User login
Unforgettable
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
A 27‐year‐old woman with a history of asthma presented to her primary care physician (PCP) with a sore throat which began after attending a party where she shared alcoholic beverages with friends. She denied any high‐risk sexual behavior. Her PCP prescribed azithromycin and methylprednisolone empirically for tonsillitis. The throat pain subsided, but in the next several days she experienced increased weakness, lethargy, poor appetite, and chills, and she returned to her PCP for reevaluation.
Two months prior she had been treated at a walk‐in clinic with a course of penicillin for a presumed streptococcal pharyngitis. Her symptoms resolved until her current presentation.
In a young woman with 2 episodes of pharyngitis in 2 months followed by an acute systemic illness, one must consider an immunocompromised state such as human immunodeficiency virus (HIV), hematologic malignancy, or autoimmune diseases. Weakness, lethargy, anorexia, and chills in the setting of pharyngitis suggest a local process in the neck, most likely infection associated with systemic toxicity. As neck abscess and bacteremia warrant early consideration, the physical examination should focus on the neck and oropharynx, as well as neurologic exam to evaluate for bacterial spreading into the central nervous system. In addition to routine laboratory studies, a chest x‐ray (CXR) would be appropriate as upper respiratory infections may be complicated by pneumonia and present with signs and symptoms of systemic illness.
On examination by her PCP, her temperature was 99.2F and her blood pressure was 118/68. She had bilateral oropharyngeal erythema without exudates and bilateral tonsillar and anterior triangle lymphadenopathy (LAD). An oropharyngeal rapid Streptococcal antigen detection test was negative, but a Monospot test was positive for heterophile antibodies. Azithromycin and methylprednisolone were discontinued, and the patient was informed she most likely had Epstein Barr Virus (EBV) infection.
The following day, the complete blood count results returned. The platelet count was 50 K/L and the white blood cell (WBC) count was 13.0 K/L. The patient stated she had developed right‐sided flank pain upon deep inspiration and used her albuterol inhaler with minimal relief. She continued to have fever, decreased appetite, chest and abdominal pain, and difficulty swallowing due to odynophagia. She was instructed to go to the emergency department (ED) for further evaluation. In the ED, she denied any shortness of breath, but reported a slight cough and right‐sided abdominal pain.
Acute tonsillar pharyngitis and fever, as well as systemic symptoms of fatigue and abdominal pain along with positive heterophile screen are highly suggestive of EBV infection in this young female. The episode of pharyngitis 2 months prior remains unexplained and may be unrelated. Right‐sided pleuritic pain and abdominal pain may be related to EBV hepatitis. Odynophagia is consistent with EBV infection as well. Profound lethargy, however, is not a common presenting feature in mononucleosis unless infected patients are profoundly dehydrated due to inability to swallow. Her pain symptoms may be secondary to other signs of EBV infection, such as hepatomegaly, splenomegaly, ascites, and/or right pleural effusion. A history of rash should be investigated. Initial assessment in this acutely ill patient should focus on evaluation for the presence of severe sepsis and for a primary source of infection. Given the severity of her illness, I would consider early computed tomography (CT) of her chest, abdomen, and pelvis, as well as CT of the neck to exclude a possibility of peritonsillar abscess. The complaint of chills indicates a possible bacteremia, so coverage with broad‐spectrum antibiotics is indicated. Symptomatic relief with acetaminophen and intravenous fluid rehydration is appropriate.
On exam, temperature was 101.9F, blood pressure was 111/74, heart rate was 140 beats per minute, respiratory rate was 18 per minute, and oxygen saturation was 99% on room air. She appeared drowsy, but answered questions appropriately. She had bilateral swollen tonsils, as well as anterior and posterior cervical adenopathy, with tenderness greater on the left side. Her chest exam had slightly diminished breath sounds at the bases bilaterally. Heart rhythm was regular, and there were no murmurs appreciated. On abdominal exam, she was tender to palpation in both right‐upper and left‐upper quadrants, without obvious hepatosplenomegaly. There were no petechiae noted on her skin.
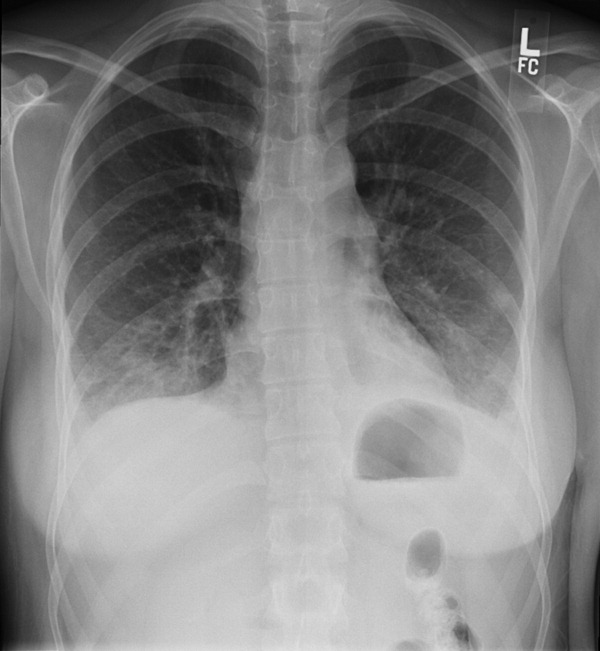
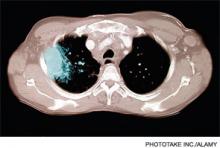
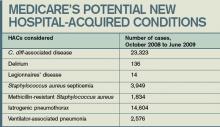
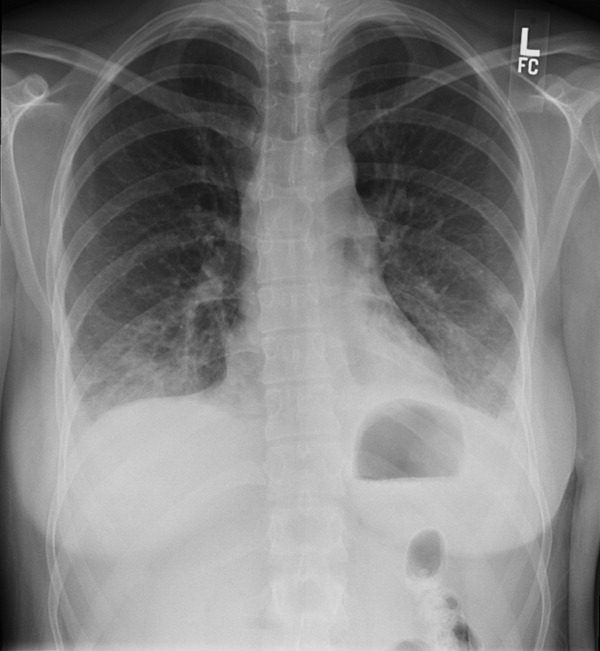
The WBC was 17.6 K/L, with 89% neutrophils and 5% lymphocytes, platelet count was 22 K/L, and hemoglobin was 13.8 g/dL. A D‐dimer test was elevated at 1344 ng/mL. Peripheral blood smear showed thrombocytopenia and neutrophilia, but demonstrated no schistocytes. The serum potassium was 3.2 mEq/L, bicarbonate was 29 mEq/L, blood urea nitrogen was 15 mg/dL and the creatinine was 1.29 mg/dL. Transaminases were within normal limits, but total bilirubin was 1.8 mg/dL. Her urinalysis was normal. Blood cultures were sent. A CXR showed bibasilar consolidations and pleural effusions (Figure 1). A CT of the chest with contrast was obtained that showed multiple confluent and patchy foci of consolidation in the lung bases, with trace bilateral pleural effusions (Figure 2). A CT of the abdomen showed a spleen at the upper limits of normal in size, measuring 13 cm in length, but was otherwise normal.
Leukocytosis with lymphopenia is not consistent with EBV infection and another process needs to be considered. This patient meets criteria for sepsis syndrome and should receive broad spectrum antibiotics, such as vancomycin and piperacillin‐tazobactam immediately after the blood cultures are sent, in addition to further evaluation to determine the source of sepsis. Depending on her mental status response to initial measures such as acetaminophen and hydration, one should consider a lumbar puncture, which would require platelet transfusion and may therefore not be done immediately. HIV serology should be performed, since acute retroviral syndrome can mimic this presentation. With neck tenderness that is more localized to her left side, a CT of her neck to evaluate for an abscess may be helpful.
She was admitted for presumed community‐acquired pneumonia complicating an upper respiratory tract infection. Her pharyngitis was thought to be of viral etiology. Moxifloxacin was started and intravenous fluids were administered. She was started on prednisone 60 mg daily for presumed immune‐mediated thrombocytopenia related to EBV infection. An HIV antibody test and quantitative polymerase chain reaction (PCR) were both negative. The EBV immunoglobulin G (IgG) titer was positive (>1:10), but the IgM titer was negative. Her mental status improved after starting moxifloxacin and fluids. Her creatinine and bilirubin normalized to 0.97 mg/dL and 0.8 mg/dL respectively. She continued to have a tender left‐sided submandibular swelling. Blood cultures grew Gram‐negative bacilli in 2 anaerobic bottles.
I am uncomfortable with moxifloxacin as initial empiric therapy because at presentation she had sepsis syndrome as well as a suspected immunocompromised state. In addition, moxifloxacin would not be adequate coverage for anaerobic organisms if a peritonsillar abscess was involved. At this point, she needs a CT of her neck to look for a focus of infection which may require surgical management and, if negative, further imaging such as a tagged white blood scan to identify the source of the anaerobes.
Moxifloxacin was switched to piperacillin‐tazobactam and prednisone was discontinued. By day 4 of hospitalization her platelet count had risen to 261 K/L. Her WBC continued to rise to a peak of 21.5 K/L and she continued to have fevers and diffuse pains, although her repeat blood cultures were negative. She continued to have tenderness of the cervical lymph nodes, left greater than right. A repeat CXR showed patchy air space disease bilaterally and pleural effusions, both of which had progressed compared with the prior film. Clindamycin was empirically added to her antibiotic regimen in light of her progressing pneumonia and evidence of anaerobic infection. A repeat CT scan of her chest revealed multiple nodular opacities scattered throughout the lung fields, some of which were cavitary, predominating in the lung bases. The CT scan of her neck revealed a left peritonsillar abscess and phlegmon in the left retropharyngeal and deep neck area along the sternocleidomastoid and internal jugular vein (IJV). There also was noted a large thrombus within the left IJV extending superiorly to involve the jugular bulb, sigmoid sinus, and distal left transverse sinus; and inferiorly to near the origin of the brachiocephalic vein (see Figure 3). An echocardiogram did not reveal any vegetations.

The combination of recent pharyngitis, septic pulmonary emboli, and IJV thrombosis is consistent with a diagnosis of Lemierre's syndrome (LS). This is a life threatening condition, even if diagnosis is made early and appropriate treatment is started. The most likely causative agent is Fusobacterium necrophorum. In this case it was important to realize that clinical presentation was not consistent with EBV infection, even though heterophile screen was positive. Early initiation of broad spectrum antibiotics as well as CT scan of the neck would have been appropriate.
The diagnosis of LS was made. The blood culture speciation revealed Fusobacterium nucleatum, which was too fastidious to perform antimicrobial sensitivities. Her symptoms improved significantly with the addition of clindamycin to piperacillin‐tazobactam, which was postulated to be the result of bacterial beta‐lactamase activity mitigating the efficacy of piperacillin‐tazobactam. Thoracentesis of her pleural effusion did not reveal an empyema. Due to her large thrombus burden, she was started on anticoagulation with heparin and transitioned to outpatient coumadin. She was switched to metronidazole as a single agent antibiotic for 6 weeks, and on outpatient follow‐up was doing well.
Commentary
LS was described by Dr. Andre Lemierre in 1936.6 The syndrome consists of a primary oropharyngeal infection, thrombosis of the IJV, bacteremia, and septic metastatic foci, usually involving the lungs.1, 2 LS is a form of necrobacillosis, which is a systemic infection resulting from F. necrophorum.3, 4 In classic LS, the initial pharyngitis is usually a tonsillar or peritonsillar abscess, and is followed by intense fever and rigors after 4 days to 2 weeks.1, 3 This is followed by a unilateral painful submaxillary LAD and IJV thrombophlebitisthe cord sign.2 Finally, bacteremia and distant metastatic pyogenic abscesses develop.1 (see Table 1).
| Lemierre's Syndrome typical features |
| Antecedent head and neck infection, typically an oropharyngeal infection prior to deterioration |
| Thrombophlebitis, typically of internal jugular vein (present in only 1/3 of cases) |
| Bacteremia (Fusobacterium necrophorum most commonly) |
| Septic metastatic foci, typically to lungs |
| Usual Presentation |
| Pharyngitis |
| Fevers |
| Rigors |
| Neck involvement: tenderness, swelling, tender internal jugular vein thrombus (cord sign) |
| Pulmonary infiltrates which cavitate |
With the advent of antibiotics, LS is now rare with an incidence of 0.9 per million persons per year. In Lemierre's time, the disease was fulminant and led to death within 2 weeks, but in the antibiotic age the mortality rate is 4.9%.1, 3 The median age of an LS patient is 19 years, with a higher incidence in males.1, 35 Although in the literature it is referred to as the forgotten disease, there is evidence the incidence is increasing.3, 4, 6, 8
There are variations of classic LS. Bacteremia may occur much later than the initial pharyngitis, the disease may be less aggressive, the thrombus may be in the external jugular vein, or there may be no identified thrombus.3, 4, 8 In fact, a thrombus is only identified in 36% of cases.9 The primary infection may be a head and neck infection that is not pharyngitis, such as an odontogenic infection,4 or may not be identified.10 Despite variations, the fundamentals of diagnosis are prior head and neck infection, presumed thrombophlebitis and bacteremia, and evidence of septic metastatic foci.
The genus Fusobacterium comprises anaerobic, nonspore forming gram negative bacilli.1, 35, 11 F. necrophorum and F. nucleatum are 2 species within this genus. F. nucleatum causes the majority of reported human bacteremias by Fusobacterium species, but it is F. necrophorum that is most associated with anaerobic oropharyngeal infections, thrombocytopenia, clot formation, and LS.35, 8, 9
It is unknown if Fusobacterium species directly cause the sore throat, or rather are bystanders which thrive once a favorable anaerobic environment is created via endotoxins and exotoxins.35 A break in oral mucosa via trauma or coinfection with bacteria/viruses (especially EBV) is also thought to play a role with infection.2, 3, 5 One‐third of LS cases have coinfection with other oropharyngeal flora. Thus, one must reexamine the anaerobic blood cultures after an organism has been identified in suspect cases.3, 4
There is an increased association of LS with EBV infection, likely due to viral‐induced and steroid‐induced immunosuppression.24 False positive heterophile tests are reported with LS, so the specific antibody tests for EBV must be checked.3, 4
Once thrombophlebitis occurs, the bacteria can metastasize to distant sites. In 80% to 92% of LS cases, the metastatic complication is a pleuro‐pulmonary infection, consisting of septic pulmonary emboli, empyema, and pleural effusions, but extra‐pulmonary lesions occur.1, 3, 9, 12 Abdominal pain usually results from abdominal microabscesses or thrombophlebitis.4 Mild renal impairment and abnormal liver function tests are common.3, 4 Cranial nerve palsies and Horner's syndrome are rare and indicate carotid sheath involvement.3, 12 An elevated C‐reactive protein can distinguish bacterial from uncomplicated viral pharyngitis.3, 4 Also, rigors are unusual in tonsillitis, and their presence indicate bacterial entry into the circulation.3
CXRs may reveal the pulmonary septic emboli. Ultrasound of the IJV is inexpensive and noninvasive, but may have limited sensitivity for an acute thrombus. CT scan allows increased visualization of anatomy, but can have decreased sensitivity and specificity for thrombosis.3 Magnetic resonance imaging (MRI) is recommended if LS results from mastoiditis, to exclude an intracerebral vein thrombosis.9
Antibiotics have both dramatically decreased the incidence of LS and improved its prognosis. The recent rise in incidence may be due to a renewed interest in restricting the use of antibiotics in cases of pharyngitis, as well as an increased use of macrolides, to which F. necrophorum is frequently resistant.3 Decreased tonsillectomies may also have a role, as LS is more common with retained tonsils.1, 3
No trials have evaluated the optimal antibiotic regimen. Fusobacterium species are sensitive to penicillin, but 23% have beta‐lactamase activity as reported clinically by several authors.3, 5 F. necrophorum is also sensitive to metronidazole, ticarcillin‐clavulanate, cefoxitin, amoxicillin‐clavulanate, imipenem, and clindamycin. There is a high resistance to macrolides and gentamicin, and the activity of tetracyclines is poor. For treatment, most authors suggest a carbapenem, a penicillin/beta‐lactamase inhibitor combination, or metronidazole. Clindamycin has weaker bactericidal activity than metronidazole or imipenem. Metronidazole is preferred because of its activity against all Fusobacterium species, good penetration into tissues, bactericidal activity, low minimum inhibitory concentration, and ability to achieve high concentration in the cerebrospinal fluid if meningitis occurs. An effective regimen is metronidazole with a penicillinase‐resistant penicillin to cover for mixed coinfection with streptococci or staphylococci.3, 4, 12 A 6‐week antibiotic course is given for adequate penetration into the protective fibrin clots.4
Reports have shown good outcomes both with and without the use of anticoagulation.3, 4, 8 Support for anticoagulation is extrapolated from experience with septic pelvic thrombophlebitis, in which anticoagulation results in more rapid resolution of symptoms.13 Given the lack of firm evidence in cases of LS, anticoagulation is typically reserved for poor clinical response despite 2 to 3 days of antibiotic therapy or propagation of thromboses into the cavernous sinus. It is generally given for 3 months.4, 13
Prior to the antibiotic era, surgical ligation or excision of the IJV was done without clear benefit. Today, surgery is reserved for cases of continued septic emboli or extension of thrombus despite aggressive medical therapy.3 If mediastinitis develops, then surgical intervention is essential.4
Lemierre stated that the symptoms and signs of LS are so characteristic that it permits diagnosis before bacteriological examination.1 However, today it may go unrecognized by physicians until a blood culture shows anaerobes or Fusobacterium species. For a young patient admitted with pneumonia preceded by pharyngitis, hospitalists must remain vigilant for the presence of LS.
Key Points for Hospitalists/Teaching Points
-
The triad of LS is pharyngitis, thrombophlebitis, and distant metastatic pyogenic emboli.
-
Suspect LS in a young, otherwise healthy patient who clinically deteriorates in the setting of a recent pharyngeal infection.
-
With the modern decrease in antibiotic use for pharyngitis, LS may be on the rise.
- .On certain septicaemias due to anaerobic organisms.Lancet.1936;1:701–703.
- ,.Lemierre's syndrome: more judicious antibiotic prescribing habits may lead to the clinical reappearance of this often forgotten disease.Am J Med.2006;119(3):e7–e9.
- .Human infection with Fusobacterium necrophorum (necrobacillosis), with a focus on Lemierre's syndrome.Clin Microbiol Rev.2007;20(4):622–659.
- ,.Human necrobacillosis, with emphasis on Lemierre's syndrome.Clin Infect Dis.2000;31(2):524–532.
- ,.Fusobacterial infections: clinical spectrum and incidence of invasive disease.J Infect.2008;57(4):283–289.
- .Human infections with Fusobacterium necrophorum.Anaerobe.2006;12(4):165–172.
- ,,, et al.Increased diagnosis of Lemierre Syndrome and other Fusobacterium necrophorum infections at a Children's Hospital.Pediatrics.2003;112(5):e380.
- ,,.Unusual presentation of Lemierre's syndrome due to Fusobacterium nucleatum.J Clin Microbiol.2003;41(7):3445–3448.
- ,,,.The evolution of Lemierre Syndrome: report of 2 cases and review of the literature.Medicine (Baltimore).2002;81(6):458–465.
- ,.An unusual case of Lemierre's syndrome presenting as pyomyositis.Am J Med Sci.2008;335(6):499–501.
- .Update on the taxonomy and clinical aspects of the genus Fusobacterium.Clin Infect Dis.2002;35(Suppl 1):S22–S27.
- ,,,,.Lemierre syndrome: two cases and a review.Laryngosope.2007;117(9):1605–1610.
- ,,.Lemierre's syndrome (necrobacillosis).Postgrad Med J.1999;75(881):141–144.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
A 27‐year‐old woman with a history of asthma presented to her primary care physician (PCP) with a sore throat which began after attending a party where she shared alcoholic beverages with friends. She denied any high‐risk sexual behavior. Her PCP prescribed azithromycin and methylprednisolone empirically for tonsillitis. The throat pain subsided, but in the next several days she experienced increased weakness, lethargy, poor appetite, and chills, and she returned to her PCP for reevaluation.
Two months prior she had been treated at a walk‐in clinic with a course of penicillin for a presumed streptococcal pharyngitis. Her symptoms resolved until her current presentation.
In a young woman with 2 episodes of pharyngitis in 2 months followed by an acute systemic illness, one must consider an immunocompromised state such as human immunodeficiency virus (HIV), hematologic malignancy, or autoimmune diseases. Weakness, lethargy, anorexia, and chills in the setting of pharyngitis suggest a local process in the neck, most likely infection associated with systemic toxicity. As neck abscess and bacteremia warrant early consideration, the physical examination should focus on the neck and oropharynx, as well as neurologic exam to evaluate for bacterial spreading into the central nervous system. In addition to routine laboratory studies, a chest x‐ray (CXR) would be appropriate as upper respiratory infections may be complicated by pneumonia and present with signs and symptoms of systemic illness.
On examination by her PCP, her temperature was 99.2F and her blood pressure was 118/68. She had bilateral oropharyngeal erythema without exudates and bilateral tonsillar and anterior triangle lymphadenopathy (LAD). An oropharyngeal rapid Streptococcal antigen detection test was negative, but a Monospot test was positive for heterophile antibodies. Azithromycin and methylprednisolone were discontinued, and the patient was informed she most likely had Epstein Barr Virus (EBV) infection.
The following day, the complete blood count results returned. The platelet count was 50 K/L and the white blood cell (WBC) count was 13.0 K/L. The patient stated she had developed right‐sided flank pain upon deep inspiration and used her albuterol inhaler with minimal relief. She continued to have fever, decreased appetite, chest and abdominal pain, and difficulty swallowing due to odynophagia. She was instructed to go to the emergency department (ED) for further evaluation. In the ED, she denied any shortness of breath, but reported a slight cough and right‐sided abdominal pain.
Acute tonsillar pharyngitis and fever, as well as systemic symptoms of fatigue and abdominal pain along with positive heterophile screen are highly suggestive of EBV infection in this young female. The episode of pharyngitis 2 months prior remains unexplained and may be unrelated. Right‐sided pleuritic pain and abdominal pain may be related to EBV hepatitis. Odynophagia is consistent with EBV infection as well. Profound lethargy, however, is not a common presenting feature in mononucleosis unless infected patients are profoundly dehydrated due to inability to swallow. Her pain symptoms may be secondary to other signs of EBV infection, such as hepatomegaly, splenomegaly, ascites, and/or right pleural effusion. A history of rash should be investigated. Initial assessment in this acutely ill patient should focus on evaluation for the presence of severe sepsis and for a primary source of infection. Given the severity of her illness, I would consider early computed tomography (CT) of her chest, abdomen, and pelvis, as well as CT of the neck to exclude a possibility of peritonsillar abscess. The complaint of chills indicates a possible bacteremia, so coverage with broad‐spectrum antibiotics is indicated. Symptomatic relief with acetaminophen and intravenous fluid rehydration is appropriate.
On exam, temperature was 101.9F, blood pressure was 111/74, heart rate was 140 beats per minute, respiratory rate was 18 per minute, and oxygen saturation was 99% on room air. She appeared drowsy, but answered questions appropriately. She had bilateral swollen tonsils, as well as anterior and posterior cervical adenopathy, with tenderness greater on the left side. Her chest exam had slightly diminished breath sounds at the bases bilaterally. Heart rhythm was regular, and there were no murmurs appreciated. On abdominal exam, she was tender to palpation in both right‐upper and left‐upper quadrants, without obvious hepatosplenomegaly. There were no petechiae noted on her skin.
The WBC was 17.6 K/L, with 89% neutrophils and 5% lymphocytes, platelet count was 22 K/L, and hemoglobin was 13.8 g/dL. A D‐dimer test was elevated at 1344 ng/mL. Peripheral blood smear showed thrombocytopenia and neutrophilia, but demonstrated no schistocytes. The serum potassium was 3.2 mEq/L, bicarbonate was 29 mEq/L, blood urea nitrogen was 15 mg/dL and the creatinine was 1.29 mg/dL. Transaminases were within normal limits, but total bilirubin was 1.8 mg/dL. Her urinalysis was normal. Blood cultures were sent. A CXR showed bibasilar consolidations and pleural effusions (Figure 1). A CT of the chest with contrast was obtained that showed multiple confluent and patchy foci of consolidation in the lung bases, with trace bilateral pleural effusions (Figure 2). A CT of the abdomen showed a spleen at the upper limits of normal in size, measuring 13 cm in length, but was otherwise normal.


Leukocytosis with lymphopenia is not consistent with EBV infection and another process needs to be considered. This patient meets criteria for sepsis syndrome and should receive broad spectrum antibiotics, such as vancomycin and piperacillin‐tazobactam immediately after the blood cultures are sent, in addition to further evaluation to determine the source of sepsis. Depending on her mental status response to initial measures such as acetaminophen and hydration, one should consider a lumbar puncture, which would require platelet transfusion and may therefore not be done immediately. HIV serology should be performed, since acute retroviral syndrome can mimic this presentation. With neck tenderness that is more localized to her left side, a CT of her neck to evaluate for an abscess may be helpful.
She was admitted for presumed community‐acquired pneumonia complicating an upper respiratory tract infection. Her pharyngitis was thought to be of viral etiology. Moxifloxacin was started and intravenous fluids were administered. She was started on prednisone 60 mg daily for presumed immune‐mediated thrombocytopenia related to EBV infection. An HIV antibody test and quantitative polymerase chain reaction (PCR) were both negative. The EBV immunoglobulin G (IgG) titer was positive (>1:10), but the IgM titer was negative. Her mental status improved after starting moxifloxacin and fluids. Her creatinine and bilirubin normalized to 0.97 mg/dL and 0.8 mg/dL respectively. She continued to have a tender left‐sided submandibular swelling. Blood cultures grew Gram‐negative bacilli in 2 anaerobic bottles.
I am uncomfortable with moxifloxacin as initial empiric therapy because at presentation she had sepsis syndrome as well as a suspected immunocompromised state. In addition, moxifloxacin would not be adequate coverage for anaerobic organisms if a peritonsillar abscess was involved. At this point, she needs a CT of her neck to look for a focus of infection which may require surgical management and, if negative, further imaging such as a tagged white blood scan to identify the source of the anaerobes.
Moxifloxacin was switched to piperacillin‐tazobactam and prednisone was discontinued. By day 4 of hospitalization her platelet count had risen to 261 K/L. Her WBC continued to rise to a peak of 21.5 K/L and she continued to have fevers and diffuse pains, although her repeat blood cultures were negative. She continued to have tenderness of the cervical lymph nodes, left greater than right. A repeat CXR showed patchy air space disease bilaterally and pleural effusions, both of which had progressed compared with the prior film. Clindamycin was empirically added to her antibiotic regimen in light of her progressing pneumonia and evidence of anaerobic infection. A repeat CT scan of her chest revealed multiple nodular opacities scattered throughout the lung fields, some of which were cavitary, predominating in the lung bases. The CT scan of her neck revealed a left peritonsillar abscess and phlegmon in the left retropharyngeal and deep neck area along the sternocleidomastoid and internal jugular vein (IJV). There also was noted a large thrombus within the left IJV extending superiorly to involve the jugular bulb, sigmoid sinus, and distal left transverse sinus; and inferiorly to near the origin of the brachiocephalic vein (see Figure 3). An echocardiogram did not reveal any vegetations.

The combination of recent pharyngitis, septic pulmonary emboli, and IJV thrombosis is consistent with a diagnosis of Lemierre's syndrome (LS). This is a life threatening condition, even if diagnosis is made early and appropriate treatment is started. The most likely causative agent is Fusobacterium necrophorum. In this case it was important to realize that clinical presentation was not consistent with EBV infection, even though heterophile screen was positive. Early initiation of broad spectrum antibiotics as well as CT scan of the neck would have been appropriate.
The diagnosis of LS was made. The blood culture speciation revealed Fusobacterium nucleatum, which was too fastidious to perform antimicrobial sensitivities. Her symptoms improved significantly with the addition of clindamycin to piperacillin‐tazobactam, which was postulated to be the result of bacterial beta‐lactamase activity mitigating the efficacy of piperacillin‐tazobactam. Thoracentesis of her pleural effusion did not reveal an empyema. Due to her large thrombus burden, she was started on anticoagulation with heparin and transitioned to outpatient coumadin. She was switched to metronidazole as a single agent antibiotic for 6 weeks, and on outpatient follow‐up was doing well.
Commentary
LS was described by Dr. Andre Lemierre in 1936.6 The syndrome consists of a primary oropharyngeal infection, thrombosis of the IJV, bacteremia, and septic metastatic foci, usually involving the lungs.1, 2 LS is a form of necrobacillosis, which is a systemic infection resulting from F. necrophorum.3, 4 In classic LS, the initial pharyngitis is usually a tonsillar or peritonsillar abscess, and is followed by intense fever and rigors after 4 days to 2 weeks.1, 3 This is followed by a unilateral painful submaxillary LAD and IJV thrombophlebitisthe cord sign.2 Finally, bacteremia and distant metastatic pyogenic abscesses develop.1 (see Table 1).
| Lemierre's Syndrome typical features |
| Antecedent head and neck infection, typically an oropharyngeal infection prior to deterioration |
| Thrombophlebitis, typically of internal jugular vein (present in only 1/3 of cases) |
| Bacteremia (Fusobacterium necrophorum most commonly) |
| Septic metastatic foci, typically to lungs |
| Usual Presentation |
| Pharyngitis |
| Fevers |
| Rigors |
| Neck involvement: tenderness, swelling, tender internal jugular vein thrombus (cord sign) |
| Pulmonary infiltrates which cavitate |
With the advent of antibiotics, LS is now rare with an incidence of 0.9 per million persons per year. In Lemierre's time, the disease was fulminant and led to death within 2 weeks, but in the antibiotic age the mortality rate is 4.9%.1, 3 The median age of an LS patient is 19 years, with a higher incidence in males.1, 35 Although in the literature it is referred to as the forgotten disease, there is evidence the incidence is increasing.3, 4, 6, 8
There are variations of classic LS. Bacteremia may occur much later than the initial pharyngitis, the disease may be less aggressive, the thrombus may be in the external jugular vein, or there may be no identified thrombus.3, 4, 8 In fact, a thrombus is only identified in 36% of cases.9 The primary infection may be a head and neck infection that is not pharyngitis, such as an odontogenic infection,4 or may not be identified.10 Despite variations, the fundamentals of diagnosis are prior head and neck infection, presumed thrombophlebitis and bacteremia, and evidence of septic metastatic foci.
The genus Fusobacterium comprises anaerobic, nonspore forming gram negative bacilli.1, 35, 11 F. necrophorum and F. nucleatum are 2 species within this genus. F. nucleatum causes the majority of reported human bacteremias by Fusobacterium species, but it is F. necrophorum that is most associated with anaerobic oropharyngeal infections, thrombocytopenia, clot formation, and LS.35, 8, 9
It is unknown if Fusobacterium species directly cause the sore throat, or rather are bystanders which thrive once a favorable anaerobic environment is created via endotoxins and exotoxins.35 A break in oral mucosa via trauma or coinfection with bacteria/viruses (especially EBV) is also thought to play a role with infection.2, 3, 5 One‐third of LS cases have coinfection with other oropharyngeal flora. Thus, one must reexamine the anaerobic blood cultures after an organism has been identified in suspect cases.3, 4
There is an increased association of LS with EBV infection, likely due to viral‐induced and steroid‐induced immunosuppression.24 False positive heterophile tests are reported with LS, so the specific antibody tests for EBV must be checked.3, 4
Once thrombophlebitis occurs, the bacteria can metastasize to distant sites. In 80% to 92% of LS cases, the metastatic complication is a pleuro‐pulmonary infection, consisting of septic pulmonary emboli, empyema, and pleural effusions, but extra‐pulmonary lesions occur.1, 3, 9, 12 Abdominal pain usually results from abdominal microabscesses or thrombophlebitis.4 Mild renal impairment and abnormal liver function tests are common.3, 4 Cranial nerve palsies and Horner's syndrome are rare and indicate carotid sheath involvement.3, 12 An elevated C‐reactive protein can distinguish bacterial from uncomplicated viral pharyngitis.3, 4 Also, rigors are unusual in tonsillitis, and their presence indicate bacterial entry into the circulation.3
CXRs may reveal the pulmonary septic emboli. Ultrasound of the IJV is inexpensive and noninvasive, but may have limited sensitivity for an acute thrombus. CT scan allows increased visualization of anatomy, but can have decreased sensitivity and specificity for thrombosis.3 Magnetic resonance imaging (MRI) is recommended if LS results from mastoiditis, to exclude an intracerebral vein thrombosis.9
Antibiotics have both dramatically decreased the incidence of LS and improved its prognosis. The recent rise in incidence may be due to a renewed interest in restricting the use of antibiotics in cases of pharyngitis, as well as an increased use of macrolides, to which F. necrophorum is frequently resistant.3 Decreased tonsillectomies may also have a role, as LS is more common with retained tonsils.1, 3
No trials have evaluated the optimal antibiotic regimen. Fusobacterium species are sensitive to penicillin, but 23% have beta‐lactamase activity as reported clinically by several authors.3, 5 F. necrophorum is also sensitive to metronidazole, ticarcillin‐clavulanate, cefoxitin, amoxicillin‐clavulanate, imipenem, and clindamycin. There is a high resistance to macrolides and gentamicin, and the activity of tetracyclines is poor. For treatment, most authors suggest a carbapenem, a penicillin/beta‐lactamase inhibitor combination, or metronidazole. Clindamycin has weaker bactericidal activity than metronidazole or imipenem. Metronidazole is preferred because of its activity against all Fusobacterium species, good penetration into tissues, bactericidal activity, low minimum inhibitory concentration, and ability to achieve high concentration in the cerebrospinal fluid if meningitis occurs. An effective regimen is metronidazole with a penicillinase‐resistant penicillin to cover for mixed coinfection with streptococci or staphylococci.3, 4, 12 A 6‐week antibiotic course is given for adequate penetration into the protective fibrin clots.4
Reports have shown good outcomes both with and without the use of anticoagulation.3, 4, 8 Support for anticoagulation is extrapolated from experience with septic pelvic thrombophlebitis, in which anticoagulation results in more rapid resolution of symptoms.13 Given the lack of firm evidence in cases of LS, anticoagulation is typically reserved for poor clinical response despite 2 to 3 days of antibiotic therapy or propagation of thromboses into the cavernous sinus. It is generally given for 3 months.4, 13
Prior to the antibiotic era, surgical ligation or excision of the IJV was done without clear benefit. Today, surgery is reserved for cases of continued septic emboli or extension of thrombus despite aggressive medical therapy.3 If mediastinitis develops, then surgical intervention is essential.4
Lemierre stated that the symptoms and signs of LS are so characteristic that it permits diagnosis before bacteriological examination.1 However, today it may go unrecognized by physicians until a blood culture shows anaerobes or Fusobacterium species. For a young patient admitted with pneumonia preceded by pharyngitis, hospitalists must remain vigilant for the presence of LS.
Key Points for Hospitalists/Teaching Points
-
The triad of LS is pharyngitis, thrombophlebitis, and distant metastatic pyogenic emboli.
-
Suspect LS in a young, otherwise healthy patient who clinically deteriorates in the setting of a recent pharyngeal infection.
-
With the modern decrease in antibiotic use for pharyngitis, LS may be on the rise.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring the patient and the discussant.
A 27‐year‐old woman with a history of asthma presented to her primary care physician (PCP) with a sore throat which began after attending a party where she shared alcoholic beverages with friends. She denied any high‐risk sexual behavior. Her PCP prescribed azithromycin and methylprednisolone empirically for tonsillitis. The throat pain subsided, but in the next several days she experienced increased weakness, lethargy, poor appetite, and chills, and she returned to her PCP for reevaluation.
Two months prior she had been treated at a walk‐in clinic with a course of penicillin for a presumed streptococcal pharyngitis. Her symptoms resolved until her current presentation.
In a young woman with 2 episodes of pharyngitis in 2 months followed by an acute systemic illness, one must consider an immunocompromised state such as human immunodeficiency virus (HIV), hematologic malignancy, or autoimmune diseases. Weakness, lethargy, anorexia, and chills in the setting of pharyngitis suggest a local process in the neck, most likely infection associated with systemic toxicity. As neck abscess and bacteremia warrant early consideration, the physical examination should focus on the neck and oropharynx, as well as neurologic exam to evaluate for bacterial spreading into the central nervous system. In addition to routine laboratory studies, a chest x‐ray (CXR) would be appropriate as upper respiratory infections may be complicated by pneumonia and present with signs and symptoms of systemic illness.
On examination by her PCP, her temperature was 99.2F and her blood pressure was 118/68. She had bilateral oropharyngeal erythema without exudates and bilateral tonsillar and anterior triangle lymphadenopathy (LAD). An oropharyngeal rapid Streptococcal antigen detection test was negative, but a Monospot test was positive for heterophile antibodies. Azithromycin and methylprednisolone were discontinued, and the patient was informed she most likely had Epstein Barr Virus (EBV) infection.
The following day, the complete blood count results returned. The platelet count was 50 K/L and the white blood cell (WBC) count was 13.0 K/L. The patient stated she had developed right‐sided flank pain upon deep inspiration and used her albuterol inhaler with minimal relief. She continued to have fever, decreased appetite, chest and abdominal pain, and difficulty swallowing due to odynophagia. She was instructed to go to the emergency department (ED) for further evaluation. In the ED, she denied any shortness of breath, but reported a slight cough and right‐sided abdominal pain.
Acute tonsillar pharyngitis and fever, as well as systemic symptoms of fatigue and abdominal pain along with positive heterophile screen are highly suggestive of EBV infection in this young female. The episode of pharyngitis 2 months prior remains unexplained and may be unrelated. Right‐sided pleuritic pain and abdominal pain may be related to EBV hepatitis. Odynophagia is consistent with EBV infection as well. Profound lethargy, however, is not a common presenting feature in mononucleosis unless infected patients are profoundly dehydrated due to inability to swallow. Her pain symptoms may be secondary to other signs of EBV infection, such as hepatomegaly, splenomegaly, ascites, and/or right pleural effusion. A history of rash should be investigated. Initial assessment in this acutely ill patient should focus on evaluation for the presence of severe sepsis and for a primary source of infection. Given the severity of her illness, I would consider early computed tomography (CT) of her chest, abdomen, and pelvis, as well as CT of the neck to exclude a possibility of peritonsillar abscess. The complaint of chills indicates a possible bacteremia, so coverage with broad‐spectrum antibiotics is indicated. Symptomatic relief with acetaminophen and intravenous fluid rehydration is appropriate.
On exam, temperature was 101.9F, blood pressure was 111/74, heart rate was 140 beats per minute, respiratory rate was 18 per minute, and oxygen saturation was 99% on room air. She appeared drowsy, but answered questions appropriately. She had bilateral swollen tonsils, as well as anterior and posterior cervical adenopathy, with tenderness greater on the left side. Her chest exam had slightly diminished breath sounds at the bases bilaterally. Heart rhythm was regular, and there were no murmurs appreciated. On abdominal exam, she was tender to palpation in both right‐upper and left‐upper quadrants, without obvious hepatosplenomegaly. There were no petechiae noted on her skin.
The WBC was 17.6 K/L, with 89% neutrophils and 5% lymphocytes, platelet count was 22 K/L, and hemoglobin was 13.8 g/dL. A D‐dimer test was elevated at 1344 ng/mL. Peripheral blood smear showed thrombocytopenia and neutrophilia, but demonstrated no schistocytes. The serum potassium was 3.2 mEq/L, bicarbonate was 29 mEq/L, blood urea nitrogen was 15 mg/dL and the creatinine was 1.29 mg/dL. Transaminases were within normal limits, but total bilirubin was 1.8 mg/dL. Her urinalysis was normal. Blood cultures were sent. A CXR showed bibasilar consolidations and pleural effusions (Figure 1). A CT of the chest with contrast was obtained that showed multiple confluent and patchy foci of consolidation in the lung bases, with trace bilateral pleural effusions (Figure 2). A CT of the abdomen showed a spleen at the upper limits of normal in size, measuring 13 cm in length, but was otherwise normal.


Leukocytosis with lymphopenia is not consistent with EBV infection and another process needs to be considered. This patient meets criteria for sepsis syndrome and should receive broad spectrum antibiotics, such as vancomycin and piperacillin‐tazobactam immediately after the blood cultures are sent, in addition to further evaluation to determine the source of sepsis. Depending on her mental status response to initial measures such as acetaminophen and hydration, one should consider a lumbar puncture, which would require platelet transfusion and may therefore not be done immediately. HIV serology should be performed, since acute retroviral syndrome can mimic this presentation. With neck tenderness that is more localized to her left side, a CT of her neck to evaluate for an abscess may be helpful.
She was admitted for presumed community‐acquired pneumonia complicating an upper respiratory tract infection. Her pharyngitis was thought to be of viral etiology. Moxifloxacin was started and intravenous fluids were administered. She was started on prednisone 60 mg daily for presumed immune‐mediated thrombocytopenia related to EBV infection. An HIV antibody test and quantitative polymerase chain reaction (PCR) were both negative. The EBV immunoglobulin G (IgG) titer was positive (>1:10), but the IgM titer was negative. Her mental status improved after starting moxifloxacin and fluids. Her creatinine and bilirubin normalized to 0.97 mg/dL and 0.8 mg/dL respectively. She continued to have a tender left‐sided submandibular swelling. Blood cultures grew Gram‐negative bacilli in 2 anaerobic bottles.
I am uncomfortable with moxifloxacin as initial empiric therapy because at presentation she had sepsis syndrome as well as a suspected immunocompromised state. In addition, moxifloxacin would not be adequate coverage for anaerobic organisms if a peritonsillar abscess was involved. At this point, she needs a CT of her neck to look for a focus of infection which may require surgical management and, if negative, further imaging such as a tagged white blood scan to identify the source of the anaerobes.
Moxifloxacin was switched to piperacillin‐tazobactam and prednisone was discontinued. By day 4 of hospitalization her platelet count had risen to 261 K/L. Her WBC continued to rise to a peak of 21.5 K/L and she continued to have fevers and diffuse pains, although her repeat blood cultures were negative. She continued to have tenderness of the cervical lymph nodes, left greater than right. A repeat CXR showed patchy air space disease bilaterally and pleural effusions, both of which had progressed compared with the prior film. Clindamycin was empirically added to her antibiotic regimen in light of her progressing pneumonia and evidence of anaerobic infection. A repeat CT scan of her chest revealed multiple nodular opacities scattered throughout the lung fields, some of which were cavitary, predominating in the lung bases. The CT scan of her neck revealed a left peritonsillar abscess and phlegmon in the left retropharyngeal and deep neck area along the sternocleidomastoid and internal jugular vein (IJV). There also was noted a large thrombus within the left IJV extending superiorly to involve the jugular bulb, sigmoid sinus, and distal left transverse sinus; and inferiorly to near the origin of the brachiocephalic vein (see Figure 3). An echocardiogram did not reveal any vegetations.

The combination of recent pharyngitis, septic pulmonary emboli, and IJV thrombosis is consistent with a diagnosis of Lemierre's syndrome (LS). This is a life threatening condition, even if diagnosis is made early and appropriate treatment is started. The most likely causative agent is Fusobacterium necrophorum. In this case it was important to realize that clinical presentation was not consistent with EBV infection, even though heterophile screen was positive. Early initiation of broad spectrum antibiotics as well as CT scan of the neck would have been appropriate.
The diagnosis of LS was made. The blood culture speciation revealed Fusobacterium nucleatum, which was too fastidious to perform antimicrobial sensitivities. Her symptoms improved significantly with the addition of clindamycin to piperacillin‐tazobactam, which was postulated to be the result of bacterial beta‐lactamase activity mitigating the efficacy of piperacillin‐tazobactam. Thoracentesis of her pleural effusion did not reveal an empyema. Due to her large thrombus burden, she was started on anticoagulation with heparin and transitioned to outpatient coumadin. She was switched to metronidazole as a single agent antibiotic for 6 weeks, and on outpatient follow‐up was doing well.
Commentary
LS was described by Dr. Andre Lemierre in 1936.6 The syndrome consists of a primary oropharyngeal infection, thrombosis of the IJV, bacteremia, and septic metastatic foci, usually involving the lungs.1, 2 LS is a form of necrobacillosis, which is a systemic infection resulting from F. necrophorum.3, 4 In classic LS, the initial pharyngitis is usually a tonsillar or peritonsillar abscess, and is followed by intense fever and rigors after 4 days to 2 weeks.1, 3 This is followed by a unilateral painful submaxillary LAD and IJV thrombophlebitisthe cord sign.2 Finally, bacteremia and distant metastatic pyogenic abscesses develop.1 (see Table 1).
| Lemierre's Syndrome typical features |
| Antecedent head and neck infection, typically an oropharyngeal infection prior to deterioration |
| Thrombophlebitis, typically of internal jugular vein (present in only 1/3 of cases) |
| Bacteremia (Fusobacterium necrophorum most commonly) |
| Septic metastatic foci, typically to lungs |
| Usual Presentation |
| Pharyngitis |
| Fevers |
| Rigors |
| Neck involvement: tenderness, swelling, tender internal jugular vein thrombus (cord sign) |
| Pulmonary infiltrates which cavitate |
With the advent of antibiotics, LS is now rare with an incidence of 0.9 per million persons per year. In Lemierre's time, the disease was fulminant and led to death within 2 weeks, but in the antibiotic age the mortality rate is 4.9%.1, 3 The median age of an LS patient is 19 years, with a higher incidence in males.1, 35 Although in the literature it is referred to as the forgotten disease, there is evidence the incidence is increasing.3, 4, 6, 8
There are variations of classic LS. Bacteremia may occur much later than the initial pharyngitis, the disease may be less aggressive, the thrombus may be in the external jugular vein, or there may be no identified thrombus.3, 4, 8 In fact, a thrombus is only identified in 36% of cases.9 The primary infection may be a head and neck infection that is not pharyngitis, such as an odontogenic infection,4 or may not be identified.10 Despite variations, the fundamentals of diagnosis are prior head and neck infection, presumed thrombophlebitis and bacteremia, and evidence of septic metastatic foci.
The genus Fusobacterium comprises anaerobic, nonspore forming gram negative bacilli.1, 35, 11 F. necrophorum and F. nucleatum are 2 species within this genus. F. nucleatum causes the majority of reported human bacteremias by Fusobacterium species, but it is F. necrophorum that is most associated with anaerobic oropharyngeal infections, thrombocytopenia, clot formation, and LS.35, 8, 9
It is unknown if Fusobacterium species directly cause the sore throat, or rather are bystanders which thrive once a favorable anaerobic environment is created via endotoxins and exotoxins.35 A break in oral mucosa via trauma or coinfection with bacteria/viruses (especially EBV) is also thought to play a role with infection.2, 3, 5 One‐third of LS cases have coinfection with other oropharyngeal flora. Thus, one must reexamine the anaerobic blood cultures after an organism has been identified in suspect cases.3, 4
There is an increased association of LS with EBV infection, likely due to viral‐induced and steroid‐induced immunosuppression.24 False positive heterophile tests are reported with LS, so the specific antibody tests for EBV must be checked.3, 4
Once thrombophlebitis occurs, the bacteria can metastasize to distant sites. In 80% to 92% of LS cases, the metastatic complication is a pleuro‐pulmonary infection, consisting of septic pulmonary emboli, empyema, and pleural effusions, but extra‐pulmonary lesions occur.1, 3, 9, 12 Abdominal pain usually results from abdominal microabscesses or thrombophlebitis.4 Mild renal impairment and abnormal liver function tests are common.3, 4 Cranial nerve palsies and Horner's syndrome are rare and indicate carotid sheath involvement.3, 12 An elevated C‐reactive protein can distinguish bacterial from uncomplicated viral pharyngitis.3, 4 Also, rigors are unusual in tonsillitis, and their presence indicate bacterial entry into the circulation.3
CXRs may reveal the pulmonary septic emboli. Ultrasound of the IJV is inexpensive and noninvasive, but may have limited sensitivity for an acute thrombus. CT scan allows increased visualization of anatomy, but can have decreased sensitivity and specificity for thrombosis.3 Magnetic resonance imaging (MRI) is recommended if LS results from mastoiditis, to exclude an intracerebral vein thrombosis.9
Antibiotics have both dramatically decreased the incidence of LS and improved its prognosis. The recent rise in incidence may be due to a renewed interest in restricting the use of antibiotics in cases of pharyngitis, as well as an increased use of macrolides, to which F. necrophorum is frequently resistant.3 Decreased tonsillectomies may also have a role, as LS is more common with retained tonsils.1, 3
No trials have evaluated the optimal antibiotic regimen. Fusobacterium species are sensitive to penicillin, but 23% have beta‐lactamase activity as reported clinically by several authors.3, 5 F. necrophorum is also sensitive to metronidazole, ticarcillin‐clavulanate, cefoxitin, amoxicillin‐clavulanate, imipenem, and clindamycin. There is a high resistance to macrolides and gentamicin, and the activity of tetracyclines is poor. For treatment, most authors suggest a carbapenem, a penicillin/beta‐lactamase inhibitor combination, or metronidazole. Clindamycin has weaker bactericidal activity than metronidazole or imipenem. Metronidazole is preferred because of its activity against all Fusobacterium species, good penetration into tissues, bactericidal activity, low minimum inhibitory concentration, and ability to achieve high concentration in the cerebrospinal fluid if meningitis occurs. An effective regimen is metronidazole with a penicillinase‐resistant penicillin to cover for mixed coinfection with streptococci or staphylococci.3, 4, 12 A 6‐week antibiotic course is given for adequate penetration into the protective fibrin clots.4
Reports have shown good outcomes both with and without the use of anticoagulation.3, 4, 8 Support for anticoagulation is extrapolated from experience with septic pelvic thrombophlebitis, in which anticoagulation results in more rapid resolution of symptoms.13 Given the lack of firm evidence in cases of LS, anticoagulation is typically reserved for poor clinical response despite 2 to 3 days of antibiotic therapy or propagation of thromboses into the cavernous sinus. It is generally given for 3 months.4, 13
Prior to the antibiotic era, surgical ligation or excision of the IJV was done without clear benefit. Today, surgery is reserved for cases of continued septic emboli or extension of thrombus despite aggressive medical therapy.3 If mediastinitis develops, then surgical intervention is essential.4
Lemierre stated that the symptoms and signs of LS are so characteristic that it permits diagnosis before bacteriological examination.1 However, today it may go unrecognized by physicians until a blood culture shows anaerobes or Fusobacterium species. For a young patient admitted with pneumonia preceded by pharyngitis, hospitalists must remain vigilant for the presence of LS.
Key Points for Hospitalists/Teaching Points
-
The triad of LS is pharyngitis, thrombophlebitis, and distant metastatic pyogenic emboli.
-
Suspect LS in a young, otherwise healthy patient who clinically deteriorates in the setting of a recent pharyngeal infection.
-
With the modern decrease in antibiotic use for pharyngitis, LS may be on the rise.
- .On certain septicaemias due to anaerobic organisms.Lancet.1936;1:701–703.
- ,.Lemierre's syndrome: more judicious antibiotic prescribing habits may lead to the clinical reappearance of this often forgotten disease.Am J Med.2006;119(3):e7–e9.
- .Human infection with Fusobacterium necrophorum (necrobacillosis), with a focus on Lemierre's syndrome.Clin Microbiol Rev.2007;20(4):622–659.
- ,.Human necrobacillosis, with emphasis on Lemierre's syndrome.Clin Infect Dis.2000;31(2):524–532.
- ,.Fusobacterial infections: clinical spectrum and incidence of invasive disease.J Infect.2008;57(4):283–289.
- .Human infections with Fusobacterium necrophorum.Anaerobe.2006;12(4):165–172.
- ,,, et al.Increased diagnosis of Lemierre Syndrome and other Fusobacterium necrophorum infections at a Children's Hospital.Pediatrics.2003;112(5):e380.
- ,,.Unusual presentation of Lemierre's syndrome due to Fusobacterium nucleatum.J Clin Microbiol.2003;41(7):3445–3448.
- ,,,.The evolution of Lemierre Syndrome: report of 2 cases and review of the literature.Medicine (Baltimore).2002;81(6):458–465.
- ,.An unusual case of Lemierre's syndrome presenting as pyomyositis.Am J Med Sci.2008;335(6):499–501.
- .Update on the taxonomy and clinical aspects of the genus Fusobacterium.Clin Infect Dis.2002;35(Suppl 1):S22–S27.
- ,,,,.Lemierre syndrome: two cases and a review.Laryngosope.2007;117(9):1605–1610.
- ,,.Lemierre's syndrome (necrobacillosis).Postgrad Med J.1999;75(881):141–144.
- .On certain septicaemias due to anaerobic organisms.Lancet.1936;1:701–703.
- ,.Lemierre's syndrome: more judicious antibiotic prescribing habits may lead to the clinical reappearance of this often forgotten disease.Am J Med.2006;119(3):e7–e9.
- .Human infection with Fusobacterium necrophorum (necrobacillosis), with a focus on Lemierre's syndrome.Clin Microbiol Rev.2007;20(4):622–659.
- ,.Human necrobacillosis, with emphasis on Lemierre's syndrome.Clin Infect Dis.2000;31(2):524–532.
- ,.Fusobacterial infections: clinical spectrum and incidence of invasive disease.J Infect.2008;57(4):283–289.
- .Human infections with Fusobacterium necrophorum.Anaerobe.2006;12(4):165–172.
- ,,, et al.Increased diagnosis of Lemierre Syndrome and other Fusobacterium necrophorum infections at a Children's Hospital.Pediatrics.2003;112(5):e380.
- ,,.Unusual presentation of Lemierre's syndrome due to Fusobacterium nucleatum.J Clin Microbiol.2003;41(7):3445–3448.
- ,,,.The evolution of Lemierre Syndrome: report of 2 cases and review of the literature.Medicine (Baltimore).2002;81(6):458–465.
- ,.An unusual case of Lemierre's syndrome presenting as pyomyositis.Am J Med Sci.2008;335(6):499–501.
- .Update on the taxonomy and clinical aspects of the genus Fusobacterium.Clin Infect Dis.2002;35(Suppl 1):S22–S27.
- ,,,,.Lemierre syndrome: two cases and a review.Laryngosope.2007;117(9):1605–1610.
- ,,.Lemierre's syndrome (necrobacillosis).Postgrad Med J.1999;75(881):141–144.
In the Literature: HM-Related Research You Need to Know
In This Edition
Literature at a Glance
A guide to this month’s studies
- Effect of early follow-up on readmission rates
- Heart rate control and outcomes in atrial fibrillation
- Pneumococcal vaccine to prevent stroke and MI
- Long-term outcomes of endovascular repair of AAA
- Insurance and outcomes in myocardial infarction
- Risk of gastrointestinal bleeding and cardiovascular outcomes with concurrent PPI and clopidogrel use
- CT in patients with suspected coronary artery disease
Reduced 30-Day Readmission Rate for Patients Discharged from Hospitals with Higher Rates of Early Follow-Up
Clinical question: Is early follow-up after discharge for heart failure associated with a reduction in readmission rates?
Background: Readmission for heart failure is very frequent and often unplanned. Early follow-up visits after discharge have been hypothesized to reduce readmissions but have been undefined.
Study design: Retrospective cohort study.
Setting: Patients with Medicare inpatient claims data linked to the OPTIMIZE-HF and GWTG-HF registries.
Synopsis: The study included 30,136 patients >65 years old with the principal discharge diagnosis of heart failure from 2003 to 2006. Hospitals were stratified into quartiles based upon the median arrival rate to “early” (within one week after discharge) follow-up appointments. Ranges of arrival rates to these appointments ranged from Quartile 1 (Q1) (<32.4% of patients) to Q4 (>44.5%). Readmission rates were highest in the lowest quartile of “early” follow-up (Q1: 23.3%; Q2: 20.5%; Q3: 20.5%; Q4: 20.5%, P<0.001). No mortality difference was seen.
The study also examined whether the physician following the patient after discharge impacted the readmission rate for these same quartiles, comparing cardiologists to generalists and comparing the same physician at discharge and follow-up (defined as “continuity”) versus different physicians. Follow-up with continuity or a cardiologist did not reduce readmissions.
Interestingly, nearly all markers of quality were best in Q1 and Q2 hospitals, which had the lowest arrival rates to appointments, which might reflect patient-centered rather than hospital-centered issues.
Bottom line: Hospitals with low “early” follow-up appointment rates after discharge have a higher readmission rate, although causality is not established.
Citation: Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303 (17):1716-1722.
Strict Heart Rate Control Is Not Necessary in Management of Chronic Atrial Fibrillation
Clinical question: Is lenient heart rate control inferior to strict heart rate control in preventing cardiovascular events in patients with chronic atrial fibrillation?
Background: Guidelines generally call for the use of medications to achieve strict heart rate control in the management of chronic atrial fibrillation, but the optimal level of heart rate control necessary to avoid cardiovascular events remains uncertain.
Study design: Prospectively randomized, noninferiority trial.
Setting: Thirty-three medical centers in the Netherlands.
Synopsis: The study looked at 614 patients with permanent atrial fibrillation; 311 patients were randomized to lenient control and 303 to strict control. Calcium channel blockers, beta-blockers, or digoxin were dose-adjusted to control heart rate below 110 beats per minute (bpm) in the lenient control group versus 80 bpm in the strict control group.
Thirty-eight patients (12.9%) in the lenient control group and 43 (14.9%) in the strict control group reached the primary composite outcome of significant cardiovascular events (death, heart failure, stroke, embolism, major bleeding, major arrhythmia, need for pacemaker, or severe drug adverse event). Although no statistical difference in the frequency of these events between groups was detected, the study was dramatically underpowered due to unanticipated low event rates.
Bottom line: Although the lenient control group had far fewer outpatient visits and a trend toward improved outcomes, no definite conclusion regarding the management of permanent atrial fibrillation can be drawn from this underpowered noninferiority trial.
Citation: Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362(15):1363-1373.
Pneumococcal Vaccine Does Not Reduce the Risk of Stroke or Myocardial Infarction
Clinical question: Does pneumococcal vaccination reduce the risk of acute myocardial infarction (MI) and stroke?
Background: Studies have demonstrated that influenza vaccination reduces the risk of cardiac and cerebrovascular events. A single study has shown similar outcomes for the pneumococcal vaccination, although the study was limited by confounders and selection bias.
Study design: Retrospective cohort.
Setting: Large HMO in California.
Synopsis: More than 84,000 men participating in the California Men’s Health Study (CMHS) and enrolled in the Kaiser Permanente health plan were categorized as unvaccinated or vaccinated with the pneumococcal vaccine. Vaccinated patients had 10.73 first MIs and 5.30 strokes per 1,000 person-years, compared with unvaccinated patients who incurred 6.07 MIs and 1.90 strokes per 1,000 unvaccinated person-years based on ICD-9 codes.
Even with propensity scoring to minimize selection bias, no clear evidence of benefit was observed. One significant limitation is that 80% of the unvaccinated patients were younger than 60 years old, whereas 74% of the vaccinated patients were 60 or older; this might represent selection bias that cannot be overcome with propensity scoring.
Bottom line: In a population of men older than age 45, pneumococcal vaccination does not appear to reduce the risk of acute MI or stroke.
Citation: Tseng HF, Slezak JM, Quinn VP, et al. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA. 2010;303(17):1699-1706.
No Durable Mortality Benefit from Endovascular Repair of Enlarged Abdominal Aortic Aneurysm
Clinical question: What is the cost and mortality benefit of endovascular versus open repair of abdominal aneurysms?
Background: Previous studies demonstrated a 30-day mortality benefit using endovascular repair over open surgical repair of large abdominal aortic aneurysms. Limited longer-term data are available assessing the durability of these findings.
Study design: Randomized controlled trial.
Setting: Thirty-seven hospitals in the United Kingdom.
Synopsis: Researchers looked at 1,252 patients who were at least 60 years old with a large abdominal aortic aneurysm (>5.5 cm) on CT scan. The patients were randomized to open versus endovascular repair and followed for a median of six years postoperatively. An early, postoperative, all-cause mortality benefit was observed for endovascular repair (1.8%) compared with open repair (4.3%), but no benefit was seen after six months of follow-up, driven by secondary aneurysm ruptures with endovascular grafts. Graft-related complications in all time periods were higher in the endovascular repair group, highest from 0 to 6 months (nearly 50% of patients), and were associated with an increased cost.
Bottom line: Immediate postoperative mortality benefit of endovascular repair is not sustained for abdominal aortic aneurysm beyond six months postoperatively.
Citation: The United Kingdom EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010:362(20):1863-1870.
Financial Constraints Delay Presentation in Patients Suffering from Acute Myocardial Infarction
Clinical question: Does being underinsured or uninsured delay individuals from seeking treatment for emergency medical care?
Background: The number of underinsured or uninsured Americans is growing. Studies have shown that patients with financial concerns avoid routine preventive and chronic medical care; however, similar avoidance has not been defined clearly for patients seeking emergent care.
Study design: Prospective cohort study.
Setting: Twenty-four urban hospitals in the U.S. included in a multisite, acute myocardial infarction (AMI) registry.
Synopsis: Of the 3,721 patients enrolled in the AMI registry, 61.7% of the cohort was insured and without financial concerns that prevented them from seeking care. These patients were less likely to have delays in care related to AMI compared with patients who were insured with financial concerns (18.5% of the cohort; OR 1.22; 95% confidence interval [CI], 1.06-1.40) or uninsured (19.8%; OR 1.30; 95% CI, 1.12-1.51) in all time frames after symptom onset. Patients were less likely to undergo PCI or thrombolysis if the delay to presentation was more than six hours.
After adjustment for confounding factors, the authors concluded that uninsured and underinsured patients were likely to delay presentation to the hospital. Despite these findings, alternative etiologies for delays in care are likely to be more significant, as insurance considerations only account for an 8% difference between the well-insured group (39.3% delayed seeking care >6 hours) and the uninsured group (48.6%). These etiologies are ill-defined.
Bottom line: Underinsured or uninsured patients have a small but significant delay in seeking treatment for AMI due to financial concerns.
Citation: Smolderen KG, Spertus JA, Nallamothu BK, et al. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. 2010;303 (14):1392-1400.
Concurrent Use of PPIs and Clopidogrel Decrease Hospitalizations for Gastroduodenal Bleeding without Significant Increase in Adverse Cardiovascular Events
Clinical question: Does concomitant use of proton-pump inhibitors (PPIs) and clopidogrel affect the risks of hospitalizations for gastroduodenal bleeding and serious cardiovascular events?
Background: PPIs commonly are prescribed with clopidogrel to reduce the risk of serious gastroduodenal bleeding. Recent observational studies suggest that concurrent PPI and clopidogrel administration might increase the risk of cardiovascular events compared with clopidogrel alone.
Study design: Retrospective cohort.
Setting: Tennessee Medicaid program.
Synopsis: Researchers identified 20,596 patients hospitalized for acute MI, revascularization, or unstable angina, and prescribed clopidogrel. Of this cohort, 7,593 were initial concurrent PPI users—62% used pantoprazole and 9% used omeprazole. Hospitalizations for gastroduodenal bleeding were reduced by 50% (HR 0.50 [95% CI, 0.39-0.65]) in concurrent users of PPIs and clopidogrel, compared with nonusers of PPIs.
Concurrent use was not associated with a statistically significant increase in serious cardiovascular diseases (HR, 0.99 [95% CI, 0.82-1.19]), defined as acute MI, sudden cardiac death, nonfatal or fatal stroke, or other cardiovascular deaths.
Subgroup analyses of individual PPIs and patients undergoing percutaneous coronary interventions also showed no increased risk of serious cardiovascular events. This study could differ from previous observational studies because far fewer patients were on omeprazole, the most potent inhibitor of clopidogrel.
Bottom line: In patients treated with clopidogrel, PPI users had 50% fewer hospitalizations for gastroduodenal bleeding compared with nonusers. Concurrent use of clopidogrel and PPIs, most of which was pantoprazole, was not associated with a significant increase in serious cardiovascular events.
Citation: Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors. Ann Intern Med. 2010;152(6):337-345.
CTCA a Promising, Noninvasive Option in Evaluating Patients with Suspected Coronary Artery Disease
Clinical question: How does computed tomography coronary angiography (CTCA) compare to noninvasive stress testing for diagnosing coronary artery disease (CAD)?
Background: CTCA is a newer, noninvasive test that has a high diagnostic accuracy for CAD, but its clinical role in the evaluation of patients with chest symptoms is unclear.
Study design: Observational study.
Setting: Single academic center in the Netherlands.
Synopsis: Five hundred seventeen eligible patients were evaluated with stress testing and CTCA. The patients were classified as having a low (<20%), intermediate (20%-80%), or high (>80%) pretest probability of CAD based on the Duke clinical score. Using coronary angiography as the gold standard, stress-testing was found to be less accurate than CTCA in all of the patient groups. In patients with low and intermediate pretest probabilities, a negative CTCA had a post-test probability of 0% and 1%, respectively. On the other hand, patients with an intermediate pretest probability and a positive CTCA had a post-test probability of 94% (CI, 89%-97%). In patients with an initial high pretest probability, stress-testing and CTCA confirmed disease in most cases.
The results of this study suggest that CTCA is particularly useful in evaluating patients with an intermediate pretest probability.
Patients were ineligible in this study if they had acute coronary syndromes, previous coronary stent placement, coronary artery bypass surgery, or myocardial infarction. It is important to note that because anatomic lesions seen on imaging (CTCA and coronary angiography) are not always functionally significant, CTCA might have seemed more accurate and clinically useful than it actually is. The investigators also acknowledge that further studies are necessary before CTCA can be accepted as a first-line diagnostic test.
Bottom line: In patients with an intermediate pretest probability of CAD, a negative CTCA is valuable in excluding coronary artery disease, thereby reducing the need for invasive coronary angiography in this group.
Citation: Weustink AC, Mollet NR, Neefjes LA, et al. Diagnostic accuracy and clinical utility of noninvasive testing for coronary artery disease. Ann Intern Med. 2010;152(10):630-639. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Effect of early follow-up on readmission rates
- Heart rate control and outcomes in atrial fibrillation
- Pneumococcal vaccine to prevent stroke and MI
- Long-term outcomes of endovascular repair of AAA
- Insurance and outcomes in myocardial infarction
- Risk of gastrointestinal bleeding and cardiovascular outcomes with concurrent PPI and clopidogrel use
- CT in patients with suspected coronary artery disease
Reduced 30-Day Readmission Rate for Patients Discharged from Hospitals with Higher Rates of Early Follow-Up
Clinical question: Is early follow-up after discharge for heart failure associated with a reduction in readmission rates?
Background: Readmission for heart failure is very frequent and often unplanned. Early follow-up visits after discharge have been hypothesized to reduce readmissions but have been undefined.
Study design: Retrospective cohort study.
Setting: Patients with Medicare inpatient claims data linked to the OPTIMIZE-HF and GWTG-HF registries.
Synopsis: The study included 30,136 patients >65 years old with the principal discharge diagnosis of heart failure from 2003 to 2006. Hospitals were stratified into quartiles based upon the median arrival rate to “early” (within one week after discharge) follow-up appointments. Ranges of arrival rates to these appointments ranged from Quartile 1 (Q1) (<32.4% of patients) to Q4 (>44.5%). Readmission rates were highest in the lowest quartile of “early” follow-up (Q1: 23.3%; Q2: 20.5%; Q3: 20.5%; Q4: 20.5%, P<0.001). No mortality difference was seen.
The study also examined whether the physician following the patient after discharge impacted the readmission rate for these same quartiles, comparing cardiologists to generalists and comparing the same physician at discharge and follow-up (defined as “continuity”) versus different physicians. Follow-up with continuity or a cardiologist did not reduce readmissions.
Interestingly, nearly all markers of quality were best in Q1 and Q2 hospitals, which had the lowest arrival rates to appointments, which might reflect patient-centered rather than hospital-centered issues.
Bottom line: Hospitals with low “early” follow-up appointment rates after discharge have a higher readmission rate, although causality is not established.
Citation: Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303 (17):1716-1722.
Strict Heart Rate Control Is Not Necessary in Management of Chronic Atrial Fibrillation
Clinical question: Is lenient heart rate control inferior to strict heart rate control in preventing cardiovascular events in patients with chronic atrial fibrillation?
Background: Guidelines generally call for the use of medications to achieve strict heart rate control in the management of chronic atrial fibrillation, but the optimal level of heart rate control necessary to avoid cardiovascular events remains uncertain.
Study design: Prospectively randomized, noninferiority trial.
Setting: Thirty-three medical centers in the Netherlands.
Synopsis: The study looked at 614 patients with permanent atrial fibrillation; 311 patients were randomized to lenient control and 303 to strict control. Calcium channel blockers, beta-blockers, or digoxin were dose-adjusted to control heart rate below 110 beats per minute (bpm) in the lenient control group versus 80 bpm in the strict control group.
Thirty-eight patients (12.9%) in the lenient control group and 43 (14.9%) in the strict control group reached the primary composite outcome of significant cardiovascular events (death, heart failure, stroke, embolism, major bleeding, major arrhythmia, need for pacemaker, or severe drug adverse event). Although no statistical difference in the frequency of these events between groups was detected, the study was dramatically underpowered due to unanticipated low event rates.
Bottom line: Although the lenient control group had far fewer outpatient visits and a trend toward improved outcomes, no definite conclusion regarding the management of permanent atrial fibrillation can be drawn from this underpowered noninferiority trial.
Citation: Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362(15):1363-1373.
Pneumococcal Vaccine Does Not Reduce the Risk of Stroke or Myocardial Infarction
Clinical question: Does pneumococcal vaccination reduce the risk of acute myocardial infarction (MI) and stroke?
Background: Studies have demonstrated that influenza vaccination reduces the risk of cardiac and cerebrovascular events. A single study has shown similar outcomes for the pneumococcal vaccination, although the study was limited by confounders and selection bias.
Study design: Retrospective cohort.
Setting: Large HMO in California.
Synopsis: More than 84,000 men participating in the California Men’s Health Study (CMHS) and enrolled in the Kaiser Permanente health plan were categorized as unvaccinated or vaccinated with the pneumococcal vaccine. Vaccinated patients had 10.73 first MIs and 5.30 strokes per 1,000 person-years, compared with unvaccinated patients who incurred 6.07 MIs and 1.90 strokes per 1,000 unvaccinated person-years based on ICD-9 codes.
Even with propensity scoring to minimize selection bias, no clear evidence of benefit was observed. One significant limitation is that 80% of the unvaccinated patients were younger than 60 years old, whereas 74% of the vaccinated patients were 60 or older; this might represent selection bias that cannot be overcome with propensity scoring.
Bottom line: In a population of men older than age 45, pneumococcal vaccination does not appear to reduce the risk of acute MI or stroke.
Citation: Tseng HF, Slezak JM, Quinn VP, et al. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA. 2010;303(17):1699-1706.
No Durable Mortality Benefit from Endovascular Repair of Enlarged Abdominal Aortic Aneurysm
Clinical question: What is the cost and mortality benefit of endovascular versus open repair of abdominal aneurysms?
Background: Previous studies demonstrated a 30-day mortality benefit using endovascular repair over open surgical repair of large abdominal aortic aneurysms. Limited longer-term data are available assessing the durability of these findings.
Study design: Randomized controlled trial.
Setting: Thirty-seven hospitals in the United Kingdom.
Synopsis: Researchers looked at 1,252 patients who were at least 60 years old with a large abdominal aortic aneurysm (>5.5 cm) on CT scan. The patients were randomized to open versus endovascular repair and followed for a median of six years postoperatively. An early, postoperative, all-cause mortality benefit was observed for endovascular repair (1.8%) compared with open repair (4.3%), but no benefit was seen after six months of follow-up, driven by secondary aneurysm ruptures with endovascular grafts. Graft-related complications in all time periods were higher in the endovascular repair group, highest from 0 to 6 months (nearly 50% of patients), and were associated with an increased cost.
Bottom line: Immediate postoperative mortality benefit of endovascular repair is not sustained for abdominal aortic aneurysm beyond six months postoperatively.
Citation: The United Kingdom EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010:362(20):1863-1870.
Financial Constraints Delay Presentation in Patients Suffering from Acute Myocardial Infarction
Clinical question: Does being underinsured or uninsured delay individuals from seeking treatment for emergency medical care?
Background: The number of underinsured or uninsured Americans is growing. Studies have shown that patients with financial concerns avoid routine preventive and chronic medical care; however, similar avoidance has not been defined clearly for patients seeking emergent care.
Study design: Prospective cohort study.
Setting: Twenty-four urban hospitals in the U.S. included in a multisite, acute myocardial infarction (AMI) registry.
Synopsis: Of the 3,721 patients enrolled in the AMI registry, 61.7% of the cohort was insured and without financial concerns that prevented them from seeking care. These patients were less likely to have delays in care related to AMI compared with patients who were insured with financial concerns (18.5% of the cohort; OR 1.22; 95% confidence interval [CI], 1.06-1.40) or uninsured (19.8%; OR 1.30; 95% CI, 1.12-1.51) in all time frames after symptom onset. Patients were less likely to undergo PCI or thrombolysis if the delay to presentation was more than six hours.
After adjustment for confounding factors, the authors concluded that uninsured and underinsured patients were likely to delay presentation to the hospital. Despite these findings, alternative etiologies for delays in care are likely to be more significant, as insurance considerations only account for an 8% difference between the well-insured group (39.3% delayed seeking care >6 hours) and the uninsured group (48.6%). These etiologies are ill-defined.
Bottom line: Underinsured or uninsured patients have a small but significant delay in seeking treatment for AMI due to financial concerns.
Citation: Smolderen KG, Spertus JA, Nallamothu BK, et al. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. 2010;303 (14):1392-1400.
Concurrent Use of PPIs and Clopidogrel Decrease Hospitalizations for Gastroduodenal Bleeding without Significant Increase in Adverse Cardiovascular Events
Clinical question: Does concomitant use of proton-pump inhibitors (PPIs) and clopidogrel affect the risks of hospitalizations for gastroduodenal bleeding and serious cardiovascular events?
Background: PPIs commonly are prescribed with clopidogrel to reduce the risk of serious gastroduodenal bleeding. Recent observational studies suggest that concurrent PPI and clopidogrel administration might increase the risk of cardiovascular events compared with clopidogrel alone.
Study design: Retrospective cohort.
Setting: Tennessee Medicaid program.
Synopsis: Researchers identified 20,596 patients hospitalized for acute MI, revascularization, or unstable angina, and prescribed clopidogrel. Of this cohort, 7,593 were initial concurrent PPI users—62% used pantoprazole and 9% used omeprazole. Hospitalizations for gastroduodenal bleeding were reduced by 50% (HR 0.50 [95% CI, 0.39-0.65]) in concurrent users of PPIs and clopidogrel, compared with nonusers of PPIs.
Concurrent use was not associated with a statistically significant increase in serious cardiovascular diseases (HR, 0.99 [95% CI, 0.82-1.19]), defined as acute MI, sudden cardiac death, nonfatal or fatal stroke, or other cardiovascular deaths.
Subgroup analyses of individual PPIs and patients undergoing percutaneous coronary interventions also showed no increased risk of serious cardiovascular events. This study could differ from previous observational studies because far fewer patients were on omeprazole, the most potent inhibitor of clopidogrel.
Bottom line: In patients treated with clopidogrel, PPI users had 50% fewer hospitalizations for gastroduodenal bleeding compared with nonusers. Concurrent use of clopidogrel and PPIs, most of which was pantoprazole, was not associated with a significant increase in serious cardiovascular events.
Citation: Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors. Ann Intern Med. 2010;152(6):337-345.
CTCA a Promising, Noninvasive Option in Evaluating Patients with Suspected Coronary Artery Disease
Clinical question: How does computed tomography coronary angiography (CTCA) compare to noninvasive stress testing for diagnosing coronary artery disease (CAD)?
Background: CTCA is a newer, noninvasive test that has a high diagnostic accuracy for CAD, but its clinical role in the evaluation of patients with chest symptoms is unclear.
Study design: Observational study.
Setting: Single academic center in the Netherlands.
Synopsis: Five hundred seventeen eligible patients were evaluated with stress testing and CTCA. The patients were classified as having a low (<20%), intermediate (20%-80%), or high (>80%) pretest probability of CAD based on the Duke clinical score. Using coronary angiography as the gold standard, stress-testing was found to be less accurate than CTCA in all of the patient groups. In patients with low and intermediate pretest probabilities, a negative CTCA had a post-test probability of 0% and 1%, respectively. On the other hand, patients with an intermediate pretest probability and a positive CTCA had a post-test probability of 94% (CI, 89%-97%). In patients with an initial high pretest probability, stress-testing and CTCA confirmed disease in most cases.
The results of this study suggest that CTCA is particularly useful in evaluating patients with an intermediate pretest probability.
Patients were ineligible in this study if they had acute coronary syndromes, previous coronary stent placement, coronary artery bypass surgery, or myocardial infarction. It is important to note that because anatomic lesions seen on imaging (CTCA and coronary angiography) are not always functionally significant, CTCA might have seemed more accurate and clinically useful than it actually is. The investigators also acknowledge that further studies are necessary before CTCA can be accepted as a first-line diagnostic test.
Bottom line: In patients with an intermediate pretest probability of CAD, a negative CTCA is valuable in excluding coronary artery disease, thereby reducing the need for invasive coronary angiography in this group.
Citation: Weustink AC, Mollet NR, Neefjes LA, et al. Diagnostic accuracy and clinical utility of noninvasive testing for coronary artery disease. Ann Intern Med. 2010;152(10):630-639. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Effect of early follow-up on readmission rates
- Heart rate control and outcomes in atrial fibrillation
- Pneumococcal vaccine to prevent stroke and MI
- Long-term outcomes of endovascular repair of AAA
- Insurance and outcomes in myocardial infarction
- Risk of gastrointestinal bleeding and cardiovascular outcomes with concurrent PPI and clopidogrel use
- CT in patients with suspected coronary artery disease
Reduced 30-Day Readmission Rate for Patients Discharged from Hospitals with Higher Rates of Early Follow-Up
Clinical question: Is early follow-up after discharge for heart failure associated with a reduction in readmission rates?
Background: Readmission for heart failure is very frequent and often unplanned. Early follow-up visits after discharge have been hypothesized to reduce readmissions but have been undefined.
Study design: Retrospective cohort study.
Setting: Patients with Medicare inpatient claims data linked to the OPTIMIZE-HF and GWTG-HF registries.
Synopsis: The study included 30,136 patients >65 years old with the principal discharge diagnosis of heart failure from 2003 to 2006. Hospitals were stratified into quartiles based upon the median arrival rate to “early” (within one week after discharge) follow-up appointments. Ranges of arrival rates to these appointments ranged from Quartile 1 (Q1) (<32.4% of patients) to Q4 (>44.5%). Readmission rates were highest in the lowest quartile of “early” follow-up (Q1: 23.3%; Q2: 20.5%; Q3: 20.5%; Q4: 20.5%, P<0.001). No mortality difference was seen.
The study also examined whether the physician following the patient after discharge impacted the readmission rate for these same quartiles, comparing cardiologists to generalists and comparing the same physician at discharge and follow-up (defined as “continuity”) versus different physicians. Follow-up with continuity or a cardiologist did not reduce readmissions.
Interestingly, nearly all markers of quality were best in Q1 and Q2 hospitals, which had the lowest arrival rates to appointments, which might reflect patient-centered rather than hospital-centered issues.
Bottom line: Hospitals with low “early” follow-up appointment rates after discharge have a higher readmission rate, although causality is not established.
Citation: Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303 (17):1716-1722.
Strict Heart Rate Control Is Not Necessary in Management of Chronic Atrial Fibrillation
Clinical question: Is lenient heart rate control inferior to strict heart rate control in preventing cardiovascular events in patients with chronic atrial fibrillation?
Background: Guidelines generally call for the use of medications to achieve strict heart rate control in the management of chronic atrial fibrillation, but the optimal level of heart rate control necessary to avoid cardiovascular events remains uncertain.
Study design: Prospectively randomized, noninferiority trial.
Setting: Thirty-three medical centers in the Netherlands.
Synopsis: The study looked at 614 patients with permanent atrial fibrillation; 311 patients were randomized to lenient control and 303 to strict control. Calcium channel blockers, beta-blockers, or digoxin were dose-adjusted to control heart rate below 110 beats per minute (bpm) in the lenient control group versus 80 bpm in the strict control group.
Thirty-eight patients (12.9%) in the lenient control group and 43 (14.9%) in the strict control group reached the primary composite outcome of significant cardiovascular events (death, heart failure, stroke, embolism, major bleeding, major arrhythmia, need for pacemaker, or severe drug adverse event). Although no statistical difference in the frequency of these events between groups was detected, the study was dramatically underpowered due to unanticipated low event rates.
Bottom line: Although the lenient control group had far fewer outpatient visits and a trend toward improved outcomes, no definite conclusion regarding the management of permanent atrial fibrillation can be drawn from this underpowered noninferiority trial.
Citation: Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362(15):1363-1373.
Pneumococcal Vaccine Does Not Reduce the Risk of Stroke or Myocardial Infarction
Clinical question: Does pneumococcal vaccination reduce the risk of acute myocardial infarction (MI) and stroke?
Background: Studies have demonstrated that influenza vaccination reduces the risk of cardiac and cerebrovascular events. A single study has shown similar outcomes for the pneumococcal vaccination, although the study was limited by confounders and selection bias.
Study design: Retrospective cohort.
Setting: Large HMO in California.
Synopsis: More than 84,000 men participating in the California Men’s Health Study (CMHS) and enrolled in the Kaiser Permanente health plan were categorized as unvaccinated or vaccinated with the pneumococcal vaccine. Vaccinated patients had 10.73 first MIs and 5.30 strokes per 1,000 person-years, compared with unvaccinated patients who incurred 6.07 MIs and 1.90 strokes per 1,000 unvaccinated person-years based on ICD-9 codes.
Even with propensity scoring to minimize selection bias, no clear evidence of benefit was observed. One significant limitation is that 80% of the unvaccinated patients were younger than 60 years old, whereas 74% of the vaccinated patients were 60 or older; this might represent selection bias that cannot be overcome with propensity scoring.
Bottom line: In a population of men older than age 45, pneumococcal vaccination does not appear to reduce the risk of acute MI or stroke.
Citation: Tseng HF, Slezak JM, Quinn VP, et al. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA. 2010;303(17):1699-1706.
No Durable Mortality Benefit from Endovascular Repair of Enlarged Abdominal Aortic Aneurysm
Clinical question: What is the cost and mortality benefit of endovascular versus open repair of abdominal aneurysms?
Background: Previous studies demonstrated a 30-day mortality benefit using endovascular repair over open surgical repair of large abdominal aortic aneurysms. Limited longer-term data are available assessing the durability of these findings.
Study design: Randomized controlled trial.
Setting: Thirty-seven hospitals in the United Kingdom.
Synopsis: Researchers looked at 1,252 patients who were at least 60 years old with a large abdominal aortic aneurysm (>5.5 cm) on CT scan. The patients were randomized to open versus endovascular repair and followed for a median of six years postoperatively. An early, postoperative, all-cause mortality benefit was observed for endovascular repair (1.8%) compared with open repair (4.3%), but no benefit was seen after six months of follow-up, driven by secondary aneurysm ruptures with endovascular grafts. Graft-related complications in all time periods were higher in the endovascular repair group, highest from 0 to 6 months (nearly 50% of patients), and were associated with an increased cost.
Bottom line: Immediate postoperative mortality benefit of endovascular repair is not sustained for abdominal aortic aneurysm beyond six months postoperatively.
Citation: The United Kingdom EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010:362(20):1863-1870.
Financial Constraints Delay Presentation in Patients Suffering from Acute Myocardial Infarction
Clinical question: Does being underinsured or uninsured delay individuals from seeking treatment for emergency medical care?
Background: The number of underinsured or uninsured Americans is growing. Studies have shown that patients with financial concerns avoid routine preventive and chronic medical care; however, similar avoidance has not been defined clearly for patients seeking emergent care.
Study design: Prospective cohort study.
Setting: Twenty-four urban hospitals in the U.S. included in a multisite, acute myocardial infarction (AMI) registry.
Synopsis: Of the 3,721 patients enrolled in the AMI registry, 61.7% of the cohort was insured and without financial concerns that prevented them from seeking care. These patients were less likely to have delays in care related to AMI compared with patients who were insured with financial concerns (18.5% of the cohort; OR 1.22; 95% confidence interval [CI], 1.06-1.40) or uninsured (19.8%; OR 1.30; 95% CI, 1.12-1.51) in all time frames after symptom onset. Patients were less likely to undergo PCI or thrombolysis if the delay to presentation was more than six hours.
After adjustment for confounding factors, the authors concluded that uninsured and underinsured patients were likely to delay presentation to the hospital. Despite these findings, alternative etiologies for delays in care are likely to be more significant, as insurance considerations only account for an 8% difference between the well-insured group (39.3% delayed seeking care >6 hours) and the uninsured group (48.6%). These etiologies are ill-defined.
Bottom line: Underinsured or uninsured patients have a small but significant delay in seeking treatment for AMI due to financial concerns.
Citation: Smolderen KG, Spertus JA, Nallamothu BK, et al. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA. 2010;303 (14):1392-1400.
Concurrent Use of PPIs and Clopidogrel Decrease Hospitalizations for Gastroduodenal Bleeding without Significant Increase in Adverse Cardiovascular Events
Clinical question: Does concomitant use of proton-pump inhibitors (PPIs) and clopidogrel affect the risks of hospitalizations for gastroduodenal bleeding and serious cardiovascular events?
Background: PPIs commonly are prescribed with clopidogrel to reduce the risk of serious gastroduodenal bleeding. Recent observational studies suggest that concurrent PPI and clopidogrel administration might increase the risk of cardiovascular events compared with clopidogrel alone.
Study design: Retrospective cohort.
Setting: Tennessee Medicaid program.
Synopsis: Researchers identified 20,596 patients hospitalized for acute MI, revascularization, or unstable angina, and prescribed clopidogrel. Of this cohort, 7,593 were initial concurrent PPI users—62% used pantoprazole and 9% used omeprazole. Hospitalizations for gastroduodenal bleeding were reduced by 50% (HR 0.50 [95% CI, 0.39-0.65]) in concurrent users of PPIs and clopidogrel, compared with nonusers of PPIs.
Concurrent use was not associated with a statistically significant increase in serious cardiovascular diseases (HR, 0.99 [95% CI, 0.82-1.19]), defined as acute MI, sudden cardiac death, nonfatal or fatal stroke, or other cardiovascular deaths.
Subgroup analyses of individual PPIs and patients undergoing percutaneous coronary interventions also showed no increased risk of serious cardiovascular events. This study could differ from previous observational studies because far fewer patients were on omeprazole, the most potent inhibitor of clopidogrel.
Bottom line: In patients treated with clopidogrel, PPI users had 50% fewer hospitalizations for gastroduodenal bleeding compared with nonusers. Concurrent use of clopidogrel and PPIs, most of which was pantoprazole, was not associated with a significant increase in serious cardiovascular events.
Citation: Ray WA, Murray KT, Griffin MR, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors. Ann Intern Med. 2010;152(6):337-345.
CTCA a Promising, Noninvasive Option in Evaluating Patients with Suspected Coronary Artery Disease
Clinical question: How does computed tomography coronary angiography (CTCA) compare to noninvasive stress testing for diagnosing coronary artery disease (CAD)?
Background: CTCA is a newer, noninvasive test that has a high diagnostic accuracy for CAD, but its clinical role in the evaluation of patients with chest symptoms is unclear.
Study design: Observational study.
Setting: Single academic center in the Netherlands.
Synopsis: Five hundred seventeen eligible patients were evaluated with stress testing and CTCA. The patients were classified as having a low (<20%), intermediate (20%-80%), or high (>80%) pretest probability of CAD based on the Duke clinical score. Using coronary angiography as the gold standard, stress-testing was found to be less accurate than CTCA in all of the patient groups. In patients with low and intermediate pretest probabilities, a negative CTCA had a post-test probability of 0% and 1%, respectively. On the other hand, patients with an intermediate pretest probability and a positive CTCA had a post-test probability of 94% (CI, 89%-97%). In patients with an initial high pretest probability, stress-testing and CTCA confirmed disease in most cases.
The results of this study suggest that CTCA is particularly useful in evaluating patients with an intermediate pretest probability.
Patients were ineligible in this study if they had acute coronary syndromes, previous coronary stent placement, coronary artery bypass surgery, or myocardial infarction. It is important to note that because anatomic lesions seen on imaging (CTCA and coronary angiography) are not always functionally significant, CTCA might have seemed more accurate and clinically useful than it actually is. The investigators also acknowledge that further studies are necessary before CTCA can be accepted as a first-line diagnostic test.
Bottom line: In patients with an intermediate pretest probability of CAD, a negative CTCA is valuable in excluding coronary artery disease, thereby reducing the need for invasive coronary angiography in this group.
Citation: Weustink AC, Mollet NR, Neefjes LA, et al. Diagnostic accuracy and clinical utility of noninvasive testing for coronary artery disease. Ann Intern Med. 2010;152(10):630-639. TH
What Should I Do If I Get a Needlestick?
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Case
While placing a central line, you sustain a needlestick. You’ve washed the area thoroughly with soap and water, but you are concerned about contracting a bloodborne pathogen. What is the risk of contracting such a pathogen, and what can be done to reduce this risk?
Overview
Needlestick injuries are a common occupational hazard in the hospital setting. According to the International Health Care Worker Safety Center (IHCWSC), approximately 295,000 hospital-based healthcare workers experience occupational percutaneous injuries annually. In 1991, Mangione et al surveyed internal-medicine house staff and found an annual incidence of 674 needlestick injuries per 1,000 participants.1 Other retrospective data estimate this risk to be as high as 839 per 1,000 healthcare workers annually.2 Evidence from the Centers for Disease Control and Prevention (CDC) in 2004 suggests that because these are only self-reported injuries, the annual incidence of such injuries is in fact much higher than the current estimates suggest.2,3,4
More than 20 bloodborne pathogens (see Table 1, right) might be transmitted from contaminated needles or sharps, including human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV). A quick and appropriate response to a needlestick injury can greatly decrease the risk of disease transmission following an occupational exposure to potentially infectious materials.
Review of the Data
After any needlestick injury, an affected healthcare worker should wash the area with soap and water immediately. There is no contraindication to using antiseptic solutions, but there is also no evidence to suggest that this reduces the rates of disease transmission.
As decisions for post-exposure prophylaxis often need to be made within hours, a healthcare worker should seek care in the facility areas responsible for managing occupational exposures. Healthcare providers should always be encouraged and supported to report all sharps-related injuries to such departments.
The source patient should be identified and evaluated for potentially transmissible diseases, including HIV, HBV, and HCV. If indicated, the source patient should then undergo appropriate serological testing, and any indicated antiviral prophylaxis should be initiated (see Table 2, p. 19).
Risk of Seroconversion
For all bloodborne pathogens, a needlestick injury carries a greater risk for transmission than other occupational exposures (e.g. mucous membrane exposure). If a needlestick injury occurs in the setting of an infected patient source, the risk of disease transmission varies for HIV, HBV, and HCV (see Table 3, p. 19). In general, risk for seroconversion is increased with a deep injury, an injury with a device visibly contaminated with the source patient’s blood, or an injury involving a needle placed in the source patient’s artery or vein.3,5,6
Human immunodeficiency virus. Contracting HIV after needlestick injury is rare. From 1981 to 2006, the CDC documented only 57 cases of HIV/AIDS in healthcare workers following occupational exposure and identified an additional “possible” 140 cases post-exposure.5,6 Of the 57 documented cases, 48 sustained a percutaneous injury.
Following needlestick injury involving a known HIV-positive source, the one-year risk of seroconversion has been estimated to be 0.3%.5,6 In 1997, Cardo and colleagues identified four factors associated with increased risk for seroconversion after a needlestick/sharps injury from a known positive-HIV source:
- Deep injury;
- Injury with a device visibly contaminated with the source patient’s blood;
- A procedure involving a needle placed in the source patient’s artery or vein; and
- Exposure to a source patient who died of AIDS in the two months following the occupational exposure.5
Hepatitis B virus. Wides-pread immunization of healthcare workers has led to a dramatic decline in occupationally acquired HBV. The CDC estimated that in 1985, approximately 12,500 new HBV infections occurred in healthcare workers.3 This estimate plummeted to approximately 500 new occupationally acquired HBV infections in 1997.3
Despite this, hospital-based healthcare personnel remain at risk for HBV transmission after a needlestick injury from a known positive patient source. Few studies have evaluated the occupational risk of HBV transmission after a needlestick injury. Buergler et al reported that following a needlestick injury involving a known HBV-positive source, the one-year risk of seroconversion was 0.76% to 7.35% for nonimmunized surgeons, and 0.23% to 2.28% for nonimmunized anesthesiologists.7
In the absence of post-exposure prophylaxis, an exposed healthcare worker has a 6% to 30% risk of becoming infected with HBV.3,8 The risk is greatest if the patient source is known to be hepatitis B e antigen-positive, a marker for greater disease infectivity. When given within one week of injury, post-exposure prophylaxis (PEP) with multiple doses of hepatitis B immune globulin (HBIG) provides an estimated 75% protection from transmission.
Healthcare workers who have received the hepatitis B vaccine and developed immunity have virtually no risk for infection.6,7
Hepatitis C virus. Prospective evaluation has demonstrated that the average risk of HCV transmission after percutaneous exposure to a known HCV-positive source is from 0% to 7%.3 The Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections evaluated HCV seroconversion within six months of a reported exposure with enzyme immunoassay and immunoblot assay. In this study, the authors found a seroconversion rate of 1.2%.9
Further, they suggested that HCV seroconversion only occurred from hollow-bore needles, as no seroconversions were noted in healthcare workers who sustained injuries with solid sharp objects.
Post-Exposure Management
The CDC does not recommend prophylaxis when source fluids make contact with intact skin. However, if a percutaneous occupational exposure has occurred, PEPs exist for HIV and HBV but not for HCV.3,6 If a source patient’s HIV, HBV, and HCV statuses are unknown, occupational-health personnel can interview the patient to evaluate his or her risks and initiate testing. Specific information about the time and nature of exposure should be documented.
When testing is indicated, it should be done following institutional and state-specific exposure-control policies and informed consent guidelines. In all situations, the decision to begin antiviral PEP should be carefully considered, weighing benefits of PEP versus the risks and toxicity of treatment.
Human immunodeficiency virus. If a source patient is known to be HIV-positive, has a positive rapid HIV test, or if HIV status cannot be quickly determined, PEP is indicated. Healthcare providers should be aware of rare cases in which the source patient initially tested HIV-seronegative but was subsequently found to have primary HIV infection.
Per 2004 CDC recommendations, PEP is indicated for all healthcare workers who sustain a percuanteous injury from a known HIV-positive source.3,8 For a less severe injury (e.g. solid needle or superficial injury), PEP with either a basic two-drug or three-drug regimen is indicated, depending on the source patient’s viral load.3,5,6,8
If the source patient has unknown HIV status, two-drug PEP is indicated based on the source patient’s HIV risk factors. In such patients, rapid HIV testing also is indicated to aid in determining the need for PEP. When the source HIV status is unknown, PEP is indicated in settings where exposure to HIV-infected persons is likely.
If PEP is indicated, it should be started as quickly as possible. The 2005 U.S. Public Health Service Recommendations for PEP recommend initiating two nucleosides for low-risk exposures and two nucleosides plus a boosted protease inhibitor for high-risk exposures.
Examples of commonly used dual nucleoside regimens are Zidovudine plus Lamivudine (coformulated as Combivir) or Tenofovir plus Emtricitabine (coformulated as Truvada). Current recommendations indicate that PEP should be continued for four weeks, with concurrent clinical and laboratory evaluation for drug toxicity.
Hepatitis B virus. Numerous prospective studies have evaluated the post-exposure effectiveness of HBIG. When administered within 24 hours of exposure, HBIG might offer immediate passive protection against HBV infection. Additionally, if initiated within one week of percutaneous injury with a known HBV-positive source, multiple doses of HGIB provide an estimated 75% protection from transmission.
Although the combination of HBIG and the hepatitis vaccine B series has not been evaluated as PEP in the occupational setting, evidence in the perinatal setting suggests this regimen is more effective than HBIG alone.3,6,8
Hepatitis C virus. No PEP exists for HCV, and current recommendations for post-exposure management focus on early identification and treatment of chronic disease. There are insufficient data for a treatment recommendation for patients with acute HCV infection with no evidence of disease; the appropriate dosing of such a regimen is unknown. Further, evidence suggests that treatment started early in the course of chronic infection could be just as effective and might eliminate the need to treat persons whose infection will spontaneously resolve.7
Back to the Case
Your needlestick occurred while using a hollow-bore needle to cannulate a source patient’s vein, placing you at higher risk for seroconversion. You immediately reported the exposure to the department of occupational health at your hospital. The source patient’s HIV, HBV, and HCV serological statuses were tested, and the patient was found to be HBV-positive. After appropriate counseling, you decide to receive HGIB prophylaxis to reduce your chances of becoming infected with HBV infection.
Bottom Line
Healthcare workers who suffer occupational needlestick injuries require immediate identification and attention to avoid transmission of such infectious diseases as HIV, HBV, and HCV. Source patients should undergo rapid serological testing to determine appropriate PEP. TH
Dr. Zehnder is a hospitalist in the Section of Hospital Medicine at the University of Colorado Denver.
References
- Mangione CM, Gerberding JL, Cummings, SR. Occupational exposure to HIV: Frequency and rates of underreporting of percutaneous and mucocutaneous exposures by medical housestaff. Am J Med. 1991;90(1):85-90.
- Lee JM, Botteman MF, Nicklasson L, et al. Needlestick injury in acute care nurses caring for patients with diabetes mellitus: a retrospective study. Curr Med Res Opinion. 2005;21(5):741-747.
- Workbook for designing, implementing, and evaluating a sharps injury prevention program. Centers for Disease Control and Prevention website. Available at: www.cdc.gov/sharpssafety/pdf/WorkbookComplete.pdf. Accessed Sept. 13, 2010.
- Lee JM, Botteman MF, Xanthakos N, Nicklasson L. Needlestick injuries in the United States. Epidemiologic, economic, and quality of life issues. AAOHN J. 2005;53(3):117-133.
- Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485-1490.
- Exposure to blood: What healthcare personnel need to know. CDC website. Available at: www.cdc.gov/ncidod /dhqp/pdf/bbp/Exp_to_Blood.pdf. Accessed Aug. 31, 2010.
- Buergler JM, Kim R, Thisted RA, Cohn SJ, Lichtor JL, Roizen MF. Risk of human immunodeficiency virus in surgeons, anesthesiologists, and medical students. Anesth Analg. 1992;75(1):118-124.
- Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. CDC website. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5011a1.htm. Accessed Aug. 31, 2010.
- Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposure in health care workers. Italian Study Group on Occupational Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23(5):273-277.
Back to Basics
Let’s examine a documentation case for hospitalists providing daily care: A 65-year-old male patient is admitted with a left hip fracture. The patient also has hypertension and Type 2 diabetes, which might complicate his care. The orthopedic surgeon manages the patient’s perioperative course for the fracture while the hospitalist provides daily post-op care for hypertension and diabetes.
A common scenario is the hospitalist will provide concurrent care, along with a varying number of specialists, depending on the complexity of the patient’s presenting problems and existing comorbidities. Payors define concurrent care as more than one physician providing care to the same patient on the same date, or during the same hospitalization. Payors often consider two key principles before reimbursing concurrent care:
- Does the patient’s condition warrant more than one physician? and
- Are the services provided by each physician reasonable and necessary?1
When more than one medical condition exists and each physician actively treats the condition related to their expertise, each physician can demonstrate medical necessity. As in the above example, the orthopedic surgeon cares for the patient’s fracture while the hospitalist oversees diabetes and hypertension management. Claim submission follows the same logic. Report each subsequent hospital care code (99231-99233) with the corresponding diagnosis each physician primarily manages (i.e., orthopedic surgeon: 9923x with 820.8; hospitalist: 9923x with 250.00, 401.1).
When each physician assigns a different primary diagnosis code to the visit code, each is more likely to receive payment. Because each of these physicians are in different specialties and different provider groups, most payors do not require modifier 25 (separately identifiable E/M service on the same day as a procedure or other service) appended to the visit code. However, some managed-care payors require each physician to append modifier 25 to the concurrent E/M visit code (i.e., 99232-25) despite claim submission under different tax identification numbers.
Unfortunately, the physicians might not realize this until a claim rejection has been issued. Furthermore, payors might want to see the proof before rendering payment. In other words, they pay the first claim received and deny any subsequent claim in order to confirm medical necessity of the concurrent visit. Appeal denied such claims rejections with supporting documentation that distinguishes each physician visit, if possible. This assists the payors in understanding each physician’s contribution to care.
Reasons for Denial
Concurrent care services are more easily distinguished when separate diagnoses are reported with each service. Conversely, payors are likely to deny services that are hard to differentiate. Furthermore, payors frequently deny concurrent care services for the following reasons:
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate or overlap those of another provider without recognizable distinction.2
For example, a hospitalist might be involved in the post-op care of patients with fractures and no other identifiable chronic or acute conditions or complications. In these cases, the hospitalist’s continued involvement might constitute a facility policy (e.g., quality of care, risk reduction, etc.) rather than active clinical management. Claim submission could erroneously occur with each physician reporting 9923x for 820.8. Payors deny medically unnecessary services, or request refunds for inappropriate payments.
Hospitalists might attempt to negotiate other terms with the facility to account for the unpaid time and effort directed toward these types of cases.
Group Practice
Physicians in the same group practice with the same specialty designation must report, and are paid, as a single physician. Multiple visits to the same patient can occur on the same day by members of the same group (e.g., hospitalist A evaluates the patient in the morning, and hospitalist B reviews test results and the resulting course of treatment in the afternoon). However, only one subsequent hospital care service can be reported for the day.
The hospitalists should select the visit level representative of the combined services and submit one appropriately determined code (e.g., 99233), thereby capturing the medically necessary efforts of each physician. To complicate matters, the hospitalists must determine which name to report on the claim: the physician who provided the first encounter, or the physician who provided the most extensive or best-documented encounter.
Tracking productivity for these cases proves challenging. Some practices develop an internal accounting system and credit each physician for their medically necessary efforts (a labor-intensive task for administrators and physicians). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Medicare Benefit Policy Manual: Concurrent Care. Chapter 15, Section 30.E. CMS website. Available at: www.cms.gov/manuals/Downloads/bp102c15.pdf. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Physicians in Group Practice. Chapter 12, Section 30.6.5. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Pohlig, C. Daily care conundrums. The Hospitalist website. Available at: www.the-hospitalist.org/details/article/188735/Daily_Care_Conundrums_.html. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Hospital Visits Same Day But by Different Physicians. Chapter 12, Section 30.6.9.C. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Abraham M, Beebe M, Dalton J, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2010:15.
Let’s examine a documentation case for hospitalists providing daily care: A 65-year-old male patient is admitted with a left hip fracture. The patient also has hypertension and Type 2 diabetes, which might complicate his care. The orthopedic surgeon manages the patient’s perioperative course for the fracture while the hospitalist provides daily post-op care for hypertension and diabetes.
A common scenario is the hospitalist will provide concurrent care, along with a varying number of specialists, depending on the complexity of the patient’s presenting problems and existing comorbidities. Payors define concurrent care as more than one physician providing care to the same patient on the same date, or during the same hospitalization. Payors often consider two key principles before reimbursing concurrent care:
- Does the patient’s condition warrant more than one physician? and
- Are the services provided by each physician reasonable and necessary?1
When more than one medical condition exists and each physician actively treats the condition related to their expertise, each physician can demonstrate medical necessity. As in the above example, the orthopedic surgeon cares for the patient’s fracture while the hospitalist oversees diabetes and hypertension management. Claim submission follows the same logic. Report each subsequent hospital care code (99231-99233) with the corresponding diagnosis each physician primarily manages (i.e., orthopedic surgeon: 9923x with 820.8; hospitalist: 9923x with 250.00, 401.1).
When each physician assigns a different primary diagnosis code to the visit code, each is more likely to receive payment. Because each of these physicians are in different specialties and different provider groups, most payors do not require modifier 25 (separately identifiable E/M service on the same day as a procedure or other service) appended to the visit code. However, some managed-care payors require each physician to append modifier 25 to the concurrent E/M visit code (i.e., 99232-25) despite claim submission under different tax identification numbers.
Unfortunately, the physicians might not realize this until a claim rejection has been issued. Furthermore, payors might want to see the proof before rendering payment. In other words, they pay the first claim received and deny any subsequent claim in order to confirm medical necessity of the concurrent visit. Appeal denied such claims rejections with supporting documentation that distinguishes each physician visit, if possible. This assists the payors in understanding each physician’s contribution to care.
Reasons for Denial
Concurrent care services are more easily distinguished when separate diagnoses are reported with each service. Conversely, payors are likely to deny services that are hard to differentiate. Furthermore, payors frequently deny concurrent care services for the following reasons:
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate or overlap those of another provider without recognizable distinction.2
For example, a hospitalist might be involved in the post-op care of patients with fractures and no other identifiable chronic or acute conditions or complications. In these cases, the hospitalist’s continued involvement might constitute a facility policy (e.g., quality of care, risk reduction, etc.) rather than active clinical management. Claim submission could erroneously occur with each physician reporting 9923x for 820.8. Payors deny medically unnecessary services, or request refunds for inappropriate payments.
Hospitalists might attempt to negotiate other terms with the facility to account for the unpaid time and effort directed toward these types of cases.
Group Practice
Physicians in the same group practice with the same specialty designation must report, and are paid, as a single physician. Multiple visits to the same patient can occur on the same day by members of the same group (e.g., hospitalist A evaluates the patient in the morning, and hospitalist B reviews test results and the resulting course of treatment in the afternoon). However, only one subsequent hospital care service can be reported for the day.
The hospitalists should select the visit level representative of the combined services and submit one appropriately determined code (e.g., 99233), thereby capturing the medically necessary efforts of each physician. To complicate matters, the hospitalists must determine which name to report on the claim: the physician who provided the first encounter, or the physician who provided the most extensive or best-documented encounter.
Tracking productivity for these cases proves challenging. Some practices develop an internal accounting system and credit each physician for their medically necessary efforts (a labor-intensive task for administrators and physicians). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Medicare Benefit Policy Manual: Concurrent Care. Chapter 15, Section 30.E. CMS website. Available at: www.cms.gov/manuals/Downloads/bp102c15.pdf. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Physicians in Group Practice. Chapter 12, Section 30.6.5. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Pohlig, C. Daily care conundrums. The Hospitalist website. Available at: www.the-hospitalist.org/details/article/188735/Daily_Care_Conundrums_.html. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Hospital Visits Same Day But by Different Physicians. Chapter 12, Section 30.6.9.C. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Abraham M, Beebe M, Dalton J, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2010:15.
Let’s examine a documentation case for hospitalists providing daily care: A 65-year-old male patient is admitted with a left hip fracture. The patient also has hypertension and Type 2 diabetes, which might complicate his care. The orthopedic surgeon manages the patient’s perioperative course for the fracture while the hospitalist provides daily post-op care for hypertension and diabetes.
A common scenario is the hospitalist will provide concurrent care, along with a varying number of specialists, depending on the complexity of the patient’s presenting problems and existing comorbidities. Payors define concurrent care as more than one physician providing care to the same patient on the same date, or during the same hospitalization. Payors often consider two key principles before reimbursing concurrent care:
- Does the patient’s condition warrant more than one physician? and
- Are the services provided by each physician reasonable and necessary?1
When more than one medical condition exists and each physician actively treats the condition related to their expertise, each physician can demonstrate medical necessity. As in the above example, the orthopedic surgeon cares for the patient’s fracture while the hospitalist oversees diabetes and hypertension management. Claim submission follows the same logic. Report each subsequent hospital care code (99231-99233) with the corresponding diagnosis each physician primarily manages (i.e., orthopedic surgeon: 9923x with 820.8; hospitalist: 9923x with 250.00, 401.1).
When each physician assigns a different primary diagnosis code to the visit code, each is more likely to receive payment. Because each of these physicians are in different specialties and different provider groups, most payors do not require modifier 25 (separately identifiable E/M service on the same day as a procedure or other service) appended to the visit code. However, some managed-care payors require each physician to append modifier 25 to the concurrent E/M visit code (i.e., 99232-25) despite claim submission under different tax identification numbers.
Unfortunately, the physicians might not realize this until a claim rejection has been issued. Furthermore, payors might want to see the proof before rendering payment. In other words, they pay the first claim received and deny any subsequent claim in order to confirm medical necessity of the concurrent visit. Appeal denied such claims rejections with supporting documentation that distinguishes each physician visit, if possible. This assists the payors in understanding each physician’s contribution to care.
Reasons for Denial
Concurrent care services are more easily distinguished when separate diagnoses are reported with each service. Conversely, payors are likely to deny services that are hard to differentiate. Furthermore, payors frequently deny concurrent care services for the following reasons:
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate or overlap those of another provider without recognizable distinction.2
For example, a hospitalist might be involved in the post-op care of patients with fractures and no other identifiable chronic or acute conditions or complications. In these cases, the hospitalist’s continued involvement might constitute a facility policy (e.g., quality of care, risk reduction, etc.) rather than active clinical management. Claim submission could erroneously occur with each physician reporting 9923x for 820.8. Payors deny medically unnecessary services, or request refunds for inappropriate payments.
Hospitalists might attempt to negotiate other terms with the facility to account for the unpaid time and effort directed toward these types of cases.
Group Practice
Physicians in the same group practice with the same specialty designation must report, and are paid, as a single physician. Multiple visits to the same patient can occur on the same day by members of the same group (e.g., hospitalist A evaluates the patient in the morning, and hospitalist B reviews test results and the resulting course of treatment in the afternoon). However, only one subsequent hospital care service can be reported for the day.
The hospitalists should select the visit level representative of the combined services and submit one appropriately determined code (e.g., 99233), thereby capturing the medically necessary efforts of each physician. To complicate matters, the hospitalists must determine which name to report on the claim: the physician who provided the first encounter, or the physician who provided the most extensive or best-documented encounter.
Tracking productivity for these cases proves challenging. Some practices develop an internal accounting system and credit each physician for their medically necessary efforts (a labor-intensive task for administrators and physicians). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Medicare Benefit Policy Manual: Concurrent Care. Chapter 15, Section 30.E. CMS website. Available at: www.cms.gov/manuals/Downloads/bp102c15.pdf. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Physicians in Group Practice. Chapter 12, Section 30.6.5. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Pohlig, C. Daily care conundrums. The Hospitalist website. Available at: www.the-hospitalist.org/details/article/188735/Daily_Care_Conundrums_.html. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Hospital Visits Same Day But by Different Physicians. Chapter 12, Section 30.6.9.C. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Abraham M, Beebe M, Dalton J, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2010:15.
28,999 and Me
How many people have to die before you’ll pay attention? Like many of you, I read the article but it didn’t really stick. Rather, I filed it in the “interesting tidbits” folder on my brain’s hard drive. Somehow 29,000 people with cancer just didn’t register as a big number.
Until I thought I could be one of them.
The Number
I was harried, running late for a meeting, questioning my decision to try to shoehorn a PCP appointment into my lunch break. Then again, this was a routine follow-up of some labs and I, of course, am the picture of health. Well, I am if you exclude my LDL. It turns out that on a check 12 months earlier, my LDL was found to be running a few heart attacks higher than normal. I took this as a sign, combined with my ballooning waist, middle-ish age, and nagging wife, that I needed to do something.
Still, I wasn’t ready for “something” to include an anticholesterol medication. Instead, I chose the masochistic route and hit the treadmill. And the bike. And a little less of the dinner plate. As a result, I had lost 30 pounds, a handful of pant sizes and, while I wasn’t exactly “in shape,” I did find myself shaped a little less like the Michelin Man.
Triumphantly, I was returning to vanquish my tormentor—the PCP who foolhardily recommended I start a medication.
Sitting in the office awaiting the news of my post-weight-loss cholesterol, my grin was wide and smug—and apparently still overflowing with LDL. I was devastated. 259? I lose weight and my LDL actually goes up!?! I could feel the foam cells in my coronary plaques twitch with delight as they mockingly gorged on chylomicrons.
Undeterred, I inquired what my options were, secretly hoping the answer would be more red wine. Emboldened by my supersaturated serum, my PCP declared it was time for a statin. Alternatively, he noted that I could get a CT angiogram of my coronaries and, if they were clean, I potentially could bypass drug therapy. Thoughts of avoided myalgias happily flittered across my mind until they stumbled onto the number 29,000. It was then that I recalled the recent Archives of Internal Medicine paper.1
The Study
Using risk models based on the known biological effects of radiation, researchers estimated that approximately 29,000 people would develop cancer from the radiation associated with CT scans in 2007 alone. To arrive at this number, the authors used data showing that 1.5% to 2% of all U.S. cancers could be traced to the radiation from CT scans.
Not surprisingly, the most commonly utilized CT scans—namely, abdominal (14,000 a year), chest (4,100 a year), and head (4,000 a year)—accounted for the most morbidity. However, CT angiography, with its super-high dose of radiation, was projected to contribute 2,700 cancers a year. Apparently, my PCP didn’t read this article.
In terms of types of malignancy, lung cancer leads the list with 6,200 projected CT-induced cancers per year, followed by colon cancer (3,500 a year) and leukemia (2,800 a year).
The Names
If the numbers from this study hold, then about 1 in every 2,000 CT scans results in a new cancer. That would mean that I’ve dished out several cancers during my practice. In fact, I’ve ordered many thousand CT scans over my career—give or take a cancer. So my pen has, statistically, caused approximately three cancers.
I wondered which three patients it was. Was it Mr. Reynolds, who would’ve very likely died had we not diagnosed his post-operative abdominal abscess? Perhaps it was Mr. Jenson, who surely would have fared poorly if his pulmonary embolism had not been diagnosed and treated. Maybe it was Mrs. Hernandez, who wouldn’t have received thrombolytics for her stroke without a head CT.
Yes, I might have played a role in causing cancer in these three patients, but I did so knowing that I also saved, or at least improved, their lives. Most patients would accept that calculus.
But what if it were a different three? What if my cancer was that head CT I ordered for Mr. Davidson’s confusion, even though I know that head scans are rarely helpful in the evaluation of delirium? Perhaps my cancer-causer was that abdominal CT scan for Mrs. Ramirez’s chronic pain, which was clearly referable to her irritable bowel syndrome. Maybe it will be that CT scan I ordered last week because the patient insisted it be done, even though I strongly suspected, correctly, that it wouldn’t alter my management.
Which three would it be?
The Questions
This triggered more questions. How many of the 70 million-plus CT scans we order every year really are necessary? How many could be avoided by a robust physical examination, crisper clinical reasoning, or an alternate test? Do our patients really know the risk of these “innocuous” tests? Do we?
And, more personally, what if my PCP was still sitting on two? Would I be his number three?
Moving forward, I vow to remember 29,000. It will remain in the forefront of my mind, constantly badgering me about the next CT scan I order. To be sure, I will continue to order CTs—a lot of CTs. However, I will do so through the prism of the following query. If a patient developed a cancer from the CT scan I was about to order, could I sincerely look them in the eye and tell them I would do the test again?
And I’m agitated by one final question. How is that it took my own carcinogenic brush with CT scans for me to realize the gravity of 29,000? It’s not that 29,000 is not a big number. In fact, it’s precisely because it is a big number that we miss its importance. It’s too easy to hide behind the anonymity of the number. Because in the end, numbers don’t have names until the name is yours. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Reference
- Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
How many people have to die before you’ll pay attention? Like many of you, I read the article but it didn’t really stick. Rather, I filed it in the “interesting tidbits” folder on my brain’s hard drive. Somehow 29,000 people with cancer just didn’t register as a big number.
Until I thought I could be one of them.
The Number
I was harried, running late for a meeting, questioning my decision to try to shoehorn a PCP appointment into my lunch break. Then again, this was a routine follow-up of some labs and I, of course, am the picture of health. Well, I am if you exclude my LDL. It turns out that on a check 12 months earlier, my LDL was found to be running a few heart attacks higher than normal. I took this as a sign, combined with my ballooning waist, middle-ish age, and nagging wife, that I needed to do something.
Still, I wasn’t ready for “something” to include an anticholesterol medication. Instead, I chose the masochistic route and hit the treadmill. And the bike. And a little less of the dinner plate. As a result, I had lost 30 pounds, a handful of pant sizes and, while I wasn’t exactly “in shape,” I did find myself shaped a little less like the Michelin Man.
Triumphantly, I was returning to vanquish my tormentor—the PCP who foolhardily recommended I start a medication.
Sitting in the office awaiting the news of my post-weight-loss cholesterol, my grin was wide and smug—and apparently still overflowing with LDL. I was devastated. 259? I lose weight and my LDL actually goes up!?! I could feel the foam cells in my coronary plaques twitch with delight as they mockingly gorged on chylomicrons.
Undeterred, I inquired what my options were, secretly hoping the answer would be more red wine. Emboldened by my supersaturated serum, my PCP declared it was time for a statin. Alternatively, he noted that I could get a CT angiogram of my coronaries and, if they were clean, I potentially could bypass drug therapy. Thoughts of avoided myalgias happily flittered across my mind until they stumbled onto the number 29,000. It was then that I recalled the recent Archives of Internal Medicine paper.1
The Study
Using risk models based on the known biological effects of radiation, researchers estimated that approximately 29,000 people would develop cancer from the radiation associated with CT scans in 2007 alone. To arrive at this number, the authors used data showing that 1.5% to 2% of all U.S. cancers could be traced to the radiation from CT scans.
Not surprisingly, the most commonly utilized CT scans—namely, abdominal (14,000 a year), chest (4,100 a year), and head (4,000 a year)—accounted for the most morbidity. However, CT angiography, with its super-high dose of radiation, was projected to contribute 2,700 cancers a year. Apparently, my PCP didn’t read this article.
In terms of types of malignancy, lung cancer leads the list with 6,200 projected CT-induced cancers per year, followed by colon cancer (3,500 a year) and leukemia (2,800 a year).
The Names
If the numbers from this study hold, then about 1 in every 2,000 CT scans results in a new cancer. That would mean that I’ve dished out several cancers during my practice. In fact, I’ve ordered many thousand CT scans over my career—give or take a cancer. So my pen has, statistically, caused approximately three cancers.
I wondered which three patients it was. Was it Mr. Reynolds, who would’ve very likely died had we not diagnosed his post-operative abdominal abscess? Perhaps it was Mr. Jenson, who surely would have fared poorly if his pulmonary embolism had not been diagnosed and treated. Maybe it was Mrs. Hernandez, who wouldn’t have received thrombolytics for her stroke without a head CT.
Yes, I might have played a role in causing cancer in these three patients, but I did so knowing that I also saved, or at least improved, their lives. Most patients would accept that calculus.
But what if it were a different three? What if my cancer was that head CT I ordered for Mr. Davidson’s confusion, even though I know that head scans are rarely helpful in the evaluation of delirium? Perhaps my cancer-causer was that abdominal CT scan for Mrs. Ramirez’s chronic pain, which was clearly referable to her irritable bowel syndrome. Maybe it will be that CT scan I ordered last week because the patient insisted it be done, even though I strongly suspected, correctly, that it wouldn’t alter my management.
Which three would it be?
The Questions
This triggered more questions. How many of the 70 million-plus CT scans we order every year really are necessary? How many could be avoided by a robust physical examination, crisper clinical reasoning, or an alternate test? Do our patients really know the risk of these “innocuous” tests? Do we?
And, more personally, what if my PCP was still sitting on two? Would I be his number three?
Moving forward, I vow to remember 29,000. It will remain in the forefront of my mind, constantly badgering me about the next CT scan I order. To be sure, I will continue to order CTs—a lot of CTs. However, I will do so through the prism of the following query. If a patient developed a cancer from the CT scan I was about to order, could I sincerely look them in the eye and tell them I would do the test again?
And I’m agitated by one final question. How is that it took my own carcinogenic brush with CT scans for me to realize the gravity of 29,000? It’s not that 29,000 is not a big number. In fact, it’s precisely because it is a big number that we miss its importance. It’s too easy to hide behind the anonymity of the number. Because in the end, numbers don’t have names until the name is yours. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Reference
- Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
How many people have to die before you’ll pay attention? Like many of you, I read the article but it didn’t really stick. Rather, I filed it in the “interesting tidbits” folder on my brain’s hard drive. Somehow 29,000 people with cancer just didn’t register as a big number.
Until I thought I could be one of them.
The Number
I was harried, running late for a meeting, questioning my decision to try to shoehorn a PCP appointment into my lunch break. Then again, this was a routine follow-up of some labs and I, of course, am the picture of health. Well, I am if you exclude my LDL. It turns out that on a check 12 months earlier, my LDL was found to be running a few heart attacks higher than normal. I took this as a sign, combined with my ballooning waist, middle-ish age, and nagging wife, that I needed to do something.
Still, I wasn’t ready for “something” to include an anticholesterol medication. Instead, I chose the masochistic route and hit the treadmill. And the bike. And a little less of the dinner plate. As a result, I had lost 30 pounds, a handful of pant sizes and, while I wasn’t exactly “in shape,” I did find myself shaped a little less like the Michelin Man.
Triumphantly, I was returning to vanquish my tormentor—the PCP who foolhardily recommended I start a medication.
Sitting in the office awaiting the news of my post-weight-loss cholesterol, my grin was wide and smug—and apparently still overflowing with LDL. I was devastated. 259? I lose weight and my LDL actually goes up!?! I could feel the foam cells in my coronary plaques twitch with delight as they mockingly gorged on chylomicrons.
Undeterred, I inquired what my options were, secretly hoping the answer would be more red wine. Emboldened by my supersaturated serum, my PCP declared it was time for a statin. Alternatively, he noted that I could get a CT angiogram of my coronaries and, if they were clean, I potentially could bypass drug therapy. Thoughts of avoided myalgias happily flittered across my mind until they stumbled onto the number 29,000. It was then that I recalled the recent Archives of Internal Medicine paper.1
The Study
Using risk models based on the known biological effects of radiation, researchers estimated that approximately 29,000 people would develop cancer from the radiation associated with CT scans in 2007 alone. To arrive at this number, the authors used data showing that 1.5% to 2% of all U.S. cancers could be traced to the radiation from CT scans.
Not surprisingly, the most commonly utilized CT scans—namely, abdominal (14,000 a year), chest (4,100 a year), and head (4,000 a year)—accounted for the most morbidity. However, CT angiography, with its super-high dose of radiation, was projected to contribute 2,700 cancers a year. Apparently, my PCP didn’t read this article.
In terms of types of malignancy, lung cancer leads the list with 6,200 projected CT-induced cancers per year, followed by colon cancer (3,500 a year) and leukemia (2,800 a year).
The Names
If the numbers from this study hold, then about 1 in every 2,000 CT scans results in a new cancer. That would mean that I’ve dished out several cancers during my practice. In fact, I’ve ordered many thousand CT scans over my career—give or take a cancer. So my pen has, statistically, caused approximately three cancers.
I wondered which three patients it was. Was it Mr. Reynolds, who would’ve very likely died had we not diagnosed his post-operative abdominal abscess? Perhaps it was Mr. Jenson, who surely would have fared poorly if his pulmonary embolism had not been diagnosed and treated. Maybe it was Mrs. Hernandez, who wouldn’t have received thrombolytics for her stroke without a head CT.
Yes, I might have played a role in causing cancer in these three patients, but I did so knowing that I also saved, or at least improved, their lives. Most patients would accept that calculus.
But what if it were a different three? What if my cancer was that head CT I ordered for Mr. Davidson’s confusion, even though I know that head scans are rarely helpful in the evaluation of delirium? Perhaps my cancer-causer was that abdominal CT scan for Mrs. Ramirez’s chronic pain, which was clearly referable to her irritable bowel syndrome. Maybe it will be that CT scan I ordered last week because the patient insisted it be done, even though I strongly suspected, correctly, that it wouldn’t alter my management.
Which three would it be?
The Questions
This triggered more questions. How many of the 70 million-plus CT scans we order every year really are necessary? How many could be avoided by a robust physical examination, crisper clinical reasoning, or an alternate test? Do our patients really know the risk of these “innocuous” tests? Do we?
And, more personally, what if my PCP was still sitting on two? Would I be his number three?
Moving forward, I vow to remember 29,000. It will remain in the forefront of my mind, constantly badgering me about the next CT scan I order. To be sure, I will continue to order CTs—a lot of CTs. However, I will do so through the prism of the following query. If a patient developed a cancer from the CT scan I was about to order, could I sincerely look them in the eye and tell them I would do the test again?
And I’m agitated by one final question. How is that it took my own carcinogenic brush with CT scans for me to realize the gravity of 29,000? It’s not that 29,000 is not a big number. In fact, it’s precisely because it is a big number that we miss its importance. It’s too easy to hide behind the anonymity of the number. Because in the end, numbers don’t have names until the name is yours. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
Reference
- Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
Should HM Redefine Its Role as Provider and Adjust Expectations for Inpatient Care?
Did you happen to read a recent New York Times article (www.nytimes.com/2010/05/27/us/27hosp.html) about hospitalists? I thought the article was great, but I was surprised by some of the negative reader feedback. What did you think?
George Eppley, MD
Reed, Ga.
Dr. Hospitalist responds: I read the NYT article by Jane Gross, “New Breed of Specialist Steps in for Family Doctor,” which was published May 26. The accompanying reader comment section is available at http://newoldage.blogs.nytimes.com/2010/05/26/in-hospitals-new-fingers -on-the-pulse/?ref=us.
The article provides the statistics that all of us in HM have come to know: HM is the fastest-growing medical specialty in the U.S. and, over the past decade, the number of hospitalists in the U.S. has grown from hundreds to 30,000. Gross talks about the care a hospitalist at the Hospital of the University of Pennsylvania provides for her patient. She highlights the challenges of transitions of care and references the work being done by hospitalists and SHM to make sure patients are making safe transitions. While the article was largely supportive of HM, she does provide a shade of balance when she mentions the risks to the patient when hospitalists fail to do their job when it comes to communication.
As a practicing hospitalist, I kind of wished I had stopped reading at the end of the article. I honestly did not like most of what I read in the reader comment section. Although the article was a feel-good story, I think it is fair to say the reader comments were largely negative. I understand that readers with negative experiences with hospitalists might be more likely to post a comment; nevertheless, some of the comments are hard to ignore—mainly because I suspect some of it is true.
One reader from Raleigh, N.C., wrote, “Hospitalists proved inept at contacting the patients’ existing doctors or even talking to the patients. Then, on discharge to nursing homes for further recovery, the ball was dropped further, with very poor communication of medication dosages, etc.” Yikes! What happened to the communication and the medication reconciliation process?
A reader from Massachusetts wrote about “the hospitalist … (who) ordered five blood draws in the space of several hours to replicate tests that had already been taken by the primary-care physician before admission.” So, not only are hospitalists poor communicators and do not do a good job with transitions of care, but their care is also driving up the cost of healthcare needlessly?
The most positive comments seem to come from outpatient providers and, quite honestly, I found them lukewarm at best. A PCP from Charleston, S.C., wrote, “I no longer have to cancel the appointments of the patients at the last minute in order to attend to an emergency occurring outside my office. It is a very efficient system.” Glad to hear the hospitalist relationship is working out for you, Dr. PCP, but as a patient in the hospital, I am worried more about the competency of this doctor, whom I have never met before this hospitalization, as opposed to how this doctor is going to make you more “efficient” in your office practice.
I came away with several thoughts after reading the article and the comments.
First, we need to set the right expectations. Is this the equivalent of the star athlete who makes a brash statement followed shortly thereafter by the statement, “I was misquoted”? Well … maybe. Are we who we say we are? The headline is “New Breed of Specialist Steps in for Family Doctor.”
As a practicing hospitalist, I never describe myself as replacing the family doctor, because this is the worst position I could put myself in. A patient might have a relationship with a family doctor for three or four decades. This family doctor might not only care for this patient, but also his children and grandchildren. The patient visits the family doctor at least once a year for a checkup. But when the patient is as sick as they have ever been in their life and needs their family doctor whom they trust, I am supposed to “step in” for this family doctor? Good luck trying to meet that standard. It’s like putting me next to Justin Timberlake on stage at a teenybopper concert. Who do you think is going to look better in that sort of comparison?
We, as hospitalists, should never allow anyone to think we are replacing their family doctor. We are here to work with the family doctor to provide the best care possible. Do surgeons, medical subspecialists, or ED doctors “replace” the family doctor? No way! They are working with the family doctor. Perhaps the problem here is that we have not set the appropriate expectations for our patients.
Next, we need to be clear in saying what we say we do or doing we what we say we do. A line in this article bothers me more than any of the reader comments: “The most compelling argument in favor of hospitalists, who are now in 5,000 institutions, from academic giants like the Hospital of the University of Pennsylvania to small community hospitals to innovators like the Mayo and Cleveland Clinics—is that they are there all the time.”
Why does it bother me so much? It is troubling because it is misleading and might simply be untrue. Many hospitalists are not there “all the time.” While many of our hospitalist programs have providers in the hospital 24 hours a day, many do not. I know a number of hospitalists who make rounds at multiple hospitals throughout the day. Are they really hospitalists or are they inpatient rounders?
Hospitalists are physicians defined by their location, not unlike ED physicians. Do we have ED doctors going from hospital to hospital, leaving nurses alone to care for patients when they are at another hospital? So what do we expect from our hospitalists? Should they be in the hospital 24/7? That would seem to be more consistent with the thought that “they are there all the time.” Remember, Gross did not say hospitalists are “reachable” all the time. She did say hospitalists are “on top of everything that happens to a patient—from entry through treatment and discharge.” It is time that we, as hospitalists, uniformly meet those expectations. Patients all over the country are figuring out that not all hospitalists are doing what they are supposed to do when it comes to communications and establishing safe transitions of care. Remember the adage: It does not take many rotten apples to spoil the barrel.
Last, let us talk more about how hospitalists can provide patient-centric care, as opposed to cost savings and carrying out President Obama’s marching orders. The article describes how a study published in the Journal of the American Medical Association found that patients have a reduced length of stay in the hospital when cared for by hospitalists; how hospitalists are being viewed as leaders in healthcare reform; and how the hospitalist spends her nonclinical time “design(ing) computer programs to contain costs.” Do not get me wrong. I am supportive as anyone of the notion that hospitalists should provide cost-effective care. But the reality is that our patients’ No. 1 priority is to believe that their doctor is providing the best care possible. They do not want to feel someone is short-changing them.
Talk all you want to insurers and hospitals about cost savings, but when speaking with patients, I think it makes more sense to discuss the quality as opposed to cost of care. Ask your next patient whether they give a hoot what you do when you are not caring for them. TH
Did you happen to read a recent New York Times article (www.nytimes.com/2010/05/27/us/27hosp.html) about hospitalists? I thought the article was great, but I was surprised by some of the negative reader feedback. What did you think?
George Eppley, MD
Reed, Ga.
Dr. Hospitalist responds: I read the NYT article by Jane Gross, “New Breed of Specialist Steps in for Family Doctor,” which was published May 26. The accompanying reader comment section is available at http://newoldage.blogs.nytimes.com/2010/05/26/in-hospitals-new-fingers -on-the-pulse/?ref=us.
The article provides the statistics that all of us in HM have come to know: HM is the fastest-growing medical specialty in the U.S. and, over the past decade, the number of hospitalists in the U.S. has grown from hundreds to 30,000. Gross talks about the care a hospitalist at the Hospital of the University of Pennsylvania provides for her patient. She highlights the challenges of transitions of care and references the work being done by hospitalists and SHM to make sure patients are making safe transitions. While the article was largely supportive of HM, she does provide a shade of balance when she mentions the risks to the patient when hospitalists fail to do their job when it comes to communication.
As a practicing hospitalist, I kind of wished I had stopped reading at the end of the article. I honestly did not like most of what I read in the reader comment section. Although the article was a feel-good story, I think it is fair to say the reader comments were largely negative. I understand that readers with negative experiences with hospitalists might be more likely to post a comment; nevertheless, some of the comments are hard to ignore—mainly because I suspect some of it is true.
One reader from Raleigh, N.C., wrote, “Hospitalists proved inept at contacting the patients’ existing doctors or even talking to the patients. Then, on discharge to nursing homes for further recovery, the ball was dropped further, with very poor communication of medication dosages, etc.” Yikes! What happened to the communication and the medication reconciliation process?
A reader from Massachusetts wrote about “the hospitalist … (who) ordered five blood draws in the space of several hours to replicate tests that had already been taken by the primary-care physician before admission.” So, not only are hospitalists poor communicators and do not do a good job with transitions of care, but their care is also driving up the cost of healthcare needlessly?
The most positive comments seem to come from outpatient providers and, quite honestly, I found them lukewarm at best. A PCP from Charleston, S.C., wrote, “I no longer have to cancel the appointments of the patients at the last minute in order to attend to an emergency occurring outside my office. It is a very efficient system.” Glad to hear the hospitalist relationship is working out for you, Dr. PCP, but as a patient in the hospital, I am worried more about the competency of this doctor, whom I have never met before this hospitalization, as opposed to how this doctor is going to make you more “efficient” in your office practice.
I came away with several thoughts after reading the article and the comments.
First, we need to set the right expectations. Is this the equivalent of the star athlete who makes a brash statement followed shortly thereafter by the statement, “I was misquoted”? Well … maybe. Are we who we say we are? The headline is “New Breed of Specialist Steps in for Family Doctor.”
As a practicing hospitalist, I never describe myself as replacing the family doctor, because this is the worst position I could put myself in. A patient might have a relationship with a family doctor for three or four decades. This family doctor might not only care for this patient, but also his children and grandchildren. The patient visits the family doctor at least once a year for a checkup. But when the patient is as sick as they have ever been in their life and needs their family doctor whom they trust, I am supposed to “step in” for this family doctor? Good luck trying to meet that standard. It’s like putting me next to Justin Timberlake on stage at a teenybopper concert. Who do you think is going to look better in that sort of comparison?
We, as hospitalists, should never allow anyone to think we are replacing their family doctor. We are here to work with the family doctor to provide the best care possible. Do surgeons, medical subspecialists, or ED doctors “replace” the family doctor? No way! They are working with the family doctor. Perhaps the problem here is that we have not set the appropriate expectations for our patients.
Next, we need to be clear in saying what we say we do or doing we what we say we do. A line in this article bothers me more than any of the reader comments: “The most compelling argument in favor of hospitalists, who are now in 5,000 institutions, from academic giants like the Hospital of the University of Pennsylvania to small community hospitals to innovators like the Mayo and Cleveland Clinics—is that they are there all the time.”
Why does it bother me so much? It is troubling because it is misleading and might simply be untrue. Many hospitalists are not there “all the time.” While many of our hospitalist programs have providers in the hospital 24 hours a day, many do not. I know a number of hospitalists who make rounds at multiple hospitals throughout the day. Are they really hospitalists or are they inpatient rounders?
Hospitalists are physicians defined by their location, not unlike ED physicians. Do we have ED doctors going from hospital to hospital, leaving nurses alone to care for patients when they are at another hospital? So what do we expect from our hospitalists? Should they be in the hospital 24/7? That would seem to be more consistent with the thought that “they are there all the time.” Remember, Gross did not say hospitalists are “reachable” all the time. She did say hospitalists are “on top of everything that happens to a patient—from entry through treatment and discharge.” It is time that we, as hospitalists, uniformly meet those expectations. Patients all over the country are figuring out that not all hospitalists are doing what they are supposed to do when it comes to communications and establishing safe transitions of care. Remember the adage: It does not take many rotten apples to spoil the barrel.
Last, let us talk more about how hospitalists can provide patient-centric care, as opposed to cost savings and carrying out President Obama’s marching orders. The article describes how a study published in the Journal of the American Medical Association found that patients have a reduced length of stay in the hospital when cared for by hospitalists; how hospitalists are being viewed as leaders in healthcare reform; and how the hospitalist spends her nonclinical time “design(ing) computer programs to contain costs.” Do not get me wrong. I am supportive as anyone of the notion that hospitalists should provide cost-effective care. But the reality is that our patients’ No. 1 priority is to believe that their doctor is providing the best care possible. They do not want to feel someone is short-changing them.
Talk all you want to insurers and hospitals about cost savings, but when speaking with patients, I think it makes more sense to discuss the quality as opposed to cost of care. Ask your next patient whether they give a hoot what you do when you are not caring for them. TH
Did you happen to read a recent New York Times article (www.nytimes.com/2010/05/27/us/27hosp.html) about hospitalists? I thought the article was great, but I was surprised by some of the negative reader feedback. What did you think?
George Eppley, MD
Reed, Ga.
Dr. Hospitalist responds: I read the NYT article by Jane Gross, “New Breed of Specialist Steps in for Family Doctor,” which was published May 26. The accompanying reader comment section is available at http://newoldage.blogs.nytimes.com/2010/05/26/in-hospitals-new-fingers -on-the-pulse/?ref=us.
The article provides the statistics that all of us in HM have come to know: HM is the fastest-growing medical specialty in the U.S. and, over the past decade, the number of hospitalists in the U.S. has grown from hundreds to 30,000. Gross talks about the care a hospitalist at the Hospital of the University of Pennsylvania provides for her patient. She highlights the challenges of transitions of care and references the work being done by hospitalists and SHM to make sure patients are making safe transitions. While the article was largely supportive of HM, she does provide a shade of balance when she mentions the risks to the patient when hospitalists fail to do their job when it comes to communication.
As a practicing hospitalist, I kind of wished I had stopped reading at the end of the article. I honestly did not like most of what I read in the reader comment section. Although the article was a feel-good story, I think it is fair to say the reader comments were largely negative. I understand that readers with negative experiences with hospitalists might be more likely to post a comment; nevertheless, some of the comments are hard to ignore—mainly because I suspect some of it is true.
One reader from Raleigh, N.C., wrote, “Hospitalists proved inept at contacting the patients’ existing doctors or even talking to the patients. Then, on discharge to nursing homes for further recovery, the ball was dropped further, with very poor communication of medication dosages, etc.” Yikes! What happened to the communication and the medication reconciliation process?
A reader from Massachusetts wrote about “the hospitalist … (who) ordered five blood draws in the space of several hours to replicate tests that had already been taken by the primary-care physician before admission.” So, not only are hospitalists poor communicators and do not do a good job with transitions of care, but their care is also driving up the cost of healthcare needlessly?
The most positive comments seem to come from outpatient providers and, quite honestly, I found them lukewarm at best. A PCP from Charleston, S.C., wrote, “I no longer have to cancel the appointments of the patients at the last minute in order to attend to an emergency occurring outside my office. It is a very efficient system.” Glad to hear the hospitalist relationship is working out for you, Dr. PCP, but as a patient in the hospital, I am worried more about the competency of this doctor, whom I have never met before this hospitalization, as opposed to how this doctor is going to make you more “efficient” in your office practice.
I came away with several thoughts after reading the article and the comments.
First, we need to set the right expectations. Is this the equivalent of the star athlete who makes a brash statement followed shortly thereafter by the statement, “I was misquoted”? Well … maybe. Are we who we say we are? The headline is “New Breed of Specialist Steps in for Family Doctor.”
As a practicing hospitalist, I never describe myself as replacing the family doctor, because this is the worst position I could put myself in. A patient might have a relationship with a family doctor for three or four decades. This family doctor might not only care for this patient, but also his children and grandchildren. The patient visits the family doctor at least once a year for a checkup. But when the patient is as sick as they have ever been in their life and needs their family doctor whom they trust, I am supposed to “step in” for this family doctor? Good luck trying to meet that standard. It’s like putting me next to Justin Timberlake on stage at a teenybopper concert. Who do you think is going to look better in that sort of comparison?
We, as hospitalists, should never allow anyone to think we are replacing their family doctor. We are here to work with the family doctor to provide the best care possible. Do surgeons, medical subspecialists, or ED doctors “replace” the family doctor? No way! They are working with the family doctor. Perhaps the problem here is that we have not set the appropriate expectations for our patients.
Next, we need to be clear in saying what we say we do or doing we what we say we do. A line in this article bothers me more than any of the reader comments: “The most compelling argument in favor of hospitalists, who are now in 5,000 institutions, from academic giants like the Hospital of the University of Pennsylvania to small community hospitals to innovators like the Mayo and Cleveland Clinics—is that they are there all the time.”
Why does it bother me so much? It is troubling because it is misleading and might simply be untrue. Many hospitalists are not there “all the time.” While many of our hospitalist programs have providers in the hospital 24 hours a day, many do not. I know a number of hospitalists who make rounds at multiple hospitals throughout the day. Are they really hospitalists or are they inpatient rounders?
Hospitalists are physicians defined by their location, not unlike ED physicians. Do we have ED doctors going from hospital to hospital, leaving nurses alone to care for patients when they are at another hospital? So what do we expect from our hospitalists? Should they be in the hospital 24/7? That would seem to be more consistent with the thought that “they are there all the time.” Remember, Gross did not say hospitalists are “reachable” all the time. She did say hospitalists are “on top of everything that happens to a patient—from entry through treatment and discharge.” It is time that we, as hospitalists, uniformly meet those expectations. Patients all over the country are figuring out that not all hospitalists are doing what they are supposed to do when it comes to communications and establishing safe transitions of care. Remember the adage: It does not take many rotten apples to spoil the barrel.
Last, let us talk more about how hospitalists can provide patient-centric care, as opposed to cost savings and carrying out President Obama’s marching orders. The article describes how a study published in the Journal of the American Medical Association found that patients have a reduced length of stay in the hospital when cared for by hospitalists; how hospitalists are being viewed as leaders in healthcare reform; and how the hospitalist spends her nonclinical time “design(ing) computer programs to contain costs.” Do not get me wrong. I am supportive as anyone of the notion that hospitalists should provide cost-effective care. But the reality is that our patients’ No. 1 priority is to believe that their doctor is providing the best care possible. They do not want to feel someone is short-changing them.
Talk all you want to insurers and hospitals about cost savings, but when speaking with patients, I think it makes more sense to discuss the quality as opposed to cost of care. Ask your next patient whether they give a hoot what you do when you are not caring for them. TH
In the Literature
In This Edition
Literature at a Glance
A guide to this month’s studies
- Antibiotics after drainage of uncomplicated skin abscesses
- Clopidogrel vs. combined aspirin-dipyridamole for acute ischemic stroke
- BNP-guided therapy in chronic heart failure outpatients
- Cognitive decline and dementia after hospitalization
- Clopidogrel delays up risks for DES implantation patients
- Clinical score identifies prolonged length of stay
- Time to therapy reduces mortality in sepsis patients
- PEEP associated with lower mortality for ARDS patients
Antibiotics Might Be Unnecessary after Drainage of Uncomplicated Skin Abscesses
Clinical question: Does trimethoprim/sulfamethoxazole (TMP/SMX) treatment after drainage of a skin abscess reduce treatment failure at seven days or development of new lesions at 30 days?
Background: Community ac-quired methicillin-resistant Staphylococcus aureus (MRSA) skin abscesses are increasing in frequency. The benefit of antibiotic treatment after incision and drainage is not clear, as there is a high cure rate without antibiotics.
Study design: Multicenter, double-blinded, randomized, placebo-controlled trial.
Setting: Four military EDs treating civilians and military patients.
Synopsis: The study enrolled a convenience sample of 220 patients, each of whom presented to EDs with uncomplicated skin abscesses from November 2007 to June 2009. Abscesses were drained in the ED, then patients were randomized to either placebo or to TMP/SMX (two DS tablets twice daily) for seven days. Re-evaluation for wound checks occurred at two days and seven days.
Treatment failure at seven days, defined as worsening infection, new lesions, or absence of clinical improvement, occurred in 26% of placebo patients and 17% of patients in the treatment arm, a nonsignificant difference (P=0.12). Fewer patients in the treatment arm had new lesions at 30 days (28% vs. 9%, P=0.02). MRSA was cultured from 53% of patients overall; all samples were sensitive to TMP/SMX.
The study was limited by the fact that only 69% of patients were evaluated at 30 days.
Bottom line: TMP/SMX treatment of uncomplicated skin abscess after drainage in EDs does not decrease treatment failure at seven days, but might decrease the development of new lesions.
Citation: Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection [published online ahead of print March 29, 2010]. Ann Emerg Med. doi:10.1016/j.annemerg med.2010.03.002.
Clopidogrel and Combined Aspirin-Dipyridamole Have Similar Safety and Efficacy Profiles for Acute Ischemic Stroke
Clinical question: What is the efficacy and safety of combined aspirin and extended-release dipyridamole (Asp/ER-DP) compared to clopidogrel in patients with acute ischemic stroke?
Background: Long-term antiplatelet therapy is effective at reducing recurrence after ischemic stroke. However, the relative safety and efficacy of Asp/ER-DP or clopidogrel is not known in patients with acute ischemic stroke.
Study design: Randomized, controlled trial.
Setting: A multicenter trial involving 695 sites in 35 countries.
Synopsis: This post-hoc subgroup analysis of the PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes) trial assessed the relative safety and efficacy of Asp/ER-DP versus clopidogrel administered within 72 hours of stroke onset in 1,360 patients. The primary endpoint was functional outcome at 30 days.
Secondary outcomes included symptomatic hemorrhagic transformation of the infarct, cerebral edema, recurrent stroke, myocardial infarction (MI), composite vascular events (combination of nonfatal stroke, nonfatal MI, and vascular death), death, cognition, bleeding, and serious adverse events studied at seven, 30, and 90 days.
Combined death or dependency did not differ between treatment groups. Nonsignificant trends to reduced recurrence and vascular events were present with Asp/ER-DP. Rates of death, major bleeding, and serious adverse events did not differ between treatment groups.
Bottom line: Either clopidogrel or combined aspirin and extended-release dipyridamole can be used to treat acute ischemic stroke, with similar outcomes and safety profiles.
Citation: Bath PM, Cotton D, Martin RH, et al. Effect of combined aspirin and extended-release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke. 2010;41(4):732-738.
BNP-Guided Therapy Reduces All-Cause Mortality in Outpatients with Chronic Heart Failure
Clinical question: Is there a clinical benefit in using B-type natriuretic peptide (BNP) to guide adjustment of proven medications in chronic heart failure?
Background: BNP is secreted by the heart in response to increased volume. It has been shown to be useful in the diagnosis of decompensated heart failure, and it can be decreased by treatment with proven heart failure medications. It is unclear if this effect provides clinical benefit on mortality and hospitalization.
Study design: Meta-analysis of prospective randomized controlled trials.
Setting: Eight studies involving 1,726 patients, published internationally from 2005-2009.
Synopsis: Study sizes ranged from 41 to 499 patients, with three- to 24-month follow-up. Patients had New York Heart Association (NYHA) class II or greater heart failure, with ejection fractions <50%.
All-cause mortality was significantly lower in BNP-guided therapy compared with clinical-guided therapy (RR=0.76; 95% CI, 0.63-0.91; P=0.003), specifically in patients younger than 75 years old (RR=0.52; 95% CI, 0.33-0.82; P=0.005).
A proposed mechanism for this result was a statistically significant increase in adjustment of most heart failure medications for BNP-guided therapy compared with clinical-guided therapy (75% vs. 58%, P<0.001 in diuretics; 49.6% vs. 30.9%, P<0.001 in ACE inhibitors or Angiotensin II receptor blockers (ARBs); and 51.1% vs. 41.6%, P=0.02 in beta-blockers) and a higher percentage reaching target doses in the BNP-guided therapy group. However, there was no significant decrease in all-cause hospitalization or survival free of hospitalization.
The study limitations include: Hospitalization for heart failure was not meta-analyzed, the pooled data were weighted toward one study, and BNP-guided titration parameters varied across studies.
Bottom line: BNP-guided therapy reduces all-cause mortality in chronic heart failure patients younger than 75 years old, but not all-cause hospitalization or survival free of hospitalization.
Citation: Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med. 2010;170(6):507-514.
Hospitalization Is Associated with Cognitive Decline and Subsequent Risk for Dementia in the Elderly
Clinical question: Is critical illness in patients 65 and older associated with long-term cognitive impairment, and does it affect the incidence of dementia?
Background: There is literature suggesting that survivors of critical illness suffer long-term cognitive impairment, but premorbid measures of cognitive function have not been researched. No studies have evaluated the risk of incident dementia among this patient population.
Study design: Prospective cohort study.
Setting: Group Health Cooperative in Seattle.
Synopsis: This study analyzed data from 2,929 community-dwelling adults older than 65 without baseline dementia. From 1994 to 2007, the individuals were screened with the Cognitive Abilities Screening Instrument (CASI) at follow-up visits every two years. CASI scores lower than 86 (out of 100) led to an examination for dementia; the diagnosis of dementia was an outcome measure. Scores were adjusted for baseline cognitive scores, age, and other risk factors.
For patients following acute-care hospitalization, adjusted CASI scores were 1.01 points lower on average than for those not hospitalized. For patients following critical-illness hospitalization, scores were 2.14 points lower. The dementia rate was 14.6 cases per 1,000 person-years among patients not hospitalized, and 33.6 among those admitted for noncritical illness.
As suspected, hospitalization might be a marker for cognitive decline in the elderly after adjusting for premorbid CASI scores and comorbid illness. Some factors in acute illness—and moreso in critical illness—might be causally related to cognitive decline.
Bottom line: In elderly patients without dementia at baseline, hospitalization for acute care and critical illness increases the likelihood of cognitive decline compared with patients who were not hospitalized. Only noncritical-illness hospitalization was not associated with the development of dementia.
Citation: Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8): 763-770.
Increased Risk of Death and Myocardial Infarction in Patients Who Delay Filling Clopidogrel Prescription after Drug-Eluting Stent Implantation
Clinical question: Is there an increased risk of death or myocardial infarction (MI) in patients with recent drug-eluting stent (DES) implantation who delayed filling their clopidogrel prescription compared with those who filled their prescription on the day of hospital discharge?
Background: Filling an initial prescription of clopidogrel on the day of discharge is important after DES implantation, as prior studies suggest that lack of thienopyridine therapy is a risk factor for early stent thrombosis.
Study design: Retrospective cohort study.
Setting: Three large, integrated healthcare systems.
Synopsis: The cohort included 7,042 patients discharged after DES implantation. Filling of a clopidogrel prescription was based on pharmacy dispensing data. Primary analysis divided patients based on whether they filled the prescription on the day of discharge or any time after discharge. Secondary analysis further characterized delays as >1 day, >3 days, or >5 days after discharge.
One in 6 patients delayed filling the initial prescription. Patients with any degree of delay had significantly higher death and MI rates during follow-up (14.2% vs. 7.9%, P<0.001), as well as an increased risk of death/MI (hazard ratio 1.53; 95% CI, 1.25-1.87). Factors associated with a delay in filling clopidogrel included older age, prior MI, diabetes, renal dysfunction, prior revascularization, cardiogenic shock, in-hospital bleeding, and use of clopidogrel upon admission.
The study was limited in that data were based on pharmacy records, and that patients might have received medication at discharge or outside the healthcare system.
Bottom line: The delay in filling a clopidogrel prescription is associated with an increased risk of death and MI in patients with recent DES implantation.
Citation: Ho PM, Tsai TT, Maddox TM, et al. Delays in filling clopidogrel prescription after hospital discharge and adverse outcomes after drug-eluting stent implantation: implications for transitions of care. Circ Cardiovasc Qual Outcomes. 2010;3(3):261-266.
Predicting Length of Stay after Stroke
Clinical question: Does a clinical score accurately predict prolonged length of stay after stroke?
Background: Stroke is a costly health problem, and length of stay is the most prominent factor contributing to the high costs. The factors leading to prolonged length of stay are varied, and there are no established tools to predict length of stay.
Study design: Prospective cohort study.
Setting: All 28 Israeli hospitals that admit stroke patients.
Synopsis: All patients admitted to Israeli hospitals during established two-month periods in 2004 (1,700 patients) and 2007 (1,648 patients) were included in the National Acute Stroke Israeli Survey (NASIS), and served as the derivation and validation cohort for development of a Prolonged Length of Stay (PLOS) score.
Using the 2004 data, investigators identified stroke severity using the National Institutes of Health Stroke Scale (NIHSS), history of congestive heart failure (CHF), history of atrial fibrillation, decreased level of consciousness on presentation, and intracerebral hemorrhage (as opposed to ischemic stroke) as predictors of prolonged length of stay. Four of these factors were expressed as dichotomous variables, whereas the stroke severity by NIHSS class was incorporated as a range; all were incorporated into a PLOS score.
Higher PLOS score correlated with longer length of stay. In the derivation cohort, 22% of patients with a PLOS score of 0 had a prolonged length of stay, whereas 85% of patients with PLOS scores of 6 or 7 had a prolonged length of stay. In the validation cohort, the corresponding figures were 19% and 72%.
Bottom line: Use of a simple score can predict risk of prolonged length of stay after stroke.
Citation: Koton S, Bornstein NM, Tsabari R, Tanne D, NASIS Investigators. Derivation and validation of the prolonged length of stay score in acute stroke patients. Neurology. 2010;74(19);1511-1516.
Earlier Administration of Appropriate Antimicrobials Decreases Mortality in Patients with Severe Sepsis and Septic Shock
Clinical question: Is the timing of antimicrobial administration an important determinant of survival in patients diagnosed with severe sepsis and septic shock?
Background: Severe sepsis and septic shock are associated with a 25% to 50% mortality rate. Early goal-directed therapy has been shown to increase survival in these patients. Antimicrobial treatment is a mainstay of this therapy, but the most effective timing of this treatment remains unclear.
Study design: Retrospective, single-center cohort study.
Setting: ED at an academic tertiary-care center.
Synopsis: Two hundred sixty-one patients in the ED in 2005-2006 presenting with severe sepsis or septic shock were enrolled in the hospital’s early goal-directed therapy (EGDT) algorithm, either at triage or later during their ED stay. Labs showed 56.7% of patients were culture-positive, with the most common sources being respiratory (30.6%), genitourinary (22.8%), and gastrointestinal (19.7%).
All patients received antibiotics and were stratified in one-hour intervals by the following categories: time from triage to antibiotics; time from qualification for EGDT to antibiotics; time from triage to appropriate antibiotics; and time from qualification for EGDT to appropriate antibiotics.
Total in-hospital mortality was 31% (35.1% for culture-positive patients vs. 25.7% for culture-negative patients, P=0.11). A significant decrease in mortality was only found when appropriate antibiotics were administered within one hour of triage, or within one hour of qualification for EGDT (OR=0.30; 95% CI, 0.11-0.83; P=0.02, and OR=0.50; 95% CI, 0.27-0.92; P=0.03, respectively).
Study limitations included the single-center site and small sample size.
Bottom line: In patients with severe sepsis and septic shock, initiating appropriate antimicrobial therapy within one hour of triage or entry into goal-directed therapy significantly reduces mortality.
Citation: Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045-1053.
Treatment with Higher Levels of Positive End-Expiratory Pressure Has Limited Affect on Hospital Survival
Clinical question: Is treatment with higher versus lower levels of positive end-expiratory pressure (PEEP) associated with improved hospital survival?
Background: In the management of patients with acute lung injury or acute respiratory distress syndrome (ARDS), a fundamental goal is to protect the lungs from ventilation-induced injury, but the optimal PEEP level has not been established.
Study design: Systematic review and meta-analysis.
Setting: N/A.
Synopsis: Three randomized-controlled trials eligible for this review included 2,299 critically ill adults with acute lung injury, as defined by the American-European Consensus Conference. The meta-analysis compared higher and lower PEEP levels with a mean difference of at least 3 cm H2O, incorporated a target tidal volume of less than 8 mL/kg of predicted body weight in both ventilation strategies, and provided patient follow-up until death or for at least 20 days.
This review demonstrated no statistically significant difference in hospital mortality between the groups. However, in patients with ARDS, higher levels of PEEP were associated with a relative reduction in mortality of 10%. This is supported by a recent cohort study in patients with acute lung injury or ARDS, which showed that the effect of PEEP on lung recruitment was associated with the proportion of potentially recruitable lung, as determined by computed tomography.
Since patients with ARDS have more pulmonary edema than those with acute lung injury without ARDS, the former have greater recruitability, and thus might benefit more from higher levels of PEEP.
Bottom line: Higher levels of PEEP might be associated with lower hospital mortality in patients with ARDS, but such a benefit is unlikely in patients with less severe lung injuries, and could actually be harmful.
Citation: Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865-873. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Antibiotics after drainage of uncomplicated skin abscesses
- Clopidogrel vs. combined aspirin-dipyridamole for acute ischemic stroke
- BNP-guided therapy in chronic heart failure outpatients
- Cognitive decline and dementia after hospitalization
- Clopidogrel delays up risks for DES implantation patients
- Clinical score identifies prolonged length of stay
- Time to therapy reduces mortality in sepsis patients
- PEEP associated with lower mortality for ARDS patients
Antibiotics Might Be Unnecessary after Drainage of Uncomplicated Skin Abscesses
Clinical question: Does trimethoprim/sulfamethoxazole (TMP/SMX) treatment after drainage of a skin abscess reduce treatment failure at seven days or development of new lesions at 30 days?
Background: Community ac-quired methicillin-resistant Staphylococcus aureus (MRSA) skin abscesses are increasing in frequency. The benefit of antibiotic treatment after incision and drainage is not clear, as there is a high cure rate without antibiotics.
Study design: Multicenter, double-blinded, randomized, placebo-controlled trial.
Setting: Four military EDs treating civilians and military patients.
Synopsis: The study enrolled a convenience sample of 220 patients, each of whom presented to EDs with uncomplicated skin abscesses from November 2007 to June 2009. Abscesses were drained in the ED, then patients were randomized to either placebo or to TMP/SMX (two DS tablets twice daily) for seven days. Re-evaluation for wound checks occurred at two days and seven days.
Treatment failure at seven days, defined as worsening infection, new lesions, or absence of clinical improvement, occurred in 26% of placebo patients and 17% of patients in the treatment arm, a nonsignificant difference (P=0.12). Fewer patients in the treatment arm had new lesions at 30 days (28% vs. 9%, P=0.02). MRSA was cultured from 53% of patients overall; all samples were sensitive to TMP/SMX.
The study was limited by the fact that only 69% of patients were evaluated at 30 days.
Bottom line: TMP/SMX treatment of uncomplicated skin abscess after drainage in EDs does not decrease treatment failure at seven days, but might decrease the development of new lesions.
Citation: Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection [published online ahead of print March 29, 2010]. Ann Emerg Med. doi:10.1016/j.annemerg med.2010.03.002.
Clopidogrel and Combined Aspirin-Dipyridamole Have Similar Safety and Efficacy Profiles for Acute Ischemic Stroke
Clinical question: What is the efficacy and safety of combined aspirin and extended-release dipyridamole (Asp/ER-DP) compared to clopidogrel in patients with acute ischemic stroke?
Background: Long-term antiplatelet therapy is effective at reducing recurrence after ischemic stroke. However, the relative safety and efficacy of Asp/ER-DP or clopidogrel is not known in patients with acute ischemic stroke.
Study design: Randomized, controlled trial.
Setting: A multicenter trial involving 695 sites in 35 countries.
Synopsis: This post-hoc subgroup analysis of the PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes) trial assessed the relative safety and efficacy of Asp/ER-DP versus clopidogrel administered within 72 hours of stroke onset in 1,360 patients. The primary endpoint was functional outcome at 30 days.
Secondary outcomes included symptomatic hemorrhagic transformation of the infarct, cerebral edema, recurrent stroke, myocardial infarction (MI), composite vascular events (combination of nonfatal stroke, nonfatal MI, and vascular death), death, cognition, bleeding, and serious adverse events studied at seven, 30, and 90 days.
Combined death or dependency did not differ between treatment groups. Nonsignificant trends to reduced recurrence and vascular events were present with Asp/ER-DP. Rates of death, major bleeding, and serious adverse events did not differ between treatment groups.
Bottom line: Either clopidogrel or combined aspirin and extended-release dipyridamole can be used to treat acute ischemic stroke, with similar outcomes and safety profiles.
Citation: Bath PM, Cotton D, Martin RH, et al. Effect of combined aspirin and extended-release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke. 2010;41(4):732-738.
BNP-Guided Therapy Reduces All-Cause Mortality in Outpatients with Chronic Heart Failure
Clinical question: Is there a clinical benefit in using B-type natriuretic peptide (BNP) to guide adjustment of proven medications in chronic heart failure?
Background: BNP is secreted by the heart in response to increased volume. It has been shown to be useful in the diagnosis of decompensated heart failure, and it can be decreased by treatment with proven heart failure medications. It is unclear if this effect provides clinical benefit on mortality and hospitalization.
Study design: Meta-analysis of prospective randomized controlled trials.
Setting: Eight studies involving 1,726 patients, published internationally from 2005-2009.
Synopsis: Study sizes ranged from 41 to 499 patients, with three- to 24-month follow-up. Patients had New York Heart Association (NYHA) class II or greater heart failure, with ejection fractions <50%.
All-cause mortality was significantly lower in BNP-guided therapy compared with clinical-guided therapy (RR=0.76; 95% CI, 0.63-0.91; P=0.003), specifically in patients younger than 75 years old (RR=0.52; 95% CI, 0.33-0.82; P=0.005).
A proposed mechanism for this result was a statistically significant increase in adjustment of most heart failure medications for BNP-guided therapy compared with clinical-guided therapy (75% vs. 58%, P<0.001 in diuretics; 49.6% vs. 30.9%, P<0.001 in ACE inhibitors or Angiotensin II receptor blockers (ARBs); and 51.1% vs. 41.6%, P=0.02 in beta-blockers) and a higher percentage reaching target doses in the BNP-guided therapy group. However, there was no significant decrease in all-cause hospitalization or survival free of hospitalization.
The study limitations include: Hospitalization for heart failure was not meta-analyzed, the pooled data were weighted toward one study, and BNP-guided titration parameters varied across studies.
Bottom line: BNP-guided therapy reduces all-cause mortality in chronic heart failure patients younger than 75 years old, but not all-cause hospitalization or survival free of hospitalization.
Citation: Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med. 2010;170(6):507-514.
Hospitalization Is Associated with Cognitive Decline and Subsequent Risk for Dementia in the Elderly
Clinical question: Is critical illness in patients 65 and older associated with long-term cognitive impairment, and does it affect the incidence of dementia?
Background: There is literature suggesting that survivors of critical illness suffer long-term cognitive impairment, but premorbid measures of cognitive function have not been researched. No studies have evaluated the risk of incident dementia among this patient population.
Study design: Prospective cohort study.
Setting: Group Health Cooperative in Seattle.
Synopsis: This study analyzed data from 2,929 community-dwelling adults older than 65 without baseline dementia. From 1994 to 2007, the individuals were screened with the Cognitive Abilities Screening Instrument (CASI) at follow-up visits every two years. CASI scores lower than 86 (out of 100) led to an examination for dementia; the diagnosis of dementia was an outcome measure. Scores were adjusted for baseline cognitive scores, age, and other risk factors.
For patients following acute-care hospitalization, adjusted CASI scores were 1.01 points lower on average than for those not hospitalized. For patients following critical-illness hospitalization, scores were 2.14 points lower. The dementia rate was 14.6 cases per 1,000 person-years among patients not hospitalized, and 33.6 among those admitted for noncritical illness.
As suspected, hospitalization might be a marker for cognitive decline in the elderly after adjusting for premorbid CASI scores and comorbid illness. Some factors in acute illness—and moreso in critical illness—might be causally related to cognitive decline.
Bottom line: In elderly patients without dementia at baseline, hospitalization for acute care and critical illness increases the likelihood of cognitive decline compared with patients who were not hospitalized. Only noncritical-illness hospitalization was not associated with the development of dementia.
Citation: Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8): 763-770.
Increased Risk of Death and Myocardial Infarction in Patients Who Delay Filling Clopidogrel Prescription after Drug-Eluting Stent Implantation
Clinical question: Is there an increased risk of death or myocardial infarction (MI) in patients with recent drug-eluting stent (DES) implantation who delayed filling their clopidogrel prescription compared with those who filled their prescription on the day of hospital discharge?
Background: Filling an initial prescription of clopidogrel on the day of discharge is important after DES implantation, as prior studies suggest that lack of thienopyridine therapy is a risk factor for early stent thrombosis.
Study design: Retrospective cohort study.
Setting: Three large, integrated healthcare systems.
Synopsis: The cohort included 7,042 patients discharged after DES implantation. Filling of a clopidogrel prescription was based on pharmacy dispensing data. Primary analysis divided patients based on whether they filled the prescription on the day of discharge or any time after discharge. Secondary analysis further characterized delays as >1 day, >3 days, or >5 days after discharge.
One in 6 patients delayed filling the initial prescription. Patients with any degree of delay had significantly higher death and MI rates during follow-up (14.2% vs. 7.9%, P<0.001), as well as an increased risk of death/MI (hazard ratio 1.53; 95% CI, 1.25-1.87). Factors associated with a delay in filling clopidogrel included older age, prior MI, diabetes, renal dysfunction, prior revascularization, cardiogenic shock, in-hospital bleeding, and use of clopidogrel upon admission.
The study was limited in that data were based on pharmacy records, and that patients might have received medication at discharge or outside the healthcare system.
Bottom line: The delay in filling a clopidogrel prescription is associated with an increased risk of death and MI in patients with recent DES implantation.
Citation: Ho PM, Tsai TT, Maddox TM, et al. Delays in filling clopidogrel prescription after hospital discharge and adverse outcomes after drug-eluting stent implantation: implications for transitions of care. Circ Cardiovasc Qual Outcomes. 2010;3(3):261-266.
Predicting Length of Stay after Stroke
Clinical question: Does a clinical score accurately predict prolonged length of stay after stroke?
Background: Stroke is a costly health problem, and length of stay is the most prominent factor contributing to the high costs. The factors leading to prolonged length of stay are varied, and there are no established tools to predict length of stay.
Study design: Prospective cohort study.
Setting: All 28 Israeli hospitals that admit stroke patients.
Synopsis: All patients admitted to Israeli hospitals during established two-month periods in 2004 (1,700 patients) and 2007 (1,648 patients) were included in the National Acute Stroke Israeli Survey (NASIS), and served as the derivation and validation cohort for development of a Prolonged Length of Stay (PLOS) score.
Using the 2004 data, investigators identified stroke severity using the National Institutes of Health Stroke Scale (NIHSS), history of congestive heart failure (CHF), history of atrial fibrillation, decreased level of consciousness on presentation, and intracerebral hemorrhage (as opposed to ischemic stroke) as predictors of prolonged length of stay. Four of these factors were expressed as dichotomous variables, whereas the stroke severity by NIHSS class was incorporated as a range; all were incorporated into a PLOS score.
Higher PLOS score correlated with longer length of stay. In the derivation cohort, 22% of patients with a PLOS score of 0 had a prolonged length of stay, whereas 85% of patients with PLOS scores of 6 or 7 had a prolonged length of stay. In the validation cohort, the corresponding figures were 19% and 72%.
Bottom line: Use of a simple score can predict risk of prolonged length of stay after stroke.
Citation: Koton S, Bornstein NM, Tsabari R, Tanne D, NASIS Investigators. Derivation and validation of the prolonged length of stay score in acute stroke patients. Neurology. 2010;74(19);1511-1516.
Earlier Administration of Appropriate Antimicrobials Decreases Mortality in Patients with Severe Sepsis and Septic Shock
Clinical question: Is the timing of antimicrobial administration an important determinant of survival in patients diagnosed with severe sepsis and septic shock?
Background: Severe sepsis and septic shock are associated with a 25% to 50% mortality rate. Early goal-directed therapy has been shown to increase survival in these patients. Antimicrobial treatment is a mainstay of this therapy, but the most effective timing of this treatment remains unclear.
Study design: Retrospective, single-center cohort study.
Setting: ED at an academic tertiary-care center.
Synopsis: Two hundred sixty-one patients in the ED in 2005-2006 presenting with severe sepsis or septic shock were enrolled in the hospital’s early goal-directed therapy (EGDT) algorithm, either at triage or later during their ED stay. Labs showed 56.7% of patients were culture-positive, with the most common sources being respiratory (30.6%), genitourinary (22.8%), and gastrointestinal (19.7%).
All patients received antibiotics and were stratified in one-hour intervals by the following categories: time from triage to antibiotics; time from qualification for EGDT to antibiotics; time from triage to appropriate antibiotics; and time from qualification for EGDT to appropriate antibiotics.
Total in-hospital mortality was 31% (35.1% for culture-positive patients vs. 25.7% for culture-negative patients, P=0.11). A significant decrease in mortality was only found when appropriate antibiotics were administered within one hour of triage, or within one hour of qualification for EGDT (OR=0.30; 95% CI, 0.11-0.83; P=0.02, and OR=0.50; 95% CI, 0.27-0.92; P=0.03, respectively).
Study limitations included the single-center site and small sample size.
Bottom line: In patients with severe sepsis and septic shock, initiating appropriate antimicrobial therapy within one hour of triage or entry into goal-directed therapy significantly reduces mortality.
Citation: Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045-1053.
Treatment with Higher Levels of Positive End-Expiratory Pressure Has Limited Affect on Hospital Survival
Clinical question: Is treatment with higher versus lower levels of positive end-expiratory pressure (PEEP) associated with improved hospital survival?
Background: In the management of patients with acute lung injury or acute respiratory distress syndrome (ARDS), a fundamental goal is to protect the lungs from ventilation-induced injury, but the optimal PEEP level has not been established.
Study design: Systematic review and meta-analysis.
Setting: N/A.
Synopsis: Three randomized-controlled trials eligible for this review included 2,299 critically ill adults with acute lung injury, as defined by the American-European Consensus Conference. The meta-analysis compared higher and lower PEEP levels with a mean difference of at least 3 cm H2O, incorporated a target tidal volume of less than 8 mL/kg of predicted body weight in both ventilation strategies, and provided patient follow-up until death or for at least 20 days.
This review demonstrated no statistically significant difference in hospital mortality between the groups. However, in patients with ARDS, higher levels of PEEP were associated with a relative reduction in mortality of 10%. This is supported by a recent cohort study in patients with acute lung injury or ARDS, which showed that the effect of PEEP on lung recruitment was associated with the proportion of potentially recruitable lung, as determined by computed tomography.
Since patients with ARDS have more pulmonary edema than those with acute lung injury without ARDS, the former have greater recruitability, and thus might benefit more from higher levels of PEEP.
Bottom line: Higher levels of PEEP might be associated with lower hospital mortality in patients with ARDS, but such a benefit is unlikely in patients with less severe lung injuries, and could actually be harmful.
Citation: Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865-873. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Antibiotics after drainage of uncomplicated skin abscesses
- Clopidogrel vs. combined aspirin-dipyridamole for acute ischemic stroke
- BNP-guided therapy in chronic heart failure outpatients
- Cognitive decline and dementia after hospitalization
- Clopidogrel delays up risks for DES implantation patients
- Clinical score identifies prolonged length of stay
- Time to therapy reduces mortality in sepsis patients
- PEEP associated with lower mortality for ARDS patients
Antibiotics Might Be Unnecessary after Drainage of Uncomplicated Skin Abscesses
Clinical question: Does trimethoprim/sulfamethoxazole (TMP/SMX) treatment after drainage of a skin abscess reduce treatment failure at seven days or development of new lesions at 30 days?
Background: Community ac-quired methicillin-resistant Staphylococcus aureus (MRSA) skin abscesses are increasing in frequency. The benefit of antibiotic treatment after incision and drainage is not clear, as there is a high cure rate without antibiotics.
Study design: Multicenter, double-blinded, randomized, placebo-controlled trial.
Setting: Four military EDs treating civilians and military patients.
Synopsis: The study enrolled a convenience sample of 220 patients, each of whom presented to EDs with uncomplicated skin abscesses from November 2007 to June 2009. Abscesses were drained in the ED, then patients were randomized to either placebo or to TMP/SMX (two DS tablets twice daily) for seven days. Re-evaluation for wound checks occurred at two days and seven days.
Treatment failure at seven days, defined as worsening infection, new lesions, or absence of clinical improvement, occurred in 26% of placebo patients and 17% of patients in the treatment arm, a nonsignificant difference (P=0.12). Fewer patients in the treatment arm had new lesions at 30 days (28% vs. 9%, P=0.02). MRSA was cultured from 53% of patients overall; all samples were sensitive to TMP/SMX.
The study was limited by the fact that only 69% of patients were evaluated at 30 days.
Bottom line: TMP/SMX treatment of uncomplicated skin abscess after drainage in EDs does not decrease treatment failure at seven days, but might decrease the development of new lesions.
Citation: Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection [published online ahead of print March 29, 2010]. Ann Emerg Med. doi:10.1016/j.annemerg med.2010.03.002.
Clopidogrel and Combined Aspirin-Dipyridamole Have Similar Safety and Efficacy Profiles for Acute Ischemic Stroke
Clinical question: What is the efficacy and safety of combined aspirin and extended-release dipyridamole (Asp/ER-DP) compared to clopidogrel in patients with acute ischemic stroke?
Background: Long-term antiplatelet therapy is effective at reducing recurrence after ischemic stroke. However, the relative safety and efficacy of Asp/ER-DP or clopidogrel is not known in patients with acute ischemic stroke.
Study design: Randomized, controlled trial.
Setting: A multicenter trial involving 695 sites in 35 countries.
Synopsis: This post-hoc subgroup analysis of the PRoFESS (Prevention Regimen for Effectively Avoiding Second Strokes) trial assessed the relative safety and efficacy of Asp/ER-DP versus clopidogrel administered within 72 hours of stroke onset in 1,360 patients. The primary endpoint was functional outcome at 30 days.
Secondary outcomes included symptomatic hemorrhagic transformation of the infarct, cerebral edema, recurrent stroke, myocardial infarction (MI), composite vascular events (combination of nonfatal stroke, nonfatal MI, and vascular death), death, cognition, bleeding, and serious adverse events studied at seven, 30, and 90 days.
Combined death or dependency did not differ between treatment groups. Nonsignificant trends to reduced recurrence and vascular events were present with Asp/ER-DP. Rates of death, major bleeding, and serious adverse events did not differ between treatment groups.
Bottom line: Either clopidogrel or combined aspirin and extended-release dipyridamole can be used to treat acute ischemic stroke, with similar outcomes and safety profiles.
Citation: Bath PM, Cotton D, Martin RH, et al. Effect of combined aspirin and extended-release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke. 2010;41(4):732-738.
BNP-Guided Therapy Reduces All-Cause Mortality in Outpatients with Chronic Heart Failure
Clinical question: Is there a clinical benefit in using B-type natriuretic peptide (BNP) to guide adjustment of proven medications in chronic heart failure?
Background: BNP is secreted by the heart in response to increased volume. It has been shown to be useful in the diagnosis of decompensated heart failure, and it can be decreased by treatment with proven heart failure medications. It is unclear if this effect provides clinical benefit on mortality and hospitalization.
Study design: Meta-analysis of prospective randomized controlled trials.
Setting: Eight studies involving 1,726 patients, published internationally from 2005-2009.
Synopsis: Study sizes ranged from 41 to 499 patients, with three- to 24-month follow-up. Patients had New York Heart Association (NYHA) class II or greater heart failure, with ejection fractions <50%.
All-cause mortality was significantly lower in BNP-guided therapy compared with clinical-guided therapy (RR=0.76; 95% CI, 0.63-0.91; P=0.003), specifically in patients younger than 75 years old (RR=0.52; 95% CI, 0.33-0.82; P=0.005).
A proposed mechanism for this result was a statistically significant increase in adjustment of most heart failure medications for BNP-guided therapy compared with clinical-guided therapy (75% vs. 58%, P<0.001 in diuretics; 49.6% vs. 30.9%, P<0.001 in ACE inhibitors or Angiotensin II receptor blockers (ARBs); and 51.1% vs. 41.6%, P=0.02 in beta-blockers) and a higher percentage reaching target doses in the BNP-guided therapy group. However, there was no significant decrease in all-cause hospitalization or survival free of hospitalization.
The study limitations include: Hospitalization for heart failure was not meta-analyzed, the pooled data were weighted toward one study, and BNP-guided titration parameters varied across studies.
Bottom line: BNP-guided therapy reduces all-cause mortality in chronic heart failure patients younger than 75 years old, but not all-cause hospitalization or survival free of hospitalization.
Citation: Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy: A meta-analysis. Arch Intern Med. 2010;170(6):507-514.
Hospitalization Is Associated with Cognitive Decline and Subsequent Risk for Dementia in the Elderly
Clinical question: Is critical illness in patients 65 and older associated with long-term cognitive impairment, and does it affect the incidence of dementia?
Background: There is literature suggesting that survivors of critical illness suffer long-term cognitive impairment, but premorbid measures of cognitive function have not been researched. No studies have evaluated the risk of incident dementia among this patient population.
Study design: Prospective cohort study.
Setting: Group Health Cooperative in Seattle.
Synopsis: This study analyzed data from 2,929 community-dwelling adults older than 65 without baseline dementia. From 1994 to 2007, the individuals were screened with the Cognitive Abilities Screening Instrument (CASI) at follow-up visits every two years. CASI scores lower than 86 (out of 100) led to an examination for dementia; the diagnosis of dementia was an outcome measure. Scores were adjusted for baseline cognitive scores, age, and other risk factors.
For patients following acute-care hospitalization, adjusted CASI scores were 1.01 points lower on average than for those not hospitalized. For patients following critical-illness hospitalization, scores were 2.14 points lower. The dementia rate was 14.6 cases per 1,000 person-years among patients not hospitalized, and 33.6 among those admitted for noncritical illness.
As suspected, hospitalization might be a marker for cognitive decline in the elderly after adjusting for premorbid CASI scores and comorbid illness. Some factors in acute illness—and moreso in critical illness—might be causally related to cognitive decline.
Bottom line: In elderly patients without dementia at baseline, hospitalization for acute care and critical illness increases the likelihood of cognitive decline compared with patients who were not hospitalized. Only noncritical-illness hospitalization was not associated with the development of dementia.
Citation: Ehlenbach WJ, Hough CL, Crane PK, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8): 763-770.
Increased Risk of Death and Myocardial Infarction in Patients Who Delay Filling Clopidogrel Prescription after Drug-Eluting Stent Implantation
Clinical question: Is there an increased risk of death or myocardial infarction (MI) in patients with recent drug-eluting stent (DES) implantation who delayed filling their clopidogrel prescription compared with those who filled their prescription on the day of hospital discharge?
Background: Filling an initial prescription of clopidogrel on the day of discharge is important after DES implantation, as prior studies suggest that lack of thienopyridine therapy is a risk factor for early stent thrombosis.
Study design: Retrospective cohort study.
Setting: Three large, integrated healthcare systems.
Synopsis: The cohort included 7,042 patients discharged after DES implantation. Filling of a clopidogrel prescription was based on pharmacy dispensing data. Primary analysis divided patients based on whether they filled the prescription on the day of discharge or any time after discharge. Secondary analysis further characterized delays as >1 day, >3 days, or >5 days after discharge.
One in 6 patients delayed filling the initial prescription. Patients with any degree of delay had significantly higher death and MI rates during follow-up (14.2% vs. 7.9%, P<0.001), as well as an increased risk of death/MI (hazard ratio 1.53; 95% CI, 1.25-1.87). Factors associated with a delay in filling clopidogrel included older age, prior MI, diabetes, renal dysfunction, prior revascularization, cardiogenic shock, in-hospital bleeding, and use of clopidogrel upon admission.
The study was limited in that data were based on pharmacy records, and that patients might have received medication at discharge or outside the healthcare system.
Bottom line: The delay in filling a clopidogrel prescription is associated with an increased risk of death and MI in patients with recent DES implantation.
Citation: Ho PM, Tsai TT, Maddox TM, et al. Delays in filling clopidogrel prescription after hospital discharge and adverse outcomes after drug-eluting stent implantation: implications for transitions of care. Circ Cardiovasc Qual Outcomes. 2010;3(3):261-266.
Predicting Length of Stay after Stroke
Clinical question: Does a clinical score accurately predict prolonged length of stay after stroke?
Background: Stroke is a costly health problem, and length of stay is the most prominent factor contributing to the high costs. The factors leading to prolonged length of stay are varied, and there are no established tools to predict length of stay.
Study design: Prospective cohort study.
Setting: All 28 Israeli hospitals that admit stroke patients.
Synopsis: All patients admitted to Israeli hospitals during established two-month periods in 2004 (1,700 patients) and 2007 (1,648 patients) were included in the National Acute Stroke Israeli Survey (NASIS), and served as the derivation and validation cohort for development of a Prolonged Length of Stay (PLOS) score.
Using the 2004 data, investigators identified stroke severity using the National Institutes of Health Stroke Scale (NIHSS), history of congestive heart failure (CHF), history of atrial fibrillation, decreased level of consciousness on presentation, and intracerebral hemorrhage (as opposed to ischemic stroke) as predictors of prolonged length of stay. Four of these factors were expressed as dichotomous variables, whereas the stroke severity by NIHSS class was incorporated as a range; all were incorporated into a PLOS score.
Higher PLOS score correlated with longer length of stay. In the derivation cohort, 22% of patients with a PLOS score of 0 had a prolonged length of stay, whereas 85% of patients with PLOS scores of 6 or 7 had a prolonged length of stay. In the validation cohort, the corresponding figures were 19% and 72%.
Bottom line: Use of a simple score can predict risk of prolonged length of stay after stroke.
Citation: Koton S, Bornstein NM, Tsabari R, Tanne D, NASIS Investigators. Derivation and validation of the prolonged length of stay score in acute stroke patients. Neurology. 2010;74(19);1511-1516.
Earlier Administration of Appropriate Antimicrobials Decreases Mortality in Patients with Severe Sepsis and Septic Shock
Clinical question: Is the timing of antimicrobial administration an important determinant of survival in patients diagnosed with severe sepsis and septic shock?
Background: Severe sepsis and septic shock are associated with a 25% to 50% mortality rate. Early goal-directed therapy has been shown to increase survival in these patients. Antimicrobial treatment is a mainstay of this therapy, but the most effective timing of this treatment remains unclear.
Study design: Retrospective, single-center cohort study.
Setting: ED at an academic tertiary-care center.
Synopsis: Two hundred sixty-one patients in the ED in 2005-2006 presenting with severe sepsis or septic shock were enrolled in the hospital’s early goal-directed therapy (EGDT) algorithm, either at triage or later during their ED stay. Labs showed 56.7% of patients were culture-positive, with the most common sources being respiratory (30.6%), genitourinary (22.8%), and gastrointestinal (19.7%).
All patients received antibiotics and were stratified in one-hour intervals by the following categories: time from triage to antibiotics; time from qualification for EGDT to antibiotics; time from triage to appropriate antibiotics; and time from qualification for EGDT to appropriate antibiotics.
Total in-hospital mortality was 31% (35.1% for culture-positive patients vs. 25.7% for culture-negative patients, P=0.11). A significant decrease in mortality was only found when appropriate antibiotics were administered within one hour of triage, or within one hour of qualification for EGDT (OR=0.30; 95% CI, 0.11-0.83; P=0.02, and OR=0.50; 95% CI, 0.27-0.92; P=0.03, respectively).
Study limitations included the single-center site and small sample size.
Bottom line: In patients with severe sepsis and septic shock, initiating appropriate antimicrobial therapy within one hour of triage or entry into goal-directed therapy significantly reduces mortality.
Citation: Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045-1053.
Treatment with Higher Levels of Positive End-Expiratory Pressure Has Limited Affect on Hospital Survival
Clinical question: Is treatment with higher versus lower levels of positive end-expiratory pressure (PEEP) associated with improved hospital survival?
Background: In the management of patients with acute lung injury or acute respiratory distress syndrome (ARDS), a fundamental goal is to protect the lungs from ventilation-induced injury, but the optimal PEEP level has not been established.
Study design: Systematic review and meta-analysis.
Setting: N/A.
Synopsis: Three randomized-controlled trials eligible for this review included 2,299 critically ill adults with acute lung injury, as defined by the American-European Consensus Conference. The meta-analysis compared higher and lower PEEP levels with a mean difference of at least 3 cm H2O, incorporated a target tidal volume of less than 8 mL/kg of predicted body weight in both ventilation strategies, and provided patient follow-up until death or for at least 20 days.
This review demonstrated no statistically significant difference in hospital mortality between the groups. However, in patients with ARDS, higher levels of PEEP were associated with a relative reduction in mortality of 10%. This is supported by a recent cohort study in patients with acute lung injury or ARDS, which showed that the effect of PEEP on lung recruitment was associated with the proportion of potentially recruitable lung, as determined by computed tomography.
Since patients with ARDS have more pulmonary edema than those with acute lung injury without ARDS, the former have greater recruitability, and thus might benefit more from higher levels of PEEP.
Bottom line: Higher levels of PEEP might be associated with lower hospital mortality in patients with ARDS, but such a benefit is unlikely in patients with less severe lung injuries, and could actually be harmful.
Citation: Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865-873. TH
What Are the Chances a Hospitalized Patient Will Survive In-Hospital Arrest?
Case
A 66-year-old woman with metastatic, non-small-cell carcinoma of the lung, chronic obstructive pulmonary disease (COPD), and hypertension presents with progressive shortness of breath and back pain. Her vital signs are normal, with the exception of tachypnea and an oxygen saturation of 84% on room air. A CT scan shows marked progression of her disease and new metastases to her spine. You begin a discussion about advance directives and code status. During the exchange, the patient asks for guidance regarding resuscitation. How can you best answer her questions about the likelihood of surviving an in-hospital arrest?
Background
Discussion regarding resuscitation status is a challenge for most hospitalists. The absence of an established relationship, limited time, patient emotion, and difficulty applying general scientific data to a single patient coalesce into a complex interaction. Further complicating matters, patients frequently have unrealistic expectations and overestimate their chance of survival.
Experience has shown that many patients pursue what physicians consider inappropriately aggressive resuscitation measures. Before you have an informed discussion about cardiopulmonary resuscitation (CPR) outcomes, patients tend to overestimate their likelihood of survival.1 In 2009, Kaldjian and colleagues found that patients’ initial mean prediction of post-arrest survival was 60.4%, compared with the actual mean of approximately 17%.2,3 Furthermore, nearly half of the patients who initially expressed a desire to receive CPR in the event of cardiac arrest opted to change their code status after they were informed of the actual survival estimates.1,2
Patient autonomy and the law, as defined by the 1990 Patient Self-Determination Act, require that physicians share responsibility with patients in making prospective resuscitation decisions.4 Shared decision-making necessitates a basic discussion on admission within the context of the patient’s prognosis and previously expressed wishes. It might simply include an acknowledgment of a previously completed advance directive. A more complex discussion might require in-depth conversation to address patient performance status, prognosis of acute and chronic illnesses, and education about the typical resuscitation procedures. After listening to the patient’s perspective, the admitting physician can provide input and an interpretation of available data regarding the patient’s likelihood of surviving an in-hospital arrest.
Review of the Data
In the past 40 years, the overall survival rates for cardiac arrest have changed little. Despite numerous advances made in the delivery of medical care, on average, only 17% of all adult arrest patients survive to hospital discharge.3 A variety of factors influence this overall survival rate, both pre-arrest and intra-arrest. Clinical experience allows most physicians to sense what probability a patient has for survival and quality of life following a cardiac arrest. However, anecdotal evidence alone does not provide a patient and their family with the information necessary to make an informed decision regarding code status.
Numerous studies have investigated the patient factors that might influence how likely one is to survive a cardiac arrest. Researchers have paid particular attention to such factors as age, race, presence or absence of a cancer diagnosis, and associated comorbidities. Not surprisingly, older age has been shown to be significantly associated with a lower likelihood of survival to discharge following cardiac arrest.5,6
Ehlenbach and colleagues examined medical data from 433,985 Medicare patients 65 and older who underwent in-hospital CPR.5 Both older age and prior residence in a skilled nursing facility were found to be associated with poorer survival rates.5 Although neither study was able to define an upper-age cutoff for certain peri-arrest mortality, age affects overall survival likelihood in an inverse fashion, with those 85 and older having only a 6% chance of surviving to hospital discharge (see Figure 1, p. 18).1,5,6
The degree of comorbid illness can be used to help predict mortality following cardiac arrest. Review of data from the National Registry of Cardiopulmonary Resuscitation (NRCPR) identified particular comorbidities that portend poor post-arrest prognosis.6 In general, the more pre-existing comorbidities a patient has, the less likely they are to survive.6 The presence of hepatic insufficiency, acute stroke, immunodeficiency, renal failure, or dialysis were associated with lower survival rates (see Figure 2, right).6,7
Poor performance status on admission, defined as severe disability, coma, or vegetative state, was predictive of worse outcomes.6 Understandably, patients with hypotension and those who required vasopressors or mechanical ventilation also tended to have lower post-arrest survival rates.6
The presence of a cancer diagnosis is another prognostic factor of interest when considering the chances of surviving an arrest. Classically, CPR was thought to be a futile intervention in this patient population. Specific characteristics within this subset of patients have been shown to influence prognosis, and multiple studies have confirmed that cancer patients generally do worse after an arrest with an overall survival rate of only 6.2%.8 Survival rates tend to be lower in patients with metastatic disease, hematologic malignancies, a history of stem cell transplant, those who arrest within an ICU, and inpatients whose cardiac arrest was anticipated.8,9
In fact, cancer patients whose hospital course followed a path of gradual deterioration showed a 0% survival rate.9 In patients with metastatic disease, poor performance status prior to arrest appeared to account for their particularly poor survival odds (this supports the intuitive, rule-of-thumb that sicker cancer patients have worse outcomes).8
Growing evidence suggests the probability of post-arrest survival is not equal between racial groups. Specifically, black or nonwhite race is associated with higher utilization of CPR and lower survival rates (see Figure 3, right).10 Among Medicare patients, Ehlenbach and colleagues found that black and nonwhite patients were much more likely to undergo CPR, presumably as a result of being less likely to opt for DNR status.5,10 Although this could account for the differences seen in survival rates among these populations, these findings also raise concerns about the possibility of racial disparities in medical care. A subsequent cohort study also suggested that blacks and nonwhites were less likely to survive following cardiac arrest.10 However, adjusted analysis revealed that these differences were strongly associated with the medical center at which these patients received care. Therefore, although being nonwhite does portend worse outcomes following an arrest, the increased risk is likely attributed to the fact that many of these patients receive care at hospitals that have poorer overall CPR performance measures.5,10
Survival is not the only outcome measure patients need to take into account when deciding whether to undergo CPR. Quality of life following resuscitation also warrants consideration. Interestingly, research has shown that neurologic outcomes among the majority of cardiac arrest survivors are generally good.3
Approximately 86% of survivors with intact pre-arrest cerebral performance maintain it on discharge, and only a minority of survivors are eventually declared brain-dead.3 Still, there are certain peri-arrest factors that pose risk for poorer neurologic and functional outcomes. For arrest from a shockable rhythm, time to defibrillation is a key determinant.11 In patients for whom time to defibrillation is greater than two minutes, there is significantly higher risk of permanent disability following cardiac arrest.11
In the event of coma following resuscitation, particular clinical findings can be used to accurately predict poor outcome.12 The absence of pupillary reflexes, corneal reflexes, or absent or extensor motor responses three days after arrest are poor prognostic indicators.12 As a general rule, if a patient does not awaken within three days, neurologic and functional impairment can be expected.12 For those patients who do survive to hospital discharge, more than 50% ultimately will be able to be discharged home.3
However, nearly a quarter will need to be newly placed in a rehabilitation or skilled nursing facility.3
Back to the Case
The patient was admitted with hypoxia secondary to both progressive lung malignancy and COPD exacerbation. She had no advanced directives, so the admitting hospitalist, in collaboration with her oncologist, had a detailed discussion regarding her understanding of her disease progression, prognosis, and goals for her remaining time. Her questions regarding survivability of cardiac arrest were answered directly with an estimate of 5% to 10%, based on her age, comorbidities, and the presence of advanced malignancy.
After hearing this information, the patient responded, “I still want everything done.” The hospitalist acknowledged her feelings of wanting to fight on, but asked her to think about what “everything” meant to her. After taking some additional time to reflect with friends and family, the patient was clear that she wanted to continue disease-focused therapies, but did not want to be resuscitated in the event of cardiac or pulmonary arrest.
Eventually, her hypoxia improved with antibiotics, steroids, and bronchodilators. She was discharged home with follow-up in the oncology clinic for additional chemotherapy and palliative radiation.
Bottom Line
For hospitalized adults, the average survival rate to discharge after cardiac arrest is about 17%. Many factors lower a patient’s chance of survival, including advanced age, performance status, malignancy, and presence of multiple comorbidities. TH
Dr. Neagle and Dr. Wachsberg are hospitalists and instructors in the division of hospital medicine at Northwestern University Medical Center in Chicago.
References
- Murphy DJ, Burrows D, Santali S, et al. The influence of the probability of survival on patients’ preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330(8):545-549.
- Kaldjian LC, Erekson ZD, Haberle TH, et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35(6):338-342.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297-308.
- La Puma J, Orentlicher D, Moss RJ. Advance directives on admission. Clinical implications and analysis of the Patient Self-Determination Act of 1990. JAMA. 1991;266(3):402-405.
- Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22-31.
- Larkin GL, Copes WS, Nathanson BH, Kaye W. Pre-resuscitation factors associated with mortality in 49,130 cases of in-hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81(3):302-311.
- de Vos R, Koster RW, De Haan RJ, Oosting H, van der Wouw PA, Lampe-Schoenmaeckers AJ. In-hospital cardiopulmonary resuscitation: prearrest morbidity and outcome. Arch Intern Med. 1999;159(8):845-850.
- Reisfield GM, Wallace SK, Munsell MF, Webb FJ, Alvarez ER, Wilson GR. Survival in cancer patients undergoing in-hospital cardiopulmonary resuscitation: a meta-analysis. Resuscitation. 2006;71(2):152-160.
- Ewer MS, Kish SK, Martin CG, Price KJ, Feeley TW. Characteristics of cardiac arrest in cancer patients as a predictor of survival after cardiopulmonary resuscitation. Cancer. 2001;92(7):1905-1912.
- Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302(11):1195-1201.
- Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17.
- Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203-210.
Case
A 66-year-old woman with metastatic, non-small-cell carcinoma of the lung, chronic obstructive pulmonary disease (COPD), and hypertension presents with progressive shortness of breath and back pain. Her vital signs are normal, with the exception of tachypnea and an oxygen saturation of 84% on room air. A CT scan shows marked progression of her disease and new metastases to her spine. You begin a discussion about advance directives and code status. During the exchange, the patient asks for guidance regarding resuscitation. How can you best answer her questions about the likelihood of surviving an in-hospital arrest?
Background
Discussion regarding resuscitation status is a challenge for most hospitalists. The absence of an established relationship, limited time, patient emotion, and difficulty applying general scientific data to a single patient coalesce into a complex interaction. Further complicating matters, patients frequently have unrealistic expectations and overestimate their chance of survival.
Experience has shown that many patients pursue what physicians consider inappropriately aggressive resuscitation measures. Before you have an informed discussion about cardiopulmonary resuscitation (CPR) outcomes, patients tend to overestimate their likelihood of survival.1 In 2009, Kaldjian and colleagues found that patients’ initial mean prediction of post-arrest survival was 60.4%, compared with the actual mean of approximately 17%.2,3 Furthermore, nearly half of the patients who initially expressed a desire to receive CPR in the event of cardiac arrest opted to change their code status after they were informed of the actual survival estimates.1,2
Patient autonomy and the law, as defined by the 1990 Patient Self-Determination Act, require that physicians share responsibility with patients in making prospective resuscitation decisions.4 Shared decision-making necessitates a basic discussion on admission within the context of the patient’s prognosis and previously expressed wishes. It might simply include an acknowledgment of a previously completed advance directive. A more complex discussion might require in-depth conversation to address patient performance status, prognosis of acute and chronic illnesses, and education about the typical resuscitation procedures. After listening to the patient’s perspective, the admitting physician can provide input and an interpretation of available data regarding the patient’s likelihood of surviving an in-hospital arrest.
Review of the Data
In the past 40 years, the overall survival rates for cardiac arrest have changed little. Despite numerous advances made in the delivery of medical care, on average, only 17% of all adult arrest patients survive to hospital discharge.3 A variety of factors influence this overall survival rate, both pre-arrest and intra-arrest. Clinical experience allows most physicians to sense what probability a patient has for survival and quality of life following a cardiac arrest. However, anecdotal evidence alone does not provide a patient and their family with the information necessary to make an informed decision regarding code status.
Numerous studies have investigated the patient factors that might influence how likely one is to survive a cardiac arrest. Researchers have paid particular attention to such factors as age, race, presence or absence of a cancer diagnosis, and associated comorbidities. Not surprisingly, older age has been shown to be significantly associated with a lower likelihood of survival to discharge following cardiac arrest.5,6
Ehlenbach and colleagues examined medical data from 433,985 Medicare patients 65 and older who underwent in-hospital CPR.5 Both older age and prior residence in a skilled nursing facility were found to be associated with poorer survival rates.5 Although neither study was able to define an upper-age cutoff for certain peri-arrest mortality, age affects overall survival likelihood in an inverse fashion, with those 85 and older having only a 6% chance of surviving to hospital discharge (see Figure 1, p. 18).1,5,6
The degree of comorbid illness can be used to help predict mortality following cardiac arrest. Review of data from the National Registry of Cardiopulmonary Resuscitation (NRCPR) identified particular comorbidities that portend poor post-arrest prognosis.6 In general, the more pre-existing comorbidities a patient has, the less likely they are to survive.6 The presence of hepatic insufficiency, acute stroke, immunodeficiency, renal failure, or dialysis were associated with lower survival rates (see Figure 2, right).6,7
Poor performance status on admission, defined as severe disability, coma, or vegetative state, was predictive of worse outcomes.6 Understandably, patients with hypotension and those who required vasopressors or mechanical ventilation also tended to have lower post-arrest survival rates.6
The presence of a cancer diagnosis is another prognostic factor of interest when considering the chances of surviving an arrest. Classically, CPR was thought to be a futile intervention in this patient population. Specific characteristics within this subset of patients have been shown to influence prognosis, and multiple studies have confirmed that cancer patients generally do worse after an arrest with an overall survival rate of only 6.2%.8 Survival rates tend to be lower in patients with metastatic disease, hematologic malignancies, a history of stem cell transplant, those who arrest within an ICU, and inpatients whose cardiac arrest was anticipated.8,9
In fact, cancer patients whose hospital course followed a path of gradual deterioration showed a 0% survival rate.9 In patients with metastatic disease, poor performance status prior to arrest appeared to account for their particularly poor survival odds (this supports the intuitive, rule-of-thumb that sicker cancer patients have worse outcomes).8
Growing evidence suggests the probability of post-arrest survival is not equal between racial groups. Specifically, black or nonwhite race is associated with higher utilization of CPR and lower survival rates (see Figure 3, right).10 Among Medicare patients, Ehlenbach and colleagues found that black and nonwhite patients were much more likely to undergo CPR, presumably as a result of being less likely to opt for DNR status.5,10 Although this could account for the differences seen in survival rates among these populations, these findings also raise concerns about the possibility of racial disparities in medical care. A subsequent cohort study also suggested that blacks and nonwhites were less likely to survive following cardiac arrest.10 However, adjusted analysis revealed that these differences were strongly associated with the medical center at which these patients received care. Therefore, although being nonwhite does portend worse outcomes following an arrest, the increased risk is likely attributed to the fact that many of these patients receive care at hospitals that have poorer overall CPR performance measures.5,10
Survival is not the only outcome measure patients need to take into account when deciding whether to undergo CPR. Quality of life following resuscitation also warrants consideration. Interestingly, research has shown that neurologic outcomes among the majority of cardiac arrest survivors are generally good.3
Approximately 86% of survivors with intact pre-arrest cerebral performance maintain it on discharge, and only a minority of survivors are eventually declared brain-dead.3 Still, there are certain peri-arrest factors that pose risk for poorer neurologic and functional outcomes. For arrest from a shockable rhythm, time to defibrillation is a key determinant.11 In patients for whom time to defibrillation is greater than two minutes, there is significantly higher risk of permanent disability following cardiac arrest.11
In the event of coma following resuscitation, particular clinical findings can be used to accurately predict poor outcome.12 The absence of pupillary reflexes, corneal reflexes, or absent or extensor motor responses three days after arrest are poor prognostic indicators.12 As a general rule, if a patient does not awaken within three days, neurologic and functional impairment can be expected.12 For those patients who do survive to hospital discharge, more than 50% ultimately will be able to be discharged home.3
However, nearly a quarter will need to be newly placed in a rehabilitation or skilled nursing facility.3
Back to the Case
The patient was admitted with hypoxia secondary to both progressive lung malignancy and COPD exacerbation. She had no advanced directives, so the admitting hospitalist, in collaboration with her oncologist, had a detailed discussion regarding her understanding of her disease progression, prognosis, and goals for her remaining time. Her questions regarding survivability of cardiac arrest were answered directly with an estimate of 5% to 10%, based on her age, comorbidities, and the presence of advanced malignancy.
After hearing this information, the patient responded, “I still want everything done.” The hospitalist acknowledged her feelings of wanting to fight on, but asked her to think about what “everything” meant to her. After taking some additional time to reflect with friends and family, the patient was clear that she wanted to continue disease-focused therapies, but did not want to be resuscitated in the event of cardiac or pulmonary arrest.
Eventually, her hypoxia improved with antibiotics, steroids, and bronchodilators. She was discharged home with follow-up in the oncology clinic for additional chemotherapy and palliative radiation.
Bottom Line
For hospitalized adults, the average survival rate to discharge after cardiac arrest is about 17%. Many factors lower a patient’s chance of survival, including advanced age, performance status, malignancy, and presence of multiple comorbidities. TH
Dr. Neagle and Dr. Wachsberg are hospitalists and instructors in the division of hospital medicine at Northwestern University Medical Center in Chicago.
References
- Murphy DJ, Burrows D, Santali S, et al. The influence of the probability of survival on patients’ preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330(8):545-549.
- Kaldjian LC, Erekson ZD, Haberle TH, et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35(6):338-342.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297-308.
- La Puma J, Orentlicher D, Moss RJ. Advance directives on admission. Clinical implications and analysis of the Patient Self-Determination Act of 1990. JAMA. 1991;266(3):402-405.
- Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22-31.
- Larkin GL, Copes WS, Nathanson BH, Kaye W. Pre-resuscitation factors associated with mortality in 49,130 cases of in-hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81(3):302-311.
- de Vos R, Koster RW, De Haan RJ, Oosting H, van der Wouw PA, Lampe-Schoenmaeckers AJ. In-hospital cardiopulmonary resuscitation: prearrest morbidity and outcome. Arch Intern Med. 1999;159(8):845-850.
- Reisfield GM, Wallace SK, Munsell MF, Webb FJ, Alvarez ER, Wilson GR. Survival in cancer patients undergoing in-hospital cardiopulmonary resuscitation: a meta-analysis. Resuscitation. 2006;71(2):152-160.
- Ewer MS, Kish SK, Martin CG, Price KJ, Feeley TW. Characteristics of cardiac arrest in cancer patients as a predictor of survival after cardiopulmonary resuscitation. Cancer. 2001;92(7):1905-1912.
- Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302(11):1195-1201.
- Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17.
- Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203-210.
Case
A 66-year-old woman with metastatic, non-small-cell carcinoma of the lung, chronic obstructive pulmonary disease (COPD), and hypertension presents with progressive shortness of breath and back pain. Her vital signs are normal, with the exception of tachypnea and an oxygen saturation of 84% on room air. A CT scan shows marked progression of her disease and new metastases to her spine. You begin a discussion about advance directives and code status. During the exchange, the patient asks for guidance regarding resuscitation. How can you best answer her questions about the likelihood of surviving an in-hospital arrest?
Background
Discussion regarding resuscitation status is a challenge for most hospitalists. The absence of an established relationship, limited time, patient emotion, and difficulty applying general scientific data to a single patient coalesce into a complex interaction. Further complicating matters, patients frequently have unrealistic expectations and overestimate their chance of survival.
Experience has shown that many patients pursue what physicians consider inappropriately aggressive resuscitation measures. Before you have an informed discussion about cardiopulmonary resuscitation (CPR) outcomes, patients tend to overestimate their likelihood of survival.1 In 2009, Kaldjian and colleagues found that patients’ initial mean prediction of post-arrest survival was 60.4%, compared with the actual mean of approximately 17%.2,3 Furthermore, nearly half of the patients who initially expressed a desire to receive CPR in the event of cardiac arrest opted to change their code status after they were informed of the actual survival estimates.1,2
Patient autonomy and the law, as defined by the 1990 Patient Self-Determination Act, require that physicians share responsibility with patients in making prospective resuscitation decisions.4 Shared decision-making necessitates a basic discussion on admission within the context of the patient’s prognosis and previously expressed wishes. It might simply include an acknowledgment of a previously completed advance directive. A more complex discussion might require in-depth conversation to address patient performance status, prognosis of acute and chronic illnesses, and education about the typical resuscitation procedures. After listening to the patient’s perspective, the admitting physician can provide input and an interpretation of available data regarding the patient’s likelihood of surviving an in-hospital arrest.
Review of the Data
In the past 40 years, the overall survival rates for cardiac arrest have changed little. Despite numerous advances made in the delivery of medical care, on average, only 17% of all adult arrest patients survive to hospital discharge.3 A variety of factors influence this overall survival rate, both pre-arrest and intra-arrest. Clinical experience allows most physicians to sense what probability a patient has for survival and quality of life following a cardiac arrest. However, anecdotal evidence alone does not provide a patient and their family with the information necessary to make an informed decision regarding code status.
Numerous studies have investigated the patient factors that might influence how likely one is to survive a cardiac arrest. Researchers have paid particular attention to such factors as age, race, presence or absence of a cancer diagnosis, and associated comorbidities. Not surprisingly, older age has been shown to be significantly associated with a lower likelihood of survival to discharge following cardiac arrest.5,6
Ehlenbach and colleagues examined medical data from 433,985 Medicare patients 65 and older who underwent in-hospital CPR.5 Both older age and prior residence in a skilled nursing facility were found to be associated with poorer survival rates.5 Although neither study was able to define an upper-age cutoff for certain peri-arrest mortality, age affects overall survival likelihood in an inverse fashion, with those 85 and older having only a 6% chance of surviving to hospital discharge (see Figure 1, p. 18).1,5,6
The degree of comorbid illness can be used to help predict mortality following cardiac arrest. Review of data from the National Registry of Cardiopulmonary Resuscitation (NRCPR) identified particular comorbidities that portend poor post-arrest prognosis.6 In general, the more pre-existing comorbidities a patient has, the less likely they are to survive.6 The presence of hepatic insufficiency, acute stroke, immunodeficiency, renal failure, or dialysis were associated with lower survival rates (see Figure 2, right).6,7
Poor performance status on admission, defined as severe disability, coma, or vegetative state, was predictive of worse outcomes.6 Understandably, patients with hypotension and those who required vasopressors or mechanical ventilation also tended to have lower post-arrest survival rates.6
The presence of a cancer diagnosis is another prognostic factor of interest when considering the chances of surviving an arrest. Classically, CPR was thought to be a futile intervention in this patient population. Specific characteristics within this subset of patients have been shown to influence prognosis, and multiple studies have confirmed that cancer patients generally do worse after an arrest with an overall survival rate of only 6.2%.8 Survival rates tend to be lower in patients with metastatic disease, hematologic malignancies, a history of stem cell transplant, those who arrest within an ICU, and inpatients whose cardiac arrest was anticipated.8,9
In fact, cancer patients whose hospital course followed a path of gradual deterioration showed a 0% survival rate.9 In patients with metastatic disease, poor performance status prior to arrest appeared to account for their particularly poor survival odds (this supports the intuitive, rule-of-thumb that sicker cancer patients have worse outcomes).8
Growing evidence suggests the probability of post-arrest survival is not equal between racial groups. Specifically, black or nonwhite race is associated with higher utilization of CPR and lower survival rates (see Figure 3, right).10 Among Medicare patients, Ehlenbach and colleagues found that black and nonwhite patients were much more likely to undergo CPR, presumably as a result of being less likely to opt for DNR status.5,10 Although this could account for the differences seen in survival rates among these populations, these findings also raise concerns about the possibility of racial disparities in medical care. A subsequent cohort study also suggested that blacks and nonwhites were less likely to survive following cardiac arrest.10 However, adjusted analysis revealed that these differences were strongly associated with the medical center at which these patients received care. Therefore, although being nonwhite does portend worse outcomes following an arrest, the increased risk is likely attributed to the fact that many of these patients receive care at hospitals that have poorer overall CPR performance measures.5,10
Survival is not the only outcome measure patients need to take into account when deciding whether to undergo CPR. Quality of life following resuscitation also warrants consideration. Interestingly, research has shown that neurologic outcomes among the majority of cardiac arrest survivors are generally good.3
Approximately 86% of survivors with intact pre-arrest cerebral performance maintain it on discharge, and only a minority of survivors are eventually declared brain-dead.3 Still, there are certain peri-arrest factors that pose risk for poorer neurologic and functional outcomes. For arrest from a shockable rhythm, time to defibrillation is a key determinant.11 In patients for whom time to defibrillation is greater than two minutes, there is significantly higher risk of permanent disability following cardiac arrest.11
In the event of coma following resuscitation, particular clinical findings can be used to accurately predict poor outcome.12 The absence of pupillary reflexes, corneal reflexes, or absent or extensor motor responses three days after arrest are poor prognostic indicators.12 As a general rule, if a patient does not awaken within three days, neurologic and functional impairment can be expected.12 For those patients who do survive to hospital discharge, more than 50% ultimately will be able to be discharged home.3
However, nearly a quarter will need to be newly placed in a rehabilitation or skilled nursing facility.3
Back to the Case
The patient was admitted with hypoxia secondary to both progressive lung malignancy and COPD exacerbation. She had no advanced directives, so the admitting hospitalist, in collaboration with her oncologist, had a detailed discussion regarding her understanding of her disease progression, prognosis, and goals for her remaining time. Her questions regarding survivability of cardiac arrest were answered directly with an estimate of 5% to 10%, based on her age, comorbidities, and the presence of advanced malignancy.
After hearing this information, the patient responded, “I still want everything done.” The hospitalist acknowledged her feelings of wanting to fight on, but asked her to think about what “everything” meant to her. After taking some additional time to reflect with friends and family, the patient was clear that she wanted to continue disease-focused therapies, but did not want to be resuscitated in the event of cardiac or pulmonary arrest.
Eventually, her hypoxia improved with antibiotics, steroids, and bronchodilators. She was discharged home with follow-up in the oncology clinic for additional chemotherapy and palliative radiation.
Bottom Line
For hospitalized adults, the average survival rate to discharge after cardiac arrest is about 17%. Many factors lower a patient’s chance of survival, including advanced age, performance status, malignancy, and presence of multiple comorbidities. TH
Dr. Neagle and Dr. Wachsberg are hospitalists and instructors in the division of hospital medicine at Northwestern University Medical Center in Chicago.
References
- Murphy DJ, Burrows D, Santali S, et al. The influence of the probability of survival on patients’ preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330(8):545-549.
- Kaldjian LC, Erekson ZD, Haberle TH, et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35(6):338-342.
- Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297-308.
- La Puma J, Orentlicher D, Moss RJ. Advance directives on admission. Clinical implications and analysis of the Patient Self-Determination Act of 1990. JAMA. 1991;266(3):402-405.
- Ehlenbach WJ, Barnato AE, Curtis JR, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22-31.
- Larkin GL, Copes WS, Nathanson BH, Kaye W. Pre-resuscitation factors associated with mortality in 49,130 cases of in-hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81(3):302-311.
- de Vos R, Koster RW, De Haan RJ, Oosting H, van der Wouw PA, Lampe-Schoenmaeckers AJ. In-hospital cardiopulmonary resuscitation: prearrest morbidity and outcome. Arch Intern Med. 1999;159(8):845-850.
- Reisfield GM, Wallace SK, Munsell MF, Webb FJ, Alvarez ER, Wilson GR. Survival in cancer patients undergoing in-hospital cardiopulmonary resuscitation: a meta-analysis. Resuscitation. 2006;71(2):152-160.
- Ewer MS, Kish SK, Martin CG, Price KJ, Feeley TW. Characteristics of cardiac arrest in cancer patients as a predictor of survival after cardiopulmonary resuscitation. Cancer. 2001;92(7):1905-1912.
- Chan PS, Nichol G, Krumholz HM, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302(11):1195-1201.
- Chan PS, Krumholz HM, Nichol G, Nallamothu BK. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358(1):9-17.
- Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203-210.
Hospital-Acquired Condition (HAC) Guidelines Produce $20M in Medicare Savings
How much has Medicare saved by not paying hospitals when patients get infections?
Hugh Black, DO
Charlotte, N.C.
Dr. Hospitalist responds: Since 2007, the Centers for Medicare and Medicaid Services (CMS) has tried to reduce the number of high-cost, hospital-acquired conditions (HACs), including infections, by encouraging providers to adhere to evidence-based guidelines. Some examples of these hospital-acquired conditions include:
- Catheter-associated urinary tract infections;
- Foreign objects retained after surgery; and
- Stage III and IV pressure ulcers.
CMS requires that acute-care hospitals, “effective with discharges occurring on or after Oct. 1, 2007, submit information on Medicare claims specifying whether diagnoses were present on admission.” Effective Oct. 1, 2008, Medicare no longer pays for charges associated with these HACs. So, if a Medicare beneficiary developed a Stage III pressure ulcer during his stay at an acute-care hospital, CMS would not pay for the incremental cost of the care associated with the “HAC.”
The U.S. government, in the May 4, 2010, edition of the Federal Register, reviewed the impact of this program. The data are based on Medicare claims data from October 2008 to June 2009. During this period of time, there were approximately 7.17 million acute-care hospital Medicare discharges.
The total net savings during this nine-month period for all HACs was $16.4 million. Three HACs (Stage III and IV pressure ulcers, DVT/PE after orthopedic procedure, and falls and trauma) accounted for more than $15.1 million in savings. Pro-rated for a 12-month period, the total net savings for all HACs would exceed $20 million.
Falls and trauma accounted for 34% of all HACs reported (11,253), followed by vascular catheter-associated infection (16%) and catheter-associated UTIs (16%). Air embolism and mediastinitis after CABG were the least recorded HACs; both were less than .01% if the total.
The goal is that, over time, with improvement in care, there would be a decrease in the number of hospital discharges where these conditions would be present. Therefore, the net savings would be expected to decline.
Medicare has considered a number of other HACs for this program, and reviewed the numbers of these conditions over the same nine-month period (see “Medicare’s Potential New Hospital-Acquired Conditions,” above). Despite some large numbers, CMS has stated it’s not proposing to add or remove HAC categories at this time. If you are interested in reviewing the entire report, visit http://edocket.access.gpo.gov/2010/pdf/ 2010-9163.pdf. TH
How much has Medicare saved by not paying hospitals when patients get infections?
Hugh Black, DO
Charlotte, N.C.
Dr. Hospitalist responds: Since 2007, the Centers for Medicare and Medicaid Services (CMS) has tried to reduce the number of high-cost, hospital-acquired conditions (HACs), including infections, by encouraging providers to adhere to evidence-based guidelines. Some examples of these hospital-acquired conditions include:
- Catheter-associated urinary tract infections;
- Foreign objects retained after surgery; and
- Stage III and IV pressure ulcers.
CMS requires that acute-care hospitals, “effective with discharges occurring on or after Oct. 1, 2007, submit information on Medicare claims specifying whether diagnoses were present on admission.” Effective Oct. 1, 2008, Medicare no longer pays for charges associated with these HACs. So, if a Medicare beneficiary developed a Stage III pressure ulcer during his stay at an acute-care hospital, CMS would not pay for the incremental cost of the care associated with the “HAC.”
The U.S. government, in the May 4, 2010, edition of the Federal Register, reviewed the impact of this program. The data are based on Medicare claims data from October 2008 to June 2009. During this period of time, there were approximately 7.17 million acute-care hospital Medicare discharges.
The total net savings during this nine-month period for all HACs was $16.4 million. Three HACs (Stage III and IV pressure ulcers, DVT/PE after orthopedic procedure, and falls and trauma) accounted for more than $15.1 million in savings. Pro-rated for a 12-month period, the total net savings for all HACs would exceed $20 million.
Falls and trauma accounted for 34% of all HACs reported (11,253), followed by vascular catheter-associated infection (16%) and catheter-associated UTIs (16%). Air embolism and mediastinitis after CABG were the least recorded HACs; both were less than .01% if the total.
The goal is that, over time, with improvement in care, there would be a decrease in the number of hospital discharges where these conditions would be present. Therefore, the net savings would be expected to decline.
Medicare has considered a number of other HACs for this program, and reviewed the numbers of these conditions over the same nine-month period (see “Medicare’s Potential New Hospital-Acquired Conditions,” above). Despite some large numbers, CMS has stated it’s not proposing to add or remove HAC categories at this time. If you are interested in reviewing the entire report, visit http://edocket.access.gpo.gov/2010/pdf/ 2010-9163.pdf. TH
How much has Medicare saved by not paying hospitals when patients get infections?
Hugh Black, DO
Charlotte, N.C.
Dr. Hospitalist responds: Since 2007, the Centers for Medicare and Medicaid Services (CMS) has tried to reduce the number of high-cost, hospital-acquired conditions (HACs), including infections, by encouraging providers to adhere to evidence-based guidelines. Some examples of these hospital-acquired conditions include:
- Catheter-associated urinary tract infections;
- Foreign objects retained after surgery; and
- Stage III and IV pressure ulcers.
CMS requires that acute-care hospitals, “effective with discharges occurring on or after Oct. 1, 2007, submit information on Medicare claims specifying whether diagnoses were present on admission.” Effective Oct. 1, 2008, Medicare no longer pays for charges associated with these HACs. So, if a Medicare beneficiary developed a Stage III pressure ulcer during his stay at an acute-care hospital, CMS would not pay for the incremental cost of the care associated with the “HAC.”
The U.S. government, in the May 4, 2010, edition of the Federal Register, reviewed the impact of this program. The data are based on Medicare claims data from October 2008 to June 2009. During this period of time, there were approximately 7.17 million acute-care hospital Medicare discharges.
The total net savings during this nine-month period for all HACs was $16.4 million. Three HACs (Stage III and IV pressure ulcers, DVT/PE after orthopedic procedure, and falls and trauma) accounted for more than $15.1 million in savings. Pro-rated for a 12-month period, the total net savings for all HACs would exceed $20 million.
Falls and trauma accounted for 34% of all HACs reported (11,253), followed by vascular catheter-associated infection (16%) and catheter-associated UTIs (16%). Air embolism and mediastinitis after CABG were the least recorded HACs; both were less than .01% if the total.
The goal is that, over time, with improvement in care, there would be a decrease in the number of hospital discharges where these conditions would be present. Therefore, the net savings would be expected to decline.
Medicare has considered a number of other HACs for this program, and reviewed the numbers of these conditions over the same nine-month period (see “Medicare’s Potential New Hospital-Acquired Conditions,” above). Despite some large numbers, CMS has stated it’s not proposing to add or remove HAC categories at this time. If you are interested in reviewing the entire report, visit http://edocket.access.gpo.gov/2010/pdf/ 2010-9163.pdf. TH
Unscripted
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was 20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal 1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, 5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was 20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal 1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, 5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
A 58‐year old man was admitted with generalized weakness and acute deep venous thrombosis (DVT). His past medical history included hypertension and polymyositis/dermatomyositis (PM/DM) with anti‐synthase syndrome, which had been diagnosed 16 months prior when his creatine kinase (CK) was greater than 12,000 U/L. At that time he also was found to have bilateral lower extremity DVT, and had been treated with warfarin for 1 year. 10 days previously, he had been discharged after a 4‐day hospitalization for a polymyositis flare which was treated with methylprednisolone at 60 mg daily for 5 days. He was discharged home with daily prednisone until this follow‐up a week later, where he reported weakness and bilateral edema. Lower extremity ultrasound demonstrated acute thrombus in the right common femoral vein.
This acute extensive DVT may be a consequence of recent hospitalization and a previously damaged venous system, or may reflect ongoing hypercoagulability from an unresolved condition, such as cancer. Bilateral lower extremity edema may suggest right‐sided heart failure due to progressive interstitial lung disease, which occurs in a subset of patients with PM/DM. Edema may alternatively reflect biventricular heart failure, or liver or kidney disease.
Generalized weakness offers little in the way of focused differential diagnosis until it is characterized as motor weakness (eg, attributed to progression of the myopathy), a dyspnea‐equivalent, or an overall sense of fatigue.
His medications included weekly methotrexate, monthly intravenous immunoglobulin (IVIG) infusions, tacrolimus, hydrochlorothiazide, and aerosolized pentamidine. He had been on varying doses of prednisone for 2 years and his present dose was 40 mg daily. He was allergic to sulfa. He was married and stopped smoking 30 years previously, and did not drink alcohol or use illicit drugs.
Various medication toxicities could account for his presentation. Methotrexate causes interstitial lung disease, and IVIG and tacrolimus may cause renal failure (and fluid overload). The heavy degree of immunosuppression renders him susceptible to a wide range of infections. Aerosolized pentamidine provides incomplete protection against Pneumocystis jirovecii, especially in the lung apices.
Evaluation of the status of his myositis with motor strength assessment is important. In addition associated rashes and signs of malignancy (eg, lymphadenopathy) and infection should be sought. Proximal motor weakness would suggest a myositis flare, although care must be given to exclude competing causes of myopathy, including infections, toxins, or endocrinopathies.
His temperature was 36.2C, pulse 103 beats per minute, blood pressure 156/83 mm Hg, and respiratory rate 18 breaths per minute. He had crackles at both lung bases, and 3+ pitting edema in both lower extremities. On neurological exam his motor strength was found to be diminished at 3/5 in the lower extremities and proximal upper extremities and 4/5 in the distal upper extremities. Reflexes were uniformly at 1+/4 and his cognition was intact. Examinations of his head, skin, heart, and abdomen were normal.
The absence of elevated jugular venous pressure argues against right heart failure. He is afebrile but that is minimally reassuring given the immunosuppression. There are no clues to suggest liver or kidney dysfunction. An unrecognized occlusion of the lower abdominal venous or lymphatic system such as upward extension of the DVT into the inferior vena cava (IVC) or a pelvic obstruction of the lower extremity lymphatic vessels could be considered. It appears that his distal weakness closely mirrors his proximal weakness in distinction to most myopathies which are predominantly proximal (with some exceptions, eg, inclusion body myositis).
The white blood cell count was 26,000/L with normal differential, hemoglobin 11.2 gm/dL, and platelet count was 191,000/L (at recent discharge these values were 23,000, 11.9, and 274,000, respectively). Chemistries were normal except for creatinine of 1.4 mg/dL (baseline 1.2), blood urea nitrogen was 42 mg/dL, albumin 2.6 gm/dL (normal, 3.55.0), and CK 3,710 U/L (20220), decreased from 6,943 U/L at recent discharge. Urine dipstick testing was positive for blood and protein; the urine sediment was unremarkable. Chest radiograph revealed normal lungs and heart.
The white blood cell count is quite elevated, perhaps more so than could be attributed to chronic steroid use, and again raises the concern of an undiagnosed infection. The presence of heme (and protein) in the urine without cells is consistent with pigment nephropathy from the recent rhabdomyolysis.
He was admitted to the hospital. Unfractionated heparin and warfarin were started. No changes were made to his immunosuppressive regimen. Blood cultures were negative after 48 hours. Transthoracic echocardiogram showed an ejection fraction of 60%, normal valves, and right ventricular systolic pressure of 32 mm Hg (normal, 1525 mmHg). On hospital day 3, his platelet count was 147,000/L, and on day 5, 101,000/L. His other laboratory values remained unchanged, and there were no new clinical developments.
A declining platelet count and extensive deep vein thrombosis suggest heparin‐induced thrombocytopenia and thrombosis (HITT), especially with the greater than 50% drop in the setting of IV heparin. His platelets have continued on a downward trajectory that was evident at admission and has progressed during this hospitalization. Assuming this is not due to laboratory error or artifact such as platelet clumping, this decline could have occurred if he was sensitized to heparin during the prior hospitalization, such as for DVT prophylaxis. It is increasingly recognized that HITT can manifest even after exposure to heparin is complete, ie, posthospitalization, and there can be an immediate drop in platelet counts if an unrecognized HITT‐mediated thrombosis is treated with IV heparin. Heparin should be discontinued in favor of a direct thrombin inhibitor and tests for heparin‐induced platelet antibodies (HIPA) and serotonin‐release assay (SRA) sent.
Antiphospholipid antibody syndrome (APLS) is associated with hypercoagulability and thrombocytopenia and is more frequent in patients with autoimmune disorders. The drug list should also be examined for associations with thrombocytopenia. The peripheral smear should be scrutinized and hemoglobin and creatinine followed to exclude thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome (TTP‐HUS).
Heparin was stopped on day 5. Warfarin was continued with a therapeutic international normalized ratio (INR). Tests for antiplatelet factor 4 antibodies, HIPA, and SRA were negative. His weakness and edema improved although his CK remained between 2000 and 4000 U/L. On day 5 he developed mild hemoptysis, and a repeat chest radiograph demonstrated a new left hilar infiltrate. Computed tomography (CT) scan of the chest with contrast demonstrated a left lower lobe consolidation, scattered ground glass opacities in both lung bases, and no pulmonary embolus. He was treated with piperacillin/tazobactam and vancomycin. He remained afebrile. The same day, he erroneously received 125 mg (instead of 12.5 mg) of subcutaneous methotrexate. High‐dose leucovorin was administered on days 5 and 6.
The hemoptysis resolved after 2 days. From days 5 to 9, the platelet count dropped to 80,000/L and his hemoglobin gradually decreased to 7.3 g/dL. Anticoagulation was stopped, vitamin K administered, and an IVC filter placed. Two units of packed red blood cells (RBCs) were transfused.
In suspected HITT (which was not verified here), warfarin is typically withheld until the platelets have recovered and thrombin‐inhibitor anticoagulation has reached a steady state, to avoid the transient hypercoagulability of warfarin initiation.
The unusual time course and the 3 negative tests make HITT unlikely. The continued platelet decline after stopping heparin further supports another etiology. The excess methotrexate dosing complicates interpretation of his thrombocytopenia and anemia, which can be explained by mucosal bleeding, microangiopathic hemolytic anemia (MAHA) such as disseminated intravascular coagulation or TTP‐HUS, or autoimmunity (Evans syndrome). Bone marrow toxicity is also a major effect of methotrexate (in addition to elevation of liver enzymes and acute renal failure); however, there is typically a lag between administration and development of cytopenias. The antibiotics could also account for the ongoing (but not original) thrombocytopenia.
With the new pulmonary infiltrate, infections remain a primary concern and should be evaluated with sputum samples and perhaps bronchoscopy. Given the abnormal urine (even without cells), a pulmonary‐renal inflammatory processes should be considered also to explain the infiltrates and hemoptysis.
Haptoglobin was 20 mg/dL (normal, 37246). The direct antiglobulin test (DAT) was negative. Serum lactate dehydrogenase (LDH) was 1657 U/L (normal, 100220), with elevated LD4 and LD5 isoenzymes. Coagulation studies normalized after the administration of vitamin K. Anti‐nuclear antibody was positive at 8.7 (normal 1.5). Tests for antineutrophil cytoplasmic antibodies were negative. No sputum could be obtained. A pathologist reviewed the blood smear and reported neutrophilic leukocytosis without left shift, and thrombocytopenia with normal platelet morphology.
Low haptoglobin in the setting of an elevated LDH is highly suggestive of hemolysis, particularly the intravascular, microangiopathic varieties. Neutrophilia may reflect infection, a primary myeloproliferative process such as chronic myeloid leukemia, steroid use, or a reactive bone marrow in the setting of acute illness. The negative DAT and significant immunosuppressive regimen makes immune‐mediated hemolysis unlikely, although the history of autoimmunity and the small DAT false‐negative rate leaves Evans syndrome as an outside possibility. Medications such as tacrolimus (causing TTP) or IVIG (given the broad spectrum of antibodies it includes) are other plausible causes of the cytopenias.
At this point, I would analyze the red blood cell (RBC) morphology and check the reticulocyte count to help differentiate between hemolysis and a myelotoxin.
After transfusion, his hemoglobin remained at approximately 8.5 gm/dL and LDH remained elevated but stable. By day 12 the platelet count had fallen to 37,000/L.
With physical therapy the patient gained strength. Antibiotics were discontinued on day 12 and a follow‐up chest x‐ray demonstrated no significant disease. From days 10 to 12, his creatinine rose from 1.5 to 1.9 mg/dL, although urine output remained normal.
A hematologist observed minimal fragmentation of red cells on the blood smear. Commenting on the thrombocytopenia, anemia, and LDH isoenzymes (representative of skeletal/hepatic origin rather than hematologic), and clinical improvement after treatment of a presumed pneumonia, he felt that the continued thrombocytopenia was likely due to drug toxicity, and recommended observation, treatment of renal failure, and discontinuation of tacrolimus.
The failure to increase the hemoglobin after transfusion is consistent with (but not specific for) hemolysis. In conjunction with the progressive thrombocytopenia and persistently elevated LDH, TTP remains a consideration. While TTP can be diagnosed with minimal evidence of schistocytes, the duration of this illness, now spanning almost 2 weeks without significant end organ damagenamely more pronounced renal failure, confusion, or feveris unusual for TTP. Therefore, I think it is reasonable to withhold plasma exchange, although if the cytopenias or renal failure progress after the methotrexate, tacrolimus, and antibiotics are stopped, it may have to be undertaken empirically.
The pulmonary process remains undefined. Edema, pneumonitis (eg, aspiration), a modest pneumonia, or pulmonary hemorrhage could normalize on chest x‐ray after 1 week.
Renal ultrasound was normal. Urinalysis dipstick demonstrated 3+ blood, 3+ protein, and no nitrate or leukocyte esterase. The urine sediment showed only granular casts. Fractional excretion of sodium was 6.7%. Urine protein‐to‐creatinine ratio was 7.5, and urine myoglobin was elevated. Serum C3 and C4 complement levels and cryoglobulins were normal. Reticulocyte count was 8.5% (normal, 0.53.2).
There is significant evidence for intrinsic renal failure, starting with the elevated fractional excretion. Marked proteinuria suggests glomerular damage; nephrotic syndrome could provide an explanation for the recurrent DVT. The 3+ blood without RBCs and the markedly elevated urine myoglobin suggest pigment nephropathy from both myoglobinuria and hemoglobinuria. The elevated reticulocyte count further confirms the impression of hemolysis.
Nephrotic syndrome may result from a primary disease process, such as diabetes, systemic lupus erythematosus (SLE), or amyloidosis, for which there is no evidence to date, or as a consequence of indolent infection, malignancy, or drugs, all of which are reasonable possibilities.
The essential elements at this point include thrombocytopenia, kidney failure with proteinuria, and likely intravascular hemolysis. I would repeat the peripheral smear (looking for schistocytes) and discuss with the rheumatologist if any other medications could be discontinued.
A nephrology consultant diagnosed acute tubular necrosis (ATN) from a combination of insults (intravenous contrast, methotrexate, tacrolimus, and myoglobinuria). Over the next several days, his platelet count rose to approximately 60,000/L. The patient continued to generally feel better but the creatinine steadily increased to 4.9 mg/dL.
The hematologist's reassessment of the smear was unchanged with minimal RBC fragmentation noted. Over the next few days the hemoglobin, creatinine, and platelet count remained stable, and there were no fevers or other clinical developments. On day 21 a kidney biopsy specimen revealed evidence of thrombotic microangiopathy (TMA) and segmental glomerular necrosis, with negative immunofluorescent findings. In addition, the glomerular basement membranes were thickened and effacement of the epithelial foot processes was noted.
TTP (or other MAHA) with only a few schistocytes would be unusual at an advanced stage where organ damage has occurred, although the clinical presentation in drug‐induced variety is variable. TTP is also generally a fatal disease, so relative stability over 3 weeks without definitive therapy is atypical, unless prednisone has served as a temporizing measure. The atypical features raise the possibility of a mimic or variant of TTP such as undiagnosed cancer causing DIC or a medication (eg, tacrolimus)‐associated TTP syndrome.
At least 2 other conditions could account for the hemolysis, thrombocytopenia, and TMA. The positive ANA, glomerular disease, and cytopenias are compatible with SLE, although such progression on an intense immunosuppressive regimen would be unusual. The renal histology in a patient with an autoimmune diathesis warrants reconsideration of antiphospholipid antibody syndrome (APLS), especially in light of the earlier DVT.
Tests for antiphospholipid antibodies were negative. After multidisciplinary deliberation, a diagnosis of TMA due to tacrolimus‐associated TTP/HUS was made. Plasmapheresis was initiated and IVIG and steroids were continued. He had a complicated hospital course and required renal replacement therapy, but with pheresis, his platelet counts and hemoglobin began to recover and he was ultimately discharged in good condition. After he was discharged, testing for ADAMTS13 (a von Willebrand factor‐cleaving protease) activity was reported as 54% (normal, >66%)
Discussion
TMA in the microcirculation is the hallmark pathology of TTP‐HUS but is not specific for this disease. TMA is also seen in disseminated intravascular coagulation, sepsis, cancer, malignant hypertension, human immunodeficiency virus infection, autoimmune disorders, pregnancy‐related conditions, and in association with certain drugs.1 The first pharmacological agent to be associated with TMA was mitomycin in 1971, and since then other drug associations have been described, including antiplatelet medications such as ticlopidine and clopidogrel, antibiotics such as quinine and rifampin, interferon, and immunosuppressants such as cyclosporine and tacrolimus.2 Drug‐induced variants of TTP and TMA are challenging to diagnose because the timing of onset, clinical features, and patient factors (eg, receipt of immunosuppressants) may vary widely and mimic other conditions.2, 3 TMA is a rare complication of tacrolimus and is mostly seen in renal transplant patients at a frequency of 1%. In these patients, renal dysfunction is usually the first herald of TMA and TTP; evidence of hemolysis may be absent.3
The clinical diagnosis of TTP has historically been based on the presence of a classic pentad: MAHA, thrombocytopenia, neurological and renal abnormalities, and fever.4 Elevated levels of LDH and indirect bilirubin and the presence of fragmented RBCs and reticulocytes point toward active intravascular hemolysis. The DAT is usually negative. This textbook illness scriptthe template of a disease that is stored in a clinician's memoryis learned by physicians during training, but undergoes little modification given the limited exposure to a rare disease.
In modern practice, the pentad is rarely seen, and the characteristics of the end‐organ findings may vary substantially. For instance, while neurological symptoms including seizures, coma, and transient confusion occur in 90% of cases, renal involvement is seen in about 50% and fever in only 25% of patients.5 Although the presence of 2 or more schistocytes on the blood smear under 100 microscopy supports the diagnosis of MAHA, cases of TTP without significant schistocytosis have been reported.6
Furthermore, TTP is typically described as acute in onset, but in a quarter of patients the symptoms and signs last for weeks before diagnosis.4 This variability in disease presentation coupled with the high mortality of untreated disease has changed the diagnostic and treatment thresholds for TTP. Trials and expert opinion use MAHA, thrombocytopenia, and the exclusion of alternative causes as sufficient criteria to diagnose TTP and begin treatment.7 The measurement of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) activity (a von Willebrand factor‐cleaving protease) for diagnostic purposes remains controversial because assay techniques are not uniform and there is insufficient correlation between levels and clinical disease.810 For instance, the presence of severe ADAMTS 13 deficiency (ie, 5%) along with the presence of an ADAMTS13 inhibitor is considered to be very specific, but not sensitive, for the laboratory diagnosis of idiopathic TTP.11 In cohort studies, the frequency of severe deficiency among patients with idiopathic TTP ranged from 18% to 100%, and the presence of severe deficiency did not predict the development of acute episodes of TTP.9 In a registry study of 142 patients diagnosed with TTP, 81% of patients with secondary TTP (ie, not classified as idiopathic) had ADAMTS13 levels that were normal to subnormal (>25%), and patients with normal ADAMTS13 levels had a higher incidence of acute renal failure, similar to the findings in this patient.10
Untreated TTP has a mortality rate of greater than 90%, but with plasma exchange, survival has improved dramatically.4, 7 Glucocorticoids are often used in addition to plasma exchange, based on case series and reports.9 The addition of cryoprecipitate or fresh frozen plasma to plasmapheresis has not been shown to be beneficial, but rituximab, an anti CD‐20 monoclonal antibody, has shown promise in a small prospective study.12, 13
TTP is a rare disorder with a classic description but substantial variation in clinical presentation. In this case, the background autoimmune myopathy, immunosuppression, coincident acute DVT, unexplained infiltrates, complex medication regimen, and nephrotic range proteinuria (attributed to focal segmental glomerular sclerosis based on the limited evidence available from the biopsy) led the clinicians to ascribe the patient's thrombocytopenia and renal injury to more common conditions and created a challenging environment for the diagnosis of TTP. TTP is a complex disorder and the simplified understanding of the disease and its time course prevented a prompt match between the patient's clinical course and his diagnosis. The combination of a rare condition with inherent variability arising in the setting of medical complexity challenges the processes of problem representation and scripting the answer for even the most seasoned clinician.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Key Teaching Points
-
The classically described pentad of TTP is seldom seen, and the findings of otherwise unexplained MAHA and thrombocytopenia should prompt consideration of TTP.
-
TTP may be acute and idiopathic, or be secondary to drugs, infections, or other conditions. Medication‐induced TTP may present with a wide range of clinical findings.
-
Therapeutic plasma exchange may be life‐saving in cases of TTP, and when appropriate, should be initiated promptly based on clinical suspicion and without waiting to perform tissue biopsy.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.
- , , .Thrombotic microangiopathies. In: Tischer CC, Brenner BM, eds.Renal Pathology.2nd ed.Philadelphia, PA:JB Lippincott;1994:1154–1184.
- , , .Drug‐induced thrombotic microangiopathy: incidence, prevention and management.Drug Saf.2001;24(7):491–501.
- , , , , .FK 506‐associated thrombotic microangiopathy: report of two cases and review of the literature.Transplantation.1999;67(4):539–544.
- , .Thrombotic Thrombocytopenic purpura: report of 16 cases and review of the literature.Medicine (Baltimore).1966;45:139–159.
- , , , .Thrombotic thrombocytopenic purpura; early and late responders.Am J Hematol.1997;54:102–107.
- .Atypical presentations of thrombotic thrombocytopenic purpura: a review.J Clin Apheresis.2009;24(1)47–52.
- , , , et al.Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura.N Engl J Med.1991;325:393–397.
- , , , , , .The incidence of thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS13deficiency.J Thromb Haemost.2005;3:1432–1436.
- .Thrombotic thrombocytopenic purpura.N Engl J Med.2006;354:1927–1935.
- , , , et al.ADAMTS13 activity in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients.Blood.2003;102:60–68.
- , , .Thrombotic thrombocytopenic purpura.J Thromb Haemost.2005;3:1663–1675.
- , , , et al.Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials.Am J Kidney Dis.2009;53:259–272.
- , , , et al.:Efficiency of curative and prophylactic treatment with rituximab in ADAMTS13‐deficient TTP: A study of 11 cases.Blood.2005;105:1932–1937.