User login
Diagnosis and Management of Acute and Chronic Graft-versus-Host Disease
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for several hematologic malignancies and other congenital diseases including immunodeficiencies or hemoglobinopathies. When the first allografts were performed, most patients given bone marrow (BM) from donors other than homozygotic twins developed skin, gut, and/or liver injury. This disease was defined by Billingham in 1966 as graft-versushost disease (GVHD). He also described 3 standard tenets for GVHD pathophysiology, which remain valid today even with rapid advances in this area: (1) donor graft must have immune-competent cells, (2) recipient must be incapable of rejecting the graft, and (3) recipient must have tissue antigens not present in the donor.
To read the full article in PDF:
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for several hematologic malignancies and other congenital diseases including immunodeficiencies or hemoglobinopathies. When the first allografts were performed, most patients given bone marrow (BM) from donors other than homozygotic twins developed skin, gut, and/or liver injury. This disease was defined by Billingham in 1966 as graft-versushost disease (GVHD). He also described 3 standard tenets for GVHD pathophysiology, which remain valid today even with rapid advances in this area: (1) donor graft must have immune-competent cells, (2) recipient must be incapable of rejecting the graft, and (3) recipient must have tissue antigens not present in the donor.
To read the full article in PDF:
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for several hematologic malignancies and other congenital diseases including immunodeficiencies or hemoglobinopathies. When the first allografts were performed, most patients given bone marrow (BM) from donors other than homozygotic twins developed skin, gut, and/or liver injury. This disease was defined by Billingham in 1966 as graft-versushost disease (GVHD). He also described 3 standard tenets for GVHD pathophysiology, which remain valid today even with rapid advances in this area: (1) donor graft must have immune-competent cells, (2) recipient must be incapable of rejecting the graft, and (3) recipient must have tissue antigens not present in the donor.
To read the full article in PDF:
Is He DISTRACTED? Tool for Assessing ADHD in an Adult
Adult attention deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
DISTRACTIBILITY
Is there a pattern of the patient getting “off track” in conversations or in school/work situations because of straying thoughts or daydreams? Is there a tendency to overrespond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
IMPULSIVITY
Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
SCHOOL HISTORY
This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
TASK COMPLETION
Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
RATING SCALES
Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are more than 12 scales that can be utilized in a clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
ACCIDENTS
Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
COMMITMENTS
Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
TIME MANAGEMENT
How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
EMPLOYMENT
Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
DECISIONS
Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed or too impatient to consider all the details?
Remember: No single component of this mnemonic suffices to diagnose adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
REFERENCES
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking Charge of Adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
Adult attention deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
DISTRACTIBILITY
Is there a pattern of the patient getting “off track” in conversations or in school/work situations because of straying thoughts or daydreams? Is there a tendency to overrespond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
IMPULSIVITY
Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
SCHOOL HISTORY
This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
TASK COMPLETION
Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
RATING SCALES
Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are more than 12 scales that can be utilized in a clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
ACCIDENTS
Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
COMMITMENTS
Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
TIME MANAGEMENT
How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
EMPLOYMENT
Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
DECISIONS
Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed or too impatient to consider all the details?
Remember: No single component of this mnemonic suffices to diagnose adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
REFERENCES
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking Charge of Adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
Adult attention deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
DISTRACTIBILITY
Is there a pattern of the patient getting “off track” in conversations or in school/work situations because of straying thoughts or daydreams? Is there a tendency to overrespond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
IMPULSIVITY
Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
SCHOOL HISTORY
This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
TASK COMPLETION
Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
RATING SCALES
Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are more than 12 scales that can be utilized in a clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
ACCIDENTS
Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
COMMITMENTS
Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
TIME MANAGEMENT
How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
EMPLOYMENT
Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
DECISIONS
Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed or too impatient to consider all the details?
Remember: No single component of this mnemonic suffices to diagnose adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
REFERENCES
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking Charge of Adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
Travelers’ Diarrhea: Prevention, Treatment, and Posttrip Evaluation
A 40-year-old woman, a childhood immigrant from India, is seeking advice regarding her upcoming two-week trip to Mumbai. She is taking her two children, ages 16 years and 16 months, to visit their grandparents for the first time. She has made this trip alone a few times and has invariably experienced short bouts of self-limited diarrheal illness. She wonders what she might do to prevent travelers’ diarrhea. Her only medical problem is rheumatoid arthritis, which has been well controlled with methotrexate. Her children are healthy. What would you recommend?
Recommendations regarding travelers’ diarrhea (TD) address prevention and management. Prevention encompasses advice about personal behaviors and the use of chemoprophylaxis (antimicrobial and nonantimicrobial) and vaccinations. Since international travelers are known to treat themselves for diarrheal illnesses during their trips,1 recommendations regarding management should assume self-treatment and include the use of both antibiotic and nonantibiotic remedies. Pretravel recommendations will of course be most effective if they account for the individual’s risk for TD.
TD RISK
TD is generally defined as the passage of three or more loose stools in a 24-hour period, with associated symptoms of enteric infection (eg, fever, nausea, vomiting, or abdominal cramping). Defined in this manner, TD is thought to occur in 60% to 70% of individuals who travel from developed countries to less-developed countries.2-4 Risk for TD is influenced both by intrinsic personal factors and by factors specific to the trip.
Personal risk factors
Individual variation in susceptibility to TD might result from a genetic predisposition arising from single nucleotide polymorphisms governing various inflammatory marker proteins.5 A history of multiple episodes of TD, especially if fellow travelers were spared, can suggest this kind of individual susceptibility. Other factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.6,7 However, the trip itself is much more important in assessing risk for TD.
Trip-related risk factors
The destination. The most salient risk factor for TD is the geographic destination. Regions of the world can be divided into TD risk strata2:
• Very high: South Asia
• High: South America, Sub-Saharan Africa
• Medium: Central America, Mexico, Caribbean, Middle East, North Africa, Southeast Asia, Oceania
• Low: Europe, North America (excluding Mexico), Australasia, Northeast Asia
Particularly notable countries, in descending order of risk, are Nepal, India, Myanmar, Bolivia, Sri Lanka, Ecuador, Peru, Kenya, and Guatemala.2
Dietary choices. Additionally, since travelers acquire TD by ingesting food or beverages contaminated with pathogenic fecal microbes, dietary behaviors during the trip affect their susceptibility. At least risk are business travelers and tourists who confine their activities to more affluent settings in which food and beverages are prepared and stored hygienically.1,4,8,9 At greater risk are travelers who immerse themselves in local culture, visiting locations that are more impoverished and not as well equipped with sanitation systems, especially if their stay lasts at least two to three weeks.1,4,8,9
Also, the older a traveler is, the lower his or her risk for TD.1,9 An exception to this might be infants whose diet consists solely of breast milk or formula prepared under sanitary conditions.
Continued on next page >>
TD PREVENTION
Emphasize food and beverage precautions
It might be reasonable to expect that travelers who are circumspect about their food and beverage choices on trips will be able to avoid TD. Indeed, this is the basis for the aphorism, “Boil it, peel it, or forget it.” Guidelines routinely recommend that travelers restrict their selection of foods to those that have been well cooked and are served while still very hot, as well as to fruits and vegetables that they peel themselves. Likewise, they should drink only beverages that have been boiled or are in sealed bottles or under carbonation and served without ice.10-12
Many travelers might find these recommendations too restrictive to follow faithfully. Moreover, studies suggest it may not be possible for even the most assiduous traveler to fully avoid the risk for TD.13,14 The hygienic characteristics of the travel destination may be more determinative, as illustrated by the successful reduction of TD rates in Jamaica by improving sanitation in tourist resorts.15
Antibiotic chemoprophylaxis: A debated practice with limited consensus
The etiologic agents of TD are multiple and vary somewhat in predominance according to geographic region.3,16,17 Table 1 depicts variance by region. The most common pathogens are strains of the bacterium Escherichia coli, particularly enterotoxigenic (ETEC), enteroaggregative, and enteropathogenic strains.16 Other bacteria of importance are Campylobacter, Salmonella, and Shigella. Viruses, particularly norovirus (notably connected with cruise ships), can also cause TD, although it is implicated in no more than 17% of cases.18 Parasitic pathogens are even less common causes of TD (4% to 10%) and mainly involve the protozoa, Giardia lamblia, and, to a lesser extent, Entamoeba histolytica and Cryptosporidium.
Although some pathogens often have a characteristic presentation—such as frothy, greasy diarrhea in the case of G lamblia—in general, they cannot be reliably distinguished from one another clinically. Notably, up to 50% of stool samples from TD patients do not yield any pathogen,16 raising the suspicion that current diagnostic technology is not sufficiently sensitive to routinely identify certain bacteria.
There is no consensus on recommending antibiotic chemoprophylaxis against TD.
Opponents of this practice point out that TD is generally a brief (three to five days), self-limited illness.10-12,19,20 Moreover, concerns about antibiotic resistance have come to pass. Previously used agents (trimethoprim-sulfamethoxazole and doxycycline) are no longer effective in preventing or treating TD. In addition, antibiotic use carries the risk for allergic reactions, as well as other adverse effects including (ironically) the development of antibiotic-associated and Clostridium difficile diarrhea.
Proponents of antibiotic chemoprophylaxis point to its demonstrated efficacy in reducing the risk for TD by 4% to 40%.11,21,22 They also argue that at least 20% to 25% of travelers who get TD must significantly curtail their activities for a day or more.1,23 This change in travel plans is associated not only with significant personal loss but also imposes a financial burden.23 Furthermore, TD is known to have longer-term effects. Up to 10% of those affected develop postinfectious irritable bowel syndrome (PI-IBS) that can last for five or six years.21,22,24,25 It is not known, however, whether the use of antibiotic chemoprophylaxis significantly reduces the incidence of PI-IBS.
Finally, the luminal antibiotic rifaximin, nonabsorbable as it is, is very well tolerated and holds promise for not inciting antibiotic resistance.22 However, while its efficacy in preventing TD has been demonstrated in various settings,22,26,27 it is not approved by the FDA for this indication. Also, concerns persist that it might not be effective in preventing TD caused by invasive pathogens.19
Indications on which all agree. Even opponents of antibiotic chemoprophylaxis grant that it is probably warranted for two groups of travelers.10-12 The first is those whose trip schedule is of such importance that any deviation would be intolerable. The second is travelers with comorbidities that would render them at high risk for serious inconvenience or illness if they developed TD. Examples of the latter include patients with enterostomies, mobility impairments, immune suppression, inflammatory bowel disease, and renal or metabolic diseases.
Chemoprophylaxis regimens. If you prescribe an antibiotic prophylactically, consider daily doses of a fluoroquinolone (eg, ciprofloxacin 500 mg orally once daily, not twice daily as for treatment) or rifaximin 200 mg orally once or twice a day, for no longer than two to three weeks.10
Nonantimicrobial chemoprophylaxis
Bismuth subsalicylate has reduced the incidence of TD from 40% to just 14% when taken in doses of two chewable tablets or 60 mL of liquid four times daily.11,19,22 However, the dosing frequency can hinder adherence. Moreover, the relatively high doses required raise the risk for adverse drug reactions, such as blackening of the tongue and stool, nausea, constipation, Reye syndrome (in children younger than 12), and possibly tinnitus. The salicylate component of the drug poses a threat to patients with aspirin allergy or renal disease and those taking anticoagulants. Drug interactions with probenecid and methotrexate are also possible. Bismuth is not recommended for use for longer than three weeks, or for children younger than 3 years or pregnant women in their third trimester.
Probiotics such as Lactobacillus and Saccharomyces are among the other nonantimicrobial chemoprophylaxis agents. These preparations of bacteria and fungi are marketed either singly or in blends of varying composition and proportion. The evidence is divided on their efficacy, and even though some meta-analyses have concluded probiotics such as Saccharomyces boulardii are useful in preventing TD, endorsement in clinical guidelines is muted.10-12,28-30
Immunizations have limited value so far
Natural immunity to E coli gastrointestinal infection among indigenous people in less developed countries has raised the possibility of a role for vaccines in preventing TD. Some strains of ETEC produce a heat-labile toxin (LT) that bears significant resemblance to the toxin produced by Vibrio cholerae. Therefore, the oral cholera vaccine has been marketed outside the United States for the prevention of TD.19,22 However, only ≤ 7% of TD cases worldwide would be prevented by routine use of this vaccine.31 A transdermal LT vaccine, which involves the antigen-presenting Langerhans cells in the superficial skin layers, is promising but not yet available for routine use.19,22
Continued on next page >>
TREATING TD AND ASSOCIATED SYMPTOMS
Antibiotic treatment
Given that most cases of TD are caused by bacterial pathogens, antibiotics are considered the mainstay of treatment. Concerns about the ill effects of antibiotic use in the case of enterohemorrhagic E coli (EHEC O157:H7) can be allayed because this strain is rarely a cause of TD.9
Consider local resistance patterns and risk for invasive infection. Which antibiotic to recommend is governed by the antibiotic resistance patterns prevalent in the travel destinations and by the risk for infection by invasive pathogens. Invasive TD is generally caused by Campylobacter, Shigella, or Salmonella and manifests clinically with bloody diarrhea, fever, or both. Rifaximin at a dose of 200 mg orally three times daily is effective for noninvasive TD.31,32
However, travelers who develop invasive TD need an alternative to rifaximin. (Those who advocate reserving antibiotic treatment only for invasive diarrhea will not see a role for rifaximin in the first place.) In most invasive cases, a fluoroquinolone will suffice.10-12,19,32 However, increasing prevalence of fluoroquinolone-resistant Campylobacter species has been reported in South and Southeast Asia. In those locations, azithromycin is an effective alternative, albeit with risk for nausea.33
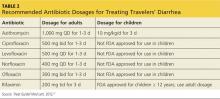
Table 2 provides details of recommended antibiotic dosages for adults and children. The duration of treatment is generally one day unless symptoms persist, in which case a three-day course is recommended.10-12,19,32 If the traveler experiences persistent, new, or worsening symptoms beyond this point, immediate evaluation by a clinician is required.
Nonantibiotic treatment
The antimotility agent loperamide is well established as an antidiarrheal agent. Its effective and safe use as an adjunct to antibiotics in the treatment of TD has been demonstrated in several studies.10-12,19,32,34 It is generally not used to treat children with TD.9
No other nonantibiotic treatment for TD has significant guideline or clinical trial support. Bismuth subsalicylate can be helpful in treating TD,35 but it is not often recommended because of the aforementioned adherence difficulties and because antibiotics and loperamide are effective.
Oral rehydration is usually a mainstay of treating gastrointestinal disease among infants and children. However, it, too, has a limited role in cases of TD, because dehydration is not usually a significant part of the clinical presentation—perhaps because vomiting is not often prominent.
Advice regarding safe food and beverage choices is essential for the patient and her children. Despite the increased risk for TD due to her history and her use of the immunosuppressant methotrexate, she decides not to pursue antibiotic prophylaxis. Bismuth is also contraindicated because of the methotrexate. Her teenage daughter declines bismuth prophylaxis, and her toddler is too young for it.
The patient does accept a prescription for azithromycin for herself and her daughters, in case they experience TD. This choice is appropriate given the destination of India and concern about Campylobacter resistance to fluoroquinolones. You also recommend loperamide for use by the mother and older child, in conjunction with the antibiotic.
Two weeks after their trip abroad, the travelers return for an office visit. On the trip, the mother and toddler experienced diarrhea, which responded well to your recommended management. The older child was well during the trip, but she developed diarrhea, abdominal pain, and anorexia one week after returning to the US. These symptoms have persisted despite a three-day course of azithromycin and loperamide.
Continued on next page >>
POSTTRAVEL EVALUATION
TD generally occurs within one to two weeks of arrival at the travel destination and usually lasts no longer than four to five days.19 This scenario is typical of a bacterial infection. When it occurs later or lasts longer (or both), consider several alternative possibilities.19,36
First, the likelihood of a protozoal parasitic infection is increased. Although giardiasis is most likely, other protozoa such as Entamoeba, Cyclospora, Isospora, and Cryptosporidium are also possibilities. Second, if diarrhea persists, it might be due, not to continued infection, but to a self-limited postinfectious enteropathy or to PI-IBS. Third, TD is known to precipitate the clinical manifestation of underlying gastrointestinal disorders, such as inflammatory bowel disease (IBD), celiac disease, or even cancer.37
With an atypical disease course, it’s advisable to send three stool samples for laboratory evaluation for ova and parasites and for antigen assays for Giardia. If results of these tests are negative, given the difficulty inherent in diagnosing Giardia, consider empiric treatment with metronidazole in lieu of duodenal sampling.36 If the diarrhea persists, investigate serologic markers for celiac disease and IBD. If these are not revealing, referral for colonoscopy is prudent.
The teenager’s three stool samples were negative for ova and parasites and for Giardia antigen. Following empiric treatment with oral metronidazole 250 mg, three times daily for seven days, the diarrhea resolved.
References on next page >>
REFERENCES
1. Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg. 2000; 62:585-589.
2. Greenwood Z, Black J, Weld L, et al for the GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221-228.
3. DuPont HL. Systematic review: the epidemiology and clinical features of travellers’ diarrhoea. Aliment Pharmacol Ther. 2009;30:187-196.
4. Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med. 2004;11:231-237.
5. de la Cabada Bauche J, DuPont HL. New developments in traveler’s diarrhea. Gastroenterol Hepatol. 2011;7:88-95.
6. Cabada MM, White AC. Travelers’ diarrhea: an update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473-479.
7. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents. 2003;21:181-188.
8. Cabada MM, Maldonado F, Quispe W, et al. Risk factors associated with diarrhea among international visitors to Cuzco, Peru. Am J Trop Med Hyg. 2006;75:968-972.
9. Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41(suppl 8):S547-S552.
10. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499-1539.
11. Connor BA. Travelers’ diarrhea. wwwnc.cdc.gov/travel/yellowbook/ 2012/chapter-2-the-pre-travel-consultation/travelers-diarrhea.htm. Accessed April 22, 2014.
12. Advice for travelers. Treat Guidel Med Lett. 2012;10:45-56.
13. Shlim DR. Looking for evidence that personal hygiene precautions prevent travelers’ diarrhea. Clin Infect Dis. 2005;41(suppl 8):S531-S535.
14. Laverone E, Boccalini S, Bechini A, et al. Travelers’ compliance to prophylactic measures and behavior during stay abroad: results of a retrospective study of subjects returning to a travel medicine center in Italy. J Travel Med. 2006;13:338-344.
15. Ashley DV, Walters C, Dockery-Brown C, et al. Interventions to prevent and control food-borne diseases associated with a reduction in traveler’s diarrhea in tourists to Jamaica. J Travel Med. 2004;11:364-367.
16. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609-614.
17. Riddle MS, Sanders JW, Putnam SD, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891-900.
18. Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010; 48:1673-1676.
19. Hill DR, Ryan ET. Management of travellers’ diarrhoea. BMJ. 2008; 337:863-867.
20. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
21. Pimentel M, Riddle MS. Prevention of traveler’s diarrhea: a call to reconvene. Clin Infect Dis. 2008;46:151-152.
22. DuPont HL. Systematic review: prevention of travellers’ diarrhoea. Aliment Pharmacol Ther. 2008;27:741-751.
23. Wang M, Szucs TD, Steffen R. Economic aspects of travelers’ diarrhea.
J Travel Med. 2008;15:110-118.
24. Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410-413.
25. Tornblom H, Holmvall P, Svenungsson B, et al. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5:461-464.
26. DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805-812.
27. Taylor DN, McKenzie R, Durbin A, et al. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis. 2006;42:1283-1288.
28. Sazawal S, Hiremath G, Dhingra U, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374-382.
29. Bri V, Buffet P, Genty S, et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: a randomized, double-blind, controlled study. Clin Infect Dis. 2006;43:1170-1175.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines—use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Taylor DN, Bourgeois AL, Ericsson CD, et al. A randomized double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060-1066.
32. DuPont HL, Ericsson CD, Farthing MJG, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009; 16:161-171.
33. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
34. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in travelers’ diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008; 47:1007-1014.
35. Steffen R. Worldwide efficacy of bismuth subsalicylate in the treatment of travelers’ diarrhea. Rev Infect Dis. 1990;12(suppl 1):S80-S86.
36. Connor BA. Persistent travelers’ diarrhea. wwwnc.cdc.gov/travel/
yellowbook/2012/chapter-5-post-travel-evaluation/persistent-travelers-diarrhea.htm. Accessed April 22 2014.
37. Landzberg BR, Connor BA. Persistent diarrhea in the returning traveler: think beyond persistent infection. Scand J Gastroenterol. 2005;40:
112-114.
A 40-year-old woman, a childhood immigrant from India, is seeking advice regarding her upcoming two-week trip to Mumbai. She is taking her two children, ages 16 years and 16 months, to visit their grandparents for the first time. She has made this trip alone a few times and has invariably experienced short bouts of self-limited diarrheal illness. She wonders what she might do to prevent travelers’ diarrhea. Her only medical problem is rheumatoid arthritis, which has been well controlled with methotrexate. Her children are healthy. What would you recommend?
Recommendations regarding travelers’ diarrhea (TD) address prevention and management. Prevention encompasses advice about personal behaviors and the use of chemoprophylaxis (antimicrobial and nonantimicrobial) and vaccinations. Since international travelers are known to treat themselves for diarrheal illnesses during their trips,1 recommendations regarding management should assume self-treatment and include the use of both antibiotic and nonantibiotic remedies. Pretravel recommendations will of course be most effective if they account for the individual’s risk for TD.
TD RISK
TD is generally defined as the passage of three or more loose stools in a 24-hour period, with associated symptoms of enteric infection (eg, fever, nausea, vomiting, or abdominal cramping). Defined in this manner, TD is thought to occur in 60% to 70% of individuals who travel from developed countries to less-developed countries.2-4 Risk for TD is influenced both by intrinsic personal factors and by factors specific to the trip.
Personal risk factors
Individual variation in susceptibility to TD might result from a genetic predisposition arising from single nucleotide polymorphisms governing various inflammatory marker proteins.5 A history of multiple episodes of TD, especially if fellow travelers were spared, can suggest this kind of individual susceptibility. Other factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.6,7 However, the trip itself is much more important in assessing risk for TD.
Trip-related risk factors
The destination. The most salient risk factor for TD is the geographic destination. Regions of the world can be divided into TD risk strata2:
• Very high: South Asia
• High: South America, Sub-Saharan Africa
• Medium: Central America, Mexico, Caribbean, Middle East, North Africa, Southeast Asia, Oceania
• Low: Europe, North America (excluding Mexico), Australasia, Northeast Asia
Particularly notable countries, in descending order of risk, are Nepal, India, Myanmar, Bolivia, Sri Lanka, Ecuador, Peru, Kenya, and Guatemala.2
Dietary choices. Additionally, since travelers acquire TD by ingesting food or beverages contaminated with pathogenic fecal microbes, dietary behaviors during the trip affect their susceptibility. At least risk are business travelers and tourists who confine their activities to more affluent settings in which food and beverages are prepared and stored hygienically.1,4,8,9 At greater risk are travelers who immerse themselves in local culture, visiting locations that are more impoverished and not as well equipped with sanitation systems, especially if their stay lasts at least two to three weeks.1,4,8,9
Also, the older a traveler is, the lower his or her risk for TD.1,9 An exception to this might be infants whose diet consists solely of breast milk or formula prepared under sanitary conditions.
Continued on next page >>
TD PREVENTION
Emphasize food and beverage precautions
It might be reasonable to expect that travelers who are circumspect about their food and beverage choices on trips will be able to avoid TD. Indeed, this is the basis for the aphorism, “Boil it, peel it, or forget it.” Guidelines routinely recommend that travelers restrict their selection of foods to those that have been well cooked and are served while still very hot, as well as to fruits and vegetables that they peel themselves. Likewise, they should drink only beverages that have been boiled or are in sealed bottles or under carbonation and served without ice.10-12
Many travelers might find these recommendations too restrictive to follow faithfully. Moreover, studies suggest it may not be possible for even the most assiduous traveler to fully avoid the risk for TD.13,14 The hygienic characteristics of the travel destination may be more determinative, as illustrated by the successful reduction of TD rates in Jamaica by improving sanitation in tourist resorts.15
Antibiotic chemoprophylaxis: A debated practice with limited consensus
The etiologic agents of TD are multiple and vary somewhat in predominance according to geographic region.3,16,17 Table 1 depicts variance by region. The most common pathogens are strains of the bacterium Escherichia coli, particularly enterotoxigenic (ETEC), enteroaggregative, and enteropathogenic strains.16 Other bacteria of importance are Campylobacter, Salmonella, and Shigella. Viruses, particularly norovirus (notably connected with cruise ships), can also cause TD, although it is implicated in no more than 17% of cases.18 Parasitic pathogens are even less common causes of TD (4% to 10%) and mainly involve the protozoa, Giardia lamblia, and, to a lesser extent, Entamoeba histolytica and Cryptosporidium.
Although some pathogens often have a characteristic presentation—such as frothy, greasy diarrhea in the case of G lamblia—in general, they cannot be reliably distinguished from one another clinically. Notably, up to 50% of stool samples from TD patients do not yield any pathogen,16 raising the suspicion that current diagnostic technology is not sufficiently sensitive to routinely identify certain bacteria.
There is no consensus on recommending antibiotic chemoprophylaxis against TD.
Opponents of this practice point out that TD is generally a brief (three to five days), self-limited illness.10-12,19,20 Moreover, concerns about antibiotic resistance have come to pass. Previously used agents (trimethoprim-sulfamethoxazole and doxycycline) are no longer effective in preventing or treating TD. In addition, antibiotic use carries the risk for allergic reactions, as well as other adverse effects including (ironically) the development of antibiotic-associated and Clostridium difficile diarrhea.
Proponents of antibiotic chemoprophylaxis point to its demonstrated efficacy in reducing the risk for TD by 4% to 40%.11,21,22 They also argue that at least 20% to 25% of travelers who get TD must significantly curtail their activities for a day or more.1,23 This change in travel plans is associated not only with significant personal loss but also imposes a financial burden.23 Furthermore, TD is known to have longer-term effects. Up to 10% of those affected develop postinfectious irritable bowel syndrome (PI-IBS) that can last for five or six years.21,22,24,25 It is not known, however, whether the use of antibiotic chemoprophylaxis significantly reduces the incidence of PI-IBS.
Finally, the luminal antibiotic rifaximin, nonabsorbable as it is, is very well tolerated and holds promise for not inciting antibiotic resistance.22 However, while its efficacy in preventing TD has been demonstrated in various settings,22,26,27 it is not approved by the FDA for this indication. Also, concerns persist that it might not be effective in preventing TD caused by invasive pathogens.19
Indications on which all agree. Even opponents of antibiotic chemoprophylaxis grant that it is probably warranted for two groups of travelers.10-12 The first is those whose trip schedule is of such importance that any deviation would be intolerable. The second is travelers with comorbidities that would render them at high risk for serious inconvenience or illness if they developed TD. Examples of the latter include patients with enterostomies, mobility impairments, immune suppression, inflammatory bowel disease, and renal or metabolic diseases.
Chemoprophylaxis regimens. If you prescribe an antibiotic prophylactically, consider daily doses of a fluoroquinolone (eg, ciprofloxacin 500 mg orally once daily, not twice daily as for treatment) or rifaximin 200 mg orally once or twice a day, for no longer than two to three weeks.10
Nonantimicrobial chemoprophylaxis
Bismuth subsalicylate has reduced the incidence of TD from 40% to just 14% when taken in doses of two chewable tablets or 60 mL of liquid four times daily.11,19,22 However, the dosing frequency can hinder adherence. Moreover, the relatively high doses required raise the risk for adverse drug reactions, such as blackening of the tongue and stool, nausea, constipation, Reye syndrome (in children younger than 12), and possibly tinnitus. The salicylate component of the drug poses a threat to patients with aspirin allergy or renal disease and those taking anticoagulants. Drug interactions with probenecid and methotrexate are also possible. Bismuth is not recommended for use for longer than three weeks, or for children younger than 3 years or pregnant women in their third trimester.
Probiotics such as Lactobacillus and Saccharomyces are among the other nonantimicrobial chemoprophylaxis agents. These preparations of bacteria and fungi are marketed either singly or in blends of varying composition and proportion. The evidence is divided on their efficacy, and even though some meta-analyses have concluded probiotics such as Saccharomyces boulardii are useful in preventing TD, endorsement in clinical guidelines is muted.10-12,28-30
Immunizations have limited value so far
Natural immunity to E coli gastrointestinal infection among indigenous people in less developed countries has raised the possibility of a role for vaccines in preventing TD. Some strains of ETEC produce a heat-labile toxin (LT) that bears significant resemblance to the toxin produced by Vibrio cholerae. Therefore, the oral cholera vaccine has been marketed outside the United States for the prevention of TD.19,22 However, only ≤ 7% of TD cases worldwide would be prevented by routine use of this vaccine.31 A transdermal LT vaccine, which involves the antigen-presenting Langerhans cells in the superficial skin layers, is promising but not yet available for routine use.19,22
Continued on next page >>
TREATING TD AND ASSOCIATED SYMPTOMS
Antibiotic treatment
Given that most cases of TD are caused by bacterial pathogens, antibiotics are considered the mainstay of treatment. Concerns about the ill effects of antibiotic use in the case of enterohemorrhagic E coli (EHEC O157:H7) can be allayed because this strain is rarely a cause of TD.9
Consider local resistance patterns and risk for invasive infection. Which antibiotic to recommend is governed by the antibiotic resistance patterns prevalent in the travel destinations and by the risk for infection by invasive pathogens. Invasive TD is generally caused by Campylobacter, Shigella, or Salmonella and manifests clinically with bloody diarrhea, fever, or both. Rifaximin at a dose of 200 mg orally three times daily is effective for noninvasive TD.31,32
However, travelers who develop invasive TD need an alternative to rifaximin. (Those who advocate reserving antibiotic treatment only for invasive diarrhea will not see a role for rifaximin in the first place.) In most invasive cases, a fluoroquinolone will suffice.10-12,19,32 However, increasing prevalence of fluoroquinolone-resistant Campylobacter species has been reported in South and Southeast Asia. In those locations, azithromycin is an effective alternative, albeit with risk for nausea.33
Table 2 provides details of recommended antibiotic dosages for adults and children. The duration of treatment is generally one day unless symptoms persist, in which case a three-day course is recommended.10-12,19,32 If the traveler experiences persistent, new, or worsening symptoms beyond this point, immediate evaluation by a clinician is required.
Nonantibiotic treatment
The antimotility agent loperamide is well established as an antidiarrheal agent. Its effective and safe use as an adjunct to antibiotics in the treatment of TD has been demonstrated in several studies.10-12,19,32,34 It is generally not used to treat children with TD.9
No other nonantibiotic treatment for TD has significant guideline or clinical trial support. Bismuth subsalicylate can be helpful in treating TD,35 but it is not often recommended because of the aforementioned adherence difficulties and because antibiotics and loperamide are effective.
Oral rehydration is usually a mainstay of treating gastrointestinal disease among infants and children. However, it, too, has a limited role in cases of TD, because dehydration is not usually a significant part of the clinical presentation—perhaps because vomiting is not often prominent.
Advice regarding safe food and beverage choices is essential for the patient and her children. Despite the increased risk for TD due to her history and her use of the immunosuppressant methotrexate, she decides not to pursue antibiotic prophylaxis. Bismuth is also contraindicated because of the methotrexate. Her teenage daughter declines bismuth prophylaxis, and her toddler is too young for it.
The patient does accept a prescription for azithromycin for herself and her daughters, in case they experience TD. This choice is appropriate given the destination of India and concern about Campylobacter resistance to fluoroquinolones. You also recommend loperamide for use by the mother and older child, in conjunction with the antibiotic.
Two weeks after their trip abroad, the travelers return for an office visit. On the trip, the mother and toddler experienced diarrhea, which responded well to your recommended management. The older child was well during the trip, but she developed diarrhea, abdominal pain, and anorexia one week after returning to the US. These symptoms have persisted despite a three-day course of azithromycin and loperamide.
Continued on next page >>
POSTTRAVEL EVALUATION
TD generally occurs within one to two weeks of arrival at the travel destination and usually lasts no longer than four to five days.19 This scenario is typical of a bacterial infection. When it occurs later or lasts longer (or both), consider several alternative possibilities.19,36
First, the likelihood of a protozoal parasitic infection is increased. Although giardiasis is most likely, other protozoa such as Entamoeba, Cyclospora, Isospora, and Cryptosporidium are also possibilities. Second, if diarrhea persists, it might be due, not to continued infection, but to a self-limited postinfectious enteropathy or to PI-IBS. Third, TD is known to precipitate the clinical manifestation of underlying gastrointestinal disorders, such as inflammatory bowel disease (IBD), celiac disease, or even cancer.37
With an atypical disease course, it’s advisable to send three stool samples for laboratory evaluation for ova and parasites and for antigen assays for Giardia. If results of these tests are negative, given the difficulty inherent in diagnosing Giardia, consider empiric treatment with metronidazole in lieu of duodenal sampling.36 If the diarrhea persists, investigate serologic markers for celiac disease and IBD. If these are not revealing, referral for colonoscopy is prudent.
The teenager’s three stool samples were negative for ova and parasites and for Giardia antigen. Following empiric treatment with oral metronidazole 250 mg, three times daily for seven days, the diarrhea resolved.
References on next page >>
REFERENCES
1. Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg. 2000; 62:585-589.
2. Greenwood Z, Black J, Weld L, et al for the GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221-228.
3. DuPont HL. Systematic review: the epidemiology and clinical features of travellers’ diarrhoea. Aliment Pharmacol Ther. 2009;30:187-196.
4. Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med. 2004;11:231-237.
5. de la Cabada Bauche J, DuPont HL. New developments in traveler’s diarrhea. Gastroenterol Hepatol. 2011;7:88-95.
6. Cabada MM, White AC. Travelers’ diarrhea: an update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473-479.
7. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents. 2003;21:181-188.
8. Cabada MM, Maldonado F, Quispe W, et al. Risk factors associated with diarrhea among international visitors to Cuzco, Peru. Am J Trop Med Hyg. 2006;75:968-972.
9. Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41(suppl 8):S547-S552.
10. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499-1539.
11. Connor BA. Travelers’ diarrhea. wwwnc.cdc.gov/travel/yellowbook/ 2012/chapter-2-the-pre-travel-consultation/travelers-diarrhea.htm. Accessed April 22, 2014.
12. Advice for travelers. Treat Guidel Med Lett. 2012;10:45-56.
13. Shlim DR. Looking for evidence that personal hygiene precautions prevent travelers’ diarrhea. Clin Infect Dis. 2005;41(suppl 8):S531-S535.
14. Laverone E, Boccalini S, Bechini A, et al. Travelers’ compliance to prophylactic measures and behavior during stay abroad: results of a retrospective study of subjects returning to a travel medicine center in Italy. J Travel Med. 2006;13:338-344.
15. Ashley DV, Walters C, Dockery-Brown C, et al. Interventions to prevent and control food-borne diseases associated with a reduction in traveler’s diarrhea in tourists to Jamaica. J Travel Med. 2004;11:364-367.
16. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609-614.
17. Riddle MS, Sanders JW, Putnam SD, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891-900.
18. Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010; 48:1673-1676.
19. Hill DR, Ryan ET. Management of travellers’ diarrhoea. BMJ. 2008; 337:863-867.
20. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
21. Pimentel M, Riddle MS. Prevention of traveler’s diarrhea: a call to reconvene. Clin Infect Dis. 2008;46:151-152.
22. DuPont HL. Systematic review: prevention of travellers’ diarrhoea. Aliment Pharmacol Ther. 2008;27:741-751.
23. Wang M, Szucs TD, Steffen R. Economic aspects of travelers’ diarrhea.
J Travel Med. 2008;15:110-118.
24. Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410-413.
25. Tornblom H, Holmvall P, Svenungsson B, et al. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5:461-464.
26. DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805-812.
27. Taylor DN, McKenzie R, Durbin A, et al. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis. 2006;42:1283-1288.
28. Sazawal S, Hiremath G, Dhingra U, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374-382.
29. Bri V, Buffet P, Genty S, et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: a randomized, double-blind, controlled study. Clin Infect Dis. 2006;43:1170-1175.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines—use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Taylor DN, Bourgeois AL, Ericsson CD, et al. A randomized double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060-1066.
32. DuPont HL, Ericsson CD, Farthing MJG, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009; 16:161-171.
33. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
34. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in travelers’ diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008; 47:1007-1014.
35. Steffen R. Worldwide efficacy of bismuth subsalicylate in the treatment of travelers’ diarrhea. Rev Infect Dis. 1990;12(suppl 1):S80-S86.
36. Connor BA. Persistent travelers’ diarrhea. wwwnc.cdc.gov/travel/
yellowbook/2012/chapter-5-post-travel-evaluation/persistent-travelers-diarrhea.htm. Accessed April 22 2014.
37. Landzberg BR, Connor BA. Persistent diarrhea in the returning traveler: think beyond persistent infection. Scand J Gastroenterol. 2005;40:
112-114.
A 40-year-old woman, a childhood immigrant from India, is seeking advice regarding her upcoming two-week trip to Mumbai. She is taking her two children, ages 16 years and 16 months, to visit their grandparents for the first time. She has made this trip alone a few times and has invariably experienced short bouts of self-limited diarrheal illness. She wonders what she might do to prevent travelers’ diarrhea. Her only medical problem is rheumatoid arthritis, which has been well controlled with methotrexate. Her children are healthy. What would you recommend?
Recommendations regarding travelers’ diarrhea (TD) address prevention and management. Prevention encompasses advice about personal behaviors and the use of chemoprophylaxis (antimicrobial and nonantimicrobial) and vaccinations. Since international travelers are known to treat themselves for diarrheal illnesses during their trips,1 recommendations regarding management should assume self-treatment and include the use of both antibiotic and nonantibiotic remedies. Pretravel recommendations will of course be most effective if they account for the individual’s risk for TD.
TD RISK
TD is generally defined as the passage of three or more loose stools in a 24-hour period, with associated symptoms of enteric infection (eg, fever, nausea, vomiting, or abdominal cramping). Defined in this manner, TD is thought to occur in 60% to 70% of individuals who travel from developed countries to less-developed countries.2-4 Risk for TD is influenced both by intrinsic personal factors and by factors specific to the trip.
Personal risk factors
Individual variation in susceptibility to TD might result from a genetic predisposition arising from single nucleotide polymorphisms governing various inflammatory marker proteins.5 A history of multiple episodes of TD, especially if fellow travelers were spared, can suggest this kind of individual susceptibility. Other factors that increase vulnerability to TD are immunodeficiency, achlorhydric states such as atrophic gastritis, and chronic use of proton pump inhibitors.6,7 However, the trip itself is much more important in assessing risk for TD.
Trip-related risk factors
The destination. The most salient risk factor for TD is the geographic destination. Regions of the world can be divided into TD risk strata2:
• Very high: South Asia
• High: South America, Sub-Saharan Africa
• Medium: Central America, Mexico, Caribbean, Middle East, North Africa, Southeast Asia, Oceania
• Low: Europe, North America (excluding Mexico), Australasia, Northeast Asia
Particularly notable countries, in descending order of risk, are Nepal, India, Myanmar, Bolivia, Sri Lanka, Ecuador, Peru, Kenya, and Guatemala.2
Dietary choices. Additionally, since travelers acquire TD by ingesting food or beverages contaminated with pathogenic fecal microbes, dietary behaviors during the trip affect their susceptibility. At least risk are business travelers and tourists who confine their activities to more affluent settings in which food and beverages are prepared and stored hygienically.1,4,8,9 At greater risk are travelers who immerse themselves in local culture, visiting locations that are more impoverished and not as well equipped with sanitation systems, especially if their stay lasts at least two to three weeks.1,4,8,9
Also, the older a traveler is, the lower his or her risk for TD.1,9 An exception to this might be infants whose diet consists solely of breast milk or formula prepared under sanitary conditions.
Continued on next page >>
TD PREVENTION
Emphasize food and beverage precautions
It might be reasonable to expect that travelers who are circumspect about their food and beverage choices on trips will be able to avoid TD. Indeed, this is the basis for the aphorism, “Boil it, peel it, or forget it.” Guidelines routinely recommend that travelers restrict their selection of foods to those that have been well cooked and are served while still very hot, as well as to fruits and vegetables that they peel themselves. Likewise, they should drink only beverages that have been boiled or are in sealed bottles or under carbonation and served without ice.10-12
Many travelers might find these recommendations too restrictive to follow faithfully. Moreover, studies suggest it may not be possible for even the most assiduous traveler to fully avoid the risk for TD.13,14 The hygienic characteristics of the travel destination may be more determinative, as illustrated by the successful reduction of TD rates in Jamaica by improving sanitation in tourist resorts.15
Antibiotic chemoprophylaxis: A debated practice with limited consensus
The etiologic agents of TD are multiple and vary somewhat in predominance according to geographic region.3,16,17 Table 1 depicts variance by region. The most common pathogens are strains of the bacterium Escherichia coli, particularly enterotoxigenic (ETEC), enteroaggregative, and enteropathogenic strains.16 Other bacteria of importance are Campylobacter, Salmonella, and Shigella. Viruses, particularly norovirus (notably connected with cruise ships), can also cause TD, although it is implicated in no more than 17% of cases.18 Parasitic pathogens are even less common causes of TD (4% to 10%) and mainly involve the protozoa, Giardia lamblia, and, to a lesser extent, Entamoeba histolytica and Cryptosporidium.
Although some pathogens often have a characteristic presentation—such as frothy, greasy diarrhea in the case of G lamblia—in general, they cannot be reliably distinguished from one another clinically. Notably, up to 50% of stool samples from TD patients do not yield any pathogen,16 raising the suspicion that current diagnostic technology is not sufficiently sensitive to routinely identify certain bacteria.
There is no consensus on recommending antibiotic chemoprophylaxis against TD.
Opponents of this practice point out that TD is generally a brief (three to five days), self-limited illness.10-12,19,20 Moreover, concerns about antibiotic resistance have come to pass. Previously used agents (trimethoprim-sulfamethoxazole and doxycycline) are no longer effective in preventing or treating TD. In addition, antibiotic use carries the risk for allergic reactions, as well as other adverse effects including (ironically) the development of antibiotic-associated and Clostridium difficile diarrhea.
Proponents of antibiotic chemoprophylaxis point to its demonstrated efficacy in reducing the risk for TD by 4% to 40%.11,21,22 They also argue that at least 20% to 25% of travelers who get TD must significantly curtail their activities for a day or more.1,23 This change in travel plans is associated not only with significant personal loss but also imposes a financial burden.23 Furthermore, TD is known to have longer-term effects. Up to 10% of those affected develop postinfectious irritable bowel syndrome (PI-IBS) that can last for five or six years.21,22,24,25 It is not known, however, whether the use of antibiotic chemoprophylaxis significantly reduces the incidence of PI-IBS.
Finally, the luminal antibiotic rifaximin, nonabsorbable as it is, is very well tolerated and holds promise for not inciting antibiotic resistance.22 However, while its efficacy in preventing TD has been demonstrated in various settings,22,26,27 it is not approved by the FDA for this indication. Also, concerns persist that it might not be effective in preventing TD caused by invasive pathogens.19
Indications on which all agree. Even opponents of antibiotic chemoprophylaxis grant that it is probably warranted for two groups of travelers.10-12 The first is those whose trip schedule is of such importance that any deviation would be intolerable. The second is travelers with comorbidities that would render them at high risk for serious inconvenience or illness if they developed TD. Examples of the latter include patients with enterostomies, mobility impairments, immune suppression, inflammatory bowel disease, and renal or metabolic diseases.
Chemoprophylaxis regimens. If you prescribe an antibiotic prophylactically, consider daily doses of a fluoroquinolone (eg, ciprofloxacin 500 mg orally once daily, not twice daily as for treatment) or rifaximin 200 mg orally once or twice a day, for no longer than two to three weeks.10
Nonantimicrobial chemoprophylaxis
Bismuth subsalicylate has reduced the incidence of TD from 40% to just 14% when taken in doses of two chewable tablets or 60 mL of liquid four times daily.11,19,22 However, the dosing frequency can hinder adherence. Moreover, the relatively high doses required raise the risk for adverse drug reactions, such as blackening of the tongue and stool, nausea, constipation, Reye syndrome (in children younger than 12), and possibly tinnitus. The salicylate component of the drug poses a threat to patients with aspirin allergy or renal disease and those taking anticoagulants. Drug interactions with probenecid and methotrexate are also possible. Bismuth is not recommended for use for longer than three weeks, or for children younger than 3 years or pregnant women in their third trimester.
Probiotics such as Lactobacillus and Saccharomyces are among the other nonantimicrobial chemoprophylaxis agents. These preparations of bacteria and fungi are marketed either singly or in blends of varying composition and proportion. The evidence is divided on their efficacy, and even though some meta-analyses have concluded probiotics such as Saccharomyces boulardii are useful in preventing TD, endorsement in clinical guidelines is muted.10-12,28-30
Immunizations have limited value so far
Natural immunity to E coli gastrointestinal infection among indigenous people in less developed countries has raised the possibility of a role for vaccines in preventing TD. Some strains of ETEC produce a heat-labile toxin (LT) that bears significant resemblance to the toxin produced by Vibrio cholerae. Therefore, the oral cholera vaccine has been marketed outside the United States for the prevention of TD.19,22 However, only ≤ 7% of TD cases worldwide would be prevented by routine use of this vaccine.31 A transdermal LT vaccine, which involves the antigen-presenting Langerhans cells in the superficial skin layers, is promising but not yet available for routine use.19,22
Continued on next page >>
TREATING TD AND ASSOCIATED SYMPTOMS
Antibiotic treatment
Given that most cases of TD are caused by bacterial pathogens, antibiotics are considered the mainstay of treatment. Concerns about the ill effects of antibiotic use in the case of enterohemorrhagic E coli (EHEC O157:H7) can be allayed because this strain is rarely a cause of TD.9
Consider local resistance patterns and risk for invasive infection. Which antibiotic to recommend is governed by the antibiotic resistance patterns prevalent in the travel destinations and by the risk for infection by invasive pathogens. Invasive TD is generally caused by Campylobacter, Shigella, or Salmonella and manifests clinically with bloody diarrhea, fever, or both. Rifaximin at a dose of 200 mg orally three times daily is effective for noninvasive TD.31,32
However, travelers who develop invasive TD need an alternative to rifaximin. (Those who advocate reserving antibiotic treatment only for invasive diarrhea will not see a role for rifaximin in the first place.) In most invasive cases, a fluoroquinolone will suffice.10-12,19,32 However, increasing prevalence of fluoroquinolone-resistant Campylobacter species has been reported in South and Southeast Asia. In those locations, azithromycin is an effective alternative, albeit with risk for nausea.33
Table 2 provides details of recommended antibiotic dosages for adults and children. The duration of treatment is generally one day unless symptoms persist, in which case a three-day course is recommended.10-12,19,32 If the traveler experiences persistent, new, or worsening symptoms beyond this point, immediate evaluation by a clinician is required.
Nonantibiotic treatment
The antimotility agent loperamide is well established as an antidiarrheal agent. Its effective and safe use as an adjunct to antibiotics in the treatment of TD has been demonstrated in several studies.10-12,19,32,34 It is generally not used to treat children with TD.9
No other nonantibiotic treatment for TD has significant guideline or clinical trial support. Bismuth subsalicylate can be helpful in treating TD,35 but it is not often recommended because of the aforementioned adherence difficulties and because antibiotics and loperamide are effective.
Oral rehydration is usually a mainstay of treating gastrointestinal disease among infants and children. However, it, too, has a limited role in cases of TD, because dehydration is not usually a significant part of the clinical presentation—perhaps because vomiting is not often prominent.
Advice regarding safe food and beverage choices is essential for the patient and her children. Despite the increased risk for TD due to her history and her use of the immunosuppressant methotrexate, she decides not to pursue antibiotic prophylaxis. Bismuth is also contraindicated because of the methotrexate. Her teenage daughter declines bismuth prophylaxis, and her toddler is too young for it.
The patient does accept a prescription for azithromycin for herself and her daughters, in case they experience TD. This choice is appropriate given the destination of India and concern about Campylobacter resistance to fluoroquinolones. You also recommend loperamide for use by the mother and older child, in conjunction with the antibiotic.
Two weeks after their trip abroad, the travelers return for an office visit. On the trip, the mother and toddler experienced diarrhea, which responded well to your recommended management. The older child was well during the trip, but she developed diarrhea, abdominal pain, and anorexia one week after returning to the US. These symptoms have persisted despite a three-day course of azithromycin and loperamide.
Continued on next page >>
POSTTRAVEL EVALUATION
TD generally occurs within one to two weeks of arrival at the travel destination and usually lasts no longer than four to five days.19 This scenario is typical of a bacterial infection. When it occurs later or lasts longer (or both), consider several alternative possibilities.19,36
First, the likelihood of a protozoal parasitic infection is increased. Although giardiasis is most likely, other protozoa such as Entamoeba, Cyclospora, Isospora, and Cryptosporidium are also possibilities. Second, if diarrhea persists, it might be due, not to continued infection, but to a self-limited postinfectious enteropathy or to PI-IBS. Third, TD is known to precipitate the clinical manifestation of underlying gastrointestinal disorders, such as inflammatory bowel disease (IBD), celiac disease, or even cancer.37
With an atypical disease course, it’s advisable to send three stool samples for laboratory evaluation for ova and parasites and for antigen assays for Giardia. If results of these tests are negative, given the difficulty inherent in diagnosing Giardia, consider empiric treatment with metronidazole in lieu of duodenal sampling.36 If the diarrhea persists, investigate serologic markers for celiac disease and IBD. If these are not revealing, referral for colonoscopy is prudent.
The teenager’s three stool samples were negative for ova and parasites and for Giardia antigen. Following empiric treatment with oral metronidazole 250 mg, three times daily for seven days, the diarrhea resolved.
References on next page >>
REFERENCES
1. Hill DR. Occurrence and self-treatment of diarrhea in a large cohort of Americans traveling to developing countries. Am J Trop Med Hyg. 2000; 62:585-589.
2. Greenwood Z, Black J, Weld L, et al for the GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221-228.
3. DuPont HL. Systematic review: the epidemiology and clinical features of travellers’ diarrhoea. Aliment Pharmacol Ther. 2009;30:187-196.
4. Steffen R, Tornieporth N, Clemens SA, et al. Epidemiology of travelers’ diarrhea: details of a global survey. J Travel Med. 2004;11:231-237.
5. de la Cabada Bauche J, DuPont HL. New developments in traveler’s diarrhea. Gastroenterol Hepatol. 2011;7:88-95.
6. Cabada MM, White AC. Travelers’ diarrhea: an update on susceptibility, prevention, and treatment. Curr Gastroenterol Rep. 2008;10:473-479.
7. Ericsson CD. Travellers with pre-existing medical conditions. Int J Antimicrob Agents. 2003;21:181-188.
8. Cabada MM, Maldonado F, Quispe W, et al. Risk factors associated with diarrhea among international visitors to Cuzco, Peru. Am J Trop Med Hyg. 2006;75:968-972.
9. Mackell S. Traveler’s diarrhea in the pediatric population: etiology and impact. Clin Infect Dis. 2005;41(suppl 8):S547-S552.
10. Hill DR, Ericsson CD, Pearson RD, et al. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499-1539.
11. Connor BA. Travelers’ diarrhea. wwwnc.cdc.gov/travel/yellowbook/ 2012/chapter-2-the-pre-travel-consultation/travelers-diarrhea.htm. Accessed April 22, 2014.
12. Advice for travelers. Treat Guidel Med Lett. 2012;10:45-56.
13. Shlim DR. Looking for evidence that personal hygiene precautions prevent travelers’ diarrhea. Clin Infect Dis. 2005;41(suppl 8):S531-S535.
14. Laverone E, Boccalini S, Bechini A, et al. Travelers’ compliance to prophylactic measures and behavior during stay abroad: results of a retrospective study of subjects returning to a travel medicine center in Italy. J Travel Med. 2006;13:338-344.
15. Ashley DV, Walters C, Dockery-Brown C, et al. Interventions to prevent and control food-borne diseases associated with a reduction in traveler’s diarrhea in tourists to Jamaica. J Travel Med. 2004;11:364-367.
16. Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg. 2009;80:609-614.
17. Riddle MS, Sanders JW, Putnam SD, et al. Incidence, etiology, and impact of diarrhea among long-term travelers (US military and similar populations): a systematic review. Am J Trop Med Hyg. 2006;74:891-900.
18. Koo HL, Ajami NJ, Jiang ZD, et al. Noroviruses as a cause of diarrhea in travelers to Guatemala, India, and Mexico. J Clin Microbiol. 2010; 48:1673-1676.
19. Hill DR, Ryan ET. Management of travellers’ diarrhoea. BMJ. 2008; 337:863-867.
20. Rendi-Wagner P, Kollaritsch H. Drug prophylaxis for travelers’ diarrhea. Clin Infect Dis. 2002;34:628-633.
21. Pimentel M, Riddle MS. Prevention of traveler’s diarrhea: a call to reconvene. Clin Infect Dis. 2008;46:151-152.
22. DuPont HL. Systematic review: prevention of travellers’ diarrhoea. Aliment Pharmacol Ther. 2008;27:741-751.
23. Wang M, Szucs TD, Steffen R. Economic aspects of travelers’ diarrhea.
J Travel Med. 2008;15:110-118.
24. Neal KR, Barker L, Spiller RC. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut. 2002;51:410-413.
25. Tornblom H, Holmvall P, Svenungsson B, et al. Gastrointestinal symptoms after infectious diarrhea: a five-year follow-up in a Swedish cohort of adults. Clin Gastroenterol Hepatol. 2007;5:461-464.
26. DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805-812.
27. Taylor DN, McKenzie R, Durbin A, et al. Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis. 2006;42:1283-1288.
28. Sazawal S, Hiremath G, Dhingra U, et al. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374-382.
29. Bri V, Buffet P, Genty S, et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: a randomized, double-blind, controlled study. Clin Infect Dis. 2006;43:1170-1175.
30. Hill DR, Ford L, Lalloo DG. Oral cholera vaccines—use in clinical practice. Lancet Infect Dis. 2006;6:361-373.
31. Taylor DN, Bourgeois AL, Ericsson CD, et al. A randomized double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers’ diarrhea. Am J Trop Med Hyg. 2006;74:1060-1066.
32. DuPont HL, Ericsson CD, Farthing MJG, et al. Expert review of the evidence base for self-therapy of travelers’ diarrhea. J Travel Med. 2009; 16:161-171.
33. Tribble DR, Sanders JW, Pang LW, et al. Traveler’s diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338-346.
34. Riddle MS, Arnold S, Tribble DR. Effect of adjunctive loperamide in combination with antibiotics on treatment outcomes in travelers’ diarrhea: a systematic review and meta-analysis. Clin Infect Dis. 2008; 47:1007-1014.
35. Steffen R. Worldwide efficacy of bismuth subsalicylate in the treatment of travelers’ diarrhea. Rev Infect Dis. 1990;12(suppl 1):S80-S86.
36. Connor BA. Persistent travelers’ diarrhea. wwwnc.cdc.gov/travel/
yellowbook/2012/chapter-5-post-travel-evaluation/persistent-travelers-diarrhea.htm. Accessed April 22 2014.
37. Landzberg BR, Connor BA. Persistent diarrhea in the returning traveler: think beyond persistent infection. Scand J Gastroenterol. 2005;40:
112-114.
2014 Update on cervical disease
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Dr. Mark Einstein anticipated final FDA approval of the first HPV test for primary cervical cancer screening and, in this UPDATE ON CERVICAL DISEASE, expands on the data behind the approval and how your practice could change
Special Operations Training: An Atypical Presentation of Aspiration Pneumonia
Empyema is a well-known sequela resulting from the extension of bacterial pneumonia or pulmonary abscess to the pleural space. This case highlights the organism Streptococcus intermedius (S intermedius), an uncommon cause of pulmonary empyema.1Streptococcus intermedius is endogenous among oral flora and is notorious for its abscess-forming capabilities when spread to alternative sites.
The patient was a healthy, active-duty male who presented in sepsis after months of worsening dyspnea and subacute hemoptysis following 2 near-drowning episodes during Special Operations training. Eight weeks after urgent surgical decortication and intensive antibiotic therapy, the patient experienced a complete resolution of his symptoms. A brief discussion follows concerning the pathogenesis and relevant literature regarding S intermedius infections.
Case History
A 21-year-old Air Force Tactical Air Control Party (TACP) trainee with no significant past medical history presented with worsening dyspnea, pleuritic chest pain, and hemoptysis after failed outpatient therapy with levofloxacin for presumed community-acquired pneumonia (CAP) 3 days prior. The chest X-ray at that time demonstrated a left lower lobe consolidation with no evidence of pleural effusion or pulmonary abscess on the lateral view (Figure 1).
The patient stated that his symptoms started about 3 months prior with fever, chills, and night sweats. His symptoms occurred episodically every few weeks, but he had no knowledge of any significant events preceding the illness. The patient developed intermittent hemoptysis 2 weeks later. This included blood-streaked mucus with productive cough and bright-red blood, ranging between a teaspoon and a tablespoon, according to the patient. The patient gradually developed increased dyspnea, which began to impact his performance during Special Operations physical training. His symptoms gradually progressed to worsening dyspnea, which began to affect daily living activities, and new-onset left-sided rib pain. The patient reported no relevant travel history and tested negative for purified protein derivatives 3 months before the initial presentation.
On further questioning, the patient disclosed 2 near-drowning incidents within the preceding year. The first occurred 10 months before presentation, when the patient was performing a 1-minute underwater swim in preparation for the TACP training. The patient stated that he came to the surface to take a breath and lost consciousness. He was immediately brought to the edge of the pool and quickly recovered with no apparent residual symptoms. The second episode occurred during an underwater buddy-breathing training exercise 4 months before presentation and just 3 weeks before symptom onset. The patient reported that he knew he was not getting enough air but remained underwater, concerned that he might fail the exercise. He had a transient syncopal episode shortly after aspirating and was brought to the surface. Afterward, the patient refused to receive medical attention following this event, fearing risk of medical disqualification from training. He reportedly did not experience symptoms after this second episode.
The patient’s past medical history included seasonal allergic rhinitis, and his past surgical history was unremarkable. The patient was not taking medication other than levofloxacin, prescribed for the suspected CAP. The patient was allergic to penicillin, did not use tobacco, and reported drinking about 5 alcoholic beverages per week. His family history included a sister with asthma and a mother with factor V Leiden deficiency and pulmonary embolism related to hormone replacement therapy.
The patient’s vital signs revealed a temperature of 103.2°F, 114 beats per minute pulse, 24 breaths per minute respiratory rate, and oxygen saturation of 89% on room air. On physical examination, the patient was noted to be in moderate respiratory distress with accessory muscle use and was diaphoretic. Breath sounds were diminished in the left upper and lower lung fields with significant egophony.
Tests revealed a white blood cell count of 23,300/mm3 with 30% bandemia. A chest radiograph showed an infiltrate/effusion of the entire left hemithorax (Figure 2). A computed tomography (CT) scan of the chest showed a large multiloculated left-side pleural effusion (Figure 3).
The patient was started on broad-spectrum antibiotics, including intravenous (IV) ceftriaxone, azithromycin, and clindamycin. He underwent thoracentesis on admission, yielding only 300 mL of purulent fluid, confirming its loculated status.
Serum total protein was 7.1 g/dL, and no serum lactic acid dehydrogenase (LDH) was obtained at the time of thoracentesis. Pleural fluid analysis revealed a protein of 5.2 g/dL, and an LDH of 5,176 units/L, meeting Light’s criteria for exudate based on a pleural fluid protein to serum protein ratio of > 0.5 (0.69) and pleural fluid LDH level > two-thirds of the upper limit of normal for serum LDH.
General surgery placed 2 thoracostomy tubes in the left hemithorax without significant drainage. On the first hospital day the patient seemed toxic and underwent a minithoracotomy with decortication. The Gram stain of the blood and blood cultures were negative over 72 hours. Another Gram stain of pleural fluid showed Gram-positive cocci in pairs. Pleural fluid cultures obtained during the procedure revealed S intermedius consistent with the patient’s history of aspiration and abscess formation. Antibiotic susceptibility tests were not performed on the sample.
A peripherally inserted central catheter line was placed for daily IV ceftriaxone infusions to be continued with oral clindamycin for the subsequent 4 weeks. A CT scan of the chest at 8 weeks posthospitalization revealed minimal postoperative scarring, and pulmonary function tests showed normal flow volume loop and maximum voluntary ventilation (Figure 4). The patient reported full recovery and was returned to full activity, including further Special Operations training.
Discussion
Pulmonary infections associated with near-drowning events are caused by a host of organisms that must be considered in the differential diagnosis. The most common include Aeromonas species, Burkholderia pseudomallei, Pseudallescheria boydii, Streptococcus pneumoniae, and Pseudomonas aeruginosa.2 However, the causative organism in this case was endogenous. Streptococcus intermedius is an anaerobic, Gram-positive cocci, a member of the Streptococcus milleri group, and is considered normal flora of the oral mucosa, upper respiratory tract, vagina, and gastrointestinal tract.3,4 This organism is innocuous in its normal habitat but may result in considerable mortality and morbidity if spread to alternative sites due to its ability to form abscesses and cause systemic infections.5
Although uncommon, respiratory infections caused by S intermedius typically result from aspiration of gastric or oral contents and may lead to pulmonary abscesses or empyema.1,6,7 It may present as a primary empyema.8 Current literature suggests a mortality rate between 2% and 14% with higher rates in older populations.9 A retrospective study looking at 72 cases of Streptococci viridans pulmonary infection from 1984 to 1996 found only 2 documented cases where S intermedius was identified as the cause of concomitant empyema and lung abscesses. This study also indicated a strong male predominance with only 7% of lung abscesses occurring in females.10,11
This patient developed a pulmonary abscess and empyema as a probable consequence of aspiration during underwater training exercises. The diagnosis was complicated, because the patient did not initially disclose the pertinent history, and he ignored his symptoms so that he could continue training. His actions delayed aggressive antibiotic therapy and likely led to the rapid progression of pneumonia and his complicated clinical course, because S intermedius has shown intermediate susceptibility or resistance to fluoroquinolone monotherapy.12
This case was also unusual given the subacute presentation and 3-month history of hemoptysis. On review of the available medical literature, hemoptysis is an unusual symptom of pulmonary infections caused by S intermedius but can likely be attributed to necrosis of the pulmonary tissue.8,13
Most patients with S intermedius pulmonary infection rapidly progress due to the virulence of this organism and predisposing comorbidities.14,15 However, this patient had a relatively indolent progression for 3 months, which speaks to the increased respiratory reserve of a healthy, young male in excellent cardiovascular condition.
Conclusion
This case highlights the potential for normal oral flora to cause advanced pulmonary disease in patients with no significant comorbidities. Streptococcus intermedius infections can be subacute in presentation but may rapidly progress to severe disease once seeded in the pleural cavity. Whereas early pleural space drainage remains fundamental, urgent surgical intervention may be required for loculated disease. Although infections with this organism may lead to irreversible pulmonary complications, complete resolution with full recovery is possible in young, healthy patients.
Primary care physicians must take a careful history to ensure optimal patient outcomes. This concept is particularly important to consider in aviators and Special Operations personnel who may be reluctant to seek medical care. Establishing a sense of trust among active-duty military is essential for mission accomplishment.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Weightman NC, Barnham MRD, Dove M. Streptococcus milleri group bacteraemia in North Yorkshire, England (1989-2000). Indian J Med Res. 2004;119(suppl):164-167.
2. Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;1(4):896-907.
3. Porta G, Rodriguez-Carballeira M, Gómez L, et al. Thoracic infection caused by Streptococcus milleri. Eur Respir J. 1998;12(2):357-362.
4. Van der Auwera P. Clinical significance of Streptococcus milleri. Eur J Clin Microbiol.1985;4(4):386-390.
5. Murray HW, Gross KC, Masur H, Roberts RB. Serious infections caused by Streptococcus milleri. Am J Med. 1978;64(5):759-764.
6. Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis. 1995;21(suppl 3):S238-S243.
7. Frankish PD, Kolbe J. Thoracic empyema due to Streptococcus milleri: Four cases. N Z Med J. 1984;97(769):849-851.
8. Iskandar SB, Al Hasan MA, Roy TM, Byrd RP Jr. Streptococcus intermedius: An unusual cause of a primary empyema. Tenn Med. 2006;99(2):37-39.
9. de Hoyos A, Sundaresan S. Thoracic empyema. Surg Clin North Am. 2002;82(3):643-671.
10. Jerng JS, Hsueh PR, Teng LJ, Lee LN, Yang PC, Luh KT. Empyema thoracis and lung abscess caused by viridans streptococci. Am J Respir Crit Care Med. 1997;156(5):1508-1514.
11. Wargo KA, McConnell VJ, Higginbotham SA. A case of Streptococcus intermedius empyema. Ann Pharmacother. 2006;40(6):1208-1210.
12. Limia A, Jiménez ML, Alarcón T, López-Brea M. Five-year analysis of antimicrobial susceptibility of the Streptococcus milleri group. Eur J Clin Microbiol Infect Dis. 1999;18(6):440-444.
13. Wong CA, Donal F, Macfarlane JT. Streptococcus milleri pulmonary disease: A review and clinical description of 25 patients. Thorax. 1995;50(10):1093-1096.
14. Shlaes DM, Lerner PI, Wolinsky E, Gopalakrishna KV. Infections due to Lancefield F and related Streptococci (S milleri, S anginosus). Medicine (Baltimore). 1981;60(3):197-207.
15. Roy WJ Jr, Roy TM, Davis GJ. Thoracic empyema due to Streptococcus intermedius. J Ky Med Assoc. 1991;89(11):558-562.
Empyema is a well-known sequela resulting from the extension of bacterial pneumonia or pulmonary abscess to the pleural space. This case highlights the organism Streptococcus intermedius (S intermedius), an uncommon cause of pulmonary empyema.1Streptococcus intermedius is endogenous among oral flora and is notorious for its abscess-forming capabilities when spread to alternative sites.
The patient was a healthy, active-duty male who presented in sepsis after months of worsening dyspnea and subacute hemoptysis following 2 near-drowning episodes during Special Operations training. Eight weeks after urgent surgical decortication and intensive antibiotic therapy, the patient experienced a complete resolution of his symptoms. A brief discussion follows concerning the pathogenesis and relevant literature regarding S intermedius infections.
Case History
A 21-year-old Air Force Tactical Air Control Party (TACP) trainee with no significant past medical history presented with worsening dyspnea, pleuritic chest pain, and hemoptysis after failed outpatient therapy with levofloxacin for presumed community-acquired pneumonia (CAP) 3 days prior. The chest X-ray at that time demonstrated a left lower lobe consolidation with no evidence of pleural effusion or pulmonary abscess on the lateral view (Figure 1).
The patient stated that his symptoms started about 3 months prior with fever, chills, and night sweats. His symptoms occurred episodically every few weeks, but he had no knowledge of any significant events preceding the illness. The patient developed intermittent hemoptysis 2 weeks later. This included blood-streaked mucus with productive cough and bright-red blood, ranging between a teaspoon and a tablespoon, according to the patient. The patient gradually developed increased dyspnea, which began to impact his performance during Special Operations physical training. His symptoms gradually progressed to worsening dyspnea, which began to affect daily living activities, and new-onset left-sided rib pain. The patient reported no relevant travel history and tested negative for purified protein derivatives 3 months before the initial presentation.
On further questioning, the patient disclosed 2 near-drowning incidents within the preceding year. The first occurred 10 months before presentation, when the patient was performing a 1-minute underwater swim in preparation for the TACP training. The patient stated that he came to the surface to take a breath and lost consciousness. He was immediately brought to the edge of the pool and quickly recovered with no apparent residual symptoms. The second episode occurred during an underwater buddy-breathing training exercise 4 months before presentation and just 3 weeks before symptom onset. The patient reported that he knew he was not getting enough air but remained underwater, concerned that he might fail the exercise. He had a transient syncopal episode shortly after aspirating and was brought to the surface. Afterward, the patient refused to receive medical attention following this event, fearing risk of medical disqualification from training. He reportedly did not experience symptoms after this second episode.
The patient’s past medical history included seasonal allergic rhinitis, and his past surgical history was unremarkable. The patient was not taking medication other than levofloxacin, prescribed for the suspected CAP. The patient was allergic to penicillin, did not use tobacco, and reported drinking about 5 alcoholic beverages per week. His family history included a sister with asthma and a mother with factor V Leiden deficiency and pulmonary embolism related to hormone replacement therapy.
The patient’s vital signs revealed a temperature of 103.2°F, 114 beats per minute pulse, 24 breaths per minute respiratory rate, and oxygen saturation of 89% on room air. On physical examination, the patient was noted to be in moderate respiratory distress with accessory muscle use and was diaphoretic. Breath sounds were diminished in the left upper and lower lung fields with significant egophony.
Tests revealed a white blood cell count of 23,300/mm3 with 30% bandemia. A chest radiograph showed an infiltrate/effusion of the entire left hemithorax (Figure 2). A computed tomography (CT) scan of the chest showed a large multiloculated left-side pleural effusion (Figure 3).
The patient was started on broad-spectrum antibiotics, including intravenous (IV) ceftriaxone, azithromycin, and clindamycin. He underwent thoracentesis on admission, yielding only 300 mL of purulent fluid, confirming its loculated status.
Serum total protein was 7.1 g/dL, and no serum lactic acid dehydrogenase (LDH) was obtained at the time of thoracentesis. Pleural fluid analysis revealed a protein of 5.2 g/dL, and an LDH of 5,176 units/L, meeting Light’s criteria for exudate based on a pleural fluid protein to serum protein ratio of > 0.5 (0.69) and pleural fluid LDH level > two-thirds of the upper limit of normal for serum LDH.
General surgery placed 2 thoracostomy tubes in the left hemithorax without significant drainage. On the first hospital day the patient seemed toxic and underwent a minithoracotomy with decortication. The Gram stain of the blood and blood cultures were negative over 72 hours. Another Gram stain of pleural fluid showed Gram-positive cocci in pairs. Pleural fluid cultures obtained during the procedure revealed S intermedius consistent with the patient’s history of aspiration and abscess formation. Antibiotic susceptibility tests were not performed on the sample.
A peripherally inserted central catheter line was placed for daily IV ceftriaxone infusions to be continued with oral clindamycin for the subsequent 4 weeks. A CT scan of the chest at 8 weeks posthospitalization revealed minimal postoperative scarring, and pulmonary function tests showed normal flow volume loop and maximum voluntary ventilation (Figure 4). The patient reported full recovery and was returned to full activity, including further Special Operations training.
Discussion
Pulmonary infections associated with near-drowning events are caused by a host of organisms that must be considered in the differential diagnosis. The most common include Aeromonas species, Burkholderia pseudomallei, Pseudallescheria boydii, Streptococcus pneumoniae, and Pseudomonas aeruginosa.2 However, the causative organism in this case was endogenous. Streptococcus intermedius is an anaerobic, Gram-positive cocci, a member of the Streptococcus milleri group, and is considered normal flora of the oral mucosa, upper respiratory tract, vagina, and gastrointestinal tract.3,4 This organism is innocuous in its normal habitat but may result in considerable mortality and morbidity if spread to alternative sites due to its ability to form abscesses and cause systemic infections.5
Although uncommon, respiratory infections caused by S intermedius typically result from aspiration of gastric or oral contents and may lead to pulmonary abscesses or empyema.1,6,7 It may present as a primary empyema.8 Current literature suggests a mortality rate between 2% and 14% with higher rates in older populations.9 A retrospective study looking at 72 cases of Streptococci viridans pulmonary infection from 1984 to 1996 found only 2 documented cases where S intermedius was identified as the cause of concomitant empyema and lung abscesses. This study also indicated a strong male predominance with only 7% of lung abscesses occurring in females.10,11
This patient developed a pulmonary abscess and empyema as a probable consequence of aspiration during underwater training exercises. The diagnosis was complicated, because the patient did not initially disclose the pertinent history, and he ignored his symptoms so that he could continue training. His actions delayed aggressive antibiotic therapy and likely led to the rapid progression of pneumonia and his complicated clinical course, because S intermedius has shown intermediate susceptibility or resistance to fluoroquinolone monotherapy.12
This case was also unusual given the subacute presentation and 3-month history of hemoptysis. On review of the available medical literature, hemoptysis is an unusual symptom of pulmonary infections caused by S intermedius but can likely be attributed to necrosis of the pulmonary tissue.8,13
Most patients with S intermedius pulmonary infection rapidly progress due to the virulence of this organism and predisposing comorbidities.14,15 However, this patient had a relatively indolent progression for 3 months, which speaks to the increased respiratory reserve of a healthy, young male in excellent cardiovascular condition.
Conclusion
This case highlights the potential for normal oral flora to cause advanced pulmonary disease in patients with no significant comorbidities. Streptococcus intermedius infections can be subacute in presentation but may rapidly progress to severe disease once seeded in the pleural cavity. Whereas early pleural space drainage remains fundamental, urgent surgical intervention may be required for loculated disease. Although infections with this organism may lead to irreversible pulmonary complications, complete resolution with full recovery is possible in young, healthy patients.
Primary care physicians must take a careful history to ensure optimal patient outcomes. This concept is particularly important to consider in aviators and Special Operations personnel who may be reluctant to seek medical care. Establishing a sense of trust among active-duty military is essential for mission accomplishment.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Empyema is a well-known sequela resulting from the extension of bacterial pneumonia or pulmonary abscess to the pleural space. This case highlights the organism Streptococcus intermedius (S intermedius), an uncommon cause of pulmonary empyema.1Streptococcus intermedius is endogenous among oral flora and is notorious for its abscess-forming capabilities when spread to alternative sites.
The patient was a healthy, active-duty male who presented in sepsis after months of worsening dyspnea and subacute hemoptysis following 2 near-drowning episodes during Special Operations training. Eight weeks after urgent surgical decortication and intensive antibiotic therapy, the patient experienced a complete resolution of his symptoms. A brief discussion follows concerning the pathogenesis and relevant literature regarding S intermedius infections.
Case History
A 21-year-old Air Force Tactical Air Control Party (TACP) trainee with no significant past medical history presented with worsening dyspnea, pleuritic chest pain, and hemoptysis after failed outpatient therapy with levofloxacin for presumed community-acquired pneumonia (CAP) 3 days prior. The chest X-ray at that time demonstrated a left lower lobe consolidation with no evidence of pleural effusion or pulmonary abscess on the lateral view (Figure 1).
The patient stated that his symptoms started about 3 months prior with fever, chills, and night sweats. His symptoms occurred episodically every few weeks, but he had no knowledge of any significant events preceding the illness. The patient developed intermittent hemoptysis 2 weeks later. This included blood-streaked mucus with productive cough and bright-red blood, ranging between a teaspoon and a tablespoon, according to the patient. The patient gradually developed increased dyspnea, which began to impact his performance during Special Operations physical training. His symptoms gradually progressed to worsening dyspnea, which began to affect daily living activities, and new-onset left-sided rib pain. The patient reported no relevant travel history and tested negative for purified protein derivatives 3 months before the initial presentation.
On further questioning, the patient disclosed 2 near-drowning incidents within the preceding year. The first occurred 10 months before presentation, when the patient was performing a 1-minute underwater swim in preparation for the TACP training. The patient stated that he came to the surface to take a breath and lost consciousness. He was immediately brought to the edge of the pool and quickly recovered with no apparent residual symptoms. The second episode occurred during an underwater buddy-breathing training exercise 4 months before presentation and just 3 weeks before symptom onset. The patient reported that he knew he was not getting enough air but remained underwater, concerned that he might fail the exercise. He had a transient syncopal episode shortly after aspirating and was brought to the surface. Afterward, the patient refused to receive medical attention following this event, fearing risk of medical disqualification from training. He reportedly did not experience symptoms after this second episode.
The patient’s past medical history included seasonal allergic rhinitis, and his past surgical history was unremarkable. The patient was not taking medication other than levofloxacin, prescribed for the suspected CAP. The patient was allergic to penicillin, did not use tobacco, and reported drinking about 5 alcoholic beverages per week. His family history included a sister with asthma and a mother with factor V Leiden deficiency and pulmonary embolism related to hormone replacement therapy.
The patient’s vital signs revealed a temperature of 103.2°F, 114 beats per minute pulse, 24 breaths per minute respiratory rate, and oxygen saturation of 89% on room air. On physical examination, the patient was noted to be in moderate respiratory distress with accessory muscle use and was diaphoretic. Breath sounds were diminished in the left upper and lower lung fields with significant egophony.
Tests revealed a white blood cell count of 23,300/mm3 with 30% bandemia. A chest radiograph showed an infiltrate/effusion of the entire left hemithorax (Figure 2). A computed tomography (CT) scan of the chest showed a large multiloculated left-side pleural effusion (Figure 3).
The patient was started on broad-spectrum antibiotics, including intravenous (IV) ceftriaxone, azithromycin, and clindamycin. He underwent thoracentesis on admission, yielding only 300 mL of purulent fluid, confirming its loculated status.
Serum total protein was 7.1 g/dL, and no serum lactic acid dehydrogenase (LDH) was obtained at the time of thoracentesis. Pleural fluid analysis revealed a protein of 5.2 g/dL, and an LDH of 5,176 units/L, meeting Light’s criteria for exudate based on a pleural fluid protein to serum protein ratio of > 0.5 (0.69) and pleural fluid LDH level > two-thirds of the upper limit of normal for serum LDH.
General surgery placed 2 thoracostomy tubes in the left hemithorax without significant drainage. On the first hospital day the patient seemed toxic and underwent a minithoracotomy with decortication. The Gram stain of the blood and blood cultures were negative over 72 hours. Another Gram stain of pleural fluid showed Gram-positive cocci in pairs. Pleural fluid cultures obtained during the procedure revealed S intermedius consistent with the patient’s history of aspiration and abscess formation. Antibiotic susceptibility tests were not performed on the sample.
A peripherally inserted central catheter line was placed for daily IV ceftriaxone infusions to be continued with oral clindamycin for the subsequent 4 weeks. A CT scan of the chest at 8 weeks posthospitalization revealed minimal postoperative scarring, and pulmonary function tests showed normal flow volume loop and maximum voluntary ventilation (Figure 4). The patient reported full recovery and was returned to full activity, including further Special Operations training.
Discussion
Pulmonary infections associated with near-drowning events are caused by a host of organisms that must be considered in the differential diagnosis. The most common include Aeromonas species, Burkholderia pseudomallei, Pseudallescheria boydii, Streptococcus pneumoniae, and Pseudomonas aeruginosa.2 However, the causative organism in this case was endogenous. Streptococcus intermedius is an anaerobic, Gram-positive cocci, a member of the Streptococcus milleri group, and is considered normal flora of the oral mucosa, upper respiratory tract, vagina, and gastrointestinal tract.3,4 This organism is innocuous in its normal habitat but may result in considerable mortality and morbidity if spread to alternative sites due to its ability to form abscesses and cause systemic infections.5
Although uncommon, respiratory infections caused by S intermedius typically result from aspiration of gastric or oral contents and may lead to pulmonary abscesses or empyema.1,6,7 It may present as a primary empyema.8 Current literature suggests a mortality rate between 2% and 14% with higher rates in older populations.9 A retrospective study looking at 72 cases of Streptococci viridans pulmonary infection from 1984 to 1996 found only 2 documented cases where S intermedius was identified as the cause of concomitant empyema and lung abscesses. This study also indicated a strong male predominance with only 7% of lung abscesses occurring in females.10,11
This patient developed a pulmonary abscess and empyema as a probable consequence of aspiration during underwater training exercises. The diagnosis was complicated, because the patient did not initially disclose the pertinent history, and he ignored his symptoms so that he could continue training. His actions delayed aggressive antibiotic therapy and likely led to the rapid progression of pneumonia and his complicated clinical course, because S intermedius has shown intermediate susceptibility or resistance to fluoroquinolone monotherapy.12
This case was also unusual given the subacute presentation and 3-month history of hemoptysis. On review of the available medical literature, hemoptysis is an unusual symptom of pulmonary infections caused by S intermedius but can likely be attributed to necrosis of the pulmonary tissue.8,13
Most patients with S intermedius pulmonary infection rapidly progress due to the virulence of this organism and predisposing comorbidities.14,15 However, this patient had a relatively indolent progression for 3 months, which speaks to the increased respiratory reserve of a healthy, young male in excellent cardiovascular condition.
Conclusion
This case highlights the potential for normal oral flora to cause advanced pulmonary disease in patients with no significant comorbidities. Streptococcus intermedius infections can be subacute in presentation but may rapidly progress to severe disease once seeded in the pleural cavity. Whereas early pleural space drainage remains fundamental, urgent surgical intervention may be required for loculated disease. Although infections with this organism may lead to irreversible pulmonary complications, complete resolution with full recovery is possible in young, healthy patients.
Primary care physicians must take a careful history to ensure optimal patient outcomes. This concept is particularly important to consider in aviators and Special Operations personnel who may be reluctant to seek medical care. Establishing a sense of trust among active-duty military is essential for mission accomplishment.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Weightman NC, Barnham MRD, Dove M. Streptococcus milleri group bacteraemia in North Yorkshire, England (1989-2000). Indian J Med Res. 2004;119(suppl):164-167.
2. Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;1(4):896-907.
3. Porta G, Rodriguez-Carballeira M, Gómez L, et al. Thoracic infection caused by Streptococcus milleri. Eur Respir J. 1998;12(2):357-362.
4. Van der Auwera P. Clinical significance of Streptococcus milleri. Eur J Clin Microbiol.1985;4(4):386-390.
5. Murray HW, Gross KC, Masur H, Roberts RB. Serious infections caused by Streptococcus milleri. Am J Med. 1978;64(5):759-764.
6. Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis. 1995;21(suppl 3):S238-S243.
7. Frankish PD, Kolbe J. Thoracic empyema due to Streptococcus milleri: Four cases. N Z Med J. 1984;97(769):849-851.
8. Iskandar SB, Al Hasan MA, Roy TM, Byrd RP Jr. Streptococcus intermedius: An unusual cause of a primary empyema. Tenn Med. 2006;99(2):37-39.
9. de Hoyos A, Sundaresan S. Thoracic empyema. Surg Clin North Am. 2002;82(3):643-671.
10. Jerng JS, Hsueh PR, Teng LJ, Lee LN, Yang PC, Luh KT. Empyema thoracis and lung abscess caused by viridans streptococci. Am J Respir Crit Care Med. 1997;156(5):1508-1514.
11. Wargo KA, McConnell VJ, Higginbotham SA. A case of Streptococcus intermedius empyema. Ann Pharmacother. 2006;40(6):1208-1210.
12. Limia A, Jiménez ML, Alarcón T, López-Brea M. Five-year analysis of antimicrobial susceptibility of the Streptococcus milleri group. Eur J Clin Microbiol Infect Dis. 1999;18(6):440-444.
13. Wong CA, Donal F, Macfarlane JT. Streptococcus milleri pulmonary disease: A review and clinical description of 25 patients. Thorax. 1995;50(10):1093-1096.
14. Shlaes DM, Lerner PI, Wolinsky E, Gopalakrishna KV. Infections due to Lancefield F and related Streptococci (S milleri, S anginosus). Medicine (Baltimore). 1981;60(3):197-207.
15. Roy WJ Jr, Roy TM, Davis GJ. Thoracic empyema due to Streptococcus intermedius. J Ky Med Assoc. 1991;89(11):558-562.
1. Weightman NC, Barnham MRD, Dove M. Streptococcus milleri group bacteraemia in North Yorkshire, England (1989-2000). Indian J Med Res. 2004;119(suppl):164-167.
2. Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;1(4):896-907.
3. Porta G, Rodriguez-Carballeira M, Gómez L, et al. Thoracic infection caused by Streptococcus milleri. Eur Respir J. 1998;12(2):357-362.
4. Van der Auwera P. Clinical significance of Streptococcus milleri. Eur J Clin Microbiol.1985;4(4):386-390.
5. Murray HW, Gross KC, Masur H, Roberts RB. Serious infections caused by Streptococcus milleri. Am J Med. 1978;64(5):759-764.
6. Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis. 1995;21(suppl 3):S238-S243.
7. Frankish PD, Kolbe J. Thoracic empyema due to Streptococcus milleri: Four cases. N Z Med J. 1984;97(769):849-851.
8. Iskandar SB, Al Hasan MA, Roy TM, Byrd RP Jr. Streptococcus intermedius: An unusual cause of a primary empyema. Tenn Med. 2006;99(2):37-39.
9. de Hoyos A, Sundaresan S. Thoracic empyema. Surg Clin North Am. 2002;82(3):643-671.
10. Jerng JS, Hsueh PR, Teng LJ, Lee LN, Yang PC, Luh KT. Empyema thoracis and lung abscess caused by viridans streptococci. Am J Respir Crit Care Med. 1997;156(5):1508-1514.
11. Wargo KA, McConnell VJ, Higginbotham SA. A case of Streptococcus intermedius empyema. Ann Pharmacother. 2006;40(6):1208-1210.
12. Limia A, Jiménez ML, Alarcón T, López-Brea M. Five-year analysis of antimicrobial susceptibility of the Streptococcus milleri group. Eur J Clin Microbiol Infect Dis. 1999;18(6):440-444.
13. Wong CA, Donal F, Macfarlane JT. Streptococcus milleri pulmonary disease: A review and clinical description of 25 patients. Thorax. 1995;50(10):1093-1096.
14. Shlaes DM, Lerner PI, Wolinsky E, Gopalakrishna KV. Infections due to Lancefield F and related Streptococci (S milleri, S anginosus). Medicine (Baltimore). 1981;60(3):197-207.
15. Roy WJ Jr, Roy TM, Davis GJ. Thoracic empyema due to Streptococcus intermedius. J Ky Med Assoc. 1991;89(11):558-562.
Pharmacist Management of Adult Asthma at an Indian Health Service Facility
According to the Centers for Disease Control and Prevention, asthma prevalence in the U.S. increased between 2001 and 2010 and is now at its highest level. In 2010, about 25.7 million people had asthma: 18.7 million adults (8%) and 7 million children (9%). Despite well-known treatment options, asthma continues to be poorly controlled. In 2009 there were 1.6 million emergency department (ED) visits, 497,300 hospitalizations, and 3,404 deaths related to asthma. Additionally, in 2008 the disease affected attendance at school and work with 10.5 million and 14.2 million missed school days and workdays, respectively.1-5
The American Indian and Alaska Native (AIAN) populations have not escaped the realities of asthma. According to a 2010 report of the National Center for Health Statistics, AIAN populations also have a high prevalence of asthma at 14.2%. This percentage is much higher than that of the general population.1
Over the past decade, pharmacists have expanded their roles from educators to clinicians with prescriptive authority in various settings. The greatest success has been seen with anticoagulation clinics, both clinically and financially.6-10 Pharmacists have also demonstrated positive outcomes when involved in cardiovascular clinics.11-13
Additionally, pharmacists have been involved with asthma clinics as both educators and prescribers with favorable results clinically and economically.14-16 A study from Taiwan done by Chan and Wang indicated that pharmacist asthma interventions in an outpatient setting improved the quality of care, reduced cost, and relieved stress on general medical resources.17 Another study indicated that education by a community pharmacist can improve asthma control in a self-managed program.18
In 2007, an asthma medication use evaluation (MUE) was completed at the Northern Navajo Medical Center (NNMC) in Shiprock, New Mexico. The results of the MUE concluded that asthma statistics for the local population differed from that of the national data (Table 1). Overall, the Navajo population served by the NNMC had a lower incidence of asthma but a higher rate of hospital admissions and ED visits.
One of the primary focus points for the MUE was short-acting beta agonist (SABA) refills. According to national guidelines, using a SABA 2 or more times per week (not for exercise-induced bronchospasms) would indicate a patient was not well controlled.19 This use equates to 2 refills of SABA per year. The MUE found that 51% of patients had ≥ 3 refills per year, and 38% of patients had 4 or more refills per year. Based on asthma prevalence and SABA history, it was determined that a specialty clinic could have a positive impact on asthma care.
This study addresses how a specialized adult asthma clinic managed by pharmacists with physician oversight can improve asthma outcomes. Since January 2010, the NNMC has had a program in place and has experienced a concurrent substantial drop in asthma-related ED visits and admissions, an improved level of control, and a decreased cost burden to the facility.
Methods
A retrospective chart review was completed on all patients currently enrolled in the clinic. The Resource and Patient Management System Visit General Retrieval (RPMS VGEN), Electronic Health Record, and the asthma clinic database were used to evaluate patients. The evaluation period began January 1, 2010, and ended December 31, 2011.
Performance improvement inclusion criteria for clinic patients were based on active status in the clinic. Active patients were defined as patients with at least 2 clinic visits and a clinic visit within 3 months of an ED visit or admission. The 3-month cutoff was chosen based on several criteria. First, most patients referred to the clinic were categorized as either not well controlled (NWC) or very poorly controlled (VPC) and required at least a 2- to 4-week follow-up based on guidelines. Second, patients who were categorized as well controlled (WC) were scheduled for clinic visits every 3 months for regular follow-up.
Using all ICD-9 codes for asthma, RPMS VGEN was used to find the number of ED visits and admissions that occurred with asthma as the primary diagnosis from both clinic and nonclinic patients. The inclusion criteria were then applied to the clinic patients, and those not meeting these criteria were returned to the nonclinic pool of patients.
Cost analysis was evaluated based on the results of a random selection of 20 patients from 2009 and 2010 ED and hospital visits at the NNMC. These numbers were averaged to determine ED and admission costs. Length of admission stay was determined from a RPMS VGEN search for each clinic and nonclinic patient admission.
Determination of the level of control was based on the 2007 national asthma guidelines. The guidelines state that the level of control can be determined by either asthma symptoms or by peak flow evaluation.19 Because of the language barrier that sometimes arises with the treated population, the use of symptom-based evaluation has been observationally superior to providing peak flow meters for home use. At each visit, patients were interviewed using tables from the asthma guidelines. Table 2 is an abbreviated portion of the guidelines representing the assessment tool used by the clinic. The level of control was determined by selecting the column with the highest severity of impairment.19
All patients seen at the clinic were tracked in a database, and their current level of control was documented at each visit. To determine the level of control, the database was reviewed, and those patients with > 1 visit were included in the analysis. The levels of control from the first visit to the most recent visit were compared.
Patient surveys were completed at each visit. These surveys included questions to assist the pharmacy provider in classifying the level of control, patient satisfaction with asthma care, and patient perception of asthma control. Approval from the Navajo Area Institutional Review Board was obtained for data publication. Odds ratios were used to determine the impact of the clinic, using a 95% confidence interval.
Results
For the review period, 2,997 patients were coded as having some form of asthma, resulting in 12,739 asthma visits within the medical center.
ED Visits and Hospital Admissions
Of these 2,997 patients, 301 visited the ED between 2010 and 2011 with 22 being active asthma clinic patients. These 22 active clinic patients accounted for 31 ED visits. The remaining 279 patients had 428 visits with a total of 459 ED visits from clinic and nonclinic patients. Sixty patients were hospitalized for asthma with 7 of them active asthma clinic patients. The 7 clinic patients admitted accounted for 8 admissions. There is a statistical significance in total ED visits and admissions as well as for individual patients (Table 3).
To determine the clinic impact, a 2-year analysis of patient pre- and postclinic enrollment was done. Search criteria for RPMS VGEN were identical to the study period search except for dates. Those patients enrolled in the clinic during the study period (2010-2011) were evaluated for the 2 years before the clinic startup (2008-2009). The results indicated a decrease in both ED visits and hospital admissions related to asthma for clinic patients (Table 4).
Cost Data and Length of Stay
Emergency department and hospital admissions costs were determined from an earlier performance improvement review of the clinic. The median cost of an ED visit was $373 with a range from $228 to $910. The cost range represented the severity of the asthma exacerbation being treated. This cost range was similar to published data that reported a cost range from $234 to $400 with an average of $339 per visit.20,21 Hospital costs (including ED visit) per day ranged from $528 to $2,470 with a median of $1,199 per day. Table 5 shows the calculated actual annual cost savings for patients pre- and postenrollment. The cost difference between clinic and nonclinic patients from 2010 to 2011 was calculated to be $111,000 annually (data not shown). The median length of the hospital stay for clinic and nonclinic patients was 2 days (range, 1-10 and 1-7 days, respectively). The national average was 4.3 days.
From 2008 to 2009, there were 123 ED visits and 20 hospital admissions related to asthma of patients who would later be enrolled in the asthma clinic study. From 2010 to 2011 there were 31 ED visits and 8 hospital asthma admissions
(Table 4). These data were used to determine the potential cost savings for the clinic (Table 5). Based on current reductions, the potential annual cost savings was $85,405 if all adult asthma patients were seen in the pharmacy managed clinic (Table 6).
Level of Control
A total of 66 patients had 3 or more visits to the asthma clinic. Of these patients, 30% had no change in control, 60% showed some measure of improvement, and 11% had a decrease in control based on the national guidelines (Table 7).
Patient Perception
At each visit, the patient’s current perception of asthma control, satisfaction with control, and clinic grade related to asthma care was determined. Of the 66 patients with 3 or more visits, a large portion of the questionnaires were missing when the data were collected. Table 8 shows the results of the data for patients with 3 more visits and 2 completed forms from different visits. These data points may not have been from the first or most recent visits.
From earliest to most recent visit, patient perception of asthma control compared with clinical guidelines improved moderately. Sixteen patients had a clinical improvement from VPC to NWC with 13 (81%) believing their symptoms were now WC.
Discussion
The results of this performance improvement evaluation are encouraging. However, not all positive data may be directly attributed to the asthma clinic. The statistical analysis for this study does not seek to remove confounding variables. Without removing potential confounding variables, questions remain about the accuracy of the outcome. However, combining the statistical data in Table 3 and the 2-year comparative data in Table 4, strong evidence exists that a positive impact from the clinic had occurred even in the presence of potential confounding variables.
The financial impact of asthma was evident with the 2010 to 2011 cost for ED visits and hospital admissions at $265,928. Asthma clinic patients made up only 8% ($21,155) of this cost and yielded an annual cost savings of $24,352. Obviously, the 8% was a direct result of the number of nonclinic vs clinic patients. However, the cost savings of $24,352 was independent of patient numbers and was calculated directly from patients pre- and postenrollment. The potential savings if all current nonclinic patients were enrolled in the clinic was $85,405 annually.
The savings was only direct cost and did not include indirect costs related to asthma, such as lost work/school days, impact on employer productivity, and so forth. This was calculated after applying the 75% and 60% reduction in ED and hospital costs. As more patients are enrolled in the clinic, the potential cost could become an actual cost savings, based on the assumption that the 75% and 60% reduction stays constant (Figure). Over 1 year, the actual savings of the clinic makes up for 31% of the current ED visits from nonclinic patients. With the addition of the potential savings, the clinic could almost negate the money that is currently spent on asthma care.
Clinic data indicated a positive impact on level of control. Of those patients with 3 or more visits to the asthma clinic, 59% had some form of improvement. Fifteen of those patients (23%) had the biggest improvement: from VPC to WC. While any form of improvement is beneficial, a jump of this magnitude in so many patients is extremely encouraging. Thirty percent of the patients had no change in level of control. Of these, 14% were WC, so it would be hoped that no change would occur. Eleven patients remained at suboptimal control. The most concerning control data were those patients who lost control of their asthma during the 2-year period.
Chronic nonadherence from a select number of clinic patients seemed to be a major problem. Thirty-one ED visits were from 22 patients, and the 8 admissions were from 7 patients. Of the 22 ED patients, 82% had poor adherence to asthma medications. The hospital data were similar with 6 out of the total 7 (86%) clinic patients reporting poor medication adherence. Additionally, the majority of patients with a decrease in level of control since enrolling in the clinic had a history of poor medication adherence (4 of 7 patients).
In rating the level of asthma care, patients indicated they received the same level of care after enrollment as they did before enrollment. Most of the care given before the clinic was by primary care providers (PCPs), the ED, and urgent care providers. Since the patients rated the level of care equal, it would suggest that pharmacists were providing the same level of care as were these providers, at least from a patient standpoint. Overall, patients were satisfied with their level of control. Most patients were satisfied at enrollment and did not have a change of opinion throughout the study period, and a large number showed increased satisfaction. The patients who showed a decrease in satisfaction of asthma control were those patients who also had no improvement in actual control. Most of these patients stayed at the VPC level. About half these same patients had a history of noncompliance.
There is significant concern regarding those patients who continue to believe they are better controlled than what the guidelines indicate. Sixteen patients moved from VPC to NWC per the guidelines. Thirteen of these patients now believe that their asthma is WC. This belief places the patients at risk for a severe asthma exacerbation. Patients who believe they are WC may be less likely to self-medicate with albuterol or seek medical help during the initial stages of an exacerbation. These patients will need further education to bring their personal perceptions and actual asthma control together.
Conclusions
Based on clinical results it seems that the NNMC Adult Asthma Clinic has made a positive impact on asthma care. Additionally, significant reduction in the financial burden to the facility is achievable. The results, both clinically and statistically significant, indicate the impact a specialty clinic can provide. Specialty clinics, pharmacy or otherwise, have a history of providing positive outcomes.
As previously noted, no confounding variables were included in the data analysis, which could bias the results, even though data for the same time frame from separate years will reduce some errors. However, there will always be a difference in pollen counts, outbreaks (ie, influenza), temperature changes, and so forth. Such variables should be reduced but not removed completely, based on this performance improvement design. If any of these variables were significantly different, it could alter the results, so a potential weakness is present in this study.
Probably the most important mechanism for the success of the clinic is education. Each visit is set at 30-minute appointments (1 hour for new patients), allowing for a significant amount of time that can be spent on education topics, including pathophysiology, trigger avoidances, and medication use. Patients are asked to bring their medications to the clinic and demonstrate inhaler technique at every visit. Patients who do not bring their inhalers to the clinic will have them filled at the clinic and given to them for demonstration. This type of show-and-tell education allows clinic providers to correct improper inhaler technique immediately. Having patients actually use their medication seems to influence the patient’s inhaler mechanics to a greater extent than does demonstration with a placebo.
In the eyes of the clinic provider, it is important for patients to understand the basic pathophysiology of the disease. The better understanding patients have of a disease, the better they can take part in the treatment. Since the clinic actively engages patients in education topics, it brings patients into an active role in the treatment. As mentioned, the inhaler technique seems to be the most effective first step. However, as patients gain trust in clinic providers due to significant improvement in symptoms secondary to inhaler technique, this trust leads to a dialogue about pathophysiology and triggers.
Another key component in the clinic success is the nature of the clinic itself. Providers in the clinic focus on only 1 disease and the guidelines to treat that disease. Therefore, providers in the clinic are trained to be extremely familiar with the treatment of asthma. This is not to imply that a patient’s PCP or usual care provider is unfamiliar with the guidelines. It simply means that specialty care involves an extra time commitment to a specific disease. Each clinic provider must attain a high level of asthma knowledge before consideration as a full-time provider. Pharmacists are encouraged to sit for the Certified Asthma Educators examination, Board Certified Pharmacotherapy Specialist examination, and/or obtain the Indian Health Service National Clinical Pharmacy Specialist certification.
Although the clinic has moved in the right direction, there are still several patients who have not had any improvement since being referred to the clinic. These patients have refractory asthma (ie, step 6) and are not able to be treated at this facility, continued poor medication adherence, or do not have asthma at all. These patients will be flagged and will be evaluated on a case-by-case basis.
In conclusion, the clinic has begun to achieve what it was intended to do: improve asthma control, reduce patient burden on ED staff, and decrease financial burden to the facility. Additionally, there is improvement in the satisfaction of the asthma care and a trend toward the patients’ perception of asthma control agreeing with medical guidelines. These findings further support the use of pharmacists in the role as provider for the management of chronic diseases.
Acknowledgements
Shail Mehta, MD, is an internal medicine provider at the NNMC. He attended the University of Pittsburgh School of Medicine in Pennsylvania and completed a residency in internal medicine at the University of Michigan in Ann Arbor. Dr. Mehta is certified with the American Board of Internal Medicine.
Erica Markovitz, MD, is an internal medicine provider at the NNMC. She attended the University of Miami School of Medicine in Florida and completed a residency at the University of Michigan. Dr. Markovitz is certified with the American Board of Internal Medicine and the American Board of Pediatrics.
Thad Koppenhafer, PharmD, is the director of pharmacy at the NNMC and the area pharmacy consultant for the Navajo Area of the IHS. He is a member of the American Society of Health-Systems Pharmacists.
CAPT Mark Strong, PharmD, MT (ASCP) is a senior supervisory pharmacist with the U.S. Public Health Commissioned Corps and is assigned to the IHS. He is currently the chief of outpatient pharmacy services at the NNMC.
CDR Clint Krestel, PharmD, is the assistant chief of pharmacy responsible for Inpatient Pharmacy Services. His professional memberships currently include the American College of Clinical Pharmacy, the American Society of Health-System Pharmacists, and the Commissioned Officers Association.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/series/sr_03/sr03_035.pdf. Accessed March 10, 2014. Vital Health Stat. 2012;3(35):1-67.
2. National Asthma Control Program. Asthma’s impact on the nation. Data from the CDC National Asthma Control Program. Centers for Disease Control and Prevention Website. http://www.cdc.gov/asthma/impacts_nation/asthmafactsheet.pdf. Accessed March 10, 2014.
3. Schappert AM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed March 10, 2014. Vital Health Stat. 2011;13(169):1-38.
4. Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: Final data for 2009. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_03.pdf. Accessed March 10, 2014. Natl Vital Statistics Rep. 2011;60(3):1-117.
5. Barnes PM, Adams PF, Powell-Griner E. Health characteristics of the American Indian or Alaska Native adult population: United States, 2004-2008. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/nhsr/nhsr020.pdf. Accessed March 10, 2014. Natl Health Stat Report. 2010;20:1-23.
6. Garwood CL, Dumo P, Baringhaus SN, Laban KM. Quality of anticoagulation care in patients discharged from a pharmacist-managed anticoagulation clinic after stabilization of warfarin therapy. Pharmacotherapy. 2008;28(1):20-26.
7. Gray DR, Garabedian-Ruffalo SM, Chretien SD. Cost-justification of a clinical pharmacist-managed anticoagulation clinic. Ann Pharmacother. 2007;41(3):496-501.
8. Ernst ME, Brandt KB. Evaluation of 4 years of clinical pharmacist anticoagulation case management in a rural, private physician office. J Am Pharm Assoc. 2003;43(5):630-636.
9. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
10. Dager WE, Branch JM, King JH, et al. Optimization of inpatient warfarin therapy: Impact of daily consultation by a pharmacist-managed anticoagulation service. Ann Pharmacother. 2000;34(5):567-572.
11. Morello CM, Zadvorny EB, Cording MA, Suemoto RT, Skog J, Harari A. Development and clinical outcomes of pharmacist-managed diabetes care clinics. Am J Health Syst Pharm. 2006;63(14):1325-1331.
12. Lowey A, Moore S, Norris C, Wright D, Silcock J, Hammond P. The cost-effectiveness of pharmacist-led treatment of cardiac risk in patients with type 2 diabetes. Pharm World Sci. 2007;29(5):541-545.
13. Erhun WO, Agbani EO, Bolaji EE. Positive benefits of a pharmacist-managed hypertension clinic in Nigeria. Public Health. 2005;119(9):792-798.
14. Hatoum HT, Witte KW, Hutchinson RA. Patient care contributions of clinical pharmacists in four ambulatory care clinics. Hosp Pharm. 1992;27(3):203-206, 208-209.
15. Pauley TR, Magee MJ, Cury JD. Pharmacist-managed, physician-directed asthma management program reduces emergency department visits. Ann Pharmacother. 1995;29(1):5-9.
16. Nack JA. Homecare management of the asthma patient. U.S. Pharmacist. 1998;23(7). http://legacy.uspharmacist.com/oldformat.asp?url=newlook/file/Home/ACF2FEE.cfm&pub_id=8&article_id=127. Accessed March 10, 2014.
17. Chan AL, Wang HY. Pharmacoeconomic assessment of clinical pharmacist interventions for patients with moderate to severe asthma in outpatient clinics: Experience in Taiwan. Clin Drug Investig. 2004;24(10):603-609.
18. Barbanel D, Eldridge S, Griffiths C. Can a self-management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax. 2003;58(10):851-854.
19. U.S. Department of Health and Human Services, National Institutes of Health, National Heart Lung and Blood Institute. National Asthma Education and Prevention Program, Expert Panel 3. Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. Bethesda, MD: National Institutes of Health; 2007. NIH publication Number 0805846.
20. Stanford R, McLaughlin T, Okamoto LJ. The cost of asthma in the emergency department and hospital. Am J Respir Crit Care Med. 1999;160(1):211-215.
21. Williams RM. The cost of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
According to the Centers for Disease Control and Prevention, asthma prevalence in the U.S. increased between 2001 and 2010 and is now at its highest level. In 2010, about 25.7 million people had asthma: 18.7 million adults (8%) and 7 million children (9%). Despite well-known treatment options, asthma continues to be poorly controlled. In 2009 there were 1.6 million emergency department (ED) visits, 497,300 hospitalizations, and 3,404 deaths related to asthma. Additionally, in 2008 the disease affected attendance at school and work with 10.5 million and 14.2 million missed school days and workdays, respectively.1-5
The American Indian and Alaska Native (AIAN) populations have not escaped the realities of asthma. According to a 2010 report of the National Center for Health Statistics, AIAN populations also have a high prevalence of asthma at 14.2%. This percentage is much higher than that of the general population.1
Over the past decade, pharmacists have expanded their roles from educators to clinicians with prescriptive authority in various settings. The greatest success has been seen with anticoagulation clinics, both clinically and financially.6-10 Pharmacists have also demonstrated positive outcomes when involved in cardiovascular clinics.11-13
Additionally, pharmacists have been involved with asthma clinics as both educators and prescribers with favorable results clinically and economically.14-16 A study from Taiwan done by Chan and Wang indicated that pharmacist asthma interventions in an outpatient setting improved the quality of care, reduced cost, and relieved stress on general medical resources.17 Another study indicated that education by a community pharmacist can improve asthma control in a self-managed program.18
In 2007, an asthma medication use evaluation (MUE) was completed at the Northern Navajo Medical Center (NNMC) in Shiprock, New Mexico. The results of the MUE concluded that asthma statistics for the local population differed from that of the national data (Table 1). Overall, the Navajo population served by the NNMC had a lower incidence of asthma but a higher rate of hospital admissions and ED visits.
One of the primary focus points for the MUE was short-acting beta agonist (SABA) refills. According to national guidelines, using a SABA 2 or more times per week (not for exercise-induced bronchospasms) would indicate a patient was not well controlled.19 This use equates to 2 refills of SABA per year. The MUE found that 51% of patients had ≥ 3 refills per year, and 38% of patients had 4 or more refills per year. Based on asthma prevalence and SABA history, it was determined that a specialty clinic could have a positive impact on asthma care.
This study addresses how a specialized adult asthma clinic managed by pharmacists with physician oversight can improve asthma outcomes. Since January 2010, the NNMC has had a program in place and has experienced a concurrent substantial drop in asthma-related ED visits and admissions, an improved level of control, and a decreased cost burden to the facility.
Methods
A retrospective chart review was completed on all patients currently enrolled in the clinic. The Resource and Patient Management System Visit General Retrieval (RPMS VGEN), Electronic Health Record, and the asthma clinic database were used to evaluate patients. The evaluation period began January 1, 2010, and ended December 31, 2011.
Performance improvement inclusion criteria for clinic patients were based on active status in the clinic. Active patients were defined as patients with at least 2 clinic visits and a clinic visit within 3 months of an ED visit or admission. The 3-month cutoff was chosen based on several criteria. First, most patients referred to the clinic were categorized as either not well controlled (NWC) or very poorly controlled (VPC) and required at least a 2- to 4-week follow-up based on guidelines. Second, patients who were categorized as well controlled (WC) were scheduled for clinic visits every 3 months for regular follow-up.
Using all ICD-9 codes for asthma, RPMS VGEN was used to find the number of ED visits and admissions that occurred with asthma as the primary diagnosis from both clinic and nonclinic patients. The inclusion criteria were then applied to the clinic patients, and those not meeting these criteria were returned to the nonclinic pool of patients.
Cost analysis was evaluated based on the results of a random selection of 20 patients from 2009 and 2010 ED and hospital visits at the NNMC. These numbers were averaged to determine ED and admission costs. Length of admission stay was determined from a RPMS VGEN search for each clinic and nonclinic patient admission.
Determination of the level of control was based on the 2007 national asthma guidelines. The guidelines state that the level of control can be determined by either asthma symptoms or by peak flow evaluation.19 Because of the language barrier that sometimes arises with the treated population, the use of symptom-based evaluation has been observationally superior to providing peak flow meters for home use. At each visit, patients were interviewed using tables from the asthma guidelines. Table 2 is an abbreviated portion of the guidelines representing the assessment tool used by the clinic. The level of control was determined by selecting the column with the highest severity of impairment.19
All patients seen at the clinic were tracked in a database, and their current level of control was documented at each visit. To determine the level of control, the database was reviewed, and those patients with > 1 visit were included in the analysis. The levels of control from the first visit to the most recent visit were compared.
Patient surveys were completed at each visit. These surveys included questions to assist the pharmacy provider in classifying the level of control, patient satisfaction with asthma care, and patient perception of asthma control. Approval from the Navajo Area Institutional Review Board was obtained for data publication. Odds ratios were used to determine the impact of the clinic, using a 95% confidence interval.
Results
For the review period, 2,997 patients were coded as having some form of asthma, resulting in 12,739 asthma visits within the medical center.
ED Visits and Hospital Admissions
Of these 2,997 patients, 301 visited the ED between 2010 and 2011 with 22 being active asthma clinic patients. These 22 active clinic patients accounted for 31 ED visits. The remaining 279 patients had 428 visits with a total of 459 ED visits from clinic and nonclinic patients. Sixty patients were hospitalized for asthma with 7 of them active asthma clinic patients. The 7 clinic patients admitted accounted for 8 admissions. There is a statistical significance in total ED visits and admissions as well as for individual patients (Table 3).
To determine the clinic impact, a 2-year analysis of patient pre- and postclinic enrollment was done. Search criteria for RPMS VGEN were identical to the study period search except for dates. Those patients enrolled in the clinic during the study period (2010-2011) were evaluated for the 2 years before the clinic startup (2008-2009). The results indicated a decrease in both ED visits and hospital admissions related to asthma for clinic patients (Table 4).
Cost Data and Length of Stay
Emergency department and hospital admissions costs were determined from an earlier performance improvement review of the clinic. The median cost of an ED visit was $373 with a range from $228 to $910. The cost range represented the severity of the asthma exacerbation being treated. This cost range was similar to published data that reported a cost range from $234 to $400 with an average of $339 per visit.20,21 Hospital costs (including ED visit) per day ranged from $528 to $2,470 with a median of $1,199 per day. Table 5 shows the calculated actual annual cost savings for patients pre- and postenrollment. The cost difference between clinic and nonclinic patients from 2010 to 2011 was calculated to be $111,000 annually (data not shown). The median length of the hospital stay for clinic and nonclinic patients was 2 days (range, 1-10 and 1-7 days, respectively). The national average was 4.3 days.
From 2008 to 2009, there were 123 ED visits and 20 hospital admissions related to asthma of patients who would later be enrolled in the asthma clinic study. From 2010 to 2011 there were 31 ED visits and 8 hospital asthma admissions
(Table 4). These data were used to determine the potential cost savings for the clinic (Table 5). Based on current reductions, the potential annual cost savings was $85,405 if all adult asthma patients were seen in the pharmacy managed clinic (Table 6).
Level of Control
A total of 66 patients had 3 or more visits to the asthma clinic. Of these patients, 30% had no change in control, 60% showed some measure of improvement, and 11% had a decrease in control based on the national guidelines (Table 7).
Patient Perception
At each visit, the patient’s current perception of asthma control, satisfaction with control, and clinic grade related to asthma care was determined. Of the 66 patients with 3 or more visits, a large portion of the questionnaires were missing when the data were collected. Table 8 shows the results of the data for patients with 3 more visits and 2 completed forms from different visits. These data points may not have been from the first or most recent visits.
From earliest to most recent visit, patient perception of asthma control compared with clinical guidelines improved moderately. Sixteen patients had a clinical improvement from VPC to NWC with 13 (81%) believing their symptoms were now WC.
Discussion
The results of this performance improvement evaluation are encouraging. However, not all positive data may be directly attributed to the asthma clinic. The statistical analysis for this study does not seek to remove confounding variables. Without removing potential confounding variables, questions remain about the accuracy of the outcome. However, combining the statistical data in Table 3 and the 2-year comparative data in Table 4, strong evidence exists that a positive impact from the clinic had occurred even in the presence of potential confounding variables.
The financial impact of asthma was evident with the 2010 to 2011 cost for ED visits and hospital admissions at $265,928. Asthma clinic patients made up only 8% ($21,155) of this cost and yielded an annual cost savings of $24,352. Obviously, the 8% was a direct result of the number of nonclinic vs clinic patients. However, the cost savings of $24,352 was independent of patient numbers and was calculated directly from patients pre- and postenrollment. The potential savings if all current nonclinic patients were enrolled in the clinic was $85,405 annually.
The savings was only direct cost and did not include indirect costs related to asthma, such as lost work/school days, impact on employer productivity, and so forth. This was calculated after applying the 75% and 60% reduction in ED and hospital costs. As more patients are enrolled in the clinic, the potential cost could become an actual cost savings, based on the assumption that the 75% and 60% reduction stays constant (Figure). Over 1 year, the actual savings of the clinic makes up for 31% of the current ED visits from nonclinic patients. With the addition of the potential savings, the clinic could almost negate the money that is currently spent on asthma care.
Clinic data indicated a positive impact on level of control. Of those patients with 3 or more visits to the asthma clinic, 59% had some form of improvement. Fifteen of those patients (23%) had the biggest improvement: from VPC to WC. While any form of improvement is beneficial, a jump of this magnitude in so many patients is extremely encouraging. Thirty percent of the patients had no change in level of control. Of these, 14% were WC, so it would be hoped that no change would occur. Eleven patients remained at suboptimal control. The most concerning control data were those patients who lost control of their asthma during the 2-year period.
Chronic nonadherence from a select number of clinic patients seemed to be a major problem. Thirty-one ED visits were from 22 patients, and the 8 admissions were from 7 patients. Of the 22 ED patients, 82% had poor adherence to asthma medications. The hospital data were similar with 6 out of the total 7 (86%) clinic patients reporting poor medication adherence. Additionally, the majority of patients with a decrease in level of control since enrolling in the clinic had a history of poor medication adherence (4 of 7 patients).
In rating the level of asthma care, patients indicated they received the same level of care after enrollment as they did before enrollment. Most of the care given before the clinic was by primary care providers (PCPs), the ED, and urgent care providers. Since the patients rated the level of care equal, it would suggest that pharmacists were providing the same level of care as were these providers, at least from a patient standpoint. Overall, patients were satisfied with their level of control. Most patients were satisfied at enrollment and did not have a change of opinion throughout the study period, and a large number showed increased satisfaction. The patients who showed a decrease in satisfaction of asthma control were those patients who also had no improvement in actual control. Most of these patients stayed at the VPC level. About half these same patients had a history of noncompliance.
There is significant concern regarding those patients who continue to believe they are better controlled than what the guidelines indicate. Sixteen patients moved from VPC to NWC per the guidelines. Thirteen of these patients now believe that their asthma is WC. This belief places the patients at risk for a severe asthma exacerbation. Patients who believe they are WC may be less likely to self-medicate with albuterol or seek medical help during the initial stages of an exacerbation. These patients will need further education to bring their personal perceptions and actual asthma control together.
Conclusions
Based on clinical results it seems that the NNMC Adult Asthma Clinic has made a positive impact on asthma care. Additionally, significant reduction in the financial burden to the facility is achievable. The results, both clinically and statistically significant, indicate the impact a specialty clinic can provide. Specialty clinics, pharmacy or otherwise, have a history of providing positive outcomes.
As previously noted, no confounding variables were included in the data analysis, which could bias the results, even though data for the same time frame from separate years will reduce some errors. However, there will always be a difference in pollen counts, outbreaks (ie, influenza), temperature changes, and so forth. Such variables should be reduced but not removed completely, based on this performance improvement design. If any of these variables were significantly different, it could alter the results, so a potential weakness is present in this study.
Probably the most important mechanism for the success of the clinic is education. Each visit is set at 30-minute appointments (1 hour for new patients), allowing for a significant amount of time that can be spent on education topics, including pathophysiology, trigger avoidances, and medication use. Patients are asked to bring their medications to the clinic and demonstrate inhaler technique at every visit. Patients who do not bring their inhalers to the clinic will have them filled at the clinic and given to them for demonstration. This type of show-and-tell education allows clinic providers to correct improper inhaler technique immediately. Having patients actually use their medication seems to influence the patient’s inhaler mechanics to a greater extent than does demonstration with a placebo.
In the eyes of the clinic provider, it is important for patients to understand the basic pathophysiology of the disease. The better understanding patients have of a disease, the better they can take part in the treatment. Since the clinic actively engages patients in education topics, it brings patients into an active role in the treatment. As mentioned, the inhaler technique seems to be the most effective first step. However, as patients gain trust in clinic providers due to significant improvement in symptoms secondary to inhaler technique, this trust leads to a dialogue about pathophysiology and triggers.
Another key component in the clinic success is the nature of the clinic itself. Providers in the clinic focus on only 1 disease and the guidelines to treat that disease. Therefore, providers in the clinic are trained to be extremely familiar with the treatment of asthma. This is not to imply that a patient’s PCP or usual care provider is unfamiliar with the guidelines. It simply means that specialty care involves an extra time commitment to a specific disease. Each clinic provider must attain a high level of asthma knowledge before consideration as a full-time provider. Pharmacists are encouraged to sit for the Certified Asthma Educators examination, Board Certified Pharmacotherapy Specialist examination, and/or obtain the Indian Health Service National Clinical Pharmacy Specialist certification.
Although the clinic has moved in the right direction, there are still several patients who have not had any improvement since being referred to the clinic. These patients have refractory asthma (ie, step 6) and are not able to be treated at this facility, continued poor medication adherence, or do not have asthma at all. These patients will be flagged and will be evaluated on a case-by-case basis.
In conclusion, the clinic has begun to achieve what it was intended to do: improve asthma control, reduce patient burden on ED staff, and decrease financial burden to the facility. Additionally, there is improvement in the satisfaction of the asthma care and a trend toward the patients’ perception of asthma control agreeing with medical guidelines. These findings further support the use of pharmacists in the role as provider for the management of chronic diseases.
Acknowledgements
Shail Mehta, MD, is an internal medicine provider at the NNMC. He attended the University of Pittsburgh School of Medicine in Pennsylvania and completed a residency in internal medicine at the University of Michigan in Ann Arbor. Dr. Mehta is certified with the American Board of Internal Medicine.
Erica Markovitz, MD, is an internal medicine provider at the NNMC. She attended the University of Miami School of Medicine in Florida and completed a residency at the University of Michigan. Dr. Markovitz is certified with the American Board of Internal Medicine and the American Board of Pediatrics.
Thad Koppenhafer, PharmD, is the director of pharmacy at the NNMC and the area pharmacy consultant for the Navajo Area of the IHS. He is a member of the American Society of Health-Systems Pharmacists.
CAPT Mark Strong, PharmD, MT (ASCP) is a senior supervisory pharmacist with the U.S. Public Health Commissioned Corps and is assigned to the IHS. He is currently the chief of outpatient pharmacy services at the NNMC.
CDR Clint Krestel, PharmD, is the assistant chief of pharmacy responsible for Inpatient Pharmacy Services. His professional memberships currently include the American College of Clinical Pharmacy, the American Society of Health-System Pharmacists, and the Commissioned Officers Association.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
According to the Centers for Disease Control and Prevention, asthma prevalence in the U.S. increased between 2001 and 2010 and is now at its highest level. In 2010, about 25.7 million people had asthma: 18.7 million adults (8%) and 7 million children (9%). Despite well-known treatment options, asthma continues to be poorly controlled. In 2009 there were 1.6 million emergency department (ED) visits, 497,300 hospitalizations, and 3,404 deaths related to asthma. Additionally, in 2008 the disease affected attendance at school and work with 10.5 million and 14.2 million missed school days and workdays, respectively.1-5
The American Indian and Alaska Native (AIAN) populations have not escaped the realities of asthma. According to a 2010 report of the National Center for Health Statistics, AIAN populations also have a high prevalence of asthma at 14.2%. This percentage is much higher than that of the general population.1
Over the past decade, pharmacists have expanded their roles from educators to clinicians with prescriptive authority in various settings. The greatest success has been seen with anticoagulation clinics, both clinically and financially.6-10 Pharmacists have also demonstrated positive outcomes when involved in cardiovascular clinics.11-13
Additionally, pharmacists have been involved with asthma clinics as both educators and prescribers with favorable results clinically and economically.14-16 A study from Taiwan done by Chan and Wang indicated that pharmacist asthma interventions in an outpatient setting improved the quality of care, reduced cost, and relieved stress on general medical resources.17 Another study indicated that education by a community pharmacist can improve asthma control in a self-managed program.18
In 2007, an asthma medication use evaluation (MUE) was completed at the Northern Navajo Medical Center (NNMC) in Shiprock, New Mexico. The results of the MUE concluded that asthma statistics for the local population differed from that of the national data (Table 1). Overall, the Navajo population served by the NNMC had a lower incidence of asthma but a higher rate of hospital admissions and ED visits.
One of the primary focus points for the MUE was short-acting beta agonist (SABA) refills. According to national guidelines, using a SABA 2 or more times per week (not for exercise-induced bronchospasms) would indicate a patient was not well controlled.19 This use equates to 2 refills of SABA per year. The MUE found that 51% of patients had ≥ 3 refills per year, and 38% of patients had 4 or more refills per year. Based on asthma prevalence and SABA history, it was determined that a specialty clinic could have a positive impact on asthma care.
This study addresses how a specialized adult asthma clinic managed by pharmacists with physician oversight can improve asthma outcomes. Since January 2010, the NNMC has had a program in place and has experienced a concurrent substantial drop in asthma-related ED visits and admissions, an improved level of control, and a decreased cost burden to the facility.
Methods
A retrospective chart review was completed on all patients currently enrolled in the clinic. The Resource and Patient Management System Visit General Retrieval (RPMS VGEN), Electronic Health Record, and the asthma clinic database were used to evaluate patients. The evaluation period began January 1, 2010, and ended December 31, 2011.
Performance improvement inclusion criteria for clinic patients were based on active status in the clinic. Active patients were defined as patients with at least 2 clinic visits and a clinic visit within 3 months of an ED visit or admission. The 3-month cutoff was chosen based on several criteria. First, most patients referred to the clinic were categorized as either not well controlled (NWC) or very poorly controlled (VPC) and required at least a 2- to 4-week follow-up based on guidelines. Second, patients who were categorized as well controlled (WC) were scheduled for clinic visits every 3 months for regular follow-up.
Using all ICD-9 codes for asthma, RPMS VGEN was used to find the number of ED visits and admissions that occurred with asthma as the primary diagnosis from both clinic and nonclinic patients. The inclusion criteria were then applied to the clinic patients, and those not meeting these criteria were returned to the nonclinic pool of patients.
Cost analysis was evaluated based on the results of a random selection of 20 patients from 2009 and 2010 ED and hospital visits at the NNMC. These numbers were averaged to determine ED and admission costs. Length of admission stay was determined from a RPMS VGEN search for each clinic and nonclinic patient admission.
Determination of the level of control was based on the 2007 national asthma guidelines. The guidelines state that the level of control can be determined by either asthma symptoms or by peak flow evaluation.19 Because of the language barrier that sometimes arises with the treated population, the use of symptom-based evaluation has been observationally superior to providing peak flow meters for home use. At each visit, patients were interviewed using tables from the asthma guidelines. Table 2 is an abbreviated portion of the guidelines representing the assessment tool used by the clinic. The level of control was determined by selecting the column with the highest severity of impairment.19
All patients seen at the clinic were tracked in a database, and their current level of control was documented at each visit. To determine the level of control, the database was reviewed, and those patients with > 1 visit were included in the analysis. The levels of control from the first visit to the most recent visit were compared.
Patient surveys were completed at each visit. These surveys included questions to assist the pharmacy provider in classifying the level of control, patient satisfaction with asthma care, and patient perception of asthma control. Approval from the Navajo Area Institutional Review Board was obtained for data publication. Odds ratios were used to determine the impact of the clinic, using a 95% confidence interval.
Results
For the review period, 2,997 patients were coded as having some form of asthma, resulting in 12,739 asthma visits within the medical center.
ED Visits and Hospital Admissions
Of these 2,997 patients, 301 visited the ED between 2010 and 2011 with 22 being active asthma clinic patients. These 22 active clinic patients accounted for 31 ED visits. The remaining 279 patients had 428 visits with a total of 459 ED visits from clinic and nonclinic patients. Sixty patients were hospitalized for asthma with 7 of them active asthma clinic patients. The 7 clinic patients admitted accounted for 8 admissions. There is a statistical significance in total ED visits and admissions as well as for individual patients (Table 3).
To determine the clinic impact, a 2-year analysis of patient pre- and postclinic enrollment was done. Search criteria for RPMS VGEN were identical to the study period search except for dates. Those patients enrolled in the clinic during the study period (2010-2011) were evaluated for the 2 years before the clinic startup (2008-2009). The results indicated a decrease in both ED visits and hospital admissions related to asthma for clinic patients (Table 4).
Cost Data and Length of Stay
Emergency department and hospital admissions costs were determined from an earlier performance improvement review of the clinic. The median cost of an ED visit was $373 with a range from $228 to $910. The cost range represented the severity of the asthma exacerbation being treated. This cost range was similar to published data that reported a cost range from $234 to $400 with an average of $339 per visit.20,21 Hospital costs (including ED visit) per day ranged from $528 to $2,470 with a median of $1,199 per day. Table 5 shows the calculated actual annual cost savings for patients pre- and postenrollment. The cost difference between clinic and nonclinic patients from 2010 to 2011 was calculated to be $111,000 annually (data not shown). The median length of the hospital stay for clinic and nonclinic patients was 2 days (range, 1-10 and 1-7 days, respectively). The national average was 4.3 days.
From 2008 to 2009, there were 123 ED visits and 20 hospital admissions related to asthma of patients who would later be enrolled in the asthma clinic study. From 2010 to 2011 there were 31 ED visits and 8 hospital asthma admissions
(Table 4). These data were used to determine the potential cost savings for the clinic (Table 5). Based on current reductions, the potential annual cost savings was $85,405 if all adult asthma patients were seen in the pharmacy managed clinic (Table 6).
Level of Control
A total of 66 patients had 3 or more visits to the asthma clinic. Of these patients, 30% had no change in control, 60% showed some measure of improvement, and 11% had a decrease in control based on the national guidelines (Table 7).
Patient Perception
At each visit, the patient’s current perception of asthma control, satisfaction with control, and clinic grade related to asthma care was determined. Of the 66 patients with 3 or more visits, a large portion of the questionnaires were missing when the data were collected. Table 8 shows the results of the data for patients with 3 more visits and 2 completed forms from different visits. These data points may not have been from the first or most recent visits.
From earliest to most recent visit, patient perception of asthma control compared with clinical guidelines improved moderately. Sixteen patients had a clinical improvement from VPC to NWC with 13 (81%) believing their symptoms were now WC.
Discussion
The results of this performance improvement evaluation are encouraging. However, not all positive data may be directly attributed to the asthma clinic. The statistical analysis for this study does not seek to remove confounding variables. Without removing potential confounding variables, questions remain about the accuracy of the outcome. However, combining the statistical data in Table 3 and the 2-year comparative data in Table 4, strong evidence exists that a positive impact from the clinic had occurred even in the presence of potential confounding variables.
The financial impact of asthma was evident with the 2010 to 2011 cost for ED visits and hospital admissions at $265,928. Asthma clinic patients made up only 8% ($21,155) of this cost and yielded an annual cost savings of $24,352. Obviously, the 8% was a direct result of the number of nonclinic vs clinic patients. However, the cost savings of $24,352 was independent of patient numbers and was calculated directly from patients pre- and postenrollment. The potential savings if all current nonclinic patients were enrolled in the clinic was $85,405 annually.
The savings was only direct cost and did not include indirect costs related to asthma, such as lost work/school days, impact on employer productivity, and so forth. This was calculated after applying the 75% and 60% reduction in ED and hospital costs. As more patients are enrolled in the clinic, the potential cost could become an actual cost savings, based on the assumption that the 75% and 60% reduction stays constant (Figure). Over 1 year, the actual savings of the clinic makes up for 31% of the current ED visits from nonclinic patients. With the addition of the potential savings, the clinic could almost negate the money that is currently spent on asthma care.
Clinic data indicated a positive impact on level of control. Of those patients with 3 or more visits to the asthma clinic, 59% had some form of improvement. Fifteen of those patients (23%) had the biggest improvement: from VPC to WC. While any form of improvement is beneficial, a jump of this magnitude in so many patients is extremely encouraging. Thirty percent of the patients had no change in level of control. Of these, 14% were WC, so it would be hoped that no change would occur. Eleven patients remained at suboptimal control. The most concerning control data were those patients who lost control of their asthma during the 2-year period.
Chronic nonadherence from a select number of clinic patients seemed to be a major problem. Thirty-one ED visits were from 22 patients, and the 8 admissions were from 7 patients. Of the 22 ED patients, 82% had poor adherence to asthma medications. The hospital data were similar with 6 out of the total 7 (86%) clinic patients reporting poor medication adherence. Additionally, the majority of patients with a decrease in level of control since enrolling in the clinic had a history of poor medication adherence (4 of 7 patients).
In rating the level of asthma care, patients indicated they received the same level of care after enrollment as they did before enrollment. Most of the care given before the clinic was by primary care providers (PCPs), the ED, and urgent care providers. Since the patients rated the level of care equal, it would suggest that pharmacists were providing the same level of care as were these providers, at least from a patient standpoint. Overall, patients were satisfied with their level of control. Most patients were satisfied at enrollment and did not have a change of opinion throughout the study period, and a large number showed increased satisfaction. The patients who showed a decrease in satisfaction of asthma control were those patients who also had no improvement in actual control. Most of these patients stayed at the VPC level. About half these same patients had a history of noncompliance.
There is significant concern regarding those patients who continue to believe they are better controlled than what the guidelines indicate. Sixteen patients moved from VPC to NWC per the guidelines. Thirteen of these patients now believe that their asthma is WC. This belief places the patients at risk for a severe asthma exacerbation. Patients who believe they are WC may be less likely to self-medicate with albuterol or seek medical help during the initial stages of an exacerbation. These patients will need further education to bring their personal perceptions and actual asthma control together.
Conclusions
Based on clinical results it seems that the NNMC Adult Asthma Clinic has made a positive impact on asthma care. Additionally, significant reduction in the financial burden to the facility is achievable. The results, both clinically and statistically significant, indicate the impact a specialty clinic can provide. Specialty clinics, pharmacy or otherwise, have a history of providing positive outcomes.
As previously noted, no confounding variables were included in the data analysis, which could bias the results, even though data for the same time frame from separate years will reduce some errors. However, there will always be a difference in pollen counts, outbreaks (ie, influenza), temperature changes, and so forth. Such variables should be reduced but not removed completely, based on this performance improvement design. If any of these variables were significantly different, it could alter the results, so a potential weakness is present in this study.
Probably the most important mechanism for the success of the clinic is education. Each visit is set at 30-minute appointments (1 hour for new patients), allowing for a significant amount of time that can be spent on education topics, including pathophysiology, trigger avoidances, and medication use. Patients are asked to bring their medications to the clinic and demonstrate inhaler technique at every visit. Patients who do not bring their inhalers to the clinic will have them filled at the clinic and given to them for demonstration. This type of show-and-tell education allows clinic providers to correct improper inhaler technique immediately. Having patients actually use their medication seems to influence the patient’s inhaler mechanics to a greater extent than does demonstration with a placebo.
In the eyes of the clinic provider, it is important for patients to understand the basic pathophysiology of the disease. The better understanding patients have of a disease, the better they can take part in the treatment. Since the clinic actively engages patients in education topics, it brings patients into an active role in the treatment. As mentioned, the inhaler technique seems to be the most effective first step. However, as patients gain trust in clinic providers due to significant improvement in symptoms secondary to inhaler technique, this trust leads to a dialogue about pathophysiology and triggers.
Another key component in the clinic success is the nature of the clinic itself. Providers in the clinic focus on only 1 disease and the guidelines to treat that disease. Therefore, providers in the clinic are trained to be extremely familiar with the treatment of asthma. This is not to imply that a patient’s PCP or usual care provider is unfamiliar with the guidelines. It simply means that specialty care involves an extra time commitment to a specific disease. Each clinic provider must attain a high level of asthma knowledge before consideration as a full-time provider. Pharmacists are encouraged to sit for the Certified Asthma Educators examination, Board Certified Pharmacotherapy Specialist examination, and/or obtain the Indian Health Service National Clinical Pharmacy Specialist certification.
Although the clinic has moved in the right direction, there are still several patients who have not had any improvement since being referred to the clinic. These patients have refractory asthma (ie, step 6) and are not able to be treated at this facility, continued poor medication adherence, or do not have asthma at all. These patients will be flagged and will be evaluated on a case-by-case basis.
In conclusion, the clinic has begun to achieve what it was intended to do: improve asthma control, reduce patient burden on ED staff, and decrease financial burden to the facility. Additionally, there is improvement in the satisfaction of the asthma care and a trend toward the patients’ perception of asthma control agreeing with medical guidelines. These findings further support the use of pharmacists in the role as provider for the management of chronic diseases.
Acknowledgements
Shail Mehta, MD, is an internal medicine provider at the NNMC. He attended the University of Pittsburgh School of Medicine in Pennsylvania and completed a residency in internal medicine at the University of Michigan in Ann Arbor. Dr. Mehta is certified with the American Board of Internal Medicine.
Erica Markovitz, MD, is an internal medicine provider at the NNMC. She attended the University of Miami School of Medicine in Florida and completed a residency at the University of Michigan. Dr. Markovitz is certified with the American Board of Internal Medicine and the American Board of Pediatrics.
Thad Koppenhafer, PharmD, is the director of pharmacy at the NNMC and the area pharmacy consultant for the Navajo Area of the IHS. He is a member of the American Society of Health-Systems Pharmacists.
CAPT Mark Strong, PharmD, MT (ASCP) is a senior supervisory pharmacist with the U.S. Public Health Commissioned Corps and is assigned to the IHS. He is currently the chief of outpatient pharmacy services at the NNMC.
CDR Clint Krestel, PharmD, is the assistant chief of pharmacy responsible for Inpatient Pharmacy Services. His professional memberships currently include the American College of Clinical Pharmacy, the American Society of Health-System Pharmacists, and the Commissioned Officers Association.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/series/sr_03/sr03_035.pdf. Accessed March 10, 2014. Vital Health Stat. 2012;3(35):1-67.
2. National Asthma Control Program. Asthma’s impact on the nation. Data from the CDC National Asthma Control Program. Centers for Disease Control and Prevention Website. http://www.cdc.gov/asthma/impacts_nation/asthmafactsheet.pdf. Accessed March 10, 2014.
3. Schappert AM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed March 10, 2014. Vital Health Stat. 2011;13(169):1-38.
4. Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: Final data for 2009. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_03.pdf. Accessed March 10, 2014. Natl Vital Statistics Rep. 2011;60(3):1-117.
5. Barnes PM, Adams PF, Powell-Griner E. Health characteristics of the American Indian or Alaska Native adult population: United States, 2004-2008. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/nhsr/nhsr020.pdf. Accessed March 10, 2014. Natl Health Stat Report. 2010;20:1-23.
6. Garwood CL, Dumo P, Baringhaus SN, Laban KM. Quality of anticoagulation care in patients discharged from a pharmacist-managed anticoagulation clinic after stabilization of warfarin therapy. Pharmacotherapy. 2008;28(1):20-26.
7. Gray DR, Garabedian-Ruffalo SM, Chretien SD. Cost-justification of a clinical pharmacist-managed anticoagulation clinic. Ann Pharmacother. 2007;41(3):496-501.
8. Ernst ME, Brandt KB. Evaluation of 4 years of clinical pharmacist anticoagulation case management in a rural, private physician office. J Am Pharm Assoc. 2003;43(5):630-636.
9. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
10. Dager WE, Branch JM, King JH, et al. Optimization of inpatient warfarin therapy: Impact of daily consultation by a pharmacist-managed anticoagulation service. Ann Pharmacother. 2000;34(5):567-572.
11. Morello CM, Zadvorny EB, Cording MA, Suemoto RT, Skog J, Harari A. Development and clinical outcomes of pharmacist-managed diabetes care clinics. Am J Health Syst Pharm. 2006;63(14):1325-1331.
12. Lowey A, Moore S, Norris C, Wright D, Silcock J, Hammond P. The cost-effectiveness of pharmacist-led treatment of cardiac risk in patients with type 2 diabetes. Pharm World Sci. 2007;29(5):541-545.
13. Erhun WO, Agbani EO, Bolaji EE. Positive benefits of a pharmacist-managed hypertension clinic in Nigeria. Public Health. 2005;119(9):792-798.
14. Hatoum HT, Witte KW, Hutchinson RA. Patient care contributions of clinical pharmacists in four ambulatory care clinics. Hosp Pharm. 1992;27(3):203-206, 208-209.
15. Pauley TR, Magee MJ, Cury JD. Pharmacist-managed, physician-directed asthma management program reduces emergency department visits. Ann Pharmacother. 1995;29(1):5-9.
16. Nack JA. Homecare management of the asthma patient. U.S. Pharmacist. 1998;23(7). http://legacy.uspharmacist.com/oldformat.asp?url=newlook/file/Home/ACF2FEE.cfm&pub_id=8&article_id=127. Accessed March 10, 2014.
17. Chan AL, Wang HY. Pharmacoeconomic assessment of clinical pharmacist interventions for patients with moderate to severe asthma in outpatient clinics: Experience in Taiwan. Clin Drug Investig. 2004;24(10):603-609.
18. Barbanel D, Eldridge S, Griffiths C. Can a self-management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax. 2003;58(10):851-854.
19. U.S. Department of Health and Human Services, National Institutes of Health, National Heart Lung and Blood Institute. National Asthma Education and Prevention Program, Expert Panel 3. Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. Bethesda, MD: National Institutes of Health; 2007. NIH publication Number 0805846.
20. Stanford R, McLaughlin T, Okamoto LJ. The cost of asthma in the emergency department and hospital. Am J Respir Crit Care Med. 1999;160(1):211-215.
21. Williams RM. The cost of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
1. Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/series/sr_03/sr03_035.pdf. Accessed March 10, 2014. Vital Health Stat. 2012;3(35):1-67.
2. National Asthma Control Program. Asthma’s impact on the nation. Data from the CDC National Asthma Control Program. Centers for Disease Control and Prevention Website. http://www.cdc.gov/asthma/impacts_nation/asthmafactsheet.pdf. Accessed March 10, 2014.
3. Schappert AM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed March 10, 2014. Vital Health Stat. 2011;13(169):1-38.
4. Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: Final data for 2009. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_03.pdf. Accessed March 10, 2014. Natl Vital Statistics Rep. 2011;60(3):1-117.
5. Barnes PM, Adams PF, Powell-Griner E. Health characteristics of the American Indian or Alaska Native adult population: United States, 2004-2008. Centers for Disease Control and Prevention Website. National Center for Health Statistics. http://www.cdc.gov/nchs/data/nhsr/nhsr020.pdf. Accessed March 10, 2014. Natl Health Stat Report. 2010;20:1-23.
6. Garwood CL, Dumo P, Baringhaus SN, Laban KM. Quality of anticoagulation care in patients discharged from a pharmacist-managed anticoagulation clinic after stabilization of warfarin therapy. Pharmacotherapy. 2008;28(1):20-26.
7. Gray DR, Garabedian-Ruffalo SM, Chretien SD. Cost-justification of a clinical pharmacist-managed anticoagulation clinic. Ann Pharmacother. 2007;41(3):496-501.
8. Ernst ME, Brandt KB. Evaluation of 4 years of clinical pharmacist anticoagulation case management in a rural, private physician office. J Am Pharm Assoc. 2003;43(5):630-636.
9. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
10. Dager WE, Branch JM, King JH, et al. Optimization of inpatient warfarin therapy: Impact of daily consultation by a pharmacist-managed anticoagulation service. Ann Pharmacother. 2000;34(5):567-572.
11. Morello CM, Zadvorny EB, Cording MA, Suemoto RT, Skog J, Harari A. Development and clinical outcomes of pharmacist-managed diabetes care clinics. Am J Health Syst Pharm. 2006;63(14):1325-1331.
12. Lowey A, Moore S, Norris C, Wright D, Silcock J, Hammond P. The cost-effectiveness of pharmacist-led treatment of cardiac risk in patients with type 2 diabetes. Pharm World Sci. 2007;29(5):541-545.
13. Erhun WO, Agbani EO, Bolaji EE. Positive benefits of a pharmacist-managed hypertension clinic in Nigeria. Public Health. 2005;119(9):792-798.
14. Hatoum HT, Witte KW, Hutchinson RA. Patient care contributions of clinical pharmacists in four ambulatory care clinics. Hosp Pharm. 1992;27(3):203-206, 208-209.
15. Pauley TR, Magee MJ, Cury JD. Pharmacist-managed, physician-directed asthma management program reduces emergency department visits. Ann Pharmacother. 1995;29(1):5-9.
16. Nack JA. Homecare management of the asthma patient. U.S. Pharmacist. 1998;23(7). http://legacy.uspharmacist.com/oldformat.asp?url=newlook/file/Home/ACF2FEE.cfm&pub_id=8&article_id=127. Accessed March 10, 2014.
17. Chan AL, Wang HY. Pharmacoeconomic assessment of clinical pharmacist interventions for patients with moderate to severe asthma in outpatient clinics: Experience in Taiwan. Clin Drug Investig. 2004;24(10):603-609.
18. Barbanel D, Eldridge S, Griffiths C. Can a self-management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax. 2003;58(10):851-854.
19. U.S. Department of Health and Human Services, National Institutes of Health, National Heart Lung and Blood Institute. National Asthma Education and Prevention Program, Expert Panel 3. Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. Bethesda, MD: National Institutes of Health; 2007. NIH publication Number 0805846.
20. Stanford R, McLaughlin T, Okamoto LJ. The cost of asthma in the emergency department and hospital. Am J Respir Crit Care Med. 1999;160(1):211-215.
21. Williams RM. The cost of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
Evaluating Patient Barriers to Tobacco Cessation Treatment
Tobacco use continues to be the single most preventable cause of death and disease in the U.S., contributing to 480,000 deaths per year, 42,000 of these associated with second-hand tobacco exposure.1 Tobacco use costs Americans over $289 billion in lost productivity and health care costs every year.1
Within the VA, where prevalence exceeds that in the general population, tobacco use among patients is as follows: 19.7% of new enrollees (compared with 19.4% of the general population), 72% of those with a psychiatric disorder, 23% of Operation Enduring Freedom/Operation Iraqi Freedom veterans, and up to 98% of substance use disorder patients in treatment.2-4 In one report, veterans with posttraumatic stress disorder (PTSD) smoked at rates 2 to 3 times that of the general veteran population.5 In 2008, the VA spent over $5.2 billion on treatment of chronic obstructive pulmonary disease alone, a disease highly correlated with smoking tobacco.6 Within the VA, it is clear that tobacco abuse is a costly issue in both health matters as well as dollars spent.
To combat this preventable loss of human life, health, and financial capital, the VA offers high-quality, evidence-based tobacco cessation counseling programs with medical adjunct therapy. In 2010, the Center for Integrated Healthcare developed a training manual to assist tobacco cessation providers in conducting integrated smoking cessation treatment across the VA.7 The Atlanta VA Medical Center (VAMC) in Georgia has had an active and highly successful tobacco cessation treatment program for many years, and in 2004, participants who completed the 5-session treatment program self-reported an abstinence rate of 69.5%, reflecting both quit (28.9%) and smoking less (40.6%) rates for the sample.8
Since that time, tobacco cessation policy within VA has transitioned to offer pharmacotherapy upon veteran request and has eliminated copays for outpatient tobacco cessation visits. In addition, the electronic medical record used within the VA Health Care System includes clinical reminders for providers to assess tobacco use and offer treatment options at several visits per year. Despite these many improvements and enhancements for tobacco cessation care, reduced attendance, including last minute cancellations and “no-shows” for tobacco cessation appointments, remain an ongoing challenge at the Atlanta VAMC.
The purpose of this investigation was to examine through a telephone survey the reasons why identified veterans had not taken advantage of smoking cessation opportunities at the Atlanta VAMC. Specifically, the study evaluated the referral completion rate for veterans referred to the program, analyzed the potential barriers behind these utilization rates, and explored possible opportunities for overcoming them.
Study Design
The VA computerized patient record system (CPRS) provides a reliable means of identifying patients who use tobacco and is replete with clinical reminders for a variety of preventive health issues, including tobacco use cessation counseling. Tobacco use screening is considered a vital sign, and this information is solicited through automatic prompts for every visit. Patients who express an interest in receiving help for tobacco cessation are referred to in-house tobacco cessation counseling services, which consist of weekly, 1-hour sessions of psycho-educational counseling and medical adjunct therapy.
Methods
This project was conducted at the Atlanta VAMC, which was recognized in 2010 by The Joint Commission as a Top Performer on Key Quality Measures. The proposed plan was presented to the Research & Development (R&D) office (an International Review Board equivalent). After careful review and consideration, it was determined to be a quality improvement initiative and did not require full R&D approval.
The CPRS was used to generate a tally of all veterans referred to the tobacco cessation treatment program from January 2008 through November 2011. A total of 3,489 consults were referred by primary care and mental health providers, of which 2,358 patients (67.6%) cancelled or did not attend the program. Names and contact information for patients who did not attend the program for the more recent period of April 1, 2011, to September 8th, 2011 (n = 229) were then selected to participate in this survey study. For the purposes of this analysis, patients were considered a “non-attend” regardless of whether they called to cancel the appointment or simply did not show up for it.
For the survey portion of this study, each of these 229 individuals were contacted by telephone to inquire about potential barriers to participation, using a close-ended survey tool. The following 4 questions were asked: (1) Are you currently using tobacco in any form?; (2) Did you recently (in the past year) receive a referral for tobacco cessation counseling or classes?; (3) Did you attend the tobacco cessation program?; and (4) If you did not attend, what was/were the reason(s)?
These participants were called over several days between October 24, 2011, and November 28, 2011. The limits of confidentiality were explained to each veteran before they were asked to participate in this initiative as an effort to improve the Atlanta VAMC’s tobacco cessation program.
Of the 229 possible participants, only 115 were accessible by phone over the survey period. One declined to participate, leaving 114 potential respondents. Of the 114, 13 reported that they either did not receive a referral for tobacco cessation over the past year or did not recall receiving such a referral. These 13 were removed from the respondent pool, leaving 101. Of these individuals, 5 reported that they did attend the tobacco cessation counseling sessions. These individuals were also removed from the respondent pool, leaving a total of 96 respondents to answer the remaining multipart question regarding barriers to attendance.
Measures and Analysis
Simple descriptive statistics were used to characterize the data from the survey portion of this study, determining frequencies of responses to different barriers. Since respondents were allowed to select as many barriers as applied to their situation, totals did not add to 100%. In addition, a separate variable, consisting of positive responses to barriers that represent accessibility (eg, distance, time, transportation, parking, gas), was used to develop a composite accessibility score to further analyze the comprehensive impact of access.
Results
The rate of cancellations remained fairly stable between January 2008 and November 2011 (67%) (Table 1). The sample was representative of the male-dominated population at the VA with 84% of the respondents being male, aged 22 to 75 years. Of the 96 respondents who did not attend the cessation counseling, 85 reported they were still using tobacco products; 11 reported having quit. Of the 85 respondents who were still using tobacco products, the majority (97%) were smoking cigarettes, while 1% each reported using smokeless tobacco products such as chew, snuff, or a combination of these (Table 2).
Of the 96 respondents who did not complete their tobacco cessation counseling referral, 45% reported that time or scheduling was a barrier to participation (Table 3). Thirty-two percent reported that distance to the counseling sessions was a barrier, and 28% reported transportation issues as a barrier. Also contributing to transportation issues, the cost of gasoline was given as a reason for not attending by 15% of the respondents. Sixteen percent reported that they were not yet ready to quit in spite of accepting a referral for cessation counseling. Smaller percentages reported that they had already quit (6%), believed that counseling did not work for them (3%), expressed that parking was a concern (1%), forgot (6%), or did not think that counseling was important to their quitting efforts (9%). Other reasons provided for nonattendance included concern that quitting is difficult, other medical and mental health priorities, discomfort in groups, and family illness.
A final analysis was conducted, where an inaccessibility score was determined for each respondent based on barriers related to inaccessibility. A single point was given for each of the following answers reported as a barrier by the respondent: (1) too far away; (2) schedule/time; (3) transportation; (4) parking; or (5) gas. Twenty-three percent of the 96 respondents had no accessibility issues, reported as an inaccessibility score of 0. Most respondents had 1 inaccessibility issue (40%); while 28% reported 2, 6% reported 3, and only 1 individual reported having ≥ 4. Of note, a majority (77%) of the respondents reported 1 or more inaccessibility issues as a barrier to their attendance (Table 4).
Discussion
Studies abound regarding barriers to provider-offered smoking cessation counseling.9,10 Even oncologists report low levels of confidence in their ability to counsel patients to quit using tobacco.11 Physicians report lack of time, training, and patient willingness as barriers that prevent them from providing counseling on critical lifestyle issues. Few studies, however, have examined patient-reported barriers to tobacco counseling services. It bears examination, though, when 67% of the patients who accept a referral for tobacco cessation counseling, with no copay, fail to utilize the opportunity.
The results of this study suggest that accessibility issues played a major role in preventing participation, indicating that 77% of the respondents reported at least 1 accessibility issue (transportation, time, or cost) as a contributing factor that kept them from their appointment. The most common accessibility issues reported by this sample were timing and scheduling (45%), distance to the counseling sessions (32%), and transportation issues (28%). The VA is actively addressing these barriers through telehealth and computer-assisted options. In addition, a new telephone mobile application based on the integrated care model for smoking cessation is now available and provides tobacco quit tips for veterans with PTSD who smoke.12
Another noteworthy finding was that 16% reported they were not ready to quit in spite of accepting a referral for counseling. In addition, 13% offered “other” reasons as barriers to tobacco cessation, suggesting that these 2 groups may not have been properly assessed as to their “readiness-to-change” status at the time the referral was generated. Another possibility is the “demand characteristics” of the referral: For example, patients did not want to disappoint their provider, although they were not fully committed to treatment at the time of their visit.
Six percent of the respondents reported they did not attend the treatment program because they had already quit tobacco between the time of the original referral and the time of the survey. This time frame could have been from 6 weeks to 7 months for the respondents. However, these responses were not verified with biomarker testing but, rather, relied on self-reported status. For this reason, these responses could be suspect and may be the result of “demand characteristics” as well.
Another category of respondents of particular interest is the 9% who reported “counseling is not important to my quitting.” This group represents a segment of respondents who failed to appreciate the evidence that demonstrates the benefits of counseling and medical adjunct therapy. Further patient education is clearly needed to ensure patients understand how important smoking cessation is to their health and how important counseling is to their quitting efforts. To accomplish this goal, patient education concerning tobacco cessation in the form of televideo programming placed in the clinic wait areas is underway at the Atlanta VAMC.
Less frequently reported as barriers were “forgot” (6%), “counseling doesn’t work for me” (3%), and “parking concerns” (1%), suggesting that in this limited sample, these were not central reasons for not utilizing these services.
Limitations
The small sample size and that it was a convenience sample pose some concerns as to whether the results are truly representative of the population under study and whether the results can be extrapolated to similar populations. In addition, the results are from self-reported replies, relying on the integrity of the respondents to provide honest answers. Prefacing the study questions with an explanation that this was an opportunity to help the VA improve the quality of its programs was intended to ward off the desire to provide “acceptable” answers.
It is important to understand that patients within the VA system in certain categories of disability and financial means are reimbursed travel expenses for attending tobacco cessation treatment. It is possible that reimbursement factors might motivate patients to accept referrals for counseling that they may not be particularly committed to attend, contributing to a higher-than-expected number of referrals for patients who were not ready to quit.
Conclusion
The results of this study highlight several patient-reported barriers to tobacco cessation treatment, including scheduling conflicts, distance, and cost of travel. Only a small percentage (16%) actually reported they were not yet ready to quit or they did not feel counseling would work for them (3%). A slightly larger percentage reported they did not feel counseling is important (9%), and since it is well established that combining medication with behavioral counseling yields the greatest results for smoking cessation, it is clear that this segment of the patient population will require more education and attention.13
Accessibility issues were the biggest reason for nonattendance to the program (77%), and these issues highlight the need for continued work, at least at the Atlanta VAMC, on providing easier patient access to tobacco cessation treatment. Since the completion of this study, many updates have been implemented at the Atlanta VAMC to improve access, including the provision of telehealth education and the use of telephone quit lines.
Telehealth education, a technique that is highly compatible with lifestyle change counseling, has been shown to be cost-effective while providing intervention and education for patients who are too distant or unable to travel for other reasons.14
Tobacco quit lines are another option for patients with accessibility conflicts and are now operational in all 50 states. Most operate 24/7, manned by counselors trained in motivational interviewing and specifically tobacco cessation counseling. A meta-analysis of quit-line efficacy performed by Stead and colleagues demonstrated that quit lines improve long-term cessation for smokers who use them and even suggested a possible dose-response effect.15 Quit-line counseling, therefore, seems to offer a useful option for veterans who cannot easily access that counseling within the VA.
Motivational interviewing principles have also been proposed by VA as a new approach with great promise for application with veterans who are unmotivated, resistant, or ambivalent about changing unhealthy habits.16 At the Atlanta VAMC, training in motivational interviewing for primary care clinicians is ongoing. It is the provider’s responsibility to strongly encourage patients who use tobacco to utilize alternative tobacco cessation resources when attending a VA treatment program is not a viable option.
This study was a first step in examining barriers to treatment. Although the sample size was small, it is representative and useful in providing a framework from which to improve access to tobacco cessation programs as well as encourage utilization of alternative resources.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
2. 2011 Survey of Veteran Enrollees’ Health and Reliance Upon VA, With Selected Comparison to the 1999-2010 Surveys. Washington, DC: Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health For Policy and Planning, Healthcare Analysis and Information Group, Enrollment and Forecasting Services; 2012.
3. Landolt K, Ajdacic-Gross V, Angst J, et al. Smoking and psychiatric disorders: Have subthreshold disorders been overlooked? Nicotine Tob Res. 2010;12(5):516-520.
4. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: What you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
5. Smoking and Tobacco Use Cessation Report. Washington, DC: Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Secretary for Health for Policy and Planning; 2010.
6. Committee on Smoking Cessation in Military and Veteran Populations, Board on Population Health and Public Health Practice, Institute of Medicine. Combating Tobacco Use in the Military and Veteran Populations. Washington, DC: The National Academies Press; 2009.
7. Dollar K, Dundon M, Kusche A. Tobacco Use Cessation: A Brief Primary Care Intervention, A Training Manual for Integrated Primary Care Behavioral Health Providers and Other Tobacco Cessation Providers. Washington, DC: Department of Veterans Affairs, Center for Integrated Healthcare; 2010.
8. Burchfield BE, Keller T, Avritt L, Wright M, Ackerman MD. A multi-disciplinary approach to smoking cessation within the Department of Veterans Affairs. Paper presented at: 2004 Georgia Psychological Association Annual Meeting; May 2004; Hilton Head, SC.
9. Raupach T, Merker J, Hasenfuss G, Andreas S, Pipe A. Knowledge gaps about smoking cessation in hospitalized patients and their doctors. Eur J Cardiovasc Prev Rehabil. 2011;18(2):334-341.
10. Huy C, Diehm C, Schneider S. Cardiovascular prevention at the general practitioner? First results of a study on attitudes, services, success and barriers in practice [in German]. Dtsch Med Wochenschr. 2012;137(1-2):17-22.
11. Weaver KE, Danhauer SC, Tooze JA, et al. Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist. 2012;17(3):455-462.
12. McFall M, Saxon AJ, Malte CA, et al; CSP 519 Study Team. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: A randomized controlled trial. JAMA. 2010;304(22):2485-2493.
13. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008.
14. Saab PG, McCalla JR, Coons HL, et al. Technological and medical advances: Implications for health psychology. Health Psychol. 2004;23(2):142-146.
15. Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16(suppl 1):i3-i8.
16. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
Tobacco use continues to be the single most preventable cause of death and disease in the U.S., contributing to 480,000 deaths per year, 42,000 of these associated with second-hand tobacco exposure.1 Tobacco use costs Americans over $289 billion in lost productivity and health care costs every year.1
Within the VA, where prevalence exceeds that in the general population, tobacco use among patients is as follows: 19.7% of new enrollees (compared with 19.4% of the general population), 72% of those with a psychiatric disorder, 23% of Operation Enduring Freedom/Operation Iraqi Freedom veterans, and up to 98% of substance use disorder patients in treatment.2-4 In one report, veterans with posttraumatic stress disorder (PTSD) smoked at rates 2 to 3 times that of the general veteran population.5 In 2008, the VA spent over $5.2 billion on treatment of chronic obstructive pulmonary disease alone, a disease highly correlated with smoking tobacco.6 Within the VA, it is clear that tobacco abuse is a costly issue in both health matters as well as dollars spent.
To combat this preventable loss of human life, health, and financial capital, the VA offers high-quality, evidence-based tobacco cessation counseling programs with medical adjunct therapy. In 2010, the Center for Integrated Healthcare developed a training manual to assist tobacco cessation providers in conducting integrated smoking cessation treatment across the VA.7 The Atlanta VA Medical Center (VAMC) in Georgia has had an active and highly successful tobacco cessation treatment program for many years, and in 2004, participants who completed the 5-session treatment program self-reported an abstinence rate of 69.5%, reflecting both quit (28.9%) and smoking less (40.6%) rates for the sample.8
Since that time, tobacco cessation policy within VA has transitioned to offer pharmacotherapy upon veteran request and has eliminated copays for outpatient tobacco cessation visits. In addition, the electronic medical record used within the VA Health Care System includes clinical reminders for providers to assess tobacco use and offer treatment options at several visits per year. Despite these many improvements and enhancements for tobacco cessation care, reduced attendance, including last minute cancellations and “no-shows” for tobacco cessation appointments, remain an ongoing challenge at the Atlanta VAMC.
The purpose of this investigation was to examine through a telephone survey the reasons why identified veterans had not taken advantage of smoking cessation opportunities at the Atlanta VAMC. Specifically, the study evaluated the referral completion rate for veterans referred to the program, analyzed the potential barriers behind these utilization rates, and explored possible opportunities for overcoming them.
Study Design
The VA computerized patient record system (CPRS) provides a reliable means of identifying patients who use tobacco and is replete with clinical reminders for a variety of preventive health issues, including tobacco use cessation counseling. Tobacco use screening is considered a vital sign, and this information is solicited through automatic prompts for every visit. Patients who express an interest in receiving help for tobacco cessation are referred to in-house tobacco cessation counseling services, which consist of weekly, 1-hour sessions of psycho-educational counseling and medical adjunct therapy.
Methods
This project was conducted at the Atlanta VAMC, which was recognized in 2010 by The Joint Commission as a Top Performer on Key Quality Measures. The proposed plan was presented to the Research & Development (R&D) office (an International Review Board equivalent). After careful review and consideration, it was determined to be a quality improvement initiative and did not require full R&D approval.
The CPRS was used to generate a tally of all veterans referred to the tobacco cessation treatment program from January 2008 through November 2011. A total of 3,489 consults were referred by primary care and mental health providers, of which 2,358 patients (67.6%) cancelled or did not attend the program. Names and contact information for patients who did not attend the program for the more recent period of April 1, 2011, to September 8th, 2011 (n = 229) were then selected to participate in this survey study. For the purposes of this analysis, patients were considered a “non-attend” regardless of whether they called to cancel the appointment or simply did not show up for it.
For the survey portion of this study, each of these 229 individuals were contacted by telephone to inquire about potential barriers to participation, using a close-ended survey tool. The following 4 questions were asked: (1) Are you currently using tobacco in any form?; (2) Did you recently (in the past year) receive a referral for tobacco cessation counseling or classes?; (3) Did you attend the tobacco cessation program?; and (4) If you did not attend, what was/were the reason(s)?
These participants were called over several days between October 24, 2011, and November 28, 2011. The limits of confidentiality were explained to each veteran before they were asked to participate in this initiative as an effort to improve the Atlanta VAMC’s tobacco cessation program.
Of the 229 possible participants, only 115 were accessible by phone over the survey period. One declined to participate, leaving 114 potential respondents. Of the 114, 13 reported that they either did not receive a referral for tobacco cessation over the past year or did not recall receiving such a referral. These 13 were removed from the respondent pool, leaving 101. Of these individuals, 5 reported that they did attend the tobacco cessation counseling sessions. These individuals were also removed from the respondent pool, leaving a total of 96 respondents to answer the remaining multipart question regarding barriers to attendance.
Measures and Analysis
Simple descriptive statistics were used to characterize the data from the survey portion of this study, determining frequencies of responses to different barriers. Since respondents were allowed to select as many barriers as applied to their situation, totals did not add to 100%. In addition, a separate variable, consisting of positive responses to barriers that represent accessibility (eg, distance, time, transportation, parking, gas), was used to develop a composite accessibility score to further analyze the comprehensive impact of access.
Results
The rate of cancellations remained fairly stable between January 2008 and November 2011 (67%) (Table 1). The sample was representative of the male-dominated population at the VA with 84% of the respondents being male, aged 22 to 75 years. Of the 96 respondents who did not attend the cessation counseling, 85 reported they were still using tobacco products; 11 reported having quit. Of the 85 respondents who were still using tobacco products, the majority (97%) were smoking cigarettes, while 1% each reported using smokeless tobacco products such as chew, snuff, or a combination of these (Table 2).
Of the 96 respondents who did not complete their tobacco cessation counseling referral, 45% reported that time or scheduling was a barrier to participation (Table 3). Thirty-two percent reported that distance to the counseling sessions was a barrier, and 28% reported transportation issues as a barrier. Also contributing to transportation issues, the cost of gasoline was given as a reason for not attending by 15% of the respondents. Sixteen percent reported that they were not yet ready to quit in spite of accepting a referral for cessation counseling. Smaller percentages reported that they had already quit (6%), believed that counseling did not work for them (3%), expressed that parking was a concern (1%), forgot (6%), or did not think that counseling was important to their quitting efforts (9%). Other reasons provided for nonattendance included concern that quitting is difficult, other medical and mental health priorities, discomfort in groups, and family illness.
A final analysis was conducted, where an inaccessibility score was determined for each respondent based on barriers related to inaccessibility. A single point was given for each of the following answers reported as a barrier by the respondent: (1) too far away; (2) schedule/time; (3) transportation; (4) parking; or (5) gas. Twenty-three percent of the 96 respondents had no accessibility issues, reported as an inaccessibility score of 0. Most respondents had 1 inaccessibility issue (40%); while 28% reported 2, 6% reported 3, and only 1 individual reported having ≥ 4. Of note, a majority (77%) of the respondents reported 1 or more inaccessibility issues as a barrier to their attendance (Table 4).
Discussion
Studies abound regarding barriers to provider-offered smoking cessation counseling.9,10 Even oncologists report low levels of confidence in their ability to counsel patients to quit using tobacco.11 Physicians report lack of time, training, and patient willingness as barriers that prevent them from providing counseling on critical lifestyle issues. Few studies, however, have examined patient-reported barriers to tobacco counseling services. It bears examination, though, when 67% of the patients who accept a referral for tobacco cessation counseling, with no copay, fail to utilize the opportunity.
The results of this study suggest that accessibility issues played a major role in preventing participation, indicating that 77% of the respondents reported at least 1 accessibility issue (transportation, time, or cost) as a contributing factor that kept them from their appointment. The most common accessibility issues reported by this sample were timing and scheduling (45%), distance to the counseling sessions (32%), and transportation issues (28%). The VA is actively addressing these barriers through telehealth and computer-assisted options. In addition, a new telephone mobile application based on the integrated care model for smoking cessation is now available and provides tobacco quit tips for veterans with PTSD who smoke.12
Another noteworthy finding was that 16% reported they were not ready to quit in spite of accepting a referral for counseling. In addition, 13% offered “other” reasons as barriers to tobacco cessation, suggesting that these 2 groups may not have been properly assessed as to their “readiness-to-change” status at the time the referral was generated. Another possibility is the “demand characteristics” of the referral: For example, patients did not want to disappoint their provider, although they were not fully committed to treatment at the time of their visit.
Six percent of the respondents reported they did not attend the treatment program because they had already quit tobacco between the time of the original referral and the time of the survey. This time frame could have been from 6 weeks to 7 months for the respondents. However, these responses were not verified with biomarker testing but, rather, relied on self-reported status. For this reason, these responses could be suspect and may be the result of “demand characteristics” as well.
Another category of respondents of particular interest is the 9% who reported “counseling is not important to my quitting.” This group represents a segment of respondents who failed to appreciate the evidence that demonstrates the benefits of counseling and medical adjunct therapy. Further patient education is clearly needed to ensure patients understand how important smoking cessation is to their health and how important counseling is to their quitting efforts. To accomplish this goal, patient education concerning tobacco cessation in the form of televideo programming placed in the clinic wait areas is underway at the Atlanta VAMC.
Less frequently reported as barriers were “forgot” (6%), “counseling doesn’t work for me” (3%), and “parking concerns” (1%), suggesting that in this limited sample, these were not central reasons for not utilizing these services.
Limitations
The small sample size and that it was a convenience sample pose some concerns as to whether the results are truly representative of the population under study and whether the results can be extrapolated to similar populations. In addition, the results are from self-reported replies, relying on the integrity of the respondents to provide honest answers. Prefacing the study questions with an explanation that this was an opportunity to help the VA improve the quality of its programs was intended to ward off the desire to provide “acceptable” answers.
It is important to understand that patients within the VA system in certain categories of disability and financial means are reimbursed travel expenses for attending tobacco cessation treatment. It is possible that reimbursement factors might motivate patients to accept referrals for counseling that they may not be particularly committed to attend, contributing to a higher-than-expected number of referrals for patients who were not ready to quit.
Conclusion
The results of this study highlight several patient-reported barriers to tobacco cessation treatment, including scheduling conflicts, distance, and cost of travel. Only a small percentage (16%) actually reported they were not yet ready to quit or they did not feel counseling would work for them (3%). A slightly larger percentage reported they did not feel counseling is important (9%), and since it is well established that combining medication with behavioral counseling yields the greatest results for smoking cessation, it is clear that this segment of the patient population will require more education and attention.13
Accessibility issues were the biggest reason for nonattendance to the program (77%), and these issues highlight the need for continued work, at least at the Atlanta VAMC, on providing easier patient access to tobacco cessation treatment. Since the completion of this study, many updates have been implemented at the Atlanta VAMC to improve access, including the provision of telehealth education and the use of telephone quit lines.
Telehealth education, a technique that is highly compatible with lifestyle change counseling, has been shown to be cost-effective while providing intervention and education for patients who are too distant or unable to travel for other reasons.14
Tobacco quit lines are another option for patients with accessibility conflicts and are now operational in all 50 states. Most operate 24/7, manned by counselors trained in motivational interviewing and specifically tobacco cessation counseling. A meta-analysis of quit-line efficacy performed by Stead and colleagues demonstrated that quit lines improve long-term cessation for smokers who use them and even suggested a possible dose-response effect.15 Quit-line counseling, therefore, seems to offer a useful option for veterans who cannot easily access that counseling within the VA.
Motivational interviewing principles have also been proposed by VA as a new approach with great promise for application with veterans who are unmotivated, resistant, or ambivalent about changing unhealthy habits.16 At the Atlanta VAMC, training in motivational interviewing for primary care clinicians is ongoing. It is the provider’s responsibility to strongly encourage patients who use tobacco to utilize alternative tobacco cessation resources when attending a VA treatment program is not a viable option.
This study was a first step in examining barriers to treatment. Although the sample size was small, it is representative and useful in providing a framework from which to improve access to tobacco cessation programs as well as encourage utilization of alternative resources.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Tobacco use continues to be the single most preventable cause of death and disease in the U.S., contributing to 480,000 deaths per year, 42,000 of these associated with second-hand tobacco exposure.1 Tobacco use costs Americans over $289 billion in lost productivity and health care costs every year.1
Within the VA, where prevalence exceeds that in the general population, tobacco use among patients is as follows: 19.7% of new enrollees (compared with 19.4% of the general population), 72% of those with a psychiatric disorder, 23% of Operation Enduring Freedom/Operation Iraqi Freedom veterans, and up to 98% of substance use disorder patients in treatment.2-4 In one report, veterans with posttraumatic stress disorder (PTSD) smoked at rates 2 to 3 times that of the general veteran population.5 In 2008, the VA spent over $5.2 billion on treatment of chronic obstructive pulmonary disease alone, a disease highly correlated with smoking tobacco.6 Within the VA, it is clear that tobacco abuse is a costly issue in both health matters as well as dollars spent.
To combat this preventable loss of human life, health, and financial capital, the VA offers high-quality, evidence-based tobacco cessation counseling programs with medical adjunct therapy. In 2010, the Center for Integrated Healthcare developed a training manual to assist tobacco cessation providers in conducting integrated smoking cessation treatment across the VA.7 The Atlanta VA Medical Center (VAMC) in Georgia has had an active and highly successful tobacco cessation treatment program for many years, and in 2004, participants who completed the 5-session treatment program self-reported an abstinence rate of 69.5%, reflecting both quit (28.9%) and smoking less (40.6%) rates for the sample.8
Since that time, tobacco cessation policy within VA has transitioned to offer pharmacotherapy upon veteran request and has eliminated copays for outpatient tobacco cessation visits. In addition, the electronic medical record used within the VA Health Care System includes clinical reminders for providers to assess tobacco use and offer treatment options at several visits per year. Despite these many improvements and enhancements for tobacco cessation care, reduced attendance, including last minute cancellations and “no-shows” for tobacco cessation appointments, remain an ongoing challenge at the Atlanta VAMC.
The purpose of this investigation was to examine through a telephone survey the reasons why identified veterans had not taken advantage of smoking cessation opportunities at the Atlanta VAMC. Specifically, the study evaluated the referral completion rate for veterans referred to the program, analyzed the potential barriers behind these utilization rates, and explored possible opportunities for overcoming them.
Study Design
The VA computerized patient record system (CPRS) provides a reliable means of identifying patients who use tobacco and is replete with clinical reminders for a variety of preventive health issues, including tobacco use cessation counseling. Tobacco use screening is considered a vital sign, and this information is solicited through automatic prompts for every visit. Patients who express an interest in receiving help for tobacco cessation are referred to in-house tobacco cessation counseling services, which consist of weekly, 1-hour sessions of psycho-educational counseling and medical adjunct therapy.
Methods
This project was conducted at the Atlanta VAMC, which was recognized in 2010 by The Joint Commission as a Top Performer on Key Quality Measures. The proposed plan was presented to the Research & Development (R&D) office (an International Review Board equivalent). After careful review and consideration, it was determined to be a quality improvement initiative and did not require full R&D approval.
The CPRS was used to generate a tally of all veterans referred to the tobacco cessation treatment program from January 2008 through November 2011. A total of 3,489 consults were referred by primary care and mental health providers, of which 2,358 patients (67.6%) cancelled or did not attend the program. Names and contact information for patients who did not attend the program for the more recent period of April 1, 2011, to September 8th, 2011 (n = 229) were then selected to participate in this survey study. For the purposes of this analysis, patients were considered a “non-attend” regardless of whether they called to cancel the appointment or simply did not show up for it.
For the survey portion of this study, each of these 229 individuals were contacted by telephone to inquire about potential barriers to participation, using a close-ended survey tool. The following 4 questions were asked: (1) Are you currently using tobacco in any form?; (2) Did you recently (in the past year) receive a referral for tobacco cessation counseling or classes?; (3) Did you attend the tobacco cessation program?; and (4) If you did not attend, what was/were the reason(s)?
These participants were called over several days between October 24, 2011, and November 28, 2011. The limits of confidentiality were explained to each veteran before they were asked to participate in this initiative as an effort to improve the Atlanta VAMC’s tobacco cessation program.
Of the 229 possible participants, only 115 were accessible by phone over the survey period. One declined to participate, leaving 114 potential respondents. Of the 114, 13 reported that they either did not receive a referral for tobacco cessation over the past year or did not recall receiving such a referral. These 13 were removed from the respondent pool, leaving 101. Of these individuals, 5 reported that they did attend the tobacco cessation counseling sessions. These individuals were also removed from the respondent pool, leaving a total of 96 respondents to answer the remaining multipart question regarding barriers to attendance.
Measures and Analysis
Simple descriptive statistics were used to characterize the data from the survey portion of this study, determining frequencies of responses to different barriers. Since respondents were allowed to select as many barriers as applied to their situation, totals did not add to 100%. In addition, a separate variable, consisting of positive responses to barriers that represent accessibility (eg, distance, time, transportation, parking, gas), was used to develop a composite accessibility score to further analyze the comprehensive impact of access.
Results
The rate of cancellations remained fairly stable between January 2008 and November 2011 (67%) (Table 1). The sample was representative of the male-dominated population at the VA with 84% of the respondents being male, aged 22 to 75 years. Of the 96 respondents who did not attend the cessation counseling, 85 reported they were still using tobacco products; 11 reported having quit. Of the 85 respondents who were still using tobacco products, the majority (97%) were smoking cigarettes, while 1% each reported using smokeless tobacco products such as chew, snuff, or a combination of these (Table 2).
Of the 96 respondents who did not complete their tobacco cessation counseling referral, 45% reported that time or scheduling was a barrier to participation (Table 3). Thirty-two percent reported that distance to the counseling sessions was a barrier, and 28% reported transportation issues as a barrier. Also contributing to transportation issues, the cost of gasoline was given as a reason for not attending by 15% of the respondents. Sixteen percent reported that they were not yet ready to quit in spite of accepting a referral for cessation counseling. Smaller percentages reported that they had already quit (6%), believed that counseling did not work for them (3%), expressed that parking was a concern (1%), forgot (6%), or did not think that counseling was important to their quitting efforts (9%). Other reasons provided for nonattendance included concern that quitting is difficult, other medical and mental health priorities, discomfort in groups, and family illness.
A final analysis was conducted, where an inaccessibility score was determined for each respondent based on barriers related to inaccessibility. A single point was given for each of the following answers reported as a barrier by the respondent: (1) too far away; (2) schedule/time; (3) transportation; (4) parking; or (5) gas. Twenty-three percent of the 96 respondents had no accessibility issues, reported as an inaccessibility score of 0. Most respondents had 1 inaccessibility issue (40%); while 28% reported 2, 6% reported 3, and only 1 individual reported having ≥ 4. Of note, a majority (77%) of the respondents reported 1 or more inaccessibility issues as a barrier to their attendance (Table 4).
Discussion
Studies abound regarding barriers to provider-offered smoking cessation counseling.9,10 Even oncologists report low levels of confidence in their ability to counsel patients to quit using tobacco.11 Physicians report lack of time, training, and patient willingness as barriers that prevent them from providing counseling on critical lifestyle issues. Few studies, however, have examined patient-reported barriers to tobacco counseling services. It bears examination, though, when 67% of the patients who accept a referral for tobacco cessation counseling, with no copay, fail to utilize the opportunity.
The results of this study suggest that accessibility issues played a major role in preventing participation, indicating that 77% of the respondents reported at least 1 accessibility issue (transportation, time, or cost) as a contributing factor that kept them from their appointment. The most common accessibility issues reported by this sample were timing and scheduling (45%), distance to the counseling sessions (32%), and transportation issues (28%). The VA is actively addressing these barriers through telehealth and computer-assisted options. In addition, a new telephone mobile application based on the integrated care model for smoking cessation is now available and provides tobacco quit tips for veterans with PTSD who smoke.12
Another noteworthy finding was that 16% reported they were not ready to quit in spite of accepting a referral for counseling. In addition, 13% offered “other” reasons as barriers to tobacco cessation, suggesting that these 2 groups may not have been properly assessed as to their “readiness-to-change” status at the time the referral was generated. Another possibility is the “demand characteristics” of the referral: For example, patients did not want to disappoint their provider, although they were not fully committed to treatment at the time of their visit.
Six percent of the respondents reported they did not attend the treatment program because they had already quit tobacco between the time of the original referral and the time of the survey. This time frame could have been from 6 weeks to 7 months for the respondents. However, these responses were not verified with biomarker testing but, rather, relied on self-reported status. For this reason, these responses could be suspect and may be the result of “demand characteristics” as well.
Another category of respondents of particular interest is the 9% who reported “counseling is not important to my quitting.” This group represents a segment of respondents who failed to appreciate the evidence that demonstrates the benefits of counseling and medical adjunct therapy. Further patient education is clearly needed to ensure patients understand how important smoking cessation is to their health and how important counseling is to their quitting efforts. To accomplish this goal, patient education concerning tobacco cessation in the form of televideo programming placed in the clinic wait areas is underway at the Atlanta VAMC.
Less frequently reported as barriers were “forgot” (6%), “counseling doesn’t work for me” (3%), and “parking concerns” (1%), suggesting that in this limited sample, these were not central reasons for not utilizing these services.
Limitations
The small sample size and that it was a convenience sample pose some concerns as to whether the results are truly representative of the population under study and whether the results can be extrapolated to similar populations. In addition, the results are from self-reported replies, relying on the integrity of the respondents to provide honest answers. Prefacing the study questions with an explanation that this was an opportunity to help the VA improve the quality of its programs was intended to ward off the desire to provide “acceptable” answers.
It is important to understand that patients within the VA system in certain categories of disability and financial means are reimbursed travel expenses for attending tobacco cessation treatment. It is possible that reimbursement factors might motivate patients to accept referrals for counseling that they may not be particularly committed to attend, contributing to a higher-than-expected number of referrals for patients who were not ready to quit.
Conclusion
The results of this study highlight several patient-reported barriers to tobacco cessation treatment, including scheduling conflicts, distance, and cost of travel. Only a small percentage (16%) actually reported they were not yet ready to quit or they did not feel counseling would work for them (3%). A slightly larger percentage reported they did not feel counseling is important (9%), and since it is well established that combining medication with behavioral counseling yields the greatest results for smoking cessation, it is clear that this segment of the patient population will require more education and attention.13
Accessibility issues were the biggest reason for nonattendance to the program (77%), and these issues highlight the need for continued work, at least at the Atlanta VAMC, on providing easier patient access to tobacco cessation treatment. Since the completion of this study, many updates have been implemented at the Atlanta VAMC to improve access, including the provision of telehealth education and the use of telephone quit lines.
Telehealth education, a technique that is highly compatible with lifestyle change counseling, has been shown to be cost-effective while providing intervention and education for patients who are too distant or unable to travel for other reasons.14
Tobacco quit lines are another option for patients with accessibility conflicts and are now operational in all 50 states. Most operate 24/7, manned by counselors trained in motivational interviewing and specifically tobacco cessation counseling. A meta-analysis of quit-line efficacy performed by Stead and colleagues demonstrated that quit lines improve long-term cessation for smokers who use them and even suggested a possible dose-response effect.15 Quit-line counseling, therefore, seems to offer a useful option for veterans who cannot easily access that counseling within the VA.
Motivational interviewing principles have also been proposed by VA as a new approach with great promise for application with veterans who are unmotivated, resistant, or ambivalent about changing unhealthy habits.16 At the Atlanta VAMC, training in motivational interviewing for primary care clinicians is ongoing. It is the provider’s responsibility to strongly encourage patients who use tobacco to utilize alternative tobacco cessation resources when attending a VA treatment program is not a viable option.
This study was a first step in examining barriers to treatment. Although the sample size was small, it is representative and useful in providing a framework from which to improve access to tobacco cessation programs as well as encourage utilization of alternative resources.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
2. 2011 Survey of Veteran Enrollees’ Health and Reliance Upon VA, With Selected Comparison to the 1999-2010 Surveys. Washington, DC: Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health For Policy and Planning, Healthcare Analysis and Information Group, Enrollment and Forecasting Services; 2012.
3. Landolt K, Ajdacic-Gross V, Angst J, et al. Smoking and psychiatric disorders: Have subthreshold disorders been overlooked? Nicotine Tob Res. 2010;12(5):516-520.
4. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: What you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
5. Smoking and Tobacco Use Cessation Report. Washington, DC: Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Secretary for Health for Policy and Planning; 2010.
6. Committee on Smoking Cessation in Military and Veteran Populations, Board on Population Health and Public Health Practice, Institute of Medicine. Combating Tobacco Use in the Military and Veteran Populations. Washington, DC: The National Academies Press; 2009.
7. Dollar K, Dundon M, Kusche A. Tobacco Use Cessation: A Brief Primary Care Intervention, A Training Manual for Integrated Primary Care Behavioral Health Providers and Other Tobacco Cessation Providers. Washington, DC: Department of Veterans Affairs, Center for Integrated Healthcare; 2010.
8. Burchfield BE, Keller T, Avritt L, Wright M, Ackerman MD. A multi-disciplinary approach to smoking cessation within the Department of Veterans Affairs. Paper presented at: 2004 Georgia Psychological Association Annual Meeting; May 2004; Hilton Head, SC.
9. Raupach T, Merker J, Hasenfuss G, Andreas S, Pipe A. Knowledge gaps about smoking cessation in hospitalized patients and their doctors. Eur J Cardiovasc Prev Rehabil. 2011;18(2):334-341.
10. Huy C, Diehm C, Schneider S. Cardiovascular prevention at the general practitioner? First results of a study on attitudes, services, success and barriers in practice [in German]. Dtsch Med Wochenschr. 2012;137(1-2):17-22.
11. Weaver KE, Danhauer SC, Tooze JA, et al. Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist. 2012;17(3):455-462.
12. McFall M, Saxon AJ, Malte CA, et al; CSP 519 Study Team. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: A randomized controlled trial. JAMA. 2010;304(22):2485-2493.
13. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008.
14. Saab PG, McCalla JR, Coons HL, et al. Technological and medical advances: Implications for health psychology. Health Psychol. 2004;23(2):142-146.
15. Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16(suppl 1):i3-i8.
16. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
2. 2011 Survey of Veteran Enrollees’ Health and Reliance Upon VA, With Selected Comparison to the 1999-2010 Surveys. Washington, DC: Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Under Secretary for Health For Policy and Planning, Healthcare Analysis and Information Group, Enrollment and Forecasting Services; 2012.
3. Landolt K, Ajdacic-Gross V, Angst J, et al. Smoking and psychiatric disorders: Have subthreshold disorders been overlooked? Nicotine Tob Res. 2010;12(5):516-520.
4. Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: What you need to know. J Subst Abuse Treat. 2009;36(2):205-219.
5. Smoking and Tobacco Use Cessation Report. Washington, DC: Department of Veterans Affairs, Veterans Health Administration, Office of the Assistant Deputy Secretary for Health for Policy and Planning; 2010.
6. Committee on Smoking Cessation in Military and Veteran Populations, Board on Population Health and Public Health Practice, Institute of Medicine. Combating Tobacco Use in the Military and Veteran Populations. Washington, DC: The National Academies Press; 2009.
7. Dollar K, Dundon M, Kusche A. Tobacco Use Cessation: A Brief Primary Care Intervention, A Training Manual for Integrated Primary Care Behavioral Health Providers and Other Tobacco Cessation Providers. Washington, DC: Department of Veterans Affairs, Center for Integrated Healthcare; 2010.
8. Burchfield BE, Keller T, Avritt L, Wright M, Ackerman MD. A multi-disciplinary approach to smoking cessation within the Department of Veterans Affairs. Paper presented at: 2004 Georgia Psychological Association Annual Meeting; May 2004; Hilton Head, SC.
9. Raupach T, Merker J, Hasenfuss G, Andreas S, Pipe A. Knowledge gaps about smoking cessation in hospitalized patients and their doctors. Eur J Cardiovasc Prev Rehabil. 2011;18(2):334-341.
10. Huy C, Diehm C, Schneider S. Cardiovascular prevention at the general practitioner? First results of a study on attitudes, services, success and barriers in practice [in German]. Dtsch Med Wochenschr. 2012;137(1-2):17-22.
11. Weaver KE, Danhauer SC, Tooze JA, et al. Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist. 2012;17(3):455-462.
12. McFall M, Saxon AJ, Malte CA, et al; CSP 519 Study Team. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: A randomized controlled trial. JAMA. 2010;304(22):2485-2493.
13. Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008.
14. Saab PG, McCalla JR, Coons HL, et al. Technological and medical advances: Implications for health psychology. Health Psychol. 2004;23(2):142-146.
15. Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16(suppl 1):i3-i8.
16. Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, NY: The Guilford Press; 2008.
What Do We Know About Opioid-Induced Hyperalgesia?
From the Massachusetts General Hospital Center for Pain Medicine, Boston, MA.
Abstract
- Objective: To review evidence from clinical and preclinical studies related to the phenomenon of opioid-induced hyperalgesia (OIH) and discuss issues relevant to clinical diagnosis and management.
- Methods: Literature review.
- Results: OIH is defined as a state of nociceptive sensitization caused by exposure to opioids such that a patient receiving opioids to treat pain could become more sensitive to painful stimuli. Interest in understanding OIH has grown over years and multiple mechanisms have been proposed. Both OIH and opioid tolerance can reduce opioid analgesic efficacy, complicating clinical management of chronic pain. When a diagnosis is uncertain, a trial of opioid dose escalation or tapering may be helpful in differentiating between tolerance and OIH. It is unclear whether certain types of opioids or routes of administration are more likely to lead to OIH.
- Conclusion: Clinical outcome of opioid therapy is a dynamic balance among the opioid analgesic effect, OIH, and worsening pain due to disease progression. While OIH has been well documented over nearly 2 decades, its exact clinical characteristics and underlying mechanisms have yet to be fully determined.
Opioids, which produce analgesia through a primarily inhibitory effect on the nociceptive system, have been used for decades for the clinical management of moderate to severe pain. Opioid analgesics act on 3 major classes of opioid receptors, including the µ, k, δ (mu, kappa, and delta) receptors. Activation of opioid receptors not only produces analgesia but also other effects, such as euphoria, respiratory depression, decreased gastrointestinal motility, and cardiovascular effects. Exposure to opioids, however, can also lead to the development of opioid tolerance and opioid-induced hyperalgesia (OIH). Both opioid tolerance and OIH can decrease opioid analgesic efficacy, making chronic pain management a challenge. OIH is a state of nociceptive sensitization caused by exposure to opioids, such that a patient receiving opioids for the treatment of pain could actually become more sensitive to painful stimulation, resulting in a paradoxical adverse response to opioid therapy. In this article, we will review evidence from preclinical and clinical studies and discuss issues relevant to clinical diagnosis and management of OIH.
Evidence of OIH in Animal Studies
In early 1990s, an original preclinical study showed that there was a progressive reduction in baseline nociceptive threshold by using a foot withdraw test in rats receiving repeated intrathecal morphine administration (10-20 mg) over a 7-day period [1]. A number of animal studies later also provided similar data. A reduced baseline nociceptive threshold was observed in animals receiving subcutaneous fentanyl boluses using the Randall-Sellitto test, in which a constantly increasing pressure was applied to a rat’s hind paw. The decreased baseline nociceptive threshold lasted 5 days after cessation of 4 fentanyl bolus injections [2]. In another study, a reduced baseline nociceptive threshold was detected in animals with repeated heroin administration [3]. In other studies, rats exposed to morphine also developed a latent sensitization of visceral pain with a shift of the morphine dose-response curve to the right [4]; exposure to methadone also induced hyperalgesia in rats, which was not prevented by a weak NMDA receptor antagonist (memantine) [5]; and a partial µ-receptor agonist buprenophine produced a dose-related OIH as well [6].
These results indicate that a progressive and lasting reduction of baseline nociceptive threshold, which was referred to as OIH, can result from repeated opioid administration [7–9]. However, different from previous preclinical observations in which a large dose of intrathecal morphine was given, these studies resulted in hyperalgesic response [10,11] in a clinically relevant opioid dose. Of interest is that OIH was observed in animals even when there was continuous opioid infusion via an implanted osmotic pump, suggesting the involvement of active cellular mechanisms in the process [12]. Therefore, prolonged opioid treatment results in not only loss of the opioid analgesic effect (anti-nociceptive effect or desensitization) but also activation of a hyperalgesic effect (a pro-nociceptive effect with reduced nociceptive threshold or increased sensitization). Although both opioid tolerance and OIH are initiated by opioid administration, two opposing cellular mechanisms (ie, desensitization versus sensitization) may be involved in the process. Subsequently, many studies explored the neural and cellular mechanisms underlying the development of OIH and their interaction with the mechanism of opioid tolerance.
Proposed Cellular Mechanisms of OIH
A significant number of recent studies have explored the neurobiological basis of OIH, revealing a divergent range of cellular elements contributory to OIH. These mechanisms include (1) N-methyl-D-aspartate (NMDA) receptor and related intracellular pathways; (2) involvement of G-protein coupled receptors including 5-HT receptors and neurokinin-1 receptors; (3) nitrix oxide and nitric oxide sunthase; (4) TRPV1 receptors; (5) calcium channels; and (6) miscellaneous mechanisms including sex differences [7–9,13–41].
In summary, an increasing number of preclinical studies in the area of OIH indicates that there is enormous interest in understanding the cellular mechanisms of OIH, and the current evidence points to a progressive sensitization process within the central nervous system that involves a constellation of cellular elements such as NMDA receptors, similar to those contributory to the mechanisms of pathological pain.
Evidence of OIH in Human Studies
In animal studies, changes in baseline nociceptive thresholds can be measured in a controlled setting. It is, however, difficult to assess changes in pain threshold in clinical environment following opioid administration [9]. It is often a challenge to distinguish opioid pharmacologic tolerance from OIH because the outcome of opioid therapy is based primarily on subjective pain scores. In the face of these challenges, an increasing number of clinical anecdotal case reports and studies suggest that OIH is likely to be a significant factor in clinical opioid therapy [47–54].
In a study of 1620 patients in which remifentanil was used for general anesthesia, the incidence of postoperative remifentanil-induced hyperalgesia was 16.1%. This study found that age younger than 16 years, male sex, operation duration longer than 2 hours, and remifentanil dose greater than 30 mg/kga were associated with higher rates of OIH [55]. On the other hand, heroin or other opioid addicts not only demonstrated OIH but also had prolonged symptoms of OIH after detoxification from opioids for at least 1 month [56]. In chronic pain patients without opioid dependence, significantly lower pain threshold and tolerance as assessed by pressure pain stimulation were detected [57]. It appears that the sensitivity of detecting OIH in the clinical setting may be influenced by the modality of sensory stimulation [58].
In a prospective preliminary study of 6 patients with chronic low back pain, hyperalgesic response was detected after 1 month of oral morphine therapy using a cold pressor test but not a heat pain test [59]. In another prospective randomized, placebo-controlled, 2-way crossover study in healthy human volunteers, the development of OIH was quantified as changes in the average radius of the area of secondary hyperalgesia generated by electrical pain stimulation. A 23.6% increase in the area of secondary hyperalgesia over baseline was detected following the remifentanil infusion. The same study showed that endogenous opioids did not seem to have an effect on OIH because a single bolus of naloxone did not change the size of secondary hyperalgesia [60].
OIH Prevention Studies
Currently, efforts have also been made to see whether OIH can be prevented with different approaches in human subjects. The following is a brief summary of these studies. In a study of adolescents undergoing scoliosis surgery, treatment with morphine (150 mg/kg) prior to commencing remifentanil infusion did not prevent the development of remifentanil-induced hyperalgesia [61]. In another study, propofol infusion alone with remifentanil both delayed and attenuated remifentanil-induced hyperalgesia [53]. In yet another study, intraoperative 70% N2O administration appeared to reduce postoperative OIH following an intraoperative remifentanil-propofol anesthesia regimen [62].
In a study of 15 healthy male volunteers, preventive administration of parecoxib significantly diminished OIH after withdrawal from remifentanil. In contrast, parecoxib given together with remifentanil did not prevent OIH, suggesting that pre-treatment, not parallel treatment, with opioid may be required to prevent OIH [63]. Other NSAIDs administered preemptively also appear to prevent remifentanil-induced hyperalgesia [64].
Another study investigated the effect of intra-operative magnesium sulfate administration in patients undergoing robot-assisted laparoscopic prostatectomy. Magnesium sulfate administration reduced postoperative opioid consumption and OIH in subjects receiving intra-operative remifentanil-based anesthesia [65,66]. Intra-operative adenosine infusion also prevented acute opioid tolerance and remifentanil-induced hyperalgesia [67]. Continuous intra-operative infusion of ketamine, an NMDA receptor antagonist, significant lowered postoperative VAS and morphine use in gynecologic surgery patients [68]. Also, in a randomized, double-blind, placebo-controlled study of 90 patients who underwent total abdominal hysterectomy, cumulative morphine consumption was significantly greater in subjects with fentanyl alone than those with saline alone, ketamine alone, ketamine with fentanyl, or fentanyl with lornoxicam at 3, 6, and 12 hours postoperatively [69].
Finally, in a double-blind, randomized, placebo-controlled study of 40 patients undergoing elective shoulder surgery, clonidine was given intra-operatively in a remifentanil/propofol-based anesthesia. The results showed that clonidine did not reduce postoperative morphine consumption and pain score in these patients [70]. However, dexmedetomidine, another α2 receptor agonist, substantially reduced baseline opioid doses in hospitalized patients with OIH [71].
Quantitative Sensory Testing and OIH
Currently, diagnostic tools for OIH are still being developed. Many clinical studies have used quantitative sensory testing (QST) as a tool to assess OIH [72,73]. In a recent study, QST was used to compare pain threshold, pain tolerance, and the degree of temporal summation of pain in response to thermal stimulation among 3 groups of subjects: Group 1 (no pain and no opioid), Group 2 (chronic pain but no opioid therapy), and Group 3 (both chronic pain and opioid therapy). Group 3 subjects displayed a decreased heat pain threshold and exacerbated temporal summation of pain to thermal stimulation as compared with both group 1 and group 2 subjects. There were no differences in cold or warm sensation among all 3 groups. Among clinical factors, daily opioid dose consistently correlated with the decreased heat pain threshold and exacerbated temporal summation of second pain in group 3 subjects [72]. Another study investigated the sensitivity to cold pain and the magnitude of diffuse noxious inhibitory control (DNIC) using QST in subjects with or without opioid therapy. Pain threshold, intensity and tolerance in response to the cold pressor (1°C) were measured. They found that oral opioid use did not result in abnormal sensitivity to cold pain but altered pain modulation as detected by DNIC [74].
Opioid Regimen and OIH
Opioid regimen features, including type of opioid and dose, may influence the development of OIH. Anecdotal clinical observations have suggested that degree of OIH may vary according to opioid regimen [75]. Although the exact relationship between the dose regimen and the development of OIH remains to be determined, it is conceivable that OIH would be more likely to develop in patients receiving high opioid doses with a prolonged treatment course, although OIH has been demonstrated in patients receiving a short course of highly potent opioid analgesics [76]. Moreover, patients with a pathological pain condition (eg, neuropathic pain) treated with opioid therapy may be more susceptible to developing opioid-induced pain, because both pathological pain and OIH may share a common cellular mechanism [77].
If OIH develops following exposure to one opioid, can switching to a different opioid diminish OIH [78]?If cross-pain sensitivity does not develop between different opioids, switching to a different opioid would be justified, a similar rationale to that for opioid rotation to overcome opioid tolerance. This issue remains to be addressed.
OIH and Pre-emptive Analgesia
There is an ongoing debate about the clinical effectiveness of pre-emptive analgesia in pain management. However, use of opioid analgesic as the sole agent for pre-emptive analgesia may not be desirable for several reasons. First, a large dose of intra-operative opioids could activate a pro-nociceptive mechanism leading to the development of postoperative OIH [79]. This may confound the assessment of postoperative pain and counteract the opioid analgesic effect. Second, pre-emptive analgesia calls for pre-emptive inhibition of neuroplastic changes mediated through multiple cellular mechanisms such as the central glutamatergic system. Paradoxically, opioid administration could activate the central glutamatergic system as discussed above. Third, the neural mechanism of opioid tolerance and OIH may interact with that of pathological pain and pathological pain could be exacerbated following opioid administration [80,81]. This issue needs to be investigated in future studies.
Clinical Implications and Management of OIH
Until recently, a decreased opioid analgesic effect associated with opioid therapy was often recognized as the presence of pharmacologic opioid tolerance (ie, desensitization of the responsiveness of the opioid receptor and its cellular mechanism) and/or a worsening of the clinical pain condition. Therefore, opioid dose escalation appeared to be a logical approach to regain analgesic effectiveness. This practice should be reconsidered in light of the information on OIH. In the clinical setting, apparent opioid tolerance may result from pharmacological tolerance, worsening pain condition due to disease progression, and/or OIH. Below are some factors to consider in forming a differential diagnosis in the clinical setting [82].
First, the quality, location, and distribution pattern of the pain related to OIH would be different from a pre-existing pain condition. Because opioid analgesics are often administered systemically, changes in pain quality would be diffuse as compared with the pre-existing pain condition. Since the mechanism of OIH is similar to that of pathological pain, such as neuropathic pain, changes in pain threshold, tolerability, and distribution patterns seen in OIH would be similar to those seen in neuropathic pain patients. Quantitative sensory testing may be a useful tool to detect such changes.
Second, OIH would possibly exacerbate a pre-existing pain condition. Overall pain intensity (VAS) would be conceivably increased above the level of pre-existing pain in the absence of disease progression. Opioid dose escalation could only transiently and minimally reduce pain intensity in such a setting, with a subsequent increase in pain intensity due to OIH.
Third, when a diagnosis is uncertain, a trial of opioid dose escalation or tapering may be helpful to differentiate between tolerance and OIH. In an undertreated, worsening pain condition due to disease progress and/or pharma-cologic opioid tolerance, improved pain control may well be seen after a trial of opioid dose escalation. On the other hand, opioid dose escalation may exacerbate pain due to OIH, while a supervised opioid tapering may reduce OIH and improve clinical pain management. In this regard, if a patient is on a low opioid dose regimen and complains of unsatisfactory pain relief, a trial of opioid dose escalation may be appropriate; if a patient is already on a high dose of opioid analgesics, further dose escalation is rarely justified and may exacerbate OIH.
It is important to remember that the clinical outcome of opioid therapy is a dynamic balance among the opioid analgesic effect, OIH, and worsening pain due to disease progression. While any opioid dose escalation may transiently increase the analgesic effect, albeit by a small degree in many cases, the real issue is whether the same dose escalation may also exacerbate OIH, which could quickly overtake the transient increase in the opioid analgesic effect. Therefore, clinical judgment is fundamentally important and all clinical conditions related to opioid therapy need to be taken into consideration in the decision making process.
Summary
Interest in understanding OIH has grown over the last decade. Many discussions and reviews have centered around several key issues: (1) opioids not only produce analgesia through their anti-nociceptive effect, but also induce hyperalgesia via a pro-nociceptive effect; (2) opioid tolerance itself may be part of sensitization of a pro-nociceptive process; (3) the onset of OIH may be later than that of opioid tolerance and OIH may be a dose-related process, although OIH has been reported following acute and chronic opioid exposure at both high and low doses; (4) it is unclear whether a certain type of opioid and route of administration may be more likely to lead to clinical presentation of OIH; and (5) although opioid tolerance, OIH, and opioid withdrawal may share some common factors and mechanisms, the mechanism underlying each of these phenomena remains unclear [83].
While OIH has been well documented and investigated over nearly 2 decades, its exact clinical characteristics and underlying mechanisms have yet to be fully determined [84]. In addition, opioid tolerance should be differentiated from OIH, although both have a similar clinical presentation with regard to change in pain intensity [85]. Clinically, OIH should be considered when the adjustment of opioid dose is contemplated if prior opioid dose escalation fails to provide the expected analgesic effect and there is unexplainable pain exacerbation following an initial period of effective opioid analgesia. Although in some cases increasing opioid dose leads to some improvement in pain management, in other cases less opioid may be more effective in pain reduction. This goal may be accomplished by initiating a trial of opioid tapering, opioid rotation, adding adjunctive medications, or combining opioid with a clinically available NMDA receptor antagonist. Continuing opioid therapy with endless dose escalation in the absence of clinical evidence of improved pain management is neither scientifically sound nor clinically justified.
Corresponding author: Lucy L. Chen, MD, MGH Center for Pain Medicine, WACC #340, 15 Parkman St., Boston, MA 02114.
Financial disclosures: None.
1. Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: Roles of excitatory amino acid receptors and protein kinase C. J Neurosci 1994;14:2301–12.
2. Celerier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology 2000;92:465–72.
3. Laulin JP, Maurette P, Corcuff JB, et al. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg 2002;94:1263–9.
4. Lian B, Vera-Portocarrero L, King T, et al. Opioid-induced latent sensitization in a model of non-inflammatory viscerosomatic hypersensitivity. Brain Res 2010;1358:64–70.
5. Hay JL, Kaboutari J, White JM, et al. Model of methadone-induced hyperalgesia in rats and effect of memantine. Eur J Pharmacol 2010;626:229–33.
6. Wala EP, Holtman JR Jr. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol. 2011;651:89–95.
7. Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: Implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 2002;22:8312–23.
8. Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: Evidence for an opioid-induced neurotoxic mechanism. J Neurosci 2002;22:7650–61.
9. Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep 2006;10:67–70.
10. Woolf CJ. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res 1981;209:491–5.
11. Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: Clinical and theoretic implications. Anesthesiology 1986;64:590–7.
12. Vanderah TW, Ossipov MH, Lai J, et al. Mechanisms of opioid-induced pain and antinociceptive tolerance: Descending facilitation and spinal dynorphin. Pain 2001;92:5–9.
13. Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology 2008;109:308–17.
14. Ghelardini C, Galeotti N, Vivoli E, et al. Molecular interaction in the mouse PAG between NMDA and opioid receptors in morphine-induced acute thermal nociception. J Neurochem 2008;105:91–100.
15. Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci 2010;30:38–46.
16. Van Elstraete AC, Sitbon P, Mazoit JX, et al. Protective effect of prior administration of magnesium on delayed hyperalgesia induced by fentanyl in rats. Can J Anaesth 2006;53:1180–5.
17. Gu X, Wu X, Liu Y, et al. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain 2009;5:76.
18. Minville V, Fourcade O, Girolami JP, Tack I. Opioid-induced hyperalgesia in a mice model of orthopaedic pain: Preventive effect of ketamine. Br J Anaesth 2010;104:231–8.
19. Cui W, Li Y, Li S, et al. Systemic lidocaine inhibits remifentanil-induced hyperalgesia via the inhibition of cPKCgamma membrane translocation in spinal dorsal horn of rats. J Neurosurg Anesthesiol 2009;21:318–25.
20. Gupta LK, Gupta R, Tripathi CD. N-methyl-D-aspartate receptor modulators block hyperalgesia induced by acute low-dose morphine. Clin Exp Pharmacol Physiol 2011;38:592–7.
21. Bianchi E, Galeotti N, Menicacci C, Ghelardini C. Contribution of G inhibitory protein alpha subunits in paradoxical hyperalgesia elicited by exceedingly low doses of morphine in mice. Life Sci 2011;89:918–25.
22. Ruiz-Medina J, Ledent C, Valverde O. GPR3 orphan receptor is involved in neuropathic pain after peripheral nerve injury and regulates morphine-induced antinociception. Neuropharmacology 2011;61:43–50.
23. Bianchi E, Norcini M, Smrcka A, Ghelardini C. Supraspinal gbetagamma-dependent stimulation of PLCbeta originating from G inhibitory protein-mu opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. J Neurochem 2009;111:171–80.
24. Liang DY, Li X, Clark JD. 5-hydroxytryptamine type 3 receptor modulates opioid-induced hyperalgesia and tolerance in mice. Anesthesiology 2011;114:1180–9.
25. Rivat C, Vera-Portocarrero LP, Ibrahim MM, et al. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci 2009;29:727–37.
26. Vera-Portocarrero LP, Zhang ET, King T, et al. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain 2007;129:35–45.
27. Simonin F, Schmitt M, Laulin JP, et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci U S A 2006;103:466–71.
28. Celerier E, Gonzalez JR, Maldonado R, et al. Opioid-induced hyperalgesia in a murine model of postoperative pain: Role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology 2006;104:546–55.
29. Vardanyan A, Wang R, Vanderah TW, et al. TRPV1 receptor in expression of opioid-induced hyperalgesia. J Pain 2009;10:243–52.
30. Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci 2010;30:4460–6.
31. Esmaeili-Mahani S, Shimokawa N, Javan M, et al. Low-dose morphine induces hyperalgesia through activation of G alphas, protein kinase C, and L-type ca 2+ channels in rats. J Neurosci Res 2008;86:471–9.
32. Esmaeili-Mahani S, Fereidoni M, Javan M, et al. Nifedipine suppresses morphine-induced thermal hyperalgesia: Evidence for the role of corticosterone. Eur J Pharmacol 2007;567:95–101.
33. Van Elstraete AC, Sitbon P, Mazoit JX, Benhamou D. Gabapentin prevents delayed and long-lasting hyperalgesia induced by fentanyl in rats. Anesthesiology 2008;108:484–94.
34. Van Elstraete AC, Sitbon P, Benhamou D, Mazoit JX. The median effective dose of ketamine and gabapentin in opioid-induced hyperalgesia in rats: An isobolographic analysis of their interaction. Anesth Analg 2011;113:634–40.
35. Wei X, Wei W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J Anesth 2012;26:236–41.
36. Bessiere B, Richebe P, Laboureyras E, Laulin JP, Contarino A, Simonnet G. Nitrous oxide (N2O) prevents latent pain sensitization and long-term anxiety-like behavior in pain and opioid-experienced rats. Neuropharmacology 2007;53:733–40.
37. Hamlin AS, McNally GP, Osborne PB. Induction of c-fos and zif268 in the nociceptive amygdala parallel abstinence hyperalgesia in rats briefly exposed to morphine. Neuropharmacology 2007;53:330–43.
38. Doyle T, Bryant L, Muscoli C, et al. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett 2010;483:85–9.
39. Liang DY, Liao G, Wang J, et al. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology 2006;104:1054–62.
40. Liang DY, Liao G, Lighthall GK, Peltz G, Clark DJ. Genetic variants of the P-glycoprotein gene Abcb1b modulate opioid-induced hyperalgesia, tolerance and dependence. Pharmacogenet Genomics 2006;16:825–35.
41. Wilson NM, Jung H, Ripsch MS, et al. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun 2011;25:565–73.
42. Terashvili M, Wu HE, Schwasinger E, Tseng LF. Paradoxical hyperalgesia induced by mu-opioid receptor agonist endomorphin-2, but not endomorphin-1, microinjected into the centromedial amygdala of the rat. Eur J Pharmacol 2007;554:137–44.
43. Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience 2007;147:439–44.
44. Waxman AR, Arout C, Caldwell M, et al. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett 2009;462:68–72.
45. Juni A, Cai M, Stankova M, et al. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology 2010;112:181–8.
46. Juni A, Klein G, Kowalczyk B, et al. Sex differences in hyperalgesia during morphine infusion: Effect of gonadectomy and estrogen treatment. Neuropharmacology 2008;54:1264–70.
47. Forero M, Chan PS, Restrepo-Garces CE. Successful reversal of hyperalgesia/myoclonus complex with low-dose ketamine infusion. Pain Pract 2012;12:154–8.
48. Vorobeychik Y, Chen L, Bush MC, Mao J. Improved opioid analgesic effect following opioid dose reduction. Pain Med 2008;9:724–7.
49. Cortinas Saenz M, Geronimo Pardo M, Cortinas Saenz ML, et al. Acute opiate tolerance and postoperative hyperalgesia after a brief infusion of remifentanil managed with multimodal analgesia. Rev Esp Anestesiol Reanim 2008;55:40–2.
50. Siniscalchi A, Piraccini E, Miklosova Z, et al. Opioid-induced hyperalgesia and rapid opioid detoxification after tacrolimus administration. Anesth Analg 2008;106:645–6.
51. Okon TR, George ML. Fentanyl-induced neurotoxicity and paradoxic pain. J Pain Symptom Manage 2008;35:327–33.
52. Axelrod DJ, Reville B. Using methadone to treat opioid-induced hyperalgesia and refractory pain. J Opioid Manag 2007;3:113–4.
53. Singler B, Troster A, Manering N, et al. Modulation of remifentanil-induced postinfusion hyperalgesia by propofol. Anesth Analg 2007;104:1397–403.
54. Hallett BR, Chalkiadis GA. Suspected opioid-induced hyperalgesia in an infant. Br J Anaesth 2012;108:116–8.
55. Ma JF, Huang ZL, Li J, et al. Cohort study of remifentanil-induced hyperalgesia in postoperative patients. Zhonghua Yi Xue Za Zhi 2011;91:977–9.
56. Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend 2006;82:218–23.
57. Fishbain DA, Lewis JE, Gao J. Are psychoactive substance (opioid)-dependent chronic pain patients hyperalgesic? Pain Pract 2011;11:337–43.
58. Hay JL, White JM, Bochner F, et al. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain 2009;10:316–22.
59. Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J Pain 2006;7:43–8.
60. Chu LF, Dairmont J, Zamora AK, et al. The endogenous opioid system is not involved in modulation of opioid-induced hyperalgesia. J Pain 2011;12:108–15.
61. McDonnell C, Zaarour C, Hull R, et al. Pre-treatment with morphine does not prevent the development of remifentanil-induced hyperalgesia. Can J Anaesth 2008;55:813–8.
62. Echevarria G, Elgueta F, Fierro C, et al. Nitrous oxide (N(2)O) reduces postoperative opioid-induced hyperalgesia after remifentanil-propofol anaesthesia in humans. Br J Anaesth 2011;107:959–65.
63. Troster A, Sittl R, Singler B, et al. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology 2006;105:1016–23.
64. Tuncer S, Yalcin N, Reisli R, Alper Y. The effects of lornoxicam in preventing remifentanil-induced postoperative hyperalgesia. Agri 2009;2:161–7.
65. Lee C, Song YK, Jeong HM, Park SN. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J Anesthesiol 2011;61:244–50.
66. Song JW, Lee YW, Yoon KB, et al. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg 2011;113:390–7.
67. Lee C, Song YK, Lee JH, Ha SM. The effects of intraoperative adenosine infusion on acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil in adult patients undergoing tonsillectomy. Korean J Pain 2011;24:7–12.
68. Hong BH, Lee WY, Kim YH, et al. Effects of intraoperative low dose ketamine on remifentanil-induced hyperalgesia in gynecologic surgery with sevoflurane anesthesia. Korean J Anesthesiol 2011;61:238–43.
69. Xuerong Y, Yuguang H, Xia J, Hailan W. Ketamine and lornoxicam for preventing a fentanyl-induced increase in postoperative morphine requirement. Anesth Analg 2008;107:2032–7.
70. Schlimp CJ, Pipam W, Wolrab C, Ohner C, Kager HI, Likar R. Clonidine for remifentanil-induced hyperalgesia: A double-blind randomized, placebo-controlled study of clonidine under intra-operative use of remifentanil in elective surgery of the shoulder. Schmerz 2011;25:290–5.
71. Belgrade M, Hall S. Dexmedetomidine infusion for the management of opioid-induced hyperalgesia. Pain Med 2010;11:1819–26.
72. Chen L, Malarick C, Seefeld L, et al. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain 2009;143:65–70.
73. Bannister K, Dickenson AH. Opioid hyperalgesia. Curr Opin Support Palliat Care 2010;4:1–5.
74. Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain 2008;139:431–8.
75. Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: Effect of long-acting maintenance agent. Drug Alcohol Depend 2001;63:139–46.
76. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg 1998;86:1307–11.
77. Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain 1995;62:259–74.
78. Sjogren P, Jensen NH, Jensen TS. Disappearance of morphine-induced hyperalgesia after discontinuing or substituting morphine with other opioid agonists. Pain 1994;59:313–6.
79. Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: Intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology 2000;93:409–17.
80. Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 2006;104:570–87.
81. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag 2006;2:277–82
82. Mao J. Opioid-induced abnormal pain sensitivity: Implications in clinical opioid therapy. Pain 2002;100:213–7.
83. Low Y, Clarke CF, Huh BK. Opioid-induced hyperalgesia: A review of epidemiology, mechanisms and management. Singapore Med J 2012;53:357–60.
84. Fishbain DA, Cole B, Lewis JE, et al. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med 2009:829–39.
85. Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain 2012;153:1583–92.
From the Massachusetts General Hospital Center for Pain Medicine, Boston, MA.
Abstract
- Objective: To review evidence from clinical and preclinical studies related to the phenomenon of opioid-induced hyperalgesia (OIH) and discuss issues relevant to clinical diagnosis and management.
- Methods: Literature review.
- Results: OIH is defined as a state of nociceptive sensitization caused by exposure to opioids such that a patient receiving opioids to treat pain could become more sensitive to painful stimuli. Interest in understanding OIH has grown over years and multiple mechanisms have been proposed. Both OIH and opioid tolerance can reduce opioid analgesic efficacy, complicating clinical management of chronic pain. When a diagnosis is uncertain, a trial of opioid dose escalation or tapering may be helpful in differentiating between tolerance and OIH. It is unclear whether certain types of opioids or routes of administration are more likely to lead to OIH.
- Conclusion: Clinical outcome of opioid therapy is a dynamic balance among the opioid analgesic effect, OIH, and worsening pain due to disease progression. While OIH has been well documented over nearly 2 decades, its exact clinical characteristics and underlying mechanisms have yet to be fully determined.
Opioids, which produce analgesia through a primarily inhibitory effect on the nociceptive system, have been used for decades for the clinical management of moderate to severe pain. Opioid analgesics act on 3 major classes of opioid receptors, including the µ, k, δ (mu, kappa, and delta) receptors. Activation of opioid receptors not only produces analgesia but also other effects, such as euphoria, respiratory depression, decreased gastrointestinal motility, and cardiovascular effects. Exposure to opioids, however, can also lead to the development of opioid tolerance and opioid-induced hyperalgesia (OIH). Both opioid tolerance and OIH can decrease opioid analgesic efficacy, making chronic pain management a challenge. OIH is a state of nociceptive sensitization caused by exposure to opioids, such that a patient receiving opioids for the treatment of pain could actually become more sensitive to painful stimulation, resulting in a paradoxical adverse response to opioid therapy. In this article, we will review evidence from preclinical and clinical studies and discuss issues relevant to clinical diagnosis and management of OIH.
Evidence of OIH in Animal Studies
In early 1990s, an original preclinical study showed that there was a progressive reduction in baseline nociceptive threshold by using a foot withdraw test in rats receiving repeated intrathecal morphine administration (10-20 mg) over a 7-day period [1]. A number of animal studies later also provided similar data. A reduced baseline nociceptive threshold was observed in animals receiving subcutaneous fentanyl boluses using the Randall-Sellitto test, in which a constantly increasing pressure was applied to a rat’s hind paw. The decreased baseline nociceptive threshold lasted 5 days after cessation of 4 fentanyl bolus injections [2]. In another study, a reduced baseline nociceptive threshold was detected in animals with repeated heroin administration [3]. In other studies, rats exposed to morphine also developed a latent sensitization of visceral pain with a shift of the morphine dose-response curve to the right [4]; exposure to methadone also induced hyperalgesia in rats, which was not prevented by a weak NMDA receptor antagonist (memantine) [5]; and a partial µ-receptor agonist buprenophine produced a dose-related OIH as well [6].
These results indicate that a progressive and lasting reduction of baseline nociceptive threshold, which was referred to as OIH, can result from repeated opioid administration [7–9]. However, different from previous preclinical observations in which a large dose of intrathecal morphine was given, these studies resulted in hyperalgesic response [10,11] in a clinically relevant opioid dose. Of interest is that OIH was observed in animals even when there was continuous opioid infusion via an implanted osmotic pump, suggesting the involvement of active cellular mechanisms in the process [12]. Therefore, prolonged opioid treatment results in not only loss of the opioid analgesic effect (anti-nociceptive effect or desensitization) but also activation of a hyperalgesic effect (a pro-nociceptive effect with reduced nociceptive threshold or increased sensitization). Although both opioid tolerance and OIH are initiated by opioid administration, two opposing cellular mechanisms (ie, desensitization versus sensitization) may be involved in the process. Subsequently, many studies explored the neural and cellular mechanisms underlying the development of OIH and their interaction with the mechanism of opioid tolerance.
Proposed Cellular Mechanisms of OIH
A significant number of recent studies have explored the neurobiological basis of OIH, revealing a divergent range of cellular elements contributory to OIH. These mechanisms include (1) N-methyl-D-aspartate (NMDA) receptor and related intracellular pathways; (2) involvement of G-protein coupled receptors including 5-HT receptors and neurokinin-1 receptors; (3) nitrix oxide and nitric oxide sunthase; (4) TRPV1 receptors; (5) calcium channels; and (6) miscellaneous mechanisms including sex differences [7–9,13–41].
In summary, an increasing number of preclinical studies in the area of OIH indicates that there is enormous interest in understanding the cellular mechanisms of OIH, and the current evidence points to a progressive sensitization process within the central nervous system that involves a constellation of cellular elements such as NMDA receptors, similar to those contributory to the mechanisms of pathological pain.
Evidence of OIH in Human Studies
In animal studies, changes in baseline nociceptive thresholds can be measured in a controlled setting. It is, however, difficult to assess changes in pain threshold in clinical environment following opioid administration [9]. It is often a challenge to distinguish opioid pharmacologic tolerance from OIH because the outcome of opioid therapy is based primarily on subjective pain scores. In the face of these challenges, an increasing number of clinical anecdotal case reports and studies suggest that OIH is likely to be a significant factor in clinical opioid therapy [47–54].
In a study of 1620 patients in which remifentanil was used for general anesthesia, the incidence of postoperative remifentanil-induced hyperalgesia was 16.1%. This study found that age younger than 16 years, male sex, operation duration longer than 2 hours, and remifentanil dose greater than 30 mg/kga were associated with higher rates of OIH [55]. On the other hand, heroin or other opioid addicts not only demonstrated OIH but also had prolonged symptoms of OIH after detoxification from opioids for at least 1 month [56]. In chronic pain patients without opioid dependence, significantly lower pain threshold and tolerance as assessed by pressure pain stimulation were detected [57]. It appears that the sensitivity of detecting OIH in the clinical setting may be influenced by the modality of sensory stimulation [58].
In a prospective preliminary study of 6 patients with chronic low back pain, hyperalgesic response was detected after 1 month of oral morphine therapy using a cold pressor test but not a heat pain test [59]. In another prospective randomized, placebo-controlled, 2-way crossover study in healthy human volunteers, the development of OIH was quantified as changes in the average radius of the area of secondary hyperalgesia generated by electrical pain stimulation. A 23.6% increase in the area of secondary hyperalgesia over baseline was detected following the remifentanil infusion. The same study showed that endogenous opioids did not seem to have an effect on OIH because a single bolus of naloxone did not change the size of secondary hyperalgesia [60].
OIH Prevention Studies
Currently, efforts have also been made to see whether OIH can be prevented with different approaches in human subjects. The following is a brief summary of these studies. In a study of adolescents undergoing scoliosis surgery, treatment with morphine (150 mg/kg) prior to commencing remifentanil infusion did not prevent the development of remifentanil-induced hyperalgesia [61]. In another study, propofol infusion alone with remifentanil both delayed and attenuated remifentanil-induced hyperalgesia [53]. In yet another study, intraoperative 70% N2O administration appeared to reduce postoperative OIH following an intraoperative remifentanil-propofol anesthesia regimen [62].
In a study of 15 healthy male volunteers, preventive administration of parecoxib significantly diminished OIH after withdrawal from remifentanil. In contrast, parecoxib given together with remifentanil did not prevent OIH, suggesting that pre-treatment, not parallel treatment, with opioid may be required to prevent OIH [63]. Other NSAIDs administered preemptively also appear to prevent remifentanil-induced hyperalgesia [64].
Another study investigated the effect of intra-operative magnesium sulfate administration in patients undergoing robot-assisted laparoscopic prostatectomy. Magnesium sulfate administration reduced postoperative opioid consumption and OIH in subjects receiving intra-operative remifentanil-based anesthesia [65,66]. Intra-operative adenosine infusion also prevented acute opioid tolerance and remifentanil-induced hyperalgesia [67]. Continuous intra-operative infusion of ketamine, an NMDA receptor antagonist, significant lowered postoperative VAS and morphine use in gynecologic surgery patients [68]. Also, in a randomized, double-blind, placebo-controlled study of 90 patients who underwent total abdominal hysterectomy, cumulative morphine consumption was significantly greater in subjects with fentanyl alone than those with saline alone, ketamine alone, ketamine with fentanyl, or fentanyl with lornoxicam at 3, 6, and 12 hours postoperatively [69].
Finally, in a double-blind, randomized, placebo-controlled study of 40 patients undergoing elective shoulder surgery, clonidine was given intra-operatively in a remifentanil/propofol-based anesthesia. The results showed that clonidine did not reduce postoperative morphine consumption and pain score in these patients [70]. However, dexmedetomidine, another α2 receptor agonist, substantially reduced baseline opioid doses in hospitalized patients with OIH [71].
Quantitative Sensory Testing and OIH
Currently, diagnostic tools for OIH are still being developed. Many clinical studies have used quantitative sensory testing (QST) as a tool to assess OIH [72,73]. In a recent study, QST was used to compare pain threshold, pain tolerance, and the degree of temporal summation of pain in response to thermal stimulation among 3 groups of subjects: Group 1 (no pain and no opioid), Group 2 (chronic pain but no opioid therapy), and Group 3 (both chronic pain and opioid therapy). Group 3 subjects displayed a decreased heat pain threshold and exacerbated temporal summation of pain to thermal stimulation as compared with both group 1 and group 2 subjects. There were no differences in cold or warm sensation among all 3 groups. Among clinical factors, daily opioid dose consistently correlated with the decreased heat pain threshold and exacerbated temporal summation of second pain in group 3 subjects [72]. Another study investigated the sensitivity to cold pain and the magnitude of diffuse noxious inhibitory control (DNIC) using QST in subjects with or without opioid therapy. Pain threshold, intensity and tolerance in response to the cold pressor (1°C) were measured. They found that oral opioid use did not result in abnormal sensitivity to cold pain but altered pain modulation as detected by DNIC [74].
Opioid Regimen and OIH
Opioid regimen features, including type of opioid and dose, may influence the development of OIH. Anecdotal clinical observations have suggested that degree of OIH may vary according to opioid regimen [75]. Although the exact relationship between the dose regimen and the development of OIH remains to be determined, it is conceivable that OIH would be more likely to develop in patients receiving high opioid doses with a prolonged treatment course, although OIH has been demonstrated in patients receiving a short course of highly potent opioid analgesics [76]. Moreover, patients with a pathological pain condition (eg, neuropathic pain) treated with opioid therapy may be more susceptible to developing opioid-induced pain, because both pathological pain and OIH may share a common cellular mechanism [77].
If OIH develops following exposure to one opioid, can switching to a different opioid diminish OIH [78]?If cross-pain sensitivity does not develop between different opioids, switching to a different opioid would be justified, a similar rationale to that for opioid rotation to overcome opioid tolerance. This issue remains to be addressed.
OIH and Pre-emptive Analgesia
There is an ongoing debate about the clinical effectiveness of pre-emptive analgesia in pain management. However, use of opioid analgesic as the sole agent for pre-emptive analgesia may not be desirable for several reasons. First, a large dose of intra-operative opioids could activate a pro-nociceptive mechanism leading to the development of postoperative OIH [79]. This may confound the assessment of postoperative pain and counteract the opioid analgesic effect. Second, pre-emptive analgesia calls for pre-emptive inhibition of neuroplastic changes mediated through multiple cellular mechanisms such as the central glutamatergic system. Paradoxically, opioid administration could activate the central glutamatergic system as discussed above. Third, the neural mechanism of opioid tolerance and OIH may interact with that of pathological pain and pathological pain could be exacerbated following opioid administration [80,81]. This issue needs to be investigated in future studies.
Clinical Implications and Management of OIH
Until recently, a decreased opioid analgesic effect associated with opioid therapy was often recognized as the presence of pharmacologic opioid tolerance (ie, desensitization of the responsiveness of the opioid receptor and its cellular mechanism) and/or a worsening of the clinical pain condition. Therefore, opioid dose escalation appeared to be a logical approach to regain analgesic effectiveness. This practice should be reconsidered in light of the information on OIH. In the clinical setting, apparent opioid tolerance may result from pharmacological tolerance, worsening pain condition due to disease progression, and/or OIH. Below are some factors to consider in forming a differential diagnosis in the clinical setting [82].
First, the quality, location, and distribution pattern of the pain related to OIH would be different from a pre-existing pain condition. Because opioid analgesics are often administered systemically, changes in pain quality would be diffuse as compared with the pre-existing pain condition. Since the mechanism of OIH is similar to that of pathological pain, such as neuropathic pain, changes in pain threshold, tolerability, and distribution patterns seen in OIH would be similar to those seen in neuropathic pain patients. Quantitative sensory testing may be a useful tool to detect such changes.
Second, OIH would possibly exacerbate a pre-existing pain condition. Overall pain intensity (VAS) would be conceivably increased above the level of pre-existing pain in the absence of disease progression. Opioid dose escalation could only transiently and minimally reduce pain intensity in such a setting, with a subsequent increase in pain intensity due to OIH.
Third, when a diagnosis is uncertain, a trial of opioid dose escalation or tapering may be helpful to differentiate between tolerance and OIH. In an undertreated, worsening pain condition due to disease progress and/or pharma-cologic opioid tolerance, improved pain control may well be seen after a trial of opioid dose escalation. On the other hand, opioid dose escalation may exacerbate pain due to OIH, while a supervised opioid tapering may reduce OIH and improve clinical pain management. In this regard, if a patient is on a low opioid dose regimen and complains of unsatisfactory pain relief, a trial of opioid dose escalation may be appropriate; if a patient is already on a high dose of opioid analgesics, further dose escalation is rarely justified and may exacerbate OIH.
It is important to remember that the clinical outcome of opioid therapy is a dynamic balance among the opioid analgesic effect, OIH, and worsening pain due to disease progression. While any opioid dose escalation may transiently increase the analgesic effect, albeit by a small degree in many cases, the real issue is whether the same dose escalation may also exacerbate OIH, which could quickly overtake the transient increase in the opioid analgesic effect. Therefore, clinical judgment is fundamentally important and all clinical conditions related to opioid therapy need to be taken into consideration in the decision making process.
Summary
Interest in understanding OIH has grown over the last decade. Many discussions and reviews have centered around several key issues: (1) opioids not only produce analgesia through their anti-nociceptive effect, but also induce hyperalgesia via a pro-nociceptive effect; (2) opioid tolerance itself may be part of sensitization of a pro-nociceptive process; (3) the onset of OIH may be later than that of opioid tolerance and OIH may be a dose-related process, although OIH has been reported following acute and chronic opioid exposure at both high and low doses; (4) it is unclear whether a certain type of opioid and route of administration may be more likely to lead to clinical presentation of OIH; and (5) although opioid tolerance, OIH, and opioid withdrawal may share some common factors and mechanisms, the mechanism underlying each of these phenomena remains unclear [83].
While OIH has been well documented and investigated over nearly 2 decades, its exact clinical characteristics and underlying mechanisms have yet to be fully determined [84]. In addition, opioid tolerance should be differentiated from OIH, although both have a similar clinical presentation with regard to change in pain intensity [85]. Clinically, OIH should be considered when the adjustment of opioid dose is contemplated if prior opioid dose escalation fails to provide the expected analgesic effect and there is unexplainable pain exacerbation following an initial period of effective opioid analgesia. Although in some cases increasing opioid dose leads to some improvement in pain management, in other cases less opioid may be more effective in pain reduction. This goal may be accomplished by initiating a trial of opioid tapering, opioid rotation, adding adjunctive medications, or combining opioid with a clinically available NMDA receptor antagonist. Continuing opioid therapy with endless dose escalation in the absence of clinical evidence of improved pain management is neither scientifically sound nor clinically justified.
Corresponding author: Lucy L. Chen, MD, MGH Center for Pain Medicine, WACC #340, 15 Parkman St., Boston, MA 02114.
Financial disclosures: None.
From the Massachusetts General Hospital Center for Pain Medicine, Boston, MA.
Abstract
- Objective: To review evidence from clinical and preclinical studies related to the phenomenon of opioid-induced hyperalgesia (OIH) and discuss issues relevant to clinical diagnosis and management.
- Methods: Literature review.
- Results: OIH is defined as a state of nociceptive sensitization caused by exposure to opioids such that a patient receiving opioids to treat pain could become more sensitive to painful stimuli. Interest in understanding OIH has grown over years and multiple mechanisms have been proposed. Both OIH and opioid tolerance can reduce opioid analgesic efficacy, complicating clinical management of chronic pain. When a diagnosis is uncertain, a trial of opioid dose escalation or tapering may be helpful in differentiating between tolerance and OIH. It is unclear whether certain types of opioids or routes of administration are more likely to lead to OIH.
- Conclusion: Clinical outcome of opioid therapy is a dynamic balance among the opioid analgesic effect, OIH, and worsening pain due to disease progression. While OIH has been well documented over nearly 2 decades, its exact clinical characteristics and underlying mechanisms have yet to be fully determined.
Opioids, which produce analgesia through a primarily inhibitory effect on the nociceptive system, have been used for decades for the clinical management of moderate to severe pain. Opioid analgesics act on 3 major classes of opioid receptors, including the µ, k, δ (mu, kappa, and delta) receptors. Activation of opioid receptors not only produces analgesia but also other effects, such as euphoria, respiratory depression, decreased gastrointestinal motility, and cardiovascular effects. Exposure to opioids, however, can also lead to the development of opioid tolerance and opioid-induced hyperalgesia (OIH). Both opioid tolerance and OIH can decrease opioid analgesic efficacy, making chronic pain management a challenge. OIH is a state of nociceptive sensitization caused by exposure to opioids, such that a patient receiving opioids for the treatment of pain could actually become more sensitive to painful stimulation, resulting in a paradoxical adverse response to opioid therapy. In this article, we will review evidence from preclinical and clinical studies and discuss issues relevant to clinical diagnosis and management of OIH.
Evidence of OIH in Animal Studies
In early 1990s, an original preclinical study showed that there was a progressive reduction in baseline nociceptive threshold by using a foot withdraw test in rats receiving repeated intrathecal morphine administration (10-20 mg) over a 7-day period [1]. A number of animal studies later also provided similar data. A reduced baseline nociceptive threshold was observed in animals receiving subcutaneous fentanyl boluses using the Randall-Sellitto test, in which a constantly increasing pressure was applied to a rat’s hind paw. The decreased baseline nociceptive threshold lasted 5 days after cessation of 4 fentanyl bolus injections [2]. In another study, a reduced baseline nociceptive threshold was detected in animals with repeated heroin administration [3]. In other studies, rats exposed to morphine also developed a latent sensitization of visceral pain with a shift of the morphine dose-response curve to the right [4]; exposure to methadone also induced hyperalgesia in rats, which was not prevented by a weak NMDA receptor antagonist (memantine) [5]; and a partial µ-receptor agonist buprenophine produced a dose-related OIH as well [6].
These results indicate that a progressive and lasting reduction of baseline nociceptive threshold, which was referred to as OIH, can result from repeated opioid administration [7–9]. However, different from previous preclinical observations in which a large dose of intrathecal morphine was given, these studies resulted in hyperalgesic response [10,11] in a clinically relevant opioid dose. Of interest is that OIH was observed in animals even when there was continuous opioid infusion via an implanted osmotic pump, suggesting the involvement of active cellular mechanisms in the process [12]. Therefore, prolonged opioid treatment results in not only loss of the opioid analgesic effect (anti-nociceptive effect or desensitization) but also activation of a hyperalgesic effect (a pro-nociceptive effect with reduced nociceptive threshold or increased sensitization). Although both opioid tolerance and OIH are initiated by opioid administration, two opposing cellular mechanisms (ie, desensitization versus sensitization) may be involved in the process. Subsequently, many studies explored the neural and cellular mechanisms underlying the development of OIH and their interaction with the mechanism of opioid tolerance.
Proposed Cellular Mechanisms of OIH
A significant number of recent studies have explored the neurobiological basis of OIH, revealing a divergent range of cellular elements contributory to OIH. These mechanisms include (1) N-methyl-D-aspartate (NMDA) receptor and related intracellular pathways; (2) involvement of G-protein coupled receptors including 5-HT receptors and neurokinin-1 receptors; (3) nitrix oxide and nitric oxide sunthase; (4) TRPV1 receptors; (5) calcium channels; and (6) miscellaneous mechanisms including sex differences [7–9,13–41].
In summary, an increasing number of preclinical studies in the area of OIH indicates that there is enormous interest in understanding the cellular mechanisms of OIH, and the current evidence points to a progressive sensitization process within the central nervous system that involves a constellation of cellular elements such as NMDA receptors, similar to those contributory to the mechanisms of pathological pain.
Evidence of OIH in Human Studies
In animal studies, changes in baseline nociceptive thresholds can be measured in a controlled setting. It is, however, difficult to assess changes in pain threshold in clinical environment following opioid administration [9]. It is often a challenge to distinguish opioid pharmacologic tolerance from OIH because the outcome of opioid therapy is based primarily on subjective pain scores. In the face of these challenges, an increasing number of clinical anecdotal case reports and studies suggest that OIH is likely to be a significant factor in clinical opioid therapy [47–54].
In a study of 1620 patients in which remifentanil was used for general anesthesia, the incidence of postoperative remifentanil-induced hyperalgesia was 16.1%. This study found that age younger than 16 years, male sex, operation duration longer than 2 hours, and remifentanil dose greater than 30 mg/kga were associated with higher rates of OIH [55]. On the other hand, heroin or other opioid addicts not only demonstrated OIH but also had prolonged symptoms of OIH after detoxification from opioids for at least 1 month [56]. In chronic pain patients without opioid dependence, significantly lower pain threshold and tolerance as assessed by pressure pain stimulation were detected [57]. It appears that the sensitivity of detecting OIH in the clinical setting may be influenced by the modality of sensory stimulation [58].
In a prospective preliminary study of 6 patients with chronic low back pain, hyperalgesic response was detected after 1 month of oral morphine therapy using a cold pressor test but not a heat pain test [59]. In another prospective randomized, placebo-controlled, 2-way crossover study in healthy human volunteers, the development of OIH was quantified as changes in the average radius of the area of secondary hyperalgesia generated by electrical pain stimulation. A 23.6% increase in the area of secondary hyperalgesia over baseline was detected following the remifentanil infusion. The same study showed that endogenous opioids did not seem to have an effect on OIH because a single bolus of naloxone did not change the size of secondary hyperalgesia [60].
OIH Prevention Studies
Currently, efforts have also been made to see whether OIH can be prevented with different approaches in human subjects. The following is a brief summary of these studies. In a study of adolescents undergoing scoliosis surgery, treatment with morphine (150 mg/kg) prior to commencing remifentanil infusion did not prevent the development of remifentanil-induced hyperalgesia [61]. In another study, propofol infusion alone with remifentanil both delayed and attenuated remifentanil-induced hyperalgesia [53]. In yet another study, intraoperative 70% N2O administration appeared to reduce postoperative OIH following an intraoperative remifentanil-propofol anesthesia regimen [62].
In a study of 15 healthy male volunteers, preventive administration of parecoxib significantly diminished OIH after withdrawal from remifentanil. In contrast, parecoxib given together with remifentanil did not prevent OIH, suggesting that pre-treatment, not parallel treatment, with opioid may be required to prevent OIH [63]. Other NSAIDs administered preemptively also appear to prevent remifentanil-induced hyperalgesia [64].
Another study investigated the effect of intra-operative magnesium sulfate administration in patients undergoing robot-assisted laparoscopic prostatectomy. Magnesium sulfate administration reduced postoperative opioid consumption and OIH in subjects receiving intra-operative remifentanil-based anesthesia [65,66]. Intra-operative adenosine infusion also prevented acute opioid tolerance and remifentanil-induced hyperalgesia [67]. Continuous intra-operative infusion of ketamine, an NMDA receptor antagonist, significant lowered postoperative VAS and morphine use in gynecologic surgery patients [68]. Also, in a randomized, double-blind, placebo-controlled study of 90 patients who underwent total abdominal hysterectomy, cumulative morphine consumption was significantly greater in subjects with fentanyl alone than those with saline alone, ketamine alone, ketamine with fentanyl, or fentanyl with lornoxicam at 3, 6, and 12 hours postoperatively [69].
Finally, in a double-blind, randomized, placebo-controlled study of 40 patients undergoing elective shoulder surgery, clonidine was given intra-operatively in a remifentanil/propofol-based anesthesia. The results showed that clonidine did not reduce postoperative morphine consumption and pain score in these patients [70]. However, dexmedetomidine, another α2 receptor agonist, substantially reduced baseline opioid doses in hospitalized patients with OIH [71].
Quantitative Sensory Testing and OIH
Currently, diagnostic tools for OIH are still being developed. Many clinical studies have used quantitative sensory testing (QST) as a tool to assess OIH [72,73]. In a recent study, QST was used to compare pain threshold, pain tolerance, and the degree of temporal summation of pain in response to thermal stimulation among 3 groups of subjects: Group 1 (no pain and no opioid), Group 2 (chronic pain but no opioid therapy), and Group 3 (both chronic pain and opioid therapy). Group 3 subjects displayed a decreased heat pain threshold and exacerbated temporal summation of pain to thermal stimulation as compared with both group 1 and group 2 subjects. There were no differences in cold or warm sensation among all 3 groups. Among clinical factors, daily opioid dose consistently correlated with the decreased heat pain threshold and exacerbated temporal summation of second pain in group 3 subjects [72]. Another study investigated the sensitivity to cold pain and the magnitude of diffuse noxious inhibitory control (DNIC) using QST in subjects with or without opioid therapy. Pain threshold, intensity and tolerance in response to the cold pressor (1°C) were measured. They found that oral opioid use did not result in abnormal sensitivity to cold pain but altered pain modulation as detected by DNIC [74].
Opioid Regimen and OIH
Opioid regimen features, including type of opioid and dose, may influence the development of OIH. Anecdotal clinical observations have suggested that degree of OIH may vary according to opioid regimen [75]. Although the exact relationship between the dose regimen and the development of OIH remains to be determined, it is conceivable that OIH would be more likely to develop in patients receiving high opioid doses with a prolonged treatment course, although OIH has been demonstrated in patients receiving a short course of highly potent opioid analgesics [76]. Moreover, patients with a pathological pain condition (eg, neuropathic pain) treated with opioid therapy may be more susceptible to developing opioid-induced pain, because both pathological pain and OIH may share a common cellular mechanism [77].
If OIH develops following exposure to one opioid, can switching to a different opioid diminish OIH [78]?If cross-pain sensitivity does not develop between different opioids, switching to a different opioid would be justified, a similar rationale to that for opioid rotation to overcome opioid tolerance. This issue remains to be addressed.
OIH and Pre-emptive Analgesia
There is an ongoing debate about the clinical effectiveness of pre-emptive analgesia in pain management. However, use of opioid analgesic as the sole agent for pre-emptive analgesia may not be desirable for several reasons. First, a large dose of intra-operative opioids could activate a pro-nociceptive mechanism leading to the development of postoperative OIH [79]. This may confound the assessment of postoperative pain and counteract the opioid analgesic effect. Second, pre-emptive analgesia calls for pre-emptive inhibition of neuroplastic changes mediated through multiple cellular mechanisms such as the central glutamatergic system. Paradoxically, opioid administration could activate the central glutamatergic system as discussed above. Third, the neural mechanism of opioid tolerance and OIH may interact with that of pathological pain and pathological pain could be exacerbated following opioid administration [80,81]. This issue needs to be investigated in future studies.
Clinical Implications and Management of OIH
Until recently, a decreased opioid analgesic effect associated with opioid therapy was often recognized as the presence of pharmacologic opioid tolerance (ie, desensitization of the responsiveness of the opioid receptor and its cellular mechanism) and/or a worsening of the clinical pain condition. Therefore, opioid dose escalation appeared to be a logical approach to regain analgesic effectiveness. This practice should be reconsidered in light of the information on OIH. In the clinical setting, apparent opioid tolerance may result from pharmacological tolerance, worsening pain condition due to disease progression, and/or OIH. Below are some factors to consider in forming a differential diagnosis in the clinical setting [82].
First, the quality, location, and distribution pattern of the pain related to OIH would be different from a pre-existing pain condition. Because opioid analgesics are often administered systemically, changes in pain quality would be diffuse as compared with the pre-existing pain condition. Since the mechanism of OIH is similar to that of pathological pain, such as neuropathic pain, changes in pain threshold, tolerability, and distribution patterns seen in OIH would be similar to those seen in neuropathic pain patients. Quantitative sensory testing may be a useful tool to detect such changes.
Second, OIH would possibly exacerbate a pre-existing pain condition. Overall pain intensity (VAS) would be conceivably increased above the level of pre-existing pain in the absence of disease progression. Opioid dose escalation could only transiently and minimally reduce pain intensity in such a setting, with a subsequent increase in pain intensity due to OIH.
Third, when a diagnosis is uncertain, a trial of opioid dose escalation or tapering may be helpful to differentiate between tolerance and OIH. In an undertreated, worsening pain condition due to disease progress and/or pharma-cologic opioid tolerance, improved pain control may well be seen after a trial of opioid dose escalation. On the other hand, opioid dose escalation may exacerbate pain due to OIH, while a supervised opioid tapering may reduce OIH and improve clinical pain management. In this regard, if a patient is on a low opioid dose regimen and complains of unsatisfactory pain relief, a trial of opioid dose escalation may be appropriate; if a patient is already on a high dose of opioid analgesics, further dose escalation is rarely justified and may exacerbate OIH.
It is important to remember that the clinical outcome of opioid therapy is a dynamic balance among the opioid analgesic effect, OIH, and worsening pain due to disease progression. While any opioid dose escalation may transiently increase the analgesic effect, albeit by a small degree in many cases, the real issue is whether the same dose escalation may also exacerbate OIH, which could quickly overtake the transient increase in the opioid analgesic effect. Therefore, clinical judgment is fundamentally important and all clinical conditions related to opioid therapy need to be taken into consideration in the decision making process.
Summary
Interest in understanding OIH has grown over the last decade. Many discussions and reviews have centered around several key issues: (1) opioids not only produce analgesia through their anti-nociceptive effect, but also induce hyperalgesia via a pro-nociceptive effect; (2) opioid tolerance itself may be part of sensitization of a pro-nociceptive process; (3) the onset of OIH may be later than that of opioid tolerance and OIH may be a dose-related process, although OIH has been reported following acute and chronic opioid exposure at both high and low doses; (4) it is unclear whether a certain type of opioid and route of administration may be more likely to lead to clinical presentation of OIH; and (5) although opioid tolerance, OIH, and opioid withdrawal may share some common factors and mechanisms, the mechanism underlying each of these phenomena remains unclear [83].
While OIH has been well documented and investigated over nearly 2 decades, its exact clinical characteristics and underlying mechanisms have yet to be fully determined [84]. In addition, opioid tolerance should be differentiated from OIH, although both have a similar clinical presentation with regard to change in pain intensity [85]. Clinically, OIH should be considered when the adjustment of opioid dose is contemplated if prior opioid dose escalation fails to provide the expected analgesic effect and there is unexplainable pain exacerbation following an initial period of effective opioid analgesia. Although in some cases increasing opioid dose leads to some improvement in pain management, in other cases less opioid may be more effective in pain reduction. This goal may be accomplished by initiating a trial of opioid tapering, opioid rotation, adding adjunctive medications, or combining opioid with a clinically available NMDA receptor antagonist. Continuing opioid therapy with endless dose escalation in the absence of clinical evidence of improved pain management is neither scientifically sound nor clinically justified.
Corresponding author: Lucy L. Chen, MD, MGH Center for Pain Medicine, WACC #340, 15 Parkman St., Boston, MA 02114.
Financial disclosures: None.
1. Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: Roles of excitatory amino acid receptors and protein kinase C. J Neurosci 1994;14:2301–12.
2. Celerier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology 2000;92:465–72.
3. Laulin JP, Maurette P, Corcuff JB, et al. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg 2002;94:1263–9.
4. Lian B, Vera-Portocarrero L, King T, et al. Opioid-induced latent sensitization in a model of non-inflammatory viscerosomatic hypersensitivity. Brain Res 2010;1358:64–70.
5. Hay JL, Kaboutari J, White JM, et al. Model of methadone-induced hyperalgesia in rats and effect of memantine. Eur J Pharmacol 2010;626:229–33.
6. Wala EP, Holtman JR Jr. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol. 2011;651:89–95.
7. Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: Implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 2002;22:8312–23.
8. Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: Evidence for an opioid-induced neurotoxic mechanism. J Neurosci 2002;22:7650–61.
9. Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep 2006;10:67–70.
10. Woolf CJ. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res 1981;209:491–5.
11. Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: Clinical and theoretic implications. Anesthesiology 1986;64:590–7.
12. Vanderah TW, Ossipov MH, Lai J, et al. Mechanisms of opioid-induced pain and antinociceptive tolerance: Descending facilitation and spinal dynorphin. Pain 2001;92:5–9.
13. Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology 2008;109:308–17.
14. Ghelardini C, Galeotti N, Vivoli E, et al. Molecular interaction in the mouse PAG between NMDA and opioid receptors in morphine-induced acute thermal nociception. J Neurochem 2008;105:91–100.
15. Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci 2010;30:38–46.
16. Van Elstraete AC, Sitbon P, Mazoit JX, et al. Protective effect of prior administration of magnesium on delayed hyperalgesia induced by fentanyl in rats. Can J Anaesth 2006;53:1180–5.
17. Gu X, Wu X, Liu Y, et al. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain 2009;5:76.
18. Minville V, Fourcade O, Girolami JP, Tack I. Opioid-induced hyperalgesia in a mice model of orthopaedic pain: Preventive effect of ketamine. Br J Anaesth 2010;104:231–8.
19. Cui W, Li Y, Li S, et al. Systemic lidocaine inhibits remifentanil-induced hyperalgesia via the inhibition of cPKCgamma membrane translocation in spinal dorsal horn of rats. J Neurosurg Anesthesiol 2009;21:318–25.
20. Gupta LK, Gupta R, Tripathi CD. N-methyl-D-aspartate receptor modulators block hyperalgesia induced by acute low-dose morphine. Clin Exp Pharmacol Physiol 2011;38:592–7.
21. Bianchi E, Galeotti N, Menicacci C, Ghelardini C. Contribution of G inhibitory protein alpha subunits in paradoxical hyperalgesia elicited by exceedingly low doses of morphine in mice. Life Sci 2011;89:918–25.
22. Ruiz-Medina J, Ledent C, Valverde O. GPR3 orphan receptor is involved in neuropathic pain after peripheral nerve injury and regulates morphine-induced antinociception. Neuropharmacology 2011;61:43–50.
23. Bianchi E, Norcini M, Smrcka A, Ghelardini C. Supraspinal gbetagamma-dependent stimulation of PLCbeta originating from G inhibitory protein-mu opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. J Neurochem 2009;111:171–80.
24. Liang DY, Li X, Clark JD. 5-hydroxytryptamine type 3 receptor modulates opioid-induced hyperalgesia and tolerance in mice. Anesthesiology 2011;114:1180–9.
25. Rivat C, Vera-Portocarrero LP, Ibrahim MM, et al. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci 2009;29:727–37.
26. Vera-Portocarrero LP, Zhang ET, King T, et al. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain 2007;129:35–45.
27. Simonin F, Schmitt M, Laulin JP, et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci U S A 2006;103:466–71.
28. Celerier E, Gonzalez JR, Maldonado R, et al. Opioid-induced hyperalgesia in a murine model of postoperative pain: Role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology 2006;104:546–55.
29. Vardanyan A, Wang R, Vanderah TW, et al. TRPV1 receptor in expression of opioid-induced hyperalgesia. J Pain 2009;10:243–52.
30. Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci 2010;30:4460–6.
31. Esmaeili-Mahani S, Shimokawa N, Javan M, et al. Low-dose morphine induces hyperalgesia through activation of G alphas, protein kinase C, and L-type ca 2+ channels in rats. J Neurosci Res 2008;86:471–9.
32. Esmaeili-Mahani S, Fereidoni M, Javan M, et al. Nifedipine suppresses morphine-induced thermal hyperalgesia: Evidence for the role of corticosterone. Eur J Pharmacol 2007;567:95–101.
33. Van Elstraete AC, Sitbon P, Mazoit JX, Benhamou D. Gabapentin prevents delayed and long-lasting hyperalgesia induced by fentanyl in rats. Anesthesiology 2008;108:484–94.
34. Van Elstraete AC, Sitbon P, Benhamou D, Mazoit JX. The median effective dose of ketamine and gabapentin in opioid-induced hyperalgesia in rats: An isobolographic analysis of their interaction. Anesth Analg 2011;113:634–40.
35. Wei X, Wei W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J Anesth 2012;26:236–41.
36. Bessiere B, Richebe P, Laboureyras E, Laulin JP, Contarino A, Simonnet G. Nitrous oxide (N2O) prevents latent pain sensitization and long-term anxiety-like behavior in pain and opioid-experienced rats. Neuropharmacology 2007;53:733–40.
37. Hamlin AS, McNally GP, Osborne PB. Induction of c-fos and zif268 in the nociceptive amygdala parallel abstinence hyperalgesia in rats briefly exposed to morphine. Neuropharmacology 2007;53:330–43.
38. Doyle T, Bryant L, Muscoli C, et al. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett 2010;483:85–9.
39. Liang DY, Liao G, Wang J, et al. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology 2006;104:1054–62.
40. Liang DY, Liao G, Lighthall GK, Peltz G, Clark DJ. Genetic variants of the P-glycoprotein gene Abcb1b modulate opioid-induced hyperalgesia, tolerance and dependence. Pharmacogenet Genomics 2006;16:825–35.
41. Wilson NM, Jung H, Ripsch MS, et al. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun 2011;25:565–73.
42. Terashvili M, Wu HE, Schwasinger E, Tseng LF. Paradoxical hyperalgesia induced by mu-opioid receptor agonist endomorphin-2, but not endomorphin-1, microinjected into the centromedial amygdala of the rat. Eur J Pharmacol 2007;554:137–44.
43. Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience 2007;147:439–44.
44. Waxman AR, Arout C, Caldwell M, et al. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett 2009;462:68–72.
45. Juni A, Cai M, Stankova M, et al. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology 2010;112:181–8.
46. Juni A, Klein G, Kowalczyk B, et al. Sex differences in hyperalgesia during morphine infusion: Effect of gonadectomy and estrogen treatment. Neuropharmacology 2008;54:1264–70.
47. Forero M, Chan PS, Restrepo-Garces CE. Successful reversal of hyperalgesia/myoclonus complex with low-dose ketamine infusion. Pain Pract 2012;12:154–8.
48. Vorobeychik Y, Chen L, Bush MC, Mao J. Improved opioid analgesic effect following opioid dose reduction. Pain Med 2008;9:724–7.
49. Cortinas Saenz M, Geronimo Pardo M, Cortinas Saenz ML, et al. Acute opiate tolerance and postoperative hyperalgesia after a brief infusion of remifentanil managed with multimodal analgesia. Rev Esp Anestesiol Reanim 2008;55:40–2.
50. Siniscalchi A, Piraccini E, Miklosova Z, et al. Opioid-induced hyperalgesia and rapid opioid detoxification after tacrolimus administration. Anesth Analg 2008;106:645–6.
51. Okon TR, George ML. Fentanyl-induced neurotoxicity and paradoxic pain. J Pain Symptom Manage 2008;35:327–33.
52. Axelrod DJ, Reville B. Using methadone to treat opioid-induced hyperalgesia and refractory pain. J Opioid Manag 2007;3:113–4.
53. Singler B, Troster A, Manering N, et al. Modulation of remifentanil-induced postinfusion hyperalgesia by propofol. Anesth Analg 2007;104:1397–403.
54. Hallett BR, Chalkiadis GA. Suspected opioid-induced hyperalgesia in an infant. Br J Anaesth 2012;108:116–8.
55. Ma JF, Huang ZL, Li J, et al. Cohort study of remifentanil-induced hyperalgesia in postoperative patients. Zhonghua Yi Xue Za Zhi 2011;91:977–9.
56. Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend 2006;82:218–23.
57. Fishbain DA, Lewis JE, Gao J. Are psychoactive substance (opioid)-dependent chronic pain patients hyperalgesic? Pain Pract 2011;11:337–43.
58. Hay JL, White JM, Bochner F, et al. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain 2009;10:316–22.
59. Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J Pain 2006;7:43–8.
60. Chu LF, Dairmont J, Zamora AK, et al. The endogenous opioid system is not involved in modulation of opioid-induced hyperalgesia. J Pain 2011;12:108–15.
61. McDonnell C, Zaarour C, Hull R, et al. Pre-treatment with morphine does not prevent the development of remifentanil-induced hyperalgesia. Can J Anaesth 2008;55:813–8.
62. Echevarria G, Elgueta F, Fierro C, et al. Nitrous oxide (N(2)O) reduces postoperative opioid-induced hyperalgesia after remifentanil-propofol anaesthesia in humans. Br J Anaesth 2011;107:959–65.
63. Troster A, Sittl R, Singler B, et al. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology 2006;105:1016–23.
64. Tuncer S, Yalcin N, Reisli R, Alper Y. The effects of lornoxicam in preventing remifentanil-induced postoperative hyperalgesia. Agri 2009;2:161–7.
65. Lee C, Song YK, Jeong HM, Park SN. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J Anesthesiol 2011;61:244–50.
66. Song JW, Lee YW, Yoon KB, et al. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg 2011;113:390–7.
67. Lee C, Song YK, Lee JH, Ha SM. The effects of intraoperative adenosine infusion on acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil in adult patients undergoing tonsillectomy. Korean J Pain 2011;24:7–12.
68. Hong BH, Lee WY, Kim YH, et al. Effects of intraoperative low dose ketamine on remifentanil-induced hyperalgesia in gynecologic surgery with sevoflurane anesthesia. Korean J Anesthesiol 2011;61:238–43.
69. Xuerong Y, Yuguang H, Xia J, Hailan W. Ketamine and lornoxicam for preventing a fentanyl-induced increase in postoperative morphine requirement. Anesth Analg 2008;107:2032–7.
70. Schlimp CJ, Pipam W, Wolrab C, Ohner C, Kager HI, Likar R. Clonidine for remifentanil-induced hyperalgesia: A double-blind randomized, placebo-controlled study of clonidine under intra-operative use of remifentanil in elective surgery of the shoulder. Schmerz 2011;25:290–5.
71. Belgrade M, Hall S. Dexmedetomidine infusion for the management of opioid-induced hyperalgesia. Pain Med 2010;11:1819–26.
72. Chen L, Malarick C, Seefeld L, et al. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain 2009;143:65–70.
73. Bannister K, Dickenson AH. Opioid hyperalgesia. Curr Opin Support Palliat Care 2010;4:1–5.
74. Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain 2008;139:431–8.
75. Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: Effect of long-acting maintenance agent. Drug Alcohol Depend 2001;63:139–46.
76. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg 1998;86:1307–11.
77. Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain 1995;62:259–74.
78. Sjogren P, Jensen NH, Jensen TS. Disappearance of morphine-induced hyperalgesia after discontinuing or substituting morphine with other opioid agonists. Pain 1994;59:313–6.
79. Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: Intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology 2000;93:409–17.
80. Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 2006;104:570–87.
81. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag 2006;2:277–82
82. Mao J. Opioid-induced abnormal pain sensitivity: Implications in clinical opioid therapy. Pain 2002;100:213–7.
83. Low Y, Clarke CF, Huh BK. Opioid-induced hyperalgesia: A review of epidemiology, mechanisms and management. Singapore Med J 2012;53:357–60.
84. Fishbain DA, Cole B, Lewis JE, et al. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med 2009:829–39.
85. Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain 2012;153:1583–92.
1. Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: Roles of excitatory amino acid receptors and protein kinase C. J Neurosci 1994;14:2301–12.
2. Celerier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology 2000;92:465–72.
3. Laulin JP, Maurette P, Corcuff JB, et al. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg 2002;94:1263–9.
4. Lian B, Vera-Portocarrero L, King T, et al. Opioid-induced latent sensitization in a model of non-inflammatory viscerosomatic hypersensitivity. Brain Res 2010;1358:64–70.
5. Hay JL, Kaboutari J, White JM, et al. Model of methadone-induced hyperalgesia in rats and effect of memantine. Eur J Pharmacol 2010;626:229–33.
6. Wala EP, Holtman JR Jr. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol. 2011;651:89–95.
7. Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: Implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 2002;22:8312–23.
8. Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: Evidence for an opioid-induced neurotoxic mechanism. J Neurosci 2002;22:7650–61.
9. Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep 2006;10:67–70.
10. Woolf CJ. Intrathecal high dose morphine produces hyperalgesia in the rat. Brain Res 1981;209:491–5.
11. Yaksh TL, Harty GJ, Onofrio BM. High dose of spinal morphine produce a nonopiate receptor-mediated hyperesthesia: Clinical and theoretic implications. Anesthesiology 1986;64:590–7.
12. Vanderah TW, Ossipov MH, Lai J, et al. Mechanisms of opioid-induced pain and antinociceptive tolerance: Descending facilitation and spinal dynorphin. Pain 2001;92:5–9.
13. Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology 2008;109:308–17.
14. Ghelardini C, Galeotti N, Vivoli E, et al. Molecular interaction in the mouse PAG between NMDA and opioid receptors in morphine-induced acute thermal nociception. J Neurochem 2008;105:91–100.
15. Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci 2010;30:38–46.
16. Van Elstraete AC, Sitbon P, Mazoit JX, et al. Protective effect of prior administration of magnesium on delayed hyperalgesia induced by fentanyl in rats. Can J Anaesth 2006;53:1180–5.
17. Gu X, Wu X, Liu Y, et al. Tyrosine phosphorylation of the N-methyl-D-aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Mol Pain 2009;5:76.
18. Minville V, Fourcade O, Girolami JP, Tack I. Opioid-induced hyperalgesia in a mice model of orthopaedic pain: Preventive effect of ketamine. Br J Anaesth 2010;104:231–8.
19. Cui W, Li Y, Li S, et al. Systemic lidocaine inhibits remifentanil-induced hyperalgesia via the inhibition of cPKCgamma membrane translocation in spinal dorsal horn of rats. J Neurosurg Anesthesiol 2009;21:318–25.
20. Gupta LK, Gupta R, Tripathi CD. N-methyl-D-aspartate receptor modulators block hyperalgesia induced by acute low-dose morphine. Clin Exp Pharmacol Physiol 2011;38:592–7.
21. Bianchi E, Galeotti N, Menicacci C, Ghelardini C. Contribution of G inhibitory protein alpha subunits in paradoxical hyperalgesia elicited by exceedingly low doses of morphine in mice. Life Sci 2011;89:918–25.
22. Ruiz-Medina J, Ledent C, Valverde O. GPR3 orphan receptor is involved in neuropathic pain after peripheral nerve injury and regulates morphine-induced antinociception. Neuropharmacology 2011;61:43–50.
23. Bianchi E, Norcini M, Smrcka A, Ghelardini C. Supraspinal gbetagamma-dependent stimulation of PLCbeta originating from G inhibitory protein-mu opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. J Neurochem 2009;111:171–80.
24. Liang DY, Li X, Clark JD. 5-hydroxytryptamine type 3 receptor modulates opioid-induced hyperalgesia and tolerance in mice. Anesthesiology 2011;114:1180–9.
25. Rivat C, Vera-Portocarrero LP, Ibrahim MM, et al. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci 2009;29:727–37.
26. Vera-Portocarrero LP, Zhang ET, King T, et al. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain 2007;129:35–45.
27. Simonin F, Schmitt M, Laulin JP, et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc Natl Acad Sci U S A 2006;103:466–71.
28. Celerier E, Gonzalez JR, Maldonado R, et al. Opioid-induced hyperalgesia in a murine model of postoperative pain: Role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology 2006;104:546–55.
29. Vardanyan A, Wang R, Vanderah TW, et al. TRPV1 receptor in expression of opioid-induced hyperalgesia. J Pain 2009;10:243–52.
30. Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci 2010;30:4460–6.
31. Esmaeili-Mahani S, Shimokawa N, Javan M, et al. Low-dose morphine induces hyperalgesia through activation of G alphas, protein kinase C, and L-type ca 2+ channels in rats. J Neurosci Res 2008;86:471–9.
32. Esmaeili-Mahani S, Fereidoni M, Javan M, et al. Nifedipine suppresses morphine-induced thermal hyperalgesia: Evidence for the role of corticosterone. Eur J Pharmacol 2007;567:95–101.
33. Van Elstraete AC, Sitbon P, Mazoit JX, Benhamou D. Gabapentin prevents delayed and long-lasting hyperalgesia induced by fentanyl in rats. Anesthesiology 2008;108:484–94.
34. Van Elstraete AC, Sitbon P, Benhamou D, Mazoit JX. The median effective dose of ketamine and gabapentin in opioid-induced hyperalgesia in rats: An isobolographic analysis of their interaction. Anesth Analg 2011;113:634–40.
35. Wei X, Wei W. Role of gabapentin in preventing fentanyl- and morphine-withdrawal-induced hyperalgesia in rats. J Anesth 2012;26:236–41.
36. Bessiere B, Richebe P, Laboureyras E, Laulin JP, Contarino A, Simonnet G. Nitrous oxide (N2O) prevents latent pain sensitization and long-term anxiety-like behavior in pain and opioid-experienced rats. Neuropharmacology 2007;53:733–40.
37. Hamlin AS, McNally GP, Osborne PB. Induction of c-fos and zif268 in the nociceptive amygdala parallel abstinence hyperalgesia in rats briefly exposed to morphine. Neuropharmacology 2007;53:330–43.
38. Doyle T, Bryant L, Muscoli C, et al. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett 2010;483:85–9.
39. Liang DY, Liao G, Wang J, et al. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology 2006;104:1054–62.
40. Liang DY, Liao G, Lighthall GK, Peltz G, Clark DJ. Genetic variants of the P-glycoprotein gene Abcb1b modulate opioid-induced hyperalgesia, tolerance and dependence. Pharmacogenet Genomics 2006;16:825–35.
41. Wilson NM, Jung H, Ripsch MS, et al. CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun 2011;25:565–73.
42. Terashvili M, Wu HE, Schwasinger E, Tseng LF. Paradoxical hyperalgesia induced by mu-opioid receptor agonist endomorphin-2, but not endomorphin-1, microinjected into the centromedial amygdala of the rat. Eur J Pharmacol 2007;554:137–44.
43. Juni A, Klein G, Pintar JE, Kest B. Nociception increases during opioid infusion in opioid receptor triple knock-out mice. Neuroscience 2007;147:439–44.
44. Waxman AR, Arout C, Caldwell M, et al. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci Lett 2009;462:68–72.
45. Juni A, Cai M, Stankova M, et al. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology 2010;112:181–8.
46. Juni A, Klein G, Kowalczyk B, et al. Sex differences in hyperalgesia during morphine infusion: Effect of gonadectomy and estrogen treatment. Neuropharmacology 2008;54:1264–70.
47. Forero M, Chan PS, Restrepo-Garces CE. Successful reversal of hyperalgesia/myoclonus complex with low-dose ketamine infusion. Pain Pract 2012;12:154–8.
48. Vorobeychik Y, Chen L, Bush MC, Mao J. Improved opioid analgesic effect following opioid dose reduction. Pain Med 2008;9:724–7.
49. Cortinas Saenz M, Geronimo Pardo M, Cortinas Saenz ML, et al. Acute opiate tolerance and postoperative hyperalgesia after a brief infusion of remifentanil managed with multimodal analgesia. Rev Esp Anestesiol Reanim 2008;55:40–2.
50. Siniscalchi A, Piraccini E, Miklosova Z, et al. Opioid-induced hyperalgesia and rapid opioid detoxification after tacrolimus administration. Anesth Analg 2008;106:645–6.
51. Okon TR, George ML. Fentanyl-induced neurotoxicity and paradoxic pain. J Pain Symptom Manage 2008;35:327–33.
52. Axelrod DJ, Reville B. Using methadone to treat opioid-induced hyperalgesia and refractory pain. J Opioid Manag 2007;3:113–4.
53. Singler B, Troster A, Manering N, et al. Modulation of remifentanil-induced postinfusion hyperalgesia by propofol. Anesth Analg 2007;104:1397–403.
54. Hallett BR, Chalkiadis GA. Suspected opioid-induced hyperalgesia in an infant. Br J Anaesth 2012;108:116–8.
55. Ma JF, Huang ZL, Li J, et al. Cohort study of remifentanil-induced hyperalgesia in postoperative patients. Zhonghua Yi Xue Za Zhi 2011;91:977–9.
56. Pud D, Cohen D, Lawental E, Eisenberg E. Opioids and abnormal pain perception: New evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend 2006;82:218–23.
57. Fishbain DA, Lewis JE, Gao J. Are psychoactive substance (opioid)-dependent chronic pain patients hyperalgesic? Pain Pract 2011;11:337–43.
58. Hay JL, White JM, Bochner F, et al. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain 2009;10:316–22.
59. Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J Pain 2006;7:43–8.
60. Chu LF, Dairmont J, Zamora AK, et al. The endogenous opioid system is not involved in modulation of opioid-induced hyperalgesia. J Pain 2011;12:108–15.
61. McDonnell C, Zaarour C, Hull R, et al. Pre-treatment with morphine does not prevent the development of remifentanil-induced hyperalgesia. Can J Anaesth 2008;55:813–8.
62. Echevarria G, Elgueta F, Fierro C, et al. Nitrous oxide (N(2)O) reduces postoperative opioid-induced hyperalgesia after remifentanil-propofol anaesthesia in humans. Br J Anaesth 2011;107:959–65.
63. Troster A, Sittl R, Singler B, et al. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology 2006;105:1016–23.
64. Tuncer S, Yalcin N, Reisli R, Alper Y. The effects of lornoxicam in preventing remifentanil-induced postoperative hyperalgesia. Agri 2009;2:161–7.
65. Lee C, Song YK, Jeong HM, Park SN. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J Anesthesiol 2011;61:244–50.
66. Song JW, Lee YW, Yoon KB, et al. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg 2011;113:390–7.
67. Lee C, Song YK, Lee JH, Ha SM. The effects of intraoperative adenosine infusion on acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil in adult patients undergoing tonsillectomy. Korean J Pain 2011;24:7–12.
68. Hong BH, Lee WY, Kim YH, et al. Effects of intraoperative low dose ketamine on remifentanil-induced hyperalgesia in gynecologic surgery with sevoflurane anesthesia. Korean J Anesthesiol 2011;61:238–43.
69. Xuerong Y, Yuguang H, Xia J, Hailan W. Ketamine and lornoxicam for preventing a fentanyl-induced increase in postoperative morphine requirement. Anesth Analg 2008;107:2032–7.
70. Schlimp CJ, Pipam W, Wolrab C, Ohner C, Kager HI, Likar R. Clonidine for remifentanil-induced hyperalgesia: A double-blind randomized, placebo-controlled study of clonidine under intra-operative use of remifentanil in elective surgery of the shoulder. Schmerz 2011;25:290–5.
71. Belgrade M, Hall S. Dexmedetomidine infusion for the management of opioid-induced hyperalgesia. Pain Med 2010;11:1819–26.
72. Chen L, Malarick C, Seefeld L, et al. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain 2009;143:65–70.
73. Bannister K, Dickenson AH. Opioid hyperalgesia. Curr Opin Support Palliat Care 2010;4:1–5.
74. Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain 2008;139:431–8.
75. Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: Effect of long-acting maintenance agent. Drug Alcohol Depend 2001;63:139–46.
76. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg 1998;86:1307–11.
77. Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain 1995;62:259–74.
78. Sjogren P, Jensen NH, Jensen TS. Disappearance of morphine-induced hyperalgesia after discontinuing or substituting morphine with other opioid agonists. Pain 1994;59:313–6.
79. Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: Intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology 2000;93:409–17.
80. Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 2006;104:570–87.
81. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag 2006;2:277–82
82. Mao J. Opioid-induced abnormal pain sensitivity: Implications in clinical opioid therapy. Pain 2002;100:213–7.
83. Low Y, Clarke CF, Huh BK. Opioid-induced hyperalgesia: A review of epidemiology, mechanisms and management. Singapore Med J 2012;53:357–60.
84. Fishbain DA, Cole B, Lewis JE, et al. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med 2009:829–39.
85. Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain 2012;153:1583–92.