User login
In the Face of It All
A 59 year‐old man was sent from urgent care clinic to the emergency room for further evaluation because of 1 month of diarrhea and an acute elevation in his serum creatinine.
Whereas acute diarrhea is commonly due to a self‐limited and often unspecified infection, diarrhea that extends beyond 23 weeks (chronic) warrants consideration of malabsorptive, inflammatory, infectious, and malignant processes. The acute renal failure likely is a consequence of dehydration, but the possibility of simultaneous gastrointestinal and renal involvement from a systemic process (eg, vasculitis) must be considered.
The patient's diarrhea began 1 month prior, shortly after having a milkshake at a fast food restaurant. The diarrhea was initially watery, occurred 8‐10 times per day, occasionally awakened him at night, and was associated with nausea. There was no mucus, blood, or steatorrhea until 1 day prior to presentation, when he developed epigastric pain and bloody stools. He denied any recent travel outside of Northern California and had no sick contacts. He had lost 10 pounds over the preceding month. He denied fevers, chills, vomiting, or jaundice, and had not taken antibiotics recently.
In the setting of chronic diarrhea, unintentional weight loss is an alarm feature but does not narrow the diagnostic possibilities significantly. The appearance of blood and pain on a single day after 1 month of symptoms renders their diagnostic value uncertain. For instance, rectal or hemorrhoidal bleeding would be a common occurrence after 1 month of frequent defecation. Sustained bloody stools might be seen in any form of erosive luminal disease, such as infection, inflammatory bowel disease, or neoplasm. Pain is compatible with inflammatory bowel disease, obstructing neoplasms, infections, or ischemia (eg, vasculitis). There are no fever or chills to support infection, and common gram‐negative enteric pathogens (such as Salmonella, Campylobacter, and Yersinia) usually do not produce symptoms for such an extended period. He has not taken antibiotics, which would predispose him to infection with Clostridum difficile, and he has no obvious exposure to parasites such as Entamoeba.
The patient had diabetes mellitus with microalbuminuria, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic low back pain, and gastritis, and had undergone a Billroth II procedure for a perforated gastric ulcer in the remote past. His medications included omeprazole, insulin glargine, simvastatin, lisinopril, amlodipine, and albuterol and beclomethasone metered‐dose inhalers. He had been married for 31 years, lived at home with his wife, was a former rigger in a shipyard and was on disability for chronic low back pain. He denied alcohol or intravenous drug use but had quit tobacco 5 years prior after more than 40 pack‐years of smoking. He had three healthy adult children and there was no family history of cancer, liver disease, or inflammatory bowel disease. There was no history of sexually transmitted diseases or unprotected sexual intercourse.
Bacterial overgrowth in the blind loop following a Billroth II operation can lead to malabsorption, but the diarrhea would not begin so abruptly this long after surgery. Medications are common causes of diarrhea. Proton‐pump inhibitors, by reducing gastric acidity, confer an increased risk of bacterial enteritis; they also are a risk factor for C difficile. Lisinopril may cause bowel angioedema months or years after initiation. Occult laxative use is a well‐recognized cause of chronic diarrhea and should also be considered. The most relevant element of his social history is the prolonged smoking and the attendant risk of cancer, although diarrhea is a rare paraneoplastic phenomenon.
On exam, temperature was 36.6C, blood pressure 125/78, pulse 88, respiratory rate 16 per minute, and oxygen saturation 97% while breathing room air. There was temporal wasting and mild scleral icterus, but no jaundice. Lungs were clear to auscultation and heart was regular in rate and rhythm without murmurs or gallops. There was no jugular venous distention. A large abdominal midline scar was present, bowel sounds were normoactive, and the abdomen was soft, nontender, and nondistended. The hard was regular in rate and rhythm the liver edge was 6 cm below costal margin; there was no splenomegaly. The patient was alert and oriented, with a normal neurologic exam.
The liver generally enlarges because of acute inflammation, congestion, or infiltration. Infiltration can be due to tumors, infections, hemochromatosis, amyloidosis, or sarcoidosis. A normal cardiac exam argues against hepatic congestion from right‐sided heart failure or pericardial disease.
The key elements of the case are diarrhea and hepatomegaly. Inflammatory bowel disease can be accompanied by sclerosing cholangitis, but this should not enlarge the liver. Mycobacterial infections and syphilis can infiltrate the liver and intestinal mucosa, causing diarrhea, but he lacks typical risk factors.
Malignancy is an increasing concern. Colon cancer commonly metastasizes to the liver and can occasionally be intensely secretory. Pancreatic cancer could account for these symptoms, especially if pancreatic exocrine insufficiency caused malabsorption. Various rare neuroendocrine tumors that arise in the pancreas can cause secretory diarrheas and liver metastases, such as carcinoid, VIPoma, and Zollinger‐Ellison syndrome.
Laboratory results revealed a serum sodium of 143 mmol/L, potassium 4.7 mmol/L, chloride 110 mmol/L, bicarbonate 25 mmol/L, urea nitrogen 24 mg/dL, and creatinine 2.5 mg/dL (baseline had been 1.2 mg/dL 2 months previously). Serum glucose was 108 mg/dL and calcium was 8.8 mg/dL. The total white blood cell count was 9300 per mm3 with a normal differential, hemoglobin was 14.4 g/dL, mean corpuscular volume was 87 fL, and the platelet count was normal. Total bilirubin was 3.7 mg/dL, and direct bilirubin was 3.1 mg/dL. Aspartate aminotransferase (AST) was 122 U/L (normal range, 831), alanine aminotransferase (ALT) 79 U/L (normal range, 731), alkaline phosphatase 1591 U/L (normal range, 39117), and gamma‐glutamyltransferase (GGT) 980 U/L (normal range, 57). Serum albumin was 2.5 mg/dL, prothrombin time was 16.4 seconds, and international normalized ratio (INR) was 1.6.
Urinalysis was normal except for trace hemoglobin, small bilirubin, and 70 mg/dL of protein; specific gravity was 1.007. Urine microscopy demonstrated no cells or casts. The ratio of protein to creatinine on a spot urine sample was less than 1. Chest x‐ray was normal. The electrocardiogram demonstrated sinus rhythm with an old right bundle branch block and normal QRS voltages.
The disproportionate elevation in alkaline phosphatase points to an infiltrative hepatopathy from a cancer originating in the gastrointestinal tract or infection. Other infiltrative processes such as sarcoidosis or amyloidosis usually have evidence of disease elsewhere before hepatic disease becomes apparent.
Mild proteinuria may be explained by diabetes. The specific gravity of 1.007 is atypical for dehydration and could suggest ischemic tubular injury. Although intrinsic renal diseases must continue to be entertained, hypovolemia (compounded by angiotensin‐converting enzyme [ACE] inhibitor use) is the leading explanation in light of the nondiagnostic renal studies. The preserved hemoglobin may simply indicate dehydration, but otherwise is somewhat reassuring in the context of bloody diarrhea.
The patient was admitted to the hospital. Three stool samples returned negative for C difficile toxin. No white cells were detected in the stool, and no ova or parasites were detected. Stool culture was negative for routine bacterial pathogens and for E coli O157. Tests for HIV and antinuclear antibodies (ANAs) and serologies for hepatitis A, B, and C were negative. Abdominal ultrasound demonstrated no intra‐ or extrahepatic bile duct dilatation; no hepatic masses were seen. Kidneys were normal in size and appearance without hydronephrosis. Computed tomography (CT) of the abdomen without intravenous contrast revealed normal‐appearing liver (with a 12‐cm span), spleen, biliary ducts, and pancreas, and there was no intra‐abdominal adenopathy.
The stool studies point away from infectious colitis. Infiltrative processes of the liver, including metastases, lymphoma, tuberculosis, syphilis, amyloidosis, and sarcoidosis, can be microscopic and therefore evade detection by ultrasound and CT scan. In conditions such as these, endoscopic retrograde cholangiopanccreatography/magnetic resonance cholangiopancreatography (ERCP/MRCP) or liver biopsy may be required. The CT is limited without contrast but does not suggest extrahepatic disease in the abdomen.
MRCP was performed, but was a technically suboptimal study due to the presence of ascites. The serum creatinine improved to 1.4 mg/dL over the next 4 days, and the patient's diarrhea decreased to two bowel movements daily with the use of loperamide. The patient was discharged home with outpatient gastroenterology follow‐up planned to discuss further evaluation of the abnormal liver enzymes.
Prior to being seen in the Gastroenterology Clinic, the patient's nonbloody diarrhea worsened. He felt weaker and continued to lose weight. He also noted new onset of bilateral lower face numbness and burning, which was followed by swelling of his lower lip 12 hours later. He returned to the hospital.
On examination, he was afebrile. His lower lip was markedly swollen and was drooping from his face. He could not move the lip to close his mouth. The upper lip and tongue were normal size and moved without restriction. Facial sensation was intact, but there was weakness when he attempted to wrinkle both of his brows and close his eyelids. The rest of his physical examination was unchanged.
The serum creatinine had risen to 3.6 mg/dL, and the complete blood count remained normal. Serum total bilirubin was 4.6 mg/dL, AST 87 U/L, ALT 76 U/L, and alkaline phosphatase 1910 U/L. The 24‐hour urine protein measurement was 86 mg.
Lip swelling suggests angioedema. ACE inhibitors are frequent offenders, and it would be important to know whether his lisinopril was restarted at discharge. ACE‐inhibitor angioedema can also affect the intestine, causing abdominal pain and diarrhea, but does not cause a systemic wasting illness or infiltrative hepatopathy. The difficulty moving the lip may reflect the physical effects of swelling, but generalized facial weakness supports a cranial neuropathy. Basilar meningitis may produce multiple cranial neuropathies, the etiologies of which are quite similar to the previously mentioned causes of infiltrative liver disease: sarcoidosis, syphilis, tuberculosis, or lymphoma.
The patient had not resumed lisinopril since his prior hospitalization. The lower lip swelling and paralysis persisted, and new sensory paresthesias developed over the right side of his chin. A consulting neurologist found normal language and speech and moderate dysarthria. Cranial nerve exam was normal except bilateral lower motor neuron facial nerve palsy was noted with bilateral facial droop, reduced strength of eyelid closure, and diminished forehead movement bilaterally; facial sensation was normal. Extremity motor exam revealed proximal iliopsoas muscle weakness bilaterally rated as 4/5 and was otherwise normal. Sensation to pinprick was diminished in a stocking/glove distribution. Deep‐tendon reflexes were normal and plantar response was down‐going bilaterally. Coordination was intact, Romberg was negative, and gait was slowed due to weakness.
Over the next several days, the patient continued to have diarrhea and facial symptoms. The serum total bilirubin increased to 14 mg/dL, alkaline phosphatase rose above 2,000 U/L, and serum creatinine increased to 5.5 mg/dL. Noncontrast CT scan of the head was normal.
Along with a mild peripheral sensory neuropathy, the exam indicates bilateral palsies of the facial nerve. Lyme disease is a frequent etiology, but this patient is not from an endemic area. I am most suspicious of bilateral infiltration of cranial nerve VII. I am thinking analogically to the numb chin syndrome, wherein lymphoma or breast cancer infiltration along the mental branch of V3 causes sensory loss, and perhaps these disorders can produce infiltrative facial neuropathy. At this point I am most concerned about lymphomatous meningitis with cranial nerve involvement. Cerebrospinal fluid (CSF) analysis (including cytology) would be informative.
Lumbar puncture demonstrated clear CSF with one white blood cell per mm3 and no red blood cells. Glucose was normal, and protein was 95.5 (normal range, 15‐45 mg/dL). Gram stain and culture for bacteria were negative, as were polymerase chain reaction (PCR) testing for herpes simplex virus, mycobacterial and fungal stains and cultures, and cytology. Transthoracic echocardiogram demonstrated severe concentric left ventricular (LV) hypertrophy, normal LV systolic function, and impaired LV relaxation. CT scan of the chest identified no adenopathy or other abnormality.
The CSF analysis does not support basilar meningitis, although the cytoalbuminologic dissociation makes me wonder whether there is some intrathecal antibody production or an autoimmune process we have yet to uncover. The absence of lymphadenopathy anywhere in the body and the negative CSF cytology now point away from lymphoma. As the case for lymphoma or an infection diminishes, systemic amyloidosis rises to the top of possibilities in this afebrile man who is losing weight, has infiltrative liver and nerve abnormalities, renal failure, cardiac enlargement, and suspected gastrointestinal luminal abnormality. Although the echocardiographic findings are most likely explained by hypertension, they are compatible with amyloid infiltration. A tissue specimen is needed, and either colonoscopy or liver biopsy should be suitable.
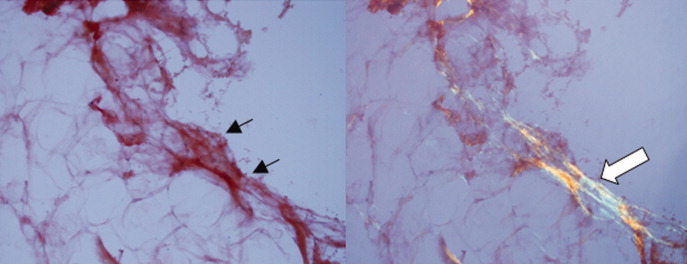
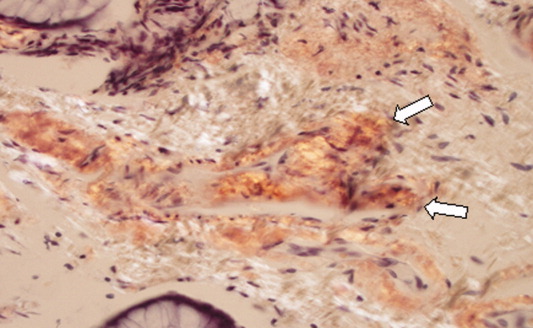
A pathologist performed a fat pad biopsy that demonstrated scant congophilic and birefringent material associated with blood vessels, suggestive of amyloid (Fig. 1). Colonoscopy demonstrated normal mucosa, and a rectal biopsy revealed congophilic material within the blood vessels consistent with amyloid (Fig. 2). No monoclonal band was present on serum protein electrophoresis. Urine protein electrophoresis identified a homogenous band in the gamma region, and urine kappa and lambda free light chains were increased: kappa was 10.7 mg/dL (normal range, 2), and lambda was 4.25 mg/dL (normal range, 2).
After extensive discussion among the patient, his wife, and a palliative care physician, the patient declined chemotherapy and elected to go home. Two days after discharge (7 weeks after his initial admission for diarrhea) he died in his sleep at home. Permission for a postmortem examination was not granted.
Discussion
Amyloidosis refers to abnormal extracellular deposition of fibril. There are many types of amyloidosis including primary amyloidosis (AL amyloidosis), secondary amyloidosis (AA amyloidosis), and hereditary causes. Systemic AL amyloidosis is a rare plasma cell disorder characterized by misfolding of insoluble extracellular fibrillar proteins derived from immunoglobulin light chains. These insoluble proteins typically deposit in the kidney, heart, and nervous system.1 Although the mechanism of organ dysfunction is debated, deposition of these proteins may disrupt the tissue architecture by interacting with local receptors and causing apoptosis.1
Table 1 indicates the most common findings in patients with AL amyloidosis.2 While our patient ultimately developed many common findings of AL amyloidosis, several features were atypical, including the marked hyperbilirubinemia, profound diarrhea, and bilateral facial diplegia.
| Organ Involvement | Incidence of Organ Involvement (%) | Symptoms | Signs | Laboratory/Test Finding |
|---|---|---|---|---|
| ||||
| General | Malaise, weight loss | |||
| Renal | 33 | Fatigue | Peripheral edema | Proteinuria with or without renal insufficiency, pleural effusion, hypercholesterolemia |
| Cardiac | 20 | Palpitations, dyspnea | Elevated jugular venous pressure, S3, peripheral edema, hepatomegaly | Low‐voltage or atrial fibrillation on electrocardiogram; echocardiogram: thickened ventricles, dilated atria |
| Neurological | 20 | Paresthesias, numbness, weakness, autonomic insufficiency | Carpal tunnel syndrome, postural hypotension | |
| Gastrointestinal and Hepatic | 16 | Diarrhea, nausea, weight loss | Macroglossia, hepatomegaly | Elevated alkaline phosphatase |
| Hematology | Rare | Bleeding | Periorbital purpura (raccoon eyes) | Prolonged prothrombin time, Factor X deficiency |
Up to 70% of patients with amyloidosis will have detectable liver deposits, typically involving portal vessels, portal stroma, central vein, and sinusoidal parenchyma.3 Clinically overt hepatic dysfunction from amyloid is less frequent,4 and the most characteristic findings are hepatomegaly with a markedly elevated serum alkaline phosphatase concentration; jaundice is rare. Palpable hepatic enlargement without abnormal liver enzymes should be interpreted with caution. The finding of a palpable liver edge correlates poorly with frank hepatomegaly, with a positive likelihood ratio of just 1.7.5 In the patient under discussion, suspected hepatomegaly was not confirmed on a subsequent CT scan. Nonetheless, the elevated alkaline phosphatase represented an important clue to potential infiltrative liver disease. In a series of amyloidosis patients from the Mayo Clinic, 81% had hepatomegaly on physical exam, and the mean alkaline phosphatase level was 1,029 U/L (normal, 250 U/L), while the mean serum bilirubin and AST levels were only modestly elevated, at 3.2 mg/dL and 72 U/L respectively. The prothrombin time was prolonged in 35% of patients.
Upper gastrointestinal tract involvement by AL amyloid may be found in up to a third of cases at autopsy, but clinically significant gastrointestinal features are seen in fewer than 5% of patients.6 Predominant intestinal manifestations are unintentional weight loss (average 7 kg) and diarrhea, nonspecific features that result in delayed diagnosis for a median of 7 months after symptom onset.7 Diarrhea in AL amyloid may stem from several mechanisms: small intestine mucosal infiltration, steatorrhea from pancreatic insufficiency, autonomic neuropathy leading to pseudo‐obstruction and bacterial overgrowth, bile acid malabsorption, or rapid transit time. Diarrhea in AL amyloid is often resistant to treatment and may be the primary cause of death.7
Systemic amyloidosis commonly produces peripheral neuropathies. Involvement of small unmyelinated fibers causes paresthesias and progressive sensory loss in a pattern that is usually distal, symmetric, and progressive.6, 9 Our patient presented with bilateral sensory paresthesias of the chin, suggesting the numb chin syndrome (NCS). NCS is characterized by facial numbness along the distribution of the mental branch of the trigeminal nerve. While dental disorders and infiltration from malignant tumors (mostly lung and breast cancer) account for most cases, amyloidosis and other infiltrative disorders are known to cause NCS as well.10, 11 Our patient's sensory paresthesias may have represented amyloid infiltration of peripheral nerves.
With the exception of carpal tunnel syndrome, motor or cranial neuropathy is uncommon in amyloid, and when present usually heralds advanced disease.12 Descriptions of bilateral facial weakness, also known as facial diplegia, from amyloidosis are limited to case reports.1315 Other causes of this rare finding include sarcoidosis, Guillain‐Barr syndrome, and Lyme disease.16
The diagnosis of primary amyloidosis requires histologic evidence of amyloid from a tissue biopsy specimen (demonstrating positive Congo red staining and pathognomonic green birefringence under cross‐polarized light microscopy), and the presence of a clonal plasma cell disorder. While biopsy of an affected organ is diagnostic, more easily obtained samples such as fat pad biopsy and rectal biopsy yield positive results in up to 80% of cases.2 Serum and urine protein electrophoresis with immunofixation identify an underlying plasma cell disorder in 90% of cases of primary amyloidosis. When these tests are inconclusive, serum or urine free light chain assays or bone marrow aspirate and biopsy are useful aids to detect underlying plasma cell dyscrasia.2 AL amyloidosis is a progressive disease with median survival of about 12 years.8 Poorer prognosis is associated with substantial echocardiographic findings, autonomic neuropathy, and liver involvement.2 Hyperbilirubinemia is associated with a poor prognosis, with a median survival of 8.5 months.4 Proteinuria or peripheral neuropathy portends a less ominous course.6
Treatment goals include reducing production and deposition of fibril proteins and contending with organ dysfunction (eg, congestive heart failure [CHF] management). Selected patients with AL amyloidosis may be candidates for high‐dose melphalan and autologous stem cell transplantation.
It would not be reasonable for clinicians to suspect amyloidosis in cases of diarrhea until two conditions are met: 1) the absence of evidence for the typical etiologies of diarrhea; and 2) the evolving picture of an infiltrative disorder. The latter was heralded by the elevated alkaline phosphatase, and was supported by the subsequent multiorgan involvement. Conceptualizing the disease as infiltrative still required a diligent exclusion of infection and invasive tumor cells, which invade disparate organs far more commonly than amyloidosis. Their absence and the organ pattern that is typical of AL amyloidosis (heart, kidney, and peripheral nerve involvement) allowed the discussant to reason by analogy that amyloidosis was also responsible for the most symptomatic phenomena, namely, the diarrhea and facial diplegia (and numb chin syndrome).
Key Teaching Points
-
Hospitalists should consider systemic amyloidosis in cases of unexplained diarrhea when other clinical features of AL amyloidosis are present, including nephrotic syndrome with or without renal insufficiency, cardiomyopathy, peripheral neuropathy, and hepatomegaly.
-
Hepatic amyloidosis should be suspected when weight loss, hepatomegaly, and elevated alkaline phosphatase are present. Although jaundice is rare in amyloidosis, liver involvement and hyperbilirubinemia portend a poorer prognosis.
-
Numb chin syndrome and bilateral facial diplegia are rare manifestations of AL amyloid deposition in peripheral nerves.
- ,.Molecular mechanisms of amyloidosis.N Engl J Med.2003;349(6):583–596.
- Guidelines Working Group of UK Myeloma Forum; British Committee for Standards in Haematology, British Society for Haematology.Guidelines on the diagnosis and management of AL amyloidosis.Br J Haematol.2004;125:681–700.
- ,.Hepatic amyloidosis: morphologic differences between systemic AL and AA types.Hum Pathol.1991;22(9):904–907.
- ,,, et al.Primary (AL) hepatic amyloidosis clinical features and natural history in 98 patients.Medicine.2003;82(5):291–298.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:WB Saunders;2001:595–599.
- ,,, et al.Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis.Am J Hematol.2005;79:319–328.
- .Primary (AL) amyloidosis with gastrointestinal involvement.Scand J Gastroenterol.2009;44(6):708–711.
- ,.Gastrointestinal manifestations of amyloid.Am J Gastroenterol.2008;103:776–787.
- ,.Primary systemic amyloidosis: clinical and laboratory features in 474 cases.Semin Hematol.1995;32:45–59.
- ,,,,.Chin numbness: a symptom that should not be underestimated: a review of 12 cases.Am J Med Sci.2009;337:407–410.
- .Numb chin syndrome: a possible clue to serious illness.Hosp Physician.2000;54–56.
- .Autonomic peripheral neuropathy.Neurol Clin.2007;25:277–301.
- ,.Facial diplegia due to amyloidosis.South Med J.1986;79(11):1458–1459.
- ,,,,.Familial amyloidosis with cranial neuropathy and corneal lattice dystrophy.Neurology.1986;36:432–435.
- ,,.Crainal neuropathy associated with primary amyloidosis.Ann Neurol.1991;29:451–454.
- .Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature.Neurology.1994;44:1198–202.
A 59 year‐old man was sent from urgent care clinic to the emergency room for further evaluation because of 1 month of diarrhea and an acute elevation in his serum creatinine.
Whereas acute diarrhea is commonly due to a self‐limited and often unspecified infection, diarrhea that extends beyond 23 weeks (chronic) warrants consideration of malabsorptive, inflammatory, infectious, and malignant processes. The acute renal failure likely is a consequence of dehydration, but the possibility of simultaneous gastrointestinal and renal involvement from a systemic process (eg, vasculitis) must be considered.
The patient's diarrhea began 1 month prior, shortly after having a milkshake at a fast food restaurant. The diarrhea was initially watery, occurred 8‐10 times per day, occasionally awakened him at night, and was associated with nausea. There was no mucus, blood, or steatorrhea until 1 day prior to presentation, when he developed epigastric pain and bloody stools. He denied any recent travel outside of Northern California and had no sick contacts. He had lost 10 pounds over the preceding month. He denied fevers, chills, vomiting, or jaundice, and had not taken antibiotics recently.
In the setting of chronic diarrhea, unintentional weight loss is an alarm feature but does not narrow the diagnostic possibilities significantly. The appearance of blood and pain on a single day after 1 month of symptoms renders their diagnostic value uncertain. For instance, rectal or hemorrhoidal bleeding would be a common occurrence after 1 month of frequent defecation. Sustained bloody stools might be seen in any form of erosive luminal disease, such as infection, inflammatory bowel disease, or neoplasm. Pain is compatible with inflammatory bowel disease, obstructing neoplasms, infections, or ischemia (eg, vasculitis). There are no fever or chills to support infection, and common gram‐negative enteric pathogens (such as Salmonella, Campylobacter, and Yersinia) usually do not produce symptoms for such an extended period. He has not taken antibiotics, which would predispose him to infection with Clostridum difficile, and he has no obvious exposure to parasites such as Entamoeba.
The patient had diabetes mellitus with microalbuminuria, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic low back pain, and gastritis, and had undergone a Billroth II procedure for a perforated gastric ulcer in the remote past. His medications included omeprazole, insulin glargine, simvastatin, lisinopril, amlodipine, and albuterol and beclomethasone metered‐dose inhalers. He had been married for 31 years, lived at home with his wife, was a former rigger in a shipyard and was on disability for chronic low back pain. He denied alcohol or intravenous drug use but had quit tobacco 5 years prior after more than 40 pack‐years of smoking. He had three healthy adult children and there was no family history of cancer, liver disease, or inflammatory bowel disease. There was no history of sexually transmitted diseases or unprotected sexual intercourse.
Bacterial overgrowth in the blind loop following a Billroth II operation can lead to malabsorption, but the diarrhea would not begin so abruptly this long after surgery. Medications are common causes of diarrhea. Proton‐pump inhibitors, by reducing gastric acidity, confer an increased risk of bacterial enteritis; they also are a risk factor for C difficile. Lisinopril may cause bowel angioedema months or years after initiation. Occult laxative use is a well‐recognized cause of chronic diarrhea and should also be considered. The most relevant element of his social history is the prolonged smoking and the attendant risk of cancer, although diarrhea is a rare paraneoplastic phenomenon.
On exam, temperature was 36.6C, blood pressure 125/78, pulse 88, respiratory rate 16 per minute, and oxygen saturation 97% while breathing room air. There was temporal wasting and mild scleral icterus, but no jaundice. Lungs were clear to auscultation and heart was regular in rate and rhythm without murmurs or gallops. There was no jugular venous distention. A large abdominal midline scar was present, bowel sounds were normoactive, and the abdomen was soft, nontender, and nondistended. The hard was regular in rate and rhythm the liver edge was 6 cm below costal margin; there was no splenomegaly. The patient was alert and oriented, with a normal neurologic exam.
The liver generally enlarges because of acute inflammation, congestion, or infiltration. Infiltration can be due to tumors, infections, hemochromatosis, amyloidosis, or sarcoidosis. A normal cardiac exam argues against hepatic congestion from right‐sided heart failure or pericardial disease.
The key elements of the case are diarrhea and hepatomegaly. Inflammatory bowel disease can be accompanied by sclerosing cholangitis, but this should not enlarge the liver. Mycobacterial infections and syphilis can infiltrate the liver and intestinal mucosa, causing diarrhea, but he lacks typical risk factors.
Malignancy is an increasing concern. Colon cancer commonly metastasizes to the liver and can occasionally be intensely secretory. Pancreatic cancer could account for these symptoms, especially if pancreatic exocrine insufficiency caused malabsorption. Various rare neuroendocrine tumors that arise in the pancreas can cause secretory diarrheas and liver metastases, such as carcinoid, VIPoma, and Zollinger‐Ellison syndrome.
Laboratory results revealed a serum sodium of 143 mmol/L, potassium 4.7 mmol/L, chloride 110 mmol/L, bicarbonate 25 mmol/L, urea nitrogen 24 mg/dL, and creatinine 2.5 mg/dL (baseline had been 1.2 mg/dL 2 months previously). Serum glucose was 108 mg/dL and calcium was 8.8 mg/dL. The total white blood cell count was 9300 per mm3 with a normal differential, hemoglobin was 14.4 g/dL, mean corpuscular volume was 87 fL, and the platelet count was normal. Total bilirubin was 3.7 mg/dL, and direct bilirubin was 3.1 mg/dL. Aspartate aminotransferase (AST) was 122 U/L (normal range, 831), alanine aminotransferase (ALT) 79 U/L (normal range, 731), alkaline phosphatase 1591 U/L (normal range, 39117), and gamma‐glutamyltransferase (GGT) 980 U/L (normal range, 57). Serum albumin was 2.5 mg/dL, prothrombin time was 16.4 seconds, and international normalized ratio (INR) was 1.6.
Urinalysis was normal except for trace hemoglobin, small bilirubin, and 70 mg/dL of protein; specific gravity was 1.007. Urine microscopy demonstrated no cells or casts. The ratio of protein to creatinine on a spot urine sample was less than 1. Chest x‐ray was normal. The electrocardiogram demonstrated sinus rhythm with an old right bundle branch block and normal QRS voltages.
The disproportionate elevation in alkaline phosphatase points to an infiltrative hepatopathy from a cancer originating in the gastrointestinal tract or infection. Other infiltrative processes such as sarcoidosis or amyloidosis usually have evidence of disease elsewhere before hepatic disease becomes apparent.
Mild proteinuria may be explained by diabetes. The specific gravity of 1.007 is atypical for dehydration and could suggest ischemic tubular injury. Although intrinsic renal diseases must continue to be entertained, hypovolemia (compounded by angiotensin‐converting enzyme [ACE] inhibitor use) is the leading explanation in light of the nondiagnostic renal studies. The preserved hemoglobin may simply indicate dehydration, but otherwise is somewhat reassuring in the context of bloody diarrhea.
The patient was admitted to the hospital. Three stool samples returned negative for C difficile toxin. No white cells were detected in the stool, and no ova or parasites were detected. Stool culture was negative for routine bacterial pathogens and for E coli O157. Tests for HIV and antinuclear antibodies (ANAs) and serologies for hepatitis A, B, and C were negative. Abdominal ultrasound demonstrated no intra‐ or extrahepatic bile duct dilatation; no hepatic masses were seen. Kidneys were normal in size and appearance without hydronephrosis. Computed tomography (CT) of the abdomen without intravenous contrast revealed normal‐appearing liver (with a 12‐cm span), spleen, biliary ducts, and pancreas, and there was no intra‐abdominal adenopathy.
The stool studies point away from infectious colitis. Infiltrative processes of the liver, including metastases, lymphoma, tuberculosis, syphilis, amyloidosis, and sarcoidosis, can be microscopic and therefore evade detection by ultrasound and CT scan. In conditions such as these, endoscopic retrograde cholangiopanccreatography/magnetic resonance cholangiopancreatography (ERCP/MRCP) or liver biopsy may be required. The CT is limited without contrast but does not suggest extrahepatic disease in the abdomen.
MRCP was performed, but was a technically suboptimal study due to the presence of ascites. The serum creatinine improved to 1.4 mg/dL over the next 4 days, and the patient's diarrhea decreased to two bowel movements daily with the use of loperamide. The patient was discharged home with outpatient gastroenterology follow‐up planned to discuss further evaluation of the abnormal liver enzymes.
Prior to being seen in the Gastroenterology Clinic, the patient's nonbloody diarrhea worsened. He felt weaker and continued to lose weight. He also noted new onset of bilateral lower face numbness and burning, which was followed by swelling of his lower lip 12 hours later. He returned to the hospital.
On examination, he was afebrile. His lower lip was markedly swollen and was drooping from his face. He could not move the lip to close his mouth. The upper lip and tongue were normal size and moved without restriction. Facial sensation was intact, but there was weakness when he attempted to wrinkle both of his brows and close his eyelids. The rest of his physical examination was unchanged.
The serum creatinine had risen to 3.6 mg/dL, and the complete blood count remained normal. Serum total bilirubin was 4.6 mg/dL, AST 87 U/L, ALT 76 U/L, and alkaline phosphatase 1910 U/L. The 24‐hour urine protein measurement was 86 mg.
Lip swelling suggests angioedema. ACE inhibitors are frequent offenders, and it would be important to know whether his lisinopril was restarted at discharge. ACE‐inhibitor angioedema can also affect the intestine, causing abdominal pain and diarrhea, but does not cause a systemic wasting illness or infiltrative hepatopathy. The difficulty moving the lip may reflect the physical effects of swelling, but generalized facial weakness supports a cranial neuropathy. Basilar meningitis may produce multiple cranial neuropathies, the etiologies of which are quite similar to the previously mentioned causes of infiltrative liver disease: sarcoidosis, syphilis, tuberculosis, or lymphoma.
The patient had not resumed lisinopril since his prior hospitalization. The lower lip swelling and paralysis persisted, and new sensory paresthesias developed over the right side of his chin. A consulting neurologist found normal language and speech and moderate dysarthria. Cranial nerve exam was normal except bilateral lower motor neuron facial nerve palsy was noted with bilateral facial droop, reduced strength of eyelid closure, and diminished forehead movement bilaterally; facial sensation was normal. Extremity motor exam revealed proximal iliopsoas muscle weakness bilaterally rated as 4/5 and was otherwise normal. Sensation to pinprick was diminished in a stocking/glove distribution. Deep‐tendon reflexes were normal and plantar response was down‐going bilaterally. Coordination was intact, Romberg was negative, and gait was slowed due to weakness.
Over the next several days, the patient continued to have diarrhea and facial symptoms. The serum total bilirubin increased to 14 mg/dL, alkaline phosphatase rose above 2,000 U/L, and serum creatinine increased to 5.5 mg/dL. Noncontrast CT scan of the head was normal.
Along with a mild peripheral sensory neuropathy, the exam indicates bilateral palsies of the facial nerve. Lyme disease is a frequent etiology, but this patient is not from an endemic area. I am most suspicious of bilateral infiltration of cranial nerve VII. I am thinking analogically to the numb chin syndrome, wherein lymphoma or breast cancer infiltration along the mental branch of V3 causes sensory loss, and perhaps these disorders can produce infiltrative facial neuropathy. At this point I am most concerned about lymphomatous meningitis with cranial nerve involvement. Cerebrospinal fluid (CSF) analysis (including cytology) would be informative.
Lumbar puncture demonstrated clear CSF with one white blood cell per mm3 and no red blood cells. Glucose was normal, and protein was 95.5 (normal range, 15‐45 mg/dL). Gram stain and culture for bacteria were negative, as were polymerase chain reaction (PCR) testing for herpes simplex virus, mycobacterial and fungal stains and cultures, and cytology. Transthoracic echocardiogram demonstrated severe concentric left ventricular (LV) hypertrophy, normal LV systolic function, and impaired LV relaxation. CT scan of the chest identified no adenopathy or other abnormality.
The CSF analysis does not support basilar meningitis, although the cytoalbuminologic dissociation makes me wonder whether there is some intrathecal antibody production or an autoimmune process we have yet to uncover. The absence of lymphadenopathy anywhere in the body and the negative CSF cytology now point away from lymphoma. As the case for lymphoma or an infection diminishes, systemic amyloidosis rises to the top of possibilities in this afebrile man who is losing weight, has infiltrative liver and nerve abnormalities, renal failure, cardiac enlargement, and suspected gastrointestinal luminal abnormality. Although the echocardiographic findings are most likely explained by hypertension, they are compatible with amyloid infiltration. A tissue specimen is needed, and either colonoscopy or liver biopsy should be suitable.
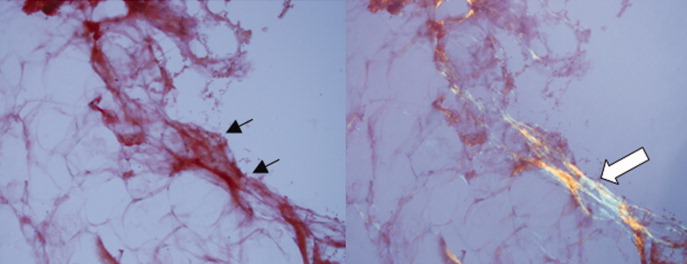
A pathologist performed a fat pad biopsy that demonstrated scant congophilic and birefringent material associated with blood vessels, suggestive of amyloid (Fig. 1). Colonoscopy demonstrated normal mucosa, and a rectal biopsy revealed congophilic material within the blood vessels consistent with amyloid (Fig. 2). No monoclonal band was present on serum protein electrophoresis. Urine protein electrophoresis identified a homogenous band in the gamma region, and urine kappa and lambda free light chains were increased: kappa was 10.7 mg/dL (normal range, 2), and lambda was 4.25 mg/dL (normal range, 2).


After extensive discussion among the patient, his wife, and a palliative care physician, the patient declined chemotherapy and elected to go home. Two days after discharge (7 weeks after his initial admission for diarrhea) he died in his sleep at home. Permission for a postmortem examination was not granted.
Discussion
Amyloidosis refers to abnormal extracellular deposition of fibril. There are many types of amyloidosis including primary amyloidosis (AL amyloidosis), secondary amyloidosis (AA amyloidosis), and hereditary causes. Systemic AL amyloidosis is a rare plasma cell disorder characterized by misfolding of insoluble extracellular fibrillar proteins derived from immunoglobulin light chains. These insoluble proteins typically deposit in the kidney, heart, and nervous system.1 Although the mechanism of organ dysfunction is debated, deposition of these proteins may disrupt the tissue architecture by interacting with local receptors and causing apoptosis.1
Table 1 indicates the most common findings in patients with AL amyloidosis.2 While our patient ultimately developed many common findings of AL amyloidosis, several features were atypical, including the marked hyperbilirubinemia, profound diarrhea, and bilateral facial diplegia.
| Organ Involvement | Incidence of Organ Involvement (%) | Symptoms | Signs | Laboratory/Test Finding |
|---|---|---|---|---|
| ||||
| General | Malaise, weight loss | |||
| Renal | 33 | Fatigue | Peripheral edema | Proteinuria with or without renal insufficiency, pleural effusion, hypercholesterolemia |
| Cardiac | 20 | Palpitations, dyspnea | Elevated jugular venous pressure, S3, peripheral edema, hepatomegaly | Low‐voltage or atrial fibrillation on electrocardiogram; echocardiogram: thickened ventricles, dilated atria |
| Neurological | 20 | Paresthesias, numbness, weakness, autonomic insufficiency | Carpal tunnel syndrome, postural hypotension | |
| Gastrointestinal and Hepatic | 16 | Diarrhea, nausea, weight loss | Macroglossia, hepatomegaly | Elevated alkaline phosphatase |
| Hematology | Rare | Bleeding | Periorbital purpura (raccoon eyes) | Prolonged prothrombin time, Factor X deficiency |
Up to 70% of patients with amyloidosis will have detectable liver deposits, typically involving portal vessels, portal stroma, central vein, and sinusoidal parenchyma.3 Clinically overt hepatic dysfunction from amyloid is less frequent,4 and the most characteristic findings are hepatomegaly with a markedly elevated serum alkaline phosphatase concentration; jaundice is rare. Palpable hepatic enlargement without abnormal liver enzymes should be interpreted with caution. The finding of a palpable liver edge correlates poorly with frank hepatomegaly, with a positive likelihood ratio of just 1.7.5 In the patient under discussion, suspected hepatomegaly was not confirmed on a subsequent CT scan. Nonetheless, the elevated alkaline phosphatase represented an important clue to potential infiltrative liver disease. In a series of amyloidosis patients from the Mayo Clinic, 81% had hepatomegaly on physical exam, and the mean alkaline phosphatase level was 1,029 U/L (normal, 250 U/L), while the mean serum bilirubin and AST levels were only modestly elevated, at 3.2 mg/dL and 72 U/L respectively. The prothrombin time was prolonged in 35% of patients.
Upper gastrointestinal tract involvement by AL amyloid may be found in up to a third of cases at autopsy, but clinically significant gastrointestinal features are seen in fewer than 5% of patients.6 Predominant intestinal manifestations are unintentional weight loss (average 7 kg) and diarrhea, nonspecific features that result in delayed diagnosis for a median of 7 months after symptom onset.7 Diarrhea in AL amyloid may stem from several mechanisms: small intestine mucosal infiltration, steatorrhea from pancreatic insufficiency, autonomic neuropathy leading to pseudo‐obstruction and bacterial overgrowth, bile acid malabsorption, or rapid transit time. Diarrhea in AL amyloid is often resistant to treatment and may be the primary cause of death.7
Systemic amyloidosis commonly produces peripheral neuropathies. Involvement of small unmyelinated fibers causes paresthesias and progressive sensory loss in a pattern that is usually distal, symmetric, and progressive.6, 9 Our patient presented with bilateral sensory paresthesias of the chin, suggesting the numb chin syndrome (NCS). NCS is characterized by facial numbness along the distribution of the mental branch of the trigeminal nerve. While dental disorders and infiltration from malignant tumors (mostly lung and breast cancer) account for most cases, amyloidosis and other infiltrative disorders are known to cause NCS as well.10, 11 Our patient's sensory paresthesias may have represented amyloid infiltration of peripheral nerves.
With the exception of carpal tunnel syndrome, motor or cranial neuropathy is uncommon in amyloid, and when present usually heralds advanced disease.12 Descriptions of bilateral facial weakness, also known as facial diplegia, from amyloidosis are limited to case reports.1315 Other causes of this rare finding include sarcoidosis, Guillain‐Barr syndrome, and Lyme disease.16
The diagnosis of primary amyloidosis requires histologic evidence of amyloid from a tissue biopsy specimen (demonstrating positive Congo red staining and pathognomonic green birefringence under cross‐polarized light microscopy), and the presence of a clonal plasma cell disorder. While biopsy of an affected organ is diagnostic, more easily obtained samples such as fat pad biopsy and rectal biopsy yield positive results in up to 80% of cases.2 Serum and urine protein electrophoresis with immunofixation identify an underlying plasma cell disorder in 90% of cases of primary amyloidosis. When these tests are inconclusive, serum or urine free light chain assays or bone marrow aspirate and biopsy are useful aids to detect underlying plasma cell dyscrasia.2 AL amyloidosis is a progressive disease with median survival of about 12 years.8 Poorer prognosis is associated with substantial echocardiographic findings, autonomic neuropathy, and liver involvement.2 Hyperbilirubinemia is associated with a poor prognosis, with a median survival of 8.5 months.4 Proteinuria or peripheral neuropathy portends a less ominous course.6
Treatment goals include reducing production and deposition of fibril proteins and contending with organ dysfunction (eg, congestive heart failure [CHF] management). Selected patients with AL amyloidosis may be candidates for high‐dose melphalan and autologous stem cell transplantation.
It would not be reasonable for clinicians to suspect amyloidosis in cases of diarrhea until two conditions are met: 1) the absence of evidence for the typical etiologies of diarrhea; and 2) the evolving picture of an infiltrative disorder. The latter was heralded by the elevated alkaline phosphatase, and was supported by the subsequent multiorgan involvement. Conceptualizing the disease as infiltrative still required a diligent exclusion of infection and invasive tumor cells, which invade disparate organs far more commonly than amyloidosis. Their absence and the organ pattern that is typical of AL amyloidosis (heart, kidney, and peripheral nerve involvement) allowed the discussant to reason by analogy that amyloidosis was also responsible for the most symptomatic phenomena, namely, the diarrhea and facial diplegia (and numb chin syndrome).
Key Teaching Points
-
Hospitalists should consider systemic amyloidosis in cases of unexplained diarrhea when other clinical features of AL amyloidosis are present, including nephrotic syndrome with or without renal insufficiency, cardiomyopathy, peripheral neuropathy, and hepatomegaly.
-
Hepatic amyloidosis should be suspected when weight loss, hepatomegaly, and elevated alkaline phosphatase are present. Although jaundice is rare in amyloidosis, liver involvement and hyperbilirubinemia portend a poorer prognosis.
-
Numb chin syndrome and bilateral facial diplegia are rare manifestations of AL amyloid deposition in peripheral nerves.
A 59 year‐old man was sent from urgent care clinic to the emergency room for further evaluation because of 1 month of diarrhea and an acute elevation in his serum creatinine.
Whereas acute diarrhea is commonly due to a self‐limited and often unspecified infection, diarrhea that extends beyond 23 weeks (chronic) warrants consideration of malabsorptive, inflammatory, infectious, and malignant processes. The acute renal failure likely is a consequence of dehydration, but the possibility of simultaneous gastrointestinal and renal involvement from a systemic process (eg, vasculitis) must be considered.
The patient's diarrhea began 1 month prior, shortly after having a milkshake at a fast food restaurant. The diarrhea was initially watery, occurred 8‐10 times per day, occasionally awakened him at night, and was associated with nausea. There was no mucus, blood, or steatorrhea until 1 day prior to presentation, when he developed epigastric pain and bloody stools. He denied any recent travel outside of Northern California and had no sick contacts. He had lost 10 pounds over the preceding month. He denied fevers, chills, vomiting, or jaundice, and had not taken antibiotics recently.
In the setting of chronic diarrhea, unintentional weight loss is an alarm feature but does not narrow the diagnostic possibilities significantly. The appearance of blood and pain on a single day after 1 month of symptoms renders their diagnostic value uncertain. For instance, rectal or hemorrhoidal bleeding would be a common occurrence after 1 month of frequent defecation. Sustained bloody stools might be seen in any form of erosive luminal disease, such as infection, inflammatory bowel disease, or neoplasm. Pain is compatible with inflammatory bowel disease, obstructing neoplasms, infections, or ischemia (eg, vasculitis). There are no fever or chills to support infection, and common gram‐negative enteric pathogens (such as Salmonella, Campylobacter, and Yersinia) usually do not produce symptoms for such an extended period. He has not taken antibiotics, which would predispose him to infection with Clostridum difficile, and he has no obvious exposure to parasites such as Entamoeba.
The patient had diabetes mellitus with microalbuminuria, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic low back pain, and gastritis, and had undergone a Billroth II procedure for a perforated gastric ulcer in the remote past. His medications included omeprazole, insulin glargine, simvastatin, lisinopril, amlodipine, and albuterol and beclomethasone metered‐dose inhalers. He had been married for 31 years, lived at home with his wife, was a former rigger in a shipyard and was on disability for chronic low back pain. He denied alcohol or intravenous drug use but had quit tobacco 5 years prior after more than 40 pack‐years of smoking. He had three healthy adult children and there was no family history of cancer, liver disease, or inflammatory bowel disease. There was no history of sexually transmitted diseases or unprotected sexual intercourse.
Bacterial overgrowth in the blind loop following a Billroth II operation can lead to malabsorption, but the diarrhea would not begin so abruptly this long after surgery. Medications are common causes of diarrhea. Proton‐pump inhibitors, by reducing gastric acidity, confer an increased risk of bacterial enteritis; they also are a risk factor for C difficile. Lisinopril may cause bowel angioedema months or years after initiation. Occult laxative use is a well‐recognized cause of chronic diarrhea and should also be considered. The most relevant element of his social history is the prolonged smoking and the attendant risk of cancer, although diarrhea is a rare paraneoplastic phenomenon.
On exam, temperature was 36.6C, blood pressure 125/78, pulse 88, respiratory rate 16 per minute, and oxygen saturation 97% while breathing room air. There was temporal wasting and mild scleral icterus, but no jaundice. Lungs were clear to auscultation and heart was regular in rate and rhythm without murmurs or gallops. There was no jugular venous distention. A large abdominal midline scar was present, bowel sounds were normoactive, and the abdomen was soft, nontender, and nondistended. The hard was regular in rate and rhythm the liver edge was 6 cm below costal margin; there was no splenomegaly. The patient was alert and oriented, with a normal neurologic exam.
The liver generally enlarges because of acute inflammation, congestion, or infiltration. Infiltration can be due to tumors, infections, hemochromatosis, amyloidosis, or sarcoidosis. A normal cardiac exam argues against hepatic congestion from right‐sided heart failure or pericardial disease.
The key elements of the case are diarrhea and hepatomegaly. Inflammatory bowel disease can be accompanied by sclerosing cholangitis, but this should not enlarge the liver. Mycobacterial infections and syphilis can infiltrate the liver and intestinal mucosa, causing diarrhea, but he lacks typical risk factors.
Malignancy is an increasing concern. Colon cancer commonly metastasizes to the liver and can occasionally be intensely secretory. Pancreatic cancer could account for these symptoms, especially if pancreatic exocrine insufficiency caused malabsorption. Various rare neuroendocrine tumors that arise in the pancreas can cause secretory diarrheas and liver metastases, such as carcinoid, VIPoma, and Zollinger‐Ellison syndrome.
Laboratory results revealed a serum sodium of 143 mmol/L, potassium 4.7 mmol/L, chloride 110 mmol/L, bicarbonate 25 mmol/L, urea nitrogen 24 mg/dL, and creatinine 2.5 mg/dL (baseline had been 1.2 mg/dL 2 months previously). Serum glucose was 108 mg/dL and calcium was 8.8 mg/dL. The total white blood cell count was 9300 per mm3 with a normal differential, hemoglobin was 14.4 g/dL, mean corpuscular volume was 87 fL, and the platelet count was normal. Total bilirubin was 3.7 mg/dL, and direct bilirubin was 3.1 mg/dL. Aspartate aminotransferase (AST) was 122 U/L (normal range, 831), alanine aminotransferase (ALT) 79 U/L (normal range, 731), alkaline phosphatase 1591 U/L (normal range, 39117), and gamma‐glutamyltransferase (GGT) 980 U/L (normal range, 57). Serum albumin was 2.5 mg/dL, prothrombin time was 16.4 seconds, and international normalized ratio (INR) was 1.6.
Urinalysis was normal except for trace hemoglobin, small bilirubin, and 70 mg/dL of protein; specific gravity was 1.007. Urine microscopy demonstrated no cells or casts. The ratio of protein to creatinine on a spot urine sample was less than 1. Chest x‐ray was normal. The electrocardiogram demonstrated sinus rhythm with an old right bundle branch block and normal QRS voltages.
The disproportionate elevation in alkaline phosphatase points to an infiltrative hepatopathy from a cancer originating in the gastrointestinal tract or infection. Other infiltrative processes such as sarcoidosis or amyloidosis usually have evidence of disease elsewhere before hepatic disease becomes apparent.
Mild proteinuria may be explained by diabetes. The specific gravity of 1.007 is atypical for dehydration and could suggest ischemic tubular injury. Although intrinsic renal diseases must continue to be entertained, hypovolemia (compounded by angiotensin‐converting enzyme [ACE] inhibitor use) is the leading explanation in light of the nondiagnostic renal studies. The preserved hemoglobin may simply indicate dehydration, but otherwise is somewhat reassuring in the context of bloody diarrhea.
The patient was admitted to the hospital. Three stool samples returned negative for C difficile toxin. No white cells were detected in the stool, and no ova or parasites were detected. Stool culture was negative for routine bacterial pathogens and for E coli O157. Tests for HIV and antinuclear antibodies (ANAs) and serologies for hepatitis A, B, and C were negative. Abdominal ultrasound demonstrated no intra‐ or extrahepatic bile duct dilatation; no hepatic masses were seen. Kidneys were normal in size and appearance without hydronephrosis. Computed tomography (CT) of the abdomen without intravenous contrast revealed normal‐appearing liver (with a 12‐cm span), spleen, biliary ducts, and pancreas, and there was no intra‐abdominal adenopathy.
The stool studies point away from infectious colitis. Infiltrative processes of the liver, including metastases, lymphoma, tuberculosis, syphilis, amyloidosis, and sarcoidosis, can be microscopic and therefore evade detection by ultrasound and CT scan. In conditions such as these, endoscopic retrograde cholangiopanccreatography/magnetic resonance cholangiopancreatography (ERCP/MRCP) or liver biopsy may be required. The CT is limited without contrast but does not suggest extrahepatic disease in the abdomen.
MRCP was performed, but was a technically suboptimal study due to the presence of ascites. The serum creatinine improved to 1.4 mg/dL over the next 4 days, and the patient's diarrhea decreased to two bowel movements daily with the use of loperamide. The patient was discharged home with outpatient gastroenterology follow‐up planned to discuss further evaluation of the abnormal liver enzymes.
Prior to being seen in the Gastroenterology Clinic, the patient's nonbloody diarrhea worsened. He felt weaker and continued to lose weight. He also noted new onset of bilateral lower face numbness and burning, which was followed by swelling of his lower lip 12 hours later. He returned to the hospital.
On examination, he was afebrile. His lower lip was markedly swollen and was drooping from his face. He could not move the lip to close his mouth. The upper lip and tongue were normal size and moved without restriction. Facial sensation was intact, but there was weakness when he attempted to wrinkle both of his brows and close his eyelids. The rest of his physical examination was unchanged.
The serum creatinine had risen to 3.6 mg/dL, and the complete blood count remained normal. Serum total bilirubin was 4.6 mg/dL, AST 87 U/L, ALT 76 U/L, and alkaline phosphatase 1910 U/L. The 24‐hour urine protein measurement was 86 mg.
Lip swelling suggests angioedema. ACE inhibitors are frequent offenders, and it would be important to know whether his lisinopril was restarted at discharge. ACE‐inhibitor angioedema can also affect the intestine, causing abdominal pain and diarrhea, but does not cause a systemic wasting illness or infiltrative hepatopathy. The difficulty moving the lip may reflect the physical effects of swelling, but generalized facial weakness supports a cranial neuropathy. Basilar meningitis may produce multiple cranial neuropathies, the etiologies of which are quite similar to the previously mentioned causes of infiltrative liver disease: sarcoidosis, syphilis, tuberculosis, or lymphoma.
The patient had not resumed lisinopril since his prior hospitalization. The lower lip swelling and paralysis persisted, and new sensory paresthesias developed over the right side of his chin. A consulting neurologist found normal language and speech and moderate dysarthria. Cranial nerve exam was normal except bilateral lower motor neuron facial nerve palsy was noted with bilateral facial droop, reduced strength of eyelid closure, and diminished forehead movement bilaterally; facial sensation was normal. Extremity motor exam revealed proximal iliopsoas muscle weakness bilaterally rated as 4/5 and was otherwise normal. Sensation to pinprick was diminished in a stocking/glove distribution. Deep‐tendon reflexes were normal and plantar response was down‐going bilaterally. Coordination was intact, Romberg was negative, and gait was slowed due to weakness.
Over the next several days, the patient continued to have diarrhea and facial symptoms. The serum total bilirubin increased to 14 mg/dL, alkaline phosphatase rose above 2,000 U/L, and serum creatinine increased to 5.5 mg/dL. Noncontrast CT scan of the head was normal.
Along with a mild peripheral sensory neuropathy, the exam indicates bilateral palsies of the facial nerve. Lyme disease is a frequent etiology, but this patient is not from an endemic area. I am most suspicious of bilateral infiltration of cranial nerve VII. I am thinking analogically to the numb chin syndrome, wherein lymphoma or breast cancer infiltration along the mental branch of V3 causes sensory loss, and perhaps these disorders can produce infiltrative facial neuropathy. At this point I am most concerned about lymphomatous meningitis with cranial nerve involvement. Cerebrospinal fluid (CSF) analysis (including cytology) would be informative.
Lumbar puncture demonstrated clear CSF with one white blood cell per mm3 and no red blood cells. Glucose was normal, and protein was 95.5 (normal range, 15‐45 mg/dL). Gram stain and culture for bacteria were negative, as were polymerase chain reaction (PCR) testing for herpes simplex virus, mycobacterial and fungal stains and cultures, and cytology. Transthoracic echocardiogram demonstrated severe concentric left ventricular (LV) hypertrophy, normal LV systolic function, and impaired LV relaxation. CT scan of the chest identified no adenopathy or other abnormality.
The CSF analysis does not support basilar meningitis, although the cytoalbuminologic dissociation makes me wonder whether there is some intrathecal antibody production or an autoimmune process we have yet to uncover. The absence of lymphadenopathy anywhere in the body and the negative CSF cytology now point away from lymphoma. As the case for lymphoma or an infection diminishes, systemic amyloidosis rises to the top of possibilities in this afebrile man who is losing weight, has infiltrative liver and nerve abnormalities, renal failure, cardiac enlargement, and suspected gastrointestinal luminal abnormality. Although the echocardiographic findings are most likely explained by hypertension, they are compatible with amyloid infiltration. A tissue specimen is needed, and either colonoscopy or liver biopsy should be suitable.
A pathologist performed a fat pad biopsy that demonstrated scant congophilic and birefringent material associated with blood vessels, suggestive of amyloid (Fig. 1). Colonoscopy demonstrated normal mucosa, and a rectal biopsy revealed congophilic material within the blood vessels consistent with amyloid (Fig. 2). No monoclonal band was present on serum protein electrophoresis. Urine protein electrophoresis identified a homogenous band in the gamma region, and urine kappa and lambda free light chains were increased: kappa was 10.7 mg/dL (normal range, 2), and lambda was 4.25 mg/dL (normal range, 2).


After extensive discussion among the patient, his wife, and a palliative care physician, the patient declined chemotherapy and elected to go home. Two days after discharge (7 weeks after his initial admission for diarrhea) he died in his sleep at home. Permission for a postmortem examination was not granted.
Discussion
Amyloidosis refers to abnormal extracellular deposition of fibril. There are many types of amyloidosis including primary amyloidosis (AL amyloidosis), secondary amyloidosis (AA amyloidosis), and hereditary causes. Systemic AL amyloidosis is a rare plasma cell disorder characterized by misfolding of insoluble extracellular fibrillar proteins derived from immunoglobulin light chains. These insoluble proteins typically deposit in the kidney, heart, and nervous system.1 Although the mechanism of organ dysfunction is debated, deposition of these proteins may disrupt the tissue architecture by interacting with local receptors and causing apoptosis.1
Table 1 indicates the most common findings in patients with AL amyloidosis.2 While our patient ultimately developed many common findings of AL amyloidosis, several features were atypical, including the marked hyperbilirubinemia, profound diarrhea, and bilateral facial diplegia.
| Organ Involvement | Incidence of Organ Involvement (%) | Symptoms | Signs | Laboratory/Test Finding |
|---|---|---|---|---|
| ||||
| General | Malaise, weight loss | |||
| Renal | 33 | Fatigue | Peripheral edema | Proteinuria with or without renal insufficiency, pleural effusion, hypercholesterolemia |
| Cardiac | 20 | Palpitations, dyspnea | Elevated jugular venous pressure, S3, peripheral edema, hepatomegaly | Low‐voltage or atrial fibrillation on electrocardiogram; echocardiogram: thickened ventricles, dilated atria |
| Neurological | 20 | Paresthesias, numbness, weakness, autonomic insufficiency | Carpal tunnel syndrome, postural hypotension | |
| Gastrointestinal and Hepatic | 16 | Diarrhea, nausea, weight loss | Macroglossia, hepatomegaly | Elevated alkaline phosphatase |
| Hematology | Rare | Bleeding | Periorbital purpura (raccoon eyes) | Prolonged prothrombin time, Factor X deficiency |
Up to 70% of patients with amyloidosis will have detectable liver deposits, typically involving portal vessels, portal stroma, central vein, and sinusoidal parenchyma.3 Clinically overt hepatic dysfunction from amyloid is less frequent,4 and the most characteristic findings are hepatomegaly with a markedly elevated serum alkaline phosphatase concentration; jaundice is rare. Palpable hepatic enlargement without abnormal liver enzymes should be interpreted with caution. The finding of a palpable liver edge correlates poorly with frank hepatomegaly, with a positive likelihood ratio of just 1.7.5 In the patient under discussion, suspected hepatomegaly was not confirmed on a subsequent CT scan. Nonetheless, the elevated alkaline phosphatase represented an important clue to potential infiltrative liver disease. In a series of amyloidosis patients from the Mayo Clinic, 81% had hepatomegaly on physical exam, and the mean alkaline phosphatase level was 1,029 U/L (normal, 250 U/L), while the mean serum bilirubin and AST levels were only modestly elevated, at 3.2 mg/dL and 72 U/L respectively. The prothrombin time was prolonged in 35% of patients.
Upper gastrointestinal tract involvement by AL amyloid may be found in up to a third of cases at autopsy, but clinically significant gastrointestinal features are seen in fewer than 5% of patients.6 Predominant intestinal manifestations are unintentional weight loss (average 7 kg) and diarrhea, nonspecific features that result in delayed diagnosis for a median of 7 months after symptom onset.7 Diarrhea in AL amyloid may stem from several mechanisms: small intestine mucosal infiltration, steatorrhea from pancreatic insufficiency, autonomic neuropathy leading to pseudo‐obstruction and bacterial overgrowth, bile acid malabsorption, or rapid transit time. Diarrhea in AL amyloid is often resistant to treatment and may be the primary cause of death.7
Systemic amyloidosis commonly produces peripheral neuropathies. Involvement of small unmyelinated fibers causes paresthesias and progressive sensory loss in a pattern that is usually distal, symmetric, and progressive.6, 9 Our patient presented with bilateral sensory paresthesias of the chin, suggesting the numb chin syndrome (NCS). NCS is characterized by facial numbness along the distribution of the mental branch of the trigeminal nerve. While dental disorders and infiltration from malignant tumors (mostly lung and breast cancer) account for most cases, amyloidosis and other infiltrative disorders are known to cause NCS as well.10, 11 Our patient's sensory paresthesias may have represented amyloid infiltration of peripheral nerves.
With the exception of carpal tunnel syndrome, motor or cranial neuropathy is uncommon in amyloid, and when present usually heralds advanced disease.12 Descriptions of bilateral facial weakness, also known as facial diplegia, from amyloidosis are limited to case reports.1315 Other causes of this rare finding include sarcoidosis, Guillain‐Barr syndrome, and Lyme disease.16
The diagnosis of primary amyloidosis requires histologic evidence of amyloid from a tissue biopsy specimen (demonstrating positive Congo red staining and pathognomonic green birefringence under cross‐polarized light microscopy), and the presence of a clonal plasma cell disorder. While biopsy of an affected organ is diagnostic, more easily obtained samples such as fat pad biopsy and rectal biopsy yield positive results in up to 80% of cases.2 Serum and urine protein electrophoresis with immunofixation identify an underlying plasma cell disorder in 90% of cases of primary amyloidosis. When these tests are inconclusive, serum or urine free light chain assays or bone marrow aspirate and biopsy are useful aids to detect underlying plasma cell dyscrasia.2 AL amyloidosis is a progressive disease with median survival of about 12 years.8 Poorer prognosis is associated with substantial echocardiographic findings, autonomic neuropathy, and liver involvement.2 Hyperbilirubinemia is associated with a poor prognosis, with a median survival of 8.5 months.4 Proteinuria or peripheral neuropathy portends a less ominous course.6
Treatment goals include reducing production and deposition of fibril proteins and contending with organ dysfunction (eg, congestive heart failure [CHF] management). Selected patients with AL amyloidosis may be candidates for high‐dose melphalan and autologous stem cell transplantation.
It would not be reasonable for clinicians to suspect amyloidosis in cases of diarrhea until two conditions are met: 1) the absence of evidence for the typical etiologies of diarrhea; and 2) the evolving picture of an infiltrative disorder. The latter was heralded by the elevated alkaline phosphatase, and was supported by the subsequent multiorgan involvement. Conceptualizing the disease as infiltrative still required a diligent exclusion of infection and invasive tumor cells, which invade disparate organs far more commonly than amyloidosis. Their absence and the organ pattern that is typical of AL amyloidosis (heart, kidney, and peripheral nerve involvement) allowed the discussant to reason by analogy that amyloidosis was also responsible for the most symptomatic phenomena, namely, the diarrhea and facial diplegia (and numb chin syndrome).
Key Teaching Points
-
Hospitalists should consider systemic amyloidosis in cases of unexplained diarrhea when other clinical features of AL amyloidosis are present, including nephrotic syndrome with or without renal insufficiency, cardiomyopathy, peripheral neuropathy, and hepatomegaly.
-
Hepatic amyloidosis should be suspected when weight loss, hepatomegaly, and elevated alkaline phosphatase are present. Although jaundice is rare in amyloidosis, liver involvement and hyperbilirubinemia portend a poorer prognosis.
-
Numb chin syndrome and bilateral facial diplegia are rare manifestations of AL amyloid deposition in peripheral nerves.
- ,.Molecular mechanisms of amyloidosis.N Engl J Med.2003;349(6):583–596.
- Guidelines Working Group of UK Myeloma Forum; British Committee for Standards in Haematology, British Society for Haematology.Guidelines on the diagnosis and management of AL amyloidosis.Br J Haematol.2004;125:681–700.
- ,.Hepatic amyloidosis: morphologic differences between systemic AL and AA types.Hum Pathol.1991;22(9):904–907.
- ,,, et al.Primary (AL) hepatic amyloidosis clinical features and natural history in 98 patients.Medicine.2003;82(5):291–298.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:WB Saunders;2001:595–599.
- ,,, et al.Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis.Am J Hematol.2005;79:319–328.
- .Primary (AL) amyloidosis with gastrointestinal involvement.Scand J Gastroenterol.2009;44(6):708–711.
- ,.Gastrointestinal manifestations of amyloid.Am J Gastroenterol.2008;103:776–787.
- ,.Primary systemic amyloidosis: clinical and laboratory features in 474 cases.Semin Hematol.1995;32:45–59.
- ,,,,.Chin numbness: a symptom that should not be underestimated: a review of 12 cases.Am J Med Sci.2009;337:407–410.
- .Numb chin syndrome: a possible clue to serious illness.Hosp Physician.2000;54–56.
- .Autonomic peripheral neuropathy.Neurol Clin.2007;25:277–301.
- ,.Facial diplegia due to amyloidosis.South Med J.1986;79(11):1458–1459.
- ,,,,.Familial amyloidosis with cranial neuropathy and corneal lattice dystrophy.Neurology.1986;36:432–435.
- ,,.Crainal neuropathy associated with primary amyloidosis.Ann Neurol.1991;29:451–454.
- .Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature.Neurology.1994;44:1198–202.
- ,.Molecular mechanisms of amyloidosis.N Engl J Med.2003;349(6):583–596.
- Guidelines Working Group of UK Myeloma Forum; British Committee for Standards in Haematology, British Society for Haematology.Guidelines on the diagnosis and management of AL amyloidosis.Br J Haematol.2004;125:681–700.
- ,.Hepatic amyloidosis: morphologic differences between systemic AL and AA types.Hum Pathol.1991;22(9):904–907.
- ,,, et al.Primary (AL) hepatic amyloidosis clinical features and natural history in 98 patients.Medicine.2003;82(5):291–298.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:WB Saunders;2001:595–599.
- ,,, et al.Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis.Am J Hematol.2005;79:319–328.
- .Primary (AL) amyloidosis with gastrointestinal involvement.Scand J Gastroenterol.2009;44(6):708–711.
- ,.Gastrointestinal manifestations of amyloid.Am J Gastroenterol.2008;103:776–787.
- ,.Primary systemic amyloidosis: clinical and laboratory features in 474 cases.Semin Hematol.1995;32:45–59.
- ,,,,.Chin numbness: a symptom that should not be underestimated: a review of 12 cases.Am J Med Sci.2009;337:407–410.
- .Numb chin syndrome: a possible clue to serious illness.Hosp Physician.2000;54–56.
- .Autonomic peripheral neuropathy.Neurol Clin.2007;25:277–301.
- ,.Facial diplegia due to amyloidosis.South Med J.1986;79(11):1458–1459.
- ,,,,.Familial amyloidosis with cranial neuropathy and corneal lattice dystrophy.Neurology.1986;36:432–435.
- ,,.Crainal neuropathy associated with primary amyloidosis.Ann Neurol.1991;29:451–454.
- .Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature.Neurology.1994;44:1198–202.
What Is the Role of BNP in Diagnosis and Management of Acutely Decompensated Heart Failure?
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
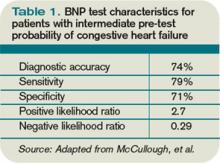
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.
What Is the Best Treatment of an Adult Patient with Hypercalcemia of Malignancy?
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Case
A 63-year-old man with hypertension, diabetes, and recently diagnosed squamous-cell lung cancer presents with diffuse abdominal pain and confusion of two-day duration. He weighs 105 Kg, his blood pressure is 105/65 mm/Hg, heart rate is 105 beats per minute, and temperature is 99.0 degrees Fahrenheit. His respirations are 18 breaths per minute, oxygen saturation is 95% on room air, and his orthostatics are positive. Dry mucus membranes with decreased skin turgor are noted on physical examination. Laboratory evaluation reveals a calcium level of 15.5 mg/dL, creatinine level of 1.2 mg/dL, albumin level of 4.3 g/dL, and a phosphorous level of 2.9 mg/dL.
What is the best treatment of this condition?
Overview
Calcium homeostasis involves complex interactions between the kidney, gastrointestinal (GI) tract, and the skeletal system via hormonal influences. Although 99% of the body’s calcium is stored in the bones, 50% of serum calcium is in the active ionized form, 40% is bound to albumin, and 10% is complexed with anions.1 It’s important to remember these percentages when evaluating a patient’s serum calcium; elevated serum calcium can be validated by using either a correction formula (corrected calcium=measured total calcium + [0.8 x (4.5-albumin)]) or by direct measurement of the ionized calcium, which is the physiologically active form.
Hypercalcemia of malignancy is the most common cause of hypercalcemia in the hospitalized patient. Twenty to 30% of patients with cancer will develop hypercalcemia at some point in their disease course.2 Overall, this portends a poor prognosis with a median survival of three to four months.3
Four general mechanisms are involved in the pathogenesis of malignant hypercalcemia; these mechanisms form the basis for available treatment strategies available:
- Osteolytic tumors, such as multiple myeloma, can directly act on bone, leading to osteoclast activation and release of calcium;
- Humoral mediators elaborated by malignant cells, such as parathyroid hormone-related peptide (PTH-RP), can effect activation of osteoclasts and decrease renal elimination of calcium, causing humoral hypercalcemia of malignancy;
- Some malignancies (most commonly lymphomas) can directly synthesize 1,25 (OH)2 vitamin D, leading to increased luminal absorption of both calcium and phosphorus from the GI tract; and
- Direct production of parathyroid hormone (PTH) by the malignant cells is rare, but has been reported.2
Other factors, including impaired mobility, might lead to further bone resorption and a worsening of the hypercalcemic state.
A patient with hypercalcemia must have a systematic workup, with knowledge of other causes of hypercalcemia that could be present, irrespective of malignancy. Examples include primary hyperparathyroidism, medications effect, and genetic etiologies. Although further discussion is beyond the scope of this article, a broad diagnostic approach is represented in Figure 1 (at right).
Effective management of hypercalcemia demands consideration of both the patient’s immediate, as well as longer-term, clinical situation in light of the patient’s prognosis. The primary aim in the acute management of hypercalcemia is to normalize serum values and decrease symptoms. However, this must be done with appreciation that the metabolic derangement was generated by an underlying malignancy. The main focus of clinical therapeutics should be aimed at this.
Review of the Data
Intravenous (IV) fluids. IV hydration with isotonic saline represents the most immediate and critical intervention in the acute management of malignant hypercalcemia. This condition has multiple, potentially deleterious effects on the kidney, including vasoconstriction, inhibition of salt absorption distally, and antagonism of anti-diuretic hormone (ADH), leading to both salt and water loss. The decrease in intravascular volume then potentiates increased sodium re-absorption proximally in the kidney.
Isotonic saline restores the volume depletion that invariably occurs in the setting of hypercalcemia-provoked urinary salt wasting. The restoration of intravascular volume results in an increase in the glomerular filtration rate and, thus, an increase in calcium filtration. Furthermore, proximal tubular sodium and calcium re-absorption decrease as the glomerular filtration rate increases. Additionally, an increase in sodium and water presentation to the distal renal tubular sites provokes a further calciuresis.
It is estimated that with saline hydration, the calcium concentration should decline, at least by the degree to which dehydration raised it, typically in the range of 1.6 mg to 2.4 mg per deciliter.4 Hydration alone, however, rarely leads to normalization of the serum calcium concentration in patients with severe hypercalcemia.
The rate of infusion is based on the severity of hypercalcemia, and the patient’s age and comorbidities, with particular attention to cardiac or renal disease. A standard approach for most patients without edema and without heart or renal failure is to begin a saline infusion at an initial rate between 200 mL/h to 300 mL/h. The goal is to maintain urine output at 100 mL/h to 150 mL/h.
Furosemide. Following the administration of intravenous fluids to re-establish a euvolemic state, furosemide historically has been used because it has a calcinuric effect with forced diuresis. It also is useful for managing and preventing the fluid overload that occurs with saline hydration. However, data does not support its routine use to lower calcium levels in hypercalcemic patients.
The majority of articles studying the use of furosemide were published in the 1970s and ’80s, and they involve a variety of doses and administration schedules ranging from 40 mg orally daily to 100 mg IV hourly with variable improvement in serum calcium levels and effects that were short-lived. Although some studies have shown that these high doses (2,400 mg/24 hours) of furosemide can decrease calcium levels, resultant severe metabolic derangements in other electrolytes were encountered. This approach required frequent and invasive monitoring to prevent such derangements.5 The clinical application of these studies have led to published recommendations that are as variable as the doses used in the initial studies more than 30 years ago.
This includes the consideration that, in light of the availability and efficacy of bisphosphonates, furosemide might no longer be clinically helpful in this endeavor.6 The current role of furosemide in the management in hypercalcemic patients remains on an as-needed basis for management of fluid overload states brought about after aggressive IV fluid resuscitation.
Bisphosphonates. Bisphosph-onates first became available for the management of hypercalcemia in the early 1990s and have dramatically changed the acute intervention and improved the long-term clinical course of patients with malignant hypercalcemia. Though first developed in the 19th century with industrial applications, it wasn’t until the 1960s that their role in bone metabolism was appreciated.
While their complex mechanism of action remains an issue of ongoing investigations, it is known that bisphosphonates are directed to the bones, where they inhibit an enzyme in the HMG-CoA reductase pathway and promote apoptotic cell death of osteoclasts.7 By blocking osteoclast-mediated bone resorption, the bisphosphonates are effective in treating the hypercalcemia that occurs with a variety of bone-resorbing disease processes, malignant hypercalcemia included. As relatively nontoxic compounds capable of conferring a profound and sustained diminution in serum calcium, these agents have become preferred in the management of acute and chronic hypercalcemia of malignancy.
There are five parenteral bisphosphonates available for the treatment of malignant hypercalcemia: pamidronate, zoledronic acid, ibandronate, etidronate, and clodronate. Etidronate and clodronate are first-generation agents, which are less potent and have more side effects than other agents and are not as commonly used. Ibandronate is a useful agent with a long half-life shown to be as effective as pamidronate, though it has not been as extensively studied as the other agents.
Pamidronate has been studied thoroughly in multiple observational and randomized trials, and has been shown to be highly efficacious and minimally toxic in the treatment of hypercalcemia due to multiple causes, including malignant hypercalcemia.8,9 A maximum calcium-lowering effect occurs at a dose of 90 mg, and the dose is often titrated based on the measured serum calcium. It is infused over two to four hours, effects a lowering of serum calcium within one to two days, and has a sustained effect lasting for up to two weeks or more.
As the most potent and most easily administered bisphosphonate, zoledronic acid is considered by many the agent of choice in the treatment of malignant hypercalcemia. It can be administered as a 4 mg-8 mg dose intravenously over 15 minutes (compared with two hours for pamidronate). Two Phase III trials comprising 275 patients have demonstrated zoledronic acid’s superior efficacy compared with pamidronate, with 88% of patients accomplishing a normalized serum calcium (compared with 70% of patients receiving a 90-mg dose of pamidronate).10
Even though these agents are relatively nontoxic, each can provoke a mild, transient flulike illness in recipients. Renal dysfunction has been noted rarely. These agents should be renally dosed and used with caution in patients with advanced renal insufficiency (serum creatinine >2.5). Osteonecrosis of the jaw has been observed in less than 2% of patients receiving IV bisphosphonates. Accordingly, it is recommended that patients undergo dental evaluation prior to receiving the agent (if feasible) and avoid invasive dental procedures around the time that they receive the agent.11
Other therapeutic interventions. The bisphosphonates represent the best studied and most efficacious pharmaceutical agents available to treat hypercalcemia. Straying from these agents should be considered only when they are contraindicated, in severe circumstances, or after the patient has failed to respond.
Calcitonin has long had FDA approval for treatment of hypercalcemia in adults. It has been shown in small, nonrandomized studies from the 1970s and ’80s to rapidly (within two hours) decrease calcium levels in hypercalcemic patients.12,13,14 However, these reductions are small (<10%) and transient (usually persisting up to 72 to 96 hours) due to the tachyphylaxsis noted with this medication. Nonetheless, calcitonin can be used as an adjuvant bridge to lower calcium levels in severely hypercalcemic patients for the first few days before other agents start taking effect.
Glucocorticoids have been used to treat hypercalcemia since the 1950s. Prednisone, dexamethasone, and methylprednisolone all carry FDA indications for hypercalcemia, but data are lacking and contradictory. A small (n=28) randomized controlled trial (RCT) conducted in 1984 showed no additional efficacy of glucocorticoids with IV fluids when compared with IV fluids alone.15 Another small (n=30) RCT done in 1992 on women with metastatic breast cancer showed a significant improvement in patients treated with prednisolone, IV fluids, and furosemide when compared with IV fluids and furosemide.16 Other nonrandomized trials have shown response to be unpredictable at best.17 Despite this, glucocorticoids likely retain a limited role for treatment in specific cases, including hypercalcemia induced by lymphomas elevating levels of 1,25(OH)2 vitamin D (as this interacts with a steroid-regulated receptor), or multiple myelomas where they potentially impact disease progression.
Gallium nitrate, an anhydrous salt of a heavy metal, has been shown in several randomized trials to be an effective therapeutic agent in lowering calcium levels in hypercalcemic patients.18,19 Furthermore, a double-blinded trial of 64 patients with hypercalcemia of malignancy showed gallium nitrate to be at least as effective as pamidronate for acute control of cancer-related hypercalcemia.20 However, the need for continuous infusion over a five-day period has limited the application of this agent.
Hemodialysis with a calcium-lacking dialysate has been shown in small, nonrandomized studies to be a temporarily effective method of reducing serum calcium levels.21,22 However, this treatment modality would best be reserved for patients with severe hypercalcemia, in whom aggressive intravascular volume repletion and bisphosphonates are not advisable (e.g. those with significant heart or kidney failure) and have an underlying etiology that is likely to be responsive to other treatment. Furthermore, consideration as to the appropriateness of such invasive temporizing procedures in patients with metastatic cancer should be undertaken.
Back to the Case
This patient had an ionized calcium level of 1.9 mmol/L (normal 1.1-1.4 mmol/L). He was started on aggressive IV hydration with normal saline and zoledronic acid. His home medications were reviewed, and it was confirmed that he was not taking such contraindicated medications as thiazides or calcium/vitamin D supplementation.
Further workup for the etiology of his hypercalcemia revealed an appropriately suppressed, intact PTH and normal 25 (OH) Vitamin D and 1,25 (OH)2 Vitamin D levels. His intact PTH-RP was elevated at 10pmol/L, and consistent with hypercalcemia of malignancy.
Oncology and palliative-care consults were requested to assist with coordination of the treatment of the patient’s underlying lung cancer; plans were made for systemic chemotherapy. His symptoms slowly improved, and 72 hours after admission, his serum calcium had normalized. He was discharged with a plan to initiate chemotherapy and continued follow-up with oncology.
Bottom Line
Acute management of hypercalcemia of malignancy focuses on lowering the serum calcium through a variety of pharmacologic agents. However, such long-term issues as treatment of the underlying malignancy and discussions about goals of care in this high-mortality patient population is paramount. TH
Dr. Hartley and Dr. Repaskey are clinical instructors in internal medicine at the University of Michigan Health System. Dr. Rohde is a clinical assistant professor of internal medicine at UMHS.
References
- Assadi F. Hypercalcemia: an evidence-based approach to clinical cases. Iran J Kidney Dis. 2009;3:(2):71-79.
- Stewart A. Hypercalcemia associated with cancer. N Engl J Med. 2005;542(4):373-379.
- Seccareccia D. Cancer-related hypercalcemia. Can Fam Physician. 2010;56:(3):244-246.
- Bilezikian JP. Management of acute hypercalcemia. N Engl J Med. 1992; 326(18):1196-1203.
- Suki WN, Yium JJ, VonMinden M, et al. Acute treatment of hypercalcemia with furosemide. N Engl J Med. 1970;283:836-840.
- LeGrand SB, Leskuski D, Zama I. Narrative review: furosemide for hypercalcemia: an unproven yet common practice. Ann Intern Med. 2008;149:259-263.
- Drake MT, Bart LC, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clinic Proc. 2008;83(9):1032-1045.
- Nussbaum SR, Younger J, Vandepol CJ, et al. Single-dose intravenous therapy with pamidronate for the treatment of hypercalcemia of malignancy: comparison of 30-, 60-, and 90-mg dosages. Am J Med. 1993; 95(3):297-304.
- Gucalp R, Ritch P, Riernik PH, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992;10(1):134-142.
- Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2): 558-567.
- Tanvetyanon T. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907.
- Wisneski LA, Croom WP, Silva OL, et al. Salmon calcitonin in hypercalcemia. Clin Pharmacol Ther. 1978; 24:219-222.
- Binstock ML, Mundy GR. Effect of calcitonin and glucocorticoids in combination on the hypercalcemia of malignancy. Ann Intern Med. 1980;93(2):269-272.
- Nilsson O, Almqvist S, Karlberg BE. Salmon calcitonin in the acute treatment of moderate and severe hypercalcemia in man. Acta Med Scand. 1978;204(4): 249-252.
- Percival RC, Yates AJ, Gray RE, et al. Role of glucocorticoids in management of malignant hypercalcemia. Br Med J. 1984;289(6440):287.
- Kristensen B, Ejlertsen B, Holmegaard SN, et al. Prednisolone in the treatment of severe malignant hypercalcemia in metastatic breast cancer: a randomized study. J Intern Med. 1992;232(3):237-245.
- Thalassinos NC, Joplin GF. Failure of corticosteroid therapy to correct the hypercalcemia of malignant disease. Lancet. 1970;2(7672):537-538.
- Warrell RP Jr, Murphy WK, Schulman P, et al. A randomized double-blind study of gallium nitrate compared with etidronate for acute control of cancer-related hypercalcemia. J Clin Oncol. 1991;9(8):1467-1475.
- Warrell RP Jr, Israel R, Frisone M, et al. Gallium nitrate for acute treatment of cancer-related hypercalcemia: a randomized, double-blinded comparison to calcitonin. Ann Intern Med. 1988;108:669-674.
- Cvitkovic F, Armand JP, Tubiana-Hulin M, et al. Randomized, double-blind, phase II trial of gallium nitrate compared with pamidronate for acute control of cancer-related hypercalcemia. Cancer J. 2006;12 (1):47-53.
- Cardella CJ, Birkin BL, Rapoport A. Role of dialysis in the treatment of severe hypercalcemia: report of two cases successfully treated with hemodialysis and review of the literature. Clin Nephrol. 1979; 12(6):285-290.
- Koo WS, Jeon DS, Ahn SJ, et al. Calcium-free hemodialysis for the management of hypercalcemia. Nephron. 1996;72(3):424-428.
Elements of Confusion
A 23‐year‐old man presented to his family physician's office with a 2‐week history of fever, chills, night sweats, anorexia, and fatigue. This was associated with a 4‐month history of a nonproductive cough and a 20‐pound involuntary weight loss. He denied shortness of breath, chest pain, headaches, abdominal pain, vomiting, diarrhea, dysuria, and rash. There was no recent travel, sick contacts, or animal exposures.
This patient's symptoms could represent an underlying infectious, neoplastic, or inflammatory process. I would ascertain any relevant personal or family history and explore whether the patient has risk factors for human immunodeficiency virus (HIV) infection or tuberculosis (TB). On physical examination, I would listen for a heart murmur and look for lymphadenopathy, hepatosplenomegaly, and arthritis. Investigations including cultures, urinalysis, and a chest radiograph would be indicated at this time.
During the 2 weeks after his initial presentation, he experienced persistent fever, and further weight loss. He was admitted to the hospital to determine the etiology of his symptoms. The patient had no previous medical problems. On initial examination, his temperature was 102 degrees Fahrenheit, blood pressure was 100/65 mmHg, heart rate was 105 per minute, respiratory rate was 22 breaths per minute and oxygen saturation was normal on ambient air. He appeared cachectic. He was oriented to person, place, and time. Head and neck examination revealed no intraoral pathology, lymphadenopathy or scleral icterus, but did reveal conjunctival pallor. The chest was clear to auscultation, and the cardiovascular examination revealed a normal apical impulse and heart sounds with no murmurs. There was peripheral edema to the level of the mid‐shins bilaterally. The abdomen was soft and non‐tender with no appreciable hepatosplenomegaly. There were no stigmata of chronic liver disease. There was no axillary or inguinal lymphadenopathy. The remainder of the examination was normal. A complete blood count showed a hemoglobin concentration of 5.2 g/dL with a mean corpuscular volume (MCV) of 89fL, white blood cells were 1,400 cells/mm3 with an absolute neutrophil count (ANC) of 800 cells/mm3 and a platelet count of 90,000 cells/mm3 The serum sodium was 124 mmol/L, potassium 3.0 mmol/L, chloride 91 mmol/L, bicarbonate 26 mmol/L, and the creatinine 1.36 mg/dL His liver enzyme profile showed aspartate aminotransferase (AST) 68 U/L (normal 35), alanine aminotransferase (ALT) 25 U/L (normal 40), alkaline phosphatase (ALP) 210 U/L (normal 110) and a total bilirubin of 1.64 mg/dL.
The patient is clearly very unwell and requires admission to the hospital for treatment and further investigation. Emergent management includes administration of intravenous fluids to correct his electrolyte abnormalities, empiric broad spectrum antibiotics (given his relative neutropenia and fever), and a transfusion for his profound anemia. I would be very concerned that he has an underlying malignancy such as lymphoma or leukemia. Pancytopenia related to decreased cell production may be secondary to infiltration (malignant or granulomatous), infection (HIV, TB, fungal, viral), or aplasia (primary or drug‐related). Less likely etiologies include B12 or folate deficiency (unlikely given the normal MCV), systemic lupus erythematosus, paroxysmal nocturnal hemoglobinuria or cell sequestration due to hypersplenism. A history of recent exposure to drugs or toxins should be elicited. The patient's pulmonary symptoms may relate to the primary disorder or may represent an infection secondary to myelosuppression. I would want an immediate review of the peripheral blood smear, a hemolysis work‐up (drawn prior to transfusion including lactate dehydrogenase [LDH], haptoglobin, fractionated bilirubin, reticulocyte count and direct antiglobulin testing), antinuclear antibody (ANA), B12 and folate levels, imaging of the chest and blood cultures.
With evidence of fever and pancytopenia, acute leukemia was suspected and the patient was admitted to a hematology service. Over the next two weeks an extensive investigation including blood and urine cultures, and computed tomograms (CT) of the chest and abdomen were performed. A bone marrow aspirate and biopsy were also were done and were submitted for histopathologic examination and culture. The CT scan of the chest revealed left axillary and supraclavicular lymphadenopathy (Figure 1), and the abdominal imaging revealed splenomegaly. The blood, urine and bone marrow cultures were all negative. A peripheral blood smear showed pancytopenia with a hematologist interpretation suggesting that an intrinsic bone marrow process may be resulting in impaired cell production. The corresponding bone marrow biopsy and aspirate showed no evidence of malignancy, but there were numerous granulomata, and the periodic acid‐Schiff (PAS) and silver staining showed cells that resembled fungal elements (Figure 2).
The absence of malignant cells in the bone marrow leaves us to consider infectious and inflammatory causes of this patient's presentation. Infectious etiologies associated with bone marrow granulomata include fungal, mycobacterial, bacterial (brucellosis, typhoid and Q fever) and viral pathogens including HIV, Epstein‐Barr virus (EBV), and cytomegalovirus (CMV). Noninfectious causes include sarcoidosis, drug effects, and autoimmune conditions. The PAS and silver staining suggests this patient has a disseminated fungal infection. Disseminated Histoplasma capsulatum is the most likely organism but blastomycosis and coccidioidomycosis should be considered. HIV and occult lymphoma are considerations as is a primary immune disorder such as common variable immunodeficiency (CVID) which can present in this age group. While there is no recent travel history, it will be critical to determine where the patient currently lives and previously resided, review the medical record for prior infections and HIV risk factors, and take a thorough occupational history.
At this point, the following investigations should be undertaken: blood, sputum, and bone marrow culture; fungal and acid‐fast bacilli (AFB) stains on sputum and bone marrow; Histoplasma urine antigen; tuberculin skin test; serology for HIV and histoplasmosis; and serum protein electrophoresis with immunofixation and quantitation of immunoglobulins.
Acid fast staining of the bone marrow as well as mycobacterial and fungal cultures were negative. He lived in eastern Ontario and worked in construction. He reported helping tear down an old cabin in a wooded area, but denied any insect bites. This project coincided with the onset of his cough. He had no history of high risk sexual activity, intravenous drug use, tattoos or blood transfusions previous to his presentation. The HIV test was negative. His clinicians at this point considered a disseminated fungal infection as a cause for his symptoms and started him empirically on itraconazole He was discharged from the hospital with a plan for close outpatient followup. Within three days of discharge on the itraconazole, the patient's fever began to diminish, but did not completely resolve.
The clinical picture including cough, geography, and recent occupational exposure is entirely consistent with disseminated histoplasmosis. However, we are still lacking microbiologic confirmation of the diagnosis. Sarcoidosis and occult malignancy must still be considered. In the absence of a definitive diagnosis, I would consider bronchoscopy with bronchoalveolar lavage (BAL) and obtaining a lymph node or liver biopsy for microbiologic and pathologic examination. With the patient now receiving antifungal therapy, a diagnosis of histoplasmosis would be supported by a response to therapy, declining Histoplasma antigen levels and clinical improvement including recovery of his bone marrow.
The urine specimen was negative for Histoplasma antigen. Seven days after initiating itraconazole, he developed jaundice and confusion and was taken back to the hospital. On presentation, he was disoriented but awake. His temperature was 103.1 degrees Fahrenheit, blood pressure was 90/60 mm Hg, heart rate was 115 per minute, and oxygen saturation was normal on room air. He was obviously jaundiced, and more cachectic than previous. The neurologic examination demonstrated disorientation with no localizing findings. The chest and cardiovascular examinations were normal. His abdomen was soft and non‐tender with no evidence of hepatomegaly, but the spleen tip was palpable. There was no ascites or any other signs of portal hypertension, but his peripheral edema was worse than before and asterixis was present. The remainder of the examination was unchanged from previous. His laboratory investigations at this point showed a bilirubin of 18.5 mg/dL, AST 269 U/L, ALT 76 U/L, ALP 165 U/L, albumin 18 g/L, fibrinogen 1.53 g/L (normal 1.5‐3.5), triglycerides 2.4 mmol/L (normal 2), ferritin 59415 ug/L (normal 22‐275) an international normalized ratio (INR) of 2.65. His complete blood count still showed pancytopenia.
The patient has now developed fulminant hepatic failure. He requires volume resuscitation, drawing of repeat cultures, initiation of empiric broad spectrum antibiotics, urgent hepatology consultation and intensive care unit (ICU) support. The most common causes of acute liver failure are drug toxicity (including acetaminophen), viral hepatitis, Wilson's disease, Budd‐Chiari syndrome, cryptogenic liver disease and fatty infiltration. The critical diagnostic issue at this point is to determine if the liver failure is a secondary process (in which case drug toxicity due to itraconazole would be the most likely cause) or if this represents evolution of his primary disease with extensive hepatic involvement. Liver failure due to itraconazole has been reported and given the lack of microbiologic confirmation of a fungal infection, this agent should clearly be discontinued. Returning to our initial differential diagnosis of this man's granulomatous bone marrow infiltration and pancytopenia, etiologies which may progress to hepatic failure include viral infections (EBV or CMV) and malignancy. This patient's presentation could be an unusual manifestation of a common illness such as EBV or a rapidly progressive lymphoma. An abdominal Doppler ultrasound is required to rule out Budd‐Chiari syndrome. Given his abrupt change in clinical status, I would repeat a CT scan of his chest and abdomen to evaluate for evidence of infection, infiltration, or malignancy. Owing to the uncertainty regarding this patient's diagnosis and the rapidly progressive nature of his disease, serious consideration must be given to a transjugular liver biopsy.
Soon after admission, he developed hematemesis. He was given multiple blood transfusions, and then intravenous fluids, broad spectrum antibiotics and lactulose. Upper gastrointestinal endoscopy showed no varices, but did reveal multiple esophageal and gastric ulcerations. He was then transferred to a liver transplant center where repeat bone marrow biopsy and a liver biopsy were done. Both revealed extensive granulomatosis and the bone marrow biopsy showed evidence of hemophagocytosis (Figure 3).
The finding of hemophagocytosis in the setting of fever, hepatosplenomegaly, and pancytopenia is consistent with a diagnosis of hemophagocytic lymphohistiocytosis (HLH). The cornerstone of therapy for patients with HLH is suppression of the severe inflammatory response with corticosteroids, etoposide and cyclosporin. Patients who respond to this are candidates for allogeneic stem cell transplant with curative intent. This patient's hepatic dysfunction precludes the use of etoposide and initial therapy should therefore include dexamethasone and cyclosporin.
All bacterial, fungal, and mycobacterial cultures again demonstrated no growth. Broad spectrum antibiotics were continued, and empiric intravenous amphotericin B was added. He became hemodynamically unstable, was intubated, put on mechanical ventilation and required vasoactive medications to maintain his blood pressure. An empiric course of pulse corticosteroids was given for the possibility of sarcoidosis. His blood pressure stabilized, though he continued to require vasopressors.
While HLH has been very rarely reported in association with sarcoidosis, the underlying pathogenesis of his clinical presentation (infectious, neoplastic, or inflammatory) has not yet been confirmed. In the meantime, I would continue with supportive care and intravenous corticosteroids.
Immunohistochemical studies of the liver biopsy returned showing CD15/30+ cells with weak‐to‐negative CD45 expression cells typical of Hodgkin lymphoma (HL) (Figure 4). He was started on chemotherapy, but over 48 hours became progressively more hypotensive. The patient died of Klebsiella and Pseudomonas sepsis on the 7th hospital day. Post‐mortem immunohistochemical examination revealed evidence of Hodgkin disease in the axillary lymph nodes, bone marrow and liver. The bone marrow showed evidence of hemophagocytosis and was also positive for Epstein‐Barr encoded RNA (EBER). Serologic studies were subsequently available and revealed positive EBV IgM against the viral capsid antigen (VCA) as well as EBV IgG VCA, which in conjunction with the marrow findings, was highly suggestive of reactivation EBV disease.
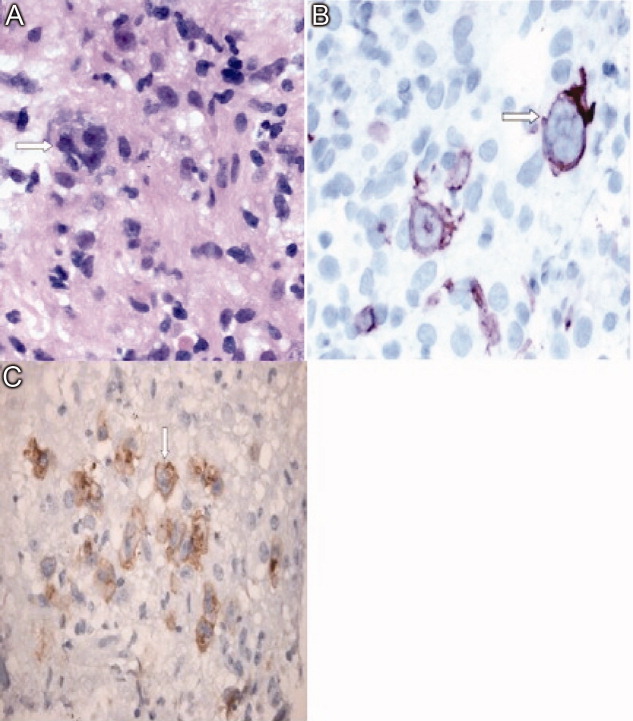
Discussion
This patient's diagnostic course led both the clinical team and discussant down a winding path, which ultimately ended in the finding of Hodgkin lymphoma, a relatively common diagnosis that had been clouded by seemingly contradictory clinical and laboratory data. The provisional diagnosis of disseminated histoplasmosis was reasonable given that H. capsulatum is endemic in Ontario and that the patient's occupation placed him at risk of infection. Given the acuity of his illness, empiric antifungal therapy based on the report of fungal elements on bone marrow examination seemed reasonable. However, Histoplasma urinary antigen testing has been shown in the literature to be 98% sensitive in immunosuppressed populations, and the negative result prompted a re‐examination of the marrow specimen. The previously described fungal elements were felt to be most likely artifact, and the underlying diagnosis was reconsidered.1 This is when the repeat bone marrow examination pointed towards the diagnosis of HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a severe, systemic hyperinflammatory disorder characterized by histiocytic proliferation that may be primary or can be triggered by infection, connective tissue diseases or malignancy.25 The central pathogenesis involves dysregulated Th1 cytokine secretion. This results in an uncontrolled accumulation of activated T‐lymphocytes and histiocytes in various organs including the liver, spleen and bone marrow. The infiltration of histiocytes into major organs can lead to disruption of function and multiorgan failure.6 Viruses are the most common infectious triggers of HLH, particularly EBV, and lymphoma is the most common associated malignancy.25 It is hypothesized that EBV can interfere with normal lymphocyte signaling pathways leading to the aforementioned over‐expression of Th1 cytokines, which can then trigger HLH.7 The diagnosis of HLH is based on a combination of clinical and laboratory parameters as outlined in Table 1.8 Our patient met all five of the major criteria.
| |
| Major criteria | 1. Fever |
| 2. Splenomegaly | |
| 3. Cytopenia in two or more cell lines | |
| 4. Hypertriglyceridemia or hypofibrinogenemia | |
| 5. Hemophagocytosis on histopathologic examination | |
| Alternative criteria | A. Low or absent natural killer cell activity |
| B. Serum ferritin level >500 ug/L | |
| C. Soluble CD‐25 level >2400 U/mL | |
The recommended treatment of HLH involves the administration of the HLH‐94 protocol consisting of corticosteroids, cyclosporine and etoposide.9, 10 Those who survive this initial phase are recommended for hematopoetic stem cell transplantation producing an overall 3‐year survival rate of 64%. However, those who do not receive early etoposide therapy fare much worse, with a mortality rate of 92%.10 This patient was not able to receive etoposide because of his decompensated liver disease.
In this case, the development of Hodgkin lymphoma involving the bone marrow and liver may have resulted in a state of immune suppression. The loss of immune function likely allowed the reactivation of Epstein‐Barr virus which triggered HLH and his fulminant presentation.35,9 Indeed, both the liver and bone marrow samples showed evidence of EBV reactivation as evidenced by the presence of EBER. The diagnosis of Hodgkin lymphoma was made from a liver biopsy specimen rather than bone marrow examination. The diagnosis of Hodgkin lymphoma is based on the presence of Reed‐Sternberg cells surrounded by an inflammatory milieu of cells including variable numbers of small lymphocytes, neutrophils eosinophils and fibroblasts. The HLH‐induced pancytopenia depleted the aforementioned inflammatory milieu in the bone marrow, which obscured the diagnosis of Hodgkin lymphoma. Unfortunately, lymphoma‐associated HLH has a very poor prognosis with a mortality rate of up to 60%.4 At the outset of this case, the reported fungal elements proved to be a source of confusion which delayed the diagnosis of Hodgkin lymphoma. Given the poor prognosis of lymphoma‐associated HLH, it is unlikely this would have had any effect on the ultimate outcome.
Teaching Points
-
HLH is a rare and complex hyperinflammatory disorder which may present as pancytopenia.
-
Triggers of HLH can include infections (particularly EBV), malignancy (particularly lymphoma) and connective tissue diseases.
-
The diagnosis of HLH is based on clinical and laboratory criteria, including cytopenias that may make the evaluation for triggering conditions such as HL more difficult.
-
Lymphoma should be included in the differential diagnosis of granulomatous inflammation.
Acknowledgements
The authors acknowledge Ralph Meyer, MD (Queen's University, Kingston, Ontario) for his comments on a draft of this paper and Drs. David Barth and Maha Guindi (Department of Laboratory Medicine, University Health Network) for their reviews of the pathology specimens.
- ,Antigen detection for diagnosis of the endemic mycoses.Curr Fung Infect Rep.2008;4:189–193.
- ,,, et al.Lymphoma‐associated hemophagocytic syndrome: Clinical features and treatment outcome.Ann Hematol.2007;86:493–498.
- ,,, et al.Hodgkin lymphoma‐associated hemophagocytic syndrome: A disorder strongly correlated with Epstein‐Barr virus.Clin Inf Dis.2008;47:531–534.
- ,.Hemophagocytic syndrome associated with Hodgkin lymphoma and pneumocystis jiroveci pneumonitis.Br J Hematol.2007;138:672.
- ,,, et al.Infections associated with haemophagocytic syndrome.Lancet Infect Dis.2007;12:814–822.
- ,,,.Hemophagocytic lymphohistiocytic syndrome: Unrecognized cause of multiple organ failure.Pediatr Crit Care Med.2000;1:51–54.
- ,,, et al.Epstein‐Barr virus LMP1 Inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome.Blood.2005;106:3090–3096.
- ,,.Diagnostic guidelines for hemophagocytic lymphohistiocytosis.Semin Oncol.1991;18:29–33
- ,,, et al.Treatment of Epstein‐Barr virus‐associated hemophagocytic lymphohistiocytosis (EBV‐HLH) in young adults: A report from the HLH Study Center.Med Pediatr Oncol.2003;41:103–109.
- ,,, et al.Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis.Br J Haematol.2005;129:622–630.
A 23‐year‐old man presented to his family physician's office with a 2‐week history of fever, chills, night sweats, anorexia, and fatigue. This was associated with a 4‐month history of a nonproductive cough and a 20‐pound involuntary weight loss. He denied shortness of breath, chest pain, headaches, abdominal pain, vomiting, diarrhea, dysuria, and rash. There was no recent travel, sick contacts, or animal exposures.
This patient's symptoms could represent an underlying infectious, neoplastic, or inflammatory process. I would ascertain any relevant personal or family history and explore whether the patient has risk factors for human immunodeficiency virus (HIV) infection or tuberculosis (TB). On physical examination, I would listen for a heart murmur and look for lymphadenopathy, hepatosplenomegaly, and arthritis. Investigations including cultures, urinalysis, and a chest radiograph would be indicated at this time.
During the 2 weeks after his initial presentation, he experienced persistent fever, and further weight loss. He was admitted to the hospital to determine the etiology of his symptoms. The patient had no previous medical problems. On initial examination, his temperature was 102 degrees Fahrenheit, blood pressure was 100/65 mmHg, heart rate was 105 per minute, respiratory rate was 22 breaths per minute and oxygen saturation was normal on ambient air. He appeared cachectic. He was oriented to person, place, and time. Head and neck examination revealed no intraoral pathology, lymphadenopathy or scleral icterus, but did reveal conjunctival pallor. The chest was clear to auscultation, and the cardiovascular examination revealed a normal apical impulse and heart sounds with no murmurs. There was peripheral edema to the level of the mid‐shins bilaterally. The abdomen was soft and non‐tender with no appreciable hepatosplenomegaly. There were no stigmata of chronic liver disease. There was no axillary or inguinal lymphadenopathy. The remainder of the examination was normal. A complete blood count showed a hemoglobin concentration of 5.2 g/dL with a mean corpuscular volume (MCV) of 89fL, white blood cells were 1,400 cells/mm3 with an absolute neutrophil count (ANC) of 800 cells/mm3 and a platelet count of 90,000 cells/mm3 The serum sodium was 124 mmol/L, potassium 3.0 mmol/L, chloride 91 mmol/L, bicarbonate 26 mmol/L, and the creatinine 1.36 mg/dL His liver enzyme profile showed aspartate aminotransferase (AST) 68 U/L (normal 35), alanine aminotransferase (ALT) 25 U/L (normal 40), alkaline phosphatase (ALP) 210 U/L (normal 110) and a total bilirubin of 1.64 mg/dL.
The patient is clearly very unwell and requires admission to the hospital for treatment and further investigation. Emergent management includes administration of intravenous fluids to correct his electrolyte abnormalities, empiric broad spectrum antibiotics (given his relative neutropenia and fever), and a transfusion for his profound anemia. I would be very concerned that he has an underlying malignancy such as lymphoma or leukemia. Pancytopenia related to decreased cell production may be secondary to infiltration (malignant or granulomatous), infection (HIV, TB, fungal, viral), or aplasia (primary or drug‐related). Less likely etiologies include B12 or folate deficiency (unlikely given the normal MCV), systemic lupus erythematosus, paroxysmal nocturnal hemoglobinuria or cell sequestration due to hypersplenism. A history of recent exposure to drugs or toxins should be elicited. The patient's pulmonary symptoms may relate to the primary disorder or may represent an infection secondary to myelosuppression. I would want an immediate review of the peripheral blood smear, a hemolysis work‐up (drawn prior to transfusion including lactate dehydrogenase [LDH], haptoglobin, fractionated bilirubin, reticulocyte count and direct antiglobulin testing), antinuclear antibody (ANA), B12 and folate levels, imaging of the chest and blood cultures.
With evidence of fever and pancytopenia, acute leukemia was suspected and the patient was admitted to a hematology service. Over the next two weeks an extensive investigation including blood and urine cultures, and computed tomograms (CT) of the chest and abdomen were performed. A bone marrow aspirate and biopsy were also were done and were submitted for histopathologic examination and culture. The CT scan of the chest revealed left axillary and supraclavicular lymphadenopathy (Figure 1), and the abdominal imaging revealed splenomegaly. The blood, urine and bone marrow cultures were all negative. A peripheral blood smear showed pancytopenia with a hematologist interpretation suggesting that an intrinsic bone marrow process may be resulting in impaired cell production. The corresponding bone marrow biopsy and aspirate showed no evidence of malignancy, but there were numerous granulomata, and the periodic acid‐Schiff (PAS) and silver staining showed cells that resembled fungal elements (Figure 2).


The absence of malignant cells in the bone marrow leaves us to consider infectious and inflammatory causes of this patient's presentation. Infectious etiologies associated with bone marrow granulomata include fungal, mycobacterial, bacterial (brucellosis, typhoid and Q fever) and viral pathogens including HIV, Epstein‐Barr virus (EBV), and cytomegalovirus (CMV). Noninfectious causes include sarcoidosis, drug effects, and autoimmune conditions. The PAS and silver staining suggests this patient has a disseminated fungal infection. Disseminated Histoplasma capsulatum is the most likely organism but blastomycosis and coccidioidomycosis should be considered. HIV and occult lymphoma are considerations as is a primary immune disorder such as common variable immunodeficiency (CVID) which can present in this age group. While there is no recent travel history, it will be critical to determine where the patient currently lives and previously resided, review the medical record for prior infections and HIV risk factors, and take a thorough occupational history.
At this point, the following investigations should be undertaken: blood, sputum, and bone marrow culture; fungal and acid‐fast bacilli (AFB) stains on sputum and bone marrow; Histoplasma urine antigen; tuberculin skin test; serology for HIV and histoplasmosis; and serum protein electrophoresis with immunofixation and quantitation of immunoglobulins.
Acid fast staining of the bone marrow as well as mycobacterial and fungal cultures were negative. He lived in eastern Ontario and worked in construction. He reported helping tear down an old cabin in a wooded area, but denied any insect bites. This project coincided with the onset of his cough. He had no history of high risk sexual activity, intravenous drug use, tattoos or blood transfusions previous to his presentation. The HIV test was negative. His clinicians at this point considered a disseminated fungal infection as a cause for his symptoms and started him empirically on itraconazole He was discharged from the hospital with a plan for close outpatient followup. Within three days of discharge on the itraconazole, the patient's fever began to diminish, but did not completely resolve.
The clinical picture including cough, geography, and recent occupational exposure is entirely consistent with disseminated histoplasmosis. However, we are still lacking microbiologic confirmation of the diagnosis. Sarcoidosis and occult malignancy must still be considered. In the absence of a definitive diagnosis, I would consider bronchoscopy with bronchoalveolar lavage (BAL) and obtaining a lymph node or liver biopsy for microbiologic and pathologic examination. With the patient now receiving antifungal therapy, a diagnosis of histoplasmosis would be supported by a response to therapy, declining Histoplasma antigen levels and clinical improvement including recovery of his bone marrow.
The urine specimen was negative for Histoplasma antigen. Seven days after initiating itraconazole, he developed jaundice and confusion and was taken back to the hospital. On presentation, he was disoriented but awake. His temperature was 103.1 degrees Fahrenheit, blood pressure was 90/60 mm Hg, heart rate was 115 per minute, and oxygen saturation was normal on room air. He was obviously jaundiced, and more cachectic than previous. The neurologic examination demonstrated disorientation with no localizing findings. The chest and cardiovascular examinations were normal. His abdomen was soft and non‐tender with no evidence of hepatomegaly, but the spleen tip was palpable. There was no ascites or any other signs of portal hypertension, but his peripheral edema was worse than before and asterixis was present. The remainder of the examination was unchanged from previous. His laboratory investigations at this point showed a bilirubin of 18.5 mg/dL, AST 269 U/L, ALT 76 U/L, ALP 165 U/L, albumin 18 g/L, fibrinogen 1.53 g/L (normal 1.5‐3.5), triglycerides 2.4 mmol/L (normal 2), ferritin 59415 ug/L (normal 22‐275) an international normalized ratio (INR) of 2.65. His complete blood count still showed pancytopenia.
The patient has now developed fulminant hepatic failure. He requires volume resuscitation, drawing of repeat cultures, initiation of empiric broad spectrum antibiotics, urgent hepatology consultation and intensive care unit (ICU) support. The most common causes of acute liver failure are drug toxicity (including acetaminophen), viral hepatitis, Wilson's disease, Budd‐Chiari syndrome, cryptogenic liver disease and fatty infiltration. The critical diagnostic issue at this point is to determine if the liver failure is a secondary process (in which case drug toxicity due to itraconazole would be the most likely cause) or if this represents evolution of his primary disease with extensive hepatic involvement. Liver failure due to itraconazole has been reported and given the lack of microbiologic confirmation of a fungal infection, this agent should clearly be discontinued. Returning to our initial differential diagnosis of this man's granulomatous bone marrow infiltration and pancytopenia, etiologies which may progress to hepatic failure include viral infections (EBV or CMV) and malignancy. This patient's presentation could be an unusual manifestation of a common illness such as EBV or a rapidly progressive lymphoma. An abdominal Doppler ultrasound is required to rule out Budd‐Chiari syndrome. Given his abrupt change in clinical status, I would repeat a CT scan of his chest and abdomen to evaluate for evidence of infection, infiltration, or malignancy. Owing to the uncertainty regarding this patient's diagnosis and the rapidly progressive nature of his disease, serious consideration must be given to a transjugular liver biopsy.
Soon after admission, he developed hematemesis. He was given multiple blood transfusions, and then intravenous fluids, broad spectrum antibiotics and lactulose. Upper gastrointestinal endoscopy showed no varices, but did reveal multiple esophageal and gastric ulcerations. He was then transferred to a liver transplant center where repeat bone marrow biopsy and a liver biopsy were done. Both revealed extensive granulomatosis and the bone marrow biopsy showed evidence of hemophagocytosis (Figure 3).

The finding of hemophagocytosis in the setting of fever, hepatosplenomegaly, and pancytopenia is consistent with a diagnosis of hemophagocytic lymphohistiocytosis (HLH). The cornerstone of therapy for patients with HLH is suppression of the severe inflammatory response with corticosteroids, etoposide and cyclosporin. Patients who respond to this are candidates for allogeneic stem cell transplant with curative intent. This patient's hepatic dysfunction precludes the use of etoposide and initial therapy should therefore include dexamethasone and cyclosporin.
All bacterial, fungal, and mycobacterial cultures again demonstrated no growth. Broad spectrum antibiotics were continued, and empiric intravenous amphotericin B was added. He became hemodynamically unstable, was intubated, put on mechanical ventilation and required vasoactive medications to maintain his blood pressure. An empiric course of pulse corticosteroids was given for the possibility of sarcoidosis. His blood pressure stabilized, though he continued to require vasopressors.
While HLH has been very rarely reported in association with sarcoidosis, the underlying pathogenesis of his clinical presentation (infectious, neoplastic, or inflammatory) has not yet been confirmed. In the meantime, I would continue with supportive care and intravenous corticosteroids.
Immunohistochemical studies of the liver biopsy returned showing CD15/30+ cells with weak‐to‐negative CD45 expression cells typical of Hodgkin lymphoma (HL) (Figure 4). He was started on chemotherapy, but over 48 hours became progressively more hypotensive. The patient died of Klebsiella and Pseudomonas sepsis on the 7th hospital day. Post‐mortem immunohistochemical examination revealed evidence of Hodgkin disease in the axillary lymph nodes, bone marrow and liver. The bone marrow showed evidence of hemophagocytosis and was also positive for Epstein‐Barr encoded RNA (EBER). Serologic studies were subsequently available and revealed positive EBV IgM against the viral capsid antigen (VCA) as well as EBV IgG VCA, which in conjunction with the marrow findings, was highly suggestive of reactivation EBV disease.

Discussion
This patient's diagnostic course led both the clinical team and discussant down a winding path, which ultimately ended in the finding of Hodgkin lymphoma, a relatively common diagnosis that had been clouded by seemingly contradictory clinical and laboratory data. The provisional diagnosis of disseminated histoplasmosis was reasonable given that H. capsulatum is endemic in Ontario and that the patient's occupation placed him at risk of infection. Given the acuity of his illness, empiric antifungal therapy based on the report of fungal elements on bone marrow examination seemed reasonable. However, Histoplasma urinary antigen testing has been shown in the literature to be 98% sensitive in immunosuppressed populations, and the negative result prompted a re‐examination of the marrow specimen. The previously described fungal elements were felt to be most likely artifact, and the underlying diagnosis was reconsidered.1 This is when the repeat bone marrow examination pointed towards the diagnosis of HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a severe, systemic hyperinflammatory disorder characterized by histiocytic proliferation that may be primary or can be triggered by infection, connective tissue diseases or malignancy.25 The central pathogenesis involves dysregulated Th1 cytokine secretion. This results in an uncontrolled accumulation of activated T‐lymphocytes and histiocytes in various organs including the liver, spleen and bone marrow. The infiltration of histiocytes into major organs can lead to disruption of function and multiorgan failure.6 Viruses are the most common infectious triggers of HLH, particularly EBV, and lymphoma is the most common associated malignancy.25 It is hypothesized that EBV can interfere with normal lymphocyte signaling pathways leading to the aforementioned over‐expression of Th1 cytokines, which can then trigger HLH.7 The diagnosis of HLH is based on a combination of clinical and laboratory parameters as outlined in Table 1.8 Our patient met all five of the major criteria.
| |
| Major criteria | 1. Fever |
| 2. Splenomegaly | |
| 3. Cytopenia in two or more cell lines | |
| 4. Hypertriglyceridemia or hypofibrinogenemia | |
| 5. Hemophagocytosis on histopathologic examination | |
| Alternative criteria | A. Low or absent natural killer cell activity |
| B. Serum ferritin level >500 ug/L | |
| C. Soluble CD‐25 level >2400 U/mL | |
The recommended treatment of HLH involves the administration of the HLH‐94 protocol consisting of corticosteroids, cyclosporine and etoposide.9, 10 Those who survive this initial phase are recommended for hematopoetic stem cell transplantation producing an overall 3‐year survival rate of 64%. However, those who do not receive early etoposide therapy fare much worse, with a mortality rate of 92%.10 This patient was not able to receive etoposide because of his decompensated liver disease.
In this case, the development of Hodgkin lymphoma involving the bone marrow and liver may have resulted in a state of immune suppression. The loss of immune function likely allowed the reactivation of Epstein‐Barr virus which triggered HLH and his fulminant presentation.35,9 Indeed, both the liver and bone marrow samples showed evidence of EBV reactivation as evidenced by the presence of EBER. The diagnosis of Hodgkin lymphoma was made from a liver biopsy specimen rather than bone marrow examination. The diagnosis of Hodgkin lymphoma is based on the presence of Reed‐Sternberg cells surrounded by an inflammatory milieu of cells including variable numbers of small lymphocytes, neutrophils eosinophils and fibroblasts. The HLH‐induced pancytopenia depleted the aforementioned inflammatory milieu in the bone marrow, which obscured the diagnosis of Hodgkin lymphoma. Unfortunately, lymphoma‐associated HLH has a very poor prognosis with a mortality rate of up to 60%.4 At the outset of this case, the reported fungal elements proved to be a source of confusion which delayed the diagnosis of Hodgkin lymphoma. Given the poor prognosis of lymphoma‐associated HLH, it is unlikely this would have had any effect on the ultimate outcome.
Teaching Points
-
HLH is a rare and complex hyperinflammatory disorder which may present as pancytopenia.
-
Triggers of HLH can include infections (particularly EBV), malignancy (particularly lymphoma) and connective tissue diseases.
-
The diagnosis of HLH is based on clinical and laboratory criteria, including cytopenias that may make the evaluation for triggering conditions such as HL more difficult.
-
Lymphoma should be included in the differential diagnosis of granulomatous inflammation.
Acknowledgements
The authors acknowledge Ralph Meyer, MD (Queen's University, Kingston, Ontario) for his comments on a draft of this paper and Drs. David Barth and Maha Guindi (Department of Laboratory Medicine, University Health Network) for their reviews of the pathology specimens.
A 23‐year‐old man presented to his family physician's office with a 2‐week history of fever, chills, night sweats, anorexia, and fatigue. This was associated with a 4‐month history of a nonproductive cough and a 20‐pound involuntary weight loss. He denied shortness of breath, chest pain, headaches, abdominal pain, vomiting, diarrhea, dysuria, and rash. There was no recent travel, sick contacts, or animal exposures.
This patient's symptoms could represent an underlying infectious, neoplastic, or inflammatory process. I would ascertain any relevant personal or family history and explore whether the patient has risk factors for human immunodeficiency virus (HIV) infection or tuberculosis (TB). On physical examination, I would listen for a heart murmur and look for lymphadenopathy, hepatosplenomegaly, and arthritis. Investigations including cultures, urinalysis, and a chest radiograph would be indicated at this time.
During the 2 weeks after his initial presentation, he experienced persistent fever, and further weight loss. He was admitted to the hospital to determine the etiology of his symptoms. The patient had no previous medical problems. On initial examination, his temperature was 102 degrees Fahrenheit, blood pressure was 100/65 mmHg, heart rate was 105 per minute, respiratory rate was 22 breaths per minute and oxygen saturation was normal on ambient air. He appeared cachectic. He was oriented to person, place, and time. Head and neck examination revealed no intraoral pathology, lymphadenopathy or scleral icterus, but did reveal conjunctival pallor. The chest was clear to auscultation, and the cardiovascular examination revealed a normal apical impulse and heart sounds with no murmurs. There was peripheral edema to the level of the mid‐shins bilaterally. The abdomen was soft and non‐tender with no appreciable hepatosplenomegaly. There were no stigmata of chronic liver disease. There was no axillary or inguinal lymphadenopathy. The remainder of the examination was normal. A complete blood count showed a hemoglobin concentration of 5.2 g/dL with a mean corpuscular volume (MCV) of 89fL, white blood cells were 1,400 cells/mm3 with an absolute neutrophil count (ANC) of 800 cells/mm3 and a platelet count of 90,000 cells/mm3 The serum sodium was 124 mmol/L, potassium 3.0 mmol/L, chloride 91 mmol/L, bicarbonate 26 mmol/L, and the creatinine 1.36 mg/dL His liver enzyme profile showed aspartate aminotransferase (AST) 68 U/L (normal 35), alanine aminotransferase (ALT) 25 U/L (normal 40), alkaline phosphatase (ALP) 210 U/L (normal 110) and a total bilirubin of 1.64 mg/dL.
The patient is clearly very unwell and requires admission to the hospital for treatment and further investigation. Emergent management includes administration of intravenous fluids to correct his electrolyte abnormalities, empiric broad spectrum antibiotics (given his relative neutropenia and fever), and a transfusion for his profound anemia. I would be very concerned that he has an underlying malignancy such as lymphoma or leukemia. Pancytopenia related to decreased cell production may be secondary to infiltration (malignant or granulomatous), infection (HIV, TB, fungal, viral), or aplasia (primary or drug‐related). Less likely etiologies include B12 or folate deficiency (unlikely given the normal MCV), systemic lupus erythematosus, paroxysmal nocturnal hemoglobinuria or cell sequestration due to hypersplenism. A history of recent exposure to drugs or toxins should be elicited. The patient's pulmonary symptoms may relate to the primary disorder or may represent an infection secondary to myelosuppression. I would want an immediate review of the peripheral blood smear, a hemolysis work‐up (drawn prior to transfusion including lactate dehydrogenase [LDH], haptoglobin, fractionated bilirubin, reticulocyte count and direct antiglobulin testing), antinuclear antibody (ANA), B12 and folate levels, imaging of the chest and blood cultures.
With evidence of fever and pancytopenia, acute leukemia was suspected and the patient was admitted to a hematology service. Over the next two weeks an extensive investigation including blood and urine cultures, and computed tomograms (CT) of the chest and abdomen were performed. A bone marrow aspirate and biopsy were also were done and were submitted for histopathologic examination and culture. The CT scan of the chest revealed left axillary and supraclavicular lymphadenopathy (Figure 1), and the abdominal imaging revealed splenomegaly. The blood, urine and bone marrow cultures were all negative. A peripheral blood smear showed pancytopenia with a hematologist interpretation suggesting that an intrinsic bone marrow process may be resulting in impaired cell production. The corresponding bone marrow biopsy and aspirate showed no evidence of malignancy, but there were numerous granulomata, and the periodic acid‐Schiff (PAS) and silver staining showed cells that resembled fungal elements (Figure 2).


The absence of malignant cells in the bone marrow leaves us to consider infectious and inflammatory causes of this patient's presentation. Infectious etiologies associated with bone marrow granulomata include fungal, mycobacterial, bacterial (brucellosis, typhoid and Q fever) and viral pathogens including HIV, Epstein‐Barr virus (EBV), and cytomegalovirus (CMV). Noninfectious causes include sarcoidosis, drug effects, and autoimmune conditions. The PAS and silver staining suggests this patient has a disseminated fungal infection. Disseminated Histoplasma capsulatum is the most likely organism but blastomycosis and coccidioidomycosis should be considered. HIV and occult lymphoma are considerations as is a primary immune disorder such as common variable immunodeficiency (CVID) which can present in this age group. While there is no recent travel history, it will be critical to determine where the patient currently lives and previously resided, review the medical record for prior infections and HIV risk factors, and take a thorough occupational history.
At this point, the following investigations should be undertaken: blood, sputum, and bone marrow culture; fungal and acid‐fast bacilli (AFB) stains on sputum and bone marrow; Histoplasma urine antigen; tuberculin skin test; serology for HIV and histoplasmosis; and serum protein electrophoresis with immunofixation and quantitation of immunoglobulins.
Acid fast staining of the bone marrow as well as mycobacterial and fungal cultures were negative. He lived in eastern Ontario and worked in construction. He reported helping tear down an old cabin in a wooded area, but denied any insect bites. This project coincided with the onset of his cough. He had no history of high risk sexual activity, intravenous drug use, tattoos or blood transfusions previous to his presentation. The HIV test was negative. His clinicians at this point considered a disseminated fungal infection as a cause for his symptoms and started him empirically on itraconazole He was discharged from the hospital with a plan for close outpatient followup. Within three days of discharge on the itraconazole, the patient's fever began to diminish, but did not completely resolve.
The clinical picture including cough, geography, and recent occupational exposure is entirely consistent with disseminated histoplasmosis. However, we are still lacking microbiologic confirmation of the diagnosis. Sarcoidosis and occult malignancy must still be considered. In the absence of a definitive diagnosis, I would consider bronchoscopy with bronchoalveolar lavage (BAL) and obtaining a lymph node or liver biopsy for microbiologic and pathologic examination. With the patient now receiving antifungal therapy, a diagnosis of histoplasmosis would be supported by a response to therapy, declining Histoplasma antigen levels and clinical improvement including recovery of his bone marrow.
The urine specimen was negative for Histoplasma antigen. Seven days after initiating itraconazole, he developed jaundice and confusion and was taken back to the hospital. On presentation, he was disoriented but awake. His temperature was 103.1 degrees Fahrenheit, blood pressure was 90/60 mm Hg, heart rate was 115 per minute, and oxygen saturation was normal on room air. He was obviously jaundiced, and more cachectic than previous. The neurologic examination demonstrated disorientation with no localizing findings. The chest and cardiovascular examinations were normal. His abdomen was soft and non‐tender with no evidence of hepatomegaly, but the spleen tip was palpable. There was no ascites or any other signs of portal hypertension, but his peripheral edema was worse than before and asterixis was present. The remainder of the examination was unchanged from previous. His laboratory investigations at this point showed a bilirubin of 18.5 mg/dL, AST 269 U/L, ALT 76 U/L, ALP 165 U/L, albumin 18 g/L, fibrinogen 1.53 g/L (normal 1.5‐3.5), triglycerides 2.4 mmol/L (normal 2), ferritin 59415 ug/L (normal 22‐275) an international normalized ratio (INR) of 2.65. His complete blood count still showed pancytopenia.
The patient has now developed fulminant hepatic failure. He requires volume resuscitation, drawing of repeat cultures, initiation of empiric broad spectrum antibiotics, urgent hepatology consultation and intensive care unit (ICU) support. The most common causes of acute liver failure are drug toxicity (including acetaminophen), viral hepatitis, Wilson's disease, Budd‐Chiari syndrome, cryptogenic liver disease and fatty infiltration. The critical diagnostic issue at this point is to determine if the liver failure is a secondary process (in which case drug toxicity due to itraconazole would be the most likely cause) or if this represents evolution of his primary disease with extensive hepatic involvement. Liver failure due to itraconazole has been reported and given the lack of microbiologic confirmation of a fungal infection, this agent should clearly be discontinued. Returning to our initial differential diagnosis of this man's granulomatous bone marrow infiltration and pancytopenia, etiologies which may progress to hepatic failure include viral infections (EBV or CMV) and malignancy. This patient's presentation could be an unusual manifestation of a common illness such as EBV or a rapidly progressive lymphoma. An abdominal Doppler ultrasound is required to rule out Budd‐Chiari syndrome. Given his abrupt change in clinical status, I would repeat a CT scan of his chest and abdomen to evaluate for evidence of infection, infiltration, or malignancy. Owing to the uncertainty regarding this patient's diagnosis and the rapidly progressive nature of his disease, serious consideration must be given to a transjugular liver biopsy.
Soon after admission, he developed hematemesis. He was given multiple blood transfusions, and then intravenous fluids, broad spectrum antibiotics and lactulose. Upper gastrointestinal endoscopy showed no varices, but did reveal multiple esophageal and gastric ulcerations. He was then transferred to a liver transplant center where repeat bone marrow biopsy and a liver biopsy were done. Both revealed extensive granulomatosis and the bone marrow biopsy showed evidence of hemophagocytosis (Figure 3).

The finding of hemophagocytosis in the setting of fever, hepatosplenomegaly, and pancytopenia is consistent with a diagnosis of hemophagocytic lymphohistiocytosis (HLH). The cornerstone of therapy for patients with HLH is suppression of the severe inflammatory response with corticosteroids, etoposide and cyclosporin. Patients who respond to this are candidates for allogeneic stem cell transplant with curative intent. This patient's hepatic dysfunction precludes the use of etoposide and initial therapy should therefore include dexamethasone and cyclosporin.
All bacterial, fungal, and mycobacterial cultures again demonstrated no growth. Broad spectrum antibiotics were continued, and empiric intravenous amphotericin B was added. He became hemodynamically unstable, was intubated, put on mechanical ventilation and required vasoactive medications to maintain his blood pressure. An empiric course of pulse corticosteroids was given for the possibility of sarcoidosis. His blood pressure stabilized, though he continued to require vasopressors.
While HLH has been very rarely reported in association with sarcoidosis, the underlying pathogenesis of his clinical presentation (infectious, neoplastic, or inflammatory) has not yet been confirmed. In the meantime, I would continue with supportive care and intravenous corticosteroids.
Immunohistochemical studies of the liver biopsy returned showing CD15/30+ cells with weak‐to‐negative CD45 expression cells typical of Hodgkin lymphoma (HL) (Figure 4). He was started on chemotherapy, but over 48 hours became progressively more hypotensive. The patient died of Klebsiella and Pseudomonas sepsis on the 7th hospital day. Post‐mortem immunohistochemical examination revealed evidence of Hodgkin disease in the axillary lymph nodes, bone marrow and liver. The bone marrow showed evidence of hemophagocytosis and was also positive for Epstein‐Barr encoded RNA (EBER). Serologic studies were subsequently available and revealed positive EBV IgM against the viral capsid antigen (VCA) as well as EBV IgG VCA, which in conjunction with the marrow findings, was highly suggestive of reactivation EBV disease.

Discussion
This patient's diagnostic course led both the clinical team and discussant down a winding path, which ultimately ended in the finding of Hodgkin lymphoma, a relatively common diagnosis that had been clouded by seemingly contradictory clinical and laboratory data. The provisional diagnosis of disseminated histoplasmosis was reasonable given that H. capsulatum is endemic in Ontario and that the patient's occupation placed him at risk of infection. Given the acuity of his illness, empiric antifungal therapy based on the report of fungal elements on bone marrow examination seemed reasonable. However, Histoplasma urinary antigen testing has been shown in the literature to be 98% sensitive in immunosuppressed populations, and the negative result prompted a re‐examination of the marrow specimen. The previously described fungal elements were felt to be most likely artifact, and the underlying diagnosis was reconsidered.1 This is when the repeat bone marrow examination pointed towards the diagnosis of HLH.
Hemophagocytic lymphohistiocytosis (HLH) is a severe, systemic hyperinflammatory disorder characterized by histiocytic proliferation that may be primary or can be triggered by infection, connective tissue diseases or malignancy.25 The central pathogenesis involves dysregulated Th1 cytokine secretion. This results in an uncontrolled accumulation of activated T‐lymphocytes and histiocytes in various organs including the liver, spleen and bone marrow. The infiltration of histiocytes into major organs can lead to disruption of function and multiorgan failure.6 Viruses are the most common infectious triggers of HLH, particularly EBV, and lymphoma is the most common associated malignancy.25 It is hypothesized that EBV can interfere with normal lymphocyte signaling pathways leading to the aforementioned over‐expression of Th1 cytokines, which can then trigger HLH.7 The diagnosis of HLH is based on a combination of clinical and laboratory parameters as outlined in Table 1.8 Our patient met all five of the major criteria.
| |
| Major criteria | 1. Fever |
| 2. Splenomegaly | |
| 3. Cytopenia in two or more cell lines | |
| 4. Hypertriglyceridemia or hypofibrinogenemia | |
| 5. Hemophagocytosis on histopathologic examination | |
| Alternative criteria | A. Low or absent natural killer cell activity |
| B. Serum ferritin level >500 ug/L | |
| C. Soluble CD‐25 level >2400 U/mL | |
The recommended treatment of HLH involves the administration of the HLH‐94 protocol consisting of corticosteroids, cyclosporine and etoposide.9, 10 Those who survive this initial phase are recommended for hematopoetic stem cell transplantation producing an overall 3‐year survival rate of 64%. However, those who do not receive early etoposide therapy fare much worse, with a mortality rate of 92%.10 This patient was not able to receive etoposide because of his decompensated liver disease.
In this case, the development of Hodgkin lymphoma involving the bone marrow and liver may have resulted in a state of immune suppression. The loss of immune function likely allowed the reactivation of Epstein‐Barr virus which triggered HLH and his fulminant presentation.35,9 Indeed, both the liver and bone marrow samples showed evidence of EBV reactivation as evidenced by the presence of EBER. The diagnosis of Hodgkin lymphoma was made from a liver biopsy specimen rather than bone marrow examination. The diagnosis of Hodgkin lymphoma is based on the presence of Reed‐Sternberg cells surrounded by an inflammatory milieu of cells including variable numbers of small lymphocytes, neutrophils eosinophils and fibroblasts. The HLH‐induced pancytopenia depleted the aforementioned inflammatory milieu in the bone marrow, which obscured the diagnosis of Hodgkin lymphoma. Unfortunately, lymphoma‐associated HLH has a very poor prognosis with a mortality rate of up to 60%.4 At the outset of this case, the reported fungal elements proved to be a source of confusion which delayed the diagnosis of Hodgkin lymphoma. Given the poor prognosis of lymphoma‐associated HLH, it is unlikely this would have had any effect on the ultimate outcome.
Teaching Points
-
HLH is a rare and complex hyperinflammatory disorder which may present as pancytopenia.
-
Triggers of HLH can include infections (particularly EBV), malignancy (particularly lymphoma) and connective tissue diseases.
-
The diagnosis of HLH is based on clinical and laboratory criteria, including cytopenias that may make the evaluation for triggering conditions such as HL more difficult.
-
Lymphoma should be included in the differential diagnosis of granulomatous inflammation.
Acknowledgements
The authors acknowledge Ralph Meyer, MD (Queen's University, Kingston, Ontario) for his comments on a draft of this paper and Drs. David Barth and Maha Guindi (Department of Laboratory Medicine, University Health Network) for their reviews of the pathology specimens.
- ,Antigen detection for diagnosis of the endemic mycoses.Curr Fung Infect Rep.2008;4:189–193.
- ,,, et al.Lymphoma‐associated hemophagocytic syndrome: Clinical features and treatment outcome.Ann Hematol.2007;86:493–498.
- ,,, et al.Hodgkin lymphoma‐associated hemophagocytic syndrome: A disorder strongly correlated with Epstein‐Barr virus.Clin Inf Dis.2008;47:531–534.
- ,.Hemophagocytic syndrome associated with Hodgkin lymphoma and pneumocystis jiroveci pneumonitis.Br J Hematol.2007;138:672.
- ,,, et al.Infections associated with haemophagocytic syndrome.Lancet Infect Dis.2007;12:814–822.
- ,,,.Hemophagocytic lymphohistiocytic syndrome: Unrecognized cause of multiple organ failure.Pediatr Crit Care Med.2000;1:51–54.
- ,,, et al.Epstein‐Barr virus LMP1 Inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome.Blood.2005;106:3090–3096.
- ,,.Diagnostic guidelines for hemophagocytic lymphohistiocytosis.Semin Oncol.1991;18:29–33
- ,,, et al.Treatment of Epstein‐Barr virus‐associated hemophagocytic lymphohistiocytosis (EBV‐HLH) in young adults: A report from the HLH Study Center.Med Pediatr Oncol.2003;41:103–109.
- ,,, et al.Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis.Br J Haematol.2005;129:622–630.
- ,Antigen detection for diagnosis of the endemic mycoses.Curr Fung Infect Rep.2008;4:189–193.
- ,,, et al.Lymphoma‐associated hemophagocytic syndrome: Clinical features and treatment outcome.Ann Hematol.2007;86:493–498.
- ,,, et al.Hodgkin lymphoma‐associated hemophagocytic syndrome: A disorder strongly correlated with Epstein‐Barr virus.Clin Inf Dis.2008;47:531–534.
- ,.Hemophagocytic syndrome associated with Hodgkin lymphoma and pneumocystis jiroveci pneumonitis.Br J Hematol.2007;138:672.
- ,,, et al.Infections associated with haemophagocytic syndrome.Lancet Infect Dis.2007;12:814–822.
- ,,,.Hemophagocytic lymphohistiocytic syndrome: Unrecognized cause of multiple organ failure.Pediatr Crit Care Med.2000;1:51–54.
- ,,, et al.Epstein‐Barr virus LMP1 Inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome.Blood.2005;106:3090–3096.
- ,,.Diagnostic guidelines for hemophagocytic lymphohistiocytosis.Semin Oncol.1991;18:29–33
- ,,, et al.Treatment of Epstein‐Barr virus‐associated hemophagocytic lymphohistiocytosis (EBV‐HLH) in young adults: A report from the HLH Study Center.Med Pediatr Oncol.2003;41:103–109.
- ,,, et al.Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis.Br J Haematol.2005;129:622–630.
In the Literature: HM-Related Research You Need to Know
In This Edition
Literature at a Glance
A guide to this month’s studies
- Continuous insulin infusion in non-ICU patients
- How hospitalists spend their day
- Outcomes of patients leaving against medical advice
- Prediction rule for readmission
- Effects of high- vs. low-dose PPIs for peptic ulcer
- Hospital utilization by generalists before hospitalists
- Effect of hospitalist fragmentation on length of stay
- Medication errors at admissions in older patients
Continuous Insulin Infusion Provides Effective Glycemic Control in Non-ICU Patients
Clinical question: Is continuous insulin infusion (CII) a safe and effective option in the management of hyperglycemia in non-ICU patients?
Background: Hyperglycemia has been associated with worse outcomes in hospitalized patients. Prior research has demonstrated the benefit of CII in managing hyperglycemia in the ICU setting. However, outcomes have not been evaluated in the general medical (non-ICU) setting, where hyperglycemia is often inadequately addressed.
Study design: Retrospective chart review.
Setting: Urban tertiary-care medical center.
Synopsis: Charts of 200 adult patients treated with CII in non-ICU areas were reviewed with the primary outcomes including mean daily blood glucose (BG) levels and number of hyper- and hypoglycemic events occurring on CII. Mean BG dropped from 323 mg/dL to 182 mg/dL by day one, with a BG≤of 150 achieved in 67% of patients by day two of therapy. Twenty-two percent of patients suffered a hypoglycemic event (BG<60), reportedly similar to prior studies of insulin use in ICU and non-ICU settings. Eighty-two percent of patients received some form of nutritional support while on CII. In multivariate analyses, receiving oral nutrition (either a solid or liquid diet) was the only factor associated with increased risk of hyperglycemia and hypoglycemia.
This study was limited by its retrospective analysis in a single center. No comparison was made with basal-bolus or sliding-scale insulin therapy regarding efficacy or safety.
Bottom line: Non-ICU patients with hyperglycemia who received CII were able to achieve effective glycemic control within 48 hours of initiation, with rates of hypoglycemia comparable to those observed in ICU settings.
Citation: Smiley D, Rhee M, Peng L, et al. Safety and efficacy of continuous insulin infusion in noncritical care settings. J Hosp Med. 2010;5(4):212-217.
Hospitalists Spend More Time on Indirect, Rather Than Direct, Patient Care
Clinical question: What are the components of the daily workflow of hospitalists working on a non-housestaff service?
Background: The use of hospitalists is associated with increased efficiency in the hospital setting. However, it is not known how this efficiency is achieved. Prior literature has attempted to address this question, but with increasing demands and patient census, the representativeness of existing data is unclear.
Study design: Observational time-motion study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Twenty-four hospitalists were directly observed for two weekday shifts. An electronic collection tool was developed using initial data on hospitalist activities and piloted prior to formal study data collection. Direct patient care was defined as involving face-to-face interaction between hospitalist and patient, while indirect patient care involved activities relevant to patient care but not performed in the patient’s presence.
Approximately 500 hours of observation were collected. Direct patient care comprised only a mean of 17.4% of the hospitalists’ daily workflow, while more was spent on indirect care, mainly electronic health record (EHR) documentation (mean 34.1%) and communication activities (mean 25.9%). Multitasking occurred 16% of the time, typically during communication or “critical documentation activities” (e.g. writing prescriptions). As patient volume increased, less time was spent in communication, and documentation was deferred to after hours.
These results were consistent with prior observational studies but were limited to a single center and might not represent the workflow of hospitalists in other settings, such as community hospitals, or nocturnists.
Bottom line: Hospitalists on non-housestaff services spend most of their time on indirect patient care and, with increasing patient census, communication is sacrificed. Multitasking is common during periods of communication and critical documentation.
Citation: Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?—a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328.
Patients Who Leave Against Medical Advice Have Higher Readmission, Mortality Rates
Clinical question: What are the 30-day hospital readmission and mortality rates for Veterans Administration (VA) patients discharged against medical advice (AMA) compared with those appropriately discharged from the hospital?
Background: Patients discharged AMA might be at increased risk of experiencing worse outcomes. Small studies have demonstrated that patients with asthma and acute myocardial infarction (MI) discharged AMA have increased risk of readmission and death. However, it is unclear whether these risks are generalizable to a wider medical population.
Study design: Five-year retrospective cohort study.
Setting: One hundred twenty-nine VA acute-care hospitals.
Synopsis: Of the nearly 2 million patients admitted to the VA from 2004 to 2008, 1.7% were discharged AMA. Patients discharged AMA generally were younger, had lower incomes, and were more likely to be black. Furthermore, patients discharged AMA had statistically significant higher rates of 30-day readmission (17.7% vs. 11%, P<0.001) and higher 30-day mortality rates (0.75% vs. 0.61%, P=0.001) compared with those who had been appropriately discharged. In hazard models, discharge AMA was a significant predictor of 30-day readmission and conferred a nonstatistically significant increase in 30-day mortality.
Because all patients were seen in VA facilities, the results might not be generalizable to other acute-care settings. Although VA patients differ from the general medical population, the characteristics of patients discharged AMA are similar to those in previously published studies. The study utilized administrative data, which are very reliable but limited by little information on clinical factors that could contribute to AMA discharges.
Bottom line: Patients discharged AMA are at increased risk of worse post-hospitalization outcomes, including hospital readmission and death.
Citation: Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med. 2010;25(9): 926-929.
Simple Model Predicts Hospital Readmission
Clinical question: Which patient-level factors can be used in a simple model to predict hospital readmission of medicine patients?
Background: Hospital readmissions are common and costly. Previously published readmission prediction models have had limited utility because they focused on a specific condition, setting, or population, or were too cumbersome for practical use.
Study design: Prospective observational cohort study.
Setting: Six academic medical centers.
Synopsis: Data from nearly 11,000 general medicine patients were included in the analysis. Overall, almost 18% of patients were readmitted within 30 days of discharge.
In the prediction model derived and validated from the data, seven factors were significant predictors of readmission within 30 days of discharge: insurance status, marital status, having a regular healthcare provider, Charlson comorbidity index, SF 12 physical component score, one or more admissions within the last year, and current length of stay greater than two days. Points assigned from each significant predictor were used to create a risk score. The 5% of patients with risk scores of 25 and higher had 30-day readmission rates of approximately 30%, compared to readmission rates of approximately 16% in patients with scores of less than 25.
These results might not be generalizable to small, rural, nonacademic settings. Planned admissions could not be excluded from the data, and readmissions to nonstudy hospitals could not be ascertained. Despite these limitations, this model is easier to use than prior models and relevant to a broad population of patients.
Bottom line: A simple prediction model using patient-level factors can be used to identify patients at higher risk of readmission within 30 days of discharge to home.
Citation: Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219.
No Difference in Outcomes Between High- and Non-High-Dose Proton Pump Inhibitors in Bleeding Peptic Ulcers
Clinical question: Do high-dose proton pump inhibitors (PPIs) decrease the rate of rebleeding, surgical intervention, or mortality in patients with bleeding peptic ulcers who have undergone endoscopic treatment?
Background: Previous studies have demonstrated superiority of both high- and low-dose PPIs to H2 receptor antagonists and placebo in reducing rebleeding rates in patients with peptic ulcers. However, no clear evidence is available to suggest that high-dose PPIs are more effective than non-high-dose PPIs for treatment of bleeding peptic ulcers.
Study design: Systematic review and meta-analysis.
Setting: Multicenter and single-site studies conducted in several countries.
Synopsis: Studies were included if they were randomized controlled trials, compared high- versus non-high-dose PPIs, evaluated endoscopically confirmed bleeding ulcers, gave PPIs after endoscopy, and documented outcomes regarding rates of rebleeding, surgical intervention, or mortality. High-dose PPIs were defined as equivalent to omeprazole 80 mg intravenous bolus followed by continuous intravenous infusion at 8 mg/hr for 72 hours.
Seven studies met inclusion criteria. The pooled odds ratios for rebleeding, surgical intervention, and mortality were 1.30 (95% CI, 0.88-1.91), 1.49 (95% CI, 0.66-3.37), and 0.89 (95% CI, 0.37-2.13), respectively, for high-dose versus non-high-dose PPIs. The authors concluded that high-dose PPIs were not superior to non-high-dose PPIs in reducing the rates of these adverse outcomes after endoscopic treatment of bleeding ulcers. Considering the cost of high-dose PPIs, further studies are indicated to help guide PPI dosing for patients with peptic ulcers.
Major limitations of this study were the small number of studies (1,157 patients in total) and their heterogeneity, and the lack of intention-to-treat analysis. The studies also included a high Asian predominance, and it has been shown that Asian populations have an enhanced PPI effect.
Bottom line: High-dose PPIs did not demonstrate reduced rates of ulcer rebleeding, surgical intervention, or mortality compared with non-high-dose PPIs in this meta-analysis, which included a small number of studies and patients.
Citation: Wang CH, Ma MH, Chou HC, et al. High-dose vs. non-high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(9):751-758.
Hospital Utilization by Practicing Generalists Declined before the Emergence of Hospitalists
Clinical question: What has been the pattern of hospital utilization by generalists before and after the emergence of hospitalists?
Background: It has been proposed that the emergence of hospitalists has “crowded out” generalist physicians from the U.S. hospital setting. This study evaluated the trends of inpatient practice by generalists both before and after the emergence of hospitalists.
Study design: Retrospective analysis of national databases.
Setting: U.S. data from 1980 to 2005.
Synopsis: Utilizing the National Hospital Discharge Survey and the American Medical Association’s Physician Characteristics and Distribution in the U.S., information was extracted to evaluate the average number of annual inpatient encounters relative to generalist workforce from 1980 to 2005. Total inpatient encounters for each year were calculated by multiplying the total number of hospital admissions by the average hospital length of stay. The emergence of hospitalists was defined as beginning in 1994.
Total inpatient encounters by generalists declined by 35% in the pre-hospitalist era but remained essentially unchanged in the hospitalist era. During the pre-hospitalist period, the number of generalists doubled, to more than 200,000 from approximately 100,000, while the number of hospital discharges remained relatively stable and the length of stay declined by a third. The decrease in average inpatient encounters in the pre-hospitalist era is thought to have been secondary to decreased length of stay and increased workforce.
Bottom line: Hospital utilization relative to generalist physician workforce was decreasing prior to the emergence of hospitalists mainly due to decreased length of hospital stay and increased numbers of physicians.
Citation: Meltzer DO, Chung JW. U.S. trends in hospitalization and generalist physician workforce and the emergence of hospitalists. J Gen Intern Med. 2010;25(5):453-459.
Fragmentation of Hospitalist Care Is Associated with Increased Length of Stay
Clinical question: Does fragmentation of care (FOC) by hospitalists affect length of stay (LOS)?
Background: Previous investigations have explored the impact of FOC provided by residency programs on LOS and quality of care. Results of these studies have been mixed. However, there have been no prior studies on the impact of the fragmentation of hospitalist care on LOS.
Study design: Concurrent control study.
Setting: Hospitalist practices all over the country managed by IPC: The Hospitalist Company.
Synopsis: Investigators looked at 10,977 patients admitted with diagnoses of pneumonia or heart failure. The primary endpoint was LOS. The independent variable of interest was a measure of FOC. The FOC was calculated as a quantitative index, by determining the percentage of hospitalist care delivered by a physician other than the primary hospitalist.
Multivariable analyses revealed a statistically significant increase in LOS of 0.39 days for each 10% increase in fragmentation level for pneumonia admissions. Similarly, for patients with heart failure, there was a significant increase in LOS of 0.30 days for each 10% increase in fragmentation level.
The study is a concurrent control study, so conclusions cannot be drawn about causality. Additionally, there are likely unmeasured differences between every hospital and hospitalist practice, which could further confound the relationships between hospitalist care and LOS.
Bottom line: Fragmentation of care provided by hospitalists is associated with an increased LOS in patients hospitalized for pneumonia or heart failure.
Citation: Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338.
Admission Medication Errors Are Common and Most Harmful in Older Patients Taking Many Medications
Clinical question: What are the risk factors and potential harm associated with medication errors at hospital admission?
Background: Obtaining a medication history from a hospitalized patient is an error-prone process. Several variables can affect the completeness and quality of medication histories, but existing data are limited regarding patient or medication risk factors associated with medication errors at admission.
Study design: Prospective cohort study.
Setting: Academic hospital in Chicago.
Synopsis: Pharmacist and admitting physician medication histories were compared with admission medication orders to identify any unexplained discrepancies. Discrepancies resulting in order changes were defined as medication errors.
Of the 651 adult medical inpatients studied, 234 (35.9%) had medication order errors identified at admission. Errors originated in the medication histories for 85% of these patients. The most frequent type of error was an omission (48.9%). An age of 65 or older (odds ratio [OR]=2.17, 95% confidence interval [CI], 1.09-4.30) and increased number of medications (OR=1.21, 95% CI, 1.14-1.29) were the only risk factors identified by multivariate analysis to be independently associated with increased risk of medication order errors potentially causing harm or requiring monitoring or intervention. Presenting a medication list upon admission was a significant protective factor (OR=0.35, 95% CI, 0.19-0.63).
Though this is the largest study to date evaluating admission medication errors in hospitalized medical patients, it remains limited by its single hospital site. Because the authors were unable to interview patients who were too ill or unwilling to participate and had no caregiver present, they might have underestimated the number of admission errors. Further, the harm assessment was based on potential and not actual harm.
Bottom line: Admission medication order errors are frequent, most commonly originate in the medication histories, and have increased potential to cause adverse outcomes in older patients and those taking higher numbers of medications.
Citation: Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications At Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
In This Edition
Literature at a Glance
A guide to this month’s studies
- Continuous insulin infusion in non-ICU patients
- How hospitalists spend their day
- Outcomes of patients leaving against medical advice
- Prediction rule for readmission
- Effects of high- vs. low-dose PPIs for peptic ulcer
- Hospital utilization by generalists before hospitalists
- Effect of hospitalist fragmentation on length of stay
- Medication errors at admissions in older patients
Continuous Insulin Infusion Provides Effective Glycemic Control in Non-ICU Patients
Clinical question: Is continuous insulin infusion (CII) a safe and effective option in the management of hyperglycemia in non-ICU patients?
Background: Hyperglycemia has been associated with worse outcomes in hospitalized patients. Prior research has demonstrated the benefit of CII in managing hyperglycemia in the ICU setting. However, outcomes have not been evaluated in the general medical (non-ICU) setting, where hyperglycemia is often inadequately addressed.
Study design: Retrospective chart review.
Setting: Urban tertiary-care medical center.
Synopsis: Charts of 200 adult patients treated with CII in non-ICU areas were reviewed with the primary outcomes including mean daily blood glucose (BG) levels and number of hyper- and hypoglycemic events occurring on CII. Mean BG dropped from 323 mg/dL to 182 mg/dL by day one, with a BG≤of 150 achieved in 67% of patients by day two of therapy. Twenty-two percent of patients suffered a hypoglycemic event (BG<60), reportedly similar to prior studies of insulin use in ICU and non-ICU settings. Eighty-two percent of patients received some form of nutritional support while on CII. In multivariate analyses, receiving oral nutrition (either a solid or liquid diet) was the only factor associated with increased risk of hyperglycemia and hypoglycemia.
This study was limited by its retrospective analysis in a single center. No comparison was made with basal-bolus or sliding-scale insulin therapy regarding efficacy or safety.
Bottom line: Non-ICU patients with hyperglycemia who received CII were able to achieve effective glycemic control within 48 hours of initiation, with rates of hypoglycemia comparable to those observed in ICU settings.
Citation: Smiley D, Rhee M, Peng L, et al. Safety and efficacy of continuous insulin infusion in noncritical care settings. J Hosp Med. 2010;5(4):212-217.
Hospitalists Spend More Time on Indirect, Rather Than Direct, Patient Care
Clinical question: What are the components of the daily workflow of hospitalists working on a non-housestaff service?
Background: The use of hospitalists is associated with increased efficiency in the hospital setting. However, it is not known how this efficiency is achieved. Prior literature has attempted to address this question, but with increasing demands and patient census, the representativeness of existing data is unclear.
Study design: Observational time-motion study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Twenty-four hospitalists were directly observed for two weekday shifts. An electronic collection tool was developed using initial data on hospitalist activities and piloted prior to formal study data collection. Direct patient care was defined as involving face-to-face interaction between hospitalist and patient, while indirect patient care involved activities relevant to patient care but not performed in the patient’s presence.
Approximately 500 hours of observation were collected. Direct patient care comprised only a mean of 17.4% of the hospitalists’ daily workflow, while more was spent on indirect care, mainly electronic health record (EHR) documentation (mean 34.1%) and communication activities (mean 25.9%). Multitasking occurred 16% of the time, typically during communication or “critical documentation activities” (e.g. writing prescriptions). As patient volume increased, less time was spent in communication, and documentation was deferred to after hours.
These results were consistent with prior observational studies but were limited to a single center and might not represent the workflow of hospitalists in other settings, such as community hospitals, or nocturnists.
Bottom line: Hospitalists on non-housestaff services spend most of their time on indirect patient care and, with increasing patient census, communication is sacrificed. Multitasking is common during periods of communication and critical documentation.
Citation: Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?—a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328.
Patients Who Leave Against Medical Advice Have Higher Readmission, Mortality Rates
Clinical question: What are the 30-day hospital readmission and mortality rates for Veterans Administration (VA) patients discharged against medical advice (AMA) compared with those appropriately discharged from the hospital?
Background: Patients discharged AMA might be at increased risk of experiencing worse outcomes. Small studies have demonstrated that patients with asthma and acute myocardial infarction (MI) discharged AMA have increased risk of readmission and death. However, it is unclear whether these risks are generalizable to a wider medical population.
Study design: Five-year retrospective cohort study.
Setting: One hundred twenty-nine VA acute-care hospitals.
Synopsis: Of the nearly 2 million patients admitted to the VA from 2004 to 2008, 1.7% were discharged AMA. Patients discharged AMA generally were younger, had lower incomes, and were more likely to be black. Furthermore, patients discharged AMA had statistically significant higher rates of 30-day readmission (17.7% vs. 11%, P<0.001) and higher 30-day mortality rates (0.75% vs. 0.61%, P=0.001) compared with those who had been appropriately discharged. In hazard models, discharge AMA was a significant predictor of 30-day readmission and conferred a nonstatistically significant increase in 30-day mortality.
Because all patients were seen in VA facilities, the results might not be generalizable to other acute-care settings. Although VA patients differ from the general medical population, the characteristics of patients discharged AMA are similar to those in previously published studies. The study utilized administrative data, which are very reliable but limited by little information on clinical factors that could contribute to AMA discharges.
Bottom line: Patients discharged AMA are at increased risk of worse post-hospitalization outcomes, including hospital readmission and death.
Citation: Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med. 2010;25(9): 926-929.
Simple Model Predicts Hospital Readmission
Clinical question: Which patient-level factors can be used in a simple model to predict hospital readmission of medicine patients?
Background: Hospital readmissions are common and costly. Previously published readmission prediction models have had limited utility because they focused on a specific condition, setting, or population, or were too cumbersome for practical use.
Study design: Prospective observational cohort study.
Setting: Six academic medical centers.
Synopsis: Data from nearly 11,000 general medicine patients were included in the analysis. Overall, almost 18% of patients were readmitted within 30 days of discharge.
In the prediction model derived and validated from the data, seven factors were significant predictors of readmission within 30 days of discharge: insurance status, marital status, having a regular healthcare provider, Charlson comorbidity index, SF 12 physical component score, one or more admissions within the last year, and current length of stay greater than two days. Points assigned from each significant predictor were used to create a risk score. The 5% of patients with risk scores of 25 and higher had 30-day readmission rates of approximately 30%, compared to readmission rates of approximately 16% in patients with scores of less than 25.
These results might not be generalizable to small, rural, nonacademic settings. Planned admissions could not be excluded from the data, and readmissions to nonstudy hospitals could not be ascertained. Despite these limitations, this model is easier to use than prior models and relevant to a broad population of patients.
Bottom line: A simple prediction model using patient-level factors can be used to identify patients at higher risk of readmission within 30 days of discharge to home.
Citation: Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219.
No Difference in Outcomes Between High- and Non-High-Dose Proton Pump Inhibitors in Bleeding Peptic Ulcers
Clinical question: Do high-dose proton pump inhibitors (PPIs) decrease the rate of rebleeding, surgical intervention, or mortality in patients with bleeding peptic ulcers who have undergone endoscopic treatment?
Background: Previous studies have demonstrated superiority of both high- and low-dose PPIs to H2 receptor antagonists and placebo in reducing rebleeding rates in patients with peptic ulcers. However, no clear evidence is available to suggest that high-dose PPIs are more effective than non-high-dose PPIs for treatment of bleeding peptic ulcers.
Study design: Systematic review and meta-analysis.
Setting: Multicenter and single-site studies conducted in several countries.
Synopsis: Studies were included if they were randomized controlled trials, compared high- versus non-high-dose PPIs, evaluated endoscopically confirmed bleeding ulcers, gave PPIs after endoscopy, and documented outcomes regarding rates of rebleeding, surgical intervention, or mortality. High-dose PPIs were defined as equivalent to omeprazole 80 mg intravenous bolus followed by continuous intravenous infusion at 8 mg/hr for 72 hours.
Seven studies met inclusion criteria. The pooled odds ratios for rebleeding, surgical intervention, and mortality were 1.30 (95% CI, 0.88-1.91), 1.49 (95% CI, 0.66-3.37), and 0.89 (95% CI, 0.37-2.13), respectively, for high-dose versus non-high-dose PPIs. The authors concluded that high-dose PPIs were not superior to non-high-dose PPIs in reducing the rates of these adverse outcomes after endoscopic treatment of bleeding ulcers. Considering the cost of high-dose PPIs, further studies are indicated to help guide PPI dosing for patients with peptic ulcers.
Major limitations of this study were the small number of studies (1,157 patients in total) and their heterogeneity, and the lack of intention-to-treat analysis. The studies also included a high Asian predominance, and it has been shown that Asian populations have an enhanced PPI effect.
Bottom line: High-dose PPIs did not demonstrate reduced rates of ulcer rebleeding, surgical intervention, or mortality compared with non-high-dose PPIs in this meta-analysis, which included a small number of studies and patients.
Citation: Wang CH, Ma MH, Chou HC, et al. High-dose vs. non-high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(9):751-758.
Hospital Utilization by Practicing Generalists Declined before the Emergence of Hospitalists
Clinical question: What has been the pattern of hospital utilization by generalists before and after the emergence of hospitalists?
Background: It has been proposed that the emergence of hospitalists has “crowded out” generalist physicians from the U.S. hospital setting. This study evaluated the trends of inpatient practice by generalists both before and after the emergence of hospitalists.
Study design: Retrospective analysis of national databases.
Setting: U.S. data from 1980 to 2005.
Synopsis: Utilizing the National Hospital Discharge Survey and the American Medical Association’s Physician Characteristics and Distribution in the U.S., information was extracted to evaluate the average number of annual inpatient encounters relative to generalist workforce from 1980 to 2005. Total inpatient encounters for each year were calculated by multiplying the total number of hospital admissions by the average hospital length of stay. The emergence of hospitalists was defined as beginning in 1994.
Total inpatient encounters by generalists declined by 35% in the pre-hospitalist era but remained essentially unchanged in the hospitalist era. During the pre-hospitalist period, the number of generalists doubled, to more than 200,000 from approximately 100,000, while the number of hospital discharges remained relatively stable and the length of stay declined by a third. The decrease in average inpatient encounters in the pre-hospitalist era is thought to have been secondary to decreased length of stay and increased workforce.
Bottom line: Hospital utilization relative to generalist physician workforce was decreasing prior to the emergence of hospitalists mainly due to decreased length of hospital stay and increased numbers of physicians.
Citation: Meltzer DO, Chung JW. U.S. trends in hospitalization and generalist physician workforce and the emergence of hospitalists. J Gen Intern Med. 2010;25(5):453-459.
Fragmentation of Hospitalist Care Is Associated with Increased Length of Stay
Clinical question: Does fragmentation of care (FOC) by hospitalists affect length of stay (LOS)?
Background: Previous investigations have explored the impact of FOC provided by residency programs on LOS and quality of care. Results of these studies have been mixed. However, there have been no prior studies on the impact of the fragmentation of hospitalist care on LOS.
Study design: Concurrent control study.
Setting: Hospitalist practices all over the country managed by IPC: The Hospitalist Company.
Synopsis: Investigators looked at 10,977 patients admitted with diagnoses of pneumonia or heart failure. The primary endpoint was LOS. The independent variable of interest was a measure of FOC. The FOC was calculated as a quantitative index, by determining the percentage of hospitalist care delivered by a physician other than the primary hospitalist.
Multivariable analyses revealed a statistically significant increase in LOS of 0.39 days for each 10% increase in fragmentation level for pneumonia admissions. Similarly, for patients with heart failure, there was a significant increase in LOS of 0.30 days for each 10% increase in fragmentation level.
The study is a concurrent control study, so conclusions cannot be drawn about causality. Additionally, there are likely unmeasured differences between every hospital and hospitalist practice, which could further confound the relationships between hospitalist care and LOS.
Bottom line: Fragmentation of care provided by hospitalists is associated with an increased LOS in patients hospitalized for pneumonia or heart failure.
Citation: Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338.
Admission Medication Errors Are Common and Most Harmful in Older Patients Taking Many Medications
Clinical question: What are the risk factors and potential harm associated with medication errors at hospital admission?
Background: Obtaining a medication history from a hospitalized patient is an error-prone process. Several variables can affect the completeness and quality of medication histories, but existing data are limited regarding patient or medication risk factors associated with medication errors at admission.
Study design: Prospective cohort study.
Setting: Academic hospital in Chicago.
Synopsis: Pharmacist and admitting physician medication histories were compared with admission medication orders to identify any unexplained discrepancies. Discrepancies resulting in order changes were defined as medication errors.
Of the 651 adult medical inpatients studied, 234 (35.9%) had medication order errors identified at admission. Errors originated in the medication histories for 85% of these patients. The most frequent type of error was an omission (48.9%). An age of 65 or older (odds ratio [OR]=2.17, 95% confidence interval [CI], 1.09-4.30) and increased number of medications (OR=1.21, 95% CI, 1.14-1.29) were the only risk factors identified by multivariate analysis to be independently associated with increased risk of medication order errors potentially causing harm or requiring monitoring or intervention. Presenting a medication list upon admission was a significant protective factor (OR=0.35, 95% CI, 0.19-0.63).
Though this is the largest study to date evaluating admission medication errors in hospitalized medical patients, it remains limited by its single hospital site. Because the authors were unable to interview patients who were too ill or unwilling to participate and had no caregiver present, they might have underestimated the number of admission errors. Further, the harm assessment was based on potential and not actual harm.
Bottom line: Admission medication order errors are frequent, most commonly originate in the medication histories, and have increased potential to cause adverse outcomes in older patients and those taking higher numbers of medications.
Citation: Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications At Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
In This Edition
Literature at a Glance
A guide to this month’s studies
- Continuous insulin infusion in non-ICU patients
- How hospitalists spend their day
- Outcomes of patients leaving against medical advice
- Prediction rule for readmission
- Effects of high- vs. low-dose PPIs for peptic ulcer
- Hospital utilization by generalists before hospitalists
- Effect of hospitalist fragmentation on length of stay
- Medication errors at admissions in older patients
Continuous Insulin Infusion Provides Effective Glycemic Control in Non-ICU Patients
Clinical question: Is continuous insulin infusion (CII) a safe and effective option in the management of hyperglycemia in non-ICU patients?
Background: Hyperglycemia has been associated with worse outcomes in hospitalized patients. Prior research has demonstrated the benefit of CII in managing hyperglycemia in the ICU setting. However, outcomes have not been evaluated in the general medical (non-ICU) setting, where hyperglycemia is often inadequately addressed.
Study design: Retrospective chart review.
Setting: Urban tertiary-care medical center.
Synopsis: Charts of 200 adult patients treated with CII in non-ICU areas were reviewed with the primary outcomes including mean daily blood glucose (BG) levels and number of hyper- and hypoglycemic events occurring on CII. Mean BG dropped from 323 mg/dL to 182 mg/dL by day one, with a BG≤of 150 achieved in 67% of patients by day two of therapy. Twenty-two percent of patients suffered a hypoglycemic event (BG<60), reportedly similar to prior studies of insulin use in ICU and non-ICU settings. Eighty-two percent of patients received some form of nutritional support while on CII. In multivariate analyses, receiving oral nutrition (either a solid or liquid diet) was the only factor associated with increased risk of hyperglycemia and hypoglycemia.
This study was limited by its retrospective analysis in a single center. No comparison was made with basal-bolus or sliding-scale insulin therapy regarding efficacy or safety.
Bottom line: Non-ICU patients with hyperglycemia who received CII were able to achieve effective glycemic control within 48 hours of initiation, with rates of hypoglycemia comparable to those observed in ICU settings.
Citation: Smiley D, Rhee M, Peng L, et al. Safety and efficacy of continuous insulin infusion in noncritical care settings. J Hosp Med. 2010;5(4):212-217.
Hospitalists Spend More Time on Indirect, Rather Than Direct, Patient Care
Clinical question: What are the components of the daily workflow of hospitalists working on a non-housestaff service?
Background: The use of hospitalists is associated with increased efficiency in the hospital setting. However, it is not known how this efficiency is achieved. Prior literature has attempted to address this question, but with increasing demands and patient census, the representativeness of existing data is unclear.
Study design: Observational time-motion study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Twenty-four hospitalists were directly observed for two weekday shifts. An electronic collection tool was developed using initial data on hospitalist activities and piloted prior to formal study data collection. Direct patient care was defined as involving face-to-face interaction between hospitalist and patient, while indirect patient care involved activities relevant to patient care but not performed in the patient’s presence.
Approximately 500 hours of observation were collected. Direct patient care comprised only a mean of 17.4% of the hospitalists’ daily workflow, while more was spent on indirect care, mainly electronic health record (EHR) documentation (mean 34.1%) and communication activities (mean 25.9%). Multitasking occurred 16% of the time, typically during communication or “critical documentation activities” (e.g. writing prescriptions). As patient volume increased, less time was spent in communication, and documentation was deferred to after hours.
These results were consistent with prior observational studies but were limited to a single center and might not represent the workflow of hospitalists in other settings, such as community hospitals, or nocturnists.
Bottom line: Hospitalists on non-housestaff services spend most of their time on indirect patient care and, with increasing patient census, communication is sacrificed. Multitasking is common during periods of communication and critical documentation.
Citation: Tipping MD, Forth VE, O’Leary KJ, et al. Where did the day go?—a time-motion study of hospitalists. J Hosp Med. 2010;5(6):323-328.
Patients Who Leave Against Medical Advice Have Higher Readmission, Mortality Rates
Clinical question: What are the 30-day hospital readmission and mortality rates for Veterans Administration (VA) patients discharged against medical advice (AMA) compared with those appropriately discharged from the hospital?
Background: Patients discharged AMA might be at increased risk of experiencing worse outcomes. Small studies have demonstrated that patients with asthma and acute myocardial infarction (MI) discharged AMA have increased risk of readmission and death. However, it is unclear whether these risks are generalizable to a wider medical population.
Study design: Five-year retrospective cohort study.
Setting: One hundred twenty-nine VA acute-care hospitals.
Synopsis: Of the nearly 2 million patients admitted to the VA from 2004 to 2008, 1.7% were discharged AMA. Patients discharged AMA generally were younger, had lower incomes, and were more likely to be black. Furthermore, patients discharged AMA had statistically significant higher rates of 30-day readmission (17.7% vs. 11%, P<0.001) and higher 30-day mortality rates (0.75% vs. 0.61%, P=0.001) compared with those who had been appropriately discharged. In hazard models, discharge AMA was a significant predictor of 30-day readmission and conferred a nonstatistically significant increase in 30-day mortality.
Because all patients were seen in VA facilities, the results might not be generalizable to other acute-care settings. Although VA patients differ from the general medical population, the characteristics of patients discharged AMA are similar to those in previously published studies. The study utilized administrative data, which are very reliable but limited by little information on clinical factors that could contribute to AMA discharges.
Bottom line: Patients discharged AMA are at increased risk of worse post-hospitalization outcomes, including hospital readmission and death.
Citation: Glasgow JM, Vaughn-Sarrazin M, Kaboli PJ. Leaving against medical advice (AMA): risk of 30-day mortality and hospital readmission. J Gen Intern Med. 2010;25(9): 926-929.
Simple Model Predicts Hospital Readmission
Clinical question: Which patient-level factors can be used in a simple model to predict hospital readmission of medicine patients?
Background: Hospital readmissions are common and costly. Previously published readmission prediction models have had limited utility because they focused on a specific condition, setting, or population, or were too cumbersome for practical use.
Study design: Prospective observational cohort study.
Setting: Six academic medical centers.
Synopsis: Data from nearly 11,000 general medicine patients were included in the analysis. Overall, almost 18% of patients were readmitted within 30 days of discharge.
In the prediction model derived and validated from the data, seven factors were significant predictors of readmission within 30 days of discharge: insurance status, marital status, having a regular healthcare provider, Charlson comorbidity index, SF 12 physical component score, one or more admissions within the last year, and current length of stay greater than two days. Points assigned from each significant predictor were used to create a risk score. The 5% of patients with risk scores of 25 and higher had 30-day readmission rates of approximately 30%, compared to readmission rates of approximately 16% in patients with scores of less than 25.
These results might not be generalizable to small, rural, nonacademic settings. Planned admissions could not be excluded from the data, and readmissions to nonstudy hospitals could not be ascertained. Despite these limitations, this model is easier to use than prior models and relevant to a broad population of patients.
Bottom line: A simple prediction model using patient-level factors can be used to identify patients at higher risk of readmission within 30 days of discharge to home.
Citation: Hasan O, Meltzer DO, Shaykevich SA, et al. Hospital readmission in general medicine patients: a prediction model. J Gen Intern Med. 2010;25(3):211-219.
No Difference in Outcomes Between High- and Non-High-Dose Proton Pump Inhibitors in Bleeding Peptic Ulcers
Clinical question: Do high-dose proton pump inhibitors (PPIs) decrease the rate of rebleeding, surgical intervention, or mortality in patients with bleeding peptic ulcers who have undergone endoscopic treatment?
Background: Previous studies have demonstrated superiority of both high- and low-dose PPIs to H2 receptor antagonists and placebo in reducing rebleeding rates in patients with peptic ulcers. However, no clear evidence is available to suggest that high-dose PPIs are more effective than non-high-dose PPIs for treatment of bleeding peptic ulcers.
Study design: Systematic review and meta-analysis.
Setting: Multicenter and single-site studies conducted in several countries.
Synopsis: Studies were included if they were randomized controlled trials, compared high- versus non-high-dose PPIs, evaluated endoscopically confirmed bleeding ulcers, gave PPIs after endoscopy, and documented outcomes regarding rates of rebleeding, surgical intervention, or mortality. High-dose PPIs were defined as equivalent to omeprazole 80 mg intravenous bolus followed by continuous intravenous infusion at 8 mg/hr for 72 hours.
Seven studies met inclusion criteria. The pooled odds ratios for rebleeding, surgical intervention, and mortality were 1.30 (95% CI, 0.88-1.91), 1.49 (95% CI, 0.66-3.37), and 0.89 (95% CI, 0.37-2.13), respectively, for high-dose versus non-high-dose PPIs. The authors concluded that high-dose PPIs were not superior to non-high-dose PPIs in reducing the rates of these adverse outcomes after endoscopic treatment of bleeding ulcers. Considering the cost of high-dose PPIs, further studies are indicated to help guide PPI dosing for patients with peptic ulcers.
Major limitations of this study were the small number of studies (1,157 patients in total) and their heterogeneity, and the lack of intention-to-treat analysis. The studies also included a high Asian predominance, and it has been shown that Asian populations have an enhanced PPI effect.
Bottom line: High-dose PPIs did not demonstrate reduced rates of ulcer rebleeding, surgical intervention, or mortality compared with non-high-dose PPIs in this meta-analysis, which included a small number of studies and patients.
Citation: Wang CH, Ma MH, Chou HC, et al. High-dose vs. non-high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(9):751-758.
Hospital Utilization by Practicing Generalists Declined before the Emergence of Hospitalists
Clinical question: What has been the pattern of hospital utilization by generalists before and after the emergence of hospitalists?
Background: It has been proposed that the emergence of hospitalists has “crowded out” generalist physicians from the U.S. hospital setting. This study evaluated the trends of inpatient practice by generalists both before and after the emergence of hospitalists.
Study design: Retrospective analysis of national databases.
Setting: U.S. data from 1980 to 2005.
Synopsis: Utilizing the National Hospital Discharge Survey and the American Medical Association’s Physician Characteristics and Distribution in the U.S., information was extracted to evaluate the average number of annual inpatient encounters relative to generalist workforce from 1980 to 2005. Total inpatient encounters for each year were calculated by multiplying the total number of hospital admissions by the average hospital length of stay. The emergence of hospitalists was defined as beginning in 1994.
Total inpatient encounters by generalists declined by 35% in the pre-hospitalist era but remained essentially unchanged in the hospitalist era. During the pre-hospitalist period, the number of generalists doubled, to more than 200,000 from approximately 100,000, while the number of hospital discharges remained relatively stable and the length of stay declined by a third. The decrease in average inpatient encounters in the pre-hospitalist era is thought to have been secondary to decreased length of stay and increased workforce.
Bottom line: Hospital utilization relative to generalist physician workforce was decreasing prior to the emergence of hospitalists mainly due to decreased length of hospital stay and increased numbers of physicians.
Citation: Meltzer DO, Chung JW. U.S. trends in hospitalization and generalist physician workforce and the emergence of hospitalists. J Gen Intern Med. 2010;25(5):453-459.
Fragmentation of Hospitalist Care Is Associated with Increased Length of Stay
Clinical question: Does fragmentation of care (FOC) by hospitalists affect length of stay (LOS)?
Background: Previous investigations have explored the impact of FOC provided by residency programs on LOS and quality of care. Results of these studies have been mixed. However, there have been no prior studies on the impact of the fragmentation of hospitalist care on LOS.
Study design: Concurrent control study.
Setting: Hospitalist practices all over the country managed by IPC: The Hospitalist Company.
Synopsis: Investigators looked at 10,977 patients admitted with diagnoses of pneumonia or heart failure. The primary endpoint was LOS. The independent variable of interest was a measure of FOC. The FOC was calculated as a quantitative index, by determining the percentage of hospitalist care delivered by a physician other than the primary hospitalist.
Multivariable analyses revealed a statistically significant increase in LOS of 0.39 days for each 10% increase in fragmentation level for pneumonia admissions. Similarly, for patients with heart failure, there was a significant increase in LOS of 0.30 days for each 10% increase in fragmentation level.
The study is a concurrent control study, so conclusions cannot be drawn about causality. Additionally, there are likely unmeasured differences between every hospital and hospitalist practice, which could further confound the relationships between hospitalist care and LOS.
Bottom line: Fragmentation of care provided by hospitalists is associated with an increased LOS in patients hospitalized for pneumonia or heart failure.
Citation: Epstein K, Juarez E, Epstein A, Loya K, Singer A. The impact of fragmentation of hospitalist care on length of stay. J Hosp Med. 2010;5(6):335-338.
Admission Medication Errors Are Common and Most Harmful in Older Patients Taking Many Medications
Clinical question: What are the risk factors and potential harm associated with medication errors at hospital admission?
Background: Obtaining a medication history from a hospitalized patient is an error-prone process. Several variables can affect the completeness and quality of medication histories, but existing data are limited regarding patient or medication risk factors associated with medication errors at admission.
Study design: Prospective cohort study.
Setting: Academic hospital in Chicago.
Synopsis: Pharmacist and admitting physician medication histories were compared with admission medication orders to identify any unexplained discrepancies. Discrepancies resulting in order changes were defined as medication errors.
Of the 651 adult medical inpatients studied, 234 (35.9%) had medication order errors identified at admission. Errors originated in the medication histories for 85% of these patients. The most frequent type of error was an omission (48.9%). An age of 65 or older (odds ratio [OR]=2.17, 95% confidence interval [CI], 1.09-4.30) and increased number of medications (OR=1.21, 95% CI, 1.14-1.29) were the only risk factors identified by multivariate analysis to be independently associated with increased risk of medication order errors potentially causing harm or requiring monitoring or intervention. Presenting a medication list upon admission was a significant protective factor (OR=0.35, 95% CI, 0.19-0.63).
Though this is the largest study to date evaluating admission medication errors in hospitalized medical patients, it remains limited by its single hospital site. Because the authors were unable to interview patients who were too ill or unwilling to participate and had no caregiver present, they might have underestimated the number of admission errors. Further, the harm assessment was based on potential and not actual harm.
Bottom line: Admission medication order errors are frequent, most commonly originate in the medication histories, and have increased potential to cause adverse outcomes in older patients and those taking higher numbers of medications.
Citation: Gleason KM, McDaniel MR, Feinglass J, et al. Results of the Medications At Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441-447.
Pediatric HM Literature Review
Clinical question: What is the relationship between duration of intravenous (IV) antibiotic therapy and treatment failure in infants <6 months of age hospitalized with urinary tract infections (UTIs)?
Background: There is an inadequate evidence base to drive decisions regarding duration of IV antibiotic therapy in young infants hospitalized with UTIs. Documented variability exists in length of stay (LOS) and resource utilization for these infants, which might be a direct result of practice variation with respect to IV therapy.
Study design: Retrospective cohort study.
Setting: Twenty-four freestanding children’s hospitals.
Synopsis: The Pediatric Health Information System (PHIS) administrative database was used to identify healthy infants <6 months of age admitted with a primary or secondary diagnosis of UTI or pyelonephritis from 1999 to 2004 to participating hospitals. Duration of IV therapy was defined as a dichotomous variable with three days (short course: three days) selected because it was the median length of therapy. Treatment failure was defined as readmission within 30 days.
More than 12,300 records were analyzed. Male gender, neonatal status, black race, Hispanic ethnicity, nonprivate insurance, severity of illness, known bacteremia, known genitourinary tract disorders, and specific hospital were independently associated with increased likelihood of long-course (four days) therapy.
Unadjusted analysis initially revealed that long-course therapy was significantly associated with a higher rate of treatment failure. After multivariate (to include propensity scores) adjustment, a significant association between treatment duration and failure was no longer identified. Treatment failure association with known genitourinary abnormalities and higher severity of illness remained.
A significant limitation of this study is the potential for multivariate analysis to fail to mitigate a bias toward sicker patients receiving longer duration of antibiotic therapy and, thus, having a higher likelihood of treatment failure. In addition, the greater question of when IV antibiotics (and hospital admission) are indicated in this population was not addressed by the study design.
Nonetheless, the data likely support a limited utility to long-course IV antibiotic therapy in this population. The study also adds to the evolving picture of considerable and widespread variation in physician practice.
Bottom line: Short-course IV therapy for infants with UTIs does not increase risk of treatment failure.
Citation: Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the relationship between duration of intravenous (IV) antibiotic therapy and treatment failure in infants <6 months of age hospitalized with urinary tract infections (UTIs)?
Background: There is an inadequate evidence base to drive decisions regarding duration of IV antibiotic therapy in young infants hospitalized with UTIs. Documented variability exists in length of stay (LOS) and resource utilization for these infants, which might be a direct result of practice variation with respect to IV therapy.
Study design: Retrospective cohort study.
Setting: Twenty-four freestanding children’s hospitals.
Synopsis: The Pediatric Health Information System (PHIS) administrative database was used to identify healthy infants <6 months of age admitted with a primary or secondary diagnosis of UTI or pyelonephritis from 1999 to 2004 to participating hospitals. Duration of IV therapy was defined as a dichotomous variable with three days (short course: three days) selected because it was the median length of therapy. Treatment failure was defined as readmission within 30 days.
More than 12,300 records were analyzed. Male gender, neonatal status, black race, Hispanic ethnicity, nonprivate insurance, severity of illness, known bacteremia, known genitourinary tract disorders, and specific hospital were independently associated with increased likelihood of long-course (four days) therapy.
Unadjusted analysis initially revealed that long-course therapy was significantly associated with a higher rate of treatment failure. After multivariate (to include propensity scores) adjustment, a significant association between treatment duration and failure was no longer identified. Treatment failure association with known genitourinary abnormalities and higher severity of illness remained.
A significant limitation of this study is the potential for multivariate analysis to fail to mitigate a bias toward sicker patients receiving longer duration of antibiotic therapy and, thus, having a higher likelihood of treatment failure. In addition, the greater question of when IV antibiotics (and hospital admission) are indicated in this population was not addressed by the study design.
Nonetheless, the data likely support a limited utility to long-course IV antibiotic therapy in this population. The study also adds to the evolving picture of considerable and widespread variation in physician practice.
Bottom line: Short-course IV therapy for infants with UTIs does not increase risk of treatment failure.
Citation: Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the relationship between duration of intravenous (IV) antibiotic therapy and treatment failure in infants <6 months of age hospitalized with urinary tract infections (UTIs)?
Background: There is an inadequate evidence base to drive decisions regarding duration of IV antibiotic therapy in young infants hospitalized with UTIs. Documented variability exists in length of stay (LOS) and resource utilization for these infants, which might be a direct result of practice variation with respect to IV therapy.
Study design: Retrospective cohort study.
Setting: Twenty-four freestanding children’s hospitals.
Synopsis: The Pediatric Health Information System (PHIS) administrative database was used to identify healthy infants <6 months of age admitted with a primary or secondary diagnosis of UTI or pyelonephritis from 1999 to 2004 to participating hospitals. Duration of IV therapy was defined as a dichotomous variable with three days (short course: three days) selected because it was the median length of therapy. Treatment failure was defined as readmission within 30 days.
More than 12,300 records were analyzed. Male gender, neonatal status, black race, Hispanic ethnicity, nonprivate insurance, severity of illness, known bacteremia, known genitourinary tract disorders, and specific hospital were independently associated with increased likelihood of long-course (four days) therapy.
Unadjusted analysis initially revealed that long-course therapy was significantly associated with a higher rate of treatment failure. After multivariate (to include propensity scores) adjustment, a significant association between treatment duration and failure was no longer identified. Treatment failure association with known genitourinary abnormalities and higher severity of illness remained.
A significant limitation of this study is the potential for multivariate analysis to fail to mitigate a bias toward sicker patients receiving longer duration of antibiotic therapy and, thus, having a higher likelihood of treatment failure. In addition, the greater question of when IV antibiotics (and hospital admission) are indicated in this population was not addressed by the study design.
Nonetheless, the data likely support a limited utility to long-course IV antibiotic therapy in this population. The study also adds to the evolving picture of considerable and widespread variation in physician practice.
Bottom line: Short-course IV therapy for infants with UTIs does not increase risk of treatment failure.
Citation: Brady PW, Conway PH, Goudie A. Length of intravenous antibiotic therapy and treatment failure in infants with urinary tract infections. Pediatrics. 2010;126(2):196-203.
Reviewed by Pediatric Editor Mark Shen, MD, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Are Stress-Dose Steroids Indicated in Patients with Adrenal Insufficiency Hospitalized with Noncritical, Nonsurgical Illness?
Case
A 46-year-old woman with Addison’s disease and type II diabetes presents with one day of right leg pain, swelling, and redness. She has had mild nausea and vomiting over the past week, with an episode of diarrhea three days prior. She takes hydrocortisone 30mg in the morning and 10mg at bedtime, as well as fludrocortisone 0.2mg in the morning. She is afebrile with a pulse of 108 beats per minute. Her initial blood pressure was 74/49 mmHg, which improved to 84/45 mmHg following one liter of normal saline. She is mentating appropriately. The physical exam is significant for a large, tender area of erythema and warmth from the right ankle to mid-calf. She is admitted for cellulitis and intravenous antibiotics are initiated.
Does she require an increase in her glucocorticoid dose during her acute illness?
Overview
Adrenal insufficiency occurs in approximately 5 out of every 10,000 people and results from primary failure of the adrenal gland, or secondary impairment of the hypothalamic-pituitary-adrenal (HPA) axis, which regulates cortisol secretion.1 In developed countries, 90% of primary adrenal insufficiency (Addison’s disease) cases are due to autoimmune adrenalitis, which might occur in isolation or as part of an autoimmune polyglandular syndrome.1,2 Secondary adrenal insufficiency is most commonly the result of chronic glucocorticoid therapy, though lesions involving the hypothalamus or pituitary gland might be implicated.2,3
In a healthy individual, cortisol is secreted in a diurnal pattern from the adrenal glands under the control of corticotropin (ACTH) produced by the pituitary gland and corticotropin-releasing hormone (CRH) produced by the hypothalamus (see Figure 1, p. 19). In the normal state, during periods of such systemic stress as illness, trauma, burns, or surgery, cortisol production increases to a degree roughly proportional to the degree of illness (as much as sixfold).4,5 Patients with adrenal insufficiency are unable to mount an appropriate cortisol response and, therefore, are at risk for adrenal crisis—a life-threatening condition characterized by hypotension and hypovolemic shock.
Although recommendations for high-dose intravenous steroids in adrenally insufficient patients who are critically ill or undergoing surgery have been extensively discussed in the literature, there are relatively few data regarding the appropriate management of adrenal insufficiency in patients hospitalized for noncritical illness.
Several recent studies have investigated the patient characteristics, situations, and conditions most likely to provoke adrenal crisis in order to establish guidelines dictating the use of supra-physiologic steroid dosing.
Review of the Data
Studies have estimated the prevalence of adrenal crisis in patients with underlying insufficiency at 3.3 to 6.3 events per 100 patient years, with 42% of patients reporting at least one crisis.2,5,6 A recent survey of 982 patients with Addison’s disease in the United Kingdom reported an 8% annual frequency.7
A retrospective Japanese study reviewed the medical charts of 137 adult patients receiving steroid replacement for established primary or secondary adrenal insufficiency. The authors noted a significant positive association between adrenal crisis and long-term steroid replacement (>4 years), concomitant mental disorder, and sex hormone deficiency. A combination of any of these factors further increased the risk.8
A more recent survey of 444 patients ages 17-81 assessed independent risk factors for adrenal crisis in the setting of primary (N=254) or secondary (N=190) adrenal insufficiency. The incidence of crisis was higher in primary versus secondary insufficiency. In patients with primary insufficiency, concomitant, non-endocrine disorders increased the risk of adrenal crisis, whereas diabetes insipidus and female gender increased risk in patients with secondary insufficiency. This same study also investigated events leading to a crisis and found gastrointestinal infection to be the most frequent factor, followed by other infectious or febrile illnesses. Overall, infection comprised 45% of all identified triggers.6
A similar study conducted by White and Arlt evaluated 841 Addison’s patients in the United Kingdom, Canada, Australia, and New Zealand.7 Again, gastrointestinal illness was the most common provoking factor, responsible for 56% of all reported crises. Flulike illness followed at 11%, followed by infections and surgical procedures at 6% each. This study found a higher risk of crisis in patients with diabetes (type I or II), premature ovarian failure, and asthma; the presence of multiple comorbidities further increased risk.
Medications. Glucocorticoid therapy is known to suppress the HPA axis. Although it was once believed that the duration and dose of therapy correlated directly with the degree of HPA suppression, more recent studies have failed to find any definite relationship.9 Patients taking the equivalent of 5mg of prednisone per day should continue to have an intact HPA axis, as this dose mimics physiologic secretion of cortisol in a healthy individual.3
However, the dose and duration of therapy at which suppression occurs is highly variable between patients. In general, patients on 7.5mg of prednisone or more per day for at least three weeks should be considered to be suppressed.3,9 Additionally, progesterone derivatives (i.e., megestrol) have glucocorticoid activity and might suppress HPA function. Other medications that might have related effects are those that inhibit enzymes involved in cortisol synthesis; ketoconazole and etomidate are common examples. Rifampin and several classes of anti-epileptic drugs induce enzymes, which increase hepatic metabolism of cortisol (see Table 1, p. 18).
Hyperthyroidism. Thyroid hormone is involved in the metabolism of cortisol, thus an increase in T4 correlates with lower levels. Adrenal insufficiency and thyroid disease might coexist within the autoimmune polyglandular syndrome. Initiation or uptitration of thyroid replacement should be avoided if acute adrenal insufficiency is suspected, as this might provoke an adrenal crisis. Conversely, any patient with adrenal insufficiency who has uncontrolled hyperthyroidism should receive two to three times their usual glucocorticoid replacement.1,2
Pregnancy. Levels of cortisol-binding globulin increase throughout pregnancy. In women with intact adrenal function, free cortisol levels also rise during the third trimester. Therefore, glucocorticoid replacement should be increased by 50% during the last three months of pregnancy.2
Acute illness. The cortisol response to stress is highly variable and dependent on a number of factors, including age, underlying health, and genetics. In general, most experts recommend doubling or tripling the daily replacement dose during mild to moderate illness until recovery (often referred to as the “sick-day rules”). What constitutes “recovery” is not clearly defined. When oral intake is compromised, as with vomiting or diarrhea, parenteral administration of steroids is recommended.1-5,9,10 Patients with adrenal insufficiency should be provided an emergency injection kit to use and further counseled to seek medical attention. Injection kits typically consist of 100mg of hydrocortisone or 4mg of dexamethasone, although other glucocorticoids may be used (see Table 2, above left, for conversions).
Limited data are available to support guidelines for glucocorticoid adjustment during acute, non-critical illness. Published guidelines vary both in illness categorization and category-specific recommendations (see Table 3, below).
Coursin and Wood devised a set of guidelines based on a literature review and consultation with experts, categorizing medical illness as minor, moderate, severe, and critical (see Table 3).3 For noncritical illness, they recommended continuation of standard replacement therapy with an additional, once-daily dose, which varied according to illness severity.
Cooper and Stewart conducted a similar review, basing their guidelines on a categorization of mild, moderate, severe/critical, or septic shock. These guidelines recommended a total daily dose of glucocorticoid supplementation, rather than an addition of a single dose to current therapy. They also stated that the least severe category of illness (defined as mild) did not require any change to a patient’s regular therapy.4
Jung et al classified illness as minimal, minor, moderate, severe, and critical.9 Under these guidelines, supplemental therapy was not advised for minimal (nonfebrile) illness. Moderate illness, including cellulitis, warranted a doubling or tripling of the outpatient dose until recovery, which was consistent with prior expert recommendation. More severe illness warranted intravenous administration of steroids.
Back to the Case
The patient had a mild case of cellulitis, classified by most experts as moderate illness, which responded well to vancomycin. Her outpatient glucocorticoid dose was doubled on admission and administered orally for the duration of her hospitalization, as she had no further episodes of vomiting or diarrhea.
Review of the patient’s records from prior hospitalizations and ambulatory visits revealed that her systolic blood pressure typically ran in the 80 mmHg to 100 mmHg range. Following initial volume resuscitation, her systolic blood pressure remained in the 90-100 mmHg range.
She was discharged home in stable condition, with instructions to complete a course of oral trimethoprim/sulfamethoxazole, resume her baseline dose of hydrocortisone the day after discharge, and follow up with her PCP for further monitoring and adjustment of her adrenal replacement therapy.
Bottom Line
For adults with adrenal insufficiency hospitalized with noncritical, nonsurgical illness, the expert recommendation is to double or triple the usual outpatient dose of glucocorticoid; however, data to support this is limited, and each patient should be assessed carefully and monitored to determine the optimal dose adjustment. TH
Dr. Shaw is a resident in the Department of Medicine at the University of Washington School of Medicine in Seattle. Dr. Best is an assistant professor of medicine in the division of general internal medicine, University of Washington School of Medicine.
References
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(4):1059-1067.
- Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003; 361:1881-1893.
- Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA. 2002;287:236-240.
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727-734.
- Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23:167-179.
- Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162:597:602.
- White K, Arlt W. Adrenal crisis in treated Addison’s disease: a predictable but under-managed event. Eur J Endocrinol. 2010;162:115-120.
- Omori K, Nomura K, Shimizu S, Omori N, Takano K. Risk factors for adrenal crises in patients with adrenal insufficiency. Endocr J. 2003;50:745-752.
- Jung C, Inder WJ. Management of adrenal insufficiency during the stress of medical illness and surgery. Med J Aust. 2008;188:409-413.
- Crown A, Lightman S. Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol. 2005;63:483-492.
Case
A 46-year-old woman with Addison’s disease and type II diabetes presents with one day of right leg pain, swelling, and redness. She has had mild nausea and vomiting over the past week, with an episode of diarrhea three days prior. She takes hydrocortisone 30mg in the morning and 10mg at bedtime, as well as fludrocortisone 0.2mg in the morning. She is afebrile with a pulse of 108 beats per minute. Her initial blood pressure was 74/49 mmHg, which improved to 84/45 mmHg following one liter of normal saline. She is mentating appropriately. The physical exam is significant for a large, tender area of erythema and warmth from the right ankle to mid-calf. She is admitted for cellulitis and intravenous antibiotics are initiated.
Does she require an increase in her glucocorticoid dose during her acute illness?
Overview
Adrenal insufficiency occurs in approximately 5 out of every 10,000 people and results from primary failure of the adrenal gland, or secondary impairment of the hypothalamic-pituitary-adrenal (HPA) axis, which regulates cortisol secretion.1 In developed countries, 90% of primary adrenal insufficiency (Addison’s disease) cases are due to autoimmune adrenalitis, which might occur in isolation or as part of an autoimmune polyglandular syndrome.1,2 Secondary adrenal insufficiency is most commonly the result of chronic glucocorticoid therapy, though lesions involving the hypothalamus or pituitary gland might be implicated.2,3
In a healthy individual, cortisol is secreted in a diurnal pattern from the adrenal glands under the control of corticotropin (ACTH) produced by the pituitary gland and corticotropin-releasing hormone (CRH) produced by the hypothalamus (see Figure 1, p. 19). In the normal state, during periods of such systemic stress as illness, trauma, burns, or surgery, cortisol production increases to a degree roughly proportional to the degree of illness (as much as sixfold).4,5 Patients with adrenal insufficiency are unable to mount an appropriate cortisol response and, therefore, are at risk for adrenal crisis—a life-threatening condition characterized by hypotension and hypovolemic shock.
Although recommendations for high-dose intravenous steroids in adrenally insufficient patients who are critically ill or undergoing surgery have been extensively discussed in the literature, there are relatively few data regarding the appropriate management of adrenal insufficiency in patients hospitalized for noncritical illness.
Several recent studies have investigated the patient characteristics, situations, and conditions most likely to provoke adrenal crisis in order to establish guidelines dictating the use of supra-physiologic steroid dosing.
Review of the Data
Studies have estimated the prevalence of adrenal crisis in patients with underlying insufficiency at 3.3 to 6.3 events per 100 patient years, with 42% of patients reporting at least one crisis.2,5,6 A recent survey of 982 patients with Addison’s disease in the United Kingdom reported an 8% annual frequency.7
A retrospective Japanese study reviewed the medical charts of 137 adult patients receiving steroid replacement for established primary or secondary adrenal insufficiency. The authors noted a significant positive association between adrenal crisis and long-term steroid replacement (>4 years), concomitant mental disorder, and sex hormone deficiency. A combination of any of these factors further increased the risk.8
A more recent survey of 444 patients ages 17-81 assessed independent risk factors for adrenal crisis in the setting of primary (N=254) or secondary (N=190) adrenal insufficiency. The incidence of crisis was higher in primary versus secondary insufficiency. In patients with primary insufficiency, concomitant, non-endocrine disorders increased the risk of adrenal crisis, whereas diabetes insipidus and female gender increased risk in patients with secondary insufficiency. This same study also investigated events leading to a crisis and found gastrointestinal infection to be the most frequent factor, followed by other infectious or febrile illnesses. Overall, infection comprised 45% of all identified triggers.6
A similar study conducted by White and Arlt evaluated 841 Addison’s patients in the United Kingdom, Canada, Australia, and New Zealand.7 Again, gastrointestinal illness was the most common provoking factor, responsible for 56% of all reported crises. Flulike illness followed at 11%, followed by infections and surgical procedures at 6% each. This study found a higher risk of crisis in patients with diabetes (type I or II), premature ovarian failure, and asthma; the presence of multiple comorbidities further increased risk.
Medications. Glucocorticoid therapy is known to suppress the HPA axis. Although it was once believed that the duration and dose of therapy correlated directly with the degree of HPA suppression, more recent studies have failed to find any definite relationship.9 Patients taking the equivalent of 5mg of prednisone per day should continue to have an intact HPA axis, as this dose mimics physiologic secretion of cortisol in a healthy individual.3
However, the dose and duration of therapy at which suppression occurs is highly variable between patients. In general, patients on 7.5mg of prednisone or more per day for at least three weeks should be considered to be suppressed.3,9 Additionally, progesterone derivatives (i.e., megestrol) have glucocorticoid activity and might suppress HPA function. Other medications that might have related effects are those that inhibit enzymes involved in cortisol synthesis; ketoconazole and etomidate are common examples. Rifampin and several classes of anti-epileptic drugs induce enzymes, which increase hepatic metabolism of cortisol (see Table 1, p. 18).
Hyperthyroidism. Thyroid hormone is involved in the metabolism of cortisol, thus an increase in T4 correlates with lower levels. Adrenal insufficiency and thyroid disease might coexist within the autoimmune polyglandular syndrome. Initiation or uptitration of thyroid replacement should be avoided if acute adrenal insufficiency is suspected, as this might provoke an adrenal crisis. Conversely, any patient with adrenal insufficiency who has uncontrolled hyperthyroidism should receive two to three times their usual glucocorticoid replacement.1,2
Pregnancy. Levels of cortisol-binding globulin increase throughout pregnancy. In women with intact adrenal function, free cortisol levels also rise during the third trimester. Therefore, glucocorticoid replacement should be increased by 50% during the last three months of pregnancy.2
Acute illness. The cortisol response to stress is highly variable and dependent on a number of factors, including age, underlying health, and genetics. In general, most experts recommend doubling or tripling the daily replacement dose during mild to moderate illness until recovery (often referred to as the “sick-day rules”). What constitutes “recovery” is not clearly defined. When oral intake is compromised, as with vomiting or diarrhea, parenteral administration of steroids is recommended.1-5,9,10 Patients with adrenal insufficiency should be provided an emergency injection kit to use and further counseled to seek medical attention. Injection kits typically consist of 100mg of hydrocortisone or 4mg of dexamethasone, although other glucocorticoids may be used (see Table 2, above left, for conversions).
Limited data are available to support guidelines for glucocorticoid adjustment during acute, non-critical illness. Published guidelines vary both in illness categorization and category-specific recommendations (see Table 3, below).
Coursin and Wood devised a set of guidelines based on a literature review and consultation with experts, categorizing medical illness as minor, moderate, severe, and critical (see Table 3).3 For noncritical illness, they recommended continuation of standard replacement therapy with an additional, once-daily dose, which varied according to illness severity.
Cooper and Stewart conducted a similar review, basing their guidelines on a categorization of mild, moderate, severe/critical, or septic shock. These guidelines recommended a total daily dose of glucocorticoid supplementation, rather than an addition of a single dose to current therapy. They also stated that the least severe category of illness (defined as mild) did not require any change to a patient’s regular therapy.4
Jung et al classified illness as minimal, minor, moderate, severe, and critical.9 Under these guidelines, supplemental therapy was not advised for minimal (nonfebrile) illness. Moderate illness, including cellulitis, warranted a doubling or tripling of the outpatient dose until recovery, which was consistent with prior expert recommendation. More severe illness warranted intravenous administration of steroids.
Back to the Case
The patient had a mild case of cellulitis, classified by most experts as moderate illness, which responded well to vancomycin. Her outpatient glucocorticoid dose was doubled on admission and administered orally for the duration of her hospitalization, as she had no further episodes of vomiting or diarrhea.
Review of the patient’s records from prior hospitalizations and ambulatory visits revealed that her systolic blood pressure typically ran in the 80 mmHg to 100 mmHg range. Following initial volume resuscitation, her systolic blood pressure remained in the 90-100 mmHg range.
She was discharged home in stable condition, with instructions to complete a course of oral trimethoprim/sulfamethoxazole, resume her baseline dose of hydrocortisone the day after discharge, and follow up with her PCP for further monitoring and adjustment of her adrenal replacement therapy.
Bottom Line
For adults with adrenal insufficiency hospitalized with noncritical, nonsurgical illness, the expert recommendation is to double or triple the usual outpatient dose of glucocorticoid; however, data to support this is limited, and each patient should be assessed carefully and monitored to determine the optimal dose adjustment. TH
Dr. Shaw is a resident in the Department of Medicine at the University of Washington School of Medicine in Seattle. Dr. Best is an assistant professor of medicine in the division of general internal medicine, University of Washington School of Medicine.
References
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(4):1059-1067.
- Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003; 361:1881-1893.
- Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA. 2002;287:236-240.
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727-734.
- Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23:167-179.
- Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162:597:602.
- White K, Arlt W. Adrenal crisis in treated Addison’s disease: a predictable but under-managed event. Eur J Endocrinol. 2010;162:115-120.
- Omori K, Nomura K, Shimizu S, Omori N, Takano K. Risk factors for adrenal crises in patients with adrenal insufficiency. Endocr J. 2003;50:745-752.
- Jung C, Inder WJ. Management of adrenal insufficiency during the stress of medical illness and surgery. Med J Aust. 2008;188:409-413.
- Crown A, Lightman S. Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol. 2005;63:483-492.
Case
A 46-year-old woman with Addison’s disease and type II diabetes presents with one day of right leg pain, swelling, and redness. She has had mild nausea and vomiting over the past week, with an episode of diarrhea three days prior. She takes hydrocortisone 30mg in the morning and 10mg at bedtime, as well as fludrocortisone 0.2mg in the morning. She is afebrile with a pulse of 108 beats per minute. Her initial blood pressure was 74/49 mmHg, which improved to 84/45 mmHg following one liter of normal saline. She is mentating appropriately. The physical exam is significant for a large, tender area of erythema and warmth from the right ankle to mid-calf. She is admitted for cellulitis and intravenous antibiotics are initiated.
Does she require an increase in her glucocorticoid dose during her acute illness?
Overview
Adrenal insufficiency occurs in approximately 5 out of every 10,000 people and results from primary failure of the adrenal gland, or secondary impairment of the hypothalamic-pituitary-adrenal (HPA) axis, which regulates cortisol secretion.1 In developed countries, 90% of primary adrenal insufficiency (Addison’s disease) cases are due to autoimmune adrenalitis, which might occur in isolation or as part of an autoimmune polyglandular syndrome.1,2 Secondary adrenal insufficiency is most commonly the result of chronic glucocorticoid therapy, though lesions involving the hypothalamus or pituitary gland might be implicated.2,3
In a healthy individual, cortisol is secreted in a diurnal pattern from the adrenal glands under the control of corticotropin (ACTH) produced by the pituitary gland and corticotropin-releasing hormone (CRH) produced by the hypothalamus (see Figure 1, p. 19). In the normal state, during periods of such systemic stress as illness, trauma, burns, or surgery, cortisol production increases to a degree roughly proportional to the degree of illness (as much as sixfold).4,5 Patients with adrenal insufficiency are unable to mount an appropriate cortisol response and, therefore, are at risk for adrenal crisis—a life-threatening condition characterized by hypotension and hypovolemic shock.
Although recommendations for high-dose intravenous steroids in adrenally insufficient patients who are critically ill or undergoing surgery have been extensively discussed in the literature, there are relatively few data regarding the appropriate management of adrenal insufficiency in patients hospitalized for noncritical illness.
Several recent studies have investigated the patient characteristics, situations, and conditions most likely to provoke adrenal crisis in order to establish guidelines dictating the use of supra-physiologic steroid dosing.
Review of the Data
Studies have estimated the prevalence of adrenal crisis in patients with underlying insufficiency at 3.3 to 6.3 events per 100 patient years, with 42% of patients reporting at least one crisis.2,5,6 A recent survey of 982 patients with Addison’s disease in the United Kingdom reported an 8% annual frequency.7
A retrospective Japanese study reviewed the medical charts of 137 adult patients receiving steroid replacement for established primary or secondary adrenal insufficiency. The authors noted a significant positive association between adrenal crisis and long-term steroid replacement (>4 years), concomitant mental disorder, and sex hormone deficiency. A combination of any of these factors further increased the risk.8
A more recent survey of 444 patients ages 17-81 assessed independent risk factors for adrenal crisis in the setting of primary (N=254) or secondary (N=190) adrenal insufficiency. The incidence of crisis was higher in primary versus secondary insufficiency. In patients with primary insufficiency, concomitant, non-endocrine disorders increased the risk of adrenal crisis, whereas diabetes insipidus and female gender increased risk in patients with secondary insufficiency. This same study also investigated events leading to a crisis and found gastrointestinal infection to be the most frequent factor, followed by other infectious or febrile illnesses. Overall, infection comprised 45% of all identified triggers.6
A similar study conducted by White and Arlt evaluated 841 Addison’s patients in the United Kingdom, Canada, Australia, and New Zealand.7 Again, gastrointestinal illness was the most common provoking factor, responsible for 56% of all reported crises. Flulike illness followed at 11%, followed by infections and surgical procedures at 6% each. This study found a higher risk of crisis in patients with diabetes (type I or II), premature ovarian failure, and asthma; the presence of multiple comorbidities further increased risk.
Medications. Glucocorticoid therapy is known to suppress the HPA axis. Although it was once believed that the duration and dose of therapy correlated directly with the degree of HPA suppression, more recent studies have failed to find any definite relationship.9 Patients taking the equivalent of 5mg of prednisone per day should continue to have an intact HPA axis, as this dose mimics physiologic secretion of cortisol in a healthy individual.3
However, the dose and duration of therapy at which suppression occurs is highly variable between patients. In general, patients on 7.5mg of prednisone or more per day for at least three weeks should be considered to be suppressed.3,9 Additionally, progesterone derivatives (i.e., megestrol) have glucocorticoid activity and might suppress HPA function. Other medications that might have related effects are those that inhibit enzymes involved in cortisol synthesis; ketoconazole and etomidate are common examples. Rifampin and several classes of anti-epileptic drugs induce enzymes, which increase hepatic metabolism of cortisol (see Table 1, p. 18).
Hyperthyroidism. Thyroid hormone is involved in the metabolism of cortisol, thus an increase in T4 correlates with lower levels. Adrenal insufficiency and thyroid disease might coexist within the autoimmune polyglandular syndrome. Initiation or uptitration of thyroid replacement should be avoided if acute adrenal insufficiency is suspected, as this might provoke an adrenal crisis. Conversely, any patient with adrenal insufficiency who has uncontrolled hyperthyroidism should receive two to three times their usual glucocorticoid replacement.1,2
Pregnancy. Levels of cortisol-binding globulin increase throughout pregnancy. In women with intact adrenal function, free cortisol levels also rise during the third trimester. Therefore, glucocorticoid replacement should be increased by 50% during the last three months of pregnancy.2
Acute illness. The cortisol response to stress is highly variable and dependent on a number of factors, including age, underlying health, and genetics. In general, most experts recommend doubling or tripling the daily replacement dose during mild to moderate illness until recovery (often referred to as the “sick-day rules”). What constitutes “recovery” is not clearly defined. When oral intake is compromised, as with vomiting or diarrhea, parenteral administration of steroids is recommended.1-5,9,10 Patients with adrenal insufficiency should be provided an emergency injection kit to use and further counseled to seek medical attention. Injection kits typically consist of 100mg of hydrocortisone or 4mg of dexamethasone, although other glucocorticoids may be used (see Table 2, above left, for conversions).
Limited data are available to support guidelines for glucocorticoid adjustment during acute, non-critical illness. Published guidelines vary both in illness categorization and category-specific recommendations (see Table 3, below).
Coursin and Wood devised a set of guidelines based on a literature review and consultation with experts, categorizing medical illness as minor, moderate, severe, and critical (see Table 3).3 For noncritical illness, they recommended continuation of standard replacement therapy with an additional, once-daily dose, which varied according to illness severity.
Cooper and Stewart conducted a similar review, basing their guidelines on a categorization of mild, moderate, severe/critical, or septic shock. These guidelines recommended a total daily dose of glucocorticoid supplementation, rather than an addition of a single dose to current therapy. They also stated that the least severe category of illness (defined as mild) did not require any change to a patient’s regular therapy.4
Jung et al classified illness as minimal, minor, moderate, severe, and critical.9 Under these guidelines, supplemental therapy was not advised for minimal (nonfebrile) illness. Moderate illness, including cellulitis, warranted a doubling or tripling of the outpatient dose until recovery, which was consistent with prior expert recommendation. More severe illness warranted intravenous administration of steroids.
Back to the Case
The patient had a mild case of cellulitis, classified by most experts as moderate illness, which responded well to vancomycin. Her outpatient glucocorticoid dose was doubled on admission and administered orally for the duration of her hospitalization, as she had no further episodes of vomiting or diarrhea.
Review of the patient’s records from prior hospitalizations and ambulatory visits revealed that her systolic blood pressure typically ran in the 80 mmHg to 100 mmHg range. Following initial volume resuscitation, her systolic blood pressure remained in the 90-100 mmHg range.
She was discharged home in stable condition, with instructions to complete a course of oral trimethoprim/sulfamethoxazole, resume her baseline dose of hydrocortisone the day after discharge, and follow up with her PCP for further monitoring and adjustment of her adrenal replacement therapy.
Bottom Line
For adults with adrenal insufficiency hospitalized with noncritical, nonsurgical illness, the expert recommendation is to double or triple the usual outpatient dose of glucocorticoid; however, data to support this is limited, and each patient should be assessed carefully and monitored to determine the optimal dose adjustment. TH
Dr. Shaw is a resident in the Department of Medicine at the University of Washington School of Medicine in Seattle. Dr. Best is an assistant professor of medicine in the division of general internal medicine, University of Washington School of Medicine.
References
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(4):1059-1067.
- Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003; 361:1881-1893.
- Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA. 2002;287:236-240.
- Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727-734.
- Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23:167-179.
- Hahner S, Loeffler M, Bleicken B, et al. Epidemiology of adrenal crisis in chronic adrenal insufficiency: the need for new prevention strategies. Eur J Endocrinol. 2010;162:597:602.
- White K, Arlt W. Adrenal crisis in treated Addison’s disease: a predictable but under-managed event. Eur J Endocrinol. 2010;162:115-120.
- Omori K, Nomura K, Shimizu S, Omori N, Takano K. Risk factors for adrenal crises in patients with adrenal insufficiency. Endocr J. 2003;50:745-752.
- Jung C, Inder WJ. Management of adrenal insufficiency during the stress of medical illness and surgery. Med J Aust. 2008;188:409-413.
- Crown A, Lightman S. Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol. 2005;63:483-492.
Gettin’ Dirty
Several months ago, my toilet broke. You should also know that I’m not particularly handy. So when I first realized that the toilet bowl seemed to fill constantly, I got a little stressed out.
How much was it going cost to call in a plumber on the weekend?
What kind of a water bill was I going to have?
Was this a serious problem?
I took a quick peek in the tank, but that just made me more confused. I was paralyzed by a lack of know-how.
Normally, I would have just Googled a local plumber. But that day, I decided to do something different. Maybe it was because it was the fantasy football offseason. Maybe it was because my wife had started to ask my father-in-law to change light bulbs around the house. Or, maybe, I wanted to learn to actually fix the problem. A few hours later, after an Internet lesson in toilet physiology, a $4.12 trip to Home Depot, and a wet pair of hands, I had replaced my first toilet flapper.
This wasn’t the rebuilding of a car engine, but it was a clear DIY step toward self-improvement. Easily the most memorable moment here was my sense of accomplishment.
I felt empowered.
One Part Science, One Part Art
It’s taken me a while to realize this, but I’ve begun to take advantage of improvement opportunities at work as well. No, I haven’t been moonlighting as a plumber for my hospital. I’ve just been fortunate to be part of a trifecta of rewarding quality-improvement (QI) projects over the past year. Before I’d gotten my hands dirty with these, my understanding of QI was fairly naive. I’d heard about Plan-Do-Study-Act many times. I had listened to a talk at a national conference. And I had kept up with the general medical literature on the subject.
But none of those activities had truly prepared me for experience of actually doing the work on my own.
By taking on a project, an ambitious attempt to reduce continuous pulse oximetry use, I experienced a crash course in both the science and the art of process improvement. I was forced to overcome my “I don’t know how” inertia. And with expert guidance in the form of a clinical safety and effectiveness class, I learned the importance of run charts (science) and a well-crafted multidisciplinary team (art) in changing established but inefficient behavior.
Our rates of continuous pulse oximetry usage dropped by 50%, and cost savings were $12,000 per year on one unit. These results made my prior attempts at change—years of complaining about ingrained nursing culture—look infantile. (OK, maybe it was ineffective, but who hasn’t complained about the overuse of continuous monitoring?)
I haven’t met a pediatric hospitalist who wouldn’t understand the symbolic importance of this success. But I know of many hospitalists who have not yet participated in meaningful QI project. Imagine calling a plumber who grasped the flush and fill mechanism of a toilet but had never touched real porcelain. Here’s an even better analogy: What if doctors could get licensed without having touched real patients?
If pediatric hospitalists are to transform the care delivery of hospitalized children, and quality learning only comes through hands-on training, then we need some more hands in the pot.
Discharge Improvement
On the heels of my first project, I was fortunate enough to augment my education through another effort—this time with a cohort of fellow pediatric hospitalists. This was a national collaborative to improve discharge handoffs, and I will admit that, at the outset, I was as puzzled as the first time I pulled the lid off the tank of the toilet. There were just too many permutations on PCP communication at the participating institutions, and some felt our aim of timely discharge handoffs was unattainable.
What carried me through, however, was the collective and infectious DIY—no, QIY (Quality Improve-it-Yourself) attitude of the group. We were all learning, and regular participation in the collaborative essentially guaranteed improvement. We achieved our aim of 90% communication with PCPs within two days of discharge. The secret was simple: The more you do, the more you learn.
Pediatric hospitalists can transform care delivery through a focus on safe and quality care, but the tools to accomplish this must come through post-residency, on-the-job learning. This QI know-how must efficiently spread among our ranks through practical and project-based educational efforts. It’s “see one, do one, teach one,” but we’re not talking about lumbar punctures anymore.
This is a journey in which we all take on the responsibility of rolling up our sleeves and simply learn by doing. And here is where the third leg of my as-yet-unfinished QI course unfolds.
Through my involvement with the Value in Inpatient Pediatrics (VIP) Network, I’ve gained a newfound vision for what the future might hold. VIP has evolved from a benchmarking project focused on bronchiolitis to an improvement network that will incorporate projects similar to the discharge handoff collaborative above.
In the process, a model for how to rapidly spread QI learning has emerged. The capacity lies in the network’s rapidly growing connectivity. The power comes from the individuals: motivated, card-carrying pediatric hospitalists from a wide array of sites. Collaborative learning harbors the potential to exponentially increase the pace at which we improve.
The future of our quality care is bright. I see an open network of improvement doers and learners. I see collaboration on quality and safety initiatives in all manner of hospitals and communities. I see that this will all be built upon a foundation of hard work and a QIY attitude.
You, too, will play a role.
Just don’t be afraid to get your hands a little dirty. TH
Dr. Shen is medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas. He is pediatric editor of The Hospitalist.
Several months ago, my toilet broke. You should also know that I’m not particularly handy. So when I first realized that the toilet bowl seemed to fill constantly, I got a little stressed out.
How much was it going cost to call in a plumber on the weekend?
What kind of a water bill was I going to have?
Was this a serious problem?
I took a quick peek in the tank, but that just made me more confused. I was paralyzed by a lack of know-how.
Normally, I would have just Googled a local plumber. But that day, I decided to do something different. Maybe it was because it was the fantasy football offseason. Maybe it was because my wife had started to ask my father-in-law to change light bulbs around the house. Or, maybe, I wanted to learn to actually fix the problem. A few hours later, after an Internet lesson in toilet physiology, a $4.12 trip to Home Depot, and a wet pair of hands, I had replaced my first toilet flapper.
This wasn’t the rebuilding of a car engine, but it was a clear DIY step toward self-improvement. Easily the most memorable moment here was my sense of accomplishment.
I felt empowered.
One Part Science, One Part Art
It’s taken me a while to realize this, but I’ve begun to take advantage of improvement opportunities at work as well. No, I haven’t been moonlighting as a plumber for my hospital. I’ve just been fortunate to be part of a trifecta of rewarding quality-improvement (QI) projects over the past year. Before I’d gotten my hands dirty with these, my understanding of QI was fairly naive. I’d heard about Plan-Do-Study-Act many times. I had listened to a talk at a national conference. And I had kept up with the general medical literature on the subject.
But none of those activities had truly prepared me for experience of actually doing the work on my own.
By taking on a project, an ambitious attempt to reduce continuous pulse oximetry use, I experienced a crash course in both the science and the art of process improvement. I was forced to overcome my “I don’t know how” inertia. And with expert guidance in the form of a clinical safety and effectiveness class, I learned the importance of run charts (science) and a well-crafted multidisciplinary team (art) in changing established but inefficient behavior.
Our rates of continuous pulse oximetry usage dropped by 50%, and cost savings were $12,000 per year on one unit. These results made my prior attempts at change—years of complaining about ingrained nursing culture—look infantile. (OK, maybe it was ineffective, but who hasn’t complained about the overuse of continuous monitoring?)
I haven’t met a pediatric hospitalist who wouldn’t understand the symbolic importance of this success. But I know of many hospitalists who have not yet participated in meaningful QI project. Imagine calling a plumber who grasped the flush and fill mechanism of a toilet but had never touched real porcelain. Here’s an even better analogy: What if doctors could get licensed without having touched real patients?
If pediatric hospitalists are to transform the care delivery of hospitalized children, and quality learning only comes through hands-on training, then we need some more hands in the pot.
Discharge Improvement
On the heels of my first project, I was fortunate enough to augment my education through another effort—this time with a cohort of fellow pediatric hospitalists. This was a national collaborative to improve discharge handoffs, and I will admit that, at the outset, I was as puzzled as the first time I pulled the lid off the tank of the toilet. There were just too many permutations on PCP communication at the participating institutions, and some felt our aim of timely discharge handoffs was unattainable.
What carried me through, however, was the collective and infectious DIY—no, QIY (Quality Improve-it-Yourself) attitude of the group. We were all learning, and regular participation in the collaborative essentially guaranteed improvement. We achieved our aim of 90% communication with PCPs within two days of discharge. The secret was simple: The more you do, the more you learn.
Pediatric hospitalists can transform care delivery through a focus on safe and quality care, but the tools to accomplish this must come through post-residency, on-the-job learning. This QI know-how must efficiently spread among our ranks through practical and project-based educational efforts. It’s “see one, do one, teach one,” but we’re not talking about lumbar punctures anymore.
This is a journey in which we all take on the responsibility of rolling up our sleeves and simply learn by doing. And here is where the third leg of my as-yet-unfinished QI course unfolds.
Through my involvement with the Value in Inpatient Pediatrics (VIP) Network, I’ve gained a newfound vision for what the future might hold. VIP has evolved from a benchmarking project focused on bronchiolitis to an improvement network that will incorporate projects similar to the discharge handoff collaborative above.
In the process, a model for how to rapidly spread QI learning has emerged. The capacity lies in the network’s rapidly growing connectivity. The power comes from the individuals: motivated, card-carrying pediatric hospitalists from a wide array of sites. Collaborative learning harbors the potential to exponentially increase the pace at which we improve.
The future of our quality care is bright. I see an open network of improvement doers and learners. I see collaboration on quality and safety initiatives in all manner of hospitals and communities. I see that this will all be built upon a foundation of hard work and a QIY attitude.
You, too, will play a role.
Just don’t be afraid to get your hands a little dirty. TH
Dr. Shen is medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas. He is pediatric editor of The Hospitalist.
Several months ago, my toilet broke. You should also know that I’m not particularly handy. So when I first realized that the toilet bowl seemed to fill constantly, I got a little stressed out.
How much was it going cost to call in a plumber on the weekend?
What kind of a water bill was I going to have?
Was this a serious problem?
I took a quick peek in the tank, but that just made me more confused. I was paralyzed by a lack of know-how.
Normally, I would have just Googled a local plumber. But that day, I decided to do something different. Maybe it was because it was the fantasy football offseason. Maybe it was because my wife had started to ask my father-in-law to change light bulbs around the house. Or, maybe, I wanted to learn to actually fix the problem. A few hours later, after an Internet lesson in toilet physiology, a $4.12 trip to Home Depot, and a wet pair of hands, I had replaced my first toilet flapper.
This wasn’t the rebuilding of a car engine, but it was a clear DIY step toward self-improvement. Easily the most memorable moment here was my sense of accomplishment.
I felt empowered.
One Part Science, One Part Art
It’s taken me a while to realize this, but I’ve begun to take advantage of improvement opportunities at work as well. No, I haven’t been moonlighting as a plumber for my hospital. I’ve just been fortunate to be part of a trifecta of rewarding quality-improvement (QI) projects over the past year. Before I’d gotten my hands dirty with these, my understanding of QI was fairly naive. I’d heard about Plan-Do-Study-Act many times. I had listened to a talk at a national conference. And I had kept up with the general medical literature on the subject.
But none of those activities had truly prepared me for experience of actually doing the work on my own.
By taking on a project, an ambitious attempt to reduce continuous pulse oximetry use, I experienced a crash course in both the science and the art of process improvement. I was forced to overcome my “I don’t know how” inertia. And with expert guidance in the form of a clinical safety and effectiveness class, I learned the importance of run charts (science) and a well-crafted multidisciplinary team (art) in changing established but inefficient behavior.
Our rates of continuous pulse oximetry usage dropped by 50%, and cost savings were $12,000 per year on one unit. These results made my prior attempts at change—years of complaining about ingrained nursing culture—look infantile. (OK, maybe it was ineffective, but who hasn’t complained about the overuse of continuous monitoring?)
I haven’t met a pediatric hospitalist who wouldn’t understand the symbolic importance of this success. But I know of many hospitalists who have not yet participated in meaningful QI project. Imagine calling a plumber who grasped the flush and fill mechanism of a toilet but had never touched real porcelain. Here’s an even better analogy: What if doctors could get licensed without having touched real patients?
If pediatric hospitalists are to transform the care delivery of hospitalized children, and quality learning only comes through hands-on training, then we need some more hands in the pot.
Discharge Improvement
On the heels of my first project, I was fortunate enough to augment my education through another effort—this time with a cohort of fellow pediatric hospitalists. This was a national collaborative to improve discharge handoffs, and I will admit that, at the outset, I was as puzzled as the first time I pulled the lid off the tank of the toilet. There were just too many permutations on PCP communication at the participating institutions, and some felt our aim of timely discharge handoffs was unattainable.
What carried me through, however, was the collective and infectious DIY—no, QIY (Quality Improve-it-Yourself) attitude of the group. We were all learning, and regular participation in the collaborative essentially guaranteed improvement. We achieved our aim of 90% communication with PCPs within two days of discharge. The secret was simple: The more you do, the more you learn.
Pediatric hospitalists can transform care delivery through a focus on safe and quality care, but the tools to accomplish this must come through post-residency, on-the-job learning. This QI know-how must efficiently spread among our ranks through practical and project-based educational efforts. It’s “see one, do one, teach one,” but we’re not talking about lumbar punctures anymore.
This is a journey in which we all take on the responsibility of rolling up our sleeves and simply learn by doing. And here is where the third leg of my as-yet-unfinished QI course unfolds.
Through my involvement with the Value in Inpatient Pediatrics (VIP) Network, I’ve gained a newfound vision for what the future might hold. VIP has evolved from a benchmarking project focused on bronchiolitis to an improvement network that will incorporate projects similar to the discharge handoff collaborative above.
In the process, a model for how to rapidly spread QI learning has emerged. The capacity lies in the network’s rapidly growing connectivity. The power comes from the individuals: motivated, card-carrying pediatric hospitalists from a wide array of sites. Collaborative learning harbors the potential to exponentially increase the pace at which we improve.
The future of our quality care is bright. I see an open network of improvement doers and learners. I see collaboration on quality and safety initiatives in all manner of hospitals and communities. I see that this will all be built upon a foundation of hard work and a QIY attitude.
You, too, will play a role.
Just don’t be afraid to get your hands a little dirty. TH
Dr. Shen is medical director of hospital medicine at Dell Children’s Medical Center in Austin, Texas. He is pediatric editor of The Hospitalist.
In the Literature: HM-Related Research You Need to Know
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk of infection with arterial and central venous catheters
- Rifaximin and prevention of hepatic encephalopathy
- Tracheotomy to prevent ventilator-associated pneumonia
- Coagulopathy and risk of VTE in patients with cirrhosis
- Use of age-adjusted D-dimer for PE diagnosis
- Continuation of anti-hypertensive medications after stroke
- Number of lumen cultures and detection of CRBSIs
- Timing of anticoagulation and outcomes in PE
Arterial and Central Venous Catheters Have Similar Rates of Colonization and Blood Stream Infections
Clinical question: Are arterial catheters (ACs) safer than central venous catheters (CVCs) in terms of colonization and catheter-related infections?
Background: Unlike CVCs, only a few studies have addressed blood-stream infections (BSI) related to AC usage, probably due to the traditional perception that ACs pose a lesser risk of colonization and BSI than CVC.
Study design: Randomized, controlled trial.
Setting: Three university hospitals and two general hospitals in France.
Synopsis: The study included 3,532 catheters (1,915 CVC and 1,617 AC) with 27,541 catheter-days from seven ICU settings. The same standard procedures were followed for catheter insertion and site dressing change at the various centers. Catheters were removed when they no longer were needed or when catheter-related infection (CRI) was suspected.
Colonization and CRI rates were similar in both arterial and venous catheters: 7.9% vs. 9.6% and 0.68% vs. 0.94%, respectively. The daily risk of colonization over time was stable for CVC, but appeared to increase for AC.
One important limitation to this study is that many patients had both arterial and venous catheters, leading to difficulty attributing infection to either one. Hospitalists caring for ICU patients should weigh the risks and benefits of prolonged use of AC due to similar rates of colonization and CRI as CVC.
Bottom line: Arterial and central venous catheters are equally prone to colonization and cause similar rates of CRI, but AC daily risk tends to increase with time; thus, AC should receive the same precautions as CVC.
Citation: Lucet JC, Bouadma L, Zahar JR, et. al. Infectious risk associated with arterial catheters compared with central venous catheters. Crit Care Med. 2010;38(4):1030-1005.
Rifaximin Prevents Recurrence of Hepatic Encephalopathy Episodes and Reduces Associated Risk for Hospitalization
Clinical question: What is the efficacy of rifaximin for the prevention of hepatic encephalopathy?
Background: Hepatic encephalopathy is a chronic, debilitating complication of liver cirrhosis. The efficacy of treatment of acute episodes with rifaximin is well documented in the literature; however, prevention of such episodes using rifaximin is poorly studied.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Seventy centers in the U.S., Canada, and Russia.
Synopsis: A total of 299 chronic liver disease patients, in remission from recurrent hepatic encephalopathy, randomly were assigned to receive either oral rifaximin (140 patients) or placebo (159 patients) for six months.
When compared to placebo, rifaximin reduced the risk of breakthrough episodes of hepatic encephalopathy over a six-month treatment period (22.1% vs 45.9%, HR 0.42; 95% confidence interval, 0.28-0.64, P<0.001), as well as risk of hospitalization involving hepatic encephalopathy (13.6% vs 22.6%, HR 0.50; 95% CI, 0.29-0.87, P=0.01).
The incidence of adverse effects was similar in both groups. More than 90% of patients received concomitant lactulose therapy.
Bottom line: Rifaximin treatment delays the first breakthrough episode of hepatic encephalopathy during a six-month period; moreover, it significantly reduces the associated risk for hospitalization.
Citation: Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
Early Tracheotomy Does Not Decrease the Incidence of Ventilator-Associated Pneumonia in ICU Patients
Clinical question: Does early tracheotomy decrease the incidence of ventilator-associated pneumonia (VAP) in mechanically ventilated adult ICU patients without existing lung infection?
Background: There is considerable variation in timing and incidence of tracheotomy across ICUs. Observational studies have reported that tracheotomy performed earlier might be associated with quicker weaning from mechanical ventilation; however, randomized, controlled trials have failed to confirm this finding.
Study design: Multicenter randomized controlled trial.
Setting: Adult ICU in Italy.
Synopsis: Between 2004 and 2008, 600 mechanically ventilated patients without lung infection were enrolled from 12 adult ICUs in Italy. Of these patients, 419 were randomized to early tracheotomy performed six to eight days after intubation (N=209) or to late tracheotomy performed 13-15 days after intubation (N=210).
VAP was diagnosed in 14% of patients in the early tracheotomy group, compared with 21% in the late tracheotomy group (P=0.07). Although the number of ventilator-free and ICU-free days was higher in the early tracheotomy group, long-term outcomes did not differ between the two groups.
Only 69% of patients in the early tracheotomy group and 57% of patients in the late tracheotomy group received tracheotomy, but all the patients were included in the final analysis due to the intention-to-treat design of the study, which might have diluted the effect of the intervention. In addition, the smaller sample size may have prevented the study from reaching statistical significance.
Bottom line: Early tracheotomy does not significantly decrease the incidence of VAP as compared to late tracheotomy.
Citation: Terragni PP, Antonelli M, Fumagalli R, et al. Early vs. late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients. JAMA. 2010;303(15): 1483-1489.
Coagulopathy in Cirrhotic Patients Is Not Protective against VTE
Clinical question: Does the degree of INR elevation affect the incidence of VTE in hospitalized patients with cirrhosis?
Background: Chronic liver disease (CLD) and subsequent development of cirrhosis renders patients coagulopathic. Historically, this has provided a sense of security to clinicians that these patients inherently possess a decreased VTE risk.
Study design: Retrospective cohort study.
Setting: University of Missouri Medical Center in Columbia.
Synopsis: Chart review of patients admitted with CLD and cirrhosis from Jan. 1, 2000, and Jan. 31, 2007, demonstrated an incidence rate of VTE of 6.3%, which is much higher than previous reports.
Most patients with CLD received no thrombosis prophylaxis; notably, there was no difference in VTE incidence between subgroups who received prophylaxis and those who did not. Five percent of VTE cases occurred in patients with an INR exceeding 1.6, with Child-Pugh class C patients having the highest thromboembolism incidence.
This retrospective chart review was limited by information and reporting bias and the inability to control confounding variables. Less than half of the patients were screened for VTE, which means that the true incidence of thrombus could actually be higher. Further studies are needed to provide proper risk assessment.
Bottom line: Patients with CLD and cirrhosis are at risk for VTE, even in the setting of coagulopathy, and might require VTE prophylaxis.
Citation: Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145-1149.
Pulmonary Embolism Can Be Safely Excluded Using Age-Adjusted D-dimer Cut-off Value
Clinical question: Does the new age-adjusted D-dimer cutoff value in older patients safely exclude pulmonary embolism (PE)?
Background: D-dimer is a useful blood test to exclude PE; however, D-dimer concentration increases with age, and hence the current cutoff of 500µg/l used in excluding a PE becomes less specific in older patients.
Study design: Retrospective multicenter cohort study.
Setting: General and teaching hospitals in Belgium, Switzerland, France, and Netherlands.
Synopsis: The study included 5,132 consecutive patients with clinically suspected PE. Patients were distributed into a derivation set (N=1,331) and two independent validation sets (N1=2,151 and N2=1,643). For patients older than 50, the use of the new age-adjusted D-dimer cutoff (patient age multiplied by 10µg/l) resulted in a combined 11% increase in the number of patients with negative results. This increase was more prominent in patients aged older than 70 (13% to 16%).
The new age-adjusted D-dimer cutoff point failed to detect PE in 0.2% of cases in the derivation set and in 0.6% and 0.3% of cases in the two validation sets, respectively. However, despite external validation, prospective studies are needed before implementing such criteria into clinical practice.
Bottom line: The age-adjusted D-dimer combined with clinical probability greatly increases the proportion of older patients in whom PE can be safely excluded.
Citation: Douma RA, Le Gal G, Söhne M, et al. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010;340:c1475.
Antihypertensive Drugs After Stroke Does Not Impact Cardiovascular Event Rate or Mortality at Six Months
Clinical question: Should antihypertensive medications be continued during the immediate post-stroke period in patients who previously were on such therapy?
Background: More than 50% of patients suffering from acute stroke are on antihypertensive therapy prior to admission. However, efficacy of such therapy in reducing cardiovascular event rates and mortality in the immediate post-stroke period is not well studied.
Study design: Prospective, randomized, open-blinded-endpoint trial.
Setting: Forty-nine UK National Institute for Health Research Stroke Centers.
Synopsis: From January 2003 and March 2009, 763 patients with pre-existing hypertension and diagnosis of mild to moderate acute stroke were recruited and assigned to continue or stop antihypertension drugs. The time limit for inclusion into the study was within 48 hours of the stroke and the endpoint was death or dependency (modified Rankin Scale >3) at the end of two weeks.
There was a statistically significant difference in the two groups at two weeks in both systolic and diastolic pressures, 13 mmHg and 8mmHg, respectively (P<0.0001). Seventy-two of 379 patients in the continuation group and 82 of 384 patients in the stop group reached the primary endpoint (P=0.3). The latter point is a major limitation to this trial, since it was underpowered because of early termination to detect differences in outcomes.
Bottom line: Antihypertensive therapy during the immediate post-stroke period did not reduce two-week death or dependency, cardiovascular event rate, or mortality at six months.
Citation: Robinson TG, Potter JF, Ford GA, et al. Effects of antihypertensive treatment after acute stroke in the continue or stop post-stroke antihypertensives collaborative study (COSSACS): a prospective, randomized, open, blinded-endpoint trial. Lancet Neurol. 2010;9:767-775.
All Lumens from Multi-Lumen Catheters Should Be Cultured to Diagnose Catheter-Related Bloodstream Infections
Clinical question: Do all lumens from multi-lumen catheters need to be cultured to best diagnose catheter-related bloodstream infections (CRBSIs)?
Background: The recent Infectious Diseases Society of America’s “Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infections” has not conclusively established the number of lumens to culture from multi-lumen catheters when attempting to diagnose CRBSIs.
Study design: Retrospective cohort study.
Setting: Large teaching institution in Spain.
Synopsis: From January 2003 until May 2009, 154 patients, mostly men, with a mean age of 58.1 years, were recruited to participate in the study. Of these, 171 episodes of proven CRBSIs were detected in 154 subjects. Of the 171 tested catheters (112 double lumen and 59 triple lumen), testing only one lumen from double catheters would have led to 27.2% of missed cases for CRBSIs. Additionally, testing only two or one lumen from triple lumen catheters would have led to 15.8% and 37.3% of missed cases for CRBSIs, respectively.
The study was limited by being conducted at a single test site and the need to withdraw catheters to perform endoluminal brushing and semi-quantitative techniques. Though diagnostic yield might significantly improve by culturing all multi-lumen sites, hospitalists should consider the time and cost expenditure for testing from more than one lumen.
Bottom line: Culturing all lumens from multi-lumen catheters could greatly increase diagnostic yield in CRBSIs.
Citation: Guembe M, Rodríguez-Créixems M, Sánchez-Carrillo C, Pérez-Parra A, Martín-Rabadán P, Bouza E. How many lumens should be cultured in the conservative diagnosis of catheter-related bloodstream infections? CID. 2010;50(12):1575-1579.
Early Anticoagulation Improves Survival after Acute PE
Clinical question: Does the timing of initial heparinization reduce mortality in patients with acute symptomatic PE?
Background: Acute PE is rapidly fatal if not diagnosed and treated. Studies have shown that intravenous heparin improves overall survival for patients with PE, and therapeutic anticoagulation reduces rates of recurrent VTE. However, studies investigating the relation between time to achieve therapeutic anticoagulation and mortality or PE recurrence are limited.
Study design: Retrospective cohort study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: From June 2002 and September 2005, 400 patients were identified with PE using retrospective data from Mayo Clinic’s electronic medical records. Patients who received heparin in the ED had lower in-hospital mortality (OR 0.20, 95% CI, 0.06-0.69) and 30-day mortality (OR 0.25, 95% CI, 0.12-0.55) compared with patients who received heparin after admission. Similarly, patients who achieved a therapeutic aPTT within 24 hours also had lower 30-day mortality (OR 0.34, 95% CI, 0.14-0.84). Patients with COPD and malignancies had higher in-hospital and 30-day mortality, respectively.
Bottom line: It is difficult to draw a causal relationship from a retrospective review, but hospitalists should start immediate anticoagulation therapy when a PE is suspected.
Citation: Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6): 1382-1390. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk of infection with arterial and central venous catheters
- Rifaximin and prevention of hepatic encephalopathy
- Tracheotomy to prevent ventilator-associated pneumonia
- Coagulopathy and risk of VTE in patients with cirrhosis
- Use of age-adjusted D-dimer for PE diagnosis
- Continuation of anti-hypertensive medications after stroke
- Number of lumen cultures and detection of CRBSIs
- Timing of anticoagulation and outcomes in PE
Arterial and Central Venous Catheters Have Similar Rates of Colonization and Blood Stream Infections
Clinical question: Are arterial catheters (ACs) safer than central venous catheters (CVCs) in terms of colonization and catheter-related infections?
Background: Unlike CVCs, only a few studies have addressed blood-stream infections (BSI) related to AC usage, probably due to the traditional perception that ACs pose a lesser risk of colonization and BSI than CVC.
Study design: Randomized, controlled trial.
Setting: Three university hospitals and two general hospitals in France.
Synopsis: The study included 3,532 catheters (1,915 CVC and 1,617 AC) with 27,541 catheter-days from seven ICU settings. The same standard procedures were followed for catheter insertion and site dressing change at the various centers. Catheters were removed when they no longer were needed or when catheter-related infection (CRI) was suspected.
Colonization and CRI rates were similar in both arterial and venous catheters: 7.9% vs. 9.6% and 0.68% vs. 0.94%, respectively. The daily risk of colonization over time was stable for CVC, but appeared to increase for AC.
One important limitation to this study is that many patients had both arterial and venous catheters, leading to difficulty attributing infection to either one. Hospitalists caring for ICU patients should weigh the risks and benefits of prolonged use of AC due to similar rates of colonization and CRI as CVC.
Bottom line: Arterial and central venous catheters are equally prone to colonization and cause similar rates of CRI, but AC daily risk tends to increase with time; thus, AC should receive the same precautions as CVC.
Citation: Lucet JC, Bouadma L, Zahar JR, et. al. Infectious risk associated with arterial catheters compared with central venous catheters. Crit Care Med. 2010;38(4):1030-1005.
Rifaximin Prevents Recurrence of Hepatic Encephalopathy Episodes and Reduces Associated Risk for Hospitalization
Clinical question: What is the efficacy of rifaximin for the prevention of hepatic encephalopathy?
Background: Hepatic encephalopathy is a chronic, debilitating complication of liver cirrhosis. The efficacy of treatment of acute episodes with rifaximin is well documented in the literature; however, prevention of such episodes using rifaximin is poorly studied.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Seventy centers in the U.S., Canada, and Russia.
Synopsis: A total of 299 chronic liver disease patients, in remission from recurrent hepatic encephalopathy, randomly were assigned to receive either oral rifaximin (140 patients) or placebo (159 patients) for six months.
When compared to placebo, rifaximin reduced the risk of breakthrough episodes of hepatic encephalopathy over a six-month treatment period (22.1% vs 45.9%, HR 0.42; 95% confidence interval, 0.28-0.64, P<0.001), as well as risk of hospitalization involving hepatic encephalopathy (13.6% vs 22.6%, HR 0.50; 95% CI, 0.29-0.87, P=0.01).
The incidence of adverse effects was similar in both groups. More than 90% of patients received concomitant lactulose therapy.
Bottom line: Rifaximin treatment delays the first breakthrough episode of hepatic encephalopathy during a six-month period; moreover, it significantly reduces the associated risk for hospitalization.
Citation: Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
Early Tracheotomy Does Not Decrease the Incidence of Ventilator-Associated Pneumonia in ICU Patients
Clinical question: Does early tracheotomy decrease the incidence of ventilator-associated pneumonia (VAP) in mechanically ventilated adult ICU patients without existing lung infection?
Background: There is considerable variation in timing and incidence of tracheotomy across ICUs. Observational studies have reported that tracheotomy performed earlier might be associated with quicker weaning from mechanical ventilation; however, randomized, controlled trials have failed to confirm this finding.
Study design: Multicenter randomized controlled trial.
Setting: Adult ICU in Italy.
Synopsis: Between 2004 and 2008, 600 mechanically ventilated patients without lung infection were enrolled from 12 adult ICUs in Italy. Of these patients, 419 were randomized to early tracheotomy performed six to eight days after intubation (N=209) or to late tracheotomy performed 13-15 days after intubation (N=210).
VAP was diagnosed in 14% of patients in the early tracheotomy group, compared with 21% in the late tracheotomy group (P=0.07). Although the number of ventilator-free and ICU-free days was higher in the early tracheotomy group, long-term outcomes did not differ between the two groups.
Only 69% of patients in the early tracheotomy group and 57% of patients in the late tracheotomy group received tracheotomy, but all the patients were included in the final analysis due to the intention-to-treat design of the study, which might have diluted the effect of the intervention. In addition, the smaller sample size may have prevented the study from reaching statistical significance.
Bottom line: Early tracheotomy does not significantly decrease the incidence of VAP as compared to late tracheotomy.
Citation: Terragni PP, Antonelli M, Fumagalli R, et al. Early vs. late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients. JAMA. 2010;303(15): 1483-1489.
Coagulopathy in Cirrhotic Patients Is Not Protective against VTE
Clinical question: Does the degree of INR elevation affect the incidence of VTE in hospitalized patients with cirrhosis?
Background: Chronic liver disease (CLD) and subsequent development of cirrhosis renders patients coagulopathic. Historically, this has provided a sense of security to clinicians that these patients inherently possess a decreased VTE risk.
Study design: Retrospective cohort study.
Setting: University of Missouri Medical Center in Columbia.
Synopsis: Chart review of patients admitted with CLD and cirrhosis from Jan. 1, 2000, and Jan. 31, 2007, demonstrated an incidence rate of VTE of 6.3%, which is much higher than previous reports.
Most patients with CLD received no thrombosis prophylaxis; notably, there was no difference in VTE incidence between subgroups who received prophylaxis and those who did not. Five percent of VTE cases occurred in patients with an INR exceeding 1.6, with Child-Pugh class C patients having the highest thromboembolism incidence.
This retrospective chart review was limited by information and reporting bias and the inability to control confounding variables. Less than half of the patients were screened for VTE, which means that the true incidence of thrombus could actually be higher. Further studies are needed to provide proper risk assessment.
Bottom line: Patients with CLD and cirrhosis are at risk for VTE, even in the setting of coagulopathy, and might require VTE prophylaxis.
Citation: Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145-1149.
Pulmonary Embolism Can Be Safely Excluded Using Age-Adjusted D-dimer Cut-off Value
Clinical question: Does the new age-adjusted D-dimer cutoff value in older patients safely exclude pulmonary embolism (PE)?
Background: D-dimer is a useful blood test to exclude PE; however, D-dimer concentration increases with age, and hence the current cutoff of 500µg/l used in excluding a PE becomes less specific in older patients.
Study design: Retrospective multicenter cohort study.
Setting: General and teaching hospitals in Belgium, Switzerland, France, and Netherlands.
Synopsis: The study included 5,132 consecutive patients with clinically suspected PE. Patients were distributed into a derivation set (N=1,331) and two independent validation sets (N1=2,151 and N2=1,643). For patients older than 50, the use of the new age-adjusted D-dimer cutoff (patient age multiplied by 10µg/l) resulted in a combined 11% increase in the number of patients with negative results. This increase was more prominent in patients aged older than 70 (13% to 16%).
The new age-adjusted D-dimer cutoff point failed to detect PE in 0.2% of cases in the derivation set and in 0.6% and 0.3% of cases in the two validation sets, respectively. However, despite external validation, prospective studies are needed before implementing such criteria into clinical practice.
Bottom line: The age-adjusted D-dimer combined with clinical probability greatly increases the proportion of older patients in whom PE can be safely excluded.
Citation: Douma RA, Le Gal G, Söhne M, et al. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010;340:c1475.
Antihypertensive Drugs After Stroke Does Not Impact Cardiovascular Event Rate or Mortality at Six Months
Clinical question: Should antihypertensive medications be continued during the immediate post-stroke period in patients who previously were on such therapy?
Background: More than 50% of patients suffering from acute stroke are on antihypertensive therapy prior to admission. However, efficacy of such therapy in reducing cardiovascular event rates and mortality in the immediate post-stroke period is not well studied.
Study design: Prospective, randomized, open-blinded-endpoint trial.
Setting: Forty-nine UK National Institute for Health Research Stroke Centers.
Synopsis: From January 2003 and March 2009, 763 patients with pre-existing hypertension and diagnosis of mild to moderate acute stroke were recruited and assigned to continue or stop antihypertension drugs. The time limit for inclusion into the study was within 48 hours of the stroke and the endpoint was death or dependency (modified Rankin Scale >3) at the end of two weeks.
There was a statistically significant difference in the two groups at two weeks in both systolic and diastolic pressures, 13 mmHg and 8mmHg, respectively (P<0.0001). Seventy-two of 379 patients in the continuation group and 82 of 384 patients in the stop group reached the primary endpoint (P=0.3). The latter point is a major limitation to this trial, since it was underpowered because of early termination to detect differences in outcomes.
Bottom line: Antihypertensive therapy during the immediate post-stroke period did not reduce two-week death or dependency, cardiovascular event rate, or mortality at six months.
Citation: Robinson TG, Potter JF, Ford GA, et al. Effects of antihypertensive treatment after acute stroke in the continue or stop post-stroke antihypertensives collaborative study (COSSACS): a prospective, randomized, open, blinded-endpoint trial. Lancet Neurol. 2010;9:767-775.
All Lumens from Multi-Lumen Catheters Should Be Cultured to Diagnose Catheter-Related Bloodstream Infections
Clinical question: Do all lumens from multi-lumen catheters need to be cultured to best diagnose catheter-related bloodstream infections (CRBSIs)?
Background: The recent Infectious Diseases Society of America’s “Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infections” has not conclusively established the number of lumens to culture from multi-lumen catheters when attempting to diagnose CRBSIs.
Study design: Retrospective cohort study.
Setting: Large teaching institution in Spain.
Synopsis: From January 2003 until May 2009, 154 patients, mostly men, with a mean age of 58.1 years, were recruited to participate in the study. Of these, 171 episodes of proven CRBSIs were detected in 154 subjects. Of the 171 tested catheters (112 double lumen and 59 triple lumen), testing only one lumen from double catheters would have led to 27.2% of missed cases for CRBSIs. Additionally, testing only two or one lumen from triple lumen catheters would have led to 15.8% and 37.3% of missed cases for CRBSIs, respectively.
The study was limited by being conducted at a single test site and the need to withdraw catheters to perform endoluminal brushing and semi-quantitative techniques. Though diagnostic yield might significantly improve by culturing all multi-lumen sites, hospitalists should consider the time and cost expenditure for testing from more than one lumen.
Bottom line: Culturing all lumens from multi-lumen catheters could greatly increase diagnostic yield in CRBSIs.
Citation: Guembe M, Rodríguez-Créixems M, Sánchez-Carrillo C, Pérez-Parra A, Martín-Rabadán P, Bouza E. How many lumens should be cultured in the conservative diagnosis of catheter-related bloodstream infections? CID. 2010;50(12):1575-1579.
Early Anticoagulation Improves Survival after Acute PE
Clinical question: Does the timing of initial heparinization reduce mortality in patients with acute symptomatic PE?
Background: Acute PE is rapidly fatal if not diagnosed and treated. Studies have shown that intravenous heparin improves overall survival for patients with PE, and therapeutic anticoagulation reduces rates of recurrent VTE. However, studies investigating the relation between time to achieve therapeutic anticoagulation and mortality or PE recurrence are limited.
Study design: Retrospective cohort study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: From June 2002 and September 2005, 400 patients were identified with PE using retrospective data from Mayo Clinic’s electronic medical records. Patients who received heparin in the ED had lower in-hospital mortality (OR 0.20, 95% CI, 0.06-0.69) and 30-day mortality (OR 0.25, 95% CI, 0.12-0.55) compared with patients who received heparin after admission. Similarly, patients who achieved a therapeutic aPTT within 24 hours also had lower 30-day mortality (OR 0.34, 95% CI, 0.14-0.84). Patients with COPD and malignancies had higher in-hospital and 30-day mortality, respectively.
Bottom line: It is difficult to draw a causal relationship from a retrospective review, but hospitalists should start immediate anticoagulation therapy when a PE is suspected.
Citation: Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6): 1382-1390. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Risk of infection with arterial and central venous catheters
- Rifaximin and prevention of hepatic encephalopathy
- Tracheotomy to prevent ventilator-associated pneumonia
- Coagulopathy and risk of VTE in patients with cirrhosis
- Use of age-adjusted D-dimer for PE diagnosis
- Continuation of anti-hypertensive medications after stroke
- Number of lumen cultures and detection of CRBSIs
- Timing of anticoagulation and outcomes in PE
Arterial and Central Venous Catheters Have Similar Rates of Colonization and Blood Stream Infections
Clinical question: Are arterial catheters (ACs) safer than central venous catheters (CVCs) in terms of colonization and catheter-related infections?
Background: Unlike CVCs, only a few studies have addressed blood-stream infections (BSI) related to AC usage, probably due to the traditional perception that ACs pose a lesser risk of colonization and BSI than CVC.
Study design: Randomized, controlled trial.
Setting: Three university hospitals and two general hospitals in France.
Synopsis: The study included 3,532 catheters (1,915 CVC and 1,617 AC) with 27,541 catheter-days from seven ICU settings. The same standard procedures were followed for catheter insertion and site dressing change at the various centers. Catheters were removed when they no longer were needed or when catheter-related infection (CRI) was suspected.
Colonization and CRI rates were similar in both arterial and venous catheters: 7.9% vs. 9.6% and 0.68% vs. 0.94%, respectively. The daily risk of colonization over time was stable for CVC, but appeared to increase for AC.
One important limitation to this study is that many patients had both arterial and venous catheters, leading to difficulty attributing infection to either one. Hospitalists caring for ICU patients should weigh the risks and benefits of prolonged use of AC due to similar rates of colonization and CRI as CVC.
Bottom line: Arterial and central venous catheters are equally prone to colonization and cause similar rates of CRI, but AC daily risk tends to increase with time; thus, AC should receive the same precautions as CVC.
Citation: Lucet JC, Bouadma L, Zahar JR, et. al. Infectious risk associated with arterial catheters compared with central venous catheters. Crit Care Med. 2010;38(4):1030-1005.
Rifaximin Prevents Recurrence of Hepatic Encephalopathy Episodes and Reduces Associated Risk for Hospitalization
Clinical question: What is the efficacy of rifaximin for the prevention of hepatic encephalopathy?
Background: Hepatic encephalopathy is a chronic, debilitating complication of liver cirrhosis. The efficacy of treatment of acute episodes with rifaximin is well documented in the literature; however, prevention of such episodes using rifaximin is poorly studied.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Seventy centers in the U.S., Canada, and Russia.
Synopsis: A total of 299 chronic liver disease patients, in remission from recurrent hepatic encephalopathy, randomly were assigned to receive either oral rifaximin (140 patients) or placebo (159 patients) for six months.
When compared to placebo, rifaximin reduced the risk of breakthrough episodes of hepatic encephalopathy over a six-month treatment period (22.1% vs 45.9%, HR 0.42; 95% confidence interval, 0.28-0.64, P<0.001), as well as risk of hospitalization involving hepatic encephalopathy (13.6% vs 22.6%, HR 0.50; 95% CI, 0.29-0.87, P=0.01).
The incidence of adverse effects was similar in both groups. More than 90% of patients received concomitant lactulose therapy.
Bottom line: Rifaximin treatment delays the first breakthrough episode of hepatic encephalopathy during a six-month period; moreover, it significantly reduces the associated risk for hospitalization.
Citation: Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081.
Early Tracheotomy Does Not Decrease the Incidence of Ventilator-Associated Pneumonia in ICU Patients
Clinical question: Does early tracheotomy decrease the incidence of ventilator-associated pneumonia (VAP) in mechanically ventilated adult ICU patients without existing lung infection?
Background: There is considerable variation in timing and incidence of tracheotomy across ICUs. Observational studies have reported that tracheotomy performed earlier might be associated with quicker weaning from mechanical ventilation; however, randomized, controlled trials have failed to confirm this finding.
Study design: Multicenter randomized controlled trial.
Setting: Adult ICU in Italy.
Synopsis: Between 2004 and 2008, 600 mechanically ventilated patients without lung infection were enrolled from 12 adult ICUs in Italy. Of these patients, 419 were randomized to early tracheotomy performed six to eight days after intubation (N=209) or to late tracheotomy performed 13-15 days after intubation (N=210).
VAP was diagnosed in 14% of patients in the early tracheotomy group, compared with 21% in the late tracheotomy group (P=0.07). Although the number of ventilator-free and ICU-free days was higher in the early tracheotomy group, long-term outcomes did not differ between the two groups.
Only 69% of patients in the early tracheotomy group and 57% of patients in the late tracheotomy group received tracheotomy, but all the patients were included in the final analysis due to the intention-to-treat design of the study, which might have diluted the effect of the intervention. In addition, the smaller sample size may have prevented the study from reaching statistical significance.
Bottom line: Early tracheotomy does not significantly decrease the incidence of VAP as compared to late tracheotomy.
Citation: Terragni PP, Antonelli M, Fumagalli R, et al. Early vs. late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients. JAMA. 2010;303(15): 1483-1489.
Coagulopathy in Cirrhotic Patients Is Not Protective against VTE
Clinical question: Does the degree of INR elevation affect the incidence of VTE in hospitalized patients with cirrhosis?
Background: Chronic liver disease (CLD) and subsequent development of cirrhosis renders patients coagulopathic. Historically, this has provided a sense of security to clinicians that these patients inherently possess a decreased VTE risk.
Study design: Retrospective cohort study.
Setting: University of Missouri Medical Center in Columbia.
Synopsis: Chart review of patients admitted with CLD and cirrhosis from Jan. 1, 2000, and Jan. 31, 2007, demonstrated an incidence rate of VTE of 6.3%, which is much higher than previous reports.
Most patients with CLD received no thrombosis prophylaxis; notably, there was no difference in VTE incidence between subgroups who received prophylaxis and those who did not. Five percent of VTE cases occurred in patients with an INR exceeding 1.6, with Child-Pugh class C patients having the highest thromboembolism incidence.
This retrospective chart review was limited by information and reporting bias and the inability to control confounding variables. Less than half of the patients were screened for VTE, which means that the true incidence of thrombus could actually be higher. Further studies are needed to provide proper risk assessment.
Bottom line: Patients with CLD and cirrhosis are at risk for VTE, even in the setting of coagulopathy, and might require VTE prophylaxis.
Citation: Dabbagh O, Oza A, Prakash S, Sunna R, Saettele TM. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145-1149.
Pulmonary Embolism Can Be Safely Excluded Using Age-Adjusted D-dimer Cut-off Value
Clinical question: Does the new age-adjusted D-dimer cutoff value in older patients safely exclude pulmonary embolism (PE)?
Background: D-dimer is a useful blood test to exclude PE; however, D-dimer concentration increases with age, and hence the current cutoff of 500µg/l used in excluding a PE becomes less specific in older patients.
Study design: Retrospective multicenter cohort study.
Setting: General and teaching hospitals in Belgium, Switzerland, France, and Netherlands.
Synopsis: The study included 5,132 consecutive patients with clinically suspected PE. Patients were distributed into a derivation set (N=1,331) and two independent validation sets (N1=2,151 and N2=1,643). For patients older than 50, the use of the new age-adjusted D-dimer cutoff (patient age multiplied by 10µg/l) resulted in a combined 11% increase in the number of patients with negative results. This increase was more prominent in patients aged older than 70 (13% to 16%).
The new age-adjusted D-dimer cutoff point failed to detect PE in 0.2% of cases in the derivation set and in 0.6% and 0.3% of cases in the two validation sets, respectively. However, despite external validation, prospective studies are needed before implementing such criteria into clinical practice.
Bottom line: The age-adjusted D-dimer combined with clinical probability greatly increases the proportion of older patients in whom PE can be safely excluded.
Citation: Douma RA, Le Gal G, Söhne M, et al. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010;340:c1475.
Antihypertensive Drugs After Stroke Does Not Impact Cardiovascular Event Rate or Mortality at Six Months
Clinical question: Should antihypertensive medications be continued during the immediate post-stroke period in patients who previously were on such therapy?
Background: More than 50% of patients suffering from acute stroke are on antihypertensive therapy prior to admission. However, efficacy of such therapy in reducing cardiovascular event rates and mortality in the immediate post-stroke period is not well studied.
Study design: Prospective, randomized, open-blinded-endpoint trial.
Setting: Forty-nine UK National Institute for Health Research Stroke Centers.
Synopsis: From January 2003 and March 2009, 763 patients with pre-existing hypertension and diagnosis of mild to moderate acute stroke were recruited and assigned to continue or stop antihypertension drugs. The time limit for inclusion into the study was within 48 hours of the stroke and the endpoint was death or dependency (modified Rankin Scale >3) at the end of two weeks.
There was a statistically significant difference in the two groups at two weeks in both systolic and diastolic pressures, 13 mmHg and 8mmHg, respectively (P<0.0001). Seventy-two of 379 patients in the continuation group and 82 of 384 patients in the stop group reached the primary endpoint (P=0.3). The latter point is a major limitation to this trial, since it was underpowered because of early termination to detect differences in outcomes.
Bottom line: Antihypertensive therapy during the immediate post-stroke period did not reduce two-week death or dependency, cardiovascular event rate, or mortality at six months.
Citation: Robinson TG, Potter JF, Ford GA, et al. Effects of antihypertensive treatment after acute stroke in the continue or stop post-stroke antihypertensives collaborative study (COSSACS): a prospective, randomized, open, blinded-endpoint trial. Lancet Neurol. 2010;9:767-775.
All Lumens from Multi-Lumen Catheters Should Be Cultured to Diagnose Catheter-Related Bloodstream Infections
Clinical question: Do all lumens from multi-lumen catheters need to be cultured to best diagnose catheter-related bloodstream infections (CRBSIs)?
Background: The recent Infectious Diseases Society of America’s “Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infections” has not conclusively established the number of lumens to culture from multi-lumen catheters when attempting to diagnose CRBSIs.
Study design: Retrospective cohort study.
Setting: Large teaching institution in Spain.
Synopsis: From January 2003 until May 2009, 154 patients, mostly men, with a mean age of 58.1 years, were recruited to participate in the study. Of these, 171 episodes of proven CRBSIs were detected in 154 subjects. Of the 171 tested catheters (112 double lumen and 59 triple lumen), testing only one lumen from double catheters would have led to 27.2% of missed cases for CRBSIs. Additionally, testing only two or one lumen from triple lumen catheters would have led to 15.8% and 37.3% of missed cases for CRBSIs, respectively.
The study was limited by being conducted at a single test site and the need to withdraw catheters to perform endoluminal brushing and semi-quantitative techniques. Though diagnostic yield might significantly improve by culturing all multi-lumen sites, hospitalists should consider the time and cost expenditure for testing from more than one lumen.
Bottom line: Culturing all lumens from multi-lumen catheters could greatly increase diagnostic yield in CRBSIs.
Citation: Guembe M, Rodríguez-Créixems M, Sánchez-Carrillo C, Pérez-Parra A, Martín-Rabadán P, Bouza E. How many lumens should be cultured in the conservative diagnosis of catheter-related bloodstream infections? CID. 2010;50(12):1575-1579.
Early Anticoagulation Improves Survival after Acute PE
Clinical question: Does the timing of initial heparinization reduce mortality in patients with acute symptomatic PE?
Background: Acute PE is rapidly fatal if not diagnosed and treated. Studies have shown that intravenous heparin improves overall survival for patients with PE, and therapeutic anticoagulation reduces rates of recurrent VTE. However, studies investigating the relation between time to achieve therapeutic anticoagulation and mortality or PE recurrence are limited.
Study design: Retrospective cohort study.
Setting: Mayo Clinic, Rochester, Minn.
Synopsis: From June 2002 and September 2005, 400 patients were identified with PE using retrospective data from Mayo Clinic’s electronic medical records. Patients who received heparin in the ED had lower in-hospital mortality (OR 0.20, 95% CI, 0.06-0.69) and 30-day mortality (OR 0.25, 95% CI, 0.12-0.55) compared with patients who received heparin after admission. Similarly, patients who achieved a therapeutic aPTT within 24 hours also had lower 30-day mortality (OR 0.34, 95% CI, 0.14-0.84). Patients with COPD and malignancies had higher in-hospital and 30-day mortality, respectively.
Bottom line: It is difficult to draw a causal relationship from a retrospective review, but hospitalists should start immediate anticoagulation therapy when a PE is suspected.
Citation: Smith SB, Geske JB, Maguire JM, Zane NA, Carter RE, Morgenthaler TI. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6): 1382-1390. TH
Concurrent Care
Let’s examine a documentation case for hospitalists providing daily care: A 65-year-old male patient is admitted with a left hip fracture. The patient also has hypertension and Type 2 diabetes, which might complicate his care. The orthopedic surgeon manages the patient’s perioperative course for the fracture while the hospitalist provides daily post-op care for hypertension and diabetes.
A common scenario is the hospitalist will provide concurrent care, along with a varying number of specialists, depending on the complexity of the patient’s presenting problems and existing comorbidities. Payors define concurrent care as more than one physician providing care to the same patient on the same date, or during the same hospitalization. Payors often consider two key principles before reimbursing concurrent care:
- Does the patient’s condition warrant more than one physician? and
- Are the services provided by each physician reasonable and necessary?1
When more than one medical condition exists and each physician actively treats the condition related to their expertise, each physician can demonstrate medical necessity. As in the above example, the orthopedic surgeon cares for the patient’s fracture while the hospitalist oversees diabetes and hypertension management. Claim submission follows the same logic. Report each subsequent hospital care code (99231-99233) with the corresponding diagnosis each physician primarily manages (i.e., orthopedic surgeon: 9923x with 820.8; hospitalist: 9923x with 250.00, 401.1).
When each physician assigns a different primary diagnosis code to the visit code, each is more likely to receive payment. Because each of these physicians are in different specialties and different provider groups, most payors do not require modifier 25 (separately identifiable E/M service on the same day as a procedure or other service) appended to the visit code. However, some managed-care payors require each physician to append modifier 25 to the concurrent E/M visit code (i.e., 99232-25) despite claim submission under different tax identification numbers.
Unfortunately, the physicians might not realize this until a claim rejection has been issued. Furthermore, payors might want to see the proof before rendering payment. In other words, they pay the first claim received and deny any subsequent claim in order to confirm medical necessity of the concurrent visit. Appeal denied such claims rejections with supporting documentation that distinguishes each physician visit, if possible. This assists the payors in understanding each physician’s contribution to care.
Reasons for Denial
Concurrent care services are more easily distinguished when separate diagnoses are reported with each service. Conversely, payors are likely to deny services that are hard to differentiate. Furthermore, payors frequently deny concurrent care services for the following reasons:
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate or overlap those of another provider without recognizable distinction.2
For example, a hospitalist might be involved in the post-op care of patients with fractures and no other identifiable chronic or acute conditions or complications. In these cases, the hospitalist’s continued involvement might constitute a facility policy (e.g., quality of care, risk reduction, etc.) rather than active clinical management. Claim submission could erroneously occur with each physician reporting 9923x for 820.8. Payors deny medically unnecessary services, or request refunds for inappropriate payments.
Hospitalists might attempt to negotiate other terms with the facility to account for the unpaid time and effort directed toward these types of cases.
Group Practice
Physicians in the same group practice with the same specialty designation must report, and are paid, as a single physician. Multiple visits to the same patient can occur on the same day by members of the same group (e.g., hospitalist A evaluates the patient in the morning, and hospitalist B reviews test results and the resulting course of treatment in the afternoon). However, only one subsequent hospital care service can be reported for the day.
The hospitalists should select the visit level representative of the combined services and submit one appropriately determined code (e.g., 99233), thereby capturing the medically necessary efforts of each physician. To complicate matters, the hospitalists must determine which name to report on the claim: the physician who provided the first encounter, or the physician who provided the most extensive or best-documented encounter.
Tracking productivity for these cases proves challenging. Some practices develop an internal accounting system and credit each physician for their medically necessary efforts (a labor-intensive task for administrators and physicians). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Medicare Benefit Policy Manual: Concurrent Care. Chapter 15, Section 30.E. CMS website. Available at: www.cms.gov/manuals/Downloads/bp102c15.pdf. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Physicians in Group Practice. Chapter 12, Section 30.6.5. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Pohlig, C. Daily care conundrums. The Hospitalist website. Available at: www.the-hospitalist.org/details/article/188735/Daily_Care_Conundrums_.html. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Hospital Visits Same Day But by Different Physicians. Chapter 12, Section 30.6.9.C. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Abraham M, Beebe M, Dalton J, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2010:15.
Let’s examine a documentation case for hospitalists providing daily care: A 65-year-old male patient is admitted with a left hip fracture. The patient also has hypertension and Type 2 diabetes, which might complicate his care. The orthopedic surgeon manages the patient’s perioperative course for the fracture while the hospitalist provides daily post-op care for hypertension and diabetes.
A common scenario is the hospitalist will provide concurrent care, along with a varying number of specialists, depending on the complexity of the patient’s presenting problems and existing comorbidities. Payors define concurrent care as more than one physician providing care to the same patient on the same date, or during the same hospitalization. Payors often consider two key principles before reimbursing concurrent care:
- Does the patient’s condition warrant more than one physician? and
- Are the services provided by each physician reasonable and necessary?1
When more than one medical condition exists and each physician actively treats the condition related to their expertise, each physician can demonstrate medical necessity. As in the above example, the orthopedic surgeon cares for the patient’s fracture while the hospitalist oversees diabetes and hypertension management. Claim submission follows the same logic. Report each subsequent hospital care code (99231-99233) with the corresponding diagnosis each physician primarily manages (i.e., orthopedic surgeon: 9923x with 820.8; hospitalist: 9923x with 250.00, 401.1).
When each physician assigns a different primary diagnosis code to the visit code, each is more likely to receive payment. Because each of these physicians are in different specialties and different provider groups, most payors do not require modifier 25 (separately identifiable E/M service on the same day as a procedure or other service) appended to the visit code. However, some managed-care payors require each physician to append modifier 25 to the concurrent E/M visit code (i.e., 99232-25) despite claim submission under different tax identification numbers.
Unfortunately, the physicians might not realize this until a claim rejection has been issued. Furthermore, payors might want to see the proof before rendering payment. In other words, they pay the first claim received and deny any subsequent claim in order to confirm medical necessity of the concurrent visit. Appeal denied such claims rejections with supporting documentation that distinguishes each physician visit, if possible. This assists the payors in understanding each physician’s contribution to care.
Reasons for Denial
Concurrent care services are more easily distinguished when separate diagnoses are reported with each service. Conversely, payors are likely to deny services that are hard to differentiate. Furthermore, payors frequently deny concurrent care services for the following reasons:
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate or overlap those of another provider without recognizable distinction.2
For example, a hospitalist might be involved in the post-op care of patients with fractures and no other identifiable chronic or acute conditions or complications. In these cases, the hospitalist’s continued involvement might constitute a facility policy (e.g., quality of care, risk reduction, etc.) rather than active clinical management. Claim submission could erroneously occur with each physician reporting 9923x for 820.8. Payors deny medically unnecessary services, or request refunds for inappropriate payments.
Hospitalists might attempt to negotiate other terms with the facility to account for the unpaid time and effort directed toward these types of cases.
Group Practice
Physicians in the same group practice with the same specialty designation must report, and are paid, as a single physician. Multiple visits to the same patient can occur on the same day by members of the same group (e.g., hospitalist A evaluates the patient in the morning, and hospitalist B reviews test results and the resulting course of treatment in the afternoon). However, only one subsequent hospital care service can be reported for the day.
The hospitalists should select the visit level representative of the combined services and submit one appropriately determined code (e.g., 99233), thereby capturing the medically necessary efforts of each physician. To complicate matters, the hospitalists must determine which name to report on the claim: the physician who provided the first encounter, or the physician who provided the most extensive or best-documented encounter.
Tracking productivity for these cases proves challenging. Some practices develop an internal accounting system and credit each physician for their medically necessary efforts (a labor-intensive task for administrators and physicians). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Medicare Benefit Policy Manual: Concurrent Care. Chapter 15, Section 30.E. CMS website. Available at: www.cms.gov/manuals/Downloads/bp102c15.pdf. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Physicians in Group Practice. Chapter 12, Section 30.6.5. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Pohlig, C. Daily care conundrums. The Hospitalist website. Available at: www.the-hospitalist.org/details/article/188735/Daily_Care_Conundrums_.html. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Hospital Visits Same Day But by Different Physicians. Chapter 12, Section 30.6.9.C. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Abraham M, Beebe M, Dalton J, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2010:15.
Let’s examine a documentation case for hospitalists providing daily care: A 65-year-old male patient is admitted with a left hip fracture. The patient also has hypertension and Type 2 diabetes, which might complicate his care. The orthopedic surgeon manages the patient’s perioperative course for the fracture while the hospitalist provides daily post-op care for hypertension and diabetes.
A common scenario is the hospitalist will provide concurrent care, along with a varying number of specialists, depending on the complexity of the patient’s presenting problems and existing comorbidities. Payors define concurrent care as more than one physician providing care to the same patient on the same date, or during the same hospitalization. Payors often consider two key principles before reimbursing concurrent care:
- Does the patient’s condition warrant more than one physician? and
- Are the services provided by each physician reasonable and necessary?1
When more than one medical condition exists and each physician actively treats the condition related to their expertise, each physician can demonstrate medical necessity. As in the above example, the orthopedic surgeon cares for the patient’s fracture while the hospitalist oversees diabetes and hypertension management. Claim submission follows the same logic. Report each subsequent hospital care code (99231-99233) with the corresponding diagnosis each physician primarily manages (i.e., orthopedic surgeon: 9923x with 820.8; hospitalist: 9923x with 250.00, 401.1).
When each physician assigns a different primary diagnosis code to the visit code, each is more likely to receive payment. Because each of these physicians are in different specialties and different provider groups, most payors do not require modifier 25 (separately identifiable E/M service on the same day as a procedure or other service) appended to the visit code. However, some managed-care payors require each physician to append modifier 25 to the concurrent E/M visit code (i.e., 99232-25) despite claim submission under different tax identification numbers.
Unfortunately, the physicians might not realize this until a claim rejection has been issued. Furthermore, payors might want to see the proof before rendering payment. In other words, they pay the first claim received and deny any subsequent claim in order to confirm medical necessity of the concurrent visit. Appeal denied such claims rejections with supporting documentation that distinguishes each physician visit, if possible. This assists the payors in understanding each physician’s contribution to care.
Reasons for Denial
Concurrent care services are more easily distinguished when separate diagnoses are reported with each service. Conversely, payors are likely to deny services that are hard to differentiate. Furthermore, payors frequently deny concurrent care services for the following reasons:
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate or overlap those of another provider without recognizable distinction.2
For example, a hospitalist might be involved in the post-op care of patients with fractures and no other identifiable chronic or acute conditions or complications. In these cases, the hospitalist’s continued involvement might constitute a facility policy (e.g., quality of care, risk reduction, etc.) rather than active clinical management. Claim submission could erroneously occur with each physician reporting 9923x for 820.8. Payors deny medically unnecessary services, or request refunds for inappropriate payments.
Hospitalists might attempt to negotiate other terms with the facility to account for the unpaid time and effort directed toward these types of cases.
Group Practice
Physicians in the same group practice with the same specialty designation must report, and are paid, as a single physician. Multiple visits to the same patient can occur on the same day by members of the same group (e.g., hospitalist A evaluates the patient in the morning, and hospitalist B reviews test results and the resulting course of treatment in the afternoon). However, only one subsequent hospital care service can be reported for the day.
The hospitalists should select the visit level representative of the combined services and submit one appropriately determined code (e.g., 99233), thereby capturing the medically necessary efforts of each physician. To complicate matters, the hospitalists must determine which name to report on the claim: the physician who provided the first encounter, or the physician who provided the most extensive or best-documented encounter.
Tracking productivity for these cases proves challenging. Some practices develop an internal accounting system and credit each physician for their medically necessary efforts (a labor-intensive task for administrators and physicians). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center in Philadelphia. She is faculty for SHM’s inpatient coding course.
References
- Medicare Benefit Policy Manual: Concurrent Care. Chapter 15, Section 30.E. CMS website. Available at: www.cms.gov/manuals/Downloads/bp102c15.pdf. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Physicians in Group Practice. Chapter 12, Section 30.6.5. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Pohlig, C. Daily care conundrums. The Hospitalist website. Available at: www.the-hospitalist.org/details/article/188735/Daily_Care_Conundrums_.html. Accessed July 9, 2010.
- Medicare Claims Processing Manual: Hospital Visits Same Day But by Different Physicians. Chapter 12, Section 30.6.9.C. CMS website. Available at: www.cms.gov/manuals/downloads/clm104c12.pdf. Accessed July 9, 2010.
- Abraham M, Beebe M, Dalton J, Evans D, Glenn R. Current Procedural Terminology Professional Edition. Chicago: American Medical Association Press; 2010:15.