User login
2023 Update on gynecologic cancer
In 2022, the most significant advances in the treatment of gynecologic cancers were achieved for patients with ovarian cancer. While ovarian cancer continues to carry the worst prognosis of all gynecologic cancers, 5-year relative survival has gradually increased, from 34.4% in 1975 to 52.4% in 2014.1
In this Update, we highlight the recent advances in our understanding of targeted therapy in ovarian cancer. We review SORAYA, a trial that demonstrated that mirvetuximab soravtansine, an antibody-drug conjugate, has promising efficacy in platinum-resistant ovarian cancers that overexpress folate receptor α. We also spotlight progress in the treatment of low-grade serous ovarian cancer, another notoriously chemotherapy-resistant disease, in GOG 281/LOGS, a phase 2 study of the MEK inhibitor trametinib. Finally, we discuss emerging long-term follow-up data on poly(ADP-ribose) polymerase (PARP) inhibitors, which are helping to refine the role of these groundbreaking drugs.
New drug approved for platinum-resistant epithelial ovarian cancer—the first since 2014
Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900.
While most patients diagnosed with advanced ovarian cancer will respond to platinum-based chemotherapy, those whose disease recurs eventually develop resistance to platinum agents. Treatment options for platinum-resistant ovarian cancer are limited and prognosis is poor. Most regimens have a response rate of only 10%. Since the approval of bevacizumab combined with chemotherapy in 2014, no new agents have been approved by the US Food and Drug Administration (FDA) for use in platinum-resistant ovarian cancer.
Efficacy shown with mirvetuximab
Recently, Matulonis and colleagues published results of the SORAYA study, a single-arm,phase 2 trial, that examined the efficacy and safety of mirvetuximab soravtansine-gynx among women with platinum-resistant ovarian cancer.2 Mirvetuximab is an antibody-drug conjugate composed of an antibody directed at the folate receptor α attached to a cytotoxic microtubule inhibitor.
The study included 106 patients with platinum-resistant ovarian cancer whose tumors expressed folate receptor α at a high level—a feature of approximately 50% of patients screened for the study. Twenty-nine patients experienced a partial response and 5 had a complete response, corresponding to a remarkable objective response rate of 32.4%. The median progression-free survival was 4.3 months.
Like other antibody-drug conjugates, ocular toxicities, including blurred vision (41%) and keratopathy (29%), were common. However, toxicity was manageable and rarely led to drug discontinuation.
The FDA has granted accelerated approval to mirvetuximab soravtansine-gynx for women with platinum-resistant ovarian cancer with high folate receptor α expression who have received 1 to 3 prior systemic treatment regimens.
Continue to: A novel agent for recurrent low-grade serous ovarian carcinoma...
A novel agent for recurrent low-grade serous ovarian carcinoma
Low-grade serous carcinoma is a histologic subtype that makes up approximately 5% of all epithelial ovarian cancers.3 Patients with low-grade serous carcinoma are often younger and, because of the indolent nature of the histology, generally have a longer overall survival compared with patients with high-grade serous carcinoma. Unlike high-grade disease, however, low-grade serous carcinoma usually is resistant to chemotherapy, making treatment options limited for patients with advanced and recurrent disease.
Trametinib: A potential option
In an international, randomized, open-label trial (GOG 281/LOGS), Gershenson and colleagues investigated the efficacy of trametinib compared with standard-of-care chemotherapy in patients with recurrent low-grade serous ovarian cancer.4 Trametinib, a mitogen-activated protein kinase MEK inhibitor, is a targeted agent that is FDA approved for treatment in BRAF-mutated melanoma, lung, and thyroid cancers.
Patients with recurrent low-grade serous ovarian cancer were randomly assigned to trametinib (n = 130) or 1 of 5 standard-of-care treatment options (n = 130), including both chemotherapy and hormonal treatments. Those assigned to trametinib were significantly less likely to have disease progression (78% vs 89%), with a median progression-free survival of 13 months, compared with7.2 months in controls (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.36–0.64). Additionally, patients who had a radiographic response to trametinib experienced a longer duration of response compared with those who responded to standard-of-care treatment (13.6 months vs 5.9 months).
While there was no statistically significant difference in overall survival (HR, 0.76; 95% CI, 0.51–1.12), crossover to trametinib from the standard-of-care group was allowed and occurred among 68% of patients, which limits the study’s ability to measure differences in overall survival.
Trametinib was well tolerated by patients, but skin rash and anemia followed by hypertension were the most common adverse effects. In the standard-of-care group, the most common toxicities were abdominal pain, nausea, and anemia. A slightly higher proportion of patients in the trametinib group discontinued the drug due to toxicity compared with the standard-of-care group (36% vs 30%), but the there was no difference between the 2 groups in scores on quality-of-life assessments.
Although trametinib is not yet FDA approved for the treatment of ovarian cancer, the National Comprehensive Cancer Network has added trametinib as a treatment option for recurrent low-grade serous ovarian carcinoma, given the significant improvement in progression-free survival compared with standard-of-care treatment.
Continue to: PARP inhibitors benefit many women with ovarian cancer, but they may hurt others...
PARP inhibitors benefit many women with ovarian cancer, but they may hurt others
Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003.
Poly(ADP-ribose) polymerase (PARP) inhibitors are a class of oral anticancer agents that target DNA repair. Since the initial FDA approval in 2014 of olaparib for the treatment of patients with recurrent BRCA-mutated ovarian cancer, PARP inhibitors have been approved for maintenance in both the frontline setting and after platinum-sensitive recurrence, and as single-agenttreatment for ovarian cancer with BRCA mutations or evidence of homologous repair deficiency (HRD), a BRCA-like molecular phenotype.5 The expanding role for PARP inhibitors in ovarian cancer seemed inexorable.
Restricted prescribing advised
In 2022, we learned that in certain settings, PARP inhibitors may be the wrong choice. Several “Dear Health Care Provider” letters were issued by AstraZeneca, Clovis, and GSK to advise physicians to restrict the prescribing of olaparib, rucaparib, and niraparib.6,7
AstraZeneca and Clovis issued letters spurred by the final analysis of ARIEL4 and SOLO3 studies, 2 randomized trials that investigated, respectively, rucaparib and olaparib monotherapy compared with chemotherapy in recurrent ovarian cancer.8,9 In both cases patients randomized to PARP inhibitors may have experienced an overall survival decrement compared with those who received chemotherapy.
At the FDA’s request, Clovis has withdrawn rucaparib as a treatment for patients with recurrent BRCA-mutant ovarian cancer who had received 2 or more lines of chemotherapy, and AstraZeneca withdrew olaparib monotherapy in germline BRCA-mutant patients with recurrent ovarian cancer. Shortly after these withdrawals, GSK also withdrew its indication for niraparib as a treatment for women with HRD, platinum-sensitive ovarian cancer who have received 3 or more prior chemotherapies. Furthermore, based on the final overall survival analysis of the NOVA study, GSK also restricted its indication for niraparib maintenance for recurrent ovarian cancer to patients with germline BRCA mutations, due to evidence of an overall survival detriment in this setting.10
Positive study results
Fortunately, 2022 was not all bad news for PARP inhibitors in ovarian cancer. In June 2022, the ATHENA-MONO trial, a phase 3 double-blind randomized controlled trial, demonstrated that rucaparib maintenance in patients with newly diagnosed epithelial ovarian cancer was associated with a significantly longer progression-free survival compared with placebo.11 The effect was most pronounced in the BRCA-mutant/HRD population, with a median progression-free survival of 28.7 months in the rucaparib group compared with 11.3 months in the placebo group (HR, 0.47; 95% CI, 0.31–0.72). Thus, rucaparib was added to the list of PARP inhibitors approved for upfront maintenance therapy in epithelial ovarian cancer.
Similarly, the long-term overall survival analysis from the upfront trials SOLO-1 and PAOLA-1 showed an overall survival advantage of PARP inhibitor, compared with placebo, maintenance in patients with BRCA mutations or HRD tumors.12,13 ●
PARP inhibitor maintenance therapy after upfront chemotherapy in women with BRCA-mutant and HRD epithelial ovarian cancer has been game changing in ovarian cancer. However, PARP inhibitors have a more limited role than previously thought for patients with recurrent ovarian cancer.
- Cancer stat facts: ovarian cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed March 11, 2023. https://seer.cancer.gov/statfacts /html/ovary.html
- Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinumresistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900
- Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11-27. doi:10.1016 /j.humpath.2018.06.018
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399:541-553. doi:10.1016/S0140-6736(21)02175-9
- Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468-3493. doi:10.1200/JCO.20.01924
- Rubraca (rucaparib) for treatment of BRCA-mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. Clovis Oncology. June 2022. Accessed May 11, 2022. chrome-extension://efaidnbmnnnibpcajpcglcle findmkaj/https://clovisoncology.com/pdfs/US_DHCPL _final_signed.pdf
- Lynparza (olaparib) for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy is voluntarily withdrawn in the US. AstraZeneca. August 26, 2022. Accessed May 11, 2023. https://www.lynparzahcp.com/content/dam /physician-services/us/590-lynparza-hcp-branded/hcp -global/pdf/solo3-dhcp-final-signed.pdf
- Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38:1164-1174. doi:10.1200/JCO.19.02745
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:465-478. doi:10.1016 /S1470-2045(22)00122-X
- Dear Health Care Provider Letter (Niraparib). GSK. November 2022. Accessed May 11, 2023. https://www.zejulahcp .com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US /pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20 November%202022.pdf
- Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. doi:10.1056 /NEJMoa1810858
- Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428. doi:10.1056/NEJMoa1911361
In 2022, the most significant advances in the treatment of gynecologic cancers were achieved for patients with ovarian cancer. While ovarian cancer continues to carry the worst prognosis of all gynecologic cancers, 5-year relative survival has gradually increased, from 34.4% in 1975 to 52.4% in 2014.1
In this Update, we highlight the recent advances in our understanding of targeted therapy in ovarian cancer. We review SORAYA, a trial that demonstrated that mirvetuximab soravtansine, an antibody-drug conjugate, has promising efficacy in platinum-resistant ovarian cancers that overexpress folate receptor α. We also spotlight progress in the treatment of low-grade serous ovarian cancer, another notoriously chemotherapy-resistant disease, in GOG 281/LOGS, a phase 2 study of the MEK inhibitor trametinib. Finally, we discuss emerging long-term follow-up data on poly(ADP-ribose) polymerase (PARP) inhibitors, which are helping to refine the role of these groundbreaking drugs.
New drug approved for platinum-resistant epithelial ovarian cancer—the first since 2014
Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900.
While most patients diagnosed with advanced ovarian cancer will respond to platinum-based chemotherapy, those whose disease recurs eventually develop resistance to platinum agents. Treatment options for platinum-resistant ovarian cancer are limited and prognosis is poor. Most regimens have a response rate of only 10%. Since the approval of bevacizumab combined with chemotherapy in 2014, no new agents have been approved by the US Food and Drug Administration (FDA) for use in platinum-resistant ovarian cancer.
Efficacy shown with mirvetuximab
Recently, Matulonis and colleagues published results of the SORAYA study, a single-arm,phase 2 trial, that examined the efficacy and safety of mirvetuximab soravtansine-gynx among women with platinum-resistant ovarian cancer.2 Mirvetuximab is an antibody-drug conjugate composed of an antibody directed at the folate receptor α attached to a cytotoxic microtubule inhibitor.
The study included 106 patients with platinum-resistant ovarian cancer whose tumors expressed folate receptor α at a high level—a feature of approximately 50% of patients screened for the study. Twenty-nine patients experienced a partial response and 5 had a complete response, corresponding to a remarkable objective response rate of 32.4%. The median progression-free survival was 4.3 months.
Like other antibody-drug conjugates, ocular toxicities, including blurred vision (41%) and keratopathy (29%), were common. However, toxicity was manageable and rarely led to drug discontinuation.
The FDA has granted accelerated approval to mirvetuximab soravtansine-gynx for women with platinum-resistant ovarian cancer with high folate receptor α expression who have received 1 to 3 prior systemic treatment regimens.
Continue to: A novel agent for recurrent low-grade serous ovarian carcinoma...
A novel agent for recurrent low-grade serous ovarian carcinoma
Low-grade serous carcinoma is a histologic subtype that makes up approximately 5% of all epithelial ovarian cancers.3 Patients with low-grade serous carcinoma are often younger and, because of the indolent nature of the histology, generally have a longer overall survival compared with patients with high-grade serous carcinoma. Unlike high-grade disease, however, low-grade serous carcinoma usually is resistant to chemotherapy, making treatment options limited for patients with advanced and recurrent disease.
Trametinib: A potential option
In an international, randomized, open-label trial (GOG 281/LOGS), Gershenson and colleagues investigated the efficacy of trametinib compared with standard-of-care chemotherapy in patients with recurrent low-grade serous ovarian cancer.4 Trametinib, a mitogen-activated protein kinase MEK inhibitor, is a targeted agent that is FDA approved for treatment in BRAF-mutated melanoma, lung, and thyroid cancers.
Patients with recurrent low-grade serous ovarian cancer were randomly assigned to trametinib (n = 130) or 1 of 5 standard-of-care treatment options (n = 130), including both chemotherapy and hormonal treatments. Those assigned to trametinib were significantly less likely to have disease progression (78% vs 89%), with a median progression-free survival of 13 months, compared with7.2 months in controls (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.36–0.64). Additionally, patients who had a radiographic response to trametinib experienced a longer duration of response compared with those who responded to standard-of-care treatment (13.6 months vs 5.9 months).
While there was no statistically significant difference in overall survival (HR, 0.76; 95% CI, 0.51–1.12), crossover to trametinib from the standard-of-care group was allowed and occurred among 68% of patients, which limits the study’s ability to measure differences in overall survival.
Trametinib was well tolerated by patients, but skin rash and anemia followed by hypertension were the most common adverse effects. In the standard-of-care group, the most common toxicities were abdominal pain, nausea, and anemia. A slightly higher proportion of patients in the trametinib group discontinued the drug due to toxicity compared with the standard-of-care group (36% vs 30%), but the there was no difference between the 2 groups in scores on quality-of-life assessments.
Although trametinib is not yet FDA approved for the treatment of ovarian cancer, the National Comprehensive Cancer Network has added trametinib as a treatment option for recurrent low-grade serous ovarian carcinoma, given the significant improvement in progression-free survival compared with standard-of-care treatment.
Continue to: PARP inhibitors benefit many women with ovarian cancer, but they may hurt others...
PARP inhibitors benefit many women with ovarian cancer, but they may hurt others
Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003.
Poly(ADP-ribose) polymerase (PARP) inhibitors are a class of oral anticancer agents that target DNA repair. Since the initial FDA approval in 2014 of olaparib for the treatment of patients with recurrent BRCA-mutated ovarian cancer, PARP inhibitors have been approved for maintenance in both the frontline setting and after platinum-sensitive recurrence, and as single-agenttreatment for ovarian cancer with BRCA mutations or evidence of homologous repair deficiency (HRD), a BRCA-like molecular phenotype.5 The expanding role for PARP inhibitors in ovarian cancer seemed inexorable.
Restricted prescribing advised
In 2022, we learned that in certain settings, PARP inhibitors may be the wrong choice. Several “Dear Health Care Provider” letters were issued by AstraZeneca, Clovis, and GSK to advise physicians to restrict the prescribing of olaparib, rucaparib, and niraparib.6,7
AstraZeneca and Clovis issued letters spurred by the final analysis of ARIEL4 and SOLO3 studies, 2 randomized trials that investigated, respectively, rucaparib and olaparib monotherapy compared with chemotherapy in recurrent ovarian cancer.8,9 In both cases patients randomized to PARP inhibitors may have experienced an overall survival decrement compared with those who received chemotherapy.
At the FDA’s request, Clovis has withdrawn rucaparib as a treatment for patients with recurrent BRCA-mutant ovarian cancer who had received 2 or more lines of chemotherapy, and AstraZeneca withdrew olaparib monotherapy in germline BRCA-mutant patients with recurrent ovarian cancer. Shortly after these withdrawals, GSK also withdrew its indication for niraparib as a treatment for women with HRD, platinum-sensitive ovarian cancer who have received 3 or more prior chemotherapies. Furthermore, based on the final overall survival analysis of the NOVA study, GSK also restricted its indication for niraparib maintenance for recurrent ovarian cancer to patients with germline BRCA mutations, due to evidence of an overall survival detriment in this setting.10
Positive study results
Fortunately, 2022 was not all bad news for PARP inhibitors in ovarian cancer. In June 2022, the ATHENA-MONO trial, a phase 3 double-blind randomized controlled trial, demonstrated that rucaparib maintenance in patients with newly diagnosed epithelial ovarian cancer was associated with a significantly longer progression-free survival compared with placebo.11 The effect was most pronounced in the BRCA-mutant/HRD population, with a median progression-free survival of 28.7 months in the rucaparib group compared with 11.3 months in the placebo group (HR, 0.47; 95% CI, 0.31–0.72). Thus, rucaparib was added to the list of PARP inhibitors approved for upfront maintenance therapy in epithelial ovarian cancer.
Similarly, the long-term overall survival analysis from the upfront trials SOLO-1 and PAOLA-1 showed an overall survival advantage of PARP inhibitor, compared with placebo, maintenance in patients with BRCA mutations or HRD tumors.12,13 ●
PARP inhibitor maintenance therapy after upfront chemotherapy in women with BRCA-mutant and HRD epithelial ovarian cancer has been game changing in ovarian cancer. However, PARP inhibitors have a more limited role than previously thought for patients with recurrent ovarian cancer.
In 2022, the most significant advances in the treatment of gynecologic cancers were achieved for patients with ovarian cancer. While ovarian cancer continues to carry the worst prognosis of all gynecologic cancers, 5-year relative survival has gradually increased, from 34.4% in 1975 to 52.4% in 2014.1
In this Update, we highlight the recent advances in our understanding of targeted therapy in ovarian cancer. We review SORAYA, a trial that demonstrated that mirvetuximab soravtansine, an antibody-drug conjugate, has promising efficacy in platinum-resistant ovarian cancers that overexpress folate receptor α. We also spotlight progress in the treatment of low-grade serous ovarian cancer, another notoriously chemotherapy-resistant disease, in GOG 281/LOGS, a phase 2 study of the MEK inhibitor trametinib. Finally, we discuss emerging long-term follow-up data on poly(ADP-ribose) polymerase (PARP) inhibitors, which are helping to refine the role of these groundbreaking drugs.
New drug approved for platinum-resistant epithelial ovarian cancer—the first since 2014
Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900.
While most patients diagnosed with advanced ovarian cancer will respond to platinum-based chemotherapy, those whose disease recurs eventually develop resistance to platinum agents. Treatment options for platinum-resistant ovarian cancer are limited and prognosis is poor. Most regimens have a response rate of only 10%. Since the approval of bevacizumab combined with chemotherapy in 2014, no new agents have been approved by the US Food and Drug Administration (FDA) for use in platinum-resistant ovarian cancer.
Efficacy shown with mirvetuximab
Recently, Matulonis and colleagues published results of the SORAYA study, a single-arm,phase 2 trial, that examined the efficacy and safety of mirvetuximab soravtansine-gynx among women with platinum-resistant ovarian cancer.2 Mirvetuximab is an antibody-drug conjugate composed of an antibody directed at the folate receptor α attached to a cytotoxic microtubule inhibitor.
The study included 106 patients with platinum-resistant ovarian cancer whose tumors expressed folate receptor α at a high level—a feature of approximately 50% of patients screened for the study. Twenty-nine patients experienced a partial response and 5 had a complete response, corresponding to a remarkable objective response rate of 32.4%. The median progression-free survival was 4.3 months.
Like other antibody-drug conjugates, ocular toxicities, including blurred vision (41%) and keratopathy (29%), were common. However, toxicity was manageable and rarely led to drug discontinuation.
The FDA has granted accelerated approval to mirvetuximab soravtansine-gynx for women with platinum-resistant ovarian cancer with high folate receptor α expression who have received 1 to 3 prior systemic treatment regimens.
Continue to: A novel agent for recurrent low-grade serous ovarian carcinoma...
A novel agent for recurrent low-grade serous ovarian carcinoma
Low-grade serous carcinoma is a histologic subtype that makes up approximately 5% of all epithelial ovarian cancers.3 Patients with low-grade serous carcinoma are often younger and, because of the indolent nature of the histology, generally have a longer overall survival compared with patients with high-grade serous carcinoma. Unlike high-grade disease, however, low-grade serous carcinoma usually is resistant to chemotherapy, making treatment options limited for patients with advanced and recurrent disease.
Trametinib: A potential option
In an international, randomized, open-label trial (GOG 281/LOGS), Gershenson and colleagues investigated the efficacy of trametinib compared with standard-of-care chemotherapy in patients with recurrent low-grade serous ovarian cancer.4 Trametinib, a mitogen-activated protein kinase MEK inhibitor, is a targeted agent that is FDA approved for treatment in BRAF-mutated melanoma, lung, and thyroid cancers.
Patients with recurrent low-grade serous ovarian cancer were randomly assigned to trametinib (n = 130) or 1 of 5 standard-of-care treatment options (n = 130), including both chemotherapy and hormonal treatments. Those assigned to trametinib were significantly less likely to have disease progression (78% vs 89%), with a median progression-free survival of 13 months, compared with7.2 months in controls (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.36–0.64). Additionally, patients who had a radiographic response to trametinib experienced a longer duration of response compared with those who responded to standard-of-care treatment (13.6 months vs 5.9 months).
While there was no statistically significant difference in overall survival (HR, 0.76; 95% CI, 0.51–1.12), crossover to trametinib from the standard-of-care group was allowed and occurred among 68% of patients, which limits the study’s ability to measure differences in overall survival.
Trametinib was well tolerated by patients, but skin rash and anemia followed by hypertension were the most common adverse effects. In the standard-of-care group, the most common toxicities were abdominal pain, nausea, and anemia. A slightly higher proportion of patients in the trametinib group discontinued the drug due to toxicity compared with the standard-of-care group (36% vs 30%), but the there was no difference between the 2 groups in scores on quality-of-life assessments.
Although trametinib is not yet FDA approved for the treatment of ovarian cancer, the National Comprehensive Cancer Network has added trametinib as a treatment option for recurrent low-grade serous ovarian carcinoma, given the significant improvement in progression-free survival compared with standard-of-care treatment.
Continue to: PARP inhibitors benefit many women with ovarian cancer, but they may hurt others...
PARP inhibitors benefit many women with ovarian cancer, but they may hurt others
Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003.
Poly(ADP-ribose) polymerase (PARP) inhibitors are a class of oral anticancer agents that target DNA repair. Since the initial FDA approval in 2014 of olaparib for the treatment of patients with recurrent BRCA-mutated ovarian cancer, PARP inhibitors have been approved for maintenance in both the frontline setting and after platinum-sensitive recurrence, and as single-agenttreatment for ovarian cancer with BRCA mutations or evidence of homologous repair deficiency (HRD), a BRCA-like molecular phenotype.5 The expanding role for PARP inhibitors in ovarian cancer seemed inexorable.
Restricted prescribing advised
In 2022, we learned that in certain settings, PARP inhibitors may be the wrong choice. Several “Dear Health Care Provider” letters were issued by AstraZeneca, Clovis, and GSK to advise physicians to restrict the prescribing of olaparib, rucaparib, and niraparib.6,7
AstraZeneca and Clovis issued letters spurred by the final analysis of ARIEL4 and SOLO3 studies, 2 randomized trials that investigated, respectively, rucaparib and olaparib monotherapy compared with chemotherapy in recurrent ovarian cancer.8,9 In both cases patients randomized to PARP inhibitors may have experienced an overall survival decrement compared with those who received chemotherapy.
At the FDA’s request, Clovis has withdrawn rucaparib as a treatment for patients with recurrent BRCA-mutant ovarian cancer who had received 2 or more lines of chemotherapy, and AstraZeneca withdrew olaparib monotherapy in germline BRCA-mutant patients with recurrent ovarian cancer. Shortly after these withdrawals, GSK also withdrew its indication for niraparib as a treatment for women with HRD, platinum-sensitive ovarian cancer who have received 3 or more prior chemotherapies. Furthermore, based on the final overall survival analysis of the NOVA study, GSK also restricted its indication for niraparib maintenance for recurrent ovarian cancer to patients with germline BRCA mutations, due to evidence of an overall survival detriment in this setting.10
Positive study results
Fortunately, 2022 was not all bad news for PARP inhibitors in ovarian cancer. In June 2022, the ATHENA-MONO trial, a phase 3 double-blind randomized controlled trial, demonstrated that rucaparib maintenance in patients with newly diagnosed epithelial ovarian cancer was associated with a significantly longer progression-free survival compared with placebo.11 The effect was most pronounced in the BRCA-mutant/HRD population, with a median progression-free survival of 28.7 months in the rucaparib group compared with 11.3 months in the placebo group (HR, 0.47; 95% CI, 0.31–0.72). Thus, rucaparib was added to the list of PARP inhibitors approved for upfront maintenance therapy in epithelial ovarian cancer.
Similarly, the long-term overall survival analysis from the upfront trials SOLO-1 and PAOLA-1 showed an overall survival advantage of PARP inhibitor, compared with placebo, maintenance in patients with BRCA mutations or HRD tumors.12,13 ●
PARP inhibitor maintenance therapy after upfront chemotherapy in women with BRCA-mutant and HRD epithelial ovarian cancer has been game changing in ovarian cancer. However, PARP inhibitors have a more limited role than previously thought for patients with recurrent ovarian cancer.
- Cancer stat facts: ovarian cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed March 11, 2023. https://seer.cancer.gov/statfacts /html/ovary.html
- Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinumresistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900
- Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11-27. doi:10.1016 /j.humpath.2018.06.018
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399:541-553. doi:10.1016/S0140-6736(21)02175-9
- Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468-3493. doi:10.1200/JCO.20.01924
- Rubraca (rucaparib) for treatment of BRCA-mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. Clovis Oncology. June 2022. Accessed May 11, 2022. chrome-extension://efaidnbmnnnibpcajpcglcle findmkaj/https://clovisoncology.com/pdfs/US_DHCPL _final_signed.pdf
- Lynparza (olaparib) for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy is voluntarily withdrawn in the US. AstraZeneca. August 26, 2022. Accessed May 11, 2023. https://www.lynparzahcp.com/content/dam /physician-services/us/590-lynparza-hcp-branded/hcp -global/pdf/solo3-dhcp-final-signed.pdf
- Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38:1164-1174. doi:10.1200/JCO.19.02745
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:465-478. doi:10.1016 /S1470-2045(22)00122-X
- Dear Health Care Provider Letter (Niraparib). GSK. November 2022. Accessed May 11, 2023. https://www.zejulahcp .com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US /pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20 November%202022.pdf
- Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. doi:10.1056 /NEJMoa1810858
- Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428. doi:10.1056/NEJMoa1911361
- Cancer stat facts: ovarian cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed March 11, 2023. https://seer.cancer.gov/statfacts /html/ovary.html
- Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinumresistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41:2436-2445. doi:10.1200/JCO.22.01900
- Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11-27. doi:10.1016 /j.humpath.2018.06.018
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. 2022;399:541-553. doi:10.1016/S0140-6736(21)02175-9
- Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:3468-3493. doi:10.1200/JCO.20.01924
- Rubraca (rucaparib) for treatment of BRCA-mutated ovarian cancer after 2 or more chemotherapies is voluntarily withdrawn in the US. Clovis Oncology. June 2022. Accessed May 11, 2022. chrome-extension://efaidnbmnnnibpcajpcglcle findmkaj/https://clovisoncology.com/pdfs/US_DHCPL _final_signed.pdf
- Lynparza (olaparib) for treatment of adult patients with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) advanced ovarian cancer who have been treated with three or more prior lines of chemotherapy is voluntarily withdrawn in the US. AstraZeneca. August 26, 2022. Accessed May 11, 2023. https://www.lynparzahcp.com/content/dam /physician-services/us/590-lynparza-hcp-branded/hcp -global/pdf/solo3-dhcp-final-signed.pdf
- Penson RT, Valencia RV, Cibula D, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38:1164-1174. doi:10.1200/JCO.19.02745
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23:465-478. doi:10.1016 /S1470-2045(22)00122-X
- Dear Health Care Provider Letter (Niraparib). GSK. November 2022. Accessed May 11, 2023. https://www.zejulahcp .com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US /pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20 November%202022.pdf
- Monk BJ, Parkinson C, Lim MC, et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964. doi:10.1200/JCO.22.01003
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. doi:10.1056 /NEJMoa1810858
- Ray-Coquard I, Pautier P, Pignata S, et al; PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416-2428. doi:10.1056/NEJMoa1911361
Postpartum IUD insertion: Best practices
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12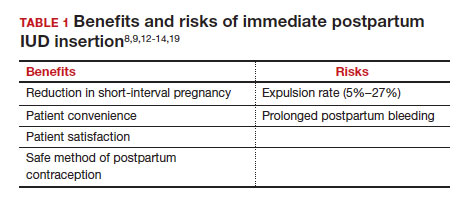
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
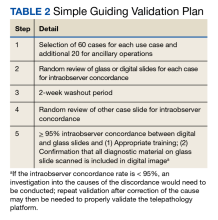
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21
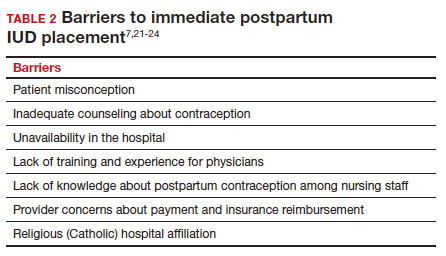
System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
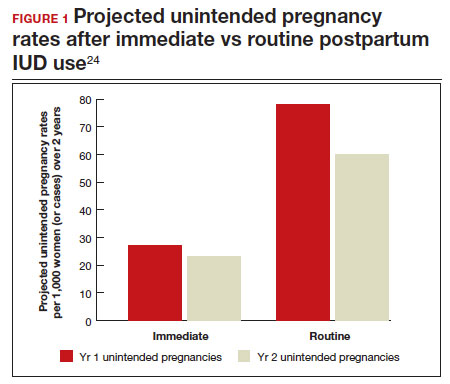
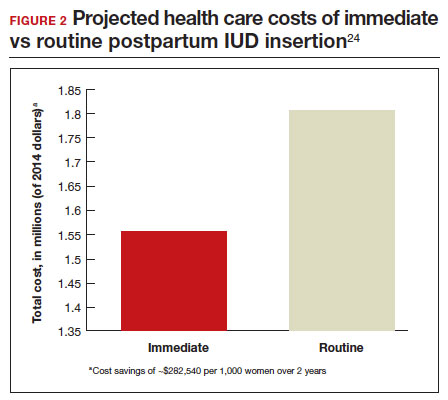
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).
Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
CASE 1 Multiparous female with short-interval pregnancies desires contraception
A 24-year-old woman (G4P3) presents for a routine prenatal visit in the third trimester. Her last 2 pregnancies have occurred within 3 months of her prior birth. She endorses feeling overwhelmed with having 4 children under the age of 5 years, and she specifies that she would like to avoid another pregnancy for several years. She plans to breast and bottle feed, and she notes that she tends to forget to take pills. When you look back at her prior charts, you note that she did not return for her last 2 postpartum visits. What can you offer her? What would be a safe contraceptive option for her?
Intrauterine devices (IUDs) are safe, effective, and reported by patients to be satisfactory methods of contraception precisely because they are prone to less user error. The Contraceptive Choice Project demonstrated that patients are more apt to choose them when barriers such as cost and access are removed and nondirective counseling is provided.1 Given that unintended pregnancy rates hover around 48%, the American College of Obstetricians and Gynecologists (ACOG) recommends them as first-line methods for pregnancy prevention.2,3
For repeat pregnancies, the postpartum period is an especially vulnerable time—non-breastfeeding women will ovulate as soon as 25 days after birth, and by 8 weeks 30% will have ovulated.4 Approximately 40% to 57% of women report having unprotected intercourse before 6 weeks postpartum, and nearly 70% of all pregnancies in the first year postpartum are unintended.3,5 Furthermore, patients at highest risk for short-interval pregnancy, such as adolescents, are less likely to return for a postpartum visit.3
Short-interval pregnancies confer greater fetal risk, including risks of low-birth weight, preterm birth, small for gestational age and increased risk of neonatal intensive care unit admission.6 Additionally, maternal health may be compromised during a short-interval pregnancy, particularly in medically complex patients due to increased risks of adverse pregnancy outcomes, such as postpartum bleeding or uterine rupture and disease progression.7 A 2006 meta-analysis by Conde-Agudelo and colleagues found that waiting at least 18 months between pregnancies was optimal for reducing these risks.6
Thus, the immediate postpartum period is an optimal time for addressing contraceptive needs and for preventing short-interval and unintended pregnancy. This article aims to provide evidence supporting the use of immediate postpartum IUDs, as well as their associated risks and barriers to use.
IUD types and routes for immediate postpartum insertion
There are several randomized controlled trials (RCTs) that examine the immediate postpartum use of copper IUDs and levonorgestrel-releasing (LNG) IUDs.8-11 In 2010, Chen and colleagues compared placement of the immediate postpartum IUD following vaginal delivery with interval placement at 6–8 weeks postpartum. Of 51 patients enrolled in each arm, 98% received an IUD immediately postpartum, and 90% received one during their postpartum visit. There were 12 expulsions (24%) in the immediate postpartum IUD group, compared with 2 (4.4%) in the interval group. Expelled IUDs were replaced, and at 6 months both groups had similar rates of IUD use.8
Whitaker and colleagues demonstrated similar findings after randomizing a small group of women who had a cesarean delivery (CD) to interval or immediate placement. There were significantly more expulsions in the post-placental group (20%) than the interval group (0%), but there were more users of the IUD in the post-placental group than in the interval group at 12 months.9
Two RCTs, by Lester and colleagues and Levi et al, demonstrated successful placement of the copper IUD or LNG-IUD following CD, with few expulsions (0% and 8%, respectively). Patients who were randomized to immediate postpartum IUD placement were more likely to receive an IUD than those who were randomized to interval insertion, mostly due to lack of postpartum follow up. Both studies followed patients out to 6 months, and rates of IUD continuation and satisfaction were higher at this time in the immediate postpartum IUD groups.10,11
Continue to: Risks, contraindications, and breastfeeding impact...
Risks, contraindications, and breastfeeding impact
What are the risks of immediate postpartum IUD placement? The highest risk of IUD placement in the immediate postpartum period appears to be expulsion (TABLE 1). In a meta-analysis conducted in 2022, which looked at 11 studies of immediate IUD insertion, the rates of expulsion were between 5% and 27%.3,8,12,13 Results of a study by Cohen and colleagues demonstrated that most expulsions occurred within the first 12 weeks following delivery; of those expulsions that occurred, only 11% went unrecognized.13 Immediate postpartum IUD insertion does not increase the IUD-associated risks of perforation, infection, or immediate postpartum bleeding (although prolonged bleeding may be more common).12
Are there contraindications to placing an IUD immediately postpartum? The main contraindication to immediate postpartum IUD use is peripartum infection, including Triple I, endomyometritis, and puerperal sepsis. Other contraindications include retained placenta requiring manual or surgical removal, uterine anomalies, and other medical contraindications to IUD use as recommended by the US Medical Eligibility Criteria.14
Does immediate IUD placement affect breastfeeding? There is theoretical risk of decreased milk supply or difficulty breastfeeding with initiation of progestin-only methods of contraception in the immediate postpartum period, as the rapid fall in progesterone levels initiates lactogenesis. However, progestin-only methods appear to have limited effect on initiation and continuation of breastfeeding in the immediate postpartum period.15
There were 2 secondary analyses of a pair of RCTs comparing immediate and delayed postpartum IUD use. Results from Levi and colleagues demonstrated no difference between immediate and interval IUD placement groups in the proportion of women who were breastfeeding at 6, 12, and 24 weeks.16 Chen and colleagues’ study was smaller; researchers found that women with interval IUD placement were more likely to be exclusively breastfeeding and continuing to breastfeed at 6 months compared with the immediate postpartum group.17
To better characterize the impact of progestin implants, in a recent meta-analysis, authors examined the use of subcutaneous levonorgestrel rods and found no difference in breastfeeding initiation and continuation rates between women who had them placed immediately versus 6 ̶ 8 weeks postpartum.12
Benefits of immediate postpartum IUD placement
One benefit of immediate postpartum IUD insertion is a reduction in short-interval pregnancies. In a study by Cohen and colleagues13 of young women aged 13 to 22 years choosing immediate postpartum IUDs (82) or implants (162), the authors found that 61% of women retained their IUDs at 12 months postpartum. Because few requested IUD removal over that time frame, the discontinuation rate at 1 year was primarily due to expulsions. Pregnancy rates at 1 year were 7.6% in the IUD group and 1.5% in the implant group. However, the 7.6% rate in the IUD group was lower than in previously studied adolescent control groups: 18.6% of control adolescents (38 of 204) using a contraceptive form other than a postpartum etonogestrel implant had repeat pregnancy at 1 year.13,18
Not only are patients who receive immediate postpartum IUDs more likely to receive them and continue their use, but they are also satisfied with the experience of receiving the IUD and with the method of contraception. A small mixed methods study of 66 patients demonstrated that women were interested in obtaining immediate postpartum contraception to avoid some of the logistical and financial challenges of returning for a postpartum visit. They also felt that the IUD placement was less painful than expected, and they didn’t feel that the insertion process imposed on their birth experience. Many described relief to know that they had a safe and effective contraceptive method upon leaving the hospital.19 Other studies have shown that even among women who expel an IUD following immediate postpartum placement, many choose to replace it in order to continue it as a contraceptive method.8,9,13
Continue to: Instructions for placement...
Instructions for placement
1. Counsel appropriately. Thoroughly counsel patients regarding their options for postpartum contraception, with emphasis on the benefits, risks, and contraindications. Current recommendations to reduce the risk of expulsion are to place the IUD in the delivery room or operating room within 10 minutes of placental delivery.
2. Post ̶ vaginal delivery. Following vaginal delivery, remove the IUD from the inserter, cut the strings to 10 cm and, using either fingers to grasp the wings of the IUD or ring forceps, advance the IUD to the fundus. Ultrasound guidance may be used, but it does not appear to be helpful in preventing expulsion.20
3. Post ̶ cesarean delivery. Once the placenta is delivered, place the IUD using the inserter or a ring forceps at the fundus and guide the strings into the cervix, then close the hysterotomy.
ACOG does recommend formal trainingbefore placing postpartum IUDs. One resource they provide is a free online webinar (https://www.acog.org/education-and-events/webinars/long-acting-reversible-contra ception-overview-and-hands-on-practice-for-residents).3
CASE 1 Resolved
The patient was counseled in the office about her options, and she was most interested in immediate postpartum LNG-IUD placement. She went on to deliver a healthy baby vaginally at 39 weeks. Within 10 minutes of placental delivery, she received an LNG-IUD. She returned to the office 3 months later for STI screening; her examination revealed correct placement and no evidence of expulsion. She expressed that she was happy with her IUD and thankful that she was able to receive it immediately after the birth of her baby.
CASE 2 Nulliparous woman desires IUD for postpartum contraception
A 33-year-old nulliparous woman presents in the third trimester for a routine prenatal visit. She had used the LNG-IUD prior to getting pregnant and reports that she was very happy with it. She knows she wants to wait at least 2 years before trying to get pregnant again, and she would like to resume contraception as soon as it is reasonably safe to do so. She has read that it is possible to get an IUD immediately postpartum and asks about it as a possible option.
What barriers will she face in obtaining an immediate postpartum IUD?
There are many barriers for patients who may be good candidates for immediate postpartum contraception (TABLE 2). Many patients are unaware that it is a safe option, and they often have concerns about such risks as infection, perforation, and effects on breastfeeding. Additionally, providers may not prioritize adequate counseling about postpartum contraception when they face time constraints and a need to counsel about other pregnancy-related topics during the prenatal visit schedule.7,21

System, hospital, and clinician barriers to immediate postpartum IUD use
Hospital implementation of a successful postpartum IUD program requires pharmacy, intrapartum and postpartum nursing staff, physicians, administration, and billing to be aligned. Hospital administration and pharmacists must stock IUDs in the pharmacy. Hospital nursing staff attitudes toward and knowledge of postpartum contraception can have profound influence on how they discuss safe and effective methods of postpartum contraception with patients who may not have received counseling during prenatal care.22 In a survey of 108 ACOG fellows, nearly 75% of ObGyn physicians did not offer immediate postpartum IUDs; lack of provider training, lack of IUD availability, and concern about cost and payment were found to be common reasons why.21 Additionally, Catholic-affiliated and rural institutions are less likely to offer it, whereas more urban, teaching hospitals are more likely to have programs in place.23 Prior to 2012, immediate postpartum IUD insertions and device costs were part of the global Medicaid obstetric fee in most states, and both hospital systems and individual providers were concerned about loss of revenue.23
In 2015, Washington and colleagues published a decision analysis that examined the cost-effectiveness and cost savings associated with immediate postpartum IUD use. Accounting for expulsion rates, they found that immediate postpartum IUD placement can save $282,540 per 1,000 women over 2 years; additionally, immediate postpartum IUD use can prevent 88 unintended pregnancies per 1,000 women over 2 years.24 Not only do immediate postpartum IUDs have great potential to prevent individual patients from undesired short-interval pregnancies (FIGURE 1), but they can also save the system substantial health care dollars (FIGURE 2).


Overcoming barriers
Immediate postpartum IUD implementation is attainable with practice, policy, and institutional changes. Education and training programs geared toward providers and nursing staff can improve understanding of the benefits and risks of immediate postpartum IUD placement. Additionally, clinicians must provide comprehensive, nondirective counseling during the antepartum period, informing patients of all safe and effective options. Expulsion risks should be disclosed, as well as the benefit of not needing to return for a separate postpartum contraception appointment.
Since 2012, many state Medicaid agencies have decoupled reimbursement for inpatient postpartum IUD insertion from the delivery fee. By 2018, more than half of states adopted this practice. Commercial insurers have followed suit in some cases, and as such, both Medicaid and commercially insured patients have had increased access to immediate postpartum IUDs.23 This has translated into increased uptake of immediate postpartum IUDs among both Medicaid and commercially insured patients. Koch et al conducted a retrospective cohort study comparing IUD use in patients 1 year before and 1 year after the policy changes, and they found a 10-fold increase in use of immediate postpartum IUDs.25
While education, counseling, access, and changes in reimbursement may increase access in many hospital systems, some barriers, such as religious affiliation of the hospital system, may be impossible to overcome. A viable alternative to immediate postpartum IUD placement may be early postpartum IUD placement, which could allow patients to coordinate this procedure with 1- or 2-week return routine postpartum visits for CD recovery, mental health screenings, and/or well-baby visits. More data are necessary before recommending this universally, but Averbach and colleagues published a promising meta-analysis that demonstrated no complete expulsions in studies in which IUDs were placed between 2 and 4 weeks postpartum, and only a pooled partial expulsion rate (of immediate postpartum, early inpatient, early outpatient, and interval placement) of 3.7%.4
CASE 2 Resolved
Although the patient was interested in receiving a postpartum LNG-IUD immediately after her vaginal birth, she had to wait until her 6-week postpartum visit. The hospital did not stock IUDs for immediate postpartum IUD use, and her provider, having not been trained on immediate postpartum insertion, did not feel comfortable trying to place it in the immediate postpartum time frame. ●
- Immediate postpartum IUD insertion is a safe and effective method for postpartum contraception for many postpartum women.
- Immediate postpartum IUD insertion can result in increased uptake of postpartum contraception, a reduction in short interval pregnancies, and the opportunity for patients to plan their ideal family size.
- Patients should be thoroughly counseled about the safety of IUD placement and risks of expulsion associated with immediate postpartum placement.
- Successful programs for immediate postpartum IUD insertion incorporate training for providers on proper insertion techniques, education for nursing staff about safety and counseling, on-site IUD supply, and reimbursement that is decoupled from the payment for delivery.
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
- Winner B, Peipert JF, Zhao Q, et al. Effectiveness of longacting reversible contraception. N Engl J Med. 2012;366:19982007. doi: 10.1056/NEJMoa1110855.
- Bearak J, Popinchalk A, Ganatra B, et al. Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990-2019. Lancet Glob Health. 2020;8:e1152-e1161. doi: 10.1016/S2214-109X(20)30315-6.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128:e32-e37. doi: 10.1097/AOG.0000000000001587.
- Averbach SH, Ermias Y, Jeng G, et al. Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223:177188. doi: 10.1016/j.ajog.2020.02.045.
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:263-267. doi: 10.1007/s00192-005-1293-6.
- Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823. doi: 10.1001 /jama.295.15.1809.
- Vricella LK, Gawron LM, Louis JM. Society for MaternalFetal Medicine (SMFM) Consult Series #48: Immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019;220:B2-B12. doi: 10.1016/j.ajog.2019.02.011.
- Chen BA, Reeves MF, Hayes JL, et al. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery: a randomized controlled trial. Obstet Gynecol. 2010;116:1079-1087. doi: 10.1097/AOG.0b013e3181f73fac.
- Whitaker AK, Endres LK, Mistretta SQ, et al. Postplacental insertion of the levonorgestrel intrauterine device after cesarean delivery vs. delayed insertion: a randomized controlled trial. Contraception. 2014;89:534-539. doi: 10.1016/j.contraception.2013.12.007.
- Lester F, Kakaire O, Byamugisha J, et al. Intracesarean insertion of the Copper T380A versus 6 weeks postcesarean: a randomized clinical trial. Contraception. 2015;91:198-203. doi: 10.1016/j.contraception.2014.12.002.
- Levi EE, Stuart GS, Zerden ML, et al. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5-11. doi: 10.1097/AOG.0000000000000882.
- Sothornwit J, Kaewrudee S, Lumbiganon P, et al. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022;10:CD011913. doi: 10.1002/14651858.CD011913.pub3.
- Cohen R, Sheeder J, Arango N, et al. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178-183. doi: 10.1016/j.contraception.2015.10.001.
- Centers for Disease Control and Prevention (CDC). US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception. 2010;82:17-37. doi: 10.1016 /j.contraception.2010.02.002.
- Levi EE, Findley MK, Avila K, et al. Placement of levonorgestrel intrauterine device at the time of cesarean delivery and the effect on breastfeeding duration. Breastfeed Med. 2018;13:674679. doi: 10.1089/bfm.2018.0060.
- Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504. doi: 10.1016/j.contraception.2011.01.022.
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Am J Obstet Gynecol. 2012;206:481.e1-7. doi: 10.1016/j.ajog.2012.04.015.
- Carr SL, Singh RH, Sussman AL, et al. Women’s experiences with immediate postpartum intrauterine device insertion: a mixed-methods study. Contraception. 2018;97:219-226. doi: 10.1016/j.contraception.2017.10.008.
- Martinez OP, Wilder L, Seal P. Ultrasound-guided compared with non-ultrasound-Guided placement of immediate postpartum intrauterine contraceptive devices. Obstet Gynecol. 2022;140:91-93. doi: 10.1097/AOG.0000000000004828.
- Holden EC, Lai E, Morelli SS, et al. Ongoing barriers to immediate postpartum long-acting reversible contraception: a physician survey. Contracept Reprod Med. 2018;3:23. doi: 10.1186/s40834-018-0078-5.
- Benfield N, Hawkins F, Ray L, et al. Exposure to routine availability of immediate postpartum LARC: effect on attitudes and practices of labor and delivery and postpartum nurses. Contraception. 2018;97:411-414. doi: 10.1016 /j.contraception.2018.01.017.
- Steenland MW, Vatsa R, Pace LE, et al. Immediate postpartum long-acting reversible contraceptive use following statespecific changes in hospital Medicaid reimbursement. JAMA Netw Open. 2022;5:e2237918. doi: 10.1001 /jamanetworkopen.2022.37918.
- Washington CI, Jamshidi R, Thung SF, et al. Timing of postpartum intrauterine device placement: a costeffectiveness analysis. Fertil Steril. 2015;103:131-137. doi: 10.1016/j.fertnstert.2014.09.032
The Critical Value of Telepathology in the COVID-19 Era
Advances in technology, including ubiquitous access to the internet and the capacity to transfer high-resolution representative images, have facilitated the adoption of telepathology by laboratories worldwide.1-5 Telepathology includes the use of telecommunication links that enable transmission of digital pathology images for primary diagnosis, quality assurance (QA), education, research, or second opinion diagnoses.3 This improvement has culminated in approvals by the US Food and Drug Administration (FDA) of whole slide imaging (WSI) systems for surgical pathology slides: specifically, the Philips IntelliSite Digital Pathology Solution in 2017 and the Leica Aperio AT2 DX in 2020.6-8 However, the approvals do not include telecytology due to lack of whole slide multiplanar scanning at different planes of focus or z-stacking capabilities.7
Long-term trends in pathology, specifically the slow reduction in the number of practicing pathologists available in the workforce compared with the total served population, along with the social distancing imperatives and disruptions brought about by the COVID-19 pandemic have made telepathology implementation pertinent to continue and improve pathology practice.8-10
Description and Definitions
The primary modes of telepathology (static image telepathology, robotic telepathology, video microscopy, WSI, and multimodality telepathology) have been defined by the American Telemedicine Association (ATA).2 WSI has been particularly suited for telepathology due to the ability to view digital slides in high resolution at various magnifications. These image files can also be viewed and shared with ease with other observers. Also, they take a shorter time to view compared with the use of a robotic microscope.3
Selection, Validation, and Implementation
WSI platforms vary in their characteristics and have several parameters, including but not limited to batch scanning vs continuous or random-access processing, throughput volume capacities, scan speed, cost, manual vs automatic loading of slides, image quality, slide capacity, flexibility for different slide sizes/features, telepathology capabilities once slide scanned, z-stacking, and regulatory approval status.8 Selection of the WSI device is dependent on need and cost considerations. For example, use for frozen section requires faster scanning speed and does not generally require a high throughput scanner.
Validation of telepathology by the testing site demonstrates that the new system performs as expected for its intended clinical use before being put into service and that the digital slides produced are acceptable for clinical diagnostic interpretation.11 The College of American Pathologists (CAP) established WSI validation guidelines are part of the published laboratory standard of care.11-13 An appropriate validation enables the benefits of telepathology while mitigating the risks.
There are 3 major CAP recommendations for validation. First, ≥ 60 cases should be included for each use case being validated with 20 additional cases for relevant ancillary applications not included in the 60 cases. Second, diagnostic concordance (ideally ≥ 95%) should be established between digital and glass slides for the same observer. Third, there should be a 2-week washout period between the viewing of digital and glass slides (Table 2).12,13
Guidelines from the ATA establish that telepathology systems should be validated for clinical use, including non-WSI platforms.2 Published validations of other non-WSI platforms (such as by robotic or multimodality telepathology) have followed the structure proposed in the guidelines by CAP for validating WSI.14,15
Ensuring that all relevant responsibilities (clinical, facility, technical, training, documentation/archiving, quality management, and operations related) for the use of telepathology are met is another aspect of validation and implementation.2 Clinical responsibilities include an agreement between the sending (referring) and receiving (consulting) parties on the information to accompany the digital material.2 From ATA clinical guidelines, this includes identification information, provision to the consulting pathologist of all relevant clinical data, provision to retrieve for access any needed and/or relevant diagnostic material, and responsibility by referrer that the correct image/metadata was sent.2 Involved parties should be trained to manage the materials being transmitted.2
Facility responsibilities include maintaining the standard of care defined by the facility and regulatory agencies.2 The maintenance of accreditation, adherence to licensure requirements, and proper management of privileges to practice telepathology are also important.2 Technical responsibilities include ensuring a proper validation that meets the standard of care and covers use cases.2,11-13
All processes, training, and competencies should be followed and documented per standard facility operating procedures.2 ATA recommends that telepathology should result in a formal report for diagnostic consultations, maintain logs of telepathology interactions or disclaimer statements, and have an appropriate retention policy.2 The CAP recommends digital images used for primary diagnosis should be kept for 10 years if the original glass slides are not available.16 Once implemented, telepathology reports must be incorporated into the pathology and laboratory medicine department’s quality management plan for both the technical performance of the telepathology system and diagnostic performance of the pathologists using the system.2 Operations responsibilities include ensuring that the telepathology system is maintained according to vendor recommendations and regulatory standards. Appropriate provisions for space and associated needs should be developed in conjunction with the information technology team of the facility to ensure appropriate security, privacy, and regulatory compliance.2
Applications and Uses
Telecytology. Rapid real-time telecytology has been documented to be useful in rapid on-site evaluations (ROSE) of the adequacy of fine needle aspirations (FNA).17-21 Nevertheless, current Medicare reimbursement is limited given that ROSE is cost prohibitive, time consuming, and affects productivity in cytology laboratories.17,22,23 Estimates of the time to provide ROSE for 1 procedure without telecytology range from 48.7 to 56.2 minutes.17,23 The use of telecytology significantly reduces pathologist ROSE time without losing quality to about 12 minutes, of which only an average of 7.5 minutes was spent by the cytopathologist for the ROSE diagnosis.17-21 ROSE also can be used for distant and remote locations to improve patient care.17-21 Multiple vendors provide real-time telecytology service. Innovations using smartphone adapters, digital cameras that could work as their own IP addresses, and connection with high-speed dedicated connections with viewing platforms on high-sensitivity monitors can facilitate ROSE to improve patient management.24,25 The successful accurate use of ROSE has been described; however, there are currently no FDA-approved telepathology ROSE platforms.17-19,21-25
To date, the FDA has not approved any telecytology whole slide scanner due to a lack of z-stacking capability in submitted scanners.7,21 Not all whole slide scanners offer z-stacking, though even in those that do offer it, the time necessary to scan the entire slide with adequate z-stacking takes too long to be clinically acceptable for many situations involving ROSE.21 WSI has also been used to develop international consensus for cytologic samples.26 Published recommendations for the validation of these other modalities before usage follow the spirit of the CAP guidelines (as far as multiple cases with high concordance rates) for validation of WSI for diagnostic purposes but vary on the exact number of slides and acceptable concordance rate.21,27 For ROSE with a robotic microscope without any on-site cytology personnel, documented standardized training of nonpathology staff members, such as the radiologist or other physician performing the FNA procedure, may be needed to enable the performance of ROSE telecytology and ensure compliance with regulations.2,21 Besides ROSE, there are published validations for telecytology in primary diagnosis and QA, indicating a role for telecytology for diagnosis for laboratories that have properly validated and implemented the laboratory-developed test.28-30
Frozen section. Telepathology has significant potential to improve access to frozen section consultation.5,31-33 Benefits to improving access to frozen section include providing frozen section consultation at remote or off-site locations, increasing access to subspecialty consultation, improving workflow by eliminating the need to travel off-site to the frozen section case, cost savings in staff work time, and providing educational opportunities for pathology trainees.5,31-33 In our experience, WSI with real-time viewing of frozen section allows for the assessment of transplant tissues, which is an evaluation that generally occurs at night. Discrepancies from frozen section telepathology using WSI to the final diagnosis may occur and those specific to WSI could result from slide or image quality, internet connectivity, and lack of training in using the telepathology system.32 Other issues that may lead to discrepancies between the frozen section diagnosis and the final diagnosis may occur with the review of glass slides by light microscopy.34 Appropriate performance of validation, training, implementation, and quality control for telepathology can help in reaping the benefits while mitigating the risks.2 In a large study comparing frozen section evaluation by telepathology with light microscopy, the sensitivity and specificity of frozen section were comparable between telepathology and light microscopy with a trend toward greater sensitivity by telepathology (0.92 and 0.99 for telepathology vs 0.90 and 0.99 by light microscopy alone, sensitivity and specificity, respectively).33
Other applications. Evidence for efficacy in surgical pathology diagnosis led to FDA approval of the Philips IntelliSite Digital Pathology in 2017 and the Leica Aperio AT2 DX in 2020 WSI platforms.6-8 The use of WSI in surgical pathology has been successfully validated or used in clinical practice at several pathology laboratory settings with documented benefits in the literature for primary and secondary diagnoses, QA, research, and education.6-8,35-45 Benefits of telepathology include improved ergonomics and access to real-time pathologic services in remote areas or during on-site pathologist absence and expert second opinions. Telepathology also may reduce risk of slide loss during transport, shortened turnaround time, reduced costs of operation through workflow efficiencies, better load balancing, improve virtual collaboration, and digital storage of slides that may be irreplaceable.3-8,35-45 Telepathology also has been shown to be useful for education, improving access to learning materials and increasing quality instructional materials at a lower cost.45 The increased ease of collaboration with remote experts and access to slide material for other pathologists improves QA capabilities.3-8,35-45 The availability of virtual slides is expected to promote further research in telepathology and pathology due to the increased availability of virtual material to researchers.1,5,46
Telehematology. Published validations have shown effectiveness for hematopathology specimens, such as the peripheral smear. Telehematology also has demonstrated potential in a laboratory after proper validation and implementation as a laboratory-developed test.37,47-49
Telemicrobiology and Computer-Assisted Pathologic Diagnosis. Telemicrobiology also has been successfully used for clinical, educational, and QA purposes.50 The digitalization of slides involved with telepathology enables further innovation in machine learning for computer-assisted pathologic diagnosis (CAPD), which is already being used clinically for cervical Pap smears.20 An artificial intelligence (AI)–based algorithm analyzes the slides to identify cells of interest, which are presented to the cytopathologist for confirmation.20 However, the expansion of CAPD to include a variety of specimen types or diagnostic situations as well as safely and effectively take initiative in completing an accurate automated diagnosis requires additional development.20,51,52 One of the key factors for machine learning to develop AI is the provision of a corpus of data.51,52 Public, open-source data sources have been limited in size while private proprietary sources have highly restricted and expensive access; to address this, there is a current effort to build the world’s largest public open-source digital pathology corpus at Temple University Hospital, which may help enable innovations in the future.52
Long-Term Trends/Applications
The COVID-19 pandemic has been unprecedented not only in its widespread morbidity and mortality, but also for the significant socioeconomic, health, lifestyle, societal, and workspace changes.53-57 Specifically, the pandemic has introduced not only a need for social distancing and staff quarantines to prevent the spread of infection, but also a reduction in the workforce due to the stresses of COVID-19 (also known as the Great Resignation).55 Before the pandemic, there was an existing downtrend in the number of pathologists in the US workforce.9-10,58,59 From 2007 to 2017, the number of active pathologists in the US declined by 17.5% despite the increasing national population, resulting in not only an absolute decrease in the number of pathologists, but also an increasing population served per pathologist ratio.59 Since 2017, this downtrend has continued; given the increasing loss of active pathologists from the workforce and the decreasing training of new pathologists, this decrease shows no signs of reversing even as the impact of the COVID-19 pandemic has begun to wane.9,10,58-60
The advantages of telepathology in enabling social distancing and reducing travel to remote sites are known.3-7,17 Given these advantages, some medical centers in the US have previously successfully validated and implemented telepathology operations earlier during the COVID-19 pandemic to ease workflow and ensure continued operations.56,57 The use of telepathology also helps in balancing workload and continuing pathology operations even in light of the workforce reduction as cases no longer need to be signed out on site with glass slides but instead can be signed out at a remote laboratory. Although the impact of the COVID-19 pandemic on operations is decreasing, the capabilities for social distancing and reducing travel remain important to both improve operations and ensure resiliency in response to similar potential events.3-7,17,60
Considering the long-term trends, the lessons of the COVID-19 pandemic, and the potential for future pandemics or other disasters, telepathology’s validation and implementation remains a reasonable choice for pathology practices looking to improve. A variety of practices not just in the general population, but also among US Department of Veterans Affairs medical centers (VAMCs) and the US Department of Defense Military Health System treating a veteran population can benefit from telepathology where it has previously been reported to have been reliable or successfully implemented.61-63 Although the veteran population differs from the general population in several characteristics, such as the severity of disease, coexisting morbidities, and other history, given proper validation and implementation, telepathology’s usefulness extends across different pathology practice settings.35-43,61-66
Limitations of Telepathology
In telepathology’s current state, there are limitations despite its immense promise.6,35 These include initial capital costs, the additional training requirement, the additional time necessary to scan slides, technical challenges (ie, laboratory information system integration, color calibration, display artifacts, potential for small particle scanner omissions, and information technology dependence), the potential for slower evaluation per slide compared with optical microscopes, limitations of slide imaging (ie, z-stacking or lack of polarization on digital pathology), and occupational concerns regarding eye strain with increased computer monitor usage (ie, computer vision syndrome).6,35 In addition, there are few telepathology scanners with FDA approval for WSI.6-8
The improving technology of telepathology has made these limitations surmountable, including faster slide scanning and increasing digital storage capacity for large WSI files. Due to this improvement in technology, an increasing number of laboratory settings, have adopted telepathology as its advantages have begun to outweigh the limitations.2-5 Additionally, the proper validation performed before implementing telepathology can help laboratories identify their unique challenges, troubleshoot, and resolve the limitations before use in clinical care.11-13 Continuing QA during its use and implementation is important to ensure that telepathology performs as expected for clinical purposes despite its limitations.2
Conclusions
Telepathology is a promising technology that may improve pathology practice once properly validated and implemented.1-8 Though there are barriers to this validation and implementation, particularly the capital costs and training, there are several potential benefits, including increased productivity, cost savings, improvement in the workflow, enhanced access to pathologic consultation, and adaptability of the pathology laboratory in an era of a decreased workforce and social distancing due to the COVID-19 pandemic.1-8,55-56 This potential applies across the wide spectrum of potential telepathology uses from frozen section, telecytology (including ROSE) to primary and second opinion diagnoses.1-8,17-33 The benefits also extends to QA, education, and research, as diagnoses can not only be rereviewed by specialty or second opinion consultation with ease, but also digital slides can be produced for educational and research purposes.3-8,35-45 Settings that treat the general population and those focused on the care of veterans or members of the armed forces have reported similar reliability or successful implementation.35-44,61-63 All in all, the use of telepathology represents an innovation that may transform the practice of pathology tomorrow.
1. Weinstein RS. Prospects for telepathology. Hum Pathol. 1986;17(5):433-434. doi:10.1016/s0046-8177(86)80028-4
2. Pantanowitz L, Dickinson K, Evans AJ, et al. American Telemedicine Association clinical guidelines for telepathology. J Pathol Inform. 2014;5(1):39. Published 2014 Oct 21. doi:10.4103/2153-3539.143329
3. Farahani N, Pantanowitz L. Overview of telepathology. Surg Pathol Clin. 2015;8(2):223-231. doi:10.1016/j.path. 2015.02.018 4. Petersen JM, Jhala D. Telepathology: a transforming practice for the efficient, safe, and best patient care at the regional Veteran Affairs medical center. Am J Clin Pathol. 2022;158(suppl 1):S97-S98. doi:10.1093/ajcp/aqac126.205
5. Bashshur RL, Krupinski EA, Weinstein RS, Dunn MR, Bashshur N. The empirical foundations of telepathology: evidence of feasibility and intermediate effects. Telemed J E Health. 2017;23(3):155-191. doi:10.1089/tmj.2016.0278
6. Jahn SW, Plass M, Moinfar F. Digital pathology: advantages, limitations and emerging perspectives. J Clin Med. 2020;9(11):3697. Published 2020 Nov 18. doi:10.3390/jcm9113697
7. Evans AJ, Bauer TW, Bui MM, et al. US Food and Drug Administration approval of whole slide imaging for primary diagnosis: a key milestone is reached and new questions are raised. Arch Pathol Lab Med. 2018;142(11):1383-1387. doi:10.5858/arpa.2017-0496-CP.
8. Patel A, Balis UGJ, Cheng J, et al. Contemporary whole slide imaging devices and their applications within the modern pathology department: a selected hardware review. J Pathol Inform. 2021;12:50. Published 2021 Dec 9. doi:10.4103/jpi.jpi_66_21
9. Association of American Medical Colleges. 2017 State Physician Workforce Data Book. November 2017. Accessed April 14, 2023. https://store.aamc.org/downloadable/download/sample/sample_id/30
10. Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7):e2010648. Published 2020 Jul 1. doi:10.1001/jamanetworkopen.2020.10648
11. Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710-1722. doi:10.5858/arpa.2013-0093-CP
12. Evans AJ, Brown RW, Bui MM, et al. Validating whole slide imaging systems for diagnostic purposes in pathology. Arch Pathol Lab Med. 2021;146(4):440-450. doi:10.5858/arpa.2020-0723-CP
13. Evans AJ, Lacchetti C, Reid K, Thomas NE. Validating whole slide imaging for diagnostic purposes in pathology: guideline update. College of American Pathologists. May 2021. Accessed April 13, 2023. https://documents.cap.org/documents/wsi-methodology.pdf
14. Chandraratnam E, Santos LD, Chou S, et al. Parathyroid frozen section interpretation via desktop telepathology systems: a validation study. J Pathol Inform. 2018;9:41. Published 2018 Dec 3. doi:10.4103/jpi.jpi_57_18
15. Thrall MJ, Rivera AL, Takei H, Powell SZ. Validation of a novel robotic telepathology platform for neuropathology intraoperative touch preparations. J Pathol Inform. 2014;5(1):21. Published 2014 Jul 28. doi:10.4103/2153-3539.137642
16. Balis UGJ, Williams CL, Cheng J, et al. Whole-Slide Imaging: Thinking Twice Before Hitting the Delete Key. AJSP: Reviews & Reports. 2018;23(6):p 249-250. doi:10.1097/PCR.0000000000000283
17. Kim B, Chhieng DC, Crowe DR, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006;3:27. Published 2006 Dec 11. doi:10.1186/1742-6413-3-27
18. Perez D, Stemmer MN, Khurana KK. Utilization of dynamic telecytopathology for rapid onsite evaluation of touch imprint cytology of needle core biopsy: diagnostic accuracy and pitfalls. Telemed J E Health. 2021;27(5):525-531. doi:10.1089/tmj.2020.0117
19. McCarthy EE, McMahon RQ, Das K, Stewart J 3rd. Internal validation testing for new technologies: bringing telecytopathology into the mainstream. Diagn Cytopathol. 2015;43(1):3-7. doi:10.1002/dc.23167
20. Marletta S, Treanor D, Eccher A, Pantanowitz L. Whole-slide imaging in cytopathology: state of the art and future directions. Diagn Histopathol (Oxf). 2021;27(11):425-430. doi:10.1016/j.mpdhp.2021.08.001
21. Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7(1):1-6. doi:10.1016/j.jasc.2017.10.002
22. Eloubeidi MA, Tamhane A, Jhala N, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101(12):2841-2847. doi:10.1111/j.1572-0241.2006.00852.x
23. Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer. 2001;93(5):319-322. doi:10.1002/cncr.9046
24. Fontelo P, Liu F, Yagi Y. Evaluation of a smartphone for telepathology: lessons learned. J Pathol Inform. 2015;6:35. Published 2015 Jun 23. doi:10.4103/2153-3539.158912
25. Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7(1):1-6. doi:10.1016/j.jasc.2017.10.002
26. Johnson DN, Onenerk M, Krane JF, et al. Cytologic grading of primary malignant salivary gland tumors: A blinded review by an international panel. Cancer Cytopathol. 2020;128(6):392-402. doi:10.1002/cncy.22271
27. Trabzonlu L, Chatt G, McIntire PJ, et al. Telecytology validation: is there a recipe for everybody? J Am Soc Cytopathol. 2022;11(4):218-225. doi:10.1016/j.jasc.2022.03.001
28. Canberk S, Behzatoglu K, Caliskan CK, et al. The role of telecytology in the primary diagnosis of thyroid fine-needle aspiration specimens. Acta Cytol. 2020;64(4):323-331. doi:10.1159/000503914.
29. Archondakis S, Roma M, Kaladelfou E. Implementation of pre-captured videos for remote diagnosis of cervical cytology specimens. Cytopathology. 2021;32(3):338-343. doi:10.1111/cyt.12948
30. Lee ES, Kim IS, Choi JS, et al. Accuracy and reproducibility of telecytology diagnosis of cervical smears. A tool for quality assurance programs. Am J Clin Pathol. 2003;119(3):356-360. doi:10.1309/7ytvag4xnr48t75h
31. Dietz RL, Hartman DJ, Pantanowitz L. Systematic review of the use of telepathology during intraoperative consultation. Am J Clin Pathol. 2020;153(2):198-209. doi:10.1093/ajcp/aqz155
32. Bauer TW, Slaw RJ, McKenney JK, Patil DT. Validation of whole slide imaging for frozen section diagnosis in surgical pathology. J Pathol Inform. 2015;6:49. Published 2015 Aug 31. doi:10.4103/2153-3539.163988

33. Vosoughi A, Smith PT, Zeitouni JA, et al. Frozen section evaluation via dynamic real-time nonrobotic telepathology system in a university cancer center by resident/faculty cooperation team. Hum Pathol. 2018;78:144-150. doi:10.1016/j.humpath.2018.04.012
34. Mahe E, Ara S, Bishara M, et al. Intraoperative pathology consultation: error, cause and impact. Can J Surg. 2013;56(3):E13-E18. doi:10.1503/cjs.011112.
35. Farahani N, Parwani AV, Pantanowitz L. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives. Pathol Lab Med Int. 2015;7:23-33. doi:10.2147/PLMI.S59826
36. Thorstenson S, Molin J, Lundström C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: digital pathology experiences 2006-2013. J Pathol Inform. 2014;5(1):14. Published 2014 Mar 28. doi:10.4103/2153-3539.129452
37. Pantanowitz L, Wiley CA, Demetris A, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi:10.4103/2153-3539.104907
38. Al Habeeb A, Evans A, Ghazarian D. Virtual microscopy using whole-slide imaging as an enabler for teledermatopathology: a paired consultant validation study. J Pathol Inform. 2012;3:2. doi:10.4103/2153-3539.93399
39. Al-Janabi S, Huisman A, Vink A, et al. Whole slide images for primary diagnostics in dermatopathology: a feasibility study. J Clin Pathol. 2012;65(2):152-158. doi:10.1136/jclinpath-2011-200277
40. Nielsen PS, Lindebjerg J, Rasmussen J, Starklint H, Waldstrøm M, Nielsen B. Virtual microscopy: an evaluation of its validity and diagnostic performance in routine histologic diagnosis of skin tumors. Hum Pathol. 2010;41(12):1770-1776. doi:10.1016/j.humpath.2010.05.015
41. Leinweber B, Massone C, Kodama K, et al. Telederma-topathology: a controlled study about diagnostic validity and technical requirements for digital transmission. Am J Dermatopathol. 2006;28(5):413-416. doi:10.1097/01.dad.0000211523.95552.86
42. Koch LH, Lampros JN, Delong LK, Chen SC, Woosley JT, Hood AF. Randomized comparison of virtual microscopy and traditional glass microscopy in diagnostic accuracy among dermatology and pathology residents. Hum Pathol. 2009;40(5):662-667. doi:10.1016/j.humpath.2008.10.009
43. Farris AB, Cohen C, Rogers TE, Smith GH. Whole slide imaging for analytical anatomic pathology and telepathology: practical applications today, promises, and perils. Arch Pathol Lab Med. 2017;141(4):542-550. doi:10.5858/arpa.2016-0265-SA
44. Chong T, Palma-Diaz MF, Fisher C, et al. The California Telepathology Service: UCLA’s experience in deploying a regional digital pathology subspecialty consultation network. J Pathol Inform. 2019;10:31. Published 2019 Sep 27. doi:10.4103/jpi.jpi_22_19
45. Meyer J, Paré G. Telepathology impacts and implementation challenges: a scoping review. Arch Pathol Lab Med. 2015;139(12):1550-1557. doi:10.5858/arpa.2014-0606-RA
46. Weinstein RS, Descour MR, Liang C, et al. Telepathology overview: from concept to implementation. Hum Pathol. 2001;32(12):1283-1299. doi:10.1053/hupa.2001.29643
47. Riley RS, Ben-Ezra JM, Massey D, Cousar J. The virtual blood film. Clin Lab Med. 2002;22(1):317-345. doi:10.1016/s0272-2712(03)00077-5
48. Garcia CA, Hanna M, Contis LC, Pantanowitz L, Hyman R. Sharing Cellavision blood smear images with clinicians via the electronic medical record. Blood. 2017;130(suppl 1):5586. doi:10.1182/blood.V130.Suppl_1.5586.5586
49. Goswami R, Pi D, Pal J, Cheng K, Hudoba De Badyn M. Performance evaluation of a dynamic telepathology system (Panoptiq) in the morphologic assessment of peripheral blood film abnormalities. Int J Lab Hematol. 2015;37(3):365-371. doi:10.1111/ijlh.12294
50. Rhoads DD, Mathison BA, Bishop HS, da Silva AJ, Pantanowitz L. Review of telemicrobiology. Arch Pathol Lab Med. 2016;140(4):362-370. doi:10.5858/arpa.2015-0116-RA51. Nam S, Chong Y, Jung CK, et al. Introduction to digital pathology and computer-aided pathology. J Pathol Transl Med. 2020;54(2):125-134. doi:10.4132/jptm.2019.12.31
52. Houser D, Shadhin G, Anstotz R, et al. The Temple University Hospital Digital Pathology Corpus. IEEE Signal Process Med Biol Symp. 2018:1-7. doi:10.1109/SPMB.2018.8615619
53. Petersen J, Dalal S, Jhala D. Criticality of in-house preparation of viral transport medium in times of shortage during COVID-19 pandemic. Lab Med. 2021;52(2):e39-e45. doi:10.1093/labmed/lmaa099
54. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382(18):e41. doi:10.1056/NEJMp2006141
55. Ksinan Jiskrova G. Impact of COVID-19 pandemic on the workforce: from psychological distress to the Great Resignation. J Epidemiol Community Health. 2022;76(6):525-526. doi:10.1136/jech-2022-218826
56. Henriksen J, Kolognizak T, Houghton T, et al. Rapid validation of telepathology by an academic neuropathology practice during the COVID-19 pandemic. Arch Pathol Lab Med. 2020;144(11):1311-1320. doi:10.5858/arpa.2020-0372-SA
57. Ardon O, Reuter VE, Hameed M, et al. Digital pathology operations at an NYC tertiary cancer center during the first 4 months of COVID-19 pandemic response. Acad Pathol. 2021;8:23742895211010276. Published 2021 Apr 28. doi:10.1177/23742895211010276
58. Jajosky RP, Jajosky AN, Kleven DT, Singh G. Fewer seniors from United States allopathic medical schools are filling pathology residency positions in the Main Residency Match, 2008-2017. Hum Pathol. 2018;73:26-32. doi:10.1016/j.humpath.2017.11.014
59. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337. Published 2019 May 3. doi:10.1001/jamanetworkopen.2019.4337
60. Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet. 2022;399(10323):417-419. doi:10.1016/S0140-6736(22)00100-3
61. Ghosh A, Brown GT, Fontelo P. Telepathology at the Armed Forces Institute of Pathology: a retrospective review of consultations from 1996 to 1997. Arch Pathol Lab Med. 2018;142(2):248-252. doi:10.5858/arpa.2017-0055-OA
62. Dunn BE, Choi H, Almagro UA, Recla DL, Davis CW. Telepathology networking in VISN-12 of the Veterans Health Administration. Telemed J E Health. 2000;6(3):349-354. doi:10.1089/153056200750040200
63. Dunn BE, Almagro UA, Choi H, et al. Dynamic-robotic telepathology: Department of Veterans Affairs feasibility study. Hum Pathol. 1997;28(1):8-12. doi:10.1016/s0046-8177(97)90271-9
64. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252

65. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
66. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448
Advances in technology, including ubiquitous access to the internet and the capacity to transfer high-resolution representative images, have facilitated the adoption of telepathology by laboratories worldwide.1-5 Telepathology includes the use of telecommunication links that enable transmission of digital pathology images for primary diagnosis, quality assurance (QA), education, research, or second opinion diagnoses.3 This improvement has culminated in approvals by the US Food and Drug Administration (FDA) of whole slide imaging (WSI) systems for surgical pathology slides: specifically, the Philips IntelliSite Digital Pathology Solution in 2017 and the Leica Aperio AT2 DX in 2020.6-8 However, the approvals do not include telecytology due to lack of whole slide multiplanar scanning at different planes of focus or z-stacking capabilities.7
Long-term trends in pathology, specifically the slow reduction in the number of practicing pathologists available in the workforce compared with the total served population, along with the social distancing imperatives and disruptions brought about by the COVID-19 pandemic have made telepathology implementation pertinent to continue and improve pathology practice.8-10
Description and Definitions
The primary modes of telepathology (static image telepathology, robotic telepathology, video microscopy, WSI, and multimodality telepathology) have been defined by the American Telemedicine Association (ATA).2 WSI has been particularly suited for telepathology due to the ability to view digital slides in high resolution at various magnifications. These image files can also be viewed and shared with ease with other observers. Also, they take a shorter time to view compared with the use of a robotic microscope.3
Selection, Validation, and Implementation
WSI platforms vary in their characteristics and have several parameters, including but not limited to batch scanning vs continuous or random-access processing, throughput volume capacities, scan speed, cost, manual vs automatic loading of slides, image quality, slide capacity, flexibility for different slide sizes/features, telepathology capabilities once slide scanned, z-stacking, and regulatory approval status.8 Selection of the WSI device is dependent on need and cost considerations. For example, use for frozen section requires faster scanning speed and does not generally require a high throughput scanner.
Validation of telepathology by the testing site demonstrates that the new system performs as expected for its intended clinical use before being put into service and that the digital slides produced are acceptable for clinical diagnostic interpretation.11 The College of American Pathologists (CAP) established WSI validation guidelines are part of the published laboratory standard of care.11-13 An appropriate validation enables the benefits of telepathology while mitigating the risks.
There are 3 major CAP recommendations for validation. First, ≥ 60 cases should be included for each use case being validated with 20 additional cases for relevant ancillary applications not included in the 60 cases. Second, diagnostic concordance (ideally ≥ 95%) should be established between digital and glass slides for the same observer. Third, there should be a 2-week washout period between the viewing of digital and glass slides (Table 2).12,13
Guidelines from the ATA establish that telepathology systems should be validated for clinical use, including non-WSI platforms.2 Published validations of other non-WSI platforms (such as by robotic or multimodality telepathology) have followed the structure proposed in the guidelines by CAP for validating WSI.14,15
Ensuring that all relevant responsibilities (clinical, facility, technical, training, documentation/archiving, quality management, and operations related) for the use of telepathology are met is another aspect of validation and implementation.2 Clinical responsibilities include an agreement between the sending (referring) and receiving (consulting) parties on the information to accompany the digital material.2 From ATA clinical guidelines, this includes identification information, provision to the consulting pathologist of all relevant clinical data, provision to retrieve for access any needed and/or relevant diagnostic material, and responsibility by referrer that the correct image/metadata was sent.2 Involved parties should be trained to manage the materials being transmitted.2
Facility responsibilities include maintaining the standard of care defined by the facility and regulatory agencies.2 The maintenance of accreditation, adherence to licensure requirements, and proper management of privileges to practice telepathology are also important.2 Technical responsibilities include ensuring a proper validation that meets the standard of care and covers use cases.2,11-13
All processes, training, and competencies should be followed and documented per standard facility operating procedures.2 ATA recommends that telepathology should result in a formal report for diagnostic consultations, maintain logs of telepathology interactions or disclaimer statements, and have an appropriate retention policy.2 The CAP recommends digital images used for primary diagnosis should be kept for 10 years if the original glass slides are not available.16 Once implemented, telepathology reports must be incorporated into the pathology and laboratory medicine department’s quality management plan for both the technical performance of the telepathology system and diagnostic performance of the pathologists using the system.2 Operations responsibilities include ensuring that the telepathology system is maintained according to vendor recommendations and regulatory standards. Appropriate provisions for space and associated needs should be developed in conjunction with the information technology team of the facility to ensure appropriate security, privacy, and regulatory compliance.2
Applications and Uses
Telecytology. Rapid real-time telecytology has been documented to be useful in rapid on-site evaluations (ROSE) of the adequacy of fine needle aspirations (FNA).17-21 Nevertheless, current Medicare reimbursement is limited given that ROSE is cost prohibitive, time consuming, and affects productivity in cytology laboratories.17,22,23 Estimates of the time to provide ROSE for 1 procedure without telecytology range from 48.7 to 56.2 minutes.17,23 The use of telecytology significantly reduces pathologist ROSE time without losing quality to about 12 minutes, of which only an average of 7.5 minutes was spent by the cytopathologist for the ROSE diagnosis.17-21 ROSE also can be used for distant and remote locations to improve patient care.17-21 Multiple vendors provide real-time telecytology service. Innovations using smartphone adapters, digital cameras that could work as their own IP addresses, and connection with high-speed dedicated connections with viewing platforms on high-sensitivity monitors can facilitate ROSE to improve patient management.24,25 The successful accurate use of ROSE has been described; however, there are currently no FDA-approved telepathology ROSE platforms.17-19,21-25
To date, the FDA has not approved any telecytology whole slide scanner due to a lack of z-stacking capability in submitted scanners.7,21 Not all whole slide scanners offer z-stacking, though even in those that do offer it, the time necessary to scan the entire slide with adequate z-stacking takes too long to be clinically acceptable for many situations involving ROSE.21 WSI has also been used to develop international consensus for cytologic samples.26 Published recommendations for the validation of these other modalities before usage follow the spirit of the CAP guidelines (as far as multiple cases with high concordance rates) for validation of WSI for diagnostic purposes but vary on the exact number of slides and acceptable concordance rate.21,27 For ROSE with a robotic microscope without any on-site cytology personnel, documented standardized training of nonpathology staff members, such as the radiologist or other physician performing the FNA procedure, may be needed to enable the performance of ROSE telecytology and ensure compliance with regulations.2,21 Besides ROSE, there are published validations for telecytology in primary diagnosis and QA, indicating a role for telecytology for diagnosis for laboratories that have properly validated and implemented the laboratory-developed test.28-30
Frozen section. Telepathology has significant potential to improve access to frozen section consultation.5,31-33 Benefits to improving access to frozen section include providing frozen section consultation at remote or off-site locations, increasing access to subspecialty consultation, improving workflow by eliminating the need to travel off-site to the frozen section case, cost savings in staff work time, and providing educational opportunities for pathology trainees.5,31-33 In our experience, WSI with real-time viewing of frozen section allows for the assessment of transplant tissues, which is an evaluation that generally occurs at night. Discrepancies from frozen section telepathology using WSI to the final diagnosis may occur and those specific to WSI could result from slide or image quality, internet connectivity, and lack of training in using the telepathology system.32 Other issues that may lead to discrepancies between the frozen section diagnosis and the final diagnosis may occur with the review of glass slides by light microscopy.34 Appropriate performance of validation, training, implementation, and quality control for telepathology can help in reaping the benefits while mitigating the risks.2 In a large study comparing frozen section evaluation by telepathology with light microscopy, the sensitivity and specificity of frozen section were comparable between telepathology and light microscopy with a trend toward greater sensitivity by telepathology (0.92 and 0.99 for telepathology vs 0.90 and 0.99 by light microscopy alone, sensitivity and specificity, respectively).33
Other applications. Evidence for efficacy in surgical pathology diagnosis led to FDA approval of the Philips IntelliSite Digital Pathology in 2017 and the Leica Aperio AT2 DX in 2020 WSI platforms.6-8 The use of WSI in surgical pathology has been successfully validated or used in clinical practice at several pathology laboratory settings with documented benefits in the literature for primary and secondary diagnoses, QA, research, and education.6-8,35-45 Benefits of telepathology include improved ergonomics and access to real-time pathologic services in remote areas or during on-site pathologist absence and expert second opinions. Telepathology also may reduce risk of slide loss during transport, shortened turnaround time, reduced costs of operation through workflow efficiencies, better load balancing, improve virtual collaboration, and digital storage of slides that may be irreplaceable.3-8,35-45 Telepathology also has been shown to be useful for education, improving access to learning materials and increasing quality instructional materials at a lower cost.45 The increased ease of collaboration with remote experts and access to slide material for other pathologists improves QA capabilities.3-8,35-45 The availability of virtual slides is expected to promote further research in telepathology and pathology due to the increased availability of virtual material to researchers.1,5,46
Telehematology. Published validations have shown effectiveness for hematopathology specimens, such as the peripheral smear. Telehematology also has demonstrated potential in a laboratory after proper validation and implementation as a laboratory-developed test.37,47-49
Telemicrobiology and Computer-Assisted Pathologic Diagnosis. Telemicrobiology also has been successfully used for clinical, educational, and QA purposes.50 The digitalization of slides involved with telepathology enables further innovation in machine learning for computer-assisted pathologic diagnosis (CAPD), which is already being used clinically for cervical Pap smears.20 An artificial intelligence (AI)–based algorithm analyzes the slides to identify cells of interest, which are presented to the cytopathologist for confirmation.20 However, the expansion of CAPD to include a variety of specimen types or diagnostic situations as well as safely and effectively take initiative in completing an accurate automated diagnosis requires additional development.20,51,52 One of the key factors for machine learning to develop AI is the provision of a corpus of data.51,52 Public, open-source data sources have been limited in size while private proprietary sources have highly restricted and expensive access; to address this, there is a current effort to build the world’s largest public open-source digital pathology corpus at Temple University Hospital, which may help enable innovations in the future.52
Long-Term Trends/Applications
The COVID-19 pandemic has been unprecedented not only in its widespread morbidity and mortality, but also for the significant socioeconomic, health, lifestyle, societal, and workspace changes.53-57 Specifically, the pandemic has introduced not only a need for social distancing and staff quarantines to prevent the spread of infection, but also a reduction in the workforce due to the stresses of COVID-19 (also known as the Great Resignation).55 Before the pandemic, there was an existing downtrend in the number of pathologists in the US workforce.9-10,58,59 From 2007 to 2017, the number of active pathologists in the US declined by 17.5% despite the increasing national population, resulting in not only an absolute decrease in the number of pathologists, but also an increasing population served per pathologist ratio.59 Since 2017, this downtrend has continued; given the increasing loss of active pathologists from the workforce and the decreasing training of new pathologists, this decrease shows no signs of reversing even as the impact of the COVID-19 pandemic has begun to wane.9,10,58-60
The advantages of telepathology in enabling social distancing and reducing travel to remote sites are known.3-7,17 Given these advantages, some medical centers in the US have previously successfully validated and implemented telepathology operations earlier during the COVID-19 pandemic to ease workflow and ensure continued operations.56,57 The use of telepathology also helps in balancing workload and continuing pathology operations even in light of the workforce reduction as cases no longer need to be signed out on site with glass slides but instead can be signed out at a remote laboratory. Although the impact of the COVID-19 pandemic on operations is decreasing, the capabilities for social distancing and reducing travel remain important to both improve operations and ensure resiliency in response to similar potential events.3-7,17,60
Considering the long-term trends, the lessons of the COVID-19 pandemic, and the potential for future pandemics or other disasters, telepathology’s validation and implementation remains a reasonable choice for pathology practices looking to improve. A variety of practices not just in the general population, but also among US Department of Veterans Affairs medical centers (VAMCs) and the US Department of Defense Military Health System treating a veteran population can benefit from telepathology where it has previously been reported to have been reliable or successfully implemented.61-63 Although the veteran population differs from the general population in several characteristics, such as the severity of disease, coexisting morbidities, and other history, given proper validation and implementation, telepathology’s usefulness extends across different pathology practice settings.35-43,61-66
Limitations of Telepathology
In telepathology’s current state, there are limitations despite its immense promise.6,35 These include initial capital costs, the additional training requirement, the additional time necessary to scan slides, technical challenges (ie, laboratory information system integration, color calibration, display artifacts, potential for small particle scanner omissions, and information technology dependence), the potential for slower evaluation per slide compared with optical microscopes, limitations of slide imaging (ie, z-stacking or lack of polarization on digital pathology), and occupational concerns regarding eye strain with increased computer monitor usage (ie, computer vision syndrome).6,35 In addition, there are few telepathology scanners with FDA approval for WSI.6-8
The improving technology of telepathology has made these limitations surmountable, including faster slide scanning and increasing digital storage capacity for large WSI files. Due to this improvement in technology, an increasing number of laboratory settings, have adopted telepathology as its advantages have begun to outweigh the limitations.2-5 Additionally, the proper validation performed before implementing telepathology can help laboratories identify their unique challenges, troubleshoot, and resolve the limitations before use in clinical care.11-13 Continuing QA during its use and implementation is important to ensure that telepathology performs as expected for clinical purposes despite its limitations.2
Conclusions
Telepathology is a promising technology that may improve pathology practice once properly validated and implemented.1-8 Though there are barriers to this validation and implementation, particularly the capital costs and training, there are several potential benefits, including increased productivity, cost savings, improvement in the workflow, enhanced access to pathologic consultation, and adaptability of the pathology laboratory in an era of a decreased workforce and social distancing due to the COVID-19 pandemic.1-8,55-56 This potential applies across the wide spectrum of potential telepathology uses from frozen section, telecytology (including ROSE) to primary and second opinion diagnoses.1-8,17-33 The benefits also extends to QA, education, and research, as diagnoses can not only be rereviewed by specialty or second opinion consultation with ease, but also digital slides can be produced for educational and research purposes.3-8,35-45 Settings that treat the general population and those focused on the care of veterans or members of the armed forces have reported similar reliability or successful implementation.35-44,61-63 All in all, the use of telepathology represents an innovation that may transform the practice of pathology tomorrow.
Advances in technology, including ubiquitous access to the internet and the capacity to transfer high-resolution representative images, have facilitated the adoption of telepathology by laboratories worldwide.1-5 Telepathology includes the use of telecommunication links that enable transmission of digital pathology images for primary diagnosis, quality assurance (QA), education, research, or second opinion diagnoses.3 This improvement has culminated in approvals by the US Food and Drug Administration (FDA) of whole slide imaging (WSI) systems for surgical pathology slides: specifically, the Philips IntelliSite Digital Pathology Solution in 2017 and the Leica Aperio AT2 DX in 2020.6-8 However, the approvals do not include telecytology due to lack of whole slide multiplanar scanning at different planes of focus or z-stacking capabilities.7
Long-term trends in pathology, specifically the slow reduction in the number of practicing pathologists available in the workforce compared with the total served population, along with the social distancing imperatives and disruptions brought about by the COVID-19 pandemic have made telepathology implementation pertinent to continue and improve pathology practice.8-10
Description and Definitions
The primary modes of telepathology (static image telepathology, robotic telepathology, video microscopy, WSI, and multimodality telepathology) have been defined by the American Telemedicine Association (ATA).2 WSI has been particularly suited for telepathology due to the ability to view digital slides in high resolution at various magnifications. These image files can also be viewed and shared with ease with other observers. Also, they take a shorter time to view compared with the use of a robotic microscope.3
Selection, Validation, and Implementation
WSI platforms vary in their characteristics and have several parameters, including but not limited to batch scanning vs continuous or random-access processing, throughput volume capacities, scan speed, cost, manual vs automatic loading of slides, image quality, slide capacity, flexibility for different slide sizes/features, telepathology capabilities once slide scanned, z-stacking, and regulatory approval status.8 Selection of the WSI device is dependent on need and cost considerations. For example, use for frozen section requires faster scanning speed and does not generally require a high throughput scanner.
Validation of telepathology by the testing site demonstrates that the new system performs as expected for its intended clinical use before being put into service and that the digital slides produced are acceptable for clinical diagnostic interpretation.11 The College of American Pathologists (CAP) established WSI validation guidelines are part of the published laboratory standard of care.11-13 An appropriate validation enables the benefits of telepathology while mitigating the risks.
There are 3 major CAP recommendations for validation. First, ≥ 60 cases should be included for each use case being validated with 20 additional cases for relevant ancillary applications not included in the 60 cases. Second, diagnostic concordance (ideally ≥ 95%) should be established between digital and glass slides for the same observer. Third, there should be a 2-week washout period between the viewing of digital and glass slides (Table 2).12,13
Guidelines from the ATA establish that telepathology systems should be validated for clinical use, including non-WSI platforms.2 Published validations of other non-WSI platforms (such as by robotic or multimodality telepathology) have followed the structure proposed in the guidelines by CAP for validating WSI.14,15
Ensuring that all relevant responsibilities (clinical, facility, technical, training, documentation/archiving, quality management, and operations related) for the use of telepathology are met is another aspect of validation and implementation.2 Clinical responsibilities include an agreement between the sending (referring) and receiving (consulting) parties on the information to accompany the digital material.2 From ATA clinical guidelines, this includes identification information, provision to the consulting pathologist of all relevant clinical data, provision to retrieve for access any needed and/or relevant diagnostic material, and responsibility by referrer that the correct image/metadata was sent.2 Involved parties should be trained to manage the materials being transmitted.2
Facility responsibilities include maintaining the standard of care defined by the facility and regulatory agencies.2 The maintenance of accreditation, adherence to licensure requirements, and proper management of privileges to practice telepathology are also important.2 Technical responsibilities include ensuring a proper validation that meets the standard of care and covers use cases.2,11-13
All processes, training, and competencies should be followed and documented per standard facility operating procedures.2 ATA recommends that telepathology should result in a formal report for diagnostic consultations, maintain logs of telepathology interactions or disclaimer statements, and have an appropriate retention policy.2 The CAP recommends digital images used for primary diagnosis should be kept for 10 years if the original glass slides are not available.16 Once implemented, telepathology reports must be incorporated into the pathology and laboratory medicine department’s quality management plan for both the technical performance of the telepathology system and diagnostic performance of the pathologists using the system.2 Operations responsibilities include ensuring that the telepathology system is maintained according to vendor recommendations and regulatory standards. Appropriate provisions for space and associated needs should be developed in conjunction with the information technology team of the facility to ensure appropriate security, privacy, and regulatory compliance.2
Applications and Uses
Telecytology. Rapid real-time telecytology has been documented to be useful in rapid on-site evaluations (ROSE) of the adequacy of fine needle aspirations (FNA).17-21 Nevertheless, current Medicare reimbursement is limited given that ROSE is cost prohibitive, time consuming, and affects productivity in cytology laboratories.17,22,23 Estimates of the time to provide ROSE for 1 procedure without telecytology range from 48.7 to 56.2 minutes.17,23 The use of telecytology significantly reduces pathologist ROSE time without losing quality to about 12 minutes, of which only an average of 7.5 minutes was spent by the cytopathologist for the ROSE diagnosis.17-21 ROSE also can be used for distant and remote locations to improve patient care.17-21 Multiple vendors provide real-time telecytology service. Innovations using smartphone adapters, digital cameras that could work as their own IP addresses, and connection with high-speed dedicated connections with viewing platforms on high-sensitivity monitors can facilitate ROSE to improve patient management.24,25 The successful accurate use of ROSE has been described; however, there are currently no FDA-approved telepathology ROSE platforms.17-19,21-25
To date, the FDA has not approved any telecytology whole slide scanner due to a lack of z-stacking capability in submitted scanners.7,21 Not all whole slide scanners offer z-stacking, though even in those that do offer it, the time necessary to scan the entire slide with adequate z-stacking takes too long to be clinically acceptable for many situations involving ROSE.21 WSI has also been used to develop international consensus for cytologic samples.26 Published recommendations for the validation of these other modalities before usage follow the spirit of the CAP guidelines (as far as multiple cases with high concordance rates) for validation of WSI for diagnostic purposes but vary on the exact number of slides and acceptable concordance rate.21,27 For ROSE with a robotic microscope without any on-site cytology personnel, documented standardized training of nonpathology staff members, such as the radiologist or other physician performing the FNA procedure, may be needed to enable the performance of ROSE telecytology and ensure compliance with regulations.2,21 Besides ROSE, there are published validations for telecytology in primary diagnosis and QA, indicating a role for telecytology for diagnosis for laboratories that have properly validated and implemented the laboratory-developed test.28-30
Frozen section. Telepathology has significant potential to improve access to frozen section consultation.5,31-33 Benefits to improving access to frozen section include providing frozen section consultation at remote or off-site locations, increasing access to subspecialty consultation, improving workflow by eliminating the need to travel off-site to the frozen section case, cost savings in staff work time, and providing educational opportunities for pathology trainees.5,31-33 In our experience, WSI with real-time viewing of frozen section allows for the assessment of transplant tissues, which is an evaluation that generally occurs at night. Discrepancies from frozen section telepathology using WSI to the final diagnosis may occur and those specific to WSI could result from slide or image quality, internet connectivity, and lack of training in using the telepathology system.32 Other issues that may lead to discrepancies between the frozen section diagnosis and the final diagnosis may occur with the review of glass slides by light microscopy.34 Appropriate performance of validation, training, implementation, and quality control for telepathology can help in reaping the benefits while mitigating the risks.2 In a large study comparing frozen section evaluation by telepathology with light microscopy, the sensitivity and specificity of frozen section were comparable between telepathology and light microscopy with a trend toward greater sensitivity by telepathology (0.92 and 0.99 for telepathology vs 0.90 and 0.99 by light microscopy alone, sensitivity and specificity, respectively).33
Other applications. Evidence for efficacy in surgical pathology diagnosis led to FDA approval of the Philips IntelliSite Digital Pathology in 2017 and the Leica Aperio AT2 DX in 2020 WSI platforms.6-8 The use of WSI in surgical pathology has been successfully validated or used in clinical practice at several pathology laboratory settings with documented benefits in the literature for primary and secondary diagnoses, QA, research, and education.6-8,35-45 Benefits of telepathology include improved ergonomics and access to real-time pathologic services in remote areas or during on-site pathologist absence and expert second opinions. Telepathology also may reduce risk of slide loss during transport, shortened turnaround time, reduced costs of operation through workflow efficiencies, better load balancing, improve virtual collaboration, and digital storage of slides that may be irreplaceable.3-8,35-45 Telepathology also has been shown to be useful for education, improving access to learning materials and increasing quality instructional materials at a lower cost.45 The increased ease of collaboration with remote experts and access to slide material for other pathologists improves QA capabilities.3-8,35-45 The availability of virtual slides is expected to promote further research in telepathology and pathology due to the increased availability of virtual material to researchers.1,5,46
Telehematology. Published validations have shown effectiveness for hematopathology specimens, such as the peripheral smear. Telehematology also has demonstrated potential in a laboratory after proper validation and implementation as a laboratory-developed test.37,47-49
Telemicrobiology and Computer-Assisted Pathologic Diagnosis. Telemicrobiology also has been successfully used for clinical, educational, and QA purposes.50 The digitalization of slides involved with telepathology enables further innovation in machine learning for computer-assisted pathologic diagnosis (CAPD), which is already being used clinically for cervical Pap smears.20 An artificial intelligence (AI)–based algorithm analyzes the slides to identify cells of interest, which are presented to the cytopathologist for confirmation.20 However, the expansion of CAPD to include a variety of specimen types or diagnostic situations as well as safely and effectively take initiative in completing an accurate automated diagnosis requires additional development.20,51,52 One of the key factors for machine learning to develop AI is the provision of a corpus of data.51,52 Public, open-source data sources have been limited in size while private proprietary sources have highly restricted and expensive access; to address this, there is a current effort to build the world’s largest public open-source digital pathology corpus at Temple University Hospital, which may help enable innovations in the future.52
Long-Term Trends/Applications
The COVID-19 pandemic has been unprecedented not only in its widespread morbidity and mortality, but also for the significant socioeconomic, health, lifestyle, societal, and workspace changes.53-57 Specifically, the pandemic has introduced not only a need for social distancing and staff quarantines to prevent the spread of infection, but also a reduction in the workforce due to the stresses of COVID-19 (also known as the Great Resignation).55 Before the pandemic, there was an existing downtrend in the number of pathologists in the US workforce.9-10,58,59 From 2007 to 2017, the number of active pathologists in the US declined by 17.5% despite the increasing national population, resulting in not only an absolute decrease in the number of pathologists, but also an increasing population served per pathologist ratio.59 Since 2017, this downtrend has continued; given the increasing loss of active pathologists from the workforce and the decreasing training of new pathologists, this decrease shows no signs of reversing even as the impact of the COVID-19 pandemic has begun to wane.9,10,58-60
The advantages of telepathology in enabling social distancing and reducing travel to remote sites are known.3-7,17 Given these advantages, some medical centers in the US have previously successfully validated and implemented telepathology operations earlier during the COVID-19 pandemic to ease workflow and ensure continued operations.56,57 The use of telepathology also helps in balancing workload and continuing pathology operations even in light of the workforce reduction as cases no longer need to be signed out on site with glass slides but instead can be signed out at a remote laboratory. Although the impact of the COVID-19 pandemic on operations is decreasing, the capabilities for social distancing and reducing travel remain important to both improve operations and ensure resiliency in response to similar potential events.3-7,17,60
Considering the long-term trends, the lessons of the COVID-19 pandemic, and the potential for future pandemics or other disasters, telepathology’s validation and implementation remains a reasonable choice for pathology practices looking to improve. A variety of practices not just in the general population, but also among US Department of Veterans Affairs medical centers (VAMCs) and the US Department of Defense Military Health System treating a veteran population can benefit from telepathology where it has previously been reported to have been reliable or successfully implemented.61-63 Although the veteran population differs from the general population in several characteristics, such as the severity of disease, coexisting morbidities, and other history, given proper validation and implementation, telepathology’s usefulness extends across different pathology practice settings.35-43,61-66
Limitations of Telepathology
In telepathology’s current state, there are limitations despite its immense promise.6,35 These include initial capital costs, the additional training requirement, the additional time necessary to scan slides, technical challenges (ie, laboratory information system integration, color calibration, display artifacts, potential for small particle scanner omissions, and information technology dependence), the potential for slower evaluation per slide compared with optical microscopes, limitations of slide imaging (ie, z-stacking or lack of polarization on digital pathology), and occupational concerns regarding eye strain with increased computer monitor usage (ie, computer vision syndrome).6,35 In addition, there are few telepathology scanners with FDA approval for WSI.6-8
The improving technology of telepathology has made these limitations surmountable, including faster slide scanning and increasing digital storage capacity for large WSI files. Due to this improvement in technology, an increasing number of laboratory settings, have adopted telepathology as its advantages have begun to outweigh the limitations.2-5 Additionally, the proper validation performed before implementing telepathology can help laboratories identify their unique challenges, troubleshoot, and resolve the limitations before use in clinical care.11-13 Continuing QA during its use and implementation is important to ensure that telepathology performs as expected for clinical purposes despite its limitations.2
Conclusions
Telepathology is a promising technology that may improve pathology practice once properly validated and implemented.1-8 Though there are barriers to this validation and implementation, particularly the capital costs and training, there are several potential benefits, including increased productivity, cost savings, improvement in the workflow, enhanced access to pathologic consultation, and adaptability of the pathology laboratory in an era of a decreased workforce and social distancing due to the COVID-19 pandemic.1-8,55-56 This potential applies across the wide spectrum of potential telepathology uses from frozen section, telecytology (including ROSE) to primary and second opinion diagnoses.1-8,17-33 The benefits also extends to QA, education, and research, as diagnoses can not only be rereviewed by specialty or second opinion consultation with ease, but also digital slides can be produced for educational and research purposes.3-8,35-45 Settings that treat the general population and those focused on the care of veterans or members of the armed forces have reported similar reliability or successful implementation.35-44,61-63 All in all, the use of telepathology represents an innovation that may transform the practice of pathology tomorrow.
1. Weinstein RS. Prospects for telepathology. Hum Pathol. 1986;17(5):433-434. doi:10.1016/s0046-8177(86)80028-4
2. Pantanowitz L, Dickinson K, Evans AJ, et al. American Telemedicine Association clinical guidelines for telepathology. J Pathol Inform. 2014;5(1):39. Published 2014 Oct 21. doi:10.4103/2153-3539.143329
3. Farahani N, Pantanowitz L. Overview of telepathology. Surg Pathol Clin. 2015;8(2):223-231. doi:10.1016/j.path. 2015.02.018 4. Petersen JM, Jhala D. Telepathology: a transforming practice for the efficient, safe, and best patient care at the regional Veteran Affairs medical center. Am J Clin Pathol. 2022;158(suppl 1):S97-S98. doi:10.1093/ajcp/aqac126.205
5. Bashshur RL, Krupinski EA, Weinstein RS, Dunn MR, Bashshur N. The empirical foundations of telepathology: evidence of feasibility and intermediate effects. Telemed J E Health. 2017;23(3):155-191. doi:10.1089/tmj.2016.0278
6. Jahn SW, Plass M, Moinfar F. Digital pathology: advantages, limitations and emerging perspectives. J Clin Med. 2020;9(11):3697. Published 2020 Nov 18. doi:10.3390/jcm9113697
7. Evans AJ, Bauer TW, Bui MM, et al. US Food and Drug Administration approval of whole slide imaging for primary diagnosis: a key milestone is reached and new questions are raised. Arch Pathol Lab Med. 2018;142(11):1383-1387. doi:10.5858/arpa.2017-0496-CP.
8. Patel A, Balis UGJ, Cheng J, et al. Contemporary whole slide imaging devices and their applications within the modern pathology department: a selected hardware review. J Pathol Inform. 2021;12:50. Published 2021 Dec 9. doi:10.4103/jpi.jpi_66_21
9. Association of American Medical Colleges. 2017 State Physician Workforce Data Book. November 2017. Accessed April 14, 2023. https://store.aamc.org/downloadable/download/sample/sample_id/30
10. Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7):e2010648. Published 2020 Jul 1. doi:10.1001/jamanetworkopen.2020.10648
11. Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710-1722. doi:10.5858/arpa.2013-0093-CP
12. Evans AJ, Brown RW, Bui MM, et al. Validating whole slide imaging systems for diagnostic purposes in pathology. Arch Pathol Lab Med. 2021;146(4):440-450. doi:10.5858/arpa.2020-0723-CP
13. Evans AJ, Lacchetti C, Reid K, Thomas NE. Validating whole slide imaging for diagnostic purposes in pathology: guideline update. College of American Pathologists. May 2021. Accessed April 13, 2023. https://documents.cap.org/documents/wsi-methodology.pdf
14. Chandraratnam E, Santos LD, Chou S, et al. Parathyroid frozen section interpretation via desktop telepathology systems: a validation study. J Pathol Inform. 2018;9:41. Published 2018 Dec 3. doi:10.4103/jpi.jpi_57_18
15. Thrall MJ, Rivera AL, Takei H, Powell SZ. Validation of a novel robotic telepathology platform for neuropathology intraoperative touch preparations. J Pathol Inform. 2014;5(1):21. Published 2014 Jul 28. doi:10.4103/2153-3539.137642
16. Balis UGJ, Williams CL, Cheng J, et al. Whole-Slide Imaging: Thinking Twice Before Hitting the Delete Key. AJSP: Reviews & Reports. 2018;23(6):p 249-250. doi:10.1097/PCR.0000000000000283
17. Kim B, Chhieng DC, Crowe DR, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006;3:27. Published 2006 Dec 11. doi:10.1186/1742-6413-3-27
18. Perez D, Stemmer MN, Khurana KK. Utilization of dynamic telecytopathology for rapid onsite evaluation of touch imprint cytology of needle core biopsy: diagnostic accuracy and pitfalls. Telemed J E Health. 2021;27(5):525-531. doi:10.1089/tmj.2020.0117
19. McCarthy EE, McMahon RQ, Das K, Stewart J 3rd. Internal validation testing for new technologies: bringing telecytopathology into the mainstream. Diagn Cytopathol. 2015;43(1):3-7. doi:10.1002/dc.23167
20. Marletta S, Treanor D, Eccher A, Pantanowitz L. Whole-slide imaging in cytopathology: state of the art and future directions. Diagn Histopathol (Oxf). 2021;27(11):425-430. doi:10.1016/j.mpdhp.2021.08.001
21. Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7(1):1-6. doi:10.1016/j.jasc.2017.10.002
22. Eloubeidi MA, Tamhane A, Jhala N, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101(12):2841-2847. doi:10.1111/j.1572-0241.2006.00852.x
23. Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer. 2001;93(5):319-322. doi:10.1002/cncr.9046
24. Fontelo P, Liu F, Yagi Y. Evaluation of a smartphone for telepathology: lessons learned. J Pathol Inform. 2015;6:35. Published 2015 Jun 23. doi:10.4103/2153-3539.158912
25. Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7(1):1-6. doi:10.1016/j.jasc.2017.10.002
26. Johnson DN, Onenerk M, Krane JF, et al. Cytologic grading of primary malignant salivary gland tumors: A blinded review by an international panel. Cancer Cytopathol. 2020;128(6):392-402. doi:10.1002/cncy.22271
27. Trabzonlu L, Chatt G, McIntire PJ, et al. Telecytology validation: is there a recipe for everybody? J Am Soc Cytopathol. 2022;11(4):218-225. doi:10.1016/j.jasc.2022.03.001
28. Canberk S, Behzatoglu K, Caliskan CK, et al. The role of telecytology in the primary diagnosis of thyroid fine-needle aspiration specimens. Acta Cytol. 2020;64(4):323-331. doi:10.1159/000503914.
29. Archondakis S, Roma M, Kaladelfou E. Implementation of pre-captured videos for remote diagnosis of cervical cytology specimens. Cytopathology. 2021;32(3):338-343. doi:10.1111/cyt.12948
30. Lee ES, Kim IS, Choi JS, et al. Accuracy and reproducibility of telecytology diagnosis of cervical smears. A tool for quality assurance programs. Am J Clin Pathol. 2003;119(3):356-360. doi:10.1309/7ytvag4xnr48t75h
31. Dietz RL, Hartman DJ, Pantanowitz L. Systematic review of the use of telepathology during intraoperative consultation. Am J Clin Pathol. 2020;153(2):198-209. doi:10.1093/ajcp/aqz155
32. Bauer TW, Slaw RJ, McKenney JK, Patil DT. Validation of whole slide imaging for frozen section diagnosis in surgical pathology. J Pathol Inform. 2015;6:49. Published 2015 Aug 31. doi:10.4103/2153-3539.163988

33. Vosoughi A, Smith PT, Zeitouni JA, et al. Frozen section evaluation via dynamic real-time nonrobotic telepathology system in a university cancer center by resident/faculty cooperation team. Hum Pathol. 2018;78:144-150. doi:10.1016/j.humpath.2018.04.012
34. Mahe E, Ara S, Bishara M, et al. Intraoperative pathology consultation: error, cause and impact. Can J Surg. 2013;56(3):E13-E18. doi:10.1503/cjs.011112.
35. Farahani N, Parwani AV, Pantanowitz L. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives. Pathol Lab Med Int. 2015;7:23-33. doi:10.2147/PLMI.S59826
36. Thorstenson S, Molin J, Lundström C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: digital pathology experiences 2006-2013. J Pathol Inform. 2014;5(1):14. Published 2014 Mar 28. doi:10.4103/2153-3539.129452
37. Pantanowitz L, Wiley CA, Demetris A, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi:10.4103/2153-3539.104907
38. Al Habeeb A, Evans A, Ghazarian D. Virtual microscopy using whole-slide imaging as an enabler for teledermatopathology: a paired consultant validation study. J Pathol Inform. 2012;3:2. doi:10.4103/2153-3539.93399
39. Al-Janabi S, Huisman A, Vink A, et al. Whole slide images for primary diagnostics in dermatopathology: a feasibility study. J Clin Pathol. 2012;65(2):152-158. doi:10.1136/jclinpath-2011-200277
40. Nielsen PS, Lindebjerg J, Rasmussen J, Starklint H, Waldstrøm M, Nielsen B. Virtual microscopy: an evaluation of its validity and diagnostic performance in routine histologic diagnosis of skin tumors. Hum Pathol. 2010;41(12):1770-1776. doi:10.1016/j.humpath.2010.05.015
41. Leinweber B, Massone C, Kodama K, et al. Telederma-topathology: a controlled study about diagnostic validity and technical requirements for digital transmission. Am J Dermatopathol. 2006;28(5):413-416. doi:10.1097/01.dad.0000211523.95552.86
42. Koch LH, Lampros JN, Delong LK, Chen SC, Woosley JT, Hood AF. Randomized comparison of virtual microscopy and traditional glass microscopy in diagnostic accuracy among dermatology and pathology residents. Hum Pathol. 2009;40(5):662-667. doi:10.1016/j.humpath.2008.10.009
43. Farris AB, Cohen C, Rogers TE, Smith GH. Whole slide imaging for analytical anatomic pathology and telepathology: practical applications today, promises, and perils. Arch Pathol Lab Med. 2017;141(4):542-550. doi:10.5858/arpa.2016-0265-SA
44. Chong T, Palma-Diaz MF, Fisher C, et al. The California Telepathology Service: UCLA’s experience in deploying a regional digital pathology subspecialty consultation network. J Pathol Inform. 2019;10:31. Published 2019 Sep 27. doi:10.4103/jpi.jpi_22_19
45. Meyer J, Paré G. Telepathology impacts and implementation challenges: a scoping review. Arch Pathol Lab Med. 2015;139(12):1550-1557. doi:10.5858/arpa.2014-0606-RA
46. Weinstein RS, Descour MR, Liang C, et al. Telepathology overview: from concept to implementation. Hum Pathol. 2001;32(12):1283-1299. doi:10.1053/hupa.2001.29643
47. Riley RS, Ben-Ezra JM, Massey D, Cousar J. The virtual blood film. Clin Lab Med. 2002;22(1):317-345. doi:10.1016/s0272-2712(03)00077-5
48. Garcia CA, Hanna M, Contis LC, Pantanowitz L, Hyman R. Sharing Cellavision blood smear images with clinicians via the electronic medical record. Blood. 2017;130(suppl 1):5586. doi:10.1182/blood.V130.Suppl_1.5586.5586
49. Goswami R, Pi D, Pal J, Cheng K, Hudoba De Badyn M. Performance evaluation of a dynamic telepathology system (Panoptiq) in the morphologic assessment of peripheral blood film abnormalities. Int J Lab Hematol. 2015;37(3):365-371. doi:10.1111/ijlh.12294
50. Rhoads DD, Mathison BA, Bishop HS, da Silva AJ, Pantanowitz L. Review of telemicrobiology. Arch Pathol Lab Med. 2016;140(4):362-370. doi:10.5858/arpa.2015-0116-RA51. Nam S, Chong Y, Jung CK, et al. Introduction to digital pathology and computer-aided pathology. J Pathol Transl Med. 2020;54(2):125-134. doi:10.4132/jptm.2019.12.31
52. Houser D, Shadhin G, Anstotz R, et al. The Temple University Hospital Digital Pathology Corpus. IEEE Signal Process Med Biol Symp. 2018:1-7. doi:10.1109/SPMB.2018.8615619
53. Petersen J, Dalal S, Jhala D. Criticality of in-house preparation of viral transport medium in times of shortage during COVID-19 pandemic. Lab Med. 2021;52(2):e39-e45. doi:10.1093/labmed/lmaa099
54. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382(18):e41. doi:10.1056/NEJMp2006141
55. Ksinan Jiskrova G. Impact of COVID-19 pandemic on the workforce: from psychological distress to the Great Resignation. J Epidemiol Community Health. 2022;76(6):525-526. doi:10.1136/jech-2022-218826
56. Henriksen J, Kolognizak T, Houghton T, et al. Rapid validation of telepathology by an academic neuropathology practice during the COVID-19 pandemic. Arch Pathol Lab Med. 2020;144(11):1311-1320. doi:10.5858/arpa.2020-0372-SA
57. Ardon O, Reuter VE, Hameed M, et al. Digital pathology operations at an NYC tertiary cancer center during the first 4 months of COVID-19 pandemic response. Acad Pathol. 2021;8:23742895211010276. Published 2021 Apr 28. doi:10.1177/23742895211010276
58. Jajosky RP, Jajosky AN, Kleven DT, Singh G. Fewer seniors from United States allopathic medical schools are filling pathology residency positions in the Main Residency Match, 2008-2017. Hum Pathol. 2018;73:26-32. doi:10.1016/j.humpath.2017.11.014
59. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337. Published 2019 May 3. doi:10.1001/jamanetworkopen.2019.4337
60. Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet. 2022;399(10323):417-419. doi:10.1016/S0140-6736(22)00100-3
61. Ghosh A, Brown GT, Fontelo P. Telepathology at the Armed Forces Institute of Pathology: a retrospective review of consultations from 1996 to 1997. Arch Pathol Lab Med. 2018;142(2):248-252. doi:10.5858/arpa.2017-0055-OA
62. Dunn BE, Choi H, Almagro UA, Recla DL, Davis CW. Telepathology networking in VISN-12 of the Veterans Health Administration. Telemed J E Health. 2000;6(3):349-354. doi:10.1089/153056200750040200
63. Dunn BE, Almagro UA, Choi H, et al. Dynamic-robotic telepathology: Department of Veterans Affairs feasibility study. Hum Pathol. 1997;28(1):8-12. doi:10.1016/s0046-8177(97)90271-9
64. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252

65. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
66. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448
1. Weinstein RS. Prospects for telepathology. Hum Pathol. 1986;17(5):433-434. doi:10.1016/s0046-8177(86)80028-4
2. Pantanowitz L, Dickinson K, Evans AJ, et al. American Telemedicine Association clinical guidelines for telepathology. J Pathol Inform. 2014;5(1):39. Published 2014 Oct 21. doi:10.4103/2153-3539.143329
3. Farahani N, Pantanowitz L. Overview of telepathology. Surg Pathol Clin. 2015;8(2):223-231. doi:10.1016/j.path. 2015.02.018 4. Petersen JM, Jhala D. Telepathology: a transforming practice for the efficient, safe, and best patient care at the regional Veteran Affairs medical center. Am J Clin Pathol. 2022;158(suppl 1):S97-S98. doi:10.1093/ajcp/aqac126.205
5. Bashshur RL, Krupinski EA, Weinstein RS, Dunn MR, Bashshur N. The empirical foundations of telepathology: evidence of feasibility and intermediate effects. Telemed J E Health. 2017;23(3):155-191. doi:10.1089/tmj.2016.0278
6. Jahn SW, Plass M, Moinfar F. Digital pathology: advantages, limitations and emerging perspectives. J Clin Med. 2020;9(11):3697. Published 2020 Nov 18. doi:10.3390/jcm9113697
7. Evans AJ, Bauer TW, Bui MM, et al. US Food and Drug Administration approval of whole slide imaging for primary diagnosis: a key milestone is reached and new questions are raised. Arch Pathol Lab Med. 2018;142(11):1383-1387. doi:10.5858/arpa.2017-0496-CP.
8. Patel A, Balis UGJ, Cheng J, et al. Contemporary whole slide imaging devices and their applications within the modern pathology department: a selected hardware review. J Pathol Inform. 2021;12:50. Published 2021 Dec 9. doi:10.4103/jpi.jpi_66_21
9. Association of American Medical Colleges. 2017 State Physician Workforce Data Book. November 2017. Accessed April 14, 2023. https://store.aamc.org/downloadable/download/sample/sample_id/30
10. Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7):e2010648. Published 2020 Jul 1. doi:10.1001/jamanetworkopen.2020.10648
11. Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710-1722. doi:10.5858/arpa.2013-0093-CP
12. Evans AJ, Brown RW, Bui MM, et al. Validating whole slide imaging systems for diagnostic purposes in pathology. Arch Pathol Lab Med. 2021;146(4):440-450. doi:10.5858/arpa.2020-0723-CP
13. Evans AJ, Lacchetti C, Reid K, Thomas NE. Validating whole slide imaging for diagnostic purposes in pathology: guideline update. College of American Pathologists. May 2021. Accessed April 13, 2023. https://documents.cap.org/documents/wsi-methodology.pdf
14. Chandraratnam E, Santos LD, Chou S, et al. Parathyroid frozen section interpretation via desktop telepathology systems: a validation study. J Pathol Inform. 2018;9:41. Published 2018 Dec 3. doi:10.4103/jpi.jpi_57_18
15. Thrall MJ, Rivera AL, Takei H, Powell SZ. Validation of a novel robotic telepathology platform for neuropathology intraoperative touch preparations. J Pathol Inform. 2014;5(1):21. Published 2014 Jul 28. doi:10.4103/2153-3539.137642
16. Balis UGJ, Williams CL, Cheng J, et al. Whole-Slide Imaging: Thinking Twice Before Hitting the Delete Key. AJSP: Reviews & Reports. 2018;23(6):p 249-250. doi:10.1097/PCR.0000000000000283
17. Kim B, Chhieng DC, Crowe DR, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006;3:27. Published 2006 Dec 11. doi:10.1186/1742-6413-3-27
18. Perez D, Stemmer MN, Khurana KK. Utilization of dynamic telecytopathology for rapid onsite evaluation of touch imprint cytology of needle core biopsy: diagnostic accuracy and pitfalls. Telemed J E Health. 2021;27(5):525-531. doi:10.1089/tmj.2020.0117
19. McCarthy EE, McMahon RQ, Das K, Stewart J 3rd. Internal validation testing for new technologies: bringing telecytopathology into the mainstream. Diagn Cytopathol. 2015;43(1):3-7. doi:10.1002/dc.23167
20. Marletta S, Treanor D, Eccher A, Pantanowitz L. Whole-slide imaging in cytopathology: state of the art and future directions. Diagn Histopathol (Oxf). 2021;27(11):425-430. doi:10.1016/j.mpdhp.2021.08.001
21. Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7(1):1-6. doi:10.1016/j.jasc.2017.10.002
22. Eloubeidi MA, Tamhane A, Jhala N, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101(12):2841-2847. doi:10.1111/j.1572-0241.2006.00852.x
23. Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer. 2001;93(5):319-322. doi:10.1002/cncr.9046
24. Fontelo P, Liu F, Yagi Y. Evaluation of a smartphone for telepathology: lessons learned. J Pathol Inform. 2015;6:35. Published 2015 Jun 23. doi:10.4103/2153-3539.158912
25. Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7(1):1-6. doi:10.1016/j.jasc.2017.10.002
26. Johnson DN, Onenerk M, Krane JF, et al. Cytologic grading of primary malignant salivary gland tumors: A blinded review by an international panel. Cancer Cytopathol. 2020;128(6):392-402. doi:10.1002/cncy.22271
27. Trabzonlu L, Chatt G, McIntire PJ, et al. Telecytology validation: is there a recipe for everybody? J Am Soc Cytopathol. 2022;11(4):218-225. doi:10.1016/j.jasc.2022.03.001
28. Canberk S, Behzatoglu K, Caliskan CK, et al. The role of telecytology in the primary diagnosis of thyroid fine-needle aspiration specimens. Acta Cytol. 2020;64(4):323-331. doi:10.1159/000503914.
29. Archondakis S, Roma M, Kaladelfou E. Implementation of pre-captured videos for remote diagnosis of cervical cytology specimens. Cytopathology. 2021;32(3):338-343. doi:10.1111/cyt.12948
30. Lee ES, Kim IS, Choi JS, et al. Accuracy and reproducibility of telecytology diagnosis of cervical smears. A tool for quality assurance programs. Am J Clin Pathol. 2003;119(3):356-360. doi:10.1309/7ytvag4xnr48t75h
31. Dietz RL, Hartman DJ, Pantanowitz L. Systematic review of the use of telepathology during intraoperative consultation. Am J Clin Pathol. 2020;153(2):198-209. doi:10.1093/ajcp/aqz155
32. Bauer TW, Slaw RJ, McKenney JK, Patil DT. Validation of whole slide imaging for frozen section diagnosis in surgical pathology. J Pathol Inform. 2015;6:49. Published 2015 Aug 31. doi:10.4103/2153-3539.163988

33. Vosoughi A, Smith PT, Zeitouni JA, et al. Frozen section evaluation via dynamic real-time nonrobotic telepathology system in a university cancer center by resident/faculty cooperation team. Hum Pathol. 2018;78:144-150. doi:10.1016/j.humpath.2018.04.012
34. Mahe E, Ara S, Bishara M, et al. Intraoperative pathology consultation: error, cause and impact. Can J Surg. 2013;56(3):E13-E18. doi:10.1503/cjs.011112.
35. Farahani N, Parwani AV, Pantanowitz L. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives. Pathol Lab Med Int. 2015;7:23-33. doi:10.2147/PLMI.S59826
36. Thorstenson S, Molin J, Lundström C. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: digital pathology experiences 2006-2013. J Pathol Inform. 2014;5(1):14. Published 2014 Mar 28. doi:10.4103/2153-3539.129452
37. Pantanowitz L, Wiley CA, Demetris A, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi:10.4103/2153-3539.104907
38. Al Habeeb A, Evans A, Ghazarian D. Virtual microscopy using whole-slide imaging as an enabler for teledermatopathology: a paired consultant validation study. J Pathol Inform. 2012;3:2. doi:10.4103/2153-3539.93399
39. Al-Janabi S, Huisman A, Vink A, et al. Whole slide images for primary diagnostics in dermatopathology: a feasibility study. J Clin Pathol. 2012;65(2):152-158. doi:10.1136/jclinpath-2011-200277
40. Nielsen PS, Lindebjerg J, Rasmussen J, Starklint H, Waldstrøm M, Nielsen B. Virtual microscopy: an evaluation of its validity and diagnostic performance in routine histologic diagnosis of skin tumors. Hum Pathol. 2010;41(12):1770-1776. doi:10.1016/j.humpath.2010.05.015
41. Leinweber B, Massone C, Kodama K, et al. Telederma-topathology: a controlled study about diagnostic validity and technical requirements for digital transmission. Am J Dermatopathol. 2006;28(5):413-416. doi:10.1097/01.dad.0000211523.95552.86
42. Koch LH, Lampros JN, Delong LK, Chen SC, Woosley JT, Hood AF. Randomized comparison of virtual microscopy and traditional glass microscopy in diagnostic accuracy among dermatology and pathology residents. Hum Pathol. 2009;40(5):662-667. doi:10.1016/j.humpath.2008.10.009
43. Farris AB, Cohen C, Rogers TE, Smith GH. Whole slide imaging for analytical anatomic pathology and telepathology: practical applications today, promises, and perils. Arch Pathol Lab Med. 2017;141(4):542-550. doi:10.5858/arpa.2016-0265-SA
44. Chong T, Palma-Diaz MF, Fisher C, et al. The California Telepathology Service: UCLA’s experience in deploying a regional digital pathology subspecialty consultation network. J Pathol Inform. 2019;10:31. Published 2019 Sep 27. doi:10.4103/jpi.jpi_22_19
45. Meyer J, Paré G. Telepathology impacts and implementation challenges: a scoping review. Arch Pathol Lab Med. 2015;139(12):1550-1557. doi:10.5858/arpa.2014-0606-RA
46. Weinstein RS, Descour MR, Liang C, et al. Telepathology overview: from concept to implementation. Hum Pathol. 2001;32(12):1283-1299. doi:10.1053/hupa.2001.29643
47. Riley RS, Ben-Ezra JM, Massey D, Cousar J. The virtual blood film. Clin Lab Med. 2002;22(1):317-345. doi:10.1016/s0272-2712(03)00077-5
48. Garcia CA, Hanna M, Contis LC, Pantanowitz L, Hyman R. Sharing Cellavision blood smear images with clinicians via the electronic medical record. Blood. 2017;130(suppl 1):5586. doi:10.1182/blood.V130.Suppl_1.5586.5586
49. Goswami R, Pi D, Pal J, Cheng K, Hudoba De Badyn M. Performance evaluation of a dynamic telepathology system (Panoptiq) in the morphologic assessment of peripheral blood film abnormalities. Int J Lab Hematol. 2015;37(3):365-371. doi:10.1111/ijlh.12294
50. Rhoads DD, Mathison BA, Bishop HS, da Silva AJ, Pantanowitz L. Review of telemicrobiology. Arch Pathol Lab Med. 2016;140(4):362-370. doi:10.5858/arpa.2015-0116-RA51. Nam S, Chong Y, Jung CK, et al. Introduction to digital pathology and computer-aided pathology. J Pathol Transl Med. 2020;54(2):125-134. doi:10.4132/jptm.2019.12.31
52. Houser D, Shadhin G, Anstotz R, et al. The Temple University Hospital Digital Pathology Corpus. IEEE Signal Process Med Biol Symp. 2018:1-7. doi:10.1109/SPMB.2018.8615619
53. Petersen J, Dalal S, Jhala D. Criticality of in-house preparation of viral transport medium in times of shortage during COVID-19 pandemic. Lab Med. 2021;52(2):e39-e45. doi:10.1093/labmed/lmaa099
54. Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382(18):e41. doi:10.1056/NEJMp2006141
55. Ksinan Jiskrova G. Impact of COVID-19 pandemic on the workforce: from psychological distress to the Great Resignation. J Epidemiol Community Health. 2022;76(6):525-526. doi:10.1136/jech-2022-218826
56. Henriksen J, Kolognizak T, Houghton T, et al. Rapid validation of telepathology by an academic neuropathology practice during the COVID-19 pandemic. Arch Pathol Lab Med. 2020;144(11):1311-1320. doi:10.5858/arpa.2020-0372-SA
57. Ardon O, Reuter VE, Hameed M, et al. Digital pathology operations at an NYC tertiary cancer center during the first 4 months of COVID-19 pandemic response. Acad Pathol. 2021;8:23742895211010276. Published 2021 Apr 28. doi:10.1177/23742895211010276
58. Jajosky RP, Jajosky AN, Kleven DT, Singh G. Fewer seniors from United States allopathic medical schools are filling pathology residency positions in the Main Residency Match, 2008-2017. Hum Pathol. 2018;73:26-32. doi:10.1016/j.humpath.2017.11.014
59. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337. Published 2019 May 3. doi:10.1001/jamanetworkopen.2019.4337
60. Murray CJL. COVID-19 will continue but the end of the pandemic is near. Lancet. 2022;399(10323):417-419. doi:10.1016/S0140-6736(22)00100-3
61. Ghosh A, Brown GT, Fontelo P. Telepathology at the Armed Forces Institute of Pathology: a retrospective review of consultations from 1996 to 1997. Arch Pathol Lab Med. 2018;142(2):248-252. doi:10.5858/arpa.2017-0055-OA
62. Dunn BE, Choi H, Almagro UA, Recla DL, Davis CW. Telepathology networking in VISN-12 of the Veterans Health Administration. Telemed J E Health. 2000;6(3):349-354. doi:10.1089/153056200750040200
63. Dunn BE, Almagro UA, Choi H, et al. Dynamic-robotic telepathology: Department of Veterans Affairs feasibility study. Hum Pathol. 1997;28(1):8-12. doi:10.1016/s0046-8177(97)90271-9
64. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252

65. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13. Published 2016 May 9.
66. Morgan RO, Teal CR, Reddy SG, Ford ME, Ashton CM. Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health Serv Res. 2005;40(5, pt 2):1573-1583. doi:10.1111/j.1475-6773.2005.00448
The diagnostic and therapeutic challenges of syringoma
Pain and pruritus are the most common complaints in patients who present to vulvar clinics.1 These symptoms can be related to a variety of conditions, including vulvar lesions. There are both common and uncommon vulvar lesions. Vulvar lesions can be skin colored, yellow, and red. Certain lesions can be diagnosed with history and physical examination alone. Some more common lesions include acrochordons (skin tags), benign growths that are common in patients with diabetes, obesity, and pregnancy.2,3 Other common vulvar lesions are papillomatosis, lichen simplex chronicus, and epidermoid cysts. Other lesions include low- and high-grade squamous intraepithelial lesions (HSIL).4 These lesions require biopsy for diagnosis as high-grade lesions require treatment. HSIL of the vulva is considered a premalignancy that necessitates treatment.5 Other lesions that can present with vulvar complaints are molluscum contagiosum, Bartholin gland duct cyst, intradermal melanocytic nevus, and squamous cell carcinoma.
Rarely, other less common conditions can present as vulvar lesions. Syringomas are benign eccrine sweat gland neoplasms. They are more commonly found on the face, neck, or chest.6 On the vulva they are generally small subcutaneous skin-colored papules.7 They may be asymptomatic and noted only on routine examination.
Vulvar syringomas also may present with symptoms. On the vulva, syringomas often present as pruritic papules that can be isolated or multifocal. Often on the labia majora they range in size from 2 to 20 mm.8
They can coalesce to form a larger lesion. They also may be described as painful. When syringomas are pruritic, the overlying skin may appear thickened from rubbing or scratching, and excoriations may be present.
Since vulvar syringomas are rare, there is no standard treatment. Biopsy is necessary for definitive diagnosis. For asymptomatic cases, expectant management is warranted. In symptomatic cases treatment can be considered. Treatment options include cryotherapy, laser ablation, and intralesional electrodissection.8 Intralesional electrodissection and curettage also has been described as treatment.9 Other treatment options include surgical excision of individual lesions or larger excisions if multifocal.
The case study described in "Case letter: Vulvar syringoma" highlights the diagnostic and therapeutic challenges associated with rare lesions of the vulva. Referral to a specialty clinic may be warranted in these challenging cases. ●
- Hansen A, Carr K, Jensen JT. Characteristics and initial diagnoses in women presenting to a referral center for vulvovaginal disorders in 1996–2000. J Reprod Med. 2002; 47: 854-860.
- Boza JC, Trindade EN, Peruzzo J, et al. Skin manifestations of obesity: a comparative study. J Eur Acad Dermatol Venereol. 2012;26:1220-1223.
- Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol. 1982;6:977-998.
- Bornstein J, Bogliatto F, Haefner HK, et al; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016;20:11-14.
- American College of Obstetricians and Gynecologists. Committee opinion no. 675: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Heller DS. Benign tumors and tumor-like lesions of the vulva. Clin Obstet Gynecol. 2015;58:526-535.
- Shalabi MMK, Homan K, Bicknell L. Vulvar syringomas. Proc (Bayl Univer Med Cent). 2022;35:113-114.
- Ozdemir O, Sari ME, Sen E, et al. Vulvar syringoma in a postmenopausal woman: a case report. J Reprod Med. 2015;60:452-454.
- Stevenson TR, Swanson NA. Syringoma: removal by electrodesiccation and curettage. Ann Plast Surg. 1985;15:151-154.
Pain and pruritus are the most common complaints in patients who present to vulvar clinics.1 These symptoms can be related to a variety of conditions, including vulvar lesions. There are both common and uncommon vulvar lesions. Vulvar lesions can be skin colored, yellow, and red. Certain lesions can be diagnosed with history and physical examination alone. Some more common lesions include acrochordons (skin tags), benign growths that are common in patients with diabetes, obesity, and pregnancy.2,3 Other common vulvar lesions are papillomatosis, lichen simplex chronicus, and epidermoid cysts. Other lesions include low- and high-grade squamous intraepithelial lesions (HSIL).4 These lesions require biopsy for diagnosis as high-grade lesions require treatment. HSIL of the vulva is considered a premalignancy that necessitates treatment.5 Other lesions that can present with vulvar complaints are molluscum contagiosum, Bartholin gland duct cyst, intradermal melanocytic nevus, and squamous cell carcinoma.
Rarely, other less common conditions can present as vulvar lesions. Syringomas are benign eccrine sweat gland neoplasms. They are more commonly found on the face, neck, or chest.6 On the vulva they are generally small subcutaneous skin-colored papules.7 They may be asymptomatic and noted only on routine examination.
Vulvar syringomas also may present with symptoms. On the vulva, syringomas often present as pruritic papules that can be isolated or multifocal. Often on the labia majora they range in size from 2 to 20 mm.8
They can coalesce to form a larger lesion. They also may be described as painful. When syringomas are pruritic, the overlying skin may appear thickened from rubbing or scratching, and excoriations may be present.
Since vulvar syringomas are rare, there is no standard treatment. Biopsy is necessary for definitive diagnosis. For asymptomatic cases, expectant management is warranted. In symptomatic cases treatment can be considered. Treatment options include cryotherapy, laser ablation, and intralesional electrodissection.8 Intralesional electrodissection and curettage also has been described as treatment.9 Other treatment options include surgical excision of individual lesions or larger excisions if multifocal.
The case study described in "Case letter: Vulvar syringoma" highlights the diagnostic and therapeutic challenges associated with rare lesions of the vulva. Referral to a specialty clinic may be warranted in these challenging cases. ●
Pain and pruritus are the most common complaints in patients who present to vulvar clinics.1 These symptoms can be related to a variety of conditions, including vulvar lesions. There are both common and uncommon vulvar lesions. Vulvar lesions can be skin colored, yellow, and red. Certain lesions can be diagnosed with history and physical examination alone. Some more common lesions include acrochordons (skin tags), benign growths that are common in patients with diabetes, obesity, and pregnancy.2,3 Other common vulvar lesions are papillomatosis, lichen simplex chronicus, and epidermoid cysts. Other lesions include low- and high-grade squamous intraepithelial lesions (HSIL).4 These lesions require biopsy for diagnosis as high-grade lesions require treatment. HSIL of the vulva is considered a premalignancy that necessitates treatment.5 Other lesions that can present with vulvar complaints are molluscum contagiosum, Bartholin gland duct cyst, intradermal melanocytic nevus, and squamous cell carcinoma.
Rarely, other less common conditions can present as vulvar lesions. Syringomas are benign eccrine sweat gland neoplasms. They are more commonly found on the face, neck, or chest.6 On the vulva they are generally small subcutaneous skin-colored papules.7 They may be asymptomatic and noted only on routine examination.
Vulvar syringomas also may present with symptoms. On the vulva, syringomas often present as pruritic papules that can be isolated or multifocal. Often on the labia majora they range in size from 2 to 20 mm.8
They can coalesce to form a larger lesion. They also may be described as painful. When syringomas are pruritic, the overlying skin may appear thickened from rubbing or scratching, and excoriations may be present.
Since vulvar syringomas are rare, there is no standard treatment. Biopsy is necessary for definitive diagnosis. For asymptomatic cases, expectant management is warranted. In symptomatic cases treatment can be considered. Treatment options include cryotherapy, laser ablation, and intralesional electrodissection.8 Intralesional electrodissection and curettage also has been described as treatment.9 Other treatment options include surgical excision of individual lesions or larger excisions if multifocal.
The case study described in "Case letter: Vulvar syringoma" highlights the diagnostic and therapeutic challenges associated with rare lesions of the vulva. Referral to a specialty clinic may be warranted in these challenging cases. ●
- Hansen A, Carr K, Jensen JT. Characteristics and initial diagnoses in women presenting to a referral center for vulvovaginal disorders in 1996–2000. J Reprod Med. 2002; 47: 854-860.
- Boza JC, Trindade EN, Peruzzo J, et al. Skin manifestations of obesity: a comparative study. J Eur Acad Dermatol Venereol. 2012;26:1220-1223.
- Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol. 1982;6:977-998.
- Bornstein J, Bogliatto F, Haefner HK, et al; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016;20:11-14.
- American College of Obstetricians and Gynecologists. Committee opinion no. 675: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Heller DS. Benign tumors and tumor-like lesions of the vulva. Clin Obstet Gynecol. 2015;58:526-535.
- Shalabi MMK, Homan K, Bicknell L. Vulvar syringomas. Proc (Bayl Univer Med Cent). 2022;35:113-114.
- Ozdemir O, Sari ME, Sen E, et al. Vulvar syringoma in a postmenopausal woman: a case report. J Reprod Med. 2015;60:452-454.
- Stevenson TR, Swanson NA. Syringoma: removal by electrodesiccation and curettage. Ann Plast Surg. 1985;15:151-154.
- Hansen A, Carr K, Jensen JT. Characteristics and initial diagnoses in women presenting to a referral center for vulvovaginal disorders in 1996–2000. J Reprod Med. 2002; 47: 854-860.
- Boza JC, Trindade EN, Peruzzo J, et al. Skin manifestations of obesity: a comparative study. J Eur Acad Dermatol Venereol. 2012;26:1220-1223.
- Winton GB, Lewis CW. Dermatoses of pregnancy. J Am Acad Dermatol. 1982;6:977-998.
- Bornstein J, Bogliatto F, Haefner HK, et al; ISSVD Terminology Committee. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. J Low Genit Tract Dis. 2016;20:11-14.
- American College of Obstetricians and Gynecologists. Committee opinion no. 675: management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2016;128:e178-e182.
- Heller DS. Benign tumors and tumor-like lesions of the vulva. Clin Obstet Gynecol. 2015;58:526-535.
- Shalabi MMK, Homan K, Bicknell L. Vulvar syringomas. Proc (Bayl Univer Med Cent). 2022;35:113-114.
- Ozdemir O, Sari ME, Sen E, et al. Vulvar syringoma in a postmenopausal woman: a case report. J Reprod Med. 2015;60:452-454.
- Stevenson TR, Swanson NA. Syringoma: removal by electrodesiccation and curettage. Ann Plast Surg. 1985;15:151-154.
Hyperlipidemia management: A calibrated approach
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
- Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
- Consider the impact of ASCVD riskenhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
- Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patientoriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimating risk for ASCVD by ascertaining LDL-C
- The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL),estimation of LDL-C is invalid.
- The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC–HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
- National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
- Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
- Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
- Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Continue to: Applying the estimate of 10-year ASCVD risk...
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
- Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
- Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention: Stratification by age
- 40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12
In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).
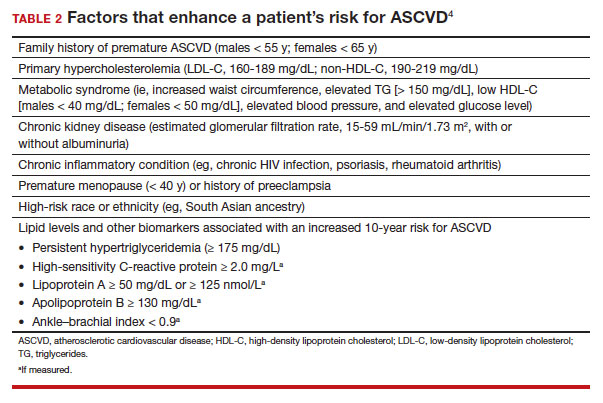
If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
- 20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
- > 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy...
Options for lipid-lowering pharmacotherapy
- Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4
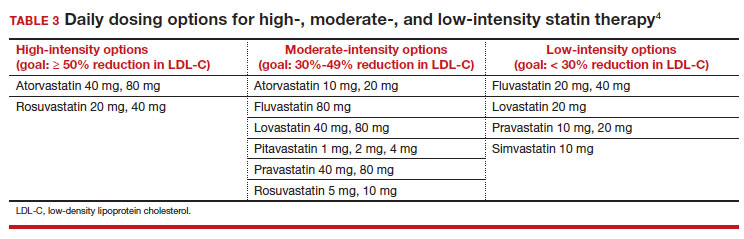
Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
- Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
- PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
- Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
- Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
- Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoicacid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
- Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL)and TG (42 mg/dL).31,32
- Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C< 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4 ●
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
- Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year followup experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
- Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001 /jama.2013.280532
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161 /CIR.0000000000000625
- Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
- Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi:10.1161/CIRCOUTCOMES.110.959247
- Framingham Heart Study. Cardiovascular disease (10year risk). Accessed February 14, 2023. www.framing hamheartstudy.org/fhs-risk-functions/cardiovascular -disease-10-year-risk/
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
- Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. Am J Clin Nutr. 2003;77:11461155. doi:10.1093/ajcn/77.5.1146
- Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi:10.1001/jama.2016.15450
- Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j. jcmg.2018.04.015
- Valenti V, O Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016 /j.jcmg.2015.01.025
- Armitage J, Baigent C, Barnes E, et al; Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407415. doi: 10.1016/S0140-6736(18)31942-1
- Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161 /CIRCULATIONAHA. 117.028271
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:23732384. doi: 10.1001/jama.2016.16951
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation Against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
- Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
- Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
- Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of highdose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
- Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001 /jamacardio.2021.1157
- US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
- Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
- Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statinintolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
- Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203212. doi: 10.1056/NEJMoa1300955
- Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
- Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001 /jamacardio.2016.4828
- Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056 /NEJMoa1001282
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
- Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
- Consider the impact of ASCVD riskenhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
- Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patientoriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimating risk for ASCVD by ascertaining LDL-C
- The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL),estimation of LDL-C is invalid.
- The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC–HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
- National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
- Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
- Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
- Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Continue to: Applying the estimate of 10-year ASCVD risk...
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
- Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
- Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention: Stratification by age
- 40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
- 20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
- > 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy...
Options for lipid-lowering pharmacotherapy
- Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
- Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
- PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
- Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
- Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
- Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoicacid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
- Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL)and TG (42 mg/dL).31,32
- Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C< 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4 ●
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
An elevated serum level of cholesterol has been recognized as a risk factor for atherosclerotic cardiovascular disease (ASCVD) since the publication of the Framingham Study in 1961.1 Although clinical outcomes related to ASCVD have improved in recent decades, ASCVD remains the leading cause of morbidity and mortality across the globe and remains, in the United States, the leading cause of death among most racial and ethnic groups. Much of this persistent disease burden can be attributed to inadequate control of ASCVD risk factors and suboptimal implementation of prevention strategies in the general population.2
The most recent (2019) iteration of the American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Primary Prevention of Cardiovascular Disease emphasizes a comprehensive, patient-centered, team-based approach to the management of ASCVD risk factors.2 In this article, I review how, first, medication to reduce ASCVD risk should be considered only when a patient’s risk is sufficiently high and, second, shared decision-making and social determinants of health should, in all cases, guide and inform optimal implementation of treatment.2
- Use an alternative to the Friedewald equation, such as the Martin–Hopkins equation, to estimate the low-density lipoprotein cholesterol (LDL-C) value; order direct measurement of LDL-C; or calculate non–high-density lipoprotein cholesterol to assess the risk for atherosclerotic cardiovascular disease (ASCVD) in patients who have a low LDL-C or a high triglycerides level. C
- Consider the impact of ASCVD riskenhancing factors and coronary artery calcium scoring in making a recommendation to begin lipid-lowering therapy in intermediate-risk patients. C
- Add ezetimibe if a statin does not sufficiently lower LDL-C or if a patient cannot tolerate an adequate dosage of the statin. C
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patientoriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimating risk for ASCVD by ascertaining LDL-C
- The Friedewald equation. Traditionally, low-density lipoprotein cholesterol (LDL-C) is estimated using the Friedewald equationa applied to a fasting lipid profile. In patients who have a low level of LDL-C (< 70 mg/dL), however, the Friedewald equation becomes less accurate; in patients with hypertriglyceridemia (TG ≥ 400 mg/dL),estimation of LDL-C is invalid.
- The Martin–Hopkins equation offers a validated estimation of LDL-C when the LDL-C value is < 70 mg/dL.3 This equation—in which the fixed factor of 5 used in the Friedewald equation to estimate very low-density lipoprotein cholesterol is replaced by an adjustable factor that is based on the patient’s non-HDL-C (ie, TC–HDL-C) and TG values—is preferred by the ACC/AHA Task Force on Clinical Practice Guidelines in this clinical circumstance.4
- National Institutes of Health equation. This newer equation provides an accurate estimate of the LDL-C level in patients whose TG value is ≤ 800 mg/dL. The equation has not been fully validated for clinical use, however.5
- Direct measurement obviates the need for an equation to estimate LDL-C, but the test is not available in all health care settings.
For adults ≥ 20 years of age who are not receiving lipid-lowering therapy, a nonfasting lipid profile can be used to estimate ASCVD risk and document the baseline LDL-C level. If the TG level is ≥ 400 mg/dL, the test should be administered in the fasting state.4
- Apolipoprotein B. Alternatively, apolipoprotein B (apoB) can be measured. Because each LDL-C particle contains 1 apoB molecule, the apoB level describes the LDL-C level more accurately than a calculation of LDL-C. Many patients with type 2 diabetes and metabolic syndrome have a relatively low calculated LDL-C (thereby falsely reassuring the testing clinician) but have an elevated apoB level. An apoB level ≥ 130 mg/dL corresponds to an LDL-C level >160 mg/dL.4
- Calculation of non-HDL-C. Because the nonfasting state does not have a significant impact on a patient’s TC and HDL-C levels, the non-HDL-C level also can be calculated from the results of a nonfasting lipid profile.
Non-HDL-C and apoB are equivalent predictors of ASCVD risk. These 2 assessments might offer better risk estimation than other available tools in patients who have type 2 diabetes and metabolic syndrome.6
Continue to: Applying the estimate of 10-year ASCVD risk...
Applying the estimate of 10-year ASCVD risk
Your recommendation for preventive intervention, such as lipid-lowering therapy, should be based on the estimated 10-year risk for ASCVD. Although multiple validated risk assessment tools are available, ACC/AHA recommends the pooled cohort risk equations (PCE), introduced in the 2013 ACC/AHA cholesterol treatment guidelines. The Framingham Heart Study now recommends the ACC/AHA PCE for risk assessment as well.7
The PCE, developed from 5 large cohorts, is based on hard atherosclerotic events: nonfatal myocardial infarction, death from coronary artery disease, and stroke. The ACC/AHA PCE is the only risk assessment tool developed using a significant percentage of patients who self-identify as Black.8 Alternatives to the ACC/AHA PCE include:
- Multi-ethnic Study of Atherosclerosis (MESA) 10-year ASCVD risk calculator, which incorporates the coronary artery calcium (CAC) score.
- Reynolds Risk Score, which incorporates high-sensitivity C-reactive protein measurement and a family history of premature ASCVD.9
How much does lifestyle modification actually matter?
The absolute impact of diet and exercise on lipid parameters is relatively modest. No studies have demonstrated a reduction in adverse cardiovascular outcomes with specific interventions regarding diet or activity.
- Diet. Nevertheless, ACC/AHA recommends that at-risk patients follow a dietary pattern that (1) emphasizes vegetables, fruits, and whole grains and (2) limits sweets, sugar-sweetened beverages, and red meat.
Saturated fat should constitute no more than 5% or 6% of total calories. In controlled-feeding trials,10 for every 1% of calories from saturated fat that are replaced with carbohydrate or monounsaturated or polyunsaturated fat, the LDL-C level was found to decline by as much as 1.8 mg/dL. Evidence is insufficient to assert that lowering dietary cholesterol reduces LDL-C.11
- Activity. Trials of aerobic physical activity, compared with a more sedentary activity pattern, have demonstrated a reduction in the LDL-C level of as much as 6 mg/dL. All adult patients should be counseled to engage in aerobic physical activity of moderate or vigorous intensity—averaging ≥ 40 minutes per session, 3 or 4 sessions per week.11
Primary prevention: Stratification by age
- 40 to 75 years. ACC/AHA recommends that you routinely assess traditional cardiovascular risk factors for these patients and calculate their 10-year risk for ASCVD using the PCE. Statin therapy as primary prevention is indicated for 3 major groups (TABLE 1).4 The US Preventive Services Task Force (USPSTF) recommends a 10-year ASCVD risk ≥ 10%, in conjunction with 1 or more additional CVD risk factors (dyslipidemia, diabetes, hypertension, smoking), as the threshold for initiating low- or moderate-intensity statin therapy in this age group.12

In adults at borderline risk (5% to < 7.5% 10-year ASCVD risk) or intermediate risk (≥ 7.5% to < 20% 10-year ASCVD risk), consider risk-enhancing factors to better inform your recommendation for preventive interventions. In these 2 groups, the presence of risk-enhancing factors might justify moderate-intensity statin therapy (TABLE 24).

If your decision regarding preventive intervention remains uncertain, measuring CAC might further guide your discussion with the patient.4 When the CAC score is:
- 0 Agatston units and higher-risk conditions (eg, diabetes, family history of premature coronary artery disease, smoking) are absent, statin therapy can be withheld; reassess ASCVD risk in 5 to 10 years.
- 1-99 Agatston units, statin therapy can be started, especially for patients ≥ 55 years of age.
- ≥ 100 Agatston units or ≥ 75th percentile, statin therapy is indicated for all patients, regardless of additional risk factors.4
Because statins promote progression from unstable, inflammatory atherosclerotic plaque to more stable, calcified plaque, CAC scoring is not valid in patients already on statin therapy.13
In primary prevention, patients who have been classified as having low or intermediate risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have an annual all-cause mortality < 1%, regardless of age and gender. Patients classified as being at high risk, based on ASCVD risk scoring, with a CAC score of 0 Agatston units, have a significantly lower annual mortality than low- or intermediate-risk patients with a CAC score > 0 Agatston units.14
- 20 to 39 years. Focus on evaluation of lifetime ASCVD risk, rather than short-term (10-year) risk. Lifestyle modification is the primary intervention for younger patients; for those with moderate hypercholesterolemia (LDL-C, 160-189 mg/dL) and a family history of premature ASCVD, however, consider statin therapy. For patients with LDL-C ≥ 190 mg/dL, lifetime ASCVD risk is markedly increased, and high-intensity statin therapy is recommended, regardless of age. In this group, reassess ASCVD risk factors every 4 to 6 years.4
- > 75 years, without ASCVD. In this group, the benefit of statin therapy is less clear and might be lessened by an increased potential for adverse effects. A meta-analysis of 28 trials demonstrated that people ages > 75 years had a 24% relative reduction in major coronary events for every 38.7 mg/dL (1.0 mmol/L) reduction in LDL-C, which is comparable to the risk reduction seen in people ages 40 to 75 years.15
With increasing age, however, the relative reduction in major coronary events with statin therapy decreased,15 although other trials have not demonstrated age heterogeneity.16 Because people > 75 years of age have a significantly higher ASCVD event rate, a comparable relative rate reduction with statin therapy results in a larger absolute rate reduction (ARR) and, therefore, a smaller number needed to treat (NNT) to prevent an event, compared to the NNT in younger people.
Secondary prevention
ACC/AHA guidelines define clinical ASCVD as a history of:
- acute coronary syndrome
- myocardial infarction
- coronary or other arterial revascularization
- cerebrovascular event
- symptomatic peripheral artery disease, including aortic aneurysm.
High-intensity statin therapy is indicated for all patients ≤ 75 years who have clinical ASCVD. In patients > 75 years, consider a taper to moderate-intensity statin therapy. An upper age limit for seeing benefit from statin therapy in secondary prevention has not been identified.4
In high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy, ezetimibe (discussed in the next section) can be added. In very-high-risk patients, if LDL-C remains ≥ 70 mg/dL despite maximally tolerated statin therapy plus ezetimibe, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor (also discussed next) can be added. Always precede initiation of a PCSK9 inhibitor with a discussion of the net benefit, safety, and cost with the patient.4
Continue to: Options for lipid-lowering pharmacotherapy...
Options for lipid-lowering pharmacotherapy
- Statins (formally, hydroxymethylglutaryl-coenzyme A reductase inhibitors) offer the most predictable reduction in ASCVD risk of any lipid-lowering therapy. The evidence report that accompanied the 2016 USPSTF guidelines on statins for the prevention of cardiovascular disease (CVD) stated that low- or moderate-dosage statin therapy is associated with approximately a 30% relative risk reduction (RRR) in CVD events and CVD deaths and a 10% to 15% RRR in all-cause mortality.17
High-intensity statin therapy reduces LDL-C by ≥ 50%. Moderate-intensity statin therapy reduces LDL-C by 30% to 49% (TABLE 3).4

Statins are not without risk: A 2016 report18 estimated that treating 10,000 patients with a statin for 5 years would cause 1 case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 cases of hemorrhagic stroke. The same treatment would, however, avert approximately 1000 CVD events among patients with preexisting disease and approximately 500 CVD events among patients at elevated risk but without preexisting disease.18
- Ezetimibe, a selective cholesterol-absorption inhibitor, lowers LDL-C by 13% to 20% and typically is well tolerated. The use of ezetimibe in ASCVD risk reduction is supported by a single randomized controlled trial of more than 18,000 patients with recent acute coronary syndrome. Adding ezetimibe to simvastatin 40 mg resulted in a 2% absolute reduction in major adverse cardiovascular events over a median follow-up of 6 years (NNT = 50), compared to simvastatin alone.19 ACC/AHA guidelines recommend adding ezetimibe to maximally tolerated statin therapy in patients with clinical ASCVD who do not reach their goal LDL reduction with a statin alone. Ezetimibe also can be considered a statin alternative in patients who are statin intolerant.4
- PCSK9 inhibitors. When added to statin therapy, evolocumab and alirocumab—monoclonal antibodies that inhibit PCSK9—offer an incremental decrease in LDL-C of approximately 60%.20-22 In a meta-analysis of 35 trials evaluating the incremental benefit of PCSK9 inhibitor therapy, a significant reduction in cardiovascular events, including myocardial infarction (ARR = 1.3%; NNT = 77), stroke (ARR = 0.4%; NNT = 250), and coronary revascularization (ARR = 1.6%; NNT = 63) was reported. No significant difference was observed in all-cause or cardiovascular mortality.21,23
- Inclisiran, an injectable small-interfering RNA that inhibits PCSK9 synthesis, provides an incremental decrease in LDL-C of > 50% in patients already receiving statin therapy. Meta-analysis of 3 small cardiovascular outcomes trials revealed no significant difference in the rate of myocardial infarction, stroke, or cardiovascular mortality with inclisiran compared to placebo. Larger outcomes trials are underway and might offer additional insight into this agent’s role in ASCVD risk management.24
- Omega-3 fatty acids. Multiple trials have demonstrated that adding omega-3 fatty acids to usual lipid-lowering therapy does not offer a consistent reduction in adverse cardiovascular outcomes, despite providing a significant reduction in TG levels. In a high-risk population with persistently elevated TG despite statin therapy, icosapent ethyl, a purified eicosapentaenoic acid ethyl ester, reduced major ASCVD outcomes by 25% over a median 4.9 years (ARR = 4.8%; NNT = 21), and cardiovascular death by 20% (ARR = 0.9%; NNT = 111), compared with a mineral oil placebo.25 Subsequent trials, using a corn oil placebo, failed to duplicate these data26—raising concern that the mineral oil comparator might have altered results of the eicosapentaenoic acid ethyl ester study.27,28
- Bempedoic acid is a small-molecule inhibitor of ATP citrate lyase that increases LDL uptake by the liver. Pooled data from studies of bempedoic acid show, on average, a 15% reduction in TC, a 23% reduction in LDL-C, and a 6% increase in HDL-C, without a significant change in TG.29 In statin-intolerant patients, bempedoicacid reduced major ASCVD outcomes by 13% over a median 40 months (ARR = 1.6%; NNT = 63), with no significant reduction in cardiovascular death.30
- Niacin. Two large trials failed to demonstrate improvement in major cardiovascular events or other clinical benefit when niacin is added to moderate-intensity statin therapy, despite a significant increase in the HDL-C level (on average, 6 mg/dL) and a decrease in the LDL-C level (10-12 mg/dL)and TG (42 mg/dL).31,32
- Fenofibrate lowers TG and increases HDL-C but does not consistently improve cardiovascular outcomes.33 In a trial of patients with type 2 diabetes and persistent dyslipidemia (serum TG > 204 mg/dL; HDL-C< 34 mg/dL) despite statin therapy, adding fenofibrate reduced CVD outcomes by 4.9%—although this absolute difference did not reach statistical significance.34
Neither niacin nor fenofibrate is considered useful for reducing ASCVD risk across broad populations.4
Follow-up to assess progress toward goals
Recheck the lipid profile 4 to 12 weeks after starting lipid-lowering therapy to verify adherence to medication and assess response. The primary goal is the percentage reduction in LDL-C based on ASCVD risk. An additional goal for very-high-risk patients is an LDL-C value ≤ 70 mg/dL. If the reduction in LDL-C is less than desired and adherence is assured, consider titrating the statin dosage or augmenting statin therapy with a nonstatin drug (eg, ezetimibe), or both.4 ●
CORRESPONDENCE
Jonathon M. Firnhaber, MD, MAEd, MBA, East Carolina University, Family Medicine Center, 101 Heart Drive, Greenville, NC 27834; [email protected]
- Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year followup experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
- Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001 /jama.2013.280532
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161 /CIR.0000000000000625
- Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
- Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi:10.1161/CIRCOUTCOMES.110.959247
- Framingham Heart Study. Cardiovascular disease (10year risk). Accessed February 14, 2023. www.framing hamheartstudy.org/fhs-risk-functions/cardiovascular -disease-10-year-risk/
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
- Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. Am J Clin Nutr. 2003;77:11461155. doi:10.1093/ajcn/77.5.1146
- Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi:10.1001/jama.2016.15450
- Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j. jcmg.2018.04.015
- Valenti V, O Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016 /j.jcmg.2015.01.025
- Armitage J, Baigent C, Barnes E, et al; Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407415. doi: 10.1016/S0140-6736(18)31942-1
- Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161 /CIRCULATIONAHA. 117.028271
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:23732384. doi: 10.1001/jama.2016.16951
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation Against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
- Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
- Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
- Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of highdose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
- Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001 /jamacardio.2021.1157
- US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
- Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
- Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statinintolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
- Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203212. doi: 10.1056/NEJMoa1300955
- Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
- Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001 /jamacardio.2016.4828
- Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056 /NEJMoa1001282
- Kannel WB, Dawber TR, Kagan A, et al. Factors of risk in the development of coronary heart disease—six-year followup experience. The Framingham Study. Ann Intern Med. 1961;55:33. doi: 10.7326/0003-4819-55-1-33
- Arnett DK, Blumenthal RS, Albert MA, et al; American Association of Cardiovascular and Pulmonary Rehabilitation, American Geriatrics Society, American Society of Preventive Cardiology, and Preventive Cardiovascular Nurses Association. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. doi: 10.1161/CIR.0000000000000678
- Martin SS, Blaha MJ, Elshazly MB, et al. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061-2068. doi: 10.1001 /jama.2013.280532
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/ AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-1143. doi: 10.1161 /CIR.0000000000000625
- Sampson M, Ling C, Sun Q, et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020;5:540-548. doi: 10.1001/jamacardio.2020.0013
- Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337-345. doi:10.1161/CIRCOUTCOMES.110.959247
- Framingham Heart Study. Cardiovascular disease (10year risk). Accessed February 14, 2023. www.framing hamheartstudy.org/fhs-risk-functions/cardiovascular -disease-10-year-risk/
- Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129(25 suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23(suppl 2):1-87. doi: 10.4158/EP171764.APPGL
- Mensink RP, Zock PL, Kester ADM, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a metaanalysis of 60 controlled trials. Am J Clin Nutr. 2003;77:11461155. doi:10.1093/ajcn/77.5.1146
- Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. doi:10.1001/jama.2016.15450
- Lee S-E, Chang H-J, Sung JM, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475-1484. doi: 10.1016/j. jcmg.2018.04.015
- Valenti V, O Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging. 2015;8:900-909. doi: 10.1016 /j.jcmg.2015.01.025
- Armitage J, Baigent C, Barnes E, et al; Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407415. doi: 10.1016/S0140-6736(18)31942-1
- Ridker PM, Lonn E, Paynter NP, et al. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979-1981. doi: 10.1161 /CIRCULATIONAHA. 117.028271
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024. doi: 10.1001/jama.2015.15629
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561. doi: 10.1016/S0140-6736(16)31357-5
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489
- Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:23732384. doi: 10.1001/jama.2016.16951
- Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-Term Evaluation Against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509. doi: 10.1056/NEJMoa1500858
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499. doi: 10.1056/NEJMoa1501031
- Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6:e006910. doi: 10.1161/JAHA.117.006910
- Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69-73. doi: 10.1016/j.amjcard.2020.08.018
- Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22. doi: 10.1056/NEJMoa1812792
- Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of highdose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268-2280. doi: 10.1001/jama.2020.22258
- Nissen SE, Lincoff AM, Wolski K, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk. JAMA Cardiol. 2021;6:1-8. doi: 10.1001 /jamacardio.2021.1157
- US Food and Drug Administration. Briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting, November 14, 2019. Accessed February 15, 2023. www.fda.gov/media/132477/download
- Cicero AFG, Fogacci F, Hernandez AV, et al. Efficacy and safety of bempedoic acid for the treatment of hypercholesterolemia: a systematic review and meta-analysis. PLOS Med. 2020;17:e1003121. doi: 10.1371/journal.pmed.1003121
- Nissen SE, Lincoff AM, Brennan D, et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statinintolerant patients. N Engl J Med. Published online March 4, 2023. doi: 10.1056/NEJMoa2215024
- Landray MJ, Haynes R, Hopewell JC, et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203212. doi: 10.1056/NEJMoa1300955
- Boden WE, Probstfield JL, Anderson T, et al; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267. doi: 10.1056/NEJMoa1107579
- Elam MB, Ginsberg HN, Lovato LC, et al; ACCORDION Study Investigators. Association of fenofibrate therapy with long-term cardiovascular risk in statin-treated patients with type 2 diabetes. JAMA Cardiol. 2017;2:370-380. doi: 10.1001 /jamacardio.2016.4828
- Ginsberg HN, Elam MB, Lovato LC, et al; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. doi: 10.1056 /NEJMoa1001282
2023 Update on genetics in fetal growth
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):
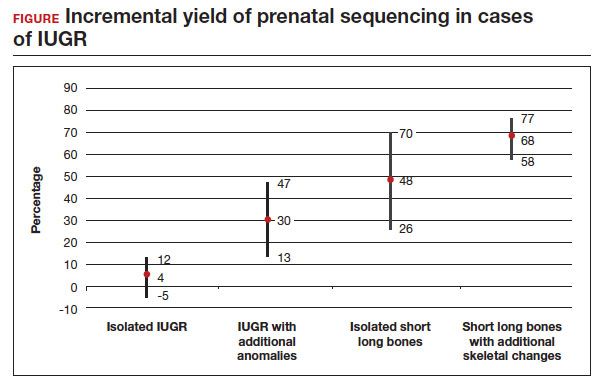
- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.
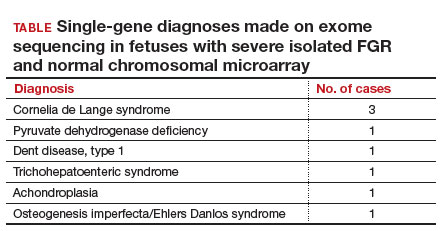
To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):

- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.

To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
Whole exome sequencing’s role in diagnosing genetic causes of FGR with and without associated anomalies
Mone F, Mellis R, Gabriel H, et al. Should we offer prenatal exome sequencing for intrauterine growth restriction or short long bones? A systematic review and meta-analysis. Am J Obstet Gynecol. Published online October 7, 2022. doi:10.1016/j.ajog.2022.09.045
Multiple factors can play a role in FGR, including inherent maternal, placental, or fetal factors; the environment; and/or nutrition. However, prenatal diagnosis is an important consideration when exploring the underlying etiology for a growth-restricted fetus, especially in severe or early-onset cases. Many genetic conditions do not result in structural anomalies but can disrupt overall growth. Additionally, phenotyping in the prenatal period is limited and can miss more subtle physical differences that could point to a genetic cause.
When compared with karyotype, chromosomal microarray (CMA) has been shown to increase the diagnostic yield in cases of isolated early FGR by 5%,1,2 and the incidence of chromosomal abnormalities has been reported to be as high as 19% in this population. Let’s explore the data on exome sequencing for prenatal diagnosis in cases of isolated FGR.
Meta-analysis details
In this meta-analysis, the authors reviewed 19 cohort studies or case series that investigated the yield of prenatal sequencing in fetuses with intrauterine growth restriction (IUGR) or short long bones, both in association with and without additional anomalies. All cases had nondiagnostic cytogenetic results. Fetal DNA in most cases was obtained through amniocentesis. Variants classified as likely pathogenic and pathogenic were considered diagnostic. The authors then calculated the incremental yield of prenatal sequencing over cytogenetic studies as a pooled value, comparing the following groups:
- isolated FGR
- growth restriction with associated anomalies
- isolated short long bones
- short long bones with additional skeletal features.
Study outcomes
The total number of cases were as follows: isolated IUGR (n = 71), IUGR associated with additional anomalies (n = 45), isolated short long bones (n = 84), and short long bones associated with additional skeletal findings (n = 252). Causative pathogenic or likely pathogenic variants were identified in 224 (50%) cases. Apparent incremental yields with prenatal sequencing were as follows for the 4 groups (as illustrated in the FIGURE):

- 4% in isolated IUGR (95% confidence interval [CI], -5%–12%)
- 30% in IUGR with additional anomalies (95% CI, 13%–47%)
- 48% in isolated short long bones (95% CI, 26%–70%)
- 68% in short long bones with additional skeletal changes (95% CI, 58%–77%).
Overall, the authors concluded that prenatal sequencing does not improve prenatal diagnosis in cases of isolated IUGR. The majority of these cases were thought to be related to placental insufficiency.
Strengths and limitations
The main limitation of this study with regard to our discussion is the small study populationof isolated growth restriction. The authors indicate that the number of cases of isolated IUGR were too small to draw firm conclusions. Another limitation was the heterogeneity of the isolated FGR population, which was not limited to severe or early-onset cases. However, the authors did demonstrate that growth restriction in association with fetal anomalies has very high genetic yield rates with prenatal sequencing.
Not surprisingly, there is a high yield of diagnosing genetic conditions in pregnancies complicated by isolated or nonisolated short long bones or in cases of growth restriction with multisystem abnormalities. Based on the results of this study, the authors advise against sending for exome sequencing in cases of isolated growth restriction with coexisting evidence of placental insufficiency.
Continue to: Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?...
Can whole exome sequencing diagnose genetic causes in cases of severe isolated FGR?
Zhou H, Fu F, Wang Y, et al. Genetic causes of isolated and severe fetal growth restriction in normal chromosomal microarray analysis. Int J Gynaecol Obstet. Published online December 10, 2022. doi:10.1002/ijgo.14620
Severe FGR is diagnosed based on an estimated fetal weight (EFW) or abdominal circumference (AC) below the third percentile. As we discussed in the above study by Mone and colleagues, it does not appear that prenatal sequencing significantly improves the diagnostic yield in all isolated FGR cases. However, this has not been previously explored in isolated severe FGR or cases of early-onset FGR (<32 weeks’ gestation). We know that several monogenic conditions are associated with severe and early-onset isolated fetal growth impairment, including but not limited to Cornelia de Lange syndrome, Smith-Lemli-Opitz syndrome, and Meier-Gorlin syndrome. Often, these syndromes can present in the prenatal period without other phenotypic findings. Therefore, this study explored the possibility that prenatal sequencing plays an important role for severe cases of FGR with nondiagnostic CMA and/or karyotype.
Retrospective study details
Zhou and colleagues retrospectively analyzed 51 cases of severe (EFW or AC below the third percentile) isolated FGR with negative CMA who underwent trio whole exome sequencing, which includes submitting fetal cells as well as both parental samples for testing. Patients with abnormal toxoplasmosis, rubella, cytomegalovirus, and herpes simplex virus (TORCH) tests; structural anomalies; and multiple gestation were excluded from the analysis. As in the study by Mone et al, variants classified as likely pathogenic and pathogenic were categorized as diagnostic.
Results
Eight of 51 cases (15.7%) with severe isolated FGR had diagnostic findings on trio whole exome sequencing as shown in the TABLE. Another 8 cases (15.7%) were found to have variants of unknown significance, of which 2 were later determined to be novel pathogenic variants. Genetic conditions uncovered in this cohort include Cornelia de Lange syndrome, pyruvate dehydrogenase deficiency, Dent disease, trichohepaticenteric syndrome, achondroplasia, osteogenesis imperfecta, Pendred syndrome, and both autosomal dominant type 3A and autosomal recessive type 1A deafness. All 10 cases with diagnostic whole exome sequencing or identified novel pathogenic variants were affected by early-onset FGR (<32 weeks’ gestation). Of these 10 cases, 7 patients underwent pregnancy termination.

To summarize, a total of 10 cases (19.6%) of severe isolated early-onset FGR with negative cytogenetic studies were subsequently diagnosed with an underlying genetic condition using prenatal trio whole exome sequencing.
Strengths and limitations
This study is retrospective and has a small sample size (n = 51) that was mostly limited to early-onset isolated severe FGR. However, the diagnostic yield (19.6%) of whole exome sequencing after negative CMA testing was noteworthy and shows that monogenic conditions are an important consideration in the evaluation of severe early-onset FGR, even in the absence of structural abnormalities.
As indications for exome sequencing during pregnancy continue to evolve, severe isolated FGR is emerging as a high-yield condition in which a subset of patients may benefit from the described testing strategy. We learned from our look at the prior study (Mone et al) that unselected isolated growth restriction with evident placental insufficiency may not benefit from exome sequencing, but this study differs in its selection of early-onset, severe cases—defined by diagnosis before 32 weeks’ gestation and an EFW or AC below the third percentile. Almost 20% of cases who met the aforementioned criteria received a genetic diagnosis from exome sequencing. We should remember to offer genetic counseling and diagnostic testing to our patients with severe growth restriction, even in the absence of additional structural anomalies.
Could epigenetic mechanisms of placental dysregulation explain low birthweight and future cardiometabolic disease?
Tekola-Ayele F, Zeng X, Chatterjee S, et al. Placental multi-omics integration identifies candidate functional genes for birthweight. Nat Commun. 2022;13:2384.
FGR has been linked to greater mortality in childhood and increased risk for cardiometabolic disease in adulthood. While genomewide associations studies (GWAS) have defined areas of interest linking genetic variants to low birthweight, their relationship to epigenetic changes in the placenta as well as biologic and functional mechanisms are not yet well understood.
Multiomics used to identify candidate functional genes for birthweight
This study analyzed the methylation and gene expression patterns of 291 placental samples, integrating findings into pathways of previously defined GWAS variants. Patient samples were obtained from participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singleton cohort. The cohort is ethnically diverse, with 97 Hispanic, 74 White, 71 Black, and 49 Asian participants. Of 286 single nucleotide polymorphisms (SNPs) found to be associated with birthweight, 273 were analyzed as part of the authors’ data set. These were found to have 7,901 unique protein-coding mRNAs (expression quantitative trait loci [eQTL]) and more than 100,000 nearby (within 1 Mb) CpG islands thought to be involved in changes in DNA methylation (methylation quantitative trait loci [mQTL]). Each functionally connected GWAS-eQTL-mQTL association is referred to as a triplet.
The next arm of the study investigatedthe connections and pathways within each triplet. Three possible scenarios were explored for birthweight GWAS SNPs using a causal interference test (CIT):
- the SNP alters placental DNA methylation, which then influences gene expression
- the SNP first alters placental DNA expression, which then influences methylation
- the SNP influences placental DNA expression and methylation independently, with no notable crossover between their pathways.
Triplets were investigated using the Mendelian randomization (MR) Steiger directionality test to validate the directionality of the pathways found by CIT. Lastly, the possibility of linkage disequilibrium was also studied using the moloc test.
Results
Using CIT, a causal relationship was predicted in 88 of 197 triplets, in which 84 (95.5%) indicated DNA methylation influences gene expression, and 4 (4.5%) indicated gene expression influences DNA methylation. The authors also used the MR Steiger test to investigate triplets to identify possible causal pathways. Using the MR Steiger test, only 3 of 45 (7%) triplets were found to have independent gene expression and methylation pathways. Thirty-eight of 45 (84%) triplets indicated that gene expression influences DNA methylation, and 7 (15%)triplets demonstrated that DNA methylation influences gene expression. Consistent predictions between CIT and the MR Steiger test revealed 3 triplets in which DNA methylation influences gene expression for the following genes: WNT3A, CTDNEP1, and RANBP2. Additionally, a strong colocalization signal was found among birthweight, DNA methylation, and gene expression for the following genes: PLEKHA1, FES, PRMT7, and CTDNEP1. Gene set enrichment analysis was performed as well and found that low birthweight is associated in substantial upregulation of genes associated with oxidative stress, immune response, adipogenesis, myogenesis, and the production of pancreatic ß cells.
Study strengths and limitations
The study is one of the first to identify regulatory targets for placental DNA methylation and gene expression in previously identified GWAS loci associated with low birthweight. For example, DNA methylation was found to influence gene expression of WNT3A, CTDNEP1, and RANBP2, which have previously been shown in animal studies to impact the vascularization and development of the placenta, embryogenesis, and fetal growth. The study also identified 4 genes (PLEKHA1, FES, PRMT7, and CTDNEP1) thought to have direct regulatory influence on placental DNA methylation and gene expression.
A limitation of the study is that it could not distinguish between whether the epigenetic changes we outlined have a maternal or fetal origin. Another limitation is that tissue used by the authors for analysis was a small placental biopsy, which does not accurately reflect the genetic heterogeneity of the placenta. Finally, this study does not establish causality between the identified epigenetic pathways and low birthweight. ●
We know that the placenta is critical to in utero development. This study begins to explore the genetic changes and programming in the placenta that may have profound effects on health and well-being both early and later in life.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
- Li LS, Li DZ. A genetic approach to the etiologic investigation of isolated intrauterine growth restriction. Am J Obstet Gynecol. 2021;225:695-696. doi: 10.1016/j.ajog.2021 .07.021.
- Borrell A, Grande M, Pauta M, et al. Chromosomal microarray analysis in fetuses with growth restriction and normal karyotype: a systematic review and meta-analysis. Fetal Diagn Ther. 2018;44:1-9. doi: 10.1159/000479506.
Vulvar syringoma
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.
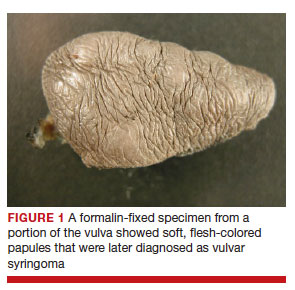
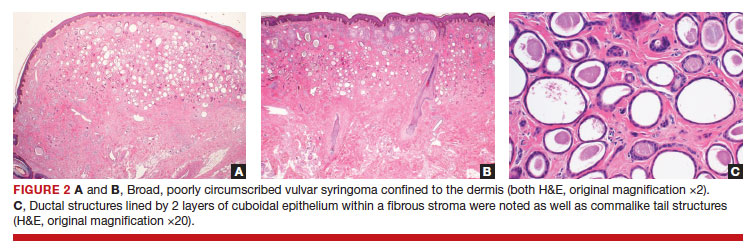
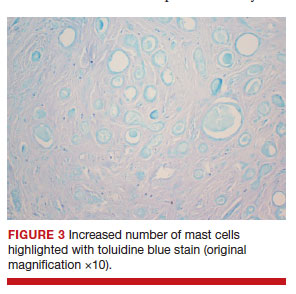
Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
Treatment of Angiosarcoma of the Head and Neck: A Systematic Review
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51
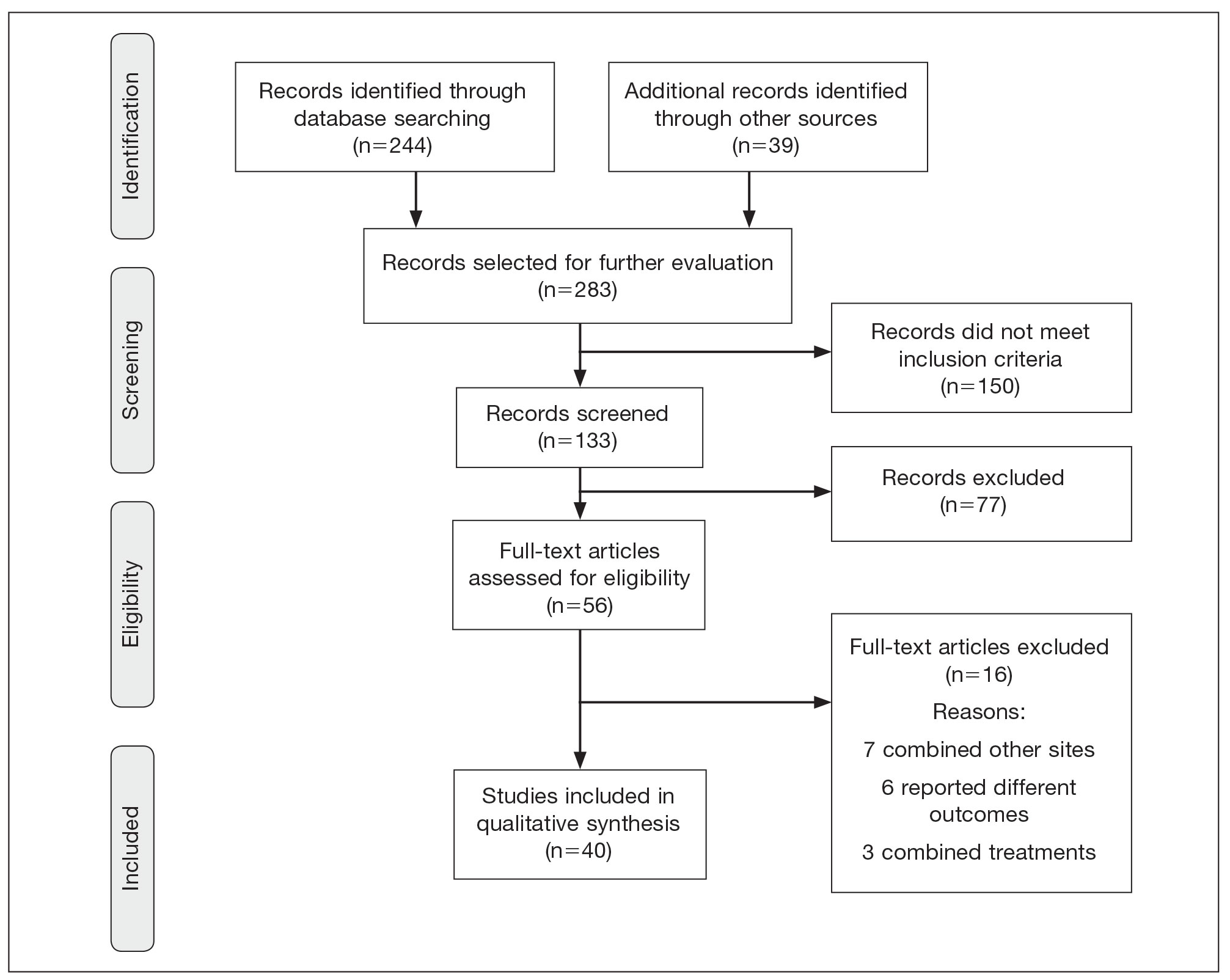
Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).
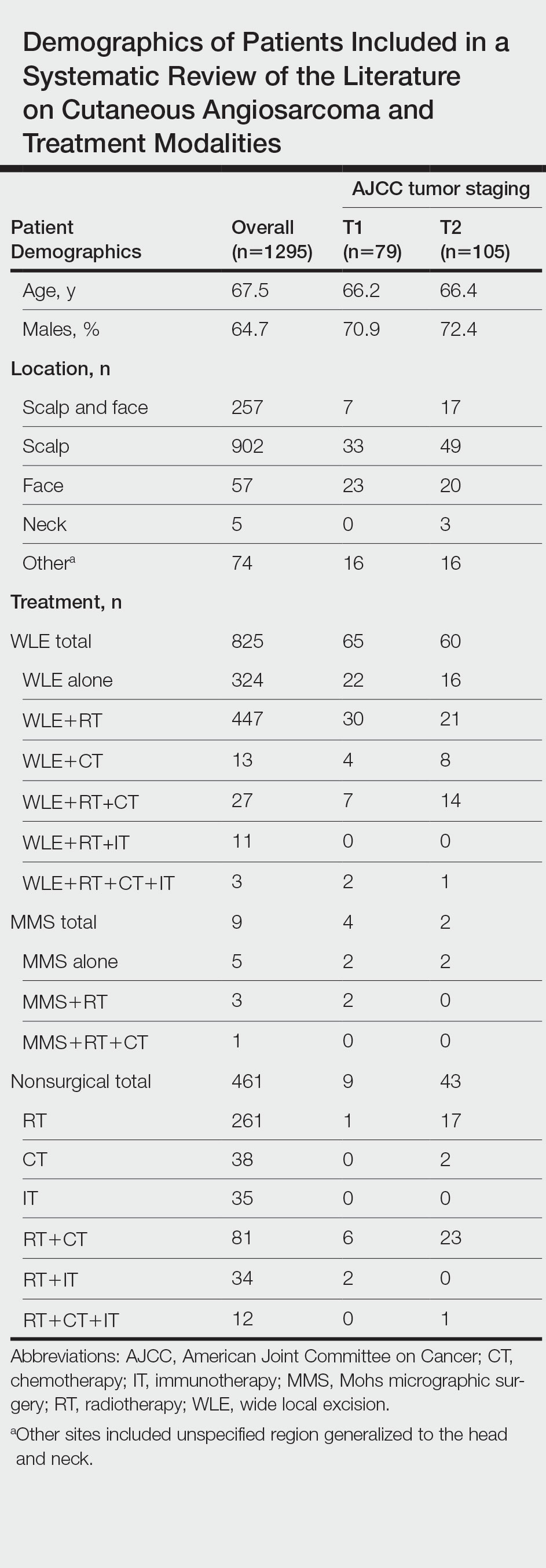
Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).
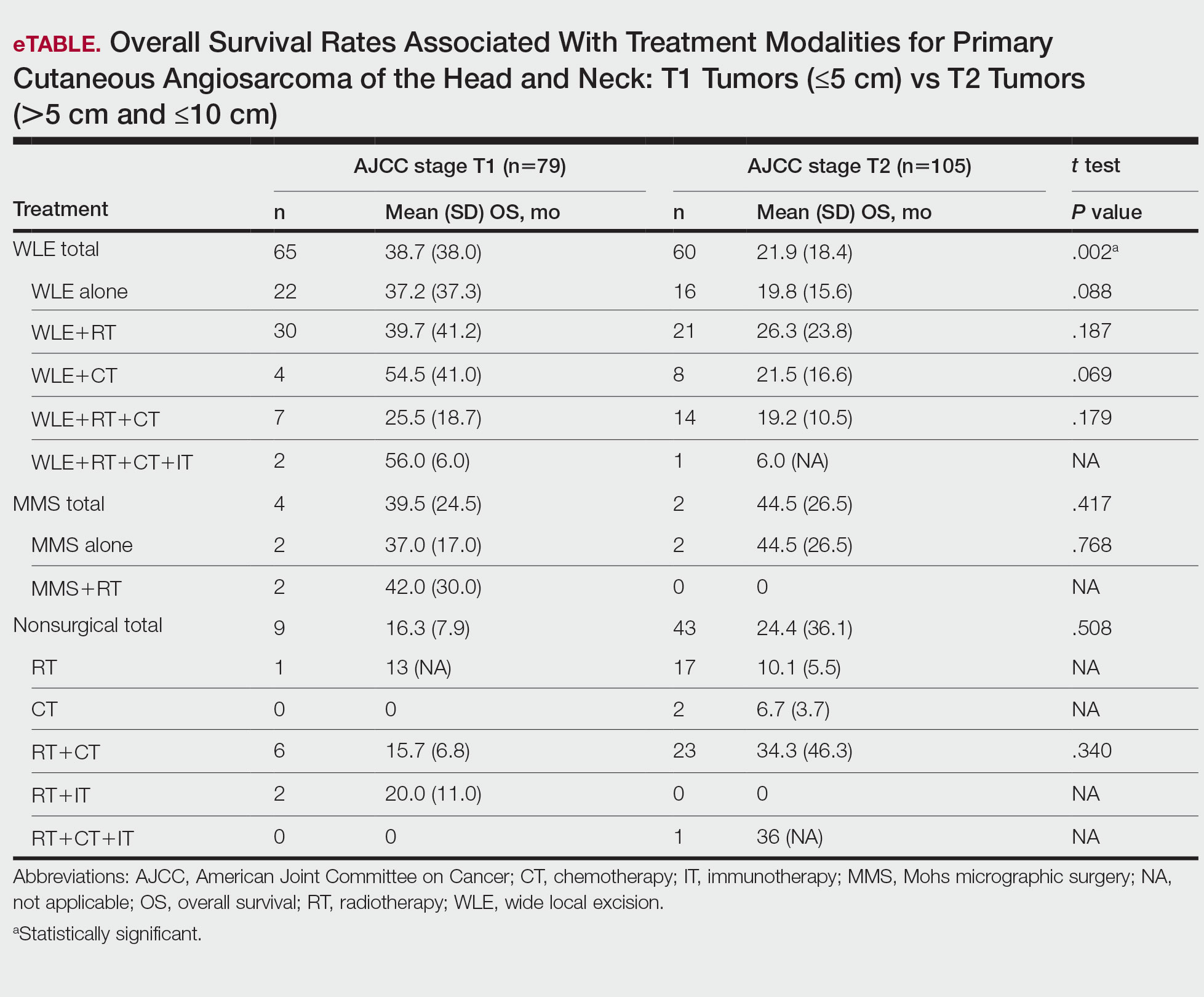
T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Practice Points
- Angiosarcoma is a rare tumor that is difficult to treat, with multiple treatment options being utilized.
- Within this systematic review, wide local excision (WLE) combined with radiotherapy (RT), chemotherapy, and immunotherapy, as well as Mohs micrographic surgery (MMS), offered the longest mean (SD) overall survival time.
- When clinicians are tasked with treating primary cutaneous angiosarcoma of the head and neck, they should consider MMS or WLE combined with RT.
ObGyn’s steady progress toward going green in the OR—but gaps persist
Have you ever looked at the operating room (OR) trash bin at the end of a case and wondered if all that waste is necessary? Since I started my residency, not a day goes by that I have not asked myself this question.
In the mid-1990s, John Elkington introduced the concept of the triple bottom line—that is, people, planet, and profit—for implementation and measurement of sustainability in businesses.1 The health care sector is no exception when it comes to the bottom line! However, “people” remain the priority. What is our role, as ObGyns, in protecting the “planet” while keeping the “people” safe?
According to the World Health Organization (WHO), climate change remains the single biggest health threat to humanity.2 The health care system is both the victim and the culprit. Studies suggest that the health care system, second to the food industry, is the biggest contributor to waste production in the United States. This sector generates more than 6,000 metric tons of waste each day and nearly 4 million tons (3.6 million metric tons) of solid waste each year.3 The health care system is responsible for an estimated 8% to 10% of total greenhouse gas emissions in the United States; the US health care system alone contributes to more than one-fourth of the global health care carbon footprint. If it were a country, the US health care system would rank 13th among all countries in emissions.4In turn, pollution produced by the health sector negatively impacts population health, further burdening the health care system. According to 2013 study data, the annual health damage caused by health care pollution was comparable to that of the deaths caused by preventable medical error.4
Aside from the environmental aspects, hospital waste disposal is expensive; reducing this cost is a potential area of interest for institutions.
As ObGyns, what is our role in reducing our waste generation and carbon footprint while keeping patients safe?
Defining health care waste, and disposal considerations
The WHO defines health care waste as including “the waste generated by health-care establishments, research facilities, and laboratories” as well as waste from scattered sources such as home dialysis and insulin injections.5 Despite representing a relatively small physical area of hospitals, labor and delivery units combined with ORs account for approximately 70% of all hospital waste.3 Operating room waste consists of disposable surgical supplies, personal protective equipment, drapes, plastic wrappers, sterile blue wraps, glass, cardboard, packaging material, medications, fluids, and other materials (FIGURE 1).

The WHO also notes that of all the waste generated by health care activities, about 85% is general, nonhazardous waste that is comparable to domestic waste.6 Hazardous waste is any material that poses a health risk, including potentially infectious materials, such as blood-soaked gauze, sharps, pharmaceuticals, or radioactive materials.6
Disposal of hazardous waste is expensiveand energy consuming as it is typically incinerated rather than disposed of in a landfill. This process produces substantial greenhouse gases, about 3 kg of carbon dioxide for every 1 kg of hazardous waste.7
Red bags are used for hazardous waste disposal, while clear bags are used for general waste. Operating rooms produce about two-thirds of the hospital red-bag waste.8 Waste segregation unfortunately is not accurate, and as much as 90% of OR general waste is improperly designated as hazardous waste.3 Drapes and uncontaminated, needleless syringes, for example, should be disposed of in clear bags, but often they are instead directed to the red-bag and sharps container (FIGURE 2).

Obstetrics and gynecology has an important role to play in accurate waste segregation given the specialty’s frequent interaction with bodily fluids. Clinicians and other staff need to recognize and appropriately separate hazardous waste from general waste. For instance, not all fabrics involved in a case should be disposed of in the red bin, only those saturated with blood or body fluids. Educating health care staff and placing instruction posters on the red trash bins potentially could aid in accurate waste segregation and reduce regulated waste while decreasing disposal costs.
Recycling in the OR
Recycling has become an established practice in many health care facilities and ORs. Studies suggest that introducing recycling programs in ORs not only reduces carbon footprints but also reduces costs.3 One study reported that US academic medical centers consume 2 million lb ($15 million) each year of recoverable medical supplies.9
Single-stream recycling, a system in which all recyclable material—including plastics, paper, metal, and glass—are placed in a single bin without segregation at the collection site, has gained in popularity. Recycling can be implemented both in ORs and in other perioperative areas where regular trash bins are located.
In a study done at Oxford University Hospitals in the United Kingdom, introducing recycling bins in every OR, as well as in recovery and staff rest areas, helped improve waste segregation such that approximately 22% of OR waste was recycled.10 Studies show that recycling programs not only decrease the health care carbon footprint but also have a considerable financial impact. Albert and colleagues demonstrated that introducing a single-stream recycling program to a 9-OR day (or ambulatory) surgery center could redirect more than 4 tons of waste each month and saved thousands of dollars.11
Despite continued improvement in recycling programs, the segregation process is still far from optimal. In a survey done at the Mayo Clinic by Azouz and colleagues, more than half of the staff reported being unclear about which OR items are recyclable and nearly half reported that lack of knowledge was the barrier to proper recycling.12 That study also showed that after implementation of a recycling education program, costs decreased 10% relative to the same time period in prior years.12
Blue wraps. One example of recycling optimization is blue wraps, the polypropylene (No. 5 plastic) material used for wrapping surgical instruments. Blue wraps account for approximately 19% of OR waste and 5% of all hospital waste.11 Blue wraps are not biodegradable and also are not widely recycled. In recent years, a resale market has emerged for blue wraps, as they can be used for production of other No. 5 plastic items.9 By reselling blue wraps, revenue can be generated by recycling a necessary packing material that would otherwise require payment for disposal.
Sterility considerations. While recycling in ORs may raise concern due to the absolute sterility required in procedural settings, technologic developments have been promising in advancing safe recycling to reduce carbon footprints and health care costs without compromising patients’ safety. Segregation of waste from recyclable packaging material prior to the case, as well as directing trash to the correct bin (regular vs red bin), is one example. Moreover, because about 80% of all OR waste is generated during the set up before the patient arrives in the OR, it is not contaminated and can be safely recycled.13
Continue to: Packaging material...
Packaging material
A substantial part of OR waste consists of packaging material; of all OR waste, 26% consists of plastics and 7%, paper and cartons.14 Increasing use of disposable or “single use” medical products in ORs, along with the intention to safeguard sterility, contributes significantly to the generation of medical waste in operating units. Containers, wraps and overwraps, cardboard, and plastic packaging are all composed of materials that when clean, can be recycled; however, these items often end up in the landfill (FIGURE 3).
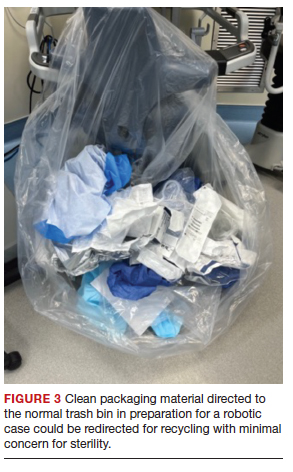
Although the segregation of packaging material to recycling versus regular trash versus red bin is of paramount importance, packaging design plays a significant role as well. In 2018, Boston Scientific introduced a new packaging design for ureteral stents that reduced plastic use in packaging by 120,000 lb each year.15 Despite the advances in the medical packaging industry to increase sustainability while safeguarding sterility for medical devices, there is still room for innovation in this area.
Reducing overage by judicious selection of surgical devices, instruments, and supplies
Overage is the term used to describe surgical inventory that is opened and prepared for surgery but ultimately not used and therefore discarded. Design of surgical carts and instrument and supply selection requires direct input from ObGyns. Opening only the needed instruments while ensuring ready availability of potentially needed supplies can significantly reduce OR waste generation as well as decrease chemical pollution generated by instrument sterilization. Decreasing OR overage reduces overall costs as well (FIGURE 4).
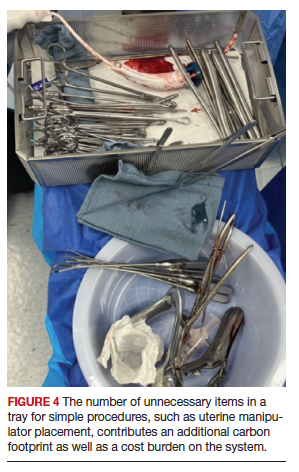
In a pilot study at the University of Massachusetts, Albert and colleagues examined the sets of disposable items and instruments designated for common plastic and hand surgery procedures.11 They identified the supplies and instruments that are routinely opened and wasted, based on surgeons’ interview responses, and redesigned the sets. Fifteen items were removed from disposable plastic surgery packs and 7 items from hand surgery packs. The authors reported saving thousands of dollars per year with these changes alone, as well as reducing waste.11 This same concept easily could be implemented in obstetrics and gynecology. We must ask ourselves: Do we always need, for example, a complete dilation and curettage kit to place the uterine manipulator prior to a minimally invasive hysterectomy?
In another pilot study, Greenberg and colleagues investigated whether cesarean deliveries consistently could be performed in a safe manner with only 20 instruments in the surgical kit.16 Obstetricians rated the 20-instrument kit an 8.7 out of 10 for performing cesarean deliveries safely.16
In addition to instrument selection, surgeons have a role in other supply use and waste generation: for instance, opening multiple pairs of surgical gloves and surgical gowns in advance when most of them will not be used during the case. Furthermore, many ObGyn surgeons routinely change gloves or even gowns during gynecologic procedures when they go back and forth between the vaginal and abdominal fields. Is the perineum “dirty” after application of a surgical prep solution?
In an observational study, Shockley and colleagues investigated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus at distinct time points during total laparoscopic hysterectomy.17 They showed that in 98.9% of cultures, the overall bacterial concentrations did not exceed the threshold for infection. There was no bacterial growth from vaginal cultures, and the only samples with some bacterial growth belonged to the surgeon’s gloves after specimen extraction; about one-third of samples showed growth after specimen extraction, but only 1 sample had a bacterial load above the infectious threshold of 5,000 colony-forming units per mL. The authors therefore suggested that if a surgeon changes gloves, doing so after specimen extraction and before turning attention back to the abdomen for vaginal cuff closure may be most effective in reducing bacterial load.17
Surgical site infection contributes to medical cost and likely medical waste as well. For example, surgical site infection may require prolonged treatments, tests, and medical instruments. In severe cases with abscesses, treatment entails hospitalization with prolonged antibiotic therapy with or without procedures to drain the collections. Further research therefore is warranted to investigate safe and environmentally friendly practices.
Myriad products are introduced to the medical system each day, some of which replace conventional tools. For instance, low-density polyethylene, or LDPE, transfer sheet is advertised for lateral patient transfer from the OR table to the bed or stretcher. This No. 4–coded plastic, while recyclable, is routinely discarded as trash in ORs. One ergonomic study found that reusable slide boards are as effective for reducing friction and staff muscle activities and are noninferior to the plastic sheets.18
Steps to making an impact
Operating rooms and labor and delivery units are responsible for a large proportion of hospital waste, and therefore they are of paramount importance in reducing waste and carbon footprint at the individual and institutional level. Reduction of OR waste not only is environmentally conscious but also decreases cost. Steps as small as individual practices to as big as changing infrastructures can make an impact. For instance:
- redesigning surgical carts
- reformulating surgeon-specific supply lists
- raising awareness about surgical overage
- encouraging recycling through education and audit
- optimizing surgical waste segregation through educational posters.
These are all simple steps that could significantly reduce waste and carbon footprint.
Bottom line
Although waste reduction is the responsibility of all health care providers, as leaders in their workplace physicians can serve as role models by implementing “green” practices in procedural units. Raising awareness and using a team approach is critical to succeed in our endeavors to move toward an environmentally friendly future. ●
- Elkington J. Towards the sustainable corporation: win-winwin business strategies for sustainable development. Calif Manage Rev. 1994;36:90-100.
- Climate change and health. October 30, 2021. World Health Organization. Accessed October 10, 2022. https://www.who .int/news-room/fact-sheets/detail/climate-change-and -health
- Kwakye G, Brat GA, Makary MA. Green surgical practices for health care. Arch Surg. 2011;146:131-136.
- Eckelman MJ, Sherman J. Environmental impacts of the US health care system and effects on public health. PloS One. 2016;11:e0157014.
- Pruss A, Giroult E, Rushbrook P. Safe management of wastes from health-care activities. World Health Organization; 1999.
- Health-care waste. February 8, 2018. World Health Organization. Accessed October 4, 2022. https://www.who. int/news-room/fact-sheets/detail/health-care-waste2
- Southorn T, Norrish AR, Gardner K, et al. Reducing the carbon footprint of the operating theatre: a multicentre quality improvement report. J Perioper Pract. 2013;23:144-146.
- Greening the OR. Practice Greenhealth. Accessed October 24, 2022. https://practicegreenhealth.org/topics/greening -operating-room/greening-or
- Babu MA, Dalenberg AK, Goodsell G, et al. Greening the operating room: results of a scalable initiative to reduce waste and recover supply costs. Neurosurgery. 2019;85:432-437.
- Oxford University Hospitals NHS Trust. Introducing recycling into the operating theatres. Mapping Greener Healthcare. Accessed October 14, 2022. https://map .sustainablehealthcare.org.uk/oxford-radcliffe-hospitals -nhs-trust/introducing-recycling-operating-theatres
- Albert MG, Rothkopf DM. Operating room waste reduction in plastic and hand surgery. Plast Surg. 2015;23:235-238.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Wyssusek KH, Keys MT, van Zundert AAJ. Operating room greening initiatives—the old, the new, and the way forward: a narrative review. Waste Manag Res. 2019;37:3-19.
- Tieszen ME, Gruenberg JC. A quantitative, qualitative, and critical assessment of surgical waste: surgeons venture through the trash can. JAMA. 1992;267:2765-2768.
- Boston Scientific 2018 Performance Report. Boston Scientific. Accessed November 19, 2022. https://www.bostonscientific. com/content/dam/bostonscientific/corporate/citizenship /sustainability/Boston_Scientific_Performance _Report_2018.pdf
- Greenberg JA, Wylie B, Robinson JN. A pilot study to assess the adequacy of the Brigham 20 Kit for cesarean delivery. Int J Gynaecol Obstet. 2012;117:157-159.
- Shockley ME, Beran B, Nutting H, et al. Sterility of selected operative sites during total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2017;24:990-997.
- Al-Qaisi SK, El Tannir A, Younan LA, et al. An ergonomic assessment of using laterally-tilting operating room tables and friction reducing devices for patient lateral transfers. Appl Ergon. 2020;87:103122.
Have you ever looked at the operating room (OR) trash bin at the end of a case and wondered if all that waste is necessary? Since I started my residency, not a day goes by that I have not asked myself this question.
In the mid-1990s, John Elkington introduced the concept of the triple bottom line—that is, people, planet, and profit—for implementation and measurement of sustainability in businesses.1 The health care sector is no exception when it comes to the bottom line! However, “people” remain the priority. What is our role, as ObGyns, in protecting the “planet” while keeping the “people” safe?
According to the World Health Organization (WHO), climate change remains the single biggest health threat to humanity.2 The health care system is both the victim and the culprit. Studies suggest that the health care system, second to the food industry, is the biggest contributor to waste production in the United States. This sector generates more than 6,000 metric tons of waste each day and nearly 4 million tons (3.6 million metric tons) of solid waste each year.3 The health care system is responsible for an estimated 8% to 10% of total greenhouse gas emissions in the United States; the US health care system alone contributes to more than one-fourth of the global health care carbon footprint. If it were a country, the US health care system would rank 13th among all countries in emissions.4In turn, pollution produced by the health sector negatively impacts population health, further burdening the health care system. According to 2013 study data, the annual health damage caused by health care pollution was comparable to that of the deaths caused by preventable medical error.4
Aside from the environmental aspects, hospital waste disposal is expensive; reducing this cost is a potential area of interest for institutions.
As ObGyns, what is our role in reducing our waste generation and carbon footprint while keeping patients safe?
Defining health care waste, and disposal considerations
The WHO defines health care waste as including “the waste generated by health-care establishments, research facilities, and laboratories” as well as waste from scattered sources such as home dialysis and insulin injections.5 Despite representing a relatively small physical area of hospitals, labor and delivery units combined with ORs account for approximately 70% of all hospital waste.3 Operating room waste consists of disposable surgical supplies, personal protective equipment, drapes, plastic wrappers, sterile blue wraps, glass, cardboard, packaging material, medications, fluids, and other materials (FIGURE 1).

The WHO also notes that of all the waste generated by health care activities, about 85% is general, nonhazardous waste that is comparable to domestic waste.6 Hazardous waste is any material that poses a health risk, including potentially infectious materials, such as blood-soaked gauze, sharps, pharmaceuticals, or radioactive materials.6
Disposal of hazardous waste is expensiveand energy consuming as it is typically incinerated rather than disposed of in a landfill. This process produces substantial greenhouse gases, about 3 kg of carbon dioxide for every 1 kg of hazardous waste.7
Red bags are used for hazardous waste disposal, while clear bags are used for general waste. Operating rooms produce about two-thirds of the hospital red-bag waste.8 Waste segregation unfortunately is not accurate, and as much as 90% of OR general waste is improperly designated as hazardous waste.3 Drapes and uncontaminated, needleless syringes, for example, should be disposed of in clear bags, but often they are instead directed to the red-bag and sharps container (FIGURE 2).

Obstetrics and gynecology has an important role to play in accurate waste segregation given the specialty’s frequent interaction with bodily fluids. Clinicians and other staff need to recognize and appropriately separate hazardous waste from general waste. For instance, not all fabrics involved in a case should be disposed of in the red bin, only those saturated with blood or body fluids. Educating health care staff and placing instruction posters on the red trash bins potentially could aid in accurate waste segregation and reduce regulated waste while decreasing disposal costs.
Recycling in the OR
Recycling has become an established practice in many health care facilities and ORs. Studies suggest that introducing recycling programs in ORs not only reduces carbon footprints but also reduces costs.3 One study reported that US academic medical centers consume 2 million lb ($15 million) each year of recoverable medical supplies.9
Single-stream recycling, a system in which all recyclable material—including plastics, paper, metal, and glass—are placed in a single bin without segregation at the collection site, has gained in popularity. Recycling can be implemented both in ORs and in other perioperative areas where regular trash bins are located.
In a study done at Oxford University Hospitals in the United Kingdom, introducing recycling bins in every OR, as well as in recovery and staff rest areas, helped improve waste segregation such that approximately 22% of OR waste was recycled.10 Studies show that recycling programs not only decrease the health care carbon footprint but also have a considerable financial impact. Albert and colleagues demonstrated that introducing a single-stream recycling program to a 9-OR day (or ambulatory) surgery center could redirect more than 4 tons of waste each month and saved thousands of dollars.11
Despite continued improvement in recycling programs, the segregation process is still far from optimal. In a survey done at the Mayo Clinic by Azouz and colleagues, more than half of the staff reported being unclear about which OR items are recyclable and nearly half reported that lack of knowledge was the barrier to proper recycling.12 That study also showed that after implementation of a recycling education program, costs decreased 10% relative to the same time period in prior years.12
Blue wraps. One example of recycling optimization is blue wraps, the polypropylene (No. 5 plastic) material used for wrapping surgical instruments. Blue wraps account for approximately 19% of OR waste and 5% of all hospital waste.11 Blue wraps are not biodegradable and also are not widely recycled. In recent years, a resale market has emerged for blue wraps, as they can be used for production of other No. 5 plastic items.9 By reselling blue wraps, revenue can be generated by recycling a necessary packing material that would otherwise require payment for disposal.
Sterility considerations. While recycling in ORs may raise concern due to the absolute sterility required in procedural settings, technologic developments have been promising in advancing safe recycling to reduce carbon footprints and health care costs without compromising patients’ safety. Segregation of waste from recyclable packaging material prior to the case, as well as directing trash to the correct bin (regular vs red bin), is one example. Moreover, because about 80% of all OR waste is generated during the set up before the patient arrives in the OR, it is not contaminated and can be safely recycled.13
Continue to: Packaging material...
Packaging material
A substantial part of OR waste consists of packaging material; of all OR waste, 26% consists of plastics and 7%, paper and cartons.14 Increasing use of disposable or “single use” medical products in ORs, along with the intention to safeguard sterility, contributes significantly to the generation of medical waste in operating units. Containers, wraps and overwraps, cardboard, and plastic packaging are all composed of materials that when clean, can be recycled; however, these items often end up in the landfill (FIGURE 3).

Although the segregation of packaging material to recycling versus regular trash versus red bin is of paramount importance, packaging design plays a significant role as well. In 2018, Boston Scientific introduced a new packaging design for ureteral stents that reduced plastic use in packaging by 120,000 lb each year.15 Despite the advances in the medical packaging industry to increase sustainability while safeguarding sterility for medical devices, there is still room for innovation in this area.
Reducing overage by judicious selection of surgical devices, instruments, and supplies
Overage is the term used to describe surgical inventory that is opened and prepared for surgery but ultimately not used and therefore discarded. Design of surgical carts and instrument and supply selection requires direct input from ObGyns. Opening only the needed instruments while ensuring ready availability of potentially needed supplies can significantly reduce OR waste generation as well as decrease chemical pollution generated by instrument sterilization. Decreasing OR overage reduces overall costs as well (FIGURE 4).

In a pilot study at the University of Massachusetts, Albert and colleagues examined the sets of disposable items and instruments designated for common plastic and hand surgery procedures.11 They identified the supplies and instruments that are routinely opened and wasted, based on surgeons’ interview responses, and redesigned the sets. Fifteen items were removed from disposable plastic surgery packs and 7 items from hand surgery packs. The authors reported saving thousands of dollars per year with these changes alone, as well as reducing waste.11 This same concept easily could be implemented in obstetrics and gynecology. We must ask ourselves: Do we always need, for example, a complete dilation and curettage kit to place the uterine manipulator prior to a minimally invasive hysterectomy?
In another pilot study, Greenberg and colleagues investigated whether cesarean deliveries consistently could be performed in a safe manner with only 20 instruments in the surgical kit.16 Obstetricians rated the 20-instrument kit an 8.7 out of 10 for performing cesarean deliveries safely.16
In addition to instrument selection, surgeons have a role in other supply use and waste generation: for instance, opening multiple pairs of surgical gloves and surgical gowns in advance when most of them will not be used during the case. Furthermore, many ObGyn surgeons routinely change gloves or even gowns during gynecologic procedures when they go back and forth between the vaginal and abdominal fields. Is the perineum “dirty” after application of a surgical prep solution?
In an observational study, Shockley and colleagues investigated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus at distinct time points during total laparoscopic hysterectomy.17 They showed that in 98.9% of cultures, the overall bacterial concentrations did not exceed the threshold for infection. There was no bacterial growth from vaginal cultures, and the only samples with some bacterial growth belonged to the surgeon’s gloves after specimen extraction; about one-third of samples showed growth after specimen extraction, but only 1 sample had a bacterial load above the infectious threshold of 5,000 colony-forming units per mL. The authors therefore suggested that if a surgeon changes gloves, doing so after specimen extraction and before turning attention back to the abdomen for vaginal cuff closure may be most effective in reducing bacterial load.17
Surgical site infection contributes to medical cost and likely medical waste as well. For example, surgical site infection may require prolonged treatments, tests, and medical instruments. In severe cases with abscesses, treatment entails hospitalization with prolonged antibiotic therapy with or without procedures to drain the collections. Further research therefore is warranted to investigate safe and environmentally friendly practices.
Myriad products are introduced to the medical system each day, some of which replace conventional tools. For instance, low-density polyethylene, or LDPE, transfer sheet is advertised for lateral patient transfer from the OR table to the bed or stretcher. This No. 4–coded plastic, while recyclable, is routinely discarded as trash in ORs. One ergonomic study found that reusable slide boards are as effective for reducing friction and staff muscle activities and are noninferior to the plastic sheets.18
Steps to making an impact
Operating rooms and labor and delivery units are responsible for a large proportion of hospital waste, and therefore they are of paramount importance in reducing waste and carbon footprint at the individual and institutional level. Reduction of OR waste not only is environmentally conscious but also decreases cost. Steps as small as individual practices to as big as changing infrastructures can make an impact. For instance:
- redesigning surgical carts
- reformulating surgeon-specific supply lists
- raising awareness about surgical overage
- encouraging recycling through education and audit
- optimizing surgical waste segregation through educational posters.
These are all simple steps that could significantly reduce waste and carbon footprint.
Bottom line
Although waste reduction is the responsibility of all health care providers, as leaders in their workplace physicians can serve as role models by implementing “green” practices in procedural units. Raising awareness and using a team approach is critical to succeed in our endeavors to move toward an environmentally friendly future. ●
Have you ever looked at the operating room (OR) trash bin at the end of a case and wondered if all that waste is necessary? Since I started my residency, not a day goes by that I have not asked myself this question.
In the mid-1990s, John Elkington introduced the concept of the triple bottom line—that is, people, planet, and profit—for implementation and measurement of sustainability in businesses.1 The health care sector is no exception when it comes to the bottom line! However, “people” remain the priority. What is our role, as ObGyns, in protecting the “planet” while keeping the “people” safe?
According to the World Health Organization (WHO), climate change remains the single biggest health threat to humanity.2 The health care system is both the victim and the culprit. Studies suggest that the health care system, second to the food industry, is the biggest contributor to waste production in the United States. This sector generates more than 6,000 metric tons of waste each day and nearly 4 million tons (3.6 million metric tons) of solid waste each year.3 The health care system is responsible for an estimated 8% to 10% of total greenhouse gas emissions in the United States; the US health care system alone contributes to more than one-fourth of the global health care carbon footprint. If it were a country, the US health care system would rank 13th among all countries in emissions.4In turn, pollution produced by the health sector negatively impacts population health, further burdening the health care system. According to 2013 study data, the annual health damage caused by health care pollution was comparable to that of the deaths caused by preventable medical error.4
Aside from the environmental aspects, hospital waste disposal is expensive; reducing this cost is a potential area of interest for institutions.
As ObGyns, what is our role in reducing our waste generation and carbon footprint while keeping patients safe?
Defining health care waste, and disposal considerations
The WHO defines health care waste as including “the waste generated by health-care establishments, research facilities, and laboratories” as well as waste from scattered sources such as home dialysis and insulin injections.5 Despite representing a relatively small physical area of hospitals, labor and delivery units combined with ORs account for approximately 70% of all hospital waste.3 Operating room waste consists of disposable surgical supplies, personal protective equipment, drapes, plastic wrappers, sterile blue wraps, glass, cardboard, packaging material, medications, fluids, and other materials (FIGURE 1).

The WHO also notes that of all the waste generated by health care activities, about 85% is general, nonhazardous waste that is comparable to domestic waste.6 Hazardous waste is any material that poses a health risk, including potentially infectious materials, such as blood-soaked gauze, sharps, pharmaceuticals, or radioactive materials.6
Disposal of hazardous waste is expensiveand energy consuming as it is typically incinerated rather than disposed of in a landfill. This process produces substantial greenhouse gases, about 3 kg of carbon dioxide for every 1 kg of hazardous waste.7
Red bags are used for hazardous waste disposal, while clear bags are used for general waste. Operating rooms produce about two-thirds of the hospital red-bag waste.8 Waste segregation unfortunately is not accurate, and as much as 90% of OR general waste is improperly designated as hazardous waste.3 Drapes and uncontaminated, needleless syringes, for example, should be disposed of in clear bags, but often they are instead directed to the red-bag and sharps container (FIGURE 2).

Obstetrics and gynecology has an important role to play in accurate waste segregation given the specialty’s frequent interaction with bodily fluids. Clinicians and other staff need to recognize and appropriately separate hazardous waste from general waste. For instance, not all fabrics involved in a case should be disposed of in the red bin, only those saturated with blood or body fluids. Educating health care staff and placing instruction posters on the red trash bins potentially could aid in accurate waste segregation and reduce regulated waste while decreasing disposal costs.
Recycling in the OR
Recycling has become an established practice in many health care facilities and ORs. Studies suggest that introducing recycling programs in ORs not only reduces carbon footprints but also reduces costs.3 One study reported that US academic medical centers consume 2 million lb ($15 million) each year of recoverable medical supplies.9
Single-stream recycling, a system in which all recyclable material—including plastics, paper, metal, and glass—are placed in a single bin without segregation at the collection site, has gained in popularity. Recycling can be implemented both in ORs and in other perioperative areas where regular trash bins are located.
In a study done at Oxford University Hospitals in the United Kingdom, introducing recycling bins in every OR, as well as in recovery and staff rest areas, helped improve waste segregation such that approximately 22% of OR waste was recycled.10 Studies show that recycling programs not only decrease the health care carbon footprint but also have a considerable financial impact. Albert and colleagues demonstrated that introducing a single-stream recycling program to a 9-OR day (or ambulatory) surgery center could redirect more than 4 tons of waste each month and saved thousands of dollars.11
Despite continued improvement in recycling programs, the segregation process is still far from optimal. In a survey done at the Mayo Clinic by Azouz and colleagues, more than half of the staff reported being unclear about which OR items are recyclable and nearly half reported that lack of knowledge was the barrier to proper recycling.12 That study also showed that after implementation of a recycling education program, costs decreased 10% relative to the same time period in prior years.12
Blue wraps. One example of recycling optimization is blue wraps, the polypropylene (No. 5 plastic) material used for wrapping surgical instruments. Blue wraps account for approximately 19% of OR waste and 5% of all hospital waste.11 Blue wraps are not biodegradable and also are not widely recycled. In recent years, a resale market has emerged for blue wraps, as they can be used for production of other No. 5 plastic items.9 By reselling blue wraps, revenue can be generated by recycling a necessary packing material that would otherwise require payment for disposal.
Sterility considerations. While recycling in ORs may raise concern due to the absolute sterility required in procedural settings, technologic developments have been promising in advancing safe recycling to reduce carbon footprints and health care costs without compromising patients’ safety. Segregation of waste from recyclable packaging material prior to the case, as well as directing trash to the correct bin (regular vs red bin), is one example. Moreover, because about 80% of all OR waste is generated during the set up before the patient arrives in the OR, it is not contaminated and can be safely recycled.13
Continue to: Packaging material...
Packaging material
A substantial part of OR waste consists of packaging material; of all OR waste, 26% consists of plastics and 7%, paper and cartons.14 Increasing use of disposable or “single use” medical products in ORs, along with the intention to safeguard sterility, contributes significantly to the generation of medical waste in operating units. Containers, wraps and overwraps, cardboard, and plastic packaging are all composed of materials that when clean, can be recycled; however, these items often end up in the landfill (FIGURE 3).

Although the segregation of packaging material to recycling versus regular trash versus red bin is of paramount importance, packaging design plays a significant role as well. In 2018, Boston Scientific introduced a new packaging design for ureteral stents that reduced plastic use in packaging by 120,000 lb each year.15 Despite the advances in the medical packaging industry to increase sustainability while safeguarding sterility for medical devices, there is still room for innovation in this area.
Reducing overage by judicious selection of surgical devices, instruments, and supplies
Overage is the term used to describe surgical inventory that is opened and prepared for surgery but ultimately not used and therefore discarded. Design of surgical carts and instrument and supply selection requires direct input from ObGyns. Opening only the needed instruments while ensuring ready availability of potentially needed supplies can significantly reduce OR waste generation as well as decrease chemical pollution generated by instrument sterilization. Decreasing OR overage reduces overall costs as well (FIGURE 4).

In a pilot study at the University of Massachusetts, Albert and colleagues examined the sets of disposable items and instruments designated for common plastic and hand surgery procedures.11 They identified the supplies and instruments that are routinely opened and wasted, based on surgeons’ interview responses, and redesigned the sets. Fifteen items were removed from disposable plastic surgery packs and 7 items from hand surgery packs. The authors reported saving thousands of dollars per year with these changes alone, as well as reducing waste.11 This same concept easily could be implemented in obstetrics and gynecology. We must ask ourselves: Do we always need, for example, a complete dilation and curettage kit to place the uterine manipulator prior to a minimally invasive hysterectomy?
In another pilot study, Greenberg and colleagues investigated whether cesarean deliveries consistently could be performed in a safe manner with only 20 instruments in the surgical kit.16 Obstetricians rated the 20-instrument kit an 8.7 out of 10 for performing cesarean deliveries safely.16
In addition to instrument selection, surgeons have a role in other supply use and waste generation: for instance, opening multiple pairs of surgical gloves and surgical gowns in advance when most of them will not be used during the case. Furthermore, many ObGyn surgeons routinely change gloves or even gowns during gynecologic procedures when they go back and forth between the vaginal and abdominal fields. Is the perineum “dirty” after application of a surgical prep solution?
In an observational study, Shockley and colleagues investigated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus at distinct time points during total laparoscopic hysterectomy.17 They showed that in 98.9% of cultures, the overall bacterial concentrations did not exceed the threshold for infection. There was no bacterial growth from vaginal cultures, and the only samples with some bacterial growth belonged to the surgeon’s gloves after specimen extraction; about one-third of samples showed growth after specimen extraction, but only 1 sample had a bacterial load above the infectious threshold of 5,000 colony-forming units per mL. The authors therefore suggested that if a surgeon changes gloves, doing so after specimen extraction and before turning attention back to the abdomen for vaginal cuff closure may be most effective in reducing bacterial load.17
Surgical site infection contributes to medical cost and likely medical waste as well. For example, surgical site infection may require prolonged treatments, tests, and medical instruments. In severe cases with abscesses, treatment entails hospitalization with prolonged antibiotic therapy with or without procedures to drain the collections. Further research therefore is warranted to investigate safe and environmentally friendly practices.
Myriad products are introduced to the medical system each day, some of which replace conventional tools. For instance, low-density polyethylene, or LDPE, transfer sheet is advertised for lateral patient transfer from the OR table to the bed or stretcher. This No. 4–coded plastic, while recyclable, is routinely discarded as trash in ORs. One ergonomic study found that reusable slide boards are as effective for reducing friction and staff muscle activities and are noninferior to the plastic sheets.18
Steps to making an impact
Operating rooms and labor and delivery units are responsible for a large proportion of hospital waste, and therefore they are of paramount importance in reducing waste and carbon footprint at the individual and institutional level. Reduction of OR waste not only is environmentally conscious but also decreases cost. Steps as small as individual practices to as big as changing infrastructures can make an impact. For instance:
- redesigning surgical carts
- reformulating surgeon-specific supply lists
- raising awareness about surgical overage
- encouraging recycling through education and audit
- optimizing surgical waste segregation through educational posters.
These are all simple steps that could significantly reduce waste and carbon footprint.
Bottom line
Although waste reduction is the responsibility of all health care providers, as leaders in their workplace physicians can serve as role models by implementing “green” practices in procedural units. Raising awareness and using a team approach is critical to succeed in our endeavors to move toward an environmentally friendly future. ●
- Elkington J. Towards the sustainable corporation: win-winwin business strategies for sustainable development. Calif Manage Rev. 1994;36:90-100.
- Climate change and health. October 30, 2021. World Health Organization. Accessed October 10, 2022. https://www.who .int/news-room/fact-sheets/detail/climate-change-and -health
- Kwakye G, Brat GA, Makary MA. Green surgical practices for health care. Arch Surg. 2011;146:131-136.
- Eckelman MJ, Sherman J. Environmental impacts of the US health care system and effects on public health. PloS One. 2016;11:e0157014.
- Pruss A, Giroult E, Rushbrook P. Safe management of wastes from health-care activities. World Health Organization; 1999.
- Health-care waste. February 8, 2018. World Health Organization. Accessed October 4, 2022. https://www.who. int/news-room/fact-sheets/detail/health-care-waste2
- Southorn T, Norrish AR, Gardner K, et al. Reducing the carbon footprint of the operating theatre: a multicentre quality improvement report. J Perioper Pract. 2013;23:144-146.
- Greening the OR. Practice Greenhealth. Accessed October 24, 2022. https://practicegreenhealth.org/topics/greening -operating-room/greening-or
- Babu MA, Dalenberg AK, Goodsell G, et al. Greening the operating room: results of a scalable initiative to reduce waste and recover supply costs. Neurosurgery. 2019;85:432-437.
- Oxford University Hospitals NHS Trust. Introducing recycling into the operating theatres. Mapping Greener Healthcare. Accessed October 14, 2022. https://map .sustainablehealthcare.org.uk/oxford-radcliffe-hospitals -nhs-trust/introducing-recycling-operating-theatres
- Albert MG, Rothkopf DM. Operating room waste reduction in plastic and hand surgery. Plast Surg. 2015;23:235-238.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Wyssusek KH, Keys MT, van Zundert AAJ. Operating room greening initiatives—the old, the new, and the way forward: a narrative review. Waste Manag Res. 2019;37:3-19.
- Tieszen ME, Gruenberg JC. A quantitative, qualitative, and critical assessment of surgical waste: surgeons venture through the trash can. JAMA. 1992;267:2765-2768.
- Boston Scientific 2018 Performance Report. Boston Scientific. Accessed November 19, 2022. https://www.bostonscientific. com/content/dam/bostonscientific/corporate/citizenship /sustainability/Boston_Scientific_Performance _Report_2018.pdf
- Greenberg JA, Wylie B, Robinson JN. A pilot study to assess the adequacy of the Brigham 20 Kit for cesarean delivery. Int J Gynaecol Obstet. 2012;117:157-159.
- Shockley ME, Beran B, Nutting H, et al. Sterility of selected operative sites during total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2017;24:990-997.
- Al-Qaisi SK, El Tannir A, Younan LA, et al. An ergonomic assessment of using laterally-tilting operating room tables and friction reducing devices for patient lateral transfers. Appl Ergon. 2020;87:103122.
- Elkington J. Towards the sustainable corporation: win-winwin business strategies for sustainable development. Calif Manage Rev. 1994;36:90-100.
- Climate change and health. October 30, 2021. World Health Organization. Accessed October 10, 2022. https://www.who .int/news-room/fact-sheets/detail/climate-change-and -health
- Kwakye G, Brat GA, Makary MA. Green surgical practices for health care. Arch Surg. 2011;146:131-136.
- Eckelman MJ, Sherman J. Environmental impacts of the US health care system and effects on public health. PloS One. 2016;11:e0157014.
- Pruss A, Giroult E, Rushbrook P. Safe management of wastes from health-care activities. World Health Organization; 1999.
- Health-care waste. February 8, 2018. World Health Organization. Accessed October 4, 2022. https://www.who. int/news-room/fact-sheets/detail/health-care-waste2
- Southorn T, Norrish AR, Gardner K, et al. Reducing the carbon footprint of the operating theatre: a multicentre quality improvement report. J Perioper Pract. 2013;23:144-146.
- Greening the OR. Practice Greenhealth. Accessed October 24, 2022. https://practicegreenhealth.org/topics/greening -operating-room/greening-or
- Babu MA, Dalenberg AK, Goodsell G, et al. Greening the operating room: results of a scalable initiative to reduce waste and recover supply costs. Neurosurgery. 2019;85:432-437.
- Oxford University Hospitals NHS Trust. Introducing recycling into the operating theatres. Mapping Greener Healthcare. Accessed October 14, 2022. https://map .sustainablehealthcare.org.uk/oxford-radcliffe-hospitals -nhs-trust/introducing-recycling-operating-theatres
- Albert MG, Rothkopf DM. Operating room waste reduction in plastic and hand surgery. Plast Surg. 2015;23:235-238.
- Azouz S, Boyll P, Swanson M, et al. Managing barriers to recycling in the operating room. Am J Surg. 2019;217:634-638.
- Wyssusek KH, Keys MT, van Zundert AAJ. Operating room greening initiatives—the old, the new, and the way forward: a narrative review. Waste Manag Res. 2019;37:3-19.
- Tieszen ME, Gruenberg JC. A quantitative, qualitative, and critical assessment of surgical waste: surgeons venture through the trash can. JAMA. 1992;267:2765-2768.
- Boston Scientific 2018 Performance Report. Boston Scientific. Accessed November 19, 2022. https://www.bostonscientific. com/content/dam/bostonscientific/corporate/citizenship /sustainability/Boston_Scientific_Performance _Report_2018.pdf
- Greenberg JA, Wylie B, Robinson JN. A pilot study to assess the adequacy of the Brigham 20 Kit for cesarean delivery. Int J Gynaecol Obstet. 2012;117:157-159.
- Shockley ME, Beran B, Nutting H, et al. Sterility of selected operative sites during total laparoscopic hysterectomy. J Minim Invasive Gynecol. 2017;24:990-997.
- Al-Qaisi SK, El Tannir A, Younan LA, et al. An ergonomic assessment of using laterally-tilting operating room tables and friction reducing devices for patient lateral transfers. Appl Ergon. 2020;87:103122.
Erythema Ab Igne: A Clinical Review
Erythema ab igne (EAI)(also known as toasted skin syndrome) was first described in the British Journal of Dermatology in the 20th century, 1 though it was known by physicians long before. Reticular netlike skin changes were seen in association with patients who spent extended time directly next to a heat source. This association led to the name of this condition, which literally means “redness by fire.” Indeed, EAI induced by chronic heat exposure has been described across the world for centuries. For example, in the cold regions of northern China, people used to sleep on beds of hot bricks called kang to stay warm at night. The people of India’s Kashmir district carried pots of hot coals called kangri next to the skin under large woven shawls to stay warm. In the past, Irish women often spent much time by a turf- or peat-burning fire. Chronic heat exposure in these cases can lead not only to EAI but also to aggressive types of cancer, often with a latency of 30 years or more. 2
More recently, the invention of home central heating led to a stark decrease in the number of cases associated with combustion-based heat, with a transition to etiologies such as use of hot water bottles, electric blankets, and electric space heaters. Over time, technological advances led to ever-increasing potential causes for EAI, such as laptops or cell phones, car heaters and heated seats, heated blankets,3,4 infrared lamps for food, and even medical devices such as ultrasound-based heating products and convective temperature management systems for hospitalized patients. As technology evolves, so do the potential causes of EAI, requiring clinicians to diagnose and deduce the cause through a thorough social and medical history as well as a workup on the present illness with considerations for the anatomical location.5-7 Herein, we describe the etiology of EAI, diagnosis, and treatment options.
Clinical Characteristics
Erythema ab igne begins as mild, transient, and erythematous macules and patches in a reticular pattern that resolve minutes to hours after removal of the heat source. With weeks to months of continued or repeated application of the heat source, the affected area eventually becomes hyperpigmented where there once was erythema (Figures 1 and 2). Sometimes papules, bullae, telangiectasia, and hyperkeratosis also form. The rash usually is asymptomatic, though pain, pruritus, and dysesthesia have been reported.7 Dermoscopy of EAI in the hyperpigmented stage can reveal diffuse superficial dark pigmentation, telangiectasia, and mild whitish scaling.8 Although the pathogenesis has remained elusive over the years, lesions do seem to be mostly associated with cumulative exposure to heat rather than length of exposure.7
Etiology of EAI
Anatomic Location—The affected site depends on the source of heat (Table). Classic examples of this condition include a patient with EAI presenting on the anterior thighs after working in front of a hot oven or a patient with chronic back pain presenting with lower-back EAI secondary to frequent use of a hot water bottle or heating pad.7 With evolving technology over the last few decades, new etiologies have become more common—teenagers are presenting with anterior thigh EAI secondary to frequent laptop use2-29; patients are holding warm cell phones in their pant pockets, leading to unilateral geometric EAI on the anterior thigh (front pocket) or buttock (back pocket)30; plug-in radiators under computer desks are causing EAI on the lower legs31-34; and automobile seat heaters have been shown to cause EAI on the posterior legs.5,35-37 Clinicians should consider anatomic location a critical clue for etiology.
Social History—There are rarer and more highly specific causes of EAI than simple heat exposure that can be parsed from a patient’s social history. Occupational exposure has been documented, such as bakers with exposure to ovens, foundry workers with exposure to heated metals, or fast-food workers with chronic exposure to infrared food lamps.6,7 There also are cultural practices that can cause EAI. For example, the practice of cupping with moxibustion was shown to create a specific pattern in the shape of the cultural tool used.38 When footbaths with Chinese herbal remedies are performed frequently with high heat, they can lead to EAI on the feet with a linear border at the ankles. There also have been reports of kotatsu (heated tables in Japan) leading to lower-body EAI.39,40 These cultural practices also are more common in patients with darker skin types, which can lead to hyperpigmentation that is difficult to treat, making early diagnosis important.7
Medical History—Case reports have shown EAI caused by patients attempting to use heat-based methods for pain relief of an underlying serious disease such as cancer, bowel pathology (abdominal EAI), spinal disc prolapse (midline back EAI),41 sickle cell anemia, and renal pathology (posterior upper flank EAI).6,7,40-49 Patients with hypothyroidism or anorexia have been noted to have generalized EAI sparing the face secondary to repeated and extended hot baths or showers.50-53 One patient with schizophrenia was shown to have associated thermophilia due to a delusion that led the patient to soak in hot baths for long periods of time, leading to EAI.54 Finally, all physicians should be aware of iatrogenic causes of EAI, such as use of warming devices, ultrasound-based warming techniques, and laser therapy for lipolysis. Inquire about the patient’s surgical history or intensive care unit stays as well as alternative medicine or chiropractic visits. Obtaining a history of medical procedures can be enlightening when an etiology is not immediately clear.7,55,56
Diagnosis
Erythema ab igne is a clinical diagnosis based on recognizable cutaneous findings and a clear history of moderate heat exposure. However, when a clinical diagnosis of EAI is not certain (eg, when unable to obtain a clear history from the patient) or when malignant transformation is suspected, a biopsy can be performed. Pathologically, hematoxylin and eosin staining of EAI classically reveals dilated small vascular channels in the superficial dermis, hence a clinically reticular rash; interface dermatitis clinically manifesting as erythema; and pigment incontinence with melanin-laden macrophages consistent with clinical hyperpigmentation. Finally, for unclear reasons, increased numbers of elastic fibers classically are seen in biopsies of EAI.7
Differential Diagnosis
The differential diagnosis for a reticular patch includes livedo reticularis (Figure 3), which usually manifests as a more generalized rash in patients with chronic disease or coagulopathy such as systemic lupus erythematosus, cryoglobulinemia, or Raynaud phenomenon. When differentiating EAI from livedo reticularis or cutis marmorata, consider that both alternative diagnoses are more vascular appearing and are associated with cold exposure rather than heat exposure. In cases that are less reticular, livedo racemosa can be considered in the differential diagnosis. Finally, poikiloderma of Civatte can be reticular, particularly on dermoscopy, but the distribution on the neck with submental sparing should help to distinguish it from EAI unless a heat source around the neck is identified while taking the patient’s history.7
In babies, a reticular generalized rash is most likely to be cutis marmorata (Figure 4), which is a physiologic response to cold exposure that resolves with rewarming of the skin. A more serious condition—cutis marmorata telangiectatica congenita (Figure 5)—usually is present at birth, most frequently involves a single extremity, and notably does not resolve with rewarming. This is an important differential for EAI in children because it can be associated with vascular and neurologic anomalies as well as limb asymmetry. Finally, port-wine stains can sometimes be reticular in appearance and can mimic the early erythematous stages of EAI. However, unlike the erythematous stage of EAI, the port-wine stains will be present at birth.7
Emerging in 2020, an important differential diagnosis to consider is a cutaneous manifestation of COVID-19 infection. An erythematous, reticular, chilblainlike or transient livedo reticularis–like rash has been described as a cutaneous manifestation of COVID-19. Although the pathophysiology is still being elucidated, it is suspected that this is caused by a major vaso-occlusive crisis secondary to COVID-19–induced thrombotic vasculopathy. Interestingly, the majority of patients with this COVID-related exanthem also displayed symptoms of COVID-19 (eg, fever, cough) at the time of presentation,57-60 but there also have been cases in patients who were asymptomatic or mildly symptomatic.60
In some cases, EAI is an indication to screen for an underlying disease. For example, uncontrolled pain is an opportunity to improve interventions such as modifying the patient’s pain-control regimen, placing a palliative care pain consultation, or checking if the patient has had age-appropriate screenings for malignancy. New focal pain in a patient with a prior diagnosis of cancer may be a sign of a new metastasis. A thermophilic patient leaves opportunity to assess for underlying medical causes such as thyroid abnormalities or social/psychological issues. Geriatric patients who are diagnosed with EAI may need to be assessed for dementia or home safety issues. Patients with a history of diabetes mellitus can unknowingly develop EAI on the lower extremities, which may signal a need to assess the patient for peripheral neuropathy. Patients with gastroparesis secondary to diabetes also may develop EAI on the abdomen secondary to heating pad use for discomfort. These examples are a reminder to consider possible secondary comorbidities in all diagnoses of EAI.7
Prognosis
Although the prognosis of EAI is excellent if caught early, failure to diagnose this condition can lead to permanent discoloration of the skin and even malignancy.6 A rare sequela includes squamous cell carcinoma, most commonly seen in chronic cases of the lower leg, which is likely related to chronic inflammation of the skin.61-65 Rare cases of poorly differentiated carcinoma,66 cutaneous marginal zone lymphoma,67 and Merkel cell carcinoma68 have been reported. Patients diagnosed with EAI should receive normal periodic surveillance of the skin based on their medical history, though the physician should have an increased suspicion and plan for biopsy of any nodules or ulcerations found on the skin of the affected area.7
Treatments
Once the diagnosis of EAI is made, treatment starts with removal of the heat source causing the rash. Because the rash usually is asymptomatic, further treatment typically is not required. The discoloration can resolve over months or years, but permanent hyperpigmentation is not uncommon. If hyperpigmentation persists despite removal of the heat source and the patient desires further treatment for discoloration, there are few treatment options, none of which are approved by the US Food and Drug Administration for this condition.7 There is some evidence for the use of Nd:YAG lasers to reduce hyperpigmentation in EAI.69 There have been some reports of treatment using topical hydroquinone and topical tretinoin in an attempt to lighten the skin. If associated hyperkeratosis or other epithelial atypia is present, the use of 5-fluorouracil may show some improvement.70 One case report has been published of successful treatment with systemic mesoglycan and topical bioflavonoids.71 It also is conceivable that medications used to treat postinflammatory hyperpigmentation may be helpful in this condition (eg, kojic acid, arbutin, mild topical steroids, azelaic acid). Patients with darker skin may experience permanent discoloration and may not be good candidates for alternative treatments such as laser therapy due to the risk for inducible hyperpigmentation.7
Conclusion
No matter the etiology, EAI usually is a benign skin condition that is treated by removal of the causative heat source. Once a diagnosis is made, the clinician must work with the patient to determine the etiology. Care must be taken to ensure that there are no underlying signs, such as chronic pain or psychiatric illness, that could point to associated conditions. Rarely, sequalae such as cancers have been documented in areas of chronic EAI. Once the heat source is identified and removed, any remaining hyperpigmentation usually will self-resolve over months to years, though this may take longer in patients with darker skin types. If more aggressive treatment is preferred by the patient, laser therapy, topical medications, and oral over-the-counter vitamins have been tried with minimal responses.
- Perry. Case of erythema ab igne. Br J Dermatol. 1900;xxiii:375.
- Bose S, Ortonee JP. Diseases affected by heat. In: Parish LC, Millikan LE, Amer M, et al. Global Dermatology Diagnosis and Management According to Geography, Climate, and Culture. Springer-Varlag; 1994:83-92.
- Leal-Lobato MM, Blasco-Morente G. Electric blanket induced erythema ab igne [in Spanish]. Semergen. 2015;41:456-457. doi:10.1016/j.semerg.2014.12.008
- Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20. doi:10.5070/D32011024689
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Smith ML. Environmental and sports-related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1569-1594.
- Errichetti E, Stinco G. Dermoscopy in general dermatology: a practical overview. Dermatol Ther (Heidelb). 2016;6:471-507. doi:10.1007/s13555-016-0141-6
- Guarneri C, Tchernev G, Wollina U, et al. Erythema ab igne caused by laptop computer. Open Access Maced J Med Sci. 2017;5:490-492. doi:10.3889/oamjms.2017.137
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Dickman J, Kessler S. Unilateral reticulated patch localized to the anterior thigh. JAAD Case Rep. 2018;4:746-748. doi:10.1016/j.jdcr.2018.06.007
- Boffa MJ. Laptop computer-induced erythema ab igne on the left breast. Cutis. 2011;87:175-176.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Andersen F. Laptop-thighs--laptop-induced erythema ab igne [in Danish]. Ugeskr Laeger. 2010;172:635.
- Jagtman BA. Erythema ab igne due to a laptop computer. Contact Dermatitis. 2004;50:105. doi:10.1111/j.0105-1873.2004.0295g.x
- Olechowska M, Kisiel K, Ruszkowska L, et al. Erythema ab igne (EAI) induced by a laptop computer: report of two cases. J Dtsch Dermatol Ges. doi:10.1111/j.1610-0387.2014.12387
- Nayak SUK, Shenoi SD, Prabhu S. Laptop induced erythema ab igne. Indian J Dermatol. 2012;57:131-132. doi:10.4103/0019-5154.94284
- Salvio AG, Nunes AJ, Angarita DPR. Laptop computer induced erythema ab igne: a new presentation of an old disease. An Bras Dermatol. 2016;91:79-80. doi:10.1590/abd1806-4841.20165139
- Schummer C, Tittelbach J, Elsner P. Right-sided laptop dermatitis [in German]. Dtsch Med Wochenschr. 2015;140:1376-1377. doi:10.1055/s-0041-103615
- Manoharan D. Erythema ab igne: usual site, unusual cause. J Pharm Bioallied Sci. 2015;7(suppl 1):S74-S75. doi:10.4103/0975-7406.155811
- Giraldi S, Diettrich F, Abbage KT, et al. Erythema ab igne induced by a laptop computer in an adolescent. An Bras Dermatol. 2011;86:128-130. doi:10.1590/S0365-05962011000100018
- Secher LLS, Vind-Kezunovic D, Zachariae COC. Side-effects to the use of laptop computers: erythema ab igne. Dermatol Reports. 2010;31:E11. doi:10.4081/dr.2010.e11
- Botten D, Langley RGB, Webb A. Academic branding: erythema ab igne and use of laptop computers. CMAJ. 2010;182:E857. doi:10.1503/cmaj.091868
- Bilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974. doi:10.1016/j.jaad.2003.08.007
- Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming - a case report. Int J Dermatol. 2012;51:716-717. doi:10.1111/j.1365-4632.2011.05033.x
- Levinbook WS, Mallett J, Grant-Kels JM. Laptop computer-associated erythema ab igne. Cutis. 2007;80:319-320.
- Mohr MR, Scott KA, Pariser RM, et al. Laptop computer-induced erythema ab igne: a case report. Cutis. 2007;79:59-60.
- Cantor AS, Bartling SJ. Laptop computer-induced hyperpigmentation. Dermatol Online J. 2018;24:13030/qt6k37r9wm.
- Kaptanog˘lu AF, Mullaaziz D. Erythema ab igne in the palmar area induced by smart phone: case report. Turkiye Klin J Med Sci. 2015;35:284-286. doi:10.5336/medsci.2015-46976
- Redding KS, Watts AN, Lee J, et al. Space heater-induced bullous erythema ab igne. Cutis. 2017;100:E9-E10.
- Goorland J, Edens MA, Baudoin TD. An emergency department presentation of erythema ab igne caused by repeated heater exposure. J La State Med Soc. 2016;168:33-34.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Brzezinski P, Ismail S, Chiriac A. Radiator-induced erythema ab igne in 8-year-old girl. Rev Chil Pediatr. 2014;85:239-240. doi:10.4067/S0370-41062014000200015
- Adams BB. Heated car seat-induced erythema ab igne. Arch Dermatol. 2012;148:265-266. doi:10.1001/archdermatol.2011.2207
- Helm TN, Spigel GT, Helm KF. Erythema ab igne caused by a car heater. Cutis. 1997;59:81-82.
- Gregory JF, Beute TC. Erythema ab igne. J Spec Oper Med. 2013;13:115-119. doi:10.55460/5AVH-NZHY
- Chua S, Chen Q, Lee HY. Erythema ab igne and dermal scarring caused by cupping and moxibustion treatment. J Dtsch Dermatol Ges. 2015;13:337-338. doi:10.1111/ddg.12581
- Chen JF, Liu YC, Chen YF, et al. Erythema ab igne after footbath with Chinese herbal remedies. J Chinese Med Assoc. 2011;74:51-53. doi:10.1016/j.jcma.2011.01.009
- Baltazar D, Brockman R, Simpson E. Kotatsu-induced erythema ab igne. An Bras Dermatol. 2019;94:253-254. doi:10.1590/abd1806-4841.20198792
- Baig M, Byrne F. Erythema ab igne and its relation to spinal pathology. Cureus. 2018;10:e2914. doi:10.7759/cureus.2914
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:e2635. doi:10.7759/cureus.2635
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;1862480. doi:10.1155/2016/1862480
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:e236-e238. doi:10.1097/PEC.0000000000001460
- Dizdarevic A, Karim OA, Bygum A. A reddish brown reticulated hyperpigmented erythema on the abdomen of a girl. Erythema ab igne, also known as toasted skin syndrome, caused by a heating pad onthe abdomen. Acta Derm Venereol. 2014;94:365-367. doi:10.2340/00015555-1722
- Chatterjee S. Erythema ab igne from prolonged use of a heating pad. Mayo Clin Proc. 2005;80:1500. doi:10.4065/80.11.1500
- Waldorf DS, Rast MF, Garofalo VJ. Heating-pad erythematous dermatitis “erythema ab igne.” JAMA. 1971;218:1704. doi:10.1001/jama.1971.03190240056023
- South AM, Crispin MK, Marqueling AL, et al. A hyperpigmented reticular rash in a patient on peritoneal dialysis. Perit Dial Int. 2016;36:677-700. doi:10.3747/pdi.2016.00042
- Ravindran R. Erythema ab igne in an individual with diabetes and gastroparesis. BMJ Case Rep. 2017;2017:bcr2014203856. doi:10.1136/bcr-2014-203856
- Dessinioti C, Katsambas A, Tzavela E, et al. Erythema ab igne in three girls with anorexia nervosa. Pediatr Dermatol. 2016;33:e149-e150. doi:10.1111/pde.12770
- Fischer J, Rein K, Erfurt-Berge C, et al. Three cases of erythema ab igne (EAI) in patients with eating disorders. Neuropsychiatr. 2010;24:141-143.
- Docx MKF, Simons A, Ramet J, et al. Erythema ab igne in an adolescent with anorexia nervosa. Int J Eat Disord. 2013;46:381-383. doi:10.1002/eat.22075
- Turan E, Cimen V, Haytoglu NSK, et al. A case of bullous erythema ab igne accompanied by anemia and subclinical hypothyroidism. Dermatol Online J. 2014;20:223366.
- Pavithran K. Erythema ab igne, schizophrenia and thermophilia. Indian J Dermatol Venereol Leprol. 1987;53:181-182.
- Dellavelle R, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Park SY, Kim SM, Yoon TJ. Erythema ab igne caused by weight loss heating pad. Korean J Dermatol. 2007;45:489-491.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Gisondi P, Plaserico S, Bordin C, et al. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020;34:2499-2504. doi:10.1111/jdv.16774
- Manalo IF, Smith MK, Cheeley J, et al. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83:700. doi:10.1016/j.jaad.2020.04.018
- Zhao Q, Fang X, Pang Z, et al. COVID‐19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:2505-2510. doi:10.1111/jdv.16778
- Akasaka T, Kon S. Two cases of squamous cell carcinoma arising from erythema ab igne. Nihon Hifuka Gakkai Zasshi. 1989;99:735-742.
- Arrington JH 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. Arch Dermatol. 1979;115:1226-1228.
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Wollina U, Helm C, Hansel G, et al. Two cases of erythema ab igne, one with a squamous cell carcinoma. G Ital Dermatol Venereol. 2007;142:415-418.
- Rudolph CM, Soyer HP, Wolf P, et al. Squamous cell carcinoma arising in erythema ab igne. Hautarzt. 2000;51:260-263. doi:10.1007/s001050051115
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Kim HW, Kim EJ, Park HC, et al. Erythema ab igne successfully treated with low fluenced 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser. J Cosmet Laser Ther. 2014;16:147-148. doi:10.3109/14764172.2013.854623
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;62:77-78.
- Gianfaldoni S, Gianfaldoni R, Tchernev G, et al. Erythema ab igne successfully treated with mesoglycan and bioflavonoids: a case-report. Open Access Maced J Med Sci. 2017;5:432-435. doi:10.3889/oamjms.2017.123
Erythema ab igne (EAI)(also known as toasted skin syndrome) was first described in the British Journal of Dermatology in the 20th century, 1 though it was known by physicians long before. Reticular netlike skin changes were seen in association with patients who spent extended time directly next to a heat source. This association led to the name of this condition, which literally means “redness by fire.” Indeed, EAI induced by chronic heat exposure has been described across the world for centuries. For example, in the cold regions of northern China, people used to sleep on beds of hot bricks called kang to stay warm at night. The people of India’s Kashmir district carried pots of hot coals called kangri next to the skin under large woven shawls to stay warm. In the past, Irish women often spent much time by a turf- or peat-burning fire. Chronic heat exposure in these cases can lead not only to EAI but also to aggressive types of cancer, often with a latency of 30 years or more. 2
More recently, the invention of home central heating led to a stark decrease in the number of cases associated with combustion-based heat, with a transition to etiologies such as use of hot water bottles, electric blankets, and electric space heaters. Over time, technological advances led to ever-increasing potential causes for EAI, such as laptops or cell phones, car heaters and heated seats, heated blankets,3,4 infrared lamps for food, and even medical devices such as ultrasound-based heating products and convective temperature management systems for hospitalized patients. As technology evolves, so do the potential causes of EAI, requiring clinicians to diagnose and deduce the cause through a thorough social and medical history as well as a workup on the present illness with considerations for the anatomical location.5-7 Herein, we describe the etiology of EAI, diagnosis, and treatment options.

Clinical Characteristics
Erythema ab igne begins as mild, transient, and erythematous macules and patches in a reticular pattern that resolve minutes to hours after removal of the heat source. With weeks to months of continued or repeated application of the heat source, the affected area eventually becomes hyperpigmented where there once was erythema (Figures 1 and 2). Sometimes papules, bullae, telangiectasia, and hyperkeratosis also form. The rash usually is asymptomatic, though pain, pruritus, and dysesthesia have been reported.7 Dermoscopy of EAI in the hyperpigmented stage can reveal diffuse superficial dark pigmentation, telangiectasia, and mild whitish scaling.8 Although the pathogenesis has remained elusive over the years, lesions do seem to be mostly associated with cumulative exposure to heat rather than length of exposure.7

Etiology of EAI
Anatomic Location—The affected site depends on the source of heat (Table). Classic examples of this condition include a patient with EAI presenting on the anterior thighs after working in front of a hot oven or a patient with chronic back pain presenting with lower-back EAI secondary to frequent use of a hot water bottle or heating pad.7 With evolving technology over the last few decades, new etiologies have become more common—teenagers are presenting with anterior thigh EAI secondary to frequent laptop use2-29; patients are holding warm cell phones in their pant pockets, leading to unilateral geometric EAI on the anterior thigh (front pocket) or buttock (back pocket)30; plug-in radiators under computer desks are causing EAI on the lower legs31-34; and automobile seat heaters have been shown to cause EAI on the posterior legs.5,35-37 Clinicians should consider anatomic location a critical clue for etiology.

Social History—There are rarer and more highly specific causes of EAI than simple heat exposure that can be parsed from a patient’s social history. Occupational exposure has been documented, such as bakers with exposure to ovens, foundry workers with exposure to heated metals, or fast-food workers with chronic exposure to infrared food lamps.6,7 There also are cultural practices that can cause EAI. For example, the practice of cupping with moxibustion was shown to create a specific pattern in the shape of the cultural tool used.38 When footbaths with Chinese herbal remedies are performed frequently with high heat, they can lead to EAI on the feet with a linear border at the ankles. There also have been reports of kotatsu (heated tables in Japan) leading to lower-body EAI.39,40 These cultural practices also are more common in patients with darker skin types, which can lead to hyperpigmentation that is difficult to treat, making early diagnosis important.7
Medical History—Case reports have shown EAI caused by patients attempting to use heat-based methods for pain relief of an underlying serious disease such as cancer, bowel pathology (abdominal EAI), spinal disc prolapse (midline back EAI),41 sickle cell anemia, and renal pathology (posterior upper flank EAI).6,7,40-49 Patients with hypothyroidism or anorexia have been noted to have generalized EAI sparing the face secondary to repeated and extended hot baths or showers.50-53 One patient with schizophrenia was shown to have associated thermophilia due to a delusion that led the patient to soak in hot baths for long periods of time, leading to EAI.54 Finally, all physicians should be aware of iatrogenic causes of EAI, such as use of warming devices, ultrasound-based warming techniques, and laser therapy for lipolysis. Inquire about the patient’s surgical history or intensive care unit stays as well as alternative medicine or chiropractic visits. Obtaining a history of medical procedures can be enlightening when an etiology is not immediately clear.7,55,56
Diagnosis
Erythema ab igne is a clinical diagnosis based on recognizable cutaneous findings and a clear history of moderate heat exposure. However, when a clinical diagnosis of EAI is not certain (eg, when unable to obtain a clear history from the patient) or when malignant transformation is suspected, a biopsy can be performed. Pathologically, hematoxylin and eosin staining of EAI classically reveals dilated small vascular channels in the superficial dermis, hence a clinically reticular rash; interface dermatitis clinically manifesting as erythema; and pigment incontinence with melanin-laden macrophages consistent with clinical hyperpigmentation. Finally, for unclear reasons, increased numbers of elastic fibers classically are seen in biopsies of EAI.7
Differential Diagnosis
The differential diagnosis for a reticular patch includes livedo reticularis (Figure 3), which usually manifests as a more generalized rash in patients with chronic disease or coagulopathy such as systemic lupus erythematosus, cryoglobulinemia, or Raynaud phenomenon. When differentiating EAI from livedo reticularis or cutis marmorata, consider that both alternative diagnoses are more vascular appearing and are associated with cold exposure rather than heat exposure. In cases that are less reticular, livedo racemosa can be considered in the differential diagnosis. Finally, poikiloderma of Civatte can be reticular, particularly on dermoscopy, but the distribution on the neck with submental sparing should help to distinguish it from EAI unless a heat source around the neck is identified while taking the patient’s history.7

In babies, a reticular generalized rash is most likely to be cutis marmorata (Figure 4), which is a physiologic response to cold exposure that resolves with rewarming of the skin. A more serious condition—cutis marmorata telangiectatica congenita (Figure 5)—usually is present at birth, most frequently involves a single extremity, and notably does not resolve with rewarming. This is an important differential for EAI in children because it can be associated with vascular and neurologic anomalies as well as limb asymmetry. Finally, port-wine stains can sometimes be reticular in appearance and can mimic the early erythematous stages of EAI. However, unlike the erythematous stage of EAI, the port-wine stains will be present at birth.7

Emerging in 2020, an important differential diagnosis to consider is a cutaneous manifestation of COVID-19 infection. An erythematous, reticular, chilblainlike or transient livedo reticularis–like rash has been described as a cutaneous manifestation of COVID-19. Although the pathophysiology is still being elucidated, it is suspected that this is caused by a major vaso-occlusive crisis secondary to COVID-19–induced thrombotic vasculopathy. Interestingly, the majority of patients with this COVID-related exanthem also displayed symptoms of COVID-19 (eg, fever, cough) at the time of presentation,57-60 but there also have been cases in patients who were asymptomatic or mildly symptomatic.60

In some cases, EAI is an indication to screen for an underlying disease. For example, uncontrolled pain is an opportunity to improve interventions such as modifying the patient’s pain-control regimen, placing a palliative care pain consultation, or checking if the patient has had age-appropriate screenings for malignancy. New focal pain in a patient with a prior diagnosis of cancer may be a sign of a new metastasis. A thermophilic patient leaves opportunity to assess for underlying medical causes such as thyroid abnormalities or social/psychological issues. Geriatric patients who are diagnosed with EAI may need to be assessed for dementia or home safety issues. Patients with a history of diabetes mellitus can unknowingly develop EAI on the lower extremities, which may signal a need to assess the patient for peripheral neuropathy. Patients with gastroparesis secondary to diabetes also may develop EAI on the abdomen secondary to heating pad use for discomfort. These examples are a reminder to consider possible secondary comorbidities in all diagnoses of EAI.7
Prognosis
Although the prognosis of EAI is excellent if caught early, failure to diagnose this condition can lead to permanent discoloration of the skin and even malignancy.6 A rare sequela includes squamous cell carcinoma, most commonly seen in chronic cases of the lower leg, which is likely related to chronic inflammation of the skin.61-65 Rare cases of poorly differentiated carcinoma,66 cutaneous marginal zone lymphoma,67 and Merkel cell carcinoma68 have been reported. Patients diagnosed with EAI should receive normal periodic surveillance of the skin based on their medical history, though the physician should have an increased suspicion and plan for biopsy of any nodules or ulcerations found on the skin of the affected area.7
Treatments
Once the diagnosis of EAI is made, treatment starts with removal of the heat source causing the rash. Because the rash usually is asymptomatic, further treatment typically is not required. The discoloration can resolve over months or years, but permanent hyperpigmentation is not uncommon. If hyperpigmentation persists despite removal of the heat source and the patient desires further treatment for discoloration, there are few treatment options, none of which are approved by the US Food and Drug Administration for this condition.7 There is some evidence for the use of Nd:YAG lasers to reduce hyperpigmentation in EAI.69 There have been some reports of treatment using topical hydroquinone and topical tretinoin in an attempt to lighten the skin. If associated hyperkeratosis or other epithelial atypia is present, the use of 5-fluorouracil may show some improvement.70 One case report has been published of successful treatment with systemic mesoglycan and topical bioflavonoids.71 It also is conceivable that medications used to treat postinflammatory hyperpigmentation may be helpful in this condition (eg, kojic acid, arbutin, mild topical steroids, azelaic acid). Patients with darker skin may experience permanent discoloration and may not be good candidates for alternative treatments such as laser therapy due to the risk for inducible hyperpigmentation.7
Conclusion
No matter the etiology, EAI usually is a benign skin condition that is treated by removal of the causative heat source. Once a diagnosis is made, the clinician must work with the patient to determine the etiology. Care must be taken to ensure that there are no underlying signs, such as chronic pain or psychiatric illness, that could point to associated conditions. Rarely, sequalae such as cancers have been documented in areas of chronic EAI. Once the heat source is identified and removed, any remaining hyperpigmentation usually will self-resolve over months to years, though this may take longer in patients with darker skin types. If more aggressive treatment is preferred by the patient, laser therapy, topical medications, and oral over-the-counter vitamins have been tried with minimal responses.
Erythema ab igne (EAI)(also known as toasted skin syndrome) was first described in the British Journal of Dermatology in the 20th century, 1 though it was known by physicians long before. Reticular netlike skin changes were seen in association with patients who spent extended time directly next to a heat source. This association led to the name of this condition, which literally means “redness by fire.” Indeed, EAI induced by chronic heat exposure has been described across the world for centuries. For example, in the cold regions of northern China, people used to sleep on beds of hot bricks called kang to stay warm at night. The people of India’s Kashmir district carried pots of hot coals called kangri next to the skin under large woven shawls to stay warm. In the past, Irish women often spent much time by a turf- or peat-burning fire. Chronic heat exposure in these cases can lead not only to EAI but also to aggressive types of cancer, often with a latency of 30 years or more. 2
More recently, the invention of home central heating led to a stark decrease in the number of cases associated with combustion-based heat, with a transition to etiologies such as use of hot water bottles, electric blankets, and electric space heaters. Over time, technological advances led to ever-increasing potential causes for EAI, such as laptops or cell phones, car heaters and heated seats, heated blankets,3,4 infrared lamps for food, and even medical devices such as ultrasound-based heating products and convective temperature management systems for hospitalized patients. As technology evolves, so do the potential causes of EAI, requiring clinicians to diagnose and deduce the cause through a thorough social and medical history as well as a workup on the present illness with considerations for the anatomical location.5-7 Herein, we describe the etiology of EAI, diagnosis, and treatment options.

Clinical Characteristics
Erythema ab igne begins as mild, transient, and erythematous macules and patches in a reticular pattern that resolve minutes to hours after removal of the heat source. With weeks to months of continued or repeated application of the heat source, the affected area eventually becomes hyperpigmented where there once was erythema (Figures 1 and 2). Sometimes papules, bullae, telangiectasia, and hyperkeratosis also form. The rash usually is asymptomatic, though pain, pruritus, and dysesthesia have been reported.7 Dermoscopy of EAI in the hyperpigmented stage can reveal diffuse superficial dark pigmentation, telangiectasia, and mild whitish scaling.8 Although the pathogenesis has remained elusive over the years, lesions do seem to be mostly associated with cumulative exposure to heat rather than length of exposure.7

Etiology of EAI
Anatomic Location—The affected site depends on the source of heat (Table). Classic examples of this condition include a patient with EAI presenting on the anterior thighs after working in front of a hot oven or a patient with chronic back pain presenting with lower-back EAI secondary to frequent use of a hot water bottle or heating pad.7 With evolving technology over the last few decades, new etiologies have become more common—teenagers are presenting with anterior thigh EAI secondary to frequent laptop use2-29; patients are holding warm cell phones in their pant pockets, leading to unilateral geometric EAI on the anterior thigh (front pocket) or buttock (back pocket)30; plug-in radiators under computer desks are causing EAI on the lower legs31-34; and automobile seat heaters have been shown to cause EAI on the posterior legs.5,35-37 Clinicians should consider anatomic location a critical clue for etiology.

Social History—There are rarer and more highly specific causes of EAI than simple heat exposure that can be parsed from a patient’s social history. Occupational exposure has been documented, such as bakers with exposure to ovens, foundry workers with exposure to heated metals, or fast-food workers with chronic exposure to infrared food lamps.6,7 There also are cultural practices that can cause EAI. For example, the practice of cupping with moxibustion was shown to create a specific pattern in the shape of the cultural tool used.38 When footbaths with Chinese herbal remedies are performed frequently with high heat, they can lead to EAI on the feet with a linear border at the ankles. There also have been reports of kotatsu (heated tables in Japan) leading to lower-body EAI.39,40 These cultural practices also are more common in patients with darker skin types, which can lead to hyperpigmentation that is difficult to treat, making early diagnosis important.7
Medical History—Case reports have shown EAI caused by patients attempting to use heat-based methods for pain relief of an underlying serious disease such as cancer, bowel pathology (abdominal EAI), spinal disc prolapse (midline back EAI),41 sickle cell anemia, and renal pathology (posterior upper flank EAI).6,7,40-49 Patients with hypothyroidism or anorexia have been noted to have generalized EAI sparing the face secondary to repeated and extended hot baths or showers.50-53 One patient with schizophrenia was shown to have associated thermophilia due to a delusion that led the patient to soak in hot baths for long periods of time, leading to EAI.54 Finally, all physicians should be aware of iatrogenic causes of EAI, such as use of warming devices, ultrasound-based warming techniques, and laser therapy for lipolysis. Inquire about the patient’s surgical history or intensive care unit stays as well as alternative medicine or chiropractic visits. Obtaining a history of medical procedures can be enlightening when an etiology is not immediately clear.7,55,56
Diagnosis
Erythema ab igne is a clinical diagnosis based on recognizable cutaneous findings and a clear history of moderate heat exposure. However, when a clinical diagnosis of EAI is not certain (eg, when unable to obtain a clear history from the patient) or when malignant transformation is suspected, a biopsy can be performed. Pathologically, hematoxylin and eosin staining of EAI classically reveals dilated small vascular channels in the superficial dermis, hence a clinically reticular rash; interface dermatitis clinically manifesting as erythema; and pigment incontinence with melanin-laden macrophages consistent with clinical hyperpigmentation. Finally, for unclear reasons, increased numbers of elastic fibers classically are seen in biopsies of EAI.7
Differential Diagnosis
The differential diagnosis for a reticular patch includes livedo reticularis (Figure 3), which usually manifests as a more generalized rash in patients with chronic disease or coagulopathy such as systemic lupus erythematosus, cryoglobulinemia, or Raynaud phenomenon. When differentiating EAI from livedo reticularis or cutis marmorata, consider that both alternative diagnoses are more vascular appearing and are associated with cold exposure rather than heat exposure. In cases that are less reticular, livedo racemosa can be considered in the differential diagnosis. Finally, poikiloderma of Civatte can be reticular, particularly on dermoscopy, but the distribution on the neck with submental sparing should help to distinguish it from EAI unless a heat source around the neck is identified while taking the patient’s history.7

In babies, a reticular generalized rash is most likely to be cutis marmorata (Figure 4), which is a physiologic response to cold exposure that resolves with rewarming of the skin. A more serious condition—cutis marmorata telangiectatica congenita (Figure 5)—usually is present at birth, most frequently involves a single extremity, and notably does not resolve with rewarming. This is an important differential for EAI in children because it can be associated with vascular and neurologic anomalies as well as limb asymmetry. Finally, port-wine stains can sometimes be reticular in appearance and can mimic the early erythematous stages of EAI. However, unlike the erythematous stage of EAI, the port-wine stains will be present at birth.7

Emerging in 2020, an important differential diagnosis to consider is a cutaneous manifestation of COVID-19 infection. An erythematous, reticular, chilblainlike or transient livedo reticularis–like rash has been described as a cutaneous manifestation of COVID-19. Although the pathophysiology is still being elucidated, it is suspected that this is caused by a major vaso-occlusive crisis secondary to COVID-19–induced thrombotic vasculopathy. Interestingly, the majority of patients with this COVID-related exanthem also displayed symptoms of COVID-19 (eg, fever, cough) at the time of presentation,57-60 but there also have been cases in patients who were asymptomatic or mildly symptomatic.60

In some cases, EAI is an indication to screen for an underlying disease. For example, uncontrolled pain is an opportunity to improve interventions such as modifying the patient’s pain-control regimen, placing a palliative care pain consultation, or checking if the patient has had age-appropriate screenings for malignancy. New focal pain in a patient with a prior diagnosis of cancer may be a sign of a new metastasis. A thermophilic patient leaves opportunity to assess for underlying medical causes such as thyroid abnormalities or social/psychological issues. Geriatric patients who are diagnosed with EAI may need to be assessed for dementia or home safety issues. Patients with a history of diabetes mellitus can unknowingly develop EAI on the lower extremities, which may signal a need to assess the patient for peripheral neuropathy. Patients with gastroparesis secondary to diabetes also may develop EAI on the abdomen secondary to heating pad use for discomfort. These examples are a reminder to consider possible secondary comorbidities in all diagnoses of EAI.7
Prognosis
Although the prognosis of EAI is excellent if caught early, failure to diagnose this condition can lead to permanent discoloration of the skin and even malignancy.6 A rare sequela includes squamous cell carcinoma, most commonly seen in chronic cases of the lower leg, which is likely related to chronic inflammation of the skin.61-65 Rare cases of poorly differentiated carcinoma,66 cutaneous marginal zone lymphoma,67 and Merkel cell carcinoma68 have been reported. Patients diagnosed with EAI should receive normal periodic surveillance of the skin based on their medical history, though the physician should have an increased suspicion and plan for biopsy of any nodules or ulcerations found on the skin of the affected area.7
Treatments
Once the diagnosis of EAI is made, treatment starts with removal of the heat source causing the rash. Because the rash usually is asymptomatic, further treatment typically is not required. The discoloration can resolve over months or years, but permanent hyperpigmentation is not uncommon. If hyperpigmentation persists despite removal of the heat source and the patient desires further treatment for discoloration, there are few treatment options, none of which are approved by the US Food and Drug Administration for this condition.7 There is some evidence for the use of Nd:YAG lasers to reduce hyperpigmentation in EAI.69 There have been some reports of treatment using topical hydroquinone and topical tretinoin in an attempt to lighten the skin. If associated hyperkeratosis or other epithelial atypia is present, the use of 5-fluorouracil may show some improvement.70 One case report has been published of successful treatment with systemic mesoglycan and topical bioflavonoids.71 It also is conceivable that medications used to treat postinflammatory hyperpigmentation may be helpful in this condition (eg, kojic acid, arbutin, mild topical steroids, azelaic acid). Patients with darker skin may experience permanent discoloration and may not be good candidates for alternative treatments such as laser therapy due to the risk for inducible hyperpigmentation.7
Conclusion
No matter the etiology, EAI usually is a benign skin condition that is treated by removal of the causative heat source. Once a diagnosis is made, the clinician must work with the patient to determine the etiology. Care must be taken to ensure that there are no underlying signs, such as chronic pain or psychiatric illness, that could point to associated conditions. Rarely, sequalae such as cancers have been documented in areas of chronic EAI. Once the heat source is identified and removed, any remaining hyperpigmentation usually will self-resolve over months to years, though this may take longer in patients with darker skin types. If more aggressive treatment is preferred by the patient, laser therapy, topical medications, and oral over-the-counter vitamins have been tried with minimal responses.
- Perry. Case of erythema ab igne. Br J Dermatol. 1900;xxiii:375.
- Bose S, Ortonee JP. Diseases affected by heat. In: Parish LC, Millikan LE, Amer M, et al. Global Dermatology Diagnosis and Management According to Geography, Climate, and Culture. Springer-Varlag; 1994:83-92.
- Leal-Lobato MM, Blasco-Morente G. Electric blanket induced erythema ab igne [in Spanish]. Semergen. 2015;41:456-457. doi:10.1016/j.semerg.2014.12.008
- Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20. doi:10.5070/D32011024689
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Smith ML. Environmental and sports-related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1569-1594.
- Errichetti E, Stinco G. Dermoscopy in general dermatology: a practical overview. Dermatol Ther (Heidelb). 2016;6:471-507. doi:10.1007/s13555-016-0141-6
- Guarneri C, Tchernev G, Wollina U, et al. Erythema ab igne caused by laptop computer. Open Access Maced J Med Sci. 2017;5:490-492. doi:10.3889/oamjms.2017.137
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Dickman J, Kessler S. Unilateral reticulated patch localized to the anterior thigh. JAAD Case Rep. 2018;4:746-748. doi:10.1016/j.jdcr.2018.06.007
- Boffa MJ. Laptop computer-induced erythema ab igne on the left breast. Cutis. 2011;87:175-176.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Andersen F. Laptop-thighs--laptop-induced erythema ab igne [in Danish]. Ugeskr Laeger. 2010;172:635.
- Jagtman BA. Erythema ab igne due to a laptop computer. Contact Dermatitis. 2004;50:105. doi:10.1111/j.0105-1873.2004.0295g.x
- Olechowska M, Kisiel K, Ruszkowska L, et al. Erythema ab igne (EAI) induced by a laptop computer: report of two cases. J Dtsch Dermatol Ges. doi:10.1111/j.1610-0387.2014.12387
- Nayak SUK, Shenoi SD, Prabhu S. Laptop induced erythema ab igne. Indian J Dermatol. 2012;57:131-132. doi:10.4103/0019-5154.94284
- Salvio AG, Nunes AJ, Angarita DPR. Laptop computer induced erythema ab igne: a new presentation of an old disease. An Bras Dermatol. 2016;91:79-80. doi:10.1590/abd1806-4841.20165139
- Schummer C, Tittelbach J, Elsner P. Right-sided laptop dermatitis [in German]. Dtsch Med Wochenschr. 2015;140:1376-1377. doi:10.1055/s-0041-103615
- Manoharan D. Erythema ab igne: usual site, unusual cause. J Pharm Bioallied Sci. 2015;7(suppl 1):S74-S75. doi:10.4103/0975-7406.155811
- Giraldi S, Diettrich F, Abbage KT, et al. Erythema ab igne induced by a laptop computer in an adolescent. An Bras Dermatol. 2011;86:128-130. doi:10.1590/S0365-05962011000100018
- Secher LLS, Vind-Kezunovic D, Zachariae COC. Side-effects to the use of laptop computers: erythema ab igne. Dermatol Reports. 2010;31:E11. doi:10.4081/dr.2010.e11
- Botten D, Langley RGB, Webb A. Academic branding: erythema ab igne and use of laptop computers. CMAJ. 2010;182:E857. doi:10.1503/cmaj.091868
- Bilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974. doi:10.1016/j.jaad.2003.08.007
- Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming - a case report. Int J Dermatol. 2012;51:716-717. doi:10.1111/j.1365-4632.2011.05033.x
- Levinbook WS, Mallett J, Grant-Kels JM. Laptop computer-associated erythema ab igne. Cutis. 2007;80:319-320.
- Mohr MR, Scott KA, Pariser RM, et al. Laptop computer-induced erythema ab igne: a case report. Cutis. 2007;79:59-60.
- Cantor AS, Bartling SJ. Laptop computer-induced hyperpigmentation. Dermatol Online J. 2018;24:13030/qt6k37r9wm.
- Kaptanog˘lu AF, Mullaaziz D. Erythema ab igne in the palmar area induced by smart phone: case report. Turkiye Klin J Med Sci. 2015;35:284-286. doi:10.5336/medsci.2015-46976
- Redding KS, Watts AN, Lee J, et al. Space heater-induced bullous erythema ab igne. Cutis. 2017;100:E9-E10.
- Goorland J, Edens MA, Baudoin TD. An emergency department presentation of erythema ab igne caused by repeated heater exposure. J La State Med Soc. 2016;168:33-34.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Brzezinski P, Ismail S, Chiriac A. Radiator-induced erythema ab igne in 8-year-old girl. Rev Chil Pediatr. 2014;85:239-240. doi:10.4067/S0370-41062014000200015
- Adams BB. Heated car seat-induced erythema ab igne. Arch Dermatol. 2012;148:265-266. doi:10.1001/archdermatol.2011.2207
- Helm TN, Spigel GT, Helm KF. Erythema ab igne caused by a car heater. Cutis. 1997;59:81-82.
- Gregory JF, Beute TC. Erythema ab igne. J Spec Oper Med. 2013;13:115-119. doi:10.55460/5AVH-NZHY
- Chua S, Chen Q, Lee HY. Erythema ab igne and dermal scarring caused by cupping and moxibustion treatment. J Dtsch Dermatol Ges. 2015;13:337-338. doi:10.1111/ddg.12581
- Chen JF, Liu YC, Chen YF, et al. Erythema ab igne after footbath with Chinese herbal remedies. J Chinese Med Assoc. 2011;74:51-53. doi:10.1016/j.jcma.2011.01.009
- Baltazar D, Brockman R, Simpson E. Kotatsu-induced erythema ab igne. An Bras Dermatol. 2019;94:253-254. doi:10.1590/abd1806-4841.20198792
- Baig M, Byrne F. Erythema ab igne and its relation to spinal pathology. Cureus. 2018;10:e2914. doi:10.7759/cureus.2914
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:e2635. doi:10.7759/cureus.2635
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;1862480. doi:10.1155/2016/1862480
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:e236-e238. doi:10.1097/PEC.0000000000001460
- Dizdarevic A, Karim OA, Bygum A. A reddish brown reticulated hyperpigmented erythema on the abdomen of a girl. Erythema ab igne, also known as toasted skin syndrome, caused by a heating pad onthe abdomen. Acta Derm Venereol. 2014;94:365-367. doi:10.2340/00015555-1722
- Chatterjee S. Erythema ab igne from prolonged use of a heating pad. Mayo Clin Proc. 2005;80:1500. doi:10.4065/80.11.1500
- Waldorf DS, Rast MF, Garofalo VJ. Heating-pad erythematous dermatitis “erythema ab igne.” JAMA. 1971;218:1704. doi:10.1001/jama.1971.03190240056023
- South AM, Crispin MK, Marqueling AL, et al. A hyperpigmented reticular rash in a patient on peritoneal dialysis. Perit Dial Int. 2016;36:677-700. doi:10.3747/pdi.2016.00042
- Ravindran R. Erythema ab igne in an individual with diabetes and gastroparesis. BMJ Case Rep. 2017;2017:bcr2014203856. doi:10.1136/bcr-2014-203856
- Dessinioti C, Katsambas A, Tzavela E, et al. Erythema ab igne in three girls with anorexia nervosa. Pediatr Dermatol. 2016;33:e149-e150. doi:10.1111/pde.12770
- Fischer J, Rein K, Erfurt-Berge C, et al. Three cases of erythema ab igne (EAI) in patients with eating disorders. Neuropsychiatr. 2010;24:141-143.
- Docx MKF, Simons A, Ramet J, et al. Erythema ab igne in an adolescent with anorexia nervosa. Int J Eat Disord. 2013;46:381-383. doi:10.1002/eat.22075
- Turan E, Cimen V, Haytoglu NSK, et al. A case of bullous erythema ab igne accompanied by anemia and subclinical hypothyroidism. Dermatol Online J. 2014;20:223366.
- Pavithran K. Erythema ab igne, schizophrenia and thermophilia. Indian J Dermatol Venereol Leprol. 1987;53:181-182.
- Dellavelle R, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Park SY, Kim SM, Yoon TJ. Erythema ab igne caused by weight loss heating pad. Korean J Dermatol. 2007;45:489-491.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Gisondi P, Plaserico S, Bordin C, et al. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020;34:2499-2504. doi:10.1111/jdv.16774
- Manalo IF, Smith MK, Cheeley J, et al. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83:700. doi:10.1016/j.jaad.2020.04.018
- Zhao Q, Fang X, Pang Z, et al. COVID‐19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:2505-2510. doi:10.1111/jdv.16778
- Akasaka T, Kon S. Two cases of squamous cell carcinoma arising from erythema ab igne. Nihon Hifuka Gakkai Zasshi. 1989;99:735-742.
- Arrington JH 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. Arch Dermatol. 1979;115:1226-1228.
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Wollina U, Helm C, Hansel G, et al. Two cases of erythema ab igne, one with a squamous cell carcinoma. G Ital Dermatol Venereol. 2007;142:415-418.
- Rudolph CM, Soyer HP, Wolf P, et al. Squamous cell carcinoma arising in erythema ab igne. Hautarzt. 2000;51:260-263. doi:10.1007/s001050051115
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Kim HW, Kim EJ, Park HC, et al. Erythema ab igne successfully treated with low fluenced 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser. J Cosmet Laser Ther. 2014;16:147-148. doi:10.3109/14764172.2013.854623
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;62:77-78.
- Gianfaldoni S, Gianfaldoni R, Tchernev G, et al. Erythema ab igne successfully treated with mesoglycan and bioflavonoids: a case-report. Open Access Maced J Med Sci. 2017;5:432-435. doi:10.3889/oamjms.2017.123
- Perry. Case of erythema ab igne. Br J Dermatol. 1900;xxiii:375.
- Bose S, Ortonee JP. Diseases affected by heat. In: Parish LC, Millikan LE, Amer M, et al. Global Dermatology Diagnosis and Management According to Geography, Climate, and Culture. Springer-Varlag; 1994:83-92.
- Leal-Lobato MM, Blasco-Morente G. Electric blanket induced erythema ab igne [in Spanish]. Semergen. 2015;41:456-457. doi:10.1016/j.semerg.2014.12.008
- Huynh N, Sarma D, Huerter C. Erythema ab igne: a case report and review of the literature. Cutis. 2011;88:290-292.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20. doi:10.5070/D32011024689
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Smith ML. Environmental and sports-related skin diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1569-1594.
- Errichetti E, Stinco G. Dermoscopy in general dermatology: a practical overview. Dermatol Ther (Heidelb). 2016;6:471-507. doi:10.1007/s13555-016-0141-6
- Guarneri C, Tchernev G, Wollina U, et al. Erythema ab igne caused by laptop computer. Open Access Maced J Med Sci. 2017;5:490-492. doi:10.3889/oamjms.2017.137
- Arnold AW, Itin PH. Laptop computer-induced erythema ab igne in a child and review of the literature. Pediatrics. 2010;126:E1227-E1230. doi:10.1542/peds.2010-1390
- Dickman J, Kessler S. Unilateral reticulated patch localized to the anterior thigh. JAAD Case Rep. 2018;4:746-748. doi:10.1016/j.jdcr.2018.06.007
- Boffa MJ. Laptop computer-induced erythema ab igne on the left breast. Cutis. 2011;87:175-176.
- Li K, Barankin B. Cutaneous manifestations of modern technology use. J Cutan Med Surg. 2011;15:347-353. doi:10.2310/7750.2011.10053
- Riahi RR, Cohen PR. Laptop-induced erythema ab igne: report and review of literature. Dermatol Online J. 2012;18:5.
- Andersen F. Laptop-thighs--laptop-induced erythema ab igne [in Danish]. Ugeskr Laeger. 2010;172:635.
- Jagtman BA. Erythema ab igne due to a laptop computer. Contact Dermatitis. 2004;50:105. doi:10.1111/j.0105-1873.2004.0295g.x
- Olechowska M, Kisiel K, Ruszkowska L, et al. Erythema ab igne (EAI) induced by a laptop computer: report of two cases. J Dtsch Dermatol Ges. doi:10.1111/j.1610-0387.2014.12387
- Nayak SUK, Shenoi SD, Prabhu S. Laptop induced erythema ab igne. Indian J Dermatol. 2012;57:131-132. doi:10.4103/0019-5154.94284
- Salvio AG, Nunes AJ, Angarita DPR. Laptop computer induced erythema ab igne: a new presentation of an old disease. An Bras Dermatol. 2016;91:79-80. doi:10.1590/abd1806-4841.20165139
- Schummer C, Tittelbach J, Elsner P. Right-sided laptop dermatitis [in German]. Dtsch Med Wochenschr. 2015;140:1376-1377. doi:10.1055/s-0041-103615
- Manoharan D. Erythema ab igne: usual site, unusual cause. J Pharm Bioallied Sci. 2015;7(suppl 1):S74-S75. doi:10.4103/0975-7406.155811
- Giraldi S, Diettrich F, Abbage KT, et al. Erythema ab igne induced by a laptop computer in an adolescent. An Bras Dermatol. 2011;86:128-130. doi:10.1590/S0365-05962011000100018
- Secher LLS, Vind-Kezunovic D, Zachariae COC. Side-effects to the use of laptop computers: erythema ab igne. Dermatol Reports. 2010;31:E11. doi:10.4081/dr.2010.e11
- Botten D, Langley RGB, Webb A. Academic branding: erythema ab igne and use of laptop computers. CMAJ. 2010;182:E857. doi:10.1503/cmaj.091868
- Bilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol. 2004;50:973-974. doi:10.1016/j.jaad.2003.08.007
- Fu LW, Vender R. Erythema ab igne caused by laptop computer gaming - a case report. Int J Dermatol. 2012;51:716-717. doi:10.1111/j.1365-4632.2011.05033.x
- Levinbook WS, Mallett J, Grant-Kels JM. Laptop computer-associated erythema ab igne. Cutis. 2007;80:319-320.
- Mohr MR, Scott KA, Pariser RM, et al. Laptop computer-induced erythema ab igne: a case report. Cutis. 2007;79:59-60.
- Cantor AS, Bartling SJ. Laptop computer-induced hyperpigmentation. Dermatol Online J. 2018;24:13030/qt6k37r9wm.
- Kaptanog˘lu AF, Mullaaziz D. Erythema ab igne in the palmar area induced by smart phone: case report. Turkiye Klin J Med Sci. 2015;35:284-286. doi:10.5336/medsci.2015-46976
- Redding KS, Watts AN, Lee J, et al. Space heater-induced bullous erythema ab igne. Cutis. 2017;100:E9-E10.
- Goorland J, Edens MA, Baudoin TD. An emergency department presentation of erythema ab igne caused by repeated heater exposure. J La State Med Soc. 2016;168:33-34.
- Kokturk A, Kaya TI, Baz K, et al. Bullous erythema ab igne. Dermatol Online J. 2003;9:18.
- Brzezinski P, Ismail S, Chiriac A. Radiator-induced erythema ab igne in 8-year-old girl. Rev Chil Pediatr. 2014;85:239-240. doi:10.4067/S0370-41062014000200015
- Adams BB. Heated car seat-induced erythema ab igne. Arch Dermatol. 2012;148:265-266. doi:10.1001/archdermatol.2011.2207
- Helm TN, Spigel GT, Helm KF. Erythema ab igne caused by a car heater. Cutis. 1997;59:81-82.
- Gregory JF, Beute TC. Erythema ab igne. J Spec Oper Med. 2013;13:115-119. doi:10.55460/5AVH-NZHY
- Chua S, Chen Q, Lee HY. Erythema ab igne and dermal scarring caused by cupping and moxibustion treatment. J Dtsch Dermatol Ges. 2015;13:337-338. doi:10.1111/ddg.12581
- Chen JF, Liu YC, Chen YF, et al. Erythema ab igne after footbath with Chinese herbal remedies. J Chinese Med Assoc. 2011;74:51-53. doi:10.1016/j.jcma.2011.01.009
- Baltazar D, Brockman R, Simpson E. Kotatsu-induced erythema ab igne. An Bras Dermatol. 2019;94:253-254. doi:10.1590/abd1806-4841.20198792
- Baig M, Byrne F. Erythema ab igne and its relation to spinal pathology. Cureus. 2018;10:e2914. doi:10.7759/cureus.2914
- Aria AB, Chen L, Silapunt S. Erythema ab igne from heating pad use: a report of three clinical cases and a differential diagnosis. Cureus. 2018;10:e2635. doi:10.7759/cureus.2635
- Milchak M, Smucker J, Chung CG, et al. Erythema ab igne due to heating pad use: a case report and review of clinical presentation, prevention, and complications. Case Rep Med. 2016;1862480. doi:10.1155/2016/1862480
- Gmuca S, Yu J, Weiss PF, et al. Erythema ab igne in an adolescent with chronic pain: an alarming cutaneous eruption from heat exposure. Pediatr Emerg Care. 2020;36:e236-e238. doi:10.1097/PEC.0000000000001460
- Dizdarevic A, Karim OA, Bygum A. A reddish brown reticulated hyperpigmented erythema on the abdomen of a girl. Erythema ab igne, also known as toasted skin syndrome, caused by a heating pad onthe abdomen. Acta Derm Venereol. 2014;94:365-367. doi:10.2340/00015555-1722
- Chatterjee S. Erythema ab igne from prolonged use of a heating pad. Mayo Clin Proc. 2005;80:1500. doi:10.4065/80.11.1500
- Waldorf DS, Rast MF, Garofalo VJ. Heating-pad erythematous dermatitis “erythema ab igne.” JAMA. 1971;218:1704. doi:10.1001/jama.1971.03190240056023
- South AM, Crispin MK, Marqueling AL, et al. A hyperpigmented reticular rash in a patient on peritoneal dialysis. Perit Dial Int. 2016;36:677-700. doi:10.3747/pdi.2016.00042
- Ravindran R. Erythema ab igne in an individual with diabetes and gastroparesis. BMJ Case Rep. 2017;2017:bcr2014203856. doi:10.1136/bcr-2014-203856
- Dessinioti C, Katsambas A, Tzavela E, et al. Erythema ab igne in three girls with anorexia nervosa. Pediatr Dermatol. 2016;33:e149-e150. doi:10.1111/pde.12770
- Fischer J, Rein K, Erfurt-Berge C, et al. Three cases of erythema ab igne (EAI) in patients with eating disorders. Neuropsychiatr. 2010;24:141-143.
- Docx MKF, Simons A, Ramet J, et al. Erythema ab igne in an adolescent with anorexia nervosa. Int J Eat Disord. 2013;46:381-383. doi:10.1002/eat.22075
- Turan E, Cimen V, Haytoglu NSK, et al. A case of bullous erythema ab igne accompanied by anemia and subclinical hypothyroidism. Dermatol Online J. 2014;20:223366.
- Pavithran K. Erythema ab igne, schizophrenia and thermophilia. Indian J Dermatol Venereol Leprol. 1987;53:181-182.
- Dellavelle R, Gillum P. Erythema ab igne following heating/cooling blanket use in the intensive care unit. Cutis. 2000;66:136-138.
- Park SY, Kim SM, Yoon TJ. Erythema ab igne caused by weight loss heating pad. Korean J Dermatol. 2007;45:489-491.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Gisondi P, Plaserico S, Bordin C, et al. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020;34:2499-2504. doi:10.1111/jdv.16774
- Manalo IF, Smith MK, Cheeley J, et al. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83:700. doi:10.1016/j.jaad.2020.04.018
- Zhao Q, Fang X, Pang Z, et al. COVID‐19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:2505-2510. doi:10.1111/jdv.16778
- Akasaka T, Kon S. Two cases of squamous cell carcinoma arising from erythema ab igne. Nihon Hifuka Gakkai Zasshi. 1989;99:735-742.
- Arrington JH 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. Arch Dermatol. 1979;115:1226-1228.
- Wharton JB, Sheehan DJ, Lesher JL Jr. Squamous cell carcinoma in situ arising in the setting of erythema ab igne. J Drugs Dermatol. 2008;7:488-489.
- Wollina U, Helm C, Hansel G, et al. Two cases of erythema ab igne, one with a squamous cell carcinoma. G Ital Dermatol Venereol. 2007;142:415-418.
- Rudolph CM, Soyer HP, Wolf P, et al. Squamous cell carcinoma arising in erythema ab igne. Hautarzt. 2000;51:260-263. doi:10.1007/s001050051115
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Wharton J, Roffwarg D, Miller J, et al. Cutaneous marginal zone lymphoma arising in the setting of erythema ab igne. J Am Acad Dermatol. 2010;62:1080-1081. doi:10.1016/j.jaad.2009.08.005
- Jones CS, Tyring SK, Lee PC, et al. Development of neuroendocrine (Merkel cell) carcinoma mixed with squamous cell carcinoma in erythema ab igne. Arch Dermatol. 1988;124:110-113.
- Kim HW, Kim EJ, Park HC, et al. Erythema ab igne successfully treated with low fluenced 1,064-nm Q-switched neodymium-doped yttrium aluminum garnet laser. J Cosmet Laser Ther. 2014;16:147-148. doi:10.3109/14764172.2013.854623
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;62:77-78.
- Gianfaldoni S, Gianfaldoni R, Tchernev G, et al. Erythema ab igne successfully treated with mesoglycan and bioflavonoids: a case-report. Open Access Maced J Med Sci. 2017;5:432-435. doi:10.3889/oamjms.2017.123
Practice Points
- Erythema ab igne (EAI) is a skin condition caused by chronic exposure to heat; removal of the heat source often will result in self-resolution of the rash.
- Erythema ab igne can be a sign of underlying illness in patients self-treating chronic pain with application of heat.
- Recognition and discontinuation of the exposure with close observation are key components in the treatment of EAI.