User login
Multi-cancer early detection liquid biopsy testing: A predictive genetic test not quite ready for prime time
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?
The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
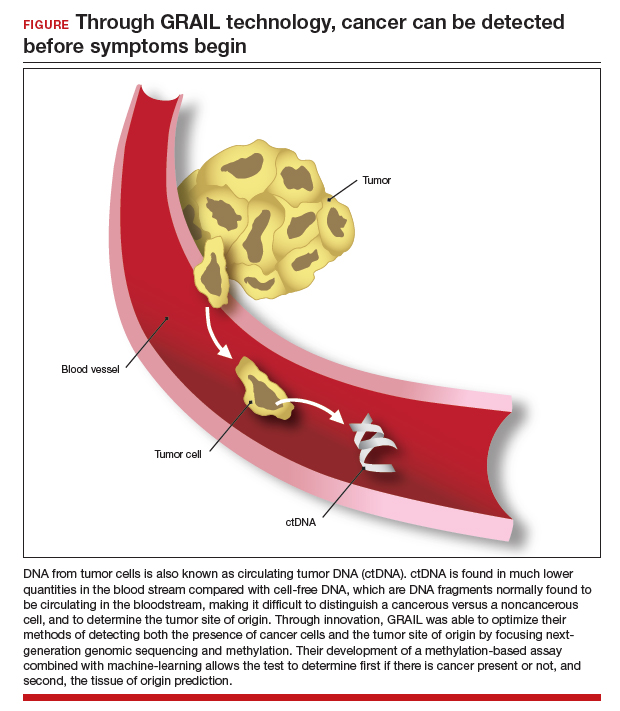
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
CASE Patient inquires about new technology to detect cancer
A 51-year-old woman (para 2) presents to your clinic for a routine gynecology exam. She is up to date on her screening mammogram and Pap testing. She has her first colonoscopy scheduled for next month. She has a 10-year remote smoking history, but she stopped smoking in her late twenties. Her cousin was recently diagnosed with skin cancer, her father had prostate cancer and is now in remission, and her paternal grandmother died of ovarian cancer. She knows ovarian cancer does not have an effective screening test, and she recently heard on the news about a new blood test that can detect cancer before symptoms start. She would like to know more about this test. Could it replace her next Pap, mammogram, and future colonoscopies? She also wants to know—How can a simple blood test detect cancer?

The power of genomics in cancer care
Since the first human genome was sequenced in 2000, the power of genomics has been evident across many aspects of medicine, including cancer care.1 Whereas the first human genome to be sequenced took more than 10 years to sequence and cost over $1 billion, sequencing of your entire genome can now be obtained for less than $400—with results in a week.2
Genomics is now an integral part of cancer care, with results having implications for both cancer risk and prevention as well as more individualized treatment. For example, a healthy 42-year-old patient with a strong family history of breast cancer may undergo genetic testing and discover she has a mutation in the tumor suppression gene BRCA1, which carries a 39% to 58% lifetime risk of ovarian cancer.3 By undergoing a risk-reducing bilateral salpingooophorectomy she will lower her ovarian cancer risk by up to 96%.4,5 A 67-year-old with a new diagnosis of stage III ovarian cancer and a BRCA2 mutation may be in remission for 5+ years due to her BRCA2 mutation, which makes her eligible for the use of the poly(ADPribose) polymerase (PARP) inhibitor olaparib.6 Genetic testing as illustrated above has led to decreased cancer-related mortality and prolonged survival.7 However, many women with such germline mutations are faced with difficult choices about surgical risk reduction, with the potential harms of early menopause and quality of life concerns. Having a test that does not just predict cancer risk but in fact quantifies that risk for the individual would greatly help in these decisions. Furthermore, more than 75% of ovarian cancers occur without a germline mutation.
Advances in genetic testing technology also have led to the ability to obtain genetic information from a simple blood test. For example, cell-free DNA (cfDNA), which is DNA fragments that are normally found to be circulating in the bloodstream, is routinely used as a screening tool for prenatal genetic testing to detect chromosomal abnormalities in the fetus.8 This technology relies on analyzing fetal free (non-cellular) DNA that is naturally found circulating in maternal blood. More recently, similar technology using cfDNA has been applied for the screening and characterization of certain cancers.9 This powerful technology can detect cancer before symptoms begin—all from a simple blood test, often referred to as a “liquid biopsy.” However, understanding the utility, supporting data, and target population for these tests is important before employing them as part of routine clinical practice.
Continue to: Current methods of cancer screening are limited...
Current methods of cancer screening are limited
Cancer is a leading cause of death worldwide, with nearly 10 million cancer-related deaths annually, and it may surpass cardiovascular disease as the leading cause over the course of the century.10,11 Many cancer deaths are in part due to late-stage diagnosis, when the cancer has already metastasized.12 Early detection of cancer improves outcomes and survival rates, but it is often difficult to detect early due to the lack of early symptoms with many cancers, which can limit cancer screening and issues with access to care.13
Currently, there are only 5 cancers: cervical, prostate, breast, colon, and lung (for high-risk adults) that are screened for in the general population (see "Cancer screening has helped save countless lives" at the end of this article).14 The Pap test to screen for cervical cancer, developed in the 1940s, has saved millions of women’s lives and reduced the mortality of cervical cancer by 70%.15 Coupled with the availability and implementation of the human papillomavirus (HPV) vaccine, cervical cancer rates are decreasing at substantial rates.16 However, there are no validated screening tests for uterine cancer, the most common gynecologic malignancy in the United States, or ovarian cancer, the most lethal.
Screening tests for cervical, prostate, breast, colon, and lung cancer have helped save millions of lives; however, these tests also come with high false-positive rates and the potential for overdiagnosis and overtreatment. For example, half of women undergoing mammograms will receive a false-positive result over a 10-year time period,17 and up to 50% of men undergoing prostate cancer screening have a positive prostate-specific antigen (PSA) test result when they do not actually have prostate cancer.18 Additionally, the positive predictive value of the current standard-of-care screening tests can be as low as <5%. Most diagnoses of cancer are made from a surgical biopsy, but these types of procedures can be difficult depending on the location or size of the tumor.19
The liquid biopsy. Given the limitations of current cancer screening and diagnostic tests, there is a great need for a more sensitive test that also can detect cancer from multiple organ sites. Liquid biopsy-based biomarkers can include circulating tumor cells, exosomes, microRNAs, and circulating tumor DNA (ctDNA). With advances in next-generation sequencing, ctDNA techniques remain the most promising.20
Methylation-based MCED testing: A new way of cancer screening
Multi-cancer early detection (MCED) technology was developed to address the need for better cancer screening and has the potential to detect up to 50 cancers with a simple blood test. This new technology opens the possibility for early detection of multiple cancers before symptoms even begin. MCED testing is sometimes referred to as “GRAIL” testing, after the American biotechnology company that developed the first commercially available MCED test, called the Galleri test (Galleri, Menlo Park, California). Although other biotechnology companies are developing similar technology (Exact Sciences, Madison, Wisconsin, and Freenome, South San Francisco, California, for example), this is the first test of its kind available to the public.21
The MCED test works by detecting the cfDNA fragments that are released into the blood passively by necrotic or apoptotic cells or secreted actively from tumor cells. The DNA from tumor cells is also known as circulating tumor DNA (ctDNA). CtDNA is found in much lower quantities in the blood stream compared with cfDNA from cells, making it difficult to distinguish a cancer versus a noncancer cell and to determine the tumor site of origin.22
Through innovation, the first example of detecting cancer through this method in fact came as a surprise result from an abnormal cfDNA test. A pregnant 37-yearold woman had a cfDNA result suggestive of aneuploidy for chromosomes 18 and 13; however, she gave birth to a normal male fetus. Shortly thereafter, a vaginal biopsy confirmed small-cell carcinoma with alterations in chromosomes 18 and 13.23 GRAIL testing for this patient was subsequently able to optimize their methods of detecting both the presence of cancer cells and the tumor site of origin by utilizing next-generation genomic sequencing and methylation. Their development of a methylation-based assay combined with 46 machine-learning allowed the test to determine, first, if there is cancer present or not, and second, the tissue of origin prediction. It is important to note that these tests are meant to be used in addition to standard-of-care screening tests, not as an alternative, and this is emphasized throughout the company’s website and the medical literature.24
Continue to: The process to develop and validate GRAIL’s blood-based cancer screening test...
The process to develop and validate GRAIL’s blood-based cancer screening test includes 4 large clinical trials of more than 180,000 participants, including those with cancer and those without. The Circulating Cell-Free Genome Atlas (CCGA) Study, was a prospective, case-controlled, observational study enrolling approximately 15,000 participants with 3 prespecified sub-studies. The first sub-study developed the machine-learning classifier for both early detection and tumor of origin detection.25,26
The highest performing assay from the first sub-study then went on to be further validated in the 2nd and 3rd sub-studies. The 3rd sub-study, published in the Annals of Oncology in 2021 looked at a cohort of 4,077 participants with and without cancer, and found the specificity of cancer signal detection to be 99.5% and the overall sensitivity to be 51.5%, with increasing sensitivity by cancer stage (stage I - 17%, stage II - 40%, stage III - 77%, and stage IV - 90.1%).24 The false-positive rate was low, at 0.7%, and the true positive rate was 88.7%. Notably, the test was able to correctly identify the tumor of origin for 93% of samples.24 The study overall demonstrated high specificity and accuracy of tumor site of origin and supported the use of this blood-based MCED assay.
The PATHFINDER study was another prospective, multicenter clinical trial that enrolled more than 6,000 participants in the United States. The participants were aged >50 years with or without additional cancer risk factors. The goal of this study was to determine the extent of testing required to achieve diagnosis after a “cancer signal detected” result. The study results found that, when MCED testing was added to the standard-of-care screening, the number of cancers detected doubled when compared with standard cancer screening alone.27,28 Of the 92 participants with positive cancer signals, 35 were diagnosed with cancer, and 71% of these cancer types did not have standard-ofcare screening. The tumor site of origin was correctly detected in 97% of cases, and there were less than 1% of false positives. Overall, the test led to diagnostic evaluation of 1.4% of patients and a cancer diagnosis in 0.5%.
Currently, there are 2 ongoing clinical trials to further evaluate the Galleri MCED test. The STRIVE trial that aims to prospectively validate the MCED test in a population of nearly 100,000 women undergoing mammography,29 and the SUMMIT trial,30 which is similarly aiming to validate the test in a group of individuals, half of whom have a significantly elevated risk of lung cancer.
With the promising results described above, the Galleri test became the first MCED test available for commercial use starting in 2022. It is only available for use in people who are aged 50 and older, have a family history of cancer, or are at an increased risk for cancer (although GRAIL does not elaborate on what constitutes increased risk). However, the Galleri test is only available through prescription—therefore, if interested, patients must ask their health care provider to register with GRAIL and order the test (https://www .galleri.com/hcp/the-galleri-test/ordering). Additionally, the test will cost the patient $949 and is not yet covered by insurances. Currently, several large health care groups such as the United States Department of Veterans Affairs, Cleveland Clinic, and Mercy hospitals have partnered with GRAIL to offer their test to certain patients for use as part of clinical trials. Currently, no MCED test, including the Galleri, is approved by the US Food and Drug Administration.
Incorporating MCED testing into clinical practice
The Galleri MCED test has promising potential to make multi-cancer screening feasible and obtainable, which could ultimately reduce late-stage cancer diagnosis and decrease mortality from all cancers. The compelling data from large cohorts and numerous clinical trials demonstrate its accuracy, reliability, reproducibility, and specificity. It can detect up to 50 different types of cancers, including cancers that affect our gynecologic patients, including breast, cervical, ovarian, and uterine. Additionally, its novel methylation-based assay accurately identifies the tumor site of origin in 97% of cases.28 Ongoing and future clinical trials will continue to validate and refine these methods and improve the sensitivity and positive-predictive value of this assay. As mentioned, although it has been incorporated into various large health care systems, it is not FDA approved and has not been validated in the general population. Additionally, it should not be used as a replacement for recommended screening.
CASE Resolved
The patient is eligible for the Galleri MCED test if ordered by her physician. However, she will need to pay for the test out-of-pocket. Due to her family history, she should consider germline genetic testing (either for herself, or if possible, for her father, who should meet criteria based on his prostate cancer).3 Panel testing for germline mutations has become much more accessible, and until MCED testing is ready for prime time, it remains one of the best ways to predict and prevent cancers. Additionally, she should continue to undergo routine screening for cervical, breast, and colon cancer as indicated. ●
- Mammography has helped reduce breast cancer mortality in the United States by nearly 40% since 19901
- Increases in screening for lung cancer with computed tomography in the United States are estimated to have saved more than 10,000 lives between 2014 and 20182
- Routine prostate specific antigen screening is no longer recommended for men at average risk for prostate cancer, and patients are advised to discuss risks and benefits of screening with their clinicians3
- Where screening programs have long been established, cervical cancer rates have decreased by as much as 65% over the past 40 years4
- 68% of colorectal cancer deaths could be prevented with increased screening, and one of the most effective ways to get screened is colonoscopy5
References
1. American College of Radiology website. https://www.acr.org/Practice-Management-Quality-Informatics/Practice-Toolkit/PatientResources/Mammography-Saves-Lives. Accessed March 1, 2023.
2. US lung cancer screening linked to earlier diagnosis and better survival. BMJ.com. https://www.bmj.com/company/newsroom/ us-lung-cancer-screening-linked-to-earlier-diagnosis-and-better-survival/. Accessed March 1, 2023.
3. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374-383.
4. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Can J Clinicians. 2015;65:87-108.
5. Colon cancer coalition website. Fact check: Do colonoscopies save lives? https://coloncancercoalition.org/2022/10/11/fact-checkdo-colonoscopies-save-lives/#:~:text=According%20to%20the%20Centers%20for,get%20screened%20is%20a%20colonoscopy. Accessed March 1, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
- Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724.
- Davies K. The era of genomic medicine. Clin Med (Lond). 2013;13:594-601.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 3.2023. February 13, 2023.
- Finch APM, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Xiao Y-L, Wang K, Liu Q, et al. Risk reduction and survival benefit of risk-reducing salpingo-oophorectomy in hereditary breast cancer: meta-analysis and systematic review. Clin Breast Cancer. 2019;19:e48-e65.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Pritchard D, Goodman C, Nadauld LD. Clinical utility of genomic testing in cancer care. JCO Precis Oncol. 2022;6:e2100349.
- Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin summary, number 226. Obstet Gynecol. 2020;136:859-867.
- Yan Y-y, Guo Q-r, Wang F-h, et al. Cell-free DNA: hope and potential application in cancer. Front Cell Dev Biol. 2021;9.
- Bray F, Laversanne M, Weiderpass E, et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71:209-249.
- Hawkes N. Cancer survival data emphasize importance of early diagnosis. BMJ. 2019;364:408.
- Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92-S107.
- Centers for Disease Control and Prevention. Screening tests. https://www.cdc.gov/cancer/dcpc/prevention/screening. htm#print. Reviewed May 19, 2022. Accessed March 1, 2023.
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930–1998. Cancer. 2003;97:3133-3275.
- Liao CI, Franceur AA, Kapp DS, et al. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001-2017. JAMA Netw Open. 2022;5:e222530. doi:10.1001/ jamanetworkopen.2022.2530.
- Ho T-QH, Bissell MCS, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Network Open. 2022;5:e222440.
- Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883-895.
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112-123.
- Dominguez-Vigil IG, Moreno-Martinez AK, Wang JY, et al. The dawn of the liquid biopsy in the fight against cancer. Oncotarget. 2018; 9:2912–2922. doi: 10.18632/ oncotarget.23131.
- GRAIL. https://grail.com/. Accessed March 1, 2023.
- Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548.
- Osborne CM, Hardisty E, Devers P, et al. Discordant noninvasive prenatal testing results in a patient subsequently diagnosed with metastatic disease. Prenat Diagn. 2013;33:609-611.
- Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncology. 2021;32:1167-1177.
- Li B, Wang C, Xu J, et al. Abstract A06: multiplatform analysis of early-stage cancer signatures in blood. Clin Cancer Res. 2020;26(11 supplement):A06-A.
- Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583.
- Nadauld LD, McDonnell CH 3rd, Beer TM, et al. The PATHFINDER Study: assessment of the implementation of an investigational multi-cancer early detection test into clinical practice. Cancers (Basel). 2021;13.
- Klein EA. A prospective study of a multi-cancer early detection blood test in a clinical practice setting. Abstract presented at ESMO conference; Portland, OR. October 18, 2022.
- The STRIVE Study: development of a blood test for early detection of multiple cancer types. https://clinicaltrials.gov /ct2/show/NCT03085888. Accessed March 2, 2023.
- The SUMMIT Study: a cancer screening study (SUMMIT). https://clinicaltrials.gov/ct2/show/NCT03934866. Accessed March 2, 2023.
CarePostRoe.com: Study seeks to document poor quality medical care due to new abortion bans
In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.

In June 2022, the US Supreme Court’s decision in Dobbs v Jackson Women’s Health Organization removed federal protections for abortion that previously had been codified in Roe v Wade. Since this removal, most abortions have been banned in at least 13 states, and about half of states are expected to attempt to ban or heavily restrict abortion.1,2 These laws banning abortion are having effects on patient care far beyond abortion, leading to uncertainty and fear among providers and denied or delayed care for patients.3,4 It is critical that research documents the harmful effects of this policy change.
Patients that are pregnant with fetuses with severe malformations have had to travel long distances to other states to obtain care.5 Others have faced delays in obtaining treatment for ectopic pregnancy, miscarriage, and even for other conditions that use medications that could potentially cause an abortion.6,7 These cases have the potential to result in serious harm or death of the patient with altered care. There is a published report from Texas showing how the change in practice due to the 6-week abortion ban imposed in 2021 was associated with a doubling of severe morbidity for patients presenting with preterm premature rupture of membranes and other complications before 22 weeks’ gestation.8
While these cases have been highlighted in the media, there has not been a resource that comprehensively documents the changes in care that clinicians have been forced to make because of abortion bans as well as the consequences for their patients’ health. The media also may not be the most desirable platform for sharing cases of substandard care if providers feel their confidentiality may be breached as they are told by their employers to avoid speaking with reporters.9 Bearing this in mind, our team of researchers at Advancing New Standards in Reproductive Health at the University of California San Francisco and the Texas Policy Evaluation Project at the University of Texas at Austin has launched a project aiming to collect stories of poor quality care post-Roe from health care professionals across the United States. The aim of the study is to document examples of the challenges in patient care that have arisen since the Dobbs decision.
The study website CarePostRoe.com was launched in October 2022 to collect narratives from health care providers who participated in the care of a patient whose management was different from the usual standard due to a need to comply with new restrictions on abortion since the Dobbs decision. These providers can include physicians, nurses, nurse practitioners, midwives, physician assistants, social workers, pharmacists, psychologists, or other allied health professionals. Clinicians can share information about a case through a brief survey linked on the website that will allow them to either submit a written narrative or a voice memo. The submissions are anonymous, and providers are not asked to submit any protected health information. If the submitter would like to share more information about the case via telephone interview, they will be taken to a separate survey which is not linked to the narrative submission to give contact information to participate in an interview.
Since October, more than 40 cases have been submitted that document patient cases from over half of the states with abortion bans. Clinicians describe pregnant patients with severe fetal malformations who have had to overcome financial and logistical barriers to travel to access abortion care. Several cases of patients with cesarean scar ectopic pregnancies have been submitted, including cases that are being followed expectantly, which is inconsistent with the standard of care.10 We also have received several submissions about cases of preterm premature rupture of membranes in the second trimester where the patient was sent home and presented several days later with a severe infection requiring management in the intensive care unit. Cases of early pregnancy loss that could have been treated safely and routinely also were delayed, increasing the risk to patients who, in addition to receiving substandard medical care, had the trauma of fearing they could be prosecuted for receiving treatment.
We hope these data will be useful to document the impact of the Court’s decision and to improve patient care as health care institutions work to update their policies and protocols to reduce delays in care in the face of legal ambiguities. If you have been involved in such a case since June 2022, including caring for a patient who traveled from another state, please consider submitting it at CarePostRoe.com, and please spread the word through your networks.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
- McCann A, Schoenfeld Walker A, Sasani A, et al. Tracking the states where abortion is now banned. New York Times. May 24, 2022. Accessed February 14, 2023. https://www.nytimes.com /interactive/2022/us/abortion-laws-roe-v-wade .html
- Nash E, Ephross P. State policy trends 2022: in a devastating year, US Supreme Court’s decision to overturn Roe leads to bans, confusion and chaos. Guttmacher Institute website. Published December 19, 2022. Accessed February 14, 2023. https://www.guttmacher.org/2022/12/state -policy-trends-2022-devastating-year-us -supreme-courts-decision-overturn-roe-leads
- Cha AE. Physicians face confusion and fear in post-Roe world. Washington Post. June 28, 2022. Accessed February 14, 2023. https://www .washingtonpost.com/health/2022/06/28 /abortion-ban-roe-doctors-confusion/
- Zernike K. Medical impact of Roe reversal goes well beyond abortion clinics, doctors say. New York Times. September 10, 2022. Accessed February 14, 2023. https://www.nytimes .com/2022/09/10/us/abortion-bans-medical -care-women.html
- Abrams A. ‘Never-ending nightmare.’ an Ohio woman was forced to travel out of state for an abortion. Time. August 29, 2022. Accessed February 14, 2023. https://time.com/6208860/ohio -woman-forced-travel-abortion/
- Belluck P. They had miscarriages, and new abortion laws obstructed treatment. New York Times. July 17, 2022. Accessed February 14, 2023. https://www.nytimes.com/2022/07/17/health /abortion-miscarriage-treatment.html
- Sellers FS, Nirappil F. Confusion post-Roe spurs delays, denials for some lifesaving pregnancy care. Washington Post. July 16, 2022. Accessed February 14, 2023. https://www.washingtonpost .com/health/2022/07/16/abortion-miscarriage -ectopic-pregnancy-care/.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1.
- Cohen E, Lape J, Herman D. “Heartbreaking” stories go untold, doctors say, as employers “muzzle” them in wake of abortion ruling. CNN website. Published October 12, 2022. Accessed February 14, 2023. https://www.cnn.com/2022/10/12 /health/abortion-doctors-talking/index.html.
- Society for Maternal-Fetal Medicine (SMFM), Miller R, Gyamfi-Bannerman C; Publications Committee. Society for Maternal-Fetal Medicine Consult Series #63: Cesarean scar ectopic pregnancy [published online July 16, 2022]. Am J Obstet Gynecol. 2022 Sep;227:B9-B20. doi:10.1016/j. ajog.2022.06.024.
Current approaches and challenges to cervical cancer prevention in the United States
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8
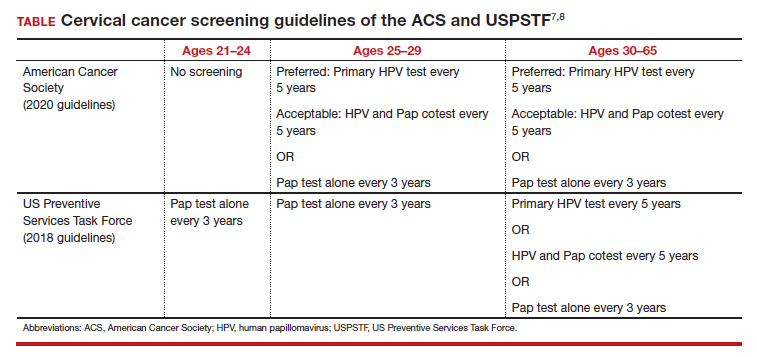
To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12
Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
CASE Intervention approaches for decreasing the risk of cervical cancer
A 25-year-old woman presents to your practice for routine examination. She has never undergone cervical cancer screening or received the human papillomavirus (HPV) vaccine series. The patient has had 3 lifetime sexual partners and currently uses condoms as contraception. What interventions are appropriate to offer this patient to decrease her risk of cervical cancer? Choose as many that may apply:
1. cervical cytology with reflex HPV testing
2. cervical cytology with HPV cotesting
3. primary HPV testing
4. HPV vaccine series (3 doses)
5. all of the above
The answer is number 5, all of the above.
Choices 1, 2, and 3 are acceptable methods of cervical cancer screening for this patient. Catch-up HPV vaccination should be offered as well.
Equitable preventive care is needed
Cervical cancer is a unique cancer because it has a known preventative strategy. HPV vaccination, paired with cervical screening and management of abnormal results, has contributed to decreased rates of cervical cancer in the United States, from 13,914 cases in 1999 to 12,795 cases in 2019.1 In less-developed countries, however, cervical cancer continues to be a leading cause of mortality, with 90% of cervical cancer deaths in 2020 occurring in low- and middle-income countries.2
Disparate outcomes in cervical cancer are often a reflection of disparities in health access. Within the United States, Black women have a higher incidence of cervical cancer, advanced-stage disease, and mortality from cervical cancer than White women.3,4 Furthermore, the incidence of cervical cancer increased among American Indian and Alaska Native people between 2000 and 2019.5 The rate for patients who are overdue for cervical cancer screening is higher among Asian and Hispanic patients compared with non-Hispanic White patients (31.4% vs 20.1%; P=.01) and among patients who identify as LGBTQ+ compared with patients who identify as heterosexual (32.0% vs 22.2%; P<.001).6 Younger patients have a significantly higher rate for overdue screening compared with their older counterparts (29.1% vs 21.1%; P<.001), as do uninsured patients compared with those who are privately insured (41.7% vs 18.1%; P<.001). Overall, the proportion of women without up-to-date screening increased significantly from 2005 to 2019 (14.4% vs 23.0%; P<.001).6
Unfortunately, despite a known strategy to eliminate cervical cancer, we are not accomplishing equitable preventative care. Barriers to care can include patient-centered issues, such as fear of cancer or of painful evaluations, lack of trust in the health care system, and inadequate understanding of the benefits of cancer prevention, in addition to systemic and structural barriers. As we assess new technologies, one of our most important goals is to consider how such innovations can increase health access—whether through increasing ease and acceptability of testing or by creating more effective screening tests.
Updates to cervical screening guidance
In 2020, the American Cancer Society (ACS) updated its cervical screening guidelines to start screening at age 25 years with the “preferred” strategy of HPV primary testing every 5 years.7 By contrast, the US Preventive Services Task Force (USPSTF) continues to recommend 1 of 3 methods: cytology alone every 3 years; cytology alone every 3 years between ages 21 and 29 followed by cytology and HPV cotesting every 5 years at age 30 or older; or high-risk HPV testing alone every 5 years (TABLE).8

To successfully prevent cervical cancer, abnormal results are managed by performing either colposcopy with biopsy, immediate treatment, or close surveillance based on the risk of developing cervical intraepithelial neoplasia (CIN) 3 or worse. A patient’s risk is determined based on both current and prior test results. The ASCCP (American Society for Colposcopy and Cervical Pathology) transitioned to risk-based management guidelines in 2019 and has both an app and a web-based risk assessment tool available for clinicians (https://www.asccp.org).9
All organizations recommend stopping screening after age 65 provided there has been a history of adequate screening in the prior 10 years (defined as 2 normal cotests or 3 normal cytology tests, with the most recent test within 5 years) and no history of CIN 2 or worse within the prior 25 years.10,11 Recent studies that examined the rate of cervical cancer diagnosed in patients older than 65 years have questioned whether patients should continue screening beyond 65.10 In the United States, 20% of cervical cancer still occurs in women older than age 65.11 One reason may be that many women have not met the requirement for adequate and normal prior screening and may still need ongoing testing.12

Continue to: Primary HPV screening...
Primary HPV screening
Primary HPV testing means that an HPV test is performed first, and if it is positive for high-risk HPV, further testing is performed to determine next steps. This contrasts with the currently used method of obtaining cytology (Pap) first with either concurrent HPV testing or reflex HPV testing. The first HPV primary screening test was approved by the US Food and Drug Administration (FDA) in 2014.13
Multiple randomized controlled trials in Europe have demonstrated the accuracy of HPV-based screening compared with cytology in the detection of cervical cancer and its precursors.14-17 The HPV FOCAL trial demonstrated increased efficacy of primary HPV screening in the detection of CIN 2+ lesions.18 This trial recruited a total of 19,000 women, ages 25 to 65, in Canada and randomly assigned them to receive primary HPV testing or liquid-based cytology. If primary HPV testing was negative, participants would return in 48 months for cytology and HPV cotesting. If primary liquid-based cytology testing was negative, participants would return at 24 months for cytology testing alone and at 48 months for cytology and HPV cotesting. Both groups had similar incidences of CIN 2+ over the study period. HPV testing was shown to detect CIN 2+ at higher rates at the time of initial screen (risk ratio [RR], 1.61; 95% confidence interval [CI], 1.24–2.09) and then significantly lower rates at the time of exit screening at 48 months (RR, 0.36; 95% CI, 0.24–0.54).18 These results demonstrated that primary HPV testing detects CIN 2+ earlier than cytology alone. In follow-up analyses, primary HPV screening missed fewer CIN 2+ diagnoses than cytology screening.19
While not as many studies have compared primary HPV testing to cytology with an HPV cotest, the current most common practice in the United States, one study performed in the United States found that a negative cytology result did not further decrease the risk of CIN 3 for HPV-negative patients (risk of CIN 3+ at 5 years: 0.16% vs 0.17%; P=0.8) and concluded that a negative HPV test was enough reassurance for a low risk of CIN 3+.20
Another study, the ATHENA trial, evaluated more than 42,000 women who were 25 years and older over a 3-year period.21 Patients underwent either primary HPV testing or combination cytology and reflex HPV (if ages 25–29) or HPV cotesting (if age 30 or older). Primary HPV testing was found to have a sensitivity and specificity of 76.1% and 93.5%, respectively, compared with 61.7% and 94.6% for cytology with HPV cotesting, but it also increased the total number of colposcopies performed.21
Subsequent management of a primary HPV-positive result can be triaged using genotyping, cytology, or a combination of both. FDA-approved HPV screening tests provide genotyping and current management guidelines use genotyping to triage positive HPV results into HPV 16, 18, or 1 of 12 other high-risk HPV genotypes.
In the ATHENA trial, the 3-year incidence of CIN 3+ for HPV 16/18-positive results was 21.16% (95% CI, 18.39%–24.01%) compared with 5.4% (95% CI, 4.5%–6.4%) among patients with an HPV test positive for 1 of the other HPV genotypes.21 While a patient with an HPV result positive for HPV 16/18 should directly undergo colposcopy, clinical guidance for an HPV-positive result for one of the other genotypes suggests using reflex cytology to triage patients. The ASCCP recommended management of primary HPV testing is included in the FIGURE.22
Many barriers remain to transitioning to primary HPV testing, including laboratory test availability as well as patient and provider acceptance. At present, 2 FDA-approved primary HPV screening tests are available: the Cobas HPV test (Roche Molecular Systems, Inc) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Changes to screening recommendations need to be accompanied by patient and provider outreach and education.
In a survey of more than 500 US women in 2015 after guidelines allowed for increased screening intervals after negative results, a majority of women (55.6%; 95% CI, 51.4%–59.8%) were aware that screening recommendations had changed; however, 74.1% (95% CI, 70.3%–77.7%) still believed that women should be screened annually.23 By contrast, participants in the HPV FOCAL trial, who were able to learn more about HPV-based screening, were surveyed about their willingness to undergo primary HPV testing rather than Pap testing at the conclusion of the trial.24 Of the participants, 63% were comfortable with primary HPV testing, and 54% were accepting of an extended screening interval of 4 to 5 years.24
Continue to: p16/Ki-67 dual-stain cytology...
p16/Ki-67 dual-stain cytology
An additional tool for triaging HPV-positive patients is the p16/Ki-67 dual stain test (CINtec Plus Cytology; Roche), which was FDA approved in March 2020. A tumor suppressor protein, p16 is found to be overexpressed by HPV oncogenic activity, and Ki-67 is a marker of cellular proliferation. Coexpression of p16 and Ki-67 indicates a loss of cell cycle regulation and is a hallmark of neoplastic transformation. When positive, this test is supportive of active HPV infection and of a high-grade lesion. While the dual stain test is not yet formally incorporated into triage algorithms by national guidelines, it has demonstrated efficacy in detecting CIN 3+
In the IMPACT trial, nearly 5,000 HPV-positive patients underwent p16/Ki-67 dual stain testing compared with cytology and HPV genotyping.25 The sensitivity of dual stain for CIN 3+ was 91.9% (95% CI, 86.1%–95.4%) in HPV 16/18–positive and 86.0% (95% CI, 77.5%–91.6%) in the 12 other genotypes. Using dual stain testing alone to triage HPV-positive results showed significantly higher sensitivity but lower specificity than using cytology alone to triage HPV-positive results. Importantly, triage with dual stain testing alone would have referred significantly fewer women to colposcopy than HPV 16/18 genotyping with cytology triage for the 12 other genotypes (48.6% vs 56.0%; P< .0001).
Self-sampling methods: An approach for potentially improving access to screening
One technology that may help bridge gaps in access to cervical cancer screening is self-collected HPV testing, which would preclude the need for a clinician-performed pelvic exam. At present, no self-sampling method is approved by the FDA. However, many studies have examined the efficacy and safety of various self-sampling kits.26
One randomized controlled trial in the Netherlands compared sensitivity and specificity of CIN 2+ detection in patient-collected versus clinician-collected swabs.27 After a median follow-up of 20 months, the sensitivity and specificity of HPV testing did not differ between the patient-collected and the clinician-collected groups (specificity 100%; 95% CI, 0.91–1.08; sensitivity 96%; 95% CI, 0.90–1.03).27 This analysis did not include patients who did not return their self-collected sample, which leaves the question of whether self-sampling may exacerbate issues with patients who are lost to follow-up.
In a study performed in the United States, 16,590 patients who were overdue for cervical cancer screening were randomly assigned to usual care reminders (annual mailed reminders and phone calls from clinics) or to the addition of a mailed HPV self-sampling test kit.28 While the study did not demonstrate significant difference in the detection of overall CIN 2+ between the 2 groups, screening uptake was higher in the self-sampling kit group than in the usual care reminders group (RR, 1.51; 95% CI, 1.43–1.60), and the number of abnormal screens that warranted colposcopy referral was similar between the 2 groups (36.4% vs 36.8%).28 In qualitative interviews of the participants of this trial, patients who were sent at-home self-sampling kits found that the convenience of at-home testing lowered barriers to scheduling an in-office appointment.29 The hope is that self-sampling methods will expand access of cervical cancer screening to vulnerable populations that face significant barriers to having an in-office pelvic exam.
It is important to note that self-collection and self-sample testing requires multidisciplinary systems for processing results and assuring necessary patient follow-up. Implementing and disseminating such a program has been well tested only in developed countries27,30 with universal health care systems or within an integrated care delivery system. Bringing such technology broadly to the United States and less developed countries will require continued commitment to increasing laboratory capacity, a central electronic health record or system for monitoring results, educational materials for clinicians and patients, and expanding insurance reimbursement for such testing.
HPV vaccination rates must increase
While we continue to investigate which screening methods will most improve our secondary prevention of cervical cancer, our path to increasing primary prevention of cervical cancer is clear: We must increase rates of HPV vaccination. The 9-valent HPV vaccine is FDA approved for use in all patients aged 9 to 45 years.
The American College of Obstetricians and Gynecologists and other organizations recommend HPV vaccination between the ages of 9 and 13, and a “catch-up period” from ages 13 to 26 in which patients previously not vaccinated should receive the vaccine.31 Initiation of the vaccine course earlier (ages 9–10) compared with later (ages 11–12) is correlated with higher overall completion rates by age 15 and has been suggested to be associated with a stronger immune response.32
A study from Sweden found that HPV vaccination before age 17 was most strongly correlated with the lowest rates of cervical cancer, although vaccination between ages 17 and 30 still significantly decreased the risk of cervical cancer compared with those who were unvaccinated.33
Overall HPV vaccination rates in the United States continue to improve, with 58.6%34 of US adolescents having completed vaccination in 2020. However, these rates still are significantly lower than those in many other developed countries, including Australia, which had a complete vaccination rate of 80.5% in 2020.35 Continued disparities in vaccination rates could be contributing to the rise in cervical cancer among certain groups, such as American Indian and Alaska Native populations.5
Work—and innovations—must continue
In conclusion, the incidence of cervical cancer in the United States continues to decrease, although at disparate rates among marginalized populations. To ensure that we are working toward eliminating cervical cancer for all patients, we must continue efforts to eliminate disparities in health access. Continued innovations, including primary HPV testing and self-collection samples, may contribute to lowering barriers to all patients being able to access the preventative care they need. ●
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
- Centers for Disease Control and Prevention. United States Cancer Statistics: data visualizations. Trends: changes over time: cervix. Accessed January 8, 2023. https://gis.cdc.gov /Cancer/USCS/#/Trends/
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi:10.3322/caac.21660.
- Francoeur AA, Liao CI, Casear MA, et al. The increasing incidence of stage IV cervical cancer in the USA: what factors are related? Int J Gynecol Cancer. 2022;32:ijgc-2022-003728. doi:10.1136/ijgc-2022-003728.
- Abdalla E, Habtemariam T, Fall S, et al. A comparative study of health disparities in cervical cancer mortality rates through time between Black and Caucasian women in Alabama and the US. Int J Stud Nurs. 2021;6:9-23. doi:10.20849/ijsn. v6i1.864.
- Bruegl AS, Emerson J, Tirumala K. Persistent disparities of cervical cancer among American Indians/Alaska natives: are we maximizing prevention tools? Gynecol Oncol. 2023;168:5661. doi:10.1016/j.ygyno.2022.11.007.
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force Guideline–Concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001/ jamanetworkopen.2021.43582.
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897.
- Nayar R, Chhieng DC, Crothers B, et al. Moving forward—the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol. 2020;9:291-303. doi:10.1016/j.jasc.2020.05.002.
- Cooley JJP, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Cervical Cancer. Accessed February 21, 2023. https://seer.cancer.gov /statfacts/html/cervix.html
- Feldman S. Screening options for preventing cervical cancer. JAMA Intern Med. 2019;179:879-880. doi:10.1001/ jamainternmed.2019.0298.
- ASCO Post Staff. FDA approves first HPV test for primary cervical cancer screening. ASCO Post. May 15, 2014. Accessed January 8, 2023. https://ascopost.com/issues/may-15-2014 /fda-approves-first-hpv-test-for-primary-cervical-cancer -screening/
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol. 2012;13:78-88. doi:10.1016/S1470-2045(11)70296-0.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249-257. doi:10.1016/S1470-2045(09)70360-2.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672-682. doi:10.1016/S1470-2045(09)70156-1.
- Bulkmans NWJ, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year followup of a randomised controlled implementation trial. Lancet. 2007;370:1764-1772. doi:10.1016/S0140-6736(07)61450-0.
- Ogilvie GS, Van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52. doi:10.1001/jama.2018.7464.
- Gottschlich A, Gondara L, Smith LW, et al. Human papillomavirus‐based screening at extended intervals missed fewer cervical precancers than cytology in the HPV For Cervical Cancer (HPV FOCAL) trial. Int J Cancer. 2022;151:897-905. doi:10.1002/ijc.34039.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663672. doi:10.1016/S1470-2045(11)70145-0.
- Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136:189-197. doi:10.1016/j.ygyno.2014.11.076
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337. doi:10.1097/AOG.0000000000000669.
- Silver MI, Rositch AF, Burke AE, et al. Patient concerns about human papillomavirus testing and 5-year intervals in routine cervical cancer screening. Obstet Gynecol. 2015;125:317-329. doi:10.1097/AOG.0000000000000638.
- Smith LW, Racey CS, Gondara L, et al. Women’s acceptability of and experience with primary human papillomavirus testing for cervical screening: HPV FOCAL trial cross-sectional online survey results. BMJ Open. 2021;11:e052084. doi:10.1136/bmjopen-2021-052084.
- Wright TC, Stoler MH, Ranger-Moore J, et al. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: results from the IMPACT trial. Int J Cancer. 2022;150:461-471. doi:10.1002/ijc.33812.
- Yeh PT, Kennedy CE, De Vuyst H, et al. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Global Health. 2019;4:e001351. doi:10.1136/bmjgh-2018-001351.
- Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229-238. doi:10.1016/S1470-2045(18)30763-0.
- Winer RL, Lin J, Tiro JA, et al. Effect of mailed human papillomavirus test kits vs usual care reminders on cervical cancer screening uptake, precancer detection, and treatment: a randomized clinical trial. JAMA Netw Open. 2019;2:e1914729. doi:10.1001/jamanetworkopen.2019.14729.
- Tiro JA, Betts AC, Kimbel K, et al. Understanding patients’ perspectives and information needs following a positive home human papillomavirus self-sampling kit result. J Womens Health (Larchmt). 2019;28:384-392. doi:10.1089/ jwh.2018.7070.
- Knauss T, Hansen BT, Pedersen K, et al. The cost-effectiveness of opt-in and send-to-all HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: the Equalscreen randomized controlled trial. Gynecol Oncol. 2023;168:39-47. doi:10.1016/j.ygyno.2022.10.027.
- ACOG committee opinion no. 809. Human papillomavirus vaccination: correction. Obstet Gynecol. 2022;139:345. doi:10.1097/AOG.0000000000004680.
- St Sauver JL, Finney Rutten LJF, Ebbert JO, et al. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327-333. doi:10.1016/j.ypmed.2016.02.039.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:13401348. doi:10.1056/NEJMoa1917338.
- Pingali C, Yankey D, Elam-Evans LD, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183-1190. doi:10.15585/ mmwr.mm7035a1.
- National Centre for Immunisation Research and Surveillance Australia. Annual Immunisation Coverage Report 2020. November 29, 2021. Accessed March 1, 2023. https://ncirs .org.au/sites/default/files/2021-11/NCIRS%20Annual%20 Immunisation%20Coverage%20Report%202020_FINAL.pdf
- Leung SOA, Feldman S. 2022 Update on cervical disease. OBG Manag. 2022;34(5):16-17, 22-24, 26, 28. doi:10.12788/ obgm.0197.
A love letter to Black birthing people from Black birth workers, midwives, and physicians
A few years ago, my partner emailed me about a consult.
“Dr. Carter, I had the pleasure of seeing Mrs. Smith today for a preconception consult for chronic hypertension. As a high-risk Black woman, she wants to know what we’re going to do to make sure that she doesn’t die in pregnancy or childbirth. I told her that you’re better equipped to answer this question.”
I was early in my career, and the only thing I could assume that equipped me to answer this question over my partners was my identity as a Black woman living in America.
Mrs. Smith was copied on the message and replied with a long list of follow-up questions and a request for an in-person meeting with me. I was conflicted. As a friend, daughter, and mother, I understood her fear and wanted to be there for her. As a newly appointed assistant professor on the tenure track with 20% clinical time, my clinical responsibilities easily exceeded 50% (in part, because I failed to set boundaries). I spent countless hours of uncompensated time serving on diversity, equity, and inclusion initiatives and mentoring and volunteering for multiple community organizations; I was acutely aware that I would be measured against colleagues who rise through the ranks, unencumbered by these social, moral, and ethical responsibilities, collectively known as the “Black tax.”1
I knew from prior experiences and the tone of Mrs. Smith’s email that it would be a tough, long meeting that would set a precedent of concierge level care that only promised to intensify once she became pregnant. I agonized over my reply. How could I balance providing compassionate care for this patient with my young research program, which I hoped to nurture so that it would one day grow to have population-level impact?
It took me 2 days to finally reply to the message with a kind, but firm, email stating that I would be happy to see her for a follow-up preconception visit. It was my attempt to balance accessibility with boundaries. She did not reply.
Did I fail her?
The fact that I still think of Mrs. Smith may indicate that I did the wrong thing. In fact, writing the first draft of this letter was a therapeutic experience, and I addressed it to Mrs. Smith. As I shared the experience and letter with friends in the field, however, everyone had similar stories. The letter continued to pass between colleagues, who each made it infinitely better. This collective process created the beautiful love letter to Black birthing people that we share here.
We call upon all of our obstetric clinician colleagues to educate themselves to be equally, ethically, and equitably equipped to care for and serve historically marginalized women and birthing people. We hope that this letter will aid in the journey, and we encourage you to share it with patients to open conversations that are too often left closed.

Continue to: Our love letter to Black women and birthing people...
Our love letter to Black women and birthing people
We see you, we hear you, we know you are scared, and we are you. In recent years, the press has amplified gross inequities in maternal care and outcomes that we, as Black birth workers, midwives, and physicians, already knew to be true. We grieve, along with you regarding the recently reported pregnancy-related deaths of Mrs. Kira Johnson,2 Dr. Shalon Irving,3 Dr. Chaniece Wallace,4 and so many other names we do not know because their stories did not receive national attention, but we know that they represented the best of us, and they are gone too soon. As Black birth workers, midwives, physicians, and more, we have a front-row seat to the United States’ serious obstetric racism, manifested in biased clinical interactions, unjust hospital policies, and an inequitable health care system that leads to disparities in maternal morbidity and mortality for Black women.
Unfortunately, this is not anything new, and the legacy dates back to slavery and the disregard for Black people in this country. What has changed is our increased awareness of these health injustices. This collective consciousness of the risk that is carried with our pregnancies casts a shadow of fear over a period that should be full of the joy and promise of new life. We fear that our personhood will be disregarded, our pain will be ignored, and our voices silenced by a medical system that has sought to dominate our bodies and experiment on them without our permission.5 While this history is reprehensible, and our collective risk as Black people is disproportionately high, our purpose in writing this letter is to help Black birthing people recapture the joy and celebration that should be theirs in pregnancy and in the journey to parenthood.
As Black birth workers, we see Black pregnant patients desperately seeking safety, security, and breaking down barriers to find us for their pregnancy care. Often, they are terrified and looking for kinship and community in our offices. In rural areas patients may drive up to 4 hours in distance for an appointment, and during appointments entrust us with their stories of feeling unheard in the medical system. When we anecdotally asked about what they feared about pregnancy, childbirth, and the postpartum period and thought was their risk of dying during pregnancy or childbirth, answers ranged from 1% to 60%. Our actual risk of dying from a pregnancy-related cause, as a Black woman, is 0.0414% (41.4 Black maternal deaths per 100,000 live births).6 To put that in perspective, our risk of dying is higher walking down the street or driving a car.7
What is the source of the fear? Based on past and present injustices inflicted on people with historically marginalized identities, we have every right to be scared; but, make no mistake that fear comes at a cost, and Black birthing people are the ones paying the bill! Stress and chronic worry are associated with poor pregnancy outcomes, and so this completely justifiable fear, at the population level, is not serving us well personally.8 Unfortunately, lost in the messaging about racial inequities in maternal mortality is the reality that the vast majority of Black people and babies will survive, thrive, and have healthy pregnancy outcomes, despite the terrifying population-level statistics and horrific stories of discrimination and neglect that make us feel like our pregnancies and personal peril are synonymous.
While it is true that our absolute individual, personal risk is lower than population-level statistics convey, let us be clear: We are furious about what is happening to Black people! It is immoral that Black patients in the richest country in the world are 3-4 times more likely to die of a pregnancy-related cause than White women,9 and we are more likely to experience pregnancy complications and “near misses” when death is narrowly avoided. Research has done an excellent job defining reproductive health disparities in this country, but prioritizing and funding meaningful strategies, policies, and programs to close this gap have not taken precedence—especially initiatives and research that are headed by Black women.10–12 This is largely because researchers and health care systems continue evaluating strategies that focus on behavior change and narratives that identify individual responsibility as a sole cause of inequity.
Let us be clear, Black people and our behaviors are not the problem.13 The problems are White supremacy, classism, sexism, heteropatriarchy, and obstetric racism.1-21 These must be recognized and addressed across all levels of power. We endorse systems-level changes that are at the root of promoting health equity in our reproductive outcomes. These changes include paid parental leave, Medicaid expansion/extension, reimbursement for doula and lactation services, increased access to perinatal mental health and wellness services, and so much more. (See the Black Mamas Matter Alliance Toolkit: https://blackmamas matter.org/our-work/toolkits/.)
Continue to: Pearls for reassurance...
Pearls for reassurance
While the inequities and their solutions are grounded in the need for systemic change,22 we realize that these population-level solutions feel abstract when our sisters and siblings ask us, “So what can I do to advocate for myself and my baby, right now in this pregnancy?” To be clear, no amount of personal hypervigilance on our part as Black pregnancy-capable people is going to fix these problems, which are systemic; however, we want to provide a few pearls that may be helpful for patient self-advocacy and reassurance:
- Seek culturally and ethnically congruent care. We intuitively want to find a clinician who looks like us, but sadly, in the United States only 5% of physicians and 2% of midwives are Black. Demand exceeds supply for Black patients who are seeking racially congruent care. Nonetheless, it is critical that you find a physician or midwife who centers you and provides support and care that affirms the strengths and assets of you, your family, and your community when cultural and ethnic congruency are not possible for you and your pregnancy.
- Ask how your clinicians are actively working to ensure optimal and equitable experiences for Black birthing individuals. We recommend asking your clinician and/or hospital what, if anything, they are doing to address health care inequities, obstetric racism, or implicit bias in their pregnancy and postpartum care. Many groups (including some authors of this letter) are working on measures to address obstetric racism. An acknowledgement of initiatives to mitigate inequities is a meaningful first step. You can suggest that they look into it while you explore your options, as this work is rapidly emerging in many areas of the country.
- Plan for well-person care. The best time to optimize pregnancy and birth outcomes is before you get pregnant. Set up an appointment with a midwife, ObGyn, or your primary care physician before you get pregnant. Discuss your concerns about pregnancy and use this time to optimize your health. This also provides an opportunity to build a relationship with your physician/ midwife and their group to evaluate whether they curate an environment where you feel seen, heard, and valued when you go for annual exams or problem visits. If you do not get that sense after a couple of visits, find a place where you do.
- Advocate for a second opinion. If something does not sound right to you or you have questions that were not adequately answered, it is your prerogative to seek a second opinion; a clinician should never be offended by this.
- Consider these factors, for those who deliver in a hospital (by choice or necessity):
a. 24/7 access to obstetricians and dedicated anesthesiologists in the hospital
b. trauma-informed medical/mental health/social services
c. lactation consultation
d. supportive trial of labor after cesarean delivery policy
e. massive blood transfusion protocol.
- Seek doula support! It always helps to have another set of eyes and ears to help advocate for you, especially when you are in pain during pregnancy, childbirth, or in the postpartum period, or are having difficulty advocating for yourself. There is also evidence that women supported by doulas have better pregnancy-related outcomes and experiences.23 Many major cities in the United States have started to provide race-concordant doula care for Black birthing people for free.24
- Don’t forget about your mental health. As stated, chronic stress from racism impacts birth outcomes. Having a mental health clinician is a great way to mitigate adverse effects of prolonged tension.25–27
- Ask your clinician, hospital, or insurance company about participating in group prenatal care and/or nurse home visiting models28 because both are associated with improved birth outcomes.29 Many institutions are implementing group care that provides race-concordant care.30,31
- Ask your clinician, hospital, or local health department for recommendations to a lactation consultant or educator who can support your efforts in breast/ chest/body-feeding.
We invite you to consider this truth
You, alone, do not carry the entire population-level risk of Black birthing people on your shoulders. We all carry a piece of it. We, along with many allies, advocates, and activists, are outraged and angered by generations of racism and mistreatment of Black birthing people in our health systems and hospitals. We are channeling our frustration and disgust to demand substantive and sustainable change.
Our purpose here is to provide love and reassurance to our sisters and siblings who are going through their pregnancies with thoughts about our nation’s past and present failures to promote health equity for us and our babies. Our purpose is neither to minimize the public health crisis of Black infant and maternal morbidity and mortality nor is it to absolve clinicians, health systems, or governments from taking responsibility for these shameful outcomes or making meaningful changes to address them. In fact, we love taking care of our community by providing the best clinical care we can to our patients. We call upon all of our clinical colleagues to educate themselves to be ethically and equitably equipped to provide health care for Black pregnant patients. Finally, to birthing Black families, please remember this: If you choose to have a baby, the outcome and experience must align with what is right for you and your baby to survive and thrive. So much of the joys of pregnancy have been stolen, but we will recapture the celebration that should be ours in pregnancy and the journey to parenthood.
Sincerely,
Ebony B. Carter, MD, MPH
Maternal Fetal Medicine
Washington University School of Medicine
St. Louis, Missouri
Karen A. Scott, MD, MPH
Birthing Cultural Rigor, LLC
Nashville, Tennessee
Andrea Jackson, MD, MAS
ObGyn
University of California,
San Francisco
Sara Whetstone, MD, MHS
ObGyn
University of California,
San Francisco
Traci Johnson, MD
ObGyn
University of Missouri
School of Medicine
Kansas City, Missouri
Sarahn Wheeler, MD
Maternal Fetal Medicine
Duke University School of Medicine
Durham, North Carolina
Asmara Gebre, CNM
Midwife
Zuckerberg San Francisco General Hospital
San Francisco, California
Joia Crear-Perry, MD
ObGyn
National Birth Equity Collaborative
New Orleans, Louisiana
Dineo Khabele, MD
Gynecologic Oncology
Washington University School of Medicine
St. Louis, Missouri
Judette Louis, MD, MPH
Maternal Fetal Medicine
University of South Florida College of Medicine
Tampa, Florida
Yvonne Smith, MSN, RN
Director
Barnes-Jewish Hospital
St. Louis, Missouri
Laura Riley, MD
Maternal Fetal Medicine
Weill Cornell Medicine
New York, New York
Antoinette Liddell, MSN, RN
Care Coordinator
Barnes-Jewish Hospital
St. Louis, Missouri
Cynthia Gyamfi-Bannerman, MD
Maternal Fetal Medicine
Columbia University Irving Medical Center
New York, New York
Rasheda Pippens, MSN, RN
Nurse Educator
Barnes-Jewish Hospital
St. Louis, Missouri
Ayaba Worjoloh-Clemens, MD
ObGyn
Atlanta, Georgia
Allison Bryant, MD, MPH
Maternal Fetal Medicine
Massachusetts General Hospital
Boston, Massachusetts
Sheri L. Foote, CNM
Midwife
Zuckerberg San Francisco General Hospital
San Francisco, California
J. Lindsay Sillas, MD
ObGyn
Bella OB/GYN
Houston, Texas
Cynthia Rogers, MD
Psychiatrist
Washington University School of Medicine
St. Louis, Missouri
Audra R. Meadows, MD, MPH
ObGyn
University of California, San Diego
AeuMuro G. Lake, MD
Urogynecologist
Urogynecology and Healing Arts
Seattle, Washington
Nancy Moore, MSN, RN, WHNP-BC
Nurse Practitioner
Barnes-Jewish Hospital
St. Louis, Missouri
Zoë Julian, MD, MPH
ObGyn
University of Alabama at Birmingham
Janice M. Tinsley, MN, RNC-OB
Zuckerberg San Francisco General Hospital
San Francisco, California
Jamila B. Perritt, MD, MPH
ObGyn
Washington, DC
Joy A. Cooper, MD, MSc
ObGyn
Culture Care
Oakland, California
Arthurine K. Zakama, MD
ObGyn
University of California,San Francisco
Alissa Erogbogbo, MD
OB Hospitalist
Los Altos, California
Sanithia L. Williams, MD
ObGyn
Huntsville, Alabama
Audra Williams, MD, MPH
ObGyn
University of Alabama, Birmingham
Hedwige “Didi” Saint Louis, MD, MPH
OB Hospitalist
Morehouse School of Medicine
Atlanta, Georgia
Cherise Cokley, MD
OB Hospitalist
Community Hospital
Munster, Indiana
J’Leise Sosa, MD, MPH
ObGyn
Buffalo, New York
- Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https ://doi.org/10.1186/s12909-015-0290-9.
- Helm A. Yet another beautiful Black woman dies in childbirth. Kira Johnson spoke 5 languages, raced cars, was daughter in law of Judge Glenda Hatchett. She still died in childbirth. October 19, 2018. https://www.theroot.com/kira-johnson-spoke- 5-languages-raced-cars-was-daughter-18298 62323. Accessed February 27, 2027.
- Shock after Black pediatrics doctor dies after giving birth to first child. November 6, 2020. https ://www.bet.com/article/rvyskv/black-pediatrics -doctor-dies-after-giving-birth#! Accessed February 24, 2023.
- Dr. Shalon’s maternal action project. https ://www.drshalonsmap.org/. Accessed February 24, 2023.
- Verdantam S, Penman M. Remembering Anarcha, Lucy, and Betsey: The mothers of modern gynecology. https://www.npr .org/2016/02/16/466942135/remembering -anarcha-lucy-and-betsey-the-mothers-of -modern-gynecology. February 16, 2016. Accessed February 24, 2023.
- Centers for Disease Control and Prevention website. Pregnancy Mortality Surveillance System. Last reviewed June 22, 2022. Accessed March 8, 2023.
- Odds of dying. NSC injury facts. https ://injuryfacts.nsc.org/all-injuries/preventable -death-overview/odds-of-dying/data-details /#:~:text=Statements%20about%20the%20 odds%20or%20chances%20of%20dying,in% 20%28value%20given%20in%20the%20lifetime %20odds%20column%29. Accessed February 24, 2023.
- Gembruch U, Baschat AA. True knot of the umbilical cord: transient constrictive effect to umbilical venous blood flow demonstrated by Doppler sonography. Ultrasound Obstet Gynecol. 1996;8:53-56. doi: 10.1046/j.14690705.1996.08010053.x.
- MacDorman MF, Thoma M, Declcerq E, et al. Racial and ethnic disparities in maternal mortality in the United States using enhanced vital records, 2016-2017. Am J Public Health. 2012;111:16731681.
- Taffe MA, Gilpin NW. Racial inequity in grant funding from the US National Institutes of Health. Elife. 2021;10. doi: 10.7554/eLife.65697.
- Black Women Scholars and Research Working Group for the Black Mamas Matter Alliance. Black maternal health research re-envisioned: best practices for the conduct of research with, for, and by Black mamas. Harvard Law Policy Rev. 2020;14:393.
- Sullivan P. In philanthropy, race is still a factor in who gets what, study shows. NY Times. https ://www.nytimes.com/2020/05/01/your-money /philanthropy-race.html. May 5, 2020.
- Scott KA, Britton L, McLemore MR. The ethics of perinatal care for Black women: dismantling the structural racism in “Mother Blame” narratives. J Perinat Neonatal Nurs. 2019;33:108-115. doi: 10.1097/jpn.0000000000000394.
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA. Racial Differences in Birth Outcomes: The Role of General, Pregnancy, and Racism Stress. Health Psychology. 2008;27(2):194203. doi: 10.1037/0278-6133.27.2.194.
- Hardeman RR, Murphy KA, Karbeah J, et al. Naming institutionalized racism in the public health literature: a systematic literature review. Public Health Rep. 2018;133:240-249. doi: 10.1177/0033354918760574.
- Hardeman RR, Karbeah J. Examining racism in health services research: a disciplinary self- critique. Health Serv Res. 2020;55 Suppl 2:777-780. doi: 10.1111/1475-6773.13558.
- Hardeman RR, Karbeah J, Kozhimannil KB. Applying a critical race lens to relationship-centered care in pregnancy and childbirth: an antidote to structural racism. Birth. 2020;47:3-7. doi: 10.1111/birt.12462.
- Scott KA, Davis D-A. Obstetric racism: naming and identifying a way out of Black women’s adverse medical experiences. Am Anthropologist. 2021;123:681-684. doi: https://doi.org/10.1111 /aman.13559.
- Mullings L. Resistance and resilience the sojourner syndrome and the social context of reproduction in central Harlem. Schulz AJ, Mullings L, eds. Gender, Race, Class, & Health: Intersectional Approaches. Jossey-Bass/Wiley: Hoboken, NJ; 2006:345-370.
- Chambers BD, Arabia SE, Arega HA, et al. Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health. 2020;36:213-219. doi: 10.1002/smi.2922.
- Chambers BD, Arega HA, Arabia SE, et al. Black women’s perspectives on structural racism across the reproductive lifespan: a conceptual framework for measurement development. Maternal Child Health J. 2021;25:402-413. doi: 10.1007 /s10995-020-03074-3.
- Julian Z, Robles D, Whetstone S, et al. Community-informed models of perinatal and reproductive health services provision: A justice-centered paradigm toward equity among Black birthing communities. Seminar Perinatol. 2020;44:151267. doi: 10.1016/j.semperi.2020.151267.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database System Rev. 2017;7:Cd003766. doi: 10.1002/14651858.CD003766.pub6.
- National Black doulas association. https://www .blackdoulas.org/. Accessed February 24, 2023.
- Therapy for Black girls. https://therapyforblack girls.com/. Accessed February 24, 2023.
- National Queer and Trans Therapists of Color Network. https://www.nqttcn.com/. Accessed February 24, 2023.
- Shades of Blue Project. http://cbww.org. Accessed February 24, 2023.
- Centering Healthcare Institute. https://www .centeringhealthcare.org/. Accessed February 24, 2023.
- Carter EB, Temming LA, Akin J, et al. Group prenatal care compared with traditional prenatal care: a systematic review and meta-analysis. Obstet Gynecol. 2016;128:551-561. doi: 10.1097 /aog.0000000000001560.
- National Center of Excellence in Women’s Health. https://womenshealth.ucsf.edu/coe/embrace -perinatal-care-black-families. Accessed February 24, 2023.
- Alameda Health System. http://www.alamedahealthsystem.org/family-birthing-center/black -centering/. Accessed February 24, 2023.
A few years ago, my partner emailed me about a consult.
“Dr. Carter, I had the pleasure of seeing Mrs. Smith today for a preconception consult for chronic hypertension. As a high-risk Black woman, she wants to know what we’re going to do to make sure that she doesn’t die in pregnancy or childbirth. I told her that you’re better equipped to answer this question.”
I was early in my career, and the only thing I could assume that equipped me to answer this question over my partners was my identity as a Black woman living in America.
Mrs. Smith was copied on the message and replied with a long list of follow-up questions and a request for an in-person meeting with me. I was conflicted. As a friend, daughter, and mother, I understood her fear and wanted to be there for her. As a newly appointed assistant professor on the tenure track with 20% clinical time, my clinical responsibilities easily exceeded 50% (in part, because I failed to set boundaries). I spent countless hours of uncompensated time serving on diversity, equity, and inclusion initiatives and mentoring and volunteering for multiple community organizations; I was acutely aware that I would be measured against colleagues who rise through the ranks, unencumbered by these social, moral, and ethical responsibilities, collectively known as the “Black tax.”1
I knew from prior experiences and the tone of Mrs. Smith’s email that it would be a tough, long meeting that would set a precedent of concierge level care that only promised to intensify once she became pregnant. I agonized over my reply. How could I balance providing compassionate care for this patient with my young research program, which I hoped to nurture so that it would one day grow to have population-level impact?
It took me 2 days to finally reply to the message with a kind, but firm, email stating that I would be happy to see her for a follow-up preconception visit. It was my attempt to balance accessibility with boundaries. She did not reply.
Did I fail her?
The fact that I still think of Mrs. Smith may indicate that I did the wrong thing. In fact, writing the first draft of this letter was a therapeutic experience, and I addressed it to Mrs. Smith. As I shared the experience and letter with friends in the field, however, everyone had similar stories. The letter continued to pass between colleagues, who each made it infinitely better. This collective process created the beautiful love letter to Black birthing people that we share here.
We call upon all of our obstetric clinician colleagues to educate themselves to be equally, ethically, and equitably equipped to care for and serve historically marginalized women and birthing people. We hope that this letter will aid in the journey, and we encourage you to share it with patients to open conversations that are too often left closed.

Continue to: Our love letter to Black women and birthing people...
Our love letter to Black women and birthing people
We see you, we hear you, we know you are scared, and we are you. In recent years, the press has amplified gross inequities in maternal care and outcomes that we, as Black birth workers, midwives, and physicians, already knew to be true. We grieve, along with you regarding the recently reported pregnancy-related deaths of Mrs. Kira Johnson,2 Dr. Shalon Irving,3 Dr. Chaniece Wallace,4 and so many other names we do not know because their stories did not receive national attention, but we know that they represented the best of us, and they are gone too soon. As Black birth workers, midwives, physicians, and more, we have a front-row seat to the United States’ serious obstetric racism, manifested in biased clinical interactions, unjust hospital policies, and an inequitable health care system that leads to disparities in maternal morbidity and mortality for Black women.
Unfortunately, this is not anything new, and the legacy dates back to slavery and the disregard for Black people in this country. What has changed is our increased awareness of these health injustices. This collective consciousness of the risk that is carried with our pregnancies casts a shadow of fear over a period that should be full of the joy and promise of new life. We fear that our personhood will be disregarded, our pain will be ignored, and our voices silenced by a medical system that has sought to dominate our bodies and experiment on them without our permission.5 While this history is reprehensible, and our collective risk as Black people is disproportionately high, our purpose in writing this letter is to help Black birthing people recapture the joy and celebration that should be theirs in pregnancy and in the journey to parenthood.
As Black birth workers, we see Black pregnant patients desperately seeking safety, security, and breaking down barriers to find us for their pregnancy care. Often, they are terrified and looking for kinship and community in our offices. In rural areas patients may drive up to 4 hours in distance for an appointment, and during appointments entrust us with their stories of feeling unheard in the medical system. When we anecdotally asked about what they feared about pregnancy, childbirth, and the postpartum period and thought was their risk of dying during pregnancy or childbirth, answers ranged from 1% to 60%. Our actual risk of dying from a pregnancy-related cause, as a Black woman, is 0.0414% (41.4 Black maternal deaths per 100,000 live births).6 To put that in perspective, our risk of dying is higher walking down the street or driving a car.7
What is the source of the fear? Based on past and present injustices inflicted on people with historically marginalized identities, we have every right to be scared; but, make no mistake that fear comes at a cost, and Black birthing people are the ones paying the bill! Stress and chronic worry are associated with poor pregnancy outcomes, and so this completely justifiable fear, at the population level, is not serving us well personally.8 Unfortunately, lost in the messaging about racial inequities in maternal mortality is the reality that the vast majority of Black people and babies will survive, thrive, and have healthy pregnancy outcomes, despite the terrifying population-level statistics and horrific stories of discrimination and neglect that make us feel like our pregnancies and personal peril are synonymous.
While it is true that our absolute individual, personal risk is lower than population-level statistics convey, let us be clear: We are furious about what is happening to Black people! It is immoral that Black patients in the richest country in the world are 3-4 times more likely to die of a pregnancy-related cause than White women,9 and we are more likely to experience pregnancy complications and “near misses” when death is narrowly avoided. Research has done an excellent job defining reproductive health disparities in this country, but prioritizing and funding meaningful strategies, policies, and programs to close this gap have not taken precedence—especially initiatives and research that are headed by Black women.10–12 This is largely because researchers and health care systems continue evaluating strategies that focus on behavior change and narratives that identify individual responsibility as a sole cause of inequity.
Let us be clear, Black people and our behaviors are not the problem.13 The problems are White supremacy, classism, sexism, heteropatriarchy, and obstetric racism.1-21 These must be recognized and addressed across all levels of power. We endorse systems-level changes that are at the root of promoting health equity in our reproductive outcomes. These changes include paid parental leave, Medicaid expansion/extension, reimbursement for doula and lactation services, increased access to perinatal mental health and wellness services, and so much more. (See the Black Mamas Matter Alliance Toolkit: https://blackmamas matter.org/our-work/toolkits/.)
Continue to: Pearls for reassurance...
Pearls for reassurance
While the inequities and their solutions are grounded in the need for systemic change,22 we realize that these population-level solutions feel abstract when our sisters and siblings ask us, “So what can I do to advocate for myself and my baby, right now in this pregnancy?” To be clear, no amount of personal hypervigilance on our part as Black pregnancy-capable people is going to fix these problems, which are systemic; however, we want to provide a few pearls that may be helpful for patient self-advocacy and reassurance:
- Seek culturally and ethnically congruent care. We intuitively want to find a clinician who looks like us, but sadly, in the United States only 5% of physicians and 2% of midwives are Black. Demand exceeds supply for Black patients who are seeking racially congruent care. Nonetheless, it is critical that you find a physician or midwife who centers you and provides support and care that affirms the strengths and assets of you, your family, and your community when cultural and ethnic congruency are not possible for you and your pregnancy.
- Ask how your clinicians are actively working to ensure optimal and equitable experiences for Black birthing individuals. We recommend asking your clinician and/or hospital what, if anything, they are doing to address health care inequities, obstetric racism, or implicit bias in their pregnancy and postpartum care. Many groups (including some authors of this letter) are working on measures to address obstetric racism. An acknowledgement of initiatives to mitigate inequities is a meaningful first step. You can suggest that they look into it while you explore your options, as this work is rapidly emerging in many areas of the country.
- Plan for well-person care. The best time to optimize pregnancy and birth outcomes is before you get pregnant. Set up an appointment with a midwife, ObGyn, or your primary care physician before you get pregnant. Discuss your concerns about pregnancy and use this time to optimize your health. This also provides an opportunity to build a relationship with your physician/ midwife and their group to evaluate whether they curate an environment where you feel seen, heard, and valued when you go for annual exams or problem visits. If you do not get that sense after a couple of visits, find a place where you do.
- Advocate for a second opinion. If something does not sound right to you or you have questions that were not adequately answered, it is your prerogative to seek a second opinion; a clinician should never be offended by this.
- Consider these factors, for those who deliver in a hospital (by choice or necessity):
a. 24/7 access to obstetricians and dedicated anesthesiologists in the hospital
b. trauma-informed medical/mental health/social services
c. lactation consultation
d. supportive trial of labor after cesarean delivery policy
e. massive blood transfusion protocol.
- Seek doula support! It always helps to have another set of eyes and ears to help advocate for you, especially when you are in pain during pregnancy, childbirth, or in the postpartum period, or are having difficulty advocating for yourself. There is also evidence that women supported by doulas have better pregnancy-related outcomes and experiences.23 Many major cities in the United States have started to provide race-concordant doula care for Black birthing people for free.24
- Don’t forget about your mental health. As stated, chronic stress from racism impacts birth outcomes. Having a mental health clinician is a great way to mitigate adverse effects of prolonged tension.25–27
- Ask your clinician, hospital, or insurance company about participating in group prenatal care and/or nurse home visiting models28 because both are associated with improved birth outcomes.29 Many institutions are implementing group care that provides race-concordant care.30,31
- Ask your clinician, hospital, or local health department for recommendations to a lactation consultant or educator who can support your efforts in breast/ chest/body-feeding.
We invite you to consider this truth
You, alone, do not carry the entire population-level risk of Black birthing people on your shoulders. We all carry a piece of it. We, along with many allies, advocates, and activists, are outraged and angered by generations of racism and mistreatment of Black birthing people in our health systems and hospitals. We are channeling our frustration and disgust to demand substantive and sustainable change.
Our purpose here is to provide love and reassurance to our sisters and siblings who are going through their pregnancies with thoughts about our nation’s past and present failures to promote health equity for us and our babies. Our purpose is neither to minimize the public health crisis of Black infant and maternal morbidity and mortality nor is it to absolve clinicians, health systems, or governments from taking responsibility for these shameful outcomes or making meaningful changes to address them. In fact, we love taking care of our community by providing the best clinical care we can to our patients. We call upon all of our clinical colleagues to educate themselves to be ethically and equitably equipped to provide health care for Black pregnant patients. Finally, to birthing Black families, please remember this: If you choose to have a baby, the outcome and experience must align with what is right for you and your baby to survive and thrive. So much of the joys of pregnancy have been stolen, but we will recapture the celebration that should be ours in pregnancy and the journey to parenthood.
Sincerely,
Ebony B. Carter, MD, MPH
Maternal Fetal Medicine
Washington University School of Medicine
St. Louis, Missouri
Karen A. Scott, MD, MPH
Birthing Cultural Rigor, LLC
Nashville, Tennessee
Andrea Jackson, MD, MAS
ObGyn
University of California,
San Francisco
Sara Whetstone, MD, MHS
ObGyn
University of California,
San Francisco
Traci Johnson, MD
ObGyn
University of Missouri
School of Medicine
Kansas City, Missouri
Sarahn Wheeler, MD
Maternal Fetal Medicine
Duke University School of Medicine
Durham, North Carolina
Asmara Gebre, CNM
Midwife
Zuckerberg San Francisco General Hospital
San Francisco, California
Joia Crear-Perry, MD
ObGyn
National Birth Equity Collaborative
New Orleans, Louisiana
Dineo Khabele, MD
Gynecologic Oncology
Washington University School of Medicine
St. Louis, Missouri
Judette Louis, MD, MPH
Maternal Fetal Medicine
University of South Florida College of Medicine
Tampa, Florida
Yvonne Smith, MSN, RN
Director
Barnes-Jewish Hospital
St. Louis, Missouri
Laura Riley, MD
Maternal Fetal Medicine
Weill Cornell Medicine
New York, New York
Antoinette Liddell, MSN, RN
Care Coordinator
Barnes-Jewish Hospital
St. Louis, Missouri
Cynthia Gyamfi-Bannerman, MD
Maternal Fetal Medicine
Columbia University Irving Medical Center
New York, New York
Rasheda Pippens, MSN, RN
Nurse Educator
Barnes-Jewish Hospital
St. Louis, Missouri
Ayaba Worjoloh-Clemens, MD
ObGyn
Atlanta, Georgia
Allison Bryant, MD, MPH
Maternal Fetal Medicine
Massachusetts General Hospital
Boston, Massachusetts
Sheri L. Foote, CNM
Midwife
Zuckerberg San Francisco General Hospital
San Francisco, California
J. Lindsay Sillas, MD
ObGyn
Bella OB/GYN
Houston, Texas
Cynthia Rogers, MD
Psychiatrist
Washington University School of Medicine
St. Louis, Missouri
Audra R. Meadows, MD, MPH
ObGyn
University of California, San Diego
AeuMuro G. Lake, MD
Urogynecologist
Urogynecology and Healing Arts
Seattle, Washington
Nancy Moore, MSN, RN, WHNP-BC
Nurse Practitioner
Barnes-Jewish Hospital
St. Louis, Missouri
Zoë Julian, MD, MPH
ObGyn
University of Alabama at Birmingham
Janice M. Tinsley, MN, RNC-OB
Zuckerberg San Francisco General Hospital
San Francisco, California
Jamila B. Perritt, MD, MPH
ObGyn
Washington, DC
Joy A. Cooper, MD, MSc
ObGyn
Culture Care
Oakland, California
Arthurine K. Zakama, MD
ObGyn
University of California,San Francisco
Alissa Erogbogbo, MD
OB Hospitalist
Los Altos, California
Sanithia L. Williams, MD
ObGyn
Huntsville, Alabama
Audra Williams, MD, MPH
ObGyn
University of Alabama, Birmingham
Hedwige “Didi” Saint Louis, MD, MPH
OB Hospitalist
Morehouse School of Medicine
Atlanta, Georgia
Cherise Cokley, MD
OB Hospitalist
Community Hospital
Munster, Indiana
J’Leise Sosa, MD, MPH
ObGyn
Buffalo, New York
A few years ago, my partner emailed me about a consult.
“Dr. Carter, I had the pleasure of seeing Mrs. Smith today for a preconception consult for chronic hypertension. As a high-risk Black woman, she wants to know what we’re going to do to make sure that she doesn’t die in pregnancy or childbirth. I told her that you’re better equipped to answer this question.”
I was early in my career, and the only thing I could assume that equipped me to answer this question over my partners was my identity as a Black woman living in America.
Mrs. Smith was copied on the message and replied with a long list of follow-up questions and a request for an in-person meeting with me. I was conflicted. As a friend, daughter, and mother, I understood her fear and wanted to be there for her. As a newly appointed assistant professor on the tenure track with 20% clinical time, my clinical responsibilities easily exceeded 50% (in part, because I failed to set boundaries). I spent countless hours of uncompensated time serving on diversity, equity, and inclusion initiatives and mentoring and volunteering for multiple community organizations; I was acutely aware that I would be measured against colleagues who rise through the ranks, unencumbered by these social, moral, and ethical responsibilities, collectively known as the “Black tax.”1
I knew from prior experiences and the tone of Mrs. Smith’s email that it would be a tough, long meeting that would set a precedent of concierge level care that only promised to intensify once she became pregnant. I agonized over my reply. How could I balance providing compassionate care for this patient with my young research program, which I hoped to nurture so that it would one day grow to have population-level impact?
It took me 2 days to finally reply to the message with a kind, but firm, email stating that I would be happy to see her for a follow-up preconception visit. It was my attempt to balance accessibility with boundaries. She did not reply.
Did I fail her?
The fact that I still think of Mrs. Smith may indicate that I did the wrong thing. In fact, writing the first draft of this letter was a therapeutic experience, and I addressed it to Mrs. Smith. As I shared the experience and letter with friends in the field, however, everyone had similar stories. The letter continued to pass between colleagues, who each made it infinitely better. This collective process created the beautiful love letter to Black birthing people that we share here.
We call upon all of our obstetric clinician colleagues to educate themselves to be equally, ethically, and equitably equipped to care for and serve historically marginalized women and birthing people. We hope that this letter will aid in the journey, and we encourage you to share it with patients to open conversations that are too often left closed.

Continue to: Our love letter to Black women and birthing people...
Our love letter to Black women and birthing people
We see you, we hear you, we know you are scared, and we are you. In recent years, the press has amplified gross inequities in maternal care and outcomes that we, as Black birth workers, midwives, and physicians, already knew to be true. We grieve, along with you regarding the recently reported pregnancy-related deaths of Mrs. Kira Johnson,2 Dr. Shalon Irving,3 Dr. Chaniece Wallace,4 and so many other names we do not know because their stories did not receive national attention, but we know that they represented the best of us, and they are gone too soon. As Black birth workers, midwives, physicians, and more, we have a front-row seat to the United States’ serious obstetric racism, manifested in biased clinical interactions, unjust hospital policies, and an inequitable health care system that leads to disparities in maternal morbidity and mortality for Black women.
Unfortunately, this is not anything new, and the legacy dates back to slavery and the disregard for Black people in this country. What has changed is our increased awareness of these health injustices. This collective consciousness of the risk that is carried with our pregnancies casts a shadow of fear over a period that should be full of the joy and promise of new life. We fear that our personhood will be disregarded, our pain will be ignored, and our voices silenced by a medical system that has sought to dominate our bodies and experiment on them without our permission.5 While this history is reprehensible, and our collective risk as Black people is disproportionately high, our purpose in writing this letter is to help Black birthing people recapture the joy and celebration that should be theirs in pregnancy and in the journey to parenthood.
As Black birth workers, we see Black pregnant patients desperately seeking safety, security, and breaking down barriers to find us for their pregnancy care. Often, they are terrified and looking for kinship and community in our offices. In rural areas patients may drive up to 4 hours in distance for an appointment, and during appointments entrust us with their stories of feeling unheard in the medical system. When we anecdotally asked about what they feared about pregnancy, childbirth, and the postpartum period and thought was their risk of dying during pregnancy or childbirth, answers ranged from 1% to 60%. Our actual risk of dying from a pregnancy-related cause, as a Black woman, is 0.0414% (41.4 Black maternal deaths per 100,000 live births).6 To put that in perspective, our risk of dying is higher walking down the street or driving a car.7
What is the source of the fear? Based on past and present injustices inflicted on people with historically marginalized identities, we have every right to be scared; but, make no mistake that fear comes at a cost, and Black birthing people are the ones paying the bill! Stress and chronic worry are associated with poor pregnancy outcomes, and so this completely justifiable fear, at the population level, is not serving us well personally.8 Unfortunately, lost in the messaging about racial inequities in maternal mortality is the reality that the vast majority of Black people and babies will survive, thrive, and have healthy pregnancy outcomes, despite the terrifying population-level statistics and horrific stories of discrimination and neglect that make us feel like our pregnancies and personal peril are synonymous.
While it is true that our absolute individual, personal risk is lower than population-level statistics convey, let us be clear: We are furious about what is happening to Black people! It is immoral that Black patients in the richest country in the world are 3-4 times more likely to die of a pregnancy-related cause than White women,9 and we are more likely to experience pregnancy complications and “near misses” when death is narrowly avoided. Research has done an excellent job defining reproductive health disparities in this country, but prioritizing and funding meaningful strategies, policies, and programs to close this gap have not taken precedence—especially initiatives and research that are headed by Black women.10–12 This is largely because researchers and health care systems continue evaluating strategies that focus on behavior change and narratives that identify individual responsibility as a sole cause of inequity.
Let us be clear, Black people and our behaviors are not the problem.13 The problems are White supremacy, classism, sexism, heteropatriarchy, and obstetric racism.1-21 These must be recognized and addressed across all levels of power. We endorse systems-level changes that are at the root of promoting health equity in our reproductive outcomes. These changes include paid parental leave, Medicaid expansion/extension, reimbursement for doula and lactation services, increased access to perinatal mental health and wellness services, and so much more. (See the Black Mamas Matter Alliance Toolkit: https://blackmamas matter.org/our-work/toolkits/.)
Continue to: Pearls for reassurance...
Pearls for reassurance
While the inequities and their solutions are grounded in the need for systemic change,22 we realize that these population-level solutions feel abstract when our sisters and siblings ask us, “So what can I do to advocate for myself and my baby, right now in this pregnancy?” To be clear, no amount of personal hypervigilance on our part as Black pregnancy-capable people is going to fix these problems, which are systemic; however, we want to provide a few pearls that may be helpful for patient self-advocacy and reassurance:
- Seek culturally and ethnically congruent care. We intuitively want to find a clinician who looks like us, but sadly, in the United States only 5% of physicians and 2% of midwives are Black. Demand exceeds supply for Black patients who are seeking racially congruent care. Nonetheless, it is critical that you find a physician or midwife who centers you and provides support and care that affirms the strengths and assets of you, your family, and your community when cultural and ethnic congruency are not possible for you and your pregnancy.
- Ask how your clinicians are actively working to ensure optimal and equitable experiences for Black birthing individuals. We recommend asking your clinician and/or hospital what, if anything, they are doing to address health care inequities, obstetric racism, or implicit bias in their pregnancy and postpartum care. Many groups (including some authors of this letter) are working on measures to address obstetric racism. An acknowledgement of initiatives to mitigate inequities is a meaningful first step. You can suggest that they look into it while you explore your options, as this work is rapidly emerging in many areas of the country.
- Plan for well-person care. The best time to optimize pregnancy and birth outcomes is before you get pregnant. Set up an appointment with a midwife, ObGyn, or your primary care physician before you get pregnant. Discuss your concerns about pregnancy and use this time to optimize your health. This also provides an opportunity to build a relationship with your physician/ midwife and their group to evaluate whether they curate an environment where you feel seen, heard, and valued when you go for annual exams or problem visits. If you do not get that sense after a couple of visits, find a place where you do.
- Advocate for a second opinion. If something does not sound right to you or you have questions that were not adequately answered, it is your prerogative to seek a second opinion; a clinician should never be offended by this.
- Consider these factors, for those who deliver in a hospital (by choice or necessity):
a. 24/7 access to obstetricians and dedicated anesthesiologists in the hospital
b. trauma-informed medical/mental health/social services
c. lactation consultation
d. supportive trial of labor after cesarean delivery policy
e. massive blood transfusion protocol.
- Seek doula support! It always helps to have another set of eyes and ears to help advocate for you, especially when you are in pain during pregnancy, childbirth, or in the postpartum period, or are having difficulty advocating for yourself. There is also evidence that women supported by doulas have better pregnancy-related outcomes and experiences.23 Many major cities in the United States have started to provide race-concordant doula care for Black birthing people for free.24
- Don’t forget about your mental health. As stated, chronic stress from racism impacts birth outcomes. Having a mental health clinician is a great way to mitigate adverse effects of prolonged tension.25–27
- Ask your clinician, hospital, or insurance company about participating in group prenatal care and/or nurse home visiting models28 because both are associated with improved birth outcomes.29 Many institutions are implementing group care that provides race-concordant care.30,31
- Ask your clinician, hospital, or local health department for recommendations to a lactation consultant or educator who can support your efforts in breast/ chest/body-feeding.
We invite you to consider this truth
You, alone, do not carry the entire population-level risk of Black birthing people on your shoulders. We all carry a piece of it. We, along with many allies, advocates, and activists, are outraged and angered by generations of racism and mistreatment of Black birthing people in our health systems and hospitals. We are channeling our frustration and disgust to demand substantive and sustainable change.
Our purpose here is to provide love and reassurance to our sisters and siblings who are going through their pregnancies with thoughts about our nation’s past and present failures to promote health equity for us and our babies. Our purpose is neither to minimize the public health crisis of Black infant and maternal morbidity and mortality nor is it to absolve clinicians, health systems, or governments from taking responsibility for these shameful outcomes or making meaningful changes to address them. In fact, we love taking care of our community by providing the best clinical care we can to our patients. We call upon all of our clinical colleagues to educate themselves to be ethically and equitably equipped to provide health care for Black pregnant patients. Finally, to birthing Black families, please remember this: If you choose to have a baby, the outcome and experience must align with what is right for you and your baby to survive and thrive. So much of the joys of pregnancy have been stolen, but we will recapture the celebration that should be ours in pregnancy and the journey to parenthood.
Sincerely,
Ebony B. Carter, MD, MPH
Maternal Fetal Medicine
Washington University School of Medicine
St. Louis, Missouri
Karen A. Scott, MD, MPH
Birthing Cultural Rigor, LLC
Nashville, Tennessee
Andrea Jackson, MD, MAS
ObGyn
University of California,
San Francisco
Sara Whetstone, MD, MHS
ObGyn
University of California,
San Francisco
Traci Johnson, MD
ObGyn
University of Missouri
School of Medicine
Kansas City, Missouri
Sarahn Wheeler, MD
Maternal Fetal Medicine
Duke University School of Medicine
Durham, North Carolina
Asmara Gebre, CNM
Midwife
Zuckerberg San Francisco General Hospital
San Francisco, California
Joia Crear-Perry, MD
ObGyn
National Birth Equity Collaborative
New Orleans, Louisiana
Dineo Khabele, MD
Gynecologic Oncology
Washington University School of Medicine
St. Louis, Missouri
Judette Louis, MD, MPH
Maternal Fetal Medicine
University of South Florida College of Medicine
Tampa, Florida
Yvonne Smith, MSN, RN
Director
Barnes-Jewish Hospital
St. Louis, Missouri
Laura Riley, MD
Maternal Fetal Medicine
Weill Cornell Medicine
New York, New York
Antoinette Liddell, MSN, RN
Care Coordinator
Barnes-Jewish Hospital
St. Louis, Missouri
Cynthia Gyamfi-Bannerman, MD
Maternal Fetal Medicine
Columbia University Irving Medical Center
New York, New York
Rasheda Pippens, MSN, RN
Nurse Educator
Barnes-Jewish Hospital
St. Louis, Missouri
Ayaba Worjoloh-Clemens, MD
ObGyn
Atlanta, Georgia
Allison Bryant, MD, MPH
Maternal Fetal Medicine
Massachusetts General Hospital
Boston, Massachusetts
Sheri L. Foote, CNM
Midwife
Zuckerberg San Francisco General Hospital
San Francisco, California
J. Lindsay Sillas, MD
ObGyn
Bella OB/GYN
Houston, Texas
Cynthia Rogers, MD
Psychiatrist
Washington University School of Medicine
St. Louis, Missouri
Audra R. Meadows, MD, MPH
ObGyn
University of California, San Diego
AeuMuro G. Lake, MD
Urogynecologist
Urogynecology and Healing Arts
Seattle, Washington
Nancy Moore, MSN, RN, WHNP-BC
Nurse Practitioner
Barnes-Jewish Hospital
St. Louis, Missouri
Zoë Julian, MD, MPH
ObGyn
University of Alabama at Birmingham
Janice M. Tinsley, MN, RNC-OB
Zuckerberg San Francisco General Hospital
San Francisco, California
Jamila B. Perritt, MD, MPH
ObGyn
Washington, DC
Joy A. Cooper, MD, MSc
ObGyn
Culture Care
Oakland, California
Arthurine K. Zakama, MD
ObGyn
University of California,San Francisco
Alissa Erogbogbo, MD
OB Hospitalist
Los Altos, California
Sanithia L. Williams, MD
ObGyn
Huntsville, Alabama
Audra Williams, MD, MPH
ObGyn
University of Alabama, Birmingham
Hedwige “Didi” Saint Louis, MD, MPH
OB Hospitalist
Morehouse School of Medicine
Atlanta, Georgia
Cherise Cokley, MD
OB Hospitalist
Community Hospital
Munster, Indiana
J’Leise Sosa, MD, MPH
ObGyn
Buffalo, New York
- Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https ://doi.org/10.1186/s12909-015-0290-9.
- Helm A. Yet another beautiful Black woman dies in childbirth. Kira Johnson spoke 5 languages, raced cars, was daughter in law of Judge Glenda Hatchett. She still died in childbirth. October 19, 2018. https://www.theroot.com/kira-johnson-spoke- 5-languages-raced-cars-was-daughter-18298 62323. Accessed February 27, 2027.
- Shock after Black pediatrics doctor dies after giving birth to first child. November 6, 2020. https ://www.bet.com/article/rvyskv/black-pediatrics -doctor-dies-after-giving-birth#! Accessed February 24, 2023.
- Dr. Shalon’s maternal action project. https ://www.drshalonsmap.org/. Accessed February 24, 2023.
- Verdantam S, Penman M. Remembering Anarcha, Lucy, and Betsey: The mothers of modern gynecology. https://www.npr .org/2016/02/16/466942135/remembering -anarcha-lucy-and-betsey-the-mothers-of -modern-gynecology. February 16, 2016. Accessed February 24, 2023.
- Centers for Disease Control and Prevention website. Pregnancy Mortality Surveillance System. Last reviewed June 22, 2022. Accessed March 8, 2023.
- Odds of dying. NSC injury facts. https ://injuryfacts.nsc.org/all-injuries/preventable -death-overview/odds-of-dying/data-details /#:~:text=Statements%20about%20the%20 odds%20or%20chances%20of%20dying,in% 20%28value%20given%20in%20the%20lifetime %20odds%20column%29. Accessed February 24, 2023.
- Gembruch U, Baschat AA. True knot of the umbilical cord: transient constrictive effect to umbilical venous blood flow demonstrated by Doppler sonography. Ultrasound Obstet Gynecol. 1996;8:53-56. doi: 10.1046/j.14690705.1996.08010053.x.
- MacDorman MF, Thoma M, Declcerq E, et al. Racial and ethnic disparities in maternal mortality in the United States using enhanced vital records, 2016-2017. Am J Public Health. 2012;111:16731681.
- Taffe MA, Gilpin NW. Racial inequity in grant funding from the US National Institutes of Health. Elife. 2021;10. doi: 10.7554/eLife.65697.
- Black Women Scholars and Research Working Group for the Black Mamas Matter Alliance. Black maternal health research re-envisioned: best practices for the conduct of research with, for, and by Black mamas. Harvard Law Policy Rev. 2020;14:393.
- Sullivan P. In philanthropy, race is still a factor in who gets what, study shows. NY Times. https ://www.nytimes.com/2020/05/01/your-money /philanthropy-race.html. May 5, 2020.
- Scott KA, Britton L, McLemore MR. The ethics of perinatal care for Black women: dismantling the structural racism in “Mother Blame” narratives. J Perinat Neonatal Nurs. 2019;33:108-115. doi: 10.1097/jpn.0000000000000394.
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA. Racial Differences in Birth Outcomes: The Role of General, Pregnancy, and Racism Stress. Health Psychology. 2008;27(2):194203. doi: 10.1037/0278-6133.27.2.194.
- Hardeman RR, Murphy KA, Karbeah J, et al. Naming institutionalized racism in the public health literature: a systematic literature review. Public Health Rep. 2018;133:240-249. doi: 10.1177/0033354918760574.
- Hardeman RR, Karbeah J. Examining racism in health services research: a disciplinary self- critique. Health Serv Res. 2020;55 Suppl 2:777-780. doi: 10.1111/1475-6773.13558.
- Hardeman RR, Karbeah J, Kozhimannil KB. Applying a critical race lens to relationship-centered care in pregnancy and childbirth: an antidote to structural racism. Birth. 2020;47:3-7. doi: 10.1111/birt.12462.
- Scott KA, Davis D-A. Obstetric racism: naming and identifying a way out of Black women’s adverse medical experiences. Am Anthropologist. 2021;123:681-684. doi: https://doi.org/10.1111 /aman.13559.
- Mullings L. Resistance and resilience the sojourner syndrome and the social context of reproduction in central Harlem. Schulz AJ, Mullings L, eds. Gender, Race, Class, & Health: Intersectional Approaches. Jossey-Bass/Wiley: Hoboken, NJ; 2006:345-370.
- Chambers BD, Arabia SE, Arega HA, et al. Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health. 2020;36:213-219. doi: 10.1002/smi.2922.
- Chambers BD, Arega HA, Arabia SE, et al. Black women’s perspectives on structural racism across the reproductive lifespan: a conceptual framework for measurement development. Maternal Child Health J. 2021;25:402-413. doi: 10.1007 /s10995-020-03074-3.
- Julian Z, Robles D, Whetstone S, et al. Community-informed models of perinatal and reproductive health services provision: A justice-centered paradigm toward equity among Black birthing communities. Seminar Perinatol. 2020;44:151267. doi: 10.1016/j.semperi.2020.151267.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database System Rev. 2017;7:Cd003766. doi: 10.1002/14651858.CD003766.pub6.
- National Black doulas association. https://www .blackdoulas.org/. Accessed February 24, 2023.
- Therapy for Black girls. https://therapyforblack girls.com/. Accessed February 24, 2023.
- National Queer and Trans Therapists of Color Network. https://www.nqttcn.com/. Accessed February 24, 2023.
- Shades of Blue Project. http://cbww.org. Accessed February 24, 2023.
- Centering Healthcare Institute. https://www .centeringhealthcare.org/. Accessed February 24, 2023.
- Carter EB, Temming LA, Akin J, et al. Group prenatal care compared with traditional prenatal care: a systematic review and meta-analysis. Obstet Gynecol. 2016;128:551-561. doi: 10.1097 /aog.0000000000001560.
- National Center of Excellence in Women’s Health. https://womenshealth.ucsf.edu/coe/embrace -perinatal-care-black-families. Accessed February 24, 2023.
- Alameda Health System. http://www.alamedahealthsystem.org/family-birthing-center/black -centering/. Accessed February 24, 2023.
- Rodríguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ. 2015;15:6. https ://doi.org/10.1186/s12909-015-0290-9.
- Helm A. Yet another beautiful Black woman dies in childbirth. Kira Johnson spoke 5 languages, raced cars, was daughter in law of Judge Glenda Hatchett. She still died in childbirth. October 19, 2018. https://www.theroot.com/kira-johnson-spoke- 5-languages-raced-cars-was-daughter-18298 62323. Accessed February 27, 2027.
- Shock after Black pediatrics doctor dies after giving birth to first child. November 6, 2020. https ://www.bet.com/article/rvyskv/black-pediatrics -doctor-dies-after-giving-birth#! Accessed February 24, 2023.
- Dr. Shalon’s maternal action project. https ://www.drshalonsmap.org/. Accessed February 24, 2023.
- Verdantam S, Penman M. Remembering Anarcha, Lucy, and Betsey: The mothers of modern gynecology. https://www.npr .org/2016/02/16/466942135/remembering -anarcha-lucy-and-betsey-the-mothers-of -modern-gynecology. February 16, 2016. Accessed February 24, 2023.
- Centers for Disease Control and Prevention website. Pregnancy Mortality Surveillance System. Last reviewed June 22, 2022. Accessed March 8, 2023.
- Odds of dying. NSC injury facts. https ://injuryfacts.nsc.org/all-injuries/preventable -death-overview/odds-of-dying/data-details /#:~:text=Statements%20about%20the%20 odds%20or%20chances%20of%20dying,in% 20%28value%20given%20in%20the%20lifetime %20odds%20column%29. Accessed February 24, 2023.
- Gembruch U, Baschat AA. True knot of the umbilical cord: transient constrictive effect to umbilical venous blood flow demonstrated by Doppler sonography. Ultrasound Obstet Gynecol. 1996;8:53-56. doi: 10.1046/j.14690705.1996.08010053.x.
- MacDorman MF, Thoma M, Declcerq E, et al. Racial and ethnic disparities in maternal mortality in the United States using enhanced vital records, 2016-2017. Am J Public Health. 2012;111:16731681.
- Taffe MA, Gilpin NW. Racial inequity in grant funding from the US National Institutes of Health. Elife. 2021;10. doi: 10.7554/eLife.65697.
- Black Women Scholars and Research Working Group for the Black Mamas Matter Alliance. Black maternal health research re-envisioned: best practices for the conduct of research with, for, and by Black mamas. Harvard Law Policy Rev. 2020;14:393.
- Sullivan P. In philanthropy, race is still a factor in who gets what, study shows. NY Times. https ://www.nytimes.com/2020/05/01/your-money /philanthropy-race.html. May 5, 2020.
- Scott KA, Britton L, McLemore MR. The ethics of perinatal care for Black women: dismantling the structural racism in “Mother Blame” narratives. J Perinat Neonatal Nurs. 2019;33:108-115. doi: 10.1097/jpn.0000000000000394.
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA. Racial Differences in Birth Outcomes: The Role of General, Pregnancy, and Racism Stress. Health Psychology. 2008;27(2):194203. doi: 10.1037/0278-6133.27.2.194.
- Hardeman RR, Murphy KA, Karbeah J, et al. Naming institutionalized racism in the public health literature: a systematic literature review. Public Health Rep. 2018;133:240-249. doi: 10.1177/0033354918760574.
- Hardeman RR, Karbeah J. Examining racism in health services research: a disciplinary self- critique. Health Serv Res. 2020;55 Suppl 2:777-780. doi: 10.1111/1475-6773.13558.
- Hardeman RR, Karbeah J, Kozhimannil KB. Applying a critical race lens to relationship-centered care in pregnancy and childbirth: an antidote to structural racism. Birth. 2020;47:3-7. doi: 10.1111/birt.12462.
- Scott KA, Davis D-A. Obstetric racism: naming and identifying a way out of Black women’s adverse medical experiences. Am Anthropologist. 2021;123:681-684. doi: https://doi.org/10.1111 /aman.13559.
- Mullings L. Resistance and resilience the sojourner syndrome and the social context of reproduction in central Harlem. Schulz AJ, Mullings L, eds. Gender, Race, Class, & Health: Intersectional Approaches. Jossey-Bass/Wiley: Hoboken, NJ; 2006:345-370.
- Chambers BD, Arabia SE, Arega HA, et al. Exposures to structural racism and racial discrimination among pregnant and early post-partum Black women living in Oakland, California. Stress Health. 2020;36:213-219. doi: 10.1002/smi.2922.
- Chambers BD, Arega HA, Arabia SE, et al. Black women’s perspectives on structural racism across the reproductive lifespan: a conceptual framework for measurement development. Maternal Child Health J. 2021;25:402-413. doi: 10.1007 /s10995-020-03074-3.
- Julian Z, Robles D, Whetstone S, et al. Community-informed models of perinatal and reproductive health services provision: A justice-centered paradigm toward equity among Black birthing communities. Seminar Perinatol. 2020;44:151267. doi: 10.1016/j.semperi.2020.151267.
- Bohren MA, Hofmeyr GJ, Sakala C, et al. Continuous support for women during childbirth. Cochrane Database System Rev. 2017;7:Cd003766. doi: 10.1002/14651858.CD003766.pub6.
- National Black doulas association. https://www .blackdoulas.org/. Accessed February 24, 2023.
- Therapy for Black girls. https://therapyforblack girls.com/. Accessed February 24, 2023.
- National Queer and Trans Therapists of Color Network. https://www.nqttcn.com/. Accessed February 24, 2023.
- Shades of Blue Project. http://cbww.org. Accessed February 24, 2023.
- Centering Healthcare Institute. https://www .centeringhealthcare.org/. Accessed February 24, 2023.
- Carter EB, Temming LA, Akin J, et al. Group prenatal care compared with traditional prenatal care: a systematic review and meta-analysis. Obstet Gynecol. 2016;128:551-561. doi: 10.1097 /aog.0000000000001560.
- National Center of Excellence in Women’s Health. https://womenshealth.ucsf.edu/coe/embrace -perinatal-care-black-families. Accessed February 24, 2023.
- Alameda Health System. http://www.alamedahealthsystem.org/family-birthing-center/black -centering/. Accessed February 24, 2023.
Iron deficiency and anemia in patients with heavy menstrual bleeding: Mechanisms and management
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.
CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10
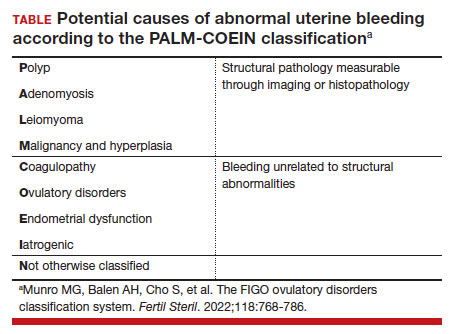
Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21 In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25 They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28 Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.

CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10

Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21 In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25 They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28 Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
Recurrent episodic blood loss from normal menstruation is not expected to result in anemia. But without treatment, chronic heavy periods will progress through the stages of low iron stores to iron deficiency and then to anemia. When iron storage levels are low, the bone marrow’s blood cell factory cannot keep up with continued losses. Every patient with heavy menstrual bleeding (HMB) or prolonged menstrual episodes should be tested and treated for iron deficiency and anemia.1,2
Particular attention should be paid to assessment of iron storage levels with serum ferritin, recognizing that low iron levels progress to anemia once the storage is depleted. Recovery from anemia is much slower in individuals with iron deficiency, so assessment for iron storage also should be included in preoperative assessments and following a diagnosis of acute blood loss anemia.
The mechanics of erythropoiesis, hemoglobin, and oxygen transport
Red blood cells (erythrocytes) have a short life cycle and require constant replacement. Erythrocytes are generated on demand in erythropoiesis by a hormonal signaling process, regardless of whether sufficient components are available.3 Hemoglobin, the main intracellular component of erythrocytes, is comprised of 4 globin chains, which each contain 1 iron atom bound to a heme molecule. After erythrocytes are assembled, they are sent out into circulation for approximately 120 days. A hemoglobin level measures the oxygen-carrying capacity of erythrocytes, and anemia is defined as hemoglobin less than 12 g/dL.
Unless erythrocytes are lost from bleeding, they are decommissioned—that is, the heme molecule is metabolized into bilirubin and excreted, and the iron atoms are recycled back to the bone marrow or to storage.4 Ferritin is the storage molecule that binds to iron, a glycoprotein with numerous subunits around a core that can contain about 4,000 iron atoms. Most ferritin is intracellular, but a small proportion is present in serum, where it can be measured.
Serum ferritin is a good marker for the iron supply in healthy individuals because it has high correlation to iron in the bone marrow and correlates to total intracellular storage unless there is inflammation, when mobilization to serum increases. The ferritin level at which the iron supply is deficient to meet demand, defined as iron deficiency, is hotly debated and ranges from less than 15 to 50 ng/mL in menstruating individuals, with higher thresholds based on onset of erythropoiesis signaling and the lower threshold being the World Health Organization recommendation.5-7 When iron atoms are in short supply, erythrocytes still are generated but they have lower amounts of intracellular hemoglobin, which makes them thinner, smaller, and paler—and less effective at oxygen transport.

CASE Patient seeks treatment for HMB-associated symptoms
A 17-year-old patient presents with HMB, fatigue, and difficulty with concentration. She reports that her periods have been regular and lasting 7 days since menarche at age 13. While they are manageable, they seem to be getting heavier, soaking pads in 2 to 3 hours. The patient reports that she would like to start treatment for her progressively heavy bleeding and prefers lighter scheduled bleeding; she currently does not desire contraception. The patient has no family history of bleeding problems and self-reports no personal history of epistaxis or bleeding with tooth extraction or tonsillectomy. Laboratory tests confirm iron deficiency with a hemoglobin level of 12.5 g/dL (reference range, 12.0–17.5 g/dL) and a serum ferritin level of 8 ng/mL (reference range, 50–420 ng/mL). Results from a coagulopathy panel are normal, as are von Willebrand factor levels.
Untreated iron deficiency will progress to anemia
This patient has iron deficiency without anemia, which warrants significant attention in HMB because without treatment it eventually will progress to anemia. The prevalence of iron deficiency, which makes up half of all causes of anemia, is at least double that of iron deficiency anemia.3
Adult bodies usually contain about 3 to 4 g of iron, with two-thirds in erythrocytes as hemoglobin.8 Approximately 40 to 60 mg of iron is recycled daily, 1 to 2 mg/day is lost from sloughed cells and sweat, and at least 1 mg/day is lost during normal menstruation. These losses are balanced with gastrointestinal uptake of 1 to 2 mg/day until bleeding exceeds about 10 mL/day. In this 17-year-old patient, iron stores have likely been on a progressive decline since menarche.
For normally menstruating individuals to maintain iron homeostasis, the daily dietary iron requirement is 18 mg/day. Iron requirements also increase during periods of illness or inflammation due to hormonal signaling in the iron absorption and transport pathway, in athletes due to sweating, foot strike hemolysis and bruising, and during growth spurts.9
Continue to: Managing iron deficiency and anemia...
Managing iron deficiency and anemia
Management of iron deficiency and iron deficiency anemia in the setting of HMB includes:
- workup for the etiology of the abnormal uterine bleeding (TABLE)
- reducing the source of blood loss, and
- iron supplementation to correct the iron deficiency state.
In most cases, workup, reduction, and repletion can occur simultaneously. The goal is not always complete cessation of menstrual bleeding; even short-term therapy can allow time to replenish iron storage. Use a shared decision-making process to assess what is important to the patient, and provide information about relative amounts of bleeding cessation that can be expected with various therapies.10

Treatment options
Medical treatments to decrease menstrual iron losses are recommended prior to proceeding with surgical interventions.11 Hormonal treatments are the most consistently recommended, with many guidelines citing the 52-mg levonorgestrel-releasing intrauterine device (LNG IUD) as first-line treatment due to its substantial reduction in the amount of bleeding, HMB treatment indication approved by the US Food and Drug Administration (FDA), and evidence of success in those with HMB.12
Any progestin or combined hormonal medication with estrogen and a progestin will result in an approximately 60% to 90% bleeding reduction, thus providing many effective options for blood loss while considering patient preferences for bleeding pattern, route of administration, and concomitant benefits. While only 1 oral product (estradiol valerate/dienogest) is FDA approved for managementof HMB, use of any of the commercially available contraceptive products will provide substantial benefit.11,13
Nonhormonal options, such as antifibrinolytics and nonsteroidal anti-inflammatory drugs (NSAIDs), tend to be listed as second-line therapies or for those who want to avoid hormonal medications. Antifibrinolytics, such as tranexamic acid, require frequent dosing of large pills and result in approximately 40% blood loss reduction, but they are a very successful and well-tolerated method for those seeking on-demand therapy.14 NSAIDs may result in a slight bleeding reduction, but they are far less effective than other therapies.15 Antifibrinolytics have a theoretical risk of thrombosis and a contraindication to use with hormonal contraceptives; therefore, concomitant use with estrogen-containing medications is reserved for patients with refractory heavy bleeding or for heavy bleeding days during the hormone-free interval, when benefits likely outweigh potential risk.16,17
Guidelines for medical management of acute HMB typically cite 3 small comparative studies with high-dose regimens of parenteral conjugated estrogen, combined ethinyl estradiol and progestin, or oral medroxyprogesterone acetate.18,19 Dosing recommendations for the oral medications include a loading dose followed by a taper regimen that is poorly tolerated and for which there is no evidence of superior effectiveness over the standard dose.20,21 In most cases, initiation of the preferred long-term hormonal medication plan will reduce bleeding significantly within 2 to 3 days. Many clinicians who commonly treat acute HMB prescribe norethindrone acetate 5 mg daily (up to 3 times daily, if needed) for effective and safe menstrual suppression.22
Iron replenishment: Dosing frequency, dietary iron, and multivitamins
Iron repletion is usually via the oral route unless surgery is imminent, anemia is severe, or the oral route is not tolerated or effective.23 Oral iron has substantial adverse effects that limit tolerance, including nausea, epigastric pain, diarrhea, and constipation. Fortunately, evidence supports lower oral iron doses than previously used.4
Iron homeostasis is controlled by the peptide hormone hepcidin, produced by the liver, which controls mobilization of iron from the gut and spleen and aids iron absorption from the diet and supplements.24 Hepcidin levels decrease in response to high circulating levels of iron, so the ideal iron repletion dose in iron-deficient nonanemic women was determined by assessing the dose response of hepcidin. Researchers compared iron 60 mg daily for 14 days versus every other day for 28 days and found that iron absorption was greater in the every-other-day group (21.8% vs 16.3%).25 They concluded that changing iron administration to 60 mg or more in a single dose every other day is most efficient in those with iron deficiency without anemia. Since study participants did not have anemia, research is pending on whether different strategies (such as daily dosing) are more effective for more severe cases. The bottom line is that conventional high-dose divided daily oral iron administration results in reduced iron bioavailability compared with alternate-day dosing.
Increasing dietary iron is insufficient to treat low iron storage, iron deficiency, and iron deficiency anemia. Likewise, multivitamins, which contain very little elemental iron, are not recommended for repletion. Any iron salt with 60 to 120 mg of elemental iron can be used (for examples, ferrous sulfate, ferrous gluconate).25 Once ingested, stomach and pancreatic acids release elemental iron from its bound form. For that reason, absorption may be improved by administering iron at least 1 hour before a meal and avoiding antacids, including milk. Meat proteins and ascorbic acid help maintain the soluble ferrous form and also aid absorption. Tea, coffee, and tannins prevent absorption when polyphenol compounds form an insoluble complex with iron (see box at end of article). Gastrointestinal adverse effects can be minimized by decreasing the dose and taking after meals, although with reduced efficacy.
Intravenous iron treatment raises hemoglobin levels significantly faster than oral administration but is limited by cost and availability, so it is reserved for individuals with a hemoglobin level less than 9 g/dL, prior gastrointestinal or bariatric surgery, imminent surgery, and intolerance, poor adherence, or nonresponse to oral iron therapy. Several approved formulations are available, all with equivalent effectiveness and similar safety profiles. Lower-dose formulations (such as iron sucrose) may require several infusions, but higher-dose intravenous iron products (ferric carboxymaltose, low-molecular weight iron dextran, etc) have a stable carbohydrate shell that inhibits free iron release and improves safety, allowing a single administration.26
Common adverse effects of intravenous iron treatment include a metallic taste and headache during administration. More serious adverse effects, such as hypotension, arthralgia, malaise, and nausea, are usually self-limited. With mild infusion reactions (1 in 200), the infusion can be stopped until symptoms improve and can be resumed at a slower rate.27
Continue to: The role of blood transfusion...
The role of blood transfusion
Blood transfusion is expensive and potentially hazardous, so its use is limited to treatment of acute blood loss or severe anemia.
A one-time red blood cell transfusion does not impact diagnostic criteria to assess for iron deficiency with ferritin, and it does not improve underlying iron deficiency.28 Patients with acute blood loss anemia superimposed on chronic blood loss should be screened and treated for iron deficiency even after receiving a transfusion.
Since ferritin levels can rise significantly as an acute phase reactant, even following a hemorrhage, iron deficiency during inflammation is defined as ferritin less than 70 ng/mL.
The potential for iron overload
Since iron is never metabolized or excreted, it is possible to have iron overload following accidental overdose, transfusion dependency, and disorders of iron transport, such as hemochromatosis and thalassemia.
While a low ferritin level always indicates iron deficiency, high ferritin levels can be an acute phase reactant. Ferritin levels greater than 150 ng/mL in healthy menstruating individuals and greater than 500 ng/mL in unhealthy individuals should raise concern for excess iron and should prompt discontinuation of iron intake or workup for conditions at risk for overload.5
Oral iron supplements should be stored away from small children, who are at particular risk of toxicity.
How long to treat?
Treatment duration depends on the individual’s degree of iron deficiency, whether anemia is present, and the amount of ongoing blood loss. The main treatment goal is normalization and maintenance of serum ferritin.
Successful treatment should be confirmed with a complete blood count and ferritin level. Hemoglobin levels improve 2 g/dL after 3 weeks of oral iron therapy, but repletion may take 4 to 6 months.23,29 The American College of Obstetricians and Gynecologists recommends 3 to 6 months of continued iron therapy after resolution of HMB.19
In a comparative study of treatment for HMB with the 52-mg LNG IUD versus hysterectomy, hemoglobin levels increased in both treatment groups but stayed lower in those with initial anemia.8 Ferritin levels normalized only after 5 years and were still lower in individuals with initial anemia.
Increase in hemoglobin is faster after intravenous iron administration but is equivalent to oral therapy by 12 weeks. If management to reduce menstrual losses is discontinued, periodic or maintenance iron repletion will be necessary.
CASE Management plan initiated
This 17-year-old patient with iron deficiency resulting from HMB requests management to reduce menstrual iron losses with a preference for predictable menses. We have already completed a basic workup, which could also include assessment for hypermobility with a Beighton score, as connective tissue disorders also are associated with HMB.30 We discuss the options of cyclic hormonal therapy, antifibrinolytic treatment, and an LNG IUD. The patient is concerned about adherence and wants to avoid unscheduled bleeding, so she opts for a trial of tranexamic acid 1,300 mg 3 times daily for 5 days during menses. This regimen results in a 50% reduction in bleeding amount, which the patient finds satisfactory. Iron repletion with oral ferrous sulfate 325 mg (containing 65 mg of elemental iron) is administered on alternating days with vitamin C taken 1 hour prior to dinner. Repeat laboratory test results at 3 weeks show improvement to a hemoglobin level of 14.2 g/dL and a ferritin level of 12 ng/mL. By 3 months, her ferritin levels are greater than 30 ng/mL and oral iron is administered only during menses.
Summing up
Chronic HMB results in a progressive net loss of iron and eventual anemia. Screening with complete blood count and ferritin and early treatment of low iron storage when ferritin is less than 30 ng/mL will help avoid symptoms. Any amount of reduction of menstrual blood loss can be beneficial, allowing a variety of effective hormonal and nonhormonal treatment options. ●
- Take 60 to 120 mg elemental iron every other day.
- To help with absorption:
—Take 1 hour before a meal, but not with coffee, tea, tannins, antacids, or milk
—Take with vitamin C or other acidic fruit juice
- Recheck complete blood count and ferritin in 2 to 3 weeks to confirm initial response.
- Continue treatment for up to 3 to 6 months until ferritin levels are greater than 30 to 50 ng/mL.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
- Munro MG, Mast AE, Powers JM, et al. The relationship between heavy menstrual bleeding, iron deficiency, and iron deficiency anemia. Am J Obstet Gynecol. 2023;S00029378(23)00024-8.
- Tsakiridis I, Giouleka S, Koutsouki G, et al. Investigation and management of abnormal uterine bleeding in reproductive aged women: a descriptive review of national and international recommendations. Eur J Contracept Reprod Health Care. 2022;27:504-517.
- Camaschella C. Iron deficiency. Blood. 2019;133:30-39.
- Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272.
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. April 21, 2020. Accessed February 17, 2023. https://www.who.int/publications/i/item/9789240000124
- Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8: e572-e582.
- Galetti V, Stoffel NU, Sieber C, et al. Threshold ferritin and hepcidin concentrations indicating early iron deficiency in young women based on upregulation of iron absorption. EClinicalMedicine. 2021;39:101052.
- Percy L, Mansour D, Fraser I. Iron deficiency and iron deficiency anaemia in women. Best Pract Res Clin Obstet Gynaecol. 2017;40:55-67.
- Brittenham GM. Short-term periods of strenuous physical activity lower iron absorption. Am J Clin Nutr. 2021;113:261-262.
- Chen M, Lindley A, Kimport K, et al. An in-depth analysis of the use of shared decision making in contraceptive counseling. Contraception. 2019;99:187-191.
- Bofill Rodriguez M, Dias S, Jordan V, et al. Interventions for heavy menstrual bleeding; overview of Cochrane reviews and network meta-analysis. Cochrane Database Syst Rev. 2022;5:CD013180.
- Mansour D, Hofmann A, Gemzell-Danielsson K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv Ther. 2021;38:201-225.
- Micks EA, Jensen JT. Treatment of heavy menstrual bleeding with the estradiol valerate and dienogest oral contraceptive pill. Adv Ther. 2013;30:1-13.
- Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;4:CD000249.
- Bofill Rodriguez M, Lethaby A, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;9:CD000400.
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: an illustrated review. Res Pract T hromb Haemost. 2021;5:e12546.
- Reid RL, Westhoff C, Mansour D, et al. Oral contraceptives and venous thromboembolism consensus opinion from an international workshop held in Berlin, Germany in December 2009. J Fam Plann Reprod Health Care. 2010;36:117-122.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 785: screening and management of bleeding disorders in adolescents with heavy menstrual bleeding. Obstet Gynecol. 2019;134:e71-e83.
- Haamid F, Sass AE, Dietrich JE. Heavy menstrual bleeding in adolescents. J Pediatr Adolesc Gynecol. 2017;30:335-340.
- Roth LP, Haley KM, Baldwin MK. A retrospective comparison of time to cessation of acute heavy menstrual bleeding in adolescents following two dose regimens of combined oral hormonal therapy. J Pediatr Adolesc Gynecol. 2022;35:294-298.
- Huguelet PS, Buyers EM, Lange-Liss JH, et al. Treatment of acute abnormal uterine bleeding in adolescents: what are providers doing in various specialties? J Pediatr Adolesc Gynecol. 2016;29:286-291.
- Elstrott B, Khan L, Olson S, et al. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. 2020;104:153-161.
- Pagani A, Nai A, Silvestri L, et al. Hepcidin and anemia: a tight relationship. Front Physiol. 2019;10:1294.
- Stoffel NU, von Siebenthal HK, Moretti D, et al. Oral iron supplementation in iron-deficient women: how much and how often? Mol Aspects Med. 2020;75:100865.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Dave CV, Brittenham GM, Carson JL, et al. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175:656-664.
- Froissart A, Rossi B, Ranque B, et al; SiMFI Group. Effect of a red blood cell transfusion on biological markers used to determine the cause of anemia: a prospective study. Am J Med. 2018;131:319-322.
- Carson JL, Brittenham GM. How I treat anemia with red blood cell transfusion and iron. Blood. 2022;blood.2022018521.
- Borzutzky C, Jaffray J. Diagnosis and management of heavy menstrual bleeding and bleeding disorders in adolescents. JAMA Pediatr. 2020;174:186-194.
Central Sleep Apnea in Adults: Diagnosis and Treatment
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring. If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring. If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
As the prevalence of obstructive sleep apnea (OSA) has steadily increased in the United States, so has the awareness of central sleep apnea (CSA). The hallmark of CSA is transient cessation of airflow during sleep due to a lack of respiratory effort triggered by the brain. This is in contrast to OSA, in which there is absence of airflow despite continued ventilatory effort due to physical airflow obstruction. The gold standard for the diagnosis and optimal treatment assessment of CSA is inlaboratory polysomnography (PSG) with esophageal manometry, but in practice, respiratory effort is generally estimated through oronasal flow and respiratory inductance plethysmography bands placed on the chest and abdomen during PSG.
Background
The literature has demonstrated a higher prevalence of moderate-to-severe OSA in the general population compared with that of CSA. While OSA is associated with worse clinical outcomes, more evidence is needed on the long-term clinical impact and optimal treatment strategies for CSA.1 CSA is overrepresented among certain clinical populations. CSA is not frequently diagnosed in the active-duty population, but is increasing in the veteran population, especially in those with heart failure (HF), stroke, neuromuscular disorders, and opioid use. It is associated with increased admissions related to comorbid cardiovascular disorders and to an increased risk of death.2-4 The clinical concerns with CSA parallel those of OSA. The absence of respiration (apneas and hypopneas due to lack of effort) results in sympathetic surge, compromise of oxygenation and ventilation, sleep fragmentation, and elevation in blood pressure. Symptoms such as excessive daytime sleepiness, morning headaches, witnessed apneas, and nocturnal arrhythmias are shared between the 2 disorders.
Ventilatory instability is the most widely accepted mechanism leading to CSA in patients. Loop gain is the concept used to explain ventilatory control. This feedback loop is influenced by controller gain (primarily represented by central and peripheral chemoreceptors causing changes in ventilation due to PaCO2 [partial pressure of CO2 in arterial blood] fluctuations), plant gain (includes lungs and respiratory muscles and their ability to eliminate CO2), and circulation time (feedback between controller and plant).5
High loop gain and narrow CO2 reserve contribute to ventilatory instability in CSA.6 Those with high loop gain have increased sensitivity to changes in CO2. These patients tend to overbreathe in response to smaller increases in PaCO2 compared with those with low loop gain. Once the PaCO2 falls below an individual’s apneic threshold (AT), an apnea will occur.7 The brainstem then pauses ventilation to allow the PaCO2 to rise back above the AT. CSAs also can occur in healthy individuals as they transition from wakefulness into non–rapid eye movement (REM) sleep in a phenomenon called sleep state oscillation, with a mechanism that is similar to hyperventilation-induced CSAs described earlier.
Primary CSA has been defined in the International Classification of Sleep Disorders 3rd edition (ICSD-3) with the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of Cheyne-Stokes breathing (CSB); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) there is no evidence of nocturnal hypoventilation; and (4) the disorder is not better explained by another medical use, substance use disorder (SUD), or other current sleep, medical, or neurologic disorder.8
A systematic clinical approach should be used to identify and treat CSA (Figure).6,7
The purpose of this review is to familiarize the primary care community with CSA to aid in the identification and management of this breathing disturbance.
Nonhypercapnic CSA
Heart Failure–Induced CSA
The leading medical diagnosis causing CSA is congestive HF (CHF), and there is a correlation between HF severity and presence of CSA. In patients with stable CHF with HF reduced ejection fraction (HFrEF), CSA is highly prevalent, occurring in 25% to 40% of patients.9 In contrast to other subtypes of CSA where literature regarding prognosis is lacking, the literature is clear that patients with HFrEF with CSA have a worse prognosis, with increased risk of death independent of the severity of HF. This may be the result of CSA promoting malignant ventricular arrhythmias. The prevalence of CSA in HF with preserved ejection fraction (HFpEF) is about 18% to 30%.10,11
A significant reduction in cardiac output results in circulatory delay between the lungs and chemoreceptors that produces CSB periodic breathing, which is characteristic of the most recognized form of CSA. Per the ICSD-3, CSA with CSB requires the following 4 findings: (1) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; there are at least 3 consecutive CSAs and/or central hypopneas separated by crescendo-decrescendo breathing with a cycle length of at least 40 seconds (ie, CSB pattern), and the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) the breathing pattern is associated with atrial fibrillation/flutter, CHF, or a neurologic disorder; and (4) the disorder is not better explained by another current sleep disorder, medication use (eg, opioids), or SUD.8
Treatment of HF-induced CSA begins with guideline-based medical management with the goal of reducing pulmonary capillary wedge pressure or increasing left ventricular ejection fraction through means that may include cardiac resynchronization therapy or left ventricular assist devices, when clinically indicated. If medical optimization is not sufficient, the next step is continuous positive airway pressure (CPAP or PAP), followed by adaptive servo-ventilation (ASV) if the apnea-hypopnea index (AHI) remains > 15 events per hour and is clinically indicated.
ASV is a second-line PAP therapy modality that uses proprietary algorithms to provide variable amounts of pressure that alternate between expiratory and inspiratory phases of the respiratory cycle in combination with physician-set or automatic backup respiratory rate designed to stabilize ventilation in patients with CSA and CSB. The inability to adjust tidal volume, potentially resulting in insufficient tidal volumes or ventilation, results in the contraindication for its use in patients with CSA with comorbid conditions that may result in hypercapnic respiratory failure. These conditions include chronic hypoventilation in obesity hypoventilation syndrome (OHS), moderate-to-severe chronic obstructive pulmonary disease, chronic elevation of PaCO2 on arterial blood gas > 45 mm Hg, and restrictive thoracic or neuromuscular disease.12
Although ASV is more effective in normalizing AHI in patients with HF and CSA than is CPAP therapy, the clinical indications for ASV in the setting of HFrEF changed drastically with the publication of the landmark SERVE-HF trial, which investigated the effects of adding ASV to guideline-based medical management on survival and cardiovascular outcomes in patients with HFrEF and predominant CSA.13 The study did not show a difference between the ASV and control groups for the primary endpoint: a composite of time to first event of death from any cause, lifesaving cardiovascular intervention (transplantation, implantation of a long-term ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening HF. However, the study showed a statistically and clinically significant increased risk of all-cause and cardiovascular mortality in the ASV group compared with the control group.13 A possible explanation for the increased all-cause and cardiovascular mortality is that CSA potentially serves a protective mechanism that when eliminated results in deleterious clinical outcomes. This resulted in significant changes in the treatment algorithm for HF-induced CSA with left ventricular ejection fraction of at least 45% becoming the cutoff for therapeutic decisions.
Treatment-Emergent CSA
Treatment-emergent CSA (TECSA, also known as complex sleep apnea) has been defined by the ICSD-3 by the following criteria: (1) diagnostic PSG with ≥ 5 events per hour of predominantly obstructive events; (2) resolution of obstructive events with PAP without a backup rate and CSA index (CAI) ≥ 5 per hour with central events ≥ 50% of the AHI; and (3) CSA not better explained by another disorder.8 Patients with TECSA can be further classified into those who have transient events that resolve within weeks to months, those with persistent events, and those with delayed events that may develop weeks to months after initiating PAP therapy.14
PAP treatment can decrease the PaCO2 below the AT due to removal of flow limitation in previously obstructed upper airways, resulting in TECSA.15,16 PAP therapy has not been the only treatment where new CSA has been identified on initiation. A 2021 systematic review identified patients who developed new-onset CSA with mandibular advancement device (MAD), hypoglossal nerve stimulator, tongue protrusion device, and nasal expiratory PAP device use, as well as after tracheostomy, maxillofacial surgery, and other surgeries, such as nasal and uvulopalatopharyngoplasty.17
The prevalence of TECSA has been noted to range between 0.6% and 20.3%, but Nigam and colleagues estimated a prevalence of 8.4% in their systematic review.11,14 The variability in prevalence between studies could be due to differences in study design (retrospective vs prospective vs cross-sectional), diagnostic and inclusion criteria, patient population, and type of study used (full-night vs split-night vs both).18,19 Risk factors for TECSA include male sex; older age; lower body mass index; higher baseline AHI, CAI, and arousal index; chronic medical issues such as CHF and coronary artery disease; opioid use; higher CPAP settings; excessive mask leak; and bilevel PAP (BiPAP) use.20 Identifying these risk factors is important, as patients with TECSA are at higher risk of discontinuing therapy and of developing PAP intolerance.15,20
Most patients with TECSA can continue CPAP therapy with resolution of events over weeks to months, but treatment of comorbid conditions should be optimized as they could be contributing factors. Zeineddine and colleagues recommend continuation of CPAP for 3 months if the patient has minor or no symptoms.19 A 2018 systematic review noted that 14.3% to 46.2% of TECSA patients will have persistent TECSA and some will develop TECSA after at least 1 month of PAP therapy.14 For these patients and those with severe symptoms in spite of therapy, a switch to BiPAP spontaneous/timed (BiPAP-S/T) or ASV should be considered, if not contraindicated based on comorbidities.21 Medications such as acetazolamide, oxygen therapy, and CO2 supplementation have also been discussed as alternative treatments, but these options should not be first-line therapies and should be used on a case-by-case basis as adjuncts to PAP therapy.16,17
Altitude-Induced CSA
Also known as CSA due to high-altitude periodic breathing (CSA-HAPB), this form of CSA occurs in nearly all lowlanders at altitudes above 3000 m, with severity increasing with altitude.15 The exact altitude at which it occurs varies based on an individual’s physiology. CSA-HAPB occurs in response to the low barometric pressure at altitude, combined with stable fraction of oxygen, resulting in decreased inspired partial pressure of oxygen and hypoxia. The normal physiologic response to hypoxia is increased ventilation, which can cause hypocapnia, suppressing respiratory drive and resulting in CSAs. The instability of the respiratory response results in cyclical CSAs followed by hyperventilation. This periodic breathing then causes arousals from sleep, driving further sleep fragmentation and exacerbation of baseline desaturation and instability in the cyclical respiratory response. There is individual variability in hypoxic chemoresponsiveness (loop gain). Compensatory mechanisms are most robust when an individual routinely dwells at high altitude, resulting in acclimatization, rather than traveling there for a brief stay. Genetics and cardiac output also contribute to the effectiveness of compensation to altitude.
CSA-HAPB is defined by the following ICSD-3 criteria: (1) Recent ascent to a high altitude (typically ≥ 2500 m, although some individuals may exhibit the disorder at altitudes as low as 1500 m); (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia; (3) symptoms are clinically attributable to HAPB, or PSG, if performed, reveals recurrent CSAs or hypopneas primarily during non-REM sleep at a frequency of ≥ 5 events per hour; (4) the disorder is not better explained by another current sleep disorder, medical or neurological disorder, medication use (eg, narcotics), or SUD.8
Treatment options to improve nocturnal oxygen saturation and reduce or eliminate CSA-HAPB in nonacclimatized individuals include oxygen-enriched air, acetazolamide, or combination treatment with acetazolamide and automatic PAP (APAP).22 A meta-analysis looking at the effectiveness of acetazolamide in 8 different randomized controlled trials demonstrated that a dose of 250 mg per day was effective in improving sleep apnea at altitude as measured by a decrease in AHI, decrease in percentage of periodic breathing, and increasing oxygenation during sleep.15 The question of superiority of combined acetazolamide with APAP to placebo with APAP in treatment of high-altitude OSA was addressed in a randomized double-blind, placebo-controlled trial. The results showed that combined APAP (5-15 cm of water pressure) and acetazolamide (250 mg morning, 500 mg evening) decreased the AHI to normal range, whereas central events persisted in the APAP and placebo group.23 In addition, Latshang and colleagues have demonstrated that ASV may not be as efficacious for controlling CSA-HAPB in nonacclimatized individuals compared with oxygen therapy and suggested that further research is warranted examining if ASV device algorithm adjustment improves efficacy of this therapeutic option.24
Comorbidity-Induced CSA
Several medical conditions may be associated with CSA, including chronic kidney disease (CKD), pulmonary hypertension, acromegaly, and hypothyroidism. The common pathophysiologic link is that these disorders may result in alteration of ventilatory responses to CO2, ultimately resulting in CSA.
As many as 10% of patients with CKD may experience CSA.25,26 The complications encountered in CKD include fluid overload with pulmonary edema, chronic metabolic acidosis, and anemia. These can provoke hyperventilation in addition to poor sleep quality, triggering arousals that further drive CSA in these patients. Additional risk factors for CSA in this population include atrial fibrillation and cardiac dysfunction. Clinical interventions that have demonstrated reduction in CSA include hemodialysis at night vs daytime and using bicarbonate buffer vs acetate for hemodialysis 22-24,26-29
Hypersecretion of growth hormone in acromegaly also results in hyperventilation contributing to CSA. While medical and surgical management of acromegaly results in a reduction in OSA, there is limited evidence on the outcome of the CSA after these interventions.
Hypothyroidism and CSA both present with similar symptoms of fatigue, daytime sleepiness, depression, and headaches. Studies suggest that respiratory muscle fatigue and decreased ventilatory response to hypercapnia and hypoxia contribute to apnea in this population. In one study, 27% of hypothyroid patients had a blunted response to hypercapnia, and 34% suffered from a blunted response to hypoxia. The same study showed universal reversal of the impairment following thyroid replacement therapy and return to euthyroid state.30 Similarly, multiple studies have shown reversal of respiratory muscle fatigue after initiation of thyroid replacement.30-32 Assessing thyroid function is an appropriate initial step during any sleep-disordered breathing workup, as it is a reversible cause of apnea. Up to 2.4% of patients presenting for PSG (and diagnosed with OSA) are found to have undiagnosed hypothyroidism.32,33 In a military population, treatment of a secondary cause of CSA, such as hypothyroidism, could remove some administrative burden as well as improve service members’ quality of life.
If CSA persists despite previous treatment strategies, then clinicians should focus on the optimization of treatment for comorbid conditions. If that does not resolve CSA, CPAP should be used when AHI remains above 15 events per hour or ASV can be used.
Idiopathic CSA
There are limited data on the pathophysiology and prevalence of idiopathic CSA. In most cases it is hypocapnic CSA, which occurs after an arousal from sleep causing hyperventilation that causes hypocapnia below the apnea threshold similar to CSA-HAPB. Therapeutic options based on addressing underlying pathophysiology include increasing CO2 by inhalation or addition of dead space. Additional therapeutic options to reduce the arousals and CSAs include hypnotics, such as zolpidem and acetazolamide, but these should be administered only with close clinical monitoring. If symptoms continue, CPAP or ASV may be trialed; however, limited clinical evidence of efficacy exists.15
For patients with moderate-to-severe CSA, an additional treatment option includes an implantable device (eg, Zoll remede¯), which stimulates the phrenic nerve to move the diaphragm and restore normal breathing. This device is not indicated for those with OSA. Based on data submitted to the US Food and Drug Administration, AHI is reduced by ≥ 50% in 51% of patients with the implanted device and by 11% in patients without the device. Five-year follow-up data show sustained improvements.34
Hypercapnic CSA
CSA due to a medication or substance requires the following criteria: (1) the patient is taking an opioid or other respiratory depressant; (2) the patient reports sleepiness, awakening with shortness of breath, snoring, witnessed apneas, or insomnia (difficulty initiating or maintaining sleep, frequent awakenings, or nonrestorative sleep); (3) PSG reveals ≥ 5 CSAs and/or central hypopneas per hour of sleep; the number of CSAs and/or central hypopneas is > 50% of the total number of apneas and hypopneas; and there is no evidence of CSB; and (4) the disorder is not better explained by another current sleep disorder.8
Drugs that affect the respiratory centers, such as opiates and opioids, γ aminobutyric acid (GABA) type A and B receptor agonists, and P2Y(12) receptor antagonists such as ticagrelor, may result in alterations in ventilatory drive in the central nervous system respiratory centers, resulting in CSA.
Opioids are prescribed either for chronic pain or to treat opiate addiction with methadone, resulting in about one-third of chronic opioid users having some form of CSA.35 CSA may be seen after opioids have been used for at least 2 months. A dose-dependent effect exists with high doses of opioids, typically resulting in hypoventilation, hypercapnia, and hypoxemia with ataxic or erratic breathing and a periodic breathing pattern similar to those described in CSA-HAPB or idiopathic CSA. About 14% to 60% of methadone patients also demonstrate CSA or ataxic breathing.35,36
Benzodiazepines (GABA-A receptor agonists) and baclofen (a GABA-B receptor agonist) depress central ventilatory drive, blunt the response to hypoxia and hypercapnia, leading to CSAs, and increase risk for OSA by increasing upper airway obstruction through reduction in tone. Use of these medications with antidepressants or opioids further exacerbates this response.
Unlike the other medications previously described, ticagrelor, a first-line dual antiplatelet therapy medication indicated for acute coronary syndrome treatment, actually increases the activity of the respiratory centers but may result in CSA.
First-line treatment, if possible, is reduction in medication dose or complete withdrawal. Additional treatment options include PAP therapies: CPAP, BiPAP, ASV, and oxygen therapy with or without PAP.37,38 The literature has demonstrated that for the treatment of opioid-associated CSA, ASV (in cases of normocapnia) and noninvasive ventilation (NIV)/BiPAP (in cases with hypercapnia or REM sleep hypoventilation) are superior treatment options when compared with conventional CPAP for elimination of respiratory events. CPAP with oxygen therapy and BiPAP with oxygen therapy are more effective than CPAP alone in reducing respiratory events. However, concerns remain that as with CSA in HF, CSA in chronic opioid users may serve as a physiologic protective mechanism to prevent further clinical injury from opioids. Similarly, as in the use of ASV in the SERVE-HF trial, focusing on elimination of respiratory events may prove detrimental. More studies are needed to determine whether reducing the number of CSA events in chronic opioid users is clinically beneficial when other health outcomes, such as cardiovascular, neurocognitive, hospital/intensive care unit admissions, and mortality risks are examined.
Neuromuscular-Induced CSA
CSA also is highly prevalent in neuromuscular conditions, such as amyotrophic lateral sclerosis, Duchenne muscular dystrophy, myotonic dystrophy, advanced multiple sclerosis, and acid maltase deficiency. There is reduced respiratory muscle strength and tone in these disorders, resulting in alveolar hypoventilation with hypercapnia. Given the hypercapnia, NIV/BiPAP is the first-line treatment to improve survival, gas exchange, symptom burden, and quality of life.
Stroke-Induced CSA
Extensive cerebrovascular events commonly precipitate sleep-related breathing disorders. The incidence increases in the acute phase of stroke and decreases 3 to 6 months poststroke; however, incidence also depends on the severity of the stroke.7,39,40 Stroke also has been shown to be a predictor of CSA (odds ratio, 1.65; 95% CI, 1.50-1.82; P < .001) in a retrospective analysis of a large cohort of US veterans.2 The location of the lesion often determines whether normocapnic or hypercapnic CSA will predominate, based on ventilatory instability resulting in normocapnia or reduced ventilatory drive resulting in hypercapnic CSA. PSG results and blood gases direct the treatment options. CSA with normocapnia is treated with ASV, and patients with hypercapnia/REM sleep hypoventilation are treated with NIV/BiPAP.
Conclusions
While much has been learned about CSA in recent decades, more evidence needs to be gathered to determine optimal treatment strategies and the impact on patient prognosis. The identification of CSA can lead to the diagnosis of previously unrecognized medical conditions. With proper diagnosis and treatment, we can optimize clinical management and improve patients’ prognosis and quality of life.
Acknowledgments
The authors thank the librarians of the Franzello Aeromedical Library in particular Sara Craycraft, Catherine Stahl, Kristen Young and Elizabeth Irvine for their support of this publication.
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
1. Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310-318. Epub 2015 Feb 12. doi:10.1016/S2213-2600(15)00043-0
2. Ratz D, Wiitala W, Safwan Badr M, Burns J, Chowdhuri S. Correlates and consequences of central sleep apnea in a national sample of US veterans. Sleep. 2018;41(9):zy058. doi:10.1093/sleep/zsyn058
3. Agrawal R, Sharafkhaneneh A, Gottlief, DJ, Nowakowski S, Razjouyan J. Mortality patterns associated with central sleep apnea among veterans: a large, retrospective, longitudinal report. Ann Am Thorac Soc. 2022;10.1513/AnnalsATS.202207-648OC. doi:10.1513/annalsATS. 202207-648OC
4. Mysliwiec V, McGraw L, Pierce R, Smith, P, Trapp, B, Roth B. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167-174. doi:10.5665/sleep.2364
5. Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14-21. Epub 2019 Mar 13. doi:10.1007/s13665-019-0221-z
6. Baillieul S, Revol B, Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Pépin JL. Diagnosis and management of central sleep apnea syndrome. Expert Rev Respir Med. 2019;13(6):545-557.1604226. Epub 2019 Apr 24. doi:10.1080/17476348.2019
7. Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J. 2017;49(1):1600959. doi:10.1183/13993003.00959-2016
8. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. American Academy of Sleep Medicine; 2014.
9. Lévy P, Pépin J-L, Tamisier R, Neuder Y, Baguet J-P, Javaheri S. Prevalence and impact of central sleep apnea in heart failure. Sleep Med Clinics. 2007;2(4):615-621. doi:10.1016/j.jsmc.2007.08.001
10. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608. doi:10.1093/eurjhf/hfp057
11. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ. 2013;22(2):104-109. Epub 2012 Oct 26. doi:10.1016/j.hlc.2012.08.006
12. Iotti GA, Polito A, Belliato M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36(8):1371-1379. Epub 2010 May 26. doi:10.1007/s00134-010-1917-2
13. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095-105. Epub 2015 Sep 1. doi:10.1056/NEJMoa1506459
14. Nigam G, Riaz M, Chang ET, Camacho M. Natural history of treatment-emergent central sleep apnea on positive airway pressure: a systematic review. Ann Thorac Med. 2018;13(2):86-91. doi:10.4103/atm.ATM_321_17
15. Orr JE, Malhotra A, Sands SA. Pathogenesis of central and complex sleep apnoea. Respirology. 2017;22(1):43-52. Epub 2016 Oct 31. doi:10.1111/resp.12927
16. Berger M, Solelhac G, Horvath C, Heinzer R, Brill AK. Treatment-emergent central sleep apnea associated with non-positive airway pressure therapies in obstructive sleep apnea patients: a systematic review. Sleep Med Rev. 2021; 58:101513. Epub 2021 Jun 5. doi:10.1016/j.smrv.2021.101513
17. Zhang J, Wang L, Guo HJ, Wang Y, Cao J, Chen BY. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J (Engl). 2020;133(22):2721-2730. doi:10.1097/CM9.0000000000001125
18. Nigam G, Pathak C, Riaz M. A systematic review on prevalence and risk factors associated with treatment- emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202-210. doi:10.4103/1817-1737.185761
19. Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449-2457. Epub 2021 Jan 23. doi:10.1016/j.hest.2021.01.036
20. Liu D, Armitstead J, Benjafield A. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751-760. Epub 2017 Jun 16. doi:10.1016/j.chest.2017.06.010
21. Moro M, Gannon K, Lovell K, Merlino M, Mojica J, Bianchi MT. Clinical predictors of central sleep apnea evoked by positive airway pressure titration. Nat Sci Sleep. 2016;8:259-266. doi:10.2147/NSS.S110032
22. Orr JE, Heinrich EC, Djokic M, et al. Adaptive servoventilation as treatment for central sleep apnea due to high-altitude periodic breathing in nonacclimatized healthy individuals. High Alt Med Biol. 2018;19(2):178-184. Epub 2018 Mar 13. doi:10.1089/ham.2017.0147
23. Liu HM, Chiang IJ, Kuo KN, Liou CM, Chen C. The effect of acetazolamide on sleep apnea at high altitude: a systematic review and meta-analysis. Ther Adv Respir Dis. 2017;11(1):20-29. Epub 2016 Nov 15. doi:10.1177/1753465816677006
24. Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390-8. doi:10.1001/jama.2012.94847
25. Nigam G, Pathak C, Riaz M. A systematic review of central sleep apnea in adult patients with chronic kidney disease. Sleep Breath. 2016;20(3):957-964. Epub 2016 Jan 27. doi:10.1007/s11325-016-1317-0
26. Nigam G, Riaz M. Pathophysiology of central sleep apnea in chronic kidney disease. Saudi J Kidney Dis Transpl. 2016;27(5):1068-1070. doi:10.4103/1319-2442.190907
27. Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102-107. doi:10.1056/NEJM200101113440204
28. Jean G, Piperno D, François B, Charra B. Sleep apnea incidence in maintenance hemodialysis patients: influence of dialysate buffer. Nephron. 1995;71(2):138-142. doi:10.1159/000188701
29. Pressman MR, Benz RL, Schleifer CR, Peterson DD. Sleep disordered breathing in ESRD: acute beneficial effects of treatment with nasal continuous positive airway pressure. Kidney Int. 1993;43(5):1134-1139. doi:10.1038/ki.1993.159
30. Ladenson PW, Goldenheim PD, Ridgway EC. Prediction and reversal of blunted ventilatory responsiveness in patients with hypothyroidism. Am J Med. 1988;84(5):877-883. doi:10.1016/0002-9343(88)90066-6
31. Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189-194. doi:10.1378/chest.102.1.189
32. Laroche CM, Cairns T, Moxham J, Green M. Hypothyroidism presenting with respiratory muscle weakness. Am Rev Respir Dis. 1988;138(2):472-474. doi:10.1164/ajrccm/138.2.472

33. Skjodt NM, Atkar R, Easton PA. Screening for hypothyroidism in sleep apnea. Am J Respir Crit Care Med. 1999;160(2):732-735. doi:10.1164/ajrccm.160.2.9802051
34. American Academy of Sleep Medicine. FDA approves Remede¯ implantable device to treat central sleep apnea. Accessed February 3, 2023. https://aasm.org/fda-approves-remede-implantable-device-treat-central-sleep-apnea
35. Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348-1356. doi:10.1378/chest.128.3.1348
36. Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD. Obstructive sleep apnea is more common than central sleep apnea in methadone maintenance patients with subjective sleep complaints. Drug Alcohol Depend. 2010;108(1-2):77-83. Epub 2010 Jan 15. doi:10.1016/j.drugalcdep.2009.11.019
37. Correa D, Farney RJ, Chung F, Prasad A, Lam D, Wong J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120:1273-1285. doi:10.1213/ANE.0000000000000672
38. Wang, D, Yee, BJ, Gunstein RR, Chung F. Chronic opioid use and central sleep apnea, where are we now and where to go? A state of the art review. Anesth Analg. 2021;132(5):1244-1253. doi:10.1213/ANE.0000000000005378
39. Schütz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304-306. Epub 2020 Aug 28. doi:10.1016/j.sleep.2020.08.025
40. Seiler A, Camilo M, Korostovtseva L, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648-e654. Epub 2019 Jan 11. doi:10.1212/WNL.0000000000006904
Epithelioma Cuniculatum (Plantar Verrucous Carcinoma): A Systematic Review of Treatment Options
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).
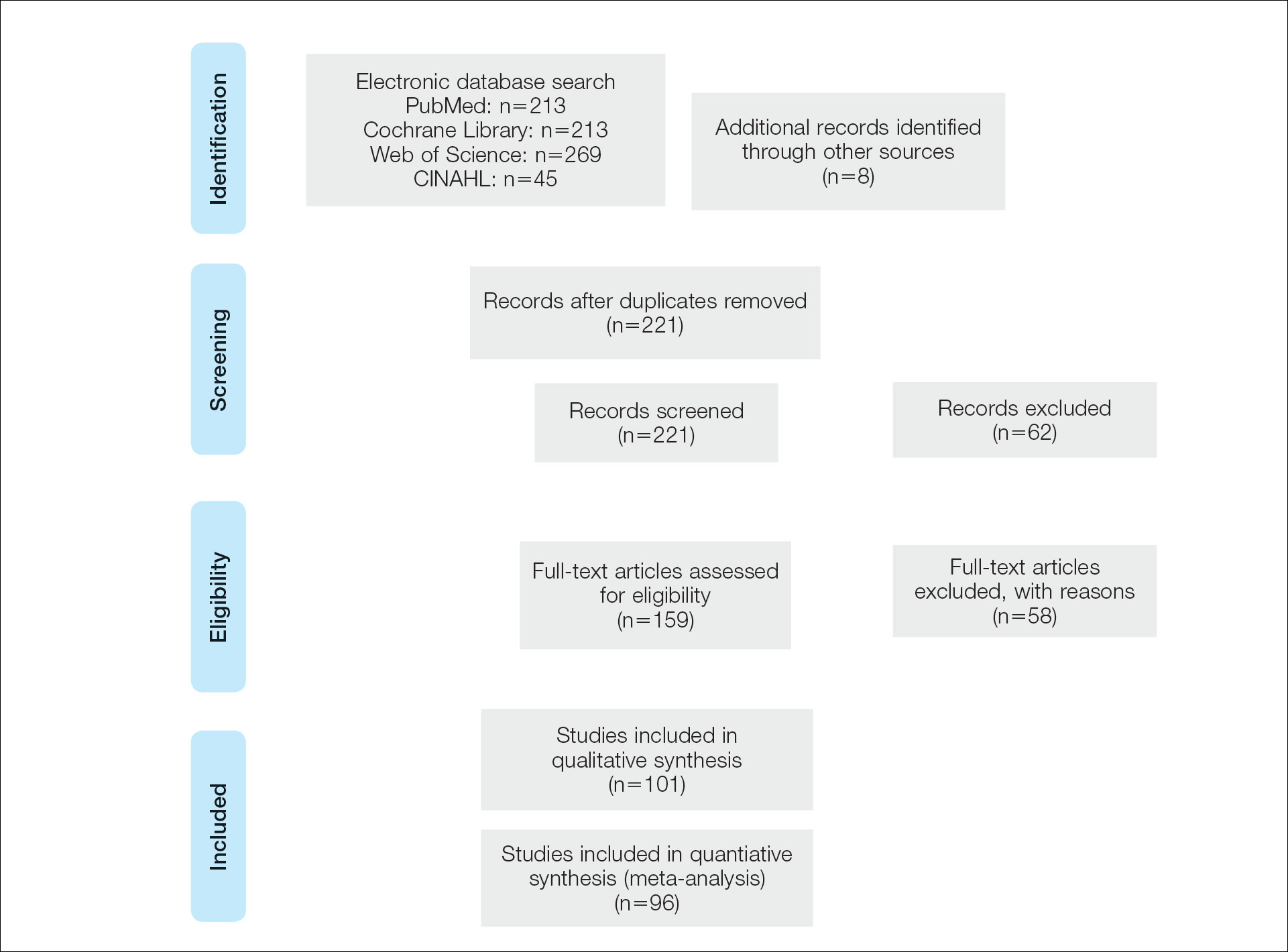
A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.
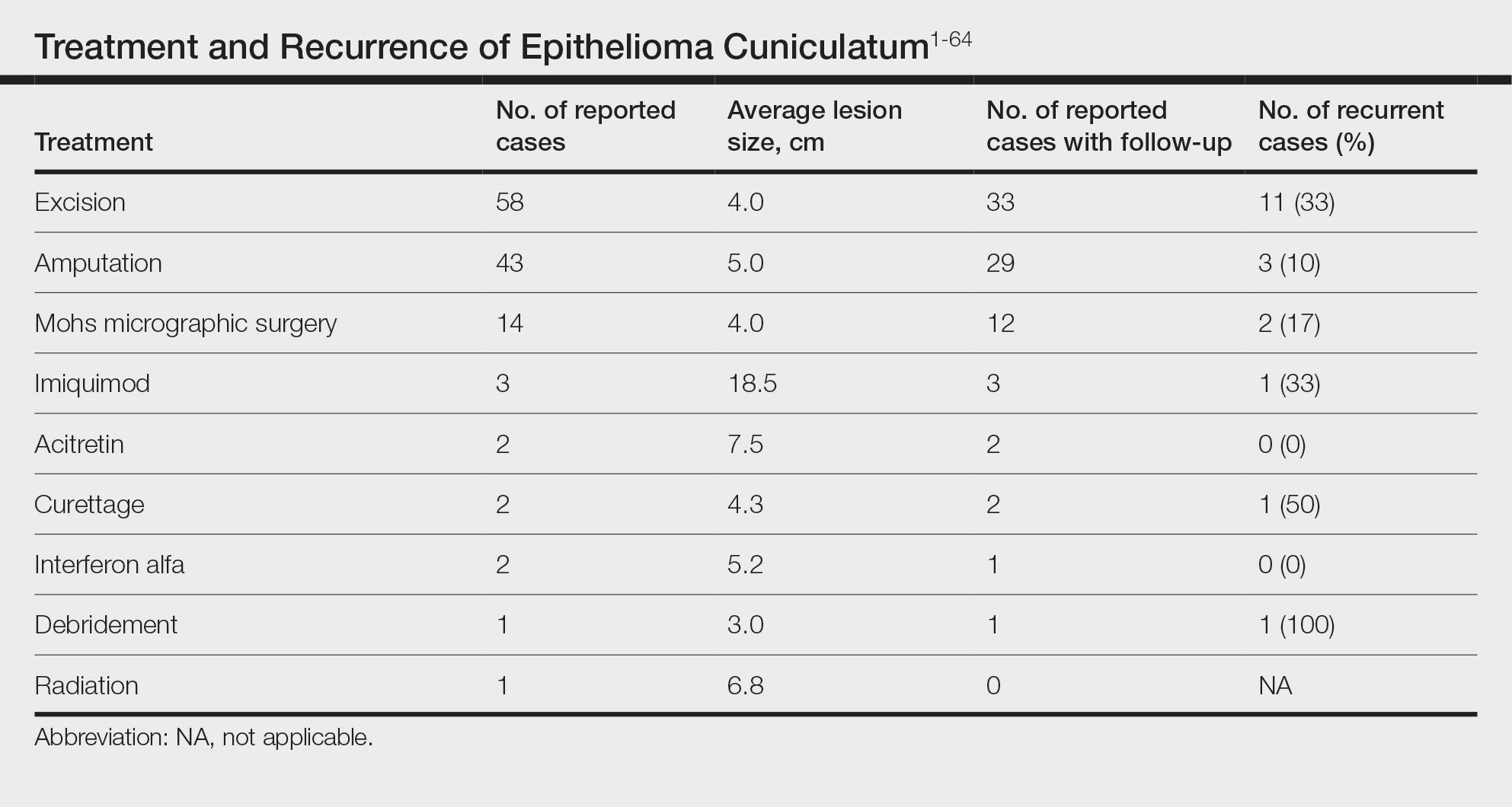
Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).

A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.

Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
Verrucous carcinoma (VC) is an uncommon type of well-differentiated squamous cell carcinoma (SCC) that most commonly affects men in the fifth to sixth decades of life. 1 The tumor grows slowly over a decade or more and does not frequently metastasize but has a high propensity for recurrence and local invasion. 2 There are 3 main subtypes of VC classified by anatomic site: oral florid papillomatosis (oral cavity), Buschke-Lowenstein tumor (anogenital region), and epithelioma cuniculatum (EC)(feet). 3 Epithelioma cuniculatum, also known as carcinoma cuniculatum or papillomatosis cutis carcinoides, most commonly presents as a solitary, warty or cauliflowerlike, exophytic mass with keratin-filled sinus tracts and malodorous discharge. 4 Diabetic foot ulcers and chronic inflammatory conditions are predisposing risk factors for EC, and it can result in difficulty walking/immobility, pain, and bleeding depending on anatomic involvement. 5-9
The differential diagnosis for VC includes refractory verruca vulgaris, clavus, SCC, keratoacanthoma, deep fungal or mycobacterial infection, eccrine poroma or porocarcinoma, amelanotic melanoma, and sarcoma.10-13 The slow-growing nature of VC, sampling error of superficial biopsies, and minimal cytological atypia on histologic examination can contribute to delayed diagnosis and appropriate treatment.14 Characteristic histologic features include hyperkeratosis, papillomatosis, marked acanthosis, broad blunt-ended rete ridges with a “bulldozing” architecture, and minimal cytologic atypia and mitoses.5,6 In some cases, pleomorphism and glassy eosinophilic cytoplasmic changes may be more pronounced than that of a common wart though less dramatic than that of conventional SCCs.15 Antigen Ki-67 and tumor protein p53 have been proposed to help differentiate between common plantar verruca, VC, and SCC, but the histologic diagnosis remains challenging, and repeat histopathologic examination often is required.16-19 Following diagnosis, computed tomography or magnetic resonance imaging may be necessary to determine tumor extension and assess for deep tissue and bony involvement.20-22
Treatment of EC is particularly challenging because of the anatomic location and need for margin control while maintaining adequate function, preserving healthy tissue, and providing coverage of defects. Surgical excision of EC is the first-line treatment, most commonly by wide local excision (WLE) or amputation. Mohs micrographic surgery (MMS) also has been utilized. One review found no recurrences in 5 cases of EC treated with MMS.23 As MMS is a tissue-sparing technique, this is a valuable modality for sites of functional importance such as the feet. Herein, we review various reported EC treatment modalities and outcomes, with an emphasis on recurrence rates for WLE and MMS.
METHODS
A systematic literature review of PubMed articles indexed for MEDLINE, as well as databases including the Cochrane Library, Web of Science, and Cumulative Index to Nursing and Allied Health Literature (CINAHL), was performed on January 14, 2020. Two authors (S.S.D. and S.V.C.) independently screened results using the search terms (plantar OR foot) AND (verrucous carcinoma OR epithelioma cuniculatum OR carcinoma cuniculatum). The search terms were chosen according to MeSH subject headings. All articles from the start date of the databases through the search date were screened, and articles pertaining to VC, EC, or carcinoma cuniculatum located on the foot were included. Of these, non–English-language articles were translated and included. Articles reporting VC on a site other than the foot (eg, the oral cavity) or benign verrucous skin lesions were excluded. The reference lists for all articles also were reviewed for additional reports that were absent from the initial search using both included and excluded articles. A full-text review was performed on 221 articles published between 1954 and 2019 per the PRISMA guidelines (Figure).

A total of 101 articles were included in the study for qualitative analysis. Nearly all articles identified were case reports, giving an evidence level of 5 by the Centre for Evidence-Based Medicine rating scale. Five articles reported data on multiple patients without individual demographic or clinical details and were excluded from analysis. Of the remaining 96 articles, information about patient characteristics, tumor size, treatment modality, and recurrence were extracted for 115 cases.
RESULTS
Of the 115 cases that were reviewed, 81 (70%) were male and 33 (29%) were female with a male-to-female ratio of 2.4:1. Ages of the patients ranged from 18 to 88 years; the mean and median age was 56 years. Nearly all reported cases of EC affected the plantar surface of one foot, with 4 reports of tumors affecting both feet.24-27 One case affecting both feet reported known exposure to lead arsenate pesticides27; all others were associated with a clinical history of chronic ulcers or warts persisting for several years to decades. Other less common sites of EC included the dorsal foot, interdigital web space, and subungual digit.28-30 The most common location reported was the anterior ball of the foot. Tumors were reported to arise within pre-existing lesions, such as hypertrophic lichen planus or chronic foot wounds associated with diabetes mellitus or leprosy.31-35 Tumor size ranged from 1 to 22 cm with a median of 4.5 cm.
Eight cases were reported to be associated with human papillomavirus; low-risk types 6 and 11 and high-risk types 16 and 18 were found in 6 cases.36-41 Two cases reported association with human papillomavirus type 2.7,42
Metastases to dermal and subdermal lymphatics, regional lymph nodes, and the lungs were reported in 3 cases, repectively.43-45 Of these, one primary tumor had received low-dose irradiation in the form of X-ray therapy.45
Treatment Modalities
The cases of EC that we reviewed included treatment with surgical and systemic therapies as well as other modalities such as acitretin, interferon alfa, topical imiquimod, curettage, debridement, electrodesiccation, and radiation. The Table includes a complete summary of the treatments we analyzed.

Surgical Therapy—The majority (91% [105/115]) of cases were treated surgically. The most common treatment modality was WLE (50% [58/115]), followed by amputation (37% [43/115]) and MMS (12% [14/115]).
Wide local excision was the most frequently reported treatment, with excision margins of at least 5 mm to 1 cm.48 Incidence of recurrence was reported for 57% (33/58) of cases treated with WLE; of these, the recurrence rate was 33% (11/33). For patients with EC recurrence, the most common secondary treatment was repeat excision with wider margins (1–2 cm) or amputation (5/11).49-52 Few postoperative complications were reported but included pain, infection, and difficulty walking, which were mostly associated with repair modality (eg, split-thickness skin grafts, rotational flaps).53 Amputation was the second most common treatment modality, with a 67% (29/43) incidence of recurrence. Types of amputation included transmetatarsal ray amputation (7/43 [16%]), foot or forefoot amputation (2/43 [5%]), above-the-knee amputation (1/43 [2%]), and below-the-knee amputation (1/43 [2%]). Complications associated with amputation included infection and requirement of prosthetics for ambulation. Split-thickness skin grafts and rotational flaps were the most common surgical repairs performed.52,53
Mohs micrographic surgery was the least frequently reported surgical treatment modality. Both traditional MMS on fresh tissue and “slow Mohs,” with formalin-fixed paraffin embedded tissue examination over several days, were performed for EC with horizontal en face sectioning.54-56 Incidence of recurrence was reported for 86% (12/14) of MMS cases. Of these, recurrence was seen in 17% (2/12) that utilized a flat horizontal processing of tissue sections coupled with saucerlike excisions to enable examination of the entire undersurface and margins. In one case, the patient was treated with MMS with recurrence noted 1 month later; thus, repeat MMS was performed, and the tumor was found to be entwined around the flexor tendon.57 The tendon was removed, and clear margins were obtained. Follow-up 3 years after the second MMS revealed no signs of recurrence.57 In the other case, the patient had a particularly aggressive course with bilateral VC in the setting of diabetic ulcers that was treated with WLE prior to MMS and recurrence still noted after MMS.26 No complications were reported with MMS.
Overall, recurrence was most frequently reported with WLE (11/33 [33%]), followed by MMS (2/12 [17%]) and amputation (3/29 [10%]). When comparing WLE and amputation, the relationship between treatment modality and recurrence was statistically significant using a χ2 test of independence (χ2=4.7; P=.03). However, results were not significant with Yates correction for continuity (χ2=3.4; P=.06). The χ2 test of independence showed no significant association between treatment method and recurrence when comparing WLE with MMS (χ2=1.2; P=.28). Reported follow-up times varied greatly from a few months to 10 years.
Systemic Therapy—Of the total cases, only 2 cases reported treatment with acitretin and 2 utilized interferon alfa.58,59 In one case, treatment of EC with interferon alfa alone required more aggressive therapy (ie, amputation).58 Neither of the 2 cases using acitretin reported recurrence.59,60 Complications of acitretin therapy included cheilitis and transaminitis.60
Other Treatment Modalities—Three cases utilized imiquimod, with 2 cases of imiquimod monotherapy and 1 case of imiquimod in combination with electrodesiccation and WLE.37 One of the cases of EC treated with imiquimod monotherapy recurred and required WLE.61
There were reports of other treatments including curettage alone (2% [2/115]),40,62 debridement alone (1% [1/115]),40 electrodesiccation (1% [1/115]),37 and radiation (1% [1/115]).43 Recurrence was found with curettage alone and debridement alone. Electrodesiccation was reported in conjunction with WLE without recurrence. Radiation was used to treat a case of VC that had metastasized to the lymph nodes; no follow-up was described.43
COMMENT
Epithelioma cuniculatum is an indolent malignancy of the plantar foot that likely is frequently underdiagnosed or misdiagnosed because of location, sampling error, and challenges in histopathologic diagnosis. Once diagnosed, surgical removal with margin control is the first-line therapy for EC. Our review found a number of surgical, systemic, and other treatment modalities that have been used to treat EC, but there remains a lack of evidence to provide clear guidelines as to which therapies are most effective. Current data on the treatment of EC largely are limited to case reports and case series. To date, there are no reports of higher-quality studies or randomized controlled trials to assess the efficacy of various treatment modalities.
Our review found that WLE is the most common treatment modality for EC, followed by amputation and MMS. Three cases43-45 that reported metastasis to lymph nodes also were treated with fine-needle aspiration or biopsy, and it is recommended that sentinel lymph node biopsy be performed when there is a history of radiation exposure or clinically and sonographically unsuspicious lymph nodes, while dissection of regional nodes should be performed if lymph node metastasis is suspected.53 Additional treatments reported included acitretin, interferon alfa, topical imiquimod, curettage, debridement, and electrodesiccation, but because of the limited number of cases and variable efficacy, no conclusions can be made on the utility of these alternative modalities.
The lowest rate of reported recurrence was found with amputation, followed by MMS and WLE. Amputation is the most aggressive treatment option, but its superiority in lower recurrence rates was not statistically significant when compared with either WLE or MMS after Yates correction. Despite treatment with radical surgery, recurrence is still possible and may be associated with factors including greater size (>2 cm) and depth (>4 mm), poor histologic differentiation, perineural involvement, failure of previous treatments, and immunosuppression.63 No statistically significant difference in recurrence rates was found among surgical methods, though data trended toward lower rates of recurrence with MMS compared with WLE, as recurrence with MMS was only reported in 2 cases.25,56
The efficacy of MMS is well documented for tumors with contiguous growth and enables maximum preservation of normal tissue structure and function with complete margin visualization. Thus, our results are in agreement with those of prior studies,54-56,64 suggesting that MMS is associated with lower recurrence rates for EC than WLE. Future studies and reporting of MMS for EC are particularly important because of the functional importance of the plantar foot.
It is important to note that there are local and systemic risk factors that increase the likelihood of developing EC and facilitate tumor growth, including antecedent trauma to the lesion site, chronic irritation or infection, and immunosuppression (HIV related or iatrogenic medication induced). These risk factors may play a role in the treatment modality utilized (eg, more aggressive EC may be treated with amputation instead of WLE). Underlying patient comorbidities could potentially affect recurrence rates, which is a variable we could not control for in our analysis.
Our findings are limited by study design, with supporting evidence consisting of case reports and series. The review is limited by interstudy variability and heterogeneity of results. Additionally, recurrence is not reported in all cases and may be a source of sampling bias. Further complicating the generalizability of these results is the lack of follow-up to evaluate morbidity and quality of life after treatment.
CONCLUSION
This review suggests that MMS is associated with lower recurrence rates than WLE for the treatment of EC. Further investigation of MMS for EC with appropriate follow-up is necessary to identify whether MMS is associated with lower recurrence and less functional impairment. Nonsurgical treatments, including topical imiquimod, interferon alfa, and acitretin, may be useful in cases where surgical therapies are contraindicated, but there is little evidence to support these treatment modalities. Treatment guidelines for EC are not established, and appropriate treatment guidelines should be developed in the future.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- McKee PH, Wilkinson JD, Black MM, et al. Carcinoma (epithelioma) cuniculatum: a clinicopathological study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Seremet S, Erdemir AT, Kiremitci U, et al. Unusually early-onset plantar verrucous carcinoma. Cutis. 2019;104:34-36.
- Spyriounis PK, Tentis D, Sparveri IF, et al. Plantar epithelioma cuniculatum. a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Ho J, Diven G, Bu J, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Zielonka E, Goldschmidt D, de Fontaine S. Verrucous carcinoma or epithelioma cuniculatum plantare. Eur J Surg Oncol. 1997;23:86-87.
- Dogan G, Oram Y, Hazneci E, et al. Three cases of verrucous carcinoma. Australas J Dermatol. 1998;39:251-254.
- Schwartz RA, Burgess GH. Verrucous carcinoma of the foot. J Surg Oncol. 1980;14:333-339.
- McKay C, McBride P, Muir J. Plantar verrucous carcinoma masquerading as toe web intertrigo. Australas J Dermatol. 2012;53:2010-2012.
- Shenoy AS, Waghmare RS, Kavishwar VS, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Lozzi G, Perris K. Carcinoma cuniculatum. CMAJ. 2007;177:249-251.
- Schein O, Orenstein A, Bar-Meir E. Plantar verrucous carcicoma (epithelioma cuniculatum): rare form of the common wart. Isr Med Assoc J. 2006;8:885.
- Rheingold LM, Roth LM. Carcinoma of the skin of the foot exhibiting some verrucous features. Plast Reconstr Surg. 1978;61:605-609.
- Klima M, Kurtis B, Jordan PH. Verrucous carcinoma of skin. J Cutan Pathol. 1980;7:88-98.
- Nakamura Y, Kashiwagi K, Nakamura A, et al. Verrucous carcinoma of the foot diagnosed using p53 and Ki-67 immunostaining in a patient with diabetic neuropathy. Am J Dermatopathol. 2015;37:257-259.
- Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Terada T. Verrucous carcinoma of the skin: a report on 5 Japanese cases. Ann Diagn Pathol. 2011;15:175-180.
- Noel JC, Heenen M, Peny MO, et al. Proliferating cell nuclear antigen distribution in verrucous carcinoma of the skin. Br J Dermatol. 1995;133:868-873.
- García-Gavín J, González-Vilas D, Rodríguez-Pazos L, et al. Verrucous carcinoma of the foot affecting the bone: utility of the computed tomography scanner. Dermatol Online J. 2010;16:3-5.
- Wasserman PL, Taylor RC, Pinillia J, et al. Verrucous carcinoma of the foot and enhancement assessment by MRI. Skeletal Radiol. 2009;38:393-395.
- Bhushan MH, Ferguson JE, Hutchinson CE. Carcinoma cuniculatum of the foot assessed by magnetic resonance scanning. Clin Exp Dermatol. 2001;26:419-422.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Suen K, Wijeratne S, Patrikios J. An unusual case of bilateral verrucous carcinoma of the foot (epithelioma cuniculatum). J Surg Case Rep. 2012;2012:rjs020.
- Riccio C, King K, Elston JB, et al. Bilateral plantar verrucous carcinoma. Eplasty. 2016;16:ic46.
- Di Palma V, Stone JP, Schell A, et al. Mistaken diabetic ulcers: a case of bilateral foot verrucous carcinoma. Case Rep Dermatol Med. 2018;2018:4192657.
- Seehafer JR, Muller SA, Dicken CH. Bilateral verrucous carcinoma of the feet. Orthop Surv. 1979;3:205.
- Tosti A, Morelli R, Fanti PA, et al. Carcinoma cuniculatum of the nail apparatus: report of three cases. Dermatology. 1993;186:217-221.
- Melo CR, Melo IS, Souza LP. Epithelioma cuniculatum, a verrucous carcinoma of the foot. report of 2 cases. Dermatologica. 1981;163:338-342.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Thakur BK, Verma S, Raphael V. Verrucous carcinoma developing in a long standing case of ulcerative lichen planus of sole: a rare case report. J Eur Acad Dermatol Venereol. 2015;29:399-401.
- Mayron R, Grimwood RE, Siegle RJ, et al. Verrucous carcinoma arising in ulcerative lichen planus of the soles. J Dermatol Surg Oncol. 1988;14:547-551.
- Boussofara L, Belajouza-Noueiri C, Ghariani N, et al. Verrucous epidermoid carcinoma as a complication in cutaneous lichen planus [article in French]. Ann Dermatol Venereol. 2006;133:404-405.
- Khullar G, Mittal S, Sharma S. Verrucous carcinoma on the foot arising in a chronic neuropathic ulcer of leprosy. Australas J Dermatol. 2019;60:245-246.
- Ochsner PE, Hausman R, Olsthoorn PGM. Epithelioma cunicalutum developing in a neuropathic ulcer of leprous etiology. Arch Orthop Trauma Surg. 1979;94:227-231.
- Ray R, Bhagat A, Vasudevan B, et al. A rare case of plantar epithelioma cuniculatum arising from a wart. Indian J Dermatol. 2015;60:485-487.
- Imko-Walczuk B, Cegielska A, Placek W, et al. Human papillomavirus-related verrucous carcinoma in a renal transplant patient after long-term immunosuppression: a case report. Transplant Proc. 2014;46:2916-2919.
- Floristán MU, Feltes RA, Sáenz JC, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 18. Actas Dermosifiliogr. 2009;100:433-435.
- Sasaoka R, Morimura T, Mihara M, et al. Detection of human pupillomavirus type 16 DNA in two cases of verriicous carcinoma of the foot. Br J Dermatol. 1996;134:983984.
- Schell BJ, Rosen T, Rády P, et al. Verrucous carcinoma of the foot associated with human papillomavirus type 16. J Am Acad Dermatol. 2001;45:49-55.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot‐blot hybridization implicates human papillomavirus type 11‐DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Jungmann J, Vogt T, Müller CSL. Giant verrucous carcinoma of the lower extremity in women with dementia. BMJ Case Rep. 2012;2012:bcr2012006357.
- McKee PH, Wilkinson JD, Corbett MF, et al. Carcinoma cuniculatum: a case metastasizing to skin and lymph nodes. Clin Exp Dermatol. 1981;6:613-618.
- Owen WR, Wolfe ID, Burnett JW, et al. Epithelioma cuniculatum. South Med J. 1978;71:477-479.
- Patel AN, Bedforth N, Varma S. Pain-free treatment of carcinoma cuniculatum on the heel using Mohs micrographic surgery and ultrasonography-guided sciatic nerve block. Clin Exp Dermatol. 2013;38:569-571.
- Padilla RS, Bailin PL, Howard WR, et al. Verrucous carcinoma of the skin and its management by Mohs’ surgery. Plast Reconstr Surg. 1984;73:442-447.
- Kotwal M, Poflee S, Bobhate S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Arefi M, Philipone E, Caprioli R, et al. A case of verrucous carcinoma (epithelioma cuniculatum) of the heel mimicking infected epidermal cyst and gout. Foot Ankle Spec. 2008;1:297-299.
- Trebing D, Brunner M, Kröning Y, et al. Young man with verrucous heel tumor [article in German]. J Dtsch Dermatol Ges. 2003;9:739-741.
- Thompson SG. Epithelioma cuniculatum: an unusual tumour of the foot. Br J Plast Surg. 1965;18:214-217.
- Thomas EJ, Graves NC, Meritt SM. Carcinoma cuniculatum: an atypical presentation in the foot. J Foot Ankle Surg. 2014;53:356-359.
- Koch H, Kowatsch E, Hödl S, et al. Verrucous carcinoma of the skin: long-term follow-up results following surgical therapy. Dermatol Surg. 2004;30:1124-1130.
- Mallatt BD, Ceilley RI, Dryer RF. Management of verrucous carcinoma on a foot by a combination of chemosurgery and plastic repair: report of a case. J Dermatol Surg Oncol. 1980;6:532-534.
- Mohs FE, Sahl WJ. Chemosurgery for verrucous carcinoma. J Dermatol Surg Oncol. 1979;5:302-306.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006;5:68-73.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Risse L, Negrier P, Dang PM, et al. Treatment of verrucous carcinoma with recombinant alfa-interferon. Dermatology. 1995;190:142-144.
- Rogozin´ski TT, Schwartz RA, Towpik E. Verrucous carcinoma in Unna-Thost hyperkeratosis of the palms and soles. J Am Acad Dermatol. 1994;31:1061-1062.
- Kuan YZ, Hsu HC, Kuo TT, et al. Multiple verrucous carcinomas treated with acitretin. J Am Acad Dermatol. 2007;56(2 suppl):S29-S32.
- Schalock PC, Kornik RI, Baughman RD, et al. Treatment of verrucous carcinoma with topical imiquimod. J Am Acad Dermatol. 2006;54:233-234.
- Brown SM, Freeman RG. Epithelioma cuniculatum. Arch Dermatol. 1976;112:1295-1296.
- Rowe DE, Carroll RJ, Day CL, et al. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Mohs’ chemosurgery technique. Arch Dermatol. 1980;116:794-797.
Practice Points
- Because of its slow-growing nature and propensity for local invasion and recurrence, diagnosis of epithelioma cuniculatum (EC) often is delayed and therefore can be associated with notable morbidity.
- Wide local excision with 5-mm to 1-cm margins is considered standard of care and is the most commonly reported treatment of EC. Amputation may be required in cases with extensive local destruction.
- Mohs micrographic surgery is a viable option for treatment of EC, with more recent cases suggesting favorable outcomes regarding recurrence rates.
Addressing OR sustainability: How we can decrease waste and emissions
In 2009, the Lancet called climate change the biggest global health threat of the 21st century, the effects of which will be experienced in our lifetimes.1 Significant amounts of data have demonstrated the negative health effects of heat, air pollution, and exposure to toxic substances.2,3 These effects have been seen in every geographic region of the United States, and in multiple organ systems and specialties, including obstetrics, pediatrics, and even cardiopulmonary and bariatric surgery.2-5
Although it does not receive the scrutiny of other industries, the global health care industry accounts for almost double the amount of carbon emissions as global aviation, and the United States accounts for 27% of this footprint despite only having 4% of the world’s population.6 It therefore serves that our own industry is an excellent target for reducing the carbon emissions that contribute to climate change. Consider the climate impact of hysterectomy, the second-most common surgery that women undergo. In this article, we will use the example of a 50-year-old woman with fibroids who plans to undergo definitive treatment via total laparoscopic hysterectomy (TLH).
Climate impact of US health care
Hospital buildings in the United States are energy intensive, consuming 10% of the energy used in commercial buildings every year, accounting for over $8 billion. Operating rooms (ORs) account for a third of this usage.7 Hospitals also use more water than any other type of commercial building, for necessary actions like cooling, sterilization, and laundry.8 Further, US hospitals generate 14,000 tons of waste per day, with a third of this coming from the ORs. Sadly, up to 15% is food waste, as we are not very good about selecting and proportioning healthy food for our staff and inpatients.6
While health care is utility intensive, the majority of emissions are created through the production, transport, and disposal of goods coming through our supply chain.6 Hospitals are significant consumers of single-use objects, the majority of which are petroleum-derived plastics—accounting for an estimated 71% of emissions coming from the health care sector. Supply chain is the second largest expense in health care, but with current shortages, it is estimated to overtake labor costs by this year. The United States is also the largest consumer of pharmaceuticals worldwide, supporting a $20 billion packaging industry,9 which creates a significant amount of waste.
Climate impact of the OR
Although ORs only account for a small portion of hospital square footage, they account for a significant amount of health care’s carbon footprint through high waste production and excessive consumption of single-use items. Just one surgical procedure in a hospital is estimated to produce about the same amount of waste as a typical family of 4 would in an entire week.10 Furthermore, the majority of these single-use items, including sterile packaging, are sorted inappropriately as regulated medical waste (RMW, “biohazardous” or “red bag” waste) (FIGURE 1a). RMW has significant effects on the environment since it must be incinerated or steam autoclaved prior to transport to the landfill, leading to high amounts of air pollution and energy usage.

We all notice the visible impacts of waste in the OR, but other contributors to carbon emissions are invisible. Energy consumption is a huge contributor to the overall carbon footprint of surgery. Heating, ventilation, and air conditioning [HVAC] is responsible for 52% of hospital energy needs but accounts for 99% of OR energy consumption.11 Despite the large energy requirements of the ORs, they are largely unoccupied in the evenings and on weekends, and thermostats are not adjusted accordingly.
Anesthetic gases are another powerful contributor to greenhouse gas emissions from the OR. Anesthetic gases alone contribute about 25% of the overall carbon footprint of the OR, and US health care emits 660,000 tons of carbon equivalents from anesthetic gas use per year.12 Desflurane is 1,600 times more potent than carbon dioxide (CO2) in its global warming potential followed by isoflurane and sevoflurane;13 this underscores the importance of working with our anesthesia colleagues on the differences between the anesthetic gases they use. Enhanced recovery after surgery recommendations in gynecology already recommend avoiding the use of volatile anesthetic gases in favor of propofol to reduce postoperative nausea and vomiting.14
In the context of a patient undergoing a TLH, the estimated carbon footprint in the United States is about 560 kg of CO2 equivalents—roughly the same as driving 1,563 miles in a gas-powered car.
Continue to: Climate impact on our patients...
Climate impact on our patients
The data in obstetrics and gynecology is clear that climate change is affecting patient outcomes, both globally and in our own country. A systematic review of 32 million births found that air pollution and heat exposure were associated with preterm birth and low birth weight, and these effects were seen in all geographic regions across the United States.1 A study of 5.9 million births in California found that patients who lived near coal- and oil-power plants had up to a 27% reduction in preterm births when those power plants closed and air pollution decreased.15 A study in Nature Sustainability on 250,000 pregnancies that ended in missed abortions at 14 weeks or less found the odds ratio of missed abortion increased with the cumulative exposure to air pollution.16 When air pollution was examined in comparison to other factors, neighborhood air pollution better predicted preterm birth, very preterm birth, and small for gestational age more than race, ethnicity, or any other socio-economic factor.17 The effects of air pollution have been demonstrated in other fields as well, including increased mortality after cardiac transplantation with exposure to air pollution,4 and for patients undergoing bariatric surgery who live near major roadways, decreased weight loss, less improvement in hemoglobin A1c, and less change in lipids compared with those with less exposure to roadway pollution.5
Air pollution and heat are not the only factors that influence health. Endocrine disrupting chemicals (EDCs) and single-use plastic polymers, which are used in significant supply in US health care, have been found in human blood,18 intestine, and all portions of the placenta.19 Phthalates, an EDC found in medical use plastics and medications to control delivery, have been associated with increasing fibroid burden in patients undergoing hysterectomy and myomectomy.20 The example case patient with fibroids undergoing TLH may have had her condition worsened by exposure to phthalates.

Specific areas for improvement
There is a huge opportunity for improvement to reduce the total carbon footprint of a TLH.
A lifecycle assessment of hysterectomy in the United States concluded that an 80% reduction in carbon emissions could be achieved by minimizing opened materials, using reusable and reprocessed instruments, reducing off-hour energy use in the OR (HVAC setbacks), and avoiding the use of volatile anesthetic gases.21 The sterilization and re-processing of reusable instruments represented the smallest proportion of carbon emissions from a TLH. Data on patient safety supports these interventions, as current practices have more to do with hospital culture and processes than evidence.
Despite a push to use single-use objects by industry and regulatory agencies in the name of patient safety, data demonstrate that single-use objects are in actuality not safer for patients and may be associated with increased surgical site infections (SSIs). A study from a cancer center in California found that when single-use head covers, shoe covers, and facemasks were eliminated due to supply shortages during the pandemic, SSIs went down by half, despite an increase in surgical volume and an increase in the number of contaminated cases.22 The authors reported an increase in hand hygiene throughout the hospital, which likely contributed to the success of reducing SSIs.
Similarly, a systematic review found no evidence to support single-use instruments over reusable or reprocessed instruments when considering instrument function, ease of use, patient safety, SSIs, or long-term patient outcomes.23 While it may be easy for regulatory agencies to focus on disposing objects as paramount to reducing infections, the Centers for Disease Control and Prevention states that the biggest factors affecting SSIs are appropriate use of prophylactic antibiotics, skin antisepsis, and patient metabolic control.24 Disposing of single-use objects in the name of patient safety will worsen patient health outcomes when considering patient proximity to waste, pollution, and EDCs.
The sterilization process for reusable items is often called out by the medical supply industry as wasteful and energy intensive; however, data refute these claims. A Swedish study researching reusable versus single-use trocars found that a reusable trocar system offers a robust opportunity to reduce both the environmental and financial costs for laparoscopic surgery.25 We can further decrease the environmental impact of reusable instruments by using sets instead of individually packed instruments and packing autoclaves more efficiently. By using rigid sterilization containers, there was an 85% reduction in carbon footprint as compared with the blue wrap system.
Electricity use can be easily reduced across all surgical spaces by performing HVAC setbacks during low occupancy times of day. On nights and weekends, when there are very few surgical cases occurring, one study found that by decreasing the ventilation rate, turning off lights, and performing the minimum temperature control in unused ORs, electricity use was cut in half.11
Waste triage and recycling
Reducing regulated medical waste is another area where hospitals can make a huge impact on carbon emissions and costs with little more than education and process change. Guidelines for regulated medical waste sorting developed out of the HIV epidemic due to the fear of blood products. Although studies show that regulated medical waste is not more infectious than household waste, state departments of public health have kept these guidelines in place for sorting fluid blood and tissue into RMW containers and bags.26 The best hospital performers keep RMW below 10% of the total waste stream, while many ORs send close to 100% of their waste as RMW (FIGURE 1b). ORs can work with nursing and environmental services staff to assess processes and divert waste into recycling and regular waste. Many OR staff are acutely aware of the huge amount of waste produced and want to make a positive impact. Success in this small area often builds momentum to tackle harder sustainability practices throughout the hospital.
Continue to: Removal of EDCs from medical products...
Removal of EDCs from medical products
Single-use medical supplies are not only wasteful but also contain harmful EDCs, such as phthalates, bisphenol A (BPA), parabens, perfluoroalkyl substances, and triclosan. Phthalates, for example, account for 30% to 40% of the weight of medical-use plastics, and parabens are ubiquitously found in ultrasound gel.3 Studies looking at exposure to EDCs within the neonatal intensive care unit reveal substantial BPA, phthalate, and paraben levels within biologic samples from premature infants, thought to be above toxicity limits. While we do not know the full extent to which EDCs can affect neonatal development, there is already mounting evidence that EDCs are associated with endocrine, metabolic, and neurodevelopmental disorders throughout our lifespan.3
30-day climate challenge
Although the example case patient undergoing TLH for fibroids will never need care for her fibroids again, the climate impact of her time in the OR represents the most carbon-intensive care she will ever need. Surgery as practiced in the United States today is unsustainable.
In 2021, the Biden administration issued an executive order requiring all federal facilities, including health care facilities and hospitals, to be carbon neutral by 2035. In order to make meaningful changes industry-wide, we should be petitioning lawmakers for stricter environmental regulations in health care, similar to regulations in the manufacturing and airline industries. We recommend a 30-day climate challenge (FIGURE 2) for bringing awareness to your circles of influence. Physicians have an ethical duty to advocate for change at the local, regional, and national level if we want to see a better future for our patients, their children, and even ourselves. Organizations such as Practice Greenhealth, Health Care without Harm, and Citizens’ Climate Lobby can help amplify our voices to reach the right people to implement sweeping policy changes. ●
- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733. doi: 10.1016/S0140-6736(09)60935-1.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3. doi:10.1001/JAMANETWORKOPEN.2020.8243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822–7. doi: 10.1210/cline2. m/dgaa358.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:3026-3035. doi: 10.1016/j.jacc.2019.09.066.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320. doi: 10.1111/ijpo.12210.
- Health Care’s Climate Footprint. Health care without harm climate-smart health care series, Green Paper Number one. September 2019. https://www.noharm.org/
ClimateFootprintReport. Accessed December 11, 2022. - Healthcare Energy End-Use Monitoring. US Department of Energy. https://www.energy.gov/eere/
buildings/downloads/ healthcare-energy-end-use- monitoring. Accessed December 11, 2022. - 2012 Commercial Buildings Energy Consumption Survey: Water Consumption in Large Buildings Summary. U.S Energy Information Administration. https://www.eia.gov/
consumption/commercial/ reports/2012/water. Accessed December 11, 2022. - Belkhir L, Elmeligi A. Carbon footprint of the global pharmaceutical industry and relative implact of its major players. J Cleaner Production. 2019;214:185-194. doi: 10.1016 /j.jclearpro.2019.11.204.
- Esaki RK, Macario A. Wastage of Supplies and Drugs in the Operating Room. 2015:8-13.
- MacNeill AJ, et al. The Impact of Surgery on Global Climate: A Carbon Footprinting Study of Operating Theatres in Three Health Systems. Lancet Planet Health.2017;1:e360–367. doi:10.1016/S2542-5196(17)30162-6.
- Shoham MA, Baker NM, Peterson ME, et al. The environmental impact of surgery: a systematic review. 2022;172:897-905. doi:10.1016/j.surg.2022.04.010.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98. doi:10.1213/ANE.0B013E3181E058D7.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573. doi: 10.1016/j.ogc.2016.04.006.
- Casey JA, Karasek D, Ogburn EL, et al. Retirements of coal and oil power plants in California: association with reduced preterm birth among populations nearby. Am J Epidemiol. 2018;187:1586-1594. doi: 10.1093/aje/kwy110.
- Zhang L, Liu W, Hou K, et al. Air pollution-induced missed abortion risk for pregnancies. Nat Sustain. 2019:1011–1017.
- Benmarhnia T, Huang J, Basu R, et al. Decomposition analysis of Black-White disparities in birth outcomes: the relative contribution of air pollution and social factors in California. Environ Health Perspect. 2017;125:107003. doi: 10.1289/EHP490.
- Leslie HA, van Velzen MJM, Brandsma SH, et al. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199.
- Ragusa A, Svelato A, Santacroce C, et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121. doi: 10.1016/j.fertnstert.2018.09.009.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786. doi: 10.1021/es504719g.
- Malhotra GK, Tran T, Stewart C, et al. Pandemic operating room supply shortage and surgical site infection: considerations as we emerge from the Coronavirus Disease 2019 Pandemic. J Am Coll Surg. 2022;234:571-578. doi: 10.1097/XCS.0000000000000087.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33. doi:10.1111/ANS.13856.
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017 [published correction appears in: JAMA Surg. 2017;152:803]. JAMA Surg. 2017;152:784-791. doi: 10.1001/jamasurg.2017.0904.
- Rizan, Chantelle, Lillywhite, et al. Minimising carbon and financial costs of steam sterilisation and packaging of reusable surgical instruments. Br J Surg. 2022;109:200-210. doi:10.1093/BJS/ZNAB406.
- Sustainability Benchmarking Report, 2010. Practice Greenhealth. https://www.practicegreenhealth.org. Accessed December 11, 2022.
In 2009, the Lancet called climate change the biggest global health threat of the 21st century, the effects of which will be experienced in our lifetimes.1 Significant amounts of data have demonstrated the negative health effects of heat, air pollution, and exposure to toxic substances.2,3 These effects have been seen in every geographic region of the United States, and in multiple organ systems and specialties, including obstetrics, pediatrics, and even cardiopulmonary and bariatric surgery.2-5
Although it does not receive the scrutiny of other industries, the global health care industry accounts for almost double the amount of carbon emissions as global aviation, and the United States accounts for 27% of this footprint despite only having 4% of the world’s population.6 It therefore serves that our own industry is an excellent target for reducing the carbon emissions that contribute to climate change. Consider the climate impact of hysterectomy, the second-most common surgery that women undergo. In this article, we will use the example of a 50-year-old woman with fibroids who plans to undergo definitive treatment via total laparoscopic hysterectomy (TLH).
Climate impact of US health care
Hospital buildings in the United States are energy intensive, consuming 10% of the energy used in commercial buildings every year, accounting for over $8 billion. Operating rooms (ORs) account for a third of this usage.7 Hospitals also use more water than any other type of commercial building, for necessary actions like cooling, sterilization, and laundry.8 Further, US hospitals generate 14,000 tons of waste per day, with a third of this coming from the ORs. Sadly, up to 15% is food waste, as we are not very good about selecting and proportioning healthy food for our staff and inpatients.6
While health care is utility intensive, the majority of emissions are created through the production, transport, and disposal of goods coming through our supply chain.6 Hospitals are significant consumers of single-use objects, the majority of which are petroleum-derived plastics—accounting for an estimated 71% of emissions coming from the health care sector. Supply chain is the second largest expense in health care, but with current shortages, it is estimated to overtake labor costs by this year. The United States is also the largest consumer of pharmaceuticals worldwide, supporting a $20 billion packaging industry,9 which creates a significant amount of waste.
Climate impact of the OR
Although ORs only account for a small portion of hospital square footage, they account for a significant amount of health care’s carbon footprint through high waste production and excessive consumption of single-use items. Just one surgical procedure in a hospital is estimated to produce about the same amount of waste as a typical family of 4 would in an entire week.10 Furthermore, the majority of these single-use items, including sterile packaging, are sorted inappropriately as regulated medical waste (RMW, “biohazardous” or “red bag” waste) (FIGURE 1a). RMW has significant effects on the environment since it must be incinerated or steam autoclaved prior to transport to the landfill, leading to high amounts of air pollution and energy usage.

We all notice the visible impacts of waste in the OR, but other contributors to carbon emissions are invisible. Energy consumption is a huge contributor to the overall carbon footprint of surgery. Heating, ventilation, and air conditioning [HVAC] is responsible for 52% of hospital energy needs but accounts for 99% of OR energy consumption.11 Despite the large energy requirements of the ORs, they are largely unoccupied in the evenings and on weekends, and thermostats are not adjusted accordingly.
Anesthetic gases are another powerful contributor to greenhouse gas emissions from the OR. Anesthetic gases alone contribute about 25% of the overall carbon footprint of the OR, and US health care emits 660,000 tons of carbon equivalents from anesthetic gas use per year.12 Desflurane is 1,600 times more potent than carbon dioxide (CO2) in its global warming potential followed by isoflurane and sevoflurane;13 this underscores the importance of working with our anesthesia colleagues on the differences between the anesthetic gases they use. Enhanced recovery after surgery recommendations in gynecology already recommend avoiding the use of volatile anesthetic gases in favor of propofol to reduce postoperative nausea and vomiting.14
In the context of a patient undergoing a TLH, the estimated carbon footprint in the United States is about 560 kg of CO2 equivalents—roughly the same as driving 1,563 miles in a gas-powered car.
Continue to: Climate impact on our patients...
Climate impact on our patients
The data in obstetrics and gynecology is clear that climate change is affecting patient outcomes, both globally and in our own country. A systematic review of 32 million births found that air pollution and heat exposure were associated with preterm birth and low birth weight, and these effects were seen in all geographic regions across the United States.1 A study of 5.9 million births in California found that patients who lived near coal- and oil-power plants had up to a 27% reduction in preterm births when those power plants closed and air pollution decreased.15 A study in Nature Sustainability on 250,000 pregnancies that ended in missed abortions at 14 weeks or less found the odds ratio of missed abortion increased with the cumulative exposure to air pollution.16 When air pollution was examined in comparison to other factors, neighborhood air pollution better predicted preterm birth, very preterm birth, and small for gestational age more than race, ethnicity, or any other socio-economic factor.17 The effects of air pollution have been demonstrated in other fields as well, including increased mortality after cardiac transplantation with exposure to air pollution,4 and for patients undergoing bariatric surgery who live near major roadways, decreased weight loss, less improvement in hemoglobin A1c, and less change in lipids compared with those with less exposure to roadway pollution.5
Air pollution and heat are not the only factors that influence health. Endocrine disrupting chemicals (EDCs) and single-use plastic polymers, which are used in significant supply in US health care, have been found in human blood,18 intestine, and all portions of the placenta.19 Phthalates, an EDC found in medical use plastics and medications to control delivery, have been associated with increasing fibroid burden in patients undergoing hysterectomy and myomectomy.20 The example case patient with fibroids undergoing TLH may have had her condition worsened by exposure to phthalates.

Specific areas for improvement
There is a huge opportunity for improvement to reduce the total carbon footprint of a TLH.
A lifecycle assessment of hysterectomy in the United States concluded that an 80% reduction in carbon emissions could be achieved by minimizing opened materials, using reusable and reprocessed instruments, reducing off-hour energy use in the OR (HVAC setbacks), and avoiding the use of volatile anesthetic gases.21 The sterilization and re-processing of reusable instruments represented the smallest proportion of carbon emissions from a TLH. Data on patient safety supports these interventions, as current practices have more to do with hospital culture and processes than evidence.
Despite a push to use single-use objects by industry and regulatory agencies in the name of patient safety, data demonstrate that single-use objects are in actuality not safer for patients and may be associated with increased surgical site infections (SSIs). A study from a cancer center in California found that when single-use head covers, shoe covers, and facemasks were eliminated due to supply shortages during the pandemic, SSIs went down by half, despite an increase in surgical volume and an increase in the number of contaminated cases.22 The authors reported an increase in hand hygiene throughout the hospital, which likely contributed to the success of reducing SSIs.
Similarly, a systematic review found no evidence to support single-use instruments over reusable or reprocessed instruments when considering instrument function, ease of use, patient safety, SSIs, or long-term patient outcomes.23 While it may be easy for regulatory agencies to focus on disposing objects as paramount to reducing infections, the Centers for Disease Control and Prevention states that the biggest factors affecting SSIs are appropriate use of prophylactic antibiotics, skin antisepsis, and patient metabolic control.24 Disposing of single-use objects in the name of patient safety will worsen patient health outcomes when considering patient proximity to waste, pollution, and EDCs.
The sterilization process for reusable items is often called out by the medical supply industry as wasteful and energy intensive; however, data refute these claims. A Swedish study researching reusable versus single-use trocars found that a reusable trocar system offers a robust opportunity to reduce both the environmental and financial costs for laparoscopic surgery.25 We can further decrease the environmental impact of reusable instruments by using sets instead of individually packed instruments and packing autoclaves more efficiently. By using rigid sterilization containers, there was an 85% reduction in carbon footprint as compared with the blue wrap system.
Electricity use can be easily reduced across all surgical spaces by performing HVAC setbacks during low occupancy times of day. On nights and weekends, when there are very few surgical cases occurring, one study found that by decreasing the ventilation rate, turning off lights, and performing the minimum temperature control in unused ORs, electricity use was cut in half.11
Waste triage and recycling
Reducing regulated medical waste is another area where hospitals can make a huge impact on carbon emissions and costs with little more than education and process change. Guidelines for regulated medical waste sorting developed out of the HIV epidemic due to the fear of blood products. Although studies show that regulated medical waste is not more infectious than household waste, state departments of public health have kept these guidelines in place for sorting fluid blood and tissue into RMW containers and bags.26 The best hospital performers keep RMW below 10% of the total waste stream, while many ORs send close to 100% of their waste as RMW (FIGURE 1b). ORs can work with nursing and environmental services staff to assess processes and divert waste into recycling and regular waste. Many OR staff are acutely aware of the huge amount of waste produced and want to make a positive impact. Success in this small area often builds momentum to tackle harder sustainability practices throughout the hospital.
Continue to: Removal of EDCs from medical products...
Removal of EDCs from medical products
Single-use medical supplies are not only wasteful but also contain harmful EDCs, such as phthalates, bisphenol A (BPA), parabens, perfluoroalkyl substances, and triclosan. Phthalates, for example, account for 30% to 40% of the weight of medical-use plastics, and parabens are ubiquitously found in ultrasound gel.3 Studies looking at exposure to EDCs within the neonatal intensive care unit reveal substantial BPA, phthalate, and paraben levels within biologic samples from premature infants, thought to be above toxicity limits. While we do not know the full extent to which EDCs can affect neonatal development, there is already mounting evidence that EDCs are associated with endocrine, metabolic, and neurodevelopmental disorders throughout our lifespan.3
30-day climate challenge
Although the example case patient undergoing TLH for fibroids will never need care for her fibroids again, the climate impact of her time in the OR represents the most carbon-intensive care she will ever need. Surgery as practiced in the United States today is unsustainable.
In 2021, the Biden administration issued an executive order requiring all federal facilities, including health care facilities and hospitals, to be carbon neutral by 2035. In order to make meaningful changes industry-wide, we should be petitioning lawmakers for stricter environmental regulations in health care, similar to regulations in the manufacturing and airline industries. We recommend a 30-day climate challenge (FIGURE 2) for bringing awareness to your circles of influence. Physicians have an ethical duty to advocate for change at the local, regional, and national level if we want to see a better future for our patients, their children, and even ourselves. Organizations such as Practice Greenhealth, Health Care without Harm, and Citizens’ Climate Lobby can help amplify our voices to reach the right people to implement sweeping policy changes. ●

In 2009, the Lancet called climate change the biggest global health threat of the 21st century, the effects of which will be experienced in our lifetimes.1 Significant amounts of data have demonstrated the negative health effects of heat, air pollution, and exposure to toxic substances.2,3 These effects have been seen in every geographic region of the United States, and in multiple organ systems and specialties, including obstetrics, pediatrics, and even cardiopulmonary and bariatric surgery.2-5
Although it does not receive the scrutiny of other industries, the global health care industry accounts for almost double the amount of carbon emissions as global aviation, and the United States accounts for 27% of this footprint despite only having 4% of the world’s population.6 It therefore serves that our own industry is an excellent target for reducing the carbon emissions that contribute to climate change. Consider the climate impact of hysterectomy, the second-most common surgery that women undergo. In this article, we will use the example of a 50-year-old woman with fibroids who plans to undergo definitive treatment via total laparoscopic hysterectomy (TLH).
Climate impact of US health care
Hospital buildings in the United States are energy intensive, consuming 10% of the energy used in commercial buildings every year, accounting for over $8 billion. Operating rooms (ORs) account for a third of this usage.7 Hospitals also use more water than any other type of commercial building, for necessary actions like cooling, sterilization, and laundry.8 Further, US hospitals generate 14,000 tons of waste per day, with a third of this coming from the ORs. Sadly, up to 15% is food waste, as we are not very good about selecting and proportioning healthy food for our staff and inpatients.6
While health care is utility intensive, the majority of emissions are created through the production, transport, and disposal of goods coming through our supply chain.6 Hospitals are significant consumers of single-use objects, the majority of which are petroleum-derived plastics—accounting for an estimated 71% of emissions coming from the health care sector. Supply chain is the second largest expense in health care, but with current shortages, it is estimated to overtake labor costs by this year. The United States is also the largest consumer of pharmaceuticals worldwide, supporting a $20 billion packaging industry,9 which creates a significant amount of waste.
Climate impact of the OR
Although ORs only account for a small portion of hospital square footage, they account for a significant amount of health care’s carbon footprint through high waste production and excessive consumption of single-use items. Just one surgical procedure in a hospital is estimated to produce about the same amount of waste as a typical family of 4 would in an entire week.10 Furthermore, the majority of these single-use items, including sterile packaging, are sorted inappropriately as regulated medical waste (RMW, “biohazardous” or “red bag” waste) (FIGURE 1a). RMW has significant effects on the environment since it must be incinerated or steam autoclaved prior to transport to the landfill, leading to high amounts of air pollution and energy usage.

We all notice the visible impacts of waste in the OR, but other contributors to carbon emissions are invisible. Energy consumption is a huge contributor to the overall carbon footprint of surgery. Heating, ventilation, and air conditioning [HVAC] is responsible for 52% of hospital energy needs but accounts for 99% of OR energy consumption.11 Despite the large energy requirements of the ORs, they are largely unoccupied in the evenings and on weekends, and thermostats are not adjusted accordingly.
Anesthetic gases are another powerful contributor to greenhouse gas emissions from the OR. Anesthetic gases alone contribute about 25% of the overall carbon footprint of the OR, and US health care emits 660,000 tons of carbon equivalents from anesthetic gas use per year.12 Desflurane is 1,600 times more potent than carbon dioxide (CO2) in its global warming potential followed by isoflurane and sevoflurane;13 this underscores the importance of working with our anesthesia colleagues on the differences between the anesthetic gases they use. Enhanced recovery after surgery recommendations in gynecology already recommend avoiding the use of volatile anesthetic gases in favor of propofol to reduce postoperative nausea and vomiting.14
In the context of a patient undergoing a TLH, the estimated carbon footprint in the United States is about 560 kg of CO2 equivalents—roughly the same as driving 1,563 miles in a gas-powered car.
Continue to: Climate impact on our patients...
Climate impact on our patients
The data in obstetrics and gynecology is clear that climate change is affecting patient outcomes, both globally and in our own country. A systematic review of 32 million births found that air pollution and heat exposure were associated with preterm birth and low birth weight, and these effects were seen in all geographic regions across the United States.1 A study of 5.9 million births in California found that patients who lived near coal- and oil-power plants had up to a 27% reduction in preterm births when those power plants closed and air pollution decreased.15 A study in Nature Sustainability on 250,000 pregnancies that ended in missed abortions at 14 weeks or less found the odds ratio of missed abortion increased with the cumulative exposure to air pollution.16 When air pollution was examined in comparison to other factors, neighborhood air pollution better predicted preterm birth, very preterm birth, and small for gestational age more than race, ethnicity, or any other socio-economic factor.17 The effects of air pollution have been demonstrated in other fields as well, including increased mortality after cardiac transplantation with exposure to air pollution,4 and for patients undergoing bariatric surgery who live near major roadways, decreased weight loss, less improvement in hemoglobin A1c, and less change in lipids compared with those with less exposure to roadway pollution.5
Air pollution and heat are not the only factors that influence health. Endocrine disrupting chemicals (EDCs) and single-use plastic polymers, which are used in significant supply in US health care, have been found in human blood,18 intestine, and all portions of the placenta.19 Phthalates, an EDC found in medical use plastics and medications to control delivery, have been associated with increasing fibroid burden in patients undergoing hysterectomy and myomectomy.20 The example case patient with fibroids undergoing TLH may have had her condition worsened by exposure to phthalates.

Specific areas for improvement
There is a huge opportunity for improvement to reduce the total carbon footprint of a TLH.
A lifecycle assessment of hysterectomy in the United States concluded that an 80% reduction in carbon emissions could be achieved by minimizing opened materials, using reusable and reprocessed instruments, reducing off-hour energy use in the OR (HVAC setbacks), and avoiding the use of volatile anesthetic gases.21 The sterilization and re-processing of reusable instruments represented the smallest proportion of carbon emissions from a TLH. Data on patient safety supports these interventions, as current practices have more to do with hospital culture and processes than evidence.
Despite a push to use single-use objects by industry and regulatory agencies in the name of patient safety, data demonstrate that single-use objects are in actuality not safer for patients and may be associated with increased surgical site infections (SSIs). A study from a cancer center in California found that when single-use head covers, shoe covers, and facemasks were eliminated due to supply shortages during the pandemic, SSIs went down by half, despite an increase in surgical volume and an increase in the number of contaminated cases.22 The authors reported an increase in hand hygiene throughout the hospital, which likely contributed to the success of reducing SSIs.
Similarly, a systematic review found no evidence to support single-use instruments over reusable or reprocessed instruments when considering instrument function, ease of use, patient safety, SSIs, or long-term patient outcomes.23 While it may be easy for regulatory agencies to focus on disposing objects as paramount to reducing infections, the Centers for Disease Control and Prevention states that the biggest factors affecting SSIs are appropriate use of prophylactic antibiotics, skin antisepsis, and patient metabolic control.24 Disposing of single-use objects in the name of patient safety will worsen patient health outcomes when considering patient proximity to waste, pollution, and EDCs.
The sterilization process for reusable items is often called out by the medical supply industry as wasteful and energy intensive; however, data refute these claims. A Swedish study researching reusable versus single-use trocars found that a reusable trocar system offers a robust opportunity to reduce both the environmental and financial costs for laparoscopic surgery.25 We can further decrease the environmental impact of reusable instruments by using sets instead of individually packed instruments and packing autoclaves more efficiently. By using rigid sterilization containers, there was an 85% reduction in carbon footprint as compared with the blue wrap system.
Electricity use can be easily reduced across all surgical spaces by performing HVAC setbacks during low occupancy times of day. On nights and weekends, when there are very few surgical cases occurring, one study found that by decreasing the ventilation rate, turning off lights, and performing the minimum temperature control in unused ORs, electricity use was cut in half.11
Waste triage and recycling
Reducing regulated medical waste is another area where hospitals can make a huge impact on carbon emissions and costs with little more than education and process change. Guidelines for regulated medical waste sorting developed out of the HIV epidemic due to the fear of blood products. Although studies show that regulated medical waste is not more infectious than household waste, state departments of public health have kept these guidelines in place for sorting fluid blood and tissue into RMW containers and bags.26 The best hospital performers keep RMW below 10% of the total waste stream, while many ORs send close to 100% of their waste as RMW (FIGURE 1b). ORs can work with nursing and environmental services staff to assess processes and divert waste into recycling and regular waste. Many OR staff are acutely aware of the huge amount of waste produced and want to make a positive impact. Success in this small area often builds momentum to tackle harder sustainability practices throughout the hospital.
Continue to: Removal of EDCs from medical products...
Removal of EDCs from medical products
Single-use medical supplies are not only wasteful but also contain harmful EDCs, such as phthalates, bisphenol A (BPA), parabens, perfluoroalkyl substances, and triclosan. Phthalates, for example, account for 30% to 40% of the weight of medical-use plastics, and parabens are ubiquitously found in ultrasound gel.3 Studies looking at exposure to EDCs within the neonatal intensive care unit reveal substantial BPA, phthalate, and paraben levels within biologic samples from premature infants, thought to be above toxicity limits. While we do not know the full extent to which EDCs can affect neonatal development, there is already mounting evidence that EDCs are associated with endocrine, metabolic, and neurodevelopmental disorders throughout our lifespan.3
30-day climate challenge
Although the example case patient undergoing TLH for fibroids will never need care for her fibroids again, the climate impact of her time in the OR represents the most carbon-intensive care she will ever need. Surgery as practiced in the United States today is unsustainable.
In 2021, the Biden administration issued an executive order requiring all federal facilities, including health care facilities and hospitals, to be carbon neutral by 2035. In order to make meaningful changes industry-wide, we should be petitioning lawmakers for stricter environmental regulations in health care, similar to regulations in the manufacturing and airline industries. We recommend a 30-day climate challenge (FIGURE 2) for bringing awareness to your circles of influence. Physicians have an ethical duty to advocate for change at the local, regional, and national level if we want to see a better future for our patients, their children, and even ourselves. Organizations such as Practice Greenhealth, Health Care without Harm, and Citizens’ Climate Lobby can help amplify our voices to reach the right people to implement sweeping policy changes. ●

- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733. doi: 10.1016/S0140-6736(09)60935-1.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3. doi:10.1001/JAMANETWORKOPEN.2020.8243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822–7. doi: 10.1210/cline2. m/dgaa358.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:3026-3035. doi: 10.1016/j.jacc.2019.09.066.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320. doi: 10.1111/ijpo.12210.
- Health Care’s Climate Footprint. Health care without harm climate-smart health care series, Green Paper Number one. September 2019. https://www.noharm.org/
ClimateFootprintReport. Accessed December 11, 2022. - Healthcare Energy End-Use Monitoring. US Department of Energy. https://www.energy.gov/eere/
buildings/downloads/ healthcare-energy-end-use- monitoring. Accessed December 11, 2022. - 2012 Commercial Buildings Energy Consumption Survey: Water Consumption in Large Buildings Summary. U.S Energy Information Administration. https://www.eia.gov/
consumption/commercial/ reports/2012/water. Accessed December 11, 2022. - Belkhir L, Elmeligi A. Carbon footprint of the global pharmaceutical industry and relative implact of its major players. J Cleaner Production. 2019;214:185-194. doi: 10.1016 /j.jclearpro.2019.11.204.
- Esaki RK, Macario A. Wastage of Supplies and Drugs in the Operating Room. 2015:8-13.
- MacNeill AJ, et al. The Impact of Surgery on Global Climate: A Carbon Footprinting Study of Operating Theatres in Three Health Systems. Lancet Planet Health.2017;1:e360–367. doi:10.1016/S2542-5196(17)30162-6.
- Shoham MA, Baker NM, Peterson ME, et al. The environmental impact of surgery: a systematic review. 2022;172:897-905. doi:10.1016/j.surg.2022.04.010.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98. doi:10.1213/ANE.0B013E3181E058D7.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573. doi: 10.1016/j.ogc.2016.04.006.
- Casey JA, Karasek D, Ogburn EL, et al. Retirements of coal and oil power plants in California: association with reduced preterm birth among populations nearby. Am J Epidemiol. 2018;187:1586-1594. doi: 10.1093/aje/kwy110.
- Zhang L, Liu W, Hou K, et al. Air pollution-induced missed abortion risk for pregnancies. Nat Sustain. 2019:1011–1017.
- Benmarhnia T, Huang J, Basu R, et al. Decomposition analysis of Black-White disparities in birth outcomes: the relative contribution of air pollution and social factors in California. Environ Health Perspect. 2017;125:107003. doi: 10.1289/EHP490.
- Leslie HA, van Velzen MJM, Brandsma SH, et al. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199.
- Ragusa A, Svelato A, Santacroce C, et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121. doi: 10.1016/j.fertnstert.2018.09.009.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786. doi: 10.1021/es504719g.
- Malhotra GK, Tran T, Stewart C, et al. Pandemic operating room supply shortage and surgical site infection: considerations as we emerge from the Coronavirus Disease 2019 Pandemic. J Am Coll Surg. 2022;234:571-578. doi: 10.1097/XCS.0000000000000087.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33. doi:10.1111/ANS.13856.
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017 [published correction appears in: JAMA Surg. 2017;152:803]. JAMA Surg. 2017;152:784-791. doi: 10.1001/jamasurg.2017.0904.
- Rizan, Chantelle, Lillywhite, et al. Minimising carbon and financial costs of steam sterilisation and packaging of reusable surgical instruments. Br J Surg. 2022;109:200-210. doi:10.1093/BJS/ZNAB406.
- Sustainability Benchmarking Report, 2010. Practice Greenhealth. https://www.practicegreenhealth.org. Accessed December 11, 2022.
- Costello A, Abbas M, Allen et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693-1733. doi: 10.1016/S0140-6736(09)60935-1.
- Bekkar B, Pacheco S, Basu R, et al. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3. doi:10.1001/JAMANETWORKOPEN.2020.8243.
- Genco M, Anderson-Shaw L, Sargis RM. Unwitting accomplices: endocrine disruptors confounding clinical care. J Clin Endocrinol Metab. 2020;105:e3822–7. doi: 10.1210/cline2. m/dgaa358.
- Al-Kindi SG, Sarode A, Zullo M, et al. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol. 2019;74:3026-3035. doi: 10.1016/j.jacc.2019.09.066.
- Ghosh R, Gauderman WJ, Minor H, et al. Air pollution, weight loss and metabolic benefits of bariatric surgery: a potential model for study of metabolic effects of environmental exposures. Pediatr Obes. 2018;13:312-320. doi: 10.1111/ijpo.12210.
- Health Care’s Climate Footprint. Health care without harm climate-smart health care series, Green Paper Number one. September 2019. https://www.noharm.org/
ClimateFootprintReport. Accessed December 11, 2022. - Healthcare Energy End-Use Monitoring. US Department of Energy. https://www.energy.gov/eere/
buildings/downloads/ healthcare-energy-end-use- monitoring. Accessed December 11, 2022. - 2012 Commercial Buildings Energy Consumption Survey: Water Consumption in Large Buildings Summary. U.S Energy Information Administration. https://www.eia.gov/
consumption/commercial/ reports/2012/water. Accessed December 11, 2022. - Belkhir L, Elmeligi A. Carbon footprint of the global pharmaceutical industry and relative implact of its major players. J Cleaner Production. 2019;214:185-194. doi: 10.1016 /j.jclearpro.2019.11.204.
- Esaki RK, Macario A. Wastage of Supplies and Drugs in the Operating Room. 2015:8-13.
- MacNeill AJ, et al. The Impact of Surgery on Global Climate: A Carbon Footprinting Study of Operating Theatres in Three Health Systems. Lancet Planet Health.2017;1:e360–367. doi:10.1016/S2542-5196(17)30162-6.
- Shoham MA, Baker NM, Peterson ME, et al. The environmental impact of surgery: a systematic review. 2022;172:897-905. doi:10.1016/j.surg.2022.04.010.
- Ryan SM, Nielsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92-98. doi:10.1213/ANE.0B013E3181E058D7.
- Kalogera E, Dowdy SC. Enhanced recovery pathway in gynecologic surgery: improving outcomes through evidence-based medicine. Obstet Gynecol Clin North Am. 2016;43:551-573. doi: 10.1016/j.ogc.2016.04.006.
- Casey JA, Karasek D, Ogburn EL, et al. Retirements of coal and oil power plants in California: association with reduced preterm birth among populations nearby. Am J Epidemiol. 2018;187:1586-1594. doi: 10.1093/aje/kwy110.
- Zhang L, Liu W, Hou K, et al. Air pollution-induced missed abortion risk for pregnancies. Nat Sustain. 2019:1011–1017.
- Benmarhnia T, Huang J, Basu R, et al. Decomposition analysis of Black-White disparities in birth outcomes: the relative contribution of air pollution and social factors in California. Environ Health Perspect. 2017;125:107003. doi: 10.1289/EHP490.
- Leslie HA, van Velzen MJM, Brandsma SH, et al. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. doi: 10.1016/j.envint.2022.107199.
- Ragusa A, Svelato A, Santacroce C, et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274.
- Zota AR, Geller RJ, Calafat AM, et al. Phthalates exposure and uterine fibroid burden among women undergoing surgical treatment for fibroids: a preliminary study. Fertil Steril. 2019;111:112-121. doi: 10.1016/j.fertnstert.2018.09.009.
- Thiel CL, Eckelman M, Guido R, et al. Environmental impacts of surgical procedures: life cycle assessment of hysterectomy in the United States. Environ Sci Technol. 2015;49:1779-1786. doi: 10.1021/es504719g.
- Malhotra GK, Tran T, Stewart C, et al. Pandemic operating room supply shortage and surgical site infection: considerations as we emerge from the Coronavirus Disease 2019 Pandemic. J Am Coll Surg. 2022;234:571-578. doi: 10.1097/XCS.0000000000000087.
- Siu J, Hill AG, MacCormick AD. Systematic review of reusable versus disposable laparoscopic instruments: costs and safety. ANZ J Surg. 2017;87:28-33. doi:10.1111/ANS.13856.
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al; Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017 [published correction appears in: JAMA Surg. 2017;152:803]. JAMA Surg. 2017;152:784-791. doi: 10.1001/jamasurg.2017.0904.
- Rizan, Chantelle, Lillywhite, et al. Minimising carbon and financial costs of steam sterilisation and packaging of reusable surgical instruments. Br J Surg. 2022;109:200-210. doi:10.1093/BJS/ZNAB406.
- Sustainability Benchmarking Report, 2010. Practice Greenhealth. https://www.practicegreenhealth.org. Accessed December 11, 2022.
How to place an IUD with minimal patient discomfort
CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8
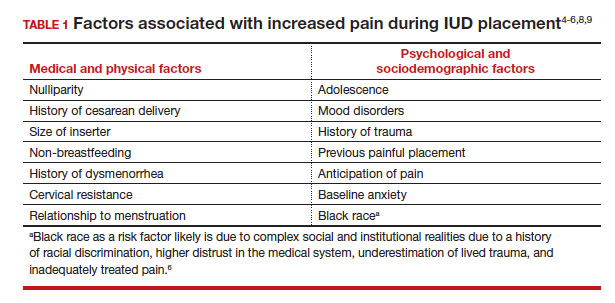
It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10
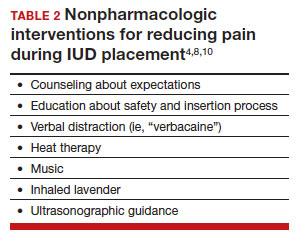
Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).
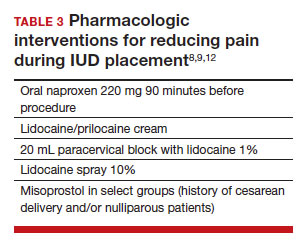
Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.

CASE Nulliparous young woman desires contraception
An 18-year-old nulliparous patient presents to your office inquiring about contraception before she leaves for college. She not only wants to prevent pregnancy but she also would like a method that can help with her dysmenorrhea. After receiving nondirective counseling about all of the methods available, she selects a levonorgestrel intrauterine device (LNG-IUD). However, she discloses that she is very nervous about placement. She has heard from friends that it can be painful to get an IUD. What are these patient’s risk factors for painful placement? How would you mitigate her experience of pain during the insertion process?
IUDs are highly effective and safe methods of preventing unwanted pregnancy. IUDs have become increasingly more common; they were the method of choice for 14% of contraception users in 2016, a rise from 12% in 2014.1 The Contraceptive CHOICE project demonstrated that IUDs were most likely to be chosen as a reversible method of contraception when unbiased counseling is provided and barriers such as cost are removed. Additionally, rates of continuation were found to be high, thus reducing the number of unwanted pregnancies.2 However, pain during IUD insertion as well as the fear and anxiety surrounding the procedure are some of the major limitations to IUD uptake and use. Specifically, fear of pain during IUD insertion is a substantial barrier; this fear is thought to also exacerbate the experience of pain during the insertion process.3
This article aims to identify risk factors for painful IUD placement and to review both nonpharmacologic and pharmacologic methods that may decrease discomfort and anxiety during IUD insertion.
What factors contribute to the experience of pain with IUD placement?
While some women do not report experiencing pain during IUD insertion, approximately 17% describe the pain as severe.4 The perception of pain during IUD placement is multifactorial; physiologic, psychological, emotional, cultural, and circumstantial factors all can play a role (TABLE 1). The biologic perception of pain results from the manipulation of the cervix and uterus; noxious stimuli activate both the sympathetic and parasympathetic nervous systems. The sympathetic system at T10-L2 mediates the fundus, the ovarian plexus at the cornua, and the uterosacral ligaments, while the parasympathetic fibers from S2-S4 enter the cervix at 3 o’clock and 9 o’clock and innervate the upper vagina, cervix, and lower uterine segment.4,5 Nulliparity, history of cesarean delivery, increased size of the IUD inserter, length of the uterine cavity, breastfeeding status, relation to timing of menstruation, and length of time since last vaginal delivery all may be triggers for pain. Other sociocultural influences on a patient’s experience of pain include young age (adolescence), Black race, and history of sexual trauma, as well as existing anxiety and beliefs about expected pain.3,5,6-8

It also is important to consider all aspects of the procedure that could be painful. Steps during IUD insertion that have been found to invoke average to severe pain include use of tenaculum on the cervix, uterine stabilization, uterine sounding, placement of the insertion tube, and deployment of the actual IUD.4-7
A secondary analysis of the Contraceptive CHOICE project confirmed that women with higher levels of anticipated pain were more likely to experience increased discomfort during placement.3 Providers tend to underestimate the anxiety and pain experienced by their patients undergoing IUD insertion. In a study about anticipated pain during IUD insertion, clinicians were asked if patients were “pleasant and appropriately engaging” or “anxious.” Only 10% of those patients were noted to be anxious by their provider; however, patients with a positive screen on the PHQ-4 depression and anxiety screen did anticipate more pain than those who did not.6 In another study, patients estimated their pain scores at 30 mm higher than their providers on a visual analog scale.7 Given these discrepancies, it is imperative to address anxiety and pain anticipation, risk factors for pain, and offerings for pain management during IUD placement to ensure a more holistic experience.
Continue to: What are nonpharmacologic interventions that can reduce anxiety and pain?...
What are nonpharmacologic interventions that can reduce anxiety and pain?
There are few formal studies on nonpharmacologic options for pain reduction at IUD insertion, with varying outcomes.4,8,10 However, many of them suggest that establishing a trusting clinician-patient relationship, a relaxing and inviting environment, and emotional support during the procedure may help make the procedure more comfortable overall (TABLE 2).4,5,10

Education and counseling
Patients should be thoroughly informed about the different IUD options, and they should be reassured regarding their contraceptive effectiveness and low risk for insertion difficulties in order to mitigate anxiety about complications and future fertility.11 This counseling session can offer the patient opportunities for relationship building with the provider and for the clinician to assess for anxiety and address concerns about the insertion and removal process. Patients who are adequately informed regarding expectations and procedural steps are more likely to have better pain management.5 Another purpose of this counseling session may be to identify any risk factors that may increase pain and tailor nonpharmacologic and pharmacologic options to the individual patient.
Environment
Examination rooms should be comfortable, private, and professional appearing. Patients prefer a more informal, unhurried, and less sterile atmosphere for procedures. Clinicians should strive to engender trust prior to the procedure by sharing information in a straightforward manner, and ensuring that staff of medical assistants, nurses, and clinicians are a “well-oiled machine” to inspire confidence in the competence of the team.4 Ultrasonography guidance also may be helpful in reducing pain during IUD placement, but this may not be available in all outpatient settings.8
Distraction techniques
Various distraction methods have been employed during gynecologic procedures, and more specifically IUD placement, with some effect. During and after the procedure, heat and ice have been found to be helpful adjuncts for uterine cramping and should be offered as first-line pain management options on the examination table. This can be in the form of reusable heating pads or chemical heat or ice packs.4 A small study demonstrated that inhaled lavender may help with lowering anxiety prior to and during the procedure; however, it had limited effects on pain.10
Clinicians and support staff should engage in conversation with the patient throughout the procedure (ie, “verbacaine”). This can be conducted via a casual chat about unrelated topics or gentle and positive coaching through the procedure with the intent to remove negative imagery associated with elements of the insertion process.5 Finally, studies have been conducted using music as a distraction for colposcopy and hysteroscopy, and results have indicated that it is beneficial, reducing both pain and anxiety during these similar types of procedures.4 While these options may not fully remove pain and anxiety, many are low investment interventions that many patients will appreciate.
What are pharmacologic interventions that can decrease pain during IUD insertion?
The literature is more robust with studies examining the benefits of pharmacologic methods for reducing pain during IUD insertion; strategies include agents that lessen uterine cramping, numb the cervix, and soften and open the cervical os. Despite the plethora of studies, there is no one standard of care for pain management during IUD insertion (TABLE 3).

Lidocaine injection
Lidocaine is an amine anesthetic that can block the nociceptive response of nerves upon administration; it has the advantages of rapid onset and low risk in appropriate doses. Multiple randomized controlled trials (RCTs) have examined the use of paracervical and intracervical block with lidocaine.9,12-15 Lopez and colleagues conducted a review in 2015, including 3 studies about injectable lidocaine and demonstrated some effect of injectable lidocaine on reduction in pain at tenaculum placement.9
Mody and colleagues conducted a pilot RCT of 50 patients comparing a 10 mL lidocaine 1% paracervical block to no block, which was routine procedure at the time.12 The authors demonstrated a reduction in pain at the tenaculum site but no decrease in pain with insertion. They also measured pain during the block administration itself and found that the block increased the overall pain of the procedure. In 2018, Mody et al13 performed another RCT, but with a higher dose of 20 mL of buffered lidocaine 1% in 64 nulliparous patients. They found that paracervical block improved pain during uterine sounding, IUD insertion, and 5 minutes following insertion, as well as the pain of the overall procedure.
De Nadai and colleagues evaluated if a larger dose of lidocaine (3.6 mL of lidocaine 2%) administered intracervically at the anterior lip was beneficial.14 They randomly assigned 302 women total: 99 to intracervical block, 101 to intracervical sham block with dry needling at the anterior lip, and 102 to no intervention. Fewer patients reported extreme pain with tenaculum placement and with IUD (levonorgestrel-releasing system) insertion. Given that this option requires less lidocaine overall and fewer injection points, it has the potential to be an easier and more reproducible technique.14
Finally, Akers and colleagues aimed to evaluate IUD insertion in nulliparous adolescents. They compared a 1% paracervical block of 10 mL with 1 mL at the anterior lip and 4.5 mL at 4 o’clock and 8 o’clock in the cervicovaginal junction versus depression of the wood end of a cotton swab at the same sites. They found that the paracervical block improved pain substantially during all steps of the procedure compared with the sham block in this young population.16
Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) show promise in reducing pain during IUD placement, as they inhibit the production of prostaglandins, which can in turn reduce uterine cramping and inflammation during IUD placement.
Lopez and colleagues evaluated the use of NSAIDs in 7 RCTs including oral naproxen, oral ibuprofen, and intramuscular ketorolac.9 While it had no effect on pain at the time of placement, naproxen administered at least 90 minutes before the procedure decreased uterine cramping for 2 hours after insertion. Women receiving naproxen also were less likely to describe the insertion as “unpleasant.” Ibuprofen was found to have limited effects during insertion and after the procedure. Intramuscular ketorolac studies were conflicting. Results of one study demonstrated a lower median pain score at 5 minutes but no differences during tenaculum placement or IUD insertion, whereas another demonstrated reduction in pain during and after the procedure.8,9
Another RCT showed potential benefit of tramadol over the use of naproxen when they were compared; however, tramadol is an opioid, and there are barriers to universal use in the outpatient setting.9
Continue to: Topical anesthetics...
Topical anesthetics
Topical anesthetics offer promise of pain relief without the pain of injection and with the advantage of self-administration for some formulations.
Several RCTs evaluated whether lidocaine gel 2% applied to the cervix or injected via flexible catheter into the cervical os improved pain, but there were no substantial differences in pain perception between topical gel and placebo groups in the insertion of IUDs.9
Rapkin and colleagues15 studied whether self-administered intravaginal lidocaine gel 2% five minutes before insertion was helpful;15 they found that tenaculum placement was less painful, but IUD placement was not. Conti et al expanded upon the Rapkin study by extending the amount of time of exposure to self-administered intravaginal lidocaine gel 2% to 15 minutes; they found no difference in perception of pain during tenaculum placement, but they did see a substantial difference in discomfort during speculum placement.17 This finding may be helpful for patients with a history of sexual trauma or anxiety about gynecologic examinations. Based on surveys conducted during their study, they found that patients were willing to wait 15 minutes for this benefit.
In Gemzell-Danielsson and colleagues’ updated review, they identified that different lidocaine formulations, such as a controlled-release lidocaine and a lidocaine-prilocaine compound, resulted in slight reduction in pain scores at multiple points during the IUD insertion process compared with controls.8 Two RCTs demonstrated substantial reduction in pain with administration of lidocaine spray 10% during tenaculum placement, sounding, and immediately after IUD placement compared with a placebo group.18,19 This may be an appealing option for patients who do not want to undergo an injection for local anesthesia.
Nitrous oxide
Nitrous oxide is an odorless colorless gas with anxiolytic, analgesic, and amnestic effects. It has several advantages for outpatient administration including rapid onset, rapid recovery, high safety profile, and no residual incapacitation, enabling a patient to safely leave the office shortly after a procedure.20
Nitrous oxide was studied in an RCT of 74 young (12-20 years of age) nulliparous patients and found to be effective for decreasing pain during IUD insertion and increasing satisfaction with the procedure.20 However, another study of 80 nulliparous patients (aged 13-45 years) did not find any reduction in pain during the insertion procedure.21
Prostaglandin analogues
Misoprostol is a synthetic prostaglandin E1 analog that causes cervical softening, uterine contractions, and cervical dilation. Dinoprostone is a synthetic prostaglandin E2 analog that has similar effects on the cervix and uterus. These properties have made it a useful tool in minor gynecologic procedures, such as first trimester uterine aspiration and hysteroscopy. However, both have the disadvantage of causing adverse effects on gastric smooth muscle, leading to nausea, vomiting, diarrhea, and uncomfortable gastric cramping.
Several RCTs have examined the use of misoprostol administration approximately 2 to 4 hours before IUD placement. No studies found any improvement in pain during IUD insertion, but this likely is due to the discomfort caused by the use of misoprostol itself.9 A meta-analysis and systematic review of 14 studies found no effect on reducing the pain associated with IUD placement but did find that providers had an easier time with cervical dilation in patients who received it. The meta-analysis also demonstrated that patients receiving vaginal misoprostol were less likely to have gastric side effects.22 In another review of 5 RCTs using 400 µg to 600 µg of misoprostol for cervical preparation, Gemzell-Danielsson et al found reductions in mean pain scores with placement specifically among patients with previous cesarean delivery and/or nulliparous patients.8
In an RCT, Ashour and colleagues looked at the use of dinoprostone 3 mg compared with placebo in 160 patients and found that those in the dinoprostone group had less pain during and 15 minutes after the procedure, as well as ease of insertion and overall higher satisfaction with the IUD placement. Dinoprostone traditionally is used for labor induction in the United States and tends to be much more expensive than misoprostol, but it shows the most promise of the prostaglandins in making IUD placement more comfortable.
Conclusion: Integrating evidence and experience
Providers tend to underestimate the pain and anxiety experienced by their patients undergoing IUD insertion. Patients’ concerns about pain and anxiety increase their risk for experiencing pain during IUD insertion. Patient anxieties, and thus, pain may be allayed by offering support and education prior to placement, offering tailored pharmacologic strategies to mitigate pain, and offering supportive and distraction measures during the insertion process. ●
- Patients should be counseled regarding the benefits and risks of the IUD, expectations for placement and removal, and offered the opportunity to ask questions and express their concerns.
- Providers should use this opportunity to assess for risk factors for increased pain during IUD placement.
- All patients should be offered premedication with naproxen 220 mg approximately 90 minutes prior to the procedure, as well as heat therapy and the opportunity to listen to music during the procedure.
- Patients with risk factors for pain should have pharmacologic strategies offered based on the available evidence, and providers should reassure patients that there are multiple strategies available that have been shown to reduce pain during IUD placement.
—Nulliparous patients and patients with a history of a cesarean delivery may be offered the option of cervical ripening with misoprostol 400 µg vaginally 2 to 4 hours prior to the procedure.
—Patients with a history of sexual trauma should be offered self-administered lidocaine 1% or lidocaine-prilocaine formulations to increase comfort during examinations and speculum placement.
—All other patients can be offered the option of a paracervical or intracervical block, with the caveat that administration of the block itself also may cause some pain during the procedure.
—For those patients who desire some sort of local anesthetic but do not want to undergo a lidocaine injection, patients should be offered the option of lidocaine spray 10%.
—Finally, for those patients who are undergoing a difficult IUD placement, ultrasound guidance should be readily available.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93.
- Piepert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105‐1113.
- Dina B, Peipert LJ, Zhao Q, et al. Anticipated pain as a predictor of discomfort with intrauterine device placement. Am J Obstet Gynecol. 2018;218:236.e1-236.e9. doi:10.1016 /j.ajog.2017.10.017.
- McCarthy C. Intrauterine contraception insertion pain: nursing interventions to improve patient experience. J Clin Nurs. 2018;27:9-21. doi:10.1111/jocn.13751.
- Ireland LD, Allen RH. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 2016;71:89-98. doi:10.1097/OGX.0000000000000272.
- Hunter TA, Sonalkar S, Schreiber CA, et al. Anticipated pain during intrauterine device insertion. J Pediatr Adolesc Gynecol. 2020;33:27-32. doi:10.1016/j.jpag.2019.09.007
- Maguire K, Morrell K, Westhoff C, Davis A. Accuracy of providers’ assessment of pain during intrauterine device insertion. Contraception. 2014;89:22-24. doi: 10.1016/j.contraception.2013.09.008.
- Gemzell-Danielsson K, Jensen JT, Monteiro I. Interventions for the prevention of pain associated with the placement of intrauterine contraceptives: an updated review. Acta Obstet Gyncol Scand. 2019;98:1500-1513.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;2015:CD007373. doi:10.1002/14651858.CD007 373.pub3.
- Nguyen L, Lamarche L, Lennox R, et al. Strategies to mitigate anxiety and pain in intrauterine device insertion: a systematic review. J Obstet Gynaecol Can. 2020;42:1138-1146.e2. doi:10.1016/j.jogc.2019.09.014.
- Akdemir Y, Karadeniz M. The relationship between pain at IUD insertion and negative perceptions, anxiety and previous mode of delivery. Eur J Contracept Reprod Health Care. 2019;24:240-245. doi:10.1080/13625187.2019.1610872.
- Mody SK, Kiley J, Rademaker A, et al. Pain control for intrauterine device insertion: a randomized trial of 1% lidocaine paracervical block. Contraception. 2012;86:704-709. doi:10.1016/j.contraception.2012.06.004.
- Mody SK, Farala JP, Jimenez B, et al. Paracervical block for intrauterine device placement among nulliparous women: a randomized controlled trial. Obstet Gynecol. 2018;132:575582. doi:10.1097/AOG.0000000000002790.
- De Nadai MN, Poli-Neto OB, Franceschini SA, et al. Intracervical block for levonorgestrel-releasing intrauterine system placement among nulligravid women: a randomized double-blind controlled trial. Am J Obstet Gynecol. 2020;222:245.e1-245.e10. doi:10.1016/j.ajog.2019.09.013.
- Rapkin RB, Achilles SL, Schwarz EB, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128:621-628. doi:10.1097/AOG.0000000000001596.
- Akers A, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion. A randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795802. doi: 10.1097/AOG.0000000000002242.
- Conti JA, Lerma K, Schneyer RJ, et al. Self-administered vaginal lidocaine gel for pain management with intrauterine device insertion: a blinded, randomized controlled trial. Am J Obstet Gynecol. 2019;220:177.e1-177.e7. doi:10.1016 /j.ajog.2018.11.1085.
- Panichyawat N, Mongkornthong T, Wongwananuruk T, et al. 10% lidocaine spray for pain control during intrauterine device insertion: a randomised, double-blind, placebocontrolled trial. BMJ Sex Reprod Health. 2021;47:159-165. doi:10.1136/bmjsrh-2020-200670.
- Karasu Y, Cömert DK, Karadağ B, et al. Lidocaine for pain control during intrauterine device insertion. J Obstet Gynaecol Res. 2017;43:1061-1066. doi:10.1111/jog.13308.
- Fowler KG, Byraiah G, Burt C, et al. Nitrous oxide use for intrauterine system placement in adolescents. J Pediatr Adolesc Gynecol. 2022;35:159-164. doi:10.1016 /j.jpag.2021.10.019.
- Singh RH, Thaxton L, Carr S, et al. A randomized controlled trial of nitrous oxide for intrauterine device insertion in nulliparous women. Int J Gynaecol Obstet. 2016;135:145-148. doi:10.1016/j.ijgo.2016.04.014.
- Ashour AS, Nabil H, Yosif MF, et al. Effect of self-administered vaginal dinoprostone on pain perception during copper intrauterine device insertion in parous women: a randomized controlled trial. Fertil Steril. 2020;114:861-868. doi: 10.1016/j. fertnstert.2020.05.004.