the manufacturer’s protocol. Genomic DNA was used to amplify the exons of the BLM gene with intron flanking sequences by polymerase chain reaction with the primer described elsewhere.6 After the amplification, the polymerase chain reaction products were purified and the BLM gene was sequenced. Sequence comparisons and analysis were performed using Phred/Phrap/Consed version 12.0.
The patient was found to carry changes in 2 heterozygous nucleotide sites, including c.2603C>T in exon 13 and c.3961G>A in exon 21 of the BLM gene. The patient’s father was found to carry c.2603C>T and her mother carried c.3961G>A (Figure 2).

Comment
Patients with Bloom syndrome have a characteristic clinical appearance that typically includes photosensitivity, telangiectatic facial erythema, and growth deficiency. Telangiectatic erythema of the face develops during infancy or early childhood as red macules or plaques and may simulate lupus erythematosus. The lesions are described as a butterfly rash affecting the bridge of the nose and cheeks but also may involve the margins of the eyelids, forehead, ears, and sometimes the dorsa of the hands and forearms. Moderate and proportionate growth deficiencies develop both in utero and postnatally. Patients with Bloom syndrome characteristically have narrow, slender, distinct facial features with micrognathism and a relatively prominent nose. They usually may have mild microcephaly, meaning the head is longer and narrower than normal.2,7-10
German and Takebe11 reported 14 Japanese patients with Bloom syndrome. The phenotype differs somewhat from most cases recognized elsewhere in that dolichocephaly was a less constant feature, the facial skin was less prominent, and life-threatening infections were less common. Our patient had typical telangiectatic facial erythema without microcephaly, dolichocephaly, or any infections. She also had some uncommon manifestations such as alopecia areata, eyebrow hair loss, flat nose, reticular pigmentation, and short sharpened distal phalanges with fingernails that were wider than they were long. Although she had no recurrent infections and laboratory tests were within reference range, the alopecia areata and eyebrow hair loss may be associated with an abnormal immune response. The reasons for the short sharpened distal phalanges and the fingernail findings are unclear. The presence of reticular pigmentation also is unclear but may be associated with photosensitivity. Since the BLM gene was discovered to be the disease-causing gene of Bloom syndrome in 1995,4,5 approximately 70 mutations were reported. The BLM gene encodes for the Bloom syndrome protein, a DNA helicase of the highly conserved RecQ subfamily of helicases, a group of nuclear proteins important in the maintenance of genomic stability.12
Mutation analysis of the BLM gene in our patient showed changes in 2 heterozygous nucleotide sites, including c.2603C>T in exon 13 and c.3961G>A in exon 21 of the BLM gene, which altered proline residue with leucine residue at 868 and valine residue with isoleucine residue at 1321, respectively. According to GenBank,13,14 c.2603C>T and c.3961G>A are single nucleotide polymorphisms of the BLM gene. The genotypic distribution of International HapMap Project15 showed that C=602/602 and T=0/602 on c.2603 in 301 unrelated Chinese patients and G=585/602 and A=17/602 on c.3961 in 301 unrelated Chinese patients. Because of the low prevalence of genotypes c.2603T and c.3961A in China, the relationship between clinical features and c.2603C>T and c.3961G>A of the BLM gene in our patient requires further study.
In conclusion, we report a patient with Bloom syndrome with uncommon clinical manifestations. Our findings indicate that c.2603C>T and c.3961G>A of the BLM gene may be the pathogenic nature for Bloom syndrome in China.
Acknowledgments
The authors would like to thank the patient and her family for their participation in the study. The authors also thank Li Qi, BA, Beijing, China, for his contribution to the review of the data in the literature.
Bloom syndrome, also called congenital telangiectatic erythema and stunted growth, was first described by David Bloom in 1954.1 It is a rare autosomal-recessive disorder (Online Mendelian Inheritance in Man 210900) characterized by specific clinical manifestations including photosensitivity, telangiectatic facial erythema, proportionate growth deficiency, hypogonadism, immunodeficiency, and a tendency to develop various malignancies.2 Linkage analysis revealed that the Bloom syndrome gene locus resides on chromosome arm 15q26.1,3 and the BLM gene in this region has been identified as being responsible for the development of Bloom syndrome.4,5 We report the case of a 12-year-old Chinese girl with Bloom syndrome and detected BLM gene. The evaluation was approved by the Institutional Ethical Review Boards of Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China).
Case Report
We evaluated a Bloom syndrome family, which consisted of the patient and her parents. The patient was a 12-year-old Chinese girl who was apparently healthy until 3 months of age when her parents noticed an erythematous eruption with blisters on the face. Exacerbation after exposure to sunlight is usual, which results in the eruption becoming prominent in summer and fainter in winter.2 Gradually, the patient’s skin lesions became more progressive, extending to the forehead, nose, and ears, with oozing, crusting, atrophy, and telangiectases developing on the face despite treatment. In the last 3 years, no blisters were present on the patient’s face because of her efforts to avoid sun exposure. She had no history of recurrent infections.
On physical examination, the patient was generally healthy with normal intelligence and short stature. She weighed 26 kg and was approximately 122-cm tall. Telangiectatic erythema and slight scaling were noted on the face, which simulated lupus erythematosus (Figures 1A and 1B). She had additional abnormalities including alopecia areata (Figure 1C), eyebrow hair loss, flat nose, reticular pigmentation on the forehead and trunk, and finger swelling. The distal phalanges on all 10 fingers became short and sharpened and the fingernails became wider than they were long (Figure 1D). Laboratory investigations, including a complete blood cell count, liver and kidney function tests, stool examination, serum complement, and albumin and globulin levels, were within reference range.

After informed consent was obtained, a mutation analysis of the BLM gene was performed in the patient and her parents. We used a genomic DNA purification kit to extract genomic DNA from peripheral blood according to the manufacturer’s protocol. Genomic DNA was used to amplify the exons of the BLM gene with intron flanking sequences by polymerase chain reaction with the primer described elsewhere.6 After the amplification, the polymerase chain reaction products were purified and the BLM gene was sequenced. Sequence comparisons and analysis were performed using Phred/Phrap/Consed version 12.0.
The patient was found to carry changes in 2 heterozygous nucleotide sites, including c.2603C>T in exon 13 and c.3961G>A in exon 21 of the BLM gene. The patient’s father was found to carry c.2603C>T and her mother carried c.3961G>A (Figure 2).

Comment
Patients with Bloom syndrome have a characteristic clinical appearance that typically includes photosensitivity, telangiectatic facial erythema, and growth deficiency. Telangiectatic erythema of the face develops during infancy or early childhood as red macules or plaques and may simulate lupus erythematosus. The lesions are described as a butterfly rash affecting the bridge of the nose and cheeks but also may involve the margins of the eyelids, forehead, ears, and sometimes the dorsa of the hands and forearms. Moderate and proportionate growth deficiencies develop both in utero and postnatally. Patients with Bloom syndrome characteristically have narrow, slender, distinct facial features with micrognathism and a relatively prominent nose. They usually may have mild microcephaly, meaning the head is longer and narrower than normal.2,7-10
German and Takebe11 reported 14 Japanese patients with Bloom syndrome. The phenotype differs somewhat from most cases recognized elsewhere in that dolichocephaly was a less constant feature, the facial skin was less prominent, and life-threatening infections were less common. Our patient had typical telangiectatic facial erythema without microcephaly, dolichocephaly, or any infections. She also had some uncommon manifestations such as alopecia areata, eyebrow hair loss, flat nose, reticular pigmentation, and short sharpened distal phalanges with fingernails that were wider than they were long. Although she had no recurrent infections and laboratory tests were within reference range, the alopecia areata and eyebrow hair loss may be associated with an abnormal immune response. The reasons for the short sharpened distal phalanges and the fingernail findings are unclear. The presence of reticular pigmentation also is unclear but may be associated with photosensitivity. Since the BLM gene was discovered to be the disease-causing gene of Bloom syndrome in 1995,4,5 approximately 70 mutations were reported. The BLM gene encodes for the Bloom syndrome protein, a DNA helicase of the highly conserved RecQ subfamily of helicases, a group of nuclear proteins important in the maintenance of genomic stability.12
Mutation analysis of the BLM gene in our patient showed changes in 2 heterozygous nucleotide sites, including c.2603C>T in exon 13 and c.3961G>A in exon 21 of the BLM gene, which altered proline residue with leucine residue at 868 and valine residue with isoleucine residue at 1321, respectively. According to GenBank,13,14 c.2603C>T and c.3961G>A are single nucleotide polymorphisms of the BLM gene. The genotypic distribution of International HapMap Project15 showed that C=602/602 and T=0/602 on c.2603 in 301 unrelated Chinese patients and G=585/602 and A=17/602 on c.3961 in 301 unrelated Chinese patients. Because of the low prevalence of genotypes c.2603T and c.3961A in China, the relationship between clinical features and c.2603C>T and c.3961G>A of the BLM gene in our patient requires further study.
In conclusion, we report a patient with Bloom syndrome with uncommon clinical manifestations. Our findings indicate that c.2603C>T and c.3961G>A of the BLM gene may be the pathogenic nature for Bloom syndrome in China.
Acknowledgments
The authors would like to thank the patient and her family for their participation in the study. The authors also thank Li Qi, BA, Beijing, China, for his contribution to the review of the data in the literature.
- Bloom D. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs; probably a syndrome entity. AMA Am J Dis Child. 1954;88:754-758.
- German J. Bloom’s syndrome, I: genetical and clinical observations in the first twenty-seven patients. Am J Hum Genet. 1969;21:196-227.
- German J, Roe AM, Leppert MF, et al. Bloom syndrome: an analysis of consanguineous families assigns the locus mutated to chromosome band 15q26.1. Proc Natl Acad Sci U S A. 1994;91:6669-6673.
- Passarge E. A DNA helicase in full Bloom. Nat Genet. 1995;11:356-357.
- Ellis NA, Groden J, Ye TZ, et al. The Bloom’s syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655-666.
- German J, Sanz MM, Ciocci S, et al. Syndrome-causing mutations of the BLM gene in persons in the Bloom’s Syndrome Registry. Hum Mutat. 2007;28:743-753.
- Landau JW, Sasaki MS, Newcomer VD, et al. Bloom’s syndrome: the syndrome of telangiectatic erythema and growth retardation. Arch Dermatol. 1966;94:687-694.
- Gretzula JC, Hevia O, Weber PJ. Bloom’s syndrome. J Am Acad Dermatol. 1987;17:479-488.
- Passarge E. Bloom’s syndrome: the German experience. Ann Genet. 1991;34:179-197.
- German J. Bloom’s syndrome. Dermatol Clin. 1995;13:7-18.
- German J, Takebe H. Bloom’s syndrome, XIV: the disorder in Japan. Clin Genet. 1989;35:93-110.
- Bennett RJ, Keck JL. Structure and function of RecQ DNA helicases. Crit Rev Biochem Mol Biol. 2004;39:79-97.
- Reference SNP (refSNP) Cluster Report: rs2227935. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=2227935. Accessed February 3, 2016.
- Reference SNP (refSNP) Cluster Report: rs7167216. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=7167216. Accessed February 3, 2016.
- Homo sapiens:GRCh37.p13 (GCF_000001405.25)Chr 1 (NC_000001.10):1 - 249.3M. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/variationtools/1000genomes/?=%EF%BC%86=. Accessed February 3, 2016.
- Bloom D. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs; probably a syndrome entity. AMA Am J Dis Child. 1954;88:754-758.
- German J. Bloom’s syndrome, I: genetical and clinical observations in the first twenty-seven patients. Am J Hum Genet. 1969;21:196-227.
- German J, Roe AM, Leppert MF, et al. Bloom syndrome: an analysis of consanguineous families assigns the locus mutated to chromosome band 15q26.1. Proc Natl Acad Sci U S A. 1994;91:6669-6673.
- Passarge E. A DNA helicase in full Bloom. Nat Genet. 1995;11:356-357.
- Ellis NA, Groden J, Ye TZ, et al. The Bloom’s syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655-666.
- German J, Sanz MM, Ciocci S, et al. Syndrome-causing mutations of the BLM gene in persons in the Bloom’s Syndrome Registry. Hum Mutat. 2007;28:743-753.
- Landau JW, Sasaki MS, Newcomer VD, et al. Bloom’s syndrome: the syndrome of telangiectatic erythema and growth retardation. Arch Dermatol. 1966;94:687-694.
- Gretzula JC, Hevia O, Weber PJ. Bloom’s syndrome. J Am Acad Dermatol. 1987;17:479-488.
- Passarge E. Bloom’s syndrome: the German experience. Ann Genet. 1991;34:179-197.
- German J. Bloom’s syndrome. Dermatol Clin. 1995;13:7-18.
- German J, Takebe H. Bloom’s syndrome, XIV: the disorder in Japan. Clin Genet. 1989;35:93-110.
- Bennett RJ, Keck JL. Structure and function of RecQ DNA helicases. Crit Rev Biochem Mol Biol. 2004;39:79-97.
- Reference SNP (refSNP) Cluster Report: rs2227935. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=2227935. Accessed February 3, 2016.
- Reference SNP (refSNP) Cluster Report: rs7167216. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=7167216. Accessed February 3, 2016.
- Homo sapiens:GRCh37.p13 (GCF_000001405.25)Chr 1 (NC_000001.10):1 - 249.3M. National Center for Biotechnology Information website. http://www.ncbi.nlm.nih.gov/variationtools/1000genomes/?=%EF%BC%86=. Accessed February 3, 2016.
Case Report: Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
Introduction
Coarctation of the aorta comprises approximately 5% to 8% of congenital heart defects and is often associated with valvular malformations.1 These defects are typically diagnosed early and are managed with surgical repair, balloon angioplasty, or endovascular stent placement. However, as the following case illustrates, complications can occur in this population despite early intervention.
Case
A 15-year-old male adolescent presented to the pediatric ED after repeated blood pressure (BP) checks by the school nurse revealed consistently elevated systolic and diastolic pressures. The patient’s hypertension was associated with symptoms of intermittent headache and light-headedness. His medical history was remarkable for a congenital aortic coarctation and a bicuspid aortic valve. The patient had undergone a subclavian flap repair prior to 1 month of age, followed by a balloon dilatation 1 year later for recurrent coarctation. The rest of the patient’s medical history was unremarkable, including normal renal function. He denied illicit drug or alcohol use, sexual activity, or trauma.
On evaluation, the patient’s cardiac examination revealed a regular rate and rhythm with normally split S2; there were no rubs, murmurs, or gallops on auscultation. He had normal and equal pulses of the upper and lower extremities bilaterally. The patient presented without cyanosis. He was alert and oriented with normal upper and lower extremity reflexes. The neurological examination, including cranial nerve, strength, and gait testing, was unremarkable. The gastrointestinal examination showed a soft, nondistended abdomen, with no pulsatile masses. There was no abnormal swelling of his extremities. Although the physical examination findings were unremarkable, the patient’s vital signs were concerning as BP in his right upper extremity was as high as 208/110 mm Hg, while BP in his right leg was 130/68 mm Hg.
The patient followed up with his cardiologist, who ordered cardiac magnetic resonance imaging (MRI). The MRI showed mild narrowing of the distal aortic arch with a minimal and clinically insignificant pressure gradient. Based on the MRI findings, the patient was referred to a pediatric nephrologist, who performed a 24-hour ambulatory BP evaluation. The results of this study showed the patient to have systolic hypertension at the 95th percentile for his age and height. Based on the patient’s athletic predilection, β-blockers were avoided, and he was instead started on the angiotensin-converting enzyme (ACE) inhibitor lisinopril, along with annual follow-up cardiac evaluation.
Discussion
The authors’ initial concern for this patient was the possibility of a recurrent coarctation causing a significant pressure gradient between the upper and lower extremities with associated symptoms. A review of the literature demonstrates such an occurrence is not uncommon in this patient population, especially in patients with a history of early intervention (ie, within the first year of life).2
Causes and Incidence
One of the factors believed to contribute to recurrent coarctation is insufficient growth versus retraction of the manipulated tissues over time. The rates of recurrence vary based on the initial technique used for repair. These recurrences have been found to be approximately 6% in patients who had subclavian flap repairs; 31% for those who had balloon angioplasty alone; and approximately 20% in patients who had aortic stenting.3-5 As seen in this case, balloon angioplasty is usually performed in patients requiring revascularization. However, up to 32% of these patients will require further intervention due to subsequent recurrence.6
Evaluation
Although emergency physicians (EPs) have numerous diagnostic modalities available to evaluate patients with suspected aortic coarctation, as long as the patient is in no acute distress, much of the work-up can be performed on an outpatient basis—in conjunction with the primary- and subspecialty-care team. Regarding appropriate imaging modalities, echocardiography with Doppler or 3D reconstruction of MR angiogram can be useful in detecting both anatomical abnormalities as well as the associated gradient dysfunction; computed tomography can be used for assessing the anatomy.7 All of these modalities can also be used to evaluate late-term complications of aortic coarctation pathology, including aortic aneurysms. To help ensure good outcome, the EP should always keep the possibility of recurrence in the differential when evaluating these patients, regardless of the number of previous interventions attempted.
Hypertension
As this case illustrates, patients with a history of coarctation repair often develop high BP. Unfortunately, up to 23% of these patients will go on to have BP above the 95th percentile.5 Moreover, a significant number of patients in this population will also suffer from exercise-induced hypertension, even when at-rest BP is controlled with antihypertensive medications.8
β-blockers, angiotensin-receptor blockers, and ACE inhibitors are considered the first-line medications for hypertension in adults and adult-sized patients with this condition.9
Since a high proportion of patients as young as age 7 years may develop high BP postrepair,10 the EP should discuss the initiation of an antihypertensive agent with the patient’s care team prior to discharge. It is also important to keep in mind that elevated BP is present to a significant degree even in patients without recurrent obstruction. The negative sequelae associated with uncontrolled hypertension is well known, and patients with congenital anatomical anomalies are at higher risk for such negative outcomes.
Conclusion
This case illustrates a common presentation of a teenaged patient with a chronic medical condition due to a corrected congenital cardiac defect. It also demonstrates the unique and early opportunity the EP has to evaluate and provide appropriate intervention for patients with potentially life-threatening diseases.
Patients with a history of corrective vascular surgery due to congenital heart malformations are an at-risk population. Therefore, during evaluation, the EP should always keep in mind that that these patients have a higher prevalence of related abnormalities at earlier ages than the general population. Steps initiated in the ED prior to discharge, in collaboration with the patient’s primary- and specialty-care team, can assist in expediting appropriate outpatient management of any sequelae. If a patient does not have a cardiologist, a referral to one should always be made prior to discharge.
Dr Smith is a postgraduate year 3 resident in the department of emergency medicine at Alpert Medical School of Brown University, Providence, Rhode Island. Dr Merritt is an assistant professor and pediatric emergency medicine attending in the department of emergency medicine, Brown Alpert Medical School, Providence, Rhode Island.
- Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
- Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg. 2015;6(2):257-265.
- Uchytil B, Ceryny J, Nicovsky J, et al. Surgery for coarctation of the aorta: long-term post-operative results. Scripta Medica. 2003;76(6):347-356.
- Jahangiri M, Shinebourne EA, Zurakowski D, Rigby ML, Redington AN, Lincoln C. Subclavian flap angioplasty: does the arch look after itself? J Thorac Cardiovasc Surg. 2000;120(2):224-229.
- Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120(6 Pt 1):1310-1304.
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010;76(4):553-563.
- Yetman AT, Nykanen D, McCrindle BW, et al. Balloon angioplasty of recurrent coarctation: a 12-year review. J Am Coll Cardiol. 1997;30(3):811-816.
- Bashore TM, Granger CB, Jackson KP, Patel MR. Heart disease. In: Current Medical Diagnosis and Treatment 2016. Papadakis MA, McPhee SJ. The McGraw-Hill Companies, Inc: New York; 2010:322,323
- Correia AS, Gonçalves A, Paiva M, et al. Long-term follow-up after aortic coarctation repair: the unsolved issue of exercise-induced hypertension. Rev Port Cardiol. 2013;32(11):879-883.
- Warnes CA, Williams RG, Bashore TM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. Circulation. 2008;111(23):e766,e767. Available at: http://circ.ahajournals.org/content/118/23/e714.full.pdf. Accessed January 12, 2016.
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88(2):163-166.
Introduction
Coarctation of the aorta comprises approximately 5% to 8% of congenital heart defects and is often associated with valvular malformations.1 These defects are typically diagnosed early and are managed with surgical repair, balloon angioplasty, or endovascular stent placement. However, as the following case illustrates, complications can occur in this population despite early intervention.
Case
A 15-year-old male adolescent presented to the pediatric ED after repeated blood pressure (BP) checks by the school nurse revealed consistently elevated systolic and diastolic pressures. The patient’s hypertension was associated with symptoms of intermittent headache and light-headedness. His medical history was remarkable for a congenital aortic coarctation and a bicuspid aortic valve. The patient had undergone a subclavian flap repair prior to 1 month of age, followed by a balloon dilatation 1 year later for recurrent coarctation. The rest of the patient’s medical history was unremarkable, including normal renal function. He denied illicit drug or alcohol use, sexual activity, or trauma.
On evaluation, the patient’s cardiac examination revealed a regular rate and rhythm with normally split S2; there were no rubs, murmurs, or gallops on auscultation. He had normal and equal pulses of the upper and lower extremities bilaterally. The patient presented without cyanosis. He was alert and oriented with normal upper and lower extremity reflexes. The neurological examination, including cranial nerve, strength, and gait testing, was unremarkable. The gastrointestinal examination showed a soft, nondistended abdomen, with no pulsatile masses. There was no abnormal swelling of his extremities. Although the physical examination findings were unremarkable, the patient’s vital signs were concerning as BP in his right upper extremity was as high as 208/110 mm Hg, while BP in his right leg was 130/68 mm Hg.
The patient followed up with his cardiologist, who ordered cardiac magnetic resonance imaging (MRI). The MRI showed mild narrowing of the distal aortic arch with a minimal and clinically insignificant pressure gradient. Based on the MRI findings, the patient was referred to a pediatric nephrologist, who performed a 24-hour ambulatory BP evaluation. The results of this study showed the patient to have systolic hypertension at the 95th percentile for his age and height. Based on the patient’s athletic predilection, β-blockers were avoided, and he was instead started on the angiotensin-converting enzyme (ACE) inhibitor lisinopril, along with annual follow-up cardiac evaluation.
Discussion
The authors’ initial concern for this patient was the possibility of a recurrent coarctation causing a significant pressure gradient between the upper and lower extremities with associated symptoms. A review of the literature demonstrates such an occurrence is not uncommon in this patient population, especially in patients with a history of early intervention (ie, within the first year of life).2
Causes and Incidence
One of the factors believed to contribute to recurrent coarctation is insufficient growth versus retraction of the manipulated tissues over time. The rates of recurrence vary based on the initial technique used for repair. These recurrences have been found to be approximately 6% in patients who had subclavian flap repairs; 31% for those who had balloon angioplasty alone; and approximately 20% in patients who had aortic stenting.3-5 As seen in this case, balloon angioplasty is usually performed in patients requiring revascularization. However, up to 32% of these patients will require further intervention due to subsequent recurrence.6
Evaluation
Although emergency physicians (EPs) have numerous diagnostic modalities available to evaluate patients with suspected aortic coarctation, as long as the patient is in no acute distress, much of the work-up can be performed on an outpatient basis—in conjunction with the primary- and subspecialty-care team. Regarding appropriate imaging modalities, echocardiography with Doppler or 3D reconstruction of MR angiogram can be useful in detecting both anatomical abnormalities as well as the associated gradient dysfunction; computed tomography can be used for assessing the anatomy.7 All of these modalities can also be used to evaluate late-term complications of aortic coarctation pathology, including aortic aneurysms. To help ensure good outcome, the EP should always keep the possibility of recurrence in the differential when evaluating these patients, regardless of the number of previous interventions attempted.
Hypertension
As this case illustrates, patients with a history of coarctation repair often develop high BP. Unfortunately, up to 23% of these patients will go on to have BP above the 95th percentile.5 Moreover, a significant number of patients in this population will also suffer from exercise-induced hypertension, even when at-rest BP is controlled with antihypertensive medications.8
β-blockers, angiotensin-receptor blockers, and ACE inhibitors are considered the first-line medications for hypertension in adults and adult-sized patients with this condition.9
Since a high proportion of patients as young as age 7 years may develop high BP postrepair,10 the EP should discuss the initiation of an antihypertensive agent with the patient’s care team prior to discharge. It is also important to keep in mind that elevated BP is present to a significant degree even in patients without recurrent obstruction. The negative sequelae associated with uncontrolled hypertension is well known, and patients with congenital anatomical anomalies are at higher risk for such negative outcomes.
Conclusion
This case illustrates a common presentation of a teenaged patient with a chronic medical condition due to a corrected congenital cardiac defect. It also demonstrates the unique and early opportunity the EP has to evaluate and provide appropriate intervention for patients with potentially life-threatening diseases.
Patients with a history of corrective vascular surgery due to congenital heart malformations are an at-risk population. Therefore, during evaluation, the EP should always keep in mind that that these patients have a higher prevalence of related abnormalities at earlier ages than the general population. Steps initiated in the ED prior to discharge, in collaboration with the patient’s primary- and specialty-care team, can assist in expediting appropriate outpatient management of any sequelae. If a patient does not have a cardiologist, a referral to one should always be made prior to discharge.
Dr Smith is a postgraduate year 3 resident in the department of emergency medicine at Alpert Medical School of Brown University, Providence, Rhode Island. Dr Merritt is an assistant professor and pediatric emergency medicine attending in the department of emergency medicine, Brown Alpert Medical School, Providence, Rhode Island.
Introduction
Coarctation of the aorta comprises approximately 5% to 8% of congenital heart defects and is often associated with valvular malformations.1 These defects are typically diagnosed early and are managed with surgical repair, balloon angioplasty, or endovascular stent placement. However, as the following case illustrates, complications can occur in this population despite early intervention.
Case
A 15-year-old male adolescent presented to the pediatric ED after repeated blood pressure (BP) checks by the school nurse revealed consistently elevated systolic and diastolic pressures. The patient’s hypertension was associated with symptoms of intermittent headache and light-headedness. His medical history was remarkable for a congenital aortic coarctation and a bicuspid aortic valve. The patient had undergone a subclavian flap repair prior to 1 month of age, followed by a balloon dilatation 1 year later for recurrent coarctation. The rest of the patient’s medical history was unremarkable, including normal renal function. He denied illicit drug or alcohol use, sexual activity, or trauma.
On evaluation, the patient’s cardiac examination revealed a regular rate and rhythm with normally split S2; there were no rubs, murmurs, or gallops on auscultation. He had normal and equal pulses of the upper and lower extremities bilaterally. The patient presented without cyanosis. He was alert and oriented with normal upper and lower extremity reflexes. The neurological examination, including cranial nerve, strength, and gait testing, was unremarkable. The gastrointestinal examination showed a soft, nondistended abdomen, with no pulsatile masses. There was no abnormal swelling of his extremities. Although the physical examination findings were unremarkable, the patient’s vital signs were concerning as BP in his right upper extremity was as high as 208/110 mm Hg, while BP in his right leg was 130/68 mm Hg.
The patient followed up with his cardiologist, who ordered cardiac magnetic resonance imaging (MRI). The MRI showed mild narrowing of the distal aortic arch with a minimal and clinically insignificant pressure gradient. Based on the MRI findings, the patient was referred to a pediatric nephrologist, who performed a 24-hour ambulatory BP evaluation. The results of this study showed the patient to have systolic hypertension at the 95th percentile for his age and height. Based on the patient’s athletic predilection, β-blockers were avoided, and he was instead started on the angiotensin-converting enzyme (ACE) inhibitor lisinopril, along with annual follow-up cardiac evaluation.
Discussion
The authors’ initial concern for this patient was the possibility of a recurrent coarctation causing a significant pressure gradient between the upper and lower extremities with associated symptoms. A review of the literature demonstrates such an occurrence is not uncommon in this patient population, especially in patients with a history of early intervention (ie, within the first year of life).2
Causes and Incidence
One of the factors believed to contribute to recurrent coarctation is insufficient growth versus retraction of the manipulated tissues over time. The rates of recurrence vary based on the initial technique used for repair. These recurrences have been found to be approximately 6% in patients who had subclavian flap repairs; 31% for those who had balloon angioplasty alone; and approximately 20% in patients who had aortic stenting.3-5 As seen in this case, balloon angioplasty is usually performed in patients requiring revascularization. However, up to 32% of these patients will require further intervention due to subsequent recurrence.6
Evaluation
Although emergency physicians (EPs) have numerous diagnostic modalities available to evaluate patients with suspected aortic coarctation, as long as the patient is in no acute distress, much of the work-up can be performed on an outpatient basis—in conjunction with the primary- and subspecialty-care team. Regarding appropriate imaging modalities, echocardiography with Doppler or 3D reconstruction of MR angiogram can be useful in detecting both anatomical abnormalities as well as the associated gradient dysfunction; computed tomography can be used for assessing the anatomy.7 All of these modalities can also be used to evaluate late-term complications of aortic coarctation pathology, including aortic aneurysms. To help ensure good outcome, the EP should always keep the possibility of recurrence in the differential when evaluating these patients, regardless of the number of previous interventions attempted.
Hypertension
As this case illustrates, patients with a history of coarctation repair often develop high BP. Unfortunately, up to 23% of these patients will go on to have BP above the 95th percentile.5 Moreover, a significant number of patients in this population will also suffer from exercise-induced hypertension, even when at-rest BP is controlled with antihypertensive medications.8
β-blockers, angiotensin-receptor blockers, and ACE inhibitors are considered the first-line medications for hypertension in adults and adult-sized patients with this condition.9
Since a high proportion of patients as young as age 7 years may develop high BP postrepair,10 the EP should discuss the initiation of an antihypertensive agent with the patient’s care team prior to discharge. It is also important to keep in mind that elevated BP is present to a significant degree even in patients without recurrent obstruction. The negative sequelae associated with uncontrolled hypertension is well known, and patients with congenital anatomical anomalies are at higher risk for such negative outcomes.
Conclusion
This case illustrates a common presentation of a teenaged patient with a chronic medical condition due to a corrected congenital cardiac defect. It also demonstrates the unique and early opportunity the EP has to evaluate and provide appropriate intervention for patients with potentially life-threatening diseases.
Patients with a history of corrective vascular surgery due to congenital heart malformations are an at-risk population. Therefore, during evaluation, the EP should always keep in mind that that these patients have a higher prevalence of related abnormalities at earlier ages than the general population. Steps initiated in the ED prior to discharge, in collaboration with the patient’s primary- and specialty-care team, can assist in expediting appropriate outpatient management of any sequelae. If a patient does not have a cardiologist, a referral to one should always be made prior to discharge.
Dr Smith is a postgraduate year 3 resident in the department of emergency medicine at Alpert Medical School of Brown University, Providence, Rhode Island. Dr Merritt is an assistant professor and pediatric emergency medicine attending in the department of emergency medicine, Brown Alpert Medical School, Providence, Rhode Island.
- Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
- Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg. 2015;6(2):257-265.
- Uchytil B, Ceryny J, Nicovsky J, et al. Surgery for coarctation of the aorta: long-term post-operative results. Scripta Medica. 2003;76(6):347-356.
- Jahangiri M, Shinebourne EA, Zurakowski D, Rigby ML, Redington AN, Lincoln C. Subclavian flap angioplasty: does the arch look after itself? J Thorac Cardiovasc Surg. 2000;120(2):224-229.
- Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120(6 Pt 1):1310-1304.
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010;76(4):553-563.
- Yetman AT, Nykanen D, McCrindle BW, et al. Balloon angioplasty of recurrent coarctation: a 12-year review. J Am Coll Cardiol. 1997;30(3):811-816.
- Bashore TM, Granger CB, Jackson KP, Patel MR. Heart disease. In: Current Medical Diagnosis and Treatment 2016. Papadakis MA, McPhee SJ. The McGraw-Hill Companies, Inc: New York; 2010:322,323
- Correia AS, Gonçalves A, Paiva M, et al. Long-term follow-up after aortic coarctation repair: the unsolved issue of exercise-induced hypertension. Rev Port Cardiol. 2013;32(11):879-883.
- Warnes CA, Williams RG, Bashore TM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. Circulation. 2008;111(23):e766,e767. Available at: http://circ.ahajournals.org/content/118/23/e714.full.pdf. Accessed January 12, 2016.
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88(2):163-166.
- Hypertension in a Pediatric Patient With Repeat Aortic Coarctation Repair
- Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg. 2015;6(2):257-265.
- Uchytil B, Ceryny J, Nicovsky J, et al. Surgery for coarctation of the aorta: long-term post-operative results. Scripta Medica. 2003;76(6):347-356.
- Jahangiri M, Shinebourne EA, Zurakowski D, Rigby ML, Redington AN, Lincoln C. Subclavian flap angioplasty: does the arch look after itself? J Thorac Cardiovasc Surg. 2000;120(2):224-229.
- Rao PS, Thapar MK, Galal O, Wilson AD. Follow-up results of balloon angioplasty of native coarctation in neonates and infants. Am Heart J. 1990;120(6 Pt 1):1310-1304.
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2010;76(4):553-563.
- Yetman AT, Nykanen D, McCrindle BW, et al. Balloon angioplasty of recurrent coarctation: a 12-year review. J Am Coll Cardiol. 1997;30(3):811-816.
- Bashore TM, Granger CB, Jackson KP, Patel MR. Heart disease. In: Current Medical Diagnosis and Treatment 2016. Papadakis MA, McPhee SJ. The McGraw-Hill Companies, Inc: New York; 2010:322,323
- Correia AS, Gonçalves A, Paiva M, et al. Long-term follow-up after aortic coarctation repair: the unsolved issue of exercise-induced hypertension. Rev Port Cardiol. 2013;32(11):879-883.
- Warnes CA, Williams RG, Bashore TM, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. Circulation. 2008;111(23):e766,e767. Available at: http://circ.ahajournals.org/content/118/23/e714.full.pdf. Accessed January 12, 2016.
- O’Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88(2):163-166.
Case Studies In Toxicology: Withdrawal: Another Danger of Diversion
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 34-year-old man with a history of polysubstance abuse presented to the ED after he had a seizure during his regular methadone-treatment program meeting. While at the clinic, attendees witnessed the patient experience a loss of consciousness accompanied by generalized shaking movements of his extremities, which lasted for several minutes.
Upon arrival in the ED, the patient stated that he had a mild headache; he was otherwise asymptomatic. Initial vital signs were: blood pressure, 126/80 mm Hg; heart rate, 82 beats/minute; respiratory rate, 16 breaths/minute; and temperature, 97.3°F. Oxygen saturation was 98% on room air, and a finger-stick glucose test was 140 mg/dL.
Physical examination revealed a small right-sided parietal hematoma. The patient had no tremors and his neurological examination, including mental status, was normal. When reviewing the patient’s medical history and medications in the health record, it was noted that the patient had a prescription for alprazolam for an anxiety disorder. On further questioning, the patient admitted that he had sold his last alprazolam prescription and had not been taking the drug for the past week.
What characterizes the benzodiazepine withdrawal syndrome?
Although introduced into clinical practice in the 1960s, the potential for dependence and a withdrawal syndrome was not appreciated until the early 1980s. This clinical syndrome can manifest with a wide variety of findings, most commonly with what are termed “rebound effects” or “rebound hyperexcitability.” These effects include anxiety, insomnia or sleep disturbance, tremulousness, irritability, sweating, psychomotor agitation, difficulty in concentration, nausea, weight loss, palpitations, headache, muscular pain and stiffness, or generalized weakness.2 More severe manifestations include delirium, seizures, or psychosis. Often, these symptoms and signs may be confused with the very manifestations that prompted the initial use of the BZD, a reemergence of which can exacerbate the withdrawal syndrome.
When does benzodiazepine withdrawal occur?
The exact time course of BZD withdrawal can vary considerably and, unlike alcohol withdrawal (which occurs from a single compound, ethanol), can be difficult to characterize. The onset of withdrawal symptoms is dependent on a number of factors, including the half-life of the BZD involved. For example, delayed onset withdrawal symptoms of up to 3 weeks after cessation of the medication are described with long-acting BZDs such as chlordiazepoxide and diazepam. Conversely, symptoms may present as early as 24 to 48 hours after abrupt termination of BZDs with shorter half-lives, alprazolam and lorazepam. This variable time of onset differs considerably from other withdrawal syndromes, notably ethanol withdrawal. While both syndromes correlate to the individual patient’s severity of dependence, alcohol withdrawal follows a more predictable time course.
Some authors distinguish a rebound syndrome from a true withdrawal syndrome, the former of which is self-limited in nature and the result of cessation of treatment for the primary disease process. In this model, rebound symptoms begin 1 to 4 days after the abrupt cessation or dose reduction of the BZD, and are relatively short-lived, lasting 2 to 3 days.2
What is the appropriate treatment for benzodiazepine withdrawal?
The standard therapy for almost all withdrawal syndromes is reinstitution of the causal agent. A number of non-BZD-based treatment strategies have been investigated, and all have met with limited success. Of these, anticonvulsant drugs such as carbamazepine and valproic acid were initially considered promising based on case reports and small case series.4 These medications ultimately proved ineffective in randomized, placebo-controlled studies.5 β-Adrenergic antagonists, such as propranolol, have been studied as a method to normalize a patient’s vital signs but also proved nonbeneficial in managing withdrawal.5,6
The safest and most effective management approach for patients with BZD withdrawal is reinstitution of the BZD followed by a prolonged and gradual tapering until cessation, if that is desired.1,2,5,6 While all BZDs share structural and mechanistic similarities, there are subtle variations within this class that can affect their pharmacologic effects. These structural differences may result in incomplete cross-tolerance, which may lead to inadequate mitigation of the withdrawal syndrome. For example, previous reports suggest that alprazolam and clonazepam are structurally unique and bind to the BZD receptor with higher affinity than other BZDs. Therefore, while in general any BZD can be used to treat withdrawal from another BZD, it is recommended to treat withdrawal from these two agents with the implicated BZD.
There are, however, limitations to this approach. Namely, some BZDs are only available in oral formulations (eg, alprazolam and clonazepam) or the BZD of choice may not be readily available or on formulary within a given institution. In a patient with a severe withdrawal syndrome where it is not feasible or potentially harmful to administer an oral medication, it is reasonable to provide parenteral (preferably intravenous [IV]) BZD therapy. The optimal approach is to start with a small “standard” dose and titrate to effect while monitoring for adverse effects (eg, oversedation, ventilatory depression). Redosing should be triggered by symptoms or signs, and not performed in a timed or standing-order fashion. If this approach proves ineffective and withdrawal symptoms persist despite adequate BZD therapy, a direct GABA agonist such as propofol is a sensible alternative or adjuvant treatment. This may sound similar to the management of patients with ethanol withdrawal; indeed, this approach is essentially the same, with the exception of the more drawn-out time course.
Case Conclusion
After arrival in the ED, the patient received diazepam 10 mg IV and was subsequently admitted to the hospital for further evaluation. During his hospitalization, the patient was re-started on his usual dose of oral alprazolam. No further withdrawal syndrome was observed, and he was discharged on hospital day 2 with a plan to slowly taper his alprazolam dose with his outpatient psychiatrist.
Dr Repplinger is a senior medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.
- Withdrawal: Another Danger of Diversion
- Marriott S, Tyrer P. Benzodiazepine dependence. Avoidance and withdrawal. Drug Saf. 1993;9(2):93-103.
- Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
- Authier N, Balayssac D, Sautereau M, et al. Benzodiazepine dependence: focus on withdrawal syndrome. Ann Pharm Fr. 2009;67(6):408-413.
- Pages KP, Ries RK. Use of anticonvulsants in benzodiazepine withdrawal. Am J Addict. 1998;7(3):198-204.
- Ashton H. The treatment of benzodiazepine dependence. Addiction. 1994;89(11):1535-1541.
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta-analysis. Addiction. 2009;104(1):13-24.