User login
Evaluating patients with breast concerns: Lump, pain, and mastitis
The vast majority of symptomatic breast conditions are benign, with the most common symptoms being palpable mass and breast pain. Clinicians, including primary care clinicians and gynecologists, play a crucial role by performing the initial assessment and subsequent therapies and referrals and serve as the mediator between the specialists and by being the patient’s spokesperson. It is therefore important for clinicians to be aware of the various possible causes of these breast symptoms, to know which imaging tests to order, and also to understand the indications for biopsies and surgical referral.
Common types of breast lumps: Imaging workup and management
Accounting for 8% of women who present with breast symptoms, breast lump is the second most common symptom after breast pain.1 The positive likelihood ratio of finding breast cancer is highest among women with breast lumps compared with any other breast symptoms. Therefore, anxiety is related to this symptom, and a thorough evaluation is recommended.1 Cysts, fibroadenoma, and fat necrosis are 3 common benign causes of breast lumps.2
In this section, we review clinical presentation, imaging workup, and management strategies for common types of breast lumps.
CASE 1 Woman with tender breast lump
A 45-year-old woman presents with a breast lump of 6 months’ duration that is associated with a change in size with the menstrual cycle and pain. Clinical examination reveals a 4 x 4.5–cm mass in the right breast in the retroareolar region, which is smooth with some tenderness on palpation.
Breast cyst
According to the American College of Radiology appropriateness criteria for an adult woman 40 years of age or older who presents with a palpable breast mass, the initial imaging study is diagnostic mammography with or without digital tomosynthesis, usually followed by a directed ultrasound. If the mammogram is suspicious or highly suggestive of malignancy, or in cases where the mammogram does not show an abnormality, the next recommended step is breast ultrasonography. Any suspicious findings on ultrasound or mammogram should be followed by an image guided biopsy. Ultrasonography also may be appropriate if the mammogram findings are benign or probably benign.
For an adult woman younger than age 30 who presents with a palpable breast mass, breast ultrasonography is the appropriate initial imaging study. If the ultrasound is suspicious or highly suggestive of malignancy, then performing diagnostic mammography with or without digital tomosynthesis or ultrasound-guided core needle biopsy of the mass are both considered appropriate. However, no further imaging is recommended if the ultrasound is benign, probably benign, or negative. Breast ultrasonography or mammography is appropriate as the initial imaging test for adult women aged 30 to 39 years who present with a palpable breast mass.3,4
Approximately 50% of women after age 30 may develop fibrocystic breast disease, and 20% of them can present with pain or lump due to a macrocysts. Simple cysts must be distinguished from complex cysts with the help of ultrasound as the latter are associated with 23% to 31% increased risk of malignancy.
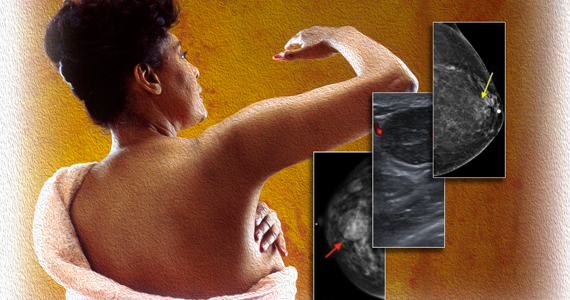
In this 45-year-old patient, the initial mammogram demonstrated a circumscribed mass underneath the area of palpable concern (FIGURE 1a, 1b). Targeted breast ultrasonography was performed for further assessment, which depicted the mass as a benign simple cyst (FIGURE 1c).
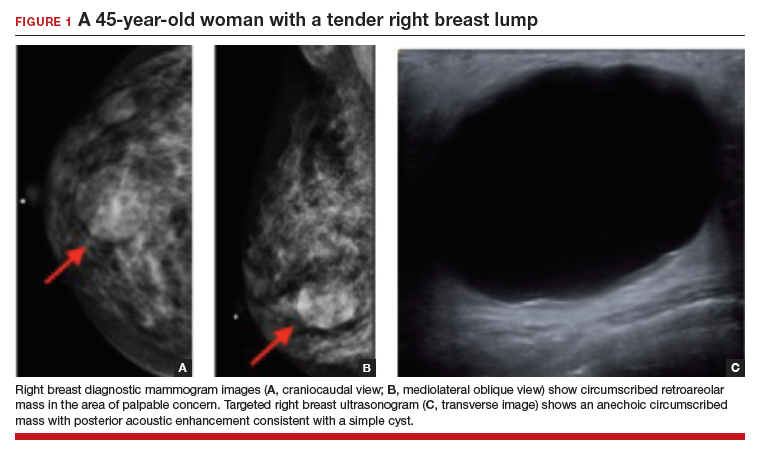
On ultrasound, a simple cyst is an anechoic, well-circumscribed mass with a thin capsule and with increased through transmission. Patients with small and asymptomatic simple cysts do not need imaging follow-up and can return for routine screening mammograms.
A breast surgeon, radiologist, or gynecologist can perform percutaneous aspiration if a cyst is large and symptomatic. A cyst with low-level internal echoes, fluid-fluid, or fluid-debris levels is considered a complicated cyst. Differential diagnosis also includes hematoma, fat necrosis, abscess, and galactocele, depending on the clinical presentation. Fine-needle aspiration or short-interval follow-up5,6 is appropriate for complicated cysts, while incision and drainage is indicated in patients with infected cysts and abscesses. A cyst with a solid component is considered a cystic, solid mass, and core needle biopsy is recommended. The differential diagnosis for cysts with solid components includes intracystic papilloma, papillary carcinoma, ductal carcinoma in situ, and necrotic cancers.5,6
Continue to: CASE 2 Painless breast mass in a young woman...
CASE 2 Painless breast mass in a young woman
A 22-year-old woman presents with a 2-month history of breast lump, which is not associated with pain or nipple discharge. On examination, there is a 2 x 2–cm mass in the right breast at 12 o’clock, 2 cm from the nipple, which is mobile, smooth, and nontender on palpation.
Fibroadenoma
In this 22-year-old, the initial imaging of choice is breast ultrasonography. Breast ultrasonography can differentiate a cystic mass from a solid mass, and it does not involve radiation. Right breast targeted ultrasound showed a circumscribed oval homogeneous hypoechoic mass that is wider than tall (FIGURE 2). The patient desired surgical removal, and a pre-lumpectomy core needle biopsy revealed a fibroadenoma.
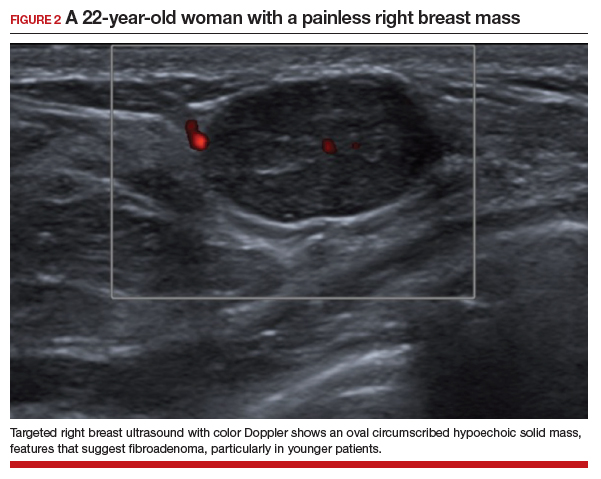
Fibroadenoma is the most common benign tumor of the breast. It is most often encountered in premenopausal women. Patients present with a painless breast lump, which is smooth and mobile on palpation. Fibroadenoma can be followed expectantly with repeat ultrasound (to assess over time for growth) if it is small and asymptomatic. No further action is needed if it remains stable. If a patient desires surgical excision, a core needle biopsy is usually performed before lumpectomy.
Excisional biopsy or removal of the mass is recommended if the mass is greater than 3 or 4 cm, is symptomatic, or if there is an increase in size that raises clinical concern for phyllodes tumor. Imaging features that are concerning for phyllodes tumors are size greater than 3 cm, indistinct or microlobulated margins, and heterogeneous echo pattern.7,8 In cases in which the imaging features are concerning for phyllodes tumor and a core needle biopsy is not definitive, wide surgical excision is recommended for definitive diagnosis.8
CASE 3 Patient develops breast mass post-surgery
A 45-year-old woman presents with a tender left breast mass that she noticed 2 months after breast reduction surgery. It has been increasing in size since. On clinical examination, a 4 x 4–cm mass is found at the surgical scar site, which is indurated on palpation and tender.
Fat necrosis
In this 45-year-old, the initial test of choice is diagnostic mammography, which showed a somewhat circumscribed area with fat under the palpable marker (FIGURE 3a). Breast ultrasonography was performed for further evaluation, which was inconclusive as the ultrasound showed ill-defined areas of mixed echogenicity (FIGURE 3b). Breast magnetic resonance imaging (MRI) clearly demonstrated fat necrosis in the area of the palpable lump (FIGURE 3c).

Fat necrosis of the breast is an inflammatory process that is seen after breast trauma or surgery. It can present as an incidental mammogram finding or a palpable mass. The patient may give a history of trauma, breast reduction surgery, or breast cancer surgery followed by radiation treatment. On clinical examination, fat necrosis occasionally can present as a firm mass with skin retraction or swelling concerning for cancer. Imaging features are variable depending on the stage of fat necrosis and inflammation.9-11
A mammogram may demonstrate a circumscribed fat-containing mass, an ill-defined mass, asymmetry or calcified oil cyst, and dystrophic calcifications. On ultrasound, fat necrosis can appear as anechoic or hypoechoic or as a complicated cyst or a mixed cystic, solid mass. MRI demonstrates a circumscribed or irregular fat-containing mass, with or without enhancement, and architectural distortion.
When the imaging features are clearly benign—for example, a circumscribed fat-containing mass on mammogram or on ultrasound or, on MRI, marked hypointensity of fat in the center of a circumscribed mass when compared with surrounding fat (keyhole sign)—no further follow-up is needed. When the imaging features are indeterminate, however, a short-interval follow-up can be considered. In cases with irregular fat-containing mass with enhancement, core needle biopsy is indicated to exclude cancer. If the workup remains inconclusive and the level of clinical suspicion is high, surgical excision can be performed for a definitive diagnosis.12
Continue to: Investigating breast pain: Imaging workup and management...
Investigating breast pain: Imaging workup and management
Breast pain, or mastalgia, is the most common concern of women presenting to a breast clinic and accounts for approximately half of such encounters.13 Causes of breast pain include hormonal changes, fibrocystic changes, musculoskeletal causes (such as costochondritis), lack of support, infection, and injury. While mastalgia often causes patient concern, the risk of malignancy in a woman presenting with breast pain alone is low. Still, it is essential to rule out other findings suspicious for cancer (mass, skin changes, or nipple discharge) with a thorough history and breast examination.
In this section, we review clinical presentation, imaging workup, and management for breast pain.
CASE 4 Woman with noncyclic breast pain
A 26-year-old woman presents to the clinic with mastalgia. The pain is noncyclic and primarily located in the upper outer quadrant of her left breast. There is no history of breast cancer in her family. She has no suspicious findings on the breast examination.
Mastalgia
The test of choice for this 26-year-old with focal left breast pain is targeted breast ultrasound. The patient’s ultrasound image showed no suspicious findings or solid or cystic mass (FIGURE 4).
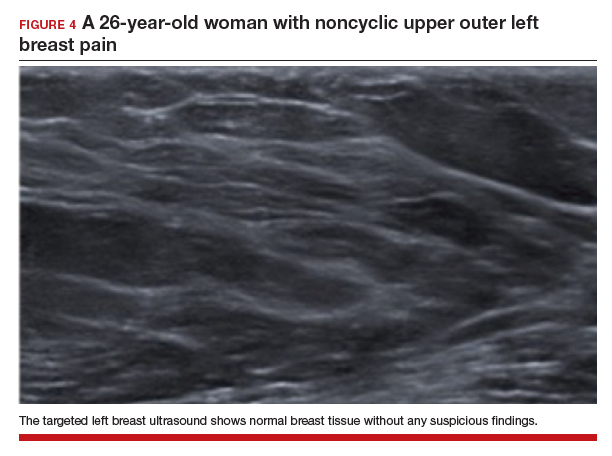
Two important characteristics of breast pain are whether it is noncyclical and whether it is focal. According to the American College of Radiology, no breast imaging is recommended for clinically insignificant cyclical, nonfocal (greater than 1 quadrant)/diffuse pain, as this type of mastalgia is not associated with malignancy.14
For patients age 40 or older, if they are not up to date with their annual screening mammogram, then a mammogram should be performed. An imaging workup is warranted for clinically significant mastalgia that is noncyclical and focal. Even then, no malignancy is identified in most patients with clinically significant mastalgia; in patients with breast pain as their only symptom, the prevalence of breast cancer is 0% to 3.0%.15-19
The initial imaging modality differs by patient age: younger than 30 years, ultrasonography; between 30 and 40 years, mammography or ultrasonography; and older than 40 years, mammography first followed by ultrasonography.14
Treatment of breast pain is primarily symptomatic, and evidence for specific treatments is generally lacking. Cyclical breast pain resolves spontaneously in 20% to 30% of women, while noncyclical pain responds poorly to treatment but resolves spontaneously in half of women.20 Reassurance is important and wearing a supportive bra often can alleviate breast pain. In addition, reducing caffeine intake can be helpful.
As a first-line treatment, both topical (diclofenac) and oral nonsteroidal anti-inflammatory drugs effectively can relieve breast pain. Supplements and herbal remedies (for example, evening primrose oil, vitamin E, flaxseed) have varying effectiveness and are of questionable benefit as few have trials to support their effectiveness.4 Danazol and tamoxifen have been shown to have some benefits but they also have adverse effects.20 Surgery does not play a role in the treatment of mastalgia.
CASE 5 Breastfeeding woman with breast pain
A 27-year-old postpartum woman presents with concerns for redness and pain in the upper inner left breast. She has been breastfeeding for the past few months. Breast examination demonstrates a 5-cm area of erythema and warmth but no fluctuance or masses.
Lactational mastitis
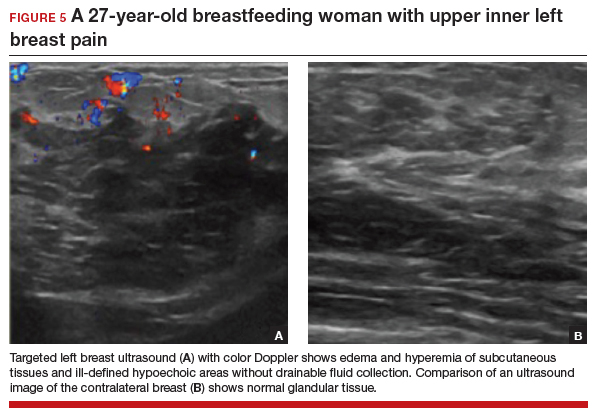
Targeted ultrasonography is the test of choice for this 27-year-old patient with focal breast pain, and the imaging revealed edema of subcutaneous tissues and ill-defined hypoechoic areas, likely inflamed fat lobules (FIGURE 5). These findings suggest uncomplicated lactational mastitis, which can be treated with antibiotics. Generally, the mastitis will improve within days of starting the antibiotics; if it does not improve, repeat examination and ultrasound should be performed to look for formation of an abscess that may require aspiration.
Continue to: CASE 6 Woman with painful periareolar mass...
CASE 6 Woman with painful periareolar mass
A 42-year-old perimenopausal woman describes having pain near the nipple of her right breast. She is a smoker and has no history of breast cancer in her family. Examination demonstrates a palpable, erythematous, painful, 3-cm periareolar fluctuant mass.
Nonpuerperal periareolar abscess
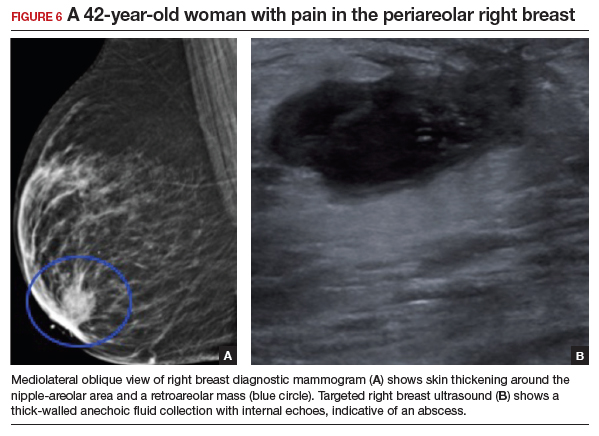
Appropriate initial imaging for this 42-year-old patient with focal pain is a diagnostic mammogram, which showed skin thickening and a retroareolar mass (FIGURE 6a). Further evaluation with targeted ultrasound showed a thick-walled anechoic collection with echoes compatible with an abscess (FIGURE 6b).
Mammographic findings in a patient with mastitis may be normal or demonstrate skin and trabecular thickening. Ultrasound imaging may show dilated ducts and heterogeneous tissue secondary to inflammation and edema without a discrete fluid collection. In cases with breast abscess, in addition to the mammographic findings described above, a mass, or an asymmetry, may be seen, most commonly in a subareolar location. On ultrasound, a hypoechoic collection with mobile debris, no internal flow on Doppler, and thick hypervascular walls can be seen with abscess, occasionally giving the appearance of a complicated cyst or a mixed cystic, solid mass.
The most important differential for mastitis is inflammatory breast cancer. Most cancers appear solid but can have central necrosis, mimicking a complicated cystic mass on ultrasound. The location for mastitis or abscess is most frequently subareolar. The presence of microcalcifications in a mass indicates the possibility of cancer.
Contrast-enhanced MRI can be helpful to differentiate between infection and cancer, with cancers showing initial early enhancement and washout kinetics compared with infected collections that show no enhancement or peripheral enhancement with a plateau or persistent enhancement curves. When clinical and imaging findings are unchanged after treatment of mastitis and abscesses, a core needle biopsy should be performed.21,22
There are 2 categories of mastitis and breast abscess: lactational and nonpuerperal (all mastitis that occurs outside the lactational period). The World Health Organization definition of puerperal mastitis includes pain, local redness, warmth and swelling of the breast (usually unilateral), fever, and malaise.4 Concerning etiology, epithelial lesions in the nipple area caused by breastfeeding can allow pathogens to enter and cause infection. The most common microorganism is Staphylococcus aureus.4 Continued emptying of the breast is important, combined with early antibiotic therapy (dicloxacillin is often the first line; if the patient is penicillin allergic, use a macrolide such as clindamycin). If no improvement is seen in 48 to 72 hours, imaging should be performed.
In most cases, continuation of breastfeeding is possible. If mastitis has evolved into an abscess in a lactating woman, it can be aspirated under ultrasound guidance. Incision and drainage should be avoided unless the abscess persists after multiple aspiration attempts, it is large, or if the overlying skin is thin or otherwise appears nonviable.
Nonpuerperal mastitis includes peripheral, periductal, and idiopathic granulomatous mastitis (IGM). Peripheral mastitis behaves like infections/abscesses in other soft tissues, responds well to treatment (antibiotics and percutaneous drainage), and is less likely to recur than periductal mastitis and IGM.21,23
Periductal mastitis and abscess, also known as Zuska disease, has a pathogenesis distinct from other forms of mastitis. Squamous metaplasia of the usual cuboidal epithelium of the breast ducts leads to keratin plugging that can cause infection.23 Risk factors include obesity, smoking, and macromastia. The typical presentation of Zuska disease is a woman with a history of chronic smoking and/or a congenital cleft in the central nipple.23 Periareolar signs of inflammation (redness, swelling, warmth) may be accompanied by an abscess. These can recur and lead to chronic fistula formation, especially if there is a history of intervention (such as aspiration, incision, and drainage).
Treatment of Zuska disease includes symptom relief and antibiotics. If S aureus is present, infection with methicillin-resistant S aureus is likely, and treatment with clindamycin or amoxicillin/clavulanic acid is preferred. If abscess is present, aspiration (preferred, often under ultrasound guidance) or incision and drainage (if the skin is compromised) may be required. If disease is recurrent or associated with a chronically draining fistula, surgical intervention may be warranted, in which resolution requires removing the keratin-plugged ducts in and immediately below the central core of the nipple. Given the association between Zuska disease and smoking, cessation should be encouraged, although there is no guarantee that this will resolve the issue.23
Continue to: CASE 7 Patient with breast pain and swelling...
CASE 7 Patient with breast pain and swelling
A 39-year-old woman presents with left breast swelling and pain of 1 month’s duration. On examination, there is a 6-cm area of edema, induration, and erythema.
Granulomatous mastitis
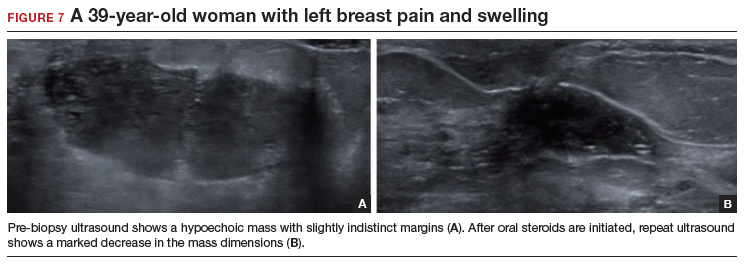
A diagnostic mammogram and ultrasound demonstrated an ill-defined hypoechoic mass (FIGURE 7a). Ultrasound-guided biopsy was performed, which showed granulomatous mastitis, negative for fungus and acid-fast bacilli. The patient was treated with prednisone and gradually improved (FIGURE 7b).
Granulomatous mastitis (GM) is a rare benign inflammatory process, with etiologies that include fungal infections, tuberculosis, Wegener granulomatosis, sarcoidosis, and idiopathic causes. Imaging can be nonspecific and show variable features. Mammograms can appear normal or show asymmetry or mass and skin thickening. Ultrasound can show heterogeneous parenchyma, ill-defined hypoechoic collection, or a mass with margins that can be circumscribed or indistinct or with tubular extensions, with or without overlying skin thickening, fistulas, and reactive lymph nodes.24
In this clinical setting, the differential diagnosis includes infectious mastitis, inflammatory breast cancer, foreign body injection granulomas, and diabetic mastopathy. Treatment involves drainage and fluid culture if there is a collection on imaging. A core biopsy is performed if imaging demonstrates a solid mass or fluid culture is negative and symptoms persist or recur. Oral steroids represent the mainstay of treatment if a core biopsy shows GM. However, immunosuppressants, including methotrexate, and surgery are options if initial treatment is not helpful.25,26
Conclusion
Breast symptoms are common reasons for patient visits to obstetricians and gynecologists. With a good understanding of the various symptomatic breast diseases and conditions, and by having a close collaboration with radiologists and breast surgeons, clinicians can provide excellent care to these patients and thereby improve patient outcomes and satisfaction. ●
- Eberl MM, Phillips RL Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med. 2008;6:528-533.
- Malherbe F, Nel D, Molabe H, et al. Palpable breast lumps: an age-based approach to evaluation and diagnosis. S Afr Fam Pract (2022). 2022;64:e1-e5.
- Expert Panel on Breast Imaging; Klein KA, Kocher M, Lourenco AP, et al. American College of Radiology ACR appropriateness criteria: palpable breast masses. Accessed February 15, 2023. https://acsearch.acr.org/docs/69495/Narrative/
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116:565574.
- Hines N, Slanetz PJ, Eisenberg RL. Cystic masses of the breast. AJR Am J Roentgenol. 2010;194:W122133.
- Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology. 2020;295:52-53.
- Duman L, Gezer NS, Balcı P, et al. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care. 2016;11:123-127.
- Lerwill MF, Lee AHS, Tan PH. Fibroepithelial tumours of the breast—a review. Virchows Arch. 2022;480:45-63.
- Vasei N, Shishegar A, Ghalkhani F, et al. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis. 2019;18:139.
- Kerridge WD, Kryvenko ON, Thompson A, et al. Fat necrosis of the breast: a pictorial review of the mammographic, ultrasound, CT, and MRI findings with histopathologic correlation. Radiol Res Pract. 2015;2015:613139.
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815-825.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318.
- Holbrook AI. Breast pain, a common grievance: guidance to radiologists. AJR Am J Roentgenol. 2020;214:259-264.
- Expert Panel on Breast Imaging; Moy L, Heller SL, Bailey L, et al. ACR appropriateness criteria: palpable breast masses. J Am Coll Radiol. 2017;14:S203-S224.
- Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging workup appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kucukerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Fariselli G, Lepera P, Viganotti G, et al. Localized mastalgia as presenting symptom in breast cancer. Eur J Surg Oncol. 1988;14:213-215.
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J. 2013;19:582-589.
- Leung JW, Kornguth PJ, Gotway MB. Utility of targeted sonography in the evaluation of focal breast pain. J Ultrasound Med. 2002;21:521-526.
- Goyal A. Breast pain. BMJ Clin Evid. 2011; 2011:0812.
- Kasales CJ, Han B, Smith Jr JS, et al. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014;202:W133-W139.
- Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014;202:W390-W399.
- Snider HC. Management of mastitis, abscess, and fistula. Surg Clin North Am. 2022;102:1103-1116.
- Oztekin PS, Durhan G, Kosar PN, et al. Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13:e33900.
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38:330-356.
- Hovanessian-Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193:574-581.
The vast majority of symptomatic breast conditions are benign, with the most common symptoms being palpable mass and breast pain. Clinicians, including primary care clinicians and gynecologists, play a crucial role by performing the initial assessment and subsequent therapies and referrals and serve as the mediator between the specialists and by being the patient’s spokesperson. It is therefore important for clinicians to be aware of the various possible causes of these breast symptoms, to know which imaging tests to order, and also to understand the indications for biopsies and surgical referral.
Common types of breast lumps: Imaging workup and management
Accounting for 8% of women who present with breast symptoms, breast lump is the second most common symptom after breast pain.1 The positive likelihood ratio of finding breast cancer is highest among women with breast lumps compared with any other breast symptoms. Therefore, anxiety is related to this symptom, and a thorough evaluation is recommended.1 Cysts, fibroadenoma, and fat necrosis are 3 common benign causes of breast lumps.2
In this section, we review clinical presentation, imaging workup, and management strategies for common types of breast lumps.
CASE 1 Woman with tender breast lump
A 45-year-old woman presents with a breast lump of 6 months’ duration that is associated with a change in size with the menstrual cycle and pain. Clinical examination reveals a 4 x 4.5–cm mass in the right breast in the retroareolar region, which is smooth with some tenderness on palpation.
Breast cyst
According to the American College of Radiology appropriateness criteria for an adult woman 40 years of age or older who presents with a palpable breast mass, the initial imaging study is diagnostic mammography with or without digital tomosynthesis, usually followed by a directed ultrasound. If the mammogram is suspicious or highly suggestive of malignancy, or in cases where the mammogram does not show an abnormality, the next recommended step is breast ultrasonography. Any suspicious findings on ultrasound or mammogram should be followed by an image guided biopsy. Ultrasonography also may be appropriate if the mammogram findings are benign or probably benign.
For an adult woman younger than age 30 who presents with a palpable breast mass, breast ultrasonography is the appropriate initial imaging study. If the ultrasound is suspicious or highly suggestive of malignancy, then performing diagnostic mammography with or without digital tomosynthesis or ultrasound-guided core needle biopsy of the mass are both considered appropriate. However, no further imaging is recommended if the ultrasound is benign, probably benign, or negative. Breast ultrasonography or mammography is appropriate as the initial imaging test for adult women aged 30 to 39 years who present with a palpable breast mass.3,4
Approximately 50% of women after age 30 may develop fibrocystic breast disease, and 20% of them can present with pain or lump due to a macrocysts. Simple cysts must be distinguished from complex cysts with the help of ultrasound as the latter are associated with 23% to 31% increased risk of malignancy.
In this 45-year-old patient, the initial mammogram demonstrated a circumscribed mass underneath the area of palpable concern (FIGURE 1a, 1b). Targeted breast ultrasonography was performed for further assessment, which depicted the mass as a benign simple cyst (FIGURE 1c).

On ultrasound, a simple cyst is an anechoic, well-circumscribed mass with a thin capsule and with increased through transmission. Patients with small and asymptomatic simple cysts do not need imaging follow-up and can return for routine screening mammograms.
A breast surgeon, radiologist, or gynecologist can perform percutaneous aspiration if a cyst is large and symptomatic. A cyst with low-level internal echoes, fluid-fluid, or fluid-debris levels is considered a complicated cyst. Differential diagnosis also includes hematoma, fat necrosis, abscess, and galactocele, depending on the clinical presentation. Fine-needle aspiration or short-interval follow-up5,6 is appropriate for complicated cysts, while incision and drainage is indicated in patients with infected cysts and abscesses. A cyst with a solid component is considered a cystic, solid mass, and core needle biopsy is recommended. The differential diagnosis for cysts with solid components includes intracystic papilloma, papillary carcinoma, ductal carcinoma in situ, and necrotic cancers.5,6
Continue to: CASE 2 Painless breast mass in a young woman...
CASE 2 Painless breast mass in a young woman
A 22-year-old woman presents with a 2-month history of breast lump, which is not associated with pain or nipple discharge. On examination, there is a 2 x 2–cm mass in the right breast at 12 o’clock, 2 cm from the nipple, which is mobile, smooth, and nontender on palpation.
Fibroadenoma
In this 22-year-old, the initial imaging of choice is breast ultrasonography. Breast ultrasonography can differentiate a cystic mass from a solid mass, and it does not involve radiation. Right breast targeted ultrasound showed a circumscribed oval homogeneous hypoechoic mass that is wider than tall (FIGURE 2). The patient desired surgical removal, and a pre-lumpectomy core needle biopsy revealed a fibroadenoma.

Fibroadenoma is the most common benign tumor of the breast. It is most often encountered in premenopausal women. Patients present with a painless breast lump, which is smooth and mobile on palpation. Fibroadenoma can be followed expectantly with repeat ultrasound (to assess over time for growth) if it is small and asymptomatic. No further action is needed if it remains stable. If a patient desires surgical excision, a core needle biopsy is usually performed before lumpectomy.
Excisional biopsy or removal of the mass is recommended if the mass is greater than 3 or 4 cm, is symptomatic, or if there is an increase in size that raises clinical concern for phyllodes tumor. Imaging features that are concerning for phyllodes tumors are size greater than 3 cm, indistinct or microlobulated margins, and heterogeneous echo pattern.7,8 In cases in which the imaging features are concerning for phyllodes tumor and a core needle biopsy is not definitive, wide surgical excision is recommended for definitive diagnosis.8
CASE 3 Patient develops breast mass post-surgery
A 45-year-old woman presents with a tender left breast mass that she noticed 2 months after breast reduction surgery. It has been increasing in size since. On clinical examination, a 4 x 4–cm mass is found at the surgical scar site, which is indurated on palpation and tender.
Fat necrosis
In this 45-year-old, the initial test of choice is diagnostic mammography, which showed a somewhat circumscribed area with fat under the palpable marker (FIGURE 3a). Breast ultrasonography was performed for further evaluation, which was inconclusive as the ultrasound showed ill-defined areas of mixed echogenicity (FIGURE 3b). Breast magnetic resonance imaging (MRI) clearly demonstrated fat necrosis in the area of the palpable lump (FIGURE 3c).

Fat necrosis of the breast is an inflammatory process that is seen after breast trauma or surgery. It can present as an incidental mammogram finding or a palpable mass. The patient may give a history of trauma, breast reduction surgery, or breast cancer surgery followed by radiation treatment. On clinical examination, fat necrosis occasionally can present as a firm mass with skin retraction or swelling concerning for cancer. Imaging features are variable depending on the stage of fat necrosis and inflammation.9-11
A mammogram may demonstrate a circumscribed fat-containing mass, an ill-defined mass, asymmetry or calcified oil cyst, and dystrophic calcifications. On ultrasound, fat necrosis can appear as anechoic or hypoechoic or as a complicated cyst or a mixed cystic, solid mass. MRI demonstrates a circumscribed or irregular fat-containing mass, with or without enhancement, and architectural distortion.
When the imaging features are clearly benign—for example, a circumscribed fat-containing mass on mammogram or on ultrasound or, on MRI, marked hypointensity of fat in the center of a circumscribed mass when compared with surrounding fat (keyhole sign)—no further follow-up is needed. When the imaging features are indeterminate, however, a short-interval follow-up can be considered. In cases with irregular fat-containing mass with enhancement, core needle biopsy is indicated to exclude cancer. If the workup remains inconclusive and the level of clinical suspicion is high, surgical excision can be performed for a definitive diagnosis.12
Continue to: Investigating breast pain: Imaging workup and management...
Investigating breast pain: Imaging workup and management
Breast pain, or mastalgia, is the most common concern of women presenting to a breast clinic and accounts for approximately half of such encounters.13 Causes of breast pain include hormonal changes, fibrocystic changes, musculoskeletal causes (such as costochondritis), lack of support, infection, and injury. While mastalgia often causes patient concern, the risk of malignancy in a woman presenting with breast pain alone is low. Still, it is essential to rule out other findings suspicious for cancer (mass, skin changes, or nipple discharge) with a thorough history and breast examination.
In this section, we review clinical presentation, imaging workup, and management for breast pain.
CASE 4 Woman with noncyclic breast pain
A 26-year-old woman presents to the clinic with mastalgia. The pain is noncyclic and primarily located in the upper outer quadrant of her left breast. There is no history of breast cancer in her family. She has no suspicious findings on the breast examination.
Mastalgia
The test of choice for this 26-year-old with focal left breast pain is targeted breast ultrasound. The patient’s ultrasound image showed no suspicious findings or solid or cystic mass (FIGURE 4).

Two important characteristics of breast pain are whether it is noncyclical and whether it is focal. According to the American College of Radiology, no breast imaging is recommended for clinically insignificant cyclical, nonfocal (greater than 1 quadrant)/diffuse pain, as this type of mastalgia is not associated with malignancy.14
For patients age 40 or older, if they are not up to date with their annual screening mammogram, then a mammogram should be performed. An imaging workup is warranted for clinically significant mastalgia that is noncyclical and focal. Even then, no malignancy is identified in most patients with clinically significant mastalgia; in patients with breast pain as their only symptom, the prevalence of breast cancer is 0% to 3.0%.15-19
The initial imaging modality differs by patient age: younger than 30 years, ultrasonography; between 30 and 40 years, mammography or ultrasonography; and older than 40 years, mammography first followed by ultrasonography.14
Treatment of breast pain is primarily symptomatic, and evidence for specific treatments is generally lacking. Cyclical breast pain resolves spontaneously in 20% to 30% of women, while noncyclical pain responds poorly to treatment but resolves spontaneously in half of women.20 Reassurance is important and wearing a supportive bra often can alleviate breast pain. In addition, reducing caffeine intake can be helpful.
As a first-line treatment, both topical (diclofenac) and oral nonsteroidal anti-inflammatory drugs effectively can relieve breast pain. Supplements and herbal remedies (for example, evening primrose oil, vitamin E, flaxseed) have varying effectiveness and are of questionable benefit as few have trials to support their effectiveness.4 Danazol and tamoxifen have been shown to have some benefits but they also have adverse effects.20 Surgery does not play a role in the treatment of mastalgia.
CASE 5 Breastfeeding woman with breast pain
A 27-year-old postpartum woman presents with concerns for redness and pain in the upper inner left breast. She has been breastfeeding for the past few months. Breast examination demonstrates a 5-cm area of erythema and warmth but no fluctuance or masses.
Lactational mastitis
Targeted ultrasonography is the test of choice for this 27-year-old patient with focal breast pain, and the imaging revealed edema of subcutaneous tissues and ill-defined hypoechoic areas, likely inflamed fat lobules (FIGURE 5). These findings suggest uncomplicated lactational mastitis, which can be treated with antibiotics. Generally, the mastitis will improve within days of starting the antibiotics; if it does not improve, repeat examination and ultrasound should be performed to look for formation of an abscess that may require aspiration.

Continue to: CASE 6 Woman with painful periareolar mass...
CASE 6 Woman with painful periareolar mass
A 42-year-old perimenopausal woman describes having pain near the nipple of her right breast. She is a smoker and has no history of breast cancer in her family. Examination demonstrates a palpable, erythematous, painful, 3-cm periareolar fluctuant mass.
Nonpuerperal periareolar abscess
Appropriate initial imaging for this 42-year-old patient with focal pain is a diagnostic mammogram, which showed skin thickening and a retroareolar mass (FIGURE 6a). Further evaluation with targeted ultrasound showed a thick-walled anechoic collection with echoes compatible with an abscess (FIGURE 6b).

Mammographic findings in a patient with mastitis may be normal or demonstrate skin and trabecular thickening. Ultrasound imaging may show dilated ducts and heterogeneous tissue secondary to inflammation and edema without a discrete fluid collection. In cases with breast abscess, in addition to the mammographic findings described above, a mass, or an asymmetry, may be seen, most commonly in a subareolar location. On ultrasound, a hypoechoic collection with mobile debris, no internal flow on Doppler, and thick hypervascular walls can be seen with abscess, occasionally giving the appearance of a complicated cyst or a mixed cystic, solid mass.
The most important differential for mastitis is inflammatory breast cancer. Most cancers appear solid but can have central necrosis, mimicking a complicated cystic mass on ultrasound. The location for mastitis or abscess is most frequently subareolar. The presence of microcalcifications in a mass indicates the possibility of cancer.
Contrast-enhanced MRI can be helpful to differentiate between infection and cancer, with cancers showing initial early enhancement and washout kinetics compared with infected collections that show no enhancement or peripheral enhancement with a plateau or persistent enhancement curves. When clinical and imaging findings are unchanged after treatment of mastitis and abscesses, a core needle biopsy should be performed.21,22
There are 2 categories of mastitis and breast abscess: lactational and nonpuerperal (all mastitis that occurs outside the lactational period). The World Health Organization definition of puerperal mastitis includes pain, local redness, warmth and swelling of the breast (usually unilateral), fever, and malaise.4 Concerning etiology, epithelial lesions in the nipple area caused by breastfeeding can allow pathogens to enter and cause infection. The most common microorganism is Staphylococcus aureus.4 Continued emptying of the breast is important, combined with early antibiotic therapy (dicloxacillin is often the first line; if the patient is penicillin allergic, use a macrolide such as clindamycin). If no improvement is seen in 48 to 72 hours, imaging should be performed.
In most cases, continuation of breastfeeding is possible. If mastitis has evolved into an abscess in a lactating woman, it can be aspirated under ultrasound guidance. Incision and drainage should be avoided unless the abscess persists after multiple aspiration attempts, it is large, or if the overlying skin is thin or otherwise appears nonviable.
Nonpuerperal mastitis includes peripheral, periductal, and idiopathic granulomatous mastitis (IGM). Peripheral mastitis behaves like infections/abscesses in other soft tissues, responds well to treatment (antibiotics and percutaneous drainage), and is less likely to recur than periductal mastitis and IGM.21,23
Periductal mastitis and abscess, also known as Zuska disease, has a pathogenesis distinct from other forms of mastitis. Squamous metaplasia of the usual cuboidal epithelium of the breast ducts leads to keratin plugging that can cause infection.23 Risk factors include obesity, smoking, and macromastia. The typical presentation of Zuska disease is a woman with a history of chronic smoking and/or a congenital cleft in the central nipple.23 Periareolar signs of inflammation (redness, swelling, warmth) may be accompanied by an abscess. These can recur and lead to chronic fistula formation, especially if there is a history of intervention (such as aspiration, incision, and drainage).
Treatment of Zuska disease includes symptom relief and antibiotics. If S aureus is present, infection with methicillin-resistant S aureus is likely, and treatment with clindamycin or amoxicillin/clavulanic acid is preferred. If abscess is present, aspiration (preferred, often under ultrasound guidance) or incision and drainage (if the skin is compromised) may be required. If disease is recurrent or associated with a chronically draining fistula, surgical intervention may be warranted, in which resolution requires removing the keratin-plugged ducts in and immediately below the central core of the nipple. Given the association between Zuska disease and smoking, cessation should be encouraged, although there is no guarantee that this will resolve the issue.23
Continue to: CASE 7 Patient with breast pain and swelling...
CASE 7 Patient with breast pain and swelling
A 39-year-old woman presents with left breast swelling and pain of 1 month’s duration. On examination, there is a 6-cm area of edema, induration, and erythema.
Granulomatous mastitis
A diagnostic mammogram and ultrasound demonstrated an ill-defined hypoechoic mass (FIGURE 7a). Ultrasound-guided biopsy was performed, which showed granulomatous mastitis, negative for fungus and acid-fast bacilli. The patient was treated with prednisone and gradually improved (FIGURE 7b).

Granulomatous mastitis (GM) is a rare benign inflammatory process, with etiologies that include fungal infections, tuberculosis, Wegener granulomatosis, sarcoidosis, and idiopathic causes. Imaging can be nonspecific and show variable features. Mammograms can appear normal or show asymmetry or mass and skin thickening. Ultrasound can show heterogeneous parenchyma, ill-defined hypoechoic collection, or a mass with margins that can be circumscribed or indistinct or with tubular extensions, with or without overlying skin thickening, fistulas, and reactive lymph nodes.24
In this clinical setting, the differential diagnosis includes infectious mastitis, inflammatory breast cancer, foreign body injection granulomas, and diabetic mastopathy. Treatment involves drainage and fluid culture if there is a collection on imaging. A core biopsy is performed if imaging demonstrates a solid mass or fluid culture is negative and symptoms persist or recur. Oral steroids represent the mainstay of treatment if a core biopsy shows GM. However, immunosuppressants, including methotrexate, and surgery are options if initial treatment is not helpful.25,26
Conclusion
Breast symptoms are common reasons for patient visits to obstetricians and gynecologists. With a good understanding of the various symptomatic breast diseases and conditions, and by having a close collaboration with radiologists and breast surgeons, clinicians can provide excellent care to these patients and thereby improve patient outcomes and satisfaction. ●
The vast majority of symptomatic breast conditions are benign, with the most common symptoms being palpable mass and breast pain. Clinicians, including primary care clinicians and gynecologists, play a crucial role by performing the initial assessment and subsequent therapies and referrals and serve as the mediator between the specialists and by being the patient’s spokesperson. It is therefore important for clinicians to be aware of the various possible causes of these breast symptoms, to know which imaging tests to order, and also to understand the indications for biopsies and surgical referral.
Common types of breast lumps: Imaging workup and management
Accounting for 8% of women who present with breast symptoms, breast lump is the second most common symptom after breast pain.1 The positive likelihood ratio of finding breast cancer is highest among women with breast lumps compared with any other breast symptoms. Therefore, anxiety is related to this symptom, and a thorough evaluation is recommended.1 Cysts, fibroadenoma, and fat necrosis are 3 common benign causes of breast lumps.2
In this section, we review clinical presentation, imaging workup, and management strategies for common types of breast lumps.
CASE 1 Woman with tender breast lump
A 45-year-old woman presents with a breast lump of 6 months’ duration that is associated with a change in size with the menstrual cycle and pain. Clinical examination reveals a 4 x 4.5–cm mass in the right breast in the retroareolar region, which is smooth with some tenderness on palpation.
Breast cyst
According to the American College of Radiology appropriateness criteria for an adult woman 40 years of age or older who presents with a palpable breast mass, the initial imaging study is diagnostic mammography with or without digital tomosynthesis, usually followed by a directed ultrasound. If the mammogram is suspicious or highly suggestive of malignancy, or in cases where the mammogram does not show an abnormality, the next recommended step is breast ultrasonography. Any suspicious findings on ultrasound or mammogram should be followed by an image guided biopsy. Ultrasonography also may be appropriate if the mammogram findings are benign or probably benign.
For an adult woman younger than age 30 who presents with a palpable breast mass, breast ultrasonography is the appropriate initial imaging study. If the ultrasound is suspicious or highly suggestive of malignancy, then performing diagnostic mammography with or without digital tomosynthesis or ultrasound-guided core needle biopsy of the mass are both considered appropriate. However, no further imaging is recommended if the ultrasound is benign, probably benign, or negative. Breast ultrasonography or mammography is appropriate as the initial imaging test for adult women aged 30 to 39 years who present with a palpable breast mass.3,4
Approximately 50% of women after age 30 may develop fibrocystic breast disease, and 20% of them can present with pain or lump due to a macrocysts. Simple cysts must be distinguished from complex cysts with the help of ultrasound as the latter are associated with 23% to 31% increased risk of malignancy.
In this 45-year-old patient, the initial mammogram demonstrated a circumscribed mass underneath the area of palpable concern (FIGURE 1a, 1b). Targeted breast ultrasonography was performed for further assessment, which depicted the mass as a benign simple cyst (FIGURE 1c).

On ultrasound, a simple cyst is an anechoic, well-circumscribed mass with a thin capsule and with increased through transmission. Patients with small and asymptomatic simple cysts do not need imaging follow-up and can return for routine screening mammograms.
A breast surgeon, radiologist, or gynecologist can perform percutaneous aspiration if a cyst is large and symptomatic. A cyst with low-level internal echoes, fluid-fluid, or fluid-debris levels is considered a complicated cyst. Differential diagnosis also includes hematoma, fat necrosis, abscess, and galactocele, depending on the clinical presentation. Fine-needle aspiration or short-interval follow-up5,6 is appropriate for complicated cysts, while incision and drainage is indicated in patients with infected cysts and abscesses. A cyst with a solid component is considered a cystic, solid mass, and core needle biopsy is recommended. The differential diagnosis for cysts with solid components includes intracystic papilloma, papillary carcinoma, ductal carcinoma in situ, and necrotic cancers.5,6
Continue to: CASE 2 Painless breast mass in a young woman...
CASE 2 Painless breast mass in a young woman
A 22-year-old woman presents with a 2-month history of breast lump, which is not associated with pain or nipple discharge. On examination, there is a 2 x 2–cm mass in the right breast at 12 o’clock, 2 cm from the nipple, which is mobile, smooth, and nontender on palpation.
Fibroadenoma
In this 22-year-old, the initial imaging of choice is breast ultrasonography. Breast ultrasonography can differentiate a cystic mass from a solid mass, and it does not involve radiation. Right breast targeted ultrasound showed a circumscribed oval homogeneous hypoechoic mass that is wider than tall (FIGURE 2). The patient desired surgical removal, and a pre-lumpectomy core needle biopsy revealed a fibroadenoma.

Fibroadenoma is the most common benign tumor of the breast. It is most often encountered in premenopausal women. Patients present with a painless breast lump, which is smooth and mobile on palpation. Fibroadenoma can be followed expectantly with repeat ultrasound (to assess over time for growth) if it is small and asymptomatic. No further action is needed if it remains stable. If a patient desires surgical excision, a core needle biopsy is usually performed before lumpectomy.
Excisional biopsy or removal of the mass is recommended if the mass is greater than 3 or 4 cm, is symptomatic, or if there is an increase in size that raises clinical concern for phyllodes tumor. Imaging features that are concerning for phyllodes tumors are size greater than 3 cm, indistinct or microlobulated margins, and heterogeneous echo pattern.7,8 In cases in which the imaging features are concerning for phyllodes tumor and a core needle biopsy is not definitive, wide surgical excision is recommended for definitive diagnosis.8
CASE 3 Patient develops breast mass post-surgery
A 45-year-old woman presents with a tender left breast mass that she noticed 2 months after breast reduction surgery. It has been increasing in size since. On clinical examination, a 4 x 4–cm mass is found at the surgical scar site, which is indurated on palpation and tender.
Fat necrosis
In this 45-year-old, the initial test of choice is diagnostic mammography, which showed a somewhat circumscribed area with fat under the palpable marker (FIGURE 3a). Breast ultrasonography was performed for further evaluation, which was inconclusive as the ultrasound showed ill-defined areas of mixed echogenicity (FIGURE 3b). Breast magnetic resonance imaging (MRI) clearly demonstrated fat necrosis in the area of the palpable lump (FIGURE 3c).

Fat necrosis of the breast is an inflammatory process that is seen after breast trauma or surgery. It can present as an incidental mammogram finding or a palpable mass. The patient may give a history of trauma, breast reduction surgery, or breast cancer surgery followed by radiation treatment. On clinical examination, fat necrosis occasionally can present as a firm mass with skin retraction or swelling concerning for cancer. Imaging features are variable depending on the stage of fat necrosis and inflammation.9-11
A mammogram may demonstrate a circumscribed fat-containing mass, an ill-defined mass, asymmetry or calcified oil cyst, and dystrophic calcifications. On ultrasound, fat necrosis can appear as anechoic or hypoechoic or as a complicated cyst or a mixed cystic, solid mass. MRI demonstrates a circumscribed or irregular fat-containing mass, with or without enhancement, and architectural distortion.
When the imaging features are clearly benign—for example, a circumscribed fat-containing mass on mammogram or on ultrasound or, on MRI, marked hypointensity of fat in the center of a circumscribed mass when compared with surrounding fat (keyhole sign)—no further follow-up is needed. When the imaging features are indeterminate, however, a short-interval follow-up can be considered. In cases with irregular fat-containing mass with enhancement, core needle biopsy is indicated to exclude cancer. If the workup remains inconclusive and the level of clinical suspicion is high, surgical excision can be performed for a definitive diagnosis.12
Continue to: Investigating breast pain: Imaging workup and management...
Investigating breast pain: Imaging workup and management
Breast pain, or mastalgia, is the most common concern of women presenting to a breast clinic and accounts for approximately half of such encounters.13 Causes of breast pain include hormonal changes, fibrocystic changes, musculoskeletal causes (such as costochondritis), lack of support, infection, and injury. While mastalgia often causes patient concern, the risk of malignancy in a woman presenting with breast pain alone is low. Still, it is essential to rule out other findings suspicious for cancer (mass, skin changes, or nipple discharge) with a thorough history and breast examination.
In this section, we review clinical presentation, imaging workup, and management for breast pain.
CASE 4 Woman with noncyclic breast pain
A 26-year-old woman presents to the clinic with mastalgia. The pain is noncyclic and primarily located in the upper outer quadrant of her left breast. There is no history of breast cancer in her family. She has no suspicious findings on the breast examination.
Mastalgia
The test of choice for this 26-year-old with focal left breast pain is targeted breast ultrasound. The patient’s ultrasound image showed no suspicious findings or solid or cystic mass (FIGURE 4).

Two important characteristics of breast pain are whether it is noncyclical and whether it is focal. According to the American College of Radiology, no breast imaging is recommended for clinically insignificant cyclical, nonfocal (greater than 1 quadrant)/diffuse pain, as this type of mastalgia is not associated with malignancy.14
For patients age 40 or older, if they are not up to date with their annual screening mammogram, then a mammogram should be performed. An imaging workup is warranted for clinically significant mastalgia that is noncyclical and focal. Even then, no malignancy is identified in most patients with clinically significant mastalgia; in patients with breast pain as their only symptom, the prevalence of breast cancer is 0% to 3.0%.15-19
The initial imaging modality differs by patient age: younger than 30 years, ultrasonography; between 30 and 40 years, mammography or ultrasonography; and older than 40 years, mammography first followed by ultrasonography.14
Treatment of breast pain is primarily symptomatic, and evidence for specific treatments is generally lacking. Cyclical breast pain resolves spontaneously in 20% to 30% of women, while noncyclical pain responds poorly to treatment but resolves spontaneously in half of women.20 Reassurance is important and wearing a supportive bra often can alleviate breast pain. In addition, reducing caffeine intake can be helpful.
As a first-line treatment, both topical (diclofenac) and oral nonsteroidal anti-inflammatory drugs effectively can relieve breast pain. Supplements and herbal remedies (for example, evening primrose oil, vitamin E, flaxseed) have varying effectiveness and are of questionable benefit as few have trials to support their effectiveness.4 Danazol and tamoxifen have been shown to have some benefits but they also have adverse effects.20 Surgery does not play a role in the treatment of mastalgia.
CASE 5 Breastfeeding woman with breast pain
A 27-year-old postpartum woman presents with concerns for redness and pain in the upper inner left breast. She has been breastfeeding for the past few months. Breast examination demonstrates a 5-cm area of erythema and warmth but no fluctuance or masses.
Lactational mastitis
Targeted ultrasonography is the test of choice for this 27-year-old patient with focal breast pain, and the imaging revealed edema of subcutaneous tissues and ill-defined hypoechoic areas, likely inflamed fat lobules (FIGURE 5). These findings suggest uncomplicated lactational mastitis, which can be treated with antibiotics. Generally, the mastitis will improve within days of starting the antibiotics; if it does not improve, repeat examination and ultrasound should be performed to look for formation of an abscess that may require aspiration.

Continue to: CASE 6 Woman with painful periareolar mass...
CASE 6 Woman with painful periareolar mass
A 42-year-old perimenopausal woman describes having pain near the nipple of her right breast. She is a smoker and has no history of breast cancer in her family. Examination demonstrates a palpable, erythematous, painful, 3-cm periareolar fluctuant mass.
Nonpuerperal periareolar abscess
Appropriate initial imaging for this 42-year-old patient with focal pain is a diagnostic mammogram, which showed skin thickening and a retroareolar mass (FIGURE 6a). Further evaluation with targeted ultrasound showed a thick-walled anechoic collection with echoes compatible with an abscess (FIGURE 6b).

Mammographic findings in a patient with mastitis may be normal or demonstrate skin and trabecular thickening. Ultrasound imaging may show dilated ducts and heterogeneous tissue secondary to inflammation and edema without a discrete fluid collection. In cases with breast abscess, in addition to the mammographic findings described above, a mass, or an asymmetry, may be seen, most commonly in a subareolar location. On ultrasound, a hypoechoic collection with mobile debris, no internal flow on Doppler, and thick hypervascular walls can be seen with abscess, occasionally giving the appearance of a complicated cyst or a mixed cystic, solid mass.
The most important differential for mastitis is inflammatory breast cancer. Most cancers appear solid but can have central necrosis, mimicking a complicated cystic mass on ultrasound. The location for mastitis or abscess is most frequently subareolar. The presence of microcalcifications in a mass indicates the possibility of cancer.
Contrast-enhanced MRI can be helpful to differentiate between infection and cancer, with cancers showing initial early enhancement and washout kinetics compared with infected collections that show no enhancement or peripheral enhancement with a plateau or persistent enhancement curves. When clinical and imaging findings are unchanged after treatment of mastitis and abscesses, a core needle biopsy should be performed.21,22
There are 2 categories of mastitis and breast abscess: lactational and nonpuerperal (all mastitis that occurs outside the lactational period). The World Health Organization definition of puerperal mastitis includes pain, local redness, warmth and swelling of the breast (usually unilateral), fever, and malaise.4 Concerning etiology, epithelial lesions in the nipple area caused by breastfeeding can allow pathogens to enter and cause infection. The most common microorganism is Staphylococcus aureus.4 Continued emptying of the breast is important, combined with early antibiotic therapy (dicloxacillin is often the first line; if the patient is penicillin allergic, use a macrolide such as clindamycin). If no improvement is seen in 48 to 72 hours, imaging should be performed.
In most cases, continuation of breastfeeding is possible. If mastitis has evolved into an abscess in a lactating woman, it can be aspirated under ultrasound guidance. Incision and drainage should be avoided unless the abscess persists after multiple aspiration attempts, it is large, or if the overlying skin is thin or otherwise appears nonviable.
Nonpuerperal mastitis includes peripheral, periductal, and idiopathic granulomatous mastitis (IGM). Peripheral mastitis behaves like infections/abscesses in other soft tissues, responds well to treatment (antibiotics and percutaneous drainage), and is less likely to recur than periductal mastitis and IGM.21,23
Periductal mastitis and abscess, also known as Zuska disease, has a pathogenesis distinct from other forms of mastitis. Squamous metaplasia of the usual cuboidal epithelium of the breast ducts leads to keratin plugging that can cause infection.23 Risk factors include obesity, smoking, and macromastia. The typical presentation of Zuska disease is a woman with a history of chronic smoking and/or a congenital cleft in the central nipple.23 Periareolar signs of inflammation (redness, swelling, warmth) may be accompanied by an abscess. These can recur and lead to chronic fistula formation, especially if there is a history of intervention (such as aspiration, incision, and drainage).
Treatment of Zuska disease includes symptom relief and antibiotics. If S aureus is present, infection with methicillin-resistant S aureus is likely, and treatment with clindamycin or amoxicillin/clavulanic acid is preferred. If abscess is present, aspiration (preferred, often under ultrasound guidance) or incision and drainage (if the skin is compromised) may be required. If disease is recurrent or associated with a chronically draining fistula, surgical intervention may be warranted, in which resolution requires removing the keratin-plugged ducts in and immediately below the central core of the nipple. Given the association between Zuska disease and smoking, cessation should be encouraged, although there is no guarantee that this will resolve the issue.23
Continue to: CASE 7 Patient with breast pain and swelling...
CASE 7 Patient with breast pain and swelling
A 39-year-old woman presents with left breast swelling and pain of 1 month’s duration. On examination, there is a 6-cm area of edema, induration, and erythema.
Granulomatous mastitis
A diagnostic mammogram and ultrasound demonstrated an ill-defined hypoechoic mass (FIGURE 7a). Ultrasound-guided biopsy was performed, which showed granulomatous mastitis, negative for fungus and acid-fast bacilli. The patient was treated with prednisone and gradually improved (FIGURE 7b).

Granulomatous mastitis (GM) is a rare benign inflammatory process, with etiologies that include fungal infections, tuberculosis, Wegener granulomatosis, sarcoidosis, and idiopathic causes. Imaging can be nonspecific and show variable features. Mammograms can appear normal or show asymmetry or mass and skin thickening. Ultrasound can show heterogeneous parenchyma, ill-defined hypoechoic collection, or a mass with margins that can be circumscribed or indistinct or with tubular extensions, with or without overlying skin thickening, fistulas, and reactive lymph nodes.24
In this clinical setting, the differential diagnosis includes infectious mastitis, inflammatory breast cancer, foreign body injection granulomas, and diabetic mastopathy. Treatment involves drainage and fluid culture if there is a collection on imaging. A core biopsy is performed if imaging demonstrates a solid mass or fluid culture is negative and symptoms persist or recur. Oral steroids represent the mainstay of treatment if a core biopsy shows GM. However, immunosuppressants, including methotrexate, and surgery are options if initial treatment is not helpful.25,26
Conclusion
Breast symptoms are common reasons for patient visits to obstetricians and gynecologists. With a good understanding of the various symptomatic breast diseases and conditions, and by having a close collaboration with radiologists and breast surgeons, clinicians can provide excellent care to these patients and thereby improve patient outcomes and satisfaction. ●
- Eberl MM, Phillips RL Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med. 2008;6:528-533.
- Malherbe F, Nel D, Molabe H, et al. Palpable breast lumps: an age-based approach to evaluation and diagnosis. S Afr Fam Pract (2022). 2022;64:e1-e5.
- Expert Panel on Breast Imaging; Klein KA, Kocher M, Lourenco AP, et al. American College of Radiology ACR appropriateness criteria: palpable breast masses. Accessed February 15, 2023. https://acsearch.acr.org/docs/69495/Narrative/
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116:565574.
- Hines N, Slanetz PJ, Eisenberg RL. Cystic masses of the breast. AJR Am J Roentgenol. 2010;194:W122133.
- Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology. 2020;295:52-53.
- Duman L, Gezer NS, Balcı P, et al. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care. 2016;11:123-127.
- Lerwill MF, Lee AHS, Tan PH. Fibroepithelial tumours of the breast—a review. Virchows Arch. 2022;480:45-63.
- Vasei N, Shishegar A, Ghalkhani F, et al. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis. 2019;18:139.
- Kerridge WD, Kryvenko ON, Thompson A, et al. Fat necrosis of the breast: a pictorial review of the mammographic, ultrasound, CT, and MRI findings with histopathologic correlation. Radiol Res Pract. 2015;2015:613139.
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815-825.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318.
- Holbrook AI. Breast pain, a common grievance: guidance to radiologists. AJR Am J Roentgenol. 2020;214:259-264.
- Expert Panel on Breast Imaging; Moy L, Heller SL, Bailey L, et al. ACR appropriateness criteria: palpable breast masses. J Am Coll Radiol. 2017;14:S203-S224.
- Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging workup appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kucukerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Fariselli G, Lepera P, Viganotti G, et al. Localized mastalgia as presenting symptom in breast cancer. Eur J Surg Oncol. 1988;14:213-215.
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J. 2013;19:582-589.
- Leung JW, Kornguth PJ, Gotway MB. Utility of targeted sonography in the evaluation of focal breast pain. J Ultrasound Med. 2002;21:521-526.
- Goyal A. Breast pain. BMJ Clin Evid. 2011; 2011:0812.
- Kasales CJ, Han B, Smith Jr JS, et al. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014;202:W133-W139.
- Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014;202:W390-W399.
- Snider HC. Management of mastitis, abscess, and fistula. Surg Clin North Am. 2022;102:1103-1116.
- Oztekin PS, Durhan G, Kosar PN, et al. Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13:e33900.
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38:330-356.
- Hovanessian-Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193:574-581.
- Eberl MM, Phillips RL Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med. 2008;6:528-533.
- Malherbe F, Nel D, Molabe H, et al. Palpable breast lumps: an age-based approach to evaluation and diagnosis. S Afr Fam Pract (2022). 2022;64:e1-e5.
- Expert Panel on Breast Imaging; Klein KA, Kocher M, Lourenco AP, et al. American College of Radiology ACR appropriateness criteria: palpable breast masses. Accessed February 15, 2023. https://acsearch.acr.org/docs/69495/Narrative/
- Stachs A, Stubert J, Reimer T, et al. Benign breast disease in women. Dtsch Arztebl Int. 2019;116:565574.
- Hines N, Slanetz PJ, Eisenberg RL. Cystic masses of the breast. AJR Am J Roentgenol. 2010;194:W122133.
- Berg WA. Reducing unnecessary biopsy and follow-up of benign cystic breast lesions. Radiology. 2020;295:52-53.
- Duman L, Gezer NS, Balcı P, et al. Differentiation between phyllodes tumors and fibroadenomas based on mammographic sonographic and MRI features. Breast Care. 2016;11:123-127.
- Lerwill MF, Lee AHS, Tan PH. Fibroepithelial tumours of the breast—a review. Virchows Arch. 2022;480:45-63.
- Vasei N, Shishegar A, Ghalkhani F, et al. Fat necrosis in the breast: a systematic review of clinical. Lipids Health Dis. 2019;18:139.
- Kerridge WD, Kryvenko ON, Thompson A, et al. Fat necrosis of the breast: a pictorial review of the mammographic, ultrasound, CT, and MRI findings with histopathologic correlation. Radiol Res Pract. 2015;2015:613139.
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The many faces of fat necrosis in the breast. AJR Am J Roentgenol. 2009;192:815-825.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318.
- Holbrook AI. Breast pain, a common grievance: guidance to radiologists. AJR Am J Roentgenol. 2020;214:259-264.
- Expert Panel on Breast Imaging; Moy L, Heller SL, Bailey L, et al. ACR appropriateness criteria: palpable breast masses. J Am Coll Radiol. 2017;14:S203-S224.
- Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging workup appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kucukerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Fariselli G, Lepera P, Viganotti G, et al. Localized mastalgia as presenting symptom in breast cancer. Eur J Surg Oncol. 1988;14:213-215.
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J. 2013;19:582-589.
- Leung JW, Kornguth PJ, Gotway MB. Utility of targeted sonography in the evaluation of focal breast pain. J Ultrasound Med. 2002;21:521-526.
- Goyal A. Breast pain. BMJ Clin Evid. 2011; 2011:0812.
- Kasales CJ, Han B, Smith Jr JS, et al. Nonpuerperal mastitis and subareolar abscess of the breast. AJR Am J Roentgenol. 2014;202:W133-W139.
- Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014;202:W390-W399.
- Snider HC. Management of mastitis, abscess, and fistula. Surg Clin North Am. 2022;102:1103-1116.
- Oztekin PS, Durhan G, Kosar PN, et al. Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13:e33900.
- Pluguez-Turull CW, Nanyes JE, Quintero CJ, et al. Idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38:330-356.
- Hovanessian-Larsen LJ, Peyvandi B, Klipfel N, et al. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193:574-581.
Telemedicine: Medicolegal aspects in ObGyn

Telemedicine (or telehealth) originated in the early 1900s, when radios were used to communicate medical advice to clinics aboard ships.1 According to the American Telemedicine Association, telemedicine is namely “the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status.”2 These communications use 2-way video, email, smartphones, wireless tools, and other forms of telecommunications technology.
During the COVID-19 pandemic, many ObGyns—encouraged and advised by professional organizations—began providing telemedicine services.3 The first reported case of COVID-19 was in late 2019; the use of telemedicine was 38 times higher in February 2021 than in February 2020,4 illustrating how many physicians quickly moved to telemedicine practices.
CASE Dr. TM’s telemedicine dream
Before COVID-19, Dr. TM (an ObGyn practi-tioner) practiced in-person medicine in his home state. With the onset of the pandemic, Dr. TM struggled to switch to primarily seeing patients online (generally using Zoom or Facebook Live), with 1 day per week in the office for essential in-person visits.
After several months, however, Dr. TM’s routine became very efficient. He could see many more patients in a shorter time than with the former, in-person system. Therefore, as staff left his practice, Dr. TM did not replace them and also laid off others. Ultimately, the practice had 1 full-time records/insurance secretary who worked from home and 1 part-time nurse who helped with the in-person day and answered some patient inquiries by email. In part as an effort to add new patients, Dr. TM built an engaging website through which his current patients could receive medical information and new patients could sign up.
In late 2022, Dr. TM offered a $100 credit to any current patient who referred a friend or family member who then became a patient. This promotion was surprisingly effective and resulted in an influx of new patients. For example, Patient Z (a long-time patient) received 3 credits for referring her 3 sisters who lived out of state and became telepatients: Patient D, who lived 200 hundred miles away; Patient E, who lived 50 miles away in the adjoining state; and Patient F, who lived 150 miles away. Patient D contacted Dr. TM because she thought she was pregnant and wanted prenatal care, Patient E thought she might have a sexually transmitted infection (STI) and wanted treatment, and Patient F wanted general care and was inquiring about a medical abortion. Dr. TM agreed to treat Patient D but required 1 in-person visit. After 1 brief telemedicine session each with Patients E and F, Dr. TM wrote prescriptions for them.
By 2023, Dr. TM was enthusiastic about telemedicine as a professional practice. However, problems would ensue.
Dos and don’ts of telemedicine2
- Do take the initiative and inform patients of the availability of telemedicine/telehealth services
- Do use the services of medical malpractice insurance companies with regard to telemedicine
- Do integrate telemedicine into practice protocols and account for their limitations
- Don’t assume there are blanket exemptions or waivers in the states where your patients are located
Medical considerations
Telemedicine is endorsed by the American College of Obstetricians and Gynecologists (ACOG) as a vehicle for delivering prenatal and postpartum care.5 This represents an effort to reduce maternal and neonatal morbidity and mortality,5 as well as expandaccess to care and address the deficit in primary care providers and services, especially in rural and underserved populations.5,6 For obstetrics, prenatal care is designed to optimize pregnancy, childbirth, and postpartum care, with a focus on nutrition and genetic consultation and patient education on pregnancy, childbearing, breastfeeding, and newborn care.7
Benefits of telemedicine include its convenience for patients and providers, its efficiency and lower costs for providers (and hopefully patients, as well), and the potential improved access to care for patients.8 It is estimated that if a woman inititates obstetric care at 6 weeks, over the course of the 40-week gestation period, 15 prenatal visits will occur.9 Ultimately, the number of visits is determined based on the specifics of the pregnancy. With telemedicine, clinicians can provide those consultations, and information related to: ultrasonography, fetal echocardiography, and postpartum care services remotely.10 Using telemedicine may reduce missed visits, and remote monitoring may improve the quality of care.11
Barriers to telemedicine care include technical limitations, time constraints, and patient concerns of telehealth (visits). Technical limitations include the lack of a high speed internet connection and/or a smart device and the initial technical set-up–related problems,12 which affect providers as well as patients. Time constraints primarly refer to the ObGyn practice’s lack of time to establish telehealth services.13 Other challenges include integrating translation services, billing-related problems,10 and reimbursement and licensing barriers.14
Before the COVID-19 pandemic, obstetrics led the way in telemedicine with the development of the OB Nest model. Designed to replace in-person obstetrics care visits with telehealth,15 it includes home management tools such as blood pressure cuffs, cardiotocography, scales for weight checks, and Doppler ultrasounds.10 Patients can be instructed to measure fundal height and receive medications by mail. Anesthesia consultation can occur via this venue by having the patient complete a questionnaire prior to arriving at the labor and delivery unit.16
Legal considerations
With the COVID-19 pandemic, temporary changes were made to encourage the rapid adoption of telemedicine, including changes to licensing laws, certain prescription requirements, Health Insurance Portability and Accountability Act (HIPAA) privacy-security regulations, and reimbursement rules that required in-person visits. Thus, many ObGyns started using telemedicine during this rarified period, in which the rules appeared to be few and far between, with limited enforcement of the law and professional obligations.17 However, now that many of the legal rules that were suspended or ignored have been (or are being) reimposed and enforced, it is important for providers to become familiar with the legal issues involved in practicing telemedicine.
First, where is the patient? When discussing the legal issues of telemedicine, it is important to remember that many legal rules for medical care (ie, liability, informed consent, and licensing) vary from state to state. If the patient resides in a different state (“foreign” state) from the physician’s practice location (the physician’s “home” state), the care is considered delivered in the state where the patient is located. Thus, the patient’s location generally establishes the law covering the telemedicine transaction. In the following discussion, the rules refer to the law and professional obligations, with commentary on some key legal issues that are relevant to ObGyn telemedicine.
Continue to: Reinforcing the rules...
Reinforcing the rules
Licensing
During the height of the COVID-19 pandemic, the federal government and almost all states temporarily modified the licensing requirement to allow telemedicine based on an existing medical license in any state—disregarding the “where is the patient” rule. As those rules begin to lapse or change with the official end of the pandemic declared by President Biden as May 2023,17 the rules under which a physician began telemedicine interstate practice in 2020 also may be changing.
Simply put, “The same standards for licensure apply to health care providers regardless of whether care is delivered in-person or virtually through telehealth services.”18 When a physician is engaged in telemedicine treatment of a patient in the physician’s home state, there is generally no licensing issue. Telemedicine generally does not require a separate specific license.19 However, when the patient is in another state (a “foreign” state), there can be a substantial licensing issue.20 Ordinarily, to provide that treatment, the physician must, in some manner, be approved to practice in the patient’s state. That may occur, for example, in the following ways: (1) the physician may hold an additional regular license in the patient’s state, which allows practice there, or (2) the physician may have received permission for “temporary practice” in another state.
Many states (often adjoining states) have formal agreements with other states that allow telemedicine practice by providers in each other’s states. There also are “compacts”, or agreements that enable providers in any of the participating states to practice in the other associated states without a separate license.18 Although several websites provide information about compact licensing and the like, clinicians should not rely on simple lists or maps. Individual states may have special provisions about applying their laws to out-of-state “compact” physicians. In addition, under the Interstate Medical Licensure Compact, “physicians have to pay licensing fees and satisfy the requirements of each medical board in the states where they wish to practice.”21
Consequences. Practicing telemedicine with a patient in a state where the physician does not have a license is generally a crime. Furthermore, it may be the basis for license discipline in the physician’s home state and result in a report to the National Practi-tioner Databank.22 In addition, reimbursement often depends on the practitioner being licensed, and the absence of a license may be a basis for denying payment for services.23 Finally, malpractice insurance generally is limited to licensed practice. Thus, the insurer may decline to defend the unlicensed clinician against a malpractice claim or pay any damages.
Prescribing privileges
Prescribing privileges usually are connected to licensing, so as the rules for licensing change postpandemic, so do the rules for prescribing. In most cases, the physician must have a license in the state where care is given to prescribe medication—which in telemedicine, as noted, typically means the state where the patient is located. Exceptions vary by state, but in general, if a physician does not have a license to provide care, the physician is unlikely to be authorized to prescribe medication.24 Failure to abide by the applicable state rules may result in civil and even criminal liability for illegal prescribing activity.
In addition, the US Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA, which enforces laws concerning controlled substances) also regulate the prescription and sale of pharmaceuticals.25 There are state and federal limits on the ability of clinicians to order controlled substances without an in-person visit. The Ryan Haight Online Pharmacy Consumer Protection Act, for example, sets limits on controlled substance prescriptions without an in-person examination.26 Federal law was modified due to COVID-19 to permit prescribing of many controlled substances by telemedicine if there is synchronous audio and visual examination of the patient. Physicians who write such prescriptions also are required to have a DEA registration in the patient’s state. This is an essential consideration for physicians considering interstate telemedicine practice.27
HIPAA and privacy
Governments waived some of the legal requirements related to health information during the pandemic, but those waivers either have expired or will do so soon. Federal and state laws regarding privacy and security—notably including HIPAA—apply to telemedicine and are of particular concern given the considerable amount of communication of protected health information with telemedicine.
HIPAA security rules essentially require making sure health information cannot be hacked or intercepted. Audio-only telemedicine by landline (not cell) is acceptable under the security rules, but almost all other remote communication requires secure communications.28
Clinicians also need to adhere to the more usual HIPAA privacy rules when practicingtelehealth. State laws protecting patient privacy vary and may be more stringent than HIPAA, so clinicians also must know the requirements in any state where they practice—whether in office or telemedicine.29
Making sure telemedicine practices are consistent with these security and privacy rules often requires particular technical expertise that is outside the realm of most practicing clinicians. However, without modification, the pre-telemedicine technology of many medical offices likely is insufficient for the full range of telemedicine services.30
Reimbursement and fraud
Before COVID-19, Medicare and Medicaid reimbursement for telemedicine was limited. Government decisions to substantially broaden those reimbursement rules (at least temporarily) provided a substantial boost to telemedicine early in the pandemic.23 Federal regulations and statutes also expanded telemedicine reimbursement for various services. Some will end shortly after the health emergency, and others will be permanent. Parts of that will not be sorted out for several years, so it will likely be a changing landscape for reimbursement.
Continue to: Rules that are evolving...
Rules that are evolving
Informed consent
The ethical and legal obligations to obtain informed consent are present in telemedicineas well as in-person care, with the same basic requirements regarding risks, benefits, alternative care, etc.32 However, with telemedicine, information related to remote care should be included and is outlined in TABLE 1.
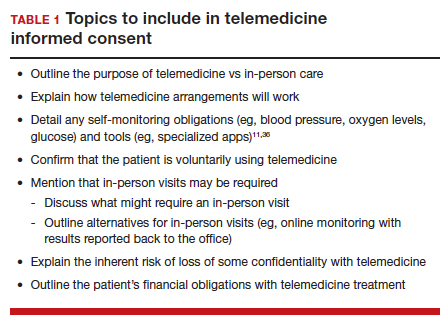
Certain states may have somewhat unique informed consent requirements—especially for reproductive care, including abortion.34 Therefore, it is important for clinicians to ensure their consent process and forms comply with any legal jurisdiction in which a patient is located.
Medical malpractice
The basics of medical malpractice (or negligence) are the same in telemedicine as in in-person care: duty, breach of duty, and injury caused by the breach. That is, there may be liability when a medical professional breaches the duty of care, causing the patient’s injury. The physician’s duty is defined by the quality of care that the profession (specialty) accepts as reasonably good. This is defined by the opinions of physicians within the specialty and formal statements from professional organizations, including ACOG.3
Maintaining the standard of care and quality. The use of telemedicine is not an excuse to lower the quality of health care. There are some circumstances for which it is medically better to have an in-person visit. In these instances, the provider should recommend the appropriate care, even if telemedicine would be more convenient for the provider and staff.35
If the patient insists and telemedicine might result in less than optimal care, the reasons for using a remote visit should be clearly documented contemporaneously with the decision. Furthermore, when the limitations of being unable to physically examine the patient result in less information than is needed for the patient’s care, the provider must find alternatives to make up for the information gap.11,36 It also may be necessary to inform patients about how to maximize telemedicine care.37 At the beginning of telemedicine care the provider should include information about the nature and limits of telehealth, and the patient’s responsibilities. (See TABLE 1) Throughout treatment of the patient, that information should be updated by the provider. That, of course, is particularly important for patients who have not previously used telemedice services.
Malpractice rules vary by state. Many states have special rules regarding malpractice cases. These differences in malpractice standards and regulations “can be problematic for physicians who use telemedicine services to provide care outside the state in which they practice.”38 Caps on noneconomic damages are an example. Those state rules would apply to telemedicine in the patient’s state.
Malpractice insurance
Malpractice insurance now commonly includes telemedicine legally practiced within the physician’s home state. Practitioners who treat patients in foreign states should carefully examine their malpractice insurance policies to confirm that the coverage extends to practice in those states.39 Malpractice carriers may require notification by a covered physician who routinely provides services to patients in another state.3
Keep in mind, malpractice insurance generally does not cover the practice of medicine that is illegal. Practicing telemedicine in a foreign state, where the physician or other provider does not have a license and where that state does not otherwise permit the practice, is illegal. Most likely, the physician’s malpractice insurance will not cover claims that arise from this illegal practice in a foreign state or provide defense for malpractice claims, including frivolous lawsuits. Thus, the physician will pay out of pocket for the costs of a defense attorney.
Telemedicine treatment of minors
Children and adolescents present special legal issues for ObGyn care, which may become more complicated with telemedicine. Historically, parents are responsible for minors (those aged <18 years): they consent to medical treatment, are responsible for paying for it, and have the right to receive information about treatment.
Over the years, though, many states have made exceptions to these principles, especially with regard to contraception and treatment of sexually transmitted diseases.40 For abortion, in particular, there is considerable variation among the states in parental consent and notification.41 The Supreme Court’s decision in Dobbs v Jackson Women’s Health42 may (depending on the state) be followed with more stringent limitations on adolescent consent to abortions, including medical abortions.43
Use of telehealth does not change any obligations regarding adolescent consent or parental notification. Because those differ considerably among states, it is important for all practitioners to know their states’ requirements and keep reasonably complete records demonstrating their compliance with state law.
Abortion
The most heated current controversy about telemedicine involves abortion—specifically medical abortion, which is the combination of mifepristone and misoprostol.44,45 The FDA approved the combination in 2000. Almost immediately, many states required in-person visits with a certified clinician to receive a prescription for mifepristone and misoprostol, and eventually, the FDA adopted similar requirements.46 However, during the pandemic from 2021 to 2022, the FDA permitted telemedicine prescriptions. Several states still require in-person physician visits, although the constitutionality of those requirements has not been established.47
With the Supreme Court’s decision in Dobbs v Jackson Women’s Health in 2022,42 disagreements have ensued about the degree to which states may regulate the prescription of FDA-approved medical abortion drugs. Thorny constitutional issues exist in the plans of both abortion opponents and proponents in the battle over medical abortion in antiabortion states. It may be that federal drug law preempts state laws limiting access to FDA-approved drugs. On the other hand, it may be that states can make it a crime within the state to possess or provide abortion-inducing drugs. Courts will probably take years to resolve the many tangled legal questions.48
Thus, while the pandemic telemedicine rules may have advanced access to abortion,34 there may be some pending downsides.49 States that prohibit abortion will likely include prohibitions on medical abortions. In addition, they may prohibit anyone in the state (including pharmacies) from selling, possessing, or obtaining any drug used for causing or inducing an abortion.50 If, for constitutional reasons, they cannot press criminal charges or undertake licensing discipline for prescribing abortion, some states will likely withdraw from telehealth licensing compacts to avoid out-of-state prescriptions. This area of telemedicine has considerable uncertainty.
Continue to: CASE Conclusion...
CASE Conclusion
Patient concerns come to the fore
By 2023, Dr. TM started receiving bad news. Patient D called complaining that after following the advice on the website, she suffered a severe reaction and had to be rushed to an emergency department. Patient E (who had only 1 in-office visit early in her pregnancy) notified the office that she developed very high blood pressure that resulted in severe placental abruption, requiring emergency care and resulting in the loss of the fetus. Patient F complained that someone hacked the TikTok direct message communication with Dr. TM and tried to “blackmail” or harass her.
Discussion. Patients D, E, and F represent potential problems of telemedicine practice. Patient D was injured because she relied on her doctor’s website (to which Dr. TM directed patients). It contained an error that caused an injury. A doctor-patient relationship existed, and bad medical advice likely caused the injury. Physicians providing advice online must ensure the advice is correct and kept current.
Patient E demonstrates the importance of monitoring patients remotely (blood pressure transmitted to the office) or with periodic in-office visits. It is not clear whether she was a no-show for office visits (and whether the office followed up on any missed appointments) or if such visits were never scheduled. Liability for failure to monitor adequately is a possibility.
Patient F’s seemingly minor complaint could be a potential problem. Dr. TM used an insecure mode of communication. Although some HIPAA security regulations were modified or suspended during the pandemic, using such an unsecure platform is problematic, especially if temporary HIPAA rules expired. The outcome of the complaint is in doubt.
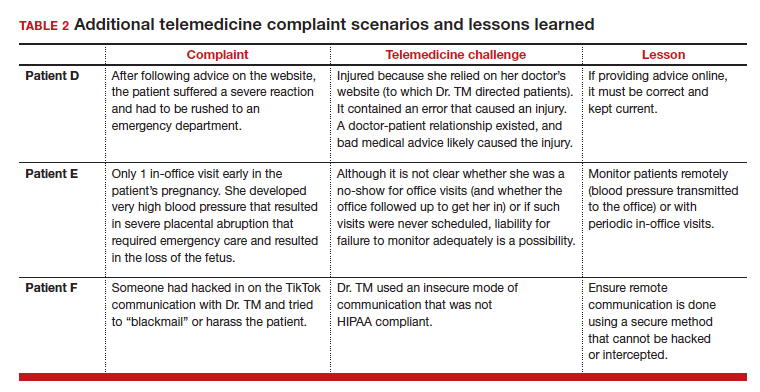
(See TABLE 2 for additional comments on patients D, E, and F.)
Out-of-state practice
Dr. TM treated 3 out-of-state residents (D, E, and F) via telemedicine. Recently Dr. TM received a complaint from the State Medical Licensure Board for practicing medicine without a license (Patient D), followed by similar charges from Patient E’s and Patient F’s state licensing boards. He has received a licensing inquiry from his home state board about those claims of illegal practice in other states and incompetent treatment.
Patient D’s pregnancy did not go well. The 1 in-person visit did not occur and she has filed a malpractice suit against Dr. TM. Patient E is threatening a malpractice case because the STI was not appropriately diagnosed and had advanced before another physician treated it.
In addition, a private citizen in Patient F’s state has filed suit against Dr. TM for abetting an illegal abortion (for Patient F).
Discussion. Patients D, E, and F illustrate the risk of even incidental out-of-state practice. The medical board inquiries arose from anonymous tips to all 4 states reporting Dr. TM was “practicing medicine without a license.” Patient E’s home state did have a licensing compact with the adjoining state (ie, Dr. TM’s home state). However, it required physicians to register and file an annual report, which Dr. TM had not done. The other 2 states did not have compacts with Dr. TM’s home state. Thus, he was illegally practicing medicine and would be subject to penalties. His home state also might impose license discipline based on his illegal practice in other states.
Continue to: What’s the verdict?...
What’s the verdict?
Dr. TM’s malpractice carrier is refusing to defend the claims of medical malpractice threatened by Patients D, E, and F. The company first notes that the terms of the malpractice policy specifically exclude the illegal practice of medicine. Furthermore, when a physician legally practices in another state, the policy requires a written notice to the insurance carrier of such practice. Dr. TM will likely have to engage and pay for a malpractice attorney for these cases. Because the claims are filed in 3 different states, more than a home-state attorney will likely be involved in the defense of these cases. Dr. TM will need to pay the attorneys and any damages from a settlement or trial.
Malpractice claims. Patient D claims that the doctor essentially abandoned her by never reaching out to her or arranging an in-person visit. Dr. TM claims the patient was responsible for scheduling the in-person visit. Patient E claims it was malpractice not to determine the specific nature of the STI and to do follow-up testing to determine that it was cured. All patients claim there was no genuine informed consent to the telemedicine. An attorney has warned Dr. TM that it is “not going to look good to the jury” that he was practicing without a license in the state and suggests he settle the cases quickly by paying damages.
Abortion-related claims. Patient F presents a different set of problems. Dr. TM’s home state is “proabortion.” Patient F’s home state is strongly “antiabortion,” making it a felony to participate in, assist, or facilitate an abortion (including medical abortion). Criminal charges have been filed against Dr. TM for the illegal practice of medicine, for aiding and facilitating an abortion, and for failure to notify a parent that a minor is seeking an abortion. For now, Dr. TM’s state is refusing to extradite on the abortion charge. Still, the patient’s state insists that it do so on the illegal practice of medicine charges and new charges of insurance fraud and failure to report suspected sexual abuse of a child. (Under the patient’s state law, anyone having sex with Patient F would have engaged in sexual abuse or “statutory rape,” so the state insists that the fact she was pregnant proves someone had sex with her.)
Patient F’s state also has a statute that allows private citizens to file civil claims against anyone procuring or assisting with an abortion (a successful private citizen can receive a minimum of $10,000 from the defendant). Several citizens from the patient’s state have already filed claims against Dr. TM in his state courts. Only one of them, probably the first to file, could succeed. Courts in the state have issued subpoenas and ordered Dr. TM to appear and reply to the civil suits. If he does not respond, there will be a default judgment.
Dr. TM’s attorney tells him that these lawsuits will not settle and will take a long time to defend and resolve. That will be expensive.
Billing and fraud. Dr. TM’s office recently received a series of notices from private health insurers stating they are investigating previously made payments as being fraudulent (unlicensed). They will not pay any new claims pending the investigation. On behalf of Medicare-Medicaid and other federal programs, the US Attorney’s office has notified Dr. TM that it has opened an investigation into fraudulent federal payments. F’s home state also is filing a (criminal) insurance fraud case, although the basis for it is unclear. (Dr. TM’s attorney believes it might be to increase pressure on the physician’s state to extradite Dr. TM for Patient F’s case.)
In addition, a disgruntled former employee of Dr. TM has filed a federal FCA case against him for filing inflated claims with various federally funded programs. The employee also made whistleblower calls to insurance companies and some state-funded medical programs. A forensic accounting investigation by Dr. TM’s accountant confirmed a pattern of very sloppy records and recurring billing for televisits that did not occur. Dr. TM believes that this was the act of one of the temporary assistants he hired in a pinch, who did not understand the system and just guessed when filing some insurance claims.
During the investigation, the federal and state attorneys are looking into a possible violation of state and federal Anti-Kickback Statutes. This is based on the original offer of a $100 credit for referrals to Dr. TM’s telemedicine practice.
The attorneys are concerned that other legal problems may present themselves. They are thoroughly reviewing Dr. TM’s practice and making several critical but somewhat modest changes to his practice. They also have insisted that Dr. TM have appropriate staff to handle the details of the practice and billing.
Conclusions
Telemedicine presents notable legal challenges to medical practice. As the pandemic status ends, ObGyn physicians practicing telemedicine need to be aware of the rules and how they are changing. For those physicians who want to continue or start a telemedicine practice, securing legal and technical support to ensure your operations are inline with the legal requirements can minimize any risk of legal troubles in the future. ●
A physician in State A, where abortion is legal, has a telemedicine patient in State B, where it is illegal to assist, provide, or procure an abortion. If the physician prescribes a medical abortion, he would violate the law of State B by using telemedicine to help the patient (located in State B) obtain an abortion. This could result in criminal charges against the prescribing physician.
- Board on Health Care Services; Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. National Academies Press: 2012. https://www.ncbi.nlm.nih.gov/books/NBK207145/. Accessed March 30, 2023.
- Bruhn HK. Telemedicine: dos and don’ts to mitigate liability risk. J APPOS. 2020;24:195-196. doi:10.1016/j.jaapos. 2020.07.002
- Implementing telehealth in practice: ACOG Committee Opinion Summary, number 798. Obstet Gynecol. 2020; 2135:493-494. doi:10.1097/AOG.0000000000003672
- Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. July 9, 2021. Accessed March 2, 2023. https://www.mckinsey.com/industries/healthcare/our-insights /telehealth-a-quarter-trillion-dollar-post-covid-19-reality
- Stanley AY, Wallace JB. Telehealth to improve perinatal care access. MCN Am J Matern Child Nurs. 2022;47:281-287. doi: 10.1097/NMC.0000000000000841
- Warshaw R. Health disparities affect millions in rural US communities. Association of American Medical Colleges. Published October 31, 2017. Accessed March 31, 2023. https://www.aamc.org/news-insights/health-disparities -affect-millions-rural-us-communities
- Almuslin H, AlDossary S. Models of incorporating telehealth into obstetric care during the COVID-19 pandemic, its benefits and barriers: a scoping review. Telemed J E Health. 2022;28:24-38. doi:10.1089/tmj.2020.0553
- Gold AE, Gilbert A, McMichael BJ. Socially distant health care. Tul L Rev. 2021;96:423-468. https://scholarship .law.ua.edu/cgi/viewcontent.cgi?article=1713&context =fac_articles. Accessed March 4, 2023.
- Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
- Odibo IN, Wendel PJ, Magann EF. Telemedicine in obstetrics. Clin Obstet Gynecol. 2013;56:422-433. doi:10.1097/ GRF.0b013e318290fef0
- Shmerling A, Hoss M, Malam N, et al. Prenatal care via telehealth. Prim Care. 2022;49:609-619. doi:10.1016/j. pop.2022.05.002
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939
- Dosaj A, Thiyagarajan D, Ter Haar C, et al. Rapid implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2020;27:116-120. doi:10.1089/ tmj.2020.0219
- Lurie N, Carr B. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;187:745-746. doi: 10.1001/jamainternmed.2018.1314
- Tobah YSB, LeBlanc A, Branda E, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638-e1-638.e8. doi:10.1016/j.ajog.2019.06.034
- Vivanti AJ, Deruelle P, Piccone O, et al. Follow-up for pregnant women during the COVID-19 pandemic: French national authority for health recommendations. J Gynecol Obstet Hum Reprod. 2020;49:101804. doi:10.1016/j. jogoh.2020.101804
- Ellimoottil C. Takeaways from 2 key studies on interstate telehealth use among Medicare fee-for-service beneficiaries. JAMA Health Forum. 2022;3:e223020-E223020. doi:10.1001/ jamahealthforum.2022.3020
- Harris J, Hartnett T, Hoagland GW, et al. What eliminating barriers to interstate telehealth taught us during the pandemic. Bipartisan Policy Center. Published November 2021. Accessed March 9, 2023. https://bipartisanpolicy .org/download/?file=/wp-content/uploads/2021/11/BPC -Health-Licensure-Brief_WEB.pdf.
- Center for Connected Health Policy. Cross-state licensing. Accessed February 21, 2023. https://www.cchpca.org/topic /cross-state-licensing-professional-requirements.
- US Department of Health & Human Services. Telehealth. Getting started with licensure. Published February 3, 2023. Accessed February 27, 2023. https://telehealth.hhs.gov /licensure/getting-started-licensure/
- US Department of Health & Human Services. Telehealth. Licensure. Accessed February 27, 2023. https://telehealth .hhs.gov/licensure
- US Department of Health & Human Services. National Practitioner Data Bank (NPDB) code lists. Published December 2022. Accessed March 9, 2023. https://www.npdb .hrsa.gov/software/CodeLists.pdf
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, telehealth. 2020. Accessed March 5, 2023. https://www.acog.org /clinical-information/physician-faqs/covid-19-faqs-for -ob-gyns-telehealth
- Gorman RK. Prescribing medication through the practice of telemedicine: a comparative analysis of federal and state online prescribing policies, and policy considerations for the future. S Cal Interdisc Law J. 2020;30:739-769. https://gould .usc.edu/why/students/orgs/ilj/assets/docs/30-3-Gorman. pdf. Accessed March 10, 2023.
- Farringer DR. A telehealth explosion: using lessons from the pandemic to shape the future of telehealth regulation. Tex A&M Law Rev. 2021;9:1-47. https://scholarship.law.tamu. edu/cgi/viewcontent.cgi?article=1232&context=lawreview. Accessed February 28, 2023.
- Sterba KR, Johnson EE, Douglas E, et al. Implementation of a women’s reproductive behavioral health telemedicine program: a qualitative study of barriers and facilitators in obstetric and pediatric clinics. BMC Pregnancy Childbirth. 2023;23:167, 1-10. doi:10.1186/s12884-023-05463-2.
- US Department of Justice. COVID-19 FAQ (telemedicine). https://www.deadiversion.usdoj.gov/faq/coronavirus_faq .htm#TELE_FAQ2. Accessed March 13, 2023.
- US Department of Health & Human Services. Guidance on how the HIPAA rules permit covered health care providers and health plans to use remote communication technologies for audio-only telehealth. Published June 13, 2022. Accessed February 22, 2023. https://www.hhs.gov/hipaa/for-professionals/privacy /guidance/hipaa-audio-telehealth/index.html.
- Gray JME. HIPAA, telehealth, and the treatment of mental illness in a post-COVID world. Okla City Uni Law Rev. 2021;46:1-26. https://law.okcu.edu/wp-content /uploads/2022/04/J-Michael-E-Gray-HIPAA-Telehealth -and-Treament.pdf. Accessed March 9, 2023.
- Kurzweil C. Telemental health care and data privacy: current HIPAA privacy pitfalls and a proposed solution. Ann Health L Adv Dir. 2022;31:165.
- US Department of Health & Human Services and US Department of Justice. Health care fraud and abuse control program FY 2020: annual report. July 2021. Accessed March 9, 2023. https://oig.hhs.gov/publications/docs/hcfac /FY2020-hcfac.pdf
- Copeland KB. Telemedicine scams. Iowa Law Rev. 2022: 108:69-126. https://ilr.law.uiowa.edu/sites/ilr.law.uiowa.edu /files/2023-01/A2_Copeland.pdf. Accessed March 10, 2023.
- Solimini R, Busardò FP, Gibelli F, et al. Ethical and legal challenges of telemedicine in the era of the COVID-19 pandemic. Medicina (Kaunas). 2021;57:13141324. doi:10.3390/medicina57121314
- Reed A. COVID: a silver linings playbook. mobilizing pandemic era success stories to advance reproductive justice. Berkeley J Gender Law Justice. 2022;37:221-266. https://lawcat.berkeley.edu/record/1237158/files/16%20 Reed_final.pdf. Accessed March 11, 2023.
- Women’s Preventive Services Initiative and The American College of Obstetricians and Gynecologists. FAQ for telehealth services. Accessed March 2, 2023. https://www .womenspreventivehealth.org/wp-content/uploads/WPSI -Telehealth-FAQ.pdf
- Warren L, Chen KT. Telehealth apps in ObGyn practice. OBG Manag. 2022;34:46-47. doi:10.12788/obgm.0178
- American College of Obstetricians and Gynecologists. 10 telehealth tips for an Ob-Gyn visit. 2020. Accessed March 2, 2023. https://www.acog.org/womens-health /infographics/10-telehealth-tips-for-an-ob-gyn-visit
- Wolf TD. Telemedicine and malpractice: creating uniformity at the national level. Wm Mary Law Rev. 2019;61:15051536. https://scholarship.law.wm.edu/cgi/viewcontent.cgi ?article=3862&context=wmlr. Accessed March 11, 2023.
- Cahan E. Lawsuits, reimbursement, and liability insurance— facing the realities of a post-Roe era. JAMA. 2022;328:515517. doi:10.1001/jama.2022.9193
- Heinrich L, Hernandez AK, Laurie AR. Telehealth considerations for the adolescent patient. Prim Care. 2022;49:597-607. doi:10.1016/j.pop.2022.04.006
- Guttmacher Institute. An overview of consent to reproductive health services by young people. Published March 1, 2023. Accessed April 1, 2023. https://www.guttmacher.org /state-policy/explore/overview-minors-consent-law.
- Dobbs v. Jackson Women’s Health. No. 19–1392. June 24, 2022. Accessed April 1, 2023. https://www.supremecourt .gov/opinions/21pdf/19-1392_6j37.pdf
- Lindgren Y. Dobbs v. Jackson Women’s Health and the post-Roe landscape. J Am Acad Matrimonial Law. 2022;35:235283. https://www.aaml.org/wp-content/uploads/MAT110-1 .pdf. Accessed March 11, 2023.
- Mohiuddin H. The use of telemedicine during a pandemic to provide access to medication abortion. Hous J Health Law Policy. 2021;21:483-525. https://houstonhealthlaw. scholasticahq.com/article/34611.pdf. Accessed March 10, 2023.
- Rebouché R. The public health turn in reproductive rights. Wash & Lee Law Rev. 2021;78:1355-1432. https:// scholarlycommons.law.wlu.edu/cgi/viewcontent .cgi?article=4743&context=wlulr. Accessed March 10, 2023.
- Fliegel R. Access to medication abortion: now more important than ever. Am J Law Med. 2022;48:286-304. doi:10.1017/amj.2022.24
- Guttmacher Institute. Medication abortion. March 1, 2023. Accessed April 1, 2023 https://www.guttmacher.org /state-policy/explore/medication-abortion#:~:text=In%20 January%202023%2C%20the%20FDA,order%20to%20 dispense%20the%20pills
- Cohen DS, Donley G, Rebouché R. The new abortion battleground. Columbia Law Rev. 2023;123:1-100. https:// columbialawreview.org/content/the-new-abortion -battleground/. Accessed March 1, 2023.
- Hunt SA. Call me, beep me, if you want to reach me: utilizing telemedicine to expand abortion access. Vanderbilt Law Rev. 2023;76:323-359. Accessed March 10, 2023. https:// vanderbiltlawreview.org/lawreview/wp-content/uploads /sites/278/2023/01/Call-Me-Beep-Me-If-You-Want-toReach-Me-Utilizing-Telemedicine-to-Expand-AbortionAccess.pdf
- Gleckel JA, Wulkan SL. Abortion and telemedicine: looking beyond COVID-19 and the shadow docket. UC Davis Law Rev Online. 2020;54:105-121. https://lawreview.law.ucdavis. edu/online/54/files/54-online-Gleckel_Wulkan.pdf. Accessed April 1, 2023.

Telemedicine (or telehealth) originated in the early 1900s, when radios were used to communicate medical advice to clinics aboard ships.1 According to the American Telemedicine Association, telemedicine is namely “the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status.”2 These communications use 2-way video, email, smartphones, wireless tools, and other forms of telecommunications technology.
During the COVID-19 pandemic, many ObGyns—encouraged and advised by professional organizations—began providing telemedicine services.3 The first reported case of COVID-19 was in late 2019; the use of telemedicine was 38 times higher in February 2021 than in February 2020,4 illustrating how many physicians quickly moved to telemedicine practices.
CASE Dr. TM’s telemedicine dream
Before COVID-19, Dr. TM (an ObGyn practi-tioner) practiced in-person medicine in his home state. With the onset of the pandemic, Dr. TM struggled to switch to primarily seeing patients online (generally using Zoom or Facebook Live), with 1 day per week in the office for essential in-person visits.
After several months, however, Dr. TM’s routine became very efficient. He could see many more patients in a shorter time than with the former, in-person system. Therefore, as staff left his practice, Dr. TM did not replace them and also laid off others. Ultimately, the practice had 1 full-time records/insurance secretary who worked from home and 1 part-time nurse who helped with the in-person day and answered some patient inquiries by email. In part as an effort to add new patients, Dr. TM built an engaging website through which his current patients could receive medical information and new patients could sign up.
In late 2022, Dr. TM offered a $100 credit to any current patient who referred a friend or family member who then became a patient. This promotion was surprisingly effective and resulted in an influx of new patients. For example, Patient Z (a long-time patient) received 3 credits for referring her 3 sisters who lived out of state and became telepatients: Patient D, who lived 200 hundred miles away; Patient E, who lived 50 miles away in the adjoining state; and Patient F, who lived 150 miles away. Patient D contacted Dr. TM because she thought she was pregnant and wanted prenatal care, Patient E thought she might have a sexually transmitted infection (STI) and wanted treatment, and Patient F wanted general care and was inquiring about a medical abortion. Dr. TM agreed to treat Patient D but required 1 in-person visit. After 1 brief telemedicine session each with Patients E and F, Dr. TM wrote prescriptions for them.
By 2023, Dr. TM was enthusiastic about telemedicine as a professional practice. However, problems would ensue.
Dos and don’ts of telemedicine2
- Do take the initiative and inform patients of the availability of telemedicine/telehealth services
- Do use the services of medical malpractice insurance companies with regard to telemedicine
- Do integrate telemedicine into practice protocols and account for their limitations
- Don’t assume there are blanket exemptions or waivers in the states where your patients are located
Medical considerations
Telemedicine is endorsed by the American College of Obstetricians and Gynecologists (ACOG) as a vehicle for delivering prenatal and postpartum care.5 This represents an effort to reduce maternal and neonatal morbidity and mortality,5 as well as expandaccess to care and address the deficit in primary care providers and services, especially in rural and underserved populations.5,6 For obstetrics, prenatal care is designed to optimize pregnancy, childbirth, and postpartum care, with a focus on nutrition and genetic consultation and patient education on pregnancy, childbearing, breastfeeding, and newborn care.7
Benefits of telemedicine include its convenience for patients and providers, its efficiency and lower costs for providers (and hopefully patients, as well), and the potential improved access to care for patients.8 It is estimated that if a woman inititates obstetric care at 6 weeks, over the course of the 40-week gestation period, 15 prenatal visits will occur.9 Ultimately, the number of visits is determined based on the specifics of the pregnancy. With telemedicine, clinicians can provide those consultations, and information related to: ultrasonography, fetal echocardiography, and postpartum care services remotely.10 Using telemedicine may reduce missed visits, and remote monitoring may improve the quality of care.11
Barriers to telemedicine care include technical limitations, time constraints, and patient concerns of telehealth (visits). Technical limitations include the lack of a high speed internet connection and/or a smart device and the initial technical set-up–related problems,12 which affect providers as well as patients. Time constraints primarly refer to the ObGyn practice’s lack of time to establish telehealth services.13 Other challenges include integrating translation services, billing-related problems,10 and reimbursement and licensing barriers.14
Before the COVID-19 pandemic, obstetrics led the way in telemedicine with the development of the OB Nest model. Designed to replace in-person obstetrics care visits with telehealth,15 it includes home management tools such as blood pressure cuffs, cardiotocography, scales for weight checks, and Doppler ultrasounds.10 Patients can be instructed to measure fundal height and receive medications by mail. Anesthesia consultation can occur via this venue by having the patient complete a questionnaire prior to arriving at the labor and delivery unit.16
Legal considerations
With the COVID-19 pandemic, temporary changes were made to encourage the rapid adoption of telemedicine, including changes to licensing laws, certain prescription requirements, Health Insurance Portability and Accountability Act (HIPAA) privacy-security regulations, and reimbursement rules that required in-person visits. Thus, many ObGyns started using telemedicine during this rarified period, in which the rules appeared to be few and far between, with limited enforcement of the law and professional obligations.17 However, now that many of the legal rules that were suspended or ignored have been (or are being) reimposed and enforced, it is important for providers to become familiar with the legal issues involved in practicing telemedicine.
First, where is the patient? When discussing the legal issues of telemedicine, it is important to remember that many legal rules for medical care (ie, liability, informed consent, and licensing) vary from state to state. If the patient resides in a different state (“foreign” state) from the physician’s practice location (the physician’s “home” state), the care is considered delivered in the state where the patient is located. Thus, the patient’s location generally establishes the law covering the telemedicine transaction. In the following discussion, the rules refer to the law and professional obligations, with commentary on some key legal issues that are relevant to ObGyn telemedicine.
Continue to: Reinforcing the rules...
Reinforcing the rules
Licensing
During the height of the COVID-19 pandemic, the federal government and almost all states temporarily modified the licensing requirement to allow telemedicine based on an existing medical license in any state—disregarding the “where is the patient” rule. As those rules begin to lapse or change with the official end of the pandemic declared by President Biden as May 2023,17 the rules under which a physician began telemedicine interstate practice in 2020 also may be changing.
Simply put, “The same standards for licensure apply to health care providers regardless of whether care is delivered in-person or virtually through telehealth services.”18 When a physician is engaged in telemedicine treatment of a patient in the physician’s home state, there is generally no licensing issue. Telemedicine generally does not require a separate specific license.19 However, when the patient is in another state (a “foreign” state), there can be a substantial licensing issue.20 Ordinarily, to provide that treatment, the physician must, in some manner, be approved to practice in the patient’s state. That may occur, for example, in the following ways: (1) the physician may hold an additional regular license in the patient’s state, which allows practice there, or (2) the physician may have received permission for “temporary practice” in another state.
Many states (often adjoining states) have formal agreements with other states that allow telemedicine practice by providers in each other’s states. There also are “compacts”, or agreements that enable providers in any of the participating states to practice in the other associated states without a separate license.18 Although several websites provide information about compact licensing and the like, clinicians should not rely on simple lists or maps. Individual states may have special provisions about applying their laws to out-of-state “compact” physicians. In addition, under the Interstate Medical Licensure Compact, “physicians have to pay licensing fees and satisfy the requirements of each medical board in the states where they wish to practice.”21
Consequences. Practicing telemedicine with a patient in a state where the physician does not have a license is generally a crime. Furthermore, it may be the basis for license discipline in the physician’s home state and result in a report to the National Practi-tioner Databank.22 In addition, reimbursement often depends on the practitioner being licensed, and the absence of a license may be a basis for denying payment for services.23 Finally, malpractice insurance generally is limited to licensed practice. Thus, the insurer may decline to defend the unlicensed clinician against a malpractice claim or pay any damages.
Prescribing privileges
Prescribing privileges usually are connected to licensing, so as the rules for licensing change postpandemic, so do the rules for prescribing. In most cases, the physician must have a license in the state where care is given to prescribe medication—which in telemedicine, as noted, typically means the state where the patient is located. Exceptions vary by state, but in general, if a physician does not have a license to provide care, the physician is unlikely to be authorized to prescribe medication.24 Failure to abide by the applicable state rules may result in civil and even criminal liability for illegal prescribing activity.
In addition, the US Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA, which enforces laws concerning controlled substances) also regulate the prescription and sale of pharmaceuticals.25 There are state and federal limits on the ability of clinicians to order controlled substances without an in-person visit. The Ryan Haight Online Pharmacy Consumer Protection Act, for example, sets limits on controlled substance prescriptions without an in-person examination.26 Federal law was modified due to COVID-19 to permit prescribing of many controlled substances by telemedicine if there is synchronous audio and visual examination of the patient. Physicians who write such prescriptions also are required to have a DEA registration in the patient’s state. This is an essential consideration for physicians considering interstate telemedicine practice.27
HIPAA and privacy
Governments waived some of the legal requirements related to health information during the pandemic, but those waivers either have expired or will do so soon. Federal and state laws regarding privacy and security—notably including HIPAA—apply to telemedicine and are of particular concern given the considerable amount of communication of protected health information with telemedicine.
HIPAA security rules essentially require making sure health information cannot be hacked or intercepted. Audio-only telemedicine by landline (not cell) is acceptable under the security rules, but almost all other remote communication requires secure communications.28
Clinicians also need to adhere to the more usual HIPAA privacy rules when practicingtelehealth. State laws protecting patient privacy vary and may be more stringent than HIPAA, so clinicians also must know the requirements in any state where they practice—whether in office or telemedicine.29
Making sure telemedicine practices are consistent with these security and privacy rules often requires particular technical expertise that is outside the realm of most practicing clinicians. However, without modification, the pre-telemedicine technology of many medical offices likely is insufficient for the full range of telemedicine services.30
Reimbursement and fraud
Before COVID-19, Medicare and Medicaid reimbursement for telemedicine was limited. Government decisions to substantially broaden those reimbursement rules (at least temporarily) provided a substantial boost to telemedicine early in the pandemic.23 Federal regulations and statutes also expanded telemedicine reimbursement for various services. Some will end shortly after the health emergency, and others will be permanent. Parts of that will not be sorted out for several years, so it will likely be a changing landscape for reimbursement.
Continue to: Rules that are evolving...
Rules that are evolving
Informed consent
The ethical and legal obligations to obtain informed consent are present in telemedicineas well as in-person care, with the same basic requirements regarding risks, benefits, alternative care, etc.32 However, with telemedicine, information related to remote care should be included and is outlined in TABLE 1.

Certain states may have somewhat unique informed consent requirements—especially for reproductive care, including abortion.34 Therefore, it is important for clinicians to ensure their consent process and forms comply with any legal jurisdiction in which a patient is located.
Medical malpractice
The basics of medical malpractice (or negligence) are the same in telemedicine as in in-person care: duty, breach of duty, and injury caused by the breach. That is, there may be liability when a medical professional breaches the duty of care, causing the patient’s injury. The physician’s duty is defined by the quality of care that the profession (specialty) accepts as reasonably good. This is defined by the opinions of physicians within the specialty and formal statements from professional organizations, including ACOG.3
Maintaining the standard of care and quality. The use of telemedicine is not an excuse to lower the quality of health care. There are some circumstances for which it is medically better to have an in-person visit. In these instances, the provider should recommend the appropriate care, even if telemedicine would be more convenient for the provider and staff.35
If the patient insists and telemedicine might result in less than optimal care, the reasons for using a remote visit should be clearly documented contemporaneously with the decision. Furthermore, when the limitations of being unable to physically examine the patient result in less information than is needed for the patient’s care, the provider must find alternatives to make up for the information gap.11,36 It also may be necessary to inform patients about how to maximize telemedicine care.37 At the beginning of telemedicine care the provider should include information about the nature and limits of telehealth, and the patient’s responsibilities. (See TABLE 1) Throughout treatment of the patient, that information should be updated by the provider. That, of course, is particularly important for patients who have not previously used telemedice services.
Malpractice rules vary by state. Many states have special rules regarding malpractice cases. These differences in malpractice standards and regulations “can be problematic for physicians who use telemedicine services to provide care outside the state in which they practice.”38 Caps on noneconomic damages are an example. Those state rules would apply to telemedicine in the patient’s state.
Malpractice insurance
Malpractice insurance now commonly includes telemedicine legally practiced within the physician’s home state. Practitioners who treat patients in foreign states should carefully examine their malpractice insurance policies to confirm that the coverage extends to practice in those states.39 Malpractice carriers may require notification by a covered physician who routinely provides services to patients in another state.3
Keep in mind, malpractice insurance generally does not cover the practice of medicine that is illegal. Practicing telemedicine in a foreign state, where the physician or other provider does not have a license and where that state does not otherwise permit the practice, is illegal. Most likely, the physician’s malpractice insurance will not cover claims that arise from this illegal practice in a foreign state or provide defense for malpractice claims, including frivolous lawsuits. Thus, the physician will pay out of pocket for the costs of a defense attorney.
Telemedicine treatment of minors
Children and adolescents present special legal issues for ObGyn care, which may become more complicated with telemedicine. Historically, parents are responsible for minors (those aged <18 years): they consent to medical treatment, are responsible for paying for it, and have the right to receive information about treatment.
Over the years, though, many states have made exceptions to these principles, especially with regard to contraception and treatment of sexually transmitted diseases.40 For abortion, in particular, there is considerable variation among the states in parental consent and notification.41 The Supreme Court’s decision in Dobbs v Jackson Women’s Health42 may (depending on the state) be followed with more stringent limitations on adolescent consent to abortions, including medical abortions.43
Use of telehealth does not change any obligations regarding adolescent consent or parental notification. Because those differ considerably among states, it is important for all practitioners to know their states’ requirements and keep reasonably complete records demonstrating their compliance with state law.
Abortion
The most heated current controversy about telemedicine involves abortion—specifically medical abortion, which is the combination of mifepristone and misoprostol.44,45 The FDA approved the combination in 2000. Almost immediately, many states required in-person visits with a certified clinician to receive a prescription for mifepristone and misoprostol, and eventually, the FDA adopted similar requirements.46 However, during the pandemic from 2021 to 2022, the FDA permitted telemedicine prescriptions. Several states still require in-person physician visits, although the constitutionality of those requirements has not been established.47
With the Supreme Court’s decision in Dobbs v Jackson Women’s Health in 2022,42 disagreements have ensued about the degree to which states may regulate the prescription of FDA-approved medical abortion drugs. Thorny constitutional issues exist in the plans of both abortion opponents and proponents in the battle over medical abortion in antiabortion states. It may be that federal drug law preempts state laws limiting access to FDA-approved drugs. On the other hand, it may be that states can make it a crime within the state to possess or provide abortion-inducing drugs. Courts will probably take years to resolve the many tangled legal questions.48
Thus, while the pandemic telemedicine rules may have advanced access to abortion,34 there may be some pending downsides.49 States that prohibit abortion will likely include prohibitions on medical abortions. In addition, they may prohibit anyone in the state (including pharmacies) from selling, possessing, or obtaining any drug used for causing or inducing an abortion.50 If, for constitutional reasons, they cannot press criminal charges or undertake licensing discipline for prescribing abortion, some states will likely withdraw from telehealth licensing compacts to avoid out-of-state prescriptions. This area of telemedicine has considerable uncertainty.
Continue to: CASE Conclusion...
CASE Conclusion
Patient concerns come to the fore
By 2023, Dr. TM started receiving bad news. Patient D called complaining that after following the advice on the website, she suffered a severe reaction and had to be rushed to an emergency department. Patient E (who had only 1 in-office visit early in her pregnancy) notified the office that she developed very high blood pressure that resulted in severe placental abruption, requiring emergency care and resulting in the loss of the fetus. Patient F complained that someone hacked the TikTok direct message communication with Dr. TM and tried to “blackmail” or harass her.
Discussion. Patients D, E, and F represent potential problems of telemedicine practice. Patient D was injured because she relied on her doctor’s website (to which Dr. TM directed patients). It contained an error that caused an injury. A doctor-patient relationship existed, and bad medical advice likely caused the injury. Physicians providing advice online must ensure the advice is correct and kept current.
Patient E demonstrates the importance of monitoring patients remotely (blood pressure transmitted to the office) or with periodic in-office visits. It is not clear whether she was a no-show for office visits (and whether the office followed up on any missed appointments) or if such visits were never scheduled. Liability for failure to monitor adequately is a possibility.
Patient F’s seemingly minor complaint could be a potential problem. Dr. TM used an insecure mode of communication. Although some HIPAA security regulations were modified or suspended during the pandemic, using such an unsecure platform is problematic, especially if temporary HIPAA rules expired. The outcome of the complaint is in doubt.

(See TABLE 2 for additional comments on patients D, E, and F.)
Out-of-state practice
Dr. TM treated 3 out-of-state residents (D, E, and F) via telemedicine. Recently Dr. TM received a complaint from the State Medical Licensure Board for practicing medicine without a license (Patient D), followed by similar charges from Patient E’s and Patient F’s state licensing boards. He has received a licensing inquiry from his home state board about those claims of illegal practice in other states and incompetent treatment.
Patient D’s pregnancy did not go well. The 1 in-person visit did not occur and she has filed a malpractice suit against Dr. TM. Patient E is threatening a malpractice case because the STI was not appropriately diagnosed and had advanced before another physician treated it.
In addition, a private citizen in Patient F’s state has filed suit against Dr. TM for abetting an illegal abortion (for Patient F).
Discussion. Patients D, E, and F illustrate the risk of even incidental out-of-state practice. The medical board inquiries arose from anonymous tips to all 4 states reporting Dr. TM was “practicing medicine without a license.” Patient E’s home state did have a licensing compact with the adjoining state (ie, Dr. TM’s home state). However, it required physicians to register and file an annual report, which Dr. TM had not done. The other 2 states did not have compacts with Dr. TM’s home state. Thus, he was illegally practicing medicine and would be subject to penalties. His home state also might impose license discipline based on his illegal practice in other states.
Continue to: What’s the verdict?...
What’s the verdict?
Dr. TM’s malpractice carrier is refusing to defend the claims of medical malpractice threatened by Patients D, E, and F. The company first notes that the terms of the malpractice policy specifically exclude the illegal practice of medicine. Furthermore, when a physician legally practices in another state, the policy requires a written notice to the insurance carrier of such practice. Dr. TM will likely have to engage and pay for a malpractice attorney for these cases. Because the claims are filed in 3 different states, more than a home-state attorney will likely be involved in the defense of these cases. Dr. TM will need to pay the attorneys and any damages from a settlement or trial.
Malpractice claims. Patient D claims that the doctor essentially abandoned her by never reaching out to her or arranging an in-person visit. Dr. TM claims the patient was responsible for scheduling the in-person visit. Patient E claims it was malpractice not to determine the specific nature of the STI and to do follow-up testing to determine that it was cured. All patients claim there was no genuine informed consent to the telemedicine. An attorney has warned Dr. TM that it is “not going to look good to the jury” that he was practicing without a license in the state and suggests he settle the cases quickly by paying damages.
Abortion-related claims. Patient F presents a different set of problems. Dr. TM’s home state is “proabortion.” Patient F’s home state is strongly “antiabortion,” making it a felony to participate in, assist, or facilitate an abortion (including medical abortion). Criminal charges have been filed against Dr. TM for the illegal practice of medicine, for aiding and facilitating an abortion, and for failure to notify a parent that a minor is seeking an abortion. For now, Dr. TM’s state is refusing to extradite on the abortion charge. Still, the patient’s state insists that it do so on the illegal practice of medicine charges and new charges of insurance fraud and failure to report suspected sexual abuse of a child. (Under the patient’s state law, anyone having sex with Patient F would have engaged in sexual abuse or “statutory rape,” so the state insists that the fact she was pregnant proves someone had sex with her.)
Patient F’s state also has a statute that allows private citizens to file civil claims against anyone procuring or assisting with an abortion (a successful private citizen can receive a minimum of $10,000 from the defendant). Several citizens from the patient’s state have already filed claims against Dr. TM in his state courts. Only one of them, probably the first to file, could succeed. Courts in the state have issued subpoenas and ordered Dr. TM to appear and reply to the civil suits. If he does not respond, there will be a default judgment.
Dr. TM’s attorney tells him that these lawsuits will not settle and will take a long time to defend and resolve. That will be expensive.
Billing and fraud. Dr. TM’s office recently received a series of notices from private health insurers stating they are investigating previously made payments as being fraudulent (unlicensed). They will not pay any new claims pending the investigation. On behalf of Medicare-Medicaid and other federal programs, the US Attorney’s office has notified Dr. TM that it has opened an investigation into fraudulent federal payments. F’s home state also is filing a (criminal) insurance fraud case, although the basis for it is unclear. (Dr. TM’s attorney believes it might be to increase pressure on the physician’s state to extradite Dr. TM for Patient F’s case.)
In addition, a disgruntled former employee of Dr. TM has filed a federal FCA case against him for filing inflated claims with various federally funded programs. The employee also made whistleblower calls to insurance companies and some state-funded medical programs. A forensic accounting investigation by Dr. TM’s accountant confirmed a pattern of very sloppy records and recurring billing for televisits that did not occur. Dr. TM believes that this was the act of one of the temporary assistants he hired in a pinch, who did not understand the system and just guessed when filing some insurance claims.
During the investigation, the federal and state attorneys are looking into a possible violation of state and federal Anti-Kickback Statutes. This is based on the original offer of a $100 credit for referrals to Dr. TM’s telemedicine practice.
The attorneys are concerned that other legal problems may present themselves. They are thoroughly reviewing Dr. TM’s practice and making several critical but somewhat modest changes to his practice. They also have insisted that Dr. TM have appropriate staff to handle the details of the practice and billing.
Conclusions
Telemedicine presents notable legal challenges to medical practice. As the pandemic status ends, ObGyn physicians practicing telemedicine need to be aware of the rules and how they are changing. For those physicians who want to continue or start a telemedicine practice, securing legal and technical support to ensure your operations are inline with the legal requirements can minimize any risk of legal troubles in the future. ●
A physician in State A, where abortion is legal, has a telemedicine patient in State B, where it is illegal to assist, provide, or procure an abortion. If the physician prescribes a medical abortion, he would violate the law of State B by using telemedicine to help the patient (located in State B) obtain an abortion. This could result in criminal charges against the prescribing physician.

Telemedicine (or telehealth) originated in the early 1900s, when radios were used to communicate medical advice to clinics aboard ships.1 According to the American Telemedicine Association, telemedicine is namely “the use of medical information exchanged from one site to another via electronic communications to improve a patient’s clinical health status.”2 These communications use 2-way video, email, smartphones, wireless tools, and other forms of telecommunications technology.
During the COVID-19 pandemic, many ObGyns—encouraged and advised by professional organizations—began providing telemedicine services.3 The first reported case of COVID-19 was in late 2019; the use of telemedicine was 38 times higher in February 2021 than in February 2020,4 illustrating how many physicians quickly moved to telemedicine practices.
CASE Dr. TM’s telemedicine dream
Before COVID-19, Dr. TM (an ObGyn practi-tioner) practiced in-person medicine in his home state. With the onset of the pandemic, Dr. TM struggled to switch to primarily seeing patients online (generally using Zoom or Facebook Live), with 1 day per week in the office for essential in-person visits.
After several months, however, Dr. TM’s routine became very efficient. He could see many more patients in a shorter time than with the former, in-person system. Therefore, as staff left his practice, Dr. TM did not replace them and also laid off others. Ultimately, the practice had 1 full-time records/insurance secretary who worked from home and 1 part-time nurse who helped with the in-person day and answered some patient inquiries by email. In part as an effort to add new patients, Dr. TM built an engaging website through which his current patients could receive medical information and new patients could sign up.
In late 2022, Dr. TM offered a $100 credit to any current patient who referred a friend or family member who then became a patient. This promotion was surprisingly effective and resulted in an influx of new patients. For example, Patient Z (a long-time patient) received 3 credits for referring her 3 sisters who lived out of state and became telepatients: Patient D, who lived 200 hundred miles away; Patient E, who lived 50 miles away in the adjoining state; and Patient F, who lived 150 miles away. Patient D contacted Dr. TM because she thought she was pregnant and wanted prenatal care, Patient E thought she might have a sexually transmitted infection (STI) and wanted treatment, and Patient F wanted general care and was inquiring about a medical abortion. Dr. TM agreed to treat Patient D but required 1 in-person visit. After 1 brief telemedicine session each with Patients E and F, Dr. TM wrote prescriptions for them.
By 2023, Dr. TM was enthusiastic about telemedicine as a professional practice. However, problems would ensue.
Dos and don’ts of telemedicine2
- Do take the initiative and inform patients of the availability of telemedicine/telehealth services
- Do use the services of medical malpractice insurance companies with regard to telemedicine
- Do integrate telemedicine into practice protocols and account for their limitations
- Don’t assume there are blanket exemptions or waivers in the states where your patients are located
Medical considerations
Telemedicine is endorsed by the American College of Obstetricians and Gynecologists (ACOG) as a vehicle for delivering prenatal and postpartum care.5 This represents an effort to reduce maternal and neonatal morbidity and mortality,5 as well as expandaccess to care and address the deficit in primary care providers and services, especially in rural and underserved populations.5,6 For obstetrics, prenatal care is designed to optimize pregnancy, childbirth, and postpartum care, with a focus on nutrition and genetic consultation and patient education on pregnancy, childbearing, breastfeeding, and newborn care.7
Benefits of telemedicine include its convenience for patients and providers, its efficiency and lower costs for providers (and hopefully patients, as well), and the potential improved access to care for patients.8 It is estimated that if a woman inititates obstetric care at 6 weeks, over the course of the 40-week gestation period, 15 prenatal visits will occur.9 Ultimately, the number of visits is determined based on the specifics of the pregnancy. With telemedicine, clinicians can provide those consultations, and information related to: ultrasonography, fetal echocardiography, and postpartum care services remotely.10 Using telemedicine may reduce missed visits, and remote monitoring may improve the quality of care.11
Barriers to telemedicine care include technical limitations, time constraints, and patient concerns of telehealth (visits). Technical limitations include the lack of a high speed internet connection and/or a smart device and the initial technical set-up–related problems,12 which affect providers as well as patients. Time constraints primarly refer to the ObGyn practice’s lack of time to establish telehealth services.13 Other challenges include integrating translation services, billing-related problems,10 and reimbursement and licensing barriers.14
Before the COVID-19 pandemic, obstetrics led the way in telemedicine with the development of the OB Nest model. Designed to replace in-person obstetrics care visits with telehealth,15 it includes home management tools such as blood pressure cuffs, cardiotocography, scales for weight checks, and Doppler ultrasounds.10 Patients can be instructed to measure fundal height and receive medications by mail. Anesthesia consultation can occur via this venue by having the patient complete a questionnaire prior to arriving at the labor and delivery unit.16
Legal considerations
With the COVID-19 pandemic, temporary changes were made to encourage the rapid adoption of telemedicine, including changes to licensing laws, certain prescription requirements, Health Insurance Portability and Accountability Act (HIPAA) privacy-security regulations, and reimbursement rules that required in-person visits. Thus, many ObGyns started using telemedicine during this rarified period, in which the rules appeared to be few and far between, with limited enforcement of the law and professional obligations.17 However, now that many of the legal rules that were suspended or ignored have been (or are being) reimposed and enforced, it is important for providers to become familiar with the legal issues involved in practicing telemedicine.
First, where is the patient? When discussing the legal issues of telemedicine, it is important to remember that many legal rules for medical care (ie, liability, informed consent, and licensing) vary from state to state. If the patient resides in a different state (“foreign” state) from the physician’s practice location (the physician’s “home” state), the care is considered delivered in the state where the patient is located. Thus, the patient’s location generally establishes the law covering the telemedicine transaction. In the following discussion, the rules refer to the law and professional obligations, with commentary on some key legal issues that are relevant to ObGyn telemedicine.
Continue to: Reinforcing the rules...
Reinforcing the rules
Licensing
During the height of the COVID-19 pandemic, the federal government and almost all states temporarily modified the licensing requirement to allow telemedicine based on an existing medical license in any state—disregarding the “where is the patient” rule. As those rules begin to lapse or change with the official end of the pandemic declared by President Biden as May 2023,17 the rules under which a physician began telemedicine interstate practice in 2020 also may be changing.
Simply put, “The same standards for licensure apply to health care providers regardless of whether care is delivered in-person or virtually through telehealth services.”18 When a physician is engaged in telemedicine treatment of a patient in the physician’s home state, there is generally no licensing issue. Telemedicine generally does not require a separate specific license.19 However, when the patient is in another state (a “foreign” state), there can be a substantial licensing issue.20 Ordinarily, to provide that treatment, the physician must, in some manner, be approved to practice in the patient’s state. That may occur, for example, in the following ways: (1) the physician may hold an additional regular license in the patient’s state, which allows practice there, or (2) the physician may have received permission for “temporary practice” in another state.
Many states (often adjoining states) have formal agreements with other states that allow telemedicine practice by providers in each other’s states. There also are “compacts”, or agreements that enable providers in any of the participating states to practice in the other associated states without a separate license.18 Although several websites provide information about compact licensing and the like, clinicians should not rely on simple lists or maps. Individual states may have special provisions about applying their laws to out-of-state “compact” physicians. In addition, under the Interstate Medical Licensure Compact, “physicians have to pay licensing fees and satisfy the requirements of each medical board in the states where they wish to practice.”21
Consequences. Practicing telemedicine with a patient in a state where the physician does not have a license is generally a crime. Furthermore, it may be the basis for license discipline in the physician’s home state and result in a report to the National Practi-tioner Databank.22 In addition, reimbursement often depends on the practitioner being licensed, and the absence of a license may be a basis for denying payment for services.23 Finally, malpractice insurance generally is limited to licensed practice. Thus, the insurer may decline to defend the unlicensed clinician against a malpractice claim or pay any damages.
Prescribing privileges
Prescribing privileges usually are connected to licensing, so as the rules for licensing change postpandemic, so do the rules for prescribing. In most cases, the physician must have a license in the state where care is given to prescribe medication—which in telemedicine, as noted, typically means the state where the patient is located. Exceptions vary by state, but in general, if a physician does not have a license to provide care, the physician is unlikely to be authorized to prescribe medication.24 Failure to abide by the applicable state rules may result in civil and even criminal liability for illegal prescribing activity.
In addition, the US Food and Drug Administration (FDA) and Drug Enforcement Administration (DEA, which enforces laws concerning controlled substances) also regulate the prescription and sale of pharmaceuticals.25 There are state and federal limits on the ability of clinicians to order controlled substances without an in-person visit. The Ryan Haight Online Pharmacy Consumer Protection Act, for example, sets limits on controlled substance prescriptions without an in-person examination.26 Federal law was modified due to COVID-19 to permit prescribing of many controlled substances by telemedicine if there is synchronous audio and visual examination of the patient. Physicians who write such prescriptions also are required to have a DEA registration in the patient’s state. This is an essential consideration for physicians considering interstate telemedicine practice.27
HIPAA and privacy
Governments waived some of the legal requirements related to health information during the pandemic, but those waivers either have expired or will do so soon. Federal and state laws regarding privacy and security—notably including HIPAA—apply to telemedicine and are of particular concern given the considerable amount of communication of protected health information with telemedicine.
HIPAA security rules essentially require making sure health information cannot be hacked or intercepted. Audio-only telemedicine by landline (not cell) is acceptable under the security rules, but almost all other remote communication requires secure communications.28
Clinicians also need to adhere to the more usual HIPAA privacy rules when practicingtelehealth. State laws protecting patient privacy vary and may be more stringent than HIPAA, so clinicians also must know the requirements in any state where they practice—whether in office or telemedicine.29
Making sure telemedicine practices are consistent with these security and privacy rules often requires particular technical expertise that is outside the realm of most practicing clinicians. However, without modification, the pre-telemedicine technology of many medical offices likely is insufficient for the full range of telemedicine services.30
Reimbursement and fraud
Before COVID-19, Medicare and Medicaid reimbursement for telemedicine was limited. Government decisions to substantially broaden those reimbursement rules (at least temporarily) provided a substantial boost to telemedicine early in the pandemic.23 Federal regulations and statutes also expanded telemedicine reimbursement for various services. Some will end shortly after the health emergency, and others will be permanent. Parts of that will not be sorted out for several years, so it will likely be a changing landscape for reimbursement.
Continue to: Rules that are evolving...
Rules that are evolving
Informed consent
The ethical and legal obligations to obtain informed consent are present in telemedicineas well as in-person care, with the same basic requirements regarding risks, benefits, alternative care, etc.32 However, with telemedicine, information related to remote care should be included and is outlined in TABLE 1.

Certain states may have somewhat unique informed consent requirements—especially for reproductive care, including abortion.34 Therefore, it is important for clinicians to ensure their consent process and forms comply with any legal jurisdiction in which a patient is located.
Medical malpractice
The basics of medical malpractice (or negligence) are the same in telemedicine as in in-person care: duty, breach of duty, and injury caused by the breach. That is, there may be liability when a medical professional breaches the duty of care, causing the patient’s injury. The physician’s duty is defined by the quality of care that the profession (specialty) accepts as reasonably good. This is defined by the opinions of physicians within the specialty and formal statements from professional organizations, including ACOG.3
Maintaining the standard of care and quality. The use of telemedicine is not an excuse to lower the quality of health care. There are some circumstances for which it is medically better to have an in-person visit. In these instances, the provider should recommend the appropriate care, even if telemedicine would be more convenient for the provider and staff.35
If the patient insists and telemedicine might result in less than optimal care, the reasons for using a remote visit should be clearly documented contemporaneously with the decision. Furthermore, when the limitations of being unable to physically examine the patient result in less information than is needed for the patient’s care, the provider must find alternatives to make up for the information gap.11,36 It also may be necessary to inform patients about how to maximize telemedicine care.37 At the beginning of telemedicine care the provider should include information about the nature and limits of telehealth, and the patient’s responsibilities. (See TABLE 1) Throughout treatment of the patient, that information should be updated by the provider. That, of course, is particularly important for patients who have not previously used telemedice services.
Malpractice rules vary by state. Many states have special rules regarding malpractice cases. These differences in malpractice standards and regulations “can be problematic for physicians who use telemedicine services to provide care outside the state in which they practice.”38 Caps on noneconomic damages are an example. Those state rules would apply to telemedicine in the patient’s state.
Malpractice insurance
Malpractice insurance now commonly includes telemedicine legally practiced within the physician’s home state. Practitioners who treat patients in foreign states should carefully examine their malpractice insurance policies to confirm that the coverage extends to practice in those states.39 Malpractice carriers may require notification by a covered physician who routinely provides services to patients in another state.3
Keep in mind, malpractice insurance generally does not cover the practice of medicine that is illegal. Practicing telemedicine in a foreign state, where the physician or other provider does not have a license and where that state does not otherwise permit the practice, is illegal. Most likely, the physician’s malpractice insurance will not cover claims that arise from this illegal practice in a foreign state or provide defense for malpractice claims, including frivolous lawsuits. Thus, the physician will pay out of pocket for the costs of a defense attorney.
Telemedicine treatment of minors
Children and adolescents present special legal issues for ObGyn care, which may become more complicated with telemedicine. Historically, parents are responsible for minors (those aged <18 years): they consent to medical treatment, are responsible for paying for it, and have the right to receive information about treatment.
Over the years, though, many states have made exceptions to these principles, especially with regard to contraception and treatment of sexually transmitted diseases.40 For abortion, in particular, there is considerable variation among the states in parental consent and notification.41 The Supreme Court’s decision in Dobbs v Jackson Women’s Health42 may (depending on the state) be followed with more stringent limitations on adolescent consent to abortions, including medical abortions.43
Use of telehealth does not change any obligations regarding adolescent consent or parental notification. Because those differ considerably among states, it is important for all practitioners to know their states’ requirements and keep reasonably complete records demonstrating their compliance with state law.
Abortion
The most heated current controversy about telemedicine involves abortion—specifically medical abortion, which is the combination of mifepristone and misoprostol.44,45 The FDA approved the combination in 2000. Almost immediately, many states required in-person visits with a certified clinician to receive a prescription for mifepristone and misoprostol, and eventually, the FDA adopted similar requirements.46 However, during the pandemic from 2021 to 2022, the FDA permitted telemedicine prescriptions. Several states still require in-person physician visits, although the constitutionality of those requirements has not been established.47
With the Supreme Court’s decision in Dobbs v Jackson Women’s Health in 2022,42 disagreements have ensued about the degree to which states may regulate the prescription of FDA-approved medical abortion drugs. Thorny constitutional issues exist in the plans of both abortion opponents and proponents in the battle over medical abortion in antiabortion states. It may be that federal drug law preempts state laws limiting access to FDA-approved drugs. On the other hand, it may be that states can make it a crime within the state to possess or provide abortion-inducing drugs. Courts will probably take years to resolve the many tangled legal questions.48
Thus, while the pandemic telemedicine rules may have advanced access to abortion,34 there may be some pending downsides.49 States that prohibit abortion will likely include prohibitions on medical abortions. In addition, they may prohibit anyone in the state (including pharmacies) from selling, possessing, or obtaining any drug used for causing or inducing an abortion.50 If, for constitutional reasons, they cannot press criminal charges or undertake licensing discipline for prescribing abortion, some states will likely withdraw from telehealth licensing compacts to avoid out-of-state prescriptions. This area of telemedicine has considerable uncertainty.
Continue to: CASE Conclusion...
CASE Conclusion
Patient concerns come to the fore
By 2023, Dr. TM started receiving bad news. Patient D called complaining that after following the advice on the website, she suffered a severe reaction and had to be rushed to an emergency department. Patient E (who had only 1 in-office visit early in her pregnancy) notified the office that she developed very high blood pressure that resulted in severe placental abruption, requiring emergency care and resulting in the loss of the fetus. Patient F complained that someone hacked the TikTok direct message communication with Dr. TM and tried to “blackmail” or harass her.
Discussion. Patients D, E, and F represent potential problems of telemedicine practice. Patient D was injured because she relied on her doctor’s website (to which Dr. TM directed patients). It contained an error that caused an injury. A doctor-patient relationship existed, and bad medical advice likely caused the injury. Physicians providing advice online must ensure the advice is correct and kept current.
Patient E demonstrates the importance of monitoring patients remotely (blood pressure transmitted to the office) or with periodic in-office visits. It is not clear whether she was a no-show for office visits (and whether the office followed up on any missed appointments) or if such visits were never scheduled. Liability for failure to monitor adequately is a possibility.
Patient F’s seemingly minor complaint could be a potential problem. Dr. TM used an insecure mode of communication. Although some HIPAA security regulations were modified or suspended during the pandemic, using such an unsecure platform is problematic, especially if temporary HIPAA rules expired. The outcome of the complaint is in doubt.

(See TABLE 2 for additional comments on patients D, E, and F.)
Out-of-state practice
Dr. TM treated 3 out-of-state residents (D, E, and F) via telemedicine. Recently Dr. TM received a complaint from the State Medical Licensure Board for practicing medicine without a license (Patient D), followed by similar charges from Patient E’s and Patient F’s state licensing boards. He has received a licensing inquiry from his home state board about those claims of illegal practice in other states and incompetent treatment.
Patient D’s pregnancy did not go well. The 1 in-person visit did not occur and she has filed a malpractice suit against Dr. TM. Patient E is threatening a malpractice case because the STI was not appropriately diagnosed and had advanced before another physician treated it.
In addition, a private citizen in Patient F’s state has filed suit against Dr. TM for abetting an illegal abortion (for Patient F).
Discussion. Patients D, E, and F illustrate the risk of even incidental out-of-state practice. The medical board inquiries arose from anonymous tips to all 4 states reporting Dr. TM was “practicing medicine without a license.” Patient E’s home state did have a licensing compact with the adjoining state (ie, Dr. TM’s home state). However, it required physicians to register and file an annual report, which Dr. TM had not done. The other 2 states did not have compacts with Dr. TM’s home state. Thus, he was illegally practicing medicine and would be subject to penalties. His home state also might impose license discipline based on his illegal practice in other states.
Continue to: What’s the verdict?...
What’s the verdict?
Dr. TM’s malpractice carrier is refusing to defend the claims of medical malpractice threatened by Patients D, E, and F. The company first notes that the terms of the malpractice policy specifically exclude the illegal practice of medicine. Furthermore, when a physician legally practices in another state, the policy requires a written notice to the insurance carrier of such practice. Dr. TM will likely have to engage and pay for a malpractice attorney for these cases. Because the claims are filed in 3 different states, more than a home-state attorney will likely be involved in the defense of these cases. Dr. TM will need to pay the attorneys and any damages from a settlement or trial.
Malpractice claims. Patient D claims that the doctor essentially abandoned her by never reaching out to her or arranging an in-person visit. Dr. TM claims the patient was responsible for scheduling the in-person visit. Patient E claims it was malpractice not to determine the specific nature of the STI and to do follow-up testing to determine that it was cured. All patients claim there was no genuine informed consent to the telemedicine. An attorney has warned Dr. TM that it is “not going to look good to the jury” that he was practicing without a license in the state and suggests he settle the cases quickly by paying damages.
Abortion-related claims. Patient F presents a different set of problems. Dr. TM’s home state is “proabortion.” Patient F’s home state is strongly “antiabortion,” making it a felony to participate in, assist, or facilitate an abortion (including medical abortion). Criminal charges have been filed against Dr. TM for the illegal practice of medicine, for aiding and facilitating an abortion, and for failure to notify a parent that a minor is seeking an abortion. For now, Dr. TM’s state is refusing to extradite on the abortion charge. Still, the patient’s state insists that it do so on the illegal practice of medicine charges and new charges of insurance fraud and failure to report suspected sexual abuse of a child. (Under the patient’s state law, anyone having sex with Patient F would have engaged in sexual abuse or “statutory rape,” so the state insists that the fact she was pregnant proves someone had sex with her.)
Patient F’s state also has a statute that allows private citizens to file civil claims against anyone procuring or assisting with an abortion (a successful private citizen can receive a minimum of $10,000 from the defendant). Several citizens from the patient’s state have already filed claims against Dr. TM in his state courts. Only one of them, probably the first to file, could succeed. Courts in the state have issued subpoenas and ordered Dr. TM to appear and reply to the civil suits. If he does not respond, there will be a default judgment.
Dr. TM’s attorney tells him that these lawsuits will not settle and will take a long time to defend and resolve. That will be expensive.
Billing and fraud. Dr. TM’s office recently received a series of notices from private health insurers stating they are investigating previously made payments as being fraudulent (unlicensed). They will not pay any new claims pending the investigation. On behalf of Medicare-Medicaid and other federal programs, the US Attorney’s office has notified Dr. TM that it has opened an investigation into fraudulent federal payments. F’s home state also is filing a (criminal) insurance fraud case, although the basis for it is unclear. (Dr. TM’s attorney believes it might be to increase pressure on the physician’s state to extradite Dr. TM for Patient F’s case.)
In addition, a disgruntled former employee of Dr. TM has filed a federal FCA case against him for filing inflated claims with various federally funded programs. The employee also made whistleblower calls to insurance companies and some state-funded medical programs. A forensic accounting investigation by Dr. TM’s accountant confirmed a pattern of very sloppy records and recurring billing for televisits that did not occur. Dr. TM believes that this was the act of one of the temporary assistants he hired in a pinch, who did not understand the system and just guessed when filing some insurance claims.
During the investigation, the federal and state attorneys are looking into a possible violation of state and federal Anti-Kickback Statutes. This is based on the original offer of a $100 credit for referrals to Dr. TM’s telemedicine practice.
The attorneys are concerned that other legal problems may present themselves. They are thoroughly reviewing Dr. TM’s practice and making several critical but somewhat modest changes to his practice. They also have insisted that Dr. TM have appropriate staff to handle the details of the practice and billing.
Conclusions
Telemedicine presents notable legal challenges to medical practice. As the pandemic status ends, ObGyn physicians practicing telemedicine need to be aware of the rules and how they are changing. For those physicians who want to continue or start a telemedicine practice, securing legal and technical support to ensure your operations are inline with the legal requirements can minimize any risk of legal troubles in the future. ●
A physician in State A, where abortion is legal, has a telemedicine patient in State B, where it is illegal to assist, provide, or procure an abortion. If the physician prescribes a medical abortion, he would violate the law of State B by using telemedicine to help the patient (located in State B) obtain an abortion. This could result in criminal charges against the prescribing physician.
- Board on Health Care Services; Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. National Academies Press: 2012. https://www.ncbi.nlm.nih.gov/books/NBK207145/. Accessed March 30, 2023.
- Bruhn HK. Telemedicine: dos and don’ts to mitigate liability risk. J APPOS. 2020;24:195-196. doi:10.1016/j.jaapos. 2020.07.002
- Implementing telehealth in practice: ACOG Committee Opinion Summary, number 798. Obstet Gynecol. 2020; 2135:493-494. doi:10.1097/AOG.0000000000003672
- Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. July 9, 2021. Accessed March 2, 2023. https://www.mckinsey.com/industries/healthcare/our-insights /telehealth-a-quarter-trillion-dollar-post-covid-19-reality
- Stanley AY, Wallace JB. Telehealth to improve perinatal care access. MCN Am J Matern Child Nurs. 2022;47:281-287. doi: 10.1097/NMC.0000000000000841
- Warshaw R. Health disparities affect millions in rural US communities. Association of American Medical Colleges. Published October 31, 2017. Accessed March 31, 2023. https://www.aamc.org/news-insights/health-disparities -affect-millions-rural-us-communities
- Almuslin H, AlDossary S. Models of incorporating telehealth into obstetric care during the COVID-19 pandemic, its benefits and barriers: a scoping review. Telemed J E Health. 2022;28:24-38. doi:10.1089/tmj.2020.0553
- Gold AE, Gilbert A, McMichael BJ. Socially distant health care. Tul L Rev. 2021;96:423-468. https://scholarship .law.ua.edu/cgi/viewcontent.cgi?article=1713&context =fac_articles. Accessed March 4, 2023.
- Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
- Odibo IN, Wendel PJ, Magann EF. Telemedicine in obstetrics. Clin Obstet Gynecol. 2013;56:422-433. doi:10.1097/ GRF.0b013e318290fef0
- Shmerling A, Hoss M, Malam N, et al. Prenatal care via telehealth. Prim Care. 2022;49:609-619. doi:10.1016/j. pop.2022.05.002
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939
- Dosaj A, Thiyagarajan D, Ter Haar C, et al. Rapid implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2020;27:116-120. doi:10.1089/ tmj.2020.0219
- Lurie N, Carr B. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;187:745-746. doi: 10.1001/jamainternmed.2018.1314
- Tobah YSB, LeBlanc A, Branda E, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638-e1-638.e8. doi:10.1016/j.ajog.2019.06.034
- Vivanti AJ, Deruelle P, Piccone O, et al. Follow-up for pregnant women during the COVID-19 pandemic: French national authority for health recommendations. J Gynecol Obstet Hum Reprod. 2020;49:101804. doi:10.1016/j. jogoh.2020.101804
- Ellimoottil C. Takeaways from 2 key studies on interstate telehealth use among Medicare fee-for-service beneficiaries. JAMA Health Forum. 2022;3:e223020-E223020. doi:10.1001/ jamahealthforum.2022.3020
- Harris J, Hartnett T, Hoagland GW, et al. What eliminating barriers to interstate telehealth taught us during the pandemic. Bipartisan Policy Center. Published November 2021. Accessed March 9, 2023. https://bipartisanpolicy .org/download/?file=/wp-content/uploads/2021/11/BPC -Health-Licensure-Brief_WEB.pdf.
- Center for Connected Health Policy. Cross-state licensing. Accessed February 21, 2023. https://www.cchpca.org/topic /cross-state-licensing-professional-requirements.
- US Department of Health & Human Services. Telehealth. Getting started with licensure. Published February 3, 2023. Accessed February 27, 2023. https://telehealth.hhs.gov /licensure/getting-started-licensure/
- US Department of Health & Human Services. Telehealth. Licensure. Accessed February 27, 2023. https://telehealth .hhs.gov/licensure
- US Department of Health & Human Services. National Practitioner Data Bank (NPDB) code lists. Published December 2022. Accessed March 9, 2023. https://www.npdb .hrsa.gov/software/CodeLists.pdf
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, telehealth. 2020. Accessed March 5, 2023. https://www.acog.org /clinical-information/physician-faqs/covid-19-faqs-for -ob-gyns-telehealth
- Gorman RK. Prescribing medication through the practice of telemedicine: a comparative analysis of federal and state online prescribing policies, and policy considerations for the future. S Cal Interdisc Law J. 2020;30:739-769. https://gould .usc.edu/why/students/orgs/ilj/assets/docs/30-3-Gorman. pdf. Accessed March 10, 2023.
- Farringer DR. A telehealth explosion: using lessons from the pandemic to shape the future of telehealth regulation. Tex A&M Law Rev. 2021;9:1-47. https://scholarship.law.tamu. edu/cgi/viewcontent.cgi?article=1232&context=lawreview. Accessed February 28, 2023.
- Sterba KR, Johnson EE, Douglas E, et al. Implementation of a women’s reproductive behavioral health telemedicine program: a qualitative study of barriers and facilitators in obstetric and pediatric clinics. BMC Pregnancy Childbirth. 2023;23:167, 1-10. doi:10.1186/s12884-023-05463-2.
- US Department of Justice. COVID-19 FAQ (telemedicine). https://www.deadiversion.usdoj.gov/faq/coronavirus_faq .htm#TELE_FAQ2. Accessed March 13, 2023.
- US Department of Health & Human Services. Guidance on how the HIPAA rules permit covered health care providers and health plans to use remote communication technologies for audio-only telehealth. Published June 13, 2022. Accessed February 22, 2023. https://www.hhs.gov/hipaa/for-professionals/privacy /guidance/hipaa-audio-telehealth/index.html.
- Gray JME. HIPAA, telehealth, and the treatment of mental illness in a post-COVID world. Okla City Uni Law Rev. 2021;46:1-26. https://law.okcu.edu/wp-content /uploads/2022/04/J-Michael-E-Gray-HIPAA-Telehealth -and-Treament.pdf. Accessed March 9, 2023.
- Kurzweil C. Telemental health care and data privacy: current HIPAA privacy pitfalls and a proposed solution. Ann Health L Adv Dir. 2022;31:165.
- US Department of Health & Human Services and US Department of Justice. Health care fraud and abuse control program FY 2020: annual report. July 2021. Accessed March 9, 2023. https://oig.hhs.gov/publications/docs/hcfac /FY2020-hcfac.pdf
- Copeland KB. Telemedicine scams. Iowa Law Rev. 2022: 108:69-126. https://ilr.law.uiowa.edu/sites/ilr.law.uiowa.edu /files/2023-01/A2_Copeland.pdf. Accessed March 10, 2023.
- Solimini R, Busardò FP, Gibelli F, et al. Ethical and legal challenges of telemedicine in the era of the COVID-19 pandemic. Medicina (Kaunas). 2021;57:13141324. doi:10.3390/medicina57121314
- Reed A. COVID: a silver linings playbook. mobilizing pandemic era success stories to advance reproductive justice. Berkeley J Gender Law Justice. 2022;37:221-266. https://lawcat.berkeley.edu/record/1237158/files/16%20 Reed_final.pdf. Accessed March 11, 2023.
- Women’s Preventive Services Initiative and The American College of Obstetricians and Gynecologists. FAQ for telehealth services. Accessed March 2, 2023. https://www .womenspreventivehealth.org/wp-content/uploads/WPSI -Telehealth-FAQ.pdf
- Warren L, Chen KT. Telehealth apps in ObGyn practice. OBG Manag. 2022;34:46-47. doi:10.12788/obgm.0178
- American College of Obstetricians and Gynecologists. 10 telehealth tips for an Ob-Gyn visit. 2020. Accessed March 2, 2023. https://www.acog.org/womens-health /infographics/10-telehealth-tips-for-an-ob-gyn-visit
- Wolf TD. Telemedicine and malpractice: creating uniformity at the national level. Wm Mary Law Rev. 2019;61:15051536. https://scholarship.law.wm.edu/cgi/viewcontent.cgi ?article=3862&context=wmlr. Accessed March 11, 2023.
- Cahan E. Lawsuits, reimbursement, and liability insurance— facing the realities of a post-Roe era. JAMA. 2022;328:515517. doi:10.1001/jama.2022.9193
- Heinrich L, Hernandez AK, Laurie AR. Telehealth considerations for the adolescent patient. Prim Care. 2022;49:597-607. doi:10.1016/j.pop.2022.04.006
- Guttmacher Institute. An overview of consent to reproductive health services by young people. Published March 1, 2023. Accessed April 1, 2023. https://www.guttmacher.org /state-policy/explore/overview-minors-consent-law.
- Dobbs v. Jackson Women’s Health. No. 19–1392. June 24, 2022. Accessed April 1, 2023. https://www.supremecourt .gov/opinions/21pdf/19-1392_6j37.pdf
- Lindgren Y. Dobbs v. Jackson Women’s Health and the post-Roe landscape. J Am Acad Matrimonial Law. 2022;35:235283. https://www.aaml.org/wp-content/uploads/MAT110-1 .pdf. Accessed March 11, 2023.
- Mohiuddin H. The use of telemedicine during a pandemic to provide access to medication abortion. Hous J Health Law Policy. 2021;21:483-525. https://houstonhealthlaw. scholasticahq.com/article/34611.pdf. Accessed March 10, 2023.
- Rebouché R. The public health turn in reproductive rights. Wash & Lee Law Rev. 2021;78:1355-1432. https:// scholarlycommons.law.wlu.edu/cgi/viewcontent .cgi?article=4743&context=wlulr. Accessed March 10, 2023.
- Fliegel R. Access to medication abortion: now more important than ever. Am J Law Med. 2022;48:286-304. doi:10.1017/amj.2022.24
- Guttmacher Institute. Medication abortion. March 1, 2023. Accessed April 1, 2023 https://www.guttmacher.org /state-policy/explore/medication-abortion#:~:text=In%20 January%202023%2C%20the%20FDA,order%20to%20 dispense%20the%20pills
- Cohen DS, Donley G, Rebouché R. The new abortion battleground. Columbia Law Rev. 2023;123:1-100. https:// columbialawreview.org/content/the-new-abortion -battleground/. Accessed March 1, 2023.
- Hunt SA. Call me, beep me, if you want to reach me: utilizing telemedicine to expand abortion access. Vanderbilt Law Rev. 2023;76:323-359. Accessed March 10, 2023. https:// vanderbiltlawreview.org/lawreview/wp-content/uploads /sites/278/2023/01/Call-Me-Beep-Me-If-You-Want-toReach-Me-Utilizing-Telemedicine-to-Expand-AbortionAccess.pdf
- Gleckel JA, Wulkan SL. Abortion and telemedicine: looking beyond COVID-19 and the shadow docket. UC Davis Law Rev Online. 2020;54:105-121. https://lawreview.law.ucdavis. edu/online/54/files/54-online-Gleckel_Wulkan.pdf. Accessed April 1, 2023.
- Board on Health Care Services; Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. National Academies Press: 2012. https://www.ncbi.nlm.nih.gov/books/NBK207145/. Accessed March 30, 2023.
- Bruhn HK. Telemedicine: dos and don’ts to mitigate liability risk. J APPOS. 2020;24:195-196. doi:10.1016/j.jaapos. 2020.07.002
- Implementing telehealth in practice: ACOG Committee Opinion Summary, number 798. Obstet Gynecol. 2020; 2135:493-494. doi:10.1097/AOG.0000000000003672
- Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. July 9, 2021. Accessed March 2, 2023. https://www.mckinsey.com/industries/healthcare/our-insights /telehealth-a-quarter-trillion-dollar-post-covid-19-reality
- Stanley AY, Wallace JB. Telehealth to improve perinatal care access. MCN Am J Matern Child Nurs. 2022;47:281-287. doi: 10.1097/NMC.0000000000000841
- Warshaw R. Health disparities affect millions in rural US communities. Association of American Medical Colleges. Published October 31, 2017. Accessed March 31, 2023. https://www.aamc.org/news-insights/health-disparities -affect-millions-rural-us-communities
- Almuslin H, AlDossary S. Models of incorporating telehealth into obstetric care during the COVID-19 pandemic, its benefits and barriers: a scoping review. Telemed J E Health. 2022;28:24-38. doi:10.1089/tmj.2020.0553
- Gold AE, Gilbert A, McMichael BJ. Socially distant health care. Tul L Rev. 2021;96:423-468. https://scholarship .law.ua.edu/cgi/viewcontent.cgi?article=1713&context =fac_articles. Accessed March 4, 2023.
- Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
- Odibo IN, Wendel PJ, Magann EF. Telemedicine in obstetrics. Clin Obstet Gynecol. 2013;56:422-433. doi:10.1097/ GRF.0b013e318290fef0
- Shmerling A, Hoss M, Malam N, et al. Prenatal care via telehealth. Prim Care. 2022;49:609-619. doi:10.1016/j. pop.2022.05.002
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939
- Dosaj A, Thiyagarajan D, Ter Haar C, et al. Rapid implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health. 2020;27:116-120. doi:10.1089/ tmj.2020.0219
- Lurie N, Carr B. The role of telehealth in the medical response to disasters. JAMA Intern Med. 2018;187:745-746. doi: 10.1001/jamainternmed.2018.1314
- Tobah YSB, LeBlanc A, Branda E, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221:638-e1-638.e8. doi:10.1016/j.ajog.2019.06.034
- Vivanti AJ, Deruelle P, Piccone O, et al. Follow-up for pregnant women during the COVID-19 pandemic: French national authority for health recommendations. J Gynecol Obstet Hum Reprod. 2020;49:101804. doi:10.1016/j. jogoh.2020.101804
- Ellimoottil C. Takeaways from 2 key studies on interstate telehealth use among Medicare fee-for-service beneficiaries. JAMA Health Forum. 2022;3:e223020-E223020. doi:10.1001/ jamahealthforum.2022.3020
- Harris J, Hartnett T, Hoagland GW, et al. What eliminating barriers to interstate telehealth taught us during the pandemic. Bipartisan Policy Center. Published November 2021. Accessed March 9, 2023. https://bipartisanpolicy .org/download/?file=/wp-content/uploads/2021/11/BPC -Health-Licensure-Brief_WEB.pdf.
- Center for Connected Health Policy. Cross-state licensing. Accessed February 21, 2023. https://www.cchpca.org/topic /cross-state-licensing-professional-requirements.
- US Department of Health & Human Services. Telehealth. Getting started with licensure. Published February 3, 2023. Accessed February 27, 2023. https://telehealth.hhs.gov /licensure/getting-started-licensure/
- US Department of Health & Human Services. Telehealth. Licensure. Accessed February 27, 2023. https://telehealth .hhs.gov/licensure
- US Department of Health & Human Services. National Practitioner Data Bank (NPDB) code lists. Published December 2022. Accessed March 9, 2023. https://www.npdb .hrsa.gov/software/CodeLists.pdf
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetrician-gynecologists, telehealth. 2020. Accessed March 5, 2023. https://www.acog.org /clinical-information/physician-faqs/covid-19-faqs-for -ob-gyns-telehealth
- Gorman RK. Prescribing medication through the practice of telemedicine: a comparative analysis of federal and state online prescribing policies, and policy considerations for the future. S Cal Interdisc Law J. 2020;30:739-769. https://gould .usc.edu/why/students/orgs/ilj/assets/docs/30-3-Gorman. pdf. Accessed March 10, 2023.
- Farringer DR. A telehealth explosion: using lessons from the pandemic to shape the future of telehealth regulation. Tex A&M Law Rev. 2021;9:1-47. https://scholarship.law.tamu. edu/cgi/viewcontent.cgi?article=1232&context=lawreview. Accessed February 28, 2023.
- Sterba KR, Johnson EE, Douglas E, et al. Implementation of a women’s reproductive behavioral health telemedicine program: a qualitative study of barriers and facilitators in obstetric and pediatric clinics. BMC Pregnancy Childbirth. 2023;23:167, 1-10. doi:10.1186/s12884-023-05463-2.
- US Department of Justice. COVID-19 FAQ (telemedicine). https://www.deadiversion.usdoj.gov/faq/coronavirus_faq .htm#TELE_FAQ2. Accessed March 13, 2023.
- US Department of Health & Human Services. Guidance on how the HIPAA rules permit covered health care providers and health plans to use remote communication technologies for audio-only telehealth. Published June 13, 2022. Accessed February 22, 2023. https://www.hhs.gov/hipaa/for-professionals/privacy /guidance/hipaa-audio-telehealth/index.html.
- Gray JME. HIPAA, telehealth, and the treatment of mental illness in a post-COVID world. Okla City Uni Law Rev. 2021;46:1-26. https://law.okcu.edu/wp-content /uploads/2022/04/J-Michael-E-Gray-HIPAA-Telehealth -and-Treament.pdf. Accessed March 9, 2023.
- Kurzweil C. Telemental health care and data privacy: current HIPAA privacy pitfalls and a proposed solution. Ann Health L Adv Dir. 2022;31:165.
- US Department of Health & Human Services and US Department of Justice. Health care fraud and abuse control program FY 2020: annual report. July 2021. Accessed March 9, 2023. https://oig.hhs.gov/publications/docs/hcfac /FY2020-hcfac.pdf
- Copeland KB. Telemedicine scams. Iowa Law Rev. 2022: 108:69-126. https://ilr.law.uiowa.edu/sites/ilr.law.uiowa.edu /files/2023-01/A2_Copeland.pdf. Accessed March 10, 2023.
- Solimini R, Busardò FP, Gibelli F, et al. Ethical and legal challenges of telemedicine in the era of the COVID-19 pandemic. Medicina (Kaunas). 2021;57:13141324. doi:10.3390/medicina57121314
- Reed A. COVID: a silver linings playbook. mobilizing pandemic era success stories to advance reproductive justice. Berkeley J Gender Law Justice. 2022;37:221-266. https://lawcat.berkeley.edu/record/1237158/files/16%20 Reed_final.pdf. Accessed March 11, 2023.
- Women’s Preventive Services Initiative and The American College of Obstetricians and Gynecologists. FAQ for telehealth services. Accessed March 2, 2023. https://www .womenspreventivehealth.org/wp-content/uploads/WPSI -Telehealth-FAQ.pdf
- Warren L, Chen KT. Telehealth apps in ObGyn practice. OBG Manag. 2022;34:46-47. doi:10.12788/obgm.0178
- American College of Obstetricians and Gynecologists. 10 telehealth tips for an Ob-Gyn visit. 2020. Accessed March 2, 2023. https://www.acog.org/womens-health /infographics/10-telehealth-tips-for-an-ob-gyn-visit
- Wolf TD. Telemedicine and malpractice: creating uniformity at the national level. Wm Mary Law Rev. 2019;61:15051536. https://scholarship.law.wm.edu/cgi/viewcontent.cgi ?article=3862&context=wmlr. Accessed March 11, 2023.
- Cahan E. Lawsuits, reimbursement, and liability insurance— facing the realities of a post-Roe era. JAMA. 2022;328:515517. doi:10.1001/jama.2022.9193
- Heinrich L, Hernandez AK, Laurie AR. Telehealth considerations for the adolescent patient. Prim Care. 2022;49:597-607. doi:10.1016/j.pop.2022.04.006
- Guttmacher Institute. An overview of consent to reproductive health services by young people. Published March 1, 2023. Accessed April 1, 2023. https://www.guttmacher.org /state-policy/explore/overview-minors-consent-law.
- Dobbs v. Jackson Women’s Health. No. 19–1392. June 24, 2022. Accessed April 1, 2023. https://www.supremecourt .gov/opinions/21pdf/19-1392_6j37.pdf
- Lindgren Y. Dobbs v. Jackson Women’s Health and the post-Roe landscape. J Am Acad Matrimonial Law. 2022;35:235283. https://www.aaml.org/wp-content/uploads/MAT110-1 .pdf. Accessed March 11, 2023.
- Mohiuddin H. The use of telemedicine during a pandemic to provide access to medication abortion. Hous J Health Law Policy. 2021;21:483-525. https://houstonhealthlaw. scholasticahq.com/article/34611.pdf. Accessed March 10, 2023.
- Rebouché R. The public health turn in reproductive rights. Wash & Lee Law Rev. 2021;78:1355-1432. https:// scholarlycommons.law.wlu.edu/cgi/viewcontent .cgi?article=4743&context=wlulr. Accessed March 10, 2023.
- Fliegel R. Access to medication abortion: now more important than ever. Am J Law Med. 2022;48:286-304. doi:10.1017/amj.2022.24
- Guttmacher Institute. Medication abortion. March 1, 2023. Accessed April 1, 2023 https://www.guttmacher.org /state-policy/explore/medication-abortion#:~:text=In%20 January%202023%2C%20the%20FDA,order%20to%20 dispense%20the%20pills
- Cohen DS, Donley G, Rebouché R. The new abortion battleground. Columbia Law Rev. 2023;123:1-100. https:// columbialawreview.org/content/the-new-abortion -battleground/. Accessed March 1, 2023.
- Hunt SA. Call me, beep me, if you want to reach me: utilizing telemedicine to expand abortion access. Vanderbilt Law Rev. 2023;76:323-359. Accessed March 10, 2023. https:// vanderbiltlawreview.org/lawreview/wp-content/uploads /sites/278/2023/01/Call-Me-Beep-Me-If-You-Want-toReach-Me-Utilizing-Telemedicine-to-Expand-AbortionAccess.pdf
- Gleckel JA, Wulkan SL. Abortion and telemedicine: looking beyond COVID-19 and the shadow docket. UC Davis Law Rev Online. 2020;54:105-121. https://lawreview.law.ucdavis. edu/online/54/files/54-online-Gleckel_Wulkan.pdf. Accessed April 1, 2023.
Which countries have made the most progress in addressing maternal mortality ratios?


Is Laundry Detergent a Common Cause of Allergic Contact Dermatitis?
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13
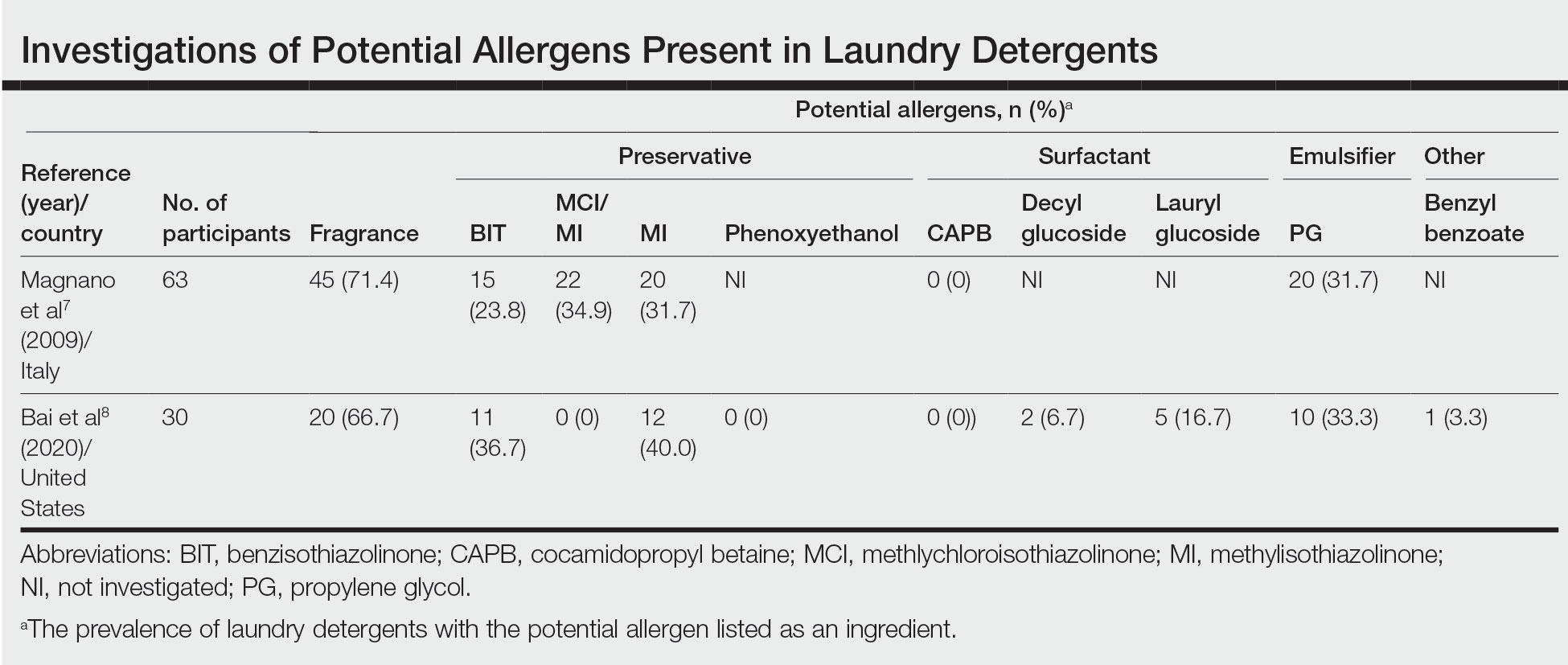
Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
Laundry detergent, a cleaning agent ubiquitous in the modern household, often is suspected as a cause of allergic contact dermatitis (ACD).
We provide a summary of the evidence for the potential allergenicity of laundry detergent, including common allergens present in laundry detergent, the role of machine washing, and the differential diagnosis for laundry detergent–associated ACD.
Allergenic Ingredients in Laundry Detergent
Potential allergens present in laundry detergent include fragrances, preservatives, surfactants, emulsifiers, bleaches, brighteners, enzymes, and dyes.6-8 In an analysis of allergens present in laundry detergents available in the United States, fragrances and preservatives were most common (eTable).7,8 Contact allergy to fragrances occurs in approximately 3.5% of the general population9 and is detected in as many as 9.2% of patients referred for patch testing in North America.10 Preservatives commonly found in laundry detergent include isothiazolinones, such as methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI), MI alone, and benzisothiazolinone (BIT). Methylisothiazolinone has gained attention for causing an ACD epidemic beginning in the early 2000s and peaking in Europe between 2013 and 2014 and decreasing thereafter due to consumer personal care product regulatory changes enacted in the European Union.11 In contrast, rates of MI allergy in North America have continued to increase (reaching as high as 15% of patch tested patients in 2017-2018) due to a lack of similar regulation.10,12 More recently, the prevalence of positive patch tests to BIT has been rising, though it often is difficult to ascertain relevant sources of exposure, and some cases could represent cross-reactions to MCI/MI.10,13

Other allergens that may be present in laundry detergent include surfactants and propylene glycol. Alkyl glucosides such as decyl glucoside and lauryl glucoside are considered gentle surfactants and often are included in products marketed as safe for sensitive skin,14 such as “free and gentle” detergents.8 However, they actually may pose an increased risk for sensitization in patients with atopic dermatitis.14 In addition to being allergenic, surfactants and emulsifiers are known irritants.6,15 Although pathologically distinct, ACD and irritant contact dermatitis can be indistinguishable on clinical presentation.
How Commonly Does Laundry Detergent Cause ACD?
The mere presence of a contact allergen in laundry detergent does not necessarily imply that it is likely to cause ACD. To do so, the chemical in question must exceed the exposure thresholds for primary sensitization (ie, induction of contact allergy) and/or elicitation (ie, development of ACD in sensitized individuals). These depend on a complex interplay of product- and patient-specific factors, among them the concentration of the chemical in the detergent, the method of use, and the amount of detergent residue remaining on clothing after washing.
In the 1990s, the North American Contact Dermatitis Group (NACDG) attempted to determine the prevalence of ACD caused by laundry detergent.1 Among 738 patients patch tested to aqueous dilutions of granular and liquid laundry detergents, only 5 (0.7%) had a possible allergic patch test reaction. It was unclear what the culprit allergens in the detergents may have been; only 1 of the patients also tested positive to fragrance. Two patients underwent further testing to additional detergent dilutions, and the results called into question whether their initial reactions had truly been allergic (positive) or were actually irritant (negative). The investigators concluded that the prevalence of laundry detergent–associated ACD in this large group of patients was at most 0.7%, and possibly lower.1
Importantly, patch testing to laundry detergents should not be undertaken in routine clinical practice. Laundry detergents should never be tested “as is” (ie, undiluted) on the skin; they are inherently irritating and have a high likelihood of producing misleading false-positive reactions. Careful dilutions and testing of control subjects are necessary if patch testing with these products is to be appropriately conducted.
Isothiazolinones in Laundry Detergent
The extremely low prevalence of laundry detergent–associated ACD reported by the NACDG was determined prior to the start of the worldwide MI allergy epidemic, raising the possibility that laundry detergents containing isothiazolinones may be associated with ACD. There is no consensus about the minimum level at which isothiazolinones pose no risk to consumers,16-19 but the US Expert Panel for Cosmetic Ingredient Safety declared that MI is “safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetic products when they are formulated to be nonsensitizing.”18,19 Although ingredient lists do not always reveal when isothiazolinones are present, analyses of commercially available laundry detergents have shown MI concentrations ranging from undetectable to 65.7 ppm.20-23
Published reports suggest that MCI/MI in laundry detergent can elicit ACD in sensitized individuals. In one case, a 7-year-old girl with chronic truncal dermatitis (atopic history unspecified) was patch tested, revealing a strongly positive reaction to MCI/MI.24 Her laundry detergent was the only personal product found to contain MI. The dermatitis completely resolved after switching detergents and flared after wearing a jacket that had been washed in the implicated detergent, further supporting the relevance of the positive patch test. The investigators suspected initial sensitization to MI from wet wipes used earlier in childhood.24 In another case involving occupational exposure, a 39-year-old nonatopic factory worker was responsible for directly adding MI to laundry detergent.25 Although he wore disposable work gloves, he developed severe hand dermatitis, eczematous pretibial patches, and generalized pruritus. Patch testing revealed positive reactions to MCI/MI and MI, and he experienced improvement when reassigned to different work duties. It was hypothesized that the leg dermatitis and generalized pruritus may have been related to exposure to small concentrations of MI in work clothes washed with an MI-containing detergent.25 Notably, this patient’s level of exposure was much greater than that encountered by individuals in day-to-day life outside of specialized occupational settings.
Regarding other isothiazolinones, a toxicologic study estimated that BIT in laundry detergent would be unlikely to induce sensitization, even at the maximal acceptable concentration, as recommended by preservative manufacturers, and accounting for undiluted detergent spilling directly onto the skin.26
Does Machine Washing Impact Allergen Concentrations?
Two recent investigations have suggested that machine washing reduces concentrations of isothiazolinones to levels that are likely below clinical relevance. In the first study, 3 fabrics—cotton, polyester, cotton-polyester—were machine washed and line dried.27 A standard detergent was used with MI added at different concentrations: less than 1 ppm, 100 ppm, and 1000 ppm. This process was either performed once or 10 times. Following laundering and line drying, MI was undetectable in all fabrics regardless of MI concentration or number of times washed (detection limit, 0.5 ppm).27 In the second study, 4 fabrics—cotton, wool, polyester, linen—were washed with standard laundry detergent in 1 of 4 ways: handwashing (positive control), standard machine washing, standard machine washing with fabric softener, and standard machine washing with a double rinse.28 After laundering and line drying, concentrations of MI, MCI, and BIT were low or undetectable regardless of fabric type or method of laundering. The highest levels detected were in handwashed garments at a maximum of 0.5 ppm of MI. The study authors postulated that chemical concentrations near these maximum residual levels may pose a risk for eliciting ACD in highly sensitized individuals. Therefore, handwashing can be considered a much higher risk activity for isothiazolinone ACD compared with machine washing.28
It is intriguing that machine washing appears to reduce isothiazolinones to low concentrations that may have limited likelihood of causing ACD. Similar findings have been reported regarding fragrances. A quantitative risk assessment performed on 24 of 26 fragrance allergens regulated by the European Union determined that the amount of fragrance deposited on the skin from laundered garments would be less than the threshold for causing sensitization.29 Although this risk assessment was unable to address the threshold of elicitation, another study conducted in Europe investigated whether fragrance residues present on fabric, such as those deposited from laundry detergent, are present at high enough concentrations to elicit ACD in previously sensitized individuals.30 When 36 individuals were patch tested with increasing concentrations of a fragrance to which they were already sensitized, only 2 (5.6%) had a weakly positive reaction and then only to the highest concentration, which was estimated to be 20-fold higher than the level of skin exposure after normal laundering. No patient reacted at lower concentrations.30
Although machine washing may decrease isothiazolinone and/or fragrance concentrations in laundry detergent to below clinically relevant levels, these findings should not necessarily be extrapolated to all chemicals in laundry detergent. Indeed, a prior study observed that after washing cotton cloths in a detergent solution for 10 minutes, detergent residue was present at concentrations ranging from 139 to 2820 ppm and required a subsequent 20 to 22 washes in water to become undetectable.31 Another study produced a mathematical model of the residual concentration of sodium dodecyl sulphate (SDS), a surfactant and known irritant, in laundered clothing.32 It was estimated that after machine washing, the residual concentration of SDS on clothes would be too low to cause irritation; however, as the clothes dry (ie, as moisture evaporates but solutes remain), the concentration of SDS on the fabric’s surface would increase to potentially irritating levels. The extensive drying that is possible with electric dryers may further enhance this solute-concentrating effect.
Differential Diagnosis of Laundry Detergent ACD
The propensity for laundry detergent to cause ACD is a question that is nowhere near settled, but the prevalence of allergy likely is far less common than is generally suspected. In our experience, many patients presenting for patch testing have already made the change to “free and clear” detergents without noticeable improvement in their dermatitis, which could possibly relate to the ongoing presence of contact allergens in these “gentle” formulations.7 However, to avoid anchoring bias, more frequent causes of dermatitis should be included in the differential diagnosis. Textile ACD presents beneath clothing with accentuation at areas of closest contact with the skin, classically involving the axillary rim but sparing the vault. The most frequently implicated allergens in textile ACD are disperse dyes and less commonly textile resins.33,34 Between 2017 and 2018, 2.3% of 4882 patients patch tested by the NACDG reacted positively to disperse dye mix.10 There is evidence to suggest that the actual prevalence of disperse dye allergy might be higher due to inadequacy of screening allergens on baseline patch test series.35 Additional diagnoses that should be distinguished from presumed detergent contact dermatitis include atopic dermatitis and cutaneous T-cell lymphoma.
Final Interpretation
Although many patients and physicians consider laundry detergent to be a major cause of ACD, there is limited high-quality evidence to support this belief. Contact allergy to laundry detergent is probably much less common than is widely supposed. Although laundry detergents can contain common allergens such as fragrances and preservatives, evidence suggests that they are likely reduced to below clinically relevant levels during routine machine washing; however, we cannot assume that we are in the “free and clear,” as uncertainty remains about the impact of these low concentrationson individuals with strong contact allergy, and large studies of patch testing to modern detergents have yet to be carried out.
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
- Belsito DV, Fransway AF, Fowler JF, et al. Allergic contact dermatitis to detergents: a multicenter study to assess prevalence. J Am Acad Dermatol. 2002;46:200-206. doi:10.1067/mjd.2002.119665
- Dallas MJ, Wilson PA, Burns LD, et al. Dermatological and other health problems attributed by consumers to contact with laundry products. Home Econ Res J. 1992;21:34-49. doi:10.1177/1077727X9202100103
- Bailey A. An overview of laundry detergent allergies. Verywell Health. September 16, 2021. Accessed March 21, 2023. https://www.verywellhealth.com/laundry-detergent-allergies-signs-symptoms-and-treatment-5198934
- Fasanella K. How to tell if you laundry detergent is messing with your skin. Allure. June 15, 2019. Accessed March 21, 2023. https://www.allure.com/story/laundry-detergent-allergy-skin-reaction
- Oykhman P, Dookie J, Al-Rammahy et al. Dietary elimination for the treatment of atopic dermatitis: a systematic review and meta-analysis. J Allergy Immunol Pract. 2022;10:2657-2666.e8. doi:10.1016/j.jaip.2022.06.044
- Kwon S, Holland D, Kern P. Skin safety evaluation of laundry detergent products. J Toxicol Environ Health A. 2009;72:1369-1379. doi:10.1080/1528739090321675
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341. doi:10.1111/j.1600-0536.2009.01647.x
- Bai H, Tam I, Yu J. Contact allergens in top-selling textile-care products. Dermatitis. 2020;31:53-58. doi:10.1097/DER.0000000000000566
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Havmose M, Thyssen JP, Zachariae C, et al. The epidemic of contact allergy to methylisothiazolinone–an analysis of Danish consecutive patients patch tested between 2005 and 2019. Contact Dermatitis. 2021;84:254-262. doi:10.1111/cod.13717
- Atwater AR, Petty AJ, Liu B, et al. Contact dermatitis associated with preservatives: retrospective analysis of North American Contact Dermatitis Group data, 1994 through 2016. J Am Acad Dermatol. 2021;84:965-976. doi:10.1016/j.jaad.2020.07.059
- King N, Latheef F, Wilkinson M. Trends in preservative allergy: benzisothiazolinone emerges from the pack. Contact Dermatitis. 2021;85:637-642. doi:10.1111/cod.13968
- Sasseville D. Alkyl glucosides: 2017 “allergen of the year.” Dermatitis. 2017;28:296. doi:10.1097/DER0000000000000290
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12. doi:10.1097/DER0000000000000307
- European Commission, Directorate-General for Health and Consumers. Opinion on methylisothiazolinone (P94) submission II (sensitisation only). Revised March 27, 2014. Accessed March 21, 2023. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_145.pdf
- Cosmetic ingredient hotlist: list of ingredients that are restricted for use in cosmetic products. Government of Canada website. Accessed March 21, 2023. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/hotlist.html#tbl2
- Burnett CL, Boyer I, Bergfeld WF, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2019;38(1 suppl):70S-84S. doi:10.1177/1091581819838792
- Burnett CL, Bergfeld WF, Belsito DV, et al. Amended safety assessment of methylisothiazolinone as used in cosmetics. Int J Toxicol. 2021;40(1 suppl):5S-19S. doi:10.1177/10915818211015795
- Aerts O, Meert H, Goossens A, et al. Methylisothiazolinone in selected consumer products in Belgium: adding fuel to the fire? Contact Dermatitis. 2015;73:142-149. doi:10.1111/cod.12449
- Garcia-Hidalgo E, Sottas V, von Goetz N, et al. Occurrence and concentrations of isothiazolinones in detergents and cosmetics in Switzerland. Contact Dermatitis. 2017;76:96-106. doi:10.1111/cod.12700
- Marrero-Alemán G, Borrego L, Antuña AG, et al. Isothiazolinones in cleaning products: analysis with liquid chromatography tandem mass spectrometry of samples from sensitized patients and markets. Contact Dermatitis. 2020;82:94-100. doi:10.1111/cod.13430
- Alvarez-Rivera G, Dagnac T, Lores M, et al. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1270:41-50. doi:10.1016/j.chroma.2012.10.063
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487. doi:10.1111/pde.13122
- Sandvik A, Holm JO. Severe allergic contact dermatitis in a detergent production worker caused by exposure to methylisothiazolinone. Contact Dermatitis. 2019;80:243-245. doi:10.1111/cod.13182
- Novick RM, Nelson ML, Unice KM, et al. Estimation of safe use concentrations of the preservative 1,2-benziosothiazolin-3-one (BIT) in consumer cleaning products and sunscreens. Food Chem Toxicol. 2013;56:60-66. doi:10.1016/j.fct.2013.02.006
- Hofmann MA, Giménez-Arnau A, Aberer W, et al. MI (2-methyl-4-isothiazolin-3-one) contained in detergents is not detectable in machine washed textiles. Clin Transl Allergy. 2018;8:1. doi:10.1186/s13601-017-0187-2
- Marrero-Alemán G, Borrego L, Atuña AG, et al. Persistence of isothiazolinones in clothes after machine washing. Dermatitis. 2021;32:298-300. doi:10.1097/DER.0000000000000603
- Corea NV, Basketter DA, Clapp C, et al. Fragrance allergy: assessing the risk from washed fabrics. Contact Dermatitis. 2006;55:48-53. doi:10.1111/j.0105-1873.2006.00872.x
- Basketter DA, Pons-Guiraud A, van Asten A, et al. Fragrance allergy: assessing the safety of washed fabrics. Contact Dermatitis. 2010;62:349-354. doi:10.1111/j.1600-0536.2010.01728.x
- Agarwal C, Gupta BN, Mathur AK, et al. Residue analysis of detergent in crockery and clothes. Environmentalist. 1986;4:240-243.
- Broadbridge P, Tilley BS. Diffusion of dermatological irritant in drying laundered cloth. Math Med Biol. 2021;38:474-489. doi:10.1093/imammb/dqab014
- Lisi P, Stingeni L, Cristaudo A, et al. Clinical and epidemiological features of textile contact dermatitis: an Italian multicentre study. Contact Dermatitis. 2014;70:344-350. doi:10.1111/cod.12179
- Mobolaji-Lawal M, Nedorost S. The role of textiles in dermatitis: an update. Curr Allergy Asthma Rep. 2015;15:17. doi:10.1007/s11882-015-0518-0
- Nijman L, Rustemeyer T, Franken SM, et al. The prevalence and relevance of patch testing with textile dyes [published online December 3, 2022]. Contact Dermatitis. doi:10.1111/cod.14260
Practice Points
- Although laundry detergent commonly is believed to be a cause of allergic contact dermatitis (ACD), the actual prevalence is quite low (<1%).
- Common allergens present in laundry detergent such as fragrances and isothiazolinone preservatives likely are reduced to clinically irrelevant levels during routine machine washing.
- Other diagnoses to consider when laundry detergent–associated ACD is suspected include textile ACD, atopic dermatitis, and cutaneous T-cell lymphoma.
Lip Reconstruction After Mohs Micrographic Surgery: A Guide on Flaps
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).
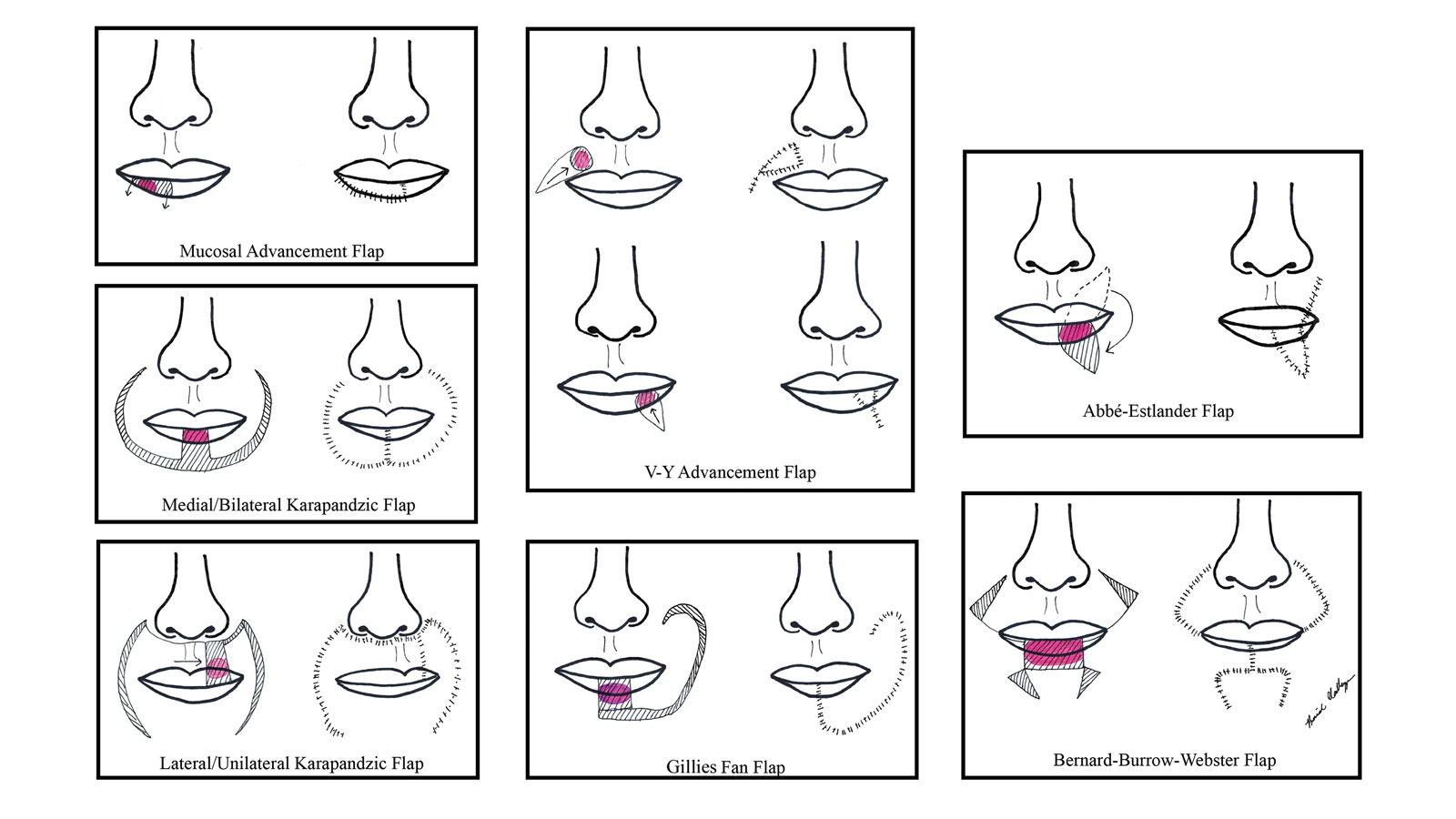
Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects.Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1
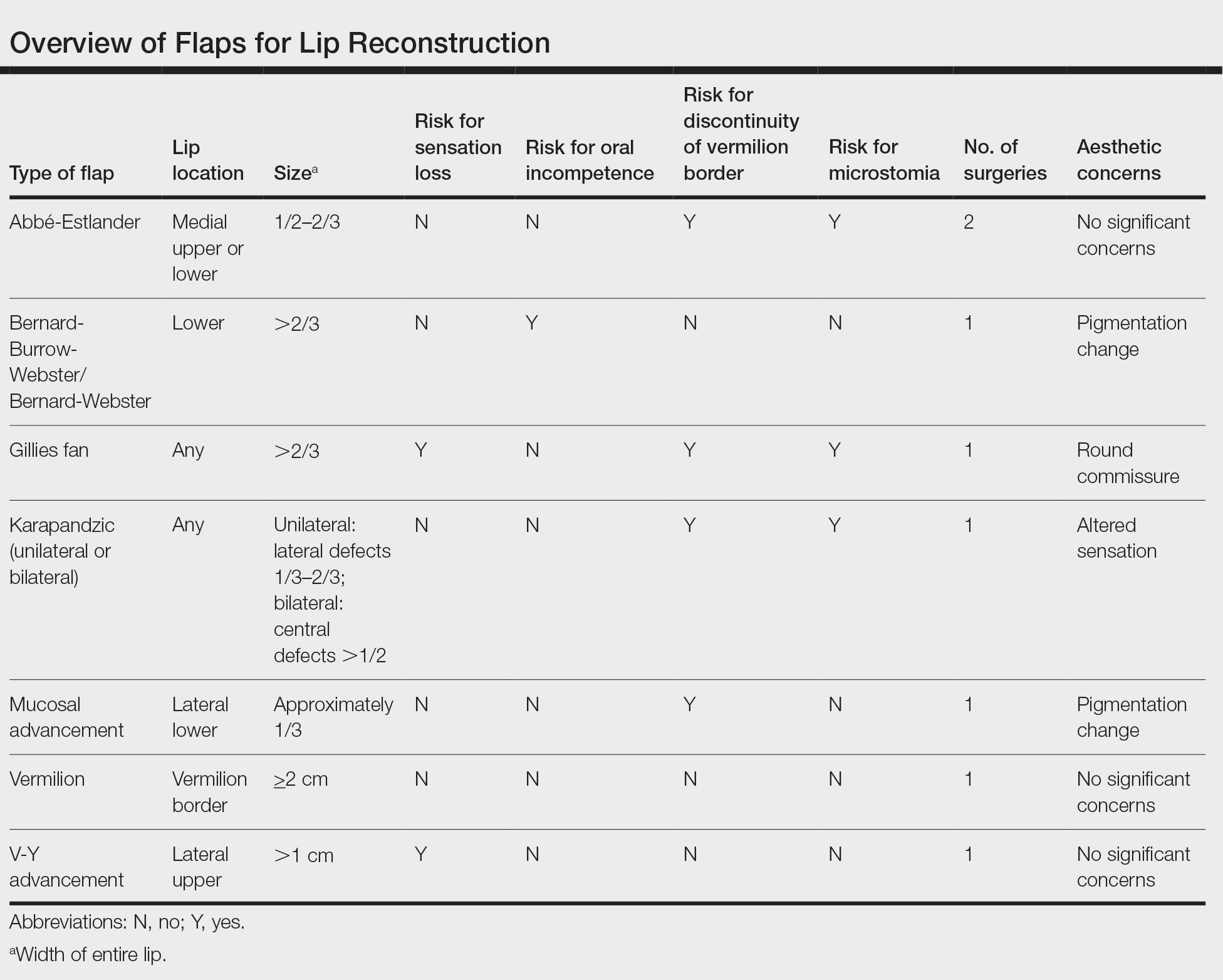
Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects.Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
The lip is commonly affected by skin cancer because of increased sun exposure and actinic damage, with basal cell carcinoma typically occurring on the upper lip and squamous cell carcinoma (SCC) on the lower lip. The risk for metastatic spread of SCC on the lip is higher than cutaneous SCC on other facial locations but lower than SCC of the oral mucosa.1,2 If the tumor is operable and the patient has no contraindications to surgery, Mohs micrographic surgery is the preferred treatment, as it allows for maximal healthy tissue preservation and has the lowest recurrence rates.1-3 Once the tumor is removed and margins are confirmed to be negative, one must consider the options for defect closure, including healing by secondary intention, primary/direct closure, full-thickness skin grafts, local flaps, or free flaps.4 Secondary intention may lead to wound contracture and suboptimal functional and cosmetic outcomes. Primary wedge closure can be utilized for optimal functional and cosmetic outcomes when the defect involves less than one-third of the horizontal width of the vermilion. For larger defects, the surgeon must consider a flap or graft. Skin grafts are less favorable than local flaps because they may have different skin color, texture, and hair-bearing properties than the recipient area.3,5 In addition, grafts require a separate donor site, which means more pain, recovery time, and risk for complications for the patient.3 Free flaps similarly utilize tissue and blood supply from a donor site to repair major tissue loss. Radial forearm free flaps commonly are used for large lip defects but are more extensive, risky, and costly compared to local flaps for smaller defects under local anesthesia or nerve blocks.6,7 With these considerations, a local lip flap often is the most ideal repair method.
When performing a local lip flap, it is important to consider the functional and aesthetic aspects of the lips. The lower face is more susceptible to distortion and wound contraction after defect repair because it lacks a substantial supportive fibrous network. The dynamics of opposing lip elevator and depressor muscles make the lips a visual focal point and a crucial structure for facial expression, mastication, oral continence, speech phonation, and mouth opening and closing.2,4,8,9 Aesthetics and symmetry of the lips also are a large part of facial recognition and self-image.9
Lip defects are classified as partial thickness involving skin and muscle or full thickness involving skin, muscle, and mucosa. Partial-thickness wounds less than one-third the width of the horizontal lip can be repaired with a primary wedge resection or left to heal by secondary intention if the defect only involves the superficial vermilion.2 For defects larger than one-third the width of the horizontal lip, local flaps are favored to allow for closely matched skin and lip mucosa to fill in the defect.9 Full-thickness defects are further classified based on defect width compared to total lip width (ie, less than one-third, between one-third and two-thirds, and greater than two-thirds) as well as location (ie, medial, lateral, upper lip, lower lip).2,10
There are several local lip flap reconstruction options available, and choosing one is based on defect size and location. We provide a succinct review of the indications, risks, and benefits of commonly utilized flaps (Table), as well as artist renderings of all of the flaps (Figure).

Vermilion Flaps
Vermilion flaps are used to close partial-thickness defects of the vermilion border, an area that poses unique obstacles of repair with blending distant tissues to match the surroundings.8 Goldstein11 developed an adjacent ipsilateral vermilion flap utilizing an arterialized myocutaneous flap for reconstruction of vermilion defects.Later, this technique was modified by Sawada et al12 into a bilateral adjacent advancement flap for closure of central vermilion defects and may be preferred for defects 2 cm in size or larger. Bilateral flaps are smaller and therefore more viable than unilateral or larger flaps, allowing for a more aesthetic alignment of the vermilion border and preservation of muscle activity because muscle fibers are not cut. This technique also allows for more efficient stretching or medial advancement of the tissue while generating less tension on the distal flap portions. Burow triangles can be utilized if necessary for improved aesthetic outcome.1
Mucosal Advancement and Split Myomucosal Advancement Flap
The mucosal advancement technique can be considered for tumors that do not involve the adjacent cutaneous skin or the orbicularis oris muscle; thus, the reconstruction involves only the superficial vermilion area.7,13 Mucosal incisions are made at the gingivobuccal sulcus, and the mucosal flap is elevated off the orbicularis oris muscle and advanced into the defect.10 A plane of dissection is maintained while preserving the labial artery. Undermining effectively advances wet mucosa into the dry mucosal lip to create a neovermilion. However, the reconstructed lip often appears thinner and will possibly be a different shade compared to the adjacent native lip. These discrepancies become more evident with deeper defects.7
There is a risk for cosmetic distortion and scar contraction with advancing the entire mucosa. Eirís et al13 described a solution—a bilateral mucosal rotation flap in which the primary incision is made along the entire vermilion border and tissue is undermined to allow advancement of the mucosa. Because the wound closure tension lays across the entire lip, there is less risk for scar contraction, even if the flap movement is unequal on either side of the defect.13
Although mucosal advancement flaps are a classic choice for reconstruction following a vermilion defect, other techniques, such as primary closure, should be considered in elderly patients and patients taking anticoagulants because of the risks for flap necrosis, swelling, bruising, hematoma, and dysesthesia, as well as a decrease in the anterior-posterior dimension of the lip. These risks can be attributed to trauma of surrounding tissue and stress secondary to longer overall operating times.14
Split myomucosal advancement flaps are used in similar scenarios as myomucosal advancement flaps but for larger red lip defects that are less than 50% the length of the upper or lower lip. Split myomucosal advancement flaps utilize an axial flap based on the labial artery, which provides robust vascular supply to the reconstructed area. This vascularity, along with lateral motor innervation of the orbicularis oris, allows for split myomucosal advancement flaps to restore the resected volume, preserve lip function, and minimize postoperative microstomia.7
V-Y Advancement Flaps
V-Y advancement flaps are based on a subcutaneous tissue pedicle and are optimal for partial- and full-thickness defects larger than 1 cm on the lateral upper lips, whereas bilateral V-Y advancement flaps are recommended for central lip defects.15-17 Advantages of V-Y advancement flaps are preserved facial symmetry and maintenance of the oral sphincter and facial nerve function. The undermining portions allow for advancement of a skin flap of similar thickness and contour into the upper or lower lip.15 Disadvantages include facial asymmetry with larger defects involving the melolabial fold as well as paresthesia after closure. However, in one study, no paresthesia was reported more than 12 months postprocedure.4 The biggest disadvantage of the V-Y advancement flap is the kite-shaped scar and possible trapdoor deformity.5,15 When working medially, the addition of the pincer modification helps avoid blunting of the philtrum and recreates a Cupid’s bow by curling the lateral flap edges medially to resemble a teardrop shape.17 V-Y advancement flaps for defects of skin and adipose tissue less than 5 mm in size have the highest need for revision surgery; thus, defects of this small size should be repaired primarily.4
When using a V-Y advancement flap to correct large defects, there are 3 common complications that may arise: fullness medial to the commissure, a depressed vermilion lip, and a standing cutaneous deformity along the trailing edge of the flap where the Y is formed upon closure of the donor site. To decrease the fullness, a skin excision from the inferior border of the flap along the vermiliocutaneous border can be made to debulk the area. A vermilion advancement can be used to optimize the vermiliocutaneous junction. Potential standing cutaneous deformity is addressed by excising a small ellipse of skin oriented along the axis of the relaxed skin tension lines.15
Abbé-Estlander Flap
The Abbé-Estlander flap (also known as a transoral cross-lip flap) is a full-thickness myocutaneous interpolation flap with blood supply from the labial artery. It is used for lower lip tumors that have deep invasion into muscle and are 30% to 60% of the horizontal lip.8,9 Abbé transposition flaps are used for defects medial to the oral commissure and are best suited for philtrum reconstruction, whereas Estlander flaps are for defects that involve the oral commissure.9,18 Interpolation flaps usually are performed in 2 stages, but some dermatologic surgeons have reported success with single-stage procedures.1 The second-stage division usually is performed 2 to 3 weeks after flap insetting to allow time for neovascularization, which is crucial for pedicle survival.8,9,19
Advantages of this type of flap are the preservation of orbicularis oris strength and a functional and aesthetic result with minimal change in appearance for defects sized from one-third to two-thirds the width of the lip.20 This aesthetic effect is particularly notable when the donor flap is taken from the mediolateral upper lip, allowing the scarred area to blend into the nasolabial fold.8 Disadvantages of this flap are a risk for microstomia, lip vermilion misalignment, and lip adhesion.21 It is important that patients are educated on the need for multiple surgeries when using this type of flap, as patients favor single-step procedures.1 The Abbé flap requires 2 surgeries, whereas the Estlander flap requires only 1. However, patients commonly require commissuroplasty with the Estlander flap alone.21
Gillies Fan Flap, Karapandzic Flap, Bernard-Webster Flap, and Bernard-Burrow-Webster Flap
The Gillies fan flap, Karapandzic flap, Bernard-Webster (BW) flap, and modified Bernard-Burrow-Webster flap are the likely choices for repair of lip defects that encompass more than two-thirds of the lip.9,10,22 The Karapandzic and BW flaps are the 2 most frequently used for reconstruction of larger lower lip defects and only require 1 surgery.
Upper lip full-thickness defects that are too big for an Abbé-Estlander flap are closed with the Gillies fan flap.18 These defects involve 70% to 80% of the horizontal lip.9 The Gillies fan flap design redistributes the remaining lip to provide similar tissue quality and texture to fill the large defects.9,23 Compared to Karapandzic and Bernard flaps, Gillies fan incision closures are hidden well in the nasolabial folds, and the degree of microstomy is decreased because of the rotation of the flaps. However, rotation of medial cheek flaps can distort the orbicular muscular fibers and the anatomy of the commissure, which may require repair with commissurotomy. Drawbacks include a risk for denervation that can result in temporary oral sphincter incompetence.23 The bilateral Gillies fan flap carries a risk for microstomy as well as misalignment of the lip vermilion and round commissures.21
The Karapandzic flap is similar to the Gillies fan flap but only involves the skin and mucosa.9 This flap can be used for lateral or medial upper lip defects greater than one-third the width of the entire lip. This single-procedure flap allows for labial continuity, preserved sensation, and motor function; however, microstomia and misalignment of the oral commissure are common.1,18,21 In a retrospective study by Nicholas et al,4 the only flap reported to have a poor functional outcome was the Karapandzic flap, with 3 patients reporting altered sensation and 1 patient reporting persistent stiffness while smiling.
The BW flap can be applied for extensive full-thickness defects greater than one-third the lower lip and for defects with limited residual lip. This flap also can be used in cases where only skin is excised, as the flap does not depend on reminiscent lip tissue for reconstruction of the new lower lip. Sensory function is maintained given adequate visualization and preservation of the local vascular, nervous, and muscular systems. Disadvantages of the BW flap include an incision notch in the region of the lower lip; blunting of the alveolobuccal sulcus; and functional deficits, such as lip incontinence to liquids during the postoperative period.21
The Bernard-Burrow-Webster flap is used for large lower lip defects and preserves the oral commissures by advancing adjacent cheek tissue and remaining lip tissue medially.10 It allows for larger site mobilization, but it is possible to see some resulting oral incontinence.1,10 The Burow wedge flap is a variant of the advancement flap, with the Burow triangle located lateral to the oral commissure. Caution must be taken to avoid intraoperative bleeding from the labial and angular arteries. In addition, there also may be downward displacement of the vermilion border.5
How to Choose a Flap
The orbicularis oris is a circular muscle that surrounds both the upper and lower lips. It is pulled into an oval, allowing for sphincter function by radially oriented muscles, all of which are innervated by the facial nerve. Other key anatomical structures of the lips include the tubercle (vermilion prominence), Cupid’s bow and philtrum, nasolabial folds, white roll, hair-bearing area, and vermilion border. The lips are divided into cutaneous, mucosal, and vermilion parts, with the vermilion area divided into dry/external and wet/internal areas. Sensation to the upper lip is provided by the maxillary division of the trigeminal nerve via the infraorbital nerve. The lower lip is innervated by the mandibular division of the trigeminal nerve via the inferior alveolar nerve. The labial artery, a branch of the facial artery, is responsible for blood supply to the lips.3,9 Because of the complex anatomy of the lips, careful reconstruction is crucial for functional and aesthetic preservation.
There are a variety of lip defect repairs, but all local flaps aim to preserve aesthetics and function. The Table summarizes the key risks and benefits of each flap. Local flap techniques can be used in combination for more complex defects.3 For example, Nadiminti et al19 described the combination of the Abbé flap and V-Y advancement flap to restore function and create a new symmetric nasolabial fold. Dermatologic surgeons will determine the most suitable technique based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.1

Other factors to consider when choosing a local flap are the patient’s age, tissue laxity, dentition/need for dentures, and any prior treatments.7 Scar revision surgery may be needed after reconstruction, especially with longer vertical scars in areas without other rhytides. In addition, paresthesia is common after Mohs micrographic surgery of the face; however, new neural networks are created postoperatively, and most paresthesia resolves within 1 year of the repair.4 Dermabrasion and Z-plasty also may be considered, as they have been shown to be successful in improving final outcomes.9 Overall, local flaps have risks for infection, flap necrosis, and bleeding, though the incidence is low in reconstructions of the face.
Final Thoughts
There are several mechanisms to repair upper and lower lip defects resulting from surgical removal of cutaneous cancers. This review of specific flaps used in lip reconstruction provides a comprehensive overview of indications, advantages, and disadvantages of available lip flaps.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
- Goldman A, Wollina U, França K, et al. Lip repair after Mohs surgery for squamous cell carcinoma by bilateral tissue expanding vermillion myocutaneous flap (Goldstein technique modified by Sawada). Open Access Maced J Med Sci. 2018;6:93-95.
- Faulhaber J, Géraud C, Goerdt S, et al. Functional and aesthetic reconstruction of full-thickness defects of the lower lip after tumor resection: analysis of 59 cases and discussion of a surgical approach. Dermatol Surg. 2010;36:859-867.
- Skaria AM. The transposition advancement flap for repair of postsurgical defects on the upper lip. Dermatology. 2011;223:203-206.
- Nicholas MN, Liu A, Chan AR, et al. Postoperative outcomes of local skin flaps used in oncologic reconstructive surgery of the upper cutaneous lip: a systematic review. Dermatol Surg. 2021;47:1047-1051.
- Wu W, Ibrahimi OA, Eisen DB. Cheek advancement flap with retained standing cone for reconstruction of a defect involving the upper lip, nasal sill, alar insertion, and medial cheek. Dermatol Surg. 2012;38:1077-1082.
- Cook JL. The reconstruction of two large full-thickness wounds of the upper lip with different operative techniques: when possible, a local flap repair is preferable to reconstruction with free tissue transfer. Dermatol Surg. 2013;39:281-289.
- Glenn CJ, Adelson RT, Flowers FP. Split myomucosal advancement flap for reconstruction of a lower lip defect. Dermatol Surg. 2012;38:1725-1728.
- Hahn HJ, Kim HJ, Choi JY, et al. Transoral cross-lip (Abbé-Estlander) flap as a viable and effective reconstructive option in middle lower lip defect reconstruction. Ann Dermatol. 2017;29:210-214.
- Larrabee YC, Moyer JS. Reconstruction of Mohs defects of the lips and chin. Facial Plast Surg Clin North Am. 2017;25:427-442.
- Campos MA, Varela P, Marques C. Near-total lower lip reconstruction: combined Karapandzic and Bernard-Burrow-Webster flap. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:19-20.
- Goldstein MH. A tissue-expanding vermillion myocutaneous flap for lip repair. Plast Reconstr Surg. 1984;73:768–770.
- Sawada Y, Ara M, Nomura K. Bilateral vermilion flap—a modification of Goldstein’s technique. Int J Oral Maxillofac Surg. 1988;17:257–259.
- Eirís N, Suarez-Valladares MJ, Cocunubo Blanco HA, et al. Bilateral mucosal rotation flap for repair of lower lip defect. J Am Acad Dermatol. 2015;72:E81-E82.
- Sand M, Altmeyer P, Bechara FG. Mucosal advancement flap versus primary closure after vermilionectomy of the lower lip. Dermatol Surg. 2010;36:1987-1992.
- Griffin GR, Weber S, Baker SR. Outcomes following V-Y advancement flap reconstruction of large upper lip defects. Arch Facial Plast Surg. 2012;14:193-197.
- Zhang WC, Liu Z, Zeng A, et al. Repair of cutaneous and mucosal upper lip defects using double V-Y advancement flaps. J Cosmet Dermatol. 2020;19:211-217.
- Tolkachjov SN. Bilateral V-Y advancement flaps with pincer modification for re-creation of large philtrum lip defect. J Am Acad Dermatol. 2021;84:E187-E188.
- García de Marcos JA, Heras Rincón I, González Córcoles C, et al. Bilateral reverse Yu flap for upper lip reconstruction after oncologic resection. Dermatol Surg. 2014;40:193-196.
- Nadiminti H, Carucci JA. Repair of a through-and-through defect on the upper cutaneous lip. Dermatol Surg. 2014;40:58-61.
- Kumar A, Shetty PM, Bhambar RS, et al. Versatility of Abbe-Estlander flap in lip reconstruction—a prospective clinical study. J Clin Diagn Res. 2014;8:NC18-NC21.
- Denadai R, Raposo-Amaral CE, Buzzo CL, et al. Functional lower lip reconstruction with the modified Bernard-Webster flap. J Plast Reconstr Aesthet Surg. 2015;68:1522-1528.
- Salgarelli AC, Bellini P, Magnoni C, et al. Synergistic use of local flaps for total lower lip reconstruction. Dermatol Surg. 2011;37:1666-1670.
- Moreno-Ramirez D, Ferrandiz L, Vasquez-Chinchay F, et al. Uncompleted fan flap for full-thickness lower lip defect. Dermatol Surg. 2009;35:1426-1429.
Practice Points
- Even with early detection, many skin cancers on the lips require surgical removal with subsequent reconstruction.
- There are several local flap reconstruction options available, and some may be used in combination for more complex defects.
- The most suitable technique should be chosen based on tumor location, tumor stage or depth of invasion (partial or full thickness), and preservation of function and aesthetics.
Mpox Update: Clinical Presentation, Vaccination Guidance, and Management
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19
Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
Practice Points
- Mpox (monkeypox) lesions typically present as well-circumscribed, painful, umbilicated papules, vesicles, or pustules, with recent cases having a predilection for an anogenital distribution accompanied by systemic viral symptoms.
- Health care workers treating suspected or confirmed cases of mpox should be familiar with current guidelines for controlling the spread of mpox, including proper personal protective equipment (gloves, disposable gowns, N95 or equivalent respirators, and eye protection) and indications for vaccination.
2023 Update on fertility
Total fertility rate and fertility care: Demographic shifts and changing demands
Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
The total fertility rate (TFR) globally is decreasing rapidly, and in the United States it is now 1.8 births per woman, well below the required replacement rate of 2.1 that maintains the population.1 These reduced TFRs result in significant demographic shifts that affect the economy, workforce, society, health care needs, environment, and geopolitical standing of every country. These changes also will shift demands for the volume and type of services delivered by women’s health care clinicians.
In addition to the TFR, mortality rates and migration rates play essential roles in determining a country’s population.2 Anticipation and planning for these population and health care service changes by each country’s government, business, professionals, and other stakeholders are imperative to manage their impact and optimize quality of life.

US standings in projected population and economic growth
The US population is predicted to peak at 364 million in 2062 and decrease to 336 million in 2100, at which time it will be the fourth largest country in the world, according to a forecasting analysis by Vollset and colleagues.1 China is expected to become the biggest economy in the world in 2035, but this is predicted to change because of its decreasing population so that by 2098 the United States will again be the country with the largest economy (FIGURE 1).1
For the United States to maintain its economic and geopolitical standing, it is important to have policies that promote families. Other countries, especially in northern Europe, have implemented such policies. These include education of the population,economic incentives to create families, extended day care, and favorable tax policies.3 They also include increased access to family-forming fertility care. Such policies in Denmark have resulted in approximately 10% of all children being born from assisted reproductive technology (ART), compared with about 1.5% in the United States. Other countries have similar policies and success in increasing the number of children born from ART.
In the United States, the American Society for Reproductive Medicine (ASRM), RESOLVE: the National Infertility Association, the American Medical Women’s Association (AMWA), and others are promoting the need for increased access to fertility care and family-forming resources, primarily through family-forming benefits provided by companies.4 Such benefits are critical since the primary reason most people do not undergo fertility care is a lack of affordability. Only 1 person in 4 in the United States who needs fertility care receives treatment. Increased access would result in more babies being born to help address the reduced TFR.
Educational access, contraceptive goals, and access to fertility care
Continued trends in women’s educational attainment and access to contraception will hasten declines in the fertility rate and slow population growth (TABLE).1 These educational and contraceptive goals also must be pursued so that every person can achieve their individual reproductive life goals of having a family if and when they want to have a family. In addition to helping address the decreasing TFR, there is a fundamental right to found a family, as stated in the United Nations charter. It is a matter of social justice and equity that everyone who wants to have a family can access reproductive care on a nondiscriminatory basis when needed.
While the need for more and better insurance coverage for infertility has been well documented for many years, the decreasing TFR in the United States is an additional compelling reason that government, business, and other stakeholders should continue to increase access to fertility benefits and care. Women’s health care clinicians are encouraged to support these initiatives that also improve quality of life, equity, and social justice.
The decreasing global and US total fertility rate causes significant demographic changes, with major socioeconomic and health care consequences. The reduced TFR impacts women’s health care services, including the need for increased access to fertility care. Government and corporate policies, including those that improve access to fertility care, will help society adapt to these changes.
Continue to: A new comprehensive ovulatory disorders classification system developed by FIGO...
A new comprehensive ovulatory disorders classification system developed by FIGO
Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
Ovulatory disorders are well-recognized and common causes of infertility and abnormal uterine bleeding (AUB). Ovulatory disorders occur on a spectrum, with the most severe form being anovulation, and comprise a heterogeneous group that has been classically categorized based on an initial monograph published by the World Health Organization (WHO) in 1973. That classification was based on gonadotropin levels and categorized these disorders into 3 groups: 1) hypogonadotropic (such as hypothalamic amenorrhea), 2) eugonadotropic (such as polycystic ovary syndrome [PCOS]), and 3) hypergonadotropic (such as primary ovarian insufficiency). This initial classification was the subject of several subsequent iterations and modifications over the past 50 years; for example, at one point, ovulatory disorder caused by hyperprolactinemia was added as a separate fourth category. However, due to advances in endocrine assays, imaging technology, and genetics, our understanding of ovulatory disorders has expanded remarkably over the past several decades.
Previous FIGO classifications
Considering the emergent complexity of these disorders and the limitations of the original WHO classification to capture these subtleties adequately, the International Federation of Gynecology and Obstetrics (FIGO) recently developed and published a new classification system for ovulatory disorders.5 This new system was designed using a meticulously followed Delphi process with inputs from a diverse group of national and international professional organizations, subspecialty societies, specialty journals, recognized experts in the field, and lay individuals interested in the subject matter.
Of note, FIGO had previously published classification systems for nongestational normal and abnormal uterine bleeding in the reproductive years (FIGO AUB System 1),as well as a subsequent classification system that described potential causes of AUB symptoms (FIGO AUB System 2), with the 9 categories arranged under the acronym PALM-COEIN (Polyp, Adenomyosis, Leiomyoma, Malignancy–Coagulopathy, Ovulatory dysfunction, Endometrial disorders, Iatrogenic, and Not otherwise classified). This new FIGO classification of ovulatory disorders can be viewed as a continuation of the previous initiatives and aims to further categorize the subgroup of AUB-O (AUB with ovulatory disorders). However, it is important to recognize that while most ovulatory disorders manifest with the symptoms of AUB, the absence of AUB symptoms does not necessarily preclude ovulatory disorders.
New system uses a 3-tier approach
The new FIGO classification system for ovulatory disorders has adopted a 3-tier system.
The first tier is based on the anatomic components of the hypothalamic-pituitary-ovarian (HPO) axis and is referred to with the acronym HyPO, for Hypothalamic-Pituitary-Ovarian. Recognizing that PCOS refers to a distinct spectrum of conditions that share a variable combination of signs and symptoms caused to varying degrees by different pathophysiologic mechanisms that involve inherent ovarian follicular dysfunction, neuroendocrine dysfunction, insulin resistance, and androgen excess, it is categorized in a separate class of its own in the first tier, referred to with the letter P.
Adding PCOS to the anatomical categories referred to by HyPO, the first tier is overall referred to with the acronym HyPO-P (FIGURE 2).5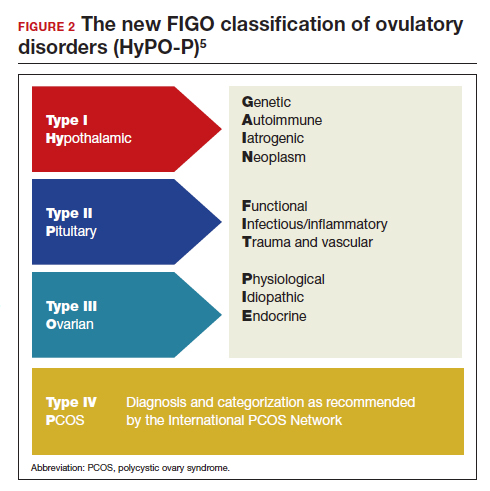
The second tier of stratification provides further etiologic details for any of the primary 3 anatomic classifications of hypothalamic, pituitary, and ovarian. These etiologies are arranged in 10 distinct groups under the mnemonic GAIN-FIT-PIE, which stands for Genetic, Autoimmune, Iatrogenic, Neoplasm; Functional, Infectious/inflammatory, Trauma and vascular; and Physiological, Idiopathic, Endocrine.
The third tier of the system refers to the specific clinical diagnosis. For example, an individual with Kallmann syndrome would be categorized as having type I (hypothalamic), Genetic, Kallmann syndrome, and an individual with PCOS would be categorized simply as having type IV, PCOS.
Our understanding of the etiology of ovulatory disorders has substantially increased over the past several decades. This progress has prompted the need to develop a more comprehensive classification system for these disorders. FIGO recently published a 3-tier classification system for ovulatory disorders that can be remembered with 2 mnemonics: HyPO-P and GAIN-FIT-PIE.
It is hoped that widespread adoption of this new classification system results in better and more concise communication between clinicians, researchers, and patients, ultimately leading to continued improvement in our understanding of the pathophysiology and management of ovulatory disorders.
Continue to: Live birth rate with conventional IVF shown noninferior to that with PGT-A...
Live birth rate with conventional IVF shown noninferior to that with PGT-A
Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
Preimplantation genetic testing for aneuploidy (PGT-A) is increasingly used in many in vitro fertilization (IVF) cycles in the United States. Based on data from the Centers for Disease Control and Prevention, 43.8% of embryo transfers in the United States in 2019 included at least 1 PGT-A–tested embryo.6 Despite this widespread use, however, there are still no robust clinical data for PGT-A’s efficacy and safety, and the guidelines published by the ASRM do not recommend its routine use in all IVF cycles.7 In the past 2 to 3 years, several large studies have raised questions about the reported benefit of this technology.8,9
Details of the trial
In a multicenter, controlled, noninferiority trial conducted by Yan and colleagues, 1,212 subfertile women were randomly assigned to either conventional IVF with embryo selection based on morphology or embryo selection based on PGT-A with next-generation sequencing. Inclusion criteria were the diagnosis of subfertility, undergoing their first IVF cycle, female age of 20 to 37, and the availability of 3 or more good-quality blastocysts.
On day 5 of embryo culture, patients with 3 or more blastocysts were randomly assigned in a 1:1 ratio to either the PGT-A group or conventional IVF. All embryos were then frozen, and patients subsequently underwent frozen embryo transfer of a single blastocyst, selected based on either morphology or euploid result by PGT-A. If the initial transfer did not result in a live birth, and there were remaining transferable embryos (either a euploid embryo in the PGT-A group or a morphologically transferable embryo in the conventional IVF group), patients underwent successive frozen embryo transfers until either there was a live birth or no more embryos were available for transfer.
The study’s primary outcome was the cumulative live birth rate per randomly assigned patient that resulted from up to 3 frozen embryo transfer cycles within 1 year. There were 606 patients randomly assigned to the PGT-A group and 606 randomly assigned to the conventional IVF group.
In the PGT-A group, 468 women (77.2%) had live births; in the conventional IVF group, 496 women (81.8%) had live births. Women in the PGT-A group had a lower incidence of pregnancy loss compared with the conventional IVF group: 8.7% versus 12.6% (absolute difference of -3.9%; 95% confidence interval [CI], -7.5 to -0.2). There was no difference in obstetric and neonatal outcomes between the 2 groups. The authors concluded that among women with 3 or more good-quality blastocysts, conventional IVF resulted in a cumulative live birth rate that was noninferior to that of the PGT-A group.
Some benefit shown with PGT-A
Although the study by Yan and colleagues did not show any benefit, and even a possible reduction, with regard to cumulative live birth rate for PGT-A, it did show a 4% reduction in clinical pregnancy loss when PGT-A was used. Furthermore, the study design has been criticized for performing PGT-A on only 3 blastocysts in the PGT-A group. It is quite conceivable that the PGT-A group would have had more euploid embryos available for transfer if the study design had included all the available embryos instead of only 3. On the other hand, one could argue that if the authors had extended the study to include all the available embryos, the conventional group would have also had more embryos for transfer and, therefore, more chances for pregnancy and live birth.
It is also important to recognize that only patients who had at least 3 embryos available for biopsy were included in this study, and therefore the results of this study cannot be extended to patients with fewer embryos, such as those with diminished ovarian reserve.
In summary, based on this study’s results, we may conclude that for the good-prognosis patients in the age group of 20 to 37 who have at least 3 embryos available for biopsy, PGT-A may reduce the miscarriage rate by about 4%, but this benefit comes at the expense of about a 4% reduction in the cumulative live birth rate. ●
Despite the lack of robust evidence for efficacy, safety, and cost-effectiveness, PGT-A has been widely adopted into clinical IVF practice in the United States over the past several years. A large randomized controlled trial has suggested that, compared with conventional IVF, PGT-A application may actually result in a slightly lower cumulative live birth rate, while the miscarriage rate may be slightly higher with conventional IVF.
PGT-A is a novel and evolving technology with the potential to improve embryo selection in IVF; however, at this juncture, there is not enough clinical data for its universal and routine use in all IVF cycles. PGT-A can potentially be more helpful in older women (>38–40) with good ovarian reserve who are likely to have a larger cohort of embryos to select from. Patients must clearly understand this technology’s pros and cons before agreeing to incorporate it into their care plan.
- Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
- Dao TH, Docquier F, Maurel M, et al. Global migration in the twentieth and twenty-first centuries: the unstoppable force of demography. Rev World Econ. 2021;157:417-449.
- Atlas of fertility treatment policies in Europe. December 2021. Fertility Europe. Accessed December 29, 2022. https:// fertilityeurope.eu/atlas/#:~:text=Fertility%20Europe%20 in%20conjunction%20with%20the%20European%20 Parliamentary,The%20Atlas%20describes%20the%20 current%20situation%20in%202021
- AMWA’s physician fertility initiative. June 2021. American Medical Women’s Association. Accessed December 29, 2022. https://www.amwa-doc.org/our-work/initiatives/physician -infertility/
- Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
- Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2021. Accessed February 24, 2023. https://www.cdc.gov/art /reports/2019/fertility-clinic.html
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
- Kucherov A, Fazzari M, Lieman H, et al. PGT-A is associated with reduced cumulative live birth rate in first reported IVF stimulation cycles age ≤ 40: an analysis of 133,494 autologous cycles reported to SART CORS. J Assist Reprod Genet. 2023;40:137-149.
Total fertility rate and fertility care: Demographic shifts and changing demands
Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
The total fertility rate (TFR) globally is decreasing rapidly, and in the United States it is now 1.8 births per woman, well below the required replacement rate of 2.1 that maintains the population.1 These reduced TFRs result in significant demographic shifts that affect the economy, workforce, society, health care needs, environment, and geopolitical standing of every country. These changes also will shift demands for the volume and type of services delivered by women’s health care clinicians.
In addition to the TFR, mortality rates and migration rates play essential roles in determining a country’s population.2 Anticipation and planning for these population and health care service changes by each country’s government, business, professionals, and other stakeholders are imperative to manage their impact and optimize quality of life.

US standings in projected population and economic growth
The US population is predicted to peak at 364 million in 2062 and decrease to 336 million in 2100, at which time it will be the fourth largest country in the world, according to a forecasting analysis by Vollset and colleagues.1 China is expected to become the biggest economy in the world in 2035, but this is predicted to change because of its decreasing population so that by 2098 the United States will again be the country with the largest economy (FIGURE 1).1

For the United States to maintain its economic and geopolitical standing, it is important to have policies that promote families. Other countries, especially in northern Europe, have implemented such policies. These include education of the population,economic incentives to create families, extended day care, and favorable tax policies.3 They also include increased access to family-forming fertility care. Such policies in Denmark have resulted in approximately 10% of all children being born from assisted reproductive technology (ART), compared with about 1.5% in the United States. Other countries have similar policies and success in increasing the number of children born from ART.
In the United States, the American Society for Reproductive Medicine (ASRM), RESOLVE: the National Infertility Association, the American Medical Women’s Association (AMWA), and others are promoting the need for increased access to fertility care and family-forming resources, primarily through family-forming benefits provided by companies.4 Such benefits are critical since the primary reason most people do not undergo fertility care is a lack of affordability. Only 1 person in 4 in the United States who needs fertility care receives treatment. Increased access would result in more babies being born to help address the reduced TFR.
Educational access, contraceptive goals, and access to fertility care
Continued trends in women’s educational attainment and access to contraception will hasten declines in the fertility rate and slow population growth (TABLE).1 These educational and contraceptive goals also must be pursued so that every person can achieve their individual reproductive life goals of having a family if and when they want to have a family. In addition to helping address the decreasing TFR, there is a fundamental right to found a family, as stated in the United Nations charter. It is a matter of social justice and equity that everyone who wants to have a family can access reproductive care on a nondiscriminatory basis when needed.

While the need for more and better insurance coverage for infertility has been well documented for many years, the decreasing TFR in the United States is an additional compelling reason that government, business, and other stakeholders should continue to increase access to fertility benefits and care. Women’s health care clinicians are encouraged to support these initiatives that also improve quality of life, equity, and social justice.
The decreasing global and US total fertility rate causes significant demographic changes, with major socioeconomic and health care consequences. The reduced TFR impacts women’s health care services, including the need for increased access to fertility care. Government and corporate policies, including those that improve access to fertility care, will help society adapt to these changes.
Continue to: A new comprehensive ovulatory disorders classification system developed by FIGO...
A new comprehensive ovulatory disorders classification system developed by FIGO
Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
Ovulatory disorders are well-recognized and common causes of infertility and abnormal uterine bleeding (AUB). Ovulatory disorders occur on a spectrum, with the most severe form being anovulation, and comprise a heterogeneous group that has been classically categorized based on an initial monograph published by the World Health Organization (WHO) in 1973. That classification was based on gonadotropin levels and categorized these disorders into 3 groups: 1) hypogonadotropic (such as hypothalamic amenorrhea), 2) eugonadotropic (such as polycystic ovary syndrome [PCOS]), and 3) hypergonadotropic (such as primary ovarian insufficiency). This initial classification was the subject of several subsequent iterations and modifications over the past 50 years; for example, at one point, ovulatory disorder caused by hyperprolactinemia was added as a separate fourth category. However, due to advances in endocrine assays, imaging technology, and genetics, our understanding of ovulatory disorders has expanded remarkably over the past several decades.
Previous FIGO classifications
Considering the emergent complexity of these disorders and the limitations of the original WHO classification to capture these subtleties adequately, the International Federation of Gynecology and Obstetrics (FIGO) recently developed and published a new classification system for ovulatory disorders.5 This new system was designed using a meticulously followed Delphi process with inputs from a diverse group of national and international professional organizations, subspecialty societies, specialty journals, recognized experts in the field, and lay individuals interested in the subject matter.
Of note, FIGO had previously published classification systems for nongestational normal and abnormal uterine bleeding in the reproductive years (FIGO AUB System 1),as well as a subsequent classification system that described potential causes of AUB symptoms (FIGO AUB System 2), with the 9 categories arranged under the acronym PALM-COEIN (Polyp, Adenomyosis, Leiomyoma, Malignancy–Coagulopathy, Ovulatory dysfunction, Endometrial disorders, Iatrogenic, and Not otherwise classified). This new FIGO classification of ovulatory disorders can be viewed as a continuation of the previous initiatives and aims to further categorize the subgroup of AUB-O (AUB with ovulatory disorders). However, it is important to recognize that while most ovulatory disorders manifest with the symptoms of AUB, the absence of AUB symptoms does not necessarily preclude ovulatory disorders.
New system uses a 3-tier approach
The new FIGO classification system for ovulatory disorders has adopted a 3-tier system.
The first tier is based on the anatomic components of the hypothalamic-pituitary-ovarian (HPO) axis and is referred to with the acronym HyPO, for Hypothalamic-Pituitary-Ovarian. Recognizing that PCOS refers to a distinct spectrum of conditions that share a variable combination of signs and symptoms caused to varying degrees by different pathophysiologic mechanisms that involve inherent ovarian follicular dysfunction, neuroendocrine dysfunction, insulin resistance, and androgen excess, it is categorized in a separate class of its own in the first tier, referred to with the letter P.
Adding PCOS to the anatomical categories referred to by HyPO, the first tier is overall referred to with the acronym HyPO-P (FIGURE 2).5
The second tier of stratification provides further etiologic details for any of the primary 3 anatomic classifications of hypothalamic, pituitary, and ovarian. These etiologies are arranged in 10 distinct groups under the mnemonic GAIN-FIT-PIE, which stands for Genetic, Autoimmune, Iatrogenic, Neoplasm; Functional, Infectious/inflammatory, Trauma and vascular; and Physiological, Idiopathic, Endocrine.
The third tier of the system refers to the specific clinical diagnosis. For example, an individual with Kallmann syndrome would be categorized as having type I (hypothalamic), Genetic, Kallmann syndrome, and an individual with PCOS would be categorized simply as having type IV, PCOS.
Our understanding of the etiology of ovulatory disorders has substantially increased over the past several decades. This progress has prompted the need to develop a more comprehensive classification system for these disorders. FIGO recently published a 3-tier classification system for ovulatory disorders that can be remembered with 2 mnemonics: HyPO-P and GAIN-FIT-PIE.
It is hoped that widespread adoption of this new classification system results in better and more concise communication between clinicians, researchers, and patients, ultimately leading to continued improvement in our understanding of the pathophysiology and management of ovulatory disorders.
Continue to: Live birth rate with conventional IVF shown noninferior to that with PGT-A...
Live birth rate with conventional IVF shown noninferior to that with PGT-A
Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
Preimplantation genetic testing for aneuploidy (PGT-A) is increasingly used in many in vitro fertilization (IVF) cycles in the United States. Based on data from the Centers for Disease Control and Prevention, 43.8% of embryo transfers in the United States in 2019 included at least 1 PGT-A–tested embryo.6 Despite this widespread use, however, there are still no robust clinical data for PGT-A’s efficacy and safety, and the guidelines published by the ASRM do not recommend its routine use in all IVF cycles.7 In the past 2 to 3 years, several large studies have raised questions about the reported benefit of this technology.8,9
Details of the trial
In a multicenter, controlled, noninferiority trial conducted by Yan and colleagues, 1,212 subfertile women were randomly assigned to either conventional IVF with embryo selection based on morphology or embryo selection based on PGT-A with next-generation sequencing. Inclusion criteria were the diagnosis of subfertility, undergoing their first IVF cycle, female age of 20 to 37, and the availability of 3 or more good-quality blastocysts.
On day 5 of embryo culture, patients with 3 or more blastocysts were randomly assigned in a 1:1 ratio to either the PGT-A group or conventional IVF. All embryos were then frozen, and patients subsequently underwent frozen embryo transfer of a single blastocyst, selected based on either morphology or euploid result by PGT-A. If the initial transfer did not result in a live birth, and there were remaining transferable embryos (either a euploid embryo in the PGT-A group or a morphologically transferable embryo in the conventional IVF group), patients underwent successive frozen embryo transfers until either there was a live birth or no more embryos were available for transfer.
The study’s primary outcome was the cumulative live birth rate per randomly assigned patient that resulted from up to 3 frozen embryo transfer cycles within 1 year. There were 606 patients randomly assigned to the PGT-A group and 606 randomly assigned to the conventional IVF group.
In the PGT-A group, 468 women (77.2%) had live births; in the conventional IVF group, 496 women (81.8%) had live births. Women in the PGT-A group had a lower incidence of pregnancy loss compared with the conventional IVF group: 8.7% versus 12.6% (absolute difference of -3.9%; 95% confidence interval [CI], -7.5 to -0.2). There was no difference in obstetric and neonatal outcomes between the 2 groups. The authors concluded that among women with 3 or more good-quality blastocysts, conventional IVF resulted in a cumulative live birth rate that was noninferior to that of the PGT-A group.
Some benefit shown with PGT-A
Although the study by Yan and colleagues did not show any benefit, and even a possible reduction, with regard to cumulative live birth rate for PGT-A, it did show a 4% reduction in clinical pregnancy loss when PGT-A was used. Furthermore, the study design has been criticized for performing PGT-A on only 3 blastocysts in the PGT-A group. It is quite conceivable that the PGT-A group would have had more euploid embryos available for transfer if the study design had included all the available embryos instead of only 3. On the other hand, one could argue that if the authors had extended the study to include all the available embryos, the conventional group would have also had more embryos for transfer and, therefore, more chances for pregnancy and live birth.
It is also important to recognize that only patients who had at least 3 embryos available for biopsy were included in this study, and therefore the results of this study cannot be extended to patients with fewer embryos, such as those with diminished ovarian reserve.
In summary, based on this study’s results, we may conclude that for the good-prognosis patients in the age group of 20 to 37 who have at least 3 embryos available for biopsy, PGT-A may reduce the miscarriage rate by about 4%, but this benefit comes at the expense of about a 4% reduction in the cumulative live birth rate. ●
Despite the lack of robust evidence for efficacy, safety, and cost-effectiveness, PGT-A has been widely adopted into clinical IVF practice in the United States over the past several years. A large randomized controlled trial has suggested that, compared with conventional IVF, PGT-A application may actually result in a slightly lower cumulative live birth rate, while the miscarriage rate may be slightly higher with conventional IVF.
PGT-A is a novel and evolving technology with the potential to improve embryo selection in IVF; however, at this juncture, there is not enough clinical data for its universal and routine use in all IVF cycles. PGT-A can potentially be more helpful in older women (>38–40) with good ovarian reserve who are likely to have a larger cohort of embryos to select from. Patients must clearly understand this technology’s pros and cons before agreeing to incorporate it into their care plan.
Total fertility rate and fertility care: Demographic shifts and changing demands
Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
The total fertility rate (TFR) globally is decreasing rapidly, and in the United States it is now 1.8 births per woman, well below the required replacement rate of 2.1 that maintains the population.1 These reduced TFRs result in significant demographic shifts that affect the economy, workforce, society, health care needs, environment, and geopolitical standing of every country. These changes also will shift demands for the volume and type of services delivered by women’s health care clinicians.
In addition to the TFR, mortality rates and migration rates play essential roles in determining a country’s population.2 Anticipation and planning for these population and health care service changes by each country’s government, business, professionals, and other stakeholders are imperative to manage their impact and optimize quality of life.

US standings in projected population and economic growth
The US population is predicted to peak at 364 million in 2062 and decrease to 336 million in 2100, at which time it will be the fourth largest country in the world, according to a forecasting analysis by Vollset and colleagues.1 China is expected to become the biggest economy in the world in 2035, but this is predicted to change because of its decreasing population so that by 2098 the United States will again be the country with the largest economy (FIGURE 1).1

For the United States to maintain its economic and geopolitical standing, it is important to have policies that promote families. Other countries, especially in northern Europe, have implemented such policies. These include education of the population,economic incentives to create families, extended day care, and favorable tax policies.3 They also include increased access to family-forming fertility care. Such policies in Denmark have resulted in approximately 10% of all children being born from assisted reproductive technology (ART), compared with about 1.5% in the United States. Other countries have similar policies and success in increasing the number of children born from ART.
In the United States, the American Society for Reproductive Medicine (ASRM), RESOLVE: the National Infertility Association, the American Medical Women’s Association (AMWA), and others are promoting the need for increased access to fertility care and family-forming resources, primarily through family-forming benefits provided by companies.4 Such benefits are critical since the primary reason most people do not undergo fertility care is a lack of affordability. Only 1 person in 4 in the United States who needs fertility care receives treatment. Increased access would result in more babies being born to help address the reduced TFR.
Educational access, contraceptive goals, and access to fertility care
Continued trends in women’s educational attainment and access to contraception will hasten declines in the fertility rate and slow population growth (TABLE).1 These educational and contraceptive goals also must be pursued so that every person can achieve their individual reproductive life goals of having a family if and when they want to have a family. In addition to helping address the decreasing TFR, there is a fundamental right to found a family, as stated in the United Nations charter. It is a matter of social justice and equity that everyone who wants to have a family can access reproductive care on a nondiscriminatory basis when needed.

While the need for more and better insurance coverage for infertility has been well documented for many years, the decreasing TFR in the United States is an additional compelling reason that government, business, and other stakeholders should continue to increase access to fertility benefits and care. Women’s health care clinicians are encouraged to support these initiatives that also improve quality of life, equity, and social justice.
The decreasing global and US total fertility rate causes significant demographic changes, with major socioeconomic and health care consequences. The reduced TFR impacts women’s health care services, including the need for increased access to fertility care. Government and corporate policies, including those that improve access to fertility care, will help society adapt to these changes.
Continue to: A new comprehensive ovulatory disorders classification system developed by FIGO...
A new comprehensive ovulatory disorders classification system developed by FIGO
Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
Ovulatory disorders are well-recognized and common causes of infertility and abnormal uterine bleeding (AUB). Ovulatory disorders occur on a spectrum, with the most severe form being anovulation, and comprise a heterogeneous group that has been classically categorized based on an initial monograph published by the World Health Organization (WHO) in 1973. That classification was based on gonadotropin levels and categorized these disorders into 3 groups: 1) hypogonadotropic (such as hypothalamic amenorrhea), 2) eugonadotropic (such as polycystic ovary syndrome [PCOS]), and 3) hypergonadotropic (such as primary ovarian insufficiency). This initial classification was the subject of several subsequent iterations and modifications over the past 50 years; for example, at one point, ovulatory disorder caused by hyperprolactinemia was added as a separate fourth category. However, due to advances in endocrine assays, imaging technology, and genetics, our understanding of ovulatory disorders has expanded remarkably over the past several decades.
Previous FIGO classifications
Considering the emergent complexity of these disorders and the limitations of the original WHO classification to capture these subtleties adequately, the International Federation of Gynecology and Obstetrics (FIGO) recently developed and published a new classification system for ovulatory disorders.5 This new system was designed using a meticulously followed Delphi process with inputs from a diverse group of national and international professional organizations, subspecialty societies, specialty journals, recognized experts in the field, and lay individuals interested in the subject matter.
Of note, FIGO had previously published classification systems for nongestational normal and abnormal uterine bleeding in the reproductive years (FIGO AUB System 1),as well as a subsequent classification system that described potential causes of AUB symptoms (FIGO AUB System 2), with the 9 categories arranged under the acronym PALM-COEIN (Polyp, Adenomyosis, Leiomyoma, Malignancy–Coagulopathy, Ovulatory dysfunction, Endometrial disorders, Iatrogenic, and Not otherwise classified). This new FIGO classification of ovulatory disorders can be viewed as a continuation of the previous initiatives and aims to further categorize the subgroup of AUB-O (AUB with ovulatory disorders). However, it is important to recognize that while most ovulatory disorders manifest with the symptoms of AUB, the absence of AUB symptoms does not necessarily preclude ovulatory disorders.
New system uses a 3-tier approach
The new FIGO classification system for ovulatory disorders has adopted a 3-tier system.
The first tier is based on the anatomic components of the hypothalamic-pituitary-ovarian (HPO) axis and is referred to with the acronym HyPO, for Hypothalamic-Pituitary-Ovarian. Recognizing that PCOS refers to a distinct spectrum of conditions that share a variable combination of signs and symptoms caused to varying degrees by different pathophysiologic mechanisms that involve inherent ovarian follicular dysfunction, neuroendocrine dysfunction, insulin resistance, and androgen excess, it is categorized in a separate class of its own in the first tier, referred to with the letter P.
Adding PCOS to the anatomical categories referred to by HyPO, the first tier is overall referred to with the acronym HyPO-P (FIGURE 2).5
The second tier of stratification provides further etiologic details for any of the primary 3 anatomic classifications of hypothalamic, pituitary, and ovarian. These etiologies are arranged in 10 distinct groups under the mnemonic GAIN-FIT-PIE, which stands for Genetic, Autoimmune, Iatrogenic, Neoplasm; Functional, Infectious/inflammatory, Trauma and vascular; and Physiological, Idiopathic, Endocrine.
The third tier of the system refers to the specific clinical diagnosis. For example, an individual with Kallmann syndrome would be categorized as having type I (hypothalamic), Genetic, Kallmann syndrome, and an individual with PCOS would be categorized simply as having type IV, PCOS.
Our understanding of the etiology of ovulatory disorders has substantially increased over the past several decades. This progress has prompted the need to develop a more comprehensive classification system for these disorders. FIGO recently published a 3-tier classification system for ovulatory disorders that can be remembered with 2 mnemonics: HyPO-P and GAIN-FIT-PIE.
It is hoped that widespread adoption of this new classification system results in better and more concise communication between clinicians, researchers, and patients, ultimately leading to continued improvement in our understanding of the pathophysiology and management of ovulatory disorders.
Continue to: Live birth rate with conventional IVF shown noninferior to that with PGT-A...
Live birth rate with conventional IVF shown noninferior to that with PGT-A
Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
Preimplantation genetic testing for aneuploidy (PGT-A) is increasingly used in many in vitro fertilization (IVF) cycles in the United States. Based on data from the Centers for Disease Control and Prevention, 43.8% of embryo transfers in the United States in 2019 included at least 1 PGT-A–tested embryo.6 Despite this widespread use, however, there are still no robust clinical data for PGT-A’s efficacy and safety, and the guidelines published by the ASRM do not recommend its routine use in all IVF cycles.7 In the past 2 to 3 years, several large studies have raised questions about the reported benefit of this technology.8,9
Details of the trial
In a multicenter, controlled, noninferiority trial conducted by Yan and colleagues, 1,212 subfertile women were randomly assigned to either conventional IVF with embryo selection based on morphology or embryo selection based on PGT-A with next-generation sequencing. Inclusion criteria were the diagnosis of subfertility, undergoing their first IVF cycle, female age of 20 to 37, and the availability of 3 or more good-quality blastocysts.
On day 5 of embryo culture, patients with 3 or more blastocysts were randomly assigned in a 1:1 ratio to either the PGT-A group or conventional IVF. All embryos were then frozen, and patients subsequently underwent frozen embryo transfer of a single blastocyst, selected based on either morphology or euploid result by PGT-A. If the initial transfer did not result in a live birth, and there were remaining transferable embryos (either a euploid embryo in the PGT-A group or a morphologically transferable embryo in the conventional IVF group), patients underwent successive frozen embryo transfers until either there was a live birth or no more embryos were available for transfer.
The study’s primary outcome was the cumulative live birth rate per randomly assigned patient that resulted from up to 3 frozen embryo transfer cycles within 1 year. There were 606 patients randomly assigned to the PGT-A group and 606 randomly assigned to the conventional IVF group.
In the PGT-A group, 468 women (77.2%) had live births; in the conventional IVF group, 496 women (81.8%) had live births. Women in the PGT-A group had a lower incidence of pregnancy loss compared with the conventional IVF group: 8.7% versus 12.6% (absolute difference of -3.9%; 95% confidence interval [CI], -7.5 to -0.2). There was no difference in obstetric and neonatal outcomes between the 2 groups. The authors concluded that among women with 3 or more good-quality blastocysts, conventional IVF resulted in a cumulative live birth rate that was noninferior to that of the PGT-A group.
Some benefit shown with PGT-A
Although the study by Yan and colleagues did not show any benefit, and even a possible reduction, with regard to cumulative live birth rate for PGT-A, it did show a 4% reduction in clinical pregnancy loss when PGT-A was used. Furthermore, the study design has been criticized for performing PGT-A on only 3 blastocysts in the PGT-A group. It is quite conceivable that the PGT-A group would have had more euploid embryos available for transfer if the study design had included all the available embryos instead of only 3. On the other hand, one could argue that if the authors had extended the study to include all the available embryos, the conventional group would have also had more embryos for transfer and, therefore, more chances for pregnancy and live birth.
It is also important to recognize that only patients who had at least 3 embryos available for biopsy were included in this study, and therefore the results of this study cannot be extended to patients with fewer embryos, such as those with diminished ovarian reserve.
In summary, based on this study’s results, we may conclude that for the good-prognosis patients in the age group of 20 to 37 who have at least 3 embryos available for biopsy, PGT-A may reduce the miscarriage rate by about 4%, but this benefit comes at the expense of about a 4% reduction in the cumulative live birth rate. ●
Despite the lack of robust evidence for efficacy, safety, and cost-effectiveness, PGT-A has been widely adopted into clinical IVF practice in the United States over the past several years. A large randomized controlled trial has suggested that, compared with conventional IVF, PGT-A application may actually result in a slightly lower cumulative live birth rate, while the miscarriage rate may be slightly higher with conventional IVF.
PGT-A is a novel and evolving technology with the potential to improve embryo selection in IVF; however, at this juncture, there is not enough clinical data for its universal and routine use in all IVF cycles. PGT-A can potentially be more helpful in older women (>38–40) with good ovarian reserve who are likely to have a larger cohort of embryos to select from. Patients must clearly understand this technology’s pros and cons before agreeing to incorporate it into their care plan.
- Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
- Dao TH, Docquier F, Maurel M, et al. Global migration in the twentieth and twenty-first centuries: the unstoppable force of demography. Rev World Econ. 2021;157:417-449.
- Atlas of fertility treatment policies in Europe. December 2021. Fertility Europe. Accessed December 29, 2022. https:// fertilityeurope.eu/atlas/#:~:text=Fertility%20Europe%20 in%20conjunction%20with%20the%20European%20 Parliamentary,The%20Atlas%20describes%20the%20 current%20situation%20in%202021
- AMWA’s physician fertility initiative. June 2021. American Medical Women’s Association. Accessed December 29, 2022. https://www.amwa-doc.org/our-work/initiatives/physician -infertility/
- Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
- Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2021. Accessed February 24, 2023. https://www.cdc.gov/art /reports/2019/fertility-clinic.html
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
- Kucherov A, Fazzari M, Lieman H, et al. PGT-A is associated with reduced cumulative live birth rate in first reported IVF stimulation cycles age ≤ 40: an analysis of 133,494 autologous cycles reported to SART CORS. J Assist Reprod Genet. 2023;40:137-149.
- Vollset SE, Goren E, Yuan C-W, et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306.
- Dao TH, Docquier F, Maurel M, et al. Global migration in the twentieth and twenty-first centuries: the unstoppable force of demography. Rev World Econ. 2021;157:417-449.
- Atlas of fertility treatment policies in Europe. December 2021. Fertility Europe. Accessed December 29, 2022. https:// fertilityeurope.eu/atlas/#:~:text=Fertility%20Europe%20 in%20conjunction%20with%20the%20European%20 Parliamentary,The%20Atlas%20describes%20the%20 current%20situation%20in%202021
- AMWA’s physician fertility initiative. June 2021. American Medical Women’s Association. Accessed December 29, 2022. https://www.amwa-doc.org/our-work/initiatives/physician -infertility/
- Munro MG, Balen AH, Cho S, et al; FIGO Committee on Menstrual Disorders and Related Health Impacts, and FIGO Committee on Reproductive Medicine, Endocrinology, and Infertility. The FIGO ovulatory disorders classification system. Fertil Steril. 2022;118:768-786.
- Centers for Disease Control and Prevention. 2019 Assisted Reproductive Technology Fertility Clinic and National Summary Report. US Dept of Health and Human Services; 2021. Accessed February 24, 2023. https://www.cdc.gov/art /reports/2019/fertility-clinic.html
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109:429-436.
- Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385:2047-2058.
- Kucherov A, Fazzari M, Lieman H, et al. PGT-A is associated with reduced cumulative live birth rate in first reported IVF stimulation cycles age ≤ 40: an analysis of 133,494 autologous cycles reported to SART CORS. J Assist Reprod Genet. 2023;40:137-149.
Trends in US colorectal cancer screening
Due to an increasing incidence of colon cancer among men and women aged 45 and younger, in 2018 the American Cancer Society, and in 2021 the US Preventive Services Task Force, recommended that screening for colon cancer begin at age 45 rather than age 50. More than half of the US population reports being up to date with these screening guidelines.
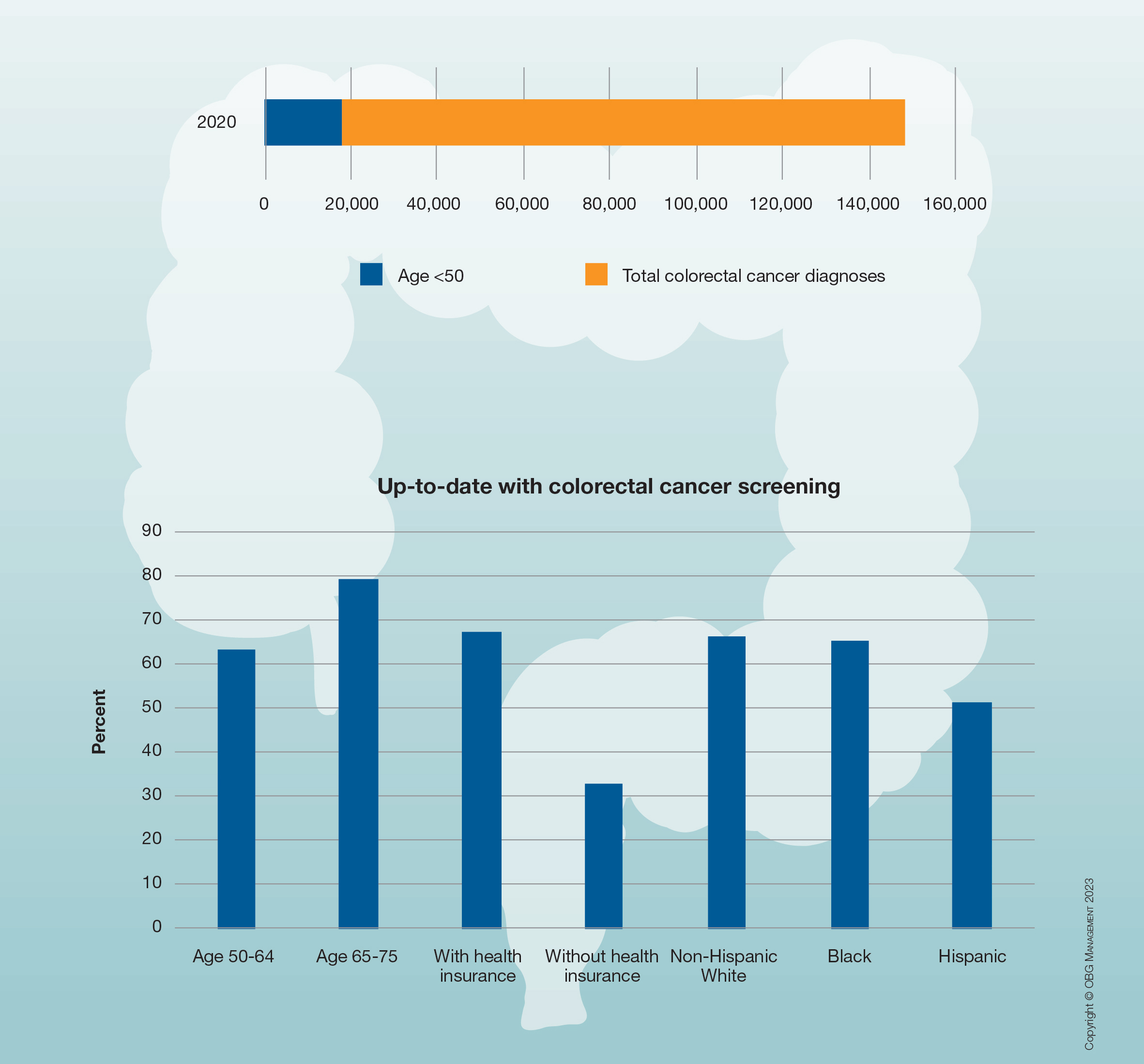
Due to an increasing incidence of colon cancer among men and women aged 45 and younger, in 2018 the American Cancer Society, and in 2021 the US Preventive Services Task Force, recommended that screening for colon cancer begin at age 45 rather than age 50. More than half of the US population reports being up to date with these screening guidelines.

Due to an increasing incidence of colon cancer among men and women aged 45 and younger, in 2018 the American Cancer Society, and in 2021 the US Preventive Services Task Force, recommended that screening for colon cancer begin at age 45 rather than age 50. More than half of the US population reports being up to date with these screening guidelines.

Multi-cancer early detection liquid biopsy testing: A predictive genetic test not quite ready for prime time
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?
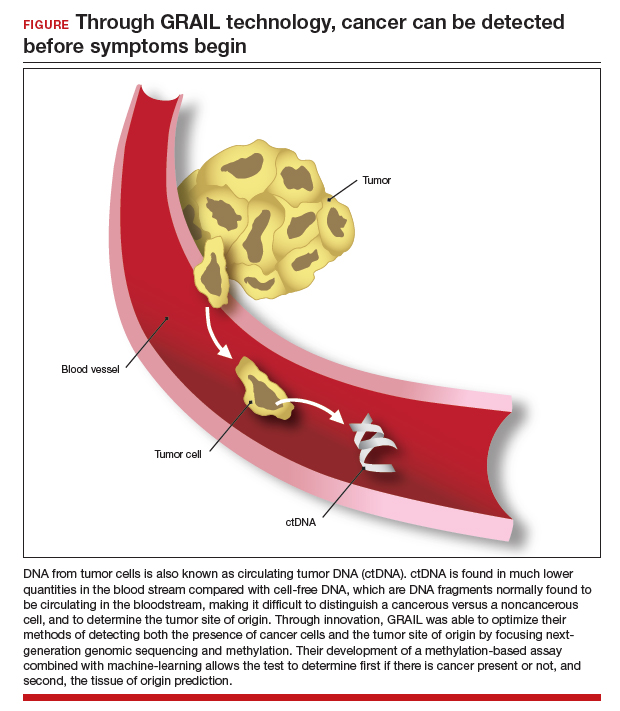
The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CarePostRoe.com: Study seeks to document poor quality medical care due to new abortion bans
In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.